Registration No._______
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NORTHWEST BIOTHERAPEUTICS, INC.
(Exact name of Registrant as specified in its charter)
|
Delaware
|
|
8731
|
|
94-3306718
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer Identification Number)
|
4800 Montgomery Lane, Suite 800
Bethesda, Maryland 20814
(240) 497-9024
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Linda F. Powers
Chief Executive Officer
Northwest Biotherapeutics, Inc.
4800 Montgomery Lane, Suite 800
Bethesda, Maryland 20814
(240) 497-9024
Copies of notices and other communications should be sent to:
Peter Campitiello, Esq.
Tarter Krinsky & Drogin LLP
1350 Broadway
New York, New York 10018
Tel: 212-216-8085
Fax: 212-216-8001
Approximate date of commencement of proposed sale to the public: From time to time after this Registration Statement is declared effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, please check the following box:
x
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
¨
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
¨
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box:
¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer
¨
|
|
Accelerated filer
¨
|
|
Non-accelerated filer
¨
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
x
|
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of
Securities to be Registered
|
|
Amount to be
Registered
|
|
|
Proposed
Maximum
Offering
Price Per
Share (1)
|
|
|
Proposed
Maximum
Aggregate
Offering Price (2)
|
|
|
Amount of
Registration Fee
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock, $0.001 par value per share, issuable pursuant to the Purchase Agreement
|
|
|
(1)7,000,000
|
|
|
$
|
0.39
|
|
|
$
|
2,730,000
|
|
|
$
|
312.86
|
|
|
TOTAL (1)
|
|
|
7,000,000
|
|
|
$
|
0.39
|
|
|
$
|
2,730,000
|
|
|
$
|
312.86
|
|
|
|
(1)
|
We are registering shares of our common stock (the “Put Shares”) that we may put to Four M Purchasers, LLC (“Four M Purchasers” or “Selling Stockholder”) pursuant to a Purchase Agreement (the “Purchase Agreement”) between the Selling Stockholder and the Registrant, entered into as of November 14, 2011. In the event of stock splits, stock dividends, or similar transactions involving the common stock, the number of common shares registered shall, unless otherwise expressly provided, automatically be deemed to cover the additional securities to be offered or issued pursuant to Rule 416 promulgated under the Securities Act of 1933, as amended (the “Securities Act”). In the event that adjustment provisions of the Purchase Agreement require the Registrant to issue more shares than are being registered in this registration statement, for reasons other than those stated in Rule 416 of the Securities Act, the Registrant will file a new registration statement to register those additional shares.
|
|
|
(2)
|
Estimated solely for purposes of calculating the registration fee under Rule 457 under the Securities Act, using the last closing price as reported on the Over-the-Counter Bulletin Board (the “OTCBB”) on January 9, 2012, which was $0.39 per share
|
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. THE SELLING STOCKHOLDER MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT, OF WHICH THIS PROSPECTUS FORMS A PART, FILED WITH THE SECURITIES AND EXCHANGE COMMISSION, IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
In this Prospectus references to “Northwest Biotherapeutics,” the “Company,” “we,” “us,” and “our” refer to Northwest Biotherapeutics, Inc. and its subsidiary.
PRELIMINARY PROSPECTUS SUBJECT TO COMPLETION DATED JANUARY 10, 2012
NORTHWEST BIOTHERAPEUTICS, INC.
7,000,000 Shares of Common Stock, par value $0.001
This Prospectus relates to resales by selling stockholder Four M Purchasers, LLC, a Delaware limited liability company (“Selling Stockholder”) of up to
7,000,000
shares (the “Put Shares”) of our common stock, par value $0.001 per share (the “Common Stock”), which are put shares that we will put to the Selling Stockholder pursuant to the Purchase Agreement dated November 14, 2011, by and between the Selling Stockholder and us (the “Purchase Agreement”).
Under the Purchase Agreement, we will notify the Selling Stockholder from time to time of our intention to sell a specified amount of Put Shares to the Selling Stockholder pursuant to the Purchase Agreement. The Selling Stockholder will then purchase such Put Shares from us on, and to the extent of, the terms and conditions set forth in the Purchase Agreement. The maximum aggregate amount of Put Shares that we may sell to the Selling Stockholder through this put arrangement, if all conditions of the Purchase Agreement are satisfied, will be an aggregate of $2.5 million of such Put Shares over a period of up to six (6) months following the effective date of this Registration Statement. For more information about the Selling Stockholder, please see the section of this prospectus entitled “Selling Stockholder”.
We will not receive any proceeds directly from the resales of the Put Shares by the Selling Stockholder. We will, however, receive proceeds from the sale of our Put Shares to the Selling Stockholder pursuant to the Purchase Agreement as described above. When we put an amount of Put Shares to the Selling Stockholder, the price per share that the Selling Stockholder will pay to us will be determined in accordance with a formula set forth in the Purchase Agreement. Generally, the price per share will be determined by a backward-looking, fixed price formula of ninety-five percent (95%) of the average of the three (3) lowest closing bid prices of our Common Stock during the five (5) trading days prior to the notification date.
The total amount of Put Shares which may be sold pursuant to this prospectus and registration statement would constitute approximately 4.7% of our issued and outstanding Common Stock as of January 10, 2012, if all of the shares had been sold by that date.
The Selling Stockholder may sell the Put Shares from time to time at the prevailing market price on the Over-the Counter Bulletin Board (“OTCBB”), or on an exchange if our shares of Common Stock become listed for trading on such an exchange, or in negotiated transactions on such terms and conditions as may be acceptable to investors purchasing the Put Shares in such resale transactions (including with respect to any price discounts and/or warrants). The Selling Stockholder is an "underwriter" within the meaning of the Securities Act of 1933, as amended (the “Securities Act”) in connection with the resale of our Common Stock under the Purchase Agreement. No other underwriter or person has been engaged, to date, to facilitate the sale of shares of our common stock in this offering; however, other parties may be so engaged during the term of the Purchase Agreement. This offering will terminate six (6) months after the registration statement to which this prospectus is made a part is declared effective by the SEC. For more information, please see the section of this prospectus entitled "Plan of Distribution."
Our Common Stock is registered under Section 12(g) of the United States Securities Exchange Act of 1934, as amended (the "Exchange Act"), and is quoted on the FINRA OTCBB under the symbol “NWBO.OB”. The last reported sale price per share of our Common Stock as reported by the OTCBB on January 9, 2012, was $0.39. These prices will fluctuate based on the demand for our Common Stock.
We will be responsible for all fees and expenses incurred in connection with the preparation and filing of this registration statement, and the implementation of the transactions contemplated herein, including, without limitation, any price discounts and/or warrants provided to investors who purchase the Put Shares, and any underwriters' discounts or commissions and other fees and expenses relating to the re-sale of the Put Shares by the Selling Stockholder.
THE PURCHASE OF THE SECURITIES OFFERED THROUGH THIS PROSPECTUS INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD INVEST IN OUR COMMON STOCK ONLY IF YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. YOU SHOULD CAREFULLY READ AND CONSIDER THE SECTION OF THIS PROSPECTUS TITLED "RISK FACTORS" BEGINNING ON PAGE [6] BEFORE BUYING ANY SHARES OF OUR COMMON STOCK.
THE SECURITIES AND EXCHANGE COMMISSION AND STATE SECURITIES REGULATORS HAVE NOT APPROVED OR DISAPPROVED OF THESE SECURITIES, OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this Prospectus is January 10, 2012.
TABLE OF CONTENTS
|
|
|
Page
|
|
Part I - Information Required in Prospectus
|
|
|
|
Forward-Looking Statements
|
|
1
|
|
Prospectus Summary
|
|
2
|
|
Risk Factors
|
|
6
|
|
Risk Factors Relating to this Offering
|
|
6
|
|
Risk Factors Relating to Operation of Northwest Biotherapeutics, Inc.
|
|
9
|
|
Risk Factors Relating to the Securities Markets and Investments in Our Common Stock
|
|
15
|
|
Use of Proceeds
|
|
18
|
|
Determination of Offering Price
|
|
19
|
|
Dilution
|
|
19
|
|
The Selling Stockholder
|
|
19
|
|
The Purchase Agreement
|
|
20
|
|
The Offering
|
|
20
|
|
Plan of Distribution
|
|
22
|
|
Description of Securities to be Registered
|
|
25
|
|
Description of Business
|
|
26
|
|
Interest of Named Experts and Counsel
|
|
43
|
|
Description of Property
|
|
43
|
|
Legal Proceedings
|
|
44
|
|
Market for Common Equity and Related Matters
|
|
44
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
46
|
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures
|
|
52
|
|
Directors, Executive Officers, Promoters and Control Persons
|
|
52
|
|
Executive Compensation
|
|
55
|
|
Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
|
|
63
|
|
Certain Relationships and Party Related Party Transactions
|
|
66
|
|
Part II - Information Not Required in Prospectus
|
|
|
|
Other Expenses of Issuance and Distribution
|
|
69
|
|
Indemnification of Directors, Officers, Employees, and Agents
|
|
70
|
|
Recent Sales of Unregistered Securities
|
|
70
|
|
Where You Can Find More Information
|
|
76
|
|
Exhibits
|
|
77
|
|
Undertakings
|
|
84
|
|
Signatures
|
|
85
|
|
Financial Statements
|
|
F-1
|
|
FORWARD-LOOKING STATEMENTS
This Prospectus contains forward-looking statements. Such forward-looking statements include statements regarding, among other things (a) our business development plans, (b) anticipated trends in our industry, (c) our future financing plans and (d) our anticipated needs for working capital. Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may”, “will”, “should”, “expect”, “anticipate”, “estimate”, “believe”, “intend” or “project” or the negative of these words or other variations on these words or comparable terminology. This information may involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from the future results, performance, or achievements expressed or implied by any forward-looking statements or otherwise. These statements may be found under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business” as well as in this Prospectus generally. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this Prospectus generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this filing will in fact occur. In addition to the information expressly required to be included in this filing, we will provide such further material information, if any, as may be necessary to make the required statements, in light of the circumstances under which they are made, not misleading.
|
PROSPECTUS SUMMARY
This Prospectus is part of a registration statement we filed with the SEC. You should rely only on the information provided in this Prospectus and incorporated by reference in this Prospectus. We have not authorized anyone to provide you with information different from that contained in or incorporated by reference into this Prospectus. This Prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities other than the Common Stock offered by this Prospectus. This Prospectus does not constitute an offer to sell or a solicitation of an offer to buy any common stock in any circumstances in which such offer or solicitation is unlawful. The Selling Stockholder is offering to sell, and seeking offers to buy, shares of our Common Stock only in jurisdictions where offers and sales are permitted.
Neither the delivery of this Prospectus nor any sale made in connection with this Prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this Prospectus or that the information contained by reference to this Prospectus is correct as of any time after its date. The information in this Prospectus is accurate only as of the date of this Prospectus, regardless of the time of delivery of this Prospectus or of any sale of common stock. The rules of the SEC may require us to update this Prospectus in the future.
The following summary highlights selected information contained in this Prospectus. This summary does not contain all the information you should consider before investing in the securities. Before making an investment decision, you should read the entire Prospectus carefully, including the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the financial statements and the notes to the financial statements included in this Prospectus, and you should conduct due diligence analysis of the Company.
The Offering
|
Common Stock Being Offered By Selling Stockholder
|
|
7,000,000 shares of Common Stock issuable to Four M Purchasers, LLC pursuant to the Purchase Agreement with us dated November 14, 2011 (the “Purchase Agreement”).
|
|
|
|
|
|
Offering Price
|
|
The offering price for shares of our Common Stock will be determined by prevailing prices established on the OTCBB, or as negotiated in private transactions, or as otherwise described in the “Plan of Distribution.”
|
|
|
|
|
|
Terms of the Offering
|
|
The Selling Stockholder will determine when and how it will sell the Put Shares of Common Stock offered in this Prospectus.
|
|
|
|
|
|
Termination of the Offering
|
|
The offering will conclude upon the earlier of (i) such time as all of the Common Stock contemplated in this Registration Statement has been sold, or (ii) six (6) months after the effective date of this Registration Statement.
|
|
|
|
|
|
Use of Proceeds
|
|
We are not selling any of the Put Shares of Common Stock in this offering and, as a result, will not receive any proceeds directly from this offering. We will, however, receive proceeds from our sale of those Put Shares to the Selling Stockholder pursuant to the Purchase Agreement. Such proceeds will be used for working capital, reduction of indebtedness, acquisitions and general corporate purposes.
|
|
|
|
|
|
OTCBB Trading Symbol
|
|
“NWBO.OB”
|
|
|
|
|
|
Risk Factors
|
|
The Common Stock offered hereby involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investment. See “Risk Factors” beginning on page [6].
|
Summary of Our Financial Information
The summary financial data set forth below as of December 31, 2009 and 2010 and September 30, 2011 and for the periods ending December 31, 2009 and 2010 and September 30, 2010 and 2011 are derived from our financial statements included elsewhere in this prospectus. The summary financial data should be read in conjunction with our audited consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and results of Operations” included elsewhere in this prospectus.
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
Condensed Consolidated Statements of Operations
(in thousands, except per share data)
(Unaudited)
|
|
|
Three months ended
|
|
|
Nine months ended
|
|
|
Period from
March 18,
1996
(Inception) to
|
|
|
|
|
September 30
|
|
|
September 30
|
|
|
September 30,
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2010
|
|
|
2011
|
|
|
2011
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research material sales
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
580
|
|
|
Contract research and development from related parties
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,128
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research grants and other
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,061
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
10
|
|
|
|
10
|
|
|
|
10
|
|
|
|
10
|
|
|
|
2,769
|
|
|
Operating cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of research material sales
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
382
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
1,606
|
|
|
|
3,565
|
|
|
|
4,791
|
|
|
|
11,474
|
|
|
|
88,286
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administration
|
|
|
1,412
|
|
|
|
2,804
|
|
|
|
4,680
|
|
|
|
10,675
|
|
|
|
72,664
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
2
|
|
|
|
2
|
|
|
|
2
|
|
|
|
6
|
|
|
|
2,359
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss on facility sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
895
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Asset impairment loss and other (gain) loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,445
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating costs and expenses
|
|
|
3,020
|
|
|
|
6,371
|
|
|
|
9,473
|
|
|
|
22,155
|
|
|
|
167,031
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(3,010
|
)
|
|
|
(6,361
|
)
|
|
|
(9,463
|
)
|
|
|
(22,145
|
)
|
|
|
(164,262
|
)
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Valuation of reclassified equity contracts
|
|
|
-
|
|
|
|
8,875
|
|
|
|
-
|
|
|
|
7,413
|
|
|
|
14,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loan conversion inducement
|
|
|
|
|
|
|
-
|
|
|
|
(4,522
|
)
|
|
|
(125
|
)
|
|
|
(10,415
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative valuation gain (loss)
|
|
|
-
|
|
|
|
338
|
|
|
|
-
|
|
|
|
29
|
|
|
|
83
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on sale of intellectual property and property and equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,664
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(2,371
|
)
|
|
|
(2,370
|
)
|
|
|
(5,968
|
)
|
|
|
(5,426
|
)
|
|
|
(39,342
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income and other
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,707
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(5,381
|
)
|
|
|
482
|
|
|
|
(19,953
|
)
|
|
|
(20,254
|
)
|
|
|
(194,393
|
)
|
|
Issuance of common stock in connection with elimination of Series A and Series A-1 preferred stock preferences
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(12,349
|
)
|
|
Modification of Series A preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,306
|
)
|
|
Modification of Series A-1 preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(16,393
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(334
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(917
|
)
|
|
Warrants issued on Series A and Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,664
|
)
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,872
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A preferred stock redemption fee
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,700
|
)
|
|
Beneficial conversion feature of Series D preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,274
|
)
|
|
Net income (loss) applicable to common stockholders
|
|
$
|
(5,381
|
)
|
|
$
|
482
|
|
|
$
|
(19,953
|
)
|
|
$
|
(20,254
|
)
|
|
$
|
(239,202
|
)
|
|
Net income (loss) per share applicable to common stockholders — basic
|
|
$
|
(0.08
|
)
|
|
$
|
0.01
|
|
|
$
|
(0.31
|
)
|
|
$
|
(0.24
|
)
|
|
|
|
|
|
Weighted average shares used in computing basic income (loss) per share
|
|
|
70,413
|
|
|
|
95,123
|
|
|
|
65,361
|
|
|
|
85,680
|
|
|
|
|
|
|
Net income (loss) per share applicable to common stockholders - diluted
|
|
$
|
(0.08
|
)
|
|
$
|
0.00
|
|
|
$
|
(0.31
|
)
|
|
$
|
(0.24
|
)
|
|
|
|
|
|
Weighted average shares used in computing diluted net income (loss) per share
|
|
|
70,413
|
|
|
|
123,136
|
|
|
|
65,361
|
|
|
|
85,680
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
As of September 30,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
2011
|
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
65
|
|
|
$
|
153
|
|
|
$
|
1,643
|
|
|
Working capital (deficit)
|
|
|
(19,329
|
)
|
|
|
(25,885
|
)
|
|
|
(44,332
|
)
|
|
Total Assets
|
|
|
103
|
|
|
|
294
|
|
|
|
1,893
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long term obligations, net of current portion and discounts
|
|
|
1,359
|
|
|
|
1,854
|
|
|
|
1,898
|
|
|
Total stockholders' equity (deficit)
|
|
|
(20,686
|
)
|
|
|
( 27,684
|
)
|
|
|
(46,150
|
)
|
RISK FACTORS
Our business, financial condition, operating results and prospects are subject to the following material risks. Additional risks and uncertainties not presently foreseeable to us may also impair our business operations. If any of the following risks actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, the trading price of our common stock could decline, and our stockholders may lose all or part of their investment in the shares of our Common Stock.
Risks Related to this Offering
There may not be an active, liquid trading market for our Common Stock.
Our Common Stock is currently listed on the Over-The-Counter Bulletin Board, or OTCBB, which is generally recognized as being a less active market than NASDAQ, the stock exchange on which our Common Stock originally was listed. Also, the pool of potential investors who may buy and sell on the OTCBB is limited. Many institutional investors have policies which preclude them from doing so. You may not be able to sell your shares at the time desired or at the price desired. There may be significant consequences associated with our stock trading on the OTCBB rather than a national exchange. The effects of not being able to list our securities on a national exchange include:
|
|
•
|
limited dissemination of the market price of our securities;
|
|
|
•
|
limited interest by investors in our securities;
|
|
|
•
|
volatility of our stock price due to low trading volume;
|
|
|
•
|
increased difficulty in selling our securities in certain states due to “blue sky” restrictions; and
|
|
|
•
|
limited ability to issue additional securities or to secure additional financing.
|
The market for our Common
Stock may be limited, because our Common Stock is subject to “penny stock” rules.
Our Common Stock is subject to the SEC’s “penny stock” rules. As a result, broker-dealers may experience difficulty in completing customer transactions, and trading activity in our securities may be adversely affected. Under the “penny stock” rules promulgated under the Exchange Act, broker-dealers who recommend such securities to persons other than institutional accredited investors must:
|
|
•
|
make a special written suitability determination for the purchaser;
|
|
|
•
|
receive the purchaser’s written agreement to a transaction prior to sale;
|
|
|
•
|
provide the purchaser with risk disclosure documents which identify certain risks associated with investing in “penny stocks” and which describe the market for these “penny stocks” as well as a purchaser’s legal remedies; and
|
|
|
•
|
obtain a signed and dated acknowledgment from the purchaser demonstrating that the purchaser has actually received the required risk disclosure document before a transaction in a “penny stock” can be completed.
|
As a result of these rules, broker-dealers may find it difficult to effectuate customer transactions, and trading activity in our Common Stock may be adversely affected. As a result, the market price of our Common Stock may be depressed, and stockholders may find it more difficult to sell our Common Stock.
Your ability to sell your shares in the secondary trading market may be limited, because our Common Stock is quoted on the “OTCBB.”
Our Common Stock is currently quoted on the over-the-counter market on the OTCBB, as described above. Consequently, the liquidity of our Common Stock is quite limited, not only in regard to the number of shares that are bought and sold, but also through delays in the timing of transactions, and lack of coverage by security analysts and the news media of our Company. As a result, prices for shares of our Common Stock may be lower than might otherwise be the case if our Common Stock were quoted and traded on NASDAQ or a national securities exchange.
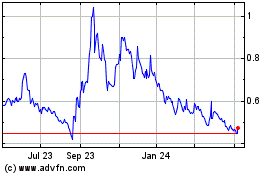
The price of our Common Stock may be highly volatile.
The share prices of publicly traded biotechnology and emerging pharmaceutical companies, particularly companies without consistent product revenues and earnings, can be highly volatile and are likely to remain highly volatile in the future. The price which investors may realize in sales of their shares of our Common Stock may be materially different than the price at which our Common Stock is quoted, and will be influenced by a large number of factors, some specific to us and our operations, and some unrelated to our operations. Such factors may cause the price of our stock to fluctuate frequently and substantially. Such factors may include large purchases or sales of our Common Stock, positive or negative events relating to other companies developing immune therapies for cancer, positive or negative events relating to healthcare and the overall pharmaceutical and biotech sector, currency fluctuations, legislative or regulatory changes, and/or general economic conditions. In the past, shareholder class action litigation has been brought against other companies that experienced volatility in the market price of their shares. Whether or not meritorious, litigation brought against a company following fluctuations in the trading price of its common stock can result in substantial costs, divert management’s attention and resources, and harm the company’s financial condition and results of operations.
Toucan Capital and its affiliates are the principal holders of our shares of
Common Stock, and this concentration of ownership may have a negative effect on the market price of our Common Stock.
As of September 30, 2011, Toucan Capital and its affiliates (including Cognate BioServices and Ms. Linda Powers, who also serves as our Chief Executive Officer and Chairperson of the Board of Directors), collectively beneficially owned an aggregate of 22,617,015 shares of our Common Stock, representing approximately 23.3 percent of our issued and outstanding Common Stock. In addition, as of September 30, 2011, Toucan Capital and its affiliates hold warrants that are exercisable for an aggregate of approximately 41,426,983 shares of our Common Stock. This concentration of ownership may adversely affect the trading price of our common stock because investors may perceive disadvantages in owning stock of companies with controlling stockholders. Toucan Capital and its affiliates have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors and any proposed merger, consolidation or sale of all or substantially all of our assets. This influence could have the effect of delaying, deferring or preventing a change in control, or impeding a merger or consolidation, takeover or other business combination that could be favorable to investors.
We do not intend to pay any cash dividends in the foreseeable future and, therefore, any return on your investment in our Common Stock must come from increases in the market price of our Common Stock.
We have not paid any cash dividends on our Common Stock to date in the Company’s history, and we do not intend to pay cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings, if any, for reinvestment in the development and expansion of our business. Also, any credit agreements which we may enter into with institutional lenders may restrict our ability to pay dividends. Therefore, any return on your investment in our capital stock must come from increases in the fair market value and trading price of our Common Stock.
Our ongoing operations will require substantial ongoing funding through equity and/or debt issuances. This may have a negative effect on the market price of our Common Stock, and will dilute existing share ownership.
Clinical trials are very expensive (especially when they involve a large numbers of patients and trial sites) and require substantial funding throughout their execution. We currently have a large, 240-patient clinical trial in brain cancer under way, and it involves many trial sites. We also have plans for additional clinical trials with other cancers. The initiation, execution and completion (if successful) of such trials is how biotech companies like ours move their products towards commercialization and build company value. At the same time, such operations require substantial amounts of ongoing funding throughout their execution. Such funding must be obtained through issuance of equity and/or incurring debt (which is usually convertible debt, convertible into equity at the investor’s option). Accordingly, we will have to obtain substantial ongoing funding throughout the execution of our clinical trials through the issuance of substantial additional equity and/or incurring substantial additional debt. This may have a negative effect on the market price of our Common Stock, and it will dilute existing share ownership.
The Purchase Agreement overall will involve registration and sale of a significant amount of our Common Stock, over a period of up to six (6) months. This may have a negative effect on the market price of our Common Stock, and will dilute existing share ownership.
Under the Purchase Agreement, we may sell up to an aggregate of $2.5 million of our Common Stock to the Selling Stockholder during the six (6) month period after this registration statement becomes effective. The actual number of shares which we may end up selling is unknown at present, as we do not yet know how much of that capacity we will choose to use, nor the timing of when we will choose to use it, nor the market price of our stock at the various times we choose to use it. However, the number of shares that we will sell under the Purchase Agreement, and that the Selling Stockholder will, in turn, re-sell in the market, is likely to be substantial. As with any small biotech company stock, our Common Stock may experience negative effects from the sale of additional stock during the course of the clinical trials, and such additional stock will dilute existing share ownership.
This registration statement is registering the first 7,000,000 shares of our Common Stock for re-sale by the Selling Stockholder pursuant to the Purchase Agreement. Such re-sale may have a negative effect on the market price of our Common Stock, and will dilute existing share ownership.
Pursuant to this registration statement, of which this Prospectus forms a part, we are registering 7,000,000 shares of our Common Stock. These shares comprise the first tranche of the total potential shares to be registered and sold pursuant to the Purchase Agreement, as described above. This first tranche of shares constitutes a substantial amount, relative to our total issued and outstanding shares at present. The sale of these shares may have a negative effect on the market price of our Common Stock, and will dilute existing share ownership.
Substantial amounts of our previously issued Common Stock are now and/or will soon be eligible for re-sale under Rule 144. This may have a negative effect on the market price of our Common Stock.
In general, under Rule 144, a person (or persons whose shares are aggregated) who has satisfied a six- month holding period may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale. In addition, under certain circumstances Rule 144 also permits the sale of securities, without any limitation, by a person who is not an affiliate of the Company (as such term is defined in Rule 144(a)(1)), and who has satisfied a one-year holding period.
As of September 30, 2011, approximately 43,727,329 shares of our Common Stock were previously issued as restricted securities as defined under Rule 144 of the Securities Act of 1933, as amended (the “Act”) and are outstanding. Of these, approximately 43,727,329 shares of such restricted stock have been outstanding for more than six (6) months and some or all of these shares may be resold without registration pursuant to Rule 144. If substantial amounts of such shares are sold pursuant to Rule 144, this may have a negative effect on the market price of our Common Stock.
The Selling Stockholder will pay less than the then-quoted market price for our Common Stock, under the formula specified in the Purchase Agreement, and this could have a negative effect on the market price of our Common Stock.
As is generally the case in stock sale arrangements of the type established in the Purchase Agreement, the Put Shares of Common Stock that we will put to the Selling Stockholder will be purchased by the Selling Stockholder at a discount price. In our case, the discount price will be equal to a formula specified in the Purchase Agreement: the price will be ninety-five percent (95%) of the average of the three (3) lowest closing bid prices of our Common Stock during the five (5) trading days prior date on which the Company delivers the Put Notice to the Selling Stockholder under the Purchase Agreement. To the extent that we (the Company) choose to exercise the put right, and sell Put Shares to the Selling Stockholder, your ownership interest will be diluted. As is generally the case in stock sale arrangements of the type established in the Purchase Agreement, it is anticipated that the Selling Stockholder, in turn, will sell the Put Shares of our Common Stock immediately upon receiving the Put Shares in order to minimize their risk and exposure in regard to the Put Shares, and in order to realize any profit involved. When the Selling Stockholder resells the Put Shares, this could have a negative effect on the market price of our Common Stock.
We may not have access to the full amount available under the Purchase Agreement.
The only way we are able to access the funding provided for in the Purchase Agreement is by selling Put Shares of our Common Stock to the Selling Stockholder. In order for us to be able to sell Put Shares, there must be an effective registration statement in place covering the resale of such Put Shares by the Selling Stockholder, and certain other conditions must be met.
So, our ability to sell Put Shares of our Common Stock to the Selling Shareholder will not begin until this Registration Statement, of which this Prospectus is a part, is declared effective by the SEC, and will only continue as long as this Registration Statement remains effective.
Our ability to sell further Put Shares to the Selling Stockholder, beyond the initial Put Shares covered in this registration statement, will only arise if and to the extent that we prepare and file one or more additional registration statements covering the re-sale of further Put Shares under the Purchase Agreement, and such registration statements become effective (and if certain other conditions are satisfied.
These subsequent registration statements may be subject to review and comment by the Staff of the SEC. Therefore, the timing of these subsequent registration statements cannot be assured, nor can their effectiveness be assured. Accordingly, there is no guarantee that we will be able to draw down all or any portion of the rest of the funding that is potentially available to us under the Purchase Agreement.
Risks Related to Operations of Northwest Biotherapeutics, Inc.
The requirements of the Sarbanes-Oxley Act of 2002 and other U.S. securities laws
impose substantial costs, and may drain our resources and distract our management.
We are subject to certain of the requirements of the Sarbanes-Oxley Act of 2002 in the U.S., as well as the reporting requirements under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The Exchange Act requires, among other things, filing of annual reports on Form 10-K, quarterly reports on Form 10-Q and periodic reports on Form 8-K following the happening of certain material events, with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls over financial reporting. Our existing controls have some weaknesses, as described below. Meeting the requirements of the Exchange Act and the Sarbanes-Oxley Act may strain our resources and may divert management's attention from other business concerns, both of which may have a material adverse effect on our business.
Our management has identified internal control deficiencies, which our management and our independent auditor believe constitute material weaknesses.
In connection with the preparation of our financial statements for the year ended December 31, 2010, and prior years, our management identified certain internal control deficiencies that, in the aggregate, represent material weaknesses, including:
|
|
•
|
lack of a sufficient number of independent directors on our audit committee
|
|
|
•
|
lack of a financial expert on our audit committee
|
|
|
•
|
insufficient segregation of duties in our finance and accounting function due to limited personnel
|
As part of our independent auditors’ communications with our Company’s audit committee with respect to audit procedures for the year ended December 31, 2010, our independent auditors informed the audit committee that these deficiencies constituted material weaknesses, as defined by Auditing Standard No. 5, “An Audit of Internal Control Over Financial Reporting that is Integrated with an Audit of Financial Statements and Related Independence Rule and Conforming Amendments,” established by the Public Company Accounting Oversight Board, or PCAOB. We intend to take appropriate and reasonable steps, in due course, to make the necessary improvements to address these deficiencies, but the timing of such steps is uncertain and the availability of funding and resources for such steps are also uncertain. Our ability to attract qualified individuals to serve on our Board and to take on key management roles within the Company is also uncertain. Our failure to successfully remedy the existing weaknesses could lead to heightened risk for financial reporting mistakes and irregularities, and/or lead to a loss of public confidence in our internal controls that could have a negative effect on the market price of our Common Stock.
We will need to continue raising substantial funding, on an ongoing basis, for general corporate purposes and operations, including our clinical trials. Such funding may not be available or may not be available on attractive terms.
As of September 30, 2011, we had approximately $1.6 million of cash on hand. We will need substantial additional funding, on an ongoing basis, in order to continue execution of our clinical trials to move our product candidates towards commercialization, to continue prosecution and maintenance of our large patent portfolio, to continue development and optimization of our manufacturing and distribution arrangements, and for other corporate purposes. We are pursuing financing with several parties, which we hope to complete later this year in addition to the sales of Put Shares under the Purchase Agreement. However, there can be no assurance that we will be able to complete any of the financings, or that the terms for such financings will be attractive. Any financing, if available, may include restrictive covenants and provisions that could limit our ability to take certain actions, preference provisions for the investors, and/or discounts, warrants or other incentives. Any financing will involve issuance of equity and/or debt, and such issuances will be dilutive to existing shareholders. If we are unable to obtain additional funds on a timely basis or on acceptable terms, we may be required to curtail or cease some or all of our operations at any time.
We are likely to continue to incur substantial losses, and may never achieve
profitability.
We have incurred net losses every year since our formation in March 1996, and had a deficit accumulated during the development stage of approximately $239.2 million as of September 30, 2011, of which $112.6 million was cash expenditures over the years and $126.6 million was non-cash accounting measures. We expect that these losses will continue, and we anticipate negative cash flows from operations for the foreseeable future. We may never achieve or sustain profitability.
Our auditors have issued a “going concern” audit opinion.
Our independent auditors have indicated, in their report on our December 31, 2010 financial statements (and in their reports on our financial statements for preceding years), that there is substantial doubt about our ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities, that may result if we do not continue as a going concern. Therefore, you should not rely on our consolidated balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to shareholders, in the event of liquidation.
As a development stage company with a novel technology and unproven business strategy,
our limited history of operations makes an evaluation of our business and prospects
difficult.
We have had a limited operating history and we are still in the process of developing our product candidates through clinical trials. Our technology is novel and involves mobilizing the immune system to fight a patient’s cancer. Immune therapies have been pursued by many parties for decades, and have experienced many failures. In addition, our technology involves personalized treatment products – a new approach to medical products that involves new product economics and business strategies, which have not yet been shown to be commercially feasible or successful. We have not yet gone through scale-up of our operations to commercial scale. This limited operating history, along with the novelty of our technology, product economics, and business strategy, and the limited scale of our operations to date, makes it difficult to assess our prospects for generating revenues commercially in the future.
We will need to expand our management and technical personnel as our operations progress, and we may not be able to recruit such additional personnel and/or retain existing personnel.
We employ eight (8) full-time employees. The rest of our personnel are retained on a consulting or contractor basis. Biotech companies would typically have a larger number of employees by the time they reach late stage clinical trials. Such trials require extensive management activities and skill sets, including scientific, medical, regulatory (FDA or foreign counterpart), manufacturing, distribution and logistics, site management, business, financial, legal and public relations outreach to both the patient community and physician community. In addition, for overall company operations, other necessary management activities and skill sets involve intellectual property, administrative, regulatory (SEC), investor relations and other.
In order to fully perform all these diverse functions, with late stage trials under way at many sites across the U.S. and outside the U.S., we will need to expand our management and technical personnel. However, the pool of such personnel with expertise and experience with living cell products, such as our DCVax
®
immune cell product, is very limited. In addition, our Company is small and has limited resources, our business prospects are uncertain and our stock price is volatile. For some or all of such reasons, we may not be able to recruit all the management and technical personnel we need, and/or we may not be able to retain all of our existing personnel. In such event, we may have to continue our operations with a smaller than usual team of personnel, and our business and financial results may suffer.
We rely at present on a single relationship with a third-party contract
manufacturer in the U.S. As a result, we may be at risk for capacity limitations and/or supply disruptions.
We currently rely upon a single contract manufacturer, Cognate BioServices, to produce all of our DCVax
®
product in the US, and to supervise the production of our DCVax
®
product candidates outside the US. The majority owners of Cognate is Toucan Capital, one of our majority stockholders, and its affiliates. We have an agreement in place with Cognate pursuant to which Cognate has agreed to provide manufacturing and other services for the next five years, in connection with our Phase II clinical trial for DCVax
®
-L and other programs. The agreement requires us to make certain minimum monthly payments to Cognate in order to have dedicated manufacturing capacity available for our products, irrespective of whether we actually order any DCVax
®
products are manufactured. The agreement also specifies the amounts we must pay for Cognate's actual manufacturing of DCVax
®
for patients.
Problems with Cognate or its facilities or processes could result in a failure to produce, or a delay in production, of adequate supplies of our DCVax
®
product candidates. A number of factors could cause interruptions or delays, including the inability of a supplier to provide raw materials, equipment malfunctions or failures, damage to a facility due to natural disasters, changes in FDA regulatory requirements or standards that require modifications to our manufacturing processes, action by the FDA or by us that results in the halting or slowdown of production of components or finished products due to regulatory issues, Cognate going out of business or failing to produce product as contractually required, and/or other similar factors. Because manufacturing processes for our DCVax
®
product candidates are highly complex, require specialized facilities and personnel that are not widely available in the industry, involve equipment and training with long lead times, and are subject to lengthy FDA approval processes, alternative qualified production capacity may not be available on a timely basis or at all. Difficulties, delays or interruptions in Cognate's manufacturing and supply of our DCVax
®
product candidates could require us to stop enrolling additional new patients into our trial, and/or require us to stop the trial or other program, increase our costs, damage our reputation and, if our product candidates are approved for sale, cause us to lose revenue or market share if Cognate is unable to timely meet market demands.
The manufacturing of our product candidates will have to be greatly scaled up for commercialization, and neither we nor other parties have experience with such scale-up.
As is usual with clinical trials, our clinical trial of DCVax
®
-L for brain cancer involves a number of patients that is a small fraction of the number of potential patients for whom DCVax
®
-L may be applicable in the commercial market. The same will be true of our other clinical programs with our other DCVax
®
product candidates. If our DCVax
®
-L (and/or other DCVax
®
product candidates) are approved for commercial sale, it will be necessary to greatly scale up the volume of manufacturing, far above its level for the trials. Neither we nor our contract manufacturer, Cognate BioServices, have experience with such scale-up. In addition, there are virtually no consultants or advisors in the industry who have such experience and can provide guidance or assistance, because active immune therapies such as DCVax
®
are a fundamentally new category of product in two major ways: these active immune therapy products consist of living cells (not chemical or biologic compounds) and the products are personalized. No such products have ever gone through and successfully completed the necessary scale-up for commercialization. Only one such product has even reached approval (Dendreon’s Provenge) and substantial difficulties have been encountered so far by Dendreon in trying to do the large-scale scale-up of manufacturing for commercialization.
The necessary specialized facilities, equipment and personnel may not be available or obtainable for the scale-up of manufacturing of our product candidates.
The manufacture of living cells requires specialized facilities, equipment and personnel which are entirely different than what is required for manufacturing of chemical or biologic compounds. Scaling up the manufacturing of living cell products to volume levels required for commercialization will require enormous amounts of these specialized facilities, equipment and personnel – especially where, as in the case of our DCVax
®
product candidates, the product is personalized and must be made for each patient individually. Since living cell products are so new, and have barely begun to reach commercialization, the supply of the specialized facilities, equipment and personnel needed for them has not yet developed. It may not be possible for us (or Cognate) to obtain all of the specialized facilities, equipment and personnel needed for commercialization of our DCVax
®
product candidates. This could delay or halt our commercialization.
Our technology is novel, involves complex immune system elements, and may not prove to be effective.
The scientific community and physician community have been trying for over 100 years to develop active immune therapies for cancer. There have been many different immune therapy product designs – and many product failures and company failures. To date, only one active immune therapy has reached approval (Dendreon’s Provenge). The immune system is complex, with many diverse elements, and the state of scientific understanding of the immune system is still limited. Some immune therapies previously developed by other parties showed surprising and unexpected toxicity in clinical trials. Other immune therapies developed by other parties delivered promising results in early clinical trials, but failed in later stage clinical trials. To date, we have only conducted early stage trials in limited numbers of patients. Although the results of those trials were quite positive, those results may not be achieved in our later stage clinical trials, such as the 240-patient trial we are now conducting in brain cancer, and our product candidates may not ultimately be found to be effective.
Clinical trials for our product candidates are expensive and time consuming, and their
outcome is uncertain.
The process of obtaining and maintaining regulatory approvals for new therapeutic products is expensive, lengthy and uncertain. It can vary substantially, based upon the type, complexity and novelty of the product involved. Late stage clinical trials, such as our 240-patient trial in brain cancer, are especially expensive (typically requiring tens of millions of dollars), and take years to reach their outcomes. Such outcomes often fail to reproduce the results of earlier trials. It is often necessary to conduct multiple late stage trials in order to obtain sufficient results to support product approval, which further increases the expense. Sometimes trials are further complicated by changes in requirements while the trials are under way (for example, when the standard of care changes for the disease that is being studied in the trial). Accordingly, any of our current or future product candidates could take a significantly longer time to gain regulatory approval than we expect, or may never gain approval, either of which could delay or stop the commercialization of our DCVax
®
product candidates.
We have limited experience in conducting and managing clinical trials.
We rely on third parties to assist us, on a contract services basis, in managing and monitoring all of our clinical trials. We do not have experience conducting late stage clinical trials ourselves, without third party service firms, nor do we have experience in supervising such third parties in managing late stage, multi-hundred patient clinical trials prior to the trials that we currently have under way. Our lack of experience and/or our reliance on these third party service firms may result in delays, or failure to complete these trials successfully and on time. If the third parties fail to perform, we may not be able to find sufficient alternative suppliers of those services in a reasonable time period, or on commercially reasonable terms, if at all. If we were unable to obtain alternative suppliers of such services, we might be forced to delay, suspend or stop our 240-patient Phase II clinical trial of DCVax
®
L for brain cancer.
Multiple late stage clinical trials of our lead product (DCVax-L for brain cancer) may be required before we can obtain regulatory approval.
Typically, companies conduct multiple late stage clinical trials of their product candidates before seeking product approval. Our current 240-patient clinical trial of DCVax
®
-L for brain cancer is our first late stage trial, and is a Phase II (not Phase III) trial. While under certain circumstances, both the FDA and the European Medicines Agency (“EMA”) will accept a Phase II study as a single study in support of approval, it is not yet known whether they will do so in this case. Even if the results are as positive and compelling as in our early stage trials, we may be required to conduct additional late stage trials before we can obtain product approval. This would substantially delay our commercialization. There is also some possibility that changes requested by the FDA could complicate the application process for product approval. In addition, a number of products are under development for brain cancer and at least one has recently been approved in the US. It is possible that the standard of care for brain cancer could change while our Phase II trial is still under way. This could necessitate further clinical trials with our DCVax
®
-L product candidate for brain cancer.
Costly and time-consuming studies of our DCVax®-L product for brain cancer, and/or additional clinical trials, may be required before we can obtain regulatory approval.
With biologics products, “the process is the product” (i.e., the manufacturing process is considered to be as integral to the product as is the composition of the product itself). If any changes are made in the manufacturing process, and such changes are considered material by the regulatory authorities, the company sponsor may be required to conduct equivalency studies to show that the product is equivalent under the changed manufacturing processes as under the original manufacturing processes, and/or the company sponsor may even be required to conduct additional clinical trials. Our manufacturing processes have undergone some changes during the early clinical trials. Accordingly, we may be required to conduct equivalency studies, and/or additional clinical trials, before we can obtain product approval, unless the regulatory authorities are satisfied that the changes in processes do not affect the quality, efficacy or safety of the product.
We may not receive regulatory approvals for our product candidates or there may be a delay in obtaining such approvals
.
Our products and our ongoing development activities are subject to regulation by regulatory authorities in the countries in which we or our collaborators and distributors wish to test, manufacture or market our products. For instance, the FDA will regulate the product in the U.S. and equivalent authorities, such as theEMA, will regulate in other jurisdictions. Regulatory approval by these authorities will be subject to the evaluation of data relating to the quality, efficacy and safety of the product for its proposed use.
The time taken to obtain regulatory approval varies between countries. In the US, for products without "Fast Track" status, it can take up to eighteen (18) months after submission of an application for product approval to receive the FDA's decision. Even with Fast Track status, FDA review and decision can take up to twelve (12) months. At present, we do not have Fast Track status for our lead product, DCVax-L for brain cancer.
Different regulators may impose their own requirements and may refuse to grant, or may require additional data before granting, an approval, notwithstanding that regulatory approval may have been granted by other regulators. Regulatory approval may be delayed, limited or denied for a number of reasons, including insufficient clinical data, the product not meeting safety or efficacy requirements or any relevant manufacturing processes or facilities not meeting applicable requirements as well as case load at the regulatory agency at the time.
We may fail to comply with regulatory requirements.
Our success will be dependent upon our ability, and our collaborative partners’ abilities, to maintain compliance with regulatory requirements, including current good manufacturing practices (“cGMP”) and safety reporting obligations. The failure to comply with applicable regulatory requirements can result in, among other things, fines, injunctions, civil penalties, total or partial suspension of regulatory approvals, refusal to approve pending applications, recalls or seizures of products, operating and production restrictions and criminal prosecutions.
Regulatory approval of our product candidates may be withdrawn at any time.
After regulatory approval has been obtained for medicinal products, the product and the manufacturer are subject to continual review and there can be no assurance that such approval will not be withdrawn or restricted. Regulators may also subject approvals to restrictions or conditions, or impose post-approval obligations on the holders of these approvals, and the regulatory status of such products may be jeopardized if such obligations are not fulfilled. If post-approval studies are required, such studies may involve significant time and expense.
Our product candidates will require a different distribution model than conventional
therapeutic products, and this may impede commercialization of our product candidates.
Our DCVax
®
product candidates consist of living human immune cells. Such products are entirely different from chemical or biologic drugs, and require different processing for the handling, distribution and delivery than chemical or biologic drugs. One crucial difference is that our DCVax
®
products must remain frozen throughout the distribution and delivery process, until the time of administration to the patient, and cannot be handled at room temperature. In addition, our DCVax
®
product candidates are personalized and they involve ongoing treatment cycles over several years for each patient. Each product shipment for each patient must be tracked and managed individually. For all of these reasons, among others, we will not be able to simply use the distribution networks and processes that already exist for conventional drugs. It may take time for shipping companies, hospitals, pharmacies and physicians to adapt to the requirements for handling, distribution and delivery of these products, which may adversely affect our commercialization.
Our product candidates will require different marketing and sales methods and personnel than conventional
therapeutic products. Also, we lack sales and marketing experience. These factors may result in significant
difficulties in commercializing our product candidates.
The commercial success of any of our product candidates will depend upon the strength of our sales and marketing efforts. We do not have a marketing or sales force and have no experience in marketing or sales of products like our lead product, DCVax
®
-L for brain cancer. To fully commercialize our product candidates, we will need to recruit and train marketing staff and a sales force with technical expertise and ability to manage the distribution of our DCVax
®
-L for brain cancer. As an alternative, we could seek assistance from a corporate partner or a third party services firm with a large distribution system and a large direct sales force. However, since our DCVax
®
living cell, immune therapy products are a fundamentally new and different type of product than are on the market today, we would still have to train such partner’s or such services firms’ personnel about our products, and would have to make changes in their distribution processes and systems to handle our products. We may be unable to recruit and train effective sales and marketing forces or our own, or of a partner or a services firm, and/or doing so may be more costly and difficult than anticipated. Such factors may result in significant difficulties in commercializing our product candidates, and we may be unable to generate significant revenues.
We may not obtain or maintain the benefits associated with orphan drug status,
including market exclusivity.
Although our lead product, DCVax
®
-L for brain cancer, has been granted orphan drug status in both the US and the European Union ("EU"), we may not receive the benefits associated with orphan drug designation (including the benefit providing for market exclusivity for a number of years). This may result from a failure to achieve or maintain orphan drug status, or result from the development of a competing product that has an orphan designation for the same disease indication. Also, in the EU, even after orphan status has been granted, that status is re-examined shortly prior to the product receiving any regulatory approval. The EMA must be satisfied that there is evidence that the product offers a significant benefit relative to existing therapies, in order for the therapeutic product to maintain its orphan drug status. Accordingly, our product candidates will have to re-qualify for orphan drug status prior to any potential product approval in the EU.
The availability and amount of potential reimbursement for our product candidates by government and private payers is uncertain and may be delayed and/or inadequate.
The availability and extent of reimbursement by governmental and/or private payers is essential for most patients to be able to afford expensive treatments, such as cancer treatments. In the US, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (“CMS”), an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payers tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products. To date, only one active immune therapy has reached the stage of a reimbursement decision (Dendreon's Provenge). Although CMS approved coverage and reimbursement for Provenge, and private payers followed suit, there remain substantial questions and concerns about reimbursement for Provenge, and such questions and concerns appear to be impeding sales.
Various factors could increase the difficulties for our DCVax
®
products to obtain reimbursement. Costs and/or difficulties associated with the reimbursement of Dendreon’s Provenge could create an adverse environment for reimbursement of other immune therapies, such as our DCVax
®
products. Approval of other competing products (drugs and/or devices) for the same disease indications could make the need for our products and the cost-benefit balance seem less compelling. The cost structure of our product is not a typical cost structure for medical products, as the majority of our costs are incurred up front, when the manufacturing of the personalized product is done. Our atypical cost structure may not be accommodated in any reimbursement for our products. If we are unable to obtain adequate levels of reimbursement, our ability to successfully market and sell our product candidates will be adversely affected.
The manner and level at which reimbursement is provided for services related to our product candidates (e.g., for administration of our product to patients) is also important. If the reimbursement for such services is inadequate, that may lead to physician resistance and adversely affect our ability to market or sell our products.
The methodology under which CMS makes coverage and reimbursement determinations is subject to change, particularly because of budgetary pressures facing the Medicare program. For example, the Medicare Prescription Drug, Improvement, and Modernization Act (the “Medicare Modernization Act”), enacted in 2003, provided for a change in reimbursement methodology that has reduced the Medicare reimbursement rates for many drugs, including oncology therapeutics.
In markets outside the US, where we plan to operate in the future, the prices of medical products are subject to direct price controls and/or to reimbursement with varying price control mechanisms, as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the US. Some jurisdictions operate positive and/or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. Accordingly, in markets outside the US, the reimbursement for our products may be reduced compared with the US and may be insufficient to generate commercially reasonable revenues and profits.
Risk Factors Relating to the Securities Markets and Investments in Our Common Stock
Competition in the biotechnology and biopharmaceutical industry is intense and most
of our competitors have substantially greater resources than we do.
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Several companies, such as Dendreon, Celldex Therapeutics, Inc., Ark Therapeutics plc, Oxford Biomedica plc, Argos Therapeutics, Inc., Agenus, Prima Biomed, Avax, Immunocellular Therapeutics, and others are actively involved in the research and development of immune therapies or cell-based therapies for cancer. (In addition, other novel technologies for cancer are under development, such as the electro-therapy device of NovoCure.) Of these companies, only one has obtained approval of such an immune therapy: Dendreon (for its Provenge treatment of prostate cancer). Additionally, several companies, such as Medarex, Inc., Amgen, Inc., Agensys, Inc., and Genentech, Inc., are actively involved in the research and development of monoclonal antibody-based cancer therapies. Currently, at least seven antibody-based products are approved for commercial sale for cancer therapy, and large number of additional ones are under development. Genentech is also engaged in several Phase III clinical trials for additional antibody-based therapeutics for a variety of cancers, and several other companies are in early stage clinical trials for such products. Many other third parties compete with us in developing alternative therapies to treat cancer, including: biopharmaceutical companies; biotechnology companies; pharmaceutical companies; academic institutions; and other research organizations, as well as some medical device companies (e.g., NovoCure and MagForce Nano).
Most of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing and sales than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly if they enter into collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to our programs, and in obtaining sites for our clinical trials and enrolling patients.
Our competitors may develop more effective or affordable products, or achieve earlier patent protection or earlier product marketing and sales. Any products developed by us may be rendered obsolete and non-competitive.
We may be prevented from using the DCVax® name in Europe.
The EMA has indicated that DCVax® might not be an acceptable name because of the suggested reference to a vaccine. Failure to obtain the approval for the use of the DCVax® name in Europe would require us to market our product candidates in Europe under a different name which could impair the successful marketing of our product candidates and may have a material adverse effect on our results of operations and financial condition.
Competing generic medicinal products may be approved.
In the EU, there exists a process for approval of generic biological medicinal products once patent protection and other forms of data and market exclusivity have expired. Arrangements for approval of generic biologics products exist and are under consideration in the US, as well. Other jurisdictions are considering adopting legislation that would allow the approval of generic biological medicinal products. If generic medicinal products are approved, competition from such products may reduce sales of our products.
We may be exposed to potential product liability claims, and insurance against these
claims may not be available to us at a reasonable rate in the future, if at all.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing, marketing and sale of therapeutic products. Insurance coverage may not be available to us at commercially reasonable terms (including acceptable cost), if at all. Insurance that we obtain may not be adequate to cover claims against us. Regardless of whether they have any merit or not, and regardless of their eventual outcome, product liability claims may result in decreased demand for our product candidates, injury to our reputation, withdrawal of clinical trial participants or physicians, and/or loss of revenues. Thus, whether or not we are insured, a product liability claim or product recall may result in losses that could be material.
We store, handle, use and dispose of controlled hazardous, radioactive and biological materials in our business. Our current use of these materials generally is below thresholds giving rise to burdensome regulatory requirements. Our development efforts, however, may result in our becoming subject to additional requirements, and if we fail to comply with applicable requirements we could be subject to substantial fines and other sanctions, delays in research and production, and increased operating costs. In addition, if regulated materials were improperly released at our current or former facilities or at locations to which we send materials for disposal, we could be liable for substantial damages and costs, including cleanup costs and personal injury or property damages, and we could incur delays in research and production and increased operating costs.
Insurance covering certain types of claims of environmental damage or injury resulting from the use of these materials is available but can be expensive and is limited in its coverage. We have no insurance specifically covering environmental risks or personal injury from the use of these materials and if such use results in liability, our business may be seriously harmed.
Our intellectual property rights may not provide sufficient commercial protection for our product candidates, or third parties may infringe upon our intellectual property.
Patent laws afford only limited protection and may not protect our rights to the extent necessary to sustain any competitive advantage we may have. In addition, the laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in those countries. Moreover patents and patent applications relating to living cell products are relatively new, involve complex factual and legal issues, and are largely untested in litigation – and as a result, are uncertain.
We have 112 patents and patent applications issued and pending in regard to our product candidates, and related matters such as the manufacturing processes. The issued patents expire at various dates from 2015 to 2026. Our issued patents may be challenged, and such challenges may result in reductions in scope or invalidations. Our pending patent applications may not result in issued patents. Moreover, our patents and patent applications may not be sufficiently broad to prevent others from using substantially similar technologies or from developing competing products. We also face the risk that others may independently develop similar or alternative technologies, or design around our patented technologies.
We have taken security measures (including execution of confidentiality agreements) to protect our proprietary information, especially proprietary information that is not covered by patents or patent applications. These measures, however, may not provide adequate protection for our trade secrets or other proprietary information. In addition, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to our trade secrets.
We may be exposed to claims or lawsuits – with or without merit – that our products infringe patents or other proprietary rights of other parties.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally. The patent landscape is especially uncertain in regard to cell therapy products, as it involves complex legal and factual questions for which important legal principles remain unresolved. Infringement and other intellectual property claims — with or without merit — can be expensive and time-consuming to litigate and can divert management’s attention. We have already been exposed to one frivolous patent lawsuit by a large company, which we vigorously defended and forced the large company to withdraw all of the claims made. We have also been exposed to frivolous claims (without a lawsuit) by a competitor asserting or implying inaccurately that a recent patent issued to them somehow covers our products (which it does not). In the future, we may again be exposed to claims by third parties – with or without merit — that our products infringe their intellectual property rights. Such claims or lawsuits may involve substantial costs and diversion of management attention to defend.
In addition, because patents can take many years to issue, and patent applications are not published until up to eighteen months after they are filed, there may be currently pending applications, unknown to us, which may later result in issued patents that our products may inadvertently infringe. There could also be existing patents of which we are not aware that one or more of our products may inadvertently infringe.
DCVax
®
is our only technology in clinical development.
Unlike many pharmaceutical companies that have a number of products in development and which utilize many different technologies, we are dependent on the success of our DCVax® platform technology. While the DCVax® technology has a wide scope of potential use, and is embodied in several different product lines for different clinical situations, if the core DCVax® technology is not effective or is not commercially viable, our business could fail. We do not currently have other technologies that could provide alternative support for our Company.
We work with scientists and medical professionals at academic and other institutions, including UCLA, among others, some of whom have conducted research for us or have assisted in developing our research and development strategy. These scientists and medical professionals are collaborators, not our employees. They may have commitments to, or contracts with, other businesses or institutions that limit the amount of time they have available to work with us. We have little control over these individuals. We can only expect that they devote time to us and our programs as required by any license, consulting or sponsored research agreements we may have with them. In addition, these individuals may have arrangements with other companies to assist in developing technologies that may compete with our products. If these individuals do not devote sufficient time and resources to our programs, or if they provide substantial assistance to our competitors, our business could be seriously harmed.
The success of our business strategy may partially depend upon our ability to develop and maintain our collaborations and to manage them effectively. Due to concerns regarding our ability to continue our operations or the commercial feasibility of our personalized DCVax
®
product candidates, these third parties may decide not to conduct business with us or may conduct business with us on terms that are less favorable than those customarily extended by them. If either of these events occurs, our business could suffer significantly.
We may have disputes with our collaborators, which could be costly and time consuming. Failure to successfully defend our rights could seriously harm our business, financial condition and operating results. We intend to continue to enter into collaborations in the future. However, we may be unable to successfully negotiate any additional collaboration and any of these relationships, if established, may not be scientifically or commercially successful.
Our business could be adversely affected by new legislation.
Changes in applicable legislation and/or regulatory policies or discovery of problems with the product, production process, site or manufacturer may result in delays in bringing products to market, the imposition of restrictions on the product’s sale or manufacture, including the possible withdrawal of the product from the market, or may otherwise have an adverse effect on our business.
Our business could be adversely affected by animal rights activists.
Our business activities have involved animal testing, as such testing is required before new medical products can be tested in clinical trials in patients. Animal testing has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to stop animal testing by pressing for legislation and regulation in these areas. To the extent that the activities of such groups are successful, our business could be adversely affected. Negative publicity about us, our pre-clinical trials and our product candidates could also adversely affect our business.
USE OF PROCEEDS
This Prospectus relates to shares of our Common Stock that may be offered and sold from time to time by the Selling Stockholder. There will be no proceeds to us from the sale of shares of our Common Stock by the Selling Stockholder. The Selling Stockholder will receive all such proceeds. However, we will receive proceeds from the sale of shares of our Common Stock to the Selling Stockholder under the Purchase Agreement.
The Purchase Agreement allows us to use the proceeds of our sales of Put Shares to the Selling Stockholder for such general corporate purposes as we may choose. We plan to use the proceeds to help fund our clinical trials (especially our ongoing 240-patient, Phase II trial in brain cancer), to fund our general corporate operations and working capital needs, to repay indebtedness and to continue strengthening our balance sheet, and for other purposes that our Board of Directors, in its good faith, deems to be in our best interest.
Our objective will be to draw down on the Purchase Agreement funding during periods of positive results for us and during stages when our stock price is rising, in order to control and minimize, as much as possible, the potential dilution for our current and future stockholders. It may not always be possible for us to meet our objective; therefore, we will continue to identify alternative sources of financing, as we always have, including additional private placements of our stock.
DETERMINATION OF OFFERING PRICE
Under the Purchase Agreement, we will sell Put Shares of our Common Stock to the Selling Stockholder at a price equal to ninety-five percent (95%) of the average of the three (3) lowest closing bid prices of our Common Stock during the five (5) trading days prior to the date on which we deliver notice to the Selling Stockholder of our desire to sell.
The Selling Stockholder is free to re-sell those Put Shares of our Common Stock pursuant to this Registration Statement at any offering price the Selling Stockholder may determine, either in the market or in privately negotiated transactions. The Selling Stockholder is likewise free to determine the number of Put Shares of our Common Stock that it will sell and the timing of those sales. However, we anticipate that, in general, the Selling Stockholder is likely to sell all of the Put Shares of our Common Stock promptly after purchasing such shares under the Purchase Agreement.
DILUTION
"Dilution” represents the difference between the Offering price of the shares of common stock and the net book value per share of common stock immediately after completion of the Offering. Net book value is the amount that results from subtracting total liabilities from total assets. Following is a table detailing dilution as of September 30, 2011 to shareholders if 100%, 75%, 50%, or 10% of the total shares proposed to be sold under the Purchase Agreement are sold.
|
Percent of Offering sold
|
|
100%
|
|
|
75%
|
|
|
50%
|
|
|
10%
|
|
|
Net Tangible Book Value Per Share Prior to Sale
|
|
$
|
(46,150
|
)
|
|
|
(46,150
|
)
|
|
|
(46,150
|
)
|
|
|
(46,150
|
)
|
|
Pro Forma Net Tangible Book Value Per Share After Sale
|
|
|
(44,120
|
)
|
|
|
(44,645
|
)
|
|
|
(45,170
|
)
|
|
|
(46,010
|
)
|
|
Increase in net book value per share due to stock sale
|
|
$
|
2,030
|
|
|
|
1,505
|
|
|
|
980
|
|
|
|
140
|
|
|
Net Dilution (Purchase price of $[0.42] less Pro Forma Net Tangible Book Value per share)
|
|
|
4.40
|
%
|
|
|
3.26
|
%
|
|
|
2.12
|
%
|
|
|
0.30
|
%
|
THE SELLING STOCKHOLDER
The table below identifies the Selling Stockholder and shows the number of shares of Common Stock beneficially owned by it before any purchases under the Purchase Agreement and any re-sales under this Prospectus and Registration Statement. No estimate can be given as to the number of shares that will be held by the Selling Stockholder after completion of the re-sales under this Prospectus and Registration Statement, as that will be determined by decisions the Selling Stockholder makes in the future. To our knowledge, there are currently no agreements, arrangements or understandings with respect to the sale of any of the Put Shares of our Common Stock by the Selling Stockholder, but we expect that the Selling Stockholder will enter into such agreements, arrangements and understandings with respect to the Put Shares. In addition, the Selling Stockholder may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, all or a portion of the shares of our Common Stock beneficially owned, in transactions exempt from the registration requirements of the Securities Act, at any time or from time to time since the date on which the information regarding its beneficial ownership of our Common Stock was determined.
|
Name of selling security holder
|
|
Amount of
securities of the
class owned by
the security
holder before this
offering
|
|
Amount to be
offered for the
security holder’s
account
|
|
Amount and (if one
percent or more)
percentage of the
class to be owned by
security holder after
the offering is
complete
|
|
|
Four M Purchasers, LLC (1)
|
|
|
0
|
|
|
|
7,000,000
|
|
|
|
0
|
%
|
|
(1)
|
Dennis Mehiel, the Sole and Managing Member of Four M Purchasers, LLC (“Four M”), holds voting and/or investment power over Four M.
|
THE PURCHASE AGREEMENT
We entered into the Purchase Agreement with the Selling Stockholder, a Delaware limited liability company on November 14, 2011. Accordingly, this prospectus relates to the resale of up to 7,000,000 shares of our common stock by the Selling Stockholder.
The Purchase Agreement was amended on December 9, 2011 to amend the aggregate investment amount to $2,500,000 and amended again on January 6, 2012 so that the Purchase Agreement could not be assigned by either party.
Pursuant to the Purchase Agreement, following the effectiveness of the Registration Statement of which this Prospectus is a part, the Selling Stockholder will purchase up to $2,500,000 of our Common Stock, in aggregate, over the course of six (6) months, on the terms and conditions set forth in the Purchase Agreement (including, without limitation, conditions that this or a subsequent Registration be and remain effective at all times, that there be no material adverse change in our business or circumstances, and that certain market conditions be met). The aggregate number of shares issuable by us and purchasable by the Selling Stockholder under the Purchase Agreement will be determined by the price of our stock over the course of the six (6) month term, and by the timing and amounts of our sales of Common Stock to the Selling Shareholder under the Purchase Agreement. In addition, the Selling Stockholder will receive fees of five percent (5%) of each sale of our Common Stock under this Registration Statement (“Commission Shares”), when such sale occurs.
We are obligated to file one or more registration statements with the SEC to register the re-sale by the Selling Stockholder of all of the shares of Common Stock which we may put to the Selling Stockholder under the Purchase Agreement. Due to the aggregate amount of our Common Stock issuable under the Purchase Agreement, we expect to file a series of registration statements over the course of the term of the Purchase Agreement. This initial Registration Statement is to cover the re-sale of first portion of the shares of Common Stock that we plan to sell to the Selling Stockholder. We may draw on the facility under the Purchase Agreement from time to time, in our discretion, as and when we determine appropriate (subject to certain limitations as described below). When we put an amount of shares to the Selling Stockholder, the per share purchase price that Selling Stockholder will pay us will be ninety-five percent (95%) of the average of the three (3) lowest closing bid prices of the Company’s Common Stock during the five (5) trading days prior to the date on which we deliver a put notice under the Purchase Agreement, provided, however, that in no event will the price be lower than a floor price of thirty five cent ($0.35) per share. (If the pricing formula would result in a price per share that it lower than the floor price, then we will make no sale of our Common Stock to the Selling Stockholder under the Purchase Agreement until such time as the pricing formula will result in a price per share that is above the floor price.)
There are certain limitations on our sales of Common Stock to the Selling Stockholder under the Purchase Agreement. For example, we must wait at least four (4) business days after each sale notice (put) before we can deliver another sale notice (put) to the Selling Stockholder. We also must limit our sale to no more than $150,000 of our Stock per transaction, until such time as the trading volume and price of our shares increases substantially.
Other limitations on our ability to put shares of our Common Stock to the Selling Shareholder under the Purchase Agreement include the following:
|
|
·
|
we will not be entitled to put shares to the Selling Stockholder unless there is an effective registration statement under the Securities Act covering the re-sale of all such Put Shares by the Selling Stockholder;
|
|
|
·
|
we will not be entitled to put shares to the Selling Stockholder unless our Common Stock continues to be quoted on the OTC Bulletin Board, or becomes listed on a national securities exchange; and
|
|
|
·
|
we will not be entitled to put shares to the Selling Stockholder prior to the closing date of the preceding put.
|
There is no guarantee that we will be able to meet the foregoing conditions, or any other conditions under the Purchase Agreement, or that we will be able to draw down any portion of the amounts available under the Purchase Agreement. In general with facilities of this type, the company usually does not end up using the full amount of the facility or even the majority of the facility.
There are no negative covenants, restrictions on future financings, or preferences in the Purchase Agreement. The Purchase Agreement may be terminated by either at any time for any reason upon(i) ten (10) days’ notice, at any time an uncured event of default exists following written notice of default and expiration of the applicable cure period hereunder or (ii) if the Commencement Date (as defined in the Purchase Agreement) has not been reached by December 31, 2011. At any time after the Commencement Date (as defined in the Purchase Agreement), the Company may terminate the Purchase Agreement for any reason or for no reason upon written notice to the Selling Shareholder, provided however, that if the Company terminates this Agreement in the absence of an uncured Event of Default (as defined in the Purchase Agreement) by the Selling Shareholder, the Company shall be required to issue to the Selling Shareholder shares of Common Stock equivalent to the number of Commission Shares which the Selling Shareholder would have received if Purchase Shares (as defined in the Purchase Agreement) equal to the Available Amount (as defined in the Purchase Agreement) then remaining, on the effective date of such termination by the Company, were all issued and sold to the Purchaser at a price equal to the Purchase Price on the effective date of the termination. In addition, the Selling Stockholder has agreed that neither it nor any of its affiliate shall engage in any direct or indirect short-selling of our Common Stock during the term of the Purchase Agreement.
The issuance of our shares of Common Stock under the Purchase Agreement will have no effect on the rights or privileges of existing holders of common stock except that the economic and voting interests of each stockholder will be diluted as a result of the issuance of our shares. Although the number of shares of Common Stock that stockholders presently own will not decrease, these shares will represent a smaller percentage of our total shares that will be outstanding after any issuances of shares of common stock to the Selling Stockholder.
We are unable to determine the exact number of shares that will actually be sold by the Selling Stockholder according to this Prospectus due to:
|
|
·
|
the uncertainty about when and in what amounts we will choose to sell shares of our Common Stock to the Selling Shareholder, and what the price of our stock will be at the time of those sales;
|
|
|
·
|
the uncertainty about when and in what amounts and at what prices the Selling Stockholder will re-sell those shares of our Common Stock..
|
The shares of our Common Stock will be offered and sold in reliance upon exemptions from registration pursuant to Section 4(2) under the Securities Act and Rule 506 promulgated thereunder. The Purchase Agreement and related documents contain representations to support our reasonable belief that the Selling Stockholder had access to information concerning our operations and financial condition, the Selling Stockholder acquired the securities for its own account and not with a view to the distribution thereof in the absence of an effective registration statement or an applicable exemption from registration, and that the Selling Stockholder are sophisticated within the meaning of Section 4(2) of the Securities Act and “accredited investors” (as defined by Rule 501 under the Securities Act). In addition, the issuances will not involve any public offering; the Company made no solicitation in connection with the sale other than communications with the Selling Stockholder; we obtained representations from Selling Stockholder regarding their investment intent, experience and sophistication; and Selling Stockholder either received or had access to adequate information about us in order to make an informed investment decision.
With respect to the Purchase Agreement, we are relying upon an exemption from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”). The transaction does not involve a public offering. The Selling Stockholder is an “accredited investor” and/or qualified institutional buyer and the Selling Stockholder has access to information about the Company and its investment.
The Selling Stockholder and any participating broker-dealers are “underwriters” within the meaning of the Securities Act. All fees and expenses and other obligations (e.g., for discounts or warrants) incurred with respect to all sales and re-sales of the Common Stock pursuant to the Purchase Agreement and this Registration Statement will be borne by us (the Company), including any underwriting fees, discounts, commissions or other expenses incurred by Selling Stockholder in connection with the re-sale of such shares.
PLAN OF DISTRIBUTION
The Selling Stockholder and any of their pledgees, donees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of Common Stock being offered under this prospectus on any stock exchange, market or trading facility on which shares of our common stock are traded, or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholder may use any one or more of the following methods when disposing of shares:
|
|
·
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
·
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and re-sell a portion of the block as principal to facilitate the transaction;
|
|
|
·
|
purchases by a broker-dealer as principal and re-sales by the broker-dealer for its account;
|
|
|
·
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
·
|
privately negotiated transactions;
|
|
|
·
|
to cover short sales made after the date that the registration statement of which this prospectus is a part is declared effective by the Securities and Exchange Commission;
|
|
|
·
|
broker-dealers may agree with the Selling Stockholder to sell a specified number of such shares at a stipulated price per share;
|
|
|
·
|
a combination of any of these methods of sale; and
|
|
|
·
|
any other method permitted pursuant to applicable law.
|
The shares may also be sold under Rule 144 under the Securities Act of 1933, as amended ("Securities Act"), if available, rather than under this prospectus. The Selling Stockholder has the sole and absolute discretion not to accept any purchase offer or make any sale of shares if they deem the purchase price (or other terms) to be unsatisfactory at any particular time.
The Selling Stockholder may pledge its shares to their brokers under the margin provisions of customer agreements. If the Selling Stockholder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares.
The Selling Stockholder may be deemed an “underwriter” within the meaning of the Securities Act of 1933 in connection with the sale of common stock under the Purchase Agreement.
Broker-dealers engaged by the Selling Stockholder may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions, discounts and/or other incentives from the Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated, which incentives as to a particular broker or dealer may be in excess of customary commissions to the extent permitted by applicable law. We will be responsible for paying or reimbursing any such commissions, discounts and/or other incentives arranged by the Selling Stockholder.
If sales of shares offered under this Prospectus are made to broker-dealers as principals, we would be required to file a post-effective amendment to this Registration Statement of which this Prospectus is a part. In the post-effective amendment, we would be required to disclose the names of any participating broker-dealers and the compensation arrangements relating to such sales.
Any broker-dealers or agents that are involved in selling the shares offered under this Prospectus may be deemed to be "underwriters" within the meaning of the Securities Act in connection with these sales. Commissions received by these broker-dealers or agents and any profit on the re-sale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Any broker-dealers or agents that are deemed to be underwriters may not sell shares offered under this Prospectus unless and until we set forth the names of the underwriters and the material details of their underwriting arrangements in a supplement to this Prospectus or, if required, in a replacement Prospectus included in a post-effective amendment to the registration statement of which this Prospectus is a part.
The Selling Stockholder and any other persons participating in the sale or distribution of the shares offered under this prospectus will be subject to applicable provisions of the Exchange Act and the rules and regulations under that act, including Regulation M. These provisions may restrict activities of and limit the timing of purchases and sales of any of the shares by the Selling Stockholder or any other person. Furthermore, under Regulation M, persons engaged in a distribution of securities are prohibited from simultaneously engaging in market making and other activities with respect to those securities for a specified period of time prior to the commencement of such distributions, subject to specified exceptions or exemptions. All of these limitations may affect the marketability of the shares.
If any of the shares of common stock offered for sale pursuant to this prospectus are transferred other than pursuant to a sale under this Prospectus, then subsequent holders could not use this prospectus until a post-effective amendment or prospectus supplement is filed, naming such holders. We offer no assurance as to whether the Selling Stockholder will sell all or any portion of the shares offered under this Prospectus.
We have agreed to pay all fees and expenses we incur incident to the registration of the shares being offered under this Prospectus, as well as all fees, expenses, discounts, commissions, warrants and other costs associated with the re-sale of the shares by the Selling Stockholder.
The Selling Stockholder and we have agreed to indemnify one another against certain losses, damages and liabilities arising in connection with this Prospectus, including liabilities under the Securities Act. Under the securities laws of certain states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. We have agreed to undertake responsibility for all state filings and compliance, and agreed to make sure that any brokers, dealers or agents effecting transactions on behalf of the Selling Stockholder are registered to sell securities in all fifty states. In addition, in certain states the shares of our Common Stock may not be sold unless the shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
The Selling Stockholder should be aware that the anti-manipulation provisions of Regulation M under the Securities Exchange Act of 1934 will apply to purchases and sales of shares of common stock by the Selling Stockholder, and that there are restrictions on market-making activities by persons engaged in the distribution of the shares. Under Regulation M, the Selling Stockholder or their agents may not bid for, purchase, or attempt to induce any person to bid for or purchase, shares of common stock of Northwest Biotherapeutics, Inc. while such Selling Stockholder is distributing shares covered by this prospectus. Accordingly, except as noted below, the Selling Stockholder is not permitted to cover short sales by purchasing shares while the offering is taking place. The Selling Stockholder is advised that if a particular offer of our Common Stock is to be made on terms constituting a material change from the information set forth above with respect to this Plan of Distribution, then, to the extent required, a post-effective amendment to the accompanying registration statement must be filed with the Securities and Exchange Commission.
Penny Stock Regulations
You should note that our stock is a penny stock. The Securities and Exchange Commission has adopted Rule 15g-9 which generally defines "penny stock" to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and "accredited investors". The term "accredited investor" refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer's account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer's confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules discourage investor interest in and limit the marketability of our common stock.
Blue Sky Restrictions on Resale
If the Selling Stockholder wants to sell shares of our Common Stock under this Registration Statement in the United States, the Selling Stockholder will also need to comply with state securities laws, also known as “Blue Sky laws,” with regard to secondary sales. All states offer a variety of exemptions from registration for secondary sales. Many states, for example, have an exemption for secondary trading of securities registered under Section 12(g) of the Securities Exchange Act of 1934 or for securities of issuers that publish continuous disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor’s. We have agreed to be responsible for all of these Blue Sky law arrangements and compliance, and all fees and expenses associated with them.
Any person who purchases shares of our Common Stock from the Selling Stockholder under this registration statement who then wants to re-sell such shares will also have to comply with Blue Sky laws regarding secondary sales.
When this Registration Statement becomes effective, and the Selling Stockholder indicates in which state(s) it desires to sell its shares, we will be able to identify what steps will need to be taken for compliance with Blue Sky laws.
DESCRIPTION OF SECURITIES TO BE REGISTERED
This Prospectus includes 7,000,000 shares of our Common Stock which may be offered by the Selling Stockholder. The following description of our Common Stock is only a summary. You should also refer to our Certificate of Incorporation and bylaws, which have been filed as exhibits incorporated by reference to this Registration Statement of which this Prospectus forms a part.
Common Stock
Number of Authorized and Outstanding Shares
The Company's Certificate of Incorporation authorizes the issuance of 150,000,000 shares of Common Stock, $0.001 par value per share, of which 149,283,254 shares 102,843,148 were issued and outstanding on January 10, 2012. These shares which were held by approximately 2,500 shareholders of record. On November 29, 2011, the holders of a majority of the Company’s Common Stock authorized an amendment to the Company’s Certificate of Incorporation to increase the number of authorized shares of Common Stock to 450,000,000 shares without amending the previously authorized 20,000,000 shares of “Blank Check” Preferred Stock.
Voting Rights
Holders of shares of Common Stock are entitled to one vote for each share on all matters to be voted on by the stockholders. Holders of Common Stock have no cumulative voting rights. Accordingly, the holders of in excess of 50% of the aggregate number of shares of Common Stock outstanding will be able to elect all of the directors of the Company and to approve or disapprove any other matter submitted to a vote of all stockholders.
Other
No shareholder has any preemptive right or other similar right to purchase or subscribe for any additional securities issued by the Company, except one investor has a right of first refusal to acquire one million dollars $1,000,000 of our stock under certain circumstances. No shareholder has any right to convert the common stock into other securities. No shares of common stock are subject to redemption or any sinking fund provisions. All the outstanding shares of the Company's common stock are fully paid and non-assessable. There is no outstanding preferred stock, and no outstanding securities convertible into or exercisable for preferred stock. The Company's Common Stock holders are entitled to dividends when, as and if declared by the Board from funds legally available therefore, although the Company does not anticipate declaring or paying any cash dividends on the common stock in the foreseeable future. Upon liquidation, the Common Stock holders are entitled to a pro-rata share in any distribution to shareholders.
Preferred Stock
The Company's Certificate of Incorporation authorizes the issuance of 20,000,000 shares of “Blank Check” Preferred Stock, par value $0.001 per share (“Preferred Stock”), in one or more series, subject to any limitations prescribed by law, without further vote or action by the stockholders. Each such series of Preferred Stock shall have such number of shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be determined by the Company's board of directors, which may include, among others, dividend rights, voting rights, liquidation preferences, conversion rights and preemptive rights.
There are no shares of Preferred Stock issued and outstanding.
Transfer Agent
Shares of our Common Stock are registered at the transfer agent and are transferable at such office by the registered holder (or duly authorized attorney) upon surrender of the Common Stock certificate, properly endorsed. No transfer shall be registered unless the Company is satisfied that such transfer will not result in a violation of any applicable federal or state securities laws. The Company's transfer agent for its Common Stock is Mellon Investor Services, Inc., c/o BNY Mellon Shareowner Services, 520 Pike Street, Suite 1220, Seattle, Washington 98101.
DESCRIPTION OF BUSINESS
Organization Within Last Five Years
Northwest Biotherapeutics, Inc. was formed in 1996 and incorporated in Delaware in July 1998. We are a development stage biotechnology company focused on discovering, developing, and commercializing immunotherapy products that safely generate and enhance immune system responses to effectively treat cancer. Currently approved cancer treatments are frequently ineffective, can cause undesirable side effects and provide marginal clinical benefits. Our approach in developing cancer therapies utilizes our expertise in the biology of dendritic cells (“DC”), which are a type of white blood cells that activate the immune system. Our primary activities since incorporation have been focused on advancing a proprietary dendritic cell immunotherapy for prostate and brain cancer together with strategic and financial planning, and raising capital to fund our operations.
We have two basic technology platforms applicable to cancer therapeutics: dendritic cell-based cancer vaccines, which we call DCVax
®
, and monoclonal antibodies for cancer therapeutics. DCVax
®
is our registered trademark. Our DCVax
®
dendritic cell-based cancer vaccine program is our main technology platform.
We completed an initial public offering of our common stock on the NASDAQ Stock market in December 2001 and an initial public offering of our common stock on the Alternative Investment Market (“AIM”) of the London Stock Exchange in June 2007. The Company voluntarily delisted from the AIM market in September 2009.
OVERVIEW
Northwest Biotherapeutics is a development stage biotechnology company, focused on developing immunotherapy products to treat cancers more effectively than current treatments, without toxicities of the kind associated with chemotherapies, and on a cost-effective affordable basis. The Company has developed a platform technology, DCVax®
1
, which uses activated dendritic cells to mobilize a patient’s own immune system to attack their cancer. The DCVax® technology is expected to be applicable to most cancers, and is embodied in several product lines designed for different cancers. The DCVax® technology has already reached late stage clinical trials for two different cancers (brain and prostate), has entered early stage trials for another cancer (ovarian) and has received clearance from the FDA for early stage trials in five other diverse cancers. The Company’s late stage clinical trial in brain cancer is already under way, and the Company believes that it can potentially reach its primary endpoint within approximately 18 months, provided that it obtains full funding as described herein (See “Further Funding”).
Dendritic cells are the master cells of the immune system, and are able to mobilize all parts of the immune system, including T cells, B cells and antibodies, natural killer cells and many others. Mobilizing the whole immune system provides a broader attack on the cancer than mobilizing just a particular component, such as T cells alone, or a particular antibody alone. Likewise, , the Company’s DCVax® technology is designed to attack the full set of biomarkers (called “antigens”) on a patient’s cancer, rather than just a particular selected target or several targets. When just one or a few biomarkers on a cancer are targeted by a drug or other treatment, sooner or later the cancer usually develops a way around that drug (called an “escape variant”), and the drug stops working. The Company believes that each of these factors – mobilizing all agents of the immune system, and targeting all biomarkers on the patient’s cancer – contribute to the effectiveness of DCVax® as explained more fully below.
In clinical trials to date, the Company’s DCVax® treatments have been achieving what the Company believes to be unprecedented results. In patients with newly diagnosed Glioblastoma multiforme (GBM), the most aggressive and lethal form of brain cancer, patients treated with full standard of care treatment today (surgery, radiation and chemotherapy), typically have recurrence of their cancer within a median of 6.9 months, and typically die within a median of 14.6 months.
N Engl J Med 352: 987-96, 2005.
In contrast, the patients who received DCVax® in addition to standard of care typically did not experience recurrence until approximately 2 years, rather than 6.9 months, and typically lived for 3 years, rather than just 14.6 months. Moreover, long-term follow-up data on the GBM patients treated with DCVax® in prior clinical trials show that, as of the most recent update, 43% of the patients have reached or exceeded 4 years’ survival so far, and 29% of the patients have reached or exceeded 6 years’ survival so far (as compared with the median survival of 14.6 months with standard of care treatment today).
1
DCVax® is a registered trademark of the Company.
Similarly striking results have been obtained in patients with late stage prostate cancer, either with or without metastases, as described below. Encouraging early results have also been seen in the initial metastatic ovarian cancer patients treated.
Nearly as importantly, DCVax® is non-toxic. Patients do not experience vomiting, hair loss or debilitating fatigue as they do with chemotherapies. In clinical trials to date, patients receiving DCVax® have been able to continue their lives on a normal basis: continuing to work, take care of their families and even travel recreationally. The broad and rapidly growing body of scientific literature about dendritic cells is consistent with the DCVax® clinical experience, and provides added support regarding the lack of toxicity.
The Company is working to develop and position DCVax® as a front line therapy – not second or third line or last resort therapy – that can potentially become standard of care and be provided to all patients with the cancers for which DCVax® obtains approvals. Accordingly, the Company is highly sensitive to the cost and affordability of DCVax®. The Company has spent more than a decade pioneering a unique method of batch manufacturing which now results in costs and pricing of DCVax® lower than most cancer drugs (especially the newer ones), even though DCVax® is a personalized product.
The Company has also worked to make DCVax® an extremely simple product for both physicians and patients. It does not involve any complex procedures for physicians or patients. It is administered to patients as a simple intra-dermal injection, similar to a flu shot.
The Company is currently under way with a large (240-patient) double blind, randomized, placebo controlled clinical trial (the gold standard) for patients with newly diagnosed GBM. This is a Phase II trial but is designed and statistically powered as though it were a Phase III trial. If the data are again positive, and the primary endpoint (measure) of the trial is met (of which there can be no assurance), the Company plans to seek early product approval.
This Phase II trial is currently open at 12 sites across the U.S. The Company has already enrolled and treated 34 patients in this Phase II trial, including 22 patients in the trial arms and 12 patients in an information arm, and the Company has similar numbers of additional patients in various stages of the screening and enrollment process. The Company plans to add a substantial number of additional clinical trial sites over the coming months.
I. COMPANY AND PRODUCT INFORMATION
A. IMMUNE THERAPIES FOR CANCER
Development of effective immune therapies for cancer has long been a goal of the medical and scientific communities. The human immune system is very powerful, and also very complex: an “army” with many divisions and many different kinds of weapons. A diagram of some key agents and weapons of the immune system is set forth below:
Diagram 1: The immune system “army” includes many diverse agents. Dendritic cells are the “General” of the army.
It has taken decades of research to identify the many different types of agents and weapons, to determine the relationships among them, and to determine how they work together to attack and defeat invaders such as bacteria, viruses – and cancers. While the research was in process, early versions of immune therapies against cancers were tried, with mixed results and a number of failures. Over the course of the 1990s and 2000s, the first commercially successful category of immune agents to treat cancers emerged: drugs that consisted of individual antibodies, such as Avastin, Herceptin and Erbitux.
Antibodies are just one category of weapon in the overall immune “army,” and there are many, many kinds of individual antibodies within this category. Each antibody drug, such as Avastin, consists of just a single one of the many kinds of antibodies within this one category of immune weapon. These drugs do not involve the numerous other important agents in the immune army, such as T cells, NK cells, and so on.
Antibody drugs have been moderate medical successes and huge commercial successes. These drugs have delivered moderate extensions of patient survival compared with traditional chemotherapy drugs, with somewhat lesser (though still significant) toxicity. On this basis, these antibody drugs are achieving multi-billion dollars per year in sales.
Now, more broad based immune therapies are starting to come of age: “therapeutic vaccines” designed to mobilize the entire immune “army,” rather than just a single agent or single category of agents. Therapeutic vaccines are similar to preventive vaccines in that they work by mobilizing the immune system. However, therapeutic vaccines are administered to patients who already have a given disease, for the purpose of preventing or delaying recurrence or progression of the existing disease.
The therapeutic vaccines that are now coming of age are mostly focusing on dendritic cells in various ways, or on T cells. The vaccines focusing on dendritic cells offer a broader potential immune response because dendritic cells are the master cells of the immune system -- the “General” of the “army.” When dendritic cells are activated against a particular pathogen (or cancer) they, in turn, mobilize all of the other agents (including T cells as well as B cells, NK cells and others) to attack that pathogen (or cancer). The process by which dendritic cells mobilize other agents takes place to a large extent in the lymph nodes.
A major challenge faced by immune therapies for cancer has been that, unlike in a healthy patient with an infectious disease, in cancer patients the dendritic cells fail to do their job, and the other immune agents also fail to do their job. Pathologists analyzing tumor tissue removed from cancer patients have long observed that there are often substantial numbers of immune cells in the surrounding tissue, but they are not infiltrating and attacking the tumor – as though the immune cells have made it to the doorstep of the tumor and then stopped.
The mechanisms by which cancer cells selectively suppress or block the immune system are still the subject of much research. It is known that cancer cells have many such mechanisms, including secretion of biochemical signals that jam normal immune signaling, that make tumor cells invisible to immune detection and/or that convey false messages to the immune system. Different therapeutic vaccines are taking different approaches to trying to overcome these cancer mechanisms and put the immune system back in action.
Many of the therapeutic vaccines for cancer (e.g., Cell Genesys, CancerVax) have targeted existing dendritic cells
in situ
in a patient’s body, by administering various compounds or factors that are designed to attract dendritic cells to the tumor or enhance the tumor signals to the dendritic cells (in essence, making the tumor signals “louder”).
The Company and a few others (e.g., Dendreon) are taking a different approach, based on the belief that existing dendritic cells
in situ
in a patient’s body are impaired and their ability to receive and process the necessary signals is blocked. Under this view, if the signaling is blocked, then no matter how “loud” the signal may be, it will not get through and will not achieve the activation needed.
B. THE DCVax® TECHNOLOGY
The Company's platform technology, DCVax®, is a personalized immune therapy: a therapeutic vaccine that uses a patient's own dendritic cells (“DCs”), the master cells of the immune system, as the therapeutic agent. The patient’s DCs are obtained through a blood draw (“leukapheresis”). The DCs are then activated and loaded with biomarkers (“antigens”) from the patient’s own tumor. The activation shifts the DCs into “attack mode.” The loading of biomarkers into the DCs “educates” the DCs about
what
to attack. The activated, educated DCs are then isolated with very high purity and constitute the DCVax personalized vaccine.
Injection of DCVax (the activated, educated dendritic cells of the patient) back into the patient, through a simple intra-dermal injection, similar to a flu shot, in the upper arm or other location near lymph nodes, initiates a potent immune response against cancer cells, mobilizing the overall immune system and doing so in the natural way, with the numerous immune agents acting in their normal roles and in combination with each other. In short, DCVax is designed to restore the potent natural functioning of the immune system which has otherwise been impaired or blocked by the cancer.
Importantly, each activated, educated dendritic cell has a large multiplier effect, mobilizing hundreds of T cells and other immune cells. As a result, small doses of such dendritic cells can mobilize large and sustained immune responses.
Diagram 2: One Educated Dendritic Cell Activates Hundreds of Anti-Cancer Cells
The Company believes that at least three key aspects of the DCVax technology contribute to the strikingly positive results (described more fully below) seen in clinical trials to date:
(1) that DCVax is personalized and thus aims at targets that are definitely present on the patient’s cancer,
(2) that DCVax targets not just one or a few biomarkers but the full set of biomarkers on a patient’s cancer, and
(3) that DCVax is designed to mobilize the entire immune system, not just a single agent or category of agent (such as T cells alone, or a single antibody alone).
First, DCVax is personalized, and targets the particular biomarkers expressed on
that patient’s
tumor. Extensive scientific evidence has shown that there is substantial variation in tumor profiles (characteristics) among patients with the “same” cancer – and the degree of variation is particularly enormous in some of the most aggressive cancers, such as Glioblastoma multiforme (GBM) brain cancer and pancreatic cancer. Cancer drugs are typically keyed to a single target which is believed to be expressed (i.e., found on the cancer cells’ surface or in one of the cancer cells’ signaling pathways) in a substantial percentage of patients with a given type of cancer. Such drugs can be of no use in patients whose cancers do not happen to express that particular target, or cease expressing that target as the disease progresses. Most cancer drugs only achieve clinical benefits in a limited percentage of the patients with the type of cancer being targeted (e.g., 25-30% of the patients). In contrast, DCVax has consistently achieved clinical benefits in over 80% of the patients who have received it in clinical trials to date. Since DCVax is personalized (made with biomarkers from the patient’s own tumor), it definitely aims at targets that are present on that patient’s cancer.
Second, DCVax is designed to target not just one but the
full set
of biomarkers on the patient’s tumor. As mentioned above, cancer drugs are typically “silver bullets” aimed at just one target on a patient’s cancer. However, cancer is a complex and variable disease. Not only do tumor profiles vary among patients with the “same” cancer and vary as the disease progresses, as described above, but when silver bullets hit individual targets on cancers, the cancers find ways around them (called “escape variants”) – and the silver bullet treatments then usually stop working. DCVax takes the opposite approach: instead of aiming at a single target, DCVax aims at the
full set
of biomarkers on a patient’s cancer. Such a treatment approach makes it far harder for tumors to develop escape variants.
Third, DCVax is designed to mobilize the
entire immune system
, not just one among the many different categories of immune agents in that overall system. As described above, DCVax is comprised of activated, educated dendritic cells, and dendritic cells are the master cells of the immune system, that mobilize or help the entire immune system. Some of the prominent cancer drugs today are composed of just one type of antibody – and antibodies themselves are just one type of agent in the overall immune “army” (see Diagram 1 above). In contrast, the full immune system involves many, many types of antibodies, and also many other kinds of agents besides antibodies. Similarly, there have been a variety of early immune therapies that failed in the past. These, too, typically involved single agents, such as a single one among the many, many types of immune signaling molecules (e.g., a particular interferon or interleukin), or a single type of agent such as T cells alone, etc. In contrast, dendritic cells mobilize all of these different categories of agents, comprising the whole immune “army,” in combination with each other and in their natural relationships to each other.
C. DCVax® PRODUCT LINES
The Company has developed several different product lines based on the DCVax technology, to address multiple different cancers and different patient situations. There are two main components to each DCVax product: the immune cells (dendritic cells) and the cancer biomarkers (antigens).
All of the DCVax product lines are made from the patient’s own dendritic cells. Furthermore, the dendritic cells are freshly isolated, newly matured and activated. The Company believes that the existing dendritic cells in a cancer patient have already been compromised by the cancer (which the Company believes is why other vaccines aimed at the existing dendritic cells in patients have largely failed). However, the patient’s body continues to produce new precursors of dendritic cells, and these precursors (monocytes) circulate in the patient’s blood stream. For all DCVax products, these precursors are obtained through a blood draw (leukapheresis), and then, through the Company’s proprietary manufacturing processes (described below), the precursors are matured into a fresh, uncompromised batch of new dendritic cells.
The antigen component, which is combined with the fresh, personalized dendritic cells, varies among the DCVax product lines.
The basic DCVax product – DCVax-L – is made with cancer antigens from tumor lysate (a processed cell mixture) from the patient’s own tumor tissue. As such, DCVax-L incorporates the
full set
of tumor antigens, making it difficult for tumors to find ways around it (“escape variants”), as described above. This is the DCVax product that has been used in the Company’s brain cancer and ovarian cancer clinical trials, and is expected to be used for most other cancers in situations in which the patient has their tumor surgically removed as part of standard of care.
Another DCVax product – DCVax-Direct – is designed for situations in which it is not feasible or not desirable for patients to have their tumors surgically removed. This includes situations in which patients have multiple metastases, or have cancers that are relatively accessible for injections (e.g., head and neck, liver and pancreatic cancers). Like DCVax-L, this DCVax product also incorporates the
full set
of tumor antigens – but it does so
in situ
in the patient’s body rather than at the manufacturing facility. With DCVax Direct, the fresh, new dendritic cells are partially matured in a special way so as to be ready to pick up antigens directly from tumor tissue in the patient’s body, and are then injected directly into the patient’s tumor(s). There, the dendritic cells pick up the antigens
in situ
rather than picking up the antigens from lysate in a lab dish at the manufacturing facility, as is done with DCVax-L.
A third DCVax product – DCVax-Prostate – is designed specifically for late stage, hormone independent prostate cancer. Such cancer involves the spread of micro-metastases beyond the prostate tissue. In most patients, there is no focal tumor which can be surgically removed and used to make lysate, or into which dendritic cells can be directly injected- the cancer cells are diffuse. So, the Company developed a DCVax product line using a particular proprietary antigen – PSMA (Prostate Specific Membrane Antigen) – which is closely associated with hormone independent prostate cancer. The PSMA is produced through recombinant manufacturing methods, and is then combined with the fresh, personalized dendritic cells to make DCVax-Prostate.
D. SIMPLICITY OF DCVax® FOR PHYSICIANS AND PATIENTS
All of the DCVax product lines are designed to be very simple for both physicians and patients, to fit within existing medical practices and procedures, and to be deliverable in virtually any clinic or doctor’s office. A number of complex and sophisticated (and proprietary) technologies are required for the production and frozen storage of DCVax, but these technologies are mostly deployed at the manufacturing facility and do not entail any effort or involvement by physicians or patients.
Front-end simplicity
For all DCVax product lines, the precursors (monocytes) for the fresh, new dendritic cells are obtained through a blood draw. This blood draw can be done not only at whatever cancer center is treating the patient, but at any ordinary blood center (Red Cross or other).
For DCVax-L, the collection of the patient’s tumor tissue (which is to be used to make lysate and provide the antigen component of the vaccine) involves a simple kit. The kit consists of a box with a vial which has a grinder top and is pre-loaded with a proprietary mix of enzymes. Such kits can be kept on hand like any inventory item at medical centers. In the operating room, after the tumor has been surgically removed, instead of disposing of the tissue in the medical waste, any nurse or technician simply coarsely chops the tissue and drops it into the vial, puts the vial back into the box, and hands the box to a courier pick-up service such as FedEx’s or UPS’ life science division, or a specialized courier such as World Courier.
For DCVax-Direct and DCVax-Prostate, there is no tumor tissue collection involved.
Back-end simplicity
For all DCVax products, administration to the patient involves a simple injection. There are no handling steps at the point of care: no preparations, no additions, no mixing – nothing except thawing the frozen DCVax product to room temperature by simply letting the product sit on a counter for a few minutes. (All DCVax products are stored frozen in single doses. Such doses are tiny, and require less than 5 minutes to thaw.) There are also no lengthy intravenous infusions. DCVax-L and DCVax-Prostate are administered through a simple intra-dermal injection, similar to a flu shot, and are just a few drops in size. With the absence of handling steps at the point of care, and the simple intra-dermal injection, these DCVax products can be delivered to patients in any clinic or doctor’s office.
The simplicity for patients also lies in the fact that DCVax is non-toxic. Patients do not have to take a second set of drugs to manage side effects. Patients do not have to quit their jobs, stop caring for their families, make special dietary efforts to avoid severe weight loss or to handle difficulty swallowing, buy wigs or undertake other cover-ups, and so on. Patients can simply go on with their lives.
E.
CLINICAL PROGRAMS AND CLINICAL TRIAL RESULTS
Overall Clinical Pipeline
Over the last ten years, the Company has built a robust clinical pipeline with DCVax products for multiple cancers, which the Company believes provides it with multiple opportunities for success. Two DCVax products have reached late stage clinical trials and are the Company’s lead products: DCVax-L for Glioblastoma multiforme brain cancer (GBM) and DCVax-Prostate for late stage prostate cancer. Very few biotech companies manage to bring two products to late stage clinical trials at the same time. In addition to these, the Company’s DCVax-L has also been applied in an early stage trial for metastatic ovarian cancer, and other DCVax products have been cleared by the FDA to begin early stage trials in multiple other cancers.
The Company’s programs in brain and prostate cancer, and the striking clinical trial results to date, are described in some detail below.
Importantly, the results seen in patients who received DCVax treatments have been quite consistent. More than 80% of patients who received DCVax in trials to date have shown clinical benefits, compared with only 25-30% of patients showing clinical benefits with typical cancer drugs. Further, the clinical effects observed (a doubling or more of the usual time to disease progression and a doubling or more of survival time) were largely consistent across diverse types of cancer, diverse patient profiles (age, gender, physical condition, etc.), and different stages of disease.
Also importantly, no toxicities have been seen in clinical trials of DCVax products to date, such as are seen with chemotherapies.
Brain Cancer (GBM)
As explained earlier, GBM is the most aggressive and lethal type of brain cancer. With full standard of care treatment today (including surgery, radiation and chemotherapy), the cancer recurs in a median of just 6.9 months and kills the patient in a median of just 14.6 months.
N Engl J Med 352: 987-96, 2005.
There has been very little improvement in clinical outcomes for GBM patients in the last 30 years. The incidence of GBM appears to be on the rise, for unknown reasons, and there is an urgent need for new and better treatments.
The Company’s Prior Clinical Trials
The Company, with its medical collaborator, Dr. Linda Liau, has conducted two prior Phase I clinical trials at UCLA with DCVax-L for GBM brain cancer. These trials included 30 patients with newly diagnosed GBM (and a number of patients with recurrent GBM). Patients who received DCVax in addition to standard of care treatment typically did not have recurrence for a median of 2 years (more than triple the usual time), and survived for a median of 3 years (2-1/2 times the usual period).
Furthermore, a substantial percentage of patients who received DCVax in the prior clinical trials have continued far beyond even the 3 year median survival. As of the latest long-term data update in July, 2010,
33%
of the patients had reached or exceeded
4 years’
survival and
27%
had reached or exceeded
6 years’
survival…. compared with just a little over 14.6 months with full standard of care treatment today.
Although the number of patients in the Company’s prior clinical trials for GBM has been limited, the difference in clinical outcomes with DCVax has been very large relative to outcomes with standard of care treatments, and it has attained a high level of statistical significance rarely seen, even in clinical trials with much larger numbers of patients.
The measure of statistical significance, or “p value,” measures the probability that a set of clinical results are a fluke. Accordingly, the smaller the “p value,” the smaller the chance that the results are a fluke and the higher the statistical significance of the results. The FDA generally requires that the results of clinical trials reach a “p value” of .05 or less, meaning that there is a 5% (5 in 100) or less chance that the trial results were due to chance or random events.
The clinical results in the Company’s two prior clinical trials with DCVax for GBM brain cancer, with a relatively small number of patients, achieved the following “p values”:
|
|
·
|
For the delay in time to recurrence, from 6.9 months with standard of care to 25 months in patients treated with DCVax, the “p value” was .00001 (i.e., a 1 in 100,000 chance that these results were random events….. a level of statistical significance far stronger than the 5 in 100 chance, or less, of random events that FDA requires for product approval).
|
|
|
·
|
For the extension of survival time, from 14.6 months with standard of care to 36.4 months in patients treated with DCVax, the “p value” was .0003 (i.e., a 3 in 10,000 chance that these results were random events…. which is also a level of statistical significance far stronger than the 5 in 100 chance, or less, of random events that FDA requires for product approval).
|
The Company believes that these results of its clinical trials to date with DCVax for GBM brain cancer are unprecedented and highly encouraging.
Following up on these results, in 2007-2008, the Company designed and began a 140-patient randomized, controlled Phase II trial but without a placebo and without blinding (which can only be achieved with a placebo that is indistinguishable from the new treatment being tested), as no placebo then existed for a living cell product like DCVax. Unfortunately, without a placebo and blinding, patients who were randomized to the control group in the trial knew that this was the case – and, not surprisingly, they tended to drop out of the trial. As a result, that 140-patient Phase II trial had to be stopped and a placebo had to be developed to enable blinding, so that patients would not know whether they were receiving DCVax or a placebo.
Placebos to look indistinguishable from various kinds of pills have been made for decades, but creating a placebo to be indistinguishable from living cells in a vial (such as the living immune cells that comprise DCVax) was a new and difficult challenge. Not only must the placebo look indistinguishable from the DCVax
visually
, it must also not have any positive
functional
action of its own that would muddy the trial results. After considerable work, the Company succeeded in developing such placebo arrangements and re-designing the Phase II trial to accommodate them, including nearly doubling the number of patients (from 140 to 240 patients). The Company obtained a new FDA clearance and re-approvals by all the clinical sites, and commenced the new Phase II trial in early 2009.
The Company’s Current, Ongoing Trial
The ongoing Phase II trial is now well under way, and offers a relatively short timeline to a major value milestone. The milestone consists of reaching the primary endpoint of the trial, measured as the time to disease progression (recurrence) in the group treated with DCVax® vs. in the control group. The milestone is based on 110 “events” among the total 240 patients (with an “event” being disease progression/recurrence or death). This key milestone is expected to be reached within 18-24 months, if the Company continues to obtain sufficient funding to proceed at the maximum rate of enrollment.
There are 16 clinical sites open and operating for the trial across the U.S. A growing number of doctors and patients have become aware of DCVax® and the prior clinical trial results, and are actively seeking access to DCVax® treatments. So, the Company believes there is a clear pathway to enrollment of the remaining patients, as long as sufficient funding is available.
The primary purpose of this Offering is to continue and ramp up new enrollment in this Phase II trial.
Prostate Cancer
Prostate cancer is the most common cancer in men in the U.S., accounting for more than 25% of all cancers in men, and nearly twice as many cases per year as lung cancer in men, according to the American Cancer Society. For late stage prostate cancer, there is a pressing unmet need for new treatments, such as DCVax®. This late stage cancer includes two subsets of patients, comprising two distinct markets: (A) about 80-85% of patients do not yet show metastases, have a last good period of life (initially still feeling well), and typically live for about 36 months; and (B) about 15-20% of patients have more aggressive disease, show metastases right away, and only live for about 18 months. Schulman et al, J Urol,: 172:141, 2004. Nearly 100,000 men reach these late stages of prostate cancer every year in the US alone (with similar numbers in Europe). Yet, even today, there is no FDA approved drug specifically for the patients in group A, who comprise the vast majority of late stage prostate cancer patients.
For the patients in group B, there are only two FDA approved drugs: taxotere (docetaxil) and Dendreon’s Provenge. Taxotere adds only about 10 weeks of survival, in only a limited percentage of patients, and has toxic side effects. Yet, this drug is generating nearly $3 billion per year in sales. The Provenge immune therapy developed by Dendreon adds about 4 months (16 weeks) of survival – and is being sold for $93,000 for just one month of treatment (three infusions).
The Company believes that DCVax®-Prostate can offer a much needed treatment for late stage prostate cancer patients in group A, for whom there is no treatment specifically approved by FDA today. In addition, for patients in group B, for whom there are now two FDA approved treatments, the Company believes that DCVax®-Prostate can offer a much longer extension of survival, and lower pricing.
The Company’s Prior Clinical Trials
Clinical experience with the Company’s DCVax®-Prostate product dates back more than a decade, and has reached the Phase III trial stage. More than one hundred patients were treated with DCVax®-Prostate in an academic clinical setting in the mid and late 1990s. Based on encouraging results from those treatments, the Company undertook a Phase I/II clinical trial with 35 patients at two leading clinical centers: MD Anderson and UCLA. Based upon strikingly positive results from that trial, the Company designed a large 612-patient, Phase III clinical trial, and previously obtained FDA clearance to proceed with this trial. The details of these clinical programs are described below.
The Phase III prostate cancer trial is the Company’s second lead program, after the brain cancer program. The Phase III trial will require funding in the range of $40 million. The Company plans to finance that trial on a non-dilutive basis, through corporate partnering or through revenue based (i.e., royalty based) financing. The Company has already received some expressions of interest and had some initial discussions relating to such potential non-dilutive financing arrangements.
The Company’s clinical development of DCVax®-Prostate has focused from the outset on patients with late stage, “hormone independent” prostate cancer. Prostate cancer typically progresses through three stages: (i) early stage, following diagnosis, when surgery or brachytherapy (radioactive seeds implanted into the prostate tissue) are typical treatments; (ii) mid stage, which can last for a number of years, when hormone therapy is used to keep the cancer under control and confined to the prostate; and (iii) late stage, when hormone treatments fail, the cancer becomes “hormone independent,” spreads and becomes lethal.
The Company’s Phase I/II clinical trial conducted at MD Anderson and UCLA included both subsets of hormone independent prostate cancer patients: group A, without visible metastases, and group B, with metastases. The results of this clinical trial were as follows:
|
Group A: Hormone Independent Prostate Cancer Patients
With
out
Metastases
|
|
|
|
Natural Course of Disease*
|
|
With DCVax-Prostate
|
|
Median time to disease progression
(appearance of bone metastases)
|
|
28-34 weeks
|
|
59 weeks
|
|
|
|
|
|
|
|
Median survival
|
|
36 months
|
|
>54 months and continuing*
*(more than half of these
patients still alive at this
long-term follow-up point)
|
* There are no FDA approved drugs specifically for this group of patients.
|
Group B: Hormone Independent Prostate Cancer Patients
With
Metastases
|
|
|
|
With Standard of Care
(Taxotere)
|
|
With Dendreon’s
Provenge
|
|
With DCVax-Prostate
|
|
Median survival
|
|
18.9 months
|
|
25.9 months
|
|
38.7 months
|
|
|
|
|
|
|
|
|
|
Overall survival at
3 years
|
|
11%
|
|
33%
|
|
64%
|
Thus, in the prior Phase I/II clinical trial, patients without metastases (group A) who were treated with DCVax-Prostate typically lived at least 1-1/2 years longer than patients going through the natural course of the disease.
Patients with metastases (group B) who were treated with DCVax-Prostate lived twice as long as patients typically do with standard of care (receiving the drug taxotere) – and more than a year longer than Dendreon has reported that such patients lived when treated with its Provenge immune therapy in the clinical trials upon which FDA approval of Provenge was based.
Following these strikingly positive results in both group A and group B patients, the Company decided to focus its Phase III clinical trial (and its application to FDA for product approval when the time comes) on the patients in group A, because 80-85% of late stage prostate cancer patients fall into this group, while only 15-20% fall into group B. In contrast, Dendreon focused its clinical trials on the group B patients, and obtained FDA approval only for that group of patients. Thus, the addressable market for the Company’s DCVax-Prostate will be at least four times the size of the addressable market for Dendreon’s Provenge.
F. TARGET MARKETS
Since DCVax® is expected, ultimately, to be applicable to most cancers, the potential market for DCVax® is enormous. According to the American Cancer Society, 1 in 2 men, and 1 in 3 women in the US will develop some form of cancer in their lifetime. There are nearly 1.5 million new cases of cancer per year in the US, and nearly 600,000 deaths from cancer. The statistics are similarly enormous in Europe (and in much of the rest of the world).
Even focusing just on the two DCVax® products which have already reached late stage clinical trials – for Glioblastoma multiforme (GBM) brain cancer and for hormone independent prostate cancer, as described above – the Company believes that the target markets for each of these have very large [billion dollar] revenue potential.
Brain cancer
:
Brain cancers fall into two broad categories: primary (meaning the cancer first originates in the brain) and metastatic (meaning the cancer first appears elsewhere in the body, but subsequently metastasizes to the brain). In the US alone, there are some 40,000 new cases of primary brain cancer, and 160,000 new cases of metastatic brain cancer. The numbers are similar in Europe and the rest of the world.
Within the category of primary brain cancer, Grade 4 -- Glioblastoma multiforme (GBM) -- is the most aggressive and lethal type. Among the 40,000 new cases of primary brain cancer per year in the US, at least 12,000 cases are GBM (with some estimates as high as 17,000) and the incidence is increasing.
In addition, brain cancer is a serious medical problem in children 18 years and under. It is the second most frequent type of childhood cancers (after leukemias) and, following progress in reducing death rates from leukemias, it is now the number one cause of childhood cancer deaths.
Very little has changed in the last 30 years in the treatment and clinical outcomes for GBM. With all standard of care treatment today – surgery, radiation and chemotherapy – patients still die within about 14.6 months from diagnosis. Senator Edward Kennedy was a sad example of this: he had access to all possible treatment options, and he died within about 15 months of diagnosis.
The one drug which has become the standard of care chemotherapy treatment for GBM – Temodar – achieved market saturation extremely rapidly, within two years of product launch. Today its sales are a billion dollars a year. And this drug added only 10 weeks of survival (extending survival from its historical 12 months to the 14.6 months typical today), and only did so in a limited percentage of patients. Other drugs approved by FDA for GBM, such as Avastin, did not extend survival at all.
Against this backdrop, the Company believes DCVax® is well positioned for this target market. Further, after seeking approval for DCVax® for the GBM subset of primary brain cancers, in the future the Company plans to conduct trials and seek approval for other primary brain cancers and for metastatic brain cancers.
The Company believes that the market potential of DCVax® for brain cancer, even under conservative assumptions, is very large. For example, if one counts only GBM cases (and not other primary brain cancers nor any metastatic brain cancers), only in the US and Europe (and not rest of world), and one assumes a 50% market share (compared with Temodar whose market share rapidly reached saturation), the number of cases to be treated with DCVax® would be 12,000 per year. At the retail price of $110,000 per patient (as full payment for all 3 years of treatment), the potential revenues would be $1.32 billion.
Prostate cancer
:
The Company also believes that the market potential of DCVax® for prostate cancer is very large, even under conservative assumptions. Prostate cancer is the most common cancer in men. At least 217,000 new cases per year are diagnosed in the US alone, according to the American Cancer Society, with similar numbers in Europe. Among these, at least 100,000 new cases reach late stage prostate cancer each year in the US (with similar numbers in Europe).
Among these 100,000 new late stage prostate cancer cases per year, 80-85% of the patients have no visible metastases (yet), and only 15-20% already have visible metastases. As noted above, in prior clinical trials, patients in both groups were treated with DCVax®, and both groups showed strikingly positive results (substantial extensions of survival, far beyond existing treatment options – including Dendreon’s Provenge). So, the Company is focusing its Phase III trial on the much larger market: patients without visible metastases, comprising 80-85% of the 100,000 new cases per year in the US.
If one counts only those 80-85,000 late stage patients, only in the US (not counting either Europe or rest of world), and one assumes only a 25% market share (compared with Taxotere, whose market share is very high despite adding only 10 weeks of survival), the number of cases to be treated with DCVax® would be 20-21,000 cases per year. At the retail price of $110,000 per patient (as full payment for all 3 years of treatment), the potential revenues would be $2.3 billion per year.
G. MANUFACTURING OF DCVax®
The Company believes that its proprietary manufacturing process for DCVax® products is the key to its favorable product economics, and its positioning of DCVax® to be a potential front line therapy that can be provided to patients everywhere. The Company has spent more than a decade honing this manufacturing process – work that received little or no attention or credit over this long period, but which now positions the Company for strong commercial prospects.
In contrast, the Company has pioneered a manufacturing model under which at least 3 years of treatments are produced in one large batch in each manufacturing cycle. Also, the Company has implemented special cryopreservation (freezing) methods which enable this multi-year quantity of product to be frozen, and kept frozen for years, while maintaining its potency.
Both of these technologies – the multi-year batch manufacturing and the cryopreservation – are essential elements of the Company’s manufacturing model and product economics. Together, they enable the Company to incur the high costs of manufacturing just one time, and then store the multi-year quantity of product, frozen, in single doses. This makes DCVax® effectively an “off the shelf” product for the patient, even though it is personalized, and enables the price of DCVax® to be at or below the price level of modern (non-personalized) cancer drugs while still achieving reasonable profit margins. This is already the case while the Company is using first generation manufacturing (without automation) and has not yet scaled up to obtain significant economies of scale. The Company believes that both automation and economies of scale will further enhance the product economics.
The Company’s manufacturing process takes about 8 days (followed by quality control and sterility testing). It involves several main steps as follows:
Isolation of Precursors. The precursors of new dendritic cells are isolated from the patient's white blood cells, which were obtained through a blood draw (leukapheresis) and sent to the manufacturing facility.
Differentiation of Precursors into Immature Dendritic Cells. Precursors are transformed into immature dendritic cells through a six-day culture period, during which specific growth factors are applied in a manner that mimics the natural process in a healthy person's body.
Maturation of Dendritic Cells. Immature dendritic cells are exposed to proprietary maturation factors and methods in order to maximize their ability to, in turn, activate Helper T-cells, Killer T-cells, and B-cells.
Antigen Display and Activation of Dendritic Cells. Cancer-associated antigens or antigen fragments obtained from the patient’s own tumor tissue (or, for prostate cancer, produced recombinantly) are added to the maturing dendritic cells. The dendritic cells ingest and process the antigen materials, and then display fragments on their outer cell surfaces (which will serve to pass along the activation signals from these dendritic cells to other agents in the immune “army,” such as T cells and B cells, when the dendritic cells are injected back into the patient).
Harvest. These matured and activated new dendritic cells are isolated with very high purity, and divided into single-dose vials. They are then frozen and stored until needed.
The Company contracts out the manufacturing of its DCVax® products to Cognate BioServices, Inc. Although there are many contract manufacturers for small molecule drugs and for biologics, Cognate is one of only three major contract manufacturers in the US that specialize in producing living cell products. The manufacturing of living cell products is highly specialized and entirely different than production of biologics: the physical facilities and equipment are different, the types of personnel and skill sets are different, and the processes are different.
In addition, the regulatory requirements for living cell products are exceptionally difficult to meet – especially for personalized living cell products, which can vary considerably from patient to patient. The Company believes that among companies developing such living cell products, nearly all cases in which the company’s clinical trials have been put on “clinical hold” by the FDA (i.e., ordered by FDA to be suspended), have been because of product or manufacturing related issues.
Cognate BioServices has a leading regulatory track record. According to Cognate, the Cognate team has been responsible for the product and manufacturing aspects of more than 20 INDs (applications for FDA approval of clinical trials) for living cells products, and all of these INDs have been approved by FDA. Moreover, the Company believes, based upon information provided by Cognate, that no client of Cognate has been put on clinical hold in connection with its product.
Cognate’s manufacturing facility for clinical-grade cell products is located in Memphis, Tennessee, near the airport. Memphis is a worldwide air shipping hub for both Federal Express and UPS. Cognate's facility is quite large, and contains substantial expansion space in addition to the portions currently built out and in use. The current manufacturing capacity is sufficient to produce DCVax® for approximately 300 patients per year – an amount in excess of what is needed for the late stage clinical trial under way. There is a large amount of expansion space, which is already planned for build -out in stages to allow for scale -up of production capacity in a modular fashion as the need increases for commercialization. This would allow Cognate's current facility to increase to a total capacity of some 5,000 patients per year. As a comparison, Dendreon, with a market capitalization in excess of $5 billion at the time, commercially launched their Provenge dendritic cell vaccine for prostate cancer with initial manufacturing capacity for only 2,000 patients per year.
H. INTELLECTUAL PROPERTY AND ORHPAN DRUG DESIGNATION
The Company has an integrated strategy for protection of its technology through both patents and other mechanisms, such as Orphan Drug status. The Company has two dozen issued patents, and more than 135 pending patents in the US and abroad. These issued and pending patents are grouped into 18 patent families. Some cover the use of dendritic cells in particular DCVax® products. Others cover key processes for manufacturing and quality control for DCVax®, as well as an automated system which the Company believes will play a major role in the scale-up of production for very large numbers of patients on a cost-effective basis.
The expiration dates of the issued patents range from 2015 to 2026. For the earlier dates, the Company plans to seek extensions of the patent life, and believes it has reasonable grounds for doing so.
A few highlights of the Company’s patent portfolio include the following:
Issued patents with composition of matter patent coverage on DCVax®-Prostate, the Company’s treatment for late stage prostate cancer.
Issued patents covering the Company’s TFF System, automating the key stages of manufacturing in which the active ingredient of DCVax® -- the immune cells – are isolated with high purity and effectiveness.
In addition to the Company’s patent portfolio, the Company has applied for and obtained Orphan Drug designation for its lead product: DCVax®-L for brain cancer. Such designation brings with it a variety of benefits, including potential market exclusivity for seven years in the US and ten years in Europe.
Many industrialized countries, including the US and the European Union, have long -established legislation to incentivize companies to develop therapies for diseases which occur in less than a specified number of patients per year, and are referred to as “Orphan Diseases.” Under the US law, Orphan Diseases can involve up to 200,000 cases (and thus can constitute a surprisingly large market). Companies who develop new treatments for such diseases can obtain substantial incentives, including enhanced access to advice from the FDA while the drug is being developed, and market exclusivity once the product reaches approval and begins sales. This market exclusivity applies regardless of patents (according product exclusivity on the market even if the company that developed it has no patent coverage on the product). In addition, the time period for such market exclusivity does not begin to run until product sales begin. In contrast, the time period of a patent begins when the patent is filed and runs down during the years while the product is going through development and clinical trials.
In order to qualify for these incentives, a company must apply for designation of its product as an “Orphan Drug” and obtain approval from the FDA (or its counterpart, abroad). In addition, for the market exclusivity, a product must be the first of its kind for a particular disease to reach the market. The Company has gone through the application processes in the US, the European Union and Switzerland for Orphan Drug designation for its DCVax®-L product for glioma brain cancer, and has been successful in obtaining this Orphan Drug designation in both the US and Europe.
I. COMPANY HISTORY
Northwest Biotherapeutics, Inc. was founded in 1996. During its first five years of operations, it advanced the DCVax® technology and began clinical trials with DCVax®. In December 2001, the Company went public on the NASDAQ market in the US – the only biotech company that was able to complete its IPO that year after 9/11. Due to the adverse circumstances at the time, including the after-effects of the bursting of the Internet bubble, the steep decline of the NASDAQ market, and the events of 9/11, the Company raised only a portion of the funding anticipated in its December 2001 IPO, and was not able to raise any further funding during 2002 or 2003. By 2004, the Company had closed and terminated its manufacturing facility, stopped its clinical trials, laid off all but a small handful of employees, and was on the verge of liquidation.
Commencing in 2004, Toucan Capital stepped in to recapitalize and rebuild the Company. In the period since then, the following have been accomplished:
Manufacturing: cGMP manufacturing arrangements (in specialized clean room facilities, for clinical grade production) have been re-developed and upgraded for late stage clinical trials. A TFF System for partial automation of the manufacturing process has been further developed, and cleared by FDA for use in the Company’s newest clinical trials.
Brain cancer program: A Phase I/II trial was designed, cleared by FDA, carried out and completed. In parallel, long-term follow-up has been continued on the first Phase I trial, that had been conducted prior to the Phase I/II trial. A 140-patient, randomized, controlled Phase II trial was designed, cleared by FDA and several clinical sites, and commenced (without a placebo and blinding). A placebo was subsequently developed, and a new 240-patient, randomized, double blind, placebo controlled Phase II trial was designed, cleared by FDA and 13 clinical sites across the US, commenced, and is currently ongoing. Orphan drug status was applied for and obtained in the US, the European Union, offering the opportunity for 7 years of market exclusivity in the US and 10 years in the EU.
Prostate cancer: Long term follow-up data was collected from the Phase I/II trial. A large, randomized, controlled Phase III trial was designed, submitted and cleared by FDA.
Metastatic ovarian cancer: A small Phase I trial was designed, cleared by FDA and carried out.
Other cancers: Phase I trials have been designed for multiple other cancers, and cleared by FDA. The first of these trials is in the process of being launched at a major cancer center in the US.
Intellectual property: The Company’s IP portfolio has been expanded to over 130 issued and pending patents. Key patents have been granted and issued, including on the DCVax®-Prostate product, [the DCVax®-Direct product] and the TFF System for partial automation of DCVax® manufacturing.
These developments within the last six years (a period which included 3 years of the worst market conditions since the Great Depression) have rebuilt and substantially advanced the Company, positioning the Company to emerge as a leader in active immune therapies for cancer.
J. MANAGEMENT
The Company currently has a small and highly experienced management team. The Company is following the business model increasingly seen in the biotech industry of keeping internal headcount and facilities low, and maximizing the use of external contract services. This business model enables biotech companies to keep their financial burn rates much lower, survive unexpected delays and difficulties that can accompany pioneering, and potentially deliver better capital efficiency for investors.
The Company’s management team includes its Chairman and Chief Executive Officer, Chief Scientific Officer, Chief Technology Officer, a controller and a regulatory and technical advisor, each of whom is profiled below.
The Company’s external teams, dedicated to the Company’s programs on a contract services basis, include at least three dozen staff. Among the existing and pending external services arrangements are the Chief Medical Officer (Dr. Vijay Hingorani), the Contract Research Organization managing the clinical trials (Synteract), the Manufacturing Manager (Dr. Mike Stella) and manufacturing staff (Cognate BioServices), and others.
As the Company expands its operations and ramps up enrollment for the Phase II brain cancer trial, the Company plans to add several management and staff positions, while still keeping internal headcount generally low. The positions to be added include: VP of Clinical Operations, senior clinical operations staff, patient liaisons, and a Chief Financial Officer.
The current senior management team is as follows:
Ms. Linda Powers has been serving as the Chair of the Board since May of 2007 and, in conjunction with this Offering, is now undertaking the role of Chief Executive Officer as well. Ms. Powers has over 25 years of corporate experience, including a decade specializing in biotechnology, with hands-on experience managing the development of novel biotech products for major diseases. Ms. Powers’ biotechnology experience includes both the US and abroad, including Europe, Israel, China, India and Korea. Ms. Powers served for several years on the Technology Advisory Board of the Moffitt Cancer Center in Tampa, Florida, one of the leading cancer centers in the U.S., and is currently on the Board of M2Gen, a joint venture between Moffitt and Merck that is developing 21st century personalized cancer care. Ms. Powers is also on the Board (with technology transfer responsibilities) at the Trudeau Research Institute in NY, which is a leader in basic research on immunology. She has been serving on the Board of the Chinese Biopharmaceutical Association for a number of years. For six years, Ms. Powers taught technology commercialization for scientists at the National Institutes of Health.
Leslie J. Goldman joined the Company as Senior Vice President Business Development in June 2011. Prior to joining the Company Mr. Goldman was a partner at the law firm of Skadden, Arps for over 30 years, specializing in a wide array of advanced technologies and their commercialization. He helped build one of the preeminent global practices in this area. He was responsible for advising on financing, regulatory strategies, and public outreach relating to his clients' development of numerous cutting edge technologies, bringing to bear a diverse range of deal-making skills. He has taken a special early retirement from Skadden, Arps to enable him to undertake an executive role at the Company. Mr. Goldman also serves as an advisor to a number of other breakthrough technology companies. In addition, for eight years, Mr. Goldman has served as Chairman of the Board of a group of TV Stations in four mid-size cities across the country.
Dr. Alton Boynton was the scientific founder of the Company, which he established in 1996. He has been the President and Chief Executive Officer since May of 2007 and is now transferring to the role of Chief Scientific Officer. Dr. Boynton has over 35 years of biotechnology and immunology experience in industry and academia. He was the Associate Director of the Cancer Center at the University of Hawaii, and was Professor of genetics and molecular biology at the University of Hawaii. He is the lead author on over 150 peer-reviewed scientific publications, and is an inventor on 15 patents.
Dr. Marnix Bosch has been with the Company for a decade, and has been the Chief Technology Officer since 1996. In that role, he serves as the director of the Company’s clinical trials, and contributes scientific, regulatory, operational, manufacturing, quality, and clinical trial expertise to the Company’s operations. He has over 25 years of biotechnology and immunology experience in industry and academia, and has extensive regulatory experience in both the US and Europe. Prior to joining the Company, he held several positions, including as the head of the Molecular Biology Lab at the Institute for Public Health in the Netherlands, as a scientist at the Regional Primate Research Center in Seattle, and as an Associate Professor at the University of Washington. Dr. Bosch is the lead author of over 50 peer-reviewed scientific publications, and is an inventor on seven issued and pending patents relating to dendritic cell technology.
Pankaj R Shah, C.A., C.P.A., joined Northwest Biotherapeutics, Inc. in 2008, and has been serving as the Financial Controller since February 2008. He has over 20 years of experience as a Chartered Accountant in finance, accounting, human resource and information technology. Mr. Shah is responsible for the accounting, financial planning, financial reporting, treasury and Human Resource functions at the Company and is a key resource responsible for preparation and submission of statutory filings with the SEC and other authorities. Prior to joining the Company, Mr. Shah served as an Assistant Controller with Qiagen, Inc., a leading provider of sample and assay technologies and as an Financial Controller with Hesperion Us Inc., a global Contract Research Organization.
Dr. Anthony Maida has been a technical and regulatory advisor to the Company for several years. He has been heavily involved in the field of cancer immune therapies for many years, and has overseen all aspects of clinical development of various cancer vaccines (pre-clinical through Phase I, II and III trials, as well as manufacturing and quality control, and other operations). He has also served in business and financial management roles, including as the CFO of a large public company, and has raised over $100 million in funding for biotechnology companies. With both an MBA and a PhD in tumor immunology, he has also served as an advisor to a number of hedge funds and institutional investors, investment banks and pharmaceutical companies.
K. RELATED PARTIES
The Company and its operations involve several related parties. The Company’s largest shareholders are Toucan Capital and Toucan Partners. Ms. Powers, who has been serving as Chair of the Company’s Board since 2007 and is now undertaking the role of CEO of the Company as well, has been a Managing Director of both Toucan entities, and is one of two major owners of both Toucan entities.
Cognate BioServices, the contract manufacturing company that produces all of the Company’s DCVax® products in the US and in Israel, is primarily owned by Toucan Capital and Toucan Partners. To date, this affiliate relationship has been greatly advantageous to the Company, as Cognate has accorded the Company priority over other client business, and has continued to provide manufacturing and related services to the Company over extended periods of time without receiving many millions of dollars in payments due from the Company. Further, as reported in the Company’s public filings, in September, 2009, Cognate agreed to an arrangement for conversion of most of the accumulated unpaid amounts into equity at whatever turned out to be the lowest conversion price that the Company provided to any other creditor.
II. USE OF PROCEEDS
The net proceeds of the Offering contemplated herein will be used for corporate obligations and general corporate purposes, particularly to fund the costs of the ongoing Phase II brain cancer trial. The Company believes that the sale of Put Shares pursuant to this Prospectus and the Registration Statement of which it forms a part will enable the Company to continue onwards with the execution of the 240-patient Phase II clinical trial in brain cancer, as well as to fund corporate operations.
Following the sale of these Put Shares, the Company estimates that additional funding in the range of $15 million (the “Further Funding”) will be needed over a 12-15 month period for the ongoing Phase II trial and corporate operations. The overall Purchase Agreement with the Selling Stockholder allows the Company to sell up to $2.53 million of Put Shares, in aggregate, including the Put Shares covered by this Prospectus over a six (6)-month term.
The Company's estimates of the funding required for the ongoing execution of the Phase II brain trial are based upon its current plans and estimates regarding anticipated capital expenditures. Actual expenditures will depend upon many factors, and may vary substantially from these estimates. Accordingly, the Company may find it necessary or advisable to reallocate the net proceeds for other purposes, and/or necessary or advisable to raise a larger amount of Further Funding.
In the event that the Company's plans or assumptions change, or its assumptions prove to be inaccurate, or the sale of Put Shares pursuant to this Prospectus, and other resources, prove to be insufficient to fund operations (due to other unanticipated expenses, delays, problems or otherwise), the Company will seek additional financing beyond that described herein, and/or may be required to curtail its activities.
INTEREST OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this Prospectus as having prepared or certified any part of this Prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis or had, or is to receive, in connection with the offering, a substantial interest, directly or indirectly, in our Company or any of our subsidiaries. Nor was any such person connected with us or any of our subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
Legal Matters
The validity of the issuance of the shares being offered hereby will be passed upon for us by Tarter Krinsky & Drogin LLP, New York, New York.
Experts
Our financial statements included in this prospectus to the extent and for the fiscal years ended December 31, 2009 and 2010 (as indicated in their reports) have been audited by Peterson Sullivan LLP, Seattle, Washington, an independent registered public accounting firm and are included herein in reliance upon the authority as experts in giving said reports.
DESCRIPTION OF PROPERTY
On November 30, 2009, the Company terminated a Sublease Agreement for the space the Company used as its headquarters at 7600 Wisconsin Avenue, Suite 750, Bethesda, Maryland. In connection with the termination, the Company is required to make monthly payments of $5,000 per month during 2011. Subsequent to 2011, the payment structure will vary.
On March 17, 2010, the Company entered into a non-cancelable operating lease for 7,097 square feet of office space in Bethesda, Maryland, which expires in September 2012. Future minimum lease payments under the lease are $163,475 in 2011 and $126,027 in 2012.
LEGAL PROCEEDINGS
On March 1, 2011, the Company entered into a transaction under Section 3(a)(10) of the Securities Act of 1933, as amended with Socius CG II, Ltd. (“Socius”). Pursuant to this 3(a)(10) transaction, Socius purchased certain claims for payment totaling $1,650,000 from Cognate. Thereafter, Socius elected to convert the claims into shares of the Company’s Common Stock. The conversion was effected through a settlement agreement between Socius and the Company. The settlement agreement was then the subject of a court proceeding (nominally brought by Socius against the Company, but handled on a cooperative basis through a Joint Stipulation by both parties) in order to obtain court approval of the settlement in accordance with the requirements of Section 3(a)(10). That Court approval was obtained on March 1, 2011. Pursuant to the settlement, the full amount of the $1,650,000 debt was converted into shares of Common Stock and the transaction with Socius was completed.
From time to time, we are involved in claims and suits that arise in the ordinary course of our business. At present, the Company is not involved in any suits other than an action by a creditor with respect to certain disputed amounts. Although management currently believes that resolving any such claims against us will not have a material adverse effect on our business, financial position or results of operations, these matters are subject to inherent uncertainties and management’s view of these matters may change in the future.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market for Common Equity and Related Stockholder Matters
The Company’s Common Stock was initially traded on NASDAQ under the symbol NWBO. From December 14, 2001, the Company’s Common Stock has been quoted on the Over The Counter Bulletin Board. The table below sets forth the high and low prices for the Company’s Common Stock for the last two recent fiscal years and the quarters described. Quotations reflect inter-dealer prices, without retail mark-up, mark-down commission, and may not represent actual transactions. No assurance can be given that an active market will exist for the Company's Common Stock. The Company does not expect to declare dividends in the foreseeable future since the Company intends to utilize all of its earnings, if any, to finance its ongoing operations and growth.
|
|
|
High
|
|
|
Low
|
|
|
|
|
|
|
|
|
|
|
Year end December 31, 2009
|
|
|
|
|
|
|
|
First Quarter
|
|
$
|
1.79
|
|
|
$
|
0.65
|
|
|
Second Quarter
|
|
|
1.00
|
|
|
|
0.55
|
|
|
Third Quarter
|
|
|
1.69
|
|
|
|
0.45
|
|
|
Fourth Quarter
|
|
|
0.85
|
|
|
|
0.32
|
|
|
|
|
|
|
|
|
|
|
|
|
Year end December 31, 2010
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
$
|
0.85
|
|
|
$
|
0.65
|
|
|
Second Quarter
|
|
|
1.35
|
|
|
|
0.67
|
|
|
Third Quarter
|
|
|
1.60
|
|
|
|
0.70
|
|
|
Fourth Quarter
|
|
|
0.95
|
|
|
|
0.72
|
|
|
|
|
|
|
|
|
|
|
|
|
Year end December 31, 2011
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
$
|
0.77
|
|
|
$
|
0.34
|
|
|
Second Quarter
|
|
|
0.89
|
|
|
|
0.38
|
|
|
Third quarter
|
|
|
0.70
|
|
|
|
0.38
|
|
As of September 30, 2011 there were approximately 2,500 holders of record of our common stock. Such holders may include any broker or clearing agencies as holders of record, and in such cases exclude the individual stockholders whose shares are held by such brokers or clearing agencies.
Penny Stock
The Commission has adopted rules that define a “penny stock” as equity securities under $5.00 per share which are not listed for trading on NASDAQ (unless the issuer (i) has a net worth of $2,000,000 if in business for more than three years or $5,000,000 if in business for less than three years or (ii) has had average annual revenue of $6,000,000 for the prior three years). The Company’s securities are characterized as penny stock, and therefore broker-dealers dealings in the securities are subject to the disclosure rules of transactions involving penny stock which require the broker-dealer, among other things, to (i) determine the suitability of purchasers of the securities and obtain the written consent of purchasers to purchase such securities and (ii) disclose the best (inside) bid and offer prices for such securities and the price at which the broker-dealer last purchased or sold the securities. The additional requirements imposed upon broker-dealers may discourage them from engaging in transactions in penny stocks, which may reduce the liquidity of the Company’s securities.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all future earnings, if any, to fund the ongoing development and growth of our business. We do not currently anticipate paying any cash dividends in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plan
We have an employee stock option plan (“2007 Stock Option Plan”) under which 7,942,430 have been reserved for issuance. The following table shows information with respect this plan as of September 30, 2011 (options in thousands).
Equity Compensation Plan Information
|
Plan category
|
|
Number of securities to
be issued upon
Exercise of outstanding
options, warrants and
Rights (a)
|
|
|
Weighted-average
exercise price of
Outstanding options,
warrants and rights (b)
|
|
|
Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a)) (c)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity compensation plans approved by security holders
|
|
|
32,763
|
|
|
$
|
0.66
|
|
|
|
7,942
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity compensation plans not approved by security holders
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
32,763,
|
|
|
$
|
0.66
|
|
|
|
7,942
|
|
2007 Stock Option Plan
The 2007 Stock Option Plan became effective on June 15, 2007. In April 2008, the Company increased the number of shares reserved for issuance by 519,132 shares for an aggregate of 6,000,000 shares of its common stock. In May 2010, the Company increased the number of shares reserved for issuance under the 2007 Stock Option Plan by an additional 10,000,000 shares of its common stock. In May 2011, the Company increased the number of shares reserved for issuance under the 2007 Stock Option Plan by an additional 20,000,000 shares of its common stock.
The plan provides for the grant to employees of the Company, its parents and subsidiaries including officers and employee directors, of “incentive stock options,” as defined, and for the grant of non-statutory stock options to the employees, officers, directors, including non-employee directors, and consultants of the Company, its parents and subsidiaries. As of September 30, 2010, net of forfeitures, a total of 7,942,430 shares remain available for issuance under this plan.
Selected Financial Data
Not required for smaller reporting companies.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and plan of operations should be read in conjunction with the financial statements and the notes to those statements included in this Registration Statement. This discussion includes forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under “
Risk Factors
” above, actual results may differ materially from those anticipated in these forward-looking statements.
Overview
We are a development stage biotechnology company focused on discovering, developing and commercializing immunotherapy products to generate and enhance immune system responses to treat cancer. Data from our clinical trials suggest that our cancer therapies significantly extend both the time to tumor recurrence and patient survival time, while providing a superior quality of life with no debilitating side effects when compared with current therapies.
Our financing activities are described below under “— Liquidity and Capital Resources”. We will need to raise additional capital to fund our operations, including our Phase II DCVax
®
-L clinical trial. If the results of our Phase II trial are positive, we plan to seek early product approval for DCVax
®
-L, at the end of this trial.
We have experienced recurring losses from operations and have a deficit accumulated during the development stage, through September 30, 2011of $239.2 million. Of this $239.2 million deficit, $112.6 million (less than half) reflects cash used in operations, and the remaining $126.6 million reflects non-cash accounting measures. In addition, our independent registered public accounting firm’s report on the financial statements for the year ended December 31, 2010, states that because of recurring operating losses, net operating cash flow deficits, and a deficit accumulated during the development stage, there is substantial doubt about the Company’s ability to continue as a going concern.
Going Concern
Our financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. Nevertheless, we have experienced recurring losses from operations and have a deficit accumulated during the development stage of $239.2 million as of September 30, 2011 that raises substantial doubt about our ability to continue as a going concern. Our auditors have issued an opinion, for the year ended December 31, 2010, which states that there is substantial doubt about our ability to continue as a going concern.
If we are unable to continue as a going concern, we would consider all opportunities for creating value in the Company, including investigating alternative ways to advance our dendritic cell-based product, such as potential corporate partnerships and/or the possible sale of some or all of our assets.
Expenses
From our inception through September 30, 2011, we incurred costs of approximately $88.3 million associated with our research and development activities. We are unable to estimate with any certainty the costs we will incur in the continued development of our product candidates for commercialization, because our technologies are unproven, the outcome of our ongoing clinical trials is uncertain, the regulatory pathway is not yet established for personalized, living cell products like DCVax, and there is no experience yet with the scale-up required for commercialization..
General and administrative expenses include salaries and benefits, cost of facilities, insurance, legal expenses and other operating costs,, as well as amortization costs of stock options granted to employees and warrants issued to consultants for their professional services.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America. In preparing these financial statements, we make assumptions, judgments and estimates that can have a significant impact on amounts reported in our financial statements. We base our assumptions, judgments and estimates on historical experience and various other factors that we believe to be reasonable under the circumstances. Actual results could differ materially from these estimates under different assumptions or conditions. On a regular basis we evaluate our assumptions, judgments and estimates and make changes accordingly. We believe that, of the significant accounting policies discussed in Note 3 to our consolidated financial statements, the following accounting policies require our most difficult, subjective and/or complex judgments:
Policy for Reclassified Equity Contracts
The Company accounts for potential shares that can be converted to common stock and if converted, will be in excess of authorized shares, as a liability that is recorded on the balance sheet (at fair value) only until the authorized number of shares is increased (at which time the whole liability will be remeasured, with changes in value included in other income/(expense), and then reclassified to additional paid-in capital).
Embedded Derivative Liability
The Company evaluates financial instruments for freestanding or embedded derivatives. Derivative instruments that have been separated from the host contract and do not qualify for hedge accounting are recorded at fair value with changes in value recognized as other income (expense) in the consolidated statements of operations in the period of change.
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
|
|
·
|
Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets;
|
|
|
·
|
Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
|
|
|
·
|
Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
|
Stock-Based Compensation
Compensation expense for all stock-based awards is measured at the grant date based on the fair value of the award and is recognized as an expense, on a straight-line basis, over the employee's requisite service period (generally the vesting period of the equity award). The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model. Stock-based compensation expense is recognized only for those awards that are expected to vest using an estimated forfeiture rate. Estimates of pre-vesting forfeiture are periodically revised in subsequent periods if actual forfeitures differ from those estimates. To the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are revised.
Results of Operations
Operating costs:
Operating costs and expenses consist primarily of research and development expenses, including clinical trial expenses which increase when we are actively participating in clinical trials, and general and administrative expenses.
Research and development:
These expenses now include predominantly scientific personnel salaries and benefits, costs of laboratory operations and supplies, travel, regulatory compliance, and expenditures for preclinical and clinical trial operation and management.
Because we are a development stage company, we do not allocate research and development costs on a project basis. We adopted this policy, in part, due to the unreasonable cost burden associated with accounting at such a level of detail and our limited number of financial and personnel resources.
General and administrative:
General and administrative expenses include administrative personnel salaries and benefits , cost of facilities, insurance, travel, legal expenses, and other operating costs, as well as property and equipment depreciation, amortization of stock options and warrants.
Nine Months Ended September 30, 2011 and 2010
The Company recognized a net loss of $20.2 million for the nine months ended September 30, 2011 compared to a net loss of $19.9 million for the nine months ended September 30, 2010. The increase in net loss was primarily attributable to increased costs associated with the ramp up of enrollment in the Company’s ongoing Phase II brain cancer clinical trial and an increase in the amortization of stock option compensation.
Research and Development Expense
Research and development expense increased from $4.8 million for the nine months ended September 30, 2010 to $11.5 million for the nine months ended September 30, 2011. The increase was primarily attributable to costs associated with the ramp up of enrollment in the ongoing Phase II brain cancer clinical trial.
General and Administrative Expense
General and administrative expense was $10.7 million for the nine months ended September 30, 2011 compared to $4.7 million for the nine months ended September 30, 2010. The increase was primarily due to amortization of initial stock option grants to the new management team.
Valuation of Reclassified Equity Contracts
At March 31, 2011 the Company valued 12.8 million shares potentially in excess of the authorized shares at that time and recognized a liability of $5,753,000. These same warrants were revalued at September 30, 2011 based on the closing stock price on September 30, 2011 with a resultant decrease in value of $7,413,000.
Loan Conversion Inducement Expense
In the nine months ended September 30, 2011 the Company incurred an expense of $125,000 for a loan conversion inducement expense to a group of non-affiliated institutional investors. Under the terms of a conversion and extension agreement, the investors converted $295,000 (representing principal and accrued interest on an unsecured 10% loan made in November 2010) into 559,480 shares of common stock. The value of the stock issued to the investors in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $125,000. In the nine months ended September 30, 2010 the Company incurred an expense of $4.5 million for a loan conversion inducement expense to the non-affiliated institutional investor. Under the terms of a conversion and extension agreement, an institutional investor converted $1,004,880 (representing principal and accrued interest on an unsecured 12% loan made in November 2008) into 5,024,400 shares of common stock. The value of the stock issued to the institutional investor in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $4,522,000.
Derivative valuation gain and loss
On September 30, 2011 the derivative liability associated with the Whitebox loans and warrants issued in connection with certain loans made in September and October 2010 was revalued resulting in a net change in the fair value of the derivative liability during the nine months ended September 30, 2011 of $29,000.
Interest (Expense)
Interest expense decreased from $6.0 million for the nine months ended September 30, 2010 to $5.4 million for the nine months ended September 30, 2011. Interest expense for both periods was primarily related to the debt discount and interest expense associated with notes payable that were outstanding. In addition, interest expense in nine months ended September, 2010 included $1.6 million of expense from the modification of warrant agreements during the 2010 period.
Year Ended December 31, 2009 Compared to the Year Ended December 31, 2010
We recognized a net loss from operations of $15.4 million for the year ended December 31, 2010 compared to a net loss from operations of $17.5 million for the year ended December 31, 2009.
Research and Development Expense
Research and development expense increased from $9.6 million for the year ended December 31, 2009 to $9.9 million for the year ended December 31, 2010. This increase which was due to recognizing a performance penalty and other unaccrued charges under the Cognate services agreement totaling $3.6 million partially offset by reduced clinical trial operations.
General and Administrative Expense.
General and administrative expense decreased from $7.5 million for the year ended December 31, 2009 to $5.5 million for the year ended December 31, 2010. This decrease was primarily related to reduced legal and consulting costs.
Depreciation and Amortization
Depreciation and amortization decreased from $7,000 during the year ended December 31, 2009 to $2,000 for the year ended December 31, 2010 as a result of all assets being fully depreciated.
Loan conversion inducement
Loan conversion inducement expense decreased from $5.6 million in the year ended December 31, 2009 to $4.7 million in the year ended December 31, 2010. The transactions resulting in these charges are as follows:
In September 2009, Toucan Partners agreed to convert $1,156,718, representing principal and accrued interest (including a default penalty of 0.25% per month) outstanding on the $1.0 million loan made to the Company on August 19, 2008, and $552,738, representing principal and accrued interest (including a default penalty of 0.25% per month) outstanding on a loan made to the Company on December 22, 2008. This total of $1,709,456 was converted into 8,547,280 shares of the Company’s common stock. Additionally in connection with the conversions the Company issued The Selling Stockholder Partners warrants to purchase 842,375 shares at an exercise price of $0.20 per share and warrants to purchase 513,841 shares at an exercise price of $0.41 per share. The value of the stock issued to Toucan Partners in excess of the carrying amount of the loans and accrued interest payable amounted to $4,701,004, and together with the fair value of the warrants, of $916,716, was charged to loan conversion inducement expense in the accompanying consolidated statements of operations during 2009.
Under the terms of a conversion and extension agreement, SDS converted $1,060,000, representing principal and accrued interest on an unsecured 12% loan made in November 2008 into 5,300,000 shares of common stock during May 2010 and December 2010. The value of the stock issued to SDS in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $4,673,000. This amount was charged to loan conversion inducement expense in the consolidated statements of operations during 2010.
Derivative valuation gain (loss)
During 2010 the Company entered into convertible loan agreements and issued certain warrants with down-round protection. As a result the Company must recognize any gains or losses arising from those agreements through the statement of operations.
Asset Impairment Loss
In July 2009 the Company’s contract manufacturer, Cognate Bioservices, Inc., consolidated its operations in its newer Memphis, Tennessee manufacturing facilities and closed the Sunnyvale facility housing the Company’s clean room. As the California clean room cannot be relocated the Company provided an impairment allowance of $389,000 reducing the carrying value of the clean room to $0.
Interest Expense, Net
Interest expense increased from $3.9 million for the year ended December 31, 2009 to $7.9 million for the year ended December 31, 2010. Interest expense includes interest payable on notes payable, debt discount amortization, warrant amortization and other financing costs. Interest expense for the year ended December 31, 2009 and December 31, 2010 was $1.0 million and $3.0 million, respectively, and debt discount amortization and other costs for the year ended December 31, 2009 and December 31, 2010 was $2.9 million and $4.9 million respectively. The change in debt discount amortization and other costs was primarily due to an increase in the average balance of notes payable outstanding during 2010 compared to 2009 and higher debt discount amortization during 2010 compared to 2009.
Liquidity and Capital Resources
At September 30, 2011, cash totaled $1.6 million, compared to $153,000 at December 31, 2010. Working capital was a deficit of $44.3 million at September 30, 2011(including the liability of $16.6 million relating to potential stock issuances in excess of the authorized number of shares, which liability will be eliminated as soon as an increase in the authorized number of shares is made), compared to a deficit of $25.9 million at December 31, 2010. Cash balances increased during the nine months ended September 30, 2011 due primarily to the financing transactions discussed below that were executed in 2011.
The change in cash for the periods presented was comprised of the following (in thousands):
|
|
|
September 30,
|
|
|
September 30,
|
|
|
|
|
|
|
|
2010
|
|
|
2011
|
|
|
Change
|
|
|
Net cash provided by (used in);
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities
|
|
$
|
(6,232
|
)
|
|
$
|
(10,152
|
)
|
|
$
|
(3,920
|
)
|
|
Investing activities
|
|
|
(41
|
)
|
|
|
(31
|
)
|
|
|
10
|
|
|
Financing activities
|
|
|
6,627
|
|
|
|
11,717
|
|
|
|
5,090
|
|
|
Effect of exchange rates on cash
|
|
|
(69
|
)
|
|
|
(44
|
)
|
|
|
25
|
|
|
Increase in cash
|
|
$
|
285
|
|
|
$
|
1,490
|
|
|
$
|
1,205
|
|
Operating Activities
We used $10.2 million in cash for operating activities during the nine months ended September 30, 2011. The increase in cash used in operating activities was a result of costs associated with ramping up enrollment in the Company’s ongoing brain cancer clinical trial.
Investing Activities
Investing activities for the periods presented are not material.
Financing Activities
Proceeds from financing activities during the nine months ended September 30, were as follows (in thousands):
|
|
|
2010
|
|
|
2011
|
|
|
Issuance of notes payable
|
|
$
|
2,667
|
|
|
$
|
6,772
|
|
|
Issuance of common stock and warrants
|
|
|
3,960
|
|
|
|
5,344
|
|
|
Repayments of notes payable
|
|
|
-
|
|
|
|
(399
|
)
|
|
|
|
$
|
6,627
|
|
|
$
|
11,717
|
|
Additionally, the Company settled the following transactions since the nine months ended September 30, 2011:
As of October 24, 2011, the Company agreed that the 750,000 shares issued to Toucan Partners on June 6, 2010, would be used to settle $150,000 of the $1.3 million note payable to Toucan Partners.
On November 14, 2011 the Company entered into a six month (6) non-convertible loan agreement with an existing non-affiliated investor for a loan in the principal amount of $2,000,000 and an annual interest rate of 6%. In connection with the loan, the Company issued the lender thirty percent (30%) warrants to purchase 1,052,632 shares of the Company’s common stock, par value $0.001 per share, at an exercise price of $.57 per share and an exercise period of five years.
On November 25, 2011 the Company issued 46,000,000 shares of common stock to Cognate, a related party, as a result of the conversion of $9.2 million of accounts payable dating back to 2008, that had previously been extended from 2008 at the previously agreed upon price of $0.20 per share.
On November 28, 2011, the Company entered into an agreement with an existing non-affiliated investor, resolving $5,046,401 of debt.This debt arose from a $4 million note which was entered into by the Company and the investor during May, 2008, with a 6-month maturity and a twelve percent (12%) annual interest rate, and which has previously been extended. Pursuant to the agreement, $2,523,201, comprising half of the total amount due, is being converted into common stock of the Company, par value $0.001, at a conversion price of $0.57 per share. The $2,523,201 comprising the other half of the total amount due, is being repaid in cash, through a series of installment payments.
On December 1,2011, the Company issued 300,000 shares of common stock as a result of the conversion of $88,416 of notes payable.
On December 6,2011, the Company issued 200,000 shares of common stock as a result of the conversion of $51,083 of notes payable.
On December 8,2011, the Company issued 51,984 shares of common stock as a result of the conversion of $15,000 of notes payable.
During the periods presented the Company received proceeds from the activities described above. The terms of the various instruments are described in notes 6 and 11 to the condensed consolidated financial statements included in this report. We intend to seek additional financing in the near term. However, there can be no assurance that external sources of financing, including the issuance of notes payable or equity securities, will be available at times and at terms acceptable to us.
Contractual Obligations
As of September 30, 2011, the Company has no contractual commitments, other than contracts entered into in the normal course of its business, which result in material financial obligations.
Recent Accounting Pronouncements
Refer to Note 3 to the Consolidated Financial Statements.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our investors.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING
AND FINANCIAL DISCLOSURE
There have not been any changes in or disagreements with accountants on accounting and financial disclosure or any other matter.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
On June 8, 2011, the Company entered into employment agreements with four persons for senior management positions in the Company: Dr. Alton Boynton, who has been serving as Chief Executive Officer since May, 2007, returned to his former position as Chief Scientific Officer; Dr. Marnix Bosch, continues to serve in his existing position as Chief Technical Officer; Ms. Linda Powers, became Chief Executive Officer in addition to continuing as Chairman of the Board, and Mr. Les Goldman, began serving as Senior Vice President, Business Development. On June 11, 2011 the Company employed Dr. Anthony Maida as Chief Operating Officer.
Officers are elected annually by the board of directors and serve at the discretion of the board.
The following table sets forth information regarding the members of the Company’s current board of directors and its executive officers. All directors hold office until the election and qualification of their successors or their earlier removal or retirement.
|
Name
|
|
Age
|
|
Position
|
|
Linda F. Powers
|
|
55
|
|
Director, Chairperson, Chief Executive Officer
|
|
Alton L. Boynton, Ph.D.
|
|
66
|
|
Director, Chief Scientific Officer
|
|
Anthony Maida, Ph.D.
|
|
59
|
|
Chief Operating Officer
|
|
Leslie Goldman
|
|
66
|
|
Senior Vice President, Business Development
|
|
Marnix Bosch, Ph.D.
|
|
52
|
|
Chief Technical Officer
|
|
Robert A. Farmer
|
|
72
|
|
Director
|
Linda F. Powers.
Ms. Powers has served as the Chairperson of our Board of Directors since her appointment on May 17, 2007. Ms. Powers has served as managing director of Toucan Capital Corporation, a provider of venture capital since 2001. She has over 15 years’ experience in corporate finance and restructurings, mergers and acquisitions joint ventures and intellectual property licensing. Ms. Powers is a board member of M2GEN (an affiliate of Moffitt Cancer Center), the Trudeau Institute (well known for its specialization in immunology), the Chinese Biopharmaceutical Association, the Rosalind Franklin Society, the Genetics Policy Institute, and a Task Force of the National Academy of Sciences . She was the Chair of the Maryland Stem Cell Research Commission for the first two years of the state’s stem cell funding program, and continues to serve on the Commission. Ms. Powers has been appointed to three Governors’ commissions created to determine how to build the respective states’ biotech and other high-tech industries. For six years, Ms. Powers taught an annual internal course at the National Institutes of Health for the bench scientists and technology transfer personnel on the development and commercialization of medical products. Ms. Powers serves on the boards of six private biotechnology companies. Ms. Powers holds a B.A. from Princeton University, where she graduated magna cum laude and Phi Beta Kappa. She also earned a JD, magna cum laude, from Harvard Law School. Ms. Powers is a member of the Company’s Audit Committee, Compensation Committee and Nominations Committee.
Ms. Powers has over 15 years of experience in corporate financings and business transactions, and 10 years specializing in biotechnology investment and company building. Ms. Powers has served for a number of years on Boards of a leading immunology research institute, a large cancer center and more than half a dozen biotechnology companies. The Company believes this background and experience makes Ms. Powers well qualified to serve as a Director and executive officer.
Alton L. Boynton, Ph.D.
Dr. Boynton co-founded the Company, has served as Secretary since August 2001, has served as our Chief Scientific Officer and a director since our inception in 1998, was appointed our Chief Operating Officer in August 2001, was appointed President in May 2003 and was appointed Chief Executive Officer in June 2007. Dr. Boynton has also served as Director of the Department of Molecular Medicine of Northwest Hospital from 1995-2003 where he coordinated the establishment of a program centered on carcinogenesis. Prior to joining Northwest Hospital, Dr. Boynton was Associate Director of the Cancer Research Center of Hawaii, The University of Hawaii, where he also held the positions of Director of Molecular Oncology of the Cancer Research Center and Professor of Genetics and Molecular Biology. Dr. Boynton received his Ph.D. in Radiation Biology from the University of Iowa in 1972.
As a result of Dr. Boynton’s significant years of service as a director and running a number of programs focusing on oncology and cancer-related research programs, including a PhD in Radiation Biology, the Company concluded that Dr. Boynton should serve as a director and executive officer.
Robert A. Farmer
. Mr. Farmer was appointed to the Board of Directors in December 2009. Mr. Farmer served as the national treasurer of four presidential campaigns, including John Kerry, Bill Clinton, Michael Dukakis and John Glenn. In these roles he led fund raising of over $800 million. He served under Ron Brown as treasurer of the Democratic National Committee, and served for eight years as treasurer of the Democratic Governor’s Association. President Clinton appointed Farmer as the United States Consul General to Bermuda where he served from 1994 to 1999. Mr. Farmer also had a successful career as an entrepreneur including building his own publishing company which he sold in 1983, Mr. Farmer currently serves on the Boards of Directors of International Data Group, Dale Carnegie Associates, Sober Steering Sensors, LLC, Charlesbridge Publishing, Haute Living Mr. Farmer is a graduate of Dartmouth College and Harvard Law School.
The Company concluded Mr. Farmer should serve as a director of the Company due to his previous business successes, his experience as treasurer in four presidential campaigns and his service on other boards of directors (including International Data Group, Dale Carnegie Associates, Sober Steering Sensors and Clark Ridge Publishing).
Anthony E. Maida
joined the Company in June 2011 as Chief Operating Officer (“COO”) bringing more than 20 years' experience in building oncology companies, with expertise in the business, financial, clinical and regulatory aspects of and the underlying science of oncology business. Over these two decades, Dr. Maida has held positions as Chairman, CEO, COO, CSO, CFO and VP Business Development, and has raised nearly $200 million in financings for oncology companies. Among these experiences, he served as CEO of CancerVax, an early leader in cancer vaccines. In that role, he raised the company's first $30 million of funding, and was responsible for conducting multi-hundred patient, multi-center clinical trials with the company's cancer vaccines. Prior to joining NWBO, Dr. Maida was serving as global head of Oncology for a leading contract research organization that manages clinical trials in the US and internationally.
Leslie J. Goldman
joined the Company as Senior Vice President Business Development in June 2011. Prior to joining NWBO Mr. Goldman was a partner at the law firm of Skadden, Arps for over 30 years, specializing in a wide array of advanced technologies and their commercialization. He helped build one of the preeminent global practices in this area. He was responsible for advising on financing, regulatory strategies, and public outreach relating to his clients' development of numerous cutting edge technologies, bringing to bear a diverse range of deal-making skills. He has taken a special early retirement from Skadden, Arps to enable him to undertake an executive role at NWBT. Mr. Goldman also serves as an advisor to a number of other breakthrough technology companies. In addition, for eight years, Mr. Goldman has served as Chairman of the Board of a group of TV Stations in four mid-size cities across the country.
Marnix L. Bosch
joined NWBO in 2000, and has been serving as Chief Technical Officer for a number of years. In this capacity, he plays a key role in the preparation and submission of the Company's regulatory applications, as well as ongoing development of the Company's product lines, and ongoing development and/or acquisition of new technologies. Dr. Bosch led the process of designing the protocols, and managed the successful preparation and submission of the Company's Investigation New Drug applications (INDs) for FDA approval to conduct clinical trials, for prostate cancer, brain cancer and five other cancers. He also led the processes for other regulatory submissions in both the US and abroad (including the successful applications for orphan drug status in both the US and Europe for DCVax-L® for brain cancer). He spearheaded the development of the Company's manufacturing and quality control processes, and is working with the Company's contract manufacturer, Cognate BioServices, on next-generation further development of these processes. Prior to joining NWBO in 2000, Dr. Bosch worked at the Dutch National Institutes of Health (RIVM) as head of the Department of Molecular Biology, as well as in academia as a professor of Pathobiology. He has authored more than 40 peer-reviewed research publications in immunology and virology, and is an inventor on several patent applications on dendritic cell product manufacturing.
Board of Directors
Our Board of Directors consists of one non-employee independent director , one director who is currently employed by us and one independent director. The Board has established the following committees:
Audit Committee
The Audit Committee has responsibility for recommending the appointment of our independent accountants, supervising our finance function (which includes, among other matters, our investment activities), reviewing our internal accounting control policies and procedures, and providing the Board such additional information and materials as it may deem necessary to make the Board aware of significant financial matters which require the attention of the Board. The Audit Committee provides the opportunity for direct contact between our independent registered public accounting firm and the Board. The Board has adopted a written charter for the Audit Committee and its current member is Linda F. Powers, a non-employee.
None of our directors meet the definition of an “audit committee financial expert” as defined by the SEC. We intend to recruit one or more additional non-executive directors in due course, including one person who qualifies as an audit committee financial expert but may not be able to do so.
Compensation Committee
The Compensation Committee is responsible for determining the overall compensation levels of our executive officers and administering our stock option plans. The Board has adopted a written charter for the Compensation Committee and its current members are Linda F. Powers and Robert A. Farmer.
Nominations Committee
The Nominations Committee is responsible for identifying and nominating members of the Board, recommending directors to be appointed to each committee of the Board and the chair of such committees, and overseeing the evaluation of the Board. The Board has adopted a written charter for the Nominations Committee. Linda F. Powers and Robert A. Farmer are currently members of the committee. The Nominations Committee will consider nominees recommended by stockholders pursuant to the procedures outlined in the Company’s Bylaws and as set forth herein. No Nominations Committee meetings were held during the year ended December 31, 2010.
We have adopted a code of ethics meeting the definition of “Code of Ethics” as defined in Item 406 of Regulation S-K. Our Code of Ethics is applicable to the chief executive officer, the chief financial officer, the principal accounting officer or persons performing similar functions. Our code of ethics is posted on our website and may be accessed at www.nwbio.com/about_code.php. We will post to our website any amendments to our code of ethics and any waivers granted under the code to any of our directors or executive officers.
EXECUTIVE COMPENSATION
Summary Compensation Table
Compensation Discussion and Analysis
Our Process
Typically, our executive compensation is comprehensively assessed and analyzed annually; however, given our limited funding since 2002, our executives have received infrequent increases in their compensation. During 2009, our executives also received equity based incentives.
Normally, the review process includes, but is not limited to, the following steps:
|
|
·
|
The Compensation Committee reviews the performance of the Chief Executive Officer and other senior executives;
|
|
|
·
|
The current annual compensation of senior management and long-term compensation grants made over the past few years are reviewed;
|
|
|
·
|
The appropriate performance metrics and attributes of annual and long-term programs for the next year are considered and discussed;
|
|
|
·
|
The entirety of our compensation program is considered;
|
|
|
·
|
For our top officers, if peer group compensation is available for their position, we use a blend of survey and peer compensation for comparison, as we compete not only in our own market, but nationally and across industries, for talent;
|
|
|
·
|
The compensation practices of our peer companies are reviewed, including their practices with respect to equity and other grants, benefits and perquisites;
|
|
|
·
|
The compensation of our management team from the standpoint of internal equity, complexity of the job, scope of responsibility and other factors is assessed; and
|
|
|
·
|
Management’s stock ownership is reviewed.
|
Management has the following involvement with the executive compensation process:
|
|
·
|
The Chief Executive Officer recommends salaries, annual and long-term incentive targets, and plan amendments and design before recommendations are submitted to the Compensation Committee for approval; and
|
|
|
·
|
The Chief Executive Officer is involved in establishing and recommending to the Compensation Committee financial goals for the incentive programs based on management’s operational goals and strategic plans.
|
Compensation Goals
Our philosophy regarding executive compensation is to attract and retain highly qualified people by paying competitive salaries, and to link the financial interests of our senior management to those of our stockholders by tying compensation to the achievement of operational and financial objectives. Our compensation package for our officers includes both short-term and long-term features in the forms of base salary and equity-based incentives in the form of stock options, which are granted periodically at the discretion of the Compensation Committee.
Elements of Executive Compensation
Base Salaries
Base salaries for all executive officers are reviewed annually. The Compensation Committee reviews the compensation of the President and Chief Executive Officer. The President and Chief Executive Officer reviews the compensation of the other executive officers. The Compensation Committee also consults with the President and Chief Executive Officer with respect to the compensation package for all other executive officers. In evaluating salaries, each officer’s individual performance during the prior year, as well as salary levels in the biotechnology industry for comparable positions are considered. In determining how the respective officer contributes to the Company, current corporate performance, as well as the potential for future performance gains, is considered. No specific weight is attributed to the foregoing for purposes of determining base salaries.
Equity-Based Incentives
We provide our executive officers with long-term incentives through Stock Option Plans, (as defined under “— Equity Plans” below), all described in more detail below. The primary objective of this plan is to provide an incentive for employees, including our executive officers, to make decisions and take actions that maximize long-term stockholder value. The plans are designed to promote this long-term focus by using discretionary grants and long-term vesting periods. Subject to the terms of the plans, the Compensation Committee determines the terms and conditions of options granted under the plans, including the exercise price, which is based on fair value of our stock on the date of grant. For various motivation and retention considerations, option awards granted subsequent to our initial public offering in December 2001 generally vest over four years. The Compensation Committee believes that stock options provide an incentive for employees, allowing us to attract and retain high quality management and staff. No stock options were issued to our executives in 2010. Stock options were issued to two employees in 2009.
Employee and Executive Benefits
Our executives participate in many of the same employee benefit programs as our other employees. The core employee benefit programs include a tax-qualified retirement plan, medical coverage, dental coverage, life insurance, disability coverage, and vacation. The tax qualified retirement plan is a 401(k) plan. We made matching contributions to each employee’s 401(k) plan account of $0.50 for each dollar contributed on the first $3,000 of compensation contributed to the plan. Our matching contribution policy was terminated effective March 2006. All of these matching contribution amounts to our Named Executive Officers are shown in the
All Other
Compensation
footnote to the Summary Compensation Table following this section.
Perquisites
Historically, we have offered only a very limited number of perquisites to our executives as an incremental benefit to recognize their position within the Company. No perquisites of any kind were offered to executives in 2010.
Compensation of the President and Chief Executive Officer
In assembling the compensation package for our President and Chief Executive Officer, the Compensation Committee considers our annual and long-term performance, the performance of the President and Chief Executive Officer, and our cash resources and needs. Although the Committee’s overall goal is to set the President and Chief Executive Officer’s salary at the median level for competitors that are similar in industry size and performance, the actual level approved by the Committee may be higher or lower based upon the Committee’s subjective evaluation of the foregoing. Consistent with the foregoing, the Compensation Committee set the base salary for the President and Chief Executive Officer at $331,250 for fiscal 2010. The President and Chief Executive Officer did not receive an increase in salary or a bonus for 2010. In connection with our initial public offering on AIM, in December 2007, the Board of Directors granted the President and Chief Executive Officer an option to purchase shares of our common stock.
On June 8, 2011 the Company entered into a two year retention agreement, with the Chief Scientific Officer, Dr. Alton Boynton. Under this agreement, Dr. Boynton will continue to receive a base salary of $335,000 per annum for the first three months of the term, $295,000 per annum for the fourth through sixth months of the term, and $250,000 per annum salary for months seven and beyond. In addition to a base salary, Dr. Boynton is eligible to receive equity based compensation of up to 1,000,000 shares at an exercise price $0.57 per share over the term, partly tied to continuing employment and partly tied to certain clinical trial milestones. At the end of each month of employment throughout the term, Dr. Boynton will receive 5,000 vested shares, up to 120,000 in total. The clinical trial milestones are specific to the progress and success of the continuing DCVax® phase II trials. Each milestone that is attained will vest a specific number of shares as shown by the following table:
|
Milestone or Event
|
|
Number of
Shares Vested
|
|
|
Maximum
Amount Eligible
|
|
|
Each month in which ≥ 15 patients, in aggregate, are enrolled in the Phase II brain cancer trial
|
|
|
20,000
|
|
|
|
240,000
|
|
|
Approval by EMA for the existing Phase II brain cancer trial to proceed in Europe under the Voluntary Harmonization Procedure
|
|
|
40,000
|
|
|
|
40,000
|
|
|
Enrollment of first patient in Europe under Voluntary Harmonization Procedure into existing Phase II brain cancer trial
|
|
|
40,000
|
|
|
|
40,000
|
|
|
Treatment of first “Hospital Exemption” patient in the United Kingdom
|
|
|
40,000
|
|
|
|
40,000
|
|
|
Treatment of first “Hospital Exemption” patient in Germany (or other country besides the U.K.)
|
|
|
40,000
|
|
|
|
40,000
|
|
|
Each of the months during the Agreement that the “Hospital Exemption” patients numbers meet or exceed the following numbers:
|
|
|
|
|
|
|
|
|
|
Months 1-6: 2 patients per month
|
|
|
10,000
|
|
|
|
120,000
|
|
|
Months 7-12: 3 patients per month
|
|
|
|
|
|
|
|
|
|
Months 13-24: 5 patients per month
|
|
|
|
|
|
|
|
|
|
Award of German Government grant of ≥ €2M
|
|
|
40,000
|
|
|
|
40,000
|
|
|
Enrollment of 1st patient in DCVax(R) Direct trial
|
|
|
40,000
|
|
|
|
40,000
|
|
|
Successfully reaching of primary endpoint in brain cancer trial
|
|
|
100,000
|
|
|
|
100,000
|
|
|
Submission of an application for product approval
|
|
|
100,000
|
|
|
|
100,000
|
|
|
Other events at the discretion of Management
|
|
|
80,000
|
|
|
|
80,000
|
|
Summary Compensation Table For 2011
On June 8 and June 21, 2011 the Company entered into employment agreements with its newly appointed management team. Each contract had a term of 2 years and provided for compensation as follows:
|
Name and Principal Position
|
|
Salary
|
|
|
Option Awards Vesting Over 2
years, # of Shares
|
|
|
Linda M. Powers (1)
Chairperson and Chief Executive Officer
|
|
$
|
360,000
|
|
|
|
14,220,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Alton L. Boynton, Ph.D. (2)
Chief Scientific Officer
|
|
$
|
335,000
|
|
|
|
2,376,562
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony Maida, Ph.D. (3)
Chief Operating Officer
|
|
$
|
300,000
|
|
|
|
1,250,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Leslie Goldman (4)
Senior Vice President, Business Development
|
|
$
|
262,000
|
|
|
|
2,250,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Marnix L. Bosch, Ph.D.(5)
Chief Technical Officer
|
|
$
|
325,000
|
|
|
|
1,831,139
|
|
|
(1)
|
In conjunction with the employment agreement entered into between the Company and Ms. Powers on June 8, 2011 the Company issued Ms. Powers an option to purchase 14,220,000 shares of the Company’s stock with an exercise price of $0.66 per share. One third of the shares vested on the grant date, one third vest in equal amounts monthly over the term of the employment agreement and the remaining third vest upon achievement of milestones relating to multiple milestones related to the Company’s clinical program. The initial one third vesting is subject to a lock up which extends to the earlier of the Company reaching the primary endpoint of its GBM brain cancer clinical trial or 18 months.
|
|
(2)
|
In conjunction with the employment agreement entered into between the Company and Dr. Boynton on June 8, 2011 the Company issued Dr. Boynton an option to purchase 1,000,000 shares of the Company’s stock with an exercise price of $0.66 per share. No shares vested on the grant date, 120,000 shares vest in equal amounts monthly over the term of the employment agreement and the remainder (880,000) vest upon achievement of milestones relating to multiple milestones related to the Company’s clinical program.
|
|
(3)
|
In conjunction with the employment agreement entered into between the Company and Dr. Maida on June 21, 2011 the Company issued Dr. Maida an option to purchase 1,250,000 shares of the Company’s stock with an exercise price of $0.66 per share. 120,000 shares vest in equal amounts monthly over the term of the employment agreement and the remainder vest upon achievement of milestones relating to multiple milestones related to the Company’s clinical program.
|
|
(4)
|
In conjunction with the employment agreement entered into between the Company and Mr. Goldman on June 8, 2011 the Company issued Mr. Goldman an option to purchase 2,250,000 shares of the Company’s stock with an exercise price of $0.66 per share. One third of the shares vested on the grant date, one third vest in equal amounts monthly over the term of the employment agreement and the remaining third vest upon achievement of milestones relating to multiple milestones related to the Company’s clinical program. The initial one third vesting is subject to a lock up which extends to the earlier of the Company reaching the primary endpoint of its GBM brain cancer clinical trial or 18 months.
|
|
(5)
|
In conjunction with the employment agreement entered into between the Company and Dr. Bosch on June 8, 2011 the Company issued Dr. Bosch an option to purchase 1,197,139 shares of the Company’s stock with an exercise price of $0.66 per share. 120,000 shares vest in equal amounts monthly over the term of the employment agreement and the remainder vest upon achievement of milestones relating to multiple milestones related to the Company’s clinical program.
|
Summary Compensation Table For 2009 and 2010
The following table sets forth certain information concerning compensation paid or accrued to our named executive officers (the “Named Executive Officers”) during the years ended December 31, 2010 and 2009.
|
Name and Principal Position
|
|
Year
|
|
Salary
|
|
|
Bonus
|
|
|
Option
Awards(1)
|
|
|
All Other
Compensation(2)
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alton L. Boynton, Ph.D. (3)
|
|
2010
|
|
$
|
359,528
|
(5)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
359,528
|
|
|
President, Chief Executive
|
|
2009
|
|
$
|
538,281
|
(4)
|
|
$
|
75,000
|
|
|
$
|
768,065
|
|
|
$
|
—
|
|
|
$
|
1,426,346
|
|
|
Officer, Chief Scientific Officer and Secretary
|
|
2008
|
|
$
|
331,250
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
504
|
|
|
$
|
331,754
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony P. Deasey (6)
|
|
2010
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Senior Vice President and Chief Financial Officer
|
|
2009
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
2008
|
|
$
|
215,331
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
378
|
|
|
$
|
215,709
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Marnix L. Bosch, Ph.D., M.B.A.
|
|
2010
|
|
$
|
431,652
|
(7)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
431,652
|
|
|
Chief Technical Officer
|
|
2009
|
|
$
|
283,750
|
|
|
$
|
50,000
|
|
|
$
|
731,892
|
|
|
$
|
—
|
|
|
$
|
1,065,642
|
|
|
|
|
2008
|
|
$
|
250,000
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
672
|
|
|
$
|
250,672
|
|
|
(1)
|
Represents the amount recognized for financial statement reporting purposes for 2010, 2009 and 2008 in respect of outstanding option awards at fair value, excluding any impact of assumed forfeiture rates. The assumptions made in valuing option awards reported in this column are discussed in Note 3, Stock-Based Compensation to our consolidated financial statements for the years ended December 31, 2010, 2009 and 2008, included elsewhere in this Annual Report on Form 10-K.
|
|
(2)
|
All Other Compensation for the year ended December 31, 2008 consisted of Company-paid premiums on term life insurance coverage up to 1.5 times the employee’s annual salary and earned but unpaid accrued vacation payments.
|
|
(3)
|
Dr. Boynton was appointed as our Chief Executive Officer in June 2007. Dr. Boynton served as our Chief Operating Officer and our principal executive officer during 2006.
|
|
(4)
|
In conjunction with a retention agreement between Dr. Boynton and the Company dated September 28, 2009 Dr. Boynton elected to have six weeks of salary paid in shares of the Company’s common stock. The salary payment was converted into stock at a price of $0.20 per share. The shares issued to Dr. Boynton had a market value at the issue date of $262,239.
|
|
(5)
|
In December 2010 Dr Boynton elected to have four pay periods of salary paid in stock at $0.61 per share. The shares issued to Dr. Boynton had a market value at the issue date of $61,543.
|
|
(6)
|
Effective October 1, 2007, Anthony P. Deasey was named as our Chief Financial Officer. Mr. Deasey resigned from this position effective August 12, 2008.
|
|
(7)
|
In conjunction with a retention agreement between Dr. Bosch and the Company dated September 28, 2009 Dr. Bosch elected to have six weeks of salary paid in shares of the Company’s common stock. The salary payment was converted into stock at a price of $0.20 per share. The shares issued to Dr. Bosch had a market value at the issue date of $105,626. In December 2010 Dr Bosch elected to have an additional two pay periods of salary paid in stock at $0.61 per share. The shares issued to Dr. Bosch had a market value at the issue date of $30,191.
|
Given our financial status, there are no regularly scheduled increases in compensation.
Grants of Plan-Based Awards in 2010
The following table provides information about equity awards granted to the Named Executive Officers during the years ended December 31, 2009 and 2010. We did not grant any stock options, stock appreciation rights or restricted stock to Named Executive Officers during the fiscal year ended December 31, 2010.
|
Name
|
|
Grant Date
|
|
All other Option
Awards: Number of
Securities
Underlying Options
|
|
|
Exercise or Base
Price of Option
Awards
|
|
|
Grant Date Closing
Price of Common
Stock
|
|
|
Grant Date Value
of Option Awards
|
|
|
Dr. Alton Boynton
|
|
08/21/09
|
|
|
1,430,486
|
(1)
|
|
$
|
0.55
|
|
|
$
|
0.55
|
|
|
|
786,055
|
|
|
Dr. Marnix Bosch
|
|
06/23/09
|
|
|
850,000
|
(2)
|
|
$
|
0.70
|
|
|
$
|
0.70
|
|
|
|
594,516
|
|
|
Dr. Marnix Bosch
|
|
08/21/09
|
|
|
250,000
|
(3)
|
|
$
|
0.55
|
|
|
$
|
0.55
|
|
|
|
137,376
|
|
|
(1)
|
This option was granted under the 2007 Stock Option Plan. This option grant vested over the balance of 2009 with 1,132,464 vesting on the grant date and the remainder vesting in equal installments on August 31, September 30, October 31, November 30 and December 31, 2009.
|
|
(2)
|
This option was granted under the 2007 Stock Option Plan. This option vests on the following schedule:
|
|
Vesting Event
|
|
# of Shares
|
|
|
June 23, 2009
|
|
|
125,000
|
|
|
May 31, 2010
|
|
|
125,000
|
|
|
May 31, 2011
|
|
|
100,000
|
|
|
May 31, 2012
|
|
|
99,996
|
|
|
May 31, 2013
|
|
|
99,996
|
|
|
Swiss Approval
|
|
|
100,000
|
|
|
Full Enrollment in Phase II Glioblastoma Multiforme Clinical Study
|
|
|
100,000
|
|
|
FDA Approval of NDA
|
|
|
100,000
|
|
(3) This option was granted under the 2007 Stock Option Plan. This option grant vested over the balance of 2009 with 125,000 vesting on the grant date and the remainder vesting on December 31, 2009.
Outstanding Equity Awards at Fiscal Year-End
The following table shows outstanding stock option awards classified as exercisable and un-exercisable as of December 31, 2010.
|
|
|
Option Awards
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name and Principal
Position
|
|
Number
of Securities
Underlying
Unexercised
Options
Exercisable
|
|
|
Number
of Securities
Underlying
Unexercised
Options
Un-exercisable
|
|
|
Option
Exercise Price
($)
|
|
Option
Expiration
Date
|
|
Number of
Shares or
Units of Stock
that Have Not
Vested
|
|
|
Market Value
of Shares or
Units of Stock
that Have not
Vested
|
|
|
Equity Incentive
Plan Awards:
Number of
Unearned Shares,
Units or Other
Rights that
Have Not vested
|
|
|
Equity
Incentive Plan
Awards:
Market or
Payout
Value of
Unearned
Shares Units
or Other
rights that
Have Not
Vested
|
|
|
Alton L. Boynton
|
|
|
5,286
|
(1)
|
|
|
|
|
|
18.75
|
|
04/18/11
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
President and Chief
|
|
|
6,666
|
(1)
|
|
|
|
|
|
1.35
|
|
02/18/13
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Executive Officer
|
|
|
125,142
|
(2)
|
|
|
41,714
|
|
|
|
0.60
|
|
12/31/11
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,430,486
|
(3)
|
|
|
|
|
|
|
0.55
|
|
08/20/19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Marnix L. Bosch
|
|
|
333
|
(4)
|
|
|
|
|
|
|
18.75
|
|
09/20//11
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Technical
|
|
|
833
|
(4)
|
|
|
|
|
|
|
75.00
|
|
01/10/12
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Officer
|
|
|
3,194
|
(4)
|
|
|
139
|
|
|
|
1.35
|
|
2/18/13
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,000
|
(4)
|
|
|
1,333
|
|
|
|
1.80
|
|
12/01/13
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
69,295
|
(5)
|
|
|
111,614
|
|
|
|
0.60
|
|
12/31/11
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
308,338
|
(6)
|
|
|
541,662
|
|
|
|
0.70
|
|
06/23/19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
250,000
|
(7)
|
|
|
|
|
|
|
0.55
|
|
08/20/19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1)
|
These options were granted under the 1999 Plan, the 2001 Plan and under Dr. Boynton’s previous employment agreement. Each of these option grants vests over a four year period. One-fourth of each option grant vests on the first anniversary of the grant date and the remaining three-fourths of each grant vests in equal monthly installments over the remaining three year vesting period.
|
|
(2)
|
This option was granted under the 2007 Stock Option Plan. This option grant vests over a three and one-half year period. Approximately 29% the option grant was vested immediately upon grant with respect to prior service performed. Approximately 17% vests on the first anniversary of the AIM offering (June 22, 2008) and the remaining portion vests in equal monthly installments over the remaining three year vesting period. These options were granted in recognition of past service to the Company and have an exercise price of $0.60 per share, which is equal to the conversion price of warrants issued to Toucan Partners under the Conversion Agreement. In accordance with Dr. Boynton’s option agreement as options to 1,430,846, 500,568 and 500,568 shares had not been exercised as of December 31, 2008, 2009 and 2010 respectively such options were forfeited.
|
|
(3)
|
This option was granted under the 2007 Stock Option Plan. This option grant vested over the balance of 2009 with 1,132,464 vesting on the grant date and the remainder vesting in equal installments on August 31, September 30, October 31, November 30 and December 31, 2009.
|
|
(4)
|
These options were granted under the 1999 Plan and the 2001 Plan. Each of these option grants vests over a four year period. One-fourth of each option grant vests on the first anniversary of the grant date and the remaining three-fourths of each grant vests in equal monthly installments over the remaining three year vesting period.
|
|
(5)
|
This option was granted under the 2007 Stock Option Plan. This option grant vests over a three and one-half year period. Approximately 19% of the option grant was vested immediately upon grant with respect to prior service performed. Approximately 21% vests on the first anniversary of the AIM offering (June 22, 2008) and the remaining portion vests in equal monthly installments over the remaining three year vesting period. These options were granted in recognition of past service to the Company and have an exercise price of $0.60 per share, which is equal to the conversion price of warrants issued to Toucan Partners under the Conversion Agreement. In accordance with Dr. Bosch’s option agreement as options to purchase 250,000 and 300,000 shares had not been exercised as of December 31, 2008 and 2009 respectively such options were forfeited.
|
|
(6)
|
This option was granted under the 2007 Stock Option Plan. This option vests on the following schedule:
|
|
Vesting Event
|
|
# of Shares
|
|
|
June 23, 2009
|
|
|
125,000
|
|
|
May 31, 2010
|
|
|
125,000
|
|
|
May 31, 2011
|
|
|
100,000
|
|
|
May 31, 2012
|
|
|
99,996
|
|
|
May 31, 2013
|
|
|
99,996
|
|
|
Swiss Approval
|
|
|
100,000
|
|
|
Full Enrollment in Phase II Glioblastoma Multiforme Clinical Study
|
|
|
100,000
|
|
|
FDA Approval of NDA
|
|
|
100,000
|
|
|
(7)
|
This option was granted under the 2007 Stock Option Plan. This option grant vested over the balance of 2009 with 125,000 vesting on the grant date and the remainder vesting on December 31, 2009.
|
Option Exercises and Stock Vested
No options were exercised by and no stock awards vested for the Named Executive Officers during 2010.
Pension Plans, Deferred Compensation and Severance Agreements
We do not currently offer any such plans or compensation or have any such agreements in place.
Director Compensation
The following table sets forth certain information concerning compensation paid or accrued to our non-executive directors during the year ended December 31, 2010.
|
Name
|
|
Year
|
|
Fees Earned
or Paid
in Cash
|
|
|
All Other
Compensation(1)
|
|
|
Total
|
|
|
Linda F. Powers
|
|
2010
|
|
$
|
100,000
|
|
|
$
|
-
|
|
|
$
|
100,000
|
|
|
Robert A. Farmer
|
|
2010
|
|
$
|
70,613
|
(1)
|
|
$
|
-
|
|
|
$
|
70,613
|
|
(1) includes directors’ fees for 2009 (previously unpaid) as well as full year 2010.
Only non-employee directors receive director fees. Effective June 22, 2007, we are required to pay Linda F. Powers, as Chairperson and a non-executive member of the Board of Directors, approximately $100,000 per annum for her services. Also effective December 10, 2009 we were required to issue Robert A. Farmer, for his services as a non-executive member of the Board of Directors, 50,000 shares of the company’s common stock per annum.
Compensation Committee Interlocks and Insider Participation
From January 1, 2007 to June 22, 2007, Dr. Boynton was the sole member of our Compensation Committee and served as our President, Chief Operating Officer and Chief Scientific Officer. In addition, as discussed further under “Transactions with Related Persons” below, in 2006, Dr. Boynton exercised warrants and convertible loans covering 126,365 and 146,385 shares of our common stock, respectively. In June 2007, Dr. Boynton was replaced by Linda F. Powers and R. Steve Harris as members of the Compensation Committee. During 2008, none of our executive officers served as a member of the compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director on our Board or as a member of our Compensation Committee. None of our executive officers served during 2010 as a director of any other entity, one of whose executive officers served as a director on our Board or as a member of our Compensation Committee.
Equity Plans
Stock Option Plans
The Company’s stock option plans are administered by the Board of Directors, which determines the terms and conditions of the options granted, including exercise price, number of options granted and vesting period of such options.
The 2007 Stock Option Plan became effective on June 15, 2007. In April 2008, the Company increased the number of shares reserved for issuance by 519,132 shares for an aggregate of 6,000,000 shares of its common stock. In May 2010, the Company increased the number of shares reserved for issuance under the 2007 Stock Option Plan by an additional 10,000,000 shares of its common stock. In May 2011, the Company increased the number of shares reserved for issuance under the 2007 Stock Option Plan by an additional 20,000,000 shares of its common stock.
Our employees, directors and consultants previously participated in the 1998 Stock Option Plan and the 1999 Executive Stock Option Plan. The 1998 Stock Option Plan and the 1999 Executive Stock Option Plan were terminated during 2008 and 2009 and no further grants may be made under the plans.
Existing stock option plans are as follows:
(a) 2001 Stock Option Plan
Under the 2001 Stock Option Plan (the “2001 Plan”), 120,000 shares of the Company’s common stock have been reserved for grant of stock options to employees and consultants. Additionally, on January 1 of each year, commencing January 1, 2002, the number of shares reserved for grant under the 2001 Plan will increase by the lesser of (i) 15% of the aggregate number of shares available for grant under the 2001 Plan or (ii) 20,000 shares. Our Board of Directors has the authority to amend or terminate this plan, but such action may not adversely affect any outstanding option previously granted under the plan. If this plan is not terminated earlier, no incentive stock options can be granted under the plan on or after the later of June 2011 or the 10th anniversary of the date when our Board of Directors adopted, subject to approval by our stockholders, the most recent increase in the number of shares available for grant under the plan.
(b) 2001 Non-employee Director Stock Incentive Plan
Under the 2001 Non-employee Director Stock Incentive Plan (the “2001 Director Plan”), 13,333 shares of the Company’s common stock have been reserved for grant of stock options to non-employee directors of the Company. As of December 31, 2009, net of forfeitures, a total of 10,500 shares remain available under this plan; however, no further grants may be made under this plan.
Employee Stock Purchase Plan
Our Employee Stock Purchase Plan (the “Employees’ Plan”) was adopted by our Board of Directors and approved by our stockholders in June 2001.
This plan is administered by the Compensation Committee of our Board of Directors and provides a mechanism for eligible employees to purchase shares of our common stock. To facilitate these purchases, eligible participants are assigned plan accounts, to which they may contribute funds via payroll deduction. The purchases are accomplished through the use of six-month offering periods. Purchases pursuant to this plan are made at a price equal to the lower of (i) 85% of the fair market value of our common stock on the last trading day in the offering period; or (ii) 85% of the fair market value of our common stock on the last trading day before the commencement of such offering period. No participant may purchase more than 67 shares of our common stock during any offering period. Additionally, purchases under the plan are limited such that no participant may purchase under the plan, in any offering period that commenced in that calendar year, shares with a fair market value in excess of $25,000 minus the fair market value of any shares that the participant previously purchased in that calendar year. In the case of shares purchased during an offering period that commenced in the preceding calendar year, the limitation is $50,000 minus the fair market value of any shares that the participant purchased during the calendar year of the purchase and the calendar year immediately preceding such purchase.
Our Board of Directors has the authority to amend or terminate this plan at any time. Amendments to the plan are subject to approval by our stockholders to the extent required by applicable law.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table presents information regarding the beneficial ownership of our common stock as of January 10, 2012 by:
|
|
·
|
each person, or group of affiliated persons, who is known by us to own beneficially 5% or more of any class of our equity securities;
|
|
|
·
|
each of our named executive officers, as defined in Item 402(a)(3) of Regulation S-K; and
|
|
|
·
|
our directors and executive officers as a group.
|
The applicable percentages of ownership are based on an aggregate of 149,283,254 shares of common stock issued and outstanding on January 10, 2012. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed shares of common stock subject to options, warrants, convertible preferred stock or convertible notes held by that person that are currently exercisable or exercisable within 60 days of January 10, 2012.
We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and the entities named in the table have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws.
Except as otherwise noted, the address of the individuals in the following table below is c/o Northwest Biotherapeutics, Inc., 4800 Montgomery Lane, Suite 800, Bethesda, MD 20814.
|
Name of Beneficial Owner
|
|
Number of Shares
Beneficially Owned
|
|
|
Percentage(1)
|
|
|
Officers and Directors
|
|
|
|
|
|
|
|
Alton L. Boynton, Ph.D.(2)
|
|
|
3,074,719
|
|
|
|
2
|
%
|
|
Marnix L. Bosch, Ph.D., M.B.A.(3)
|
|
|
1,820,056
|
|
|
|
1.2
|
%
|
|
Linda F. Powers(4)
|
|
|
112,174,363
|
|
|
|
59.1
|
%
|
|
Robert A. Farmer (5)
|
|
|
1,300,968
|
|
|
|
0.9
|
%
|
|
Les Goldman (6)
|
|
|
3,220,823
|
|
|
|
2.1
|
%
|
|
All executive officers and directors as a group (5 persons)(7)
|
|
|
121,590,929
|
|
|
|
61.6
|
%
|
|
5% Security Holders
|
|
|
|
|
|
|
|
|
|
Toucan Capital Fund II, L.P.(8)
|
|
|
27,162,363
|
|
|
|
16.6
|
%
|
|
7600 Wisconsin Avenue, Suite 700, Bethesda, MD 20814
|
|
|
|
|
|
|
|
|
|
Toucan Partners, LLC(9)
|
|
|
28,609,290
|
|
|
|
16.8
|
%
|
|
7600 Wisconsin Avenue, Suite 700, Bethesda, MD 20814
|
|
|
|
|
|
|
|
|
|
Regen Med Acquisition Corp (10)
|
|
|
11,144,165
|
|
|
|
7.1
|
%
|
|
1313 N. Market Street, Suite 5100
Wilmington, DE 19801
|
|
|
|
|
|
|
|
|
|
Al Rajhi Holdings (11)
|
|
|
6,353,872
|
|
|
|
4.3
|
%
|
|
Rue Maurice 3, 1204 Geneve, Switzerland
|
|
|
|
|
|
|
|
|
|
Al Salam Opportunities Limited
4
th
Floor Harbour Center
P.O. Box 613 George Town
Grand Cayman, British West Indies, KY1-1001
|
|
|
2,590,270
|
|
|
|
1.7
|
%
|
|
The Richard M. Schulze Family Trust (12)
|
|
|
8,902,175
|
|
|
|
6
|
%
|
|
8500 Normandale Lake Blvd, Suite 1750,
|
|
|
|
|
|
|
|
|
|
Minneapolis, MN 55347
|
|
|
|
|
|
|
|
|
|
Cognate BioServices, Inc.(13)
|
|
|
48,500,000
|
|
|
|
32.5
|
%
|
|
4800 East Shelby Drive, Suite 108, Memphis, TN
|
|
|
|
|
|
|
|
|
|
(1)
|
Percentage represents beneficial ownership percentage of common stock calculated in accordance with SEC rules and does not equate to voting percentages.
|
|
(2)
|
Includes 2,833,715 shares of common stock issuable upon exercise of options that are exercisable within 60.
|
|
(3)
|
Includes 1,486,039 shares of common stock issuable upon exercise of options that are exercisable within 60 days.
|
|
(4)
|
Includes (i) 12,866,324 shares of common stock held by Toucan Capital; (ii) 14,296,039 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days held by Toucan Capital; (iii) 7,946,447shares of common stock held by Toucan Partners; (iv) 12,516,924 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days of September 6, 2011; (v 8,145,919 shares of common stock currently issuable upon conversion of convertible loans held by Toucan Partners; (vi) 5,530,000 shares of common stock issuable upon exercise of options that are exercisable within 60 days; and (vii) 48,500,000 shares of common stock issued upon conversion of amounts due to Cognate. Ms. Powers is a managing member of Toucan Management, LLC, which is the manager of Toucan Capital; is a managing member of Toucan Partners and controls a majority of the stock of Cognate BioServices, Inc.
|
|
(5)
|
Includes 328,397 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days of September 6, 2011 and 140,000 shares issuable on conversion of a loan held by Mr. Farmer convertible within 60 days.
|
|
(6)
|
Includes 1,824,262 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days; 875,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of September 6, 2011 and 224,000 shares of common stock issuable upon conversion of loans held by Mr. Goldman convertible within 60 days.
|
|
(7)
|
Includes 10,724,754 shares issuable to officers and directors upon exercise of options that are exercisable within 60 days; 28,965,622 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days; and 8,509,919 shares of common stock issuable upon conversion of convertible notes and amounts due to Toucan Partners.
|
|
(8)
|
Includes 14,296,039 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days held by Toucan Capital.
|
|
(9)
|
Includes 12,516,924 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days and 8,145,919 shares of common stock currently issuable upon conversion of convertible loans held by Toucan Partners.
|
|
(10)
|
Includes 7,416,703 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days held by Regen Med.
|
|
(11)
|
Includes 1,743,411 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days held by Al Rajhi.
|
|
(12)
|
Includes 2,307,972 shares of common stock currently issuable upon exercise of warrants that are exercisable within 60 days held by the Richard M. Schulze Family Trust.
|
|
(13)
|
Includes 48,500,000 shares of common stock issued upon conversion of amounts due to Cognate.
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Toucan Capital and Toucan Partners
Toucan Capital Fund II, L.P. loaned the Company $6.75 million during 2004 and 2005. The Company’s Chief Executive Officer and the Board’s Chairperson is the managing director of Toucan Capital. In April 2006, the $6.75 million of notes payable plus all accrued interest were converted into shares of Series A-1 cumulative convertible Preferred Stock (the “Series A-1 Preferred Stock”). In connection with these loans the Company issued Toucan Capital a warrant to purchase 8,166,667 shares of Series A-1 Preferred Stock. The warrants to purchase Series A-1 Preferred Stock were later converted into warrants to purchase 17,256,888 shares of common stock in connection with the Conversion Agreement, described below.
On January 26, 2005, Toucan Capital purchased 32.5 million shares of Series A cumulative convertible preferred stock (the “Series A Preferred Stock”) at $0.04 per share, for a total of $1.276 million. In connection with the securities purchase agreement, the Company issued Toucan Capital a warrant to purchase 2,166,667 million shares of Series A Preferred Stock. The warrants to purchase Series A Preferred Stock were later converted into warrants to purchase 4,778,201 shares of common stock in connection with the Conversion Agreement, described below.
From November 14, 2005 through May 25, 2007, Toucan Partners, LLC loaned the Company $4.825 million under various promissory note agreements. The Company’s Chief Executive Officer and the Board’s Chairperson is the managing member of Toucan Partners. The promissory note agreements were amended and restated into the 2007 Convertible Notes. The 2007 Convertible Notes also included warrants to purchase shares of Series A-1 Preferred Stock ("2007 Warrants"). The Company repaid $5.3 million of principal and accrued interest due to Toucan Partners during 2007. The warrants to purchase Series A-1 Preferred Stock were later converted into warrants to purchase 8,832,541 shares of common stock in connection with the Conversion Agreement, described below.
Under the June 22, 2007 Conversion Agreement, Toucan Capital and Toucan Partners agreed to eliminate a number of rights, preferences and protections associated with the Series A Preferred Stock and the Series A-1 Preferred Stock and Toucan Capital received 4,287,851 shares of common stock and Toucan Partners received 2,572,710 shares of common stock. Also, Toucan Capital converted its preferred shares into 15,011,635 shares of common stock. Additionally under the conversion agreement the Company exchanged the warrants to purchase Series A-1 Preferred Stock and Series A Preferred Stock (discussed above) for warrants to purchase common stock. As a result of the conversion Toucan Capital received warrants to purchase 14,150,732 shares of Common Stock at an exercise price of $0.60 per share and warrants to purchase 7,884,357 shares of Common Stock at an exercise price of $0.15 per share and Toucan Partners received warrants to purchase 8,832,541 shares of Common Stock at an exercise price of $0.60 per share.
Toucan Partners loaned the Company $1.0 million on August 19, 2008 under the terms of an unsecured promissory note (the “Toucan Partners August Loan”) with a principal amount of $1,060,000 (reflecting an original issue discount of $60,000). On September 28, 2009, the note principal and accrued interest (including a default penalty of 0.25% per month) amounting to $1,156,718 was converted to 5,783,589 shares of common stock at a conversion price of $0.20. In connection with the conversion, the Company issued Toucan Partners a warrant to purchase 690,000 shares of common stock at an exercise price of $0.20 per share.
Toucan Partners loaned the Company $500,000 on December 22, 2008 under the terms of an unsecured 12% promissory note (the “Toucan Partners December Loan”). In connection with the promissory note, the Company issued to Toucan Partners a warrant to purchase 132,500 shares of common stock at an exercise price of $0.40 per share and a term of 5 years. On September 28, 2009, the note principal and accrued interest (including a default penalty of 0.25% per month) amounting to $552,738 was converted to 2,763,691 shares of common stock at a conversion rate of $0.20. To bring the December Loan into conformity with the SDS and Private Lender notes issued in October and November 2008, as agreed by the parties at the time of the Toucan Partners December Loan, the Company issued Toucan Partners a warrant to purchase 513,841 shares of common stock at an exercise price of $0.41 per share. In connection with the conversion, the Company issued Toucan Partners a warrant to purchase 152,375 shares of common stock at an exercise price of $0.20 per share.
Toucan Partners and the Board's Chairperson also received a total of 2,504,034 shares of common stock as compensation for services rendered during 2008 and 2009.
Toucan Partners loaned the Company a total of $1,300,000 on June 30, 2009, July 2, 2009 and July 17, 2009 under unsecured 6% convertible promissory notes due June 29, 2011, July 1, 2011 and July 16, 2011. The conversion feature of the notes allows Toucan Partners to convert the principal into shares of common stock at a conversion price of $0.20.
On July 2, 2010 the Company entered into a securities purchase agreement with Toucan Partners, under which Toucan Partners purchased 866,667 shares of common stock for $650,000. In connection with this private placement the Company issued warrants to purchase 86,667 shares of common stock at an exercise price of $0.75 per share with an exercise period of three years.
On October 1, 2010 Toucan Partners loaned the Company $900,000 by repaying a portion of the Bridge Funding Note payable to Regen Med Acquisition Corporation (“Regen Med”) originating on July 14, 2010 under a 6% convertible promissory note on the same terms and conditions as the September Notes that the Company had negotiated and executed with non-affiliated investors on September 28, 2010, secured by an interest in all the Company’s assets, due on December 1, 2010. The conversion feature of the note allows Toucan Partners to convert the principal into shares of common stock at a conversion price of $0.75. Additionally, Toucan Partners received 100% warrant coverage, on the same terms and conditions as the September Notes (including the same market formula for the warrant exercise price), at $0.82 per share. In the event of default, Toucan Partners was entitled to adjust the interest rate to 9% per annum for the default period and to be granted an additional 100% warrant coverage at $0.82 per share. The Company did not repay the loan on December 1, 2010 however Toucan waived its right to increase the interest rate and waived its right to the additional warrant. The loan was repaid in full by December 31, 2010.
In December 2010 Toucan Capital transferred 6,433,162 shares of common stock and 7,345,030 warrants to purchase shares of the Company’s common stock to Regen Med Acquisition Corp (“Regen Med”), a non-affiliate third party.
In March 2011, the Company received $550,000 from Toucan Capital as a convertible note payable. The note was issued with a 10% original issue discount and a 10% one time interest charge payable to Toucan Capital.
As a result of the financings described above, as of September 30, 2011 Toucan Capital held:
|
|
•
|
an aggregate of 12,866,324 shares of Common Stock;
|
|
|
•
|
warrants to purchase 9,433,821 shares of Common Stock at an exercise price of $0.60 per share (net of 4,716,911 transferred to Regen Med);
|
|
|
•
|
warrants to purchase 5,256,238 shares of Common Stock at an exercise price of $0.60 per share (net of 2,628,119 transferred to Regen Med);
|
As a result of the financings described above, and other open market transactions, as of September 30, 2011, Toucan Partners and its managing member Ms. Linda Powers held:
|
|
•
|
an aggregate of 9,750,691 shares of Common Stock;
|
|
|
•
|
warrants to purchase 18,266,362 shares of Common Stock at an exercise price of $0.60 per share;
|
|
|
•
|
warrants to purchase 513,841 shares of common stock at an exercise price of $0.41 per share;
|
|
|
•
|
warrants to purchase 132,500 shares of common stock at an exercise price of $0.40 per share;
|
|
|
•
|
Warrants to purchase 2,195,667 shares of common stock at an exercise price of $0.75 per share
|
|
|
•
|
warrants to purchase 842,375 shares of common stock at an exercise price of $0.20 per share;
|
|
|
•
|
Warrants to purchase 5,256,238 shares of common stock at an exercise price of $0.15 per share
|
As of September 30, 2011, Toucan Capital, including the holdings of Toucan Partners, held 22,617,015 shares of common stock, representing approximately 23.3% of the common stock outstanding. Further, as of September 30, 2011, Toucan Capital, including the holdings of Toucan Partners, beneficially owned (including unexercised warrants) 41,426,953 shares of common stock, representing a beneficial ownership interest of approximately 36.1%.
On March 21, 2008, the Company executed a Sublease Agreement (the “Sublease Agreement”) with Toucan Capital Corporation for the space the Company used as its headquarters at 7600 Wisconsin Avenue, Suite 750, Bethesda, Maryland. The Sublease Agreement is effective as of July 1, 2007 and expires on October 31, 2016, unless sooner terminated according to its terms. The Company was and remains obligated to pay operating expenses allocable to the subleased premises under Toucan Capital Corporation’s master lease. Effective November 30, 2009, the Sublease was terminated in connection with termination and buyout of the overall lease of this space. (The overall lease and the Company’s Sublease had 7 years left to run at that time). The termination and buyout did not require any lump sum exit payment. Instead, it requires a partial payout over several years. During 2011the obligation for the Company is less than $5,000 per month.
Cognate
During the quarter ended June 30, 2011, the Company entered into a new service agreement with Cognate Therapeutics, Inc. (“Cognate”), a contract manufacturing and services organization in which Toucan Capital has a majority interest. In addition, two of the principals of Toucan Capital are members of Cognate’s board of directors and Linda Powers who is a director of Cognate and managing director of Toucan Capital is Chairperson of the Company’s Board of Directors and Chief Executive of the Company. This agreement replaces the agreement dated May 17, 2007 between the Company and Cognate, which had expired. Under the service agreement, the Company agreed to continue to utilize Cognate’s services, for certain consulting and manufacturing services to the Company for its ongoing DCVax®-Brain Phase II clinical trial. The scope of services and the economics are comparable to the prior agreement, and the structure and process for payments are simplified. Under the terms of the new agreement the Company will pay Cognate a monthly facility fee of $400,000 per month (the same amount as under the prior agreement) and a fixed fee (in lieu of cost-plus charges) for each patient enrolled in the study, subject to specified minimum number of patients per month, plus charges for certain patient and product data services if such services are requested by the Company. The current service agreement expires on March 31, 2016. Additionally the Company has agreed to reimburse Cognate for enrollment ramp up costs, foreign program costs and costs of facilities and equipment dedicated to Company programs (which were also previously reimbursable).
Cognate has a cGMP (clean room manufacturing under current Good Manufacturing Practices) facility with a capacity for approximately 600 patients per year, which we believe will be sufficient for our Phase II clinical trial for DCVax -Brain. We have a plan with Cognate to accommodate an increase in production capacity based on demand and have detailed plans and cost analysis for additional modular expansions which should increase the capacity of the current facilities from approximately 600 patients to over 9,000 patients per year. We believe that Cognate’s current facilities are sufficient to cover additional agreements for our initial commercialization efforts
During 2009, the Company and Cognate agreed that most of the accounts payable owed by the Company to Cognate, will be converted into shares of common stock instead of paid in cash. The conversion price will be no less favorable than the conversion price applied to any other creditor of the Company. The lowest conversion price applied to any other creditor of the Company to date following the agreement is $0.20 per share. Accordingly, if no lower conversion price is applied to any other creditor prior to completion of the Cognate conversion, the Cognate conversion will take place at $0.20 per share. The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of accounts payable converted, if any, as a charge to operations when the conversion takes place. Initial review of the payables by both parties indicated a difference in the parties’ respective understanding of the amounts due. The parties have now agreed that the amount due to Cognate at December 31, 2010, was $10.2 million and the Company has recognized $3.6 million in additional expense in 2010. Finalization of the conversion arrangements are in process.
Also during 2009, the Company agreed with Toucan Capital, Toucan Partners and Linda Powers (the Board’s Chairperson) that accrued expenses owed by the Company to these parties for certain expense reimbursements will be converted into shares of common stock instead of paid in cash. The parties agreed that these accrued expenses will be converted into common stock at a conversion rate equal to the price per share paid by unrelated investors at that time, and no less favorable than the conversion rate applied to any other creditor of the Company ($0.20 per share). The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of the accrued expenses converted, if any, as a charge to operations when the conversion takes place. Finalization of these arrangements is in process.
During the nine months ending September 30, 2010 and 2011, respectively, the Company recognized approximately $7.8 million and $10.2 million of research and development costs related to this service agreement. As of December 31, 2009 and 2010, the Company owed Cognate approximately $5.9 million and $10.2 million, respectively. In February 2011 Cognate sold $1,650,000 of the amount due to the Company to a non-affiliated third party.
Director Independence
We have one independent director on our Board of Directors and will insure that all transactions are on terms at least as favorable to the Company as we would negotiate with unrelated third parties
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the expenses in connection with the issuance and distribution of the securities being registered hereby. All such expenses will be borne by the Company; none shall be borne by any Selling Stockholder.
|
SEC Registration Fee
|
|
$
|
304.84
|
|
|
Legal Fees and Expenses
|
|
|
40,000.00
|
|
|
Accounting Fees and Expenses
|
|
|
20,000.00
|
|
|
Miscellaneous
|
|
|
10,000.00
|
|
|
|
|
|
|
|
|
TOTAL
|
|
$
|
70,304.84
|
|
INDEMNIFICATION OF DIRECTORS, OFFICERS, EMPLOYEES AND AGENTS
The Certificate of Incorporation of our company provides that no director will be personally liable to the company or its stockholders for monetary damages for breach of a fiduciary duty as a director, except to the extent such exemption or limitation of liability is not permitted under the Delaware General Corporation Law. The effect of this provision in the Certificate of Incorporation is to eliminate the rights of the company and its stockholders, either directly or through stockholders’ derivative suits brought on behalf of the company, to recover monetary damages from a director for breach of the fiduciary duty of care as a director except in those instances described under the Delaware General Corporation Law.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 ("the "Act") may be permitted to directors, officers and controlling persons of the small business issuer pursuant to the foregoing provisions, or otherwise, the small business issuer has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the small business issuer of expenses incurred or paid by a director, officer or controlling person of the small business issuer in the successful defense of any action, suit or proceeding) is asserted by a director, officer or controlling person in connection with the securities being registered, the small business issuer will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
RECENT SALES OF UNREGISTERED SECURITIES
Below is information as to all securities sold by the Registrant within the past three years which were not registered under the Securities act of 1933:
On January 16, 2009 we entered into a securities purchase agreement for $700,000 with Al Rajhi Holdings who purchased 1,000,000 shares of our common stock at $0.70 per share.
During March 2009, we entered into loan agreements with a group of private lenders for $760,000 for a term of two years at 6% per annum.
On March 27, 2009, we sold approximately 1.4 million shares of common stock at a purchase price of $0.53 per share and raised aggregate gross proceeds of approximately $0.7 million in a closed equity financing with unrelated investors.
In July 2009 we entered into a loan agreement with Toucan Partners for $1,300,000 with a term of two years at 6% interest.
In August and September 2009 we entered into a series of small loan agreements with a group of Private Investors for an aggregate of $580,000 with a term of two years at 6% interest.
In October through December 2009 we entered into a series of small loan agreements with a group of private investors for an aggregate of $720,000 with a term of two years at 6% interest.
Between February 22, 2010 and March 31, 2010, the Company sold 1,451,666 shares of common stock at $0.75 per share for net proceeds of $1,088,750.
During the three months ended June 30, 2010, the Company sold to private investors 2,333,333 shares of common stock at $0.75 per share for net proceeds of $1,750,000. In connection with this private placement, the Company issued warrants to purchase 233,333 shares of common stock, as described below.
On July 2, 2010, the Company completed execution of a Conversion and Extension Agreement with SDS Capital (“SDS”) for the conversion or extension of all outstanding debt held by SDS, and new investment of two hundred and fifty thousand dollars ($250,000) in restricted Common Stock. Prior to this Agreement, SDS was the second largest holder of investment debt of the Company. The largest holder of investment debt is the Al Rajhi Group. As previously reported, the Company already entered into an agreement with the Al Rajhi Group in March, 2010, for partial conversion into Common Stock and partial extension of the maturity of the debt held by Al Rajhi.
The Conversion and Extension Agreement with SDS Capital relates to investment debt of $2 million plus accumulated interest. In October 2008, SDS loaned the Company $1 million, with an original maturity of six months and an annual interest rate of twelve percent. In November 2008, SDS loaned the Company an additional $1 million, also with an initial maturity of six months and an annual interest rate of twelve percent. SDS previously agreed to extend the maturity of the two loans on terms to be negotiated with the Company.
Under the terms of a conversion and extension agreement, SDS converted $1,060,000, representing principal and accrued interest on an unsecured 12% loan made in November 2008 into 5,300,000 shares of common stock during May 2010 and December 2010. The value of the stock issued to SDS in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $4,673,000. This amount was charged to loan conversion inducement expense in the consolidated statements of operations during the quarters ended June 30, 2010 and December 31, 2010.
In July 2010, the Company sold to Toucan Partners 866,667 shares of common stock at $0.75 per share for net proceeds of $650,000. In connection with this private placement, the Company issued warrants to purchase 86,667 shares of common stock, as described below.
On July 14, 2010, the Company entered into unsecured 6% Loan Agreements and Convertible Promissory Notes with Regen Med. Under these Notes, Regen Med provided bridge funding (“Bridge Funding”) to the Company in the amount of $1,750,000. The Bridge Funding carried an interest rate of 6% with a term of 60 days maturing on September 14, 2010 and was convertible at the discretion of the lender at a conversion price of at $0.75 per share. On September 28, 2010 $350,000 of the principal was repaid directly by the holders of the notes described below. On October 1, 2010 Toucan Partners and the holder of the note also described below repaid the remaining $1,400,000 payable to Regen Med.
On September 28, 2010 the Company entered into unsecured 6% Loan Agreements and Promissory Notes (the “September Notes”) with private lenders in the amount of $350,000 and used the proceeds to partially repay the Bridge Funding. The notes had a term of 60 days and were convertible into shares of the Company’s common stock at $0.75 per share.
On October 1, 2010 the Company entered into unsecured 6% Loan Agreements and Promissory Notes (the “October Note”) with Toucan Partners in the amount of $900,000 when Toucan Partners partially repaid the Regen Med Notes. The terms of the October Note and related warrants conformed to the terms of the September Notes previously negotiated by the Company with unrelated investors, and executed by the Company with such investors prior to these October Notes with Toucan Partners. Accordingly, the October Notes had a term of 60 days and were convertible into shares of the Company’s common stock at $0.75 per share.
On October 1, 2010 the Company entered into unsecured 6% Loan Agreements and Promissory Notes (the “Additional October Note”) with an unrelated third party in the amount of $500,000, on the same terms and conditions as the September Notes with non-affiliated investors, and used the proceeds to partially repay the Bridge Funding. The notes had a term of 60 days and were convertible into shares of the Company’s common stock at $0.75 per share.
On October 19, 2010, we sold approximately 200,000 shares of common stock at a purchase price of $0.75 per share and raised aggregate gross proceeds of approximately $150,000 in a closed equity financing with an unrelated investor.
On November 29, 2010 the Company received $295,000 upon issuing unsecured 10% convertible loan agreements and promissory notes due on May 29, 2011 to a group of non-affiliated investors. The terms promissory notes have a principal amount of $324,500 (reflecting an original issue discount of $29,500).
On December 16, 2010 the Company entered into a $100,000 unsecured 10% convertible loan agreement with a non affiliated party due March 17, 2011. Warrants to purchase 133,333 shares of common stock at an exercise price of $0.75 per share were issued with the loan agreements.
On December 16, 2010, the Company entered into an agreement to potentially borrow up to $3,050,000 in tranches from a non affiliated third party (the “Lender”). As of December 31, 2010 formal agreements have been closed for a borrowing of $1,450,000. Under this arrangement, the Lender provides secured collateralized promissory notes to the Company for the funding tranches ("Lender Notes"). The notes are secured by certain assets of the Lender, which makes the funding eligible for certain securities law treatment for the Lender (the Lender investment is a recourse loan to the Company) which the Lender considers beneficial. When the Lender advances a tranche of funding under a Lender Note to the Company, that funding becomes a repayment obligation of the Company, which is embodied in a convertible promissory note from the Company to the Lender (the "Company Note"). The Company may repay any advance from the Lender at any time with either cash, surrender of the collateral, or surrender of the Company Note which would terminate the Company’s obligation under such Lender Notes.
As of December 31, 2010, the Lender had executed Lender Notes to the Company with principal amounts of $950,000 and $500,000. These Lender Notes carry a one-time interest charge equal to 11 percent of the principal payable at maturity. The Lender Notes have a maturity date of December 16, 2013. In the event that the Lender fails to meet its obligations under its notes, the Company has recourse to liquidate the security collateralizing the loan. The proceeds generated from liquidating the collateral will also be treated as Lender Note.
As of December 31, 2010 $350,000 had been advanced against the $950,000 Lender Note.
As of December 31, 2010, the Company had executed corresponding Company Notes, payable to the Lender, with principal amounts of $1,050,000 (reflecting the $950,000 principal amount in the Lender’s note, plus an original issue discount of $100,000) and $550,000 (reflecting the $500,000 principal amount in the Lender’s note, plus original issue discount of $50,000). The Company Notes also carry a one-time interest charge of 10 percent payable at maturity. The Company Notes have a maturity date of December 16, 2013. As noted above, the Company Notes may be surrendered to terminate the Company’s obligation under the Lender Notes. The Company has not incurred an obligation under the Company Notes until they are surrendered.
As of December 31, 2010, SDS sold a promissory note in the original principal amount of $1,000,000 to three non-affiliated, third parties. The Company exchanged the note for amended convertible notes with the following terms:
|
|
(1)
|
A convertible note in the amount of $370,000. The note matures on December 20, 2011 and carries a one-time interest charge payable at maturity of $37,000. The note is convertible into shares of the Company’s stock at a conversion price equal to 70 percent of the average daily closing bid price for the ten trading day period preceding the date of the conversion notice which was $0.47 on the date the note was issued.
|
|
|
(2)
|
A convertible note in the amount of $130,000. The note matures on June 2, 2011 and carries zero interest. The note is convertible into shares of the Company’s stock at a conversion price equal to 80 percent of the average of the five lowest closing prices in the 25 days previous to the conversion date which was $0.54 on the date the note was issued. Any amount that is still outstanding at maturity is repayable in cash.
|
|
|
(3)
|
A convertible note in the amount of $500,000. The note matures on June 2, 2011 and carries zero interest. The note is convertible into shares of the Company’s stock at a conversion price equal to the lesser of (i) the average of the closing prices for the ten trading days preceding the issue date of this note which was $0.67, or (ii) seventy percent of the volume weighted average price for the fifteen trading days prior to a conversion date. The conversion price is adjustable for down rounds as long as any amount due is outstanding under the note, subject to substantial exceptions.
|
On December 31, 2010, the Company received $790,000 from an existing investor, SDS Capital (“SDS”), as of December 31, 2010 in the form of a Note with a maturity of July 2011, and an annual interest rate of twelve percent (12%). SDS also received ten percent (10%) warrant coverage. The warrants have an exercise period of five years, and an exercise price of seventy-five cents ($0.75) per share.
In January 2011 the Company entered into securities purchase agreements with two non-affiliated investors, under which the investors purchased 280,000 shares of common stock for $210,000.
On January 21, 2011, the Company consummated a Note Purchase Agreement with a single investor in the aggregate amount of $2,220,000 for the purchase and sale of two Secured Convertible Promissory Notes, consisting of (i) a principal amount of $1,120,000, with an original issue discount (“OID”) of ten percent (10%) and interest at the rate of 9% per annum, payable on June 30, 2012and (ii) a principal amount of $1,100.000, with OID of ten percent (10%) and interest at the rate of 6% per annum, payable on December 31, 2013.
On March 1, 2011, the Company into a transaction under Section 3(a)(10) of the Securities Act of 1933, as amended (the “Act”) with Socius CG II, Ltd. (“Socius”). Pursuant to this initial 3(a)(10) transaction, Socius purchased certain claims for payment totaling $1,650,000 from Cognate BioServices, Inc.(“Cognate”) which Cognate had with respect to the Company. Thereafter, Socius elected to convert the claims into shares of the Company’s common stock. The conversion was effected through a settlement agreement between Socius and the Company. The settlement agreement was then the subject of a court proceeding (nominally brought by Socius against the Company, but handled on a cooperative basis through a Joint Stipulation by both parties) in order to obtain court approval of the settlement in accordance with the requirements of Section 3(a)(10). That Court approval was obtained on March 1, 2011. Pursuant to the settlement, the full amount of the $1,650,000 debt will be converted into shares of common stock at a conversion price equal to a thirty percent discount from the market price, with the market price being determined over a reference period of up to twenty (20) trading days from the date hereof (the “Reference Period”). In addition, the settlement provided that an equal amount of shares of common stock are to be delivered by the Company to Socius on a temporary basis, as a form of security during the Reference Period. At the end of the Reference Period, a true-up mechanism will be applied to confirm the final number of conversion shares based on the market price.
On March 30, 2011, a private non-affiliated investor provided $500,000 of funding to the Company. This funding was provided pursuant to a convertible note due three years after the date of issuance. The note is convertible into the Company’s common stock at any time during its term at a conversion price equal to a twenty percent (20%) discount from the market price at the time of conversion. The note carries an annual interest rate of ten percent (10%), and Original Issue Discount of ten percent (10%). The note comprises a tranche of the funding arrangement entered into by the Company and the private investor on December 14, 2010, as previously reported.
On March 31, 2011, Toucan Partners provided a matching amount of funding ($500,000) to the Company, in the same form (convertible note) and on the same terms and conditions as the private investor’s funding mentioned immediately above.
On March 30, 2011, at the Company’s request, the investor agreed to cancel a note in the amount of $500,000 that was to be included in future stages of the funding arrangement entered into on December 14, 2010. The effect of this cancellation was to remove the Company’s obligation in that amount, and correspondingly reduce that funding arrangement.
On May 2, 2011, the Company entered into securities purchase agreements with a three non-affiliated investor, under which the investors purchased 312,500 shares of common stock for $150,000.
On May 2, 2011, the Company entered into securities purchase agreements with a three non-affiliated investor, under which the investors purchased 115,741 shares of common stock for $50,000.
On May 5, 2011, a private non-affiliated investor provided $600,000 of funding to the Company. This funding was provided pursuant to a convertible note due in June 2012. The note is convertible into the Company’s common stock at any time during its term at a conversion price equal to a 30% discount from the market price at the time of conversion. The note carries an annual interest rate of six percent, and Original Issue Discount of 10%. The note comprises a tranche of the funding arrangement entered into by the Company and the private investor on January 19, 2011, as previously reported.
On May 16, 2011, the Company entered into securities purchase agreements with a non-affiliated investor, under which the investors purchased 53,333 shares of common stock for $40,000.
On May 16 and May 25, 2011, a private non-affiliated investor provided $500,000 and $50,000, respectively, of funding to the Company. This funding was provided pursuant to a convertible note due three years after the date of issuance. The note is convertible into the Company’s common stock at any time during its term at a conversion price equal to a 20% discount from the market price at the time of conversion. The note carries a one-time interest charge of ten percent 10%, and Original Issue Discount of 10%. The notes comprise tranches of the funding arrangement entered into by the Company and the private investor on December 14, 2010, as previously reported.
On May 31, 2011, a private non-affiliated investor provided $3,000,000 of funding to the Company. This funding was provided pursuant to convertible note due in November 2012. The notes are convertible into the Company’s common stock upon maturity of the notes at a conversion price $0.57 per share. The notes carry an annual interest rate of 10%. The notes fulfill the funding arrangement entered into by the Company and the private investor on May 31, 2911, as reported in Note 6 above.
On June 9, 2011, the Company entered into securities purchase agreements with a non-affiliated investor, under which the investors purchased 438,597 shares of common stock for $250,000.
On June 14, 2011, the Company entered into securities purchase agreements with a non-affiliated investor, under which the investors purchased 104,167 shares of common stock for $75,000.
On June 24, 2011, the Company entered into securities purchase agreements with a non-affiliated investor, under which the investors purchased 21,127 shares of common stock for $15,000.
On June 28, 2011, the Company entered into securities purchase agreements with The Richard M. Schulze Family Foundation, under which the Foundation purchased 6,594,203 shares of common stock for $4,550,000.
In September 2011 the company issued a warrant to purchase 182,640 shares of common stock at an exercise price of $0.75 per share with an exercise period of five years in connection with an extension of a loan of $115,000 made to the Company by an Officer of the Company in November 2010.
In September 2011 the Company issued a warrant to purchase 505,000 shares of common stock at an exercise price of $0.75 per share with an exercise period of five years in connection with an extension of a loan of $500,000 made to the Company by an Officer of the Company in October 2010.
In September 2011 the Company issued a warrant to purchase 909,000 shares of common stock at an exercise price of $0.75 per share with an exercise period of five years in connection with an extension of a loan of $900,000 originally made to the Company by Toucan Partners in October 2010.
In September 2011 the Company issued a warrant to purchase 84,000 shares of common stock at an exercise price of $0.50 per share with an exercise period of five years in connection with an extension of a loan of $150,000 made to the Company by a an Officer of the Company in March 2009.
In September 2011 the Company issued a warrant to purchase 255,000 shares of common stock at an exercise price of $0.50 per share with an exercise period of five years in connection with an extension of loans in the amount of $200,000 made to the Company by Officers of the Company in Oct 2008.
On September 30, 2011 the Company converted a $605,000 note held by a related party to 2,016,667 shares of common stock using a conversion price of $0.30 per share.
On October 7, 2011, the Company issued 550,000 shares of common stock as a result of the conversion of $143,790 of notes payable.
On October 12, 2011, the Company issued 263,158 shares of common stock for $150,000 as a result of an equity purchase.
On October 20, 2011, the Company issued 1,000,000 shares of common stock as a result of a conversion of $263,312 of notes payable.
As of October 24, 2011, the Company agreed that the 750,000 shares issued to Toucan Partners on June 6, 2010, would be used to settle $150,000 of the $1.3 million note payable to Toucan Partners.
On October 27, 2011, the Company issued 99,687 shares of common stock as a result of the conversion of $30,000 of notes payable.
On October 27, 2011, the Company issued 500,000 shares of common stock as a result of the conversion of $150,800 of notes payable.
On October 27, 2011, the Company issued 156,144 shares of common stock as a result of the conversion of $50,000 of notes payable.
On November 1, 2011, the Company issued 2,016,667 shares of common stock as a result of the conversion of $605,000 of related party notes payable.
On November 14, 2011 the Registrant entered into a six month (6) non-convertible loan agreement with an existing non-affiliate investor for a loan in the principal amount of $2,000,000 and an annual interest rate of 6%. In connection with the loan, the Company issued the lender warrants to purchase 1,052,632 shares of the Company’s common stock, par value $0.001 per share, at an exercise price of $.57 per share and an exercise period of five years.
On November 25, 2011 the Company issued 46,000,000 shares of common stock as a result of the conversion of 9.2M of related party accounts payable.
On November 28, 2011, the Company entered into an agreement with an existing non-affiliated investor, resolving $5,046,401 of debt (principal and accumulated interest and Original Interest Discount). This debt arose from a $4 million Note which was entered into by the Company and the investor during May, 2008, with a 6-month maturity and a twelve percent annual interest rate, and which has previously been extended. Pursuant to the agreement, $2,523,201, comprising half of the total amount due, is being converted into common stock of the Company, par value $0.001 (the “Common Stock”), and $2,523,201, comprising the other half of the total amount due, is being repaid in cash, through a series of installment payments.
On December 1,2011, the Company issued 300,000 shares of common stock as a result of the conversion of $88,416 of notes payable.
On December 6,2011, the Company issued 200,000 shares of common stock as a result of the conversion of $51,083 of notes payable.
On December 8,2011, the Company issued 51,984 shares of common stock as a result of the conversion of $15,000 of notes payable.
The sales of the securities identified above were made pursuant to privately negotiated transactions that did not involve a public offering of securities and, accordingly, we believe that these transactions were exempt from the registration requirements of the Securities Act pursuant to Section 4(2) of the Securities Act and Rule 506 of Regulation D. The agreements executed in connection with this sale contain representations to support the Company’s reasonable belief that the Investor had access to information concerning the Company’s operations and financial condition, the Investor acquired the securities for their own account and not with a view to the distribution thereof in the absence of an effective registration statement or an applicable exemption from registration, and that the Investor are sophisticated within the meaning of Section 4(2) of the Securities Act and are “accredited investors” (as defined by Rule 501 under the Securities Act). In addition, the issuances did not involve any public offering; the Company made no solicitation in connection with the sale other than communications with the Investor; the Company obtained representations from the Investor regarding their investment intent, experience and sophistication; and the Investor either received or had access to adequate information about the Company in order to make an informed investment decision. All of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
Except as noted above, the sales of the securities identified above were made pursuant to privately negotiated transactions that did not involve a public offering of securities and, accordingly, we believe that these transactions were exempt from the registration requirements of the Securities Act pursuant to Section 4(2) of the Securities Act and Rule 506 of Regulation D and Regulation S promulgated thereunder. The agreements executed in connection with this sale contain representations to support the Registrant’s reasonable belief that the Investor had access to information concerning the Registrant’s operations and financial condition, the Investor acquired the securities for their own account and not with a view to the distribution thereof in the absence of an effective registration statement or an applicable exemption from registration, and that the Investor are sophisticated within the meaning of Section 4(2) of the Securities Act and are “accredited investors” (as defined by Rule 501 under the Securities Act). In addition, the issuances did not involve any public offering; the Registrant made no solicitation in connection with the sale other than communications with the Investor; the Registrant obtained representations from the Investor regarding their investment intent, experience and sophistication; and the Investor either received or had access to adequate information about the Registrant in order to make an informed investment decision. All of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
FINANCIAL STATEMENTS
Our consolidated unaudited financial statements are presented beginning at page F-1.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the Securities Act with the SEC for the securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules which are part of the registration statement. For additional information about us and our securities, we refer you to the registration statement and the accompanying exhibits and schedules. Statements contained in this prospectus regarding the contents of any contract or any other documents to which we refer are not necessarily complete. In each instance, reference is made to the copy of the contract or document filed as an exhibit to the registration statement, and each statement is qualified in all respects by that reference. Copies of the registration statement and the accompanying exhibits and schedules may be inspected without charge (and copies may be obtained at prescribed rates) at the public reference facility of the SEC at Room 1024, 100 F Street, N.E. Washington, D.C. 20549.
You can request copies of these documents upon payment of a duplicating fee by writing to the SEC. You may call the SEC at 1-800-SEC-0330 for further information on the operation of its public reference rooms. Our filings, including the registration statement, will also be available to you on the Internet web site maintained by the SEC at
http://www.sec.gov
.
Part I — Financial Information
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
Condensed Consolidated Balance Sheets
(in thousands)
|
|
|
December 31, 2010
|
|
|
September 30, 2011
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
Assets
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
Cash
|
|
$
|
153
|
|
|
$
|
1,643
|
|
|
Prepaid expenses and other current assets
|
|
|
86
|
|
|
|
170
|
|
|
Total current assets
|
|
|
239
|
|
|
|
1,813
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment:
|
|
|
|
|
|
|
|
|
|
Laboratory equipment
|
|
|
29
|
|
|
|
29
|
|
|
Office furniture and other equipment
|
|
|
123
|
|
|
|
154
|
|
|
|
|
|
152
|
|
|
|
183
|
|
|
Less accumulated depreciation and amortization
|
|
|
(113
|
)
|
|
|
(119
|
)
|
|
Property and equipment, net
|
|
|
39
|
|
|
|
64
|
|
|
Deposit and other non-current assets
|
|
|
16
|
|
|
|
16
|
|
|
Total assets
|
|
$
|
294
|
|
|
$
|
1,893
|
|
|
Liabilities And Stockholders’ Equity (Deficit)
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
2,835
|
|
|
$
|
2,284
|
|
|
Accounts payable, related party
|
|
|
10,527
|
|
|
|
9,879
|
|
|
Accrued expenses
|
|
|
2,074
|
|
|
|
2,982
|
|
|
Accrued expenses, related party
|
|
|
1,749
|
|
|
|
2,265
|
|
|
Notes payable
|
|
|
1,364
|
|
|
|
1,384
|
|
|
Note payable to related parties
|
|
|
4,000
|
|
|
|
4,000
|
|
|
Convertible notes payable, net
|
|
|
2,736
|
|
|
|
3,671
|
|
|
Convertible notes payable to related party, net
|
|
|
-
|
|
|
|
1,300
|
|
|
Embedded derivative liability
|
|
|
839
|
|
|
|
1,818
|
|
|
Liability for reclassified equity contracts
|
|
|
-
|
|
|
|
16,562
|
|
|
Total current liabilities
|
|
|
26,124
|
|
|
|
46,145
|
|
|
Long term liabilities:
|
|
|
|
|
|
|
|
|
|
Notes payable, net
|
|
|
350
|
|
|
|
200
|
|
|
Convertible notes payable, net
|
|
|
555
|
|
|
|
1,513
|
|
|
Convertible notes payable to related party, net
|
|
|
949
|
|
|
|
185
|
|
|
Total long term liabilities
|
|
|
1,854
|
|
|
|
1,898
|
|
|
Total liabilities
|
|
|
27,978
|
|
|
|
48,043
|
|
|
Stockholders’ equity (deficit):
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 20,000,000 shares authorized and none issued and outstanding
|
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par value; 150,000,000 shares authorized, 73,118,471 and 97,112,036 shares issued and outstanding at December 31, 2010 and September 30, 2011, respectively
|
|
|
73
|
|
|
|
97
|
|
|
Additional paid-in capital
|
|
|
191,344
|
|
|
|
193,152
|
|
|
Deficit accumulated during the development stage
|
|
|
(218,948
|
)
|
|
|
(239,202
|
)
|
|
Cumulative translation adjustment
|
|
|
(153
|
)
|
|
|
(197
|
)
|
|
Total stockholders’ equity (deficit)
|
|
|
(27,684
|
)
|
|
|
(46,150
|
)
|
|
Total liabilities and
stockholders’ equity (deficit)
|
|
$
|
294
|
|
|
$
|
1,893
|
|
See accompanying notes to condensed consolidated financial statements
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
Condensed Consolidated Statements of Operations
(in thousands, except per share data)
(Unaudited)
|
|
|
Three months ended
|
|
|
Nine months ended
|
|
|
Period from March
18, 1996 (Inception)
|
|
|
|
|
September 30
|
|
|
September 30
|
|
|
to September 30,
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2010
|
|
|
2011
|
|
|
2011
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research material sales
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
580
|
|
|
Contract research and development from related parties
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,128
|
|
|
Research grants and other
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,061
|
|
|
Total revenues
|
|
|
10
|
|
|
|
10
|
|
|
|
10
|
|
|
|
10
|
|
|
|
2,769
|
|
|
Operating cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of research material sales
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
382
|
|
|
Research and development
|
|
|
1,606
|
|
|
|
3,565
|
|
|
|
4,791
|
|
|
|
11,474
|
|
|
|
88,286
|
|
|
General and administration
|
|
|
1,412
|
|
|
|
2,804
|
|
|
|
4,680
|
|
|
|
10,675
|
|
|
|
72,664
|
|
|
Depreciation and amortization
|
|
|
2
|
|
|
|
2
|
|
|
|
2
|
|
|
|
6
|
|
|
|
2,359
|
|
|
Loss on facility sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
895
|
|
|
Asset impairment loss and other (gain) loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,445
|
|
|
Total operating costs and expenses
|
|
|
3,020
|
|
|
|
6,371
|
|
|
|
9,473
|
|
|
|
22,155
|
|
|
|
167,031
|
|
|
Loss from operations
|
|
|
(3,010
|
)
|
|
|
(6,361
|
)
|
|
|
(9,463
|
)
|
|
|
(22,145
|
)
|
|
|
(164,262
|
)
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Valuation of reclassified equity contracts
|
|
|
-
|
|
|
|
8,875
|
|
|
|
-
|
|
|
|
7,413
|
|
|
|
14,172
|
|
|
Loan conversion inducement
|
|
|
|
|
|
|
-
|
|
|
|
(4,522
|
)
|
|
|
(125
|
)
|
|
|
(10,415
|
)
|
|
Derivative valuation gain (loss)
|
|
|
-
|
|
|
|
338
|
|
|
|
-
|
|
|
|
29
|
|
|
|
83
|
|
|
Gain on sale of intellectual property and property and equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,664
|
|
|
Interest expense
|
|
|
(2,371
|
)
|
|
|
(2,370
|
)
|
|
|
(5,968
|
)
|
|
|
(5,426
|
)
|
|
|
(39,342
|
)
|
|
Interest income and other
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,707
|
|
|
Net income (loss)
|
|
|
(5,381
|
)
|
|
|
482
|
|
|
|
(19,953
|
)
|
|
|
(20,254
|
)
|
|
|
(194,393
|
)
|
|
Issuance of common stock in connection with elimination of Series A and Series A-1 preferred stock preferences
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(12,349
|
)
|
|
Modification of Series A preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,306
|
)
|
|
Modification of Series A-1 preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(16,393
|
)
|
|
Series A preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(334
|
)
|
|
Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(917
|
)
|
|
Warrants issued on Series A and Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,664
|
)
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,872
|
)
|
|
Series A preferred stock redemption fee
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,700
|
)
|
|
Beneficial conversion feature of Series D preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,274
|
)
|
|
Net income (loss) applicable to common stockholders
|
|
$
|
(5,381
|
)
|
|
$
|
482
|
|
|
$
|
(19,953
|
)
|
|
$
|
(20,254
|
)
|
|
$
|
(239,202
|
)
|
|
Net income (loss) per share applicable to common stockholders — basic
|
|
$
|
(0.08
|
)
|
|
$
|
0.01
|
|
|
$
|
(0.31
|
)
|
|
$
|
(0.24
|
)
|
|
|
|
|
|
Weighted average shares used in computing basic income (loss) per share
|
|
|
70,413
|
|
|
|
95,123
|
|
|
|
65,361
|
|
|
|
85,680
|
|
|
|
|
|
|
Net income (loss) per share applicable to common stockholders - diluted
|
|
$
|
(0.08
|
)
|
|
$
|
0.00
|
|
|
$
|
(0.31
|
)
|
|
$
|
(0.24
|
)
|
|
|
|
|
|
Weighted average shares used in computing diluted net income (loss) per share
|
|
|
70,413
|
|
|
|
123,136
|
|
|
|
65,361
|
|
|
|
85,680
|
|
|
|
|
|
See accompanying notes to condensed consolidated financial statements.
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
Condensed Consolidated Statements of Cash Flows
(in thousands)(Unaudited)
|
|
|
Nine Months Ended
|
|
|
Period from
March 18, 1996
(Inception) to
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2011
|
|
|
Cash Flows from Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$
|
(19,953
|
)
|
|
$
|
(20,254
|
)
|
|
$
|
(194,393
|
)
|
|
Reconciliation of net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
2
|
|
|
|
6
|
|
|
|
2,359
|
|
|
Amortization of deferred financing costs
|
|
|
-
|
|
|
|
-
|
|
|
|
320
|
|
|
Amortization debt discount
|
|
|
2,853
|
|
|
|
4,058
|
|
|
|
28,267
|
|
|
Derivative valuation (gain) loss
|
|
|
-
|
|
|
|
(29
|
)
|
|
|
(83
|
)
|
|
Accrued interest converted to stock
|
|
|
1,047
|
|
|
|
-
|
|
|
|
260
|
|
|
Accreted interest on convertible promissory note
|
|
|
-
|
|
|
|
-
|
|
|
|
1,484
|
|
|
Stock-based compensation costs
|
|
|
1,512
|
|
|
|
7,354
|
|
|
|
18,768
|
|
|
Stock and warrants issued for services and other expenses
|
|
|
3,593
|
|
|
|
3,470
|
|
|
|
12,805
|
|
|
Loan conversion inducement
|
|
|
4,522
|
|
|
|
125
|
|
|
|
10,415
|
|
|
Valuation of reclassified equity contracts
|
|
|
-
|
|
|
|
(7,413
|
)
|
|
|
(14,172
|
)
|
|
Asset impairment loss and loss (gain) on sale of properties
|
|
|
-
|
|
|
|
-
|
|
|
|
(936
|
)
|
|
Loss on facility sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
895
|
|
|
Increase (decrease) in cash resulting from changes in assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets
|
|
|
(129
|
)
|
|
|
(84
|
)
|
|
|
540
|
|
|
Accounts payable and accrued expenses
|
|
|
380
|
|
|
|
1,097
|
|
|
|
6,887
|
|
|
Related party accounts payable and accrued expenses
|
|
|
(59
|
)
|
|
|
1,518
|
|
|
|
13,794
|
|
|
Accrued loss on sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
(265
|
)
|
|
Deferred rent
|
|
|
-
|
|
|
|
-
|
|
|
|
410
|
|
|
Net Cash used in Operating Activities
|
|
|
(6,232
|
)
|
|
|
(10,152
|
)
|
|
|
(112,645
|
)
|
|
Cash Flows from Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment, net
|
|
|
(41
|
)
|
|
|
(31
|
)
|
|
|
(5,075
|
)
|
|
Proceeds from sale of property and equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
258
|
|
|
Proceeds from sale of intellectual property
|
|
|
-
|
|
|
|
-
|
|
|
|
1,816
|
|
|
Proceeds from sale of marketable securities
|
|
|
-
|
|
|
|
-
|
|
|
|
2,000
|
|
|
Refund of security deposit
|
|
|
-
|
|
|
|
-
|
|
|
|
(3
|
)
|
|
Transfer of restricted cash
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,035
|
)
|
|
Net Cash used in Investing Activities
|
|
|
(41
|
)
|
|
|
(31
|
)
|
|
|
(2,039
|
)
|
|
Cash Flows from Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of note payable
|
|
|
-
|
|
|
|
6,272
|
|
|
|
12,122
|
|
|
Proceeds from issuance of convertible notes payable to related parties
|
|
|
2,667
|
|
|
|
500
|
|
|
|
1,800
|
|
|
Proceeds from issuance of note payable to related parties
|
|
|
-
|
|
|
|
-
|
|
|
|
11,250
|
|
|
Repayment of note payable to related party
|
|
|
-
|
|
|
|
-
|
|
|
|
(7,600
|
)
|
|
Proceeds from issuance of convertible promissory note and warrants, net of issuance costs
|
|
|
-
|
|
|
|
-
|
|
|
|
16,091
|
|
|
Repayment of convertible promissory note
|
|
|
-
|
|
|
|
(399
|
)
|
|
|
(1,069
|
)
|
|
Borrowing under line of credit, Northwest Hospital
|
|
|
-
|
|
|
|
-
|
|
|
|
2,834
|
|
|
Repayment of line of credit, Northwest Hospital
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,834
|
)
|
|
Payment on capital lease obligations
|
|
|
-
|
|
|
|
-
|
|
|
|
(323
|
)
|
|
Payments on note payable
|
|
|
-
|
|
|
|
-
|
|
|
|
(420
|
)
|
|
Proceeds from issuance preferred stock, net
|
|
|
-
|
|
|
|
-
|
|
|
|
28,708
|
|
|
Proceeds from exercise of stock options and warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
228
|
|
|
Proceeds from issuance common stock, net
|
|
|
3,960
|
|
|
|
5,340
|
|
|
|
58,914
|
|
|
Proceeds from sale of stock warrant
|
|
|
-
|
|
|
|
4
|
|
|
|
94
|
|
|
Payment of preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,251
|
)
|
|
Series A preferred stock redemption fee
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,700
|
)
|
|
Deferred financing costs
|
|
|
-
|
|
|
|
-
|
|
|
|
(320
|
)
|
|
Net Cash provided by Financing Activities
|
|
|
6,627
|
|
|
|
11,717
|
|
|
|
116,524
|
|
|
Effect of exchange rates on cash
|
|
|
(69
|
)
|
|
|
(44
|
)
|
|
|
(276
|
)
|
|
Net increase in cash
|
|
|
285
|
|
|
|
1,490
|
|
|
|
1,564
|
|
|
Cash at beginning of period
|
|
|
65
|
|
|
|
153
|
|
|
|
-
|
|
|
Cash at end of period
|
|
$
|
350
|
|
|
$
|
1,643
|
|
|
$
|
1,564
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information — Cash paid during the period for interest
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
1,879
|
|
|
Supplemental schedule of non-cash financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equipment acquired through capital leases
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
285
|
|
|
Issuance of common stock in connection with elimination of Series A and Series A-1 preferred stock preferences
|
|
|
-
|
|
|
|
-
|
|
|
|
12,349
|
|
|
Issuance of common stock in connection with conversion of notes payable and accrued interest
|
|
|
1,004
|
|
|
|
2,790
|
|
|
|
6,076
|
|
|
Modification of Series A preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
2,306
|
|
|
Modification of Series A-1 preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
16,393
|
|
|
Warrants issued on Series A and Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
4,664
|
|
|
Common stock warrant liability
|
|
|
-
|
|
|
|
-
|
|
|
|
11,841
|
|
|
Accretion of mandatorily redeemable Series A preferred stock redemption obligation
|
|
|
-
|
|
|
|
-
|
|
|
|
1,872
|
|
|
Debt discount on promissory notes
|
|
|
2,490
|
|
|
|
4,891
|
|
|
|
18,982
|
|
|
Conversion of convertible promissory notes and accrued interest to Series D preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
5,324
|
|
|
Conversion of convertible promissory notes and accrued interest to Series A-1 preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
7,707
|
|
|
Conversion of convertible promissory notes and accrued interest to common stock
|
|
|
-
|
|
|
|
-
|
|
|
|
269
|
|
|
Issuance of Series C preferred stock warrants in connection with lease agreement
|
|
|
-
|
|
|
|
-
|
|
|
|
43
|
|
|
Issuance of common stock to settle accounts payable
|
|
|
-
|
|
|
|
-
|
|
|
|
4
|
|
|
Liability for and issuance of common stock and warrants to Medarex
|
|
|
-
|
|
|
|
-
|
|
|
|
840
|
|
|
Issuance of common stock to landlord
|
|
|
-
|
|
|
|
-
|
|
|
|
35
|
|
|
Deferred compensation on issuance of stock options and restricted stock grants
|
|
|
-
|
|
|
|
-
|
|
|
|
759
|
|
|
Cancellation of options and restricted stock
|
|
|
-
|
|
|
|
-
|
|
|
|
849
|
|
|
Financing of prepaid insurance through note payable
|
|
|
-
|
|
|
|
-
|
|
|
|
491
|
|
|
Stock subscription receivable
|
|
|
-
|
|
|
|
-
|
|
|
|
480
|
|
See accompanying notes to condensed consolidated financial statements.
Northwest Biotherapeutics, Inc.
(A Development Stage Company)
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Basis of Presentation
The accompanying unaudited condensed consolidated financial statements include the accounts of Northwest Biotherapeutics, Inc. and its subsidiary, NW Bio Europe Sarl (collectively, the “Company”, “we”, “us”, and “our”). All material intercompany balances and transactions have been eliminated. The accompanying unaudited condensed consolidated financial statements should be read in conjunction with the financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2010. The year-end condensed balance sheet data was derived from audited financial statements. All normal recurring adjustments which are necessary for the fair presentation of the results for the interim periods are reflected herein.
The independent registered public accounting firm’s report on the financial statements for the fiscal year ended December 31, 2010 states that because of recurring operating losses, net operating cash flow deficits, and a deficit accumulated during the development stage, there is substantial doubt about the Company’s ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming the Company will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
2. Summary of Significant Accounting Policies
The Company implemented the following accounting policy during 2011:
|
|
·
|
Liability for reclassified equity contracts - The Company accounts for potential shares that can be converted to common stock that were in excess of authorized shares, as a liability that is recorded at fair value.
|
The other significant accounting policies used in the preparation of the Company’s condensed consolidated financial statements are disclosed in Note 3 to our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2010.
Recent Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board issued ASU No. 2011-05, Comprehensive Income or ASU 2011-05. The guidance in ASU 2011-05 revises the manner in which entities present comprehensive income in their financial statements. An entity is required to report the components of comprehensive income in either one or two consecutive financial statements:
|
|
·
|
A single, continuous statement must present the components of net income and total net income, the components of other comprehensive income and total other comprehensive income, and a total for comprehensive income.
|
|
|
·
|
In a two-statement approach, an entity must present the components of net income and total net income in the first statement. That statement must be immediately followed by a financial statement that presents the components of other comprehensive income, a total for other comprehensive income, and a total for comprehensive income.
|
ASU 2011-05 does not change the items that must be reported in other comprehensive income. The amendments in ASU 2011-05 are effective for fiscal years beginning after December 15, 2011. The Company does not believe the adoption of ASU 2011-05 will have a material impact on the presentation of information in its financial statements.
3. Stock-Based Compensation
Compensation expense for all stock-based awards is measured at the grant date based on the fair value of the award and is recognized as an expense, on a straight-line basis, over the employee's requisite service period (generally the vesting period of the equity award). The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model. Stock-based compensation expense is recognized only for those awards that are expected to vest using an estimated forfeiture rate. We estimate pre-vesting option forfeitures at the time of grant and reflect the impact of estimated pre-vesting option forfeitures in compensation expense recognized. For options and warrants issued to non-employees, the Company recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit.
The following key weighted average assumptions were employed in the Black-Scholes option valuation model for the three and nine month periods ended September 30, 2011 (no options were granted in the three or nine month periods ended September 30, 2010 and no calculation was performed):
|
|
|
Three Months
Ended
September 30,
2011
|
|
|
Nine Months
Ended
September
30, 2011
|
|
|
Risk-free interest rate
|
|
|
2.27
|
%
|
|
|
2.27
|
%
|
|
Expected dividend yield
|
|
|
-
|
%
|
|
|
-
|
%
|
|
Volatility
|
|
|
193.6
|
%
|
|
|
193.6
|
%
|
|
Expected life
|
|
7 Years
|
|
|
7 Years
|
|
|
Weighted average Black - Scholes value of options granted
|
|
$
|
0.65
|
|
|
$
|
0.65
|
|
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The Company does not anticipate declaring dividends in the foreseeable future. Volatility is based on historical data. The expected life of an option is based on assumptions about future option vesting and exercises.
A summary of the Company’s year-to-date stock option activity and related information follows (options in thousands):
|
|
|
Options
|
|
|
Weighted
Average
Exercise price
|
|
Weighted Average
remaining Contractual
Term
|
|
Outstanding at December 31, 2010
|
|
|
3,256
|
|
|
$
|
0.71
|
|
|
|
Grants
|
|
|
22,128
|
|
|
|
0.66
|
|
|
|
Expired
|
|
|
(5
|
)
|
|
|
10.62
|
|
|
|
Cancellations
|
|
|
(557
|
)
|
|
|
0.60
|
|
|
|
Outstanding at September 30, 2011
|
|
|
24,822
|
|
|
$
|
0.67
|
|
9.8 years
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at September 30, 2011
|
|
|
10,574
|
|
|
$
|
0.66
|
|
9.6 years
|
|
|
|
|
|
|
|
|
|
|
|
|
Available for grant at September 30, 2011
|
|
|
7,942
|
|
|
|
|
|
|
In May 2011, the Company increased the number of shares reserved for issuance under the 2007 Stock Option Plan by an additional 20,000,000 shares of its common stock. As of September 30, 2011, the aggregate intrinsic value of outstanding stock options was $0. Intrinsic value represents the total pretax intrinsic value for all “in-the-money” options (i.e., the difference between the Company’s closing stock price on the last trading day of its third quarter of 2011 and the exercise price, multiplied by the number of shares of common stock underlying the stock options) that would have been received by the option holders had all option holders exercised their options on September 30, 2011.
Stock-based compensation expense was as follows for the three and nine months ended September 30, (in thousands):
|
|
|
Three months ended
September 30,
|
|
|
Nine months ended
September 30,
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2010
|
|
|
2011
|
|
|
Research and development
|
|
$
|
163
|
|
|
$
|
226
|
|
|
$
|
525
|
|
|
$
|
2,172
|
|
|
General and administrative
|
|
|
329
|
|
|
|
1,178
|
|
|
|
987
|
|
|
|
5,182
|
|
|
Total Stock-based compensation
|
|
$
|
492
|
|
|
$
|
1,404
|
|
|
$
|
1,512
|
|
|
$
|
7,354
|
|
At September 30, 2011, the unrecognized compensation expense related to stock options was $5.9 million which is to be recognized over a weighted average period of 2.9 years (approximately $2 million per year).
4. Fair Value Measurement
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets;
Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
The Company's embedded derivative liability and liability for reclassified equity contracts were measured using significant unobservable (Level 3) inputs. There were no assets measured at fair value using unobservable inputs either as of or during the nine months ended September 30, 2011 or as of or during the nine months ended September 30, 2010. There were no liabilities measured at fair value using unobservable inputs as of or during the nine months ended September 30, 2010.
As a result of the Company entering into convertible promissory notes and issuing warrants to purchase common stock, the Company's total potential outstanding common stock exceeded the Company’s authorized shares by approximately 61.0 million shares as of September 30, 2011, as also discussed in Note 7. As a result the Company is currently required to value a number of shares equal to the excess (61.0 million) issuable on exercise of warrants and options and on conversion of convertible notes and recognize the value as a liability. Once an increase in the number of authorized shares sufficient to cover the excess has been approved by stockholders, the liability will be remeasured, with changes in value included in other income/(expense), and then reclassified to additional paid-in capital. The Company intends to obtain authorization of an increase during the quarter ending December 31, 2011.
At June 30, 2011, the Company valued 53.5 million shares potentially in excess of the authorized shares at that date and recognized a liability of $19,991,000. These same shares were revalued at September 30, 2011 based on the closing stock price on September 30, 2011 with a resultant decrease in value of $8,875,000. The liability was correspondingly decreased by $8,875,000 with an offsetting change being included in income. The additional 7.5 million shares required to cover the total of 61.0 million potential excess shares at September 30, 2011 were valued at $5,446,000 and that amount was recognized as a liability at September 30, 2011. The total fair value of the shares potentially issuable in excess of the authorized shares at September 30, 2011 totaled approximately $16,562,000 and was recognized as a liability on September 30, 2011.
The following table represents the activity for the Company’s liability for reclassified equity contracts for the period ended September 30, 2011:
|
|
|
Liability for
reclassified
equity contracts
|
|
|
Balance , January 1, 2011
|
|
$
|
-
|
|
|
Liabilities reclassified at inception
|
|
|
23,975,000
|
|
|
Change in value of liabilities reclassified
|
|
|
(7,413,000
|
)
|
|
Balance, September 30, 2011
|
|
$
|
16,562,000
|
|
In the third and fourth quarters of 2010 the Company entered into loan agreements and issued warrants in connection with other loans. The terms of the loan agreement and warrants allowed for the conversion price for the loan and the warrants’ strike price to be adjusted downwards (but no additional warrants will be issued) in the event that stock or warrants were issued at a lower price or with a lower strike price during that 12 month period (“down round price protection”). As a result the warrants must be valued as of their issuance date with the value being recognized as a derivative liability on the balance sheet and revalued at the end of each reporting period with the net change in the value during that reporting period being charged against income as a derivative gain or (loss). At the end of the 12 month adjustment period the final value of the warrants is charged against additional paid-in capital.
The Company recognizes embedded derivative liabilities at their respective fair values at inception and on each reporting date. The Company measures fair value using a binomial model adjusted for the probability of issuance using a Monte Carlo simulation. Changes in fair value are recorded in “Derivative valuation gain (loss)” in the consolidated statement of operations. Key assumptions for determining fair values included volatility of 123% and risk-free interest rate of 0.3%.
As of January 1, 2011, the embedded derivative liability related to certain loan agreements and warrants issued in September and October 2010 with down round price protection amounted to $839,000. During the quarter ended March 31, 2011 the principal outstanding under one agreement was converted into stock and the residual embedded derivative liability of $169,000 was reclassified to stockholders equity. During the quarter ended September 30, 2011 the down round protection related to certain warrants issued in September 2010 expired and the residual embedded derivative liability of $212,000 was reclassified to stockholders equity. The derivative liability associated with the conversion feature in the May 31, 2011 $3,000,000 note from Whitebox Advisors and its affiliates was initially recorded in the consolidated balance sheet upon issuance as of May 31, 2011 at a fair value of $1,389,000. On September 30, 2011 the embedded derivative liabilities were revalued based on the closing price of the Company’s stock resulting in a decrease in the fair value of the derivative liability of $29,000 for the period ended September 30, 2011.
The following table represents the activity for the Company’s embedded derivative liability for the period ended September 30, 2011:
|
|
|
Embedded
Derivative
Liability
|
|
|
Balance , January 1, 2011
|
|
$
|
839,000
|
|
|
Reclassification to stockholders' equity
|
|
|
(381,000
|
)
|
|
Embedded derivative liability recognized
|
|
|
1,389,000
|
|
|
Net change in fair value of embedded derivative liabilities
|
|
|
(29,000
|
)
|
|
Balance, September 30, 2011
|
|
$
|
1,818,000
|
|
5. Liquidity
The Company has experienced recurring losses from operations, and, as of September 30, 2011, had a working capital deficit of $44.3 million (including the $16.6 million recorded as a liability relating to securities in excess of the authorized number, which will be eliminated as soon as the authorized number of common stock is increased), and a deficit accumulated during the 15 years of Company operations to date of $239.2 million. Of this $239.2 million deficit, $112.7 million (less than half) reflects cash used in operations, and the remaining $126.5 million reflects non-cash accounting measures.
Since 2004, Toucan Capital Fund II, L.P. (“Toucan Capital”), Toucan Partners LLC (“Toucan Partners”), entities controlled by Ms. Linda Powers, the managing director of Toucan Capital and managing member of Toucan Partners, and Ms. Linda Powers (collectively “Toucan”) have provided substantial funding to the Company. During the period from 2004 to 2007, the funding consisted of various loans and the purchase of preferred stock and common stock. Under a Conversion Agreement during 2007, all loans payable to Toucan outstanding at the time were converted to preferred stock and all of the preferred stock was converted to common stock. As a result of additional loans, as of September 30, 2011, notes payable include a $1.3 million convertible note with an annual interest rate of 12% and a $0.5 million convertible note with a 10% original issue discount and a 10% one time interest charge payable to Toucan. The $1.3 million notes payable to Toucan outstanding as of September 30, 2011, are convertible at $0.20 per share and the $0.5 million note payable is convertible at a conversion price equal to 80% of the average of the five lowest closing prices for the Company's Stock over the 25 days immediately preceding conversion. In September 2011 Toucan Partners agreed to terms related to the extension of a 60 day note originally entered into by Toucan Partners in October 2010, which was repaid after the maturity date in December 2010. Under the terms of this subsequent extension agreement, the Company issued in the third quarter of 2011 to Toucan Partners a warrant to purchase 909,000 shares at an exercise price of $0.75 over a term of five years. As a result of this financing activity, as of September 30, 2011, Toucan held 22,617,015 shares of common stock, representing approximately 23.3% of the common stock outstanding. Further, as of September 30, 2011, Toucan, beneficially owned (including unexercised warrants) 64,043,998 shares of common stock, representing a beneficial ownership interest of approximately 36.1%.
On June 2, 2011, the Company entered into an agreement with Toucan Partners to establish an equity facility pursuant to which the Company will issue registered, tradable shares of its common stock (the “Common Stock”) for future financings of up to $25 million over a 30-month period in aggregate. The Company expects the shares involved in this equity facility will be registered in several tranches through a series of registration statements over the 30-month term. The 30-month period will begin on the effective date of the first registration statement. Any use of this equity facility will be entirely in the Company’s discretion.
Under the terms of the facility, the Company may issue and sell shares of its Common Stock and deliver a sales notice to Toucan. Upon such notification, Toucan will be obligated to purchase a specified amount of stock based upon the then current price level of the Company’s stock. The price per share at which Toucan will be obligated to make such purchase will be determined by a backward-looking, fixed price formula of 95% of the average of the three lowest closing bid prices of the Company’s Common Stock during the 15 trading days prior to the Company’s notification date. The price per share is also subject to a floor price of $0.35 per share throughout the term of the Agreement. The purchase price formula does not include any discount and/or warrants that may be provided to an investor who purchases shares that have been registered pursuant to this facility. Such discounts and/or warrants are the Company's responsibility.
The amount of stock the Company can elect to issue in each sale will be determined by the then current price level of the Company’s common stock, ranging from $150,000 to $1 million. The Company is required to pay or reimburse all expenses associated with the establishment and use of this equity facility and all transactions in connection therewith, including legal fees, broker fees and placement agent fees. The Company may terminate the facility at any time for any reason upon thirty days notice; however, if the Company does so and Toucan is not in default, the Company may not enter into or use a similar facility for eight months after such termination. Toucan may terminate the facility if the Company is in default. Toucan will receive a one percent commitment fee for the facility and a five percent fee on the sales of shares under the facility when the sales are executed.
In addition to financing obtained from Toucan, the Company has raised additional capital by issuing common stock and debt securities. As of September 30, 2011, the Company had approximately $1.6 million of cash on hand. The Company expects to begin accessing the $25 million equity facility with Toucan Partners described above during the fourth quarter of 2011. If the Company’s capital raising efforts are unsuccessful, its inability to obtain additional cash as needed could have a material adverse effect on the Company’s financial position, results of operations and the Company’s ability to continue its existence.
6. Notes Payable
Notes payable originating during 2011
In January 2011, the Company received $50,000 upon issuing an unsecured 10% convertible loan agreement and promissory note due in April 2011 to private, non-affiliated investors. The note is convertible at maturity into shares of the Company’s common stock at a conversion price of $0.75 per share. Warrants to purchase 66,667 shares of common stock at an exercise price of $0.75 per share were issued with the loan agreement. The Company did not repay the promissory note on or before the maturity date, and as a result, the note holder became entitled to receive a warrant to purchase an additional 66,667 shares of common stock.
On January 19, 2011, the Company entered into an agreement to receive up to $2,220,000 from a non affiliated third party (the “Investor”). Under the agreement the Company entered into two promissory notes with principal amounts of $1,120,000 and $1,100,000, which are secured by certain notes and assets provided by the Investor (representing future tranches of funding under the Agreement), but are not secured by any assets of the Company. The $1,120,000 note carries an original issue discount of 10% and annual interest of 9% per annum, payable at maturity on June 30, 2012, and the $1,100,000 note carries an original issue discount of 10% and annual interest of 6% per annum, payable at maturity on December 31, 2013. Both notes were initially convertible into shares of the Company’s common stock, at a conversion price equal to 80% of the average of the daily volume weighted price of the Company’s common stock for the five lowest trading days during the 10 days immediately preceding conversion. The notes have down-round protection upon the occurrence of specified events. The proceeds of Note 1 were received at closing on January 21, 2011. During the three months ended September 30, 2011 $645,000 of the principal and interest outstanding on Note 1 was converted into 2,000,000 shares of the Company’s Common Stock. As of September 30, 2011 the balance outstanding, including principal, original issue discount and interest, was $509,000. The beneficial conversion feature related to Note 1 was determined to be approximately $276,000. As a result, the total discount on the Note 1 (including the original issue discount) totaled $396,000, and is being amortized over the term of the note. The proceeds of Note 2 initially were to be received in four equal monthly tranches starting on July 19, 2011. Note 2 was subsequently amended on May 4, 2011 as described in the paragraph below.
On January 20, 2011, the Company entered into a $130,000 unsecured 12% Loan Agreement with SDS (the “SDS Loan”) due August 2, 2011.
On February 11, 2011, the Company received $50,000 upon issuing unsecured 6% convertible loan agreement and promissory note due in February 11, 2012 to a private, non-affiliated investor. The note is convertible at maturity into shares of the Company’s common stock at a conversion price of $0.50 per share. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $25,000 which was recorded as a debt discount to be amortized over the term of the note.
On February 18, 2011, the Company entered into a transaction under which amounts due to a service provider were converted into a Convertible Promissory note in the amount of $192,195. The note was assigned to an unrelated, non-affiliated third party and amended (the “Amended Note”). The Amended Note carries an original issue discount of 10% and an annual interest rate of 4% payable at maturity on February 18, 2013. The Amended Note is convertible into shares of the Company’s common stock, at a conversion price equal to 80% of the market price at the time of conversion (the average price comprised of the average of the daily volume weighted average prices for the 5 trading days with the lowest average prices during the 15 trading days immediately preceding conversion). During the six months ended June 30, 2011 the note was converted into 749,365 shares of the Company’s common stock. The Company also entered into an additional agreement with the same investor to receive $190,000 pursuant to an additional Convertible Promissory Note under the same terms and with the same maturity date as the Amended Note. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $122,000. As a result, the total discount on the notes (including original issue discount) totaled $176,000 and is being amortized over the term of the note. ). During the three months ended September 30, 2011 $15,000 of the note was converted into 46,780 shares of the Company’s common stock.
During the three months ended March 31, 2011, the Company received an additional $650,000 under the $3,050,000 financing agreement which originated on December 14, 2010. During 2010, the Company received $350,000, and as of March 31, 2011, the cumulative amount advanced under the agreement was $1,000,000. The advances include a one-time interest charge of 11% and are repayable on December 16, 2013. The principal and interest payable under the agreement of $1,102,000 at March 31, 2011, is effectively convertible into common stock, at a conversion price equal to 80% of the average of the five lowest closing prices for the Company's stock, over the 25 trading days immediately preceding conversion. The beneficial conversion feature related to the agreement was determined to be approximately $675,000, and is being amortized over the term of the note. On March 30, 2011, at the Company’s request, the Investor agreed to cancel a note in the amount of $500,000 that was to be included in future stages of the funding arrangement entered into on December 14, 2010. The effect of this cancellation was to remove the Company’s obligation in that amount, and correspondingly reduce that funding arrangement.
On March 31, 2011, the Company entered into an unsecured Loan Agreement and Promissory Note with Toucan Partners in the amount of $500,000. The note matures on December 14, 2013, carries an original issue discount of approximately 10% and a one-time interest charge of 10%. The note is convertible into shares of the Company’s stock at a conversion price equal to 80% of the average of the five lowest closing prices in the 25 days previous to the conversion date which was $0.28 on the date the note was issued. Any amount that is still outstanding at maturity is repayable in cash. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $436,000. As a result, the total discount on the combined notes (including original issue discount) totaled $489,000 and is being amortized over the term of the notes.
On May 4, 2011, the Company received funding of $600,000 pursuant to the January 19, 2011 $2,220,000 funding agreement described above. Initial funding of $1,000,000 was received in January 2011 and under the terms of the agreement the second tranche of $1,000,000 was to be provided in four equal monthly installments, starting six months after the closing of the initial funding in January 2011. On May 4, 2011 the Company and the Investor agreed to modify the terms of the original agreement to accelerate the Investor’s advance of $600,000 of the second $1,000,000 tranche. The Company issued a convertible promissory note in the amount of $600,000 to the Investor, with a maturity of December 31, 2013, an original issue discount of 10% and an annual interest rate of 6%. In consideration of the acceleration of the $600,000 funding, the Company agreed to amend the conversion price of convertible promissory Notes 1 and 2, on a going forward basis, thereby reducing it from 80% to 70% of the market price at the time of conversion. The beneficial conversion feature related to Note 2 was determined to be approximately $152,000. As a result, the total discount on the note (including the original issue discount) totaled $343,000, and is being amortized over the term of the note.
On May 31, 2011, the Company accepted financing in the aggregate amount of $3,000,000 from Whitebox Advisors a large unaffiliated institutional investor and its affiliates (“Whitebox”). In consideration of the funding received from Whitebox, the Company issued convertible promissory notes (the “Notes”) to Whitebox, in accordance with their respective investment. The maturity date of the Notes is November 30, 2012 (the “Maturity Date”) and the Notes bear interest at the annualized rate of 10% per annum. The Notes are only convertible on the Maturity Date, at the election of Whitebox, and are only convertible into shares of the Company's unregistered, restricted common stock at that time. The Notes are convertible at $0.57 per share (the “Conversion Price”). So long as more than 50% of the original principal amount of the Notes is still outstanding, the Conversion Price is subject to anti-dilution adjustment based on subsequent offerings; however, in no event shall the Conversion Price be adjusted to a price less than $0.35 (“down round protection”). When at least 50% of the original principal amount has been repaid, the anti-dilution provision shall terminate and cease to have any applicability. The Company may prepay the Notes in whole or in part at any time or times after August 31, 2011, provided that the Company pays the full amount of interest that would have been due up to the Maturity Date on any prepaid amounts. Whitebox has the right to elect whether to receive such prepayment in cash and/or in shares of the Company’s common stock. The Company has no obligation to register any of the shares of common stock underlying the Notes, other than an obligation to allow piggyback registration when capacity is available in the Company’s reasonable commercial discretion. The Company determined that the down round protection on the conversion price should be accounted for as an embedded derivative. The derivate was valued at $1,389,000 using a binomial model and recorded an embedded derivative liability with the offsetting charge recorded as a debt discount.
The Company also issued to Whitebox warrants to purchase 5,263,159 shares of the Company’s common stock, at an exercise price of $0.57 per share (the same price per share as the Conversion Price under the Notes). The Warrants expire five years after issuance. The Warrants do not allow any cashless exercise, and require the holder to pay for all exercises in cash. The Company has agreed to file a registration statement for the resale of the shares of common stock underlying the Warrants by the second anniversary of the issuance of the warrants. In June 2011 Whitebox effectively transferred a sizeable portion of these warrants (for 725,363 shares) in conjunction with the investment of the Richard Schulze Family Foundation in the Company (see Note 11). The relative fair value of the Warrants is $1,611,000 and is being amortized over the term of the note.
On May 26, 2011, the Company reached an agreement with the holders of convertible promissory notes dated November 29, 2010 in the amount of $295,000 with a maturity date of May 29, 2011. Under the terms of the notes, the notes were convertible into shares of the Company’s stock at a conversion price equal to the first $1 million of equity raised during the term of the notes. Since May 26, 2011, the Company had not raised $1 million in equity, during the term of the notes, the parties negotiated to convert the outstanding principal and interest into shares of the Company’s common stock at the average of the volume weighted average price for the 10 days prior to the conversion. The Company issued the note holders 559,480 shares of the Company’s common stock in full and final settlement of the Company’s obligations under the notes. As a result of the negotiated conversion terms, the Company recognized the excess of the fair value of the securities transferred over the securities issuable pursuant to the original terms as a charge to operations amounting to $125,000 during the three months ended June 30, 2011.
During the three months ended June 30, 2011, the Company received an additional $550,000 under the $3,050,000 financing agreement which originated on December 14, 2010. During 2010, the Company received $350,000 and during 2011 the Company has received $1.2 million.
As of June 30, 2011, the cumulative amount advanced under the agreement was $1,550,000. The advances include an original issue discount of 10% and a one-time interest charge of 11% and are repayable on December 16, 2013. The principal and interest payable under the agreement is convertible into common stock, at a conversion price equal to 80% of the average of the five lowest closing prices for the Company's stock, over the 25 trading days immediately preceding conversion. On June 27, 2011, $574,000 of the advances made to date was converted into 2,000,000 shares of common stock.
During the three months ended September 30, 2011, the Company received an additional $50,000 under the $3,050,000 financing agreement which originated on December 14, 2010. During 2010, the Company received $350,000 and during 2011 the Company has received $1,250,000. As of September 30, 2011, the cumulative amount advanced under the agreement was $1,600,000. The advances include an original issue discount of 10% and a one-time interest charge of 10% and are repayable on December 16, 2013. The principal and interest payable under the agreement is convertible into common stock, at a conversion price equal to 80% of the average of the five lowest closing prices for the Company's stock, over the 25 trading days immediately preceding conversion. As of September 30, 2011 a balance, including principal, original issue discount and interest of $1,362,000 was outstanding under the agreement. The value of the beneficial conversion related to the funding provided in the quarter was $28,000. As a result, the total discount on the note (including the original issue discount) totaled $917,000 is being amortized over the term of the note.
On July 22, 2011 the Company issued 300,000 shares of common stock at $0.75 per share in conversion of a note payable amounting to $136,618.
On August 11, 2011 the Company issued 350,000 shares of common stock at $0.34 per share in conversion of a note payable amounting to $119,315.
On August 18, 2011 the Company issued 400,000 shares of common stock at $0.33 per share in conversion of a note payable amounting to $134,417.
On September 1, 2011 the Company issued 450,000 shares of common stock at $0.32 per share in conversion of a note payable amounting to $142,966.
On September 14, 2011 the Company issued 420,000 shares of common stock at $0.20 per share in conversion of a note payable amounting to $100,000.
On September 15, 2011 the Company issued 560,000 shares of common stock at $0.20 per share in conversion of a note payable amounting to $75,000.
On September 21, 2011 the Company issued 500,000 shares of common stock at $0.28 per share in conversion of a note payable amounting to $141,008.
On September 23, 2011 the Company issued 46,783 shares of common stock at $0.32 per share in conversion of a note payable amounting to $15,000.
During the three months ended September 30, 2011, a note payable to an Officer of the Company was repaid in the amount of $49,000.
|
|
|
December 31,
2010
|
|
|
September 30,
2011
|
|
|
Notes payable - current
|
|
|
|
|
|
|
|
12% unsecured due July 2011 (net of warrant discount and original issue discount $38 in 2010 and $0 in 2011)
|
|
$
|
714
|
|
|
$
|
934
|
|
|
12% unsecured originally due March 2011
|
|
|
650
|
|
|
|
450
|
|
|
|
|
$
|
1,364
|
|
|
$
|
1,384
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes payable related parties - current
|
|
|
|
|
|
|
|
|
|
12% unsecured due December 31, 2011
|
|
$
|
4,000
|
|
|
$
|
4,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes payable, net - current
|
|
|
|
|
|
|
|
|
|
0% unsecured due June 2011 (net of discount related to beneficial conversion feature $357 in 2010 and $0 in 2011)
|
|
$
|
360
|
|
|
$
|
-
|
|
|
6% unsecured due November 2010
|
|
|
300
|
|
|
|
50
|
|
|
6% unsecured originally due March 2011
|
|
|
110
|
|
|
|
110
|
|
|
6% unsecured due between March 2011 and March 2012 (net of discount related to beneficial conversion feature $424 in 2010 and $139 in 2011)
|
|
|
1,526
|
|
|
|
2,509
|
|
|
10% unsecured due between March and May 2011 (net of discount related to beneficial conversion feature $57 in 2010 and $0 in 2011)
|
|
|
338
|
|
|
|
-
|
|
|
11% unsecured due December 2011 (net of discount related to beneficial conversion feature $143 in 2010 and $38 in 2011)
|
|
|
102
|
|
|
|
49
|
|
|
6% unsecured due June 2012 (net of discount related to beneficial conversion feature $0 in 2010 and $182 in 2011)
|
|
|
-
|
|
|
|
953
|
|
|
|
|
$
|
2,736
|
|
|
$
|
3,671
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Notes payable related party, net - current
|
|
|
|
|
|
|
|
|
|
6% due July 2011 and November 2011 (net of discount reated to beneficial conversion feature $351 in 2010 and $0 in 2011)
|
|
$
|
-
|
|
|
$
|
1,300
|
|
|
|
|
|
|
|
|
|
|
|
|
Long term notes payable
|
|
|
|
|
|
|
|
|
|
20% unsecured convertible note due December 2013
|
|
$
|
350
|
|
|
$
|
-
|
|
|
6% unsecured note due October 2012
|
|
|
-
|
|
|
|
200
|
|
|
|
|
$
|
350
|
|
|
$
|
200
|
|
|
|
|
|
|
|
|
|
|
|
|
Long term convertible notes, net
|
|
|
|
|
|
|
|
|
|
6% unsecured due March 2012 (net of discount related to beneficial conversion feature $321 in 2010 and $0 in 2011)
|
|
$
|
555
|
|
|
$
|
-
|
|
|
20% unsecured convertible note due December 2013 (net of discount related to beneficial conversion feature $0 in 2010 and $492 in 2011)
|
|
|
-
|
|
|
|
695
|
|
|
4% unsecured convertible note due February 2013 (net of discount related to beneficial conversion feature $0 in 2010 and $55 in 2011)
|
|
|
-
|
|
|
|
151
|
|
|
10% unsecured convertible note due November 2012 (net of discount related to beneficial conversion feature $0 in 2010 and $2,333 in 2011)
|
|
|
-
|
|
|
|
667
|
|
|
|
|
$
|
555
|
|
|
$
|
1,513
|
|
|
Long term convertible notes related party, net
|
|
|
|
|
|
|
|
|
|
20% unsecured convertible note due December 2013 (net of discount reated to beneficial conversion feature and original issue discount $367 in 2011)
|
|
$
|
949
|
|
|
$
|
185
|
|
|
|
|
|
|
|
|
|
|
|
|
Total notes payable, net
|
|
$
|
9,954
|
|
|
$
|
12,253
|
|
7. Liability for Reclassified Equity Contracts
The Company accounts for potential shares that can be converted to common stock and if converted, will be in excess of authorized shares, as a liability that is recorded on the balance sheet (at fair value) only until the authorized number of shares is increased (at which time the whole liability will be remeasured, with changes in value included in other income/(expense), and then reclassified to additional paid-in capital). As of September 30, 2011 total potential issuable common stock exceeded the Company’s authorized shares by 61,000,000 shares. The fair value of these shares totaled approximately $16,562,000 and was recognized as a liability on September 30, 2011. The value of the liability was computed by valuing the securities that management believed were most likely to be converted. As of September 30, 2011, those securities consisted of warrants and options to purchase common stock and shares issuable upon conversion of convertible notes payable which have been issued over the last 7 years and which have exercise and conversion prices ranging from $0.15 to $0.60 per share. The fair value of the warrants as of September 30, 2011 was determined using the Black-Scholes option pricing model based on the following assumptions: expected dividend yield of 0%, risk-free interest rates between 0.13 and 0.97%, volatility between 108 and 194 and contractual lives between of one and five years. The fair value of the shares issuable upon conversion of convertible notes was calculated based on the anticipated conversion price relative to the closing price of the Company’s common stock on September 30, 2011. Until an increase in authorized shares is approved by stockholders, future issuances of common stock and instruments convertible or exercisable into common stock will have the impact of increasing the number of potential shares used to value the liability for reclassified equity contracts. This liability is revalued at each reporting date with any change in value included in other income/(expense) until such time as enough shares are authorized to cover all potentially convertible instruments. When the Company’s stockholders authorize an increase in the authorized capital sufficient to cover this excess the liability will be remeasured, with changes in value included in other income/(expense), and then reclassified to additional paid-in capital. The Company intends to obtain shareholder approval to increase the authorized share capital during the fourth quarter of 2011.
8. Net Income (Loss) Per Share Applicable to Common Stockholders
For the three months ended September 30, 2011, options and warrants to purchase 6.7 million shares of common stock were included in the computation of diluted net income (loss) per share because they were dilutive. Convertible debt having a face value of $5.4 million is also considered dilutive for purposes of computing diluted net income (loss) per share. The conversion of the convertible debt increases the number of shares outstanding for purposes of computing diluted net income (loss) per share by 21,270,891 shares for the three month period ended September 30, 2011. In determining the amount of net income used to compute diluted earnings per share, the Company applied the “if converted method.” Accordingly, net income for the three months ended September 30, 2011, has been increased by approximately $54,000, representing interest expense that would have been avoided if the debt had been converted as of July 1, 2011. Options, warrants, and convertible debt outstanding were all considered anti-dilutive for the three and nine months ended September 30, 2010, and for the nine months ended September 30, 2011, due to net losses.
The following securities were not included in the diluted net income (loss) per share calculation because their effect was antidilutive as of the periods presented (in thousands):
|
|
|
Three months endedSeptember
30,
|
|
|
Nine Months endedSeptember
30,
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2010
|
|
|
2011
|
|
|
Common stock options
|
|
|
4,110
|
|
|
|
25,186
|
|
|
|
4,110
|
|
|
|
25,186
|
|
|
Common stock warrants
|
|
|
38,200
|
|
|
|
48,606
|
|
|
|
38,200
|
|
|
|
53,215
|
|
|
Convertible notes
|
|
|
20,000
|
|
|
|
12,111
|
|
|
|
20,000
|
|
|
|
31,651
|
|
|
Excluded potentially dilutive securities
|
|
|
62,310
|
|
|
|
85,903
|
|
|
|
62,310
|
|
|
|
110,052
|
|
9. Related Party Transactions
Cognate Agreement
During the quarter ended June 30, 2011, the Company entered into a new service agreement with Cognate Therapeutics, Inc. (“Cognate”), a contract manufacturing and services organization in which Toucan Capital has a majority interest. In addition, two of the principals of Toucan Capital are members of Cognate’s board of directors and Linda Powers who is a director of Cognate and managing director of Toucan Capital is Chairperson of the Company’s Board of Directors and Chief Executive of the Company. This agreement replaces the agreement dated May 17, 2007 between the Company and Cognate, which had expired. Under the service agreement, the Company agreed to continue to utilize Cognate’s services, for certain consulting and manufacturing services to the Company for its ongoing DCVax®-Brain Phase II clinical trial. The scope of services and the economics are comparable to the prior agreement, and the structure and process for payments are simplified. Under the terms of the new agreement the Company will pay Cognate a monthly facility fee, dependent on the number of patients, ranging from $150,000 to $225,000 per month and a fee (in lieu of cost-plus charges) for each patient enrolled in the study, subject to specified minimum number of patients per month, plus charges for certain patient and product data services if such services are requested by the Company. The current service agreement expires on March 31, 2016. Additionally the Company has agreed to reimburse Cognate for enrollment ramp up costs, foreign program costs and costs of facilities and equipment dedicated to Company programs (which were also previously reimbursable). The amount of ramp up costs during the three months ended June 30, 2011 was $275,000.
During the quarters ended September 30, 2010 and 2011, respectively, the Company recognized approximately $1.1 million and $2.4 million of research and development costs related to these service agreements. As of December 31, 2010 and September 30, 2011 the Company owed Cognate approximately $10.2 million and $10.2, respectively.
During 2009, the Company and Cognate agreed that most of the accounts payable owed by the Company to Cognate will be converted into shares of common stock instead of paid in cash. The Company and Cognate agreed on a conversion price of $0.20 per share based on conversion prices applied to other creditors of the Company to date. The parties have agreed that this conversion will be applied to the accounts payable balance due to Cognate as of September 30, 2011 at which date the amount due to Cognate was $10.2 million. Finalization of the conversion arrangements are in process. The Company will recognize the value of common stock issued in excess of the amount of accounts payable converted, if any, as a charge to operations when the conversion takes place.
Toucan Capital Management
Toucan Capital on occasion incurs costs on behalf of the Company. These costs primarily relate to legal and consulting fees and costs and travel expenses incurred in support of the Company’s financing and international expansion efforts.
During the three months ended September 30, 2010 and 2011, respectively, the Company recognized approximately $15,000 and $0 of general and administrative costs for legal, travel and other costs incurred by Toucan Capital, Toucan Partners and Linda Powers on the Company’s behalf. At December 31, 2010 and September 30, 2011, expenses payable to Toucan Capital and related parties accrued to date amounted to $1.5 million and $0.3 million, respectively, and are included as part of accounts payable to related parties in the accompanying consolidated balance sheets.
Also, during 2009, the Company agreed with Toucan Capital, Toucan Partners and Linda Powers (the company's chairperson) that some or all of the accrued expenses owed by the Company to these parties for certain expense reimbursements will be converted into shares of common stock instead of paid in cash. The Company and Toucan agreed on a conversion price of $0.20 per share based on conversion prices applied to other creditors of the Company in the same time frame in 2009. The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of the accrued expenses converted, if any, as a charge to operations when the conversion takes place. Finalization of these arrangements is in process.
10. Contingencies and Commitments
The Company terminated its lease with Toucan Capital Corporation on December 31, 2009. The Company is obligated to make monthly payments of $5,000 during 2011. Subsequent to 2011, the payment structure will vary.
On March 17, 2010, the Company entered into a non-cancelable operating lease for 7,097 square feet of office space in Bethesda, Maryland, which expires in September 2012. Future minimum lease payments under the lease are $43,402 and $126,027 for 2011 and 2012, respectively. Rent expense for the nine months ended September 30, 2010 and 2011 amounted to $41,649 and $94,817, respectively. The Company expects to lease part of this space to Toucan and proceeds of this sublease, if any, will be offset against the minimum lease payments specified above.
As of September 30, 2011, the Company has no contractual commitments, other than contracts entered into in the normal course of its business, which result in material financial obligations.
11. Stockholders’ Equity (Deficit)
Common Stock Issuances
Issuances of common stock during 2011 were as follows:
During the three months ended March 31, 2011, the Company sold to private investors 280,000 shares of common stock at $0.75 per share for net proceeds of $210,000.
During the three months ended March 31, 2011, the Company issued 1,760,486 shares of common stock as a result of the conversion of notes payable.
During three months ended March 31, 2011, the Company issued 1,169,000 shares of common stock for services valued at $728,000 based on the closing market price on the date of issuance of the shares.
On March 1, 2011, the Company issued 6,852,228 shares of common stock in conjunction with the settlement agreement between the Company and Socius CG II, Ltd. The settlement occurred when Socius purchased accounts payable amounting to $1,650,000 from Cognate, and the Company became indebted to Socius. The Company recognized the fair value of the common stock issued to Socius in excess of the accounts payable owed amounting to $2,378,000, as research and development expense in the accompanying consolidated financial statements.
On May 2, 2011, the Company issued 312,500 shares of common stock to a private investor at $0.48 per share for net proceeds of $150,000.
On May 2, 2011, the Company issued 115,741 shares of common stock to a private investor at $0.43 per share for net proceeds of $50,000.
On May 16, 2011, the Company issued 53,333 shares of common stock to a private investor at $0.75 per share for net proceeds of $40,000.
On June 9, 2011, the Company issued 438,597 shares of common stock to a private investor at $0.57 per share for net proceeds of $250,000.
On June 14, 2011, the Company issued 104,167 shares of common stock to a private investor at $0.72 per share for net proceeds of $75,000.
On June 24, 2011, the Company issued 21,127 shares of common stock to a private investor at $0.71 per share for net proceeds of $15,000.
On June 28, 2011, Socius CG II, Ltd returned to the Company 1,310,075 shares of common stock in fulfillment of a true-up mechanism associated with the March 1, 2011 transaction under which Socius acquired $1,650,000 of trade debt from the Company for 6,852,228 shares of the Company’s common stock. The Company valued the true-up at the same price per share as the original issuance and reduced research and development expense by $455,000.
On June 30, 2011, the Company issued 6,594,203 shares of common stock to The Richard M. Schulze Family Foundation at $0.69 per share for net proceeds of $4,550,000.
During three months ended June 30, 2011, the Company issued 669,316 shares of common stock for services valued at $440,000 based on the closing market price on the date of issuance of the shares.
On July 15, 2011, the Company issued 666,667 shares of common stock in settlement of accounts payable amounting to $500,000.
During the three months ended September 30, 2011, the Company issued 3,023,783 shares of common stock as a result of the conversion of notes payable.
Stock Purchase Warrants
Issuances of warrants during 2011 were as follows:
In January 2011, the Company issued warrants to purchase 66,667 shares of common stock at an exercise price of $0.75 per share connection with the 90 day unsecured 10% convertible loan agreement and promissory note entered into in January 2011.
In connection with the January 2011 securities purchase agreements with two non-affiliated investors, under which the investors purchased 280,000 shares of common stock for $210,000, the Company issued warrants to purchase 262,500 shares of common stock at an exercise price of $0.80 per share with an exercise period of five years. Additionally, one of the investors purchased a warrant to acquire 93,750 shares of common stock at a price of $0.80 per share for $3,750.
In January 2011, the Company issued warrants to purchase 250,000 shares of common stock at an exercise price of $0.72 with a four year exercise period to a consultant. The fair value of the warrants amounting to $155,696 was charged to general and administrative expense in the accompanying consolidated financial statements and was determined using the Black-Scholes option pricing model with the following assumptions: expected dividend yield of 0%, risk-free interest rate of 1.48%, volatility of 178%, and a contractual life of 4 years.
In connection with the May 2, 2011 securities purchase agreement with a non-affiliated investor, under which the investors purchased 115,741 shares of common stock for $50,000, the Company issued warrants to purchase 11,574 shares of common stock at an exercise price of $0.43 per share with an exercise period of three years.
In connection with a May 31, 2011 loan agreement with a non-affiliated investor, under which the investors provided funding of $3,000,000 the Company issued warrants to purchase 4,537,796 shares of common stock at an exercise price of $0.57 per share with an exercise period of five years.
In connection with a June 9, 2011 securities purchase agreement with a non-affiliated investor, under which the investors purchased 438,597 shares of common stock for $250,000, the Company issued warrants to purchase 87,719 shares of common stock at an exercise price of $0.57 per share with an exercise period of three years.
In connection with the June 14, 2011 securities purchase agreement with a non-affiliated investor, under which the investors purchased 104,167 shares of common stock for $75,000, the Company issued warrants to purchase 10,417 shares of common stock at an exercise price of $0.72 per share with an exercise period of three years.
In connection with a June 24, 2011 securities purchase agreement with a non-affiliated investor, under which the investors purchased 21,127 shares of common stock for $15,000, the Company issued warrants to purchase 2,127 shares of common stock at an exercise price of $0.71 per share with an exercise period of three years.
In connection with a June 28, 2011 securities purchase agreement with The Richard M. Schulze Family Foundation, under which the investors purchased 6,594,203 shares of common stock for $4,550,000, the Company issued a warrant to purchase 1,582,609 shares of common stock at an exercise price of $0.69 per share with an exercise period of three years. Additionally, a warrant to purchase 725,363 shares at an exercise price of $0.57 per share was issued in conjunction with the Whitebox warrant return described above (see Note 6).
In September 2011 the company issued a warrant to purchase 182,640 shares of common stock at an exercise price of $0.75 per share with an exercise period of five years in connection with an extension of a loan of $115,000 made to the Company by an Officer of the Company in November 2010.
In September 2011 the Company issued a warrant to purchase 505,000 shares of common stock at an exercise price of $0.75 per share with an exercise period of five years in connection with an extension of a loan of $500,000 made to the Company by an Officer of the Company in October 2010.
In September 2011 the Company issued a warrant to purchase 909,000 shares of Common Stock at an exercise price of $0.75 per share with an exercise period of five years in connection with an extension of a loan of $900,000 originally made to the Company by Toucan Partners in October 2010.
In September 2011 the Company issued a warrant to purchase 84,000 shares of common stock at an exercise price of $0.50 per share with an exercise period of five years in connection with an extension of a loan of $150,000 made to the Company by an Officer of the Company in March 2009.
In September 2011 the Company issued a warrant to purchase 255,000 shares of common stock at an exercise price of $0.50 per share with an exercise period of five years in connection with an extension of loans in the amount of $200,000 made to the Company by Officers of the Company in October 2008.
The fair value of the warrants issued during September 2011, in connection with the extensions of loans amounted to $797,275 and was charged to interest expense in the accompanying consolidated financial statements. The fair value was determined using the Black-Scholes option pricing model with the following assumptions: expected dividend yield of 0%, risk-free interest rates of 0.13% - 0.97%, volatility of 108% - 194% and contractual lives of 1 - 5 years.
12. Subsequent Events
On October 7, 2011, the Company issued 550,000 shares of common stock as a result of the conversion of $143,790 of notes payable.
On October 12, 2011, the Company issued 263,158 shares of common stock for $150,000 as a result of an equity purchase.
On October 20, 2011, the Company issued 1,000,000 shares of common stock as a result of a conversion of $263,312 of notes payable.
On October 27, 2011, the Company issued 99,687 shares of common stock as a result of the conversion of $30,000 of notes payable.
On October 27, 2011, the Company issued 500,000 shares of common stock as a result of the conversion of $150,800 of notes payable.
On October 27, 2011, the Company issued 156,144 shares of common stock as a result of the conversion of $50,000 of notes payable.
On November 1, 2011, the Company issued 2,016,667 shares of common stock as a result of the conversion of $605,000 of related party notes payable.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Northwest Biotherapeutics, Inc.
Bethesda, Maryland
We have audited the accompanying consolidated balance sheets of Northwest Biotherapeutics, Inc. and Subsidiary (a development stage company) ("the Company") as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity (deficit), and cash flows for the years then ended, and for the period from March 18, 1996 (date of inception) to December 31, 2010. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company has determined that it is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Northwest Biotherapeutics, Inc. and Subsidiary (a development stage company) as of December 31, 2010 and 2009, and the results of their operations and their cash flows for the years then ended, and for the period from March 18, 1996 (date of inception) to December 31, 2010, in conformity with accounting principles generally accepted in the United States.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has experienced recurring losses from operations since inception, net operating cash flow deficits and has a deficit accumulated during the development stage. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans regarding these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/S/ PETERSON SULLIVAN LLP
Seattle, Washington
April 21, 2011
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
CONSOLIDATED BALANCE SHEETS
(in thousands)
|
|
|
December 31,
2009
|
|
|
December 31,
2010
|
|
|
|
|
(In thousands)
|
|
|
ASSETS
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
Cash
|
|
$
|
65
|
|
|
$
|
153
|
|
|
Prepaid expenses and other current assets
|
|
|
36
|
|
|
|
86
|
|
|
Total current assets
|
|
|
101
|
|
|
|
239
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment:
|
|
|
|
|
|
|
|
|
|
Laboratory equipment
|
|
|
29
|
|
|
|
29
|
|
|
Office furniture and other equipment
|
|
|
82
|
|
|
|
123
|
|
|
|
|
|
111
|
|
|
|
152
|
|
|
Less accumulated depreciation and amortization
|
|
|
(111
|
)
|
|
|
(113
|
)
|
|
Property and equipment, net
|
|
|
-
|
|
|
|
39
|
|
|
Deposit and other non-current assets
|
|
|
2
|
|
|
|
16
|
|
|
Total assets
|
|
$
|
103
|
|
|
$
|
294
|
|
|
|
|
|
|
|
|
|
|
|
|
LAIBILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
3,249
|
|
|
$
|
2,835
|
|
|
Accounts payable, related party
|
|
|
6,328
|
|
|
|
10,527
|
|
|
Accrued expenses
|
|
|
1,874
|
|
|
|
2,074
|
|
|
Accrued expenses, related party
|
|
|
1,329
|
|
|
|
1,749
|
|
|
Notes payable
|
|
|
2,650
|
|
|
|
1,364
|
|
|
Note payable to related parties
|
|
|
4,000
|
|
|
|
4,000
|
|
|
Convertible notes payable, net
|
|
|
-
|
|
|
|
2,736
|
|
|
Embedded derivative liability
|
|
|
-
|
|
|
|
839
|
|
|
Total current liabilities
|
|
|
19,430
|
|
|
|
26,124
|
|
|
|
|
|
|
|
|
|
|
|
|
Long term liabilities:
|
|
|
|
|
|
|
|
|
|
Notes payable
|
|
|
-
|
|
|
|
350
|
|
|
Convertible notes payable, net
|
|
|
1,061
|
|
|
|
555
|
|
|
Convertible notes payable to related party, net
|
|
|
298
|
|
|
|
949
|
|
|
Total long term liabilities
|
|
|
1,359
|
|
|
|
1,854
|
|
|
Total liabilities
|
|
|
20,789
|
|
|
|
27,978
|
|
|
Stockholders’ equity (deficit):
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 20,000,000 shares authorized and none issued and outstanding
|
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par value; 150,000,000 shares authorized, 58,877,087 and 73,118,471 shares issued and outstanding at December 31, 2009 and 2010, respectively
|
|
|
58
|
|
|
|
73
|
|
|
Additional paid-in capital
|
|
|
170,885
|
|
|
|
191,344
|
|
|
Deficit accumulated during the development stage
|
|
|
(191,580
|
)
|
|
|
(218,948
|
)
|
|
Cumulative translation adjustment
|
|
|
(49
|
)
|
|
|
(153
|
)
|
|
Total stockholders’ equity (deficit)
|
|
|
(20,686
|
)
|
|
|
(27,684
|
)
|
|
Total liabilities and
stockholders’ equity (deficit)
|
|
$
|
103
|
|
|
$
|
294
|
|
See accompanying notes to the consolidated financial statements
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF OPERATIONS
(in thousands, except per share data)
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
March 18,
|
|
|
|
|
|
|
|
1996
|
|
|
|
|
|
|
|
(Inception)
|
|
|
|
|
Year Ended December 31
|
|
|
to December
|
|
|
|
|
2009
|
|
|
2010
|
|
|
31, 2010
|
|
|
|
|
(in thousands, except per share data)
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
Research material sales
|
|
$
|
10
|
|
|
$
|
10
|
|
|
$
|
570
|
|
|
Contract research and development from related parties
|
|
|
-
|
|
|
|
-
|
|
|
|
1,128
|
|
|
Research grants and other
|
|
|
-
|
|
|
|
-
|
|
|
|
1,061
|
|
|
Total revenues
|
|
|
10
|
|
|
|
10
|
|
|
|
2,759
|
|
|
Operating cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of research material sales
|
|
|
-
|
|
|
|
-
|
|
|
|
382
|
|
|
Research and development
|
|
|
9,588
|
|
|
|
9,899
|
|
|
|
76,812
|
|
|
General and administration
|
|
|
7,482
|
|
|
|
5,463
|
|
|
|
61,989
|
|
|
Depreciation and amortization
|
|
|
7
|
|
|
|
2
|
|
|
|
2,353
|
|
|
Loss on facility sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
895
|
|
|
Asset impairment loss and other (gain) loss
|
|
|
389
|
|
|
|
-
|
|
|
|
2,445
|
|
|
Total operating costs and expenses
|
|
|
17,466
|
|
|
|
15,364
|
|
|
|
144,876
|
|
|
Loss from operations
|
|
|
(17,456
|
)
|
|
|
(15,354
|
)
|
|
|
(142,117
|
)
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant valuation
|
|
|
-
|
|
|
|
-
|
|
|
|
6,759
|
|
|
Loan conversion inducement
|
|
|
(5,617
|
)
|
|
|
(4,673
|
)
|
|
|
(10,290
|
)
|
|
Derivative valuation gain (loss)
|
|
|
-
|
|
|
|
54
|
|
|
|
54
|
|
|
Gain on sale of intellectual property and property and equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
3,664
|
|
|
Interest expense
|
|
|
(3,881
|
)
|
|
|
(7,884
|
)
|
|
|
(33,916
|
)
|
|
Interest income and other
|
|
|
-
|
|
|
|
489
|
|
|
|
1,707
|
|
|
Net loss
|
|
|
(26,954
|
)
|
|
|
(27,368
|
)
|
|
|
(174,139
|
)
|
|
Issuance of common stock in connection with elimination of Series A and Series A-1 preferred stock preferences
|
|
|
-
|
|
|
|
-
|
|
|
|
(12,349
|
)
|
|
Modification of Series A preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,306
|
)
|
|
Modification of Series A-1 preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
(16,393
|
)
|
|
Series A preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
(334
|
)
|
|
Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
(917
|
)
|
|
Warrants issued on Series A and Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,664
|
)
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,872
|
)
|
|
Series A preferred stock redemption fee
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,700
|
)
|
|
Beneficial conversion feature of Series D preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,274
|
)
|
|
Net loss applicable to common stockholders
|
|
$
|
(26,954
|
)
|
|
$
|
(27,368
|
)
|
|
$
|
(218,948
|
)
|
|
Net loss per share applicable to common stockholders — basic and diluted
|
|
$
|
(0.56
|
)
|
|
$
|
(0.41
|
)
|
|
|
|
|
|
Weighted average shares used in computing basic and diluted net loss per share
|
|
|
47,961
|
|
|
|
67,063
|
|
|
|
|
|
See accompanying notes to the consolidated financial statements.
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT) AND COMPREHENSIVE LOSS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock
|
|
Preferred Stock
|
|
Additional
|
|
|
|
During the
|
|
Cumulative
|
|
Total
|
|
|
|
|
Common Stock
|
|
Series A
|
|
Series A-1
|
|
|
|
|
|
Paid-In
|
|
Deferred
|
|
Development
|
|
Translation
|
|
Stockholders’
|
|
|
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Capital
|
|
Compensation
|
|
Stage
|
|
Adjustment
|
|
Equity (Deficit)
|
|
|
|
|
(In thousands)
|
|
|
Balances at March 18, 1996
|
|
|
—
|
|
$
|
—
|
|
|
—
|
|
$
|
—
|
|
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|
Accretion of membership units mandatory redemption obligation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(106
|
)
|
|
—
|
|
|
(106
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(1,233
|
)
|
|
—
|
|
|
(1,233
|
)
|
|
Balances at December 31, 1996
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(1,339
|
)
|
|
—
|
|
|
(1,339
|
)
|
|
Accretion of membership units mandatory redemption obligation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(275
|
)
|
|
—
|
|
|
(275
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(2,560
|
)
|
|
—
|
|
|
(2,560
|
)
|
|
Balances at December 31, 1997
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(4,174
|
)
|
|
—
|
|
|
(4,174
|
)
|
|
Conversion of membership units to common stock
|
|
|
2,203
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(2
|
)
|
|
—
|
|
|
—
|
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(329
|
)
|
|
—
|
|
|
(329
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(4,719
|
)
|
|
—
|
|
|
(4,719
|
)
|
|
Balances at December 31, 1998
|
|
|
2,203
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(9,224
|
)
|
|
—
|
|
|
(9,222
|
)
|
|
Issuance of Series C preferred stock warrants for services related to sale of Series C preferred shares
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
394
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
394
|
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(354
|
)
|
|
—
|
|
|
(354
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(5,609
|
)
|
|
—
|
|
|
(5,609
|
)
|
|
Balances at December 31, 1999
|
|
|
2,203
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
394
|
|
|
—
|
|
|
(15,187
|
)
|
|
—
|
|
|
(14,791
|
)
|
|
Issuance of Series C preferred stock warrants in connection with lease agreement
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
43
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
43
|
|
|
Exercise of stock options for cash
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1
|
|
|
Issuance of common stock at $0.85 per share for license rights
|
|
|
5
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4
|
|
|
Issuance of Series D preferred stock warrants in convertible promissory note offering
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,039
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,039
|
|
|
Beneficial conversion feature of convertible promissory notes
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,026
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,026
|
|
|
Issuance of Series D preferred stock warrants for services related to sale of Series D preferred shares
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
368
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
368
|
|
|
Issuance of common stock warrants in conjunction with issuance of promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3
|
|
|
Cancellation of common stock
|
|
|
(275
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(430
|
)
|
|
—
|
|
|
(430
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(12,779
|
)
|
|
—
|
|
|
(12,779
|
)
|
|
Balances at December 31, 2000
|
|
|
1,935
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
5,878
|
|
|
—
|
|
|
(28,396
|
)
|
|
—
|
|
|
(22,516
|
)
|
|
Issuance of Series D preferred stock warrants in conjunction with refinancing of note payable to stockholder
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
225
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
225
|
|
|
Beneficial conversion feature of convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
456
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
456
|
|
|
Beneficial conversion feature of Series D preferred stock
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,274
|
|
|
—
|
|
|
(4,274
|
)
|
|
—
|
|
|
—
|
|
|
Issuance of Series D preferred stock warrants for services related to the sale of Series D preferred shares
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,287
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,287
|
|
|
Exercises of stock options and warrants for cash
|
|
|
1,158
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
407
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
408
|
|
|
Issuance of common stock in initial public offering for cash, net of offering costs of $2,845
|
|
|
4,000
|
|
|
4
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
17,151
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
17,155
|
|
|
Conversion of preferred stock into common stock
|
|
|
9,776
|
|
|
10
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
31,569
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
31,579
|
|
|
Series A preferred stock redemption fee
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(1,700
|
)
|
|
—
|
|
|
(1,700
|
)
|
|
Issuance of stock options to nonemployees for services
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
45
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
45
|
|
|
Deferred compensation related to employee stock options
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,330
|
|
|
(1,330
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Amortization of deferred compensation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
314
|
|
|
—
|
|
|
—
|
|
|
314
|
|
|
Accretion of Series A preferred stock mandatory redemption obligation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(379
|
)
|
|
—
|
|
|
(379
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(10,940
|
)
|
|
—
|
|
|
(10,940
|
)
|
|
Balances at December 31, 2001
|
|
|
16,869
|
|
|
17
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
63,622
|
|
|
(1,016
|
)
|
|
(45,689
|
)
|
|
—
|
|
|
16,934
|
|
|
Issuance of unregistered common stock
|
|
|
1,000
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
199
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
200
|
|
|
Issuance of common stock, Employee Stock Purchase Plan
|
|
|
9
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
6
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
6
|
|
|
Issuance of common stock warrants to Medarex
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
80
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
80
|
|
|
Issuance of restricted stock to nonemployees
|
|
|
8
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
34
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
34
|
|
|
Issuance of stock options to nonemployees for service
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
57
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
57
|
|
|
Issuance of stock options to employees
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
22
|
|
|
(22
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Cancellation of employee stock options
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(301
|
)
|
|
301
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Exercise of stock options and warrants for cash
|
|
|
32
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
18
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
18
|
|
|
Deferred compensation related to employee restricted stock option
|
|
|
99
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
449
|
|
|
(449
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Cancellation of employee restricted stock grants
|
|
|
(87
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(392
|
)
|
|
392
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Amortization of deferred compensation, net
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
350
|
|
|
—
|
|
|
—
|
|
|
350
|
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(12,804
|
)
|
|
—
|
|
|
(12,804
|
)
|
|
Balances at December 31, 2002
|
|
|
17,930
|
|
|
18
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
63,794
|
|
|
(444
|
)
|
|
(58,493
|
)
|
|
—
|
|
|
4,875
|
|
|
Issuance of unregistered common stock to Medarex
|
|
|
1,000
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
199
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
200
|
|
|
Issuance of unregistered common stock to Nexus
|
|
|
90
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
35
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
35
|
|
|
Issuance of common stock warrants to Medarex
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
80
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
80
|
|
|
Issuance of warrants with convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
221
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
221
|
|
|
Beneficial conversion feature of convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
114
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
114
|
|
|
Issuance of common stock, Employee Stock Purchase Plan
|
|
|
4
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Exercise of stock options and warrants for cash
|
|
|
8
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Cancellation of employee restricted stock grants
|
|
|
(4
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(20
|
)
|
|
20
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Cancellation of employee stock options
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(131
|
)
|
|
131
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Amortization of deferred compensation, net
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
240
|
|
|
—
|
|
|
—
|
|
|
240
|
|
|
Non-employee stock compensation
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2
|
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(5,752
|
)
|
|
—
|
|
|
(5,752
|
)
|
|
Balances at December 31, 2003
|
|
|
19,028
|
|
|
19
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
64,294
|
|
|
(53
|
)
|
|
(64,245
|
)
|
|
—
|
|
|
15
|
|
|
Issuance of warrants with convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,711
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,711
|
|
|
Beneficial conversion feature of convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,156
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,156
|
|
|
Issuance of common stock, Employee Stock Purchase Plan
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Cancellation of employee stock options
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(5
|
)
|
|
5
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Amortization of deferred compensation, net
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
41
|
|
|
—
|
|
|
—
|
|
|
41
|
|
|
Warrant valuation
|
|
|
|
|
|
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
368
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
368
|
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(8,508
|
)
|
|
—
|
|
|
(8,508
|
)
|
|
Balances at December 31, 2004
|
|
|
19,029
|
|
|
19
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
67,524
|
|
|
(7
|
)
|
|
(72,753
|
)
|
|
—
|
|
|
(5,217
|
)
|
|
Issuance of unregistered common stock and preferred stock to Toucan Capital
|
|
|
—
|
|
|
—
|
|
|
32,500
|
|
|
33
|
|
|
—
|
|
|
—
|
|
|
1,243
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,276
|
|
|
Issuance of stock options to non-employees for services
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3
|
|
|
Issuance of warrants with convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,878
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,878
|
|
|
Exercise of stock options and warrants for cash
|
|
|
49
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4
|
|
|
Amortization of deferred compensation, net
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
7
|
|
|
—
|
|
|
—
|
|
|
7
|
|
|
Beneficial conversion feature of convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,172
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,172
|
|
|
Common Stock warrant liability
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(604
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(604
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(9,937
|
)
|
|
—
|
|
|
(9,937
|
)
|
|
Balances at December 31, 2005
|
|
|
19,078
|
|
|
19
|
|
|
32,500
|
|
|
33
|
|
|
—
|
|
|
—
|
|
|
71,220
|
|
|
—
|
|
|
(82,690
|
)
|
|
—
|
|
|
(11,418
|
)
|
|
Issuance of common stock to PIPE Investors for cash, net of cash and non-cash offering costs of $837
|
|
|
39,468
|
|
|
39
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,649
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,688
|
|
|
Issuance of warrants to PIPE investment bankers
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
395
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
395
|
|
|
Conversion of notes payable due to Toucan Capital to Series A-1 preferred stock
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,817
|
|
|
5
|
|
|
7,702
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
7,707
|
|
|
Conversion of notes payable due to management to common stock
|
|
|
2,688
|
|
|
3
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
266
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
269
|
|
|
Issuance of warrants with convertible promissory notes
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
236
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
236
|
|
|
Exercise of stock options and warrants for cash
|
|
|
66
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
9
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
9
|
|
|
Exercise of stock options and warrants — cashless
|
|
|
3,942
|
|
|
4
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(4
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Stock compensation expense
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
19
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
19
|
|
|
Beneficial conversion feature of convertible promissory note
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
64
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
64
|
|
|
Common Stock warrant liability
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(6,523
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(6,523
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(1,395
|
)
|
|
—
|
|
|
(1,395
|
)
|
|
Balances at December 31, 2006
|
|
|
65,241
|
|
|
65
|
|
|
32,500
|
|
|
33
|
|
|
4,817
|
|
|
5
|
|
|
78,033
|
|
|
—
|
|
|
(84,085
|
)
|
|
—
|
|
|
(5,949
|
)
|
|
Conversion of common stock at par related to the reverse stock split
|
|
|
(60,892
|
)
|
|
(61
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
61
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Conversion of Series A and A-1 preferred stock into common stock
|
|
|
15,012
|
|
|
15
|
|
|
(32,500
|
)
|
|
(33
|
)
|
|
(4,817
|
)
|
|
(5
|
)
|
|
23
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Issuance of common stock in connection with elimination of Series A and Series A-1 preferred stock preferences
|
|
|
6,861
|
|
|
7
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
12,342
|
|
|
—
|
|
|
(12,349
|
)
|
|
—
|
|
|
—
|
|
|
Modification of preferred stock Series A and Series A-1 warrants
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
18,699
|
|
|
—
|
|
|
(18,699
|
)
|
|
—
|
|
|
—
|
|
|
Series A and Series A-1 preferred stock dividend payment
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(1,251
|
)
|
|
—
|
|
|
(1,251
|
)
|
|
Warrants issued on Series A and Series A-1 preferred stock dividends
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,664
|
|
|
—
|
|
|
(4,664
|
)
|
|
—
|
|
|
—
|
|
|
Issuance of common stock in initial public offering on the AIM London market for cash, net of offering costs of $3,965
|
|
|
15,789
|
|
|
16
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
25,870
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
25,886
|
|
|
Remeasurement of warrants issued in connection with convertible promissory notes
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,495
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
4,495
|
|
|
Remeasurement of beneficial conversion feature related to convertible promissory notes
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,198
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,198
|
|
|
Exercise of warrants — cashless
|
|
|
335
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Stock compensation expense
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,679
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,679
|
|
|
Cumulative translation adjustment
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(4
|
)
|
|
(4
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(21,247
|
)
|
|
—
|
|
|
(21,247
|
)
|
|
Total comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(21,251
|
)
|
|
Balances at December 31, 2007
|
|
|
42,346
|
|
|
42
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
148,064
|
|
|
—
|
|
|
(142,295
|
)
|
|
(4
|
)
|
|
5,807
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issuance in exchange for license option
|
|
|
122
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
225
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
225
|
|
|
Exercise of stock options — cashless
|
|
|
25
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1
|
|
|
Stock compensation expense
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3,001
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3,001
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of warrants with promissory notes
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,017
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,017
|
|
|
Cumulative translation adjustment
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(20
|
)
|
|
(20
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(22,331
|
)
|
|
—
|
|
|
(22,331
|
)
|
|
Total comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(22,351
|
)
|
|
Balances at December 31, 2008
|
|
|
42,493
|
|
|
42
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
152,308
|
|
|
—
|
|
|
(164,626
|
)
|
|
(24
|
)
|
|
(12,300
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock options — cashless
|
|
|
20
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Exercise of warrants — cashless
|
|
|
1,214
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Issuance of common stock in private placements
|
|
|
2,378
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,391
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,393
|
|
|
Stock compensation expense
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,712
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,618
|
|
|
Debt Discount related to beneficial conversion
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,578
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,578
|
|
|
Warrants issued for services
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,645
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
1,645
|
|
|
Stock and warrants issued for services
|
|
|
3,662
|
|
|
3
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,819
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,916
|
|
|
Loan conversion
|
|
|
563
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
111
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
112
|
|
|
Loan conversion and conversion inducement
|
|
|
8,547
|
|
|
10
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
7,321
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
7,331
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cumulative translation adjustment
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(25
|
)
|
|
(25
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(26,954
|
)
|
|
—
|
|
|
(26,954
|
)
|
|
Total comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(26,979
|
)
|
|
Balances at December 31, 2009
|
|
|
58,877
|
|
|
58
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
170,885
|
|
|
—
|
|
|
(191,580
|
)
|
|
(49
|
)
|
|
(20,686
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock compensation expense
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,004
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,004
|
|
|
Debt discount related to beneficial conversion and warrants
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3,254
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3,254
|
|
|
Stock and warrants issued for services
|
|
|
2,264
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,246
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,248
|
|
|
Interest extensions and warrant valuations
|
|
|
1,139
|
|
|
1
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,627
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,628
|
|
|
Issuance of common stock in private placements
|
|
|
5,118
|
|
|
6
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3,832
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
3,838
|
|
|
Loan conversion and conversion inducement
|
|
|
5,720
|
|
|
6
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
6,406
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
6,412
|
|
|
Sale of warrants
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
90
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
90
|
|
|
Cumulative translation adjustment
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(104
|
)
|
|
(104
|
)
|
|
Net loss
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(27,368
|
)
|
|
—
|
|
|
(27,368
|
)
|
|
Total comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(27,472
|
)
|
|
Balance at December 31, 2010
|
|
|
73,118
|
|
$
|
73
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
191,344
|
|
$
|
—
|
|
$
|
(218,948
|
)
|
$
|
(153
|
)
|
$
|
(27,684
|
)
|
See accompanying notes to the consolidated financial statements.
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOW
|
|
|
|
|
|
Period from
March 18,
|
|
|
|
|
|
|
|
1996 (Inception) to
|
|
|
|
|
Year Ended December 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
(in thousands)
|
|
|
Cash Flows from Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$
|
(26,954
|
)
|
|
$
|
(27,368
|
)
|
|
$
|
(174,139
|
)
|
|
Reconciliation of net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
7
|
|
|
|
2
|
|
|
|
2,353
|
|
|
Amortization of deferred financing costs
|
|
|
-
|
|
|
|
-
|
|
|
|
320
|
|
|
Amortization debt discount
|
|
|
1,337
|
|
|
|
4,508
|
|
|
|
24,209
|
|
|
Derivative valuation (gain) loss
|
|
|
-
|
|
|
|
(54
|
)
|
|
|
(54
|
)
|
|
Accrued interest converted to preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
260
|
|
|
Accreted interest on convertible promissory note
|
|
|
-
|
|
|
|
-
|
|
|
|
1,484
|
|
|
Stock-based compensation costs
|
|
|
2,618
|
|
|
|
2,004
|
|
|
|
11,414
|
|
|
Stock and warrants issued for services and other expenses
|
|
|
4,776
|
|
|
|
4,559
|
|
|
|
9,335
|
|
|
Loan conversion inducement
|
|
|
5,617
|
|
|
|
4,673
|
|
|
|
10,290
|
|
|
Warrant valuation
|
|
|
-
|
|
|
|
-
|
|
|
|
(6,759
|
)
|
|
Asset impairment loss and loss (gain) on sale of properties
|
|
|
389
|
|
|
|
-
|
|
|
|
(936
|
)
|
|
Loss on facility sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
895
|
|
|
Increase (decrease) in cash resulting from changes in assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
1
|
|
|
|
-
|
|
|
|
-
|
|
|
Prepaid expenses and other current assets
|
|
|
1,031
|
|
|
|
(64
|
)
|
|
|
624
|
|
|
Accounts payable and accrued expenses
|
|
|
405
|
|
|
|
745
|
|
|
|
5,790
|
|
|
Related party accounts payable and accrued expenses
|
|
|
6,096
|
|
|
|
4,619
|
|
|
|
12,276
|
|
|
Accrued loss on sublease
|
|
|
-
|
|
|
|
-
|
|
|
|
(265
|
)
|
|
Deferred rent
|
|
|
-
|
|
|
|
-
|
|
|
|
410
|
|
|
Net Cash used in Operating Activities
|
|
|
(4,677
|
)
|
|
|
(6,376
|
)
|
|
|
(102,493
|
)
|
|
Cash Flows from Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment, net
|
|
|
(2
|
)
|
|
|
(41
|
)
|
|
|
(5,044
|
)
|
|
Proceeds from sale of property and equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
258
|
|
|
Proceeds from sale of intellectual property
|
|
|
-
|
|
|
|
-
|
|
|
|
1,816
|
|
|
Proceeds from sale of marketable securities
|
|
|
-
|
|
|
|
-
|
|
|
|
2,000
|
|
|
Refund of security deposit
|
|
|
-
|
|
|
|
-
|
|
|
|
(3
|
)
|
|
Transfer of restricted cash
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,035
|
)
|
|
Net Cash used in Investing Activities
|
|
|
(2
|
)
|
|
|
(41
|
)
|
|
|
(2,008
|
)
|
|
Cash Flows from Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of note payable
|
|
|
2,060
|
|
|
|
1,140
|
|
|
|
5,850
|
|
|
Proceeds from issuance of convertible notes payable to related parties
|
|
|
1,300
|
|
|
|
-
|
|
|
|
1,300
|
|
|
Proceeds from issuance of note payable to related parties
|
|
|
-
|
|
|
|
-
|
|
|
|
11,250
|
|
|
Repayment of note payable to related party
|
|
|
-
|
|
|
|
(900
|
)
|
|
|
(7,600
|
)
|
|
Proceeds from issuance of convertible promissory note and warrants, net of issuance costs
|
|
|
-
|
|
|
|
2,992
|
|
|
|
16,091
|
|
|
Repayment of convertible promissory note
|
|
|
-
|
|
|
|
(551)
|
|
|
|
(670
|
)
|
|
Borrowing under line of credit, Northwest Hospital
|
|
|
-
|
|
|
|
-
|
|
|
|
2,834
|
|
|
Repayment of line of credit, Northwest Hospital
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,834
|
)
|
|
Payment on capital lease obligations
|
|
|
-
|
|
|
|
-
|
|
|
|
(323
|
)
|
|
Payments on note payable
|
|
|
-
|
|
|
|
-
|
|
|
|
(420
|
)
|
|
Proceeds from issuance preferred stock, net
|
|
|
-
|
|
|
|
-
|
|
|
|
28,708
|
|
|
Proceeds from exercise of stock options and warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
228
|
|
|
Proceeds from issuance common stock, net
|
|
|
1,393
|
|
|
|
3,838
|
|
|
|
53,574
|
|
|
Proceeds for sale of stock warrant
|
|
|
-
|
|
|
|
90
|
|
|
|
90
|
|
|
Payment of preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,251
|
)
|
|
Series A preferred stock redemption fee
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,700
|
)
|
|
Deferred financing costs
|
|
|
-
|
|
|
|
-
|
|
|
|
(320
|
)
|
|
Net Cash provided by Financing Activities
|
|
|
4,753
|
|
|
|
6,609
|
|
|
|
104,807
|
|
|
Effect of exchange rates on cash
|
|
|
(25
|
)
|
|
|
(104
|
)
|
|
|
(153
|
)
|
|
Net increase in cash
|
|
|
49
|
|
|
|
88
|
|
|
|
153
|
|
|
Cash at beginning of period
|
|
|
16
|
|
|
|
65
|
|
|
|
-
|
|
|
Cash at end of period
|
|
$
|
65
|
|
|
$
|
153
|
|
|
$
|
153
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information — Cash paid during the period for interest
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
1,879
|
|
|
Supplemental schedule of non-cash financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equipment acquired through capital leases
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
285
|
|
|
Issuance of common stock in connection with elimination of Series A and Series A-1 preferred stock preferences
|
|
|
-
|
|
|
|
-
|
|
|
|
12,349
|
|
|
Issuance of common stock in connection conversion of notes payable and accrued interest
|
|
|
1,500
|
|
|
|
1,786
|
|
|
|
3,286
|
|
|
Modification of Series A preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
2,306
|
|
|
Modification of Series A-1 preferred stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
16,393
|
|
|
Warrants issued on Series A and Series A-1 preferred stock dividends
|
|
|
-
|
|
|
|
-
|
|
|
|
4,664
|
|
|
Common stock warrant liability
|
|
|
-
|
|
|
|
-
|
|
|
|
11,841
|
|
|
Accretion of mandatorily redeemable Series A preferred stock redemption obligation
|
|
|
-
|
|
|
|
-
|
|
|
|
1,872
|
|
|
Debt discount on promissory notes
|
|
|
2,578
|
|
|
|
3,254
|
|
|
|
14,091
|
|
|
Conversion of convertible promissory notes and accrued interest to Series D preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
5,324
|
|
|
Conversion of convertible promissory notes and accrued interest to Series A-1 preferred stock
|
|
|
-
|
|
|
|
-
|
|
|
|
7,707
|
|
|
Conversion of convertible promissory notes and accrued interest to common stock
|
|
|
-
|
|
|
|
-
|
|
|
|
269
|
|
|
Issuance of Series C preferred stock warrants in connection with lease agreement
|
|
|
-
|
|
|
|
-
|
|
|
|
43
|
|
|
Issuance of common stock for license rights
|
|
|
-
|
|
|
|
-
|
|
|
|
4
|
|
|
Liability for and issuance of common stock and warrants to Medarex
|
|
|
-
|
|
|
|
-
|
|
|
|
840
|
|
|
Issuance of common stock to landlord
|
|
|
-
|
|
|
|
-
|
|
|
|
35
|
|
|
Deferred compensation on issuance of stock options and restricted stock grants
|
|
|
-
|
|
|
|
-
|
|
|
|
759
|
|
|
Cancellation of options and restricted stock
|
|
|
-
|
|
|
|
-
|
|
|
|
849
|
|
|
Financing of prepaid insurance through note payable
|
|
|
-
|
|
|
|
-
|
|
|
|
491
|
|
|
Stock subscription receivable
|
|
|
-
|
|
|
|
-
|
|
|
|
480
|
|
See accompanying notes to the consolidated financial statements.
NORTHWEST BIOTHERAPEUTICS, INC.
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(1) Organization and Description of Business
Northwest Biotherapeutics, Inc. and its majority owned subsidiary NW Bio Europe Sarl (collectively, the “Company”, “we”, “us” and “our”) was organized to discover and develop innovative diagnostics and immunotherapies for prostate and brain cancer. During 1998, the Company incorporated as a Delaware corporation. Prior to 1998, the Company was a limited liability company, which was formed on March 18, 1996. The Company is a development stage company, has yet to generate significant revenues from its intended business purpose and has no assurance of future revenues. While in the development stage, the Company’s principal activities have included defining and conducting research programs, conducting clinical trials, raising capital and recruiting scientific and management personnel.
(2) Operations and Financing
Liquidity
The Company has experienced recurring losses from operations, and, as of December 31, 2010, had a working capital deficit of $25.9 million and a deficit accumulated during the development stage of $218.9 million. Of this $218.9 million deficit, $102.5 million (less than half) reflects cash used in operations, and the remaining $116.4 million reflects non-cash accounting measures.
Between 2004 and 2010, the Company has undergone a significant recapitalization through the transactions described below.
Toucan Capital and Toucan Partners
Toucan Capital Fund II, L.P. (“Toucan Capital”) loaned the Company $6.75 million during 2004 and 2005. The Board’s Chairperson is the managing director of Toucan Capital. In April 2006, the $6.75 million of notes payable plus all accrued interest were converted into shares of Series A-1 cumulative convertible Preferred Stock (the “Series A-1 Preferred Stock”). In connection with these loans the Company issued Toucan Capital a warrant to purchase 8,166,667 shares of Series A-1 Preferred Stock. The warrants to purchase Series A-1 Preferred Stock were later converted into warrants to purchase 17,256,888 shares of common stock in connection with the Conversion Agreement, described below.
On January 26, 2005, Toucan Capital purchased 32.5 million shares of Series A cumulative convertible preferred stock (the “Series A Preferred Stock”) at $0.04 per share, for a total of $1.276 million. In connection with the securities purchase agreement, the Company issued Toucan Capital a warrant to purchase 2,166,667 million shares of Series A Preferred Stock. The warrants to purchase Series A Preferred Stock were later converted into warrants to purchase 4,778,201 shares of common stock in connection with the Conversion Agreement, described below.
From November 14, 2005 through May 25, 2007, Toucan Partners, LLC (“Toucan Partners”) loaned the Company $4.825 million under various promissory note agreements. The Board’s Chairperson is the managing member of Toucan Partners. The promissory note agreements were amended and restated into the 2007 Convertible Notes. The 2007 Convertible Notes also included warrants to purchase shares of Series A-1 Preferred Stock ("2007 Warrants"). The Company repaid $5.3 million of principal and accrued interest due to Toucan Partners during 2007. The warrants to purchase Series A-1 Preferred Stock were later converted into warrants to purchase 8,832,541 shares of common stock in connection with the Conversion Agreement, described below.
Under the June 22, 2007 Conversion Agreement, Toucan Capital and Toucan Partners agreed to eliminate a number of rights, preferences and protections associated with the Series A Preferred Stock and the Series A-1 Preferred Stock and Toucan Capital received 4,287,851 shares of common stock and Toucan Partners received 2,572,710 shares of common stock. Also, Toucan Capital converted its preferred shares into 15,011,635 shares of common stock. Additionally under the conversion agreement the Company exchanged the warrants to purchase Series A-1 Preferred Stock and Series A Preferred Stock (discussed above) for warrants to purchase common stock. As a result of the conversion Toucan Capital received warrants to purchase 14,150,732 shares of Common Stock at an exercise price of $0.60 per share and warrants to purchase 7,884,357 shares of Common Stock at an exercise price of $0.15 per share and Toucan Partners received warrants to purchase 8,832,541 shares of Common Stock at an exercise price of $0.60 per share.
Toucan Partners loaned the Company $1.0 million on August 19, 2008 under the terms of an unsecured promissory note (the “Toucan Partners August Loan”) with a principal amount of $1,060,000 (reflecting an original issue discount of $60,000). On September 28, 2009, the note principal and accrued interest (including a default penalty of 0.25% per month) amounting to $1,156,718 was converted to 5,783,589 shares of common stock at a conversion price of $0.20. In connection with the conversion, the Company issued Toucan Partners a warrant to purchase 690,000 shares of common stock at an exercise price of $0.20 per share.
Toucan Partners loaned the Company $500,000 on December 22, 2008 under the terms of an unsecured 12% promissory note (the “Toucan Partners December Loan”). In connection with the promissory note, the Company issued to Toucan Partners a warrant to purchase 132,500 shares of common stock at an exercise price of $0.40 per share and a term of 5 years. On September 28, 2009, the note principal and accrued interest (including a default penalty of 0.25% per month) amounting to $552,738 was converted to 2,763,691 shares of common stock at a conversion rate of $0.20. To bring the December Loan into conformity with the SDS and Private Lender notes issued in October and November 2008, as agreed by the parties at the time of the Toucan Partners December Loan, the Company issued Toucan Partners a warrant to purchase 513,841 shares of common stock at an exercise price of $0.41 per share. In connection with the conversion, the Company issued Toucan Partners a warrant to purchase 152,375 shares of common stock at an exercise price of $0.20 per share.
Toucan Partners and the Board's Chairperson also received a total of 2,504,034 shares of common stock as compensation for services rendered during 2008 and 2009.
Toucan Partners loaned the Company a total of $1,300,000 on June 30, 2009, July 2, 2009 and July 17, 2009 under unsecured 6% convertible promissory notes due June 29, 2011, July 1, 2011 and July 16, 2011. The conversion feature of the notes allows Toucan Partners to convert the principal into shares of common stock at a conversion price of $0.20.
On July 2, 2010 the Company entered into a securities purchase agreement with Toucan Partners, under which Toucan Partners purchased 866,667 shares of common stock for $650,000. In connection with this private placement the Company issued warrants to purchase 86,667 shares of common stock at an exercise price of $0.75 per share with an exercise period of three years.
On October 1, 2010 Toucan Partners loaned the Company $900,000 by repaying a portion of the Bridge Funding Note payable to Regen Med Acquisition Corporation (“Regen Med”) originating July 14, 2010 under a 6% convertible promissory note on the same terms and conditions as the September Notes that the Company had negotiated and executed with non-affiliated investors on September 28, 2010, secured by an interest in all the Company’s assets, due on December 1, 2010. The conversion feature of the note allows Toucan Partners to convert the principal into shares of common stock at a conversion price of $0.75. Additionally, Toucan Partners received 100% warrant coverage, on the same terms and conditions as the September Notes (including the same market formula for the warrant exercise price), at $0.82 per share. In the event of default, Toucan Partners was entitled to adjust the interest rate to 9% per annum for the default period and to be granted an additional 100% warrant coverage at $0.82 per share. The Company did not repay the loan on December 1, 2010 however Toucan Partners waived its right to increase the interest rate and waived its right to the additional warrant. The loan was repaid in full by December 31, 2010.
In December 2010 Toucan Capital transferred 6,433,162 shares of common stock and 7,345,030 warrants to purchase shares of the Company’s common stock to Regen Med, a non-affiliate third party.
As a result of the financings described above, as of December 31, 2010 Toucan Capital held:
|
|
•
|
an aggregate of 12,866,324 shares of Common Stock;
|
|
|
•
|
warrants to purchase 9,433,821 shares of Common Stock at an exercise price of $0.60 per share (net of 4,716,911 warrants transferred to Regen Med stockholders); and
|
|
|
•
|
warrants to purchase 5,256,238 shares of Common Stock at an exercise price of $0.15 per share (net of 2,628,119 warrants transferred to Regen Med stockholders).
|
As a result of the financings described above, and other open market transactions, as of December 31, 2010, Toucan Partners and its managing member Ms. Linda Powers held:
|
|
•
|
an aggregate of 10,510,691 shares of Common Stock;
|
|
|
•
|
warrants to purchase 8,832,541 shares of Common Stock at an exercise price of $0.60 per share;
|
|
|
•
|
warrants to purchase 1,097,561 shares of Common Stock at an exercise price of $0.82 per share;
|
|
|
•
|
warrants to purchase 132,500 shares of common stock at an exercise price of $0.40;
|
|
|
•
|
warrants to purchase 86,667 shares of common stock at an exercise price of $0.75 per share; and
|
|
|
•
|
warrants to purchase 842,375 shares of common stock at an exercise price of $0.20.
|
As of December 31, 2010, Toucan Capital, including the holdings of Toucan Partners, and its managing member Ms Linda Powers held 23,377,015 shares of common stock, representing approximately 32.0% of the common stock outstanding. Further, as of December 31, 2010, Toucan Capital, including the holdings of Toucan Partners, and its managing member Ms Linda Powers beneficially owned (including unexercised warrants) 49,572,559 shares of common stock, representing a beneficial ownership interest of approximately 40.5%.
Other Financings
In April 2006, the Company completed the PIPE Financing and raised approximately $5.5 million from the issuance of 2.6 million shares of common stock.
On June 22, 2007, we placed 15,789,473 shares of common stock with foreign institutional investors at a price of £0.95 per share. The gross proceeds from the placement were approximately £15.0 million, or $29.9 million, while net proceeds from the offering, after deducting commissions and expenses, were approximately £13.0 million, or $25.9 million.
On January 16, 2009 we entered into a securities purchase agreement for $700,000 with Al Rajhi Holdings who purchased 1,000,000 shares of our common stock at $0.70 per share.
On March 27, 2009, we completed a private placement of 1.4 million shares of our common stock and received $0.7 million.
Between February 22, 2010 and March 31, 2010, the Company sold 1,451,666 shares of common stock at $0.75 per share for net proceeds of $1,088,750.
During the three months ended June 30, 2010, the Company sold to private investors 2,333,333 shares of common stock at $0.75 per share for net proceeds of $1,750,000. In connection with this private placement, the Company issued warrants to purchase 233,333 shares of common stock, as described below.
During September, 2010, the Company sold to a private investor 266,667 shares of common stock at $0.75 per share for net proceeds of $200,000. In connection with this private placement, the Company issued warrants to purchase 40,000 shares of common stock.
On October 19, 2010, the Company sold approximately 200,000 shares of common stock at a purchase price of $0.75 per share and raised aggregate gross proceeds of approximately $150,000 in a closed equity financing with an unrelated investor. In connection with the private placement, the Company issued warrants to purchase 20,000 shares of common stock at an exercise price of $0.75 per share and a term of 3 years.
On November 2, 2010 the Company received $90,000 as proceeds from the sale of a warrant to purchase 3,250,000 shares of the Company’s common stock. The warrants have an exercise price of $1.50 per share and a term of 3 years.
Shareholder Loan
Al Rajhi loaned the Company $4.0 million on May 12, 2008 under the terms of an unsecured promissory note with a principal amount of $4,240,000 (reflecting an original issue discount of $240,000). The note was initially due on November 12, 2008. Al Rajhi agreed to extend the term of the note of the loan until December 31, 2009. On February 22, 2010, Al Rajhi agreed to extend the term of the note to December 31, 2010. The Company and Al Rajhi are currently negotiating new terms for the note. Additionally, Al Rajhi agreed to convert the interest accrued pursuant to the promissory note into shares of common stock. A total of $853,952 was converted into 1,138,603 shares of common stock at a conversion price of $0.75. In consideration of the extension the Company agreed to extend the term of the warrants issued to Al Rajhi to September 30, 2013.
Other Loans
On October 1, 2008, the Company entered into a $1 million unsecured 12% Loan Agreement with SDS (the “SDS Loan”). The SDS Loan was initially due April 1, 2009. On May 27, 2010 SDS agreed to extend the term of the note to June 2, 2011. In consideration of the extension the Company issued SDS a warrant to purchase 500,000 shares of the Company’s stock with an exercise price of $0.53 per share. The warrants have a five year term.
On dates between October 21, 2008 and November 6, 2008, the Company entered into unsecured 12% Loan Agreements (the “Private Investor Loans”) and Promissory Notes (the “Private Investor Promissory Notes”) with SDS and a group of private investors (the “Private Investors”). Under the Private Investor Promissory Notes, SDS loaned the Company $1 million and the Private Investors loaned the Company $650,000 for a total of $1.65 million. The Private Investor Promissory Notes were initially due in April 2009, and the Private Investors (excluding SDS) agreed to extend the maturity date to June 2010. The Private Investors agreed to further extend the maturity of the loans, maturing in June 2010, on terms that are currently being negotiated. Under the terms of a conversion and extension agreement, SDS converted $1,060,000, representing principal and accrued interest on an unsecured 12% loan made in November 2008 into 5,300,000 shares of common stock during May 2010 and December 2010. The value of the stock issued to SDS in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $4,673,000. This amount was charged to loan conversion inducement expense in the consolidated statements of operations during 2010.
During March 2009, the Company received $650,000 upon issuing an unsecured 6% convertible loan agreements and promissory notes due in March 2011 to a group of private lenders (“Private Lenders”). The Company and the Private Lenders are currently negotiating new terms for the notes.
During March 2009, the Company received $110,000 upon issuing an unsecured 6% convertible loan agreement and promissory note due in March 2011 to a private lender (“Private Lender”). The Company and the Private Lenders are currently negotiating new terms for the note.
On dates between August 13, 2009 and September 24, 2009, the Company received $580,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due in August and September 2011 to a group of Private Lenders.
On dates between October 6, 2009 and December 31, 2009, the Company received $720,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due in August and September 2011 to a group of Private Lenders.
On dates between January 8, 2010 and March 29, 2010, the Company received $875,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due between January and March 2012 to a group of Private Lenders.
Between July and December, 2010, the Company borrowed $1,750,000 under a series of unsecured 6% convertible Loan Agreements and Promissory Notes with several affiliated and non- affiliated lenders. The notes were originally payable to Regen Med.
In November 2010, the Company received $295,000 upon issuing unsecured convertible loan agreements and promissory notes due in May 2011 to a group of Private Lenders. The notes carried an original issue discount of 10%.
On December 16, 2010, the Company entered into a loan agreement totaling $3,050,000 with a private non-affiliated investor due in December 2013. The notes carry an original issue discount of 10% in addition to one-time interest charge of 10%. As of December 31, 2010 the Company had received $350,000 under this agreement.
On December 16, 2010, the Company received $100,000 upon issuing unsecured 10% convertible loan agreements and promissory notes due on March 17, 2011 to a private non-affiliated investor. The note was repaid on March 17, 2011.
In December 2010, SDS and its principals sold a promissory note issued by the Company in the principal amount of $1 million to non-affiliated third parties. As of December 31, 2010 two of these non affiliated investors had exchanged a portion of the notes for restated convertible notes in the amount of $500,000 and $407,000 inclusive of an original interest discount of $37,000 in addition to an annual interest rate of 10% and are due in June 2011 and December 2011 respectively.
On December 31, 2010, the Company received $790,000 from SDS Capital, an existing investor, upon issuing two unsecured loan agreements and promissory notes due in July 2011.
Going Concern
The Company has raised an aggregate of approximately $1.6 million in a series of small loans and stock purchases since December 31, 2010. We need to raise additional capital to fund our clinical trials and other operating activities and repay various note payable and loan agreements. The amount of additional funding required will depend on many factors, including the speed with which we are able to identify and hire people to fill key positions, the speed of patient enrollment in our DCVax®-Brain cancer trial, and unanticipated developments, including any litigation matters. However, without additional capital, we will not be able to complete our DCVax®-Brain clinical trial or move forward with any of our other product candidates for which investigational new drug applications have been cleared by the U.S. Food and Drug Administration, or FDA. We will also not be able to develop our second generation manufacturing processes, which offer substantial product cost reductions.
During 2009, the Company and Cognate BioServices ("Cognate") agreed that most of the accounts payable owed by the Company to Cognate, will be converted into shares of common stock instead of paid in cash. The conversion price will be no less favorable than the conversion price applied to any other creditor of the Company. The lowest conversion price applied to any other creditor of the Company to date following the agreement is $0.20 per share. Accordingly, if no lower conversion price is applied to any other creditor prior to completion of the Cognate conversion, the Cognate conversion will take place at $0.20 per share. The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of accounts payable converted, if any, as a charge to operations when the conversion takes place. Initial review of the payables by both parties indicated a difference in the parties’ respective understanding of the amounts due. The parties have now agreed that as of December 31, 2010 the amount due to Cognate is $10.2 million and the Company has recognized $3.6 million in additional expense in 2010. Finalization of the conversion arrangements are in process.
Also during 2009, the Company agreed with Toucan Capital, Toucan Partners and Linda Powers that a portion of the accrued expenses owed by the Company to these parties for certain expense reimbursements will be converted into shares of common stock instead of paid in cash. Toucan Capital, Toucan Partners and Linda Powers have paid certain expenses on behalf of the Company. The parties agreed that these accrued expenses will be converted into common stock at a conversion rate equal to the price per share paid by unrelated investors at that time, and no less favorable than the conversion rate applied to any other creditor of the Company ($0.20 per share). The parties are in the process of determining the amounts of unbilled accrued expenses. The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of the accrued expenses converted, if any, as a charge to operations when the conversion takes place. Finalization of these arrangements is in process.
We will require additional funding before we achieve profitability. We are in late stage discussions with several parties in regard to additional financing transactions, which we hope to complete later in the year. There can be no assurance that our efforts to seek such funding will be successful. We may raise additional funds by issuing additional common stock or securities (equity or debt) convertible into shares of Common Stock, in which case, the ownership interest of our stockholders will be diluted. Any debt financing, if available, is likely to include restrictive covenants that could limit our ability to take certain actions. Further, we may seek funding from Toucan Capital or Toucan Partners or their affiliates or other third parties. Such parties are under no obligation to provide us any additional funds, and any such funding may be dilutive to stockholders and may contain restrictive covenants. We currently are exploring additional financings with several other parties; however, there can be no assurance that we will be able to complete any such financings or that the terms of such financings will be attractive to us. If our capital raising efforts are unsuccessful, our inability to obtain additional cash as needed could have a material adverse effect on our financial position, results of operations and our ability to continue our existence. Our independent registered public accounting firm has indicated in its report on our consolidated financial statements included in the Annual Report on Form 10-K for the year ended December 31, 2010 that there is substantial doubt about our ability to continue as a going concern.
(
3) Summary of Significant Accounting Policies
(a) References to Authoritative Accounting Literature
In June 2009, the Financial Accounting Standards Board ("FASB") issued the Accounting Standards Codification ("ASC") as the single source of authoritative U.S. GAAP recognized by the FASB to be applied by non-governmental entities in preparation of financial statements in conformity with U.S. GAAP, except for additional authoritative rules and interpretative releases issued by the SEC. While the adoption of the ASC changes how we reference accounting standards, the adoption did not have an impact on our consolidated financial statements.
(b) Principles of Consolidation and Basis of Presentation
The accompanying consolidated financial statements of the Company were prepared in accordance with U.S. GAAP and include the assets, liabilities, revenues and expenses of the Company’s majority-owned subsidiary over which the Company exercises control. Intercompany transactions and balances are eliminated in consolidation. The subsidiary was established in Switzerland during third quarter 2007. The Company contributed 95% of the initial share capital in this subsidiary and Cognate, a related party to the Company, contributed the remaining 5%. Noncontrolling interest is not material for all periods presented.
(c) Foreign Currency Translation
For operations outside the U.S. that prepare financial statements in currencies other than U.S. dollars, we translate the financial statements into U.S. dollars. Results of operations and cash flows are translated at average exchange rates during the period, and assets and liabilities are translated at end of period exchange rates, except for equity transactions and advances not expected to be repaid in the foreseeable future, which are translated at historical cost. The effects of exchange rate fluctuations on translating foreign currency assets and liabilities into U.S. dollars are accumulated as a separate component in other comprehensive income (loss).
(d) Use of Estimates in Preparation of Financial Statements
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
(e) Embedded Derivative Liability
The Company evaluates financial instruments for freestanding or embedded derivatives. Derivative instruments that have been separated from the host contract and do not qualify for hedge accounting are recorded at fair value with changes in value recognized as other income (expense) in the consolidated statements of operations in the period of change.
(f) Fair Value of Financial Instruments
The fair value of financial instruments other than liabilities payable to related parties approximate the recorded value based on the short term nature of these financial instruments. The fair value of liabilities payable to related parties is presently undeterminable due to the related party nature of the obligations. The fair value of derivative liabilities is estimated using a binomial model or Monte Carlo simulation depending on the complexity of the derivative being measured. See Note 4.
(g) Cash
Cash consists of checking and money market accounts. While cash held by financial institutions may at times exceed federally insured limits, management believes that no material credit or market risk exposure exists due to the high quality of the institutions. The Company has not experienced any losses on such accounts.
(h) Property and Equipment
Property and equipment are stated at cost, as adjusted for any prior impairments. Property and equipment are depreciated on a straight-line basis over the estimated useful lives which range from between three and seven years.
Expenditures for maintenance and repairs are expensed as incurred. Gains and losses from disposal representing the difference between any proceeds received from the sale of property and equipment and the recorded values of the asset disposed are recorded in total operating costs and expenses.
(i) Impairment of Long-Lived Assets
Long-lived assets including property and equipment are reviewed for possible impairment whenever significant events or changes in circumstances, including changes in our business strategy and plans, indicate that impairment may have occurred. An impairment is indicated when the sum of the expected future undiscounted net cash flows identifiable to that asset or asset group is less than its carrying value. Long-lived assets to be held and used, including assets to be disposed of other than by sale, for which the carrying amount is not recoverable are adjusted to their estimated fair value at the date an impairment is indicated, which establishes a new basis for the assets for depreciation purposes. Long-lived assets to be disposed of by sale are reported at the lower of carrying amount or fair value less cost to sell. Impairment losses are determined from actual or estimated fair values, which are based on market values, net realizable values or projections of discounted net cash flows, as appropriate.
(j) Operating Leases
The Company recognizes lease expense on a straight-line basis over the initial lease term. For leases that contain rent holidays or escalation clauses, the Company recognizes rent expense on a straight-line basis and records the difference between the rent expense and rental amount payable as deferred rent. As of December 31, 2009 and 2010, we did not have any deferred rent.
(k) Revenue Recognition
The Company has earned revenues through sale of research materials, providing research services to third parties and through research grants in the past. Revenues from sale of research materials are to multiple customers with whom there is no other contractual relationship and are recognized when shipped to the customer and title has passed.
Research contracts and grants require the Company to perform research activities as specified in each respective contract or grant on a best efforts basis, and the Company is paid based on the fees stipulated in the respective contracts and grants which approximate the costs incurred by the Company in performing such activities. The Company recognizes revenue under the research contracts and grants based on completion of performance under the respective contracts and grants where no ongoing obligation on the part of the Company exists. Direct costs related to these contracts and grants are reported as research and development expenses.
(l) Research and Development Expenses
Research and development costs are expensed as incurred. These costs include, but are not limited to, contract manufacturing costs, personnel costs, lab supplies, depreciation, amortization and other indirect costs directly related to the Company’s research and development activities.
(m) Income Taxes
We recognize income taxes on an accrual basis based on tax positions taken or expected to be taken in our tax returns. A tax position is defined as a position in a previously filed tax return or a position expected to be taken in a future tax filing that is reflected in measuring current or deferred income tax assets and liabilities. Tax positions are recognized only when it is more likely than not (i.e., likelihood of greater than 50%), based on technical merits, that the position would be sustained upon examination by taxing authorities. Tax positions that meet the more likely than not threshold are measured using a probability-weighted approach as the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized. Should they occur, our policy is to classify interest and penalties related to tax positions as income tax expense. Since our inception, no such interest or penalties have been incurred, however. Prior to 1998, the Company was a limited liability company and the Company’s tax losses and credits generally flowed directly to the members.
(n) Stock-Based Compensation
Compensation expense for all stock-based awards is measured at the grant date based on the fair value of the award and is recognized as an expense, on a straight-line basis, over the employee's requisite service period (generally the vesting period of the equity award). The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model. Stock-based compensation expense is recognized only for those awards that are expected to vest using an estimated forfeiture rate. We estimate pre-vesting option forfeitures at the time of grant and reflect the impact of estimated pre-vesting option forfeitures in compensation expense recognized. For options and warrants issued to non-employees, the Company recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit.
Stock-based compensation expense was as follows ($ in thousands):
|
|
|
2009
|
|
|
2010
|
|
|
Research and development
|
|
$
|
770
|
|
|
$
|
593
|
|
|
General and administrative expenses
|
|
|
1,848
|
|
|
|
1,411
|
|
|
Total stock- based compensation expense
|
|
$
|
2,618
|
|
|
$
|
2,004
|
|
The assumptions used to estimate the fair value of awards granted for the periods presented are noted in the table below. Expected volatility is based on the separate historical volatility of the market prices of our common stock over the most recent period commensurate with the estimated expected life of the award. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant.
|
|
|
2009
|
|
|
2010
|
|
|
Risk free interest rate
|
|
|
3.6
|
%
|
|
|
3.6
|
%
|
|
Volatility
|
|
|
208
|
%
|
|
|
208
|
%
|
|
Expected term
|
|
10 years
|
|
|
10 years
|
|
|
Expected dividends
|
|
|
0
|
%
|
|
|
0
|
%
|
(o) Loss per Share
Basic loss per share is computed on the basis of the weighted average number of shares outstanding for the reporting period. Diluted loss per share is computed on the basis of the weighted average number of common shares plus dilutive potential common shares outstanding using the treasury stock method. Any potentially dilutive securities are antidilutive due to the Company’s net losses. For the years presented, there is no difference between the basic and diluted net loss per share.
(p) Operating Segments
The Company is principally engaged in the discovery and development of innovative immunotherapies for cancer and has a single operating segment as management reviews all financial information together for the purposes of making decisions and assessing the financial performance of the Company.
Operating costs:
Operating costs and expenses consist primarily of research and development expenses, including clinical trial expenses which arise when we are actively participating in clinical trials, and general and administrative expenses.
Research and development:
Discovery and preclinical research and development expenses include scientific personnel related salary and benefit expenses, stock-based compensation, costs of laboratory supplies used in our internal research and development projects, travel, regulatory compliance, and expenditures for preclinical and clinical trial operation and management when we are actively engaged in clinical trials.
Because the Company is a development stage company, it does not allocate research and development costs on a project basis. The Company adopted this policy, in part, due to the unreasonable cost burden associated with accounting at such a level of detail and its limited number of financial and personnel resources. The Company’s business judgment continues to be that there is little value associated with evaluating expenditures at the project level since the Company is focusing primarily on its lead clinical trial programs as most of the Company’s expenditures relate to those programs.
For the year ended December 31, 2010, of the Company’s operating expenses of approximately $15.4 million, approximately 64% of its expended resources were apportioned to its two DCVax clinical trial programs. From its inception through December 31, 2010, the Company incurred costs of approximately $76.8 million associated with its research and development activities. Because its technologies are novel and unproven, the Company is unable to estimate with any certainty the costs it will incur in the continued development of its product candidates for commercialization.
General and administrative:
General and administrative expenses include administrative personnel related salary and benefit expenses, cost of facilities, insurance, travel, legal support, property and equipment depreciation, stock-based compensation, and amortization of debt discounts and beneficial conversion costs associated with the Company’s debt financing.
(q) Reclassifications
Certain reclassifications have been made to prior period financial statements and footnotes in order to conform to the current period's presentation.
(r) Recent and Adopted Accounting Pronouncements
From time to time new accounting guidance is issued by the FASB that the Company adopts as of an effective date. If not discussed, management believes that the impact of the new accounting guidance, which is not yet effective, will not have a material impact on the Company’s financial statements.
(4) Fair Value Measurements
Effective January 1, 2008 the Company adopted the new accounting guidance relating to fair value measurements as required by accounting guidance for financial instruments measured at fair value on a recurring basis and effective on January 1, 2009 on a non-recurring basis. The new accounting guidance defines fair value, establishes a framework for measuring fair value in accordance with U.S. GAAP and expands disclosures about fair value measurements.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
|
|
·
|
Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets;
|
|
|
·
|
Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
|
|
|
·
|
Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
|
Effective January 1, 2009, the Company adopted new accounting guidance for determining whether a financial instrument is indexed to its own stock. The adoption of the new accounting guidance can affect accounting for equity or debt instruments with down round-round provisions that protect holders from a decline in the stock price. For example, equity or debt instruments with such provisions will no longer be recorded in equity. Down round provisions reduce the exercise price of equity or debt instruments if a company either issues equity shares for a price that is lower than the exercise price of those instruments or issues new warrants or convertible instruments that have a lower exercise price. The Company evaluated whether any warrants or other agreements to acquire shares of the Company’s common stock contain down- round provisions based on a variable that is not an input to the fair value of a “fixed-for-fixed” option. As discussed in Note 10, the Company determined that the September Note warrants, October Note warrants and conversion feature for the $500,000 note payable issued on December 31, 2010 contained such provisions. As a result the Company concluded that the down-round provisions associated with these instruments were not indexed to the Company’s stock and are therefore recorded as derivative liabilities.
The Company recognizes the derivative liabilities at their respective fair values at inception and on each reporting date. The Company measures fair values using a binomial model adjusted for the probability of issuance using a Monte Carlo simulation.
The derivative liabilities associated with the September Note warrants were initially recorded in the consolidated balance sheet upon issuance as of September 28, 2010 at a fair value of $189,000.
The derivative liabilities associated with the warrants issued with the October 1, 2010 $900,000 note from Toucan Partners were initially recorded in the consolidated balance sheet upon issuance as of October 1, 2010 at a fair value of $329,000.
The derivative liabilities associated with the warrants issued with the October 1, 2010 $500,000 note from an unaffiliated third party were initially recorded in the consolidated balance sheet upon issuance as of October 1, 2010 at a fair value of $161,000.
The derivative liabilities associated with the conversion feature in the December 31, 2010 $500,000 note from an unaffiliated third party were initially recorded in the consolidated balance sheet upon issuance as of December 31, 2010 at a fair value of $214,000.
The derivative liabilities continue to be recorded in the consolidated balance sheet at fair value with changes in fair value also recorded in “Derivative valuation gain (loss)” in the consolidated statement of operations. As of December 31, 2010, the fair value of the derivative liabilities was $839,000 with a change in fair value during the period from issuance to December 31, 2010 of $54,000. Key assumptions for determining fair values included expected terms ranging from between 6 and 9 months, volatility ranging between 76% and 131% and risk-free interest rate of 0.25%.
The derivative liabilities are considered Level 3 liabilities on the fair value hierarchy as the determination of fair value includes various assumptions about the future activities and the Company’s stock prices and historical volatility inputs.
The table below provides a reconciliation of the beginning and ending balances for the liabilities measured using fair significant unobservable inputs (Level 3). There were no assets as of or during the year ended December 31, 2010 and no assets or liabilities as of or during the year ended December 31, 2009 measured at fair value using unobservable inputs (Level 3).
Fair Value Measurements Using Significant Unobservable Inputs (Level 3):
|
Balance, January 1, 2010
|
|
$
|
-
|
|
|
Fair value upon issuance, Down-round protection on September Note warrants
|
|
|
189,000
|
|
|
Fair value upon issuance, Down-round protection on October Note warrants
|
|
|
329,000
|
|
|
Fair value upon issuance, Down-round protection on October Note warrants
|
|
|
161,000
|
|
|
Fair value upon issuance, Down-round protection on note payable conversion feature
|
|
|
214,000
|
|
|
Net change in fair value of derivative liabilities (an unrealized gain)
|
|
|
(54,000
|
)
|
|
Balance at December 31, 2010
|
|
$
|
839,000
|
|
(5) Stock-Based Compensation Plans
Stock Option Plans
The Company’s stock option plans are administered by the Board of Directors, which determines the terms and conditions of the options granted, including exercise price, number of options granted and vesting period of such options.
Our employees, directors and consultants previously participated in the 1998 Stock Option Plan and the 1999 Executive Stock Option Plan. The 1998 Stock Option Plan and the 1999 Executive Stock Option Plan were terminated during 2008 and 2009 and no further grants may be made under the plans.
Existing stock option plans are as follows:
(a) 2001 Stock Option Plan
Under the 2001 Stock Option Plan (the “2001 Plan”), 120,000 shares of the Company’s common stock have been reserved for grant of stock options to employees and consultants. Additionally, on January 1 of each year, commencing January 1, 2002, the number of shares reserved for grant under the 2001 Plan will increase by the lesser of (i) 15% of the aggregate number of shares available for grant under the 2001 Plan or (ii) 20,000 shares. Our Board of Directors has the authority to amend or terminate this plan, but such action may not adversely affect any outstanding option previously granted under the plan. If this plan is not terminated earlier, no incentive stock options can be granted under the plan on or after the later of June 2011 or the 10th anniversary of the date when our Board of Directors adopted, subject to approval by our stockholders, the most recent increase in the number of shares available for grant under the plan.
As of December 31, 2010, net of forfeitures, a total of 162,603 shares remain available under this plan; however, effective June 22, 2007, the Company amended the 2001 Stock Option Plan, such that no further option grants may be made under the plan.
(b) 2001 Non-employee Director Stock Incentive Plan
Under the 2001 Non-employee Director Stock Incentive Plan (the “2001 Director Plan”), 13,333 shares of the Company’s common stock have been reserved for grant of stock options to non-employee directors of the Company. As of December 31, 2010, net of forfeitures, a total of 10,500 shares remain available under this plan; however, no further grants may be made under this plan.
(c) 2007 Stock Option Plan
The 2007 Stock Option Plan became effective on June 15, 2007 (the “2007 Stock Option Plan”). In April 2008, the Company increased the number of shares reserved for issuance by 519,132 shares for an aggregate of 6,000,000 shares of its common stock. In May 2010, the Company increased the number of shares reserved for issuance under the 2007 Stock Option Plan by an additional 10,000,000 shares of its common stock. The plan provides for the grant to employees of the Company, its parents and subsidiaries, including officers and employee directors, of “incentive stock options,” as defined, and for the grant of non-statutory stock options to the employees, officers, directors, including non-employee directors, and consultants of the Company, its parents and subsidiaries. As of December 31, 2010, net of forfeitures, a total of 10,070,528 shares remain available for issuance under this plan.
Stock Option Activity
A summary of activity relating to our stock options is as follows (options in thousands):
|
|
|
Options
|
|
|
Weighted-
Average
Exercise
Price
|
|
|
Weighted-
Average
Remaining
Contractual
Term (Years)
|
|
|
Aggregate
Intrinsic
Value
|
|
|
Outstanding as of December 31, 2009
|
|
|
4,108
|
|
|
$
|
0.69
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
100
|
|
|
|
0.75
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(99
|
)
|
|
|
0.75
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
(2
|
)
|
|
|
15.15
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(851
|
)
|
|
|
0.60
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2010
|
|
|
3,256
|
|
|
|
0.71
|
|
|
|
8.3
|
|
|
|
|
|
|
Exercisable as of December 31, 2010
|
|
|
2,305
|
|
|
|
0.71
|
|
|
|
8.5
|
|
|
$
|
—
|
|
A summary of the Company’s unvested stock option grants and changes during 2010 was as follows (options in thousands):
|
|
|
|
|
|
Weighted-
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
Grant Date
|
|
|
|
|
Options
|
|
|
Fair Value
|
|
|
Outstanding at December 31, 2009
|
|
|
1,816
|
|
|
$
|
0.68
|
|
|
Granted during 2010
|
|
|
100
|
|
|
|
0.75
|
|
|
Expired/Forfeited during 2010
|
|
|
(2
|
)
|
|
|
15.15
|
|
|
Vested during 2010
|
|
|
(963
|
)
|
|
|
0.67
|
|
|
Outstanding at December 31, 2010
|
|
|
951
|
|
|
|
0.70
|
|
Additional information regarding stock options outstanding and exercisable at December 31, 2010 is as follows, in thousands, except option price and weighted average exercise price.
|
|
|
Options Outstanding
|
|
|
Options Exercisable
|
|
|
Range of
Exercise
Prices
|
|
Number
Outstanding
|
|
|
Weighted-
Average
Remaining
Contractual
Life (Years)
|
|
|
Weighted
Average
Exercise
Price
|
|
|
Number
Exercisable
|
|
|
Weighted
Average
Exercise
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.55 - 0.60
|
|
|
2,237
|
|
|
|
8.37
|
|
|
$
|
0.56
|
|
|
|
1,875
|
|
|
$
|
0.56
|
|
|
$ 0.61 - 2.40
|
|
|
1,011
|
|
|
|
8.23
|
|
|
$
|
0.88
|
|
|
|
421
|
|
|
$
|
1.03
|
|
|
$ 2.41 - 75.00
|
|
|
8
|
|
|
|
1.24
|
|
|
$
|
21.43
|
|
|
|
8
|
|
|
$
|
21.43
|
|
|
Total
|
|
|
3,256
|
|
|
|
8.30
|
|
|
$
|
0.71
|
|
|
|
2,304
|
|
|
$
|
0.70
|
|
Options granted under the plans are generally priced at or above the estimated fair market value of the Company’s common stock on the date of grant and generally vest over between four and nine years. Compensation expense, if any, is charged over the period of vesting. All options, if not previously exercised or canceled, expire ten years from the date of grant, or the expiration date specified in the individual option agreement, if earlier.
During 2010 and 2009, the Company granted options to purchase 100,000 and 2,581,000, respectively, shares of common stock. The weighted average exercise price of options granted in 2010 and 2009 was $0.75 and $0.60, respectively. Stock compensation expense amounted to $2,004,000 and $2,618,000 during 2010 and 2009, respectively.
The aggregate intrinsic value of options exercised during 2010 and 2009 was $74,241 and $64,025, respectively. Our policy is to issue new shares to fulfill the requirements for options that are exercised.
The aggregate fair value of options vested during 2010 and 2009 was $1,998,778 and $2,919,671, respectively.
As of December 31, 2010 the total unrecognized compensation expense related to unvested stock option awards was $1,702,175, which is expected to be recognized over a weighted average term of approximately 9 years.
(6) Stockholders’ Equity (Deficit)
(a) Stock Purchase Warrants
Toucan Capital and Toucan Partners Warrants
The Company has issued the following warrants to Toucan Capital and Toucan Partners:
|
Date of Issue
|
|
Related transaction
|
|
Warrants Issued
|
|
|
Exercise Price
|
|
|
Warrants
outstanding at
December 31,
2010
|
|
|
Issued to Toucan Capital
|
|
|
|
|
|
|
|
|
|
|
June 2007
|
|
See Conversion Agreement in Note 2
|
|
|
14,150,732
|
|
|
$
|
0.60
|
|
|
|
9,433,821
|
|
|
June 2007
|
|
See Conversion Agreement in Note 3
|
|
|
7,884,357
|
|
|
|
0.15
|
|
|
|
5,256,238
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued to Toucan Partners
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 2007
|
|
See Conversion Agreement in Note 2
|
|
|
8,832,541
|
|
|
|
0.60
|
|
|
|
8,832,541
|
|
|
December 2008
|
|
December 2008 Loan warrants
|
|
|
132,500
|
|
|
|
0.40
|
|
|
|
132,500
|
|
|
September 2009
|
|
August and December 2008 Loan Conversions
|
|
|
513,841
|
|
|
|
0.41
|
|
|
|
513,841
|
|
|
September 2009
|
|
Consulting services
|
|
|
842,375
|
|
|
|
0.2
|
|
|
|
842,375
|
|
|
July 2010
|
|
Equity Investment ($650,000)
|
|
|
86,667
|
|
|
|
0.75
|
|
|
|
86,667
|
|
|
October 2010
|
|
October $900,000 loan
|
|
|
1,097,561
|
|
|
|
0.82
|
|
|
|
1,097,561
|
|
Other Warrant Issues
|
Date of Issue
|
|
Related transaction
|
|
Warrants
Issued
|
|
|
Exercise
Price
|
|
|
Warrants
outstanding
at
December
31,
2010
|
|
|
September 2009
|
|
Al Rajhi loan extension
|
|
|
1,743,111
|
|
|
$
|
0.63
|
|
|
|
1,743,111
|
|
|
September 2009
|
|
Loan extensions
|
|
|
861,520
|
|
|
|
0.20
|
|
|
|
861,520
|
|
|
January 2010
|
|
Consultant compensation (1)
|
|
|
100,000
|
|
|
|
0.75
|
|
|
|
100,000
|
|
|
February 2010
|
|
Consultant compensation (2)
|
|
|
250,000
|
|
|
|
1.00
|
|
|
|
250,000
|
|
|
April 2010
|
|
Private Placement
|
|
|
233,333
|
|
|
|
0.75
|
|
|
|
176,996
|
|
|
May 2010
|
|
SDS loan extension
|
|
|
500,000
|
|
|
|
0.53
|
|
|
|
500,000
|
|
|
July 2010
|
|
Regen Med July 2010 $1,750,000 Loan
|
|
|
2,333,333
|
|
|
|
0.75
|
|
|
|
1,949,678
|
|
|
July 2010
|
|
Consultant compensation (3)
|
|
|
300,000
|
|
|
|
0.75
|
|
|
|
300,000
|
|
|
September 2010
|
|
September 28, 2010 $350,000 Loan
|
|
|
421,687
|
|
|
|
0.83
|
|
|
|
421,687
|
|
|
October 2010
|
|
October 1, 2010 $500,000 Loan
|
|
|
609,756
|
|
|
|
0.82
|
|
|
|
609,756
|
|
|
October 2010
|
|
Consultant compensation (4)
|
|
|
20,000
|
|
|
|
0.75
|
|
|
|
20,000
|
|
|
November 2010
|
|
Consultant compensation (5)
|
|
|
75,833
|
|
|
|
0.75
|
|
|
|
75,833
|
|
|
November 2010
|
|
Warrant issued for cash
|
|
|
3,250,000
|
|
|
|
1.50
|
|
|
|
3,250,000
|
|
|
December 2010
|
|
December 16, 2010 $100,000 loan
|
|
|
133,333
|
|
|
|
0.75
|
|
|
|
133,333
|
|
|
December 2010
|
|
December SDS loan warrants
|
|
|
118,165
|
|
|
|
0.75
|
|
|
|
118,165
|
|
(1) The fair value of the warrants amounting to $72,000 was recognized as general and administrative expense.
(2) The fair value of the warrants amounting to $169,000 was recognized as general and administrative expense.
(3) The fair value of the warrants amounting to $203,000 was recognized as general and administrative expense.
(4) The fair value of the warrants amounting to $14,000 was recognized as general and administrative expense.
(5) The fair value of the warrants amounting to $48,000 was recognized as general and administrative expense.
A summary of the warrants outstanding at December 31, 2010 is as follows:
|
Date of Issue
|
|
Warrants
Outstanding
as of
December 31,
2010
|
|
|
Exercise
Price
|
|
Expiration
|
|
June 1, 2007
|
|
|
7,628,587
|
|
|
$
|
0.15
|
|
May 31, 2015
|
|
December 3, 2010
|
|
|
255,750
|
|
|
|
0.15
|
|
December 2, 2013
|
|
September 30, 2009
|
|
|
1,703,625
|
|
|
|
0.20
|
|
September 29, 2012
|
|
December 23, 2008
|
|
|
132,500
|
|
|
|
0.40
|
|
December 22, 2016
|
|
November 6, 2008
|
|
|
1,354,083
|
|
|
|
0.41
|
|
November 5, 2012
|
|
May 26, 2010
|
|
|
500,000
|
|
|
|
0.53
|
|
November 17, 2015
|
|
June 1, 2007
|
|
|
21,064,997
|
|
|
|
0.60
|
|
May 31, 2015
|
|
December 3, 2010
|
|
|
1,918,275
|
|
|
|
0.60
|
|
December 2, 2013
|
|
September 28, 2009
|
|
|
1,743,111
|
|
|
|
0.63
|
|
September 27, 2012
|
|
January 1, 2010
|
|
|
100,000
|
|
|
|
0.75
|
|
December 31, 2012
|
|
March 23, 2010 to May 26, 2010
|
|
|
233,329
|
|
|
|
0.75
|
|
May 25, 2013
|
|
July 7, 2010
|
|
|
300,000
|
|
|
|
0.75
|
|
July 6, 2013
|
|
July 2, 2010
|
|
|
86,667
|
|
|
|
0.75
|
|
July 1, 2013
|
|
July 14, 2010
|
|
|
1,949,678
|
|
|
|
0.75
|
|
July 13, 2015
|
|
October 13, 2010
|
|
|
80,000
|
|
|
|
0.75
|
|
October 12, 2013
|
|
November 23, 2010
|
|
|
75,833
|
|
|
|
0.75
|
|
November 22, 2015
|
|
December 3, 2010
|
|
|
383,655
|
|
|
|
0.75
|
|
December 2, 2010
|
|
December 13, 2010
|
|
|
133,333
|
|
|
|
0.75
|
|
December 12, 2015
|
|
December 29, 2010
|
|
|
118,165
|
|
|
|
0.75
|
|
December 28, 2015
|
|
November 29, 2010
|
|
|
36,875
|
|
|
|
0.80
|
|
November 23, 2013
|
|
October 1, 2010
|
|
|
1,707,317
|
|
|
|
0.82
|
|
September 30, 2015
|
|
November 29,2010
|
|
|
421,687
|
|
|
|
0.83
|
|
November 28, 2010
|
|
February 1, 2010
|
|
|
250,000
|
|
|
|
1.00
|
|
January 31, 2013
|
|
November 5, 2010
|
|
|
3,250,000
|
|
|
|
1.50
|
|
November 4, 2013
|
|
February 9, 2003
|
|
|
13,333
|
|
|
|
1.53
|
|
February 8, 2013
|
|
March 30, 2006
|
|
|
677,389
|
|
|
|
2.10
|
|
March 29, 2011
|
|
January 8, 2003
|
|
|
13,333
|
|
|
|
2.66
|
|
January 7, 2013
|
|
December 26, 2002
|
|
|
26,666
|
|
|
|
3.24
|
|
December 25, 2012
|
|
|
|
|
46,158,188
|
|
|
|
|
|
|
(b) Common Stock Equivalents
The following common stock equivalents on an as-converted basis were excluded from the calculation of diluted net loss per share, as the effect would be antidilutive (in thousands):
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
Common stock options
|
|
|
4,108
|
|
|
|
3,256
|
|
|
Common stock warrants
|
|
|
36,599
|
|
|
|
46,158
|
|
|
Common stock issuable on conversion of notes payable
|
|
|
14,177
|
|
|
|
18,606
|
|
(c) Common Stock Issuances
Issuances of common stock during 2010 and 2009 were as follows:
|
Date
|
|
Transaction
|
|
# of Shares
|
|
|
Price per
share
|
|
|
Gross
Cash
Proceeds
|
|
|
January 2009
|
|
Private Placement
|
|
|
1,000,000
|
|
|
$
|
0.70
|
|
|
$
|
700,000
|
|
|
March 2010
|
|
Consultant fees paid in stock (1)
|
|
|
199,661
|
|
|
|
0.69
|
|
|
|
|
|
|
March 2009
|
|
Private Placement
|
|
|
1,377,358
|
|
|
|
0.50
|
|
|
|
700,000
|
|
|
September 2009
|
|
Toucan Partners Loan Conversion (2)
|
|
|
8,547,280
|
|
|
|
0.20
|
|
|
|
|
|
|
September 2009
|
|
Toucan Partners consulting services (1)
|
|
|
2,504,034
|
|
|
|
0.75
|
|
|
|
|
|
|
September 2009
|
|
Consultant fees paid in stock (1)
|
|
|
397,500
|
|
|
|
0.75
|
|
|
|
|
|
|
September 2009
|
|
Note conversion
|
|
|
562,500
|
|
|
|
0.20
|
|
|
|
|
|
|
December 2009
|
|
Consultant fees paid in stock (1)
|
|
|
146,306
|
|
|
|
0.82
|
|
|
|
|
|
|
December 2009
|
|
Exercise of employee stock options and warrants (cashless basis)
|
|
|
1,236,055
|
|
|
|
|
|
|
|
|
|
|
December 2009
|
|
Stock issued in lieu of salaries (1)
|
|
|
413,540
|
|
|
|
0.93
|
|
|
|
|
|
|
Total issued in 2009
|
|
|
|
|
16,384,234
|
|
|
|
|
|
|
$
|
1,400,000
|
|
|
March 2010
|
|
Private Placement
|
|
|
1,451,667
|
|
|
|
0.75
|
|
|
$
|
1,088,750
|
|
|
March 2010
|
|
Al Rajhi interest conversion (3)
|
|
|
1,138,603
|
|
|
|
0.75
|
|
|
|
|
|
|
May 2010
|
|
SDS Loan conversion (4)
|
|
|
5,300,000
|
|
|
|
0.20
|
|
|
|
|
|
|
June 2010
|
|
Private Placement
|
|
|
2,333,333
|
|
|
|
0.75
|
|
|
|
1,750,000
|
|
|
July 2010
|
|
Private Placement
|
|
|
866,667
|
|
|
|
0.75
|
|
|
|
650,000
|
|
|
Jul;y 2010
|
|
Toucan Partners consulting fees (1)
|
|
|
8,466
|
|
|
|
0.20
|
|
|
|
|
|
|
September 2010
|
|
Private Placement
|
|
|
266,667
|
|
|
|
0.75
|
|
|
|
200,000
|
|
|
October 2010
|
|
Note conversion
|
|
|
58,971
|
|
|
|
0.77
|
|
|
|
|
|
|
December 2010
|
|
Stock issued in lieu of salaries (1)
|
|
|
704,752
|
|
|
|
0.73
|
|
|
|
|
|
|
December 2010
|
|
Consultant fees paid in stock (1)
|
|
|
1,559,071
|
|
|
|
0.77
|
|
|
|
|
|
|
December 2010
|
|
Loan conversion
|
|
|
353,187
|
|
|
|
0.46
|
|
|
|
|
|
|
December 2010
|
|
Private Placement
|
|
|
200,000
|
|
|
|
0.75
|
|
|
|
150,000
|
|
|
Total issued in 2010
|
|
|
|
|
14,241,384
|
|
|
|
|
|
|
$
|
3,838,750
|
|
(1) Common stock valued at closing price at date of issue.
(2) The value of the stock issued to Toucan Partners in excess of the carrying amount of the loans and accrued interest payable amounted to $4,701,004, and together with the fair value of the warrants, of $916,716, was charged to loan conversion inducement expense in the accompanying consolidated statements of operations during 2009.
(3) The fair value of the common stock issued in excess of the accrued interest payable converted into common stock amounted to $194,761 and was recorded in interest expense in the accompanying consolidated financial statements.
(4) The value of the stock issued to SDS in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $4,673,568. This amount was charged to loan conversion inducement expense in the accompanying consolidated statements of operations during 2010.
(d) Employee 401(k) Plan
On August 19, 1999, the Company adopted a 401(k) Plan for certain eligible employees. Under the plan, an eligible employee may elect to contribute to the plan. In addition, the Company may elect to contribute matching contributions. Effective March 1, 2006, the Company no longer matches employee contributions.
(e) Stockholder Rights Agreement
On March 6, 2002, the Company adopted a Stockholder Rights Agreement, under which each common stockholder received a dividend of one right per share of common stock held. Each right entitles the holder to purchase one share of common stock at a price equal to $19.25 per share, subject to certain anti-dilution provisions, and is exercisable only in the event that a third party acquires beneficial ownership of, or announces a tender or exchange offer for, at least 15% of the Company’s outstanding common stock and such acquisition or offer is determined by the Board of Directors to not be in the best interests of the stockholders. If the acquisition or offer were determined by the Board of Directors to be in the best interests of the stockholders, the rights may be redeemed by the Company for $0.0001 per right. The rights will expire on February 25, 2012, unless earlier terminated in accordance with the rights agreement. The Board of Directors and Mellon Investor Services LLC, its Rights Agent, on April 26, 2004, amended the Stockholder Rights Agreement. The definition of an “Acquiring Person” was amended to exclude Toucan Capital Fund II, L.P. and other investors selected by Toucan from the definition of “Acquiring Person” for those shares of the Company’s capital stock they acquire, or are deemed to beneficially own, in connection with the Recapitalization Agreement.
(7) Related Party Transactions
(a) Notes Payable to Related Parties
Convertible promissory notes have been issued to Toucan Capital and Toucan Partners. As of December 31, 2010 all of the notes issued prior to December 31, 2008 have either been converted or repaid.
In June and July 2009 we entered into Loan Agreements and Promissory Notes with Toucan Partners for an aggregate of $1,300,000 with a term of two years at 6% interest.
In September 2010 we entered into Convertible Loan Agreements and Promissory Notes with Toucan Partners for an aggregate of $900,000 with a term of sixty days at 6% interest. The loan was repaid in 2010.
Notes payable to related parties are more fully described in Notes (2) and (10).
(b) Cognate Agreement
On July 30, 2004, the Company entered into a service agreement with Cognate Therapeutics, Inc. (now known as Cognate BioServices, Inc., or Cognate), a contract manufacturing and services organization in which Toucan Capital has a majority interest. In addition, two of the principals of Toucan Capital are members of Cognate’s board of directors and, on May 17, 2007, the managing director of Toucan Capital was appointed to serve as a director of the Company and to serve as the non-executive Chairperson of the Company’s Board of Directors. Under the service agreement, the Company agreed to utilize Cognate’s services for an initial two-year period, related primarily to manufacturing DCVax product candidates, regulatory advice, research and development preclinical activities and managing clinical trials. The agreement expired on July 30, 2006; however, the Company continued to utilize Cognate’s services under the same terms as set forth in the expired agreement. On May 17, 2007, the Company entered into a new service agreement with Cognate pursuant to which Cognate will provide certain consulting and, when needed, manufacturing services to the Company for its DCVax -Brain Phase II clinical trial. Under the terms of the new contract, the Company paid a non-refundable contract initiation fee of $250,000 and committed to pay budgeted monthly service fees of $400,000, subject to quarterly true-ups, and monthly facility fees of $150,000. Under the terms of the contract unless the contract is terminated earlier the contract will expire at the earlier of (i) the submission of an FDA biological license application/new drug application on the Company’s brain cancer clinical trial or (ii) July 1, 2010. The Company may terminate this agreement with 180 days notice and payment of all reasonable wind-up costs and Cognate may terminate the contract in the event that the brain cancer clinical trial fails to complete enrollment by July 1, 2009. However, if such termination by the Company occurs at any time prior to the earlier of the submission of an FDA biological license application/new drug application on the Company’s brain cancer clinical trial or July 1, 2010 or, such termination by Cognate results from failure of the brain cancer clinical trial to complete patient enrollment by July 1, 2009, the Company is obligated to make an additional termination fee payment to Cognate equal to $2 million. Although the Company failed to complete enrollment of the brain cancer clinical trial by July 1, 2009, Cognate reserved its rights but did not trigger termination as of December 31, 2010. Since July 1, 2009 with the mutual agreement of Cognate and the Company the agreement has continued on a month to month basis on the same terms as included in the original agreement.
If and to the extent we wish to engage Cognate to manufacture any of our product candidates for clinical trials or commercialization, we will need to enter into a new agreement with Cognate or another third-party manufacturer which might not be feasible on a timely or favorable basis. Cognate’s facility in Memphis, Tennessee has the capacity for approximately 600 patients per year, which we believe will be sufficient for our Phase II clinical trial for DCVax -Brain. We have a plan with Cognate to accommodate an increase in production capacity based on demand and have detailed plans and cost analysis for additional modular expansions which should increase the capacity of the current facilities from approximately 600 patients to over 9,000 patients per year.
During the years ending December 31, 2009 and 2010, respectively, the Company recognized approximately $7.3 million and $7.8 million of research and development costs related to these service agreements. As of December 31, 2009 and 2010, the Company owed Cognate approximately $5.9 and $10.2 million, respectively.
During 2009, the Company and Cognate agreed that most of the accounts payable owed by the Company to Cognate, will be converted into shares of common stock instead of paid in cash. The conversion price will be no less favorable than the conversion price applied to any other creditor of the Company. The lowest conversion price applied to any other creditor of the Company to date following the agreement is $0.20 per share. Accordingly, if no lower conversion price is applied to any other creditor prior to completion of the Cognate conversion, the Cognate conversion will take place at $0.20 per share. The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of accounts payable converted, if any, as a charge to operations when the conversion takes place. Initial review of the payables by both parties indicated a difference in the parties’ respective understanding of the amounts due. The parties have now agreed that as of December 31, 2010 the amount due to Cognate is $10.2 million, and the Company has recognized $3.6 million in additional expense in 2010. Finalization of the conversion arrangements are in process.
(c) Toucan Capital Management
In accordance with a recapitalization agreement dated April 26, 2004 between the Company and Toucan Capital, as amended and restated on July 30, 2004 and further amended ten times between October 22, 2004 and November 14, 2005, pursuant to which Toucan Capital agreed to recapitalize the Company by making loans to the Company, the Company accrued and paid certain legal and other administrative costs on Toucan Capital’s behalf. Pursuant to the terms of the Conversion Agreement discussed above, the recapitalization agreement was terminated on June 22, 2007. Subsequent to the termination of the recapitalization agreement, Toucan Capital continues to incur costs on behalf of the Company. These costs primarily relate to consulting costs and travel expenses incurred in support of the Company’s international expansion efforts. In addition, since July 1, 2007 the Company has accrued and recorded rent expense due to Toucan Capital Corp. an affiliate of Toucan Capital for its office space in Bethesda, Maryland.
During the years ending December 31, 2009 and 2010, respectively, the Company recognized approximately $1.1 million and $0.9 million of general and administrative costs related to this recapitalization agreement, rent expense, as well as legal, travel and other costs incurred by Toucan Capital, Toucan Partners and Linda Powers on the Company’s behalf. At December 31, 2009 and 2010, accrued expenses payable to Toucan Capital and related parties amounted to $0.4 million and $1.5 million, respectively, and are included as part of accounts payable to related parties in the accompanying consolidated balance sheets.
Also during 2009, the Company agreed with Toucan Capital, Toucan Partners and Linda Powers that a portion of the accrued expenses owed by the Company to these parties for certain expense reimbursements will be converted into shares of common stock instead of paid in cash. Toucan Capital, Toucan Partners and Linda Powers have paid certain expenses on behalf of the Company. The parties agreed that these accrued expenses will be converted into common stock at a conversion rate equal to the price per share paid by unrelated investors at that time, and no less favorable than the conversion rate applied to any other creditor of the Company ($0.20 per share). The parties are in the process of determining the amounts of unbilled accrued expenses. The impact of the conversion will result in a reduction of liabilities for the amount converted. In addition, the Company will recognize the value of common stock issued in excess of the amount of the accrued expenses converted, if any, as a charge to operations when the conversion takes place. Finalization of these arrangements is in process.
On March 21, 2008, the Company executed a Sublease Agreement (the “Sublease Agreement”) with Toucan Capital for the space the Company uses as its headquarters at 7600 Wisconsin Avenue, Suite 750, Bethesda, Maryland. The Sublease Agreement is effective as of July 1, 2007 and expires on October 31, 2016, unless sooner terminated according to its terms. The Company was and remains obligated to pay operating expenses allocable to the subleased premises under Toucan Capital master lease. Effective November 30, 2009, the Sublease was terminated in connection with termination and buyout of the overall lease of this space. (The overall lease and the Company’s Sublease had 7 years left to run at that time). The termination and buyout did not require any lump sum exit payment. Instead, it requires a partial payout over several years. The obligation for the Company will be less than $5,000 per month during 2011.
(8) Income Taxes
There was no income tax benefit attributable to net losses for 2010 and 2009. The difference between taxes computed by applying the U.S. federal corporate rate of 34% and the actual income tax provisions in 2010 and 2009 is primarily the result of establishing a valuation allowance on the Company’s deferred tax assets arising primarily from tax loss carry forwards.
The tax effects of temporary differences and tax loss and credit carry forwards that give rise to significant portions of deferred tax assets and liabilities at December 31 are comprised of the following (in thousands):
|
|
|
2009
|
|
|
2010
|
|
|
Net operating loss carry forwards
|
|
$
|
36,666
|
|
|
$
|
41,175
|
|
|
Research and development credit carry forwards
|
|
|
2,549
|
|
|
|
2,821
|
|
|
Other
|
|
|
165
|
|
|
|
25
|
|
|
Gross deferred tax assets
|
|
|
39,380
|
|
|
|
44,021
|
|
|
Less valuation allowance
|
|
|
(39,380
|
)
|
|
|
(44,021
|
)
|
|
Net deferred tax assets
|
|
$
|
—
|
|
|
$
|
—
|
|
The increase in the valuation allowance for deferred tax assets for 2010 and 2009 of $4.6 million and $5.0 million, respectively, was due to the inability to utilize net operating losses and research and development credits.
At December 31, 2010, the Company had net operating loss carry forwards for income tax purposes of approximately $121.1 million and unused research and development tax credits of approximately $2.8 million available to offset future taxable income and income taxes, respectively, expiring beginning 2019 through 2030. The Company’s ability to utilize net operating loss and credit carry forwards is limited pursuant to the Tax Reform Act of 1986, due to cumulative changes in stock ownership in excess of 50% such that some net operating losses may never be utilized. The tax years 2007 through 2010 remain open to examination by federal agencies and other jurisdictions in which the Company operates.
(9) Notes Payable
Toucan Partners Loans
Toucan Partners loaned the Company a total of $1,300,000 on June 30, 2009, July 2, 2009 and July 17, 2009 under unsecured 6% convertible promissory notes due June 29, 2011, July 1, 2011 and July 16, 2011. The conversion feature of the notes allows Toucan Partners to receive shares of common stock, at a conversion price of $0.20. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $1,300,000 which was recorded as a debt discount to be amortized over the term of the notes.
On October 1, 2010 the Company entered into unsecured 6% Loan Agreements and Promissory Notes (the “October Note”) with Toucan Partners in the amount of $900,000 when Toucan Partners partially repaid the Regen Med Notes. The terms of the October Note and related warrants conformed to the terms of the September Notes previously negotiated by the Company with unrelated investors, and executed by the Company with such investors prior to these October Notes with Toucan Partners. Accordingly, the October Notes had a term of 60 days and were convertible into shares of the Company’s common stock at $0.75 per share. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $285,366 which was recorded as a debt discount to be amortized over the term of the notes. The notes also carried 100% warrant coverage with an exercise price, based on the same market formula as the September Notes, of $0.82 per share. The warrants also provided the same price protection as in the September Notes, in that if the Company issues stock or warrants at a price lower than the $0.82 per share during the 1-year period following the effective date of these notes, the price of the warrants will be reset to such lower price (other than on issuances, if any, pursuant to arrangements entered into reported prior to the effective date). In the event that lower priced stock or warrants are issued during the protected period all the warrants pursuant to the October Note will be re-priced but no additional warrants will be due. The Company determined that the warrants should be accounted for as an embedded derivative. The derivate was valued at $329,000 using a binomial model and accounted for as an embedded derivative liability with the offsetting charge booked as debt discount.
Other
Al Rajhi loaned the Company $4.0 million on May 12, 2008 under the terms of an unsecured promissory note with a principal amount of $4,240,000 (reflecting an original issue discount of $240,000). The note was initially due on November 12, 2008. Al Rajhi agreed to extend the term of the note of the loan until December 31, 2009. On February 22, 2010, Al Rajhi agreed to extend the term of the note to December 31, 2010. The Company and Al Rajhi are currently negotiating new terms for the note. Additionally, Al Rajhi agreed to convert the interest accrued pursuant to the promissory note into shares of common stock. A total of $853,952 was converted into 1,138,603 shares of common stock at a conversion price of $0.75. In consideration of the extension the Company agreed to extend the term of the warrants issued to Al Rajhi to September 30, 2013.
On October 1, 2008, the Company entered into a $1 million unsecured 12% Loan Agreement with SDS (the “SDS Loan”). The SDS Loan was initially due April 1, 2009. On May 27, 2010 SDS agreed to extend the term of the note to June 2, 2011. In consideration of the extension the Company issued SDS a warrant to purchase 500,000 shares of the Company’s stock with an exercise price of $0.53 per share. The warrants have a five year term.
On dates between October 21, 2008 and November 6, 2008, the Company entered into unsecured 12% Loan Agreements (the “Private Investor Loans”) and Promissory Notes (the “Private Investor Promissory Notes”) with SDS and a group of private investors (the “Private Investors”). Under the Private Investor Promissory Notes, SDS loaned the Company $1 million and the Private Investors loaned the Company $650,000 for a total of $1.65 million. The Private Investor Promissory Notes were initially due in April 2009, and the Private Investors (excluding SDS) agreed to extend the maturity date to June 2010. The Private Investors agreed to further extend the maturity of the loans, maturing in June 2010, on terms that are currently being negotiated. Under the terms of a conversion and extension agreement, SDS converted the November 2008 $1 million unsecured 12% loan and accrued interest payable into 5,024,400 shares of common stock during May 2010. The value of the stock issued to SDS in excess of the carrying amount of the loan principal and accrued interest payable that was converted was $4,521,960. This amount was charged to loan conversion inducement expense in the accompanying consolidated statements of operations during 2010.
During March 2009, the Company received $650,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due in March 2011 to a group of private lenders (“Private Lenders”). The Company and the Private Lenders are currently negotiating new terms for the notes. The conversion feature allows the holders to receive shares of common stock only, and not cash or other consideration, at a conversion price of $0.63. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $73,000 which was recorded as a debt discount to be amortized over the term of the notes.
During March 2009, the Company received $110,000 upon issuing a unsecured 6% convertible loan agreement and promissory note due on March 25, 2011 to a Private Lender. The Company and Private lender are currently negotiating new terms for the note. Upon receiving a request for conversion, the Company may elect to settle the instrument in cash or pay the total principal and accrued interest in shares of common stock, at a conversion price of $0.63. The Company has assessed the note and concluded that the amount of the loan attributable to equity is immaterial. The intrinsic value of the note did not result in a material beneficial conversion feature.
On dates between August 13, 2009 and September 24, 2009, the Company received $580,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due in August and September 2011 to a group of Private Lenders. The conversion feature allows the holders to receive shares of common stock at a conversion price of $0.20. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $580,000 which was recorded as a debt discount to be amortized over the term of the notes.
On dates between October 6, 2009 and December 31, 2009, the Company received $215,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due in August and September 2011 to a group of Private Lenders. The conversion feature allows the holders to receive shares of common stock at a conversion price of $0.20. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $205,000 which was recorded as a debt discount to be amortized over the term of the notes.
On dates between October 6, 2009 and December 31, 2009, the Company received $505,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due in August and September 2011 to a group of Private Lenders. The conversion feature allows the holders to receive shares of common stock at a conversion price of $0.50. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $295,000 which was recorded as a debt discount to be amortized over the term of the notes.
On dates between January 8, 2010 and March 29, 2010, the Company received $875,000 upon issuing unsecured 6% convertible loan agreements and promissory notes due between January and March 2012 to a group of Private Lenders. The conversion feature allows the holders to elect in their discretion to receive shares of common stock at a conversion price of $0.50. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $579,000 which was recorded as a debt discount to be amortized over the term of the notes.
On July 14, 2010, the Company entered into unsecured 6% Loan Agreements and Convertible Promissory Notes with Regen Med. Under these Notes, Regen Med provided bridge funding (“Bridge Funding”) to the Company in the amount of $1,750,000. The Bridge Funding carried an interest rate of 6% with a term of 60 days maturing on September 14, 2011 and was convertible at the discretion of the lender at a conversion price of at $0.75 per share. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $844,667 which was recorded as a debt discount which was expensed during 2010. The Bridge Funding carried 100% warrant coverage at $0.75 per share. The $1,750,000 proceeds from the issuance of the Bridge Funding Notes and warrants were allocated on a relative fair value basis. The relative fair value of the promissory notes was estimated to be $844,667. The relative fair value of the warrants was estimated to be $905,333, which was expensed during 2010. On the maturity date, September 14, 2010, the term of the Bridge Funding was extended to October 1, 2010. On September 28, 2010 $350,000 of the principal was repaid directly by the holders of the notes described below. On October 1, 2010, Toucan Partners and the holder of the note also described below repaid the remaining $1,400,000 payable to Regen Med.
On September 28, 2010 the Company entered into unsecured 6% Loan Agreements and Promissory Notes (the “September Notes”) with private lenders in the amount of $350,000 and used the proceeds to partially repay the Bridge Funding. The notes had a term of 60 days and were convertible into shares of the Company’s common stock at $0.75 per share. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $161,000 which was recorded as a debt discount to be amortized over the term of the notes. The September Notes also carried 100% warrant coverage with an exercise price, pursuant to a market formula, of $0.83 per share. The warrants also featured price protection in that if the Company issues stock or warrants at a price lower than the $0.83 per share during the 1-year period following the effective date of these notes, the price of the September Note warrants will be reset to such lower price (other than on issuances, if any, pursuant to arrangements entered into and reported prior to the effective date). In the event that lower priced stock or warrants are issued during the protected period all the September Note warrants will be priced but no additional warrants will be due. The Company determined that the warrants should be accounted for as an embedded derivative. The derivate was valued at $189,000 using a binomial model and accounted for as an embedded derivative liability with the offsetting charge booked as debt discount
On October 1, 2010 the Company entered into unsecured 6% Loan Agreements and Promissory Notes (the “Additional October Note”) with an unrelated third party in the amount of $500,000, on the same terms and conditions as the September Notes with non-affiliated investors, and used the proceeds to partially repay the Bridge Funding. The notes had a term of 60 days and were convertible into shares of the Company’s common stock at $0.75 per share. The intrinsic value of the convertible notes resulted in a beneficial conversion feature amounting to $137,000 which was recorded as a debt discount to be amortized over the term of the notes. The notes also carried 100% warrant coverage with an exercise price, based on the same market formula as in the September Notes of the non-affiliated investors, of $0.82 per share. The warrants also featured price protection in that if the Company issues stock or warrants at a price lower than the $0.82 per share during the 1-year period following the effective date of these notes, the price of the warrants will be reset to such lower price (other than on issuances, if any, pursuant to arrangements entered into reported prior to the effective date). In the event that lower priced stock or warrants are issued during the protected period, all of the Additional October Note warrants will be priced but no additional warrants will be due. The Company determined that the warrants should be accounted for as an embedded derivative. The derivate was valued at $161,000 using a binomial model and accounted for as an embedded derivative liability with the offsetting charge booked as a debt discount. The Company repaid $201,000 of this note during 2010.
On November 29, 2010 the Company received $295,000 upon issuing unsecured 10% convertible loan agreements and promissory notes due on May 29, 2011 to a group of non-affiliated investors. The terms promissory notes have a principal amount of $324,500 (reflecting an original issue discount of $29,500). The notes are convertible at the same price per share as equity issued on the closing of a sale of newly issued, unregistered common stock of the Company equal to $1 million including the value of the notes issued in this offering. Warrants to purchase 36,875 shares of common stock at an exercise price of $0.80 per share were issued with the loan agreements The $295,000 proceeds from the issuance of the promissory notes and warrants were allocated on a relative fair value basis. The relative fair value of the promissory notes was estimated to be $272,663. The relative fair value of the warrants was estimated to be $22,337, which was recorded as a debt discount to be amortized over the term of the notes.
On December 31, 2010, SDS sold a promissory note issued to SDS in October 2008 in the original principal amount of $1,000,000 to three non-affiliated, third parties. The Company exchanged the note for amended convertible notes with the following terms:
|
|
(1)
|
A convertible note in the amount of $370,000. The note matures on December 20, 2011 and carries a one-time interest charge payable at maturity of $37,000. The note is convertible into shares of the Company’s stock at a conversion price equal to 70 percent of the average daily closing bid price for the ten trading day period preceding the date of the conversion notice which was $0.47 on the date the note was issued.
|
|
|
(2)
|
A convertible note in the amount of $130,000. The note matures on June 2, 2011 and carries zero interest. The note is convertible into shares of the Company’s stock at a conversion price equal to 80 percent of the average of the five lowest closing prices in the 25 days previous to the conversion date which was $0.54 on the date the note was issued. Any amount that is still outstanding at maturity is repayable in cash.
|
|
|
(3)
|
A convertible note in the amount of $500,000. The note matures on June 2, 2011 and carries zero interest. The note is convertible into shares of the Company’s stock at a conversion price equal to the lesser of (i) the average of the closing prices for the ten trading days preceding the issue date of this note which was $0.67, or (ii) seventy percent of the volume weighted average price for the fifteen trading days prior to a conversion date. The conversion price is adjustable for down rounds as long as any amount due is outstanding under the note subject to substantial exceptions.
|
On December 31, 2010, the Company entered into a $790,000 unsecured 12% Loan Agreement with SDS (the “SDS Loan”) due June 2, 2011. In connection with this loan the Company issued SDS a warrant to purchase 118,165 shares of the Company’s stock with an exercise price of $0.75 per share. The $790,000 proceeds from the issuance of the promissory notes and warrants were allocated on a relative fair value basis. The relative fair value of the promissory notes was estimated to be $713,944. The relative fair value of the warrants was estimated to be $76,056, which was recorded as a debt discount to be amortized over the term of the notes.
On December 16, 2010, the Company entered into an agreement to potentially borrow up to $3,050,000 in tranches from a non affiliated third party (the “Lender”). As of December 31, 2010 formal agreements have been closed for a borrowing of $1,450,000. Under this arrangement, the Lender provides secured collateralized promissory notes to the Company for the funding tranches ("Lender Notes"). The notes are secured by certain assets of the Lender, which makes the funding eligible for certain securities law treatment for the Lender (the Lender investment is a recourse loan to the Company) which the Lender considers beneficial. When the Lender advances a tranche of funding under a Lender Note to the Company, that funding becomes a repayment obligation of the Company, which is embodied in a convertible promissory note from the Company to the Lender (the "Company Note"). The Company may repay any advance from the Lender at any time with either cash, surrender of the collateral, or surrender of the Company Note which would terminate the Company’s obligation under such Lender Notes.
As of December 31, 2010, the Lender had executed Lender Notes to the Company with principal amounts of $950,000 and $500,000. These Lender Notes carry a one-time interest charge equal to 11 percent of the principal payable at maturity. The Lender Notes have a maturity date of December 16, 2013. In the event that the Lender fails to meet its obligations under its notes, the Company has recourse to liquidate the security collateralizing the loan. The proceeds generated from liquidating the collateral will also be treated as Lender Note.
As of December 31, 2010 $350,000 had been advanced against the $950,000 Lender Note.
As of December 31, 2010, the Company had executed corresponding Company Notes, payable to the Lender, with principal amounts of $1,050,000 (reflecting the $950,000 principal amount in the Lender’s note, plus an original issue discount of $100,000) and $550,000 (reflecting the $500,000 principal amount in the Lender’s note, plus original issue discount of $50,000). The Company Notes also carry a one-time interest charge of 10 percent payable at maturity. The Company Notes have a maturity date of December 16, 2013. As noted above, the Company Notes may be surrendered to terminate the Company’s obligation under the Lender Notes. The Company has not incurred an obligation under the Company Notes until they are surrendered.
On December 16, 2010 the Company entered into a $100,000 unsecured 10% convertible loan agreement with a non affiliated party due March 17, 2011. Warrants to purchase 133,333 shares of common stock at an exercise price of $0.75 per share were issued with the loan agreements. The $100,000 proceeds from the issuance of the promissory notes and warrants were allocated on a relative fair value basis. The relative fair value of the promissory notes was estimated to be $53,454. The relative fair value of the warrants was estimated to be $46,546 which was recorded as a debt discount to be amortized over the term of the notes. The note was repaid on March 17, 2011.
Notes payable to related parties at December 31, 2009 and December 31, 2010 (in thousands):
|
|
|
December
31, 2009
|
|
|
December
31, 2010
|
|
|
12% note payable to Al Rajhi
|
|
$
|
4,000
|
|
|
$
|
4,000
|
|
|
6% unsecured convertible note payable to Toucan Partners, due July, 2011 and November, 2011 (net of discount related to beneficial conversion feature $1,002 in 2009 and $351 in 2010)
|
|
|
298
|
|
|
|
949
|
|
|
Total notes payable (net)
|
|
|
4,298
|
|
|
|
4,949
|
|
|
|
|
|
|
|
|
|
|
|
|
Less current portion
|
|
|
(4,000
|
)
|
|
|
(4,000
|
)
|
|
Long-term notes payable to related parties (net)
|
|
$
|
298
|
|
|
$
|
949
|
|
Notes payable consist of the following at December 31, 2009 and December 31, 2010 (in thousands):
|
|
|
December
31, 2009
|
|
|
December
31,
2010
|
|
|
12% unsecured note payable to SDS
|
|
$
|
1,000
|
|
|
$
|
-
|
|
|
12% unsecured note payable to SDS (net of warrant related discount $76 in 2010)
|
|
|
-
|
|
|
|
714
|
|
|
12% unsecured notes payable to SDS and Private Investors,
|
|
|
1,650
|
|
|
|
650
|
|
|
6% unsecured convertible notes payable to Private Lenders, due in August and September 2011, (net of discount related to beneficial conversion feature $ 485 in 2009 and $195 in 2010)
|
|
|
95
|
|
|
|
385
|
|
|
6% unsecured convertible notes payable to Private Lenders, due March 25, 2011, (net of discount related to beneficial conversion feature $46 in 2009 and $9 in 2010)
|
|
|
604
|
|
|
|
641
|
|
|
6% unsecured convertible notes payable to Private Lenders, due October, 2011, (net of discount related to beneficial conversion feature $194 in 2009 and $87 in 2010)
|
|
|
21
|
|
|
|
128
|
|
|
6% unsecured convertible notes payable to Private Lenders, due October and December 2011, (net of discount related to beneficial conversion feature $274 in 2009 and $133 in 2010)
|
|
|
231
|
|
|
|
372
|
|
|
6% unsecured convertible note payable to Private Lender, due March 25, 2011
|
|
|
110
|
|
|
|
110
|
|
|
6% secured convertible note payable to Private Lender due November 27, 2010
|
|
|
–
|
|
|
|
300
|
|
|
6% unsecured convertible notes payable to Private Lenders, due March 2012, (net of discount related to beneficial conversion feature $321 in 2010)
|
|
|
–
|
|
|
|
555
|
|
|
0% unsecured convertible note to Private Lender due December 20, 2011 (net of discount related to beneficial conversion feature of $143 in 2010)
|
|
|
–
|
|
|
|
102
|
|
|
0% unsecured convertible note to Private Lender due June 2, 2011(net of discount related to beneficial conversion feature $214 in 2010)
|
|
|
–
|
|
|
|
286
|
|
|
11% unsecured convertible note to Private Lender due June 2, 2011 (net of discount related to beneficial conversion feature of $56 in 2010)
|
|
|
–
|
|
|
|
74
|
|
|
20% unsecured convertible note to Private Lender due December 14, 2013
|
|
|
–
|
|
|
|
350
|
|
|
10% unsecured convertible note to Private Lender due March 17, 2011 (net of discount related to beneficial conversion feature $37 in 2010)
|
|
|
–
|
|
|
|
63
|
|
|
10% unsecured convertible note to Private Investors due May 29, 2011 (net of discount related to beneficial conversion feature $20 in 2010)
|
|
|
–
|
|
|
|
275
|
|
|
|
|
|
3,711
|
|
|
|
5,005
|
|
|
Less current portion
|
|
|
(2,650
|
)
|
|
|
(4,100
|
)
|
|
Total long-term debt (net)
|
|
$
|
1,061
|
|
|
$
|
905
|
|
The current portion of notes payable at December 31, 2010, is reported in the accompanying consolidated balance sheet as follows (in thousands):
|
Notes payable
|
|
$
|
1,364
|
|
|
Convertible notes payable
|
|
|
2,736
|
|
|
|
|
$
|
4,100
|
|
The long-term portion of notes payable at December 31, 2010, is reported in the accompanying consolidated balance sheet as follows (in thousands):
|
Notes payable
|
|
$
|
350
|
|
|
Convertible notes payable
|
|
|
555
|
|
|
|
|
$
|
905
|
|
(10) Commitments and Contingencies
(a) Lease Obligations
The Company terminated its lease with Toucan Capital Corporation on December 31, 2009. The Company is obligated to pay monthly payments of approximately $5,000 per month during 2011 under the terminated lease.
On March 17, 2010, the Company entered into a non-cancelable operating lease for 7,097 square feet of office space in Bethesda, Maryland, which expires in September 2012. Future minimum lease payments under the lease are $163,475, $126,027, in 2011 and 2012, respectively. Rent expense for the years ended December 31, 2009 and 2010 amounted to $0 and $100,085, respectively. The Company expects to lease part of this space to Toucan and proceeds of this sublease will be offset against the minimum lease payments specified above.
(11) Unaudited Quarterly Financial Information (in thousands, except loss
per share data)
The following table contains selected unaudited statement of operations information for each of the quarters in 2010 and 2009. The Company believes that the following information reflects all adjustments, consisting of only normal recurring adjustments, necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
|
|
|
First
Quarter
2010
|
|
|
Second
Quarter
2010
|
|
|
Third
Quarter
2010
|
|
|
Fourth
Quarter
2010
|
|
|
Total revenues
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
10
|
|
|
$
|
—
|
|
|
Net loss applicable to common stockholders
|
|
$
|
(5,845
|
)
|
|
$
|
(8,727
|
)
|
|
$
|
(5,381
|
)
|
|
$
|
(7,415
|
)
|
|
Net loss per share applicable to common stockholders — basic and diluted
|
|
$
|
(0.10
|
)
|
|
$
|
(0.14
|
)
|
|
$
|
(0.08
|
)
|
|
$
|
(0.09
|
)
|
|
Weighted average shares used in computing basic and diluted loss per share
|
|
|
59,928
|
|
|
|
63,853
|
|
|
|
70,413
|
|
|
|
72,028
|
|
|
|
|
First
Quarter
2009
|
|
|
Second
Quarter
2009
|
|
|
Third
Quarter
2009
|
|
|
Fourth
Quarter
2009
|
|
|
Total revenues
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
10
|
|
|
$
|
—
|
|
|
Net loss applicable to common stockholders
|
|
$
|
(4,620
|
)
|
|
$
|
(4,095
|
)
|
|
$
|
(13,837
|
)
|
|
$
|
(4,402
|
)
|
|
Net loss per share applicable to common stockholders — basic and diluted
|
|
$
|
(0.11
|
)
|
|
$
|
(0.09
|
)
|
|
$
|
(0.31
|
)
|
|
$
|
(0.05
|
)
|
|
Weighted average shares used in computing basic and diluted loss per share
|
|
|
43,385
|
|
|
|
45,069
|
|
|
|
45,276
|
|
|
|
57,966
|
|
(12) Subsequent Events
In January 2011, the Company received $50,000 upon issuing unsecured 10% convertible loan agreement and promissory note due in April 2011 to a private, non-affiliated investors. Warrants to purchase 66,667 shares of common stock at an exercise price of $0.75 per share were issued with the loan agreement. The $50,000 proceeds from the issuance of the promissory note and warrant were allocated on a relative fair value basis. The relative fair value of the promissory note was estimated to be $25,011. The relative fair value of the warrant was estimated to be $24,989, which was recorded as a debt discount to be amortized over the term of the note.
In January 2011 the Company entered into securities purchase agreements with two non-affiliated investors, under which the investors purchased 280,000 shares of common stock for $210,000. In connection with this private placement the Company issued warrants to purchase 262,500 shares of common stock at an exercise price of $0.80 per share with an exercise period of five years. Additionally, one of the investors purchased a warrant to acquire 93,750 shares of common stock at a price of $0.80 per share for $3,750.
On January 18, 2011 the Company received $100,000 as a second advance under the $950,000 facility agreement entered into on December 14, 2010 which is fully described in Note 10 above.
On January 20, 2011, the Company entered into a $130,000 unsecured 12% Loan Agreement with SDS (the “SDS Loan”) due June 2, 2011. In connection with this loan the Company issued SDS a warrant to purchase 17,333 shares of the Company’s stock with an exercise price of $0.75 per share. The $130,000 proceeds from the issuance of the promissory notes and warrants were allocated on a relative fair value basis. The relative fair value of the promissory notes was estimated to be $120,024. The relative fair value of the warrants was estimated to be $9,976, which was recorded as a debt discount to be amortized over the term of the notes.
On January 19, 2011, the Company entered into an agreement to borrow up to $2,220,000 from a non affiliated third party (the “Investor”). Under the Agreement the Company entered into two secured and collateralized promissory notes with principal amounts of $1,120,000 and $1,100,000. The $1,120,000 note carries an original issue discount of ten percent and annual interest of nine percent per annum, payable at maturity on June 30, 2012, and the $1,100,000 note carries an original issue discount of ten percent and annual interest of six percent per annum, payable at maturity on December 31, 2013. Both notes are convertible into shares of the Company’s common stock, at a conversion price equal to 80% of the average of the daily volume weighted price of the Company’s common stock. The notes have down-round protection upon the occurrence of specified events. The proceeds of Note 1 were received at closing on January 21, 2011. The proceeds of Note 2 will be received in four equal monthly tranches starting on July 19, 2011.
On January 21, 2011, The Company consummated a Note Purchase Agreement with a single investor in the aggregate amount of $2,220,000 for the purchase and sale of two Secured Convertible Promissory Notes (the “Notes”).
On March 1, 2011, the Company entered into a transaction under Section 3(a)(10) of the Securities Act of 1933, as amended with Socius CG II, Ltd. (“Socius”). Pursuant to this initial 3(a)(10) transaction, Socius purchased certain claims for payment totaling $1,650,000 from Cognate. Thereafter, Socius elected to convert the claims into shares of the Company’s common stock. The conversion was effected through a settlement agreement between Socius and the Company. The settlement agreement was then the subject of a court proceeding (nominally brought by Socius against the Company, but handled on a cooperative basis through a Joint Stipulation by both parties) in order to obtain court approval of the settlement in accordance with the requirements of Section 3(a)(10). That Court approval was obtained on March 1, 2011. Pursuant to the settlement, the full amount of the $1,650,000 debt will be converted into shares of common stock at a conversion price equal to a thirty percent discount from the market price, with the market price being determined over a reference period of up to twenty (20) trading days from the date hereof (the “Reference Period”). In addition, the settlement provided that an equal amount of shares of common stock are to be delivered by the Company to Socius on a temporary basis, as a form of security during the Reference Period. At the end of the Reference Period, a true-up mechanism will be applied to confirm the final number of conversion shares based on the market price. As of March 31, 2010 The Company had issued 6,852,228 shares of the Company’s common stock to Socius in settlement of the Socius claims.
During the quarter ended March 31, 2011 the Company issued 2,930,228 shares in addition to the share issuances described above. Approximately 1,521,000 the shares were issued in settlement of loan conversions and 1,408,000 were issued in respect of consulting and other services rendered.
EXHIBITS
The following exhibits are filed as part of this registration statement:
EXHIBIT INDEX
|
Exhibit
Number
|
|
Description
|
|
|
|
|
|
3.1
|
|
Seventh Amended and Restated Certificate of Incorporation.(3.1)(22)
|
|
|
|
|
|
3.2
|
|
Third Amended and Restated Bylaws of the Company.(3.1)(29)
|
|
|
|
|
|
3.3
|
|
Amendment to Seventh Amended and Restated Certificate of Incorporation.(3.2)(29)
|
|
|
|
|
|
3.4
|
|
Amendment to Seventh Amended and Restated Certificate of Incorporation.(3.4)(33)
|
|
|
|
|
|
4.1
|
|
Form of common stock certificate.(4.1)(2)
|
|
|
|
|
|
4.2
|
|
Northwest Biotherapeutics, Inc. Stockholders Rights Agreement dated February 26, 2002 between the Company and Mellon Investors Services, LLC.(4.2)(3)
|
|
|
|
|
|
4.3
|
|
Form of Rights Certificate.(4.1)(3)
|
|
|
|
|
|
4.4
|
|
Amendment to Northwest Biotherapeutics, Inc. Stockholders Rights Agreement dated April 26, 2004.(4.2)(4)
|
|
|
|
|
|
5.1
|
|
Opinion of Tarter Krinsky and Drogin, LLP as to the legality of the securities being registered.
|
|
|
|
|
|
10.1
|
|
Amended and Restated Loan Agreement and 10% Promissory Note dated November 14, 2005 in the principal amount of $400,000 as amended and restated on April 14, 2007 between the Company and Toucan Partners, LLC.(10.1)(23)
|
|
|
|
|
|
10.2
|
|
Second Amended and Restated Loan Agreement and 10% Promissory Note originally dated December 30, 2005, and amended and restated on April 17, 2006 and April 14, 2007 in the principal amount of $250,000 between the Company and Toucan Partners, LLC.(10.2)(23)
|
|
|
|
|
|
10.3
|
|
Second Amended and Restated Loan Agreement and 10% Promissory Note originally dated March 9, 2006, and as amended and restated on April 17, 2006 and April 14, 2007 in the principal amount of $300,000 between the Company and Toucan Partners, LLC.(10.3)(23)
|
|
|
|
|
|
10.4
|
|
Form of Loan Agreement and 10% Convertible, Promissory Note between the Company and Toucan Partners, LLC.(10.4)(23)
|
|
|
|
|
|
10.5
|
|
Amended and Restated Investor Rights Agreement dated April 17, 2006.(10.4)(18)
|
|
|
|
|
|
10.6
|
|
Second Amended and Restated Investor Rights Agreement dated June 22, 2007 between the Company and Toucan Capital Fund II, LLP.(10.3)(29)
|
|
|
|
|
|
10.7
|
|
Securities Purchase Agreement, dated March 30, 2006 by and among the Company and the Investors identified therein.(10.1)(6)
|
|
|
|
|
|
10.8
|
|
Form of Warrant.(10.2)(6)
|
|
|
|
|
|
10.9
|
|
Warrant to purchase securities of the Company dated April 26, 2004 issued to Toucan Capital Fund II, L.P.(10.9)(7)
|
|
10.10
|
|
Warrant to purchase securities of the Company dated June 11, 2004 issued to Toucan Capital Fund II, L.P.(10.8)(7)
|
|
|
|
|
|
10.11
|
|
Warrant to purchase securities of the Company dated July 30, 2004 issued to Toucan Capital Fund II, L.P.(10.7)(7)
|
|
|
|
|
|
10.12
|
|
Warrant to purchase securities of the Company dated October 22, 2004 issued to Toucan Capital Fund II, L.P.(10.3)(8)
|
|
|
|
|
|
10.13
|
|
Warrant to purchase securities of the Company dated November 10, 2004 issued to Toucan Capital Fund II, L.P.(10.3)(9)
|
|
|
|
|
|
10.14
|
|
Warrant to purchase securities of the Company dated December 27, 2004 issued to Toucan Capital Fund II, L.P.(10.3)(10)
|
|
|
|
|
|
10.15
|
|
First Amendment to Warrants between Northwest Biotherapeutics, Inc. and Toucan Capital Fund II, L.P. dated January 26, 2005.(10.5)(1)
|
|
|
|
|
|
10.16
|
|
Warrant to purchase Series A Preferred Stock dated January 26, 2005 issued to Toucan Capital Fund II, L.P.(10.2)(1)
|
|
|
|
|
|
10.17
|
|
Warrant to purchase securities of the Company dated April 12, 2005 issued to Toucan Capital Fund II, L.P.(10.39)(11)
|
|
|
|
|
|
10.18
|
|
Warrant to purchase securities of the Company dated May 13, 2005 issued to Toucan Capital Fund II, L.P.(10.3)(12)
|
|
10.19
|
|
Warrant to purchase securities of the Company dated June 16, 2005 issued to Toucan Capital Fund II, L.P.(10.3)(13)
|
|
|
|
|
|
10.20
|
|
Warrant to purchase securities of the Company dated July 26, 2005 issued to Toucan Capital Fund II, L.P.(10.3)(14)
|
|
|
|
|
|
10.21
|
|
Warrant to purchase securities of the Company dated September 7, 2005 issued to Toucan Capital Fund II, L.P.(10.3)(15)
|
|
|
|
|
|
10.22
|
|
Amended Form of Warrant to purchase securities of the Company dated November 14, 2005 and April 17, 2006, as amended April 14, 2007, issued to Toucan Partners, LLC.(10.21)(23)
|
|
|
|
|
|
10.23
|
|
Form of Warrant to purchase securities of the Company dated April 14, 2007 issued to Toucan Partners, LLC.(10.22)(23)
|
|
|
|
|
|
10.24
|
|
Loan Agreement and 10% Convertible Promissory Note in the principal amount of $100,000 between the Company and Toucan Partners, LLC, dated April 27, 2007.(10.1)(24)
|
|
|
|
|
|
10.25
|
|
Warrant to purchase securities of the Company issued to Toucan Partners, LLC, dated April 27, 2007. (10.2)(24)
|
|
|
|
|
|
10.26
|
|
Form of Toucan Partners Loan Agreement and 10% Convertible Note, dated as of June 1, 2007.(10.1)(27)
|
|
|
|
|
|
10.27
|
|
Form of Toucan Partners Warrant, dated as of June 1, 2007.(10.2)(27)
|
|
|
|
|
|
10.28
|
|
Amended and Restated Warrant to purchase Series A Preferred Stock issued to Toucan Capital Fund II, L.P., dated as of June 1, 2007.(10.3)(27)
|
|
10.29
|
|
Warrant to purchase Series A-1 Preferred Stock issued to Toucan Capital Fund II, L.P., dated as of June 1, 2007.(10.4)(27)
|
|
|
|
|
|
10.30
|
|
Warrant to purchase Series A-1 Preferred Stock issued to Toucan Capital Fund II, L.P., dated as of June 1, 2007.(10.5)(27)
|
|
|
|
|
|
10.31
|
|
Northwest Biotherapeutics, Inc. $225,000 Demand Note dated June 13, 2007.(10.1)(28)
|
|
|
|
|
|
10.32
|
|
Conversion Agreement dated June 15, 2007 and effective June 22, 2007 between the Company and Toucan Capital Fund II, LLP.(10.1)(29)
|
|
|
|
|
|
10.33
|
|
Termination Agreement dated June 22, 2007 between the Company and Toucan Capital Fund II, LLP.(10.2)(29)
|
|
|
|
|
|
10.34
|
|
NOMAD Agreement dated June 15, 2007 and effective June 22, 2007 between the Company and Collins Stewart Europe Limited.(10.4)(29)
|
|
|
|
|
|
10.35***
|
|
Employment Agreement dated June 18, 2007 between Dr. Alton L. Boynton and the Company. (10.6)(29)
|
|
|
|
|
|
10.36***
|
|
Employment Agreement dated October 1, 2007 between Anthony P. Deasey and the Company.(10.1)(30)
|
|
|
|
|
|
10.37***
|
|
Letter of Appointment for Linda F. Powers.(10.8)(29)
|
|
|
|
|
|
10.38***
|
|
Letter of Appointment for R. Steve Harris.(10.9)(29)
|
|
|
|
|
|
10.39
|
|
Form of Warrant to purchase common stock of the Company, as amended.(10.27)(18)
|
|
|
|
|
|
10.40**
|
|
Northwest Biotherapeutics DCVax — Brain Services Agreement with Cognate BioServices, Inc. dated May 17, 2007.(10.1)(25)
|
|
|
|
|
|
10.41***
|
|
1998 Stock Option Plan.(10.15)(2)
|
|
|
|
|
|
10.42***
|
|
1999 Executive Stock Option Plan.(10.16)(2)
|
|
|
|
|
|
10.43***
|
|
2001 Stock Option Plan.(10.17)(2)
|
|
|
|
|
|
10.44***
|
|
2001 Nonemployee Director Stock Incentive Plan.(10.18)(2)
|
|
|
|
|
|
10.45***
|
|
Employee Stock Purchase Plan.(10.19)(2)
|
|
|
|
|
|
10.46***
|
|
2007 Stock Option Plan.(10.5)(29)
|
|
|
|
|
|
10.47***
|
|
Form of Stock Option Agreement under the 2007 Stock Option Plan.(10.2)(31)
|
|
|
|
|
|
10.48
|
|
Lease Agreement.(10.34)(18)
|
|
|
|
|
|
10.49
|
|
Lease Extension between the Company and the International Union of Operating Engineers Local 302, dated May 31, 2007(10.1)(26).
|
|
10.50
|
|
Clinical Study Agreement between the Company and the Regents of the University of California dated February 14, 2006.(10.35)(18)
|
|
10.51***
|
|
Employment Agreement dated June 18, 2007, by and between Jim Johnston and the Company.(10.7)(29)
|
|
|
|
|
|
10.52***
|
|
Form of Stock Option Agreement, dated December 31, 2007, by and between Dr. Alton L. Boynton and the Company.(99.1)(32)
|
|
|
|
|
|
10.53***
|
|
Form of Stock Option Agreement, dated December 31, 2007, by and between Dr. Marnix Bosch and the Company.(99.2)(32)
|
|
|
|
|
|
10.54
|
|
Sublease Agreement, dated as of March 21, 2008, between the Company and Toucan Capital Corporation.(10.1)(34)
|
|
|
|
|
|
10.55
|
|
Loan Agreement and Promissory Note, dated May 9, 2008 between the Company and Al Rajhi Holdings WLL (4.5)(36)
|
|
|
|
|
|
10.56
|
|
Loan Agreement and Promissory Note, dated August 19, 2008 between the Company and Toucan Partners LLC (10.1)(37)
|
|
|
|
|
|
10.57
|
|
Loan Agreement and Promissory Note, dated October 1, 2008 between the Company and SDS Capital Group SPC, Ltd (10.2)(38)
|
|
|
|
|
|
10.58
|
|
Warrant, dated October 1, 2008, between the Company and SDS Capital Group SPC, Ltd (10.3)(38)
|
|
|
|
|
|
10.59
|
|
Loan Agreement and Promissory Note, dated October 21, 2008, between the Company and SDS Capital Group SPC, Ltd (10.4)(39)
|
|
|
|
|
|
10.60
|
|
Form of Loan Agreement and Promissory Note, dated November 6, 2008, between the Company and a Group of Private Investors (10.5)(39)
|
|
|
|
|
|
10.61
|
|
Form of Warrant, dated November 6, 2008, between the Company and SDS Capital Group SPC. Ltd and a Group of Private Investors (10.5)(39)
|
|
|
|
|
|
10.62
|
|
Loan Agreement and Promissory Note, dated December 22, 2008, between the Company and Toucan Partners LLC (10.62)(40)
|
|
|
|
|
|
10.63
|
|
Form of Warrant, dated December 22, 2008, between the Company and Toucan Partners LLC (10.62)(40)
|
|
|
|
|
|
10.64
|
|
Form of Securities Purchase Agreement, dated January 16, 2009, by and among the Company and Al Rajhi Holdings (10.62)(40)
|
|
10.65
|
|
Securities Purchase Agreement, dated March 27, 2009 by and among the Company and a Group of Equity Investors (10.62)(40)
|
|
10.66
|
|
Form of Warrant, dated March 27,2009, between the Company and a Group of Equity Investors (10.62)(40)
|
|
10.67
|
|
Form of Loan Agreement and Promissory Note, dated March 27 2009, between the Company and a Group of Private Lenders (10.62)(40)
|
|
10.68
|
|
Conversion Agreement effective September 28, 2009 between the Company and Toucan Partners, LLC (10.1) (41)
|
|
10.69
|
|
Form of Loan Extension Agreement dated September 28, 2009 (10.2) (42)
|
|
10.70***
|
|
Retention Agreement between Dr. Alton L. Boynton and the Company dated September 28, 2009 (10.3) (42)
|
|
10.71
|
|
Purchase Agreement, dated November 14, 2011 between the Company and Four M Purchasers, LLC.
|
|
10.72
|
|
Amendment to Purchase Agreement, dated December 6, 2011 between the Company and Four M Purchasers, LLC.
|
|
10.73
|
|
Second Amendment to Purchase Agreement, dated January 6, 2012 between the Company and Four M Purchasers, LLC.
|
|
11
|
|
Computation of net loss per share included within the Northwest Biotherapeutics, Inc. audited financial statements for the year ended December 31, 2010 included in this Annual Report on Form 10-K.
|
|
21
|
|
Subsidiary of the Registrant.(21.1)(35)
|
|
23*
|
|
Consent of Peterson Sullivan LLP, Independent Registered Public Accounting Firm.
|
|
*
|
Filed or furnished herewith.
|
|
**
|
Portions of this exhibit have been omitted and filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment.
|
|
***
|
Denotes management contract or compensation plan or arrangement.
|
|
(1)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K, February 1, 2005.
|
|
(2)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001.
|
|
(3)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Registration Statement on Form 8-A on July 8, 2002.
|
|
(4)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Form 10-K on May 14, 2004.
|
|
(5)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Quarterly Report on Form 10-Q on November 14, 2005.
|
|
(6)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on March 31, 2006.
|
|
(7)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on August 6, 2004.
|
|
(8)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on October 28, 2004.
|
|
(9)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on November 16, 2004.
|
|
(10)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on December 30, 2004.
|
|
(11)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Annual Report on Form 10-K on April 15, 2005.
|
|
(12)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8- K on May 18, 2005.
|
|
(13)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on June 21, 2005.
|
|
(14)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on August 1, 2005.
|
|
(15)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on September 9, 2005.
|
|
(16)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Quarterly Report on Form 10-Q on November 14, 2003.
|
|
(17)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on August 5, 2005.
|
|
(18)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 10-K on April 18, 2006.
|
|
(19)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on April 26, 2006.
|
|
(20)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Form 10-K/A on June 30, 2006.
|
|
(21)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant Registration Statement on Form S-1 (File No. 33-134320) on May 19, 2006.
|
|
(22)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1(File No. 333-134320) on July 17, 2006.
|
|
(23)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Form 10-K on April 17, 2007.
|
|
(24)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on May 3, 2007.
|
|
(25)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on May 21, 2007.
|
|
(26)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on June 4, 2007.
|
|
(27)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on June 7, 2007.
|
|
(28)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on June 18, 2007.
|
|
(29)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on June 22, 2007.
|
|
(30)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on October 2, 2007.
|
|
(31)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Registration Statement on Form S-8 on November 21, 2007.
|
|
(32)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on January 3, 2008.
|
|
(33)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Post-Effective Amendment No. 2 to the Registrant’s Registration Statement on Form S-1 on January 28, 2008.
|
|
(34)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on March 24, 2008.
|
|
(35)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Post-Effective Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 on December 17, 2007.
|
|
(36)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on May 15, 2008, 2008.
|
|
(37)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Quarterly Report on Form 10-Q on August 19, 2008.
|
|
(38)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on October 6, 2008.
|
|
(39)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Current Report on Form 8-K on November 11, 2008.
|
|
(40)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Form 10-K on April 15, 2008
|
|
(41)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Quarterly Report on Form 10-Q on August 14, 2008.
|
|
(42)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Quarterly Report on Form 10-Q on November 23, 2009.
|
|
(43)
|
Incorporated by reference to the exhibit shown in the preceding parentheses filed with the Registrant’s Quarterly Report on Form 10-Q on May 21, 2010.
|
UNDERTAKINGS
The undersigned Registrant hereby undertakes:
1. To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(a) To include any Prospectus required by Section 10(a)(3) of the Securities Act;
(b) To reflect in the Prospectus any facts or events arising after the effective date of this Registration Statement, or most recent post-effective amendment, which, individually or in the aggregate, represent a fundamental change in the information set forth in this Registration Statement; and notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospects filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in the volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(c) To include any material information with respect to the plan of distribution not previously disclosed in this Registration Statement or any material change to such information in the registration statement.
2. That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3. To remove from registration by means of a post-effective amendment any of the securities being registered hereby which remain unsold at the termination of the offering.
4. For determining liability of the undersigned registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(a) Any preliminary Prospectus or Prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (Sec. 230.424);
(b) Any free writing Prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the registrant;
(c) The portion of any other free writing Prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(d) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the provisions above, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by one of our directors, officers, or controlling persons in the successful defense of any action, suit or proceeding, is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification is against public policy as expressed in the Securities Act, and we will be governed by the final adjudication of such issue.
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing this Form S-1 and has authorized this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Bethesda, State of Maryland, on January 10
, 2012.
|
|
NORTHWEST BIOTHERAPEUTICS, INC.
|
|
|
|
|
|
By:
|
/s/ Linda Powers
|
|
|
|
Name: Linda Powers
|
|
|
|
Title: Chief Executive Officer and Chairperson
|
|
|
|
(Principal Executive Officer and
Principal Accounting Officer)
|
POWER OF ATTORNEY
Know all persons by these presents that each individual whose signature appears below constitutes and appoints Linda Powers, our Chairperson, Chief Executive Officer and a director, as a true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing under Rule 462 promulgated under the Securities Act of 1933, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any one of them, or his or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ Linda Powers
|
|
Director, Chairperson and Chief Executive Officer
|
|
January 10, 2012.
|
|
Linda Powers
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Alton Boynton
|
|
Chief Scientific Officer, Director
|
|
January 10, 2012.
|
|
Alton Boynton
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Robert Farmer
|
|
Director
|
|
January 10, 2012.
|
|
Robert Farmer
|
|
|
|
|
Northwest Biotherapeutics (QB) (USOTC:NWBO)
Historical Stock Chart
From Jun 2024 to Jul 2024
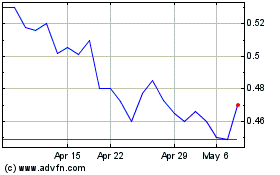
Northwest Biotherapeutics (QB) (USOTC:NWBO)
Historical Stock Chart
From Jul 2023 to Jul 2024