Filed pursuant to Rule 253(g)(2)
File No. 024-11617
OFFERING CIRCULAR
Dated: September 10, 2021
PURSUANT TO REGULATION A OF THE SECURITIES ACT OF 1933
U.S. STEM CELL, INC.
1560 Sawgrass Corporate Pkwy, 4th FL
Sunrise, Florida 33323
(954) 835-1500
250,000,000 Shares of Common Stock
at a price of $0.01 per Share
Minimum Investment: $25,000
Maximum Offering: $2,500,000
See The Offering - Page 11 and Securities Being Offered - Page 54 For Further Details. None of the Securities Offered Are Being Sold By Present Security Holders. This Offering Will Commence Upon Qualification of this Offering by the Securities and Exchange Commission and Will Terminate 365 days from the date of qualification by the Securities And Exchange Commission, Unless Extended or Terminated Earlier By The Issuer
PLEASE REVIEW ALL RISK FACTORS ON PAGES 12 THROUGH PAGE 17 BEFORE MAKING AN INVESTMENT IN THIS COMPANY. AN INVESTMENT IN THIS COMPANY SHOULD ONLY BE MADE IF YOU ARE CAPABLE OF EVALUATING THE RISKS AND MERITS OF THIS INVESTMENT AND IF YOU HAVE SUFFICIENT RESOURCES TO BEAR THE ENTIRE LOSS OF YOUR INVESTMENT, SHOULD THAT OCCUR.
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SELLING LITERATURE. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED HEREUNDER ARE EXEMPT FROM REGISTRATION.
Because these securities are being offered on a “best efforts” basis, the following disclosures are hereby made:
|
|
Price to Public
|
Commissions (1)
|
Proceeds to
Company (2)
|
Proceeds to
Other Persons (3)
|
|
Per Share
|
$0.01
|
$0
|
$0.01
|
None
|
|
Minimum Investment
|
$25,000
|
$0
|
$25,000
|
None
|
|
Maximum Offering
|
$2,500,000
|
$0
|
$2,500,000
|
None
|
(1) The Company shall pay no commissions to underwriters for the sale of securities under this Offering.
(2) Does not reflect payment of expenses of this Offering, which are estimated to not exceed $25,000 and which include, among other things, legal fees, accounting costs, reproduction expenses, due diligence, marketing, consulting, administrative services other costs of blue sky compliance, and actual out-of-pocket expenses incurred by the Company selling the Shares. This amount represents the proceeds of the offering to the Company, which will be used as set out in “USE OF PROCEEDS TO ISSUER.”
(3) There are no finder's fees or other fees being paid to third parties from the proceeds. See “PLAN OF DISTRIBUTION.”
This Offering (the “Offering”) consists of Common Stock (the “Shares” or individually, each a “Share”) that is being offered on a “best efforts” basis, which means that there is no guarantee that any minimum amount will be sold. The Shares are being offered and sold by U.S. Stem Cell, Inc., a Florida Corporation (the “Company”). There are 250,000,000 Shares being offered at a price of $0.01 per Share with a minimum purchase of $25,000 per investor. The Shares are being offered only by the Company on a best efforts basis to an unlimited number of accredited investors and to non-accredited investors based on the limitations of Regulation A. Under Rule 251(d)(2)(i)(C) of Regulation of Regulation A+, non-accredited, non-natural investors are subject to the investment limitation and may only invest funds which do not exceed 10% of the greater of the purchaser’s revenue or net assets (as of the purchaser’s most recent fiscal year end). A non-accredited, natural person may only invest funds which do not exceed 10% of the greater of the purchaser’s annual income or net worth (please see below on how to calculate your net worth). The maximum aggregate amount of the Shares offered is 250,000,000 Shares of Common Stock ($2,500,000 at the maximum offering price). There is no minimum number of Shares that needs to be sold in order for funds to be released to the Company and for this Offering to close.
The Shares are being offered pursuant to Regulation A of Section 3(b) of the Securities Act of 1933, as amended, for Tier II offerings. The Shares will only be issued to purchasers who satisfy the requirements set forth in Regulation A. The offering is expected to expire on the first of: (i) all of the Shares offered are sold; or (ii) the close of business 365 days from the date of qualification by the Commission, unless sooner terminated or extended by the Company's CEO. Pending each closing, payments for the Shares will be paid directly to the Company. Funds will be immediately transferred to the Company where they will be available for use in the operations of the Company's business in a manner consistent with the “USE OF PROCEEDS TO ISSUER” in this Offering Circular.
THIS OFFERING CIRCULAR DOES NOT CONSTITUTE AN OFFER OR SOLICITATION IN ANY JURISDICTION IN WHICH SUCH AN OFFER OR SOLICITATION WOULD BE UNLAWFUL. NO PERSON HAS BEEN AUTHORIZED TO GIVE ANY INFORMATION OR TO MAKE ANY REPRESENTATIONS CONCERNING THE COMPANY OTHER THAN THOSE CONTAINED IN THIS OFFERING CIRCULAR, AND IF GIVEN OR MADE, SUCH OTHER INFORMATION OR REPRESENTATION MUST NOT BE RELIED UPON.
PROSPECTIVE INVESTORS ARE NOT TO CONSTRUE THE CONTENTS OF THIS OFFERING CIRCULAR, OR OF ANY PRIOR OR SUBSEQUENT COMMUNICATIONS FROM THE COMPANY OR ANY OF ITS EMPLOYEES, AGENTS OR AFFILIATES, AS INVESTMENT, LEGAL, FINANCIAL OR TAX ADVICE.
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO www.investor.gov (WHICH IS NOT INCORPORATED BY REFERENCE INTO THIS OFFERING CIRCULAR).
This Offering is inherently risky. See “Risk Factors” beginning on page 12.
Sales of these securities will commence three calendar days of the qualification date and the filing of a Form 253(g)(2) Offering Circular AND it will be a continuous Offering pursuant to Rule 251(d)(3)(i)(F).
The Company is following the “Offering Circular” format of disclosure under Regulation A.
AN OFFERING STATEMENT PURSUANT TO REGULATION A RELATING TO THESE SECURITIES HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. INFORMATION CONTAINED IN THIS PRELIMINARY OFFERING CIRCULAR IS SUBJECT TO COMPLETION OR AMENDMENT. THESE SECURITIES MAY NOT BE SOLD NOR MAY OFFERS TO BUY BE ACCEPTED BEFORE THE OFFERING STATEMENT FILED WITH THE COMMISSION IS QUALIFIED. THIS PRELIMINARY OFFERING CIRCULAR SHALL NOT CONSTITUTE AN OFFER TO SELL OR THE SOLICITATION OF AN OFFER TO BUY NOR MAY THERE BE ANY SALES OF THESE SECURITIES IN ANY STATE IN WHICH SUCH OFFER, SOLICITATION OR SALE WOULD BE UNLAWFUL BEFORE REGISTRATION OR QUALIFICATION UNDER THE LAWS OF SUCH STATE. THE COMPANY MAY ELECT TO SATISFY ITS OBLIGATION TO DELIVER A FINAL OFFERING CIRCULAR BY SENDING YOU A NOTICE WITHIN TWO BUSINESS DAYS AFTER THE COMPLETION OF THE COMPANY’S SALE TO YOU THAT CONTAINS THE URL WHERE THE FINAL OFFERING CIRCULAR OR THE OFFERING STATEMENT IN WHICH SUCH FINAL OFFERING CIRCULAR WAS FILED MAY BE OBTAINED.
NASAA UNIFORM LEGEND
FOR RESIDENTS OF ALL STATES: THE PRESENCE OF A LEGEND FOR ANY GIVEN STATE REFLECTS ONLY THAT A LEGEND MAY BE REQUIRED BY THAT STATE AND SHOULD NOT BE CONSTRUED TO MEAN AN OFFER OR SALE MAY BE MADE IN A PARTICULAR STATE. IF YOU ARE UNCERTAIN AS TO WHETHER OR NOT OFFERS OR SALES MAY BE LAWFULLY MADE IN ANY GIVEN STATE, YOU ARE HEREBY ADVISED TO CONTACT THE COMPANY. THE SECURITIES DESCRIBED IN THIS OFFERING CIRCULAR HAVE NOT BEEN REGISTERED UNDER ANY STATE SECURITIES LAWS (COMMONLY CALLED 'BLUE SKY' LAWS).
IN MAKING AN INVESTMENT DECISION INVESTORS MUST RELY ON THEIR OWN EXAMINATION OF THE PERSON OR ENTITY CREATING THE SECURITIES AND THE TERMS OF THE OFFERING, INCLUDING THE MERITS AND RISKS INVOLVED. THESE SECURITIES HAVE NOT BEEN RECOMMENDED BY ANY FEDERAL OR STATE SECURITIES COMMISSION OR REGULATORY AUTHORITY. FURTHERMORE, THE FOREGOING AUTHORITIES HAVE NOT CONFIRMED THE ACCURACY OR DETERMINED THE ADEQUACY OF THIS DOCUMENT. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
NOTICE TO FOREIGN INVESTORS
IF THE PURCHASER LIVES OUTSIDE THE UNITED STATES, IT IS THE PURCHASER'S RESPONSIBILITY TO FULLY OBSERVE THE LAWS OF ANY RELEVANT TERRITORY OR JURISDICTION OUTSIDE THE UNITED STATES IN CONNECTION WITH ANY PURCHASE OF THE SECURITIES, INCLUDING OBTAINING REQUIRED GOVERNMENTAL OR OTHER CONSENTS OR OBSERVING ANY OTHER REQUIRED LEGAL OR OTHER FORMALITIES. THE COMPANY RESERVES THE RIGHT TO DENY THE PURCHASE OF THE SECURITIES BY ANY FOREIGN PURCHASER.
PATRIOT ACT RIDER
The Investor hereby represents and warrants that Investor is not, nor is it acting as an agent, representative, intermediary or nominee for, a person identified on the list of blocked persons maintained by the Office of Foreign Assets Control, U.S. Department of Treasury. In addition, the Investor has complied with all applicable U.S. laws, regulations, directives, and executive orders relating to anti-money laundering , including but not limited to the following laws: (1) the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001, Public Law 107-56, and (2) Executive Order 13224 (Blocking Property and Prohibiting Transactions with Persons Who Commit, Threaten to Commit, or Support Terrorism) of September 23, 2001.
NO DISQUALIFICATION EVENT (“BAD BOY” DECLARATION)
NONE OF THE COMPANY, ANY OF ITS PREDECESSORS, ANY AFFILIATED ISSUER, ANY DIRECTOR, EXECUTIVE OFFICER, OTHER OFFICER OF THE COMPANY PARTICIPATING IN THE OFFERING CONTEMPLATED HEREBY, ANY BENEFICIAL OWNER OF 20% OR MORE OF THE COMPANY'S OUTSTANDING VOTING EQUITY SECURITIES, CALCULATED ON THE BASIS OF VOTING POWER, NOR ANY PROMOTER (AS THAT TERM IS DEFINED IN RULE 405 UNDER THE SECURITIES ACT OF 1933) CONNECTED WITH THE COMPANY IN ANY CAPACITY AT THE TIME OF SALE (EACH, AN “ISSUER COVERED PERSON”) IS SUBJECT TO ANY OF THE “BAD ACTOR” DISQUALIFICATIONS DESCRIBED IN RULE 506(D)(1)(I) TO (VIII) UNDER THE SECURITIES ACT OF 1933 (A “DISQUALIFICATION EVENT”), EXCEPT FOR A DISQUALIFICATION EVENT COVERED BY RULE 506(D)(2) OR (D)(3) UNDER THE SECURITIES ACT. THE COMPANY HAS EXERCISED REASONABLE CARE TO DETERMINE WHETHER ANY ISSUER COVERED PERSON IS SUBJECT TO A DISQUALIFICATION EVENT.
Continuous Offering
Under Rule 251(d)(3) to Regulation A, the following types of continuous or delayed Offerings are permitted, among others: (1) securities offered or sold by or on behalf of a person other than the issuer or its subsidiary or a person of which the issuer is a subsidiary; (2) securities issued upon conversion of other outstanding securities; or (3) securities that are part of an Offering which commences within two calendar days after the qualification date. These may be offered on a continuous basis and may continue to be offered for a period in excess of 30 days from the date of initial qualification. They may be offered in an amount that, at the time the Offering statement is qualified, is reasonably expected to be offered and sold within one year from the initial qualification date. No securities will be offered or sold “at the market.” The supplement will not, in the aggregate, represent any change from the maximum aggregate Offering price calculable using the information in the qualified Offering statement. This information will be filed no later than two business days following the earlier of the date of determination of such pricing information or the date of first use of the Offering circular after qualification.
Sale of these shares will commence within three calendar days of the qualification date and it will be a continuous Offering pursuant to Rule 251(d)(3)(i)(F).
Subscriptions are irrevocable and the purchase price is non-refundable as expressly stated in this Offering Circular. The Company, by determination of the Board of Directors, in its sole discretion, may issue the Securities under this Offering for cash, promissory notes, services, and/or other consideration without notice to subscribers. All proceeds received by the Company from subscribers for this Offering will be available for use by the Company upon acceptance of subscriptions for the Securities by the Company.
Forward Looking Statement Disclosure
This Form 1-A, Offering Circular, and any documents incorporated by reference herein or therein contain forward-looking statements and are subject to risks and uncertainties. All statements other than statements of historical fact or relating to present facts or current conditions included in this Form 1-A, Offering Circular, and any documents incorporated by reference are forward-looking statements. Forward-looking statements give the Company's current reasonable expectations and projections relating to its financial condition, results of operations, plans, objectives, future performance, and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as 'anticipate,' 'estimate,' 'expect,' 'project,' 'plan,' 'intend,' 'believe,' 'may,' 'should,' 'can have,' 'likely' and other words and terms of similar meaning in connection with any discussion of the timing or nature of future operating or financial performance or other events. The forward-looking statements contained in this Form 1-A, Offering Circular, and any documents incorporated by reference herein or therein are based on reasonable assumptions the Company has made in light of its industry experience, perceptions of historical trends, current conditions, expected future developments and other factors it believes are appropriate under the circumstances. As you read and consider this Form 1-A, Offering Circular, and any documents incorporated by reference, you should understand that these statements are not guarantees of performance or results. They involve risks, uncertainties (many of which are beyond the Company's control) and assumptions. Although the Company believes that these forward-looking statements are based on reasonable assumptions, you should be aware that many factors could affect its actual operating and financial performance and cause its performance to differ materially from the performance anticipated in the forward-looking statements. Should one or more of these risks or uncertainties materialize, or should any of these assumptions prove incorrect or change, the Company's actual operating and financial performance may vary in material respects from the performance projected in these forward- looking statements. Any forward-looking statement made by the Company in this Form 1-A, Offering Circular or any documents incorporated by reference herein speaks only as of the date of this Form 1-A, Offering Circular or any documents incorporated by reference herein. Factors or events that could cause our actual operating and financial performance to differ may emerge from time to time, and it is not possible for the Company to predict all of them. The Company undertakes no obligation to update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.
About This Form 1-A and Offering Circular
In making an investment decision, you should rely only on the information contained in this Form 1-A and Offering Circular. The Company has not authorized anyone to provide you with information different from that contained in this Form 1-A and Offering Circular. We are offering to sell, and seeking offers to buy the Shares only in jurisdictions where offers and sales are permitted. You should assume that the information contained in this Form 1-A and Offering Circular is accurate only as of the date of this Form 1-A and Offering Circular, regardless of the time of delivery of this Form 1-A and Offering Circular. Our business, financial condition, results of operations, and prospects may have changed since that date. Statements contained herein as to the content of any agreements or other documents are summaries and, therefore, are necessarily selective and incomplete and are qualified in their entirety by the actual agreements or other documents.
TABLE OF CONTENTS
USE OF MARKET AND INDUSTRY DATA
This Offering Circular includes market and industry data that we have obtained from third-party sources, including industry publications, as well as industry data prepared by our management on the basis of its knowledge of and experience in the industries in which we operate (including our management’s estimates and assumptions relating to such industries based on that knowledge). Management has developed its knowledge of such industries through its experience and participation in these industries. While our management believes the third-party sources referred to in this Offering Circular are reliable, neither we nor our management have independently verified any of the data from such sources referred to in this Offering Circular or ascertained the underlying economic assumptions relied upon by such sources. Furthermore, internally prepared and third-party market prospective information, in particular, are estimates only and there will usually be differences between the prospective and actual results, because events and circumstances frequently do not occur as expected, and those differences may be material. Also, references in this Offering Circular to any publications, reports, surveys or articles prepared by third parties should not be construed as depicting the complete findings of the entire publication, report, survey or article. The information in any such publication, report, survey or article is not incorporated by reference in this Offering Circular.
We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where such offers and sales are permitted. You should rely only on the information contained in this Offering Circular. We have not authorized anyone to provide you with any information other than the information contained in this Offering Circular. The information contained in this Offering Circular is accurate only as of its date, regardless of the time of its delivery or of any sale or delivery of our securities. Neither the delivery of this Offering Circular nor any sale or delivery of our securities shall, under any circumstances, imply that there has been no change in our affairs since the date of this Offering Circular. This Offering Circular will be updated and made available for delivery to the extent required by the federal securities laws.
Unless otherwise indicated or the context otherwise requires, all references in this Offering Circular to “we,” “us,” “our,” “our company,” “U. S. Stem Cell, Inc.” or the “Company” refer to U.S. Stem Cell, Inc. and its subsidiaries.
OFFERING CIRCULAR SUMMARY
This summary highlights selected information contained elsewhere in this Offering Circular. This summary is not complete and does not contain all the information that you should consider before deciding whether to invest in our Common Stock. You should carefully read the entire Offering Circular, including the risks associated with an investment in the company discussed in the “Risk Factors” section of this Offering Circular, before making an investment decision. Some of the statements in this Offering Circular are forward-looking statements. See the section entitled “ Forward-Looking Statement Disclosure.”
Corporate Information
U.S. Stem Cell, Inc. was incorporated in the State of Florida in August 1999 as Bioheart, Inc. In 2015, we changed our name to U.S. Stem Cell, Inc. Our principal executive offices are located at 1560 Sawgrass Corporate Pkwy, 4th Floor, Sunrise, FL 33323 and our telephone number is (954) 835-1500. Information about us is available on our corporate website at www. us-stemcell.com. We include our website address in the Offering Circular on Form 1- only as an interactive textual reference and do not intend it to be an active link to our website. The information on our websites is expressly not incorporated by reference in this Offering Circular.
Mission Statement
We are a biotechnology company focused on the discovery, development and, subject to regulatory approval, commercialization of autologous cell therapies for the treatment of disease and injury. We are also a regenerative medicine company specializing in physician/veterinary training and certification and stem cell products.
Going Concern
As of June 30, 2021, the Company had cash on hand of $137,962 and a working capital deficit (current liabilities in excess of current assets) of $11,086,870. During the six months ended June 30, 2021, the net loss was $1,973,950 and net cash used in operating activities was $635,233. Our independent registered public accounting firm has issued its report in connection with the audit of our annual financial statements as of December 31, 2020, that included an explanatory paragraph describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern and the unaudited financial statements for the period ended June 30, 2021 also describes the existence of conditions that raise substantial doubt about our ability to continue as a going concern. These factors raise substantial doubt about our ability to continue as a going concern within one year after the date the financial statements are issued. The Company’s financial statements do not include any adjustments that might result from the outcome of this uncertainty should we be unable to continue as a going concern.
Management estimates that the current funds on hand will be sufficient to continue operations through the next six months. Our ability to continue as a going concern is dependent upon our ability to continue to implement our business plan. Our management intends to continue the current business strategy, to the extent possible, to finance their clinical development pipeline through revenue (cash in-flows) generated through the marketing and sales of unique educational and training services, animal health products and distribution of products in the industry as well as evaluate and act upon opportunities to increase our top line revenue position and that correspondingly increase cash in-flows. These opportunities include but are not limited to the development and marketing of new products and services, mergers and acquisitions, joint ventures, licensing deals and more.
Trading Market
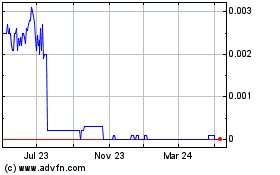
Our Common Stock trades in the OTC Market under the symbol “USRM”.
We are Offering, on a best-efforts, a number of shares of our common stock at a per share price of $0.01 to be sold up to a maximum of 250,000,000 shares. The fixed price per share determined upon qualification shall be fixed for the duration of the Offering unless a post-effective amendment is filed to reset the price per share and approved by the Securities and Exchange Commission. There is a minimum investment of $25,000 per investor. The shares are intended to be sold directly through the efforts of our officers and directors.
We have two billion (2,000,000,000) authorized common stock shares, of which there are 456,217,501 issued and outstanding as of August 27, 2021. We have 20,000,000 authorized Preferred Shares, of which none are outstanding.
We are quoted on the OTC Pink Sheet Market and there is a limited established market for our stock. The Offering price of the Shares has been determined arbitrarily by us. The price does not bear any relationship to our assets, book value, earnings, or other established criteria for valuing a privately held company. In determining the number of shares to be offered and the Offering price, we took into consideration our capital structure and the amount of money we would need to implement our business plans. Accordingly, the Offering price should not be considered an indication of the actual value of our securities.
The Offering
This is a public Offering of securities of U.S. Stem Cell, Inc., a Florida corporation. We are offering 250,000,000 shares of our Common Stock at an Offering price of $0.01 per share (the “Offered Shares” or “Shares”). This Offering will terminate on twelve months from the day the Offering is qualified or the date on which the maximum Offering amount is sold (such earlier date, the “Termination Date”). The minimum purchase requirement per investor is $25,000.
These securities are speculative securities. Investment in the Company’s stock involves significant risk. You should purchase these securities only if you can afford a complete loss of your investment. See “Risk Factors” on page 12 to read about factors you should consider before buying shares of Common Stock.
Our Common Stock currently trades on the OTC Market under the symbol “USRM” and the closing price of our Common Stock on August 24, 2021 was $0.012.
We are offering our shares without the use of an exclusive placement agent. We expect to commence the sale of the shares as of the date on which the Offering Statement is Qualified by the SEC.
As there is no minimum Offering, upon the approval of any subscription to this Offering Circular, we shall immediately deposit said proceeds into our bank account and may dispose of the proceeds in accordance with the Use of Proceeds.
This Offering will be conducted on a “best-efforts” basis, which means our Officers will use their commercially reasonable best efforts to offer and sell the Shares. Our Officers will not receive any commission or any other remuneration for these sales. Any Officer offering the securities on our behalf will rely on the safe harbor from broker-dealer registration set out in Rule 3a4-1 under the Securities Exchange Act of 1934, as amended.
This Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sales of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful, prior to registration or qualification under the laws of any such state.
As there is no minimum Offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit said proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds.
Completion of this Offering is not subject to us raising a minimum Offering amount. We do not have an arrangement to place the proceeds from this Offering in an escrow, trust or similar account. Any funds raised from the Offering will be immediately available to us for our immediate use. We have provided an estimate below of the gross proceeds to be received by the Company if 25%, 50%, 75%, and 100% of the Shares registered in the Offering are sold.
In order to subscribe to purchase the shares, a prospective investor must complete a subscription agreement and send payment by check, wire transfer or ACH. We have not currently engaged any party for the public relations or promotion of this Offering. As of the date of this filing, there are no additional offers for shares, nor any options, warrants, or other rights for the issuance of additional shares except those described herein.
We are Offering to sell, and seeking offers to buy, our securities only in jurisdictions where such offers and sales are permitted. You should rely only on the information contained in this Offering Circular. We have not authorized anyone to provide you with any information other than the information contained in this Offering Circular. The information contained in this Offering Circular is accurate only as of its date, regardless of the time of its delivery or of any sale or delivery of our securities. Neither the delivery of this Offering Circular nor any sale or delivery of our securities shall, under any circumstances, imply that there has been no change in our affairs since the date of this Offering Circular. This Offering Circular will be updated and made available for delivery to the extent required by the federal securities laws.
Section 15(g) of the Securities Exchange Act of 1934
Our shares are covered by section 15(g) of the Securities Exchange Act of 1934, as amended that imposes additional sales practice requirements on broker/dealers who sell such securities to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 or individuals with net worth in excess of $1,000,000, excluding their primary residences or annual income exceeding $200,000 or $300,000 jointly with their spouses). For transactions covered by the Rule, the broker/dealer must make a special suitability determination for the purchase and have received the purchaser’s written agreement to the transaction prior to the sale. Consequently, the Rule may affect the ability of broker/dealers to sell our securities and may affect your ability to sell your shares in the secondary market.
Section 15(g) also imposes additional sales practice requirements on broker/dealers who sell penny securities. These rules require a one-page summary of certain essential items. The items include the risk of investing in penny stocks in both public Offerings and secondary marketing; terms important to in understanding of the function of the penny stock market, such as bid and offer quotes, a dealers spread and broker/dealer compensation; the broker/dealer compensation, the broker/dealers’ duties to its customers, including the disclosures required by any other penny stock disclosure rules; the customers’ rights and remedies in cases of fraud in penny stock transactions; and, FINRA’s toll free telephone number and the central number of the North American Administrators Association, for information on the disciplinary history of broker/dealers and their associated persons.
The Offering
This Offering Circular relates to the sale of up to 250,000,000 shares of our Common Stock, through the efforts of our executive officers and directors, at a price of $0.01 per share, for total Offering proceeds of up to $2,500,000, if all Offered Shares are sold. The minimum amount established for investors is $25,000 unless such minimum is waived by the Company, in its sole discretion, on a case-by-case basis. There is no minimum aggregate Offering amount and the Company will not escrow or return investor funds if any minimum number of shares is not sold. All money we receive from the Offering will be immediately available to us for the uses set forth in the “Use of Proceeds” section of this Offering Circular.
|
Type of Stock Offering:
|
Common Stock
|
|
|
|
|
Price Per Share:
|
$0.01
|
|
|
|
|
Minimum Investment:
|
$25,000 per investor (250,000 Shares of Common Stock at the Maximum Offering Price)
|
|
|
|
|
Maximum Offering:
|
$2,500,000. The Company will not accept investments greater than the Maximum Offering amount.
|
|
|
|
|
Maximum Shares Offered:
|
250,000,000 Shares of Common Stock
|
|
|
|
|
Use of Proceeds:
|
See the description in section entitled “USE OF PROCEEDS TO ISSUER” on page 24 herein.
|
|
|
|
|
Voting Rights:
|
The Shares have full voting rights.
|
|
|
|
|
Length of Offering:
|
Shares will be offered on a continuous basis until either (1) the maximum number of Shares or sold; (2) 365 days from the date of qualification by the Commission; or (3) the Company in its sole discretion withdraws this
Offering.
|
|
|
|
|
Risk factors:
|
Investing in our Common Stock involves risks. See “Risk Factors” for a discussion of certain factors that you should carefully consider before making an investment decision.
|
|
|
|
|
Dividend policy:
|
Holders of our Common Stock are only entitled to receive dividends when, as and if declared by our board of directors out of funds legally available for dividends. We do not intend to pay dividends for the foreseeable future. Our ability to pay dividends to our stockholders in the future will depend on regulatory restrictions, liquidity and capital requirements, our earnings and financial condition, the general economic climate, our ability to service any equity or debt obligations senior to our Common Stock and other factors deemed relevant by our board of directors. For additional information, see “Dividend Policy.”
|
|
|
|
|
Use of proceeds:
|
See “Use of Proceeds” beginning on page 25.
|
|
Common Stock Outstanding as of August 27, 2021 (1)
|
456,217,501 Shares
|
|
Common Stock in this Offering
|
250,000,000 Shares
|
|
Stock to be outstanding after the offering (2)
|
706,217,501 Shares
|
|
|
(1)
|
The Company has also authorized 20,000,000 shares of preferred stock, of which 0 are issued and outstanding. No preferred stock is being sold in this Offering.
|
|
|
(2)
|
The total number of Shares of Common Stock assumes that the maximum number of Shares are sold in this Offering. The Company may not be able to sell the Maximum Offering Amount. The Company will conduct one or more closings on a rolling basis as funds are received from investors. The net proceeds of the Offering will be the gross proceeds of the Shares sold minus the expenses of the offering. Investors should not assume that the Offered Shares will be listed. A consistent public trading market for the shares may not develop.
|
Investment Analysis
There is no assurance U.S. Stem Cell, Inc. will be profitable, or that management's opinion of the Company's future prospects will not be outweighed by the unanticipated losses, adverse regulatory developments and other risks. Investors should carefully consider the various risk factors below before investing in the Shares.
ABOUT THIS OFFERING CIRCULAR
We have prepared this Offering Circular to be filed with the Securities and Exchange Commission for our Offering of securities. The Offering Circular includes exhibits that provide more detailed descriptions of the matters discussed in this circular. You should rely only on the information contained in this circular and its exhibits. The Company has not authorized any person to provide you with any information different from that contained in this Offering Circular. The information contained in this Offering Circular is complete and accurate only as of the date of this Offering Circular, regardless of the time of delivery of this circular or sale of our Shares. This Offering Circular contains summaries of certain other documents, but reference is hereby made to the full text of the actual documents for complete information concerning the rights and obligations of the parties thereto. All documents relating to this Offering and related documents and agreements, if readily available to us, will be made available to a prospective investor or its representatives upon request.
TAX CONSIDERATIONS
No information contained herein, nor in any prior, contemporaneous or subsequent communication should be construed by a prospective investor as legal or tax advice. We are not providing any tax advice as to the acquisition, holding or disposition of the securities offered herein. In making an investment decision, investors are strongly encouraged to consult their own tax advisor to determine the U.S. Federal, state and any applicable foreign tax consequences relating to their investment in our securities. This written communication is not intended to be “written advice,” as defined in Circular 230 published by the U.S. Treasury Department.
RISK FACTORS
The purchase of the Company's Common Stock involves substantial risks. You should carefully consider the following risk factors in addition to any other risks associated with this investment. The Shares offered by the Company constitute a highly speculative investment and you should be in an economic position to lose your entire investment. The risks listed do not necessarily comprise all those associated with an investment in the Shares and are not set out in any particular order of priority. Additional risks and uncertainties may also have an adverse effect on the Company's business and your investment in the Shares. An investment in the Company may not be suitable for all recipients of this Offering Circular. You are advised to consult an independent professional adviser or attorney who specializes in investments of this kind before making any decision to invest. You should consider carefully whether an investment in the Company is suitable in the light of your personal circumstances and the financial resources available to you.
The discussions and information in this Offering Circular may contain both historical and forward- looking statements. To the extent that the Offering Circular contains forward-looking statements regarding the financial condition, operating results, business prospects, or any other aspect of the Company's business, please be advised that the Company's actual financial condition, operating results, and business performance may differ materially from that projected or estimated by the Company in forward-looking statements. The Company has attempted to identify, in context, certain of the factors it currently believes may cause actual future experience and results may differ from the Company's current expectations.
SHOULD ONE OR MORE OF THE FOREGOING RISKS OR UNCERTAINTIES MATERIALIZE, OR SHOULD THE UNDERLYING ASSUMPTIONS OF OUR BUSINESS PROVE INCORRECT, ACTUAL RESULTS MAY DIFFER SIGNIFICANTLY FROM THOSE ANTICIPATED, BELIEVED, ESTIMATED, EXPECTED, INTENDED OR PLANNED.
Before investing, you should carefully read and carefully consider the following risk factors:
Risks Relating to the Company and Its Business
The Company is dependent upon its management, key personnel, and consultants to execute its business plan.
The Company's success is heavily dependent upon the continued active participation of the Company's current executive officer, Mike Tomas. Loss of this individual could have a material adverse effect upon the Company's business, financial condition, or results of operations. Further, the Company's success and achievement of the Company's growth plans depend on the Company's ability to recruit, hire, train and retain other highly qualified technical and managerial personnel. Competition for qualified employees among companies in the health care, and the loss of any of such persons, or an inability to attract, retain and motivate any additional highly skilled employees required for the expansion of the Company's activities, could have a materially adverse effect on its ability to operate. The inability to attract and retain the necessary personnel, consultants and advisors could have a material adverse effect on the Company's business, financial condition, or results of operations.
The Company's bank accounts will not be fully insured.
The Company's regular bank accounts have federal insurance that is limited to a certain amount of coverage. It is anticipated that the account balances in each account may exceed those limits at times. In the event that any of the Company's banks should fail, the Company may not be able to recover all amounts deposited in these bank accounts.
The Company's business plan is speculative.
The Company's present business and planned business are speculative and subject to numerous risks and uncertainties. There is no assurance that the Company will generate significant revenues or profits.
The Company's expenses could increase without a corresponding increase in revenues.
The Company's operating and other expenses could increase without a corresponding increase in revenues, which could have a material adverse effect on the Company's financial results and on your investment. Factors which could increase operating and other expenses include, but are not limited to (1) increases in the rate of inflation, (2) increases in taxes and other statutory charges, (3) changes in laws, regulations, or government policies which increase the costs of compliance with such laws, regulations, or policies, (4) significant increases in insurance premiums, and (5) increases in borrowing costs.
Risks Related to our Intellectual Property
Our success will depend on our ability to obtain and maintain meaningful intellectual property protection for any such intellectual property. The names and/or logos of Company brands (whether owned by the Company or licensed to us) may be challenged by holders of trademarks who file opposition notices, or otherwise contest trademark applications by the Company for its brands. Similarly, domains owned and used by the Company may be challenged by others who contest the ability of the Company to use the domain name or URL. Current or future patent applications may not result in issued patents which could hinder ability to obtain and maintain other rights to technology required or desirable for the conduct of our business. Such challenges could have a material adverse effect on the Company's financial results as well as your investment.
Changes in the economy could have a detrimental impact on the Company.
Changes in the general economic climate could have a detrimental impact on consumer expenditure and, therefore, on the Company's revenue. It is possible that recessionary pressures and other economic factors (such as declining incomes, future potential rising interest rates, higher unemployment and tax increases) may adversely affect customers' confidence and willingness to spend. Any of such events or occurrences could have a material adverse effect on the Company's financial results and on your investment.
Limitation on director liability.
The Company may provide for the indemnification of directors to the fullest extent permitted by law and, to the extent permitted by such law, eliminate or limit the personal liability of directors to the Company and its shareholders for monetary damages for certain breaches of fiduciary duty. Such indemnification may be available for liabilities arising in connection with this Offering. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the Company pursuant to the foregoing provisions, the Company has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Risks Related to Our Financial Position and Need for Additional Financing
We will need to secure additional financing in 2021 in order to continue to finance our operations. If we are unable to secure additional financing on acceptable terms, or at all, we may be forced to curtail or cease our operations.
As of December 31, 2020, we had cash and cash equivalents of $18,570 and an accumulated capital deficit of $136,514,508. At June 30, 2021, we had cash and cash equivalents totaling $137,962. However, our accumulated capital deficit as of June 30, 2021 was $138,488,458. As such, our existing cash resources are insufficient to finance even our immediate operations. Accordingly, we will need to secure additional sources of capital to develop our business and product candidates as planned. We are seeking substantial additional financing through public and/or private financing, which may include equity and/or debt financings, research grants and through other arrangements, including collaborative arrangements.
As part of such efforts, we may seek loans from certain of our executive officers, directors and/or current shareholders. We may also seek to satisfy some of our obligations to the guarantors of our loan with Seaside National Bank & Trust, or the Guarantors, through the issuance of various forms of securities or debt on negotiated terms. The Company renewed the loan with Seaside National Bank and Trust during the first quarter of 2020 to extend the maturity date to May 18, 2022, all other terms and conditions remain unchanged. However, financing and/or alternative arrangements with the Guarantors may not be available when we need it, or may not be available on acceptable terms.
If we are unable to secure additional financing in the near term, we may be forced to:
|
|
●
|
curtail or abandon our existing business plans;
|
|
|
●
|
reduce our headcount;
|
|
|
●
|
default on our debt obligations;
|
|
|
●
|
file for bankruptcy;
|
|
|
●
|
seek to sell some or all of our assets; and/or
|
|
|
●
|
Cease our operations.
|
If we are forced to take any of these steps, any investment in our common stock may be worthless.
If we raise additional capital and/or secure alternative arrangements, with the Guarantors or otherwise, by issuing equity, equity-related or convertible securities, the economic, voting and other rights of our existing shareholders may be diluted, and those newly issued securities may be issued at prices that are a significant discount to current and/or then prevailing market prices. In addition, any such newly issued securities may have rights superior to those of our common stock. If we obtain additional capital through collaborative arrangements, we may be required to relinquish greater rights to our technologies or product candidates than we might otherwise have or become subject to restrictive covenants that may affect our business.
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern.
Our independent registered public accounting firm issued its report in connection with the audit of our financial statements as of December 31, 2020, which included an explanatory paragraph describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern. In addition, the notes to our financial statements for the quarter ended June 30, 2021 included an explanatory paragraph describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern for a reasonable period of time. If we are not able to continue as a going concern, it is likely that holders of our common stock will lose all of their investment. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We are a development stage life sciences company with a limited operating history and a history of net losses and negative cash flows from operations. We may never be profitable, and if we incur operating losses and generate negative cash flows from operations for longer than expected, we may be unable to continue operations.
We are a development stage life sciences company and have a limited operating history, limited capital, limited sources of revenue, and have incurred losses since inception. Our operations to date have been limited to organizing our company, developing and engaging in clinical trials of our MyoCell™ product candidate, expanding our pipeline of complementary product candidates through internal development and third party licenses, expanding and strengthening our intellectual property position through internal programs and third party licenses and recruiting management, research and clinical personnel. Consequently, it may be difficult to predict our future success or viability due to our lack of operating history. As of December 31, 2020, we have accumulated a deficit of $136,514,508 million and as of June 30, 2021, we have an accumulated deficit of $138,488,458. Our MyoCell™ product candidate has not received regulatory approval or generated any material revenues and is not expected to generate any material revenues until commercialization of MyoCell, if ever.
Our ability to continue generating revenues from any of our product candidates will depend on a number of factors, including our ability to successfully complete clinical trials, obtain necessary regulatory approvals and implement our commercialization strategy. In addition, even if we are successful in obtaining necessary regulatory approvals and bringing one or more product candidates to market, we will be subject to the risk that the marketplace will not accept those products. We may, and anticipate that we will need to, transition from a company with a research and development focus to a company capable of supporting commercial activities and we may not succeed in such a transition.
Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict the extent of our future losses or when or if we will become profitable. Our failure to successfully commercialize our product candidates or to become and remain profitable could impair our ability to raise capital, expand our business, diversify our product offerings and continue our operations.
The amount of capital the Company is attempting to raise in this Offering is not enough to sustain the Company’s current business plan.
In order to achieve the Company's near and long-term goals, the Company will need to procure funds in addition to the amount raised in the Offering. There is no guarantee the Company will be able to raise such funds on acceptable terms, if at all. If we are not able to raise sufficient capital in the future, we will not be able to execute our business plan, our continued operations will be in jeopardy and we may be forced to cease operations and sell or otherwise transfer all or substantially all of our remaining assets, which could cause you to lose all or a portion of your investment.
Additional financing may be necessary for the implementation of our growth strategy.
The Company may require additional debt and/or equity financing to pursue our growth and business strategies. These include, but are not limited to enhancing our operating infrastructure and otherwise respond to competitive pressures. Given our limited operating history and existing losses, there can be no assurance that additional financing will be available, or, if available, that the terms will be acceptable to us. Lack of additional funding could force us to substantially curtail our growth plans. Furthermore, the issuance by us of any additional securities pursuant to any future fundraising activities undertaken by us would dilute the ownership of existing shareholders and may reduce the price of our Shares.
Risks Relating to This Offering and Investment
The Company may undertake additional equity or debt financing that may dilute the Shares in this Offering.
The Company may undertake further equity or debt financing, which may be dilutive to existing shareholders, including you, or result in an issuance of securities whose rights, preferences and privileges are senior to those of existing shareholders, including you, and also reducing the value of Shares subscribed for under this Offering.
An investment in the Shares is speculative and there can be no assurance of any return on such investment.
An investment in the Company's Shares is speculative, and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
The Shares are offered on a “best efforts” basis and the Company may not raise the maximum amount being offered.
Since the Company is offering the Shares on a “best efforts” basis, there is no assurance that the Company will sell enough Shares to meet its capital needs. If you purchase Shares in this Offering, you will do so without any assurance that the Company will raise enough money to satisfy the full Use Of Proceeds To Issuer which the Company has outlined in this Offering Circular or to meet the Company's working capital needs. If the maximum Offering amount is not sold, we may need to incur additional debt or raise additional equity in order to finance our operations. Increasing the amount of debt will increase our debt service obligations and make less cash available for distribution to our shareholders. Increasing the amount of additional equity that we will have to seek in the future will further dilute those investors participating in this Offering.
We have not paid dividends in the past and do not anticipate paying them in the future. You return on investment, if any, will be limited to the market value of the Shares you purchase.
We have never paid cash dividends on our Shares and do not anticipate paying cash dividends in the future. Since we do not pay dividends, our Shares may be less valuable because a return on your investment will only occur if the market value of the Shares appreciates beyond your purchase price. While the Company may choose to pay dividends at some point in the future to its shareholders, there can be no assurance that cash flow and profits will allow such distributions to ever be made.
The Company may not be able to obtain additional financing.
Even if the Company is successful in selling the maximum number of Shares in the Offering, the Company may require additional funds to continue and grow its business. The Company may not be able to obtain additional financing as needed, on acceptable terms, or at all, which would force the Company to delay its plans for growth and implementation of its strategy which could seriously harm its business, financial condition and results of operations. If the Company needs additional funds, the Company may seek to obtain them primarily through additional equity or debt financings. Those additional financings could result in dilution to the Company's current shareholders and to you if you invest in this Offering.
The offering price has been arbitrarily determined.
The offering price of the Shares has been arbitrarily established by the Company based upon its present and anticipated financing needs and bears no relationship to the Company's present financial condition, assets, book value, projected earnings, or any other generally accepted valuation criteria. The offering price of the Shares may not be indicative of the value of the Shares or the Company, now or in the future.
The management of the Company has broad discretion in application and use of Offering proceeds.
The management of the Company has broad discretion to adjust the application and allocation of the net proceeds of this Offering in order to address changed circumstances and opportunities. As such, the success of the Company will be substantially dependent upon the discretion and judgment of the management of the Company with respect to the application and allocation of the net proceeds of the Offering.
An investment in the Company Shares could result in a loss of your entire investment.
An investment in this Offering involves a high degree of risk and you should not purchase the Shares if you cannot afford the loss of your entire investment. You may not be able to liquidate your investment for any reason in the near future.
Sales of a substantial number of shares of our Common Stock may cause the price of our Common Stock to decline.
If our shareholders sell substantial amounts of our Shares in the public market, Shares sold may cause the price to decrease below the current offering price. These sales may also make it more difficult for us to sell equity or equity-related securities at a time and price that we deem reasonable or appropriate.
The Shares in this Offering have no protective provisions.
The Shares in this Offering have no protective provisions. As such, you will not be afforded protection by any provision of the Shares or as a Shareholder in the event of a transaction that may adversely affect you, including a reorganization, restructuring, merger or other similar transaction involving the Company. If there is a 'liquidation event' or 'change of control' the Shares being offered do not provide you with any protection. In addition, there are no provisions attached to the Shares in the Offering that would permit you to require the Company to repurchase the Shares in the event of a takeover, recapitalization or similar transaction.
You will not have significant influence on the management of the Company.
Substantially all decisions with respect to the management of the Company will be made exclusively by the officers, directors, managers or employees of the Company. You will have a very limited ability, if at all, to vote on issues of Company management and will not have the right or power to take part in the management of the Company and will not be represented on the board of directors or by managers of the Company. Accordingly, no person should purchase Shares unless he or she is willing to entrust all aspects of management to the Company.
No guarantee of return on investment.
There is no assurance that you will realize a return on your investment or that you will not lose your entire investment. For this reason, you should read this Form 1-A, Offering Circular, and all exhibits and referenced materials carefully and should consult with your own attorney and business advisor prior to making any investment decision.
Our subscription agreement provides that Florida will be the governing law and forum for substantially all disputes between us and our investors, which could limit an investors’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees but does not preclude the investor for federal or state securities law litigation.
By becoming an investor in this Offering, you are deemed to have notice of and have consented to the provisions of our Subscription Agreement related to governing law and choice of forum. This forum provision may limit an investor’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage lawsuits against us and our directors, officers and other employees. This provision does not apply to claims arising under the Securities Act of 1933, the Securities Exchange Act of 1934, or other federal securities laws for which there is exclusive federal or concurrent federal and state jurisdiction. In addition, investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. In that regard, we note that Section 22 of the Securities Act of 1933 creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Exchange Act 27 creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. If a court were to find the exclusive forum provision in our Subscription Agreement to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving the dispute in other jurisdictions, which could seriously harm our business. In addition, an Investors’ ability to seek relief in the state courts as a more favorable jurisdiction, will likely fail because the Courts will defer to federal jurisdiction.
Risks Related to Product Development
All of our product candidates are in an early stage of development and we may never succeed in developing and/or commercializing them. We depended heavily on the success of our MyoCell™ product candidate. If we are unable to commercialize MyoCell™ or any of our other product candidates or experience significant delays in doing so, our business may fail.
|
|
●
|
We have invested a significant portion of our efforts and financial resources in our MyoCell™ product candidate and depended heavily on its success. MyoCell™ was currently in the clinical testing stage of development, although we have suspended work under our clinical trials.
|
|
|
●
|
We need to devote significant additional research and development, financial resources and personnel to develop commercially viable products, obtain regulatory approvals and establish a sales and marketing infrastructure.
|
|
|
●
|
We are likely to encounter hurdles and unexpected issues as we proceed in the development of our other product candidates. There are many reasons that we may not succeed in our efforts to develop our product candidates, including the possibility that:
|
|
|
●
|
our product candidates will be deemed ineffective, unsafe or will not receive regulatory approvals;
|
|
|
●
|
our product candidates will be too expensive to manufacture or market or will not achieve broad market acceptance
|
|
|
●
|
others will hold proprietary rights that will prevent us from marketing our product candidates; or
|
|
|
●
|
our competitors will market products that are perceived as equivalent or superior.
|
Our approach of using cell-based therapy for the treatment of heart damage is risky and unproven and no products using this approach have received regulatory approval in the United States or Europe.
No company, to our knowledge, has yet been successful in its efforts to obtain regulatory approval in the United States or Europe of a cell-based therapy product for the treatment of heart damage. Cell-based therapy products, in general, may be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy or other characteristics that may prevent or limit their approval by regulators or commercial use. Many companies in the industry have suffered significant setbacks in advanced clinical trials, despite promising results in earlier trials.
One of our competitors exploring the use of skeletal myoblasts ceased enrolling new patients in its European Phase II clinical trial based on the determination of its monitoring committee that there was a low likelihood that the trial would result in the hypothesized improvement in heart function. Although our clinical research to date suggests that MyoCell™ may improve the contractile function of the heart, we have not yet been able to demonstrate a mechanism of action and additional research is needed to precisely identify such mechanism.
U.S. Food and Drug Administration injunction has curtailed our business.
On May 9, 2018, the U.S. Department of Justice filed an injunctive action, specifically United States of America v. U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., Kristin C. Comella, and Theodore Gradel. The Complaint was filed at the request of the U.S. Food and Drug Administration (FDA) and alleges that the respective defendants manufacture “stromal vascular fraction” (SVF) products from patient adipose (fat) tissue, which the companies then market as stem cell-based treatments without first obtaining what the government alleges are necessary FDA approvals. The Company has retained counsel to defend in this action. . On June 25, 2019, the federal court for the Southern District of Florida ruled in favor of the government, enjoining the Company and the other defendants from certain product sales and processes. The Company filed an appeal on August 23, 2019 and attended oral argument on January 13th, 2021. On June 2nd, 2021, the Eleventh Circuit Court ruled to affirm lower courts’ judgement. The Company did not challenge the district court’s judgment upon any other ground. The Company is not able to predict the duration, scope, results, or consequences of the U.S. Department of Justice actions and final rulings and management is assessing its options on a going forward basis.
Healthcare reform could substantially reduce our revenues, earnings and cash flows.
We cannot predict how employers, private payors or persons buying insurance might react to the changes brought on by broad U.S. healthcare reform legislation or what form many of these regulations will take before implementation. The healthcare reform legislation, enacted in 2010, introduced healthcare insurance exchanges which provide a marketplace for eligible individuals and small employers to purchase healthcare insurance. While patients have begun receiving insurance coverage through these exchanges, the business and regulatory environment for these exchanges continues to evolve as the exchanges mature. Additionally, there is uncertainty about how the applicable state and federal agencies will enforce regulations relating to the exchanges. There is also a considerable amount of uncertainty as to the prospective implementation of the federal healthcare reform legislation and what similar measures might be enacted at the state level. There have been multiple attempts through legislative action and legal challenges to repeal or amend the Patient Protection and Affordable Care Act of 2010, as modified by the Health Reform Acts, including the case that was recently heard by the U.S. Supreme Court, King v. Burwell. Although the Supreme Court upheld the provision by the federal government of subsidies to individuals in federally facilitated healthcare exchanges in Burwell, which ultimately did not disrupt significantly the implementation of the healthcare reform legislation, we cannot predict whether other current or future efforts to repeal or amend these laws will be successful, nor can we predict the impact that such a repeal or amendment would have on our business and operations, or on our revenues and earnings. In addition, in the last year, the executive branch and Congress have taken actions to weaken or modify the Affordable Care Act. The enacted reforms, future legislative changes, as well as current ongoing uncertainty in matters related to the Affordable Care Act, could have a material adverse effect on our results of operations.
Risks Related to Our Common Stock
Our common stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make it more difficult to sell.
Our common stock is considered to be a “penny stock.” It does not qualify for one of the exemptions from the definition of “penny stock” under Section 3a51-1 of the Exchange Act. Our common stock is a “penny stock” because it meets one or more of the following conditions (i) the stock trades at a price less than $5.00 per share; (ii) it is not traded on a “recognized” national exchange or (iii) it is not quoted on the NASDAQ Global Market, or has a price less than $5.00 per share. The principal result or effect of being designated a “penny stock” is that securities broker-dealers participating in sales of our common stock are subject to the “penny stock” regulations set forth in Rules 15-2 through 15g-9 promulgated under the Securities Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
FINRA sales practice requirements may limit a shareholder’s ability to buy and sell our common shares.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common shares, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares. In addition, there are a limited number of clearing houses that clear “penny stocks” and those that will clear such stocks may enforce internal time consuming administrative requirements and may arbitrarily determine to refuse to clear any stock.
Rule 144 sales in the future may have a depressive effect on the company’s stock price as an increase in supply of shares for sale, with no corresponding increase in demand will cause prices to fall.
All of the outstanding shares of common stock held by the present officers, directors, and affiliate stockholders are “restricted securities” within the meaning of Rule 144 under the Securities Act of 1933, as amended. As restricted shares, these shares may be resold only pursuant to an effective registration statement or under the requirements of Rule 144 or other applicable exemptions from registration under the Securities Act of 1933 and as required under applicable state securities laws. Rule 144 provides in essence that a person who is an affiliate or officer or director who has held restricted securities for six months may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed the greater of 1.0% of a Company’s issued and outstanding common stock. There is no limit on the amount of restricted securities that may be sold by a non-affiliate after the owner has held the restricted securities for a period of six months if the Company is a current reporting company under the Securities Exchange Act of 1934. The Company, as of the date of this report, not current in its filings but is making efforts to bring the filings current. A sale under Rule 144 or under any other exemption from the Securities Act of 1933, if available, or pursuant to subsequent registration of shares of common stock of present stockholders, may have a depressive effect upon the price of the common stock in any market that may develop. In addition, if we are deemed a shell company pursuant to Section 12(b)-2 of the Act, our “restricted securities”, whether held by affiliates or non-affiliates, may not be re-sold for a period of 12 months following the filing of a Form 10 level disclosure or registration pursuant to the Securities Act of 1933. Currently, we are not current in our report and the exemption to registration required pursuant to Rule 144 is unavailable to our shareholders. While we intend to bring our filings current to permit the use of the exemption to registration required pursuant to Rule 144, there can be no assurances as to timing and subsequent continued filings.
Failure to achieve and maintain effective internal controls in accordance with section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our business and operating results.
It is time consuming, difficult and costly for us to develop and maintain the internal controls, processes and reporting procedures required by the Sarbanes-Oxley Act, and as our business develops, we may need to hire additional financial reporting, internal auditing and other finance staff in order to remain compliant. The cost of compliance will adversely affect our financial results, while, if we are unable to comply, we may not be able to obtain the independent accountant certifications that the Sarbanes-Oxley Act requires of publicly traded companies.
If we fail to comply in a timely manner with the requirements of Section 404 of the Sarbanes-Oxley Act regarding internal control over financial reporting or to remedy any material weaknesses in our internal controls that we may identify, such failure could result in material misstatements in our financial statements, cause investors to lose confidence in our reported financial information and have a negative effect on the trading price of our common stock.
Pursuant to Section 404 of the Sarbanes-Oxley Act and current SEC regulations, we are required to prepare assessments regarding internal controls over financial reporting and furnish a report by our management on our internal control over financial reporting. Failure to achieve and maintain an effective internal control environment or complete our Section 404 certifications could have a material adverse effect on our stock price.
In addition, in connection with our on-going assessment of the effectiveness of our internal control over financial reporting, we may discover “material weaknesses” in our internal controls as defined in standards established by the Public Company Accounting Oversight Board, or the PCAOB. A material weakness is a significant deficiency, or combination of significant deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected. The PCAOB defines “significant deficiency” as a deficiency that results in more than a remote likelihood that a misstatement of the financial statements that is more than inconsequential will not be prevented or detected.
In the event that a material weakness is identified, upon receiving sufficient financing or generating sufficient revenues, we will employ qualified personnel and adopt and implement policies and procedures to address any such material weaknesses. However, the process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. We cannot assure you that the measures we will take will remediate any material weaknesses that we may identify or that we will implement and maintain adequate controls over our financial process and reporting in the future.
Any failure to complete our assessment of our internal control over financial reporting, to remediate any material weaknesses that we may identify or to implement new or improved controls, or difficulties encountered in their implementation, could harm our operating results, cause us to fail to meet our reporting obligations or result in material misstatements in our financial statements. Any such failure could also adversely affect the results of the periodic management evaluations of our internal controls and, in the case of a failure to remediate any material weaknesses that we may identify, would adversely affect the annual auditor attestation reports regarding the effectiveness of our internal control over financial reporting that are required under Section 404 of the Sarbanes-Oxley Act. Inadequate internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
The systems of internal controls and procedures that we have developed and implemented to date are adequate in a research and development business. The current transaction volume and limited transaction channels mean that operating management, financial management, board members and auditor can, and do, efficiently perform a very extensive and detailed transaction review to ensure compliance with the Company’s established procedures and controls. If our business grows rapidly, we may not be able to keep up with recruiting and training personnel, and enhancing our systems of internal control in line with the growth in transaction volumes and compliance risks which could result in loss of assets, profit, and ability to manage the daily operations of our Company.
Public disclosure requirements and compliance with changing regulation of corporate governance pose challenges for our management team and result in additional expenses and costs which may reduce the focus of management and the profitability of our company.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated thereunder, the Sarbanes-Oxley Act and SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the U.S. public markets. Our management team will need to devote significant time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
COVID-19.
The outbreak of the coronavirus may negatively impact our ability to provide services as well as consumer spending, which could adversely affect our business, results of operations and financial condition.
In December 2019, a novel strain of coronavirus was reported to have surfaced in Wuhan, China, which has and is continuing to spread throughout China and other parts of the world, including the United States. On January 30, 2020, the World Health Organization declared the outbreak of the coronavirus disease (COVID-19) a “Public Health Emergency of International Concern.” On January 31, 2020, U.S. Health and Human Services Secretary Alex M. Azar II declared a public health emergency for the United States to aid the U.S. healthcare community in responding to COVID-19, and on March 11, 2020 the World Health Organization characterized the outbreak as a “pandemic”. The significant outbreak of COVID-19 has resulted in a widespread health crisis that could adversely affect the economies and financial markets worldwide, and could adversely affect our business, results of operations and financial condition.
We cannot, at this point, determine the extent to which COVID-19 outbreak will impact business or the economy as both are highly uncertain and cannot be predicted. The ability of to provide services that adhere to COVID protocals and how long the pandemic lasts, will have a direct impact on the scope and duration of the services we provide, even once the pandemic subsides. This factor, coupled with the possibility of economic recession or runaway inflation, could have a deleterious impact on proceeds from services for a significant period that could negatively impact our revenues. Additionally, there is a risk that government responses to thwart the spread of the virus, in the form of local or regional quarantine or shelter-in-place orders, could require temporary curtailment of operations.
The global impact of COVID-19 and actions taken to reduce its spread continues to rapidly evolve and we will continue to monitor the situation and the effects on our business and operations closely. We do not yet know the full extent of potential impacts on our business or operations or on the global economy as a whole, particularly if the COVID-19 pandemic continues and persists for an extended period of time. The length of time it may take for global vaccine distribution and more normal economic and operating conditions to resume remains uncertain and the economic recovery period could continue for a prolonged period even after the health risks of the pandemic subside. Given the uncertainty, we cannot reasonably estimate the impact on our future results of operations, cash flows or financial condition. To the extent the ongoing COVID-19 pandemic adversely affects our business and results of operations, it may also have the effect of heightening many of the other risks and uncertainties described in this “Risk Factors” section of this prospectus.
THE OUTBREAK OF COVID-19 HAS RESULTED IN A WIDESPREAD HEALTH CRISIS THAT COULD ADVERSELY AFFECT THE ECONOMIES AND FINANCIAL MARKETS WORLDWIDE AND COULD EXPONENTIALLY INCREASE THE RISK FACTORS DESCRIBED HEREIN AND IN OUR PRIOR FILINGS.
IN ADDITION TO THE RISKS LISTED ABOVE, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY THE MANAGEMENT. IT IS NOT POSSIBLE TO FORESEE ALL RISKS THAT MAY AFFECT THE COMPANY. MOREOVER, THE COMPANY CANNOT PREDICT WHETHER THE COMPANY WILL SUCCESSFULLY EFFECTUATE THE COMPANY'S CURRENT BUSINESS PLAN. EACH PROSPECTIVE PURCHASER IS ENCOURAGED TO CAREFULLY ANALYZE THE RISKS AND MERITS OF AN INVESTMENT IN THE SECURITIES AND SHOULD TAKE INTO CONSIDERATION WHEN MAKING SUCH ANALYSIS, AMONG OTHER FACTORS, THE RISK FACTORS DISCUSSED ABOVE.
DETERMINATION OF OFFERING PRICE
This Offering is a self-underwritten offering, which means that it does not involve the participation of an underwriter to market. Our Offering Price is arbitrary with no relation to value of the Company.
DILUTION
The term 'dilution' refers to the reduction (as a percentage of the aggregate Shares outstanding) that occurs for any given share of stock when additional Shares are issued. If all of the Shares in this Offering are fully subscribed and sold, the Shares offered herein will constitute approximately 36.47% of the total Shares of stock of the Company. Because The Company anticipates that subsequent to this Offering the Company may require additional capital and such capital may take the form of Common Stock, other stock or securities or debt convertible into stock. Such future fund raising will further dilute the percentage ownership of the Shares sold herein in the Company.
If you purchase shares in this Offering, your ownership interest in our Common Stock will be diluted immediately, to the extent of the difference between the price to the public charged for each share in this Offering and the net tangible book value per share of our Common Stock after this Offering.
Our historical net tangible book value (total stockholders’ deficit) as of June 30, 2021 was $(11,886,270) or $(0.0273) per share based on 456,217,501 outstanding shares of our Common Stock outstanding on August 24, 2021. Historical net tangible book value per share equals the amount of our total tangible assets, less total liabilities, divided by the total number of shares of our Common Stock outstanding, all as of the date specified.
The following table illustrates the per share dilution to new investors discussed above, assuming the sale of 100% of the shares offered for sale in this Offering at the Maximum Offering Price (before deducting estimated offering expenses of $25,000):
|
Percentage of shares offered that are sold
|
|
100%
|
|
|
|
|
|
|
|
|
Price to the public charged for each share in this Offering
|
|
$
|
0.01
|
|
|
|
|
|
|
|
|
Historical net tangible book value per share (1)
|
|
$
|
(0.0273
|
)
|
|
|
|
|
|
|
|
Increase in net tangible book value per share attributable to new investors in this Offering (2)
|
|
$
|
0.0136
|
|
|
|
|
|
|
|
|
Net tangible book value per share, after this Offering
|
|
$
|
(0.0137
|
)
|
|
|
|
|
|
|
|
Dilution per share to new investors
|
|
$
|
0.0237
|
|
|
|
(1)
|
Based on net tangible shareholders equity book value as of June 30, 2021 of $(11,886,270) or ($0.0273) based on 456,217,501 outstanding shares of Common Stock on August 24, 2021.
|
|
|
(2)
|
Before deducting estimated offering expenses of $25,000.
|
There is no material disparity between the price of the Shares in this Offering and the effective cash cost to officers, directors, promoters and affiliated persons for shares acquired by them in a transaction during the past year, or that they have a right to acquire.
The following table illustrates the per share dilution to new investors discussed above, assuming the sale of, respectively, 100%, 75%, 50% and 25% of the Shares offered for sale in this Offering (after deducting our offering expenses):
|
|
|
100%
|
|
|
75%
|
|
|
50%
|
|
|
25%
|
|
|
Funding Level
|
|
$
|
2,500,000
|
|
|
$
|
1,875,000
|
|
|
$
|
1,250,000
|
|
|
$
|
625,000
|
|
|
Offering Price
|
|
$
|
0.01
|
|
|
$
|
0.01
|
|
|
$
|
0.01
|
|
|
$
|
0.01
|
|
|
Net tangible book value per share of Common Stock before this Offering
|
|
$
|
(0.0273
|
)
|
|
$
|
(0.0273
|
)
|
|
$
|
(0.0273
|
)
|
|
$
|
(0.0273
|
)
|
|
Increase in net tangible book value per share attributable to new investors in this Offering
|
|
$
|
0.0136
|
|
|
$
|
0.0112
|
|
|
$
|
0.0083
|
|
|
$
|
0.0047
|
|
|
Net tangible book value per share of Common Stock, after this Offering
|
|
$
|
(0.0137
|
)
|
|
$
|
(0.0161
|
)
|
|
$
|
(0.0190
|
)
|
|
$
|
(0.0227
|
)
|
|
Dilution to investors in the Offering
|
|
$
|
0.0237
|
|
|
$
|
0.0261
|
|
|
$
|
0.0290
|
|
|
$
|
0.0327
|
|
PLAN OF DISTRIBUTION
We are offering a Maximum Offering of up to 250,000,000 in Shares of our Common Stock for $0.01 per share, for a total of up to $2,500,000. The offering is being conducted on a best-efforts basis without any minimum number of shares or amount of proceeds required to be sold. There is no minimum subscription amount required (other than a per investor minimum purchase) to distribute funds to the Company. The Company will not initially sell the Shares through commissioned broker-dealers, but may do so after the commencement of the offering. Any such arrangement will add to our expenses in connection with the offering. If we engage one or more commissioned sales agents or underwriters, we will supplement this Form 1-A to describe the arrangement. Subscribers have no right to a return of their funds. The Company may terminate the offering at any time for any reason at its sole discretion, and may extend the Offering past the termination date of 365 days from the date of qualification by the Commission in the absolutely discretion of the Company and in accordance with the rules and provisions of Regulation A of the JOBS Act. None of the Shares being sold in this Offering are being sold by existing securities holders.
After the Offering Statement has been qualified by the Securities and Exchange Commission (the “SEC”), the Company will accept tenders of funds to purchase the Shares. No escrow agent is involved and the Company will receive the proceeds directly from any subscription. You will be required to complete a subscription agreement in order to invest.
Our officers and directors are not statutorily disqualified, are not being compensated, and are not associated with a broker-dealer. They are and will continue to hold their positions as officers or directors following the completion of the Offering and have not been during the past 12 months and are currently not brokers or dealers or associated with brokers or dealers. They have not nor will they participate in the sale of securities of any issuer more than once every 12 months.
All subscription agreements and checks received by the Company for the purchase of shares are irrevocable until accepted or rejected by the Company and should be delivered to the Company as provided in the subscription agreement. A subscription agreement executed by a subscriber is not binding on the Company until it is accepted on our behalf by the Company’s Chief Executive Officer or by specific resolution of our board of directors. Any subscription not accepted within 30 days will be automatically deemed rejected. Once accepted, the Company will deliver a stock certificate to a purchaser within five days from request by the purchaser; otherwise, purchasers’ shares will be noted and held on the book records of the Company.
At this time no broker-dealer registered with the SEC and a member of the Financial Industry Regulatory Authority (“FINRA”), is being engaged as an underwriter or for any other purpose in connection with this Offering. This Offering will commence on the qualification of this Offering Circular, as determined by the Securities and Exchange Commission and continue for a period of 365 days. The Company may extend the Offering for an additional time period unless the Offering is completed or otherwise terminated by us, or unless we are required to terminate by application of Regulation A of the JOBS Act. Funds received from investors will be counted towards the Offering only if the form of payment, such as a check or wire transfer, clears the banking system and represents immediately available funds held by us prior to the termination of the subscription period, or prior to the termination of the extended subscription period if extended by the Company.
If you decide to subscribe for any Common Stock in this Offering, you must deliver a funds for acceptance or rejection. The minimum investment amount for a single investor is a principal amount of $25,000 equal to 2,500,000 Shares of Common Stock at the offering price. All subscription checks should be sent to the following address:
U.S. Stem Cell, Inc.
1560 Sawgrass Corporate Pkwy, 4th FL
Sunrise, Florida 33323
In such case, subscription checks should be made payable to U.S. Stem Cell, Inc. If a subscription is rejected, all funds will be returned to subscribers within ten days of such rejection without deduction or interest. Upon acceptance by the Company of a subscription, a confirmation of such acceptance will be sent to the investor. The Company maintains the right to accept or reject subscriptions in whole or in part, for any reason or for no reason. The Company maintains the right to accept subscriptions below the minimum investment amount or minimum per share investment amount in its discretion. All monies from rejected subscriptions will be returned by the Company to the investor, without interest or deductions.
This is an offering made under “Tier II” of Regulation A, and the shares will not be listed on a registered national securities exchange upon qualification but are listed on the OTC Markets OTCQB market. For non-accredited investors, the shares will be sold only to a person if the aggregate purchase price paid by such person is no more than 10% of the greater of such person's annual income or net worth, not including the value of his primary residence, as calculated under Rule 501 of Regulation D promulgated under Section 4(a)(2) of the Securities Act of 1933, as amended. In the case of sales to fiduciary accounts (Keogh Plans, Individual Retirement Accounts (IRAs) and Qualified Pension/Profit Sharing Plans or Trusts), the above suitability standards must be met by the fiduciary account, the beneficiary of the fiduciary account, or by the donor who directly or indirectly supplies the funds for the purchase of the shares. Investor suitability standards in certain States may be higher than those described in this Form 1-A and/or Offering Circular. These standards represent minimum suitability requirements for prospective investors, and the satisfaction of such standards does not necessarily mean that an investment in the Company is suitable for such persons. Different rules apply to accredited investors.
Each investor must represent in writing that he/she/it meets the applicable requirements set forth above and in the Subscription Agreement, including, among other things, that (i) he/she/it is purchasing the shares for his/her/its own account and (ii) he/she/it has such knowledge and experience in financial and business matters that he/she/it is capable of evaluating without outside assistance the merits and risks of investing in the shares, or he/she/it and his/her/its purchaser representative together have such knowledge and experience that they are capable of evaluating the merits and risks of investing in the shares. Broker-dealers and other persons participating in the offering must make a reasonable inquiry in order to verify an investor's suitability for an investment in the Company. Transferees of the shares will be required to meet the above suitability standards.
The shares may not be offered, sold, transferred, or delivered, directly or indirectly, to any person who (i) is named on the list of “specially designated nationals” or “blocked persons” maintained by the U.S. Office of Foreign Assets Control (“OFAC”) at www.ustreas.gov/offices/enforcement/ofac/sdn or as otherwise published from time to time, (ii) an agency of the government of a Sanctioned Country, (iii) an organization controlled by a Sanctioned Country, or (iv) is a person residing in a Sanctioned Country, to the extent subject to a sanctions program administered by OFAC. A “Sanctioned Country” means a country subject to a sanctions program identified on the list maintained by OFAC and available at www.ustreas.gov/offices/enforcement/ofac/sdn or as otherwise published from time to time. Furthermore, the shares may not be offered, sold, transferred, or delivered, directly or indirectly, to any person who (i) has more than fifteen percent (15%) of its assets in Sanctioned Countries or (ii) derives more than fifteen percent (15%) of its operating income from investments in, or transactions with, sanctioned persons or Sanctioned Countries.
The sale of other securities of the same class as those to be offered for the period of distribution will be limited and restricted to those sold through this Offering. Because the Shares being sold are not publicly or otherwise traded, the market for the securities offered is presently stabilized.
OTC Markets Considerations
The OTC Markets is separate and distinct from the New York Stock Exchange and Nasdaq stock market or other national exchange. Neither the New York Stock Exchange nor Nasdaq has a business relationship with issuers of securities quoted on the OTC Markets. The SEC’s order handling rules, which apply to New York Stock Exchange and Nasdaq-listed securities, do not apply to securities quoted on the OTC Markets.
Although other national stock markets have rigorous listing standards to ensure the high quality of their issuers and can delist issuers for not meeting those standards; the OTC Markets has no listing standards. Rather, it is the market maker who chooses to quote a security on the system, files the application, and is obligated to comply with keeping information about the issuer in its files.
Investors may have greater difficulty in getting orders filled than if we were on Nasdaq or other exchanges. Trading activity in general is not conducted as efficiently and effectively on OTC Markets as with exchange-listed securities. Also, because OTC Markets stocks are usually not followed by analysts, there may be lower trading volume than New York Stock Exchange and Nasdaq-listed securities.
USE OF PROCEEDS TO ISSUER
The Use of Proceeds is an estimate based on the Company's current business plan. We may find it necessary or advisable to reallocate portions of the net proceeds reserved for one category to another, or to add additional categories, and we will have broad discretion in doing so.
The maximum gross proceeds from the sale of the Shares in this Offering are $2,500,000. The net proceeds from the offering, assuming it is fully subscribed, are expected to be approximately $2,475,000 after the payment of offering costs such as printing, mailing, marketing, legal and accounting costs, and other compliance and professional fees that may be incurred. The estimate of the budget for offering costs is an estimate only and the actual offering costs may differ from those expected by management.
Management of the Company has wide latitude and discretion in the use of proceeds from this Offering. Ultimately, management of the Company intends to use substantially all of the net proceeds for general working capital. At present, management's best estimate of the use of proceeds, at various funding milestones, is set out in the chart below. However, potential investors should note that this chart contains only the best estimates of the Company's management based upon information available to them at the present time, and that the actual use of proceeds is likely to vary from this chart based upon circumstances as they exist in the future, various needs of the Company at different times in the future, and the discretion of the Company's management at all times.
A portion of the proceeds from this Offering may be used to compensate or otherwise make payments to officers or directors of the issuer. The officers and directors of the Company may be paid salaries and receive benefits that are commensurate with similar companies, and a portion of the proceeds may be used to pay these ongoing business expenses.
USE OF PROCEEDS
|
Offering Price: $0.01 per share
|
|
10%
|
|
|
25%
|
|
|
50%
|
|
|
75%
|
|
|
100%
|
|
|
Working Capital
|
|
$
|
187,500
|
|
|
$
|
468,750
|
|
|
$
|
937,500
|
|
|
$
|
1,406,250
|
|
|
$
|
1,875,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisitions
|
|
$
|
62,500
|
|
|
$
|
156,250
|
|
|
$
|
312,500
|
|
|
$
|
468,750
|
|
|
$
|
625,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
250,000
|
|
|
$
|
625,000
|
|
|
$
|
1,250,000
|
|
|
$
|
1,875,000
|
|
|
$
|
2,500,000
|
|
As indicated in the table above, if we sell only 75%, or 50%, or 25% or 10% of the shares offered for sale in this Offering, we would expect to use the resulting net proceeds for the same purposes as we would use the net proceeds from a sale of 100% of the shares, and in approximately the same proportions, until such time as such use of proceeds would leave us without working capital reserve. At that point we would expect to modify our use of proceeds by limiting our expansion, leaving us with the working capital reserve indicated.
The expected use of net proceeds from this Offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve and change. The amounts and timing of our actual expenditures, specifically with respect to working capital, may vary significantly depending on numerous factors. The precise amounts that we will devote to each of the foregoing items, and the timing of expenditures, will vary depending on numerous factors. As a result, our management will retain broad discretion over the allocation of the net proceeds from this Offering.
In the event we do not sell all the shares being offered, we may seek additional financing from other sources in order to support the intended use of proceeds indicated above. If we secure additional equity funding, investors in this Offering would be diluted. In all events, there can be no assurance that additional financing would be available to us when wanted or needed and, if available, on terms acceptable to us.
The allocation of the use of proceeds among the categories of anticipated expenditures represents management’s best estimates based on the current status of the Company’s proposed operations, plans, investment objectives, capital requirements, and financial conditions. No assurances can be provided that any milestone represented herein will be achieved. Future events, including changes in economic or competitive conditions of our business plan or the completion of less than the total Offering amount, may cause the Company to modify the above-described allocation of proceeds. The Company’s use of proceeds may vary significantly in the event any of the Company’s assumptions prove inaccurate. We reserve the right to change the allocation of net proceeds from the Offering as unanticipated events or opportunities arise. Additionally, the Company may from time to time need to raise more capital to address future needs.
The Company reserves the right to change the use of proceeds set out herein based on the needs of the ongoing business of the Company and the discretion of the Company's management. The Company may reallocate the estimated use of proceeds among the various categories or for other uses if management deems such a reallocation to be appropriate.
DESCRIPTION OF BUSINESS
Company Overview and Plan of Operation
U.S. Stem Cell, Inc. (“Company,” “We,” “Our,” “Us”) was incorporated under the laws of the State of Florida in August 1999. We are a biotechnology company focused on the discovery, development and, subject to regulatory approval, commercialization of autologous cell therapies for the treatment of disease and injury. We are also a regenerative medicine company specializing in physician/veterinary training and certification and stem cell products.. Our lead cardiac product candidate is MyoCell™, an innovative clinical therapy designed to populate regions of scar tissue within a patient’s heart with autologous muscle cells, or cells from a patient’s body, for the purpose of improving cardiac function in chronic heart failure patients.
Biotechnology Product Candidates
We are an enterprise in the regenerative medicine/cellular therapy industry. Our prior focus was on the discovery, development, and commercialization of cell based therapeutics. Our business included the development of proprietary cell therapy products, distribution of regenerative medicine products, as well as revenue generating physician and patient based regenerative medicine/cell therapy training services.
US Stem Cell Training, Inc. (“SCT”), an operating division of our company, is a content developer of regenerative medicine/cell therapy informational and training materials for physicians and patients. SCT also provides in-person and online training courses which are delivered through in-person presentations at SCT’s state of the art facilities and globally at university, hospital and physician’s office locations as well as through online webinars. Additionally, SCT provides hands-on clinical application training for physicians and health care professionals interested in providing regenerative medicine / cell therapy procedures.
Vet biologics, (“VBI”), an operating division of our company, is a veterinary regenerative medicine company committed to providing veterinarians with the ability to deliver the highest quality regenerative medicine therapies to dogs, cats and horses. VBI provides veterinarians with extensive regenerative medicine capabilities including the ability to isolate regenerative stem cells from a patient’s own adipose (fat) tissue directly on-site within their own clinic or stall-side.
During fiscal 2019, we had interests in US Stem Cell Clinic, LLC, (“SCC”), Regenerative Wellness Clinic, LLC, and US Stem Cell Clinic of the Villages, LLC as partially owned investments of our company (in which we had a 49.9%, 49.9% and 49% respectively member interests), which were physician run regenerative medicine/cell therapy clinics providing cellular treatments for patients afflicted with neurological, autoimmune, orthopedic and degenerative diseases. During the last quarter of 2019 (and in early 2021 in the case of SCC), we divested ourselves of our Member Interests in SCC and Regenerative Wellness Clinic, LLC, while US Stem Cell Clinic of the Villages, LLC is currently dormant.
U.S. Stem Cell’s comprehensive map of products and services:
U.S. Stem Cell, Inc. was incorporated in the State of Florida in August 1999 as Bioheart, Inc. In 2015, we changed our name to U.S. Stem Cell, Inc. Our principal executive offices are located at 1560 Sawgrass Corporate Pkwy, 4th FL, Sunrise, Florida 33323 and our telephone number is (954) 835-1500. Information about us is available on our corporate websites at www.us-stemcell.com, www.usstemcelltraining.com, www.vetbiologics.com, www.regenerativewellnessclinic.com and www.usstemcellclinic.com. We include our website addresses in this Offering Circular only as an interactive textual reference and do not intend it to be an active link to our website. The information on our websites is expressly not incorporated by reference in this Offering Circular.
This Offering Circular includes the following trademarks, service marks and trade names owned by the Company: U.S. Stem Cell, Inc. ™, US Stem Cell Training, Vetbiologics, US Stem Cell Clinic, LLC. ™, MyoCell ™ and Adipocell ™. These trademarks, service marks and trade names are the property of U.S. Stem Cell, Inc. and its affiliates.
Regenerative Medicine/Cell Therapy Industry
Regenerative medicine is defined as the process of replacing or regenerating human cells, tissues or organs to restore normal function. Among the categories of therapeutic technology platforms within this field are cell therapy; tissue engineering; tools, devices and diagnostics; and aesthetic medicine. U.S. Stem Cell’s business model is focused on two of these areas. First, cell therapy, in which we introduce cells (adult, donor or patient, stem cell or differentiated) into the body to prevent and treat disease; and second, we are a provider of services and products to physicians and veterinaries who provide or seek to provide cellular therapies and direct patient care for individuals and animals who may benefit from cellular therapy.
All living complex organisms start as a single cell that replicates, differentiates (matures) and perpetuates in an adult organism through its lifetime. Cellular therapy is the process that uses cells to prevent, treat or cure disease, or regenerate damaged or aged tissue. To date, the most common type of cell therapy has been the replacement of mature, functioning cells such as through blood and platelet transfusions. Since the 1970s, first bone marrow and then blood and umbilical cord-derived stem cells have been used to restore bone marrow, as well as blood and immune system cells damaged by the chemotherapy and radiation that are used to treat many cancers. These types of cell therapies are standard practice world-wide and are typically reimbursed by insurance.
Within the field of cell therapy, research and development using stem cells to treat a host of diseases and conditions has greatly expanded. Stem cells (in either embryonic or adult forms) are primitive and undifferentiated cells that have the unique ability to transform into or otherwise affect many different cells, such as white blood cells, nerve cells or heart muscle cells. U.S. Stem Cell’s cell therapy development efforts are focused on the use of adult stem cells; those cells which are found in the muscle, fat tissue and peripheral blood.
There are two general classes of cell therapies: Patient Specific Cell Therapies (“PSCTs”) and Off-the-Shelf Cell Therapies (“OSCTs”). In PSCTs, cells collected from a person (“donor”) are transplanted, with or without modification, to a patient (“recipient”). In cases where the donor and the recipient are the same individual, these procedures are referred to as “autologous”. In cases in which the donor and the recipient are not the same individual, these procedures are referred to as “allogeneic.” Autologous cells offer a low likelihood of rejection by the patient and we believe the long-term benefits of these PSCTs can best be achieved with an autologous product. In the case of OSCT, donor cells are expanded many fold in tissue culture, and large banks of cells are frozen in individual aliquots that may result in treatments, in our observation, for as many as 10,000 people from a single donor tissue. By definition, OSCTs are always allogeneic in nature.
Cellular Therapy Product Development Pipeline
Specific to cellular therapy, we are focused on the discovery, development and commercialization of autologous cellular therapies for the treatment of chronic and acute heart damage as well as vascular and autoimmune diseases.
At present, our development pipeline is on hold.
Status of Cellular Therapy Product Development Clinical Trials.
MyoCell/MyoCell SDF-1
MyoCell™ is a regenerative, cellular therapy intended to improve cardiac function for those with congestive heart failure and is designed to be utilized months or even years after a patient has suffered severe heart damage due to a heart attack or other cause. We believe that MyoCell has the potential to become a leading treatment for severe, chronic damage to the heart due to its perceived ability to satisfy, at least in part, what we believe to be an unmet demand for more effective and/or more affordable therapies for chronic heart damage. MyoCell™ uses myoblasts, cells that are precursors to muscle cells, from the patient’s own body. The myoblasts are removed from a patient’s thigh muscle, isolated, grown through our proprietary cell culturing process, and injected directly in the scar tissue of a patient’s heart. A qualified physician performs this minimally invasive procedure using an endoventricular catheter. We entered into an agreement with Biosense Webster (a Johnson & Johnson company) to use its NOGA® Cardiac Navigation System along with its MyoStar™ injection catheter for the delivery of MyoCell™ in the MARVEL Trial.
When injected into scar tissue within the heart wall, myoblasts have been shown to be capable of engrafting in the damaged tissue and differentiating into mature skeletal muscle cells. In a number of clinical and animal studies, the engrafted skeletal muscle cells have been shown to express various proteins that are important components of contractile function. By using myoblasts obtained from a patient’s own body, we believe MyoCell™ is able to avoid certain challenges currently faced by other types of cell-based clinical therapies including tissue rejection and instances of the cells differentiating into cells other than muscle. Although a number of therapies have proven to improve the cardiac function of a damaged heart, no currently available competing treatment, to our knowledge, has demonstrated an ability to generate new muscle tissue within the scarred regions of a heart as MyoCell™ has demonstrated.
Our completed clinical trials of MyoCell™ to date have been primarily targeted to patients with severe, chronic damage to the heart, who are in Class II or Class III heart failure according to the New York Heart Association, or NYHA, heart failure classification system. The NYHA system classifies patients in one of four categories based on how limited they are during physical activity. NYHA Class II heart failure patients have a mild limitation of activity and are generally comfortable at rest or with mild exertion while NYHA Class III heart failure patients suffer from a marked limitation of activity and are generally comfortable only at rest.
We believe the market for treating patients in NYHA Class II or NYHA Class III heart failure is significant. According to the American Heart Association (“AHA”) Statistics and the European Society of Cardiology Task Force for the Treatment of Chronic Heart Failure, in the United States and Europe there are approximately 5.2 million and 9.6 million, respectively, patients with heart failure. The AHA Statistics further indicate that, after heart failure is diagnosed, the one-year mortality rate is high, with one in five dying and that 80% of men and 70% of women under age 65 who have heart failure will die within eight years.
We believe that approximately 60% of heart failure patients are in either NYHA Class II or NYHA Class III heart failure based upon a 1999 study entitled “Congestive Heart Failure Due to Diastolic or Systolic Dysfunction – Frequency and Patient Characteristics in an Ambulatory Setting” by Diller, PM, et. al.
MyoCell SDF-1 is intended to be an improvement to MyoCell™. MyoCell SDF-1 is similar to MyoCell but the myoblast cells to be injected for use in MyoCell SDF-1 are modified prior to injection by an adenovirus vector or non-viral vector so that they will release extra quantities of the SDF-1 protein, which expresses angiogenic factors.
Advancement of the MyoCell™ and MyoCell™ SDF-1 clinical development programs is contingent, among many factors, upon the Company obtaining access to sufficient funding to execute the necessary clinical trials to achieve proof of efficacy and regulatory authorization to market such products. No assurances can be provided that such development programs will be realized.
Adipocell
Adipocell, a proprietary kit for the isolation of adipose derived stem cells which, as of the date of this filing, is currently on hold.
Business Strategy
U.S. Stem Cell’s mission is to advance to market novel regenerative medicine and cellular therapy products that substantially benefit humankind. Our business strategy is, to the extent possible, finance our clinical development pipeline through revenue (cash in-flows) generated through the marketing and sales of unique educational and training services, animal health products and personalized cellular therapeutic treatments.
A fundamental shift in venture capital investment strategies where, management believes, financial sponsorship is now directed toward commercial or near commercial enterprises has required U.S. Stem Cell to adapt its mission combining immediate revenue generating opportunities with longer-term development programs. Accordingly, U.S. Stem Cell has developed a multifaceted portfolio of revenue generating products and services in its US Stem Cell Training, Vetbiologics, and US Stem Cell Clinic, operating divisions that will, if successful, financially support its clinical development programs. Our goal is to maximize shareholder value through the generation of short-term profits that increase cash in-flows and decrease the need venture financings – a modern biotechnology company development strategy.
We will continue to evaluate and act upon opportunities to increase our top line revenue position and that correspondingly increase cash in-flows. These opportunities include but are not limited to the development and marketing of new products and services, mergers and acquisitions, joint ventures, licensing deals and more.
Further, if the opportunity presents itself whereby the Company can raise additional capital at a reasonable fair market value, the Company will do so. Accordingly, we plan to continue in our efforts to restructure, equitize or eliminate legacy balance sheet issues that are obstacles to market capitalization appreciation and capital fund raising.
US STEM CELL TRAINING
US Stem Cell Training offers a variety of courses for physicians and other health care professionals. These courses include didactic lecture series and hands-on clinical techniques in the field of regenerative medicine. We are currently hosting these courses throughout the United States and in multiple countries. These courses are also available in an online format. Pricing currently ranges from $500-$7,500 depending on the location and modules.
U.S. STEM CELL, INC.
U.S. Stem Cell markets several products to physicians for in clinic regenerative medicine use. These products include equipment (centrifuges, heating block, laminar hood, autoclave) for laboratory use. We are also providing a variety of materials necessary to obtain bone marrow including, trocars, syringes and other supplies.
VETBIOLOGICS
Vetbiologics is focused on providing regenerative medicine therapies to veterinarians for use in both small and large animals. We provide a complete regenerative medicine package which includes training, equipment and supplies necessary for in clinic cell therapy. We sell kits for isolating stem cells from bone marrow and fat. We also provide kits for isolating platelet rich plasma. The kits include all of the disposables and reagents necessary. Vetbiologics is also working on several off the shelf type products including an allogeneic stem cell source.
Royalty Agreement/Middle East
On November 9, 2016, the Company entered into an Intellectual Property License Agreement whereby the Company granted High Rise Group Company the exclusive right to the Company’s intellectual property (as defined) for the licensed use and development in Kuwait and other GCC/Middle East countries for 25 years in exchange for a payment of $75,000 and a 5% royalty generated under the agreement. The royalty payment is recorded as deferred revenue and amortized over the term of the agreement. The carrying balance as of December 31, 2020 and 2019 was $62,500 and $65,500, respectively.
The intent is for U.S. Stem Cell Middle East to offer regenerative treatment options to patients, based on U.S. Stem Cell, Inc. products and technologies like MyoCell™. To date, the first clinic in Kuwait City has been completed but has not begun operations as High Rising Group has not yet been able to secure regulatory approvals to operate.
Patents and Proprietary Rights
We own or hold licenses or sublicenses to an intellectual property portfolio consisting of numerous patents and patent applications in the United States, and in foreign countries, for use in the field of heart muscle regeneration. References in this report to “our” patents and patent applications and other similar references include the patents and patent applications that are owned by us, and references to patents and patent applications that are “licensed” to us and other similar references refer to patents, patent applications and other intellectual property that are licensed or sublicensed to us.
Patent life determination depends on the date of filing of the application or the date of patent issuance and other factors as promulgated under the patent laws. Under the U.S. Drug Price Competition and Patent Term Restoration Act of 1984, as amended, a patent which claims a product, use or method of manufacture covering drugs and certain other products, including biologic products, may be extended for up to five years to compensate the patent holder for a portion of the time required for research and FDA review of the product. Only one patent applicable to an approved drug or biologic product is eligible for a patent term extension. This law also establishes a period of time following approval of a drug or biologic product during which the FDA may not accept or approve applications for certain similar or identical drugs or biologic products from other sponsors unless those sponsors provide their own safety and efficacy data.
MyoCell™ is no longer protected by patents, which means that competitors will be free to sell products that incorporate the same or similar technologies that are used in MyoCell™ without infringing our patent rights. As a result, MyoCell, if approved for use, may be vulnerable to competition. In addition, many of the patent and patent applications that have been licensed to us that pertain to our other product candidates do not cover certain countries within Europe.
Our commercial success will depend to a significant degree on our ability to:
|
●
|
defend and enforce our patents and/or compel the owners of the patents licensed to us to defend and enforce such patents, to the extent such patents may be applicable to our products and material to their commercialization;
|
|
●
|
obtain additional patent and other proprietary protection for MyoCell™ and our other product candidates;
|
|
●
|
obtain and/or maintain appropriate licenses to patents, patent applications or other proprietary rights held by others with respect to our technology, both in the United States and other countries; and
|
|
●
|
preserve company trade secrets and other intellectual property rights relating to our product candidates; and operate without infringing the patents and proprietary rights of third parties.
|
In addition to patented intellectual property, we also rely on our own trade secrets and proprietary know-how to protect our technology and maintain our competitive position, since patent protection may not be available or applicable to our technology. Our policy is to require each of our employees, consultants and advisors to execute a confidentiality and inventions assignment agreement before beginning their employment, consulting or advisory relationship with us. The agreements generally provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of the individual’s relationship with us except in limited circumstances. These agreements generally also provide that we shall own all inventions conceived by the individual in the course of rendering services to us. Moreover, some of our academic institution licensors, collaborators and scientific advisors have rights to publish data and information to which we have rights, which may impair our ability to protect our proprietary information or obtain patent protection in the future.
We work with others in our research and development activities and one of our strategies is to enter into collaborative agreements with third parties to develop our proposed products. Disputes may arise about inventorship and corresponding rights in know-how and inventions resulting from the joint creation or use of intellectual property by us and our licensors, collaborators, consultants and others. In addition, other parties may circumvent any proprietary protection we do have. As a result, we may not be able to maintain our proprietary position.
We are not currently a party to any litigation or other adverse proceeding challenging our patents, patent licenses or intellectual property rights. However, if we become involved in litigation or any other adverse intellectual property proceeding, for example, as a result of an alleged infringement, or a third party alleging an earlier date of invention, we may have to spend significant amounts of money and time and, in the event of an adverse ruling, we could be subject to liability for damages, including treble damages, invalidation of our intellectual property and injunctive relief that could prevent us from using technologies or developing products, any of which could have a significant adverse effect on our business, financial condition and results of operation.
In addition, any claims relating to the infringement of third party proprietary rights, or earlier date of invention, even if not meritorious, could result in costly litigation, lengthy governmental proceedings, divert management’s attention and resources and require us to enter royalty or license agreements which are not advantageous, if available at all.
See the Section on page 13 entitled “Risk Factors — Risks Related to Our Intellectual Property” for a discussion of additional risks we face with respect to our intellectual property rights.
Government Claim
On May 9, 2018, the U.S. Department of Justice filed an injunctive action, specifically United States of America v. U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., Kristin C. Comella, and Theodore Gradel. The Complaint alleges, among other matters that the defendants manufacture “stromal vascular fraction” (SVF) products from patient adipose (fat) tissue, which the companies then market as stem cell-based treatments, and which U.S. Stem Cell Clinic, LLC administers to patients, without first obtaining what the government alleges are necessary FDA approvals. Although Theodore Gradel was initially listed as a defendant, he subsequently entered into a consent agreement and is no longer party to this case.
The U.S. and the defendants filed cross motions for summary judgment, each asking for a ruling in its favor. On June 3, 2019, the Court entered an order granting Summary Judgment for the government and denying the defendants’ motion for summary judgment. The order focused on the defendants’ actions in providing and marketing SVF therapy. In an order dated June 4, 2019, the Court granted the defendants’ request to allow it the opportunity to work out the language of the form of injunction with the government, and if unsuccessful, to provide a status report to the Court by June 14, 2019, outlining areas of disagreement. The Court further ordered that the defendants (U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., and Kristin C. Comella) ‘not sell, provide or otherwise engage in any SVF therapy or any other activities to be regulated by the FDA as explained in the Court’s Order on the Parties’ Motions for Summary Judgment.” On June 25, 2019, the Court entered an Order of Permanent Injunction, generally enjoining the defendants with respect to the SVF Product and requiring other actions. The Company filed an appeal on August 23, 2019 and attended oral argument on January 13, 2021. On June 2, 2021, the Eleventh Circuit Court ruled to affirm lower courts’ judgement. The Company did not challenge the district court’s judgment upon any other ground. The Company is not able to predict the duration, scope, results, or consequences of the U.S. Department of Justice actions and final rulings and management is assessing its options on a going forward basis. The Company, in divesting certain equipment and other assets and assigning its lease, has and will continue to experience a decrease in revenues as the Company both maintains the remainder of the business and transitions into similar or unrelated business opportunities as determined by management. However, management is not able to predict the duration, scope, results, or consequences of the summary judgment and any transition of the business plan.
DESCRIPTION OF PROPERTY
Our headquarters are located in Sunrise, Florida which we currently lease on a month-to-month basis at a monthly lease rate of $99.00. We believe the space available at our headquarters will be sufficient to meet the needs of our operations for the foreseeable future.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
Forward-Looking Statements
Certain statements, other than purely historical information, including estimates, projections, statements relating to our business plans, objectives, and expected operating results, and the assumptions upon which those statements are based, are forward-looking statements. These forward-looking statements generally are identified by the words believes, project, expects, anticipates, estimates, intends, strategy, plan, may, will, would, will be, will continue, will likely result, and similar expressions. Forward-looking statements are based on current expectations and assumptions that are subject to risks and uncertainties which may cause actual results to differ materially from the forward-looking statements. Our ability to predict results or the actual effect of future plans or strategies is inherently uncertain. Factors which could have a material adverse effect on our operations and future prospects on a consolidated basis include, but are not limited to: changes in economic conditions, legislative/regulatory changes, availability of capital, interest rates, competition, and generally accepted accounting principles. These risks and uncertainties should also be considered in evaluating forward-looking statements and undue reliance should not be placed on such statements.
The following discussion and analysis should be read in conjunction with our financial statements and the related notes thereto and other financial information contained elsewhere in this Form 1-A.
Our MD&A is comprised of significant accounting estimates made in the normal course of its operations, overview of our business conditions, results of operations, liquidity and capital resources and contractual obligations. We did not have any off balance sheet arrangements as of December 31, 2020 or 2019.
The discussion and analysis of our financial condition and results of operations is based upon its financial statements, which have been prepared in accordance with generally accepted accounting principles generally accepted in the United States (or “GAAP”). The preparation of those financial statements requires us to make estimates and judgments that affect the reported amount of assets and liabilities at the date of its financial statements. Actual results may differ from these estimates under different assumptions or conditions.
Results of Operations
Year Ended December 31, 2020 Compared to Year Ended December 31, 2019.
Revenues
Our primary source of revenue is from the sale of test kits and equipment, training services, patient treatments and laboratory services, and cell banking. Our revenue may vary substantially from quarter to quarter and from year to year. We believe that period-to-period comparisons of our results of operations are not meaningful and should not be relied upon as indicative of our future performance. We do not expect to generate substantial revenues until we obtain regulatory approval for and commercialize our product candidates.
We recognized revenues of $277,087 in 2020 compared to revenues of $3,072,293 in 2019. Our revenue in 2020 was generated from the sale of test kits and equipment, training services, patient treatments and laboratory services, and cell banking. Our revenues for 2019 were generated from the sale, test kits and equipment, training services, patient treatments and laboratory services, and cell banking. The decrease in revenue was a consequence of the Court Order resulting from the FDA court case result.
Cost of Sales
Cost of sales consists of the costs associated with the production of MyoCath and test kits, product costs, labor for production and training and lab and banking costs consistent with products and services provided.
Cost of sales was $64,117 in the year ended December 31, 2020 compared to $1,335,237 in the year ended December 31, 2019. The decrease is due to the decrease in revenues.
Research and Development
Research and development expenses were $0 in 2020, a decrease of $263 from research and development expenses of $263 in 2019. The decrease was primarily attributable to a decrease in the amount of available funds.
The timing and amount of our planned research and development expenditures is dependent on our ability to obtain additional financing.
Selling, General and Administrative
Selling, general and administrative expenses were $2,613,211 in 2020, a decrease of $1,841,501 in selling, general and administrative expenses of $4,454,712 in 2019. The decrease is due to reduced operations due to the Court order.
Gain (loss) on settlement of debt
During the year ended December 31, 2020, we recognized a net gain of $182 primarily related to the settlement of accounts payable and accrued interest. In 2019, we recognized a gain of $214,883 primarily related to the dissolved GACP transaction and divestiture of assets.
Gain on sale of equipment
In March 2017, we entered a sale/leaseback transaction whereby we sold our lab and other medical equipment and re-leased the equipment back for 36 months. In connection with the sale/leaseback, we realized a gain on sale of equipment of $386,536 which we will recognize to operations over the term of the lease (36 months). During the year ended December 31, 2020, we recognized $21,474 in current period operations as compared to $128,845 for the previous year.
Income from equity investment
Our investment of a 49.9% member interest ownership of U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic as well as a 49% interest in U.S. Stem Cell Clinic of the Villages LLC are accounted for using the equity method of accounting. As such, we report our pro rata share of income (loss) from equity investments for the period. For the year ended December 31, 2020 and 2019 our pro rata share of income (loss) was $(23,539) and $117,318, respectively. We divested ourselves of our member interests in Regenerative Wellness Clinic, LLC and U.S. Stem Cell Clinic, LLC in October 2019 and March 2021, respectively. U.S. Stem Cell Clinic of the Villages, LLC is currently dormant.
Interest Expense
Interest expense during the year ended December 31, 2020 was $488,369 compared to $981,001 for the year ended December 31, 2019. Interest expense primarily consists of interest incurred on the principal amount of the Northstar loan, the Seaside National Bank loan, the Capital Lease with GACP, accrued fees and interest payable to the Guarantors, imputed interest on non-interest bearing debt, the amortization of debt discounts and non-cash interest incurred relating to our issued convertible notes payable. There was nominal change in interest year over year.
On January 3, 2018, we renewed the loan with Seaside National Bank and Trust extend the maturity date to May 18, 2020 all other terms and conditions remain unchanged. On May 18, 2020, the Seaside loan was turned into a Demand Note with no fixed maturity date but with a re-documentation requirement every four years. The new re-documentation deadline is May 2022.
Inflation
Our opinion is that inflation has not had, and is not expected to have, a material effect on our operations.
Climate Change
Our opinion is that neither climate change, nor governmental regulations related to climate change, have had, or are expected to have, any material effect on our operations.
Three Months Ended June 30, 2021 as compared to the Three Months Ended June 30, 2020.
Revenues
We recognized revenues of $35,442 for the three months ended June 30, 2021. These revenues were generated from the sales of laboratory supplies and equipment, and services. We recognized revenues of $39,654 for the three months ended June 30, 2020 from the sale of MyoCath catheters, physician training, patient studies and veterinary sales. Due to the Injunction, as described in Note 11- Government Claim in our financial statements, our revenue has been impaired but for 2021 has moderately increased.
Cost of Sales
Cost of sales consists of the costs associated with the production of MyoCath, laboratory supplies necessary for laboratory services, physician course materials. Cost of sales were $13,677 and $16,877 in in the three-month periods ended June 30 2021 and 2020, respectively. Associated gross margins were $21,765 (61.4%) and $22,777 (46.0%) for the three months periods ended June 30 2021 and 2020, respectively.
Research and Development
Our research and development expenses consist of costs incurred in identifying, developing, and testing, our products and services. Research and development expenses were $0 in the three-month period ended June 30, 2021, the same as the research and development expenses of $0 in the three-month period ended June 30, 2020. Current management focus is towards on sales in addition to research and development and its corresponding ongoing costs. The timing and amount of our planned research and development expenditures is dependent on our ability to obtain additional financing.
Marketing, General and Administrative
Our marketing, general and administrative costs were $802,970 for the three-month period ended June 30, 2021 compared to $491,358 for the three-month period ended June 30, 2020, an increase of $311,312. The increase in cost due to increase in activity in new avenues of growth.
Our marketing, general and administrative expenses primarily consist of the costs associated with our general management and product and service marketing programs, including, but not limited to, salaries and related expenses for executive, administrative and marketing personnel, rent, insurance, legal and accounting fees, consulting fees, travel and entertainment expenses, conference costs and other clinical marketing and trade program expenses.
(Gain) Loss on settlement of debt
During the three months ended June 30, 2021, we incurred a net loss of $45,995 primarily related to accounts payable and debt restructured during the current period as compared to a net aggregate loss of $5,387 for the same period last year.
Income from equity investment
Our investment of a 49.9% (33.3% in 2018) member interest ownership of U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic as well as a 49% interest in U.S. Stem Cell Clinic of the Villages LLC are accounted for using the equity method of accounting. As such, we report our pro rata share of its income or loss for the period. For the three months ended June 30, 2021, our pro rata share of its income (loss) was $0 as we divested the assets in the first quarter of 2021. For the three month ended June 20, 2020 the pro rata share of income (loss) was $9,718. During the last quarter of 2019 (and in early 2021 in the case of SCC), we divested ourselves of our Member Interests in U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic, LLC , while and US Stem Cell Clinic of the Villages, LLC is currently dormant.
Interest Expense
Interest expenses during the three months ended June 30, 2021 were $166,994 compared to $104,993 for the three months ended June 30, 2020. Interest expenses primarily consists of interest incurred on the principal amount of the Northstar loan, our former Bank of America loan, the Seaside National Bank loan, accrued fees and interest payable to the Guarantors, our capital lease and the amortization of debt discounts and non-cash interest incurred relating to our issued convertible notes payable.
Six Months Ended June 30, 2021 as compared to the Six Months Ended June 30, 2020.
Revenues
We recognized revenues of $119,822 for the six months ended June 30, 2021. These revenues were generated from the sales of laboratory supplies and equipment, and services. We recognized revenues of $97,146 for the six months ended June 30, 2020 from the sale of MyoCath catheters, physician training, sale of product and services and veterinary division. Due to the Injunction, as described in Note 11- Government Claim in our financial statements, our revenue has been impaired but for 2021 has moderately increased.
Cost of Sales
Cost of sales consists of the costs associated with the production of MyoCath, laboratory supplies necessary for laboratory services, physician course materials. Cost of sales were $29,198 and $34,974 in in the six-month periods ended June 30, 2021 and 2020, respectively. Associated gross margins were $90,624 (75.6%) and $62,172 (64.0%) for the six months periods ended June 30, 2021 and 2020 respectively.
Research and Development
Our research and development expenses consist of costs incurred in identifying, developing, and testing, our products and services. Research and development expenses were $0 in the six-month period ended June 30, 2021, same as the six-month period ended June 30, 2020. Current management focus is towards on sales in addition to research and development and its corresponding ongoing costs. The timing and amount of our planned research and development expenditures is dependent on our ability to obtain additional financing.
Marketing, General and Administrative
Our marketing, general and administrative costs were $1,358,985 for the six-month period ended June 30, 2021 compared to $1,028,893 for the six-month period ended June 30, 2020, an increase of $330,092. The increase in cost due to increase in activity in new avenues of growth.
Our marketing, general and administrative expenses primarily consist of the costs associated with our general management and product and service marketing programs, including, but not limited to, salaries and related expenses for executive, administrative and marketing personnel, rent, insurance, legal and accounting fees, consulting fees, travel and entertainment expenses, conference costs and other clinical marketing and trade program expenses.
(Gain) Loss on settlement of debt
During the six months ended June 30, 2021, we incurred a net loss of $(383,870) primarily related to accounts payable and debt restructured during the current period as compared to a net aggregate gain of $481 for the same period last year.
Income from equity investment
Our investment of a 49.9% (33.3% in 2018) member interest ownership of U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic as well as a 49% interest in U.S. Stem Cell Clinic of the Villages LLC are accounted for using the equity method of accounting. As such, we report our pro rata share of its income or loss for the period. For the six months ended June 30, 2021, our pro rata share of its income (loss) was $0 as we divested the assets in the first quarter of 2021. For the six month ended June 20, 2020 the pro rata share of income (loss) was $9,718. During the last quarter of 2019 (and in early 2021 in the case of SCC), we divested ourselves of our Member Interests in U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic, LLC , while and US Stem Cell Clinic of the Villages, LLC is currently dormant.
Interest Expense
Interest expenses during the six months ended June 30, 2021 were $321,719 compared to $186,447 for the six months ended June 30, 2020. Interest expenses primarily consists of interest incurred on the principal amount of the Northstar loan, our former Bank of America loan, the Seaside National Bank loan, accrued fees and interest payable to the Guarantors, our capital lease and the amortization of debt discounts and non-cash interest incurred relating to our issued convertible notes payable.
Stock-Based Compensation
Stock-based compensation reflects our recognition as an expense of the value of stock options and other equity instruments issued to our employees and non-employees over the vesting period of the options and other equity instruments. We have granted to our employee’s options to purchase shares of common stock at exercise prices equal to the fair market value of the underlying shares of common stock at the time of each grant, as determined by our Board of Directors, with input from management.
We follow Accounting Standards Codification subtopic 718-10. Compensation (“ASC 718-10”) which requires that all share-based payments to both employee and non-employees be recognized in the income statement based on their fair values.
In awarding our common stock, our Board of Directors considered a number of factors, including, but not limited to:
|
|
●
|
our financial position and historical financial performance;
|
|
|
●
|
arm’s length sales of our common stock;
|
|
|
●
|
the development status of our product candidates;
|
|
|
●
|
the business risks we face;
|
|
|
●
|
vesting restrictions imposed upon the equity awards; and
|
|
|
●
|
an evaluation and benchmark of our competitors; and
|
|
|
●
|
prospects of a liquidity event.
|
On April 1, 2013, the Board of Directors approved, subject to subsequently received shareholder approval, the establishment of the Bioheart 2013 Omnibus Equity Compensation Plan, or the “2013 Omnibus Plan”. The 2013 Omnibus Plan initially reserved up to fifty thousand (50,000) shares of common stock for issuance. On August 4, 2014, the Board of Directors approved to set the reserve to one hundred thousand (100,000) shares of common stock for issuance and to close the 1999 Officers and Employees Stock Option Plan. On February 2, 2015, at the annual meeting of shareholders, the majority of shareholders approved the 2013 Omnibus Equity Compensation Plan. On November 2, 2015, the Board of Directors approved the increase of the reserve under the 2013 Omnibus Plan to five hundred million (500,000,000) shares of common stock for issuance, effective September 16, 2016, approved an addition of twenty five million (25,000,000) shares of common stock to the reserve, effective April 21, 2017, approved an addition of twenty five million (25,000,000) shares of common stock to the reserve, effective August 7, 2017, approved an addition of thirty million (30,000,000) shares of common stock to the reserve and effective May 7, 2018, approved an addition of one hundred million (100,000,000) shares of common stock to reserve.
Liquidity and Capital Resources
Net cash used in operating activities was $478,886 in 2020 as compared to $1,210,994 of net cash used in operations in 2019. Our net cash used in operations in 2020 resulted primarily from a net loss of $2,890,493, partially offset by non-cash items including related party notes payable issued for services rendered of $1,119,615, stock-based compensation of $701,939 and interest and amortization of debt discount of $447,380 as well as an increase in accounts payable of $149,521.
In the six months ended June 30, 2021, we incurred negative cash flow from operations of $635,233 and will continue to finance our considerable operational cash needs with cash generated from financing activities and revenues.
Investing Activities
Net cash provided by investing activities was $0 for the year ended December 31, 2020. Net cash provided by investing activities was $0 for the three-months ended March 31, 2021 represented proceeds from our equity investment as compared to cash provided by investing activities of $0 from our equity investments for the same period last year. Net cash provided by investing activities was $0 for the six-months ended June 30, 2021 represented proceeds from our equity investment as compared to cash provided by investing activities of $0 from our equity investments for the same period last year.
Financing Activities
Net cash provided by financing activities was $497,456 in the year ended December 31, 2020 as compared to net cash used of $312,986 in the year ended December 31, 2019. Our net cash provided by financing activities in 2020 resulted primarily from proceeds of $349,688 received from related party advances, $150,000 received from the issuance of notes payable and $45,000 received from the issuance of convertible notes payable, partially offset by repayments of related party notes payable of 45,712.
Net cash provided in financing activities was an aggregate of $754,625 in the six-month period ended June 30, 2021 as compared to cash provided of $315,371 in the six-month period ended in June 30, 2020.
Existing Capital Resources and Future Capital Requirements
Our MyoCell™ product candidate has not received regulatory approval or generated any material revenues. We do not expect to generate any material revenues or cash from sales of our MyoCell™ product candidate until commercialization of MyoCell, if ever. We have generated substantial net losses and negative cash flow from operations since inception and anticipate incurring significant net losses and negative cash flows from operations for the foreseeable future. Historically, we have relied on proceeds from the sale of our common stock and our incurrence of debt to provide the funds necessary to conduct our research and development activities and to meet our other cash needs.
At December 31, 2020, we had cash and cash equivalents totaling $18,570; our working capital deficit as of such date was $10,759,372.
As of December 31, 2020, we had $8,346,367 in outstanding debt, net of debt discount of $43,111.
At June 30, 2021, we had cash and cash equivalents totaling $137,962. However, our working capital deficit as of such date was $11,086,870.
Economic Injury Disaster Loan (EIDL)
On June 20, 2020, the Company executed the standard loan documents for an EIDL from the U.S. Small Business Administration in light of the impact of the COVID-19 pandemic on our business. Pursuant to that certain Loan Authorization and Agreement (the “SBA Loan Agreement”), the principal amount of the EIDL received was $150,000, with proceeds to be used for working capital purposes. Interest accrues at the rate of 3.75% per annum. Installment payments, including principal and interest, are due monthly beginning June 20, 2021 (twelve months from the date of the SBA Loan Agreement) in the amount of $731. The balance of principal and interest is payable thirty years from the date of the SBA Loan Agreement. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $150,000. At June 30, 2021 and December 31, 2020, accrued interest on the note was $5,779 and $2,990, respectively
Along with diversifying the portfolio of products distributed by our company, including equipment and biologics, it is the intention of our Company to both continue to adhere to the Court Order resulting from the FDA court case as well as re -establish its good standing with the Agency (FDA). These points are not mutually exclusive nor negotiable and we believe that there are still business and patient goodness opportunities while still abiding by all legal requirements As a result, management shall be continuing with the development of US Stem Cell Training, Inc. , an operating division of our company, that is a content developer of regenerative medicine/cell therapy informational and training materials for physicians and patients and complies with both requirements--as well as Vetbiologics, an operating division of our company, that is a veterinary regenerative medicine company committed to providing veterinarians with the ability to deliver the highest quality regenerative medicine therapies to dogs, cats and horses. In addition, our company is transitioning the current clinics to a more diversified regenerative medicine platform, while complying with recent court rulings. While not providing legal advice, our company may also engage in managing third-party clinics to ensure they too abide by recent regulatory requirements
As the Company continues to incur losses, achieving profitability is dependent on achieving a level of revenues adequate to support the Company's cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital. Management intends to fund future operations through additional private or public equity offering and may seek additional capital through arrangements with strategic partners of from other sources. There can be no assurances, however, that additional funding will be available on terms acceptable to the Company, or at all. Any equity financing may be dilutive to existing shareholders.
Concentrations of Credit Risk
As of June 30, 2021, two customers represented 69% and 26%, representing an aggregate of 85% of the Company’s accounts receivable. As of December 31, 2020, two customers represented 50% and 44% respectively, representing an aggregate of 94% of the Company’s accounts receivable.
Off Balance Sheet Arrangements
As of June 30, 2021, there were no off balance sheet arrangements.
Our Ability To Continue as a Going Concern
The Company’s management has evaluated whether there is substantial doubt about the Company’s ability to continue as a going concern and has determined that substantial doubt existed as of the date of this Offering Circular. This determination was based on the following factors, as of December 31, 2020, the Company had cash on hand of $18,570 and a working capital deficit (current liabilities in excess of current assets) of $10,759,372. During the year ended December 31, 2020, the net loss was $2,890,493 and net cash used in operating activities was $478,886. As of June 30, 2021, the Company had cash on hand of $137,962 and a working capital deficit (current liabilities in excess of current assets) of $11,086,870. During the six months ended June 30, 2021, the net loss was $1,973,950 and net cash used in operating activities was $635,233. The Company’s existence is dependent upon management’s ability to develop profitable operations and to obtain additional funding sources. There can be no assurance that the Company’s financing efforts will result in profitable operations or the resolution of the Company’s liquidity problems. The accompanying statements do not include any adjustments that might result should the Company be unable to continue as a going concern. Along with diversifying the portfolio of products distributed by the Company, including equipment and biologics, it is the intention of the Company management to both continue to adhere to the Court Order Order resulting from the FDA court case as well as re-establish its good standing with the Agency (FDA). These points are not mutually exclusive nor negotiable and management believes that there are still business and patient goodness opportunities while still abiding by all legal requirements As a result, management shall be continuing with the development of US Stem Cell Training, Inc., an operating division of the Company, that is a content developer of regenerative medicine/cell therapy informational and training materials for physicians and patients and complies with both requirements--as well as Vetbiologics, an operating division of the Company, that is a veterinary regenerative medicine company committed to providing veterinarians with the ability to deliver the highest quality regenerative medicine therapies to dogs, cats and horses.
Critical Accounting Policies
We have identified the policies outlined below as critical to our business operations and an understanding of our results of operations. The list is not intended to be a comprehensive list of all of our accounting policies. In many cases, the accounting treatment of a particular transaction is specifically dictated by accounting principles generally accepted in the United States, with no need for management's judgment in their application. The impact and any associated risks related to these policies on our business operations is discussed throughout Management's Discussion and Analysis of Financial Condition and Results of Operation where such policies affect our reported and expected financial results. Note that our preparation of the financial statements requires us to make estimates and assumptions that affect the reported amount of assets and liabilities, disclosure of contingent assets and liabilities at the date of our financial statements, and the reported amounts of revenue and expenses during the reporting period. There can be no assurance that actual results will not differ from those estimates.
While our critical accounting policies are described in our financial statements appearing elsewhere in this Offering Circular, we believe the following policies are important to understanding and evaluating our reported financial results:
Revenue Recognition
Effective January 1, 2018, the Company recognizes revenue in accordance with Accounting Standards Codification 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes the revenue recognition requirements in Topic 605, Revenue Recognition, and most industry-specific revenue recognition guidance throughout the Industry Topics of the Accounting Standards Codification. The updated guidance states that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also provides for additional disclosures with respect to revenues and cash flows arising from contracts with customers.
At the time of each transaction, management assesses whether the fee associated with the transaction is fixed or determinable and whether or not collection is reasonably assured. The assessment of whether the fee is fixed or determinable is based upon the payment terms of the transaction. Collectability is assessed based on a number of factors, including past transaction history with the client and the creditworthiness of the client.
The Company’s primary sources of revenue are from the sale of test kits and equipment, training services, patient treatments, laboratory services and cell banking.
Revenues for kits and equipment sold are not recorded until kits and equipment are received by the customer. Revenues from in-person trainings are recognized when the training occurs and revenues from on demand online trainings are recognized when the customer purchases the rights to the training course. Any cash received as a deposit for trainings are recorded by the Company as a liability.
Patient treatments and laboratory services revenue are recognized when those services have been completed or satisfied.
Research and Development Activities
We account for research and development costs in accordance with Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and development costs are expensed when the contracted work has been performed or as milestone results have been achieved as defined under the applicable agreement. Our company-sponsored research and development costs related to both present and future products are expensed in the period incurred.
Inflation
Our opinion is that inflation has not had, and is not expected to have, a material effect on our operations.
Climate Change
Our opinion is that neither climate change, nor governmental regulations related to climate change, have had, or are expected to have, any material effect on our operations.
Additional Company Matters
The Company has not filed for bankruptcy protection nor has it ever been involved in receivership or similar proceedings.
Litigation
On September 17, 2015, a product liability lawsuit was filed in Broward County, specifically Patsy Bade v. Bioheart, Inc. US Stem Cell Clinics LLC, Alejandro Perez, ARNP, and Shareen Greenbaum, M.D., and on November 30, 2015, a product liability lawsuit was filed in Broward County, specifically Elizabeth Noble v. Bioheart, Inc. US Stem Cell Clinics LLC, Alejandro Perez, ARNP, and Shareen Greenbaum, M.D. During the year ended December 31, 2016, both matters settled by the Company’s insurance policy with no additional cost to the Company, excluding the Company payment of the $100,000 insurance company deductible of which $11,000 was paid in fiscal 2017. As a result of the final settlement and determination of insurance coverage, the Company recognized $100,000 of expense due to litigation for the year ended December 31, 2017. The remaining amount due under this settlement was $27,350 as of June 30, 2021.
On December 12, 2017, a product liability lawsuit was filed in Broward County, specifically Jeannine Mallard v. U.S. Stem Cell, Inc., US Stem Cell Clinics LLC., Regenestem, LLC., Regenestem Network, LLC., and Kristin C. Comella. The Company will continue to defend it vigorously. On December 6, 2019, the Company was one of the parties to a Settlement Agreement and General Release (the “Agreement”) related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year non-interest bearing unsecured promissory note, dated December 6, 2019, in the principal amount of $250,000, payable in monthly increments of $750 per month, with a final balloon payment of $205,000 due on January 1, 2025.
On June 3, 2019, the Company was one of the parties to a Settlement Agreement and General Release (the “Agreement”) related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year 5.25% Promissory Note, dated June 15, 2019, in the principal amount of Five Hundred Thousand Dollars ($500,000), payable in monthly increments of Five Thousand ($5,000) per month. . The remaining amount due under this settlement was $428,281 as of June 30, 2021.
The Company is subject at times to other legal proceedings and claims, which arise in the ordinary course of its business. Although occasional adverse decisions or settlements may occur, the Company believes that the final disposition of such matters should not have a material adverse effect on its financial position, results of operations or liquidity. There was no outstanding litigation as of June 30, 2021 other than that described above.
Government Claim
On May 9, 2018, the U.S. Department of Justice filed an injunctive action, specifically United States of America v. U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., Kristin C. Comella, and Theodore Gradel. The Complaint alleges, among other matters that the defendants manufacture “stromal vascular fraction” (SVF) products from patient adipose (fat) tissue, which the companies then market as stem cell-based treatments, and which U.S. Stem Cell Clinic, LLC administers to patients, without first obtaining what the government alleges are necessary FDA approvals. Although Theodore Gradel was initially listed as a defendant, he subsequently entered into a consent agreement and is no longer party to this case.
The U.S. and the defendants filed cross motions for summary judgment, each asking for a ruling in its favor. On June 3, 2019, the Court entered an order granting Summary Judgment for the government and denying the defendants’ motion for summary judgment. The order focused on the defendants’ actions in providing and marketing SVF therapy. In an order dated June 4, 2019, the Court granted the defendants’ request to allow it the opportunity to work out the language of the form of injunction with the government, and if unsuccessful, to provide a status report to the Court by June 14, 2019, outlining areas of disagreement. The Court further ordered that the defendants (U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., and Kristin C. Comella) “not sell, provide or otherwise engage in any SVF therapy or any other activities to be regulated by the FDA as explained in the Court’s Order on the Parties’ Motions for Summary Judgment.” On June 25, 2019, the Court entered an Order of Permanent Injunction, generally enjoining the defendants with respect to the SVF Product and requiring other actions. The Company filed an appeal on August 23, 2019 and attended oral argument on January 13, 2021. On June 2, 2021, the Eleventh Circuit Court ruled to affirm lower courts’ judgement. The Company did not challenge the district court’s judgment upon any other ground. The Company is not able to predict the duration, scope, results, or consequences of the U.S. Department of Justice actions and final rulings and management is assessing its options on a going forward basis.
The Company, in divesting certain equipment and other assets and assigning its lease, has and will continue to experience a decrease in revenues as the Company both maintains the remainder of the business and transitions into similar or unrelated business opportunities as determined by management . However, management is not able to predict the duration, scope, results, or consequences of the summary judgment and any transition of the business plan.
After the Court’s issuance of the Order of Permanent Injunction, the Company has received demand letters for compensation from persons who store their SVF Product and/or other tissue product with the tissue bank (several of the persons have requested refunds of the monies paid to the tissue bank and one person has requested a full refund of monies paid to an altogether separate company due to her not receiving the full amount of treatments she requested; such requests for compensation, to date, have not been material) and requests that the Company preserve cells in the Company’s possession. The Company sought guidance from the Court, which entered an order generally staying the requirement to destroy any SVF Product, pending a decision on the Company’s appeal. Many of the tissue bank depositors attempted to intervene in the FDA action, and filed an appeal when their intervention was denied. Their appeal was dismissed. It is anticipated that these depositors will present their position on tissue/SVF preservation to the trial court now that the appeal has been decided. As disclosed by the Company previously, the Company entered into a transaction in 2019 in which it divested itself of the operation of the tissue bank.
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
Directors and Executive Officers
Set forth below is information regarding our current executive officers and directors:
|
Miguel (Mike) Tomas
|
56
|
Director, President and Chief Executive Officer, Chief Financial Officer
|
|
William P. Murphy, Jr., M.D.
|
97
|
Director, Chairman of the Board
|
|
Mark P. Borman
|
66
|
Director
|
|
Sheldon T. Anderson
|
70
|
Director
|
|
Greg Knutson
|
69
|
Director
|
Our Bylaws provide that we shall have that number of directors determined by the majority vote of the board of directors. Currently we have five directors. Each director will serve until our next annual shareholder meeting. Directors are elected for one-year terms. Our Board of Directors elects our officers at the regular annual meeting of the Board of Directors following the annual meeting of shareholders. Vacancies may be filled by a majority vote of the remaining directors then in office. Our directors and executive officers are as follows:
Executive Officers and Directors
Mike Tomas. Mike Tomas, President & CEO of U.S. Stem Cell Inc, is considered by many in the industry as one of the most experienced marketers and operating executives for IT/Communications and Biotech/Life Sciences private equity and venture groups portfolio companies. The son of a serial entrepreneur, he spent nearly 20 years driving the evolution of telecommunications technology in the U.S. and Mexico in leadership roles ranging from sales, marketing, customer service, telemarketing, engineering, and operations. Upon retiring as Chief Marketing Officer of Avantel, MCI/Worldcom’s Global Ventures $1B investment with Banamex (at the time, the largest bank in Latin America), Mr. Tomás joined other former-MCI executives (including MCI CEO Jerry Taylor) and helped form an integrated customer communications software solution that was named on Red Herring magazine’s “Top Ten to Watch” list.
Upon the successful sale of that company in 2001, Mr. Tomas helped launch The ASTRI Group, an early-stage private equity investment company providing capital, business development and strategic marketing support to emerging private companies. Mr. Tomas sits on the board of U.S. Stem Cell Inc. (adult stem cell development and applications) and has sat on the boards of The IDEA Center (Miami Dade College’s entrepreneurial institute), Career Source Florida (appointed by Florida Governor Rick Scott to his statewide workforce investment board) and is the past chairman of Florida International University’s Global Entrepreneurship Center. Mr. Tomas is an inductee into the Miami-Dade College and WACE Halls of Fame for business, an FIU Torch Award winner--and winner of top communications, medical innovations, education and entrepreneurial awards. An avid athlete, Mr. Tomas was also a Miami-Dade County Sports Commissioner.
William P. Murphy, Jr., M.D. Dr. Murphy has served as a member of our Board of Directors since June 2003. Dr. Murphy founded Small Parts, Inc., a supplier of high quality mechanical components for design engineers, in 1964 and served as its Chairman until his retirement in April 2005. Small Parts, Inc. was acquired by Amazon.com, Inc. in March 2005. From October 1999 until October 2004, Dr. Murphy served as the Chairman and Chief Executive Officer of Hyperion, Inc., a medical diagnosis company which had an involuntary bankruptcy filed against it in December 2003. Dr. Murphy is the founder of Cordis Corporation (now Cordis Johnson & Johnson) which he led as President, Chairman and Chief Executive Officer at various times during his 28 years at Cordis until his retirement in October 1985. Cordis Johnson & Johnson is a leading firm in cardiovascular instrumentation.
Dr. Murphy received an M.D. in 1947 from the University of Illinois and a B.S. in pre-medicine from Harvard College in 1946. He also studied physiologic instrumentation at Massachusetts Institute of Technology, or MIT. After a two year rotating internship at St. Francis Hospital in Honolulu, he became a Research Fellow in Medicine at the Peter Bent Brigham Hospital in Boston where he was the dialysis engineer on the first clinical dialysis team in the United States. He continued as an Instructor in Medicine and then a research associate in Medicine at Harvard Medical School. Dr. Murphy is the author of numerous papers and owns 17 patents.
He is the recipient of a number of honors, including the prestigious Lemelson-MIT Lifetime Achievement Award, the MIT Corporate Leadership Award, the Distinguished Service Award from North American Society of Pacing and Electrophysiology, and the Jay Malina Award from the Beacon Council of Miami, Florida. He is also a member of the Inventors Hall of Fame.
Mark P. Borman. Mr. Borman has served as a member of the Company’s Board of Directors since May 2009. He is a seasoned financial officer with more than 30 years of broad-based financial and investor relations experience. Mr. Borman brings small-company entrepreneurial passion and larger-company disciplines. In addition to the valuable experience he gained working with entrepreneurs and their startups from 2009 to present, Mr. Borman has experience with global, NASDAQ- and NYSE-listed companies in various executive and financial roles. He is currently a board member with public and private technology companies and serves on advisory and non-profit boards. During his career, Mr. Borman has held positions with ADC Telecommunications, General Instrument Corporation, First Chicago Corporation, FMC Corporation, Price Waterhouse, and KPMG. Mr. Borman received his B.A. in Accounting from Michigan State University and his M.B.A. in Finance from the University of Chicago Booth School of Business. He is an Audit Committee Financial Expert under SEC rules, a Certified Public Accountant and Chartered Financial Analyst. Mr. Borman is a Board Leadership Fellow and member of the National Association of Corporate Directors.
Sheldon T. Anderson. Mr. Anderson is Chairman of the Florida Advisory Board of Northern Trust Corporation. From 1992 through December 31, 2012, Mr. Anderson served in a variety of executive capacities with Northern Trust Corporation, including his most recent position as Chairman and Chief executive Officer Southeast Region of Northern Trust Corporation. Mr. Anderson is the Chair-elect of the Beacon Council, Miami-Dade County’s economic development agency. He is a Board member of the Miami-Dade College Foundation, Inc.; Museum of Contemporary Art (MOCA); the New World Symphony; Baptist Health Systems Governing Board and Carrollton School of the Sacred Heart. He is Past Chair and a member of the Advisory Council of the United Way of Miami-Dade County. Anderson is President of the Board of Cleveland Orchestra Miami / Miami Music Association and also serves on the Advisory Board of the University of Miami School of Law for Ethics& Public Service. He is a member of the Orange Bowl Committee and the President’s Council of Florida International University. A Miami native, Sheldon holds a degree in International Studies from Ohio State University.
Greg Knutson. Mr. Knutson founded Concrete Specialists, Inc. in 1985 and continues to serve as its President. Mr. Knutson founded Sunwood Properties in 2009 and continues to serve as its President. Mr. Knutson founded G&G Land Development, LLC and continues to serve as its Managing Partner. Mr. Knutson, a holder of Member Interests in Northstar, was appointed as a Manager of Northstar Biotech Group, LLC in late 2014.
Compensation of Directors and Executive Officers
On July 1, 2019, the Company’s Board of Directors approved the 2019/2020 salary for Mike Tomas, Chief Executive Officer, for $750,000 per year, beginning July 1, 2019 with an incentive bonus ranging from $150,000 to $500,000. In addition, the Board of Directors approved a bonus of $500,000 and options to acquire 20,000,000 shares of the Company’s common stock for ten years with four-year vesting and a cashless exercise provision. The cash bonus may be paid in the form a six-month promissory note bearing interest at 5% per annum. No compensatory package has been approved for 2021.
Stock Incentive Plan
Stock Options
On April 1, 2013, the Board of Directors approved, subject to subsequently received shareholder approval, the establishment of the Bioheart 2013 Omnibus Equity Compensation Plan, or the “2013 Omnibus Plan”. The 2013 Omnibus Plan initially reserved up to fifty thousand (50,000) shares of common stock for issuance. On August 4, 2014, the Board of Directors approved to set the reserve to one hundred thousand (100,000) shares of common stock for issuance and to close the 1999 Officers and Employees Stock Option Plan. On February 2, 2015, at the annual meeting of shareholders, the majority of shareholders approved the 2013 Omnibus Equity Compensation Plan. On November 2, 2015, the Board of Directors approved the increase of the reserve under the 2013 Omnibus Plan to five hundred million (500,000,000) shares of common stock for issuance, effective September 16, 2016, approved an addition of twenty five million (25,000,000) shares of common stock to the reserve, effective April 21, 2017, approved an addition of twenty five million (25,000,000) shares of common stock to the reserve, effective August 7, 2017, approved an addition of thirty million (30,000,000) shares of common stock to the reserve and effective May 7, 2018, approved an addition of one hundred million (100,000,000) shares of common stock to reserve.
Board of Directors
Our Board of Directors currently consists of five directors. [4 of our directors are “independent” as defined in Rule 4200 of FINRA's listing standards]. We may appoint additional independent directors to our Board of Directors in the future, particularly to serve on committees should they be established.
Audit Committee
The Board of Directors has a separately-designated standing Audit Committee established in accordance with Section 3(a) (58) (A) of the Exchange Act. The members of our Audit Committee are Mr. Borman, who serves as Chairperson of the Audit Committee, Dr. Murphy, and Mr. Anderson. Our Board of Directors has determined that Mr. Borman qualifies as a “financial expert” as that term is defined in the rules of the SEC implementing requirements of the Sarbanes-Oxley Act of 2002.
Director Compensation
We currently do not pay our directors any compensation for their services as board members, with the exception of reimbursing and board related expenses. In the future, we may compensate directors, particularly those who are not also employees and who act as independent board members, on either a per meeting or fixed compensation basis.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than ten percent (10%) of our outstanding Common Stock, or the Reporting Persons, to file with the SEC initial reports of ownership on Form 3 and reports of changes in ownership of Common Stock on Forms 4 or 5. Such persons are required by SEC regulation to furnish us with copies of all such reports they file. Based solely on a review of Forms 3 and 4 furnished to us by the Reporting Persons or prepared on behalf of the Reporting Persons by the Company, the Company believes that the Reporting Persons have complied with reporting requirements applicable to them.
Involvement in Certain Legal Proceedings
None of the following events have occurred during the past ten years and are material to an evaluation of the ability or integrity of any director or officer of the Company:
|
|
1.
|
A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
|
|
|
2.
|
Such person was convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
|
|
3.
|
Such person was the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
|
|
|
|
a.
|
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
|
|
|
|
b.
|
Engaging in any type of business practice; or
|
|
|
|
c.
|
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
|
|
|
4.
|
Such person was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph (f)(3)(i) of this section, or to be associated with persons engaged in any such activity;
|
|
|
5.
|
Such person was found by a court of competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has not been subsequently reversed, suspended, or vacated;
|
|
|
6.
|
Such person was found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
|
|
|
7.
|
Such person was the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
|
|
|
|
a.
|
Any Federal or State securities or commodities law or regulation; or
|
|
|
|
b.
|
Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
|
|
|
|
c.
|
Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
|
|
|
8.
|
Such person was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29)), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
Code of Ethics
We have adopted a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Our code of ethics contains standards that are reasonably designed to deter wrongdoing and to promote:
|
o
|
Honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships;
|
|
o
|
Full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with, or submits to, the Commission and in other public communications made by us;
|
|
o
|
Compliance with applicable governmental laws, rules and regulations;
|
|
o
|
The prompt internal reporting of violations of the code to the board of directors or another appropriate person or persons; and
|
|
o
|
Accountability for adherence to the code.
|
Limitation of Liability and Indemnification of Officers and Directors
Our Bylaws limit the liability of directors and officers of the Company to the maximum extent permitted by Florida law. The Bylaws state that the Company shall indemnify and hold harmless each person who was or is a party or is threatened to be made a party to, or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such person is or was a director or an officer of the Company or such director or officer is or was serving at the request of the Company as a director, officer, partner, member, manager, trustee, employee or agent of another company or of a partnership, limited liability company, joint venture, trust or other enterprise.
The Company believes that indemnification under our Bylaws covers at least negligence and gross negligence on the part of indemnified parties. The Company also may secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our Bylaws permit such indemnification.
The Company may also enter into separate indemnification agreements with its directors and officers, in addition to the indemnification provided for in our Bylaws. These agreements, among other things, may provide that we will indemnify our directors and officers for certain expenses (including attorneys' fees), judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of such person's services as one of our directors or officers, or rendering services at our request, to any of its subsidiaries or any other company or enterprise. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and officers.
There is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
For additional information on indemnification and limitations on liability of our directors and officers, please review the Company's Bylaws, which are attached to this Offering Circular.
COMPENSATION OF DIRECTORS AND EXECUTIVE OFFICERS
Summary Compensation Table
The following table sets forth, for the fiscal years ended December 31, 2020 and 2019, the aggregate compensation awarded to/earned by or paid to our Chief Executive Officer and our two most highly compensated officers (other than the Chief Executive Officer), who were serving as executive officers as of December 31, 2020, or the Named Executive Officers.
|
Name and
Principal
Position
|
|
Fiscal
Year
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
|
Stock
Awards
($)
|
|
|
Option
Awards ($)
|
|
|
|
Non-
Equity
Incentive Plan
Compensation
($)
|
|
|
Change in
Pension
Value
and Non-
Qualified
Deferred
Compensation
Earnings
($)
|
|
|
All Other
Compensation
($)
|
|
|
Total
($)
|
|
|
Mike Tomas
CEO, President,
|
|
2020
|
|
$
|
1,305,837
|
|
|
$
|
500,000
|
(1)
|
|
|
$
|
—
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
2,797,439
|
|
|
CFO and Director
|
|
2019
|
|
$
|
880,603
|
|
|
$
|
500,000
|
(2)
|
|
|
$
|
—
|
|
|
$
|
121,979
|
(3)(4)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
2,797,439
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,264,699
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,264,699
|
|
|
(1)
|
On July 1, 2020, Mr. Tomas received a $500,000 promissory note for a bonus awarded. The promissory note bears 5% interest per annum, is unsecured and is due on demand.
|
|
(2)
|
On July 1, 2019, Mr. Tomas received a $500,000 promissory note for a bonus awarded. The promissory note bears 5% interest per annum, is unsecured and is due on demand.
|
|
(3)
|
On September 1, 2019, Mr. Tomas was granted 20,000,000 options to purchase the Company’s common stock at $0.00557 per share for ten years, vesting over four years at each anniversary.
|
|
(4)
|
On November 18, 2019, Mr. Tomas was granted 1,500,000 options to purchase the Company’s common stock at $0.00495 per share for ten years, vesting immediately.
|
Employment Agreements
Employment Agreements
On July 1, 2019, the Company’s Board of Directors approved the 2019/2020 salary for Mike Tomas, Chief Executive Officer, for $750,000 per year, beginning July 1, 2019 with an incentive bonus ranging from $150,000 to $500,000. In addition, the Board of Directors approved a bonus of $500,000 and options to acquire 20,000,000 shares of the Company’s common stock for ten years with four-year vesting and a cashless exercise provision. The cash bonus may be paid in the form a six-month promissory note bearing interest at 5% per annum. On May 7, 2018, the Company’s Board of Directors approved the 2018/2019 salary for Mike Tomas, Chief Executive Officer, for $750,000 per year, beginning July 1, 2018 with an incentive bonus ranging from $150,000 to $500,000. In addition, the Board of Directors approved a bonus of $500,000 and options to acquire 20,000,000 shares of the Company’s common stock for ten years with four-year vesting and a cashless exercise provision. The cash bonus may be paid in the form a six-month promissory note bearing interest at 5% per annum. No compensatory package has been approved for 2021.
Outstanding Equity Awards at Fiscal Year End
The following table sets forth outstanding equity awards held by our Named Executive Officers as of December 31, 2020
|
|
|
Number of Securities Underlying
|
|
|
Option
|
|
Option
|
|
|
|
Unexercised Options and Warrants
|
|
|
Exercise Price
|
|
Expiration
|
|
Name
|
|
Total (#)
|
|
|
Unexercisable (#)
|
|
|
($/per share)
|
|
Date
|
|
Mike Tomas
|
|
|
500
|
|
|
|
-
|
|
|
|
0.15402
|
|
8/12/2021
|
|
|
|
|
500
|
|
|
|
-
|
|
|
|
0.15402
|
|
1/16/2022
|
|
|
|
|
2,000
|
|
|
|
-
|
|
|
|
0.15402
|
|
8/6/2022
|
|
|
|
|
10,000
|
|
|
|
-
|
|
|
|
0.15402
|
|
8/1/2023
|
|
|
|
|
400
|
|
|
|
-
|
|
|
|
0.15402
|
|
9/1/2023
|
|
|
|
|
10,800
|
|
|
|
-
|
|
|
|
0.15402
|
|
2/24/2024
|
|
|
|
|
4,299
|
|
|
|
-
|
|
|
|
0.15402
|
|
5/12/2024
|
|
|
|
|
10,000
|
|
|
|
-
|
|
|
|
0.15402
|
|
8/1/2024
|
|
|
|
|
400
|
|
|
|
-
|
|
|
|
0.15402
|
|
11/3/2024
|
|
|
|
|
291,885
|
|
|
|
-
|
|
|
|
0.15402
|
|
11/2/2025
|
|
|
|
|
10,000
|
|
|
|
-
|
|
|
|
0.15402
|
|
11/2/2025
|
|
|
|
|
11,500,000
|
|
|
|
-
|
|
|
|
0.01960
|
|
9/19/2026
|
|
|
|
|
10,000,000
|
|
|
|
2,500,000
|
|
|
|
0.00430
|
|
2/6/2027
|
|
|
|
|
16,500,000
|
|
|
|
3,750,000
|
|
|
|
0.03626
|
|
8/7/2027
|
|
|
|
|
20,000,000
|
|
|
|
10,000,000
|
|
|
|
0.05360
|
|
5/7/2028
|
|
|
|
|
1,500,000
|
|
|
|
-
|
|
|
|
0.02511
|
|
12/3/2028
|
|
|
|
|
20,000,000
|
|
|
|
15,000,000
|
|
|
|
0.00557
|
|
9/1//2029
|
|
|
|
|
1,500,000
|
|
|
|
-
|
|
|
|
0.00495
|
|
11/18/2029
|
Options Exercises and Stocks Vested
Options exercised and stocks vested as at December 31, 2020 are as follows:
|
|
|
Option awards
|
|
|
Stock awards
|
|
|
Name
|
|
Number of Shares
Acquired
on Exercise (#)
|
|
|
Value Realized
on Exercise ($)
|
|
|
Number of Shares
Acquired on
Vesting (#)
|
|
|
Value
Realized on
Investing ($)
|
|
|
Mike Tomas, CEO
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Grants of Plan-Based Awards
Grants of plan-based awards as at December 31, 2020 are as follows:
|
|
|
Estimated future payouts
under non-equity incentive
plan awards
|
|
|
Estimated future payouts
under equity incentive
plan awards
|
|
|
All other stock
awards:
Number of shares of stock
or units
(#)
|
|
|
All other option
awards:
Number of securities underlying
options
(#)
|
|
|
Exercise or
base price
of option
awards
($/Sh)
|
|
|
Grant date
fair value
of stock and
option awards
|
|
|
Name
|
|
Grant
date
|
|
|
Threshold ($)
|
|
|
Target
($)
|
|
|
Maximum
($)
|
|
|
Threshold
($)
|
|
|
Target
($)
|
|
|
Maximum
($)
|
|
|
|
|
|
|
|
|
|
|
Mike Tomas, CEO
|
|
|
n/a
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Reference – Grant Date - n/a = not applicable.
Non-Qualified Deferred Compensation
As at December 31, 2020 the Company had no formalized deferred compensation plan.
|
Name
|
|
Executive
contributions
in last FY ($)
|
|
|
Registrant
contributions in
last FY ($)
|
|
|
Aggregate
earnings in last
FY ($)
|
|
|
Aggregate
withdrawals/
distributions ($)
|
|
|
Aggregate
balance at last
FYE ($)
|
|
|
Mike Tomas, CEO
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Golden Parachute Compensation
As at December 31, 2020, the Company had no arrangements in place relating to the termination of employees.
|
Name
|
|
Cash
($)
|
|
|
Equity
($)
|
|
|
Pension/NQDC
($)
|
|
|
Perquisites/benefits
($)
|
|
|
Tax
reimbursement
($)
|
|
|
Other
($)
|
|
|
Total
($)
|
|
|
Mike Tomas, CEO
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Compensation of Directors
The following table sets forth summary information concerning the total compensation paid to our non-employee directors during the fiscal year ended December 31, 2020 for services to our company.
|
Name
|
|
Fees Earned
or Paid in
Cash ($)
|
|
|
Equity
Awards ($)
|
|
|
Total ($)
|
|
|
William P Murphy, Jr.
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Sheldon T. Andersen
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Mark Borman
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Gregory Knutson
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Total:
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
Pension Benefits
As of December 31, 2020, the Company had no pension or retirement plans.
|
Name
|
|
Plan name
|
|
Number of years
credited service
(#)
|
|
|
Present value of
accumulated benefit
($)
|
|
|
Payments during last
fiscal year ($)
|
|
|
Mike Tomas,CEO
|
|
not applicable
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity Compensation Plan Information
The following table sets forth information as of December 31, 2020 for all compensation plans under the Company’s Stock Option Plan:
|
Name
|
|
No. of
Shares of Common
Stock Underlying
Unexercised
Common Stock
Purchase Options
Exercisable (#)
|
|
Date of
Grant
|
|
Additional
Consideration
to be Received
Upon Exercise
or Material
Conditions
Required to
Exercise
|
|
|
Option
Exercise
Price ($)
|
|
|
Value
Realized if
Exercised ($)
|
|
Option
Expiration
Date
|
|
Mike Tomas, CEO
|
|
|
500
|
|
6/18/2010
|
|
$
|
—
|
|
|
$
|
0.15402
|
|
|
$
|
—
|
|
6/18/2020
|
|
|
|
|
500
|
|
8/12/2011
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
8/12/2021
|
|
|
|
|
500
|
|
1/16/2012
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
1/16/2022
|
|
|
|
|
2,000
|
|
8/6/2012
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
8/6/2022
|
|
|
|
|
10,000
|
|
8/01/2013
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
8/1/2023
|
|
|
|
|
400
|
|
9/1/2013
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
9/1/2023
|
|
|
|
|
10,000
|
|
2/24/2014
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
2/24/2024
|
|
|
|
|
800
|
|
2/24/2014
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
2/24/2024
|
|
|
|
|
3,225
|
|
5/12/2014
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
5/12/2014
|
|
|
|
|
10,000
|
|
8/01/2014
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
8/1/2024
|
|
|
|
|
400
|
|
11/3/2014
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
11/3/2024
|
|
|
|
|
291,885
|
|
11/2/2015
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
11/2/2025
|
|
|
|
|
5,000
|
|
11/2/2015
|
|
|
—
|
|
|
|
0.15402
|
|
|
|
—
|
|
11/2/2025
|
|
|
|
|
1,500,000
|
|
9/19/2016
|
|
|
—
|
|
|
|
0.01960
|
|
|
|
—
|
|
9/19/2026
|
|
|
|
|
2,500,000
|
|
9/19/2016
|
|
|
—
|
|
|
|
0.01960
|
|
|
|
—
|
|
9/19/2026
|
|
|
|
|
1,500,000
|
|
8/7/2017
|
|
|
—
|
|
|
|
0.03626
|
|
|
|
—
|
|
8/7/2027
|
|
|
|
|
20,000,000
|
|
5/7/2018
|
|
|
—
|
|
|
|
0.05360
|
|
|
|
—
|
|
5/7/2028
|
|
|
|
|
1,500,000
|
|
12/3/2018
|
|
|
—
|
|
|
|
0.02511
|
|
|
|
—
|
|
12/3/2028
|
|
|
|
|
20,000,000
|
|
9/1/2019
|
|
|
—
|
|
|
|
0.00557
|
|
|
|
—
|
|
09/01/2029
|
|
|
|
|
1,500,000
|
|
11/18/2019
|
|
|
—
|
|
|
|
0.00495
|
|
|
|
—
|
|
11/18/2029
|
Director Compensation
As of December 31, 2020, we had five directors that qualified for compensation. Our non-employee directors do not receive cash compensation for their services as directors. However, it is generally our policy to annually grant each non-employee director options to purchase shares of our common stock provided that he or she has served as a member of our Board of Directors for at least six months and one day of the twelve month period immediately preceding the date of grant. Our one employee director, Mike Tomas, is also granted director options to purchase shares of our common stock In addition, we reimburse non-employee directors for actual out-of-pocket expenses incurred.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table sets forth the beneficial ownership (1) of our common stock as of June 30, 2021 based on an aggregate of 452,413,153 common shares issued, and 46,597,915 shares underlying exercisable options, 7,384,398 issuable upon exercise of a promissory note and 20,000 common stock purchase warrants, for an aggregate of 506,415,466, for each of our greater than 5% shareholders, directors, named executive officers that continue to serve as executive officers of U.S. Stem Cell and by all of our directors and named executive officers as a group as of June 30, 2021. Unless otherwise indicated, the address of each of the individuals and entities named below is: c/o U.S. Stem Cell, Inc., 1560 Sawgrass Corporate Parkway, 4th FL Sunrise FL 33323. Except as noted below, to our knowledge, each person named in the table has sole voting and investment power with respect to all shares of our common stock beneficially owned by them.
The number and percentage of shares beneficially owned is determined in accordance with Rule 13d-3 under the Exchange Act, and the information is not necessarily indicative of beneficial ownership for any other purpose. The Company believes that each individual or entity named has sole investment and voting power with respect to the securities indicated as beneficially owned by them, subject to community property laws, where applicable, except where otherwise noted. The “Amount of Beneficial Ownership” in calculated based on total shares held plus warrants held (plus stock options entitled to exercise). The aggregate of these items will be used as the denominator each for the percentage calculation below.
|
Name and Address of Beneficial Owner
|
|
Amount of Beneficial Ownership
|
|
|
|
Percent of Class
|
|
|
Mike Tomas, President, CEO, CFO, and Director
|
|
|
61,350,577
|
(1)
|
|
|
|
13.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
William P. Murphy, Director**
|
|
|
18,448,262
|
(2)
|
|
|
|
4.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark P. Borman, Director
|
|
|
6,529,732
|
(3)
|
|
|
|
1.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
Sheldon T. Anderson, Director
|
|
|
6,003,341
|
(4)
|
|
|
|
1.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
Greg Knutson**
|
|
|
44,553,942
|
(5)
|
|
|
|
9.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
All officers and directors as a group (5 persons)
|
|
|
136,885,854
|
(6)
|
|
|
|
30.2
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
Northstar Biotechnology Group, LLC
|
|
|
35,494,544
|
(7)
|
|
|
|
7.8
|
%
|
|
(1)
|
Includes shares are held by The Astri Group over which Mr. Tomas has shared voting and investment power and includes (i) includes 9,793 shares of common stock and (ii) 61,340,784 shares of common stock issuable upon exercise of presently exercisable stock options .
|
|
(2)
|
Includes (i) 12,445,322 shares of common stock and (ii) 6,002,940 shares of common stock issuable upon exercise of presently exercisable stock options. Shares are directly owned by trusts controlled by Dr. Murphy and his spouse.
|
|
(3)
|
Includes (i) 526,942 shares of common stock and (ii) 6,002,790 shares of common stock issuable upon exercise of presently exercisable stock options
|
|
(4)
|
Includes (i) 1,941 shares of common stock and (ii) 6,001,400 shares of common stock issuable upon exercise of presently exercisable options.
|
|
(5)
|
Includes 1,675,000 shares held directly, 35,474,544 shares of common stock held by Northstar Biotechnology Group, LLC, an entity in which Mr. Knutson is the Manger, and 7,384,398 issuable upon exercise of a promissory note and 20,000 common stock purchase warrants held by Northstar Biotechnology Group, LLC, an entity in which Mr. Knutson is the Manger.
|
|
(6)
|
Includes an aggregate of (i) 47,040,823 shares of common stock and (ii) 46,597,915 shares underlying exercisable options, 7,384,398 issuable upon exercise of a promissory note and 20,000 common stock purchase warrants.
|
|
(7)
|
Includes 35,474,544 shares of common stock and (ii) 20,000 shares of common stock issuable upon exercise of warrants.
|
DESCRIPTION OF SECURITIES
The following statements relating to the capital stock set forth the material terms of our securities; however, reference is made to the more detailed provisions of, and such statements are qualified in their entirety by reference to, the Certificate of Incorporation, amendment to the Certificate of Incorporation and the By-laws, copies of which are filed as exhibits to this registration statement.
COMMON STOCK
The holders of our Common Stock are entitled to one vote per share on all matters to be voted on by our stockholders, including the election of directors. Our stockholders are not entitled to cumulative voting rights, and, accordingly, the holders of a majority of the shares voting for the election of directors can elect the entire board of directors if they choose to do so and, in that event, the holders of the remaining shares will not be able to elect any person to our board of directors.
On February 4, 2013, effective with the filing of the amendment to the Company’s Articles of Incorporation with the Florida Secretary of State (confirmed as filed on February 11, 2013), the Company amended its Articles of Incorporation to increase the authorized shares of capital stock of the Company to nine hundred and seventy million (970,000,000) shares of capital stock consisting of nine hundred and fifty million (950,000,000) shares of common stock and twenty million (20,000,000) shares of preferred stock, both $.001 par value respectively.
Effective May 22, 2014, the Company amended its articles of incorporation to increase the authorized shares of capital stock of the Company from nine hundred and fifty million (950,000,000) shares of common stock and twenty million (20,000,000) shares of preferred stock, both $.001 par value respectively, to two billion (2,000,000,000) shares of shares of common stock and twenty million (20,000,000) shares of preferred stock, both $.001 par value respectively.
On October 12, 2015, the Company filed an amendment to its Articles of Incorporation and affected a 1-for-1,000 reverse stock split of its issued and outstanding shares of common stock, $0.001 par value, and effective November 19, 2015. The Financial Industry Regulatory Authority (“FINRA”) declared the ex-dividend date for the dividend date as November 4, 2015 (the “2015 Reverse Split”).
The holders of the Company’s Common Stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by the board of directors, in its discretion, from funds legally available there for and subject to prior dividend rights of holders of any shares of our Preferred Stock which may be outstanding and any contractual limitations. Upon the Company’s liquidation, dissolution or winding up, subject to prior liquidation rights of the holders of our Preferred Stock, if any, the holders of our Common Stock are entitled to receive on a pro rata basis our remaining assets available for distribution. Holders of the Company’s Common Stock have no preemptive or other subscription rights, and there are no conversion rights or redemption or sinking fund provisions with respect to such shares. All outstanding shares of the Company’s Common Stock are, fully paid and not liable to further calls or assessment by the Company.
Preferred Stock
The Company is authorized to issue 20,000,000 shares of preferred stock, par value $0.001. The designations, rights, and preferences of such preferred stock are to be determined by the Board of Directors. Subsequently and prior to the 2015 “Reverse Split”, 20,000,000 shares were designated as Series A Preferred Stock.
The Series A Preferred Stock collectively has voting rights equal to 25 votes on all matters presented to be voted by the holders of common stock per share of preferred stock and the right to convert to one share of common stock for each share of preferred stock. Northstar Biotechnology Group, LLC was issued, prior to the 2015 “Reverse Split” , an aggregate of 20,000,000 shares of Series A Preferred Stock which were converted to common stock pursuant to a Settlement Agreement dated March 1, 2017. As of the date of this report, no shares of preferred stock are issued and outstanding.
DIVIDENDS
Dividends, if any, will be contingent upon our revenues and earnings, if any, capital requirements and financial conditions. The payment of dividends, if any, will be within the discretion of our Board of Directors. We presently intend to retain all earnings, if any, for use in its business operations and accordingly, the Board of Directors does not anticipate declaring any dividends prior to a business combination.
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
As of June 30, 2021 and December 31, 2020, the Company’s officers and directors have provided advances that are unsecured, non-interest bearing and due on demand. During the six months ended June 30, 2021 and 2020, the Company received aggregate proceeds from advances of $30,000 and $155,904, respectively. As of June 30, 2021 and December 31, 2020, the Company owed $891,432 and $861,432, respectively, for related party advances.
Notes Payable – Related Parties
Northstar Biotechnology Group, LLC
On February 29, 2012, a promissory note issued to BlueCrest Master Fund Limited (“BlueCrest”) was assigned to Northstar Biotechnology Group, LLC (“Northstar”), owned partly by certain directors and existing shareholders of the Company at the time, including Dr. William P. Murphy Jr., Dr. Samuel Ahn and Charles Hart. At the date of the assignment, the principal amount of the BlueCrest note was $544,267 (the “Note”).
On March 30, 2012, the Company and Northstar agreed to extend until May 1, 2012 the initial payment date for any and all required monthly under the Note, such that the first of the four monthly payments required under the Note will be due and payable on May 1, 2012 and all subsequent payments will be due on a monthly basis thereafter commencing on June 1, 2012, and to waive any and all defaults and/or events of default under the Note with respect to such payments. The Company did not make the required payment, and as a result, was in default of the revised agreement. The Company renegotiated the terms of the Note and Northstar agreed to suspend the requirement of principal payments by the Company and allow payment of interest-only in common stock.
On September 21, 2012, the Company issued 5,000 common stock purchase warrants to Northstar that was treated as additional interest expense upon issuance.
On October 1, 2012, the Company and Northstar entered into a limited waiver and forbearance agreement providing a recapitalized new note balance comprised of all sums due Northstar with a maturity date extended perpetually. The Company agreed to issue 5,000,000 shares of Series A Convertible Preferred Stock and 10,000 shares of common stock in exchange for $210,000 as payment towards outstanding debt, default interest, penalties, professional fees outstanding and due Northstar. In addition, the Company executed a security agreement granting Northstar a lien on all patents, patent applications, trademarks, service marks, copyrights and intellectual property rights of any nature, as well as the results of all clinical trials, know-how for preparing Myoblasts, old and new clinical data, existing approved trials, all right and title to Myoblasts, clinical trial protocols and other property rights.
In addition, the Company granted Northstar a perpetual license on products as described for resale, relicensing, and commercialization outside the United States. In connection with the granted license, Northstar shall pay the Company a royalty of up to 8% on revenues generated.
Effective October 1, 2012, the interest rate was 12.85% per annum. The parties agreed, as of February 28, 2013, to reduce the interest rate to 7% per annum.
In connection with the consideration paid, Northstar waived, from the effective date through the earlier of termination or expiration of the agreement, satisfaction of the obligations as described in the forbearance agreement.
In 2012, 5,000,000 shares of Series A Convertible Preferred Stock were approved to be issued, which was subsequently increased to 20,000,000 shares of preferred stock as Series A Convertible Preferred Stock. In addition, the Company was obligated to issue additional preferred stock equal in lieu of payment of cash of accrued and unpaid interest on each six-month anniversary of the effective date (October 1, 2012). In lieu of the initial two payments in preferred stock, the parties agreed to modify the voting rights of the subsequently cancelled Series A Convertible Preferred Stock from 20 votes per share on matters to be voted on by the common stockholders to 25 votes per share on matters to be voted on by the common stockholders and all prior and subsequent payments of interest will be in common stock. The Company is required to issue additional shares of its common stock (as amended), in lieu of cash, each six-month anniversary of the effective date for any accrued and unpaid interest.
On September 30, 2013, the Company issued 8,772 shares of its common stock as payment of $100,000 towards principal.
On December 24, 2013, the Company issued 3,916 shares of its common stock as payment of accrued interest through June 30, 2013 of $85,447.
On April 2, 2014, the Company issued 275 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2014 per the forbearance agreement.
On September 17, 2014, the limited waiver and forbearance agreement entered into on October 1, 2012 to provide that the perpetual license on products as described for resale, relicensing and commercialization outside the United States was amended as such on the condition that Northstar provide certain financing, which financing the Company, in its sole discretion, could decline and retain the license.
On October 3, 2014, the Company issued 515 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2014 per the forbearance agreement.
On April 3, 2015, the Company issued 1,363 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2015 per the forbearance agreement.
On October 2, 2015, the Company issued 4,156 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2015 per the forbearance agreement.
On October 7, 2015, the Company issued 34,522 shares of its common stock in settlement of $100,000 principal payment towards the outstanding debt.
On April 7, 2016, the Company issued 57,778 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due April 1, 2016 per the forbearance agreement.
On October 6, 2016, the Company issued 848,490 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2016 per the forbearance agreement.
On March 1, 2017, Northstar and the Company entered into a settlement agreement (“Settlement Agreement “) related to then pending litigation. Pursuant to the terms and conditions of the Settlement Agreement, Northstar converted its outstanding Series A Convertible preferred stock, into twenty million (20,000,000) shares of common stock according to the original conversion terms. In addition, and separate and apart from the conversion, Northstar received eleven million (11,000,000) shares of the Company’s common stock. Northstar will receive ten percent (10%) of all Company international sales (based on a gross sales basis). There was no effect of the 10% obligation as there were no international sales in 2017 or through 2019. Furthermore, a Northstar designee, Greg Knutson, was appointed as a member of the Board of Directors of the Company and two Company directors, Michael Tomas and Kristin Comella, each exercised their prior Northstar options to each receive a five percent (5%) member interest in Northstar. The parties agreed to a mutual release and Northstar agreed to terminate any UCC lien on the Company assets previously filed for the benefit of Northstar. On March 9, 2017 and April 1, 2017, the Company issued 30,000,000 and 1,000,000 shares of its common stock, respectively, as described above. In connection with the settlement, the Company recorded a loss on litigation settlement of $316,800.
On April 1, 2017, the Company issued 286,315 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,703.
On October 2, 2017, the Company issued 559,187 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705.
On October 19, 2018, the Company issued 164,523 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,195.
On April 19, 2019, the Company issued 379,141 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,145.
On October 1, 2019, the Company issued 1,692,353 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,195.
On April 1, 2020, the Company issued 1,445,647 shares of its common stock, having a fair value of $11,565, in lieu of payment in cash of accrued and unpaid interest of $9,145, resulting in a loss on settlement of $2,420.
On October 1, 2020, the Company issued 2,035,820 shares of its common stock, having a fair value of $10,179, in lieu of payment in cash of accrued and unpaid interest of $9,195, resulting in a loss on settlement of $984.
On April 1, 2021, the Company issued 187,575 shares of its common stock, having a fair value of $10,879, in lieu of payment in cash of accrued and unpaid interest of $9,145, resulting in a loss on settlement of $1,735.
As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $262,000. At June 30, 2021 and December 31, 2020, accrued interest on the note was $8,700 and $8,751, respectively, and is included in accrued expenses on the accompanying balance sheet.
Notes Payable - Mr. Tomas, President and Chief Executive Officer
On August 7, 2017, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due one year from date of issuance. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $161,786.
On May 7, 2018, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due six months from date of issuance. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $500,000.
On July 1, 2019, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due November 7, 2019. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $500,000.
On December 31, 2019, the Company issued a $178,077 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $178,077.
On March 31, 2020, the Company issued a $187,500 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $187,500.
On June 30, 2020, the Company issued a $187,500 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $187,500.
On July 1, 2020, the Company issued a $500,000 promissory note as payment of an annual bonus awarded. The promissory note bears interest of 5% per annum and is due on demand. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $500,000.
On September 30, 2020, the Company issued a $100,962 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $100,962.
On December 31, 2020, the Company issued a $143,654 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of March 31, 2021 and December 31, 2020, the remaining carrying value of the note was $143,654.
On June 30, 2021, the Company issued a $43,269 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021, the remaining carrying value of the note was $43,269.
At June 30, 2021 and December 31, 2021, accrued interest on the notes was $544,584 and $482,468, respectively, and is included in accrued expenses on the accompanying balance sheet.
|
|
|
June 30,
2021
|
|
|
December 31,
2020
|
|
|
Northstar
|
|
$
|
262,000
|
|
|
$
|
262,000
|
|
|
Note payable, Mr. Tomas
|
|
|
161,786
|
|
|
|
161,786
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
178,077
|
|
|
|
178,077
|
|
|
Note payable, Mr. Tomas
|
|
|
187,500
|
|
|
|
187,500
|
|
|
Note payable, Mr. Tomas
|
|
|
187,500
|
|
|
|
187,500
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
100,962
|
|
|
|
100,962
|
|
|
Note payable, Mr. Tomas
|
|
|
143,654
|
|
|
|
143,653
|
|
|
Note payable, Mr. Tomas
|
|
|
90,990
|
|
|
|
-
|
|
|
Note payable, Mr. Tomas
|
|
|
43,269
|
|
|
|
-
|
|
|
Total notes payable - related parties
|
|
$
|
2,855,738
|
|
|
$
|
2,721,478
|
|
Notes Payable - William P. Murphy Jr., M.D
On February 26, 2021, Dr. Murphy purchased an unsecured convertible promissory notes in the aggregate principal amount of $200,000 that mature 12 months after the respective issuance date. The note was non-interest bearing and Dr. Murphy retained the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0266. On April 8, 2021, Dr Murphy converted the full value of the note into 7,518,797 shares of the Company’s common stock.
SECURITIES BEING OFFERED
The Company is offering Shares of its Common Stock. Except as otherwise required by law, the Company's Articles of Incorporation or Bylaws, each Shareholder shall be entitled to one vote for each Share held by such Shareholder on the record date of any vote of Shareholders of the Company. The Shares of Common Stock, when issued, will be fully paid and non-assessable. Since it is anticipated that at least for the next 12 months the majority of the Company's voting power will be held by Management, the holders of Common Stock issued pursuant to this Offering Circular should not expect to be able to influence any decisions by management of the Company through the voting power of such Common Stock.
The Company does not expect to create any additional classes of Common Stock during the next 12 months, but the Company is not limited from creating additional classes which may have preferred dividend, voting and/or liquidation rights or other benefits not available to holders of its common stock.
The Company does not expect to declare dividends for holders of Common Stock in the foreseeable future. Dividends will be declared, if at all (and subject to rights of holders of additional classes of securities, if any), in the discretion of the Company's Board of Directors. Dividends, if ever declared, may be paid in cash, in property, or in shares of the capital stock of the Company, subject to the provisions of law, the Company's Bylaws and the Certificate of Incorporation. Before payment of any dividend, there may be set aside out of any funds of the Company available for dividends such sums as the Board of Directors, in its absolute discretion, deems proper as a reserve for working capital, to meet contingencies, for equalizing dividends, for repairing or maintaining any property of the Company, or for such other purposes as the Board of Directors shall deem in the best interests of the Company.
There is no minimum number of Shares that needs to be sold in order for funds to be released to the Company and for this Offering to hold its first closing.
The minimum subscription that will be accepted from an investor is $250.00 for the purchase of 8,334 Shares at the Maximum Offering Price (the 'Minimum Subscription').
A subscription for $250.00 or more in the Shares may be made only by tendering to the Company the executed Subscription Agreement (electronically or in writing) delivered with the subscription price in a form acceptable to the Company, via check, wire, credit or debit card, or ACH. The execution and tender of the documents required, as detailed in the materials, constitutes a binding offer to purchase the number of Shares stipulated therein and an agreement to hold the offer open until the Expiration Date or until the offer is accepted or rejected by the Company, whichever occurs first.
The Company reserves the unqualified discretionary right to reject any subscription for Shares, in whole or in part. The Company reserves the unqualified discretionary right to accept any subscription for Shares, in an amount less than the Minimum Subscription. If the Company rejects any offer to subscribe for the Shares, it will return the subscription payment, without interest or reduction. The Company's acceptance of your subscription will be effective when an authorized representative of the Company issues you written or electronic notification that the subscription was accepted.
There are no liquidation rights, preemptive rights, conversion rights, redemption provisions, sinking fund provisions, impacts on classification of the Board of Directors where cumulative voting is permitted or required related to the Common Stock, provisions discriminating against any existing or prospective holder of the Common Stock as a result of such Shareholder owning a substantial amount of securities, or rights of Shareholders that may be modified otherwise than by a vote of a majority or more of the shares outstanding, voting as a class defined in any corporate document as of the date of filing. The Common Stock will not be subject to further calls or assessment by the Company. There are no restrictions on alienability of the Common Stock in the corporate documents other than those disclosed in this Offering Circular. The Company has engaged Transfer Online to serve as the transfer agent and registrant for the Shares. For additional information regarding the Shares, please review the Company's Bylaws, which are attached to this Offering Circular.
Excepting matters arising under federal securities laws, any disputes between the Company and shareholders shall be governed in reliance on the laws of the state of Florida. Furthermore, the Subscription Agreement for this Regulation A offering appoints the state and federal courts located in Sunrise, Florida as having jurisdiction over any disputes related to this Regulation A offering between the Company and shareholders.
DISQUALIFYING EVENTS DISCLOSURE
Recent changes to Regulation A promulgated under the Securities Act prohibit an issuer from claiming an exemption from registration of its securities under such rule if the issuer, any of its predecessors, any affiliated issuer, any director, executive officer, other officer participating in the offering of the interests, general partner or managing member of the issuer, any beneficial owner of 20% or more of the voting power of the issuer's outstanding voting equity securities, any promoter connected with the issuer in any capacity as of the date hereof, any investment manager of the issuer, any person that has been or will be paid (directly or indirectly) remuneration for solicitation of purchasers in connection with such sale of the issuer's interests, any general partner or managing member of any such investment manager or solicitor, or any director, executive officer or other officer participating in the offering of any such investment manager or solicitor or general partner or managing member of such investment manager or solicitor has been subject to certain “Disqualifying Events” described in Rule 506(d)(1) of Regulation D subsequent to September 23, 2013, subject to certain limited exceptions. The Company is required to exercise reasonable care in conducting an inquiry to determine whether any such persons have been subject to such Disqualifying Events and is required to disclose any Disqualifying Events that occurred prior to September 23, 2013 to investors in the Company. The Company believes that it has exercised reasonable care in conducting an inquiry into Disqualifying Events by the foregoing persons and is aware of the no such Disqualifying Events.
It is possible that (a) Disqualifying Events may exist of which the Company is not aware and (b) the SEC, a court or other finder of fact may determine that the steps that the Company has taken to conduct its inquiry were inadequate and did not constitute reasonable care. If such a finding were made, the Company may lose its ability to rely upon exemptions under Regulation A, and, depending on the circumstances, may be required to register the Offering of the Company's Common Stock with the SEC and under applicable state securities laws or to conduct a rescission offer with respect to the securities sold in the Offering.
ERISA CONSIDERATIONS
Trustees and other fiduciaries of qualified retirement plans or IRAs that are set up as part of a plan sponsored and maintained by an employer, as well as trustees and fiduciaries of Keogh Plans under which employees, in addition to self-employed individuals, are participants (together, “ERISA Plans”), are governed by the fiduciary responsibility provisions of Title 1 of the Employee Retirement Income Security Act of 1974 (“ERISA”). An investment in the Shares by an ERISA Plan must be made in accordance with the general obligation of fiduciaries under ERISA to discharge their duties (i) for the exclusive purpose of providing benefits to participants and their beneficiaries; (ii) with the same standard of care that would be exercised by a prudent man familiar with such matters acting under similar circumstances; (iii) in such a manner as to diversify the investments of the plan, unless it is clearly prudent not do so; and (iv) in accordance with the documents establishing the plan. Fiduciaries considering an investment in the Shares should accordingly consult their own legal advisors if they have any concern as to whether the investment would be inconsistent with any of these criteria.
Fiduciaries of certain ERISA Plans which provide for individual accounts (for example, those which qualify under Section 401(k) of the Code, Keogh Plans and IRAs) and which permit a beneficiary to exercise independent control over the assets in his individual account, will not be liable for any investment loss or for any breach of the prudence or diversification obligations which results from the exercise of such control by the beneficiary, nor will the beneficiary be deemed to be a fiduciary subject to the general fiduciary obligations merely by virtue of his exercise of such control. On October 13, 1992, the Department of Labor issued regulations establishing criteria for determining whether the extent of a beneficiary's independent control over the assets in his account is adequate to relieve the ERISA Plan's fiduciaries of their obligations with respect to an investment directed by the beneficiary. Under the regulations, the beneficiary must not only exercise actual, independent control in directing the particular investment transaction, but also the ERISA Plan must give the participant or beneficiary a reasonable opportunity to exercise such control, and must permit him to choose among a broad range of investment alternatives.
Trustees and other fiduciaries making the investment decision for any qualified retirement plan, IRA or Keogh Plan (or beneficiaries exercising control over their individual accounts) should also consider the application of the prohibited transactions provisions of ERISA and the Code in making their investment decision. Sales and certain other transactions between a qualified retirement plan, IRA or Keogh Plan and certain persons related to it (e.g., a plan sponsor, fiduciary, or service provider) are prohibited transactions. The particular facts concerning the sponsorship, operations and other investments of a qualified retirement plan, IRA or Keogh Plan may cause a wide range of persons to be treated as parties in interest or disqualified persons with respect to it. Any fiduciary, participant or beneficiary considering an investment in Shares by a qualified retirement plan IRA or Keogh Plan should examine the individual circumstances of that plan to determine that the investment will not be a prohibited transaction. Fiduciaries, participants or beneficiaries considering an investment in the Shares should consult their own legal advisors if they have any concern as to whether the investment would be a prohibited transaction.
Regulations issued on November 13, 1986, by the Department of Labor (the “Final Plan Assets Regulations”) provide that when an ERISA Plan or any other plan covered by Code Section 4975 (e.g., an IRA or a Keogh Plan which covers only self-employed persons) makes an investment in an equity interest of an entity that is neither a “publicly offered security” nor a security issued by an investment company registered under the Investment Company Act of 1940, the underlying assets of the entity in which the investment is made could be treated as assets of the investing plan (referred to in ERISA as “plan assets”). Programs which are deemed to be operating companies or which do not issue more than 25% of their equity interests to ERISA Plans are exempt from being designated as holding “plan assets.” Management anticipates that we would clearly be characterized as an “operating” for the purposes of the regulations, and that it would therefore not be deemed to be holding “plan assets.”
Classification of our assets of as “plan assets” could adversely affect both the plan fiduciary and management. The term “fiduciary” is defined generally to include any person who exercises any authority or control over the management or disposition of plan assets. Thus, classification of our assets as plan assets could make the management a “fiduciary” of an investing plan. If our assets are deemed to be plan assets of investor plans, transactions which may occur in the course of its operations may constitute violations by the management of fiduciary duties under ERISA. Violation of fiduciary duties by management could result in liability not only for management but also for the trustee or other fiduciary of an investing ERISA Plan. In addition, if our assets are classified as “plan assets,” certain transactions that we might enter into in the ordinary course of our business might constitute “prohibited transactions” under ERISA and the Code.
Under Code Section 408(i), as amended by the Tax Reform Act of 1986, IRA trustees must report the fair market value of investments to IRA holders by January 31 of each year. The Service has not yet promulgated regulations defining appropriate methods for the determination of fair market value for this purpose. In addition, the assets of an ERISA Plan or Keogh Plan must be valued at their “current value” as of the close of the plan's fiscal year in order to comply with certain reporting obligations under ERISA and the Code. For purposes of such requirements, “current value” means fair market value where available. Otherwise, current value means the fair value as determined in good faith under the terms of the plan by a trustee or other named fiduciary, assuming an orderly liquidation at the time of the determination. We do not have an obligation under ERISA or the Code with respect to such reports or valuation although management will use good faith efforts to assist fiduciaries with their valuation reports. There can be no assurance, however, that any value so established (i) could or will actually be realized by the IRA, ERISA Plan or Keogh Plan upon sale of the Shares or upon liquidation of us, or (ii) will comply with the ERISA or Code requirements.
The income earned by a qualified pension, profit sharing or stock bonus plan (collectively, “Qualified Plan”) and by an individual retirement account (“IRA”) is generally exempt from taxation. However, if a Qualified Plan or IRA earns “unrelated business taxable income” (“UBTI”), this income will be subject to tax to the extent it exceeds $1,000 during any fiscal year. The amount of unrelated business taxable income in excess of $1,000 in any fiscal year will be taxed at rates up to 36%. In addition, such unrelated business taxable income may result in a tax preference, which may be subject to the alternative minimum tax. It is anticipated that income and gain from an investment in the Shares will not be taxed as UBTI to tax exempt shareholders, because they are participating only as passive financing sources.
Transfer Agent
Our transfer agent is Continental Stock Transfer & Trust Company, One State Street, 30th Floor, New York, NY 10004. Our transfer agent is registered with the Securities and Exchange Commission.
DIVIDEND POLICY
Subject to preferences that may be applicable to any then-outstanding shares of Preferred Stock, if any, and any other restrictions, holders of Common Stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. We and our predecessors have not declared any dividends in the past. Further, we do not presently contemplate that there will be any future payment of any dividends on Common Stock.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this Offering, there has been a limited market for our Common Stock on the OTC Markets. Future sales of substantial amounts of our Common Stock, or securities or instruments convertible into our Common Stock, in the public market, or the perception that such sales may occur, could adversely affect the market price of our Common Stock prevailing from time to time. Furthermore, because there will be limits on the number of shares available for resale shortly after this Offering due to contractual and legal restrictions described below, there may be resales of substantial amounts of our Common Stock in the public market after those restrictions lapse. This could adversely affect the market price of our Common Stock prevailing at that time.
Upon completion of this Offering, assuming the maximum amount of shares of Common Stock offered in this Offering are sold, there will be 706,217,501shares of our Common Stock outstanding.
Rule 144
In general, a person who has beneficially owned restricted shares of our Common Stock for at least twelve months, in the event we are a reporting company under Regulation A, or at least six months, in the event we have been a reporting company under the Exchange Act for at least 90 days before the sale, would be entitled to sell such securities, provided that such person is not deemed to be an affiliate of ours at the time of sale or to have been an affiliate of ours at any time during the 90 days preceding the sale. A person who is an affiliate of ours at such time would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of shares that does not exceed the greater of the following:
|
|
•
|
1% of the number of shares of our Common Stock then outstanding; or
|
|
|
•
|
the average weekly trading volume of our Common Stock during the four calendar weeks preceding the filing by such person of a notice on Form 144 with respect to the sale;
|
provided that, in each case, we are subject to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Rule 144 trades must also comply with the manner of sale, notice and other provisions of Rule 144, to the extent applicable.
INVESTOR ELIGIBILITY STANDARDS
ADDITIONAL INFORMATION ABOUT THE OFFERING
Investment Limitations
Generally, no sale may be made to you in this Offering if the aggregate purchase price you pay is more than 10% of the greater of your annual income or net worth (please see below on how to calculate your net worth). Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A+. For general information on investing, we encourage you to refer to www.investor.gov.
Because this is a Tier 2, Regulation A+ offering, most investors must comply with the 10% limitation on investment in the Offering. The only investor in this Offering exempt from this limitation is an “accredited investor” as defined under Rule 501 of Regulation D under the Securities Act. If you meet one of the following tests you should qualify as an accredited investor:
|
|
(i)
|
You are a natural person who has had individual income in excess of $200,000 in each of the two most recent years, or joint income with your spouse in excess of $300,000 in each of these years, and have a reasonable expectation of reaching the same income level in the current year;
|
|
|
|
|
|
|
(ii)
|
You are a natural person and your individual net worth, or joint net worth with your spouse, exceeds $1,000,000 at the time you purchase Shares (please see below on how to calculate your net worth);
|
|
|
|
|
|
|
(iii)
|
You are an executive officer or general partner of the issuer or a manager or executive officer of the general partner of the issuer;
|
|
|
|
|
|
|
(iv)
|
You are an organization described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, or the Code, a corporation, a Massachusetts or similar business trust or a partnership, not formed for the specific purpose of acquiring the Shares, with total assets in excess of $5,000,000;
|
|
|
(v)
|
You are a bank or a savings and loan association or other institution as defined in the Securities Act, a broker or dealer registered pursuant to Section 15 of the Exchange Act, an insurance company as defined by the Securities Act, an investment company registered under the Investment Company Act of 1940 (Investment Company Act), or a business development company as defined in that act, any Small Business Investment Company licensed by the Small Business Investment Act of 1958 or a private business development company as defined in the Investment Advisers Act of 1940;
|
|
|
(v)
|
You are an entity (including an Individual Retirement Account trust) in which each equity owner is an accredited
|
|
|
|
|
|
|
(vii)
|
You are a trust with total assets in excess of $5,000,000, your purchase of Shares is directed by a person who either alone or with his purchaser representative(s) (as defined in Regulation D promulgated under the Securities Act) has such knowledge and experience in financial and business matters that he is capable of evaluating the merits and risks of the prospective investment, and you were not formed for the specific purpose of investing in the Shares; or
|
|
|
|
|
|
|
(viii)
|
You are a plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has assets in excess of $5,000,000.
|
Offering Period and Expiration Date
This Offering will start on the date on which the SEC initially qualifies this Offering Statement (the Qualification Date) and will terminate on the Termination Date.
Procedures for Subscribing
If you decide to subscribe for our Common Stock shares in this Offering, you should:
|
1.
|
Electronically receive, review, execute and deliver to us a Subscription Agreement; and
|
|
|
|
|
2.
|
Deliver funds directly to the Company’s designated bank account via bank wire transfer (pursuant to the wire transfer instructions set forth in our Subscription Agreement) or electronic funds transfer via wire transfer or via personal check mailed to the Company, at 1090 King Georges Post Road, Suite 603, Edison, NJ 08837.
|
Any potential investor will have ample time to review the subscription agreement, along with their counsel, prior to making any final investment decision. We shall only deliver such subscription agreement upon request after a potential investor has had ample opportunity to review this Offering Circular.
Right to Reject Subscriptions. After we receive your complete, executed subscription agreement and the funds required under the subscription agreement have been transferred to our designated account, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions. Upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the shares subscribed at closing. Once you submit the subscription agreement, you may not revoke or change your subscription or request your subscription funds. All submitted subscription agreements are irrevocable.
Under Rule 251 of Regulation A+, non-accredited, non-natural investors are subject to the investment limitation and may only invest funds which do not exceed 10% of the greater of the purchaser’s revenue or net assets (as of the purchaser’s most recent fiscal year end). A non-accredited, natural person may only invest funds which do not exceed 10% of the greater of the purchaser’s annual income or net worth (please see below on how to calculate your net worth).
NOTE: For the purposes of calculating your net worth, it is defined as the difference between total assets and total liabilities. This calculation must exclude the value of your primary residence and may exclude any indebtedness secured by your primary residence (up to an amount equal to the value of your primary residence). In the case of fiduciary accounts, net worth and/or income suitability requirements may be satisfied by the beneficiary of the account or by the fiduciary, if the fiduciary directly or indirectly provides funds for the purchase of the Shares.
In order to purchase our Common Stock shares and prior to the acceptance of any funds from an investor, an investor will be required to represent, to the Company’s satisfaction, that such investor is either an accredited investor or is in compliance with the 10% of net worth or annual income limitation on investment in this Offering.
LEGAL MATTERS
Certain legal matters with respect to the shares of common stock offered hereby will be passed upon by Joseph I. Emas, P.A.
EXPERTS
The financial statements of U.S. Stem Cell, Inc. as of and for the years ended December 31, 2020 and 2019 appearing in this Regulation A Offering Circular have been audited by RBSM LLP, an independent registered public accounting firm, as stated in its report thereon, included therein, and are included in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
REPORTS
Following this Tier II, Regulation A offering, we will be required to comply with certain ongoing disclosure requirements under Rule 257 of Regulation A which will be incorporated into our filings under the Securities Exchange Act of 1934, as amended.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a Regulation A Offering Statement on Form 1-A under the Securities Act with respect to the shares of common stock offered hereby. This Offering Circular, which constitutes a part of the Offering Statement, does not contain all of the information set forth in the Offering Statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the Offering Statement and the exhibits and schedules filed therewith. Statements contained in this Offering Circular regarding the contents of any contract or other document that is filed as an exhibit to the Offering Statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the Offering Statement. Upon the completion of this Offering, we will be required to file periodic reports, proxy statements, and other information with the SEC pursuant to the Securities Exchange Act of 1934. You may read and copy this information at the SEC's Public Reference Room, 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about issuers, including us, that file electronically with the SEC. The address of this site is www.sec.gov.
FINANCIAL STATEMENTS
OF U.S. STEM CELL, INC.
DECEMBER 31, 2019 AND DECEMBER 31, 2020
INDEX TO FINANCIAL STATEMENTS
|
Reports of Independent Registered Public Accounting Firms
|
F-2
|
|
|
|
|
Balance Sheets as of December 31, 2020 and 2019
|
F-3
|
|
|
|
|
Statements of Operations for the years ended December 31, 2020 and 2019
|
F-4
|
|
|
|
|
Statement of Stockholders’ Deficit for the two years ended December 31, 2020
|
F-5
|
|
|
|
|
Statements of Cash Flows for the years ended December 31, 2020 and 2019
|
F-6
|
|
|
|
|
Notes to Financial Statements
|
F-7
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of U.S Stem Cell, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of U.S Stem Cell, Inc. (the Company) as of December 31, 2020 and 2019, and the related statements of operations, stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
The Company's Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations, generated negative cash flows from operating activities, will require additional capital to fund its current operating plan, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management's evaluation of the events and conditions and management’s plans in regarding these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there were no critical audit matters.
/s/ RBSM LLP
We have served as the Company’s auditor since 2018.
New York, NY
July 15, 2021
U.S. STEM CELL, INC.
BALANCE SHEETS
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
18,570
|
|
|
$
|
-
|
|
|
Accounts receivable, net
|
|
|
54,164
|
|
|
|
48,208
|
|
|
Inventories
|
|
|
5,418
|
|
|
|
8,096
|
|
|
Prepaid expenses and other current assets
|
|
|
10,000
|
|
|
|
10,000
|
|
|
Total current assets
|
|
|
88,152
|
|
|
|
66,304
|
|
|
|
|
|
|
|
|
|
|
|
|
Investments
|
|
|
-
|
|
|
|
23,539
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
88,152
|
|
|
$
|
89,843
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Bank overdraft
|
|
$
|
-
|
|
|
$
|
1,520
|
|
|
Accounts payable
|
|
|
1,282,010
|
|
|
|
1,187,989
|
|
|
Accrued expenses
|
|
|
1,511,281
|
|
|
|
1,115,526
|
|
|
Advances - related parties
|
|
|
861,432
|
|
|
|
511,744
|
|
|
Deferred revenue, current portion
|
|
|
3,000
|
|
|
|
23,800
|
|
|
Deferred gain on sale of equipment, current portion
|
|
|
-
|
|
|
|
21,474
|
|
|
Deposits
|
|
|
465,286
|
|
|
|
465,286
|
|
|
Notes payable - related parties
|
|
|
3,877,057
|
|
|
|
2,757,442
|
|
|
Notes payable, current portion, net of debt discount of $9,610 and $9,057, respectively
|
|
|
1,406,570
|
|
|
|
1,297,477
|
|
|
Promissory note payable, net of debt discount of $0 and $29,296, respectively
|
|
|
1,397,762
|
|
|
|
1,368,467
|
|
|
Convertible notes payable, net of debt discount of $1,874 and $0, respectively
|
|
|
43,126
|
|
|
|
-
|
|
|
Total current liabilities
|
|
|
10,847,524
|
|
|
|
8,750,725
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Deferred revenue
|
|
|
59,500
|
|
|
|
62,500
|
|
|
Notes payable, net of debt discount of $31,627 and $41,391, respectively
|
|
|
760,420
|
|
|
|
756,014
|
|
|
Total long-term liabilities
|
|
|
819,920
|
|
|
|
818,514
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
11,667,444
|
|
|
|
9,569,239
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (See Note 13)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' deficit:
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001; 20,000,000 shares authorized, -0- issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common stock, par value $0.001; 2,000,000,000 shares authorized, 435,560,794 and 417,724,767 shares issued and outstanding, respectively
|
|
|
435,561
|
|
|
|
417,725
|
|
|
Additional paid-in capital
|
|
|
124,499,655
|
|
|
|
123,726,894
|
|
|
Accumulated deficit
|
|
|
(136,514,508
|
)
|
|
|
(133,624,015
|
)
|
|
Total stockholders' deficit
|
|
|
(11,579,292
|
)
|
|
|
(9,479,396
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders' deficit
|
|
$
|
88,152
|
|
|
$
|
89,843
|
|
The accompanying notes are an integral part of these financial statements.
U.S. STEM CELL, INC.
STATEMENTS OF OPERATIONS
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Revenue:
|
|
|
|
|
|
|
|
|
|
Products
|
|
$
|
209,696
|
|
|
$
|
378,621
|
|
|
Services
|
|
|
67,391
|
|
|
|
2,642,921
|
|
|
Management fees - related party
|
|
|
-
|
|
|
|
50,751
|
|
|
Total revenue
|
|
|
277,087
|
|
|
|
3,072,293
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales
|
|
|
64,117
|
|
|
|
1,335,238
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
212,970
|
|
|
|
1,737,055
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
-
|
|
|
|
263
|
|
|
Selling, general and administrative
|
|
|
2,613,211
|
|
|
|
4,454,712
|
|
|
Pre-litigation settlement
|
|
|
-
|
|
|
|
698,937
|
|
|
Total operating expenses
|
|
|
2,613,211
|
|
|
|
5,153,912
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(2,400,241
|
)
|
|
|
(3,416,857
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expenses):
|
|
|
|
|
|
|
|
|
|
Gain (loss) on settlement of accounts payable and accrued interest, net
|
|
|
182
|
|
|
|
214,883
|
|
|
Gain on sale of equipment
|
|
|
21,474
|
|
|
|
128,845
|
|
|
Miscellaneous income
|
|
|
-
|
|
|
|
101,475
|
|
|
Income (loss) from equity investments
|
|
|
(23,539
|
)
|
|
|
117,318
|
|
|
Interest expense
|
|
|
(488,369
|
)
|
|
|
(981,001
|
)
|
|
Total other income (expenses)
|
|
|
(490,252
|
)
|
|
|
(418,480
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before income taxes
|
|
|
(2,890,493
|
)
|
|
|
(3,835,337
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes (benefit)
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(2,890,493
|
)
|
|
$
|
(3,835,337
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share, basic and diluted
|
|
$
|
(0.01
|
)
|
|
$
|
(0.01
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding, basic and diluted
|
|
|
429,291,011
|
|
|
|
398,037,479
|
|
The accompanying notes are an integral part of these financial statements.
U.S. STEM CELL, INC.
STATEMENT OF STOCKHOLDERS' DEFICIT
FOR THE TWO YEARS ENDED DECEMBER 31, 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance, December 31, 2018
|
|
|
-
|
|
|
$
|
-
|
|
|
|
378,076,976
|
|
|
$
|
378,077
|
|
|
$
|
122,528,391
|
|
|
$
|
(129,788,678
|
)
|
|
$
|
(6,882,210
|
)
|
|
Common stock issued in settlement of accounts payable and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
22,371,084
|
|
|
|
22,371
|
|
|
|
326,089
|
|
|
|
-
|
|
|
|
348,460
|
|
|
Common stock issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
4,000,000
|
|
|
|
4,000
|
|
|
|
85,250
|
|
|
|
-
|
|
|
|
89,250
|
|
|
Contribution to equity investment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
15,265
|
|
|
|
-
|
|
|
|
15,265
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
735,176
|
|
|
|
-
|
|
|
|
735,176
|
|
|
Common shares issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
13,276,707
|
|
|
|
13,277
|
|
|
|
36,723
|
|
|
|
-
|
|
|
|
50,000
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,835,337
|
)
|
|
|
(3,835,337
|
)
|
|
Balance, December 31, 2019
|
|
|
-
|
|
|
|
-
|
|
|
|
417,724,767
|
|
|
|
417,725
|
|
|
|
123,726,894
|
|
|
|
(133,624,015
|
)
|
|
|
(9,479,396
|
)
|
|
Common stock issued in settlement of accounts payable and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
13,836,027
|
|
|
|
13,836
|
|
|
|
59,822
|
|
|
|
-
|
|
|
|
73,658
|
|
|
Common stock issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
4,000,000
|
|
|
|
4,000
|
|
|
|
12,000
|
|
|
|
-
|
|
|
|
16,000
|
|
|
Beneficial conversion feature recognized on convertible note
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
15,000
|
|
|
|
-
|
|
|
|
15,000
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
685,939
|
|
|
|
-
|
|
|
|
685,939
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,890,493
|
)
|
|
|
(2,890,493
|
)
|
|
Balance, December 31, 2020
|
|
|
-
|
|
|
$
|
-
|
|
|
|
435,560,794
|
|
|
$
|
435,561
|
|
|
$
|
124,499,655
|
|
|
$
|
(136,514,508
|
)
|
|
$
|
(11,579,292
|
)
|
The accompanying notes are an integral part of these financial statements.
U.S. STEM CELL, INC.
STATEMENTS OF CASH FLOWS
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(2,890,493
|
)
|
|
$
|
(3,835,337
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
-
|
|
|
|
242,615
|
|
|
Bad debt (recoveries)
|
|
|
-
|
|
|
|
46,121
|
|
|
Interest and amortization of debt discount
|
|
|
447,380
|
|
|
|
198,355
|
|
|
Gain on settlement of accounts payable and accrued interest
|
|
|
(182
|
)
|
|
|
(214,883
|
)
|
|
Gain on sale of equipment
|
|
|
(21,474
|
)
|
|
|
(128,845
|
)
|
|
Related party notes payable issued for services rendered
|
|
|
1,119,615
|
|
|
|
978,077
|
|
|
Loss (income) on equity investments
|
|
|
23,539
|
|
|
|
(117,318
|
)
|
|
Note payable issued in pre-trial settlement
|
|
|
-
|
|
|
|
698,937
|
|
|
Stock-based compensation
|
|
|
701,939
|
|
|
|
824,426
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(5,956
|
)
|
|
|
(76,294
|
)
|
|
Inventories
|
|
|
2,678
|
|
|
|
85,119
|
|
|
Prepaid expenses and other current assets
|
|
|
-
|
|
|
|
28,128
|
|
|
Deposits
|
|
|
-
|
|
|
|
10,160
|
|
|
Accounts payable
|
|
|
149,521
|
|
|
|
344,536
|
|
|
Accrued expenses
|
|
|
18,347
|
|
|
|
(21,926
|
)
|
|
Deferred revenue
|
|
|
(23,800
|
)
|
|
|
(272,865
|
)
|
|
Net cash used in operating activities
|
|
|
(478,886
|
)
|
|
|
(1,210,994
|
)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from equity investments
|
|
|
-
|
|
|
|
166,834
|
|
|
Net cash provided by investing activities
|
|
|
-
|
|
|
|
166,834
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from (repayments to) overdraft protection
|
|
|
(1,520
|
)
|
|
|
1,520
|
|
|
Proceeds from sale of common shares
|
|
|
-
|
|
|
|
50,000
|
|
|
Proceeds from related party advances
|
|
|
349,688
|
|
|
|
276,843
|
|
|
Proceeds from related party notes
|
|
|
-
|
|
|
|
107,868
|
|
|
Repayments of related party notes
|
|
|
-
|
|
|
|
(321,607
|
)
|
|
Proceeds from notes payable
|
|
|
150,000
|
|
|
|
84,876
|
|
|
Repayments of notes payable
|
|
|
(45,712
|
)
|
|
|
(512,486
|
)
|
|
Proceeds from convertible note payable
|
|
|
45,000
|
|
|
|
-
|
|
|
Net cash provided by (used in) financing activities
|
|
|
497,456
|
|
|
|
(312,986
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
18,570
|
|
|
|
(1,357,146
|
)
|
|
Cash and cash equivalents, beginning of year
|
|
|
-
|
|
|
|
1,357,146
|
|
|
Cash and cash equivalents, end of year
|
|
$
|
18,570
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION:
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
40,989
|
|
|
$
|
782,646
|
|
|
Income taxes paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL NON-CASH INVESTING AND FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Common shares issued in settlement of accounts payable and accrued interest
|
|
$
|
73,658
|
|
|
$
|
348,460
|
|
|
Beneficial conversion feature recognized on convertible note
|
|
$
|
15,000
|
|
|
$
|
-
|
|
|
Equity contributed to investment
|
|
$
|
-
|
|
|
$
|
15,265
|
|
The accompanying notes are an integral part of these financial statements.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
NOTE 1 — NATURE OF OPERATIONS
U.S. Stem Cell, Inc. was incorporated under the laws of the State of Florida in August 1999. The Company is in the cardiovascular sector of the cell technology industry delivering cell therapies and biologics that help address congestive heart failure, lower limb ischemia, chronic heart ischemia, acute myocardial infarctions and other issues. The business includes the development of proprietary cell therapy products as well as revenue generating physician and patient-based regenerative medicine/cell therapy training services, cell collection and cell storage services, the sale of cell collection and treatment kits for humans and animals, and the operation of cell therapy clinics. To date, the Company has not generated significant revenues in that they remain less than their total operating expenses, has incurred expenses, and has sustained losses. Consequently, its operations are subject to all the risks inherent in the establishment of a research and development business enterprise.
NOTE 2 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
As shown in the accompanying financial statements, as of December 31, 2020, the Company had cash on hand of $18,570 and a working capital deficit (current liabilities in excess of current assets) of $10,759,372. During the year ended December 31, 2020, the net loss was $2,890,493 and net cash used in operating activities was $478,886. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for one year from the issuance of the financial statements.
The Company’s primary source of operating funds has been from revenue generated from sales with additional cash proceeds from the sale of common stock and the issuances of promissory notes and other debt. The Company has experienced net losses from operations since inception, but it expects these conditions to improve in the future as it develops its business model. The Company had a stockholders’ deficit at December 31, 2020 and requires additional financing to fund future operations.
The Company’s existence is dependent upon management’s ability to develop profitable operations and to obtain additional funding sources. There can be no assurance that the Company’s financing efforts will result in profitable operations or the resolution of the Company’s liquidity problems. The accompanying statements do not include any adjustments that might result should the Company be unable to continue as a going concern.
NOTE 3 – SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include, stock-based compensation, debt discounts and the valuation allowance related to deferred tax assets. Actual results may differ from these estimates.
Fair Value
Accounting Standards Codification subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable, accrued liabilities, and short-term borrowings, as reflected in the balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities and equity instruments of the Company are either recognized or disclosed in the financial statements together with other information relevant for making a reasonable assessment of future cash flows, interest rate risk and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
The Company follows Accounting Standards Codification subtopic 820-10, Fair Value Measurements and Disclosures (“ASC 820-10”) and Accounting Standards Codification subtopic 825-10, Financial Instruments (“ASC 825-10”), which permits entities to choose to measure many financial instruments and certain other items at fair value.
Cash
The Company considers cash to consist of cash on hand and temporary investments having an original maturity of 90 days or less that are readily convertible into cash.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are non-interest bearing and are stated at gross invoice amounts less an allowance for doubtful accounts. Credit is extended to customers based on an evaluation of their financial condition, industry reputation, and other judgmental factors considered by the Company’s management. The Company generally does not require collateral or other security interest to support accounts receivable. Based on trends and specific factors, the customer’s credit terms may be modified, including required payment upon delivery.
The Company performs regular on-going credit evaluations of its customers as deemed relevant. As events, trends, and circumstance warrant, the Company’s management estimates the amounts that are more likely than not to be uncollectible. These amounts are recognized as bad debt expense and are reflected within selling, general, administrative and other expenses on the Company’s accompanying statement of operations.
Any charges to the allowance for doubtful accounts on accounts receivable are charged to operations in amounts sufficient to maintain the allowance for uncollectible accounts at a level management believes is adequate to cover any probable losses. Management determines the adequacy of the allowance based on historical write-off percentages and the current status of accounts receivable. Accounts receivable are charged off against the allowance when collectability is determined to be permanently impaired. As of December 31, 2020 and 2019, the allowance for doubtful accounts was $13,203.
Inventories
Inventories are stated at the lower of cost or market with cost being determined on a first-in, first-out (FIFO) basis. The Company writes down its inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required. During the periods presented, there were no inventory write-downs.
Investments
The Company follows Accounting Standards Codification subtopic 323-10, Investments-Equity Methods and Joint Ventures (“ASC 323-10) which requires the accounting for investments where the Company can exert significant influence, but not control of a joint venture or equity investment. The Company accounted for its 49.9% member interest ownerships of U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic, LLC, respectively, and its 49% member interest ownership of U.S. Stem Cell Clinic of the Villages utilizing the equity method of accounting (See Note 5).
Property and Equipment
Property and equipment are stated at cost. When retired or otherwise disposed, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition, is reflected in earnings. For financial statement purposes, property and equipment are recorded at cost and depreciated using the straight-line method over their estimated useful lives of 3 to 15 years. Equipment under capital leases is recorded at the estimated present value of the minimum lease payments. Equipment under capital leases is amortized over the term of the lease, which is three years.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
Long-Lived Assets
The Company follows FASB ASC 360-10-15-3, “Impairment or Disposal of Long-lived Assets.” Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. The Company determined that there was no impairment on its long-lived assets during the period presented.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Codification 2014-09, Revenue from Contracts with Customers (Topic 606), which states that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. Topic 606 also provides for additional disclosures with respect to revenues and cash flows arising from contracts with customers.
At the time of each transaction, management assesses whether the fee associated with the transaction is fixed or determinable and whether or not collection is reasonably assured. The assessment of whether the fee is fixed or determinable is based upon the payment terms of the transaction. Collectability is assessed based on a number of factors, including past transaction history with the client and the creditworthiness of the client.
The Company’s primary sources of revenue are from the sale of test kits and equipment, training services, patient treatments, laboratory services and cell banking.
Revenues for kits and equipment sold are not recorded until kits and equipment are received by the customer. Revenues from in-person trainings are recognized when the training occurs and revenues from on demand online trainings are recognized when the customer purchases the rights to the training course. Any cash received as a deposit for trainings are recorded by the Company as a liability.
Patient treatments and laboratory services revenue are recognized when those services have been completed or satisfied.
Revenues for cell banking are accounted for as multiple performance obligations as described in ASC 606 and addresses accounting for arrangements that may involve the delivery or performance of multiple products, services and/or rights to use assets. Because the Company sells its services separately, on more than a limited basis and at a price within a narrow range, the Company was able to allocate revenue based on stand-alone pricing. The multiple performance obligations include stem cell banking, dose retrieval and yearly storage fees. Revenues for stem cell banking and dose retrieval is recognized at the point of service and revenues for the yearly storage fees is recognized over the term of the banking contract, which is typically one year with annual renewals.
At December 31, 2020 and 2019, the Company had deferred revenues of $62,500 and $86,300, respectively, which includes $62,500 and $65,500, respectively, to the Intellectual Property Licensing Agreement.
Research and Development
The Company accounts for research and development costs in accordance with Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and development costs are expensed when the contracted work has been performed or as milestone results have been achieved as defined under the applicable agreement. Company-sponsored research and development costs related to both present and future products are expensed in the period incurred. The Company incurred research and development expenses of $0 and $263 for the year ended December 31, 2020 and 2019, respectively.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
Stock-Based Compensation
Stock-based compensation expense is measured at the grant date fair value of the award and is expensed over the requisite service period. For stock-based awards to employees, non-employees and directors, the Company calculates the fair value of the award on the date of grant using the Black-Scholes option pricing model. Determining the fair value of stock-based awards at the grant date under this model requires judgment, including estimating volatility, employee stock option exercise behaviors and forfeiture rates. The assumptions used in calculating the fair value of stock-based awards represent the Company’s best estimates, but these estimates involve inherent uncertainties and the application of management’s judgment.
Income Taxes
The Company follows Accounting Standards Codification subtopic 740-10, Income Taxes (“ASC 740-10”) for recording the provision for income taxes. Deferred tax assets and liabilities are computed based upon the difference between the financial statement and income tax basis of assets and liabilities using the enacted marginal tax rate applicable when the related asset or liability is expected to be realized or settled. Deferred income tax expenses or benefits are based on the changes in the asset or liability during each period. If available evidence suggests that it is more likely than not that some portion or all of the deferred tax assets will not be realized, a valuation allowance is required to reduce the deferred tax assets to the amount that is more likely than not to be realized. Future changes in such valuation allowance are included in the provision for deferred income taxes in the period of change. Deferred income taxes may arise from temporary differences resulting from income and expense items reported for financial accounting and tax purposes in different periods.
Deferred taxes are classified as current or non-current, depending on the classification of assets and liabilities to which they relate. Deferred taxes arising from temporary differences that are not related to an asset or liability are classified as current or non-current depending on the periods in which the temporary differences are expected to reverse and are considered immaterial.
Net Loss per Common Share
The Company computes earnings (loss) per share under Accounting Standards Codification subtopic 260-10, Earnings Per Share (“ASC 260-10”). Net loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share, if presented, would include the dilution that would occur upon the exercise or conversion of all potentially dilutive securities into common stock using the “treasury stock” and/or “if converted” methods as applicable.
The computation of basic and diluted income (loss) per share as of December 31, 2020 and 2019 excludes potentially dilutive securities when their inclusion would be anti-dilutive, or if their exercise prices were greater than the average market price of the common stock during the period.
Potentially dilutive securities excluded from the computation of basic and diluted net loss per share are as follows:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Options
|
|
|
111,119,914
|
|
|
|
111,120,474
|
|
|
Warrants
|
|
|
1,110,468
|
|
|
|
1,110,468
|
|
|
Convertible note
|
|
|
7,309,676
|
|
|
|
-
|
|
|
Total potentially dilutive shares
|
|
|
119,540,058
|
|
|
|
112,230,942
|
|
Recent Accounting Pronouncements
FASB Accounting Standards Updates (“ASU”) 2017-04 (Topic 350), “Intangibles – Goodwill and Others” – Issued in January 2017, ASU 2017-04 simplifies how an entity is required to test goodwill for impairment by eliminating Step 2 from the goodwill impairment test. Step 2 measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill. This guidance was effective for the Company in the first fiscal quarter of 2020. The adoption of this standard did not have a material impact on the Company’s financial statements and related disclosures.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
In February 2016, the FASB established ASC Topic 842, Leases (Topic 842), by issuing ASU No. 2016-02, which requires lessees to recognize leases on-balance sheet and disclose key information about leasing arrangements. Topic 842 was subsequently amended by ASU No. 2018-01, Land Easement Practical Expedient for Transition to Topic 842; ASU No. 2018-10, Codification Improvements to Topic 842, Leases; and ASU No. 2018-11, Targeted Improvements. The new standard establishes a right-of-use (ROU) model that requires a lessee to recognize a ROU asset and lease liability on the balance sheet. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the statement of operations. The Company adopted the new standard on January 1, 2019. The new standard provides a number of optional practical expedients in transition. The Company has elected the ‘package of practical expedients’, which permit it not to reassess under the new standard its prior conclusions about lease identification, lease classification and initial direct costs. The Company did not elect the use-of-hindsight or the practical expedient pertaining to land easements; the latter is not applicable to the Company. The new standard had a material effect on the Company’s financial statements. The most significant effects of adoption relate to (1) the recognition of new ROU assets and lease liabilities on its balance sheet for real estate operating leases; and (2) providing significant new disclosures about its leasing activities. Upon adoption, the Company recognized additional operating lease liabilities, net of deferred rent, of approximately $57,000 based on the present value of the remaining minimum rental payments under current leasing standards for existing operating leases. The Company also recognized corresponding ROU assets of approximately $57,000. On October 24, 2019, the Company entered into an Assignment and Assumption of Lease by and between the Company, American Cell Technology, LLC, and Sawgrass Business Plaza, LLC. Subsequently, the Company relocated to a new location within the same city and entered into a month-to-month lease. Accordingly, the right of use assets and lease liabilities were eliminated. The new standard also provides practical expedients for an entity’s ongoing accounting. The Company elected the short-term lease recognition exemption for all leases that qualify. This means, for those leases that qualify, the Company will not recognize ROU assets or lease liabilities, and this includes not recognizing ROU assets or lease liabilities for existing short-term leases of those assets in transition. Beginning in 2019, the Company changed to its disclosed lease recognition policies and practices, as well as to other related financial statement disclosures due to the adoption of this standard.
FASB ASU No. 2018-07 (Topic 718), “Compensation – Stock Compensation: Improvements to Nonemployee Share-Based Payment Accounting” – Issued in June 2018, ASU 2018-07 expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from non-employees. The amendments also clarify that Topic 718 does not apply to share-based payments used to effectively provide (1) financing to the issuer or (2) awards granted in conjunction with selling goods or services to customers as part of a contract accounted for under Topic 606. The Company adopted the new standard on January 1, 2019. The adoption of this guidance did not have a material impact on the Company’s financial condition or results of operations.
In August 2020, the FASB issued ASU 2020-06, which simplifies the guidance on accounting for convertible debt instruments by removing the separation models for: (1) convertible debt with a cash conversion feature; and (2) convertible instruments with a beneficial conversion feature. As a result, the Company will not separately present in equity an embedded conversion feature in such debt. Instead, we will account for a convertible debt instrument wholly as debt, unless certain other conditions are met. We expect the elimination of these models will reduce reported interest expense and increase reported net income for the Company’s convertible instruments falling under the scope of those models before the adoption of ASU 2020-06. Also, ASU 2020-06 requires the application of the if-converted method for calculating diluted earnings per share and the treasury stock method will be no longer available. The provisions of ASU 2020-06 are applicable for fiscal years beginning after December 15, 2021, with early adoption permitted no earlier than fiscal years beginning after December 15, 2020. The Company is currently evaluating the impact of ASU 2020-06 on its financial statements.
In August 2018, the FASB issued Accounting Standards Update (“ASU”) 2018-13, “Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement” (“ASU 2018-13”). ASU 2018-13 removes certain disclosure requirements, including the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, the policy for timing of transfers between levels, and the valuation processes for Level 3 fair value measurements. ASU 2018-13 also adds disclosure requirements, including changes in unrealized gains and losses for the period included in other comprehensive income for recurring Level 3 fair value measurements, and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. The amendments on changes in unrealized gains and losses, and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements, should be applied prospectively for only the most recent interim or annual period presented in the initial fiscal year of adoption. All other amendments should be applied retrospectively to all periods presented upon their effective date. This guidance was effective for the Company in the first fiscal quarter of 2020. The adoption of this standard did not have a material impact on the Company’s financial statements and related disclosures.
There are various other updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on the Company’s financial position, results of operations or cash flows.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
NOTE 4 — PROPERTY AND EQUIPMENT
Property and equipment as of December 31, 2020 and 2019 is summarized as follows:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Furniture, fixtures and equipment
|
|
$
|
5,598
|
|
|
$
|
5,598
|
|
|
Computer equipment
|
|
|
1,809
|
|
|
|
1,809
|
|
|
Property and equipment, cost
|
|
|
7,407
|
|
|
|
7,407
|
|
|
Less: accumulated depreciation and amortization
|
|
|
(7,407
|
)
|
|
|
(7,407
|
)
|
|
Property and equipment, net
|
|
$
|
-
|
|
|
$
|
-
|
|
On March 3, 2017, the Company entered into an asset sale and lease agreement (sale/leaseback transaction, the “Asset Sale and Lease Agreement”) with GACP, whereby the Company sold certain lab, medical and other equipment relating to the cell banking business for $400,000 and leased back the sold equipment over a three-year term.
The Company determined that the transaction was a capitalized lease and accordingly recorded the leased assets and liability based on the estimated present value of the minimum lease payments.
As a consequence of the Court Order (see Note 13 “Government Claim”), the Company resolved to divest itself of certain equipment and other assets (the “Equipment Assets”) used in connection with the Company’s human tissue banking business, but consistent however with the requirements of the Court Order, and to adjust the business plan and operations to accommodate this potential divesture. To facilitate the above, the Company entered into a Termination and Release Agreement and a Letter Agreement intended to divest itself of certain equipment and other assets underlying the related equipment lease transaction (see Note 6). In addition, on October 24, 2019, the Company entered into an Assignment and Assumption of Lease by and between the Company, American Cell Technology, LLC, and Sawgrass Business Plaza, LLC. Subsequently, the Company relocated to a new location within the same city and entered into a month-to-month lease. As part of the termination of the operating lease, the Company left certain property and equipment (all of which had been fully depreciated) at the old location.
In connection with the sale of the lab, medical and other equipment, the Company realized a gain on sale of equipment of $386,535. The gain is recognized ratably over the term of the lease to operations. During the year ended December 31, 2020 and 2019, the Company recognized $21,474 and $128,845, as the gain on sale of equipment, respectively. As of December 31, 2020 and 2019, deferred gain on sale of equipment was $0 and $21,474, respectively.
Depreciation expense was $0 and $242,615 of which $0 and $242,615 was included in cost of sales for the year ended December 31, 2020 and 2019, respectively.
NOTE 5 — INVESTMENTS
During the last quarter of 2019 (and in early 2021 in the case of SCC), we divested ourselves of our Member Interests in U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic, LLC , while US Stem Cell Clinic of the Villages, LLC is currently dormant.
U.S. Stem Cell Clinic, LLC
The investment in U.S. Stem Cell Clinic, LLC is comprised of a 49.9% (increased from 33.3% on January 29, 2019) member interest ownership and is accounted for using the equity method of accounting. The Company’s income (loss) earned by U.S. Stem Cell Clinic, LLC member interest was ($23,539) and $75,054 for the year ended December 31, 2020 and 2019, respectively (inception to date income of $599,721) and is included in other income (expense) in the accompanying Statements of Operations. In addition, during the year ended December 31, 2020 and 2019, the Company received distributions totaling $0 and $64,870, respectively, from U.S. Stem Cell Clinic, LLC (inception to date of $663,870). The carrying value of the investment at December 31, 2020 and 2019 is $0 and $23,539, respectively (See also Note 16).
At December 31, 2020 and 2019, accounts receivable for sales of product and services to U.S. Stem Cell Clinic, LLC was $28,763. Revenues earned from sales to U.S. Stem Clinic, LLC for the year ended December 31, 2020 were $2,182 and $337,108, respectively.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
In January 2019, a member of U.S. Stem Cell Clinic, LLC contributed 16.6% of his ownership interest to the Company increasing the Company’s member interest from 33.3% to 49.9%. The Company recorded the contribution to equity of $4,435.
An affiliate of one of the Company’s officers is a minority investor in the U.S. Stem Cell Clinic, LLC.
Regenerative Wellness Clinic, LLC
The investment in Regenerative Wellness Clinic, LLC is comprised of a 49.9% (increased from 33.3% on January 29, 2019) member interest ownership and is accounted for using the equity method of accounting. The Company has provided technical expertise, but no cash investment with Regenerative Wellness Clinic, LLC’s startup in 2017. The Company’s income earned by Regenerative Wellness Clinic, LLC member interest was $0 and $69,345 for the year ended December 31, 2020 and 2019, respectively (inception to date income of $113,047) and is included in other income (expense) in the accompanying Statements of Operations. In addition, during the year ended December 31, 2020 and 2019, the Company received distributions totaling $0 and $101,963, respectively, from Regenerative Wellness Clinic, LLC (inception to date of $101,963). The carrying value of the investment at December 31, 2020 and 2019 is $0. In October 2019, the Company divested its entire interest in U.S. Stem Cell Clinic, LLC.
At December 31, 2020 accounts receivable for sales of products and services to Regenerative Wellness Clinic, LLC was $0. Revenues earned from sales to Regenerative Wellness Clinic, LLC for the year ended December 31, 2020 and 2019 was $0 and $132,984, respectively.
In January 2019, a member of Regenerative Wellness Clinic, LLC contributed 16.6% of his ownership interest to the Company increasing the Company’s member interest from 33.3% to 49.9%. The Company recorded the contribution to equity of $10,830.
An affiliate of one of the Company’s officers is an investor in the Regenerative Wellness Clinic, LLC.
U.S. Stem Cell of the Villages LLC
On January 30, 2018, Greg Knutson, a director of the Company (“Knutson”) and the Company agreed to open and operate a regenerative medicine/cell therapy clinic providing cellular treatments for patients afflicted with neurological, autoimmune, orthopedic and degenerative diseases in Florida. To that end, U.S. Stem Cell Clinic of The Villages LLC (the “LLC”) was formed January 30, 2018. Knutson provided the Company with the sum of Three Hundred Thousand Dollars ($300,000) (the “Investment”) to be utilized for the formation and initial operation of the LLC. Currently, Knutson holds a 51% member interest in the LLC and the Company holds a 49% member interest. The Company will provide operating assistance as well as management services, the latter to be compensated at fee of five percent (5%) of the LLC gross revenues.
As of December 31, 2018, upon completion of U.S. Stem Cell of the Villages LLC, the Company received $189,909 from Greg Knutson, the holder of the 51% member interest. Accordingly, this was recognized as additional paid-in capital. Subsequently, the Company contributed $86,750 as its initial investment in the U.S. Stem Cell of the Villages, LLC. The Company’s 49% income (loss) incurred by U.S. Stem Cell of the Villages LLC member interest was $0 and ($27,081) for the year ended December 31, 2020 and 2019, respectively (inception to date loss of $23,050) and is included in other income (expense) in the accompanying Statements of Operations. In addition, during the year ended December 31, 2020 and 2019, the Company received distributions totaling $0 from U.S. Stem Cell of the Villages LLC. The carrying value of the investment at December 31, 2020 and 2019 was $0.
At December 31, 2020 and 2019, accounts receivable for sales of products and services to U.S. Stem Cell of the Villages LLC was $0. Revenues earned from sales to U.S. Stem Cell of the Villages LLC for the year ended December 31, 2020 and 2019 was $0 and $150,328, respectively.
During the year ended December 31, 2020 and 2019, the Company received $0 and $50,751, respectively, in management fees from the LLC.
As of the date of this filing, US Stem Cell Clinic of the Villages, LLC is currently dormant.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
NOTE 6 — RIGHT TO USE ASSETS AND LEASE LIABILITY
The Company leased its headquarters in Sunrise, Florida which consisted of 4,860 square feet of space at a rate of approximately $82,620 per year. On February 4, 2016, the Company extended its facility lease to extend the term of the lease until August 31, 2019 at a monthly base rent of $7,306 plus a pro rata share of landlord’s operating expenses. Effective September 1, 2019, the Company entered into a second amendment to extend the lease through August 31, 2024 with base rent beginning at $87,674 per year and escalating to $98,678 per year plus a pro rata share of the landlord’s operating expenses.
On September 1, 2019, the lease commencement date, the Company estimated the lease liability and the right of use assets at present value using the Company’s estimated incremental borrowing rate of 8% and determined their initial present values, at inception, of $383,351. In determining the length of the lease term to its long-term lease, the Company determined there was not an option to extend the lease.
In determining the length of the lease term for this long-term lease, the Company determined there was not an option to extend the lease. At lease commencement date, the Company estimated the lease liability and the right of use assets at present value using the Company’s estimated incremental borrowing rate of 8% and determined the initial present value, at inception, of $274,180. On January 1, 2019, upon adoption of ASC Topic 842, the Company recorded right to use assets of $56,734 and lease liability of $56,734.
On October 24, 2019, the Company entered into an Assignment and Assumption of Lease by and between the Company, American Cell Technology, LLC, and Sawgrass Business Plaza, LLC. Subsequently, the Company relocated to a new location within the same city and entered into a month-to-month lease. Accordingly, the capital lease liabilities and right of use assets and lease liabilities were eliminated, resulting in a $189,062 net gain on settlement of debt (See Note 4 and Note 8).
During the year ended December 31, 2020 and 2019, the Company recognized $5,582 and $107,156, respectively, as lease expense. Lease expense was comprised of the following:
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Operating lease expense
|
|
$
|
5,582
|
|
|
$
|
106,471
|
|
|
Variable lease expense
|
|
|
-
|
|
|
|
685
|
|
|
Total lease expense
|
|
$
|
5,582
|
|
|
$
|
107,156
|
|
NOTE 7 — ACCRUED EXPENSES
Accrued expenses consisted of the following as of December 31, 2020 and 2019:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Interest and fees payable to the Guarantors of the Company’s loan agreement with Seaside Bank
|
|
$
|
549,628
|
|
|
$
|
456,190
|
|
|
Accrued interest payable
|
|
|
882,515
|
|
|
|
580,204
|
|
|
Vendor accruals and other
|
|
|
79,138
|
|
|
|
79,132
|
|
|
Accrued expenses and other current liabilities
|
|
$
|
1,511,281
|
|
|
$
|
1,115,526
|
|
During the year ended December 31, 2020, the Company issued 3,481,467 shares of its common stock, having a fair value of $21,744, in lieu of payment in cash of accrued and unpaid interest of $18,340, resulting in a loss on settlement of $3,404.
During the year ended December 31, 2019, the Company issued 2,071,494 shares of its common stock, having a fair value of $18,340, in lieu of payment in cash of accrued and unpaid interest of $18,340, resulting in a loss on settlement of $0.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
NOTE 8 — NOTES PAYABLE
Notes and capital leases payable were comprised of the following as of December 31, 2020 and 2019:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Seaside Bank note payable
|
|
$
|
980,000
|
|
|
$
|
980,000
|
|
|
Hunton & Williams note payable
|
|
|
386,000
|
|
|
|
391,000
|
|
|
Weider note payable
|
|
|
450,477
|
|
|
|
482,939
|
|
|
Mallard note payable
|
|
|
241,750
|
|
|
|
250,000
|
|
|
EIDL note payable
|
|
|
150,000
|
|
|
|
-
|
|
|
Total notes payable
|
|
|
2,208,227
|
|
|
|
2,103,939
|
|
|
Less unamortized debt discount
|
|
|
(41,237
|
)
|
|
|
(50,448
|
)
|
|
Total notes payable net of unamortized debt discount
|
|
|
2,166,990
|
|
|
|
2,053,491
|
|
|
Less current portion
|
|
|
(1,406,570
|
)
|
|
|
(1,297,477
|
)
|
|
Long-term portion
|
|
$
|
760,420
|
|
|
$
|
756,014
|
|
Seaside Bank
On October 25, 2010, the Company entered into a Loan Agreement with Seaside National Bank and Trust for a $980,000 loan at 4.25% per annum interest that was used to refinance the Company’s loan with Bank of America. The obligation is guaranteed by certain stockholders of the Company. The Company renewed the loan with Seaside National Bank and Trust during the first quarter of 2018 to extend the maturity date to May 18, 2020. The Company renewed the loan with Seaside National Bank and Trust during the first quarter of 2020 to extend the maturity date to May 18, 2022.
Hunton & Williams
At December 31, 2016, the Company has two outstanding notes payable with interest at 8% per annum due at maturity. The two notes, $61,150 and $323,822, are payable in one balloon payment upon the date the Noteholder provides written demand, however the Company is not obligated to make payments until the Northstar Biotech Group, LLC (or successor) Loan is paid off.
On August 31, 2017, the Company and the noteholder entered into a Note Forbearance, Modification and Repayment Agreement (“Agreement”). The two notes, $61,150 and $323,822, were payable in one balloon payment upon the date of a written demand and upon certain triggering events occurring. The sum of unpaid principal and accumulated interest for both notes as of August 31, 2017 of $747,680 and an accounts payable of $40,596 result in an aggregate balance due of $788,276.
The noteholder agreed to accept full payment of their obligation over a four (4) year period in 48 monthly installments on an adjusted debt obligation in aggregate of $624,000 (reducing the outstanding balance), with such payments staggered in amounts such that the Company will pay $10,000 monthly the first year, $12,000 monthly the second year, $14,000 monthly the third year, and $16,000 monthly the final year. In addition, the noteholder agreed to suspend accrual interest on the notes commencing September 1, 2017.
The Agreement remains in full force and effect provided the Company continues to make the monthly payments, there is no event of default as defined in the notes and an agreement to a subordination agreement by Northstar Biotech Group, LLC, which has been provided. In May 2019, the Company did not make the required scheduled payment. In September 2019 the noteholder agreed to waive their default rights under the agreement provided a minimum of $5,000 was paid by the end of 2019 and to reduce the required monthly payment to $500 per month commencing in January 2020. The Company satisfied the $5,000 payment requirement by the end of 2019 and commenced making the required $500 monthly payments in January 2020.
The Company imputed an interest rate of 5% and discounted the note accordingly. The imputed debt discount of $69,700 was amortized to interest expense using the effective interest method. For the years ended December 31, 2020 and 2019, the Company amortized $0 and $31,201, respectively, of debt discount to interest expense. In September 2019, the Company was in default and was negotiating a revised payment structure. Thus, the remaining unamortized debt discount was charged to interest expense at September 30, 2019. As of December 31, 2020 and 2019, the remaining carrying value of the note was $386,000 and $391,000, respectively.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
PowerUp Lending Group, Ltd
On November 8, 2018, the Company entered into a revenue based factoring agreement and received an aggregate of $137,200 (less origination fees of $2,800) in exchange for $187,600 of future receipts relating to monies collected from customers or other third-party payors. Under the terms of the factoring agreement, the Company is required to make daily payments of $1,276 for 147 business days. The Company received net proceeds of $93,809 along with cancellation of the previous revenue based factoring agreement issued in May 2018. In connection with the cancellation of the May 2018 revenue based factoring agreement, the Company incurred a loss in settlement of debt of $37,604 in 2018.
On April 16, 2019, the Company entered into a revenue based factoring agreement and received an aggregate of $137,200 (less origination fees of $2,800) in exchange for $187,600 of future receipts relating to monies collected from customers or other third-party payors. Under the terms of the factoring agreement, the Company is required to make daily payments of $1,276 for 147 business days. The Company received net proceeds of $84,876 along with cancellation of the previous revenue based factoring agreement issued in November 2018. In connection with the cancellation of the November 2018 revenue based factoring agreement, the Company incurred a loss in settlement of debt of $1,276 in 2019.
As of December 31, 2020 and 2019, the remaining carrying value of the note was $0.
Weider
The Company, as one of the parties entered into a Settlement Agreement and General Release (the “Agreement”) dated June 3, 2019 related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year 5.25% unsecured promissory note, dated June 15, 2019, in the principal amount of $500,000, payable in monthly increments of $5,000 per month, with a final balloon payment due on June 15, 2024. Accordingly, the Company recognized Pre-litigation expense of $500,000 in the accompanying statement of operations. As of December 31, 2020 and 2019, the remaining carrying value of the note was $450,477 and $482,939, respectively.
Mallard
The Company, as one of the parties entered into a Settlement Agreement and General Release (the “Agreement”) dated December 6, 2019 related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year non-interest bearing unsecured promissory note, dated December 6, 2019, in the principal amount of $250,000, payable in monthly increments of $750 per month, with a final balloon payment of $205,000 due on January 1, 2025. The Company imputed an interest rate of 5% and discounted the note accordingly. The imputed debt discount of $51,063 is being amortized to interest expense using the effective interest method. Accordingly, the Company recognized Pre-litigation expense of $198,937 in the accompanying statement of operations. For the year ended December 31, 2020 and 2019, the Company amortized $9,211 and $614, respectively, of debt discount to interest expense. As of December 31, 2020 and 2019, the remaining carrying value of the note was $208,763 and $199,552, net of debt discount of $41,237 and $50,448, respectively.
Lab and Medical Equipment Capitalized Leases
On March 3, 2017, the Company entered into an asset sale and lease agreement (sale/leaseback transaction; “Asset Sale and Lease Agreement”) with GACP, whereby the Company sold certain lab, medical and other equipment relating to the cell banking business for $400,000 and leased back the sold equipment over a three-year term. The Company recognized the arrangement as a capital lease. The Company initially recorded the equipment and the capitalized lease liability at the estimated present value of the minimum lease payments of $619,825.
The lease includes a base monthly rental payment of $20,000, due the first day of each calendar month plus contingent rent equal to 2.3%, 22.5%, and 31.6% of revenues collected on deposits arising from cell banking business for years 1, 2 and 3, respectively. The contingent rent is recognized as a period expense and as interest expense at the time of collection. At the expiration of the lease, the Company is required to return all leased equipment and along with any maintenance records, logs, etc. in the Company’s possession to the lessor with no right of repurchase.
The Company determined that the present value of the minimum lease payments exceeded 90% of the estimated fair value of the equipment and therefore classified the equipment sale/lease as a capitalized lease. The effective interest rate of the capitalized lease is estimated at 10.00% based on the Company estimated incremental borrowing rate.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
The following summarizes the assets under capital leases:
|
|
|
December 31,
|
|
|
|
|
2019
|
|
|
Lab, medical and other equipment
|
|
$
|
619,825
|
|
|
Office equipment
|
|
|
4,777
|
|
|
Less: accumulated depreciation
|
|
|
(624,602
|
)
|
|
Equipment under capital lease
|
|
$
|
-
|
|
On October 24, 2019, the Company entered into an Assignment and Assumption of Lease by and between the Company, American Cell Technology, LLC, and Sawgrass Business Plaza, LLC. Accordingly, the capital lease liabilities and right of use assets and lease liabilities were eliminated, resulting in a $189,062 net gain on settlement of debt (See Note 4 and Note 6).
NOTE 9 — PROMISSORY NOTE PAYABLE
On June 1, 2015, the Company issued an amended and restated promissory note of $1,697,762 in settlement of the $1,500,000 outstanding subordinated debt, related accrued interest of $373,469 and accumulated and unpaid guarantor fees of $624,737.
The note is unsecured and non-interest bearing and requires four semi-annual payments of $75,000 beginning on December 31, 2015 with the remaining unpaid balance due June 1, 2020. On June 1, 2020, the Company defaulted on the promissory note. Upon default, the note became due in full and the Company began accruing interest at the default interest rate of 18%.
The Company imputed an interest rate of 5% and discounted the promissory note accordingly. The imputed debt discount of $368,615 was amortized to interest expense using the effective interest method. For the year ended December 31, 2020 and 2019, the Company amortized $29,295 and $87,503, respectively of debt discount to interest expense. As of December 31, 2020 and 2019, the remaining carrying value of the note was $1,397,762 and $1,368,467, net of debt discount of $0 and $29,295, respectively.
NOTE 10 — CONVERTIBLE NOTE PAYABLE
On February 5, 2020, the Company issued an unsecured convertible promissory note in the principal amount of $35,000 that matures on February 5, 2021 and bears interest at a rate of 5% per annum. The investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price equal to a thirty percent (30%) discount of the average closing price of the Company’s common stock on the OTC Markets electronic exchange for the prior thirty (30) trading days prior to conversion, subject to adjustment. Upon the occurrence of an event of default, the investor may accelerate the note pursuant to which the outstanding balance will become, at the noteholder’s election, immediately due and payable. As a result of the beneficial conversion feature of the note, debt discount of $15,000 was recognized with a corresponding increase in additional paid-in capital. The debt discount is being amortized to interest expense using the effective interest method. For the year ended December 31, 2020, the Company amortized 13,126 of debt discount to interest expense. As of December 31, 2020, the remaining carrying value of the note was $33,126, net of debt discount of $1,874. At December 31, 2020, accrued interest on the note was $1,582, and is included in accrued expenses on the accompanying balance sheet.
On September 8, 2020, the Company issued an unsecured convertible promissory note in the principal amount of $10,000 that is due on demand and bears interest at a rate of 5% per annum. The investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0467. Upon the occurrence of an event of default, the remaining principal and accrued interest become immediately due and payable, with interest accruing at 18% per annum on any unpaid amounts. As of December 31, 2020, the remaining carrying value of the note was $10,000. At December 31, 2020, accrued interest on the note was $156, and is included in accrued expenses on the accompanying balance sheet.
NOTE 11 — RELATED PARTY TRANSACTIONS
Advances – Related Parties
As of December 31, 2020 and 2019, the Company’s officers and directors have provided advances that are unsecured, non-interest bearing and due on demand. During the year ended December 31, 2020 and 2019, the Company received aggregate proceeds from advances of $349,688 and $276,843, respectively. As of December 31, 2020 and 2019, the Company owed $861,432 and $511,744, respectively, for related party advances.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
Notes Payable – Related Parties
Northstar Biotechnology Group, LLC
On February 29, 2012, a promissory note issued to BlueCrest Master Fund Limited (“BlueCrest”) was assigned to Northstar Biotechnology Group, LLC (“Northstar”), owned partly by certain directors and existing shareholders of the Company at the time, including Dr. William P. Murphy Jr., Dr. Samuel Ahn and Charles Hart. At the date of the assignment, the principal amount of the BlueCrest note was $544,267 (the “Note”).
On March 30, 2012, the Company and Northstar agreed to extend until May 1, 2012 the initial payment date for any and all required monthly under the Note, such that the first of the four monthly payments required under the Note will be due and payable on May 1, 2012 and all subsequent payments will be due on a monthly basis thereafter commencing on June 1, 2012, and to waive any and all defaults and/or events of default under the Note with respect to such payments. The Company did not make the required payment, and as a result, was in default of the revised agreement. The Company renegotiated the terms of the Note and Northstar agreed to suspend the requirement of principal payments by the Company and allow payment of interest-only in common stock.
On September 21, 2012, the Company issued 5,000 common stock purchase warrants to Northstar that was treated as additional interest expense upon issuance.
On October 1, 2012, the Company and Northstar entered into a limited waiver and forbearance agreement providing a recapitalized new note balance comprised of all sums due Northstar with a maturity date extended perpetually. The Company agreed to issue 5,000,000 shares of Series A Convertible Preferred Stock and 10,000 shares of common stock in exchange for $210,000 as payment towards outstanding debt, default interest, penalties, professional fees outstanding and due Northstar. In addition, the Company executed a security agreement granting Northstar a lien on all patents, patent applications, trademarks, service marks, copyrights and intellectual property rights of any nature, as well as the results of all clinical trials, know-how for preparing Myoblasts, old and new clinical data, existing approved trials, all right and title to Myoblasts, clinical trial protocols and other property rights.
In addition, the Company granted Northstar a perpetual license on products as described for resale, relicensing, and commercialization outside the United States. In connection with the granted license, Northstar shall pay the Company a royalty of up to 8% on revenues generated.
Effective October 1, 2012, the interest rate was 12.85% per annum. The parties agreed, as of February 28, 2013, to reduce the interest rate to 7% per annum.
In connection with the consideration paid, Northstar waived, from the effective date through the earlier of termination or expiration of the agreement, satisfaction of the obligations as described in the forbearance agreement.
In 2012, 5,000,000 shares of Series A Convertible Preferred Stock were approved to be issued, which was subsequently increased to 20,000,000 shares of preferred stock as Series A Convertible Preferred Stock. In addition, the Company was obligated to issue additional preferred stock equal in lieu of payment of cash of accrued and unpaid interest on each six-month anniversary of the effective date (October 1, 2012). In lieu of the initial two payments in preferred stock, the parties agreed to modify the voting rights of the subsequently cancelled Series A Convertible Preferred Stock from 20 votes per share on matters to be voted on by the common stockholders to 25 votes per share on matters to be voted on by the common stockholders and all prior and subsequent payments of interest will be in common stock. The Company is required to issue additional shares of its common stock (as amended), in lieu of cash, each six-month anniversary of the effective date for any accrued and unpaid interest.
On September 30, 2013, the Company issued 8,772 shares of its common stock as payment of $100,000 towards principal.
On December 24, 2013, the Company issued 3,916 shares of its common stock as payment of accrued interest through June 30, 2013 of $85,447.
On April 2, 2014, the Company issued 275 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2014 per the forbearance agreement.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
On September 17, 2014, the limited waiver and forbearance agreement entered into on October 1, 2012 to provide that the perpetual license on products as described for resale, relicensing and commercialization outside the United States was amended as such on the condition that Northstar provide certain financing, which financing the Company, in its sole discretion, could decline and retain the license.
On October 3, 2014, the Company issued 515 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2014 per the forbearance agreement.
On April 3, 2015, the Company issued 1,363 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2015 per the forbearance agreement.
On October 2, 2015, the Company issued 4,156 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2015 per the forbearance agreement.
On October 7, 2015, the Company issued 34,522 shares of its common stock in settlement of $100,000 principal payment towards the outstanding debt.
On April 7, 2016, the Company issued 57,778 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due April 1, 2016 per the forbearance agreement.
On October 6, 2016, the Company issued 848,490 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2016 per the forbearance agreement.
On March 1, 2017, Northstar and the Company entered into a settlement agreement (“Settlement Agreement “) related to then pending litigation. Pursuant to the terms and conditions of the Settlement Agreement, Northstar converted its outstanding Series A Convertible preferred stock, into twenty million (20,000,000) shares of common stock according to the original conversion terms. In addition, and separate and apart from the conversion, Northstar received eleven million (11,000,000) shares of the Company’s common stock. Northstar will receive ten percent (10%) of all Company international sales (based on a gross sales basis). There was no effect of the 10% obligation as there were no international sales in 2017 or through 2019. Furthermore, a Northstar designee, Greg Knutson, was appointed as a member of the Board of Directors of the Company and two Company directors, Michael Tomas and Kristin Comella, each exercised their prior Northstar options to each receive a five percent (5%) member interest in Northstar. The parties agreed to a mutual release and Northstar agreed to terminate any UCC lien on the Company assets previously filed for the benefit of Northstar. On March 9, 2017 and April 1, 2017, the Company issued 30,000,000 and 1,000,000 shares of its common stock, respectively, as described above. In connection with the settlement, the Company recorded a loss on litigation settlement of $316,800.
On April 1, 2017, the Company issued 286,315 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,703.
On October 2, 2017, the Company issued 559,187 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705.
On October 19, 2018, the Company issued 164,523 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,195.
On April 19, 2019, the Company issued 379,141 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,145.
On October 1, 2019, the Company issued 1,692,353 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,195.
On April 1, 2020, the Company issued 1,445,647 shares of its common stock, having a fair value of $11,565, in lieu of payment in cash of accrued and unpaid interest of $9,145, resulting in a loss on settlement of $2,420.
On October 1, 2020, the Company issued 2,035,820 shares of its common stock, having a fair value of $10,179, in lieu of payment in cash of accrued and unpaid interest of $9,195, resulting in a loss on settlement of $984.
As of December 31, 2020 and 2019, the remaining carrying value of the note was $262,000. At December 31, 2020 and 2019, accrued interest on the note was $8,751 and $8,700, respectively, and is included in accrued expenses on the accompanying balance sheet.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
Notes Payable - Mr. Tomas, President and Chief Executive Officer
On August 7, 2017, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due one year from date of issuance. During the nine months ended September 30, 2020 and 2019, the Company paid principal of $0 and $321,607, respectively, of this note. As of December 31, 2020 and 2019, the remaining carrying value of the note was $161,786.
On May 7, 2018, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due six months from date of issuance. As of December 31, 2020 and 2019, the remaining carrying value of the note was $500,000.
On July 1, 2019, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due November 7, 2019. As of December 31, 2020 and 2019, the remaining carrying value of the note was $500,000.
On December 31, 2019, the Company issued a $178,077 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of December 31, 2020 and 2019, the remaining carrying value of the note was $178,077.
On March 31, 2020, the Company issued a $187,500 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of December 31, 2020, the remaining carrying value of the note was $187,500.
On June 30, 2020, the Company issued a $187,500 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of December 31, 2020, the remaining carrying value of the note was $187,500.
On July 1, 2020, the Company issued a $500,000 promissory note as payment of an annual bonus awarded. The promissory note bears interest of 5% per annum and is due on demand. As of December 31, 2020, the remaining carrying value of the note was $500,000.
On September 30, 2020, the Company issued a $100,962 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of December 31, 2020, the remaining carrying value of the note was $100,962.
On December 31, 2020, the Company issued a $143,654 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of December 31, 2020, the remaining carrying value of the note was $143,654.
At December 31, 2020 and 2019, accrued interest on the notes was 482,468 and $389,695, respectively, and is included in accrued expenses on the accompanying balance sheet.
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Northstar
|
|
$
|
262,000
|
|
|
$
|
262,000
|
|
|
Note payable, Mr. Tomas
|
|
|
161,786
|
|
|
|
161,786
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
178,077
|
|
|
|
178,077
|
|
|
Note payable, Mr. Tomas
|
|
|
187,500
|
|
|
|
-
|
|
|
Note payable, Mr. Tomas
|
|
|
187,500
|
|
|
|
-
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
-
|
|
|
Note payable, Mr. Tomas
|
|
|
100,962
|
|
|
|
-
|
|
|
Note payable, Mr. Tomas
|
|
|
143,653
|
|
|
|
-
|
|
|
Note payable, Dr. Comella*
|
|
|
255,579
|
|
|
|
255,579
|
|
|
Note payable, Dr. Comella*
|
|
|
300,000
|
|
|
|
300,000
|
|
|
Note payable, Dr. Comella*
|
|
|
300,000
|
|
|
|
300,000
|
|
|
Note payable, Dr. Comella*
|
|
|
300,000
|
|
|
|
300,000
|
|
|
Total officer and director notes
|
|
$
|
3,877,057
|
|
|
$
|
2,757,442
|
|
|
|
|
|
|
|
|
|
|
|
|
* Dr. Comella is no longer a member of the Board of Directors effective September 1, 2019.
|
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
Other Transactions – Related Parties
Transactions with GACP
On March 3, 2017, the Company entered into an asset sale and lease agreement (sale/leaseback transaction, the “Asset Sale and Lease Agreement”) with General American Capital Partners (GACP), whereby the Company sold certain lab, medical and other equipment relating to the cell banking business for $400,000 and leased back the sold equipment over a three-year term. The Company determined that the transaction was a capitalized lease and accordingly recorded the leased assets and liabilities based on the estimated present value of the minimum lease payments. In connection with the Asset Sale and Lease Agreement, the Company is obligated to accrue 10% of cell-banking revenue as for marketing, offset by any incurred costs of the Company. At September 30, 2020 and December 31, 2019, the outstanding accrued marketing obligation is $0.
On March 3, 2017, the Company also entered into a customer purchase agreement with GACP whereby the Company received a deposit $50,000 (included in long-term liabilities at December 31, 2018) in exchange for the sale of the first 5,000 future customers of the cell-banking business after the effective date of the agreement; GACP also had the right to purchase additional customers at a price of $20 per customer. There is no reduction in the selling price should the new customers be fewer than 5,000. The effective date of the sale is upon the expiry or early termination of the aforementioned Asset Sale and Lease Agreement.
March 3, 2017, the Company also entered into an asset purchase agreement of intellectual property with GACP whereby the Company received a deposit of $50,000 (included in long-term liabilities at December 31, 2018) for the sale of all of the Company’s worldwide rights, title or interest in certain intellectual and other property (as defined) associated with the cell-banking business. The effective date of the sale is upon the expiry or early termination of the aforementioned Asset Sale and Lease Agreement.
Effective May 9, 2018, pursuant to an Amendment to the Asset Sale and Lease Agreement, GACP suspended their obligation to open additional clinics (tolling such obligation to a mutually agreeable date in the future) and it has suspended the monthly aggregate number of stem cell kits set forth for purchase in a given month arising from such clinics.
As a consequence of the Court Order dated June 4, 2019 (see Note 13 “Government Claim”), the Company resolved to divest itself of certain equipment and other assets (the “Equipment Assets”) used in connection with the Company’s human tissue banking business, but consistent however with the requirements of the Court Order, and to adjust the business plan and operations to accommodate this potential divesture. To facilitate the above, the Company entered into the following agreements:
|
|
●
|
Termination and Release Agreement by and between GACP, the Company, and Michael Tomas and Kristin Comella dated September 24, 2019 (terminating the Non-Competition and Non-Solicitation Agreement between U.S. Stem Cell, Inc. and GACP Stem Cell Bank LLC., dated March 3, 2017).
|
|
|
●
|
Letter Agreement on Stem Cell Processing and Storage by and between the Company and American Cell Technology, LLC
|
Effective October 9, 2019, the Company terminated the Asset Sale and Lease Agreement by and between the Company and GACP. Accordingly, the long-term deposit liabilities were written off, resulting in a gain on debt settlement of $100,000. In addition, the capital lease liabilities and right of use assets and lease liabilities were also written off (See Note 6 and Note 8).
NOTE 12 — FAIR VALUE MEASUREMENT
The Company adopted the provisions of ASC 825-10. ASC 825-10 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of non-performance. ASC 825-10 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825-10 establishes three levels of inputs that may be used to measure fair value:
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
|
●
|
Level 1 – Quoted prices in active markets for identical assets or liabilities.
|
|
●
|
Level 2 – Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
●
|
Level 3 – Unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities.
|
All items required to be recorded or measured on a recurring basis are based upon Level 3 inputs.
To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes, the level in the fair value hierarchy within which the fair value measurement is disclosed and is determined based on the lowest level input that is significant to the fair value measurement.
As of December 31, 2020 and 2019, the Company did not have any items that would be classified as level 1, 2 or 3 disclosures.
As of December 31, 2020 and 2019, the Company did not have any derivative instruments that were designated as hedges.
NOTE 13 — COMMITMENTS AND CONTINGENCIES
Employment Agreements
On July 1, 2019, the Company’s Board of Directors approved the 2019/2020 salary for Mike Tomas, Chief Executive Officer, for $750,000 per year, beginning July 1, 2019 with an incentive bonus ranging from $150,000 to $500,000. In addition, the Board of Directors approved a bonus of $500,000 and options to acquire 20,000,000 shares of the Company’s common stock for ten years with four-year vesting and a cashless exercise provision. The cash bonus may be paid in the form a six-month promissory note bearing interest at 5% per annum.
Royalty Agreement / Middle East
On November 9, 2016, the Company entered into an Intellectual Property License Agreement whereby the Company granted High Rise Group Company the exclusive right to the Company’s intellectual property (as defined) for the licensed use and development in Kuwait and other GCC/Middle East countries for 25 years in exchange for a payment of $75,000 and a 5% royalty generated under the agreement. The licensing agreement is recorded as deferred revenue and amortized over the term of the agreement. The carrying balance as of December 31, 2020 and 2019 was $62,500 and $65,500, respectively.
The intent is for U.S. Stem Cell Middle East to offer regenerative treatment options to patients, based on U.S. Stem Cell, Inc. products and technologies like MyoCell™. To date, the first clinic in Kuwait City has been completed but has not begun operations as High Rising Group has not yet been able to secure regulatory approvals to operate.
Litigation
On December 12, 2017, a product liability lawsuit was filed in Broward County, specifically Jeannine Mallard v. U.S. Stem Cell, Inc., US Stem Cell Clinics LLC., Regenestem, LLC., Regenestem Network, LLC., and Kristin C. Comella. The Company will continue to defend it vigorously. On December 6, 2019, the Company was one of the parties to a Settlement Agreement and General Release (the “Agreement”) related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year non-interest bearing unsecured promissory note, dated December 6, 2019, in the principal amount of $250,000, payable in monthly increments of $750 per month, with a final balloon payment of $205,000 due on January 1, 2025 (see Note 8, “Mallard”).
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
On September 17, 2015, a product liability lawsuit was filed in Broward County, specifically Patsy Bade v. Bioheart, Inc. US Stem Cell Clinics LLC, Alejandro Perez, ARNP, and Shareen Greenbaum, M.D., and on November 30, 2015, a product liability lawsuit was filed in Broward County, specifically Elizabeth Noble v. Bioheart, Inc. US Stem Cell Clinics LLC, Alejandro Perez, ARNP, and Shareen Greenbaum, M.D. During the year ended December 31, 2016, both matters settled by the Company’s insurance policy with no additional cost to the Company, except for the obligation to pay the insurance company deductible of $100,000, of which $11,000 was paid in fiscal 2017. The remaining amount due under this settlement is $28,850 and $30,050 as of December 31, 2020 and 2019, respectively, and is included in accounts payable.
On June 3, 2019, the Company was one of the parties to a Settlement Agreement and General Release (the “Agreement”) related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year 5.25% Promissory Note, dated June 15, 2019, in the principal amount of Five Hundred Thousand Dollars ($500,000), payable in monthly increments of Five Thousand ($5,000) per month (see Note 8, “Weider”).
The Company is subject at times to other legal proceedings and claims, which arise in the ordinary course of its business. Although occasional adverse decisions or settlements may occur, the Company believes that the final disposition of such matters should not have a material adverse effect on its financial position, results of operations or liquidity. There was no outstanding litigation as of December 31, 2020 other than that described above.
Effective September 4, 2020, the Company settled an outstanding obligation underlying the engagement letter with Venable LLP, the law firm representing the Company and others in the lawsuit filed by the United States Department of Justice in the Southern District of Florida. The Company paid Venable LLP the sum of Fifteen Thousand Dollars ($15,000.00) and executed, jointly and severally with Kristin Comella, a Promissory Note in the principal amount of One Hundred Thousand Dollars ($100,000.00) which provides that, commencing on January 15, 2021 through December 15, 2021, the Company and Kristin Comella will be responsible to pay principle of the Note in the amount of Two Thousand Five Hundred and 00/100 Dollars ($2,500.00) each month, and commencing on January 15, 2022 through November 15, 2022, the Company and Kristin Comella will be responsible to pay principle of the Note in the amount of Five Thousand and 00/100 Dollars ($5,000.00) each month. A final installment of Fifteen Thousand and 00/100 Dollars ($15,000.00) shall be due and payable on December 15, 2022 (“Maturity Date”). As of the date of this filing, the Company is current in its payments.
Government Claim
On May 9, 2018, the U.S. Department of Justice filed an injunctive action, specifically United States of America v. U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., Kristin C. Comella, and Theodore Gradel. The Complaint alleges, among other matters that the defendants manufacture “stromal vascular fraction” (SVF) products from patient adipose (fat) tissue, which the companies then market as stem cell-based treatments, and which U.S. Stem Cell Clinic, LLC administers to patients, without first obtaining what the government alleges are necessary FDA approvals. Although Theodore Gradel was initially listed as a defendant, he subsequently entered into a consent agreement and is no longer party to this case.
The U.S. and the defendants filed cross motions for summary judgment, each asking for a ruling in its favor. On June 3, 2019, the Court entered an order granting Summary Judgment for the government and denying the defendants’ motion for summary judgment. The order focused on the defendants’ actions in providing and marketing SVF therapy. In an order dated June 4, 2019, the Court granted the defendants’ request to allow it the opportunity to work out the language of the form of injunction with the government, and if unsuccessful, to provide a status report to the Court by June 14, 2019, outlining areas of disagreement. The Court further ordered that the defendants (U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., and Kristin C. Comella) ‘not sell, provide or otherwise engage in any SVF therapy or any other activities to be regulated by the FDA as explained in the Court’s Order on the Parties’ Motions for Summary Judgment.” On June 25, 2019, the Court entered an Order of Permanent Injunction, generally enjoining the defendants with respect to the SVF Product and requiring other actions. The Company filed an appeal on August 23, 2019 and attended oral argument on January 13, 2021. On June 2, 2021, the Eleventh Circuit Court ruled to affirm lower courts’ judgement. The Company did not challenge the district court’s judgment upon any other ground. The Company is not able to predict the duration, scope, results, or consequences of the U.S. Department of Justice actions and final rulings and management is assessing its options on a going forward basis.
The Company, in divesting certain equipment and other assets and assigning its lease, has and will continue to experience a decrease in revenues as the Company both maintains the remainder of the business and transitions into similar or unrelated business opportunities as determined by management (see Item 2 Existing Capital Resources and Future Capital Requirements and Plan of Operations below); However, management is not able to predict the duration, scope, results, or consequences of the summary judgment and any transition of the business plan.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
After the Court’s issuance of the Order of Permanent Injunction, the Company has received demand letters for compensation from persons who store their SVF Product and/or other tissue product with the tissue bank (several of the persons have requested refunds of the monies paid to the tissue bank and one person has requested a full refund of monies paid to an altogether separate company due to her not receiving the full amount of treatments she requested; such requests for compensation, to date, have not been material) and requests that the Company preserve cells in the Company’s possession. The Company sought guidance from the Court, which entered an order generally staying the requirement to destroy any SVF Product, pending a decision on the Company’s appeal. Many of the tissue bank depositors attempted to intervene in the FDA action, and filed an appeal when their intervention was denied. Their appeal was dismissed. It is anticipated that these depositors will present their position on tissue/SVF preservation to the trial court now that the appeal has been decided. As disclosed by the Company previously, the Company entered into a transaction in 2019 in which it divested itself of the operation of the tissue bank
NOTE 13 — STOCKHOLDERS’ DEFICIT
Common Stock
During the year ended December 31, 2019, the Company issued an aggregate of 22,371,084 shares of its common stock, having a fair value of $348,460, in settlement of outstanding accounts payable and accrued expenses. In connection with the issuance, the Company incurred a $27,097 net gain on settlement of debt.
During the year ended December 31, 2019, the Company issued an aggregate of 4,000,000 shares of its common stock, having a fair value of $89,250, for services.
During the year ended December 31, 2019, the Company issued an aggregate of 13,276,707 shares of its common stock for cash proceeds of $50,000.
During the year ended December 31, 2020, the Company issued an aggregate of 10,354,560 shares of its common stock, having a fair value of $51,914, in settlement of outstanding accounts payable. In connection with the issuances, the Company incurred a $3,586 net gain on settlement.
During the year ended December 31, 2020, the Company issued 3,481,467 shares of its common stock, having a fair value of $21,744, in lieu of payment in cash of accrued and unpaid interest of $18,340, resulting in a loss on settlement of $3,404.
During the year ended December 31, 2020, the Company issued an aggregate of 4,000,000 shares of its common stock, having a fair value of $16,000, for services.
Stock Options
On April 1, 2013, the Board of Directors approved, subject to subsequently received stockholder approval, the establishment of the Bioheart 2013 Omnibus Equity Compensation Plan, or the “2013 Omnibus Plan” (replacing the 1999 Officers and Employees Stock Option Plan, or the Employee Plan, and the 1999 Directors and Consultants Stock Option Plan). The 2013 Omnibus Plan initially reserved up to fifty thousand (50,000) shares of common stock for issuance. On August 4, 2014, the Board of Directors approved to set the reserve to one hundred thousand (100,000) shares of common stock for issuance and to close the 1999 Officers and Employees Stock Option Plan. On February 2, 2015, at the annual meeting of stockholders, the 2013 Omnibus Equity Compensation Plan was approved.
On November 2, 2015, the Company increased the shares reserved under the 2013 Omnibus Plan to five hundred million (500,000,000) shares of common stock for issuance. Effective September 16, 2016, the Company approved an additional twenty five million (25,000,000) shares of common stock to the reserve; effective April 21, 2017, the Company approved an additional twenty five million (25,000,000) shares of common stock to the reserve; effective August 7, 2017, the Company approved an additional thirty million (30,000,000) shares of common stock to the reserve; and effective May 7, 2018, the Company approved an additional one hundred million (100,000,000) shares of common stock to reserve.
On September 1, 2019, the Company granted an aggregate 33,400,000 options to purchase the Company’s common stock at $0.0056 per share to key employees, vesting over 4 years, at grant date anniversary and exercisable over 10 years. The aggregate fair value of $192,189, was determined using the Black Scholes option pricing model with the following assumptions: Dividend yield: 0%; Volatility: 215.10% and Risk-free rate: 1.45%.
On November 18, 2019, the Company granted to its directors an aggregate of 7,500,000 options to purchase the Company’s common stock at $0.0049 per share that vested immediately and are exercisable for 10 years. The aggregate fair value of $34,477, was determined using the Black Scholes option pricing model with the following assumptions: Dividend yield: 0%; Volatility: 213.97% and Risk-free rate: 1.81%.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
A summary of the stock option activity for the year ended December 31, 2020 and 2019 is as follows:
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Average
|
|
|
|
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining Life
|
|
|
Intrinsic
|
|
|
|
|
Options
|
|
|
Price
|
|
|
In Years
|
|
|
Value
|
|
|
Outstanding, December 31, 2018
|
|
|
112,970,693
|
|
|
$
|
0.0329
|
|
|
|
8.7
|
|
|
$
|
189,540
|
|
|
Granted
|
|
|
40,900,000
|
|
|
$
|
0.0055
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited/Expired
|
|
|
(42,750,219
|
)
|
|
$
|
0.0280
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2019
|
|
|
111,120,474
|
|
|
$
|
0.0247
|
|
|
|
8.3
|
|
|
$
|
-
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited/Expired
|
|
|
(560
|
)
|
|
$
|
0.1540
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2020
|
|
|
111,119,914
|
|
|
$
|
0.0247
|
|
|
|
7.2
|
|
|
$
|
296,636
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, December 31, 2020
|
|
|
75,737,414
|
|
|
$
|
0.0255
|
|
|
|
7.0
|
|
|
$
|
158,802
|
|
|
Options Outstanding
|
|
|
Options Exercisable
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Outstanding
|
|
|
Average
|
|
|
Average
|
|
|
Exercisable
|
|
|
Average
|
|
|
Exercise
|
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining Life
|
|
|
Number of
|
|
|
Exercise
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
In Years
|
|
|
Options
|
|
|
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.004 to $0.010
|
|
|
|
41,900,000
|
|
|
$
|
0.0051
|
|
|
|
8.0
|
|
|
|
21,650,000
|
|
|
$
|
0.0049
|
|
|
$0.011 to $0.020
|
|
|
|
16,300,000
|
|
|
$
|
0.0196
|
|
|
|
5.7
|
|
|
|
16,300,000
|
|
|
$
|
0.0196
|
|
|
$0.021 to $0.030
|
|
|
|
9,710,000
|
|
|
$
|
0.0253
|
|
|
|
7.9
|
|
|
|
8,605,000
|
|
|
$
|
0.0252
|
|
|
$0.0363
|
|
|
|
22,735,000
|
|
|
$
|
0.0363
|
|
|
|
6.6
|
|
|
|
18,707,500
|
|
|
$
|
0.0363
|
|
|
$0.0536
|
|
|
|
20,000,000
|
|
|
$
|
0.0536
|
|
|
|
7.4
|
|
|
|
10,000,000
|
|
|
$
|
0.0536
|
|
|
$0.1540
|
|
|
|
474,914
|
|
|
$
|
0.1540
|
|
|
|
4.8
|
|
|
|
474,914
|
|
|
$
|
0.1540
|
|
|
|
|
|
|
111,119,914
|
|
|
$
|
0.0247
|
|
|
|
7.2
|
|
|
|
75,737,414
|
|
|
$
|
0.0255
|
|
The aggregate intrinsic value of outstanding stock options was $296,636, based on options with an exercise price less than the Company’s stock price of $0.0122 as of December 31, 2020, which would have been received by the option holders had those option holders exercised their options as of that date.
The fair value of all options that vested during the years ended December 31, 2020 and 2019 was $685,939 and $735,176, respectively. As of December 31, 2020, the Company had $729,612 of total unrecognized compensation cost related to non-vested awards granted under the 2013 Omnibus Plan, which the Company expects to recognize over a weighted average period of 0.82 years.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
Warrants
A summary of the warrant activity for the year ended December 31, 2020 and 2019 is as follows:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
|
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Life
|
|
|
Intrinsic
|
|
|
|
|
Warrants
|
|
|
Price
|
|
|
In Years
|
|
|
Value
|
|
|
Outstanding, December 31, 2018
|
|
|
1,114,019
|
|
|
$
|
14.74
|
|
|
|
9.1
|
|
|
$
|
-
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
(3,551
|
)
|
|
$
|
607.31
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2019
|
|
|
1,110,468
|
|
|
$
|
12.84
|
|
|
|
8.2
|
|
|
$
|
-
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2020
|
|
|
1,110,468
|
|
|
$
|
12.84
|
|
|
|
7.1
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, December 31, 2020
|
|
|
1,108,923
|
|
|
$
|
2.14
|
|
|
|
7.2
|
|
|
$
|
-
|
|
|
Warrants Outstanding
|
|
|
Warrants Exercisable
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
Outstanding
|
|
|
Average
|
|
|
Average
|
|
|
Exercisable
|
|
|
Average
|
|
|
Exercise
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining Life
|
|
|
Number of
|
|
|
Exercise
|
|
|
Price
|
|
Warrants
|
|
|
Price
|
|
|
In Years
|
|
|
Warrants
|
|
|
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.03 to $20.00
|
|
|
1,086,536
|
|
|
$
|
1.27
|
|
|
|
7.2
|
|
|
|
1,086,536
|
|
|
$
|
1.27
|
|
|
$20.01 to $30.00
|
|
|
19,543
|
|
|
$
|
25.06
|
|
|
|
3.2
|
|
|
|
19,543
|
|
|
$
|
25.06
|
|
|
$40.01 to $50.00
|
|
|
2,253
|
|
|
$
|
48.83
|
|
|
|
1.8
|
|
|
|
2,253
|
|
|
$
|
48.83
|
|
|
$50.01 to $60.00
|
|
|
543
|
|
|
$
|
60.00
|
|
|
|
0.6
|
|
|
|
543
|
|
|
$
|
60.00
|
|
|
>$60.00
|
|
|
1,593
|
|
|
$
|
7,690.00
|
|
|
|
5.8
|
|
|
|
48
|
|
|
$
|
7,690.00
|
|
|
|
|
|
1,110,468
|
|
|
$
|
12.84
|
|
|
|
7.1
|
|
|
|
1,108,923
|
|
|
$
|
2.14
|
|
The aggregate intrinsic value of the issued and exercisable warrants of $-0- represents the total pretax intrinsic value, based on warrants with an exercise price less than the Company’s stock price of $0.0122 as of December 31, 2020, which would have been received by the warrant holders had those warrants holders exercised their warrants as of that date.
NOTE 14 — INCOME TAXES
The Company’s provision (benefit) for income taxes consists of the following United States federal and state components:
|
|
|
For the Year Ended
|
|
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Current:
|
|
|
|
|
|
|
|
|
|
Federal
|
|
$
|
-
|
|
|
$
|
-
|
|
|
State
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred:
|
|
|
|
|
|
|
|
|
|
Federal
|
|
|
962,911
|
|
|
|
(69,163
|
)
|
|
State
|
|
|
199,230
|
|
|
|
(14,310
|
)
|
|
|
|
|
1,162,141
|
|
|
|
(83,473
|
)
|
|
Change in valuation allowance
|
|
|
(1,162,141
|
)
|
|
|
83,473
|
|
|
Income tax provision (benefit)
|
|
$
|
-
|
|
|
$
|
-
|
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
The deferred tax expense (benefit) is the change in the deferred tax assets and liabilities representing the tax consequences of changes in the amounts of temporary differences, net operating loss carryforwards and changes in tax rates during the year. The Company’s deferred tax assets and liabilities are comprised of the following:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
Net operating losses
|
|
$
|
23,317,369
|
|
|
$
|
24,929,613
|
|
|
Share-based compensation
|
|
|
4,263,112
|
|
|
|
4,089,261
|
|
|
Deferred compensation
|
|
|
916,236
|
|
|
|
632,470
|
|
|
Pre-litigation settlement
|
|
|
164,993
|
|
|
|
172,977
|
|
|
Other
|
|
|
260,494
|
|
|
|
260,024
|
|
|
Total deferred tax assets
|
|
|
28,922,204
|
|
|
|
30,084,345
|
|
|
Valuation allowance
|
|
|
(28,922,204
|
)
|
|
|
(30,084,345
|
)
|
|
Net deferred tax assets
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|
|
|
Total deferred tax liabilities
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
-
|
|
|
$
|
-
|
|
As of December 31, 2020 and 2019, the Company had U.S. federal net operating loss carryforwards of approximately $92.0 million and $98.4 million, respectively, of which $3.1 million do not expire, but are instead limited to 80% of taxable income in the year utilized. The remaining loss carryforwards expire at various dates from 2021 through 2037. These net operating loss carryforwards may be used to offset future taxable income and thereby reduce the Company’s U.S. federal income taxes. Section 382 of the Internal Revenue Code of 1986 (the “Code”) imposes an annual limit on the ability of a corporation that undergoes a greater than 50% ownership change to use its net operating loss carry forwards to reduce its tax liability. If in the future the Company issues common stock or additional equity instruments convertible in common shares which result in an ownership change exceeding the 50% limitation threshold imposed by Section 382 of the Code, the Company’s net operating loss carryforwards may be significantly limited as to the amount of use in a particular year. In addition, all or a portion of the Company’s net operating loss carryforwards may expire unutilized. As of December 31, 2020 and 2019, the Company had net operating loss carryforwards for state income tax purposes of approximately $92.0 million and $98.4 million, respectively, of which $3.1 million do not expire, but are instead limited to 80% of taxable income in the year utilized. The remaining loss carryforwards expire at various dates from 2021 through 2037.
For U.S. purposes, the Company has not completed its evaluation of NOL utilization limitations under Internal Revenue Code, as amended (the “Code”) Section 382/383, change of ownership rules. If the Company has had a change in ownership, the NOL’s would be limited as to the amount that could be utilized each year, based on the Code or might be eliminated.
The Company has provided a full valuation allowance against its net deferred tax assets, since in the opinion of management based upon the earnings history of the Company; it is more likely than not that the benefits of these assets will not be realized.
The Company complies with the provisions of FASB ASC 740-10 in accounting for its uncertain tax positions. ASC 740-10 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740-10, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely that not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. Management has determined that the Company has no significant uncertain tax positions requiring recognition under ASC 740-10.
The Company is subject to income tax in the U.S., and certain state jurisdictions. The Company has not been audited by the U.S. Internal Revenue Service, or any states in connection with income taxes. The Company’s tax years generally remain open to examination for all federal and state tax matters until its net operating loss carryforwards are utilized and the applicable statutes of limitation have expired. The federal and state tax authorities can generally reduce a net operating loss (but not create taxable income) for a period outside the statute of limitations in order to determine the correct amount of net operating loss which may be allowed as a deduction against income for a period within the statute of limitations.
The Company recognizes interest and penalties related to unrecognized tax benefits, if incurred, as a component of income tax expense.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2020 AND 2019
The significant elements contributing to the difference between the United States federal statutory tax rate and the Company’s effective tax rate are as follows:
|
|
|
For the Year Ended
|
|
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
US federal statutory rate
|
|
$
|
(607,004
|
)
|
|
$
|
(805,421
|
)
|
|
State tax rate, net of federal benefit
|
|
|
(124,658
|
)
|
|
|
(156,467
|
)
|
|
Tax effect of expiring net operating loss
|
|
|
1,889,289
|
|
|
|
181,704
|
|
|
Other
|
|
|
4,514
|
|
|
|
696,711
|
|
|
Change in valuation allowance
|
|
|
(1,162,141
|
)
|
|
|
83,473
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax provision (benefit)
|
|
$
|
-
|
|
|
$
|
-
|
|
NOTE 15 — CONCENTRATIONS
Concentrations of Credit Risk
The Company’s financial instruments that are exposed to a concentration of credit risk are cash and accounts receivable. Generally, the Company’s cash and cash equivalents in interest-bearing accounts does not exceed FDIC insurance limits. The financial stability of these institutions is periodically reviewed by senior management.
Concentrations of Revenues
For the year ended December 31, 2020 and 2019, the following customers accounted for more than 10% of the Company’s net revenues:
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Customer 1
|
|
|
22
|
%
|
|
|
-
|
|
|
Customer 2
|
|
|
-
|
|
|
|
11
|
%
|
|
Totals
|
|
|
22
|
%
|
|
|
11
|
%
|
Customer 2 is U.S. Stem Cell Clinic, LLC, a related party, a partly owned investment in which the Company holds a 49.9% member interest.
Concentrations of Accounts Receivable
As of December 31, 2020 and 2019, the following customers represented more than 10% of the Company’s accounts receivable:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Customer 1
|
|
|
50
|
%
|
|
|
47
|
%
|
|
Customer 2
|
|
|
44
|
%
|
|
|
-
|
|
|
Customer 3
|
|
|
-
|
|
|
|
47
|
%
|
|
Totals
|
|
|
94
|
%
|
|
|
94
|
%
|
Customer 1 is U.S. Stem Cell Clinic, LLC, a related party, a partly owned investment in which the Company holds a 49.9% member interest.
During the last quarter of 2019,
NOTE 16 — SUBSEQUENT EVENTS
In March 2021, the Company divested its entire interest in U.S. Stem Cell Clinic, LLC.
FINANCIAL STATEMENTS
U.S. STEM CELL, INC.
JUNE 30, 2020 AND JUNE 30, 2021
(UNAUDITED)
CONDENSED BALANCE SHEETS
|
|
|
June 30,
|
|
|
December 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
(unaudited)
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
137,962
|
|
|
$
|
18,570
|
|
|
Accounts receivable, net of allowance of $75,000 and $13,203, respectively
|
|
|
33,962
|
|
|
|
54,164
|
|
|
Inventories
|
|
|
4,693
|
|
|
|
5,418
|
|
|
Prepaid expenses and other current assets
|
|
|
22,089
|
|
|
|
10,000
|
|
|
Total current assets
|
|
|
198,706
|
|
|
|
88,152
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
198,706
|
|
|
$
|
88,152
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,293,333
|
|
|
$
|
1,282,010
|
|
|
Accrued expenses
|
|
|
1,689,033
|
|
|
|
1,511,281
|
|
|
Advances - related parties
|
|
|
891,432
|
|
|
|
861,432
|
|
|
Deferred revenue, current portion
|
|
|
3,000
|
|
|
|
3,000
|
|
|
Deposits
|
|
|
465,286
|
|
|
|
465,286
|
|
|
Notes payable - related parties
|
|
|
2,855,738
|
|
|
|
2,721,478
|
|
|
Notes payable, current portion, net of debt discount of $9,827 and $9,610, respectively
|
|
|
2,561,505
|
|
|
|
2,562,149
|
|
|
Promissory note payable
|
|
|
1,397,762
|
|
|
|
1,397,762
|
|
|
Convertible notes payable, net of debt discount of $465,513 and $1,874, respectively
|
|
|
128,487
|
|
|
|
43,126
|
|
|
Total current liabilities
|
|
|
11,285,576
|
|
|
|
10,847,524
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Deferred revenue
|
|
|
58,000
|
|
|
|
59,500
|
|
|
Notes payable, net of debt discount of $26,699 and $31,627, respectively
|
|
|
741,400
|
|
|
|
760,420
|
|
|
Total long-term liabilities
|
|
|
799,400
|
|
|
|
819,920
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
12,084,976
|
|
|
|
11,667,444
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (See Note 11)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' deficit:
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001; 20,000,000 shares authorized, -0- issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common stock, par value $0.001; 2,000,000,000 shares authorized, 451,738,971 and 435,560,794 shares issued and outstanding, respectively
|
|
|
451,739
|
|
|
|
435,561
|
|
|
Additional paid-in capital
|
|
|
126,150,449
|
|
|
|
124,499,655
|
|
|
Accumulated deficit
|
|
|
(138,488,458
|
)
|
|
|
(136,514,508
|
)
|
|
Total stockholders' deficit
|
|
|
(11,886,270
|
)
|
|
|
(11,579,292
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders' deficit
|
|
$
|
198,706
|
|
|
$
|
88,152
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
U.S. STEM CELL, INC.
CONDENSED STATEMENTS OF OPERATIONS
(unaudited)
|
|
|
For the Three Months
Ended June 30,
|
|
|
For the Six Months
Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Products
|
|
$
|
29,639
|
|
|
$
|
26,993
|
|
|
$
|
108,730
|
|
|
$
|
71,377
|
|
|
Services
|
|
|
5,803
|
|
|
|
12,661
|
|
|
|
11,092
|
|
|
|
25,769
|
|
|
Total revenue
|
|
|
35,442
|
|
|
|
39,654
|
|
|
|
119,822
|
|
|
|
97,146
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales
|
|
|
13,677
|
|
|
|
16,877
|
|
|
|
29,198
|
|
|
|
34,974
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
21,765
|
|
|
|
22,777
|
|
|
|
90,624
|
|
|
|
62,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
802,970
|
|
|
|
491,358
|
|
|
|
1,358,985
|
|
|
|
1,028,893
|
|
|
Total operating expenses
|
|
|
802,970
|
|
|
|
491,358
|
|
|
|
1,358,985
|
|
|
|
1,028,893
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(781,205
|
)
|
|
|
(468,581
|
)
|
|
|
(1,268,361
|
)
|
|
|
(966,721
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain (loss) on settlement of accounts payable and accrued interest, net
|
|
|
(45,995
|
)
|
|
|
(5,387
|
)
|
|
|
(383,870
|
)
|
|
|
481
|
|
|
Gain on sale of equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
21,474
|
|
|
Income (loss) from equity investments
|
|
|
-
|
|
|
|
9,718
|
|
|
|
-
|
|
|
|
(13,821
|
)
|
|
Interest expense
|
|
|
(166,994
|
)
|
|
|
(104,993
|
)
|
|
|
(321,719
|
)
|
|
|
(186,447
|
)
|
|
Total other income (expense)
|
|
|
(212,989
|
)
|
|
|
(100,662
|
)
|
|
|
(705,589
|
)
|
|
|
(178,313
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before income taxes
|
|
|
(994,194
|
)
|
|
|
(569,243
|
)
|
|
|
(1,973,950
|
)
|
|
|
(1,145,034
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes (benefit)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(994,194
|
)
|
|
$
|
(569,243
|
)
|
|
$
|
(1,973,950
|
)
|
|
$
|
(1,145,034
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share, basic and diluted
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding, basic and diluted
|
|
|
451,724,735
|
|
|
|
427,968,284
|
|
|
|
448,303,465
|
|
|
|
425,955,114
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
U.S. STEM CELL, INC.
CONDENSED STATEMENT OF CHANGES IN STOCKHOLDERS' DEFICIT
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance, March 31, 2021 (unaudited)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
450,443,462
|
|
|
$
|
450,444
|
|
|
$
|
125,916,818
|
|
|
$
|
(137,494,264
|
)
|
|
$
|
(11,127,002
|
)
|
|
Common stock issued in settlement of accounts payable and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
1,295,509
|
|
|
|
1,295
|
|
|
|
73,844
|
|
|
|
-
|
|
|
|
75,139
|
|
|
Beneficial conversion feature recognized on convertible notes
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,400
|
|
|
|
-
|
|
|
|
2,400
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
157,387
|
|
|
|
-
|
|
|
|
157,387
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(994,194
|
)
|
|
|
(994,194
|
)
|
|
Balance, June 30, 2021 (unaudited)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
451,738,971
|
|
|
$
|
451,739
|
|
|
$
|
126,150,449
|
|
|
$
|
(138,488,458
|
)
|
|
$
|
(11,886,270
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance, December 31, 2020
|
|
|
-
|
|
|
$
|
-
|
|
|
|
435,560,794
|
|
|
$
|
435,561
|
|
|
$
|
124,499,655
|
|
|
$
|
(136,514,508
|
)
|
|
$
|
(11,579,292
|
)
|
|
Common stock issued in settlement of accounts payable and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
4,659,380
|
|
|
|
4,659
|
|
|
|
208,399
|
|
|
|
-
|
|
|
|
213,058
|
|
|
Common stock issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
4,000,000
|
|
|
|
4,000
|
|
|
|
124,000
|
|
|
|
-
|
|
|
|
128,000
|
|
|
Beneficial conversion feature recognized on convertible notes
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
634,250
|
|
|
|
-
|
|
|
|
634,250
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
315,724
|
|
|
|
-
|
|
|
|
315,724
|
|
|
Common shares issued upon conversion of convertible notes
|
|
|
-
|
|
|
|
-
|
|
|
|
7,518,797
|
|
|
|
7,519
|
|
|
|
368,421
|
|
|
|
-
|
|
|
|
375,940
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,973,950
|
)
|
|
|
(1,973,950
|
)
|
|
Balance, June 30, 2021 (unaudited)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
451,738,971
|
|
|
$
|
451,739
|
|
|
$
|
126,150,449
|
|
|
$
|
(138,488,458
|
)
|
|
$
|
(11,886,270
|
)
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
U.S. STEM CELL, INC.
CONDENSED STATEMENT OF CHANGES IN STOCKHOLDERS' DEFICIT
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance, March 31, 2020 (unaudited)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
424,935,588
|
|
|
$
|
424,936
|
|
|
$
|
123,935,713
|
|
|
$
|
(134,199,806
|
)
|
|
$
|
(9,839,157
|
)
|
|
Common stock issued in settlement of accounts payable and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
3,066,393
|
|
|
|
3,066
|
|
|
|
21,465
|
|
|
|
-
|
|
|
|
24,531
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
175,018
|
|
|
|
-
|
|
|
|
175,018
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(569,243
|
)
|
|
|
(569,243
|
)
|
|
Balance, June 30, 2020 (unaudited)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
428,001,981
|
|
|
$
|
428,002
|
|
|
$
|
124,132,196
|
|
|
$
|
(134,769,049
|
)
|
|
$
|
(10,208,851
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance, December 31, 2019
|
|
|
-
|
|
|
$
|
-
|
|
|
|
417,724,767
|
|
|
$
|
417,725
|
|
|
$
|
123,726,894
|
|
|
$
|
(133,624,015
|
)
|
|
$
|
(9,479,396
|
)
|
|
Common stock issued in settlement of accounts payable and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
6,277,214
|
|
|
|
6,277
|
|
|
|
27,886
|
|
|
|
-
|
|
|
|
34,163
|
|
|
Common stock issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
4,000,000
|
|
|
|
4,000
|
|
|
|
12,000
|
|
|
|
-
|
|
|
|
16,000
|
|
|
Beneficial conversion feature recognized on convertible note
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
15,000
|
|
|
|
-
|
|
|
|
15,000
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
350,416
|
|
|
|
-
|
|
|
|
350,416
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,145,034
|
)
|
|
|
(1,145,034
|
)
|
|
Balance, June 30, 2020 (unaudited)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
428,001,981
|
|
|
$
|
428,002
|
|
|
$
|
124,132,196
|
|
|
$
|
(134,769,049
|
)
|
|
$
|
(10,208,851
|
)
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
U.S. STEM CELL, INC.
CONDENSED STATEMENTS OF CASH FLOWS
(unaudited)
|
|
|
For the Six Months Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(1,973,950
|
)
|
|
$
|
(1,145,034
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Bad debt (recoveries)
|
|
|
61,797
|
|
|
|
-
|
|
|
Interest and amortization of debt discount
|
|
|
310,094
|
|
|
|
165,413
|
|
|
Loss (gain) on settlement of accounts payable and accrued interest
|
|
|
383,870
|
|
|
|
(481
|
)
|
|
Gain on sale of equipment
|
|
|
-
|
|
|
|
(21,474
|
)
|
|
Related party notes payable issued for services rendered
|
|
|
134,260
|
|
|
|
375,000
|
|
|
Loss on equity investments
|
|
|
-
|
|
|
|
13,821
|
|
|
Stock-based compensation
|
|
|
431,635
|
|
|
|
364,727
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(41,595
|
)
|
|
|
25,913
|
|
|
Inventories
|
|
|
725
|
|
|
|
797
|
|
|
Accounts payable
|
|
|
54,153
|
|
|
|
52,571
|
|
|
Accrued expenses
|
|
|
5,278
|
|
|
|
8,446
|
|
|
Deferred revenue
|
|
|
(1,500
|
)
|
|
|
(1,805
|
)
|
|
Net cash used in operating activities
|
|
|
(635,233
|
)
|
|
|
(162,106
|
)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from (repayments to) overdraft protection
|
|
|
-
|
|
|
|
(1,520
|
)
|
|
Proceeds from related party advances
|
|
|
30,000
|
|
|
|
155,904
|
|
|
Proceeds from notes payable
|
|
|
-
|
|
|
|
150,000
|
|
|
Repayments of notes payable
|
|
|
(24,375
|
)
|
|
|
(24,013
|
)
|
|
Proceeds from convertible note payable
|
|
|
749,000
|
|
|
|
35,000
|
|
|
Net cash provided by financing activities
|
|
|
754,625
|
|
|
|
315,371
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
119,392
|
|
|
|
153,265
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
18,570
|
|
|
|
-
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
137,962
|
|
|
$
|
153,265
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION:
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
11,625
|
|
|
$
|
21,034
|
|
|
Income taxes paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL NON-CASH INVESTING AND FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Common shares issued in settlement of accounts payable and accrued interest
|
|
$
|
213,058
|
|
|
$
|
34,163
|
|
|
Beneficial conversion feature recognized on convertible note
|
|
$
|
634,250
|
|
|
$
|
15,000
|
|
|
Common shares issued for prepaid services
|
|
$
|
12,089
|
|
|
$
|
1,689
|
|
|
Common shares issued upon conversion of convertible notes
|
|
$
|
375,940
|
|
|
$
|
-
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
NOTE 1 — NATURE OF OPERATIONS
Overview
U.S. Stem Cell, Inc. was incorporated under the laws of the State of Florida in August 1999. The Company is in the cardiovascular sector of the cell technology industry delivering cell therapies and biologics that help address congestive heart failure, lower limb ischemia, chronic heart ischemia, acute myocardial infarctions and other issues. The business includes the development of proprietary cell therapy products as well as revenue generating physician and patient-based regenerative medicine/cell therapy training services, cell collection and cell storage services, and the sale of cell collection and treatment kits for humans and animals., To date, the Company has not generated significant revenues in that they remain less than their total operating expenses, has incurred expenses, and has sustained losses. Consequently, its operations are subject to all the risks inherent in the establishment of a research and development business enterprise.
Basis of Presentation
The interim unaudited condensed financial statements included herein reflect all material adjustments (consisting of normal recurring adjustments and reclassifications and non-recurring adjustments) which, in the opinion of the Company’s management, are ordinary and necessary for a fair presentation of results for the interim periods. Certain information and footnote disclosures required under generally accepted accounting principles in the United States of America (“GAAP”) have been condensed or omitted pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). The Company’s management believes the disclosures are adequate to make the information presented not misleading.
The condensed balance sheet information as of December 31, 2020 was derived from the Company’s annual report on Form 10-K for the fiscal year ended December 31, 2020 (“2020 Annual Report”), filed with the SEC pursuant to Section 13 or 15(d) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), on July 16, 2021. These interim unaudited condensed financial statements should be read in conjunction with the 2020 Annual Report. The results of operations for the three and six months ended June 30, 2021 are not necessarily indicative of the results to be expected for the entire fiscal year or for any other period.
NOTE 2 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
As shown in the accompanying financial statements, as of June 30, 2021, the Company had cash on hand of $137,962 and a working capital deficit (current liabilities in excess of current assets) of $11,086,870. During the six months ended June 30, 2021, the net loss was $1,973,950 and net cash used in operating activities was $635,233. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for one year from the issuance of the unaudited condensed financial statements.
The Company’s primary source of operating funds has been from revenue generated from sales with additional cash proceeds from the sale of common stock and the issuances of promissory notes and other debt. The Company has experienced net losses from operations since inception, but it expects these conditions to improve in the future as it develops its business model. The Company had a stockholders’ deficit at June 30, 2021 and requires additional financing to fund future operations.
The Company’s existence is dependent upon management’s ability to develop profitable operations and to obtain additional funding sources. There can be no assurance that the Company’s financing efforts will result in profitable operations or the resolution of the Company’s liquidity problems. The accompanying statements do not include any adjustments that might result should the Company be unable to continue as a going concern. Our management intends to continue the current business strategy, to the extent possible, to finance their clinical development pipeline through revenue (cash in-flows) generated through the marketing and sales of unique educational and training services, animal health products and distribution of products in the industry as well as evaluate and act upon opportunities to increase our top line revenue position and that correspondingly increase cash in-flows. These opportunities include but are not limited to the development and marketing of new products and services, mergers and acquisitions, joint ventures, licensing deals and more.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
NOTE 3 – SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include stock-based compensation, debt discounts and the valuation allowance related to deferred tax assets. Actual results may differ from these estimates.
Cash
The Company considers cash to consist of cash on hand and temporary investments having an original maturity of 90 days or less that are readily convertible into cash.
Fair Value Measurements
Fair value is the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. The Company classifies assets and liabilities recorded at fair value under the fair value hierarchy based upon the observability of inputs used in valuation techniques. Observable inputs (highest level) reflect market data obtained from independent sources, while unobservable inputs (lowest level) reflect internally developed market assumptions. The fair value measurements are classified under the following hierarchy:
|
●
|
Level 1 – Quoted prices in active markets for identical assets or liabilities.
|
|
●
|
Level 2 – Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
●
|
Level 3 – Unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities.
|
The carrying value of cash and cash equivalents, accounts payable, accrued liabilities, and short-term borrowings, as reflected in the balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities and equity instruments of the Company are either recognized or disclosed in the financial statements together with other information relevant for making a reasonable assessment of future cash flows, interest rate risk and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are non-interest bearing and are stated at gross invoice amounts less an allowance for doubtful accounts. Credit is extended to customers based on an evaluation of their financial condition, industry reputation, and other judgmental factors considered by the Company’s management. The Company generally does not require collateral or other security interest to support accounts receivable. Based on trends and specific factors, the customer’s credit terms may be modified, including required payment upon delivery.
The Company performs regular on-going credit evaluations of its customers as deemed relevant. As events, trends, and circumstance warrant, the Company’s management estimates the amounts that are more likely than not to be uncollectible. These amounts are recognized as bad debt expense and are reflected within selling, general, administrative and other expenses on the Company’s accompanying statement of operations.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
Any charges to the allowance for doubtful accounts on accounts receivable are charged to operations in amounts sufficient to maintain the allowance for uncollectible accounts at a level management believes is adequate to cover any probable losses. Management determines the adequacy of the allowance based on historical write-off percentages and the current status of accounts receivable. Accounts receivable are charged off against the allowance when collectability is determined to be permanently impaired.
Inventories
Inventories are stated at the lower of cost or market with cost being determined on a first-in, first-out (FIFO) basis. The Company writes down its inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required. During the periods presented, there were no inventory write-downs.
Investments
The Company follows Accounting Standards Codification subtopic 323-10, Investments-Equity Methods and Joint Ventures (“ASC 323-10) which requires the accounting for investments where the Company can exert significant influence, but not control of a joint venture or equity investment. The Company accounted for its 49.9% member interest ownerships of U.S. Stem Cell Clinic, LLC and Regenerative Wellness Clinic, LLC, respectively, and its 49% member interest ownership of U.S. Stem Cell Clinic of the Villages utilizing the equity method of accounting (See Note 5).
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Codification 606, ”Revenue from Contracts with Customers” (“ASC 606”). ASC 606 is based on the principle that revenue is recognized to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASC also requires additional disclosure about the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer purchase orders, including significant judgments.
At the time of each transaction, management assesses whether the fee associated with the transaction is fixed or determinable and whether or not collection is reasonably assured. The assessment of whether the fee is fixed or determinable is based upon the payment terms of the transaction. Collectability is assessed based on a number of factors, including past transaction history with the client and the creditworthiness of the client.
The Company’s primary sources of revenue are from the sale of test kits and equipment, training services, patient treatments, laboratory services and cell banking.
Revenues for kits and equipment sold are not recorded until kits and equipment are received by the customer. Revenues from in-person trainings are recognized when the training occurs and revenues from on demand online trainings are recognized when the customer purchases the rights to the training course. Any cash received as a deposit for trainings are recorded by the Company as a liability.
Patient treatments and laboratory services revenue are recognized when those services have been completed or satisfied.
Revenues for cell banking are accounted for as multiple performance obligations as described in ASC 606 and addresses accounting for arrangements that may involve the delivery or performance of multiple products, services and/or rights to use assets. Because the Company sells its services separately, on more than a limited basis and at a price within a narrow range, the Company was able to allocate revenue based on stand-alone pricing. The multiple performance obligations include stem cell banking, dose retrieval and yearly storage fees. Revenues for stem cell banking and dose retrieval is recognized at the point of service and revenues for the yearly storage fees is recognized over the term of the banking contract, which is typically one year with annual renewals.
At June 30, 2021 and December 31, 2020, the Company had deferred revenues of $61,000 and $62,500, respectively, all of which pertains to the Intellectual Property Licensing Agreement.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
Research and Development
The Company accounts for research and development costs in accordance with Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and development costs are expensed when the contracted work has been performed or as milestone results have been achieved as defined under the applicable agreement. Company-sponsored research and development costs related to both present and future products are expensed in the period incurred. The Company did not incur any research and development expenses during the period presented.
Stock-Based Compensation
Stock-based compensation expense is measured at the grant date fair value of the award and is expensed over the requisite service period. For stock-based awards to employees, non-employees and directors, the Company calculates the fair value of the award on the date of grant using the Black-Scholes option pricing model. Determining the fair value of stock-based awards at the grant date under this model requires judgment, including estimating volatility, employee stock option exercise behaviors and forfeiture rates. The assumptions used in calculating the fair value of stock-based awards represent the Company’s best estimates, but these estimates involve inherent uncertainties and the application of management’s judgment.
Income Taxes
The Company follows Accounting Standards Codification subtopic 740-10, Income Taxes (“ASC 740-10”) for recording the provision for income taxes. Deferred tax assets and liabilities are computed based upon the difference between the financial statement and income tax basis of assets and liabilities using the enacted marginal tax rate applicable when the related asset or liability is expected to be realized or settled. Deferred income tax expenses or benefits are based on the changes in the asset or liability during each period. If available evidence suggests that it is more likely than not that some portion or all of the deferred tax assets will not be realized, a valuation allowance is required to reduce the deferred tax assets to the amount that is more likely than not to be realized. Future changes in such valuation allowance are included in the provision for deferred income taxes in the period of change. Deferred income taxes may arise from temporary differences resulting from income and expense items reported for financial accounting and tax purposes in different periods.
Deferred taxes are classified as current or non-current, depending on the classification of assets and liabilities to which they relate. Deferred taxes arising from temporary differences that are not related to an asset or liability are classified as current or non-current depending on the periods in which the temporary differences are expected to reverse and are considered immaterial.
Net Loss per Common Share
The Company computes earnings (loss) per share under Accounting Standards Codification subtopic 260-10, Earnings Per Share (“ASC 260-10”). Net loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share, if presented, would include the dilution that would occur upon the exercise or conversion of all potentially dilutive securities into common stock using the “treasury stock” and/or “if converted” methods as applicable.
The computation of basic and diluted income (loss) per share as of June 30, 2021 and 2020 excludes potentially dilutive securities when their inclusion would be anti-dilutive, or if their exercise prices were greater than the average market price of the common stock during the period.
Potentially dilutive securities excluded from the computation of basic and diluted net loss per share are as follows:
|
|
|
June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Options
|
|
|
110,644,914
|
|
|
|
111,119,914
|
|
|
Warrants
|
|
|
1,110,468
|
|
|
|
1,110,468
|
|
|
Convertible note
|
|
|
36,259,837
|
|
|
|
7,820,647
|
|
|
Total potentially dilutive shares
|
|
|
148,015,219
|
|
|
|
120,051,029
|
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
Reclassifications
Certain reclassifications have been made to the prior years’ data to conform to the current year presentation. These reclassifications had no effect on reported income (losses).
Recent Accounting Pronouncements
FASB Accounting Standards Updates (“ASU”) 2017-04 (Topic 350), “Intangibles – Goodwill and Others” – Issued in January 2017, ASU 2017-04 simplifies how an entity is required to test goodwill for impairment by eliminating Step 2 from the goodwill impairment test. Step 2 measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill. This guidance was effective for the Company in the first fiscal quarter of 2020. The adoption of this standard did not have a material impact on the Company’s financial statements and related disclosures.
In August 2020, the FASB issued ASU 2020-06, which simplifies the guidance on accounting for convertible debt instruments by removing the separation models for: (1) convertible debt with a cash conversion feature; and (2) convertible instruments with a beneficial conversion feature. As a result, the Company will not separately present in equity an embedded conversion feature in such debt. Instead, we will account for a convertible debt instrument wholly as debt, unless certain other conditions are met. We expect the elimination of these models will reduce reported interest expense and increase reported net income for the Company’s convertible instruments falling under the scope of those models before the adoption of ASU 2020-06. Also, ASU 2020-06 requires the application of the if-converted method for calculating diluted earnings per share and the treasury stock method will be no longer available. The provisions of ASU 2020-06 are applicable for fiscal years beginning after December 15, 2021, with early adoption permitted no earlier than fiscal years beginning after December 15, 2020. The Company is currently evaluating the impact of ASU 2020-06 on its financial statements.
In August 2018, the FASB issued Accounting Standards Update (“ASU”) 2018-13, “Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement” (“ASU 2018-13”). ASU 2018-13 removes certain disclosure requirements, including the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, the policy for timing of transfers between levels, and the valuation processes for Level 3 fair value measurements. ASU 2018-13 also adds disclosure requirements, including changes in unrealized gains and losses for the period included in other comprehensive income for recurring Level 3 fair value measurements, and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. The amendments on changes in unrealized gains and losses, and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements, should be applied prospectively for only the most recent interim or annual period presented in the initial fiscal year of adoption. All other amendments should be applied retrospectively to all periods presented upon their effective date. This guidance was effective for the Company in the first fiscal quarter of 2020. The adoption of this standard did not have a material impact on the Company’s financial statements and related disclosures.
There are various other updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on the Company’s financial position, results of operations or cash flows.
NOTE 4 — PROPERTY AND EQUIPMENT
Property and equipment as of June 30, 2021 and December 31, 2020 is summarized as follows:
|
|
|
June 30,
2021
|
|
|
December 31,
2020
|
|
|
Furniture, fixtures and equipment
|
|
$
|
5,598
|
|
|
$
|
5,598
|
|
|
Computer equipment
|
|
|
1,809
|
|
|
|
1,809
|
|
|
Property and equipment, cost
|
|
|
7,407
|
|
|
|
7,407
|
|
|
Less: accumulated depreciation and amortization
|
|
|
(7,407
|
)
|
|
|
(7,407
|
)
|
|
Property and equipment, net
|
|
$
|
-
|
|
|
$
|
-
|
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
As a consequence of the Court Order (see Note 11 “Government Claim”), the Company resolved to divest itself of certain equipment and other assets (the “Equipment Assets”) used in connection with the Company’s human tissue banking business, but consistent however with the requirements of the Court Order, and to adjust the business plan and operations to accommodate this potential divesture. To facilitate the above, the Company entered into a Termination and Release Agreement and a Letter Agreement intended to divest itself of certain equipment and other assets underlying the related equipment lease transaction. In addition, on October 24, 2019, the Company entered into an Assignment and Assumption of Lease by and between the Company, American Cell Technology, LLC, and Sawgrass Business Plaza, LLC. Subsequently, the Company relocated to a new location within the same city and entered into a month-to-month lease. As part of the termination of the operating lease, the Company left certain property and equipment (all of which had been fully depreciated) at the old location.
In connection with the sale of the lab, medical and other equipment, the Company realized a gain on sale of equipment of $386,535. The gain is recognized ratably over the term of the lease to operations. During the three and six months ended June 30, 2020, the Company recognized $0 and $21,474, respectively, as gain on sale of equipment. As of June 30, 2021, the gain has been fully recognized and deferred gain on sale of equipment was $0.
Depreciation expense was $0 for the three and six months ended June 30, 2021 and 2020.
NOTE 5 — INVESTMENTS
U.S. Stem Cell Clinic, LLC
The investment in U.S. Stem Cell Clinic, LLC was comprised of a 49.9% (increased from 33.3% on January 29, 2019) member interest ownership and is accounted for using the equity method of accounting. The Company’s income (loss) earned by U.S. Stem Cell Clinic, LLC member interest was $0 for the three and six months ended June 30, 2021; and $9,718 and ($13,821) for the three and six months ended June 30, 2020, respectively (inception to date income of $599,721) and is included in other income (expense) in the accompanying Statements of Operations. In addition, during the six months ended June 30, 2021 and 2020, the Company received distributions totaling $0 from U.S. Stem Cell Clinic, LLC (inception to date of $663,870). On February 10, 2021, as part of a settlement agreement, the Company transferred its entire member interest in U.S. Stem Cell, LLC to Dr. Kristen Comella as settlement for $100,000 of accrued interest owed to Dr. Comella (See Note 6, Note 7 and Note 11 “Litigation”). The carrying value of the investment at June 30, 2021 and December 31, 2020 is $0.
At June 30, 2021 and December 31, 2020, accounts receivable for sales of product and services to U.S. Stem Cell Clinic, LLC was $28,763 (prior to divesture on February 10, 2021). Revenues earned from sales to U.S. Stem Clinic, LLC for the three and six months ended June 30, 2021 were $250 and $2,531, respectively (prior to divesture on February 10, 2021) and $0 and $1,441, for the three and six months ended June 30, 2020, respectively.
U.S. Stem Cell of the Villages LLC
On January 30, 2018, Greg Knutson, a director of the Company (“Knutson”) and the Company agreed to open and operate a regenerative medicine/cell therapy clinic providing cellular treatments for patients afflicted with neurological, autoimmune, orthopedic and degenerative diseases in Florida. To that end, U.S. Stem Cell Clinic of The Villages LLC (the “LLC”) was formed January 30, 2018. Knutson provided the Company with the sum of Three Hundred Thousand Dollars ($300,000) (the “Investment”) to be utilized for the formation and initial operation of the LLC. Currently, Knutson holds a 51% member interest in the LLC and the Company holds a 49% member interest. The Company will provide, if requested, operating assistance as well as management services, the latter to be compensated at fee of five percent (5%) of the LLC gross revenues.
As of December 31, 2018, upon completion of U.S. Stem Cell of the Villages LLC, the Company received $189,909 from Greg Knutson, the holder of the 51% member interest. Accordingly, this was recognized as additional paid-in capital. Subsequently, the Company contributed $86,750 as its initial investment in the U.S. Stem Cell of the Villages, LLC. The Company’s 49% income (loss) incurred by U.S. Stem Cell of the Villages LLC member interest was $0 for the three and six months ended June 30, 2021 and 2020 (inception to date loss of $23,050) and is included in other income (expense) in the accompanying Statements of Operations. In addition, during the six months ended June 30, 2021 and 2020, the Company received distributions totaling $0 from U.S. Stem Cell of the Villages LLC. The carrying value of the investment at June 30, 2021 and December 31, 2020 is $0.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
At June 30, 2021 and December 31, 2020, accounts receivable for sales of products and services to U.S. Stem Cell of the Villages LLC was $0. Revenues earned from sales to U.S. Stem Cell of the Villages LLC for the three and six months ended June 30, 2021 and 2020 was $0.
As of the date of this filing, US Stem Cell Clinic of the Villages, LLC is currently dormant.
NOTE 6 — ACCRUED EXPENSES
Accrued expenses consisted of the following as of June 30, 2021 and December 31, 2020:
|
|
|
June 30,
2021
|
|
|
December 31,
2020
|
|
|
Interest and fees payable to the Guarantors of the Company’s loan agreement with Seaside Bank
|
|
$
|
595,731
|
|
|
$
|
549,628
|
|
|
Accrued interest payable
|
|
|
1,003,793
|
|
|
|
882,515
|
|
|
Vendor accruals and other
|
|
|
89,509
|
|
|
|
79,138
|
|
|
Total Accrued expenses
|
|
$
|
1,689,033
|
|
|
$
|
1,511,281
|
|
On April 1, 2021, the Company issued 187,575 shares of its common stock, having a fair value of $10,879, in lieu of payment in cash of accrued and unpaid interest of $9,145, resulting in a loss on settlement of $1,735 (See Note 10).
On February 10, 2021, as part of a settlement agreement, the Company transferred its entire member interest in U.S. Stem Cell, LLC to Dr. Kristen Comella as settlement for $100,000 of accrued interest owed to Dr. Comella, resulting in a gain on settlement of $100,000 (See Note 5, Note 7 and Note 11 “Litigation”).
NOTE 7 — NOTES PAYABLE
Notes payable were comprised of the following as of June 30, 2021 and December 31, 2020:
|
|
|
June 30,
2021
|
|
|
December 31,
2020
|
|
|
Seaside Bank note payable
|
|
$
|
980,000
|
|
|
$
|
980,000
|
|
|
Dr. Comella note payable*
|
|
|
255,579
|
|
|
|
255,579
|
|
|
Dr. Comella note payable*
|
|
|
300,000
|
|
|
|
300,000
|
|
|
Dr. Comella note payable*
|
|
|
300,000
|
|
|
|
300,000
|
|
|
Dr. Comella note payable*
|
|
|
300,000
|
|
|
|
300,000
|
|
|
Hunton & Williams note payable
|
|
|
384,500
|
|
|
|
386,000
|
|
|
Weider note payable
|
|
|
432,102
|
|
|
|
450,477
|
|
|
Mallard note payable
|
|
|
237,250
|
|
|
|
241,750
|
|
|
EIDL note payable
|
|
|
150,000
|
|
|
|
150,000
|
|
|
Total notes payable
|
|
|
3,339,431
|
|
|
|
3,363,806
|
|
|
Less unamortized debt discount
|
|
|
(36,526
|
)
|
|
|
(41,237
|
)
|
|
Total notes payable net of unamortized debt discount
|
|
|
3,302,905
|
|
|
|
3,322,569
|
|
|
Less current portion
|
|
|
(2,561,505
|
)
|
|
|
(2,562,149
|
)
|
|
Long-term portion
|
|
$
|
741,400
|
|
|
$
|
760,420
|
|
|
|
|
|
|
|
|
|
|
|
|
* Dr. Comella is a former member of the Board of Directors and resigned on December 1, 2019. This note was previously included in notes payable - related parties.
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
Seaside Bank
On October 25, 2010, the Company entered into a Loan Agreement with Seaside National Bank and Trust for a $980,000 loan at 4.25% per annum interest that was used to refinance the Company’s loan with Bank of America. The obligation is guaranteed by certain stockholders of the Company. The Company renewed the loan with Seaside National Bank and Trust during the first quarter of 2018 to extend the maturity date to May 18, 2020. The Company renewed the loan with Seaside National Bank and Trust during the first quarter of 2020 to extend the maturity date to May 18, 2022.
Dr. Comella, former Chief Science Officer
On September 6, 2016, the Company issued a $300,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due upon demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $255,579.
On August 7, 2017, the Company issued a $300,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due one year from date of issuance. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $300,000.
On May 7, 2018, the Company issued a $300,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due six months from date of issuance. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $300,000.
On July 1, 2019, the Company issued a $300,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due November 7, 2019. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $300,000.
On February 10, 2021, as part of a settlement agreement, the Company transferred its entire member interest in U.S. Stem Cell, LLC to Dr. Kristen Comella as settlement for $100,000 of accrued interest owed to Dr. Comella, resulting in a gain on settlement of $100,000 (See Note 5, Note 6 and Note 11 “Litigation”). At June 30, 2021 and December 31, 2020, accrued interest on the notes was $137,297 and $208,645, respectively, and is included in accrued expenses on the accompanying balance sheet.
Dr. Comella has not served as member of the Board of Directors since September 1, 2019.
Hunton & Williams
At December 31, 2016, the Company has two outstanding notes payable with interest at 8% per annum due at maturity. The two notes, $61,150 and $323,822, are payable in one balloon payment upon the date the Noteholder provides written demand, however the Company is not obligated to make payments until the Northstar Biotech Group, LLC (or successor) Loan is paid off.
On August 31, 2017, the Company and the noteholder entered into a Note Forbearance, Modification and Repayment Agreement (“Agreement”). The two notes, $61,150 and $323,822, were payable in one balloon payment upon the date of a written demand and upon certain triggering events occurring. The sum of unpaid principal and accumulated interest for both notes as of August 31, 2017 of $747,680 and an accounts payable of $40,596 result in an aggregate balance due of $788,276.
The noteholder agreed to accept full payment of their obligation over a four (4) year period in 48 monthly installments on an adjusted debt obligation in aggregate of $624,000 (reducing the outstanding balance), with such payments staggered in amounts such that the Company will pay $10,000 monthly the first year, $12,000 monthly the second year, $14,000 monthly the third year, and $16,000 monthly the final year. In addition, the noteholder agreed to suspend accrual interest on the notes commencing September 1, 2017.
The Agreement remains in full force and effect provided the Company continues to make the monthly payments, there is no event of default as defined in the notes and an agreement to a subordination agreement by Northstar Biotech Group, LLC, which has been provided. In May 2019, the Company did not make the required scheduled payment. In September 2010, the noteholder agreed to waive their default rights under the agreement provided a minimum of $5,000 was paid by the end of 2019 and to reduce the required monthly payment to $500 per month commencing in January 2020. The Company satisfied the $5,000 payment requirement by the end of 2019 and commenced making the required $500 monthly payments in January 2020.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
The Company imputed an interest rate of 5% and discounted the note accordingly. The imputed debt discount of $69,700 was amortized to interest expense using the effective interest method. At September 30, 2019, the Company was in default and renegotiating the payment structure. Thus, the remaining unamortized debt discount was charged to interest expense at September 30, 2019. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $384,500 and $386,000, respectively.
Weider
The Company, as one of the parties entered into a Settlement Agreement and General Release (the “Agreement”) dated June 3, 2019 related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year 5.25% unsecured promissory note, dated June 15, 2019, in the principal amount of $500,000, payable in monthly increments of $5,000 per month, with a final balloon payment due on June 15, 2024. Accordingly, the Company recognized Pre-litigation expense of $500,000. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $432,102 and $450,477, respectively. At present, the Company has been noticed that it is delinquent if the last two monthly payments and, if not cured, will be considered in default of the promissory note underlying the Agreement.
Mallard
The Company, as one of the parties entered into a Settlement Agreement and General Release (the “Agreement”) dated December 6, 2019 related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year non-interest bearing unsecured promissory note, dated December 6, 2019, in the principal amount of $250,000, payable in monthly increments of $750 per month, with a final balloon payment of $205,000 due on January 1, 2025. The Company imputed an interest rate of 5% and discounted the note accordingly. The imputed debt discount of $51,063 is being amortized to interest expense using the effective interest method. Accordingly, the Company recognized Pre-litigation expense of $198,937. For the three and six months ended June 30, 2021, the Company amortized $2,382 and $4,711, respectively; and $2,277 and $4,529 during the three and six months ended June 30, 2020, respectively, of debt discount to interest expense. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $200,724 and $200,513, net of debt discount of $36,526 and $41,237, respectively.
Economic Injury Disaster Loan (EIDL)
On June 20, 2020, the Company executed the standard loan documents for an EIDL from the U.S. Small Business Administration in light of the impact of the COVID-19 pandemic on our business. Pursuant to that certain Loan Authorization and Agreement (the “SBA Loan Agreement”), the principal amount of the EIDL received was $150,000, with proceeds to be used for working capital purposes. Interest accrues at the rate of 3.75% per annum. Installment payments, including principal and interest, are due monthly beginning June 20, 2021 (twelve months from the date of the SBA Loan Agreement) in the amount of $731. On March 15, 2021, the initial payment date was extended 12 months to June 20, 2022. The balance of principal and interest is payable thirty years from the date of the SBA Loan Agreement. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $150,000. At June 30, 2021 and December 31, 2020, accrued interest on the note was $5,779 and $2,990, respectively, and is included in accrued expenses on the accompanying balance sheet.
NOTE 8 — PROMISSORY NOTE PAYABLE
On June 1, 2015, the Company issued an amended and restated promissory note of $1,697,762 in settlement of the $1,500,000 outstanding subordinated debt, related accrued interest of $373,469 and accumulated and unpaid guarantor fees of $624,737.
The note is unsecured and non-interest bearing and requires four semi-annual payments of $75,000 beginning on December 31, 2015 with the remaining unpaid balance due June 1, 2020. On June 1, 2020, the Company defaulted on the promissory note. Upon default, the note became due in full and the Company began accruing interest at the default interest rate of 18%.
The Company imputed an interest rate of 5% and discounted the promissory note accordingly. The imputed debt discount of $368,615 was amortized to interest expense using the effective interest method. For the three and six months ended June 30, 2021, the Company amortized $0; and $11,871 and $29,295 during the three and six months ended June 30, 2020, respectively, of debt discount to interest expense. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $1,397,762.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
NOTE 9 — CONVERTIBLE NOTE PAYABLE
On February 5, 2020, the Company issued an unsecured convertible promissory note in the principal amount of $35,000 that matured on February 5, 2021 and bears interest at a rate of 5% per annum. The investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price equal to a thirty percent (30%) discount of the average closing price of the Company’s common stock on the OTC Markets electronic exchange for the prior thirty (30) trading days prior to conversion, subject to adjustment. Upon the occurrence of an event of default, the investor may accelerate the note pursuant to which the outstanding balance will become, at the noteholder’s election, immediately due and payable. As a result of the beneficial conversion feature of the note, debt discount of $15,000 was recognized with a corresponding increase in additional paid-in capital. The debt discount was amortized to interest expense using the effective interest method. For the three and six months ended June 30, 2021, the Company amortized $0 and $1,874, respectively; and $3,247 and $5,002 during the three and six months ended June 30, 2020, respectively, of debt discount to interest expense. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $35,000 and $33,126, net of debt discount of $0 and $1,874, respectively. At June 30, 2021 and December 31, 2020, accrued interest on the note was $2,450 and $1,582, respectively, and is included in accrued expenses on the accompanying balance sheet. As of February 5, 2021, the maturity date, the note is in default. The investor converted the full value of the note into 3,804,348 shares of the Company’s common stock on August 4, 2021.
On September 8, 2020, the Company issued an unsecured convertible promissory note in the principal amount of $10,000 that is due on demand and bears interest at a rate of 5% per annum. The investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0467. Upon the occurrence of an event of default, the remaining principal and accrued interest become immediately due and payable, with interest accruing at 18% per annum on any unpaid amounts. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $10,000. As of June 30, 2021 and December 31, 2020, accrued interest on the note was $404 and $156, respectively, and is included in accrued expenses on the accompanying balance sheet.
From February 17, 2021 through February 26, 2021, the Company issued unsecured convertible promissory notes in the aggregate principal amount of $619,000 that mature 12 months after the respective issuance date. The notes are non-interest bearing and the investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0266. Upon the occurrence of an event of default, the remaining principal and accrued interest become immediately due and payable. As a result of the beneficial conversion feature of the notes, an aggregate of $521,850 of debt discount was recognized with a corresponding increase in additional paid-in capital. The debt discount is being amortized to interest expense using the effective interest method. On March 23, 2021, one of the holders, a related party, converted a convertible note with a face value of $200,000, dated February 26, 2021, having a net book value of $54,887 at the date of conversion, into 7,518,797 shares of the Company’s common stock, having a fair value of $375,940, resulting in a loss on conversion of $321,053 (See Note 10 and Note 12). For the three and six months ended June 30, 2021, the Company amortized $18,289 and $23,541 of debt discount to interest expense. As of June 30, 2021, the remaining carrying value of the notes was $65,804, net of debt discount of $353,196.
On March 24, 2021, the Company issued an unsecured convertible promissory note in the principal amount of $110,000 that matures 12 months after the issuance date. The note is non-interest bearing and the investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0070. Upon the occurrence of an event of default, the remaining principal and accrued interest become immediately due and payable. As a result of the beneficial conversion feature of the note, $110,000 of debt discount was recognized with a corresponding increase in additional paid-in capital. The debt discount is being amortized to interest expense using the effective interest method. For the three and six months ended June 30, 2021, the Company amortized $22 of debt discount to interest expense. As of June 30, 2021, the remaining carrying value of the note was $22, net of debt discount of $109,978.
On June 20, 2021, the Company issued an unsecured convertible promissory note in the principal amount of $20,000 that matures 12 months after the issuance date. The note is non-interest bearing and the investor has the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0125. Upon the occurrence of an event of default, the remaining principal and accrued interest become immediately due and payable. As a result of the beneficial conversion feature of the note, $2,400 of debt discount was recognized with a corresponding increase in additional paid-in capital. The debt discount is being amortized to interest expense using the effective interest method. For the three and six months ended June 30, 2021, the Company amortized $61 of debt discount to interest expense. As of June 30, 2021, the remaining carrying value of the note was $17,661, net of debt discount of $2,339.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
NOTE 10 — RELATED PARTY TRANSACTIONS
Advances – Related Parties
As of June 30, 2021 and December 31, 2020, the Company’s officers and directors have provided advances that are unsecured, non-interest bearing and due on demand. During the six months ended June 30, 2021 and 2020, the Company received aggregate proceeds from advances of $30,000 and $155,904, respectively. As of June 30, 2021 and December 31, 2020, the Company owed $891,432 and $861,432, respectively, for related party advances.
Notes Payable – Related Parties
Northstar Biotechnology Group, LLC
On February 29, 2012, a promissory note issued to BlueCrest Master Fund Limited (“BlueCrest”) was assigned to Northstar Biotechnology Group, LLC (“Northstar”), owned partly by certain directors and existing shareholders of the Company at the time, including Dr. William P. Murphy Jr., Dr. Samuel Ahn and Charles Hart. At the date of the assignment, the principal amount of the BlueCrest note was $544,267 (the “Note”).
On March 30, 2012, the Company and Northstar agreed to extend until May 1, 2012 the initial payment date for any and all required monthly under the Note, such that the first of the four monthly payments required under the Note will be due and payable on May 1, 2012 and all subsequent payments will be due on a monthly basis thereafter commencing on June 1, 2012, and to waive any and all defaults and/or events of default under the Note with respect to such payments. The Company did not make the required payment, and as a result, was in default of the revised agreement. The Company renegotiated the terms of the Note and Northstar agreed to suspend the requirement of principal payments by the Company and allow payment of interest-only in common stock.
On September 21, 2012, the Company issued 5,000 common stock purchase warrants to Northstar that was treated as additional interest expense upon issuance.
On October 1, 2012, the Company and Northstar entered into a limited waiver and forbearance agreement providing a recapitalized new note balance comprised of all sums due Northstar with a maturity date extended perpetually. The Company agreed to issue 5,000,000 shares of Series A Convertible Preferred Stock and 10,000 shares of common stock in exchange for $210,000 as payment towards outstanding debt, default interest, penalties, professional fees outstanding and due Northstar. In addition, the Company executed a security agreement granting Northstar a lien on all patents, patent applications, trademarks, service marks, copyrights and intellectual property rights of any nature, as well as the results of all clinical trials, know-how for preparing Myoblasts, old and new clinical data, existing approved trials, all right and title to Myoblasts, clinical trial protocols and other property rights.
In addition, the Company granted Northstar a perpetual license on products as described for resale, relicensing, and commercialization outside the United States. In connection with the granted license, Northstar shall pay the Company a royalty of up to 8% on revenues generated.
Effective October 1, 2012, the interest rate was 12.85% per annum. The parties agreed, as of February 28, 2013, to reduce the interest rate to 7% per annum.
In connection with the consideration paid, Northstar waived, from the effective date through the earlier of termination or expiration of the agreement, satisfaction of the obligations as described in the forbearance agreement.
In 2012, 5,000,000 shares of Series A Convertible Preferred Stock were approved to be issued, which was subsequently increased to 20,000,000 shares of preferred stock as Series A Convertible Preferred Stock. In addition, the Company was obligated to issue additional preferred stock equal in lieu of payment of cash of accrued and unpaid interest on each six-month anniversary of the effective date (October 1, 2012). In lieu of the initial two payments in preferred stock, the parties agreed to modify the voting rights of the subsequently cancelled Series A Convertible Preferred Stock from 20 votes per share on matters to be voted on by the common stockholders to 25 votes per share on matters to be voted on by the common stockholders and all prior and subsequent payments of interest will be in common stock. The Company is required to issue additional shares of its common stock (as amended), in lieu of cash, each six-month anniversary of the effective date for any accrued and unpaid interest.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
On September 30, 2013, the Company issued 8,772 shares of its common stock as payment of $100,000 towards principal.
On December 24, 2013, the Company issued 3,916 shares of its common stock as payment of accrued interest through June 30, 2013 of $85,447.
On April 2, 2014, the Company issued 275 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2014 per the forbearance agreement.
On September 17, 2014, the limited waiver and forbearance agreement entered into on October 1, 2012 to provide that the perpetual license on products as described for resale, relicensing and commercialization outside the United States was amended as such on the condition that Northstar provide certain financing, which financing the Company, in its sole discretion, could decline and retain the license.
On October 3, 2014, the Company issued 515 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2014 per the forbearance agreement.
On April 3, 2015, the Company issued 1,363 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2015 per the forbearance agreement.
On October 2, 2015, the Company issued 4,156 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2015 per the forbearance agreement.
On October 7, 2015, the Company issued 34,522 shares of its common stock in settlement of $100,000 principal payment towards the outstanding debt.
On April 7, 2016, the Company issued 57,778 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due April 1, 2016 per the forbearance agreement.
On October 6, 2016, the Company issued 848,490 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2016 per the forbearance agreement.
On March 1, 2017, Northstar and the Company entered into a settlement agreement (“Settlement Agreement “) related to then pending litigation. Pursuant to the terms and conditions of the Settlement Agreement, Northstar converted its outstanding Series A Convertible preferred stock, into twenty million (20,000,000) shares of common stock according to the original conversion terms. In addition, and separate and apart from the conversion, Northstar received eleven million (11,000,000) shares of the Company’s common stock. Northstar will receive ten percent (10%) of all Company international sales (based on a gross sales basis). There was no effect of the 10% obligation as there were no international sales in 2017 or through 2019. Furthermore, a Northstar designee, Greg Knutson, was appointed as a member of the Board of Directors of the Company and two Company directors, Michael Tomas and Kristin Comella, each exercised their prior Northstar options to each receive a five percent (5%) member interest in Northstar. The parties agreed to a mutual release and Northstar agreed to terminate any UCC lien on the Company assets previously filed for the benefit of Northstar. On March 9, 2017 and April 1, 2017, the Company issued 30,000,000 and 1,000,000 shares of its common stock, respectively, as described above. In connection with the settlement, the Company recorded a loss on litigation settlement of $316,800.
On April 1, 2017, the Company issued 286,315 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,703.
On October 2, 2017, the Company issued 559,187 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705.
On October 19, 2018, the Company issued 164,523 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,195.
On April 19, 2019, the Company issued 379,141 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,145.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
On October 1, 2019, the Company issued 1,692,353 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $9,195.
On April 1, 2020, the Company issued 1,445,647 shares of its common stock, having a fair value of $11,565, in lieu of payment in cash of accrued and unpaid interest of $9,145, resulting in a loss on settlement of $2,420.
On October 1, 2020, the Company issued 2,035,820 shares of its common stock, having a fair value of $10,179, in lieu of payment in cash of accrued and unpaid interest of $9,195, resulting in a loss on settlement of $984.
On April 1, 2021, the Company issued 187,575 shares of its common stock, having a fair value of $10,879, in lieu of payment in cash of accrued and unpaid interest of $9,145, resulting in a loss on settlement of $1,735.
As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $262,000. At June 30, 2021 and December 31, 2020, accrued interest on the note was $8,700 and $8,751, respectively, and is included in accrued expenses on the accompanying balance sheet.
Notes Payable - Mr. Tomas, President and Chief Executive Officer
On August 7, 2017, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due one year from date of issuance. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $161,786.
On May 7, 2018, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due six months from date of issuance. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $500,000.
On July 1, 2019, the Company issued a $500,000 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due November 7, 2019. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $500,000.
On December 31, 2019, the Company issued a $178,077 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $178,077.
On June 30, 2021, the Company issued a $187,500 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $187,500.
On June 30, 2020, the Company issued a $187,500 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $187,500.
On July 1, 2020, the Company issued a $500,000 promissory note as payment of an annual bonus awarded. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $500,000.
On September 30, 2020, the Company issued a $100,962 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $100,962.
On December 31, 2020, the Company issued a $143,654 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $143,654.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
On March 31, 2021, the Company issued a $90,990 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021, the remaining carrying value of the note was $90,990.
On June 30, 2021, the Company issued a $43,269 promissory note in exchange for compensation earned. The promissory note bears interest of 5% per annum and is due on demand. As of June 30, 2021, the remaining carrying value of the note was $43,269.
At June 30, 2021 and December 31, 2021, accrued interest on the notes was $544,584 and $482,468, respectively, and is included in accrued expenses on the accompanying balance sheet.
|
|
|
June 30,
2021
|
|
|
December 31,
2020
|
|
|
Northstar
|
|
$
|
262,000
|
|
|
$
|
262,000
|
|
|
Note payable, Mr. Tomas
|
|
|
161,786
|
|
|
|
161,786
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
178,077
|
|
|
|
178,077
|
|
|
Note payable, Mr. Tomas
|
|
|
187,500
|
|
|
|
187,500
|
|
|
Note payable, Mr. Tomas
|
|
|
187,500
|
|
|
|
187,500
|
|
|
Note payable, Mr. Tomas
|
|
|
500,000
|
|
|
|
500,000
|
|
|
Note payable, Mr. Tomas
|
|
|
100,962
|
|
|
|
100,962
|
|
|
Note payable, Mr. Tomas
|
|
|
143,654
|
|
|
|
143,653
|
|
|
Note payable, Mr. Tomas
|
|
|
90,990
|
|
|
|
-
|
|
|
Note payable, Mr. Tomas
|
|
|
43,269
|
|
|
|
-
|
|
|
Total notes payable - related parties
|
|
$
|
2,855,738
|
|
|
$
|
2,721,478
|
|
Note Payable - William P. Murphy Jr., M.D
On February 26, 2021, Dr. Murphy purchased an unsecured convertible promissory note in the aggregate principal amount of $200,000 maturing 12 months after the issuance date. The note was non-interest bearing and Dr. Murphy had the right to convert the outstanding balance of the note at any time into shares of common stock of the Company at a conversion price of $0.0266. On March 23, 2021, Dr. Murphy converted the full value of the note into 7,518,797 shares of the Company’s common stock (See Note 9 and Note 12).
NOTE 11 — COMMITMENTS AND CONTINGENCIES
Leases
On October 24, 2019, the Company entered into an Assignment and Assumption of Lease by and between the Company, American Cell Technology, LLC, and Sawgrass Business Plaza, LLC. Subsequently, the Company relocated to a new location within the same city and entered into a month-to-month lease. Accordingly, the right of use assets and lease liabilities were eliminated.
During the three and six months ended June 30, 2021 and 2020, lease expense was comprised of the following:
|
|
|
For the Three Months Ended June 30,
|
|
|
For the Six Months Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Operating lease expense
|
|
$
|
1,001
|
|
|
$
|
1,395
|
|
|
$
|
2,433
|
|
|
$
|
2,202
|
|
|
Total lease expense
|
|
$
|
1,001
|
|
|
$
|
1,395
|
|
|
$
|
2,433
|
|
|
$
|
2,202
|
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
Royalty Agreement / Middle East
On November 9, 2016, the Company entered into an Intellectual Property License Agreement whereby the Company granted High Rise Group Company the exclusive right to the Company’s intellectual property (as defined) for the licensed use and development in Kuwait and other GCC/Middle East countries for 25 years in exchange for a payment of $75,000 and a 5% royalty generated under the agreement. The licensing agreement is recorded as deferred revenue and amortized over the term of the agreement. The carrying balance as of June 30, 2021 and December 31, 2020 was $61,000 and $62,500, respectively.
The intent is for U.S. Stem Cell Middle East to offer regenerative treatment options to patients, based on U.S. Stem Cell, Inc. products and technologies like MyoCell™. To date, the first clinic in Kuwait City has been completed but has not begun operations as High Rising Group has not yet been able to secure regulatory approvals to operate.
Litigation
On September 17, 2015, a product liability lawsuit was filed in Broward County, specifically Patsy Bade v. Bioheart, Inc. US Stem Cell Clinics LLC, Alejandro Perez, ARNP, and Shareen Greenbaum, M.D., and on November 30, 2015, a product liability lawsuit was filed in Broward County, specifically Elizabeth Noble v. Bioheart, Inc. US Stem Cell Clinics LLC, Alejandro Perez, ARNP, and Shareen Greenbaum, M.D. During the year ended December 31, 2016, both matters settled by the Company’s insurance policy with no additional cost to the Company, except for the obligation to pay the insurance company deductible of $100,000, of which $11,000 was paid in fiscal 2017. The remaining amount due under this settlement is $27,350 and $28,850 as of June 30, 2021 and December 31, 2020, respectively, and is included in accounts payable.
On July 27, 2020, Brenda Leonhardt filed a lawsuit against U.S. Stem Cell, Inc., Mike Tomas, Dr. William P. Murphy, Jr., Richard T. Spencer, III, Mark Borman, Dr. Samuel S. Ahn, Charles Hart, Sheldon T. Anderson, Greg Knutson, and Kristin Comella in Broward County Court, Case No. CACE-10-012095. The lawsuit alleges breach of a settlement agreement, breach of contract with respect to failure to make a balloon payment under a promissory note, and several tort theories such as misrepresentation and fraudulent transfer. The Company denies most of the allegations in the lawsuit, and moved to dismiss almost all of the claims. The motions to dismiss remain pending. U.S. Stem Cell, Inc. does note that it provided a promissory note to Ms. Leonhardt, which has not been fully satisfied.
The Company, as one of the parties entered into a Settlement Agreement and General Release (the “Agreement”) dated June 3, 2019 related to certain medical procedures. Without admitting any liability, and as part of that Agreement, the Company agreed to provide a five-year 5.25% unsecured promissory note, dated June 15, 2019, in the principal amount of $500,000, payable in monthly increments of $5,000 per month, with a final balloon payment due on June 15, 2024. Accordingly, the Company recognized Pre-litigation expense of $500,000. As of June 30, 2021 and December 31, 2020, the remaining carrying value of the note was $432,102 and $450,477, respectively. At present, the Company has been noticed that it is delinquent if the last two monthly payments and, if not cured, will be considered in default of the promissory note underlying the Agreement.
On February 10, 2021, as part of a settlement agreement, the Company transferred its entire member interest in U.S. Stem Cell, LLC to Dr. Kristen Comella as settlement for $100,000 of accrued interest owed to Dr. Comella (See Note 5, Note 6 and Note 7).
The Company is subject at times to other legal proceedings and claims, which arise in the ordinary course of its business. Although occasional adverse decisions or settlements may occur, the Company believes that the final disposition of such matters should not have a material adverse effect on its financial position, results of operations or liquidity. There was no outstanding litigation as of June 30, 2021 other than that described above.
Government Claim
On May 9, 2018, the U.S. Department of Justice filed an injunctive action, specifically United States of America v. U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., Kristin C. Comella, and Theodore Gradel. The Complaint alleges, among other matters that the defendants manufacture “stromal vascular fraction” (SVF) products from patient adipose (fat) tissue, which the companies then market as stem cell-based treatments, and which U.S. Stem Cell Clinic, LLC administers to patients, without first obtaining what the government alleges are necessary FDA approvals. Although Theodore Gradel was initially listed as a defendant, he subsequently entered into a consent agreement and is no longer party to this case.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
The U.S. and the defendants filed cross motions for summary judgment, each asking for a ruling in its favor. On June 3, 2019, the Court entered an order granting Summary Judgment for the government and denying the defendants’ motion for summary judgment. The order focused on the defendants’ actions in providing and marketing SVF therapy. In an order dated June 4, 2019, the Court granted the defendants’ request to allow it the opportunity to work out the language of the form of injunction with the government, and if unsuccessful, to provide a status report to the Court by June 14, 2019, outlining areas of disagreement. The Court further ordered that the defendants (U.S. Stem Clinic, LLC, U.S. Stem Cell, Inc., and Kristin C. Comella) ‘not sell, provide or otherwise engage in any SVF therapy or any other activities to be regulated by the FDA as explained in the Court’s Order on the Parties’ Motions for Summary Judgment.” On June 25, 2019, the Court entered an Order of Permanent Injunction, generally enjoining the defendants with respect to the SVF Product and requiring other actions. The Company filed an appeal on August 23, 2019 and attended oral argument on January 13, 2021. On June 2, 2021, the Eleventh Circuit Court ruled to affirm lower courts’ judgement. The Company did not challenge the district court’s judgment upon any other ground. The Company is not able to predict the duration, scope, results, or consequences of the U.S. Department of Justice actions and final rulings and management is assessing its options on a going forward basis. The Company, in divesting certain equipment and other assets and assigning its lease, has and will continue to experience a decrease in revenues as the Company both maintains the remainder of the business and transitions into similar or unrelated business opportunities as determined by management. However, management is not able to predict the duration, scope, results, or consequences of the summary judgment and any transition of the business plan.
Since the Court’s issuance of the Order of Permanent Injunction, the Company has received demand letters for compensation from persons who store their SVF Product and/or other tissue product with the tissue bank (several of the persons have requested refunds of the monies paid to the tissue bank and one person has requested a full refund of monies paid to an altogether separate company due to her not receiving the full amount of treatments she requested; such requests for compensation, to date, have not been material) and requests that the Company preserve cells in the Company’s possession. The Company sought guidance from the Court, which entered an order generally staying the requirement to destroy any SVF Product, pending a decision on the Company’s appeal.
NOTE 12 — STOCKHOLDERS’ DEFICIT
Common Stock
During the six months ended June 30, 2021, the Company issued an aggregate of 4,659,380 shares of its common stock, having a fair value of $213,058, in settlement of outstanding accounts payable of $40,000 and accrued interest of $9,145. In connection with the issuances, the Company incurred a $163,914 net loss on settlement.
During the six months ended June 30, 2021, the Company issued an aggregate of 4,000,000 shares of its common stock, having a fair value of $128,000, for services, of which $12,089 remains in prepaid expenses as of June 30, 2021.
On March 23, 2021, a convertible note with a face value of $200,000, having a net book value of $54,887 at the date of conversion, was converted into 7,518,797 shares of the Company’s common stock, having a fair value of $375,940, resulting in a loss on conversion of $321,053 (See Note 9 and Note 10).
Stock Options
On April 1, 2013, the Board of Directors approved, subject to subsequently received stockholder approval, the establishment of the Bioheart 2013 Omnibus Equity Compensation Plan, or the “2013 Omnibus Plan” (replacing the 1999 Officers and Employees Stock Option Plan, or the Employee Plan, and the 1999 Directors and Consultants Stock Option Plan). The 2013 Omnibus Plan initially reserved up to fifty thousand (50,000) shares of common stock for issuance. On August 4, 2014, the Board of Directors approved to set the reserve to one hundred thousand (100,000) shares of common stock for issuance and to close the 1999 Officers and Employees Stock Option Plan. On February 2, 2015, at the annual meeting of stockholders, the 2013 Omnibus Equity Compensation Plan was approved.
On November 2, 2015, the Company increased the shares reserved under the 2013 Omnibus Plan to five hundred million (500,000,000) shares of common stock for issuance. Effective September 16, 2016, the Company approved an additional twenty five million (25,000,000) shares of common stock to the reserve; effective April 21, 2017, the Company approved an additional twenty five million (25,000,000) shares of common stock to the reserve; effective August 7, 2017, the Company approved an additional thirty million (30,000,000) shares of common stock to the reserve; and effective May 7, 2018, the Company approved an addition of one hundred million (100,000,000) shares of common stock to reserve.
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
A summary of the stock option activity for the six months ended June 30, 2021 is as follows:
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Average
|
|
|
|
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining Life
|
|
|
Intrinsic
|
|
|
|
|
Options
|
|
|
Price
|
|
|
In Years
|
|
|
Value
|
|
|
Outstanding, December 31, 2020
|
|
|
111,119,914
|
|
|
$
|
0.0247
|
|
|
|
7.2
|
|
|
$
|
296,636
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited/Expired
|
|
|
(475,000
|
)
|
|
$
|
0.0296
|
|
|
|
|
|
|
|
|
|
|
Outstanding, June 30, 2021
|
|
|
110,644,914
|
|
|
$
|
0.0247
|
|
|
|
6.8
|
|
|
$
|
429,606
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, June 30, 2021
|
|
|
83,162,414
|
|
|
$
|
0.0264
|
|
|
|
6.5
|
|
|
$
|
257,775
|
|
|
Options Outstanding
|
|
|
Options Exercisable
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Outstanding
|
|
|
Average
|
|
|
Average
|
|
|
Exercisable
|
|
|
Average
|
|
|
Exercise
|
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining Life
|
|
|
Number of
|
|
|
Exercise
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
In Years
|
|
|
Options
|
|
|
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.004 to $0.010
|
|
|
|
41,800,000
|
|
|
$
|
0.0051
|
|
|
|
7.5
|
|
|
|
24,325,000
|
|
|
$
|
0.0048
|
|
|
$0.011 to $0.020
|
|
|
|
16,250,000
|
|
|
$
|
0.0196
|
|
|
|
5.2
|
|
|
|
16,250,000
|
|
|
$
|
0.0196
|
|
|
$0.021 to $0.030
|
|
|
|
9,510,000
|
|
|
$
|
0.0252
|
|
|
|
7.4
|
|
|
|
8,505,000
|
|
|
$
|
0.0252
|
|
|
$0.0363
|
|
|
|
22,635,000
|
|
|
$
|
0.0363
|
|
|
|
6.1
|
|
|
|
18,632,500
|
|
|
$
|
0.0363
|
|
|
$0.0536
|
|
|
|
20,000,000
|
|
|
$
|
0.0536
|
|
|
|
6.9
|
|
|
|
15,000,000
|
|
|
$
|
0.0536
|
|
|
$0.1540
|
|
|
|
449,914
|
|
|
$
|
0.1540
|
|
|
|
4.3
|
|
|
|
449,914
|
|
|
$
|
0.1540
|
|
|
|
|
|
|
110,644,914
|
|
|
$
|
0.0247
|
|
|
|
6.8
|
|
|
|
83,162,414
|
|
|
$
|
0.0264
|
|
The aggregate intrinsic value of outstanding stock options was $429,606, based on options with an exercise price less than the Company’s stock price of $0.0154 as of June 30, 2021, which would have been received by the option holders had those option holders exercised their options as of that date.
The fair value of all options that vested during the six months ended June 30, 2021 and 2020 was $315,724 and $350,416, respectively. As of June 30, 2021, the Company had $413,889 of total unrecognized compensation cost related to non-vested awards granted under the 2013 Omnibus Plan, which the Company expects to recognize over a weighted average period of 0.66 years.
Warrants
A summary of the warrant activity for the six months ended June 30, 2021 is as follows:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
|
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Life
|
|
|
Intrinsic
|
|
|
|
|
Warrants
|
|
|
Price
|
|
|
In Years
|
|
|
Value
|
|
|
Outstanding, December 31, 2020
|
|
|
1,110,468
|
|
|
$
|
12.84
|
|
|
|
7.1
|
|
|
$
|
-
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, June 30, 2021
|
|
|
1,110,468
|
|
|
$
|
12.84
|
|
|
|
6.7
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, June 30, 2021
|
|
|
1,108,923
|
|
|
$
|
2.14
|
|
|
|
6.7
|
|
|
$
|
-
|
|
U.S. STEM CELL, INC.
NOTES TO FINANCIAL STATEMENTS
JUNE 30, 2021
|
Warrants Outstanding
|
|
|
Warrants Exercisable
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
Outstanding
|
|
|
Average
|
|
|
Average
|
|
|
Exercisable
|
|
|
Average
|
|
|
Exercise
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining Life
|
|
|
Number of
|
|
|
Exercise
|
|
|
Price
|
|
Warrants
|
|
|
Price
|
|
|
In Years
|
|
|
Warrants
|
|
|
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.03 to $20.00
|
|
|
1,086,536
|
|
|
$
|
1.27
|
|
|
|
6.7
|
|
|
|
1,086,536
|
|
|
$
|
1.27
|
|
|
$20.01 to $30.00
|
|
|
19,543
|
|
|
$
|
25.06
|
|
|
|
2.7
|
|
|
|
19,543
|
|
|
$
|
25.06
|
|
|
$40.01 to $50.00
|
|
|
2,253
|
|
|
$
|
48.83
|
|
|
|
1.3
|
|
|
|
2,253
|
|
|
$
|
48.83
|
|
|
$50.01 to $60.00
|
|
|
543
|
|
|
$
|
60.00
|
|
|
|
0.1
|
|
|
|
543
|
|
|
$
|
60.00
|
|
|
>$60.00
|
|
|
1,593
|
|
|
$
|
7,690.00
|
|
|
|
5.3
|
|
|
|
48
|
|
|
$
|
7,690.00
|
|
|
|
|
|
1,110,468
|
|
|
$
|
12.84
|
|
|
|
6.7
|
|
|
|
1,108,923
|
|
|
$
|
2.14
|
|
The aggregate intrinsic value of the issued and exercisable warrants of $0 represents the total pretax intrinsic value, based on warrants with an exercise price less than the Company’s stock price of $0.0154 as of June 30, 2021, which would have been received by the warrant holders had those warrants holders exercised their warrants as of that date.
NOTE 13 — CONCENTRATIONS
Concentrations of Credit Risk
The Company’s financial instruments that are exposed to a concentration of credit risk are cash and accounts receivable. Generally, the Company’s cash and cash equivalents in interest-bearing accounts does not exceed FDIC insurance limits. The financial stability of these institutions is periodically reviewed by senior management.
Concentrations of Revenues
For the three and six months ended June 30, 2021 and 2020, the following customers accounted for more than 10% of the Company’s net revenues:
|
|
|
For the Three Months Ended June 30,
|
|
|
For the Six Months Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Customer 1
|
|
|
21
|
%
|
|
|
-
|
|
|
|
11
|
%
|
|
|
-
|
|
|
Customer 2
|
|
|
17
|
%
|
|
|
25
|
%
|
|
|
-
|
|
|
|
10
|
%
|
|
Customer 3
|
|
|
11
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Customer 4
|
|
|
10
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
19
|
%
|
|
Customer 5
|
|
|
-
|
|
|
|
-
|
|
|
|
33
|
%
|
|
|
-
|
|
|
Customer 6
|
|
|
-
|
|
|
|
20
|
%
|
|
|
-
|
|
|
|
-
|
|
|
Customer 7
|
|
|
-
|
|
|
|
10
|
%
|
|
|
-
|
|
|
|
-
|
|
|
Totals
|
|
|
59
|
%
|
|
|
55
|
%
|
|
|
44
|
%
|
|
|
29
|
%
|
Concentrations of Accounts Receivable
As of June 30, 2021 and December 31, 2020, the following customers represented more than 10% of the Company’s accounts receivable:
|
|
|
June 30,
|
|
|
December 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Customer 1
|
|
|
69
|
%
|
|
|
44
|
%
|
|
Customer 2
|
|
|
26
|
%
|
|
|
50
|
%
|
|
Totals
|
|
|
95
|
%
|
|
|
94
|
%
|
Customer 2 is U.S. Stem Cell Clinic, LLC, a related party, an investment in which the Company held a 49.9% member interest through February 10, 2021 (See Note 5).
NOTE 14 — SUBSEQUENT EVENTS
On July 1, 2021, the Company issued an aggregate of 674,182 shares of common stock for services rendered.
On August 4, 2021, a promissory note dated February 5, 2020 (see Note 9, Convertible Note Payable) was fully converted to 3,804,348 shares of Common Stock.
US Stem Cell (CE) (USOTC:USRM)
Historical Stock Chart
From Aug 2024 to Sep 2024
US Stem Cell (CE) (USOTC:USRM)
Historical Stock Chart
From Sep 2023 to Sep 2024