false
0001720893
0001720893
2024-02-14
2024-02-14
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) February 14, 2024
BioXcel
Therapeutics, Inc.
(Exact name of registrant as specified in its
charter)
| Delaware |
|
001-38410 |
|
82-1386754 |
(State
or other jurisdiction of
incorporation) |
|
(Commission
File Number) |
|
(IRS
Employer
Identification No.) |
555
Long Wharf Drive
New
Haven, CT 06511
(Address of principal executive offices, including
Zip Code)
(475)
238-6837
(Registrant’s telephone number, including
area code)
N/A
(Former name or former address, if changed since
last report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ¨ | Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant
to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which
registered |
| Common
Stock, par value $0.001 |
|
BTAI |
|
The Nasdaq
Capital Market |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 | Regulation FD Disclosure. |
On February 14,
2024, BioXcel Therapeutics, Inc. (the “Company”) issued an updated corporate presentation, including its clinical development
programs and business strategy. A copy of the presentation is furnished hereto as Exhibit 99.1 and is incorporated herein by reference,
and will also be available through the “Investors & Media” page of the Company’s website at http://www.bioxceltherapeutics.com.
The information in this
Current Report on Form 8-K, including Exhibit 99.1 hereto, shall not be deemed “filed” for purposes of Section 18
of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section,
nor shall it be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or
the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits:
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: February 14, 2024 |
BIOXCEL THERAPEUTICS, INC. |
| |
|
| |
/s/ Richard Steinhart |
| |
Richard Steinhart |
| |
Chief Financial Officer |
Exhibit 99.1

® BioXcel Therapeutics | 555 Long Wharf Drive, 12th Floor | New Haven, CT 06511 | bioxceltherapeutics.com NASDAQ: BTAI February 2024 AI - Driven Transformative Medicines in Neuroscience

This presentation includes "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 19 95. Forward - looking statements in this presentation include but are not limited to: statements regarding BioXcel Therapeutics’ expected timing of, and data res ult s from, trials and clinical studies, and other milestones involving its product candidates including BXCL501, BXCL502 , BXCL503, BXCL504, BXCL701 and BXCL702; paths to potential FDA approvals for BXCL501 ; the potential for the results from the Company's completed, ongoing and proposed clinical trials to support regulatory approv al s for its product candidates; its commercial plan, targets, and strategy for IGALMI Ρ ; strategic options for OnkosXcel; potential benefits of treatment with BXCL501 and BXCL701, potential market size and opportunity for products and product candidates; and its future financial and operational res ults. When used herein, words including "anticipate," "being," "will," "plan," "may," "continue," and similar expressions are intended to identify forward - loo king statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance, or other characte riz ations of future events or circumstances, including any underlying assumptions, are forward - looking. All forward - looking statements are based upon BioXcel Therapeutics’ current expectations and various assumptions. BioXcel Therapeutics believes there is a reasonable basis for its expectations and beli efs , but they are inherently uncertain. BioXcel Therapeutics may not realize its expectations, and its beliefs may not prove correct. Actual results could differ mat eri ally from those described or implied by such forward - looking statements as a result of various important factors, including, without limitation: its limited operatin g history; its incurrence of significant losses; its need for substantial additional funding and ability to raise capital when needed; its limited experience in drug dis covery and drug development; risks related to the TRANQUILITY II Phase 3 trial; its dependence on the success and commercialization of IGALMI Ρ , BXCL501, BXCL502, BXCL701 and BXCL702, and other product candidates; the Company has no experience in marketing and selling drug products; IGALMI Ρ or the Company’s product candidates may not be accepted by physicians or the medical community in general; the failure of preliminary data from its clinical studies to p red ict final study results; failure of its early clinical studies or preclinical studies to predict future clinical studies; its ability to receive regulatory approval for its product candidates; its ability to enroll patients in its clinical trials; undesirable side effects caused by the Company’s product candidates; its novel approach to t he discovery and development of product candidates based on EvolverAI; its exposure to patent infringement lawsuits; its ability to comply with the extensive re gulations applicable to it; impacts from the COVID - 19 pandemic; risks associated with the increased scrutiny related to environmental, social and governance (ESG) m atters, its ability to commercialize its product candidates; and the other important factors discussed under the caption "Risk Factors" in its Quart erl y Report on Form 10 - Q for the quarterly period ended September 30, 2023, as such factors may be further updated from time to time in its other filings with th e SEC, which are accessible on the SEC's website at www.sec.gov and the Investors section of our website at www.bioxceltherapeutics.com . These and other important factors could cause actual results to differ materially from those indicated by the forward - looking st atements made in this presentation. Any such forward - looking statements represent management's estimates as of the date of this presentation. While BioXcel Therapeu tics may elect to update such forward - looking statements at some point in the future, except as required by law, it disclaims any obligation to do so, ev en if subsequent events cause our views to change. These forward - looking statements should not be relied upon as representing BioXcel Therapeutics’ views as of an y date subsequent to the date of this presentation. Forward - Looking Statements 2

® Corporate Overview ® 3 ®

About BioXcel Therapeutics 4 Founded: 2017 IPO: 2018 Ticker: BTAI (Nasdaq) Headquarters: New Haven, CT

® 5 ® Develop transformative medicines in neuroscience utilizing artificial intelligence Our Mission
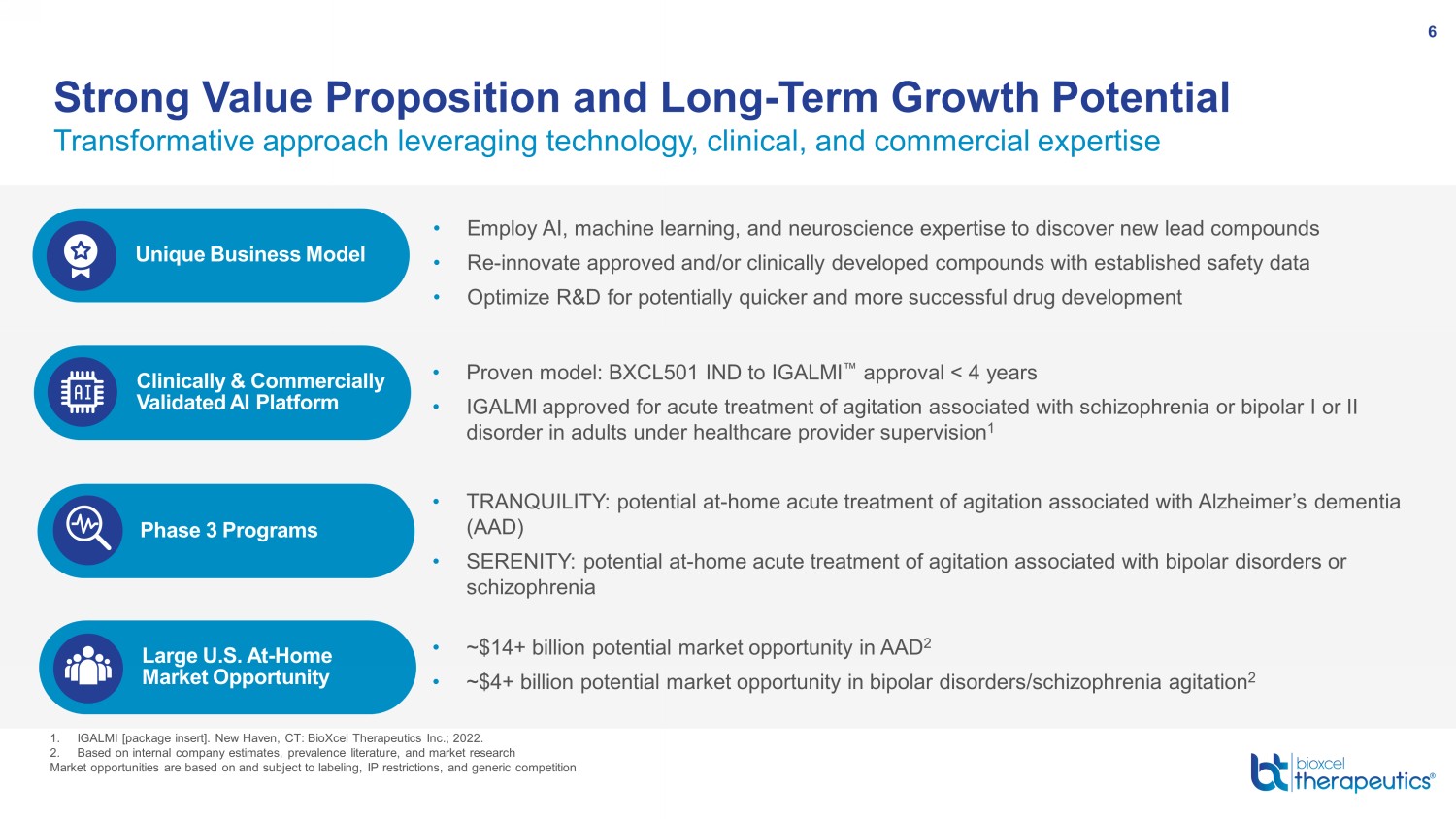
Transformative approach leveraging technology, clinical, and commercial expertise Strong Value Proposition and Long - Term Growth Potential 6 Unique Business Model • Employ AI , machine learning, and neuroscience expertise to discover new lead compounds • Re - innovate approved and/or clinically developed compounds with established safety data • Optimize R&D for potentially quicker and more successful drug development Large U.S. At - Home Market Opportunity Clinically & Commercially Validated AI Platform • Proven model: BXCL501 IND to IGALMI Œ approval < 4 years • IGALMI approved for acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults under healthcare provider supervision 1 Phase 3 Programs • TRANQUILITY: potential at - home a cute treatment of agitation associated with Alzheimer’s dementia (AAD) • SERENITY: potential at - home acute treatment of agitation associated with bipolar disorders or schizophrenia 1. IGALMI [package insert]. New Haven, CT: BioXcel Therapeutics Inc.; 2022. 2. Based on internal company estimates, prevalence literature, and market research Market opportunities are based on and subject to labeling, IP restrictions, and generic competition • ~$14+ billion potential market opportunity in AAD 2 • ~$4+ billion potential market opportunity in bipolar disorders/ schizophrenia agitation 2

Transformative drug re - innovation approach resulted in rapid development and approval of IGALMI Œ Corporate Growth Drivers Phase 3 TRANQUILITY and SERENITY Programs BXCL502 BXCL503 7 Land Agitation Associated w ith Bipolar Disorders or Schizophrenia Healthcare Provider Supervision The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. Expand BXCL501 Potential At - Home Expansion Agitation Associated With: 2024 Focus • Alzheimer’s Dementia • Bipolar Disorders or Schizophrenia Developing an Emerging Alzheimer's Symptoms - Based Pipeline 2024 & Beyond

R&D Strategy: Build Pipeline Depth with Innovation and Expansion Compound Indication/Proposed Indication Preclinical Phase 1 Phase 2 Phase 3 Registration Marketed APPROVED APRIL 5, 2022 Acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults under healthcare provider supervision BXCL501 TRANQUILITY PROGRAM Acute treatment of agitation associated with Alzheimer’s dementia (at home) SERENITY PROGRAM Acute treatment of agitation associated with bipolar disorders/schizophrenia (at home) Opioid Use Disorder (OUD) * Post Traumatic Stress Disorder (PTSD) * BXCL502 Neuropsychiatric symptoms Chronic agitation in Alzheimer’s dementia Candidate BXCL503 Apathy in dementia Candidate BXCL504 Aggression in dementia Pipeline as of February 14 , 202 4 8 *Government - funded, investigator - sponsored trials The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established

Leadership Expertise 9 Vimal Mehta, Ph.D. Chief Executive Officer & Founder Richard I. Steinhart Senior Vice President & Chief Financial Officer Frank D. Yocca, Ph.D. Senior Vice President & Chief Scientific Officer Vincent J. O’Neill, M.D. Executive Vice President, Chief of Product Development and Medical Officer Chetan D. Lathia, Ph.D. Senior Vice President & Head of Regulatory Affairs Matt Wiley Senior Vice President & Chief Commercial Officer Dusan Kostic, Ph.D. Vice President, Head of Medical Affairs Robert Risinger, M.D. Chief Medical Officer, Neuroscience

® Acute Treatment of Agitation Associated with Alzheimer’s Dementia (AAD) ® 10 ® TRANQUILITY Program
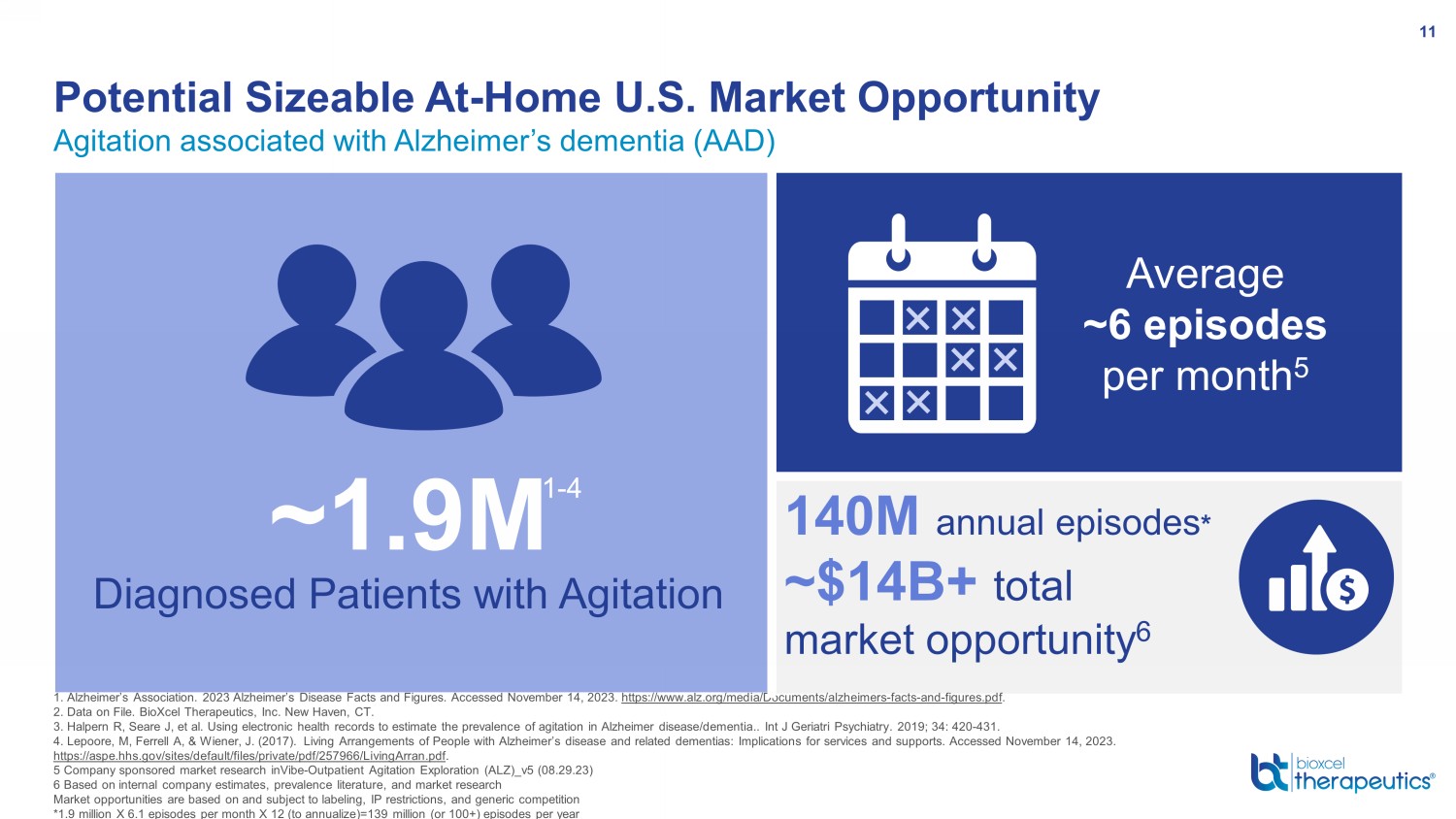
140M annual episodes * ~$14B+ total market opportunity 6 Agitation associated with Alzheimer’s dementia (AAD) Potential Sizeable At - Home U.S. Market Opportunity 11 1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Accessed November 14, 2023. https://www.alz.org/media/Documents/alzheimers - facts - and - figures.pdf . 2. Data on File. BioXcel Therapeutics, Inc. New Haven, CT. 3. Halpern R, Seare J, et al. Using electronic health records to estimate the prevalence of agitation in Alzheimer disease/de men tia.. Int J Geriatri Psychiatry. 2019; 34: 420 - 431. 4. Lepoore, M, Ferrell A, & Wiener, J. (2017). Living Arrangements of People with Alzheimer’s disease and related dementias: Implications for services and supports. Accessed November 14, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/257966/LivingArran.pdf . 5 Company sponsored market research inVibe - Outpatient Agitation Exploration (ALZ)_v5 (08.29.23) 6 Based on internal company estimates, prevalence literature, and market research Market opportunities are based on and subject to labeling, IP restrictions, and generic competition *1.9 million X 6.1 episodes per month X 12 (to annualize)=139 million (or 100+) episodes per year Average ~6 episodes per month 5 ~1.9M Diagnosed Patients with Agitation 1 - 4
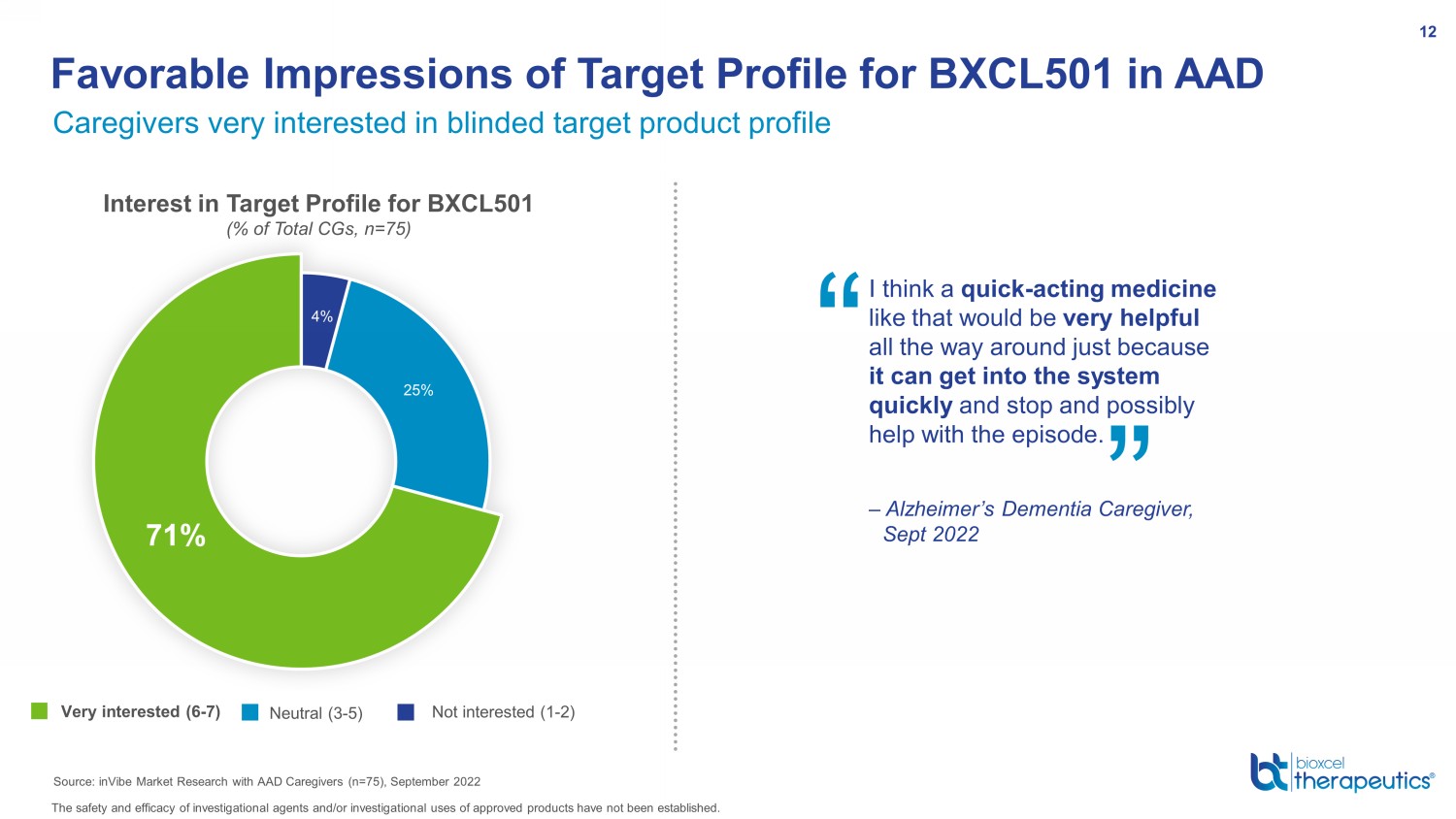
Interest in Target Profile for BXCL501 (% of Total CGs, n=75) 4% 25% 71% Not interested (1 - 2) Neutral (3 - 5) Very interested (6 - 7) Caregivers very interested in blinded target product profile Favorable Impressions of Target Profile for BXCL501 in AAD Source: inVibe Market Research with AAD Caregivers (n=75), September 2022 I think a quick - acting medicine like that would be very helpful all the way around just because it can get into the system quickly and stop and possibly help with the episode. – Alzheimer’s Dementia Caregiver, Sept 2022 “ ” 12 The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established.
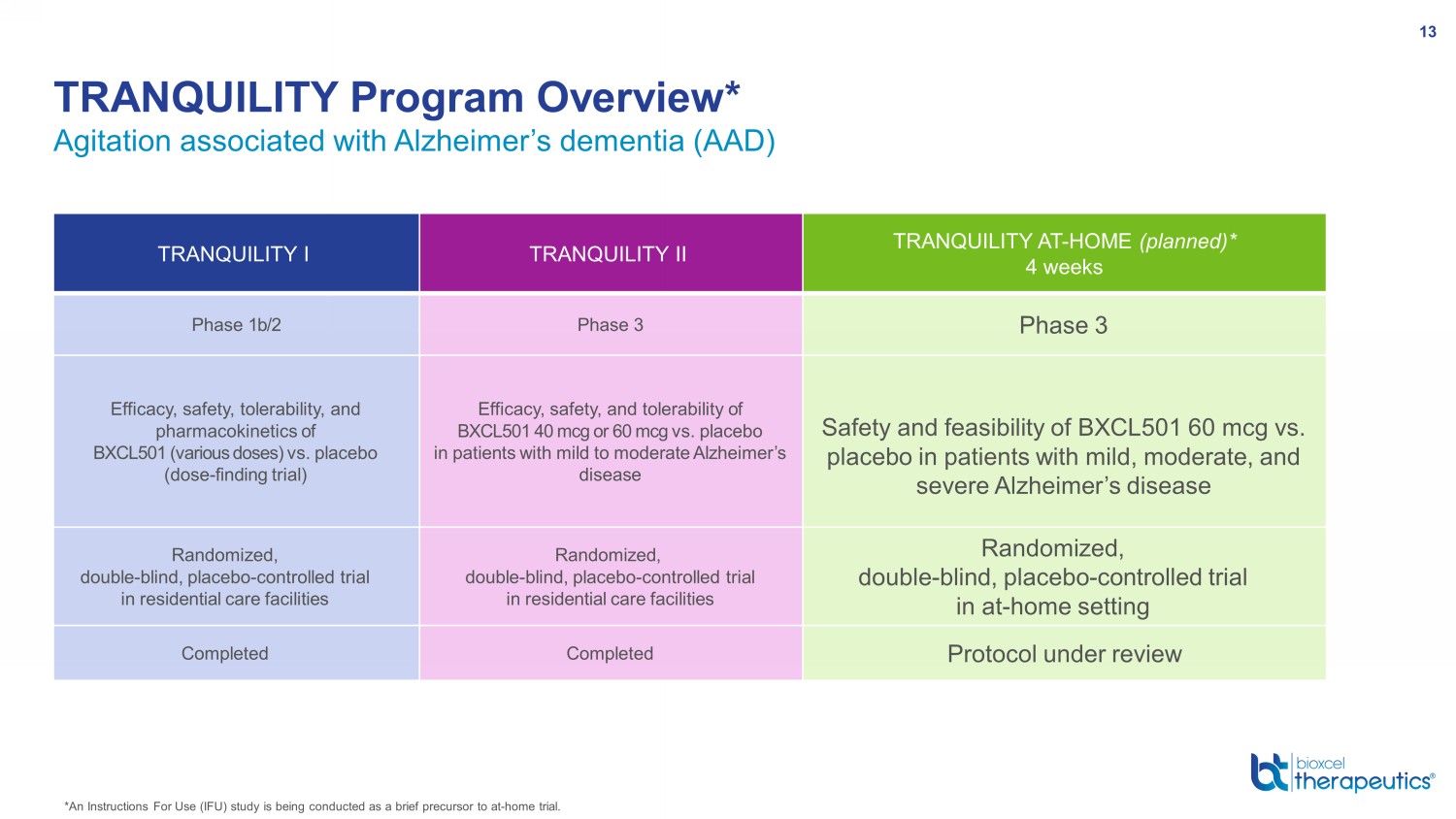
Agitation associated with Alzheimer’s dementia (AAD) TRANQUILITY Program Overview* 13 *An Instructions For Use (IFU) study is being conducted as a brief precursor to at - home trial. TRANQUILITY I TRANQUILITY II TRANQUILITY AT - HOME (planned)* 4 weeks Phase 1b/2 Phase 3 Phase 3 Efficacy, safety, tolerability, and pharmacokinetics of BXCL501 (various doses) vs. placebo (dose - finding trial) Efficacy , safety , and tolerability of BXCL501 40 mcg or 60 mcg vs. placebo in patients with mild to moderate Alzheimer’s disease Safety and feasibility of BXCL501 60 mcg vs. placebo in patients with mild, moderate, and severe Alzheimer’s disease Randomized, double - blind, placebo - controlled trial in residential care facilities Randomized, double - blind, placebo - controlled trial in residential care facilities Randomized, double - blind, placebo - controlled trial in at - home setting Completed Completed Protocol under review
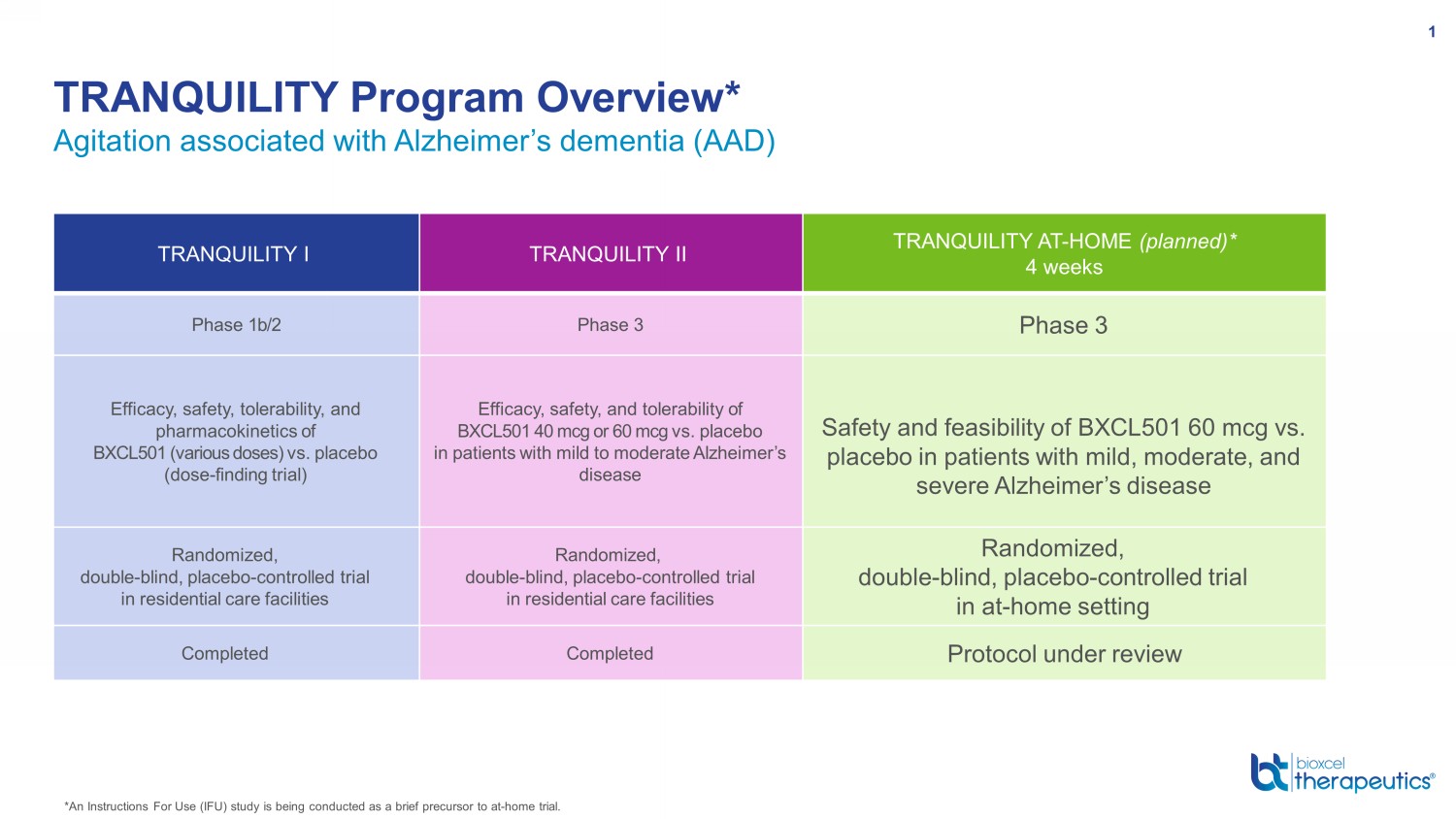
Agitation associated with Alzheimer’s dementia (AAD) TRANQUILITY Program Overview* 1 *An Instructions For Use (IFU) study is being conducted as a brief precursor to at - home trial. TRANQUILITY I TRANQUILITY II TRANQUILITY AT - HOME (planned)* 4 weeks Phase 1b/2 Phase 3 Phase 3 Efficacy, safety, tolerability, and pharmacokinetics of BXCL501 (various doses) vs. placebo (dose - finding trial) Efficacy , safety , and tolerability of BXCL501 40 mcg or 60 mcg vs. placebo in patients with mild to moderate Alzheimer’s disease Safety and feasibility of BXCL501 60 mcg vs. placebo in patients with mild, moderate, and severe Alzheimer’s disease Randomized, double - blind, placebo - controlled trial in residential care facilities Randomized, double - blind, placebo - controlled trial in residential care facilities Randomized, double - blind, placebo - controlled trial in at - home setting Completed Completed Protocol under review
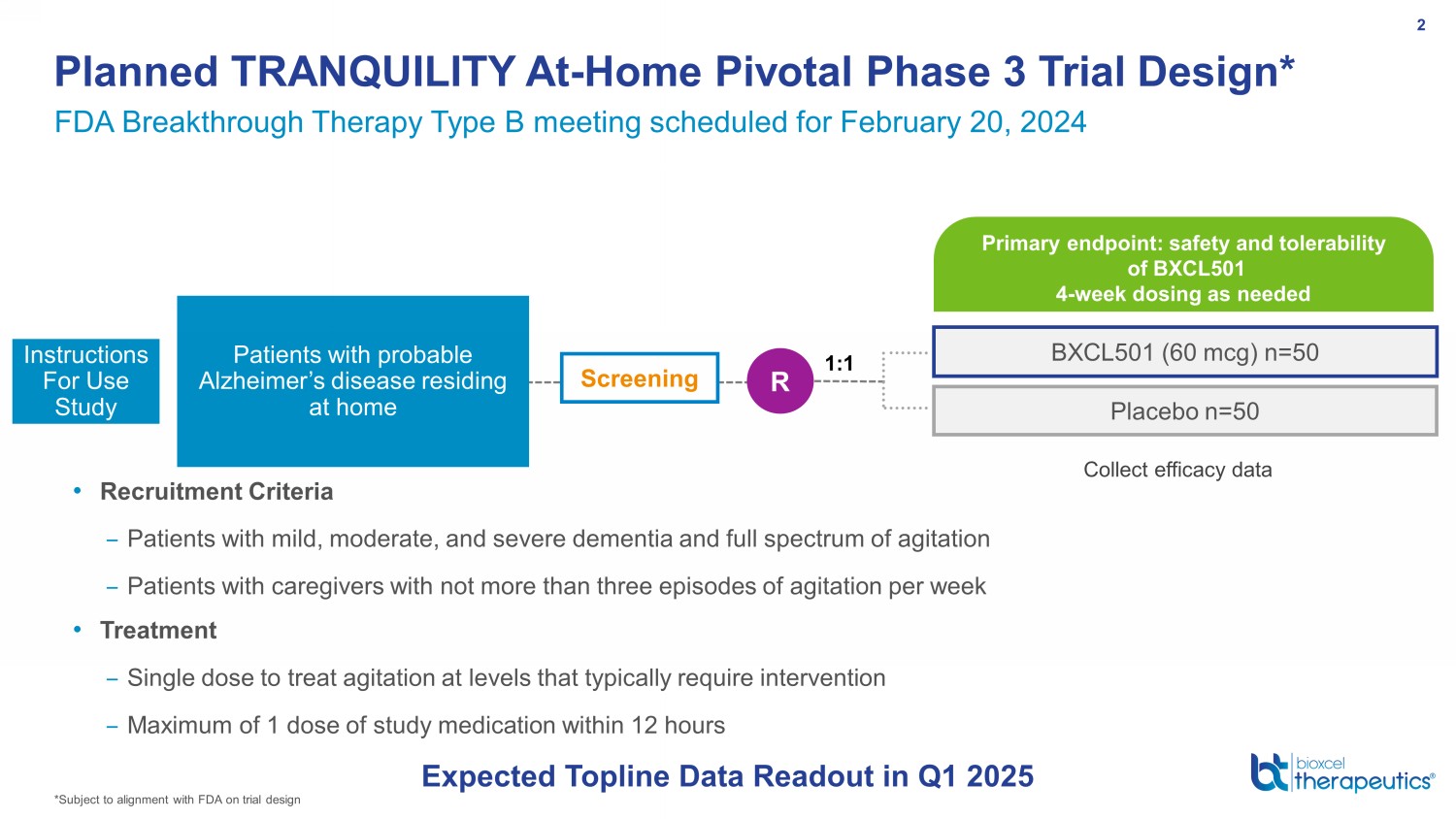
BXCL501 (60 mcg) n=50 Placebo n=50 Screening R Patients with probable Alzheimer’s disease residing at home Primary endpoint: safety and tolerability of BXCL501 4 - week dosing as needed Planned TRANQUILITY At - Home Pivotal Phase 3 Trial Design* 2 Instructions For Use Study • Recruitment Criteria – Patients with mild, moderate, and severe dementia and full spectrum of agitation – Patients with caregivers with not more than three episodes of agitation per week • Treatment – Single dose to treat agitation at levels that typically require intervention – Maximum of 1 dose of study medication within 12 hours 1:1 Collect efficacy data FDA Breakthrough Therapy Type B meeting scheduled for February 20, 2024 Expected Topline Data Readout in Q1 2025 *Subject to alignment with FDA on trial design

BXCL501 Clinical Foundation: Expansion Into At - Home Setting 3 • 11 double - blind, placebo - controlled Phase 2 and 3 clinical trials evaluating safety and efficacy • 1,100+ patients enrolled across multiple neuropsychiatric conditions and in healthy volunteers • 273 were over 60 years of age and 204 were over 65 years of age who have received doses of BXCL501 • No unexpected safety signals – No reports of serious adverse events or falls related to study drug – No drug - related deaths Vast amounts of data from thousands of patients in clinical and real - world settings

® Acute Treatment of Agitation Associated with Bipolar Disorders or Schizophrenia (at - home setting) ® 4 ® SERENITY Program
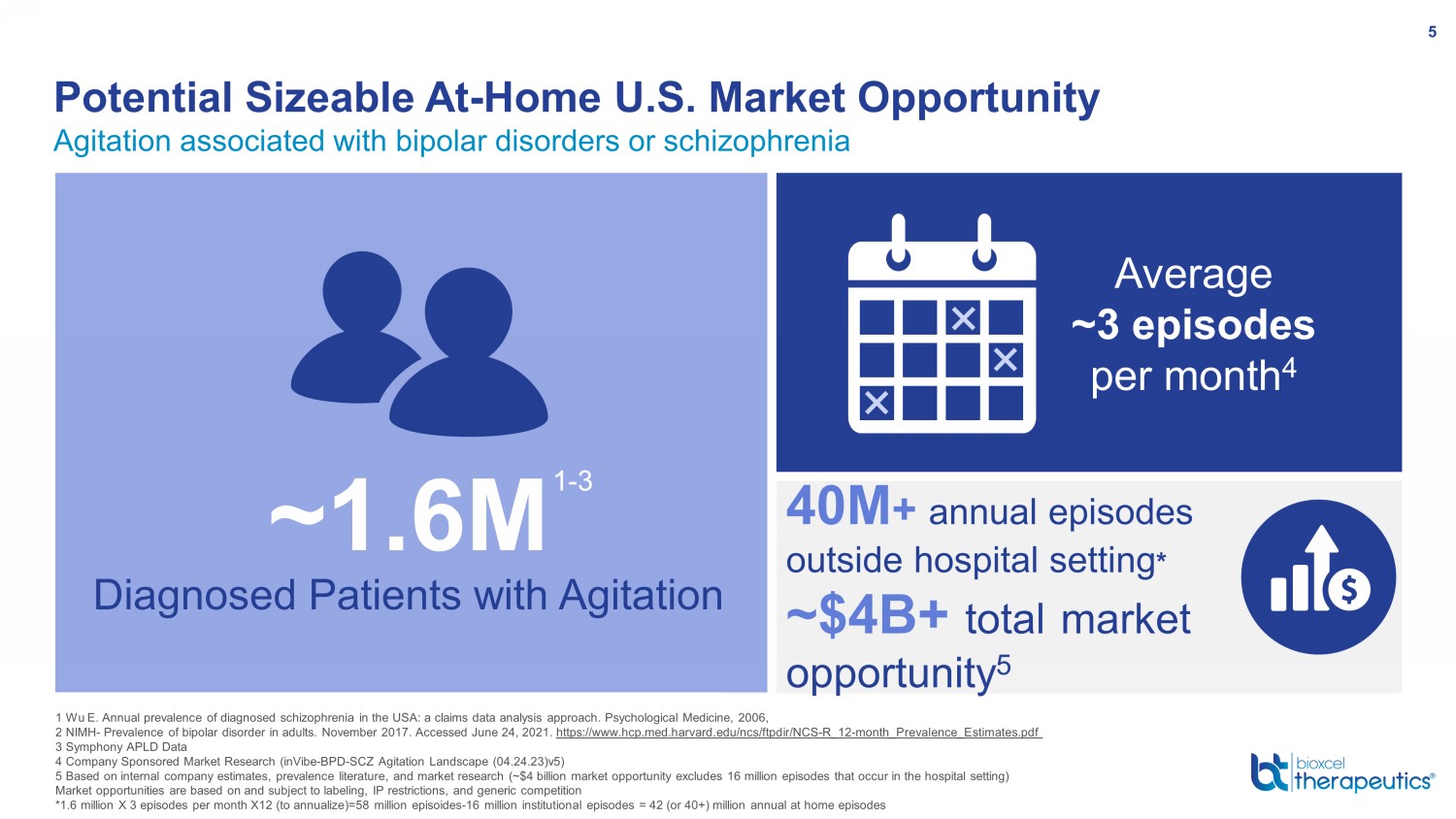
40M + annual episodes outside hospital setting * ~$4B+ total market opportunity 5 Agitation associated with bipolar disorders or schizophrenia Potential Sizeable At - Home U.S. Market Opportunity 5 Average ~3 episodes per month 4 ~1.6M Diagnosed Patients with Agitation 1 - 3 1 Wu E. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychological Medicine, 2006, 2 NIMH - Prevalence of bipolar disorder in adults. November 2017. Accessed June 24, 2021. https://www.hcp.med.harvard.edu/ncs/ftpdir/NCS - R_12 - month_Prevalence_Estimates.pdf 3 Symphony APLD Data 4 Company Sponsored Market Research (inVibe - BPD - SCZ Agitation Landscape (04.24.23)v5) 5 Based on internal company estimates, prevalence literature, and market research (~$4 billion market opportunity excludes 16 mi llion episodes that occur in the hospital setting) Market opportunities are based on and subject to labeling, IP restrictions, and generic competition *1.6 million X 3 episodes per month X12 (to annualize)=58 million episoides - 16 million institutional episodes = 42 (or 40+) mill ion annual at home episodes

80% 33% 17% 14% 13% 6% 4% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% BXCL501 Anti-anxiety Anti-depressants Mood stabilizers Anti-psychotic Other None Potential Uptake for BXCL501 (if approved for at - home use) (% of Episodes, n=240 ) Q22. You previously indicated that you used the following medications to manage your last 3 agitation episodes. Now please im agi ne that Igalmi was also available for you to use. Please indicate what treatment you would have chosen to treat the last 3 episodes if Igalmi were also available to you. We have provided your prev iou s below for reference. Source: InVibe Feb 2023 When shown product profile stimulus, patients said they would use the targeted product for 80% of their bipolar/schizophrenia agitation episodes, and for those on therapy it would be additive. Potential for Patient Use of BXCL501 At Home 6
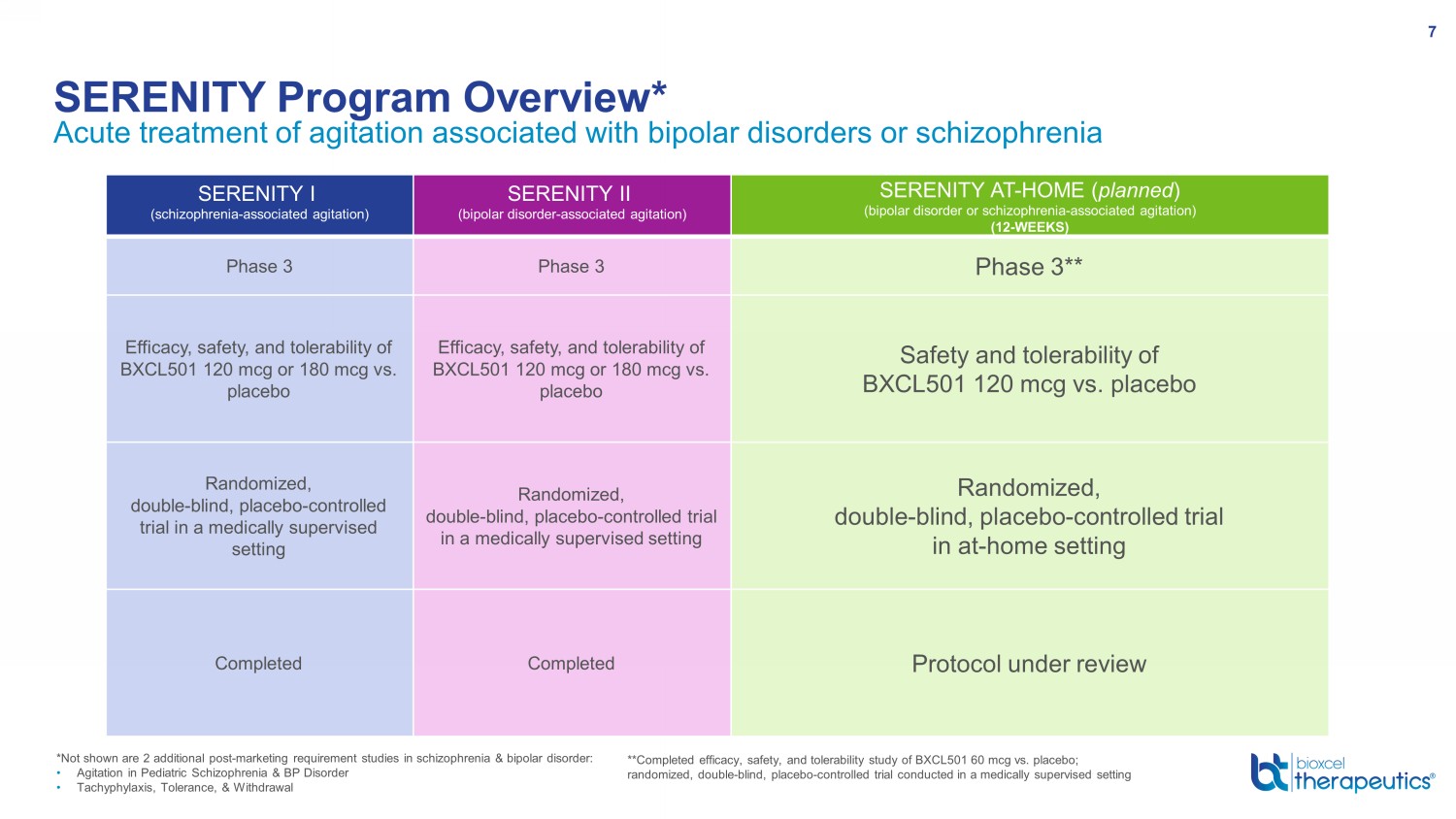
Acute treatment of agitation associated with bipolar disorders or schizophrenia (bipolar disorder - associated agitation) SERENITY Program Overview* 7 PART 1 | CLINIC PART 2 | HOME *Not shown are 2 additional post - marketing requirement studies in schizophrenia & bipolar disorder: • Agitation in Pediatric Schizophrenia & BP Disorder • Tachyphylaxis, Tolerance, & Withdrawal SERENITY I (schizophrenia - associated agitation) SERENITY II (bipolar disorder - associated agitation) SERENITY AT - HOME ( planned ) (bipolar disorder or schizophrenia - associated agitation) (12 - WEEKS) Phase 3 Phase 3 Phase 3** Efficacy, safety, and tolerability of BXCL501 120 mcg or 180 mcg vs. placebo Efficacy, safety, and tolerability of BXCL501 120 mcg or 180 mcg vs. placebo Safety and tolerability of BXCL501 120 mcg vs. placebo Randomized, double - blind, placebo - controlled trial in a medically supervised setting Randomized, double - blind, placebo - controlled trial in a medically supervised setting Randomized, double - blind, placebo - controlled trial in at - home setting Completed Completed Protocol under review **Completed efficacy, safety, and tolerability study of BXCL501 60 mcg vs. placebo; randomized, double - blind, placebo - controlled trial conducted in a medically supervised setting

• Recruitment Criteria – Patients alone or with informants (as dyads) with at least 1 treated episode of agitation • Treatment – Single dose to treat agitation at levels that typically require intervention – Maximum of 1 dose of study medication within 12 hours Planned SERENITY At - home Pivotal Phase 3 Trial Design* Placebo n=100 BXCL501 120 mcg n=100 Screening R Schiz/BPD patients residing at home Primary endpoint: safety and tolerability of BXCL501 12 - Week At - Home PRN Treatment 1:1 8 Collect efficacy data FDA Type C meeting scheduled for March 6, 2024 *Subject to alignment with FDA on trial design

® Upcoming Expected Milestones 9 ® 9
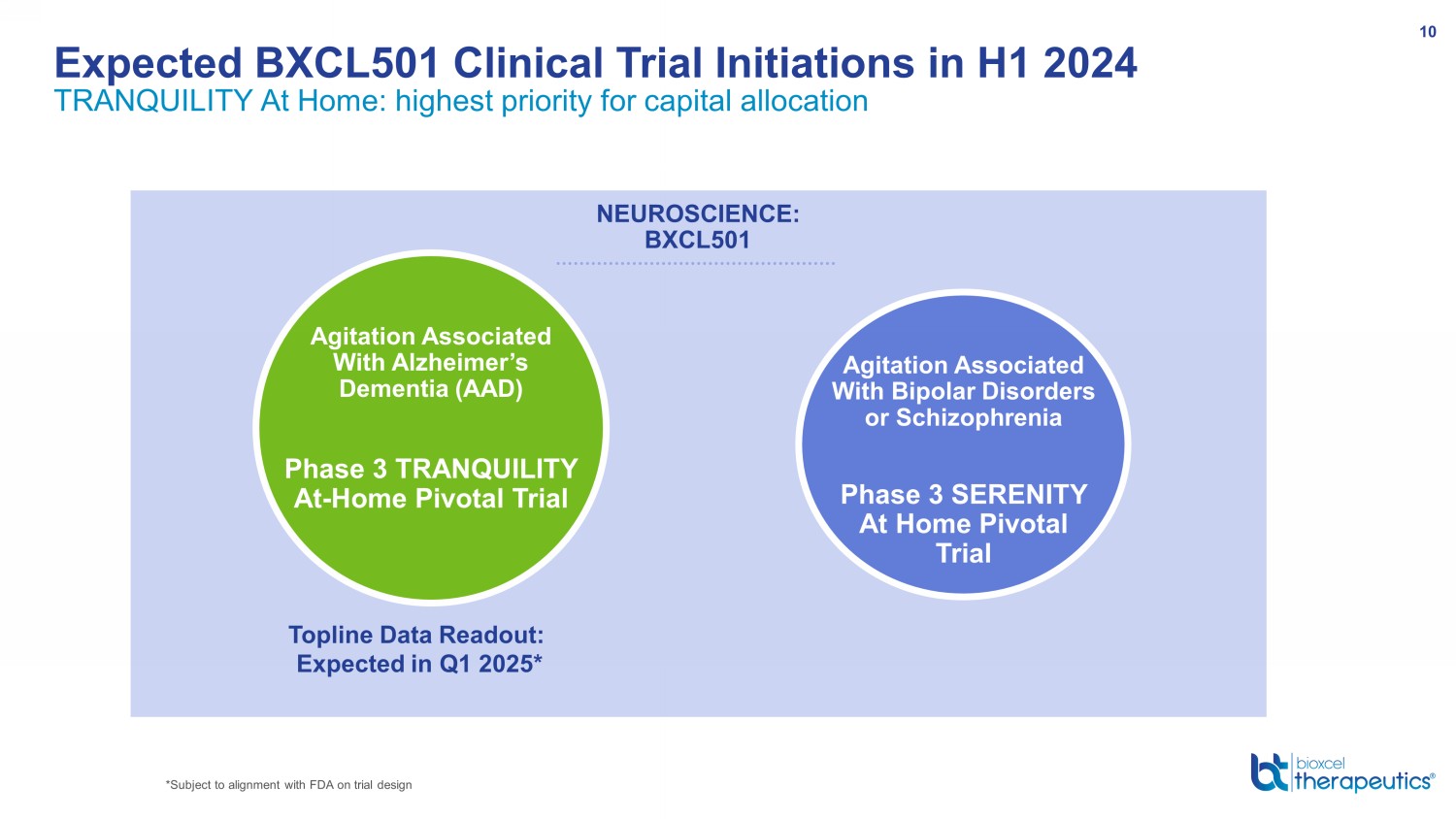
Expected BXCL501 Clinical Trial Initiations in H1 2024 TRANQUILITY At Home: highest priority for capital allocation 10 NEUROSCIENCE: BXCL501 Agitation Associated With Bipolar Disorders or Schizophrenia Phase 3 SERENITY At Home Pivotal Trial Topline Data Readout: Expected in Q1 2025* Agitation Associated With Alzheimer’s Dementia (AAD) P hase 3 TRANQUILITY At - Home Pivotal Trial *Subject to alignment with FDA on trial design
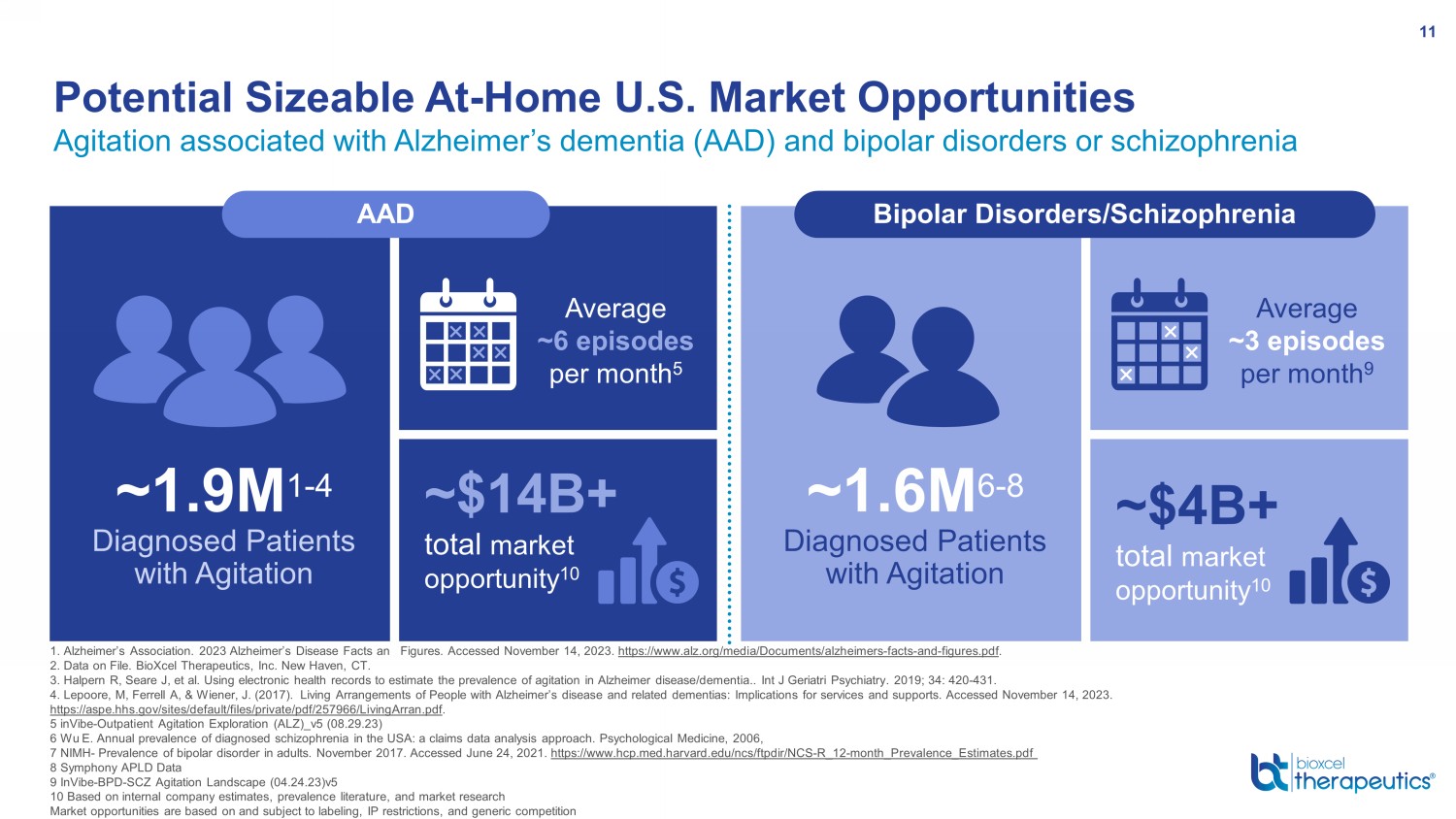
Agitation associated with Alzheimer’s dementia (AAD) and bipolar disorders or schizophrenia Potential Sizeable At - Home U.S. Market Opportunities 11 1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Accessed November 14, 2023. https://www.alz.org/media/Documents/alzheimers - facts - and - figures.pdf . 2. Data on File. BioXcel Therapeutics, Inc. New Haven, CT. 3. Halpern R, Seare J, et al. Using electronic health records to estimate the prevalence of agitation in Alzheimer disease/de men tia.. Int J Geriatri Psychiatry. 2019; 34: 420 - 431. 4. Lepoore, M, Ferrell A, & Wiener, J. (2017). Living Arrangements of People with Alzheimer’s disease and related dementias: Im plications for services and supports. Accessed November 14, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/257966/LivingArran.pdf . 5 inVibe - Outpatient Agitation Exploration (ALZ)_v5 (08.29.23) 6 Wu E. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychological Medicine, 2006, 7 NIMH - Prevalence of bipolar disorder in adults. November 2017. Accessed June 24, 2021. https://www.hcp.med.harvard.edu/ncs/ftpdir/NCS - R_12 - month_Prevalence_Estimates.pdf 8 Symphony APLD Data 9 InVibe - BPD - SCZ Agitation Landscape (04.24.23)v5 10 Based on internal company estimates, prevalence literature, and market research Market opportunities are based on and subject to labeling, IP restrictions, and generic competition ~$14B+ total market opportunity 10 ~1.9M 1 - 4 Diagnosed Patients with Agitation AAD Average ~6 episodes per month 5 ~$4B+ total market opportunity 10 ~1.6M 6 - 8 Diagnosed Patients with Agitation Average ~3 episodes per month 9 Bipolar Disorders/Schizophrenia

® IGALMI Œ Commercialization 12 Following commercial field workforce reduction in August 2023

First and only orally dissolving sublingual film currently in use under healthcare provider supervision for acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults 1 IGALMI Œ (dexmedetomidine) Sublingual Film 13 Noninvasive, self - administered film 1 - 4 covering mild, moderate, and severe agitation • Rapid absorption of dexmedetomidine into the bloodstream via oral mucosa 1 • Mucoadhesive film, designed so it cannot be spit out or swallowed 1 - 3 • Sublingual or buccal placement 1 • Mint - flavored 1 IGALMI was not studied for longer than 24 hours after the first dose. There may be a risk of physical dependence, a withdrawa l s yndrome, tolerance, and/or tachyphylaxis if IGALMI is used in a manner other than indicated. 1. IGALMI [package insert]. New Haven, CT: BioXcel Therapeutics Inc.; 2022. 2. Data on file. BXCL501 - 301 CSR (SERENITY I). BioXcel Therapeutics, Inc.; January 2021 3. Data on file. BXCL501 - 302 CSR (SERENITY II). BioXcel Therapeutics, Inc.; January 2021. 4.Preskorn SH, et al. Effect of Sublingual Dexmedetomidine vs Placebo on Acute Agitation Associated With Bipolar Disorder A Randomized Clinical Trial. JAMA . 2022;327(8):727 - 736. Please see Important Safety Information at the end of this presentation.
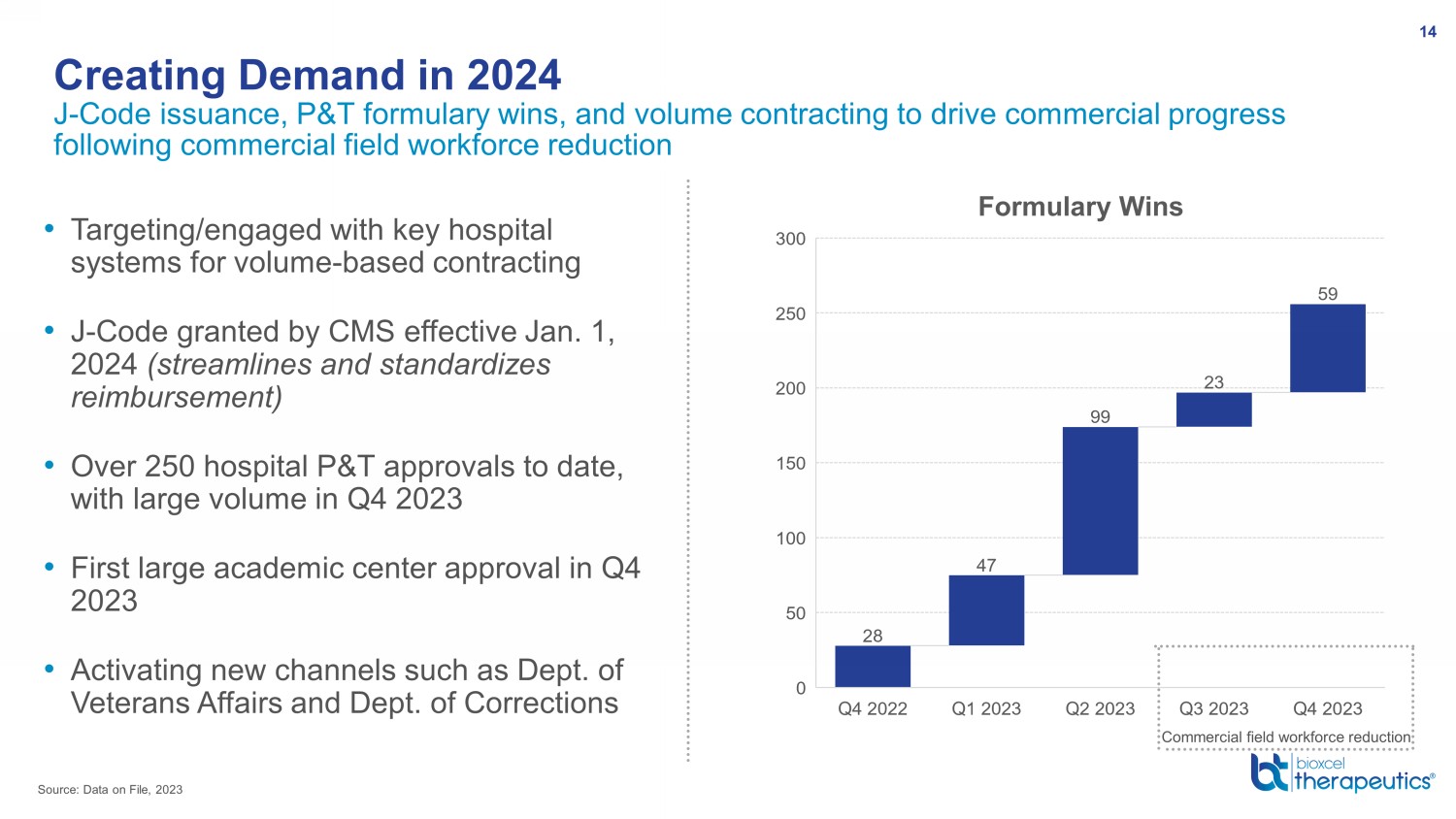
• Targeting/engaged with key hospital systems for volume - based contracting • J - Code granted by CMS effective Jan. 1, 2024 (streamlines and standardizes reimbursement) • Over 250 hospital P&T approvals to date, with large volume in Q4 2023 • First large academic center approval in Q4 2023 • Activating new channels such as Dept. of Veterans Affairs and Dept. of Corrections J - Code issuance, P&T formulary wins, and volume contracting to drive commercial progress following commercial field workforce reduction Creating Demand in 2024 14 Source: Data on File, 2023 Commercial field workforce reduction
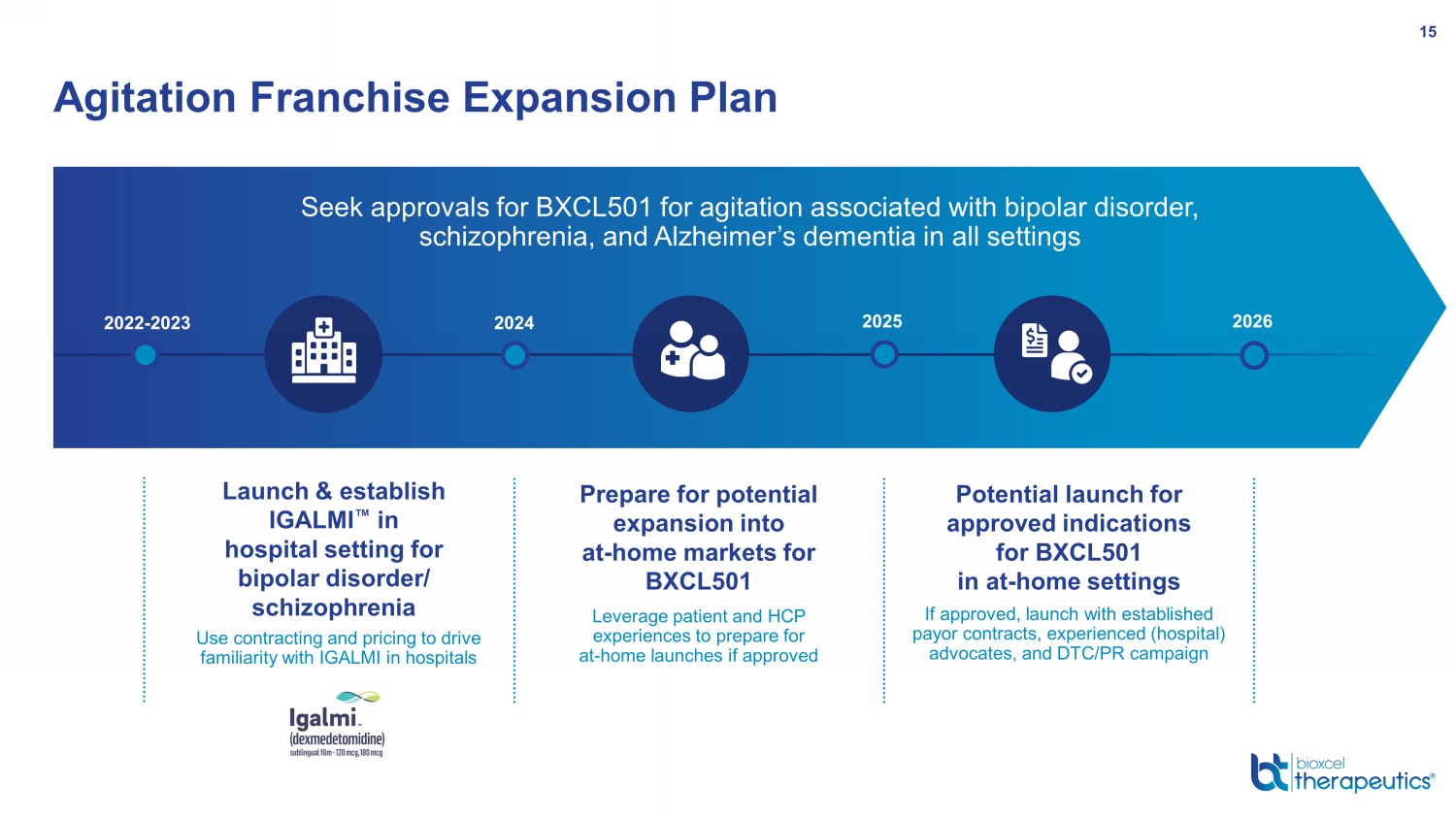
Agitation Franchise Expansion Plan 15 Launch & establish IGALMI Œ in hospital setting for bipolar disorder/ schizophrenia Use contracting and pricing to drive familiarity with IGALMI in hospitals 2022 - 2023 2024 2025 2026 Prepare for potential expansion into at - home markets for BXCL501 Leverage patient and HCP experiences to prepare for at - home launches if approved Potential launch for approved indications for BXCL501 in at - home settings If approved, launch with established payor contracts, experienced (hospital) advocates, and DTC/PR campaign Seek approvals for BXCL501 for agitation associated with bipolar disorder, schizophrenia, and Alzheimer’s dementia in all settings

® AI - Driven Drug Re - innovation Platform ® ® 16
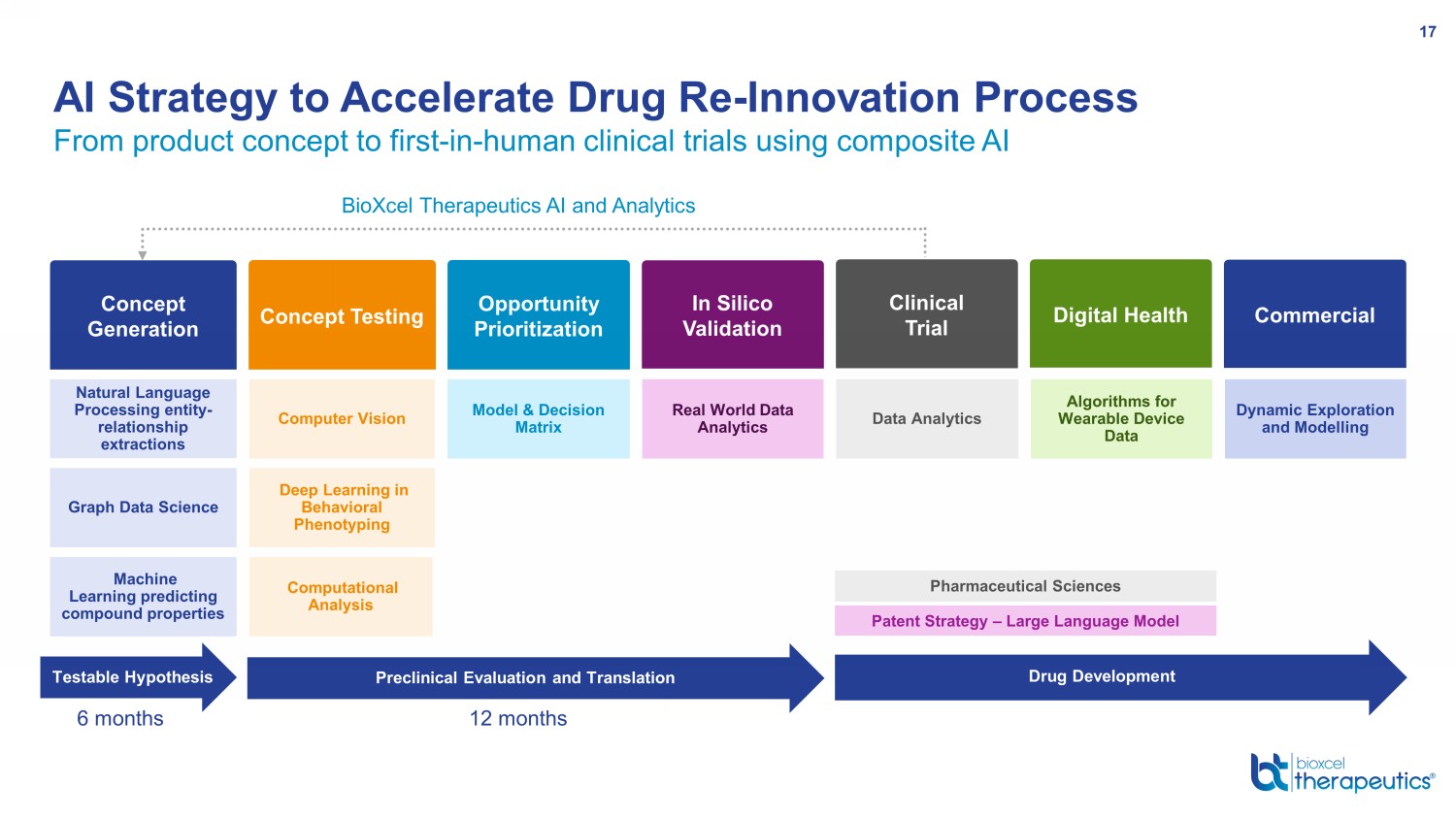
From product concept to first - in - human clinical trials using composite AI AI Strategy to Accelerate Drug Re - Innovation Process 17 Concept Generation BioXcel Therapeutics AI and Analytics Natural Language Processing entity - relationship extractions Graph Data Science Machine Learning predicting compound properties Concept Testing Opportunity Prioritization Digital Health Data Analytics Algorithms for Wearable Device Data Model & Decision Matrix Computer Vision Deep Learning in Behavioral Phenotyping Computational Analysis In Silico Validation Real World Data Analytics Commercial Dynamic Exploration and Modelling Preclinical Evaluation and Translation Testable Hypothesis 6 months 12 months Drug Development Patent Strategy – Large Language Model Pharmaceutical Sciences Clinical Trial

Pre - Clinical Platforms Indications Targets Compounds BTAI Re - Innovation Library Opportunity Prioritization Stress Axis Pre - Clinical Validation Mid/Late - Stage Assets BTAI Knowledge Graphs NovareAI: Ecosystem for Drug Discovery and Development AI 18 AI AI AI

Identifying targets and compounds designed to address unmet medical needs in dementia Behavioral and Psychological Symptoms in Alzheimer’s Disease 19 Sleep Disturbances Hallucinations Apathy Depression Agitation/Aggression Anxiety NovareAI 42.3% 38.8% 26.8% 57.4% 27.5% Delusions 24.8% 35.6% BXCL501/502 Disinhibition 26.3% Irritability 50.5% BXCL501 - Norepinephrine BXCL502 - Serotonin BXCL503 - Dopamine BXCL501/502 BXCL501/502 BXCL503 * Denotes a potential role for BXCL502 and BXCL503 in addressing these symptoms The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. Prevalences derived from Laganà et al., Neuropsychiatric or Behavioral and Psychological Symptoms of Dementia (BPSD): Focus on P revalence and Natural History in Alzheimer's Disease and Frontotemporal Dementia; Front Neurol 2022;13 83219 9 * * * *

® BXCL502: A Novel Agent for Treatment of Chronic Agitation in Dementia ® 20 ®
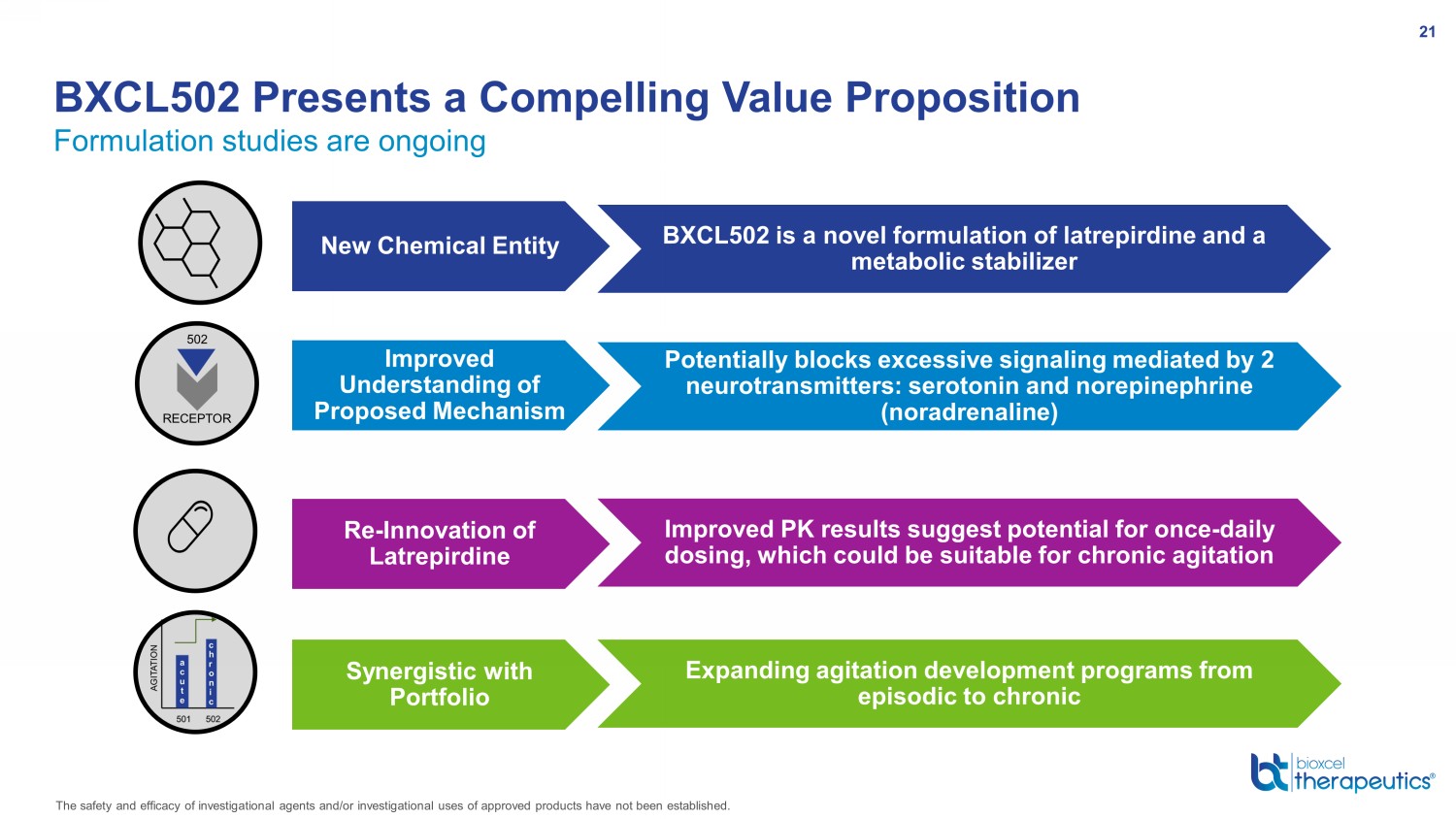
Formulation studies are ongoing BXCL502 Presents a Compelling Value Proposition 21 BXCL502 is a novel formulation of latrepirdine and a metabolic stabilizer Potentially blocks excessive signaling mediated by 2 neurotransmitters: serotonin and norepinephrine (noradrenaline) New Chemical Entity Improved Understanding of Proposed Mechanism Re - Innovation of Latrepirdine Improved PK results suggest potential for once - daily dosing, which could be suitable for chronic agitation Synergistic with Portfolio Expanding agitation development programs from episodic to chronic The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established.

Latrepirdine (Dimebon): Clinical Safety Results, Preclinical Confidence in Rationale, and Early Sign of Potential Efficacy 22 CLINICAL Early Sign of Potential Efficacy PRECLINICAL Confidence in Rationale SAFETY RESULTS IN PATIENTS Lower risk VALUE ● Over 1000 patients with AD exposed for 26 weeks and 500 up to 52 weeks ( Trials conducted by Pfizer and Medivation ) ● Showed activity in 5 preclinical models of neuropsychiatric symptoms ( Trials conducted by BioXcel Therapeutics ) ● Secondary efficacy endpoint, changes in Neuropsychiatric Inventory (NPI) , showed statistical superiority over placebo in 3 trials ( Trials conducted by Pfizer and Medivation ) The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. Data support development for treatment of neuropsychiatric symptoms associated with dementia
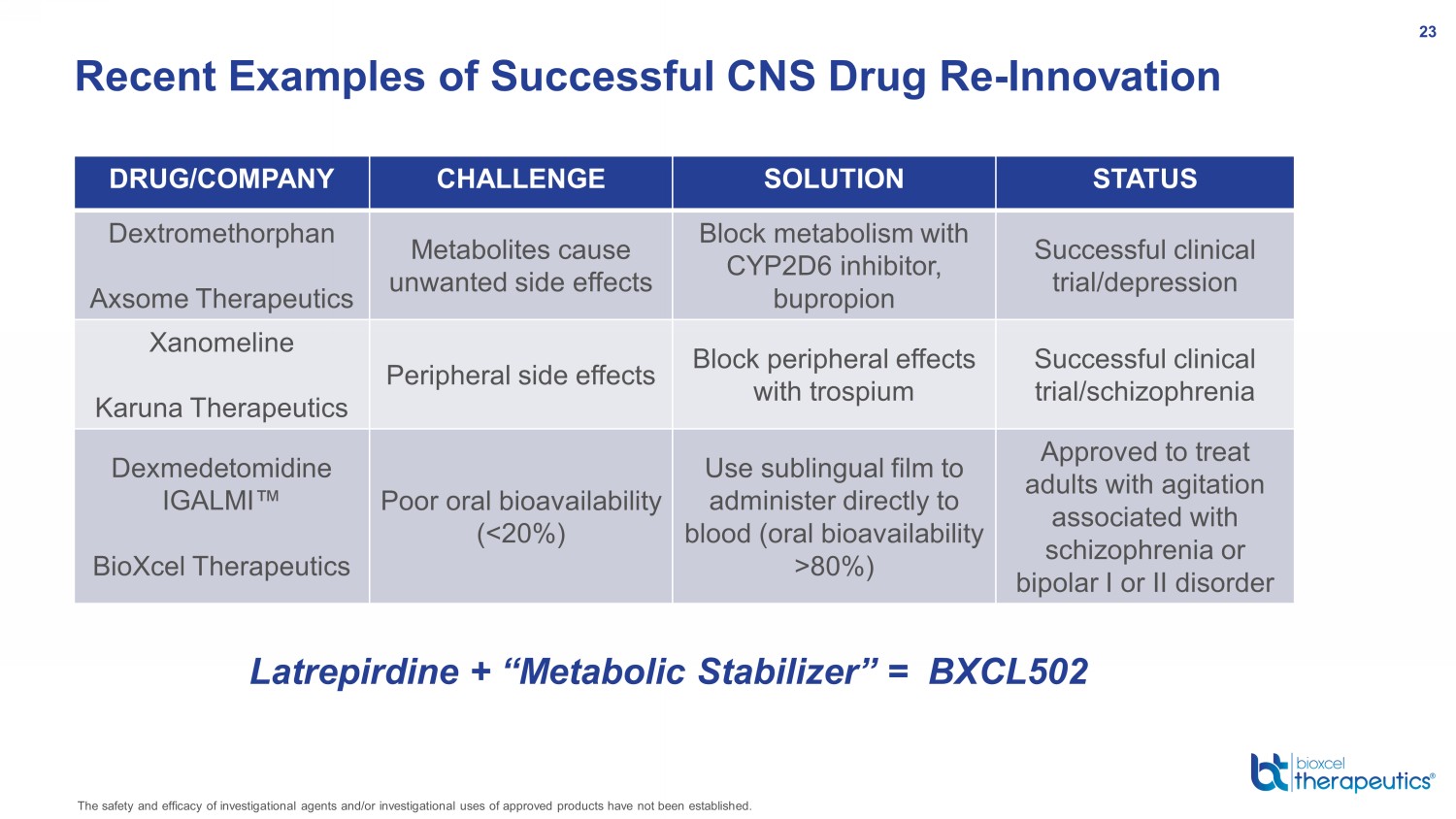
Recent Examples of Successful CNS Drug Re - Innovation DRUG/COMPANY CHALLENGE SOLUTION STATUS Dextromethorphan Axsome Therapeutics Metabolites cause unwanted side effects Block metabolism with CYP2D6 inhibitor, bupropion Successful clinical trial/depression Xanomeline Karuna Therapeutics Peripheral side effects Block peripheral effects with trospium Successful clinical trial/schizophrenia Dexmedetomidine IGALMI Œ BioXcel Therapeutics Poor oral bioavailability (<20%) Use sublingual film to administer directly to blood (oral bioavailability >80%) Approved to treat adults with agitation associated with schizophrenia or bipolar I or II disorder Latrepirdine + “Metabolic Stabilizer” = BXCL502 The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. 23

® Immuno - Oncology 24
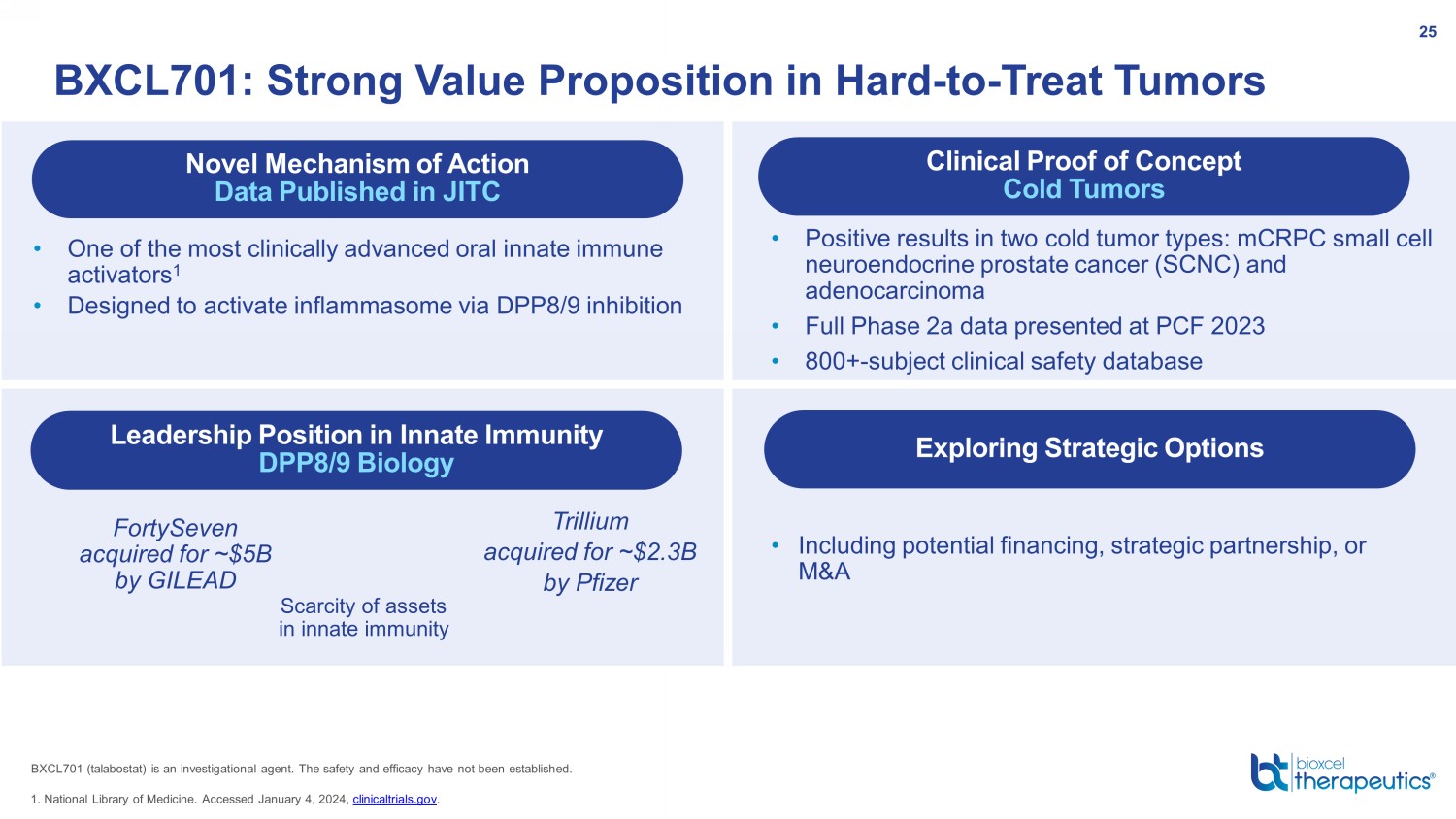
BXCL701: Strong Value Proposition in Hard - to - Treat Tumors 25 Novel Mechanism of Action Data Published in JITC Leadership Position in Innate Immunity DPP8/9 Biology Clinical Proof of Concept Cold Tumors Exploring Strategic Options • One of the most clinically advanced oral innate immune activators 1 • Designed to activate inflammasome via DPP8/9 inhibition • Including potential financing, strategic partnership, or M&A • Positive results in two cold tumor types: mCRPC small cell neuroendocrine prostate cancer (SCNC) and adenocarcinoma • Full Phase 2a data presented at PCF 2023 • 800+ - subject clinical safety database 1. National Library of Medicine. Accessed January 4, 2024, clinicaltrials.gov . BXCL701 ( talabostat ) is an investigational agent. The safety and efficacy have not been established. Scarcity of assets in innate immunity FortySeven acquired for ~$5B by GILEAD Trillium acquired for ~$2.3B by Pfizer

Immuno - Oncology Clinical Development Compound Proposed Indication Preclinical Phase 1 Phase 2 Phase 3 Expected Upcoming Milestone Collaborator BXCL701 Company - sponsored trials Small Cell Neuroendocrine Prostate Cancer (SCNC) FDA Meeting 13 centers US / UK Small Cell Lung Cancer (SCLC) Initiate Phase 1b/2 BXCL701 Investigator - sponsored trials Metastatic Pancreatic Ductal Adenocarcinoma Phase 2 readout Acute Myeloid Leukemia (AML) Phase 1b readout BXCL702 BXCL701 follow - on/ novel DPP inhibitor Solid Tumors Candidate nomination 26 As of February 14, 2024 The safety and efficacy of these investigational agents have not been established. Dana - Farber Cancer Institute Georgetown Lombardi Comprehensive Cancer Center Supply agreement: Merck

BioXcel Therapeutics | 555 Long Wharf Drive, 12th Floor | New Haven, CT 06511 | bioxceltherapeutics.com ® Thank you! 27

® Appendix 28

IGALMI Œ Indication and Important Safety Information 29 INDICATION IGALMI Ρ (dexmedetomidine) sublingual film is a prescription medicine, administered under the supervision of a health care provider, t ha t is placed under the tongue or behind the lower lip and is used for the acute treatment of agitation associated with schizophrenia and bipolar disorder I or II in adults. The sa fet y and effectiveness of IGALMI has not been studied beyond 24 hours from the first dose. It is not known if IGALMI is safe and effective in children. IMPORTANT SAFETY INFORMATION IGALMI can cause serious side effects, including: • Decreased blood pressure, low blood pressure upon standing, and slower than normal heart rate , which may be more likely in patients with low blood volume, diabetes, chronic high blood pressure, and older patients. IGALMI is taken under the supervision of a healthcare provider who will monitor vita l s igns (like blood pressure and heart rate) and alertness after IGALMI is administered to help prevent falling or fainting. Patients should be adequately hydrated and sit or lie down aft er taking IGALMI and instructed to tell their healthcare provider if they feel dizzy, lightheaded, or faint. • Heart rhythm changes (QT interval prolongation). IGALMI should not be given to patients with an abnormal heart rhythm, a history of an irregular heartbeat, slow heart rate, l ow potassium, low magnesium, or taking other drugs that could affect heart rhythm. Taking IGALMI with a history of abnormal hear t r hythm can increase the risk of torsades de pointes and sudden death. Patients should be instructed to tell their healthcare provider immediately if they feel faint or have heart pa lpi tations. • Sleepiness/drowsiness. Patients should not perform activities requiring mental alertness, such as driving or operating hazardous machinery, for at l ea st 8 hours after taking IGALMI. • Withdrawal reactions, tolerance, and decreased response/efficacy . IGALMI was not studied for longer than 24 hours after the first dose. Physical dependence, withdrawal symptoms (e.g., nausea, vomiting, agitation), and decreased response to IGALMI may occur if IGALMI is used longer than 24 hours. The most common side effects of IGALMI in clinical studies were sleepiness or drowsiness, a prickling or tingling sensation or numbness of the mouth, dizz in ess, dry mouth, low blood pressure, and low blood pressure upon standing. These are not all the possible side effects of IGALMI. Patients should speak with their healthcare provider for medical advic e a bout side effects. Patients should tell their healthcare provider about their medical history, including if they suffer from any known heart problems, low potassium, low magnesium, low blood pressure, low heart rate, diabetes, high blood pressure, history of fainting, or liver impairment. They should also tell their healthca re provider if they are pregnant or breastfeeding or take any medicines, including prescription and over - the - counter medicines, vitamins, and herbal supplements. Patients should especially t ell their healthcare provider if they take any drugs that lower blood pressure, change heart rate, or take anesthetics, sedatives, hypnotics, and opioids. Everyone is encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1 - 800 - FDA - 1088. You can also contact BioXcel Therapeutics, Inc. at 1 - 833 - 201 - 1088 or medinfo@bioxceltherapeutics.com . Please see full Prescribing Information .
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
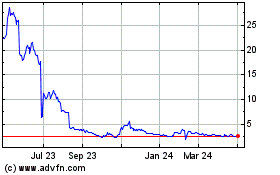
BioXcel Therapeutics (NASDAQ:BTAI)
Historical Stock Chart
From Mar 2024 to Apr 2024
BioXcel Therapeutics (NASDAQ:BTAI)
Historical Stock Chart
From Apr 2023 to Apr 2024