0000880242
BIOLARGO, INC.
false
--12-31
Q2
2023
0.00067
0.00067
50,000,000
50,000,000
0
0
0
0
0.00067
0.00067
550,000,000
550,000,000
287,220,661
278,462,706
39
77
35
10
27
10
17
10
10
10
10
11
10
31
10
15
0
0
0
0
0
0
0
0
0
6
18
0
9
6
5
5
154,000
4
1
10
178,000
4
0.28
0.28
0.28
0.35
0.35
6
5
6
5
5
3
161,000
51,571
1,352
66,000
5
5
0
135,000
5
4
10
3
00008802422023-01-012023-06-30
xbrli:shares
00008802422023-08-11
thunderdome:item
iso4217:USD
00008802422023-06-30
00008802422022-12-31
0000880242blgo:EntitiesExcludingPartiallyOwnedSubsidiaryMember2023-06-30
0000880242blgo:EntitiesExcludingPartiallyOwnedSubsidiaryMember2022-12-31
0000880242blgo:PartiallyOwnedSubsidiaryMember2023-06-30
0000880242blgo:PartiallyOwnedSubsidiaryMember2022-12-31
iso4217:USDxbrli:shares
0000880242us-gaap:ProductMember2023-04-012023-06-30
0000880242us-gaap:ProductMember2022-04-012022-06-30
0000880242us-gaap:ProductMember2023-01-012023-06-30
0000880242us-gaap:ProductMember2022-01-012022-06-30
0000880242us-gaap:ServiceMember2023-04-012023-06-30
0000880242us-gaap:ServiceMember2022-04-012022-06-30
0000880242us-gaap:ServiceMember2023-01-012023-06-30
0000880242us-gaap:ServiceMember2022-01-012022-06-30
00008802422023-04-012023-06-30
00008802422022-04-012022-06-30
00008802422022-01-012022-06-30
0000880242us-gaap:CommonStockMember2022-12-31
0000880242us-gaap:AdditionalPaidInCapitalMember2022-12-31
0000880242us-gaap:RetainedEarningsMember2022-12-31
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-31
0000880242us-gaap:NoncontrollingInterestMember2022-12-31
0000880242us-gaap:CommonStockMember2023-01-012023-03-31
0000880242us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-31
0000880242us-gaap:RetainedEarningsMember2023-01-012023-03-31
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-31
0000880242us-gaap:NoncontrollingInterestMember2023-01-012023-03-31
00008802422023-01-012023-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:CommonStockMember2023-01-012023-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:RetainedEarningsMember2023-01-012023-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:NoncontrollingInterestMember2023-01-012023-03-31
0000880242blgo:ClyraMedicalTechnologiesMember2023-01-012023-03-31
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberus-gaap:CommonStockMember2023-01-012023-03-31
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberus-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-31
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberus-gaap:RetainedEarningsMember2023-01-012023-03-31
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberus-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-31
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberus-gaap:NoncontrollingInterestMember2023-01-012023-03-31
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-01-012023-03-31
0000880242us-gaap:CommonStockMember2023-03-31
0000880242us-gaap:AdditionalPaidInCapitalMember2023-03-31
0000880242us-gaap:RetainedEarningsMember2023-03-31
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-31
0000880242us-gaap:NoncontrollingInterestMember2023-03-31
00008802422023-03-31
0000880242us-gaap:CommonStockMember2023-04-012023-06-30
0000880242us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-30
0000880242us-gaap:RetainedEarningsMember2023-04-012023-06-30
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-30
0000880242us-gaap:NoncontrollingInterestMember2023-04-012023-06-30
0000880242us-gaap:CommonStockMember2023-06-30
0000880242us-gaap:AdditionalPaidInCapitalMember2023-06-30
0000880242us-gaap:RetainedEarningsMember2023-06-30
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-30
0000880242us-gaap:NoncontrollingInterestMember2023-06-30
0000880242us-gaap:CommonStockMember2021-12-31
0000880242us-gaap:AdditionalPaidInCapitalMember2021-12-31
0000880242us-gaap:RetainedEarningsMember2021-12-31
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-31
0000880242us-gaap:NoncontrollingInterestMember2021-12-31
00008802422021-12-31
0000880242us-gaap:CommonStockMember2022-01-012022-03-31
0000880242us-gaap:AdditionalPaidInCapitalMember2022-01-012022-03-31
0000880242us-gaap:RetainedEarningsMember2022-01-012022-03-31
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-03-31
0000880242us-gaap:NoncontrollingInterestMember2022-01-012022-03-31
00008802422022-01-012022-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:CommonStockMember2022-01-012022-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:AdditionalPaidInCapitalMember2022-01-012022-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:RetainedEarningsMember2022-01-012022-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-03-31
0000880242blgo:ClyraMedicalTechnologiesMemberus-gaap:NoncontrollingInterestMember2022-01-012022-03-31
0000880242blgo:ClyraMedicalTechnologiesMember2022-01-012022-03-31
0000880242us-gaap:CommonStockMember2022-03-31
0000880242us-gaap:AdditionalPaidInCapitalMember2022-03-31
0000880242us-gaap:RetainedEarningsMember2022-03-31
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-31
0000880242us-gaap:NoncontrollingInterestMember2022-03-31
00008802422022-03-31
0000880242us-gaap:CommonStockMember2022-04-012022-06-30
0000880242us-gaap:AdditionalPaidInCapitalMember2022-04-012022-06-30
0000880242us-gaap:RetainedEarningsMember2022-04-012022-06-30
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012022-06-30
0000880242us-gaap:NoncontrollingInterestMember2022-04-012022-06-30
0000880242us-gaap:CommonStockMember2022-06-30
0000880242us-gaap:AdditionalPaidInCapitalMember2022-06-30
0000880242us-gaap:RetainedEarningsMember2022-06-30
0000880242us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-30
0000880242us-gaap:NoncontrollingInterestMember2022-06-30
00008802422022-06-30
0000880242blgo:EntitiesExcludingPartiallyOwnedSubsidiaryMember2023-01-012023-06-30
0000880242blgo:EntitiesExcludingPartiallyOwnedSubsidiaryMember2022-01-012022-06-30
0000880242blgo:PartiallyOwnedSubsidiaryMember2023-01-012023-06-30
0000880242blgo:PartiallyOwnedSubsidiaryMember2022-01-012022-06-30
0000880242blgo:BetiCommonStockMember2023-01-012023-06-30
0000880242blgo:BetiCommonStockMember2022-01-012022-06-30
0000880242blgo:ConversionOfIntercompanyReceivableIntoClyraSharesMember2023-01-012023-06-30
0000880242blgo:ConversionOfIntercompanyReceivableIntoClyraSharesMember2022-01-012022-06-30
0000880242blgo:ConversionOfClyraCommonStockToBiolargoCommonStockMember2023-01-012023-06-30
0000880242blgo:ConversionOfClyraCommonStockToBiolargoCommonStockMember2022-01-012022-06-30
xbrli:pure
0000880242blgo:BiolargoEngineeringScienceTechnologiesLLCMemberblgo:ApproximationMember2023-06-30
0000880242blgo:ClyraMedicalTechnologiesMember2023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-06-30
0000880242blgo:LincolnParkCapitalFundLLCMember2023-01-012023-06-30
0000880242blgo:The2020UnitOfferingMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalTechnologiesMember2023-01-012023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-01-012023-06-30
0000880242blgo:VehicleLoanMember2023-06-30
0000880242blgo:SBACARESActPaycheckProtectionProgramMember2023-06-30
0000880242blgo:EconomicInjuryDisasterLoanMember2023-06-30
0000880242blgo:ClyraMedicalTechnologiesMemberblgo:InventoryLineOfCreditMember2023-06-30
0000880242srt:ParentCompanyMember2023-06-30
0000880242srt:ParentCompanyMember2022-12-31
0000880242us-gaap:NoncontrollingInterestMember2023-06-30
0000880242us-gaap:NoncontrollingInterestMember2022-12-31
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerAMember2023-04-012023-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerAMember2022-04-012022-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerAMember2023-01-012023-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerAMember2022-01-012022-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerBMember2023-04-012023-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerBMember2022-04-012022-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerBMember2023-01-012023-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerBMember2022-01-012022-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerCMember2023-04-012023-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerCMember2022-04-012022-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerCMember2023-01-012023-06-30
0000880242us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerCMember2022-01-012022-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMember2023-01-012023-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:OneCustomerMember2023-01-012023-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMember2022-01-012022-12-31
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:ThreeCustomersMember2022-01-012022-12-31
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerAMember2023-01-012023-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerAMember2022-01-012022-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerBMember2023-01-012023-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerBMember2022-01-012022-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerDMember2023-01-012023-06-30
0000880242us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberblgo:CustomerDMember2022-01-012022-06-30
0000880242us-gaap:PatentsMember2021-01-22
0000880242blgo:OdinCoLtdMember2020-03-202020-03-20
0000880242blgo:TomorrowWaterMemberblgo:OdinCoLtdMember2020-03-202020-03-20
0000880242blgo:BktAndTomorrowWaterMemberblgo:OdinCoLtdMember2020-03-202020-03-20
0000880242blgo:OdinCoLtdMember2023-01-012023-06-30
0000880242blgo:OdinCoLtdMember2023-04-012023-06-30
0000880242blgo:OdinCoLtdMember2022-04-012022-06-30
0000880242blgo:OdinCoLtdMember2022-01-012022-06-30
0000880242blgo:NonPlanMembersrt:MinimumMember2023-01-012023-06-30
0000880242blgo:NonPlanMembersrt:MaximumMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MinimumMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MaximumMember2023-01-012023-06-30
0000880242blgo:NonPlanMembersrt:MinimumMember2022-01-012022-06-30
0000880242blgo:NonPlanMembersrt:MaximumMember2022-01-012022-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MinimumMember2022-01-012022-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MaximumMember2022-01-012022-06-30
0000880242blgo:NonPlanMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Member2023-01-012023-06-30
0000880242blgo:NonPlanMember2022-01-012022-06-30
0000880242blgo:EquityIncentivePlan2018Member2022-01-012022-06-30
utr:Y
utr:M
0000880242blgo:CanadianGovernmentGrantsMembersrt:MinimumMember2023-01-012023-06-30
0000880242blgo:CanadianGovernmentGrantsMembersrt:MaximumMember2023-01-012023-06-30
0000880242srt:MinimumMember2023-06-30
0000880242srt:MaximumMember2023-06-30
0000880242blgo:LincolnParkCapitalFundLLCMember2022-12-31
0000880242blgo:LincolnParkCapitalFundLLCMember2023-04-012023-06-30
0000880242blgo:LincolnParkCapitalFundLLCMember2022-04-012022-06-30
0000880242blgo:LincolnParkCapitalFundLLCMember2022-01-012022-06-30
0000880242blgo:The2020UnitOfferingMember2023-04-012023-06-30
0000880242blgo:WarrantsIssuedWith2020UnitOfferingMembersrt:MinimumMember2023-06-30
0000880242blgo:WarrantsIssuedWith2020UnitOfferingMembersrt:MaximumMember2023-06-30
0000880242blgo:PaycheckProtectionProgramCaresActMember2023-06-30
0000880242blgo:PaycheckProtectionProgramCaresActMember2022-12-31
0000880242blgo:VehicleLoanMember2023-06-30
0000880242blgo:VehicleLoanMember2022-12-31
0000880242blgo:ConvertibleNoteMaturingOnMarch12023Member2023-06-30
0000880242blgo:ConvertibleNoteMaturingOnMarch12023Member2022-12-31
0000880242blgo:EconomicInjuryDisasterLoanMember2022-12-31
0000880242blgo:VehicleLoanMember2023-02-07
0000880242blgo:VehicleLoanMember2023-02-072023-02-07
0000880242blgo:ConversionOfNoteIntoBetiCommonSharesMember2023-03-062023-03-06
0000880242blgo:WarrantIssuedWithConversionOfNoteMember2023-03-06
0000880242blgo:PaycheckProtectionProgramCaresActMemberblgo:ONMMember2022-02-072022-02-07
0000880242blgo:PaycheckProtectionProgramCaresActMemberblgo:ONMMember2023-06-30
0000880242blgo:PaycheckProtectionProgramCaresActMemberblgo:BELSTMember2022-05-122022-05-12
0000880242blgo:EconomicInjuryDisasterLoanMemberblgo:ONMMember2020-07-31
0000880242blgo:EconomicInjuryDisasterLoanMemberblgo:ONMMember2020-04-012020-07-31
0000880242srt:OfficerMember2023-04-012023-06-30
0000880242srt:OfficerMember2023-06-30
0000880242srt:OfficerMember2023-01-012023-03-31
0000880242srt:OfficerMember2023-03-31
0000880242srt:OfficerMember2022-01-012022-06-30
0000880242srt:OfficerMember2022-06-30
0000880242srt:OfficerMember2023-01-012023-06-30
0000880242blgo:ConsultantsMember2023-04-012023-06-30
0000880242blgo:ConsultantsMember2023-06-30
0000880242blgo:ConsultantsMember2023-03-312023-03-31
0000880242blgo:ConsultantsMember2023-03-31
0000880242blgo:ConsultantsMember2022-04-012022-06-30
0000880242blgo:ConsultantsMember2022-06-30
0000880242blgo:ConsultantsMember2022-03-312022-03-31
0000880242blgo:ConsultantsMember2022-03-31
0000880242us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-04-012023-06-30
0000880242us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-01-012023-06-30
0000880242us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-04-012022-06-30
0000880242us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-01-012022-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalTechnologiesMember2023-04-012023-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalTechnologiesMember2023-01-012023-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalTechnologiesMember2022-04-012022-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalTechnologiesMember2022-01-012022-06-30
0000880242blgo:EquityIncentivePlan2018Member2018-06-222018-06-22
0000880242blgo:EquityIncentivePlan2018Member2018-06-22
0000880242blgo:EquityIncentivePlan2018Member2023-06-30
0000880242blgo:EquityIncentivePlan2018Member2022-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:MinimumMember2022-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:MaximumMember2022-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:WeightedAverageMember2022-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:WeightedAverageMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MinimumMember2023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MaximumMember2023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:WeightedAverageMember2023-06-30
0000880242blgo:EquityIncentivePlan2018Member2021-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:MinimumMember2021-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:MaximumMember2021-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:WeightedAverageMember2021-12-31
0000880242blgo:EquityIncentivePlan2018Membersrt:WeightedAverageMember2022-01-012022-06-30
0000880242blgo:EquityIncentivePlan2018Member2022-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MinimumMember2022-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:MaximumMember2022-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:WeightedAverageMember2022-06-30
0000880242blgo:OfficerBoardOfDirectorsEmployeesAndAConsultantMember2023-01-012023-06-30
0000880242blgo:OfficerBoardOfDirectorsEmployeesAndAConsultantMember2023-01-012023-03-31
0000880242blgo:OfficerBoardOfDirectorsEmployeesAndAConsultantMember2023-04-012023-06-30
0000880242blgo:BoardOfDirectorsMember2023-01-012023-06-30
0000880242blgo:EmployeeRetentionProgramMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Memberblgo:EmployeesMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Memberblgo:ConsultantsMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:ChiefFinancialOfficerMember2023-01-012023-06-30
0000880242blgo:EquityIncentivePlan2018Membersrt:ChiefFinancialOfficerMember2022-03-222022-03-22
0000880242blgo:OfficersBoardMembersAndVendorsMember2022-01-012022-06-30
0000880242blgo:ChiefFinancialOfficerAndPresidentMember2022-01-012022-06-30
0000880242srt:ChiefFinancialOfficerMember2022-01-012022-06-30
0000880242blgo:BoardOfDirectorsMember2022-01-012022-06-30
0000880242srt:MinimumMemberblgo:BoardOfDirectorsMember2022-01-012022-06-30
0000880242srt:MaximumMemberblgo:BoardOfDirectorsMember2022-01-012022-06-30
0000880242blgo:EmployeeRetentionProgramMember2022-01-012022-06-30
0000880242blgo:EmployeeRetentionProgramMembersrt:MinimumMember2022-01-012022-06-30
0000880242blgo:EmployeeRetentionProgramMembersrt:MaximumMember2022-01-012022-06-30
0000880242blgo:ConsultantsMember2022-01-012022-06-30
0000880242blgo:The2007EquityIncentivePlanMember2017-09-072017-09-07
0000880242blgo:The2007EquityIncentivePlanMember2022-12-31
0000880242blgo:The2007EquityIncentivePlanMembersrt:MinimumMember2022-12-31
0000880242blgo:The2007EquityIncentivePlanMembersrt:MaximumMember2022-12-31
0000880242blgo:The2007EquityIncentivePlanMember2023-01-012023-06-30
0000880242blgo:The2007EquityIncentivePlanMember2023-06-30
0000880242blgo:The2007EquityIncentivePlanMembersrt:MinimumMember2023-06-30
0000880242blgo:The2007EquityIncentivePlanMembersrt:MaximumMember2023-06-30
0000880242blgo:The2007EquityIncentivePlanMember2021-12-31
0000880242blgo:The2007EquityIncentivePlanMembersrt:MinimumMember2021-12-31
0000880242blgo:The2007EquityIncentivePlanMembersrt:MaximumMember2021-12-31
0000880242blgo:The2007EquityIncentivePlanMember2022-01-012022-06-30
0000880242blgo:The2007EquityIncentivePlanMember2022-06-30
0000880242blgo:The2007EquityIncentivePlanMembersrt:MinimumMember2022-06-30
0000880242blgo:The2007EquityIncentivePlanMembersrt:MaximumMember2022-06-30
0000880242blgo:NonPlanMember2022-12-31
0000880242blgo:NonPlanMembersrt:MinimumMember2022-12-31
0000880242blgo:NonPlanMembersrt:MaximumMember2022-12-31
0000880242blgo:NonPlanMembersrt:WeightedAverageMember2022-12-31
0000880242blgo:NonPlanMembersrt:WeightedAverageMember2023-01-012023-06-30
0000880242blgo:NonPlanMember2023-06-30
0000880242blgo:NonPlanMembersrt:MinimumMember2023-06-30
0000880242blgo:NonPlanMembersrt:MaximumMember2023-06-30
0000880242blgo:NonPlanMembersrt:WeightedAverageMember2023-06-30
0000880242blgo:NonPlanMember2021-12-31
0000880242blgo:NonPlanMembersrt:MinimumMember2021-12-31
0000880242blgo:NonPlanMembersrt:MaximumMember2021-12-31
0000880242blgo:NonPlanMembersrt:WeightedAverageMember2021-12-31
0000880242blgo:NonPlanMembersrt:WeightedAverageMember2022-01-012022-06-30
0000880242blgo:NonPlanMember2022-06-30
0000880242blgo:NonPlanMembersrt:MinimumMember2022-06-30
0000880242blgo:NonPlanMembersrt:MaximumMember2022-06-30
0000880242blgo:NonPlanMembersrt:WeightedAverageMember2022-06-30
0000880242blgo:NonPlanMemberblgo:VendorsMember2023-01-012023-06-30
0000880242blgo:NonPlanMembersrt:MinimumMemberblgo:VendorsMember2023-01-012023-06-30
0000880242blgo:NonPlanMembersrt:MaximumMemberblgo:VendorsMember2023-01-012023-06-30
0000880242blgo:NonPlanMemberblgo:VendorsMember2022-01-012022-06-30
0000880242srt:MinimumMember2022-12-31
0000880242srt:MaximumMember2022-12-31
0000880242srt:WeightedAverageMember2022-12-31
0000880242srt:MinimumMember2023-01-012023-06-30
0000880242srt:MaximumMember2023-01-012023-06-30
0000880242srt:WeightedAverageMember2023-01-012023-06-30
0000880242srt:WeightedAverageMember2023-06-30
0000880242srt:MinimumMember2021-12-31
0000880242srt:MaximumMember2021-12-31
0000880242srt:WeightedAverageMember2021-12-31
0000880242srt:MinimumMember2022-01-012022-06-30
0000880242srt:MaximumMember2022-01-012022-06-30
0000880242srt:WeightedAverageMember2022-01-012022-06-30
0000880242srt:MinimumMember2022-06-30
0000880242srt:MaximumMember2022-06-30
0000880242srt:WeightedAverageMember2022-06-30
0000880242blgo:SixMonthWarrantsInConnectionWithThe2020UnitOfferingMember2023-06-30
0000880242blgo:FiveyearWarrantsInConnectionWithThe2020UnitOfferingMember2023-06-30
0000880242blgo:SixMonthWarrantsInConnectionWithThe2020UnitOfferingMember2022-06-30
0000880242blgo:SixMonthWarrantsInConnectionWithThe2020UnitOfferingMembersrt:MinimumMember2022-06-30
0000880242blgo:SixMonthWarrantsInConnectionWithThe2020UnitOfferingMembersrt:MaximumMember2022-06-30
0000880242blgo:FiveyearWarrantsInConnectionWithThe2020UnitOfferingMember2022-06-30
0000880242blgo:FiveyearWarrantsInConnectionWithThe2020UnitOfferingMembersrt:MinimumMember2022-06-30
0000880242blgo:FiveyearWarrantsInConnectionWithThe2020UnitOfferingMembersrt:MaximumMember2022-06-30
0000880242blgo:NotePayableMaturingMarch82023Member2023-03-06
0000880242blgo:WarrantsIssuedInConnectionWithConversionOfInterestOnNotePayableMaturingMarch82023Member2023-03-06
0000880242us-gaap:MeasurementInputRiskFreeInterestRateMembersrt:MinimumMember2023-06-30
0000880242us-gaap:MeasurementInputRiskFreeInterestRateMembersrt:MaximumMember2023-06-30
0000880242us-gaap:MeasurementInputRiskFreeInterestRateMembersrt:MinimumMember2022-12-31
0000880242us-gaap:MeasurementInputRiskFreeInterestRateMember2022-12-31
0000880242us-gaap:MeasurementInputPriceVolatilityMembersrt:MinimumMember2023-06-30
0000880242us-gaap:MeasurementInputPriceVolatilityMembersrt:MaximumMember2023-06-30
0000880242us-gaap:MeasurementInputPriceVolatilityMember2022-12-31
0000880242us-gaap:MeasurementInputExpectedTermMembersrt:MinimumMember2023-06-30
0000880242us-gaap:MeasurementInputExpectedTermMembersrt:MaximumMember2023-06-30
0000880242us-gaap:MeasurementInputExpectedTermMembersrt:MinimumMember2022-12-31
0000880242us-gaap:CorporateNonSegmentMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:OdorNoMoreMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BLESTMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoWaterMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEnergyTechnologiesIncBETIMember2023-06-30
0000880242srt:ConsolidationEliminationsMember2023-06-30
0000880242us-gaap:CorporateNonSegmentMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:OdorNoMoreMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:BLESTMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoWaterMember2022-12-31
0000880242srt:ConsolidationEliminationsMember2022-12-31
0000880242blgo:ClyraMedicalMemberus-gaap:NotesPayableOtherPayablesMember2022-04-08
0000880242us-gaap:RevolvingCreditFacilityMemberblgo:InventoryLineOfCreditMemberblgo:ClyraMedicalMemberblgo:VernalBayCapitalGroupLLCMember2020-06-30
0000880242us-gaap:RevolvingCreditFacilityMemberblgo:InventoryLineOfCreditMemberblgo:ClyraMedicalMemberblgo:VernalBayCapitalGroupLLCMember2020-06-302020-06-30
0000880242us-gaap:RevolvingCreditFacilityMemberblgo:InventoryLineOfCreditMemberblgo:ClyraMedicalMemberblgo:VernalBayCapitalGroupLLCMember2022-12-31
0000880242us-gaap:RevolvingCreditFacilityMemberblgo:InventoryLineOfCreditMemberblgo:ClyraMedicalMemberblgo:VernalBayCapitalGroupLLCMember2022-01-012022-12-31
0000880242blgo:ConversionOfClyraMedicalStockIntoBiolargoCommonStockMemberblgo:VernalBayCapitalGroupLLCMember2023-01-012023-03-31
0000880242us-gaap:RevolvingCreditFacilityMemberblgo:InventoryLineOfCreditMemberblgo:ClyraMedicalMemberblgo:VernalBayCapitalGroupLLCMember2023-06-30
0000880242blgo:ClyraMedicalMember2023-06-30
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMember2023-06-30
0000880242blgo:ClyraMedicalMember2022-12-31
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMember2022-12-31
0000880242us-gaap:CommonStockMemberblgo:ClyraMedicalMember2022-12-31
0000880242us-gaap:CommonStockMemberblgo:ClyraMedicalMember2023-06-30
0000880242blgo:PreferredStockSeriesAMemberblgo:ClyraMedicalMember2022-12-31
0000880242blgo:PreferredStockSeriesAMemberblgo:ClyraMedicalMember2023-06-30
0000880242blgo:SharesIssuedForDebtOwedToBiolargoMemberblgo:ClyraMedicalMember2022-03-022022-03-02
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMember2023-04-012023-06-30
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMember2023-01-012023-06-30
0000880242blgo:WarrantsIssuedInConjunctionWithTheSaleOfSeriesAPreferredStockMemberblgo:ClyraMedicalMember2022-12-20
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMember2022-12-20
0000880242blgo:WarrantsIssuedInConjunctionWithTheSaleOfSeriesAPreferredStockMemberblgo:ClyraMedicalMember2023-06-30
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMember2022-12-202022-12-20
0000880242blgo:ClyraMedicalMember2022-12-20
0000880242blgo:ClyraMedicalMembersrt:MinimumMember2022-12-31
0000880242blgo:ClyraMedicalMembersrt:MaximumMember2022-12-31
0000880242blgo:ClyraMedicalMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalMembersrt:MinimumMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalMembersrt:MaximumMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalMembersrt:MinimumMember2023-06-30
0000880242blgo:ClyraMedicalMembersrt:MaximumMember2023-06-30
0000880242blgo:ClyraMedicalMember2021-12-31
0000880242blgo:ClyraMedicalMember2022-01-012022-06-30
0000880242blgo:ClyraMedicalMember2022-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalMemberblgo:VendorsAndEmployeesMember2023-04-012023-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalMemberblgo:VendorsAndEmployeesMember2023-01-012023-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalMemberblgo:VendorsAndEmployeesMember2022-04-012022-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalMemberblgo:VendorsAndEmployeesMember2022-01-012022-06-30
0000880242blgo:TheRemainingOptionsMemberblgo:ClyraMedicalMemberblgo:EmployeesAndConsultantsMember2023-01-012023-06-30
0000880242us-gaap:EmployeeStockOptionMemberblgo:ClyraMedicalMemberblgo:EmployeesAndConsultantsMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalMemberblgo:NonPlanMembersrt:MinimumMemberblgo:VendorsAndEmployeesMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalMembersrt:MaximumMemberblgo:VendorsAndEmployeesMember2023-01-012023-06-30
0000880242blgo:ClyraMedicalMemberblgo:VendorsAndEmployeesMember2022-01-012022-12-31
0000880242blgo:ClyraMedicalMemberblgo:VendorsAndEmployeesMember2023-01-012023-06-30
0000880242blgo:BiolargoEngineeringScienceTechnologiesLLCMember2017-09-30
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2017-09-012017-09-30
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2017-09-30
0000880242blgo:NonqualifiedStockOptionMemberblgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2017-09-012017-09-30
0000880242blgo:NonqualifiedStockOptionMemberblgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2017-09-012019-09-30
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2021-12-31
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMemberus-gaap:ShareBasedCompensationAwardTrancheOneMember2021-01-012021-12-31
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMemberus-gaap:ShareBasedCompensationAwardTrancheTwoMember2021-01-012021-12-31
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2021-01-012021-12-31
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2022-12-31
0000880242blgo:SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember2022-01-012022-12-31
0000880242blgo:BiolargoEngineeringScienceTechnologiesLLCMember2023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2017-09-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-04-012023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-01-012023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberblgo:BiolargoMember2023-01-012023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-06-30
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMember2023-06-30
0000880242us-gaap:CorporateNonSegmentMember2023-04-012023-06-30
0000880242us-gaap:CorporateNonSegmentMember2022-04-012022-06-30
0000880242us-gaap:CorporateNonSegmentMember2023-01-012023-06-30
0000880242us-gaap:CorporateNonSegmentMember2022-01-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:OdorNoMoreMember2023-04-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:OdorNoMoreMember2022-04-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:OdorNoMoreMember2023-01-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:OdorNoMoreMember2022-01-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEngineeringScienceTechnologiesLLCMember2023-04-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEngineeringScienceTechnologiesLLCMember2022-04-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEngineeringScienceTechnologiesLLCMember2023-01-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEngineeringScienceTechnologiesLLCMember2022-01-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEnergyTechnologiesIncBETIMember2023-04-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEnergyTechnologiesIncBETIMember2022-04-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEnergyTechnologiesIncBETIMember2023-01-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoEnergyTechnologiesIncBETIMember2022-01-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoWaterMember2023-04-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoWaterMember2022-04-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoWaterMember2023-01-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:BiolargoWaterMember2022-01-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:ClyraSegmentMember2023-04-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:ClyraSegmentMember2022-04-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:ClyraSegmentMember2023-01-012023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:ClyraSegmentMember2022-01-012022-06-30
0000880242srt:ConsolidationEliminationsMember2023-04-012023-06-30
0000880242srt:ConsolidationEliminationsMember2022-04-012022-06-30
0000880242srt:ConsolidationEliminationsMember2023-01-012023-06-30
0000880242srt:ConsolidationEliminationsMember2022-01-012022-06-30
0000880242us-gaap:IntersegmentEliminationMember2023-04-012023-06-30
0000880242us-gaap:IntersegmentEliminationMember2022-04-012022-06-30
0000880242us-gaap:IntersegmentEliminationMember2023-01-012023-06-30
0000880242us-gaap:IntersegmentEliminationMember2022-01-012022-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:ClyraMedicalMember2023-06-30
0000880242us-gaap:CorporateNonSegmentMemberblgo:InvestmentInSouthKoreanJointVentureMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:OdorNoMoreMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:ClyraMedicalMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:BLESTMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:BiolargoWaterMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:BiolargoEnergyTechnologiesIncBETIMember2023-06-30
0000880242srt:ConsolidationEliminationsMemberblgo:InvestmentInSouthKoreanJointVentureMember2023-06-30
0000880242blgo:InvestmentInSouthKoreanJointVentureMember2023-06-30
0000880242us-gaap:OperatingSegmentsMemberblgo:ClyraMedicalMember2022-12-31
0000880242us-gaap:CorporateNonSegmentMemberblgo:InvestmentInSouthKoreanJointVentureMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:OdorNoMoreMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:ClyraMedicalMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:BLESTMember2022-12-31
0000880242us-gaap:OperatingSegmentsMemberblgo:InvestmentInSouthKoreanJointVentureMemberblgo:BiolargoWaterMember2022-12-31
0000880242srt:ConsolidationEliminationsMemberblgo:InvestmentInSouthKoreanJointVentureMember2022-12-31
0000880242blgo:InvestmentInSouthKoreanJointVentureMember2022-12-31
0000880242blgo:WestminsterCaliforniaFacilityLeaseMember2023-06-30
0000880242blgo:OakRidgeTennesseeFacilityLeaseMember2022-09-30
0000880242blgo:LincolnParkCapitalFundLLCMemberus-gaap:SubsequentEventMember2023-07-012023-08-10
0000880242blgo:ClyraMedicalMemberus-gaap:SeriesAPreferredStockMemberus-gaap:SubsequentEventMember2023-07-012023-08-10
0000880242us-gaap:WarrantMemberblgo:ClyraMedicalMemberus-gaap:SubsequentEventMember2023-08-10
0000880242blgo:BiolargoEnergyTechnologiesIncBETIMemberus-gaap:SubsequentEventMember2023-07-012023-08-10
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2023.
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 000-19709
BIOLARGO, INC.
(Exact name of registrant as specified in its charter)
| Delaware | | 65-0159115 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
14921 Chestnut St.
Westminster, CA 92683
(Address of principal executive offices)
(888) 400-2863
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading symbol(s) | Name of each exchange on which registered |
| Common stock | BLGO | OTC Markets (OTCQB) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| | |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| | |
| | Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of shares of the Registrant’s Common Stock outstanding as of August 11, 2023 was 288,073,533 shares.
BIOLARGO, INC.
FORM 10-Q
INDEX
PART I
PART II
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
AS OF JUNE 30, 2023 AND DECEMBER 31, 2022
(in thousands, except for share and per share data)
| | | JUNE 30, | | | DECEMBER 31, | |
| | | 2023 (unaudited) | | | 2022 | |
| | | | | | | | | |
| Assets | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 3,575 | | | $ | 1,851 | |
| Accounts receivable, net of allowance | | | 1,037 | | | | 1,064 | |
| Inventories, net of allowance | | | 127 | | | | 120 | |
| Prepaid expenses and other current assets | | | 108 | | | | 118 | |
| Total current assets | | | 4,847 | | | | 3,153 | |
| | | | | | | | | |
| Property and equipment, net of depreciation | | | 542 | | | | 287 | |
| Other non-current assets | | | 69 | | | | 124 | |
| Investment in South Korean joint venture | | | 21 | | | | 33 | |
| Right of use, operating lease, net of amortization | | | 807 | | | | 867 | |
| Clyra Medical prepaid marketing | | | 394 | | | | 394 | |
| Total assets | | $ | 6,680 | | | $ | 4,858 | |
| | | | | | | | | |
| Liabilities and stockholders’ equity | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 720 | | | $ | 940 | |
| Clyra Medical accounts payable and accrued expenses | | | 288 | | | | 238 | |
| Debt obligations | | | 66 | | | | 100 | |
| Deferred revenue | | | 10 | | | | 17 | |
| Lease liability | | | 97 | | | | 97 | |
| Customer deposits | | | 113 | | | | 184 | |
| Total current liabilities | | | 1,294 | | | | 1,576 | |
| | | | | | | | | |
| Long-term liabilities: | | | | | | | | |
| Debt obligations, net of current | | | 299 | | | | 237 | |
| Lease liability, net of current | | | 721 | | | | 773 | |
| Clyra Medical debt obligations | | | 243 | | | | 261 | |
| Total long-term liabilities | | | 1,263 | | | | 1,271 | |
| Total liabilities | | | 2,557 | | | | 2,847 | |
| | | | | | | | | |
| COMMITMENTS AND CONTINGENCIES (Note 12) | | | | | | | | |
| | | | | | | | | |
| STOCKHOLDERS’ EQUITY: | | | | | | | | |
| Preferred Series A, $0.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding, at June 30, 2023 and December 31, 2022 | | | — | | | | — | |
| Common stock, $0.00067 Par Value, 550,000,000 Shares Authorized, 287,220,661 and 278,462,706 Shares Issued, at June 30, 2023 and December 31, 2022, respectively | | | 192 | | | | 186 | |
| Additional paid-in capital | | | 152,507 | | | | 148,435 | |
| Accumulated deficit | | | (145,169 | ) | | | (143,594 | ) |
| Accumulated other comprehensive loss | | | (162 | ) | | | (149 | ) |
| Total BioLargo Inc. and subsidiaries stockholders’ equity | | | 7,368 | | | | 4,878 | |
| Non-controlling interest | | | (3,245 | ) | | | (2,867 | ) |
| Total stockholders’ equity | | | 4,123 | | | | 2,011 | |
| Total liabilities and stockholders’ equity | | $ | 6,680 | | | $ | 4,858 | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2023 AND 2022
(in thousands, except for share and per share data)
(unaudited)
| | | THREE MONTHS | | | SIX MONTHS | |
| | | JUNE 30, 2023 | | | JUNE 30, 2022 | | | JUNE 30, 2023 | | | JUNE 30, 2022 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Revenues | | | | | | | | | | | | | | | | |
| Product revenue | | $ | 1,315 | | | $ | 707 | | | $ | 4,864 | | | $ | 1,317 | |
| Service revenue | | | 131 | | | | 616 | | | | 324 | | | | 970 | |
| Total revenue | | | 1,446 | | | | 1,323 | | | | 5,188 | | | | 2,287 | |
| | | | | | | | | | | | | | | | | |
| Cost of revenue | | | | | | | | | | | | | | | | |
| Cost of goods sold | | | (567 | ) | | | (364 | ) | | | (2,364 | ) | | | (659 | ) |
| Cost of service | | | (60 | ) | | | (336 | ) | | | (194 | ) | | | (487 | ) |
| Gross profit | | | 819 | | | | 623 | | | | 2,630 | | | | 1,141 | |
| | | | | | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 1,791 | | | | 1,589 | | | | 3,515 | | | | 3,425 | |
| Research and development | | | 587 | | | | 355 | | | | 1,152 | | | | 747 | |
| Operating loss: | | | (1,559 | ) | | | (1,321 | ) | | | (2,037 | ) | | | (3,031 | ) |
| Other (expense) income: | | | | | | | | | | | | | | | | |
| Interest expense | | | (12 | ) | | | (15 | ) | | | (60 | ) | | | (28 | ) |
| PPP loan forgiveness | | | — | | | | — | | | | — | | | | 174 | |
| Tax credit reversal | | | (55 | ) | | | — | | | | (55 | ) | | | — | |
| Grant income | | | — | | | | 3 | | | | 32 | | | | 8 | |
| Total other expense: | | | (67 | ) | | | (12 | ) | | | (83 | ) | | | 154 | |
| Net loss | | | (1,626 | ) | | | (1,333 | ) | | | (2,120 | ) | | | (2,877 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to noncontrolling interest | | | (298 | ) | | | (105 | ) | | | (545 | ) | | | 3 | |
| Net loss attributable to common shareholders | | $ | (1,328 | ) | | $ | (1,228 | ) | | $ | (1,575 | ) | | $ | (2,880 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss per share attributable to common shareholders: | | | | | | | | | | | | | | | | |
| Loss per share attributable to shareholders – basic and diluted | | $ | (0.01 | ) | | $ | (0.01 | ) | | $ | (0.01 | ) | | $ | (0.01 | ) |
| Weighted average number of common shares outstanding: | | | 284,944,366 | | | | 265,856,970 | | | | 282,839,515 | | | | 263,345,148 | |
| | | | | | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | | | | | |
| Net loss | | $ | (1,626 | ) | | $ | (1,333 | ) | | $ | (2,120 | ) | | $ | (2,877 | ) |
| Foreign currency translation | | | (7 | ) | | | (3 | ) | | | (13 | ) | | | (11 | ) |
| Comprehensive loss | | | (1,633 | ) | | | (1,336 | ) | | | (2,133 | ) | | | (2,888 | ) |
| Comprehensive loss attributable to noncontrolling interest | | | (298 | ) | | | (105 | ) | | | (545 | ) | | | 3 | |
| Comprehensive loss attributable to common stockholders | | $ | (1,335 | ) | | $ | (1,231 | ) | | $ | (1,588 | ) | | $ | (2,891 | ) |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2023 AND 2022
(in thousands, except for share and per share data)
(unaudited)
| |
|
Common stock |
|
|
Additional paid-in |
|
|
Accumulated |
|
|
Accumulated other comprehensive |
|
|
Non- controlling |
|
|
Total stockholders’ |
|
| |
|
Shares |
|
|
Amount |
|
|
capital |
|
|
deficit |
|
|
Loss |
|
|
interest |
|
|
equity (deficit) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2022 |
|
|
278,462,706 |
|
|
$ |
186 |
|
|
$ |
148,435 |
|
|
$ |
(143,594 |
) |
|
$ |
(149 |
) |
|
$ |
(2,867 |
) |
|
$ |
2,011 |
|
| Sale of stock for cash |
|
|
4,201,402 |
|
|
|
3 |
|
|
|
797 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
800 |
|
| Issuance of common stock for services |
|
|
930,490 |
|
|
|
1 |
|
|
|
206 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
207 |
|
| Issuance of common stock in exchange for Clyra shares |
|
|
527,983 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Stock option compensation expense |
|
|
— |
|
|
|
— |
|
|
|
195 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
195 |
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
|
|
— |
|
|
|
— |
|
|
|
61 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
61 |
|
| Warrant issued for interest |
|
|
— |
|
|
|
— |
|
|
|
30 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
30 |
|
| Clyra – sales of Series A Preferred Stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
225 |
|
|
|
225 |
|
| Clyra – Series A Preferred Stock – dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(27 |
) |
|
|
(27 |
) |
| Biolargo Energy Technology Inc. (BETI) offering |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
550 |
|
|
|
550 |
|
| Noncontrolling interest allocation |
|
|
— |
|
|
|
— |
|
|
|
467 |
|
|
|
— |
|
|
|
— |
|
|
|
(467 |
) |
|
|
— |
|
| Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(247 |
) |
|
|
— |
|
|
|
(247 |
) |
|
|
(494 |
) |
| Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6 |
) |
|
|
— |
|
|
|
(6 |
) |
| Balance, March 31, 2023 |
|
|
284,122,581 |
|
|
$ |
190 |
|
|
$ |
150,191 |
|
|
$ |
(143,841 |
) |
|
$ |
(155 |
) |
|
$ |
(2,833 |
) |
|
$ |
3,552 |
|
| Sale of stock for cash |
|
|
2,677,169 |
|
|
|
2 |
|
|
|
492 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
494 |
|
| Issuance of common stock for services |
|
|
420,911 |
|
|
|
— |
|
|
|
75 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
75 |
|
| Stock option compensation expense |
|
|
— |
|
|
|
— |
|
|
|
222 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
222 |
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
|
|
— |
|
|
|
— |
|
|
|
66 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
66 |
|
| Clyra – sales of Series A Preferred Stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,062 |
|
|
|
1,062 |
|
| Clyra – Series A Preferred Stock – dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(45 |
) |
|
|
(45 |
) |
| Biolargo Energy Technology Inc. (BETI) offering |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
330 |
|
|
|
330 |
|
| Noncontrolling interest allocation |
|
|
— |
|
|
|
— |
|
|
|
1,461 |
|
|
|
— |
|
|
|
— |
|
|
|
(1,461 |
) |
|
|
— |
|
| Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,328 |
) |
|
|
— |
|
|
|
(298 |
) |
|
|
(1,626 |
) |
| Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7 |
) |
|
|
— |
|
|
|
(7 |
) |
| Balance, June 30, 2023 |
|
|
287,220,661 |
|
|
$ |
192 |
|
|
$ |
152,507 |
|
|
$ |
(145,169 |
) |
|
$ |
(162 |
) |
|
$ |
(3,245 |
) |
|
$ |
4,123 |
|
| |
|
Common stock |
|
|
Additional paid-in |
|
|
Accumulated |
|
|
Accumulated other comprehensive |
|
|
Non- controlling |
|
|
Total stockholders’ |
|
| |
|
Shares |
|
|
Amount |
|
|
capital |
|
|
deficit |
|
|
Loss |
|
|
interest |
|
|
equity (deficit) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2021 |
|
|
255,893,726 |
|
|
$ |
171 |
|
|
$ |
143,718 |
|
|
$ |
(139,121 |
) |
|
$ |
(115 |
) |
|
$ |
(3,720 |
) |
|
$ |
933 |
|
| Sale of stock for cash |
|
|
6,703,789 |
|
|
|
4 |
|
|
|
1,198 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,202 |
|
| Issuance of common stock for services |
|
|
86,752 |
|
|
|
— |
|
|
|
17 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
17 |
|
| Stock option compensation expense |
|
|
— |
|
|
|
— |
|
|
|
660 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
660 |
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
|
|
— |
|
|
|
— |
|
|
|
141 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
141 |
|
| Noncontrolling interest allocation |
|
|
— |
|
|
|
— |
|
|
|
(528 |
) |
|
|
— |
|
|
|
— |
|
|
|
528 |
|
|
|
— |
|
| Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,652 |
) |
|
|
— |
|
|
|
108 |
|
|
|
(1,544 |
) |
| Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(8 |
) |
|
|
— |
|
|
|
(8 |
) |
| Balance, March 31, 2022 |
|
|
262,684,267 |
|
|
$ |
175 |
|
|
$ |
145,206 |
|
|
$ |
(140,773 |
) |
|
$ |
(123 |
) |
|
$ |
(3,084 |
) |
|
$ |
1,401 |
|
| Sale of stock for cash |
|
|
5,011,570 |
|
|
|
4 |
|
|
|
944 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
948 |
|
| Issuance of common stock for services |
|
|
340,891 |
|
|
|
— |
|
|
|
59 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
59 |
|
| Stock option compensation expense |
|
|
— |
|
|
|
— |
|
|
|
234 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
234 |
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
|
|
— |
|
|
|
— |
|
|
|
82 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
82 |
|
| Noncontrolling interest allocation |
|
|
— |
|
|
|
— |
|
|
|
(103 |
) |
|
|
— |
|
|
|
— |
|
|
|
103 |
|
|
|
— |
|
| Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,228 |
) |
|
|
— |
|
|
|
(105 |
) |
|
|
(1,333 |
) |
| Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3 |
) |
|
|
— |
|
|
|
(3 |
) |
| Balance, June 30, 2022 |
|
|
268,036,728 |
|
|
$ |
179 |
|
|
$ |
146,422 |
|
|
$ |
(142,001 |
) |
|
$ |
(126 |
) |
|
$ |
(3,086 |
) |
|
$ |
1,388 |
|
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED JUNE 30, 2023 AND 2022
(in thousands, except for share and per share data)
(unaudited)
| | | JUNE 30, 2023 | | | JUNE 30, 2022 | |
| Cash flows from operating activities | | | | | | | | |
| Net loss | | $ | (2,120 | ) | | $ | (2,877 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Stock option compensation expense | | | 544 | | | | 1,117 | |
| Common stock issued for services | | | 282 | | | | 76 | |
| Bad debt expense | | | 46 | | | | — | |
| Excess and obsolete inventory | | | 54 | | | | — | |
| Amortization of right-of-use operating lease assets | | | 60 | | | | — | |
| Interest expense related to amortization of the discount on note payable | | | 3 | | | | 8 | |
| Fair value of warrant issued for interest | | | 30 | | | | — | |
| PPP loan forgiveness | | | — | | | | (174 | ) |
| Loss on investment in South Korean joint venture | | | 12 | | | | 15 | |
| Depreciation expense | | | 48 | | | | 6 | |
| Changes in assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (19 | ) | | | (66 | ) |
| Inventories | | | (63 | ) | | | (15 | ) |
| Prepaid expenses and other assets | | | 66 | | | | 4 | |
| Accounts payable and accrued expenses | | | (391 | ) | | | 105 | |
| Clyra accounts payable and accrued expenses | | | 51 | | | | (32 | ) |
| Deferred revenue | | | (7 | ) | | | (89 | ) |
| Lease liability, net | | | (52 | ) | | | — | |
| Customer deposits | | | (71 | ) | | | 58 | |
| Net cash used in operating activities | | | (1,527 | ) | | | (1,864 | ) |
| Cash flows from investing activities | | | | | | | | |
| Equipment purchases | | | (127 | ) | | | (101 | ) |
| Net cash used in investing activities | | | (127 | ) | | | (101 | ) |
| Cash flows from financing activities | | | | | | | | |
| Proceeds from sale of common stock | | | 1,294 | | | | 2,150 | |
| Proceeds from sale of BETI common stock | | | 880 | | | | — | |
| Repayment of note payable and vehicle loan | | | (52 | ) | | | — | |
| Repayment by Clyra on inventory line of credit | | | (18 | ) | | | (10 | ) |
| Proceeds from sale of Clyra Medical preferred stock | | | 1,287 | | | | — | |
| Proceeds from Clyra Medical convertible note | | | — | | | | 100 | |
| Net cash provided by financing activities | | | 3,391 | | | | 2,240 | |
| Net effect of foreign currency translation | | | (13 | ) | | | (11 | ) |
| Net change in cash | | | 1,724 | | | | 264 | |
| Cash at beginning of year | | | 1,851 | | | | 962 | |
| Cash at end of period | | $ | 3,575 | | | $ | 1,226 | |
| Supplemental disclosures of cash flow information | | | | | | | | |
| Cash paid during the year for: | | | | | | | | |
| Interest | | $ | 27 | | | $ | 7 | |
| Income taxes | | $ | 5 | | | $ | — | |
| Short-term lease payments not included in lease liability | | $ | 24 | | | $ | 78 | |
| Non-cash investing and financing activities | | | | | | | | |
| Equipment added via vehicle loan | | $ | 80 | | | $ | — | |
| Leasehold improvements included in accounts payable | | $ | 102 | | | $ | — | |
| Allocation of noncontrolling interest | | $ | 1,927 | | | $ | 631 | |
| Conversion of Clyra common stock to BioLargo common stock | | $ | 100 | | | $ | — | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Business and Organization
Description of Business
BioLargo, Inc. (“BioLargo”, or the “Company”) invents, develops, and commercializes innovative platform technologies to solve challenging environmental problems like PFAS contamination (per- and polyfluoroalkyl substances), advanced water and wastewater treatment, industrial odor control, air quality control, infection control, and myriad environmental remediation challenges. Our business strategy is straightforward: we invent or acquire technologies that we believe have the potential to be disruptive in large commercial markets; we develop and validate these technologies to advance and promote their commercial success as we leverage our considerable scientific, engineering, and entrepreneurial talent; we then monetize these technical assets through a variety of business structures that may include licensure, joint venture, sale, spin off, or by deploying direct to market strategies.
Organization
We are a Delaware corporation formed in 1991. We have six wholly-owned subsidiaries: BioLargo Life Technologies, Inc., organized under the laws of the State of California in 2006; ONM Environmental, Inc., organized under the laws of the State of California in 2009; BioLargo Equipment and Technologies, Inc., organized under the laws of the State of California in 2022; BioLargo Water, Inc. (“Water”), organized under the laws of Canada in 2014; BioLargo Equipment and Solutions Technologies, Inc., organized under the laws of the State of California in 2022; and BioLargo Development Corp., organized under the laws of the State of California in 2016. Additionally, we own 82% (see Note 9) of BioLargo Engineering Science and Technologies, LLC (“BLEST”), organized under the laws of the State of Tennessee in 2017, 56% of Clyra Medical Technologies, Inc. (“Clyra” or “Clyra Medical”), organized under the laws of the State of California in 2012, and 96% of BioLargo Energy Technologies, Inc. (“BETI”) organized under the laws of the State of California in 2022. We consolidate the financial statements of our partially owned subsidiaries (see Note 2, subheading “Principles of Consolidation,” and Note 8).
Liquidity / Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the six months ended June 30, 2023, we generated revenues of $5,188,000 through our business segments (see Note 11), had a net loss of $2,120,000, used $1,527,000 cash in operations, and at June 30, 2023, we had working capital of $3,553,000, and current assets of $4,847,000.
During the six months ended June 30, 2023, we (i) sold $299,000 of our common stock to Lincoln Park Capital Fund, LLC (“Lincoln Park”) (see Note 3), (ii) sold $995,000 of our common stock and warrants to accredited investors (see Notes 3 and 6), (iii) sold $1,287,000 of Clyra Medical Series A Preferred Stock (see Note 8), and (iv) sold $880,000 of BETI common stock (see Note 10). Subsequent to June 30, 2023, we continued these financing activities (see Note 13). As of June 30, 2023, our cash and cash equivalents totaled $3,575,000. Our total liabilities included a $75,000 vehicle loan, $140,000 due in U.S. Small Business Administration (SBA) loans issued pursuant to the Paycheck Protection Program (see Note 4), $150,000 due to the SBA issued pursuant to the Economic Injury Disaster program (see Note 4), and $243,000 owed by Clyra Medical due in 2024 (see Note 8). We have been, and anticipate that we will continue to be, limited in terms of our capital resources, and expect to continue to need further investment capital to fund operations. Such activities have continued subsequent to June 30, 2023.
If we are unable to rely on our current arrangement with Lincoln Park to fund our working capital requirements, we will have to rely on other forms of financing, and there is no assurance that we will be able to do so, or if we do so, it will be on favorable terms.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The foregoing factors raise substantial doubt about our ability to continue as a going concern, unless we are able to continue to raise funds through stock sales to Lincoln Park or other private financings, and in the long term, our ability to attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and partially owned subsidiaries BETI, BLEST and Clyra Medical. All intercompany accounts and transactions have been eliminated.
Foreign Currency
The Company has designated the functional currency of BioLargo Water, Inc., our Canadian subsidiary, to be the Canadian dollar. Therefore, translation gains and losses resulting from differences in exchange rates are recorded in accumulated other comprehensive income.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when acquired to be cash equivalents. Substantially all cash equivalents are held in short-term money market accounts at one of the largest financial institutions in the United States, Bank of America. From time to time, our cash account balances are greater than the Federal Deposit Insurance Corporation insurance limit of $250,000 per owner per bank, and during such times, we are exposed to credit loss for amounts in excess of insured limits in the event of non-performance by the financial institution. We do not anticipate non-performance by our financial institution.
As of June 30, 2023, and December 31, 2022, our cash balances were made up of the following (in thousands):
| | | June 30, 2023 | | | December 31, 2022 | |
| BioLargo, Inc. and subsidiaries | | $ | 2,762 | | | $ | 1,681 | |
| Clyra Medical Technologies, Inc. | | | 813 | | | | 170 | |
| Total | | $ | 3,575 | | | $ | 1,851 | |
Accounts Receivable
In June 2016, the FASB issued ASU 2016-13, which sets out the principles for the recognition of measurement of credit losses on financial instruments, including trade receivables. The standard eliminates the probable initial recognition threshold and requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. The new standard was effective for the Company beginning January 1, 2023 and primarily impacted trade accounts receivable.
Accounts receivable are customer obligations that are unconditional. Accounts receivable are presented net of an allowance for doubtful accounts for expected credit losses, which represents an estimate of amounts that may not be collectible. The Company performs ongoing credit evaluations of its customers and, if necessary, provides an allowance for doubtful accounts and expected credit losses. A provision to the allowances for doubtful accounts for expected credit losses is recorded based on factors including the length of time the receivables are past due, the current business environment, and the Company’s historical experience. Provisions to the allowances for doubtful accounts for expected credit losses are recorded to general and administrative expenses. The Company writes off accounts receivable against the allowance when it determines a balance is uncollectible and no longer actively pursues collection of the receivable. The Company does not have any off-balance-sheet credit exposure related to customers. As of June 30, 2023, and December 31, 2022, the allowance for doubtful accounts for expected credit losses was $58,000 and $12,000.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Credit Concentration
We have a limited number of customers that account for significant portions of our revenue. During the three and six months ended June 30, 2023, and 2022, the following customers accounted for more than 10% of consolidated revenues:
| | | Three Months | | | Six Months | |
| | | June 30, 2023 | | | June 30, 2022 | | | June 30, 2023 | | | June 30, 2022 | |
| | | | | | | | | | | | | | | | | |
| Customer A | | | 55 | % | | | 39 | % | | | 77 | % | | | 35 | % |
| Customer B | | <10 | % | | | 27 | % | | <10 | % | | | 17 | % |
| Customer C | | <10 | % | | <10 | % | | <10 | % | | | 10 | % |
At June 30, 2023, one customer accounted for more than 10% of consolidated accounts receivable, and at December 31, 2022, three customers accounted for more than 10% of consolidated accounts receivable:
| | | June 30, 2023 | | | December 31, 2022 | |
| Customer A | | | 49 | % | | | 11 | % |
| Customer B | | <10 | % | | | 31 | % |
| Customer D | | <10 | % | | | 15 | % |
Inventory
Inventories are stated at the lower of cost or net realizable value using the average cost method. The allowance for obsolete inventory as of June 30, 2023, and December 31, 2022, was $212,000 and $158,000. Inventories consisted of (in thousands):
| | | June 30, 2023 | | | December 31, 2022 | |
| Raw material | | $ | 83 | | | $ | 46 | |
| Finished goods | | | 44 | | | | 74 | |
| Total | | $ | 127 | | | $ | 120 | |
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Other Non-Current Assets
Other non-current assets consisted of (i) security deposits related to our business offices, (ii) three patents acquired on October 22, 2021, for $34,000, and (iii) tax credit receivables from the Canadian government related to a research and development credit from our Canadian subsidiary for which we’ve applied for and received in prior periods.
| | | June 30, 2023 | | | December 31, 2022 | |
| Patents | | $ | 34 | | | $ | 34 | |
| Security deposits | | | 35 | | | | 35 | |
| Tax credit receivable | | | -- | | | | 55 | |
| Total | | $ | 69 | | | $ | 124 | |
Equity Method of Accounting
On March 20, 2020, we invested $100,000 into a South Korean entity (Odin Co. Ltd., “Odin”) pursuant to a Joint Venture agreement we had entered into with BKT Co. Ltd. and its U.S. based subsidiary, Tomorrow Water. We received a 40% non-dilutive equity interest, and BKT and Tomorrow Water each received 30% equity interests for an aggregate $150,000 investment.
We account for our investment in the joint venture under the equity method of accounting. We have determined that while we have significant influence over the joint venture through our technology license and our position on the Board of Directors, we do not control the joint venture or are otherwise involved in managing the entity and we own less than a majority of the equity. Therefore, we record the asset on our consolidated balance sheet and record an increase or decrease of the recorded balance by our percentage ownership of the profits or losses in the joint venture. The joint venture has incurred a loss since inception and our 40% ownership share reduced our investment interest. For the three and six months ended June 30, 2023, the reduction of our investment interest totaled $6,000 and $12,000, respectively, and for the same periods in 2022, reduced our investment interest $8,000 and $15,000, respectively.
Impairment
Long-lived and definite lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows from the use of the asset and its eventual disposition is less than the carrying amount of the asset, then an impairment loss is recognized. The impairment loss is measured based on the fair value of the asset. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. There were no impairment losses related to intangible assets during the three or six months ended June 30, 2023 or 2022.
Earnings (Loss) Per Share
We report basic and diluted earnings (loss) per share (“EPS”) for common and common share equivalents. Basic EPS is computed by dividing reported earnings by the weighted average shares outstanding. Diluted EPS is computed by adding to the weighted average shares the dilutive effect if convertible notes payable, stock options and warrants were exercised into common stock. For the three and six months ended June 30, 2023, and 2022, the denominator in the diluted EPS computation is the same as the denominator for basic EPS due to the Company’s net loss which creates an anti-dilutive effect of the convertible notes payable, warrants and stock options.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and revenues and expenses during the period reported. Actual results could differ from those estimates. Estimates are used when accounting for stock-based transactions, debt transactions, derivative liabilities, allowance for bad debt, asset depreciation and amortization, impairment expense, among others.
The methods, estimates and judgments we use in applying these most critical accounting policies have a significant impact on the results of our financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Share-Based Compensation Expense
We recognize compensation expense for stock option awards on a straight-line basis over the applicable service period of the award, which is the vesting period. Fair value is determined on the grant date. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes Option Pricing Model.
For stock and stock options issued to consultants and other non-employees for services, the Company measures and records an expense as of the earlier of the date at which either: a commitment for performance by the non-employee has been reached or the non-employee’s performance is complete. The equity instruments are measured at the current fair value, and for stock options, the instruments are measured at fair value using the Black Scholes option model.
The following methodology and assumptions were used to calculate share-based compensation for the six months ended June 30, 2023, and 2022:
| | | 2023 | | | 2022 | |
| | | Non Plan | | | 2018 Plan | | | Non Plan | | | 2018 Plan | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | 3.48 | – | 3.58% | | | 2.32 | – | 3.83% | | | 2.32 | – | 2.98% | |
| Expected volatility | | 113 | - | 114% | | | 113 | - | 114% | | | 114 | – | 117% | | | 116 | – | 117% | |
| Expected dividend yield | | — | | | — | | | — | | | — | |
| Forfeiture rate | | — | | | — | | | — | | | — | |
| Life in years | | 10 | | | 10 | | | 10 | | | 10 | |
Expected price volatility is the measure by which our stock price is expected to fluctuate during the expected term of an option. The expected volatility is derived from the historical daily change in the market price of our common stock, as we believe that historical volatility is the best indicator of future volatility.
The risk-free interest rate used in the Black-Scholes calculation is based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. We have never paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future.
Warrants
Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method. The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”). If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note. Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. The warrant relative fair values are also recorded as a discount to the convertible promissory notes.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Non-Cash Transactions
We determine the value assigned to each intangible we acquire, and/or services or products received for non-cash consideration of our common stock based on the market price of our common stock issued as consideration, at the date of the agreement of each transaction or when the service is rendered or product is received.
Revenue Recognition
We account for revenue in accordance with ASC 606, “Revenue from Contacts with Customers”. The guidance focuses on the core principle for revenue recognition, which is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, the guidance provides that an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
The Company’s products are sold through a contract with the customer and a written purchase order, in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product, and each product has separate pricing. Revenue is recognized at a point in time when the goods are shipped if the agreement is FOB manufacturer, and when goods are delivered if FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order.
Service contracts are performed through a written contract, which specifies the performance obligations and the rate at which the services will be billed, typically by time and materials. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed, or, for services related to product installations, at the completion of the installation. A few contracts have called for milestone or fixed cost payments, where we invoice an agreed-to amount per month for the life of the contract. In these instances, completed work, billed hourly, is recognized as revenue. If the billing amount is greater or lesser than the completed work, a receivable or payable is created. These accounts are adjusted upon additional billings as the work is completed. To date, there have been no discounts or other financing terms for the contracts.
The Company has outstanding contract liability obligations of $10,000 as of June 30, 2023, recorded as deferred revenue. We recognized $7,000 in revenue and reduced the deferred revenue balance by the same amount as performance obligations were satisfied in the six months ended June 30, 2023. The outstanding balance will be recognized over the remaining life of the contracts. Our Canadian subsidiary had a customer deposit outstanding at June 30, 2023, totaling $113,000, that was awarded as part of a grant for a particular project that has been delayed.
As we generate revenues from royalties or license fees from our intellectual property, a licensee will pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. We have entered into a licensing agreement for the CupriDyne Clean product, and we recognize royalty and license fees on a quarterly basis as the product is sold through to third parties and reported to us.
Government Grants
We have been awarded multiple research grants from the private and public Canadian research programs. The income we receive directly from grants is recorded as other income. We have been awarded over 80 grants since our first in 2015. Some of the funds from these grants are given directly to third parties (such as the University of Alberta or a third-party research scientist) to support research on our technology. The grants have terms generally ranging between six and eighteen months and support a majority, but not all, of the related research budget costs. This cooperative research allows us to utilize (i) a depth of resources and talent to accomplish highly skilled work, (ii) financial aid to support research and development costs, (iii) independent and credible validation of our technical claims.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The grants typically provide for (i) recurring monthly amounts, (ii) reimbursement of costs for research talent for which we invoice to request payment, and (iii) ancillary cost reimbursement for research talent travel related costs. All awarded grants have specific requirements on how the money is spent, typically to employ researchers. None of the funds may be used for general administrative expenses or overhead in the United States. These grants have substantially increased our level of research and development activities in Canada. We continue to apply for Canadian government and agency grants to fund research and development activities. Not all of our grant applications have been awarded, and no assurance can be made that any pending grant application, or any future grant applications, will be awarded.
Income Taxes
The asset and liability approach is used to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of asset and liabilities. Deferred tax assets and liabilities are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
We account for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation, and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by generally accepted accounting principles (“GAAP”). Under GAAP, the tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized. Management believes there are no unrecognized tax benefits or uncertain tax positions as of June 30, 2023, and December 31, 2022.
The Company assessed its earnings history, trends and estimates of future earnings and determined that the deferred tax asset could not be realized as of June 30, 2023, and December 31, 2022. Accordingly, a 100% valuation allowance was recorded against the net deferred tax asset.
The Company recognizes interest and penalties on income taxes as a component of income tax expense, should such an expense be realized.
Fair Value of Financial Instruments
Management believes the carrying amounts of the Company’s financial instruments as of June 30, 2023, and December 31, 2022 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, line of credit, and other assets and liabilities. The carrying amount of debt instruments are believed to approximate fair value as the stated interest rates are reflective of the prevailing market rates.
Tax Credits
Our research and development activities in Canada may entitle our Canadian subsidiary to claim benefits under the “Scientific Research and Experimental Development Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses of all sizes and in all sectors to conduct research and development in Canada. Benefits under the program include credits to taxable income. If our Canadian subsidiary does not have taxable income in a reporting period, we instead receive a tax refund from the Canadian Revenue Authority. Those refunds are classified in Other Income on our Consolidated Statement of Operations and Comprehensive Loss.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Leases
At inception of a lease contract, we assess whether the contract is, or contains, a lease. Our assessment is based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether we obtain the right to substantially all the economic benefit from the use of the asset throughout the period of the contract, and (3) whether we have the right to direct the use of the asset during such time period. At inception of a lease, we allocate the consideration in the contract to each lease component based on its relative stand-alone price to determine the lease payments. Leases are classified as either finance leases or operating leases. A lease must be classified as a finance lease if any of the following criteria are met: the lease transfers ownership of the asset by the end of the lease term, the lease contains an option to purchase the asset that is reasonably certain to be exercised, the lease term is for a major part of the remaining useful life of the asset or the present value of the lease payments equals or exceeds substantially all of the fair value of the asset. A lease is classified as an operating lease if it does not meet any of these criteria. We have no leases classified as finance leases. As of June 30, 2023, the weighted average remaining lease term for our operating leases was nine years. The weighted average discount rate for our operating leases was 18%. For all leases at the lease commencement date, a right-of-use asset and a lease liability are recognized. The right-of-use asset represents the right to use the leased asset for the lease term. The lease liability represents the present value of the lease payments under the lease. The right-of-use asset is initially measured at cost, which primarily comprises the initial amount of the lease liability, plus any initial direct costs incurred, consisting mainly of brokerage commissions, less any lease incentives received. All right-of-use assets are reviewed for impairment. The lease liability is initially measured at the present value of the lease payments, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, management estimates the incremental borrowing rate, which currently is estimated to be 18%. Lease payments included in the measurement of the lease liability comprise the following: the fixed noncancelable lease payments, payments for optional renewal periods where it is reasonably certain the renewal period will be exercised, and payments for early termination options unless it is reasonably certain the lease will not be terminated early. Lease components are included in the measurement of the initial lease liability. Additional payments based on a change in our portion of the operating expenses, including real estate taxes and insurance, are recorded as a period expense when incurred. Lease modifications result in remeasurement of the lease liability. Lease expense for operating leases consists of the lease payments plus any initial direct costs, primarily brokerage commissions, and is recognized on a straight-line basis over the lease term. We have elected not to recognize right-of-use assets and lease liabilities for short-term leases that have a term of 12 months or less. The effect of short-term leases on our right-of-use asset and lease liability was not material.
As of June 30, 2023, the right-of-use assets totaled $807,000 and the lease liability totaled $818,000 on our balance sheet related to our operating leases.
Property and Equipment
Property and equipment includes machinery and leasehold improvements and is stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, which range from 3 - 5 years or the remaining lease term. Newly built leaseholds, additions, renewals, and betterments that significantly extend the life of the asset are capitalized. The Company is currently constructing a facility and purchasing equipment for producing sodium sulfur batteries. Expenditures for repairs and maintenance are charged to expense as incurred. For assets sold or otherwise disposed of, the cost and related accumulated depreciation and amortization are removed from the accounts, and any related gain or loss is reflected in income for the period.
Recent Accounting Pronouncements
Currently there are no recently released pronouncements that are considered applicable to the Company’s financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 3. Sale of Stock for Cash
Lincoln Park Financing
On December 13, 2022, we entered into a stock purchase agreement (the “2022 LPC Purchase Agreement”) with Lincoln Park, pursuant to which Lincoln Park agreed to purchase from us at our request up to an aggregate of $10,000,000 of our common stock (subject to certain limitations) from time to time over a period of three years. The agreement allows us, at our sole discretion, to direct Lincoln Park to purchase shares of our common stock, subject to limitations in both volume and dollar amount. The purchase price of the shares that may be sold to Lincoln Park under the agreement is the lower of (i) the lowest sale price on the date of purchase, or (ii) the average of the three lowest closing prices in the prior 12 business days. There are no restrictions on future financings, rights of first refusal, participation rights, penalties, or liquidated damages other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the agreement. Concurrently with the 2022 LPC Purchase Agreement, we entered into a Registration Rights Agreement, pursuant to which we filed a registration statement on Form S-1 with the SEC on December 23, 2022. This registration statement was declared effective on January 19, 2023.
During the three and six months ended June 30, 2023, we sold 1,098,221 and 1,643,623 shares of our common stock to Lincoln Park, and received $194,000 and $299,000, respectively, in gross and net proceeds.
During the three and six months ended June 30, 2022, pursuant to a prior (similar) arrangement, we sold 406,140 and 1,912,961 shares of our common stock to Lincoln Park, and received $72,000 and $418,000, respectively, in gross and net proceeds.
Unit Offerings
During the three and six months ended June 30, 2023, we sold 1,578,948 and 5,234,948 shares of our common stock and received $300,000 and $995,000 in gross and net proceeds from accredited investors. In addition to the shares, we issued each investor a six-month and a five-year warrant to purchase additional shares. (See Note 6, “Warrants Issued in Unit Offering”.)
Note 4. Debt Obligations
The following table summarizes our debt obligations outstanding as of June 30, 2023, and December 31, 2022 (in thousands). The table does not include debt obligations of our partially owned subsidiary Clyra Medical (see Note 8, “Debt Obligations of Clyra Medical”).
| | | June 30, 2023 | | |
December 31,
2022 | |
| Current portion of debt: | | | | | | | | |
| SBA Paycheck Protection Program loan | | $ | 43 | | | $ | 43 | |
| Vehicle loan, current portion | | | 13 | | | | — | |
| Convertible note payable, matures March 1, 2023 | | | — | | | | 50 | |
| SBA EIDL Loan, matures July 2053, current portion | | | 10 | | | | 10 | |
| Debt discount, net of amortization | | | — | | | | (3 | ) |
| Total current portion of debt | | $ | 66 | | | $ | 100 | |
| | | | | | | | | |
| Long-term debt: | | | | | | | | |
| SBA Paycheck Protection Program loans, matures May 2025 | | $ | 97 | | | $ | 97 | |
| Vehicle loan, matures March 2029 | | | 62 | | | | — | |
| SBA EIDL Loan, matures July 2050 | | | 140 | | | | 140 | |
| Total long-term debt, net of current | | $ | 299 | | | $ | 237 | |
| | | | | | | | | |
| Total | | $ | 365 | | | $ | 337 | |
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the three and six months ended June 30, 2023, we recorded $12,000 and $60,000 of interest expense related to the amortization of discounts on convertible notes payable and coupon interest from our convertible notes and lines of credit.
For the three and six months ended June 30, 2022, we recorded $15,000 and $28,000 of interest expense related to the amortization of discounts on convertible notes payable and coupon interest from our convertible notes and lines of credit.
Vehicle loan
On February 7, 2023, we entered a loan agreement with Bank of America for the purchase of a vehicle used in operations totaling $80,000, at 5.29% annual interest which matures March 7, 2029. The loan agreement requires monthly payments of $1,000.
Convertible note payable, matures March 1, 2023
On March 6, 2023, we entered into an agreement with the holder of a $50,000 note to convert that note into common stock of BETI (see note 10). As payment for interest, a warrant to purchase 200,000 shares of BioLargo common stock at $0.21 was issued to the investor, expiring five years from the grant date. (See Note 6).
SBA Program Loans
On February 7, 2022, we received notice that the SBA had forgiven $174,000 of ONM Environmental's $217,000 Paycheck Protection Program (PPP) loan. As of June 30, 2023, the outstanding balance on this loan totals $43,000. The partial forgiveness decision has been appealed, and during such time, loan payments are deferred.
On May 12, 2022, we received notice that the SBA had denied the forgiveness application of BLEST’s $97,000 PPP loan. We have appealed that decision. During the period upon which a forgiveness decision is on appeal, loan payments are deferred. The maturity date of the BLEST PPP loan was officially extended on our request to May 2025.
In July 2020, ONM Environmental received an Economic Injury Disaster Loan from the SBA in the amount of $150,000. The note has a 3.75% annual interest rate, requires monthly payments of $731, and matures July 2050.
Note 5. Share-Based Compensation
Issuance of Common Stock in exchange for Services
Payment of Officer Salaries
On June 30, 2023, an officer agreed to convert an aggregate $12,000 of accrued and unpaid salary into 68,541 shares of our common stock at $0.18 per share. On March 31, 2023, an officer agreed to convert an aggregate $6,000 of accrued and unpaid salary into 30,747 shares of our common stock at $0.20 per share.
On June 30, 2022, we issued 263,895 shares of our common stock at $0.18 per share in lieu of $47,000 of accrued and unpaid salary to our officers.
Shares issued to Officers are unvested at the date of grant and subject to a lock-up agreement restricting vesting and sale until the earlier of (i) the consummation of a sale (in a single transaction or in a series of related transactions) of BioLargo by means of a sale of (a) a majority of the then outstanding common stock of BioLargo (whether by merger, consolidation, sale or transfer of common stock, reorganization, recapitalization or otherwise) or (b) all or substantially all of the assets of BioLargo; and (ii) the successful commercialization of BioLargo’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Officer and resulting in Officer’s termination.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Payment of Consultant and Vendor Fees
On June 30, 2023, we issued 352,370 shares of our common stock at $0.18 per share in lieu of $63,000 of accrued and unpaid obligations to consultants and vendors. On March 31, 2023, we issued 899,743 shares of our common stock at $0.20 per share in lieu of $201,000 of accrued and unpaid obligations to consultants and vendors.
On June 30, 2022, we issued 76,996 shares of our common stock at $0.18 per share in lieu of $12,000 of accrued and unpaid obligations to consultants and vendors. On March 31, 2022, we issued 86,752 shares of our common stock at $0.23 per share in lieu of $17,000 of accrued and unpaid obligations to consultants and vendors.
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Stock Option Expense
During the three and six months ended June 30, 2023, we recorded an aggregate $288,000 and $544,000, and during the three and six months ended June 30, 2022, we recorded an aggregate $316,000 and $1,117,000, in selling general and administrative expense related to the issuance of stock options. We issued options through our 2018 Equity Incentive Plan, and outside of this plan. Included in these totals is option expense related to issuances by our subsidiary, Clyra Medical, totaling $66,000 and $127,000 in the three and six months ended June 30, 2023, and $82,000 and $223,000 in the three and six months ended June 30, 2022. (See Note 8.)
2018 Equity Incentive Plan
On June 22, 2018, our stockholders adopted the BioLargo 2018 Equity Incentive Plan (“2018 Plan”) as a means of providing our directors, key employees, and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years. It is set to expire on its terms on June 22, 2028. Our Board of Director’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The plan authorizes the following types of awards: (i) incentive and non-qualified stock options, (ii) restricted stock awards, (iii) stock bonus awards, (iv) stock appreciation rights, (v) restricted stock units, and (vi) performance awards. The total number of shares reserved and available for awards pursuant to this Plan as of the date of adoption of this 2018 Plan by the Board is 40 million shares. The number of shares available to be issued under the 2018 Plan increases automatically each January 1st by the lesser of (a) 2 million shares, or (b) such number of shares determined by our Board. As of June 30, 2023, 50,000,000 shares are authorized under the plan.
Activity for our stock options under the 2018 Plan during the six months ended June 30, 2023, and 2022, is as follows:
| | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | Average | | | Aggregate | |
| | | Options | | | Exercise | | | Price per | | | intrinsic | |
| | | Outstanding | | | Price per share | | | share | | | Value(1) | |
| Balance, December 31, 2022 | | | 28,484,549 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| Granted | | | 2,636,712 | | | $0.18 | – | 0.20 | | | $ | 0.19 | | | | | |
| Balance, June 30, 2023 | | | 31,121,261 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| Unvested | | | (4,121,892 | ) | | $0.12 | – | 0.32 | | | $ | 0.19 | | | | | |
| Vested, June 30, 2023 | | | 26,999,369 | | | 0.12 | – | 0.43 | | | $ | 0.19 | | | $ | 402,000 | |
| Balance, December 31, 2021 | | | 23,186,142 | | | $0.16 | – | 0.43 | | | $ | 0.19 | | | | | |
| Granted | | | 3,782,923 | | | $0.18 | – | 0.24 | | | $ | 0.22 | | | | | |
| Balance, June 30, 2022 | | | 26,969,065 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The options granted to purchase 2,636,712 shares during the six months ended June 30, 2023 with an aggregate fair value of $474,000 were issued to an officer, board of directors, employees and a consultant. The exercise price for all options were issued on their respective grant date of March 31, 2023 ($0.20 per share) and June 30, 2023 ($0.18 per share): (i) we issued options to purchase 864,290 shares of our common stock to members of our board of directors for services performed, in lieu of cash; the fair value of these options totaled $154,000; (ii) we issued options to purchase 1,137,301 shares of our common stock to employees as part of an employee retention plan; the fair value of employee retention plan options totaled $203,000 and will vest quarterly over four years as long as they are retained as employees; (iii) we issued options to purchase 335,121 shares of our common stock to consultants in lieu of cash and for expiring options at $0.20 per share totaling $61,000, and (iv) we issued 300,000 options to our Chief Financial Officer with a fair value of $56,000 (see “Chief Financial Officer Contract Extension” immediately below). All stock option expense is recorded on our consolidated statement of operations as selling, general and administrative expense.
Chief Financial Officer Contract Extension
On March 21, 2023, we and our Chief Financial Officer Charles K. Dargan, II formally agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times), pursuant to which Mr. Dargan has been and continues to serve as the Company’s Chief Financial Officer. The Engagement Extension Agreement dated as of March 21, 2023 (the “Engagement Extension Agreement”) provides for an additional one-year term to expire January 31, 2024 (the “Extended Term”), at which time Mr. Dargan will continue to serve as CFO, unless and until either party terminates the agreement.
As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 25,000 shares of the Company’s common stock for each month during the Extended Term (thus, an option to purchase 300,000 shares reflecting an extended term of 12 months). The Option vests over the period of the Extended Term, with 25,000 shares having vested as of March 21, 2023, and the remaining shares to vest 25,000 shares monthly beginning March 31, 2023, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.20 per share, the closing price of BioLargo’s common stock on the March 21, 2023, grant date, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan.
The Option is Mr. Dargan’s sole compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
The options granted to purchase 3,782,923 shares during the six months ended June 30, 2022 were issued to officers, board of directors, employees and consultants: (i) we issued options to purchase 251,551 shares of our common stock at an exercise price on the respective grant date of $0.23 per share to our CFO and President to replace options that had expired and we issued an option to purchase 300,000 shares of our common stock at an exercise price on the respective grant date of $0.24 per share to our CFO (details below); (ii) we issued options to purchase 884,356 shares of our common stock at an exercise price on the respective grant date ranging between $0.18 – $0.23 per share to members of our board of directors for services performed, in lieu of cash; the fair value of these options totaled $178,000; (iii) we issued options to purchase 1,764,025 shares of our common stock to employees as part of an employee retention plan at an exercise price on the respective date ranging between $0.18 – $0.23 per share; the fair value of employee retention plan options totaled $360,000 and will vest quarterly over four years as long as they are retained as employees; and (iv) we issued options to purchase 582,991 shares of our common stock to consultants in lieu of cash for expiring options and per agreement totaling $127,000. All stock option expense is recorded on our consolidated statement of operations as selling, general and administrative expense.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2007 Equity Incentive Plan
On September 7, 2007, and as amended April 29, 2011, the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Plan”) was adopted as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years, which expired on September 7, 2017. The Board’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. As of September 2017, the Plan was closed to further stock option grants.
Activity for our stock options under the 2007 Plan for the six months ended June 30, 2023, and 2022 is as follows:
| | | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | | Average | | | Aggregate | |
| | | Options | | | Exercise | | | Price per | | | intrinsic | |
| | | Outstanding | | | price per share | | | share | | | Value(1) | |
| Balance, December 31, 2022 | | | 1,904,085 | | | | $0.28 | – | 0.69 | | | $ | 0.56 | | | | | |
| Expired | | | (40,000 | ) | | | 0.28 | | | | 0.28 | | | | | |
| Balance, June 30, 2023 | | | 1,864,085 | | | | $0.28 | – | 0.69 | | | $ | 0.56 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 2,879,246 | | | | $0.23 | – | 0.94 | | | $ | 0.49 | | | | | |
| Expired | | | (300,000 | ) | | | 0.35 | | | | 0.35 | | | | | |
| Balance, June 30, 2022 | | | 2,579,246 | | | | $0.23 | – | 0.94 | | | $ | 0.49 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
Non-Plan Options
Activity of our non-plan stock options issued for the six months ended June 30, 2023 and 2022 is as follows:
| | | | | | | | | | | | | Weighted | | | | | |
| | | Non-plan | | | | | | | | | average | | | Aggregate | |
| | | Options | | | Exercise | | | price per | | | intrinsic | |
| | | outstanding | | | price per share | | | share | | | value(1) | |
| Balance, December 31, 2022 | | | 19,023,829 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Granted | | | 60,040 | | | | 0.18 | – | 0.20 | | | | 0.20 | | | | | |
| Balance, June 30, 2023 | | | 19,083,869 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Unvested | | | (507,500 | ) | | | 0.45 | | | | 0.45 | | | | | |
| Vested, June 30, 2023 | | | 18,576,369 | | | | $0.12 | – | 0.83 | | | $ | 0.38 | | | $ | 46,000 | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 20,119,207 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Granted | | | 39,130 | | | | 0.23 | | | | 0.23 | | | | | |
| Balance, June 30, 2022 | | | 20,158,337 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
During the six months ended June 30, 2023, we issued options to purchase an aggregate 60,040 shares of our common stock at $0.18 and $0.20 per share to vendors for fees for services. The fair value of the options issued totaled an aggregate $11,000 and is recorded in our selling, general and administrative expense.
During the six months ended June 30, 2022, we issued options to purchase an aggregate 39,130 shares of our common stock at $0.23 per share to vendors for fees for services. The fair value of the options issued totaled an aggregate $8,000 and is recorded in our selling, general and administrative expense.
Note 6. Warrants
We have certain warrants outstanding to purchase our common stock, at various prices, as described in the following table:
| | | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | | average | | | Aggregate | |
| | | Warrants | | | Exercise | | | price per | | | intrinsic | |
| | | outstanding | | | price per share | | | share | | | value(1) | |
| Balance, December 31, 2022 | | | 49,023,398 | | | | $0.13 | – | 1.00 | | | $ | 0.26 | | | | | |
| Granted | | | 10,669,896 | | | | 0.21 | – | 0.29 | | | | 0.25 | | | | | |
| Expired | | | (5,173,209 | ) | | | 0.19 | – | 0.35 | | | | 0.21 | | | | | |
| Balance, June 30, 2023 | | | 54,520,085 | | | | $0.13 | – | 1.00 | | | $ | 0.26 | | | $ | 47,000 | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 36,765,502 | | | | $0.16 | – | 1.00 | | | $ | 0.27 | | | | | |
| Granted | | | 19,604,796 | | | | 0.20 | – | 0.29 | | | | 0.24 | | | | | |
| Expired | | | (4,016,754 | ) | | | 0.19 | – | 0.48 | | | | 0.35 | | | | | |
| Balance, June 30, 2022 | | | 52,353,544 | | | | $0.14 | – | 1.00 | | | $ | 0.25 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
Warrants issued in Unit Offerings
During the six months ended June 30, 2023, pursuant to our Unit Offerings (see Note 3), we issued six-month stock purchase warrants to purchase an aggregate 5,234,948 shares of our common stock at $0.23 per share, and five-year stock purchase warrants to purchase an aggregate 5,234,948 shares of our common stock at $0.29 per share. The fair value of these warrants totaled $913,000.
During the six months ended June 30, 2022, pursuant to our Unit Offering (see Note 3), we issued six-month stock purchase warrants to purchase an aggregate 9,802,398 shares of our common stock at $0.20 - $0.23 per share, and five-year stock purchase warrants to purchase an aggregate 9,802,398 shares of our common stock at $0.25 - $0.29 per share. The fair value of these warrants totaled $2,134,000.
Warrant issued in conjunction with amendment to note payable
On March 6, 2023, we entered into an agreement with the holder of a $50,000 note (see Note 4, “Convertible note payable, matures March 1, 2023”) to convert that note into common stock of BETI. As payment for interest, a warrant to purchase 200,000 shares of BioLargo common stock at $0.21 was issued to the investor, expiring five years from the grant date. The fair value of this warrant totaled $30,000 and was recorded as interest expense on our statement of operations.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Fair Value – Interest Expense
To determine interest expense related to our outstanding warrants issued in conjunction with debt offerings, the fair value of each award grant is estimated on the date of grant using the Black-Scholes option pricing model and the relative fair values are amortized over the life of the warrant. For the determination of expense of warrants issued for services, extinguishment of debt and settlement management also uses the option-pricing model. The principal assumptions we used in applying this model were as follows:
| | | 2023 | | | 2022 | |
| Risk free interest rate | | 3.88 | – | 4.27% | | | 3.69 | – | 3.88% | |
| Expected volatility | | 40 | – | 95% | | | 40% | |
| Expected dividend yield | | — | | | — | |
| Forfeiture rate | | — | | | — | |
| Expected life in years | | 3 | - | 5 | | | 3 | |
The risk-free interest rate is based on U.S. Treasury yields in effect at the time of grant. Expected volatilities are based on historical volatility of our common stock. The expected life in years is based on the contract term of the warrant.
Note 7. Accounts Payable and Accrued Expenses
As of June 30, 2023, accounts payable and accrued expenses included the following (in thousands):
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | BETI | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 142 | | | $ | 168 | | | $ | 119 | | | $ | 106 | | | $ | 29 | | | $ | (82 | ) | | $ | 482 | |
| Accrued payroll | | | 50 | | | | 92 | | | | 71 | | | | — | | | | — | | | | — | | | | 213 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 720 | |
As of December 31, 2022, accounts payable and accrued expenses included the following (in thousands):
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 187 | | | $ | 486 | | | $ | 7 | | | $ | 119 | | | $ | (82 | ) | | $ | 717 | |
| Accrued payroll | | | 20 | | | | 58 | | | | 120 | | | | — | | | | — | | | | 198 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | $ | 940 | |
See Note 8, “Accounts Payable and Accrued Expenses”, for the accounts payable and accrued expenses of Clyra Medical.
Note 8. Noncontrolling Interest – Clyra Medical
As discussed in Note 2 above, we consolidate the operations of our partially owned subsidiary Clyra Medical, of which we owned 56% of its outstanding shares as of June 30, 2023.
Debt Obligations of Clyra Medical
Promissory Note
On April 8, 2022, Clyra Medical issued a promissory note in the principal amount of $100,000 to an individual investor, payable April 8, 2024, and bearing 8% annual interest. The note may be converted by its holder at any time prior to the maturity date, and automatically converts to stock upon (i) Clyra’s sale of $5,000,000 or more of its common or preferred stock, or (ii) the maturity date, at a conversion price equal to 70% of the lowest price-per-share of shares sold to a future investor prior to the maturity date.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Line of Credit
On June 30, 2020, Clyra Medical entered into a Revolving Line of Credit Agreement whereby Vernal Bay Capital Group, LLC committed to provide a $1,000,000 inventory line of credit. Clyra Medical received $260,000 in draws and made repayments totaling $117,000. Clyra issued Vernal Bay 322 shares of its common stock as a commitment fee for the line of credit, valued at $70,000. A security agreement of the same date grants Vernal Bay a security interest in Clyra’s inventory, as that term is defined in the Uniform Commercial Code. Clyra may prepay the note at any time.
On December 13, 2022, we entered into an amendment of the Revolving Line of Credit Agreement whereby the maturity date of the line of credit was extended to September 30, 2024, and the payment terms were modified such that amounts of principal due in each month are capped at a maximum of 15% of the principal amount then due under the note. Additionally, BioLargo agreed to allow Vernal Bay to elect to convert, any time prior to the note’s maturity date, the 322 shares of Clyra common stock it received as consideration for the line of credit into shares of Biolargo common stock at the then market price of BioLargo’s common stock. On January 9, 2023, Vernal Bay elected to convert Clyra shares to 527,983 BioLargo shares of common stock.
As of June 30, 2023, the balance outstanding on this line of credit totals $143,000. As of December 31, 2022, the balance outstanding on this line of credit totaled $161,000.
Equity Transactions
As of June 30, 2023, Clyra had 95,301 shares issued and outstanding, of which 89,070 were common shares, and 6,231 were preferred shares. As of December 31, 2022, Clyra had 91,145 shares issued and outstanding, of which 89,070 were common shares, and 2075 were preferred shares. As of June 30, 2023 and December 31, 2022, of the outstanding amount, BioLargo owned 51,571 common shares and 1,352 Series A Preferred shares.
BioLargo Conversion of Intercompany Balances
On March 2, 2022, BioLargo converted $633,000 owed to it by Clyra into 2,042 shares of Clyra common stock.
Sales of Series A Preferred Stock
During the three months ended June 30, 2023, Clyra sold 3,427 shares of its Series A Preferred Stock, and in exchange received $1,062,000 in gross and net proceeds from 26 accredited investors. During the six months ended June 30, 2023, Clyra sold 4,879 shares of its Series A Preferred Stock, and in exchange received $1,287,000 in gross and net proceeds from 28 accredited investors. Purchasers of the Series A Preferred Stock also received a 3-year warrant to purchase the same number of additional shares of common stock for $372 per share. The fair value of the warrants issued totaled $324,000, and is limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the Series A Preferred Stock. Shares of Series A Preferred Stock earn a dividend of 15% each year, compounding annually; the company is under no obligation to pay such dividends in cash, and such dividends automatically convert to common stock upon conversion of the Series A Preferred Stock to common stock. Each share of Series A Preferred stock can be converted by the holder at any time for one share of common stock, and automatically convert upon the completion of a public offering of shares in which at least $5,000,000 of gross proceeds is received by the company. Accrued dividends may be converted to common stock at a conversion rate of $310 per share.
Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its Series A Preferred stock, plus accrued dividends, into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 30 prior trading days. Elections must be made during the 18-month period that begins 18 months after the closing of the Series A Preferred offering (which has not yet taken place), or June 30, 2023, whichever is earlier.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Clyra Stock Options
| | | | | | | | | | | | | Weighted | |
| | | Clyra | | | | | | | | | average | |
| | | Options | | | Exercise | | | price per | |
| | | Outstanding | | | price per share | | | share | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2022 | | | 15,833 | | | | $1.00 | - | 310 | | | $ | 5.53 | |
| Granted | | | 858 | | | | 1.00 | - | 271 | | | | 146.06 | |
| Balance, June 30, 2023 | | | 16,691 | | | | $1.00 | - | 310 | | | $ | 12.76 | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 14,004 | | | | $1.00 | | | $ | 1.00 | |
| Granted | | | 1,026 | | | | 1.00 | | | | 1.00 | |
| Balance, June 30, 2022 | | | 15,030 | | | | $1.00 | | | $ | 1.00 | |
Clyra issues options to its employees and consultants in lieu of compensation owed on a regular basis. The fair value of the options issued totaled $66,000 and $127,000 in the three and six months ended June 30, 2023, and $82,000 and $223,000 in the three and six months ended June 30, 2022. We used the Black-Scholes model to calculate the initial fair value, assuming a stock price on date of grant of $310 per share. Because Clyra is a private company with no secondary market for its common stock, the resulting fair value was discounted by 30%.
| | | June 30, 2023 | | | December 31, 2022 | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | | 2.32 | % |
| Expected volatility | | 40 | - | 48% | | | | 40 | % |
| Expected dividend yield | | — | | | | — | |
| Forfeiture rate | | — | | | | — | |
| Expected life in years | | 10 | | | | 10 | |
Accounts Payable and Accrued Expenses
At June 30, 2023, and December 31, 2022, Clyra had the following accounts payable and accrued expenses (in thousands):
| Category | | 2023 | | | 2022 | |
| Accounts payable | | $ | 199 | | | $ | 186 | |
| Accrued payroll | | | 10 | | | | 45 | |
| Accrued interest | | | 5 | | | | 7 | |
| Accrued dividend | | | 74 | | | | --- | |
| Total | | $ | 288 | | | $ | 238 | |
Note 9. BioLargo Engineering, Science and Technologies, LLC
In September 2017, we commenced a full-service environmental engineering firm and formed a Tennessee entity named BioLargo Engineering, Science & Technologies, LLC (“BLEST”). In conjunction with the start of this subsidiary we entered into employment agreements with six scientists and engineers. (See Note 11 “Business Segment Information”.) BLEST was capitalized with two classes of membership units: Class A, 100% owned by BioLargo, and Class B, held by management of BLEST, and which initially have no “profit interest,” as that term is defined in Tennessee law. However, over the succeeding five years, the Class B members can earn up to a 30% profit interest. They also have been granted options to purchase up to an aggregate 1,750,000 shares of BioLargo, Inc. common stock. The profit interest and option shares are subject to a five year vesting schedule tied to the performance of the subsidiary, including gross revenue targets that increase over time, obtaining positive cash flow by March 31, 2018 (which was not met), collecting 90% of its account receivables, obtaining a profit of 10% in its first year (and increasing in subsequent years) (which was not met), making progress in the scale-up and commercialization of our AOS system, and using BioLargo research scientists (such as our Canadian team) for billable work on client projects. These criteria are to be evaluated annually by BLEST’s compensation committee (which includes BioLargo’s president, CFO, and BLEST’s president), beginning September 2018. Given the significant performance criteria, the Class B units and the stock options will only be recognized in compensation expense if or when the criteria are satisfied.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The BLEST Compensation Committee has met regularly since the subsidiary commenced operations. In 2018, it reviewed the operating performance and determined that the performance metrics were not met and as a result, did not award any Class B units or stock options. As of December 31, 2021, BLEST employees had earned 13% in profits interests and had earned (vested) 455,000 option shares. In January 2022, the committee again reviewed the operating performance and determined that a portion of the performance metrics were met. It was agreed that an additional one-half and one-quarter of the eligible profits interests would be vested (11% in the aggregate), and therefore an additional half of the option interests would be vested (525,000 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $65,000, recorded on our consolidated statement of operations as selling, general and administrative expense for the year ended December 31, 2021. In December 2022, the committee again reviewed the operating performance and determined that a portion of the performance metrics were met. It was agreed that an additional one-half and one-quarter of the eligible profits interests would be vested (18% in the aggregate), and therefore an additional half of the option interests would be vested (1,242,500 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $135,000 and is recorded on our consolidated statement of operations as selling, general and administrative expense for the year ended December 31, 2022.
At June 30, 2023, BioLargo owns 82% of BLEST.
Note 10. BioLargo Energy Technologies, Inc.
Subsidiary BioLargo Energy Technologies, Inc. (“BETI”) was formed for the purpose of commercializing a sodium-sulfur battery technology. BioLargo purchased 9,000,000 shares of its common stock upon its formation.
During the three months ended June 30, 2023, BETI sold 132,000 shares of its common stock to nine accredited investors and received $330,000 in gross and net proceeds. During the six months ended June 30, 2023, BETI sold 450,000 shares of its common stock to 15 accredited investors, and in exchange received gross and net proceeds totaling $980,000. Of that amount, $100,000 in shares were purchased by BioLargo.
Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its shares of BETI common stock into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 20 trading days prior to the election to exchange. Elections must be made during calendar year 2024.
As of June 30, 2023, there are 9,457,000 shares outstanding, of which BioLargo holds 9,050,000 (96%).
Note 11. Business Segment Information
BioLargo has five operating business segments, plus its corporate entity which is responsible for general corporate operations, including administrative functions, finance, human resources, marketing, legal, etc. The operational business segments are:
| | 1. | ONM Environmental -- which sells odor and volatile organic control products and services (located in Westminster, California); |
| | 2. | Clyra Medical Technologies (“Clyra Medical”) -- which develops and sells medical products based on our technologies; |
| | 3. | BLEST -- which provides professional engineering services on a time and materials basis for outside clients and supports our internal operations as needed (located in Oak Ridge, Tennessee); |
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | 4. | BioLargo Water (“Water”) – our research, development and innovation group operating out of the University of Alberta, Edmonton, Canada; and |
| | 5. | BioLargo Energy Technologies, Inc. (“BETI”) – formed to commercialize our sodium-sulfur battery technology. |
Other than ONM Environmental, during the last three quarterly periods none of our operating business units have operated at a profit, and therefore each required additional cash to meet its monthly expenses, funded through BioLargo’s sales of debt or equity, research grants, and tax credits. BETI and Clyra Medical have also been funded by third party investors who invest directly in exchange for equity ownership in that entity.
The segment information for the three and six months ended June 30, 2023, and 2022, is as follows (in thousands):
| | | Three months ended June 30, | | | Six months ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| Revenue | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | — | | | $ | — | | | $ | — | | | $ | 2 | |
| ONM Environmental | | | 1,316 | | | | 700 | | | | 4,859 | | | | 1,300 | |
| BLEST | | | 538 | | | | 670 | | | | 901 | | | | 1,213 | |
| BETI | | | — | | | | — | | | | — | | | | — | |
| Water | | | 9 | | | | — | | | | — | | | | — | |
| Clyra Medical | | | — | | | | 6 | | | | 6 | | | | 17 | |
| Intersegment revenue | | | (417 | ) | | | (53 | ) | | | (587 | ) | | | (245 | ) |
| Total | | $ | 1,446 | | | $ | 1,323 | | | $ | 5,188 | | | $ | 2,287 | |
| | | | | | | | | | | | | | | | | |
| Research and development expense | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (243 | ) | | $ | (165 | ) | | $ | (432 | ) | | $ | (430 | ) |
| BLEST | | | (292 | ) | | | (80 | ) | | | (537 | ) | | | (188 | ) |
| BETI | | | (271 | ) | | | — | | | | (303 | ) | | | — | |
| Water | | | (138 | ) | | | (130 | ) | | | (273 | ) | | | (327 | ) |
| Clyra Medical | | | (60 | ) | | | (26 | ) | | | (194 | ) | | | (42 | ) |
| Intersegment R&D | | | 417 | | | | 46 | | | | 587 | | | | 240 | |
| Total | | $ | (587 | ) | | $ | (355 | ) | | $ | (1,152 | ) | | $ | (747 | ) |
| | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (635 | ) | | $ | (923 | ) | | $ | (1,434 | ) | | $ | (2,143 | ) |
| ONM Environmental | | | 428 | | | | 11 | | | | 1,815 | | | | 18 | |
| BLEST | | | (450 | ) | | | 56 | | | | (818 | ) | | | 21 | |
| BETI | | | (297 | ) | | | — | | | | (384 | ) | | | — | |
| Water | | | (209 | ) | | | (209 | ) | | | (393 | ) | | | (431 | ) |
| Clyra Medical | | | (396 | ) | | | (256 | ) | | | (823 | ) | | | (496 | ) |
| Total | | $ | (1,559 | ) | | $ | (1,321 | ) | | $ | (2,037 | ) | | $ | (3,031 | ) |
| | | | | | | | | | | | | | | | | |
| Interest expense | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (4 | ) | | $ | (6 | ) | | $ | (40 | ) | | $ | (12 | ) |
| ONM Environmental | | | (2 | ) | | | — | | | | (4 | ) | | | — | |
| Clyra Medical | | | (6 | ) | | | (9 | ) | | | (16 | ) | | | (16 | ) |
| Total | | $ | (12 | ) | | $ | (15 | ) | | $ | (60 | ) | | $ | (28 | ) |
| | | | | | | | | | | | | | | | | |
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| As of June 30, 2023 | | BioLargo | | | ONM | | | Clyra | | | BLEST | | | Water | | | BETI | | | Elimination | | | Total | |
| Tangible assets | | $ | 731 | | | $ | 2,705 | | | $ | 1,265 | | | $ | 414 | | | $ | 92 | | | $ | 686 | | | $ | (41 | ) | | $ | 5,852 | |
| Right of use leased asset | | | 93 | | | | — | | | | — | | | | 714 | | | | — | | | | — | | | | — | | | | 807 | |
| Investment in South Korean joint venture | | | 21 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 21 | |
| Total | | $ | 845 | | | $ | 2,705 | | | $ | 1,265 | | | $ | 1,128 | | | $ | 92 | | | $ | 686 | | | $ | (41 | ) | | $ | 6,680 | |
| As of December 31, 2022 | | BioLargo | | | ONM | | | Clyra | | | BLEST | | | Water | | | Elimination | | | Total | |
| Tangible assets | | $ | 669 | | | $ | 2,064 | | | $ | 631 | | | $ | 441 | | | $ | 194 | | | $ | (41 | ) | | $ | 3,958 | |
| Right of use | | | 136 | | | | — | | | | — | | | | 731 | | | | — | | | | — | | | | 867 | |
| Investment in South Korean joint venture | | | 33 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 33 | |
| Total | | $ | 838 | | | $ | 2,064 | | | $ | 631 | | | $ | 1,172 | | | $ | 194 | | | $ | (41 | ) | | $ | 4,858 | |
Note 12. Commitments and Contingencies
Office Leases
We have long-term operating leases for office, industrial and laboratory space in Westminster, California, Oak Ridge, Tennessee, and Alberta, Canada. Payments made under operating leases are charged to the Consolidated Statement of Operations and Comprehensive Loss on a straight-line basis over the term of the operating lease agreement. Short-term leases less than one-year are not included in our analysis. For the three and six months ended June 30, 2023, rental expense was $83,000 and $166,000 and for the three and six months ended June 30, 2022, rental expense was $106,000 and $169,000. The lease of our Westminster facility expires August 2024. Management has not yet determined whether it will exercise its option to extend the lease four years; therefore the four-year extension is not included in the analysis. In September 2022, the lease of our Oak Ridge, Tennessee facility was extended for ten years. The lease of our Canadian facility is less than one year. None of our leases have additional terms related to the payments or mechanics of the lease. The leases have no additional payment terms such as common area maintenance payments, tax sharing payments or other allocable expenses. Likewise, the leases do not contain other terms and conditions of use, such as variable lease payments, residual value guaranties or other restrictive financial terms. Since there is no explicit interest rate in our leases, management used its incremental borrowing rate, which is estimated to be 18% to determine lease liability.
Note 13. Subsequent Events.
Management has evaluated subsequent events through the date of the filing of this quarterly report and management noted the following for disclosure.
Lincoln Park Capital Purchase of Shares
From July 1, 2023, through August 10, 2023, we sold 782,553 shares of common stock to Lincoln Park pursuant to the 2022 LPC Purchase Agreement (see Note 3) and received $136,000 in gross and net proceeds. These sales were registered with the SEC on Form S-1 (file number 333-268973).
Clyra Medical – Sales of Series A Preferred Stock Units
From July 1, 2023, through August 10, 2023, Clyra Medical sold 81 shares of its Series A Preferred Stock, and in exchange received $25,000 in gross and net proceeds. Purchasers of the Series A Preferred Stock also received a 3-year warrant to purchase the same number of additional shares of common stock for $372 per share. (See Note 8, “Sales of Series A Preferred Stock”.)
BETI – Sales of Common Stock
From July 1, 2023, through August 10, 2023, BETI sold 10,000 shares of its common stock, and in exchange received $25,000 in gross and net proceeds. (See Note 10.)
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This quarterly report on Form 10-Q contains forward-looking statements. These forward-looking statements involve risks and uncertainties, including statements regarding BioLargo’s capital needs, business plans and expectations. Such forward-looking statements involve risks and uncertainties regarding BioLargo’s ability to carry out its planned development and production of products. Forward-looking statements are made, without limitation, in relation to BioLargo’s operating plans, BioLargo’s liquidity and financial condition, availability of funds, operating and exploration costs and the market in which BioLargo competes. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict”, “potential” or “continue”, the negative of such terms or other comparable terminology. Actual events or results may differ materially. In evaluating these statements, you should consider various factors, including the risks outlined in our Form most recent annual report on Form 10-K, and, from time to time, in other reports BioLargo files with the SEC. These factors may cause BioLargo’s actual results to differ materially from any forward-looking statement. BioLargo disclaims any obligation to publicly update these statements, or disclose any difference between its actual results and those reflected in these statements. The information constitutes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Given these uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
Unless otherwise expressly stated herein, all statements, including forward-looking statements, set forth in this Form 10-Q are as of June 30, 2023, unless expressly stated otherwise, and we undertake no duty to update this information.
As used in this report, “we” and “Company” refers to (i) BioLargo, Inc., a Delaware corporation; and (ii) its partially or wholly-owned subsidiaries BioLargo Life Technologies, Inc., a California corporation which holds our registered patents, ONM Environmental, Inc., a California corporation which manufactures, markets, sells and distributes our odor and volatile organic compound control products, BioLargo Water Investment Group, Inc., a California corporation (which wholly owns BioLargo Water, Inc., a Canadian corporation), BioLargo Development Corp., a California corporation which employs and provides benefits to our employees, BioLargo Engineering, Science & Technologies, LLC, a Tennessee limited liability company (“BLEST”) that provides professional engineering services out of Oak Ridge Tennessee, BioLargo Energy Technologies, Inc., a California corporation (“BETI”) formed to commercialize our proprietary battery technology, BioLargo Equipment Solutions & Technologies, Inc., a California corporation, and Clyra Medical Technologies, Inc., a California corporation (“Clyra Medical”) which commercializes our technologies in the medical and dental fields. All subsidiaries are wholly owned, except for BETI, BLEST and Clyra Medical.
The following discussion and analysis should be read in conjunction with our unaudited consolidated financial statements and the related notes to the consolidated financial statements included elsewhere in this report.
Our Business - Innovator and Solution Provider
BioLargo is in the business of creating new cleantech technologies to solve tough problems. We invent, develop, then commercialize disruptive technologies to tackle global challenges in air quality, water, environmental engineering, long duration energy storage, and advanced antimicrobial medical device platforms. Our model is to invent new technologies that solve specific problems, prove them out and develop them, then commercialize them with purpose-suited subsidiaries, identify and secure the right partnerships to increase their commercial reach, and potentially sell them or spin them out later.
Why do we do this work? Every member of our team – including PhD scientists, engineers, and entrepreneurs – has a passion for seeking new, never-before-seen innovations that can make life better around the world. We care about safeguarding the environment and human health for future generations. We care about making technologies that are affordable and flexible enough to be accessed around the world. And we care about being the best at what we do – creating best-in-class technologies to solve big, tough cleantech challenges.
Some of our areas of focus include environmental problems like PFAS contamination (per- and polyfluoroalkyl substances), water pollution by pharmaceuticals and micropollutants, air pollution by VOCs, hard-to-treat odors from landfills and sewage plants, and infection and wound healing.
Below you’ll read about the cleantech ventures and projects we are most excited about. Behind those, however, is a pipeline of other cleantech innovations in various stages of development associated with our expansive array of issued and pending patents, and having been funded in part by over 90 government grants.
We operate our business in distinct business segments:
| |
● |
Odor and VOC control products, including consumer products, such as the Pooph branded pet-odor control product, and our flagship industrial odor control product, CupriDyne Clean, sold by our subsidiary ONM Environmental, Inc.; |
| |
● |
Water treatment equipment and solutions, including our PFAS removal system the AEC, our co-developed minimal liquid discharge water treatment system the AROS, and our micro-pollutant treatment solution, the AOS, all sold by our subsidiary BioLargo Equipment Solutions & Technologies, Inc.; |
| |
● |
Long-term battery storage solutions, being developed by our subsidiary BioLargo Energy Technologies, Inc. (97% owned by BioLargo, Inc.); |
| |
● |
Medical products based on our technologies, including the FDA-cleared Bioclynse surgical wound irrigation solution sold by our partially owned subsidiary Clyra Medical Technologies, Inc.; and |
| |
● |
Our professional engineering services division, which, in addition to serving outside clients on a fee for service basis, supports our internal business units, through our partially owned subsidiary BioLargo Engineering, Science & Technologies, LLC ("BLEST"). |
In addition to the foregoing, we have scientists working in a research and support facility on campus at the University of Alberta, Edmonton, Canada, and corporate offices in Westminster, California that support the operating segments with legal, accounting, human resources, and other services.
Odor Control (Consumer and Industrial)
Pooph - Consumer Private-Label Products
We sell the Pooph branded pet odor-control product to Ikigai Marketing Works, LLC, pursuant to license and manufacturing agreements. Ikigai advertises and sells Pooph to consumers and major retailers, including Walmart, Amazon, and Chewy.com, and is currently expanding to more than a dozen large retailers. Our agreement with Ikigai grants them an exclusive license to sell the Pooph pet odor-control product, and requires, in addition to purchasing product from us at an agreed-upon manufacturing margin, they are required to pay us an additional six percent of their sales.
As Ikigai has expanded their national television advertising, their sales have increased, and correspondingly, their purchase of product from us has increased, such that for the six months ended June 30, 2023, it comprised 77% of our company-wide revenue. However, in the three months ended June 30, 2023, revenues from sales of Pooph to Ikigai were down significantly compared to the first quarter of 2023, as Ikigai managed inventory purchased for initial stocking orders at retailers (primarily Walmart).
Ikigai management informs us that, over the coming months, there will be some shift of focus in their advertising from direct-to-consumer to driving sales to national retailers, and that they expect to secure contracts for more than 20,000 retail stores by the end of 2023, representing an approximately ten times increase in the number of stores carrying the product as were at June 30, 2023.
Industrial Odor and VOC Solutions
ONM Environmental, Inc. is BioLargo’s subsidiary that delivers robust and comprehensive products and services to control and mitigate odor and volatile organic compounds (“VOCs”) emitted from a variety of industrial activities, including landfills and other waste handling facilities. Its flagship product – CupriDyne® Clean – is delivered through misting systems, sprayers, water trucks and similar water delivery systems designed, manufactured and installed by ONM. We believe the product is the number-one performing odor-control product in the market, and that it offers substantial savings to our customers compared with competing products. ONM Environmental holds General, Electrical, Plumbing and Low Voltage contractor licenses issued by the California Contractors State License Board, and offers a menu of services to landfills, transfer stations, wastewater treatment facilities as well as facilities in non-waste related industries. These services include engineering design, construction, installation, ongoing maintenance and on-site support services to assist our clients in the implementation and continued use of the various systems that deliver our liquid products in the field (such as misting systems).
We have been and expect to continue selling product to the largest solid waste handling companies in the country, with a portion of chemistry product sales resulting from national purchasing agreements (NPAs), and are also serving municipalities. In the past quarter, ONM Environmental focused on securing more contracts with municipal customers like sanitation agencies, securing new construction projects for municipal customers that will be completed over the coming months. ONM also onboarded two new wastewater treatment plant customers, as well as one more manufacturing facility customer, representing encouraging advancements in expanding into non-solid-waste markets.
In addition to its goal to keep growing its revenues organically through the sale of odor and VOC control chemistry and air quality control systems to its primary market segment (municipal solid waste handling in California), ONM Environmental aims to accelerate its growth through development of new sales and distribution channels without being limited by our own sales and distribution infrastructure, such as through our partnership with Ikigai Marketing Works, LLC (see “Consumer Packaged Goods Products” below).
South Korean Joint Venture
We are partners with a leading wastewater treatment solution provider based in South Korea in a joint venture to commercialize our CupriDyne® Clean products in South Korea. We own 40% of the joint venture. Although the joint venture established manufacturing and is marketing the product, the COVID-19 pandemic significantly impacted the expected growth of the company. While the local management team continues to market the product to industrial clients, their efforts have struggled to gain a foothold. We are not obligated to contribute additional funds to the venture, and cannot predict its future success.
Innovative Water Treatment Solutions
Over the years, we have developed multiple innovative technologies and equipment platforms that focus on challenging issues in the water treatment industry, including the AOS developed to remove micro-pollutants, the AEC developed to remove per- and polyfluoroalkyl substances (PFAS), and along with Garratt-Callahan, the AROS to allow for minimal water discharge. As a result of increase in interest from potential customers for our PFAS solutions, we believe we will be better able to serve this market with a uniform identity and operating unit called BioLargo Equipment Solutions & Technologies, Inc. (“BEST”), which will manage the sales and distribution of our water treatment products and related services. As we transition this venture from incubation to commercialization, we are focusing staff and resources we believe necessary for success. Ultimately, BEST will be reflected as a new operating segment in our consolidated financial statements. Although BEST is primarily focused on AEC, AROS and AOS, discussed below, it will offer comprehensive water treatment solutions, related equipment, and services.
AEC, a solution for the PFAS “Forever-Chemicals” crisis – and more
One of the most significant and timely innovations in our portfolio is our per- and polyfluoroalkyl substances (PFAS) removal and collection/disposal solution we call the Aqueous Electrostatic Concentrator (AEC), a novel water treatment system that removes PFAS from water at a lower operating cost and while generating only a fraction of the PFAS-laden waste of the most common currently used solutions (carbon filtration, ion exchange, and reverse osmosis). PFAS chemicals have been linked to cancer, immune disorders, liver dysfunction, and many other human health problems, and are contained in a vast range of manufactured goods, common household products (e.g., cleaning products, cookware), and electronics, and contaminate drinking water in unsafe levels all over the globe.
PFAS is often referred to as the “contaminant of the decade.” The EPA proposed new drinking water standards on March 14, 2023, limiting certain PFAS chemicals to four parts per trillion – a standard our AEC can meet. We believe these proposed rules will continue to push the market to find and adopt commercially viable solutions to remove PFAS chemicals from water. Additionally, some emerging regulations on PFAS in the U.S. are expected to skew the market toward seeking treatment technologies that produce as little PFAS-laden solid waste as possible, a favorable trend for our AEC that generates very little PFAS-laden waste. Detection of unsafe levels of PFAS around the world has given rise to a number of market opportunities, including in drinking water, industrial wastewater, municipal wastewater, solid waste, organic foods and more.
We have successfully validated the AEC as an effective system to selectively extract and collect PFAS chemicals from contaminated water including performance testing that shows “non-detect” levels of removal, which meets new EPA standards. We have demonstrated more than 10,000 hours of continuous operation showing no materially significant degradation of the AEC system’s components or performance over time. As a modular system, we believe the AEC is scalable to small portable commercial units as well as very large commercial operations, and we believe that our engineering team has the experience to deliver systems to meet the needs of any sized commercial installation. In order to provide a full turn-key solution for our customers, we have developed an expanded offering whereby we can bundle a service package with each customer project that includes a membrane exchange program, the collection of PFAS, and transport and destruction of the PFAS.
Our strategy to market our PFAS treatment technology and related engineering services is as follows: 1) focus on demonstrating our technology’s efficacy in first demonstration projects, trials, and early customer deployments with the understanding that this early success can be leveraged to secure larger and more numerous subsequent projects, 2) market our PFAS expertise and our technology by presenting at industry events and conferences around the country, cultivating our status as “thought leaders” in the space (we are taking part in seven such events over the next three months), 3) use our network of manufacturer’s representatives and channel selling partners to maximize the number of potential opportunities with early adopters, and 4) engage in discussions with credible distribution partners at established water treatment technology companies.
The AEC’s commercial roll-out will be executed with the help of a network of sales representative organizations whose role will be to market and sell the treatment system, related equipment, and the Company’s engineering services to municipal and industrial customers across the country. We have secured channel partner agreements with several sales representative organizations ensuring coverage for most of the continental United States. In August 2022, we began engineering a comprehensive PFAS mitigation plan for client operating an industrial site. Having completed initial preliminary engineering and project budgeting, in early 2023 we delivered a proposal for a mobile commercial pilot, as well as scoping, preliminary design and budget for the building of the full-scale system. We are awaiting client feedback and engagement on the proposal. We are currently in negotiations with multiple prospective industrial and municipal customers to treat PFAS contaminated water. These opportunities include small to medium sized municipalities, waste facilities, Air Force bases, remediation sites, and industrial sites.
AROS Minimal Liquid Discharge Water Treatment
In association with Garratt-Callahan, a national industrial water treatment company, BLEST developed a “minimal liquid discharge” wastewater treatment system, called AROS, based on Garratt-Callahan’s patented technology that is able to reduce industrial wastewater discharge and therefore reduce wastewater discharge fees for customers. Garratt-Callahan is currently marketing the AROS system to its customers. BLEST will serve as the manufacturing partner and Garratt-Callahan will serve as the selling distributor to leverage their national sales force and over one hundred years of providing services and products to customers. Garratt-Callahan has identified multiple customer prospects, as has BioLargo, through its own marketing efforts. We remain confident that this innovation and partnership will find commercial success.
Advanced Oxidation System (AOS)
The Advanced Oxidation water treatment system (AOS) is our patented water treatment device that generates highly oxidative and energetic species of iodine and other molecules which allow it to eliminate pathogenic organisms and organic contaminants rapidly and effectively as water passes through the device. The key value proposition of the AOS is its ability to reduce or eliminate a wide variety of waterborne contaminants with high performance, including the normally hard-to-treat class of recalcitrant water contaminants called “micropollutants”, while using very little electricity and input chemicals.
Our proof-of-concept studies and on-site pilot projects have generated results that project the AOS will be more cost- and energy-efficient than commonly used advanced water treatment technologies such as UV, electro-chlorination, and ozonation. Furthermore, our technology has been proven capable of removing hard-to-treat organic micropollutants such as pharmaceuticals from water more quickly and energy-efficiently than other technologies. Together, these characteristics make the AOS an economical and versatile tool to enable wastewater treatment and reuse in the face of emerging water contaminants and increasing regulatory scrutiny on industrial wastewater discharge.
Our current sales and marketing efforts for the AOS focus on: 1) domestic water and wastewater treatment markets in agriculture, pharmaceutical, and small municipal where the technology’s claims of low energy usage and proven pharmaceutical/micropollutant removal is especially valuable, and 2) opportunities to treat drinking water in Europe, where pharmaceuticals/micropollutants in water and wastewater are currently regulated in many countries. To achieve goal 2, we are focusing on cultivating partnerships with established European organizations.
The AOS has been included as a component of treatment train (comprehensive system) for a number of projects being scoped and budgeted through our engineering subsidiary. In addition, it has been included in the catalog of offerings being sold through our independent representatives as well as channel partners.
BioLargo Energy Technologies, Inc.
BioLargo acquired a proprietary sodium-sulfur battery technology and has formed and secured initial seed capital for a subsidiary – BioLargo Energy Technologies, Inc. (“BETI”) – designed to address the ongoing shift toward renewable energy production and the growth in global electricity demand, and the consequent drastic expansion in energy storage capacity in the US and world-wide that will be needed to accommodate increased demand and the intermittent nature of renewable energy sources like wind and solar.
The capital raised – $980,000 during the six months ended June 30, 2023 – is being used to build a pilot-scale production facility located in our building in Oak Ridge Tennessee in order to construct prototype batteries. These batteries will be tested to confirm energy efficiency, useful life expectancy, energy density, safety profile, number of charge/discharge cycles, and other technical claims that differentiate the battery from incumbent technologies. Batteries built based on the underlying technology a decade ago demonstrated features that far surpass comparable lithium-ion batteries, the dominant incumbent technology in the market:
| |
● |
This sodium-sulfur battery technology demonstrates increased safety, no runaway fire risks, and a more sustainable design – with no rare-earth elements – that is capable of being manufactured completely from the domestic supply chain. |
| |
● |
Unlike lithium-ion batteries, BioLargo’s battery can charge and discharge completely, with no degradation of performance, ensuring virtually unlimited charge/discharge cycles, without self-discharge. |
| |
● |
BioLargo’s battery technology also demonstrates increased energy efficiency and energy density in comparison to lithium-ion batteries, and a longer useful life expectancy of at least 10 years. |
BioLargo’s battery uses common, inexpensive, domestically available materials, and through its unique design and manufacturing process, creates an energy storage solution that has higher energy density than lithium virtually unlimited charge/discharge cycles, stable and secure supply chain management, and which is far safer than lithium-ion batteries, the current dominant energy storage technology. While the concept of sodium-sulfur salt batteries is not new (a concept conceived more than 80 years ago), we are not aware of any known ‘salt’ battery that can match the performance metrics of our battery.
Our battery technology operates at higher temperatures, and its casing and materials when combined, are heavier than lithium-ion, making it more suitable for stationary energy storage applications like grid-scale energy storage, electric vehicle charging stations, and commercial and residential energy storage, and believed to be less suitable for placement into electric vehicles or portable electronics.
Clyra Medical Technologies, Inc. - Bioclynse Wound Irrigation Solution
Clyra Medical Technologies, Inc. is our partially owned subsidiary creating medical products based on our technologies. It is launching a product, called Bioclynse, to be used as a surgical wound irrigation solution and to help manage patient care and outcomes. The first target market for this product is orthopedics, including for hip and knee replacements. Management believes Bioclynse outperforms competing products as it has proven performance in biofilm disruption and inhibition, is non-toxic and non-cytotoxic, is non-sensitizing to tissue, and unlike competing products, does not require it to be rinsed and/or removed from a surgical cavity.
Clyra has secured manufacturing with independent third parties, a a robust quality control system for FDA compliance, recruited a national director of sales, secured independent manufacturer’s representatives and is actively expanding these efforts to build out a national rep network. Simultaneously, Clyra is focused on developing partnerships with large, well-established distributors who can help rapidly accelerate the product’s access to clinicians and surgeons in hospitals around the country.
During the six months ended June 30, 2023, Clyra sold $1,287,000 in preferred stock to support these efforts.
Full Service Environmental Engineering
BioLargo Engineering, Science & Technologies, LLC (“BLEST”) offers full service environmental engineering to third parties and provides engineering support services to our internal teams to accelerate the commercialization of our technologies.
BLEST focuses its efforts in three areas:
| |
● |
providing engineering services to third-party clients as well as affiliated BioLargo entities; |
| |
● |
supporting internal product development; and |
| |
● |
advancing their own technical innovations such as the AEC PFAS treatment technology and the battery energy storage system. |
BLEST operates out of an engineering facility in Oak Ridge (a suburb of Knoxville), Tennessee), and employs a group of scientists and engineers, many of whom are owners of the entity (BioLargo owns 82%). The team is led by Randall Moore, who served as Manager of Operations for Consulting and Engineering for the Knoxville office of CB&I Environmental & Infrastructure and was formerly a leader at The Shaw Group, Inc., a Fortune 500 global engineering firm. Many of the other team members are also former employees of CB&I and Shaw, with the exception of more recent staff hires. The team is highly experienced across multiple industries and we believe are considered experts in their respective fields, including: chemical engineering, wastewater treatment (including design, operations, data gathering and data evaluation), process safety, energy efficiency, air pollution, design and control, technology evaluation, technology integration, air quality management & testing, engineering management, permitting, industrial hygiene, applied research and development, air testing, environmental permitting, HAZOP review, chemical processing, thermal design, computational fluid dynamics, mechanical engineering, mechanical design, NEPDES permitting, RCRA/TSCA compliance and permitting, project management, storm water design & permitting, computer assisted design (CAD), bench chemistry, continuous emission monitoring system operator, data handling and evaluation and decommissioning and decontamination of radiological and chemical contaminated facilities. The team has decades of high-level experience in the energy industry. The engineering team has also developed an extended network of trusted engineering subcontractors that assist in serving specific client projects as needed.
BLEST engineers generate revenue through services to third party clients, as well as for internal BioLargo projects such as the AEC and battery (revenues from internal projects are eliminated in the consolidation of our financial statements and are designed “intersegment revenue”). Third party contracts include ongoing work at U.S. Air Force bases for air quality control. Efforts to expand this work as well as with other clients are consistently ongoing.
Results of Operations
Our revenues increased 9% and 127% in the three and six months ended June 30, 2023, as compared with the same period in 2022. Revenues from the sale of products for the six months ended June 30, 2023, were $4,864,000, a 269% increase over the same period of 2022, primarily due to sales of the pet odor product (under the brand name “Pooph”) to our consumer-packaged goods partners at Ikigai Marketing Works, which comprised 77% of our total revenues in the six months ended June 30, 2023.
ONM Environmental
Our wholly-owned subsidiary ONM Environmental generates revenues through (i) sales of our flagship product CupriDyne Clean, including related design, installation, and maintenance services on the systems that deliver CupriDyne Clean at its clients’ facilities, and (ii) sale of private-label products to third parties, including the Pooph branded pet product.
Revenue (ONM Environmental)
ONM Environmental’s revenues increased 88% (to $1,316,000) and 274% (to $4,859,000) in the three and six months ended June 30, 2023, compared with the same periods in the prior year. The increase in revenues was almost entirely due to an increase in the volume of sales of private label odor-control products, specifically the Pooph branded pet-odor product, sold to Ikigai, which comprised 65% and 77% of ONM Environmental’s total revenues for the three and six months ended June 30, 2023. On a quarter-to-quarter basis, revenues from sales to Ikigai decreased from $3,143,385 in the first quarter of 2023 to $797,640 in the second quarter of 2023. The second quarter decrease was due to Ikigai’s purchase of initial stocking inventory in the first quarter to ready for a national rollout of Pooph at Walmart, and the resulting lack of need for product during the second quarter as that inventory was distributed. As Ikigai shifts its business model from primarily direct-to-consumer (primarily through their website and Amazon), to retailers like Walmart, we expect both continued overall growth in sales and similar fluctuations in the amount of product ordered from quarter-to-quarter. Although Ikigai management has expressed confidence in the increase of the number of retail stores nationwide, we have no control over those efforts and cannot predict their ultimate success. Any downturn in purchases of the Pooph product from Ikigai will affect our results of operations, as it did so for this quarter. (See also our risk factor titled “A significant portion of our revenue is concentrated with one customer” set forth in our Form 10-Q filed May 16, 2023.)
Cost of Goods Sold (ONM Environmental)
ONM Environmental’s cost of goods sold includes costs of raw materials, contract manufacturing, and portions of depreciation, salaries and expenses related to the manufacturing and installation of its products. As a percentage of revenue, ONM Environmental’s costs of goods decreased 7% and 0% for the three and six months ended June 30, 2023, to 39% and 47%, compared to the same periods in 2022, due to increased sales. The fluctuation in cost of goods is due to our efforts to improve margins in our private-label products, and increases in raw material costs.
Selling, General and Administrative Expense (ONM Environmental)
ONM Environmental’s selling, general and administrative expenses increased by 2% and 11% during the three and six months ended June 30, 2023, as compared with the same period in 2022. These expenses increased due to an increase of sales and support staff. We expect these expenses to increase to add support if revenue continues to increase in the year ending December 31, 2023.
Operating Income (ONM Environmental)
ONM Environmental generated operating income of $428,000 and $1,815,000 in the three and six months ended June 30, 2023, compared to operating income of $11,000 and $18,000 for the three and six months ended June 30, 2022. The generation of operating income for the three and six months ended June 30, 2023, was entirely dependent on sale of Pooph branded products to Ikigai.
BLEST (engineering division)
Revenue (BLEST)
Our engineering segment (BLEST) generated $131,000 and $324,000 of revenue, net of intersegment revenue, in the three and six months ended June 30, 2023, compared to $616,000 and $970,000 in the three and six months ended June 30, 2022. In addition to providing engineering services to third party clients, BLEST supports BioLargo’s internal projects such as the AEC PFAS treatment system and the buildout of manufacturing facilities for the battery prototype. These services are billed internally, are considered intersegment revenue, and are eliminated in the consolidation of our financial statements. In the three and six months ended June 30, 2023, intersegment revenue for BLEST totaled $408,000 and $578,000 and for the three and six months ended June 30, 2022, intersegment revenue for BLEST totaled $53,000 and $241,000. The decrease in BLEST’s revenue net of intersegment revenue is due to a decrease in the number of client contracts, and its focus on supporting internal projects.
Cost of Services Sold (BLEST)
BLEST’s cost of services includes employee labor as well as subcontracted costs. In the three and six months ended June 30, 2023, cost of services were 56% and 64% of its revenues, versus 61% and 46% in the same period in 2022. These fluctuations are due to changes in the number of flat-fee monthly service accounts, as well as the recognition of deferred revenue in the three and six months ended June 30, 2022, which did not occur in the same period in 2023.
Selling, General and Administrative Expense (BLEST)
BLEST's SG&A expenses were $214,000 and $399,000, in the three and six months ended June 30, 2023, compared to $105,000 and $239,000, in the three and six months ended June 30, 2022, primarily due to increased rental costs and staff.
Operating Loss (BLEST)
BLEST incurred an operating loss of $450,000 and $818,000, in the three and six months ended June 30, 2023, compared to an operating income of $56,000 and $21,000 in the three and six months ended June 30, 2022. The increase in operating loss was due to lower third party-revenues.
Because the subsidiary had an operating loss, we invested cash during the year to allow it to maintain operations. BLEST’s need for a cash subsidy to support its operations has decreased over time. Our goal for this operation is that it produces a profit and contributes to corporate overhead in a significant way, although predicting when that will happen and other uncertainties in the market, and our limited resources, is difficult.
Clyra Medical
In the three and six months ended June 30, 2023, Clyra Medical generated zero and $6,000 in revenues, had $393,000 and $825,000 in total costs and expenses which included $60,000 and $194,000 in research and development expenses, and an operating loss of $396,000 and $823,000, respectively. In the same periods in 2022, it generated zero and $17,000 in revenue, had $257,000 and $508,000 in total costs and expenses, including $26,000 and $42,000 in research and development expenses, and an operating loss of $256,000 and $496,000. We expect these losses to continue until Clyra can increase its sales of its Bioclynse wound irrigation solution.
BETI
Formed to develop and commercialize a sodium-sulfur battery technology, BETI raised $330,000 and $880,000 in investment capital in the three and six months ended June 30, 2023, respectively, did not generate revenue, and incurred $297,000 and $384,000 in expenses, respectively, primarily related to the development of battery prototypes.
Selling, General and Administrative Expense – consolidated
Our Selling, General and Administrative expense (“SG&A”) include both cash (for example, salaries to employees) and non-cash expenses (for example, stock option compensation expense). Our consolidated SG&A increased in the aggregate by 3% and 13% in the three and six months ended June 30, 2023, to $1,791,000 and $3,515,000, respectively. The largest components of our SG&A expenses included (in thousands):
| |
|
Three months ended: |
|
|
Six months ended: |
|
| |
|
June 30, 2023 |
|
|
June 30, 2022 |
|
|
June 30, 2023 |
|
|
June 30, 2022 |
|
| Salaries and payroll related |
|
$ |
574 |
|
|
$ |
633 |
|
|
$ |
1,180 |
|
|
$ |
1,442 |
|
| Professional fees |
|
|
203 |
|
|
|
198 |
|
|
|
414 |
|
|
|
344 |
|
| Consulting |
|
|
186 |
|
|
|
133 |
|
|
|
384 |
|
|
|
450 |
|
| Office expense |
|
|
494 |
|
|
|
420 |
|
|
|
952 |
|
|
|
730 |
|
| Sales and marketing |
|
|
118 |
|
|
|
75 |
|
|
|
211 |
|
|
|
134 |
|
| Investor relations |
|
|
86 |
|
|
|
62 |
|
|
|
179 |
|
|
|
147 |
|
| Board of director expense |
|
|
130 |
|
|
|
68 |
|
|
|
195 |
|
|
|
178 |
|
In the three and six months ended June 30, 2023, our non-cash expenses from stock for service, stock options and warrant expense total $363,000 and $859,000, respectively, and $375,000 and $809,000 in the same periods in the prior year. Office expense included $163,000 and $307,000 of insurance expenses in the three and six months ended June 30, 2023, and $124,000 and $243,000 in the same periods in the prior year. Insurance expenses increased due to market conditions unrelated to the Company’s activities.
Research and Development
In the three and six months ended June 30, 2023, we spent $587,000 and $1,152,000, respectively, in the research and development of our technologies and products. This was an increase of 63% and 54% compared to the three and six months ended June 30, 2022. The increase is related to increased headcount and the work at BETI.
Interest expense
Our interest expense for the three and six months ended June 30, 2023, was $12,000 and $60,000, respectively, compared with $15,000 and $28,000, respectively, in the same periods in 2022. Of our total interest expense in 2023, $27,000 was paid in cash, and the remainder was comprised primarily of non-cash debt discounts related to warrants issued in conjunction with debt instruments being amortized over the life of the debt instrument; for the six months ended June 30, 2023, non-cash interest expense totaled $33,000.
Other Income and Expense
The amount of grant income increased $24,000 in the six months ended June 30, 2023, to $32,000. For the three months ended June 30, 2023, grant income was zero, compared with $3,000 in the prior year period. Grant income is primarily generated through our wholly owned Canadian subsidiary, we have been awarded more than 80 research grants over the years from various public and private agencies, including the Canadian National Research Institute – Industrial Research Assistance Program (NRC-IRAP), the National Science and Engineering Research Council of Canada (NSERC), and the Metropolitan Water District of Southern California’s Innovative Conservation Program “ICP”. The research grants received are considered reimbursement grants related to costs we incur and therefore are included as Other Income. Grant funds paid directly to third parties are not included as income in our financial statements.
During the three and six months ended June 30, 2022, we recorded $174,000 in income related to the forgiveness of Paycheck Protection Act loan to ONM Environmental.
Net Loss
Net loss for the three and six months ended June 30, 2023, was $1,626,000 and $2,120,000 a loss of $0.01 per share, compared to a net loss for the three and six months ended June 30, 2022, of $1,333,000 and $2,897,000, a loss of $0.01 per share, an increase of 22% and decrease of 33%, respectively. Our net loss for the three months ended June 30, 2023, increased because of the decrease in engineering service revenue and an increase in total costs and expenses, offset by the increase in product revenue. Our net loss decreased during the six months ended June 30, 2023, primarily due to the increase of product revenue, offset by a decrease of engineering service revenue and increase in total costs and expenses.
The net income (loss) per business segment is as follows (in thousands):
| |
|
Three months ended |
|
|
Six months ended |
|
| |
|
June 30, 2023 |
|
|
June 30, 2022 |
|
|
June 30, 2023 |
|
|
June 30, 2022 |
|
| BioLargo corporate |
|
$ |
(639 |
) |
|
$ |
(929 |
) |
|
$ |
(1,474 |
) |
|
$ |
(2,155 |
) |
| ONM |
|
|
426 |
|
|
|
11 |
|
|
|
1,811 |
|
|
|
192 |
|
| Clyra Medical |
|
|
(404 |
) |
|
|
(265 |
) |
|
|
(840 |
) |
|
|
(512 |
) |
| BLEST |
|
|
(450 |
) |
|
|
56 |
|
|
|
(818 |
) |
|
|
21 |
|
| BETI |
|
|
(297 |
) |
|
|
-- |
|
|
|
(384 |
) |
|
|
-- |
|
| BioLargo Water |
|
|
(262 |
) |
|
|
(206 |
) |
|
|
(415 |
) |
|
|
(423 |
) |
| Net loss |
|
|
(1,626 |
) |
|
|
(1,333 |
) |
|
|
(2,120 |
) |
|
|
(2,877 |
) |
Liquidity and Capital Resources
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the six months ended June 30, 2023, we generated revenues of $5,188,000 through our business segments (see Note 11), had a net loss of $2,120,000, used $1,527,000 cash in operations, and at June 30, 2023, we had working capital of $3,553,000, and current assets of $4,847,000.
During the six months ended June 30, 2023, we (i) sold $299,000 of our common stock to Lincoln Park Capital Fund, LLC (“Lincoln Park”) (see Note 3), (ii) sold $995,000 of our common stock and warrants to accredited investors (see Notes 3 and 6), (iii) sold $1,287,000 of Clyra Medical Series A Preferred Stock (see Note 8), and (iv) sold $880,000 of BETI common stock (see Note 10). Subsequent to June 30, 2023, we continued these financing activities (see Note 13). As of June 30, 2023, our cash and cash equivalents totaled $3,575,000. Our total liabilities included a $75,000 vehicle loan, $140,000 due in U.S. Small Business Administration (SBA) loans issued pursuant to the Paycheck Protection Program (see Note 4), $150,000 due to the SBA issued pursuant to the Economic Injury Disaster program (see Note 4), and $243,000 owed by Clyra Medical due in 2024 (see Note 8). We have been, and anticipate that we will continue to be, limited in terms of our capital resources, and expect to continue to need further investment capital to fund operations. Such activities have continued subsequent to June 30, 2023.
If we are unable to rely on our current arrangement with Lincoln Park to fund our working capital requirements, we will have to rely on other forms of financing, and there is no assurance that we will be able to do so, or if we do so, it will be on favorable terms.
The foregoing factors raise substantial doubt about our ability to continue as a going concern, unless we are able to continue to raise funds through stock sales to Lincoln Park or other private financings, and in the long term, our ability to attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Critical Accounting Policies
Our discussion and analysis of our results of operations and liquidity and capital resources are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, valuation of offerings of debt with equity or derivative features which include the valuation of the warrant component, any beneficial conversion feature and potential derivative treatment, and share-based payments. We base our estimates on anticipated results and trends and on various other assumptions that we believe are reasonable under the circumstances, including assumptions as to future events. These estimates form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. By their nature, estimates are subject to an inherent degree of uncertainty. Actual results that differ from our estimates could have a significant adverse effect on our operating results and financial position.
Our significant accounting policies and methods used in the preparation of the Company’s consolidated financial statements are described in (i) in Part I, Item 1 of this Form 10-Q, Note 2, “Summary of Significant Accounting Policies” and (ii) in the Form 10-K for the year ended December 31, 2022, filed with the SEC on March 31, 2023, in the Notes to Consolidated Financial Statements in Part II, Item 8, and “Critical Accounting Policies and Estimates” in Part II, Item 7. There have been no material changes to the Company’s critical accounting policies and estimates since the filing of its Form 10-K.
Item 4. Controls and Procedures
We conducted an evaluation, under the supervision and with the participation of management, including our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended – the “Exchange Act”) as of the end of the period covered by this Report. There were no changes in our internal control over financial reporting during the quarter ended June 30, 2023, which were identified in connection with management’s evaluation required by paragraph (d) of Rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
Our procedures have been designed to ensure that the information relating to our company, including our consolidated subsidiaries, required to be disclosed in our SEC reports is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow for timely decisions regarding required disclosure. However, our Company is continuing to grow and evolve, as our product and services sales continues to grow, and as we diversify our clients to include municipalities, increasing strain on our accounting systems. These activities put stress on our overall controls and procedures. As our operations do not yet generate enough cash to fund operations, and we rely on financing activities to maintain our level of operations and fund our anticipated growth, we do not yet have the ability to implement the more sophisticated control systems used by larger companies. Although we have made some improvements, our chief executive officer and chief financial officer have concluded that, as of the evaluation date, our disclosure controls and procedures were not effective, due to the material weakness identified below.
It should be noted that the design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our chief executive officer and the chief financial officer, we have established internal control procedures in accordance with the guidelines established in the 2013 Framework —Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Management evaluated the effectiveness of our internal controls, and concluded that due to our limited financial and personnel resources, the fact that we operate our business in three distinct locations in the U.S. and Canada, and the lack of sophisticated reporting systems, we continue to have a material weakness in our internal controls with respect to the closing our financial statements. Until the Company has the financial resources to implement more robust automated systems, or to hire additional dedicated accounting personnel, we expect this material weakness to continue.
Our management, including our chief executive officer and chief financial officer, does not expect that our disclosure controls or our internal control over financial reporting, or any system we design or implement in the future, will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
PART II
OTHER INFORMATION
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
The following is a report of sales of unregistered securities during the period covered by this report not previously reported in an annual report on Form 10-K, a Quarterly Report on Form 10-Q, or a Current Report on Form 8-K.
Unit Offering
During the three months ended June 30, 2023, we sold 1,578,948 shares of our common stock and received $300,000 in gross and net proceeds from eleven accredited investors. In addition to the shares, we issued the investors six-month warrants to purchase an aggregate 1,578,948 additional shares at $0.23 per share, and five-year warrants to purchase an aggregate 1,578,948 additional shares at $0.29 per share.
Clyra Medical
During the three months ended June 30, 2023, Clyra sold 3,427 shares of its Series A Preferred Stock, and in exchange received $1,062,000 in gross and net proceeds. Purchasers of the Series A Preferred Stock also received a 3-year warrant to purchase the same number of additional shares of common stock for $372 per share. Shares of Series A Preferred Stock earn a dividend of 15% each year. Each share of Series A Preferred stock can be converted by the holder at any time for one share of common stock. Accrued dividends may be converted to common stock at a conversion rate of $310 per share.
Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its Series A Preferred stock, plus accrued dividends, into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 30 prior trading days. Elections must be made during the 18-month period that begins 18 months after the closing of the Series A Preferred offering (which has not yet taken place), or June 30, 2023, whichever is earlier.
BETI
During the three months ended June 30, 2023, BETI sold 132,000 shares of its common stock to nine accredited investors and received $330,000 in gross and net proceeds. Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its shares of BETI common stock into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 20 trading days prior to the election to exchange. Elections must be made during calendar year 2024.
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Item 5. Other Information
Lincoln Park
On December 13, 2022, we entered into a purchase agreement (the “Purchase Agreement”) and a registration rights agreement (the “Registration Rights Agreement”), with Lincoln Park Capital Fund, LLC (“Lincoln Park”), pursuant to which Lincoln Park has committed to purchase up to $10.0 million of the Company’s common stock, par value $0.00067 per share (the “Common Stock”), subject to certain limitations and the satisfaction of the conditions set forth in the Purchase Agreement. (See Item 9B titled “Other Information”, and the subheading “Lincoln Park”, for additional information.)
During the three months ended June 30, 2023, we sold 1,098,221 shares of our common stock to Lincoln Park, and received $194,000 in gross and net proceeds.
Item 6. Exhibits
See the Exhibit Index for a list of exhibits filed as part of this report and incorporated herein by reference.
Exhibit Index
| |
|
Incorporated by Reference Herein |
| Exhibit Number |
Exhibit Description |
Form |
File Date |
| 3.1 |
Amended and Restated Bylaws |
Form 10-KSB |
5/23/2003 |
| 3.2 |
Amended and Restated Certificate of Incorporation for BioLargo, Inc. filed March 16, 2007 |
Form 10-KSB |
5/4/2007 |
| 3.3 |
Certificate of Amendment to Certificate of Incorporation, filed May 25, 2018 |
Pos Am |
6/22/2018 |
| 3.4 |
Certificate of Amendment to Certificate of Incorporation, filed August 30, 2022 |
Form 10-Q |
11/14/2022 |
| 3.5 |
Amended and Restated Articles of Incorporation of Clyra Medical Technologies, Inc. (filed December 30, 2022) |
Form 10-K |
3/31/2023 |
| 4.1 |
BioLargo, Inc. 2007 Equity Incentive Plan |
Form 10-QSB |
11/19/2007 |
| 4.2 |
Amendment No. 1 to BioLargo 2007 Equity Incentive Plan |
Def 14C (Exhibit A) |
5/2/2011 |
| 4.3 |
Form of Stock Options issued in exchange for reduction in accounts payable. |
Form 10-K |
3/31/2015 |
| 4.4 |
$50,000 convertible note, matures March 8, 2020 |
Form 10-Q |
5/14/2018 |
| 4.5 |
2018 Equity Incentive Plan |
Form S-8 |
6/22/2018 |
| 4.6 |
Stock Option Award Agreement under 2018 Equity Incentive Plan |
Form S-8 |
6/22/2018 |
| 4.7 |
Notice of Stock Option Grant under 2018 Equity Incentive Plan |
Form S-8 |
6/22/2018 |
| 4.8 |
Restricted Stock Unit Award Agreement under 2018 Equity Incentive Plan |
Form S-8 |
6/22/2018 |
| 4.9 |
Notice of Restricted Stock Unit Award under 2018 Equity Incentive Plan |
Form S-8 |
6/22/2018 |
| 4.10 |
Revolving Line of Credit Agreement dated June 30, 2020, between Clyra Medical and Vernal Bay |
Form 8-K |
7/7/2020 |
| 4.11 |
Security Agreement dated June 30, 2020, between Clyra Medical and Vernal Bay |
Form 8-K |
7/7/2020 |
| 4.12 |
Revolving Line of Credit Note issued by Clyra Medical to Vernal Bay on June 30, 2020 |
Form 8-K |
7/7/2020 |
| 4.13 |
Warrant issued in 2020 Unit Offering |
Form 10-Q |
8/14/2020 |
| 4.14 |
Amendment to $50,000 Convertible Note dated March 8, 2018 |
Form 10-K |
3/30/2021 |
| 4.15 |
Warrant issued to $50,000 Convertible Noteholder on March 1, 2020 |
Form 10-K |
3/30/2021 |
| 4.16 |
Satisfaction of Convertible Note dated March 8, 2018 |
Form 10-K |
3/31/2023 |
| 10.1 |
Form of indemnity agreement between the Company at its officers and directors |
Form 10-K |
3/31/2023 |
| 10.2 |
License Agreement to Clyra Medical Technologies, Inc., dated December 17, 2012 |
Form 8-K |
1/6/2016 |
| 10.3 |
December 30, 2015 amendment to License Agreement with Clyra Medical Technologies, Inc. |
Form 8-K |
1/6/2016 |
| 10.4 |
Consulting Agreement dated December 30, 2015 with Beach House Consulting LLC |
Form 8-K |
1/6/2016 |
| 10.5 |
Commercial Office Lease Agreement for 14921 Chestnut St., Westminster, CA 92683 |
Form 8-K |
8/24/2016 |
| 10.6† |
Employment Agreement with Dennis P. Calvert dated May 2, 2017. |
Form 8-K |
5/4/2017 |
| 10.7† |
Lock-Up Agreement with Dennis P. Calvert dated April 30, 2017 |
Form 8-K |
5/4/2017 |
| 10.8 |
Commercial Office Lease Agreement for Oak Ridge Tennessee |
Form 8-K |
9/8/2017 |
| 10.9 |
Form of Employment Agreement for Engineering Subsidiary |
Form 8-K |
9/8/2017 |
| 10.10 |
Form of Option issued to founding employees of Engineering subsidiary (BLEST) |
Form 8-K |
9/8/2017 |
| 10.11† |
Employment Agreement with Joseph L. Provenzano dated May 28, 2019 |
Form 8-K |
6/24/2019 |
| 10.12† |
Lock-Up Agreement dated May 28, 2019 |
Form 8-K |
6/24/2019 |
| 10.13 |
Purchase Agreement, dated as of March 30, 2020 by and between BioLargo, Inc. and Lincoln Park Capital Fund, LLC. |
Form 8-K |
3/31/2020 |
| 10.14 |
Amendment dated June 30, 2020 to License Agreement with Clyra Medical Technologies, Inc. |
Form 8-K |
7/7/2020 |
| 10.15 |
Amendment dated June 30, 2020 to Consulting Agreement dated December 30, 2015 between Clyra Medical and Beach House Consulting LLC |
Form 8-K |
7/7/2020 |
| 10.16† |
2022 Engagement Extension Agreement with CFO |
Form 8-K |
3/24/2022 |
| 10.17 |
Purchase Agreement, dated as of December 13, 2022, by and between BioLargo, Inc. and Lincoln Park Capital Fund, LLC. |
Form 8-K |
12/19/2022 |
| 10.18 |
Registration Rights Agreement, dated as of December 13, 2022, by and between BioLargo, Inc. and Lincoln Park Capital Fund, LLC |
Form 8-K |
12/19/2022 |
| 10.19† |
2023 Engagement Extension Agreement with CFO |
Form 8-K |
3/27/2023 |
| 10.20 |
Form of Share Exchange Agreement between BioLargo, Inc., and purchasers of Clyra Medical Series A Preferred Stock |
Form 10-Q |
5/17/2023 |
* Filed herewith
** Furnished herewith
† Management contract or compensatory plan, contract or arrangement
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Date: August 14, 2023 |
|
BIOLARGO, INC. By: /s/ DENNIS P. CALVERT |
| |
|
Dennis P. Calvert Chief Executive Officer |
| |
|
|
| |
|
|
| Date: August 14, 2023 |
|
By: /s/ CHARLES K. DARGAN, II |
| |
|
Chief Financial Officer |
EXHIBIT 31.1
Certification of Chief Executive Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Dennis P. Calvert, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q of BioLargo, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation;
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting;
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| |
|
|
|
|
|
|
| |
|
|
|
|
Date: August 14, 2023
|
|
|
|
By:
|
|
/s/ DENNIS P. CALVERT
|
| |
|
|
|
|
|
Dennis P. Calvert
|
| |
|
|
|
|
|
Chief Executive Officer
|
EXHIBIT 31.2
Certification of Chief Financial Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Charles K. Dargan II, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q of BioLargo, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation;
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting;
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| |
|
|
|
|
|
|
| |
|
|
|
|
Date: August 14, 2023
|
|
|
|
By:
|
|
/s/ CHARLES K. DARGAN II
|
| |
|
|
|
|
|
Charles K. Dargan II
|
| |
|
|
|
|
|
Chief Financial Officer
|
EXHIBIT 32
CERTIFICATION OF CHIEF EXECUTIVE OFFICER
AND CHIEF FINANCIAL OFFICER
PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
I, Dennis P. Calvert, Chief Executive Officer of BioLargo, Inc., certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that the Quarterly Report of BioLargo, Inc. on Form 10-Q for the quarter ended June 30, 2023, fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and that information contained in such Quarterly Report on Form 10-Q fairly presents in all material respects the financial condition and results of operations of BioLargo, Inc.
| |
|
|
|
|
|
|
| |
|
|
|
|
Dated: August 14, 2023
|
|
|
|
By:
|
|
/s/ DENNIS P. CALVERT
|
| |
|
|
|
|
|
Dennis P. Calvert
|
| |
|
|
|
|
|
President and Chief Executive Officer
|
A signed original of this written statement required by Section 906 of the Sarbanes-Oxley Act of 2002 has been provided to BioLargo, Inc. and will be retained by BioLargo, Inc. and furnished to the Securities and Exchange Commission or its staff upon request.
I, Charles K. Dargan II, Chief Financial Officer of BioLargo, Inc., certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge that the Quarterly Report of BioLargo, Inc. on Form 10-Q for the quarter ended June 30, 2023, fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and that information contained in such Quarterly Report on Form 10-Q fairly presents in all material respects the financial condition and results of operations of BioLargo, Inc.
| |
|
|
|
|
|
|
| |
|
|
|
|
Dated: August 14, 2023
|
|
|
|
By:
|
|
/s/ CHARLES K. DARGAN II
|
| |
|
|
|
|
|
Charles K. Dargan II
|
| |
|
|
|
|
|
Chief Financial Officer
|
A signed original of this written statement required by Section 906 of the Sarbanes-Oxley Act of 2002 has been provided to BioLargo, Inc. and will be retained by BioLargo, Inc. and furnished to the Securities and Exchange Commission or its staff upon request.
v3.23.2
Document And Entity Information - shares
|
6 Months Ended |
|
Jun. 30, 2023 |
Aug. 11, 2023 |
| Document Information [Line Items] |
|
|
| Entity Central Index Key |
0000880242
|
|
| Entity Registrant Name |
BIOLARGO, INC.
|
|
| Amendment Flag |
false
|
|
| Current Fiscal Year End Date |
--12-31
|
|
| Document Fiscal Period Focus |
Q2
|
|
| Document Fiscal Year Focus |
2023
|
|
| Document Type |
10-Q
|
|
| Document Quarterly Report |
true
|
|
| Document Period End Date |
Jun. 30, 2023
|
|
| Document Transition Report |
false
|
|
| Entity File Number |
000-19709
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
| Entity Tax Identification Number |
65-0159115
|
|
| Entity Address, Address Line One |
14921 Chestnut St.
|
|
| Entity Address, City or Town |
Westminster
|
|
| Entity Address, State or Province |
CA
|
|
| Entity Address, Postal Zip Code |
92683
|
|
| City Area Code |
888
|
|
| Local Phone Number |
400-2863
|
|
| Title of 12(b) Security |
Common stock
|
|
| Trading Symbol |
BLGO
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Small Business |
true
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Shell Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
288,073,533
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Consolidated Balance Sheets (Current Period Unaudited) - USD ($)
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 3,575,000
|
$ 1,851,000
|
| Accounts receivable, net of allowance |
1,037,000
|
1,064,000
|
| Inventories, net of allowance |
127,000
|
120,000
|
| Prepaid expenses and other current assets |
108,000
|
118,000
|
| Total current assets |
4,847,000
|
3,153,000
|
| Property and equipment, net of depreciation |
542,000
|
287,000
|
| Other non-current assets |
69,000
|
124,000
|
| Investment in South Korean joint venture |
21,000
|
33,000
|
| Right of use, operating lease, net of amortization |
807,000
|
867,000
|
| Clyra Medical prepaid marketing |
394,000
|
394,000
|
| Total assets |
6,680,000
|
4,858,000
|
| Current liabilities: |
|
|
| Accounts payable and accrued expenses |
720,000
|
940,000
|
| Debt obligations |
66,000
|
100,000
|
| Deferred revenue |
10,000
|
17,000
|
| Lease liability |
97,000
|
97,000
|
| Customer deposits |
113,000
|
184,000
|
| Total current liabilities |
1,294,000
|
1,576,000
|
| Long-term liabilities: |
|
|
| Debt obligations, net of current |
299,000
|
237,000
|
| Lease liability, net of current |
721,000
|
773,000
|
| Total long-term liabilities |
1,263,000
|
1,271,000
|
| Total liabilities |
2,557,000
|
2,847,000
|
| COMMITMENTS AND CONTINGENCIES (Note 12) |
|
|
| STOCKHOLDERS’ EQUITY: |
|
|
| Preferred Series A, $0.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding, at June 30, 2023 and December 31, 2022 |
0
|
0
|
| Common stock, $0.00067 Par Value, 550,000,000 Shares Authorized, 287,220,661 and 278,462,706 Shares Issued, at June 30, 2023 and December 31, 2022, respectively |
192,000
|
186,000
|
| Additional paid-in capital |
152,507,000
|
148,435,000
|
| Accumulated deficit |
(145,169,000)
|
(143,594,000)
|
| Accumulated other comprehensive loss |
(162,000)
|
(149,000)
|
| Total BioLargo Inc. and subsidiaries stockholders’ equity |
7,368,000
|
4,878,000
|
| Non-controlling interest |
(3,245,000)
|
(2,867,000)
|
| Total stockholders’ equity |
4,123,000
|
2,011,000
|
| Total liabilities and stockholders’ equity |
6,680,000
|
4,858,000
|
| Entities, Excluding Partially Owned Subsidiary [Member] |
|
|
| Current liabilities: |
|
|
| Accounts payable and accrued expenses |
720,000
|
940,000
|
| Partially Owned Subsidiary [Member] |
|
|
| Current liabilities: |
|
|
| Accounts payable and accrued expenses |
288,000
|
238,000
|
| Long-term liabilities: |
|
|
| Debt obligations, net of current |
$ 243,000
|
$ 261,000
|
| X |
- DefinitionAmount of customer deposit liability, classified as current.
| Name: |
blgo_CustomerDepositLiabilityCurrent |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of obligation to transfer good or service to customer for which consideration has been received or is receivable, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479837/606-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-8
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479837/606-10-45-2
| Name: |
us-gaap_ContractWithCustomerLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis item represents the carrying amount on the entity's balance sheet of its investment in common stock of an equity method investee. This is not an indicator of the fair value of the investment, rather it is the initial cost adjusted for the entity's share of earnings and losses of the investee, adjusted for any distributions (dividends) and other than temporary impairment (OTTI) losses recognized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481664/323-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
| Name: |
us-gaap_EquityMethodInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of obligation due after one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 23: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as current. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as noncurrent. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_MinorityInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of amounts paid in advance for expenses which will be charged against earnings in periods after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 34: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 38: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 40: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 41: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-15
Reference 42: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-16
Reference 43: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4I
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_EntitiesExcludingPartiallyOwnedSubsidiaryMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_PartiallyOwnedSubsidiaryMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Consolidated Balance Sheets (Current Period Unaudited) (Parentheticals) - $ / shares
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Convertible Preferred Stock, Par Value (in dollars per share) |
$ 0.00067
|
$ 0.00067
|
| Convertible Preferred Stock, Shares Authorized (in shares) |
50,000,000
|
50,000,000
|
| Convertible Preferred Stock, Shares Issued (in shares) |
0
|
0
|
| Convertible Preferred Stock, Shares Outstanding (in shares) |
0
|
0
|
| Common stock, par value (in dollars per share) |
$ 0.00067
|
$ 0.00067
|
| Common stock, shares authorized (in shares) |
550,000,000
|
550,000,000
|
| Common stock, shares issued (in shares) |
287,220,661
|
278,462,706
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.2
Consolidated Statements of Operations and Comprehensive Loss (Unaudited) - USD ($)
|
3 Months Ended |
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Revenue from Contract with Customer, Including Assessed Tax |
$ 1,446,000
|
$ 1,323,000
|
$ 5,188,000
|
$ 2,287,000
|
| Cost of revenue |
|
|
|
|
| Gross profit |
819,000
|
623,000
|
2,630,000
|
1,141,000
|
| Selling, general and administrative expenses |
1,791,000
|
1,589,000
|
3,515,000
|
3,425,000
|
| Research and development |
587,000
|
355,000
|
1,152,000
|
747,000
|
| Operating loss |
(1,559,000)
|
(1,321,000)
|
(2,037,000)
|
(3,031,000)
|
| Other (expense) income: |
|
|
|
|
| Interest expense |
(12,000)
|
(15,000)
|
(60,000)
|
(28,000)
|
| PPP loan forgiveness |
0
|
0
|
0
|
174,000
|
| Tax credit reversal |
(55,000)
|
0
|
(55,000)
|
0
|
| Grant income |
0
|
3,000
|
32,000
|
8,000
|
| Total other expense: |
(67,000)
|
(12,000)
|
(83,000)
|
154,000
|
| Net loss |
(1,626,000)
|
(1,333,000)
|
(2,120,000)
|
(2,877,000)
|
| Net loss attributable to noncontrolling interest |
(298,000)
|
(105,000)
|
(545,000)
|
3,000
|
| Net loss attributable to common shareholders |
$ (1,328,000)
|
$ (1,228,000)
|
$ (1,575,000)
|
$ (2,880,000)
|
| Net loss per share attributable to common shareholders: |
|
|
|
|
| Loss per share attributable to shareholders – basic and diluted (in dollars per share) |
$ (0.01)
|
$ (0.01)
|
$ (0.01)
|
$ (0.01)
|
| Weighted average number of common shares outstanding: (in shares) |
284,944,366
|
265,856,970
|
282,839,515
|
263,345,148
|
| Comprehensive loss: |
|
|
|
|
| Net loss |
$ (1,626,000)
|
$ (1,333,000)
|
$ (2,120,000)
|
$ (2,877,000)
|
| Foreign currency translation |
(7,000)
|
(3,000)
|
(13,000)
|
(11,000)
|
| Comprehensive loss |
(1,633,000)
|
(1,336,000)
|
(2,133,000)
|
(2,888,000)
|
| Comprehensive loss attributable to noncontrolling interest |
(298,000)
|
(105,000)
|
(545,000)
|
3,000
|
| Comprehensive loss attributable to common stockholders |
(1,335,000)
|
(1,231,000)
|
(1,588,000)
|
(2,891,000)
|
| Product [Member] |
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
1,315,000
|
707,000
|
4,864,000
|
1,317,000
|
| Cost of revenue |
|
|
|
|
| Cost of Goods and Services Sold |
(567,000)
|
(364,000)
|
(2,364,000)
|
(659,000)
|
| Service [Member] |
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
131,000
|
616,000
|
324,000
|
970,000
|
| Cost of revenue |
|
|
|
|
| Cost of Goods and Services Sold |
$ (60,000)
|
$ (336,000)
|
$ (194,000)
|
$ (487,000)
|
| X |
- DefinitionRepresents the amount of gain recognized during the period from Paycheck Protection Program (CARES Act) loan forgiveness.
| Name: |
blgo_GainFromPPPLoanForgiveness |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of nonoperating income from grants.
| Name: |
blgo_NonoperatingIncomeGrantIncome |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the amount of tax credit income during the period.
| Name: |
blgo_TaxCreditIncome |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income (loss) and other comprehensive income (loss), attributable to noncontrolling interests. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs related to goods produced and sold and services rendered by an entity during the reporting period. This excludes costs incurred during the reporting period related to financial services rendered and other revenue generating activities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2(a),(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfGoodsAndServicesSold |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CostOfRevenueAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of Net Income (Loss) attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
| Name: |
us-gaap_NetIncomeLossAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-5
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-11
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherIncomeAndExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ProductMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ServiceMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Consolidated Statement of Stockholders' Equity (Deficit) (Unaudited) - USD ($)
|
Clyra Medical Technologies [Member]
Common Stock [Member]
|
Clyra Medical Technologies [Member]
Additional Paid-in Capital [Member]
|
Clyra Medical Technologies [Member]
Retained Earnings [Member]
|
Clyra Medical Technologies [Member]
AOCI Attributable to Parent [Member]
|
Clyra Medical Technologies [Member]
Noncontrolling Interest [Member]
|
Clyra Medical Technologies [Member] |
BioLargo Energy Technologies, Inc (BETI) [Member]
Common Stock [Member]
|
BioLargo Energy Technologies, Inc (BETI) [Member]
Additional Paid-in Capital [Member]
|
BioLargo Energy Technologies, Inc (BETI) [Member]
Retained Earnings [Member]
|
BioLargo Energy Technologies, Inc (BETI) [Member]
AOCI Attributable to Parent [Member]
|
BioLargo Energy Technologies, Inc (BETI) [Member]
Noncontrolling Interest [Member]
|
BioLargo Energy Technologies, Inc (BETI) [Member] |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
AOCI Attributable to Parent [Member] |
Noncontrolling Interest [Member] |
Total |
| Balance (in shares) at Dec. 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
255,893,726
|
|
|
|
|
|
| Balance at Dec. 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 171,000
|
$ 143,718,000
|
$ (139,121,000)
|
$ (115,000)
|
$ (3,720,000)
|
$ 933,000
|
| Sale of stock for cash (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
6,703,789
|
|
|
|
|
|
| Sale of stock for cash |
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,000
|
1,198,000
|
0
|
0
|
0
|
1,202,000
|
| Issuance of common stock for services (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
86,752
|
|
|
|
|
|
| Issuance of common stock for services |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
17,000
|
0
|
0
|
0
|
17,000
|
| Stock option compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
660,000
|
0
|
0
|
0
|
660,000
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
$ 0
|
$ 141,000
|
$ 0
|
$ 0
|
$ 0
|
$ 141,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Noncontrolling interest allocation |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
(528,000)
|
0
|
0
|
528,000
|
0
|
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
(1,652,000)
|
0
|
108,000
|
(1,544,000)
|
| Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
0
|
0
|
(8,000)
|
0
|
(8,000)
|
| Balance (in shares) at Mar. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
262,684,267
|
|
|
|
|
|
| Balance at Mar. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 175,000
|
145,206,000
|
(140,773,000)
|
(123,000)
|
(3,084,000)
|
1,401,000
|
| Balance (in shares) at Dec. 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
255,893,726
|
|
|
|
|
|
| Balance at Dec. 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 171,000
|
143,718,000
|
(139,121,000)
|
(115,000)
|
(3,720,000)
|
933,000
|
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,877,000)
|
| Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(11,000)
|
| Balance (in shares) at Jun. 30, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
268,036,728
|
|
|
|
|
|
| Balance at Jun. 30, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 179,000
|
146,422,000
|
(142,001,000)
|
(126,000)
|
(3,086,000)
|
1,388,000
|
| Balance (in shares) at Mar. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
262,684,267
|
|
|
|
|
|
| Balance at Mar. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 175,000
|
145,206,000
|
(140,773,000)
|
(123,000)
|
(3,084,000)
|
1,401,000
|
| Sale of stock for cash (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
5,011,570
|
|
|
|
|
|
| Sale of stock for cash |
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,000
|
944,000
|
0
|
0
|
0
|
948,000
|
| Issuance of common stock for services (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
340,891
|
|
|
|
|
|
| Issuance of common stock for services |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
59,000
|
0
|
0
|
0
|
59,000
|
| Stock option compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
234,000
|
0
|
0
|
0
|
234,000
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
82,000
|
0
|
0
|
0
|
82,000
|
| Noncontrolling interest allocation |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
(103,000)
|
0
|
0
|
103,000
|
0
|
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
(1,228,000)
|
0
|
(105,000)
|
(1,333,000)
|
| Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
0
|
0
|
(3,000)
|
0
|
(3,000)
|
| Balance (in shares) at Jun. 30, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
268,036,728
|
|
|
|
|
|
| Balance at Jun. 30, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 179,000
|
146,422,000
|
(142,001,000)
|
(126,000)
|
(3,086,000)
|
1,388,000
|
| Balance (in shares) at Dec. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
278,462,706
|
|
|
|
|
|
| Balance at Dec. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 186,000
|
148,435,000
|
(143,594,000)
|
(149,000)
|
(2,867,000)
|
2,011,000
|
| Sale of stock for cash (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
4,201,402
|
|
|
|
|
|
| Sale of stock for cash |
|
|
|
|
|
|
|
|
|
|
|
|
$ 3,000
|
797,000
|
0
|
0
|
0
|
800,000
|
| Issuance of common stock for services (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
930,490
|
|
|
|
|
|
| Issuance of common stock for services |
|
|
|
|
|
|
|
|
|
|
|
|
$ 1,000
|
206,000
|
0
|
0
|
0
|
207,000
|
| Issuance of common stock in exchange for Clyra shares (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
527,983
|
|
|
|
|
|
| Stock option compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
195,000
|
0
|
0
|
0
|
195,000
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
0
|
61,000
|
0
|
0
|
0
|
61,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrant issued for interest |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
30,000
|
0
|
0
|
0
|
30,000
|
| Clyra – sales of Series A Preferred Stock |
0
|
0
|
0
|
0
|
225,000
|
225,000
|
$ 0
|
$ 0
|
$ 0
|
$ 0
|
$ 550,000
|
$ 550,000
|
|
|
|
|
|
|
| Clyra – Series A Preferred Stock – dividend |
$ 0
|
$ 0
|
$ 0
|
$ 0
|
$ (27,000)
|
(27,000)
|
|
|
|
|
|
|
|
|
|
|
|
|
| Noncontrolling interest allocation |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
467,000
|
0
|
0
|
(467,000)
|
0
|
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
(247,000)
|
0
|
(247,000)
|
(494,000)
|
| Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
0
|
0
|
(6,000)
|
0
|
(6,000)
|
| Balance (in shares) at Mar. 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
284,122,581
|
|
|
|
|
|
| Balance at Mar. 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 190,000
|
150,191,000
|
(143,841,000)
|
(155,000)
|
(2,833,000)
|
3,552,000
|
| Balance (in shares) at Dec. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
278,462,706
|
|
|
|
|
|
| Balance at Dec. 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 186,000
|
148,435,000
|
(143,594,000)
|
(149,000)
|
(2,867,000)
|
2,011,000
|
| Clyra – sales of Series A Preferred Stock |
|
|
|
|
|
$ 1,287,000
|
|
|
|
|
|
$ 880,000
|
|
|
|
|
|
|
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,120,000)
|
| Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(13,000)
|
| Balance (in shares) at Jun. 30, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
287,220,661
|
|
|
|
|
|
| Balance at Jun. 30, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 192,000
|
152,507,000
|
(145,169,000)
|
(162,000)
|
(3,245,000)
|
4,123,000
|
| Balance (in shares) at Mar. 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
284,122,581
|
|
|
|
|
|
| Balance at Mar. 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 190,000
|
150,191,000
|
(143,841,000)
|
(155,000)
|
(2,833,000)
|
3,552,000
|
| Sale of stock for cash (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
2,677,169
|
|
|
|
|
|
| Sale of stock for cash |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,000
|
492,000
|
0
|
0
|
0
|
494,000
|
| Issuance of common stock for services (in shares) |
|
|
|
|
|
|
|
|
|
|
|
|
420,911
|
|
|
|
|
|
| Issuance of common stock for services |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
75,000
|
0
|
0
|
0
|
75,000
|
| Stock option compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
222,000
|
0
|
0
|
0
|
222,000
|
| Clyra Medical Technologies, Inc. (Clyra) stock options issued for services |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
66,000
|
0
|
0
|
0
|
66,000
|
| Clyra – sales of Series A Preferred Stock |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
0
|
0
|
1,062,000
|
1,062,000
|
| Clyra – Series A Preferred Stock – dividend |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
0
|
0
|
(45,000)
|
(45,000)
|
| Noncontrolling interest allocation |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
1,461,000
|
0
|
0
|
(1,461,000)
|
0
|
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
(1,328,000)
|
0
|
(298,000)
|
(1,626,000)
|
| Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
|
0
|
0
|
0
|
(7,000)
|
0
|
(7,000)
|
| Biolargo Energy Technology Inc. (BETI) offering |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
0
|
0
|
0
|
330,000
|
330,000
|
| Balance (in shares) at Jun. 30, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
287,220,661
|
|
|
|
|
|
| Balance at Jun. 30, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
$ 192,000
|
$ 152,507,000
|
$ (145,169,000)
|
$ (162,000)
|
$ (3,245,000)
|
$ 4,123,000
|
| X |
- DefinitionAmount of increase in additional paid in capital from subsidiary issuance of equity interests for service.
| Name: |
blgo_AdjustmentsToAdditionalPaidInCapitalIncreaseFromSubsidiaryEquityIssuedForService |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares exchanged for shares of noncontrolling interest during the period.
| Name: |
blgo_StockIssuedDuringPeriodSharesExchangedForNoncontrollingInterest |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis element represents movements included in the statement of changes in stockholders' equity in connection with the allocation of noncontrolling interest from subsidiary's equity issuance.
| Name: |
blgo_StockholdersEquityAllocationOfNoncontrollingInterestFromSubsidiarysEquityIssuance |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for option under share-based payment arrangement.
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalShareBasedCompensationStockOptionsRequisiteServicePeriodRecognition |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in additional paid in capital (APIC) resulting from the issuance of warrants. Includes allocation of proceeds of debt securities issued with detachable stock purchase warrants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 20
-Section 25
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481284/470-20-25-2
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalWarrantIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDecrease in noncontrolling interest balance from payment of dividends or other distributions by the non-wholly owned subsidiary or partially owned entity, included in the consolidation of the parent entity, to the noncontrolling interest holders. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_MinorityInterestDecreaseFromDistributionsToNoncontrollingInterestHolders |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in noncontrolling interest from sale of a portion of the parent's controlling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-23
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(2)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_NoncontrollingInterestIncreaseFromSaleOfParentEquityInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in noncontrolling interest from subsidiary issuance of equity interests to noncontrolling interest holders. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-23
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(2)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_NoncontrollingInterestIncreaseFromSubsidiaryEquityIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued in lieu of cash for services contributed to the entity. Value of the stock issued includes, but is not limited to, services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodValueIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 34: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 38: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 40: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 41: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-15
Reference 42: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-16
Reference 43: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4I
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Consolidated Statements of Cash Flows (Unaudited) - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Cash flows from operating activities |
|
|
| Net loss |
$ (2,120,000)
|
$ (2,877,000)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Stock option compensation expense |
544,000
|
1,117,000
|
| Common stock issued for services |
282,000
|
76,000
|
| Bad debt expense |
46,000
|
0
|
| Excess and obsolete inventory |
54,000
|
0
|
| Amortization of right-of-use operating lease assets |
60,000
|
0
|
| Interest expense related to amortization of the discount on note payable |
3,000
|
8,000
|
| Fair value of warrant issued for interest |
30,000
|
0
|
| PPP loan forgiveness |
0
|
(174,000)
|
| Loss on investment in South Korean joint venture |
12,000
|
15,000
|
| Depreciation expense |
48,000
|
6,000
|
| Changes in assets and liabilities: |
|
|
| Accounts receivable |
(19,000)
|
(66,000)
|
| Inventories |
(63,000)
|
(15,000)
|
| Prepaid expenses and other assets |
66,000
|
4,000
|
| Deferred revenue |
(7,000)
|
(89,000)
|
| Lease liability, net |
(52,000)
|
0
|
| Customer deposits |
(71,000)
|
58,000
|
| Net cash used in operating activities |
(1,527,000)
|
(1,864,000)
|
| Cash flows from investing activities |
|
|
| Equipment purchases |
(127,000)
|
(101,000)
|
| Net cash used in investing activities |
(127,000)
|
(101,000)
|
| Cash flows from financing activities |
|
|
| Proceeds from sale of common stock |
1,294,000
|
2,150,000
|
| Repayment of note payable and vehicle loan |
(52,000)
|
0
|
| Repayment by Clyra on inventory line of credit |
(18,000)
|
(10,000)
|
| Proceeds from sale of Clyra Medical preferred stock |
1,287,000
|
0
|
| Proceeds from Clyra Medical convertible note |
0
|
100,000
|
| Net cash provided by financing activities |
3,391,000
|
2,240,000
|
| Net effect of foreign currency translation |
(13,000)
|
(11,000)
|
| Net change in cash |
1,724,000
|
264,000
|
| Cash at beginning of year |
1,851,000
|
962,000
|
| Cash at end of period |
3,575,000
|
1,226,000
|
| Supplemental disclosures of cash flow information |
|
|
| Interest |
27,000
|
7,000
|
| Income taxes |
5,000
|
0
|
| Short-term lease payments not included in lease liability |
24,000
|
78,000
|
| Non-cash investing and financing activities |
|
|
| Equipment added via vehicle loan |
80,000
|
0
|
| Leasehold improvements included in accounts payable |
102,000
|
0
|
| Conversion of Clyra Common Stock to BioLargo Common Stock [Member] |
|
|
| Non-cash investing and financing activities |
|
|
| Conversion of Clyra common stock to BioLargo common stock |
100,000
|
0
|
| Conversion of Intercompany Receivable into Clyra Shares [Member] |
|
|
| Non-cash investing and financing activities |
|
|
| Allocation of noncontrolling interest |
1,927,000
|
631,000
|
| BETI Common Stock [Member] |
|
|
| Cash flows from financing activities |
|
|
| Proceeds from sale of common stock |
880,000
|
0
|
| Entities, Excluding Partially Owned Subsidiary [Member] |
|
|
| Changes in assets and liabilities: |
|
|
| Accounts payable and accrued expenses |
(391,000)
|
105,000
|
| Partially Owned Subsidiary [Member] |
|
|
| Changes in assets and liabilities: |
|
|
| Accounts payable and accrued expenses |
$ 51,000
|
$ (32,000)
|
| X |
- DefinitionAmount of fair value of warrant issued for interest.
| Name: |
blgo_FairValueOfWarrantIssuedForInterest |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the amount of gain recognized during the period from Paycheck Protection Program (CARES Act) loan forgiveness.
| Name: |
blgo_GainFromPPPLoanForgiveness |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in customer deposit liability.
| Name: |
blgo_IncreaseDecreaseInCustomerDepositLiability |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents The increase (decrease) during the reporting period in right of use and lease liability, net
| Name: |
blgo_IncreaseDecreaseInRightOfUseAndLeaseLiabilityNet |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of leasehold improvements included in accounts payable.
| Name: |
blgo_LeaseholdImprovementsIncludedInAccountsPayable |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNoncash short-term lease payments not included in lease liability.
| Name: |
blgo_ShorttermLeasePaymentsNotIncludedInLeaseLiability |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense attributable to debt discount (premium) and debt issuance costs. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69F
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
| Name: |
us-gaap_AmortizationOfFinancingCostsAndDiscounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFuture cash outflow to pay for purchases of fixed assets that have occurred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_CapitalExpendituresIncurredButNotYetPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe value of the stock converted in a noncash (or part noncash) transaction. Noncash is defined as transactions during a period that do not result in cash receipts or cash payments in the period. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_ConversionOfStockAmountConverted1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of the original debt being converted in a noncash (or part noncash) transaction. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionOriginalDebtAmount1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (loss) for proportionate share of equity method investee's income (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481664/323-10-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(13)(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_IncomeLossFromEquityMethodInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation to transfer good or service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482312/912-310-45-11
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInContractWithCustomerLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of loss from reductions in inventory due to subsequent measurement adjustments, including, but not limited to, physical deterioration, obsolescence, or changes in price levels. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-2
| Name: |
us-gaap_InventoryWriteDown |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of share-based compensation granted to nonemployees as payment for services rendered or acknowledged claims. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IssuanceOfStockAndWarrantsForServicesOrClaims |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for acquisition of machinery and equipment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireMachineryAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from issuance of capital stock which provides for a specific dividend that is paid to the shareholders before any dividends to common stockholders and which takes precedence over common stockholders in the event of liquidation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPreferredStockAndPreferenceStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense related to estimated loss from loan and lease transactions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11B
-Subparagraph (c)(2)
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-11B
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.11)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_ProvisionForLoanAndLeaseLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for the settlement of obligation drawn from a contractual arrangement with the lender, including letter of credit, standby letter of credit and revolving credit arrangements, under which borrowings can be made up to a specific amount at any point in time with maturities due beyond one year or the operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfLongTermLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for a borrowing supported by a written promise to pay an obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConversionOfStockByUniqueDescriptionAxis=blgo_ConversionOfClyraCommonStockToBiolargoCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtConversionByUniqueDescriptionAxis=blgo_ConversionOfIntercompanyReceivableIntoClyraSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=blgo_BetiCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_EntitiesExcludingPartiallyOwnedSubsidiaryMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_PartiallyOwnedSubsidiaryMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 1 - Business and Organization
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Organization, Consolidation and Presentation of Financial Statements Disclosure [Text Block] |
Note 1. Business and Organization
Description of Business
BioLargo, Inc. (“BioLargo”, or the “Company”) invents, develops, and commercializes innovative platform technologies to solve challenging environmental problems like PFAS contamination (per- and polyfluoroalkyl substances), advanced water and wastewater treatment, industrial odor control, air quality control, infection control, and myriad environmental remediation challenges. Our business strategy is straightforward: we invent or acquire technologies that we believe have the potential to be disruptive in large commercial markets; we develop and validate these technologies to advance and promote their commercial success as we leverage our considerable scientific, engineering, and entrepreneurial talent; we then monetize these technical assets through a variety of business structures that may include licensure, joint venture, sale, spin off, or by deploying direct to market strategies.
Organization
We are a Delaware corporation formed in 1991. We have six wholly-owned subsidiaries: BioLargo Life Technologies, Inc., organized under the laws of the State of California in 2006; ONM Environmental, Inc., organized under the laws of the State of California in 2009; BioLargo Equipment and Technologies, Inc., organized under the laws of the State of California in 2022; BioLargo Water, Inc. (“Water”), organized under the laws of Canada in 2014; BioLargo Equipment and Solutions Technologies, Inc., organized under the laws of the State of California in 2022; and BioLargo Development Corp., organized under the laws of the State of California in 2016. Additionally, we own 82% (see Note 9) of BioLargo Engineering Science and Technologies, LLC (“BLEST”), organized under the laws of the State of Tennessee in 2017, 56% of Clyra Medical Technologies, Inc. (“Clyra” or “Clyra Medical”), organized under the laws of the State of California in 2012, and 96% of BioLargo Energy Technologies, Inc. (“BETI”) organized under the laws of the State of California in 2022. We consolidate the financial statements of our partially owned subsidiaries (see Note 2, subheading “Principles of Consolidation,” and Note 8).
Liquidity / Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the six months ended June 30, 2023, we generated revenues of $5,188,000 through our business segments (see Note 11), had a net loss of $2,120,000, used $1,527,000 cash in operations, and at June 30, 2023, we had working capital of $3,553,000, and current assets of $4,847,000.
During the six months ended June 30, 2023, we (i) sold $299,000 of our common stock to Lincoln Park Capital Fund, LLC (“Lincoln Park”) (see Note 3), (ii) sold $995,000 of our common stock and warrants to accredited investors (see Notes 3 and 6), (iii) sold $1,287,000 of Clyra Medical Series A Preferred Stock (see Note 8), and (iv) sold $880,000 of BETI common stock (see Note 10). Subsequent to June 30, 2023, we continued these financing activities (see Note 13). As of June 30, 2023, our cash and cash equivalents totaled $3,575,000. Our total liabilities included a $75,000 vehicle loan, $140,000 due in U.S. Small Business Administration (SBA) loans issued pursuant to the Paycheck Protection Program (see Note 4), $150,000 due to the SBA issued pursuant to the Economic Injury Disaster program (see Note 4), and $243,000 owed by Clyra Medical due in 2024 (see Note 8). We have been, and anticipate that we will continue to be, limited in terms of our capital resources, and expect to continue to need further investment capital to fund operations. Such activities have continued subsequent to June 30, 2023.
If we are unable to rely on our current arrangement with Lincoln Park to fund our working capital requirements, we will have to rely on other forms of financing, and there is no assurance that we will be able to do so, or if we do so, it will be on favorable terms.
The foregoing factors raise substantial doubt about our ability to continue as a going concern, unless we are able to continue to raise funds through stock sales to Lincoln Park or other private financings, and in the long term, our ability to attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Significant Accounting Policies [Text Block] |
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and partially owned subsidiaries BETI, BLEST and Clyra Medical. All intercompany accounts and transactions have been eliminated.
Foreign Currency The Company has designated the functional currency of BioLargo Water, Inc., our Canadian subsidiary, to be the Canadian dollar. Therefore, translation gains and losses resulting from differences in exchange rates are recorded in accumulated other comprehensive income.
Cash and Cash Equivalents The Company considers all highly liquid investments with maturities of three months or less when acquired to be cash equivalents. Substantially all cash equivalents are held in short-term money market accounts at one of the largest financial institutions in the United States, Bank of America. From time to time, our cash account balances are greater than the Federal Deposit Insurance Corporation insurance limit of $250,000 per owner per bank, and during such times, we are exposed to credit loss for amounts in excess of insured limits in the event of non-performance by the financial institution. We do not anticipate non-performance by our financial institution. As of June 30, 2023, and December 31, 2022, our cash balances were made up of the following (in thousands):
| | | June 30, 2023 | | | December 31, 2022 | |
| BioLargo, Inc. and subsidiaries | | $ | 2,762 | | | $ | 1,681 | |
| Clyra Medical Technologies, Inc. | | | 813 | | | | 170 | |
| Total | | $ | 3,575 | | | $ | 1,851 | |
Accounts Receivable In June 2016, the FASB issued ASU 2016-13, which sets out the principles for the recognition of measurement of credit losses on financial instruments, including trade receivables. The standard eliminates the probable initial recognition threshold and requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. The new standard was effective for the Company beginning January 1, 2023 and primarily impacted trade accounts receivable. Accounts receivable are customer obligations that are unconditional. Accounts receivable are presented net of an allowance for doubtful accounts for expected credit losses, which represents an estimate of amounts that may not be collectible. The Company performs ongoing credit evaluations of its customers and, if necessary, provides an allowance for doubtful accounts and expected credit losses. A provision to the allowances for doubtful accounts for expected credit losses is recorded based on factors including the length of time the receivables are past due, the current business environment, and the Company’s historical experience. Provisions to the allowances for doubtful accounts for expected credit losses are recorded to general and administrative expenses. The Company writes off accounts receivable against the allowance when it determines a balance is uncollectible and no longer actively pursues collection of the receivable. The Company does not have any off-balance-sheet credit exposure related to customers. As of June 30, 2023, and December 31, 2022, the allowance for doubtful accounts for expected credit losses was $58,000 and $12,000.
Credit Concentration We have a limited number of customers that account for significant portions of our revenue. During the three and six months ended June 30, 2023, and 2022, the following customers accounted for more than 10% of consolidated revenues:
| | | Three Months | | | Six Months | |
| | | June 30, 2023 | | | June 30, 2022 | | | June 30, 2023 | | | June 30, 2022 | |
| | | | | | | | | | | | | | | | | |
| Customer A | | | 55 | % | | | 39 | % | | | 77 | % | | | 35 | % |
| Customer B | | <10 | % | | | 27 | % | | <10 | % | | | 17 | % |
| Customer C | | <10 | % | | <10 | % | | <10 | % | | | 10 | % |
At June 30, 2023, one customer accounted for more than 10% of consolidated accounts receivable, and at December 31, 2022, three customers accounted for more than 10% of consolidated accounts receivable:
| | | June 30, 2023 | | | December 31, 2022 | |
| Customer A | | | 49 | % | | | 11 | % |
| Customer B | | <10 | % | | | 31 | % |
| Customer D | | <10 | % | | | 15 | % |
Inventory Inventories are stated at the lower of cost or net realizable value using the average cost method. The allowance for obsolete inventory as of June 30, 2023, and December 31, 2022, was $212,000 and $158,000. Inventories consisted of (in thousands):
| | | June 30, 2023 | | | December 31, 2022 | |
| Raw material | | $ | 83 | | | $ | 46 | |
| Finished goods | | | 44 | | | | 74 | |
| Total | | $ | 127 | | | $ | 120 | |
Other Non-Current Assets Other non-current assets consisted of (i) security deposits related to our business offices, (ii) three patents acquired on October 22, 2021, for $34,000, and (iii) tax credit receivables from the Canadian government related to a research and development credit from our Canadian subsidiary for which we’ve applied for and received in prior periods.
| | | June 30, 2023 | | | December 31, 2022 | |
| Patents | | $ | 34 | | | $ | 34 | |
| Security deposits | | | 35 | | | | 35 | |
| Tax credit receivable | | | -- | | | | 55 | |
| Total | | $ | 69 | | | $ | 124 | |
Equity Method of Accounting On March 20, 2020, we invested $100,000 into a South Korean entity (Odin Co. Ltd., “Odin”) pursuant to a Joint Venture agreement we had entered into with BKT Co. Ltd. and its U.S. based subsidiary, Tomorrow Water. We received a 40% non-dilutive equity interest, and BKT and Tomorrow Water each received 30% equity interests for an aggregate $150,000 investment. We account for our investment in the joint venture under the equity method of accounting. We have determined that while we have significant influence over the joint venture through our technology license and our position on the Board of Directors, we do not control the joint venture or are otherwise involved in managing the entity and we own less than a majority of the equity. Therefore, we record the asset on our consolidated balance sheet and record an increase or decrease of the recorded balance by our percentage ownership of the profits or losses in the joint venture. The joint venture has incurred a loss since inception and our 40% ownership share reduced our investment interest. For the three and six months ended June 30, 2023, the reduction of our investment interest totaled $6,000 and $12,000, respectively, and for the same periods in 2022, reduced our investment interest $8,000 and $15,000, respectively.
Impairment Long-lived and definite lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows from the use of the asset and its eventual disposition is less than the carrying amount of the asset, then an impairment loss is recognized. The impairment loss is measured based on the fair value of the asset. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. There were no impairment losses related to intangible assets during the three or six months ended June 30, 2023 or 2022.
Earnings (Loss) Per Share We report basic and diluted earnings (loss) per share (“EPS”) for common and common share equivalents. Basic EPS is computed by dividing reported earnings by the weighted average shares outstanding. Diluted EPS is computed by adding to the weighted average shares the dilutive effect if convertible notes payable, stock options and warrants were exercised into common stock. For the three and six months ended June 30, 2023, and 2022, the denominator in the diluted EPS computation is the same as the denominator for basic EPS due to the Company’s net loss which creates an anti-dilutive effect of the convertible notes payable, warrants and stock options.
Use of Estimates The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and revenues and expenses during the period reported. Actual results could differ from those estimates. Estimates are used when accounting for stock-based transactions, debt transactions, derivative liabilities, allowance for bad debt, asset depreciation and amortization, impairment expense, among others. The methods, estimates and judgments we use in applying these most critical accounting policies have a significant impact on the results of our financial statements.
Share-Based Compensation Expense We recognize compensation expense for stock option awards on a straight-line basis over the applicable service period of the award, which is the vesting period. Fair value is determined on the grant date. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes Option Pricing Model. For stock and stock options issued to consultants and other non-employees for services, the Company measures and records an expense as of the earlier of the date at which either: a commitment for performance by the non-employee has been reached or the non-employee’s performance is complete. The equity instruments are measured at the current fair value, and for stock options, the instruments are measured at fair value using the Black Scholes option model. The following methodology and assumptions were used to calculate share-based compensation for the six months ended June 30, 2023, and 2022:
| | | 2023 | | | 2022 | |
| | | Non Plan | | | 2018 Plan | | | Non Plan | | | 2018 Plan | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | 3.48 | – | 3.58% | | | 2.32 | – | 3.83% | | | 2.32 | – | 2.98% | |
| Expected volatility | | 113 | - | 114% | | | 113 | - | 114% | | | 114 | – | 117% | | | 116 | – | 117% | |
| Expected dividend yield | | — | | | — | | | — | | | — | |
| Forfeiture rate | | — | | | — | | | — | | | — | |
| Life in years | | 10 | | | 10 | | | 10 | | | 10 | |
Expected price volatility is the measure by which our stock price is expected to fluctuate during the expected term of an option. The expected volatility is derived from the historical daily change in the market price of our common stock, as we believe that historical volatility is the best indicator of future volatility. The risk-free interest rate used in the Black-Scholes calculation is based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. We have never paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future.
Warrants Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method. The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”). If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note. Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. The warrant relative fair values are also recorded as a discount to the convertible promissory notes.
Non-Cash Transactions We determine the value assigned to each intangible we acquire, and/or services or products received for non-cash consideration of our common stock based on the market price of our common stock issued as consideration, at the date of the agreement of each transaction or when the service is rendered or product is received.
Revenue Recognition We account for revenue in accordance with ASC 606, “Revenue from Contacts with Customers”. The guidance focuses on the core principle for revenue recognition, which is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, the guidance provides that an entity should apply the following steps: Step 1: Identify the contract(s) with a customer. Step 2: Identify the performance obligations in the contract. Step 3: Determine the transaction price. Step 4: Allocate the transaction price to the performance obligations in the contract. Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation. The Company’s products are sold through a contract with the customer and a written purchase order, in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product, and each product has separate pricing. Revenue is recognized at a point in time when the goods are shipped if the agreement is FOB manufacturer, and when goods are delivered if FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order. Service contracts are performed through a written contract, which specifies the performance obligations and the rate at which the services will be billed, typically by time and materials. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed, or, for services related to product installations, at the completion of the installation. A few contracts have called for milestone or fixed cost payments, where we invoice an agreed-to amount per month for the life of the contract. In these instances, completed work, billed hourly, is recognized as revenue. If the billing amount is greater or lesser than the completed work, a receivable or payable is created. These accounts are adjusted upon additional billings as the work is completed. To date, there have been no discounts or other financing terms for the contracts. The Company has outstanding contract liability obligations of $10,000 as of June 30, 2023, recorded as deferred revenue. We recognized $7,000 in revenue and reduced the deferred revenue balance by the same amount as performance obligations were satisfied in the six months ended June 30, 2023. The outstanding balance will be recognized over the remaining life of the contracts. Our Canadian subsidiary had a customer deposit outstanding at June 30, 2023, totaling $113,000, that was awarded as part of a grant for a particular project that has been delayed. As we generate revenues from royalties or license fees from our intellectual property, a licensee will pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. We have entered into a licensing agreement for the CupriDyne Clean product, and we recognize royalty and license fees on a quarterly basis as the product is sold through to third parties and reported to us.
Government Grants We have been awarded multiple research grants from the private and public Canadian research programs. The income we receive directly from grants is recorded as other income. We have been awarded over 80 grants since our first in 2015. Some of the funds from these grants are given directly to third parties (such as the University of Alberta or a third-party research scientist) to support research on our technology. The grants have terms generally ranging between six and eighteen months and support a majority, but not all, of the related research budget costs. This cooperative research allows us to utilize (i) a depth of resources and talent to accomplish highly skilled work, (ii) financial aid to support research and development costs, (iii) independent and credible validation of our technical claims.
The grants typically provide for (i) recurring monthly amounts, (ii) reimbursement of costs for research talent for which we invoice to request payment, and (iii) ancillary cost reimbursement for research talent travel related costs. All awarded grants have specific requirements on how the money is spent, typically to employ researchers. None of the funds may be used for general administrative expenses or overhead in the United States. These grants have substantially increased our level of research and development activities in Canada. We continue to apply for Canadian government and agency grants to fund research and development activities. Not all of our grant applications have been awarded, and no assurance can be made that any pending grant application, or any future grant applications, will be awarded.
Income Taxes The asset and liability approach is used to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of asset and liabilities. Deferred tax assets and liabilities are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. We account for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation, and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by generally accepted accounting principles (“GAAP”). Under GAAP, the tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized. Management believes there are no unrecognized tax benefits or uncertain tax positions as of June 30, 2023, and December 31, 2022. The Company assessed its earnings history, trends and estimates of future earnings and determined that the deferred tax asset could not be realized as of June 30, 2023, and December 31, 2022. Accordingly, a 100% valuation allowance was recorded against the net deferred tax asset. The Company recognizes interest and penalties on income taxes as a component of income tax expense, should such an expense be realized.
Fair Value of Financial Instruments Management believes the carrying amounts of the Company’s financial instruments as of June 30, 2023, and December 31, 2022 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, line of credit, and other assets and liabilities. The carrying amount of debt instruments are believed to approximate fair value as the stated interest rates are reflective of the prevailing market rates.
Tax Credits Our research and development activities in Canada may entitle our Canadian subsidiary to claim benefits under the “Scientific Research and Experimental Development Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses of all sizes and in all sectors to conduct research and development in Canada. Benefits under the program include credits to taxable income. If our Canadian subsidiary does not have taxable income in a reporting period, we instead receive a tax refund from the Canadian Revenue Authority. Those refunds are classified in Other Income on our Consolidated Statement of Operations and Comprehensive Loss.
Leases At inception of a lease contract, we assess whether the contract is, or contains, a lease. Our assessment is based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether we obtain the right to substantially all the economic benefit from the use of the asset throughout the period of the contract, and (3) whether we have the right to direct the use of the asset during such time period. At inception of a lease, we allocate the consideration in the contract to each lease component based on its relative stand-alone price to determine the lease payments. Leases are classified as either finance leases or operating leases. A lease must be classified as a finance lease if any of the following criteria are met: the lease transfers ownership of the asset by the end of the lease term, the lease contains an option to purchase the asset that is reasonably certain to be exercised, the lease term is for a major part of the remaining useful life of the asset or the present value of the lease payments equals or exceeds substantially all of the fair value of the asset. A lease is classified as an operating lease if it does not meet any of these criteria. We have no leases classified as finance leases. As of June 30, 2023, the weighted average remaining lease term for our operating leases was nine years. The weighted average discount rate for our operating leases was 18%. For all leases at the lease commencement date, a right-of-use asset and a lease liability are recognized. The right-of-use asset represents the right to use the leased asset for the lease term. The lease liability represents the present value of the lease payments under the lease. The right-of-use asset is initially measured at cost, which primarily comprises the initial amount of the lease liability, plus any initial direct costs incurred, consisting mainly of brokerage commissions, less any lease incentives received. All right-of-use assets are reviewed for impairment. The lease liability is initially measured at the present value of the lease payments, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, management estimates the incremental borrowing rate, which currently is estimated to be 18%. Lease payments included in the measurement of the lease liability comprise the following: the fixed noncancelable lease payments, payments for optional renewal periods where it is reasonably certain the renewal period will be exercised, and payments for early termination options unless it is reasonably certain the lease will not be terminated early. Lease components are included in the measurement of the initial lease liability. Additional payments based on a change in our portion of the operating expenses, including real estate taxes and insurance, are recorded as a period expense when incurred. Lease modifications result in remeasurement of the lease liability. Lease expense for operating leases consists of the lease payments plus any initial direct costs, primarily brokerage commissions, and is recognized on a straight-line basis over the lease term. We have elected not to recognize right-of-use assets and lease liabilities for short-term leases that have a term of 12 months or less. The effect of short-term leases on our right-of-use asset and lease liability was not material. As of June 30, 2023, the right-of-use assets totaled $807,000 and the lease liability totaled $818,000 on our balance sheet related to our operating leases.
Property and Equipment Property and equipment includes machinery and leasehold improvements and is stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, which range from 3 - 5 years or the remaining lease term. Newly built leaseholds, additions, renewals, and betterments that significantly extend the life of the asset are capitalized. The Company is currently constructing a facility and purchasing equipment for producing sodium sulfur batteries. Expenditures for repairs and maintenance are charged to expense as incurred. For assets sold or otherwise disposed of, the cost and related accumulated depreciation and amortization are removed from the accounts, and any related gain or loss is reflected in income for the period.
Recent Accounting Pronouncements Currently there are no recently released pronouncements that are considered applicable to the Company’s financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 3 - Sale of Stock for Cash
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Stock Purchase Agreement [Text Block] |
Note 3. Sale of Stock for Cash
Lincoln Park Financing
On December 13, 2022, we entered into a stock purchase agreement (the “2022 LPC Purchase Agreement”) with Lincoln Park, pursuant to which Lincoln Park agreed to purchase from us at our request up to an aggregate of $10,000,000 of our common stock (subject to certain limitations) from time to time over a period of three years. The agreement allows us, at our sole discretion, to direct Lincoln Park to purchase shares of our common stock, subject to limitations in both volume and dollar amount. The purchase price of the shares that may be sold to Lincoln Park under the agreement is the lower of (i) the lowest sale price on the date of purchase, or (ii) the average of the three lowest closing prices in the prior 12 business days. There are no restrictions on future financings, rights of first refusal, participation rights, penalties, or liquidated damages other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the agreement. Concurrently with the 2022 LPC Purchase Agreement, we entered into a Registration Rights Agreement, pursuant to which we filed a registration statement on Form S-1 with the SEC on December 23, 2022. This registration statement was declared effective on January 19, 2023.
During the three and six months ended June 30, 2023, we sold 1,098,221 and 1,643,623 shares of our common stock to Lincoln Park, and received $194,000 and $299,000, respectively, in gross and net proceeds.
During the three and six months ended June 30, 2022, pursuant to a prior (similar) arrangement, we sold 406,140 and 1,912,961 shares of our common stock to Lincoln Park, and received $72,000 and $418,000, respectively, in gross and net proceeds.
Unit Offerings
During the three and six months ended June 30, 2023, we sold 1,578,948 and 5,234,948 shares of our common stock and received $300,000 and $995,000 in gross and net proceeds from accredited investors. In addition to the shares, we issued each investor a six-month and a five-year warrant to purchase additional shares. (See Note 6, “Warrants Issued in Unit Offering”.)
|
| X |
- DefinitionThe textual disclosure of information pertaining to a stock purchase agreement.
| Name: |
blgo_StockPurchaseAgreementTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 4 - Debt Obligations
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Debt Disclosure [Text Block] |
Note 4. Debt Obligations
The following table summarizes our debt obligations outstanding as of June 30, 2023, and December 31, 2022 (in thousands). The table does not include debt obligations of our partially owned subsidiary Clyra Medical (see Note 8, “Debt Obligations of Clyra Medical”).
| | | June 30, 2023 | | |
December 31,
2022 | |
| Current portion of debt: | | | | | | | | |
| SBA Paycheck Protection Program loan | | $ | 43 | | | $ | 43 | |
| Vehicle loan, current portion | | | 13 | | | | — | |
| Convertible note payable, matures March 1, 2023 | | | — | | | | 50 | |
| SBA EIDL Loan, matures July 2053, current portion | | | 10 | | | | 10 | |
| Debt discount, net of amortization | | | — | | | | (3 | ) |
| Total current portion of debt | | $ | 66 | | | $ | 100 | |
| | | | | | | | | |
| Long-term debt: | | | | | | | | |
| SBA Paycheck Protection Program loans, matures May 2025 | | $ | 97 | | | $ | 97 | |
| Vehicle loan, matures March 2029 | | | 62 | | | | — | |
| SBA EIDL Loan, matures July 2050 | | | 140 | | | | 140 | |
| Total long-term debt, net of current | | $ | 299 | | | $ | 237 | |
| | | | | | | | | |
| Total | | $ | 365 | | | $ | 337 | |
For the three and six months ended June 30, 2023, we recorded $12,000 and $60,000 of interest expense related to the amortization of discounts on convertible notes payable and coupon interest from our convertible notes and lines of credit.
For the three and six months ended June 30, 2022, we recorded $15,000 and $28,000 of interest expense related to the amortization of discounts on convertible notes payable and coupon interest from our convertible notes and lines of credit.
Vehicle loan
On February 7, 2023, we entered a loan agreement with Bank of America for the purchase of a vehicle used in operations totaling $80,000, at 5.29% annual interest which matures March 7, 2029. The loan agreement requires monthly payments of $1,000.
Convertible note payable, matures March 1, 2023
On March 6, 2023, we entered into an agreement with the holder of a $50,000 note to convert that note into common stock of BETI (see note 10). As payment for interest, a warrant to purchase 200,000 shares of BioLargo common stock at $0.21 was issued to the investor, expiring five years from the grant date. (See Note 6).
SBA Program Loans
On February 7, 2022, we received notice that the SBA had forgiven $174,000 of ONM Environmental's $217,000 Paycheck Protection Program (PPP) loan. As of June 30, 2023, the outstanding balance on this loan totals $43,000. The partial forgiveness decision has been appealed, and during such time, loan payments are deferred.
On May 12, 2022, we received notice that the SBA had denied the forgiveness application of BLEST’s $97,000 PPP loan. We have appealed that decision. During the period upon which a forgiveness decision is on appeal, loan payments are deferred. The maturity date of the BLEST PPP loan was officially extended on our request to May 2025.
In July 2020, ONM Environmental received an Economic Injury Disaster Loan from the SBA in the amount of $150,000. The note has a 3.75% annual interest rate, requires monthly payments of $731, and matures July 2050.
|
| X |
- DefinitionThe entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//470/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 5 - Share-based Compensation
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Compensation and Employee Benefit Plans [Text Block] |
Note 5. Share-Based Compensation
Issuance of Common Stock in exchange for Services
Payment of Officer Salaries
On June 30, 2023, an officer agreed to convert an aggregate $12,000 of accrued and unpaid salary into 68,541 shares of our common stock at $0.18 per share. On March 31, 2023, an officer agreed to convert an aggregate $6,000 of accrued and unpaid salary into 30,747 shares of our common stock at $0.20 per share.
On June 30, 2022, we issued 263,895 shares of our common stock at $0.18 per share in lieu of $47,000 of accrued and unpaid salary to our officers.
Shares issued to Officers are unvested at the date of grant and subject to a lock-up agreement restricting vesting and sale until the earlier of (i) the consummation of a sale (in a single transaction or in a series of related transactions) of BioLargo by means of a sale of (a) a majority of the then outstanding common stock of BioLargo (whether by merger, consolidation, sale or transfer of common stock, reorganization, recapitalization or otherwise) or (b) all or substantially all of the assets of BioLargo; and (ii) the successful commercialization of BioLargo’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Officer and resulting in Officer’s termination.
Payment of Consultant and Vendor Fees
On June 30, 2023, we issued 352,370 shares of our common stock at $0.18 per share in lieu of $63,000 of accrued and unpaid obligations to consultants and vendors. On March 31, 2023, we issued 899,743 shares of our common stock at $0.20 per share in lieu of $201,000 of accrued and unpaid obligations to consultants and vendors.
On June 30, 2022, we issued 76,996 shares of our common stock at $0.18 per share in lieu of $12,000 of accrued and unpaid obligations to consultants and vendors. On March 31, 2022, we issued 86,752 shares of our common stock at $0.23 per share in lieu of $17,000 of accrued and unpaid obligations to consultants and vendors.
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Stock Option Expense
During the three and six months ended June 30, 2023, we recorded an aggregate $288,000 and $544,000, and during the three and six months ended June 30, 2022, we recorded an aggregate $316,000 and $1,117,000, in selling general and administrative expense related to the issuance of stock options. We issued options through our 2018 Equity Incentive Plan, and outside of this plan. Included in these totals is option expense related to issuances by our subsidiary, Clyra Medical, totaling $66,000 and $127,000 in the three and six months ended June 30, 2023, and $82,000 and $223,000 in the three and six months ended June 30, 2022. (See Note 8.)
2018 Equity Incentive Plan
On June 22, 2018, our stockholders adopted the BioLargo 2018 Equity Incentive Plan (“2018 Plan”) as a means of providing our directors, key employees, and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years. It is set to expire on its terms on June 22, 2028. Our Board of Director’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The plan authorizes the following types of awards: (i) incentive and non-qualified stock options, (ii) restricted stock awards, (iii) stock bonus awards, (iv) stock appreciation rights, (v) restricted stock units, and (vi) performance awards. The total number of shares reserved and available for awards pursuant to this Plan as of the date of adoption of this 2018 Plan by the Board is 40 million shares. The number of shares available to be issued under the 2018 Plan increases automatically each January 1st by the lesser of (a) 2 million shares, or (b) such number of shares determined by our Board. As of June 30, 2023, 50,000,000 shares are authorized under the plan.
Activity for our stock options under the 2018 Plan during the six months ended June 30, 2023, and 2022, is as follows:
| | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | Average | | | Aggregate | |
| | | Options | | | Exercise | | | Price per | | | intrinsic | |
| | | Outstanding | | | Price per share | | | share | | | Value(1) | |
| Balance, December 31, 2022 | | | 28,484,549 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| Granted | | | 2,636,712 | | | $0.18 | – | 0.20 | | | $ | 0.19 | | | | | |
| Balance, June 30, 2023 | | | 31,121,261 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| Unvested | | | (4,121,892 | ) | | $0.12 | – | 0.32 | | | $ | 0.19 | | | | | |
| Vested, June 30, 2023 | | | 26,999,369 | | | 0.12 | – | 0.43 | | | $ | 0.19 | | | $ | 402,000 | |
| Balance, December 31, 2021 | | | 23,186,142 | | | $0.16 | – | 0.43 | | | $ | 0.19 | | | | | |
| Granted | | | 3,782,923 | | | $0.18 | – | 0.24 | | | $ | 0.22 | | | | | |
| Balance, June 30, 2022 | | | 26,969,065 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
The options granted to purchase 2,636,712 shares during the six months ended June 30, 2023 with an aggregate fair value of $474,000 were issued to an officer, board of directors, employees and a consultant. The exercise price for all options were issued on their respective grant date of March 31, 2023 ($0.20 per share) and June 30, 2023 ($0.18 per share): (i) we issued options to purchase 864,290 shares of our common stock to members of our board of directors for services performed, in lieu of cash; the fair value of these options totaled $154,000; (ii) we issued options to purchase 1,137,301 shares of our common stock to employees as part of an employee retention plan; the fair value of employee retention plan options totaled $203,000 and will vest quarterly over four years as long as they are retained as employees; (iii) we issued options to purchase 335,121 shares of our common stock to consultants in lieu of cash and for expiring options at $0.20 per share totaling $61,000, and (iv) we issued 300,000 options to our Chief Financial Officer with a fair value of $56,000 (see “Chief Financial Officer Contract Extension” immediately below). All stock option expense is recorded on our consolidated statement of operations as selling, general and administrative expense.
Chief Financial Officer Contract Extension
On March 21, 2023, we and our Chief Financial Officer Charles K. Dargan, II formally agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times), pursuant to which Mr. Dargan has been and continues to serve as the Company’s Chief Financial Officer. The Engagement Extension Agreement dated as of March 21, 2023 (the “Engagement Extension Agreement”) provides for an additional one-year term to expire January 31, 2024 (the “Extended Term”), at which time Mr. Dargan will continue to serve as CFO, unless and until either party terminates the agreement.
As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 25,000 shares of the Company’s common stock for each month during the Extended Term (thus, an option to purchase 300,000 shares reflecting an extended term of 12 months). The Option vests over the period of the Extended Term, with 25,000 shares having vested as of March 21, 2023, and the remaining shares to vest 25,000 shares monthly beginning March 31, 2023, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.20 per share, the closing price of BioLargo’s common stock on the March 21, 2023, grant date, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan.
The Option is Mr. Dargan’s sole compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
The options granted to purchase 3,782,923 shares during the six months ended June 30, 2022 were issued to officers, board of directors, employees and consultants: (i) we issued options to purchase 251,551 shares of our common stock at an exercise price on the respective grant date of $0.23 per share to our CFO and President to replace options that had expired and we issued an option to purchase 300,000 shares of our common stock at an exercise price on the respective grant date of $0.24 per share to our CFO (details below); (ii) we issued options to purchase 884,356 shares of our common stock at an exercise price on the respective grant date ranging between $0.18 – $0.23 per share to members of our board of directors for services performed, in lieu of cash; the fair value of these options totaled $178,000; (iii) we issued options to purchase 1,764,025 shares of our common stock to employees as part of an employee retention plan at an exercise price on the respective date ranging between $0.18 – $0.23 per share; the fair value of employee retention plan options totaled $360,000 and will vest quarterly over four years as long as they are retained as employees; and (iv) we issued options to purchase 582,991 shares of our common stock to consultants in lieu of cash for expiring options and per agreement totaling $127,000. All stock option expense is recorded on our consolidated statement of operations as selling, general and administrative expense.
2007 Equity Incentive Plan
On September 7, 2007, and as amended April 29, 2011, the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Plan”) was adopted as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years, which expired on September 7, 2017. The Board’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. As of September 2017, the Plan was closed to further stock option grants.
Activity for our stock options under the 2007 Plan for the six months ended June 30, 2023, and 2022 is as follows:
| | | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | | Average | | | Aggregate | |
| | | Options | | | Exercise | | | Price per | | | intrinsic | |
| | | Outstanding | | | price per share | | | share | | | Value(1) | |
| Balance, December 31, 2022 | | | 1,904,085 | | | | $0.28 | – | 0.69 | | | $ | 0.56 | | | | | |
| Expired | | | (40,000 | ) | | | 0.28 | | | | 0.28 | | | | | |
| Balance, June 30, 2023 | | | 1,864,085 | | | | $0.28 | – | 0.69 | | | $ | 0.56 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 2,879,246 | | | | $0.23 | – | 0.94 | | | $ | 0.49 | | | | | |
| Expired | | | (300,000 | ) | | | 0.35 | | | | 0.35 | | | | | |
| Balance, June 30, 2022 | | | 2,579,246 | | | | $0.23 | – | 0.94 | | | $ | 0.49 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
Non-Plan Options
Activity of our non-plan stock options issued for the six months ended June 30, 2023 and 2022 is as follows:
| | | | | | | | | | | | | Weighted | | | | | |
| | | Non-plan | | | | | | | | | average | | | Aggregate | |
| | | Options | | | Exercise | | | price per | | | intrinsic | |
| | | outstanding | | | price per share | | | share | | | value(1) | |
| Balance, December 31, 2022 | | | 19,023,829 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Granted | | | 60,040 | | | | 0.18 | – | 0.20 | | | | 0.20 | | | | | |
| Balance, June 30, 2023 | | | 19,083,869 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Unvested | | | (507,500 | ) | | | 0.45 | | | | 0.45 | | | | | |
| Vested, June 30, 2023 | | | 18,576,369 | | | | $0.12 | – | 0.83 | | | $ | 0.38 | | | $ | 46,000 | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 20,119,207 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Granted | | | 39,130 | | | | 0.23 | | | | 0.23 | | | | | |
| Balance, June 30, 2022 | | | 20,158,337 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
During the six months ended June 30, 2023, we issued options to purchase an aggregate 60,040 shares of our common stock at $0.18 and $0.20 per share to vendors for fees for services. The fair value of the options issued totaled an aggregate $11,000 and is recorded in our selling, general and administrative expense.
During the six months ended June 30, 2022, we issued options to purchase an aggregate 39,130 shares of our common stock at $0.23 per share to vendors for fees for services. The fair value of the options issued totaled an aggregate $8,000 and is recorded in our selling, general and administrative expense.
|
| X |
- DefinitionThe entire disclosure for an entity's employee compensation and benefit plans, including, but not limited to, postemployment and postretirement benefit plans, defined benefit pension plans, defined contribution plans, non-qualified and supplemental benefit plans, deferred compensation, share-based compensation, life insurance, severance, health care, unemployment and other benefit plans. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 710
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//710/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 712
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//712/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 715
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//715/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
| Name: |
us-gaap_CompensationAndEmployeeBenefitPlansTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 6 - Warrants
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Warrants [Text Block] |
Note 6. Warrants
We have certain warrants outstanding to purchase our common stock, at various prices, as described in the following table:
| | | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | | average | | | Aggregate | |
| | | Warrants | | | Exercise | | | price per | | | intrinsic | |
| | | outstanding | | | price per share | | | share | | | value(1) | |
| Balance, December 31, 2022 | | | 49,023,398 | | | | $0.13 | – | 1.00 | | | $ | 0.26 | | | | | |
| Granted | | | 10,669,896 | | | | 0.21 | – | 0.29 | | | | 0.25 | | | | | |
| Expired | | | (5,173,209 | ) | | | 0.19 | – | 0.35 | | | | 0.21 | | | | | |
| Balance, June 30, 2023 | | | 54,520,085 | | | | $0.13 | – | 1.00 | | | $ | 0.26 | | | $ | 47,000 | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 36,765,502 | | | | $0.16 | – | 1.00 | | | $ | 0.27 | | | | | |
| Granted | | | 19,604,796 | | | | 0.20 | – | 0.29 | | | | 0.24 | | | | | |
| Expired | | | (4,016,754 | ) | | | 0.19 | – | 0.48 | | | | 0.35 | | | | | |
| Balance, June 30, 2022 | | | 52,353,544 | | | | $0.14 | – | 1.00 | | | $ | 0.25 | | | | | |
(1) – Aggregate intrinsic value based on closing common stock price of $0.18 at June 30, 2023.
Warrants issued in Unit Offerings
During the six months ended June 30, 2023, pursuant to our Unit Offerings (see Note 3), we issued six-month stock purchase warrants to purchase an aggregate 5,234,948 shares of our common stock at $0.23 per share, and five-year stock purchase warrants to purchase an aggregate 5,234,948 shares of our common stock at $0.29 per share. The fair value of these warrants totaled $913,000.
During the six months ended June 30, 2022, pursuant to our Unit Offering (see Note 3), we issued six-month stock purchase warrants to purchase an aggregate 9,802,398 shares of our common stock at $0.20 - $0.23 per share, and five-year stock purchase warrants to purchase an aggregate 9,802,398 shares of our common stock at $0.25 - $0.29 per share. The fair value of these warrants totaled $2,134,000.
Warrant issued in conjunction with amendment to note payable
On March 6, 2023, we entered into an agreement with the holder of a $50,000 note (see Note 4, “Convertible note payable, matures March 1, 2023”) to convert that note into common stock of BETI. As payment for interest, a warrant to purchase 200,000 shares of BioLargo common stock at $0.21 was issued to the investor, expiring five years from the grant date. The fair value of this warrant totaled $30,000 and was recorded as interest expense on our statement of operations.
Fair Value – Interest Expense
To determine interest expense related to our outstanding warrants issued in conjunction with debt offerings, the fair value of each award grant is estimated on the date of grant using the Black-Scholes option pricing model and the relative fair values are amortized over the life of the warrant. For the determination of expense of warrants issued for services, extinguishment of debt and settlement management also uses the option-pricing model. The principal assumptions we used in applying this model were as follows:
| | | 2023 | | | 2022 | |
| Risk free interest rate | | 3.88 | – | 4.27% | | | 3.69 | – | 3.88% | |
| Expected volatility | | 40 | – | 95% | | | 40% | |
| Expected dividend yield | | — | | | — | |
| Forfeiture rate | | — | | | — | |
| Expected life in years | | 3 | - | 5 | | | 3 | |
The risk-free interest rate is based on U.S. Treasury yields in effect at the time of grant. Expected volatilities are based on historical volatility of our common stock. The expected life in years is based on the contract term of the warrant.
|
| X |
- DefinitionDisclosure of notes that entitle the holder to buy stock of the company at a specified price, which is much higher than the stock price at the time of issue.
| Name: |
blgo_WarrantsTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 7 - Accounts Payable and Accrued Expenses
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Accounts Payable and Accrued Liabilities Disclosure [Text Block] |
Note 7. Accounts Payable and Accrued Expenses
As of June 30, 2023, accounts payable and accrued expenses included the following (in thousands):
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | BETI | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 142 | | | $ | 168 | | | $ | 119 | | | $ | 106 | | | $ | 29 | | | $ | (82 | ) | | $ | 482 | |
| Accrued payroll | | | 50 | | | | 92 | | | | 71 | | | | — | | | | — | | | | — | | | | 213 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 720 | |
As of December 31, 2022, accounts payable and accrued expenses included the following (in thousands):
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 187 | | | $ | 486 | | | $ | 7 | | | $ | 119 | | | $ | (82 | ) | | $ | 717 | |
| Accrued payroll | | | 20 | | | | 58 | | | | 120 | | | | — | | | | — | | | | 198 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | $ | 940 | |
See Note 8, “Accounts Payable and Accrued Expenses”, for the accounts payable and accrued expenses of Clyra Medical.
|
| X |
- DefinitionThe entire disclosure for accounts payable and accrued liabilities at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a),20,24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 8 - Noncontrolling Interest - Clyra Medical
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Noncontrolling Interest Disclosure [Text Block] |
Note 8. Noncontrolling Interest – Clyra Medical
As discussed in Note 2 above, we consolidate the operations of our partially owned subsidiary Clyra Medical, of which we owned 56% of its outstanding shares as of June 30, 2023.
Debt Obligations of Clyra Medical
Promissory Note
On April 8, 2022, Clyra Medical issued a promissory note in the principal amount of $100,000 to an individual investor, payable April 8, 2024, and bearing 8% annual interest. The note may be converted by its holder at any time prior to the maturity date, and automatically converts to stock upon (i) Clyra’s sale of $5,000,000 or more of its common or preferred stock, or (ii) the maturity date, at a conversion price equal to 70% of the lowest price-per-share of shares sold to a future investor prior to the maturity date.
Line of Credit
On June 30, 2020, Clyra Medical entered into a Revolving Line of Credit Agreement whereby Vernal Bay Capital Group, LLC committed to provide a $1,000,000 inventory line of credit. Clyra Medical received $260,000 in draws and made repayments totaling $117,000. Clyra issued Vernal Bay 322 shares of its common stock as a commitment fee for the line of credit, valued at $70,000. A security agreement of the same date grants Vernal Bay a security interest in Clyra’s inventory, as that term is defined in the Uniform Commercial Code. Clyra may prepay the note at any time.
On December 13, 2022, we entered into an amendment of the Revolving Line of Credit Agreement whereby the maturity date of the line of credit was extended to September 30, 2024, and the payment terms were modified such that amounts of principal due in each month are capped at a maximum of 15% of the principal amount then due under the note. Additionally, BioLargo agreed to allow Vernal Bay to elect to convert, any time prior to the note’s maturity date, the 322 shares of Clyra common stock it received as consideration for the line of credit into shares of Biolargo common stock at the then market price of BioLargo’s common stock. On January 9, 2023, Vernal Bay elected to convert Clyra shares to 527,983 BioLargo shares of common stock.
As of June 30, 2023, the balance outstanding on this line of credit totals $143,000. As of December 31, 2022, the balance outstanding on this line of credit totaled $161,000.
Equity Transactions
As of June 30, 2023, Clyra had 95,301 shares issued and outstanding, of which 89,070 were common shares, and 6,231 were preferred shares. As of December 31, 2022, Clyra had 91,145 shares issued and outstanding, of which 89,070 were common shares, and 2075 were preferred shares. As of June 30, 2023 and December 31, 2022, of the outstanding amount, BioLargo owned 51,571 common shares and 1,352 Series A Preferred shares.
BioLargo Conversion of Intercompany Balances
On March 2, 2022, BioLargo converted $633,000 owed to it by Clyra into 2,042 shares of Clyra common stock.
Sales of Series A Preferred Stock
During the three months ended June 30, 2023, Clyra sold 3,427 shares of its Series A Preferred Stock, and in exchange received $1,062,000 in gross and net proceeds from 26 accredited investors. During the six months ended June 30, 2023, Clyra sold 4,879 shares of its Series A Preferred Stock, and in exchange received $1,287,000 in gross and net proceeds from 28 accredited investors. Purchasers of the Series A Preferred Stock also received a 3-year warrant to purchase the same number of additional shares of common stock for $372 per share. The fair value of the warrants issued totaled $324,000, and is limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the Series A Preferred Stock. Shares of Series A Preferred Stock earn a dividend of 15% each year, compounding annually; the company is under no obligation to pay such dividends in cash, and such dividends automatically convert to common stock upon conversion of the Series A Preferred Stock to common stock. Each share of Series A Preferred stock can be converted by the holder at any time for one share of common stock, and automatically convert upon the completion of a public offering of shares in which at least $5,000,000 of gross proceeds is received by the company. Accrued dividends may be converted to common stock at a conversion rate of $310 per share.
Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its Series A Preferred stock, plus accrued dividends, into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 30 prior trading days. Elections must be made during the 18-month period that begins 18 months after the closing of the Series A Preferred offering (which has not yet taken place), or June 30, 2023, whichever is earlier.
Clyra Stock Options
| | | | | | | | | | | | | Weighted | |
| | | Clyra | | | | | | | | | average | |
| | | Options | | | Exercise | | | price per | |
| | | Outstanding | | | price per share | | | share | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2022 | | | 15,833 | | | | $1.00 | - | 310 | | | $ | 5.53 | |
| Granted | | | 858 | | | | 1.00 | - | 271 | | | | 146.06 | |
| Balance, June 30, 2023 | | | 16,691 | | | | $1.00 | - | 310 | | | $ | 12.76 | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 14,004 | | | | $1.00 | | | $ | 1.00 | |
| Granted | | | 1,026 | | | | 1.00 | | | | 1.00 | |
| Balance, June 30, 2022 | | | 15,030 | | | | $1.00 | | | $ | 1.00 | |
Clyra issues options to its employees and consultants in lieu of compensation owed on a regular basis. The fair value of the options issued totaled $66,000 and $127,000 in the three and six months ended June 30, 2023, and $82,000 and $223,000 in the three and six months ended June 30, 2022. We used the Black-Scholes model to calculate the initial fair value, assuming a stock price on date of grant of $310 per share. Because Clyra is a private company with no secondary market for its common stock, the resulting fair value was discounted by 30%.
| | | June 30, 2023 | | | December 31, 2022 | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | | 2.32 | % |
| Expected volatility | | 40 | - | 48% | | | | 40 | % |
| Expected dividend yield | | — | | | | — | |
| Forfeiture rate | | — | | | | — | |
| Expected life in years | | 10 | | | | 10 | |
Accounts Payable and Accrued Expenses
At June 30, 2023, and December 31, 2022, Clyra had the following accounts payable and accrued expenses (in thousands):
| Category | | 2023 | | | 2022 | |
| Accounts payable | | $ | 199 | | | $ | 186 | |
| Accrued payroll | | | 10 | | | | 45 | |
| Accrued interest | | | 5 | | | | 7 | |
| Accrued dividend | | | 74 | | | | --- | |
| Total | | $ | 288 | | | $ | 238 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for noncontrolling interest in consolidated subsidiaries, which could include the name of the subsidiary, the ownership percentage held by the parent, the ownership percentage held by the noncontrolling owners, the amount of the noncontrolling interest, the location of this amount on the balance sheet (when not reported separately), an explanation of the increase or decrease in the amount of the noncontrolling interest, the noncontrolling interest share of the net Income or Loss of the subsidiary, the location of this amount on the income statement (when not reported separately), the nature of the noncontrolling interest such as background information and terms, the amount of the noncontrolling interest represented by preferred stock, a description of the preferred stock, and the dividend requirements of the preferred stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
| Name: |
us-gaap_MinorityInterestDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 9 - BioLargo Engineering, Science and Technologies, LLC
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Wholly-Owned Subsidiary [Text Block] |
Note 9. BioLargo Engineering, Science and Technologies, LLC
In September 2017, we commenced a full-service environmental engineering firm and formed a Tennessee entity named BioLargo Engineering, Science & Technologies, LLC (“BLEST”). In conjunction with the start of this subsidiary we entered into employment agreements with six scientists and engineers. (See Note 11 “Business Segment Information”.) BLEST was capitalized with two classes of membership units: Class A, 100% owned by BioLargo, and Class B, held by management of BLEST, and which initially have no “profit interest,” as that term is defined in Tennessee law. However, over the succeeding five years, the Class B members can earn up to a 30% profit interest. They also have been granted options to purchase up to an aggregate 1,750,000 shares of BioLargo, Inc. common stock. The profit interest and option shares are subject to a five year vesting schedule tied to the performance of the subsidiary, including gross revenue targets that increase over time, obtaining positive cash flow by March 31, 2018 (which was not met), collecting 90% of its account receivables, obtaining a profit of 10% in its first year (and increasing in subsequent years) (which was not met), making progress in the scale-up and commercialization of our AOS system, and using BioLargo research scientists (such as our Canadian team) for billable work on client projects. These criteria are to be evaluated annually by BLEST’s compensation committee (which includes BioLargo’s president, CFO, and BLEST’s president), beginning September 2018. Given the significant performance criteria, the Class B units and the stock options will only be recognized in compensation expense if or when the criteria are satisfied.
The BLEST Compensation Committee has met regularly since the subsidiary commenced operations. In 2018, it reviewed the operating performance and determined that the performance metrics were not met and as a result, did not award any Class B units or stock options. As of December 31, 2021, BLEST employees had earned 13% in profits interests and had earned (vested) 455,000 option shares. In January 2022, the committee again reviewed the operating performance and determined that a portion of the performance metrics were met. It was agreed that an additional one-half and one-quarter of the eligible profits interests would be vested (11% in the aggregate), and therefore an additional half of the option interests would be vested (525,000 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $65,000, recorded on our consolidated statement of operations as selling, general and administrative expense for the year ended December 31, 2021. In December 2022, the committee again reviewed the operating performance and determined that a portion of the performance metrics were met. It was agreed that an additional one-half and one-quarter of the eligible profits interests would be vested (18% in the aggregate), and therefore an additional half of the option interests would be vested (1,242,500 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $135,000 and is recorded on our consolidated statement of operations as selling, general and administrative expense for the year ended December 31, 2022.
At June 30, 2023, BioLargo owns 82% of BLEST.
|
| X |
- DefinitionThe textual disclosure of a wholly-owned subsidiary.
| Name: |
blgo_WhollyOwnedSubsidiaryTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 10 - BioLargo Energy Technologies, Inc.
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Noncontrolling Interest Disclosure [Text Block] |
Note 8. Noncontrolling Interest – Clyra Medical
As discussed in Note 2 above, we consolidate the operations of our partially owned subsidiary Clyra Medical, of which we owned 56% of its outstanding shares as of June 30, 2023.
Debt Obligations of Clyra Medical
Promissory Note
On April 8, 2022, Clyra Medical issued a promissory note in the principal amount of $100,000 to an individual investor, payable April 8, 2024, and bearing 8% annual interest. The note may be converted by its holder at any time prior to the maturity date, and automatically converts to stock upon (i) Clyra’s sale of $5,000,000 or more of its common or preferred stock, or (ii) the maturity date, at a conversion price equal to 70% of the lowest price-per-share of shares sold to a future investor prior to the maturity date.
Line of Credit
On June 30, 2020, Clyra Medical entered into a Revolving Line of Credit Agreement whereby Vernal Bay Capital Group, LLC committed to provide a $1,000,000 inventory line of credit. Clyra Medical received $260,000 in draws and made repayments totaling $117,000. Clyra issued Vernal Bay 322 shares of its common stock as a commitment fee for the line of credit, valued at $70,000. A security agreement of the same date grants Vernal Bay a security interest in Clyra’s inventory, as that term is defined in the Uniform Commercial Code. Clyra may prepay the note at any time.
On December 13, 2022, we entered into an amendment of the Revolving Line of Credit Agreement whereby the maturity date of the line of credit was extended to September 30, 2024, and the payment terms were modified such that amounts of principal due in each month are capped at a maximum of 15% of the principal amount then due under the note. Additionally, BioLargo agreed to allow Vernal Bay to elect to convert, any time prior to the note’s maturity date, the 322 shares of Clyra common stock it received as consideration for the line of credit into shares of Biolargo common stock at the then market price of BioLargo’s common stock. On January 9, 2023, Vernal Bay elected to convert Clyra shares to 527,983 BioLargo shares of common stock.
As of June 30, 2023, the balance outstanding on this line of credit totals $143,000. As of December 31, 2022, the balance outstanding on this line of credit totaled $161,000.
Equity Transactions
As of June 30, 2023, Clyra had 95,301 shares issued and outstanding, of which 89,070 were common shares, and 6,231 were preferred shares. As of December 31, 2022, Clyra had 91,145 shares issued and outstanding, of which 89,070 were common shares, and 2075 were preferred shares. As of June 30, 2023 and December 31, 2022, of the outstanding amount, BioLargo owned 51,571 common shares and 1,352 Series A Preferred shares.
BioLargo Conversion of Intercompany Balances
On March 2, 2022, BioLargo converted $633,000 owed to it by Clyra into 2,042 shares of Clyra common stock.
Sales of Series A Preferred Stock
During the three months ended June 30, 2023, Clyra sold 3,427 shares of its Series A Preferred Stock, and in exchange received $1,062,000 in gross and net proceeds from 26 accredited investors. During the six months ended June 30, 2023, Clyra sold 4,879 shares of its Series A Preferred Stock, and in exchange received $1,287,000 in gross and net proceeds from 28 accredited investors. Purchasers of the Series A Preferred Stock also received a 3-year warrant to purchase the same number of additional shares of common stock for $372 per share. The fair value of the warrants issued totaled $324,000, and is limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the Series A Preferred Stock. Shares of Series A Preferred Stock earn a dividend of 15% each year, compounding annually; the company is under no obligation to pay such dividends in cash, and such dividends automatically convert to common stock upon conversion of the Series A Preferred Stock to common stock. Each share of Series A Preferred stock can be converted by the holder at any time for one share of common stock, and automatically convert upon the completion of a public offering of shares in which at least $5,000,000 of gross proceeds is received by the company. Accrued dividends may be converted to common stock at a conversion rate of $310 per share.
Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its Series A Preferred stock, plus accrued dividends, into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 30 prior trading days. Elections must be made during the 18-month period that begins 18 months after the closing of the Series A Preferred offering (which has not yet taken place), or June 30, 2023, whichever is earlier.
Clyra Stock Options
| | | | | | | | | | | | | Weighted | |
| | | Clyra | | | | | | | | | average | |
| | | Options | | | Exercise | | | price per | |
| | | Outstanding | | | price per share | | | share | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2022 | | | 15,833 | | | | $1.00 | - | 310 | | | $ | 5.53 | |
| Granted | | | 858 | | | | 1.00 | - | 271 | | | | 146.06 | |
| Balance, June 30, 2023 | | | 16,691 | | | | $1.00 | - | 310 | | | $ | 12.76 | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 14,004 | | | | $1.00 | | | $ | 1.00 | |
| Granted | | | 1,026 | | | | 1.00 | | | | 1.00 | |
| Balance, June 30, 2022 | | | 15,030 | | | | $1.00 | | | $ | 1.00 | |
Clyra issues options to its employees and consultants in lieu of compensation owed on a regular basis. The fair value of the options issued totaled $66,000 and $127,000 in the three and six months ended June 30, 2023, and $82,000 and $223,000 in the three and six months ended June 30, 2022. We used the Black-Scholes model to calculate the initial fair value, assuming a stock price on date of grant of $310 per share. Because Clyra is a private company with no secondary market for its common stock, the resulting fair value was discounted by 30%.
| | | June 30, 2023 | | | December 31, 2022 | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | | 2.32 | % |
| Expected volatility | | 40 | - | 48% | | | | 40 | % |
| Expected dividend yield | | — | | | | — | |
| Forfeiture rate | | — | | | | — | |
| Expected life in years | | 10 | | | | 10 | |
Accounts Payable and Accrued Expenses
At June 30, 2023, and December 31, 2022, Clyra had the following accounts payable and accrued expenses (in thousands):
| Category | | 2023 | | | 2022 | |
| Accounts payable | | $ | 199 | | | $ | 186 | |
| Accrued payroll | | | 10 | | | | 45 | |
| Accrued interest | | | 5 | | | | 7 | |
| Accrued dividend | | | 74 | | | | --- | |
| Total | | $ | 288 | | | $ | 238 | |
|
| BioLargo Energy Technologies, Inc (BETI) [Member] |
|
| Notes to Financial Statements |
|
| Noncontrolling Interest Disclosure [Text Block] |
Note 10. BioLargo Energy Technologies, Inc.
Subsidiary BioLargo Energy Technologies, Inc. (“BETI”) was formed for the purpose of commercializing a sodium-sulfur battery technology. BioLargo purchased 9,000,000 shares of its common stock upon its formation.
During the three months ended June 30, 2023, BETI sold 132,000 shares of its common stock to nine accredited investors and received $330,000 in gross and net proceeds. During the six months ended June 30, 2023, BETI sold 450,000 shares of its common stock to 15 accredited investors, and in exchange received gross and net proceeds totaling $980,000. Of that amount, $100,000 in shares were purchased by BioLargo.
Each investor also entered into an agreement with BioLargo whereby the investor may exchange some or all of its shares of BETI common stock into shares of BioLargo common stock, at a price equal to a 20% discount of the volume weighted average price over the 20 trading days prior to the election to exchange. Elections must be made during calendar year 2024.
As of June 30, 2023, there are 9,457,000 shares outstanding, of which BioLargo holds 9,050,000 (96%).
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for noncontrolling interest in consolidated subsidiaries, which could include the name of the subsidiary, the ownership percentage held by the parent, the ownership percentage held by the noncontrolling owners, the amount of the noncontrolling interest, the location of this amount on the balance sheet (when not reported separately), an explanation of the increase or decrease in the amount of the noncontrolling interest, the noncontrolling interest share of the net Income or Loss of the subsidiary, the location of this amount on the income statement (when not reported separately), the nature of the noncontrolling interest such as background information and terms, the amount of the noncontrolling interest represented by preferred stock, a description of the preferred stock, and the dividend requirements of the preferred stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
| Name: |
us-gaap_MinorityInterestDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 11 - Business Segment Information
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Segment Reporting Disclosure [Text Block] |
Note 11. Business Segment Information
BioLargo has five operating business segments, plus its corporate entity which is responsible for general corporate operations, including administrative functions, finance, human resources, marketing, legal, etc. The operational business segments are:
| | 1. | ONM Environmental -- which sells odor and volatile organic control products and services (located in Westminster, California); |
| | 2. | Clyra Medical Technologies (“Clyra Medical”) -- which develops and sells medical products based on our technologies; |
| | 3. | BLEST -- which provides professional engineering services on a time and materials basis for outside clients and supports our internal operations as needed (located in Oak Ridge, Tennessee); |
| | 4. | BioLargo Water (“Water”) – our research, development and innovation group operating out of the University of Alberta, Edmonton, Canada; and |
| | 5. | BioLargo Energy Technologies, Inc. (“BETI”) – formed to commercialize our sodium-sulfur battery technology. |
Other than ONM Environmental, during the last three quarterly periods none of our operating business units have operated at a profit, and therefore each required additional cash to meet its monthly expenses, funded through BioLargo’s sales of debt or equity, research grants, and tax credits. BETI and Clyra Medical have also been funded by third party investors who invest directly in exchange for equity ownership in that entity.
The segment information for the three and six months ended June 30, 2023, and 2022, is as follows (in thousands):
| | | Three months ended June 30, | | | Six months ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| Revenue | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | — | | | $ | — | | | $ | — | | | $ | 2 | |
| ONM Environmental | | | 1,316 | | | | 700 | | | | 4,859 | | | | 1,300 | |
| BLEST | | | 538 | | | | 670 | | | | 901 | | | | 1,213 | |
| BETI | | | — | | | | — | | | | — | | | | — | |
| Water | | | 9 | | | | — | | | | — | | | | — | |
| Clyra Medical | | | — | | | | 6 | | | | 6 | | | | 17 | |
| Intersegment revenue | | | (417 | ) | | | (53 | ) | | | (587 | ) | | | (245 | ) |
| Total | | $ | 1,446 | | | $ | 1,323 | | | $ | 5,188 | | | $ | 2,287 | |
| | | | | | | | | | | | | | | | | |
| Research and development expense | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (243 | ) | | $ | (165 | ) | | $ | (432 | ) | | $ | (430 | ) |
| BLEST | | | (292 | ) | | | (80 | ) | | | (537 | ) | | | (188 | ) |
| BETI | | | (271 | ) | | | — | | | | (303 | ) | | | — | |
| Water | | | (138 | ) | | | (130 | ) | | | (273 | ) | | | (327 | ) |
| Clyra Medical | | | (60 | ) | | | (26 | ) | | | (194 | ) | | | (42 | ) |
| Intersegment R&D | | | 417 | | | | 46 | | | | 587 | | | | 240 | |
| Total | | $ | (587 | ) | | $ | (355 | ) | | $ | (1,152 | ) | | $ | (747 | ) |
| | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (635 | ) | | $ | (923 | ) | | $ | (1,434 | ) | | $ | (2,143 | ) |
| ONM Environmental | | | 428 | | | | 11 | | | | 1,815 | | | | 18 | |
| BLEST | | | (450 | ) | | | 56 | | | | (818 | ) | | | 21 | |
| BETI | | | (297 | ) | | | — | | | | (384 | ) | | | — | |
| Water | | | (209 | ) | | | (209 | ) | | | (393 | ) | | | (431 | ) |
| Clyra Medical | | | (396 | ) | | | (256 | ) | | | (823 | ) | | | (496 | ) |
| Total | | $ | (1,559 | ) | | $ | (1,321 | ) | | $ | (2,037 | ) | | $ | (3,031 | ) |
| | | | | | | | | | | | | | | | | |
| Interest expense | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (4 | ) | | $ | (6 | ) | | $ | (40 | ) | | $ | (12 | ) |
| ONM Environmental | | | (2 | ) | | | — | | | | (4 | ) | | | — | |
| Clyra Medical | | | (6 | ) | | | (9 | ) | | | (16 | ) | | | (16 | ) |
| Total | | $ | (12 | ) | | $ | (15 | ) | | $ | (60 | ) | | $ | (28 | ) |
| | | | | | | | | | | | | | | | | |
| As of June 30, 2023 | | BioLargo | | | ONM | | | Clyra | | | BLEST | | | Water | | | BETI | | | Elimination | | | Total | |
| Tangible assets | | $ | 731 | | | $ | 2,705 | | | $ | 1,265 | | | $ | 414 | | | $ | 92 | | | $ | 686 | | | $ | (41 | ) | | $ | 5,852 | |
| Right of use leased asset | | | 93 | | | | — | | | | — | | | | 714 | | | | — | | | | — | | | | — | | | | 807 | |
| Investment in South Korean joint venture | | | 21 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 21 | |
| Total | | $ | 845 | | | $ | 2,705 | | | $ | 1,265 | | | $ | 1,128 | | | $ | 92 | | | $ | 686 | | | $ | (41 | ) | | $ | 6,680 | |
| As of December 31, 2022 | | BioLargo | | | ONM | | | Clyra | | | BLEST | | | Water | | | Elimination | | | Total | |
| Tangible assets | | $ | 669 | | | $ | 2,064 | | | $ | 631 | | | $ | 441 | | | $ | 194 | | | $ | (41 | ) | | $ | 3,958 | |
| Right of use | | | 136 | | | | — | | | | — | | | | 731 | | | | — | | | | — | | | | 867 | |
| Investment in South Korean joint venture | | | 33 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 33 | |
| Total | | $ | 838 | | | $ | 2,064 | | | $ | 631 | | | $ | 1,172 | | | $ | 194 | | | $ | (41 | ) | | $ | 4,858 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for reporting segments including data and tables. Reportable segments include those that meet any of the following quantitative thresholds a) it's reported revenue, including sales to external customers and intersegment sales or transfers is 10 percent or more of the combined revenue, internal and external, of all operating segments b) the absolute amount of its reported profit or loss is 10 percent or more of the greater, in absolute amount of 1) the combined reported profit of all operating segments that did not report a loss or 2) the combined reported loss of all operating segments that did report a loss c) its assets are 10 percent or more of the combined assets of all operating segments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//280/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 26
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-26
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 34
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-34
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_SegmentReportingDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 12 - Commitments and Contingencies
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Commitments and Contingencies Disclosure [Text Block] |
Note 12. Commitments and Contingencies
Office Leases
We have long-term operating leases for office, industrial and laboratory space in Westminster, California, Oak Ridge, Tennessee, and Alberta, Canada. Payments made under operating leases are charged to the Consolidated Statement of Operations and Comprehensive Loss on a straight-line basis over the term of the operating lease agreement. Short-term leases less than one-year are not included in our analysis. For the three and six months ended June 30, 2023, rental expense was $83,000 and $166,000 and for the three and six months ended June 30, 2022, rental expense was $106,000 and $169,000. The lease of our Westminster facility expires August 2024. Management has not yet determined whether it will exercise its option to extend the lease four years; therefore the four-year extension is not included in the analysis. In September 2022, the lease of our Oak Ridge, Tennessee facility was extended for ten years. The lease of our Canadian facility is less than one year. None of our leases have additional terms related to the payments or mechanics of the lease. The leases have no additional payment terms such as common area maintenance payments, tax sharing payments or other allocable expenses. Likewise, the leases do not contain other terms and conditions of use, such as variable lease payments, residual value guaranties or other restrictive financial terms. Since there is no explicit interest rate in our leases, management used its incremental borrowing rate, which is estimated to be 18% to determine lease liability.
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 13 - Subsequent Events
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Subsequent Events [Text Block] |
Note 13. Subsequent Events.
Management has evaluated subsequent events through the date of the filing of this quarterly report and management noted the following for disclosure.
Lincoln Park Capital Purchase of Shares
From July 1, 2023, through August 10, 2023, we sold 782,553 shares of common stock to Lincoln Park pursuant to the 2022 LPC Purchase Agreement (see Note 3) and received $136,000 in gross and net proceeds. These sales were registered with the SEC on Form S-1 (file number 333-268973).
Clyra Medical – Sales of Series A Preferred Stock Units
From July 1, 2023, through August 10, 2023, Clyra Medical sold 81 shares of its Series A Preferred Stock, and in exchange received $25,000 in gross and net proceeds. Purchasers of the Series A Preferred Stock also received a 3-year warrant to purchase the same number of additional shares of common stock for $372 per share. (See Note 8, “Sales of Series A Preferred Stock”.)
BETI – Sales of Common Stock
From July 1, 2023, through August 10, 2023, BETI sold 10,000 shares of its common stock, and in exchange received $25,000 in gross and net proceeds. (See Note 10.)
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies (Policies)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Consolidation, Policy [Policy Text Block] |
Principles of Consolidation The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and partially owned subsidiaries BETI, BLEST and Clyra Medical. All intercompany accounts and transactions have been eliminated.
|
| Foreign Currency Transactions and Translations Policy [Policy Text Block] |
Foreign Currency The Company has designated the functional currency of BioLargo Water, Inc., our Canadian subsidiary, to be the Canadian dollar. Therefore, translation gains and losses resulting from differences in exchange rates are recorded in accumulated other comprehensive income.
|
| Cash and Cash Equivalents, Policy [Policy Text Block] |
Cash and Cash Equivalents The Company considers all highly liquid investments with maturities of three months or less when acquired to be cash equivalents. Substantially all cash equivalents are held in short-term money market accounts at one of the largest financial institutions in the United States, Bank of America. From time to time, our cash account balances are greater than the Federal Deposit Insurance Corporation insurance limit of $250,000 per owner per bank, and during such times, we are exposed to credit loss for amounts in excess of insured limits in the event of non-performance by the financial institution. We do not anticipate non-performance by our financial institution. As of June 30, 2023, and December 31, 2022, our cash balances were made up of the following (in thousands):
| | | June 30, 2023 | | | December 31, 2022 | |
| BioLargo, Inc. and subsidiaries | | $ | 2,762 | | | $ | 1,681 | |
| Clyra Medical Technologies, Inc. | | | 813 | | | | 170 | |
| Total | | $ | 3,575 | | | $ | 1,851 | |
|
| Receivable [Policy Text Block] |
Accounts Receivable In June 2016, the FASB issued ASU 2016-13, which sets out the principles for the recognition of measurement of credit losses on financial instruments, including trade receivables. The standard eliminates the probable initial recognition threshold and requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. The new standard was effective for the Company beginning January 1, 2023 and primarily impacted trade accounts receivable. Accounts receivable are customer obligations that are unconditional. Accounts receivable are presented net of an allowance for doubtful accounts for expected credit losses, which represents an estimate of amounts that may not be collectible. The Company performs ongoing credit evaluations of its customers and, if necessary, provides an allowance for doubtful accounts and expected credit losses. A provision to the allowances for doubtful accounts for expected credit losses is recorded based on factors including the length of time the receivables are past due, the current business environment, and the Company’s historical experience. Provisions to the allowances for doubtful accounts for expected credit losses are recorded to general and administrative expenses. The Company writes off accounts receivable against the allowance when it determines a balance is uncollectible and no longer actively pursues collection of the receivable. The Company does not have any off-balance-sheet credit exposure related to customers. As of June 30, 2023, and December 31, 2022, the allowance for doubtful accounts for expected credit losses was $58,000 and $12,000.
|
| Concentration Risk, Credit Risk, Policy [Policy Text Block] |
Credit Concentration We have a limited number of customers that account for significant portions of our revenue. During the three and six months ended June 30, 2023, and 2022, the following customers accounted for more than 10% of consolidated revenues:
| | | Three Months | | | Six Months | |
| | | June 30, 2023 | | | June 30, 2022 | | | June 30, 2023 | | | June 30, 2022 | |
| | | | | | | | | | | | | | | | | |
| Customer A | | | 55 | % | | | 39 | % | | | 77 | % | | | 35 | % |
| Customer B | | <10 | % | | | 27 | % | | <10 | % | | | 17 | % |
| Customer C | | <10 | % | | <10 | % | | <10 | % | | | 10 | % |
At June 30, 2023, one customer accounted for more than 10% of consolidated accounts receivable, and at December 31, 2022, three customers accounted for more than 10% of consolidated accounts receivable:
| | | June 30, 2023 | | | December 31, 2022 | |
| Customer A | | | 49 | % | | | 11 | % |
| Customer B | | <10 | % | | | 31 | % |
| Customer D | | <10 | % | | | 15 | % |
|
| Inventory, Policy [Policy Text Block] |
Inventory Inventories are stated at the lower of cost or net realizable value using the average cost method. The allowance for obsolete inventory as of June 30, 2023, and December 31, 2022, was $212,000 and $158,000. Inventories consisted of (in thousands):
| | | June 30, 2023 | | | December 31, 2022 | |
| Raw material | | $ | 83 | | | $ | 46 | |
| Finished goods | | | 44 | | | | 74 | |
| Total | | $ | 127 | | | $ | 120 | |
|
| Other Assets, Policy [Policy Text Block] |
Other Non-Current Assets Other non-current assets consisted of (i) security deposits related to our business offices, (ii) three patents acquired on October 22, 2021, for $34,000, and (iii) tax credit receivables from the Canadian government related to a research and development credit from our Canadian subsidiary for which we’ve applied for and received in prior periods.
| | | June 30, 2023 | | | December 31, 2022 | |
| Patents | | $ | 34 | | | $ | 34 | |
| Security deposits | | | 35 | | | | 35 | |
| Tax credit receivable | | | -- | | | | 55 | |
| Total | | $ | 69 | | | $ | 124 | |
|
| Equity Method Investments [Policy Text Block] |
Equity Method of Accounting On March 20, 2020, we invested $100,000 into a South Korean entity (Odin Co. Ltd., “Odin”) pursuant to a Joint Venture agreement we had entered into with BKT Co. Ltd. and its U.S. based subsidiary, Tomorrow Water. We received a 40% non-dilutive equity interest, and BKT and Tomorrow Water each received 30% equity interests for an aggregate $150,000 investment. We account for our investment in the joint venture under the equity method of accounting. We have determined that while we have significant influence over the joint venture through our technology license and our position on the Board of Directors, we do not control the joint venture or are otherwise involved in managing the entity and we own less than a majority of the equity. Therefore, we record the asset on our consolidated balance sheet and record an increase or decrease of the recorded balance by our percentage ownership of the profits or losses in the joint venture. The joint venture has incurred a loss since inception and our 40% ownership share reduced our investment interest. For the three and six months ended June 30, 2023, the reduction of our investment interest totaled $6,000 and $12,000, respectively, and for the same periods in 2022, reduced our investment interest $8,000 and $15,000, respectively.
|
| Impairment or Disposal of Long-Lived Assets, Policy [Policy Text Block] |
Impairment Long-lived and definite lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows from the use of the asset and its eventual disposition is less than the carrying amount of the asset, then an impairment loss is recognized. The impairment loss is measured based on the fair value of the asset. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. There were no impairment losses related to intangible assets during the three or six months ended June 30, 2023 or 2022.
|
| Earnings Per Share, Policy [Policy Text Block] |
Earnings (Loss) Per Share We report basic and diluted earnings (loss) per share (“EPS”) for common and common share equivalents. Basic EPS is computed by dividing reported earnings by the weighted average shares outstanding. Diluted EPS is computed by adding to the weighted average shares the dilutive effect if convertible notes payable, stock options and warrants were exercised into common stock. For the three and six months ended June 30, 2023, and 2022, the denominator in the diluted EPS computation is the same as the denominator for basic EPS due to the Company’s net loss which creates an anti-dilutive effect of the convertible notes payable, warrants and stock options.
|
| Use of Estimates, Policy [Policy Text Block] |
Use of Estimates The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and revenues and expenses during the period reported. Actual results could differ from those estimates. Estimates are used when accounting for stock-based transactions, debt transactions, derivative liabilities, allowance for bad debt, asset depreciation and amortization, impairment expense, among others. The methods, estimates and judgments we use in applying these most critical accounting policies have a significant impact on the results of our financial statements.
|
| Share-Based Payment Arrangement [Policy Text Block] |
Share-Based Compensation Expense We recognize compensation expense for stock option awards on a straight-line basis over the applicable service period of the award, which is the vesting period. Fair value is determined on the grant date. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes Option Pricing Model. For stock and stock options issued to consultants and other non-employees for services, the Company measures and records an expense as of the earlier of the date at which either: a commitment for performance by the non-employee has been reached or the non-employee’s performance is complete. The equity instruments are measured at the current fair value, and for stock options, the instruments are measured at fair value using the Black Scholes option model. The following methodology and assumptions were used to calculate share-based compensation for the six months ended June 30, 2023, and 2022:
| | | 2023 | | | 2022 | |
| | | Non Plan | | | 2018 Plan | | | Non Plan | | | 2018 Plan | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | 3.48 | – | 3.58% | | | 2.32 | – | 3.83% | | | 2.32 | – | 2.98% | |
| Expected volatility | | 113 | - | 114% | | | 113 | - | 114% | | | 114 | – | 117% | | | 116 | – | 117% | |
| Expected dividend yield | | — | | | — | | | — | | | — | |
| Forfeiture rate | | — | | | — | | | — | | | — | |
| Life in years | | 10 | | | 10 | | | 10 | | | 10 | |
Expected price volatility is the measure by which our stock price is expected to fluctuate during the expected term of an option. The expected volatility is derived from the historical daily change in the market price of our common stock, as we believe that historical volatility is the best indicator of future volatility. The risk-free interest rate used in the Black-Scholes calculation is based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. We have never paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future.
|
| Warrant Policy [Policy Text Block] |
Warrants Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method. The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”). If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note. Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. The warrant relative fair values are also recorded as a discount to the convertible promissory notes.
|
| Non Cash Transactions [Policy Text Block] |
Non-Cash Transactions We determine the value assigned to each intangible we acquire, and/or services or products received for non-cash consideration of our common stock based on the market price of our common stock issued as consideration, at the date of the agreement of each transaction or when the service is rendered or product is received.
|
| Revenue from Contract with Customer [Policy Text Block] |
Revenue Recognition We account for revenue in accordance with ASC 606, “Revenue from Contacts with Customers”. The guidance focuses on the core principle for revenue recognition, which is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, the guidance provides that an entity should apply the following steps: Step 1: Identify the contract(s) with a customer. Step 2: Identify the performance obligations in the contract. Step 3: Determine the transaction price. Step 4: Allocate the transaction price to the performance obligations in the contract. Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation. The Company’s products are sold through a contract with the customer and a written purchase order, in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product, and each product has separate pricing. Revenue is recognized at a point in time when the goods are shipped if the agreement is FOB manufacturer, and when goods are delivered if FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order. Service contracts are performed through a written contract, which specifies the performance obligations and the rate at which the services will be billed, typically by time and materials. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed, or, for services related to product installations, at the completion of the installation. A few contracts have called for milestone or fixed cost payments, where we invoice an agreed-to amount per month for the life of the contract. In these instances, completed work, billed hourly, is recognized as revenue. If the billing amount is greater or lesser than the completed work, a receivable or payable is created. These accounts are adjusted upon additional billings as the work is completed. To date, there have been no discounts or other financing terms for the contracts. The Company has outstanding contract liability obligations of $10,000 as of June 30, 2023, recorded as deferred revenue. We recognized $7,000 in revenue and reduced the deferred revenue balance by the same amount as performance obligations were satisfied in the six months ended June 30, 2023. The outstanding balance will be recognized over the remaining life of the contracts. Our Canadian subsidiary had a customer deposit outstanding at June 30, 2023, totaling $113,000, that was awarded as part of a grant for a particular project that has been delayed. As we generate revenues from royalties or license fees from our intellectual property, a licensee will pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. We have entered into a licensing agreement for the CupriDyne Clean product, and we recognize royalty and license fees on a quarterly basis as the product is sold through to third parties and reported to us.
|
| Government Grants [Policy Text Block] |
Government Grants We have been awarded multiple research grants from the private and public Canadian research programs. The income we receive directly from grants is recorded as other income. We have been awarded over 80 grants since our first in 2015. Some of the funds from these grants are given directly to third parties (such as the University of Alberta or a third-party research scientist) to support research on our technology. The grants have terms generally ranging between six and eighteen months and support a majority, but not all, of the related research budget costs. This cooperative research allows us to utilize (i) a depth of resources and talent to accomplish highly skilled work, (ii) financial aid to support research and development costs, (iii) independent and credible validation of our technical claims.
The grants typically provide for (i) recurring monthly amounts, (ii) reimbursement of costs for research talent for which we invoice to request payment, and (iii) ancillary cost reimbursement for research talent travel related costs. All awarded grants have specific requirements on how the money is spent, typically to employ researchers. None of the funds may be used for general administrative expenses or overhead in the United States. These grants have substantially increased our level of research and development activities in Canada. We continue to apply for Canadian government and agency grants to fund research and development activities. Not all of our grant applications have been awarded, and no assurance can be made that any pending grant application, or any future grant applications, will be awarded.
|
| Income Tax, Policy [Policy Text Block] |
Income Taxes The asset and liability approach is used to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of asset and liabilities. Deferred tax assets and liabilities are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. We account for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation, and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by generally accepted accounting principles (“GAAP”). Under GAAP, the tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized. Management believes there are no unrecognized tax benefits or uncertain tax positions as of June 30, 2023, and December 31, 2022. The Company assessed its earnings history, trends and estimates of future earnings and determined that the deferred tax asset could not be realized as of June 30, 2023, and December 31, 2022. Accordingly, a 100% valuation allowance was recorded against the net deferred tax asset. The Company recognizes interest and penalties on income taxes as a component of income tax expense, should such an expense be realized.
|
| Fair Value of Financial Instruments, Policy [Policy Text Block] |
Fair Value of Financial Instruments Management believes the carrying amounts of the Company’s financial instruments as of June 30, 2023, and December 31, 2022 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, line of credit, and other assets and liabilities. The carrying amount of debt instruments are believed to approximate fair value as the stated interest rates are reflective of the prevailing market rates.
|
| Tax Credits [Policy Text Block] |
Tax Credits Our research and development activities in Canada may entitle our Canadian subsidiary to claim benefits under the “Scientific Research and Experimental Development Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses of all sizes and in all sectors to conduct research and development in Canada. Benefits under the program include credits to taxable income. If our Canadian subsidiary does not have taxable income in a reporting period, we instead receive a tax refund from the Canadian Revenue Authority. Those refunds are classified in Other Income on our Consolidated Statement of Operations and Comprehensive Loss.
|
| Lessee, Leases [Policy Text Block] |
Leases At inception of a lease contract, we assess whether the contract is, or contains, a lease. Our assessment is based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether we obtain the right to substantially all the economic benefit from the use of the asset throughout the period of the contract, and (3) whether we have the right to direct the use of the asset during such time period. At inception of a lease, we allocate the consideration in the contract to each lease component based on its relative stand-alone price to determine the lease payments. Leases are classified as either finance leases or operating leases. A lease must be classified as a finance lease if any of the following criteria are met: the lease transfers ownership of the asset by the end of the lease term, the lease contains an option to purchase the asset that is reasonably certain to be exercised, the lease term is for a major part of the remaining useful life of the asset or the present value of the lease payments equals or exceeds substantially all of the fair value of the asset. A lease is classified as an operating lease if it does not meet any of these criteria. We have no leases classified as finance leases. As of June 30, 2023, the weighted average remaining lease term for our operating leases was nine years. The weighted average discount rate for our operating leases was 18%. For all leases at the lease commencement date, a right-of-use asset and a lease liability are recognized. The right-of-use asset represents the right to use the leased asset for the lease term. The lease liability represents the present value of the lease payments under the lease. The right-of-use asset is initially measured at cost, which primarily comprises the initial amount of the lease liability, plus any initial direct costs incurred, consisting mainly of brokerage commissions, less any lease incentives received. All right-of-use assets are reviewed for impairment. The lease liability is initially measured at the present value of the lease payments, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, management estimates the incremental borrowing rate, which currently is estimated to be 18%. Lease payments included in the measurement of the lease liability comprise the following: the fixed noncancelable lease payments, payments for optional renewal periods where it is reasonably certain the renewal period will be exercised, and payments for early termination options unless it is reasonably certain the lease will not be terminated early. Lease components are included in the measurement of the initial lease liability. Additional payments based on a change in our portion of the operating expenses, including real estate taxes and insurance, are recorded as a period expense when incurred. Lease modifications result in remeasurement of the lease liability. Lease expense for operating leases consists of the lease payments plus any initial direct costs, primarily brokerage commissions, and is recognized on a straight-line basis over the lease term. We have elected not to recognize right-of-use assets and lease liabilities for short-term leases that have a term of 12 months or less. The effect of short-term leases on our right-of-use asset and lease liability was not material. As of June 30, 2023, the right-of-use assets totaled $807,000 and the lease liability totaled $818,000 on our balance sheet related to our operating leases.
|
| Property, Plant and Equipment, Policy [Policy Text Block] |
Property and Equipment Property and equipment includes machinery and leasehold improvements and is stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, which range from 3 - 5 years or the remaining lease term. Newly built leaseholds, additions, renewals, and betterments that significantly extend the life of the asset are capitalized. The Company is currently constructing a facility and purchasing equipment for producing sodium sulfur batteries. Expenditures for repairs and maintenance are charged to expense as incurred. For assets sold or otherwise disposed of, the cost and related accumulated depreciation and amortization are removed from the accounts, and any related gain or loss is reflected in income for the period.
|
| New Accounting Pronouncements, Policy [Policy Text Block] |
Recent Accounting Pronouncements Currently there are no recently released pronouncements that are considered applicable to the Company’s financial statements.
|
| X |
- DefinitionThe disclosure of policies relating to government grants.
| Name: |
blgo_GovernmentGrantsPolicyTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAccounting policy disclosure for non cash transactions.
| Name: |
blgo_NonCashTransactionsPolicyTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for assets classified as other.
| Name: |
blgo_OtherAssetsPolicyPolicyTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for tax credits.
| Name: |
blgo_TaxCreditsPolicyTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for warrant.
| Name: |
blgo_WarrantPolicyTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480981/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for equity method of accounting for investments and other interests. Investment includes, but is not limited to, unconsolidated subsidiary, corporate joint venture, noncontrolling interest in real estate venture, limited partnership, and limited liability company. Information includes, but is not limited to, ownership percentage, reason equity method is or is not considered appropriate, and accounting policy election for distribution received. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 21D
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-21D
| Name: |
us-gaap_EquityMethodInvestmentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482105/912-330-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for receivable. Includes, but is not limited to, accounts receivable and financing receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481569/310-20-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481569/310-20-50-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481569/310-20-50-2
| Name: |
us-gaap_ReceivablesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue from contract with customer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-18
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-18
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 9: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org//606/tableOfContent
| Name: |
us-gaap_RevenueFromContractWithCustomerPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for award under share-based payment arrangement. Includes, but is not limited to, methodology and assumption used in measuring cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.C.Q3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.1.Q5)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.3.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.2.Q6)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationOptionAndIncentivePlansPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Cash and Cash Equivalents [Table Text Block] |
| | | June 30, 2023 | | | December 31, 2022 | |
| BioLargo, Inc. and subsidiaries | | $ | 2,762 | | | $ | 1,681 | |
| Clyra Medical Technologies, Inc. | | | 813 | | | | 170 | |
| Total | | $ | 3,575 | | | $ | 1,851 | |
|
| Schedules of Concentration of Risk, by Risk Factor [Table Text Block] |
| | | Three Months | | | Six Months | |
| | | June 30, 2023 | | | June 30, 2022 | | | June 30, 2023 | | | June 30, 2022 | |
| | | | | | | | | | | | | | | | | |
| Customer A | | | 55 | % | | | 39 | % | | | 77 | % | | | 35 | % |
| Customer B | | <10 | % | | | 27 | % | | <10 | % | | | 17 | % |
| Customer C | | <10 | % | | <10 | % | | <10 | % | | | 10 | % |
| | | June 30, 2023 | | | December 31, 2022 | |
| Customer A | | | 49 | % | | | 11 | % |
| Customer B | | <10 | % | | | 31 | % |
| Customer D | | <10 | % | | | 15 | % |
|
| Schedule of Inventory, Current [Table Text Block] |
| | | June 30, 2023 | | | December 31, 2022 | |
| Raw material | | $ | 83 | | | $ | 46 | |
| Finished goods | | | 44 | | | | 74 | |
| Total | | $ | 127 | | | $ | 120 | |
|
| Schedule of Other Assets, Noncurrent [Table Text Block] |
| | | June 30, 2023 | | | December 31, 2022 | |
| Patents | | $ | 34 | | | $ | 34 | |
| Security deposits | | | 35 | | | | 35 | |
| Tax credit receivable | | | -- | | | | 55 | |
| Total | | $ | 69 | | | $ | 124 | |
|
| Schedule of Share-Based Payment Award, Stock Options, Valuation Assumptions [Table Text Block] |
| | | 2023 | | | 2022 | |
| | | Non Plan | | | 2018 Plan | | | Non Plan | | | 2018 Plan | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | 3.48 | – | 3.58% | | | 2.32 | – | 3.83% | | | 2.32 | – | 2.98% | |
| Expected volatility | | 113 | - | 114% | | | 113 | - | 114% | | | 114 | – | 117% | | | 116 | – | 117% | |
| Expected dividend yield | | — | | | — | | | — | | | — | |
| Forfeiture rate | | — | | | — | | | — | | | — | |
| Life in years | | 10 | | | 10 | | | 10 | | | 10 | |
|
| X |
- DefinitionTabular disclosure of the components of cash and cash equivalents.
| Name: |
us-gaap_ScheduleOfCashAndCashEquivalentsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the carrying amount as of the balance sheet date of merchandise, goods, commodities, or supplies held for future sale or to be used in manufacturing, servicing or production process. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
| Name: |
us-gaap_ScheduleOfInventoryCurrentTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of noncurrent assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ScheduleOfOtherAssetsNoncurrentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the nature of a concentration, a benchmark to which it is compared, and the percentage that the risk is to the benchmark. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-16
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-21
| Name: |
us-gaap_SchedulesOfConcentrationOfRiskByRiskFactorTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 4 - Debt Obligations (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Debt [Table Text Block] |
| | | June 30, 2023 | | |
December 31,
2022 | |
| Current portion of debt: | | | | | | | | |
| SBA Paycheck Protection Program loan | | $ | 43 | | | $ | 43 | |
| Vehicle loan, current portion | | | 13 | | | | — | |
| Convertible note payable, matures March 1, 2023 | | | — | | | | 50 | |
| SBA EIDL Loan, matures July 2053, current portion | | | 10 | | | | 10 | |
| Debt discount, net of amortization | | | — | | | | (3 | ) |
| Total current portion of debt | | $ | 66 | | | $ | 100 | |
| | | | | | | | | |
| Long-term debt: | | | | | | | | |
| SBA Paycheck Protection Program loans, matures May 2025 | | $ | 97 | | | $ | 97 | |
| Vehicle loan, matures March 2029 | | | 62 | | | | — | |
| SBA EIDL Loan, matures July 2050 | | | 140 | | | | 140 | |
| Total long-term debt, net of current | | $ | 299 | | | $ | 237 | |
| | | | | | | | | |
| Total | | $ | 365 | | | $ | 337 | |
|
| X |
- DefinitionTabular disclosure of information pertaining to short-term and long-debt instruments or arrangements, including but not limited to identification of terms, features, collateral requirements and other information necessary to a fair presentation.
| Name: |
us-gaap_ScheduleOfDebtTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 5 - Share-based Compensation (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Share-Based Payment Arrangement, Option, Activity [Table Text Block] |
| | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | Average | | | Aggregate | |
| | | Options | | | Exercise | | | Price per | | | intrinsic | |
| | | Outstanding | | | Price per share | | | share | | | Value(1) | |
| Balance, December 31, 2022 | | | 28,484,549 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| Granted | | | 2,636,712 | | | $0.18 | – | 0.20 | | | $ | 0.19 | | | | | |
| Balance, June 30, 2023 | | | 31,121,261 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| Unvested | | | (4,121,892 | ) | | $0.12 | – | 0.32 | | | $ | 0.19 | | | | | |
| Vested, June 30, 2023 | | | 26,999,369 | | | 0.12 | – | 0.43 | | | $ | 0.19 | | | $ | 402,000 | |
| Balance, December 31, 2021 | | | 23,186,142 | | | $0.16 | – | 0.43 | | | $ | 0.19 | | | | | |
| Granted | | | 3,782,923 | | | $0.18 | – | 0.24 | | | $ | 0.22 | | | | | |
| Balance, June 30, 2022 | | | 26,969,065 | | | $0.12 | – | 0.43 | | | $ | 0.19 | | | | | |
| | | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | | Average | | | Aggregate | |
| | | Options | | | Exercise | | | Price per | | | intrinsic | |
| | | Outstanding | | | price per share | | | share | | | Value(1) | |
| Balance, December 31, 2022 | | | 1,904,085 | | | | $0.28 | – | 0.69 | | | $ | 0.56 | | | | | |
| Expired | | | (40,000 | ) | | | 0.28 | | | | 0.28 | | | | | |
| Balance, June 30, 2023 | | | 1,864,085 | | | | $0.28 | – | 0.69 | | | $ | 0.56 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 2,879,246 | | | | $0.23 | – | 0.94 | | | $ | 0.49 | | | | | |
| Expired | | | (300,000 | ) | | | 0.35 | | | | 0.35 | | | | | |
| Balance, June 30, 2022 | | | 2,579,246 | | | | $0.23 | – | 0.94 | | | $ | 0.49 | | | | | |
| | | | | | | | | | | | | Weighted | | | | | |
| | | Non-plan | | | | | | | | | average | | | Aggregate | |
| | | Options | | | Exercise | | | price per | | | intrinsic | |
| | | outstanding | | | price per share | | | share | | | value(1) | |
| Balance, December 31, 2022 | | | 19,023,829 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Granted | | | 60,040 | | | | 0.18 | – | 0.20 | | | | 0.20 | | | | | |
| Balance, June 30, 2023 | | | 19,083,869 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Unvested | | | (507,500 | ) | | | 0.45 | | | | 0.45 | | | | | |
| Vested, June 30, 2023 | | | 18,576,369 | | | | $0.12 | – | 0.83 | | | $ | 0.38 | | | $ | 46,000 | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 20,119,207 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
| Granted | | | 39,130 | | | | 0.23 | | | | 0.23 | | | | | |
| Balance, June 30, 2022 | | | 20,158,337 | | | | $0.12 | – | 0.83 | | | $ | 0.39 | | | | | |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 6 - Warrants (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Stockholders' Equity Note, Warrants or Rights [Table Text Block] |
| | | | | | | | | | | | | Weighted | | | | | |
| | | | | | | | | | | | | average | | | Aggregate | |
| | | Warrants | | | Exercise | | | price per | | | intrinsic | |
| | | outstanding | | | price per share | | | share | | | value(1) | |
| Balance, December 31, 2022 | | | 49,023,398 | | | | $0.13 | – | 1.00 | | | $ | 0.26 | | | | | |
| Granted | | | 10,669,896 | | | | 0.21 | – | 0.29 | | | | 0.25 | | | | | |
| Expired | | | (5,173,209 | ) | | | 0.19 | – | 0.35 | | | | 0.21 | | | | | |
| Balance, June 30, 2023 | | | 54,520,085 | | | | $0.13 | – | 1.00 | | | $ | 0.26 | | | $ | 47,000 | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 36,765,502 | | | | $0.16 | – | 1.00 | | | $ | 0.27 | | | | | |
| Granted | | | 19,604,796 | | | | 0.20 | – | 0.29 | | | | 0.24 | | | | | |
| Expired | | | (4,016,754 | ) | | | 0.19 | – | 0.48 | | | | 0.35 | | | | | |
| Balance, June 30, 2022 | | | 52,353,544 | | | | $0.14 | – | 1.00 | | | $ | 0.25 | | | | | |
|
| Schedule Of Assumptions Used To Determine Fair Value Of Warrants [Table Text Block] |
| | | 2023 | | | 2022 | |
| Risk free interest rate | | 3.88 | – | 4.27% | | | 3.69 | – | 3.88% | |
| Expected volatility | | 40 | – | 95% | | | 40% | |
| Expected dividend yield | | — | | | — | |
| Forfeiture rate | | — | | | — | |
| Expected life in years | | 3 | - | 5 | | | 3 | |
|
| X |
- DefinitionThe tabular disclosure of assumptions used to determine the fair value of warrants.
| Name: |
blgo_ScheduleOfAssumptionsUsedToDetermineFairValueOfWarrantsTableTextBlock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of warrants or rights issued. Warrants and rights outstanding are derivative securities that give the holder the right to purchase securities (usually equity) from the issuer at a specific price within a certain time frame. Warrants are often included in a new debt issue to entice investors by a higher return potential. The main difference between warrants and call options is that warrants are issued and guaranteed by the company, whereas options are exchange instruments and are not issued by the company. Also, the lifetime of a warrant is often measured in years, while the lifetime of a typical option is measured in months. Disclose the title of issue of securities called for by warrants and rights outstanding, the aggregate amount of securities called for by warrants and rights outstanding, the date from which the warrants or rights are exercisable, and the price at which the warrant or right is exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
| Name: |
us-gaap_ScheduleOfStockholdersEquityNoteWarrantsOrRightsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 7 - Accounts Payable and Accrued Expenses (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Accounts Payable and Accrued Liabilities [Table Text Block] |
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | BETI | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 142 | | | $ | 168 | | | $ | 119 | | | $ | 106 | | | $ | 29 | | | $ | (82 | ) | | $ | 482 | |
| Accrued payroll | | | 50 | | | | 92 | | | | 71 | | | | — | | | | — | | | | — | | | | 213 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 720 | |
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 187 | | | $ | 486 | | | $ | 7 | | | $ | 119 | | | $ | (82 | ) | | $ | 717 | |
| Accrued payroll | | | 20 | | | | 58 | | | | 120 | | | | — | | | | — | | | | 198 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | $ | 940 | |
|
| X |
- DefinitionTabular disclosure of the (a) carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business (accounts payable); (b) other payables; and (c) accrued liabilities. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). An alternative caption includes accrued expenses.
| Name: |
us-gaap_ScheduleOfAccountsPayableAndAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 8 - Noncontrolling Interest - Clyra Medical (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Other Ownership Interests [Table Text Block] |
| | | | | | | | | | | | | Weighted | |
| | | Clyra | | | | | | | | | average | |
| | | Options | | | Exercise | | | price per | |
| | | Outstanding | | | price per share | | | share | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2022 | | | 15,833 | | | | $1.00 | - | 310 | | | $ | 5.53 | |
| Granted | | | 858 | | | | 1.00 | - | 271 | | | | 146.06 | |
| Balance, June 30, 2023 | | | 16,691 | | | | $1.00 | - | 310 | | | $ | 12.76 | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 14,004 | | | | $1.00 | | | $ | 1.00 | |
| Granted | | | 1,026 | | | | 1.00 | | | | 1.00 | |
| Balance, June 30, 2022 | | | 15,030 | | | | $1.00 | | | $ | 1.00 | |
|
| Schedule of Share-Based Payment Award, Stock Options, Valuation Assumptions [Table Text Block] |
| | | 2023 | | | 2022 | |
| | | Non Plan | | | 2018 Plan | | | Non Plan | | | 2018 Plan | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | 3.48 | – | 3.58% | | | 2.32 | – | 3.83% | | | 2.32 | – | 2.98% | |
| Expected volatility | | 113 | - | 114% | | | 113 | - | 114% | | | 114 | – | 117% | | | 116 | – | 117% | |
| Expected dividend yield | | — | | | — | | | — | | | — | |
| Forfeiture rate | | — | | | — | | | — | | | — | |
| Life in years | | 10 | | | 10 | | | 10 | | | 10 | |
|
| Schedule of Accounts Payable and Accrued Liabilities [Table Text Block] |
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | BETI | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 142 | | | $ | 168 | | | $ | 119 | | | $ | 106 | | | $ | 29 | | | $ | (82 | ) | | $ | 482 | |
| Accrued payroll | | | 50 | | | | 92 | | | | 71 | | | | — | | | | — | | | | — | | | | 213 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 720 | |
| Category | | BioLargo | | | ONM | | | BLEST | | | Water | | | Intercompany amounts | | | Totals | |
| Accounts payable | | $ | 187 | | | $ | 486 | | | $ | 7 | | | $ | 119 | | | $ | (82 | ) | | $ | 717 | |
| Accrued payroll | | | 20 | | | | 58 | | | | 120 | | | | — | | | | — | | | | 198 | |
| Accrued interest | | | 25 | | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Total | | | | | | | | | | | | | | | | | | | | | | $ | 940 | |
|
| Clyra Medical [Member] |
|
| Notes Tables |
|
| Schedule of Share-Based Payment Award, Stock Options, Valuation Assumptions [Table Text Block] |
| | | June 30, 2023 | | | December 31, 2022 | |
| Risk free interest rate | | 3.48 | – | 3.58% | | | | 2.32 | % |
| Expected volatility | | 40 | - | 48% | | | | 40 | % |
| Expected dividend yield | | — | | | | — | |
| Forfeiture rate | | — | | | | — | |
| Expected life in years | | 10 | | | | 10 | |
|
| Schedule of Accounts Payable and Accrued Liabilities [Table Text Block] |
| Category | | 2023 | | | 2022 | |
| Accounts payable | | $ | 199 | | | $ | 186 | |
| Accrued payroll | | | 10 | | | | 45 | |
| Accrued interest | | | 5 | | | | 7 | |
| Accrued dividend | | | 74 | | | | --- | |
| Total | | $ | 288 | | | $ | 238 | |
|
| X |
- DefinitionTabular disclosure of the (a) carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business (accounts payable); (b) other payables; and (c) accrued liabilities. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). An alternative caption includes accrued expenses.
| Name: |
us-gaap_ScheduleOfAccountsPayableAndAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of other units or shares or classes of ownership in a partnership. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section S99
-Paragraph 5
-Subparagraph (SAB TOPIC 4.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-5
| Name: |
us-gaap_ScheduleOfOtherOwnershipInterestsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 11 - Business Segment Information (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Segment Reporting Information, by Segment [Table Text Block] |
| | | Three months ended June 30, | | | Six months ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| Revenue | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | — | | | $ | — | | | $ | — | | | $ | 2 | |
| ONM Environmental | | | 1,316 | | | | 700 | | | | 4,859 | | | | 1,300 | |
| BLEST | | | 538 | | | | 670 | | | | 901 | | | | 1,213 | |
| BETI | | | — | | | | — | | | | — | | | | — | |
| Water | | | 9 | | | | — | | | | — | | | | — | |
| Clyra Medical | | | — | | | | 6 | | | | 6 | | | | 17 | |
| Intersegment revenue | | | (417 | ) | | | (53 | ) | | | (587 | ) | | | (245 | ) |
| Total | | $ | 1,446 | | | $ | 1,323 | | | $ | 5,188 | | | $ | 2,287 | |
| | | | | | | | | | | | | | | | | |
| Research and development expense | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (243 | ) | | $ | (165 | ) | | $ | (432 | ) | | $ | (430 | ) |
| BLEST | | | (292 | ) | | | (80 | ) | | | (537 | ) | | | (188 | ) |
| BETI | | | (271 | ) | | | — | | | | (303 | ) | | | — | |
| Water | | | (138 | ) | | | (130 | ) | | | (273 | ) | | | (327 | ) |
| Clyra Medical | | | (60 | ) | | | (26 | ) | | | (194 | ) | | | (42 | ) |
| Intersegment R&D | | | 417 | | | | 46 | | | | 587 | | | | 240 | |
| Total | | $ | (587 | ) | | $ | (355 | ) | | $ | (1,152 | ) | | $ | (747 | ) |
| | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (635 | ) | | $ | (923 | ) | | $ | (1,434 | ) | | $ | (2,143 | ) |
| ONM Environmental | | | 428 | | | | 11 | | | | 1,815 | | | | 18 | |
| BLEST | | | (450 | ) | | | 56 | | | | (818 | ) | | | 21 | |
| BETI | | | (297 | ) | | | — | | | | (384 | ) | | | — | |
| Water | | | (209 | ) | | | (209 | ) | | | (393 | ) | | | (431 | ) |
| Clyra Medical | | | (396 | ) | | | (256 | ) | | | (823 | ) | | | (496 | ) |
| Total | | $ | (1,559 | ) | | $ | (1,321 | ) | | $ | (2,037 | ) | | $ | (3,031 | ) |
| | | | | | | | | | | | | | | | | |
| Interest expense | | | | | | | | | | | | | | | | |
| BioLargo corporate | | $ | (4 | ) | | $ | (6 | ) | | $ | (40 | ) | | $ | (12 | ) |
| ONM Environmental | | | (2 | ) | | | — | | | | (4 | ) | | | — | |
| Clyra Medical | | | (6 | ) | | | (9 | ) | | | (16 | ) | | | (16 | ) |
| Total | | $ | (12 | ) | | $ | (15 | ) | | $ | (60 | ) | | $ | (28 | ) |
| | | | | | | | | | | | | | | | | |
| As of June 30, 2023 | | BioLargo | | | ONM | | | Clyra | | | BLEST | | | Water | | | BETI | | | Elimination | | | Total | |
| Tangible assets | | $ | 731 | | | $ | 2,705 | | | $ | 1,265 | | | $ | 414 | | | $ | 92 | | | $ | 686 | | | $ | (41 | ) | | $ | 5,852 | |
| Right of use leased asset | | | 93 | | | | — | | | | — | | | | 714 | | | | — | | | | — | | | | — | | | | 807 | |
| Investment in South Korean joint venture | | | 21 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 21 | |
| Total | | $ | 845 | | | $ | 2,705 | | | $ | 1,265 | | | $ | 1,128 | | | $ | 92 | | | $ | 686 | | | $ | (41 | ) | | $ | 6,680 | |
| As of December 31, 2022 | | BioLargo | | | ONM | | | Clyra | | | BLEST | | | Water | | | Elimination | | | Total | |
| Tangible assets | | $ | 669 | | | $ | 2,064 | | | $ | 631 | | | $ | 441 | | | $ | 194 | | | $ | (41 | ) | | $ | 3,958 | |
| Right of use | | | 136 | | | | — | | | | — | | | | 731 | | | | — | | | | — | | | | 867 | |
| Investment in South Korean joint venture | | | 33 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 33 | |
| Total | | $ | 838 | | | $ | 2,064 | | | $ | 631 | | | $ | 1,172 | | | $ | 194 | | | $ | (41 | ) | | $ | 4,858 | |
|
| X |
- DefinitionTabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 1 - Business and Organization (Details Textual)
|
3 Months Ended |
6 Months Ended |
|
|
|
Jun. 30, 2023
USD ($)
|
Mar. 31, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Mar. 31, 2022
USD ($)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Dec. 31, 2022
USD ($)
|
Sep. 30, 2017 |
| Number of Wholly-Owned Subsidiaries |
6
|
|
|
|
6
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
$ 1,446,000
|
|
$ 1,323,000
|
|
$ 5,188,000
|
$ 2,287,000
|
|
|
| Net Income (Loss), Including Portion Attributable to Noncontrolling Interest |
(1,626,000)
|
$ (494,000)
|
(1,333,000)
|
$ (1,544,000)
|
(2,120,000)
|
(2,877,000)
|
|
|
| Net Cash Provided by (Used in) Operating Activities |
|
|
|
|
(1,527,000)
|
$ (1,864,000)
|
|
|
| Working Capital (Deficit) |
3,553,000
|
|
|
|
3,553,000
|
|
|
|
| Assets, Current |
4,847,000
|
|
|
|
4,847,000
|
|
$ 3,153,000
|
|
| Stock Issued During Period, Value, New Issues |
494,000
|
800,000
|
$ 948,000
|
$ 1,202,000
|
|
|
|
|
| Noncontrolling Interest, Increase from Subsidiary Equity Issuance |
1,062,000
|
|
|
|
|
|
|
|
| Cash and Cash Equivalents, at Carrying Value |
3,575,000
|
|
|
|
3,575,000
|
|
$ 1,851,000
|
|
| Vehicle Loan [Member] |
|
|
|
|
|
|
|
|
| Long-Term Debt |
75,000
|
|
|
|
75,000
|
|
|
|
| SBA CARES Act Paycheck Protection Program [Member] |
|
|
|
|
|
|
|
|
| Long-Term Debt |
140,000
|
|
|
|
140,000
|
|
|
|
| Economic Injury Disaster Loan [Member] |
|
|
|
|
|
|
|
|
| Long-Term Debt |
150,000
|
|
|
|
150,000
|
|
|
|
| Inventory Line of Credit [Member] | Clyra Medical Technologies [Member] |
|
|
|
|
|
|
|
|
| Long-Term Line of Credit |
$ 243,000
|
|
|
|
243,000
|
|
|
|
| The 2020 Unit Offering [Member] |
|
|
|
|
|
|
|
|
| Stock Issued During Period, Value, New Issues |
|
|
|
|
995,000
|
|
|
|
| Lincoln Park Capital Fund, LLC [Member] |
|
|
|
|
|
|
|
|
| Stock Issued During Period, Value, New Issues |
|
|
|
|
$ 299,000
|
|
|
|
| BioLargo Engineering, Science & Technologies, LLC [Member] |
|
|
|
|
|
|
|
|
| Noncontrolling Interest, Ownership Percentage by Parent |
82.00%
|
|
|
|
82.00%
|
|
|
100.00%
|
| BioLargo Engineering, Science & Technologies, LLC [Member] | Approximation [Member] |
|
|
|
|
|
|
|
|
| Noncontrolling Interest, Ownership Percentage by Parent |
82.00%
|
|
|
|
82.00%
|
|
|
|
| Clyra Medical Technologies [Member] |
|
|
|
|
|
|
|
|
| Noncontrolling Interest, Ownership Percentage by Parent |
56.00%
|
|
|
|
56.00%
|
|
|
|
| Noncontrolling Interest, Increase from Subsidiary Equity Issuance |
|
225,000
|
|
|
$ 1,287,000
|
|
|
|
| BioLargo Energy Technologies, Inc (BETI) [Member] |
|
|
|
|
|
|
|
|
| Noncontrolling Interest, Ownership Percentage by Parent |
96.00%
|
|
|
|
96.00%
|
|
|
|
| Noncontrolling Interest, Increase from Subsidiary Equity Issuance |
|
$ 550,000
|
|
|
$ 880,000
|
|
|
|
| X |
- DefinitionRepresents the number of wholly-owned subsidiaries operated by the company.
| Name: |
blgo_NumberOfWhollyOwnedSubsidiaries |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:pureItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the value of total current assets net of current liabilities as of the balance sheet date.
| Name: |
blgo_WorkingCapitalDeficit |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current and noncurrent portions of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_LineOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe parent entity's interest in net assets of the subsidiary, expressed as a percentage.
| Name: |
us-gaap_MinorityInterestOwnershipPercentageByParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in noncontrolling interest from subsidiary issuance of equity interests to noncontrolling interest holders. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-23
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(2)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_NoncontrollingInterestIncreaseFromSubsidiaryEquityIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_VehicleLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_SBACARESActPaycheckProtectionProgramMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_EconomicInjuryDisasterLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_InventoryLineOfCreditMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_ClyraMedicalTechnologiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=blgo_The2020UnitOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=blgo_LincolnParkCapitalFundLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_BiolargoEngineeringScienceTechnologiesLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=blgo_ApproximationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_ClyraMedicalTechnologiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies (Details Textual)
|
|
3 Months Ended |
6 Months Ended |
12 Months Ended |
|
|
Mar. 20, 2020
USD ($)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Dec. 31, 2022
USD ($)
|
Jan. 22, 2021
USD ($)
|
| Accounts Receivable, Allowance for Credit Loss, Current |
|
$ 58,000
|
|
$ 58,000
|
|
$ 12,000
|
|
| Inventory Valuation Reserves |
|
212,000
|
|
212,000
|
|
158,000
|
|
| Income (Loss) from Equity Method Investments |
|
|
|
(12,000)
|
$ (15,000)
|
|
|
| Impairment of Intangible Assets (Excluding Goodwill) |
|
0
|
$ 0
|
0
|
0
|
|
|
| Contract with Customer, Liability |
|
10,000
|
|
10,000
|
|
|
|
| Contract with Customer, Liability, Revenue Recognized |
|
|
|
7,000
|
|
|
|
| Customer Deposit Liability, Current |
|
113,000
|
|
113,000
|
|
184,000
|
|
| Unrecognized Tax Benefits, Ending Balance |
|
$ 0
|
|
$ 0
|
|
0
|
|
| Operating Lease, Weighted Average Remaining Lease Term (Year) |
|
9 years
|
|
9 years
|
|
|
|
| Operating Lease, Weighted Average Discount Rate, Percent |
|
18.00%
|
|
18.00%
|
|
|
|
| Operating Lease, Right-of-Use Asset |
|
$ 807,000
|
|
$ 807,000
|
|
$ 867,000
|
|
| Operating Lease, Liability |
|
$ 818,000
|
|
$ 818,000
|
|
|
|
| Minimum [Member] |
|
|
|
|
|
|
|
| Property, Plant and Equipment, Useful Life (Year) |
|
3 years
|
|
3 years
|
|
|
|
| Maximum [Member] |
|
|
|
|
|
|
|
| Property, Plant and Equipment, Useful Life (Year) |
|
5 years
|
|
5 years
|
|
|
|
| Canadian Government Grants [Member] | Minimum [Member] |
|
|
|
|
|
|
|
| Grant Term (Month) |
|
|
|
6 months
|
|
|
|
| Canadian Government Grants [Member] | Maximum [Member] |
|
|
|
|
|
|
|
| Grant Term (Month) |
|
|
|
18 months
|
|
|
|
| Odin Co Ltd [Member] |
|
|
|
|
|
|
|
| Payments to Acquire Interest in Joint Venture |
$ 100,000
|
|
|
|
|
|
|
| Variable Interest Entity, Qualitative or Quantitative Information, Ownership Percentage |
40.00%
|
|
|
40.00%
|
|
|
|
| Income (Loss) from Equity Method Investments |
|
$ 6,000
|
$ 8,000
|
$ 12,000
|
$ 15,000
|
|
|
| Odin Co Ltd [Member] | Tomorrow Water [Member] |
|
|
|
|
|
|
|
| Variable Interest Entity, Qualitative or Quantitative Information, Ownership Percentage |
30.00%
|
|
|
|
|
|
|
| Odin Co Ltd [Member] | BKT and Tomorrow Water [Member] |
|
|
|
|
|
|
|
| Payments to Acquire Interest in Joint Venture |
$ 150,000
|
|
|
|
|
|
|
| Patents [Member] |
|
|
|
|
|
|
|
| Intangible Assets, Net (Excluding Goodwill), Total |
|
|
|
|
|
|
$ 34,000
|
| Customer Concentration Risk [Member] | Accounts Receivable [Member] |
|
|
|
|
|
|
|
| Number of Major Customers |
|
|
|
1
|
|
3
|
|
| Customer Concentration Risk [Member] | Accounts Receivable [Member] | One Customer [Member] |
|
|
|
|
|
|
|
| Concentration Risk, Percentage |
|
|
|
10.00%
|
|
|
|
| Customer Concentration Risk [Member] | Accounts Receivable [Member] | Three Customers [Member] |
|
|
|
|
|
|
|
| Concentration Risk, Percentage |
|
|
|
|
|
10.00%
|
|
| X |
- DefinitionAmount of customer deposit liability, classified as current.
| Name: |
blgo_CustomerDepositLiabilityCurrent |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod for grants earned during the period from non-repayable sum of money awarded to an entity to carry out a specific purpose as provided in grant agreements.
| Name: |
blgo_GrantTerm |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the number of major customers accounting for 10% or more of the specified concentration risk benchmark, which includes, but not limited to, sales revenue, accounts receivable, etc.
| Name: |
blgo_NumberOfMajorCustomers |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of allowance for credit loss on accounts receivable, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479344/326-20-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-4
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of obligation to transfer good or service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479837/606-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-8
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479837/606-10-45-2
| Name: |
us-gaap_ContractWithCustomerLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of revenue recognized that was previously included in balance of obligation to transfer good or service to customer for which consideration from customer has been received or is due. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-8
| Name: |
us-gaap_ContractWithCustomerLiabilityRevenueRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of impairment loss recognized in the period resulting from the write-down of the carrying amount of an intangible asset (excluding goodwill) to fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-SubTopic 30
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ImpairmentOfIntangibleAssetsExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (loss) for proportionate share of equity method investee's income (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481664/323-10-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(13)(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_IncomeLossFromEquityMethodInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph ((a)(1),(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of valuation reserve for inventory. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 330
-SubTopic 10
-Section S99
-Paragraph 2
-Subparagraph (SAB TOPIC 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryValuationReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash outflow associated with the investment in or advances to an entity in which the reporting entity shares control of the entity with another party or group. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireInterestInJointVenture |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of unrecognized tax benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-10B
| Name: |
us-gaap_UnrecognizedTaxBenefits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of the Variable Interest Entity's (VIE) voting interest owned by (or beneficial interest in) the reporting entity (directly or indirectly). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 5A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-5A
| Name: |
us-gaap_VariableInterestEntityOwnershipPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=blgo_CanadianGovernmentGrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_OdinCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_TomorrowWaterMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_BktAndTomorrowWaterMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_PatentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_CustomerConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_AccountsReceivableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=blgo_OneCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=blgo_ThreeCustomersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies - Summary of Cash Balances (Details) - USD ($)
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Cash and Cash Equivalents, at Carrying Value |
$ 3,575,000
|
$ 1,851,000
|
| Parent Company [Member] |
|
|
| Cash and Cash Equivalents, at Carrying Value |
2,762,000
|
1,681,000
|
| Noncontrolling Interest [Member] |
|
|
| Cash and Cash Equivalents, at Carrying Value |
$ 813,000
|
$ 170,000
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=srt_ParentCompanyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=us-gaap_NoncontrollingInterestMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=blgo_CustomerAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_CustomerConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_RevenueFromContractWithCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_AccountsReceivableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=blgo_CustomerBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=blgo_CustomerCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=blgo_CustomerDMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies - Inventory (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Dec. 31, 2022 |
| Raw material |
$ 83
|
$ 46
|
| Finished goods |
44
|
74
|
| Total |
$ 127
|
$ 120
|
| X |
- DefinitionCarrying amount, net of valuation reserves and adjustments, as of the balance sheet date of merchandise or goods held by the company that are readily available for sale. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryFinishedGoodsNetOfReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount, net of valuation reserves and adjustments, as of the balance sheet date of unprocessed items to be consumed in the manufacturing or production process. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryRawMaterialsNetOfReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe value of patents classified as noncurrent.
| Name: |
blgo_PatentsNoncurrent |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of security deposits classified as noncurrent.
| Name: |
blgo_SecurityDepositsNoncurrent |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionRepresents expected forfeiture rate for share-based payment awards.
| Name: |
blgo_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedForfeitureRate |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_NonPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_EquityIncentivePlan2018Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 3 - Sale of Stock for Cash (Details Textual) - USD ($)
|
3 Months Ended |
6 Months Ended |
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
| Proceeds from Issuance of Common Stock |
|
|
$ 1,294,000
|
$ 2,150,000
|
|
| Warrants Issued with 2020 Unit Offering [Member] | Maximum [Member] |
|
|
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
5 years
|
|
5 years
|
|
|
| Warrants Issued with 2020 Unit Offering [Member] | Minimum [Member] |
|
|
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
6 months
|
|
6 months
|
|
|
| The 2020 Unit Offering [Member] |
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
1,578,948
|
|
5,234,948
|
|
|
| Proceeds from Issuance of Common Stock |
$ 300,000
|
|
$ 995,000
|
|
|
| Lincoln Park Capital Fund, LLC [Member] |
|
|
|
|
|
| Stock Purchase Agreement, Maximum Amount of Common Stock |
|
|
|
|
$ 10,000,000
|
| Stock Issued During Period, Shares, New Issues (in shares) |
1,098,221
|
406,140
|
1,643,623
|
1,912,961
|
|
| Proceeds from Issuance of Common Stock |
$ 194,000
|
$ 72,000
|
$ 299,000
|
$ 418,000
|
|
| X |
- DefinitionThe maximum value available of the common stock for sale in a stock purchase agreement.
| Name: |
blgo_StockPurchaseAgreementMaximumAmountOfCommonStock |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=blgo_WarrantsIssuedWith2020UnitOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=blgo_The2020UnitOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=blgo_LincolnParkCapitalFundLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 4 - Debt Obligations (Details Textual) - USD ($)
|
|
|
|
|
3 Months Ended |
4 Months Ended |
6 Months Ended |
Mar. 06, 2023 |
Feb. 07, 2023 |
May 12, 2022 |
Feb. 07, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Jul. 31, 2020 |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Interest Expense, Debt |
|
|
|
|
$ 12,000
|
$ 15,000
|
|
$ 60,000
|
$ 28,000
|
| Paycheck Protection Program CARES Act [Member] | ONM [Member] |
|
|
|
|
|
|
|
|
|
| Extinguishment of Debt, Amount |
|
|
|
$ 174,000
|
|
|
|
|
|
| Proceeds from Issuance of Debt |
|
|
|
$ 217,000
|
|
|
|
|
|
| Long-Term Debt |
|
|
|
|
43,000
|
|
|
43,000
|
|
| Paycheck Protection Program CARES Act [Member] | BELST [Member] |
|
|
|
|
|
|
|
|
|
| Proceeds from Issuance of Debt |
|
|
$ 97,000
|
|
|
|
|
|
|
| Economic Injury Disaster Loan [Member] |
|
|
|
|
|
|
|
|
|
| Long-Term Debt |
|
|
|
|
$ 150,000
|
|
|
$ 150,000
|
|
| Economic Injury Disaster Loan [Member] | ONM [Member] |
|
|
|
|
|
|
|
|
|
| Debt Instrument, Face Amount |
|
|
|
|
|
|
$ 150,000
|
|
|
| Debt Instrument, Interest Rate, Stated Percentage |
|
|
|
|
|
|
3.75%
|
|
|
| Debt Instrument, Periodic Payment |
|
|
|
|
|
|
$ 731
|
|
|
| Warrant Issued With Conversion of Note [Member] |
|
|
|
|
|
|
|
|
|
| Class of Warrant or Right, Number of Securities Called by Warrants or Rights (in shares) |
200,000
|
|
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
$ 0.21
|
|
|
|
|
|
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
5 years
|
|
|
|
|
|
|
|
|
| Conversion of Note into BETI Common Shares [Member] |
|
|
|
|
|
|
|
|
|
| Debt Conversion, Original Debt, Amount |
$ 50,000
|
|
|
|
|
|
|
|
|
| Vehicle Loan [Member] |
|
|
|
|
|
|
|
|
|
| Debt Instrument, Face Amount |
|
$ 80,000
|
|
|
|
|
|
|
|
| Debt Instrument, Interest Rate, Stated Percentage |
|
5.29%
|
|
|
|
|
|
|
|
| Debt Instrument, Periodic Payment |
|
$ 1,000
|
|
|
|
|
|
|
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of the original debt being converted in a noncash (or part noncash) transaction. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionOriginalDebtAmount1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of the required periodic payments including both interest and principal payments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
| Name: |
us-gaap_DebtInstrumentPeriodicPayment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionGross amount of debt extinguished.
| Name: |
us-gaap_ExtinguishmentOfDebtAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense for debt. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69F
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.8)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-6
| Name: |
us-gaap_InterestExpenseDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow during the period from additional borrowings in aggregate debt. Includes proceeds from short-term and long-term debt. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_PaycheckProtectionProgramCaresActMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ONMMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_BELSTMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_EconomicInjuryDisasterLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=blgo_WarrantIssuedWithConversionOfNoteMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtConversionByUniqueDescriptionAxis=blgo_ConversionOfNoteIntoBetiCommonSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=blgo_VehicleLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 4 - Debt Obligations - Schedule of Debt (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Dec. 31, 2022 |
| Long-term debt, current |
$ 66
|
$ 100
|
| Debt discount, net of amortization |
0
|
(3)
|
| Total current portion of debt |
66
|
100
|
| Total long-term debt, net of current |
299
|
237
|
| Total |
365
|
337
|
| Vehicle Loan [Member] |
|
|
| Long-term debt, current |
13
|
0
|
| Total long-term debt, net of current |
62
|
0
|
| Paycheck Protection Program CARES Act [Member] |
|
|
| SBA Paycheck Protection Program loan |
43
|
43
|
| SBA Paycheck Protection Program loans, matures May 2025 |
97
|
97
|
| Convertible Note, Maturing On March 1, 2023 [Member] |
|
|
| Convertible notes |
0
|
50
|
| Economic Injury Disaster Loan [Member] |
|
|
| Long-term debt, current |
10
|
10
|
| Total long-term debt, net of current |
$ 140
|
$ 140
|
| X |
- DefinitionCarrying value as of the balance sheet date of the portion of long-term debt due within one year or the operating cycle if longer identified as Convertible Notes Payable. Convertible Notes Payable is a written promise to pay a note which can be exchanged for a specified amount of another, related security, at the option of the issuer and the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ConvertibleNotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of debt and lease obligation, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of debt discount to be amortized within one year or within the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-1A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentUnamortizedDiscountCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the aggregate of total long-term debt, including current maturities and short-term debt.
| Name: |
us-gaap_DebtLongtermAndShorttermCombinedAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as current. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as noncurrent. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of notes payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of the portions of long-term notes payable due within one year or the operating cycle if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_NotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=blgo_VehicleLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_PaycheckProtectionProgramCaresActMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_ConvertibleNoteMaturingOnMarch12023Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_EconomicInjuryDisasterLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 5 - Share-based Compensation (Details Textual) - USD ($)
|
|
|
|
|
|
3 Months Ended |
6 Months Ended |
Mar. 31, 2023 |
Mar. 31, 2022 |
Mar. 22, 2022 |
Jun. 22, 2018 |
Sep. 07, 2017 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Jun. 30, 2022 |
Mar. 31, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Stock Issued During Period, Value, Issued for Services |
|
|
|
|
|
$ 75,000
|
$ 207,000
|
$ 59,000
|
$ 17,000
|
|
|
| Share Price (in dollars per share) |
|
|
|
|
|
$ 0.18
|
|
|
|
$ 0.18
|
|
| 2018 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period (Year) |
|
|
|
10 years
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized (in shares) |
|
|
|
40,000,000
|
|
50,000,000
|
|
|
|
50,000,000
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Additional Shares Authorized Per Year (in shares) |
|
|
|
2,000,000
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
2,636,712
|
3,782,923
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
|
| Employee Retention Program [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
1,137,301
|
1,764,025
|
| Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Fair Value |
|
|
|
|
|
|
|
|
|
$ 203,000
|
$ 360,000
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period (Year) |
|
|
|
|
|
|
|
|
|
|
4 years
|
| Employee Retention Program [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.18
|
| Employee Retention Program [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.23
|
| The 2007 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period (Year) |
|
|
|
|
10 years
|
|
|
|
|
|
|
| Non Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
60,040
|
39,130
|
| Share-Based Payment Arrangement, Option [Member] | Clyra Medical Technologies [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Payment Arrangement, Expense |
|
|
|
|
|
$ 66,000
|
|
82,000
|
|
$ 127,000
|
$ 223,000
|
| Selling, General and Administrative Expenses [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Payment Arrangement, Expense |
|
|
|
|
|
288,000
|
|
$ 316,000
|
|
$ 544,000
|
1,117,000
|
| Officer [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Stock Issued During Period, Value, Issued for Services |
|
|
|
|
|
$ 12,000
|
$ 6,000
|
|
|
|
$ 47,000
|
| Stock Issued During Period, Shares, Issued for Services (in shares) |
|
|
|
|
|
68,541
|
30,747
|
|
|
|
263,895
|
| Shares Issued, Price Per Share (in dollars per share) |
$ 0.20
|
|
|
|
|
$ 0.18
|
$ 0.20
|
$ 0.18
|
|
$ 0.18
|
$ 0.18
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Rights, Minimum Cash Amount Receipt |
|
|
|
|
|
|
|
|
|
$ 3,000,000
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Rights, Minimum Revenue Required |
|
|
|
|
|
|
|
|
|
$ 3,000,000
|
|
| Consultants [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Stock Issued During Period, Value, Issued for Services |
$ 201,000
|
$ 17,000
|
|
|
|
$ 63,000
|
|
$ 12,000
|
|
|
|
| Stock Issued During Period, Shares, Issued for Services (in shares) |
899,743
|
86,752
|
|
|
|
352,370
|
|
76,996
|
|
|
|
| Shares Issued, Price Per Share (in dollars per share) |
$ 0.20
|
$ 0.23
|
|
|
|
$ 0.18
|
0.20
|
$ 0.18
|
$ 0.23
|
$ 0.18
|
$ 0.18
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
|
582,991
|
| Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Fair Value |
|
|
|
|
|
|
|
|
|
|
$ 127,000
|
| Consultants [Member] | 2018 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
335,121
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Fair Value |
|
|
|
|
|
|
|
|
|
$ 61,000
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
$ 0.20
|
|
| Officer, Board of Directors, Employees, and a Consultant [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
2,636,712
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Fair Value |
|
|
|
|
|
|
|
|
|
$ 474,000
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
$ 0.18
|
$ 0.20
|
|
|
|
|
| Board Of Directors [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
864,290
|
884,356
|
| Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Fair Value |
|
|
|
|
|
|
|
|
|
$ 154,000
|
$ 178,000
|
| Board Of Directors [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.18
|
| Board Of Directors [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.23
|
| Employees [Member] | 2018 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period (Year) |
|
|
|
|
|
|
|
|
|
4 years
|
|
| Chief Financial Officer [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
|
300,000
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.24
|
| Chief Financial Officer [Member] | 2018 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period (Year) |
|
|
10 years
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
300,000
|
|
|
|
|
|
|
300,000
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Fair Value |
|
|
|
|
|
|
|
|
|
$ 56,000
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
$ 0.20
|
|
|
|
|
|
|
|
|
| Term Extension (Year) |
|
|
1 year
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement By Share-based Payment Award, Options Grants Per Month, Gross (in shares) |
|
|
25,000
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Vested, Number of Shares (in shares) |
|
|
25,000
|
|
|
|
|
|
|
|
|
| Officers, Board Members, and Vendors [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
|
3,782,923
|
| Chief Financial Officer and President [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
|
251,551
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.23
|
| Vendors [Member] | Non Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Payment Arrangement, Expense |
|
|
|
|
|
|
|
|
|
$ 11,000
|
$ 8,000
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
|
|
|
|
|
60,040
|
39,130
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 0.23
|
| Vendors [Member] | Non Plan [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
$ 0.18
|
|
| Vendors [Member] | Non Plan [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
|
|
|
$ 0.20
|
|
| X |
- DefinitionFair value of options granted during the period. Excludes equity instruments other than options, for example, but not limited to, share units, stock appreciation rights, restricted stock.
| Name: |
blgo_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodFairValue |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionMinimum amount of cash receipt required to vest share-based compensation plan.
| Name: |
blgo_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsMinimumCashAmountReceipt |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionMinimum revenue required to vest share-based compensation award.
| Name: |
blgo_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsMinimumRevenueRequired |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the number of additional shares authorized per year for issuance under an established share-based compensation plan.
| Name: |
blgo_SharebasedCompensationArrangementBySharebasedPaymentAwardNumberOfAdditionalSharesAuthorizedPerYear |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted per month.
| Name: |
blgo_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsGrantsPerMonthGross |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTerm extension, in years.
| Name: |
blgo_TermExtension |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares authorized for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options vested.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedNumberOfShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued in lieu of cash for services contributed to the entity. Value of the stock issued includes, but is not limited to, services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodValueIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_EquityIncentivePlan2018Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_EmployeeRetentionProgramMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_The2007EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_NonPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalTechnologiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_SellingGeneralAndAdministrativeExpensesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_OfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_ConsultantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_OfficerBoardOfDirectorsEmployeesAndAConsultantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_BoardOfDirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_EmployeesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefFinancialOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_OfficersBoardMembersAndVendorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_ChiefFinancialOfficerAndPresidentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_VendorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 5 - Share-based Compensation - Stock Options (Details) - $ / shares
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
| 2018 Equity Incentive Plan [Member] |
|
|
|
| Options outstanding, balance (in shares) |
28,484,549
|
23,186,142
|
23,186,142
|
| Weighted average exercise price per share, balance (in dollars per share) |
|
|
|
| Options granted (in shares) |
2,636,712
|
3,782,923
|
|
| Weighted average exercise price per share, granted (in dollars per share) |
|
|
|
| Options Unvested (in shares) |
(4,121,892)
|
|
|
| Weighted average exercise price per share, unvested (in dollars per share) |
|
|
|
| Options Vested (in shares) |
26,999,369
|
|
|
| Weighted average exercise price per share, vested (in dollars per share) |
$ 402,000
|
|
|
| Options outstanding, balance (in shares) |
31,121,261
|
26,969,065
|
28,484,549
|
| Weighted average exercise price per share, balance (in dollars per share) |
|
|
|
| 2018 Equity Incentive Plan [Member] | Minimum [Member] |
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.12
|
0.16
|
0.16
|
| Exercise price per share, granted (in dollars per share) |
0.18
|
0.18
|
|
| Exercise price per share, Unvested (in dollars per share) |
0.12
|
|
|
| Exercise price per share, Vested (in dollars per share) |
0.12
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.12
|
0.12
|
0.12
|
| 2018 Equity Incentive Plan [Member] | Maximum [Member] |
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.43
|
0.43
|
0.43
|
| Exercise price per share, granted (in dollars per share) |
0.20
|
0.24
|
|
| Exercise price per share, Unvested (in dollars per share) |
0.32
|
|
|
| Exercise price per share, Vested (in dollars per share) |
0.43
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.43
|
0.43
|
0.43
|
| 2018 Equity Incentive Plan [Member] | Weighted Average [Member] |
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.19
|
0.19
|
0.19
|
| Exercise price per share, granted (in dollars per share) |
0.19
|
0.22
|
|
| Exercise price per share, Unvested (in dollars per share) |
0.19
|
|
|
| Exercise price per share, Vested (in dollars per share) |
0.19
|
|
|
| Exercise price per share, balance (in dollars per share) |
$ 0.19
|
$ 0.19
|
$ 0.19
|
| The 2007 Equity Incentive Plan [Member] |
|
|
|
| Options outstanding, balance (in shares) |
1,904,085
|
2,879,246
|
2,879,246
|
| Weighted average exercise price per share, balance (in dollars per share) |
$ 0.56
|
$ 0.49
|
$ 0.49
|
| Options outstanding, balance (in shares) |
1,864,085
|
2,579,246
|
1,904,085
|
| Weighted average exercise price per share, balance (in dollars per share) |
$ 0.56
|
$ 0.49
|
$ 0.56
|
| Options outstanding, Expired (in shares) |
(40,000)
|
(300,000)
|
|
| Weighted average exercise price per share, Expired (in dollars per share) |
$ 0.28
|
$ 0.35
|
|
| The 2007 Equity Incentive Plan [Member] | Minimum [Member] |
|
|
|
| Weighted average exercise price per share, balance (in dollars per share) |
0.28
|
0.23
|
0.23
|
| Weighted average exercise price per share, balance (in dollars per share) |
0.28
|
0.23
|
0.28
|
| The 2007 Equity Incentive Plan [Member] | Maximum [Member] |
|
|
|
| Weighted average exercise price per share, balance (in dollars per share) |
0.69
|
0.94
|
0.94
|
| Weighted average exercise price per share, balance (in dollars per share) |
$ 0.69
|
$ 0.94
|
$ 0.69
|
| Non Plan [Member] |
|
|
|
| Options outstanding, balance (in shares) |
19,023,829
|
20,119,207
|
20,119,207
|
| Options granted (in shares) |
60,040
|
39,130
|
|
| Exercise price per share, granted (in dollars per share) |
|
$ 0.23
|
|
| Options Unvested (in shares) |
(507,500)
|
|
|
| Exercise price per share, Unvested (in dollars per share) |
$ 0.45
|
|
|
| Options Vested (in shares) |
18,576,369
|
|
|
| Weighted average exercise price per share, vested (in dollars per share) |
$ 46,000
|
|
|
| Options outstanding, balance (in shares) |
19,083,869
|
20,158,337
|
19,023,829
|
| Non Plan [Member] | Minimum [Member] |
|
|
|
| Exercise price per share, balance (in dollars per share) |
$ 0.12
|
$ 0.12
|
$ 0.12
|
| Exercise price per share, granted (in dollars per share) |
0.18
|
|
|
| Exercise price per share, Vested (in dollars per share) |
0.12
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.12
|
0.12
|
0.12
|
| Non Plan [Member] | Maximum [Member] |
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.83
|
0.83
|
0.83
|
| Exercise price per share, granted (in dollars per share) |
0.20
|
|
|
| Exercise price per share, Vested (in dollars per share) |
0.83
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.83
|
0.83
|
0.83
|
| Non Plan [Member] | Weighted Average [Member] |
|
|
|
| Exercise price per share, balance (in dollars per share) |
0.39
|
0.39
|
0.39
|
| Exercise price per share, granted (in dollars per share) |
0.20
|
0.23
|
|
| Exercise price per share, Vested (in dollars per share) |
0.38
|
|
|
| Exercise price per share, balance (in dollars per share) |
$ 0.39
|
$ 0.39
|
$ 0.39
|
| X |
- DefinitionStock options exercise price range.
| Name: |
blgo_StockOptionsExercisePriceRange |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the exercise price range of stock options granted.
| Name: |
blgo_StockOptionsGrantedExercisePriceRange |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock options non-vested exercise price range.
| Name: |
blgo_StockOptionsNonvestedExercisePriceRange |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exercise price range for stock options vested and outstanding.
| Name: |
blgo_StockOptionsVestedAndOutstandingExercisePriceRange |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-vested options outstanding.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsNonvestedNumberOfShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average grant-date fair value of non-vested options outstanding.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_EquityIncentivePlan2018Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_WeightedAverageMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_The2007EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_NonPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 6 - Warrants (Details Textual) - USD ($)
|
Jun. 30, 2023 |
Mar. 06, 2023 |
Jun. 30, 2022 |
| Share Price (in dollars per share) |
$ 0.18
|
|
|
| Note Payable, Maturing March 8, 2023 [Member] |
|
|
|
| Debt Instrument, Face Amount |
|
$ 50,000
|
|
| Six-month Warrants in Connection With the 2020 Unit Offering [Member] |
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
6 months
|
|
6 months
|
| Class of Warrant or Right, Number of Securities Called by Each Warrant or Right (in shares) |
5,234,948
|
|
9,802,398
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
$ 0.23
|
|
|
| Six-month Warrants in Connection With the 2020 Unit Offering [Member] | Minimum [Member] |
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
|
|
$ 0.20
|
| Six-month Warrants in Connection With the 2020 Unit Offering [Member] | Maximum [Member] |
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
|
|
$ 0.23
|
| Five-year Warrants in Connection With the 2020 Unit Offering [Member] |
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
5 years
|
|
5 years
|
| Class of Warrant or Right, Number of Securities Called by Each Warrant or Right (in shares) |
5,234,948
|
|
9,802,398
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
$ 0.29
|
|
|
| Warrants and Rights Outstanding |
$ 913,000
|
|
$ 2,134,000
|
| Five-year Warrants in Connection With the 2020 Unit Offering [Member] | Minimum [Member] |
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
|
|
$ 0.25
|
| Five-year Warrants in Connection With the 2020 Unit Offering [Member] | Maximum [Member] |
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
|
|
$ 0.29
|
| Warrants Issued in Connection with Conversion of Interest on Note Payable Maturing March 8, 2023 [Member] |
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
|
5 years
|
|
| Class of Warrant or Right, Number of Securities Called by Each Warrant or Right (in shares) |
|
200,000
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in dollars per share) |
|
$ 0.21
|
|
| Warrants and Rights Outstanding |
|
$ 30,000
|
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which each warrant or right may be converted. For example, but not limited to, each warrant may be converted into two shares.
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByEachWarrantOrRight |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionValue of outstanding derivative securities that permit the holder the right to purchase securities (usually equity) from the issuer at a specified price.
| Name: |
us-gaap_WarrantsAndRightsOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_NotePayableMaturingMarch82023Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=blgo_SixMonthWarrantsInConnectionWithThe2020UnitOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=blgo_FiveyearWarrantsInConnectionWithThe2020UnitOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=blgo_WarrantsIssuedInConnectionWithConversionOfInterestOnNotePayableMaturingMarch82023Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 6 - Warrants - Warrants Outstanding (Details) - $ / shares
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Balance, outstanding (in shares) |
49,023,398
|
36,765,502
|
| Granted (in shares) |
10,669,896
|
19,604,796
|
| Expired (in shares) |
(5,173,209)
|
(4,016,754)
|
| Expired, price range (in dollars per share) |
|
$ 0.48
|
| Balance, outstanding (in shares) |
54,520,085
|
52,353,544
|
| Minimum [Member] |
|
|
| Balance, outstanding, price range (in dollars per share) |
$ 0.13
|
$ 0.16
|
| Granted, price range (in dollars per share) |
0.21
|
0.20
|
| Expired, price range (in dollars per share) |
0.19
|
|
| Balance, outstanding, price range (in dollars per share) |
0.13
|
0.14
|
| Maximum [Member] |
|
|
| Balance, outstanding, price range (in dollars per share) |
0.26
|
0.27
|
| Granted, price range (in dollars per share) |
0.25
|
0.24
|
| Expired, price range (in dollars per share) |
0.21
|
|
| Balance, outstanding, price range (in dollars per share) |
0.26
|
0.25
|
| Weighted Average [Member] |
|
|
| Balance, outstanding, price range (in dollars per share) |
|
|
| Granted, price range (in dollars per share) |
|
|
| Expired, price range (in dollars per share) |
|
|
| Balance, outstanding, price range (in dollars per share) |
$ 47,000
|
|
| X |
- DefinitionThe number of warrants or rights expired during period.
| Name: |
blgo_ClassOfWarrantOrRightExpiredDuringPeriod |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of warrants or rights issued during period.
| Name: |
blgo_ClassOfWarrantOrRightIssuedDuringPeriod |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the price of warrants that expired during the period.
| Name: |
blgo_PriceRangeWarrantsExpired |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the price of warrants that were issued during the period.
| Name: |
blgo_PriceRangeWarrantsIssued |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the price of warrants outstanding.
| Name: |
blgo_PriceRangeWarrantsOutstanding |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_WeightedAverageMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 6 - Warrants - Assumptions Used to Determine Fair Value of Warrants (Details)
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Measurement Input, Risk Free Interest Rate [Member] |
|
|
| Risk free interest rate |
|
0.0388
|
| Measurement Input, Price Volatility [Member] |
|
|
| Risk free interest rate |
|
0.40
|
| Minimum [Member] | Measurement Input, Risk Free Interest Rate [Member] |
|
|
| Risk free interest rate |
0.0388
|
0.0369
|
| Minimum [Member] | Measurement Input, Price Volatility [Member] |
|
|
| Risk free interest rate |
0.40
|
|
| Minimum [Member] | Measurement Input, Expected Term [Member] |
|
|
| Risk free interest rate |
3
|
3
|
| Maximum [Member] | Measurement Input, Risk Free Interest Rate [Member] |
|
|
| Risk free interest rate |
0.0427
|
|
| Maximum [Member] | Measurement Input, Price Volatility [Member] |
|
|
| Risk free interest rate |
0.95
|
|
| Maximum [Member] | Measurement Input, Expected Term [Member] |
|
|
| Risk free interest rate |
5
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of [accrued] interest payable on all forms of debt, including trade payables, that has been incurred and is unpaid. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_CorporateNonSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_OperatingSegmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_OdorNoMoreMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BLESTMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BiolargoWaterMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=srt_ConsolidationEliminationsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 8 - Noncontrolling Interest - Clyra Medical (Details Textual) - USD ($)
|
|
|
|
3 Months Ended |
6 Months Ended |
12 Months Ended |
|
Dec. 20, 2022 |
Mar. 02, 2022 |
Jun. 30, 2020 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
Apr. 08, 2022 |
| Preferred Stock, Shares Outstanding (in shares) |
|
|
|
0
|
|
|
0
|
|
0
|
|
| Proceeds from Issuance of Preferred Stock and Preference Stock |
|
|
|
|
|
|
$ 1,287,000
|
$ 0
|
|
|
| Clyra Medical [Member] |
|
|
|
|
|
|
|
|
|
|
| Shares, Outstanding (in shares) |
|
|
|
95,301
|
|
|
95,301
|
|
91,145
|
|
| Common Stock, Shares, Outstanding (in shares) |
|
|
|
89,070
|
|
|
89,070
|
|
89,070
|
|
| Shares Issued, Price Per Share (in dollars per share) |
$ 310
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
$ 146.06
|
$ 1.00
|
|
|
| Clyra Medical [Member] | Vendors and Employees [Member] | Share-Based Payment Arrangement, Option [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Payment Arrangement, Expense |
|
|
|
$ 66,000
|
|
$ 82,000
|
$ 127,000
|
$ 223,000
|
|
|
| Clyra Medical [Member] | Employees and Consultants [Member] | Share-Based Payment Arrangement, Option [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Fair Value Assumptions, Discount Rate |
|
|
|
|
|
|
30.00%
|
|
|
|
| Clyra Medical [Member] | Employees and Consultants [Member] | The Remaining Options [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
|
|
|
|
$ 310
|
|
|
|
| Clyra Medical [Member] | Warrants Issued in Conjunction With the Sale of Series A Preferred Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
3 years
|
|
|
|
|
|
|
|
|
|
| Warrants and Rights Outstanding |
|
|
|
$ 324,000
|
|
|
$ 324,000
|
|
|
|
| Clyra Medical [Member] | Shares Issued for Debt Owed to Biolargo [Member] |
|
|
|
|
|
|
|
|
|
|
| Debt Conversion, Converted Instrument, Amount |
|
$ 633,000
|
|
|
|
|
|
|
|
|
| Debt Conversion, Converted Instrument, Shares Issued (in shares) |
|
2,042
|
|
|
|
|
|
|
|
|
| Clyra Medical [Member] | Series A Preferred Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Preferred Stock, Shares Outstanding (in shares) |
|
|
|
6,231
|
|
|
6,231
|
|
2,075
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
|
|
|
3,427
|
|
|
4,879
|
|
|
|
| Proceeds from Issuance of Preferred Stock and Preference Stock |
|
|
|
$ 1,062,000
|
|
|
$ 1,287,000
|
|
|
|
| Shares Issued, Price Per Share (in dollars per share) |
$ 372
|
|
|
|
|
|
|
|
|
|
| Preferred Stock, Dividend Rate, Percentage |
15.00%
|
|
|
|
|
|
|
|
|
|
| Preferred Stock, Convertible, Sale of Stock Amount |
$ 5,000,000
|
|
|
|
|
|
|
|
|
|
| Clyra Medical [Member] | Revolving Credit Facility [Member] | Vernal Bay Capital Group, LLC [Member] | Inventory Line of Credit [Member] |
|
|
|
|
|
|
|
|
|
|
| Line of Credit Facility, Maximum Borrowing Capacity |
|
|
$ 1,000,000
|
|
|
|
|
|
|
|
| Proceeds from Lines of Credit, Total |
|
|
260,000
|
|
|
|
|
|
|
|
| Repayments of Lines of Credit |
|
|
$ 117,000
|
|
|
|
|
|
|
|
| Stock Issued During Period, Shares, Commitment Fee (in shares) |
|
|
322
|
|
|
|
|
|
322
|
|
| Stock Issued During Period, Value, Commitment Fee |
|
|
$ 70,000
|
|
|
|
|
|
|
|
| Debt Instrument, Percentage of Principal Payment, Cap |
|
|
|
|
|
|
|
|
15.00%
|
|
| Long-Term Line of Credit |
|
|
|
$ 143,000
|
|
|
$ 143,000
|
|
$ 161,000
|
|
| Clyra Medical [Member] | Notes Payable, Other Payables [Member] |
|
|
|
|
|
|
|
|
|
|
| Debt Instrument, Face Amount |
|
|
|
|
|
|
|
|
|
$ 100,000
|
| Debt Instrument, Interest Rate, Stated Percentage |
|
|
|
|
|
|
|
|
|
8.00%
|
| Debt Instrument, Convertible, Sale of Stock Amount |
|
|
|
|
|
|
|
|
|
$ 5,000,000
|
| Debt Instrument, Convertible, Conversion Percentage |
|
|
|
|
|
|
|
|
|
70.00%
|
| Vernal Bay Capital Group, LLC [Member] | Conversion of Clyra Medical Stock Into Biolargo Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Conversion of Stock, Shares Issued (in shares) |
|
|
|
|
527,983
|
|
|
|
|
|
| Clyra Medical Technologies [Member] |
|
|
|
|
|
|
|
|
|
|
| Noncontrolling Interest, Ownership Percentage by Parent |
|
|
|
56.00%
|
|
|
56.00%
|
|
|
|
| Clyra Medical [Member] | Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Investment Owned, Balance, Shares (in shares) |
|
|
|
51,571
|
|
|
51,571
|
|
51,571
|
|
| Clyra Medical [Member] | Preferred Stock, Series A [Member] |
|
|
|
|
|
|
|
|
|
|
| Investment Owned, Balance, Shares (in shares) |
|
|
|
1,352
|
|
|
1,352
|
|
1,352
|
|
| X |
- DefinitionThe percentage of lowest price per share of shares sold for conversion price of the debt instrument.
| Name: |
blgo_DebtInstrumentConvertibleConversionPercentage |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of sale of stock to trigger conversion under the debt instrument.
| Name: |
blgo_DebtInstrumentConvertibleSaleOfStockAmount |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the maximum percentage of principal due monthly.
| Name: |
blgo_DebtInstrumentPercentageOfPrincipalPaymentCap |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of proceeds in sale of stock for preferred stock to be convertible.
| Name: |
blgo_PreferredStockConvertibleSaleOfStockAmount |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe discount rate assumption that is used in valuing an option on its own shares.
| Name: |
blgo_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsDiscountRate |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of commitment fee stock issued during the period.
| Name: |
blgo_StockIssuedDuringPeriodSharesCommitmentFee |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of commitment fee stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering.
| Name: |
blgo_StockIssuedDuringPeriodValueCommitmentFee |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of new shares issued in the conversion of stock in a noncash (or part noncash) transaction. Noncash is defined as transactions during a period that do not result in cash receipts or cash payments in the period. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_ConversionOfStockSharesIssued1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe value of the financial instrument(s) that the original debt is being converted into in a noncash (or part noncash) transaction. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionConvertedInstrumentAmount1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued in exchange for the original debt being converted in a noncash (or part noncash) transaction. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionConvertedInstrumentSharesIssued1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of investment owned. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480493/946-210-55-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_InvestmentOwnedBalanceShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current and noncurrent portions of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_LineOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMaximum borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(b),22(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityMaximumBorrowingCapacity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe parent entity's interest in net assets of the subsidiary, expressed as a percentage.
| Name: |
us-gaap_MinorityInterestOwnershipPercentageByParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe percentage rate used to calculate dividend payments on preferred stock. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_PreferredStockDividendRatePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionProceeds from issuance of capital stock which provides for a specific dividend that is paid to the shareholders before any dividends to common stockholders and which takes precedence over common stockholders in the event of liquidation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPreferredStockAndPreferenceStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from contractual arrangement with the lender, including but not limited to, letter of credit, standby letter of credit and revolving credit arrangements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for payment of an obligation from a lender, including but not limited to, letter of credit, standby letter of credit and revolving credit arrangements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of outstanding derivative securities that permit the holder the right to purchase securities (usually equity) from the issuer at a specified price.
| Name: |
us-gaap_WarrantsAndRightsOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_VendorsAndEmployeesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_EmployeesAndConsultantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=blgo_TheRemainingOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=blgo_WarrantsIssuedInConjunctionWithTheSaleOfSeriesAPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtConversionByUniqueDescriptionAxis=blgo_SharesIssuedForDebtOwedToBiolargoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesAPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CreditFacilityAxis=us-gaap_RevolvingCreditFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LineOfCreditFacilityAxis=blgo_VernalBayCapitalGroupLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=blgo_InventoryLineOfCreditMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=us-gaap_NotesPayableOtherPayablesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_VernalBayCapitalGroupLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConversionOfStockByUniqueDescriptionAxis=blgo_ConversionOfClyraMedicalStockIntoBiolargoCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_ClyraMedicalTechnologiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=blgo_PreferredStockSeriesAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 8 - Noncontrolling Interest - Clyra Medical - Common Shares Outstanding (Details) - Clyra Medical [Member] - $ / shares
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
| Options outstanding, balance (in shares) |
15,833
|
14,004
|
14,004
|
| Balance, Exercise Price Range (in shares) |
|
1.00
|
1.00
|
| Weighted average exercise price per share, balance (in dollars per share) |
$ 5.53
|
$ 1.00
|
$ 1.00
|
| Options granted (in shares) |
858
|
1,026
|
|
| Granted, Exercise Price (in dollars per share) |
|
$ 1.00
|
|
| Weighted average exercise price per share, granted (in dollars per share) |
$ 146.06
|
$ 1.00
|
|
| Options outstanding, balance (in shares) |
16,691
|
15,030
|
15,833
|
| Balance, Exercise Price Range (in shares) |
|
1.00
|
|
| Weighted average exercise price per share, balance (in dollars per share) |
$ 12.76
|
$ 1.00
|
$ 5.53
|
| Minimum [Member] |
|
|
|
| Balance, Exercise Price Range (in shares) |
1.00
|
|
|
| Granted, Exercise Price (in dollars per share) |
$ 1.00
|
|
|
| Balance, Exercise Price Range (in shares) |
1.00
|
|
1.00
|
| Maximum [Member] |
|
|
|
| Balance, Exercise Price Range (in shares) |
310
|
|
|
| Granted, Exercise Price (in dollars per share) |
$ 271
|
|
|
| Balance, Exercise Price Range (in shares) |
310
|
|
310
|
| X |
- DefinitionPer share amount at which grantees can acquire shares of common stock by exercise of options.
| Name: |
blgo_SharebasedPaymentArrangementOptionExercisePriceRangeSharesGrantsInPeriod |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares reserved for issuance pertaining to the outstanding stock options as of the balance sheet date for all option plans in the customized range of exercise prices. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)-(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeNumberOfOutstandingOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_VendorsAndEmployeesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=blgo_NonPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of dividends declared but unpaid on equity securities issued by the entity and outstanding. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DividendsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of [accrued] interest payable on all forms of debt, including trade payables, that has been incurred and is unpaid. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 9 - BioLargo Engineering, Science and Technologies, LLC (Details Textual) - USD ($)
|
1 Months Ended |
6 Months Ended |
12 Months Ended |
25 Months Ended |
Sep. 30, 2017 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
Sep. 30, 2019 |
| Share-based Payment Arrangement, Noncash Expense, Total |
|
$ 544,000
|
$ 1,117,000
|
|
|
|
| Seven Employees Working at BioLargo Engineering, Science & Technologies, LLC [Member] |
|
|
|
|
|
|
| Deferred Compensation Arrangement with Individual, Requisite Service Period (Year) |
5 years
|
|
|
|
|
|
| Potential Ownership Percentage of Subsidiary Held by Subsidiary Employees Based on Performance |
30.00%
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
1,242,500
|
|
|
| Incentive Issuance Stipulations for Subsidiary Employees, Accounts Receivable Collected by Year One of Operation |
90.00%
|
|
|
|
|
|
| Incentive Issuance Stipulations for Subsidiary Employees, Profit Earned in Year One of Operation |
10.00%
|
|
|
|
|
|
| Percentage of Profit Interest |
|
|
|
|
13.00%
|
|
| Percentage of Profits Interests Vested |
|
|
|
18.00%
|
11.00%
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Vested in Period, Fair Value |
|
|
|
$ 135,000
|
$ 65,000
|
|
| Seven Employees Working at BioLargo Engineering, Science & Technologies, LLC [Member] | Share-Based Payment Arrangement, Tranche One [Member] |
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
455,000
|
|
| Seven Employees Working at BioLargo Engineering, Science & Technologies, LLC [Member] | Share-Based Payment Arrangement, Tranche Two [Member] |
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
|
|
525,000
|
|
| Seven Employees Working at BioLargo Engineering, Science & Technologies, LLC [Member] | Non-Qualified Stock Option [Member] |
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
1,750,000
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period (Year) |
5 years
|
|
|
|
|
|
| Share-based Payment Arrangement, Noncash Expense, Total |
|
|
|
|
|
$ 0
|
| BioLargo Engineering, Science & Technologies, LLC [Member] |
|
|
|
|
|
|
| Noncontrolling Interest, Ownership Percentage by Parent |
100.00%
|
82.00%
|
|
|
|
|
| X |
- DefinitionThe required accounts receivable collected percentage of the total accounts receivable necessary for incentives to be issued to subsidiary employees.
| Name: |
blgo_IncentiveIssuanceStipulationsForSubsidiaryEmployeesAccountsReceivableCollectedByYearOneOfOperation |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe required profit percentage necessary for incentives to be issued to subsidiary employees.
| Name: |
blgo_IncentiveIssuanceStipulationsForSubsidiaryEmployeesProfitEarnedInYearOneOfOperation |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents percentage of profit interest.
| Name: |
blgo_PercentageOfProfitInterest |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents percentage of profits interest vested.
| Name: |
blgo_PercentageOfProfitsInterestsVested |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionMinimum period the individual is required to perform services to be fully vested under the deferred compensation arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 710
-SubTopic 10
-Section 55
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482943/710-10-55-7
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DeferredCompensationArrangementWithIndividualRequisiteServicePeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe parent entity's interest in net assets of the subsidiary, expressed as a percentage.
| Name: |
us-gaap_MinorityInterestOwnershipPercentageByParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of options vested. Excludes equity instruments other than options, for example, but not limited to, share units, stock appreciation rights, restricted stock. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedInPeriodFairValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=blgo_SevenEmployeesWorkingAtBiolargoEngineeringScienceTechnologiesLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DeferredCompensationArrangementWithIndividualShareBasedPaymentsByTypeOfDeferredCompensationAxis=blgo_NonqualifiedStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_BiolargoEngineeringScienceTechnologiesLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 10 - BioLargo Energy Technologies, Inc. (Details Textual) - USD ($)
|
3 Months Ended |
6 Months Ended |
|
Jun. 30, 2023 |
Jun. 30, 2023 |
Sep. 30, 2017 |
| BioLargo Energy Technologies, Inc (BETI) [Member] |
|
|
|
| Investment Owned, Balance, Shares (in shares) |
9,457,000
|
9,457,000
|
|
| BioLargo Energy Technologies, Inc (BETI) [Member] |
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
132,000
|
450,000
|
|
| Proceeds from Issuance or Sale of Equity |
$ 330,000
|
$ 980,000
|
|
| Stock Conversion, Discount on Volume Weighted average Price |
20.00%
|
20.00%
|
|
| BioLargo Energy Technologies, Inc (BETI) [Member] | Biolargo [Member] |
|
|
|
| Proceeds from Issuance or Sale of Equity |
|
$ 100,000
|
|
| BioLargo Energy Technologies, Inc (BETI) [Member] |
|
|
|
| Investment Owned, Balance, Shares (in shares) |
9,050,000
|
9,050,000
|
9,000,000
|
| Subsidiary, Parent Ownership Interest |
96.00%
|
96.00%
|
|
| X |
- DefinitionPercentage discount on volume weighed average price upon conversion of stock.
| Name: |
blgo_StockConversionDiscountOnVolumeWeightedAveragePrice |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe percentage ownership interest of a subsidiary owned by the parent.
| Name: |
blgo_SubsidiaryParentOwnershipInterest |
| Namespace Prefix: |
blgo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of investment owned. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480493/946-210-55-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_InvestmentOwnedBalanceShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=blgo_BiolargoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionNumber of operating segments. An operating segment is a component of an enterprise: (a) that engages in business activities from which it may earn revenues and incur expenses (including revenues and expenses relating to transactions with other components of the same enterprise), (b) whose operating results are regularly reviewed by the enterprise's chief operating decision maker to make decisions about resources to be allocated to the segment and assess its performance, and (c) for which discrete financial information is available. An operating segment may engage in business activities for which it has yet to earn revenues, for example, start-up operations may be operating segments before earning revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfOperatingSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Note 11 - Business Segment Information - Segment Information (Details) - USD ($)
|
3 Months Ended |
6 Months Ended |
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
| Revenue from Contract with Customer, Including Assessed Tax |
$ 1,446,000
|
$ 1,323,000
|
$ 5,188,000
|
$ 2,287,000
|
|
| Research and development |
(587,000)
|
(355,000)
|
(1,152,000)
|
(747,000)
|
|
| Operating loss |
(1,559,000)
|
(1,321,000)
|
(2,037,000)
|
(3,031,000)
|
|
| Interest expense |
(12,000)
|
(15,000)
|
(60,000)
|
(28,000)
|
|
| Tangible assets |
5,852,000
|
|
5,852,000
|
|
$ 3,958,000
|
| Operating Lease, Right-of-Use Asset |
807,000
|
|
807,000
|
|
867,000
|
| Investment in South Korean joint venture |
21,000
|
|
21,000
|
|
33,000
|
| Total |
6,680,000
|
|
6,680,000
|
|
4,858,000
|
| Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
21,000
|
|
21,000
|
|
33,000
|
| Corporate, Non-Segment [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
0
|
0
|
0
|
2,000
|
|
| Research and development |
(243,000)
|
(165,000)
|
(432,000)
|
(430,000)
|
|
| Operating loss |
(635,000)
|
(923,000)
|
(1,434,000)
|
(2,143,000)
|
|
| Interest expense |
(4,000)
|
(6,000)
|
(40,000)
|
(12,000)
|
|
| Tangible assets |
731,000
|
|
731,000
|
|
669,000
|
| Operating Lease, Right-of-Use Asset |
93,000
|
|
93,000
|
|
136,000
|
| Total |
845,000
|
|
845,000
|
|
838,000
|
| Corporate, Non-Segment [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
21,000
|
|
21,000
|
|
33,000
|
| Operating Segments [Member] | Odor-No-More [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
1,316,000
|
700,000
|
4,859,000
|
1,300,000
|
|
| Operating loss |
428,000
|
11,000
|
1,815,000
|
18,000
|
|
| Interest expense |
(2,000)
|
0
|
(4,000)
|
0
|
|
| Tangible assets |
2,705,000
|
|
2,705,000
|
|
2,064,000
|
| Operating Lease, Right-of-Use Asset |
0
|
|
0
|
|
0
|
| Total |
2,705,000
|
|
2,705,000
|
|
2,064,000
|
| Operating Segments [Member] | Odor-No-More [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
0
|
|
0
|
|
0
|
| Operating Segments [Member] | Clyra Medical [Member] |
|
|
|
|
|
| Tangible assets |
1,265,000
|
|
1,265,000
|
|
631,000
|
| Operating Lease, Right-of-Use Asset |
0
|
|
0
|
|
0
|
| Total |
1,265,000
|
|
1,265,000
|
|
631,000
|
| Operating Segments [Member] | Clyra Medical [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
0
|
|
0
|
|
0
|
| Operating Segments [Member] | BLEST [Member] |
|
|
|
|
|
| Tangible assets |
414,000
|
|
414,000
|
|
441,000
|
| Operating Lease, Right-of-Use Asset |
714,000
|
|
714,000
|
|
731,000
|
| Total |
1,128,000
|
|
1,128,000
|
|
1,172,000
|
| Operating Segments [Member] | BLEST [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
0
|
|
0
|
|
0
|
| Operating Segments [Member] | BioLargo Water [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
9,000
|
0
|
0
|
0
|
|
| Research and development |
(138,000)
|
(130,000)
|
(273,000)
|
(327,000)
|
|
| Operating loss |
(209,000)
|
(209,000)
|
(393,000)
|
(431,000)
|
|
| Tangible assets |
92,000
|
|
92,000
|
|
194,000
|
| Operating Lease, Right-of-Use Asset |
0
|
|
0
|
|
0
|
| Total |
92,000
|
|
92,000
|
|
194,000
|
| Operating Segments [Member] | BioLargo Water [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
0
|
|
0
|
|
0
|
| Operating Segments [Member] | BioLargo Energy Technologies, Inc (BETI) [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
0
|
0
|
0
|
0
|
|
| Research and development |
(271,000)
|
0
|
(303,000)
|
0
|
|
| Operating loss |
(297,000)
|
0
|
(384,000)
|
0
|
|
| Tangible assets |
686,000
|
|
686,000
|
|
|
| Operating Lease, Right-of-Use Asset |
0
|
|
0
|
|
|
| Total |
686,000
|
|
686,000
|
|
|
| Operating Segments [Member] | BioLargo Energy Technologies, Inc (BETI) [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
0
|
|
0
|
|
|
| Operating Segments [Member] | BioLargo Engineering, Science & Technologies, LLC [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
538,000
|
670,000
|
901,000
|
1,213,000
|
|
| Research and development |
(292,000)
|
(80,000)
|
(537,000)
|
(188,000)
|
|
| Operating loss |
(450,000)
|
56,000
|
(818,000)
|
21,000
|
|
| Operating Segments [Member] | Clyra Segment [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
0
|
6,000
|
6,000
|
17,000
|
|
| Research and development |
(60,000)
|
(26,000)
|
(194,000)
|
(42,000)
|
|
| Operating loss |
(396,000)
|
(256,000)
|
(823,000)
|
(496,000)
|
|
| Interest expense |
(6,000)
|
(9,000)
|
(16,000)
|
(16,000)
|
|
| Consolidation, Eliminations [Member] |
|
|
|
|
|
| Revenue from Contract with Customer, Including Assessed Tax |
(417,000)
|
(53,000)
|
(587,000)
|
(245,000)
|
|
| Tangible assets |
(41,000)
|
|
(41,000)
|
|
(41,000)
|
| Operating Lease, Right-of-Use Asset |
0
|
|
0
|
|
0
|
| Total |
(41,000)
|
|
(41,000)
|
|
(41,000)
|
| Consolidation, Eliminations [Member] | Investment in South Korean Joint Venture [Member] |
|
|
|
|
|
| Investment in South Korean joint venture |
0
|
|
0
|
|
$ 0
|
| Intersegment Eliminations [Member] |
|
|
|
|
|
| Research and development |
$ 417,000
|
$ 46,000
|
$ 587,000
|
$ 240,000
|
|
| X |
- DefinitionAmount after amortization of assets which have physical substance with a finite life.
| Name: |
blgo_FinitelivedTangibleAssetsNet |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal amount of tangible and intangible assets, right-of-use assets and equity method investment.
| Name: |
blgo_TangibleAndIntangibleAssetsRightofuseAssetsAndEquityMethodInvestmentTotal |
| Namespace Prefix: |
blgo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThis item represents the carrying amount on the entity's balance sheet of its investment in common stock of an equity method investee. This is not an indicator of the fair value of the investment, rather it is the initial cost adjusted for the entity's share of earnings and losses of the investee, adjusted for any distributions (dividends) and other than temporary impairment (OTTI) losses recognized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481664/323-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
| Name: |
us-gaap_EquityMethodInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ScheduleOfEquityMethodInvestmentEquityMethodInvesteeNameAxis=blgo_InvestmentInSouthKoreanJointVentureMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_CorporateNonSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_OperatingSegmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_OdorNoMoreMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BLESTMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BiolargoWaterMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_BiolargoEngineeringScienceTechnologiesLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=blgo_ClyraSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=srt_ConsolidationEliminationsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_IntersegmentEliminationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 12 - Commitments and Contingencies (Details Textual) - USD ($)
|
3 Months Ended |
6 Months Ended |
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Sep. 30, 2022 |
| Operating Lease, Expense |
$ 83,000
|
$ 106,000
|
$ 166,000
|
$ 169,000
|
|
| Operating Lease, Weighted Average Remaining Lease Term (Year) |
9 years
|
|
9 years
|
|
|
| Lessee, Operating Lease, Discount Rate |
18.00%
|
|
18.00%
|
|
|
| Westminster, California Facility Lease [Member] |
|
|
|
|
|
| Lessee, Operating Lease, Renewal Term (Year) |
4 years
|
|
4 years
|
|
|
| Oak Ridge, Tennessee Facility Lease [Member] |
|
|
|
|
|
| Operating Lease, Weighted Average Remaining Lease Term (Year) |
|
|
|
|
10 years
|
| X |
- DefinitionDiscount rate used by lessee to determine present value of operating lease payments. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseDiscountRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease renewal, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseRenewalTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating lease expense. Excludes sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-4
| Name: |
us-gaap_OperatingLeaseExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=blgo_WestminsterCaliforniaFacilityLeaseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=blgo_OakRidgeTennesseeFacilityLeaseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 13 - Subsequent Events (Details Textual) - USD ($)
|
1 Months Ended |
3 Months Ended |
6 Months Ended |
|
Aug. 10, 2023 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 20, 2022 |
| Proceeds from Issuance of Common Stock |
|
|
|
$ 1,294,000
|
$ 2,150,000
|
|
| Proceeds from Issuance of Preferred Stock and Preference Stock |
|
|
|
$ 1,287,000
|
$ 0
|
|
| Clyra Medical [Member] |
|
|
|
|
|
|
| Shares Issued, Price Per Share (in dollars per share) |
|
|
|
|
|
$ 310
|
| Series A Preferred Stock [Member] | Clyra Medical [Member] |
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
|
3,427
|
|
4,879
|
|
|
| Proceeds from Issuance of Preferred Stock and Preference Stock |
|
$ 1,062,000
|
|
$ 1,287,000
|
|
|
| Shares Issued, Price Per Share (in dollars per share) |
|
|
|
|
|
$ 372
|
| Subsequent Event [Member] | Clyra Medical [Member] | Warrant [Member] |
|
|
|
|
|
|
| Warrants and Rights Outstanding, Term (Year) |
3 years
|
|
|
|
|
|
| Shares Issued, Price Per Share (in dollars per share) |
$ 372
|
|
|
|
|
|
| Subsequent Event [Member] | Series A Preferred Stock [Member] | Clyra Medical [Member] |
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
81
|
|
|
|
|
|
| Proceeds from Issuance of Preferred Stock and Preference Stock |
$ 25,000
|
|
|
|
|
|
| Lincoln Park Capital Fund, LLC [Member] |
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
|
1,098,221
|
406,140
|
1,643,623
|
1,912,961
|
|
| Proceeds from Issuance of Common Stock |
|
$ 194,000
|
$ 72,000
|
$ 299,000
|
$ 418,000
|
|
| Lincoln Park Capital Fund, LLC [Member] | Subsequent Event [Member] |
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
782,553
|
|
|
|
|
|
| Proceeds from Issuance of Common Stock |
$ 136,000
|
|
|
|
|
|
| BioLargo Energy Technologies, Inc (BETI) [Member] | Subsequent Event [Member] |
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
10,000
|
|
|
|
|
|
| Proceeds from Issuance of Common Stock |
$ 25,000
|
|
|
|
|
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from issuance of capital stock which provides for a specific dividend that is paid to the shareholders before any dividends to common stockholders and which takes precedence over common stockholders in the event of liquidation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPreferredStockAndPreferenceStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=blgo_ClyraMedicalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesAPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=blgo_LincolnParkCapitalFundLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=blgo_BiolargoEnergyTechnologiesIncBETIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
BioLargo (QB) (USOTC:BLGO)
Historical Stock Chart
From Mar 2024 to Apr 2024
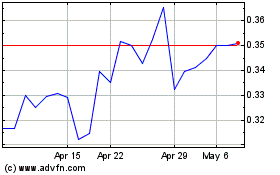
BioLargo (QB) (USOTC:BLGO)
Historical Stock Chart
From Apr 2023 to Apr 2024