false000137469000013746902023-07-252023-07-25
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): July 25, 2023 |
Larimar Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-36510 |
20-3857670 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
Three Bala Plaza East |
|
Bala Cynwyd, Pennsylvania |
|
19004 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (844) 511-9056 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 per share |
|
lrmr |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
Press Release
On July 25, 2023, Larimar Therapeutics, Inc. (the “Company”) issued a press release announcing that the U.S. Food and Drug Administration has (i) cleared the Company’s four-week, placebo-controlled, Phase 2 dose exploration trial of CTI-1601 in patients with Friedreich’s ataxia to proceed to a 50 mg cohort in which participants will be dosed daily for the first 14 days, and then every other day until day 28 and (ii) cleared for initiation the Company’s open label extension trial in which participants will receive 25 mg of CTI-1601 daily. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Investor Presentation
On July 25, 2023, the Company posted on its website an updated slide presentation, which is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference. Representatives of the Company will use the presentation in various meetings with investors, analysts and other parties from time to time.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Below is a list of exhibits included with this Current Report on Form 8-K.
* Filed herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Larimar Therapeutics, Inc. |
|
|
|
|
Date: |
July 25, 2023 |
By: |
/s/ Carole S. Ben-Maimon, M.D. |
|
|
|
Name: Carole S. Ben-Maimon, M.D.
Title: President and Chief Executive Officer |

Larimar Therapeutics Receives FDA Clearance to Proceed to 50 mg Cohort in CTI-1601’s Phase 2 Friedreich's Ataxia Trial and to Initiate Open Label Extension Trial
•Top-line safety, pharmacokinetic, and pharmacodynamic (frataxin level) data from Phase 2 trial’s 50 mg cohort expected in 1H 2024
•Initiation of open label extension trial with 25 mg daily dosing expected in Q1 2024; interim data expected in Q4 2024
•Company management hosting a webcast and conference call today at 8:00 a.m. ET
Bala Cynwyd, PA, July 25, 2023 – Larimar Therapeutics, Inc. (“Larimar”) (Nasdaq: LRMR), a clinical-stage biotechnology company focused on developing treatments for complex rare diseases, today announced that the U.S. Food and Drug Administration (FDA) has cleared the Company’s four-week, placebo-controlled, Phase 2 dose exploration trial of CTI-1601 in patients with Friedreich's ataxia (FA) to proceed to a 50 mg cohort in which participants will be dosed daily for the first 14 days, and then every other day until day 28. In addition, Larimar’s open label extension (OLE) trial was also cleared for initiation by the FDA. Participants in the OLE will receive 25 mg of CTI-1601 daily. CTI-1601 is a novel protein replacement therapy designed to deliver frataxin to the mitochondria of patients with FA who have low levels of frataxin.
Larimar received clearance to advance its Phase 2 trial to a 50 mg cohort and initiate its OLE trial following a review by the FDA of Larimar’s complete response to its partial clinical hold that included unblinded safety, pharmacokinetic (PK), and pharmacodynamic (PD) data from the Phase 2 trial’s completed 25 mg cohort. Data from the completed 25 mg cohort (n = 13) indicated that CTI-1601 was generally well tolerated and showed increases in frataxin (FXN) levels from baseline compared to placebo in all evaluated tissues (skin and buccal cells) at day 14 (the final day of daily dosing in the trial). Further dose escalation in the Phase 2 and OLE trials and the initiation of additional U.S. clinical trials evaluating CTI-1601 are contingent on FDA review of results from the Phase 2 trial’s 50 mg cohort in accordance with a partial clinical hold.
“Gaining clearance to advance to a 50 mg cohort in our Phase 2 trial and initiate the OLE trial are crucial steps in CTI-1601’s development as potentially the first therapy to increase frataxin levels in patients with FA,” said Carole Ben-Maimon, MD, President, and Chief Executive Officer of Larimar. “Given the inability of current treatments to address the frataxin deficiency underlying Friedreich's ataxia, we believe CTI-1601 has the potential to improve the treatment paradigm for this devastating disease. We now look forward to data from our Phase 2 trial’s 50 mg cohort in the first half of 2024, which will help us further characterize the safety and PK profiles of CTI-1601 and its ability to increase frataxin levels in a dose-dependent fashion as seen in our earlier Phase 1 studies.”
Participants who complete treatment in the Phase 2 dose exploration trial, or who previously completed a prior clinical trial of CTI-1601, are eligible to screen for Larimar’s OLE trial. Participants in the OLE trial will receive subcutaneous injections of 25 mg of CTI-1601 administered daily. The trial is expected to begin in Q1 2024 with interim data expected in Q4 2024.
Dr. Ben-Maimon continued, “We are pleased to have clearance to begin our OLE trial and look forward to what we expect will be important interim data from the study in Q4 2024. Alongside our efforts to advance both our OLE and Phase 2 trials in the United States, we have also begun to engage with regulators and investigators outside the U.S. as we prepare to expand our clinical
program to additional geographies. With approximately 75% of individuals with FA living outside the U.S., establishing global clinical trial capabilities is important for addressing the pressing unmet needs of the FA community.”
CTI-1601 has been granted Orphan Drug (U.S. and Europe), Rare Pediatric Disease (U.S.), Fast Track (U.S.), and PRIME (Europe) designations for FA.
Webcast and Conference Call
Larimar will host a conference call and webcast today, July 25, 2023 at 8:00 a.m. ET. To access the webcast please visit this link to the event. To participate by phone, please dial 1-877-407-9716 (domestic) or 1-201-493-6779 (international) and refer to conference ID 13740205 or click on this link and request a return call. Following the live event, the archived webcast will be available on the “Events & Presentations” page of the Larimar website. Following the live event, the archived webcast will be available for 90 days.
About the Phase 2 Trial
The Phase 2 trial is a placebo-controlled, four-week, dose exploration trial designed to further characterize CTI-1601’s safety, pharmacokinetic, and pharmacodynamic profiles. Eligible participants include ambulatory and non-ambulatory individuals with FA who are at least 18 years old. Participants enrolled in each cohort of the trial are randomized 2:1 to receive CTI-1601 or placebo daily via subcutaneous injections for the first 14 days, and then every other day until day 28. The trial currently includes two cohorts. Cohort 1 included 13 participants (9 on active treatment and 4 on placebo) and evaluated a 25 mg dose of CTI-1601. Cohort 2, which is initiating, will include 12-15 participants randomized 2:1 to CTI-1601 or placebo and will evaluate a 50 mg dose of CTI-1601. Key endpoints include safety assessments, pharmacokinetic assessments, as well as measures of frataxin levels and other pharmacodynamic markers (e.g., lipid profiles and gene expression data) in peripheral tissues. For more information on the trial, please visit clinicaltrials.gov under the identifier: NCT05579691.
About the Open Label Extension Trial
The open label extension trial is a multi-center study designed to enroll patients with FA who have previously completed a clinical trial of CTI-1601. Participants in the trial will receive daily subcutaneous injections of 25 mg of CTI-1601 with injections being self-administered or administered by a caregiver. The safety and pharmacodynamic objectives of the open-label extension trial are to evaluate the safety, tolerability, and pharmacokinetics of long-term subcutaneous administration of CTI-1601 as well as measures of frataxin levels and other pharmacodynamic markers (e.g., lipid profiles and gene expression data) in peripheral tissues. Other objectives include evaluation of the effects of long-term subcutaneous administration of CTI-1601 on measures of clinical function. Data collected during the trial will be compared to a matched set of untreated patients derived from participants in the Friedreich’s Ataxia Clinical Outcome Measures Study (FACOMS) database.
About Larimar Therapeutics
Larimar Therapeutics, Inc. (Nasdaq: LRMR), is a clinical-stage biotechnology company focused on developing treatments for complex rare diseases. Larimar’s lead compound, CTI-1601, is being developed as a potential treatment for Friedreich's ataxia. Larimar also plans to use its intracellular delivery platform to design other fusion proteins to target additional rare diseases characterized by deficiencies in intracellular bioactive compounds. For more information, please visit: https://larimartx.com.
Forward-Looking Statements
This press release contains forward-looking statements that are based on Larimar’s management’s beliefs and assumptions and on information currently available to management. All statements contained in this release other than statements of historical fact are forward-looking statements, including but not limited to Larimar’s ability to develop and commercialize CTI-1601 and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its CTI-1601 clinical trials and overall development plan and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations.
In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including CTI-1601 clinical milestones and continued interactions with the FDA regarding the partial clinical hold; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of CTI-1601 may not be predictive of the results or success of later clinical trials, and assessments; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale CTI-1601’s manufacturing process; Larimar’s ability to obtain regulatory approvals for CTI-1601 and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this press release represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law.
Investor Contact:
Joyce Allaire
LifeSci Advisors
jallaire@lifesciadvisors.com
(212) 915-2569
Company Contact:
Michael Celano
Chief Financial Officer
mcelano@larimartx.com
(484) 414-2715

July 2023 Larimar Therapeutics CTI-1601 Program Update

This presentation contains forward-looking statements that are based on the beliefs and assumptions of Larimar Therapeutics, Inc. ( “Company”) and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to Larimar’s ability to develop and commercialize CTI-1601 and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its CTI-1601 clinical trials and overall development plan and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, , the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including CTI-1601 clinical milestones and continued interactions with the FDA regarding the partial clinical hold; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of CTI-1601 may not be predictive of the results or success of later clinical trials, and assessments; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale CTI-1601’s manufacturing process; Larimar’s ability to obtain regulatory approvals for CTI-1601 and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law. Forward-Looking Statements
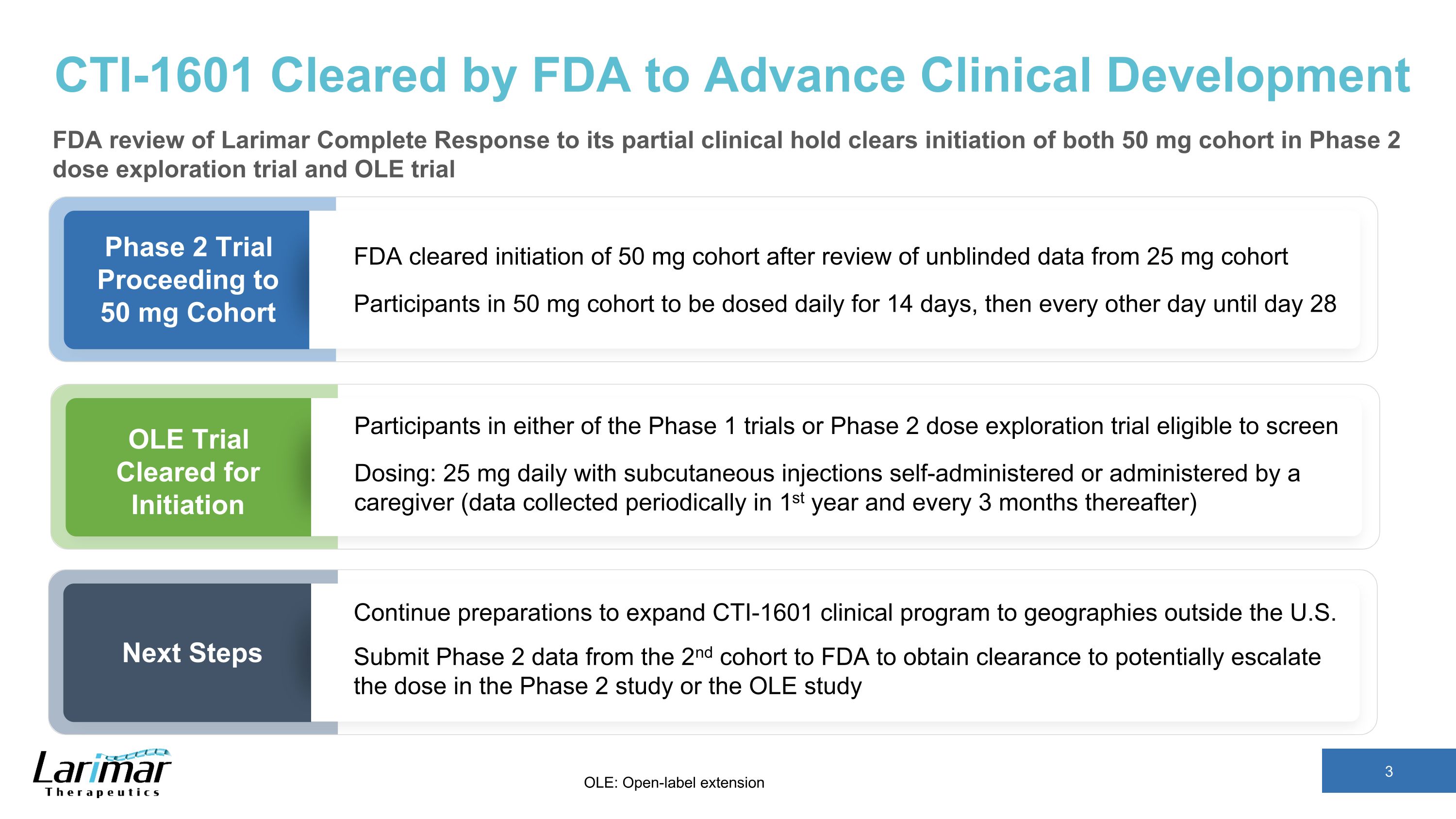
CTI-1601 Cleared by FDA to Advance Clinical Development FDA review of Larimar Complete Response to its partial clinical hold clears initiation of both 50 mg cohort in Phase 2 dose exploration trial and OLE trial Phase 2 Trial Proceeding to 50 mg Cohort OLE Trial Cleared for Initiation Next Steps FDA cleared initiation of 50 mg cohort after review of unblinded data from 25 mg cohort Participants in 50 mg cohort to be dosed daily for 14 days, then every other day until day 28 Participants in either of the Phase 1 trials or Phase 2 dose exploration trial eligible to screen Dosing: 25 mg daily with subcutaneous injections self-administered or administered by a caregiver (data collected periodically in 1st year and every 3 months thereafter) Continue preparations to expand CTI-1601 clinical program to geographies outside the U.S. Submit Phase 2 data from the 2nd cohort to FDA to obtain clearance to potentially escalate the dose in the Phase 2 study or the OLE study OLE: Open-label extension

Investment Highlights Clinical-stage biotechnology company focused on addressing unmet needs in Friedreich's ataxia (FA) and potentially other complex rare diseases based on a platform technology backed by a strong intellectual property portfolio Lead candidate CTI-1601 is a recombinant fusion protein designed to directly address frataxin deficiency by delivering the protein to mitochondria. CTI-1601 has received Orphan Drug (US & EU), Rare Pediatric Disease (US), Fast Track (US), & PRIME (EU) designations Two double-blind, placebo-controlled Phase 1 trials in FA demonstrating CTI-1601 was generally well tolerated when dosed daily for up to 13 days; dose-dependent increases in frataxin (FXN) levels from baseline vs. placebo were observed in all evaluated tissues Ongoing Phase 2, placebo-controlled, 4-week dose exploration study in FA; 25 mg cohort data show CTI-1601 is generally well tolerated, increasing FXN levels from baseline vs. placebo in skin and buccal cells; trial advancing to 50 mg cohort with data expected in 1H 2024; OLE trial with 25 mg daily dosing cleared for initiation in Q1 2024. To potentially escalate dose in Phase 2 study or the OLE study, submit Phase 2 data from 50 mg cohort to FDA due to continued partial clinical hold. $111.5 M cash balance (March 31, 2023) with projected cash runway into Q4 2024 Novel protein replacement therapy platform Potential first-ever therapy to increase frataxin levels Completed Phase 1 proof-of-concept Phase 2 and OLE studies with near-term catalysts Strong financial foundation

Caused by genetic defect resulting in low levels of frataxin Patients with FA only produce ~20-40% of normal frataxin levels depending on the tissue, sampling technique, and assay considered1 Affects ~20,000 patients globally, with ~5,000 patients in the U.S. and majority of the remaining patients in the EU Approximately 70% of patients present before age 14 Initial symptoms may include unsteady posture, frequent falling and progressive difficulty in walking. By the time symptoms occur, heart damage may have already occurred. Progressive disease: symptoms worsen and patients are eventually confined to a wheelchair with speech becoming hesitant and jerky (often referred to as “scanning speech”) Life expectancy of 30-50 years Early death usually caused by heart disease No available therapies increase frataxin levels Only treatment approved for FA does not address frataxin deficiency Friedreich’s Ataxia (FA) Rare and Progressive Disease 5 1. E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245 LRMR continues to have a strong relationship with Friedreich’s Ataxia Research Alliance Dedicated FA patient advocacy group focused on treatments for FA
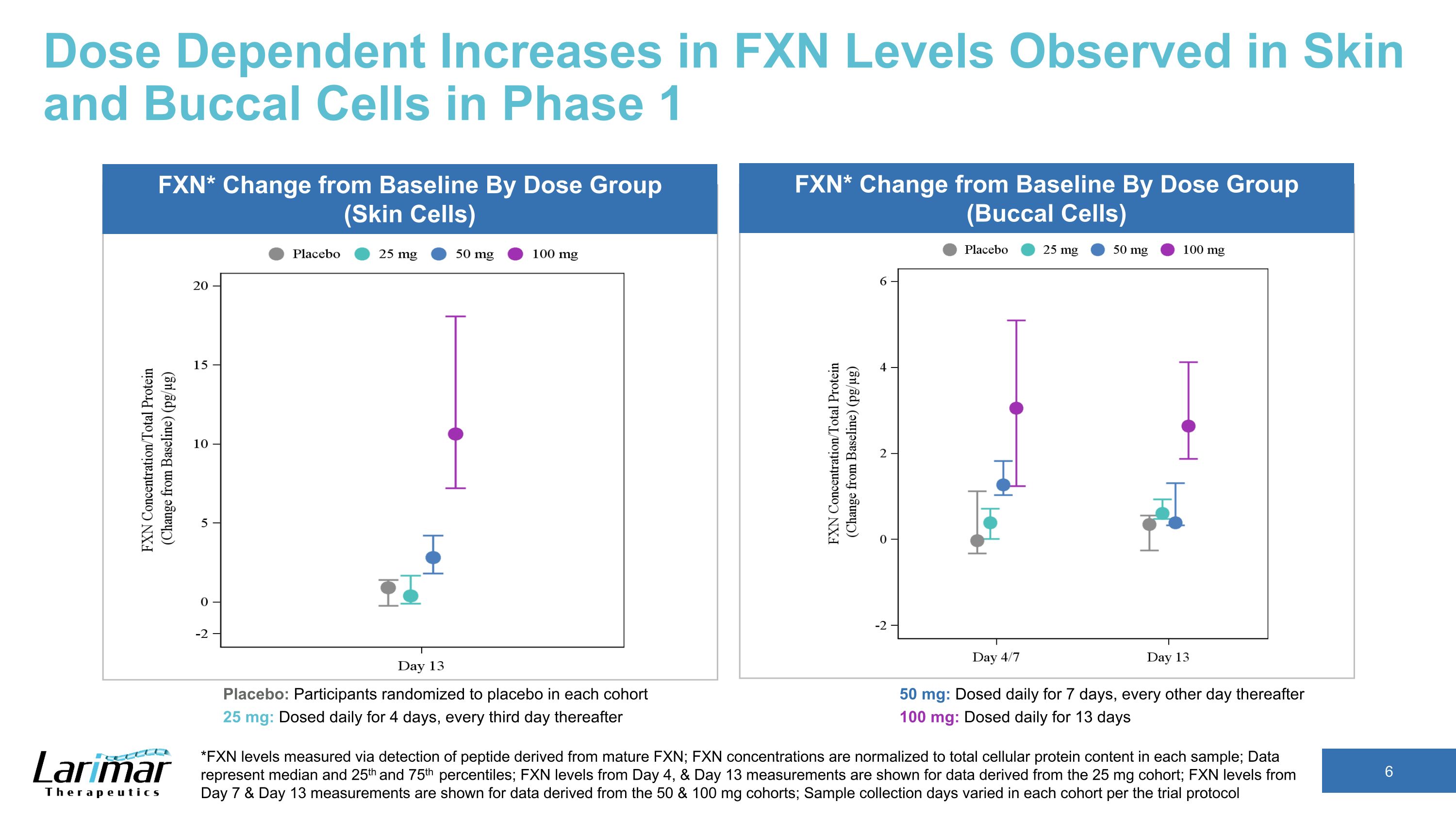
Dose Dependent Increases in FXN Levels Observed in Skin and Buccal Cells in Phase 1 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data represent median and 25th and 75th percentiles; FXN levels from Day 4, & Day 13 measurements are shown for data derived from the 25 mg cohort; FXN levels from Day 7 & Day 13 measurements are shown for data derived from the 50 & 100 mg cohorts; Sample collection days varied in each cohort per the trial protocol FXN* Change from Baseline By Dose Group (Skin Cells) FXN* Change from Baseline By Dose Group (Buccal Cells) Placebo: Participants randomized to placebo in each cohort 25 mg: Dosed daily for 4 days, every third day thereafter 50 mg: Dosed daily for 7 days, every other day thereafter 100 mg: Dosed daily for 13 days
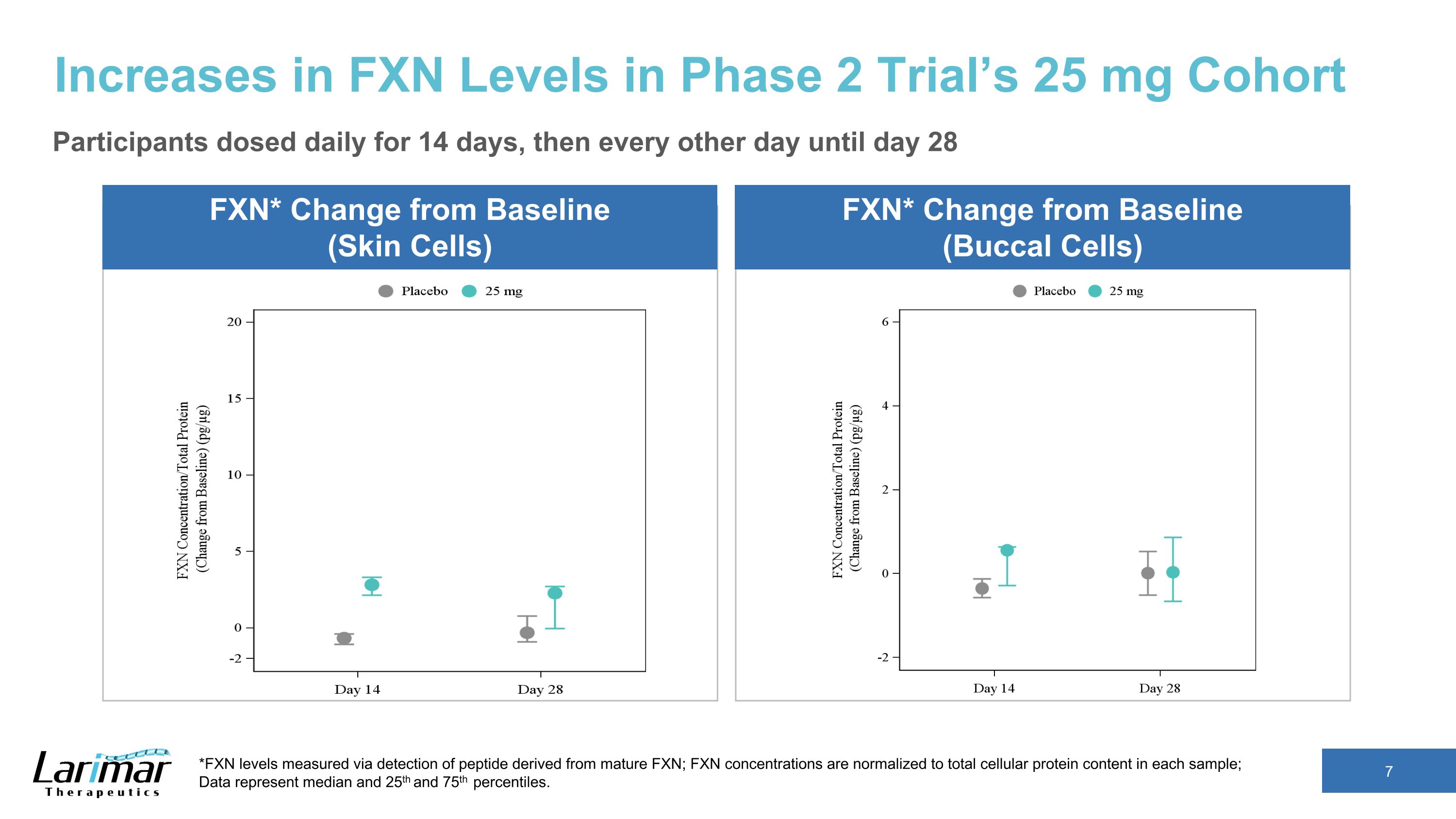
Increases in FXN Levels in Phase 2 Trial’s 25 mg Cohort FXN* Change from Baseline (Skin Cells) *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; �Data represent median and 25th and 75th percentiles. FXN* Change from Baseline (Buccal Cells) Participants dosed daily for 14 days, then every other day until day 28
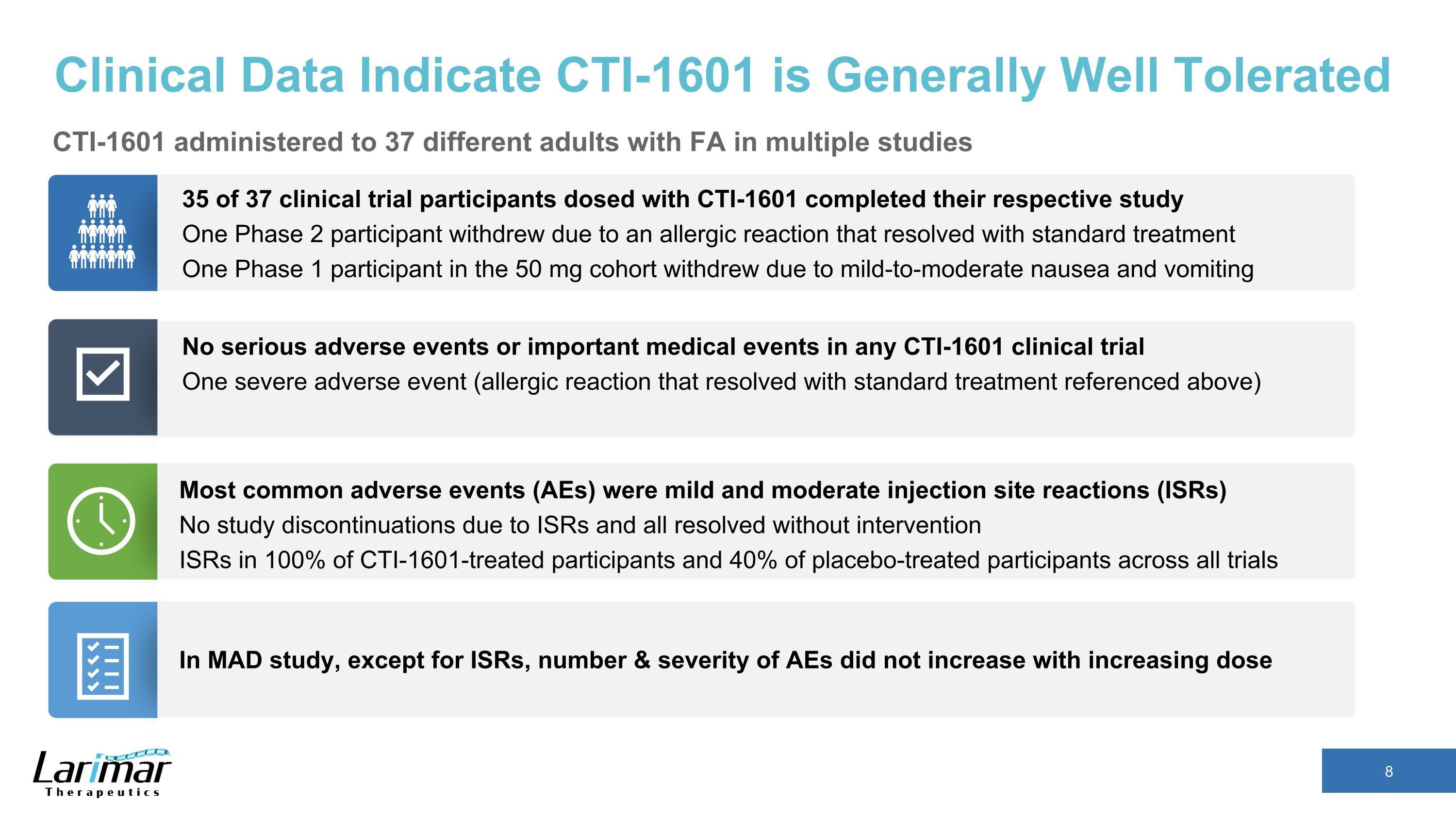
Clinical Data Indicate CTI-1601 is Generally Well Tolerated CTI-1601 administered to 37 different adults with FA in multiple studies 35 of 37 clinical trial participants dosed with CTI-1601 completed their respective study One Phase 2 participant withdrew due to an allergic reaction that resolved with standard treatment One Phase 1 participant in the 50 mg cohort withdrew due to mild-to-moderate nausea and vomiting No serious adverse events or important medical events in any CTI-1601 clinical trial One severe adverse event (allergic reaction that resolved with standard treatment referenced above) Most common adverse events (AEs) were mild and moderate injection site reactions (ISRs) No study discontinuations due to ISRs and all resolved without intervention ISRs in 100% of CTI-1601-treated participants and 40% of placebo-treated participants across all trials In MAD study, except for ISRs, number & severity of AEs did not increase with increasing dose
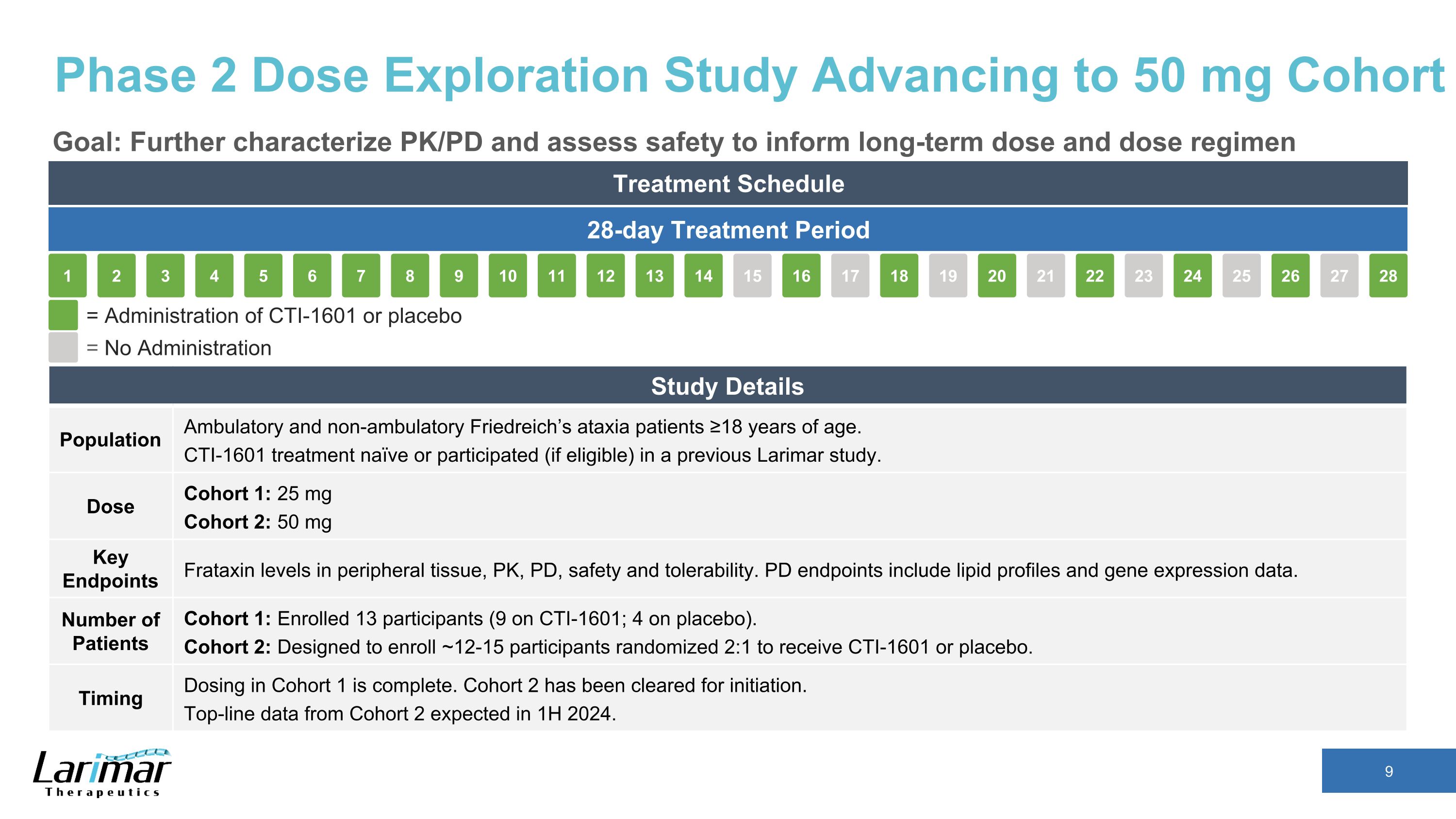
Phase 2 Dose Exploration Study Advancing to 50 mg Cohort Goal: Further characterize PK/PD and assess safety to inform long-term dose and dose regimen Treatment Schedule 28-day Treatment Period 16 17 18 19 15 20 21 22 23 24 25 26 27 28 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of CTI-1601 or placebo = No Administration Study Details Population Ambulatory and non-ambulatory Friedreich’s ataxia patients ≥18 years of age. CTI-1601 treatment naïve or participated (if eligible) in a previous Larimar study. Dose Cohort 1: 25 mg Cohort 2: 50 mg Key Endpoints Frataxin levels in peripheral tissue, PK, PD, safety and tolerability. PD endpoints include lipid profiles and gene expression data. Number of Patients Cohort 1: Enrolled 13 participants (9 on CTI-1601; 4 on placebo). Cohort 2: Designed to enroll ~12-15 participants randomized 2:1 to receive CTI-1601 or placebo. Timing Dosing in Cohort 1 is complete. Cohort 2 has been cleared for initiation. Top-line data from Cohort 2 expected in 1H 2024.
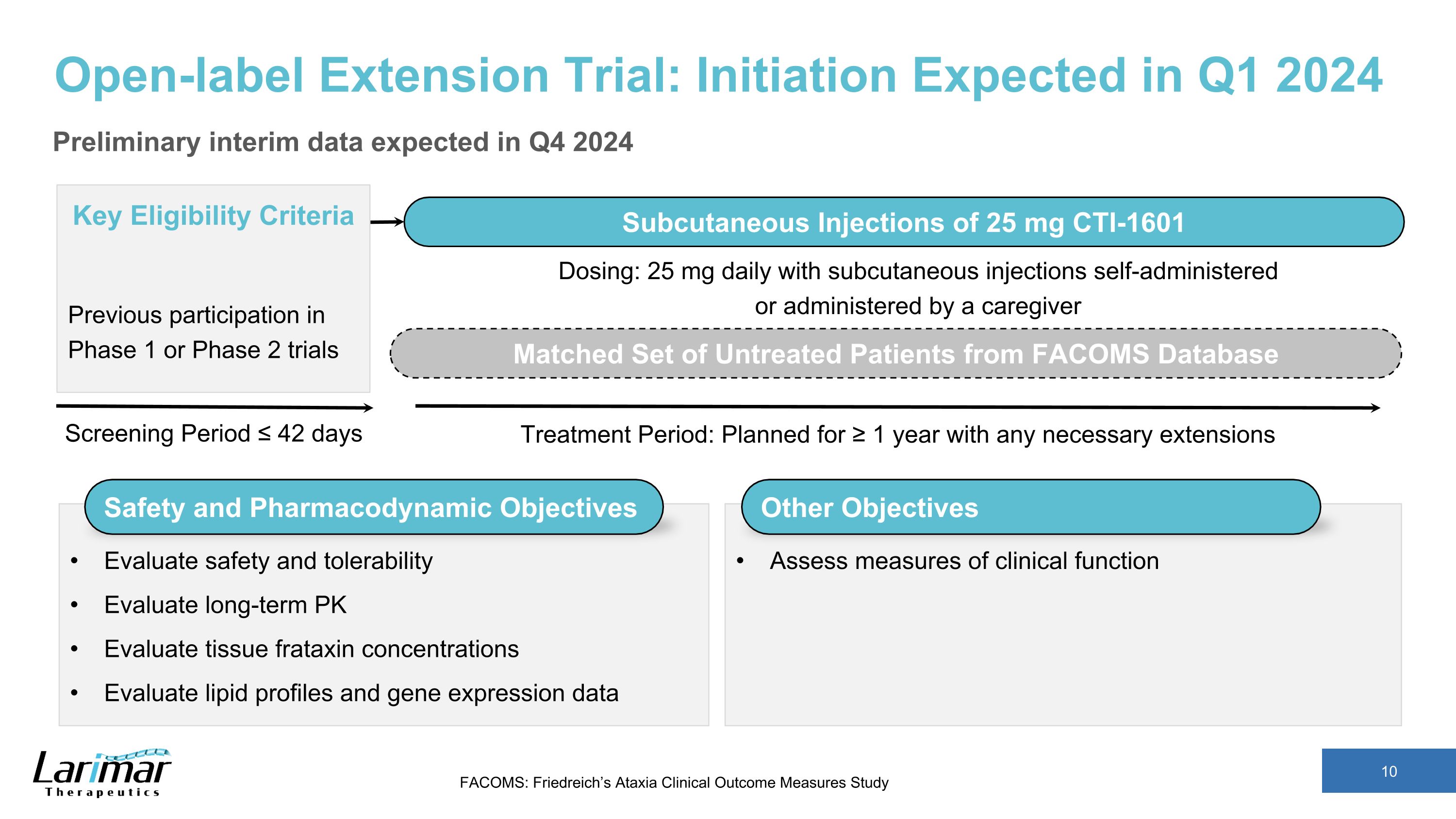
Open-label Extension Trial: Initiation Expected in Q1 2024 Preliminary interim data expected in Q4 2024 Screening Period ≤ 42 days Key Eligibility Criteria Previous participation in Phase 1 or Phase 2 trials Subcutaneous Injections of 25 mg CTI-1601 Treatment Period: Planned for ≥ 1 year with any necessary extensions Matched Set of Untreated Patients from FACOMS Database Safety and Pharmacodynamic Objectives Evaluate safety and tolerability Evaluate long-term PK Evaluate tissue frataxin concentrations Evaluate lipid profiles and gene expression data Dosing: 25 mg daily with subcutaneous injections self-administered or administered by a caregiver Other Objectives Assess measures of clinical function FACOMS: Friedreich’s Ataxia Clinical Outcome Measures Study

CTI-1601 Clinical Development Plan Ongoing and Planned Trials* Include: Global double-blind placebo-controlled pivotal trial*** Open-label extension trial with 25 mg daily dosing for eligible patients who participated in SAD, MAD, and/or four-week dose exploration studies. Initiation expected Q1 2024. MAD trial in patients 2 to 17 years of age**. Participants eligible to screen for OLE trial. Phase 2, four-week dose exploration study intended to identify dose and dose regimen for long-term studies. Advancing to 50 mg cohort. Top-line data from 50 mg cohort of Phase 2 dose exploration trial expected in 1H 2024 *Initiation of additional U.S. clinical trials are contingent on submission to FDA of additional data from adult patients due to partial clinical hold. **Company is planning discussions with FDA on how to best include patients 2 to 17 years of age in clinical development ***Company plans to initiate discussions with FDA on appropriate pivotal trial endpoints, including value of FXN levels. Also, the Company is planning discussions with regulators & investigators outside the U.S. to expand clinical program to international geographies.

Regulatory Updates Q3 2023: Initiation of 50 mg cohort in Phase 2 dose exploration trial Q1 2024: Initiation of open-label extension trial 1H 2024: Top-line data from Phase 2 trial’s 50 mg cohort Q4 2024: Interim data from open-label extension trial Expected Milestones CTI-1601 Phase 2 dose exploration trial of CTI-1601 in FA cleared to proceed to 50 mg cohort Open label extension trial with 25 mg daily dosing cleared for initiation Beginning preparations to expand CTI-1601 clinical program to ex-U.S. geographies Summary: CTI-1601 Cleared by FDA to Advance Clinical Development Generally well tolerated Dose-dependent increases in FXN levels in all evaluated tissues in Phase 1 Increases in FXN from baseline compared to placebo in all evaluated tissues (skin and buccal cells) in Phase 2
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Larimar Therapeutics (NASDAQ:LRMR)
Historical Stock Chart
From Apr 2024 to May 2024
Larimar Therapeutics (NASDAQ:LRMR)
Historical Stock Chart
From May 2023 to May 2024