Intercept Reports More Positive Phase 3 Data From NASH Study
July 07 2022 - 8:29AM
Dow Jones News
By Colin Kellaher
Intercept Pharmaceuticals Inc. on Thursday reported positive
topline results from an interim analysis of its pivotal Phase 3
study of its proposed treatment of liver fibrosis due to
nonalcoholic steatohepatitis, a chronic liver condition known as
NASH.
The New York biopharmaceutical company said obeticholic acid
25-milligram met the agreed-upon primary endpoint of improvement in
liver fibrosis without worsening of NASH at 18 months and showed
double the response rate in reduction of fibrosis without worsening
of NASH compared with placebo.
Intercept said that the analysis is the second in which
obeticholic acid met the primary endpoint for the intent-to-treat
population in the study, and that based on the results, it plans to
resubmit its application seeking U.S. Food and Drug Administration
approval of the drug.
The FDA in 2020 rejected Intercept's initial application for
obeticholic acid, saying the company's efficacy data didn't
sufficiently outweigh potential risks to support accelerated
approval.
Intercept is slated to meet with the agency later this month to
discuss the resubmission.
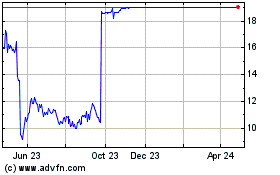
Trading in shares of Intercept, which closed Wednesday at
$15.29, was halted premarket Thursday.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
July 07, 2022 08:14 ET (12:14 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Intercept Pharmaceuticals (NASDAQ:ICPT)
Historical Stock Chart
From Mar 2024 to Apr 2024
Intercept Pharmaceuticals (NASDAQ:ICPT)
Historical Stock Chart
From Apr 2023 to Apr 2024