false
0001850119
0001850119
2023-11-09
2023-11-09
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
November 9, 2023
Century Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
|
001-40498 |
|
84-2040295 |
(State or other jurisdiction of
incorporation or organization) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
|
25
North 38th Street, 11th Floor
Philadelphia, Pennsylvania |
|
19104 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (267) 817-5790
Not Applicable
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2.
below):
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
|
Trading Symbol |
|
Name
of Exchange on Which Registered |
| Common Stock, par value $0.0001 per share |
|
IPSC |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange
Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act.
| Item 2.02 |
Results of Operations and Financial Condition |
On November 9, 2023, Century Therapeutics, Inc. (the “Company”)
issued a press release announcing its financial results for the quarter ended September 30, 2023. A copy of the press release is
furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information contained in this Item 2.02 (including Exhibit 99.1)
is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934,
as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and shall not be deemed to
be incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or
the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 7.01 |
Regulation FD Disclosure |
On November 9, 2023, the Company updated information reflected
in a slide presentation, which is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by
reference. Representatives of the Company will use the updated presentation in various meetings with investors from time to time.
The information contained in this Item 7.01 (including Exhibit 99.2)
is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject
to the liabilities of that section and shall not be deemed incorporated by reference in any filing under the Securities Act or the Exchange
Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01 |
Financial Statements and Exhibits |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
CENTURY THERAPEUTICS, INC. |
| |
|
|
| |
By: |
/s/
Gregory Russotti, Ph.D. |
| |
Name: |
Gregory Russotti, Ph.D. |
| |
Title: |
Interim President and Chief
Executive Officer |
Date: November 9, 2023
Exhibit 99.1

Century Therapeutics Reports Third Quarter 2023
Financial Results and Provides Business Updates
- Brent Pfeiffenberger, Pharm.D., MBA, appointed
Chief Executive Officer of Century Therapeutics -
- Initial data from the ongoing Phase 1 ELiPSE-1
trial evaluating CNTY-101 in relapsed or refractory CD19 positive B-cell lymphomas to be presented at the American Society of Hematology
(ASH) Annual Meeting -
- Announced expanded license agreements with
FUJIFILM Cellular Dynamics (FCDI) for the development and commercialization of iPSC-derived cell therapies in autoimmune and inflammatory
diseases -
- Ended third quarter 2023 with cash, cash equivalents,
and investments of $284.3 million; Cash runway into 2026 -
PHILADELPHIA, November 9, 2023 -- Century
Therapeutics, Inc. (NASDAQ: IPSC), an innovative biotechnology company developing induced pluripotent stem cell (iPSC)-derived cell
therapies in immuno-oncology, today reported financial results and business highlights for the third quarter ended September 30, 2023.
“The last several months have been marked
by immense progress for Century, and we are thrilled to welcome Brent to the team as our new leader at this exciting period in the Company’s
history,” said Joe Jimenez, Chairman of the Board of Directors of Century. Greg Russotti, Interim Chief Executive Officer, Century
Therapeutics added, “We continued to advance our Phase 1 ELiPSE-1 trial evaluating CNTY-101 in relapsed or refractory CD19 positive
B-cell lymphomas. Building on the exciting case study featured in our ASH abstract earlier this month, we look forward to sharing additional
data in December which we believe continue to support the potential for a multi-dosing strategy for CAR iNK enabled by Allo-Evasion™.”
Dr. Russotti added, “Furthermore, we were
extremely excited to announce this morning that we have expanded our license agreements with our partners at FCDI. These expanded agreements
provide us new and continued access to technology that we believe will aid in our mission to bring curative cell therapy products to patients
in need, including patients with autoimmune and inflammatory diseases.”
Business Highlights and Upcoming Milestones
| · | This morning, Century announced the appointment of Brent Pfeiffenberger, Pharm.D., MBA, as Chief Executive
Officer and member of the Board of Directors, effective December 4, 2023. He brings to Century over 20 years of broad-ranging global leadership
experience across the healthcare industry, most recently serving as Chief Operating Officer at Neogene Therapeutics. Also effective December
4, 2023, Greg Russotti, Ph.D., who has served as Century’s Interim Chief Executive Officer since April 2023, will assume the role
of Chief Technology and Manufacturing Officer, an expanded role from his previous position as Chief Technology Officer. |

| · | During
a poster session at the upcoming ASH Annual Meeting being held December 9-12 in San Diego,
the Company will present initial data from the ongoing first-in-human Phase 1 ELiPSE-1 trial
of CNTY-101 in relapsed/refractory CD19 positive B-cell lymphomas. As previously announced,
preliminary clinical data from a case study featured in the recently published ASH abstract
shows a complete response maintained in a Dose Cohort 1 (100 million cell dose level) patient
with high risk relapsed/refractory follicular lymphoma following completion of four 28-day
cycles of CNTY-101, the two most recent of which did not include lymphodepletion. Updated
data to be announced in December will include additional results from patients treated in
Dose Cohort 1 as of a more recent cutoff date, as well as preliminary data from patients
in Dose Cohort 2 (300 million cell dose level). |
| · | This morning, Century and FUJI Cellular Dynamics (FCDI) announced that they have entered into a worldwide
license agreement whereby FCDI will grant the Company non-exclusive licenses for certain patent rights and know-how related to cell differentiation
and reprogramming for the development and commercialization of iPSC-derived therapies for the treatment of autoimmune and inflammatory
diseases. The Company also shared that it expanded its existing 2018 license agreement with FCDI related to the development and commercialization
of iPSC-derived cancer immunotherapeutics to also include inflammatory and autoimmune diseases. |
Third Quarter 2023 Financial Results
| · | Cash Position: Cash, cash equivalents, and investments were $284.3 million as of September
30, 2023, as compared to $367.4 million as of December 31, 2022. Net cash used in operations was $61.8 million for the nine months ended
September 30, 2022 compared to net cash provided by operations of $37.0 million for the nine months ended September 30, 2022 (which includes
deferred revenue from the Bristol Myers Squibb (BMS) collaboration of $118.5 million). |
| | | |
| | · | Collaboration Revenue: Collaboration revenue generated through the Company’s
collaboration, option, and license agreement with BMS was $0.1 million for the three months ended September 30, 2023 compared to $2.2
million for the same period in 2022. Revenue recognized under the collaboration agreement fluctuates based on the amount and timing of
expenses incurred under the agreement. |
| · | Research and Development (R&D) expenses: R&D expenses were $22.8 million for the three
months ended September 30, 2023 compared to $25.9 million for the same period in 2022. The decrease in R&D expenses was primarily
due to the Company’s 2023 reorganization and reprioritization of early-stage programs and discovery platforms as well as a decline
in sponsored research activities. |
| · | General and Administrative (G&A) expenses: G&A expenses were $9.0 million for the three
months ended September 30, 2023, compared to $8.1 million for the same period in 2022. The increase was primarily due increases in stock-based
compensation and recruiting fees. |
| · | Net
loss: Net loss was $32.7 million for the three months ended September 30, 2023, compared to $30.7 million for the same period in
2022. |

Financial Guidance
| · | The
Company expects full year GAAP Operating Expenses to be between $135 million and $145 million including non-cash stock-based compensation
expense of $12 million to $17 million. |
| · | The
Company expects its cash, cash equivalents, and investments will support operations into 2026. |
About Century Therapeutics
Century Therapeutics (NASDAQ: IPSC) is harnessing
the power of adult stem cells to develop curative cell therapy products for cancer and autoimmune and inflammatory diseases that we believe
will allow us to overcome the limitations of first-generation cell therapies. Our genetically engineered, iPSC-derived iNK and iT cell
product candidates are designed to specifically target hematologic and solid tumor cancers with a broadening application to inflammatory
and autoimmune diseases. We are leveraging our expertise in cellular reprogramming, genetic engineering, and manufacturing to develop
therapies with the potential to overcome many of the challenges inherent to cell therapy and provide a significant advantage over existing
cell therapy technologies. We believe our commitment to developing off-the-shelf cell therapies will expand patient access and provide
an unparalleled opportunity to advance the course of care for cancer and autoimmune and inflammatory diseases. For more information on
Century Therapeutics please visit www.centurytx.com.

Century Therapeutics Forward-Looking Statement
This press release contains forward-looking
statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act
of 1995. All statements contained in this press release, other than statements of historical facts or statements that relate to
present facts or current conditions, including but not limited to, statements regarding our clinical development plans and
timelines, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important
factors that may cause our actual results, performance, or achievements to be materially different from any future results,
performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking
statements by terms such as “may,” “might,” “will,” “should,” “expect,”
“plan,” “aim,” “seek,” “anticipate,” “could,” “intend,”
“target,” “project,” “contemplate,” “believe,” “estimate,”
“predict,” “forecast,” “potential” or “continue” or the negative of these terms or
other similar expressions. The forward-looking statements in this press release are only predictions. We have based these
forward-looking statements largely on our current expectations and projections about future events and financial trends that we
believe may affect our business, financial condition, and results of operations. These forward-looking statements speak only as of
the date of this press release and are subject to a number of risks, uncertainties and assumptions, some of which cannot be
predicted or quantified and some of which are beyond our control, including, among others: our ability to successfully advance our
current and future product candidates through development activities, preclinical studies, and clinical trials; our dependence on
the success of our lead product candidate, CNTY-101, our ability to obtain FDA acceptance for our future IND submissions and
commence clinical trials on expected timelines, or at all; our reliance on the maintenance of certain key collaborative
relationships for the manufacturing and development of our product candidates; the timing, scope and likelihood of regulatory
filings and approvals, including final regulatory approval of our product candidates; the impact of the effects of the COVID-19
pandemic, geopolitical issues, banking instability and inflation on our business and operations, supply chain and labor force; the
performance of third parties in connection with the development of our product candidates, including third parties conducting our
clinical trials as well as third-party suppliers and manufacturers; our ability to successfully commercialize our product candidates
and develop sales and marketing capabilities, if our product candidates are approved; our ability to recruit and maintain key
members of management and our ability to maintain and successfully enforce adequate intellectual property protection. These and
other risks and uncertainties are described more fully in the “Risk Factors” section of our most recent filings with the
Securities and Exchange Commission and available at www.sec.gov. You should not rely on these forward-looking statements as
predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur,
and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in a dynamic
industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to
predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update
or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed
circumstances or otherwise.
For More Information:
Investors and Media: Melissa Forst/Maghan Meyers – century@argotpartners.com

Century Therapeutics, Inc
Condensed Balance Sheets
(unaudited, in thousands)
| | |
September 30, | | |
December 31, | |
| |
2023 | | |
2022 | |
| Assets | |
|
| | |
|
| |
| Current Assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 55,307 | | |
$ | 84,265 | |
| Short-term investments | |
| 114,198 | | |
| 231,233 | |
| Prepaid expenses and other current assets | |
| 4,198 | | |
| 4,223 | |
| Total current assets | |
| 173,703 | | |
| 319,721 | |
| Property and equipment, net | |
| 81,993 | | |
| 82,785 | |
| Operating lease right-of-use assets, net | |
| 24,551 | | |
| 28,945 | |
| Long-term investments | |
| 114,762 | | |
| 51,854 | |
| Other long-term assets | |
| 2,542 | | |
| 3,239 | |
| Total assets | |
$ | 397,551 | | |
$ | 486,544 | |
| | |
| | | |
| | |
| Liabilities, convertible preferred stock, and stockholders' equity | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 5,927 | | |
$ | 5,454 | |
| Accrued expenses and other liabilities | |
| 10,637 | | |
| 10,707 | |
| Long-term debt, current | |
| - | | |
| 6,502 | |
| Deferred revenue, current | |
| 3,871 | | |
| 7,154 | |
| Total current liabilities | |
| 20,435 | | |
| 29,817 | |
| Operating lease liability, noncurrent | |
| 45,535 | | |
| 38,698 | |
| Long-term debt, net | |
| - | | |
| 3,739 | |
| Other long-term liabilities | |
| 201 | | |
| 718 | |
| Deferred revenue | |
| 112,150 | | |
| 110,834 | |
| Total liabilities | |
| 178,321 | | |
| 183,806 | |
| Stockholders' equity | |
| | | |
| | |
| Common stock | |
| 6 | | |
| 6 | |
| Additional paid-in capital | |
| 836,901 | | |
| 824,292 | |
| Accumulated deficit | |
| (616,373 | ) | |
| (519,098 | ) |
| Accumulated other comprehensive loss | |
| (1,304 | ) | |
| (2,462 | ) |
| Total stockholders' equity | |
| 219,230 | | |
| 302,738 | |
| Total liabilities and stockholders' equity | |
$ | 397,551 | | |
$ | 486,544 | |

Century Therapeutics, Inc
Condensed consolidated statements of operations
(unaudited, in thousands, except share and per share amounts)
| | |
Three months Ended | | |
Nine months Ended | |
| | |
September 30, | | |
September 30, | | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Collaboration Revenue | |
$ | 148 | | |
$ | 2,224 | | |
$ | 1,967 | | |
$ | 4,678 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating Expenses | |
| | | |
| | | |
| | | |
| | |
| Research and development | |
$ | 22,788 | | |
$ | 25,898 | | |
$ | 70,414 | | |
$ | 71,588 | |
| General and administrative | |
| 8,986 | | |
| 8,064 | | |
| 26,117 | | |
| 23,615 | |
| In-process research and development | |
| 4,000 | | |
| - | | |
| 4,000 | | |
| 10,000 | |
| Impairment on long-lived assets | |
| - | | |
| - | | |
| 4,220 | | |
| - | |
| Total operating expenses | |
$ | 35,774 | | |
$ | 33,962 | | |
$ | 104,751 | | |
$ | 105,203 | |
| | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
| (35,626 | ) | |
| (31,738 | ) | |
| (102,784 | ) | |
| (100,525 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Interest expense | |
| - | | |
| (373 | ) | |
| (540 | ) | |
| (1,017 | ) |
| Interest income | |
| 3,486 | | |
| 1,411 | | |
| 9,167 | | |
| 2,370 | |
| Other income, net | |
| 12 | | |
| (24 | ) | |
| (368 | ) | |
| (19 | ) |
| Loss before provision for income taxes | |
$ | (32,128 | ) | |
$ | (30,724 | ) | |
$ | (94,525 | ) | |
$ | (99,191 | ) |
| Provision for income taxes | |
| (592 | ) | |
| (25 | ) | |
| (2,750 | ) | |
| (59 | ) |
| Net Loss | |
$ | (32,720 | ) | |
$ | (30,749 | ) | |
$ | (97,275 | ) | |
$ | (99,250 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Unrealized loss on investments | |
| (95 | ) | |
| (165 | ) | |
| 1,157 | | |
| (2,931 | ) |
| Foreign currency translation adjustment | |
| (2 | ) | |
| (5 | ) | |
| (1 | ) | |
| (23 | ) |
| Comprehensive loss | |
| (32,817 | ) | |
| (30,919 | ) | |
| (96,119 | ) | |
| (102,204 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per common share - Basic and Diluted | |
| (0.55 | ) | |
| (0.53 | ) | |
| (1.65 | ) | |
| (1.72 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average common shares outstanding | |
| 59,448,229 | | |
| 57,973,541 | | |
| 59,087,374 | | |
| 57,573,406 | |
Exhibit 99.2

Corporate Overview November 2023

2 Forward - looking statements This presentation contains forward - looking statements within the meaning of, and made pursuant to the safe harbour provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this document, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding possible or assumed future results of operations, business strategies, research and development plans, regulatory activities, market opportunity, competitive position and potential growth opportunities are forward - looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. In some cases, you can identify forward - looking statements by terms such as “may,” “might,” “will,” “should,” “expect,” “plan,” “aim,” “seek,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward - looking statements in this presentation are only predictions. We have based these forward - looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward - looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among others: our ability to successfully advance our current and future product candidates through development activities, preclinical studies, and clinical trials; our reliance on the maintenance on certain key collaborative relationships for the manufacturing and development of our product candidates; the timing, scope and likelihood of regulatory filings and approvals, including final regulatory approval of our product candidates; the impact of the COVID - 19 pandemic, geopolitical issues and inflation on our business and operations, supply chain and labor force; the performance of third parties in connection with the development of our product candidates, including third parties conducting our future clinical trials as well as third - party suppliers and manufacturers; our ability to successfully commercialize our product candidates and develop sales and marketing capabilities, if our product candidates are approved; and our ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainties are described more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission and available at www.sec.gov. You should not rely on these forward - looking statements as predictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

3 Investment thesis Next generation platforms for iNK and gamma delta iT candidates Foundational investments in iPSC technology, genetic editing, and manufacturing Experienced team in R&D, immuno - oncology, manufacturing and commercialization Exemplified by execution of ongoing first - in - human Phase 1 ELiPSE - 1 trial Well capitalized with cash runway into 2026 $284.3M in cash, cash equivalents and investments at the end of 3Q23; operational efficiencies designed to enable delivery on key milestones, clinical data

iPSC Platform

5 Building a next generation allogeneic cell therapy platform Gene Editing • Proprietary gene editing platform • CRISPR MAD7 - derived gene editing for precise transgene integration Protein Engineering • Developing proprietary next - generation CARs • Universal tumor targeting platform iPSC Differentiation/Manufacturing • Scalable protocols and processes to produce highly functional iNK and iT cell products iPSC Reprogramming • Comprehensive collection of clinical grade lines (CD34+ HSC, αβ T cell , γδ T cell derived) Vertically integrated capabilities differentiate Century’s approach

6 Foundational investments in iPSC know - how and manufacturing Established in - house manufacturing • Accelerates learnings and enables faster product iteration • 53,000 ft 2 facility • Designed to produce multiple immune cell types • Two sites provides optionality and maximizes flexibility iPSC license and collaboration agreement • Access to clinical grade iPSC lines • Exclusive IP and know - how to generate immune effector cells using feeder - free methods (NK, T, Mono/ Mac, DC) • Expanded to include development and commercialization of cell therapies for the treatment of autoimmune and inflammatory diseases (2023) • FCDI GMP manufacturing capacity for Century’s product candidates • Leveraging two decades of research & investment at University of Wisconsin and FCDI

7 Multiple gene edits (KO/KI) iPSC bank Precision CRISPR MAD7 mediated sequential gene editing of iPSC cells generates uniform product candidates Engineered iPSC Master Cell Bank (MCB) Advantages of Century’s Platform P recise CRISPR mediated homology directed repair reduces off - target integration Stepwise and efficient gene editing avoids risky multiplex modification and structural variants Quality control through generation of homogenous MCB establishes genomic product integrity Manufacturing begins at the MCB, confirmed to be free from genetic aberrations Sequential selection steps iSPC Precision Engineering CRISPR - mediated HDR (MAD 7)

8 Potential to drive durable responses with engineering to resist immune rejection Allo - Evasion TM edits + repeat dosing = potential greater durability Next - wave of allogeneic cell therapies must solve for challenge of rejection With Allo - Evasion TM engineering Without Allo - Evasion TM engineering
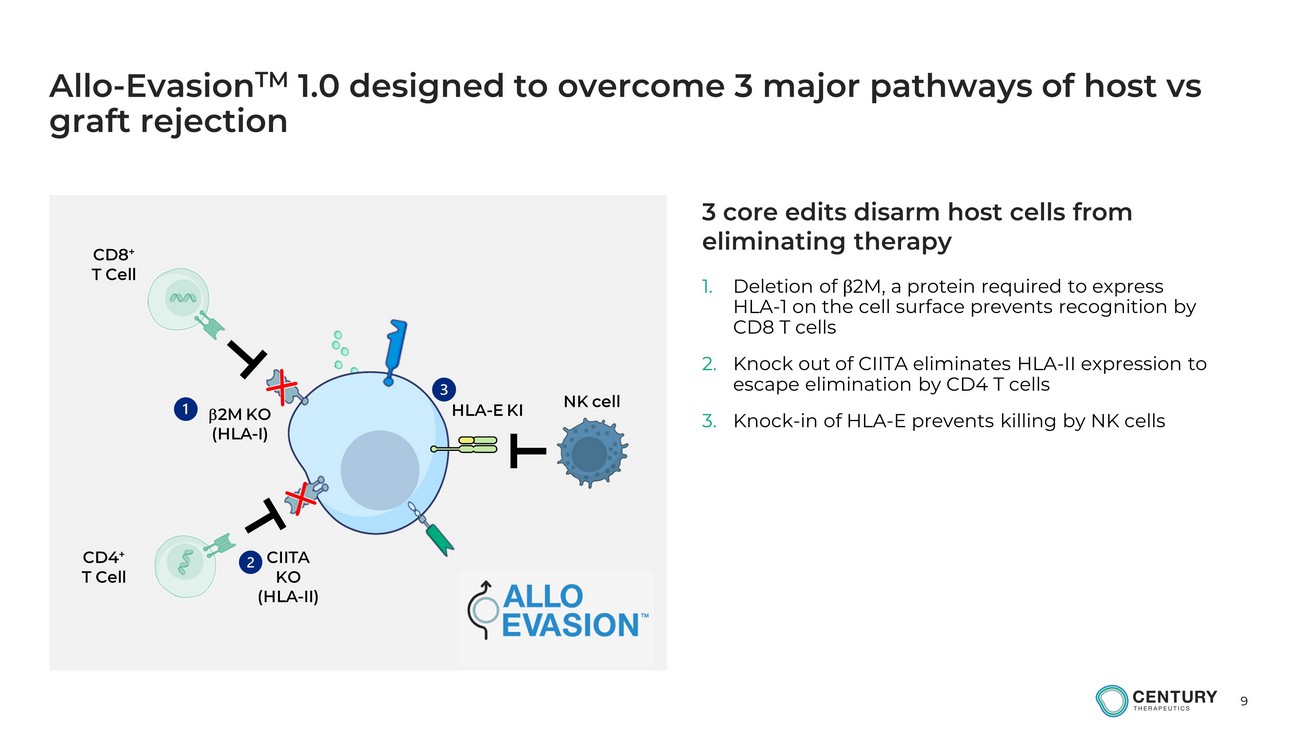
9 Allo - Evasion TM 1.0 designed to overcome 3 major pathways of host vs graft rejection 1. Deletion of β 2M, a protein required to express HLA - 1 on the cell surface prevents recognition by CD8 T cells 2. Knock out of CIITA eliminates HLA - II expression to escape elimination by CD4 T cells 3. Knock - in of HLA - E prevents killing by NK cells b 2M KO (HLA - I) HLA - E KI CIITA KO (HLA - II) CD8 + T Cell CD4 + T Cell NK cell 3 core edits disarm host cells from eliminating therapy

10 Allo - Evasion TM 3.0 provides additional protection against NK cell killing 1. Deletion of β 2M, a protein required to express HLA - 1 on the cell surface prevents recognition by CD8 T cells 2. Knock out of CIITA eliminates HLA - II expression to escape elimination by CD4 T cells 3. Knock - in of HLA - E prevents killing by NK cells 4. Knock - in of HLA - G improves protection against killing by NK cells b 2M KO (HLA - I) HLA - E KI CIITA KO (HLA - II) CD8 + T Cell CD4 + T Cell NK cell 4 core edits disarm host cells from eliminating therapy HLA - G KI

11 Expression of HLA - E + HLA - G further protects from NK cell killing HLA - E HLA - G NKG2A KIRs, LIRs Activating ligand Activating receptor Proof - of - Concept Study with HLA - I Null K562 Cells Engineered with HLA - E and HLA - G 0 . 1 2 5 0 . 2 5 0 . 5 1 2 4 8 1 6 3 2 0 20 40 60 80 100 Donor RC01 Ratio (E:T) % K i l l i n g Parental K562 HLA-G K562 HLA-E K562 HLA-E+G K562 The Combination of HLA - E + HLA - G Improved Protection to Killing by Allogeneic NK Cells • HLA - E and HLA - G engage different receptors on NK cells including NKG2A, KIRs, and LIRs • The expression of NKG2A, KIRs, and LIRs varies among NK cells from different donors Agglomerated Data from 22 NK Cell Donors N o e d i t H L A - G H L A - E H L A - E / G 0.0 0.2 0.4 0.6 0.8 1.0 R e l a t i v e c y t o l y s i s

Pipeline
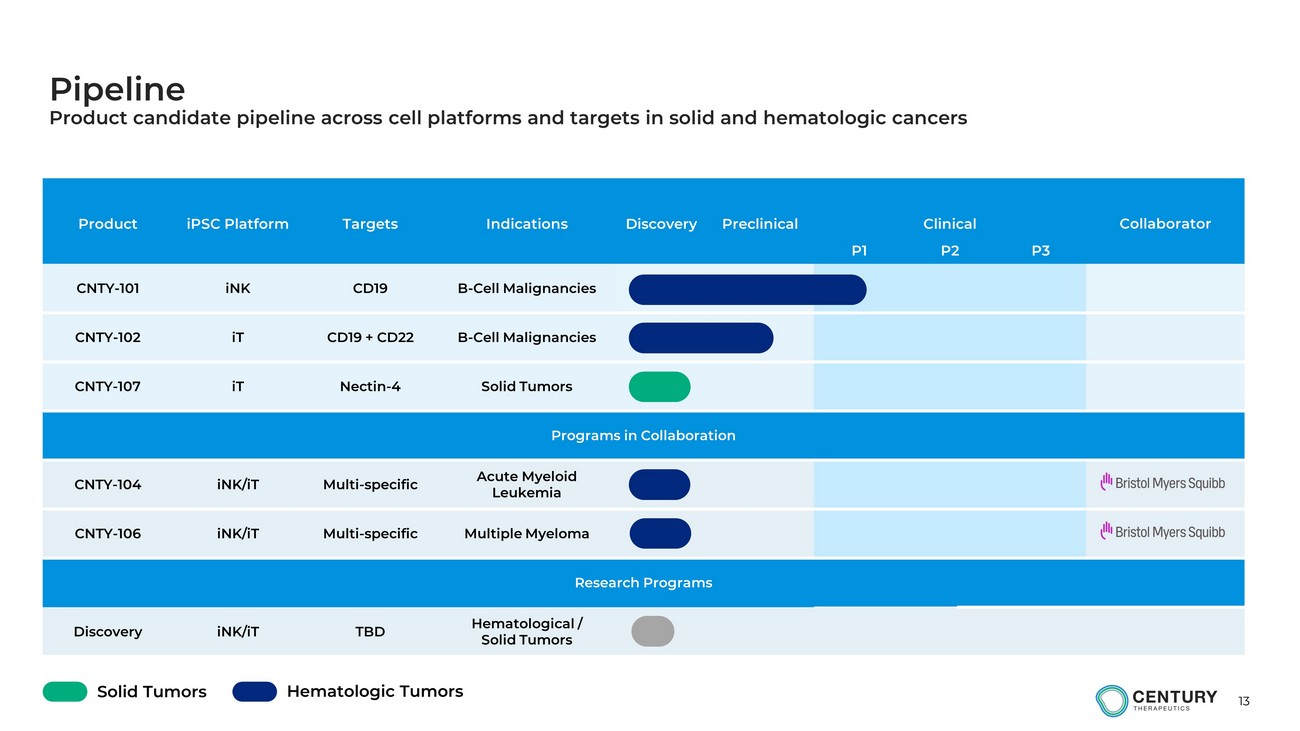
13 Product iPSC Platform Targets Indications Discovery Preclinical Clinical Collaborator P1 P2 P3 CNTY - 101 iNK CD19 B - Cell Malignancies CNTY - 102 iT CD19 + CD22 B - Cell Malignancies CNTY - 107 iT Nectin - 4 Solid Tumors Programs in Collaboration CNTY - 104 iNK/iT Multi - specific Acute Myeloid Leukemia CNTY - 106 iNK/iT Multi - specific Multiple Myeloma Research Programs Discovery iNK/iT TBD Hematological / Solid Tumors Pipeline Product candidate pipeline across cell platforms and targets in solid and hematologic cancers Hematologic Tumors Solid Tumors

CNTY - 101 Clinical Program

15 CNTY - 101 aims to deliver durable responses in R/R B - cell NHL via repeat dosing facilitated by Allo - Evasion TM R/R: relapsed or refractory, NHL: non - Hodgkin lymphoma, CAR - T: chimeric antigen receptor T cell therapy Potential solution from Century’s platform: • Off - the - shelf product offers immediate access and consistency • Multiple doses to increase pharmacological pressure to increase durability • Host rejection addressed by Allo - Evasion TM edits Unmet need: • Autologous CD19 CAR - T is curative in 40 percent of patients • Autologous CD19 CAR - T access is limited and/or can fail in manufacturing as quality is dependent on patient - derived starting material • Limited options and poor prognosis for patients who fail autologous CAR - T Aim: extending the period of pharmacologic pressure on tumor cells

16 Delivering on our vision to change the cell therapy treatment paradigm • Goal to improve durability, tolerability and ease of outpatient administration • Potential to e liminate need for lymphodepletion with subsequent cycles of therapy • First CD19 - targeted agent to test durability benefit of repeat dosing enabled by Allo - Evasion TM edits CNTY - 101: differentiated next - gen CD19 targeted product CNTY - 101 Allo - Evasion TM edits HLA - I Knockout IL - 15 HLA - II Knockout CD19 CAR HLA - E Safety Switch

17 CNTY - 101 shows strong pre - clinical anti - tumor activity In Vitro Serial killing assay Robust activity against lymphoma xenograft Borges, et al, ASH 202 1

18 CNTY - 101: ELiPSE - 1 (NCT05336409) Phase 1 BOIN 1 Design Initial clinical data to be presented at ASH 2023 Annual Meeting in December Clinical data providing initial proof - of - concept expected in 2024 1 BOIN: Bayesian Optimal Interval 2 Standard lymphodepletion regimen: fludarabine (30 mg/m2/d) and cyclophosphamide IV (300 mg/m2/d) for 3 days 3 Subjects who are assessed as stable disease or better may receive additional cycles of CNTY - 101 Patients with CD19+ aggressive and high - risk indolent R/R B - NHL ▪ DLBCL, HGBL, MCL, PMBCL, FL3B, FL, MZL ▪ ≥ 2 prior lines of therapy ▪ Prior CD19 - targeted cell therapy allowed Part 1 - Schedule A: single dose escalation Schedule B: 1 dose per week x 3 weeks Part 2 - Dose expansion Initial Dose Additional Cycles 3 First Additional Cycle: lymphodepletion at investigator discretion No lymphodepletion for following cycles Schedule A Dose level 1: 100 x 10 6 Dose level 2: 300 x 10 6 Dose level 3: 1000 x 10 6 LYMPHO - DEPLETION 2 IL - 2 x 22 days Schedule B Dose level 2: 300 x 10 6 x3 Dose level 3: 1000 x 10 6 x3 28 - DAY DLT PERIOD RESPONSE ASSESSMENT Patient enrollment CNTY - 101 DAY 1 101 DAY 1 DAY 8 101 101 DAY 15 101 DAY 1 101 DAY 1 DAY 8 101 101 DAY 15 IL - 2 x 8 days IL - 2 x 8 days IL - 2 x 22 days

19 Initial ELiPSE - 1 data support the potential for a multi - dosing strategy for CAR iNK enabled by Allo - Evasion ۲ 65 th Annual Meeting 2023 Title: Multiple Doses of CNTY - 101, an iPSC - Derived Allogeneic CD19 Targeting CAR - NK Product, are Safe and Result in Tumor Microenvironment Changes Associated with Response: A Case Study Abstract: 1654 Session Name : 622. Lymphomas: Translational – Non - Genetic: Poster I Date : Saturday, December 9, 2023 Session Time : 5:30 PM - 7:30 PM PT Summary of Case Study Featured in ASH Abstract* • Patient with high risk relapsed/refractory follicular lymphoma • Completed four 28 - day cycles of CNTY - 101 at the 100 million cell dose (Dose Level 1 ), first two administered following lymphodepletion, while the most recent two were administered without lymphodepletion • All doses of CNTY - 101 with and without IL - 2 or LDC were well tolerated and demonstrated clinical benefit as defined as stable disease or better per Lugano 2014 criteria • Responses were associated with tumor shrinkage and an ongoing CR of a duration of 5 months since the first CNTY - 101 infusion • PK showed that CNTY - 101 cells were detected after each infusion with comparable kinetics , with a limited duration in circulation • No measurable CDC - inducing functional ADA detected in any samples by data cutoff (including the first three cycles) • T reatment was associated with changes in tumor microenvironment within 8 days post - infusion, augmentation of adaptive T cell responses, and tumor shrinkage *Data featured in abstract as of a July 10, 2023 cutoff date Additional data from Dose Level 1 patients, as well as preliminary data from Dose Level 2, to be featured at ASH Annual Meeting in December

20 ELiPSE - 1 translational approach MRD= minimum residual disease; CDC=complement - dependent cytotoxicity; ADCC= antibody - dependent cellular cytotoxicity; CAR=chimer ic antigen receptor; cf - DNA=cell free deoxyribonucleic acid Identify optimal dosing regimen Cellular Pharmacokinetics (PK) Assess impact of Allo - Evasion TM PK after redosing Humoral Cellular Functional anti - drug antibodies • CAR Neutralization • ADCC Competence • CDC Competence Evaluate mechanism of action PK in blood Persistence in the body Pharmacodynamics B cell kinetics MRD Adaptive immunity engagement iNK cf - DNA Key Methods Readouts iNK cells Immunogenicity

Discovery Programs

22 CNTY - 102: leveraging the γδ iT platform designed to deliver best - in - class potential HLA - II KO Illustrative construct γδ TCR/CD3 IL - 15/IL15RA HLA - E HLA - G HLA - I KO CD19/CD22 loop CAR Designed to address factors that limit durability of cell therapy in B - cell malignancies • γδ iT cells demonstrate high proliferation, persistence, trafficking leading to potentially sustained anti - tumor activity • Dual targeting designed to counter antigen escape relapse - a major limiting factor for durability of CD19 CAR T therapies • Armed with Allo - Evasion TM edits to enable repeat dosing to potentially deliver durable responses CNTY - 102 Allo - Evasion TM
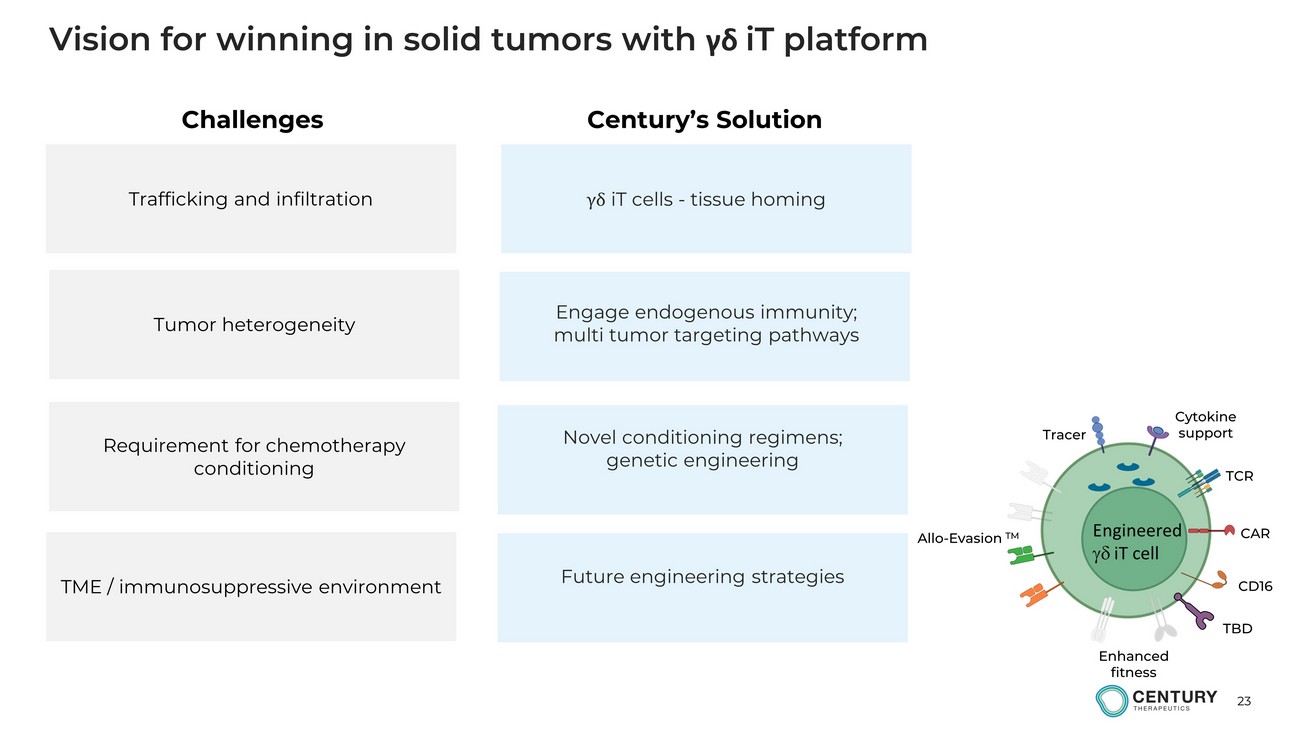
23 Vision for winning in solid tumors with γδ iT platform Trafficking and infiltration γδ iT cells - tissue homing TME / immunosuppressive environment Requirement for chemotherapy conditioning Tumor heterogeneity Novel conditioning regimens; g enetic engineering Challenges Century’s Solution Future engineering strategies Engage endogenous immunity; m ulti tumor targeting pathways CAR CD16 TCR Cytokine support Allo - Evasion TM Enhanced fitness Tracer TBD

24 iPSC - derived γδ T cells effective at tumor control as monotherapy and in combination with antibody Millar, et al, SITC 2022 γδ - EGFR - CAR - T cells demonstrate significant CAR killing of ovarian spheroids γδ CAR - T demonstrate additive efficacy in combination with trastuzumab Treatment % TGI Significance trastuzumab 0 P=0.9980 γδ - CAR - T 18 P=0.7073 γδ - CAR - T + trastuzumab 42 P=0.0358 TGI = Tumor Growth Inhibition

25 CNTY - 107: first in class Nectin - 4 targeted γδ iT cell therapy Leveraging the power of the γδ iT cell platform for solid tumors Nectin - 4 has been validated by ADC approaches • Opportunity to address multiple Nectin - 4 positive solid tumors • Potential indications include bladder, breast, pancreatic, non - small cell lung cancer, esophageal/gastric, head and neck, and/or ovarian cancers 1 GD iT allogeneic therapies provide potential to improve upon ADC toxicity profile and efficacy • Intrinsic homing of GD iT cells to tissues and solid malignancies • Multi - tumor killing modalities to tackle heterogeneity Tumor cell killing Allo - Evasion TM Cell Fitness CNTY - 107 Illustrative construct Nectin - 4 CAR CD16 Tracer TCR Cytokine support 1. Cancer Res . 2016 May 15;76(10):3003 - 13

26 Investment thesis Next generation platforms for iNK and gamma delta iT candidates Foundational investments in iPSC technology, genetic editing, and manufacturing Experienced team in R&D, immuno - oncology, manufacturing and commercialization Exemplified by execution of ongoing first - in - human Phase 1 ELiPSE - 1 trial Well capitalized with cash runway into 2026 $284.3M in cash, cash equivalents and investments at the end of 3Q23; operational efficiencies designed to enable delivery on key milestones, clinical data

Emerging leader in cell therapies for cancer Comprehensive iPSC cell platform For immune effector cells Technical Expertise Genetic and protein engineering, process development and immuno - oncology Foundation in Science Continuing investment in innovation drives R&D State - of - the - art GMP manufacturing facility Fully operational, enabling improved and faster product iteration Financial Strength Cash runway into 2026, Ended 3Q23 with cash, cash equivalents, and investments of $284.3M ~ 165 Employees including experienced leaders and entrepreneurs Emerging pipeline of candidates Product engine anticipated to deliver additional candidates and INDs in the coming years BMS Discovery Collaboration Initial focus on AML (CNTY - 104) and Multiple Myeloma (CNTY - 106)

Thank you.
v3.23.3
Cover
|
Nov. 09, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Nov. 09, 2023
|
| Entity File Number |
001-40498
|
| Entity Registrant Name |
Century Therapeutics, Inc.
|
| Entity Central Index Key |
0001850119
|
| Entity Tax Identification Number |
84-2040295
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
25
North 38th Street,
|
| Entity Address, Address Line Two |
11th Floor
|
| Entity Address, City or Town |
Philadelphia
|
| Entity Address, State or Province |
PA
|
| Entity Address, Postal Zip Code |
19104
|
| City Area Code |
267
|
| Local Phone Number |
817-5790
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
IPSC
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Century Therapeutics (NASDAQ:IPSC)
Historical Stock Chart
From Mar 2024 to Apr 2024
Century Therapeutics (NASDAQ:IPSC)
Historical Stock Chart
From Apr 2023 to Apr 2024