false000178534500017853452024-01-042024-01-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 04, 2024 |
Landos Biopharma, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39971 |
81-5085535 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
P.O. Box 11239 |
|
Blacksburg, Virginia |
|
24062 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 540 218-2232 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.01 per share |
|
LABP |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 4, 2024, Landos Biopharma, Inc. (the “Company”) updated its corporate presentation for use in meetings with investors, analysts and others. The presentation is available on the Company’s website and is furnished as Exhibit 99.1 hereto.
The information in this Item 7.01 and Exhibit 99.1 hereto are being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Landos Biopharma, Inc. |
|
|
|
|
Date: |
January 4, 2024 |
By: |
/s/ Gregory Oakes |
|
|
|
Gregory Oakes
President and Chief Executive Officer |

January 2024 Clinical stage biopharmaceutical company focused on developing first-in-class, oral therapeutics for autoimmune disease Corporate Overview Exhibit 99.1

Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Annual Report on Form 10-K for the year ended December 31, 2022. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this prospectus involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions.
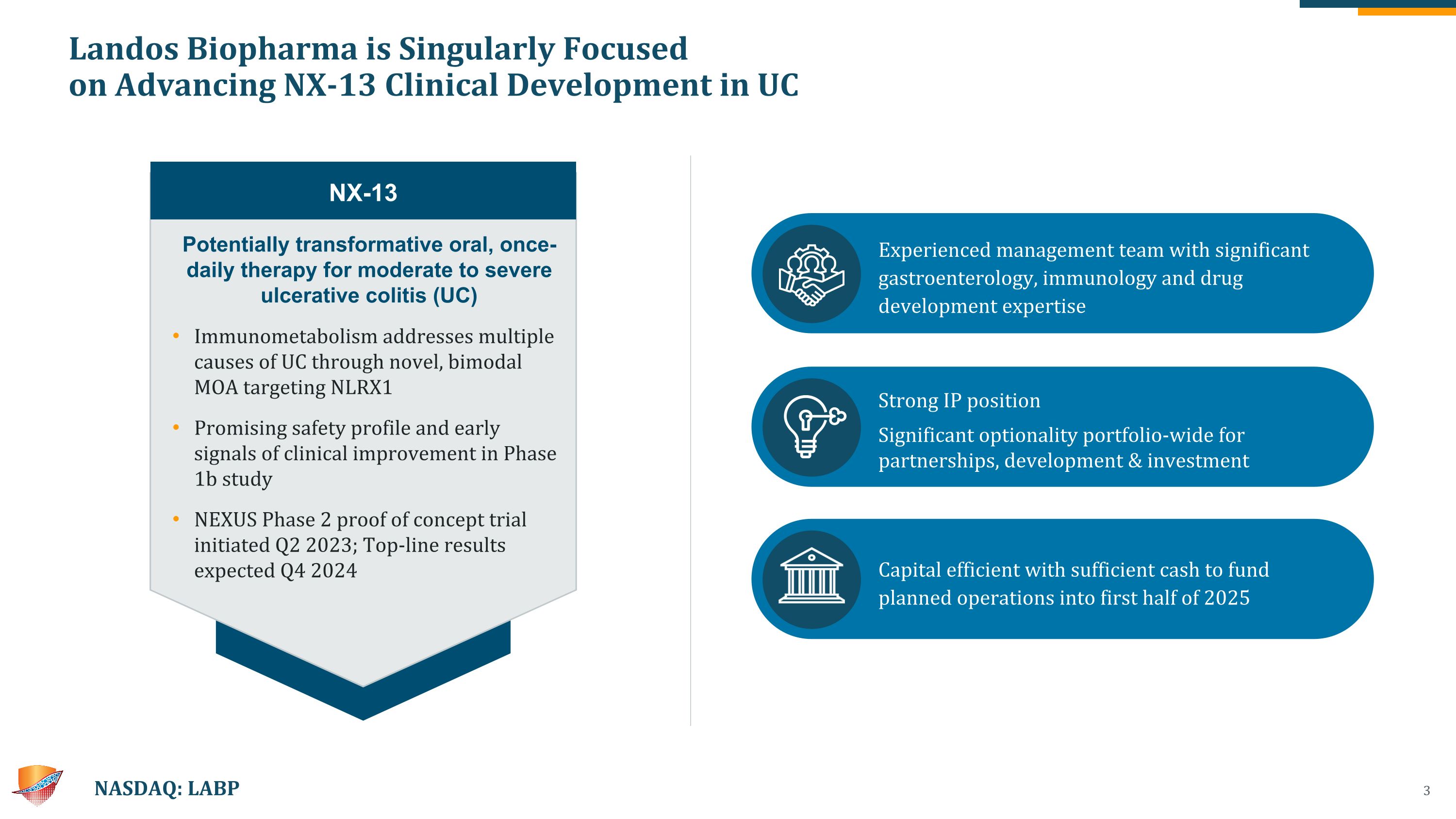
Landos Biopharma is Singularly Focused �on Advancing NX-13 Clinical Development in UC NASDAQ: LABP Potentially transformative oral, once-daily therapy for moderate to severe ulcerative colitis (UC) Immunometabolism addresses multiple causes of UC through novel, bimodal MOA targeting NLRX1 Promising safety profile and early signals of clinical improvement in Phase 1b study NEXUS Phase 2 proof of concept trial initiated Q2 2023; Top-line results expected Q4 2024 NX-13 Experienced management team with significant gastroenterology, immunology and drug development expertise Strong IP position Significant optionality portfolio-wide for partnerships, development & investment Capital efficient with sufficient cash to fund planned operations into first half of 2025
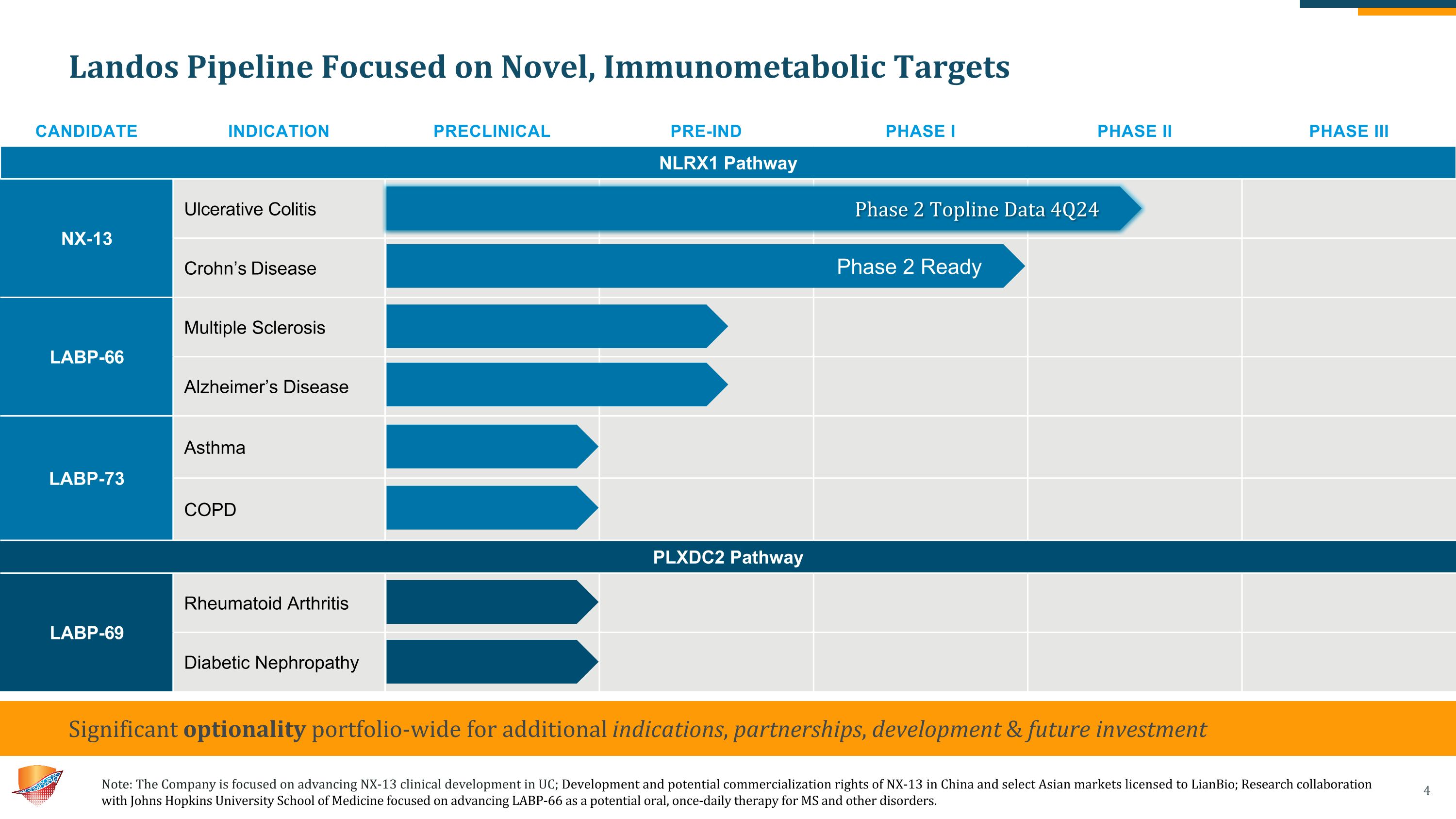
Landos Pipeline Focused on Novel, Immunometabolic Targets Need a footnote describing the relationship with JH, also likely need a footnote for LianBio CANDIDATE INDICATION PRECLINICAL PRE-IND PHASE I PHASE II PHASE III NLRX1 Pathway NX-13 Ulcerative Colitis Crohn’s Disease LABP-66 Multiple Sclerosis Alzheimer’s Disease LABP-73 Asthma COPD PLXDC2 Pathway LABP-69 Rheumatoid Arthritis Diabetic Nephropathy Significant optionality portfolio-wide for additional indications, partnerships, development & future investment Phase 2 Ready Phase 2 Topline Data 4Q24 Note: The Company is focused on advancing NX-13 clinical development in UC; Development and potential commercialization rights of NX-13 in China and select Asian markets licensed to LianBio; Research collaboration with Johns Hopkins University School of Medicine focused on advancing LABP-66 as a potential oral, once-daily therapy for MS and other disorders.
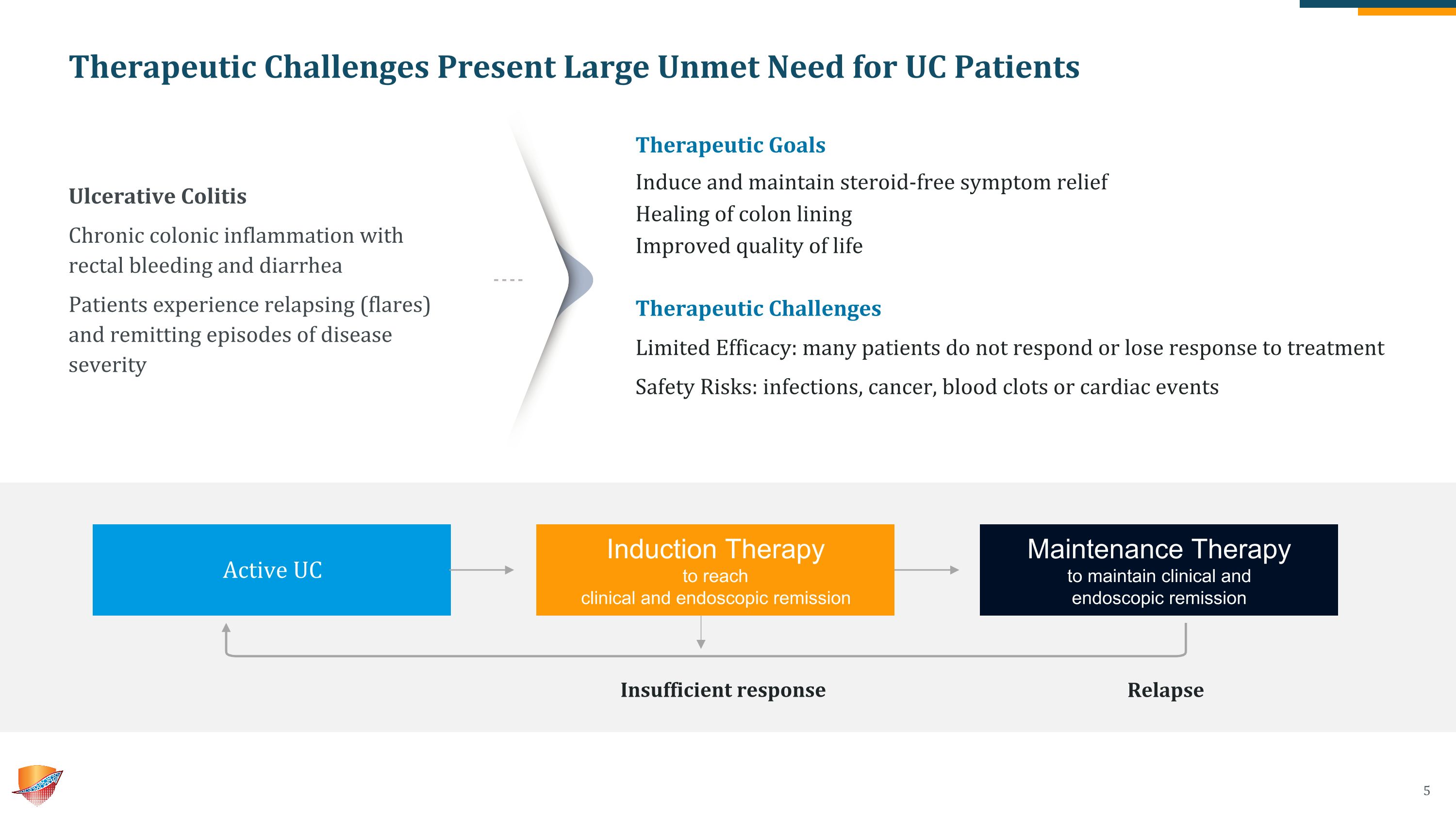
Therapeutic Challenges Present Large Unmet Need for UC Patients Relapse Ulcerative Colitis Chronic colonic inflammation with rectal bleeding and diarrhea Patients experience relapsing (flares) and remitting episodes of disease severity Therapeutic Goals Induce and maintain steroid-free symptom relief Healing of colon lining Improved quality of life Therapeutic Challenges Limited Efficacy: many patients do not respond or lose response to treatment Safety Risks: infections, cancer, blood clots or cardiac events Active UC Induction Therapy to reach �clinical and endoscopic remission Maintenance Therapy to maintain clinical and �endoscopic remission Insufficient response
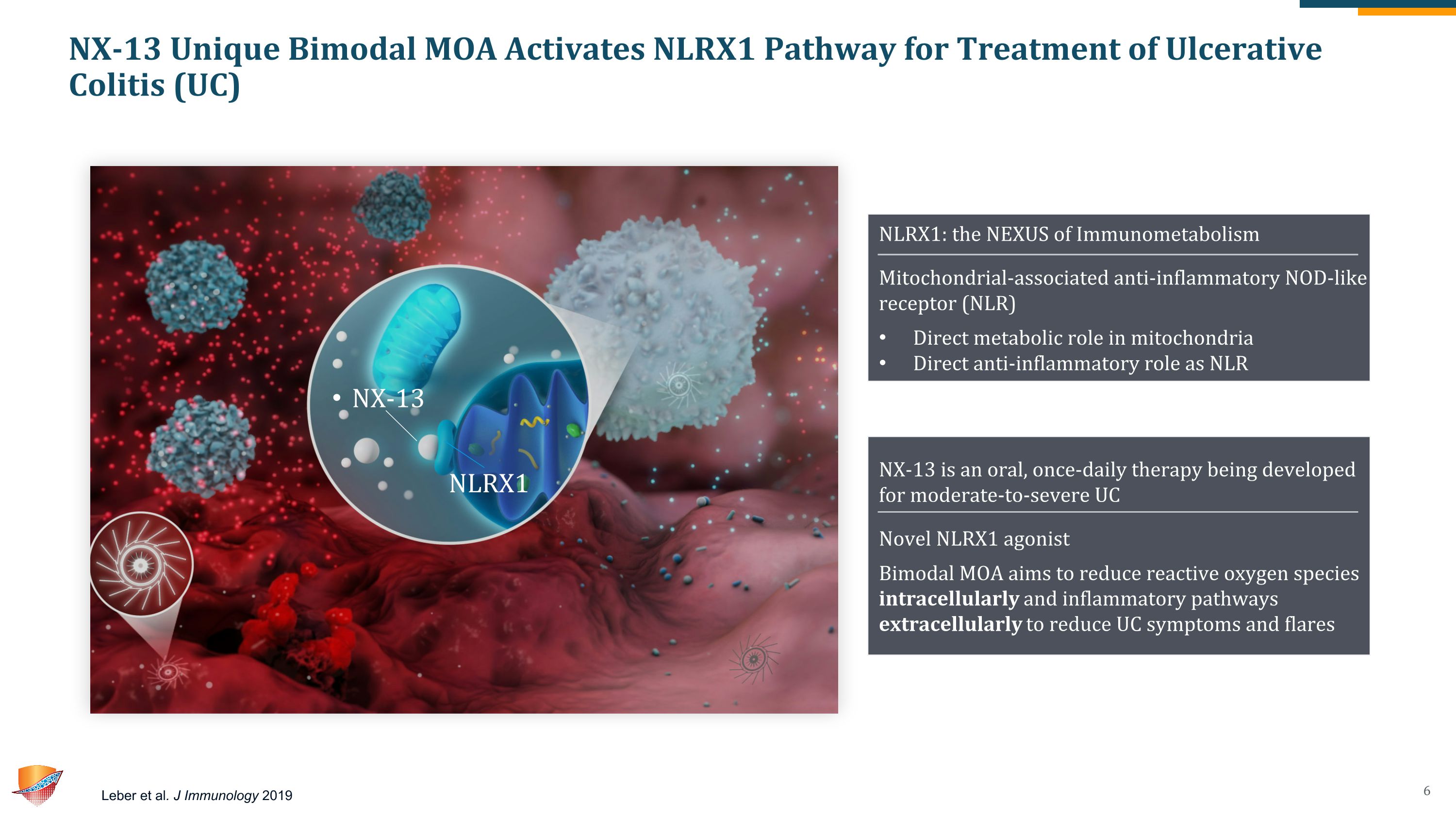
NX-13 Unique Bimodal MOA Activates NLRX1 Pathway for Treatment of Ulcerative Colitis (UC) Leber et al. J Immunology 2019 NX-13 is an oral, once-daily therapy being developed for moderate-to-severe UC Novel NLRX1 agonist Bimodal MOA aims to reduce reactive oxygen species intracellularly and inflammatory pathways extracellularly to reduce UC symptoms and flares NLRX1: the NEXUS of Immunometabolism Mitochondrial-associated anti-inflammatory NOD-like receptor (NLR) Direct metabolic role in mitochondria Direct anti-inflammatory role as NLR NX-13 NLRX1
Mechanism of Action
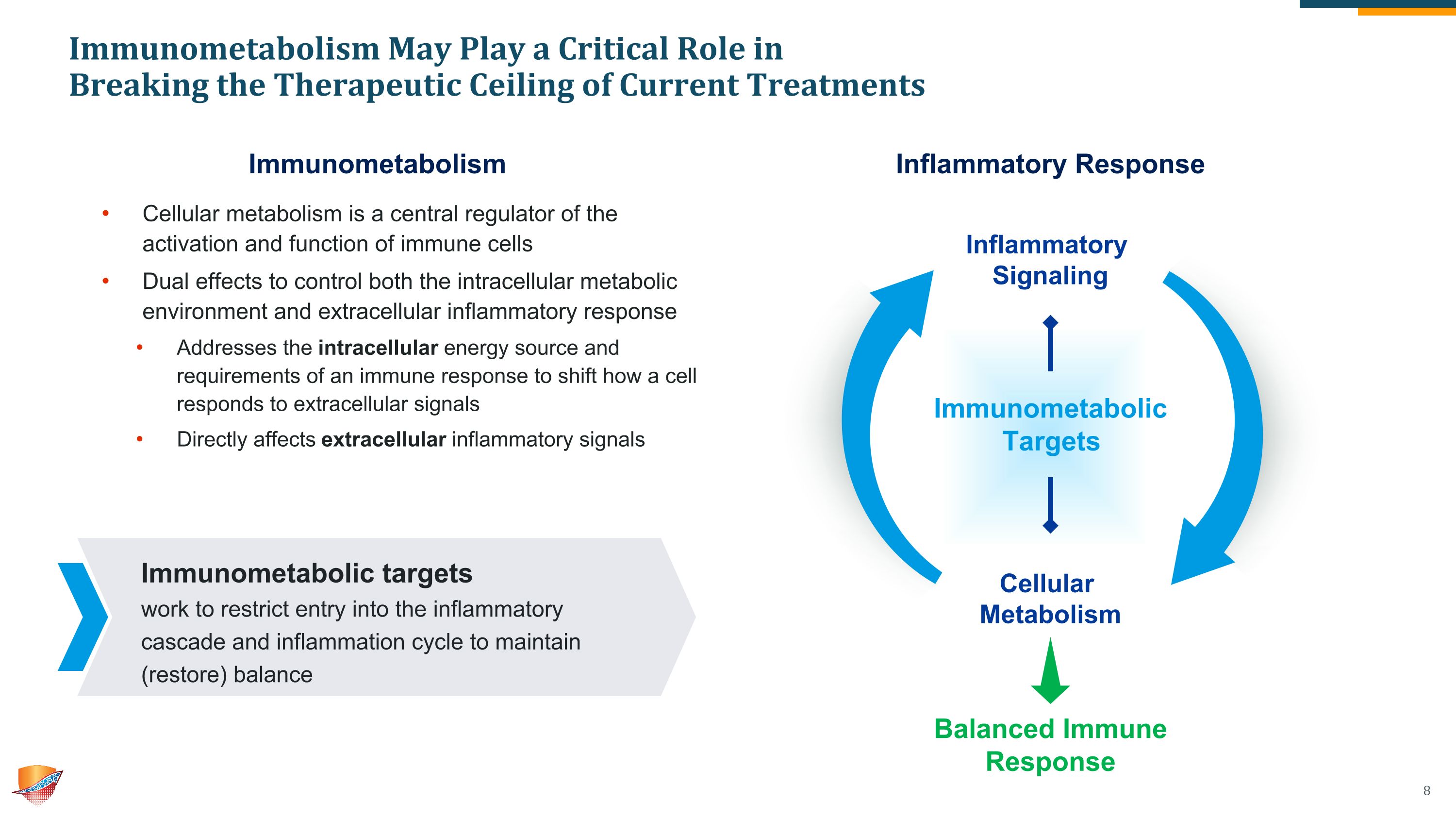
Inflammatory Signaling Cellular Metabolism Immunometabolism May Play a Critical Role in �Breaking the Therapeutic Ceiling of Current Treatments Cellular metabolism is a central regulator of the activation and function of immune cells Dual effects to control both the intracellular metabolic environment and extracellular inflammatory response Addresses the intracellular energy source and requirements of an immune response to shift how a cell responds to extracellular signals Directly affects extracellular inflammatory signals Immunometabolic targets �work to restrict entry into the inflammatory cascade and inflammation cycle to maintain (restore) balance Immunometabolism Inflammatory Response Immunometabolic Targets Balanced Immune Response
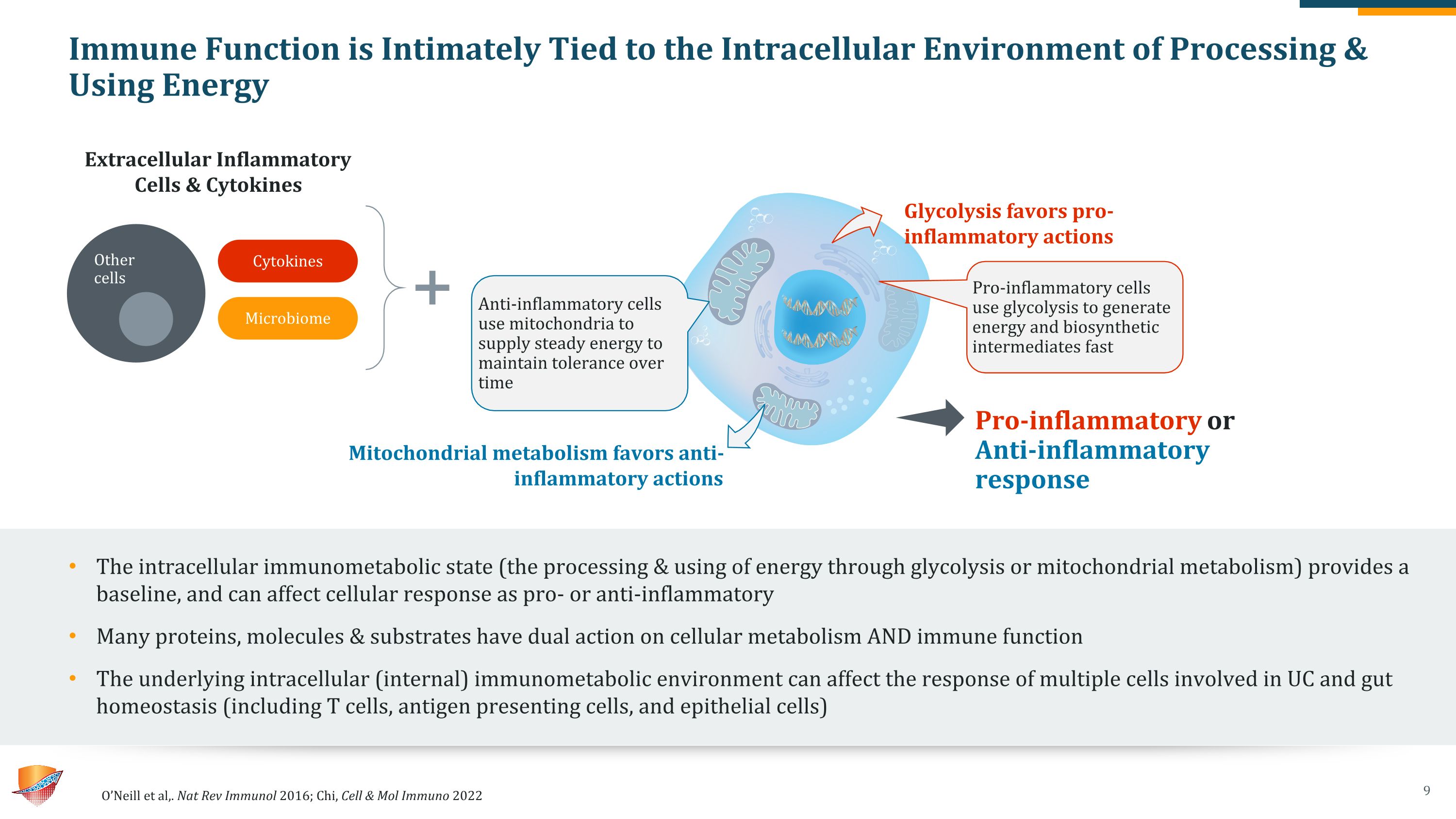
Immune Function is Intimately Tied to the Intracellular Environment of Processing & Using Energy The intracellular immunometabolic state (the processing & using of energy through glycolysis or mitochondrial metabolism) provides a baseline, and can affect cellular response as pro- or anti-inflammatory Many proteins, molecules & substrates have dual action on cellular metabolism AND immune function The underlying intracellular (internal) immunometabolic environment can affect the response of multiple cells involved in UC and gut homeostasis (including T cells, antigen presenting cells, and epithelial cells) O’Neill et al,. Nat Rev Immunol 2016; Chi, Cell & Mol Immuno 2022 Pro-inflammatory cells use glycolysis to generate energy and biosynthetic intermediates fast Anti-inflammatory cells use mitochondria to supply steady energy to maintain tolerance over time Glycolysis favors pro-inflammatory actions Extracellular Inflammatory Cells & Cytokines Mitochondrial metabolism favors anti-inflammatory actions Pro-inflammatory or Anti-inflammatory response Cytokines Microbiome Other cells
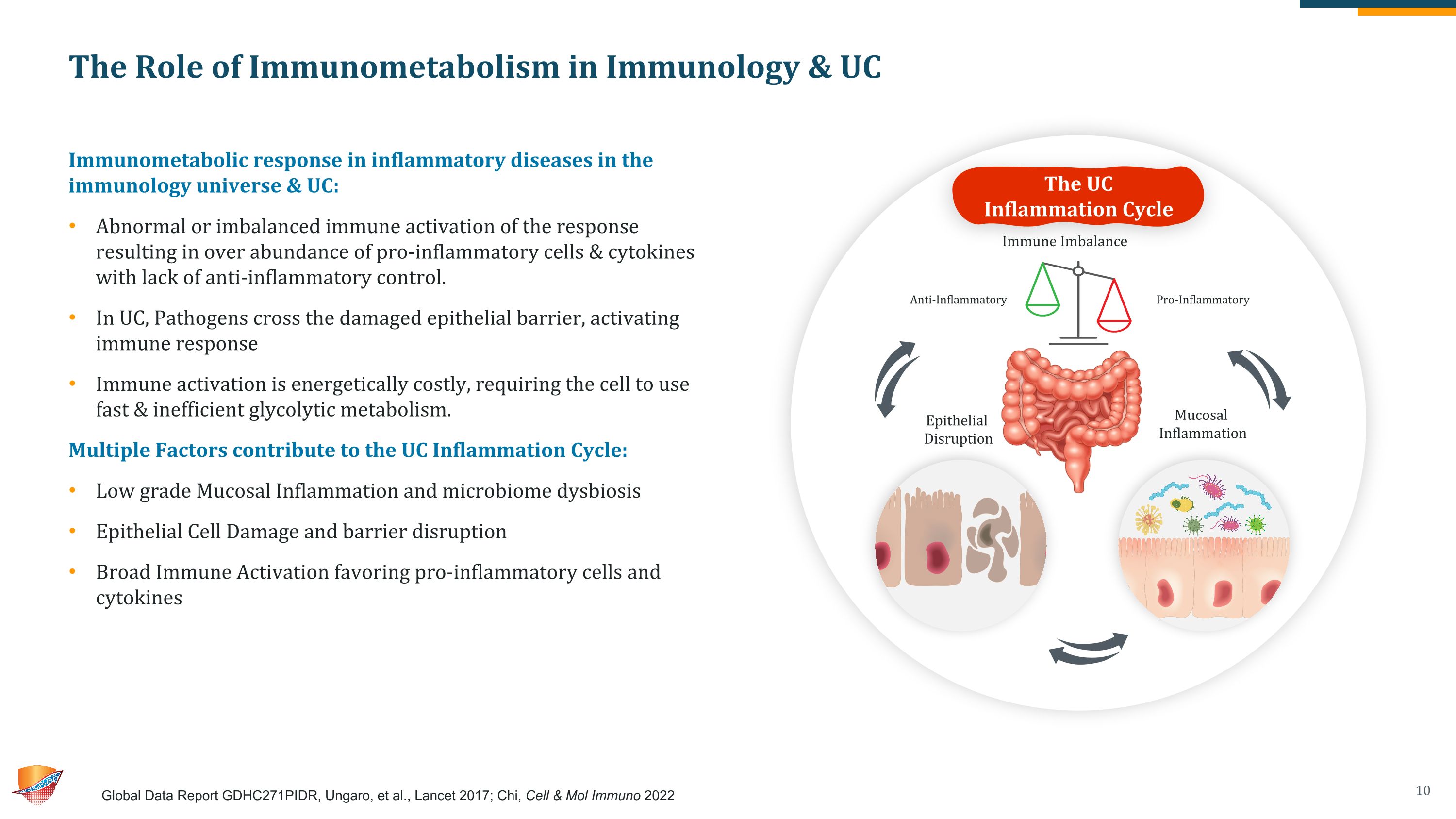
The Role of Immunometabolism in Immunology & UC Immunometabolic response in inflammatory diseases in the immunology universe & UC: Abnormal or imbalanced immune activation of the response resulting in over abundance of pro-inflammatory cells & cytokines with lack of anti-inflammatory control. In UC, Pathogens cross the damaged epithelial barrier, activating immune response Immune activation is energetically costly, requiring the cell to use fast & inefficient glycolytic metabolism. Multiple Factors contribute to the UC Inflammation Cycle: Low grade Mucosal Inflammation and microbiome dysbiosis Epithelial Cell Damage and barrier disruption Broad Immune Activation favoring pro-inflammatory cells and cytokines Global Data Report GDHC271PIDR, Ungaro, et al., Lancet 2017; Chi, Cell & Mol Immuno 2022 The UC Inflammation Cycle Immune Imbalance Epithelial Disruption Mucosal Inflammation Anti-Inflammatory Pro-Inflammatory
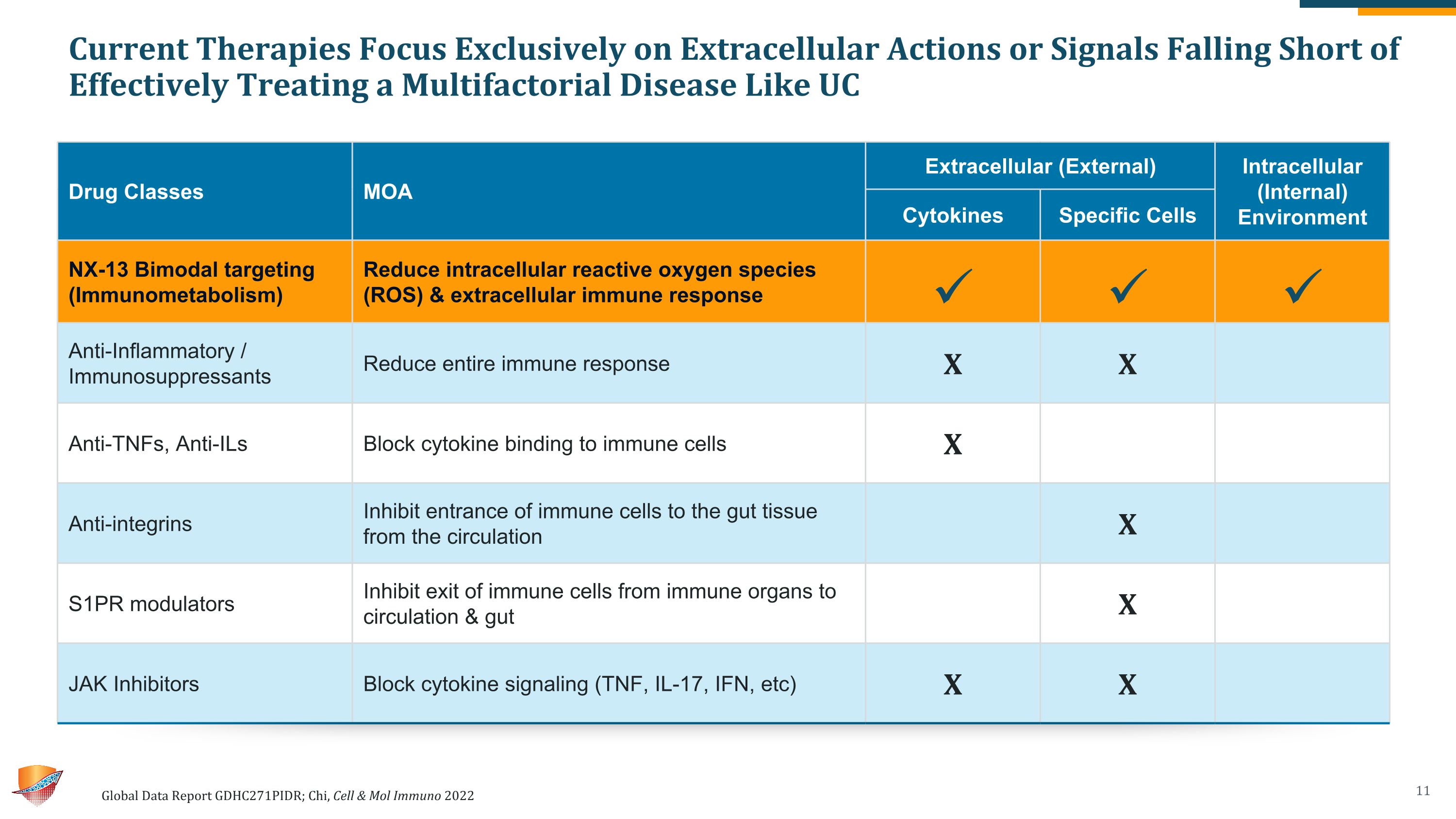
Current Therapies Focus Exclusively on Extracellular Actions or Signals Falling Short of Effectively Treating a Multifactorial Disease Like UC Global Data Report GDHC271PIDR; Chi, Cell & Mol Immuno 2022 Drug Classes MOA Extracellular (External) Intracellular (Internal) Environment Drug Classes MOA Cytokines Specific Cells Intracellular (Internal) Environment NX-13 Bimodal targeting (Immunometabolism) Reduce intracellular reactive oxygen species (ROS) & extracellular immune response Anti-Inflammatory / Immunosuppressants Reduce entire immune response X X Anti-TNFs, Anti-ILs Block cytokine binding to immune cells X Anti-integrins Inhibit entrance of immune cells to the gut tissue from the circulation X S1PR modulators Inhibit exit of immune cells from immune organs to circulation & gut X JAK Inhibitors Block cytokine signaling (TNF, IL-17, IFN, etc) X X
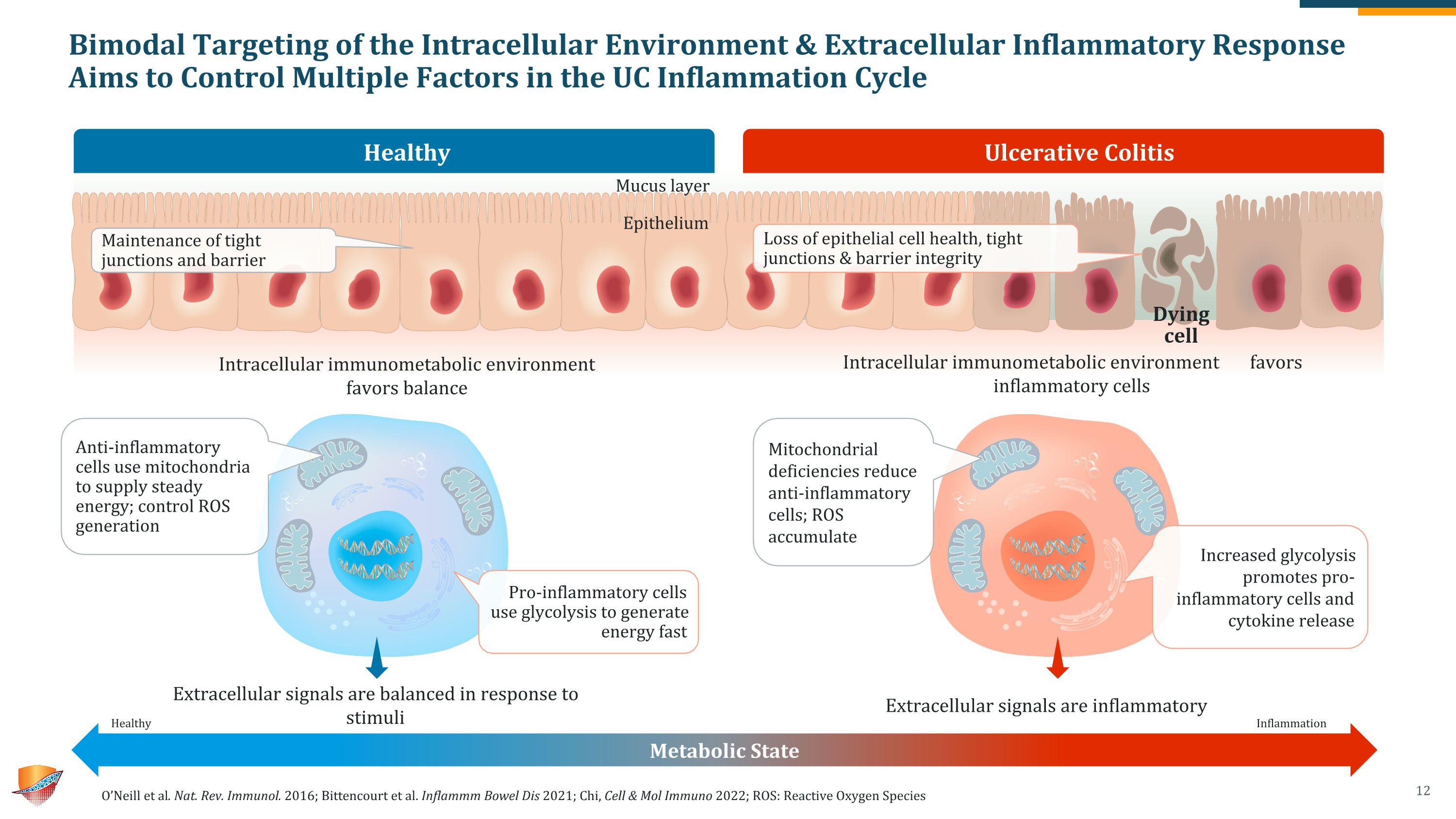
Bimodal Targeting of the Intracellular Environment & Extracellular Inflammatory Response Aims to Control Multiple Factors in the UC Inflammation Cycle O’Neill et al. Nat. Rev. Immunol. 2016; Bittencourt et al. Inflammm Bowel Dis 2021; Chi, Cell & Mol Immuno 2022; ROS: Reactive Oxygen Species Metabolic State Extracellular signals are balanced in response to stimuli Pro-inflammatory cells use glycolysis to generate energy fast Anti-inflammatory cells use mitochondria to supply steady energy; control ROS generation Intracellular immunometabolic environment favors inflammatory cells Increased glycolysis promotes pro-inflammatory cells and cytokine release Intracellular immunometabolic environment favors balance Extracellular signals are inflammatory Maintenance of tight junctions and barrier Loss of epithelial cell health, tight junctions & barrier integrity Healthy Ulcerative Colitis Inflammation Healthy Dying cell Mucus layer Epithelium Mitochondrial deficiencies reduce anti-inflammatory cells; ROS accumulate
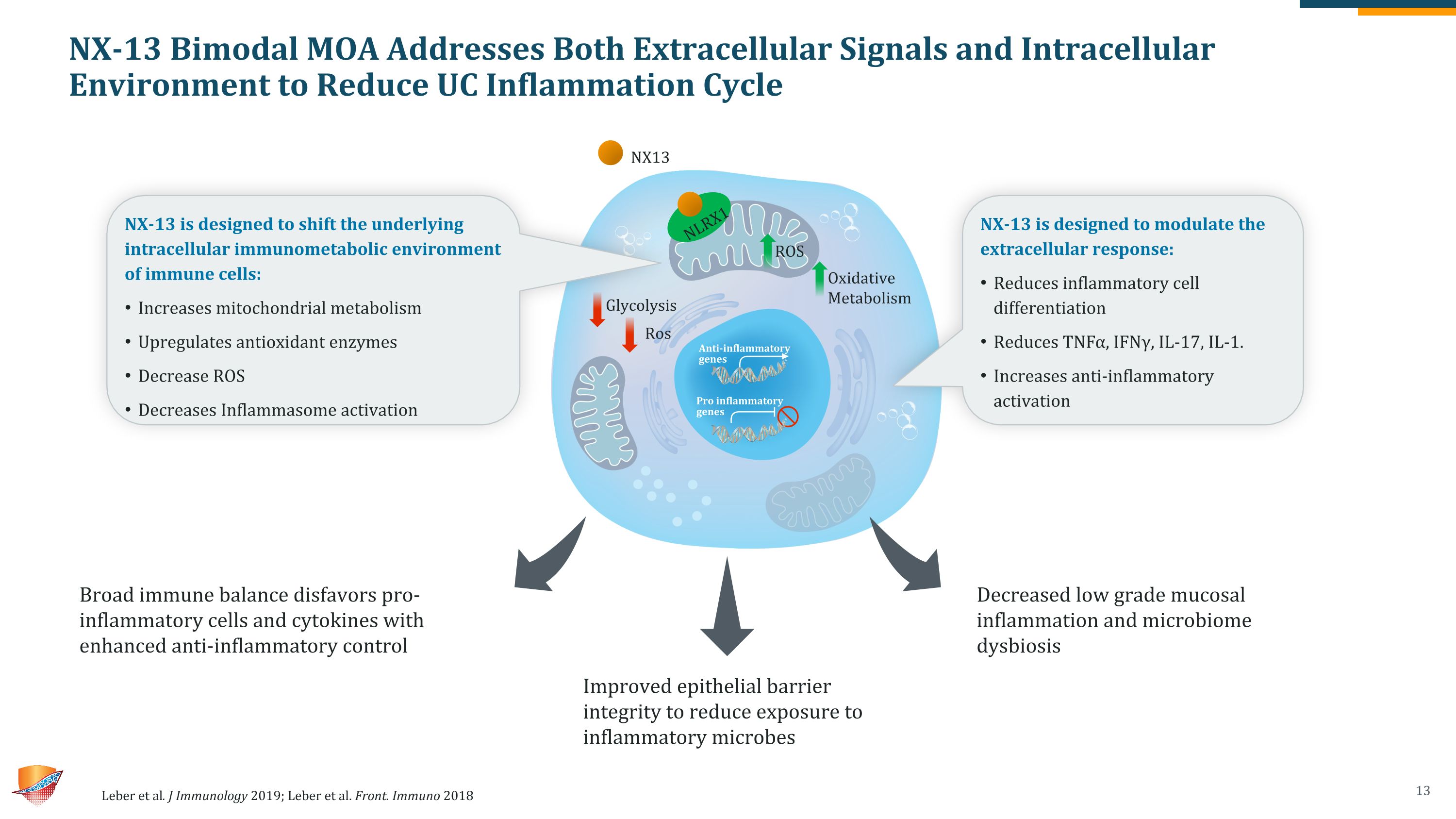
NX-13 Bimodal MOA Addresses Both Extracellular Signals and Intracellular Environment to Reduce UC Inflammation Cycle Leber et al. J Immunology 2019; Leber et al. Front. Immuno 2018 Broad immune balance disfavors pro-inflammatory cells and cytokines with enhanced anti-inflammatory control Improved epithelial barrier integrity to reduce exposure to inflammatory microbes Decreased low grade mucosal inflammation and microbiome dysbiosis NX-13 is designed to shift the underlying intracellular immunometabolic environment of immune cells: Increases mitochondrial metabolism Upregulates antioxidant enzymes Decrease ROS Decreases Inflammasome activation NX-13 is designed to modulate the extracellular response: Reduces inflammatory cell differentiation Reduces TNFα, IFNγ, IL-17, IL-1. Increases anti-inflammatory activation NX13 ROS Oxidative Metabolism Glycolysis Ros Anti-inflammatory genes Pro inflammatory genes NLRX1
NX-13 Pre-Clinical / Clinical Data & Phase 2 Trial Design
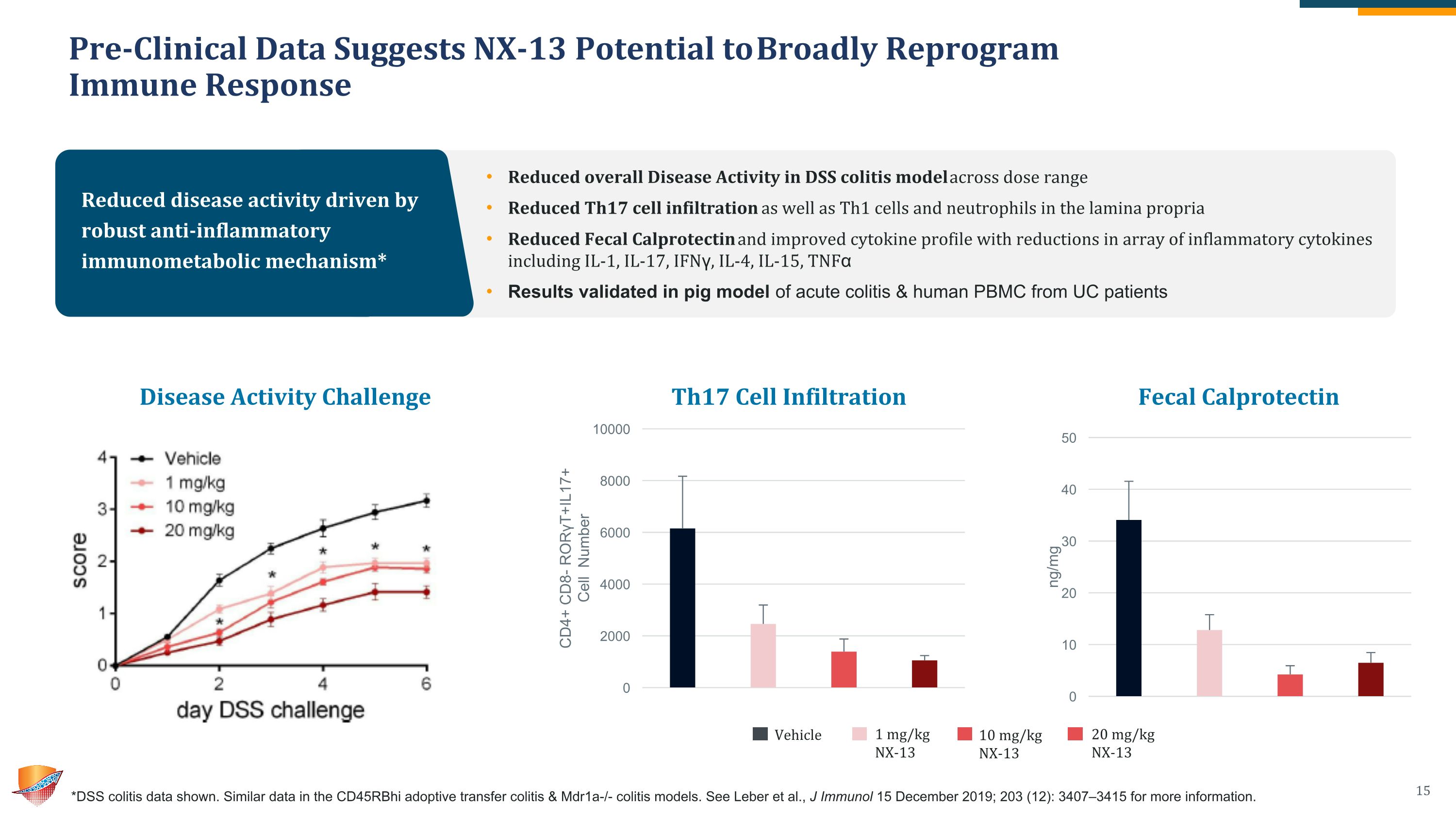
Pre-Clinical Data Suggests NX-13 Potential to Broadly Reprogram �Immune Response *DSS colitis data shown. Similar data in the CD45RBhi adoptive transfer colitis & Mdr1a-/- colitis models. See Leber et al., J Immunol 15 December 2019; 203 (12): 3407–3415 for more information. Reduced disease activity driven by robust anti-inflammatory immunometabolic mechanism* Reduced overall Disease Activity in DSS colitis model across dose range Reduced Th17 cell infiltration as well as Th1 cells and neutrophils in the lamina propria Reduced Fecal Calprotectin and improved cytokine profile with reductions in array of inflammatory cytokines including IL-1, IL-17, IFNγ, IL-4, IL-15, TNFα Results validated in pig model of acute colitis & human PBMC from UC patients Disease Activity Challenge Vehicle 1 mg/kg �NX-13 10 mg/kg�NX-13 20 mg/kg�NX-13 Th17 Cell Infiltration Fecal Calprotectin
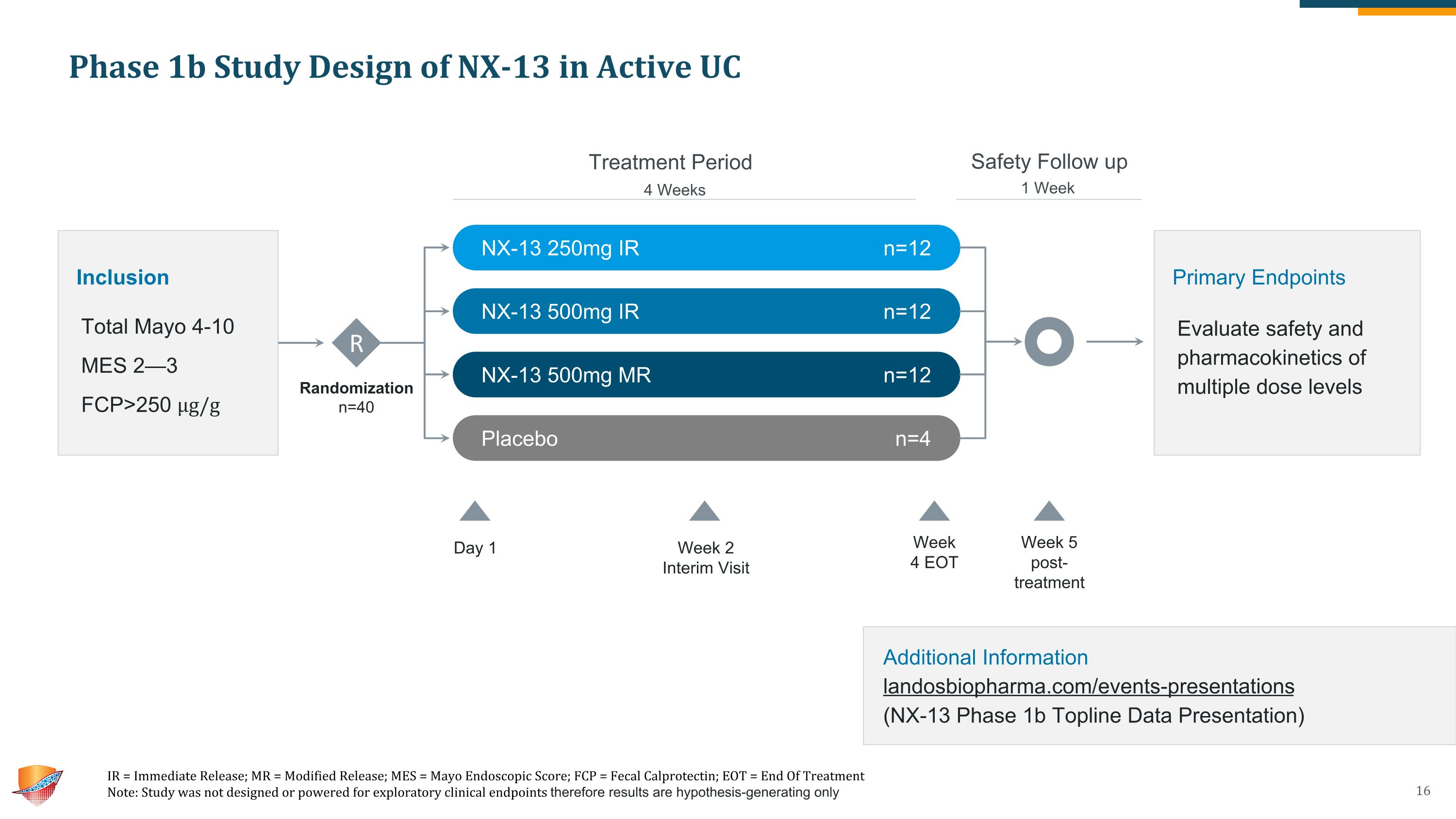
Phase 1b Study Design of NX-13 in Active UC IR = Immediate Release; MR = Modified Release; MES = Mayo Endoscopic Score; FCP = Fecal Calprotectin; EOT = End Of Treatment Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only 4 Weeks 1 Week Additional Information�landosbiopharma.com/events-presentations �(NX-13 Phase 1b Topline Data Presentation) Treatment Period Safety Follow up Randomization n=40 Inclusion Total Mayo 4-10 MES 2—3 FCP>250 µg/g R NX-13 250mg IR n=12 NX-13 500mg IR n=12 NX-13 500mg MR n=12 Placebo n=4 Week 2 Interim Visit Week �4 EOT Week 5 �post-�treatment Primary Endpoints Evaluate safety and pharmacokinetics of multiple dose levels Day 1
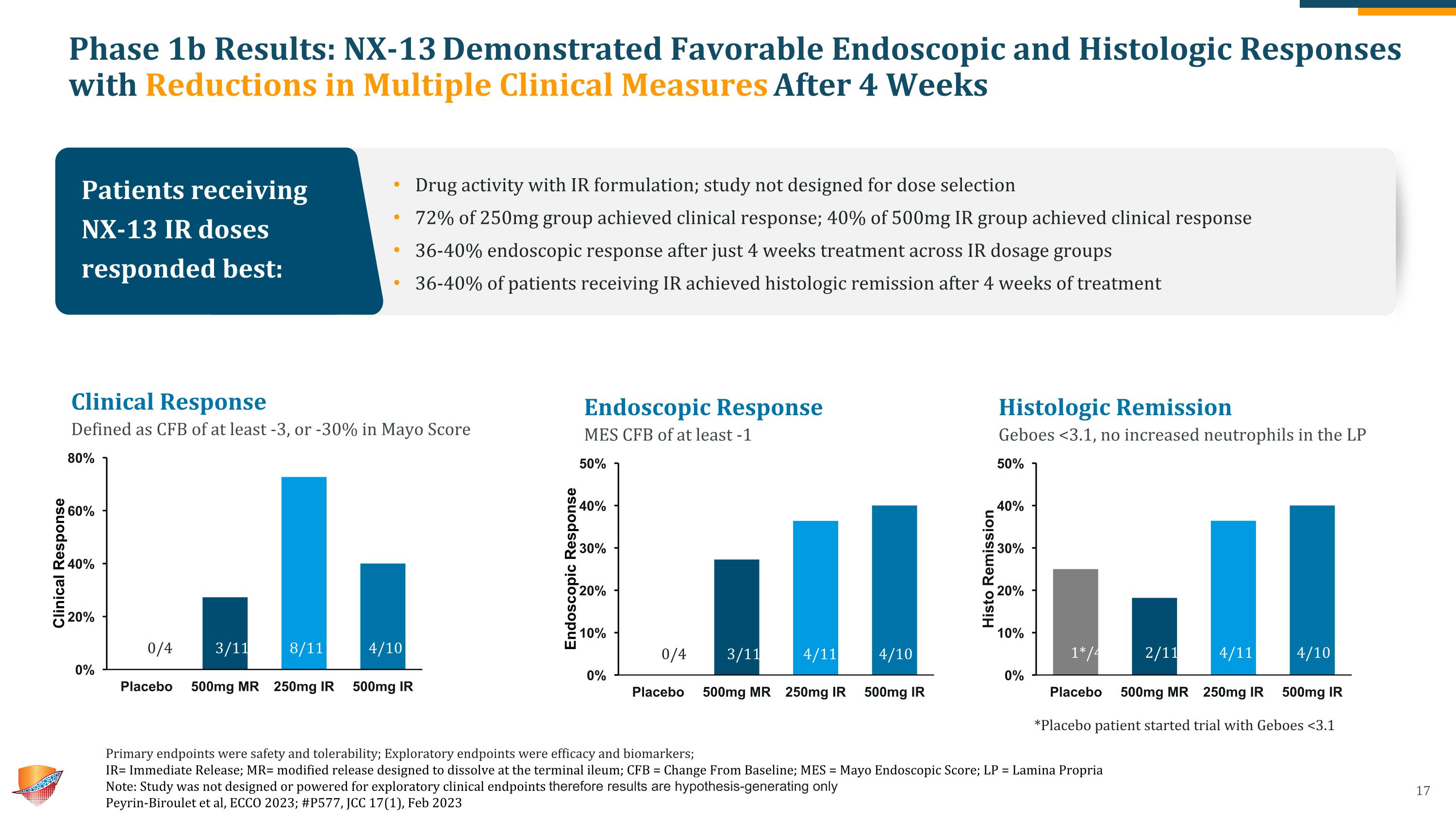
Phase 1b Results: NX-13 Demonstrated Favorable Endoscopic and Histologic Responses with Reductions in Multiple Clinical Measures After 4 Weeks Primary endpoints were safety and tolerability; Exploratory endpoints were efficacy and biomarkers; �IR= Immediate Release; MR= modified release designed to dissolve at the terminal ileum; CFB = Change From Baseline; MES = Mayo Endoscopic Score; LP = Lamina Propria Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Peyrin-Biroulet et al, ECCO 2023; #P577, JCC 17(1), Feb 2023 Endoscopic Response MES CFB of at least -1 Histologic Remission Geboes <3.1, no increased neutrophils in the LP 0/4 4/11 4/10 3/11 1*/4 4/11 4/10 2/11 Clinical Response Defined as CFB of at least -3, or -30% in Mayo Score 4/10 3/11 8/11 0/4 Patients receiving NX-13 IR doses responded best: Drug activity with IR formulation; study not designed for dose selection 72% of 250mg group achieved clinical response; 40% of 500mg IR group achieved clinical response 36-40% endoscopic response after just 4 weeks treatment across IR dosage groups 36-40% of patients receiving IR achieved histologic remission after 4 weeks of treatment *Placebo patient started trial with Geboes <3.1
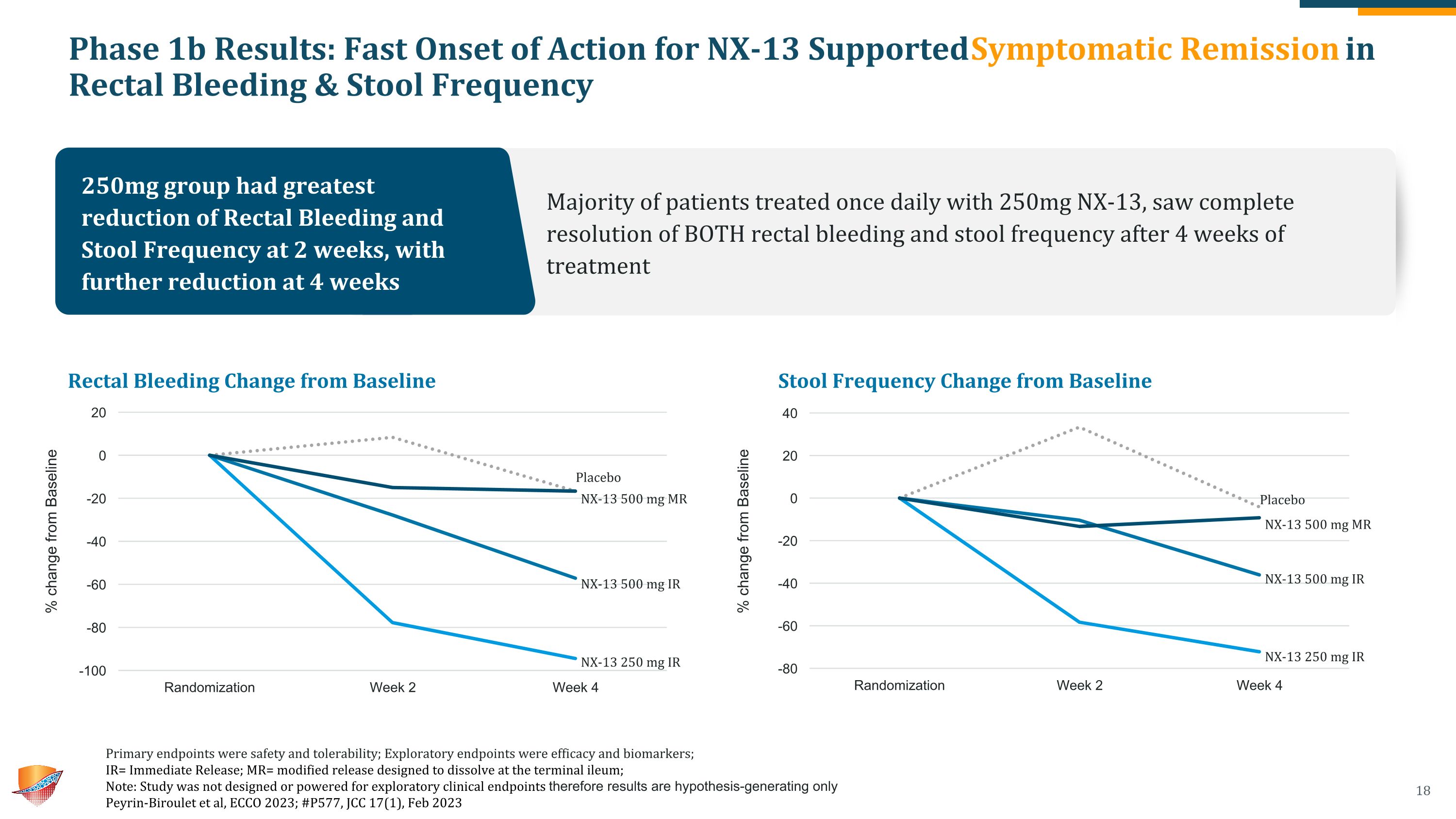
Phase 1b Results: Fast Onset of Action for NX-13 Supported Symptomatic Remission in Rectal Bleeding & Stool Frequency Rectal Bleeding Change from Baseline Stool Frequency Change from Baseline Placebo NX-13 500 mg MR NX-13 500 mg IR NX-13 250 mg IR Placebo NX-13 500 mg MR NX-13 500 mg IR NX-13 250 mg IR 250mg group had greatest reduction of Rectal Bleeding and Stool Frequency at 2 weeks, with further reduction at 4 weeks Majority of patients treated once daily with 250mg NX-13, saw complete resolution of BOTH rectal bleeding and stool frequency after 4 weeks of treatment Primary endpoints were safety and tolerability; Exploratory endpoints were efficacy and biomarkers; �IR= Immediate Release; MR= modified release designed to dissolve at the terminal ileum; Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Peyrin-Biroulet et al, ECCO 2023; #P577, JCC 17(1), Feb 2023
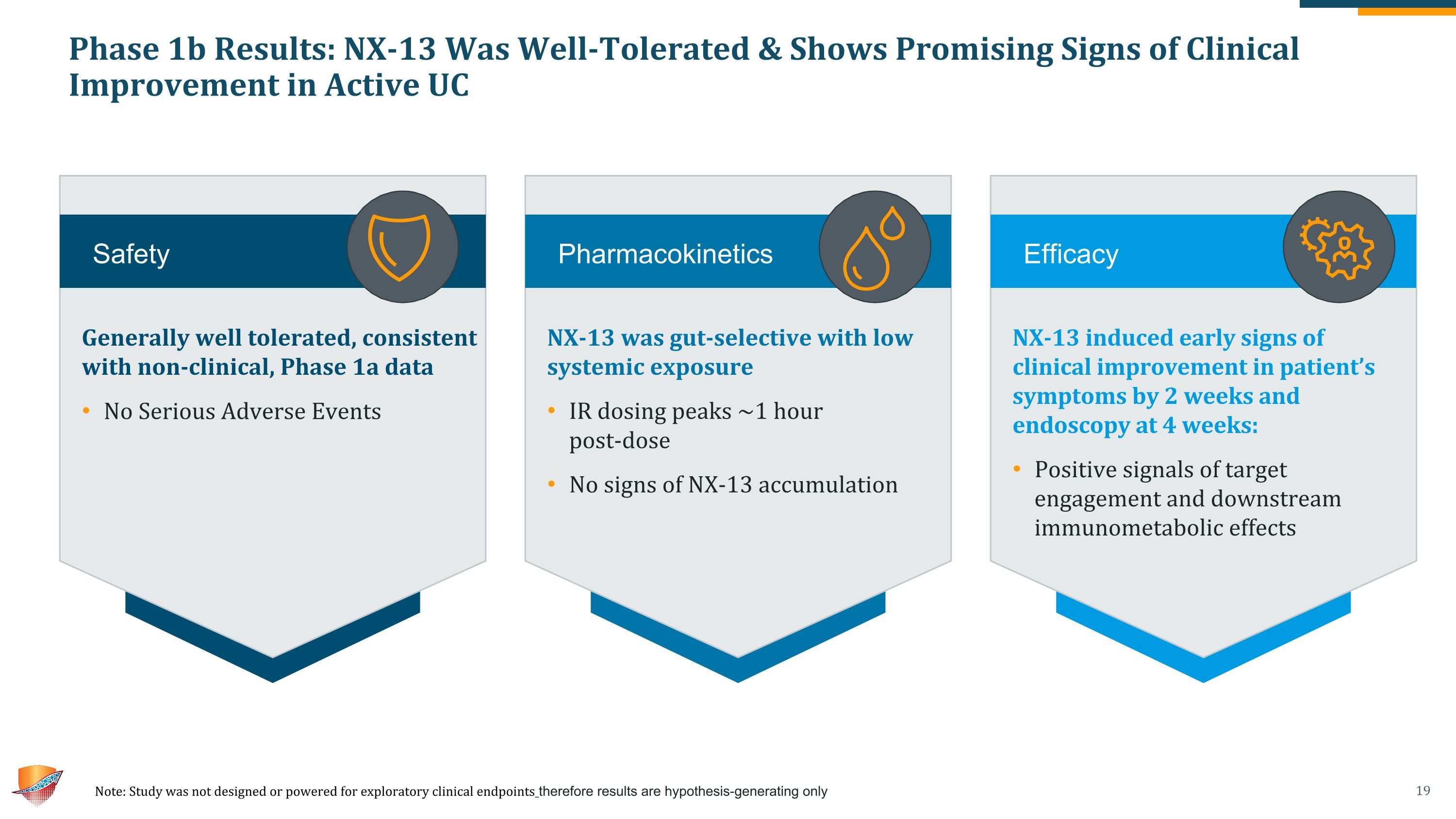
Phase 1b Results: NX-13 Was Well-Tolerated & Shows Promising Signs of Clinical Improvement in Active UC Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only NX-13 induced early signs of clinical improvement in patient’s symptoms by 2 weeks and endoscopy at 4 weeks: Positive signals of target engagement and downstream immunometabolic effects NX-13 was gut-selective with low systemic exposure IR dosing peaks ~1 hour �post-dose No signs of NX-13 accumulation Generally well tolerated, consistent with non-clinical, Phase 1a data No Serious Adverse Events Safety Efficacy Pharmacokinetics
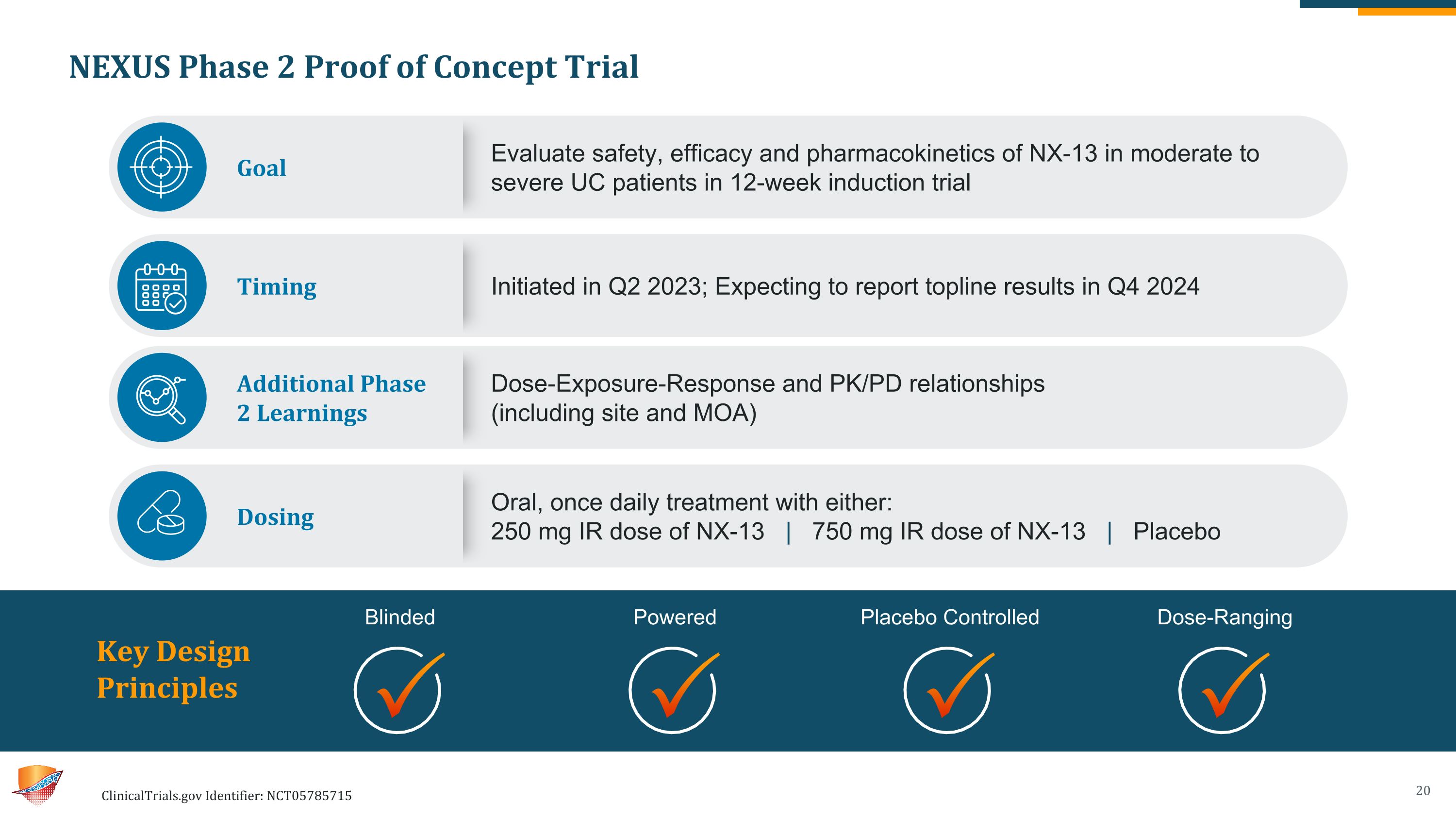
NEXUS Phase 2 Proof of Concept Trial ClinicalTrials.gov Identifier: NCT05785715 Key Design �Principles Blinded Powered Placebo Controlled Dose-Ranging Goal Evaluate safety, efficacy and pharmacokinetics of NX-13 in moderate to severe UC patients in 12-week induction trial Timing Initiated in Q2 2023; Expecting to report topline results in Q4 2024 Additional Phase �2 Learnings Dose-Exposure-Response and PK/PD relationships �(including site and MOA) Dosing Oral, once daily treatment with either: 250 mg IR dose of NX-13 | 750 mg IR dose of NX-13 | Placebo
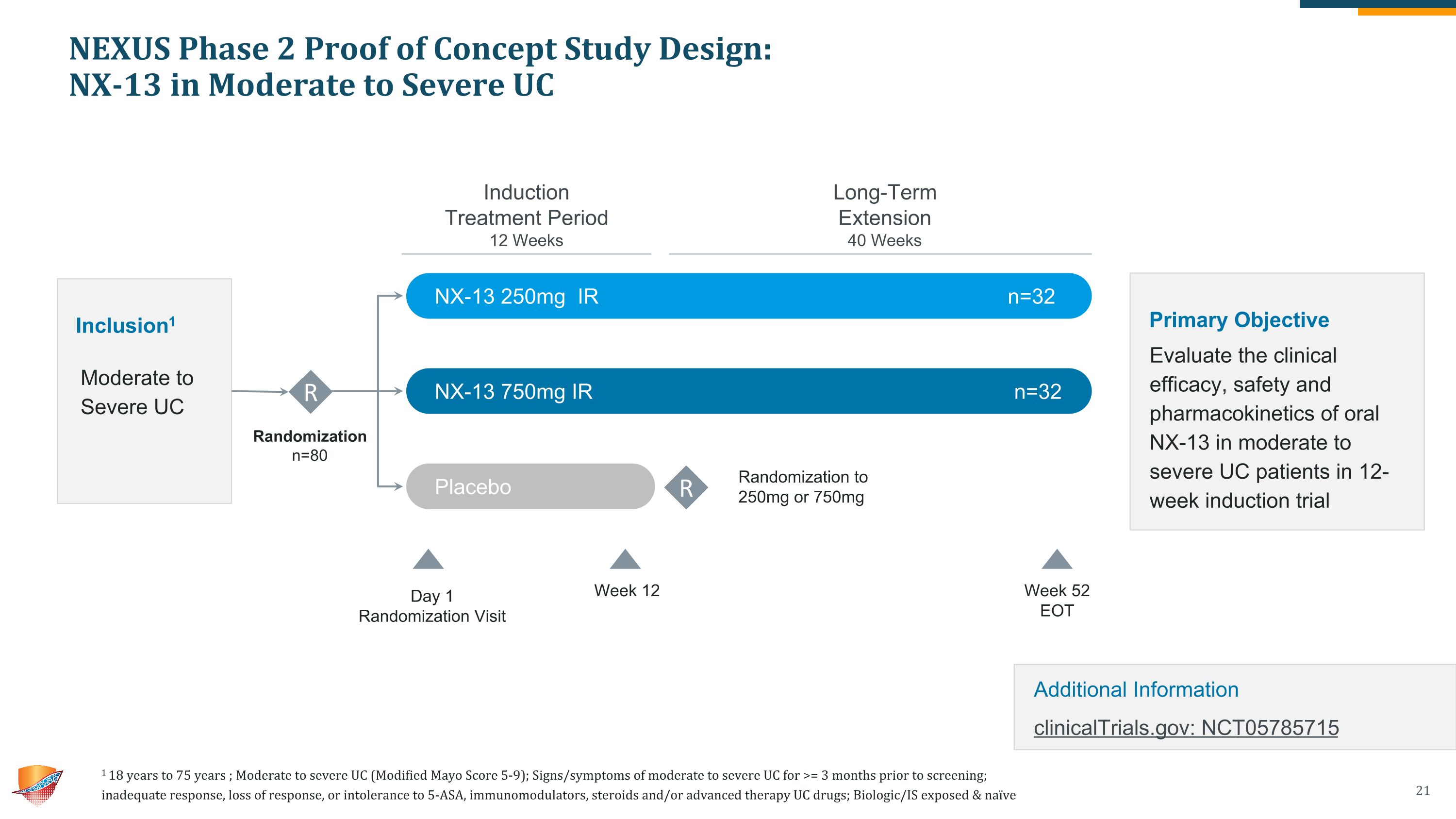
NEXUS Phase 2 Proof of Concept Study Design: �NX-13 in Moderate to Severe UC Randomization n=80 Inclusion1 Moderate to Severe UC R NX-13 250mg IR n=32 NX-13 750mg IR n=32 Placebo n=16 Week 12 Week 52 �EOT Day 1 Randomization Visit Induction �Treatment Period 12 Weeks Long-Term �Extension 40 Weeks R Randomization to 250mg or 750mg Primary Objective Evaluate the clinical efficacy, safety and pharmacokinetics of oral NX-13 in moderate to severe UC patients in 12-week induction trial Additional Information�clinicalTrials.gov: NCT05785715 1 18 years to 75 years ; Moderate to severe UC (Modified Mayo Score 5-9); Signs/symptoms of moderate to severe UC for >= 3 months prior to screening; �inadequate response, loss of response, or intolerance to 5-ASA, immunomodulators, steroids and/or advanced therapy UC drugs; Biologic/IS exposed & naïve

Market & NX-13 Positioning
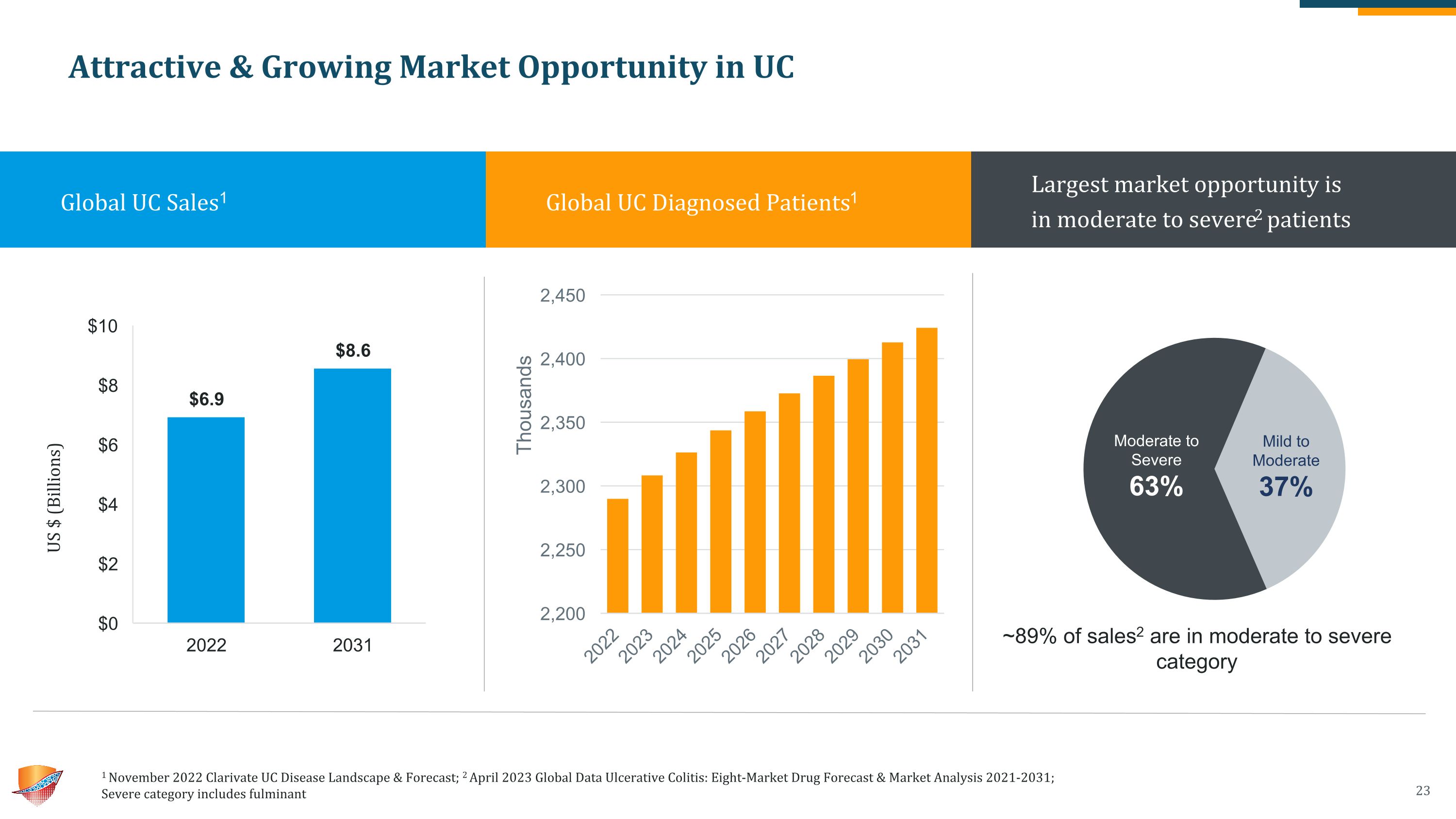
Attractive & Growing Market Opportunity in UC 1 November 2022 Clarivate UC Disease Landscape & Forecast; 2 April 2023 Global Data Ulcerative Colitis: Eight-Market Drug Forecast & Market Analysis 2021-2031;�Severe category includes fulminant Global UC Sales1 Global UC Diagnosed Patients1 Largest market opportunity is �in moderate to severe2 patients ~89% of sales2 are in moderate to severe category US $ (Billions)
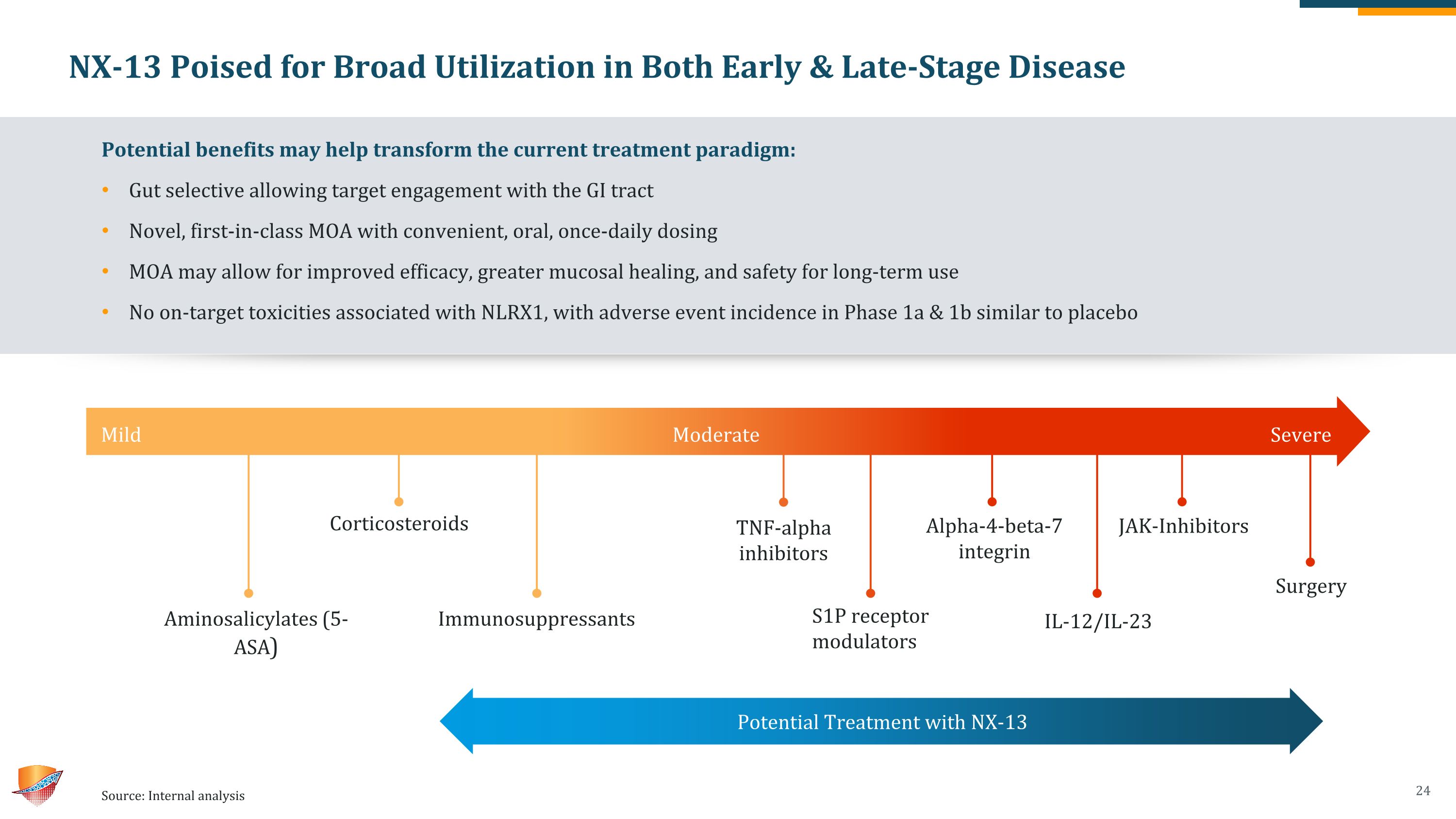
NX-13 Poised for Broad Utilization in Both Early & Late-Stage Disease Potential benefits may help transform the current treatment paradigm: Gut selective allowing target engagement with the GI tract Novel, first-in-class MOA with convenient, oral, once-daily dosing MOA may allow for improved efficacy, greater mucosal healing, and safety for long-term use No on-target toxicities associated with NLRX1, with adverse event incidence in Phase 1a & 1b similar to placebo Source: Internal analysis Aminosalicylates (5-ASA) Immunosuppressants Corticosteroids Alpha-4-beta-7 �integrin TNF-alpha inhibitors IL-12/IL-23 JAK-Inhibitors Mild Moderate Severe S1P receptor modulators Potential Treatment with NX-13 Surgery
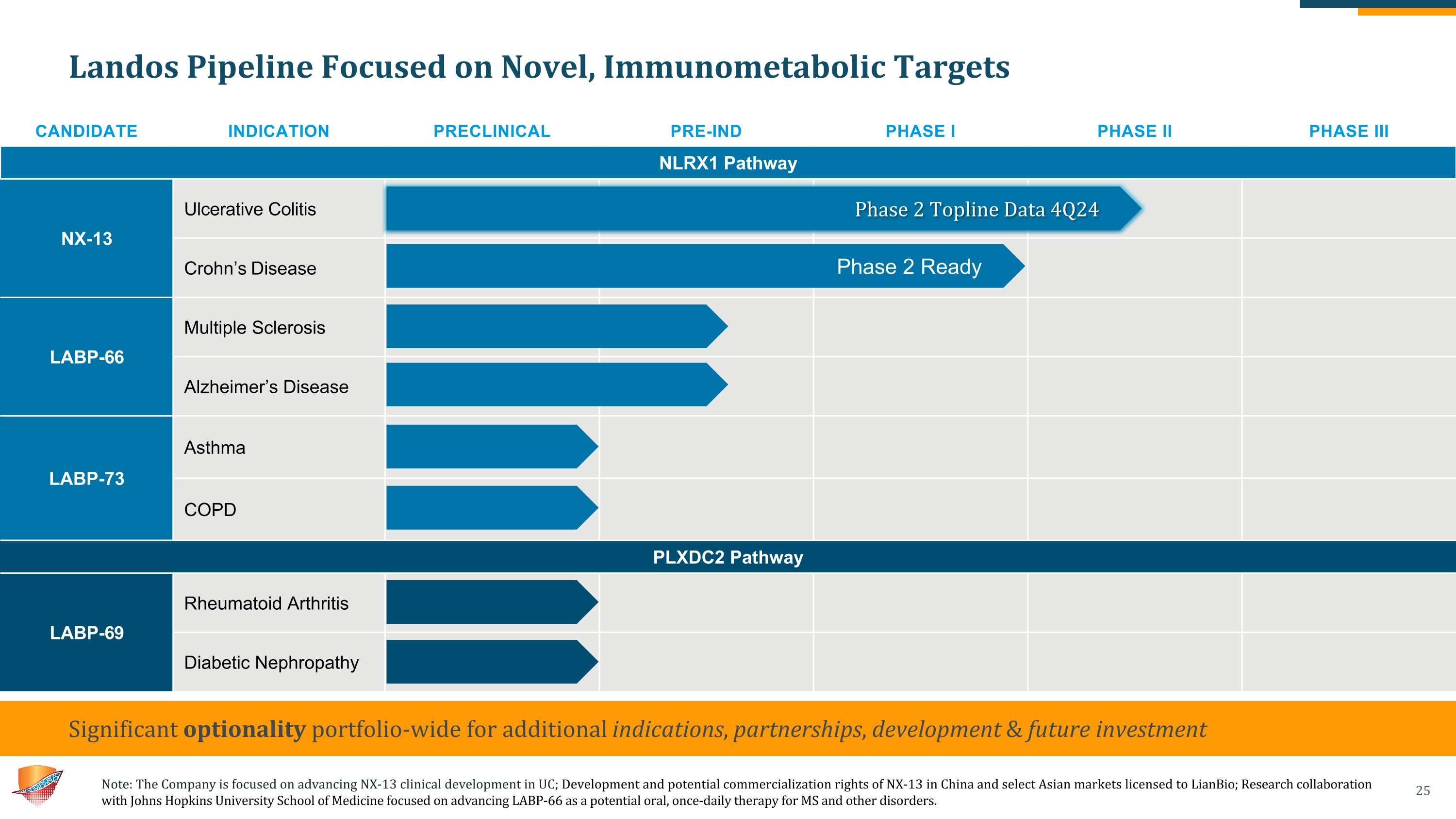
Landos Pipeline Focused on Novel, Immunometabolic Targets Need a footnote describing the relationship with JH, also likely need a footnote for LianBio CANDIDATE INDICATION PRECLINICAL PRE-IND PHASE I PHASE II PHASE III NLRX1 Pathway NX-13 Ulcerative Colitis Crohn’s Disease LABP-66 Multiple Sclerosis Alzheimer’s Disease LABP-73 Asthma COPD PLXDC2 Pathway LABP-69 Rheumatoid Arthritis Diabetic Nephropathy Significant optionality portfolio-wide for additional indications, partnerships, development & future investment Phase 2 Ready Phase 2 Topline Data 4Q24 Note: The Company is focused on advancing NX-13 clinical development in UC; Development and potential commercialization rights of NX-13 in China and select Asian markets licensed to LianBio; Research collaboration with Johns Hopkins University School of Medicine focused on advancing LABP-66 as a potential oral, once-daily therapy for MS and other disorders.
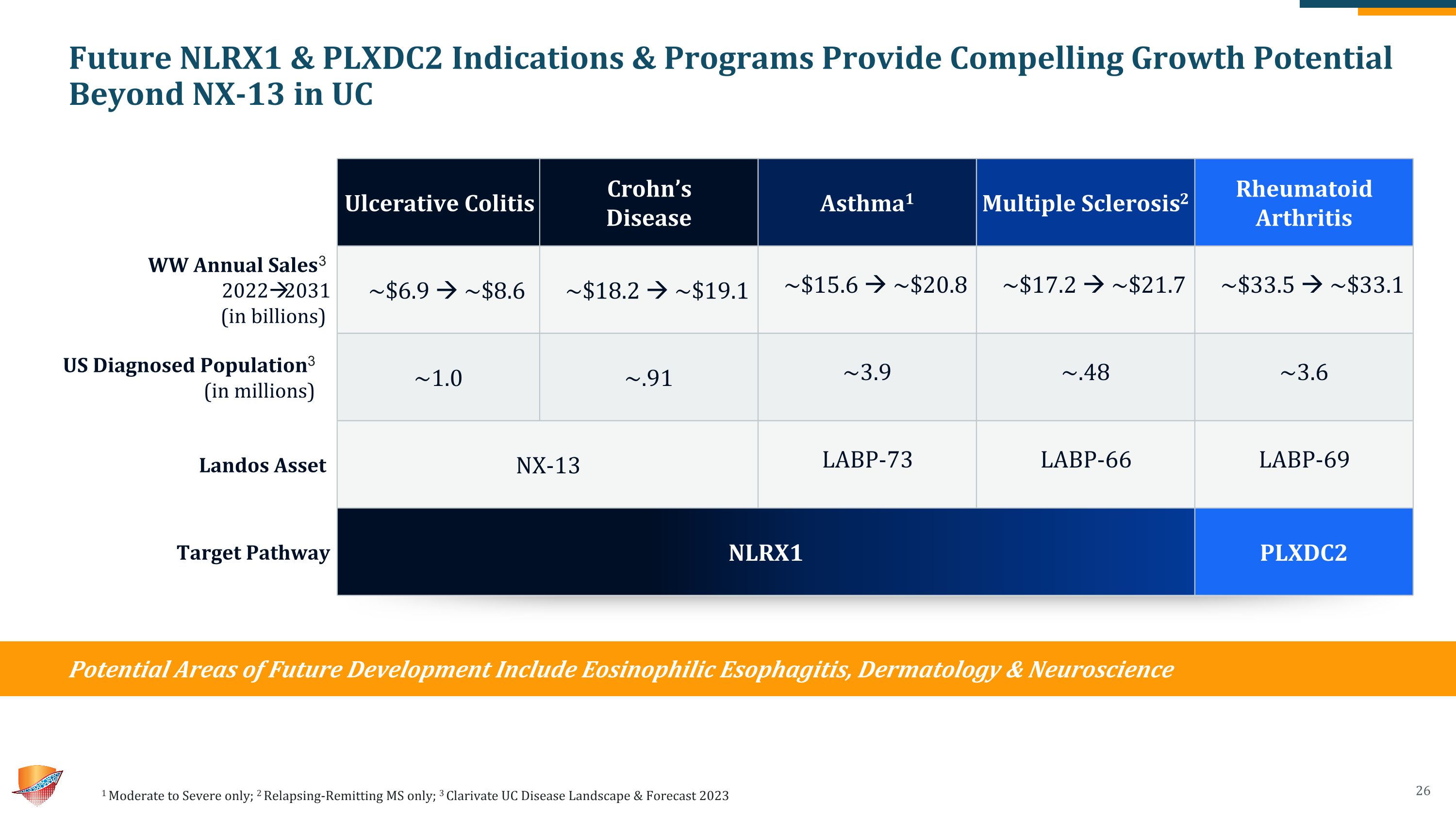
Future NLRX1 & PLXDC2 Indications & Programs Provide Compelling Growth Potential Beyond NX-13 in UC 1 Moderate to Severe only; 2 Relapsing-Remitting MS only; 3 Clarivate UC Disease Landscape & Forecast 2023 Ulcerative Colitis Crohn’s Disease Asthma1 Multiple Sclerosis2 Rheumatoid Arthritis WW Annual Sales3 2022 2031�(in billions) ~$6.9 ~$8.6 ~$18.2 ~$19.1 ~$15.6 ~$20.8 ~$17.2 ~$21.7 ~$33.5 ~$33.1 US Diagnosed Population3�(in millions) ~1.0 ~.91 ~3.9 ~.48 ~3.6 Landos Asset NX-13 NLRX1 / NX-13 LABP-73 LABP-66 LABP-69 Target Pathway NLRX1 PLXDC2 Potential Areas of Future Development Include Eosinophilic Esophagitis, Dermatology & Neuroscience

Experienced Management Team in Immunology & Drug Development FABIO CATALDI, MD Executive Vice President & Chief Medical Officer DAWN LOURO Vice President, Clinical Operations CLAUDIA LOPEZ, DVM Vice President, Clinical Development GREGORY OAKES President & Chief Executive Officer DAVID PEREIRA, PHD Vice President, CMC AMY PLACE, PHD Vice President, Project Leadership & Site Engagement REBECCA MOSIG, PHD Executive Director, Corporate Development JENN CREEL Interim Chief Financial Officer

Top-Tier Advisory Teams GREGORY OAKES�President & Chief Executive Officer CHRIS GARABEDIAN�Chairman Xontogeny, Perceptive Advisors ROGER ADSETT�Chief Operating Officer of Insmed, Inc. ALKA BATYCKY, PH.D.�Director FRED CALLORI�Xontogeny, Perceptive Advisors TIAGO GIRÃO�CFO of Televant Therapeutics TIM M. MAYLEBEN�Director JEAN-FREDERIC COLOMBEL, MD�Icahn School of Medicine at Mount Sinai GEERT D’HAENS, MD, PHD�Amsterdam UMC, University of Amsterdam SILVIO DANESE, MD, PHD�IRCCS San Raffaele Hospital, Vita-Salute San Raffaele University MARLA DUBINSKY, MD�Icahn School of Medicine at Mount Sinai BRIAN G. FEAGAN, MD, FRCPC�Western University, Ontario, Canada REMO PANACCIONE, MD, FRCPC�University of Calgary Board of Directors Scientific & Steering Committee LAURENT PEYRIN-BIROULET, MD, PHD�Nancy University Hospital, University of Lorraine FLORIAN REIDER, MD�Cleveland Clinic STEFAN SCHREIBER, MD�UKSH-Campus Kiel BRITTA SIEGMUND, MD, PHD�Charité – Universitätsmedizin, Berlin BRAM VERSTOCKT, MD, PHD�University Hospitals Leuven, KU Leuven ANDRES YARUR, MD�Cedars Sinai Medical Center
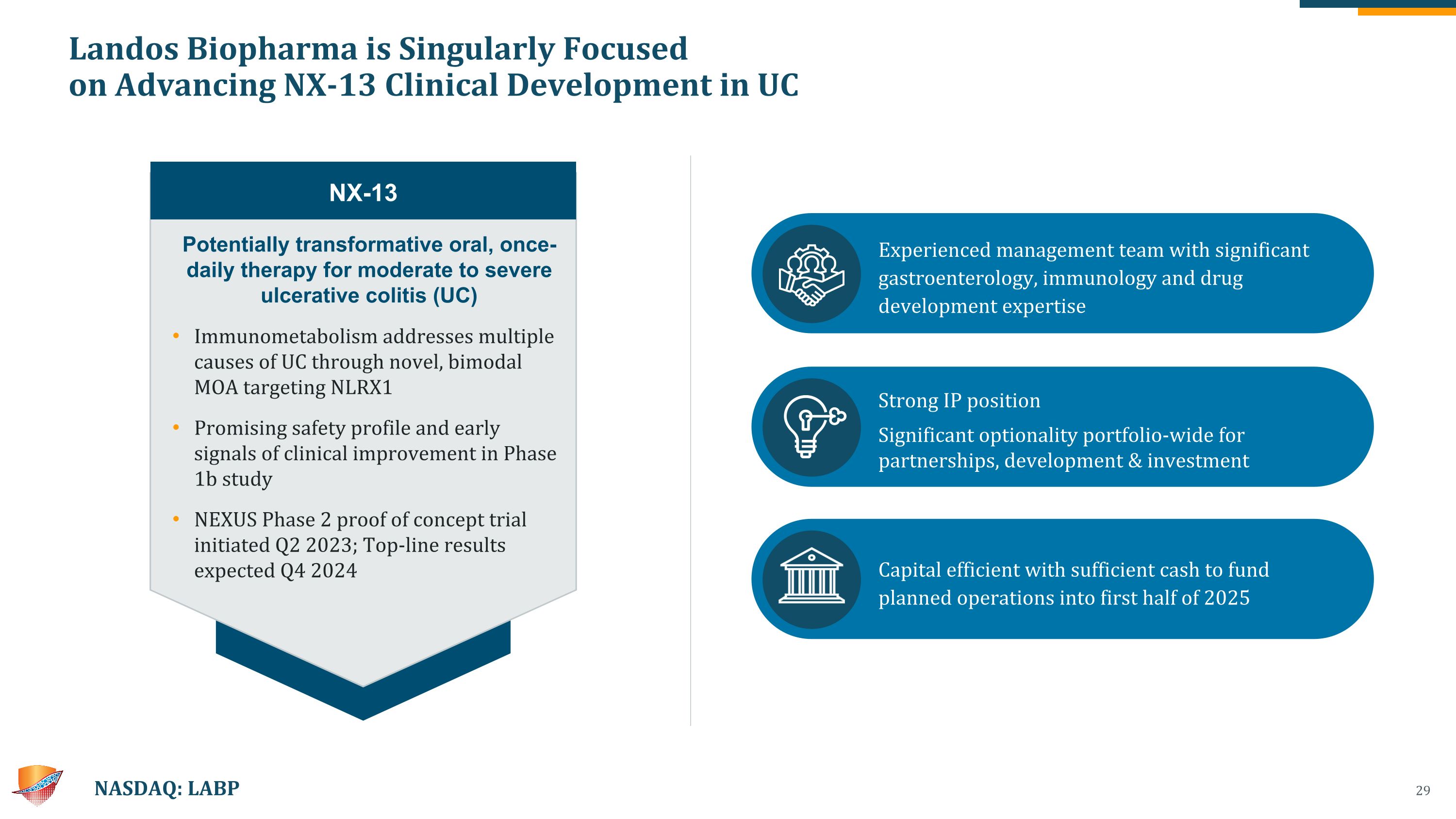
Landos Biopharma is Singularly Focused �on Advancing NX-13 Clinical Development in UC NASDAQ: LABP Potentially transformative oral, once-daily therapy for moderate to severe ulcerative colitis (UC) Immunometabolism addresses multiple causes of UC through novel, bimodal MOA targeting NLRX1 Promising safety profile and early signals of clinical improvement in Phase 1b study NEXUS Phase 2 proof of concept trial initiated Q2 2023; Top-line results expected Q4 2024 NX-13 Experienced management team with significant gastroenterology, immunology and drug development expertise Strong IP position Significant optionality portfolio-wide for partnerships, development & investment Capital efficient with sufficient cash to fund planned operations into first half of 2025

Thank you Contact: IR@landosbiopharma.com

Appendix: Key Publications (11/23) The Safety, Tolerability, Pharmacokinetics and Clinical Efficacy of the NLRX1 agonist NX-13 in Active Ulcerative Colitis: Results of a Phase 1b Study. Journal of Crohn's and Colitis, e-published ahead of print (10/23) The Nucleotide-Binding Oligomerization Domain, Leucine Rich Repeat Containing X1 (NLRX1) Agonist NX-13 Demonstrates Rapid Symptomatic and Biomarkers Improvement in Ulcerative Colitis: Results In a Phase 1b Study. UEG Week Journal Abstracts 2023; Poster Presentations – United European Gastroenterology Journal (11) S8 (Publication OP078 / p76) (10/23) Symptomatic Relief Is Correlated with Early Endoscopic Response to the Nucleotide-Binding Oligomerization Domain, Leucine Rich Repeat Containing X1 (NLRX1) Agonist NX-13 In Ulcerative Colitis: Results in a Phase 1b Study. UEG Week Journal Abstracts 2023; Poster Presentations – United European Gastroenterology Journal (11) S8 (Publication OP104 / p103) (10/23) Target Engagement And Pharmacodynamic Molecular Mechanism Evaluation In A Phase 1b Study of the Nucleotide-binding Oligomerization Domain, Leucine Rich Repeat Containing X1 (NLRX1) Agonist NX-13 in Ulcerative Colitis. UEG Week Journal Abstracts 2023; Poster Presentations – United European Gastroenterology Journal (11) S8 (Publication PP785 / p975) (2/23) A Phase 1b Study to Evaluate Safety, Tolerability, Pharmacokinetics and Clinical Efficacy of the Nucleotide-binding oligomerization domain, Leucine rich Repeat containing X1 (NLRX1) agonist NX-13 in Ulcerative Colitis. Journal of Crohn's and Colitis, Volume 17, Issue Supplement 1 (Publication P577) (10/21) Safety and Tolerability of NX-13 in a Randomized, Double-Blind Placebo Controlled Phase I Study in Normal Healthy Volunteers. UEG Week 2021 Poster Presentations - United European Gastroenterology Journal (9) S8 (Publication P0480) (11/19) Activation of NLRX1 by NX-13 Alleviates Inflammatory Bowel Disease through Immunometabolic Mechanisms in CD4+ T Cells. The Journal of Immunology (November 6, 2019) (6/19) Exploratory studies with NX-13: oral toxicity and pharmacokinetics in rodents of an orally active, gut-restricted first-in-class therapeutic for IBD that targets NLRX1. Drug and Chemical Toxicology (June 10, 2019) (5/19) Preclinical Efficacy and Safety of NX-13: A Novel Nlrx1-Targeting Immunometabolic Therapeutic for Crohn’s Disease and Ulcerative Colitis. AGA Journals (May 2019) (2/18) NLRX1 Modulates Immunometabolic Mechanisms Controlling the Host-Gut Microbiota Interactions during Inflammatory Bowel Disease. Front Immunol (February 2018) (3/17) NLRX1 Regulates Effector and Metabolic Functions of CD4+ T Cells. J Immunol (March 2017) (5/21) PLXDC2 activation by PX-69 ameliorates rheumatoid arthritis through activation of novel immunometabolic mechanisms. J Immunol (May 1, 2021)
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
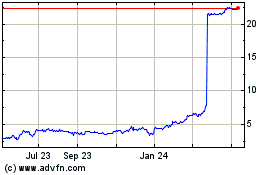
Landos Biopharma (NASDAQ:LABP)
Historical Stock Chart
From Mar 2024 to Apr 2024
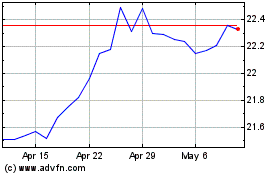
Landos Biopharma (NASDAQ:LABP)
Historical Stock Chart
From Apr 2023 to Apr 2024