UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1-A
Tier
1 offering
Offering
Statement UNDER THE SECURITIES ACT OF 1933 CURRENT REPORT
LifeQuest
World Corp.
(Exact name of registrant as specified in its
charter)
Date: February __, 2024
| Minnesota |
4950 |
88-0407679 |
|
(State or Other Jurisdiction
of Incorporation) |
(Primary Standard Classification Code) |
(IRS Employer
Identification No.)
|
100 Challenger Road, 8th Floor
Ridgefield Park, NJ 07660
Phone: 646-201-5242
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
CT Corporation System Inc.
100 S 5th Str #1075
Minneapolis, MN 55402
Phone: 562-986-8043
(Name, address, including zip code, and telephone
number,
including area code, of agent for service)
THIS OFFERING STATEMENT SHALL ONLY BE QUALIFIED UPON ORDER OF THE
COMMISSION, UNLESS A SUBSEQUENT AMENDMENT IS FILED INDICATING THE INTENTION TO BECOME QUALIFIED BY OPERATION OF THE TERMS OF REGULATION
A.
PART I - NOTIFICATION
Part I should be read in conjunction with the
attached XML Document for Items 1-6
PART I - END
Please send copies of all correspondence to:
The Doney Law Firm
4955 S. Durango Rd. Ste. 165
Las Vegas, NV 89113
(702) 982-5686
PRELIMINARY OFFERING CIRCULAR DATED FEBRUARY ____,
2024
An offering statement pursuant to Regulation A relating to
these securities has been filed with the U.S. Securities and Exchange Commission, which we refer to as the Commission. Information
contained in this Preliminary Offering Circular is subject to completion or amendment. These securities may not be sold nor may
offers to buy be accepted before the offering statement filed with the Commission is qualified. This Preliminary Offering Circular shall
not constitute an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in
which such offer, solicitation or sale would be unlawful before registration or qualification under the laws of any such state. We may
elect to satisfy our obligation to deliver a Final Offering Circular by sending you a notice within two business days after the completion
of our sale to you that contains the URL where the Final Offering Circular or the offering statement in which such Final Offering Circular
was filed may be obtained.
LifeQuest
World Corp.
13,000,000 SHARES OF COMMON STOCK
$0.001 PAR VALUE PER SHARE
In this public offering of 13,000,000 shares of our common stock, we are
offering 10,000,000 shares of our common stock and the selling shareholders are offering 3,000,000 shares of our common stock underlying
warrants. We will not receive any of the proceeds from the sale of shares by the selling shareholders unless the warrants are exercised.
This offering is being conducted on a “best efforts” basis, which means that there is no guarantee that any minimum amount
will be sold.
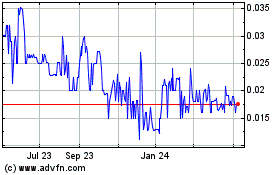
Our Common Stock trades in the OTCMarket Pink Open Market under the symbol
LQWC. There is currently no active trading market for our securities. There is no assurance that a regular trading market will develop,
or if developed, that it will be sustained. Therefore, shareholders may be unable to resell their securities in our company.
All of the shares being registered for sale by us will be sold at a fixed
price, which will be within a range of $0.05 to $0.10 per share, established at qualification for the duration of the offering. There
is no minimum amount we are required to raise from the shares being offered by the Company. There is no minimum amount we are required
to raise from the shares being offered by the Company. There are no arrangements to place the funds received in an escrow, trust, or similar
arrangement and the funds will be available to us following deposit into the Company’s bank account. There is no guarantee that
we will sell any of the securities being offered in this offering. Additionally, there is no guarantee that this offering will successfully
raise enough funds to institute our company’s business plan.
Resale shares may be sold to or through underwriters or dealers, directly
to purchasers or through agents designated from time to time by the selling shareholders. For additional information regarding the methods
of sale, you should refer to the section entitled “Plan of Distribution” in this offering. There is no minimum number of shares
required to be purchased by each investor.
This primary offering will terminate upon the earliest of (i) such time
as all of the common stock has been sold pursuant to the Offering Statement or (ii) 365 days
from the qualified date of this offering circular, unless extended by our directors for an additional 90 days. We may, however, at any
time and for any reason terminate the offering.
| SHARES
OFFERED BY COMPANY |
|
PRICE
TO PUBLIC(1) |
|
SELLING
AGENT COMMISSIONS |
|
PROCEEDS
TO THE COMPANY(2) |
| Per Share |
|
$ |
0.10 |
|
|
$ |
Not applicable |
|
$ |
0.10 |
| Minimum Purchase |
|
|
None |
|
|
|
Not applicable |
|
|
Not applicable |
| Total (10,000,000 shares) |
|
$ |
1,000,000 |
|
|
$ |
Not applicable |
|
$ |
1,000,000 |
| |
(1) |
Price range of offering being estimated
pursuant to Rule 253(b). Estimate includes a maximum offering price of $0.10 and a maximum number of shares offered in this offering
of 10,000,000 shares for an estimated maximum aggregate offering of $1,000,000. |
| |
(2) |
Does not include expenses of the offering, estimated
to be $65,000 including legal, accounting and other costs of registration. |
| SHARES
OFFERED SELLING SHAREHOLDERS |
|
PRICE
TO PUBLIC(1) |
|
SELLING
AGENT COMMISSIONS |
|
PROCEEDS
TO THE SELLING SHAREHOLDERS |
| Per
Share |
|
$ |
0.10 |
|
Not
applicable |
|
$ |
0.10 |
| Minimum
Purchase |
|
None |
|
Not
applicable |
|
Not
applicable |
| Total
(3,000,000 shares) |
|
$ |
300,000 |
|
Not
applicable |
|
$ |
300,000 |
| |
(1) |
Price range of offering being estimated
pursuant to Rule 253(b). Estimate includes a maximum offering price of $0.10 and a maximum number of shares offered in this
offering of 3,000,000 shares for an estimated maximum aggregate offering of $300,000. |
We
expect to commence the offer and sale of the shares of common stock being offered pursuant to this offering circular within two
calendar days of the qualification date, but only after filing a supplement to include the offering price per share within the range
presented.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES
COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE OFFERING CIRCULAR. ANY REPRESENTATION
TO THE CONTRARY IS A CRIMINAL OFFENSE.
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE
PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS
AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU
TO REVIEW RULE 251(D)(2)(I)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO WWW.INVESTOR.GOV.
THESE SECURITIES ARE SPECULATIVE AND INVOLVE A HIGH DEGREE OF RISK.
YOU SHOULD PURCHASE SHARES ONLY IF YOU CAN AFFORD THE COMPLETE LOSS OF YOUR INVESTMENT. PLEASE REFER TO ‘RISK FACTORS’ BEGINNING
ON PAGE 14.
The date of this offering circular is February ____, 2024
The following table of contents has been designed to help you find important
information contained in this offering circular. We encourage you to read the entire offering circular.
TABLE OF CONTENTS
PART II – OFFERING CIRCULAR
You should rely only on the information contained in this offering
circular or contained in any free writing offering circular filed with the Securities and Exchange Commission. We have not authorized
anyone to provide you with additional information or information different from that contained in this offering circular filed with the
Securities and Exchange Commission. We take no responsibility for, and can provide no assurance as to the reliability of, any other information
that others may give you. We are offering to sell, and seeking offers to buy, our common stock only in jurisdictions where offers and
sales are permitted. The information contained in this offering circular is accurate only as of the date of this offering circular, regardless
of the time of delivery of this offering circular or any sale of shares of our common stock. Our business, financial condition, results
of operations and prospects may have changed since that date.
PART - II
offering
circular SUMMARY
This summary highlights information contained elsewhere
in this Offering Circular and does not contain all of the information that you should consider in making your investment decision. Before
investing in our common stock, you should carefully read this entire Offering Circular, including our consolidated financial statements
and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion
and Analysis of Financial Condition and Results of Operations,” in each case included elsewhere in this Offering Circular. Unless
otherwise stated, all references to “us,” “our,” “we,” the “Company” and similar designations
refer to LifeQuest World Corp and its wholly owned subsidiary, BioPipe Global Corp.
This offering circular, and any supplement to this offering circular include
“forward-looking statements.” To the extent that the information presented in this offering circular discusses financial projections,
information or expectations about our business plans, results of operations, products or markets, or otherwise makes statements about
future events, such statements are forward-looking. Such forward-looking statements can be identified by the use of words such as “intends”,
“anticipates”, “believes”, “estimates”, “projects”, “forecasts”, “expects”,
“plans” and “proposes”. Although we believe that the expectations reflected in these forward-looking statements
are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially
from such forward-looking statements. These include, among others, the cautionary statements in the “Risk Factors” section
and the “Management’s Discussion and Analysis of Financial Position and Results of Operations” section in this offering
circular.
This summary only highlights selected information contained in greater
detail elsewhere in this offering circular. This summary may not contain all of the information that you should consider before investing
in our common stock. You should carefully read the entire offering circular, including “Risk Factors” beginning on Page 14,
and the financial statements, before making an investment decision.
Generally, no sale may be made to you in this offering if the aggregate
purchase price you pay is more than 10% of the greater of your annual income or net worth. Different rules apply to accredited investors
and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, we encourage you
to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
Sale of these shares will commence within two calendar days of the qualification
date and it will be a continuous offering pursuant to Rule 251(d)(3)(i)(F).
The Company
LifeQuest World Corp., through its our wholly owned subsidiary, BioPipe
Global Corp., is a wastewater treatment company that treats domestic sewage (wastewater from toilets, kitchens, showers, laundry, etc.)
into clean water ready for secondary purposes like flushing of toilets or irrigation, without producing sludge.
On April 17, 2019, we entered into an Agreement and Plan of Merger (the
“Merger Agreement”) with BioPipe Acquisition, Inc., a New Jersey corporation (“Merger Sub”) and BioPipe Global
Corp., a privately held New Jersey corporation (“BioPipe Global”). In connection with the closing of this merger transaction,
Merger Sub merged with and into BioPipe Global (the “Merger”) on April 30, 2019, with the filing of Articles of Merger with
the New Jersey Secretary of State.
In addition, pursuant to the terms and conditions of the Merger Agreement:
| |
• |
All of the outstanding shares of BioPipe Global was
exchanged for the right to receive an aggregate of 75,000,000 share of the Company’s common stock, par value $0.001 per share,
which was issued to certain shareholders in connection with an Intellectual Property Purchase Agreement set forth below; |
| |
• |
BioPipe Global provided customary representations and
warranties and closing conditions, including approval of the Merger by a majority of its voting shareholders; and |
| |
• |
Bradford Brock, our prior officer and director, was
required to cancel 55,000,000 shares of his Common Stock in the Company but permitted to retain 1,000,000 shares in the Company.
|
From the date of the Merger Agreement, we have been engaged in eco-friendly
decentralized wastewater treatment. The Company’s mission is to become a singular platform for highly efficient, scalable, low footprint
onsite treatment technologies for treatment sewage wastewater and industrial wastewater. Our flagship product is Biopipe STP, (sewage
wastewater treatment), which is a highly scalable and biological sewage wastewater system that treats domestic sewage into clean water
ready for secondary purposes.
On May 7, 2019, under an Intellectual Property Purchase Agreement,
BioPipe Global acquired all the assets of BioPipe Global AG, a Swiss company, and its wholly-owned Turkish subsidiary, BioPipe Cevre Teknolojileri
A.S. We acquired all trade receivables, income, royalties, damages, rights to sue, rights to enforce and any and all payments unpaid and
due now or hereafter due or payable with respect to the patented BioPipe System.
In the last five years, the BioPipe STP system has been installed
in Bangladesh, India, Ethiopia, the Philippines, South Africa, Turkey, UAE, Qatar, Saudi Arabia, Oman, and Maldives. These BioPipe systems
are running successfully at hospitals, resorts, hotels, commercial and government buildings, labor camps, ports and individual homes.
On
September 26, 2019, the Company entered into a 50-50 joint venture and technology transfer agreement with South Africa based, Abrimix
Pty Ltd. The purpose of the agreement was to introduce our Biopipe STP into southern Africa and eventually introduce Abrimix in the countries
we operate in. Abrimix is a patented, low footprint, scalable industrial wastewater treatment technology with the ability to treat a
wide variety of industrial wastewater. Due to disruptions related to Covid-19 and a lack of funding, the parties to the joint venture
dissolved the venture and, on May 30, 2022 , a technology license agreement was put in place which includes royalty payment
of 7.5% of Net Revenue.
Traditional centralized wastewater treatment systems are expensive
to install and operate, energy-intensive and are dependent on chemicals. The world is seeking sustainable solutions through decentralized
wastewater treatment which “get back to nature” while using 21st century technologies and management. Reuse of Biopipe treated
water may include irrigation of gardens and agricultural fields or replenishing surface water and groundwater. Reused water may also be
directed toward fulfilling certain needs in residences (e.g. toilet flushing), businesses and industry, and where necessary, treated to
reach potable standards.
The circular water economy as a business model provides sustainable production
and consumption of treated water and recovery of resources. Recycled water can meet some of this need, benefited by the nutrient content
inherent in wastewater. Only mid-range treatment levels would be required, as irrigation can be accomplished while minimizing the potential
for human contact with the recycled water. Reusing wastewater as part of sustainable water management allows water to remain as an alternative
water source for human activities. This can reduce scarcity and alleviate pressures on groundwater and other natural water bodies.
Circular Economy of Water
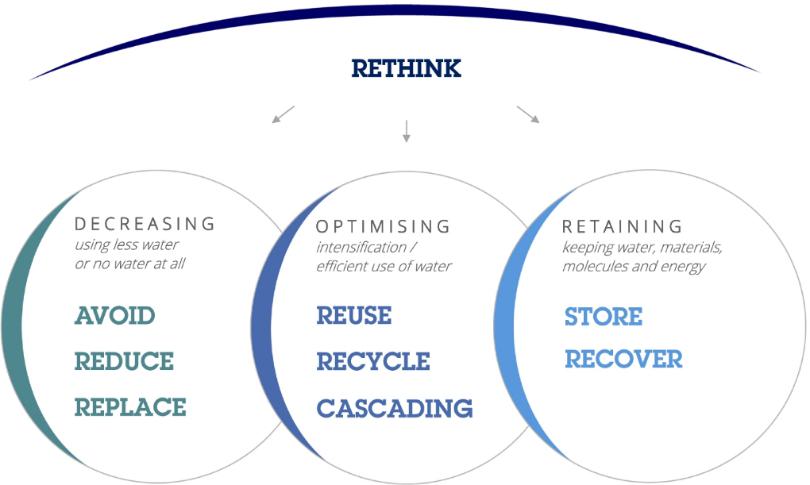
Lifequest is seeking to build a platform of disruptive decentralized
wastewater treatment systems (DEWATS) that are highly efficient, scalable, cost effective with small footprint. Our flagship biological
treatment system, Biopipe STP, can be adapted to treat all types of wastewaters, but our current focus is on domestic wastewater. Our
Abrimix process through our technology partnership is a versatile primary treatment for all types of industrial wastewater with a small
footprint and is superior to the nearest Dissolve Air Floatation technology. The treated water from our Biopipe system can be reused for
irrigation, exterior washing, irrigation and re-flushing of toilets. Both solutions offer a pathway to zero liquid discharge solutions.
Reclaiming water from waste for reuse applications (instead of using
freshwater supplies) can be a water-saving measure. When treated waste water is eventually discharged back into natural water sources,
it still has significant benefits to the ecosystem, such as improving streamflow, nourishing plant life and recharging aquifers, as part
of the natural water cycle. With the number of water-stressed countries rising, treatment and reuse of wastewater is becoming increasingly
necessary.
BioPipe is a revolutionary sewage wastewater treatment system.
Biopipe is a highly scalable, eco-friendly and extremely cost-effective wastewater treatment with a broad installed base. It is the
planet’s first biological wastewater treatment system where the process takes place entirely inside the pipe and:
| | ☐ | | |
100% biological system with NO
chemicals used |
| | ☐ | | |
There is absolutely no sludge produced and
no odor |
| | ☐ | | |
Lower energy consumption versus competing systems |
| | ☐ | | |
Very low operation and maintenance cost |
| | ☐ | | |
Virtually silent |
| | ☐ | | |
Modular design |
| | ☐ | | |
Low footprint |
| | ☐ | | |
Meets or exceeds discharge standards globally |
Biopipe currently has around 44 plants installed around the globe,
and we expect significant number of projects in India and Ethiopia and limited numbers in South Africa and the Philippines. Some of these
plants were installed prior to the merger agreement. We have now set up joint ventures, licensing and distribution in five countries.
Abrimix is an industrial effluent treatment technology.
It is an innovative technology that applies “shear” to increase the kinetics and completeness of reactions within aqueous
solutions. The Abrimix Technology can be applied to various influents where certain reactions are desired to achieve the dissolution,
oxidation, and/or precipitation of certain elements, the phase separation of ultra-fine suspended solids and the breaking of emulsions
within a liquid. Abrimix (Pty) Ltd. has several plants running in South Africa and Biopipe’s subsidiary in India has done two plants
in India to date under the joint venture.
The key component to the Abrimix Technology is the powerful static in-line
mixer or “Reaction Enhancing Unit.” This patented unit improves the speed and thoroughness of liquid and gaseous chemical
interactions within aqueous solutions. The unit, most commonly referred to as a High Shear Reactor (HSR), utilizes a specifically designed
and researched hydrodynamic flow path that is derived from proven and sound fluid engineering mechanics.
Glanris Media is a green hybrid media made from rice hull and is
extremely effective in removing heavy metals, color/turbidity, chlorine and chloramine, suspended solids and colloids, gas and oil, solvents
and low molecular weight organics, as well as odors. The media can be regenerated with a weak acid. Glanris media is produced by Glanris
Corp and the Company is a distributor in India.
Our Strategy
Our initial focus is on countries that have high water stress
and a large gap between production of sewage and industrial wastewater versus installed capacity or simply lag adequate wastewater treatment
infrastructure.
Our core strategy is to introduce Biopipe and Abrimix through joint
ventures, license agreements partners or distribution partners with strategic and well-established partners in the top 10 countries with
sewage problems. The JV partner, license partner or distribution partner handles sales marketing, installation, operations, and maintenance.
This includes India, Bangladesh, the Philippines, Ethiopia, and South Africa.
Our Market
Packaged Treatment
Our decentralized wastewater treatment falls into the category of packaged
wastewater technology. According to Ken Research estimates, the Global Water and Wastewater Treatment Equipment Market is valued around
~US$ 50 billion by 2022 and it is further expected to reach a market size of ~US$ 75 billion opportunity by 2028. The key drivers are
(a) rising population and rapid urbanization. According to The World Bank Group, the urban population has seen a rise from 55% of the
world population in 2018 to 57% of the world population in 2021, as many people from rural area shift to the urban region for better employment
opportunities.
Biological Treatment
According to a report published by Research Dive, the global biological
wastewater treatment market is anticipated to reach $15 billion and rising at a CAGR of 5.2% through 2031. Our Biopipe biological treatment
technology, currently being applied to domestic sewage wastewater, can be applied to many other types of wastewaters. Abrimix wastewater
treatment technology has the ability to treat a wide variety of industrial wastewaters.
Countries We Operate In:
India
Biopipe Global has a 99% owned subsidiary in Biopipe India Private
Ltd. for our operations in India.
India possesses 4% of the world’s water resources and is home
to 17.7% of the world’s population and ranks 13th for overall water stress. India is also recognized as one of the world’s
“very highly water-stressed countries” on the Aqueduct’s Water Risk Atlas, ranking 13th. (World Resources Institute,
2019).
Water stress has developed in most places due to the imbalance between
local supplies and demand from people, agriculture, and industry—in 26 of India’s 32 major cities, 10%–60% of total
water demand remains unmet. Two facts exacerbate the water scarcity: (i) most of India’s water sources are highly polluted; and
(ii) very little wastewater is actually treated and reused, making water the other single-use resource wreaking havoc on the climate with
its pollution.
It has also made it mandatory by certain state pollution control
boards for apartments and commercial complexes to install STPs. India generates 72,368 million liters (MLD) of sewage every day, only
28% is treated and reused. A new report by the Council on Energy, Environment and Water (CEEW), states that 80% of wastewater generated
by urban India has the potential to be treated and reused for non-potable purposes like irrigation, which can relieve the immense pressure
on water bodies, lower pollution levels and provide water security in the face of climate crisis-induced weather events that can render
water bodies unreliable. The Indian water and wastewater treatment market is on a dynamic trajectory, projected to rise from USD 1.31
billion in 2020 to an impressive USD 2.08 billion by 2025, showcasing a compelling CAGR of 9.7%.
Bangladesh
We currently have nine Biopipe STPs running in Bangladesh and we
are in the process of introducing our Abrimix industrial wastewater solution under our license agreement.
Bangladesh is an important market for our solutions. According to Bangladesh
Institute of Planners, capital Dhaka along dumps 1.16 million m3 of sewage per day into the local rivers. According to www.water.org,
out of its population of 165 million people, 68 million people (41% of the population) lack access to a reliable, safely managed source
of water, and 100 million people (61%) lack access to safely managed household sanitation facilities. https://bdnews24.com/environment/2019/05/25/dhaka-pumps-1.1-million-cubic-metres-of-sewage-into-rivers-daily-study-says
According to official records of the Bangladesh Inland Water Transport
Authority (BIWTA), around 350,000 kilograms (350 metric tons) of toxic waste is dumped into rivers every day from about 7,000 industries
and other residential areas in greater Dhaka and adjacent areas. Bangladesh annually discharges 1.03 billion tons of textile wastewater
without treatment. The government has implemented a Zero Liquid Discharge (ZLD) policy to address industrial wastewater discharge. Under
this policy, industries are required to treat and recycle their wastewater to ensure zero discharge into water bodies.
South Africa
We currently have one Biopipe sewage treatment plant in South Africa
under the 50-50 joint venture. Abrimix Pty Ltd. has eleven of its own industrial wastewater treatment plants in South Africa. The Company
teamed with Abrimix to introduce Biopipe in South Africa and Abrimix’s technology in the countries we currently operate in. The
agreement is for 50-50 distribution of profits.
South Africa is another water stressed country with massive sewage
problem. More than half of the sewage treatment installed capacity is not working and close to 50,000 liters of sewage is being
dumped every second - https://mg.co.za/article/2017-07-21-south-africas-shit-has-hit-the-fan . The local governments are asking
developers to have their own decentralized sewage wastewater systems before any development is approved. This creates what we
believe is a highly scalable Build Own & Operate (BOO) or leasing opportunity whereby we will provide the system and charge the
customers for the amount of water reused. BOO or Leasing is expected to produce a long term stable cashflow.
South Africa is heavily dependent on centralized wastewater treatment
plants and most are not functioning well or not meeting the standards. A total of 334 (39%) of municipal wastewater systems were identified
to be in a critical state in 2021, compared to 248 (29%) in 2013. A total of 102 (89%) out of the 115 DPW systems were identified in critical
state, compared to 84% in 2013.
South Africa represents a huge opportunity for decentralized wastewater
systems or packaged treatment plants like Biopipe. There was only one packaged treatment plant installed by the government sector. However,
due to budget constraints, as well as Covid, we have not been able to penetrate the market in South Africa as we had planned. Also, we
have experienced operated plant issues with Morgan Beef and we have to upgrade the plant to produce better quality effluent. For South
Africa, we expect business activity to pick up in the second half of fiscal year 2024.

Source: Green Drop 2022
Capex Advantage
Biopipe has considerable advantages over other packaged or decentralized
wastewater treatment technologies when it comes to operating cost. It currently costs circa $2,550,000 per 1000m3 for a centralized
system which involves $1,481,000 per 1000m3 capex on reticulated sewerage, circa $162,000 per 1000m3 for main sewer
lines and $891,890 per 1000m3 on conventional treatment plant. Biopipe offers considerably lower capex.
Opex Advantage
The average cost to treat 1 m3 of wastewater is circa
$1/m3 and median cost is $0.48/m3, with the latter giving a more representative estimate of treatment cost. Biopipe
does not use energy hogging blowers for aeration, dosing of chemicals and minimal maintenance.
Source: Green Drop 2022
Ethiopia
On
October 1, 2019, we entered into an Exclusive Distribution Agreement with Tesurcon Trading PLC .
Under the agreement, we have been working in Ethiopia for the past 3 years to develop the market for our Biopipe system. The exercise
started with a shipment of a demo plant in 2021 to the Ministry of Water & Irrigation. Due to political uncertainty and longer government
approval process, we were able to finally install and commission the demo plant at the Ministry of Water & Irrigation facility in
2023. Since then, we have concluded one commercial sale and currently have a robust pipeline of projects.
Ethiopia is considered water-stressed because the rapid population growth
over the last decade has put a strain on its abundant water sources. Despite estimations showing that 13.5 to 28 billion cubic meters
of renewable annual groundwater is available per year, only 2.6 billion cubic meters is usable. According to the UNICEF/WHO Joint Monitoring
Program, only half of the population in Ethiopia has access to basic water services, while less than 10 percent of the population can
access basic sanitation services. Access to basic WASH services is substantially lower among rural populations than urban. As of 2020,
only 40 percent of Ethiopia’s rural population could access basic water, compared with 84 percent in urban areas. Rural and urban
access to at least basic sanitation reflects a similarly large gap with only five percent of the rural population having access compared
to 21 percent of the urban. Since 78 percent (93.6 million people) of Ethiopia’s total population resides in rural areas, overcoming
these access gaps is key to Ethiopia’s national development. On the other hand, rapid urban population growth is estimated to be
nearly five percent per year. This puts pressure on the need to maintain and expand water and sanitation infrastructure in Ethiopia’s
cities and small towns.
Sanitation and water deficit remains a problem. According to a 2021 Joint
Monitoring Program, only 7 percent of the households in Ethiopia have an individual toilet where fecal waste ends up safely disposed of
and treated in situ or offsite.
The Philippines
We have a joint venture with Bpipe Corporation on a 40-60 basis.
The Philippines
has the two factors that cause us to operate there (a) water deficit and (b) sanitation deficit. According to UNICEF, around 50.3 million
Filipinos (around 10 million families) do not have access to safely managed sanitation services and of these some 24 million use limited/unimproved
toilets or none at all. The main sources of water pollution include:
|
• |
untreated domestic
(also called ‘municipal’) wastewater discharges (33%); |
|
• |
industrial sources (27%); |
| |
• |
agriculture and livestock (29%); |
| |
• |
and non-point sources such as agricultural farms (11%). |
The pollution of Philippine waters comes for a significant part from untreated
domestic and industrial wastewater. Only about five percent of households are connected to sewerage networks and treatment facilities.
In Metro Manila alone, approximately 2,000 cubic meters of solvent wastes, 22,000 tons of heavy metals, infectious wastes, biological
sludge, lubricants, and intractable wastes, as well as 25 million cubic meters of acid/alkaline liquid wastes are improperly disposed
of annually.
Our principal place of business is located at
100 Challenger Road, 8th Floor Ridgefield Park, NJ 07660. General information about us can be found at http://biopipe.co/. The information
contained on or connected to our website is not incorporated by reference into this Offering Circular on Form 1-A and should not be considered
part of this or any other report filed with the SEC.
Risks Affecting Us
Our business will be subject to numerous risks and uncertainties, including
those described in “Risk Factors” immediately following this offering circular summary and elsewhere in this offering circular.
These risks represent challenges to the successful implementation of our strategy and to the growth and future profitability of our business.
These risks include, but are not limited to, the following:
| |
• |
Company has failed to generate profits; |
| |
• |
Disruption due to Covid-19 disrupted our
business development and resulted is loss of sales pipeline; |
| |
• |
Significant competition; |
| |
• |
The business risks of international operations,
particularly in emerging markets; |
| |
• |
Geopolitical situation in countries we
operate in; |
| |
• |
Risk of not getting paid by customers; |
| |
• |
Inability to finance fulfilment of orders; |
| |
• |
failure of manufacturers to deliver products
or provide services in a cost effective and timely manner; |
| |
• |
Risk of exposure of our intellectual property
and confidential information; |
| |
• |
Risk associated with water treatment industry; |
| |
• |
Failure of Build Own Operate plants; |
| |
• |
Risk of repatriation of profits from overseas
operations; and |
| |
• |
we are not a 12g reporting company. |
The Offering
| Securities being offered by the Company |
10,000,000 shares of common stock, at a fixed price of $____ offered by us on a “best efforts” basis, which means that there is no guarantee that any minimum amount will be sold, through our officers and directors. |
| |
|
| Securities being offered by the Selling Stockholders |
3,000,000 shares of common stock underlying warrants, at a fixed price of $____ offered by selling stockholder in a resale offering. This fixed price applies at all times for the duration of the offering. |
| |
|
| Offering price per share |
We and the selling shareholder will sell the shares at a fixed price per share of $___ for the duration of this Offering. |
| |
|
| Number of shares of common stock outstanding before the offering of common stock |
120,984,150 common shares are currently issued and outstanding. |
| |
|
| Number of shares of common stock outstanding after the offering of common stock |
130,984,150 common shares will be issued and outstanding if we sell all of the shares we are offering herein. |
| |
|
|
The minimum number of shares to be
sold in this offering |
None |
| |
|
| Use of Proceeds |
We intend to use the gross proceeds to us for working capital and for other corporate purposes. |
| |
|
| Termination of the Offering |
The offering will terminate upon the earliest of (i) such time as all of the common stock has been sold pursuant to the Offering Statement or (ii) 365 days from the qualified date of this offering circular, unless extended by our Board of Directors for an additional 90 days. We may however, at any time and for any reason terminate the offering. |
| |
|
| Subscriptions |
All subscriptions once accepted by us are irrevocable. |
| |
|
| Registration
Costs |
We estimate our total offering registration costs and selling expenses
to be approximately $65,000. |
| |
|
| Risk Factors |
See “Risk Factors” and the other information in this offering circular for a discussion of the factors you should consider before deciding to invest in shares of our common stock. |
You should rely only upon the information contained in this offering circular.
We have not authorized anyone to provide you with information different from that which is contained in this offering circular. We are
offering to sell common stock and seeking offers to common stock only in jurisdictions where offers and sales are permitted.
MANAGEMENT’S DISCUSSION AND ANALYSIS
You should read the following discussion and analysis
of our financial condition and results of our operations together with our financial statements and related notes appearing at the end
of this Offering Circular. This discussion contains forward-looking statements reflecting our current expectations that involve risks
and uncertainties. Actual results and the timing of events may differ materially from those contained in these forward-looking statements
due to a number of factors, including those discussed in the section entitled "Risk Factors" and elsewhere in this Offering
Circular.
Results of Operations for the Years Ended May 31,
2022 and 2023
Revenues
Our total revenue reported for the year ended May
31, 2023, was $149,806, compared with $349,297 for the year ended May 31, 2022.
Our revenues fiscal year ended 2023 revenue declined
largely due to delays in a major government tender that was pushed out into fiscal year 2024, lack of sales activity in Bangladesh due
to shortage of hard currency and delays in getting our demo plant commissioned on time. We could not generate any revenue from our South
African operated plant due to issues with Morgan Beef and need for upgrading the plant to produce better quality effluent. The key issues
involved generation of wastewater at twice the capacity of the designed plant, high variability in incoming wastewater, management turnover
at Morgan Beef and suspension of its export license due to outbreak of foot and mouth disease. Additionally, the client would like the
water to be treated to point where the treated water can be used for washing the inside of the abattoir. Unless we experience continued
setbacks, such as those described above, we expect business activity to pick up in the second half of fiscal year 2024 after the plant
upgrade is completed. We have selected an engineering procurement and construction (EPC) company to provide the secondary and tertiary
treatments to meet the client’s quality and volume needs. Our Biopipe sewage treatment technology was selected as the technology
of choice in a municipal tender involving twenty-one plants. We are in the middle of final negotiations of the terms with the general
contractor who has won the tender. All plants have to delivered within the next twelve months. Additionally, we have entered into an agreement
with a channel partner in India which has already secured one contract and upon successful commissioning of the 200 m3/day
plant, expect additional orders of two 400 m3/day plants. We are also seeing strong activity in Ethiopia where Biopipe has
been approved by the government. We shipped our first commercial plant after successfully demonstrating the efficiency of Biopipe system
at Ministry of Water and Irrigation.
Cost of Revenues
Our total cost of revenues for the year ended May
31, 2023, was to $111,936 compared with $258,336 for the year ended May 31, 2022. Our gross margins were relatively stable year over year.
Operating Expenses
Operating expenses declined to $307,324 for the year
ended May 31, 2023, from $378,564 for the year ended May 31, 2022. The decrease in operating expenses is largely the result of a decline
in professional fees and wages.
We expect our operating expenses will increase in
future quarters with the expenses for professional fees in connection with this offering, as well as increased operational activity.
Other Expenses/Other Income
We had other expenses of $41,959 for the year ended May 31, 2023, as compared
with other expenses of $24,641 for the year ended 2022. We booked an expense on the Bangladesh joint venture, which was dissolved and
our relationship with restructured into a licensing agreement. We also booked an expense based on fluctuations in foreign currency rates.
Each foreign entity maintains the books in the foreign currency and then translates them to USD for the financial statements, which results
in fluctuations throughout the year.
Net Loss
We finished the year ended May 31, 2023, with a loss of $379,714, as compared
to a net loss of $217,338 during the year ended May 31, 2022.
Results
of Operations for the Six Months Ended November 30, 2023 and 2022
Revenues
Our total revenue reported for the six months
ended November 30, 2023, was $42,251, compared with $45,591 for the six months ended November 30, 2022. The decrease in revenues is the
result of delays in delivery and commissioning of two new plants. Additionally, a tender in which our Biopipe STP was selected as the
system of choice was delayed and was opened in April of 2023.
Cost of Revenues
Our total cost of revenues for the six months
ended November 30, 2023, was to $23,464 compared with $6,441 for the six months ended November 30, 2022. The increase in cost of revenues
is the result of purchases of high sheer reactors from Abrimix Pty Ltd.
Operating Expenses
Operating expenses declined to $100,730 for the
six months ended November 30, 2023, from $150,374 for the six months ended November 30, 2022. The decrease in operating expenses is the
result of decline in SG&A.
We expect our operating expenses will increase in
future quarters with the expenses for professional fees in connection with this offering, as well as increased operational activity.
Other Expenses/Other Income
We had other income of $79 for the six months ended November 30, 2023,
as compared with other expenses of $88,570 for the six months ended November 30, 2022. The other expenses in 2022 were the result of
loss of $92,227 from dissolution of the joint venture in Bangladesh.
Net Loss
We finished the six months ended November 30, 2023, with a loss of $89,725,
as compared to a net loss of $261,393 during the six months ended November 30, 2022.
Liquidity and Capital Resources
As of November 30, 2023, we had total current assets of $345,591 and current
liabilities of $101,349, resulting in working capital of $244,242 as of November 20, 2023, as compared with working capital of $228,480
at May 31, 2023.
Our operating activities used $259,141 in cash for the year ended May 31,
2023, as compared with cash used of $242,929 from operating activities in the year ended May 31, 2022. Our negative operating cash flow
for 2023 was mainly the result of changes in accounts receivable, net operating loss and net loss allocated to noncontrolling interest.
Our operating activities used $52,865 in cash for the six months ended
November 30, 2023, as compared with cash used of $143,009 from operating activities in the six months ended November 30, 2022. Our negative
operating cash flow for 2023 was mainly the result of our net loss for the period, offset by adjustments to operating activities.
Our investing activities provided $38,679 for the year ended May 31, 2023,
as compared with net cash used of $397,847 for the year ended May 31, 2022. Our positive investing cash flow in 2023 is the result of
changes to the basis of purchased fixed assets from foreign currency.
Our investing activities used $750 for the six months ended November 30,
2023, as compared with net cash provided of $38,849 for the six months ended November 30, 2022. Our negative investing cash flow in 2023
is the result of changes in the fixed asset basis from foreign currency.
Financing activities provided $11,500 in cash for the six months ended
November 30, 2023, compared with $12,942 in cash provided in the six months ended November 30, 2022. Our positive financing cash flow
was mainly the result of sale of common sure pursuant to Reg D.
Based upon our current financial condition, we have sufficient capital
to operate our business for the next twelve months at the current levels, but we lack funds for our expansion efforts. We are
seeking financing to upgrade our operating plant at Morgan Beef Pty Ltd. in South Africa, to finance government tenders and work orders
received from Eagle Infrastructure Ltd. where we must supply 21 Biopipe sewage wastewater treatment plants in India and to ramp up the
marketing of our products. We intend to fund these expansion efforts through this offering, or through increased sales and debt and/or
equity financing arrangements, which may be insufficient to fund expenditures or other cash requirements. There can be no assurance that
we will be successful in raising additional funding. If we are not able to secure additional funding, the implementation of our business
plan will be impaired. There can be no assurance that such additional financing will be available to us on acceptable terms or at all.
Inflation
Although our operations are influenced by general economic conditions,
we do not believe that inflation had a material effect on our results of operations during the years ended May 31, 2023 and 2022 or for
the six months ended November 30, 2023.
Critical Accounting Polices
In December 2001, the SEC requested that all registrants list their most
“critical accounting polices” in the Management Discussion and Analysis. The SEC indicated that a “critical accounting
policy” is one which is both important to the portrayal of a company’s financial condition and results, and requires management’s
most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are
inherently uncertain. Our critical accounting policies are disclosed in Note 2 of our unaudited consolidated financial statements included
in this Offering Circular with the Securities and Exchange Commission.
Off Balance Sheet Arrangements
As of November 30, 2023, there were no off-balance sheet arrangements.
Recent Accounting Pronouncements
The recent accounting pronouncements that are material to our financial
statements are disclosed in Note 2 of our consolidated unaudited yearend financial statements included in this Offering Circular filed
with the Securities and Exchange Commission and in Note 2 of our unaudited consolidated financial statements included herein.
RISK FACTORS
Please consider the following risk factors and other information in this
offering circular relating to our business before deciding to invest in our common stock.
This offering and any investment in our common stock involves a high degree
of risk. You should carefully consider the risks described below and all of the information contained in this offering circular before
deciding whether to purchase our common stock. If any of the following risks actually occur, our business, financial condition and results
of operations could be harmed. The trading price of our common stock could decline due to any of these risks, and you may lose all or
part of your investment.
We consider the following to be the material risks for an investor regarding
this offering. Our company should be viewed as a high-risk investment and speculative in nature. An investment in our common stock may
result in a complete loss of the invested amount.
An investment in our common stock is highly speculative, and should only
be made by persons who can afford to lose their entire investment in us. You should carefully consider the following risk factors and
other information in this report before deciding to become a holder of our common stock. If any of the following risks actually occur,
our business and financial results could be negatively affected to a significant extent.
Risk Factors Related to Our Financial Condition
We have limited cash on hand and there is substantial doubt as to
our ability to continue as a going concern.
As at November 30, 2023, we had cash on hand of $303,531 and we had working
capital of $244,242. While this is enough to operate at present levels for the next 12 months, our current capital resources will limit
our ability to make capital investments in Build Own & Operate or Leased projects. We also expect to continue to incur significant
operating and capital expenditures for the next twelve months as we ramp our marketing and funding of purchase orders, government tenders,
upgrading of Morgan Beef Build Own & Operate plant, and other initiatives. We also expect to experience negative cash flow in the
foreseeable future as we fund our operating losses and capital expenditures. As a result, we will need to generate significant revenues
in order to achieve and thereafter maintain profitability. As of November 30, 2023, we have an accumulated deficit of $1,401,511and we
may not be able to generate sufficient revenues or achieve profitability in the future. Our failure to generate significant revenues,
achieve or maintain profitability could negatively impact the value of our Common Stock.
Risk Factors Related to Our Business
We have a limited operating history upon which investors can evaluate
our future prospects.
Our operating subsidiary, BioPipe Global Corp., was incorporated in the
State of New Jersey on April 23, 2019. Therefore, we have limited operating history upon which an evaluation of our business plan or performance
and prospects can be made. The business and prospects of the Company must be considered in the light of the potential problems, delays,
uncertainties and complications encountered in connection with a newly established business. To successfully introduce and market our
products and services at a profit, we must establish brand name recognition and competitive advantages for our products. There are no
assurances that the Company can successfully address these challenges. If it is unsuccessful, the Company and its business, financial
condition and operating results could be materially and adversely affected.
Given the limited operating history, management has little basis on which
to forecast future demand for our products and services from our existing customer base, much less new customers. We are depended on our
in-country joint venture, license and distribution partners. It is difficult to accurately forecast future revenues because the business
of the Company is new and its market has not been developed. If the forecasts for the Company prove incorrect, the business, operating
results and financial condition of the Company will be materially and adversely affected. Moreover, the Company may be unable to adjust
its spending in a timely manner to compensate for any unanticipated reduction in revenue. As a result, any significant reduction in revenues
would immediately and adversely affect the business, financial condition and operating results of the Company.
We are dependent on our in-country Joint Venture partners, Licensors
and Distributors
Our success depends on the ability of our joint venture, license and distribution
partners to effectively market, install and support our wastewater treatment systems. Acquisitions, strategic investments, partnerships,
or alliances could be difficult to identify, pose integration challenges, divert the attention of management, disrupt our business, dilute
stockholder value, and adversely affect our business, financial condition, and results of operations.
We have in the past and may in the future seek to obtain joint venture,
license, and distribution partners that we believe could complement or expand product offerings, enhance our technology, or otherwise
offer growth opportunities.
Any such transaction may divert the attention of management and cause us
to incur various expenses in identifying, investigating, and pursuing suitable opportunities, whether or not the transactions are completed,
and may result in unforeseen operating difficulties and expenditures. Any such transactions that we are able to complete may not result
in synergies or other benefits we expect to achieve, which could result in substantial impairment charges. These transactions could also
result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our results of operations.
We may not be able to compete successfully with current and future
competitors.
We have many potential competitors in the wastewater equipment and services
industry. We will compete, in our current and proposed businesses, with other companies, most of which have far greater marketing and
financial resources and experience than we do. We cannot guarantee that we will be able to penetrate our intended market and be able to
compete profitably, if at all.
If we do not continually upgrade our technology, it may become obsolete
and we may not be able to compete with other companies.
We cannot assure you that we will be able to keep pace with advances in
technology for wastewater treatment or that our services will not become obsolete. We cannot assure you that competitors will not develop
related or similar wastewater treatment systems.
Defects in our products or failures in quality control could impair
our ability to sell our products or could result in product liability claims, litigation and other significant events involving substantial
costs.
Detection of any significant defects in our products or failure in our
quality control procedures may result in, among other things, delay in time-to-market, loss of sales and market acceptance of our products,
diversion of development resources, and injury to our reputation. The costs we may incur in correcting any product defects may be substantial.
Additionally, errors, defects or other performance problems could result in financial or other damages to our customers, which could result
in litigation. Product liability litigation, even if we prevail, would be time consuming and costly to defend, and if we do not prevail,
could result in the imposition of a damages award. Our offshore operating entities carry general liability insurance. The company uses
off-the-shelf components which our covered by manufacturer warranties.
There can be no assurances of protection for proprietary rights or
reliance on trade secrets.
The ownership and protection of the Company’s trademarks, patents,
trade secrets and intellectual property rights are significant aspects of its future success. Unauthorized parties may attempt to replicate
or otherwise obtain and use the Company’s products and technology. Policing the unauthorized use of current or future trademarks,
patents, trade secrets or intellectual property rights could be difficult, expensive, time-consuming and unpredictable, as may be enforcing
these rights against unauthorized use by others. Identifying unauthorized use of intellectual property rights is difficult as the Company
may be unable to effectively monitor and evaluate the intellectual property used by its competitors, including parties such as unlicensed
dispensaries, and the processes used to produce such products. In addition, in any infringement proceeding, some or all of the trademarks,
patents or other intellectual property rights or other proprietary know-how, or arrangements or agreements seeking to protect the same
may be found invalid, unenforceable, anti-competitive or not infringed. An adverse result in any litigation or defense proceedings could
put one or more of the trademarks, patents or other intellectual property rights at risk of being invalidated or interpreted narrowly
and could put existing intellectual property applications at risk of not being issued. Any or all of these events could materially and
adversely affect the Company’s business, financial condition and results of operations.
In addition, other parties may claim that the Company’s products
infringe on their proprietary and perhaps patent protected rights. Such claims, whether or not meritorious, may result in the expenditure
of significant financial and managerial resources, legal fees, result in injunctions, temporary restraining orders and/or require the
payment of damages.
We may not be able to manage our growth effectively.
We must continually implement and improve our products and/or services,
operations, operating procedures and quality controls on a timely basis, as well as expand, train, motivate and manage our work force
in order to accommodate anticipated growth and compete effectively in our market segment. Successful implementation of our strategy also
requires that we establish and manage a competent, dedicated work force and employ additional key employees in corporate management, product
development, client service and sales. We can give no assurance that our personnel, systems, procedures and controls will be adequate
to support our existing and future operations. If we fail to implement and improve these operations, there could be a material, adverse
effect on our business, operating results and financial condition.
Risks associated with operating abroad may negatively affect our
ability to implement our business plan.
The Company’s expansion into jurisdictions outside of the United
States is subject to risks. In addition, in jurisdictions outside of the United States, there can be no assurance that any market for
the Company’s products or services will develop or be maintained. The Company may face new or unexpected risks or significantly
increase its exposure to one or more existing risks, including economic instability, changes in laws and regulations, and the effects
of competition. These factors may limit the Company’s ability to successfully expand its operations into such jurisdictions and
may have a material adverse effect on the Company’s business, financial condition and results of operations.
There might be unanticipated obstacles to the execution of our business
plan.
The Company’s business plans may change significantly. The Company’s
Build Own & Operate (BOO), municipal projects requiring bank guarantees and purchase order financing are capital intensive. Management
believes that the Company’s chosen activities and strategies are achievable in light of current water stress and wastewater problem
around the world.
Failure to comply with local laws and regulations.
We may incur substantial costs on an ongoing basis to comply with all laws
and regulations. New or stricter laws and/or regulations could increase our costs and make us less competitive.
Wastewater operations entail significant risks and may impose significant
costs.
Wastewater treatment systems fail or do not operate properly, or if there
is a spill, untreated or partially treated wastewater could discharge onto property or into nearby streams and rivers, causing various
damages and injuries, including environmental damage. Liabilities resulting from such damages and injuries could harm our business,
financial condition, and results of operations.
Significant or prolonged disruptions in the supply of important goods
or services from third parties could harm our business, financial condition, and results of operations.
We are dependent on procuring all our components from third parties and
any deterioration in quality or disruption or prolonged delays in obtaining important supplies such as water pipe, valves, pumps, control
panels, or other materials, could harm our ability to deliver and commission plants on time.
Risks Related to Insiders and Control Persons
Insiders will continue to have substantial control over us and our
policies after this offering and will be able to influence corporate matters.
Enes Kutluca, our COO and director, owns 20,802,342 shares of our common
stock representing 20.93% of our outstanding shares. Mr. Kutluca also has an option to purchase 10% of our outstanding common stock. The
option is exercisable at market price and subject to vesting. Max Khan, our CEO and director, has the same option as Mr. Kutluca. These
options commence, as stated in their respective employment agreements, upon the company adopting a stock option plan, which has not yet
occurred and the board of directors has stated that it does not expect to adopt a plan until second half of 2024. Fifty percent (50%)
of the options shall vest on the date a stock option plan is adopted and the remaining fifty percent (50%) of the options shall vest and
become exercisable on the first anniversary of the date a stock option plan is adopted by the company.
Once the stock option plan is adopted, these shareholders will have the
right to acquire approximately 20% of our outstanding common stock. Even after the offering, these officers and shareholders, whose interests
may differ from other stockholders, have the ability to exercise significant control over us. They are able to exercise significant influence
over all matters requiring approval by our stockholders, including the election of directors, the approval of significant corporate transactions,
and any change of control of our company. They could prevent transactions, which would be in the best interests of the other shareholders.
Their interests may not necessarily be in the best interests of the shareholders in general.
We may engage in transactions that present conflicts of interest.
The Company’s officers and directors may enter into agreements with
the Company from time to time which may not be equivalent to similar transactions entered into with an independent third party. A conflict
of interest arises whenever a person has an interest on both sides of a transaction. While we believe that it will take prudent steps
to ensure that all transactions between the Company and any officer or director are fair, reasonable, and no more than the amount it would
otherwise pay to a third party in an “arms’-length” transaction, there can be no assurance that any transaction will
meet these requirements in every instance.
We have agreed to indemnify our officers and directors against lawsuits
to the fullest extent of the law.
The Company is a Minnesota corporation. Minnesota law permits the indemnification
of officers and directors against expenses incurred in successfully defending against a claim. Minnesota law also authorizes corporations
to indemnify their officers and directors against expenses and liabilities incurred because of their being or having been an officer or
director. Our organizational documents provide for this indemnification to the fullest extent permitted by law.
We currently do not maintain any insurance coverage. In the event that
we are found liable for damage or other losses, we would incur substantial and protracted losses in paying any such claims or judgments.
Although we intend to acquire such coverage immediately upon resources becoming available, there is no guarantee that we can secure such
coverage or that any insurance coverage would protect us from any damages or loss claims filed against us.
We depend significantly on the services of the members of our management
team, and the departure of any of those persons could cause our operating results to suffer.
Our success depends significantly on the continued individual and collective
contributions of our management team. The loss of the services of any member of our management team or the inability to hire
and retain experienced management personnel could harm our business, financial condition, and results of operations.
Members of our Board and our executive officers may have other business
interests and obligations to other entities.
Neither our directors nor our executive officers will be required to manage
our company as their sole and exclusive function and they may have other business interests and may engage in other activities in addition
to those relating to our company, provided that such activities do not compete with the business of our company. We are dependent on our
directors and executive officers to successfully operate our company, and in particular Max Khan and Mehmet Enes Kutluca. Their other
business interests and activities could divert time and attention from operating our business.
Risks Related to the Offering and the Market for our Stock
Because there is no minimum offering amount, funds raised may not
be sufficient to complete the plans of the Company as set forth in “Use of Proceeds” in this Offering Circular.
There is no minimum offering amount. If we do not raise the maximum proceeds,
funds raised may not be sufficient to complete all plans of the Company as set forth in “Use of Proceeds” in this Offering
Circular, which could inhibit our ability to commence to generate revenue.
We have the right to issue shares of preferred stock. If we were
to issue preferred stock, it is likely to have rights, preferences and privileges that may adversely affect the common stock.
We are authorized to issue 50,000,000 shares of “blank check”
preferred stock, with such rights, preferences and privileges as may be determined from time-to-time by our board of directors. Our board
of directors is empowered, without stockholder approval, to issue preferred stock in one or more series, and to fix for any series the
dividend rights, dissolution or liquidation preferences, redemption prices, conversion rights, voting rights, and other rights, preferences
and privileges for the preferred stock.
The issuance of shares of preferred stock, depending on the rights, preferences
and privileges attributable to the preferred stock, could reduce the voting rights and powers of the common stock and the portion of our
assets allocated for distribution to common stockholders in a liquidation event, and could also result in dilution in the book value per
share of the common stock we are offering. The preferred stock could also be utilized, under certain circumstances, as a method for raising
additional capital or discouraging, delaying or preventing a change in control of the Company, to the detriment of the investors in the
common stock offered hereby.
New investors in our common stock will experience immediate and substantial
dilution after this Offering.
If you purchase shares of common stock in this Offering, you will experience
immediate dilution, because the price that you pay will be substantially greater than the adjusted pro forma net tangible book value per
share that you acquire. This dilution is due in large part to our negative book value.
If a market for our common stock does not develop, shareholders may
be unable to sell their shares.
Our common stock is quoted under the symbol “LQWC” on the OTCPink
operated by OTC Markets Group, Inc., an electronic inter-dealer quotation medium for equity securities. We do not currently have an active
trading market. There can be no assurance that an active and liquid trading market will develop or, if developed, that it will be sustained.
Our securities are very thinly traded. Accordingly, it may be difficult
to sell shares of our common stock without significantly depressing the value of the stock. Unless we are successful in developing continued
investor interest in our stock, sales of our stock could continue to result in major fluctuations in the price of the stock.
The market price of our common stock is likely to be highly volatile
and could fluctuate widely in price in response to various factors, many of which are beyond our control.
Our stock price is subject to a number of factors, including:
| |
• |
Technological innovations or new products and services
by us or our competitors; |
| |
• |
Government regulation of our products and services; |
| |
• |
The establishment of partnerships with other environmental
companies; |
| |
• |
Intellectual property disputes; |
| |
• |
Additions or departures of key personnel; |
| |
• |
Issuances of our common stock; |
| |
• |
Our ability to execute our business plan; |
| |
• |
Operating results below or exceeding expectations; |
| |
• |
Financial condition of our joint venture partners; |
| |
• |
Whether we achieve profits or not; |
| |
• |
Loss or addition of any strategic relationship; |
| |
• |
Economic and other external factors; and |
| |
• |
Period-to-period fluctuations in our financial results. |
Our stock price may fluctuate widely as a result of any of the above. In
addition, the securities markets have from time-to-time experienced significant price and volume fluctuations that are unrelated to the
operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of
our common stock.
Because we are subject to the “Penny Stock” rules, the
level of trading activity in our stock may be reduced.
The Securities and Exchange Commission has adopted regulations which generally
define "penny stock" to be any listed, trading equity security that has a market price less than $5.00 per share or an exercise
price of less than $5.00 per share, subject to certain exemptions. The penny stock rules require a broker-dealer, prior to a transaction
in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about
penny stocks and the risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations
for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing
the market value of each penny stock held in the customer’s account. In addition, the penny stock rules generally require that prior
to a transaction in a penny stock, the broker-dealer make a special written determination that the penny stock is a suitable investment
for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect
of reducing the level of trading activity in the secondary market for a stock that becomes subject to the penny stock rules which may
increase the difficulty Purchasers may experience in attempting to liquidate such securities.
We do not expect to pay dividends in the foreseeable future. Any
return on investment may be limited to the value of our common stock.
We do not anticipate paying cash dividends on our common stock in the foreseeable
future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors
affecting it at such time as the board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable
because a return on your investment will occur only if our stock price appreciates.
We will have broad discretion in applying the net proceeds of this
Offering and we may not use those proceeds in ways that will enhance our business operations.
We have significant flexibility in applying the net proceeds we will receive
in this Offering. We will use the proceeds that we receive from the sale of shares in this Offering for legal fees, accounting expenses,
research and development, capital investments and working capital. As part of your investment decision, you will not be able to assess
or direct how we apply these net proceeds. If we do not apply these funds effectively, we may lose significant business opportunities.
The securities laws may restrict transferability of the securities
sold in the Offering.
The shares in this Offering have not been registered under the Securities
Act or registered or qualified under any state or foreign securities laws. Such securities are being issued based upon the Company’s
reliance upon an exemption from registration under the Securities Act for an offer and sale of securities that does not involve a public
offering. Unless such securities are so registered, they may not be offered or sold except pursuant to an exemption from, or in a transaction
not subject to, the registration requirements of the Securities Act and applicable state or foreign securities laws.
You must make an independent investment analysis in connection with
this Offering.
No independent legal, accounting or business advisors have been appointed
to represent the interests of prospective Investors in connection with this Offering. Neither the Company nor any of its officers, directors,
employees or agents makes any representation or expresses any opinion with respect to the merits of an investment in the shares offered
hereby. Each prospective Investor is therefore encouraged to engage independent accountants, appraisers, attorneys and other advisors
to (i) conduct due diligence review as the prospective investor may deem necessary and advisable, and (ii) provide advice with respect
to the merits of an investment in the shares offered hereby and applicable risk factors as a prospective investor may deem necessary and
advisable to rely upon. We will fully cooperate with any prospective Investor who desires to conduct an independent analysis, so long
as it determines, in our sole discretion, that cooperation is not unduly burdensome. Each prospective Investor acknowledges that he, she
or it has been informed and understands.
Because we lack certain internal controls over financial reporting
in that we do not have an audit committee and our Board of Directors has no technical knowledge of U.S. GAAP and internal control of financial
reporting and relies upon the Company's financial personnel to advise the Board on such matters, we are subject to increased risk related
to financial statement disclosures.
We lack certain internal controls over financial reporting
in that we do not yet have an audit committee and our Board of Directors has little technical knowledge of U.S. GAAP and internal control
of financial reporting and relies upon the Company's financial personnel and accounting firm to advise the Board on such matters. Accordingly,
we are subject to increased risk related to financial statement disclosures.
FORWARD LOOKING STATEMENTS
This offering circular contains forward-looking statements that involve
risk and uncertainties. We use words such as “anticipate”, “believe”, “plan”, “expect”,
“future”, “intend”, and similar expressions to identify such forward-looking statements. Investors should be aware
that all forward-looking statements contained within this filing are good faith estimates of management as of the date of this filing.
Our actual results could differ materially from those anticipated in these forward-looking statements for many reasons, including the
risks faced by us as described in the “Risk Factors” section and elsewhere in this offering circular.
DESCRIPTION OF BUSINESS
Overview
LifeQuest World Corp., through its our wholly owned subsidiary, BioPipe
Global Corp., is a wastewater treatment company with world’s only sludge free onsite wastewater system.
On April 17, 2019, we entered into an Agreement and Plan of Merger (the
“Merger Agreement”) with BioPipe Acquisition, Inc., a New Jersey corporation (“Merger Sub”) and BioPipe Global
Corp., a privately held New Jersey corporation (“BioPipe Global”). In connection with the closing of this merger transaction,
Merger Sub merged with and into BioPipe Global (the “Merger”) on April 30, 2019, with the filing of Articles of Merger with
the New Jersey Secretary of State.
As a result of the Merger Agreement, we are now engaged in eco-friendly
decentralized wastewater treatment. The Company’s mission is to become a singular platform for highly efficient, scalable, low footprint
onsite treatment technologies for treatment sewage wastewater and industrial wastewater. Our flagship product is Biopipe STP, (sewage
wastewater treatment), which is a highly scalable and biological sewage wastewater system that treats domestic sewage into clean water
ready for secondary purposes.
On May 7, 2019, under an Intellectual Property Purchase Agreement,
BioPipe Global acquired all the assets of BioPipe Global AG, a Swiss company, and its wholly-owned Turkish subsidiary, BioPipe Cevre Teknolojileri
A.S. We acquired all trade receivables, income, royalties, damages, rights to sue, rights to enforce and any and all payments unpaid and
due now or hereafter due or payable with respect to the patented BioPipe System.
In the last five years, the BioPipe STP system has been installed
in Bangladesh, India, Ethiopia, the Philippines, South Africa, Turkey, UAE, Qatar, Saudi Arabia, Oman, and Maldives. These BioPipe systems
are running successfully at hospitals, resorts, hotels, commercial and government buildings, labor camps, ports and individual homes.
On September 26, 2019, the Company entered into a 50-50 joint venture
and technology transfer agreement with South Africa based, Abrimix Pty Ltd. The purpose of the agreement was to introduce our Biopipe
STP into southern Africa and eventually introduce Abrimix in the countries we operate in. Abrimix is a patented, low footprint, scalable
industrial wastewater treatment technology with the ability to treat a wide variety of industrial wastewater. Due to disruptions related
to Covid-19 and a lack of funding, the parties to the joint venture dissolved the venture and, on June 1, 2022, a technology license agreement
was put in place. Under the licensing agreement, the prior joint venture partner, Biotech Innovations Ltd. is required to pay 7.5% of
net revenues.
Traditional centralized wastewater treatment systems are expensive, energy-intensive
and chemical-dependent. The world is seeking sustainable solutions through decentralized wastewater treatment which “get back to
nature” while using 21st century technologies and management. Reuse may include irrigation of gardens and agricultural
fields or replenishing surface water and groundwater. Reused water may also be directed toward fulfilling certain needs in residences
(e.g. toilet flushing), businesses and industry, and where necessary, treated to reach drinking water standards.
The reuse of wastewater has long been established as critically important
for irrigation, especially in arid countries. According to the World Bank, there will be a 40 percent global shortfall between supply
and demand of water by 2030. And by 2025, approximately 1.8 billion people will be living in regions with “absolute water scarcity.”
The World Bank also estimates that 70 percent of water use today is for agriculture. A projected global population of 9 billion by 2050
is expected to require a 60 percent increase in agricultural production and a 15 percent increase in water withdrawals. Recycled water
can meet some of this need, benefited by the nutrient content inherent in wastewater. And only mid-range treatment levels would be required,
as irrigation can be accomplished while minimizing the potential for human contact with the recycled water. Reusing wastewater as part
of sustainable water management allows water to remain as an alternative water source for human activities. This can reduce scarcity and
alleviate pressures on groundwater and other natural water bodies. At the nexus between unusable wastewater and clean water, lies BioPipe:
We are a company seeking, and finding, opportunities to supply wastewater
treatment systems in countries that have water and sanitation deficits and low sewerage connections. This is the process of converting
wastewater into water that can be repurposed for other uses. Reclaiming water from waste for reuse applications (instead of using freshwater
supplies) can be a water-saving measure. When treated wastewater is eventually discharged back into natural water sources, it still has
significant benefits to ecosystems; improving streamflow, nourishing plant life and replenishing aquifers, as part of the natural water
cycle.
BioPipe is a revolutionary onsite wastewater treatment system which
is a highly scalable, eco-friendly and extremely cost-effective wastewater treatment with a broad installed base. It is the planet’s
first biological wastewater treatment system where the process takes place entirely inside the pipe and:
| |
• |
has an extremely small footprint which allows it to
be installed in places of high population density and commercial buildings; |
| |
• |
very low energy consumption; and |
| |
• |
discharge meets strict European Union standards. |
Industry Drivers
According to Water Resource Institute water shortages affect 2.7 billion
people now and 2.4 billion people lack proper wastewater treatment. An additional 2.1 billion people need upgraded treatment. Population
is expected to grow from 7.4 billion in 2016 to 9.1 billion in 2050 which will require 60% increase in global food production by 2050.
Manufacturing water demand will grow 400% by 2050 and global water consumption is expected to double by 2050. This will result in 40%
water deficit by 2030 and by 2025, two-thirds of the world will face water shortages. Governments and private sectors are rapidly moving
towards treatment and re-use.
Sustainable Development Goals (SDG). Due to climate change,
there is an ever-increasing focus on sustainability and the circular economy. As a result, green capital investment is expected to reach
$6 trillion with nearly $400 billion expected to be spent on water.
$2.8 trillion of Green Capex is
needed per year in the 2020s to support Net Zero, Infrastructure and Clean Water
Source: World Bank, OECD, McKinsey,
Goldman Sachs
Regulations:
Governments throughout the world are increasingly tightening
environmental regulations. Other than renewable energy, treatment and reuse of water is becoming critical due to water deficits, tightening
discharge standards and mortality caused by water pollution.
Corporates and investors also see regulations as potentially
helping to drive water demand efficiency optimization solutions.
The United Nations estimates that some 1.8 billion people
hailing from countries around the globe drink water that is contaminated with fecal matter. Drinking poop-infused water can result in
serious illnesses like dysentery, typhoid, and polio rearing their ugly heads. Globally, nearly 1.2 trillion gallons of untreated wastewater
back into bodies of water, ranging from the vast ocean to tiny streams that go on to form rivers. It is now estimated that circa 47 percent
of people around the globe will struggle to find sufficient stores of drinking water by 2050.
According to the study conducted by Acumen Research and
Consulting (ARC), (2019), the market size of the global industrial wastewater treatment is poised to increase up to the US $16.5 billion
by 2026 with an average growth rate of 4.5% CAGR. A report published by UN Water, UNESCO in 2017, highlighted that approximately 70% of
the wastewater both municipal and industrial is treated in high-income countries as compared to upper-middle-income countries (38%) and
lower-middle-income countries (28%), whereas, the lower-income countries are limited to only 8%. Considering the existing patterns, it
is anticipated that approximately 80% of the global wastewater is discharged without any treatment.
Approximately 3,575,000 people die from water related diseases
each year and nearly 5.2 billion people do not have access to sewage system.
High capex: Although rapid progress is being made in treatment
of industrial wastewater, high capital investment requirement, inefficient treatment options, and lack of infrastructure remain a high
hurdle. The concepts, such as the circular economy (CE), partitions-release-recover (PRR), and transforming wastewater into bio factory
are anticipated to be more convenient options to tackle the industrial wastewater problem.
Rising water consumption:
According to the United Nations, global freshwater demand
predicted to exceed supply by 40% by 2030 with agriculture accounting for 70% of the total consumption. The problem has been exacerbated
by rising salinity throughout the world due to rising see levels and catastrophic storms.
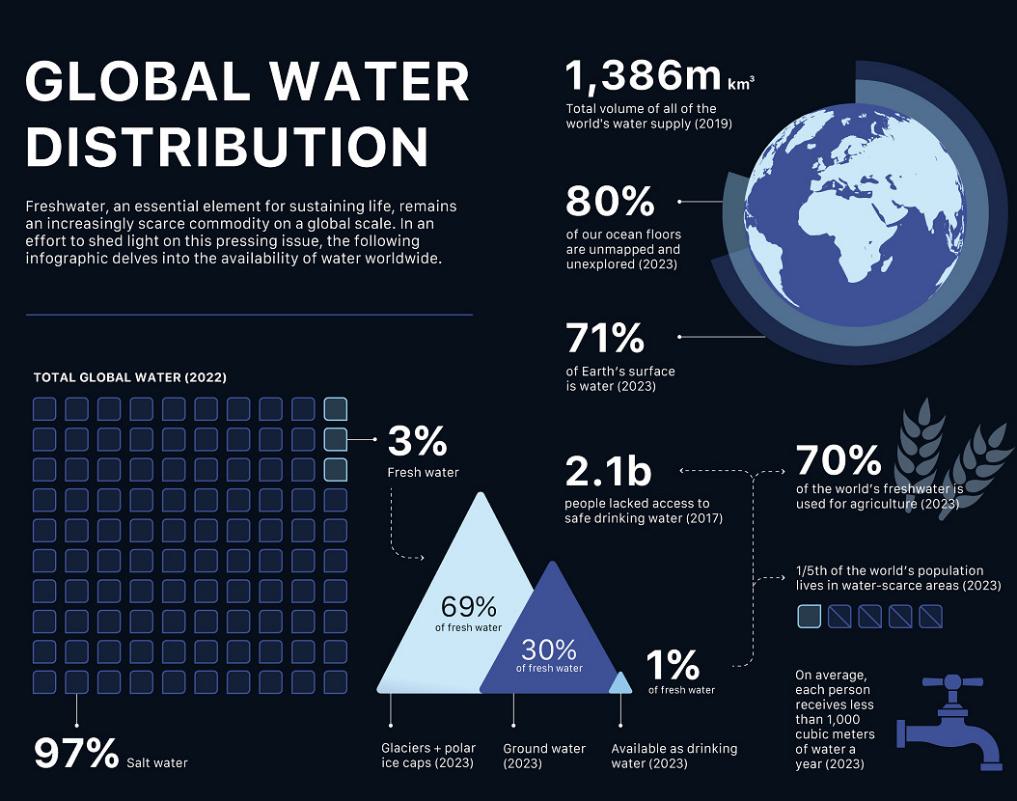
Source www.weforum.org
Our Solutions

SEWAGE WASTEWATER TREATEMENT
Biopipe Global AG (Switzerland), established in 2014 and acquired by Lifequest
World Corp in 2019 via its wholly owned operating subsidiary, Biopipe Global Corp., is the 1st patented and world’s only sludge
free onsite wastewater treatment system. Biopipe is 100% sludge free, odor free, silent, easy to assemble and install, scalable, low cost,
ecological and virtually maintenance free (if 1000 gallons of sewage wastewater (grey water and black water) goes in, 1000 gallons of
clean water is discharged). The treated water is clean enough to exceed EU Standards for discharge and reuse. The entire treatment takes
place within a series of pipes and the only output is non-potable clean water that can be used for irrigation, flushing or cleaning.
Biopipe currently has around 44 plants installed around the
globe, and we expect significant number of projects in India and Ethiopia and limited numbers in South Africa and the Philippines. Some
of these plants were installed prior to the merger agreement. We have now set up joint ventures, licensing and distribution in five countries
at government and commercial buildings, hotels, resorts, labor camps and residences.
Example 1: 73m3/day system running
at a government building in Sharjah- UAE

Example 2: 100m3/day system running
a government residential building in Dhaka, Bangladesh

The system has a very small footprint and can treat a single residential
home (replaces expensive septic tank), large commercial properties like hotels, offices, labor camps, and community housing. It does not
use any chemicals, low cost, easy to design and install and the only output is clean water that meets European Union standards and can
be discharged into the ground. Throughout Middle East the treated water is used for irrigation and can certainly be used for re-flushing
and cleaning.
The Abrimix ETP

Abrimix is a versatile technology for treating a wide variety of industrial
wastewater. The Abrimix Wastewater Treatment Plant is a proven and patented technology that effectively separates suspended solids and
organic/inorganic contaminants from the wastewaters of processing plants. The Abrimix Plant is also able to effectively separate and break
emulsions within liquids allowing for the separation of suspended solids.
The Abrimix Treatment Plant utilizes a proprietary technology mixer, the
Abrimix HSR, and a gravity assisted separation column, the Abrimix SSF column, to achieve desired treated water results with high efficiency.
The Abrimix HSR imparts a high-speed mixing regime within an elevated pressure
environment, allowing reagents to be quickly and efficiently dispersed into the wastewater. As the water travels from the Abrimix HSR
to the Abrimix SSF column, the utilization of custom sized micro bubbles added to the elevated pressure environment allows for the distinct
separation of suspended solids and water, pushing moisture-reduced suspended solids to the top of the Abrimix SSF column and treated water
to the bottom. Treated water can be further polished, depending on end-use, by the addition of a tertiary filter. This gravity assisted
system reduces electricity consumption and eliminates the need for sludge dewatering equipment. However, to be on safer side we have considered
the dewatering unit in this process.
The rapid rate of reaction and separation achieved with the use of the
Abrimix treatment plant through which the wastewater passes accounts for the benefits obtained with improved results and reduced reagent
consumptions.
The Main Benefits of the Abrimix Treatment Plant are:
|
• | |
Can handle variable water contamination qualities |
| |
• | |
Can handle variable suspended solids loading |
| |
• | |
Can handle variable flow rates |
| |
• | |
Reduced treatment time due to high efficiency |
| |
• | |
Reduced Reagent consumption |
| |
• | |
Reduced OPEX costs |
| |
• | |
Reusable Water Discharge |
| |
• | |
Small Plant Footprint |
We have sold two plants in India- a 5m3/hour gooseberry processing
plant and a 350m3/day for a pharmaceutical API manufacturer. Both plants were done on a capex basis and operations and maintenance
is done by our subsidiary in India. The pharma plant is expected to be commissioned in first quarter of 2024.
VP Foods Gooseberry Wastewater Treatment Plant

Decentralized (Onsite) Wastewater Treatment Market
A centralized system requires a far-reaching network of pumps and pipelines
to transport the collected wastewater to a central installation for treatment prior to discharge and/or reuse. Wastewater treatment systems
that have small foot print, mobile, plug and play solutions like Biopipe and Abrimix are highly desirable. They require very little onsite
infrastructure and low capex. These systems can be remotely monitored and operated substantially lower cost than centralized systems.
Additionally, localized treatment and reuse reduces water and energy demand. Biopipe uses very low power to operate because it does not
use blowers in its system.
Competition
The packaged wastewater treatment market is highly competitive. We compete
against the following established technologies:
MBBRs biologically treat wastewater by circulating moving
media in aerobic, activated-sludge environments. The moving media is typically a floating plastic substrate colonized by a community
of bacteria. These bacteria form a biofilm on the plastic surface. Increased levels of biofilm enhance the biological treatment
process by introducing a more robust microbial community. In addition to the biofilm attached to the plastic carriers, biomass in
the system also exists in the form of suspended flocks. At smaller scales*in India, MBBR tanks were found to be constructed of
reinforced concrete or mild steel. Some suppliers also offer fiber reinforced polymer (FRP) tanks.
SBR (Sequence Batch Reactor)
SBRs are based on the activated-sludge treatment process. SBRs use a batch approach in which secondary sewage treatment occurs in a single
tank. In the first stage of SBR treatment, influent is added to the batch-reactor tank and aerated. Following aeration, the wastewater
is allowed to settle. Finally, the treated wastewater is removed from the top of the tank using a decanter valve, pump, or airlift tube.
At smaller scale SBR tanks can be constructed with reinforced concrete or mild steel.
MBR (Membrane Bioreactor)
Membrane bioreactors (MBRs) combine two treatments processes: the activated-sludge process and membrane filtration. Wastewater is first
aerated in a bioreactor tank, where microorganisms are present in the form of suspended flocks. Then, a microporous membrane is used for
solid/liquid separation. This configuration eliminates the need for secondary clarifiers. For smaller plants steel tanks are used, which
reduces the civil costs (or costs associated with constructing reinforced concrete structures—e.g., tanks, foundations). Note that
MBRs can also be added to existing MBBRs as retrofits.
DAF (Dissolved Air Floatation) INDUSTRIAL ETP
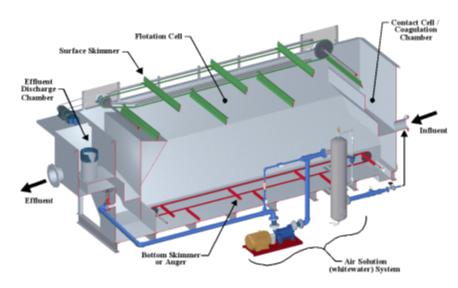
Dissolved
Air Flotation (DAF) clarifier systems are advanced separators for the treatment of industrial wastewater. DAF systems separate suspended
solids, oils, and fats together with all the pollutants associated with them. This technology applies dissolved air in the form of microbubbles
that drag the buoyant solids to the surface of the equipment where skimmers eliminate them. After this process, a clear effluent is discharged
from the DAF system.
Environmental, Health and Safety Regulation
Different countries have different discharge standards
for treated wastewater. We meet or exceed the discharge standards in countries we are now operating or intend to operate in. We are not
subject to EPA regulations.
USE OF PROCEEDS
We
estimate that, at a per share price of $0.10, the net proceeds from the sale of the 10,000,000 shares in this offering will be approximately
$1,000,000 less estimated offering expenses of approximately $65,000.
We intend to use the proceeds for working capital and to invest to upgrade
and recommission our Morgan Beef in Build Own Operate & Transfer (BOOT) plant in South Africa, short term financing for municipal
plants in India, purchase orders from Ethiopia, purchase orders from government tenders and marketing efforts. Accordingly, we expect
to use the proceeds of the Maximum Offering as follows:
Maximum
Offering
| | |
Amount | |
Percentage |
| Corporate | |
Each | |
Each |
| Offering Expense | |
$ | 65,000 | | |
| 6.50% |
| Municipal Plants | |
$ | 400,000 | | |
| 40.00% |
| Build Own Operate Transfer Upgrade | |
$ | 180,000 | | |
| 18.00% |
| Finance Purchase Orders | |
$ | 250,000 | | |
| 25.00% |
| Working Capital | |
$ | 105,000 | | |
| 10.50% |
| TOTAL | |
$ | 1,000,000 | | |
| 100% |
75% Offering
| | |
Amount | |
Percentage |
| Corporate | |
Each | |
Each |
| Offering Expense | |
$ | 65,000 | | |
| 8.67% |
| Municipal Plants | |
$ | 300,000 | | |
| 40.00% |
| Build Own Operate Transfer Upgrade | |
$ | 180,000 | | |
| 24.00% |
| Finance Purchase Orders | |
$ | 125,000 | | |
| 16.67% |
| Working Capital | |
$ | 80,000 | | |
| 10.67% |
| TOTAL | |
$ | 750,000 | | |
| 100% |
50% Offering
| | |
Amount | |
Percentage |
| Corporate | |
Each | |
Each |
| Offering Expense | |
$ | 65,000 | | |
| 13.00% |
| Municipal Plants | |
$ | 200,000 | | |
| 40.00% |
| Build Own Operate Transfer Upgrade | |
$ | 180,000 | | |
| 36.00% |
| Finance Purchase Orders | |
$ | 55,000 | | |
| 11.00% |
| TOTAL | |
$ | 500,000 | | |
| 100% |
25% Offering
| | |
Amount | |
Percentage |
| Corporate | |
Each | |
Each |
| Offering Expense | |
$ | 65,000 | | |
| 26.00% |
| Municipal Plants | |
$ | — | | |
| 0.00% |
| Build Own Operate Transfer Upgrade | |
$ | 185,000 | | |
| 74.00% |
| Finance Purchase Orders | |
$ | — | | |
| 0.00% |
| TOTAL | |
$ | 250,000 | | |
| 100% |
The expected use of net proceeds from this offering represents our intentions
based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The
amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including negotiations with the other
parties in the merge and acquisitions process of the target companies, the amount of cash available from other sources and any unforeseen
cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
DILUTION
If you invest in our shares, your interest will be
diluted to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible
book value per share of our capital stock after this Offering. Our net tangible book value as of November 30, 2023 was $0.00462 per share
of outstanding common stock. Without giving effect to any changes in the net tangible book value after November 30, 2023 other than the
sale of 13,000,000 shares in this offering at the initial public offering price of $0.10 per share less direct costs of the offering,
our pro forma net tangible book value as of November 30, 2023 would have been $1,441,295 or $0.012 per share of outstanding capital stock.
Dilution in net tangible book value per share represents the difference between the amount per share paid by the purchasers of our shares
in this offering and the net tangible book value per share of our capital stock immediately afterwards. This represents an immediate
increase of $0.0073 per share of capital stock to existing shareholders and an immediate dilution of $0.088 per share of common stock
to the new investors, or approximately 100% of the assumed initial public offering price of $0.10 per share. The following table illustrates
this per share dilution:
| Net Proceeds (net of commission & offering cost) | |
| |
$210,000 | |
$435,000 | |
$660,000 | |
$885,000 |
| Offering - Primary Shares | |
10,000,000 | |
2,500,000 | |
5,000,000 | |
7,500,000 | |
10,000,000 |
| Offering - Secondary Shares | |
3,000,000 | |
— | |
— | |
— | |
— |
| Offering Cost | |
$15,000 | |
| |
| |
| |
|
| Commission | |
10% | |
25% | |
50% | |
75% | |
100% |
| Initial
price to public | |
|
| | |
$ | 0.10 | | |
$ | 0.10 | | |
$ | 0.10 | | |
$ | 0.10 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
Net
tangible book value per share as of November 31, 2023 | |
| | | |
$ | 0.00462 | | |
$ | 0.00462 | | |
$ | 0.00462 | | |
$ | 0.00462 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Increase
in net tangible book value per share attributable to new investors | |
| | | |
$ | 0.0017 | | |
$ | 0.0036 | | |
$ | 0.0055 | | |
$ | 0.0073 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| As adjusted net tangible
book value per share after the offering | |
| | | |
$ | 0.0064 | | |
$ | 0.0082 | | |
$ | 0.0101 | | |
$ | 0.0120 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Dilution in net tangible
book value per share to new investors | |
| | | |
$ | 0.0936 | | |
$ | 0.0918 | | |
$ | 0.0899 | | |
$ | 0.0880 | |
The following table summarizes the differences between
the existing shareholders and the new investors with respect to the number of shares of common stock purchased, the total consideration
paid, and the average price per share paid, on a maximum offering basis:
Maximum
Offering :
| | |
Shares
Purchased | |
Total
Consideration | |
|
| | |
Number | |
Percent | |
Amount | |
Percent | |
Average
Price Per Share |
| | |
| |
| |
| |
| |
|
| Existing
Shareholders | |
| 120,484,150 | | |
| 90.261 | % | |
$ | 1,876,218 | | |
| 65.23 | % | |
$ | 0.0156 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
New
Investors - Primary | |
| 10,000,000 | | |
| 7.492 | % | |
$ | 1,000,000 | | |
| 34.77 | % | |
$ | 0.1000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Selling Shareholders
- Secondary* | |
| 3,000,000 | | |
| 2.247 | % | |
$ | — | | |
| — | | |
$ | — | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total | |
| 133,484,150 | | |
| 100.00 | % | |
$ | 2,876,218 | | |
| 100.0 | % | |
| | |
*
Exercise of warrants
SELLING SHAREHOLDERS
The shares being offered for resale by the selling stockholders consist
of 3,000,000 shares of our common stock underlying warrants held by 2 (two) shareholders.
On July 6, 2022, a Business and Financial Consulting Agreement was signed
between the Company and Ivest Consulting GmbH for consulting services which involves identifying build own operate and build own operate
and transfer projects in various countries. In consideration of consulting services rendered the Company issued warrants to purchase 1,500,000
shares of common stock at a strike price of $0.05 per share with a 48-month expiration. The contract also has a provision that additional
warrants for 10,500,000 shares of common stock with a strike price of $0.10 with a 48-month expiration are issued and held in escrow if
certain debt/equity capital is raised. As of November 30, 2023, the conditions have not been met to release the warrants from escrow and
no warrants have been exercised.
On October 1, 2023, a Business and Financial Consulting Agreement was signed
between the Company and Berkshire Finance Holdings, Ltd. In consideration of the consulting services rendered the Company issued warrants
for 1,500,000 shares of common stock at a strike price of $0.05 with a 24-month expiration. As of November 30, 2023, none of the warrants
have been exercised.
The following table sets forth the name of the selling stockholder,
the number of shares of common stock beneficially owned by each of the selling stockholder as of February 15, 2024 and the number of
shares of common stock being offered by the selling stockholder. The shares being offered hereby are being registered to permit public
secondary trading, and the selling stockholders may offer all or part of the shares for resale from time to time. However, the selling
stockholder is under no obligation to sell all or any portion of such shares nor is the selling stockholder obligated to sell any shares
immediately upon effectiveness of this offering circular. All information with respect to share ownership has been furnished by the selling
stockholder
| Name of selling stockholder |
|
|
Shares of Common
stock owned prior to offering |
|
|
|
Shares of Common stock
Underlying Warrants owned prior to offering |
|
|
|
Shares
of Common stock to be sold |
|
|
|
Shares of Common
stock owned after offering (if all shares are sold) |
|
|
|
Percent of common stock
owned after offering (if all shares are sold) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Berkshire Finance Holdings, LTD1 |
|
|
3,306,355 |
|
|
|
1,500,000 |
|
|
|
1,500,000 |
|
|
|
3,306,355 |
|
|
|
0.0275% |
Ivest
Consulting2 |
|
|
1,000,000 |
|
|
|
1,500,000 |
|
|
|
1,500,000 |
|
|
|
1,000,000 |
|
|
|
0.008% |
| Total |
|
|
4,306,355 |
|
|
|
3,000,000 |
|
|
|
3,000,000 |
|
|
|
4,306,355 |
|
|
|
0.0358% |
| |
|
(1) |
John Figliolini has voting and dispositive control over the shares held. |
| |
|
(2) |
Robert Kaufmann has voting and dispositive control over the shares held. |
PLAN OF DISTRIBUTION
This Offering Statement is part the Form 1-A that
we filed with the SEC, using a continuous offering process. Periodically, as we have material developments, we will provide an Offering
Statement supplement that may add, update or change information contained in this Offering Statement. Any statement that we make in this
Offering Statement will be modified or superseded by any inconsistent statement made by us in a subsequent Offering Statement supplement.
Quotation
Our Common Stock is traded on the OTCMarkets.com Pink Sheet Open Market
under the symbol “LQWC.”
Pricing of the Offering
Prior to the offering, there has been a limited public market for our common
shares. The initial public offering price was determined by the Company. The principal factors considered in determining the initial public
offering price include:
| |
• |
the information set forth in this Offering Statement
and otherwise available; |
| |
• |
our history and prospects and the history of and prospects
for the industry in which we compete; |
| |
• |
our past and present financial performance; |
| |
• |
our prospects for future earnings and the present state
of our development; |
| |
• |
the general condition of the securities markets at
the time of this offering; |
| |
• |
the recent market prices of, and demand for, publicly
traded common stock of generally comparable companies; and |
| |
• |
other factors deemed relevant by us. |
Investment Limitations
Generally, no sale may be made to you in this offering if the aggregate
purchase price you pay is more than 10% of the greater of your annual income or net worth (please see below on how to calculate your net
worth). Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does
not exceed applicable thresholds, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing,
we encourage you to refer to www.investor.gov.
Because this is a Tier 1, Regulation A Offering, most investors must comply
with the 10% limitation on investment in the Offering. The only investor in this offering exempt from this limitation is an “accredited
investor” as defined under Rule 501 of Regulation D under the Securities Act (an “Accredited Investor”). If you meet
one of the following tests you should qualify as an Accredited Investor:
| |
• |
You are a natural person who has had individual income
in excess of $200,000 in each of the two most recent years, or joint income with your spouse in excess of $300,000 in each of these
years, and have a reasonable expectation of reaching the same income level in the current year; |
| |
• |
You are a natural person and your individual net worth,
or joint net worth with your spouse, exceeds $1,000,000 at the time you purchase Offered Shares (please see below on how to calculate
your net worth); |
| |
• |
You are an executive officer or general partner of
the issuer or a manager or executive officer of the general partner of the issuer; |
| |
• |
You are an organization described in Section 501(c)(3)
of the Internal Revenue Code of 1986, as amended, or the Code, a corporation, a Massachusetts or similar business trust or a partnership,
not formed for the specific purpose of acquiring the Offered Shares, with total assets in excess of $5,000,000; |
| |
• |
You are a bank or a savings and loan association or
other institution as defined in the Securities Act, a broker or dealer registered pursuant to Section 15 of the Exchange Act, an
insurance company as defined by the Securities Act, an investment company registered under the Investment Company Act of 1940 (the
“Investment Company Act”), or a business development company as defined in that act, any Small Business Investment Company
licensed by the Small Business Investment Act of 1958 or a private business development company as defined in the Investment Advisers
Act of 1940; |
| |
• |
You are an entity (including an Individual Retirement
Account trust) in which each equity owner is an accredited investor; |
| |
• |
You are a trust with total assets in excess of $5,000,000,
your purchase of Offered Shares is directed by a person who either alone or with his purchaser representative(s) (as defined in Regulation
D promulgated under the Securities Act) has such knowledge and experience in financial and business matters that he is capable of
evaluating the merits and risks of the prospective investment, and you were not formed for the specific purpose of investing in the
Offered Shares; or |
| |
• |
You are a plan established and maintained by a state,
its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees,
if such plan has assets in excess of $5,000,000. |
Offering Period and Expiration Date
Our offering will terminate upon the earliest of (i) such time as all of
the common stock has been sold pursuant to the Offering Statement or (ii) 365 days from the qualified date of this offering circular unless
extended by our Board of Directors for an additional 90 days. We may however, at any time and for any reason terminate the offering.
Procedures for Subscribing
Any potential investor will have ample time to review the subscription
agreement, along with their counsel, prior to making any final investment decision. We shall only deliver such subscription agreement
upon request after a potential investor has had ample opportunity to review this Offering Statement.
Right to Reject Subscriptions. After we receive your complete, executed
subscription agreement and the funds required under the subscription agreement have been transferred to our bank account, we have the
right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies
from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions. Upon our acceptance of a subscription
agreement, we will countersign the subscription agreement and issue the shares subscribed at closing. Once you submit the subscription
agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription
agreements are irrevocable.
Under Rule 251 of Regulation A, non-accredited, non-natural investors are
subject to the investment limitation and may only invest funds which do not exceed 10% of the greater of the purchaser’s revenue
or net assets (as of the purchaser’s most recent fiscal year end). A non-accredited, natural person may only invest funds which
do not exceed 10% of the greater of the purchaser’s annual income or net worth (please see below on how to calculate your net worth).
NOTE: For the purposes of calculating your net worth, it is defined as
the difference between total assets and total liabilities. This calculation must exclude the value of your primary residence and may exclude
any indebtedness secured by your primary residence (up to an amount equal to the value of your primary residence). In the case of fiduciary
accounts, net worth and/or income suitability requirements may be satisfied by the beneficiary of the account or by the fiduciary, if
the fiduciary directly or indirectly provides funds for the purchase of the Offered Shares.
In order to purchase offered Shares and prior to the acceptance of any
funds from an investor, an investor will be required to represent, to the Company’s satisfaction, that he is either an accredited
investor or is in compliance with the 10% of net worth or annual income limitation on investment in this offering.
No Escrow
The proceeds of this offering will not be placed into an escrow account.
We will offer our shares of common stock on a best-efforts basis primarily through an online platform. As there is no minimum offering,
upon the approval of any subscription to this Offering Statement, the Company shall immediately deposit said proceeds into the bank account
of the Company and may dispose of the proceeds in accordance with the Use of Proceeds.
DESCRIPTION OF SECURITIES
The following is a summary of the rights of our capital stock as provided
in our articles of incorporation and bylaws. For more detailed information, please see our articles of incorporation and bylaws, which
have been filed as exhibits to the offering statement of which this Offering Circular is a part.
Our authorized capital stock consists of 500,000,000 shares of common stock,
with a par value of $0.001 per share, and 50,000,000 shares of preferred stock, par value $0.001 per share. As of February 15, 2024, there
were 120,484,150 shares of our common stock issued and outstanding. Our shares are currently held by 66 stockholders of record with others
in street name. As of February 15, 2024, 50,000,000 authorized shares of preferred stock have been designated as Series B Preferred Stock.
The Series B Preferred Stock has a stated value of $0.001 per share, super voting rights and every share of Series B Preferred Stock may
convert into 240 shares of common stock. To date, the company has not issued any shares of Series B Preferred Stock.
Common Stock
Our common stock is entitled to one vote per share on all matters submitted
to a vote of the stockholders, including the election of directors. Except as otherwise required by law or provided in any resolution
adopted by our board of directors with respect to any series of preferred stock, the holders of our common stock will possess all voting
power. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors,
by a plurality) of the votes entitled to be cast by all shares of our common stock that are present in person or represented by proxy,
subject to any voting rights granted to holders of any preferred stock. Holders of our common stock representing fifty percent (50%) of
our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at
any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental
corporate changes such as liquidation, merger or an amendment to our Articles of Incorporation. Our Articles of Incorporation do not provide
for cumulative voting in the election of directors.
Subject to any preferential rights of any outstanding series of preferred
stock created by our board of directors from time to time, the holders of shares of our common stock will be entitled to such cash dividends
as may be declared from time to time by our board of directors from funds available therefore.
Subject to any preferential rights of any outstanding series of preferred
stock created from time to time by our board of directors, upon liquidation, dissolution or winding up, the holders of shares of our common
stock will be entitled to receive pro rata all assets available for distribution to such holders.
In the event of any merger or consolidation with or into another company
in connection with which shares of our common stock are converted into or exchangeable for shares of stock, other securities or property
(including cash), all holders of our common stock will be entitled to receive the same kind and number of shares of stock and other securities
and property (including cash). Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption
provisions applicable to our common stock.
Preferred Stock
Our board of directors is authorized by our articles of incorporation to
divide the authorized shares of our preferred stock into one or more series, each of which must be so designated as to distinguish the
shares of each series of preferred stock from the shares of all other series and classes. Our board of directors is authorized, within
any limitations prescribed by law and our articles of incorporation, to fix and determine the designations, rights, qualifications, preferences,
limitations and terms of the shares of any series of preferred stock including, but not limited to, the following:
| |
1. |
The number of shares constituting that series and the distinctive designation of that series, which may be by distinguishing number, letter or title; |
| |
2. |
The dividend rate on the shares of that series, whether dividends will be cumulative, and if so, from which date(s), and the relative rights of priority, if any, of payment of dividends on shares of that series; |
| |
3. |
Whether that series will have voting rights, in addition to the voting rights provided by law, and, if so, the terms of such voting rights; |
| |
4. |
Whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the Board of Directors determines; |
| |
5. |
Whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption, including the date or date upon or after which they are redeemable, and the amount per share payable in case of redemption, which amount may vary under different conditions and at different redemption dates; |
| |
6. |
Whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund; |
| |
7. |
The rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation, and the relative rights of priority, if any, of payment of shares of that series; |
| |
8. |
Any other relative rights, preferences and limitations of that series. |
Provisions in Our Articles of Incorporation and By-Laws That Would Delay,
Defer or Prevent a Change in Control
Our articles of incorporation authorize our board of directors to issue
a class of preferred stock commonly known as a "blank check" preferred stock. Specifically, the preferred stock may be issued
from time to time by the board of directors as shares of one or more classes or series. Our board of directors, subject to the provisions
of our Articles of Incorporation and limitations imposed by law, is authorized to adopt resolutions; to issue the shares; to fix the number
of shares; to change the number of shares constituting any series; and to provide for or change the following: the voting powers; designations;
preferences; and relative, participating, optional or other special rights, qualifications, limitations or restrictions, including the
following: dividend rights, including whether dividends are cumulative; dividend rates; terms of redemption, including sinking fund provisions;
redemption prices; conversion rights and liquidation preferences of the shares constituting any class or series of the preferred stock.
In each such case, we will not need any further action or vote by our shareholders.
One of the effects of undesignated preferred stock may be to enable the board of directors to render more difficult or to discourage an
attempt to obtain control of us by means of a tender offer, proxy contest, merger or otherwise, and thereby to protect the continuity
of our management. The issuance of shares of preferred stock pursuant to the board of director's authority described above may adversely
affect the rights of holders of common stock. For example, preferred stock issued by us may rank prior to the common stock as to dividend
rights, liquidation preference or both, may have full or limited voting rights and may be convertible into shares of common stock. Accordingly,
the issuance of shares of preferred stock may discourage bids for the common stock at a premium or may otherwise adversely affect the
market price of the common stock.
Dividend Policy
We have never declared or paid any cash dividends
on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we
do not anticipate paying any cash dividends in the foreseeable future.
Options
We have not issued and do not have outstanding any
options to purchase shares of our common stock but we do anticipate establishing employee stock option plans in the second half of 2024.
Warrants
On July 6, 2022, a
Business and Financial Consulting Agreement was signed between the Company and Ivest Consulting GmbH for consulting services which involves
identifying build own operate and build own operate and transfer projects in various countries. In consideration of consulting services
rendered the Company issued warrants to purchase 1,500,000 shares of common stock at a strike price of $0.05 per share with a 48-month
expiration. The contract also has a provision that additional warrants for 10,500,000 shares of common stock with a strike price of $0.10
with a 48-month expiration are issued and held in escrow if certain debt/equity capital is
raised. As of November 30, 2023, the conditions have not been met to release the warrants from escrow and no warrants have been exercised.
On August 19, 2022, a Business and Financial Consulting
Agreement was signed between the Company and Tirante Finance AG for consulting services. In consideration of the consulting services
rendered the Company issued warrants for 1,500,000 shares of common stock at a strike price of $0.05 with a 24-month expiration (which
have been extended by 12 months). As of November 30, 2023, none of the warrants have been exercised.
On October 1, 2023, a Business and Financial Consulting
Agreement was signed between the Company and Berkshire Finance Holdings, Ltd. In consideration
of the consulting services rendered the Company issued warrants for 1,500,000 shares of common stock at a strike price of $0.05 with
a 24-month expiration. As of November 30, 2023, none of the warrants have been exercised.
As of August 31, 2023, the Company had warrants outstanding
to purchase 4,500,000 shares of the Company’s common stock.
Market Information
Our common stock is quoted under the symbol “LQWC”
on the OTC Pink operated by OTC Markets Group, Inc.
Our securities are thinly traded and there is no assurance
that a regular trading market will develop, or if developed, that it will be sustained. Therefore, a shareholder may be unable to resell
his or her securities in our company.
Penny Stock
The Securities Exchange Commission has adopted
rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities
with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system,
provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. The
penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document
prepared by the Commission, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public
offerings and secondary trading;(b) contains a description of the broker's or dealer's duties to the customer and of the rights and remedies
available to the customer with respect to a violation to such duties or other requirements of Securities' laws; (c) contains a brief,
clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between
the bid and ask price;(d) contains a toll-free telephone number for inquiries on disciplinary actions;(e) defines significant
terms in the disclosure document or in the conduct of trading in penny stocks; and;(f) contains such other information and is in such
form, including language, type, size and format, as the Commission shall require by rule or regulation.
The broker-dealer also must provide, prior to
effecting any transaction in a penny stock, the customer with; (a) bid and offer quotations for the penny stock;(b) the compensation of
the broker-dealer and its salesperson in the transaction;(c) the number of shares to which such bid and ask prices apply, or other comparable
information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statements showing the market
value of each penny stock held in the customer's account.
In addition, the penny stock rules require that
prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination
that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgment of the receipt of a
risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitability
statement.
These disclosure requirements may have the effect
of reducing the trading activity in the secondary market for our stock if it becomes subject to these penny stock rules. Therefore, because
our common stock is subject to the penny stock rules, stockholders may have difficulty selling those securities.
Recent Sales of Unregistered Securities
During the three months ended May 31, 2023, the Company issued as compensation,
1,000,000 shares to Mehmet Enes Kutluca, the Company’s officer and director; 225,000 shares to Tanmay Pawale, Chief Operating Officer
and CEO of Biopipe India and 50,000 shares to Prathamesh Jadhav, Project Manager, Biopipe
On September 8, 2023, the Company entered into an agreement with Beyond
Media SEZC to provide investor relations services. Under this agreement, the Company issued 2,250,000 shares on September 28, 2023.
On November 14, 2023, the Company sold 200,000 shares of its common stock
to Berkshire Finance Holdings, Ltd. at $0.02 cents per share for a total of $4,000.
On November 20, 2023, the Company sold 500,000 shares of its common stock
to Berkshire Finance Holdings, Ltd. at $0.015 cents per share for a total of $7,500.
On November 28, 2023 the Company sold 300,000 shares of its common stock
to C. Robert Walford at $0.025 cents per share for a total of $7,500.
On February 5, 2024, the Company sold 500,000 shares of its common stock
to Berkshire Finance Holdings, Ltd. at $0.01 cents per share for a total of $5,000.
These securities were issued pursuant to Section 4(2) of the Securities
Act and/or Rule 506 promulgated thereunder. The investor represented his intention to acquire the securities for investment only and not
with a view towards distribution. The investor was given adequate information about us to make an informed investment decision. We did
not engage in any general solicitation or advertising. We directed our transfer agent to issue the stock certificates with the appropriate
restrictive legend affixed to the restricted stock.
Securities Authorized for Issuance under Equity Compensation Plans
We do not have any equity compensation plans. We plan to adopt an equity
incentive plan in the second half of 2024.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having
prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon
other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or
is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or
subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal
underwriter, voting trustee, director, officer, or employee.
The Doney Law Firm, our independent legal counsel,
has provided an opinion on the validity of our common stock.
DESCRIPTION OF FACILITIES
The Company is headquartered at 100 Challenger Road, 8th Floor, Ridgefield
Park, NJ 07660. The agreement is for a shared office space at $2,000 per annum.
LEGAL PROCEEDINGS
From time to time, we may become party to litigation or other legal proceedings
that we consider to be a part of the ordinary course of our business. We are not currently involved in legal proceedings that could reasonably
be expected to have a material adverse effect on our business, prospects, financial condition or results of operations. We may become
involved in material legal proceedings in the future.
DIRECTORS AND EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following information sets forth the names, ages, and positions of
our current directors and executive officers.
| Name |
|
Age |
|
Positions
and Offices Held |
| Max Khan |
|
57 |
|
President, Chief Executive Officer, Secretary, Treasurer and Director |
| Mehmet Enes Kutluca |
|
34 |
|
COO and Director |
Set forth below is a brief description of the background and business experience
of each of our current executive officers and directors.
Max Khan
Max has over 25 years of corporate finance and advisory experience. Over
the past five years Max has been providing strategic consulting services to companies across various industries. Khan served as Director,
President and CEO of PwrCor Inc. (PWCO) from 2004 until June 2014. He currently oversees investment in Compaction & Recycling Equipment,
Inc. However, nearly 100% of Max’s time is devoted to the Company. He brings a deep insight into cleantech related to wastewater
and solid waste management. Khan owned FINRA registered broker deal Thor Capital LLC from April 2011 through May 2013. Max Khan received
his BA in Accounting and Economics from the City University of New York, and his MBA from Pace University, also in New York.
Aside from that provided above, Mr. Khan does not hold and has not held
over the past five years any other directorships in any company with a class of securities registered pursuant to Section 12 of the Exchange
Act or subject to the requirements of Section 15(d) of the Exchange Act or any company registered as an investment company under the Investment
Company Act of 1940.
Mr. Khan is qualified to serve on our Board of Directors because he has
been an officer and director of a both public and private company and his financial and corporate skills are important for executing the
Company’s business plan.
Enes Kutluca
Enes
is the inventor and founder of Biopipe and devotes 100% of his time to Biopipe since its establishment. at his second year at. All plant
design is overseen by Enes. He continues to focus on research and development to improving the technology for treating a variety of wastewater.
In 2012 he was named the best entrepreneur in Turkey by USA, and the most outstanding young businessman in Turkey. Kutluca was invited
to US by Visitor Leadership Program (IVLP) by U.S. Department of State's in 2013. .
Prior to the merger, Enes was critical in forming a partnership with Metito (Overseas) Limited which is majority owned by Mitsubishi and
IFC. Enes has an engineering degree from Bahcesehir University, Istanbul
Aside from that provided above, Mr. Kutluca does not hold and has not held
over the past five years any other directorships in any company with a class of securities registered pursuant to Section 12 of the Exchange
Act or subject to the requirements of Section 15(d) of the Exchange Act or any company registered as an investment company under the Investment
Company Act of 1940.
Mr. Kutluca is qualified to serve on our Board of Directors because he
the inventor of the Biopipe system and is critical for our success, improvement of our technology and development of our intellectual
property. Enes is also the largest shareholder of the Company.
Term of Office
Our directors are appointed for a one-year term and automatically re-appointed
until removed from office in accordance with our bylaws. Our officers are appointed by our board of directors and hold office until removed
by the board, subject to their respective employment agreements.
Third Party Providers
Securities Legal Counsel:
The Doney Law Firm
4955 S. Durango Dr. Ste. 165 | Las Vegas, NV 89113
Office: (702) 982-5686
Scott@DoneyLawFirm.com
www.doneylawfirm.com
Accounting Firm:
Benjamin Young, CPA
Transfer Agent:
16801 ADDISON ROAD ~ SUITE 247
ADDISON, TEXAS 75001
Telephone ~ 972 612-4120
www.signaturestocktransfer.com
Employees
The Company has seven full-time employees.
Significant Employees
We have no significant employees other than our officers and directors.
We have 5 employees in our subsidiary in India and expect to hire additional personnel in South Africa once the upgraded build own operate
abattoir plant is commissioned again. We rely on short labor for our installations. and country head in India. Mr. Tanmay Pawale, is the
CEO and Director of 99% owned subsidiary in India, Biopipe India Pvt Ltd. (BIPL) Max Khan is also a director of Biopipe India Pvt Ltd.
He devotes 100% of his time to BIPL.
Tanmay brings over 9 years of experience to the techno-commercial domain
with experience in Project Management, Business Development and Consulting in the Solar and Wind Energy sector. He also has extensive
experience in research, advisory, and consultancy in sectors such as renewable energy, energy management, rural development, climate change,
and sustainability. Tanmay is a capable team player with effective team leading and management skills coupled with strong communications
and interpersonal skills. He has worked in countries like the United Kingdom, Thailand, Sri Lanka, Philippines, and Turkey.
Tanmay has completed his M Tech in Rural Development. He has also completed
MS in Engineering Management from NTU, UK with Entrepreneurship and Project Management as a major subject of study. He has completed his
Mechanical Engineering from Kolhapur Institute of Technology. Passionate about Green Project Management,
Nina Aquino is the CMO of Biopipe Global Corp. She is a trained Architect
from California with over 8 years of experience leading teams of engineers, designers and consultants in the Hospitality Sector for companies
such as renowned London-based hospitality giant Soho House & Chipotle Mexican Grill.
She served as Project Architect at Soho House, overseeing project teams
to plan, design, budget and execute: members only clubs, spas and hotels. At Chipotle, working under both the Chief Development Officer
& Chief Marketing Officer, she was instrumental in the fast launch of a new, modularized design. Within the U.S. Northeast market
& Europe, she partnered with internal Marketing teams to deploy the company's strong brand messaging. She graduated Cum Laude with
a Bachelor of Architecture from Syracuse University in New York.
Freddie Canta is our SVP and country head for the Philippines. Freddie
is a finance and operations leader with over 20 years of experience in managing global operations. He has numerous accomplishments in
leading financial operations, driving complex international projects, conversions and programs, implementing/upgrading accounting systems,
creating timely and effective financial reports, managing internal audits and conducting effective financial analysis.
Family Relationships
There are no family relationships between or among the directors, executive
officers, our significant employee, or persons nominated or chosen by us to become directors or executive officers.
Involvement in Certain Legal Proceedings
During the past 10 years, none of our current directors, nominees for directors
or current executive officers has been involved in any legal proceeding identified in Item 401(f) of Regulation S-K, including:
1. Any petition under the Federal bankruptcy laws or any state insolvency
law filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such
person, or any partnership in which he or she was a general partner at or within two years before the time of such filing, or any corporation
or business association of which he or she was an executive officer at or within two years before the time of such filing;
2. Any conviction in a criminal proceeding or being named a subject of
a pending criminal proceeding (excluding traffic violations and other minor offenses);
3. Being subject to any order, judgment, or decree, not subsequently reversed,
suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him or her from, or otherwise limiting,
the following activities:
i. Acting as a futures commission merchant,
introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person
regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter,
broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association
or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
ii. Engaging in any type of business practice;
or
iii. Engaging in any activity in connection
with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal
commodities laws;
4. Being subject to any order, judgment or decree, not subsequently reversed,
suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such
person to engage in any type of business regulated by the Commodity Futures Trading Commission, securities, investment, insurance or banking
activities, or to be associated with persons engaged in any such activity;
5. Being found by a court of competent jurisdiction in a civil action or
by the SEC to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has
not been subsequently reversed, suspended, or vacated;
6. Being found by a court of competent jurisdiction in a civil action or
by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding
by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
7. Being subject to, or a party to, any Federal or State judicial or administrative
order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
i. Any Federal or State securities or commodities
law or regulation; or
ii. Any law or regulation respecting financial
institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution,
civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
iii. Any law or regulation prohibiting mail
or wire fraud or fraud in connection with any business entity; or
8. Being subject to, or a party to, any sanction or order, not subsequently
reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))),
any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange,
association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Director Independence
The Board of Directors reviews the independence of our directors on the
basis of standards adopted by the NASDAQ Stock Market (“NASDAQ”). As a part of this review, the Board of Directors considers
transactions and relationships between our company, on the one hand, and each director, members of the director’s immediate family,
and other entities with which the director is affiliated, on the other hand. The purpose of such a review is to determine which, if any,
of such transactions or relationships were inconsistent with a determination that the director is independent under NASDAQ rules. As a
result of this review, the Board of Directors has determined that none of our directors is an “independent director” within
the meaning of applicable NASDAQ listing standards.
Committees of the Board
Our full board serves the functions that would normally be served by a
separately-designated Audit Committee, Nominating Committee, and Compensation Committee. Further, we do not have an audit committee financial
expert on the Board.
Code of Ethics
We do not have a code of ethics but we plan to adopt one this fiscal year.
Conflicts of Interest
No member of management will be required by us to work on a full-time basis.
Accordingly, certain conflicts of interest may arise between us and our officers and directors in that they may have other business interests
currently and in the future to which they devote their attention, and they may be expected to continue to do so although management time
must also be devoted to our business. Our officers and directors have other business interests in companies other than ours. As a result,
conflicts of interest may arise that can be resolved only through exercise of such judgment as is consistent with each officer's understanding
of his fiduciary duties to the Company.
Currently we have only two officers and directors. We will seek to add
additional officers and/or directors as and when the proper personnel are located and terms of employment are mutually negotiated and
agreed, and we have sufficient capital resources and cash flow to make such offers.
In an effort to resolve such potential conflicts of interest, our officers
and directors have agreed that any opportunities that they are aware of independently or directly through their association with us (as
opposed to disclosure to them of such business opportunities by management or consultants associated with other entities) would be presented
by them solely to us.
We cannot provide assurances that our efforts to eliminate the potential
impact of conflicts of interest will be effective.
EXECUTIVE COMPENSATION
The table below summarizes all compensation awarded to, earned by, or paid
to our former or current executive officers for the fiscal years ended May 31, 2023 and 2022.
Name
and principal
Position |
Year |
Salary
($) |
Bonus
($) |
Stock
Awards
($) |
Option
Awards
($) |
All
Other
Compensation
($) |
Total
($) |
Max
Khan
President,
CEO, Secretary, Treasurer and Director |
2022
2023 |
60,000 |
-
- |
-
- |
-
- |
-
- |
60,000
- |
Enes
Kutluca
COO
and Director |
2022
2023 |
30,000 |
-
- |
-
- |
-
- |
-
- |
30,000
- |
Employment Agreements for New Management
On December 31, 2019, we entered into an employment
agreement with Max Khan to serve as President and CEO for a five-year term. We agreed to compensate Mr. Khan with an annual salary of
$80,000 and he shall be entitled to an annual bonus of $20,000. He is also entitled to an option to purchase 10% of the fully diluted
shares of the company at market value. To date, the Company has not paid salaries as stated in the employment agreement and has unpaid
salary has been accruing at the rate of $5,000 per month. The salary was reduced to preserve cash due to Covid-19 related disruptions
and to preserve capital until the company begins generating meaningful revenue.
On December 31, 2019,
we entered into an employment agreement with Enes Kutluca to serve as COO for a five-year term. We agreed to compensate Mr. Kutluca with
an annual salary of $60,000 and he shall be entitled to an annual bonus of $20,000. He is also entitled to an option to purchase 10% of
the fully diluted shares of the company at market value. The compensation was changed to $2,500 per month and now he is being paid $2,000
per month. The salary was reduced to preserve cash due to Covid-19 related disruptions and to preserve
capital until the company begins generating meaningful revenue.
As of May 31, 2023, we have accrued compensation of
$0 and $64,000 owing to Mr. Kutluca and Mr. Khan, respectively.
Option Grants
We have not granted any options or stock appreciation
rights to our named executive officers or directors since inception. We do not have any stock option plans, but we plan to adopt a plan
in the second half of 2024.
Compensation of Directors
None.
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide
pension, retirement or similar benefits to our directors or executive officers. We have no material bonus or profit-sharing plans pursuant
to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted
at the discretion of the board of directors or a committee thereof.
Compensation Committee
We do not currently have a compensation committee
of the board of directors or a committee performing similar functions. The board of directors as a whole participates in the consideration
of executive officer and director compensation.
Board Committees and Director Independence
Our securities are not quoted on an exchange that
has requirements that a majority of our Board members be independent and we are not currently otherwise subject to any law, rule or regulation
requiring that all or any portion of our Board of Directors include "independent" directors, nor are we required to establish
or maintain an Audit Committee or other committee of our Board of Directors.
The Board does not have standing audit, compensation
or nominating committees. The Board does not believe these committees are necessary based on the size of our company and the current levels
of compensation to corporate officers. We will consider establishing audit, compensation and nominating committees at the appropriate
time
Indebtedness of Directors, Senior Officers, Executive Officers and Other
Management
None of our directors or executive officers or any
associate or affiliate of our company during the last two fiscal years is or has been indebted to our company by way of guarantee, support
agreement, letter of credit or other similar agreement or understanding currently outstanding.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND MANAGEMENT
The following table sets forth, as of February
15, 2024 certain information as to shares of our voting stock owned by (i) each person known by us to beneficially own more than 5% of
our outstanding voting stock, (ii) each of our directors, and (iii) all of our executive officers and directors as a group.
Unless otherwise indicated below, to our knowledge,
all persons listed below have sole voting and investment power with respect to their shares of voting stock, except to the extent authority
is shared by spouses under applicable law. Unless otherwise indicated below, each entity or person listed below maintains an address of
100 Challenger Road, 8th Floor, Ridgefield Park, NJ 07660.
The
number of shares beneficially owned by each stockholder is determined under rules promulgated by the SEC. The information is not necessarily
indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares as to which the
individual or entity has sole or shared voting or investment power and any shares as to which the individual or entity has the right to
acquire beneficial ownership within 60 days through the exercise of any stock option, warrant or other right. The inclusion in the following
table of those shares, however, does not constitute an admission that the named stockholder is a direct or indirect beneficial owner.
| Name
of Officer/Director and Control Person |
Affiliation
with Company (e.g. Officer/Director/Owner of more than 5%) |
Residential
Address (City / State Only) |
Number
of shares owned |
Share
type/class |
Ownership
Percentage of Class Outstanding |
Mehmet
Kutluca
|
5%
Owner, COO & Director |
Bengi
Sokak Erenkoy Mahllesi Sayan Hanim Apartmani No. 4Daire 20 Istanbul, Turkey |
23,302,342 |
Common |
19.34% |
| Max
Khan |
CEO,
Director |
Ridgefield,
New Jersey |
NA |
NA |
NA |
| Enver
Mısırlı |
5%
Owner |
Yesilvadi
Sokak Yesilvadi Konaklari, Fatih Sultan Mehmet mahallesi E20 Blok Daire 1 Istanbul, Turkey |
13,713,297 |
Common |
11.38% |
| Nilgün
Sebnem Berker |
5%
Owner |
Ulus
Mahallesi Kör Kadi Sokak Tekfen Evleri G Blok, Istanbul, Turkey |
10,280,468 |
Common
|
8.53% |
| Erinç
Alper |
5%
Owner |
Seefeldstrasse
129
Zurich,
8008
Switzerland |
7,964,849 |
Common |
6.61% |
| |
(1) |
Unless otherwise indicated, each person or entity named in the table has sole voting power and investment power (or shares that power with that person’s spouse) with respect to all shares of voting stock listed as owned by that person or entity. |
| |
(2) |
Pursuant to Rules 13d-3
and 13d-5 of the Exchange Act, beneficial ownership includes any shares as to which a shareholder has sole or shared voting power
or investment power, and also any shares which the shareholder has the right to acquire within 60 days, including upon exercise of
common shares purchase options or warrants. The percent of class is based on 120,484,150 voting shares as of February 15, 2024 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Other than described below or the transactions described under the heading
“Executive Compensation” (or with respect to which such information is omitted in accordance with SEC regulations), there
have not been, and there is not currently proposed, any transaction or series of similar transactions to which we were or will be a participant
in which the amount involved exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets at year-end
for the last two completed fiscal years, and in which any director, executive officer, holder of 5% or more of any class of our capital
stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
On April 17, 2019, we entered into an Agreement and Plan of Merger (the
“Merger Agreement”) with BioPipe Acquisition, Inc., a New Jersey corporation (“Merger Sub”) and BioPipe Global
Corp., a privately held New Jersey corporation (“BioPipe Global”). In connection with the closing of this merger transaction,
Merger Sub merged with and into BioPipe Global (the “Merger”) on April 30, 2019, with the filing of Articles of Merger with
the New Jersey Secretary of State.
In addition, pursuant to the terms and conditions of the Merger Agreement:
| |
• |
All of the outstanding shares of BioPipe Global was
exchanged for the right to receive an aggregate of 75,000,000 share of the Company’s common stock, par value $0.001 per share
(the “Common Stock”), which was issued to certain shareholders in connection with an Intellectual Property Purchase Agreement
set forth below; |
| |
• |
BioPipe Global provided customary representations and
warranties and closing conditions, including approval of the Merger by a majority of its voting shareholders; and |
| |
• |
Bradford Brock, our prior officer and director, was
required to cancel 55,000,000 shares of his Common Stock in the Company but permitted to retain 1,000,000 shares in the Company.
|
On May 7, 2019, BioPipe Global acquired all the assets of BioPipe Global
AG, a Swiss company, and BioPipe Cevre Teknolojileri A.S. a Turkish company. We acquired all patents, trade receivables, income, royalties,
damages, rights to sue, rights to enforce and any and all payments unpaid and due now or hereafter due or payable with respect to the
BioPipe System.
The Company issued Seventy-One Million Eight Hundred Forty-Six Thousand
Six hundred and Sixty-Seven shares (71,846,667) duly authorized, validly issued, fully paid and nonassessable shares of common stock
to the Shareholders of Biopipe Cevre Teknolojileri A.S and a Three million One Hundred and Fifty-three Thousand and Three Hundred and
Thirty-Three (3,153,333) duly authorized, validly issued, fully paid and nonassessable shares of common stock to Biopipe Global AG.
ADDITIONAL INFORMATION
This Offering Circular does not purport to restate
all of the relevant provisions of the documents referred to or pertinent to the matters discussed herein, all of which must be read for
a complete description of the terms relating to an investment in us. Such documents are available for inspection during regular business
hours at our office by appointment, and upon written request, copies of documents not annexed to this Offering Circular will be provided
to prospective investors. Each prospective investor is invited to ask questions of, and receive answers from, our representatives. Each
prospective investor is invited to obtain such information concerning us and this offering, to the extent we possess the same or can acquire
it without unreasonable effort or expense, as such prospective investor deems necessary to verify the accuracy of the information referred
to into their Offering Circular. Arrangements to ask such questions or obtain such information should be made by contacting Max Khan -
CEO at our executive offices. The telephone number is 646-201-5242. We reserve the right, however, in our sole discretion, to condition
access to information that management deems proprietary in nature, on the execution by each prospective investor of appropriate confidentiality
agreements prior to having access to such information.
The offering of the common stock is made solely by
this Offering Circular and the exhibits hereto. The prospective investors have a right to inquire about and request and receive any additional
information they may deem appropriate or necessary to further evaluate this offering and to make an investment decision. Our representatives
may prepare written responses to such inquiries or requests if the information requested is available. The use of any documents other
than those prepared and expressly authorized by us in connection with this offering is not permitted, and should not be relied upon by
any prospective investor.
ONLY INFORMATION OR REPRESENTATIONS CONTAINED HEREIN
MAY BE RELIED UPON AS HAVING BEEN AUTHORIZED BY US. NO PERSON HAS BEEN AUTHORIZED TO GIVE ANY INFORMATION OR TO MAKE ANY REPRESENTATIONS
OTHER THAN THOSE CONTAINED IN THIS OFFERING CIRCULAR IN CONNECTION WITH THE OFFER BEING MADE HEREBY, AND IF GIVEN OR MADE, SUCH INFORMATION
OR REPRESENTATIONS MUST NOT BE RELIED UPON AS HAVING BEEN AUTHORIZED BY US. INVESTORS ARE CAUTIONED NOT TO RELY UPON ANY INFORMATION NOT
EXPRESSLY SET FORTH IN THIS OFFERING CIRCULAR. THE INFORMATION PRESENTED IS AS OF THE DATE ON THE COVER HEREOF UNLESS ANOTHER DATE IS
SPECIFIED, AND NEITHER THE DELIVERY OF THIS OFFERING CIRCULAR NOR ANY SALE HEREUNDER SHALL CREATE ANY IMPLICATION THAT THERE HAS BEEN
NO CHANGE IN THE INFORMATION PRESENTED SUBSEQUENT TO SUCH DATES(S).
FINANCIAL
STATEMENTS AND EXHIBITS.
LifeQuest
World Corp.
INDEX TO YEAR
END FINANCIAL STATEMENTS
| |
|
Page |
| |
|
|
| Balance Sheets |
|
F-1 |
| |
|
|
| Statement of Operations |
|
F-2 |
| |
|
|
| Statement of Changes in Stockholders’ Deficit |
|
F-3 |
| |
|
|
| Statement of Cash Flows |
|
F-4 |
| |
|
|
| Notes to Financial Statements |
|
F-5 - F-11 |
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(unaudited)
| | |
May
31, 2023 | |
May
31, 2022 |
| ASSETS | |
| | | |
| | |
| CURRENT ASSETS | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 309,552 | | |
$ | 517,072 | |
| Subscription
receivable | |
| — | | |
| 10,000 | |
| Accounts
receivable | |
| — | | |
| 183,200 | |
| Inventory | |
| — | | |
| 10,000 | |
| Other current
assets | |
| — | | |
| 31,543 | |
| | |
| | | |
| | |
| Total Current
Assets | |
| 309,552 | | |
| 751,815 | |
| | |
| | | |
| | |
| FIXED ASSETS | |
| | | |
| | |
| Aquity
Plant | |
| 286,500 | | |
| 370,511 | |
| Machinery
and equipment, net | |
| 8,250 | | |
| 18,224 | |
| | |
| | | |
| | |
| Total Fixed
Assets | |
| 294,750 | | |
| 388,735 | |
| | |
| | | |
| | |
| INTANGIBLE ASSETS | |
| | | |
| | |
| Intellectual
property | |
| 45,000 | | |
| 52,500 | |
| | |
| | | |
| | |
| Total Other
Assets | |
| 45,000 | | |
| 52,500 | |
| | |
| | | |
| | |
| TOTAL ASSETS | |
$ | 649,302 | | |
$ | 1,193,050 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’
EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| Accounts
payable and accrued liabilities | |
$ | 8,750 | | |
$ | 128,207 | |
| Accrued
compensation | |
| 64,000 | | |
| 60,000 | |
| Credit
card payable | |
| 8,322 | | |
| 1,540 | |
| | |
| | | |
| | |
| Total Current
Liabilities | |
| 81,072 | | |
| 189,747 | |
| | |
| | | |
| | |
| STOCKHOLDERS' EQUITY | |
| | | |
| | |
| Common
stock (Par $0.001), 550,000,000 authorized, 116,234,150 and 114,959,150 issued and outstanding | |
| 116,234 | | |
| 114,959 | |
| Paid-in
capital in excess of par value | |
| 1,852,718 | | |
| 1,841,051 | |
| Non-controlling
interest | |
| (14,785 | ) | |
| 53,516 | |
| Retained
deficit | |
| (1,385,937 | ) | |
| (1,006,223 | ) |
| | |
| | | |
| | |
| Total Stockholders'
Equity | |
| 568,230 | | |
| 1,003,303 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES
AND STOCKHOLDERS' EQUITY | |
$ | 649,302 | | |
| 1,193,050 | |
The accompanying consolidated financials
were not subject to an audit, review, or compilation. The accompanying notes are an integral part of these consolidated financial statements.
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
(unaudited)
| | |
For the year
ended | |
For the year
ended |
| | |
May
31, 2023 | |
May
31, 2022 |
| | |
| |
|
| INCOME | |
$ | 149,806 | | |
$ | 349,297 | |
| | |
| | | |
| | |
| COST OF SALES | |
| 111,936 | | |
| 258,336 | |
| | |
| | | |
| | |
| GROSS PROFIT | |
| 37,870 | | |
| 90,961 | |
| | |
| | | |
| | |
| OPERATING EXPENSES | |
| | | |
| | |
| | |
| | | |
| | |
| Amortization
expense | |
| 7,500 | | |
| 7,500 | |
| Depreciation
expense | |
| 55,306 | | |
| 34,836 | |
| Wages
expense | |
| 46,725 | | |
| 98,526 | |
| Postage
and shipping | |
| 1,057 | | |
| 6,353 | |
| Professional
fees | |
| 78,323 | | |
| 118,594 | |
| Rent expense | |
| 2,056 | | |
| 9,115 | |
| Advertising
expense | |
| 1,027 | | |
| 1,755 | |
| Travel
expense | |
| 29,150 | | |
| 6,055 | |
| Utilities | |
| 11,845 | | |
| 6,863 | |
| Audit
fees | |
| — | | |
| 17,500 | |
| General
and administrative | |
| 74,335 | | |
| 71,467 | |
| | |
| | | |
| | |
| OPERATING
EXPENSES | |
| 307,324 | | |
| 378,564 | |
| | |
| | | |
| | |
| OTHER INCOME (EXPENSE) | |
| | | |
| | |
| | |
| | | |
| | |
| Foreign
currency gains and losses | |
| 50,186 | | |
| (24,641 | ) |
| Loss on
disposal of subsidiary | |
| (92,227 | ) | |
| — | |
| Interest
income | |
| 82 | | |
| 288 | |
| Interest
expense | |
| — | | |
| 130 | |
| | |
| | | |
| | |
| TOTAL
OTHER EXPENSE | |
| (41,959 | ) | |
| (24,223 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | |
| NET INCOME (LOSS) | |
| (311,413 | ) | |
| (311,826 | ) |
| | |
| | | |
| | |
| LESS NET (INCOME) LOSS ALLOCATED | |
| | | |
| | |
| TO NONCONTROLLING
INTEREST | |
| (68,301 | ) | |
| 94,488 | |
| | |
| | | |
| | |
| NET
INCOME (LOSS) | |
$ | (379,714 | ) | |
$ | (217,338 | ) |
The accompanying consolidated financials
were not subject to an audit, review, or compilation. The accompanying notes are an integral part of these consolidated financial statements.
LIFEQUEST WORLD CORPORATION, INC.
AND SUBSIDIARIES
Consolidated Statement of Stockholders’ Equity (Deficit)
(unaudited)
| | |
Common
Stock | |
| |
| |
| |
|
| | |
Shares | |
Amount | |
Paid
in Capital in Excess of Par Value | |
Noncontrolling
Interest | |
Retained
Deficit | |
Total
Stockholders' Equity |
| Balance, May 31, 2022 | |
| 114,959,150 | | |
$ | 114,959 | | |
$ | 1,841,051 | | |
$ | 53,516 | | |
$ | (1,006,223 | ) | |
$ | 1,003,303 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for services
rendered | |
| 1,275,000 | | |
| 1,275 | | |
| (1,275 | ) | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Entry of new joint venture | |
| — | | |
| — | | |
| 12,942 | | |
| — | | |
| — | | |
| 12,942 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss
for the year ended May 31, 2023 | |
| — | | |
| — | | |
| — | | |
| (68,301 | ) | |
| (379,714 | ) | |
| (448,015 | ) |
| Balance,
May, 31 2023 | |
| 116,234,150 | | |
$ | 116,234 | | |
$ | 1,852,718 | | |
$ | (14,785 | ) | |
$ | (1,385,937 | ) | |
$ | 568,230 | |
The accompanying consolidated financials
were not subject to an audit, review, or compilation. The accompanying notes are an integral part of these consolidated financial statements.
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
(unaudited)
| | |
For
the year | |
For
the year |
| | |
May
31, 2023 | |
May
31, 2022 |
| CASH FLOWS FROM OPERATING
ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (379,714 | ) | |
$ | (217,338 | ) |
| Adjustments to reconcile net
loss to net cash | |
| | | |
| | |
| used in operating activities: | |
| | | |
| | |
| Net Loss
allocated to noncontrolling interest | |
| (68,301 | ) | |
| 81,646 | |
| Depreciation
expense | |
| 55,306 | | |
| 34,836 | |
| Amortization
expense | |
| 7,500 | | |
| 7,500 | |
| Change
in accounts receivable | |
| 183,200 | | |
| (143,416 | ) |
| Change
in subscriptions receivable | |
| 10,000 | | |
| — | |
| Change
in inventory | |
| 10,000 | | |
| 8,204 | |
| Change
in underbillings | |
| — | | |
| 30,372 | |
| Change
in other current assets | |
| 31,543 | | |
| 9,319 | |
| Change
in accounts payable and accrued expenses | |
| (119,457 | ) | |
| 35,023 | |
| Change
in accrued compensation | |
| 4,000 | | |
| 30,000 | |
| Change
in overbillings | |
| — | | |
| (113,822 | ) |
| Change
in credit cards payable | |
| 6,782 | | |
| (5,250 | ) |
| Net
Cash Used in Operating Activities | |
| (259,141 | ) | |
| (242,926 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING
ACTIVITIES: | |
| | | |
| | |
| Purchases
of fixed assets | |
| — | | |
| (397,847 | ) |
| Change
in fixed asset basis from foreign currency | |
| 38,679 | | |
| — | |
| Net
Cash Provided by (Used in) Financing Activities | |
| 38,679 | | |
| (397,847 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING
ACTIVITIES: | |
| | | |
| | |
| Contributed
capital | |
| 12,942 | | |
| 50,000 | |
| Proceeds
from notes payable - related party | |
| — | | |
| (30,588 | ) |
| Net
Cash Provided by Financing Activities | |
| 12,942 | | |
| 19,412 | |
| | |
| | | |
| | |
| NET DECREASE IN CASH | |
| (207,520 | ) | |
| (621,361 | ) |
| | |
| | | |
| | |
| CASH AT BEGINNING OF PERIOD | |
| 517,072 | | |
| 1,138,433 | |
| | |
| | | |
| | |
| CASH AT END OF PERIOD | |
$ | 309,552 | | |
$ | 517,072 | |
| | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURES | |
| | | |
| | |
| | |
| | | |
| | |
| Cash
Paid For: | |
| | | |
| | |
| | |
| | | |
| | |
| Interest | |
$ | — | | |
$ | — | |
| Income
taxes | |
$ | — | | |
$ | — | |
The accompanying consolidated financials
were not subject to an audit, review, or compilation. The accompanying notes are an integral part of these consolidated financial statements.
NOTE
1 - ORGANIZATION AND DESCRIPTION OF BUSINESS
LifeQuest
World Corporation was incorporated under the laws of the State of Minnesota on November 1, 1997. The Company develops and distributes
dietary supplements. The shares of the Company trade on the Over-the-Counter Bulletin Board under the symbol, “LQWC.”
On
October 20, 2017, the Company entered into a Share Exchange Agreement with Amagon ApS. The Company acquired 100% interest in Amagon ApS
in exchange for 50,000,000 shares of the Company’s Series B Preferred Stock. Since the shareholders of Amagon ApS control the Company
upon consummation of the Share Exchange through the voting rights in the preferred stock, the transaction has been recorded as a reverse
merger and resulted in a recapitalization with Amagon ApS being the acquirer for accounting purposes. Accordingly, the historical financial
statements are those of Amagon ApS and have been prepared to give retroactive effect to the reverse acquisition.
On
September 26, 2019, Biopipe Africa Ltd., 50% owned joint venture was established. As of May 31, 2023 the other 50% joint owner has not
contributed required capital, the subscription receivable is therefore considered impaired. See Note 6 for additional discussion.
On
April 19, 2022, Biopipe India, 99% owned subsidiary was established. See Note 6 for additional discussion.
Collectively
LifeQuest World Corporation and its wholly owned subsidiary are collectively referred herein as “the Company.”
NOTE
2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This
summary of significant accounting policies of the Company is presented to assist in understanding the Company's financial statements
which conform to U.S. generally accepted accounting principles. The consolidated financial statements and notes are representations of
the Company's management, which is responsible for their integrity and objectivity. These accounting policies conform to generally accepted
accounting principles and have been consistently applied in the preparation of the consolidated financial statements. The following policies
are considered to be significant:
Principles
of Consolidation
The
accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the
United States of America and include the accounts of LifeQuest World Corporation, and its 50% owned subsidiary BioPipe Africa LTD, and
its 70% owned subsidiary Aquity Capital Ltd, and its 99% owned subsidiary Biopipe India Private Limited. All significant intercompany
transactions and balances have been eliminated.
Basis
of Accounting
The
consolidated financial statements of the Company are prepared using the accrual method of accounting in accordance with accounting principles
generally accepted in the United States of America. The Company has elected a May 31 year-end.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents, unless
held for reinvestment as part of the investment portfolio, pledged to secure loan agreements or otherwise encumbered. The carrying amount
approximates the fair value because of the short maturities of those instruments.
Property
and Equipment
Property
and equipment are stated at cost less accumulated depreciation. Repairs and maintenance are expensed as incurred, whereas major improvements
are capitalized. If donated, property and equipment are recorded at the approximate fair value on the date of donation. Depreciation
is computed using the straight-line method over the estimated useful lives of the related assets.
Impairment
of Long-Lived Assets
The
Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset
may not be recoverable. The Company evaluates the recoverability of long-lived assets by measuring the carrying amounts of the assets
against the estimated undiscounted cash flows associated with these assets. At the time such evaluation indicates that the future undiscounted
cash flows of certain long-lived assets are not sufficient to recover the assets’ carrying value, the assets are adjusted to their
fair value (based upon discounted cash flows). No impairment losses were recognized for the year ended May 31, 2023 and 2022, respectively.
Use
of Estimates
The
preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires
management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses, including functional
allocations during the reporting period. Actual results could differ from those estimates. Management bases its estimates on historical
experience and on various other assumptions that are believed to be reasonable under the circumstances in making judgments about the
carrying value of assets and liabilities that are not readily apparent from other sources. While actual results could differ from those
estimates, management believes that the estimates are reasonable.
Key
estimates made in the accompanying financial statements include, among others, the economic useful lives and recovery of long-lived assets
and contingencies.
Concentrations
of Risk
The
Company maintains its cash in bank deposit accounts which, at times, may exceed the federally insured limits. Accounts are guaranteed
by the Federal Deposit Insurance Corporation (FDIC) up to certain limits. The Company has not experienced any losses in such accounts
or lack of access to its cash, and believes it is not exposed to significant risk of loss with respect to cash. However, no assurance
can be provided that access to the Company’s cash will not be impacted by adverse economic conditions in the financial markets.
At
May 31, 2023, the Company had in its bank accounts $57,235 in excess of the $250,000 per depository institution that is federally insured.
Contingencies
Certain
conditions may exist as of the date that these financial statements are issued which may result in a loss to the Company, but which will
only be resolved when one or more future events occur or fail to occur. The Company’s management and its legal counsel assess such
contingent liabilities and such assessments inherently involve the exercise of judgment. In assessing loss contingencies related to legal
proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal
counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of
relief sought or expected to be sought therein.
If
the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability
can be estimated, then the estimated liability is accrued in the Company's financial statements. If the assessment indicates that a potentially
material loss contingency is not probable, but is reasonably possible, or is probable but cannot be estimated, then the nature of the
contingent liability, together with an estimate of the range of possible loss if determinable and material, is disclosed. Loss contingencies
considered remote are generally not disclosed unless they involve guarantees, in which case the nature of the guarantee is disclosed.
Fair
Value of Financial Instruments
The
Company’s financial instruments consist of cash and cash equivalents, accounts payable and accrued expenses, and shareholder loans.
The carrying amount of these financial instruments approximates fair value due either to length of maturity or interest rates that approximate
prevailing market rates unless otherwise disclosed in these financial statements.
Financial
assets and liabilities recorded at fair value on the balance sheets are categorized based upon a fair value hierarchy established by
GAAP, which prioritizes the inputs used to measure fair value into the following levels:
Level
1— Quoted market prices in active markets for identical assets or liabilities at the measurement date.
Fair
Value of Financial Instruments (Continued)
Level
2— Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets and liabilities
in markets that are not active; or other inputs that are observable and can be corroborated by observable market data.
Level
3— Inputs reflecting management’s best estimates and assumptions of what market participants would use in pricing assets
or liabilities at the measurement date. The inputs are unobservable in the market and significant to the valuation of the instruments.
Financial
instrument’s categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair
value measurement.
Revenue
Recognition
The
Company recognizes revenue on products based on a percentage of completion methodology.
Recent
Accounting Pronouncements
Other
recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force) did not or are not believed to have a
material impact on the Company’s present or future financial statements.
Accounts
Receivable
Accounts
receivables are stated at the amount billed to the Company’s customer. The Company provides an allowance for doubtful accounts,
which is based upon a review of outstanding receivables, historical collection information, and existing economic conditions.
Accounts
receivable is ordinarily due 30 days after the issuance of the invoice. Accounts past due more than 120 days are considered delinquent
unless determined otherwise by Company’s management. Delinquent receivables are written off based on individual credit evaluation
and specific circumstances of the customer.
NOTE
3 - LIQUIDITY AND GOING CONCERN
The
Company has incurred losses since inception and incurred an operating loss for the year ended May 31, 2023 due to Covid-19 related disruption
which disrupted business through first half of 2022, the Company had an operating loss of $379,714.
The
ability of the Company to continue as a going concern is dependent on the Company generating cash from the sale of its common stock and/or
obtaining debt financing and attaining future profitable operations. Management’s plans include selling its equity securities and
obtaining debt financing to fund its capital requirement and ongoing operations; however, here can be no assurance the Company will be
successful in these efforts. The Company has adequate working capital for the foreseeable future.
NOTE
4 - CONVERTIBLE NOTES AND SETTLEMENT AGREEMENT
Convertible
Notes
On
November 7, 2018, the Company issued a convertible promissory note in the amount of $25,000. The note is due on demand and bears interest
at 12% per annum. The loan and any accrued interest can then be converted into shares of the Company’s common stock at a rate of
$0.01 per share of common stock. Because of the discounted conversion price, a beneficial conversion feature of $25,000 was recorded
as a discount to the notes with the offset to Additional Paid-in Capital. As of August 30, 2019, $25,000 of the debt discount was amortized.
On
December 4, 2018, the Company issued a convertible promissory note in the amount of $23,000. The note is due on demand and bears interest
at 12% per annum. The loan and any accrued interest can then be converted into shares of the Company’s common stock at a rate of
$0.01 per share of common stock. Because of the discounted conversion price, a beneficial conversion feature of $22,000 was recorded
as a discount to the notes with the offset to Additional Paid-in Capital. As of August 30, 2019, $23,000 of the debt discount was amortized.
On
February 20, 2019, the Company issued a convertible promissory note in the amount of $40,000. The note is due on demand and bears interest
at 12% per annum. The loan and any accrued interest can then be converted into shares of the Company’s common stock at a rate of
$0.01 per share of common stock. Because of the discounted conversion price, a beneficial conversion feature of $40,000 was recorded
as a discount to the notes with the offset to Additional Paid-in Capital. As of August 30, 2019, $40,000 of the debt discount was amortized.
All
three of the above notes were settled on June 18, 2019, as part of the stock issuance mentioned in the settlement agreement below.
Settlement
Agreements
On
June 18, 2019, the Company entered into a certain settlement agreement with a shareholder of certain note payables in the amount of $88,000
including accrued interest for 12,571,000 shares of common stock. As of August 31, 2022, the Company had issued 12,571,000 shares of
common stock. The value of the notes and the accrued interest is written off against additional paid-in capital until the shares are
issued.
NOTE
5 – STOCKHOLDERS’ EQUITY
During
the three months ended August 31, 2020, the Company received $142,500 in exchange for the issuance of 950,000 shares of common stock
which were issued in exchange of warrants.
During
the three months ended August 31, 2020, the Company received $365,000 in exchange for the issuance of 2,500,000 shares of common stock
which were issued in exchange of
warrants.
In addition to that issuance 2,000,000 shares were issued on the court settlement reserved shares as explained in Note 4.
During
the three months ended February 28, 2021, the Company received $205,000 in exchange for the issuance of 4,025,000 shares of common stock
which were issued in exchange of warrants.
During
the three months ended May 31, 2021, the Company received $50,000 in exchange for the issuance of 2,250,000 shares of common stock which
were issued in exchange of warrants.
During
the three months ended May 31, 2021, the Company issued 7,071,000 shares on the court settlement reserved shares as explained in Note
4.
During
the three months ended May 31, 2021, the Company issued 250,000 shares for services rendered.
During
the three months ended August 31, 2021, the Company received $50,000 in exchange for the issuance of 250,000 shares of common stock which
were issued in exchange of warrants.
During
the three months ended May 31, 2021, the Company received $50,000 in exchange for the issuance of 2,250,000 shares of common stock which
were issued in exchange of warrants.
During
the three months ended May 31, 2021, the Company issued 7,071,000 shares on the court settlement reserved shares as explained in Note
4.
During
the three months ended May 31, 2021, the Company issued 250,000 shares for services rendered.
During
the three months ended August 31, 2021, the Company received $50,000 in exchange for the issuance of 250,000 shares of common stock which
were issued in exchange of warrants.
During
the three months ended August 31, 2021, the Company issued 1,000,000 shares on the court settlement reserved shares as explained in Note
4.
During
the three months ended February 28, 2022, the Company issued 1,500,000 shares on the court settlement reserved shares as explained in
Note 4.
On
July 6, 2022, a Business and Financial Consulting Agreement was signed between the Company and another entity for consulting services
which involves identifying build own operate and build own operate and transfer projects in various countries. In consideration of consulting
services rendered the Company issued warrants for 1,500,000 shares of common stock at a strike price of $0.05 per share with a 48-month
expiration. The contract also has a provision that additional warrants for 10,500,000 shares of common stock with a strike price of $0.10
with a 48-month expiration are issued and held in escrow if certain debt/equity capital is raised. As of November 30, 2022, the conditions
have not been met to release the warrants from escrow and no warrants have been exercised.
On
August 19, 2022, a Business and Financial Consulting Agreement was signed between the Company and another entity for consulting services.
In consideration of the consulting services rendered the Company issued warrants for 1,500,000 shares of common stock at a strike price
of $0.05 with a 24-month expiration. As of November 30, 2022, none of the warrants have been exercised.
During
the three months ended May 31, 2023, the Company issued as compensation, 1,000,000 shares to Mehmet Enes Kutluca, officer and director;
225,000 shares to Tanmay Pawale, Chief Operating Officer and CEO of Biopipe India and 50,000 shares to Prathamesh Jadhav, Project Manager,
Biopipe
As
of May 31, 2023 and 2022, there were 116,234,150 and 113,459,150 of common stock issued and outstanding, respectively. There were also
-0- shares of preferred stock issued and outstanding.
NOTE
6 – JOINT VENTURES
During
June 2019 the Company entered into a 50-50 Joint Venture Agreement between Biopipe Global Corp and Biotech Innovation for the purpose
of commercialization of Biopipe’s technology in Bangladesh and any and all activities related or incidental thereto and any other
business as mutually agreed upon within the ambit of the objects of the Company as determined in the Memorandum of Association of the
same. During June 2022 that Joint Venture Agreement was terminated. All ownership in the joint venture by the Company was transferred
to the non-controlling interest and a Licensing/Royalty Agreement was signed between the entity and the Company.
On
September 26, 2019, the Company entered into a 50-50 Joint Venture Agreement with Abrimix (PTY) Ltd. for the purpose of commercialization
of Biopipe’s technology in South Africa and several other countries in Africa and any and all activities related or incidental
thereto and any other business as mutually agreed upon within the ambit of the objects of the Company as determined in the Memorandum
of Association of the same.
On
October 2, 2019, the Company entered into a 50-50 Joint Venture Agreement with Environest Private Global Ltd. for the purpose of commercialization
of Biopipe’s technology in India and any and all activities related or incidental thereto and any other business as mutually agreed
upon within the ambit of the objects of the Company as determined in the Memorandum of Association of the same. The Joint Venture was
dissolved and the Company has set up a 99% owned subsidiary in India in 2022.
On
March 1, 2020, the Company entered into a 50-50 Joint Venture Agreement with Hydros Agritech Inc. for the purpose of commercialization
of Biopipe’s technology in the USA and any and all activities related or incidental thereto and any other business as mutually
agreed upon within the ambit of the objects of the Company as determined in the Memorandum of Association of the same. This Joint Venture
was not formalized due to the inability of Hydros Agritech to make full payment for the two plants that were shipped to them.
On
September 28, 2020, the Company entered into a 40-60 Joint Venture Agreement with Biopipe Corporation for the purpose of commercialization
of Biopipe’s technology in the Philippines and any and all activities related or incidental thereto and any other business as mutually
agreed upon within the ambit of the objects of the Company as determined in the
Memorandum
of Association of the same. Although the Joint Venture is 40-60, the shareholders will split profits on a 50-50 basis.
On
July 2, 2021, Lifequest World Corp acquired 70% of Aquity Capital Pty Ltd. a company domiciled in South Africa for ZAR 1,400,000 ($104,000)
and Biopipe Global Corp entered into a credit agreement and provided fully secured project finance debt of $350,000 at South African
prime (7%) + 2% with month amortization and seven-year term. The debt can be prepaid without any penalty. The debt was eliminated in
consolidation. The $454,000 was used for engineering, procurement, construction, installation, and commissioning of a wastewater treatment
plant at an abattoir. The abattoir owner has entered into a 10+10-year water-as-a-service agreement. The plant has not been operating
since May 2022 and requires an upgrade which the Company is currently in the process of doing. The plant is expected back on line in
the 4th quarter of 2024 fiscal year.
On
June 10, 2022, the Company entered into a 99-1 Joint Venture Agreement with Biopipe India for the purpose of commercialization of Biopipe’s
technology in India and any and all activities related or incidental thereto and any other business as mutually agreed upon within the
ambit of the objects of the Company as determined in the Memorandum of Association of the same. The Joint Venture is 99-1, and the shareholders
will split profits on a 99-1 basis.
NOTE
7 – FIXED ASSETS
As
of May 31, 2023 and 2022, machinery and equipment had a basis of $31,892 and $35,900, respectively, and an accumulated depreciation balance
of $23,642 and $17,676, respectively.
As
of May 31, 2023 and 2022, Aquity plant had a basis of $364,637 and $399,012, respectively, and an accumulated depreciation balance of
$78,137 and $28,501, respectively.
Depreciation
expense for the year ended May 31, 2023 and 2022 was $55,306 and $34,836, respectively.
NOTE
8 – INTANGIBLE ASSETS
As
of May 31, 2023 and 2022 intellectual property had a basis of $75,000, and an accumulated amortization balance of $30,000 and $22,500,
respectively. Amortization expense for the year ended May 31, 2023 and 2022, was $7,500, respectively.
During
the three months that ended November 30, 2021, the Company recognized an impairment for the full net book value of the goodwill and trade
names.
NOTE
9 – SUBSEQUENT EVENTS
The
Company has evaluated subsequent events through September 8, 2023, the date which the consolidated financial statements were available
to be issued, and noted no material subsequent events that would require adjustment in or disclosure to these financial statements as
of May 31, 2023.
LifeQuest
World Corp.
INDEX
TO INTERIM FINANCIAL STATEMENTS
FOR SIX MONTHS
ENDED NOVEMBER 30, 2023 AND 2022
| |
|
Page |
| |
|
|
| Balance
Sheets |
|
F-14 |
| |
|
|
| Statement
of Operations |
|
F-15 |
| |
|
|
| Statement
of Changes in Stockholders’ Deficit |
|
F-16 |
| |
|
|
| Statement
of Cash Flows |
|
F-17 |
| |
|
|
| Notes
to Financial Statements |
|
F-18
- F-23 |
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(unaudited)
| | |
November
30, 2023 | |
November
30, 2022 |
| ASSETS | |
| | | |
| | |
| CURRENT ASSETS | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 303,531 | | |
$ | 425,854 | |
| Subscription
receivable | |
| — | | |
| 14,500 | |
| Accounts
receivable | |
| 14,898 | | |
| — | |
| Inventory | |
| 25,542 | | |
| 5,000 | |
| Other
current assets | |
| 1,620 | | |
| — | |
| | |
| | | |
| | |
| Total
Current Assets | |
| 345,591 | | |
| 445,354 | |
| | |
| | | |
| | |
| FIXED ASSETS | |
| | | |
| | |
| Aquity
Plant | |
| 260,455 | | |
| 312,546 | |
| Machinery
and equipment, net | |
| 10,348 | | |
| 11,000 | |
| | |
| | | |
| | |
| Total
Fixed Assets | |
| 270,803 | | |
| 323,546 | |
| | |
| | | |
| | |
| INTANGIBLE ASSETS | |
| | | |
| | |
| Intellectual
property | |
| 41,250 | | |
| 48,750 | |
| Goodwill | |
| — | | |
| — | |
| Trade
Names | |
| — | | |
| — | |
| BioPipe
Africa JV | |
| — | | |
| — | |
| Aquity
Equity | |
| — | | |
| — | |
| Aquity
Debt | |
| — | | |
| — | |
| Biopipe
India | |
| — | | |
| — | |
| BioPipe
Global JV | |
| — | | |
| — | |
| | |
| | | |
| | |
| Total
Other Assets | |
| 41,250 | | |
| 48,750 | |
| | |
| | | |
| | |
| TOTAL
ASSETS | |
$ | 657,644 | | |
$ | 817,650 | |
| | |
| | | |
| | |
| LIABILITIES
AND STOCKHOLDERS' EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| Accounts
payable and accrued liabilities | |
$ | 21,889 | | |
$ | 41,148 | |
| Accrued
compensation | |
| 75,500 | | |
| 79,000 | |
| Overbillings | |
| — | | |
| — | |
| Credit
card payable | |
| 3,960 | | |
| 4,249 | |
| Convertible
promissory note | |
| — | | |
| — | |
| Notes
payable | |
| — | | |
| — | |
| Notes
payable - related party | |
| — | | |
| — | |
| | |
| | | |
| | |
| Total
Current Liabilities | |
| 101,349 | | |
| 124,397 | |
| | |
| | | |
| | |
| STOCKHOLDERS' EQUITY | |
| | | |
| | |
| Common
stock (Par $0.001), 550,000,000 authorized, 118,984,150 and 114,959,150 issued and outstanding | |
| 118,984 | | |
| 114,959 | |
| Paid-in capital in excess
of par value | |
| 1,861,468 | | |
| 1,853,993 | |
| Non-controlling
interest | |
| (22,646 | ) | |
| (8,083 | ) |
| Retained
deficit | |
| (1,401,511 | ) | |
| (1,267,616 | ) |
| | |
| | | |
| | |
| Total
Stockholders' Equity | |
| 556,295 | | |
| 693,253 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES AND STOCKHOLDERS' EQUITY | |
$ | 657,644 | | |
$ | 817,650 | |
The accompanying consolidated financials were not subject
to an audit, review, or compilation.
The accompanying notes are an integral
part of these consolidated financial statements.
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
(unaudited)
| | |
For the six
months ended | |
For the six
months ended |
| | |
November
30, 2023 | |
November
30, 2022 |
| | |
| |
|
| INCOME | |
$ | 42,251 | | |
$ | 45,591 | |
| | |
| | | |
| | |
| COST OF SALES | |
| 23,464 | | |
| 6,441 | |
| | |
| | | |
| | |
| GROSS PROFIT | |
| 18,787 | | |
| 39,150 | |
| | |
| | | |
| | |
| OPERATING EXPENSES | |
| | | |
| | |
| | |
| | | |
| | |
| Amortization
expense | |
| 3,750 | | |
| 3,750 | |
| Depreciation
expense | |
| 28,994 | | |
| 26,340 | |
| Wages
expense | |
| 42 | | |
| 30,000 | |
| Postage
and shipping | |
| 5,088 | | |
| — | |
| Professional
fees | |
| 51,770 | | |
| 42,029 | |
| Rent expense | |
| 1,242 | | |
| — | |
| Advertising
expense | |
| 614 | | |
| 389 | |
| Travel
expense | |
| 3,352 | | |
| 3,278 | |
| Utilities | |
| 2,042 | | |
| 3,252 | |
| Audit
fees | |
| — | | |
| — | |
| General
and administrative | |
| 3,836 | | |
| 41,336 | |
| | |
| | | |
| | |
| OPERATING
EXPENSES | |
| 100,730 | | |
| 150,374 | |
| | |
| | | |
| | |
| OTHER INCOME (EXPENSE) | |
| | | |
| | |
| | |
| | | |
| | |
| Foreign
currency gains and losses | |
| 59 | | |
| 3,595 | |
| Loss on
disposal of subsidiary | |
| — | | |
| (92,227 | ) |
| Interest
income | |
| 20 | | |
| 62 | |
| | |
| | | |
| | |
| TOTAL
OTHER EXPENSE | |
| 79 | | |
| (88,570 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | |
| NET INCOME (LOSS) | |
| (81,864 | ) | |
| (199,794 | ) |
| | |
| | | |
| | |
| LESS
NET (INCOME) LOSS ALLOCATED TO NONCONTROLLING INTEREST | |
| (7,861 | ) | |
| (61,599 | ) |
| | |
| | | |
| | |
| NET
INCOME (LOSS) | |
$ | (89,725 | ) | |
$ | (261,393 | ) |
The accompanying consolidated financials were not subject
to an audit, review, or compilation.
The accompanying notes are an integral
part of these consolidated financial statements.
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Statement of Stockholders’ Equity (Deficit)
(unaudited)
| | |
Common
Stock | |
| |
| |
| |
|
| | |
Shares | |
Amount | |
Paid
in Capital in Excess of Par Value | |
Noncontrolling
Interest | |
Retained
Deficit | |
Total
Stockholders' Equity |
| Balance, May 31, 2023 | |
| 116,234,150 | | |
$ | 116,234 | | |
$ | 1,852,718 | | |
$ | (14,785 | ) | |
$ | (1,311,786 | ) | |
$ | 642,381 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for services
rendered | |
| 2,250,000 | | |
| 2,250 | | |
| (2,250 | ) | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for cash | |
| 500,000 | | |
| 500 | | |
| 11,000 | | |
| — | | |
| — | | |
| 11,500 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss for the six months
ended November 30, 2023 | |
| — | | |
| — | | |
| — | | |
| (7,861 | ) | |
| (89,725 | ) | |
| (97,586 | ) |
| Balance,
November, 30 2023 | |
| 118,984,150 | | |
$ | 118,984 | | |
$ | 1,861,468 | | |
$ | (22,646 | ) | |
$ | (1,401,511 | ) | |
$ | 556,295 | |
The
accompanying consolidated financials were not subject to an audit, review, or compilation.
The
accompanying notes are an integral part of these consolidated financial statements.
LIFEQUEST WORLD CORPORATION, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
(unaudited)
| | |
For the six
months ended | |
For the six months
ended |
| | |
November
30, 2023 | |
November
30, 2022 |
| CASH FLOWS FROM OPERATING
ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (89,725 | ) | |
$ | (261,393 | ) |
| Adjustments to reconcile net
loss to net cash | |
| | | |
| | |
| used in operating activities: | |
| | | |
| | |
| Net Loss
allocated to noncontrolling interest | |
| (7,861 | ) | |
| (61,599 | ) |
| Depreciation
expense | |
| 28,994 | | |
| 26,340 | |
| Amortization
expense | |
| 3,750 | | |
| 3,750 | |
| Change
in accounts receivable | |
| (7,071 | ) | |
| 183,200 | |
| Change
in subscriptions receivable | |
| — | | |
| (4,500 | ) |
| Change
in inventory | |
| 1,956 | | |
| 5,000 | |
| Change
in underbillings | |
| — | | |
| — | |
| Change
in other current assets | |
| (1,063 | ) | |
| 31,543 | |
| Change
in accounts payable and accrued expenses | |
| 11,017 | | |
| (87,059 | ) |
| Change
in accrued compensation | |
| 11,500 | | |
| 19,000 | |
| Change
in credit cards payable | |
| (4,362 | ) | |
| 2,709 | |
| Net
Cash Used in Operating Activities | |
| (52,865 | ) | |
| (143,009 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING
ACTIVITIES: | |
| | | |
| | |
| Change
in fixed asset basis from foreign currency | |
| (750 | ) | |
| 38,849 | |
| Net
Cash Provided by (Used in) Financing Activities | |
| (750 | ) | |
| 38,849 | |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING
ACTIVITIES: | |
| | | |
| | |
| Contributed
capital | |
| 11,500 | | |
| 12,942 | |
| Net
Cash Provided by Financing Activities | |
| 11,500 | | |
| 12,942 | |
| | |
| | | |
| | |
| NET DECREASE IN CASH | |
| (42,115 | ) | |
| (91,218 | ) |
| | |
| | | |
| | |
| CASH AT BEGINNING OF PERIOD | |
| 345,646 | | |
| 517,072 | |
| | |
| | | |
| | |
| CASH AT END OF PERIOD | |
$ | 303,531 | | |
$ | 425,854 | |
| | |
| | | |
| | |
| SUPPLEMENTAL
DISCLOSURES | |
| | | |
| | |
| | |
| | | |
| | |
| Cash
Paid For: | |
| | | |
| | |
| | |
| | | |
| | |
| Interest | |
$ | — | | |
$ | — | |
| Income
taxes | |
$ | — | | |
$ | — | |
The accompanying consolidated financials were not subject
to an audit, review, or compilation.
The accompanying notes are an integral part of these consolidated financial
statements.
LifeQuest World Corporation, Inc. and subsidiaries
Notes to the Consolidated Financial Statements
November 30, 2023 and 2022
NOTE 1
- ORGANIZATION AND DESCRIPTION OF BUSINESS
LifeQuest
World Corporation was incorporated under the laws of the State of Minnesota on November 1, 1997. The Company originally developed and
distributed dietary supplements. The shares of the Company trade on the OTC Markets under the symbol, “LQWC.”
On
April 17, 2019, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with BioPipe Acquisition,
Inc., a New Jersey corporation (“Merger Sub”) and BioPipe Global Corp., a privately held New Jersey corporation (“BioPipe
Global”). In connection with the closing of this merger transaction, Merger Sub merged with and into BioPipe Global (the “Merger”)
on April 30, 2019, with the filing of Articles of Merger with the New Jersey Secretary of State.
In
addition, pursuant to the terms and conditions of the Merger Agreement:
| • | All
of the outstanding shares of BioPipe Global was exchanged for the right to receive an aggregate
of 75,000,000 share of the Company’s common stock, par value $0.001 per share, which
was issued to certain shareholders in connection with an Intellectual Property Purchase Agreement
set forth below; |
| • | BioPipe
Global provided customary representations and warranties and closing conditions, including
approval of the Merger by a majority of its voting shareholders; and |
| • | The
Company’s prior officer and director, was required to cancel 55,000,000 shares of his
Common Stock in the Company but permitted to retain 1,000,000 shares in the Company. |
As
a result of the Merger Agreement, the Company is now engaged in eco-friendly decentralized wastewater treatment. The Company’s
mission is to become a singular platform for highly efficient, scalable, low footprint onsite treatment technologies for treatment sewage
wastewater and industrial wastewater. The Company’s flagship product is Biopipe STP, (sewage wastewater treatment), which is a
highly scalable and biological sewage wastewater system that treats domestic sewage into clean water ready for secondary purposes.
On
May 7, 2019, under an Intellectual Property Purchase Agreement, BioPipe Global acquired all the assets of BioPipe Global AG, a Swiss
company, and its wholly-owned Turkish subsidiary, BioPipe Cevre Teknolojileri A.S. The Company acquired all trade receivables, income,
royalties, damages, rights to sue, rights to enforce and any and all payments unpaid and due now or hereafter due or payable with respect
to the patented BioPipe System.
In
the last five years, the BioPipe STP system has been installed in Bangladesh, India, Ethiopia, the Philippines, South Africa, Turkey,
UAE, Qatar, Saudi Arabia, Oman, and Maldives. These BioPipe systems are running successfully at hospitals, resorts, hotels, commercial
and government buildings, labor camps, ports and individual homes.
On
September 26, 2019, Biopipe Africa Ltd., 50% owned joint venture was established. As of May 31, 2023, as a result of the lack of payment,
the subscription receivable was therefore considered impaired. See Note 5 for additional discussion.
On
April 19, 2022, Biopipe India, 99% owned subsidiary was established. See Note 5 for additional discussion.
Collectively
LifeQuest World Corporation and its wholly owned subsidiary are collectively referred herein as “the Company.”
NOTE
2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This
summary of significant accounting policies of the Company is presented to assist in understanding the Company's financial statements
which conform to U.S. generally accepted accounting principles. The consolidated financial statements and notes are representations of
the Company's management, which is responsible for their integrity and objectivity. These accounting policies conform to generally accepted
accounting principles and have been consistently applied in the preparation of the consolidated financial statements. The following policies
are considered to be significant:
Principles
of Consolidation
The
accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the
United States of America and include the accounts of LifeQuest World Corporation, its 100% owned subsidiary, BioPipe Global Corp., its
50% owned subsidiary BioPipe Africa LTD, and its 70% owned subsidiary Aquity Capital Ltd, and its 99% owned subsidiary Biopipe India
Private Limited. All significant intercompany transactions and balances have been eliminated.
Basis
of Accounting
The
consolidated financial statements of the Company are prepared using the accrual method of accounting in accordance with accounting principles
generally accepted in the United States of America. The Company has elected a May 31 year-end.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents, unless
held for reinvestment as part of the investment portfolio, pledged to secure loan agreements or otherwise encumbered. The carrying amount
approximates the fair value because of the short maturities of those instruments.
Property
and Equipment
Property
and equipment are stated at cost less accumulated depreciation. Repairs and maintenance are expensed as incurred, whereas major improvements
are capitalized. If donated, property and equipment are recorded at the approximate fair value on the date of donation. Depreciation
is computed using the straight-line method over the estimated useful lives of the related assets.
Impairment
of Long-Lived Assets
The
Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset
may not be recoverable. The Company evaluates the recoverability of long-lived assets by measuring the carrying amounts of the assets
against the estimated undiscounted cash flows associated with these assets. At the time such evaluation indicates that the future undiscounted
cash flows of certain long-lived assets are not sufficient to recover the assets’ carrying value, the assets are adjusted to their
fair value (based upon discounted cash flows). No impairment losses were recognized for the quarters s ended November 30, 2023 and 2022,
respectively.
NOTE
2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Use
of Estimates
The
preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires
management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses, including functional
allocations during the reporting period. Actual results could differ from those estimates. Management bases its estimates on historical
experience and on various other assumptions that are believed to be reasonable under the circumstances in making judgments about the
carrying value of assets and liabilities that are not readily apparent from other sources. While actual results could differ from those
estimates, management believes that the estimates are reasonable.
Key
estimates made in the accompanying financial statements include, among others, the economic useful lives and recovery of long-lived assets
and contingencies.
Concentrations
of Risk
The
Company maintains its cash in bank deposit accounts which, at times, may exceed the federally insured limits. Accounts are guaranteed
by the Federal Deposit Insurance Corporation (FDIC) up to certain limits. The Company has not experienced any losses in such accounts
or lack of access to its cash, and believes it is not exposed to significant risk of loss with respect to cash. However, no assurance
can be provided that access to the Company’s cash will not be impacted by adverse economic conditions in the financial markets.
At
November 30, 2023, the Company had in its bank accounts $37,743 in excess of the $250,000 per depository institution that is federally
insured.
Contingencies
Certain
conditions may exist as of the date that these financial statements are issued which may result in a loss to the Company, but which
will only be resolved when one or more future events occur or fail to occur. The Company’s management and its legal counsel
assess such contingent liabilities and such assessments inherently involve the exercise of judgment. In assessing loss contingencies
related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the
Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived
merits of the amount of relief sought or expected to be sought therein.
If
the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability
can be estimated, then the estimated liability is accrued in the Company's financial statements. If the assessment indicates that a potentially
material loss contingency is not probable, but is reasonably possible, or is probable but cannot be estimated, then the nature of the
contingent liability, together with an estimate of the range of possible loss if determinable and material, is disclosed. Loss contingencies
considered remote are generally not disclosed unless they involve guarantees, in which case the nature of the guarantee is disclosed.
NOTE
2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair
Value of Financial Instruments
The
Company’s financial instruments consist of cash and cash equivalents, accounts payable and accrued expenses, and shareholder loans.
The carrying amount of these financial instruments approximates fair value due either to length of maturity or interest rates that approximate
prevailing market rates unless otherwise disclosed in these financial statements.
Financial
assets and liabilities recorded at fair value on the balance sheets are categorized based upon a fair value hierarchy established by
GAAP, which prioritizes the inputs used to measure fair value into the following levels:
Level
1— Quoted market prices in active markets for identical assets or liabilities at the measurement date.
Level
2— Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets and liabilities
in markets that are not active; or other inputs that are observable and can be corroborated by observable market data.
Level
3— Inputs reflecting management’s best estimates and assumptions of what market participants would use in pricing assets
or liabilities at the measurement date. The inputs are unobservable in the market and significant to the valuation of the instruments.
Financial
instrument’s categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair
value measurement.
Revenue
Recognition
The
Company recognizes revenue on products based on a percentage of completion methodology.
Recent
Accounting Pronouncements
Other
recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force) did not or are not believed to have a
material impact on the Company’s present or future financial statements.
Accounts
Receivable
Accounts
receivables are stated at the amount billed to the Company’s customer. The Company provides an allowance for doubtful accounts,
which is based upon a review of outstanding receivables, historical collection information, and existing economic conditions.
Accounts
receivables are ordinarily due 30 days after the issuance of the invoice. Accounts past due more than 120 days are considered delinquent
unless determined otherwise by Company’s management. Delinquent receivables are written off based on individual credit evaluation
and specific circumstances of the customer.
NOTE
3 - LIQUIDITY AND GOING CONCERN
The
Company has incurred losses since inception and incurred an operating loss for the six months ended November 30, 2023, the Company had
an operating loss of $89,725.
The
ability of the Company to continue as a going concern is dependent on the Company generating cash from the sale of its common stock and/or
obtaining debt financing and attaining future profitable operations. Management’s plans include selling its equity securities and
obtaining debt financing to fund its capital requirement and ongoing operations; however,
there
can be no assurance the Company will be successful in these efforts. The Company has adequate working capital for the foreseeable future.
NOTE
4 – STOCKHOLDERS’ EQUITY
As
of August 31, 2022, the Company had 114,959,150 shares of common stock.
On
July 6, 2022, a Business and Financial Consulting Agreement was signed between the Company and another entity for consulting services.
In consideration of consulting services rendered the Company issued warrants for 1,500,000 shares of common stock at a strike price of
$0.05 per share with a 48-month expiration. The contract also has a provision that additional warrants for 10,500,000 shares of common
stock with a strike price of $0.10 with a 48-month expiration are issued and held in escrow if certain debt/equity capital is raised.
As of August 31, 2023, the conditions have not been met to release the warrants from escrow and no warrants have been exercised.
On
August 19, 2022, a Business and Financial Consulting Agreement was signed between the Company and another entity for consulting services.
In consideration of the consulting services rendered the Company issued warrants for 1,500,000 shares of common stock at a strike price
of $0.05 with a 24-month expiration. As of November 30, 2023, none of the warrants have been exercised.
During
the three months ended November 30, 2024 the Company issued 2,250,000 shares for services rendered and sold 500,000 shares for $11,500
cash.
As
of November 30, 2023 and 2022, there were 118,984,150 and 114,959,150 of common stock issued and outstanding, respectively. There were
also -0- shares of preferred stock issued and outstanding.
NOTE
5 – JOINT VENTURES
During
June 2019 the Company entered into a 50-50 Joint Venture Agreement between Biopipe Global Corp and Biotech Innovation for the purpose
of commercialization of Biopipe’s technology in Bangladesh and any and all activities related or incidental thereto and any other
business as mutually agreed upon within the ambit of the objects of the Company as determined in the Memorandum of Association of the
same. During June 2022 that Joint Venture Agreement was terminated. All ownership in the joint venture by the Company was transferred
to the non-controlling interest and a Licensing/Royalty Agreement was signed between the entity and the Company.
On
September 26, 2019, the Company entered into a 50-50 joint venture and technology transfer agreement with South Africa based, Abrimix
Pty Ltd. The purpose of the agreement was to introduce our Biopipe STP into southern Africa and eventually introduce Abrimix in the countries
we operate in. Abrimix is a patented, low footprint, scalable industrial wastewater treatment technology with the ability to treat a
wide variety of industrial wastewater. Due to disruptions related to Covid-19 and a lack of funding, the parties to the joint venture
dissolved the venture and, in lieu of a joint venture, a technology license agreement was put in place that includes royalty payment
of 7.5% of net revenue.
NOTE
5 – JOINT VENTURES (Continued)
On
October 2, 2019, the Company entered into a 50-50 Joint Venture Agreement with Environest Private Global Ltd. for the purpose of commercialization
of Biopipe’s technology in India and any and all activities related or incidental thereto and any other business as mutually agreed
upon within the ambit of the objects of the Company as determined in the Memorandum of Association of the same. The Joint Venture was
dissolved and the Company has set up a 99% owned subsidiary in India in 2022.
On
March 1, 2020, the Company entered into a 50-50 Joint Venture Agreement with Hydros Agritech Inc. for the purpose of commercialization
of Biopipe’s technology in the USA and any and all activities related or incidental thereto and any other business as mutually
agreed upon within the ambit of the objects of the Company as determined in the Memorandum of Association of the same. This Joint Venture
was not formalized.
On
September 28, 2020, the Company entered into a 40-60 Joint Venture Agreement with Biopipe Corporation for the purpose of commercialization
of Biopipe’s technology in the Philippines and any and all activities related or incidental thereto and any other business as mutually
agreed upon within the ambit of the objects of the Company as determined in the
Memorandum
of Association of the same. Although the Joint Venture is 40-60, the shareholders will split profits on a 50-50 basis.
On
July 2, 2021, Lifequest World Corp acquired 70% of Aquity Capital Pty Ltd. a company domiciled in South Africa for ZAR 1,400,000 ($104,000)
and Biopipe Global Corp entered into a credit agreement and provided fully secured project finance debt of $350,000 at South African
prime (7%) + 2% with month amortization and seven-year term. The debt can be prepaid without any penalty. The debt was eliminated in
consolidation. The $454,000 was used for engineering, procurement, construction, installation, and commissioning of a wastewater treatment
plant at an abattoir. The abattoir owner has entered into a 10+10-year water-as-a-service agreement. The plant has not been operating
since May 2022 and requires an upgrade which the Company is currently in the process of doing. The Company entered into a revised water-as-a-serive
agreement on October 10, 2023 under which it will upgrade the plant to higher capacity at circa 42% higher tariff. The upgraded plant
is expected to come online in the first quarter of 2024.
On
June 10, 2022, the Company entered into a 99-1 Joint Venture Agreement with Biopipe India for the purpose of commercialization of Biopipe’s
technology in India and any and all activities related or incidental thereto and any other business as mutually agreed upon within the
ambit of the objects of the Company as determined in the Memorandum of Association of the same. Although the Joint Venture is 99-1, the
shareholders will split profits on a 99-1 basis.
NOTE
6 – FIXED ASSETS
As
of November 30, 2023 and 2022, machinery and equipment had a basis of $32,889 and $32,693, respectively, and an accumulated depreciation
balance of $22,541 and $21,213, respectively.
As
of November 30, 2023 and 2022, Aquity plant had a basis of $364,637, respectively, and an accumulated depreciation balance of $104,182
and $91,159, respectively.
Depreciation
expense for the six months ended November 30, 2023 and 2022 was $28,994 and $26,340, respectively.
NOTE
7 – INTANGIBLE ASSETS
As
of November 30, 2023 and 2022 intellectual property had a basis of $75,000, and an accumulated amortization balance of $33,750 and $31,875,
respectively. Amortization expense for the six months ended November 30, 2023 and 2022, was $3,750, respectively.
NOTE
8 – SUBSEQUENT EVENTS
The
Company has evaluated subsequent events through January 19, 2024, the date which the consolidated financial statements were available
to be issued, and noted no material subsequent events that would require adjustment in or disclosure to these financial statements as
of November 30, 2023.
PART III
| EXHIBITS TO Offering Statement |
|
Exhibit No. |
|
Description |
| |
|
|
| |
|
|
| Exhibit 1A-2A |
|
Articles of Incorporation and Amendments of the Registrant* |
| Exhibit 1A-2B |
|
Bylaws of the Registrant* |
| Exhibit 1A-3 |
|
Amended Stock Designation for Series B Preferred Stock, dated October 24, 2017 |
| Exhibit 1A-4 |
|
Subscription Agreement* |
| Exhibit 1A-4 |
|
Warrant Agreement, dated April 11, 2019* |
| Exhibit 1A-4 |
|
Warrant Agreement, dated February 21, 2022 |
| Exhibit 1A-4 |
|
Warrant Agreement, dated August 1, 2022 |
| Exhibit 1A-4 |
|
Warrant Agreement, dated October 1, 2023 |
| Exhibit 1A-6 |
|
Settlement
Agreement and Stipulation* |
| Exhibit 1A-6 |
|
Settlement Agreement and Stipulation* |
| Exhibit 1A-6 |
|
Convertible Promissory Note, dated November 7, 2018* |
| Exhibit 1A-6 |
|
Convertible Promissory Note, dated December 4, 2018* |
| Exhibit 1A-6 |
|
Convertible Promissory Note, dated February 20, 2018* |
| Exhibit 1A-6 |
|
Senior Convertible Note, dated December 18, 2019* |
| Exhibit 1A-6 |
|
Employment Agreement, Max Khan* |
| Exhibit 1A-6 |
|
Employment Agreement, Enus Kutluca* |
| Exhibit 1A-6 |
|
Amended
and Restated Employment Agreement, Max Khan* |
| Exhibit 1A-6 |
|
Amended
and Restated Employment Agreement, Enes Kutluca* |
| Exhibit 1A-7 |
|
Agreement and Plan of Merger* |
| Exhibit 1A-7 |
|
Agreement of Conveyance, Transfer and Assignment of Subsidiary and Assumption of Obligations* |
| Exhibit 1A-7 |
|
Share Purchase Agreement, dated April 2, 2019* |
| Exhibit 1A-7 |
|
Receivables & Share Purchase Agreement, dated April 2, 2019* |
| Exhibit 1A-7 |
|
Intellectual Property & Receivable Purchase Agreement, dated April 2, 2019* |
| Exhibit 1A-12 |
|
Opinion of The Doney Law Firm with consent to use |
* Previously Filed
SIGNATURES
Pursuant to the requirements of Regulation A, the
issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused
this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Ridgefield Park,
State of New Jersey, on February 21, 2024.
LifeQuest World Corp.
| By: |
/s/ Max Khan |
| |
Max Khan |
| Title: |
President, Chief Executive
Officer, Principal Executive Officer, Principal Financial Officer, Principal Accounting Officer, Secretary, Treasurer and Director |
| |
|
| By: |
/s/
Enes Kutluca |
| |
Enes Kutluca |
| Title: |
Chief Operating Officer
and Director |
This
Offering Circular has been signed below by the following persons in the capacities and on the date indicated.
| By: |
/s/
Max Khan |
| |
Max Khan |
| Title: |
President, Chief Executive
Officer, Principal Executive Officer, Principal Financial Officer, Principal Accounting Officer, Secretary, Treasurer and Director |
| Date: |
February 21, 2024 |
| |
|
| By: |
/s/
Enes Kutluca |
| |
Enes Kutluca |
| Title: |
Chief Operating Officer
and Director |
| Date: |
February 21, 2024 |
AMENDED AND RESTATED CERTIFICATE OF DESIGNATION
OF SERIES B PREFERRED STOCK
OF
LIFEQUEST WORLD CORPORATION
It is hereby certified that:
1. The name
of the corporation (hereinafter called the “Corporation” is LIFEQUEST WORLD CORPORATION.
2. The certificate
of incorporation of the Corporation authorizes the issuance of 50,000,000 shares of Preferred Stock with a one tenth of one cent ($0.001)
par value and expressly vests the Board of Directors of the Corporation the authority provided therein to issue any or all of said shares
in one or more series and by resolution or resolutions, the designation, the number, full or limited voting powers, or the denial of voting
powers, preferences and relative participating, optional, and other special rights and the qualifications, limitations, restrictions,
and other distinguishing characteristics of each series to be issued.
3. On October
15, 2008, the Corporation filed a Certificate of Designation of Series B Preferred Stock of the Corporation. This document is purposed
to amend and restate the original Certificate of Designation to increase the allowance of shares of Series B Preferred Stock and to provide
the same with conversion rights.
4. The Board
of Directors of the Corporation, pursuant to the authority expressly vested in it, has adopted the following resolutions for the Corporation’s
Series B Preferred Stock:
RESOLVED, that fifty million (50,000,000) shares of the Preferred Stock
(par value $0.001 per share) are authorized to be issued by this Corporation pursuant to its certificate of incorporation or the laws
of the state of Minnesota, and that there be and hereby is authorized and created a series of preferred stock, hereby designated as the
Series B Preferred Stock, which shall have the voting powers, designations, preferences, and relative participating, optional or other
rights, if any, of the qualifications, limitations, or restrictions, set forth in such certificate of incorporation and in addition thereto,
those following:
| (a) | DESIGNATION: The Preferred Stock subject hereof shall be designated Series B Preferred Stock (“Series B Preferred Stock”).
No other shares of Preferred Stock shall be designated as Series B Preferred Stock. |
| (b) | DIVIDENDS. The holders of the Series B Preferred Stock shall not be entitled to receive dividends. |
| (c) | NO IMPAIRMENT. The Corporation will not, by amendment of its Certificate of Incorporation or through any reorganization, transfer
of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance
or performance of any of the terms to be observed or performed hereunder by the Corporation, but it will at all times in good faith assist
in the carrying out all of the provisions of this Certificate and in the taking of all such action as may be necessary or appropriate
in order to protect the conversion rights of the holders of the Series B Preferred Stock against impairment. |
| (d) | LIQUIDATION RIGHTS. In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Corporation, the holders
of the Series B Preferred Stock shall not be entitled to receive preference upon liquidation in preference to the holders of common stock
or any other class or series of preferred stock. Rather, the Series B Preferred Stock shall not be entitled to receive any distribution
upon liquidation unless and until all shareholders of common stock shall have received full and fair liquidation. |
| (e) | VOTING. As long as at least 500,000 shares of the Corporation’s Series B Preferred Stock remain outstanding, the separate consent
of the holders of at least 51% of the outstanding Series B Preferred Stock shall be required for any action which (i) alters or change
the rights, preferences or privileges of the Series B Preferred Stock, or (ii) increases or decreases the authorized number of Series
B Preferred Stock. On all other matters, the Series B Preferred Stock shall entitle the holder thereof to such number of votes per share
as shall equal ten (10) shares of Common Stock as of the record date for the determination of stockholders entitled to vote on or consent
to such matters or, if no such record date is established, as of the date on which notice of such meeting is mailed or the date any written
consent of stockholders is solicited. Except as required by law, the holders of shares of Series B Preferred Stock and the common stock
shall vote together as a single class on all matters. |
| (f) | Optional Conversion of Series B Preferred Stock. The holders of Series B Preferred
Stock shall have conversion rights as follows: |
| (i) | Conversion Right. Each share of Series B Preferred Stock shall be convertible at the option of the holder thereof and without the
payment of additional consideration by the holder thereof, at any time, into shares of Common Stock on the Optional Conversion Date (as
hereinafter defined) at a conversion rate of two hundred and forty (240) shares of Common Stock (the “Conversion Rate”) for
every one (1) share of Series B Preferred Stock, subject to adjustment as provided herein. |
| (ii) | No Fractional Shares. No fractional shares of Common Stock or scrip shall be issued upon conversion of shares of Series B Preferred
Stock. In lieu of any fractional share to which the holder would be entitled but for the provisions of this Section based on the number
of shares of Series B Preferred Stock held by such holder, the Corporation shall issue a number of shares to such holder rounded up to
the nearest whole number of shares of Common Stock. No cash shall be paid to any holder of Series B Preferred Stock by the Corporation
upon conversion of Series B Preferred Stock by such Holder. |
| (iii) | Reservation of Stock. The Corporation shall at all times when any shares of Series B Preferred Stock shall be outstanding, reserve
and keep available out of its authorized but unissued Common Stock, such number of shares of Common Stock as shall from time to time be
sufficient to effect the conversion of all outstanding shares of Series B Preferred Stock. If at any time the number of authorized but
unissued shares of Common Stock shall not be sufficient to effect the conversion of all outstanding shares of the Series B Preferred Stock,
the Corporation will take such corporate action as may, in the opinion of its counsel, be necessary to increase its authorized but unissued
shares of Common Stock to such number of shares as shall be sufficient for such purpose. |
| (iv) | Stock Dividends, Splits, Combinations and Reclassifications. If the Corporation shall (i) declare a dividend or other distribution
payable in securities, (ii) split its outstanding shares of Common Stock into a larger number, (iii) combine its outstanding shares of
Common Stock into a smaller number, or (iv) increase or decrease the number of shares of its capital stock in a reclassification of the
Common Stock including any such reclassification in connection with a merger, consolidation or other business combination in which the
Corporation is the continuing entity (any such corporate event, an “Event”), then in each instance the Conversion Rate shall
be proportionately adjusted such that the number of shares issued upon conversion of one share of Series B Preferred Stock will take into
account such Event. |
| (v) | Certificate as to Adjustments. Upon the occurrence of each adjustment or readjustment of the Conversion Rate, the Corporation at its
expense shall promptly compute such adjustment or readjustment in accordance with the terms hereof and cause its principal financial officer
to verify such computation and prepare and furnish to each holder of Series B Preferred Stock a certificate setting forth such adjustment
or readjustment and setting forth in reasonable detail the facts upon which such adjustment or readjustment is based. The Corporation
shall, upon the written request at any time of any holder of Series B Preferred Stock, furnish or cause to be furnished to such holder
a like certificate setting forth: (i) such adjustments and readjustments; (ii) the Conversion Rate in effect at such time for the Series
B Preferred Stock; and (iii) the number of shares of Common Stock and the amount, if any, of other property that at such time would be
received upon the conversion of the Series B Preferred Stock. |
| (vi) | Issue Taxes. The converting holder shall pay any and all issue and other non-income taxes that may be payable in respect of any issue
or delivery of shares of Common Stock on conversion of shares of Series B Preferred Stock.” |
| (g) | STATED VALUE. The shares of Series B Preferred Stock shall have a stated value of $0.001 per share. |
| (h) | OTHER PREFERENCES. The shares of the Series B Preferred Stock shall have no other preferences, rights, restrictions, or qualifications,
except as otherwise provided by law or the certificate of incorporation of the Corporation. |
FURTHER RESOLVED, that the statements contained in the foregoing resolution
creating and designating the said Series B Preferred Stock and fixing the number, powers, preferences and relative, optional, participating,
and other special rights and the qualifications, limitations, restrictions, and other distinguishing characteristics thereof shall, upon
the effective date of said series, be deemed to be included in and be a part of the certificate of incorporation of the Corporation.
Signed on October 24, 2017,
By Unanimous Written Consent of the Board of Directors and the Unanimous
Written Consent of the Holders of Series B Preferred Stock
Director/Series B Preferred Stockholder:
/s/ Bradford Brock
Bradford Brock
Work Item 97841550027
Original File Number 9W-503
STATE OF MINNESOTA
OFFICE OF THE SECRETARY OF STATE
FILED
11/07/2017 11:59 PM
/s/
Steve Simon
Steve Simon
Secretary of State
THE SECURITIES REPRESENTED
BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR REGISTERED OR QUALIFIED UNDER ANY STATE
SECURITIES LAWS. THE SECURITIES MAY NOT BE SOLD, TRANSFERRED, PLEDGED OR HYPOTHECATED UNLESS SUCH SALE, TRANSFER, PLEDGE OR HYPOTHECATION
IS IN ACCORDANCE WITH SUCH ACT AND APPLICABLE STATE SECURITIES LAWS.
Warrant No. 002
No. of Shares of Common Stock: 1,500,000
shares
WARRANT
to Purchase Common Stock of
LifeQuest World Corp
a Minnesotta Corporation
This Warrant
certifies that Berkshire Finance Holdings, Ltd. (“Purchaser”), is entitled to purchase from LifeQuest World Corp. a Minnesotta
Corporation (the “Company”), 1,500,000 shares of Common Stock (or any portion thereof) at an exercise price of $0.05 per share
of Common Stock on a cashless exercise basis, all on the terms and conditions hereinafter provided.
Section 1. Certain Definitions. As used in this Warrant,
unless the context otherwise requires:
“Articles” shall mean the Articles of
Incorporation of the Company, as in effect from time to time.
“Cashless Exercise of Warrant”
shall mean: A cashless exercise enables the investor to pay the exercise price by having the issuer withhold stock otherwise issuable
under the warrant.
“Common Stock” shall mean the Company’s
authorized common stock, $0.001 par value per share.
“Exercise Price”
shall mean the exercise price per share of Common Stock set forth above, as adjusted from time to time pursuant to Section 3 hereof.
“Securities Act” shall mean the Securities
Act of 1933, as amended.
“Warrant”
shall mean this Warrant and all additional or new warrants issued upon division or combination of, or in substitution for, this Warrant.
All such additional or new warrants shall at all times be identical as to terms and conditions and date, except as to the number of shares
of Common Stock for which they may be exercised.
“Warrant
Stock” shall mean the shares of Common Stock purchasable by the holder of this Warrant upon the exercise of such Warrant.
“Warrantholder”
shall mean the Purchaser, as the initial holder of this Warrant, and its nominees, successors or assigns, including any subsequent holder
of this Warrant to whom it has been legally transferred.
Section 2. Exercise of Warrant; Vesting.
(a)
Purchaser may, at any time and from time to time, exercise this Warrant, in whole or in part. This warrant expires in two
year from the date hereof. In addition, the Company shall have the right to call for payment of the Warrant at any time.
(b)
(i) The Warrantholder shall exercise this Warrant by means of delivering to the Company at its office identified in Section
14 hereof (i) a written notice of exercise, including the number of shares of Warrant Stock to be delivered pursuant to such exercise,
(ii) this Warrant and (iii) payment equal to the Exercise Price in accordance with Section 2(b)(ii). In the event that any exercise shall
not be for all shares of Warrant Stock purchasable hereunder, the Company shall deliver to the Warrantholder a new Warrant registered
in the name of the Warrantholder, of like tenor to this Warrant and for the remaining shares of Warrant Stock purchasable hereunder, within
ten (10) days of any such exercise. Such notice of exercise shall be in the Subscription Form set out at the end of this Warrant.
(ii) The Warrantholder may elect to
pay the Exercise Price to the Company either by cash, certified check or wire transfer.
(c)
Upon exercise of this Warrant and delivery of the Subscription Form with proper payment relating thereto, the Company shall
cause to be executed and delivered to the Warrantholder a certificate or certificates representing the aggregate number of fully-paid
and nonassessable shares of Common Stock issuable upon such exercise.
(d)
The stock certificate or certificates for Warrant Stock to be delivered in accordance with this Section 2 shall be in such
denominations as may be specified in said notice of exercise and shall be registered in the name of the Warrantholder or such other name
or names as shall be designated in said notice. Such certificate or certificates shall be deemed to have been issued and the Warrantholder
or any other person so designated to be named therein shall be deemed to have become the holder of record of such shares, including to
the extent permitted by law the right to vote such shares or to consent or to receive notice as stockholders, as of the time said notice
is delivered to the Company as aforesaid.
(e)
The Company shall pay all expenses payable in connection with the preparation, issue and delivery of stock certificates
under this Section 2, resulting from the exercise of the Warrant and the issuance of Warrant Stock hereunder.
(f)
All shares of Warrant Stock issuable upon the exercise of this Warrant in accordance with the terms hereof shall be validly
issued, fully paid and nonassessable, and free from all liens and other encumbrances thereon, other than liens or other encumbrances created
by the Warrantholder.
(g)
In no event shall any fractional share of Common Stock of the Company be issued upon any exercise of this Warrant. If, upon
any exercise of this Warrant, the Warrantholder would, except as provided in this paragraph, be entitled to receive a fractional share
of Common Stock, then the Company shall deliver in cash to such holder an amount equal to such fractional interest.
(h)
Cashless Exercise If at any time after the Commencement Date there is no effective registration statement registering,
or no current prospectus available for, the resale of the Shares by the Holder, then in lieu of exercising this Purchase Warrant by payment
of cash or check payable to the order of the Company pursuant to Section 2.1 above, Holder may elect to receive the number of Shares equal
to the value of this Purchase Warrant (or the portion thereof being exercised), by surrender of this Purchase Warrant to the Company,
together with the exercise form attached hereto, in which event the Company shall issue to Holder, Shares in accordance with the following
formula:
X = Y(A-B)
A
Where,
X = The number of Shares to be issued to Holder;
Y = The number of Shares for which the Purchase
Warrant is being exercised; A = The fair market value of one Share; and
B = The Exercise Price.
For purposes of this Section 2.2, the fair market value
of a Share is defined as follows:
(i)
if the Company’s common stock is traded on a securities exchange, the value shall be deemed to be the closing price
on such exchange prior to the exercise form being submitted in connection with the exercise of the Purchase Warrant; or
(ii)
if the Company’s common stock is actively traded over-the-counter, the value shall be deemed to be the closing bid
price prior to the exercise form being submitted in connection with the exercise of the Purchase Warrant; if there is no active public
market, the value shall be the fair market value thereof, as determined in good faith by the Company’s Board of Directors.
Section 3. Adjustment of Exercise Price and Warrant Stock.
(a)
If, at any time prior to the Expiration Date, the number of outstanding shares of Common Stock is
(i) increased by a stock dividend payable in shares of Common Stock or by a subdivision or split-up of shares of Common Stock, or
(ii) decreased by a combination of shares of Common Stock, then, following the record date fixed for the determination of holders of
Common Stock entitled to receive the benefits of such stock dividend, subdivision, split-up, or combination, the Exercise Price
shall be adjusted to a new amount equal to the product of (I) the Exercise Price in effect on such record date and (II) the quotient
obtained by dividing (x) the number of shares of Common Stock outstanding on such record date (without giving effect to the event
referred to in the foregoing clause (i) or (ii)), by (y) the number of shares of Common Stock which would be outstanding immediately
after the event referred to in the foregoing clause (i) or (ii), if such event had occurred immediately following such record date.
In addition, the Exercise Price may be adjusted in other circumstances set forth in Article 5 of Exhibit A of the
Articles.
(b)
Upon each adjustment of the Exercise Price as provided in Section 3 (a), the Warrantholder shall thereafter be entitled
to subscribe for and purchase, at the Exercise Price resulting from such adjustment, the number of shares of Warrant Stock equal to the
product of (i) the number of shares of Warrant Stock existing prior to such adjustment and (ii) the quotient obtained by dividing (I)
the Exercise Price existing prior to such adjustment by (II) the new Exercise Price resulting from such adjustment.
(c)
If, at any time prior to the Expiration Date, there occurs an event which would cause the automatic conversion (“Automatic
Conversion”) of the Warrant Stock into shares of the Company’s common stock (“Common Stock”) in accordance with
the Articles, then any Warrant shall thereafter be exercisable, prior to the Expiration Date, into the number of shares of Common Stock
into which the Warrant Stock would have been convertible pursuant to the Charter if the Automatic Conversion had not taken place.
Section 4.
Division and Combination. This Warrant may be divided or combined with other Warrants upon presentation at the aforesaid office
of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by
the Warrantholder or its agent or attorney. The Company shall pay all expenses in connection with the preparation, issue and delivery
of Warrants under this Section 4, including any transfer taxes resulting from the division or combination hereunder. The Company agrees
to maintain at its aforesaid office books for the registration of the Warrants.
Section
5. Reclassification, Etc. In case of any reclassification or change of the outstanding Common of the Company (other than as a result
of a subdivision, combination or stock dividend), or in case of any consolidation of the Company with, or merger of the Company into,
another corporation or other business organization (other than a consolidation or merger in which the Company is the continuing corporation
and which does not result in any reclassification or change of the outstanding Common Stock of the Company) at any time prior to the Expiration
Date, then, as a condition of such reclassification, reorganization, change, consolidation or merger, lawful provision shall be made,
and duly executed documents evidencing the same from the Company or its successor shall be delivered to the Warrantholder, so that the
Warrantholder shall have the right prior to the Expiration Date to purchase, at a total price not to exceed that payable upon the exercise
of this Warrant, the kind and amount of shares of stock and other securities and property receivable upon such reclassification, reorganization,
change, consolidation or merger by a holder of the number of shares of Common Stock of the Company which might have been purchased by
the Warrantholder immediately prior to such reclassification, reorganization, change, consolidation or merger, in any such case appropriate
provisions shall be made with respect to the rights and interest of the Warrantholder to the end that the provisions hereof (including
provisions for the adjustment of the Exercise Price and of the number of shares purchasable upon exercise of this Warrant) shall thereafter
be applicable in relation to any shares of stock and other securities and property thereafter deliverable upon exercise hereof.
Section
6. Reservation and Authorization of Capital Stock. The Company shall at all times reserve and keep available for issuance such
number of its authorized but unissued shares of Common Stock as will be sufficient to permit the exercise in full of all outstanding Warrants.
Section
7. Stock and Warrant Books. The Company will not at any time, except upon dissolution, liquidation or winding up, close its stock
books or Warrant books so as to result in preventing or delaying the exercise of any Warrant.
Section
8. Limitation of Liability. No provisions hereof, in the absence of affirmative action by the Warrantholder to purchase Warrant
Stock hereunder, shall give rise to any liability of the Warrantholder to pay the Exercise Price or as a stockholder of the Company (whether
such liability is asserted by the Company or creditors of the Company).
Section 9.
Registration Rights. For as long as the Warrant remains outstanding, in the event of a business combination with the Company, the
Company covenants that it shall at all times maintain one or more current and effective registration statements or offering circulars
that the Warrantholder may utilize for the resale of all Warrant Shares.
Section
10. Transfer. Subject to compliance with the Securities Act and the applicable rules and regulations promulgated thereunder, this
Warrant and all rights hereunder shall be transferable in whole or in part. Any such transfer shall be made at the office or agency of
the Company at which this Warrant is exercisable, by the registered holder hereof in person or by its duly authorized attorney, upon surrender
of this Warrant together with the assignment hereof properly endorsed, and promptly thereafter a new warrant shall be issued and delivered
by the Company, registered in the name of the assignee. Until registration of transfer hereof on the books of the Company, the Company
may treat the Purchaser as the owner hereof for all purposes.
Section
11. Investment Representations; Restrictions on Transfer of Warrant Stock. Unless a current registration statement under the Securities
Act shall be in effect with respect to the Warrant Stock to be issued upon exercise of this Warrant, the Warrantholder, by accepting this
Warrant, covenants and agrees that, at the time of exercise hereof, and at the time of any proposed transfer of Warrant Stock acquired
upon exercise hereof, such Warrantholder will deliver to the Company a written statement that the securities acquired by the Warrantholder
upon exercise hereof are for the account of the Warrantholder or are being held by the Warrantholder as trustee, investment manager, investment
advisor or as any other fiduciary for the account of the beneficial owner or owners for investment and are not acquired with a view to,
or for sale in connection with, any distribution thereof (or any portion thereof) and with no present intention (at any such time) of
offering and distributing such securities (or any portion thereof).
Section
12. Loss, Destruction of Warrant Certificates. Upon receipt of evidence satisfactory to the Company of the loss, theft, destruction
or mutilation of any Warrant and, in the case of any such loss, theft or destruction, upon receipt of indemnity and/or security satisfactory
to the Company or, in the case of any such mutilation, upon surrender and cancellation of such Warrant, the Company will make and deliver,
in lieu of such lost, stolen, destroyed or mutilated Warrant, a new Warrant of like tenor and representing the right to purchase the same
aggregate number of shares of Common Stock.
Section
13. Amendments. The terms of this Warrant may be amended, and the observance of any term herein may be waived, but only with the
written consent of the Company and the Warrantholder.
Section
14. Notices Generally. Any notice, request, consent, other communication or delivery pursuant to the provisions hereof shall be
in writing and shall be sent by one of the following means: (i) by registered or certified first class mail, postage prepaid, return receipt
requested; (ii) by facsimile transmission with confirmation of receipt; (iii) by nationally recognized courier service guaranteeing overnight
delivery; or (iv) by personal delivery, and shall be properly addressed to the Warrantholder at the last known address or facsimile number
appearing on the books of the Company, or, except as herein otherwise expressly provided, to the Company at its principal executive office,
or such other address or facsimile number as shall have been furnished to the party giving or making such notice, demand or delivery.
Section
15. Successors and Assigns. This Warrant shall bind and inure to the benefit of and be enforceable by the parties hereto and their
respective permitted successors and assigns.
Section
16. Governing Law. In all respects, including all matters of construction, validity and performance, this Warrant and the obligations
arising hereunder shall be governed by, and construed and enforced in accordance with the laws of the State of Nevada.
IN WITNESS WHEREOF, the Company has caused this Warrant
to be signed in its name by its Chief Executive Officer.
Dated: February 21, 2022
LifeQuest
World Corp.
a Minnesotta
Corporation
By:/s/
Max Khan
Name:
Max Khan
Title:
CEO
THE
SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR REGISTERED OR QUALIFIED
UNDER ANY STATE SECURITIES LAWS. THE SECURITIES MAY NOT BE SOLD, TRANSFERRED, PLEDGED OR HYPOTHECATED UNLESS SUCH SALE, TRANSFER, PLEDGE
OR HYPOTHECATION IS IN ACCORDANCE WITH SUCH ACT AND APPLICABLE STATE SECURITIES LAWS.
Warrant No. 003
No. of Shares of Common Stock:
1,500,000 shares
WARRANT
to Purchase
Common Stock of
LifeQuest
World Corp
a Minnesotta
Corporation
This
Warrant certifies that Tirante Finance AG (“Purchaser”), is entitled to purchase from LifeQuest World Corp. a Minnesotta
Corporation (the “Company”), 1,500,000 shares of Common Stock (or any portion thereof) at an exercise price of $0.05 per
share of Common Stock on a cashless exercise basis, all on the terms and conditions hereinafter provided.
Section
1. Certain Definitions. As used in this Warrant, unless the context otherwise requires: “Articles” shall mean
the Articles of Incorporation of the Company, as in effect from time to time.
“Cashless
Exercise of Warrant” shall mean: A cashless exercise enables the investor to pay the exercise price by having the issuer withhold
stock otherwise issuable under the warrant.
“Common
Stock” shall mean the Company’s authorized common stock, $0.001 par value per share. “Exercise Price”
shall mean the exercise price per share of Common Stock set forth above, as adjusted from
time to time
pursuant to Section 3 hereof.
“Securities
Act” shall mean the Securities Act of 1933, as amended.
“Warrant”
shall mean this Warrant and all additional or new warrants issued upon division or combination of, or in substitution for, this Warrant.
All such additional or new warrants shall at all times be identical as to terms and conditions and date, except as to the number of shares
of Common Stock for which they may be exercised.
“Warrant
Stock” shall mean the shares of Common Stock purchasable by the holder of this Warrant upon the exercise of such Warrant.
“Warrantholder”
shall mean the Purchaser, as the initial holder of this Warrant, and its nominees, successors or assigns, including any subsequent holder
of this Warrant to whom it has been legally transferred.
Section
2. Exercise of Warrant; Vesting.
(a)
Purchaser may, at any time and from time to time, exercise this Warrant, in whole or in part.
This warrant expires in two year from the date hereof. In addition, the Company shall have the right to call for payment of the Warrant
at any time.
(b)
(i) The Warrantholder shall exercise this Warrant by means of delivering to the Company at its
office identified in Section 14 hereof (i) a written notice of exercise, including the number of shares of Warrant Stock to be delivered
pursuant to such exercise, (ii) this Warrant and (iii) payment equal to the Exercise Price in accordance with Section 2(b)(ii). In the
event that any exercise shall not be for all shares of Warrant Stock purchasable hereunder, the Company shall deliver to the Warrantholder
a new Warrant registered in the name of the Warrantholder, of like tenor to this Warrant and for the remaining shares of Warrant Stock
purchasable hereunder, within ten (10) days of any such exercise. Such notice of exercise shall be in the Subscription Form set out at
the end of this Warrant.
(ii)
The Warrantholder may elect to pay the Exercise Price to the Company either by cash, certified check or wire transfer.
(c)
Upon exercise of this Warrant and delivery of the Subscription Form with proper payment relating
thereto, the Company shall cause to be executed and delivered to the Warrantholder a certificate or certificates representing the aggregate
number of fully-paid and nonassessable shares of Common Stock issuable upon such exercise.
(d)
The stock certificate or certificates for Warrant Stock to be delivered in accordance with this
Section 2 shall be in such denominations as may be specified in said notice of exercise and shall be registered in the name of the Warrantholder
or such other name or names as shall be designated in said notice. Such certificate or certificates shall be deemed to have been issued
and the Warrantholder or any other person so designated to be named therein shall be deemed to have become the holder of record of such
shares, including to the extent permitted by law the right to vote such shares or to consent or to receive notice as stockholders, as
of the time said notice is delivered to the Company as aforesaid.
(e)
The Company shall pay all expenses payable in connection with the preparation, issue and delivery
of stock certificates under this Section 2, resulting from the exercise of the Warrant and the issuance of Warrant Stock hereunder.
(f)
All shares of Warrant Stock issuable upon the exercise of this Warrant in accordance with the
terms hereof shall be validly issued, fully paid and nonassessable, and free from all liens and other encumbrances thereon, other than
liens or other encumbrances created by the Warrantholder.
(g)
In no event shall any fractional share of Common Stock of the Company be issued upon any exercise
of this Warrant. If, upon any exercise of this Warrant, the Warrantholder would, except as provided in this paragraph, be entitled to
receive a fractional share of Common Stock, then the Company shall deliver in cash to such holder an amount equal to such fractional
interest.
(h)
Cashless Exercise If at any time after the Commencement Date there is no effective registration
statement registering, or no current prospectus available for, the resale of the Shares by the Holder, then in lieu of exercising this
Purchase Warrant by payment of cash or check payable to the order of the Company pursuant to Section 2.1 above, Holder may elect to receive
the number of Shares equal to the value of this Purchase Warrant (or the portion thereof being exercised), by surrender of this Purchase
Warrant to the Company, together with the exercise form attached hereto, in which event the Company shall issue to Holder, Shares in
accordance with the following formula:
X
= Y(A-B)
A
Where,
X
= The number of Shares to be issued to Holder;
Y
= The number of Shares for which the Purchase Warrant is being exercised; A = The fair market value of one Share; and
B
= The Exercise Price.
For
purposes of this Section 2.2, the fair market value of a Share is defined as follows:
(i)
if the Company’s common stock is traded on a securities exchange, the value shall be deemed
to be the closing price on such exchange prior to the exercise form being submitted in connection with the exercise of the Purchase Warrant;
or
(ii)
if the Company’s common stock is actively traded over-the-counter, the value shall be
deemed to be the closing bid price prior to the exercise form being submitted in connection with the exercise of the Purchase Warrant;
if there is no active public market, the value shall be the fair market value thereof, as determined in good faith by the Company’s
Board of Directors.
Section 3. Adjustment
of Exercise Price and Warrant Stock.
(a)
If, at any time prior to the Expiration Date, the number of outstanding shares of Common Stock is
(i) increased by a stock dividend payable in shares of Common Stock or by a subdivision or split-up of shares of Common Stock, or (ii)
decreased by a combination of shares of Common Stock, then, following the record date fixed for the determination of holders of Common
Stock entitled to receive the benefits of such stock dividend, subdivision, split-up, or combination, the Exercise Price shall be adjusted
to a new amount equal to the product of (I) the Exercise Price in effect on such record date and (II) the quotient obtained by
dividing (x) the number of shares of Common Stock outstanding on such record date (without giving effect to the event referred to in
the foregoing clause (i) or (ii)), by (y) the number of shares of Common Stock which would be outstanding immediately after the event
referred to in the foregoing clause (i) or (ii), if such event had occurred immediately following such record date. In addition, the
Exercise Price may be adjusted in other circumstances set forth in Article 5 of Exhibit A of the Articles.
(b)
Upon each adjustment of the Exercise Price as provided in Section 3 (a), the Warrantholder shall
thereafter be entitled to subscribe for and purchase, at the Exercise Price resulting from such adjustment, the number of shares of Warrant
Stock equal to the product of (i) the number of shares of Warrant Stock existing prior to such adjustment and (ii) the quotient obtained
by dividing (I) the Exercise Price existing prior to such adjustment by (II) the new Exercise Price resulting from such adjustment.
(c)
If, at any time prior to the Expiration Date, there occurs an event which would cause the automatic
conversion (“Automatic Conversion”) of the Warrant Stock into shares of the Company’s common stock (“Common Stock”)
in accordance with the Articles, then any Warrant shall thereafter be exercisable, prior to the Expiration Date, into the number of shares
of Common Stock into which the Warrant Stock would have been convertible pursuant to the Charter if the Automatic Conversion had not
taken place.
Section
4. Division and Combination. This Warrant may be divided or combined with other Warrants upon presentation at the aforesaid office
of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed
by the Warrantholder or its agent or attorney. The Company shall pay all expenses in connection with the preparation, issue and delivery
of Warrants under this Section 4, including any transfer taxes resulting from the division or combination hereunder. The Company agrees
to maintain at its aforesaid office books for the registration of the Warrants.
Section
5. Reclassification, Etc. In case of any reclassification or change of the outstanding Common of the Company (other than as a
result of a subdivision, combination or stock dividend), or in case of any consolidation of the Company with, or merger of the Company
into, another corporation or other business organization (other than a consolidation or merger in which the Company is the continuing
corporation and which does not result in any reclassification or change of the outstanding Common Stock of the Company) at any time prior
to the Expiration Date, then, as a condition of such reclassification, reorganization, change, consolidation or merger, lawful provision
shall be made, and duly executed documents evidencing the same from the Company or its successor shall be delivered to the Warrantholder,
so that the Warrantholder shall have the right prior to the Expiration Date to purchase, at a total price not to exceed that payable
upon the exercise of this Warrant, the kind and amount of shares of stock and other securities and property receivable upon such reclassification,
reorganization, change, consolidation or merger by a holder of the number of shares of Common Stock of the Company which might have been
purchased by the Warrantholder immediately prior to such reclassification, reorganization, change, consolidation or merger, in any such
case appropriate provisions shall be made with respect to the rights and interest of the Warrantholder to the end that the provisions
hereof (including provisions for the adjustment of the Exercise Price and of the number of shares purchasable upon exercise of this Warrant)
shall thereafter be applicable in relation to any shares of stock and other securities and property thereafter deliverable upon exercise
hereof.
Section
6. Reservation and Authorization of Capital Stock. The Company shall at all times reserve and keep available for issuance such
number of its authorized but unissued shares of Common Stock as will be sufficient to permit the exercise in full of all outstanding
Warrants.
Section
7. Stock and Warrant Books. The Company will not at any time, except upon dissolution, liquidation or winding up, close its stock
books or Warrant books so as to result in preventing or delaying the exercise of any Warrant.
Section
8. Limitation of Liability. No provisions hereof, in the absence of affirmative action by the Warrantholder to purchase Warrant
Stock hereunder, shall give rise to any liability of the Warrantholder to pay the Exercise Price or as a stockholder of the Company (whether
such liability is asserted by the Company or creditors of the Company).
Section
9. Registration Rights. For as long as the Warrant remains outstanding, in the event of a business combination with the Company,
the Company covenants that it shall at all times maintain one or more current and effective registration statements or offering circulars
that the Warrantholder may utilize for the resale of all Warrant Shares.
Section
10. Transfer. Subject to compliance with the Securities Act and the applicable rules and regulations promulgated thereunder, this
Warrant and all rights hereunder shall be transferable in whole or in part. Any such transfer shall be made at the office or agency of
the Company at which this Warrant is exercisable, by the registered holder hereof in person or by its duly authorized attorney, upon
surrender of this Warrant together with the assignment hereof properly endorsed, and promptly thereafter a new warrant shall be issued
and delivered by the Company, registered in the name of the assignee. Until registration of transfer hereof on the books of the Company,
the Company may treat the Purchaser as the owner hereof for all purposes.
Section
11. Investment Representations; Restrictions on Transfer of Warrant Stock. Unless a current registration statement under the Securities
Act shall be in effect with respect to the Warrant Stock to be issued upon exercise of this Warrant, the Warrantholder, by accepting
this Warrant, covenants and agrees that, at the time of exercise hereof, and at the time of any proposed transfer of Warrant Stock acquired
upon exercise hereof, such Warrantholder will deliver to the Company a written statement that the securities acquired by the Warrantholder
upon exercise hereof are for the account of the Warrantholder or are being held by the Warrantholder as trustee, investment manager,
investment advisor or as any other fiduciary for the account of the beneficial owner or owners for investment and are not acquired with
a view to, or for sale in connection with, any distribution thereof (or any portion thereof) and with no present intention (at any such
time) of offering and distributing such securities (or any portion thereof).
Section
12. Loss, Destruction of Warrant Certificates. Upon receipt of evidence satisfactory to the Company of the loss, theft, destruction
or mutilation of any Warrant and, in the case of any such loss, theft or destruction, upon receipt of indemnity and/or security satisfactory
to the Company or, in the case of any such mutilation, upon surrender and cancellation of such Warrant, the Company will make and deliver,
in lieu of such lost, stolen, destroyed or mutilated Warrant, a new Warrant of like tenor and representing the right to purchase the
same aggregate number of shares of Common Stock.
Section
13. Amendments. The terms of this Warrant may be amended, and the observance of any term herein may be waived, but only with the
written consent of the Company and the Warrantholder.
Section
14. Notices Generally. Any notice, request, consent, other communication or delivery pursuant to the provisions hereof shall be
in writing and shall be sent by one of the following means: (i) by registered or certified first class mail, postage prepaid, return
receipt requested; (ii) by facsimile transmission with confirmation of receipt; (iii) by nationally recognized courier service guaranteeing
overnight delivery; or (iv) by personal delivery, and shall be properly addressed to the Warrantholder at the last known address or facsimile
number appearing on the books of the Company, or, except as herein otherwise expressly provided, to the Company at its principal executive
office, or such other address or facsimile number as shall have been furnished to the party giving or making such notice, demand or delivery.
Section
15. Successors and Assigns. This Warrant shall bind and inure to the benefit of and be enforceable by the parties hereto and their
respective permitted successors and assigns.
Section
16. Governing Law. In all respects, including all matters of construction, validity and performance, this Warrant and the obligations
arising hereunder shall be governed by, and construed and enforced in accordance with the laws of the State of Nevada.
IN WITNESS
WHEREOF, the Company has caused this Warrant to be signed in its name by its Chief Executive Officer.
Dated: August
1, 2022
LifeQuest
World Corp.
a Minnesotta
Corporation
By:/s/
Max Khan
Name:
Max Khan
Title:
CEO
THE
SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR REGISTERED OR QUALIFIED
UNDER ANY STATE SECURITIES LAWS. THE SECURITIES MAY NOT BE SOLD, TRANSFERRED, PLEDGED OR HYPOTHECATED UNLESS SUCH SALE, TRANSFER, PLEDGE
OR HYPOTHECATION IS IN ACCORDANCE WITH SUCH ACT AND APPLICABLE STATE SECURITIES LAWS.
Warrant
No. 005
No.
of Shares of Common Stock: 1,500,000 shares
WARRANT
to
Purchase Common Stock of
LifeQuest
World Corp
a
Minnesotta Corporation
This
Warrant certifies that Berkshire Finance Holdings, Ltd.("Purchaser"), is entitled to purchase from LifeQuest World Corp. a
Minnesotta Corporation (the '·Company''), 1,500,000 shares of Common Stock (or any portion thereof) at an exercise price of $0.05
per share of Common Stock for a period of one year from the Date of this Agreement.
Section
I. Certain Definitions. As used in this Warrant, unless the context otherwise requires:
"Articles"
shall mean the Articles of Incorporation of the Company, as in effect from time to time.
"Common
Stock" shall mean the Company's authorized common stock, $0.001 par value per share.
"Exercise
Price" shall mean the exercise price per share of Common Stock set forth above, as adjusted from time to time pursuant to Section
3 hereof.
"Securities
Act" shall mean the Securities Act of 1933, as amended.
"Warrant''
shall mean this Warrant and all additional or new warrants issued upon division or combination of, or in substitution for, this Warrant.
All such additional or new warrants shall at all times be identical as to terms and conditions and date, except as to the number of shares
of Common Stock for which they may be exercised.
"Warrant
Stock" shall mean the shares of Common Stock purchasable by the holder of this Warrant upon the exercise of such Warrant.
"Warrantholder"
shall mean the Purchaser, as the initial holder of this Warrant, and its nominees, successors or assigns, including any subsequent
holder of this Warrant to whom it has been legally transferred.
Section
2. Exercise of Warrant; Vesting.
(a)
Purchaser may, at any time and from time
to time, exercise this Warrant, in whole or in part. This warrant expires in two year from the date hereof. In addition, the Company
shall have the right to call for payment of the Warrant at any time.
(b)
(i) The Warrantholder shall exercise this
Warrant by means of delivering to the Company at its office identified in Section 14 hereof (i) a written notice of exercise, including
the number of shares of Warrant Stock to be delivered pursuant to such exercise, (ii) this Warrant and (iii) payment equal to the Exercise
Price in accordance with Section 2(b)(ii). In the event that any exercise shall not be for all shares of Warrant Stock purchasable hereunder,
the Company shall deliver to the Warrantholder a new Warrant registered in the name of the Warrantholder, of like tenor to this Warrant
and for the remaining shares of Warrant Stock purchasable hereunder, within ten (10) days of any such exercise. Such notice of exercise
shall be in the Subscription Form set out at the end of this Warrant.
(ii)
The Warrantholder may elect to pay the Exercise Price to the Company either by cash, certified check or wire transfer.
(c)
Upon exercise of this Warrant and delivery
of the Subscription Form with proper payment relating thereto, the Company shall cause to be executed and delivered to the Warrantholder
a certificate or certificates representing the aggregate number of fully-paid and nonassessable shares of Common Stock issuable upon
such exercise.
(d)
The stock certificate or certificates
for Warrant Stock to be delivered in accordance with this Section 2 shall be in such denominations as may be specified in said notice
of exercise and shall be registered in the name of the Warrantholder or such other name or names as shall be designated in said notice.
Such certificate or certificates shall be deemed to have been issued and the Warrantholder or any other person so designated to be named
therein shall be deemed to have become the holder of record of such shares, including to the extent permitted by law the right to vote
such shares or to consent or to receive notice as stockholders, as of the time said notice is delivered to the Company as aforesaid.
(e)
The Company shall pay all expenses payable
in connection with the preparation, issue and delivery of stock certificates under this Section 2, resulting from the exercise of the
Warrant and the issuance of Warrant Stock hereunder.
(f)
All shares of Warrant Stock issuable upon
the exercise of this Warrant in accordance with the terms hereof shall be validly issued, fully paid and nonassessable, and free from
all liens and other encumbrances thereon, other than liens or other encumbrances created by the Warrantholder.
(g)
In no event shall any fractional share
of Common Stock of the Company be issued upon any exercise of this Warrant. If, upon any exercise of this Warrant, the Warrantholder
would, except as provided in this paragraph, be entitled to receive a fractional share of Common Stock, then the Company shall deliver
in cash to such holder an amount equal to such fractional interest.
Section
3. Adjustment of Exercise Price and Warrant Stock.
(a)
If, at any time prior to the Expiration Date, the number of outstanding shares of Common Stock is
(i) increased by a stock dividend payable in shares of Common Stock or by a subdivision or split-up of shares of Common Stock, or
(ii) decreased by a combination of shares of Common Stock, then, following the record date fixed for the determination of holders of
Common Stock entitled to receive the benefits of such stock dividend, subdivision, split-up, or combination, the Exercise Price
shall be adjusted to a new amount equal to the product of (I) the Exercise Price in effect on such record date and (11) the
quotient obtained by dividing (x) the number of shares of Common Stock outstanding on such record date (without giving effect to the
event referred to in the
foregoing
clause (i) or (ii)), by (y) the number of shares of Common Stock which would be outstanding immediately after the event referred to in
the foregoing clause (i) or (ii), if such event had occurred immediately following such record date. In addition, the Exercise Price
may be adjusted in other circumstances set forth in Article 5 of Exhibit A of the Articles.
(b)
Upon each adjustment of the Exercise Price
as provided in Section 3 (a), the Warrantholder shall thereafter be entitled to subscribe for and purchase, at the Exercise Price resulting
from such adjustment, the number of shares of Warrant Stock equal to the product of (i) the number of shares of Warrant Stock existing
prior to such adjustment and (ii) the quotient obtained by dividing (I) the Exercise Price existing prior to such adjustment by (II)
the new Exercise Price resulting from such adjustment.
(c)
If, at any time prior to the Expiration
Date, there occurs an event which would cause the automatic conversion ("Automatic Conversion") of the Warrant Stock into shares
of the Company's common stock ("Common Stock") in accordance with the Articles, then any Warrant shall thereafter be exercisable,
prior to the Expiration Date, into the number of shares of Common Stock into which the Warrant Stock would have been convertible pursuant
to the Charter if the Automatic Conversion had not taken place.
Section
4. Division and Combination. This Warrant may be divided or combined with other Warrants upon presentation at the aforesaid office
of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed
by the Warrantholder or its agent or attorney. The Company shall pay all expenses in connection with the preparation, issue and delivery
of Warrants under this Section 4, including any transfer taxes resulting from the division or combination hereunder. The Company agrees
to maintain at its aforesaid office books for the registration of the Warrants.
Section
5. Reclassification, Etc. In case of any reclassification or change of the outstanding Common of the Company (other than as a
result of a subdivision, combination or stock dividend), or in case of any consolidation of the Company with, or merger of the Company
into, another corporation or other business organization (other than a consolidation or merger in which the Company is the continuing
corporation and which does not result in any reclassification or change of the outstanding Common Stock of the Company) at any time prior
to the Expiration Date, then, as a condition of such reclassification, reorganization, change, consolidation or merger, lawful provision
shall be made, and duly executed documents evidencing the same from the Company or its successor shall be delivered to the Warrantholder,
so that the Warrantholder shall have the right prior to the Expiration Date to purchase; at a total price not to exceed that payable
upon the exercise of this Warrant, the kind and amount of shares of stock and other securities and property receivable upon such reclassification,
reorganization, change, consolidation or merger by a holder of the number of shares of Common Stock of the Company which might have been
purchased by the Warrantholder immediately prior to such reclassification, reorganization, change, consolidation or merger, in any such
case appropriate provisions shall be made with respect to the rights and interest of the Warrantholder to the end that the provisions
hereof (including provisions for the adjustment of the Exercise Price and of the number of shares purchasable upon exercise of this Warrant)
shall thereafter be applicable in relation to any shares of stock and other securities and property thereafter deliverable upon exercise
hereof.
Section
6. Reservation and Authorization of Capital Stock. The Company shall at all times reserve and keep available for issuance such
number of its authorized but unissued shares of Common Stock as will be sufficient to permit the exercise in full of all outstanding
Warrants.
Section
7. Stock and Warrant Books. The Company will not at any time, except upon dissolution, liquidation or winding up, close its stock
books or Warrant books so as to result in preventing or delaying the exercise of any Warrant.
Section
8. Limitation of Liability. No provisions hereof, in the absence of affirmative action by the Warrantholder to purchase Warrant
Stock hereunder, shall give rise to any liability of the Warrantholder to pay the Exercise Price or as a stockholder of the Company (whether
such liability is asserted by the Company or creditors of the Company).
Section
9. Registration Rights. For as long as the Warrant remains outstanding, in the event of a business combination with the Company,
the Company covenants that it shall at all times maintain one or more current and effective registration statements or offering circulars
that the Warrantholder may utilize for the resale of all Warrant Shares.
Section
I 0. Transfer. Subject to compliance with the Securities Act and the applicable rules and regulations promulgated thereunder,
this Warrant and all rights hereunder shall be transferable in whole or in part. Any such transfer shall be made at the office or agency
of the Company at which this Warrant is exercisable, by the registered holder hereof in person or by its duly authorized attorney, upon
surrender of this Warrant together with the assignment hereof properly endorsed, and promptly thereafter a new warrant shall be issued
and delivered by the Company, registered in the name of the assignee. Until registration of transfer hereof on the books of the Company,
the Company may treat the Purchaser as the owner hereof for all purposes.
Section
11. Investment Representations: Restrictions on Transfer of Warrant Stock. Unless a current registration statement under the Securities
Act shall be in effect with respect to the Warrant Stock to be issued upon exercise of this Warrant, the Warrantholder, by accepting
this Warrant, covenants and agrees that, at the time of exercise hereof, and at the time of any proposed transfer of Warrant Stock acquired
upon exercise hereof, such Warrantholder will deliver to the Company a written statement that the securities acquired by the Warrantholder
upon exercise hereof are for the account of the Warrantholder or are being held by the Warrantholder as trustee, investment manager,
investment advisor or as any other fiduciary for the account of the beneficial owner or owners for investment and are not acquired with
a view to, or for sale in connection with, any distribution thereof (or any portion thereof) and with no present intention (at any such
time) of offering and distributing such securities (or any portion thereof).
Section
12. Loss, Destruction of Warrant Certificates. Upon receipt of evidence satisfactory to the Company of the loss, theft, destruction
or mutilation of any Warrant and, in the case of any such loss, theft or destruction, upon receipt of indemnity and/or security satisfactory
to the Company or, in the case of any such mutilation, upon surrender and cancellation of such Warrant, the Company will make and deliver,
in lieu of such lost, stolen, destroyed or mutilated Warrant, a new Warrant of like tenor and representing the right to purchase the
same aggregate number of shares of Common Stock.
Section
13. Amendments. The terms of this Warrant may be amended, and the observance of any term herein may be waived, but only with the
written consent of the Company and the Warrantholder.
Section
14. Notices Generally. Any notice, request, consent, other communication or delivery pursuant to the provisions hereof shall be
in writing and shall be sent by one of the following means: (i) by registered or certified first class mail, postage prepaid, return
receipt requested; (ii) by facsimile transmission with confirmation of receipt; (iii) by nationally recognized courier service guaranteeing
overnight delivery; or (iv) by personal delivery, and shall be properly addressed to the Warrantholder at the last known address or facsimile
number appearing on the books of the Company, or, except as herein otherwise expressly provided, to the Company at its principal executive
office, or such other address or facsimile number as shall have been furnished to the party giving or making such notice, demand or delivery.
Section
15. Successors and Assigns. This Warrant shall bind and inure to the benefit of and be enforceable by the parties hereto and their
respective permitted successors and assigns.
Section
16. Governing Law. In all respects, including all matters of construction, validity and performance, this Warrant and the obligations
arising hereunder shall be governed by, and construed and enforced in accordance with the laws of the State of Minnessota.
IN
WITNESS WHEREOF, the Company has caused this Warrant to be signed in its name by its Chief Executive Officer.
Dated:
October 1, 2023
LifeQuest
World Corp.
a Minnesotta
Corporation
By:/s/
Max Khan
Name:
Max Khan
Title:
CEO

February 21, 2024
LifeQuest World Corp.
100 Challenger Road, 8th Floor
Ridgefield Park, NJ 07660
Re: LifeQuest World Corp. Registration Statement on Form 1-A
Ladies and Gentlemen:
We have acted as counsel to LifeQuest World Corp., a Minnesota corporation
(the “Company”), in connection with the preparation of a Registration Statement on Form 1-A (the “Registration Statement”)
filed with the Securities and Exchange Commission (the “Commission”) for the registration for sale from time to time of up
to 10,000,000 shares of the Company’s common stock, par value $0.001 per share (the “Shares”), issued or issuable pursuant
to subscription agreements (the “Subscription Agreements”), and for the resale of up to 3,000,000 shares of common stock underlying
warrants.
For purposes of rendering this opinion, we have made such legal and factual
examinations as we have deemed necessary under the circumstances and, as part of such examination, we have examined, among other things,
originals and copies, certified or otherwise, identified to our satisfaction, of such documents, corporate records and other instruments
as we have deemed necessary or appropriate. For the purposes of such examination, we have assumed the genuineness of all signatures on
original documents and the conformity to original documents of all copies submitted to us. We have relied, without independent investigation,
on certificates of public officials and, as to matters of fact material to the opinion set forth below, on certificates of officers of
the Company.
On the basis of and in reliance upon the foregoing examination and assumptions,
we are of the opinion that assuming the Registration Statement shall have become qualified pursuant to the provisions of the Securities
Act of 1933, as amended (the “Act”), the Shares, when issued by the Company against payment therefore (not less than par value)
and in accordance with the Registration Statement and the provisions of the Subscription Agreements, and when duly registered on the books
of the Company’s transfer agent and registrar therefor in the name or on behalf of the purchasers, will be validly issued, fully
paid and non-assessable.
We are also of the opinion that the 3,000,000 shares of common stock underlying
the warrants to be sold by the Selling Shareholders will be validly issued, fully paid and non-assessable and will be a binding obligation
of the Company when issued by the Company if the exercise price is received by the Company.
We express no opinion as to the laws of any state or jurisdiction other
than the laws of the State of Minnesota, as currently in effect.
We hereby consent to the filing of this opinion as an exhibit to the Registration
Statement and to the reference to us under the caption “Legal Matters” in the Offering Circular constituting a part of the
Registration Statement. This opinion is for your benefit in connection with the Registration Statement and may be relied upon by you and
by persons entitled to rely upon it pursuant to the applicable provisions of the Act. In giving this consent, we do not thereby admit
that we are in the category of persons whose consent is required under Section 7 of the Act or the rules and regulations of the Commission.
Very truly yours,

The Doney Law Firm
Scott P. Doney, Esq.
LifeQuest World (PK) (USOTC:LQWC)
Historical Stock Chart
From Mar 2024 to Apr 2024
LifeQuest World (PK) (USOTC:LQWC)
Historical Stock Chart
From Apr 2023 to Apr 2024