
TIA, Living with OCD AND HELPING RECRUIT IN BIOHAVEN CLINICAL TRIALS 42nd Annual J.P. Morgan Healthcare Conference Vlad Coric, M.D. Chairman and Chief Executive Officer January 8, 2024 © 2024 Biohaven, Ltd. All rights reserved.

Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Biohaven Ltd. (the “Company”) and our planned and ongoing clinical trials, the timing of and the availability of data from those trials, the timing and our decisions to proceed with our planned regulatory filings, the timing of and our ability to obtain regulatory approvals for our product candidates, the clinical potential utility of our product candidates, alone and as compared to other existing potential treatment options, and the potential advancement of our early phase programs. The use of certain words, including “continue”, “plan”, “will”, “believe”, “may”, “expect”, “anticipate” and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of our development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven’s planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven’s product candidates; the potential for Biohaven’s product candidates to be first in class and best in class therapies; the anticipated consummation of the Trop2 transaction, and the effectiveness and safety of Biohaven’s product candidates. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the Company’s filings with the Securities and Exchange Commission, including within the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. The forward-looking statements are made as of the date of this presentation, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. This presentation also contains market data and other information based on industry publications, reports by market research firms or published independent sources. Some market data and information is also based on the Company’s good faith estimates, which are derived from management’s knowledge of its industry and such independent sources referred to above. January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference2

GROUNDBREAKING LEGACY OF SUCCESS IN MIGRAINE Biohaven has reemerged for countless patients and is growing one of the most innovative portfolios in life sciences. NEUROSCIENCE | IMMUNOLOGY | ONCOLOGY

Top Areas of Innovation BIOHAVEN PORTFOLIO Positioned for Future Value Creation for Patients and Investors IgG Degrader TYK2/JAK1 Kv7 Activator TRPM3 Antagonist Troriluzole Taldefgrobep Alfa Trop2 CD30 β1-AR Degrader IgA Degrader RARE DISEASE RENAL CARDIOVASCULAR ONCOLOGY OBESITY NEUROLOGY IMMUNOLOGY & INFLAMMATION 1. Adapted from BioCentury survey: https://www.biocentury.com/article/650883/move-over-oncology-i-i-will-write-the-next-big-stories-in-innovation# January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference4

PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET GLUTAMATE Troriluzole BHV-4157 Obsessive-Compulsive Disorder MYOSTATIN Taldefgrobep Alfa BHV-2000 Spinal Muscular Atrophy Obesity ION CHANNEL Kv7 Activator BHV-7000 Focal Epilepsy Generalized Epilepsy Bipolar Disorder Major Depressive Disorder TRPM3 Antagonist BHV-2100 Migraine Neuropathic Pain INFLAMMATION & IMMUNOLOGY TYK2/JAK1 Inhibitor (brain penetrant) BHV-8000 Prevention of Amyloid Therapy Induced ARIA Early Alzheimer’s Disease Early Parkinson’s Disease Multiple Sclerosis IgG Degrader BHV-1300 Rheumatoid Arthritis BHV-1310 Myasthenia Gravis IgA Degrader BHV-1400 IgA Nephropathy β1-AR Degrader BHV-1600 Dilated Cardiomyopathy ONCOLOGY CD38 BHV-1100 Multiple Myeloma Trop2 BHV-1510 Carcinoma CD30 (next-gen brentuximab vedotin) BHV-1500 Hodgkin’s Lymphoma ARIA, Amyloid-related imaging abnormalities
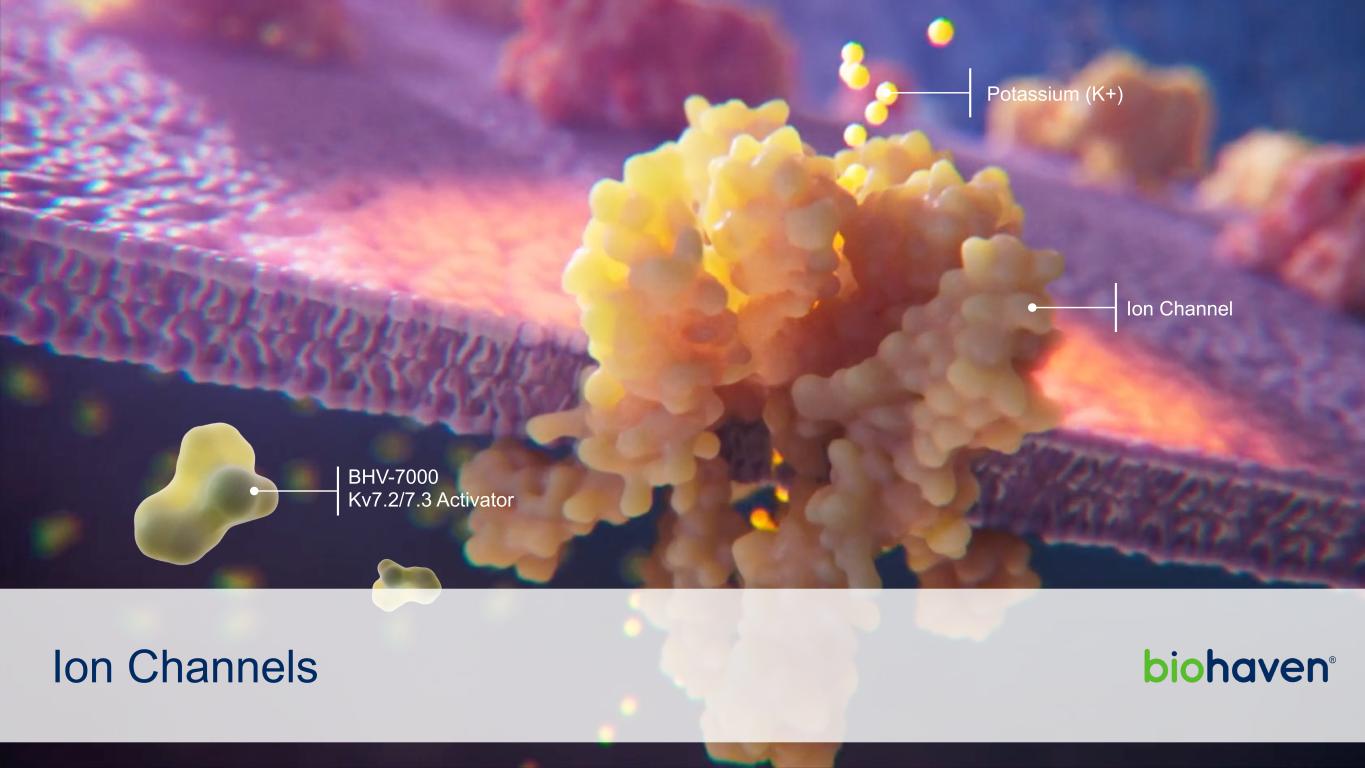
Ion Channels BHV-7000 Kv7.2/7.3 Activator Potassium (K+) Ion Channel

BHV-7000 SELECTIVE Kv7 ACTIVATOR Kv7 is Breakthrough Target in Neurology and Neuropsychiatry • Selective Kv7 activation avoids unwanted CNS side effects • Clinically validated in epilepsy and major depressive disorder BHV-7000 is Potentially Best-in-class Selective Kv7 Activator with Blockbuster Potential • Rationally designed to eliminate GABAA receptor activation • No dose-limiting CNS side effects in Phase 1 studies • CNS target engagement confirmed in a dose proportional manner in Phase 1 EEG study BHV-7000 Has Compelling Preclinical Efficacy Profile • Highly effective in epilepsy model • Ketamine-like efficacy in neuropsychiatry model • Wide therapeutic index to explore full dose range 7 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 Phase 2/3 Epilepsy Update: >110 global clinical sites selected, FPFV 1Q24 Phase 2 MDD and Bipolar Studies expected to initiate FPFV 1Q24 BREAKING NEWS
BHV-7000: Epilepsy Update ION CHANNELS
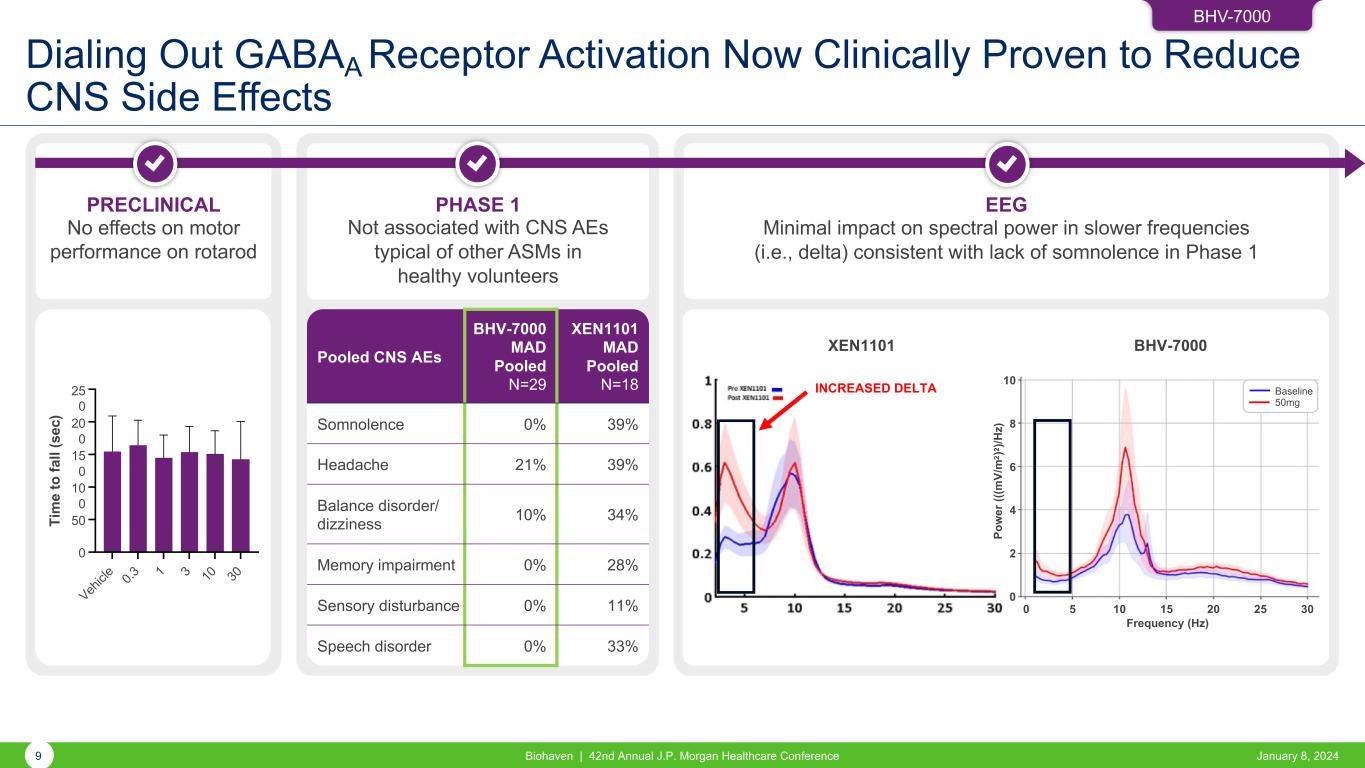
Dialing Out GABAA Receptor Activation Now Clinically Proven to Reduce CNS Side Effects Pooled CNS AEs BHV-7000 MAD Pooled N=29 XEN1101 MAD Pooled N=18 Somnolence 0% 39% Headache 21% 39% Balance disorder/ dizziness 10% 34% Memory impairment 0% 28% Sensory disturbance 0% 11% Speech disorder 0% 33% BHV-7000 9 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 PHASE 1 Not associated with CNS AEs typical of other ASMs in healthy volunteers EEG Minimal impact on spectral power in slower frequencies (i.e., delta) consistent with lack of somnolence in Phase 1 PRECLINICAL No effects on motor performance on rotarod Veh icl e 0.3 1 3 10 30 0 50 100 150 200 250 BHV-7000 (mg/kg) Ti m e to fa ll (s ec ): R ot ar od 25 0 2 0 15 0 1 0 Ti m e to fa ll (s ec ) i le 0.3 10 30 BHV-7000XEN1101 INCREASED DELTA Baseline 50mg 10 8 6 4 2 0 Po w er (( (m V/ m 2 ) 2 )/ H z) 0 5 10 15 20 25 30 Frequency (Hz)
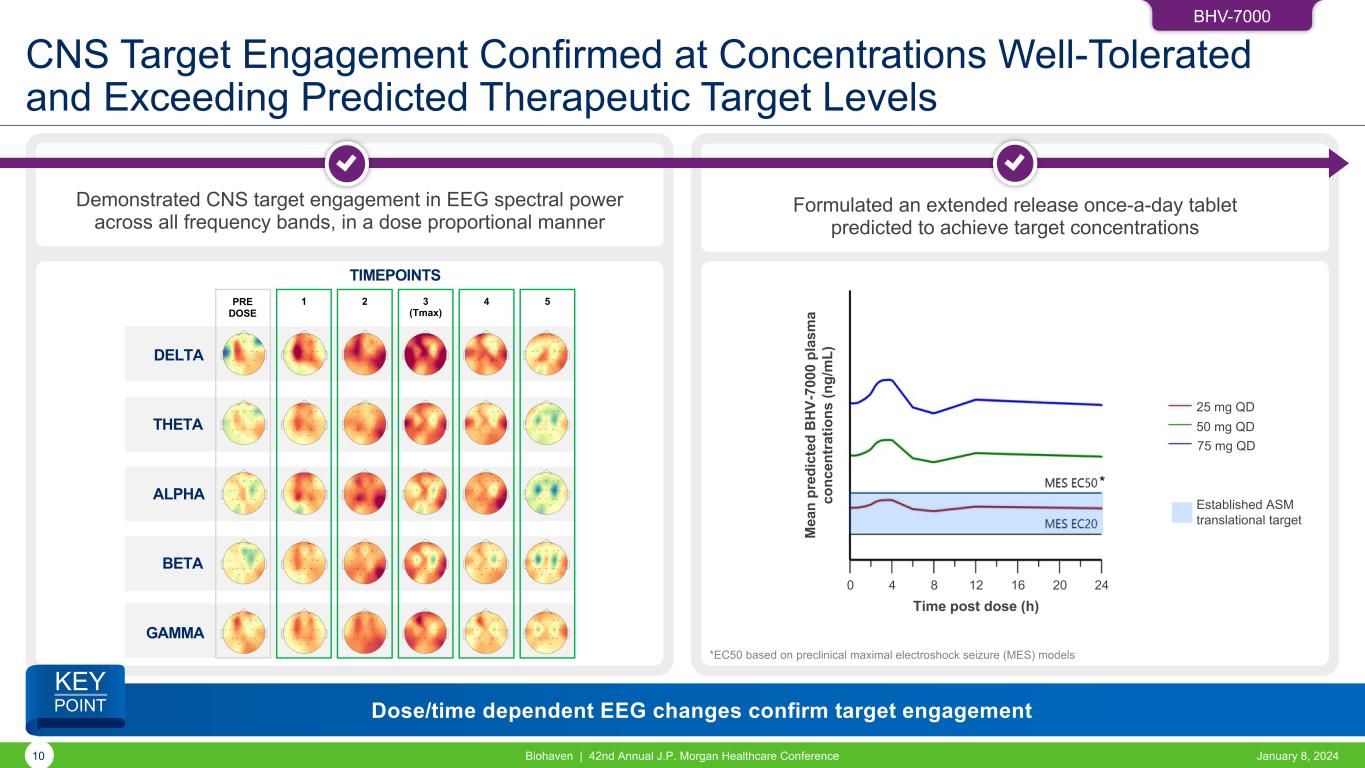
BHV-7000 CNS Target Engagement Confirmed at Concentrations Well-Tolerated and Exceeding Predicted Therapeutic Target Levels 10 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 Demonstrated CNS target engagement in EEG spectral power across all frequency bands, in a dose proportional manner Formulated an extended release once-a-day tablet predicted to achieve target concentrations TIMEPOINTS GAMMA BETA ALPHA THETA DELTA PRE DOSE 1 2 3 (Tmax) 4 5 MES EC50 MES EC20M ea n pr ed ic te d B H V- 70 00 p la sm a co nc en tr at io ns (n g/ m L) 0 4 8 12 16 20 24 Time post dose (h) 25 mg QD 50 mg QD 75 mg QD Established ASM translational target * *EC50 based on preclinical maximal electroshock seizure (MES) models Dose/time dependent EEG changes confirm target engagement KEY POINT

Epilepsy Phase 3 Studies in Focal and Idiopathic Generalized Epilepsy January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference11 BHV-7000 Focal Design Generalized Design Focal Epilepsy Study — 110 global clinical sites selected, FPFV 1Q24 KEY POINT DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Subjects 18–75 years old with intractable focal epilepsy SAMPLE SIZE 390 subjects (randomized 1:1:1) TREATMENT BHV-7000 (75/50 mg) and (50/25 mg) vs. placebo TREATMENT DURATION 12- or 8-week treatment phase ENDPOINTS Change in seizure frequency, 50% seizure reduction, seizure freedom, safety DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Subjects 18–75 years old with idiopathic generalized epilepsy with intractable generalized tonic-clonic seizures SAMPLE SIZE 242 subjects (randomized 1:1:), study ends with the 127th seizure event TREATMENT BHV-7000 75 mg vs. placebo TREATMENT DURATION Up to 24-week double-blind phase, subject will transition to open label extension ENDPOINTS Time to event (2nd day with generalize tonic-clonic seizure)

BHV-7000: Neuropsychiatry Updates ION CHANNELS

Kv7 Activation Validated in the Clinic for Major Depressive Disorder 13 0 1 2 3 4 5 -20 -15 -10 -5 0 C ha ng e in M AD R S sc or e Weeks • 7.9-point benefit vs. placebo on MADRS (p<0.001) • 6.9-point benefit vs. placebo on SHAPS (p<0.001) • Dose-limiting side effects in 20% of study subjects Ezogabine Demonstrated Robust Clinical Benefit (n=45)1 • Benefit on MADRS (p=0.135) vs. placebo in 20 mg group • Benefit on MADRS at week 1 (p<0.05) vs. placebo in 20 mg group • Efficacy not optimized likely due to dose limiting tolerability concerns XEN1101 Demonstrated Rapid Onset of Clinical Benefit With a Clear Dose Response (n=167)2 Randomized clinical trials in MDD with two nonselective Kv7 activators have demonstrated potential for rapid onset of clinically meaningful benefit in symptoms of depression and anhedonia BHV-7000 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 BHV-7000 has ideal profile for potential in MDD due to low rates of CNS AEs vs. nonselective Kv7 activators KEY POINT

BHV-7000: Potential for Ketamine and Psilocybin-Like Anti-Depressant Effect January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference14 BHV-7000 Kv7 (KCNQ2) Mediates Therapeutic Benefits of Ketamine1 • Chronically stressed mice show downregulation of Kv7 gene expression • Kv7 mediated ketamine anti-depressant effects abolished when Kv7 is inhibited or Kv7 expression reduced Lopez et al. Neuron. 2022 Jul 20;110(14):2283-2298.e9 Ketamine, psilocybin, and BHV-7000 all enhance motivation in poor performing rats in operant model Ketamine and Psilocybin BHV-7000 BHV-7000 shows similar or greater magnitude of anti-depressant behavioral effects to ketamine and psilocybin KEY POINT Biohaven data on file.Higgins et al. Front Pharmacol. 2021 Feb 26;12:640241
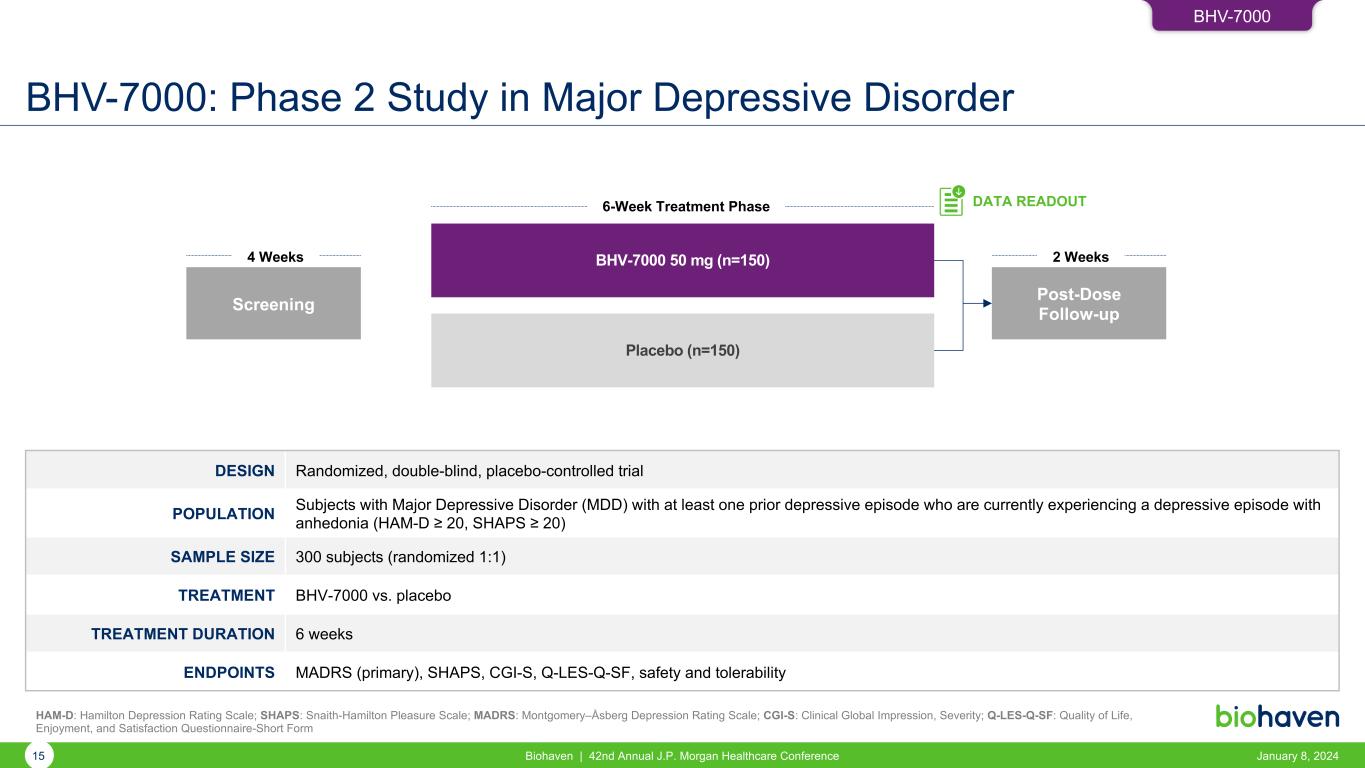
BHV-7000: Phase 2 Study in Major Depressive Disorder January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference15 DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Subjects with Major Depressive Disorder (MDD) with at least one prior depressive episode who are currently experiencing a depressive episode with anhedonia (HAM-D ≥ 20, SHAPS ≥ 20) SAMPLE SIZE 300 subjects (randomized 1:1) TREATMENT BHV-7000 vs. placebo TREATMENT DURATION 6 weeks ENDPOINTS MADRS (primary), SHAPS, CGI-S, Q-LES-Q-SF, safety and tolerability BHV-7000 50 mg (n=150) Placebo (n=150) Screening 4 Weeks 6-Week Treatment Phase DATA READOUT Post-Dose Follow-up 2 Weeks HAM-D: Hamilton Depression Rating Scale; SHAPS: Snaith-Hamilton Pleasure Scale; MADRS: Montgomery–Åsberg Depression Rating Scale; CGI-S: Clinical Global Impression, Severity; Q-LES-Q-SF: Quality of Life, Enjoyment, and Satisfaction Questionnaire-Short Form BHV-7000

Human Genetics Links Kv7 to Risk of Bipolar Disorder ASMs Such as Lamotrigine Are Cornerstone Bipolar Treatments Kv7 Activation With Ezogabine Normalizes Hyperactive Locomotion and Brain Hypermetabolism in Mania Model Compelling Evidence for Targeting Kv7 in Bipolar Disorder • HUMAN GENETICS ANK3 gene link to Kv7 and disease risk1, 2, 3, 4 • MOLECULAR PROFILING OF BIPOLAR DISORDER PATIENT TISSUES demonstrating epigenetic, transcriptomic and proteomic Kv7 deregulation • PRECLINICAL MODELS Kv7 activation corrects disease-related phenotypes and behaviors • ANTISEIZURE MEDICINES ARE CORNERSTONE BIPOLAR TREATMENTS Bowden et al. 2003;60:392-400Feng et al., 2019. 1. Pan et al. Journal of Neuroscience, 2006. 2. Ferreira et al. Nat. Genet. 40, 1056–1058. 3. Psychiatric GWAS Consortium Bipolar Disorder Working Group. (2011). 4. Judy et al. Front Genet (2013). BHV-7000 16 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
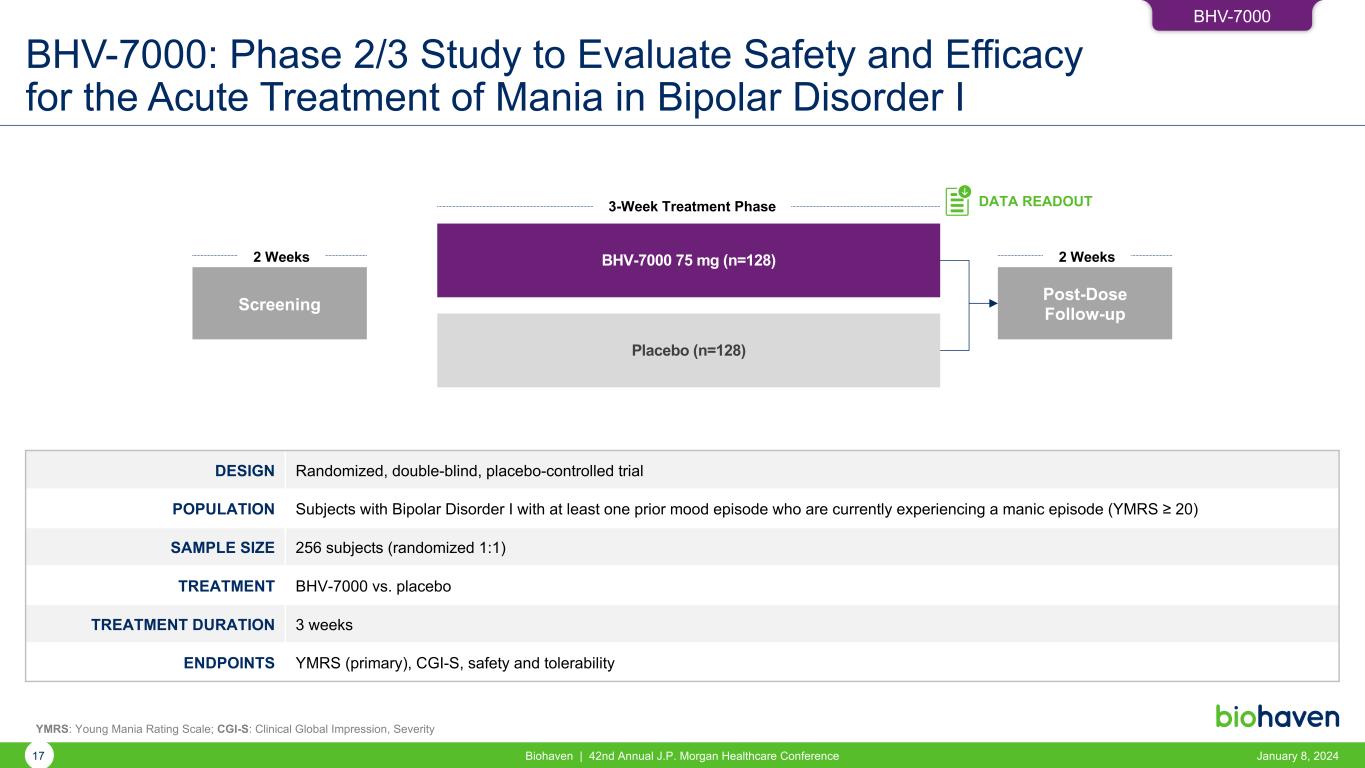
BHV-7000: Phase 2/3 Study to Evaluate Safety and Efficacy for the Acute Treatment of Mania in Bipolar Disorder I January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference17 DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Subjects with Bipolar Disorder I with at least one prior mood episode who are currently experiencing a manic episode (YMRS ≥ 20) SAMPLE SIZE 256 subjects (randomized 1:1) TREATMENT BHV-7000 vs. placebo TREATMENT DURATION 3 weeks ENDPOINTS YMRS (primary), CGI-S, safety and tolerability BHV-7000 75 mg (n=128) Placebo (n=128) Screening 2 Weeks 3-Week Treatment Phase DATA READOUT Post-Dose Follow-up 2 Weeks YMRS: Young Mania Rating Scale; CGI-S: Clinical Global Impression, Severity BHV-7000

BHV-2100 TRPM3 ANTAGONIST • Biohaven is back in migraine with novel agent, BHV-2100 • Phase 1 SAD study ongoing • Phase 2 in migraine and neuropathic pain planned 2H 2024 BREAKING NEWS Despite the CGRP Revolution, Significant Unmet Need Remains for 40M Migraine Sufferers in the US and 1B Worldwide • 30–40% of patients do not respond to treatments that block CGRP or its receptor • Migraine is 2nd leading cause of disability worldwide and 1st among young women1 First-in-Class TRPM3 Antagonist — Novel Mechanism for the Treatment of Migraine and Pain • BHV-2100 is the only TRPM3 antagonist in clinical development • Preclinical data shows robust efficacy in pain models • Highly differentiated from programs that target TRPV1 and TRPA1, avoids TRP family liabilities such as hyperthermia Phase 1 Study Preliminary Data Supports Evaluation in Acute Migraine • SAD study: 2 cohorts completed dosing (25 and 75 mg) • MAD study: initiating • Rapidly absorbed (Tmax 1–2 hours) • Projected therapeutic concentrations achieved (IC90 exceeded within 1 hour) • Well tolerated with only mild adverse events (flatulence, constipation, upper respiratory tract infection, dysesthesia) and no evidence of temperature dysregulation to date 1. Steiner. J Headache Pain 2020 18 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
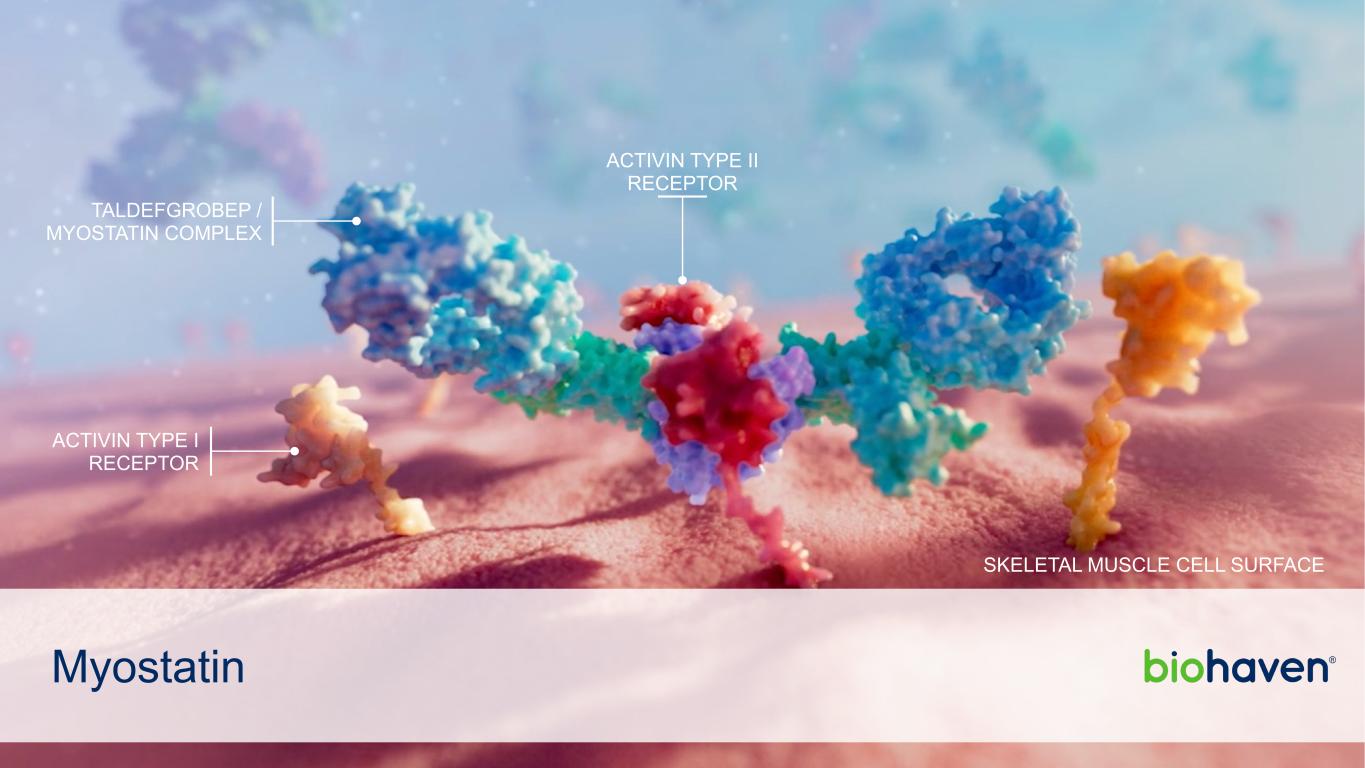
Myostatin TALDEFGROBEP / MYOSTATIN COMPLEX ACTIVIN TYPE II RECEPTOR ACTIVIN TYPE I RECEPTOR SKELETAL MUSCLE CELL SURFACE

TALDEFGROBEP ALFA (Anti-myostatin) Differentiated Profile Balancing Both Efficacy and Safety • Taldefgrobep alfa inactivates free myostatin (GDF-8), an inhibitor of muscle growth • Pharmacology uniquely sustained by taldefgrobep alfa/myostatin complex • Taldefgrobep alfa/myostatin complex inhibits skeletal muscle ActRIIB receptor signaling, and thus, enhances muscle mass Potential Paradigm Shift in the Treatment of Obesity • Taldefgrobep alfa treatment of >350 subjects with favorable safety and tolerability observed in children, adolescents, and adults • Reductions in fat mass while increasing lean mass in healthy adults • Maintains muscle gains after cessation of administration • Weekly SC administration with the potential for extended dosing intervals Phase 3 in SMA • Global Phase 3 study in broad-population of SMA patients now fully enrolled • Weekly SC taldefgrobep alfa on top of stand of care continues to be well tolerated • Orphan designation granted in the US and EU, along with Fast Track designation by FDA 20 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 • Obesity Phase 2 to initiate in 2Q 2024 • Topline Phase 3 Results in SMA in 2H 2024 BREAKING NEWS
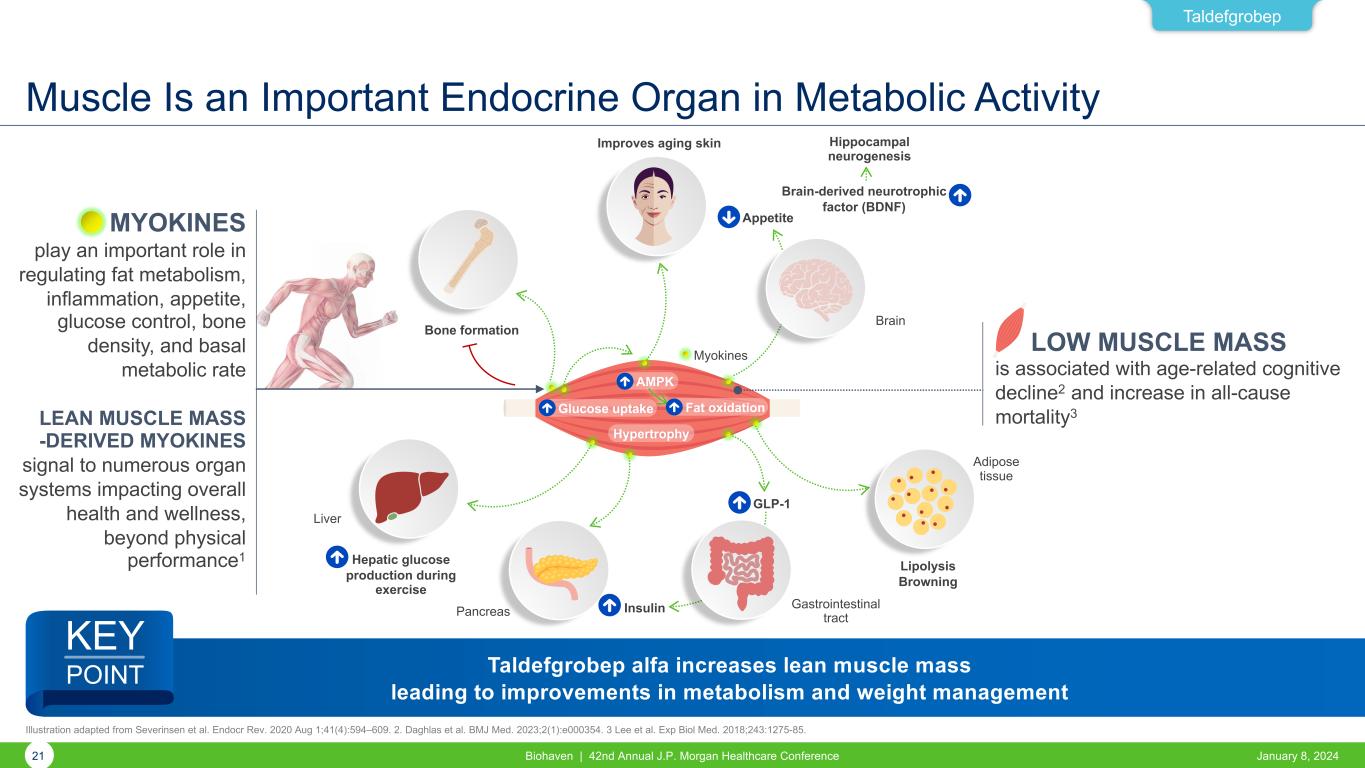
Muscle Is an Important Endocrine Organ in Metabolic Activity January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference21 Taldefgrobep MYOKINES play an important role in regulating fat metabolism, inflammation, appetite, glucose control, bone density, and basal metabolic rate LEAN MUSCLE MASS -DERIVED MYOKINES signal to numerous organ systems impacting overall health and wellness, beyond physical performance1 LOW MUSCLE MASS is associated with age-related cognitive decline2 and increase in all-cause mortality3 Bone formation Improves aging skin Adipose tissue Hippocampal neurogenesis Appetite Brain-derived neurotrophic factor (BDNF) Lipolysis Browning GLP-1 Gastrointestinal tractPancreas Hepatic glucose production during exercise Liver Insulin AMPK Glucose uptake Fat oxidation Hypertrophy Brain Myokines Illustration adapted from Severinsen et al. Endocr Rev. 2020 Aug 1;41(4):594–609. 2. Daghlas et al. BMJ Med. 2023;2(1):e000354. 3 Lee et al. Exp Biol Med. 2018;243:1275-85. Taldefgrobep alfa increases lean muscle mass leading to improvements in metabolism and weight management KEY POINT

Increased lean mass Reduction in total body fat mass Improved insulin sensitivity Reduction in visceral fat Reduction in intramuscular fat Reduction in intrahepatic fat Increased basal metabolic rate Improved bone mineral density Inhibiting Myostatin Increases Muscle Mass and Metabolic Health Taldefgrobep Myostatin as a Pharmacologic Target 22 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
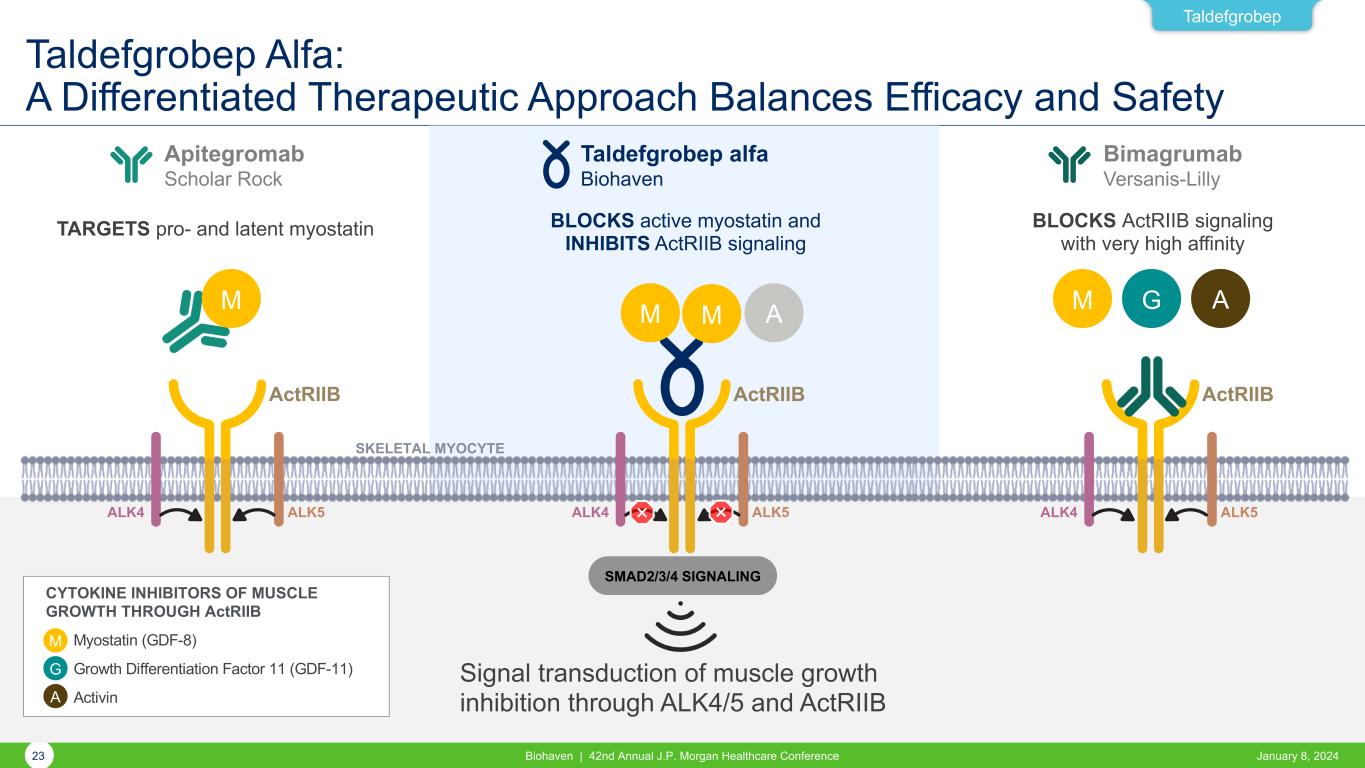
Taldefgrobep Alfa: A Differentiated Therapeutic Approach Balances Efficacy and Safety January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference23 Taldefgrobep ALK4 ALK5 ActRIIB SKELETAL MYOCYTE SMAD2/3/4 SIGNALING Signal transduction of muscle growth inhibition through ALK4/5 and ActRIIB TARGETS pro- and latent myostatin ALK4 ALK5 ActRIIB ALK4 ALK5 ActRIIB M M G AM AM Apitegromab Scholar Rock Taldefgrobep alfa Biohaven Bimagrumab Versanis-Lilly BLOCKS active myostatin and INHIBITS ActRIIB signaling BLOCKS ActRIIB signaling with very high affinity M G A Myostatin (GDF-8) Growth Differentiation Factor 11 (GDF-11) Activin CYTOKINE INHIBITORS OF MUSCLE GROWTH THROUGH ActRIIB

SC Taldefgrobep Effectively Suppresses Free Myostatin in Healthy Adults January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference24 Free Myostatin Levels Myostatin Complex Levels Taldefgrobep LAST DOSE LAST DOSE Complex sustains activity • Robust lowering of free myostatin after administration of taldefgrobep alfa 45 mg Q1W • Taldefgrobep-myostatin complex continues to exert activity for weeks after dosing stops • Continued improvement in muscle mass after cessation of dosing KEY POINTS Taldefgrobep alfa activity sustained by circulating taldefgrobep-myostatin complex Biohaven Phase 1 data on file Biohaven Phase 1 data on file
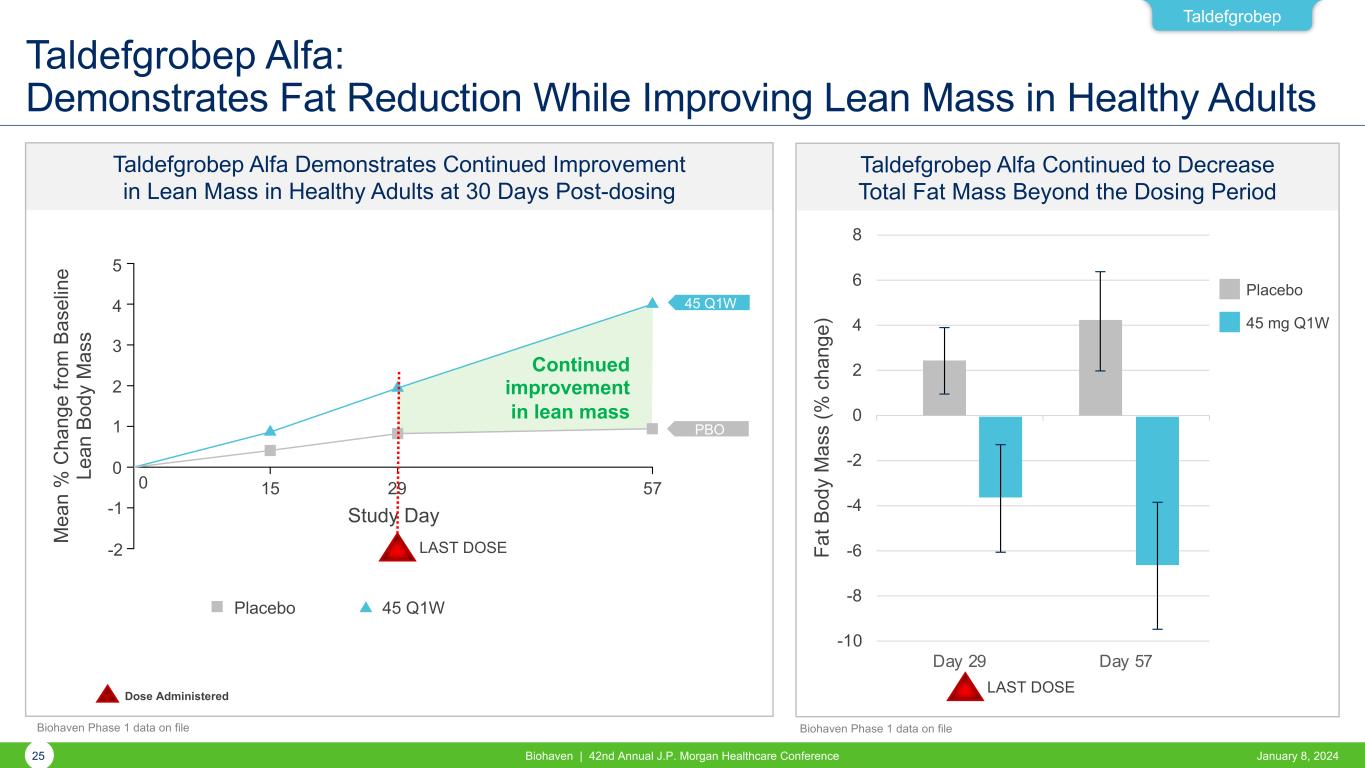
Taldefgrobep Taldefgrobep Alfa: Demonstrates Fat Reduction While Improving Lean Mass in Healthy Adults Taldefgrobep Alfa Demonstrates Continued Improvement in Lean Mass in Healthy Adults at 30 Days Post-dosing Taldefgrobep Alfa Continued to Decrease Total Fat Mass Beyond the Dosing Period -10 -8 -6 -4 -2 0 2 4 6 8 Day 29 Day 57 Fa t B od y M as s (% c ha ng e) Placebo 45 mg Q1W 45 Q1W 5 4 3 2 1 0 -1 -2 0 15 29 57 M ea n % C ha ng e fro m B as el in e Le an B od y M as s Study Day Placebo 45 Q1W LAST DOSE Continued improvement in lean mass PBO LAST DOSEDose Administered 25 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 Biohaven Phase 1 data on file Biohaven Phase 1 data on file

Taldefgrobep Alfa: Phase 2 Study to Evaluate Taldefgrobep +/- Semaglutide in the Treatment of Overweight and Obesity Innovative study design allows for early insight into a number of key clinical questions • Impact of Taldefgrobep monotherapy on changes in body composition, total body weight, and metabolic parameters • Ability of Taldefgrobep to augment fat mass loss when used as adjunct to anti-obesity standard of care (GLP-1 agonist) • Potential for Taldefgrobep to prevent against GLP-1-induced lean muscle loss • Influence of Taldefgrobep on weight regain following discontinuation of GLP-1 agonist January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference26 Post-Dose Follow-up Taldefgrobep (n=30) Taldefgrobep + Semaglutide (n=30) Semaglutide (n=30) Screening 4 Weeks PRIMARY DATA READOUT Week 18 Taldefgrobep + Semaglutide (n=30) Semaglutide (n=30) Semaglutide (n=30) Week 36 Taldefgrobep Phase 2 Proof of Concept Study Initiation in 1H24 KEY UPDATE

3M+ OCD Patients in US With High Unmet Medical Need • 40–60% do not respond to first line treatment • 10–40% are treatment refractory potentially requiring ablative neurosurgery or deep brain stimulation Phase 2 Troriluzole Trial in OCD Demonstrated Efficacy Signal Consistent numerical benefits vs. placebo on Y-BOCS (primary endpoint) at all timepoints (weeks 4 to 12); p < 0.05 at week 8 and p = 0.22 at week 12 Global Phase 3 Program (2 Identical Studies) Currently Ongoing TRORILUZOLE OCD Database Lock for Interim Efficacy Analysis in 1Q 2024 BREAKING NEWS Population: Subjects with moderate-to-severe OCD and inadequate response to SOC Primary Outcome: Y-BOCS (FDA accepted outcome measure) Interim Efficacy Analysis: Planned when 70% of subjects reach primary endpoint SOC + Troriluzole 280 mg QD SOC + Placebo QD Screening Phase 42 days Randomization Phase 10 weeks Extension Study 48 weeks SOC + Troriluzole 280 mg QDR OCD, obsessive-compulsive disorder; SOC, standard of care; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale 27 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
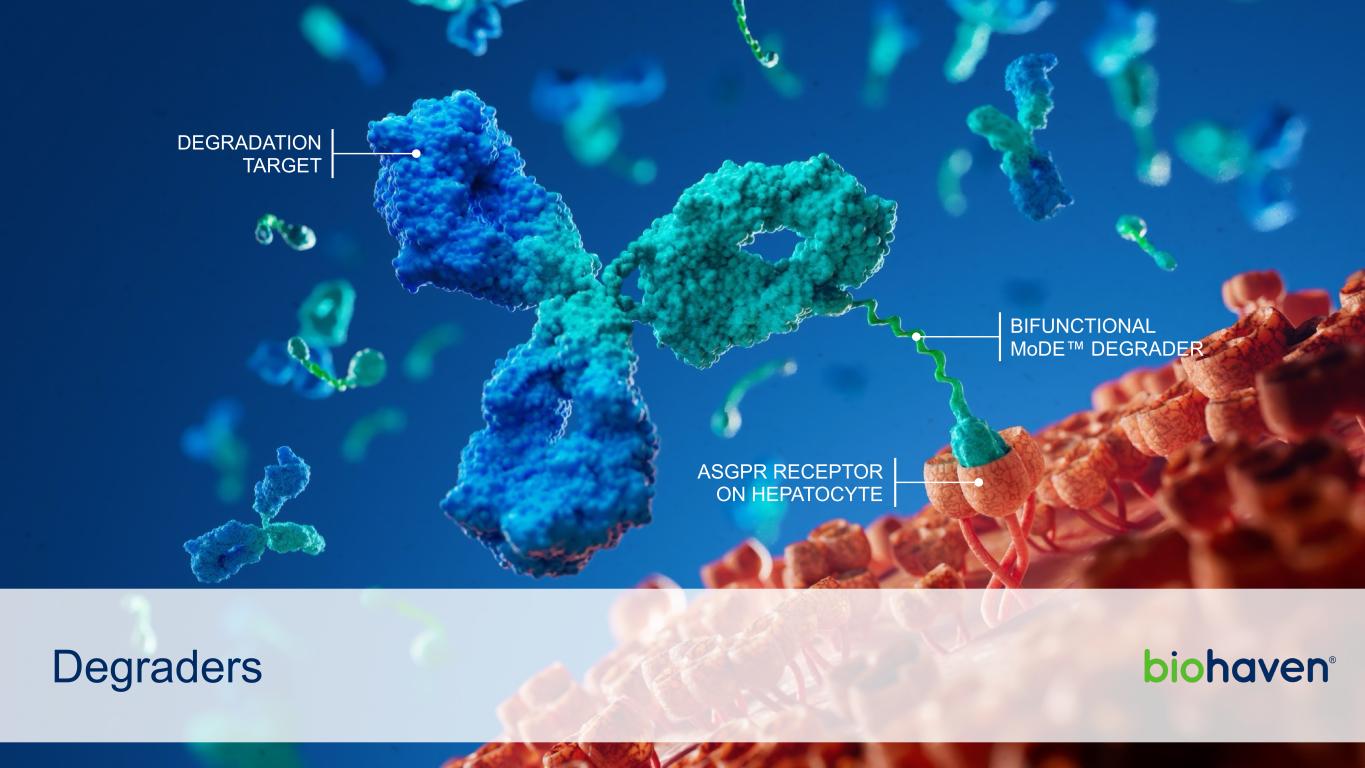
Degraders DEGRADATION TARGET BIFUNCTIONAL MoDE™ DEGRADER ASGPR RECEPTOR ON HEPATOCYTE

Potent Extracellular Pan-IgG Lowering Agents • Degrading and depleting pathogenic IgG presents multiple disease opportunities • BHV-1310 has further optimized properties over first-generation BHV-1300 Innovative Mechanism of Action • Protein degradation rather than inhibition • Low projected human dose range • Small molecule allows for small-volume subcutaneous dosing • Next-gen technology allows for selective targeting of a variety of proteins Faster and Deeper Depletion • NHP studies showed 80% IgG depletion with a single dose of BHV-1300; increasing to ~90% after multiple doses • Safe in doses up to 500 mg/kg • More rapid IgG reduction vs. competitors • Allows for co-administration with biologics Potential in Multiple Diseases • Common diseases — RA, lupus erythematosus, lupus nephritis • Rare diseases — Generalized myasthenia gravis, transplant, oncology, etc. PAN IgG DEGRADERS • BHV-1300: First-in-human Phase 1 start and data expected 1Q 2024 • BHV-1310: ~90% IgG depletion with a single dose • New NHP data showing that Biohaven’s IgG Degrader technology allows for co-administration with biologics (Humira® — PK unaltered) BREAKING NEWS 29 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

A First-in-Class Mechanism: Hepatic ASGPR Receptor Harnessed for Efficient and Safe Removal of Circulating Pathogenic Targets subcutaneous or intravenous injection MoDEs™ are administered via *Stylistic representation ASGPR, asialoglycoprotein receptor; MoDE™, molecular degrader of extracellular proteins • Internalized target is rapidly degraded in lysosomes • Degree of target degradation is precisely controlled3 1 Legend Degradation Target Bifunctional MoDE™ Degrader Hepatocyte Asialoglycoprotein Receptor (ASGPR)* ASGPR receptors are rapidly recycled. Optimized safety and efficacy is achieved through balancing of relative affinities for ASGPR and target protein. 4 MoDE™ binds circulating target and efficiently delivers it to ASGPR on hepatocytes 2 30 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

Precisely balanced components selected for optimal efficacy, safety and product profile A Transformational Drug Platform: Molecular Degraders of Extracellular Proteins (MoDE™) January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference31 TARGET BINDER STABLE LINKER ASGPR BINDER COMPOUND LIBRARY BINDER COMPOUND LIBRARY MoDE™ Platform allows for new compound generation in only 12–18 months! KEY POINT Accelerated new drug candidate timelines (12–18 months) Efficiently removes immune targets causing disease Fast onset and potential for > 90% deep reduction in target Allows for selective targeting of proteins to avoid broad immunosuppression Ability to adjunctively dose Fc biologics
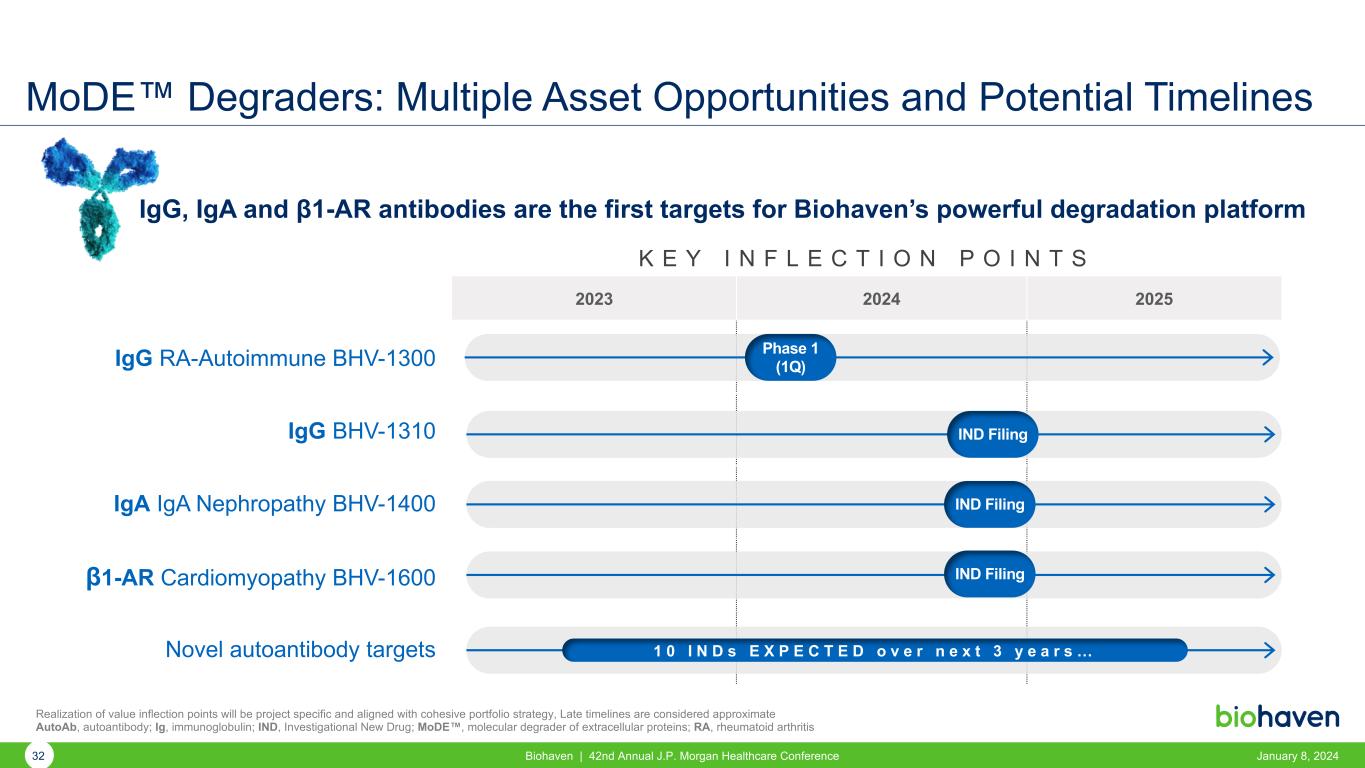
K E Y I N F L E C T I O N P O I N T S 2023 2024 2025 IgG RA-Autoimmune BHV-1300 IgG BHV-1310 IgA IgA Nephropathy BHV-1400 β1-AR Cardiomyopathy BHV-1600 Novel autoantibody targets MoDE™ Degraders: Multiple Asset Opportunities and Potential Timelines Realization of value inflection points will be project specific and aligned with cohesive portfolio strategy, Late timelines are considered approximate AutoAb, autoantibody; Ig, immunoglobulin; IND, Investigational New Drug; MoDE™, molecular degrader of extracellular proteins; RA, rheumatoid arthritis Phase 1 (1Q) IND Filing IND Filing IND Filing 1 0 I N D s E X P E C T E D o v e r n e x t 3 y e a r s … IgG, IgA and β1-AR antibodies are the first targets for Biohaven’s powerful degradation platform 32 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

BHV-1300 DEGRADERS

BHV-1300: Shows Potential for Superiority Over Competition BHV-1300 demonstrated faster depletion of IgG in non-human primates 0 50 100 150 0 5 10 15 % Ig G , b as el in e Days after start of infusion 10 mg/kg 75 mg/kg 250 mg/kg Vehicle BHV-1300 NHP Pharmacodynamics 2–3 days to reach 80% depletion Efgartigimod NHP Pharmacodynamics 5–7 days to reach 50% depletion 0 5 10 15 0 50 100 150 Days after start of infusion % Ig G , b as el in e Vehicle 0.2 mg/kg 2 mg/kg 20 mg/kg 70 mg/kg 200 mg/kg Immunovant NHP Pharmacodynamics 0 7 14 21 28 35 42 56 Days % c ha ng e / T 0 0 10 25 50 100 Batoclimab 50 mg/kg (n=3) IMVT-1402 50 mg/kg (n=7) IMVT-1402 5 mg/kg (n=7) Placebo (n=7) ~21 days to reach 75% depletion BHV-1300 Dose Administered Ulrichts P et al, J Clin Invest. 2018 Oct 1;128(10):4372-4386. doi: 10.1172/JCI97911. Epub 2018 Jul 24. PMID: 30040076; PMCID: PMC6159959. Excerpted from Immunovant Corporate Presentation, August 2023. 34 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

Unique Properties of BHV-1300 and BHV-1310 Matched to Indications BHV-1300 35 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 BHV-1310 Preclinical Pharmacodynamics (single dose) BHV-1300 Preclinical Pharmacodynamics (multiple dose) Dose Administered 60% DEPLETION 90% DEPLETION Dose Administered 60% DEPLETION 90% DEPLETION Optimization of degrader technology (BHV-1310) allows for deeper reductions in IgG after single dose KEY POINT ACUTE indications — e.g., myasthenia gravisCHRONIC indications — e.g., rheumatoid arthritis BHV-1300 pharmacodynamics in NHP and BHV-1310 pharmacodynamics in rabbit
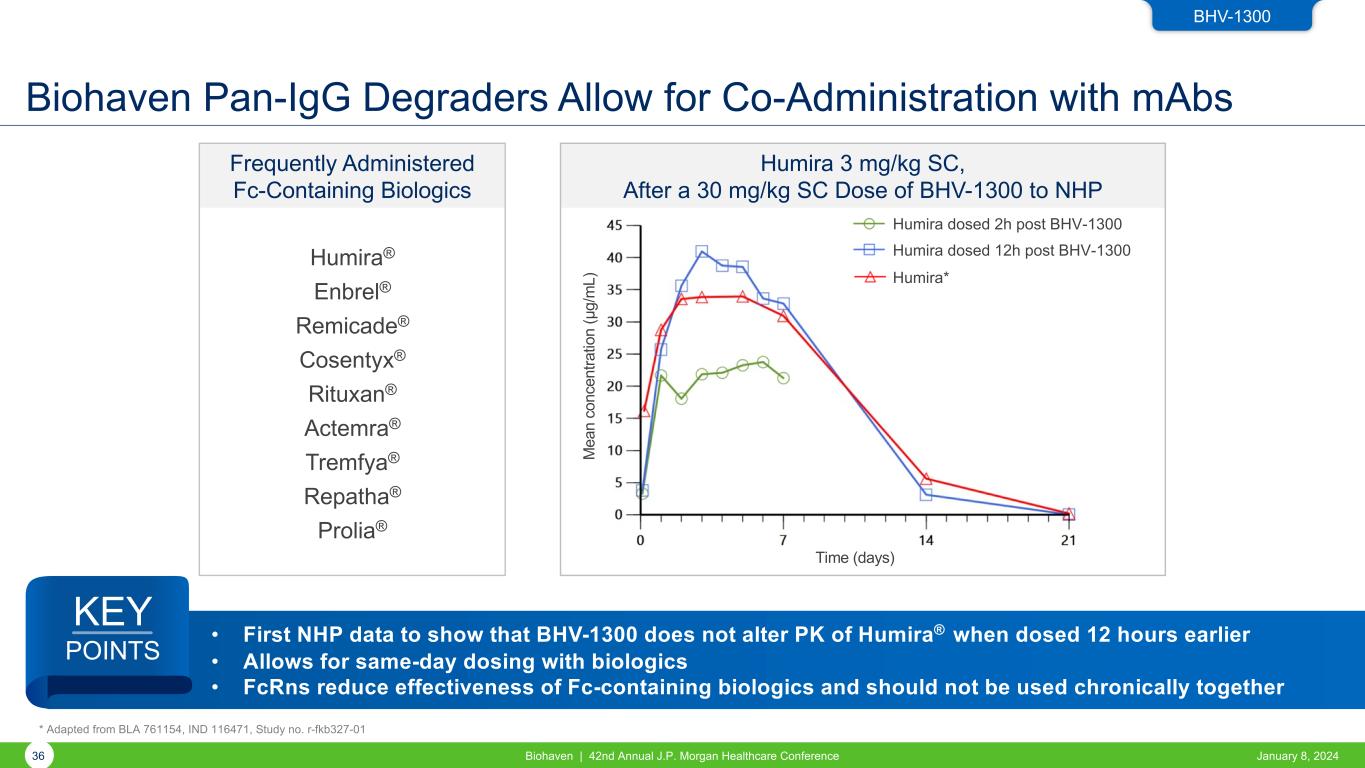
Biohaven Pan-IgG Degraders Allow for Co-Administration with mAbs January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference36 BHV-1300 * Adapted from BLA 761154, IND 116471, Study no. r-fkb327-01 Humira 3 mg/kg SC, After a 30 mg/kg SC Dose of BHV-1300 to NHP Time (days) M ea n co nc en tra tio n (µ g/ m L) Humira dosed 2h post BHV-1300 Humira dosed 12h post BHV-1300 Humira* Frequently Administered Fc-Containing Biologics Humira® Enbrel® Remicade® Cosentyx® Rituxan® Actemra® Tremfya® Repatha® Prolia® • First NHP data to show that BHV-1300 does not alter PK of Humira® when dosed 12 hours earlier • Allows for same-day dosing with biologics • FcRns reduce effectiveness of Fc-containing biologics and should not be used chronically together KEY POINTS
BHV-1600, Next-Generation Selective Degrader Targeting β1-AR Autoantibodies DEGRADERS

Selective Targeting of β1-AR Autoantibodies for Dilated Cardiomyopathy BHV-1600 Cardiac beta-adrenergic receptors (β1-AR) Inducibly increases heart rate, contractility and cardiac output Agonistic autoantibodies to β1-AR increase basal heart rate Sustained β1-AR agonism ▶ dilated cardiomyopathy ▶ heart failure Adenylyl cyclase PKA GS P Caspase L-type Ca2+ Channel ATP cAMP Ca2+ Apoptosis NORMAL HEART DILATED CARDIOMYOPATHY β1-AR autoantibodies β1-AR autoantibodies CURRENT TREATMENT FOR β1-AR AUTOANTIBODY-DRIVEN CARDIAC DISEASE: • BETA BLOCKERS: Ineffective treatment limited to supportive treatment, diuresis, etc. • REMOVAL OF ANTIBODIES: Plasmapheresis1,2 demonstrates POC but requires hospitalization 1. Eur J Heart Fail. 2013; 15(7): 724–729. 2. Nat. Rev. Nephrol. 2014; 10(3): 125-125. Illustration adapted from European Journal of Heart Failure (2013) 15, 724–729. Heart image adapted from https://thoracickey.com/clinical-presentation-and-therapy-of-cardiomyopathies/ BHV-1600 targeted hepatic degradation of autoantibodies 38 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

IND Filing and FIH Phase 1 Study 2H 2024 KEY UPDATE Ternary Complex and Endocytosis Marked Degradation of Anti-β-1AR Antibody in Mice BHV-1600: In Vitro and In Vivo Properties Ideal for Degrading β-1AR Abs BHV-1600 High Affinity to the Target High affinity for monoclonal mouse anti-b1-AR antibody and ASGPR protein construct by SPR • Rapid ASGPR-mediated hepatic clearance in mouse and rat • Stoichiometric degradation of exogenously administered anti-b-1AR Ab in mice compared to controls Formation of ternary complex confirmed in TR-FRET assay Cellular internalization of anti-b-1AR Ab demonstrated in HEK293 (hASGPR) cells 0 6 12 18 24 0 20 40 60 80 100 Time (Hrs) % a nt i-β 1A R PBS Peptide Alone BHV-1600 January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference39 KD B1AR = 18 nM BHV-1600

First-in-Class Oral, Selective, Brain-Penetrant TYK2/JAK1 Inhibitor • Uniquely potent, TYK2/JAK1 selective, brain-penetrant inhibitor • Selectivity profile should avoid class risks associated with JAK2/3 inhibition Breaks the Cycle of Neuroinflammation Reduces inflammatory impacts of microglia, astrocytes, and infiltrating T-lymphocytes Potential to Treat Multiple Neuroinflammatory Disorders Evidence supports efficacy in prevention of amyloid therapy induced ARIA, Alzheimer’s disease, Parkinson’s disease, Multiple Sclerosis and other disorders Encouraging Preliminary Results from Ongoing Phase 1 Trial • Projected therapeutic concentrations achieved • Well tolerated with only mild adverse events to date (loose bowel movements, headache, and constipation) Upcoming Milestones Anticipate initiating multiple clinical trials in 2024 BHV-8000 TYK2/JAK1 INHIBITOR (brain-penetrant) ARIA, Amyloid-related imaging abnormalities • SAD study: SAD cohorts completed dosing (10, 20 and 30 mg) • MAD study: Completed 10 mg dose cohort and began 20 mg dose PROGRAM UPDATE 40 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

BHV-8000: TYK2/JAK1 in Neuroinflammatory Disorders Cytokine Receptor JAK FAMILY Microglia IFN-γ, IFN-⍺, IFN-β Astrocytes IFN-γ, IFN-⍺ and IFN-β Lymphocytes and Other Leukocytes IL-23, IL-17 downstream of IL-23 CELLULAR DRIVERS IN NEUROINFLAMMATION Inflammation plays a key role in the pathogenesis of neurodegenerative diseases Nonclinical, clinical, genetic and epidemiological data show that interrupting chronic inflammation may slow disease progression ARIA Alzheimer’s Parkinson’s Multiple Sclerosis Other Neuroinflammatory & Neurodegenerative Disorders JAK2 JAK1 TYK2 ST A T SI G N A LI N G BHV-8000 JAK3 BHV-8000 A dual, brain-penetrant inhibitor of TYK2 and JAK1 that can effectively block Th17 cell generation, Type I IFN signaling and inflammation 41 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024

Biohaven's Real-World Analytics of Large Healthcare Database: Parkinson's Disease Risk Reduction with IL-17/TNF Targeting Therapies • Biohaven conducted analysis using Komodo Health database (over 320 million patients since 2012) examining treatment with Anti-TNF or Anti-IL17 and incidence of PD • Millions of patients over 8+ years of dosing captured key epidemiologic confirmation of the neuroinflammatory hypothesis • Result provides MOA rationale for the effectiveness of a TYK/JAK inhibitor in PD Treatment PD Events Person-years Rate (per 100 person-years) Adjusted IRR (95% CI) P-value Anti-TNF or Anti-IL-17 exposure 2,957 393,114 0.66 0.77 (0.74 – 0.80) <0.0001 No Treatment 50,562 5,328,307 0.95 Anti-TNF exposure 2,471 371,867 0.66 0.64 (0.52 – 0.80) <0.0001 No Treatment 50,562 5,328,307 0.95 Anti-IL-17 exposure 81 15,598 0.52 0.77 (0.78 – 0.81) <0.0001 No Treatment 50,562 5,328,307 0.95 BHV-8000 42 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
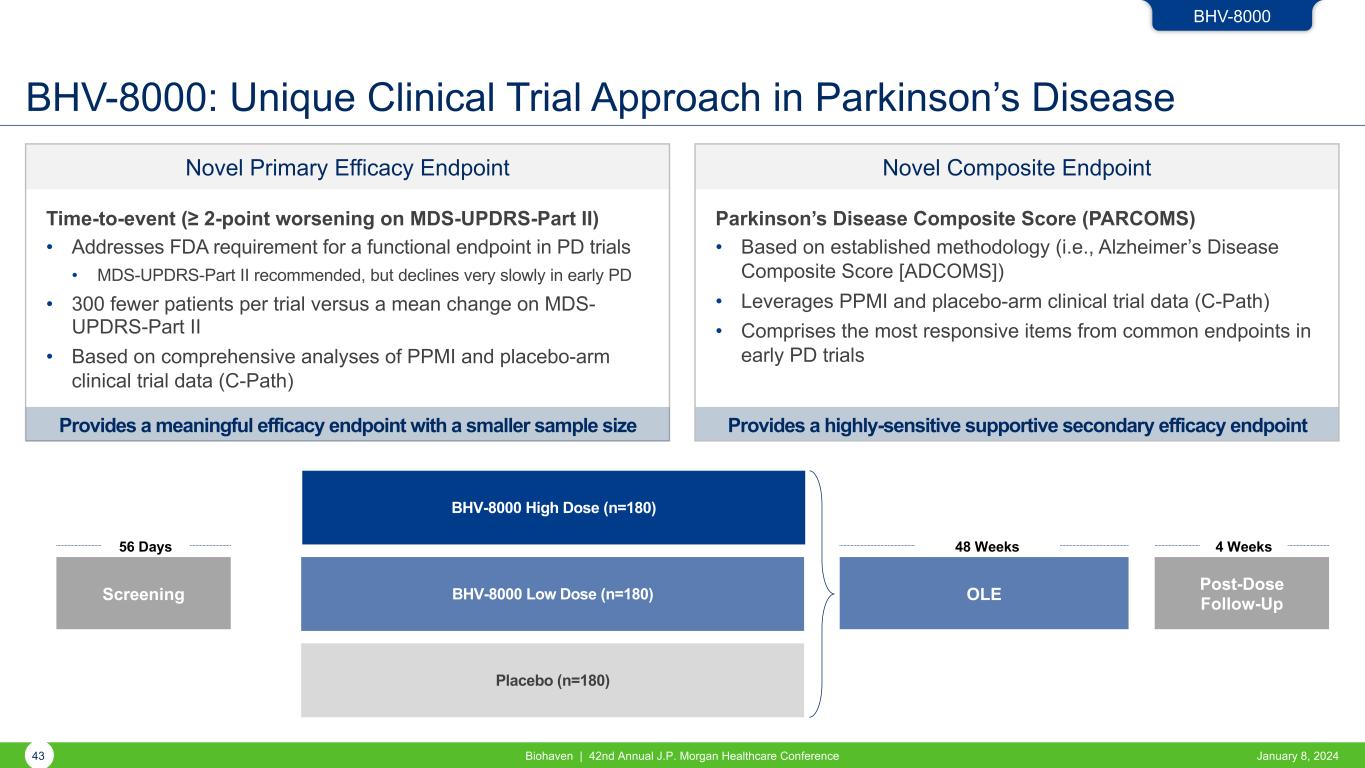
Provides a highly-sensitive supportive secondary efficacy endpoint Novel Composite Endpoint Parkinson’s Disease Composite Score (PARCOMS) • Based on established methodology (i.e., Alzheimer’s Disease Composite Score [ADCOMS]) • Leverages PPMI and placebo-arm clinical trial data (C-Path) • Comprises the most responsive items from common endpoints in early PD trials Novel Primary Efficacy Endpoint BHV-8000: Unique Clinical Trial Approach in Parkinson’s Disease BHV-8000 High Dose (n=180) BHV-8000 Low Dose (n=180) Placebo (n=180) Screening 56 Days OLE 48 Weeks Post-Dose Follow-Up 4 Weeks BHV-8000 Time-to-event (≥ 2-point worsening on MDS-UPDRS-Part II) • Addresses FDA requirement for a functional endpoint in PD trials • MDS-UPDRS-Part II recommended, but declines very slowly in early PD • 300 fewer patients per trial versus a mean change on MDS- UPDRS-Part II • Based on comprehensive analyses of PPMI and placebo-arm clinical trial data (C-Path) Provides a meaningful efficacy endpoint with a smaller sample size 43 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
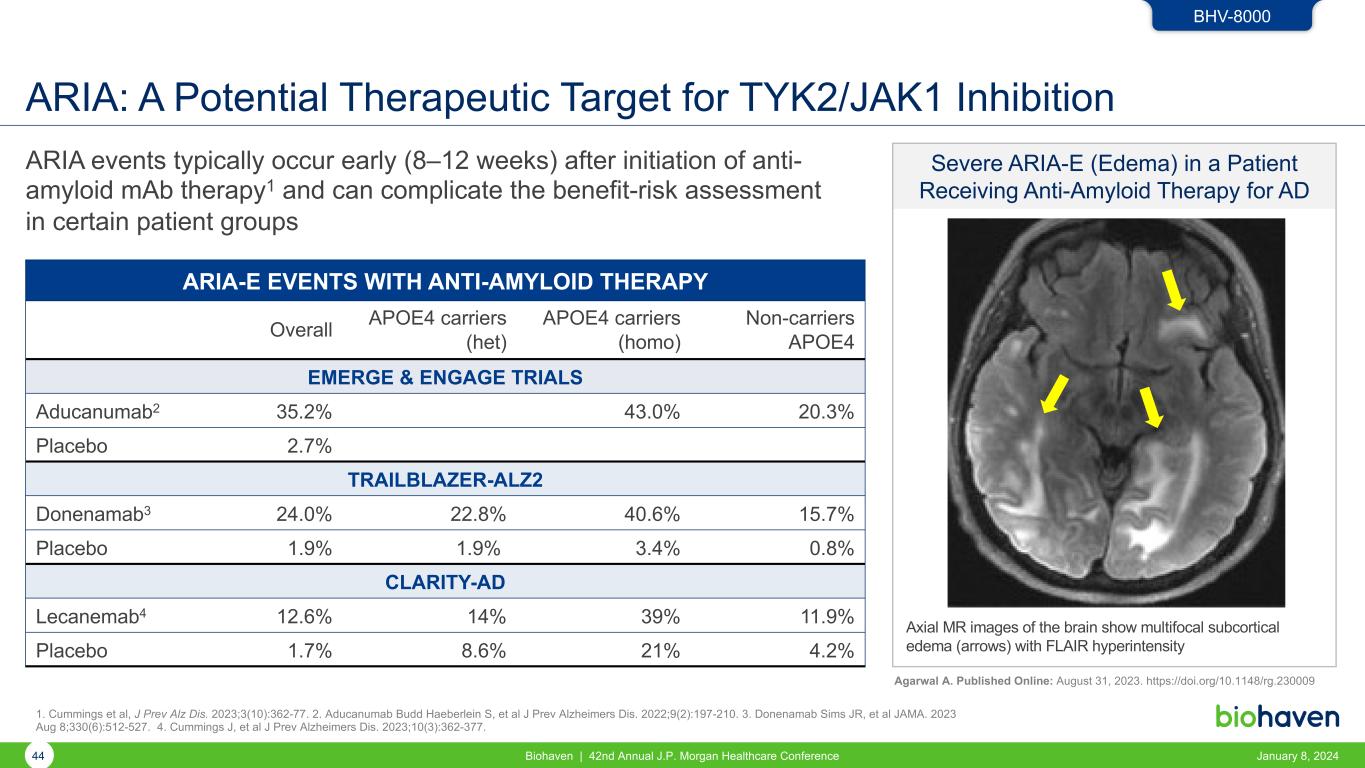
Severe ARIA-E (Edema) in a Patient Receiving Anti-Amyloid Therapy for AD ARIA: A Potential Therapeutic Target for TYK2/JAK1 Inhibition ARIA events typically occur early (8–12 weeks) after initiation of anti- amyloid mAb therapy1 and can complicate the benefit-risk assessment in certain patient groups ARIA-E EVENTS WITH ANTI-AMYLOID THERAPY Overall APOE4 carriers (het) APOE4 carriers (homo) Non-carriers APOE4 EMERGE & ENGAGE TRIALS Aducanumab2 35.2% 43.0% 20.3% Placebo 2.7% TRAILBLAZER-ALZ2 Donenamab3 24.0% 22.8% 40.6% 15.7% Placebo 1.9% 1.9% 3.4% 0.8% CLARITY-AD Lecanemab4 12.6% 14% 39% 11.9% Placebo 1.7% 8.6% 21% 4.2% BHV-8000 Agarwal A. Published Online: August 31, 2023. https://doi.org/10.1148/rg.230009 Axial MR images of the brain show multifocal subcortical edema (arrows) with FLAIR hyperintensity 44 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 1. Cummings et al, J Prev Alz Dis. 2023;3(10):362-77. 2. Aducanumab Budd Haeberlein S, et al J Prev Alzheimers Dis. 2022;9(2):197-210. 3. Donenamab Sims JR, et al JAMA. 2023 Aug 8;330(6):512-527. 4. Cummings J, et al J Prev Alzheimers Dis. 2023;10(3):362-377.
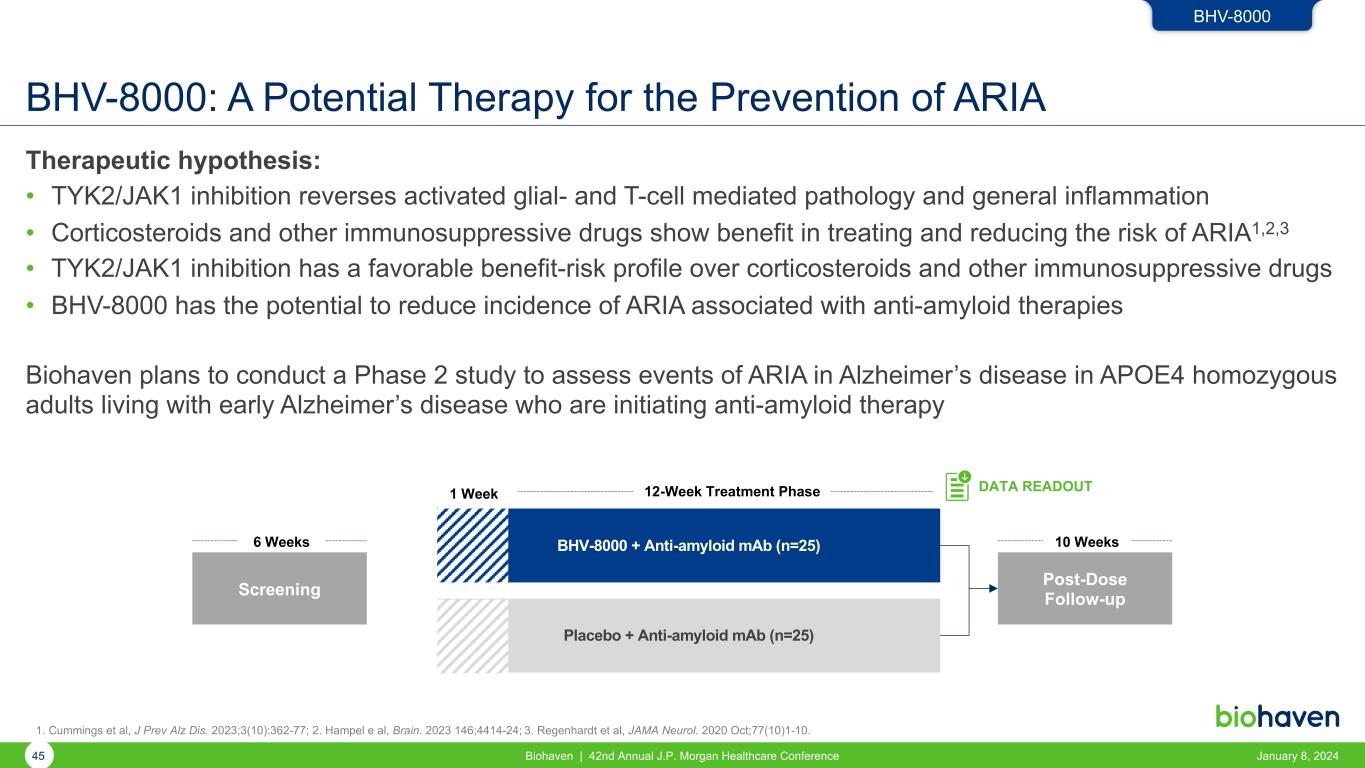
BHV-8000: A Potential Therapy for the Prevention of ARIA Therapeutic hypothesis: • TYK2/JAK1 inhibition reverses activated glial- and T-cell mediated pathology and general inflammation • Corticosteroids and other immunosuppressive drugs show benefit in treating and reducing the risk of ARIA1,2,3 • TYK2/JAK1 inhibition has a favorable benefit-risk profile over corticosteroids and other immunosuppressive drugs • BHV-8000 has the potential to reduce incidence of ARIA associated with anti-amyloid therapies Biohaven plans to conduct a Phase 2 study to assess events of ARIA in Alzheimer’s disease in APOE4 homozygous adults living with early Alzheimer’s disease who are initiating anti-amyloid therapy BHV-8000 + Anti-amyloid mAb (n=25) Placebo + Anti-amyloid mAb (n=25) Screening 6 Weeks 12-Week Treatment Phase DATA READOUT1 Week Post-Dose Follow-up 10 Weeks BHV-8000 1. Cummings et al, J Prev Alz Dis. 2023;3(10):362-77; 2. Hampel e al, Brain. 2023 146;4414-24; 3. Regenhardt et al, JAMA Neurol. 2020 Oct;77(10)1-10. 45 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
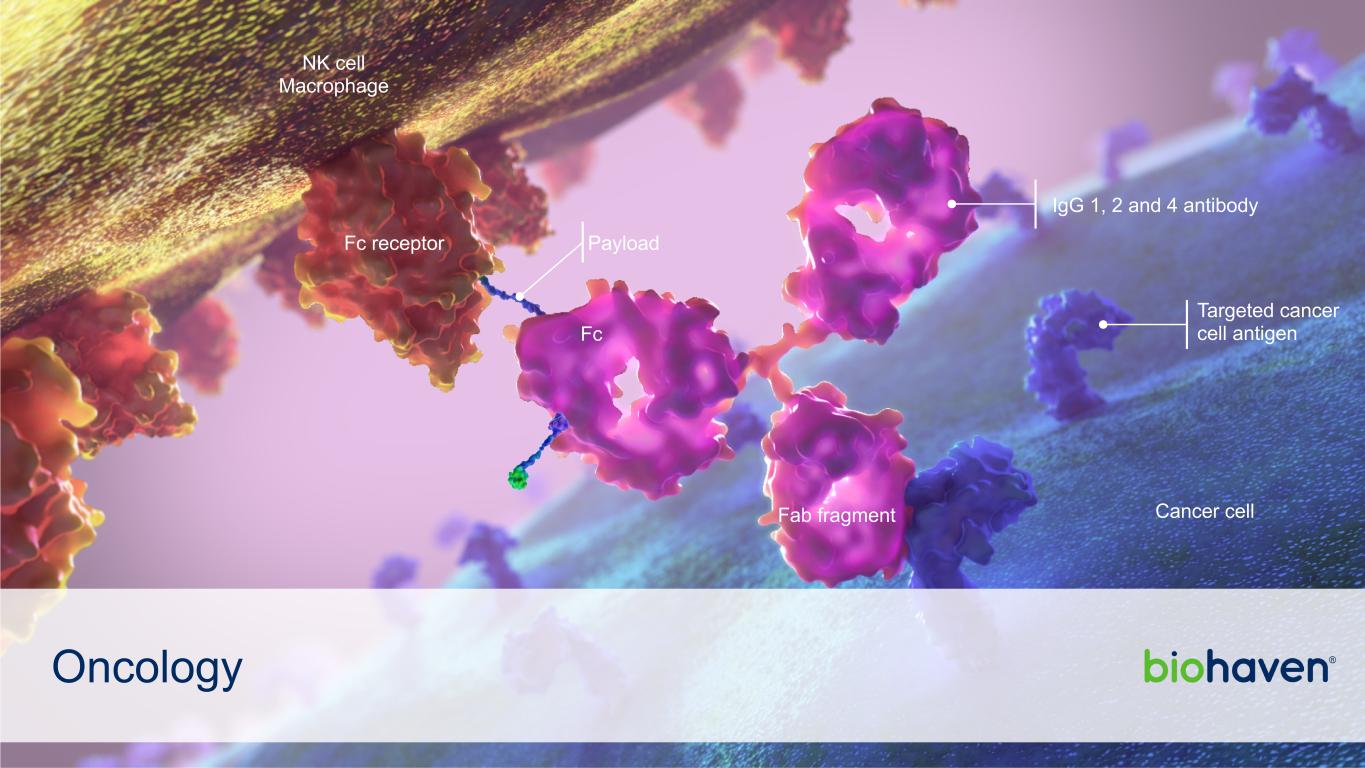
Oncology Payload IgG 1, 2 and 4 antibody NK cell Macrophage Cancer cell Targeted cancer cell antigen Fab fragment Fc Fc receptor

Biohaven chemistry Stable, physicochemically benign amide linkage O Payload N H N PayloadS O O Poorly stable linkage Industry standard maleimide Conjugation Chemistry Superior to Industry Standard Maleimide and lipophilic click chemistry Attached to Two Specific Lysines Provides stable and consistent drug antibody ratio (DAR) ü ADAPTABLE Complements and improves multiple existing ADC payload-linker technologies ü STABLE Improved ADC plasma stability with controlled DAR potentially improves therapeutic index ü EFFECTIVE Improved efficacy in mouse tumor model also suggests potential for increased therapeutic index ü MULTIPURPOSE Conjugates IgG1, 2 and 4; Single step conjugation with predictable favorable yields, low aggregation ü NOVEL IP filed globally in key markets Current Status • Two INDs planned for 2024 ADC PLATFORM • Two INDs planned for 2024 • TROP2 Phase 1 2Q 2024 • 5–7 new ADCs in next two years BREAKING NEWS 47 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
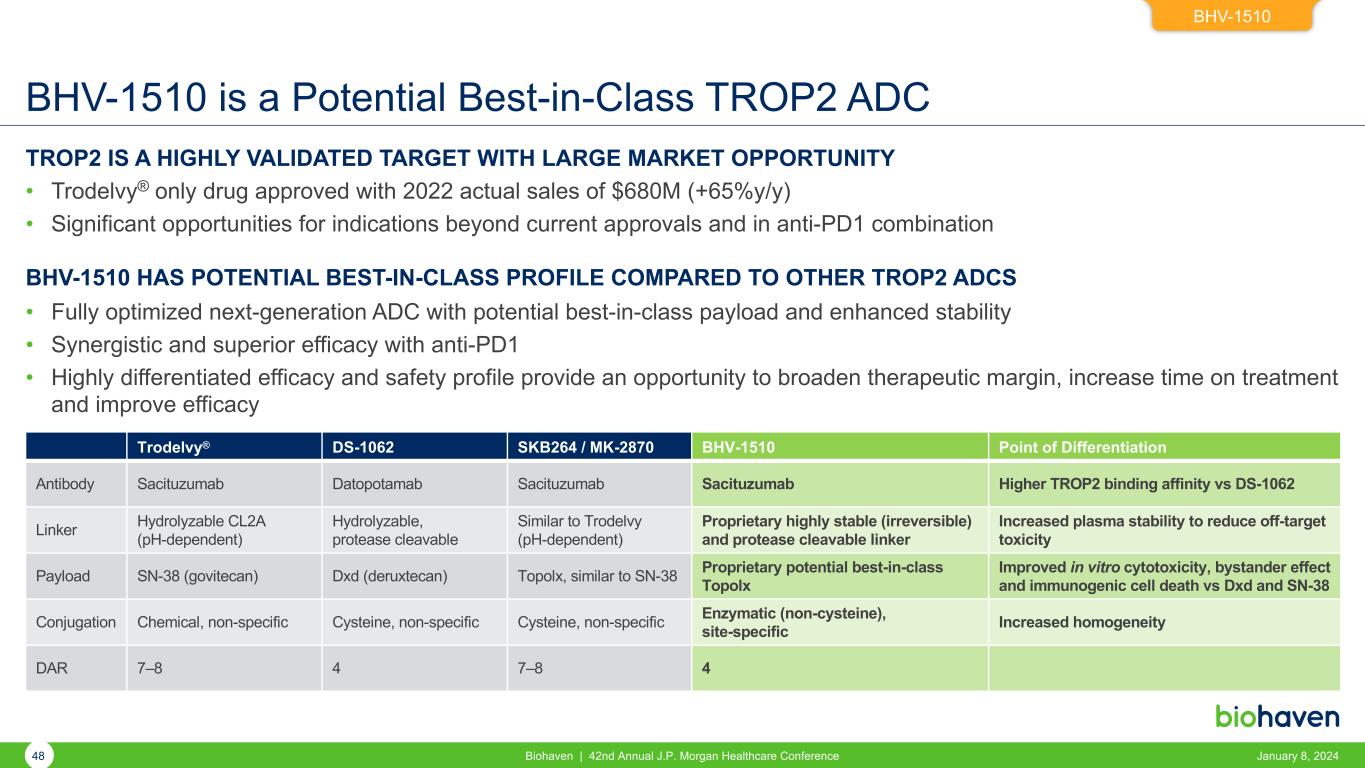
BHV-1510 is a Potential Best-in-Class TROP2 ADC TROP2 IS A HIGHLY VALIDATED TARGET WITH LARGE MARKET OPPORTUNITY • Trodelvy® only drug approved with 2022 actual sales of $680M (+65%y/y) • Significant opportunities for indications beyond current approvals and in anti-PD1 combination BHV-1510 HAS POTENTIAL BEST-IN-CLASS PROFILE COMPARED TO OTHER TROP2 ADCS • Fully optimized next-generation ADC with potential best-in-class payload and enhanced stability • Synergistic and superior efficacy with anti-PD1 • Highly differentiated efficacy and safety profile provide an opportunity to broaden therapeutic margin, increase time on treatment and improve efficacy BHV-1510 Trodelvy® DS-1062 SKB264 / MK-2870 BHV-1510 Point of Differentiation Antibody Sacituzumab Datopotamab Sacituzumab Sacituzumab Higher TROP2 binding affinity vs DS-1062 Linker Hydrolyzable CL2A (pH-dependent) Hydrolyzable, protease cleavable Similar to Trodelvy (pH-dependent) Proprietary highly stable (irreversible) and protease cleavable linker Increased plasma stability to reduce off-target toxicity Payload SN-38 (govitecan) Dxd (deruxtecan) Topolx, similar to SN-38 Proprietary potential best-in-class Topolx Improved in vitro cytotoxicity, bystander effect and immunogenic cell death vs Dxd and SN-38 Conjugation Chemical, non-specific Cysteine, non-specific Cysteine, non-specific Enzymatic (non-cysteine), site-specific Increased homogeneity DAR 7–8 4 7–8 4 48 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
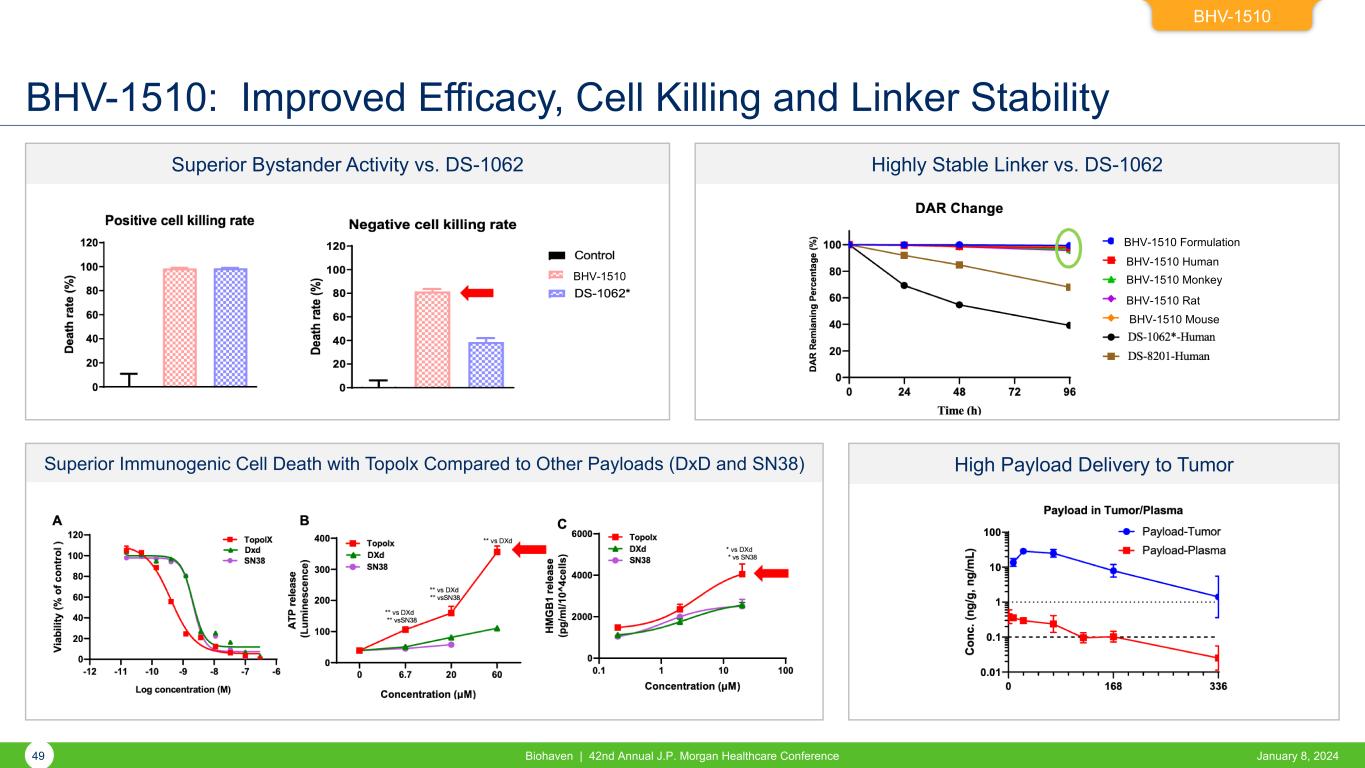
Superior Bystander Activity vs. DS-1062 Superior Immunogenic Cell Death with Topolx Compared to Other Payloads (DxD and SN38) Highly Stable Linker vs. DS-1062 High Payload Delivery to Tumor BHV-1510: Improved Efficacy, Cell Killing and Linker Stability BHV-1510 49 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 BHV-1510 BHV-1510 Formulation BHV-1510 Human BHV-1510 Monkey BHV-1510 Rat BHV-1510 Mouse
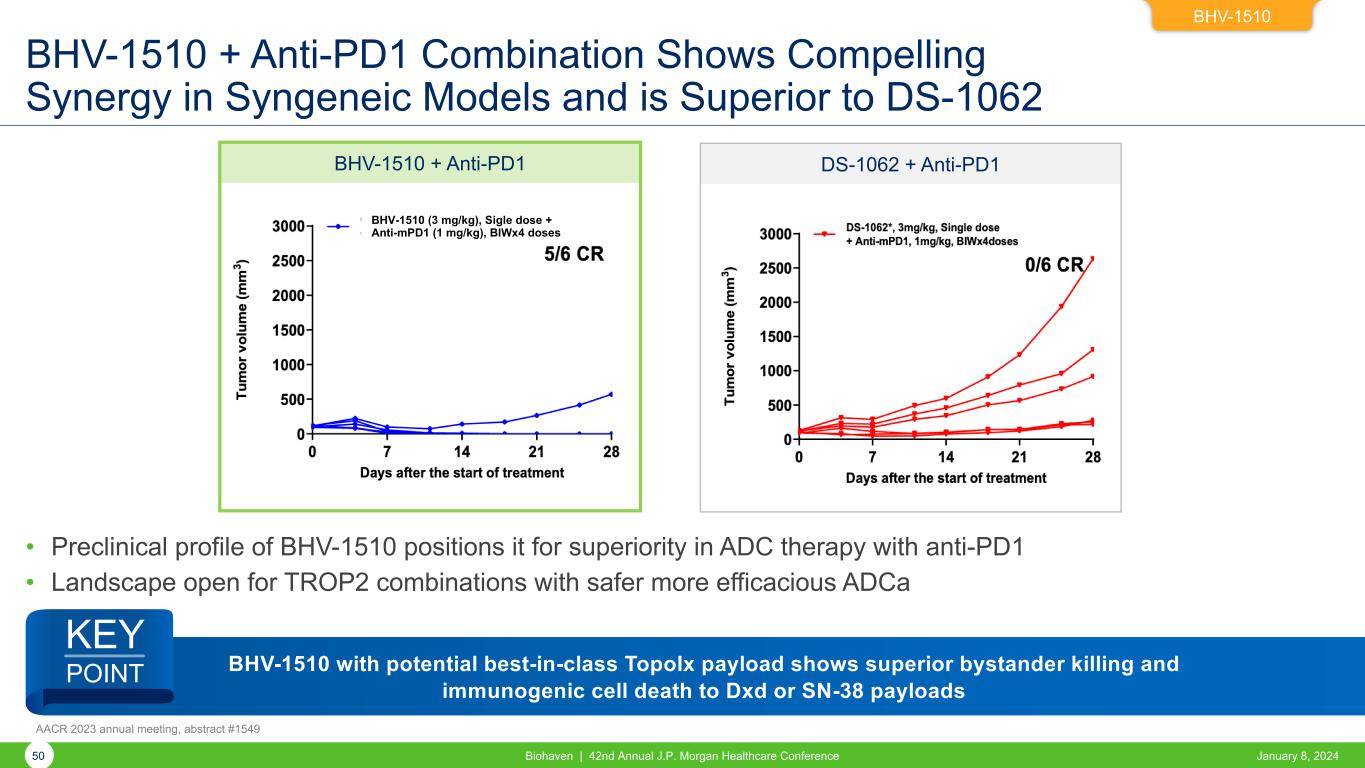
BHV-1510 BHV-1510 + Anti-PD1 Combination Shows Compelling Synergy in Syngeneic Models and is Superior to DS-1062 • Preclinical profile of BHV-1510 positions it for superiority in ADC therapy with anti-PD1 • Landscape open for TROP2 combinations with safer more efficacious ADCa January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference50 AACR 2023 annual meeting, abstract #1549 BHV-1510 + Anti-PD1 DS-1062 + Anti-PD1 BHV-1510 (3 mg/kg), Sigle dose + Anti-mPD1 (1 mg/kg), BIWx4 doses BHV-1510 with potential best-in-class TopoIx payload shows superior bystander killing and immunogenic cell death to Dxd or SN-38 payloads KEY POINT
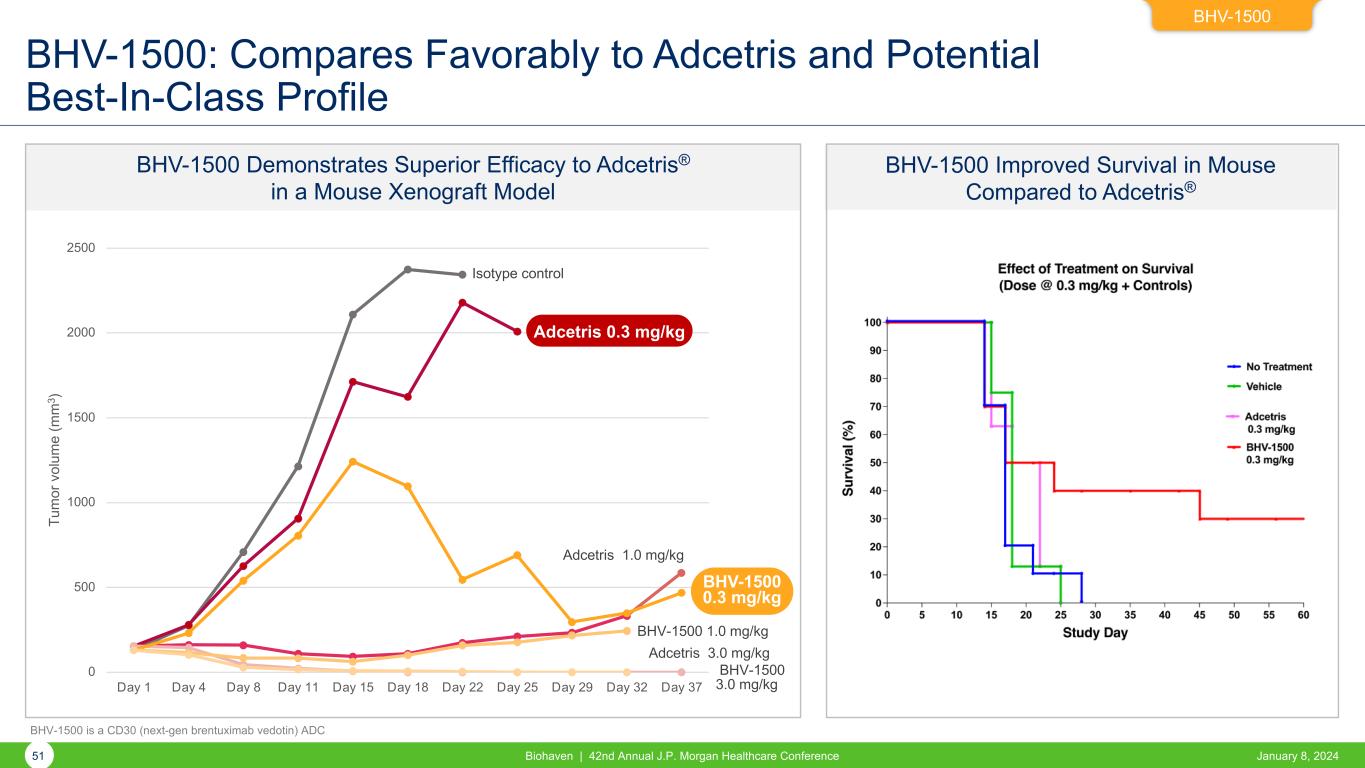
BHV-1500 Improved Survival in Mouse Compared to Adcetris® BHV-1500 BHV-1500: Compares Favorably to Adcetris and Potential Best-In-Class Profile BHV-1500 Demonstrates Superior Efficacy to Adcetris® in a Mouse Xenograft Model 0 500 1000 1500 2000 2500 Day 1 Day 4 Day 8 Day 11 Day 15 Day 18 Day 22 Day 25 Day 29 Day 32 Day 37 Tu m or v ol um e (m m 3 ) Adcetris 1.0 mg/kg BHV-1500 1.0 mg/kg BHV-1500 3.0 mg/kg Adcetris 0.3 mg/kg BHV-1500 0.3 mg/kg Isotype control Adcetris 3.0 mg/kg 51 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 BHV-1500 is a CD30 (next-gen brentuximab vedotin) ADC
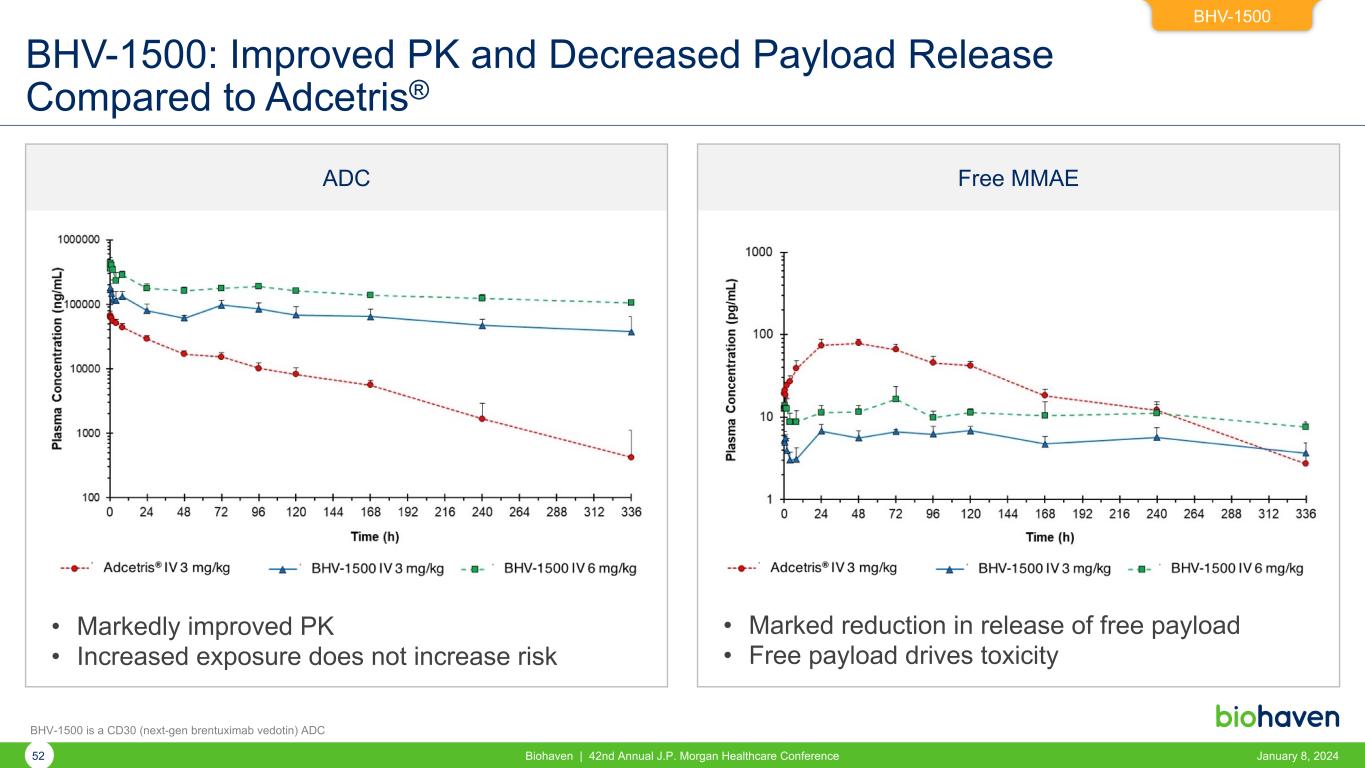
Free MMAEADC BHV-1500: Improved PK and Decreased Payload Release Compared to Adcetris® • Markedly improved PK • Increased exposure does not increase risk • Marked reduction in release of free payload • Free payload drives toxicity BHV-1500 52 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 BHV-1500 is a CD30 (next-gen brentuximab vedotin) ADC
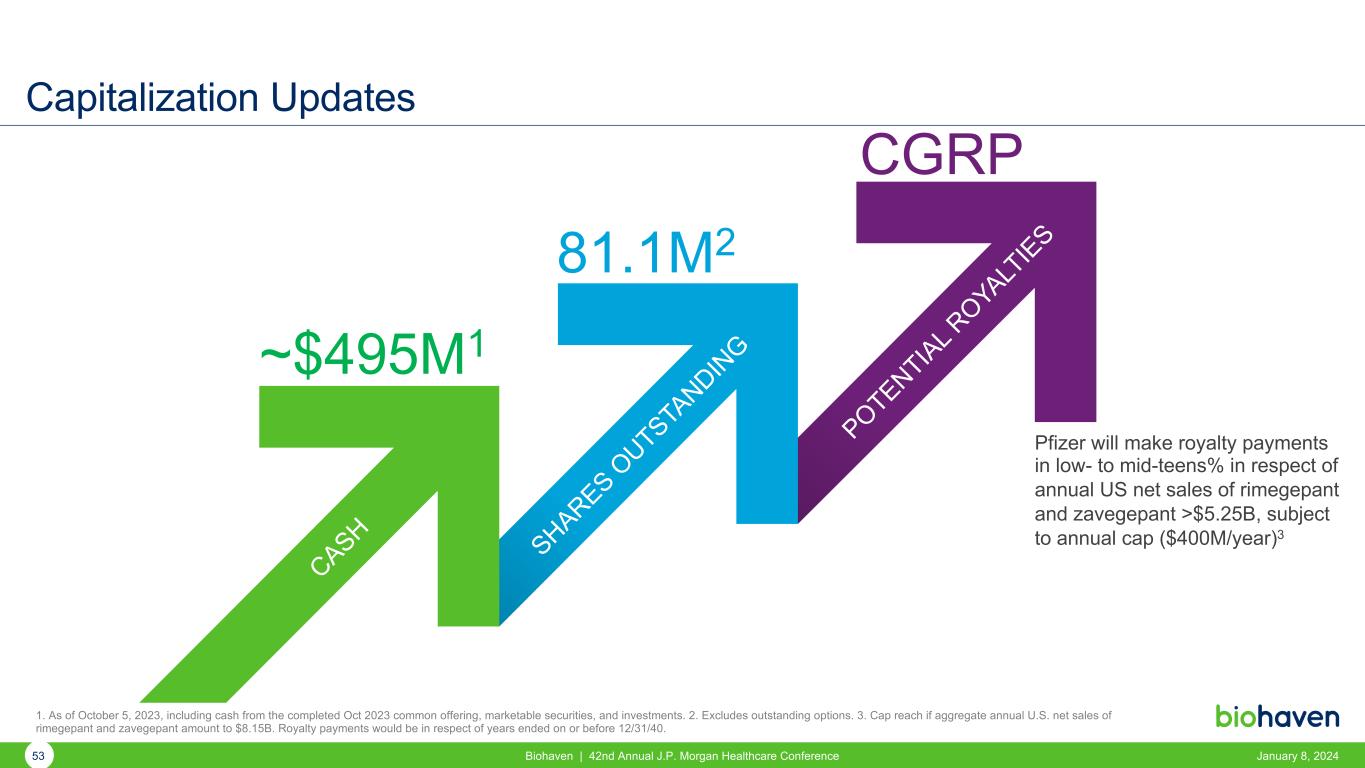
Capitalization Updates CASH SHARES O UTSTA NDIN G POTENTIAL R OYA LT IES ~$495M1 81.1M2 CGRP Pfizer will make royalty payments in low- to mid-teens% in respect of annual US net sales of rimegepant and zavegepant >$5.25B, subject to annual cap ($400M/year)3 1. As of October 5, 2023, including cash from the completed Oct 2023 common offering, marketable securities, and investments. 2. Excludes outstanding options. 3. Cap reach if aggregate annual U.S. net sales of rimegepant and zavegepant amount to $8.15B. Royalty payments would be in respect of years ended on or before 12/31/40. 53 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024
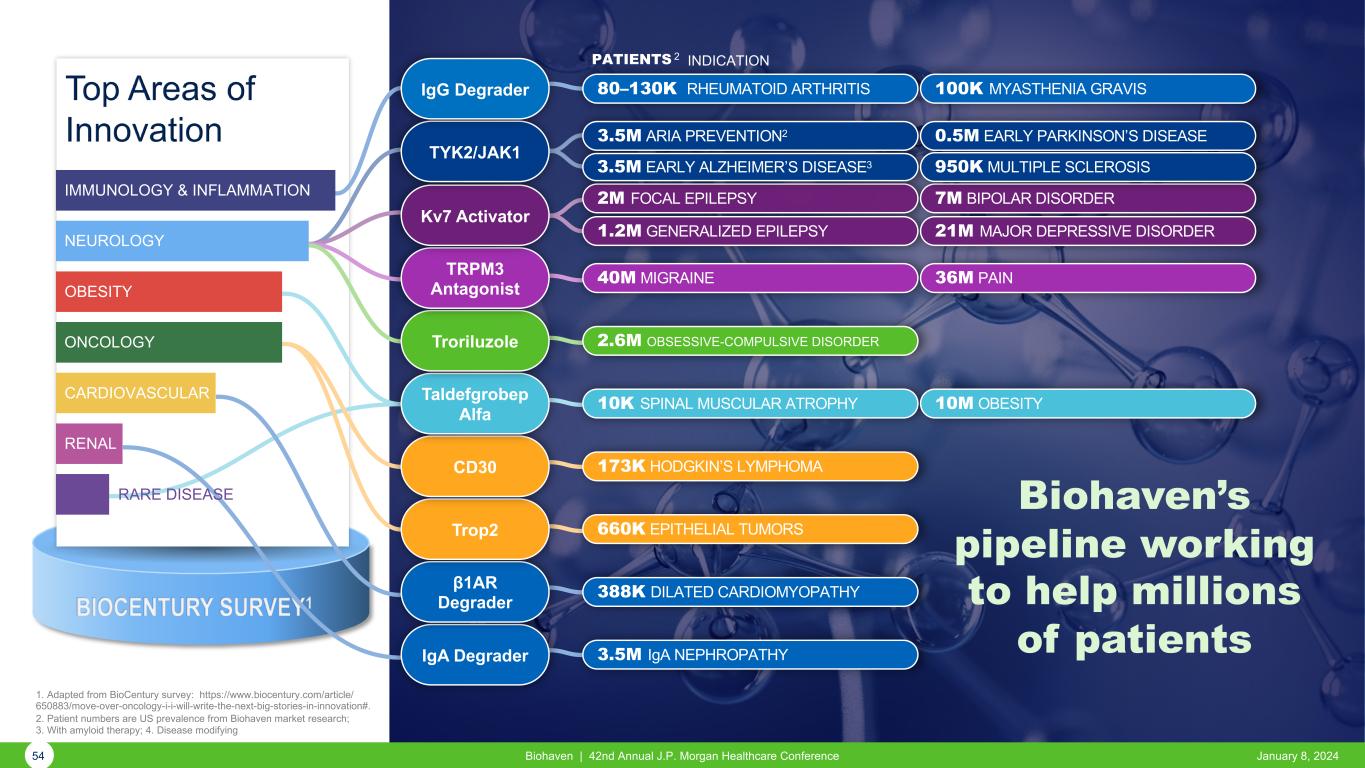
BIOCENTURY SURVEY1 1. Adapted from BioCentury survey: https://www.biocentury.com/article/ 650883/move-over-oncology-i-i-will-write-the-next-big-stories-in-innovation#. 2. Patient numbers are US prevalence from Biohaven market research; 3. With amyloid therapy; 4. Disease modifying PATIENTS 2 INDICATION IgA Degrader 3.5M IgA NEPHROPATHY RARE DISEASE RENAL CARDIOVASCULAR ONCOLOGY OBESITY NEUROLOGY IMMUNOLOGY & INFLAMMATION 54 Biohaven | 42nd Annual J.P. Morgan Healthcare Conference January 8, 2024 β1AR Degrader 388K DILATED CARDIOMYOPATHY Trop2 660K EPITHELIAL TUMORS CD30 173K HODGKIN’S LYMPHOMA Taldefgrobep Alfa 10K SPINAL MUSCULAR ATROPHY 10M OBESITY Troriluzole 2.6M OBSESSIVE-COMPULSIVE DISORDER TRPM3 Antagonist 40M MIGRAINE 36M PAIN Kv7 Activator 2M FOCAL EPILEPSY 7M BIPOLAR DISORDER 1.2M GENERALIZED EPILEPSY 21M MAJOR DEPRESSIVE DISORDER TYK2/JAK1 0.5M EARLY PARKINSON’S DISEASE3.5M ARIA PREVENTION2 950K MULTIPLE SCLEROSIS3.5M EARLY ALZHEIMER’S DISEASE3 80–130K RHEUMATOID ARTHRITIS 100K MYASTHENIA GRAVISIgG Degrader Biohaven’s pipeline working to help millions of patients

1Q 2024 2Q 2024 2H 2024 Troriluzole | BHV-4157 Obsessive-Compulsive Disorder Taldefgrobep Alfa | BHV-2000 Spinal Muscular Atrophy Obesity Kv7 Activator | BHV-7000 Focal Epilepsy Generalized Epilepsy Bipolar Disorder Major Depressive Disorder TRPM3 Antagonist | BHV-2100 Migraine Neuropathic Pain TYK2/JAK1 | BHV-8000 (brain-penetrant) Prevention of Amyloid Therapy Induced ARIA Early Alzheimer’s Disease Early Parkinson’s Disease Multiple Sclerosis IgG Degrader | BHV-1300 Rheumatoid Arthritis IgG Degrader | BHV-1310 Myasthenia Gravis IgA Degrader | BHV-1400 IgA Nephropathy β1-AR Degrader | BHV-1600 Dilated Cardiomyopathy CD30 | BHV-1500 Hodgkin’s Lymphoma Trop2 | BHV-1510 Carcinoma 2024 Milestones: Potential for Multiple Value Inflection Points Initiate Phase 2/3 Initiate Phase 2 Initiate Phase 2/3 Initiate Phase 2 Initiate Phase 2 Initiate Phase 2/3 Initiate Phase 2/3 Phase 1 IgG Lowering Data Initiate Phase 1 Initiate Phase 1 File IND Phase 3 IA Topline Initiate Phase 2 Phase 3 Topline Initiate Phase 2/3 Initiate Phase 1 Initiate POC Initiate Phase 2a January 8, 2024Biohaven | 42nd Annual J.P. Morgan Healthcare Conference55 Initiate Phase 1 Database Lock

Our Commitment: Building Value for Patients and Shareholders