Filed Pursuant to Rule 424(b)(4)
Registration Statement No. 333-275697
Prospectus

BLUEJAY
DIAGNOSTICS, INC.
537,768 Shares of Common
Stock
Prefunded Warrants to purchase up to 2,154,540
Shares of Common Stock
Common Warrants to purchase up to 2,692,308
Shares of Common Stock
4,846,848 Shares of Common Stock underlying
Prefunded Warrants and Common Warrants
Placement Agent Warrants to Purchase up to 188,462
Shares of Common Stock
188,462 Shares of Common
Stock Underlying the Placement Agent Warrants
We are offering 537,768 shares of common stock,
together with warrants to purchase up to537,768 shares of common stock, each a Common Warrant, at a combined public offering price of
$1.30 per share and Common Warrant (and the shares issuable from time to time upon exercise of the Common Warrants), pursuant to this
prospectus. The shares of common stock and Common Warrants will be separately issued but must be purchased together in this offering.
Each share of common stock is being offered together with a Common Warrant to purchase share of common stock. Each Common Warrant will
have an exercise price of $1.30 per share, (representing 100% of the price at which a share of common stock and accompanying Common Warrant
are sold to the public in this offering), will be exercisable upon issuance and will expire five years from the date of issuance.
We are also offering prefunded warrants, or Prefunded Warrants, to
purchase up to an aggregate of 2,154,540 shares of common stock, together with Common Warrants to purchase up to 2,154,540 shares of common
stock, to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with its
affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding
common stock following the consummation of this offering in lieu of the shares of our common stock that would result in ownership in excess
of 4.99% (or, at the election of the purchaser, 9.99%). Each Prefunded Warrant will be exercisable for one share of common stock at an
exercise price of $0.0001 per share. Each Prefunded Warrant is being offered together with the same Common Warrant described above being
offered with each share of common stock. The combined public offering price for each such Prefunded Warrant, together with the Common
Warrant, is $1.2999, which is equal to the combined public offering price per share of common stock and Common Warrant, less the $0.0001
per share exercise price of each such Prefunded Warrant. Each Prefunded Warrant will be exercisable upon issuance and will expire when
exercised in full. The Prefunded Warrants and Common Warrants are immediately separable and will be issued separately in this offering,
but must be purchased together in this offering. This prospectus also relates to the shares of common stock issuable upon the exercise
of the Prefunded Warrants and the Common Warrants, and the Placement Agent Warrants (as defined below) to purchase up to 188,462
shares of common stock and 188,462 shares of common stock issuable upon exercise of the Placement Agent Warrants.
We will have one closing for all the securities
purchased in this offering. The combined public offering price per share (or Prefunded Warrant) and Common Warrant will be fixed for
the duration of this offering.
We have engaged H.C. Wainwright & Co., LLC,
or the placement agent, to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use
its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing
or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific
number or dollar amount of securities. We have agreed to pay to the placement agent the placement agent fees set forth in the table below,
which assumes that we sell all of the securities offered by this prospectus. Since we will deliver the securities to be issued in this
offering upon our receipt of investor funds, there is no arrangement for funds to be received in escrow, trust or similar arrangement.
There is no minimum offering requirement as a condition of closing of this offering. Because there is no minimum offering amount required
as a condition to closing this offering, we may sell fewer than all of the securities offered hereby, which may significantly reduce the
amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount
of securities sufficient to pursue our business goals described in this prospectus. In addition, because there is no escrow account and
no minimum offering amount, investors could be in a position where they have invested in our company, but we are unable to fulfill all
of our contemplated objectives due to a lack of interest in this offering. Further, any proceeds from the sale of securities offered by
us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement
our business plan. We will bear all costs associated with the offering. See “Plan of Distribution” on page 24 of this prospectus
for more information regarding these arrangements.
Our common stock is listed on Nasdaq under the
symbol “BJDX.” The closing price of our common stock on Nasdaq on December 27, 2023 was $1.755 per share.
There is no established trading market for the
Prefunded Warrants or the Common Warrants, and we do not expect a market to develop. We do not intend to apply for a listing of the Prefunded
Warrants or the Common Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market,
the liquidity of the Prefunded Warrants and the Common Warrants will be limited.
We are an “emerging growth company”
as that term is used in the Jumpstart Our Business Startups Act of 2012 and as such, are subject to reduced public company disclosure
standards for this prospectus supplement, the accompanying prospectus and our filings with the Securities and Exchange Commission. See
“Prospectus Summary—Implications of Being an Emerging Growth Company.”
You should read this prospectus, together with
additional information described under the headings “Information Incorporated by Reference” and “Where You Can Find
Additional Information,” carefully before you invest in any of our securities.
Investing in our securities involves a high
degree of risk. See “Risk Factors” beginning on page 8 of this prospectus for a discussion of risks that should be considered
in connection with an investment in our securities.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or the accuracy of this
prospectus. Any representation to the contrary is a criminal offense.
| | |
Per Share and
Accompanying
Common
Warrant | | |
Per Prefunded
Warrant and
Accompanying
Common
Warrant | | |
Total | |
| Public offering price | |
$ | 1.3000 | | |
$ | 1.2999 | | |
$ | 3,500,000.40 | (3) |
| Placement agent’s fees(1) | |
$ | 0.0910 | | |
$ | 0.0910 | | |
$ | 245,000.03 | |
| Proceeds to us, before expenses(2) | |
$ | 1.2090 | | |
$ | 1.2089 | | |
$ | 3,255,000.37 | |
| (1) | In addition, we have also agreed
to pay the placement agent a management fee of 1.0% of the aggregate gross proceeds raised in this offering and to pay the placement
agent for certain of its offering-related expenses. In addition, we have agreed to issue the placement agent or its designees, as compensation
in connection with this offering, warrants, or the Placement Agent Warrants, to purchase a number of shares of common stock equal to
7.0% of the shares of common stock sold in this offering (including the shares of common stock issuable upon the exercise of the Prefunded
Warrants), at an exercise price of $1.6250 per share, which represents 125% of the combined public offering price per share of common
stock and accompanying Common Warrant. See “Plan of Distribution” for a description of the compensation to be received by
the placement agent. |
| (2) | We estimate the total offering
expenses of this offering that will be payable by us, excluding the placement agent fees and expenses, will be approximately $220,000.
For more information, see “Plan of Distribution.” |
| (3) | The total public offering price
assumes the full exercise of the Prefunded Warrants. |
Delivery of the securities offered hereby is expected
to be made on or about January 2, 2024, subject to satisfaction of customary closing conditions.
H.C. Wainwright
& Co.
The date of this prospectus is December 27, 2023
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
The registration statement of which this prospectus
forms a part that we filed with the Securities and Exchange Commission (the “SEC”) includes exhibits that provide more detail
of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together with
the additional information described under the headings “Where You Can Find Additional Information” and “Information
Incorporated by Reference” before making your investment decision. You should rely only on the information provided in or incorporated
by reference in this prospectus, in any prospectus supplement or in a related free writing prospectus, or documents to which we otherwise
refer you. In addition, this prospectus contains summaries of certain provisions contained in some of the documents described herein,
but reference is made to the actual documents for complete information.
This prospectus includes important information
about us, the securities being offered and other information you should know before investing in our securities. You should not assume
that the information contained in this prospectus is accurate on any date subsequent to the date set forth on the front cover of this
prospectus, even though this prospectus is delivered or securities are sold or otherwise disposed of on a later date. It is important
for you to read and consider all information contained in this prospectus in making your investment decision. All of the summaries in
this prospectus are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been
filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part,
and you may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
We have not, and the placement agent has not,
authorized anyone to provide any information or to make any representations other than those contained or incorporated by reference in
this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility
for, and can provide no assurance as to the reliability of, any other information that others may give you. The information contained
in this prospectus or incorporated by reference in this prospectus or contained in any applicable free writing prospectus is current only
as of its date, regardless of its time of delivery or any sale of our securities. Our business, financial condition, results of operations
and prospects may have changed since that date.
For investors outside the United States: We have
not, and the placement agent has not, done anything that would permit this offering or possession or distribution of this prospectus in
any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come
into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities
and the distribution of this prospectus outside the United States.
Unless otherwise indicated, information contained
in this prospectus or incorporated by reference in this prospectus concerning our industry, including our general expectations and market
opportunity, is based on information from our own management estimates and research, as well as from industry and general publications
and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our
knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. In addition, assumptions
and estimates of our and our industry’s future performance are necessarily uncertain due to a variety of factors, including those
described in “Risk Factors” beginning on page 8 of this prospectus. These and other factors could cause our future
performance to differ materially from our assumptions and estimates.
This prospectus is an offer to sell only the
securities offered hereby, and only under circumstances and in jurisdictions where it is lawful to do so. We are not, and the placement
agent is not, making an offer to sell these securities in any state or jurisdiction where the offer or sale is not permitted.
Industry and Market Data
This prospectus and the documents incorporated
by reference contain estimates, projections and other information concerning our industry, our business, the science of our products and
the markets for our products, including data regarding the incidence of certain medical conditions and the scientific basis of our products.
We obtained the industry, science, market and similar data set forth in this prospectus from our internal estimates and research and from
academic and industry research, publications, surveys, and studies conducted by third parties. While we believe that these industry publications
and third-party research, surveys and studies are reliable, we have not independently verified such data and we do not make any representation
as to the accuracy of the information. The content of the above sources, except to the extent specifically set forth in this prospectus,
does not constitute a portion of this prospectus and is not incorporated herein. Information that is based on estimates, forecasts, projections,
market research, scientific research, or similar methodologies is inherently subject to uncertainties and actual events or circumstances
may differ materially from events and circumstances that are assumed in this information.
Note Regarding Trademarks
Unless the context otherwise requires, references in this prospectus
to “Bluejay,” “the Company,” “we,” “us” and “our” refer to Bluejay Diagnostics,
Inc. Our logo and all product names are our common law trademarks. Solely for convenience, trademarks and tradenames referred to in this
prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate in any way that we will not
assert, to the fullest extent under applicable law, our rights, or that the applicable owner will not assert its rights, to these trademarks
and tradenames. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with,
or endorsement or sponsorship of us by, any other companies, products or services.
Basis of Presentation
On July 21, 2023, we filed a Certificate of Amendment
to our restated certificate of incorporation with the Secretary of State of the State of Delaware to effect a 1-for-20 reverse stock split
of our issued and outstanding shares of common stock, par value $0.0001 per share (the “Reverse Stock Split”), which
became effective on July 24, 2023. All historical share and per share amounts reflected throughout this prospectus have been adjusted
to reflect the Reverse Stock Split. However, our periodic and current reports, and all other documents incorporated by reference into
this prospectus that were filed prior to July 24, 2023, do not give effect to the Reverse Stock Split.
PROSPECTUS SUMMARY
This summary highlights information contained
in greater detail elsewhere in this prospectus or incorporated by reference into this prospectus from our filings with the SEC. This summary
is not complete and does not contain all of the information you should consider in making your investment decision. You should read the
entire prospectus and the information incorporated by reference herein carefully before making an investment in our securities. You should
carefully consider, among other things, our financial statements and the related notes and the sections entitled “Risk Factors”
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in, or
incorporated by reference into, this prospectus. When we use the terms “Bluejay,” “the Company,” “us,”
“we” and “our,” we refer to Bluejay Diagnostics, Inc., and its wholly owned subsidiary Bluejay SpinCo, LLC, taken
as a whole.
Overview
We are a medical diagnostics company developing rapid tests using whole
blood, plasma, and serum on our Symphony technology platform, (“Symphony”), to improve patient outcomes in critical care settings.
Our Symphony platform is a combination of our intellectual property (“IP”), and exclusively licensed and patented IP that
consists of a mobile analyzer and single-use test cartridges that if cleared, authorized, or approved by the U.S. Food and Drug Administration(the
“FDA”), can provide a solution to a significant market need in the United States. Clinical trials indicate the Symphony analyzer
produces laboratory-quality results in less than 20 minutes, important in intensive care units (ICUs) and emergency rooms (ERs) where
rapid and reliable results are required.
Our first product, the Symphony IL-6 test, is
for the monitoring of disease progression in critical care settings. A current challenge for healthcare professionals is the excessive
time and cost associated with triage and risk stratifying patients. IL-6 is a clinically established biomarker, and is considered a ‘first-responder’
biomarker in the inflammatory cascade which can be used for the assessment of patient prognosis for many diseases and conditions, including
sepsis. The Symphony IL-6 test has the ability to consistently monitor this important and clinically informative biomarker with rapid
results.
In the future we plan to develop additional tests
for the Symphony platform, including two cardiac biomarkers (hsTNT and NT pro-BNP) among others. We do not yet have regulatory clearance
for our Symphony products, and our Symphony products will need to receive regulatory authorization from the FDA in order to be marketed
as a diagnostic product in the United States.
Our operations to date have been funded primarily
through the proceeds of (i) our initial public offering in November 2021, and (ii) the registered direct offering of common stock and
concurrent private placement of warrants that we completed on August 28, 2023, which is described further below.
Our Market
The Symphony platform and our initial biomarker
test, Symphony IL-6 test, is well suited to address a subset of the global in vitro diagnostics devices (“IVDs”), market,
including sepsis, cardio-metabolic diseases, cancer and other diseases that require rapid tests. Symphony targets critical care markets
where physicians must quickly determine patient acuity to identify optimal treatment regimens.
Our Business Model
Our goal is to become the first provider of rapid
tests for infectious, inflammatory, and metabolic diseases by leveraging the strengths of our Symphony platform. We intend to target sales
and marketing of Symphony to large critical care facilities in the United States. Our business model includes the following:
| ● | Attractive Financing Model. We intend to offer various financing options for the analyzer itself. As such,
our business model should not require customers to incur a significant capital outlay. |
| ● | Recurring Revenue. We intend to sell single-use diagnostic test cartridges. We believe that our cartridges
can create a growing and recurring revenue stream, as adoption and utilization increases, and as we develop tests for additional indications.
We expect the sale of our test cartridges will generate the majority of our revenue and gross profit. |
| ● | Expand our Menu of Diagnostic Products. As adoption increases, the customer use of the Symphony platform
should also increase. As we expand our test menu to include more biomarkers, we hope to be able to increase our annual revenue per customer
through the resulting increase in utilization. |
The Symphony Platform
The Symphony platform is an innovative and proprietary
technology platform that provides rapid and accurate measurements of key diagnostic biomarkers found in biological fluids, such as whole
blood. Symphony is compact and can be deployed mobile as compared to current laboratory diagnostic platforms. Symphony incorporates a
user-friendly interface where all sample preparation and reagents are integrated into disposable Symphony cartridges. Symphony a very
small amount of blood (0.15cc or 0.15mL) to provide a measurement in less than 20 minutes.
The Symphony analyzer orchestrates whole blood
processing, biomarker isolation, and immunoassay preparation using non-contact centrifugal force. All necessary reagents and components
are integrated into the Symphony cartridges. Utilizing precision microchannel technology and high-specificity antibodies, whole blood
is processed, and the biomarkers are isolated within the Symphony cartridge. Intermitted centrifugation cycles enable complex fluid movements,
allowing sequential reagent additions and independent reaction steps inside the sealed Symphony cartridge. At the conclusion of the test,
the Symphony analyzer measures the fluorescence signature correlating to a highly sensitive quantitation of the biomarker.
To perform a Symphony test, the test operator
adds 0.15cc or 0.15mL (approximately three drops) of blood to the Symphony cartridge. After scanning in the patient ID, the Symphony cartridge
is inserted into the Symphony analyzer and the test runs automatically. Each analyzer can run up to six cartridges simultaneously, either
with six different patient samples or six different tests, in less than 20 minutes, providing quantitative measurements used for improved
patient management and clinical decision-making.
Manufacturing
We plan to manufacture both our analyzers and cartridges through Contract
Manufacturing Organizations (“CMOs”). We have contracts with Toray Industries, Inc (“Toray”), to license the intellectual
property rights needed to manufacture our cartridges and Sanyoseiko Co. Ltd. (“Sanyoseiko”), to manufacture both our analyzers
and cartridges. Each of our partners are well-established global manufacturing companies with capabilities to scale up, re-design and
supply our analyzers and cartridges.
Sanyoseiko had been selected as our CMO, though
in the near-term Toray will continue to manufacture certain product intermediary components for use in cartridges being manufactured for
the Company by Sanyoseiko. These cartridges made using Toray intermediates are for the purpose of obtaining FDA approval and not for commercial
sale. We expect to meet the demands of our global market. Both Toray’s and Sanyoseiko’s facilities are located in Japan. We
license the technology for the Symphony cartridges from Toray. Our license grants us exclusive global use, with the exception of Japan.
FDA Regulatory Strategy
Our current regulatory strategy is designed
to support commercialization of Symphony in the United States pending marketing authorization from the FDA. Previously, our regulatory
strategy involved clinical studies involving COVID-19 patients. However, we have shifted our focus away from COVID-19 patients due to
a significant decline in the number of COVID-19 related hospitalizations. Pursuant to this revised strategy, we are beginning to conduct
a clinical study to support an FDA regulatory submission with an initial indication for risk stratification of hospitalized sepsis patients.
We submitted a pre-submission application to the FDA presenting the new study design in May 2023 and participated in a pre-submission
meeting on August 11, 2023. At the meeting, the FDA provided feedback on the new study design, determined that the submission of a 510(k)
is the appropriate premarket submission pathway, and requested that certain data be provided in the 510(k). Based on this feedback, we
determined to proceed on this basis, which considers the FDA’s feedback.
In December 2023, we initiated the study at
an initial site, which will use the Symphony IL-6 test to monitor IL-6 concentrations in patients who are diagnosed with sepsis or septic
shock and are admitted or intended to be admitted to the ICU. The objective of this study is to establish IL-6 concentrations in these
sepsis patients that best predict 28-day mortality. We except to bring several additional sites into the study in the coming months.
We are working towards a Symphony IL-6 regulatory submission timeline of the first half of 2024.
We maintain contracts with Toray to manufacture
our cartridges and Sanyoseiko to manufacture both our analyzers and cartridges.
Sales and Marketing
Until Symphony products are authorized by the
FDA, we expect to focus our sales and marketing efforts on brand awareness and market education to potential customers, emphasizing the
value of monitoring a critical care patient’s IL-6 levels to improve decision making and patient outcomes. If cleared or approved
by the FDA, we intend to target sales to ERs and ICUs at United States hospitals, as well as to long-term acute care facilities. We plan
to establish a market presence by selling Symphony analyzers and tests both directly and through various distribution channels to maximize
sales volume and market penetration.
License Agreement
On October 6, 2020, we entered into a License
and Supply Agreement, as amended (the “License Agreement”), with Toray, providing us with an exclusive global license with
Toray, excluding Japan, to use their patents and know-how related to the Symphony detection cartridges for the manufacturing, marketing
and sale of the products (as defined in the License Agreement).
On October 23, 2023, we entered into an Amended
and Restated License Agreement (the “New Toray License Agreement”) and a Master Supply Agreement (the “New Toray Supply
Agreement” and, together, the “Toray Agreements”) with Toray. Under the New Toray License Agreement, we continue to
license from Toray intellectual property rights needed to manufacture single-use test cartridges, and we have received the right to sublicense
certain Toray intellectual property to Sanyoseiko in connection with our ongoing agreement with Sanyoseiko to manufacture our Symphony
analyzers and cartridges. In addition, the New Toray License Agreement provides for the transfer of certain technology related to the
cartridges to Sanyoseiko. The royalty payments we are required to pay Toray have been reduced under the New Toray License Agreement from
15% to 7.5% (or less in certain circumstances) of net sales of certain cartridges for a term of 10 years. A 50% reduction in the royalty
rate applies upon expiry of applicable Toray patents on a product-by-product and country-by-country basis. The New Toray License Agreement
contemplates that applicable royalty payment obligations from us to Toray for other products will be determined separately in the future.
Under the New Toray Supply Agreement, Toray
will manufacture in the near-term (through its wholly owned subsidiary Kamakura Techno-Science, Inc.) certain product intermediary
components for use in cartridges being manufactured for the Company by Sanyoseiko. These cartridges made using Toray intermediates
are for the purpose of obtaining FDA approval and not for commercial sale. The New Toray Supply Agreement has a term ending on the
earlier of October 23, 2025 or the date that we obtain FDA approval for our product, and may be extended for up to six months by
mutual agreement. Once FDA approval has been obtained, the intermediates and cartridges will be manufactured by Sanyoseiko under a
separate supply agreement between us and Sanyoseiko. The FDA may not clear or approve these product submissions or applications on a timely basis or at all. Such delays or refusals could
have a material adverse effect on our business, financial condition, and results of operations.
Intellectual Property, Proprietary Technology
We do not currently hold any patents directly.
We rely on a combination either directly or through the New Toray License Agreement with Toray of patent, copyright, trade secret, trademark,
confidentiality agreements, and contractual protection to establish and protect our proprietary rights.
Competition
Our primary competition in the IL-6 market is
laboratory size equipment including the Roche Cobas®, Siemens ADVIA Centaur® and Beckman Coulter Access 2®, which require
pre-processing of whole blood prior to performing their test. We believe that our technology, which uses whole blood, provides us with
a substantial competitive advantage over our existing competition that will sustain through commercialization, despite the major life
science companies and consistent entry of innovative start-ups that define our competitive landscape.
Employees
As of December 20, 2023, we have 10 full-time
employees. We also contract with several consultants and contractors performing accounting, finance, regulatory advisory, investor relations
and manufacturing scale-up support. None of our employees are represented by labor unions or covered by collective bargaining agreements.
August 2023 Registered Direct Offering and Concurrent Private Placement
On August 24, 2023, we entered into a securities
purchase agreement with certain institutional and accredited investors (the “August 2023 Purchase Agreement”) relating to
the registered direct offering and sale of 216,000 shares (the “August 2023 Offering”) of our common stock, and a concurrent
private placement (the “August 2023 Private Placement”) of unregistered warrants to purchase up to 216,000 shares of our common
stock (the “August 2023 Warrants”). Each August 2023 Warrant is exercisable for one share of common stock (the “August
2023 Warrant Shares”) at an exercise price of $7.24 per share, will be immediately exercisable upon issuance and will expire five
years from the date of issuance.
The common stock sold in the August 2023 Offering
was sold at a purchase price of $7.365 per share (which amount included a purchase price of $0.125 per accompanying August 2023 Warrant).
Our gross proceeds from the August 2023 Offering and the August 2023 Private Placement were approximately $1.59 million, before deducting
placement agent fees and offering expenses.
Pursuant to an engagement letter, dated as of August 7, 2023, by and
between us and H.C. Wainwright & Co., LLC (“Wainwright”), as a part of compensation, we issued to Wainwright or its designees,
warrants to purchase up to 15,120 shares of common stock (the “August 2023 Placement Agent Warrants”). The August 2023 Placement
Agent Warrants have substantially the same terms as the August 2023 Warrants, except that the August 2023 Placement Agent Warrants have
an exercise price equal to $9.2063 per share and a term of five years from the commencement of the sales pursuant to the August 2023 Offering.
The August 2023 Offering and August 2023 Private Placement closed on
August 28, 2023.
Reverse Stock Split
On July 24, 2023, we effected a reverse stock split of our shares of
common stock at a ratio of 1-for-20 (the “Reverse Stock Split”), with a corresponding reduction in the number of authorized
outstanding number of shares of common stock from 100,000,000 to 7,500,000. The Reverse Stock Split became effective on July 24, 2023,
when the Company’s common stock opened for trading on Nasdaq on a post-split basis under the Company’s existing trading symbol,
“BJDX.” All historical share and per share amounts reflected throughout this prospectus have been adjusted to reflect the
Reverse Stock Split. However, our periodic and current reports, and all other documents incorporated by reference into this prospectus
that were filed prior to July 24, 2023, do not give effect to the Reverse Stock Split.
Nasdaq Minimum Bid Price Requirement
On October 25, 2022, we received a notification
letter from the Nasdaq Listing Qualifications Staff of Nasdaq notifying us that the closing bid price for our common stock had been below
$1.00 for the previous 30 consecutive business days and that we therefore were not in compliance with the minimum bid price requirement
for continued listing on Nasdaq under Nasdaq Listing Rule 5550(a)(2). On April 25, 2023, at our request, Nasdaq’s Listing Qualifications
Staff notified us that it has extended the time for the Company to regain compliance with the Minimum Bid Requirement until October 23,
2023.
On August 8, 2023, we received a letter from the
Listing Qualifications Department of Nasdaq notifying us that, based on the closing bid price of our common stock having been at least
$1.00 per share for the required period, we have regained compliance with Nasdaq Listing Rule 5550(a)(2) and the minimum bid price deficiency
matter is now closed.
Risks Associated with Our Business
Our business is subject to a number of risks of
which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors”
section of this prospectus immediately following this prospectus summary and in Part I, Item 1A “Risk Factors” of our Annual
Report on Form 10-K for the fiscal year ended December 31, 2022 and in Part II, Item 1A “Risk Factors” of our Quarterly Reports
on Form 10-Q for the fiscal quarters ended March 31, 2023, June 30, 2023 and September 30, 2023, which are each incorporated by reference
in this prospectus. These risks include the following:
| ● | We have incurred significant losses since inception and may not be able to achieve significant revenues or profitability. |
| ● | We will require substantial additional funding, which may not be available to us on acceptable terms, or at all, and, if not so available,
may require us to delay, limit, reduce or cease our operations. |
| ● | The New License Agreement with Toray, which covers the license of the
core technology used in our Symphony Cartridges, and the New Supply Agreement with Toray, which covers the supply of cartridge intermediates
from Toray to Sanyoseiko for Sanyoseiko to manufacture our cartridges, contain significant risks that may threaten our viability or otherwise
have a material adverse effect on us and our business, assets and prospects. |
| ● | We depend on, and are liable for, Sanyoseiko as our primary contract
manufacturing organization (CMO), so its inability or failure to perform appropriately in that capacity may threaten our viability or
have a material adverse effect on us and our business, assets and its prospects. |
| ● | We cannot accurately predict the volume or timing of any sales, making the timing of any revenues difficult to predict. |
| ● | If third-party payors do not provide coverage and reimbursement for the use of our platform, our business and prospects may be negatively
impacted. |
| ● | If we are not able to attract and retain highly skilled managerial, scientific and technical personnel, we may not be able to implement
our business model successfully. |
| ● | Significant raw material shortages, supplier capacity constraints, supplier disruptions, and sourcing issues may adversely impact
or limited our products sales and or impact our product margins. |
| ● | The regulatory approval process which we may be required to navigate may be expensive, time-consuming, and uncertain and may prevent
us from obtaining clearance for our planned products |
| ● | Product clearances and approvals can often be denied or significantly delayed. |
| ● | Clinical data obtained in the future may not meet the required objectives, which could delay, limit or prevent any regulatory approval. |
| ● | We may be unable to complete required clinical evaluations, or we may experience significant delays in completing such clinical evaluations,
which could prevent or significantly delay our targeted product launch timeframe and impair our viability and business plan. |
| ● | We may be liable if the FDA or another regulatory agency concludes that we have engaged in the off-label promotion of our products. |
| ● | We depend on intellectual property licensed from Toray, and any dispute over the license would significantly harm our business. |
| ● | We face intense competition in the diagnostic testing market, particularly in the IL-6 space, and as a result we may be unable to
effectively compete in our industry. |
| ● | If we or Toray fail to respond quickly to technological developments, our products may become uncompetitive and obsolete. |
| ● | Shares eligible for future sale may adversely affect the market for our common stock. |
Implications of Being an Emerging Growth Company
We are an emerging growth company as defined in
the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). We may remain an “emerging growth company” until
as late as December 31, 2027 (the fiscal year-end following the fifth anniversary of the completion of our initial public offering, though
we may cease to be an “emerging growth company” earlier under certain circumstances, including (1) if the market value of
our common stock that is held by nonaffiliates exceeds $700 million as of any June 30, in which case we would cease to be an “emerging
growth company” as of the following December 31, or (2) if our gross revenue exceeds $1.235 billion in any fiscal year. “Emerging
growth companies” may take advantage of certain exemptions from various reporting requirements that are applicable to other public
companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of
2002, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from
the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments
not previously approved. Investors could find our common stock less attractive because we may rely on these exemptions. If some investors
find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price
may be more volatile.
In addition, Section 102 of the JOBS Act also
provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B)
of the Securities Act, for complying with new or revised accounting standards. An “emerging growth company” can therefore
delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
Corporate Information
We were incorporated under the laws of the State
of Delaware on March 20, 2015. Our principal executive offices are located at 360 Massachusetts Avenue, Suite 203, Acton, MA 01720
and our telephone number is (844) 327-7078. Our website address is www.bluejaydx.com. We do not incorporate the information on, or accessible
through, our website into this prospectus, and you should not consider any information on, or accessible through, our website as part
of this prospectus.
THE OFFERING
| Common Stock Offered |
|
537,768 shares based on the sale of our common stock at a combined public offering price of $1.30 per share of common stock and accompanying Common Warrant. |
| |
|
|
| Prefunded Warrants Offered |
|
We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, Prefunded Warrants to purchase up to 2,154,540 shares of common stock, in lieu of shares of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each Prefunded Warrant will be exercisable for one share of our common stock. The purchase price of each Prefunded Warrant and accompanying Common Warrant will equal the price at which the share of common stock and accompanying Common Warrant are being sold to the public in this offering, minus $0.0001, and the exercise price of each Prefunded Warrant will be $0.0001 per share. The Prefunded Warrants will be exercisable immediately and may be exercised at any time until all of the Prefunded Warrants are exercised in full. See “Description of Securities We Are Offering—Prefunded Warrants.” This offering also relates to the shares of common stock issuable upon exercise of the Prefunded Warrants sold in this offering. |
| |
|
|
| Common Warrants Offered |
|
Each share of our common stock and each Prefunded
Warrant to purchase one share of our common stock is being sold together with a Common Warrant to purchase share of our common stock.
Each Common Warrant will have an exercise price of $1.30 per share (representing 100% of the combined price at which a share of common
stock and accompanying Common Warrant are sold to the public in this offering), will be immediately exercisable and will expire on the
fifth anniversary of the original issuance date.
The shares of common stock and Prefunded Warrants,
and the accompanying Common Warrants, as the case may be, can only be purchased together in this offering but will be issued separately
and will be immediately separable upon issuance. See “Description of Securities We Are Offering—Common Warrants.”
This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the Common Warrants. |
| |
|
|
| Lock-up Agreements |
|
We and all of our executive officers, directors and 10% or greater stockholders have entered into lock-up agreements with the placement agent. Under these agreements, we and each of these persons may not, without the prior written approval of the placement agent, offer, sell, contract to sell or otherwise dispose of or hedge common stock or securities convertible into or exchangeable for common stock, subject to certain exceptions. The restrictions contained in these agreements will be in effect for a period of 60 days after the date of the securities purchase agreement. For more information, see “Plan of Distribution.” |
| |
|
|
| Placement Agent Warrants |
|
We have agreed to issue to the placement agent or its designees as
compensation in connection with this offering, the Placement Agent Warrants to purchase up to 7.0% of the aggregate number of
shares of common stock sold in this offering (including the shares of common stock issuable upon the exercise of the Prefunded
Warrants) at an exercise price equal to 125% of the combined public offering price per share and accompanying Common Warrant to be
sold in this offering. The Placement Agent Warrants will be exercisable upon issuance
and will expire five years from the commencement of sales under this offering. |
| |
|
|
| Common Stock Outstanding After This Offering (1) |
|
3,931,448 shares (assuming the full exercise of the Prefunded Warrants and no exercise of the Common Warrants) |
| Use of Proceeds |
|
We estimate that the net proceeds from this offering
will be approximately $2.8 million, based on a combined public offering price of $1.30 per share of common stock and accompanying Common
Warrant, after deducting the placement agent fees and estimated offering expenses payable by us, and excluding the proceeds, if any, from
the exercise of the Common Warrants in this offering.
We currently intend to use the net proceeds from
the offering to fund matters related to obtaining FDA approval (including clinical studies related thereto), as well as for other research
and development activities, and for general working capital needs. We may also use a portion of the net proceeds to acquire or invest
in complementary businesses, products and technologies or to fund the development of any such complementary businesses, products or technologies.
We currently have no plans for any such acquisitions or investments. See “Use of Proceeds” beginning on page 15. |
| |
|
|
| Risk Factors |
|
See “Risk Factors” beginning on page 8 of this prospectus and other information included and incorporated by reference in this prospectus for a discussion of the risk factors you should consider carefully when making an investment decision. |
| |
|
|
| Nasdaq Symbol |
|
Our common stock is listed on Nasdaq under the symbol “BJDX.” There is no established trading market for the Common Warrants or the Prefunded Warrants, and we do not expect a trading market to develop. We do not intend to list the Common Warrants or the Prefunded Warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the Common Warrants and the Prefunded Warrants will be extremely limited. |
| (1) |
The number of shares of our common stock to be outstanding after this
offering is based on 1,239,140 shares of our common stock outstanding as of December 20, 2023, and, unless otherwise indicated, excludes,
as of that date: |
| |
● |
31,361 shares of common stock issuable upon the exercise of stock options
outstanding as of December 20, 2023 at a weighted average exercise price of $36.28 per share; |
| |
● |
216,000 shares of common stock issuable upon the exercise of the August
2023 Warrants outstanding as of as of December 20, 2023 at an exercise price of $7.24 per share; |
| |
● |
15,120 shares of common stock issuable upon the exercise of the August
2023 Placement Agent Warrants outstanding as of as of December 20, 2023 at an exercise price of $9.2063 per share; |
| |
● |
40,594 shares of common
stock issuable upon the exercise of additional Common Stock warrants outstanding as of December 20, 2023 at a weighted average exercise
price of $64.73 per share; |
| |
● |
124,200 shares of common
stock issuable upon the exercise of Class A warrants outstanding as of December 20, 2023 at an exercise price of $140.00; |
| |
● |
3,770 shares of common
stock issuable upon the exercise of Class B warrants outstanding as of December 20, 2023 at an exercise price of $200.00; |
| |
● |
13,113 shares of common
stock available for future issuance under our 2018 Stock Incentive Plan as of December 20, 2023; |
| |
● |
40,377 shares of common
stock available for grant under the 2021 Stock Incentive Plan as of December 20, 2023; and |
| |
● |
7,875 unvested restricted
stock units outstanding as of December 20, 2023. |
Except as otherwise indicated, the information
in this prospectus assumes: (i) no exercise of the Prefunded Warrants in this offering; (ii) no exercise of any Common Warrants to be
issued in this offering; (iii) no exercise of the Placement Agent Warrants to be issued to the placement agent or its designees as compensation
in connection with this offering; and (iv) no exercise of the options or warrants described above.
RISK FACTORS
An investment in our securities involves a
high degree of risk. Before deciding whether to invest in our securities, you should carefully consider the risks described below and
those discussed under the section captioned “Risk Factors” contained in our Annual Report on Form 10-K for the fiscal year
ended December 31, 2022 and our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023, June 30, 2023 and September 30,
2023, which are incorporated by reference in this prospectus, together with the information included in this prospectus and documents
incorporated by reference herein, and in any free writing prospectus that we have authorized for use in connection with this offering.
If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be harmed. In such
event, the trading price of our common stock and value of the Prefunded Warrants and Common Warrants could decline and you might lose
all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties
not presently known to us or that we currently believe to be immaterial may also adversely affect our business. Certain statements below
are forward-looking statements.
Risks Related to Our Financial Condition and
Capital Requirements
We have incurred significant losses since
our inception and may continue to incur losses and thus may never achieve or maintain profitability.
We have incurred substantial losses since our
inception, and we expect to continue to incur additional losses for the next several years. For the three months ended September 30, 2023,
we had a net loss of approximately $2.3 million. From our inception through September 30, 2023, we had an accumulated deficit of $24.6
million. We had cash and cash equivalents of $5.1 million as of September 30, 2023. We continue to develop the Symphony analyzer and its
first test for the measurement of IL-6. We remain committed to obtaining FDA clearance and plan to conduct clinical trials to obtain sufficient
data to support its FDA submission, while also continuing to build its manufacturing operations with its contract manufacturing organizations.
Expected future operating losses will have an adverse effect on our cash resources, stockholders’ equity and working capital. Our
liquidity position raises substantial doubt about our ability to continue as a going concern.
Our failure to become and remain profitable could
depress the value of our common stock and impair our ability to raise capital, expand our business, maintain our development efforts,
or continue our operations. A decline in the value of our common stock could also cause you to lose all or part of your investment.
The report of our independent registered public
accounting firm on our financial statements for the year ended December 31, 2022 included an emphasis of matter paragraph stating that
our recurring losses from operations and continued cash outflows from operating activities raised substantial doubt about our ability
to continue as a going concern. Our consolidated financial statements do not include any adjustments
that might result from the outcome of this going concern uncertainty and have been prepared under the assumption that we will continue
to operate as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of
business. If we are unable to continue as a going concern, we may be forced to liquidate our assets which would have an adverse impact
on our business and developmental activities. In such a scenario, the values we receive for our assets in liquidation or dissolution could
be significantly lower than the values reflected in our financial statements. The reaction of investors to the inclusion of a going concern
statement by our independent registered public accounting firm and our potential inability to continue as a going concern may materially
adversely affect our stock price and our ability to raise new capital.
We will need to
raise additional funding to fund our working capital needs. Additional financing may not be available on acceptable terms, or at all.
Failure to obtain additional capital may force us to limit or terminate our operations.
Even if we sell all securities offered hereby,
the expected net proceeds of this offering may not be sufficient for us to fund the working capital needs of our business. We will continue
to seek funds through equity or debt financings, collaborative or other arrangements with corporate sources, or through other sources
of financing. Additional funding may not be available to us on acceptable terms, or at all. If adequate funds are not available or are
not available on acceptable terms, we may be required to delay our FDA regulatory strategy, and
to delay or reduce the scope of our research or development programs, our commercialization efforts or our manufacturing commitments and
capacity. Such inability to obtain additional financing when needed could have a material adverse effect on our business, results
of operations, cash flow, financial condition and prospects.
Risks Related to this Offering and our Common
Stock
Purchasers who purchase our securities in
this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit
of a securities purchase agreement.
In addition to rights and remedies available
to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement
will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those
investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including: (i) timely
delivery of shares; (ii) agreement to not enter into variable rate financings for one year from closing, subject to certain exceptions;
(iii) agreement to not enter into any financings for 60 days from closing; and (iv) indemnification for breach of contract.
This is a best efforts offering, with no
minimum amount of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business
plans, including our near-term business plans, nor will investors in this offering receive a refund in the event that we do not sell an
amount of securities sufficient to pursue the business goals outlined in this prospectus.
The placement agent has agreed to use its reasonable
best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities
from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. We may sell fewer than all of
the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will
not receive a refund in the event that we do not sell an amount of securities sufficient to support our business goals, continued operations,
including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required for our operations in
the short-term and may need to raise additional funds to complete such short-term operations. Such additional fundraises may not be available
or available on terms acceptable to us, or at all.
There is no required minimum number of securities
that must be sold as a condition to completion of this offering, and we have not, nor will we, establish an escrow account in connection
with this offering. Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering
amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts
set forth herein. Because there is no escrow account and no minimum offering amount, investors could be in a position where they have
invested in us, but we are unable to fulfill our objectives due to a lack of interest in this offering. Further, because there is no escrow
account in operation and no minimum investment amount, any proceeds from the sale of securities offered by us will be available for our
immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. Investor
funds will not be returned under any circumstances whether during or after the offering.
We have broad discretion in how we use the
net proceeds of this offering, and we may not use these proceeds effectively or in ways with which you agree.
Our management will have broad discretion as to
the application of the net proceeds of this offering and could use them for purposes other than those contemplated at the time of the
offering. We currently intend to use the net proceeds from the offering to fund matters related to obtaining FDA approval (including clinical
studies related thereto), as well as for other research and development activities, and for general working capital needs. We may also
use a portion of the net proceeds to acquire or invest in complementary businesses, products and technologies or to fund the development
of any such complementary businesses, products or technologies, though we currently have no plans for any such acquisitions or investments.
Our stockholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Moreover, our management
may use the net proceeds for corporate purposes that may not increase the market price of our common stock.
If you purchase our securities in this offering,
you may experience future dilution as a result of future equity offerings or other equity issuances.
In
order to raise additional capital, we believe that we will offer and issue additional shares
of our common stock or other securities convertible into or exchangeable for our common stock
in the future. We are generally not restricted from issuing additional securities, including
shares of common stock, securities that are convertible into or exchangeable for, or that
represent the right to receive, common stock or substantially similar securities. The issuance
of securities in future offerings may cause dilution to our stockholders, including investors
in this offering. We cannot assure you that we will be able to sell shares or other securities
in any other offering at a price per share that is equal to or greater than the price per
share paid by investors in this offering, and investors purchasing other securities in the
future could have rights superior to existing stockholders. The price per share at which
we sell additional shares of our common stock or other securities convertible into or exchangeable
for our common stock in future transactions may be higher or lower than the price per share
in this offering.
In addition, we have a significant number
of stock options, restricted stock units and warrants outstanding. To the extent that outstanding stock options or warrants have been
or may be exercised or other shares issued, you may experience dilution. Further, we may choose to raise additional capital due to market
conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
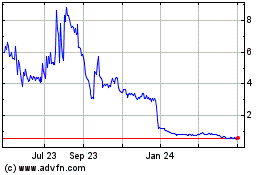
The market price of our common stock and
the trading volume of our common stock has been and may continue to be, highly volatile, and such volatility could cause the market price
of our common stock to decrease.
During 2022 and 2023, the market price of our
common stock was volatile, and our stock price continues to fluctuate. The market price and trading volume of our common stock may continue
to fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
| ● | the success of competitive products or technologies; |
| ● | regulatory actions with respect to our product candidates or our competitors’ products; |
| ● | the ability of our third-party manufacturers and suppliers to meet our demand volume; |
| ● | actual or anticipated changes in our growth rate relative to our competitors; |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures,
collaborations or capital commitments; |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| ● | the level of expenses related to our clinical development programs; |
| ● | the results of our efforts to in-license or acquire additional product candidates or products; |
| ● | actual or anticipated changes in estimates as to financial results, development timelines or recommendations
by securities analysts; |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| ● | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| ● | announcement or expectation of additional financing efforts; |
| ● | sales of our common stock by us, our insiders or our other stockholders; and |
| ● | other events or factors, many of which are beyond our control. |
Further, the stock market in general, and the
market for health care and life sciences companies in particular, has recently experienced extreme price and volume fluctuations. The
volatility of our common stock is further exacerbated due to its low trading volume. Continued market fluctuations could result in extreme
volatility in the price of our common stock, which could cause a decline in the value of our common stock and the loss of some or all
of your investment.
Trading of our common stock is limited,
making it difficult for our stockholders to sell their shares, and future sales of common stock could reduce our stock price.
Trading of our common stock is currently conducted
on Nasdaq. The liquidity of our common stock is limited, including in terms of the number of shares that can be bought and sold at a given
price and reduction in security analysts’ and the media’s coverage of us, if any. These factors may result in different prices
for our common stock than might otherwise be obtained in a more liquid market and could also result in a larger spread between the bid
and asked prices for our common stock. In addition, in the absence of a large market capitalization, our common stock is less liquid than
the stock of companies with broader public ownership, and, as a result, the trading prices of our common stock may be more volatile. In
the absence of an active public trading market, an investor may be unable to liquidate his investment in our common stock. Trading of
a relatively small volume of our common stock may have a greater impact on the trading price of our stock. We cannot predict the prices
at which our common stock will trade in the future, if at all.
The exercise of outstanding common stock
purchase warrants and stock options and the settlement of outstanding restricted stock units will have a dilutive effect on the percentage
ownership of our capital stock by existing stockholders.
As of December 20, 2023, we had outstanding
warrants to acquire 399,684 shares of our common stock, stock options to purchase 31,361 shares of our common stock, and restricted stock
units to acquire 7,875 shares of our common stock. All such warrants have exercise prices above our common stock’s recent trading
prices, but certain holders have the right to effect a cashless exercise of such warrants. If a significant number of such warrants and
stock options are exercised by the holders, the percentage of our common stock owned by our existing stockholders will be diluted. Further,
settlement of the outstanding restricted stock units will cause dilution to our existing stockholders.
We do not currently intend to pay dividends
on our common stock in the foreseeable future, and consequently, your ability to achieve a return on your investment will depend on appreciation
in the price of our common stock.
We do not anticipate paying any cash dividends
to holders of our common stock in the foreseeable future. Consequently, investors must rely on sales of their common stock after price
appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that shares
of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
There is no public market for the Common
Warrants or Prefunded Warrants to purchase shares of our common stock being offered by us in this offering.
There is no established public trading market
for the Common Warrants or the Prefunded Warrants to purchase shares of our common stock that are being offered as part of this offering,
and we do not expect a market to develop. In addition, we do not intend to apply to list the Common Warrants or Prefunded Warrants on
any national securities exchange or other nationally recognized trading system, including Nasdaq. Without an active market, the liquidity
of the Common Warrants and Prefunded Warrants will be limited.
The Common Warrants
are speculative in nature.
The Common Warrants offered hereby do not confer
any rights of share of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely
represent the right to acquire shares of common stock at a fixed price. Specifically, commencing on the date of issuance, holders of the
Common Warrants may acquire the shares of common stock issuable upon exercise of such warrants at an exercise price of $1.30 per share
of common stock. Moreover, following this offering, the market value of the Common Warrants is uncertain and there can be no assurance
that the market value of the Common Warrants, if any, will equal or exceed their public offering prices. There can be no assurance that
the market price of the shares of common stock will ever equal or exceed the exercise price of the Common Warrants, and consequently,
whether it will ever be profitable for holders of Common Warrants to exercise the Common Warrants.
Holders of the Prefunded Warrants and the
Common Warrants offered hereby will have no rights as common stockholders with respect to the shares our common stock underlying the warrants
until such holders exercise their warrants and acquire our common stock, except as otherwise provided in the Prefunded Warrants and the
Common Warrants.
Until holders of the Common Warrants and the Prefunded
Warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our
common stock underlying such warrants, except to the extent that holders of such Common Warrants and Prefunded Warrants will have certain
rights to participate in distributions or dividends paid on our common stock as set forth in the Common Warrants and the Prefunded Warrants.
Upon exercise of the Common Warrants and the Prefunded Warrants, the holders will be entitled to exercise the rights of a common stockholder
only as to matters for which the record date occurs after the exercise date.
This offering may
cause the trading price of our shares of common stock to decrease.
The price per share,
together with the number of shares of common stock we propose to issue and ultimately will issue if this offering is completed, may result
in an immediate decrease in the market price of our shares. This decrease may continue after the completion of this offering.
Resales of our shares of common stock in
the public market by our stockholders as a result of this offering may cause the market price of our shares of common stock to fall.
We are registering 537,768 shares of common
stock, as well as 5,035,310 shares of common stock, in the aggregate, issuable upon the exercise of the Prefunded Warrants, the Common
Warrants and the Placement Agent Warrants offered under this prospectus. Sales of substantial amounts of our shares of common stock in
the public market, or the perception that such sales might occur, could adversely affect the market price of our shares of common stock.
The issuance of new shares of common stock could result in resales of our shares of common stock by our current shareholders concerned
about the potential ownership dilution of their holdings. Furthermore, in the future, we may issue additional shares of common stock or
other equity or debt securities exercisable or convertible into shares of common stock. Any such issuance could result in substantial
dilution to our existing shareholders and could cause our stock price to decline.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated
herein by reference contain forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the
Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. In some
cases, you can identify these statements by forward-looking words such as “may,” “might,” “should,”
“would,” “could,” “expect,” “plan,” “anticipate,” “intend,” “believe,”
“estimate,” “predict,” “potential” or “continue,” and the negative of these terms and
other comparable terminology. These forward-looking statements, which are subject to known and unknown risks, uncertainties and assumptions
about us, may include projections of our future financial performance based on our growth strategies and anticipated trends in our business.
These statements are only predictions based on our current expectations and projections about future events. There are important factors
that could cause our actual results, level of activity, performance or achievements to differ materially from the results, level of activity,
performance or achievements expressed or implied by the forward-looking statements. We have included important factors in the cautionary
statements included in this prospectus, particularly under “Risk Factors” on page 8 of this prospectus and the documents
incorporated herein that we believe could cause actual results or events to differ materially from the forward-looking statements that
we make.
While we believe we have identified material risks
in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and our Quarterly Reports on Form 10-Q for the quarters
ended March 31, 2023, June 30, 2023 and September 30, 2023, which are incorporated by reference in this prospectus, together with the
information included in this prospectus and the documents incorporated by reference herein, and in any free writing prospectus that we
have authorized for use in connection with this offering, these risks and uncertainties are not exhaustive. Other sections of this prospectus
and the documents incorporated herein by reference may describe additional factors that could adversely impact our business and financial
performance. Moreover, we operate in a very competitive and rapidly changing environment. New risks and uncertainties emerge from time
to time, and it is not possible to predict all risks and uncertainties, nor can we assess the impact of all factors on our business or
the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking
statements.
Although we believe the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee future results, level of activity, performance or achievements.
Moreover, neither we nor any other person assumes responsibility for the accuracy or completeness of any of these forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. You should read this prospectus and any free writing
prospectus and the documents that we have incorporated by reference to this prospectus and filed as exhibits to this prospectus completely
and with the understanding that our actual future results may be materially different from what we expect.
We caution you not to place undue reliance on
the forward-looking statements, which speak only as of the date of this prospectus in the case of forward-looking statements contained
in this prospectus.
You should not rely upon forward-looking statements
as predictions of future events. Our actual results and financial condition may differ materially from those indicated in the forward-looking
statements. We qualify all of our forward-looking statements by these cautionary statements. Although we believe that the expectations
reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
Therefore, you should not rely on any of the forward-looking statements. In addition, with respect to all of our forward-looking statements,
we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of
1995.
Except as required by law, we undertake no obligation
to update or revise any forward-looking statements to reflect new information or future events or developments. You should not assume
that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. Before
deciding to purchase our securities, you should carefully consider the risk factors discussed and incorporated by reference in this prospectus
and the documents incorporated herein.
SELECTED
FINANCIAL DATA
Reverse Stock Split
On July 24, 2023, we effected the Reverse
Stock Split, with a corresponding reduction in the number of authorized outstanding number of shares of common stock from 100,000,000
to 7,500,000. The Reverse Stock Split became effective on July 24, 2023.The par value per share of our common stock also remained unchanged.
The following selected financial data has
been derived from our audited financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, filed
with the SEC on March 20, 2023, and our unaudited financial statements included in our Quarterly Report on Form 10-Q for the quarterly
period ended March 30, 2023, filed with the SEC on May 11, 2023, as adjusted to reflect the Reverse Stock Split for all periods presented.
The as adjusted information reflecting the Reverse Stock Split for the quarterly periods ended June 30, 2023 and September 30, 2023,
have been included in our Quarterly Reports on Form 10-Q filed with the SEC on August 14, 2023 and November 9, 2023, respectively, and
should be read together with this summary. See the sections entitled Information Incorporated by Reference and Where to Find Additional
Information and Incorporation.
Our historical results are not indicative
of the results that may be expected in the future, and results of interim periods are not indicative of the results for the entire year.
As Reported
| | |
Years Ended December 31, | |
| |
2022 | | |
2021 | |
| Net loss | |
$ | (9,296,948 | ) | |
$ | (3,488,298 | ) |
| Net loss per share - basic and diluted | |
$ | (0.46 | ) | |
$ | (0.41 | ) |
| Weighted average common shares outstanding, basic and
diluted | |
| 20,163,915 | | |
| 8,522,422 | |
| Common shares outstanding at year end | |
| 20,215,288 | | |
| 20,112,244 | |
| | |
Three Months Ended
March 31, | |
| | |
2023 | | |
2022 | |
| | |
(Unaudited) | |
| Net loss | |
$ | (2,539,843 | ) | |
$ | (2,013,403 | ) |
| Net loss per share - basic and diluted | |
$ | (0.12 | ) | |
$ | (0.10 | ) |
| Weighted average common shares outstanding, basic and
diluted | |
| 20,375,092 | | |
| 20,142,300 | |
| Common shares outstanding at period end | |
| 20,459,057 | | |
| 20,151,244 | |
As Adjusted For The Reverse Stock Split
| | |
Years Ended December 31, | |
| |
2022 | | |
2021 | |
| | |
(Unaudited) | |
| Net loss | |
$ | (9,296,948 | ) | |
$ | (3,488,298 | ) |
| Net loss per share - basic and diluted | |
$ | (9.22 | ) | |
$ | (8.19 | ) |
| Weighted average common shares outstanding, basic and
diluted | |
| 1,008,196 | | |
| 426,121 | |
| Common shares outstanding at year end | |
| 1,010,560 | | |
| 1,005,612 | |
| | |
Three Months Ended
March 31, | |
| | |
2023 | | |
2022 | |
| | |
(Unaudited) | |
| Net loss | |
$ | (2,539,843 | ) | |
$ | (2,013,403 | ) |
| Net loss per share - basic and diluted | |
$ | (2.49 | ) | |
$ | (2.00 | ) |
| Weighted average common shares outstanding, basic and
diluted | |
| 1,018,755 | | |
| 1,007,115 | |
| Common shares outstanding at period end | |
| 1,022,748 | | |
| 1,007,562 | |
USE OF PROCEEDS
We estimate that the net proceeds from this offering,
after deducting placement agent’s fees and estimated offering expenses payable by us, will be approximately $2.8 million. We
intend to use the net proceeds from the offering to fund matters related to obtaining FDA approval (including clinical studies related
thereto), as well as for other research and development activities, and for general working capital needs. We may also use a portion of
the net proceeds to acquire or invest in complementary businesses, products and technologies or to fund the development of any such complementary
businesses, products or technologies. We currently have no plans for any such acquisitions or investments.
This expected use of net proceeds from this offering
represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business
conditions evolve. We cannot currently allocate specific percentages of the net proceeds to us from this offering that we may use for
the purposes specified above. Our management will have broad discretion in the application of the net proceeds from this offering and
could use them for purposes other than those contemplated at the time of this offering. Our stockholders may not agree with the manner
in which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate
purposes that may not result in our being profitable or that increases our market value.
Pending our
use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including
short-term, investment-grade, interest-bearing instruments and U.S. government securities.
DIVIDEND POLICY
We have never declared or paid any cash dividends on
our capital stock. We currently intend to retain earnings, if any, to finance the growth and development of our business. We do not expect
to pay any cash dividends on our common stock in the foreseeable future. Payment of future dividends, if any, will be at the discretion
of our Board of Directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained
in any financing instruments, provisions of applicable law and other factors our Board of Directors deems relevant. On June 7, 2021, our
Board of Directors declared a stock dividend of 2.15 shares of common stock for every share of common stock. This stock dividend was deemed
a large stock dividend and was treated as a 1-for-3.15 stock split.
DESCRIPTION OF CAPITAL STOCK
The summary of general terms and provisions of
our capital stock set forth below does not purport to be complete and is subject to and qualified by reference to the Company’s
Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) and Amended and Restated Bylaws (the
“Bylaws,” and together with the Certificate of Incorporation, the “Charter Documents”), each of which is included
as an exhibit to the Company’s most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission and incorporated
by reference herein. For additional information, please read the Charter Documents and the applicable provisions of the Delaware General
Corporation Law (the “DGCL”).
Authorized Capital Stock
We are authorized to issue up to 12,500,000
shares, of which (i) 7,500,000 have been designated common stock, par value $0.0001 per share, and (ii) 5,000,000 have been designated
preferred stock, par value $0.0001 per share. As of December 20, 2023, there were 1,239,140 shares of our common stock outstanding, held
by 15 stockholders of record. This figure does not reflect the number of beneficial owners of shares of our common stock as a single
stockholder of record often holds shares in nominee name (also referred to as, in “street name”) on behalf of multiple beneficial
owners.
Common Stock
Voting
Each holder of common stock is entitled to one vote
for each share of common stock held on all matters submitted to a vote of stockholders. Any action at a meeting at which a quorum is present
will be decided by a majority of the voting power present in person or represented by proxy, except in the case of any election of directors,
which will be decided by a plurality of votes cast. There is no cumulative voting.
Dividends
Holders of our common stock are entitled to receive
dividends when, as and if declared by our Board of Directors out of funds legally available for payment, subject to the rights of holders,
if any, of any class of stock having preference over the common stock. Any decision to pay dividends on our common stock will be at the
discretion of our Board of Directors. Our Board of Directors may or may not determine to declare dividends in the future. See “Dividend
Policy.” The board’s determination to issue dividends will depend upon our profitability and financial condition, any contractual
restrictions, restrictions imposed by applicable law and the SEC, and other factors that our Board of Directors deems relevant.
Liquidation Rights
In the event of a voluntary or involuntary liquidation,
dissolution or winding up of the Company, the holders of our common stock will be entitled to share ratably on the basis of the number
of shares held in any of the assets available for distribution after we have paid in full, or provided for payment of, all of our debts
and after the holders of all outstanding series of any class of stock have preference over the common stock, if any, have received their
liquidation preferences in full.
Other
Our issued and outstanding shares of common stock
are fully paid and nonassessable. Holders of shares of our common stock are not entitled to preemptive rights. Shares of our common stock
are not convertible into shares of any other class of capital stock, nor are they subject to any redemption or sinking fund provisions.
Preferred Stock
We are authorized to issue up to 5,000,000 shares
of preferred stock. Our amended and restated certificate of incorporation authorizes the board to issue these shares in one or more series,
to determine the designations and the powers, preferences and relative, participating, optional or other special rights and the qualifications,
limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number
of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting
the series. Our Board of Directors could, without stockholder approval, issue preferred stock with voting and other rights that could
adversely affect the voting power and other rights of the holders of common stock and which could have the effect of making it more difficult
for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
We have no shares of preferred stock outstanding.
Outstanding Warrants to Acquire
Common Stock
As of December 20,
2023, we had outstanding:
| ● | 216,000
shares of common stock issuable upon the exercise of the August 2023 Warrants at an exercise
price of $7.24 per share; |
| ● | 15,120
shares of common stock issuable upon the exercise of the August 2023 Placement Agent Warrants
at an exercise price of $9.2063 per share; |
| ● | 40,594
shares of common stock issuable upon the exercise of additional Common Stock warrants at
a weighted average exercise price of $64.73 per share; |
| |
● |
124,200 shares of common stock issuable upon the exercise of Class A warrants at an exercise price of $140.00; and |
| |
● |
3,770 shares of common stock issuable upon the exercise of Class B warrants at an exercise price of $200.00. |
Outstanding Stock Options to Purchase our Common Stock
As of December 20, 2023, options to purchase
an aggregate of 31,361 shares of our common stock, at a weighted average exercise price of $36.28 per share, were outstanding.
Unvested Restricted Stock Units
As of December 20, 2023, 7,875 unvested restricted stock units
were outstanding.
Anti-Takeover Effects of Provisions of Our Certificate of Incorporation,
Our Bylaws and Delaware Law
Some provisions of Delaware law, our amended and restated
certificate of incorporation and our amended and restated bylaws contain provisions that could make hostile takeovers, including the following
transactions, more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise;
or the removal of our incumbent officers and directors. As a consequence, they may also inhibit temporary fluctuations in the market price
of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing
changes in the composition of our board and management. It is possible that these provisions could make it more difficult to accomplish
or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including
transactions which provide for payment of a premium over the market price for our shares.
These provisions, summarized below, are intended to
discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to
acquire control of us to first negotiate with our Board of Directors. We believe that the benefits of the increased protection of our
potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages
of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General
Corporation Law, which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination”
with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the business
combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another
prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates,
owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s
voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in
a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect
to transactions not approved in advance by the Board of Directors. A Delaware corporation may “opt out” of these provisions
with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or
bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have not
opted out of these provisions. As a result, mergers or other takeover or change in control attempts of us may be discouraged or prevented.
Undesignated Preferred Stock
The ability of our Board of Directors, without action
by the stockholders, to issue undesignated shares of preferred stock with voting or other rights or preferences as designated by our Board
of Directors could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring
hostile takeovers or delaying changes in control or management of our company.
Authorized Common Stock
Our authorized but unissued shares of common stock
will be available for future issuance without stockholder approval. These additional shares may be utilized for a variety of corporate
purposes, including future public offerings to raise additional capital and corporate acquisitions. The existence of authorized but unissued
shares of common stock could render more difficult or discourage an attempt to obtain control of a majority of our common stock by means
of a proxy contest, tender offer, merger or otherwise.
Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our amended and restated bylaws will provide advance
notice procedures for stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for
election as directors at any meeting of stockholders. Our amended and restated bylaws also will specify certain requirements regarding
the form and content of a stockholder’s notice. These provisions may preclude our stockholders from bringing matters before our
annual meeting of stockholders or from making nominations for directors at our meetings of stockholders.
No Cumulative Voting; No Action Without a Meeting; Special Meeting of
Stockholders
Stockholders will not be permitted to cumulate their
votes for the election of directors. In addition, stockholders will not be able to take action by written consent and will only be able
to take action at annual or special meetings of our stockholders. Furthermore, special meetings of our stockholders may be called only
by our Chief Executive Officer, our President or our Board of Directors.
Exclusive Forum Selection
Our amended and restated certificate of incorporation
will require, to the fullest extent permitted by law, subject to limited exceptions, that derivative actions brought in our name, actions
against directors, officers and employees for breach of fiduciary duty and other similar actions may be brought only in the Court of Chancery
in the State of Delaware and, if brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service
of process on such stockholder’s counsel in any action brought to enforce the exclusive forum provision. Any person or entity purchasing
or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum provisions
in our amended and restated certificate of incorporation.
Notwithstanding the foregoing, Section 27 of the Exchange
Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the
rules and regulations thereunder. In addition, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state
courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
As a result, the exclusive forum provision will provide that the Court of Chancery and the federal district court for the District of
Delaware will have concurrent jurisdiction over any action arising under the Securities Act or the rules and regulations thereunder, and
the exclusive forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or the rules
and regulations thereunder or any other claim for which the federal courts have exclusive jurisdiction. To the extent the exclusive forum
provision restricts the courts in which our stockholders may bring claims arising under the Securities Act and the rules and regulations
thereunder, there is uncertainty as to whether a court would enforce such provision. Investors cannot waive compliance with the federal
securities laws and the rules and regulations promulgated thereunder.
Although we believe this provision benefits our company
by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, a court may determine
that this provision is unenforceable, and to the extent it is enforceable, the provision may have the effect of discouraging lawsuits
against our directors and officers and increasing the cost to stockholders of bringing such lawsuits.
Listing
The common stock is listed on Nasdaq under the symbol
“BJDX.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Continental
Stock Transfer & Trust Company, 1 State Street, 30th Floor, New York, New York 10004.
DESCRIPTION OF SECURITIES WE ARE OFFERING
The following is a summary
of the material terms of our common stock. For additional information about our authorized capital, including our common stock and our
outstanding warrants to purchase common stock, we refer you to our amended and restated certificate of incorporation and amended and restated
bylaws that are currently in effect, which are filed as Exhibit 3.1, and Exhibit 3.3, respectively, to the registration statement of which
this prospectus forms a part, and our filings with the SEC that are incorporated by reference in this prospectus, including our Annual
Report on Form 10-K for the year ended December 31, 2022. For instructions on how to find copies of these documents, please read “Where
You Can Find Additional Information” and “Information Incorporated by Reference.”
Common Stock
The material terms and provisions of our common stock
and each other class of our securities which qualifies or limits our common stock are described under the caption “Description of
Capital Stock” in this prospectus.
Common Warrants
The following summary of certain terms and provisions
of the Common Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions
of the Common Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective
investors should carefully review the terms and provisions of the form of Common Warrant for a complete description of the terms and conditions
of the Common Warrants.
Duration, Exercise Price and Form
Each Common Warrant offered hereby will have an
exercise price equal to $1.30 per share. The Common Warrants will be immediately exercisable and may be exercised until the five
year anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject
to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock
and the exercise price. The Common Warrants will be issued separately from the common stock or the Prefunded Warrants, as the case may
be, and may be transferred separately immediately thereafter. The Common Warrants will be issued in certificated form only.
Exercisability
The Common Warrants will be exercisable, at the option
of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number
of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together
with its affiliates) may not exercise any portion of such holder’s warrants to the extent that the holder would own more than 4.99%
of the outstanding common stock (or at the election of a holder prior to the date of issuance, 9.99%) immediately after exercise, except
that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock
after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving
effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Common Warrants.
Cashless Exercise
If at the time of exercise there is no effective registration
statement registering, or the prospectus contained therein is not available for the issuance of the underlying shares to the holder, in
lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price,
the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined
according to a formula set forth in the Common Warrant.
Fundamental Transactions
In the event of a fundamental transaction, as described in the Common Warrants
and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition
of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more
than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented
by our outstanding common stock, the holders of the Common Warrants will be entitled to receive upon exercise of the Common Warrants the
kind and amount of securities, cash or other property that the holders would have received had they exercised the Common Warrants immediately
prior to such fundamental transaction. In addition, in certain circumstances, upon a fundamental transaction, the holder of a Common Warrant
will have the right to require us to repurchase its Common Warrants at the Black-Scholes value; provided, however, that, if the fundamental
transaction is not within our control, including not approved by our Board, then the holder will only be entitled to receive the same
type or form of consideration (and in the same proportion), at the Black-Scholes value of the unexercised portion of the Common Warrant
that is being offered and paid to the holders of our common stock in connection with the fundamental transaction.
Transferability
Subject to applicable laws, a Common Warrant may be
transferred at the option of the holder upon surrender of the Common Warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the Common Warrants. Rather, the number of shares of common stock to be issued will, at our election, either be rounded
up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the exercise price.
Trading Market
There is no established trading market for the Common
Warrants, and we do not expect a market to develop. We do not intend to apply for a listing of the Common Warrants on any securities exchange
or other nationally recognized trading system. Without an active trading market, the liquidity of the Common Warrants will be limited.
The common stock issuable upon exercise of the Common Warrants is currently listed on Nasdaq.
Rights as a Stockholder
Except as otherwise provided in the Common Warrants
or by virtue of the holders’ ownership of shares of common stock, the holders of the Common Warrants do not have the rights or privileges
of holders of our shares of common stock, including any voting rights, until such Common Warrant holders exercise their Common Warrants.
Waivers and Amendments
No term of the Common Warrants may be amended or waived
without the written consent of the majority of the holders of the Common Warrants purchased in this offering.
Prefunded Warrants
The following summary of certain terms and provisions
of the Prefunded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions
of the Prefunded Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Prospective investors should carefully review the terms and provisions of the form of Prefunded Warrant for a complete description of
the terms and conditions of the Prefunded Warrants.
Duration, Exercise Price and Form
The Prefunded Warrants offered hereby will have an exercise price of $0.0001
per share. The Prefunded Warrants will be immediately exercisable and may be exercised at any time after their original issuance until
such Prefunded Warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise are subject
to appropriate adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our shares of common
stock. The Prefunded Warrants and Common Warrants are immediately separable and will be issued separately in this offering, but must be
purchased together in this offering. The Prefunded Warrants will be issued in certificated form only.
Exercisability
The Prefunded Warrants will be exercisable, at the
option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the
number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder
(together with its affiliates) may not exercise any portion of the Prefunded Warrant to the extent that the holder would own more than
4.99% (or at the election of a holder prior to the date of issuance, 9.99%) of the outstanding common stock immediately after exercise;
provided, however, that upon 61 days’ notice to us, the holder may increase or decrease such beneficial ownership limitation, provided
that in no event shall the beneficial ownership limitation exceed 9.99% and any increase in the beneficial ownership limitation will not
be effective until 61 days following notice of such increase from the holder to us.
Cashless Exercise
At the time a holder exercises its Prefunded Warrants,
in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price,
the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined
according to a formula set forth in the Prefunded Warrant.
Fundamental Transactions
In the event of a fundamental transaction, as described
in the Prefunded Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale,
transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another
person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50%
of the voting power represented by our outstanding common stock, the holders of the Prefunded Warrants will be entitled to receive upon
exercise of the Prefunded Warrants the kind and amount of securities, cash or other property that the holders would have received had
they exercised the Prefunded Warrants immediately prior to such fundamental transaction.
Transferability
Subject to applicable laws, a Prefunded Warrant may
be transferred at the option of the holder upon surrender of the Prefunded Warrant to us together with the appropriate instruments of
transfer.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the Prefunded Warrants. Rather, the number of shares of common stock to be issued will, at our election, either be
rounded up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the exercise price.
Trading Market
There is no established trading market for the Prefunded
Warrants, and we do not expect a market to develop. We do not intend to apply for a listing of the Prefunded Warrants on any securities
exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Prefunded Warrants will
be limited. The common stock issuable upon exercise of the Prefunded Warrants is currently listed on Nasdaq.
Rights as a Stockholder
Except as otherwise provided in the Prefunded Warrants
or by virtue of the holders’ ownership of shares of common stock, the holders of Prefunded Warrants do not have the rights or privileges
of holders of our shares of common stock, including any voting rights, until such Prefunded Warrant holders exercise their warrants.
Waivers and Amendments
No term of the Prefunded Warrants may be amended or
waived without the written consent of the majority of the holders of the Prefunded Warrants purchased in this offering.
Placement Agent Warrants
We have also agreed to issue to the
placement agent or its designees, the Placement Agent Warrants as compensation in connection with this offering to purchase up to
188,462 shares of common stock as compensation in connection with this offering. The Placement Agent Warrants will be exercisable
immediately and will have substantially the same terms as the Common Warrants described above, except that the Placement Agent
Warrants will have an exercise price of $1.6250 per share (representing 125% of the combined public offering price per share and
accompanying Common Warrant) and a termination date that will be five years from the commencement of the sales pursuant to this
offering. See “Plan of Distribution” below.
Anti-Takeover Effects of Provisions of Our Certificate of Incorporation,
Our Bylaws and Delaware Law
Some provisions of Delaware law, our amended and restated
certificate of incorporation and our amended and restated bylaws contain provisions that could make hostile takeovers, including the following
transactions, more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise;
or the removal of our incumbent officers and directors. As a consequence, they may also inhibit temporary fluctuations in the market price
of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing
changes in the composition of our board and management. It is possible that these provisions could make it more difficult to accomplish
or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including
transactions which provide for payment of a premium over the market price for our shares.
These provisions, summarized below, are intended to
discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to
acquire control of us to first negotiate with our Board of Directors. We believe that the benefits of the increased protection of our
potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages
of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General
Corporation Law, which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination”
with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the business
combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another
prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates,
owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s
voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in
a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions
not approved in advance by the Board of Directors. A Delaware corporation may “opt out” of these provisions with an express
provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting
from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have not opted out of these
provisions. As a result, mergers or other takeover or change in control attempts of us may be discouraged or prevented.
Undesignated Preferred Stock
The ability of our Board of Directors, without action
by the stockholders, to issue undesignated shares of preferred stock with voting or other rights or preferences as designated by our Board
of Directors could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring
hostile takeovers or delaying changes in control or management of our company.
Authorized Common Stock
Our authorized but unissued shares of common stock
will be available for future issuance without stockholder approval. These additional shares may be utilized for a variety of corporate
purposes, including future public offerings to raise additional capital and corporate acquisitions. The existence of authorized but unissued
shares of common stock could render more difficult or discourage an attempt to obtain control of a majority of our common stock by means
of a proxy contest, tender offer, merger or otherwise.
Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our amended and restated bylaws will provide advance
notice procedures for stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for
election as directors at any meeting of stockholders. Our amended and restated bylaws also will specify certain requirements regarding
the form and content of a stockholder’s notice. These provisions may preclude our stockholders from bringing matters before our
annual meeting of stockholders or from making nominations for directors at our meetings of stockholders.
No
Cumulative Voting; No Action Without a Meeting; Special Meeting of Stockholders
Stockholders
will not be permitted to cumulate their votes for the election of directors. In addition, stockholders will not be able to take action
by written consent and will only be able to take action at annual or special meetings of our stockholders. Furthermore, special meetings
of our stockholders may be called only by our Chief Executive Officer, our President or our Board of Directors.
Exclusive
Forum Selection
Our
amended and restated certificate of incorporation will require, to the fullest extent permitted by law, subject to limited exceptions,
that derivative actions brought in our name, actions against directors, officers and employees for breach of fiduciary duty and other
similar actions may be brought only in the Court of Chancery in the State of Delaware and, if brought outside of Delaware, the stockholder
bringing the suit will be deemed to have consented to service of process on such stockholder’s counsel in any action brought to
enforce the exclusive forum provision. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock
shall be deemed to have notice of and consented to the forum provisions in our amended and restated certificate of incorporation.
Notwithstanding
the foregoing, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability
created by the Exchange Act or the rules and regulations thereunder. In addition, Section 22 of the Securities Act creates
concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities
Act or the rules and regulations thereunder. As a result, the exclusive forum provision will provide that the Court of Chancery
and the federal district court for the District of Delaware will have concurrent jurisdiction over any action arising under the Securities
Act or the rules and regulations thereunder, and the exclusive forum provision will not apply to suits brought to enforce any duty
or liability created by the Exchange Act or the rules and regulations thereunder or any other claim for which the federal courts
have exclusive jurisdiction. To the extent the exclusive forum provision restricts the courts in which our stockholders may bring claims
arising under the Securities Act and the rules and regulations thereunder, there is uncertainty as to whether a court would enforce
such provision. Investors cannot waive compliance with the federal securities laws and the rules and regulations promulgated thereunder.
Although
we believe this provision benefits our company by providing increased consistency in the application of Delaware law in the types of
lawsuits to which it applies, a court may determine that this provision is unenforceable, and to the extent it is enforceable, the provision
may have the effect of discouraging lawsuits against our directors and officers and increasing the cost to stockholders of bringing such
lawsuits.
PLAN
OF DISTRIBUTION
Pursuant to an engagement agreement, dated August
7, 2023 (as amended, the “Engagement Agreement”), we have engaged H.C. Wainwright & Co., LLC to act as our exclusive placement
agent to solicit offers to purchase the securities offered pursuant to this prospectus on a reasonable best efforts basis. The Engagement
Agreement does not give rise to any commitment by the placement agent to purchase any of our securities, and the placement agent will
have no authority to bind us by virtue of the Engagement Agreement. The placement agent is not purchasing or selling any of the securities
offered by us under this prospectus, nor is it required to arrange for the purchase or sale of any specific number or dollar amount of
securities. This is a best efforts offering and there is no minimum offering amount required as a condition to the closing of this offering.
The placement agent has agreed to use reasonable best efforts to arrange for the sale of the securities by us.
Certain institutional investors purchasing securities
offered hereby have entered into a securities purchase agreement with us. In addition to rights and remedies available to all purchasers
in this offering under federal securities and state law, the purchasers which entered into the securities purchase agreement will also
be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract is material to larger purchasers
in this offering as a means to enforce the following covenants uniquely available to them under the securities purchase agreement: (i)
a covenant to not enter into variable rate financings for a period of one year following the closing of the offering, subject to an exception;
and (ii) a covenant to not enter into any equity financings for 60 days from closing of the offering, subject to certain exceptions. The
nature of the representations, warranties and covenants in the securities purchase agreements shall include:
| ● | standard
issuer representations and warranties on matters such as organization, qualification, authorization,
no conflict, no governmental filings required, current in SEC filings, no litigation, labor
or other compliance issues, environmental, intellectual property and title matters and compliance
with various laws such as the Foreign Corrupt Practices Act; and |
| ● | covenants
regarding matters such as registration of warrant shares, no integration with other offerings,
filing of an 8-K to disclose entering into these securities purchase agreements, no stockholder
rights plans, no material nonpublic information, use of proceeds, indemnification of purchasers,
reservation and listing of shares of common stock, and no subsequent equity sales for 60 days. |
We expect to deliver the securities being offered
pursuant to this prospectus on or about January 2, 2024. There is no minimum number of securities or amount of proceeds that is a
condition to closing of this offering.
Fees
and Expenses
We
have agreed to pay the placement agent a total cash fee equal to 7.0% of the aggregate gross proceeds raised in the offering and a management
fee equal to 1.0% of the gross proceeds raised in this offering. We will also pay the placement agent a non-accountable expense allowance
of $50,000, its legal fees and expenses in an amount up to $100,000 and its clearing fees in an amount up to $15,950 in connection with
this offering. We estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent fees
and expenses, will be approximately $220,000.
Placement
Agent Warrants
In addition, we have agreed to issue to the placement
agent or its designees the Placement Agent Warrants as compensation in connection with this offering, to purchase up to 7.0% of the aggregate
number of shares of common stock sold in this offering (including shares underlying any Prefunded Warrants), at an exercise price equal
to 125% of the combined public offering price per share and accompanying Common Warrant to be sold in this offering. The Placement Agent
Warrants will be exercisable upon issuance and will expire five years from the commencement of sales under this offering.
If at the time of exercise there is no effective
registration statement registering, or the prospectus contained therein is not available for the resale of warrant shares by the holders
of the Placement Agent Warrants, then the Placement Agent Warrants may be exercised, in whole or in part, at such time by means of a “cashless
exercise” in which the holders shall be entitled to receive a number of warrant shares as calculated in the Placement Agent Warrants.
The Placement Agent Warrants provide for customary
anti-dilution provisions (for share dividends, splits and recapitalizations and the like) consistent with FINRA Rule 5110.
Tail
In
the event that any investors that were contacted by the placement agent or were introduced to the Company by the placement agent during
the term of our engagement agreement with the placement agent provide any capital to us in a public or private offering or capital-raising
transaction within 12 months following the termination or expiration of our engagement agreement with the placement agent, we shall pay
the placement agent the cash and warrant compensation provided above on the gross proceeds from such investors. The placement agent will
only be entitled to such fee to the extent that the parties are directly introduced to us by the placement agent, in accordance with
FINRA Rule 2010.
Right
of First Refusal
If,
from the date of the Engagement Agreement until the 12-month anniversary following consummation of each offering of our securities during
the term of the Engagement Agreement, we or any of our subsidiaries decides to raise funds by means of a public offering (excluding an
at-the-market facility) or a private placement or any other capital-raising financing of equity, equity-linked or debt securities, the
placement agent (or any affiliate designated by the placement agent) shall have the right to act as sole book-running manager, sole underwriter
or sole placement agent for such financing. If the placement agent or one of its affiliates decides to accept any such engagement, the
agreement governing such engagement will contain, among other things, provisions for customary fees for transactions of similar size
and nature and the provisions of the Engagement Agreement, including indemnification, which are appropriate to such a transaction.
Lock-Up
Agreements
Our officers, directors and 10% or greater stockholders representing
beneficial ownership of 38% of our outstanding shares of common stock as of December 20, 2023, have agreed with the placement agent to
be subject to a lock-up period of 60 days following the date of the securities purchase agreement. This means that, during the applicable
lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase,
pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into,
or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if the
transferee agrees to these lock-up restrictions. We have also agreed to similar lock-up restrictions on the issuance and sale of our securities
for 60 days following the closing of this offering, although we will be permitted to issue stock options or stock awards to directors,
officers and employees under our existing plans. The lock-up period is subject to an additional extension to accommodate for our reports
of financial results or material news releases. The placement agent may, in its sole discretion and without notice, waive the terms of
any of these lock-up agreements.
In
addition, subject to certain exceptions, we have agreed to not issue any securities that are subject to a price reset based on the trading
prices of our common stock or upon a specified or contingent event in the future, or enter into any agreement to issue securities at
a future determined price for a period of one year following the closing date of this offering.
Indemnification
We
have agreed to indemnify the placement agent against certain liabilities, including certain liabilities under the Securities Act, or
to contribute to payments that the placement agent may be required to make in respect of those liabilities.
In
addition, we will indemnify the purchasers of securities in this offering against liabilities arising out of or relating to (i) any breach
of any of the representations, warranties, covenants or agreements made by us in the securities purchase agreement or related documents
or (ii) any action instituted against a purchaser by a third party (other than a third party who is affiliated with such purchaser) with
respect to the securities purchase agreement or related documents and the transactions contemplated thereby, subject to certain exceptions
Regulation
M Compliance
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any fees received
by it and any profit realized on the sale of our securities offered hereby by it while acting as principal might be deemed to be underwriting
discounts or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities
Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations
may limit the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement
agent may not (i) engage in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities
or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed
their participation in the distribution.
Other
Relationships
The
placement agent and its affiliates have engaged, and may in the future engage, in investment banking transactions and other commercial
dealings in the ordinary course of business with us or our affiliates. The placement agent has received, or may in the future receive,
customary fees and commissions for these transactions.
In
addition, in the ordinary course of their business activities, the placement agent and its affiliates may make or hold a broad array
of investments and actively trade debt and equity securities (or related derivative securities) for their own account and for the accounts
of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The
placement agent and its affiliates may also make investment recommendations and/or publish or express independent research views in respect
of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such
securities and instruments.
The
placement agent acted as the exclusive placement agent for the August 2023 Offering and it received as compensation cash fees and the
August 2023 Placement Agent Warrants to purchase up to 15,120 shares of common stock. The August 2023 Placement Agent Warrants have substantially
the same terms as the August 2023 Warrants, except that the August 2023 Placement Agent Warrants have an exercise price equal to $9.2063
per share and a term of five years from the commencement of the sales pursuant to the August 2023 Offering. Except as disclosed in this
prospectus, we have no present arrangements with the placement agent for any further services.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the placement agent and the placement agent may distribute
prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus
or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent
and should not be relied upon by investors.
Transfer
Agent
The
transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company.
Nasdaq
listing
Our
shares of common stock are listed on Nasdaq under the symbol “BJDX.”
LEGAL
MATTERS
The
validity of the securities offered hereby is being passed upon for us by Hogan Lovells US LLP. Haynes and Boone, LLP, New York, New York
is acting as counsel for the placement agent in connection with this offering.
EXPERTS
Wolf &
Company P.C., independent registered public accounting firm, has audited our consolidated financial statements included in our Annual
Report on Form 10-K, as amended, for the year ended December 31, 2022, as set forth in their report (which contains an explanatory paragraph
describing conditions that raise substantial doubt about our ability to continue as a going concern as described in Note 1 to the consolidated
financial statements), which is incorporated by reference in this prospectus supplement and elsewhere in the registration statement.
Our financial statements are incorporated by reference in reliance on Wolf & Company P.C.’s report, given on their authority
as experts in accounting and auditing.
INFORMATION
INCORPORATED BY REFERENCE
The
SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important
information to you by referring you to another document filed separately with the SEC. The SEC file number for the documents incorporated
by reference in this prospectus is 001-41031. The documents incorporated by reference into this prospectus contain important information
that you should read about us. All documents incorporated by reference into this prospectus that were filed prior to July 24, 2023, do
not give effect to the Reverse Stock Split.
The
following documents are incorporated by reference into this document:
| ● | our
Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March
20, 2023, as amended by Amendment No. 1 thereto, filed on May
1, 2023; |
| ● | our
Quarterly Report on Form
10-Q for the fiscal quarter ended March 31, 2023, filed on May 11, 2023, our Quarterly
Report on Form
10-Q for the fiscal quarter ended June 30, 2023, filed on August 14, 2023, and our Quarterly
Report on Form
10-Q for the fiscal quarter ended September 30, 2023, filed on November 9, 2023; |
| ● | those
portions of our Definitive Proxy Statement on Schedule
14A filed on May 18, 2023 that are deemed “filed” with the SEC; |
| ● | our
Current Reports on Form 8-K (other than portions thereof furnished under Item 2.02 or Item
7.01 of Form 8-K and exhibits accompanying such reports that relate to such items) filed
with the SEC on January
27, 2023, April
27, 2023, May
19, 2023, June
20, 2023, July
21, 2023, August
10, 2023, August
28, 2023, October
2, 2023, October
16, 2023, October
26, 2023 and December 27, 2023;
and |
| ● | The
description of our common stock, par value $0.0001 per share contained in its Registration
Statement on Form
8-A, dated and filed with the SEC on November 5, 2021, as amended by the description
of our common stock contained in Exhibit
4.6 to our Annual Report on Form
10-K for the year ended December 31, 2022, including all amendments and reports updating
that description. |
We
also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of
Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a),
13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement of which this prospectus is a part and prior
to the effectiveness of such registration statement and all documents that are filed by us with the SEC pursuant to Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act after the date of this prospectus but prior to the termination of the offering. These documents include
periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy
statements.
Any
statement contained herein or in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed
to be modified or superseded for purposes of the document to the extent that a statement contained in this prospectus or any other subsequently
filed document that is deemed to be incorporated by reference into this document modifies or supersedes the statement.
We
will provide without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral
request, a copy of any or all documents that are incorporated by reference into this prospectus, but not delivered with the prospectus,
other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus
incorporates. You should direct oral or written requests by one of the following methods. Attention: Investor Relations, Bluejay Diagnostics,
Inc., 360 Massachusetts Avenue, Suite 203, Acton, MA, 01720, (844) 327-7078. You may also access these documents, free of charge on the
SEC’s website at www.sec.gov or on the “Investors” page of our website at www.bluejaydx.com. The information found
on our website, or that may be accessed by links on our website, is not part of this prospectus. We have included our website address
solely as an inactive textual reference. Investors should not rely on any such information in deciding whether to purchase our common
stock.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
We filed with the SEC a registration statement
under the Securities Act for the securities offered by this prospectus. This prospectus does not contain all of the information in the
registration statement and the exhibits and schedule that were filed with the registration statement. For further information with respect
to us and our securities, we refer you to the registration statement and the exhibits and schedule that were filed with the registration
statement. Statements contained in this prospectus about the contents of any contract or any other document that is filed as an exhibit
to the registration statement of which this prospectus forms a part are not necessarily complete, and we refer you to the full text of
the contract or other document filed as an exhibit to the registration statement. The SEC maintains an Internet website at http://www.sec.gov
that contains reports, proxy and information statements, and other information regarding registrants that file electronically with the
SEC.
We
file periodic reports and current reports under the Exchange Act, including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q
and Current Reports on Form 8-K, and other information with the Securities and Exchange Commission. These periodic reports and other
information are available for inspection and copying at the SEC regional offices, public reference facilities and on the website of the
SEC referred to above.
We
make available free of charge on or through our internet website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current
reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange
Act of 1934 as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The information
found on our website, www.bluejaydx.com, other than as specifically incorporated by reference in this prospectus, is not part of this
prospectus.

BLUEJAY
DIAGNOSTICS, INC.
537,768 Shares of Common
Stock
Prefunded Warrants to purchase up to 2,154,540
Shares of Common Stock
Common Warrants to purchase up to 2,692,308
Shares of Common Stock
4,846,848 Shares of Common Stock underlying
Prefunded Warrants and Common Warrants
Placement Agent Warrants to Purchase up to 188,462
Shares of Common Stock
188,462 Shares of Common
Stock Underlying the Placement Agent Warrants
Prospectus
December
27, 2023
H.C.
Wainwright & Co.
Bluejay Diagnostics (NASDAQ:BJDX)
Historical Stock Chart
From Mar 2024 to Apr 2024
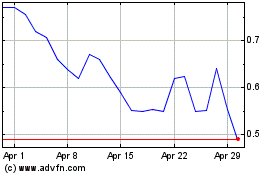
Bluejay Diagnostics (NASDAQ:BJDX)
Historical Stock Chart
From Apr 2023 to Apr 2024