0001659323false00-000000000016593232024-01-302024-01-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 30, 2024 |
Iterum Therapeutics plc
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Ireland |
001-38503 |
Not applicable |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
Fitzwilliam Court 1st Floor Leeson Close |
|
Dublin 2, Ireland, |
|
Not applicable |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: +353 1 6694820 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Ordinary Shares, par value $0.01 per share |
|
ITRM |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 30, 2024, Iterum Therapeutics plc (the “Company”), in connection with the positive topline results for its Phase 3 REASSURE Clinical Trial of Oral Sulopenem in uncomplicated urinary tract infections (“uUTI”), issued a press release and provided an investor presentation, which will be made available on the Company’s website.
A copy of the press release and investor presentation are attached as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K (this “Current Report”). The information set forth in this Item 7.01 and in Exhibit 99.1 and Exhibit 99.2 attached hereto is “furnished” and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall such information be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended.
Item 8.01 Other Events.
On January 30, 2024, the Company announced positive topline results from its Phase 3 REASSURE Clinical Trial of Oral Sulopenem in uUTIs. The trial was conducted under special protocol assessment (“SPA”) agreement with the U.S. Food and Drug Administration (“FDA”). Results demonstrate that oral sulopenem was non-inferior to Augmentin® with respect to the trial’s primary endpoint, overall response (combined clinical cure plus microbiologic eradication) at the test-of-cure (“TOC”) visit in the microbiological-modified-intent-to-treat susceptible (“m-MITTS”) population. Oral sulopenem showed overall success in 61.7% of patients compared to 55.0% for Augmentin®, demonstrating statistically significant superiority of oral sulopenem versus Augmentin®. Oral sulopenem showed overall success in 61.7% of patients compared to 55.0% for Augmentin® demonstrating statistically significant superiority of oral sulopenem versus Augmentin®. Favorable overall response rates at TOC were 61.7% versus 55.0% for oral sulopenem and Augmentin®, respectively.
Both oral sulopenem and Augmentin® were well tolerated in this trial with discontinuations due to adverse events occurring in <1% of patients on both regimens. No serious adverse events (“SAE”) were reported in patients receiving oral sulopenem, while five SAEs occurred in patients receiving Augmentin®, with no drug-related SAEs. The safety profile for oral sulopenem was consistent with those observed in each of the previously conducted Phase 3 trials, with no new safety signals observed beyond those associated with β-lactams.
In addition to achieving non-inferiority for the primary endpoint of overall response at the TOC visit in the Augmentin®-susceptible population in the REASSURE trial, the lower limit of the 95% confidence interval around the treatment difference was above zero, indicating statistical superiority of oral sulopenem over Augmentin® for the treatment of uUTI. Furthermore, consistent results were observed for all key secondary efficacy endpoints in this population.
The REASSURE trial is designed as a non-inferiority (10% margin) trial comparing oral sulopenem and Augmentin® in the Augmentin®-susceptible population and is entitled “A prospective, Phase 3, randomized, multi-center, double-blind study of the efficacy, tolerability, and safety of oral sulopenem etzadroxil/probenecid versus oral amoxicillin/clavulanate for treatment of uncomplicated urinary tract infections (“uUTI”) in adult women.” If the lower bound of the 95% CI is greater than -10%, non-inferiority of oral sulopenem over Augmentin® would be concluded under the trial's statistical analysis plan. If the lower bound of the 95% CI is greater than 0%, superiority of oral sulopenem over Augmentin® would be concluded under the trial's statistical analysis plan. Patients were randomized to receive either oral sulopenem twice daily for five days or Augmentin® twice daily for five days. The primary endpoint was the overall response (clinical and microbiologic combined response) at Day 12 (+/- 1 day) (TOC) of the trial. The trial enrolled 2,222 patients and is being conducted under a SPA agreement with the FDA.
The Company expects to resubmit the New Drug Application (“NDA”) for oral sulopenem to the U.S. Food and Drug Administration (“FDA”) in the second quarter of 2024. Provided that the resubmitted NDA addresses all of the deficiencies identified in the Complete Response Letter (“CRL”) the Company received from the FDA in July 2021, the Company expects that the FDA will complete its review and take action in the fourth quarter of 2024 (six months from the date the FDA receives the resubmitted NDA). At the same time, the Company plans to focus on a strategic process to sell, license, or otherwise dispose of its rights to sulopenem with the goal of maximizing value for its stakeholders. The Company cannot provide any commitment regarding when or if this strategic process will result in any type of transaction and no assurance can be given that the Company will determine to pursue a potential sale, licensing arrangement or other disposition of its rights to sulopenem.
Cautionary Note Regarding Forward-looking Statements
This Current Report contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation the Company's ability to address the deficiencies set out in the complete response letter received in July 2021, the expected timing of resubmission of the NDA, the expected timing of review by the FDA and the Company’s strategic process to sell, license, or otherwise dispose of its rights to sulopenem to maximise value for its stakeholders. In some cases, forward-looking statements can be identified by words such as “may,” “believes,” “intends,” “seeks,” “anticipates,” “plans,” “estimates,” “expects,” “should,” “assumes,” “continues,” “could,” “would,” “will,” “future,” “potential” or the negative of these or similar terms and phrases. Forward-looking statements involve known and unknown risks, uncertainties and other factors that
may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include all matters that are not historical facts. Actual future results may be materially different from what is expected due to factors largely outside the Company’s control, including uncertainties inherent in the conduct of clinical and non-clinical development, changes in regulatory requirements or decisions of regulatory authorities, the timing or likelihood of regulatory filings and approvals, including the potential resubmission of the NDA for oral sulopenem, changes in public policy or legislation, commercialization plans and timelines, if oral sulopenem is approved, the actions of third-party clinical research organizations, suppliers and manufacturers, the accuracy of the Company’s expectations regarding how far into the future the Company’s cash on hand will fund the Company’s ongoing operations, the Company’s ability to maintain its listing on the Nasdaq Capital Market, risks and uncertainties concerning the outcome, impact, effects and results of the Company’s pursuit of strategic alternatives, including the terms, timing, structure, value, benefits and costs of any strategic process and the Company’s ability to complete one at all and other factors discussed under the caption “Risk Factors” in its Quarterly Report on Form 10-Q filed with the SEC on November 14, 2023, and other documents filed with the U.S. Securities and Exchange Commission (“SEC”) from time to time. Forward-looking statements represent the Company’s beliefs and assumptions only as of the date of this press release. Except as required by law, the Company assumes no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
|
|
Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
The following exhibits relate to Items 7.01 and 8.01, and shall be deemed to be furnished, and not filed:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Iterum Therapeutics plc |
|
|
|
|
Date: |
January 30, 2024 |
By: |
/s/ Corey N. Fishman |
|
|
|
Corey N. Fishman
Chief Executive Officer |
Exhibit 99.1
Iterum Therapeutics Announces Positive Topline Results from its Phase 3 REASSURE Clinical Trial of Oral Sulopenem in Uncomplicated Urinary Tract Infections
Phase 3 REASSURE Trial Met Primary Endpoint of Non-Inferiority to Augmentin®; Demonstrated Statistical Superiority
Re-submission of NDA to FDA Expected in Q2 2024
Potential to be First Oral Penem Approved in the U.S.
Management to host a conference call at 8:30 a.m. ET today
DUBLIN, Ireland and CHICAGO, January 30, 2024 -- Iterum Therapeutics plc (Nasdaq: ITRM) (Iterum), a clinical-stage pharmaceutical company focused on developing next-generation oral antibiotics to treat infections caused by multi-drug resistant pathogens in community settings, today announced positive topline results from its REASSURE (REnewed ASsessment of Sulopenem in uUTI caused by Resistant Enterobacterales) Phase 3 clinical trial comparing oral sulopenem (sulopenem etzadroxil combined with probenecid in a bilayer tablet) to oral Augmentin® (amoxicillin/clavulanate) in adult women with uncomplicated urinary tract infections (uUTIs).
“We are very pleased to announce positive data from this confirmatory trial, which was conducted under special protocol assessment (SPA) agreement with the U.S. Food and Drug Administration (FDA),” said Corey Fishman, Iterum’s Chief Executive Officer. “With the positive data from this trial, we plan to resubmit our New Drug Application (NDA) for oral sulopenem for the treatment of uUTI in the second quarter of 2024. At the same time, with these results in hand, we will be focusing on a strategic process to sell, license, or otherwise dispose of our rights to sulopenem with the goal of maximizing value for our stakeholders. We believe there is tremendous value in sulopenem as a potential new, oral antibiotic for the uUTI indication which has over 30 million infections annually in the U.S., rising resistance to all currently prescribed oral antibiotics, and a complete lack of new product innovation over the last 20 years.”
Results demonstrate that oral sulopenem was non-inferior to Augmentin® with respect to the trial’s primary endpoint, overall response (combined clinical cure plus microbiologic eradication) at the test-of-cure (TOC) visit in the microbiological-modified-intent-to-treat susceptible (m-MITTS) population. Oral sulopenem showed overall success in 61.7% of patients compared to 55.0% for Augmentin®, demonstrating statistically significant superiority of oral sulopenem versus Augmentin®.
The table below summarizes the key efficacy data from the REASSURE trial at the TOC visit:
|
|
|
|
|
Sulopenem/probenecid
500 mg/500 mg BID N=480 n (%) |
Augmentin® (Amoxicillin/clavulanate)875 mg/125 mg BID
N=442 n (%) |
Treatment Differencei
(95% CI) |
Overall Responseii |
296 (61.7) |
243 (55.0) |
6.7 (0.3, 13.0) |
Clinical Successiii |
371 (77.3) |
339 (76.7) |
0.6 (-4.8, 6.1) |
Microbiological Successiv |
361 (75.2) |
295 (66.7) |
8.5 (2.6, 14.3) |
[i] Difference in oral sulopenem versus Augmentin® in the m-MITTS population
[ii] Combined clinical and microbiological success (primary endpoint)
[iii] Clinical success at TOC = symptom resolution + no new uUTI symptoms
[iv] Eradication of qualifying uropathogen to <103 CFU/mL at TOC visit
Both oral sulopenem and Augmentin® were well tolerated in this study with discontinuations due to adverse events occurring in <1% of patients on both regimens. No serious adverse events (SAE) were reported in patients receiving oral sulopenem, while five SAEs occurred in patients receiving Augmentin®, with no drug-related SAEs. The safety profile for oral sulopenem was consistent with those observed in each of the previously conducted Phase 3 trials, with no new safety signals noted beyond those associated with β-lactams.
Iterum expects to present complete results from the REASSURE trial at an upcoming scientific meeting.
“In addition to achieving non-inferiority for the primary endpoint of overall response at the TOC visit in the Augmentin®-susceptible population in the REASSURE trial, the lower limit of the 95% confidence interval around the treatment difference was above zero, indicating statistical superiority of oral sulopenem over Augmentin® for the treatment of uUTI. Furthermore, consistent results were observed for all key secondary efficacy endpoints in this population,” said Sailaja Puttagunta, M.D., Iterum’s Chief Medical Officer. “These results bring us one step closer to delivering a much-needed oral treatment option for women suffering from uUTIs. In addition, we believe these results, along with evidence from our prior Phase 3 studies, support the potential of sulopenem in other indications, such as complicated urinary tract infections (cUTI).”
Iterum expects to resubmit its NDA for oral sulopenem to the FDA in the second quarter of 2024. Provided that the resubmitted NDA addresses all of the deficiencies identified in the Complete Response Letter (CRL) Iterum received from the FDA in July 2021, Iterum expects that the FDA will complete its review and take action six months from the date the FDA receives the resubmitted NDA (or during the fourth quarter of 2024).
Conference Call and Webcast Details
Iterum will host a conference call and webcast today, Tuesday, January 30, 2024, at 8:30 a.m. Eastern Time. The dial-in information for the call is as follows:
United States: 1 833 470 1428 / International: 1 404 975 4839
Access code: 781689
The conference call will also be webcast live. The webcast can be accessed here.
About REASSURE
The REASSURE trial is designed as a non-inferiority (10% margin) trial comparing oral sulopenem and Augmentin® in the Augmentin®-susceptible population and is entitled “A prospective, Phase 3, randomized, multi-center, double-blind study of the efficacy, tolerability, and safety of oral sulopenem etzadroxil/probenecid versus oral amoxicillin/clavulanate for treatment of uncomplicated urinary tract infections (uUTI) in adult women.” If the lower bound of the 95% CI is greater than -10%, non-inferiority of oral sulopenem over Augmentin would be concluded. If the lower bound of the 95% CI is greater than 0%, superiority of oral sulopenem over Augmentin would be concluded. Patients were randomized to receive either oral sulopenem twice daily for five days or Augmentin® twice daily for five days. The primary endpoint was the overall response (clinical and microbiologic combined response) at Day 12 (+/- 1 day) (TOC visit) of the trial. The trial enrolled 2,222 patients and is being conducted under a SPA agreement with the FDA.
About Urinary Tract Infections (UTIs)
UTIs are among the most common bacterial infections encountered in the community. There are approximately 15 million emergency room and office visits for symptoms of UTIs and over 30 million uUTIs treated in the United States annually, with approximately 30% of those infections caused by a quinolone non-susceptible organism, and approximately 1% of those infections caused by pathogens that are resistant to all commonly available classes of oral antibiotics. As a result, the treatment of UTIs has become more challenging because of the development of resistance by pathogens responsible for these infections. uUTIs are infections of the bladder occurring mainly in women. Half (50%) of all women experience at least one uUTI at some point in their lives.
About Iterum Therapeutics plc
Iterum Therapeutics plc is a clinical-stage pharmaceutical company dedicated to developing differentiated anti-infectives aimed at combatting the global crisis of multi-drug resistant pathogens to significantly improve the lives of people affected by serious and life-threatening diseases around the world. Iterum is currently advancing its first compound – sulopenem – a novel penem anti-infective compound, in Phase 3 clinical development with an oral formulation.
Sulopenem also has an IV formulation. Sulopenem has demonstrated potent in vitro activity against a wide variety of gram-negative, gram-positive and anaerobic bacteria resistant to other antibiotics. Iterum has received Qualified Infectious Disease Product (QIDP) and Fast Track designations for its oral and IV formulations of sulopenem in seven indications. For more information, please visit www.iterumtx.com.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements regarding the development, therapeutic and market potential of sulopenem, our ability to address the deficiencies set out in the complete response letter received in July 2021, the expected timing of resubmission of the NDA, the expected timing of review by the FDA and Iterum’s strategic process to sell, license, or otherwise dispose of its rights to sulopenem. In some cases, forward-looking statements can be identified by words such as “may,” “believes,” “intends,” “seeks,” “anticipates,” “plans,” “estimates,” “expects,” “should,” “assumes,” “continues,” “could,” “would,” “will,” “future,” “potential” or the negative of these or similar terms and phrases. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Iterum’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include all matters that are not historical facts. Actual future results may be materially different from what is expected due to factors largely outside Iterum’s control, including uncertainties inherent in the conduct of clinical and non-clinical development, changes in regulatory requirements or decisions of regulatory authorities, the timing or likelihood of regulatory filings and approvals, including the potential resubmission of the NDA for oral sulopenem, changes in public policy or legislation, commercialization plans and timelines, if oral sulopenem is approved, the actions of third-party clinical research organizations, suppliers and manufacturers, the accuracy of Iterum’s expectations regarding how far into the future Iterum’s cash on hand will fund Iterum’s ongoing operations, Iterum’s ability to maintain its listing on the Nasdaq Capital Market, risks and uncertainties concerning the outcome, impact, effects and results of Iterum’s pursuit of strategic alternatives, including the terms, timing, structure, value, benefits and costs of any strategic process and Iterum’s ability to complete one at all and other factors discussed under the caption “Risk Factors” in its Quarterly Report on Form 10-Q filed with the SEC on November 14, 2023, and other documents filed with the SEC from time to time. Forward-looking statements represent Iterum’s beliefs and assumptions only as of the date of this press release. Except as required by law, Iterum assumes no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
Investor Contact:
Judy Matthews
Chief Financial Officer
312-778-6073
IR@iterumtx.com

REASSURE Phase 3 Topline Data�Conference Call��January 30, 2024 EXHIBIT 99.2

This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements regarding the development, therapeutic and market potential of sulopenem, the Company’s ability to address the deficiencies set out in the complete response letter received in July 2021, the expected timing of resubmission of the NDA, the expected timing of review by the FDA, and the Company’s strategic process to sell, license or otherwise dispose of its rights to sulopenem. In some cases, forward-looking statements can be identified by words such as “may,” “believes,” “intends,” “seeks,” “anticipates,” “plans,” “estimates,” “expects,” “should,” “assumes,” “continues,” “could,” “would,” “will,” “future,” “potential” or the negative of these or similar terms and phrases. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include all matters that are not historical facts. Actual future results may be materially different from what is expected due to factors largely outside the Company’s control, including uncertainties inherent in the conduct of clinical and non-clinical development, changes in regulatory requirements or decisions of regulatory authorities, the timing or likelihood of regulatory filings and approvals, including the potential resubmission of the NDA for oral sulopenem, changes in public policy or legislation, commercialization plans and timelines, if oral sulopenem is approved, the actions of third-party clinical research organizations, suppliers and manufacturers, the accuracy of Iterum’s expectations regarding how far into the future Iterum’s cash on hand will fund Iterum’s ongoing operations, Iterum’s ability to maintain its listing on the Nasdaq Capital Market, risks and uncertainties concerning the outcome, impact, effects and results of the Company’s pursuit of strategic alternatives, including the terms, timing, structure, value, benefits and costs of any strategic process and the Company’s ability to complete one at all and other factors discussed under the caption “Risk Factors” in its Quarterly Report on Form 10-Q filed with the SEC on November 14, 2023, and other documents filed with the SEC from time to time. Forward-looking statements contained herein represent the Company’s beliefs and assumptions only as of January 30, 2024. Except as required by law, neither we, nor the Company, assume any obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not been independently verified, and neither we nor the Company make any representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its own internal research is reliable, such research has not been verified by any independent source. Forward-looking Statements & Disclaimer
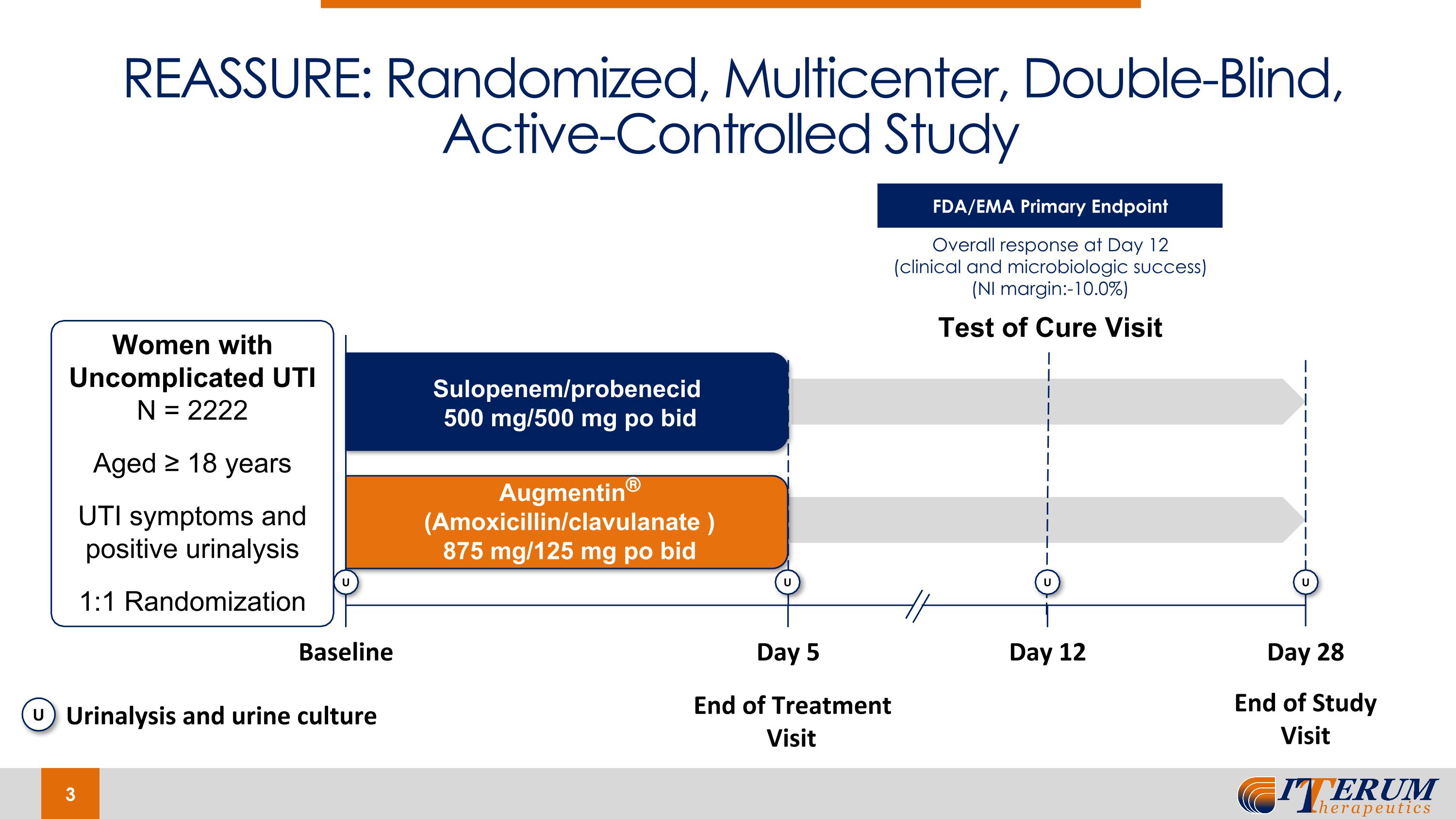
REASSURE: Randomized, Multicenter, Double-Blind, Active-Controlled Study Test of Cure Visit Baseline Day 5 Day 12 Day 28 End of Treatment Visit End of Study Visit Augmentin® (Amoxicillin/clavulanate ) 875 mg/125 mg po bid Sulopenem/probenecid 500 mg/500 mg po bid Women with Uncomplicated UTI N = 2222 Aged ≥ 18 years UTI symptoms and positive urinalysis 1:1 Randomization U U U U U Urinalysis and urine culture FDA/EMA Primary Endpoint Overall response at Day 12 �(clinical and microbiologic success) (NI margin:-10.0%)
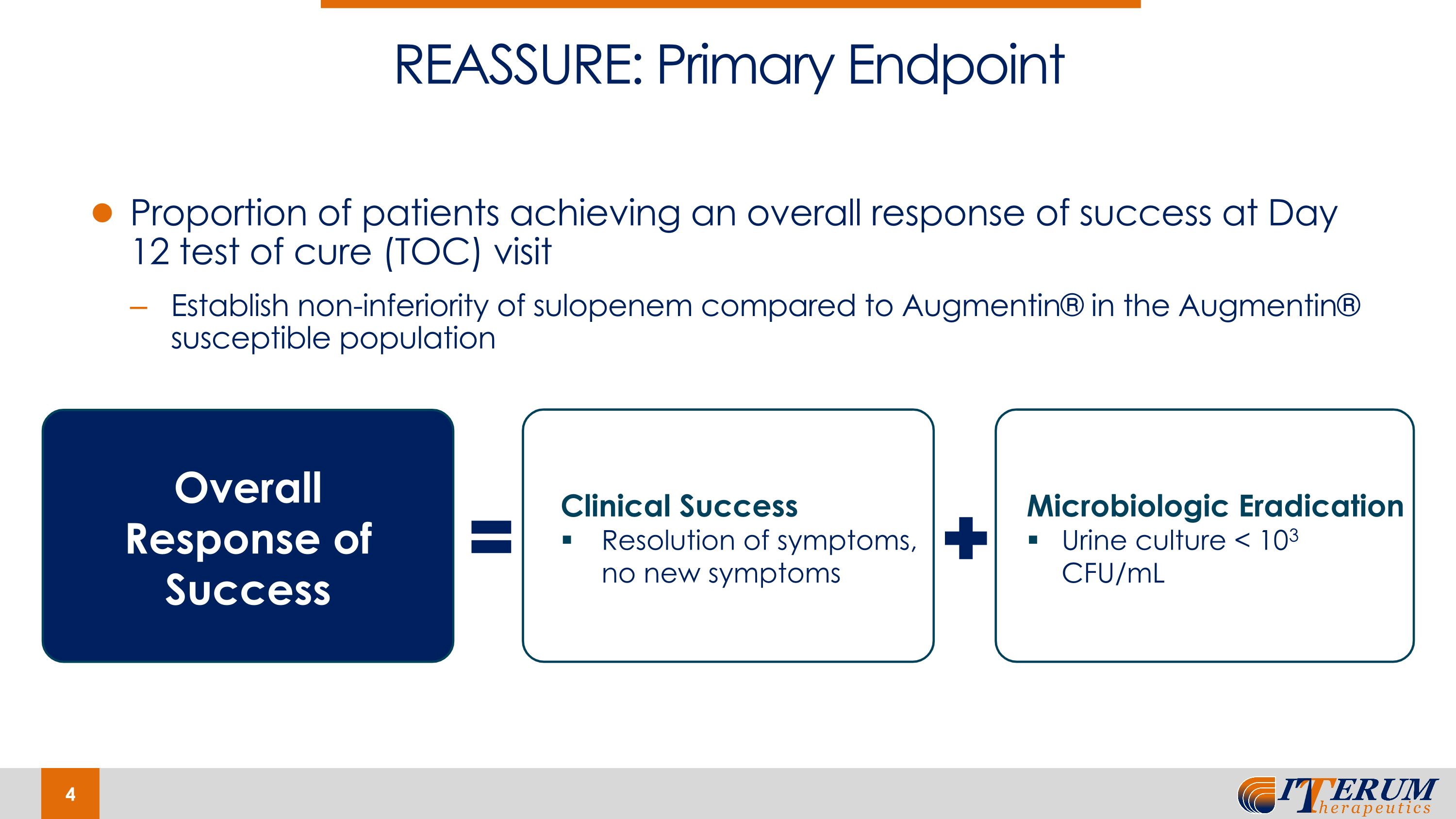
Proportion of patients achieving an overall response of success at Day 12 test of cure (TOC) visit Establish non-inferiority of sulopenem compared to Augmentin® in the Augmentin® susceptible population REASSURE: Primary Endpoint Microbiologic Eradication Urine culture < 103 CFU/mL Overall Response of Success Clinical Success Resolution of symptoms, no new symptoms
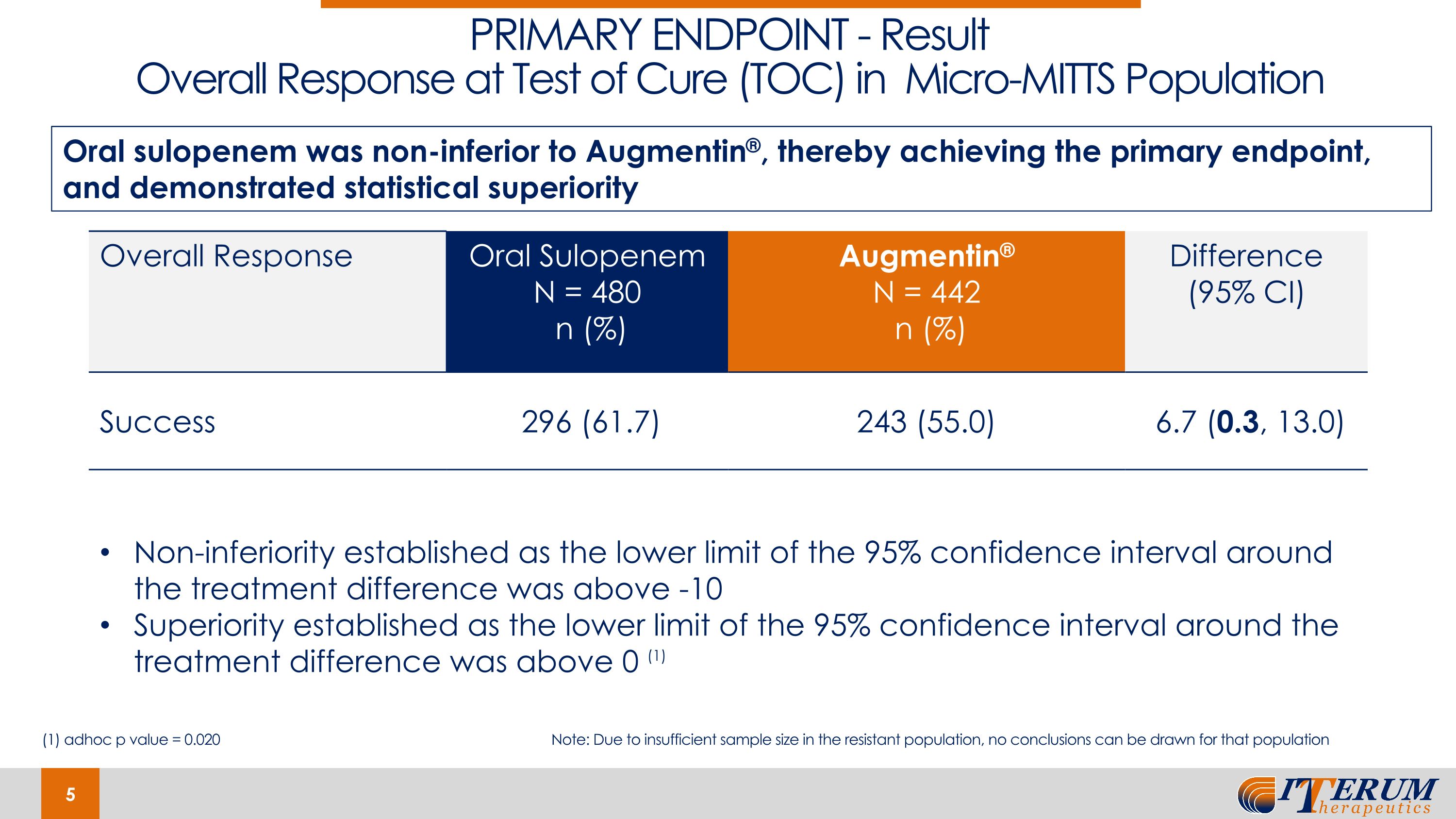
PRIMARY ENDPOINT - Result�Overall Response at Test of Cure (TOC) in Micro-MITTS Population Overall Response Oral Sulopenem N = 480 n (%) Augmentin® N = 442 n (%) Difference (95% CI) Success 296 (61.7) 243 (55.0) 6.7 (0.3, 13.0) Oral sulopenem was non-inferior to Augmentin®, thereby achieving the primary endpoint, and demonstrated statistical superiority Non-inferiority established as the lower limit of the 95% confidence interval around the treatment difference was above -10 Superiority established as the lower limit of the 95% confidence interval around the treatment difference was above 0 (1) (1) adhoc p value = 0.020 Note: Due to insufficient sample size in the resistant population, no conclusions can be drawn for that population
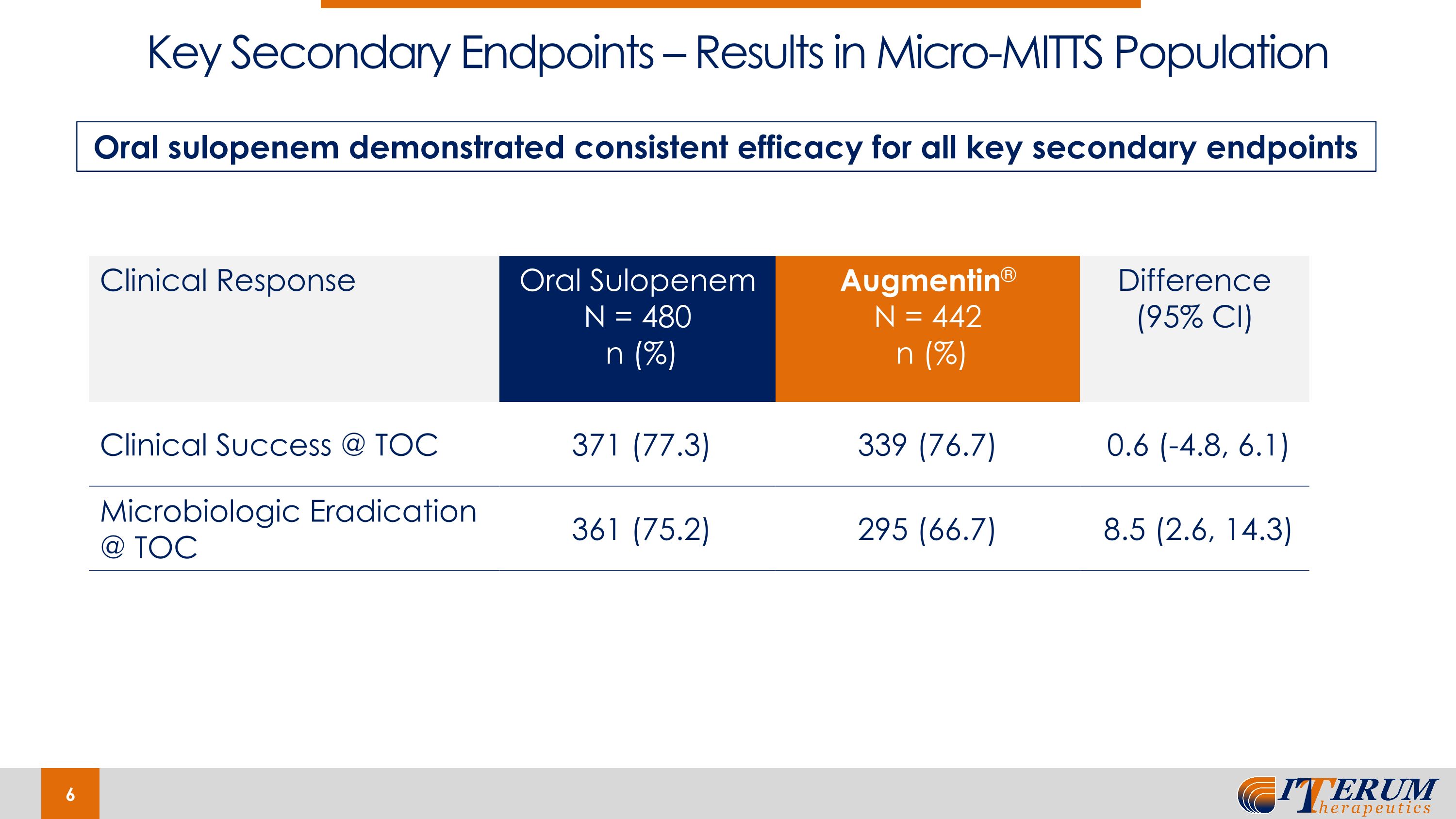
Key Secondary Endpoints – Results in Micro-MITTS Population Clinical Response Oral Sulopenem N = 480 n (%) Augmentin® N = 442 n (%) Difference (95% CI) Clinical Success @ TOC 371 (77.3) 339 (76.7) 0.6 (-4.8, 6.1) Microbiologic Eradication @ TOC 361 (75.2) 295 (66.7) 8.5 (2.6, 14.3) Oral sulopenem demonstrated consistent efficacy for all key secondary endpoints
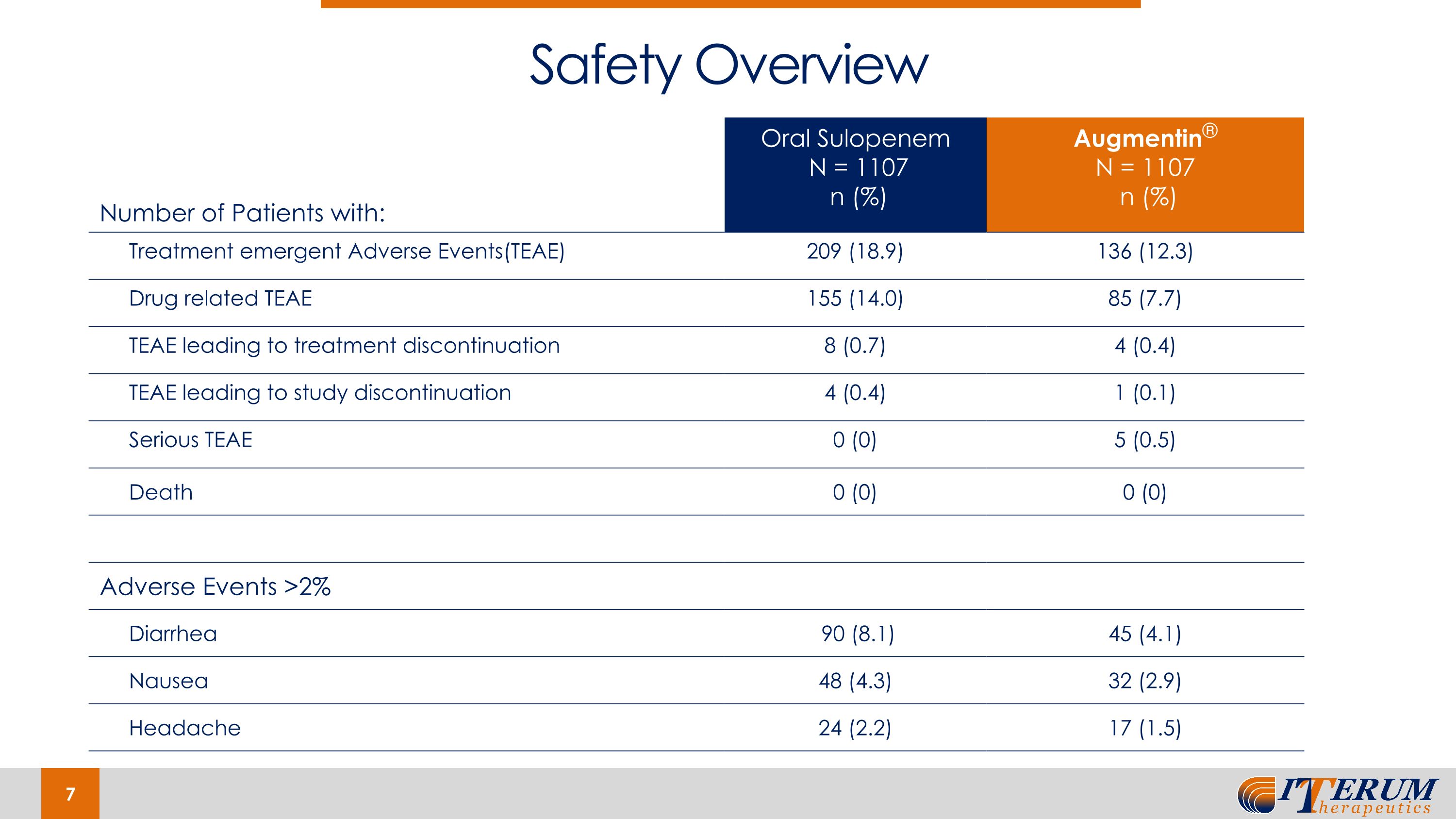
Number of Patients with: Oral Sulopenem N = 1107 n (%) Augmentin® N = 1107 n (%) Treatment emergent Adverse Events(TEAE) 209 (18.9) 136 (12.3) Drug related TEAE 155 (14.0) 85 (7.7) TEAE leading to treatment discontinuation 8 (0.7) 4 (0.4) TEAE leading to study discontinuation 4 (0.4) 1 (0.1) Serious TEAE 0 (0) 5 (0.5) Death 0 (0) 0 (0) Adverse Events >2% Diarrhea 90 (8.1) 45 (4.1) Nausea 48 (4.3) 32 (2.9) Headache 24 (2.2) 17 (1.5) Safety Overview

REASSURE Study Results In the overall response at the test of cure in the Augmentin susceptible population, oral sulopenem was non-inferior to Augmentin®, thereby achieving the primary endpoint; in this population, sulopenem also demonstrated statistical superiority Additionally, oral sulopenem demonstrated consistent efficacy for key secondary/additional endpoints Very solid safety profile Timing/Next Steps Expect to resubmit NDA Q2 2024 Expect FDA to complete its review and take action within six months from resubmission or in Q4 2024* Market Dynamics The uUTI market is quite large, with an estimated 30 million infections annually Antibiotic resistance and the safety profiles of existing older products currently in the market are driving a substantial need for new, efficacious products to treat these infections If approved, sulopenem would be the first oral penem to be approved in the United States Additionally, if approved, sulopenem would be one of the first new oral products approved for uncomplicated urinary tract infections since the turn of the century With positive data now in hand, we will focus on a strategic process to sell, license, or otherwise dispose of our rights to sulopenem with the goal of maximizing value for our stakeholders Summary

v3.24.0.1
Document And Entity Information
|
Jan. 30, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 30, 2024
|
| Entity Registrant Name |
Iterum Therapeutics plc
|
| Entity Central Index Key |
0001659323
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-38503
|
| Entity Incorporation, State or Country Code |
L2
|
| Entity Tax Identification Number |
00-0000000
|
| Entity Address, Address Line One |
Fitzwilliam Court
|
| Entity Address, Address Line Two |
1st Floor
|
| Entity Address, Address Line Three |
Leeson Close
|
| Entity Address, City or Town |
Dublin 2
|
| Entity Address, Country |
IE
|
| Entity Address, Postal Zip Code |
Not applicable
|
| City Area Code |
+353
|
| Local Phone Number |
1 6694820
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Ordinary Shares, par value $0.01 per share
|
| Trading Symbol |
ITRM
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Iterum Therapeutics (NASDAQ:ITRM)
Historical Stock Chart
From Mar 2024 to Apr 2024
Iterum Therapeutics (NASDAQ:ITRM)
Historical Stock Chart
From Apr 2023 to Apr 2024