06-300001262104Q1false2024DE2028-03-310001262104us-gaap:SubsequentEventMember2023-10-012023-10-010001262104meip:KkcAgreementsMembermeip:KyowaKirinCoMember2023-07-012023-09-300001262104meip:TwoThousandAndEightOmnibusPlanMemberus-gaap:EmployeeStockOptionMember2023-09-300001262104us-gaap:MeasurementInputExpectedDividendRateMember2023-06-300001262104us-gaap:FairValueMeasurementsRecurringMember2023-06-300001262104meip:PotentialPaymentsOnAchievementOfDevelopmentRegulatoryAndCommercialMilestonesMembermeip:PresageLicenseAgreementMembermeip:PresageBiosciencesIncMember2023-09-300001262104us-gaap:RetainedEarningsMember2022-09-300001262104meip:TwoThousandAndEightOmnibusPlanMember2023-09-300001262104us-gaap:ContractTerminationMember2022-07-012022-12-310001262104us-gaap:RevenueFromContractWithCustomerMemberus-gaap:TransferredAtPointInTimeMember2022-07-012022-09-300001262104us-gaap:GeneralAndAdministrativeExpenseMember2022-07-012022-09-300001262104us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2022-06-300001262104us-gaap:WarrantMembermeip:TorreyaPartnersMember2023-09-300001262104meip:SanDiegoCaliforniaMember2022-07-0100012621042022-09-300001262104us-gaap:MeasurementInputRiskFreeInterestRateMember2023-06-300001262104us-gaap:EmployeeStockOptionMember2023-09-300001262104us-gaap:LeaseholdImprovementsMember2023-06-300001262104us-gaap:WarrantMember2023-07-012023-09-300001262104us-gaap:AdditionalPaidInCapitalMember2023-07-012023-09-300001262104us-gaap:SubsequentEventMember2023-10-0100012621042022-07-012023-06-300001262104us-gaap:OneTimeTerminationBenefitsMember2023-06-300001262104meip:ServicesPerformedOverTimeMemberus-gaap:RevenueFromContractWithCustomerMember2022-07-012022-09-300001262104us-gaap:AccountingStandardsUpdate201602Membermeip:SanDiegoCaliforniaMember2020-07-310001262104us-gaap:WarrantMember2022-07-012022-09-300001262104us-gaap:ContractTerminationMember2021-07-012022-06-300001262104us-gaap:RetainedEarningsMember2022-07-012022-09-3000012621042022-07-012022-09-300001262104us-gaap:EmployeeStockOptionMember2023-07-012023-09-300001262104meip:InducementPlanMember2023-09-300001262104meip:PresageLicenseAgreementMembermeip:PresageBiosciencesIncMember2023-09-300001262104meip:RevenueFromCollaborationAgreementsMember2023-07-012023-09-300001262104meip:IncrementalPaymentMembermeip:PresageLicenseAgreementMembermeip:PresageBiosciencesIncMember2023-09-300001262104us-gaap:AdditionalPaidInCapitalMember2022-07-012022-09-300001262104us-gaap:AdditionalPaidInCapitalMember2023-06-300001262104meip:KyowaKirinCoMember2020-04-300001262104us-gaap:RetainedEarningsMember2022-06-300001262104meip:ServicesPerformedOverTimeMemberus-gaap:RevenueFromContractWithCustomerMember2023-07-012023-09-300001262104meip:UsLicenseMembermeip:KyowaKirinCoMember2023-06-300001262104us-gaap:RetainedEarningsMember2023-09-3000012621042023-07-012023-09-3000012621042022-06-3000012621042022-12-310001262104meip:FurnitureAndEquipmentMember2023-09-300001262104us-gaap:MeasurementInputExpectedTermMember2023-06-300001262104us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2022-09-300001262104us-gaap:OneTimeTerminationBenefitsMember2023-09-300001262104us-gaap:SubsequentEventMember2023-10-312023-10-310001262104us-gaap:AdditionalPaidInCapitalMember2023-09-300001262104us-gaap:MeasurementInputPriceVolatilityMember2023-06-300001262104us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2022-07-012022-09-300001262104us-gaap:SubsequentEventMember2023-11-062023-11-060001262104meip:FurnitureAndEquipmentMember2023-06-3000012621042023-06-300001262104us-gaap:RestrictedStockUnitsRSUMember2023-07-012023-09-300001262104us-gaap:CommonStockMember2023-09-300001262104us-gaap:EmployeeStockOptionMembersrt:DirectorMember2023-07-012023-09-300001262104us-gaap:RestrictedStockUnitsRSUMember2022-07-012022-09-300001262104meip:RevenueFromCollaborationAgreementsMember2022-07-012022-09-300001262104us-gaap:OneTimeTerminationBenefitsMember2023-07-012023-09-300001262104meip:KyowaKirinCoMember2022-11-012022-11-300001262104us-gaap:CommonStockMember2022-06-300001262104meip:PresageLicenseAgreementMember2023-09-300001262104us-gaap:WarrantMember2023-09-3000012621042023-09-300001262104us-gaap:GeneralAndAdministrativeExpenseMember2023-07-012023-09-300001262104meip:PresageLicenseAgreementMembermeip:PresageBiosciencesIncMember2017-09-012017-09-300001262104us-gaap:AccountingStandardsUpdate201602Member2022-07-310001262104meip:MeasurementInputWeightedAverageGrantDateFairValueMember2023-06-300001262104us-gaap:ResearchAndDevelopmentExpenseMember2022-07-012022-09-300001262104meip:PassThroughServicesMember2023-07-012023-09-300001262104us-gaap:RevenueFromContractWithCustomerMemberus-gaap:TransferredAtPointInTimeMember2023-07-012023-09-300001262104meip:InducementPlanMemberus-gaap:EmployeeStockOptionMember2023-09-300001262104us-gaap:AdditionalPaidInCapitalMember2022-09-300001262104us-gaap:AdditionalPaidInCapitalMember2022-06-300001262104us-gaap:WarrantMember2023-05-310001262104meip:InducementPlanMember2021-05-310001262104us-gaap:CommonStockMember2022-09-300001262104us-gaap:ResearchAndDevelopmentExpenseMember2023-07-012023-09-300001262104us-gaap:RevenueFromContractWithCustomerMember2023-07-012023-09-3000012621042023-10-310001262104us-gaap:LeaseholdImprovementsMember2023-09-300001262104us-gaap:CommonStockMember2022-07-012022-09-300001262104us-gaap:RevenueFromContractWithCustomerMember2022-07-012022-09-300001262104us-gaap:CommonStockMember2023-06-300001262104us-gaap:EmployeeStockOptionMember2022-07-012022-09-300001262104us-gaap:RetainedEarningsMember2023-06-300001262104us-gaap:RetainedEarningsMember2023-07-012023-09-300001262104meip:EmployeesMemberus-gaap:EmployeeStockOptionMember2023-07-012023-09-300001262104us-gaap:FairValueMeasurementsRecurringMember2023-09-30meip:Employeexbrli:pureutr:sqftxbrli:sharesiso4217:USDutr:Y
Table of Contents
ROC
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
|
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2023
OR
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
|
|
|
For the transition period from |
|
to |
|
|
Commission File Number: 001-41827
MEI Pharma, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
DELAWARE |
|
51-0407811 |
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
11455 El Camino Real Suite 250, San Diego, CA 92130
(Address of principal executive offices) (Zip Code)
(858) 369-7100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.00000002 par value |
|
MEIP |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
Emerging growth company |
|
☐ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☒ No ☐
As of October 31, 2023, the number of shares outstanding of the issuer’s common stock, $0.00000002 par value, was 6,662,857.
Table of Contents
MEI PHARMA, INC.
Table of Contents
2
Table of Contents
PART I FINANCIAL INFORMATION
Item 1. Condensed Consolidated Financial Statements
MEI PHARMA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except par value data)
|
|
|
|
|
|
|
|
|
|
|
September 30, |
|
|
June 30, |
|
|
|
2023 |
|
|
2023 |
|
ASSETS |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
3,372 |
|
|
$ |
16,906 |
|
Short-term investments |
|
|
78,830 |
|
|
|
83,787 |
|
Unbilled receivables |
|
|
— |
|
|
|
85 |
|
Prepaid expenses and other current assets |
|
|
6,220 |
|
|
|
6,750 |
|
Total current assets |
|
|
88,422 |
|
|
|
107,528 |
|
Operating lease right-of-use asset |
|
|
11,600 |
|
|
|
11,972 |
|
Property and equipment, net |
|
|
1,229 |
|
|
|
1,309 |
|
Total assets |
|
$ |
101,251 |
|
|
$ |
120,809 |
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
3,220 |
|
|
$ |
6,134 |
|
Accrued liabilities |
|
|
4,289 |
|
|
|
12,461 |
|
Deferred revenue |
|
|
— |
|
|
|
317 |
|
Operating lease liability |
|
|
1,055 |
|
|
|
1,428 |
|
Total current liabilities |
|
|
8,564 |
|
|
|
20,340 |
|
Deferred revenue, long-term |
|
|
— |
|
|
|
64,545 |
|
Operating lease liability, long-term |
|
|
11,326 |
|
|
|
11,300 |
|
Total liabilities |
|
|
19,890 |
|
|
|
96,185 |
|
Commitments and contingencies (Note 6) |
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
Preferred stock, $0.01 par value; 100 shares authorized; none outstanding |
|
|
— |
|
|
|
— |
|
Common stock, $0.00000002 par value; 226,000 shares authorized; 6,663
shares issued and outstanding at September 30, 2023 and June 30, 2023. |
|
|
— |
|
|
|
— |
|
Additional paid-in capital |
|
|
430,984 |
|
|
|
430,621 |
|
Accumulated deficit |
|
|
(349,623 |
) |
|
|
(405,997 |
) |
Total stockholders’ equity |
|
|
81,361 |
|
|
|
24,624 |
|
Total liabilities and stockholders’ equity |
|
$ |
101,251 |
|
|
$ |
120,809 |
|
See accompanying notes to condensed consolidated financial statements.
3
Table of Contents
MEI PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Revenues: |
|
|
|
|
|
|
Revenue from customers |
|
$ |
752 |
|
|
$ |
8,730 |
|
Revenue from collaboration agreements |
|
|
64,545 |
|
|
|
— |
|
Total revenues |
|
|
65,297 |
|
|
|
8,730 |
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
3,485 |
|
|
|
19,463 |
|
General and administrative |
|
|
6,531 |
|
|
|
7,486 |
|
Total operating expenses |
|
|
10,016 |
|
|
|
26,949 |
|
Income (loss) from operations |
|
|
55,281 |
|
|
|
(18,219 |
) |
Other income (expense): |
|
|
|
|
|
|
Change in fair value of warrant liability |
|
|
— |
|
|
|
1,117 |
|
Interest and dividend income |
|
|
1,094 |
|
|
|
480 |
|
Other expense, net |
|
|
(1 |
) |
|
|
(2 |
) |
Total other income, net |
|
|
1,093 |
|
|
|
1,595 |
|
Net income (loss) |
|
$ |
56,374 |
|
|
$ |
(16,624 |
) |
|
|
|
|
|
|
|
Net income (loss) per share - basic and diluted |
|
$ |
8.46 |
|
|
$ |
(2.49 |
) |
|
|
|
|
|
|
|
Weighted-average shares used in computing net income (loss) per share - basic and diluted |
|
|
6,663 |
|
|
|
6,663 |
|
See accompanying notes to condensed consolidated financial statements.
4
Table of Contents
MEI PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
Shares |
|
|
Additional
Paid-In
Capital |
|
|
Accumulated
Deficit |
|
|
Total
Stockholders’
Equity |
|
Balance at June 30, 2023 |
|
|
6,663 |
|
|
$ |
430,621 |
|
|
$ |
(405,997 |
) |
|
$ |
24,624 |
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
56,374 |
|
|
|
56,374 |
|
Share-based compensation expense |
|
|
— |
|
|
|
363 |
|
|
|
— |
|
|
|
363 |
|
Balance at September 30, 2023 |
|
|
6,663 |
|
|
$ |
430,984 |
|
|
$ |
(349,623 |
) |
|
$ |
81,361 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
Shares |
|
|
Additional
Paid-In
Capital |
|
|
Accumulated
Deficit |
|
|
Total
Stockholders’
Equity |
|
Balance at June 30, 2022 |
|
|
6,658 |
|
|
$ |
426,572 |
|
|
$ |
(374,159 |
) |
|
$ |
52,413 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
(16,624 |
) |
|
|
(16,624 |
) |
Issuance of common stock for vested restricted stock units |
|
|
5 |
|
|
|
(40 |
) |
|
|
— |
|
|
|
(40 |
) |
Share-based compensation expense |
|
|
— |
|
|
|
1,559 |
|
|
|
— |
|
|
|
1,559 |
|
Balance at September 30, 2022 |
|
|
6,663 |
|
|
$ |
428,091 |
|
|
$ |
(390,783 |
) |
|
$ |
37,308 |
|
See accompanying notes to condensed consolidated financial statements.
5
Table of Contents
MEI PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Cash flows from operating activities: |
|
|
|
|
|
|
Net income (loss) |
|
$ |
56,374 |
|
|
$ |
(16,624 |
) |
Adjustments to reconcile net income (loss) to net cash used in operating activities: |
|
|
|
|
|
|
Change in fair value of warrant liability |
|
|
— |
|
|
|
(1,117 |
) |
Share-based compensation |
|
|
363 |
|
|
|
1,559 |
|
Non-cash lease expense |
|
|
372 |
|
|
|
349 |
|
Depreciation expense |
|
|
87 |
|
|
|
99 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Unbilled receivables |
|
|
85 |
|
|
|
2,286 |
|
Prepaid expenses and other current assets |
|
|
530 |
|
|
|
1,048 |
|
Accounts payable |
|
|
(2,921 |
) |
|
|
500 |
|
Accrued liabilities |
|
|
(8,172 |
) |
|
|
(1,604 |
) |
Deferred revenue |
|
|
(64,862 |
) |
|
|
(971 |
) |
Operating lease liability |
|
|
(347 |
) |
|
|
(307 |
) |
Net cash used in operating activities |
|
|
(18,491 |
) |
|
|
(14,782 |
) |
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
Purchases of property and equipment |
|
|
— |
|
|
|
(63 |
) |
Purchases of short-term investments |
|
|
(24,197 |
) |
|
|
(34,052 |
) |
Proceeds from maturity of short-term investments |
|
|
29,154 |
|
|
|
47,850 |
|
Net cash provided by investing activities |
|
|
4,957 |
|
|
|
13,735 |
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
Payments of tax withholdings related to vesting of restricted stock units |
|
|
— |
|
|
|
(40 |
) |
Net cash (used in) provided by financing activities |
|
|
— |
|
|
|
(40 |
) |
Net decrease in cash and cash equivalents |
|
|
(13,534 |
) |
|
|
(1,087 |
) |
Cash and cash equivalents at beginning of the period |
|
|
16,906 |
|
|
|
15,740 |
|
Cash and cash equivalents at end of the period |
|
$ |
3,372 |
|
|
$ |
14,653 |
|
|
|
|
|
|
|
|
Supplemental cash flow information: |
|
|
|
|
|
|
Purchases of property and equipment included in accounts payable |
|
$ |
7 |
|
|
$ |
— |
|
Operating lease right-of-use assets obtained in exchange for operating lease liabilities |
|
$ |
— |
|
|
$ |
4,347 |
|
|
|
|
|
|
|
|
See accompanying notes to condensed consolidated financial statements.
6
Table of Contents
MEI PHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Description of Business and Basis of Presentation
Description of Business
MEI Pharma, Inc. (Nasdaq: MEIP) is a clinical-stage pharmaceutical company committed to developing novel and differentiated cancer therapies. We build our pipeline by acquiring promising cancer agents and creating value in programs through development, strategic partnerships, and out-licensing or commercialization, as appropriate. Our approach to oncology drug development is to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. Our pipeline includes voruciclib, an oral cyclin-dependent kinase 9 (“CDK9”) inhibitor, and ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway.
Reverse Stock Split
On April 14, 2023, we amended our Certificate of Incorporation to affect a combination of our issued and outstanding common stock at a ratio of one-for-twenty (“Reverse Stock Split”). The par value and authorized shares of our common stock were not adjusted as a result of the Reverse Stock Split. The Reverse Stock Split was effective on April 14, 2023, with a market effective date of April 17, 2023. All historical share and per share amounts have been adjusted to reflect the Reverse Stock Split for all periods presented. All stock options, restricted stock units and warrants outstanding were ratably adjusted to give effect to the Reverse Stock Split.
Current Events
Cooperation Agreement
On October 31, 2023, we announced a Cooperation Agreement (“Cooperation Agreement”) with Anson Funds and Cable Car Capital of which, among other non-financial related items as described within the overview section of Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations, contained a capital return to stockholders in the form of a dividend in the amount of $1.75 per share of common stock to all stockholders. Additionally, a potential second return of capital of approximately $9.33 million in the aggregate will be authorized by the board of directors ("Board") should our ongoing ME-344 Phase 1b trial fail to meet certain defined endpoints or our Board determines not to proceed with a second cohort, both as further described in the Cooperation Agreement. The potential second return of capital may take the form of a dividend or tender offer and is subject to Board approval as well as modification associated with applicable requirements under Delaware law, both as detailed in the Cooperation Agreement. The $1.75 dividend is anticipated to be distributed during the second quarter of fiscal year 2024.
As part of the Cooperation Agreement, Anson and Cable Car withdrew their consent solicitation and agreed to abide by customary standstill provisions. Additionally, we will reimburse Anson and Cable Car’s fees and expenses related to their engagement with us to date, in an amount not to exceed $1.2 million. As of September 30, 2023, no amounts were expensed or accrued in connection with the Cooperation Agreement.
Cash Dividend
On November 6, 2023, we announced that pursuant to the Cooperation Agreement, the Board declared a special cash dividend of $1.75 per share of common stock, payable on December 6, 2023, to stockholders of record at the close of business on November 17, 2023.
Liquidity
We have accumulated losses of $349.6 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of September 30, 2023, we had $82.2 million in cash and cash equivalents and short-term investments. We believe that these resources will be sufficient to meet our obligations and fund our liquidity and capital expenditure requirements for at least the next 12 months from the issuance of these condensed consolidated financial statements. Our current business operations are focused on continuing the clinical development of our drug candidates. Changes to our research and development plans or other changes affecting our operating expenses may affect actual future use of existing cash resources. Our research and development expenses are expected to increase in the foreseeable future. We cannot determine with certainty costs associated with ongoing and future clinical trials or the regulatory approval process. The duration, costs and timing associated with the development of our product candidates will depend on a variety of factors, including uncertainties associated with the results of our clinical trials.
7
Table of Contents
To date, we have obtained cash and funded our operations primarily through equity financings and license agreements. In order to continue the development of our drug candidates, at some point in the future we expect to pursue one or more capital transactions, whether through the sale of equity securities, debt financing, license agreements or entry into strategic partnerships. There can be no assurance that we will be able to continue to raise additional capital in the future.
Basis of Presentation and Principles of Consolidation
The condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the U.S. (“U.S. GAAP”) for interim financial information and in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, the accompanying financial statements do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, the accompanying condensed consolidated financial statements reflect all adjustments (consisting of normal recurring adjustments) that are necessary for a fair statement of the financial position, results of operations and cash flows for the periods presented.
The accompanying unaudited condensed consolidated financial statements include the accounts of MEI Pharma, Inc. and our wholly owned subsidiary, Meadow Merger Sub, Inc. We have eliminated all significant intercompany accounts and transactions in consolidation.
The accompanying unaudited condensed consolidated financial statements for the quarterly period ended September 30, 2023 should be read in conjunction with the audited financial statements and notes thereto as of and for the fiscal year ended June 30, 2023, included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on September 26, 2023 ("2023 Annual Report"). Interim results are not necessarily indicative of results for a full year.
2. Summary of Significant Accounting Policies
There have been no material changes to our significant accounting policies from those described in the notes to our audited consolidated financial statements contained in the 2023 Annual Report.
Risks and Uncertainties
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of share-based awards, the discount rate used in estimating the present value of the right-of-use assets and lease liabilities, the useful lives of property and equipment, the recoverability of long-lived assets, clinical trial accruals, periods over which revenue should be recognized, relative stand-alone selling price, deferred income taxes and related valuation allowances, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these condensed consolidated financial statements. We base our estimates on historical experience and other market-specific or other relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each reporting period and updated to reflect current information. As future events and their effects cannot be determined with precision, actual results may materially differ from those estimates or assumptions.
Concentrations of Credit Risk
Financial instruments that potentially subject us to significant concentrations of credit risk consist primarily of cash, cash equivalents and short-term investments. Deposits in our checking and money market accounts are maintained in federally insured financial institutions and are subject to federally insured limits or limits set by the Securities Investor Protection Corporation.
We attempt to minimize credit risk associated with our cash, cash equivalents and short-term investments by periodically evaluating the credit quality of our primary financial institutions. Our investment portfolio is maintained in accordance with our investment policy, which is designed to preserve capital, safeguard funds and limit exposure to risk. While we maintain cash deposits in Federal Deposit Insurance Corporation insured financial institutions in excess of federally insured limits, we do not believe that we are exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. We have not experienced any losses on such accounts.
Short-term Investments
Short-term investments are marketable securities with maturities greater than three months but less than one year from date of purchase. As of September 30, 2023 and June 30, 2023, our short-term investments consisted of $78.8 million and $83.8 million, respectively, in United States government securities. The short-term investments held as of September 30, 2023 and June 30, 2023 are considered to be “held to maturity” and are carried at amortized cost. As of September 30, 2023 and June 30, 2023, the gross unrealized gains and losses were immaterial.
8
Table of Contents
Revenue Recognition
Revenues from Customers
In accordance with ASC Topic 606, Revenue from Contracts with Customers (“Topic 606”), we recognize revenue when control of the promised goods or services is transferred to our customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. For enforceable contracts with our customers, we first identify the distinct performance obligations – or accounting units – within the contract. Performance obligations are commitments in a contract to transfer a distinct good or service to the customer.
Payments received under commercial arrangements, such as licensing technology rights, may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, and royalties on the sale of products. At the inception of arrangements that include milestone payments, we use judgment to evaluate whether the milestones are probable of being achieved, and we estimate the amount, if any, to include in the transaction price using the most likely method. If it is probable that a significant revenue reversal will not occur, the estimated amount is included in the transaction price. Milestone payments that are not within our or the licensee’s control, such as regulatory approvals, are not included in the transaction price until those approvals are received. At the end of each reporting period, we re-evaluate the probability of achievement of development milestones and any related constraint and, as necessary, we adjust our estimate of the overall transaction price.
We may enter into arrangements that consist of multiple performance obligations. Such arrangements may include any combination of our deliverables. To the extent a contract includes multiple promised deliverables, we apply judgment to determine whether promised deliverables are capable of being distinct and are distinct within the context of the contract. If these criteria are not met, the promised deliverables are accounted for as a combined performance obligation. For arrangements with multiple distinct performance obligations, we allocate variable consideration related to our 50-50 cost share for development services directly to the associated performance obligation and then allocate the remaining consideration among the performance obligations based on their relative stand-alone selling price.
Stand-alone selling price is the price at which we would sell a promised good or service separately to the customer. When not directly observable, we typically estimate the stand-alone selling price for each distinct performance obligation. Variable consideration that relates specifically to our efforts to satisfy specific performance obligations is allocated entirely to those performance obligations. Other components of the transaction price are allocated based on the relative stand-alone selling price, over which management has applied significant judgment. We develop assumptions that require judgment to determine the stand-alone selling price for license-related performance obligations, which may include forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical, regulatory and commercial success. We estimate stand-alone selling price for research and development performance obligations by forecasting the expected costs of satisfying a performance obligation plus an appropriate margin.
In the case of a license that is a distinct performance obligation, we recognize revenue allocated to the license from non-refundable, up-front fees at the point in time when the license is transferred to the licensee and the licensee can use and benefit from the license. For licenses that are bundled with other distinct or combined obligations, we use judgment to assess the nature of the performance obligation to determine whether the performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. If the performance obligation is satisfied over time, we evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition.
The selection of the method to measure progress towards completion requires judgment and is based on the nature of the products or services to be provided. Revenue is recorded proportionally as costs are incurred. We generally use the cost-to-cost measure of progress because it best depicts the transfer of control to the customer which occurs as we incur costs. Under the cost-to-cost measure of progress, the extent of progress towards completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation (an “input method” under Topic 606). We use judgment to estimate the total cost expected to complete the research and development performance obligations, which include subcontractors’ costs, labor, materials, other direct costs and an allocation of indirect costs. We evaluate these cost estimates and the progress each reporting period and, as necessary, we adjust the measure of progress and related revenue recognition.
For arrangements that include sales-based or usage-based royalties, we recognize revenue at the later of (i) when the related sales occur or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied or partially satisfied. To date, we have not recognized any sales-based or usage-based royalty revenue from license agreements.
In connection with our April 2020 License, Development and Commercialization Agreement (the "KKC Commercialization Agreement") described in Note 7. License Agreements, we perform development services related to our 50-50 cost sharing arrangement for which revenue is recognized over time. Additionally, we perform services for KKC at their request, the costs of which are fully reimbursed to us. We record the reimbursement for such pass through services as revenue at 100% of reimbursed
9
Table of Contents
costs, as control of the additional services for KKC is transferred at the time we incur such costs. The costs of these services are recognized in the consolidated statements of operations as research and development expense. From time to time, we perform additional services for Kyowa Kirin Co., Ltd. ("KKC") at their request, the costs of which are fully reimbursed to us. The cost of these services is recognized in the consolidated statements of operations as research and development expense.
We recognized revenue associated with the KKC Commercialization Agreement for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Timing of Revenue Recognition: |
|
|
|
|
|
|
Services performed over time |
|
$ |
743 |
|
|
$ |
8,359 |
|
Pass through services at a point in time |
|
|
9 |
|
|
|
371 |
|
|
|
$ |
752 |
|
|
$ |
8,730 |
|
Contract Balances
Accounts receivables are included in prepaid expenses and other current assets, and contract liabilities are included in deferred revenue and deferred revenue, long-term in our condensed consolidated balance sheets. Our contract liabilities accounted for under Topic 606 relate to the amount of initial upfront consideration allocated to the development services performance obligations. Contract liabilities are recognized over the duration of the performance obligations based on the costs incurred relative to total expected costs.
As of September 30, 2023 and June 30, 2023, we had no balances in accounts receivable. The following table presents changes in unbilled receivables and contract liabilities accounted for under Topic 606 for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Unbilled receivables |
|
$ |
— |
|
|
$ |
85 |
|
Contract liabilities |
|
|
|
|
|
|
Contract liabilities, beginning of period |
|
$ |
317 |
|
|
$ |
30,900 |
|
Revenue recognized |
|
|
(317 |
) |
|
|
(5,411 |
) |
Revenue recognized from change in estimate for performance obligations that are being closed |
|
|
— |
|
|
|
(16,565 |
) |
Revenue recognized for performance obligations that will no longer commence |
|
|
— |
|
|
|
(8,607 |
) |
Contract liabilities, end of period |
|
$ |
— |
|
|
$ |
317 |
|
The timing of revenue recognition, invoicing and cash collections results in billed accounts receivable and unbilled receivables (contract assets) and deferred revenue (contract liabilities). We invoice our customers in accordance with agreed-upon contractual terms, typically at periodic intervals or upon achievement of contractual milestones. Invoicing may occur subsequent to revenue recognition, resulting in unbilled receivables. We may receive advance payments from our customers before revenue is recognized, resulting in contract liabilities. The unbilled receivables and deferred revenue reported on the consolidated balance sheets relate to the KKC Commercialization Agreement.
Revenues from Collaborators
At contract inception, we assess whether the collaboration arrangements are within the scope of Topic 808, to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed based on the responsibilities of all parties in the arrangement. For collaboration arrangements within the scope of Topic 808 that contain multiple units of account, we first determine which units of account within the arrangement are within the scope of Topic 808 and which elements are within the scope of Topic 606. For units of account within collaboration arrangements that are accounted for pursuant to Topic 808, an appropriate recognition method is determined and applied consistently, by analogy to authoritative accounting literature. For elements of collaboration arrangements that are accounted for pursuant to Topic 606, we recognize revenue as discussed above. Consideration received that does not meet the requirements to satisfy Topic 606 revenue recognition criteria is recorded as deferred revenue in the accompanying consolidated balance sheets, classified as either current or long-term deferred revenue based on our best estimate of when such amounts will be recognized.
10
Table of Contents
Net Income (Loss) Per Share
Basic and diluted net income (loss) per share is computed using the weighted-average number of shares of common stock outstanding during the period, less any shares subject to repurchase or forfeiture. There were no shares of common stock subject to repurchase or forfeiture for the three months ended September 30, 2023 and 2022. Diluted net income (loss) per share is computed based on the sum of the weighted-average number of common shares and potentially dilutive common shares outstanding during the period determined using the treasury-stock and if-converted methods.
For purposes of the diluted net income per share calculation for the three months ended September 30, 2023, potentially dilutive securities are excluded from the calculation of diluted net income per share because their weighted-average exercise prices were above our weighted-average share price as of September 30, 2023; therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2023. For purposes of the diluted net loss per share calculation for the three months ended September 30, 2022, potentially dilutive securities are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive and, therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2022.
The following table presents potentially dilutive shares excluded from the calculation of diluted net income (loss) per share (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Stock options |
|
|
1,447 |
|
|
|
1,388 |
|
Warrants |
|
|
103 |
|
|
|
803 |
|
Restricted stock units |
|
|
— |
|
|
|
1 |
|
Total anti-dilutive shares |
|
|
1,550 |
|
|
|
2,192 |
|
Recent Accounting Pronouncement
Recently Adopted
In June 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update 2016-13, Financial Instruments–Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments ("ASU 2016-13"), as amended. The amendments in ASU 2016-13 require, among other things, financial assets measured at amortized cost basis to be presented at the net amount expected to be collected as compared to previous U.S. GAAP which delayed recognition until it was probable a loss had been incurred. The amendments in ASU 2016-13 are effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years, with early adoption permitted. The adoption of ASU 2016-13 did not have a material impact on our financial statements and related disclosures.
Recently Issued
From time to time, new accounting pronouncements are issued by the FASB or other standards setting bodies that are adopted as of the specified effective date. The Company believes the impact of recently issued standards and any issued but not yet effective standards will not have a material impact on its condensed consolidated financial statements upon adoption.
3. Balance Sheet Details
Property and Equipment
Property and equipment consisted of the following, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Furniture and equipment |
|
$ |
1,381 |
|
|
$ |
1,374 |
|
Leasehold improvements |
|
|
969 |
|
|
|
969 |
|
|
|
|
2,350 |
|
|
|
2,343 |
|
Less: accumulated depreciation |
|
|
(1,121 |
) |
|
|
(1,034 |
) |
Property and equipment, net |
|
$ |
1,229 |
|
|
$ |
1,309 |
|
Depreciation expense of property and equipment for the three months ended September 30, 2023 and 2022 are presented in the condensed consolidated statements of cash flows.
11
Table of Contents
Accrued Liabilities
Accrued liabilities consisted of the following, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Accrued pre-clinical and clinical trial expenses |
|
$ |
730 |
|
|
$ |
3,663 |
|
Accrued compensation and benefits(1) |
|
|
1,515 |
|
|
|
7,189 |
|
Accrued legal and professional services |
|
|
1,044 |
|
|
|
1,423 |
|
Accrued reimbursement to KKC |
|
|
892 |
|
|
|
— |
|
Other |
|
|
108 |
|
|
|
186 |
|
Total accrued liabilities |
|
$ |
4,289 |
|
|
$ |
12,461 |
|
(1) Includes $0.1 million and $1.0 million of one-time termination employee benefits as of September 30, 2023 and June 30, 2023, respectively, as more fully described in Note 5. One-time Termination Benefits.
4. Fair Value Measurements
The carrying amounts of financial instruments such as cash equivalents, short-term investments and accounts payable approximate the related fair values due to the short-term maturities of these instruments. We invest our excess cash in financial instruments which are readily convertible into cash, such as money market funds and U.S. government securities. Cash equivalents and short-term investments are classified as Level 1 as defined by the fair value hierarchy. As of September 30, 2023 and June 30, 2023, we had no assets or liabilities measured on a recurring or non-recurring basis.
In May 2018, we issued warrants in connection with a private placement of our shares of common stock. Pursuant to the terms of the warrants, we could have been required to settle the warrants in cash in the event of an acquisition of us and, as a result, the warrants were required to be measured at fair value and reported as a liability in the condensed consolidated balance sheets. We recorded the fair value of the warrants upon issuance using the Black-Scholes valuation model and were required to revalue the warrants at each reporting date with any changes in fair value recorded on our condensed consolidated statement of operations through their expiration in May 2023. The valuation of the warrants is considered under Level 3 of the fair value hierarchy due to the need to use assumptions in the valuation that are both significant to the fair value measurement and unobservable. Inputs used to determine estimated fair value of the warrant liabilities include the estimated fair value of the underlying stock at the valuation date, the estimated term of the warrants, risk-free interest rates, expected dividends and the expected volatility of the underlying stock. The significant unobservable inputs used in the fair value measurement of the warrant liabilities were the volatility rate and the estimated term of the warrants. Generally, increases (decreases) in the fair value of the underlying stock and estimated term would result in a directionally similar impact to the fair value measurement. The change in the fair value of the Level 3 warrant liability is reflected in the condensed consolidated statements of operations for the three months ended September 30, 2022. During the three months ended September 30, 2023 and the year ended June 30,2023, there were no transfers into or out of Level 3 of the fair value hierarchy.
To calculate the fair value of the warrant liability as of June 30, 2023, the following assumptions were used:
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
4.4 |
% |
Expected life (years) |
|
|
0.5 |
|
Expected volatility |
|
|
128.7 |
% |
Dividend yield |
|
|
— |
% |
Weighted-average grant date fair value |
|
$ |
0.02 |
|
The following table sets forth a summary of changes in the estimated fair value of our Level 3 warrant liability for the three months ended September 30, 2022 (in thousands):
|
|
|
|
|
|
|
|
|
Balance as of June 30, 2022 |
|
$ |
1,603 |
|
Change in estimated fair value of liability classified warrants |
|
|
(1,117 |
) |
Balance as of September 30, 2022 |
|
$ |
486 |
|
12
Table of Contents
5. One-Time Termination Benefits
In connection with our joint decision to discontinue development of zandelisib outside of Japan, in December 2022, we announced a realignment of our clinical development efforts that streamlined our organization towards the continued clinical development of our two earlier clinical-stage assets, voruciclib and ME-344. As a result, also in December 2022, our Board approved a staggered workforce reduction (the "Reduction in Force"), affecting 28 employees in December 2022 and an additional 26 employees through June 2023, representing an aggregate 51% Reduction in Force. For the three months ended September 30, 2023, we recorded additional one-time employee termination benefits of $28,000 within research and development expense as a result of an additional termination.
The following table summarizes our activity related to one-time employee termination benefits included in accrued liabilities (in thousands):
|
|
|
|
|
|
|
One-Time Employee Termination Benefits |
|
Balance at June 30, 2023 |
|
$ |
993 |
|
Increase in accrued restructuring |
|
|
28 |
|
Cash payments |
|
|
(917 |
) |
Balance at September 30, 2023 |
|
$ |
104 |
|
6. Commitments and Contingencies
We have contracted with various consultants and third parties to assist us in pre-clinical research and development and clinical trials work for our leading drug compounds. The contracts are terminable at any time, but obligate us to reimburse the providers for any time or costs incurred through the date of termination. We also have employment agreements with certain of our current employees that provide for severance payments and accelerated vesting for share-based awards if their employment is terminated under specified circumstances.
Litigation
From time to time, the Company may be involved in various lawsuits, legal proceedings, or claims that arise in the ordinary course of business. Management believes there are no claims or actions pending against the Company as of September 30, 2023 which will have, individually or in the aggregate, a material adverse effect on its business, liquidity, financial position, or results of operations. Litigation, however, is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
Indemnification
In accordance with our amended and restated memorandum and articles of association, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving in such capacity. There have been no claims to date, and the Company has a directors and officers liability insurance policy that may enable it to recover a portion of any amounts paid for future claims.
Presage License Agreement
As discussed in Note 8. Other License Agreements, we are party to a license agreement with Presage under which we may be required to make future payments upon the achievement of certain development, regulatory and commercial milestones, as well as potential future royalties based upon net sales. As of September 30, 2023, we had not accrued any amounts for potential future payments as achievement of the milestones had not been met.
7. License Agreements
Kyowa Kirin Co., Ltd. License, Development and Commercialization Agreement
In April 2020, we entered into the KKC Commercialization Agreement under which we granted to KKC a co-exclusive, sublicensable, payment-bearing license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in the U.S. (the "U.S. License"), and an exclusive (subject to certain retained rights to perform obligations under the KKC Commercialization Agreement), sublicensable, payment-bearing, license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in countries outside of the U.S.. KKC granted to us a co-exclusive, sublicensable, license under certain patents and know-how controlled by KKC to develop and commercialize zandelisib for all human indications in the U.S., and a co-exclusive, sublicensable, royalty-free, fully paid license under certain patents and
13
Table of Contents
know-how controlled by KKC to perform our obligations in the Ex-U.S. under the KKC Commercialization Agreement. KKC paid us an initial nonrefundable payment of $100.0 million.
In November 2022, we and KKC jointly decided to discontinue zandelisib development in the U.S. and in May 2023, KKC decided to discontinue development of zandelisib in Japan. Considering the decisions to discontinue worldwide development of zandelisib the parties entered into a Termination Agreement on July 14, 2023, agreeing to mutually terminate the global License, Development and Commercialization Agreement executed in April 2020. Pursuant to the Termination Agreement, we regained full, global rights to develop, manufacture and commercialize zandelisib, subject to KKCs limited rights to use for “compassionate use” (as more specifically defined in the Termination Agreement) in certain expanded access programs for the existing patients who have been enrolled in Japanese clinical trials sponsored by KKC until November 30, 2027, and for which KKC is fully liable; each party released the other party from any and all claims or demands arising from the original KKC Commercialization Agreement excluding certain surviving claims; however, we are obligated to deliver a discrete quantity of materials to facilitate KKCs compassionate use activities.
We determined the KKC Commercialization Agreement was a collaborative arrangement in accordance with Topic 808 which contained multiple units of account, as we and KKC were both active participants in the development and commercialization activities and were exposed to significant risks and rewards dependent on commercial success of the activities of the arrangement. We determined the U.S. License was a separate unit of account under the scope of Topic 808 and was not a deliverable under Topic 606, while the license issued to KKC within its territory and related development services were within the scope of Topic 606. See discussion within the Revenue Recognition subsection of Note 2. Summary of Significant Accounting Policies.
We evaluated the Termination Agreement under ASC 606 and determined it met the requirements of a contract modification which changed the scope of the KKC Commercialization Agreement, and the remaining goods and services associated with the wind-down activities to be transferred. The cost of satisfying our performance obligation to provide compassionate use supply to KKC was determined to be de minimis and therefore immaterial within the context of the KKC Commercialization Agreement. As of September 30, 2023, activities associated with the compassionate use supply were completed.
With the execution of the Termination Agreement, we regained full, global rights (subject to KKC's limited rights for compassionate use) and KKC has no further rights to develop, use or commercialize zandelisib in the U.S., nor do we have any remaining performance obligations with all consideration received from KKC being nonrefundable. Therefore, the remaining long-term deferred revenue as of June 30, 2023, of $64.5 million that was allocated to the U.S. License obligation accounted for under Topic 808 at inception of the KKC Commercialization Agreement was recognized as revenue from collaboration agreements in the three months ended September 30, 2023, utilizing contract termination analogous to guidance provided in Topic 606.
We recognized the remaining transaction price of $317,000 of deferred revenue during the three months ended September 30, 2023, as any remaining performance obligations under the KKC Commercialization Agreement were determined to be de minimis as of September 30, 2023. Therefore, as of September 30, 2023, all deferred revenue associated with the KKC Commercialization Agreement has been recognized.
8. Other License Agreements
Presage License Agreement
In September 2017, we, as licensee, entered into a license agreement with Presage Biosciences, Inc. (“Presage”). Under the terms of the license agreement, Presage granted to us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. In exchange, we paid $2.9 million to Presage. With respect to the first indication, an incremental $2.0 million payment, due upon dosing of the first subject in the first registration trial, will be owed to Presage, for total payments of $4.9 million prior to receipt of marketing approval of the first indication in the U.S., EU or Japan. Additional potential payments of up to $179 million will be due upon the achievement of certain development, regulatory and commercial milestones. We will also pay mid-single digit tiered royalties on the net sales of any product successfully developed. As an alternative to milestone and royalty payments related to countries in which we sublicense product rights, we will pay to Presage a tiered percentage (which decreases as product development progresses) of amounts received from such sublicensees. During the three months ended September 30, 2023 and 2022, we made no payments under the Presage license agreement.
BeiGene Collaboration
In October 2018, we entered into a clinical collaboration with BeiGene, Ltd. (“BeiGene”) to evaluate the safety and efficacy of zandelisib in combination with BeiGene’s zanubrutinib (marketed as Brukinsa®), an inhibitor of Bruton’s tyrosine kinase, for the treatment of patients with B-cell malignancies. Under the terms of the clinical collaboration agreement, we amended our ongoing Phase 1b trial to include evaluation of zandelisib in combination with zanubrutinib in patients with B-cell malignancies. Study costs are being shared equally by the parties, and we agreed to supply zandelisib and BeiGene agreed to supply zanubrutinib. We record the costs reimbursed by BeiGene as a reduction of our research and development expenses. We retained full commercial rights for
14
Table of Contents
zandelisib and BeiGene retained full commercial rights for zanubrutinib. With the discontinuation of the zandelisib program outside of Japan, this clinical collaboration was terminated on September 28, 2023.
9. Leases
In July 2020, we entered into a lease agreement (the "Initial Lease Agreement") for approximately 32,800 square feet of office space in San Diego, California. The Lease Agreement was scheduled to expire in March 2028 but was extended by 20 months to November 2029 in accordance with the amended lease agreement we entered into in January 2022 (the "Amended Lease Agreement"). The Initial and Amended Lease Agreements are collectively referred to as the "Lease Agreements". The Lease Agreements contain rent escalations over the lease term. In addition, the Lease Agreements contain an option to renew and extend the lease term, which is not included in the determination of the right-of-use ("ROU") asset and operating lease liability, as it was not reasonably certain to be exercised. Upon commencement of the Amended Lease Agreement, to extend the lease term, we recognized an additional operating lease ROU asset and a corresponding operating lease liability. The Lease Agreements include variable non-lease components (e.g., common area maintenance, maintenance, etc.) that are not included in the ROU asset and operating lease liability and are reflected as an expense in the period incurred as a component of the lease cost.
The Amended Lease Agreements also provides for an additional 12,300 square feet of office space adjacent to our current office in San Diego. Upon taking control of the additional office space on July 1, 2022, we recognized operating lease ROU assets obtained in exchange for operating lease liabilities of $4.3 million.
The total operating lease costs for the Lease Agreements were as follows for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
2023 |
|
|
2022 |
|
|
Operating lease cost |
|
$ |
608 |
|
|
$ |
608 |
|
|
Variable lease costs |
|
|
12 |
|
|
|
18 |
|
|
Total lease costs included in general and administrative expenses |
|
$ |
620 |
|
|
$ |
626 |
|
|
Supplemental cash flow information related to our operating leases was as follows for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
|
|
2023 |
|
|
2022 |
|
|
Cash paid for amount included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
Operating cash flows from operating leases |
|
$ |
584 |
|
|
$ |
567 |
|
|
|
|
|
|
|
|
|
|
The following is a schedule of the future minimum lease payments under the Lease Agreements, reconciled to the operating lease liability, as of September 30, 2023 (in thousands):
|
|
|
|
|
|
|
September 30, 2023 |
|
Remainder of fiscal year ending June 30, 2024 |
|
$ |
1,751 |
|
Years ending June 30, |
|
|
|
2025 |
|
|
1,913 |
|
2026 |
|
|
2,477 |
|
2027 |
|
|
2,551 |
|
2028 |
|
|
2,715 |
|
Thereafter |
|
|
4,385 |
|
Total lease payments |
|
|
15,792 |
|
Less: Present value discount |
|
|
(3,411 |
) |
Total operating lease liability |
|
$ |
12,381 |
|
|
|
|
|
Balance Sheet Classification - Operating Leases |
|
|
|
Operating lease liability |
|
$ |
1,055 |
|
Operating lease liability, long-term |
|
|
11,326 |
|
Total operating lease liability |
|
$ |
12,381 |
|
|
|
|
|
Other Balance Sheet Information - Operating Leases |
|
|
|
Weighted-average remaining lease term (in years) |
|
|
6.2 |
|
Weighted-average discount rate |
|
|
7.50 |
% |
15
Table of Contents
10. Stockholders’ Equity
Equity Transactions
Warrants
In May 2023, outstanding warrants to purchase 802,949 shares of our common stock expired. The warrants were fully vested, exercisable at a price of $50.80 per share. Prior to their expiration, the warrants had been previously revalued to zero, as of December 31, 2022. All corresponding changes in fair value were recorded as a component of other income (expense) in our condensed consolidated statements of operations. No warrants were exercised during the three months ended September 30, 2022.
As of September 30, 2023, we also have outstanding warrants to purchase 102,513 shares of our common stock issued to Torreya Partners. The warrants are fully vested, exercisable at a price of $6.80 per share and expire in October 2027. No warrants were exercised during the three months ended September 30, 2023.
Description of Capital Stock
Our total authorized share capital is 226,100,000 shares consisting of 226,000,000 shares of common stock, $0.00000002 par value per share, and 100,000 shares of preferred stock, $0.01 par value per share.
Common Stock
The holders of common stock are entitled to one vote per share. In the event of a liquidation, dissolution or winding up of our affairs, holders of the common stock will be entitled to share ratably in all our assets that are remaining after payment of our liabilities and the liquidation preference of any outstanding shares of preferred stock. All outstanding shares of common stock are fully paid and non-assessable. The rights, preferences and privileges of holders of common stock are subject to any series of preferred stock that we have issued or that we may issue in the future. The holders of common stock have no preemptive rights and are not subject to future calls or assessments by us.
Preferred Stock
Our Board has the authority to issue up to 100,000 shares of preferred stock with a par value of $0.01 per share in one or more series and to fix the rights, preferences, privileges and restrictions in respect of that preferred stock, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption (including sinking fund provisions), redemption prices and liquidation preferences, and the number of shares constituting such series and the designation of any such series, without future vote or action by the stockholders. Therefore, the Board, without the approval of the stockholders, could authorize the issuance of preferred stock with voting, conversion and other rights that could affect the voting power, dividend and other rights of the holders of shares or that could have the effect of delaying, deferring or preventing a change of control. There were no shares of preferred stock outstanding as of September 30, 2023 and June 30, 2023.
11. Share-based Compensation
We use equity-based compensation programs to provide long-term performance incentives for our employees. These incentives consist primarily of stock options and RSUs. In December 2008, we adopted the MEI Pharma, Inc. 2008 Stock Omnibus Equity Compensation Plan (“Omnibus Plan”), as amended and restated from time-to-time, under which 1,850,739 shares of common stock are currently authorized for issuance. The Omnibus Plan provides for the grant of options and/or other stock-based or stock-denominated awards to our non-employee directors, officers, and employees. As of September 30, 2023, there were 398,718 shares available for future grant under the Omnibus Plan.
In May 2021, we adopted the 2021 Inducement Plan ("Inducement Plan"), under which 125,000 shares of common stock are authorized for issuance. The Inducement Plan is intended to assist us in attracting and retaining selected individuals to serve as employees who are expected to contribute to our success, by providing an inducement for such individuals to enter into employment with us, and to achieve long-term objectives that will benefit our stockholders. As of September 30, 2023, there were 107,738 shares available for future grant under the Inducement Plan.
Total share-based compensation expense for all stock awards consisted of the following for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Research and development |
|
$ |
(69 |
) |
|
$ |
649 |
|
General and administrative |
|
|
432 |
|
|
|
910 |
|
Total share-based compensation |
|
$ |
363 |
|
|
$ |
1,559 |
|
16
Table of Contents
Stock Options
Stock options granted to employees vest 25% one year from the date of grant and ratably each month thereafter for a period of 36 months and expire ten years from the date of grant. Stock options granted to directors vest ratably each month for a period of 12 months from the date of grant and expire ten years from the date of grant. Of the total options outstanding of 1,447,127 as of September 30, 2023, 1,337,865 were granted under the Omnibus Plan and 109,262 were granted under the Inducement Plan.
A summary of our stock option activity and related data follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
Options |
|
|
Weighted-Average
Exercise Price |
|
|
Weighted-Average
Remaining Contractual
Term (in years) |
|
|
Aggregate
Intrinsic Value |
|
Outstanding at June 30, 2023 |
|
|
1,284,907 |
|
|
$ |
38.32 |
|
|
|
|
|
|
|
Granted |
|
|
227,037 |
|
|
$ |
7.01 |
|
|
|
|
|
|
|
Forfeited |
|
|
(64,817 |
) |
|
$ |
36.35 |
|
|
|
|
|
|
|
Outstanding at September 30, 2023 |
|
|
1,447,127 |
|
|
$ |
33.50 |
|
|
|
7.6 |
|
|
$ |
789 |
|
Vested and exercisable at September 30, 2023 |
|
|
819,813 |
|
|
$ |
49.16 |
|
|
|
6.3 |
|
|
$ |
— |
|
As of September 30, 2023, the aggregate intrinsic value of outstanding options was calculated as the difference between the exercise price of the underlying options and the closing price of our common stock of $7.01 on that date.
Unrecognized compensation expense related to non-vested stock options totaled $3.1 million as of September 30, 2023. Such compensation expense is expected to be recognized over a weighted-average period of 1.6 years. As of September 30, 2023, we expect all options to vest.
We use the Black-Scholes valuation model to estimate the grant date fair value of stock options. To calculate these fair values, the following weighted-average assumptions were used for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Risk-free interest rate |
|
|
4.6 |
% |
|
|
2.8 |
% |
Expected life (years) |
|
|
5.7 |
|
|
|
6.0 |
|
Expected volatility |
|
|
89.8 |
% |
|
|
84.1 |
% |
Dividend yield |
|
|
— |
% |
|
|
— |
% |
Weighted-average grant date fair value |
|
$ |
5.27 |
|
|
$ |
7.80 |
|
12. Subsequent Events
On October 1, 2023, our Board approved and adopted a Rights Agreement, ("Rights Agreement") by and between us and Computershare, Inc., as Rights Agent (as defined in the Rights Agreement). Pursuant to the Rights Agreement, the Board declared a dividend of one preferred share purchase right (each a "Right") for each outstanding share of our common stock, par value $0.00000002 (each a "Common Share" and collectively, the "Common Shares"). The Rights are distributable to stockholders of record as of the close of business on October 12, 2023. One Right also will be issued together with each Common Share issued by the Company after October 12, 2023, but before the Distribution Date, as defined in the Rights Agreement, (or the earlier of the redemption or expiration of the Rights) and, in certain circumstances, after the Distribution Date.
As more fully described in Note 1. Description of Business and Basis of Presentation, we announced a Cooperation Agreement (“Cooperation Agreement”) with Anson Funds and Cable Car Capital effective as of October 31, 2023. On November 6, 2023, pursuant to the Cooperation Agreement, the Board declared a special cash dividend of $1.75 per share of common stock, payable on December 6, 2023, to stockholders of record at the close of business on November 17, 2023.
17
Table of Contents
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Quarterly Report on Form 10-Q ("Quarterly Report") includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical facts contained in this Quarterly Report, including statements regarding the future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, without limitation, those described in “Risk Factors” in our 2023 Annual Report on Form 10-K ("2023 Annual Report"), as filed with the Securities and Exchange Commission on September 26, 2023. Set forth below is a summary of the principal risks we face:
Summary Risk Factors
Our business is subject to numerous risks and uncertainties that you should consider before investing in our common stock. Set forth below is a summary of the principal risks we face:
•We are currently operating in a period of capital markets disruption and economic uncertainty;
•We will need substantial additional funds to progress the clinical trial programs for our drug candidates, to commercialize our drug candidates and to develop new compounds. The actual amount of funds we will need will be determined by a number of factors, some of which are beyond our control;
•We may be required to seek additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties at terms which maybe unfavorable to us;
•We are a clinical-stage pharmaceutical company focused on developing potential new therapies for cancer and are likely to incur operating losses for the foreseeable future;
•The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current drug candidates may not have favorable results in later studies or trials;
•Changes in drug candidate manufacturing or formulation may result in additional costs or delay;
•If third parties with whom we collaborate on the development and commercialization of our drug candidates do not satisfy their obligations, do not otherwise pursue development or commercialization of our drug candidates or if they terminate their agreements with us, we may not be able to develop or commercialize our drug candidates;
•We are subject to significant obligations to Presage in connection with our license of voruciclib, and we may become subject to significant obligations in connection with future licenses we obtain, which could adversely affect the overall profitability of any products we may seek to commercialize, and such licenses of drug candidates, the development and commercialization for which we are solely responsible, may never become profitable;
•Our business strategy may include entry into additional collaborative or license agreements. We may not be able to enter into collaborative or license agreements or may not be able to negotiate commercially acceptable terms for these agreements;
•Final approval by regulatory authorities of our drug candidates for commercial use may be delayed, limited or prevented, any of which would adversely affect our ability to generate operating revenues;
•The FDA may determine that our drug candidates have undesirable side effects that could delay or prevent regulatory approval or commercialization;
•If we experience delays or difficulties in the enrollment of patients in clinical trials, our completion of clinical trials and receipt of necessary regulatory approvals could be delayed or prevented;
•Changes in funding for the FDA and other government agencies or future government shutdowns could cause delays in the submission and regulatory review of marketing applications, which could negatively impact our business or prospects;
•Failure to obtain regulatory approval in foreign jurisdictions would prevent us from marketing our products internationally;
18
Table of Contents
•Any designation granted by the FDA for any of our product candidates may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidates will receive marketing approval. We may also not be able to obtain or maintain any such designation;
•Any orphan drug designations we receive may not confer marketing exclusivity or other benefits;
•Even if we or our licensees receive regulatory approval to commercialize our drug candidates, our ability to generate revenues from any resulting products will be subject to a variety of risks, many of which are out of our control;
•If any products we develop become subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, our ability to successfully commercialize our products will be impaired;
•Our drug candidates are subject to ongoing government regulation both before and after regulatory approval;
•We may not be able to establish the contractual arrangements necessary to develop, market and distribute our drug candidates;
•Our commercial opportunity will be reduced or eliminated if competitors develop and market products that are more effective, have fewer side effects or are less expensive than our drug candidates;
•Our product candidates may face competition sooner than anticipated;
•We rely on third parties to conduct our clinical trials and pre-clinical studies. If those parties do not successfully carry out their contractual duties or meet expected deadlines, our drug candidates may not advance in a timely manner or at all;
•We will depend on third party suppliers and contract manufacturers for the manufacturing of our drug candidates and have no direct control over the cost and timing of manufacturing our drug candidates. Increases in the cost of manufacturing our drug candidates or delays in manufacturing would increase our costs of conducting clinical trials and could adversely affect our future profitability;
•We rely on acquisitions or licenses from third parties to expand our pipeline of drug candidates;
•Our commercial success is dependent, in part, on obtaining and maintaining patent protection and preserving trade secrets, which cannot be guaranteed;
•Claims by other companies that we infringe on their proprietary technology may result in liability for damages or stop our development and commercialization efforts;
•We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property;
•We may be subject to substantial costs stemming from our defense against third party intellectual property infringement claims;
•We face a risk of product liability claims and claims may exceed our insurance limits;
•Our employees, independent contractors, consultants, commercial partners, principal investigators, or clinical contract research organizations ("CROs") may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business;
•Our business and operations would suffer in the event of system failures;
•Our efforts will be seriously jeopardized if we are unable to retain and attract key employees;
•Negative U.S. and global economic conditions may pose challenges to our business strategy, which relies on funding from the financial markets or collaborators;
•Laws, rules and regulations relating to public companies may be costly and impact our ability to attract and retain directors and executive officers;
•Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer;
•If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business;
19
Table of Contents
•We or the third parties upon whom we depend may be adversely affected by natural disasters and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster;
•Limitations on the tax deductibility of net operating losses could adversely affect our business and financial condition;
•Our business could be negatively impacted as a result of actions by activist investors;
•The trading price of the shares of our common stock has been and may continue to be highly volatile and could decline in value and we may incur significant costs from class action litigation;
•Future sales of our common stock, including common stock issued upon exercise of outstanding warrants or options, may depress the market price of our common stock and cause stockholders to experience dilution;
•We will have broad discretion over the use of the net proceeds from any exercise of outstanding warrants and options;
•We are authorized to issue blank check preferred stock, which could adversely affect the holders of our common stock;
•Anti-takeover provisions contained in our amended and restated certificate of incorporation and fifth amended and restated bylaws, as well as provisions of Delaware law, could impair a takeover attempt;
•Our fifth amended and restated bylaws require, to the fullest extent permitted by law, that derivative actions brought in our name, actions against our directors, officers, other employees or stockholders for breach of fiduciary duty and other similar actions may be brought only in the Court of Chancery in the State of Delaware and, if brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service of process on such stockholder’s counsel, which may have the effect of discouraging lawsuits against our directors, officers, other employees or stockholders; and
•Our executive officers and directors may sell shares of their stock, and these sales could adversely affect our stock price.
These risks are not exhaustive. Other sections of this report and our other filings with the Securities and Exchange Commission ("SEC") include additional factors which could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for us to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Past performance may not be an indicator of future results. The following discussion is qualified in its entirety by, and should be read in conjunction with, the more detailed information set forth in the condensed consolidated financial statements and the notes thereto appearing elsewhere in this Quarterly Report and the audited financial statements and notes thereto included in our 2023 Annual Report, as filed with the SEC. Operating results are not necessarily indicative of results that may occur in future periods.
Overview
MEI Pharma, Inc. (Nasdaq: MEIP) is a clinical-stage pharmaceutical company committed to developing novel and differentiated cancer therapies. We build our pipeline by acquiring promising cancer agents and creating value in programs through clinical development, strategic partnerships, and out-licensing or commercialization, as appropriate. Our approach to oncology drug development is to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. The drug candidate pipeline includes voruciclib, an oral cyclin-dependent kinase 9 ("CDK9") inhibitor, and ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway. Our common stock is listed on the Nasdaq Capital Market under the symbol “MEIP.”
Following our announcement in December 2022 to realign our clinical development efforts to focus on our two clinical assets, voruciclib and ME-344 that are currently in Phase 1 and Phase 1b clinical programs, respectively, we initiated a staggered workforce reduction to streamline our operations that has resulted in a 61% reduction in full-time employees since our announcement. We believe our cash is sufficient to fund operations for at least 12 months and through the reporting of clinical data readouts from the ongoing and planned voruciclib and ME-344 Phase 1 and Phase 1b clinical programs, respectively.
20
Table of Contents
On October 31, 2023, we announced a Cooperation Agreement (“Cooperation Agreement”) with Anson Funds ("Anson") and Cable Car Capital ("Cable Car") of which contained the following key terms:
•Capital Return to Stockholders: Payment of a dividend in the amount of $1.75 per share of common stock to all stockholders. Additionally, a second return of capital of approximately $9.33 million in the aggregate will be authorized by the board of directors ("Board") should our ongoing ME-344 phase 1b trial fail to meet certain endpoints or our Board determines not to proceed with a second cohort, both as further described in the Cooperation Agreement. The second return of capital may take the form of a dividend or tender offer and is subject to Board approval as well as modification associated with applicable requirements under Delaware law, both as detailed in the Cooperation Agreement. The $1.75 dividend is anticipated to be distributed during the second quarter of fiscal year 2024.
•Three of our current directors resigned from the Board concurrently with the execution of the Cooperation Agreement and will not seek reelection at the 2024 Annual Meeting of Stockholders ("2024 Annual Meeting").
•Stockholder Designees Added to the Board: The appointment of two directors designated by Anson and Cable Car, with an additional director appointment mutually agreed upon by us and Anson and Cable Car. These appointments were effective immediately and the new directors will be nominated for election by us in connection with our upcoming fiscal 2024 Annual Meeting to serve for a three-year term if elected.
•Formation of a Capital Allocation Committee: The formation of a Capital Allocation Committee, comprising of five directors including the three new directors. The Capital Allocation Committee will advise the Board on the strategic allocation of capital to support (i) the development of our drug candidate programs and (ii) other value creation or preservation measures, with a view toward maximizing stockholder value.
As part of the Cooperation Agreement, Anson and Cable Car withdrew their consent solicitation and agreed to abide by customary standstill provisions. Additionally, we will reimburse Anson and Cable Car’s fees and expenses related to their engagement with us to date, in an amount not to exceed $1.2 million. As of September 30, 2023, no amounts were expensed or accrued in connection with the Cooperation Agreement.
On November 6, 2023, we announced that pursuant to the Cooperation Agreement, the Board declared a special cash dividend of $1.75 per share of common stock, payable on December 6, 2023, to stockholders of record at the close of business on November 17, 2023.
Clinical Development Programs
Our clinical-stage drug candidate pipeline includes voruciclib, an oral CDK9 inhibitor, and ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway in the mitochondria.
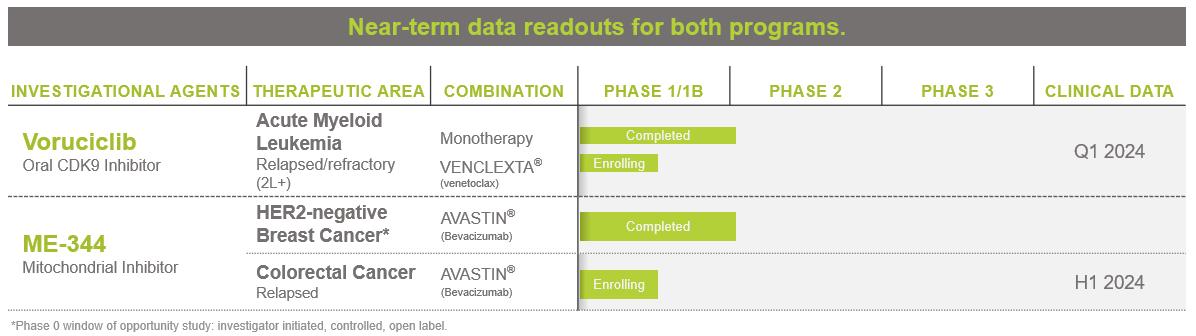
Voruciclib: Potent Orally Administered CDK9 Inhibitor in Phase 1 Studies
Voruciclib is a potent and selective orally administered CDK9 inhibitor. Voruciclib is being studied in a Phase 1 trial evaluating dose and schedule in patients with acute myeloid leukemia (“AML”) and B-cell malignancies as a single-agent, and in combination with the B-cell lymphoma 2 (“BCL2”) inhibitor venetoclax (marketed as Venclexta®) in patients with AML. Voruciclib is also being evaluated in pre-clinical studies to explore potential activity in various solid tumor cancers including in combination with therapies that target the RAS signaling pathway, such as KRAS inhibitors.
21
Table of Contents
Voruciclib Scientific Overview: Cell Cycle Signaling
CDK9 has important functions in cell cycle regulation, including the modulation of two therapeutic targets in cancer:
•CDK9 is a transcriptional regulator of the myeloid leukemia cell differentiation protein (“MCL1”), a member of the family of anti-apoptotic proteins which, when elevated, may prevent the cell from undergoing cell death and result in poor prognosis in cancer. Inhibition of CDK9 blocks the production of MCL1, which is also an established resistance mechanism to the BCL2 inhibitor venetoclax.
•CDK9 is a transcriptional regulator of the MYC proto-oncogene protein (“MYC”) which regulates cell proliferation and growth. Up regulation of MYC is implicated in many human cancers and is frequently associated with poor prognosis and unfavorable patient survival. CDK9, in addition to being a transcription factor for MYC, also decreases phosphorylation of MYC protein that is implicated in stabilizing MYC in KRAS mutant cancers.
Directly inhibiting MCL1 and MYC has historically been difficult, but CDK9 is a promising approach to indirectly target these oncogenes.
Voruciclib: Inhibition of MCL1
Over expression of MCL1 is frequently observed in many tumor types and is closely associated with tumorigenesis, poor prognosis and drug resistance. Further, up regulation of MCL1 is understood to play a role in resistance to venetoclax. CDK9 is a known transcriptional regulator of MCL1.
In pre-clinical studies voruciclib shows dose-dependent suppression of MCL1; in December 2017, a study of voruciclib published in the journal Nature Scientific Reports reported that the combination of voruciclib plus the BCL-2 inhibitor venetoclax was capable of inhibiting two master regulators of cell survival, MCL-1 and BCL-2, and achieved synergistic antitumor effect in an aggressive subset of DLBCL cells.
In a peer reviewed manuscript published in 2020, it was reported that the inhibition of CDK9 by voruciclib synergistically enhances cell death induced by the BCL-2 inhibitor venetoclax in preclinical models of AML. The data demonstrated that voruciclib synergizes with venetoclax to induce programmed cell death, or apoptosis, in both AML cell lines and primary patient samples. It was also demonstrated that voruciclib downregulates MCL1, which is relevant for the synergy between voruciclib and venetoclax, and further that voruciclib downregulates MYC, which also contributes to the synergies with venetoclax.
The research suggests that voruciclib is potentially an attractive therapeutic agent for treating cancers in combination with venetoclax or other BCL-2 inhibitors, to address potential resistance associated with MCL1, and is supportive of our ongoing clinical evaluation of voruciclib in B-cell malignancies and AML.
Voruciclib: Inhibition of MYC
Many cancers are associated with over expression of MYC, a transcription factor regulating cell proliferation and growth. CDK9 is a known regulator of MYC transcription and a modulator of MYC protein phosphorylation. Data reported at the American Association for Cancer Research (“AACR”) Annual Meeting 2021 in preclinical models demonstrated that voruciclib:
•Results in a rapid decrease in the phosphorylation of proteins that promote MYC transcription;
•Rapidly decreases phosphorylation of MYC protein on Ser62, a site implicated in stabilizing MYC in KRAS mutant cancers;
•Possesses single agent activity against multiple KRAS mutant cancer cell lines both in vitro and in vivo; and
•Synergistically inhibits KRAS G12C mutant cancer cell lines in combination with KRAS G12C inhibitors, both in vitro and in vivo.
The research presented suggests that voruciclib could be an attractive therapeutic agent for both hematological cancers, as well as solid tumors, dependent on the activity of MYC.
Clinical Programs
We are evaluating patients with hematological malignancies in a Phase 1 clinical trial evaluating the dose and schedule of voruciclib. The trial started with the evaluation of dose and schedule of voruciclib as a monotherapy in patients with relapsed and refractory B-cell malignancies and AML after failure of prior standard therapies to determine the safety, preliminary efficacy and maximum tolerated dose. After completing the monotherapy dose escalation stage of the study, we are now evaluating the dose and schedule of voruciclib in combination with venetoclax, a BCL-2 inhibitor, initially in patients with AML. The primary goal of the Phase 1 study is to assess the safety, and possible synergies, of voruciclib administered in combination with venetoclax. Clinical data is expected to be reported from the voruciclib Phase 1 study early in calendar 2024.
22
Table of Contents
As we reported in May 2023, the voruciclib monotherapy dose escalation/expansion stage of the study, which enrolled 40 patients with relapsed and refractory (“R/R”) AML and B-cell malignancies, is complete. Of the 40 patients enrolled, the first 16 were dosed daily continuously at 50 and 100 mg and the following 24 were dosed on an intermittent schedule (14 consecutive days on therapy in a 28-day cycle) at 100, 150 and 200 mg. All patients were heavily pre-treated with a median of 3 prior therapies (range 1-8). The most common (≥5% of all patients) adverse events related to voruciclib were diarrhea (15%), nausea (10%) and fatigue (7.5%), all graded 1 or 2. On the intermittent dosing schedule selected for further development, no dose-limiting toxicities (DLT) were observed, there were no grade three or higher drug related toxicities, and dose escalation was stopped at 200 mg before reaching the maximum tolerated dose because plasma concentrations reached levels considered sufficient for target inhibition. Of the 10 AML patients treated at the highest dose evaluated, 200 mg daily on the intermittent schedule, the disease control rate among these patients was 50%, with a median duration on therapy of 72 days (range 27-127) at the time of evaluation.
As further reported in our May 2023 update, the second stage of the study evaluating the combination of voruciclib and venetoclax in patients with R/R AML is ongoing. The first cohort in the dose escalation stage enrolled 6 patients administered 50 mg of voruciclib every other day for 14 days followed by 14 days without any therapy in a 28-day cycle, plus standard dose venetoclax. All patients were heavily pre-treated with a median of three prior therapies. Notably, all patients previously progressed after receiving treatment with venetoclax. No DLTs or overlapping bone marrow toxicities were observed. The disease control rate was 50%, including one patient who received five prior therapies including stem cell transplant and who achieved a partial response after the 1st cycle of therapy and a 2nd patient with stable disease and a reduction in transfusion requirement. Subsequently, the study Safety Review Committee has cleared enrollment in the three dose levels: 50 mg, 100 mg, 150 mg and 200 mg administered daily for 14 consecutive days followed by 14 days without any therapy in a 28-day cycle.
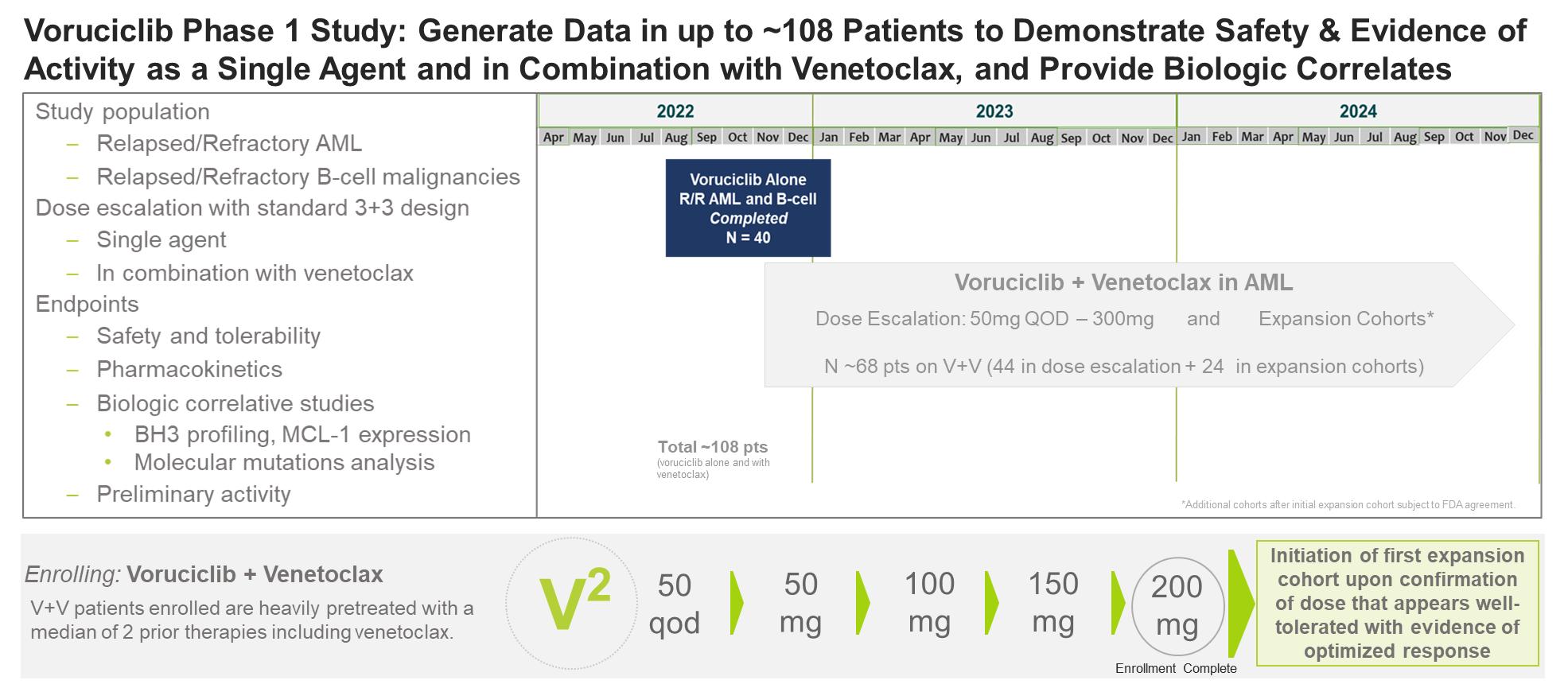
Voruciclib was also previously evaluated in more than 70 patients with solid tumors in multiple Phase 1 studies. The totality of the clinical data, along with data from pre-clinical studies, suggests voruciclib’s ability to inhibit its molecular target at a projected dose as low as 150 mg daily. In one clinical study, voruciclib was evaluated in combination with vemurafenib (marketed as Zelboraf®) in nine patients with BRAF mutated advanced/ inoperable malignant melanoma. All three BRAF/MEK naive patients achieved a response: two partial responses and one complete response. In this study voruciclib was dosed at 150 mg daily plus vemurafenib 720 mg or 960 mg twice daily in 28-day cycles. The most common adverse events were fatigue, constipation, diarrhea, arthralgia and headache. One instance of grade 3 fatigue was dose limiting and no serious adverse events related to voruciclib were reported. Other clinical studies evaluated voruciclib at doses up to 850 mg in patients with solid tumors, demonstrating additional evidence of potential biologic activity and an adverse event profile generally consistent with other drugs in its class.
ME-344: Clinical-stage Mitochondrial Inhibitor with Combinatorial Potential
ME-344 is a novel mitochondrial inhibitor drug candidate that demonstrates tumor selective activity in pre-clinical studies. It targets the OXPHOS pathway involved in the production of adenosine triphosphate, or ATP. By disrupting the production of ATP, the main source of energy for cells, ME-344 has been shown pre-clinically to induce cancer cell death through the induction of DNA fragmentation and through a process known as autophagy, whereby a cell consumes itself. ME-344 has also demonstrated evidence of antitumor activity in preclinical and clinical studies.
23
Table of Contents
ME-344 Scientific Overview: Cancer Metabolism
Energy supplied in the form of ATP fuels tumor metabolism supporting cell division and growth. Accordingly, tumor cells often display a high metabolic rate to support tumor cell survival and proliferation. This heightened metabolism requires a continual supply of energy in the form of ATP. The two major sources of ATP are oxidative phosphorylation in specialized cellular organelles termed mitochondria and through the metabolism of carbohydrates via the glycolysis pathway, which is frequently unregulated in cancer cells in a phenomenon called the Warburg Effect.
It is understood that anti-angiogenics, like the vascular endothelial growth factor (“VEGF”) inhibitor bevacizumab (marketed as Avastin®), may reduce the rate of glycolysis in tumors. As a result, tumor metabolism may then shift to mitochondria for energy production. In such cases of tumor plasticity in the presence of treatment with anti-angiogenics, contemporaneously targeting the alternative metabolic source by also inhibiting ATP production with the mitochondrial drug inhibitor ME-344 presents an important therapeutic opportunity for clinical evaluation.
MEI is pursuing evaluation of ME-344 in combination with VEGF inhibition and obtained preliminary clinical validation of the approach in a completed investigator-initiated, multi-center, randomized, controlled, window of opportunity clinical trial in combination with bevacizumab that enrolled a total of 42 patients with human epidermal growth factor receptor 2 (“HER2”) negative breast cancer. Additionally, we are currently evaluating the combination of ME-344 and bevacizumab in patients with metastatic colorectal cancer.
Clinical Program
ME-344 has been evaluated pre-clinically and clinically as a single agent and in combination with anti-angiogenics such as bevacizumab. When evaluated as a single agent, ME-344 demonstrated evidence of activity against refractory solid tumors in a Phase 1b trial, and in pre-clinical studies tumor cells treated with ME-344 resulted in a rapid loss of ATP and cancer cell death. In addition to single agent activity, ME-344 has also demonstrated significant potential in combination with anti-angiogenic therapeutics.
Pre-clinical studies, have shown that one outcome of anti-angiogenics is a reduced rate of glycolysis in tumors as a mechanism to slow tumor growth. However, when faced with reduced glycolysis and reduced ATP production, tumor metabolism was able to shift to mitochondrial metabolism for energy production to support continued tumor proliferation. In such cases of tumor plasticity in the presence of treatment with anti-angiogenics, contemporaneously targeting the mitochondria as an alternative metabolic source of ATP with ME-344 may open an important therapeutic opportunity.
Support for this combinatorial use of ME-344 was first published in the June 2016 edition of Cell Reports; pre-clinical data from a collaboration with the Spanish National Cancer Research Centre in Madrid demonstrated mitochondria-specific effects of ME-344 in cancer cells, including substantially enhanced anti-tumor activity when combined with agents that inhibit the activity of VEGF. These data demonstrating the potential anti-cancer effects of combining ME-344 with a VEGF inhibitor due to an inhibition of both mitochondrial and glycolytic metabolism provided a basis for commencement of an investigator-initiated trial of ME-344 in combination with bevacizumab in HER2 negative breast cancer patients.
Results published in the November 2019 issue of Clinical Cancer Research from a multi-center, investigator-initiated, randomized, controlled, clinical trial that evaluated the combination of ME-344 and bevacizumab in 42 women with early HER2-negative breast cancer further support the combinatorial use of ME-344 with anti-angiogenic therapeutics.
The primary objective of the trial was to show proof of ME-344 biologic activity as measured by reductions in the nuclear protein Ki67 (expression of which is strongly associated with tumor cell proliferation and growth) from days 0 to 28 compared to the control group who received bevacizumab alone. Secondary objectives included determining whether ME-344 biologic activity correlates with vascular normalization. The data demonstrated significant biologic activity in the ME-344 treatment group:
•In ME-344 treated patients, mean absolute Ki67 decreases were 13.3 compared to an increase of 1.1 in the bevacizumab monotherapy group (P=0.01).
•In ME-344 treated patients, mean relative Ki67 decreases were 23% compared to an increase of 186% in the bevacizumab monotherapy group (P < 0.01).
•The mean relative Ki67 reduction in patients experiencing vascular normalization in the ME-344 treated patients was 33%, compared to an increase of 11.8% in normalized patients from the bevacizumab monotherapy group (P=0.09). Approximately one-third of patients in each arm had vascular normalization.
Treatment was generally well tolerated; three grade 3 adverse events of high blood pressure were reported, two in the ME-344 arm and one in the bevacizumab monotherapy arm.
Results from our earlier, first-in-human, single-agent Phase 1 clinical trial of ME-344 in patients with refractory solid tumors were published in the April 1, 2015 edition of Cancer. The results indicated that eight of 21 evaluable patients (38%) treated with
24
Table of Contents
ME-344 achieved stable disease or better, including five who experienced progression-free survival that was at least twice the duration of their last prior treatment before entry into the trial. In addition, one of these patients, a heavily pre-treated patient with small cell lung cancer, achieved a confirmed partial response and remained on study for two years. ME-344 was generally well tolerated at doses equal to or less than 10 mg/kg delivered on a weekly schedule for extended durations. Treatment-related adverse events included nausea, dizziness and fatigue. Dose-limiting toxicities were observed at both the 15 mg/kg and 20 mg/kg dose levels, consisting primarily of grade 3 peripheral neuropathy.
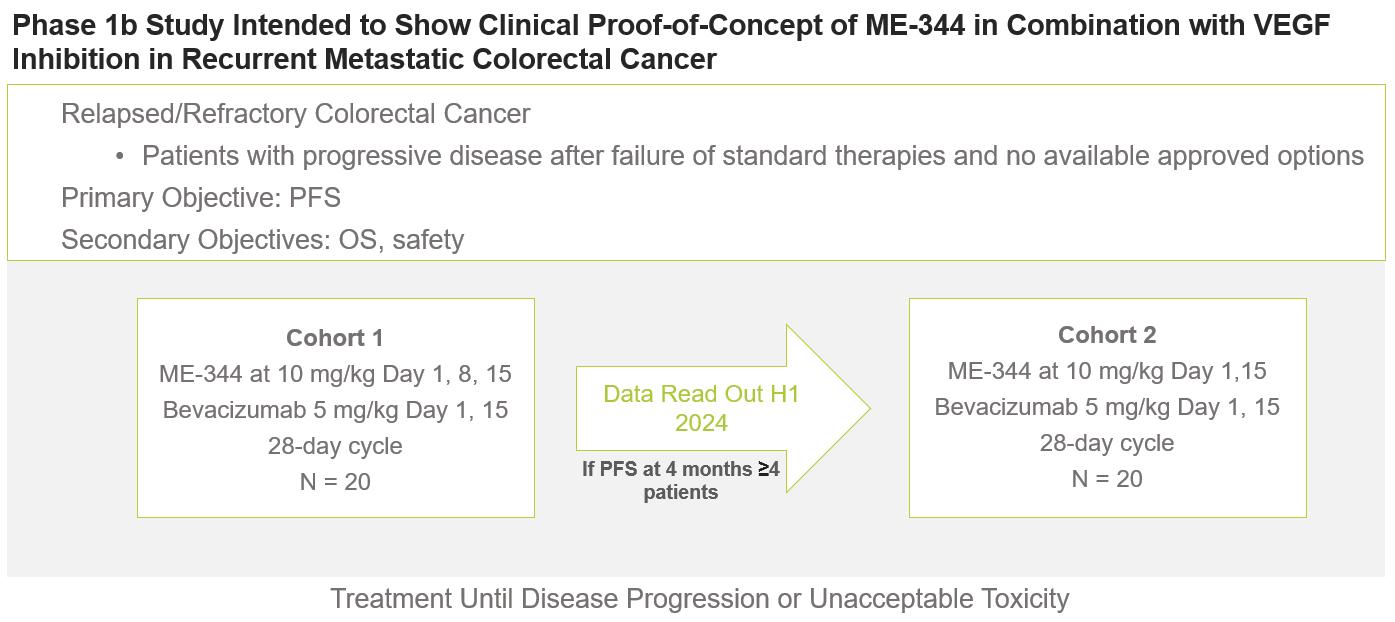
We are advancing ME-344 in combination with the anti-angiogenic antibody bevacizumab in a Phase 1b study evaluating patients with relapsed colorectal cancer. The study is enrolling patients with progressive disease after failure of standard therapies with patients treated until disease progression or intolerance. The primary objective is progression free survival. Secondary endpoints include overall response rate, duration of response, overall survival and safety. Safety and efficacy data from the first cohort of 20 patients in the ongoing ME-344 Phase 1b study is expected to be reported in the first half of calendar 2024. Additionally, ME-344 may also have clinical potential against hematological malignancies. At the AACR Annual Meeting 2022, a poster presentation reported results from preclinical studies exploring the ability of ME-344 to enhance the activity of venetoclax against AML. Data from the in vitro and in vivo preclinical studies evaluating the combination of ME-344 with venetoclax in standard-of-care-resistant AML cell lines and relapsed or refractory AML patient samples suggest that ME-344, both alone and in combination with venetoclax, inhibits purine biosynthesis, suppresses oxidative phosphorylation, induces apoptosis and decreases MCL-1, which together target metabolic vulnerabilities of AML cells. The data demonstrated that ME-344 and venetoclax prolong survival in MV4-11 and MV4-11/AraC-R-derived xenograft AML models. The poster concluded that ME-344 enhances venetoclax activity against AML cells including resistant AML.
Zandelisib: PI3Kδ Inhibitor Overview
Zandelisib is an oral, once-daily, selective PI3Kδ inhibitor that we were jointly developing with KKC under a global license, development and commercialization agreement entered into in April 2020.
Zandelisib completed the global Phase 2 TIDAL study evaluating it as a single agent in adults with relapsed/refractory (“r/r”) follicular lymphoma (“FL”) after failure of at least two prior systemic therapies, including chemotherapy and an anti-CD20 antibody. A total of 121 patients with r/r FL were enrolled in the intermittent dosing arm, 91 of which were enrolled in the primary efficacy population for the evaluation of objective response rate (“ORR”) and duration of response (“DOR”). The median age of patients with FL was 64 years old; 45% of patients had disease refractory to last therapy, and patients received a median of 3 prior lines of treatment (range: 2-8). Patients were administered zandelisib once daily for two 28-day cycles as response induction therapy, followed thereafter by once daily dosing for the first seven days of each subsequent 28-day cycle, a schedule called Intermittent Dosing Therapy (IDT).
By independent review, the ORR was 73% (95% CI, 63.9 to 80.4%), the complete response rate 38% (95% CI, 29.3 to 47.3%), and the median DOR 16.4 months (95% CI, 9.5 months to not reached). With a median follow up of 14.3 months (range, 1 to 30.5 months), the median PFS was 11.6 months (95% CI, 8.3 to not reached). Twenty-one patients (17%) discontinued zandelisib due to an adverse event. Grade 3-4 class-related toxicities were diarrhea in 6% of patients, lung infections 5 %, rash 3%, colitis 3%, AST elevation 2%, and non-infectious pneumonitis 1%.
25
Table of Contents
In March 2022, we and KKC reported the outcome of an end of Phase 2 meeting with the FDA wherein the agency discouraged a filing based on data from a single-arm Phase 2 TIDAL trial. At this meeting, the FDA stated that data generated from single arm studies such as the Phase 2 TIDAL trial are insufficient to adequately assess the risk/benefit of PI3Kδ inhibitors evaluating indolent non-Hodgkin lymphoma. At that time, the FDA emphasized that the company continue efforts with the ongoing randomized Phase 3 COASTAL trial evaluating patients with relapsed or refractory follicular or marginal zone lymphomas. Subsequently, at an April 2022 meeting of the FDA Oncology Drugs Advisory Committee, the committee voted that future approvals of PI3Kδ inhibitors for hematologic malignancies should be supported by randomized data.
In November 2022, we and KKC met with the FDA in a follow-up meeting to the March 2022 end of Phase 2 meeting. At this meeting, the FDA provided further guidance regarding the design and statistical analysis for the Phase 3 COASTAL trial. Following the November meeting, the companies jointly concluded that a clinical trial consistent with the recent FDA guidance, including modification of the ongoing COASTAL trial, would likely not be feasible to complete within a time period that would support further investment or with sufficient certainty of the regulatory requirements for approval to justify continued global development efforts. As a result, we and KKC jointly decided to discontinue global development of zandelisib for indolent forms of non-Hodgkin lymphoma outside of Japan. The discontinuation of zandelisib development outside of Japan was a business decision based on the most recent regulatory guidance from the FDA and is not related to the zandelisib clinical data generated to date. After making the joint decision to terminate development outside of Japan, we and KKC began closing all ongoing zandelisib clinical studies outside of Japan, including the Phase 3 COASTAL trial, the Phase 2 TIDAL trial, and the Phase 2 CORAL trial.
Subsequently, in May 2023, KKC decided to discontinue development of zandelisib in Japan. The discontinuation of zandelisib in Japan was a business decision by KKC based on the most recent regulatory guidance from the PMDA and was not related to the zandelisib clinical data generated to date.
On July 14, 2023, we entered into a Termination Agreement (the “Termination Agreement”) with KKC to terminate all agreements between the parties and cease further zandelisib clinical development globally. As of September 30, 2023, activities associated with the compassionate use supply were completed. We anticipate completing the wind-down activities associated with the KKC Commercialization Agreement to be completed in fiscal year 2024.
KKC License, Development and Commercialization Agreement
In April 2020, we entered into the KKC Commercialization Agreement under which we granted to KKC a co-exclusive, sublicensable, payment-bearing license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in the U.S. (the “U.S. License”), and an exclusive (subject to certain retained rights to perform obligations under the KKC Commercialization Agreement), sublicensable, payment- bearing, license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in countries outside of the U.S. (the “Ex-U.S.” and the “Ex-U.S. License”). Also under the KKC Commercialization Agreement, we were granted a co-exclusive, sublicensable, license under certain patents and know-how controlled by KKC to develop and commercialize zandelisib for all human indications in the U.S., and a co-exclusive, sublicensable, royalty-free, fully paid license under certain patents and know-how controlled by KKC to perform our obligations in the Ex-U.S. and were paid an initial non-refundable payment of $100.0 million. Additionally, in Japan, the KKC Commercialization Agreement included potential regulatory and commercialization milestone payments plus royalties on net sales of zandelisib in Japan, which are tiered beginning in the teens. Prior to the execution of the Termination Agreement on July 14, 2023, KKC was responsible for the development and commercialization of zandelisib in the Ex-U.S. and, subject to certain exceptions, solely responsible for all costs related thereto. We also provided to KKC certain drug supplies necessary for the development and commercialization of zandelisib in the Ex-U.S., with the understanding that KKC would have assumed responsibility for manufacturing for the Ex-U.S. as soon as practicable.
As noted above, on July 14, 2023, we entered into a Termination Agreement with KKC to mutually terminate the original KKC Commercialization Agreement and all other related agreements between the parties. Pursuant to the Termination Agreement:
•We regained full, global rights to develop, manufacture and commercialize ME-401, subject to KKC's limited rights to use ME-401 for “compassionate use” (as more specifically defined in the Termination Agreement) in certain expanded access programs for the existing patients who have been enrolled in Japanese clinical trial sponsored by KKC until November 30, 2027, and for which KKC is fully liable;
•each party released the other party from any and all claims, demands, etc. arising from the original KKC Commercialization Agreement, excluding certain surviving claims; and
•we are obligated to deliver a discrete quantity of materials to facilitate KKC's activities.
As of June 30, 2023, we had $64.9 million of deferred revenue associated with the KKC Commercialization Agreement, of which $64.5 million was allocated to the U.S. License and $317,000 was allocated to the Development Services performance
26
Table of Contents
obligations that continue to be recognized based on the proportional performance of these development activities through wind-down of the associated trials. As further discussed in Note 7. License Agreements, in connection with the execution of the Termination Agreement during the three months ended September 30, 2023, we recognized the $64.5 million of deferred revenue associated with the U.S. License as well as the remaining $317,000 noncash deferred revenue associated with the completion of the underlying proportional performance activities. As of September 30, 2023, all deferred revenue associated with the KKC Commercialization Agreement has been recognized.
Results of Operations
Comparison of Three Months Ended September 30, 2023 and 2022
Revenue: We recognized revenue of $65.3 million for the three months ended September 30, 2023 compared to $8.7 million for the three months ended September 30, 2022. The increase in revenue is due to the recognition of deferred revenue associated with the U.S. License that was terminated in July 2023, offset by a decrease in revenue recognized during the three months ending September 30, 2023, related to cost sharing from the KKC Commercialization Agreement.
Research and Development: The following is a summary of our research and development expenses to supplement the more detailed discussion below (in thousands).
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
zandelisib |
|
$ |
449 |
|
|
$ |
11,606 |
|
voruciclib |
|
|
(335 |
) |
|
|
733 |
|
ME-344 |
|
|
1,220 |
|
|
|
785 |
|
Other |
|
|
2,151 |
|
|
|
6,339 |
|
Total research and development expenses |
|
$ |
3,485 |
|
|
$ |
19,463 |
|
Research and development expenses consist primarily of clinical trial costs (including payments to contract research organizations “CROs”), pre-clinical study costs, and costs to manufacture our drug candidates for non-clinical and clinical studies. Other research and development expenses consist primarily of salaries and personnel costs, share-based compensation, legal costs, and other costs not allocated to specific drug programs. Costs related to zandelisib decreased $11.2 million, primarily as a result of the discontinuation of the program during fiscal year 2023 and the completion of wind-down related activities. Costs related to voruciclib decreased $1.1 million mainly due to lower recognized clinical costs in the Phase 1 study. Costs related to ME-344 increased $0.4 million due to increased clinical costs related to the Phase 1b study. Other research and development costs decreased $4.2 million primarily due to a decrease of $3.4 million in personnel costs related to the reduced headcount and a $0.7 million decrease in noncash stock-based compensation.
General and Administrative: General and administrative expenses decreased by $1.0 million to $6.5 million for the three months ended September 30, 2023 compared to $7.5 million for the three months ended September 30, 2022. The decrease is primarily due to $1.0 million lower personnel costs due to reduced headcount, $0.5 million lower noncash stock-based compensation, and $0.3 million lower corporate overhead costs partially offset by a $0.8 million increase in external professional services primarily related to advisory and legal fees associated with various stockholder-related activities, including stockholder-initiated consent solicitation efforts.
Other Income, net: Other income, net decreased by $0.5 million to $1.1 million for the three months ended September 30, 2023 compared to $1.6 million for the three months ended September 30, 2022. We recorded a non-cash gain of $1.1 million during the three months ended September 30, 2022 due to a change in the fair value of our warrant liability with no similar gain during the three months ended September 30, 2023 as a result of the warrants expiring in May 2023. Additionally, we received interest and dividend income of $1.1 million for the three months ended September 30, 2023 compared to $0.5 million for the three months ended September 30, 2022. The increase in interest and dividend income is primarily due to higher yields during the three months ended September 30, 2023 compared to the three months ended September 30, 2022.
Liquidity and Capital Resources
We have accumulated losses of $349.6 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of September 30, 2023, we had $82.2 million in cash and cash equivalents, and short-term investments. We believe that these resources will be sufficient to fund our operations for at least 12 months from the issuance of this Quarterly Report. Our current business operations are focused on continuing the clinical development of our drug candidates. Changes to our research and development plans or other changes affecting our operations and operating expenses may affect actual future use of existing cash resources. We cannot determine with certainty costs associated with ongoing and future
27
Table of Contents
clinical trials or the regulatory approval process. The duration, costs and timing associated with the development of our product candidates will depend on a variety of factors, including uncertainties associated with the results of our clinical trials.
To date, we have obtained cash and funded our operations primarily through equity financings and license agreements. In order to continue the development of our drug candidates, at some point in the future we expect to pursue one or more capital transactions, whether through the sale of equity securities, debt financing, license agreements or entry into strategic partnerships. There can be no assurance that we will be able to continue to raise additional capital in the future.
Sources and Uses of Our Cash
Net cash used in operating activities for the three months ended September 30, 2023 of $18.5 million consisted of our net income of $56.4 million offset by $75.7 million in changes in our operating assets and liabilities primarily due to recognition of $64.9 million in deferred revenue, partially offset by $0.8 million for noncash items. Net cash used in operating activities for the three months ended September 30, 2022, of $14.8 million consisted of our net loss of $16.6 million partially offset by changes in our operating assets and liabilities of $1.0 million and $0.9 million in noncash items.
Net cash provided by investing activities for the three months ended September 30, 2023 was $5.0 million as compared to $13.7 million cash provided by investing activities for the three months ended September 30, 2022. The decrease was primarily due to less proceeds from maturities of short-term investments during the three months ended September 30, 2023, net of purchases.
Net cash used in financing activities during the three months ended September 30, 2022 was $40,000 due to the payment of RSU withholding in exchange for common shares surrendered by RSU holders. There were no cash flows from financing activities during the three months ended September 30, 2023.
Capital Resource Requirements
As previously discussed in the overview section above, in conjunction with our Cooperation Agreement with Anson and Cable Car, we agreed to reimburse up to $1.2 million of their legal fees. In addition, we also agreed to at least one capital return to shareholders in the form of a dividend in the amount of $1.75 per share of common stock to all stockholders. Lastly, a potential second return of capital of approximately $9.33 million in the aggregate could be authorized by the Board if our ongoing ME-344 Phase 1b trial fails to meet certain endpoints or our Board determines not to proceed with a second cohort, both as further described in the Cooperation Agreement. The second return of capital may take the form of a dividend or tender offer and is subject to Board approval as well as modification associated with applicable requirements under Delaware law, both as detailed in the Cooperation Agreement. The $1.75 dividend is anticipated to be distributed during the second quarter of fiscal year 2024.
In January 2022, we amended our facility lease for an additional 20 months through November 2029. The amended lease agreement also provided for additional lease space that we took control over on July 1, 2022. Under the terms of the lease, we are obligated to make aggregate remaining lease payments as of September 30, 2023, of $15.8 million, excluding common area maintenance and other variable consideration due under the lease agreement. Estimated lease payments for the remainder of our fiscal year ended June 30, 2024, are expected to be $1.8 million, excluding common area maintenance and other variable consideration due under the lease agreement.
As of September 30, 2023, we have the following potential purchase obligations for which the timing and/or likelihood of occurrence is unknown; however, if such claims arise in the future, they could have a material effect on our financial position, results of operations, and cash flows.
•Under our remaining license agreements, we have payment obligations, which are contingent upon future events such as our achievement of specified development, regulatory and commercial milestones and are required to make royalty payments in connection with the sales of products developed under those agreements. For additional details regarding these agreements, see the section titled Note 8—Other License Agreements and Note 6—Commitments and Contingencies to our condensed consolidated financial statements and related notes included elsewhere in this Quarterly Report;
•Obligations under contracts which are cancelable without significant penalty;
•Purchase orders issued in the ordinary course of business as they represent authorizations to purchase the items rather than binding agreements; and
•Contracts in the normal course of business with clinical supply manufactures and with vendors for preclinical studies, research supplies and other services and products for operating purposes. These contracts are cancelable and generally provide for termination after a notice period.
Our future capital requirements will depend on many factors, including:
•the scope, progress, results and costs of drug discovery, preclinical development, laboratory testing and clinical trials for our product candidates;
28
Table of Contents
•the costs, timing and outcome of regulatory review of our product candidates;
•the costs of establishing or contracting for sales, marketing and distribution capabilities if we obtain regulatory approvals to market our product candidates;
•the costs of securing and producing drug substance and drug product material for use in preclinical studies, clinical trials and for use as commercial supply;
•the costs of securing manufacturing arrangements for development activities and commercial production;
•the scope, prioritization and number of our research and development programs;
•the extent to which we are obligated to reimburse, or entitled to reimbursement of, clinical trial costs under future collaboration agreements, if any; and
•the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims.
Estimate Considerations Related to Macroeconomic Conditions and other Geopolitical Conditions
Due to recent disruptions in access to bank deposits and lending commitments associated with bank failures, the COVID-19 pandemic and macroeconomic and geopolitical conditions, there has been uncertainty and disruption in the global economy and financial markets. We are not aware of any specific event or circumstance that would require an update to its estimates or judgments or a revision of the carrying value of its assets or liabilities as of September 30, 2023. While there was no material impact to our condensed consolidated financial statements as of and for the three months ended September 30, 2023, these estimates may change, as new events occur and additional information is obtained, which could materially impact our condensed consolidated financial statements in future reporting periods.
Critical Accounting Policies and Management Estimates
We describe our significant accounting policies in Note 2. The Company and Summary of Significant Accounting Policies, of the notes to the financial statements included in our 2023 Annual Report. We discuss our critical accounting estimates in Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations, in our 2023 Annual Report. There have been no changes in our significant accounting policies or critical accounting estimates since June 30, 2023.
Recent Accounting Pronouncement
See Note 2.Summary of Significant Accounting Policies in the Notes to Condensed Consolidated Financial Statements in this Quarterly Report.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our exposure to market interest rates relates primarily to the investments of cash balances and short-term investments. We have cash reserves held in U.S. dollars and we place funds on deposit with financial institutions, which are readily available. Our short-term investments consist solely of U.S. government securities with a maturity of three to twelve months.
We place our cash deposits with high credit quality financial institutions and by policy limit the amount of credit exposure to any one corporation or bank. These deposits are in excess of the Federal Deposit Insurance Corporation (“FDIC”) insurance limits. We are adverse to principal loss and we ensure the safety and preservation of our invested funds by limiting default risk, market risk and reinvestment risk. We seek to mitigate default risk by depositing funds with high credit quality financial institutions, by limiting the amount of credit exposure to any one corporation or bank, by purchasing short-term investments consisting of U.S. government securities, and by positioning our portfolio to respond appropriately to a significant reduction in a credit rating of any such financial institution.
We do not consider the effects of interest rate movements to be a material risk to our financial condition.
Item 4. Controls and Procedures
(a) Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer, or CEO, and Chief Financial Officer, or CFO, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as
29
Table of Contents
of September 30, 2023. Based on such evaluation, our CEO and CFO have concluded that, as of September 30, 2023, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the U.S. Securities and Exchange Commission, or SEC’s, rules and forms, and is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
Under the supervision and with the participation of our management, including our CEO and CFO, we carried out an evaluation of any potential changes in our internal control over financial reporting during the fiscal quarter ended September 30, 2023. There were no changes in our internal control over financial reporting (as defined in Rule 13a‑15(f) of the Exchange Act) that occurred during the fiscal quarter ended September 30, 2023, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations of Internal Controls
Our management, including our CEO and CFO, does not expect that our disclosure controls and procedures or our internal controls over financial reporting will prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Due to the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
30
Table of Contents
PART II OTHER INFORMATION
Item 1. Legal Proceedings
None.
Item 1A. Risk Factors
There have been no material changes in our risk factors from those included in our 2023 Annual Report
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
31
Table of Contents
Item 6. Exhibits
Exhibit Index
|
|
|
Exhibits |
|
|
3.1 |
|
Certificate of Designation of Series A Junior Participating Preferred Stock of MEI Pharma, Inc. effective as of October 1, 2023 (incorporated by reference to Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on October 3, 2023 (File No. 000-50484)) |
4.1 |
|
Rights Agreement between MEI Pharma, Inc. and Computershare, Inc. (as Rights Agent) dated as of October 1, 2023 (incorporated by reference to the Registrant's Current Report on Form 8-K filed on October 3, 2023 (File No. 000-50484)) |
10.1 |
|
Termination Agreement by and between MEI Pharma, Inc. and Kyowa Kirin Co., Ltd. (formerly known as Kyowa Hakko Kirin Co., Ltd.) dated as of July 14, 2023 (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on July 19, 2023 (File No. 000-50484)) |
10.2 |
|
Termination Letter from MEI Pharma, Inc. to Infinity Pharmaceuticals, Inc. dated July 23, 2023 (incorporated by reference to the Registrant's Current Report on Form 8-K filed on July 24, 2023 (File No. 000-0050484)) |
10.3 |
|
Cooperation Agreement dated as of October 31, 2023 by and among the Investors and the Company (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on November 1, 2023 (File No. 001-41827)) |
31.1 |
|
Rule 13a-14(a) or Rule 15d-14(a) Certification of Principal Executive Officer. |
31.2 |
|
Rule 13a-14(a) or Rule 15d-14(a) Certification of Principal Financial Officer. |
32.1 |
|
Certification of Principal Executive Officer and Principal Financial Officer required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C 1350). |
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
32
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Quarterly Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
MEI Pharma, Inc. |
|
|
|
/s/ Justin J. File |
|
Justin J. File Chief Financial Officer and Secretary |
|
|
|
Date: November 9, 2023 |
33
Exhibit 31.1
CERTIFICATION
I, David M. Urso, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of MEI Pharma, Inc.;
2.Based on my knowledge, this Quarterly Report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this Quarterly Report;
3.Based on my knowledge, the financial statements, and other financial information included in this Quarterly Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this Quarterly Report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this Quarterly Report is being prepared;
(b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in the Quarterly Report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this Quarterly Report based on such evaluation; and
(d)disclosed in this Quarterly Report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting.
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors:
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
Date: November 9, 2023 |
|
|
|
/s/ David M. Urso |
|
|
|
|
David M. Urso President and Chief Executive Officer (Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION
I, Justin J. File, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of MEI Pharma, Inc.;
2.Based on my knowledge, this Quarterly Report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this Quarterly Report;
3.Based on my knowledge, the financial statements, and other financial information included in this Quarterly Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this Quarterly Report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this Quarterly Report is being prepared;
(b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in the Quarterly Report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this Quarterly Report based on such evaluation; and
(d)disclosed in this Quarterly Report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting.
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors:
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
Date: November 9, 2023 |
|
|
|
/s/ Justin J. File |
|
|
|
|
Justin J. File Chief Financial Officer and Secretary (Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION
Pursuant to the requirement set forth in Rule 13a-14(b) or Rule 15d-14(b) of the Securities Exchange Act of 1934, as amended, and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. § 1350), David M. Urso, the President and Chief Executive Officer of MEI Pharma, Inc. (the “Registrant”), and Justin J. File, the Chief Financial Officer of the Registrant, each hereby certifies that, to his knowledge:
1.The Registrant’s Quarterly Report on Form 10-Q for the period ended September 30, 2023, (the “Form 10-Q”) to which this Certification is attached as Exhibit 32.1, fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Form 10-Q fairly presents, in all material respects, the financial condition of the Registrant at the end of the period covered by the Form 10-Q and results of operations of the registrant for the period covered by the Form 10-Q.
These certifications accompanying the Form 10-Q to which they relate, are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of the registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-Q), irrespective of any general incorporation language contained in such filing.
Dated: November 9, 2023
|
|
|
/s/ David. M. Urso |
|
/s/ Justin J. File |
David. M. Urso |
|
Justin J. File |
President and Chief Executive Officer (Principal Executive Officer) |
|
Chief Financial Officer and Secretary (Principal Financial Officer) |
v3.23.3
Cover Page - shares
|
3 Months Ended |
|
Sep. 30, 2023 |
Oct. 31, 2023 |
| Cover [Abstract] |
|
|
| Document Type |
10-Q
|
|
| Amendment Flag |
false
|
|
| Document Quarterly Report |
true
|
|
| Document Transition Report |
false
|
|
| Document Period End Date |
Sep. 30, 2023
|
|
| Document Fiscal Year Focus |
2024
|
|
| Document Fiscal Period Focus |
Q1
|
|
| Securities Act File Number |
001-41827
|
|
| Trading Symbol |
MEIP
|
|
| Entity Registrant Name |
MEI Pharma, Inc.
|
|
| Entity Tax Identification Number |
51-0407811
|
|
| Entity Central Index Key |
0001262104
|
|
| Current Fiscal Year End Date |
--06-30
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Shell Company |
false
|
|
| City Area Code |
858
|
|
| Local Phone Number |
369-7100
|
|
| Entity Small Business |
true
|
|
| Title of 12(b) Security |
Common Stock, $0.00000002 par value
|
|
| Security Exchange Name |
NASDAQ
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
| Entity Address, Address Line One |
11455 El Camino Real
|
|
| Entity Address, Address Line Two |
Suite 250
|
|
| Entity Address, City or Town |
San Diego
|
|
| Entity Address, State or Province |
CA
|
|
| Entity Address, Postal Zip Code |
92130
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Bankruptcy Proceedings, Reporting Current |
true
|
|
| Entity Common Stock, Shares Outstanding |
|
6,662,857
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor registrants involved in bankruptcy proceedings during the preceding five years, the value Yes indicates that the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court; the value No indicates the registrant has not. Registrants not involved in bankruptcy proceedings during the preceding five years should not report this element. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12, 13, 15d
| Name: |
dei_EntityBankruptcyProceedingsReportingCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
CONDENSED CONSOLIDATED BALANCE SHEETS - USD ($)
$ in Thousands |
Sep. 30, 2023 |
Jun. 30, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 3,372
|
$ 16,906
|
| Short-term investments |
78,830
|
83,787
|
| Unbilled receivables |
0
|
85
|
| Prepaid expenses and other current assets |
6,220
|
6,750
|
| Total current assets |
88,422
|
107,528
|
| Operating lease right-of-use asset |
11,600
|
11,972
|
| Property and equipment, net |
1,229
|
1,309
|
| Total assets |
101,251
|
120,809
|
| Current liabilities: |
|
|
| Accounts payable |
3,220
|
6,134
|
| Accrued liabilities |
4,289
|
12,461
|
| Deferred revenue |
0
|
317
|
| Operating lease liability |
1,055
|
1,428
|
| Total current liabilities |
8,564
|
20,340
|
| Deferred revenue, long-term |
0
|
64,545
|
| Operating lease liability, long-term |
11,326
|
11,300
|
| Total liabilities |
19,890
|
96,185
|
| Commitments and contingencies (Note 6) |
|
|
| Stockholders' equity: |
|
|
| Preferred stock, $0.01 par value; 100 shares authorized; none outstanding |
0
|
0
|
| Common stock, $0.00000002 par value; 226,000 shares authorized; 6,663 shares issued and outstanding at September 30, 2023 and June 30, 2023. |
0
|
0
|
| Additional paid-in capital |
430,984
|
430,621
|
| Accumulated deficit |
(349,623)
|
(405,997)
|
| Total stockholders' equity |
81,361
|
24,624
|
| Total liabilities and stockholders' equity |
$ 101,251
|
$ 120,809
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionValue received from shareholders in common stock-related transactions that are in excess of par value or stated value and amounts received from other stock-related transactions. Includes only common stock transactions (excludes preferred stock transactions). May be called contributed capital, capital in excess of par, capital surplus, or paid-in capital. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapitalCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred income and obligation to transfer product and service to customer for which consideration has been received or is receivable, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredRevenueCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred income and obligation to transfer product and service to customer for which consideration has been received or is receivable, classified as noncurrent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredRevenueNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investments including trading securities, available-for-sale securities, held-to-maturity securities, and short-term investments classified as other and current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_ShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount received for services rendered and products shipped, but not yet billed, for non-contractual agreements due within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_UnbilledReceivablesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
CONDENSED CONSOLIDATED BALANCE SHEETS (parenthetical) - $ / shares
|
Sep. 30, 2023 |
Jun. 30, 2023 |
| Preferred stock, par value |
$ 0.01
|
$ 0.01
|
| Preferred stock, shares authorized |
100,000
|
100,000
|
| Preferred stock, shares outstanding |
0
|
0
|
| Common stock, par value |
$ 0.00000002
|
$ 0.00000002
|
| Common stock, shares authorized |
226,000,000
|
226,000,000
|
| Common stock, shares issued |
6,663,000
|
6,663,000
|
| Common stock, shares outstanding |
6,663,000
|
6,663,000
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.3
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS - USD ($)
shares in Thousands, $ in Thousands |
3 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Revenues |
$ 65,297
|
$ 8,730
|
| Operating expenses: |
|
|
| Research and development |
3,485
|
19,463
|
| General and administrative |
6,531
|
7,486
|
| Total operating expenses |
10,016
|
26,949
|
| Income (loss) from operations |
55,281
|
(18,219)
|
| Other income (expense): |
|
|
| Change in fair value of warrant liability |
0
|
1,117
|
| Interest and dividend income |
1,094
|
480
|
| Other expense, net |
(1)
|
(2)
|
| Total other income, net |
1,093
|
1,595
|
| Net income (loss) |
$ 56,374
|
$ (16,624)
|
| Net income (loss) per share - basic and diluted: |
|
|
| Basic |
$ 8.46
|
$ (2.49)
|
| Diluted |
$ 8.46
|
$ (2.49)
|
| Weighted-average shares used in computing net income (loss) per share - basic and diluted: |
|
|
| Basic |
6,663
|
6,663
|
| Diluted |
6,663
|
6,663
|
| Revenue from customers |
|
|
| Revenues |
$ 752
|
$ 8,730
|
| Revenue from collaboration agreements |
|
|
| Revenues |
$ 64,545
|
$ 0
|
| X |
- DefinitionTotal costs of sales and operating expenses for the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostsAndExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CostsAndExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) recognized in income from liability measured at fair value on recurring basis using unobservable input (level 3) and still held. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisChangeInUnrealizedGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income and dividend income on nonoperating securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7(a),(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeInterestAndDividend |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_RevenueFromContractWithCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=meip_RevenueFromCollaborationAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY - USD ($)
$ in Thousands |
Total |
Common Shares |
Additional paid in capital |
Accumulated Deficit |
| Beginning Balance at Jun. 30, 2022 |
$ 52,413
|
$ 6,658
|
$ 426,572
|
$ (374,159)
|
| Net income (loss) |
(16,624)
|
|
|
(16,624)
|
| Issuance of common stock for vested restricted stock units |
(40)
|
5
|
(40)
|
|
| Share-based compensation expense |
1,559
|
|
1,559
|
|
| Ending Balance at Sep. 30, 2022 |
37,308
|
6,663
|
428,091
|
(390,783)
|
| Beginning Balance at Jun. 30, 2023 |
24,624
|
6,663
|
430,621
|
(405,997)
|
| Net income (loss) |
56,374
|
|
|
56,374
|
| Share-based compensation expense |
363
|
|
363
|
|
| Ending Balance at Sep. 30, 2023 |
$ 81,361
|
$ 6,663
|
$ 430,984
|
$ (349,623)
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock related to Restricted Stock Awards issued during the period, net of the stock value of such awards forfeited. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueRestrictedStockAwardNetOfForfeitures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS - USD ($)
$ in Thousands |
3 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Cash flows from operating activities: |
|
|
| Net income (loss) |
$ 56,374
|
$ (16,624)
|
| Adjustments to reconcile net income (loss) to net cash used in operating activities: |
|
|
| Change in fair value of warrant liability |
0
|
(1,117)
|
| Share-based compensation |
363
|
1,559
|
| Non-cash lease expense |
372
|
349
|
| Depreciation expense |
87
|
99
|
| Changes in operating assets and liabilities: |
|
|
| Unbilled receivables |
85
|
2,286
|
| Prepaid expenses and other current assets |
530
|
1,048
|
| Accounts payable |
(2,921)
|
500
|
| Accrued liabilities |
(8,172)
|
(1,604)
|
| Deferred revenue |
(64,862)
|
(971)
|
| Operating lease liability |
(347)
|
(307)
|
| Net cash used in operating activities |
(18,491)
|
(14,782)
|
| Cash flows from investing activities: |
|
|
| Purchases of property and equipment |
0
|
(63)
|
| Purchases of short-term investments |
(24,197)
|
(34,052)
|
| Proceeds from maturity of short-term investments |
29,154
|
47,850
|
| Net cash provided by investing activities |
4,957
|
13,735
|
| Cash flows from financing activities: |
|
|
| Payments of tax withholdings related to vesting of restricted stock units |
0
|
(40)
|
| Net cash (used in) provided by financing activities |
0
|
(40)
|
| Net decrease in cash and cash equivalents |
(13,534)
|
(1,087)
|
| Cash and cash equivalents at beginning of the period |
16,906
|
15,740
|
| Cash and cash equivalents at end of the period |
3,372
|
14,653
|
| Supplemental cash flow information: |
|
|
| Purchases of property and equipment included in accounts payable |
7
|
0
|
| Operating lease right-of-use assets obtained in exchange for operating lease liabilities |
$ 0
|
$ 4,347
|
| X |
- DefinitionIncrease Decrease In Unbilled Receivable
| Name: |
meip_IncreaseDecreaseInUnbilledReceivable |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFuture cash outflow to pay for purchases of fixed assets that have occurred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_CapitalExpendituresIncurredButNotYetPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) recognized in income from liability measured at fair value on recurring basis using unobservable input (level 3) and still held. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisChangeInUnrealizedGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionChange in recurring obligations of a business that arise from the acquisition of merchandise, materials, supplies and services used in the production and sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableTrade |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation to transfer good or service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482312/912-310-45-11
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInContractWithCustomerLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for securities or other assets acquired, which qualify for treatment as an investing activity and are to be liquidated, if necessary, within the current operating cycle. Includes cash flows from securities classified as trading securities that were acquired for reasons other than sale in the short-term. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from maturities, prepayments, calls and collections of all investments, including securities and other assets, having ready marketability and intended by management to be liquidated, if necessary, within the current operating cycle. Includes cash flows from securities classified as trading securities that were acquired for reasons other than sale in the short-term. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromMaturitiesPrepaymentsAndCallsOfShorttermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for operating lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Description of Business and Basis of Presentation
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Description of Business and Basis of Presentation |
1. Description of Business and Basis of Presentation Description of Business MEI Pharma, Inc. (Nasdaq: MEIP) is a clinical-stage pharmaceutical company committed to developing novel and differentiated cancer therapies. We build our pipeline by acquiring promising cancer agents and creating value in programs through development, strategic partnerships, and out-licensing or commercialization, as appropriate. Our approach to oncology drug development is to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. Our pipeline includes voruciclib, an oral cyclin-dependent kinase 9 (“CDK9”) inhibitor, and ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway. Reverse Stock Split On April 14, 2023, we amended our Certificate of Incorporation to affect a combination of our issued and outstanding common stock at a ratio of one-for-twenty (“Reverse Stock Split”). The par value and authorized shares of our common stock were not adjusted as a result of the Reverse Stock Split. The Reverse Stock Split was effective on April 14, 2023, with a market effective date of April 17, 2023. All historical share and per share amounts have been adjusted to reflect the Reverse Stock Split for all periods presented. All stock options, restricted stock units and warrants outstanding were ratably adjusted to give effect to the Reverse Stock Split. Current Events Cooperation Agreement On October 31, 2023, we announced a Cooperation Agreement (“Cooperation Agreement”) with Anson Funds and Cable Car Capital of which, among other non-financial related items as described within the overview section of Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations, contained a capital return to stockholders in the form of a dividend in the amount of $1.75 per share of common stock to all stockholders. Additionally, a potential second return of capital of approximately $9.33 million in the aggregate will be authorized by the board of directors ("Board") should our ongoing ME-344 Phase 1b trial fail to meet certain defined endpoints or our Board determines not to proceed with a second cohort, both as further described in the Cooperation Agreement. The potential second return of capital may take the form of a dividend or tender offer and is subject to Board approval as well as modification associated with applicable requirements under Delaware law, both as detailed in the Cooperation Agreement. The $1.75 dividend is anticipated to be distributed during the second quarter of fiscal year 2024. As part of the Cooperation Agreement, Anson and Cable Car withdrew their consent solicitation and agreed to abide by customary standstill provisions. Additionally, we will reimburse Anson and Cable Car’s fees and expenses related to their engagement with us to date, in an amount not to exceed $1.2 million. As of September 30, 2023, no amounts were expensed or accrued in connection with the Cooperation Agreement. Cash Dividend On November 6, 2023, we announced that pursuant to the Cooperation Agreement, the Board declared a special cash dividend of $1.75 per share of common stock, payable on December 6, 2023, to stockholders of record at the close of business on November 17, 2023. Liquidity We have accumulated losses of $349.6 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of September 30, 2023, we had $82.2 million in cash and cash equivalents and short-term investments. We believe that these resources will be sufficient to meet our obligations and fund our liquidity and capital expenditure requirements for at least the next 12 months from the issuance of these condensed consolidated financial statements. Our current business operations are focused on continuing the clinical development of our drug candidates. Changes to our research and development plans or other changes affecting our operating expenses may affect actual future use of existing cash resources. Our research and development expenses are expected to increase in the foreseeable future. We cannot determine with certainty costs associated with ongoing and future clinical trials or the regulatory approval process. The duration, costs and timing associated with the development of our product candidates will depend on a variety of factors, including uncertainties associated with the results of our clinical trials. To date, we have obtained cash and funded our operations primarily through equity financings and license agreements. In order to continue the development of our drug candidates, at some point in the future we expect to pursue one or more capital transactions, whether through the sale of equity securities, debt financing, license agreements or entry into strategic partnerships. There can be no assurance that we will be able to continue to raise additional capital in the future. Basis of Presentation and Principles of Consolidation The condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the U.S. (“U.S. GAAP”) for interim financial information and in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, the accompanying financial statements do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, the accompanying condensed consolidated financial statements reflect all adjustments (consisting of normal recurring adjustments) that are necessary for a fair statement of the financial position, results of operations and cash flows for the periods presented. The accompanying unaudited condensed consolidated financial statements include the accounts of MEI Pharma, Inc. and our wholly owned subsidiary, Meadow Merger Sub, Inc. We have eliminated all significant intercompany accounts and transactions in consolidation. The accompanying unaudited condensed consolidated financial statements for the quarterly period ended September 30, 2023 should be read in conjunction with the audited financial statements and notes thereto as of and for the fiscal year ended June 30, 2023, included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on September 26, 2023 ("2023 Annual Report"). Interim results are not necessarily indicative of results for a full year. |
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the organization, consolidation and basis of presentation of financial statements disclosure, and significant accounting policies of the reporting entity. May be provided in more than one note to the financial statements, as long as users are provided with an understanding of (1) the significant judgments and assumptions made by an enterprise in determining whether it must consolidate a VIE and/or disclose information about its involvement with a VIE, (2) the nature of restrictions on a consolidated VIE's assets reported by an enterprise in its statement of financial position, including the carrying amounts of such assets, (3) the nature of, and changes in, the risks associated with an enterprise's involvement with the VIE, and (4) how an enterprise's involvement with the VIE affects the enterprise's financial position, financial performance, and cash flows. Describes procedure if disclosures are provided in more than one note to the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureAndSignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
2. Summary of Significant Accounting Policies There have been no material changes to our significant accounting policies from those described in the notes to our audited consolidated financial statements contained in the 2023 Annual Report. Risks and Uncertainties Use of Estimates The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of share-based awards, the discount rate used in estimating the present value of the right-of-use assets and lease liabilities, the useful lives of property and equipment, the recoverability of long-lived assets, clinical trial accruals, periods over which revenue should be recognized, relative stand-alone selling price, deferred income taxes and related valuation allowances, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these condensed consolidated financial statements. We base our estimates on historical experience and other market-specific or other relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each reporting period and updated to reflect current information. As future events and their effects cannot be determined with precision, actual results may materially differ from those estimates or assumptions. Concentrations of Credit Risk Financial instruments that potentially subject us to significant concentrations of credit risk consist primarily of cash, cash equivalents and short-term investments. Deposits in our checking and money market accounts are maintained in federally insured financial institutions and are subject to federally insured limits or limits set by the Securities Investor Protection Corporation. We attempt to minimize credit risk associated with our cash, cash equivalents and short-term investments by periodically evaluating the credit quality of our primary financial institutions. Our investment portfolio is maintained in accordance with our investment policy, which is designed to preserve capital, safeguard funds and limit exposure to risk. While we maintain cash deposits in Federal Deposit Insurance Corporation insured financial institutions in excess of federally insured limits, we do not believe that we are exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. We have not experienced any losses on such accounts. Short-term Investments Short-term investments are marketable securities with maturities greater than three months but less than one year from date of purchase. As of September 30, 2023 and June 30, 2023, our short-term investments consisted of $78.8 million and $83.8 million, respectively, in United States government securities. The short-term investments held as of September 30, 2023 and June 30, 2023 are considered to be “held to maturity” and are carried at amortized cost. As of September 30, 2023 and June 30, 2023, the gross unrealized gains and losses were immaterial. Revenue Recognition Revenues from Customers In accordance with ASC Topic 606, Revenue from Contracts with Customers (“Topic 606”), we recognize revenue when control of the promised goods or services is transferred to our customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. For enforceable contracts with our customers, we first identify the distinct performance obligations – or accounting units – within the contract. Performance obligations are commitments in a contract to transfer a distinct good or service to the customer. Payments received under commercial arrangements, such as licensing technology rights, may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, and royalties on the sale of products. At the inception of arrangements that include milestone payments, we use judgment to evaluate whether the milestones are probable of being achieved, and we estimate the amount, if any, to include in the transaction price using the most likely method. If it is probable that a significant revenue reversal will not occur, the estimated amount is included in the transaction price. Milestone payments that are not within our or the licensee’s control, such as regulatory approvals, are not included in the transaction price until those approvals are received. At the end of each reporting period, we re-evaluate the probability of achievement of development milestones and any related constraint and, as necessary, we adjust our estimate of the overall transaction price. We may enter into arrangements that consist of multiple performance obligations. Such arrangements may include any combination of our deliverables. To the extent a contract includes multiple promised deliverables, we apply judgment to determine whether promised deliverables are capable of being distinct and are distinct within the context of the contract. If these criteria are not met, the promised deliverables are accounted for as a combined performance obligation. For arrangements with multiple distinct performance obligations, we allocate variable consideration related to our 50-50 cost share for development services directly to the associated performance obligation and then allocate the remaining consideration among the performance obligations based on their relative stand-alone selling price. Stand-alone selling price is the price at which we would sell a promised good or service separately to the customer. When not directly observable, we typically estimate the stand-alone selling price for each distinct performance obligation. Variable consideration that relates specifically to our efforts to satisfy specific performance obligations is allocated entirely to those performance obligations. Other components of the transaction price are allocated based on the relative stand-alone selling price, over which management has applied significant judgment. We develop assumptions that require judgment to determine the stand-alone selling price for license-related performance obligations, which may include forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical, regulatory and commercial success. We estimate stand-alone selling price for research and development performance obligations by forecasting the expected costs of satisfying a performance obligation plus an appropriate margin. In the case of a license that is a distinct performance obligation, we recognize revenue allocated to the license from non-refundable, up-front fees at the point in time when the license is transferred to the licensee and the licensee can use and benefit from the license. For licenses that are bundled with other distinct or combined obligations, we use judgment to assess the nature of the performance obligation to determine whether the performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. If the performance obligation is satisfied over time, we evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition. The selection of the method to measure progress towards completion requires judgment and is based on the nature of the products or services to be provided. Revenue is recorded proportionally as costs are incurred. We generally use the cost-to-cost measure of progress because it best depicts the transfer of control to the customer which occurs as we incur costs. Under the cost-to-cost measure of progress, the extent of progress towards completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation (an “input method” under Topic 606). We use judgment to estimate the total cost expected to complete the research and development performance obligations, which include subcontractors’ costs, labor, materials, other direct costs and an allocation of indirect costs. We evaluate these cost estimates and the progress each reporting period and, as necessary, we adjust the measure of progress and related revenue recognition. For arrangements that include sales-based or usage-based royalties, we recognize revenue at the later of (i) when the related sales occur or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied or partially satisfied. To date, we have not recognized any sales-based or usage-based royalty revenue from license agreements. In connection with our April 2020 License, Development and Commercialization Agreement (the "KKC Commercialization Agreement") described in Note 7. License Agreements, we perform development services related to our 50-50 cost sharing arrangement for which revenue is recognized over time. Additionally, we perform services for KKC at their request, the costs of which are fully reimbursed to us. We record the reimbursement for such pass through services as revenue at 100% of reimbursed costs, as control of the additional services for KKC is transferred at the time we incur such costs. The costs of these services are recognized in the consolidated statements of operations as research and development expense. From time to time, we perform additional services for Kyowa Kirin Co., Ltd. ("KKC") at their request, the costs of which are fully reimbursed to us. The cost of these services is recognized in the consolidated statements of operations as research and development expense. We recognized revenue associated with the KKC Commercialization Agreement for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Timing of Revenue Recognition: |
|
|
|
|
|
|
Services performed over time |
|
$ |
743 |
|
|
$ |
8,359 |
|
Pass through services at a point in time |
|
|
9 |
|
|
|
371 |
|
|
|
$ |
752 |
|
|
$ |
8,730 |
|
Contract Balances Accounts receivables are included in prepaid expenses and other current assets, and contract liabilities are included in deferred revenue and deferred revenue, long-term in our condensed consolidated balance sheets. Our contract liabilities accounted for under Topic 606 relate to the amount of initial upfront consideration allocated to the development services performance obligations. Contract liabilities are recognized over the duration of the performance obligations based on the costs incurred relative to total expected costs. As of September 30, 2023 and June 30, 2023, we had no balances in accounts receivable. The following table presents changes in unbilled receivables and contract liabilities accounted for under Topic 606 for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Unbilled receivables |
|
$ |
— |
|
|
$ |
85 |
|
Contract liabilities |
|
|
|
|
|
|
Contract liabilities, beginning of period |
|
$ |
317 |
|
|
$ |
30,900 |
|
Revenue recognized |
|
|
(317 |
) |
|
|
(5,411 |
) |
Revenue recognized from change in estimate for performance obligations that are being closed |
|
|
— |
|
|
|
(16,565 |
) |
Revenue recognized for performance obligations that will no longer commence |
|
|
— |
|
|
|
(8,607 |
) |
Contract liabilities, end of period |
|
$ |
— |
|
|
$ |
317 |
|
The timing of revenue recognition, invoicing and cash collections results in billed accounts receivable and unbilled receivables (contract assets) and deferred revenue (contract liabilities). We invoice our customers in accordance with agreed-upon contractual terms, typically at periodic intervals or upon achievement of contractual milestones. Invoicing may occur subsequent to revenue recognition, resulting in unbilled receivables. We may receive advance payments from our customers before revenue is recognized, resulting in contract liabilities. The unbilled receivables and deferred revenue reported on the consolidated balance sheets relate to the KKC Commercialization Agreement. Revenues from Collaborators At contract inception, we assess whether the collaboration arrangements are within the scope of Topic 808, to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed based on the responsibilities of all parties in the arrangement. For collaboration arrangements within the scope of Topic 808 that contain multiple units of account, we first determine which units of account within the arrangement are within the scope of Topic 808 and which elements are within the scope of Topic 606. For units of account within collaboration arrangements that are accounted for pursuant to Topic 808, an appropriate recognition method is determined and applied consistently, by analogy to authoritative accounting literature. For elements of collaboration arrangements that are accounted for pursuant to Topic 606, we recognize revenue as discussed above. Consideration received that does not meet the requirements to satisfy Topic 606 revenue recognition criteria is recorded as deferred revenue in the accompanying consolidated balance sheets, classified as either current or long-term deferred revenue based on our best estimate of when such amounts will be recognized. Net Income (Loss) Per Share Basic and diluted net income (loss) per share is computed using the weighted-average number of shares of common stock outstanding during the period, less any shares subject to repurchase or forfeiture. There were no shares of common stock subject to repurchase or forfeiture for the three months ended September 30, 2023 and 2022. Diluted net income (loss) per share is computed based on the sum of the weighted-average number of common shares and potentially dilutive common shares outstanding during the period determined using the treasury-stock and if-converted methods. For purposes of the diluted net income per share calculation for the three months ended September 30, 2023, potentially dilutive securities are excluded from the calculation of diluted net income per share because their weighted-average exercise prices were above our weighted-average share price as of September 30, 2023; therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2023. For purposes of the diluted net loss per share calculation for the three months ended September 30, 2022, potentially dilutive securities are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive and, therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2022. The following table presents potentially dilutive shares excluded from the calculation of diluted net income (loss) per share (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Stock options |
|
|
1,447 |
|
|
|
1,388 |
|
Warrants |
|
|
103 |
|
|
|
803 |
|
Restricted stock units |
|
|
— |
|
|
|
1 |
|
Total anti-dilutive shares |
|
|
1,550 |
|
|
|
2,192 |
|
Recent Accounting Pronouncement Recently Adopted In June 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update 2016-13, Financial Instruments–Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments ("ASU 2016-13"), as amended. The amendments in ASU 2016-13 require, among other things, financial assets measured at amortized cost basis to be presented at the net amount expected to be collected as compared to previous U.S. GAAP which delayed recognition until it was probable a loss had been incurred. The amendments in ASU 2016-13 are effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years, with early adoption permitted. The adoption of ASU 2016-13 did not have a material impact on our financial statements and related disclosures. Recently Issued From time to time, new accounting pronouncements are issued by the FASB or other standards setting bodies that are adopted as of the specified effective date. The Company believes the impact of recently issued standards and any issued but not yet effective standards will not have a material impact on its condensed consolidated financial statements upon adoption.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Balance Sheet Details
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Balance Sheet Related Disclosures [Abstract] |
|
| Balance Sheet Details |
3. Balance Sheet Details Property and Equipment Property and equipment consisted of the following, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Furniture and equipment |
|
$ |
1,381 |
|
|
$ |
1,374 |
|
Leasehold improvements |
|
|
969 |
|
|
|
969 |
|
|
|
|
2,350 |
|
|
|
2,343 |
|
Less: accumulated depreciation |
|
|
(1,121 |
) |
|
|
(1,034 |
) |
Property and equipment, net |
|
$ |
1,229 |
|
|
$ |
1,309 |
|
Depreciation expense of property and equipment for the three months ended September 30, 2023 and 2022 are presented in the condensed consolidated statements of cash flows. Accrued Liabilities Accrued liabilities consisted of the following, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Accrued pre-clinical and clinical trial expenses |
|
$ |
730 |
|
|
$ |
3,663 |
|
Accrued compensation and benefits(1) |
|
|
1,515 |
|
|
|
7,189 |
|
Accrued legal and professional services |
|
|
1,044 |
|
|
|
1,423 |
|
Accrued reimbursement to KKC |
|
|
892 |
|
|
|
— |
|
Other |
|
|
108 |
|
|
|
186 |
|
Total accrued liabilities |
|
$ |
4,289 |
|
|
$ |
12,461 |
|
(1) Includes $0.1 million and $1.0 million of one-time termination employee benefits as of September 30, 2023 and June 30, 2023, respectively, as more fully described in Note 5. One-time Termination Benefits. |
| X |
- DefinitionThe entire disclosure for supplemental balance sheet disclosures, including descriptions and amounts for assets, liabilities, and equity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//210/tableOfContent
| Name: |
us-gaap_SupplementalBalanceSheetDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Fair Value Measurements
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value Measurements |
4. Fair Value Measurements The carrying amounts of financial instruments such as cash equivalents, short-term investments and accounts payable approximate the related fair values due to the short-term maturities of these instruments. We invest our excess cash in financial instruments which are readily convertible into cash, such as money market funds and U.S. government securities. Cash equivalents and short-term investments are classified as Level 1 as defined by the fair value hierarchy. As of September 30, 2023 and June 30, 2023, we had no assets or liabilities measured on a recurring or non-recurring basis. In May 2018, we issued warrants in connection with a private placement of our shares of common stock. Pursuant to the terms of the warrants, we could have been required to settle the warrants in cash in the event of an acquisition of us and, as a result, the warrants were required to be measured at fair value and reported as a liability in the condensed consolidated balance sheets. We recorded the fair value of the warrants upon issuance using the Black-Scholes valuation model and were required to revalue the warrants at each reporting date with any changes in fair value recorded on our condensed consolidated statement of operations through their expiration in May 2023. The valuation of the warrants is considered under Level 3 of the fair value hierarchy due to the need to use assumptions in the valuation that are both significant to the fair value measurement and unobservable. Inputs used to determine estimated fair value of the warrant liabilities include the estimated fair value of the underlying stock at the valuation date, the estimated term of the warrants, risk-free interest rates, expected dividends and the expected volatility of the underlying stock. The significant unobservable inputs used in the fair value measurement of the warrant liabilities were the volatility rate and the estimated term of the warrants. Generally, increases (decreases) in the fair value of the underlying stock and estimated term would result in a directionally similar impact to the fair value measurement. The change in the fair value of the Level 3 warrant liability is reflected in the condensed consolidated statements of operations for the three months ended September 30, 2022. During the three months ended September 30, 2023 and the year ended June 30,2023, there were no transfers into or out of Level 3 of the fair value hierarchy. To calculate the fair value of the warrant liability as of June 30, 2023, the following assumptions were used:
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
4.4 |
% |
Expected life (years) |
|
|
0.5 |
|
Expected volatility |
|
|
128.7 |
% |
Dividend yield |
|
|
— |
% |
Weighted-average grant date fair value |
|
$ |
0.02 |
|
The following table sets forth a summary of changes in the estimated fair value of our Level 3 warrant liability for the three months ended September 30, 2022 (in thousands):
|
|
|
|
|
|
|
|
|
Balance as of June 30, 2022 |
|
$ |
1,603 |
|
Change in estimated fair value of liability classified warrants |
|
|
(1,117 |
) |
Balance as of September 30, 2022 |
|
$ |
486 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
One-Time Termination Benefits
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Restructuring Reserve [Abstract] |
|
| One-Time Termination Benefits |
5. One-Time Termination Benefits In connection with our joint decision to discontinue development of zandelisib outside of Japan, in December 2022, we announced a realignment of our clinical development efforts that streamlined our organization towards the continued clinical development of our two earlier clinical-stage assets, voruciclib and ME-344. As a result, also in December 2022, our Board approved a staggered workforce reduction (the "Reduction in Force"), affecting 28 employees in December 2022 and an additional 26 employees through June 2023, representing an aggregate 51% Reduction in Force. For the three months ended September 30, 2023, we recorded additional one-time employee termination benefits of $28,000 within research and development expense as a result of an additional termination. The following table summarizes our activity related to one-time employee termination benefits included in accrued liabilities (in thousands):
|
|
|
|
|
|
|
One-Time Employee Termination Benefits |
|
Balance at June 30, 2023 |
|
$ |
993 |
|
Increase in accrued restructuring |
|
|
28 |
|
Cash payments |
|
|
(917 |
) |
Balance at September 30, 2023 |
|
$ |
104 |
|
|
| X |
- DefinitionThe entire disclosure for restructuring and related activities. Description of restructuring activities such as exit and disposal activities, include facts and circumstances leading to the plan, the expected plan completion date, the major types of costs associated with the plan activities, total expected costs, the accrual balance at the end of the period, and the periods over which the remaining accrual will be settled. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//420/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringAndRelatedActivitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RestructuringReserveAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments and Contingencies
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
6. Commitments and Contingencies We have contracted with various consultants and third parties to assist us in pre-clinical research and development and clinical trials work for our leading drug compounds. The contracts are terminable at any time, but obligate us to reimburse the providers for any time or costs incurred through the date of termination. We also have employment agreements with certain of our current employees that provide for severance payments and accelerated vesting for share-based awards if their employment is terminated under specified circumstances. Litigation From time to time, the Company may be involved in various lawsuits, legal proceedings, or claims that arise in the ordinary course of business. Management believes there are no claims or actions pending against the Company as of September 30, 2023 which will have, individually or in the aggregate, a material adverse effect on its business, liquidity, financial position, or results of operations. Litigation, however, is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. Indemnification In accordance with our amended and restated memorandum and articles of association, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving in such capacity. There have been no claims to date, and the Company has a directors and officers liability insurance policy that may enable it to recover a portion of any amounts paid for future claims. Presage License Agreement As discussed in Note 8. Other License Agreements, we are party to a license agreement with Presage under which we may be required to make future payments upon the achievement of certain development, regulatory and commercial milestones, as well as potential future royalties based upon net sales. As of September 30, 2023, we had not accrued any amounts for potential future payments as achievement of the milestones had not been met.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
License Agreements
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Related Party Transactions [Abstract] |
|
| License Agreements |
7. License Agreements Kyowa Kirin Co., Ltd. License, Development and Commercialization Agreement In April 2020, we entered into the KKC Commercialization Agreement under which we granted to KKC a co-exclusive, sublicensable, payment-bearing license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in the U.S. (the "U.S. License"), and an exclusive (subject to certain retained rights to perform obligations under the KKC Commercialization Agreement), sublicensable, payment-bearing, license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in countries outside of the U.S.. KKC granted to us a co-exclusive, sublicensable, license under certain patents and know-how controlled by KKC to develop and commercialize zandelisib for all human indications in the U.S., and a co-exclusive, sublicensable, royalty-free, fully paid license under certain patents and know-how controlled by KKC to perform our obligations in the Ex-U.S. under the KKC Commercialization Agreement. KKC paid us an initial nonrefundable payment of $100.0 million. In November 2022, we and KKC jointly decided to discontinue zandelisib development in the U.S. and in May 2023, KKC decided to discontinue development of zandelisib in Japan. Considering the decisions to discontinue worldwide development of zandelisib the parties entered into a Termination Agreement on July 14, 2023, agreeing to mutually terminate the global License, Development and Commercialization Agreement executed in April 2020. Pursuant to the Termination Agreement, we regained full, global rights to develop, manufacture and commercialize zandelisib, subject to KKCs limited rights to use for “compassionate use” (as more specifically defined in the Termination Agreement) in certain expanded access programs for the existing patients who have been enrolled in Japanese clinical trials sponsored by KKC until November 30, 2027, and for which KKC is fully liable; each party released the other party from any and all claims or demands arising from the original KKC Commercialization Agreement excluding certain surviving claims; however, we are obligated to deliver a discrete quantity of materials to facilitate KKCs compassionate use activities. We determined the KKC Commercialization Agreement was a collaborative arrangement in accordance with Topic 808 which contained multiple units of account, as we and KKC were both active participants in the development and commercialization activities and were exposed to significant risks and rewards dependent on commercial success of the activities of the arrangement. We determined the U.S. License was a separate unit of account under the scope of Topic 808 and was not a deliverable under Topic 606, while the license issued to KKC within its territory and related development services were within the scope of Topic 606. See discussion within the Revenue Recognition subsection of Note 2. Summary of Significant Accounting Policies. We evaluated the Termination Agreement under ASC 606 and determined it met the requirements of a contract modification which changed the scope of the KKC Commercialization Agreement, and the remaining goods and services associated with the wind-down activities to be transferred. The cost of satisfying our performance obligation to provide compassionate use supply to KKC was determined to be de minimis and therefore immaterial within the context of the KKC Commercialization Agreement. As of September 30, 2023, activities associated with the compassionate use supply were completed. With the execution of the Termination Agreement, we regained full, global rights (subject to KKC's limited rights for compassionate use) and KKC has no further rights to develop, use or commercialize zandelisib in the U.S., nor do we have any remaining performance obligations with all consideration received from KKC being nonrefundable. Therefore, the remaining long-term deferred revenue as of June 30, 2023, of $64.5 million that was allocated to the U.S. License obligation accounted for under Topic 808 at inception of the KKC Commercialization Agreement was recognized as revenue from collaboration agreements in the three months ended September 30, 2023, utilizing contract termination analogous to guidance provided in Topic 606. We recognized the remaining transaction price of $317,000 of deferred revenue during the three months ended September 30, 2023, as any remaining performance obligations under the KKC Commercialization Agreement were determined to be de minimis as of September 30, 2023. Therefore, as of September 30, 2023, all deferred revenue associated with the KKC Commercialization Agreement has been recognized.
|
| X |
- DefinitionThe entire disclosure of revenue from contract with customer to transfer good or service and to transfer nonfinancial asset. Includes, but is not limited to, disaggregation of revenue, credit loss recognized from contract with customer, judgment and change in judgment related to contract with customer, and asset recognized from cost incurred to obtain or fulfill contract with customer. Excludes insurance and lease contracts. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-9
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-15
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-12
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-12
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-13
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org//606/tableOfContent
| Name: |
us-gaap_RevenueFromContractWithCustomerTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Other License Agreements
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Licensing Arrangements [Abstract] |
|
| Other License Agreements |
8. Other License Agreements Presage License Agreement In September 2017, we, as licensee, entered into a license agreement with Presage Biosciences, Inc. (“Presage”). Under the terms of the license agreement, Presage granted to us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. In exchange, we paid $2.9 million to Presage. With respect to the first indication, an incremental $2.0 million payment, due upon dosing of the first subject in the first registration trial, will be owed to Presage, for total payments of $4.9 million prior to receipt of marketing approval of the first indication in the U.S., EU or Japan. Additional potential payments of up to $179 million will be due upon the achievement of certain development, regulatory and commercial milestones. We will also pay mid-single digit tiered royalties on the net sales of any product successfully developed. As an alternative to milestone and royalty payments related to countries in which we sublicense product rights, we will pay to Presage a tiered percentage (which decreases as product development progresses) of amounts received from such sublicensees. During the three months ended September 30, 2023 and 2022, we made no payments under the Presage license agreement. BeiGene Collaboration In October 2018, we entered into a clinical collaboration with BeiGene, Ltd. (“BeiGene”) to evaluate the safety and efficacy of zandelisib in combination with BeiGene’s zanubrutinib (marketed as Brukinsa®), an inhibitor of Bruton’s tyrosine kinase, for the treatment of patients with B-cell malignancies. Under the terms of the clinical collaboration agreement, we amended our ongoing Phase 1b trial to include evaluation of zandelisib in combination with zanubrutinib in patients with B-cell malignancies. Study costs are being shared equally by the parties, and we agreed to supply zandelisib and BeiGene agreed to supply zanubrutinib. We record the costs reimbursed by BeiGene as a reduction of our research and development expenses. We retained full commercial rights for zandelisib and BeiGene retained full commercial rights for zanubrutinib. With the discontinuation of the zandelisib program outside of Japan, this clinical collaboration was terminated on September 28, 2023.
|
| X |
- DefinitionLicensing arrangements [Abstract].
| Name: |
meip_LicensingArrangementsAbstract |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionOther licensing agreements.
| Name: |
meip_OtherLicensingArrangementsTextBlock |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Leases
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Disclosure Text Block [Abstract] |
|
| Leases |
9. Leases In July 2020, we entered into a lease agreement (the "Initial Lease Agreement") for approximately 32,800 square feet of office space in San Diego, California. The Lease Agreement was scheduled to expire in March 2028 but was extended by 20 months to November 2029 in accordance with the amended lease agreement we entered into in January 2022 (the "Amended Lease Agreement"). The Initial and Amended Lease Agreements are collectively referred to as the "Lease Agreements". The Lease Agreements contain rent escalations over the lease term. In addition, the Lease Agreements contain an option to renew and extend the lease term, which is not included in the determination of the right-of-use ("ROU") asset and operating lease liability, as it was not reasonably certain to be exercised. Upon commencement of the Amended Lease Agreement, to extend the lease term, we recognized an additional operating lease ROU asset and a corresponding operating lease liability. The Lease Agreements include variable non-lease components (e.g., common area maintenance, maintenance, etc.) that are not included in the ROU asset and operating lease liability and are reflected as an expense in the period incurred as a component of the lease cost. The Amended Lease Agreements also provides for an additional 12,300 square feet of office space adjacent to our current office in San Diego. Upon taking control of the additional office space on July 1, 2022, we recognized operating lease ROU assets obtained in exchange for operating lease liabilities of $4.3 million. The total operating lease costs for the Lease Agreements were as follows for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
2023 |
|
|
2022 |
|
|
Operating lease cost |
|
$ |
608 |
|
|
$ |
608 |
|
|
Variable lease costs |
|
|
12 |
|
|
|
18 |
|
|
Total lease costs included in general and administrative expenses |
|
$ |
620 |
|
|
$ |
626 |
|
|
Supplemental cash flow information related to our operating leases was as follows for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
|
|
2023 |
|
|
2022 |
|
|
Cash paid for amount included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
Operating cash flows from operating leases |
|
$ |
584 |
|
|
$ |
567 |
|
|
|
|
|
|
|
|
|
|
The following is a schedule of the future minimum lease payments under the Lease Agreements, reconciled to the operating lease liability, as of September 30, 2023 (in thousands):
|
|
|
|
|
|
|
September 30, 2023 |
|
Remainder of fiscal year ending June 30, 2024 |
|
$ |
1,751 |
|
Years ending June 30, |
|
|
|
2025 |
|
|
1,913 |
|
2026 |
|
|
2,477 |
|
2027 |
|
|
2,551 |
|
2028 |
|
|
2,715 |
|
Thereafter |
|
|
4,385 |
|
Total lease payments |
|
|
15,792 |
|
Less: Present value discount |
|
|
(3,411 |
) |
Total operating lease liability |
|
$ |
12,381 |
|
|
|
|
|
Balance Sheet Classification - Operating Leases |
|
|
|
Operating lease liability |
|
$ |
1,055 |
|
Operating lease liability, long-term |
|
|
11,326 |
|
Total operating lease liability |
|
$ |
12,381 |
|
|
|
|
|
Other Balance Sheet Information - Operating Leases |
|
|
|
Weighted-average remaining lease term (in years) |
|
|
6.2 |
|
Weighted-average discount rate |
|
|
7.50 |
% |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Stockholders' Equity
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Federal Home Loan Banks [Abstract] |
|
| Stockholders' Equity |
10. Stockholders’ Equity Equity Transactions Warrants In May 2023, outstanding warrants to purchase 802,949 shares of our common stock expired. The warrants were fully vested, exercisable at a price of $50.80 per share. Prior to their expiration, the warrants had been previously revalued to zero, as of December 31, 2022. All corresponding changes in fair value were recorded as a component of other income (expense) in our condensed consolidated statements of operations. No warrants were exercised during the three months ended September 30, 2022. As of September 30, 2023, we also have outstanding warrants to purchase 102,513 shares of our common stock issued to Torreya Partners. The warrants are fully vested, exercisable at a price of $6.80 per share and expire in October 2027. No warrants were exercised during the three months ended September 30, 2023. Description of Capital Stock Our total authorized share capital is 226,100,000 shares consisting of 226,000,000 shares of common stock, $0.00000002 par value per share, and 100,000 shares of preferred stock, $0.01 par value per share. Common Stock The holders of common stock are entitled to one vote per share. In the event of a liquidation, dissolution or winding up of our affairs, holders of the common stock will be entitled to share ratably in all our assets that are remaining after payment of our liabilities and the liquidation preference of any outstanding shares of preferred stock. All outstanding shares of common stock are fully paid and non-assessable. The rights, preferences and privileges of holders of common stock are subject to any series of preferred stock that we have issued or that we may issue in the future. The holders of common stock have no preemptive rights and are not subject to future calls or assessments by us. Preferred Stock Our Board has the authority to issue up to 100,000 shares of preferred stock with a par value of $0.01 per share in one or more series and to fix the rights, preferences, privileges and restrictions in respect of that preferred stock, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption (including sinking fund provisions), redemption prices and liquidation preferences, and the number of shares constituting such series and the designation of any such series, without future vote or action by the stockholders. Therefore, the Board, without the approval of the stockholders, could authorize the issuance of preferred stock with voting, conversion and other rights that could affect the voting power, dividend and other rights of the holders of shares or that could have the effect of delaying, deferring or preventing a change of control. There were no shares of preferred stock outstanding as of September 30, 2023 and June 30, 2023.
|
| X |
- References
+ Details
| Name: |
us-gaap_FederalHomeLoanBanksAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Share-based Compensation
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Share-based Compensation |
11. Share-based Compensation We use equity-based compensation programs to provide long-term performance incentives for our employees. These incentives consist primarily of stock options and RSUs. In December 2008, we adopted the MEI Pharma, Inc. 2008 Stock Omnibus Equity Compensation Plan (“Omnibus Plan”), as amended and restated from time-to-time, under which 1,850,739 shares of common stock are currently authorized for issuance. The Omnibus Plan provides for the grant of options and/or other stock-based or stock-denominated awards to our non-employee directors, officers, and employees. As of September 30, 2023, there were 398,718 shares available for future grant under the Omnibus Plan. In May 2021, we adopted the 2021 Inducement Plan ("Inducement Plan"), under which 125,000 shares of common stock are authorized for issuance. The Inducement Plan is intended to assist us in attracting and retaining selected individuals to serve as employees who are expected to contribute to our success, by providing an inducement for such individuals to enter into employment with us, and to achieve long-term objectives that will benefit our stockholders. As of September 30, 2023, there were 107,738 shares available for future grant under the Inducement Plan. Total share-based compensation expense for all stock awards consisted of the following for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Research and development |
|
$ |
(69 |
) |
|
$ |
649 |
|
General and administrative |
|
|
432 |
|
|
|
910 |
|
Total share-based compensation |
|
$ |
363 |
|
|
$ |
1,559 |
|
Stock Options Stock options granted to employees vest 25% one year from the date of grant and ratably each month thereafter for a period of 36 months and expire ten years from the date of grant. Stock options granted to directors vest ratably each month for a period of 12 months from the date of grant and expire ten years from the date of grant. Of the total options outstanding of 1,447,127 as of September 30, 2023, 1,337,865 were granted under the Omnibus Plan and 109,262 were granted under the Inducement Plan. A summary of our stock option activity and related data follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
Options |
|
|
Weighted-Average
Exercise Price |
|
|
Weighted-Average
Remaining Contractual
Term (in years) |
|
|
Aggregate
Intrinsic Value |
|
Outstanding at June 30, 2023 |
|
|
1,284,907 |
|
|
$ |
38.32 |
|
|
|
|
|
|
|
Granted |
|
|
227,037 |
|
|
$ |
7.01 |
|
|
|
|
|
|
|
Forfeited |
|
|
(64,817 |
) |
|
$ |
36.35 |
|
|
|
|
|
|
|
Outstanding at September 30, 2023 |
|
|
1,447,127 |
|
|
$ |
33.50 |
|
|
|
7.6 |
|
|
$ |
789 |
|
Vested and exercisable at September 30, 2023 |
|
|
819,813 |
|
|
$ |
49.16 |
|
|
|
6.3 |
|
|
$ |
— |
|
As of September 30, 2023, the aggregate intrinsic value of outstanding options was calculated as the difference between the exercise price of the underlying options and the closing price of our common stock of $7.01 on that date. Unrecognized compensation expense related to non-vested stock options totaled $3.1 million as of September 30, 2023. Such compensation expense is expected to be recognized over a weighted-average period of 1.6 years. As of September 30, 2023, we expect all options to vest. We use the Black-Scholes valuation model to estimate the grant date fair value of stock options. To calculate these fair values, the following weighted-average assumptions were used for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Risk-free interest rate |
|
|
4.6 |
% |
|
|
2.8 |
% |
Expected life (years) |
|
|
5.7 |
|
|
|
6.0 |
|
Expected volatility |
|
|
89.8 |
% |
|
|
84.1 |
% |
Dividend yield |
|
|
— |
% |
|
|
— |
% |
Weighted-average grant date fair value |
|
$ |
5.27 |
|
|
$ |
7.80 |
|
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Subsequent Events
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
12. Subsequent Events On October 1, 2023, our Board approved and adopted a Rights Agreement, ("Rights Agreement") by and between us and Computershare, Inc., as Rights Agent (as defined in the Rights Agreement). Pursuant to the Rights Agreement, the Board declared a dividend of one preferred share purchase right (each a "Right") for each outstanding share of our common stock, par value $0.00000002 (each a "Common Share" and collectively, the "Common Shares"). The Rights are distributable to stockholders of record as of the close of business on October 12, 2023. One Right also will be issued together with each Common Share issued by the Company after October 12, 2023, but before the Distribution Date, as defined in the Rights Agreement, (or the earlier of the redemption or expiration of the Rights) and, in certain circumstances, after the Distribution Date. As more fully described in Note 1. Description of Business and Basis of Presentation, we announced a Cooperation Agreement (“Cooperation Agreement”) with Anson Funds and Cable Car Capital effective as of October 31, 2023. On November 6, 2023, pursuant to the Cooperation Agreement, the Board declared a special cash dividend of $1.75 per share of common stock, payable on December 6, 2023, to stockholders of record at the close of business on November 17, 2023.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Description of Business and Basis of Presentation (Policies)
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Description of Business |
Description of Business MEI Pharma, Inc. (Nasdaq: MEIP) is a clinical-stage pharmaceutical company committed to developing novel and differentiated cancer therapies. We build our pipeline by acquiring promising cancer agents and creating value in programs through development, strategic partnerships, and out-licensing or commercialization, as appropriate. Our approach to oncology drug development is to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. Our pipeline includes voruciclib, an oral cyclin-dependent kinase 9 (“CDK9”) inhibitor, and ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway. Reverse Stock Split On April 14, 2023, we amended our Certificate of Incorporation to affect a combination of our issued and outstanding common stock at a ratio of one-for-twenty (“Reverse Stock Split”). The par value and authorized shares of our common stock were not adjusted as a result of the Reverse Stock Split. The Reverse Stock Split was effective on April 14, 2023, with a market effective date of April 17, 2023. All historical share and per share amounts have been adjusted to reflect the Reverse Stock Split for all periods presented. All stock options, restricted stock units and warrants outstanding were ratably adjusted to give effect to the Reverse Stock Split.
|
| Current Events |
Current Events Cooperation Agreement On October 31, 2023, we announced a Cooperation Agreement (“Cooperation Agreement”) with Anson Funds and Cable Car Capital of which, among other non-financial related items as described within the overview section of Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations, contained a capital return to stockholders in the form of a dividend in the amount of $1.75 per share of common stock to all stockholders. Additionally, a potential second return of capital of approximately $9.33 million in the aggregate will be authorized by the board of directors ("Board") should our ongoing ME-344 Phase 1b trial fail to meet certain defined endpoints or our Board determines not to proceed with a second cohort, both as further described in the Cooperation Agreement. The potential second return of capital may take the form of a dividend or tender offer and is subject to Board approval as well as modification associated with applicable requirements under Delaware law, both as detailed in the Cooperation Agreement. The $1.75 dividend is anticipated to be distributed during the second quarter of fiscal year 2024. As part of the Cooperation Agreement, Anson and Cable Car withdrew their consent solicitation and agreed to abide by customary standstill provisions. Additionally, we will reimburse Anson and Cable Car’s fees and expenses related to their engagement with us to date, in an amount not to exceed $1.2 million. As of September 30, 2023, no amounts were expensed or accrued in connection with the Cooperation Agreement. Cash Dividend On November 6, 2023, we announced that pursuant to the Cooperation Agreement, the Board declared a special cash dividend of $1.75 per share of common stock, payable on December 6, 2023, to stockholders of record at the close of business on November 17, 2023.
|
| Liquidity |
Liquidity We have accumulated losses of $349.6 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of September 30, 2023, we had $82.2 million in cash and cash equivalents and short-term investments. We believe that these resources will be sufficient to meet our obligations and fund our liquidity and capital expenditure requirements for at least the next 12 months from the issuance of these condensed consolidated financial statements. Our current business operations are focused on continuing the clinical development of our drug candidates. Changes to our research and development plans or other changes affecting our operating expenses may affect actual future use of existing cash resources. Our research and development expenses are expected to increase in the foreseeable future. We cannot determine with certainty costs associated with ongoing and future clinical trials or the regulatory approval process. The duration, costs and timing associated with the development of our product candidates will depend on a variety of factors, including uncertainties associated with the results of our clinical trials. To date, we have obtained cash and funded our operations primarily through equity financings and license agreements. In order to continue the development of our drug candidates, at some point in the future we expect to pursue one or more capital transactions, whether through the sale of equity securities, debt financing, license agreements or entry into strategic partnerships. There can be no assurance that we will be able to continue to raise additional capital in the future.
|
| Basis of Presentation and Principles of Consolidation |
Basis of Presentation and Principles of Consolidation The condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the U.S. (“U.S. GAAP”) for interim financial information and in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, the accompanying financial statements do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, the accompanying condensed consolidated financial statements reflect all adjustments (consisting of normal recurring adjustments) that are necessary for a fair statement of the financial position, results of operations and cash flows for the periods presented. The accompanying unaudited condensed consolidated financial statements include the accounts of MEI Pharma, Inc. and our wholly owned subsidiary, Meadow Merger Sub, Inc. We have eliminated all significant intercompany accounts and transactions in consolidation. The accompanying unaudited condensed consolidated financial statements for the quarterly period ended September 30, 2023 should be read in conjunction with the audited financial statements and notes thereto as of and for the fiscal year ended June 30, 2023, included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on September 26, 2023 ("2023 Annual Report"). Interim results are not necessarily indicative of results for a full year.
|
| X |
- DefinitionCurrent events and reduction in force.
| Name: |
meip_CurrentEventsAndReductionInForcePolicyTextBlock |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Disclosure In Its Entirety Pertaining to the Accounting Policy In Respect Of Liquid Resources Held By The Entity.
| Name: |
meip_LiquidityPolicyTextBlock |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and accounting policies concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Policies)
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Use of Estimates |
Use of Estimates The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of share-based awards, the discount rate used in estimating the present value of the right-of-use assets and lease liabilities, the useful lives of property and equipment, the recoverability of long-lived assets, clinical trial accruals, periods over which revenue should be recognized, relative stand-alone selling price, deferred income taxes and related valuation allowances, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these condensed consolidated financial statements. We base our estimates on historical experience and other market-specific or other relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each reporting period and updated to reflect current information. As future events and their effects cannot be determined with precision, actual results may materially differ from those estimates or assumptions.
|
| Concentrations of Credit Risk |
Concentrations of Credit Risk Financial instruments that potentially subject us to significant concentrations of credit risk consist primarily of cash, cash equivalents and short-term investments. Deposits in our checking and money market accounts are maintained in federally insured financial institutions and are subject to federally insured limits or limits set by the Securities Investor Protection Corporation. We attempt to minimize credit risk associated with our cash, cash equivalents and short-term investments by periodically evaluating the credit quality of our primary financial institutions. Our investment portfolio is maintained in accordance with our investment policy, which is designed to preserve capital, safeguard funds and limit exposure to risk. While we maintain cash deposits in Federal Deposit Insurance Corporation insured financial institutions in excess of federally insured limits, we do not believe that we are exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. We have not experienced any losses on such accounts.
|
| Short-term Investments |
Short-term Investments Short-term investments are marketable securities with maturities greater than three months but less than one year from date of purchase. As of September 30, 2023 and June 30, 2023, our short-term investments consisted of $78.8 million and $83.8 million, respectively, in United States government securities. The short-term investments held as of September 30, 2023 and June 30, 2023 are considered to be “held to maturity” and are carried at amortized cost. As of September 30, 2023 and June 30, 2023, the gross unrealized gains and losses were immaterial.
|
| Revenue Recognition |
Revenue Recognition Revenues from Customers In accordance with ASC Topic 606, Revenue from Contracts with Customers (“Topic 606”), we recognize revenue when control of the promised goods or services is transferred to our customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. For enforceable contracts with our customers, we first identify the distinct performance obligations – or accounting units – within the contract. Performance obligations are commitments in a contract to transfer a distinct good or service to the customer. Payments received under commercial arrangements, such as licensing technology rights, may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, and royalties on the sale of products. At the inception of arrangements that include milestone payments, we use judgment to evaluate whether the milestones are probable of being achieved, and we estimate the amount, if any, to include in the transaction price using the most likely method. If it is probable that a significant revenue reversal will not occur, the estimated amount is included in the transaction price. Milestone payments that are not within our or the licensee’s control, such as regulatory approvals, are not included in the transaction price until those approvals are received. At the end of each reporting period, we re-evaluate the probability of achievement of development milestones and any related constraint and, as necessary, we adjust our estimate of the overall transaction price. We may enter into arrangements that consist of multiple performance obligations. Such arrangements may include any combination of our deliverables. To the extent a contract includes multiple promised deliverables, we apply judgment to determine whether promised deliverables are capable of being distinct and are distinct within the context of the contract. If these criteria are not met, the promised deliverables are accounted for as a combined performance obligation. For arrangements with multiple distinct performance obligations, we allocate variable consideration related to our 50-50 cost share for development services directly to the associated performance obligation and then allocate the remaining consideration among the performance obligations based on their relative stand-alone selling price. Stand-alone selling price is the price at which we would sell a promised good or service separately to the customer. When not directly observable, we typically estimate the stand-alone selling price for each distinct performance obligation. Variable consideration that relates specifically to our efforts to satisfy specific performance obligations is allocated entirely to those performance obligations. Other components of the transaction price are allocated based on the relative stand-alone selling price, over which management has applied significant judgment. We develop assumptions that require judgment to determine the stand-alone selling price for license-related performance obligations, which may include forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical, regulatory and commercial success. We estimate stand-alone selling price for research and development performance obligations by forecasting the expected costs of satisfying a performance obligation plus an appropriate margin. In the case of a license that is a distinct performance obligation, we recognize revenue allocated to the license from non-refundable, up-front fees at the point in time when the license is transferred to the licensee and the licensee can use and benefit from the license. For licenses that are bundled with other distinct or combined obligations, we use judgment to assess the nature of the performance obligation to determine whether the performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. If the performance obligation is satisfied over time, we evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition. The selection of the method to measure progress towards completion requires judgment and is based on the nature of the products or services to be provided. Revenue is recorded proportionally as costs are incurred. We generally use the cost-to-cost measure of progress because it best depicts the transfer of control to the customer which occurs as we incur costs. Under the cost-to-cost measure of progress, the extent of progress towards completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation (an “input method” under Topic 606). We use judgment to estimate the total cost expected to complete the research and development performance obligations, which include subcontractors’ costs, labor, materials, other direct costs and an allocation of indirect costs. We evaluate these cost estimates and the progress each reporting period and, as necessary, we adjust the measure of progress and related revenue recognition. For arrangements that include sales-based or usage-based royalties, we recognize revenue at the later of (i) when the related sales occur or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied or partially satisfied. To date, we have not recognized any sales-based or usage-based royalty revenue from license agreements. In connection with our April 2020 License, Development and Commercialization Agreement (the "KKC Commercialization Agreement") described in Note 7. License Agreements, we perform development services related to our 50-50 cost sharing arrangement for which revenue is recognized over time. Additionally, we perform services for KKC at their request, the costs of which are fully reimbursed to us. We record the reimbursement for such pass through services as revenue at 100% of reimbursed costs, as control of the additional services for KKC is transferred at the time we incur such costs. The costs of these services are recognized in the consolidated statements of operations as research and development expense. From time to time, we perform additional services for Kyowa Kirin Co., Ltd. ("KKC") at their request, the costs of which are fully reimbursed to us. The cost of these services is recognized in the consolidated statements of operations as research and development expense. We recognized revenue associated with the KKC Commercialization Agreement for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Timing of Revenue Recognition: |
|
|
|
|
|
|
Services performed over time |
|
$ |
743 |
|
|
$ |
8,359 |
|
Pass through services at a point in time |
|
|
9 |
|
|
|
371 |
|
|
|
$ |
752 |
|
|
$ |
8,730 |
|
Contract Balances Accounts receivables are included in prepaid expenses and other current assets, and contract liabilities are included in deferred revenue and deferred revenue, long-term in our condensed consolidated balance sheets. Our contract liabilities accounted for under Topic 606 relate to the amount of initial upfront consideration allocated to the development services performance obligations. Contract liabilities are recognized over the duration of the performance obligations based on the costs incurred relative to total expected costs. As of September 30, 2023 and June 30, 2023, we had no balances in accounts receivable. The following table presents changes in unbilled receivables and contract liabilities accounted for under Topic 606 for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Unbilled receivables |
|
$ |
— |
|
|
$ |
85 |
|
Contract liabilities |
|
|
|
|
|
|
Contract liabilities, beginning of period |
|
$ |
317 |
|
|
$ |
30,900 |
|
Revenue recognized |
|
|
(317 |
) |
|
|
(5,411 |
) |
Revenue recognized from change in estimate for performance obligations that are being closed |
|
|
— |
|
|
|
(16,565 |
) |
Revenue recognized for performance obligations that will no longer commence |
|
|
— |
|
|
|
(8,607 |
) |
Contract liabilities, end of period |
|
$ |
— |
|
|
$ |
317 |
|
The timing of revenue recognition, invoicing and cash collections results in billed accounts receivable and unbilled receivables (contract assets) and deferred revenue (contract liabilities). We invoice our customers in accordance with agreed-upon contractual terms, typically at periodic intervals or upon achievement of contractual milestones. Invoicing may occur subsequent to revenue recognition, resulting in unbilled receivables. We may receive advance payments from our customers before revenue is recognized, resulting in contract liabilities. The unbilled receivables and deferred revenue reported on the consolidated balance sheets relate to the KKC Commercialization Agreement. Revenues from Collaborators At contract inception, we assess whether the collaboration arrangements are within the scope of Topic 808, to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed based on the responsibilities of all parties in the arrangement. For collaboration arrangements within the scope of Topic 808 that contain multiple units of account, we first determine which units of account within the arrangement are within the scope of Topic 808 and which elements are within the scope of Topic 606. For units of account within collaboration arrangements that are accounted for pursuant to Topic 808, an appropriate recognition method is determined and applied consistently, by analogy to authoritative accounting literature. For elements of collaboration arrangements that are accounted for pursuant to Topic 606, we recognize revenue as discussed above. Consideration received that does not meet the requirements to satisfy Topic 606 revenue recognition criteria is recorded as deferred revenue in the accompanying consolidated balance sheets, classified as either current or long-term deferred revenue based on our best estimate of when such amounts will be recognized.
|
| Net Income (Loss) Per Share |
Net Income (Loss) Per Share Basic and diluted net income (loss) per share is computed using the weighted-average number of shares of common stock outstanding during the period, less any shares subject to repurchase or forfeiture. There were no shares of common stock subject to repurchase or forfeiture for the three months ended September 30, 2023 and 2022. Diluted net income (loss) per share is computed based on the sum of the weighted-average number of common shares and potentially dilutive common shares outstanding during the period determined using the treasury-stock and if-converted methods. For purposes of the diluted net income per share calculation for the three months ended September 30, 2023, potentially dilutive securities are excluded from the calculation of diluted net income per share because their weighted-average exercise prices were above our weighted-average share price as of September 30, 2023; therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2023. For purposes of the diluted net loss per share calculation for the three months ended September 30, 2022, potentially dilutive securities are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive and, therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2022. The following table presents potentially dilutive shares excluded from the calculation of diluted net income (loss) per share (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Stock options |
|
|
1,447 |
|
|
|
1,388 |
|
Warrants |
|
|
103 |
|
|
|
803 |
|
Restricted stock units |
|
|
— |
|
|
|
1 |
|
Total anti-dilutive shares |
|
|
1,550 |
|
|
|
2,192 |
|
|
| Recent Accounting Pronouncement |
Recent Accounting Pronouncement Recently Adopted In June 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update 2016-13, Financial Instruments–Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments ("ASU 2016-13"), as amended. The amendments in ASU 2016-13 require, among other things, financial assets measured at amortized cost basis to be presented at the net amount expected to be collected as compared to previous U.S. GAAP which delayed recognition until it was probable a loss had been incurred. The amendments in ASU 2016-13 are effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years, with early adoption permitted. The adoption of ASU 2016-13 did not have a material impact on our financial statements and related disclosures. Recently Issued From time to time, new accounting pronouncements are issued by the FASB or other standards setting bodies that are adopted as of the specified effective date. The Company believes the impact of recently issued standards and any issued but not yet effective standards will not have a material impact on its condensed consolidated financial statements upon adoption.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480981/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Tables)
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Schedule of Revenue Associated with License Agreement |
We recognized revenue associated with the KKC Commercialization Agreement for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Timing of Revenue Recognition: |
|
|
|
|
|
|
Services performed over time |
|
$ |
743 |
|
|
$ |
8,359 |
|
Pass through services at a point in time |
|
|
9 |
|
|
|
371 |
|
|
|
$ |
752 |
|
|
$ |
8,730 |
|
|
| Schedule of Changes in Unbilled Receivables and Contract Liabilities |
The following table presents changes in unbilled receivables and contract liabilities accounted for under Topic 606 for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Unbilled receivables |
|
$ |
— |
|
|
$ |
85 |
|
Contract liabilities |
|
|
|
|
|
|
Contract liabilities, beginning of period |
|
$ |
317 |
|
|
$ |
30,900 |
|
Revenue recognized |
|
|
(317 |
) |
|
|
(5,411 |
) |
Revenue recognized from change in estimate for performance obligations that are being closed |
|
|
— |
|
|
|
(16,565 |
) |
Revenue recognized for performance obligations that will no longer commence |
|
|
— |
|
|
|
(8,607 |
) |
Contract liabilities, end of period |
|
$ |
— |
|
|
$ |
317 |
|
|
| Antidilutive Securities |
The following table presents potentially dilutive shares excluded from the calculation of diluted net income (loss) per share (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Stock options |
|
|
1,447 |
|
|
|
1,388 |
|
Warrants |
|
|
103 |
|
|
|
803 |
|
Restricted stock units |
|
|
— |
|
|
|
1 |
|
Total anti-dilutive shares |
|
|
1,550 |
|
|
|
2,192 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of receivable, contract asset, and contract liability from contract with customer. Includes, but is not limited to, change in contract asset and contract liability. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-10
| Name: |
us-gaap_ContractWithCustomerAssetAndLiabilityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of disaggregation of revenue into categories depicting how nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factor. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
| Name: |
us-gaap_DisaggregationOfRevenueTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Balance Sheet Details (Tables)
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Balance Sheet Related Disclosures [Abstract] |
|
| Schedule of Property and Equipment |
Property and equipment consisted of the following, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Furniture and equipment |
|
$ |
1,381 |
|
|
$ |
1,374 |
|
Leasehold improvements |
|
|
969 |
|
|
|
969 |
|
|
|
|
2,350 |
|
|
|
2,343 |
|
Less: accumulated depreciation |
|
|
(1,121 |
) |
|
|
(1,034 |
) |
Property and equipment, net |
|
$ |
1,229 |
|
|
$ |
1,309 |
|
|
| Accrued Liabilities |
Accrued liabilities consisted of the following, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
|
June 30, 2023 |
|
Accrued pre-clinical and clinical trial expenses |
|
$ |
730 |
|
|
$ |
3,663 |
|
Accrued compensation and benefits(1) |
|
|
1,515 |
|
|
|
7,189 |
|
Accrued legal and professional services |
|
|
1,044 |
|
|
|
1,423 |
|
Accrued reimbursement to KKC |
|
|
892 |
|
|
|
— |
|
Other |
|
|
108 |
|
|
|
186 |
|
Total accrued liabilities |
|
$ |
4,289 |
|
|
$ |
12,461 |
|
(1) Includes $0.1 million and $1.0 million of one-time termination employee benefits as of September 30, 2023 and June 30, 2023, respectively, as more fully described in Note 5. One-time Termination Benefits.
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionFair Value of Warrant Liability Assumptions [Table Text Block]
| Name: |
meip_FairValueOfWarrantLiabilityAssumptionsTableTextBlock |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSchedule Of Changes In Fair Value Of Warrant Liability [Table Text Block]
| Name: |
meip_ScheduleOfChangesInFairValueOfWarrantLiabilityTableTextBlock |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_RestructuringReserveAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of costs incurred for restructuring including, but not limited to, exit and disposal activities, remediation, implementation, integration, asset impairment, and charges against earnings from the write-down of assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 420
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 5.P.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 420
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 420
-SubTopic 10
-Section S99
-Paragraph 2
-Subparagraph (SAB TOPIC 5.P.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_ScheduleOfRestructuringAndRelatedCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Leases (Tables)
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Leases [Abstract] |
|
| Schedule of Total Operating Costs |
The total operating lease costs for the Lease Agreements were as follows for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
2023 |
|
|
2022 |
|
|
Operating lease cost |
|
$ |
608 |
|
|
$ |
608 |
|
|
Variable lease costs |
|
|
12 |
|
|
|
18 |
|
|
Total lease costs included in general and administrative expenses |
|
$ |
620 |
|
|
$ |
626 |
|
|
|
| Schedule of Supplemental Cash Flow Information Related to Operating Leases |
Supplemental cash flow information related to our operating leases was as follows for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
|
|
2023 |
|
|
2022 |
|
|
Cash paid for amount included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
Operating cash flows from operating leases |
|
$ |
584 |
|
|
$ |
567 |
|
|
|
|
|
|
|
|
|
|
|
| Schedule of Future Minimum Rental Payments |
The following is a schedule of the future minimum lease payments under the Lease Agreements, reconciled to the operating lease liability, as of September 30, 2023 (in thousands):
|
|
|
|
|
|
|
September 30, 2023 |
|
Remainder of fiscal year ending June 30, 2024 |
|
$ |
1,751 |
|
Years ending June 30, |
|
|
|
2025 |
|
|
1,913 |
|
2026 |
|
|
2,477 |
|
2027 |
|
|
2,551 |
|
2028 |
|
|
2,715 |
|
Thereafter |
|
|
4,385 |
|
Total lease payments |
|
|
15,792 |
|
Less: Present value discount |
|
|
(3,411 |
) |
Total operating lease liability |
|
$ |
12,381 |
|
|
|
|
|
Balance Sheet Classification - Operating Leases |
|
|
|
Operating lease liability |
|
$ |
1,055 |
|
Operating lease liability, long-term |
|
|
11,326 |
|
Total operating lease liability |
|
$ |
12,381 |
|
|
|
|
|
Other Balance Sheet Information - Operating Leases |
|
|
|
Weighted-average remaining lease term (in years) |
|
|
6.2 |
|
Weighted-average discount rate |
|
|
7.50 |
% |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of supplemental cash flow information for the periods presented.
| Name: |
us-gaap_ScheduleOfCashFlowSupplementalDisclosuresTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Share-based Compensation (Tables)
|
3 Months Ended |
Sep. 30, 2023 |
|---|
| Share-Based Compensation Expense for Stock Awards |
Total share-based compensation expense for all stock awards consisted of the following for the periods presented (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Research and development |
|
$ |
(69 |
) |
|
$ |
649 |
|
General and administrative |
|
|
432 |
|
|
|
910 |
|
Total share-based compensation |
|
$ |
363 |
|
|
$ |
1,559 |
|
|
| Summary of Stock Option Activity and Related Data |
A summary of our stock option activity and related data follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
Options |
|
|
Weighted-Average
Exercise Price |
|
|
Weighted-Average
Remaining Contractual
Term (in years) |
|
|
Aggregate
Intrinsic Value |
|
Outstanding at June 30, 2023 |
|
|
1,284,907 |
|
|
$ |
38.32 |
|
|
|
|
|
|
|
Granted |
|
|
227,037 |
|
|
$ |
7.01 |
|
|
|
|
|
|
|
Forfeited |
|
|
(64,817 |
) |
|
$ |
36.35 |
|
|
|
|
|
|
|
Outstanding at September 30, 2023 |
|
|
1,447,127 |
|
|
$ |
33.50 |
|
|
|
7.6 |
|
|
$ |
789 |
|
Vested and exercisable at September 30, 2023 |
|
|
819,813 |
|
|
$ |
49.16 |
|
|
|
6.3 |
|
|
$ |
— |
|
|
| Fair Value of Stock Options Weighted-Average Assumptions Used |
following weighted-average assumptions were used for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
Risk-free interest rate |
|
|
4.6 |
% |
|
|
2.8 |
% |
Expected life (years) |
|
|
5.7 |
|
|
|
6.0 |
|
Expected volatility |
|
|
89.8 |
% |
|
|
84.1 |
% |
Dividend yield |
|
|
— |
% |
|
|
— |
% |
Weighted-average grant date fair value |
|
$ |
5.27 |
|
|
$ |
7.80 |
|
|
| X |
- DefinitionTabular disclosure of share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Description of Business and Basis of Presentation - Additional Information (Detail) - USD ($)
$ / shares in Units, $ in Thousands |
Nov. 06, 2023 |
Oct. 31, 2023 |
Sep. 30, 2023 |
Jun. 30, 2023 |
| Targeted or Tracking Stock, Stock [Line Items] |
|
|
|
|
| Accumulated Losses Since Inception |
|
|
$ (349,623)
|
$ (405,997)
|
| Cash and cash equivalents and short term investments |
|
|
$ 82,200
|
|
| Subsequent Event |
|
|
|
|
| Targeted or Tracking Stock, Stock [Line Items] |
|
|
|
|
| Unpaid common stock dividend |
$ 1.75
|
$ 1.75
|
|
|
| Return of capital authorised |
|
$ 9,330
|
|
|
| Expenses related to an agreement |
|
$ 1,200
|
|
|
| Closing agreement date |
Nov. 17, 2023
|
|
|
|
| X |
- DefinitionCash includes currency on hand as well as demand deposits with banks or financial institutions. It also includes other kinds of accounts that have the general characteristics of demand deposits in that the customer may deposit additional funds at any time and effectively may withdraw funds at any time without prior notice or penalty. Cash equivalents, excluding items classified as marketable securities, include short-term, highly liquid Investments that are both readily convertible to known amounts of cash, and so near their maturity that they present minimal risk of changes in value because of changes in interest rates. Generally, only investments with original maturities of three months or less qualify under that definition. Original maturity means original maturity to the entity holding the investment. For example, both a three-month US Treasury bill and a three-year Treasury note purchased three months from maturity qualify as cash equivalents. However, a Treasury note purchased three years ago does not become a cash equivalent when its remaining maturity is three months. Short-term investments, exclusive of cash equivalents, generally consist of marketable securities intended to be sold within one year (or the normal operating cycle if longer) and may include trading securities, available-for-sale securities, or held-to-maturity securities (if maturing within one year), as applicable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashCashEquivalentsAndShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate dividends declared during the period for each share of common stock outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_CommonStockDividendsPerShareDeclared |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of paid and unpaid cash dividends declared for classes of stock, for example, but not limited to, common and preferred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-SubTopic 405
-Topic 942
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481071/942-405-45-2
| Name: |
us-gaap_DividendsCash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_TargetedOrTrackingStockStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
meip_RecognizedRevenueAssociatedWithTheFollowingLicenseAgreementsLineItems |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_RevenueFromContractWithCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TimingOfTransferOfGoodOrServiceAxis=us-gaap_TransferredAtPointInTimeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionContract with customer asset and liability Line items.
| Name: |
meip_ContractWithCustomerAssetAndLiabilityLineItems |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of obligation to transfer good or service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479837/606-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-8
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479837/606-10-45-2
| Name: |
us-gaap_ContractWithCustomerLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of revenue recognized that was previously included in balance of obligation to transfer good or service to customer for which consideration from customer has been received or is due. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-8
| Name: |
us-gaap_ContractWithCustomerLiabilityRevenueRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount received for services rendered and products shipped, but not yet billed, for non-contractual agreements due within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_UnbilledReceivablesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
| X |
- DefinitionPotentially Dilutive Securities Outstanding [Line Items]
| Name: |
meip_PotentiallyDilutiveSecuritiesOutstandingLineItems |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionPercentage Of Reimbursement Of Pass Through Costs As Revenue
| Name: |
meip_PercentageOfReimbursementOfPassThroughCostsAsRevenue |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 50
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147477123/405-50-65-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482477/820-10-65-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482477/820-10-65-13
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483482/832-10-65-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483482/832-10-65-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479845/805-20-65-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479845/805-20-65-3
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479845/805-20-65-3
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 40: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 41: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 42: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 43: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 44: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 45: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 46: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 47: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 48: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 49: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 50: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 51: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 11.M.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480530/250-10-S99-5
Reference 52: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 53: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 54: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 55: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 56: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 57: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 58: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 59: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 60: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 61: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 62: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 63: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 64: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 65: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 66: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 67: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 68: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 69: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 70: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 71: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 72: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 73: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 74: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-6
Reference 75: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 76: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 77: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 78: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 79: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 80: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 81: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 82: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 83: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 84: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 85: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 86: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 87: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 88: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 89: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 90: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481925/310-20-65-2
Reference 91: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481925/310-20-65-2
Reference 92: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-3
| Name: |
us-gaap_NewAccountingPronouncementsOrChangeInAccountingPrincipleLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investments including trading securities, available-for-sale securities, held-to-maturity securities, and short-term investments classified as other and current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_ShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock subject to repurchase or cancellation determined by relating the portion of time within a reporting period that these shares have been outstanding to the total time in that period. Common stock subject to repurchase are outstanding common shares that are contingently returnable (that is, subject to recall). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-13
| Name: |
us-gaap_WeightedAverageNumberOfSharesCommonStockSubjectToRepurchaseOrCancellation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TimingOfTransferOfGoodOrServiceAxis=meip_PassThroughServicesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Balance Sheet Details - Schedule of Property and Equipment (Detail) - USD ($)
$ in Thousands |
Sep. 30, 2023 |
Jun. 30, 2023 |
| Property, Plant and Equipment [Line Items] |
|
|
| Property, Plant and Equipment, Gross |
$ 2,350
|
$ 2,343
|
| Less: accumulated depreciation |
(1,121)
|
(1,034)
|
| Property and equipment, net |
1,229
|
1,309
|
| Furniture And Equipment |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, Plant and Equipment, Gross |
1,381
|
1,374
|
| Leasehold Improvements |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, Plant and Equipment, Gross |
$ 969
|
$ 969
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=meip_FurnitureAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LeaseholdImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAccrued Liabilities [Line Items]
| Name: |
meip_AccruedLiabilitiesLineItems |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAccrued pre-clinical and clinical trial expenses.
| Name: |
meip_AccruedPreclinicalAndClinicalCosts |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedProfessionalFeesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
| X |
- DefinitionCarrying amount (including both current and noncurrent portions of the accrual) as of the balance sheet date pertaining to a specified type of cost associated with exit from or disposal of business activities or restructuring pursuant to a duly authorized plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB TOPIC 5.P.4(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringReserve |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_RestructuringCostAndReserveAxis=us-gaap_OneTimeTerminationBenefitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Fair Value Measurements - Additional Information (Detail) - USD ($)
$ in Thousands |
3 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Jun. 30, 2023 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair value, measurement with unobservable inputs reconciliation, recurring basis, liability, transfers |
$ 0
|
$ 0
|
| Fair Value, Measurements, Recurring [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Liabilities |
0
|
0
|
| Assets |
$ 0
|
$ 0
|
| X |
- DefinitionFair value portion of probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_AssetsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of financial and nonfinancial obligations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_LiabilitiesFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionAmount of unrealized gain (loss) recognized in income from liability measured at fair value on recurring basis using unobservable input (level 3) and still held. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisChangeInUnrealizedGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFair value portion of warrants not settleable in cash classified as equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsNotSettleableInCashFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
One-Time Termination Benefits - Additional Information (Detail)
|
3 Months Ended |
6 Months Ended |
12 Months Ended |
|
Sep. 30, 2023
USD ($)
|
Dec. 31, 2022
Employee
|
Jun. 30, 2022
Employee
|
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
| Number of workforce reduction percentage |
51.00%
|
|
|
| Termination Benefits |
|
|
|
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
| Number of workforce reduction | Employee |
|
28
|
26
|
| Research and Development |
|
|
|
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
| Severance Costs | $ |
$ 28,000
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses for special or contractual termination benefits provided to current employees involuntarily terminated under a benefit arrangement associated exit or disposal activities pursuant to an authorized plan. Excludes expenses related to one-time termination benefits, a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_SeveranceCosts1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_RestructuringCostAndReserveAxis=us-gaap_ContractTerminationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAmount of cash payments made as the result of exit or disposal activities. Excludes payments associated with a discontinued operation or an asset retirement obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
| Name: |
us-gaap_PaymentsForRestructuring |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
| Name: |
us-gaap_RestructuringCostAndReserveLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount (including both current and noncurrent portions of the accrual) as of the balance sheet date pertaining to a specified type of cost associated with exit from or disposal of business activities or restructuring pursuant to a duly authorized plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB TOPIC 5.P.4(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringReserve |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in the accrual for restructuring costs. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringReservePeriodIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_RestructuringCostAndReserveAxis=us-gaap_OneTimeTerminationBenefitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAccrued milestone payments.
| Name: |
meip_AccruedMilestonePayments |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_PurchaseCommitmentExcludingLongtermCommitmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=meip_PresageLicenseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
License Agreements - Additional Information (Detail) - USD ($)
|
1 Months Ended |
3 Months Ended |
12 Months Ended |
|
Nov. 30, 2022 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Apr. 30, 2020 |
| Related Party Transaction [Line Items] |
|
|
|
|
| Revenue recognized |
|
$ (317,000)
|
$ (5,411,000)
|
|
| Kyowa Kirin Co. Ltd |
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
| Termination Agreement |
Jul. 14, 2023
|
|
|
|
| Total upfront payment receivable for grant of rights |
|
|
|
$ 100,000,000
|
| Kkc Agreements [Member] | Kyowa Kirin Co. Ltd |
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
| Transaction price of deferred revenue remaining performance obligations |
|
$ 317,000
|
|
|
| US License [Member] | Kyowa Kirin Co. Ltd |
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
| Deferred revenue |
|
|
$ 64,500,000
|
|
| X |
- DefinitionLicense obligation account transaction price allocated deferred revenue.
| Name: |
meip_LicenseObligationAccountTransactionPriceAllocatedDeferredRevenue |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionTotal upfront payment receivable for grant of rights.
| Name: |
meip_TotalUpfrontPaymentReceivableForGrantOfRights |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of revenue recognized that was previously included in balance of obligation to transfer good or service to customer for which consideration from customer has been received or is due. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-8
| Name: |
us-gaap_ContractWithCustomerLiabilityRevenueRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of deferred income and obligation to transfer product and service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=meip_KyowaKirinCoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=meip_KkcAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=meip_UsLicenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionPayment for license agreement.
| Name: |
meip_PaymentForLicenseAgreement |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPotential milestone payments receivable.
| Name: |
meip_PotentialMilestonePaymentsReceivable |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_OtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=meip_PresageLicenseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionDate which lease or group of leases is set to expire, in YYYY-MM-DD format.
| Name: |
us-gaap_LeaseExpirationDate1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of terms and conditions of option to extend lessor's operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479773/842-30-50-3
| Name: |
us-gaap_LessorOperatingLeaseOptionToExtend |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=meip_SanDiegoCaliforniaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AdjustmentsForNewAccountingPronouncementsAxis=us-gaap_AccountingStandardsUpdate201602Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAmount of lease cost recognized by lessee for lease contract. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of variable lease cost, excluded from lease liability, recognized when obligation for payment is incurred for finance and operating leases. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_VariableLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
Schedule of Future Minimum Rental Payments (Detail) - USD ($)
$ in Thousands |
Sep. 30, 2023 |
Jun. 30, 2023 |
| Leases [Abstract] |
|
|
| Remainder of fiscal year ending June 30, 2024 |
$ 1,751
|
|
| 2025 |
1,913
|
|
| 2026 |
2,477
|
|
| 2027 |
2,551
|
|
| 2028 |
2,715
|
|
| Thereafter |
4,385
|
|
| Total lease payments |
15,792
|
|
| Less: Present value discount |
(3,411)
|
|
| Total operating lease liability |
12,381
|
|
| Operating lease liability |
1,055
|
$ 1,428
|
| Operating lease liability, long-term |
11,326
|
$ 11,300
|
| Total operating lease liability |
$ 12,381
|
|
| Weighted-average remaining lease term (in years) |
6 years 2 months 12 days
|
|
| Weighted-average discount rate |
7.50%
|
|
| X |
- DefinitionLessee operating lease liability payments due after year four.
| Name: |
meip_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFour |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease having initial or remaining lease term in excess of one year to be paid in remainder of current fiscal year. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.3
| X |
- DefinitionPotentially Dilutive Securities Outstanding [Line Items]
| Name: |
meip_PotentiallyDilutiveSecuritiesOutstandingLineItems |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of common stock subject to repurchase or cancellation determined by relating the portion of time within a reporting period that these shares have been outstanding to the total time in that period. Common stock subject to repurchase are outstanding common shares that are contingently returnable (that is, subject to recall). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-13
| Name: |
us-gaap_WeightedAverageNumberOfSharesCommonStockSubjectToRepurchaseOrCancellation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Stockholders' Equity - Additional Information (Detail) - USD ($)
$ / shares in Units, $ in Billions |
3 Months Ended |
|
|
|
Sep. 30, 2023 |
Sep. 30, 2022 |
Jun. 30, 2023 |
May 31, 2023 |
Dec. 31, 2022 |
| Class of Stock [Line Items] |
|
|
|
|
|
| Fair value of warrants |
|
|
|
|
$ 0
|
| Number of warrants exercised |
0
|
0
|
|
|
|
| Total authorized share capital |
226,100,000
|
|
|
|
|
| Common stock, shares authorized |
226,000,000
|
|
226,000,000
|
|
|
| Common stock, par value |
$ 0.00000002
|
|
$ 0.00000002
|
|
|
| Preferred stock, shares authorized |
100,000
|
|
100,000
|
|
|
| Preferred stock, par value |
$ 0.01
|
|
$ 0.01
|
|
|
| Common stock voting rights |
one vote per share
|
|
|
|
|
| Preferred stock, shares outstanding |
0
|
|
0
|
|
|
| Warrants |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Exercise price |
|
|
|
$ 50.80
|
|
| Warrants outstanding |
102,513
|
|
|
802,949
|
|
| Warrants | Torreya partners |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Exercise price |
$ 6.80
|
|
|
|
|
| X |
- DefinitionNumber Of Warrants Exercised
| Name: |
meip_NumberOfWarrantsExercised |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of authorized capital units or capital shares. This element is relevant to issuers of face-amount certificates and registered investment companies.
| Name: |
us-gaap_CapitalUnitsAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which each warrant or right may be converted. For example, but not limited to, each warrant may be converted into two shares.
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByEachWarrantOrRight |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDescription of voting rights of common stock. Includes eligibility to vote and votes per share owned. Include also, if any, unusual voting rights. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_CommonStockVotingRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFair value portion of warrants not settleable in cash classified as equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsNotSettleableInCashFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=meip_TorreyaPartnersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Share-based Compensation - Additional Information (Detail) - USD ($)
$ / shares in Units, $ in Millions |
3 Months Ended |
|
|
Sep. 30, 2023 |
Jun. 30, 2023 |
May 31, 2021 |
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Outstanding Options |
1,447,127
|
1,284,907
|
|
| Unrecognized compensation expense related to unvested stock options |
$ 3.1
|
|
|
| Expected weighted average period for recognition of compensation expense |
1 year 7 months 6 days
|
|
|
| Employee Stock Option |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Outstanding Options |
1,447,127,000
|
|
|
| Closing price of common stock |
$ 7.01
|
|
|
| Employee Stock Option | Directors |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Share-based compensation arrangement by share-based payment award, award vesting period |
12 months
|
|
|
| Share-based compensation arrangement by share-based payment award, expiration period |
10 years
|
|
|
| Employee Stock Option | Employees |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Stock option vested percentage |
25.00%
|
|
|
| Share-based compensation arrangement by share-based payment award, award vesting period |
36 months
|
|
|
| 2008 Omnibus Plan |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Common stock authorized |
1,850,739,000
|
|
|
| Shares available for future grant |
398,718,000
|
|
|
| 2008 Omnibus Plan | Employee Stock Option |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Outstanding Options |
1,337,865,000
|
|
|
| Inducement Plan |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Common stock authorized |
|
|
125,000,000
|
| Shares available for future grant |
107,738,000
|
|
|
| Inducement Plan | Employee Stock Option |
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
| Outstanding Options |
109,262,000
|
|
|
| X |
- DefinitionClosing price of common stock.
| Name: |
meip_Sharebasedcompensationarrangementbysharebasedpaymentawardoptionsclosingstockprice |
| Namespace Prefix: |
meip_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost to be recognized for option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares authorized for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of vesting of award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_DirectorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=meip_EmployeesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=meip_TwoThousandAndEightOmnibusPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=meip_InducementPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionShare-based compensation arrangement by share-based payment award, options, weighted average remaining contractual term.
| Name: |
meip_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsWeightedAverageRemainingContractualTermAbstract |
| Namespace Prefix: |
meip_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAdditionalGeneralDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionAggregate dividends declared during the period for each share of common stock outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_CommonStockDividendsPerShareDeclared |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
MEI Pharma (NASDAQ:MEIP)
Historical Stock Chart
From Mar 2024 to Apr 2024
MEI Pharma (NASDAQ:MEIP)
Historical Stock Chart
From Apr 2023 to Apr 2024