
Corporate Presentation January 2024

Cautionary Note Regarding Forward Looking Statements and Disclaimers This presentation contains certain forward-looking statements about Curis, Inc. (“we,” “us,” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as “expect(s),” “believe(s),” “will,” “may,” “anticipate(s),” “focus(es),” “plans,” “mission,” “strategy,” “potential,” “estimate(s)”, "intend," "project," "seek," "should," "would" and similar expressions are intended to identify forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this presentation, and involve important risks and uncertainties. Forward-looking statements herein include, but are not limited to, statements with respect to the timing and results of future clinical and pre-clinical milestones; the timing of future preclinical studies and clinical trials and results of these studies and trials; the clinical and therapeutic potential of our drug candidates; our cash runway; the proposed focus on emavusertib and management’s ability to successfully achieve its goals. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of important factors including, without limitation, risks relating to: whether and when the U.S. Food and Drug Administration may take further regulatory action with regard to our trials, whether any of our drug candidates will advance further in the clinical development process and whether and when, if at all, they will receive approval from the FDA or equivalent foreign regulatory agencies; whether historical preclinical results will be predictive of future clinical trial results; whether historical clinical trial results will be predictive of future trial results; whether any of our drug candidate development efforts will be successful; whether any of our drug candidates will be successfully marketed if approved; our ability to achieve the benefits contemplated by our collaboration agreements; management’s ability to successfully achieve its goals; the sufficiency of our cash resources; our ability to raise additional capital to fund our operations on terms acceptable to us or the use of proceeds of any offering of securities or other financing; general economic conditions; competition; and the other risk factors contained in our periodic reports filed with the Securities and Exchange Commission, including the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and the Company's Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023, June 30, 2023 and September 30, 2023, which are available on the SEC website at www.sec.gov. You are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events, except as required by law. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties as well as our own estimates. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. slide 2

Investment Overview Emavusertib is a novel, highly-active IRAK4 inhibitor with potential cornerstone utility in heme and solid tumors Full development program underway in front-line and end-stage AML/MDS; BTKi combination PoC study in PCNSL Single agent and combination study results near-term (2024); Cash runway into 2025 – $68.5M as of Sept 30, 2023 Recent Update Initial PCNSL data released at ASH 2023 extends thesis for IRAK4/BTK combination in lymphoma, 3 of 5 patients – who had progressed on BTKi – achieved CR when dosed with combination Key Indications TakeAim Leukemia: Near-term, potentially registration-directed, PoC studies with monotherapy in AML/MDS Emavusertib inhibits IRAK4 and FLT3, the two most prevalent disease drivers in AML/MDS1,2 TakeAim Lymphoma: Near-term, potentially registration-directed, combination study with ibrutinib in PCNSL Complementary blockade of the two key pathways driving NF-ĸB mediated proliferation in NHL Solid Tumors: Multiple investigator-sponsored studies expected to enroll in 2024 Preclinical studies have shown IRAK4 potentiates chemo- and immunotherapies in combination in solid tumor malignancies Market Opportunity AML/MDS: 333K patients3 all patients addressable with either front-line combination or salvage-line monotherapy NHL/CLL: 1.9M patients3 all patients addressable with emavusertib in combination with BTKi Solid Tumors: tbd potential opportunities currently being explored in metastatic melanoma, bladder, colorectal, and others Corporate Overview 1) Smith et al. Nat Cell Biol 2019; 2) Saygin, et al. J Hematol Oncol. 2017 Apr 18; 3) 2022 Prevalence Data DRG Clarivate slide 3 Leader of IRAK4 Inhibition; Developing Emavusertib with Broad Application in Oncology

IRAK4 is a Novel Anti-Cancer Target Oncogenic IRAK4 activity promotes inflammation and impairs T cell function slide 4 Koziczak-Holbro M 2008 • Upregulation of IRAK4 in tumors leads to activation of NF-κB, JNK, and MAPK1,2 • IRAK-4 upregulation is associated with increased phenotypically exhausted TILs, MDSCs, increased CD4+ T regs, and resistance to aPD-1 therapy4,5 • Tumor and stromal IRAK4 activation drives collagen and hyaluronan deposition, promoting inflammation in the TME3 • Targeting IRAK4 has demonstrated anti-cancer activity as a single agent and the ability to potentiate chemo- and immunotherapies in combination3 1. Rhyasen GW and Starczynowski DT. Br J Cancer. 2015; 112(2): 232-237. 2. Madonna G, et al. J Transl Med. 2012; 10(1):53. 3. Lim et al, 2023 IRAK4 Symposium 4. Martin Lasola JJ., et al. Cancer Immunol Res. 5(3_suppl):B31. 5. Somani VK, et al. Gastroenterology. 2022; 162(7): 2047-2062.

Haspin Emavusertib Has A Unique Molecular Fingerprint Targeted design is specifically engineered to be best-in-class Emavusertib Kinase Interaction Map % Inhibition at 0.1 nM Illustration reproduced courtesy of Cell Signaling Technology Target Kd nM IRAK1 12,000 IRAK2 >20,000 IRAK3 8,500 IRAK4 23 DYRK1A 25 FLT3 WT FLT3 (D835H) 31 5 FLT3 (D835V) 44 FLT3 (D835Y) 3 FLT3 (ITD) 8 FLT3 (K663Q) 47 FLT3 (N841I) 16 Haspin (GSG2) 32 CLK1 10 CLK2 20 CLK3 >20,000 CLK4 14 TrkA 130 Emavusertib Binding Affinity DiscoverX Kinase Panel (378 kinases screened) The NCI selected emavusertib for NCI-sponsored research and clinical studies of IRAK4 high binding affinity to FLT3 contributes additional anti-cancer activity, differentiating emavusertib from other IRAK4-directed therapies high binding affinity to IRAK4 (>97% inhibition achieved at Ph2 dose concentrations) IRAK4 FLT3 CLK 1, 2, 4 slide 5

Emavusertib in Leukemia

Emavusertib Has a Unique Mechanism of Action The two primary targets of emavusertib are independent drivers of cancer 1) Guillamot et al. Nat Cell Biol 2019; 2) Smith et al. Nat Cell Biol 2019; 3) American Cancer Society, Cancer Facts & Figures 2020; 4) Leukemia & Lymphoma Society, Facts and Statistics Overview spliceosome mutations drive overexpression of IRAK4-Long, which constitutively activates the myddosome, driving NF-ĸB overactivity IRAK4 P P PI3K AKT MTOR STAT5 RAS RAF MEK ERK C-terminus cell growth, proliferation, survival cell membrane FLT3 Ligand genetic mutations cause constitutive activation of FLT3 and downstream cell growth, proliferation, and survival pathways FLT3 Spliceosome Ex3 Ex5Ex4 IRAK1 MYD88 NF-ĸB overactivity2 emavusertib Myddosome Ex4 constitutive activation IRAK4-Long ITD mutation P P TKD point mutation U2AF1 mutation slide 7

0% p<0.05 p<0.05 50% 100% -50% Preclinical Data Rationale for monotherapy and combination with azacitidine/venetoclax 1) Choudhary et al. AACR 2017; 2) Melgar, Sci Transl Med. 2019; 3) Curis AML MDS poster, EHA 2021 Control Re la tiv e Ce ll G ro w th Aza Aza + emavusertib Aza/Ven emavusertib demonstrates synergy with both azacitidine and venetoclax in THP-1 model3 AML cell lines treated for 96 hrs (values presented as mean ± SE) Aza/Ven + emavusertib Ve hi cl e Em av us er tib emavusertib demonstrates monotherapy activity in patient-derived xenografts1 clear reduction of leukemic blasts in models with high IRAK4-L expression IRAK/FLT3 combination demonstrates synergy in pre-clinical studies2 FLT3-ITD cells treated for 3 days with DMSO (control), quizartinib (0.5 μM), IRAKi (10 μM), and quizartinib + IRAKi Combination with Aza/VenMonotherapy in IRAK4 Monotherapy in FLT3 0 2 4 6 8 10 FLT3i + IRAK1/4i FLT3i Control IRAK1/4i 100 – 75 – 50 – 25 – 0 – Pe rc en t V ia bl e Ce lls (A nn ex in V- ne ga tiv e) “Concomitant targeting of IRAK1 or IRAK4, alongside FLT3, is the most effective means to overcome the adaptive resistance incurred when targeting FLT3” - Melgar 2019 Days slide 8

mCR mCR mCR CR mCR mCR mCR CR CR CRh MLFS -100% -80% -60% -40% -20% 0% 20% 40% 60% 80% 100% Emavusertib Compelling Initial Clinical Data Showing clear single agent activity where expected in AML/MDS clinical studies Note: 84 total AML/MDS patients enrolled in monotherapy as of Feb 9, 2023 with data cut-off as of Mar 17, 2023; * Three AML patients had both FLT3 and Spliceosome mutation and are counted in both populations; evaluable patients include all patients whose disease was determined to be evaluable for objective response with baseline and post-treatment marrow assessments ** Denotes blast percent increase > 10% CR CR MLFS -100% -80% -60% -40% -20% 0% All comers, All dose levels CR CRh -100% -80% -60% -40% -20% 0% 8 Targeted AML Patients w/ Spliceosome Mutation* ** ** ** % C ha ng e in M ar ro w B la st fr om B as el in e % C ha ng e in M ar ro w B la st fr om B as el in e Patients with ≤ 2 prior lines treated at 300 mg BID % C ha ng e in M ar ro w B la st fr om B as el in e Strongest monotherapy signal observed where expected (in Spliceosome and FLT3 patients) 7 Targeted AML Patients w/ FLT3 Mutation* Duration of Responses: 5.6 – 7.0 months slide 9

Emavusertib in Leukemia (AML/MDS) The ideal candidate to address the two largest genetically-defined populations in AML/MDS Next Steps TakeAim Leukemia Study • Monotherapy: R/R AML with FLT3 R/R AML with Spliceosome • Combination: AML/MDS in combination with azacitidine/venetoclax Emavusertib addresses the two largest genetically-defined populations in AML/MDS: 1) IRAK4-L (>50% of population)1 2) FLT3 (>25% of population)2 • Demonstrated single-agent activity • Genetically-defined patient population • Dual targeting of IRAK4 and FLT3 may prevent adaptive resistance observed with current FLT3 inhibitors 3 1) Smith et al. Nat Cell Biol 2019; 2) Saygin, et al. J Hematol Oncol. 2017 Apr 18; 3) Melgar, Sci Transl Med. 2019 slide 10

Emavusertib in Lymphoma

Emavusertib in Lymphoma Combination therapy provides complimentary inhibition of two pathways that drive NF-κB NFκB Biology: Two Pathways Drive NHL/CLL BCR and TLR Pathways independently drive NF-κB overactivity (and NF-κB drives NHL/CLL) Clinical Strategy: Block both pathways with Combination Therapy In preclinical testing, blocking both IRAK4 and BTK drove tumor reduction better than blocking either one alone 2) Booher et al. Waldenström Roadmap Symposium 20191) IMBRUVICA Package Insert. Rev 08/2018 TLR1B cell receptor BTK CARD11 MALT1 BCL10 ibrutinib TLR2 TLR4 TLR5 TLR6 TLR7 TLR8 TLR9 BCR Pathway TLR Pathway Endosome Lymphoma1 (NHL,CLL) NF-κB Myddosome IRAK1 IRAK4 MYD88 emavusertib overactivity vehicle ibrutinib emavusertib 0 5 10 15 20 25 30 0 200 400 600 800 1000 1200 1400 Days of treatment M ea n Tu m or V ol um e (m m 3 ) + S EM M ea n Tu m or V ol um e (m m 3 ) ± S EM Monotherapy vs. Combination in OCI-Ly10 model2 emavusertib + ibrutinib slide 12
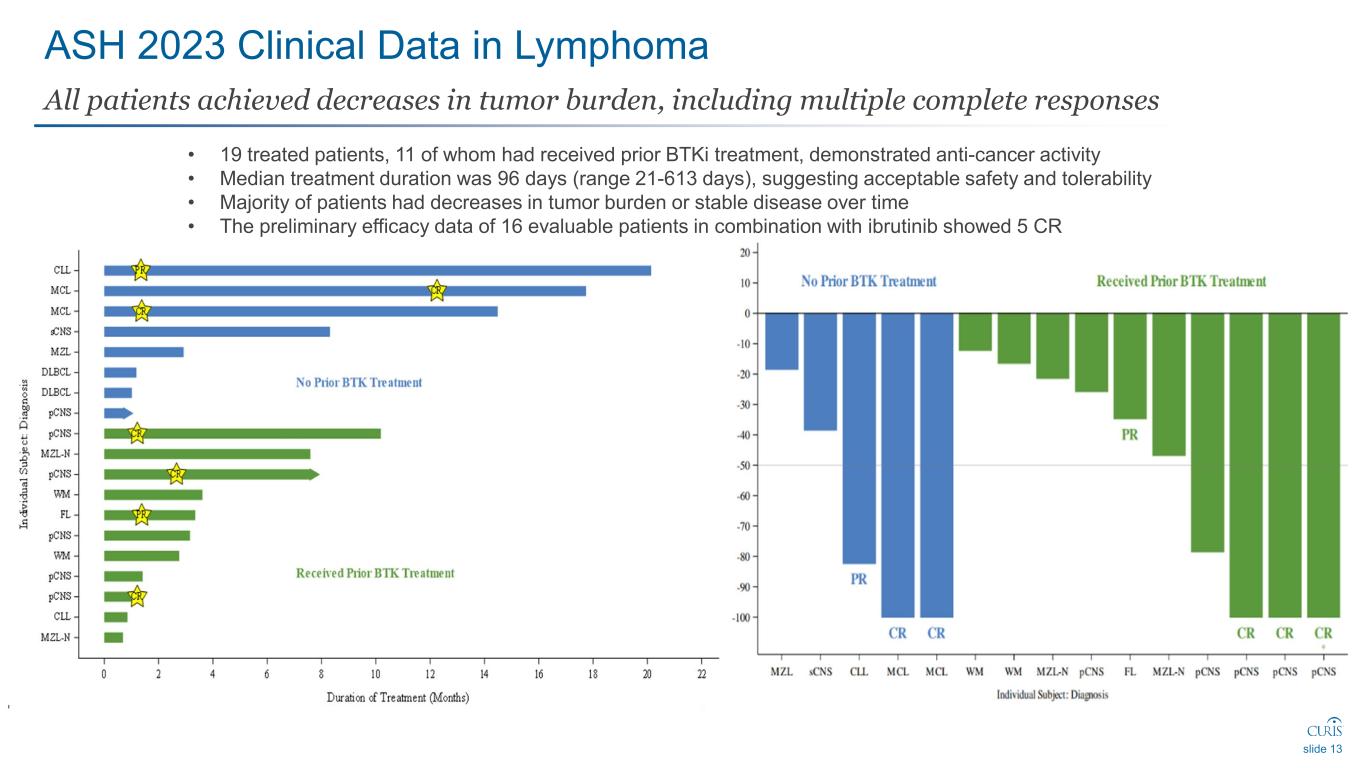
ASH 2023 Clinical Data in Lymphoma All patients achieved decreases in tumor burden, including multiple complete responses slide 13 • 19 treated patients, 11 of whom had received prior BTKi treatment, demonstrated anti-cancer activity • Median treatment duration was 96 days (range 21-613 days), suggesting acceptable safety and tolerability • Majority of patients had decreases in tumor burden or stable disease over time • The preliminary efficacy data of 16 evaluable patients in combination with ibrutinib showed 5 CR

ASH 2023 Clinical Data in PCNSL All patients achieved decreases in tumor burden, including multiple complete responses slide 14 -100% -80% -60% -40% -20% 0% % C ha ng e fr om B as el in e PCNSL combination with ibrutinib BTK-experienced CRCRCR 5 evaluable PCNSL patients previously treated with BTKi demonstrated anti-cancer activity, with 3 patients achieving CR and one patient having a durable response for approximately 7 months

Clinical Strategy in Lymphoma Emavusertib is the ideal candidate to combine with BTKi to maximize downregulation of NF-κB • Patients are currently treated with BTKi because it downregulates NF-κB • Two pathways drive NF-κB: 1) BCR Pathway: addressed by blocking BTK 2) TLR Pathway: addressed by blocking IRAK4 • Initial clinical data suggest blocking both pathways may overcome resistance to ibrutinib Next Steps TakeAim Lymphoma Study • Combination with BTKi: R/R PCNSL slide 15

Investment Overview Emavusertib is a novel, highly-active IRAK4 inhibitor with potential cornerstone utility in heme and solid tumors Full development program underway in front-line and end-stage AML/MDS; BTKi combination PoC study in PCNSL Single agent and combination study results near-term (2024); Cash runway into 2025 – $68.5M as of Sept 30, 2023 Recent Update Initial PCNSL data released at ASH 2023 extends thesis for IRAK4/BTK combination in lymphoma, 3 of 5 patients – who had progressed on BTKi – achieved CR when dosed with combination Key Indications TakeAim Leukemia: Near-term, potentially registration-directed, PoC studies with monotherapy in AML/MDS Emavusertib inhibits IRAK4 and FLT3, the two most prevalent disease drivers in AML/MDS1,2 TakeAim Lymphoma: Near-term, potentially registration-directed, combination study with ibrutinib in PCNSL Complementary blockade of the two key pathways driving NF-ĸB mediated proliferation in NHL Solid Tumors: Multiple investigator-sponsored studies expected to enroll in 2024 Preclinical studies have shown IRAK4 potentiates chemo- and immunotherapies in combination in solid tumor malignancies Market Opportunity AML/MDS: 333K patients3 all patients addressable with either front-line combination or salvage-line monotherapy NHL/CLL: 1.9M patients3 all patients addressable with emavusertib in combination with BTKi Solid Tumors: tbd potential opportunities currently being explored in metastatic melanoma, bladder, colorectal, and others Corporate Overview 1) Smith et al. Nat Cell Biol 2019; 2) Saygin, et al. J Hematol Oncol. 2017 Apr 18; 3) 2022 Prevalence Data DRG Clarivate slide 16 Leader of IRAK4 Inhibition; Developing Emavusertib with Broad Application in Oncology

End of Corporate Presentation NASDAQ: CRIS