0001553643
false
FL
0001553643
2023-08-10
2023-08-10
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported): August
10, 2023
RELMADA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Nevada |
|
001-39082 |
|
45-5401931 |
(State or other jurisdiction
of incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
2222 Ponce de Leon Blvd., Floor 3
Coral Gables, FL |
|
33134 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (786) 629 1376
| |
| (Former name or former address, if changed since last report) |
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General
Instruction A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each class |
|
Trading Symbol |
|
Name of each exchange on which registered |
| Common stock, $0.001 par value per share |
|
RLMD |
|
The NASDAQ Global Select Market |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On August 10, 2023, the Company updated its corporate
presentation, a copy of which is filed herewith as Exhibit 99.1 and is incorporated herein by reference.
Item 9.01 Financial Statements and
Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: August 10, 2023 |
RELMADA THERAPEUTICS, INC. |
| |
|
|
| |
By: |
/s/ Sergio Traversa |
| |
Name: |
Sergio Traversa |
| |
Title: |
Chief Executive Officer |
2
Exhibit 99.1

Targeting Major Advances in the Treatment of CNS Disorders August 10 th , 2023

©2023 Relmada - All rights reserved Disclosures 2 Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - looking statements.” These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. These statements are based upon the current beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties. Statements regarding future action, future performance and/or future results, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the forward - looking statements. Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate. Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and financial performance. The Company assumes no obligation to update forward - looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports.
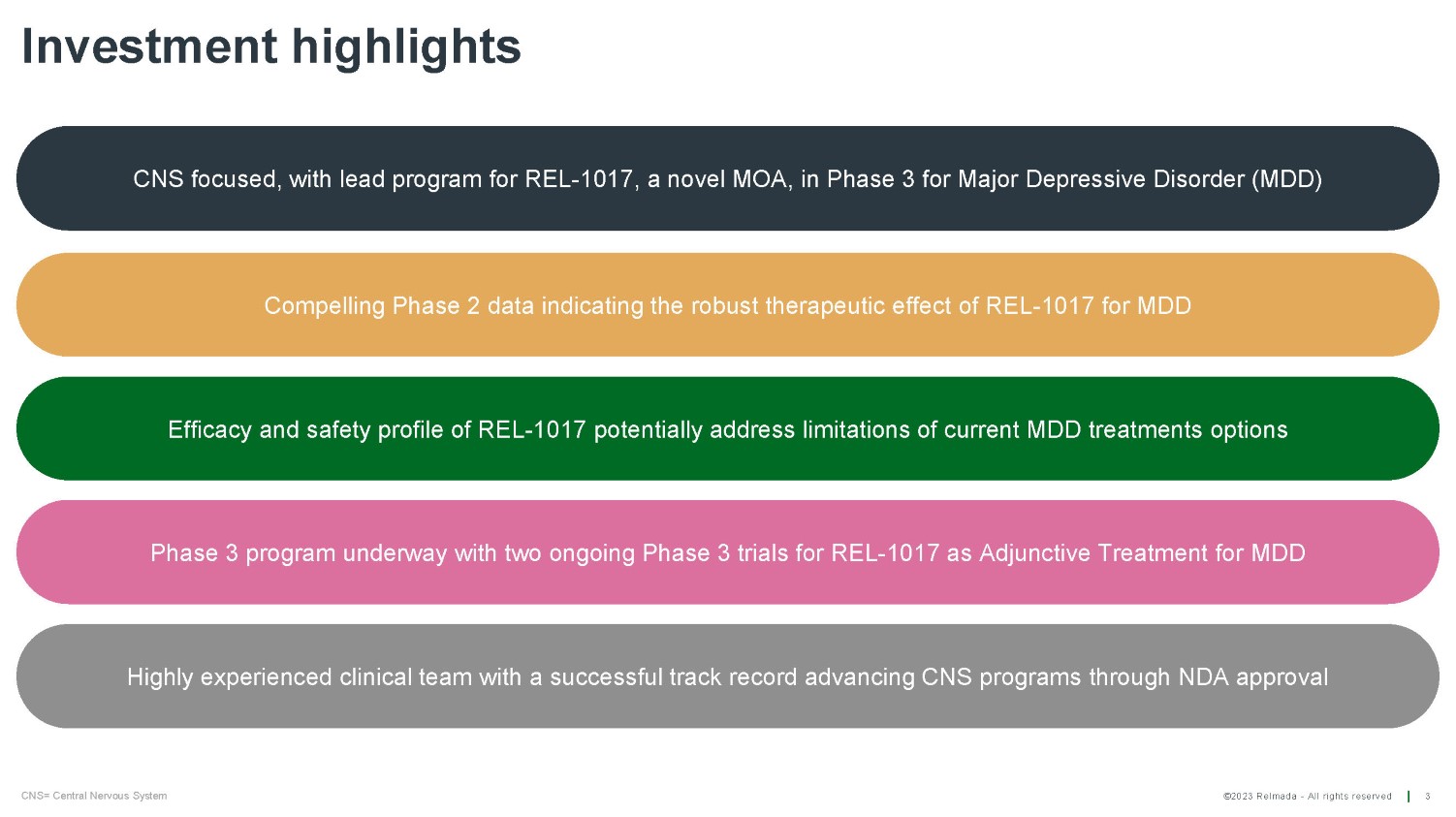
©2023 Relmada - All rights reserved Highly experienced clinical team with a successful track record advancing CNS programs through NDA approval Phase 3 program underway with two ongoing Phase 3 trials for REL - 1017 as Adjunctive Treatment for MDD Efficacy and safety profile of REL - 1017 potentially address limitations of current MDD treatments options Compelling Phase 2 data indicating the robust therapeutic effect of REL - 1017 for MDD Investment highlights 3 CNS= Central Nervous System CNS focused, with lead program for REL - 1017, a novel MOA, in Phase 3 for Major Depressive Disorder (MDD)

©2023 Relmada - All rights reserved 4 The unique profile of esmethadone (REL - 1017) addresses the limitations of current treatment options for MDD
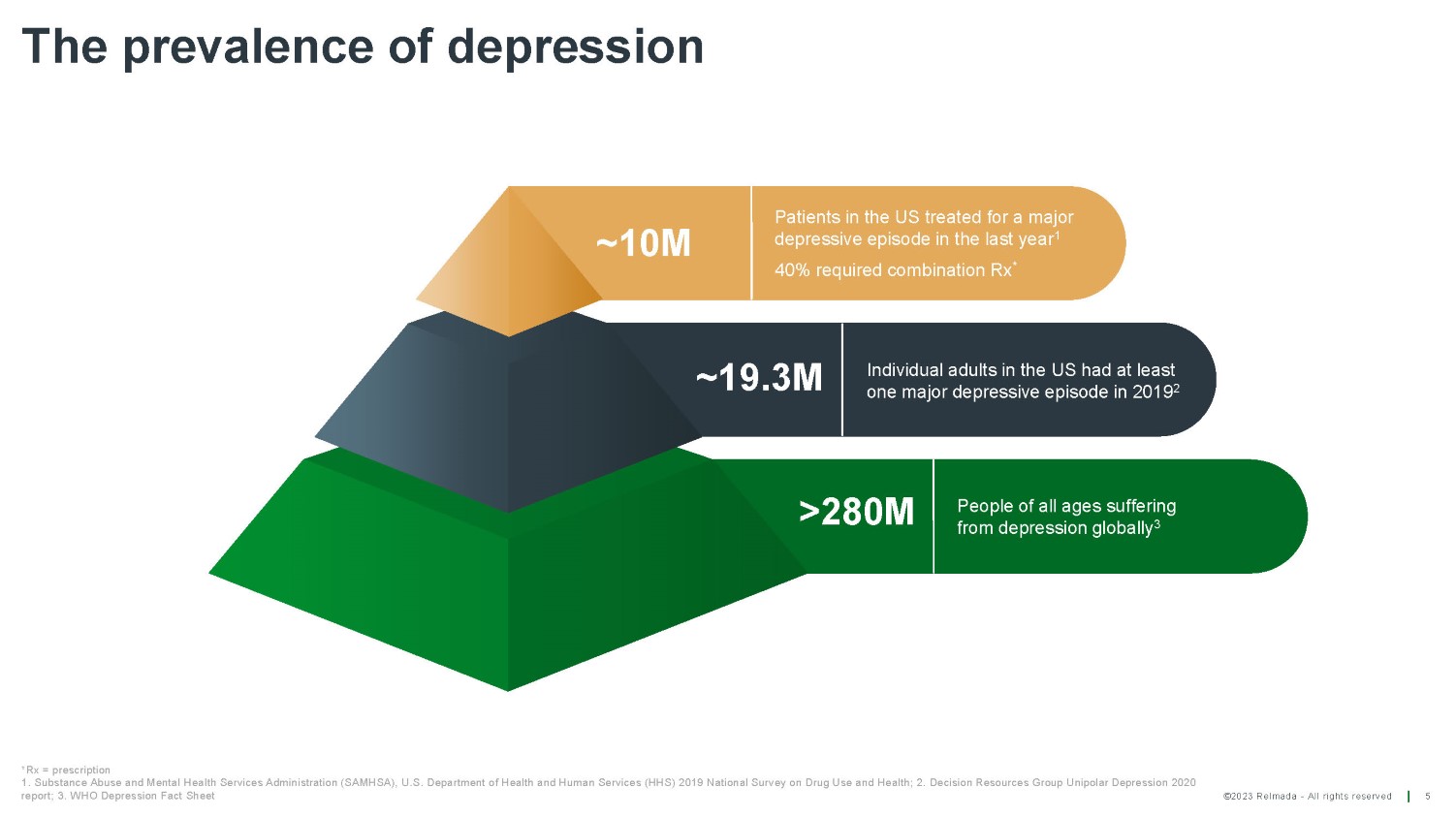
©2023 Relmada - All rights reserved The prevalence of depression 5 ~10M Patients in the US treated for a major depressive episode in the last year 1 40% required combination Rx * ~19.3M Individual adults in the US had at least one major depressive episode in 2019 2 >280M People of all ages suffering from depression globally 3 *Rx = prescription 1. Substance Abuse and Mental Health Services Administration (SAMHSA), U.S. Department of Health and Human Services (HHS) 2019 National Survey on Drug Use and Health; 2. Decision Resources Group Unipolar Depression 2020 report; 3. WHO Depression Fact Sheet

©2023 Relmada - All rights reserved Limitations of current treatments for MDD 6 MDD = major depressive disorder 1. Trivedi MH, et al. Am J Psychiatry. 2006;163:28 - 40; 2. Ashton AK, et al. Curr Ther Res. 2005;66(2):97 - 106; 3. US Prescribing Information, brexpiprazole, quetiapine, aripiprazole Limited efficacy Slow onset of action Safety and tolerability challenges ~65% MDD patients do not respond to first antidepressant treatment 1 Side effects of atypical antipsychotics approved as adjunctive treatments for MDD include tardive dyskinesia, metabolic syndrome, cognitive impairment and stroke 3 Standard antidepressants, even when effective, may take up to 8 weeks to reach efficacy 2
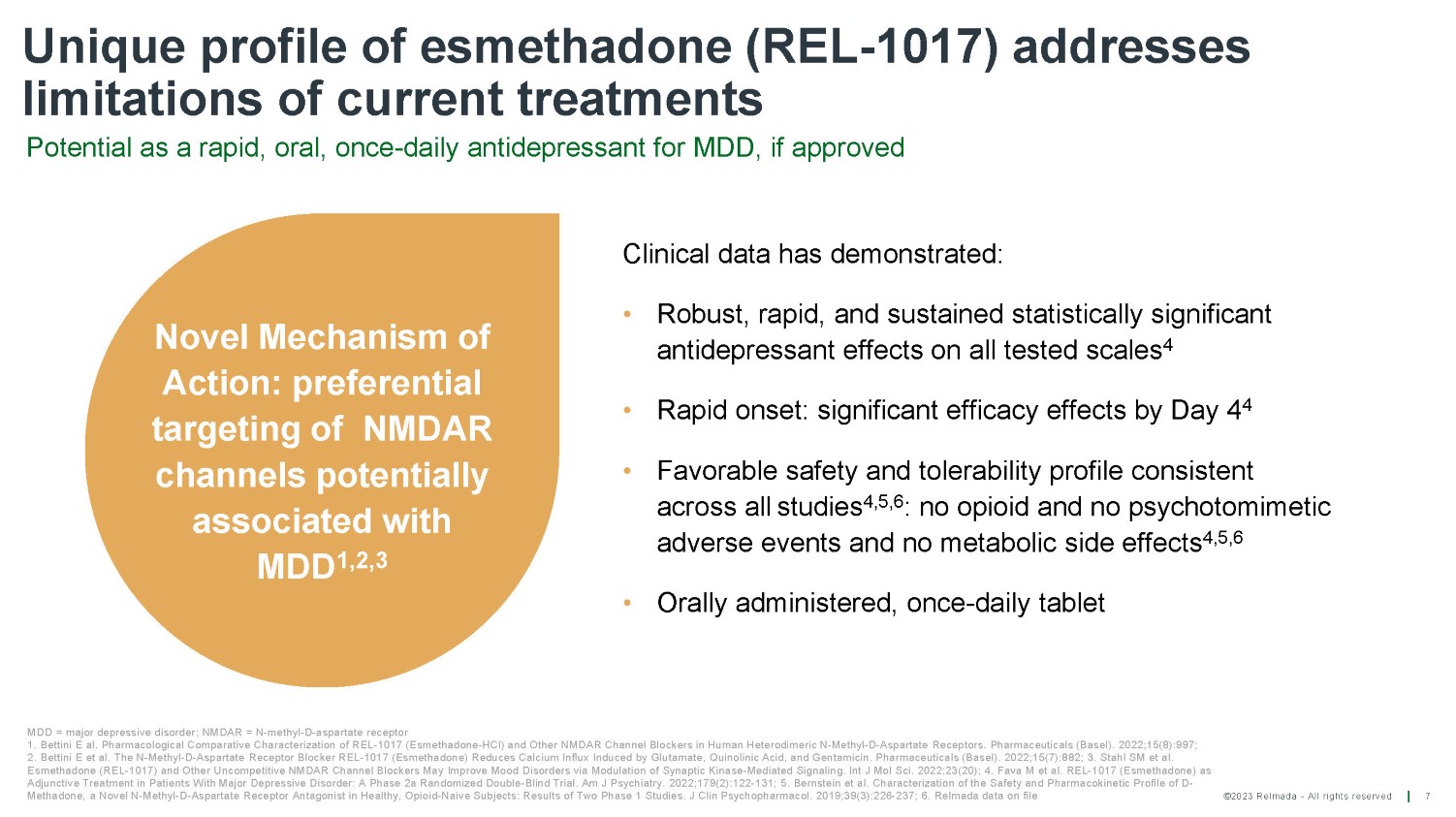
©2023 Relmada - All rights reserved Unique profile of esmethadone (REL - 1017) addresses limitations of current treatments Potential as a rapid, oral, once - daily antidepressant for MDD, if approved 7 MDD = major depressive disorder; NMDAR = N - methyl - D - aspartate receptor 1. Bettini E al. Pharmacological Comparative Characterization of REL - 1017 (Esmethadone - HCl) and Other NMDAR Channel Blockers in Human Heterodimeric N - Methyl - D - Aspartate Receptors. Pharmaceuticals (Basel). 2022;15(8):997; 2. Bettini E et al. The N - Methyl - D - Aspartate Receptor Blocker REL - 1017 (Esmethadone) Reduces Calcium Influx Induced by Glutamate, Quinolinic Acid, and Gentamicin. Pharmaceuticals (Basel). 2022;15(7):882; 3. Stahl SM et al. Esmethadone (REL - 1017) and Other Uncompetitive NMDAR Channel Blockers May Improve Mood Disorders via Modulation of Synaptic Kinase - Mediated Signaling. Int J Mol Sci. 2022;23(20); 4. Fava M et al. REL - 1017 (Esmethadone) as Adjunctive Treatment in Patients With Major Depressive Disorder: A Phase 2a Randomized Double - Blind Trial. Am J Psychiatry. 2022;179(2):122 - 131; 5. Bernstein et al. Characterization of the Safety and Pharmacokinetic Profile of D - Methadone, a Novel N - Methyl - D - Aspartate Receptor Antagonist in Healthy, Opioid - Naive Subjects: Results of Two Phase 1 Studies. J Clin Psychopharmacol. 2019;39(3):226 - 237; 6. Relmada data on file Clinical data has demonstrated: • Robust, rapid, and sustained statistically significant antidepressant effects on all tested scales 4 • Rapid onset: significant efficacy effects by Day 4 4 • Favorable safety and tolerability profile consistent across all studies 4,5,6 : no opioid and no psychotomimetic adverse events and no metabolic side effects 4,5,6 • Orally administered, once - daily tablet Novel Mechanism of Action: preferential targeting of NMDAR channels potentially associated with MDD 1,2,3
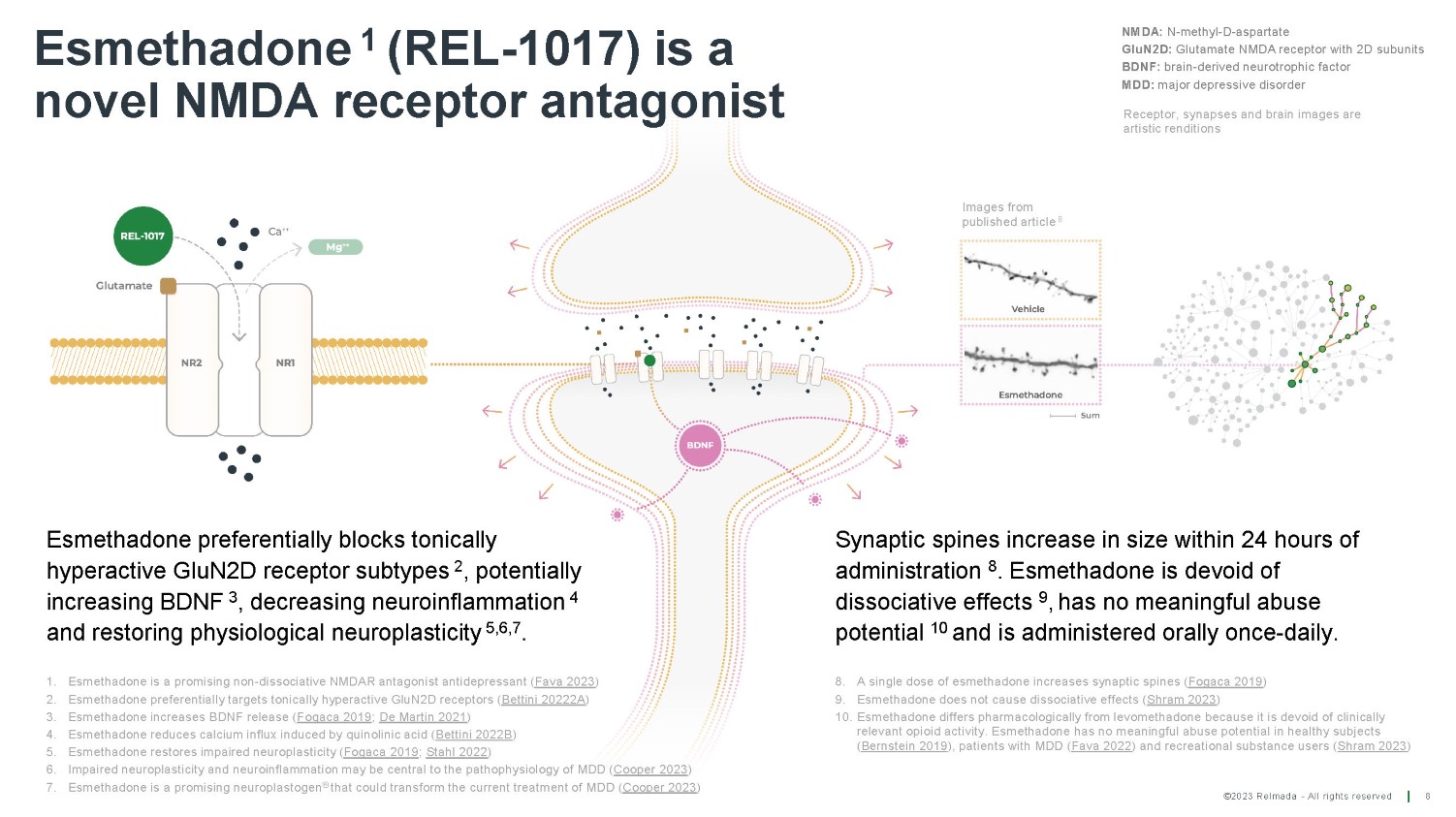
Esmethadone 1 (REL - 1017) is a novel NMDA receptor antagonist Esmethadone preferentially blocks tonically hyperactive GluN2D receptor subtypes 2 , potentially increasing BDNF 3 , decreasing neuroinflammation 4 and restoring physiological neuroplasticity 5,6,7 . NMDA: N - methyl - D - aspartate GluN2D: Glutamate NMDA receptor with 2D subunits BDNF: brain - derived neurotrophic factor MDD: major depressive disorder Synaptic spines increase in size within 24 hours of administration 8 . Esmethadone is devoid of dissociative effects 9 , has no meaningful abuse potential 10 and is administered orally once - daily. Images from published article 8 Receptor, synapses and brain images are artistic renditions 8 ©2023 Relmada - All rights reserved 8. A single dose of esmethadone increases synaptic spines ( Fogaca 2019 ) 9. Esmethadone does not cause dissociative effects ( Shram 2023 ) 10. Esmethadone differs pharmacologically from levomethadone because it is devoid of clinically relevant opioid activity. Esmethadone has no meaningful abuse potential in healthy subjects ( Bernstein 2019 ), patients with MDD ( Fava 2022 ) and recreational substance users ( Shram 2023 ) 1. Esmethadone is a promising non - dissociative NMDAR antagonist antidepressant ( Fava 2023 ) 2. Esmethadone preferentially targets tonically hyperactive GluN2D receptors ( Bettini 20222A ) 3. Esmethadone increases BDNF release ( Fogaca 2019 ; De Martin 2021 ) 4. Esmethadone reduces calcium influx induced by quinolinic acid ( Bettini 2022B ) 5. Esmethadone restores impaired neuroplasticity ( Fogaca 2019 ; Stahl 2022 ) 6. Impaired neuroplasticity and neuroinflammation may be central to the pathophysiology of MDD ( Cooper 2023 ) 7. Esmethadone is a promising neuroplastogen ® that could transform the current treatment of MDD ( Cooper 2023 )

©2023 Relmada - All rights reserved 9 The clinical development of esmethadone (REL - 1017) is steadily progressing as Adjunctive Treatment for MDD
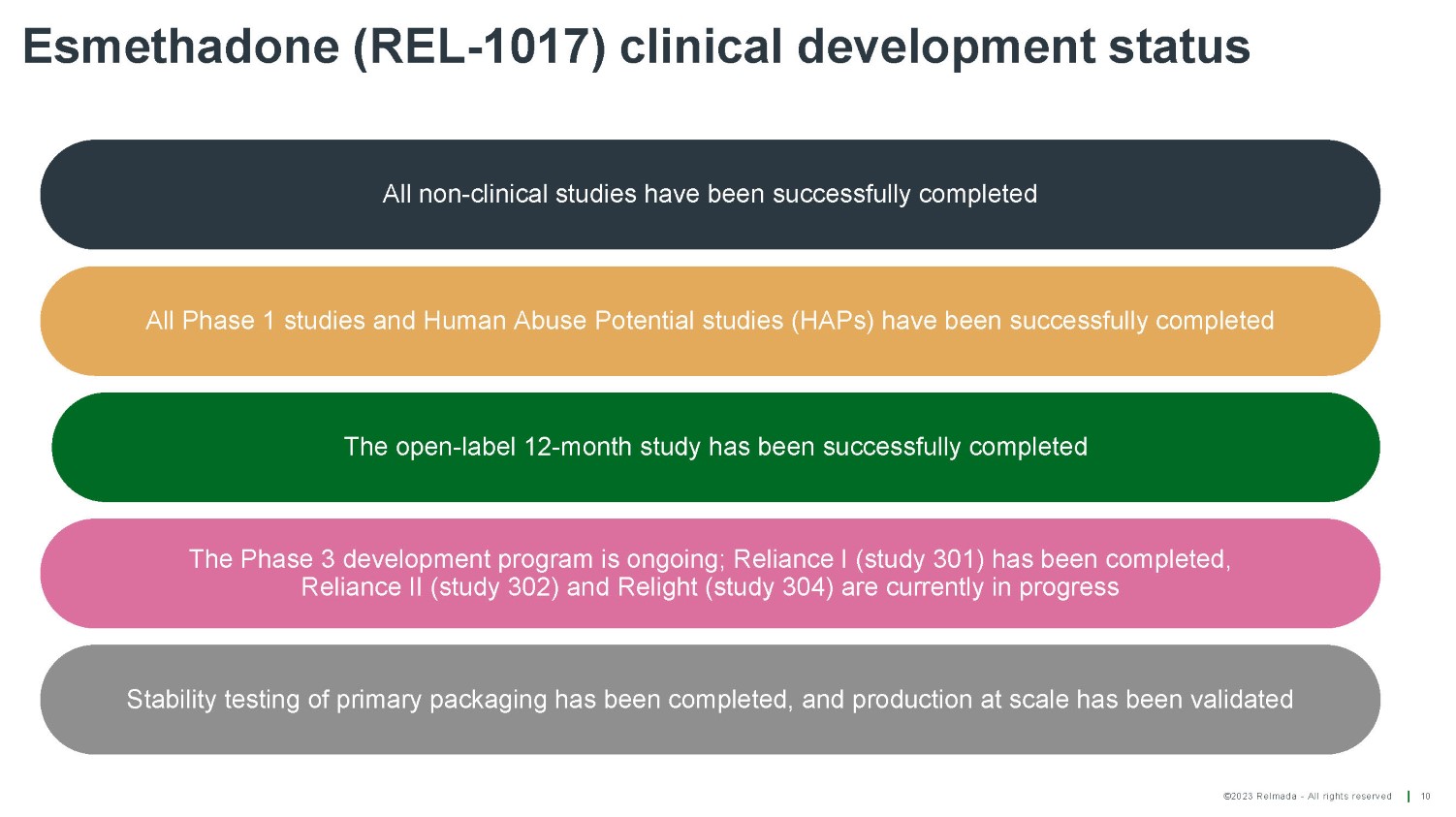
©2023 Relmada - All rights reserved Esmethadone (REL - 1017) clinical development status 10 All non - clinical studies have been successfully completed Stability testing of primary packaging has been completed, and production at scale has been validated The Phase 3 development program is ongoing; Reliance I (study 301) has been completed, Reliance II (study 302) and Relight (study 304) are currently in progress All Phase 1 studies and Human Abuse Potential studies (HAPs) have been successfully completed The open - label 12 - month study has been successfully completed

©2023 Relmada - All rights reserved 11 Data from the Phase 1, Phase 2, and Human Abuse Potential studies indicate favorable safety and tolerability of esmethadone (REL - 1017)

©2023 Relmada - All rights reserved All Phase 1 studies have been successfully completed 12 Multiple Ascending Dose (MAD) study Single Ascending Dose (SAD) study Oxycodone Human Abuse Potential (HAP) study Intravenous Ketamine Human Abuse Potential (HAP) study Renal Impairment study and Hepatic Impairment study Drug - Drug Interaction (DDI) study Absorption, distribution, metabolism, excretion (ADME) study

©2023 Relmada - All rights reserved The Human Abuse Potential studies have been successfully completed and indicate no abuse potential of REL - 1017 13 “The d - isomer lacks significant respiratory depressant action and addiction liability...” US Drug Enforcement Administration December 2019 4 1. Henningfield, et al. REL - 1017 (esmethadone; D - methadone) does not cause reinforcing effect, physical dependence and withdrawal signs in Sprague Dawley rats. Sci Rep 12, 11389 (2022); 2. Shram M, et al., No meaningful abuse potential in recreational opioid users of REL - 1017 (esmethadone hydrochloride), a new NMDAR antagonist and potential rapid - acting antidepressant American Society of Clinical Psychopharmacology (ASCP) 2022; 3. Shram M, et al., No meaningful abuse potential in recreational ketamine users of REL - 1017 (esmethadone hydrochloride), a new NMDAR antagonist and potential rapid - acting antidepressant. American Society of Clinical Psychopharmacology (ASCP) 2022; 4. US DEA Statement on Methadone, December 2019 February 2022 The results of experimental studies predictive of human abuse potential 1 and the results of human abuse potential studies in recreational opioid users 2 and in recreational ketamine users 3 indicate no meaningful abuse potential and support the DEA statement below:
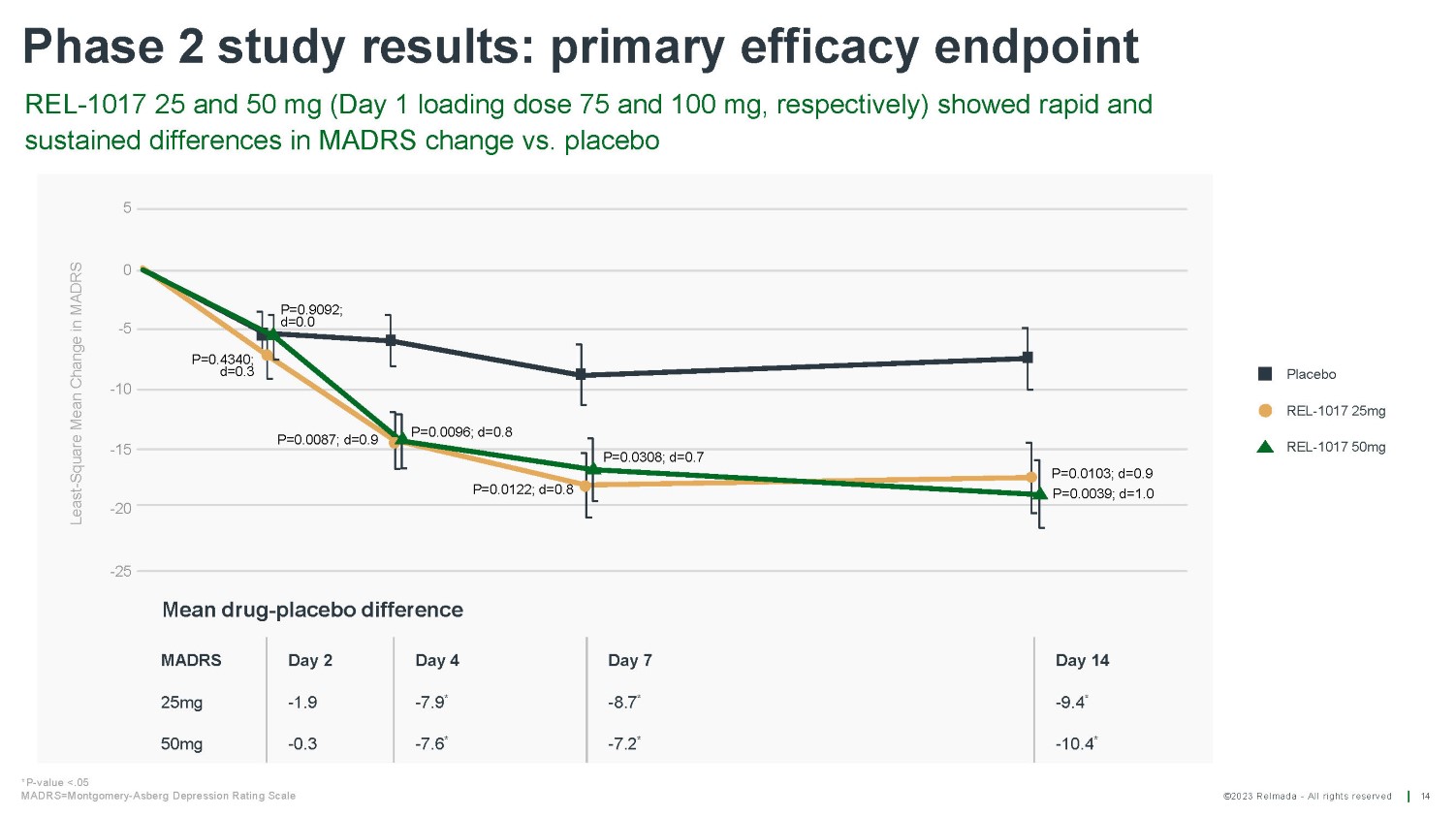
©2023 Relmada - All rights reserved Phase 2 study results: primary efficacy endpoint REL - 1017 25 and 50 mg (Day 1 loading dose 75 and 100 mg, respectively) showed rapid and sustained differences in MADRS change vs. placebo 14 Least - Square Mean Change in MADRS 5 0 - 5 - 10 - 15 - 20 - 25 P=0.0103; d=0.9 P=0.0039; d=1.0 P=0.0308; d=0.7 P=0.0122; d=0.8 P=0.0096; d=0.8 P=0.0087; d=0.9 P=0.9092; d=0.0 P=0.4340; d=0.3 Day 7 Day 14 - 8.7 * - 9.4 * - 7.6 * - 7.2 * - 10.4 * 50mg - 0.3 *P - value <.05 MADRS=Montgomery - Asberg Depression Rating Scale Placebo REL - 1017 25mg REL - 1017 50mg Mean drug - placebo difference MADRS Day 2 Day 4 25mg - 1.9 - 7.9 *
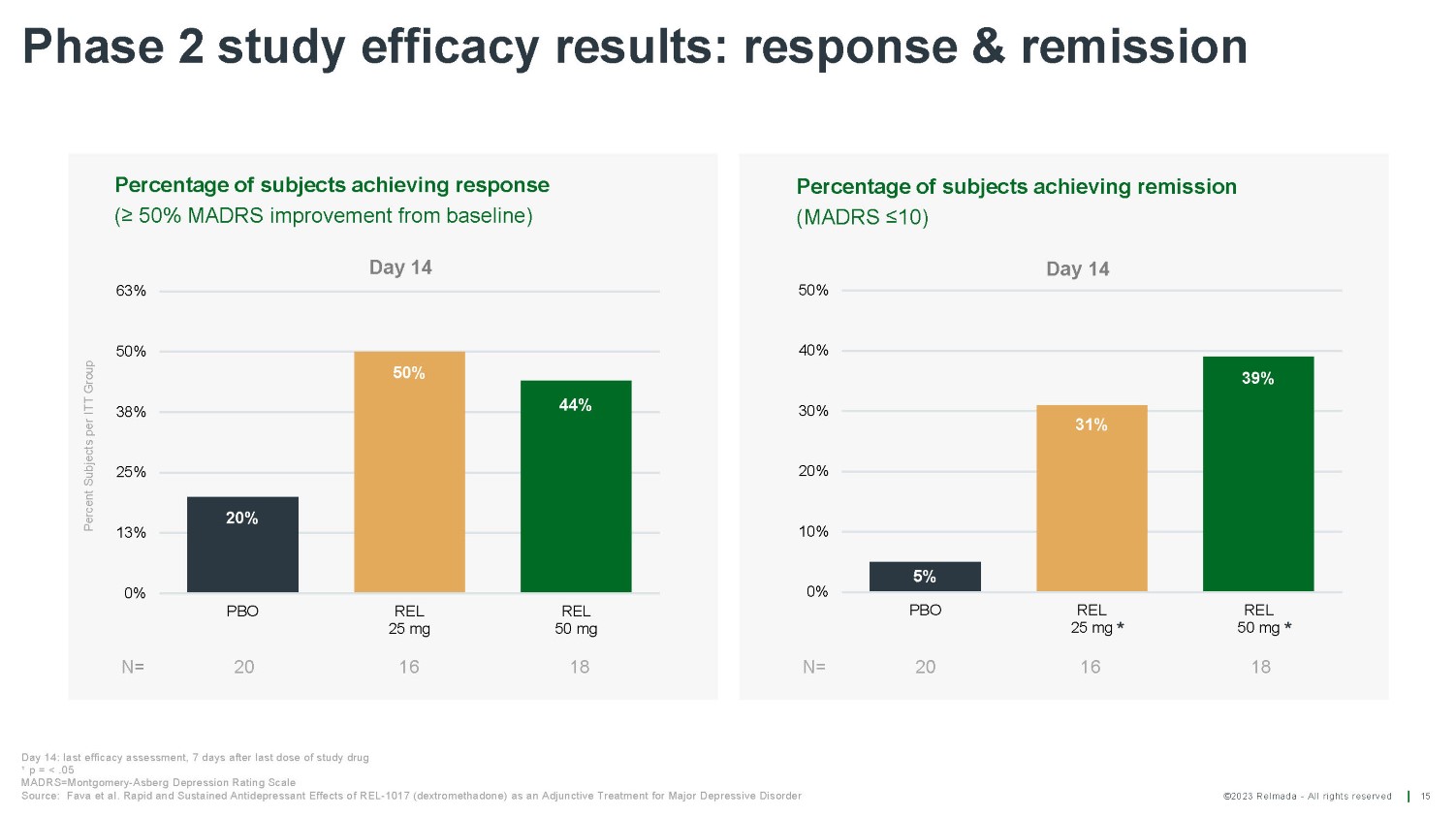
©2023 Relmada - All rights reserved Phase 2 study efficacy results: response & remission 15 50% 44% 20% 0% 13% 25% 38% 50% 63% PBO REL 25 mg REL 50 mg Day 14 Percent Subjects per ITT Group Percentage of subjects achieving response (≥ 50% MADRS improvement from baseline) N = 20 16 18 5% 31% 39% 0% 10% 20% 30% 40% PBO Percentage of subjects achieving remission (MADRS ≤10) Day 14 50% N= 20 REL 25 mg * 16 REL 50 mg * 18 Day 14: last efficacy assessment, 7 days after last dose of study drug * p = < .05 MADRS=Montgomery - Asberg Depression Rating Scale Source: Fava et al. Rapid and Sustained Antidepressant Effects of REL - 1017 (dextromethadone) as an Adjunctive Treatment for Major Depressive Disorder

©2023 Relmada - All rights reserved 16 The Phase 3 program as Adjunctive Treatment for MDD is currently ongoing
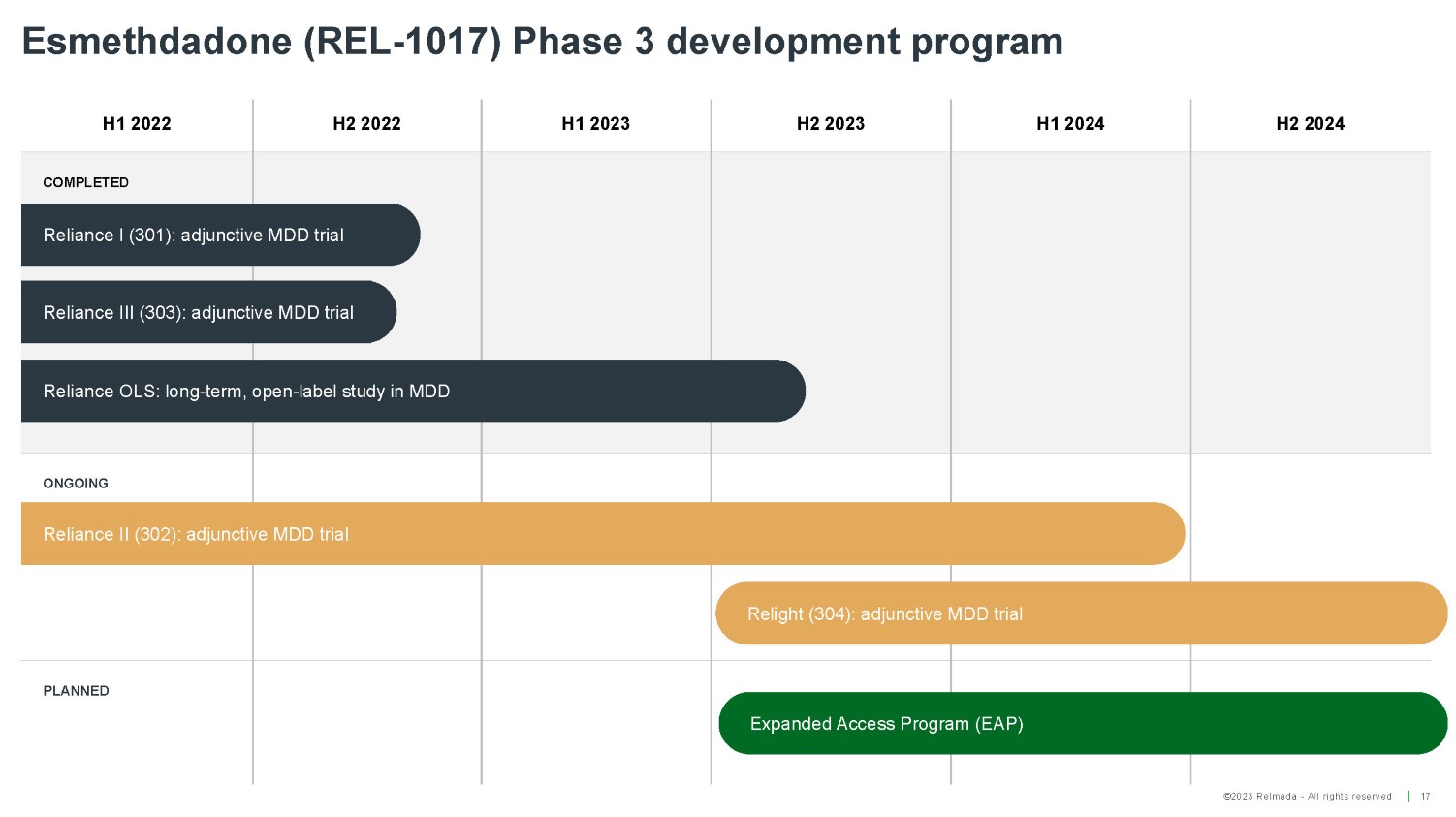
©2023 Relmada - All rights reserved H1 2022 H2 2022 H1 2023 H2 2023 H1 2024 H2 2024 COMPLETED ONGOING PLANNED Esmethdadone (REL - 1017) Phase 3 development program 17 Relight (304): adjunctive MDD trial Expanded Access Program (EAP) Reliance I (301): adjunctive MDD trial Reliance III (303): adjunctive MDD trial Reliance OLS: long - term, open - label study in MDD Reliance II (302): adjunctive MDD trial
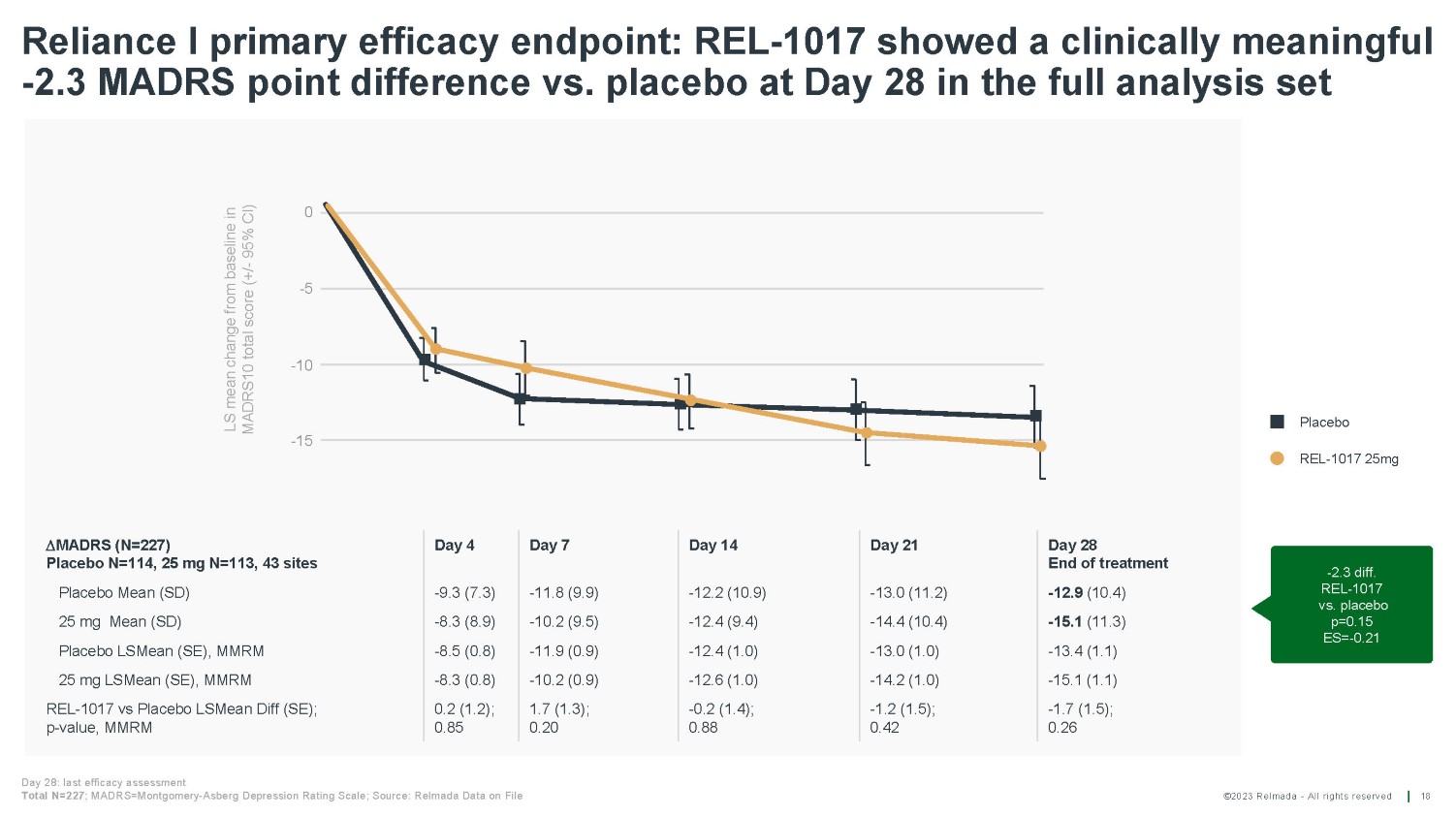
©2023 Relmada - All rights reserved LS mean change from baseline in MADRS10 total score (+/ - 95% CI) 0 - 5 - 10 - 15 Reliance I primary efficacy endpoint: REL - 1017 showed a clinically meaningful - 2.3 MADRS point difference vs. placebo at Day 28 in the full analysis set 18 Day 28: last efficacy assessment Total N=227 ; MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File MADRS (N=227) Placebo N=114, 25 mg N=113, 43 sites Day 4 Day 7 Day 14 Day 21 Day 28 End of treatment Placebo Mean (SD) - 9.3 (7.3) - 11.8 (9.9) - 12.2 (10.9) - 13.0 (11.2) - 12.9 (10.4) 25 mg Mean (SD) - 8.3 (8.9) - 10.2 (9.5) - 12.4 (9.4) - 14.4 (10.4) - 15.1 (11.3) Placebo LSMean (SE), MMRM - 8.5 (0.8) - 11.9 (0.9) - 12.4 (1.0) - 13.0 (1.0) - 13.4 (1.1) 25 mg LSMean (SE), MMRM - 8.3 (0.8) - 10.2 (0.9) - 12.6 (1.0) - 14.2 (1.0) - 15.1 (1.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM 0.2 (1.2); 0.85 1.7 (1.3); 0.20 - 0.2 (1.4); 0.88 - 1.2 (1.5); 0.42 - 1.7 (1.5); 0.26 Placebo REL - 1017 25mg - 2.3 diff. REL - 1017 vs. placebo p=0.15 ES= - 0.21
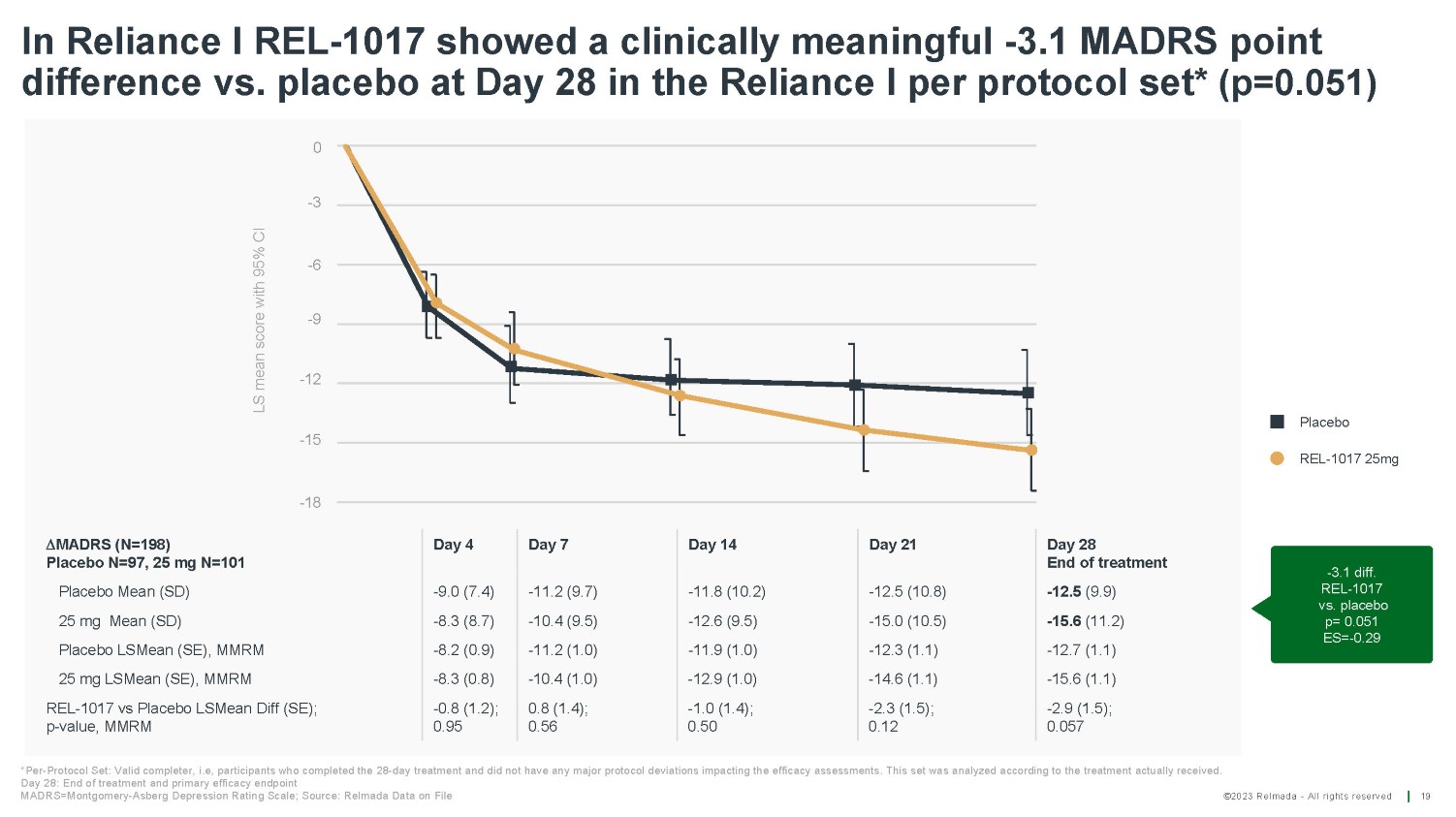
©2023 Relmada - All rights reserved In Reliance I REL - 1017 showed a clinically meaningful - 3.1 MADRS point difference vs. placebo at Day 28 in the Reliance I per protocol set* (p=0.051) 19 - 3.1 diff. REL - 1017 vs. placebo p= 0.051 ES= - 0.29 LS mean score with 95% CI Placebo REL - 1017 25mg *Per - Protocol Set: Valid completer, i.e, participants who completed the 28 - day treatment and did not have any major protocol deviations impacting the efficacy assessments. This set was analyzed according to the treatment actually received. Day 28: End of treatment and primary efficacy endpoint MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File MADRS (N=198) Placebo N=97, 25 mg N=101 Day 4 Day 7 Day 14 Day 21 Day 28 End of treatment Placebo Mean (SD) - 9.0 (7.4) - 11.2 (9.7) - 11.8 (10.2) - 12.5 (10.8) - 12.5 (9.9) 25 mg Mean (SD) - 8.3 (8.7) - 10.4 (9.5) - 12.6 (9.5) - 15.0 (10.5) - 15.6 (11.2) Placebo LSMean (SE), MMRM - 8.2 (0.9) - 11.2 (1.0) - 11.9 (1.0) - 12.3 (1.1) - 12.7 (1.1) 25 mg LSMean (SE), MMRM - 8.3 (0.8) - 10.4 (1.0) - 12.9 (1.0) - 14.6 (1.1) - 15.6 (1.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM - 0.8 (1.2); 0.95 0.8 (1.4); 0.56 - 1.0 (1.4); 0.50 - 2.3 (1.5); 0.12 - 2.9 (1.5); 0.057 - 15 0 - 3 - 6 - 9 - 12 - 18
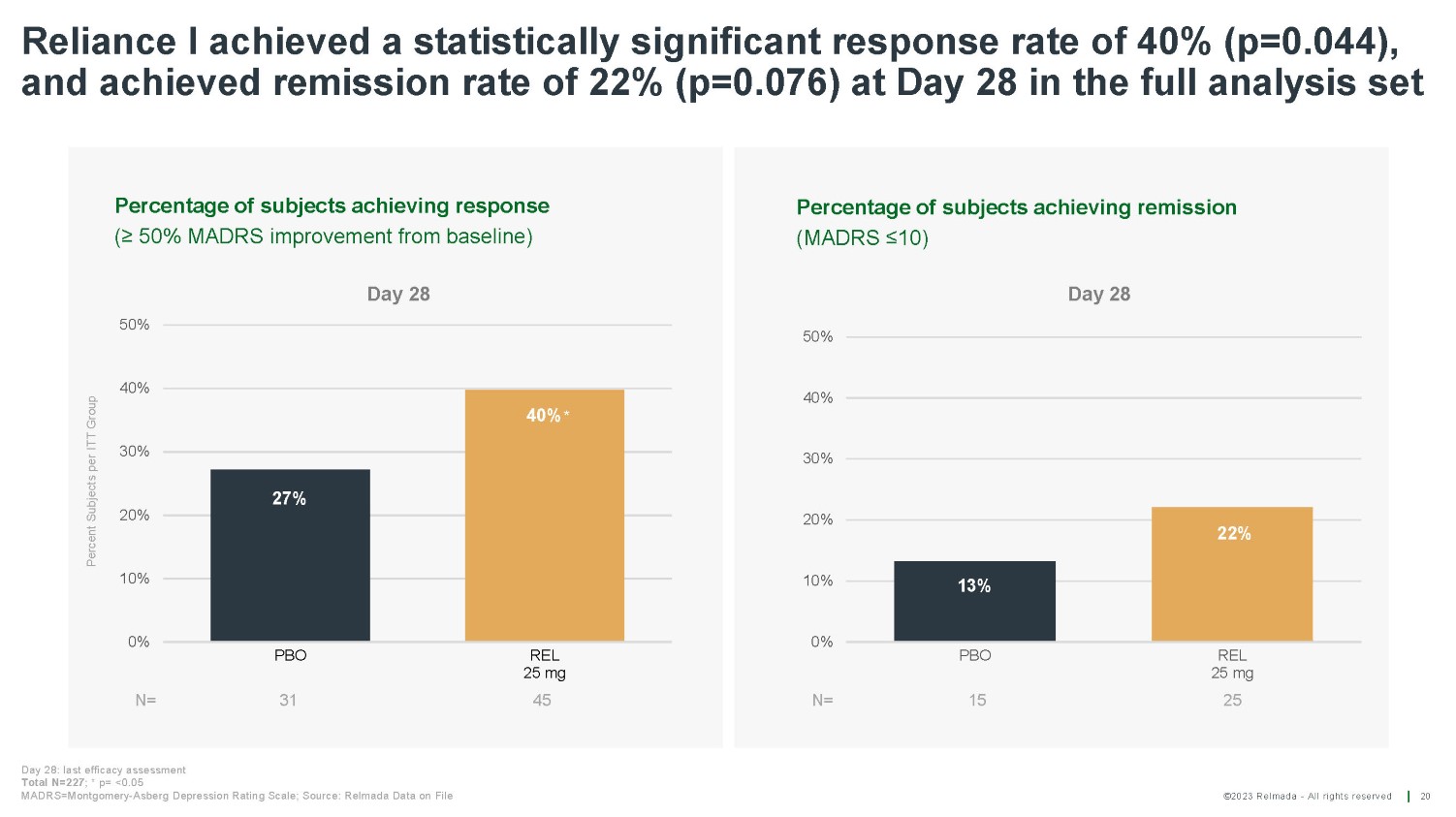
©2023 Relmada - All rights reserved Percentage of subjects achieving remission (MADRS ≤10) Percentage of subjects achieving response (≥ 50% MADRS improvement from baseline) Reliance I achieved a statistically significant response rate of 40% (p=0.044), and achieved remission rate of 22% (p=0.076) at Day 28 in the full analysis set 20 N= 0% 10% 20% 30% 40% 50% PBO REL 25 mg 45 13% 22% 0% 10% 20% 30% 40% 50% PBO 15 REL 25 mg 25 N= 31 40% * 27% Day 28: last efficacy assessment Total N=227 ; * p= <0.05 MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File Percent Subjects per ITT Group Day 28 Day 28

©2023 Relmada - All rights reserved Patient sources: verifiable vs. unverifiable 21 Verifiable sources • Past patient at site • Current patient • Site database • HCP referral • Radio/TV AD • Banner/Pop AD • Social media • Internet search • Friend • Recruitment company • Referral from other patients Unverifiable sources
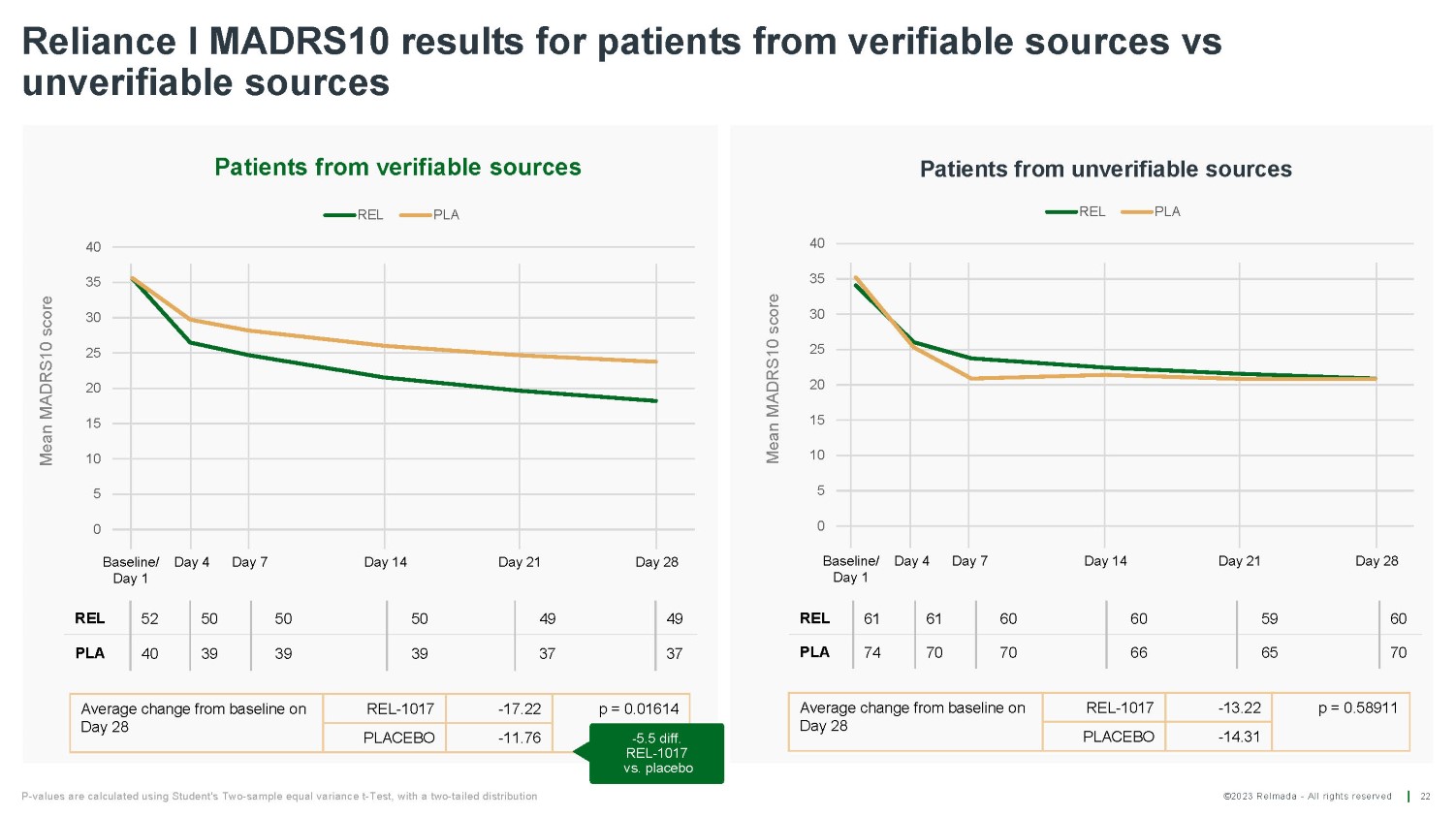
©2023 Relmada - All rights reserved Baseline/ Day 1 Day 4 Day 7 Day 14 Day 21 Day 28 Reliance I MADRS10 results for patients from verifiable sources vs unverifiable sources 22 REL 52 50 50 50 49 49 PLA 40 39 39 39 37 37 Average change from baseline on Day 28 REL - 1017 - 17.22 PLACEBO - 11.76 Average change from baseline on Day 28 REL - 1017 - 13.22 p = 0.58911 PLACEBO - 14.31 Mean MADRS10 score Mean MADRS10 score Baseline/ Day 4 Day 1 Day 7 Day 14 Day 21 Day 28 REL 61 61 60 60 59 60 PLA 74 70 70 66 65 70 P - values are calculated using Student's Two - sample equal variance t - Test, with a two - tailed distribution 0 5 10 15 20 25 30 35 40 Patients from verifiable sources REL PLA p = 0.01614 - 5.5 diff. REL - 1017 vs. placebo 0 5 10 15 20 25 30 35 40 Patients from unverifiable sources REL PLA

©2023 Relmada - All rights reserved In Reliance I no serious treatment related treatment - emergent adverse event (TEAE)* and no opioid like effects were observed 23 Variable Placebo (N=114) REL - 1017 25 mg (N=113) All patients (N=227) N % N % N % Patients with at least one TEAE 61 53.5 55 48.7 116 51.1 Patients with at least one treatment related TEAE 28 24.6 30 26.5 58 25.6 Patients with at least one serious treatment related TEAE 0 0.0 0 0.0 0 0.0 Headache 9 7.9 13 11.5 22 9.7 COVID19 10 8.8 6 5.3 16 7.0 Upper respiratory tract infection 6 5.3 8 7.1 14 6.2 Nausea 5 4.4 8 7.1 13 5.7 Diarrhea 7 6.1 5 4.4 12 5.3 Constipation 7 6.1 3 2.7 10 4.4 Dizziness 2 1.8 7 6.2 9 4.0 Treatment - emergent adverse events* occurring in 5% or more patients per treatment arm Treatment - emergent adverse events by preferred term in patients with MDD in the safety analysis set *Treatment - emergent adverse event was defined as an adverse event that started or worsened after treatment with the trial drug
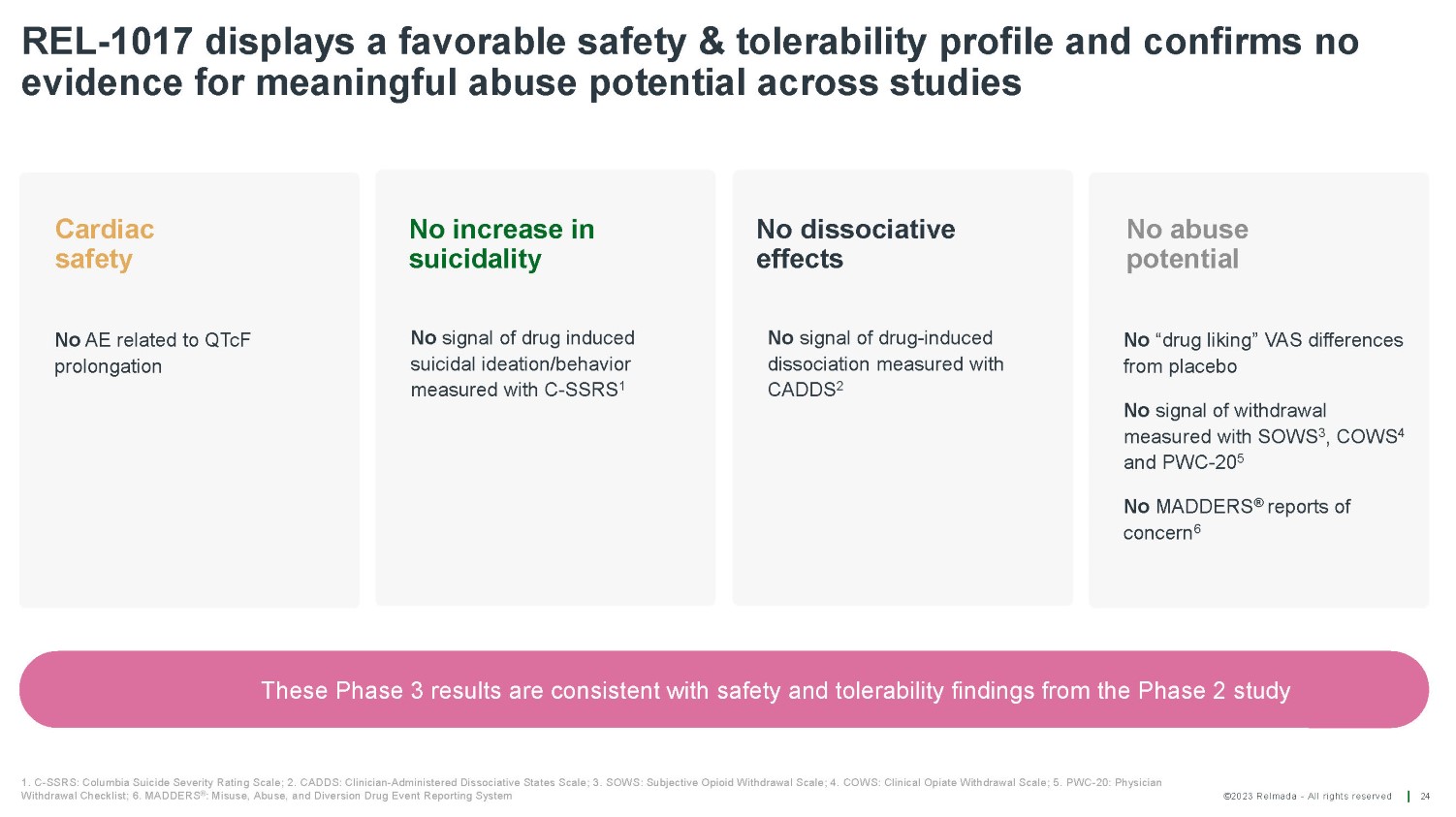
©2023 Relmada - All rights reserved REL - 1017 displays a favorable safety & tolerability profile and confirms no evidence for meaningful abuse potential across studies 24 1. C - SSRS: Columbia Suicide Severity Rating Scale; 2. CADDS: Clinician - Administered Dissociative States Scale; 3. SOWS: Subjective Opioid Withdrawal Scale; 4. COWS: Clinical Opiate Withdrawal Scale; 5. PWC - 20: Physician Withdrawal Checklist; 6. MADDERS ® : Misuse, Abuse, and Diversion Drug Event Reporting System No AE related to QTcF prolongation No signal of drug induced suicidal ideation/behavior measured with C - SSRS 1 No signal of drug - induced dissociation measured with CADDS 2 No “drug liking” VAS differences from placebo No signal of withdrawal measured with SOWS 3 , COWS 4 and PWC - 20 5 No MADDERS ® reports of concern 6 Cardiac safety No increase in suicidality No dissociative effects No abuse potential These Phase 3 results are consistent with safety and tolerability findings from the Phase 2 study

©2023 Relmada - All rights reserved 25 Relmada is conducting two Phase 3 trials Phase 3 studies, currently ongoing in the United States, to evaluate the efficacy and safety of REL - 1017 as an adjunctive treatment for MDD.
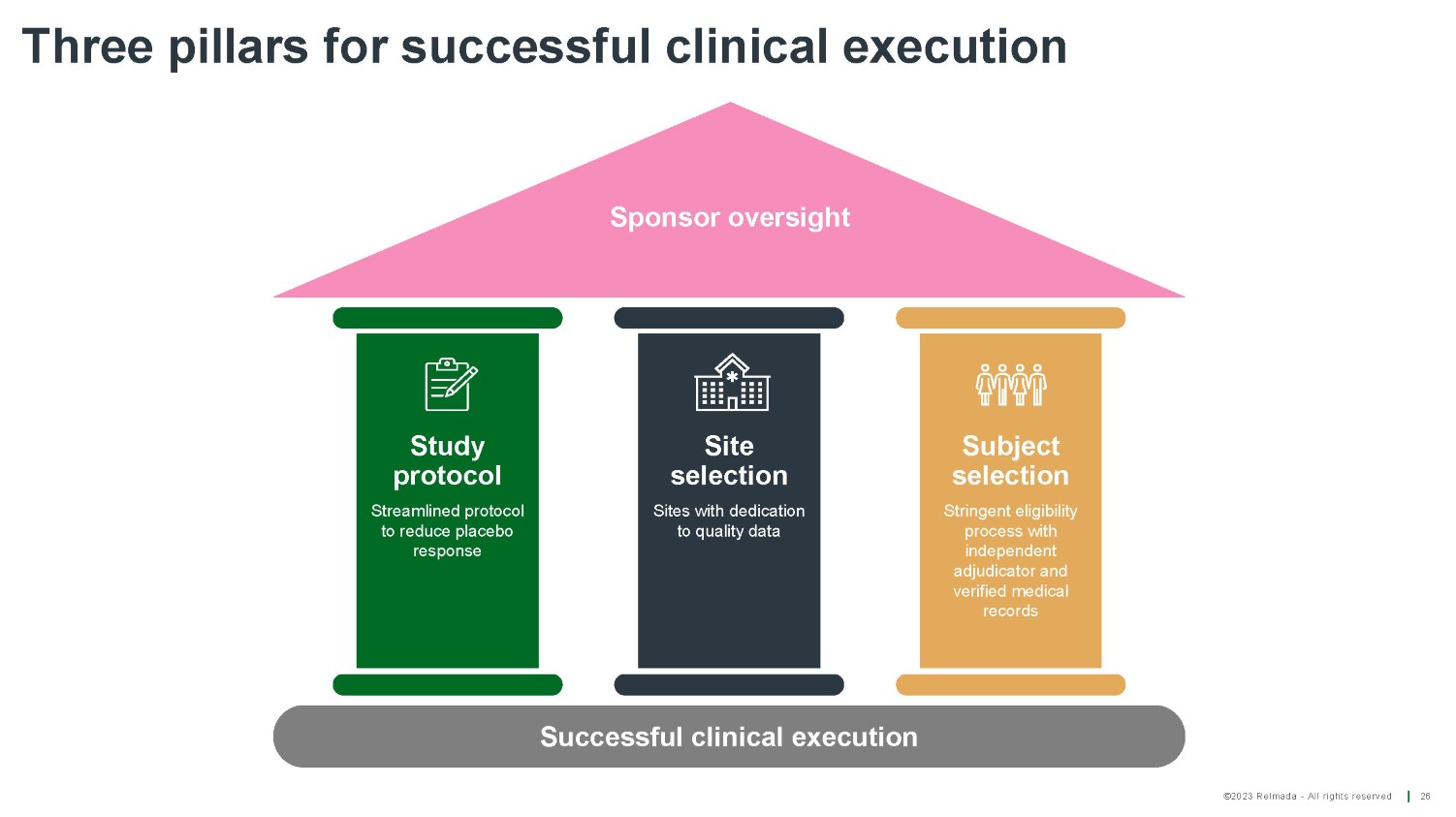
©2023 Relmada - All rights reserved Three pillars for successful clinical execution 26 Successful clinical execution Sponsor oversight Study protocol Streamlined protocol to reduce placebo response Site selection Sites with dedication to quality data Subject selection Stringent eligibility process with independent adjudicator and verified medical records
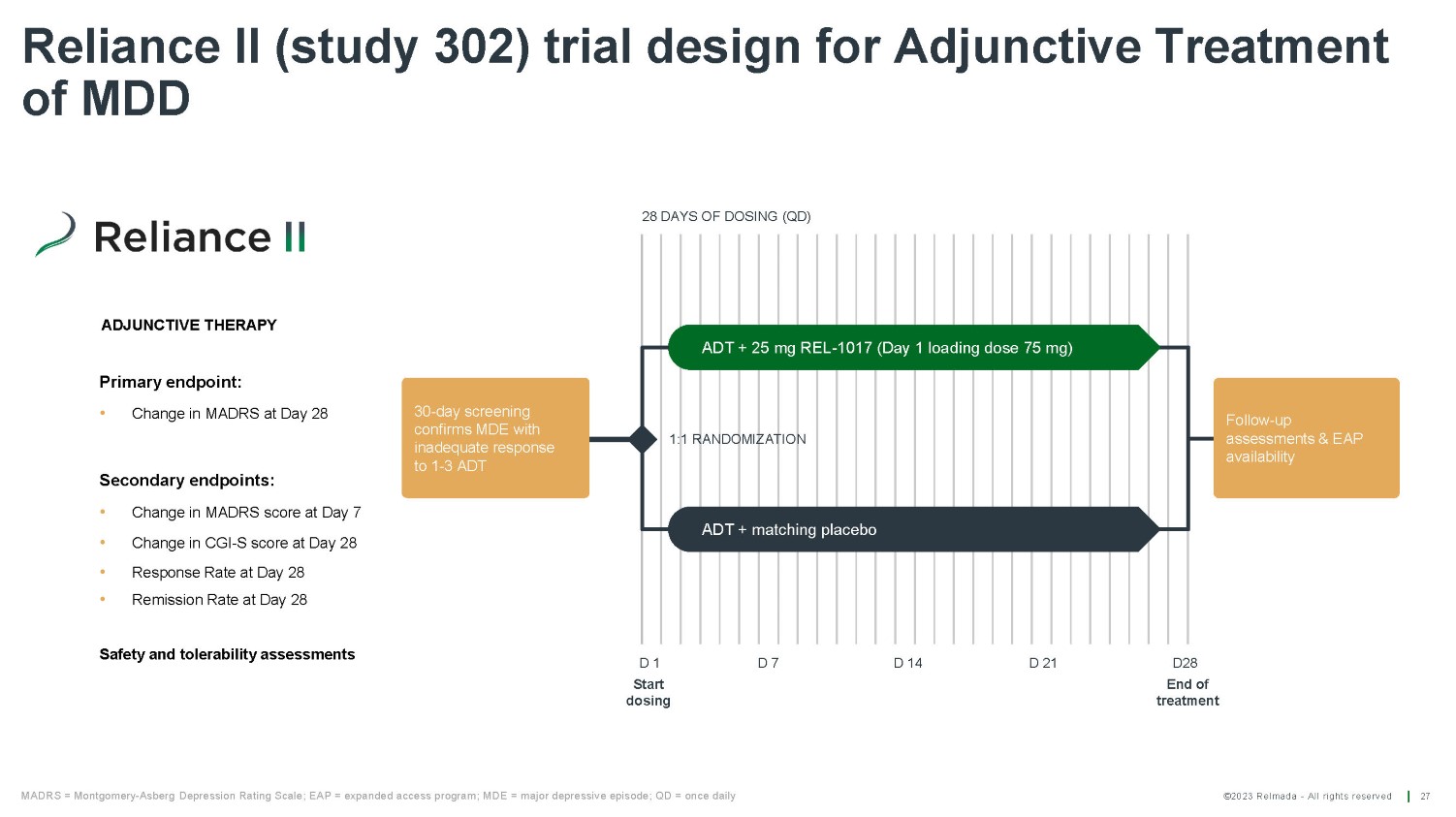
©2023 Relmada - All rights reserved Reliance II (study 302) trial design for Adjunctive Treatment of MDD 27 Follow - up assessments & EAP availability ADT + matching placebo ADT + 25 mg REL - 1017 (Day 1 loading dose 75 mg) 1:1 RANDOMIZATION 28 DAYS OF DOSING (QD) D 1 Start dosing D 7 D 14 D28 End of treatment D 21 MADRS = Montgomery - Asberg Depression Rating Scale; EAP = expanded access program; MDE = major depressive episode; QD = once daily 30 - day screening confirms MDE with inadequate response to 1 - 3 ADT Primary endpoint: • Change in MADRS at Day 28 Secondary endpoints: • Change in MADRS score at Day 7 • Change in CGI - S score at Day 28 • Response Rate at Day 28 • Remission Rate at Day 28 Safety and tolerability assessments ADJUNCTIVE THERAPY
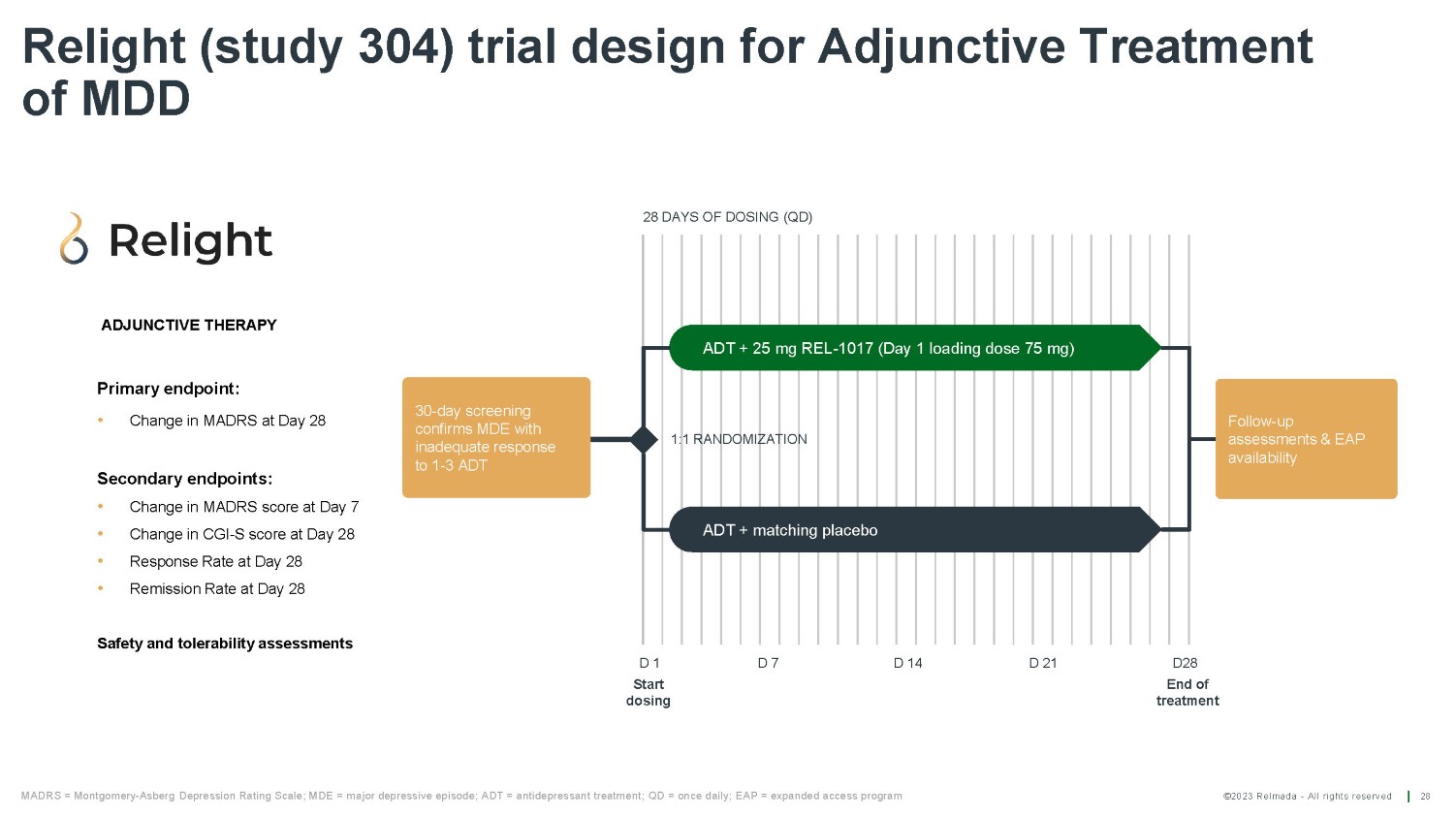
©2023 Relmada - All rights reserved Relight (study 304) trial design for Adjunctive Treatment of MDD 28 Follow - up assessments & EAP availability ADT + matching placebo ADT + 25 mg REL - 1017 (Day 1 loading dose 75 mg) 1:1 RANDOMIZATION 28 DAYS OF DOSING (QD) MADRS = Montgomery - Asberg Depression Rating Scale; MDE = major depressive episode; ADT = antidepressant treatment; QD = once daily; EAP = expanded access program Primary endpoint: • Change in MADRS at Day 28 Secondary endpoints: • Change in MADRS score at Day 7 • Change in CGI - S score at Day 28 • Response Rate at Day 28 • Remission Rate at Day 28 Safety and tolerability assessments 30 - day screening confirms MDE with inadequate response to 1 - 3 ADT ADJUNCTIVE THERAPY D 1 Start dosing D 7 D 14 D28 End of treatment D 21

©2023 Relmada - All rights reserved Data generated for esmethadone (REL - 1017) support efficacy, safety, and tolerability for adjunctive treatment of depression 29 Phase 2 trial reached significance p= 0.0122 (25 mg) for the primary endpoint in the Intent - to - treat (ITT) analysis Reliance I, the first adjunctive Phase 3 trial, showed a 40% response rate (p = 0.044) in the ITT analysis and 3.1 MADRS - points CFB difference compared with placebo (p = 0.0510) in the Per Protocol (PP) analysis All studies to date have shown a consistent favorable safety and tolerability profile with no evidence of abuse potential or withdrawal Reliance II (study 302) and Relight (study 304) are currently ongoing in the US to evaluate the efficacy of REL - 1017 25mg MADRS = Montgomery - Asberg Depression Rating Scale; CFB = change from baseline

©2023 Relmada - All rights reserved 30 Corporate information
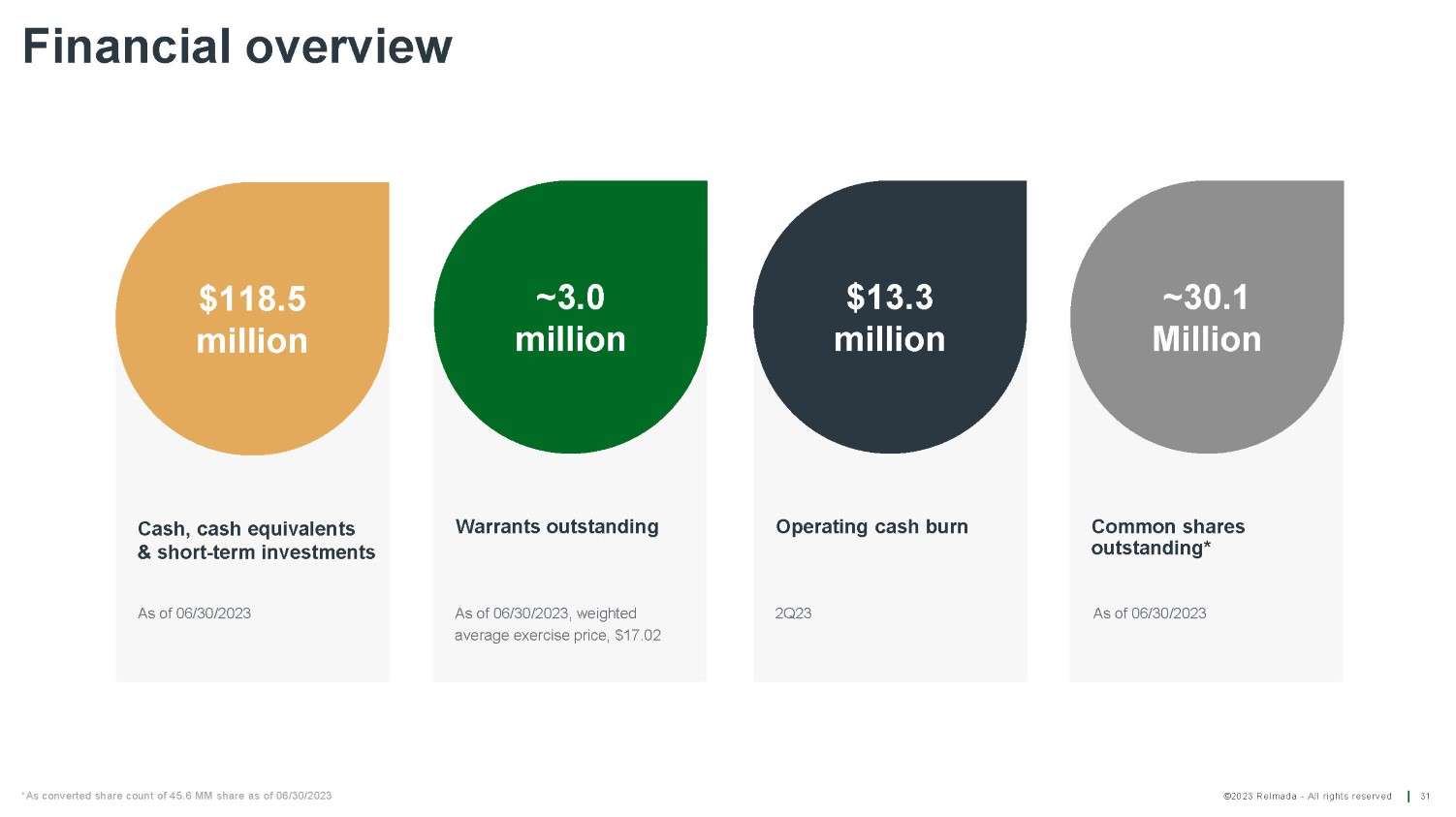
©2023 Relmada - All rights reserved Warrants outstanding Operating cash burn Common shares outstanding* Financial overview 31 Cash, cash equivalents & short - term investments $118.5 million ~3.0 million $13.3 million ~30.1 Million As of 06/30/2023, weighted average exercise price, $17.02 2Q23 As of 06/30/2023 As of 06/30/2023 *As converted share count of 45.6 MM share as of 06/30/2023

©2023 Relmada - All rights reserved Through our Neuroplastogen TM Program we are developing a pipeline of molecules with neuroplastic modulating activity for a variety of indications 32 Novel esmethadone derivatives We have synthesized a series of novel esmethadone derivatives with NMDAR regulating activity. We are in the process of selecting the most promising candidates to advance into development for the treatment of CNS disorders. CNS = Central Nervous System; NMDAR = N - methyl - D - aspartate receptor Novel psilocybin formulation and novel psilocybin derivatives We are developing a novel psilocybin formulation and we are synthetizing psilocybin derivatives with promising activity for the treatment of CNS disorders.
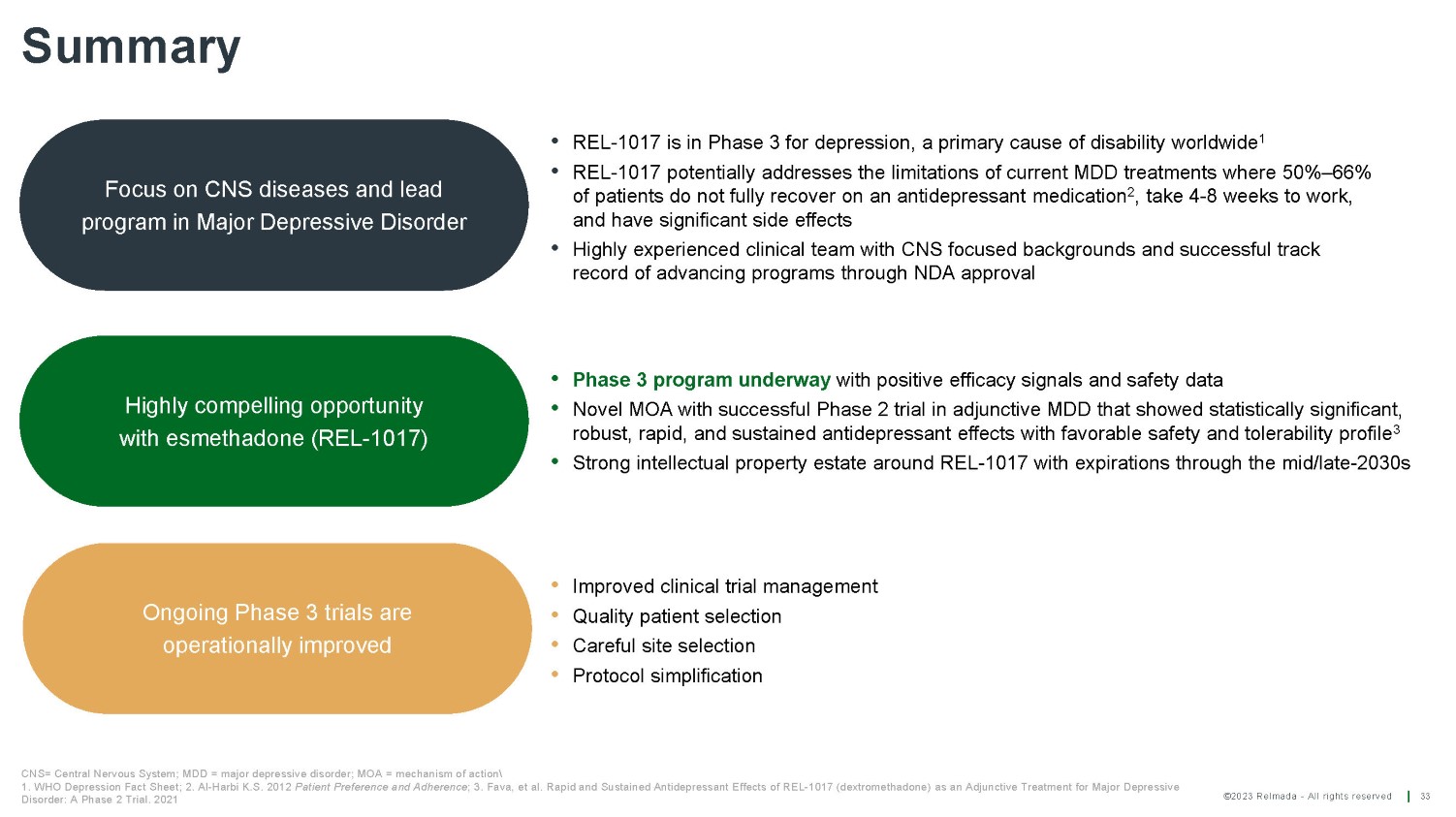
©2023 Relmada - All rights reserved Summary 33 Focus on CNS diseases and lead program in Major Depressive Disorder CNS= Central Nervous System; MDD = major depressive disorder; MOA = mechanism of action 1. WHO Depression Fact Sheet; 2. Al - Harbi K.S. 2012 Patient Preference and Adherence ; 3. Fava, et al. Rapid and Sustained Antidepressant Effects of REL - 1017 (dextromethadone) as an Adjunctive Treatment for Major Depressive Disorder: A Phase 2 Trial. 2021 Highly compelling opportunity with esmethadone (REL - 1017) Ongoing Phase 3 trials are operationally improved • Phase 3 program underway with positive efficacy signals and safety data • Novel MOA with successful Phase 2 trial in adjunctive MDD that showed statistically significant, robust, rapid, and sustained antidepressant effects with favorable safety and tolerability profile 3 • Strong intellectual property estate around REL - 1017 with expirations through the mid/late - 2030s • REL - 1017 is in Phase 3 for depression, a primary cause of disability worldwide 1 • REL - 1017 potentially addresses the limitations of current MDD treatments where 50% – 66% of patients do not fully recover on an antidepressant medication 2 , take 4 - 8 weeks to work, and have significant side effects • Highly experienced clinical team with CNS focused backgrounds and successful track record of advancing programs through NDA approval • Improved clinical trial management • Quality patient selection • Careful site selection • Protocol simplification

Thank you 34
v3.23.2
Cover
|
Aug. 10, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Aug. 10, 2023
|
| Entity File Number |
001-39082
|
| Entity Registrant Name |
RELMADA THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001553643
|
| Entity Tax Identification Number |
45-5401931
|
| Entity Incorporation, State or Country Code |
NV
|
| Entity Address, Address Line One |
2222 Ponce de Leon Blvd.
|
| Entity Address, Address Line Two |
Floor 3
|
| Entity Address, City or Town |
Coral Gables
|
| Entity Address, State or Province |
FL
|
| Entity Address, Postal Zip Code |
33134
|
| City Area Code |
786
|
| Local Phone Number |
629 1376
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common stock, $0.001 par value per share
|
| Trading Symbol |
RLMD
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Relmada Therapeutics (NASDAQ:RLMD)
Historical Stock Chart
From Mar 2024 to Apr 2024
Relmada Therapeutics (NASDAQ:RLMD)
Historical Stock Chart
From Apr 2023 to Apr 2024