Intercept Says FDA Sets AdCom Meeting on Obeticholic Acid
March 10 2023 - 10:05AM
Dow Jones News
By Colin Kellaher
Intercept Pharmaceuticals Inc. on Friday said the U.S. Food and
Drug Administration will hold an advisory committee meeting to
discuss the biopharmaceutical company's resubmitted application
seeking approval of obeticholic acid.
The Morristown, N.J., company, which is once again seeking an
FDA green light for obeticholic acid as a treatment for
pre-cirrhotic liver fibrosis due to the chronic liver condition
nonalcoholic steatohepatitis, commonly known as NASH, said the
panel plans to meet on May 19.
The FDA in 2020 rejected Intercept's initial application for
obeticholic acid, saying the company's efficacy data didn't
sufficiently outweigh potential risks.
Intercept conducted additional studies and resubmitted the
application in December, and the agency in January set a target
action date of June 22 for a decision.
The FDA often relies on advisory committees to provide
independent advice when a scientific, technical or policy question
arises, such as whether an unapproved product is safe and
effective, and the panels make non-binding recommendations that the
agency generally follows, although it isn't legally bound to do
so.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
March 10, 2023 09:50 ET (14:50 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
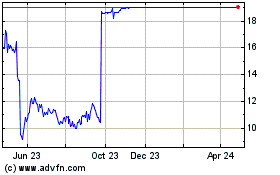
Intercept Pharmaceuticals (NASDAQ:ICPT)
Historical Stock Chart
From Mar 2024 to Apr 2024
Intercept Pharmaceuticals (NASDAQ:ICPT)
Historical Stock Chart
From Apr 2023 to Apr 2024