false000137469000013746902023-08-142023-08-14
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): August 14, 2023 |
Larimar Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-36510 |
20-3857670 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
Three Bala Plaza East |
|
Bala Cynwyd, Pennsylvania |
|
19004 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (844) 511-9056 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 per share |
|
lrmr |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On August 14, 2023, Larimar Therapeutics, Inc. (the “Company”) posted on its website an updated slide presentation, which is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference. Representatives of the Company will use the presentation in various meetings with investors, analysts and other parties from time to time.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Below is a list of exhibits included with this Current Report on Form 8-K.
* Filed herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Larimar Therapeutics, Inc. |
|
|
|
|
Date: |
August 14, 2023 |
By: |
/s/ Carole S. Ben-Maimon, M.D. |
|
|
|
Name: Carole S. Ben-Maimon, M.D.
Title: President and Chief Executive Officer |

August 2023 Larimar Therapeutics Corporate Presentation Exhibit 99.1

This presentation contains forward-looking statements that are based on the beliefs and assumptions of Larimar Therapeutics, Inc. ( “Company”) and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to Larimar’s ability to develop and commercialize CTI-1601 and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its CTI-1601 clinical trials and overall development plan and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including CTI-1601 clinical milestones and continued interactions with the FDA regarding the partial clinical hold; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of CTI-1601 may not be predictive of the results or success of later clinical trials, and assessments; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale CTI-1601’s manufacturing process; Larimar’s ability to obtain regulatory approvals for CTI-1601 and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law. Forward-Looking Statements

Investment Highlights Clinical-stage biotechnology company focused on addressing unmet needs in Friedreich's ataxia (FA) and potentially other complex rare diseases based on a platform technology backed by a strong intellectual property portfolio Lead candidate CTI-1601 is a recombinant fusion protein designed to directly address frataxin deficiency by delivering the protein to mitochondria. CTI-1601 has received Orphan Drug (US & EU), Rare Pediatric Disease (US), Fast Track (US), & PRIME (EU) designations Two double-blind, placebo-controlled Phase 1 trials in FA demonstrating CTI-1601 was generally well tolerated when dosed daily for up to 13 days; dose-dependent increases in frataxin (FXN) levels from baseline vs. placebo were observed in all evaluated tissues Ongoing Phase 2, placebo-controlled, 4-week dose exploration study in FA; 25 mg cohort data show CTI-1601 is generally well tolerated, increasing FXN levels from baseline vs. placebo in skin and buccal cells; trial advancing to 50 mg cohort with data expected in 1H 2024; OLE trial with 25 mg daily dosing cleared for initiation in Q1 2024. To potentially further escalate dose in Phase 2 study or the OLE study, submit Phase 2 data from 50 mg cohort to FDA due to continued partial clinical hold. $104.2 Million cash balance (June 30, 2023) with projected cash runway into Q4 2024 Novel protein replacement therapy platform Potential first-ever therapy to increase frataxin levels Completed Phase 1 proof-of-concept Phase 2 and OLE studies with near-term catalysts Strong financial foundation
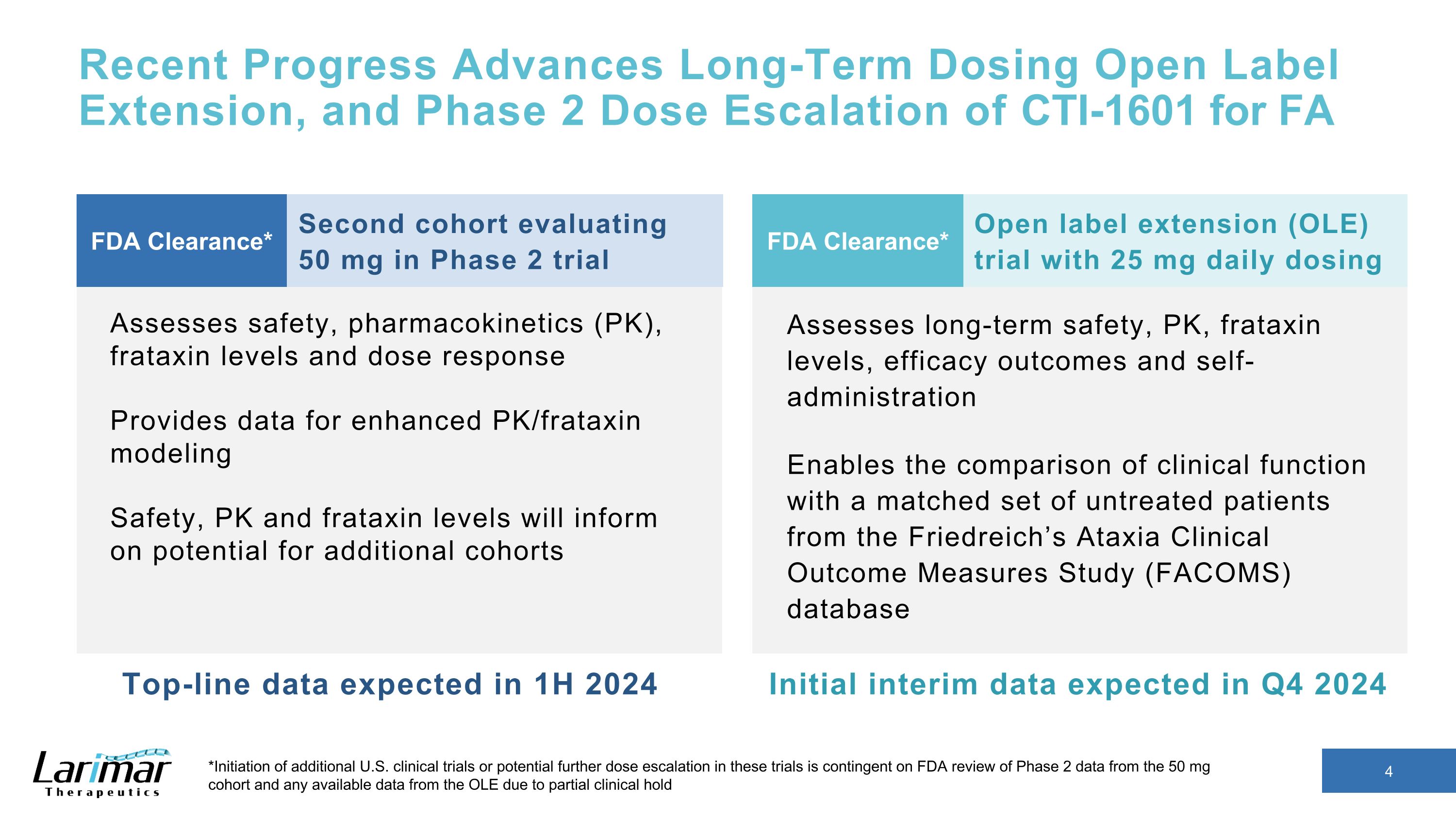
Recent Progress Advances Long-Term Dosing Open Label Extension, and Phase 2 Dose Escalation of CTI-1601 for FA Second cohort evaluating �50 mg in Phase 2 trial Assesses long-term safety, PK, frataxin levels, efficacy outcomes and self-administration Enables the comparison of clinical function with a matched set of untreated patients from the Friedreich’s Ataxia Clinical Outcome Measures Study (FACOMS) database FDA Clearance* Assesses safety, pharmacokinetics (PK), frataxin levels and dose response Provides data for enhanced PK/frataxin modeling Safety, PK and frataxin levels will inform on potential for additional cohorts Open label extension (OLE) trial with 25 mg daily dosing FDA Clearance* Top-line data expected in 1H 2024 Initial interim data expected in Q4 2024 *Initiation of additional U.S. clinical trials or potential further dose escalation in these trials is contingent on FDA review of Phase 2 data from the 50 mg cohort and any available data from the OLE due to partial clinical hold
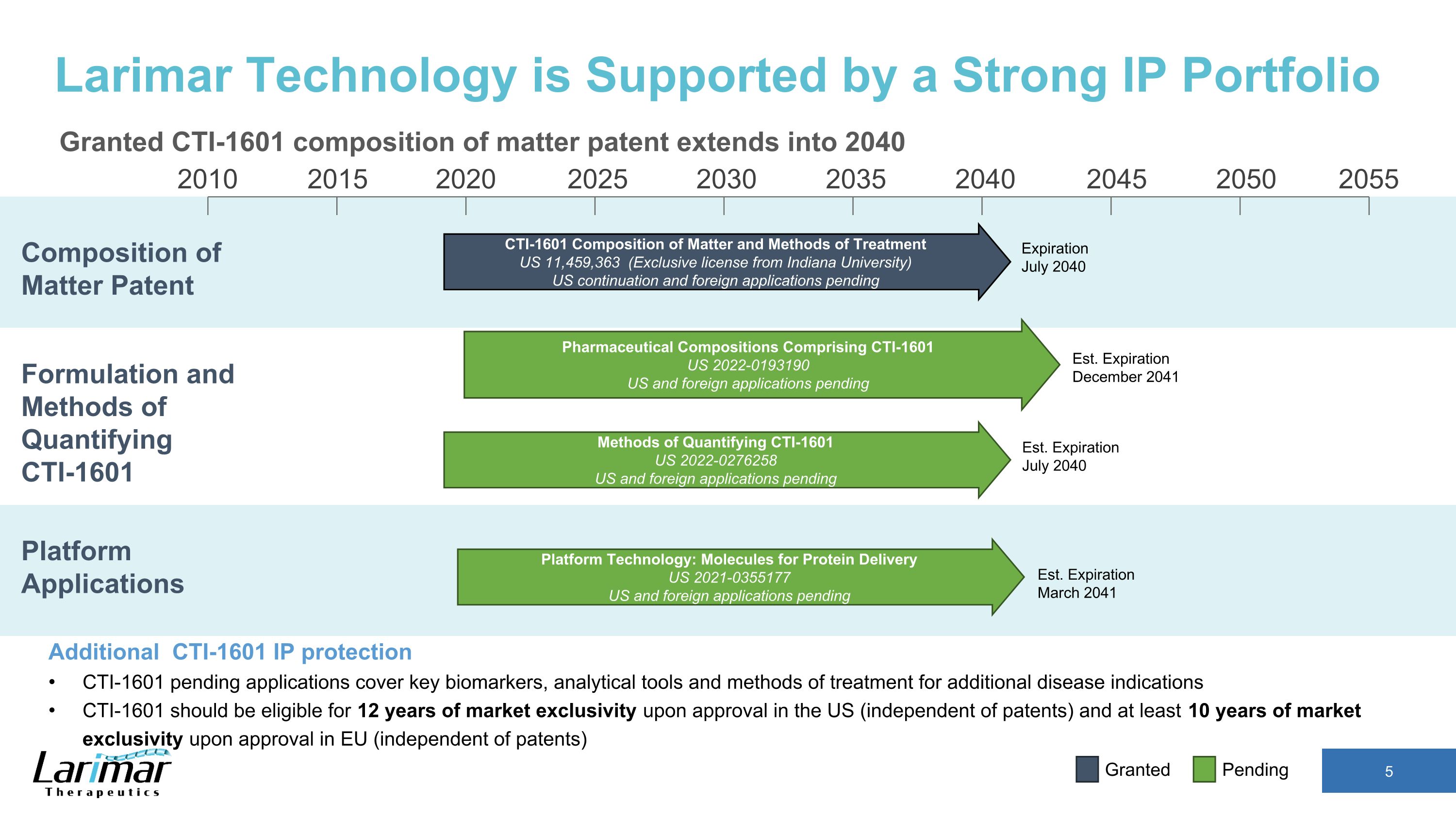
2015 2020 2030 2035 2040 2045 2050 2055 2010 2025 Est. Expiration March 2041 CTI-1601 Composition of Matter and Methods of Treatment US 11,459,363 (Exclusive license from Indiana University) US continuation and foreign applications pending Expiration July 2040 Composition of Matter Patent Larimar Technology is Supported by a Strong IP Portfolio Granted CTI-1601 composition of matter patent extends into 2040 Additional CTI-1601 IP protection CTI-1601 pending applications cover key biomarkers, analytical tools and methods of treatment for additional disease indications CTI-1601 should be eligible for 12 years of market exclusivity upon approval in the US (independent of patents) and at least 10 years of market exclusivity upon approval in EU (independent of patents) Pending Granted Platform Applications Formulation and Methods of Quantifying CTI-1601 Platform Technology: Molecules for Protein Delivery US 2021-0355177 US and foreign applications pending Pharmaceutical Compositions Comprising CTI-1601 US 2022-0193190 US and foreign applications pending Methods of Quantifying CTI-1601 US 2022-0276258 US and foreign applications pending Est. Expiration December 2041 Est. Expiration July 2040

Caused by genetic defect resulting in low levels of frataxin Patients with FA only produce ~20-40% of normal frataxin levels depending on the tissue, sampling technique, and assay considered1 Affects ~20,000 patients globally, with ~5,000 patients in the U.S. and majority of the remaining patients in the EU Approximately 70% of patients present before age 14 Initial symptoms may include unsteady posture, frequent falling and progressive difficulty in walking. By the time symptoms occur, heart damage may have already occurred. Progressive disease: symptoms worsen and patients are eventually confined to a wheelchair with speech becoming hesitant and jerky (often referred to as “scanning speech”) Life expectancy of 30-50 years Early death usually caused by heart disease No available therapies increase frataxin levels Only treatment approved for FA does not address frataxin deficiency Friedreich’s Ataxia (FA) Rare and Progressive Disease 6 1. E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245 LRMR continues to have a strong relationship with Friedreich’s Ataxia Research Alliance Dedicated FA patient advocacy group focused on treatments for FA
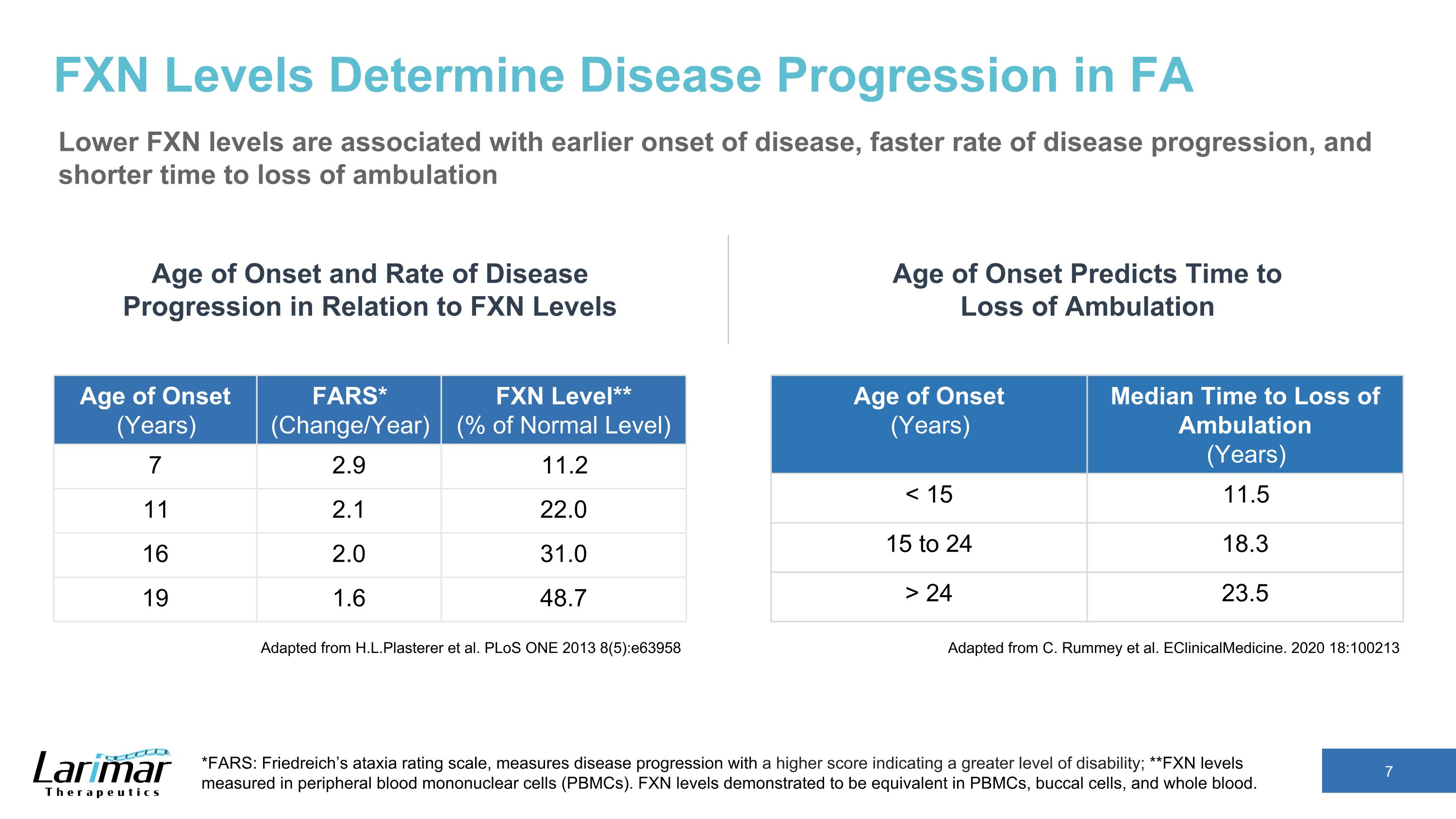
FXN Levels Determine Disease Progression in FA Lower FXN levels are associated with earlier onset of disease, faster rate of disease progression, and shorter time to loss of ambulation Adapted from H.L.Plasterer et al. PLoS ONE 2013 8(5):e63958 Age of Onset (Years) Median Time to Loss of Ambulation (Years) < 15 11.5 15 to 24 18.3 > 24 23.5 Age of Onset and Rate of Disease Progression in Relation to FXN Levels *FARS: Friedreich’s ataxia rating scale, measures disease progression with a higher score indicating a greater level of disability; **FXN levels measured in peripheral blood mononuclear cells (PBMCs). FXN levels demonstrated to be equivalent in PBMCs, buccal cells, and whole blood. Age of Onset (Years) FARS* (Change/Year) FXN Level** (% of Normal Level) 7 2.9 11.2 11 2.1 22.0 16 2.0 31.0 19 1.6 48.7 Adapted from C. Rummey et al. EClinicalMedicine. 2020 18:100213 Age of Onset Predicts Time to Loss of Ambulation
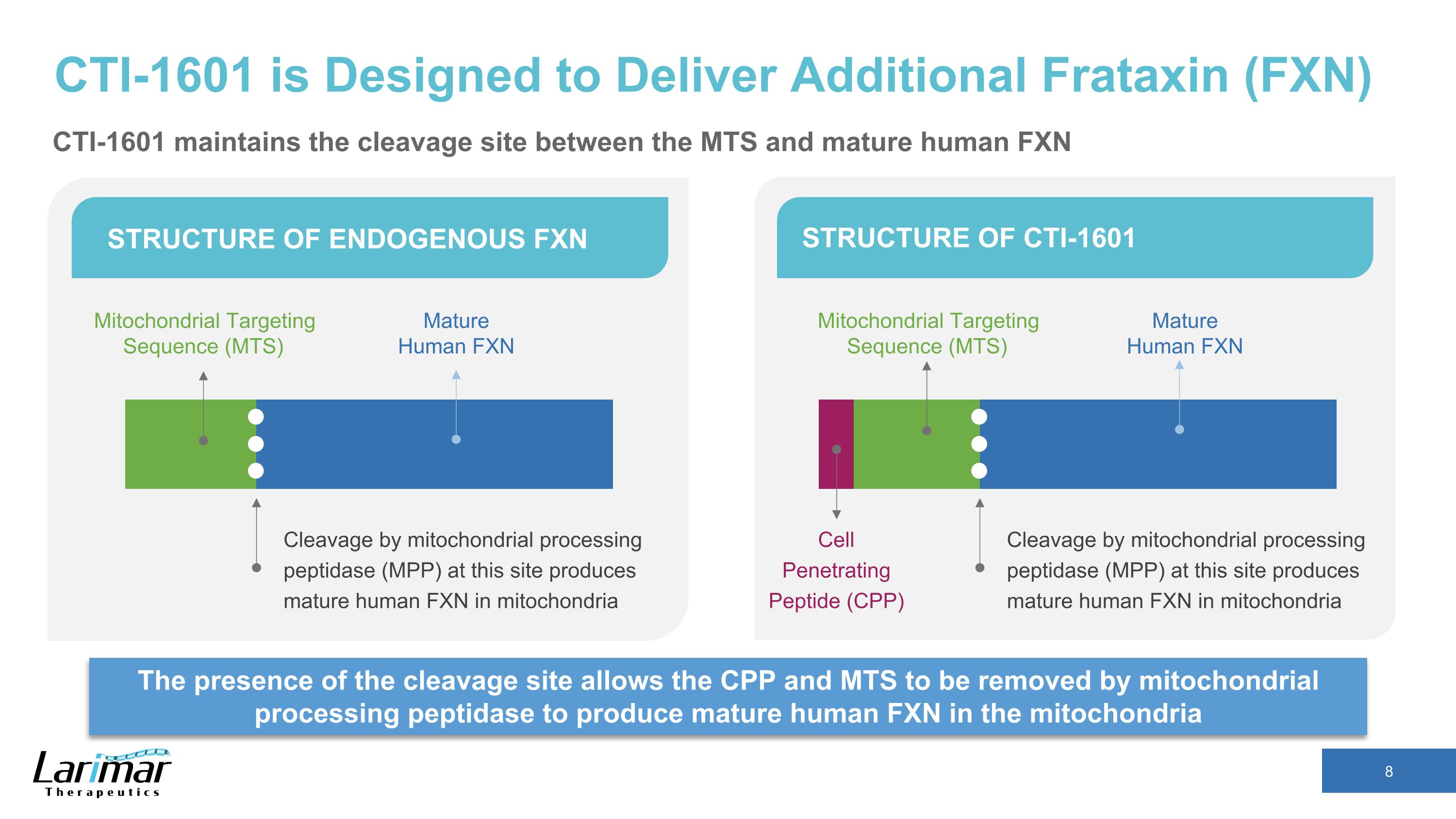
CTI-1601 is Designed to Deliver Additional Frataxin (FXN) The presence of the cleavage site allows the CPP and MTS to be removed by mitochondrial processing peptidase to produce mature human FXN in the mitochondria STRUCTURE OF ENDOGENOUS FXN STRUCTURE OF CTI-1601 Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mitochondrial Targeting Sequence (MTS) Mature Human FXN Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mature Human FXN Cell Penetrating Peptide (CPP) Mitochondrial Targeting Sequence (MTS) CTI-1601 maintains the cleavage site between the MTS and mature human FXN
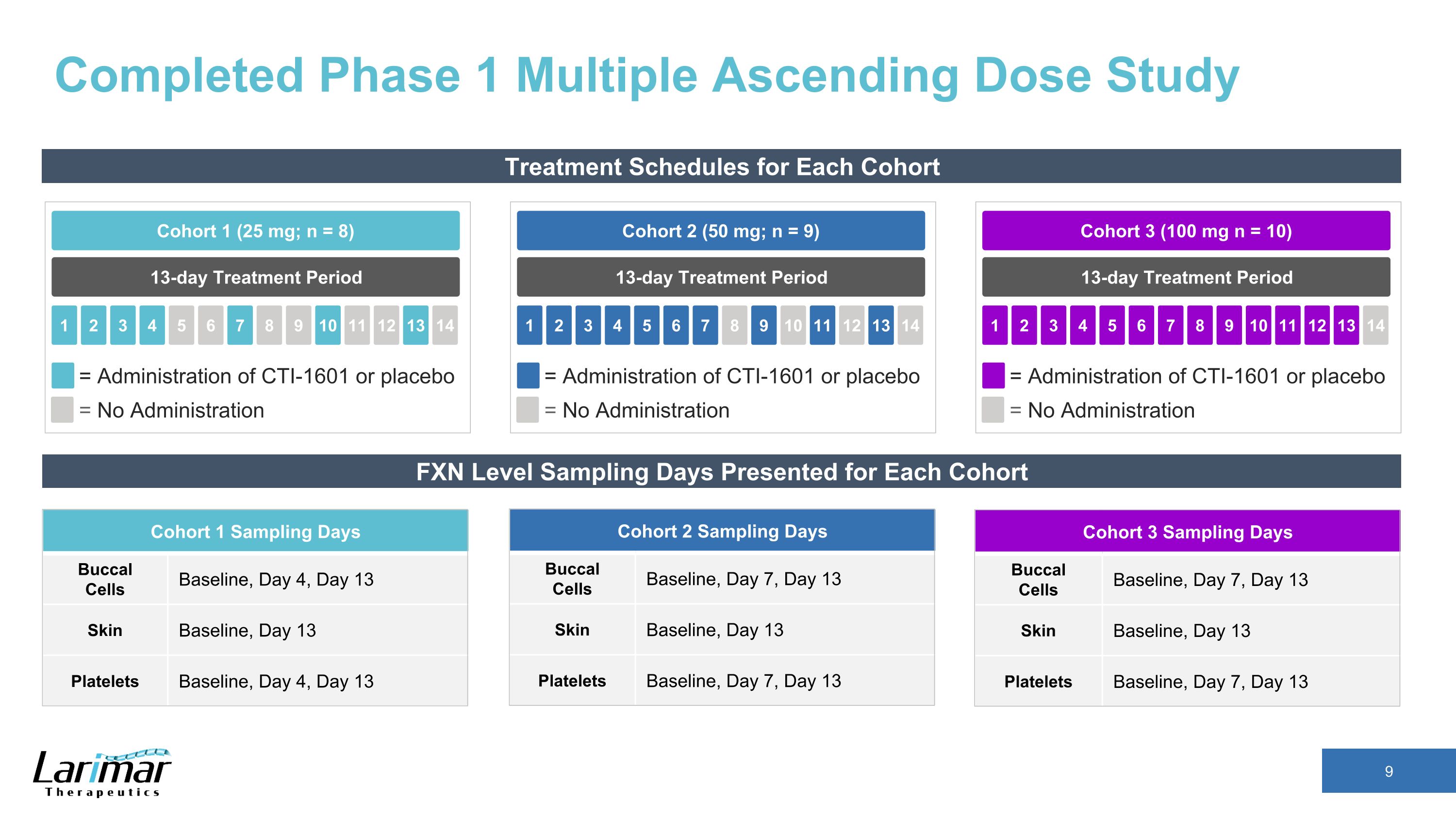
Completed Phase 1 Multiple Ascending Dose Study Treatment Schedules for Each Cohort 13-day Treatment Period Cohort 2 (50 mg; n = 9) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration 13-day Treatment Period Cohort 1 (25 mg; n = 8) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration 13-day Treatment Period Cohort 3 (100 mg n = 10) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration FXN Level Sampling Days Presented for Each Cohort Cohort 1 Sampling Days Buccal Cells Baseline, Day 4, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 4, Day 13 Cohort 2 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13 Cohort 3 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13
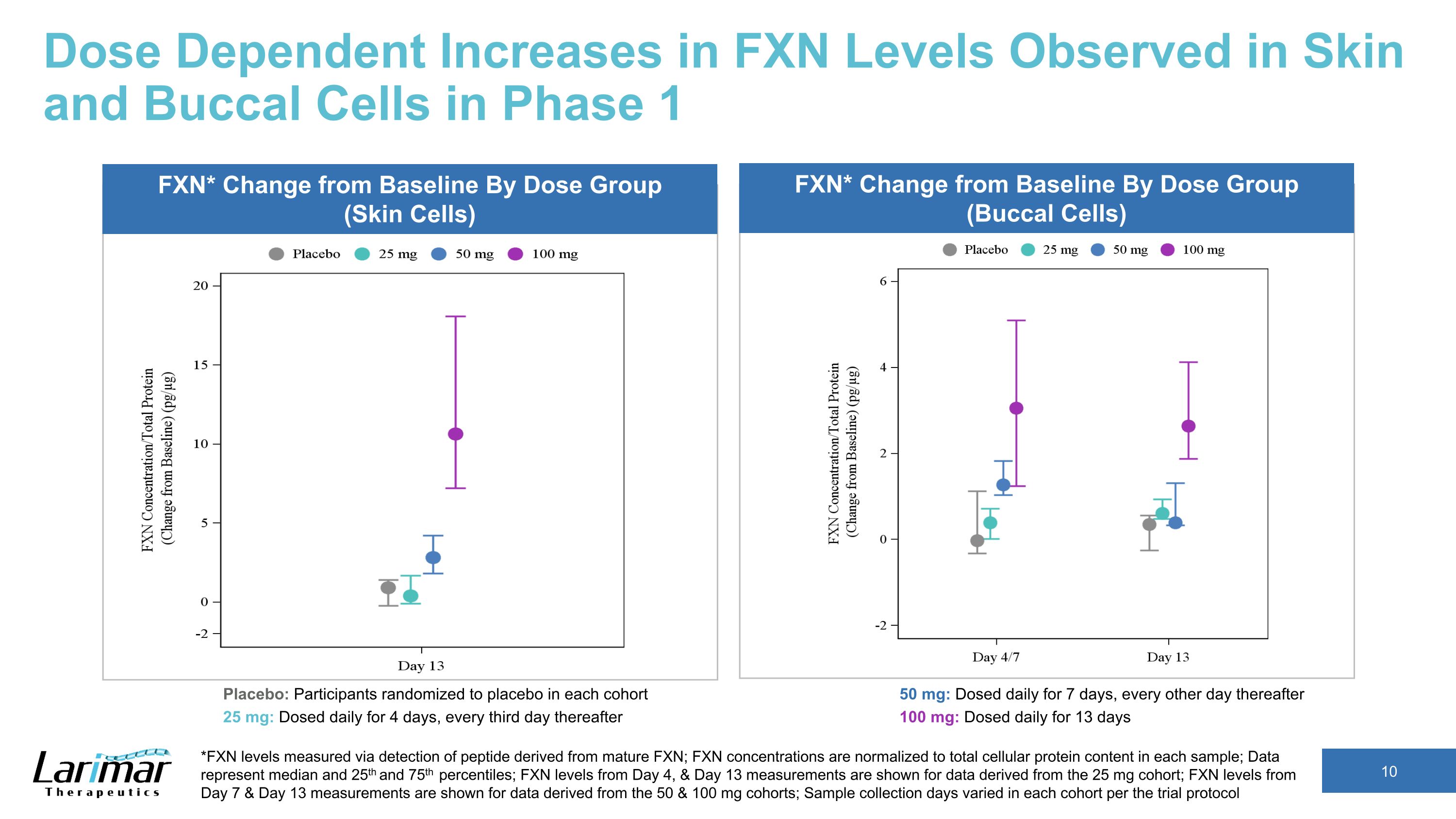
Dose Dependent Increases in FXN Levels Observed in Skin and Buccal Cells in Phase 1 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data represent median and 25th and 75th percentiles; FXN levels from Day 4, & Day 13 measurements are shown for data derived from the 25 mg cohort; FXN levels from Day 7 & Day 13 measurements are shown for data derived from the 50 & 100 mg cohorts; Sample collection days varied in each cohort per the trial protocol FXN* Change from Baseline By Dose Group (Skin Cells) FXN* Change from Baseline By Dose Group (Buccal Cells) Placebo: Participants randomized to placebo in each cohort 25 mg: Dosed daily for 4 days, every third day thereafter 50 mg: Dosed daily for 7 days, every other day thereafter 100 mg: Dosed daily for 13 days
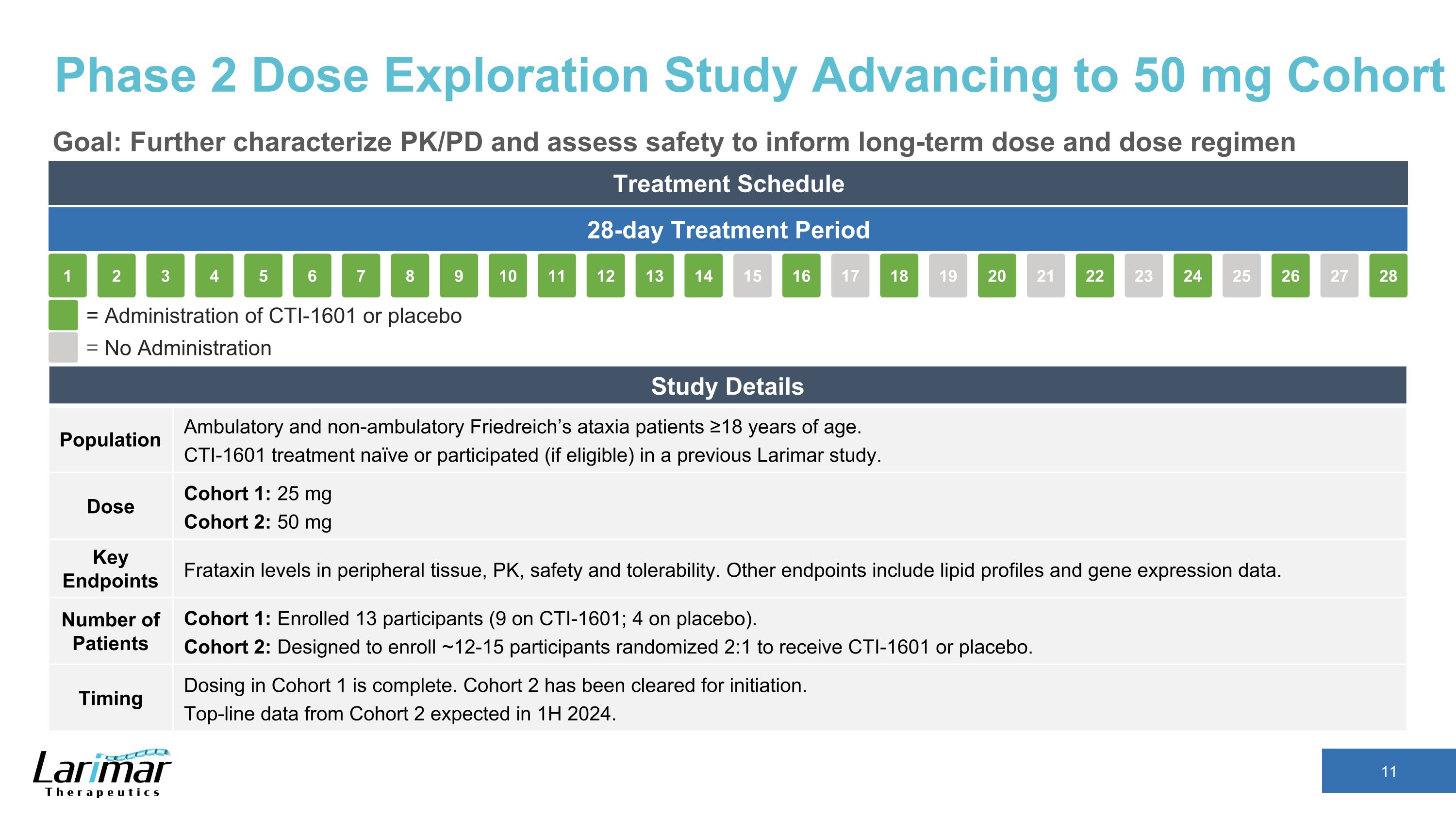
Phase 2 Dose Exploration Study Advancing to 50 mg Cohort Goal: Further characterize PK/PD and assess safety to inform long-term dose and dose regimen Treatment Schedule 28-day Treatment Period 16 17 18 19 15 20 21 22 23 24 25 26 27 28 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration Study Details Population Ambulatory and non-ambulatory Friedreich’s ataxia patients ≥18 years of age. CTI-1601 treatment naïve or participated (if eligible) in a previous Larimar study. Dose Cohort 1: 25 mg Cohort 2: 50 mg Key Endpoints Frataxin levels in peripheral tissue, PK, safety and tolerability. Other endpoints include lipid profiles and gene expression data. Number of Patients Cohort 1: Enrolled 13 participants (9 on CTI-1601; 4 on placebo). Cohort 2: Designed to enroll ~12-15 participants randomized 2:1 to receive CTI-1601 or placebo. Timing Dosing in Cohort 1 is complete. Cohort 2 has been cleared for initiation. Top-line data from Cohort 2 expected in 1H 2024.
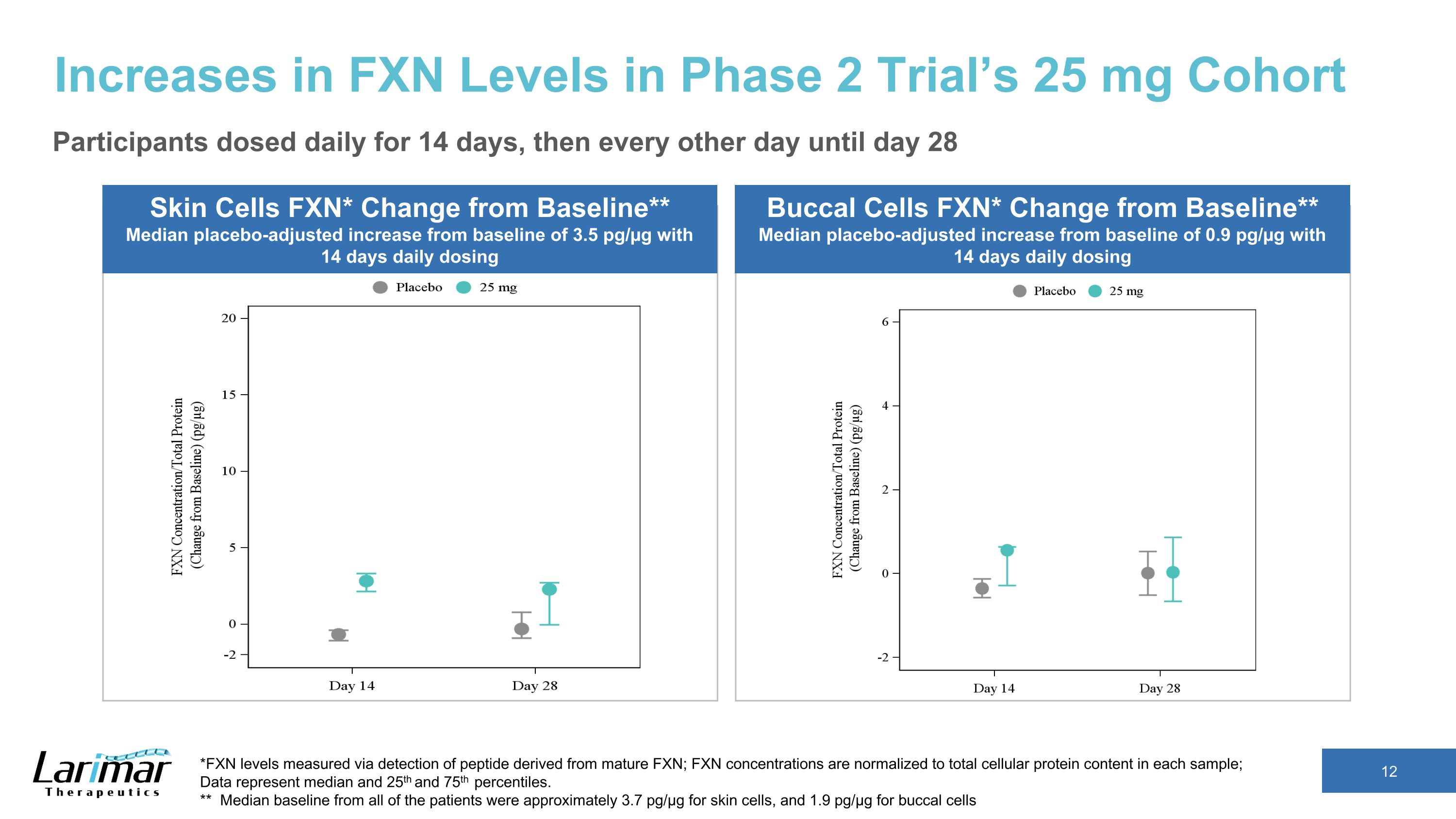
Increases in FXN Levels in Phase 2 Trial’s 25 mg Cohort Skin Cells FXN* Change from Baseline** Median placebo-adjusted increase from baseline of 3.5 pg/µg with 14 days daily dosing *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; �Data represent median and 25th and 75th percentiles. ** Median baseline from all of the patients were approximately 3.7 pg/µg for skin cells, and 1.9 pg/µg for buccal cells Buccal Cells FXN* Change from Baseline** Median placebo-adjusted increase from baseline of 0.9 pg/µg with 14 days daily dosing Participants dosed daily for 14 days, then every other day until day 28
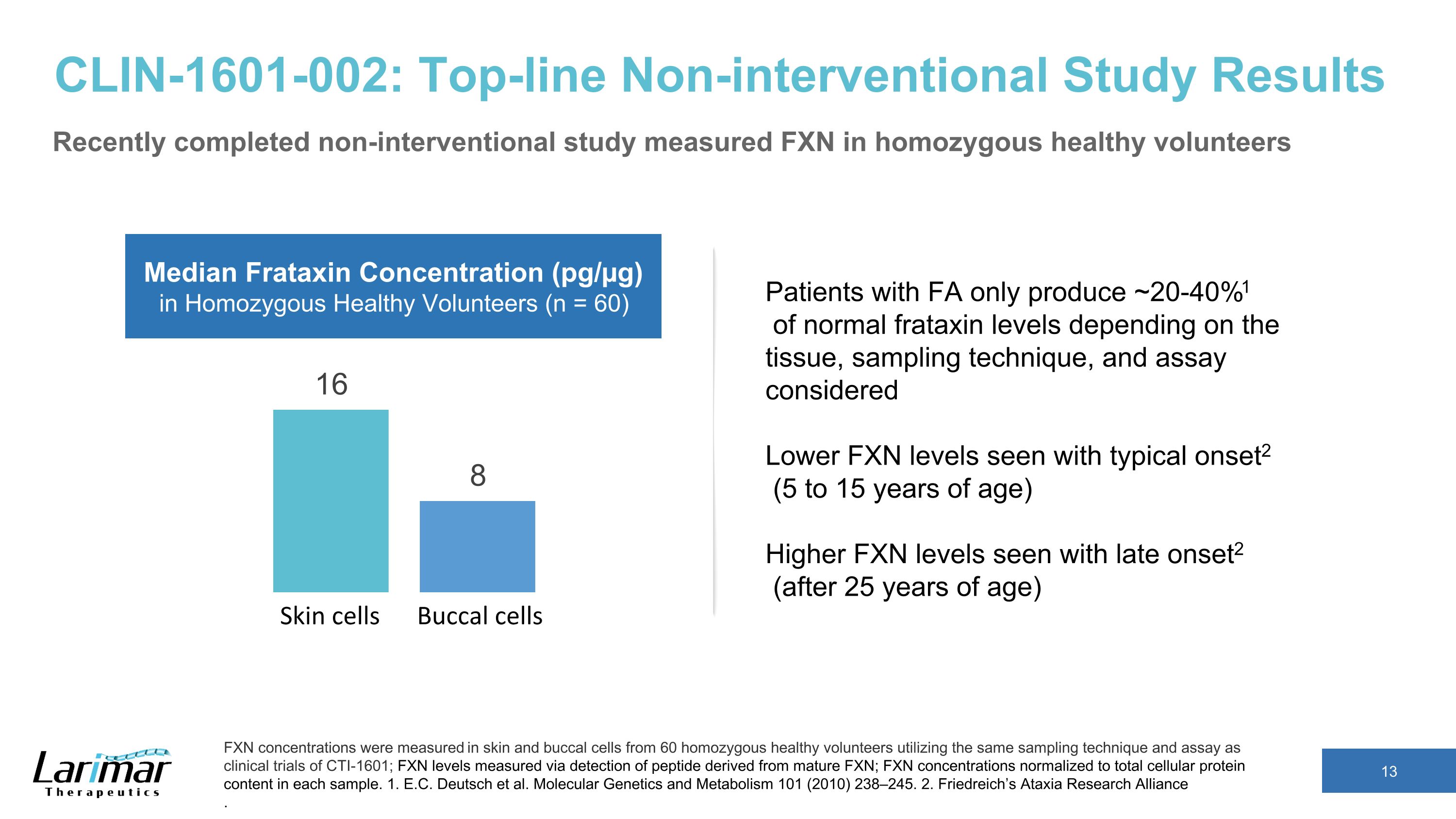
CLIN-1601-002: Top-line Non-interventional Study Results Recently completed non-interventional study measured FXN in homozygous healthy volunteers FXN concentrations were measured in skin and buccal cells from 60 homozygous healthy volunteers utilizing the same sampling technique and assay as clinical trials of CTI-1601; FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations normalized to total cellular protein content in each sample. 1. E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. 2. Friedreich’s Ataxia Research Alliance . Skin cells Buccal cells Median Frataxin Concentration (pg/µg) in Homozygous Healthy Volunteers (n = 60) Patients with FA only produce ~20-40%1 of normal frataxin levels depending on the tissue, sampling technique, and assay considered Lower FXN levels seen with typical onset2 (5 to 15 years of age) Higher FXN levels seen with late onset2 (after 25 years of age)
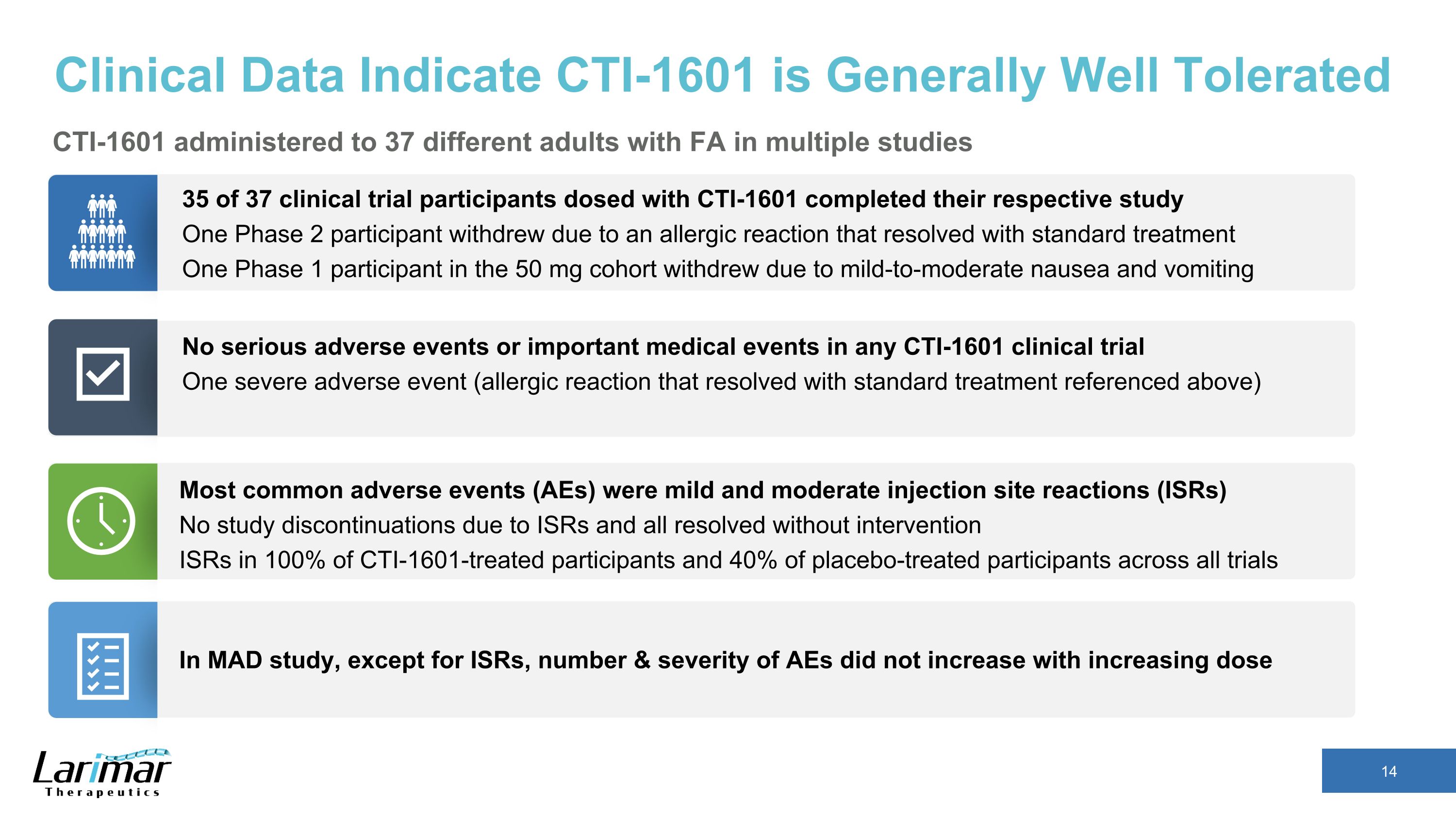
Clinical Data Indicate CTI-1601 is Generally Well Tolerated CTI-1601 administered to 37 different adults with FA in multiple studies 35 of 37 clinical trial participants dosed with CTI-1601 completed their respective study One Phase 2 participant withdrew due to an allergic reaction that resolved with standard treatment One Phase 1 participant in the 50 mg cohort withdrew due to mild-to-moderate nausea and vomiting No serious adverse events or important medical events in any CTI-1601 clinical trial One severe adverse event (allergic reaction that resolved with standard treatment referenced above) Most common adverse events (AEs) were mild and moderate injection site reactions (ISRs) No study discontinuations due to ISRs and all resolved without intervention ISRs in 100% of CTI-1601-treated participants and 40% of placebo-treated participants across all trials In MAD study, except for ISRs, number & severity of AEs did not increase with increasing dose
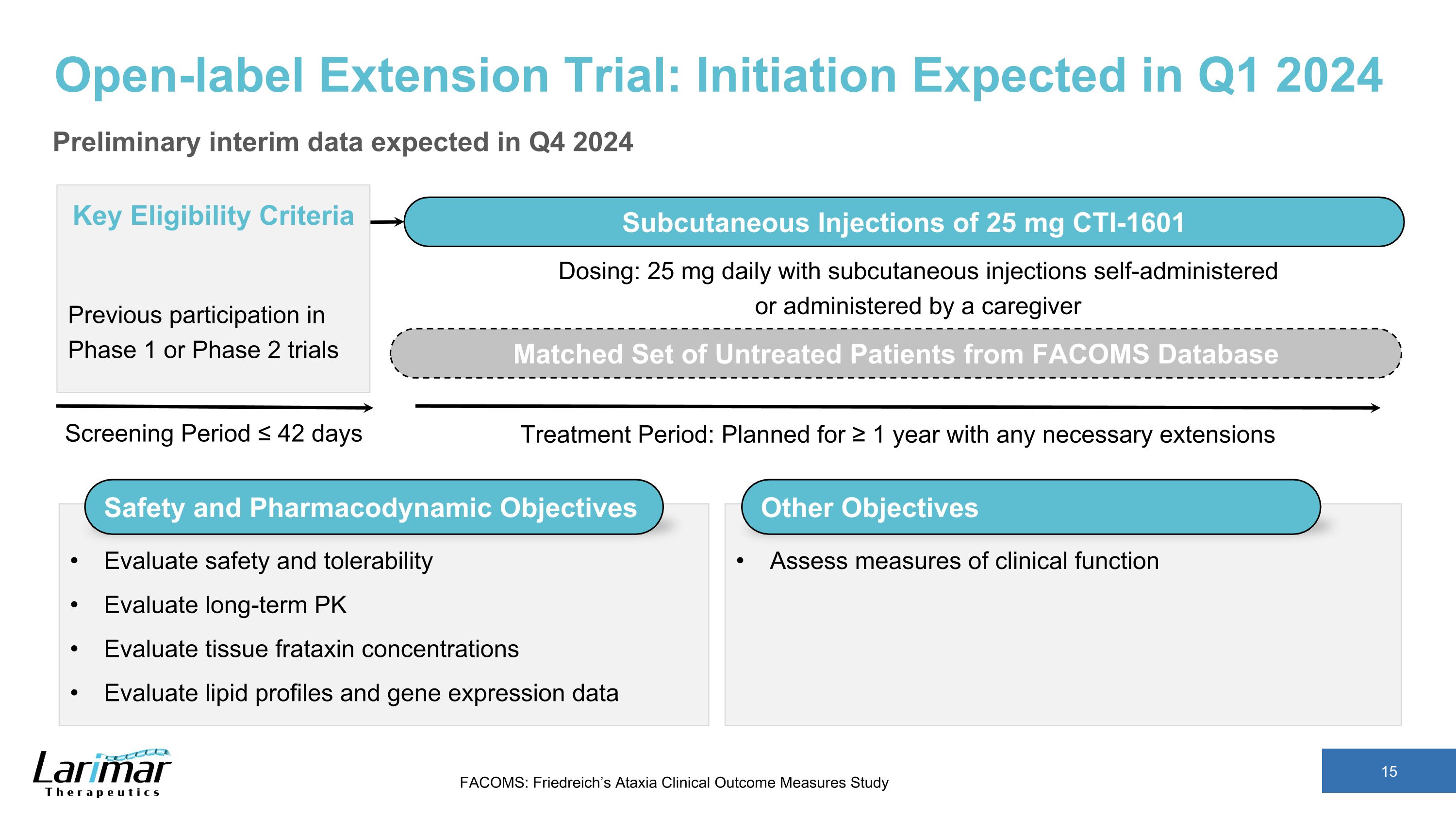
Open-label Extension Trial: Initiation Expected in Q1 2024 Preliminary interim data expected in Q4 2024 Screening Period ≤ 42 days Key Eligibility Criteria Previous participation in Phase 1 or Phase 2 trials Subcutaneous Injections of 25 mg CTI-1601 Treatment Period: Planned for ≥ 1 year with any necessary extensions Matched Set of Untreated Patients from FACOMS Database Safety and Pharmacodynamic Objectives Evaluate safety and tolerability Evaluate long-term PK Evaluate tissue frataxin concentrations Evaluate lipid profiles and gene expression data Dosing: 25 mg daily with subcutaneous injections self-administered or administered by a caregiver Other Objectives Assess measures of clinical function FACOMS: Friedreich’s Ataxia Clinical Outcome Measures Study

CTI-1601 Clinical Development Plan Ongoing and Planned Trials* Include: Global double-blind placebo-controlled pivotal trial*** Open-label extension trial with 25 mg daily dosing for eligible patients who participated in SAD, MAD, and/or four-week dose exploration studies. Initiation expected Q1 2024. MAD trial in patients 2 to 17 years of age**. Participants eligible to screen for OLE trial. Phase 2, four-week dose exploration study intended to identify dose and dose regimen for long-term studies. Advancing to 50 mg cohort. Top-line data from 50 mg cohort of Phase 2 dose exploration trial expected in 1H 2024 *Initiation of additional U.S. clinical trials or potential further dose escalation in the Phase 2 or OLE trials is contingent on submission to FDA of additional data from adult patients due to partial clinical hold. **Company is planning discussions with FDA on how to best include patients 2 to 17 years of age in clinical development ***Company plans to initiate discussions with FDA on appropriate pivotal trial endpoints, including value of FXN levels. Also, the Company is planning discussions with regulators & investigators outside the U.S. to expand clinical program to international geographies. OLE
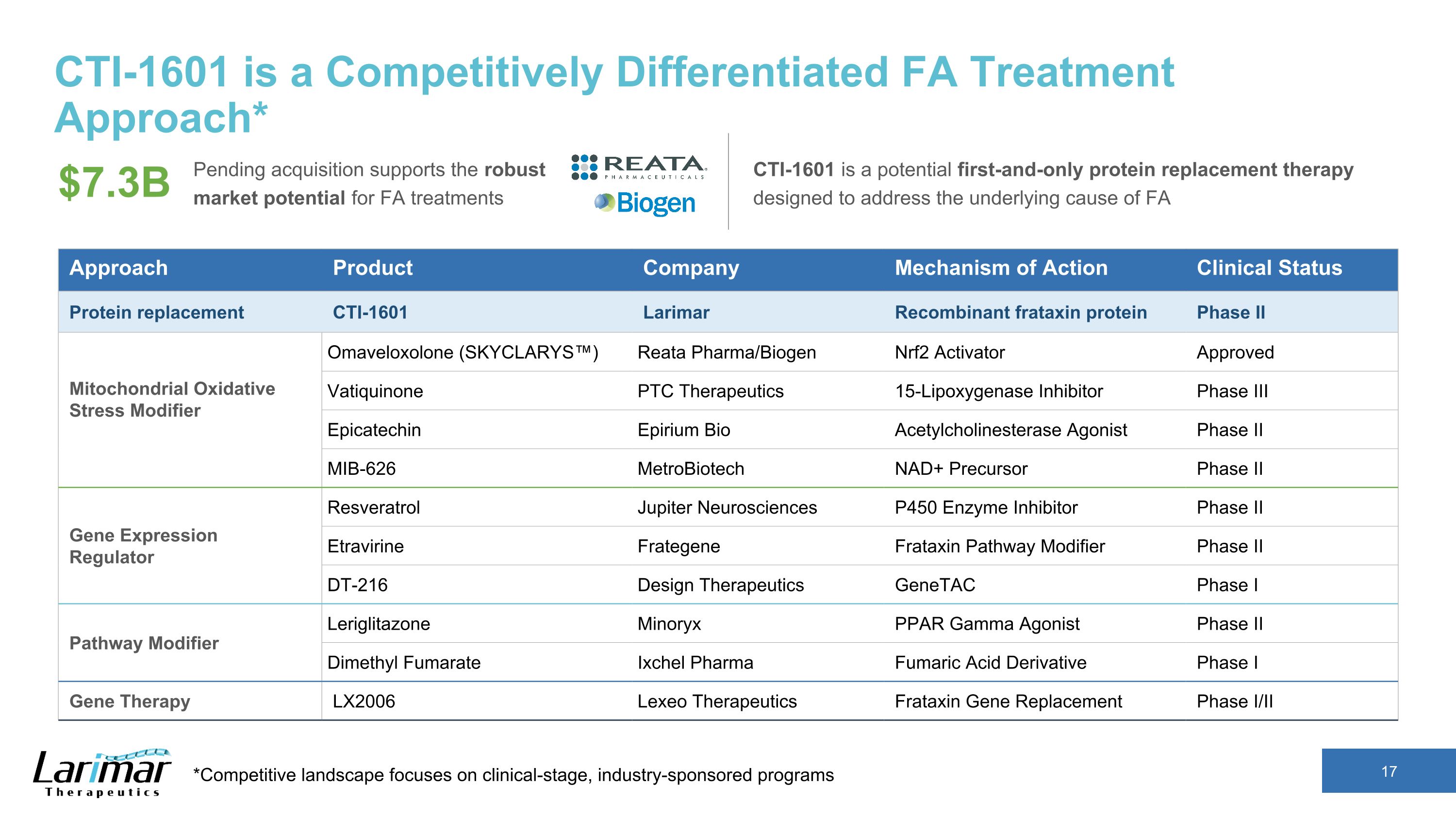
CTI-1601 is a Competitively Differentiated FA Treatment Approach* *Competitive landscape focuses on clinical-stage, industry-sponsored programs Pending acquisition supports the robust market potential for FA treatments CTI-1601 is a potential first-and-only protein replacement therapy designed to address the underlying cause of FA $7.3B Approach Product Company Mechanism of Action Clinical Status Protein replacement CTI-1601 Larimar Recombinant frataxin protein Phase II Mitochondrial Oxidative Stress Modifier Omaveloxolone (SKYCLARYS™) Reata Pharma/Biogen Nrf2 Activator Approved Vatiquinone PTC Therapeutics 15-Lipoxygenase Inhibitor Phase III Epicatechin Epirium Bio Acetylcholinesterase Agonist Phase II MIB-626 MetroBiotech NAD+ Precursor Phase II Gene Expression Regulator Resveratrol Jupiter Neurosciences P450 Enzyme Inhibitor Phase II Etravirine Frategene Frataxin Pathway Modifier Phase II DT-216 Design Therapeutics GeneTAC Phase I Pathway Modifier Leriglitazone Minoryx PPAR Gamma Agonist Phase II Dimethyl Fumarate Ixchel Pharma Fumaric Acid Derivative Phase I Gene Therapy LX2006 Lexeo Therapeutics Frataxin Gene Replacement Phase I/II

Regulatory Updates Q3 2023: Initiation of 50 mg cohort in Phase 2 dose exploration trial Q1 2024: Initiation of open-label extension trial 1H 2024: Top-line data from Phase 2 trial’s 50 mg cohort Q4 2024: Interim data from open-label extension trial Expected Milestones CTI-1601 Phase 2 dose exploration trial of CTI-1601 in FA cleared to proceed to 50 mg cohort Open label extension trial with 25 mg daily dosing cleared for initiation Beginning preparations to expand CTI-1601 clinical program to ex-U.S. geographies Summary: CTI-1601 Cleared by FDA to Advance Clinical Development Generally well tolerated Dose-dependent increases in FXN levels in all evaluated tissues in Phase 1 Increases in FXN from baseline compared to placebo in all evaluated tissues (skin and buccal cells) in Phase 2

Investment Highlights Clinical-stage biotechnology company focused on addressing unmet needs in Friedreich's ataxia (FA) and potentially other complex rare diseases based on a platform technology backed by a strong intellectual property portfolio Lead candidate CTI-1601 is a recombinant fusion protein designed to directly address frataxin deficiency by delivering the protein to mitochondria. CTI-1601 has received Orphan Drug (US & EU), Rare Pediatric Disease (US), Fast Track (US), & PRIME (EU) designations Two double-blind, placebo-controlled Phase 1 trials in FA demonstrating CTI-1601 was generally well tolerated when dosed daily for up to 13 days; dose-dependent increases in frataxin (FXN) levels from baseline vs. placebo were observed in all evaluated tissues Ongoing Phase 2, placebo-controlled, 4-week dose exploration study in FA; 25 mg cohort data show CTI-1601 is generally well tolerated, increasing FXN levels from baseline vs. placebo in skin and buccal cells; trial advancing to 50 mg cohort with data expected in 1H 2024; OLE trial with 25 mg daily dosing cleared for initiation in Q1 2024. To potentially further escalate dose in Phase 2 study or the OLE study, submit Phase 2 data from 50 mg cohort to FDA due to continued partial clinical hold. $104.2 Million cash balance (June 30, 2023) with projected cash runway into Q4 2024 Novel protein replacement therapy platform Potential first-ever therapy to increase frataxin levels Completed Phase 1 proof-of-concept Phase 2 and OLE studies with near-term catalysts Strong financial foundation

THANK YOU Larimar Therapeutics August 2023 Corporate Update

Appendix Larimar Therapeutics

Scientific Advisory Board Co-founder of Chondrial Therapeutics, which became Larimar Therapeutics, Inc. Professor of Pediatrics at Indiana University School of Medicine Mark Payne, MD Executive Director of the Mitochondrial Medicine Frontier Program at The Children’s Hospital of Philadelphia (CHOP) Professor in the Division of Human Genetics, Department of Pediatrics at University of Pennsylvania Perelman School of Medicine Marni J. Falk, MD Medical Director and Division Chief of the University of California San Francisco (UCSF) Movement Disorders and Neuromodulation Center. Carlin and Ellen Wiegner Endowed Professor of Neurology Jill Ostrem, MD Giovanni Manfredi, MD, PhD Finbar and Marianne Kenny Professor in Clinical and Research Neurology at Weill Cornell Medicine. Professor of Neuroscience at Weill Cornell Medicine.

Company has strong relationship with Friedreich’s Ataxia Research Alliance (FARA) National, non-profit organization dedicated to the pursuit of scientific research leading to treatments and a cure for FA FARA provides industry with several key items Assistance with patient recruitment and education Access to Global Patient Registry with demographic and clinical information on more than 1,000 FA patients Sponsored a Patient-Focused Drug Development Meeting in 2017 resulting in a publication titled “The Voice of the Patient” Strong Relationship with FARA

Additional Phase 1 Data
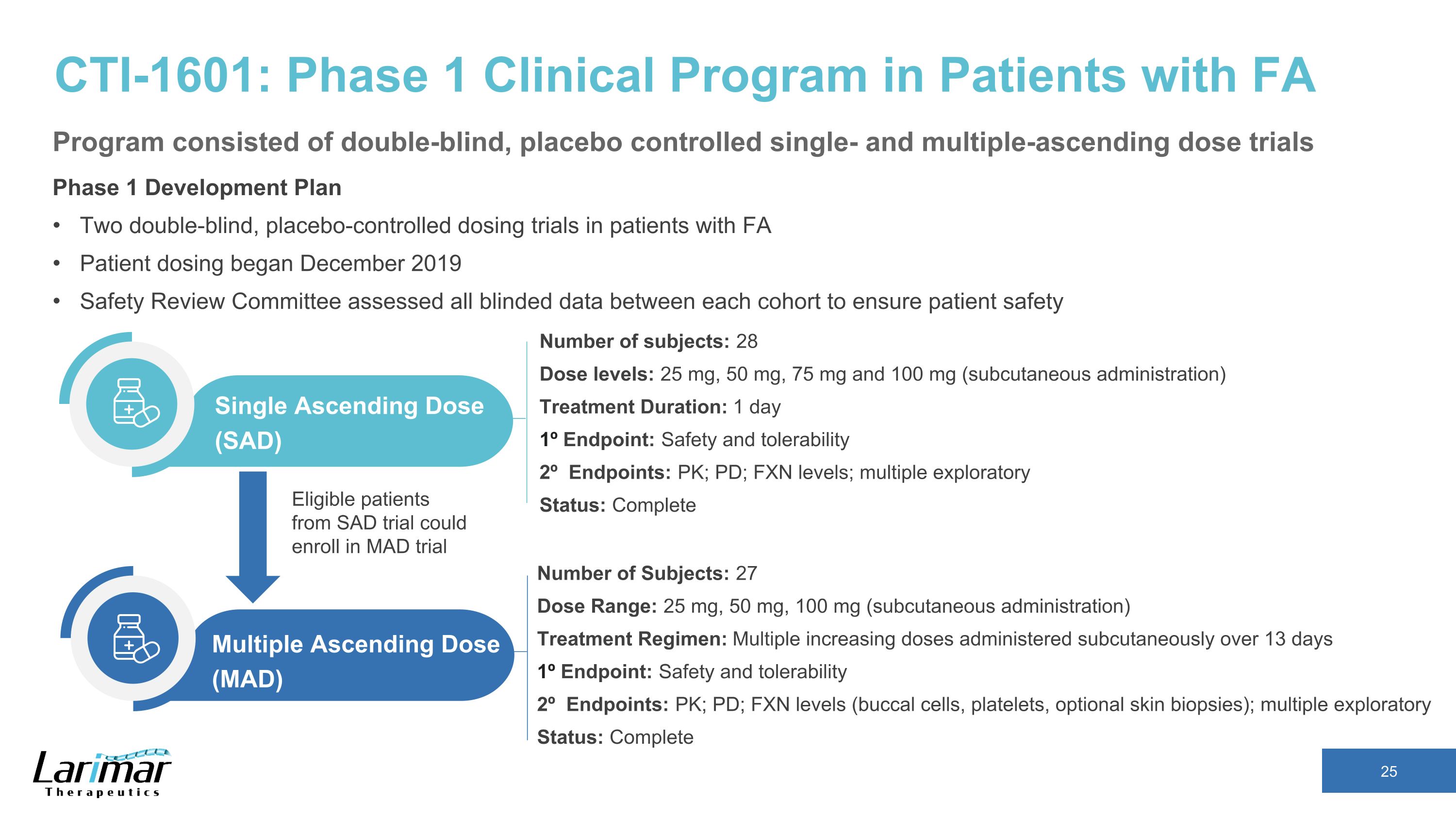
CTI-1601: Phase 1 Clinical Program in Patients with FA Phase 1 Development Plan Two double-blind, placebo-controlled dosing trials in patients with FA Patient dosing began December 2019 Safety Review Committee assessed all blinded data between each cohort to ensure patient safety Number of subjects: 28 Dose levels: 25 mg, 50 mg, 75 mg and 100 mg (subcutaneous administration) Treatment Duration: 1 day 1º Endpoint: Safety and tolerability 2º Endpoints: PK; PD; FXN levels; multiple exploratory Status: Complete Single Ascending Dose (SAD) Number of Subjects: 27 Dose Range: 25 mg, 50 mg, 100 mg (subcutaneous administration) Treatment Regimen: Multiple increasing doses administered subcutaneously over 13 days 1º Endpoint: Safety and tolerability 2º Endpoints: PK; PD; FXN levels (buccal cells, platelets, optional skin biopsies); multiple exploratory Status: Complete Multiple Ascending Dose (MAD) Eligible patients from SAD trial could enroll in MAD trial Program consisted of double-blind, placebo controlled single- and multiple-ascending dose trials
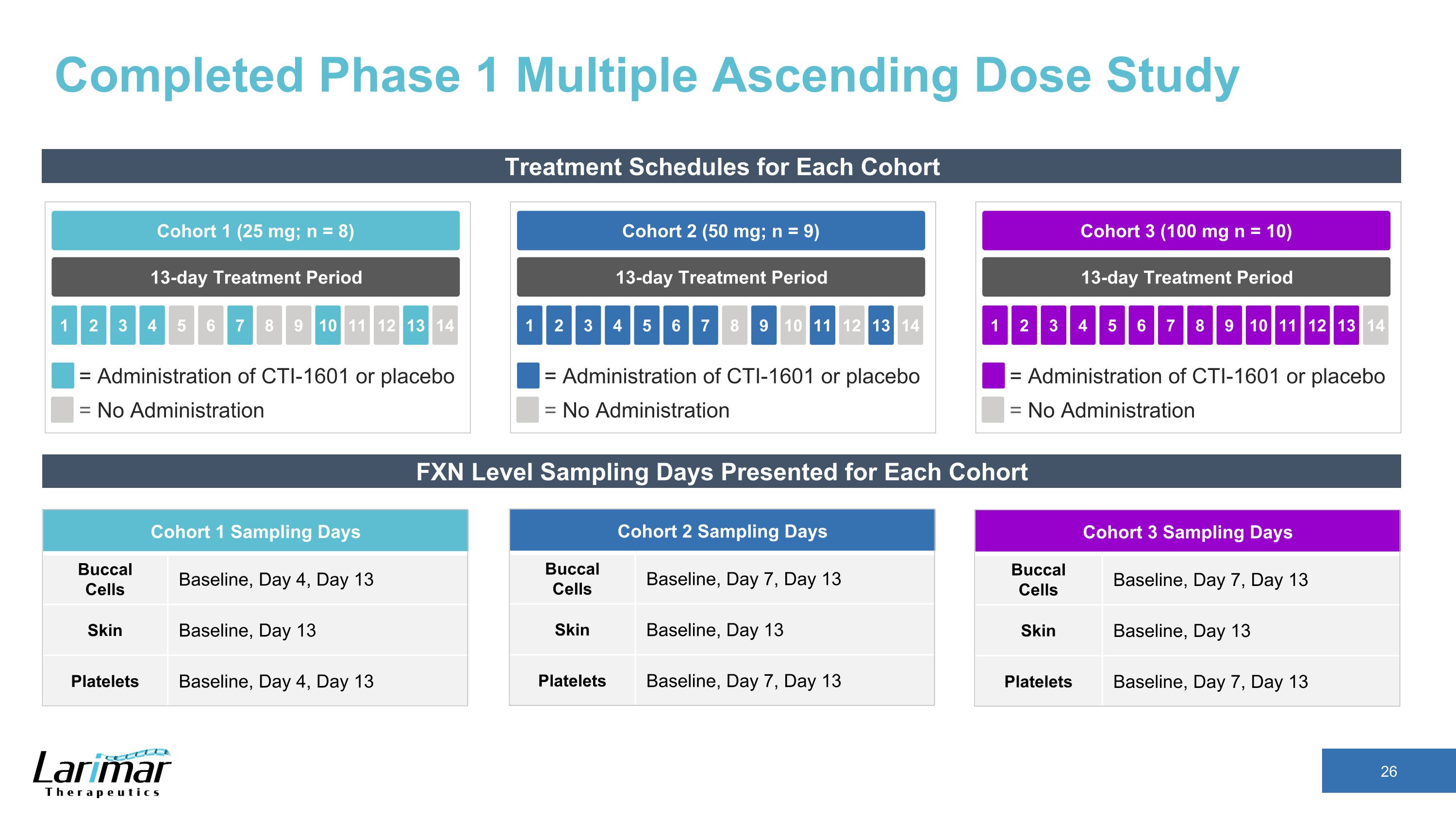
Completed Phase 1 Multiple Ascending Dose Study Treatment Schedules for Each Cohort 13-day Treatment Period Cohort 2 (50 mg; n = 9) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration 13-day Treatment Period Cohort 1 (25 mg; n = 8) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration 13-day Treatment Period Cohort 3 (100 mg n = 10) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration FXN Level Sampling Days Presented for Each Cohort Cohort 1 Sampling Days Buccal Cells Baseline, Day 4, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 4, Day 13 Cohort 2 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13 Cohort 3 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13
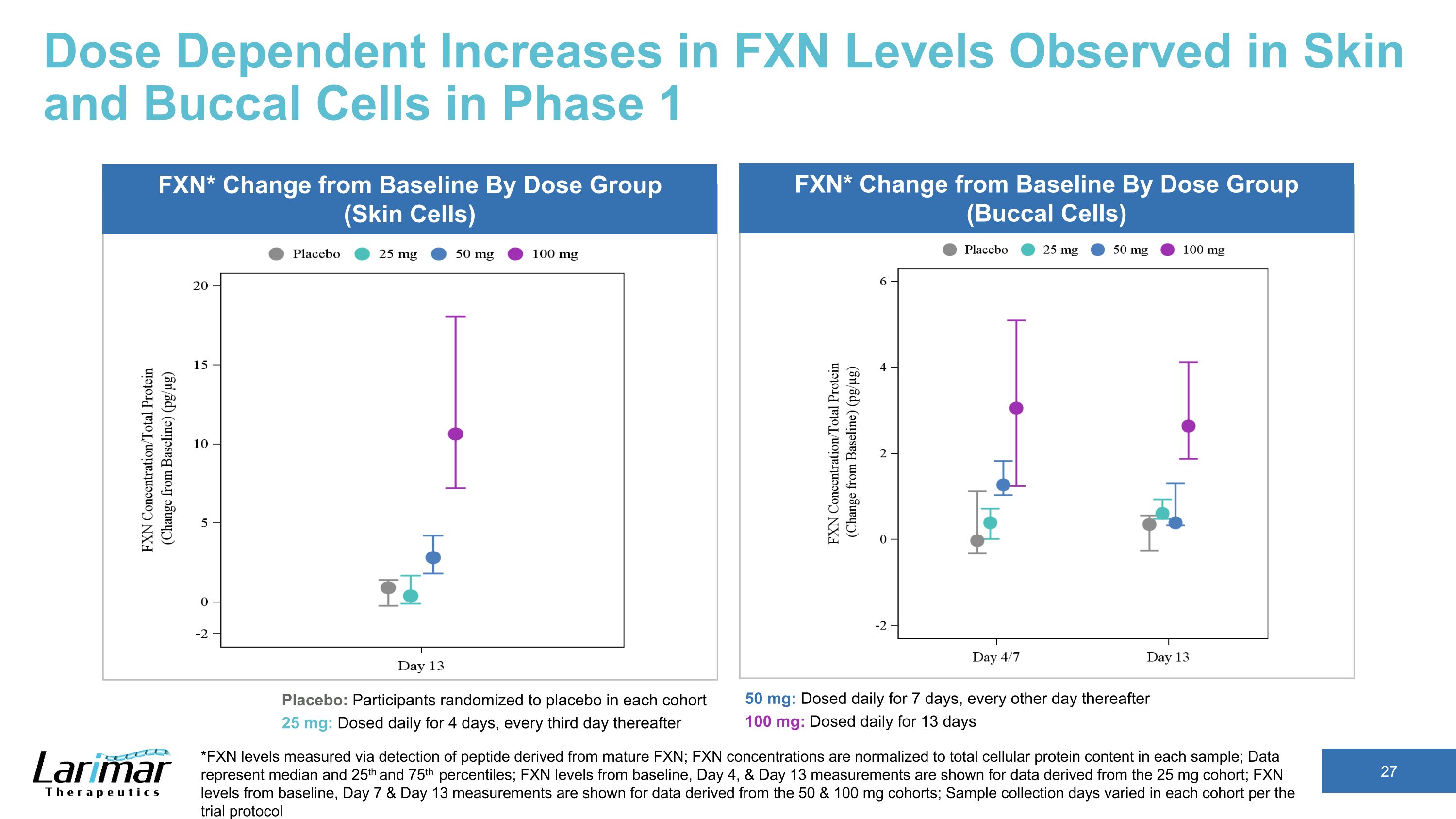
Dose Dependent Increases in FXN Levels Observed in Skin and Buccal Cells in Phase 1 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data represent median and 25th and 75th percentiles; FXN levels from baseline, Day 4, & Day 13 measurements are shown for data derived from the 25 mg cohort; FXN levels from baseline, Day 7 & Day 13 measurements are shown for data derived from the 50 & 100 mg cohorts; Sample collection days varied in each cohort per the trial protocol Placebo: Participants randomized to placebo in each cohort 25 mg: Dosed daily for 4 days, every third day thereafter 50 mg: Dosed daily for 7 days, every other day thereafter 100 mg: Dosed daily for 13 days FXN* Change from Baseline By Dose Group (Skin Cells) FXN* Change from Baseline By Dose Group (Buccal Cells)
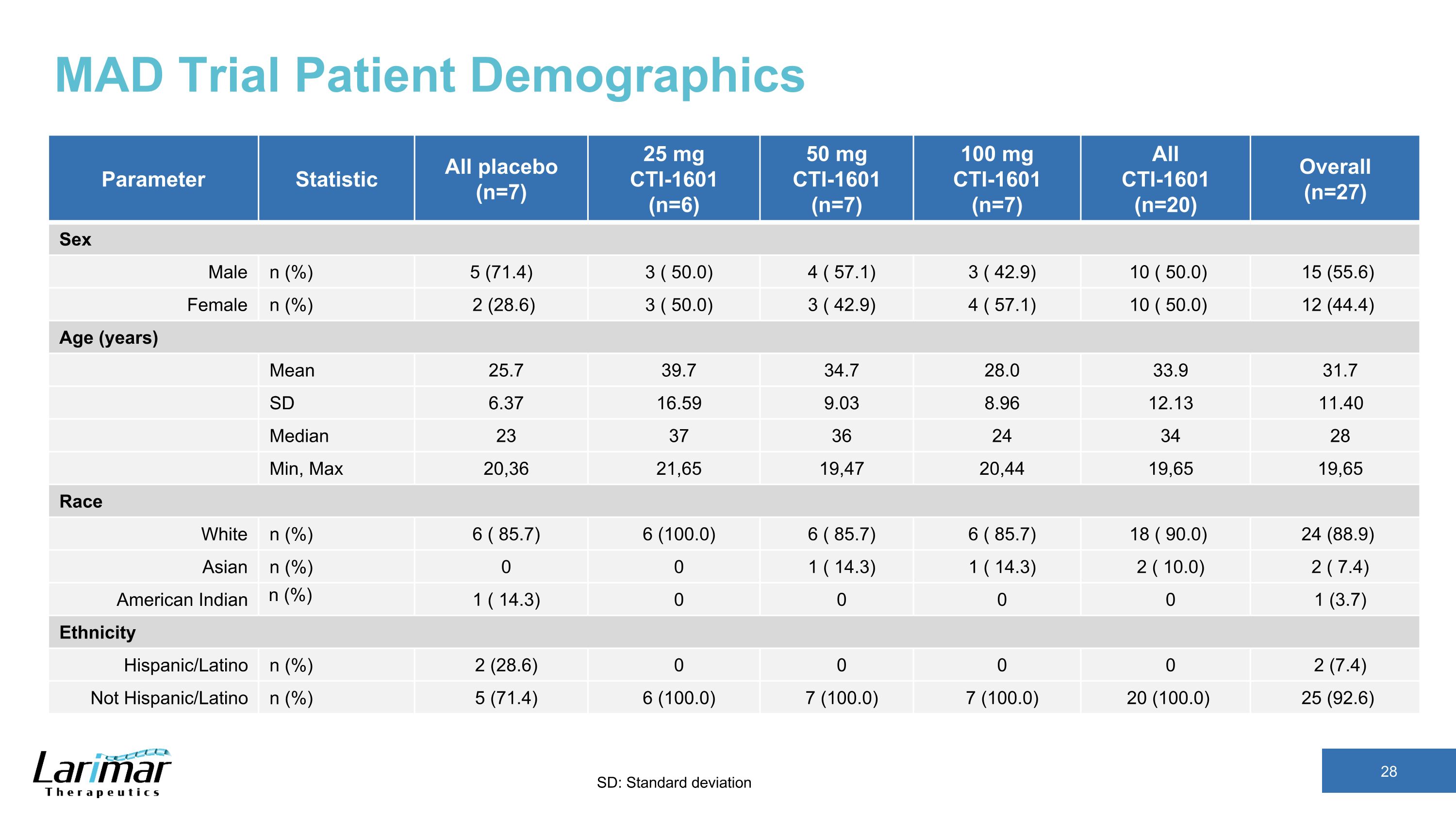
MAD Trial Patient Demographics Parameter Statistic All placebo (n=7) 25 mg CTI-1601 (n=6) 50 mg CTI-1601 (n=7) 100 mg CTI-1601 (n=7) All CTI-1601 (n=20) Overall (n=27) Sex Male n (%) 5 (71.4) 3 ( 50.0) 4 ( 57.1) 3 ( 42.9) 10 ( 50.0) 15 (55.6) Female n (%) 2 (28.6) 3 ( 50.0) 3 ( 42.9) 4 ( 57.1) 10 ( 50.0) 12 (44.4) Age (years) Mean 25.7 39.7 34.7 28.0 33.9 31.7 SD 6.37 16.59 9.03 8.96 12.13 11.40 Median 23 37 36 24 34 28 Min, Max 20,36 21,65 19,47 20,44 19,65 19,65 Race White n (%) 6 ( 85.7) 6 (100.0) 6 ( 85.7) 6 ( 85.7) 18 ( 90.0) 24 (88.9) Asian n (%) 0 0 1 ( 14.3) 1 ( 14.3) 2 ( 10.0) 2 ( 7.4) American Indian n (%) 1 ( 14.3) 0 0 0 0 1 (3.7) Ethnicity Hispanic/Latino n (%) 2 (28.6) 0 0 0 0 2 (7.4) Not Hispanic/Latino n (%) 5 (71.4) 6 (100.0) 7 (100.0) 7 (100.0) 20 (100.0) 25 (92.6) SD: Standard deviation
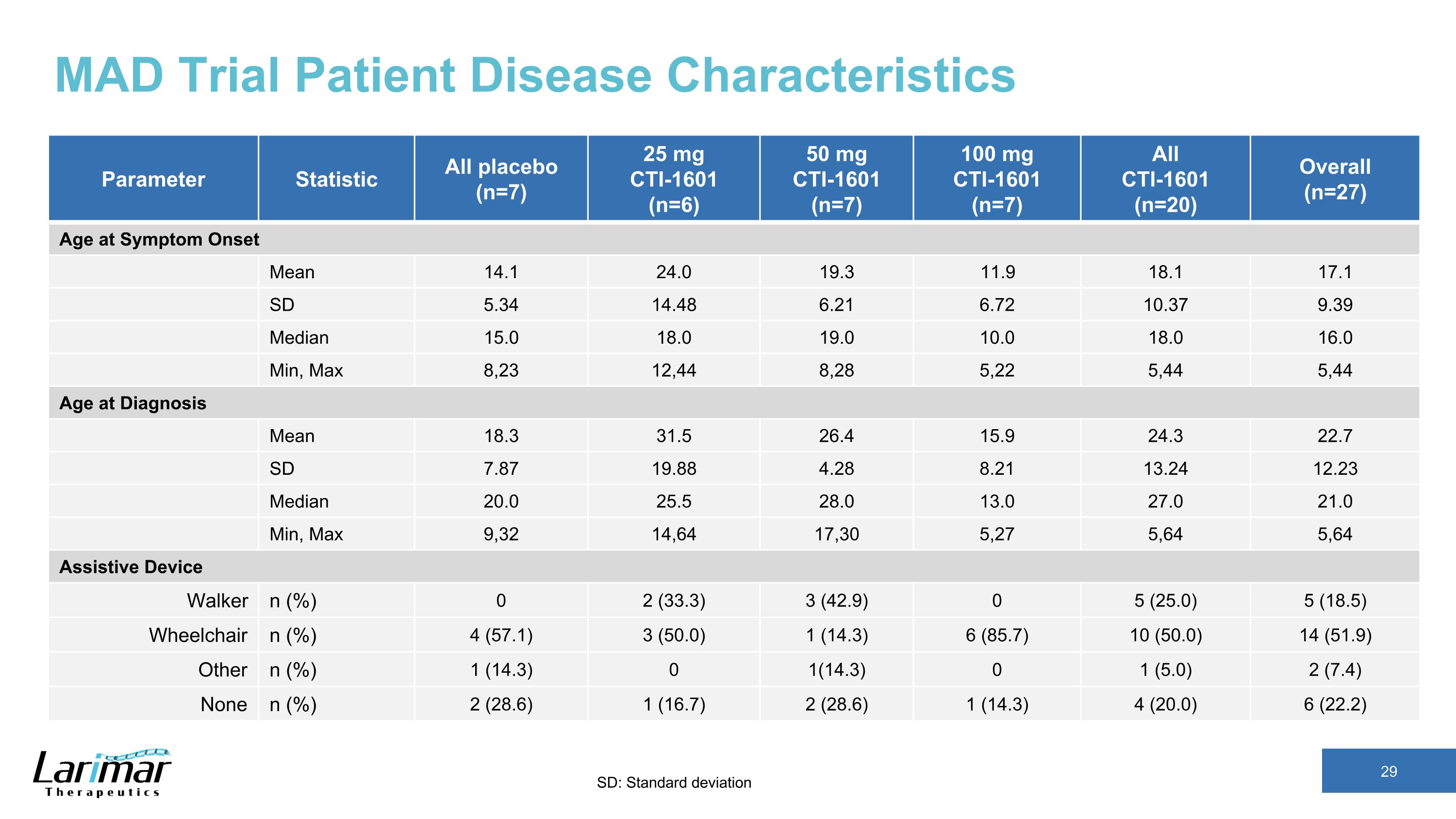
MAD Trial Patient Disease Characteristics Parameter Statistic All placebo (n=7) 25 mg CTI-1601 (n=6) 50 mg CTI-1601 (n=7) 100 mg CTI-1601 (n=7) All CTI-1601 (n=20) Overall (n=27) Age at Symptom Onset Mean 14.1 24.0 19.3 11.9 18.1 17.1 SD 5.34 14.48 6.21 6.72 10.37 9.39 Median 15.0 18.0 19.0 10.0 18.0 16.0 Min, Max 8,23 12,44 8,28 5,22 5,44 5,44 Age at Diagnosis Mean 18.3 31.5 26.4 15.9 24.3 22.7 SD 7.87 19.88 4.28 8.21 13.24 12.23 Median 20.0 25.5 28.0 13.0 27.0 21.0 Min, Max 9,32 14,64 17,30 5,27 5,64 5,64 Assistive Device Walker n (%) 0 2 (33.3) 3 (42.9) 0 5 (25.0) 5 (18.5) Wheelchair n (%) 4 (57.1) 3 (50.0) 1 (14.3) 6 (85.7) 10 (50.0) 14 (51.9) Other n (%) 1 (14.3) 0 1(14.3) 0 1 (5.0) 2 (7.4) None n (%) 2 (28.6) 1 (16.7) 2 (28.6) 1 (14.3) 4 (20.0) 6 (22.2) SD: Standard deviation
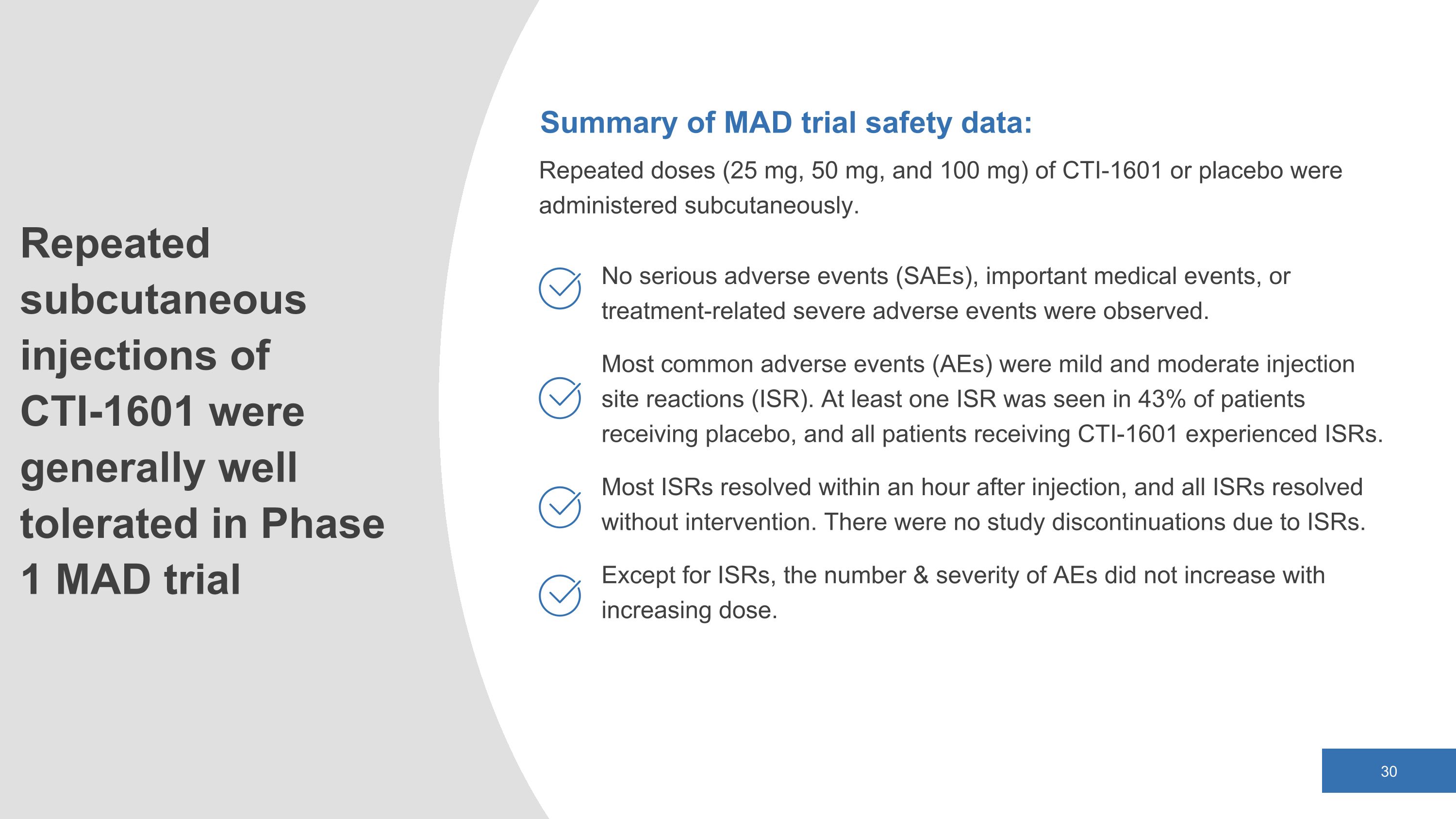
Repeated subcutaneous injections of CTI-1601 were generally well tolerated in Phase 1 MAD trial Summary of MAD trial safety data: Repeated doses (25 mg, 50 mg, and 100 mg) of CTI-1601 or placebo were administered subcutaneously. No serious adverse events (SAEs), important medical events, or treatment-related severe adverse events were observed. Most common adverse events (AEs) were mild and moderate injection site reactions (ISR). At least one ISR was seen in 43% of patients receiving placebo, and all patients receiving CTI-1601 experienced ISRs. Most ISRs resolved within an hour after injection, and all ISRs resolved without intervention. There were no study discontinuations due to ISRs. Except for ISRs, the number & severity of AEs did not increase with increasing dose.
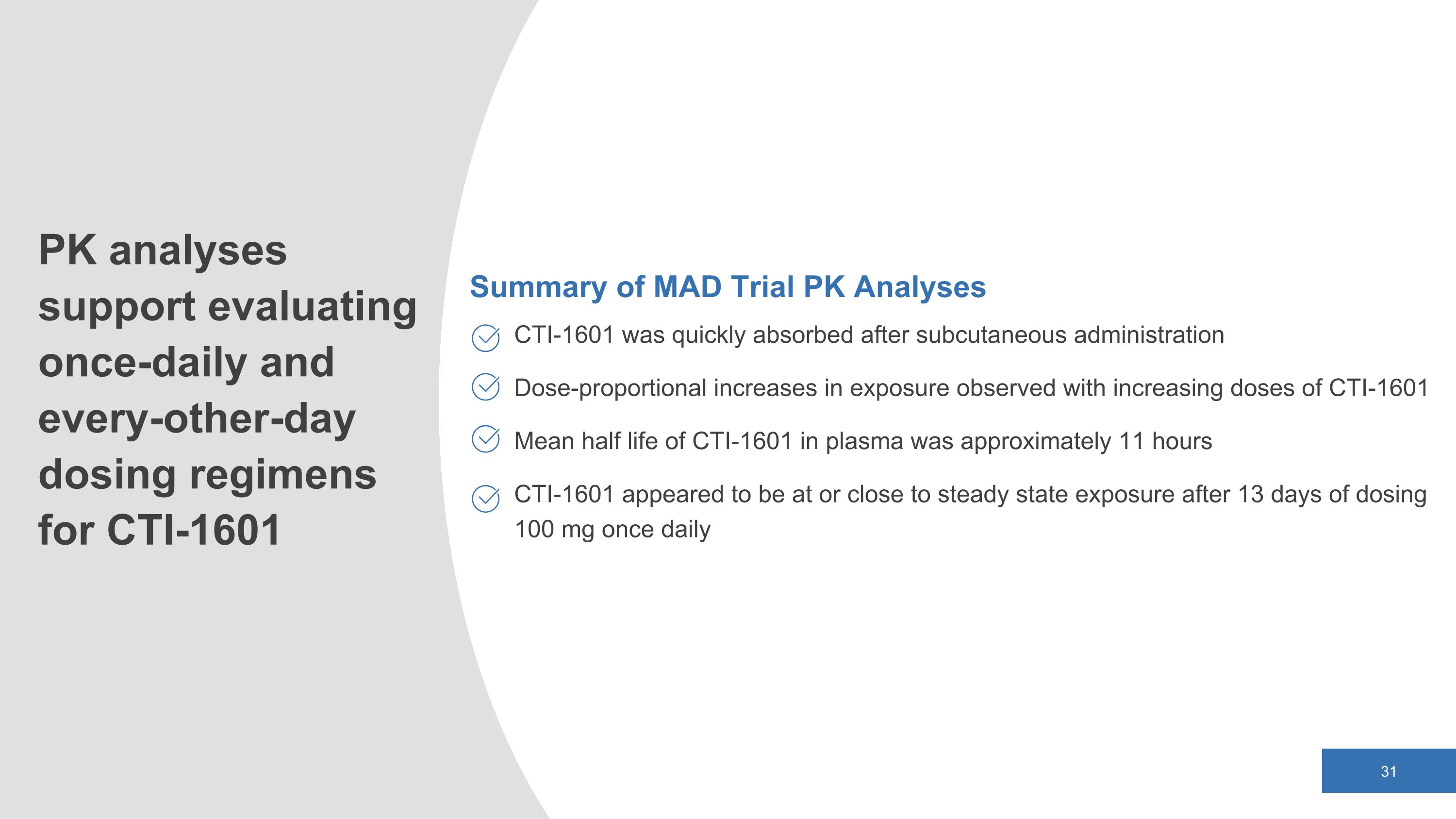
Summary of MAD Trial PK Analyses CTI-1601 was quickly absorbed after subcutaneous administration Dose-proportional increases in exposure observed with increasing doses of CTI-1601 Mean half life of CTI-1601 in plasma was approximately 11 hours CTI-1601 appeared to be at or close to steady state exposure after 13 days of dosing 100 mg once daily PK analyses support evaluating once-daily and every-other-day dosing regimens for CTI-1601

Additional Phase 2 Cohort 1 Data
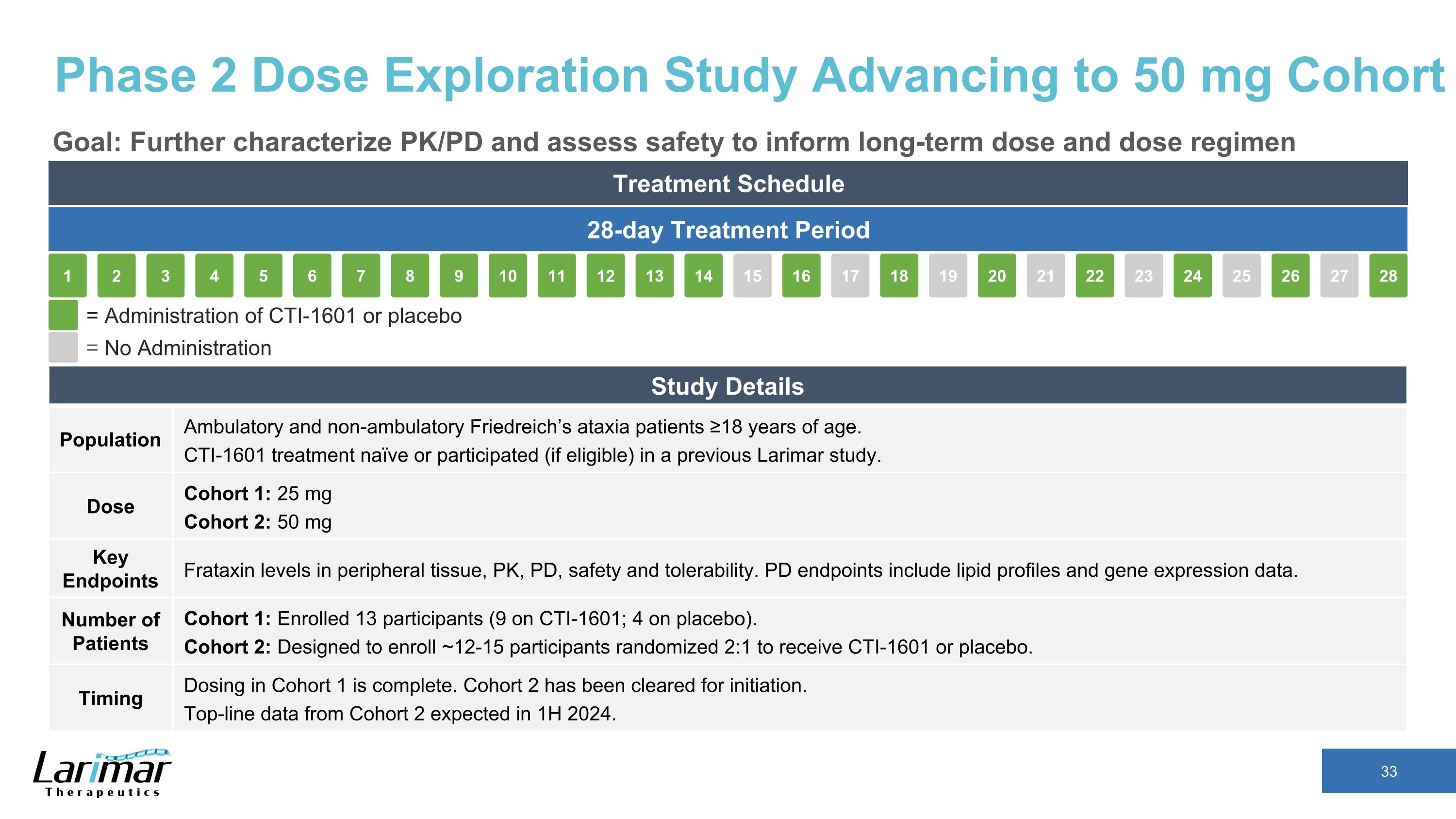
Phase 2 Dose Exploration Study Advancing to 50 mg Cohort Goal: Further characterize PK/PD and assess safety to inform long-term dose and dose regimen Treatment Schedule 28-day Treatment Period 16 17 18 19 15 20 21 22 23 24 25 26 27 28 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration Study Details Population Ambulatory and non-ambulatory Friedreich’s ataxia patients ≥18 years of age. CTI-1601 treatment naïve or participated (if eligible) in a previous Larimar study. Dose Cohort 1: 25 mg Cohort 2: 50 mg Key Endpoints Frataxin levels in peripheral tissue, PK, PD, safety and tolerability. PD endpoints include lipid profiles and gene expression data. Number of Patients Cohort 1: Enrolled 13 participants (9 on CTI-1601; 4 on placebo). Cohort 2: Designed to enroll ~12-15 participants randomized 2:1 to receive CTI-1601 or placebo. Timing Dosing in Cohort 1 is complete. Cohort 2 has been cleared for initiation. Top-line data from Cohort 2 expected in 1H 2024.
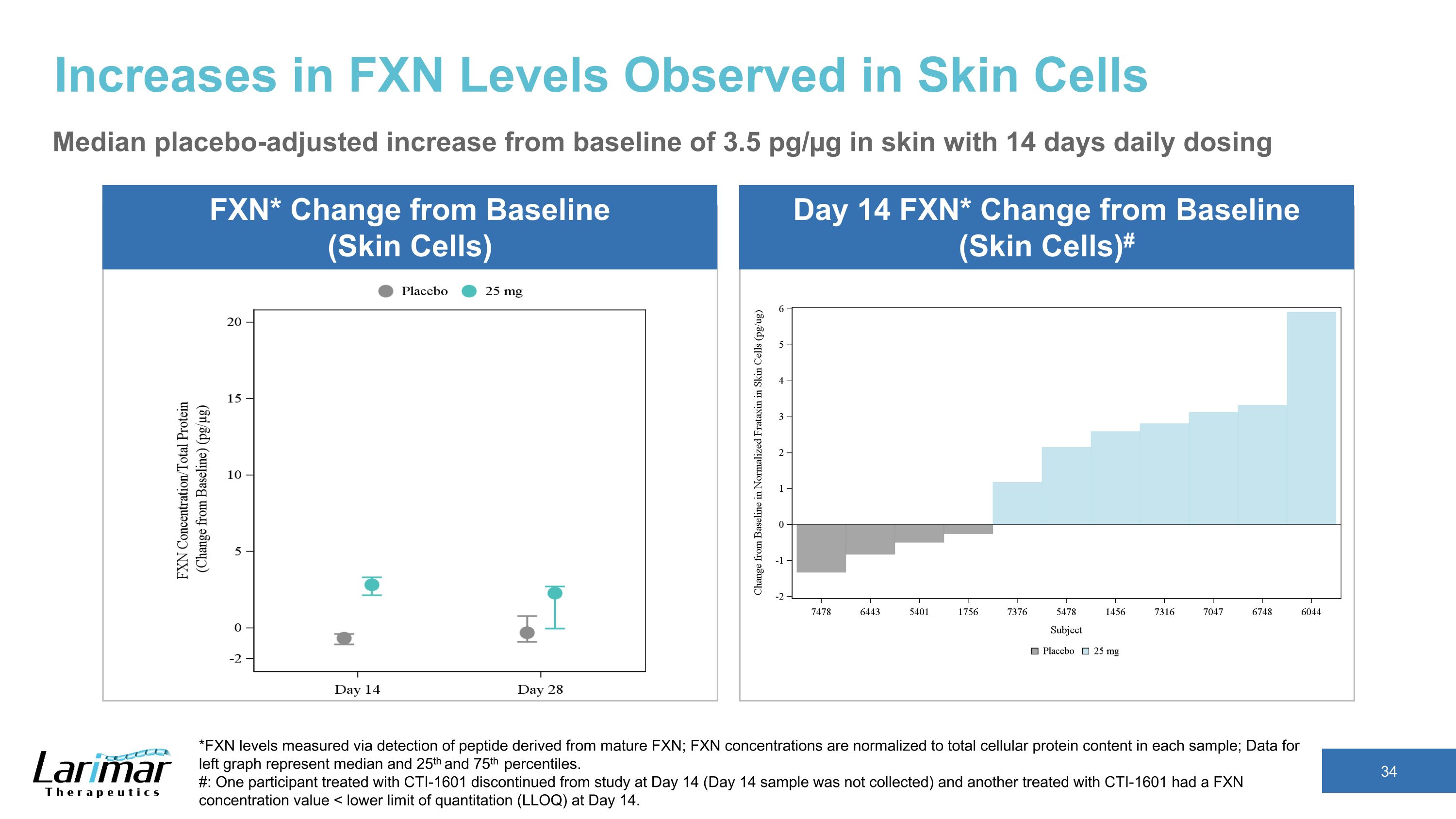
Increases in FXN Levels Observed in Skin Cells Median placebo-adjusted increase from baseline of 3.5 pg/µg in skin with 14 days daily dosing *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data for left graph represent median and 25th and 75th percentiles. #: One participant treated with CTI-1601 discontinued from study at Day 14 (Day 14 sample was not collected) and another treated with CTI-1601 had a FXN concentration value < lower limit of quantitation (LLOQ) at Day 14. FXN* Change from Baseline (Skin Cells) Day 14 FXN* Change from Baseline (Skin Cells)#
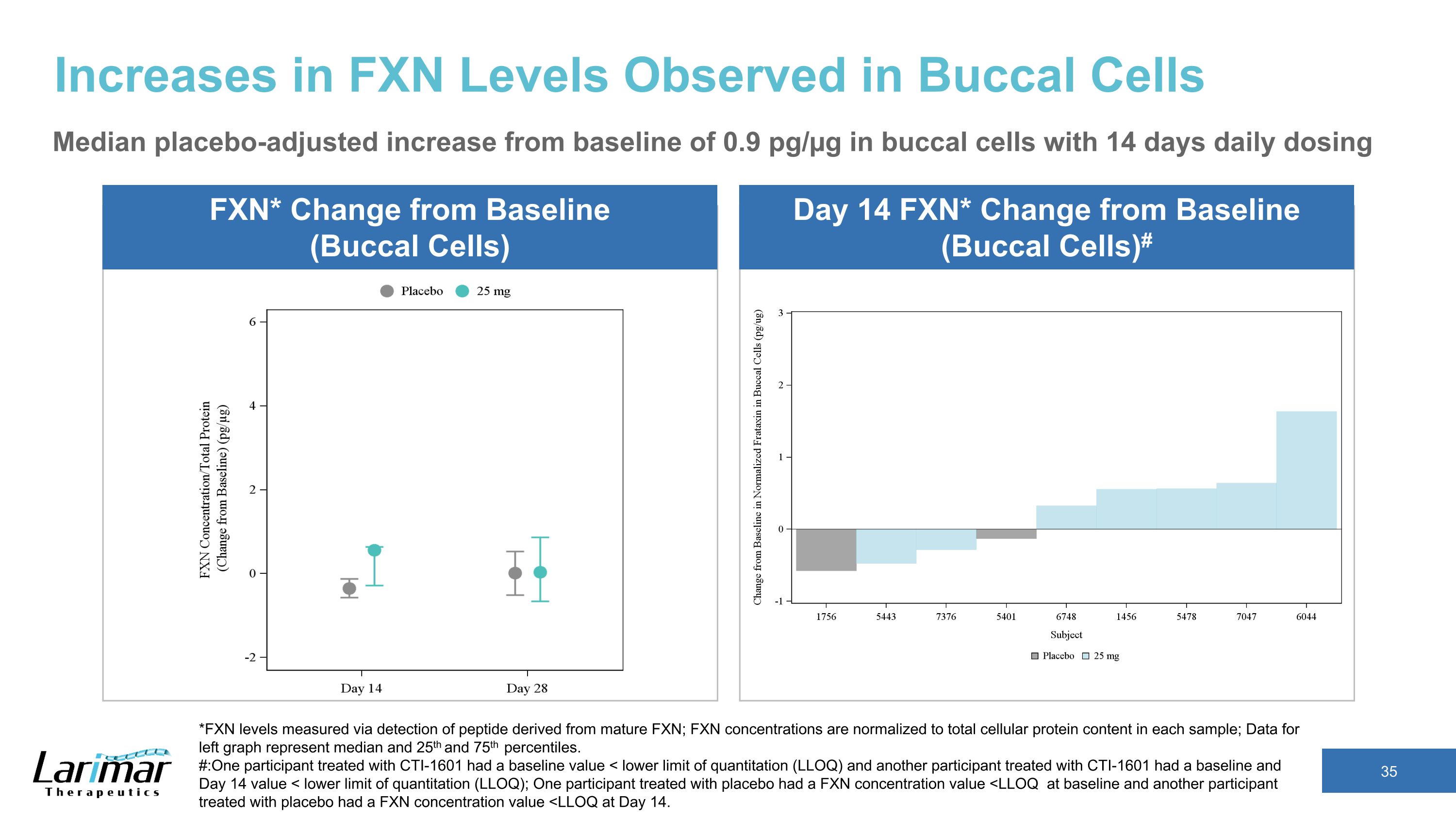
Increases in FXN Levels Observed in Buccal Cells Median placebo-adjusted increase from baseline of 0.9 pg/µg in buccal cells with 14 days daily dosing *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data for left graph represent median and 25th and 75th percentiles. #:One participant treated with CTI-1601 had a baseline value < lower limit of quantitation (LLOQ) and another participant treated with CTI-1601 had a baseline and Day 14 value < lower limit of quantitation (LLOQ); One participant treated with placebo had a FXN concentration value <LLOQ at baseline and another participant treated with placebo had a FXN concentration value <LLOQ at Day 14. FXN* Change from Baseline (Buccal Cells) Day 14 FXN* Change from Baseline (Buccal Cells)#
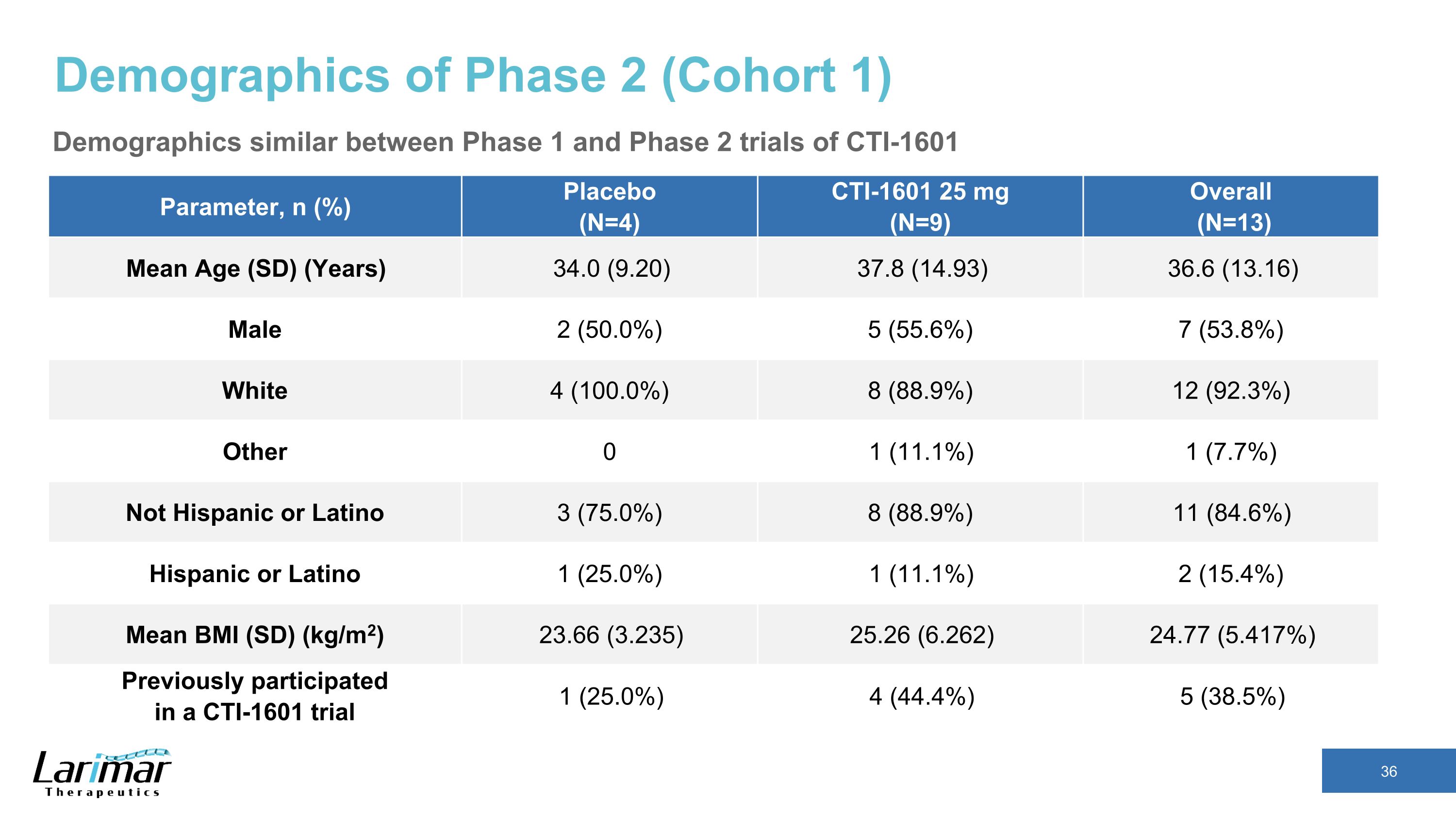
Demographics of Phase 2 (Cohort 1) Demographics similar between Phase 1 and Phase 2 trials of CTI-1601 Parameter, n (%) Placebo �(N=4) CTI-1601 25 mg �(N=9) Overall� (N=13) Mean Age (SD) (Years) 34.0 (9.20) 37.8 (14.93) 36.6 (13.16) Male 2 (50.0%) 5 (55.6%) 7 (53.8%) White 4 (100.0%) 8 (88.9%) 12 (92.3%) Other 0 1 (11.1%) 1 (7.7%) Not Hispanic or Latino 3 (75.0%) 8 (88.9%) 11 (84.6%) Hispanic or Latino 1 (25.0%) 1 (11.1%) 2 (15.4%) Mean BMI (SD) (kg/m2) 23.66 (3.235) 25.26 (6.262) 24.77 (5.417%) Previously participated �in a CTI-1601 trial 1 (25.0%) 4 (44.4%) 5 (38.5%)
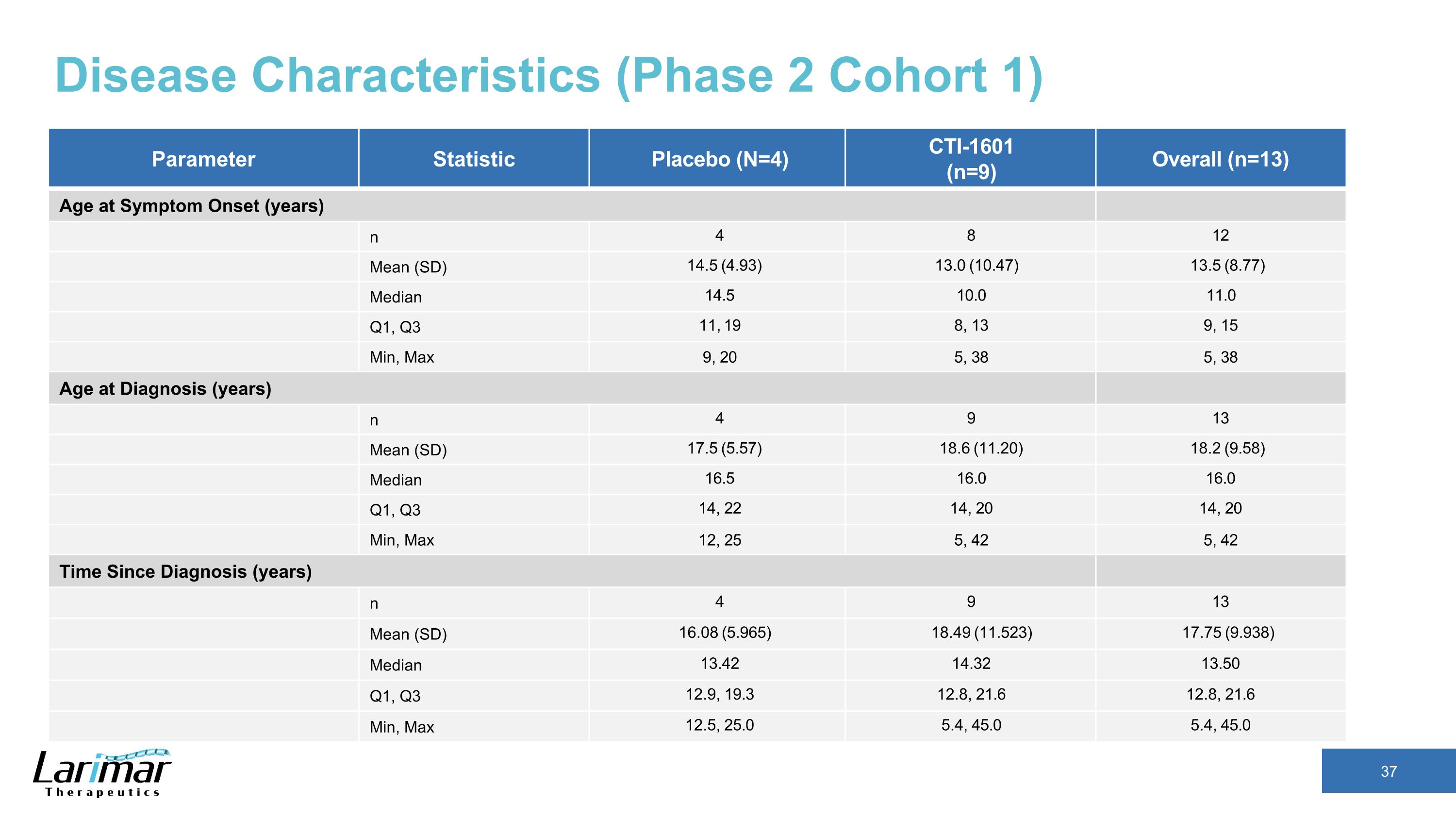
Disease Characteristics (Phase 2 Cohort 1) Parameter Statistic Placebo (N=4) CTI-1601 (n=9) Overall (n=13) Age at Symptom Onset (years) n 4 8 12 Mean (SD) 14.5 (4.93) 13.0 (10.47) 13.5 (8.77) Median 14.5 10.0 11.0 Q1, Q3 11, 19 8, 13 9, 15 Min, Max 9, 20 5, 38 5, 38 Age at Diagnosis (years) n 4 9 13 Mean (SD) 17.5 (5.57) 18.6 (11.20) 18.2 (9.58) Median 16.5 16.0 16.0 Q1, Q3 14, 22 14, 20 14, 20 Min, Max 12, 25 5, 42 5, 42 Time Since Diagnosis (years) n 4 9 13 Mean (SD) 16.08 (5.965) 18.49 (11.523) 17.75 (9.938) Median 13.42 14.32 13.50 Q1, Q3 12.9, 19.3 12.8, 21.6 12.8, 21.6 Min, Max 12.5, 25.0 5.4, 45.0 5.4, 45.0
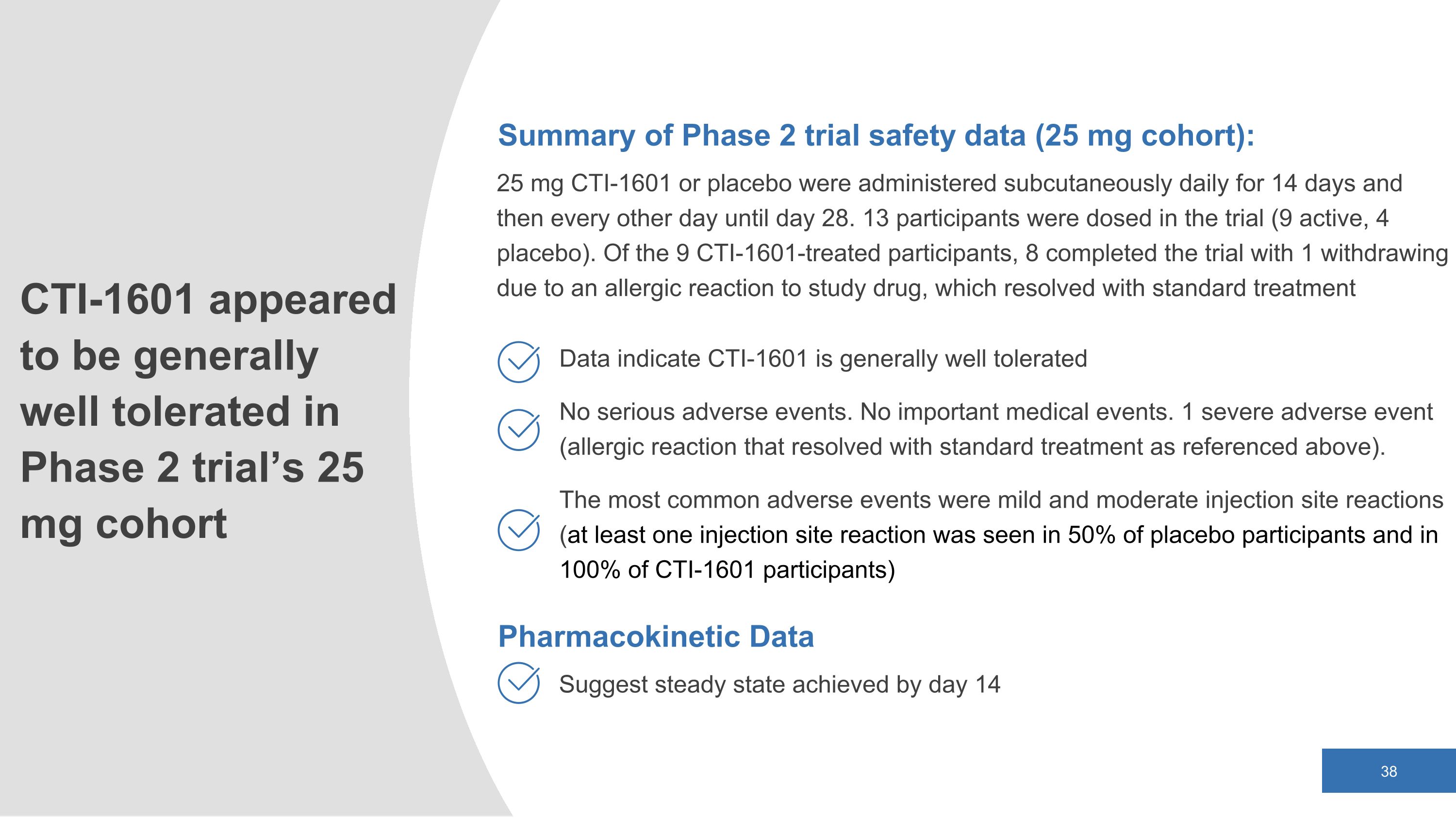
Summary of Phase 2 trial safety data (25 mg cohort): 25 mg CTI-1601 or placebo were administered subcutaneously daily for 14 days and then every other day until day 28. 13 participants were dosed in the trial (9 active, 4 placebo). Of the 9 CTI-1601-treated participants, 8 completed the trial with 1 withdrawing due to an allergic reaction to study drug, which resolved with standard treatment Data indicate CTI-1601 is generally well tolerated No serious adverse events. No important medical events. 1 severe adverse event (allergic reaction that resolved with standard treatment as referenced above). The most common adverse events were mild and moderate injection site reactions (at least one injection site reaction was seen in 50% of placebo participants and in 100% of CTI-1601 participants) CTI-1601 appeared to be generally well tolerated in Phase 2 trial’s 25 mg cohort Pharmacokinetic Data Suggest steady state achieved by day 14

Preclinical Data
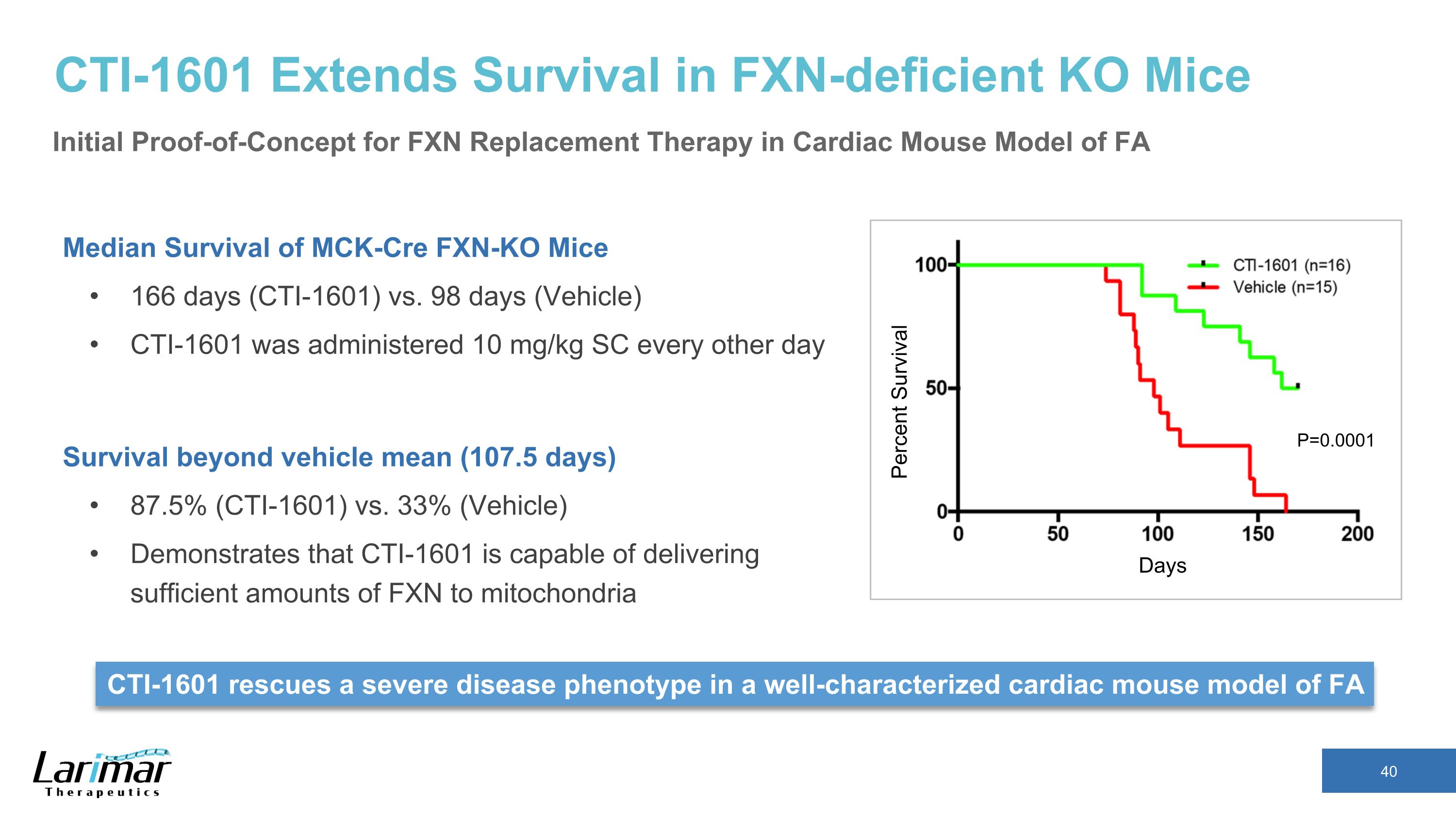
CTI-1601 Extends Survival in FXN-deficient KO Mice Median Survival of MCK-Cre FXN-KO Mice 166 days (CTI-1601) vs. 98 days (Vehicle) CTI-1601 was administered 10 mg/kg SC every other day Survival beyond vehicle mean (107.5 days) 87.5% (CTI-1601) vs. 33% (Vehicle) Demonstrates that CTI-1601 is capable of delivering sufficient amounts of FXN to mitochondria Days Percent Survival CTI-1601 rescues a severe disease phenotype in a well-characterized cardiac mouse model of FA P=0.0001 Initial Proof-of-Concept for FXN Replacement Therapy in Cardiac Mouse Model of FA
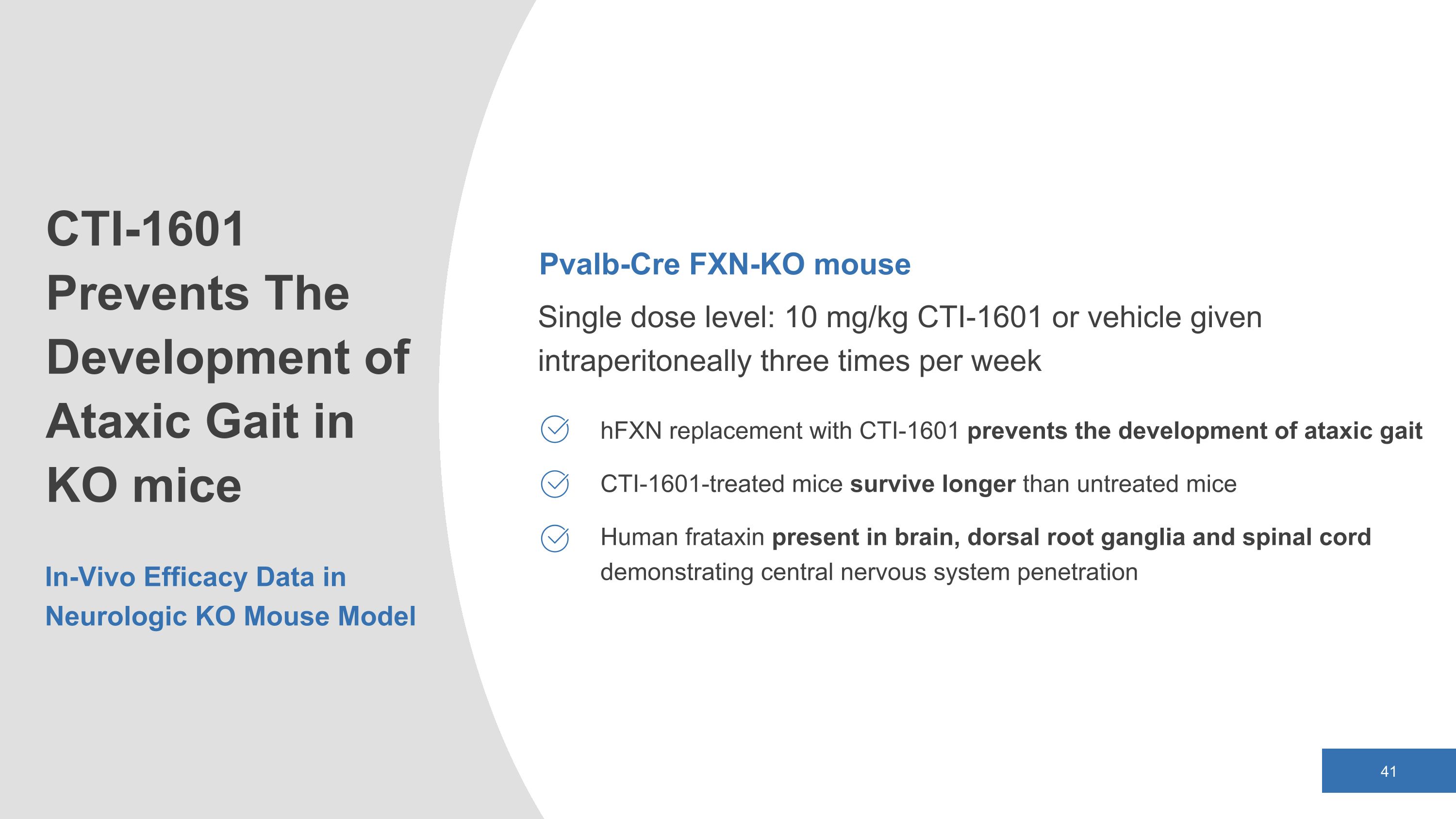
Pvalb-Cre FXN-KO mouse Single dose level: 10 mg/kg CTI-1601 or vehicle given intraperitoneally three times per week hFXN replacement with CTI-1601 prevents the development of ataxic gait CTI-1601-treated mice survive longer than untreated mice Human frataxin present in brain, dorsal root ganglia and spinal cord demonstrating central nervous system penetration CTI-1601 Prevents The Development of Ataxic Gait in KO mice In-Vivo Efficacy Data in Neurologic KO Mouse Model
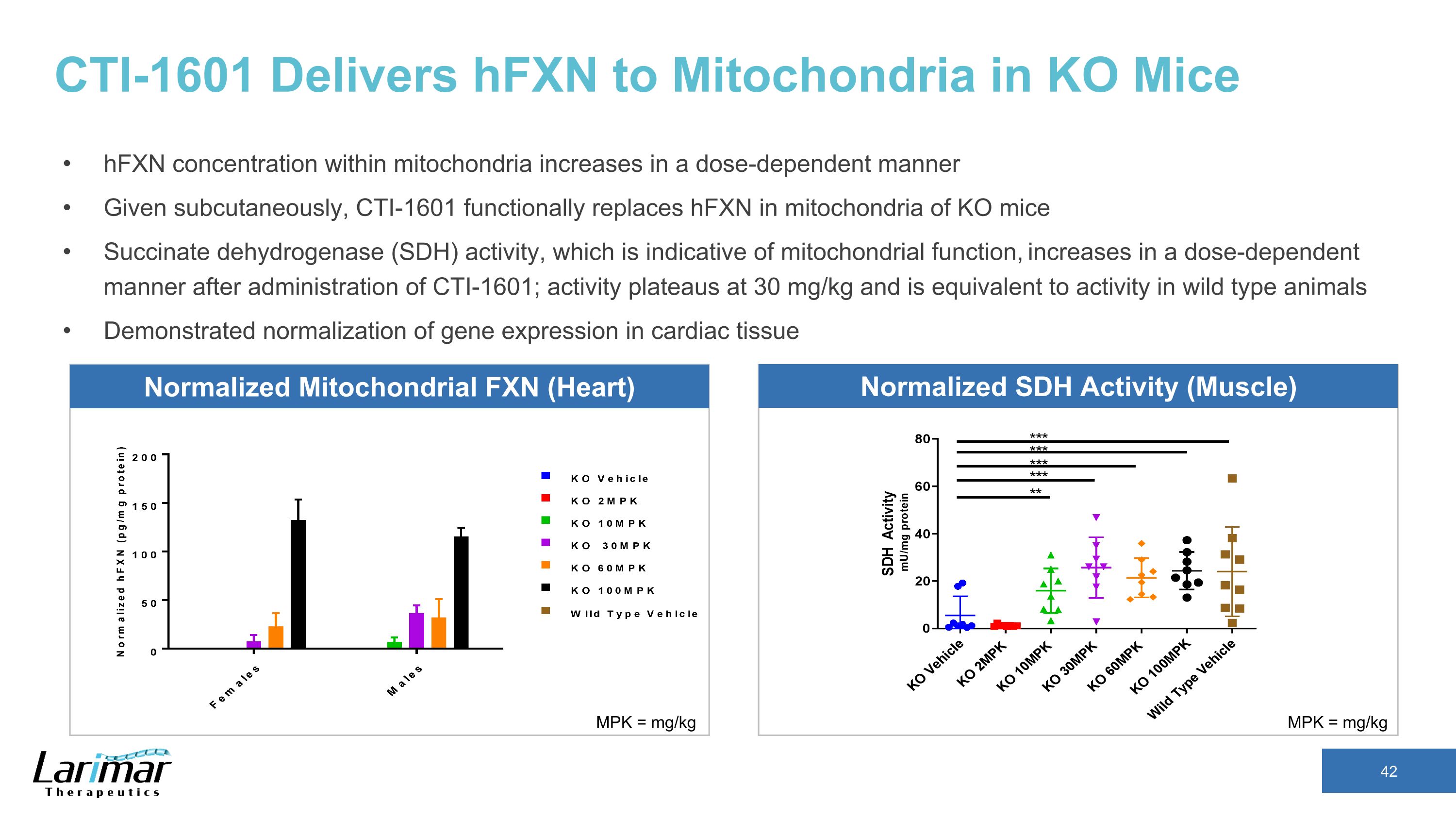
CTI-1601 Delivers hFXN to Mitochondria in KO Mice hFXN concentration within mitochondria increases in a dose-dependent manner Given subcutaneously, CTI-1601 functionally replaces hFXN in mitochondria of KO mice Succinate dehydrogenase (SDH) activity, which is indicative of mitochondrial function, increases in a dose-dependent manner after administration of CTI-1601; activity plateaus at 30 mg/kg and is equivalent to activity in wild type animals Demonstrated normalization of gene expression in cardiac tissue MPK = mg/kg MPK = mg/kg Normalized Mitochondrial FXN (Heart) Normalized SDH Activity (Muscle)
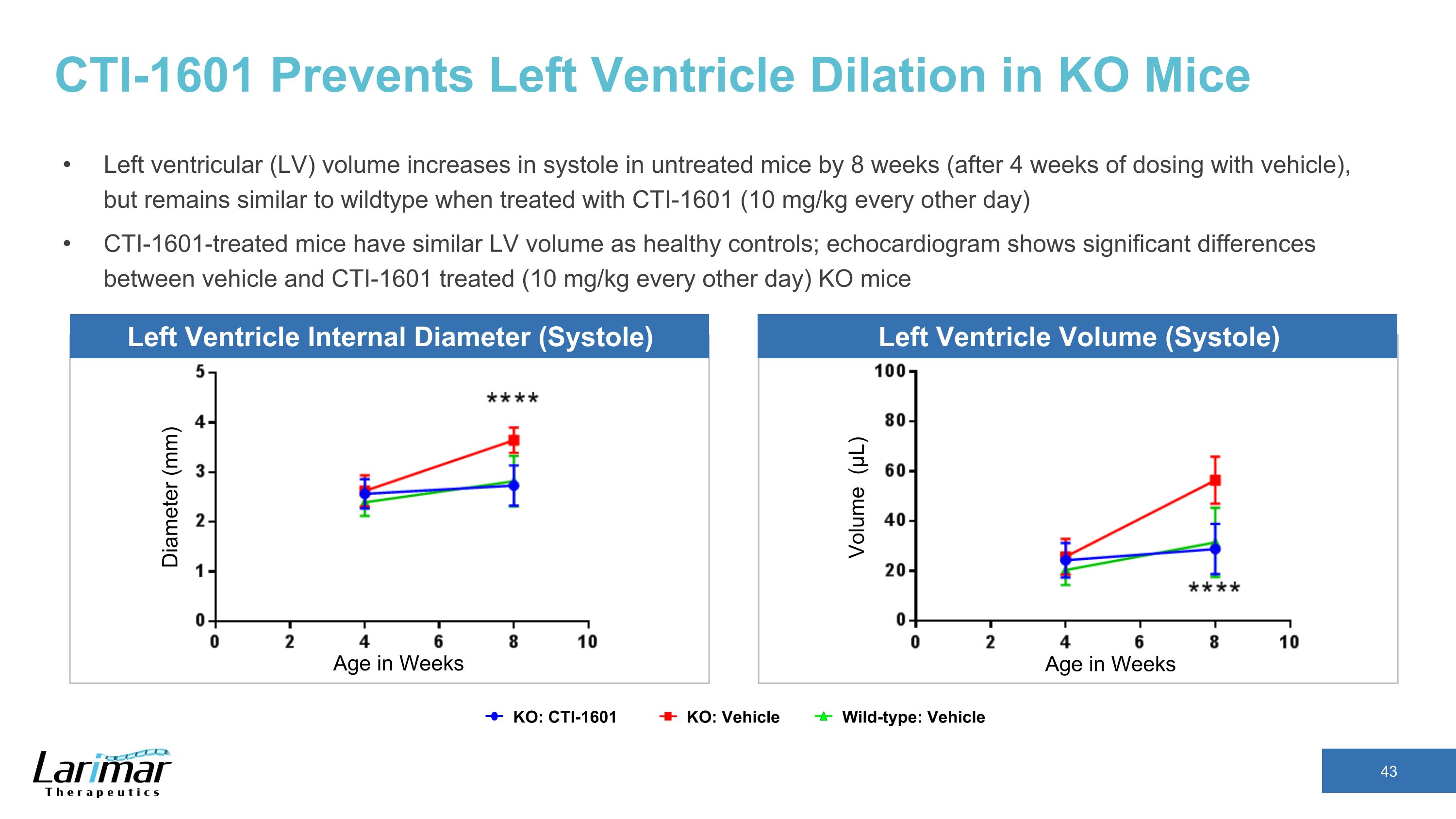
CTI-1601 Prevents Left Ventricle Dilation in KO Mice Left ventricular (LV) volume increases in systole in untreated mice by 8 weeks (after 4 weeks of dosing with vehicle), but remains similar to wildtype when treated with CTI-1601 (10 mg/kg every other day) CTI-1601-treated mice have similar LV volume as healthy controls; echocardiogram shows significant differences between vehicle and CTI-1601 treated (10 mg/kg every other day) KO mice Diameter (mm) Age in Weeks Age in Weeks Volume (μL) KO: CTI-1601 Wild-type: Vehicle KO: Vehicle Left Ventricle Internal Diameter (Systole) Left Ventricle Volume (Systole)
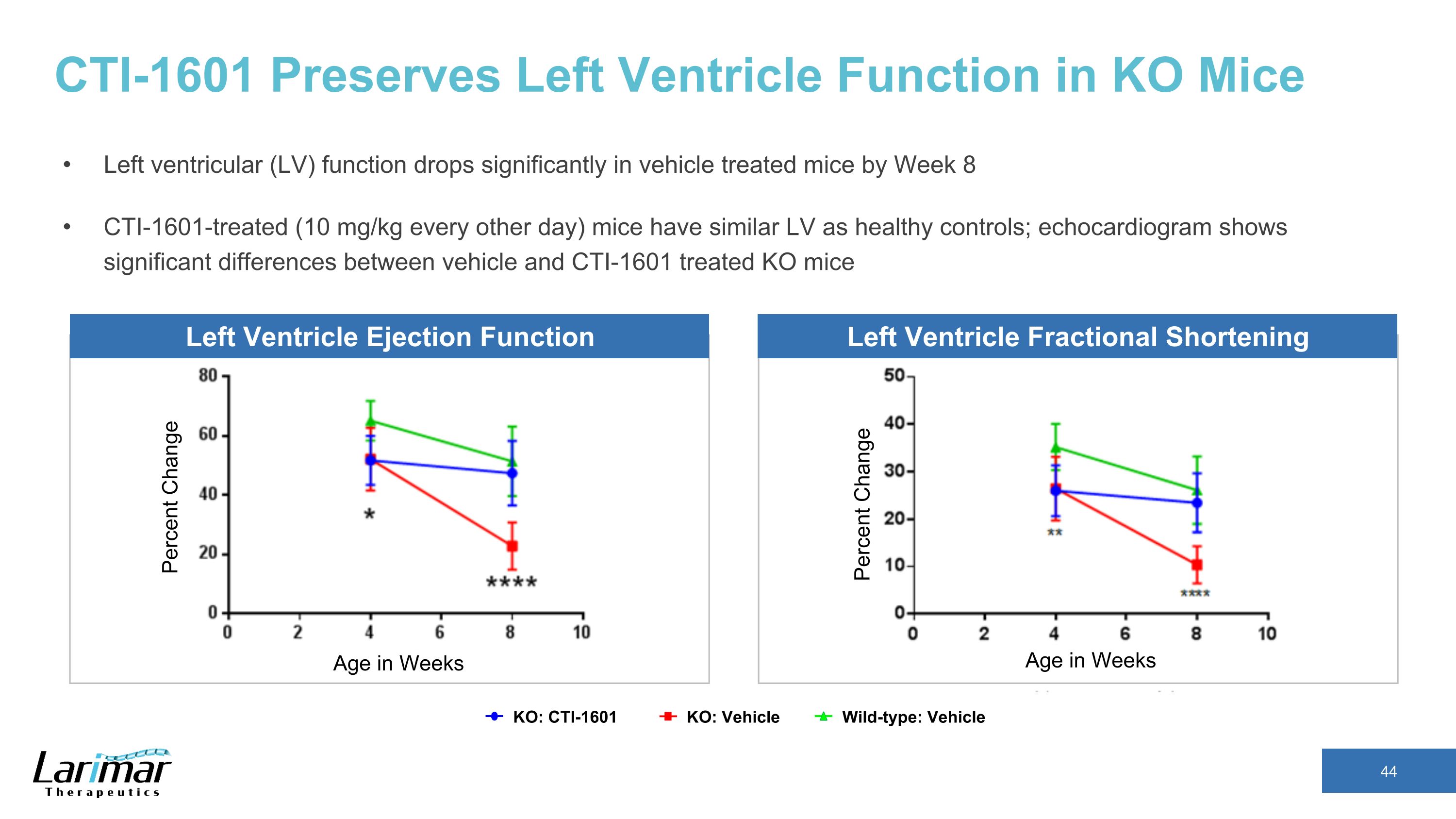
CTI-1601 Preserves Left Ventricle Function in KO Mice Left ventricular (LV) function drops significantly in vehicle treated mice by Week 8 CTI-1601-treated (10 mg/kg every other day) mice have similar LV as healthy controls; echocardiogram shows significant differences between vehicle and CTI-1601 treated KO mice Percent Change Age in Weeks KO: CTI-1601 Wild-type: Vehicle KO: Vehicle Left Ventricle Ejection Function Left Ventricle Fractional Shortening Percent Change Age in Weeks
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Larimar Therapeutics (NASDAQ:LRMR)
Historical Stock Chart
From Apr 2024 to May 2024
Larimar Therapeutics (NASDAQ:LRMR)
Historical Stock Chart
From May 2023 to May 2024