Form 8-K - Current report
November 09 2023 - 8:02AM
Edgar (US Regulatory)
false000137229900013722992023-11-092023-11-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________________________________
FORM 8-K
___________________________________________________________
CURRENT REPORT
Pursuant to Section 13 OR 15 (d)
of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): November 9, 2023
___________________________________________________________
OCUGEN, INC.
(Exact Name of Registrant as Specified in its Charter)
___________________________________________________________
| | | | | | | | | | | | | | |
| Delaware | | 001-36751 | | 04-3522315 |
(State or Other Jurisdiction of
Incorporation) | | (Commission
File Number) | | (I.R.S. Employer
Identification Number) |
11 Great Valley Parkway
Malvern, Pennsylvania 19355
(484) 328-4701
(Address, including zip code, and telephone number, including area code, of principal executive office)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8–K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a–12 under the Exchange Act (17 CFR 240.14a–12)
☐ Pre–commencement communications pursuant to Rule 14d–2(b) under the Exchange Act (17 CFR 240.14d–2(b))
☐ Pre–commencement communications pursuant to Rule 13e–4(c) under the Exchange Act (17 CFR 240.13e–4(c))
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, $0.01 par value per share | | OCGN | | The Nasdaq Stock Market LLC (The Nasdaq Capital Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
Attached as Exhibit 99.1 hereto and incorporated herein by reference is a presentation that Ocugen, Inc. will post on its website on November 9, 2023 and may use from time to time in presentations or discussions with investors, analysts, and other parties.
Item 9.01 Financial Statements and Exhibits.
The following exhibits are being filed herewith:
(d) Exhibits
| | | | | | | | |
| Exhibit No. | | Document |
| 99.1 | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: November 9, 2023
| | | | | | | | |
| OCUGEN, INC. |
| |
| By: | /s/ Shankar Musunuri |
| | Name: Shankar Musunuri |
| | Title: Chairman, Chief Executive Officer, & Co-Founder |

Courageous Innovation Dedicated to Bringing Game-Changing Gene & Cell Therapies and Vaccines to Market and Working Even Harder to Provide Access to Patients Globally November 2023 NASDAQ: OCGN

Forward Looking Statements 2 This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, which are subject to risks and uncertainties. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements include, but are not limited to, statements regarding our clinical development activities and related anticipated timelines. Such statements are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from our current expectations. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Exchange Commission (SEC), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual reports that we file with the SEC. Any forward-looking statements that we make in this presentation speak only as of the date of this presentation. Except as required by law, we assume no obligation to update forward-looking statements contained in this presentation whether as a result of new information, future events, or otherwise, after the date of this presentation.

Through Courageous Innovation, We are Leveraging Our First- in-Class Platforms to Address Serious Unmet Medical Needs Modifier Gene Therapy Platform First-in-Class • Therapeutic Focus: inherited retinal diseases and larger blindness diseases with unmet need • Differentiator: “master gene regulator”; gene- agnostic approach • Pipeline: o OCU400 (Ph1/2): RP* & LCA**; Orphan drug designation from FDA/EMA ⎼ Ph3 target: early 2024 o OCU410 (Ph1/2): dry AMD o OCU410ST (Ph1/2): Stargardt; Orphan drug designation from FDA Inhalation Vaccines Platform First-in-Class • Therapeutic Focus: Flu and COVID-19 • Differentiator: inhalation for improved durability and transmission control • Pipeline: o OCU500 (Preclin): COVID-19 vaccine (NIH/NIAID Nextgen Collaboration - Ph1 planned for early 2024) o OCU510 (Preclin): flu quadrivalent o OCU520 (Preclin): COVID-19 + flu combo Regenerative Cell Therapy Platform First-in-Class • Therapeutic Focus: articular cartilage lesions • Differentiator: 3-D scaffold • Pipeline: o NeoCart (Ph3): articular cartilage defects in the knee o Ph3 target: 2H2024 o RMAT Designation by FDA *RP, retinitis pigmentosa **LCA, Leber congenital amaurosis

4 Pipeline Overview Current StatusIndicationAsset/Program • Phase 1/2 • Completed dosing of RP and LCA patients • Phase 3 trial for the treatment of RP expected to be initiated in early 2024 Retinitis pigmentosa (RP)--NR2E3 MutationOCU400 * AAV-hNR2E3 Gene mutation-associated retinal degeneration Gene therapies RP—RHO Mutation Leber congenital amaurosis (LCA)—CEP290 Mutation • Phase 1/2Dry Age-Related Macular Degeneration (Dry AMD)OCU410 AAV-hRORA • Phase 1/2Stargardt disease (orphan disease)OCU410ST AAV-hRORA • Continue to work on the Company’s response to the FDA regarding the IND application • Expect to initiate the Phase 1 clinical trial in the first half of 2024, contingent on the lift of the FDA hold and adequate availability of funding Diabetic Macular EdemaOCU200 Transferrin – TumstatinBiologics • IND-readyDiabetic Retinopathy • IND-readyWet Age-Related Macular Degeneration (Wet AMD) • Phase 3 clinical trial is planned for 2H 2024Treatment of Articular Cartilage Defects in the KneeNeoCart® (Autologous chondrocyte-derived neocartilage) RMAT** Cell therapies (Regenerative Medicine) OCU500 SeriesVaccines • OCU500 IND planned for early 2024 in collaboration with NIAIDFor Prevention of Disease Caused by COVID-19 OCU500: COVID-19 (Bivalent) For Prevention of Disease Caused by FluOCU510: Flu (Quadrivalent) For Prevention of Diseases Caused by Flu and COVID-19 OCU520: Flu + COVID-19 **Regenerative Medicine Advanced Therapy Designation *Broad , gene-agnostic, ORPHAN DRUG DESIGNATIONS FOR RP/LCA FROM FDA AND EMA

5 Modifier Gene Therapy Platform Breakthrough technology designed to address many rare diseases as well as complex diseases that affect millions

6 Modifier Gene Therapy: A Broader Reach Gene modifier therapy can potentially address multiple genetic defects with a single product utilizing a gene agnostic approach. In patients with IRDs, this could mean: Improved visual outcomes and quality of life GENE X GENE M cell NR2E3 Delivery “Molecular reset” of health & survival gene networks Cell with normal function cell Restored retinal cell homeostasis O N L ONL/photoreceptor survival GENE X cell GENE M Cell with mutated/ nonfunctioning gene(s) other than modifier gene M O D I F I E R G E N E M https://www.nature.com/articles/s41434-020-0134-z

FDA granted expanded Orphan Drug Designations for all retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA) mutations Despite its prevalence, RP and LCA patients have limited treatment options • US: RP & LCA affect 110,000 and 15,000 people, respectively • Worldwide: conditions affect approximately 1.6M people Current approved and in-development gene therapies focus on individual gene • More than 125 mutated genes associated with RP and LCA • Developing a single therapy to treat each mutation is not feasible OCU400 addresses shortcomings of current gene therapy approaches • Broad-spectrum, gene-agnostic approach to genetically diverse inherited retinal diseases • Potential one-time, curative therapy with a single sub-retinal injection, using NR2E3 Study summary: • Completed dosing of RP patients and three LCA patients including a pediatric patient • Phase 3 adult trial for the treatment of RP to be initiated in early 2024 following FDA concurrence on study design • Expanding the OCU400 Phase 3 clinical trial for LCA patients in the second half of 2024 based on Phase 1/2 study results in LCApatients and alignment with the FDA 7 OCU400: Phase 1/2 Clinical Trial Progressing as Planned, Developing a Novel Gene Therapy in Ophthalmic Areas of High Unmet Need

OCU400 Clinical Program A Phase 1/2 Study to Assess the Safety and Efficacy of OCU400 for Retinitis Pigmentosa associated with NR2E3 and RHO mutations and Leber Congenital Amaurosis with mutation(s) in CEP290 gene Clinical Trials.gov Identifier: NCT05203939 Safety of subretinal administration of OCU400 Immune responses Systemic Distribution Best Corrected Visual Acuity (BCVA) Multi-Luminance Mobility Test (MLMT) Low Luminance Visual Acuity (LLVA) Primary: Safety Exploratory: Efficacy 48

Safety Summary for OCU400—Clinical Study Update • The Phase 1/2 clinical trial demonstrated that OCU400 continued to be generally safe and well-tolerated in subjects across different mutations and dose levels • There were no serious adverse events (SAEs) related to the investigational product in the low and medium dose cohorts • In the high dose and open-enrollment cohorts, SAEs were reported for two subjects. None of them were related to the study drug. • Adverse events were mostly deemed related to the surgical procedure and resolved within a few days to weeks 79

RP and LCA—Unmet need and Treatment Benefit Target 8 • IRDs, such as RP and LCA, are a group of heterogenous genetic disorders that affect the retina, the light-sensitive tissue at the back of the eye • They often lead to a gradual loss of vision over time and can ultimately result in blindness • Stabilization of vision is crucial for patients with RP and LCA due to the progressive and degenerative nature of these diseases • Preservation of remaining vision, slowing disease progression, or improving the vision can significantly impact patients’ quality of life. Such outcomes not only can enhance the quality of life for affected individuals but also provide hope that future treatments could ultimately lead to vision restoration. • Comprehensive care, early diagnosis, and access to emerging therapies are essential components of a strategy to stabilize vision in RP and LCA patients 10
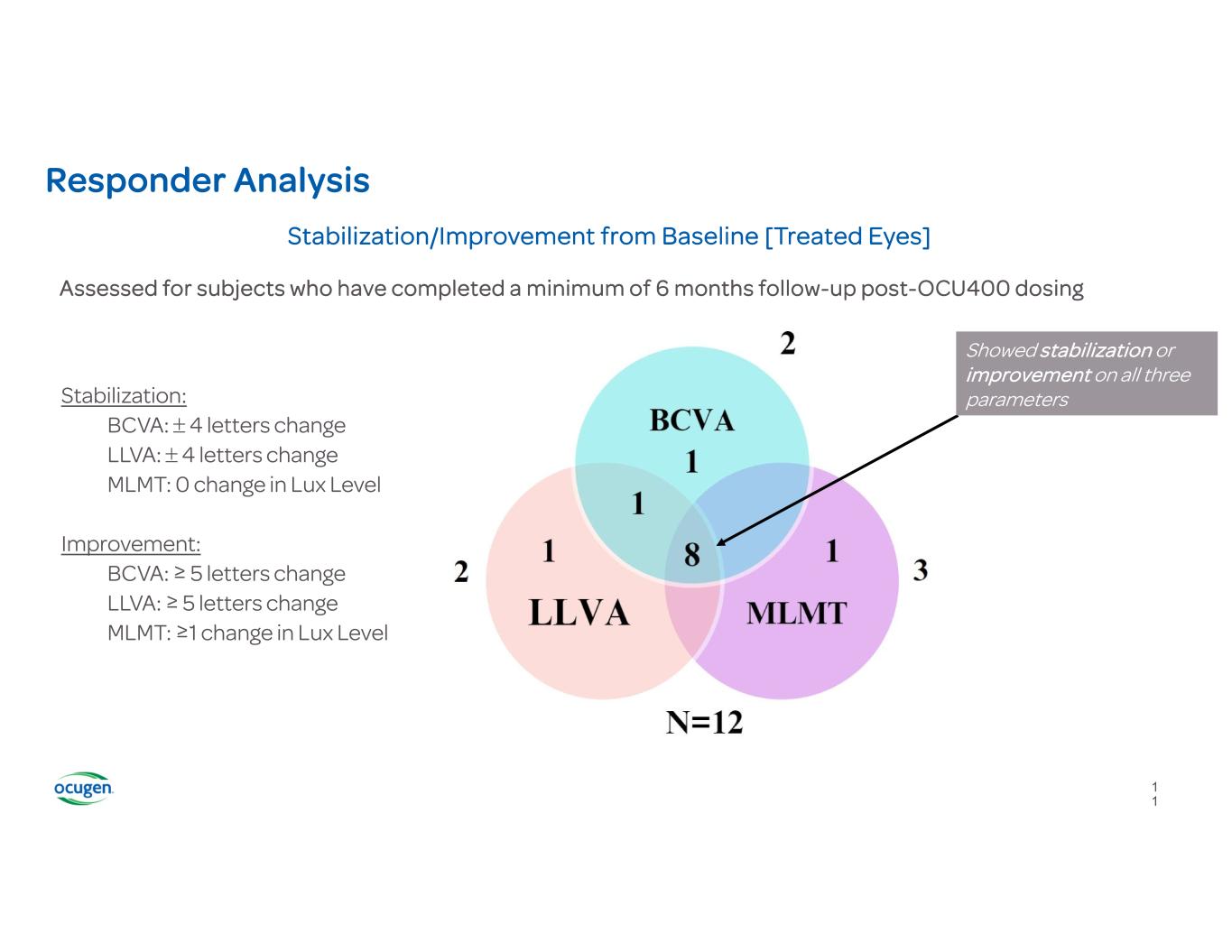
Responder Analysis Stabilization/Improvement from Baseline [Treated Eyes] Showed stabilization or improvement on all three parametersStabilization: BCVA: 4 letters change LLVA: 4 letters change MLMT: 0 change in Lux Level Improvement: BCVA: ≥ 5 letters change LLVA: ≥ 5 letters change MLMT: ≥1 change in Lux Level Assessed for subjects who have completed a minimum of 6 months follow-up post-OCU400 dosing 1 1

Conclusions from Latest Clinical Study Results • OCU400 continues to demonstrate a favorable safety and tolerability profile • Clinical study update suggests continued positive trends in Best-Corrected Visual Acuity (BCVA) and Multi-Luminance Mobility Testing (MLMT), as well as positive trends in Low-Luminance Visual Acuity (LLVA) among treated eyes • 83% (10/12) of subjects demonstrated stabilization or improvement in the treated eye either on BCVA or LLVA or MLMT scores from baseline • 75% (9/12) of subjects demonstrated stabilization or improvement in treated eyes in MLMT scores from baseline • 86% (6/7) of RHO mutation subjects experienced either stabilization or improvement in MLMT scores from baseline, among which 29% (2/7) demonstrated 3 Lux luminance level improvement • Treatment effect in RHO patients supports the gene-agnostic mechanism of action of OCU400 12 12
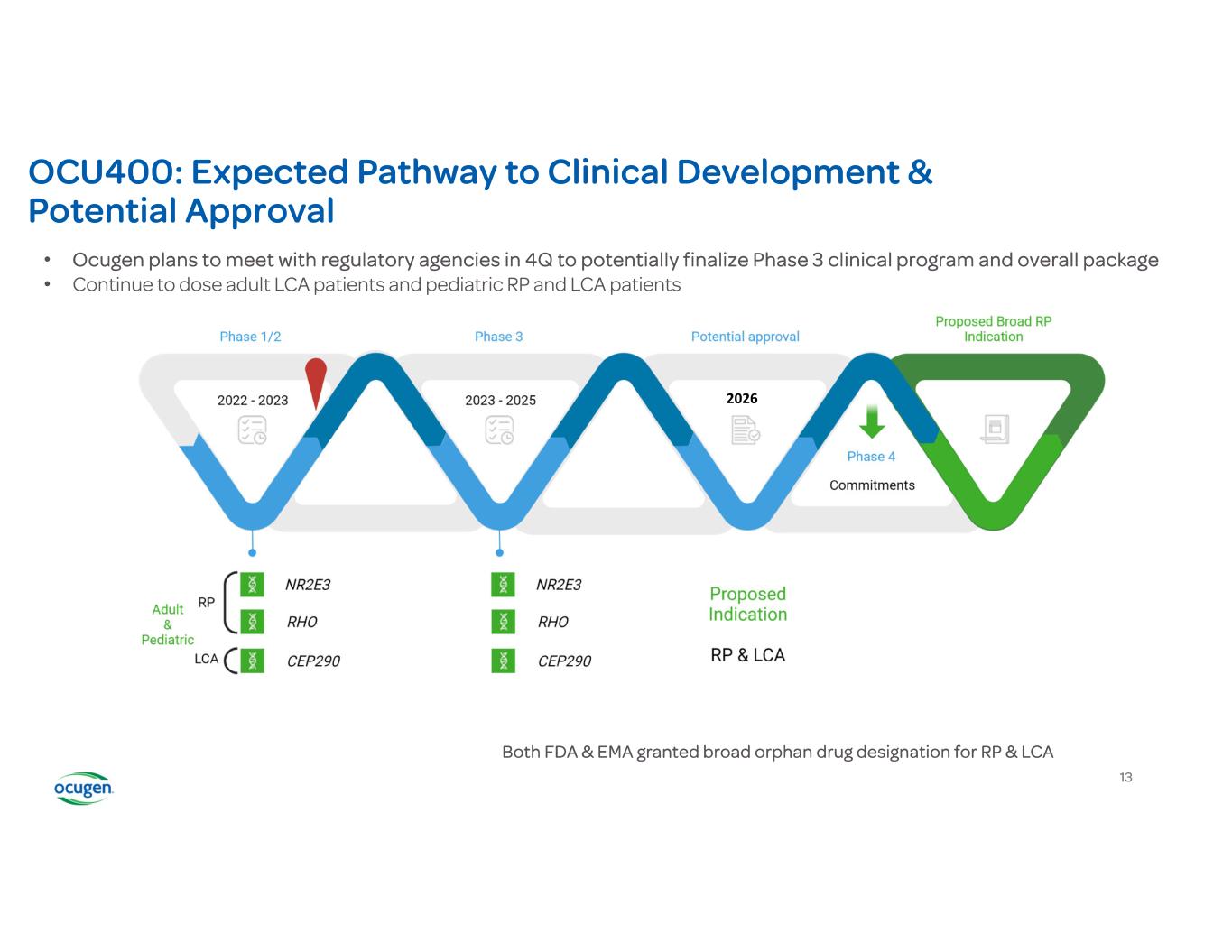
OCU400: Expected Pathway to Clinical Development & Potential Approval Both FDA & EMA granted broad orphan drug designation for RP & LCA • Ocugen plans to meet with regulatory agencies in 4Q to potentially finalize Phase 3 clinical program and overall package • Continue to dose adult LCA patients and pediatric RP and LCA patients 13 2026

* As demonstrated in Peak scotopic B-wave amplitudes. N≥5 biological replicates 14 Advancement from recently approved therapies for GA: Potential to address limitations of recently approved therapies for GA focused only on the complement system, including: Patient Compliance • Frequent intravitreal injections (~6-12 doses per year) Observed Structural Impact • Limited effect of GA lesion growth rate Safety Considerations • 12% of patients experienced nAMD when therapy is administered every month for two years (Syfovre®) OCU410 Phase 1/2 study currently underway Limited options for dAMD, presenting significant unmet medical need • U.S.: 10M (GA: 1M) • Worldwide: 266M Distinct 4-Way MOA: Addresses multiple regulator pathways involved with the disease including: • Lipid Metabolism • Regulation of Inflammation • Oxidative Stress • Membrane Attack Complex (Complement) Optimal Delivery and Durability: • A single subretinal injection designed to eliminate patient compliance concerns and the treatment burden associated with multiple injections Improved Retinal function: • Improved photoreceptor function in OCU410 treated eyes with all doses* The potential for a one-time curative therapy with a single sub-retinal injection to address the unmet needs and treatment burden in patients with dAMD OCU410 (RORA): A Single-Injection Approach to Addressing Unmet Needs in dAMD BEYOND the Complement System

50 60 70 80 90 1e61E51E41E31E2Cells N or m al iz ed C el l S ur vi va l RORA NR2E3 NR1D1 Anti-oxidative: Improves ARPE19 cells survival Anti-complement: Increased anti-compliment (Cd59) protein Anti-inflammatory: Suppresses inflammation in HMC3 cellsAnti-drusen activity and improves retinal function 0 50 100 150 200 Months 1 7 1 7 1 7 Control Abca4-/- Uninjected Abca4-/- OCU410 ✱✱ ✱✱✱ Macular Degeneration Model Reduced Drusen 15 OCU410 (RORA): A Potential Modifier Therapeutic for Dry-AMD and STGD
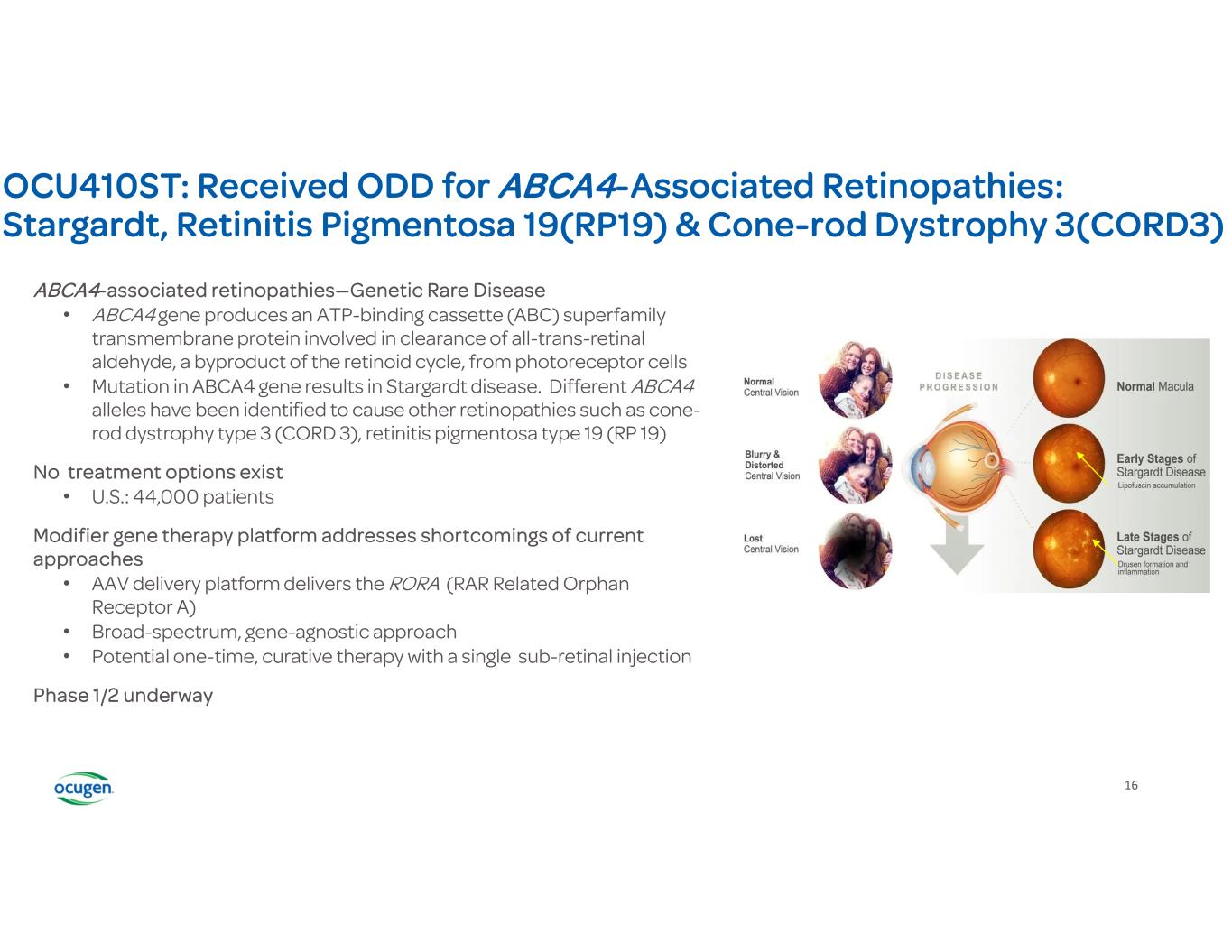
16 OCU410ST: Received ODD for ABCA4-Associated Retinopathies: Stargardt, Retinitis Pigmentosa 19(RP19) & Cone-rod Dystrophy 3(CORD3) ABCA4-associated retinopathies—Genetic Rare Disease • ABCA4 gene produces an ATP-binding cassette (ABC) superfamily transmembrane protein involved in clearance of all-trans-retinal aldehyde, a byproduct of the retinoid cycle, from photoreceptor cells • Mutation in ABCA4 gene results in Stargardt disease. Different ABCA4 alleles have been identified to cause other retinopathies such as cone- rod dystrophy type 3 (CORD 3), retinitis pigmentosa type 19 (RP 19) No treatment options exist • U.S.: 44,000 patients Modifier gene therapy platform addresses shortcomings of current approaches • AAV delivery platform delivers the RORA (RAR Related Orphan Receptor A) • Broad-spectrum, gene-agnostic approach • Potential one-time, curative therapy with a single sub-retinal injection Phase 1/2 underway

17 OCU200 Novel biologic for treating Diabetic Macular Edema (DME), Diabetic Retinopathy (DR) and Wet Age-Related Macular Degeneration (Wet AMD)

18 OCU200 is our novel biologics candidate for sight-threatening conditions • A recombinant fusion protein of transferrin and tumstatin • Potential to address diabetic macular edema (DME), diabetic retinopathy (DR), wet AMD High prevalence of DME, DR and wet AMD patients • DME: 21M worldwide • DR: 162M worldwide • Wet AMD: 30M worldwide Limited treatment options available for the above patients • Current therapies target only one pathway, either angiogenesis or inflammation • Up to 50% of patient population are not responsive to current treatments OCU200 potentially addresses shortcomings of current treatments • Intended to target multiple causative pathways such as angiogenesis, oxidation, inflammation • Potential to offer better treatment options for all patients Company submitted an IND application* • Initially targeting DME Diabetic Macular Edema: bulges protrude from the blood vessels, leading to leakage of fluid and blood into the retina; leakage results in swelling (or “edema”), promoting vision loss. OCU200: Submitted an IND with the U.S. FDA to Initiate a Phase 1 Clinical Trial Targeting Diabetic Macular Edema (DME) * Continue to work on the Company’s response to the FDA regarding the IND application and expect to initiate the Phase 1 clinical trial in the first half of 2024, contingent on the lift of the FDA hold and adequate availability of funding.

19 NeoCart® (Autologous chondrocyte-derived neocartilage)

20 *The Journal of Bone & Joint Surgery: June 1, 2011 - Volume 93 - Issue 11 - p 994-1000 **https://www.biospace.com/article/cell-therapy-market-size-cagr-trends-forecast-report-2022-2030/ NeoCart is a regenerative cell therapy • Received RMAT designation • Combines bioengineering and cell processing to enhance autologous cartilage repair • Potential to accelerate healing and reduce pain through reconstructing damaged knee cartilage High prevalence of knee cartilage damage, with progression to osteoarthritis (OA) • Arthroscopic knee procedures: over 1M annually* • OA: 528M diagnosed worldwide • Cell therapy global revenue forecast: $45B+, with North America expected to hold largest share** Current therapies to treat cartilage damage in the knee suboptimal • Varying outcomes due to variable cellular responses • Current standard of care suffers from one or more of the following: pain, reduced knee function, failure to address cartilage damage, donor tissue availability, open surgery NeoCart potentially addresses shortcomings of current treatments • Treat pain, improve function, and prevent progression to OA • Potential for improved efficacy, long-term benefits Program advancing on several fronts • Received FDA concurrence on confirmatory trial design of Phase 3 (initiate in 2H 2024) • Construction of cGMP manufacturing facility to be completed by the end of 2023 Follow-up Arthroscopy Demonstrates NeoCart® Progression and Integration** Initial Lesion Time Zero Implantation 8 Weeks 6 Months NeoCart®: U.S. FDA Agreed to Proposed Control and Overall Design for Phase 3 Trial

21 OCU500 Series: OCU500: COVID-19 Mucosal Vaccine OCU510: Flu OCU520: COVID-19/Flu

22 OCU500 Series: Next-Generation Vaccine Technology Inhaled mucosal vaccine platform based on ChAd vector OCU500 A bivalent COVID-19 vaccine OCU510 A seasonal quadrivalent flu vaccine OCU520 A combination quadrivalent flu and bivalent COVID19 vaccine Inhalation technology as a differentiator • Multiple preclinical studies using Ocugen’s vector demonstrated vaccine-induced high neutralizing and effector responses • Clinical studies using a similar vector administered via the inhalation platform showed mucosal antibodies, systemic antibodies, and durable immune response up to 1 year with 1/5 of the dose compared to the same vaccine given via intramuscular administration • The inhaled method offers the potential for broad, durable protection from severe disease and reduction in transmission Alignment with American Pandemic Preparedness Plan to transform U.S. capabilities to rapidly and effectively respond to existing and emerging infectious diseases via: • Legislative advocacy for next-generation mucosal vaccine development • OCU500 was selected by the NIH/NIAID Project NextGen for inclusion in clinical trials. NIAID is planning to initiate the Phase 1 clinical trials in early 2024.

23 Ocugen™ Vision Fully integrated, patient-centric biotech company focused on vaccines in support of public health and gene and cell therapies targeting unmet medical needs through Courageous Innovation
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
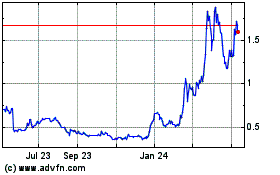
Ocugen (NASDAQ:OCGN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Ocugen (NASDAQ:OCGN)
Historical Stock Chart
From Apr 2023 to Apr 2024