TABLE OF CONTENTS
You should read this prospectus, any applicable prospectus supplement and the information incorporated by
reference in this prospectus before making an investment in the securities of BG Medicine, Inc. See “Where You Can Find More Information” on page 23 for more information. You should rely only on the information contained in or incorporated
by reference in this prospectus or a prospectus supplement. The Company has not authorized anyone to provide you with different information. This document may be used only in jurisdictions where offers and sales of these securities are permitted.
You should assume that information contained in this prospectus, or in any document incorporated by reference, is accurate only as of any date on the front cover of the applicable document. Our business, financial condition, results of operations
and prospects may have changed since that date. Unless otherwise noted in this prospectus, (1) the term “BG Medicine” refers to BG Medicine, Inc., a Delaware corporation, (2) the terms “BG Medicine,” the
“Company,” “we,” “us,” and “our,” refer to the ongoing business operations of BG Medicine, and (3) the term “Common Stock” refers to shares of BG Medicine, Inc.’s Common Stock and the term
“stockholder(s)” refers to the holders of Common Stock or securities exercisable for Common Stock.
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference into it contain, in addition to historical information, certain information,
assumptions and discussions that may constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as
amended, or the Exchange Act. We have made these statements in reliance on the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements are subject to certain risks and uncertainties, which could cause actual
results to differ materially from those projected or anticipated. Although we believe our assumptions underlying our forward-looking statements are reasonable as of the date of this prospectus, we cannot assure you that the forward-looking
statements set out in this prospectus will prove to be accurate. We typically identify these forward-looking statements by the use of forward-looking words such as “expect,” “potential,” “continue,” “may,”
“will,” “should,” “could,” “would,” “seek,” “intend,” “plan,” “estimate,” “anticipate” or the negative version of those words or other comparable words.
Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
|
|
•
|
|
our estimates of future performance, including the commercialization and sales of our galectin-3 test;
|
|
|
•
|
|
our ability to provide sufficient evidence of clinical utility for our galectin-3 test and to differentiate it from competing cardiovascular
diagnostics tests;
|
|
|
•
|
|
our ability to obtain regulatory clearance from the U.S. Food and Drug Administration, or FDA, for our CardioSCORE test and certain additional
indications for our galectin-3 test;
|
|
|
•
|
|
our ability to successfully market, commercialize and achieve widespread market penetration for our cardiovascular diagnostic tests;
|
|
|
•
|
|
our ability to conduct the clinical studies required for regulatory clearance or approval and to demonstrate the clinical benefits and
cost-effectiveness to support commercial acceptance of our products;
|
|
|
•
|
|
the timing, costs and other limitations involved in obtaining regulatory clearance or approval for any of our products;
|
|
|
•
|
|
the potential benefits of our cardiovascular diagnostic tests over current medical practices or other diagnostics;
|
|
|
•
|
|
willingness of third-party payers to reimburse for the cost of our tests;
|
|
|
•
|
|
the ability of our automated partners to develop and obtain regulatory clearance of galectin-3 tests that can be performed on their automated platforms
and to commercialize any such tests;
|
|
|
•
|
|
estimates of market sizes and anticipated uses of our cardiovascular diagnostic tests;
|
|
|
•
|
|
our ability to enter into collaboration and distribution agreements with respect to our cardiovascular diagnostic tests, the performance of our
partners under such agreements and the potential benefits of these arrangements;
|
|
|
•
|
|
our ability to obtain and maintain intellectual property protections for our products and operate our business without infringing upon the intellectual
property rights of others;
|
|
|
•
|
|
the expected timing, progress or success of our development and commercialization efforts;
|
|
|
•
|
|
our ability to successfully obtain sufficient and appropriate blood samples for our validation tests in support of our regulatory filings for our
cardiovascular tests;
|
|
|
•
|
|
our ability to continue as a going concern;
|
|
|
•
|
|
our ability to obtain additional financing on terms acceptable to us;
|
ii
|
|
•
|
|
our expectations regarding the use of our Purchase Agreement to obtain additional capital through sales of our common stock to Aspire Capital and the
trading price of our common stock being above the $1.00 minimum floor price that is required for us to use this facility;
|
|
|
•
|
|
our ability to maintain compliance with the continued listing requirements of The NASDAQ Capital Market;
|
|
|
•
|
|
the success of our efforts to remediate the material weakness we identified in fiscal 2012 and to satisfactorily improve our internal controls over
financial reporting;
|
|
|
•
|
|
the success of competing cardiovascular diagnostic tests that are or become available;
|
|
|
•
|
|
regulatory developments in the United States and other countries in which we sell or plan to sell our tests;
|
|
|
•
|
|
the performance of our third-party suppliers and the manufacturer of our galectin-3 tests;
|
|
|
•
|
|
our ability to service the principal and interest amounts payable under our secured term loan facility; and
|
|
|
•
|
|
our estimates regarding anticipated operating losses, future revenue, expenses, capital requirements and our needs for additional financing.
|
This prospectus also contains estimates and other statistical data provided by independent parties and by us relating to
market size and growth and other industry data. These and other forward-looking statements made in this prospectus are presented as of the date on which the statements are made. We have included important factors in the cautionary statements
included in this prospectus, particularly in the section entitled “Risk Factors,” that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do
not reflect the potential impact of any new information, future events or circumstances that may affect our business after the date of this prospectus. Except as required by law, we do not intend to update any forward-looking statements after the
date on which the statement is made, whether as a result of new information, future events or circumstances or otherwise.
iii
PROSPECTUS SUMMARY
This summary highlights some information from this prospectus. It may not contain all the information important to making an
investment decision. You should read the following summary together with the more detailed information regarding our Company and the securities being sold in this offering, including “Risk Factors” and other information incorporated by
reference herein.
Overview
We are developing and commercializing diagnostic products that may be used to help guide the care and management of patients who suffer from heart failure and related disorders.
Our BGM Galectin-3
®
Test is our first U.S. Food and Drug Administration, or FDA, cleared and CE Marked diagnostic product. It is currently available as a blood test in the United States
and the European Union, or EU. Our BGM Galectin-3 Test was included in the 2013 American College of Cardiology Foundation and the American Heart Association (ACCF/AHA) Guideline for the Management of Heart Failure.
We market and sell our BGM Galectin-3 Test kits to clinical laboratories, hospitals, and health care providers. We hope to accelerate the
clinical and commercial adoption of galectin-3 testing by generating, publishing and publicizing data derived from clinical research studies that have incorporated our BGM
Galectin-3
Test and by expanding our
BGM Galectin-3 Test’s indications for use. We have entered into licensing agreements with leading diagnostic instrument manufacturers to develop and commercialize galectin-3 assays that will be performed on automated platforms that have been
incorporated into routine practice in laboratories throughout the world.
We are developing a pipeline of diagnostic products
by leveraging our intellectual property and the mining of data generated from a patient cohort and specimen repository to which we have exclusive access for the development of diagnostic products. Among the products in development is our BGM
CardioSCORE™ Test, a biomarker-based blood test designed as an aid in the assessment of near-term risk for significant cardiovascular events, such as heart attack and stroke.
Our BGM Galectin-3 Test
Our BGM Galectin-3 Test is our first FDA
cleared and CE Marked diagnostic product. It is an
in vitro
diagnostic device that quantitatively measures galectin-3 in serum or plasma by enzyme linked immunosorbent assay (ELISA) on a microtiter plate platform. Heart failure patients with
elevated galectin-3 levels as measured using our BGM Galectin-3 Test have been found to be at significantly greater risk of adverse outcomes, including death or hospitalization. Measurement of this protein biomarker is intended to be used in
conjunction with clinical evaluation.
Galectins are a family of proteins that play many important roles in inflammation,
immunity and cancer. Galectin-3, a member of this family of proteins, is a protein biomarker that has been shown to play an important role in heart failure in both animal and human studies. In animal experiments, administration of galectin-3 to the
heart led to the development of cardiac fibrosis, or stiffening, in the heart muscle, a process that is often referred to as cardiac remodeling. In these animal studies, adverse remodeling reduced the heart’s ability to pump normally, causing
the animals to develop heart failure. This link between galectin-3 and cardiac remodeling is significant and suggests that galectin-3 may be a useful biomarker for adverse cardiac remodeling, an important determinant of the clinical outcome of
patients suffering from heart failure. We have obtained an exclusive worldwide license to certain galectin-3 rights that relate to the association of this protein biomarker with heart failure. We have also filed several of our own patent
applications related to galectin-3.
1
Our BGM Galectin-3 Test is currently available as a blood test in the United States and the
EU. The following table summarizes the current indications for use and commercial status of our BGM Galectin-3 Test:
|
|
|
|
|
|
|
Test / Disease Area
|
|
Indications
|
|
Regulatory and Commercial Status
|
|
BGM Galectin-3 Test
|
|
An aid in assessing the prognosis of chronic heart failure
|
|
— Marketed in the U.S. and Europe
— FDA 510(k) cleared
November 2010
— CE Mark obtained October 2009
|
|
|
|
|
|
|
|
An aid in assessing the prognosis of acute heart failure
|
|
— Marketed in Europe
— CE Mark obtained October
2009
|
|
|
|
|
|
|
|
An aid to identify adults at elevated risk of developing heart failure
|
|
— Marketed in Europe
— CE Mark obtained May
2012
|
Automated Testing For Galectin-3
Overview
We believe that automation of our galectin-3 test will
broaden its acceptance by laboratory customers and, as a result, accelerate its clinical adoption. To that end, we have entered into licensing and commercialization agreements with four leading diagnostic instrument manufacturers to develop and
commercialize automated instrument versions of our galectin-3 test. We have entered into worldwide license, development and commercialization agreements with Abbott Laboratories, or Abbott, bioMérieux SA, or bioMérieux, Siemens
Healthcare Diagnostics Inc., or Siemens, and Alere Inc., or Alere. These diagnostic instrument manufacturers account for a significant percentage of the automated laboratory testing instruments that are used throughout the world. The installed
customer base of these automated partners reflects all major segments of the diagnostics market, including hospital laboratories, national reference laboratories, regional laboratories and others.
Progress to Date
In January 2013, bioMérieux obtained a CE Mark for its
VIDAS
®
Galectin-3 assay and initiated its commercial launch in the EU. The VIDAS
®
Galectin-3 assay was developed by bioMérieux for the quantitative measurement of galectin-3 levels in blood
using the bioMérieux VIDAS
®
automated and multiparametric immunoassay testing system.
In April 2013, Abbott obtained a CE mark for its ARCHITECT
®
Galectin-3 assay and initiated its commercial launch in the EU. Abbott is offering the ARCHITECT
®
Galectin-3 assay on its
ARCHITECT
®
immunoassay platform. In the United States, Fujirebio Diagnostics, Incorporated, or Fujirebio, on
behalf of Abbott, is the first of our automated partners to have filed for 510(k) regulatory clearance of an automated version of the galectin-3 test. Fujirebio is developing the test for use on Abbott’s ARCHITECT
®
immunochemistry instrument platform. Fujirebio initially submitted its 510(k) to the FDA in July 2012. Subsequently,
Fujirebio received a letter from the FDA requesting additional information on various matters, including the geographic composition of the patient cohort that provided the blood samples used to support the 510(k) submission. Due to the nature of the
additional information requested and the time required to address the FDA’s questions, Fujirebio was unable to submit a complete response to the FDA by the FDA-designated deadline on February 25, 2013 and withdrew the submission. Fujirebio
submitted its new 510(k) to the FDA in February 2014.
2
Recognition of Galectin-3 Testing in U.S. Guideline for the Management of Heart Failure
In June 2013, galectin-3 testing was recognized for the first time in the newly issued 2013 American College of Cardiology Foundation and
the American Heart Association Guideline for the Management of Heart Failure. The ACCF/AHA Guideline is designed to assist clinicians in selecting the best management strategy for individual patients and provides expert analysis of data on
prevention, diagnosis, risk stratification, and treatment. Galectin-3 testing has been assigned a Level of Evidence of ‘A’, multiple populations evaluated, and a Class of Recommendation corresponding to ‘May Be Considered’ for
the purpose of additive risk stratification of acute heart failure patients, and a Level of Evidence of ‘B’, limited populations evaluated, and a Class of Recommendation of ‘May Be Considered’ for risk stratification of
ambulatory heart failure patients. The guideline further notes that testing for galectin-3 is predictive of risk of adverse outcomes in heart failure, including hospitalization, and is additive to BNP and NT-proBNP testing for heart failure patient
risk stratification. The American College of Cardiology Foundation and the American Heart Association have jointly produced guidelines in the area of cardiovascular disease since 1980. The ACCF/AHA Task Force on Practice Guidelines is charged with
developing, updating and revising practice guidelines for cardiovascular diseases and procedures. Writing committees are charged with regularly reviewing and evaluating all available evidence to develop balanced, patient-centric recommendations for
clinical practice. The guidelines for heart failure management were last revised in 2009.
Reimbursement for Galectin-3 Testing
Approximately 70% of heart failure patients in the United States are of Medicare age. Therefore,
reimbursement by Medicare is of considerable interest to our laboratory customers. In the United States, for the 2014 calendar year, the Centers for Medicare and Medicaid Services (CMS) published a 2014 Medicare national limitation amount for the
galectin-3 blood test (analyte-specific CPT
®
Code 82777) at the amount of a crosswalked test (analyte-specific
CPT
®
Code 84244) whose 2014 national limitation amount is $30.01. This national limitation replaces the
galectin-3 blood test’s national limitation amount of $17.80 that was effective in 2013. The 2014 national limitation amount applies across the U.S. except in Ohio and West Virginia where rates of $23.99 and $26.40, respectively, will apply. In
Europe, the Company’s sales efforts are currently directed to hospital situated emergency departments whose reimbursement is covered under the hospital budgeting process.
Our Product Pipeline
New Clinical Claims and Indications for the BGM Galectin-3 Test
We believe that the clinical and commercial value of our BGM Galectin-3 Test may extend beyond its current indications
for use. We expect to pursue new clinical claims and indications for its use in assessing heart failure, as well as, in related disorders. Expansion of the product label to include new clinical claims and indications for use will require additional
clinical studies and clearance, or approval, by regulatory bodies, such as the FDA, and inclusion in our CE Mark for use in the EU.
CardioSCORE™ Test
CardioSCORE test is a multi-analyte biomarker-based blood test that is designed as an aid in the assessment of near-term risk for significant atherothrombotic cardiovascular events, such as heart attack
and ischemic stroke. The CardioSCORE test is a proprietary
in vitro
diagnostic multi-analyte assay in which the levels of multiple biomarkers are measured in blood and the results are mathematically integrated to yield a single numerical
score that is predictive of an individual’s near-term atherothrombotic cardiovascular risk. Our development work indicates that the CardioSCORE test has the potential to offer significant improvement over conventional risk factor-based
diagnostics, such as the Framingham Risk Score, to identify near-term cardiovascular risk.
In December 2012, we obtained a CE
Mark for the CardioSCORE test, which will enable us to market the test in Europe and other countries that recognize CE Mark. However, as a result of our decision to focus our
3
efforts on increasing the adoption and sales of our galectin-3 test, we have decided to redirect investments from a launch of the CardioSCORE test in Europe to support our galectin-3 efforts. We
may move forward with a European launch in test markets, when and if appropriate partnership opportunities arise.
In December
2011, we submitted a 510(k) to the FDA in order to obtain regulatory clearance to market the CardioSCORE test in the United States as an aid in the assessment of near-term risk for significant cardiovascular events, such as heart attack and stroke.
In response to this submission, FDA requested that we engage an independent committee of physicians to conduct a medical review and adjudication of clinical endpoints reported in the submission. Due to the time involved in responding to this
request, we withdrew the 510(k) on August 8, 2012. Our medical review also included the assessment of sample stability and the evaluation of other technical issues raised by the FDA. We are currently analyzing the results of the medical review.
When completed, the results obtained from our analysis of data collected from the medical review will guide our go-forward regulatory, commercial and investment strategy for the CardioSCORE test in the United States.
BioImage Study Patient Cohort and Banked Specimens
We have exclusive rights to diagnostic inventions arising from our analysis of a proprietary observational and community-based cohort of over 6,800 individuals who have been followed since 2009. Baseline
blood serum, plasma, DNA, and RNA samples collected from all participants have been stored and are available for our analysis. In addition, insurance claims data, including information regarding diagnoses, procedures, and therapies related to over
1,200 non-fatal cardiovascular events that were experienced by participants in the cohort over the more than four years since follow-up was initiated is available to us for data mining. We believe that this asset provides us with a unique and
proprietary platform from which we may develop new diagnostic products.
Risk Factors
Our business is subject to numerous risks as discussed more fully in the section entitled “Risk Factors” beginning on page 8.
Principal risks of our business include, but are not limited to, the following: our history of operating losses and our need for additional financing to fund our operations; our ability to generate sufficient product revenue to sustain our
commercial diagnostics business; our estimates of future performance, including the expected timing of the launch of our products; our expectations regarding the impact on our galectin-3 test sales as a result of focusing our sales efforts on the
hospital readmissions problem and associated penalties facing our customers; our ability to conduct the clinical studies required for regulatory clearance or approval and to demonstrate the clinical benefits and cost-effectiveness to support
commercial acceptance of our products; the timing, costs and other limitations involved in obtaining regulatory clearance or approval for any of our products; the potential benefits of our products over current medical practices or other
diagnostics; our ability to successfully develop, receive regulatory clearance or approval, commercialize and achieve market acceptance for any of our products; the willingness of third-party payers to reimburse for the cost of our tests at prices
that allow us to generate sufficient profit margins; our reliance on third parties to develop and distribute our products, including our ability to enter into collaboration agreements with respect to our products and the performance of our
collaborative partners under such agreements; our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; the expected timing, progress or success of our research and
development and commercialization efforts; our estimates regarding anticipated operating losses, future revenue, expenses, capital requirements and our needs for additional financing; our ability to recruit, hire and retain qualified personnel; and
the limited public float and trading volume for our common stock and volatility in our stock price.
Corporate History and Available
Information
We were incorporated in Delaware in February 2000 and later that year chose the name Beyond Genomics, Inc. In
October 2004, we changed our name to BG Medicine, Inc. We maintain our operations at 880 Winter Street, Suite 210, Waltham, Massachusetts 02451, and our phone number is (781) 890-1199. Our
4
Internet website address is www.bg-medicine.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports filed or
furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free of charge through the investor relations page of our internet website as soon as reasonably practicable
after we electronically file such material with, or furnish it to, the Securities and Exchange Commission.
5
THE OFFERING
Common stock covered
|
by this Prospectus:
|
Up to 4,106,071 shares of Common Stock, including 132,743 shares currently outstanding.
|
Common stock outstanding as of
|
June 30, 2014:
|
34,417,249 shares, including 132,743 shares issued to Aspire Capital on January 24, 2013.
|
|
Use of proceeds:
|
Aspire Capital will receive all of the proceeds from the sale of the shares offered for sale by it under this prospectus. We will not receive proceeds from the sale of the shares by Aspire
Capital. However, we may receive up to $12 million in gross proceeds from the sale of our Common Stock to Aspire Capital under the Purchase Agreement described below, which we currently intend to use to fund our operations, advance commercialization
of our cardiovascular diagnostic tests in the United States and Europe; and other general corporate purposes, including, but not limited to, capital expenditures, licensing of intellectual property, repayment of indebtedness and working capital. See
“Use of Proceeds.”
|
|
Risk factors:
|
The shares offered hereby involve a high degree of risk. See “Risk Factors” beginning on page 8.
|
|
Dividend policy:
|
We have not paid dividends to our stockholders since our inception and we are currently prohibited from making any dividend payments under the terms of the term loan facility with our lenders.
We currently intend to retain all available funds and any future earnings to fund the development and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future.
|
|
Trading Symbol:
|
Our Common Stock currently trades on the NASDAQ Capital Market under the symbol “BGMD”.
|
Our Purchase Agreement with Aspire Capital Fund, LLC
On January 24,
2013, we entered into a common stock purchase agreement, or the Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital
is committed to purchase up to an aggregate of $12 million of shares of our Common Stock, or the Purchase Shares, over the 24-month period ending on May 24, 2015.
In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued to Aspire Capital 132,743 shares of our Common Stock, or the Commitment
Shares, as a commitment fee. We refer to the Commitment Shares and the Purchase Shares collectively as the Shares. As of the filing date of this prospectus, we had not issued any shares to Aspire Capital under the Purchase Agreement, other than the
Commitment Shares. Other terms and conditions of the Purchase Agreement are described below.
Concurrently with our entering
into the Purchase Agreement, we also entered into a registration rights agreement with Aspire Capital, or the Registration Rights Agreement. The Registration Rights Agreement provides, among other things, that we will file one or more registration
statements, as necessary, to register under
6
the Securities Act, the sale of the Shares that have been and may be issued to Aspire Capital under the Purchase Agreement. We further agreed to keep the registration statement effective and to
indemnify Aspire Capital for certain liabilities in connection with the sale of the Shares under the terms of the Registration Rights Agreement.
Pursuant to the Purchase Agreement and the Registration Rights Agreement, we are registering under the Securities Act 4,106,071 shares of our Common Stock, which includes the 132,743 Commitment Shares
that have already been issued to Aspire Capital, as well as an additional 3,973,328 Purchase Shares that we may issue to Aspire Capital. As of the filing date of this prospectus, we had not issued any shares to Aspire Capital under the Purchase
Agreement, other than the Commitment Shares. All 4,106,071 shares of Common Stock are being offered pursuant to this prospectus.
As of June 30, 2014, there were 34,417,249 shares of our Common Stock outstanding, including the 132,743 Commitment Shares, but excluding the 3,973,328 Purchase Shares that we may sell to Aspire
Capital pursuant to the Purchase Agreement. If all of the 4,106,071 shares of our Common Stock offered hereby were issued and outstanding as of June 30, 2014, such shares would represent approximately 10.7% of our Common Stock outstanding or
approximately 14.9% of the non-affiliate shares of our Common Stock, or our public float, outstanding as of June 30, 2014. The number of shares of our Common Stock ultimately offered for sale by Aspire Capital is dependent upon the number of
shares we choose to sell to Aspire Capital under the Purchase Agreement.
As described in more detail below, generally under
the Purchase Agreement we have two ways we can elect to sell shares of Common Stock to Aspire Capital on any business day we select: (1) through a regular purchase of up to 100,000 shares (but not to exceed $500,000) at a known price based on
the market price of our Common Stock prior to the time of each sale, and (2) through a volume-weighted average price, or VWAP, purchase of a number of shares up to 30% of the volume traded on the purchase date at a price equal to the lesser of
the closing sale price or 95% of the VWAP for such purchase date.
On any business day on which the closing sale price of our
Common Stock equals or exceeds $1.00 per share, over the 24-month period ending on May 24, 2015, we have the right, in our sole discretion, to present Aspire Capital with a purchase notice directing Aspire Capital to purchase up to 100,000
shares of our Common Stock per business day; however, no sale pursuant to such purchase notice may exceed $500,000 per business day. The purchase price per share is the lower of (i) the lowest sale price for our Common Stock on the purchase
date or (ii) the arithmetic average of the three lowest closing sale prices for our Common Stock during the 12 consecutive business days ending on the business day immediately preceding the purchase date. The applicable purchase price will
be determined prior to delivery of any purchase notice.
In addition, on any date on which we have submitted a purchase notice
to Aspire Capital in the amount of 100,000 shares, we also have the right, in our sole discretion, to present Aspire Capital with a volume-weighted average price purchase notice, or a VWAP Purchase Notice, directing Aspire Capital to purchase an
amount of our Common Stock equal to a percentage (not to exceed 30%) of the aggregate shares of Common Stock traded on the NASDAQ Capital Market on the next business day subject to a maximum number of shares determined by us. The purchase price per
share pursuant to such VWAP Purchase Notice shall be generally the lower of (i) the closing sale price on the purchase date and (ii) 95% of the VWAP of our Common Stock traded on the NASDAQ Capital Market on the purchase date.
The number of Purchase Shares covered by, and the timing of, each purchase notice are determined by us, at our sole discretion. We may
deliver multiple purchase notices to Aspire Capital from time to time during the term of the Purchase Agreement, so long as the most recent purchase has been completed. There are no trading volume requirements or other restrictions under the
Purchase Agreement. Aspire Capital has no right to require any sales from us, but is obligated to make purchases as directed in accordance with the Purchase Agreement.
The Purchase Agreement contains customary representations, warranties, covenants, closing conditions and indemnification and termination provisions. The Purchase Agreement may be terminated by us at any
time, at our
7
discretion, without any cost or penalty. Aspire Capital has covenanted not to cause or engage in any manner whatsoever, any direct or indirect short selling or hedging of our Common Stock. We did
not pay any additional amounts to reimburse or otherwise compensate Aspire Capital in connection with the transaction other than the Commitment Shares. There are no limitations on use of proceeds, financial or business covenants, restrictions on
future financings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement.
Our gross proceeds will depend on the purchase prices and the frequency of sales of shares to Aspire Capital;
provided
,
however
, that the maximum aggregate proceeds from sales of shares is $12 million. Our delivery of purchase notices will be made subject to market conditions, in light of our anticipated capital needs from time to time and under the
limitations contained in the Purchase Agreement. We expect to use proceeds from sales of shares for general corporate purposes and working capital requirements.
The issuance of the shares to Aspire Capital under the Purchase Agreement is exempt from registration under the Securities Act, pursuant to the exemption for transactions by an issuer not involving any
public offering under Section 4(a)(2) of the Securities Act.
Effect of Issuances of Shares to Aspire Capital; Dilution
The issuances of the shares to Aspire Capital under the Purchase Agreement will have no effect on the rights or privileges
of existing holders of our Common Stock, except that the economic and voting interests of each stockholder will be diluted as a result of any such issuances. What this means is that, although the number of shares of our Common Stock that current
stockholders presently own will not decrease, the shares that are held by our current stockholders will represent a smaller percentage of our total shares that will be outstanding after any issuances of shares of our Common Stock to Aspire Capital.
RISK FACTORS
A purchase of shares of our Common Stock is an investment in our securities and involves a high degree of risk. You should carefully consider the risks and uncertainties and all other information
contained in or incorporated by reference in this prospectus, including the risks and uncertainties discussed under “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on
Form 10-Q for the quarter ended March 31, 2014. All of these risk factors are incorporated by reference herein in their entirety. If any of these risks actually occur, our business, financial condition and results of operations would
likely suffer. In that case, the market price of the Common Stock could decline, and you may lose part or all of your investment in our company. Additional risks of which we are not presently aware or that we currently believe are immaterial may
also harm our business and results of operations.
USE OF PROCEEDS
The Selling Stockholder will receive all of the proceeds from the sale of the shares offered for sale by it under this prospectus. We
will not receive proceeds from the sale of the shares by the Selling Stockholder. However, we may receive up to an aggregate of $12 million in proceeds from the sale of our Common Stock to the Selling Stockholder under the Purchase Agreement. We
will bear all reasonable expenses incident to the registration of the shares under federal and state securities laws other than expenses incident to the delivery of the shares to be sold by the Selling Stockholder. Any transfer taxes payable on
these shares and any commissions and discounts payable to underwriters, agents, brokers or dealers will be paid by the Selling Stockholder.
Assuming the sale by us of all $12 million of shares of our Common Stock to the Selling Stockholder and estimated expenses of approximately $77,964, the total net proceeds to us under the Purchase
Agreement would
8
be approximately $11.92 million. We currently expect to use the net proceeds to us from the sale of shares to the Selling Stockholder, if any, to fund our operations, advance commercialization of
our cardiovascular diagnostic tests in the United States and Europe; and other general corporate purposes, including, but not limited to, capital expenditures, licensing of intellectual property, repayment of indebtedness and working capital. We
expect from time to time to evaluate the acquisition of businesses, products and technologies for which a portion of the net proceeds may be used, although we currently are not planning or negotiating any such transactions. As of the date of this
prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from the sale of shares to the Selling Stockholder. Accordingly, we will retain broad discretion over the use of these proceeds, if any. Pending
application of the net proceeds as described above, we may initially invest the net proceeds in short-term, investment-grade, interest-bearing securities or apply them to the reduction of short-term indebtedness.
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND
ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
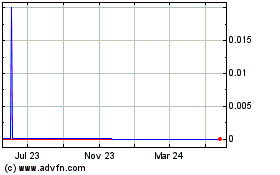
Our common stock began trading on The NASDAQ Global
Market on February 4, 2011 under the symbol “BGMD” and was transferred to The NASDAQ Capital Market on January 27, 2014 where it continues to trade under the same symbol. The following table sets forth the high and low sales
prices of our common stock as quoted on The NASDAQ Global Market or The NASDAQ Capital Market, as applicable, for the periods indicated.
|
|
|
|
|
|
|
|
|
|
|
2014:
|
|
High
|
|
|
Low
|
|
|
First Quarter
|
|
$
|
2.41
|
|
|
$
|
0.99
|
|
|
Second Quarter
|
|
$
|
2.02
|
|
|
$
|
0.90
|
|
|
|
|
|
|
2013:
|
|
High
|
|
|
Low
|
|
|
First Quarter
|
|
$
|
3.15
|
|
|
$
|
1.11
|
|
|
Second Quarter
|
|
$
|
2.17
|
|
|
$
|
1.21
|
|
|
Third Quarter
|
|
$
|
1.40
|
|
|
$
|
0.83
|
|
|
Fourth Quarter
|
|
$
|
1.70
|
|
|
$
|
0.55
|
|
|
|
|
|
|
2012:
|
|
High
|
|
|
Low
|
|
|
First Quarter
|
|
$
|
12.80
|
|
|
$
|
4.49
|
|
|
Second Quarter
|
|
$
|
7.50
|
|
|
$
|
3.75
|
|
|
Third Quarter
|
|
$
|
7.05
|
|
|
$
|
3.35
|
|
|
Fourth Quarter
|
|
$
|
4.48
|
|
|
$
|
1.00
|
|
Stockholders
As of June 30, 2014, there were approximately 33 stockholders of record of the 34,417,249 outstanding shares of our common stock.
DIVIDEND POLICY
We have not paid dividends to our stockholders since our inception and we are currently prohibited from making any dividend payments under the terms of the term loan facility with our lenders. We
currently intend to retain all available funds and any future earnings to fund the development and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future.
9
SELLING STOCKHOLDER
We have included in this prospectus 132,743 shares of Common Stock issued to the Selling Stockholder, Aspire Capital Fund, LLC, on
January 24, 2013 and up to an additional 3,973,328 shares of Common Stock that may be issued in the future to Aspire Capital pursuant to the Purchase Agreement. Prior to entering into the Purchase Agreement on January 24, 2013, Aspire
Capital did not own any shares of our Common Stock.
The following table sets forth certain information regarding the Selling
Stockholder and the shares of Common Stock beneficially owned by it, which information is available to us as of June 30, 2014. The Selling Stockholder may offer shares under this prospectus from time to time and may elect to sell none, some or
all of the shares set forth below. As a result, we cannot estimate the number of shares of Common Stock that the Selling Stockholder will beneficially own after termination of sales under this prospectus. However, for the purposes of the table
below, we have assumed that the Selling Stockholder will sell all shares covered by this prospectus.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling Stockholder
|
|
Shares
Beneficially
Owned
Before
Offering(1)
|
|
|
Percentage of
Outstanding
Shares
Beneficially
Owned
Before
Offering
|
|
|
Maximum
Shares to
Be Sold in the
Offering
|
|
|
Shares
Beneficially
Owned
After
Offering
|
|
|
Percentage
of
Outstanding
Shares
Beneficially
Owned
After
Offering
|
|
|
Aspire Capital Fund, LLC(2)
|
|
|
382,743
|
(3)
|
|
|
1.1
|
%
|
|
|
4,106,071
|
|
|
|
565,000
|
|
|
|
2.0
|
%
|
|
(1)
|
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In general, a person is deemed to be the beneficial owner of (i) any
shares of our Common Stock over which such person has sole or shared voting power or investment power, plus (ii) any shares which such person has the right to acquire beneficial ownership of within 60 days, whether through the exercise of
options, warrants or otherwise. The percentage of ownership set forth above assumes the sale by the Company to Aspire Capital of all shares being offered pursuant to this prospectus and is based on 34,417,249 shares of our Common Stock outstanding
as of June 30, 2014, which includes the 132,743 shares previously issued to Aspire Capital pursuant to the Purchase Agreement, together with securities exercisable or convertible into shares of Common Stock within 60 days of the date hereof for
the Selling Stockholder. The Company may elect in its sole discretion to sell to Aspire Capital up to an additional number of shares under the Purchase Agreement equal to $12 million in value, but Aspire Capital does not presently beneficially own
those shares as determined in accordance with the rules of the SEC.
|
|
(2)
|
As of the date of the Purchase Agreement, Aspire Capital beneficially owned no shares of Common Stock of the Company. Steven G. Martin, Erik J. Brown and Christos
Komissopoulos, the principals of Aspire Capital, are deemed to be beneficial owners of all of the shares of Common Stock owned by Aspire Capital. Although Messrs. Martin, Brown and Komissopoulos are deemed to have shared voting and investment power
over the shares being offered under the prospectus filed with the SEC in connection with the transactions contemplated under the Purchase Agreement, each disclaims beneficial ownership of these shares except to the extent of their pecuniary interest
therein. Aspire Capital is not a licensed broker dealer or an affiliate of a licensed broker dealer.
|
|
(3)
|
As of the date of the Purchase Agreement, 132,743 shares of our Common Stock were issued to Aspire Capital as a commitment fee under the Purchase Agreement. Pursuant to
the terms of the Purchase Agreement, the Company may elect in its sole discretion to sell to Aspire Capital up to an additional number of shares under the Purchase Agreement equal to $12 million in value, but Aspire Capital does not presently
beneficially own those shares as determined in accordance with the rules of the SEC.
|
10
THE ASPIRE CAPITAL TRANSACTION
General
On
January 24, 2013, we entered into the Purchase Agreement, which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $12 million of shares
of our Common Stock, or the Purchase Shares, over the 24-month period ending on May 24, 2015. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued to Aspire Capital the
Commitment Shares as a commitment fee. Concurrently with our entering into the Purchase Agreement, we also entered into the Registration Rights Agreement, in which we agreed to file one or more registration statements, as necessary, to register
under the Securities Act, the sale of the Shares that have been and may be issued to Aspire Capital under the Purchase Agreement.
Pursuant to the Purchase Agreement and the Registration Rights Agreement, we are registering under the Securities Act 4,106,071 shares of our Common Stock, which includes the 132,743 Commitment Shares
that have already been issued to Aspire Capital, as well as an additional 3,973,328 Purchase Shares that we may issue to Aspire Capital. As of the filing date of this prospectus, we had not issued any shares to Aspire Capital under the Purchase
Agreement, other than the Commitment Shares. All 4,106,071 shares of Common Stock are being offered pursuant to this prospectus.
As of June 30, 2014, there were 34,417,249 shares of our Common Stock outstanding, including the 132,743 Commitment Shares, but excluding the 3,973,328 Purchase Shares that we may sell to Aspire
Capital pursuant to the Purchase Agreement. If all of the 4,106,071 shares of our Common Stock offered hereby were issued and outstanding as of June 30, 2014, such shares would represent approximately 10.7% of our Common Stock outstanding or
approximately 14.9% of our public float as of June 30, 2014. The number of shares of our Common Stock ultimately offered for sale by Aspire Capital is dependent upon the number of shares we choose to sell to Aspire Capital under the Purchase
Agreement.
Under the Purchase Agreement, we have the right, but not the obligation, to sell more than the 4,106,071 shares of
our Common Stock offered by this prospectus. The Purchase Agreement provides that the number of shares that may be sold pursuant to the Purchase Agreement shall be limited to 4,106,071, or the “Exchange Cap,” which represents 19.99% of our
outstanding shares as of January 24, 2013, the date we entered into the Purchase Agreement, unless we obtain shareholder approval or identify an exception to the rules of the NASDAQ Capital Market to issue more than 19.99%. This limitation
shall not apply if, at any time the Exchange Cap is reached and at all times thereafter, the average price paid for all shares issued and sold under the Purchase Agreement is equal to or greater than $2.30, which was the closing sale price of our
Common Stock on January 23, 2013. We are not required or permitted to issue any shares of Common Stock under the Purchase Agreement if such issuance would breach our obligations under the rules or regulations of the NASDAQ Capital Market. If we
elect to sell more than the 4,106,071 shares of Common Stock offered hereby, we must first obtain the approval of our stockholders to do so, if necessary, and register under the Securities Act the sale of any additional shares we may elect to sell
to Aspire Capital before we can sell such additional shares to Aspire Capital under the Purchase Agreement.
After the SEC has
declared effective the registration statement of which this prospectus is a part, we have the right, in our sole discretion, to present Aspire Capital with a Purchase Notice, directing Aspire Capital (as principal) to purchase up to 100,000 shares
of our Common Stock per business day, up to $12 million of our Common Stock in the aggregate at a Purchase Price calculated by reference to the prevailing market price of our Common Stock over a preceding 12-business day period (as more specifically
described below); however, no sale pursuant to a Purchase Notice may exceed $500,000 per trading day.
In addition, on any
date on which we submit a Purchase Notice to Aspire Capital in an amount equal to 100,000 shares, we also have the right, in our sole discretion, to present Aspire Capital with a VWAP Purchase
11
Notice directing Aspire Capital to purchase an amount of stock equal to up to 30% of the aggregate shares of the Company’s Common Stock traded on the NASDAQ Capital Market on the purchase
date, subject to the VWAP Purchase Share Volume Maximum and the VWAP Minimum Price Threshold. The VWAP Purchase Price is calculated by reference to the prevailing market price of our Common Stock (as more specifically described below).
The Purchase Agreement provides that in no event will any shares of Common Stock be sold at a Purchase Price less than $1.00, or the
Floor Price. This Floor Price and the respective prices and share numbers in the preceding paragraphs shall be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar
transaction. Additionally, the Purchase Agreement provides that the Company and Aspire Capital shall not effect any sales under the Purchase Agreement if such shares proposed to be issued and sold, when aggregated with all other shares of the
Company’s Common Stock that Aspire Capital and its affiliates beneficially own, would result in Aspire Capital and its affiliates beneficially owning more than 19.99% of the Company’s then issued and outstanding Common Stock.
There are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any
sales of our Common Stock to Aspire Capital. Aspire Capital has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Purchase Agreement. There are no limitations on use of proceeds,
financial or business covenants, restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our
discretion, without any penalty or cost to us. The rights and obligations of Aspire Capital under the Purchase Agreement are not assignable or transferable.
Purchase of shares under the Purchase Agreement
Under the Purchase
Agreement, on any trading day selected by us on which the closing price of our Common Stock is not less than $1.00 per share, we may direct Aspire Capital to purchase up to 100,000 shares of our Common Stock per trading day so long as sales pursuant
to such Purchase Notice do not exceed $500,000 per trading day. The Purchase Price of such shares is equal to the lesser of:
|
|
•
|
|
the lowest sale price of our Common Stock on the purchase date; or
|
|
|
•
|
|
the arithmetic average of the three lowest closing sale prices for our Common Stock during the twelve consecutive trading days ending on the trading
day immediately preceding the purchase date.
|
In addition, on any date on which we submit a Purchase Notice
to Aspire Capital in an amount equal to 100,000 shares we also have the right to direct Aspire Capital to purchase an amount of stock equal to up to 30% of the aggregate shares of the Company’s Common Stock traded on the NASDAQ Capital Market
on the purchase date, subject to the VWAP Purchase Share Volume Maximum and the VWAP Minimum Price Threshold, which is equal to the greater of (a) 90% of the closing price on the NASDAQ Capital Market on the business day immediately preceding
the VWAP Purchase Date or (b) such higher price as set forth by the Company in the VWAP Purchase Notice. The VWAP Purchase Price of such shares is the lower of:
|
|
•
|
|
the closing sale price on the VWAP Purchase Date; or
|
|
|
•
|
|
95% of the volume-weighted average price for our Common Stock traded on the NASDAQ Capital Market during normal trading hours:
|
|
|
a.
|
on the VWAP Purchase Date, if the aggregate shares traded on the NASDAQ Capital Market have not exceeded the VWAP Purchase Share Volume Maximum; or
|
|
|
b.
|
the portion of the VWAP Purchase Date until such time as the sooner to occur of (i) the time at which the aggregate shares traded on the NASDAQ Capital Market has
exceeded the VWAP Purchase Share Volume Maximum or (ii) the time at which the sale price of the Common Stock falls below the VWAP Minimum Price Threshold.
|
12
The Purchase Price and VWAP Purchase Price will be adjusted for any reorganization,
recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction occurring during the period(s) used to compute such price. We may deliver multiple Purchase Notices and VWAP Purchase Notices to Aspire Capital from
time to time during the term of the Purchase Agreement, so long as the most recent purchase has been completed.
Minimum Share Price
Under the Purchase Agreement, the Company and Aspire Capital may not effect any sales of shares of our Common Stock on any
trading day that the closing sale price of our Common Stock is less than $1.00 per share.
Compliance with the NASDAQ Capital Market Price
The Purchase Agreement provides that the number of shares that may be sold pursuant to the Purchase Agreement shall be
limited to 4,106,071 shares, or the Exchange Cap, which represents 19.99% of our outstanding shares as of January 24, 2013, the date we entered into the Purchase Agreement, unless we obtain shareholder approval or identify an exception to the
rules of the NASDAQ Capital Market to issue more than 19.99%, to be in compliance with the applicable listing maintenance rules of the NASDAQ Capital Market. This limitation shall not apply if, at any time the Exchange Cap is reached and at all
times thereafter, the average price paid for all shares issued and sold under the Purchase Agreement is equal to or greater than $2.30, which was the closing sale price of our Common Stock on January 23, 2013. We are not permitted to issue any
shares of Common Stock under the Purchase Agreement if such issuance would breach our obligations under the rules or regulations of the NASDAQ Capital Market.
Beneficial Ownership Limitation
Under the Purchase Agreement, we may not
effect any sales of shares of our Common Stock to Aspire Capital if such shares proposed to be issued and sold, when aggregated with all other shares of our Common Stock then beneficially owned by Aspire Capital and its affiliates, would result in
the beneficial ownership by Aspire Capital and its affiliates of more than 19.99% of our then issued and outstanding shares of Common Stock.
Events of Default
The
following are events of default under the Purchase Agreement. If any of the following events of default occur, we may not require Aspire Capital, and Aspire Capital shall not be obligated or permitted, to purchase any shares of our Common Stock
pursuant to the Purchase Agreement. In addition, upon the occurrence of any of the following events of default, Aspire Capital may terminate the Purchase Agreement.
|
|
•
|
|
the effectiveness of any registration statement that is required to be maintained effective pursuant to the terms of the Registration Rights Agreement
between us and Aspire Capital lapses for any reason (including, without limitation, the issuance of a stop order) or is unavailable to Aspire Capital for sale of our shares of Common Stock, and such lapse or unavailability continues for a period of
ten consecutive business days or for more than an aggregate of thirty business days in any 365-day period, which is not in connection with a post-effective amendment to any such registration statement; provided, however, that in connection with any
post-effective amendment to such registration statement that is required to be declared effective by the SEC, such lapse or unavailability may continue for a period of no more than twenty consecutive business days, which such period shall be
extended for up to an additional twenty business days if we receive a comment letter from the SEC in connection therewith;
|
|
|
•
|
|
the suspension from trading or failure of our Common Stock to be listed on a Principal Market (as defined in the Purchase Agreement) for a period of
three consecutive business days;
|
13
|
|
•
|
|
the delisting of our Common Stock from the NASDAQ Capital Market, provided our Common Stock is not immediately thereafter trading on the New York Stock
Exchange, the NYSE MKT, the NASDAQ Global Select Market, the NASDAQ Global Market, the OTCQB or OTCQX market places of the OTC markets;
|
|
|
•
|
|
our transfer agent’s failure to issue to Aspire Capital shares of our Common Stock which Aspire Capital is entitled to receive under the Purchase
Agreement within five business days after an applicable purchase date;
|
|
|
•
|
|
a breach by us of the representations, warranties, covenants or other term or condition contained in the Purchase Agreement or any related agreements
that could reasonably be expected to have a material adverse effect except, in the case of a breach of a covenant which is reasonably curable, only if such breach continues for a period of at least five business days;
|
|
|
•
|
|
if at any time the issuance of shares of Common Stock upon the submission of a Purchase Notice or VWAP Purchase Notice under the Purchase Agreement
would result in the issuance of an aggregate of number of shares of Common Stock that would exceed the number of shares of Common Stock that we may issue under this agreement without breaching our obligations under the rules or regulations of the
NASDAQ Capital Market;
|
|
|
•
|
|
if we become insolvent or are generally unable to pay our debts as they become due; or
|
|
|
•
|
|
any participation or threatened participation in insolvency or bankruptcy proceedings by or against us.
|
Our Termination Rights
The Purchase Agreement may be terminated by us at any time, at our discretion, without any cost to us.
No Short-Selling or Hedging by Aspire Capital
Aspire Capital has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging, which establishes a net short position with
respect to our Common Stock during any time prior to the termination of the Purchase Agreement.
Effect of Sales under the Purchase
Agreement on Our Stockholders
The Purchase Agreement does not limit the ability of Aspire Capital to sell any or all of
the 4,106,071 shares registered in this offering. It is anticipated that shares registered in this offering will be sold over a period of up to approximately 24 months ending on May 24, 2015. The sale by Aspire Capital of a significant amount
of shares registered in this offering at any given time could cause the market price of our Common Stock to decline or to be highly volatile. Sales to Aspire Capital by us pursuant to the Purchase Agreement also may result in dilution to the
interests of other holders of our Common Stock. However, we have the right to control the timing and amount of sales of our shares to Aspire Capital, and the Purchase Agreement may be terminated by us at any time at our discretion without any
penalty or cost to us.
Amount of Potential Proceeds to be Received under the Purchase Agreement
In connection with entering into the Purchase Agreement, we authorized the sale to Aspire Capital of up to $12 million of shares of our
Common Stock. However, we estimate that we will sell no more than 4,106,071 shares to Aspire Capital under the Purchase Agreement (inclusive of the Commitment Shares and Purchase Shares), all of which are included in this offering. Subject to
any required approval by our Board of Directors and our stockholders, we have the right but not the obligation to issue more than the 4,106,071 shares included in this prospectus to Aspire Capital under the Purchase Agreement. In the event we elect
to issue more than 4,106,071 shares under the Purchase Agreement, we will be required to file a new registration statement and
14
have it declared effective by the SEC. The number of shares ultimately offered for sale by Aspire Capital in this offering is dependent upon the number of shares we choose to sell to Aspire
Capital under the Purchase Agreement. The following table sets forth the number and percentage of outstanding shares to be held by Aspire Capital after giving effect to the sale of shares of Common Stock issued to Aspire Capital covered by the
registration statement, of which this prospectus is a part, at varying purchase prices in addition to the Commitment Shares.
|
|
|
|
|
|
|
|
|
Assumed Average
Purchase Price of the
Additional Shares Sold
Under the Purchase
Agreement
|
|
Number of Additional
Shares to be Sold(1)
|
|
Percentage of
Outstanding Shares
After Giving Effect to the
Aspire Capital
Transaction(2)
|
|
Proceeds from the
Sale of Shares to
Aspire Capital Under the
Purchase Agreement
|
|
$1.00
|
|
3,973,328
|
|
10.3%
|
|
$ 3,973,328
|
|
$1.50
|
|
3,973,328
|
|
10.3%
|
|
$ 5,959,992
|
|
$2.00
|
|
3,973,328
|
|
10.3%
|
|
$ 7,946,656
|
|
$2.50
|
|
3,973,328
|
|
10.3%
|
|
$ 9,933,320
|
|
$3.00
|
|
3,973,328
|
|
10.3%
|
|
$11,919,984
|
|
$3.50
|
|
3,428,571
|
|
9.1%
|
|
$11,999,999
|
|
$4.00
|
|
3,000,000
|
|
8.0%
|
|
$12,000,000
|
|
(1)
|
Based on total aggregate sales of the lesser of (a) $12 million of shares of Common Stock and (b) the 3,973,328 Purchase Shares registered herein. Excludes
the Commitment Shares.
|
|
(2)
|
The denominator is based on 34,417,249 shares outstanding on June 30, 2014, plus the number of shares set forth in the adjacent column which we would have sold to
Aspire Capital at the assumed price in the first column. The numerator is based on the number of shares which we would have sold under the Purchase Agreement at the corresponding assumed purchase price set forth in the first column and assuming a
maximum of $12 million of shares are sold to Aspire Capital.
|
PLAN OF DISTRIBUTION
The shares may be sold or distributed from time to time by the Selling Stockholder directly to one or more purchasers or
through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of
the shares offered by this prospectus may be effected in one or more of the following methods:
|
|
•
|
|
ordinary brokers’ transactions;
|
|
|
•
|
|
transactions involving cross or block trades;
|
|
|
•
|
|
through brokers, dealers, or underwriters who may act solely as agents;
|
|
|
•
|
|
“at the market” into an existing market for the Common Stock;
|
|
|
•
|
|
in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents in
privately negotiated transactions; or any combination of the foregoing.
|
In order to comply with the
securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the
state or an exemption from the registration or qualification requirement is available and complied with.
The Selling
Stockholder may also sell shares of Common Stock under Rule 144 promulgated under the Securities Act, if available, rather than under this prospectus. In addition, the Selling Stockholder may transfer the shares of Common Stock by other means not
described in this prospectus.
15
Brokers, dealers, underwriters, or agents participating in the distribution of the shares as
agents may receive compensation in the form of commissions, discounts, or concessions from the Selling Stockholder and/or purchasers of the Common Stock for whom the broker-dealers may act as agent. The Selling Stockholder has informed us that each
such broker-dealer will receive commissions from Aspire Capital which will not exceed customary brokerage commissions.
The
Selling Stockholder and its affiliates have agreed not to engage in any direct or indirect short selling or hedging of our Common Stock during the term of the Purchase Agreement.
The Selling Stockholder is an “underwriter” within the meaning of the Securities Act.
We have advised Selling Stockholder that while it is engaged in a distribution of the shares included in this prospectus it is required
to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes the Selling Stockholder, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from
bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to
stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the shares offered hereby this prospectus.
We may suspend the sale of shares by the Selling Stockholder pursuant to this prospectus for certain periods of time for certain reasons,
including if the prospectus is required to be supplemented or amended to include additional material information.
This
offering will terminate on the date that all shares offered by this prospectus have been sold by the Selling Stockholder.
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
The following is a description of the transactions in which we
have engaged, over the past three years, since January 1, 2011 with our directors and officers and then beneficial owners of more than five percent of our voting securities and their affiliates.
Participation in Follow-on Underwritten Public Offering
In January 2013, we closed a follow-on underwritten public offering of 6,900,000 shares of our common stock at a price to the public of $2.00 per share, including an aggregate of 2,250,000 shares to the
following directors and beneficial owners of more than five percent of our voting securities, and their affiliates:
|
|
|
|
|
|
|
|
|
|
|
Name
|
|
Number of
Shares of
Common
Stock
|
|
|
Aggregate
Purchase
Price
|
|
|
Entities affiliated with Flagship Ventures(1)
|
|
|
2,000,000
|
|
|
$
|
4,000,000
|
|
|
Stelios Papadopoulos(2)
|
|
|
250,000
|
|
|
|
500,000
|
|
|
(1)
|
Includes 75,000 shares of common stock purchased by AGTC Advisors Fund, L.P., 500,000 shares of common stock purchased by Applied Genomic Technology Fund, L.P.,
1,050,000 shares of common stock purchased by Flagship Ventures Fund 2007, L.P., 125,000 shares of common stock purchased by NewcoGen Equity Investors LLC and 250,000 shares of common stock purchased by NewcoGen Group LLC. Noubar B. Afeyan, Ph.D.,
one of our directors, is affiliated with all entities affiliated with Flagship Ventures.
|
|
(2)
|
Dr. Papadopoulos is a director of the Company.
|
16
Participation in Initial Public Offering
In February 2011, we issued an aggregate of 5,750,000 shares of our common stock in connection with our initial public offering at an
initial public offering price of $7.00 per share, including an aggregate of 3,592,069 shares to the following directors and beneficial owners of more than five percent of our voting securities, and their affiliates:
|
|
|
|
|
|
|
|
|
|
|
Name
|
|
Number of
Shares of
Common
Stock
|
|
|
Aggregate
Purchase
Price
|
|
|
Entities affiliated with Flagship Ventures(1)
|
|
|
1,142,857
|
|
|
$
|
7,999,999
|
|
|
General Electric Pension Trust
|
|
|
428,571
|
|
|
|
2,999,997
|
|
|
Gilde Europe Food & Agribusiness Fund, B.V.(2)
|
|
|
142,857
|
|
|
|
999,999
|
|
|
Legg Mason Capital Management Special Investment Trust
|
|
|
1,428,571
|
|
|
|
9,999,997
|
|
|
Stelios Papadopoulos(3)
|
|
|
75,000
|
|
|
|
525,000
|
|
|
SMALLCAP World Fund, Inc.
|
|
|
374,213
|
|
|
|
2,619,491
|
|
|
(1)
|
Includes 14,285 shares of common stock purchased by AGTC Advisors Fund, L.P., 271,429 shares of common stock purchased by Applied Genomic Technology Fund, L.P., 714,286
shares of common stock purchased by Flagship Ventures Fund 2007, L.P., 21,428 shares of common stock purchased by NewcoGen-Long Reign Holding LLC, 10,714 shares of common stock purchased by NewcoGen-Elan LLC, 35,715 shares of common stock purchased
by NewcoGen Equity Investors LLC, 42,858 shares of common stock purchased by NewcoGen Group LLC, 10,714 shares of common stock purchased by NewcoGen-PE LLC and 21,428 shares of common stock purchased by ST NewcoGen LLC. Noubar B. Afeyan, Ph.D., one
of our directors, is affiliated with all entities affiliated with Flagship Ventures.
|
|
(2)
|
Pieter van der Meer, one of our former directors, is affiliated with Gilde Europe Food & Agribusiness Fund, B.V.
|
|
(3)
|
Dr. Papadopoulos is a director of the Company.
|
The initial public offering price of $7.00 per share was determined through negotiations between us and the representatives of the underwriters of the offering based on several factors.
Investor Rights Agreement
In connection with the Series D redeemable convertible preferred stock financing, we entered into the Fourth Amended and Restated Investor Rights Agreement, dated as of July 10, 2008, with entities
affiliated with Flagship; Gilde; Stelios Papadopoulos; Humana; Legg Mason; GE; SMALLCAP; and certain of our other stockholders. This agreement terminated upon our initial public offering, other than the portions relating to registration rights,
which will continue in effect and entitle the holders of such rights to have us register their shares of our common stock for sale in the United States. These registration rights are subject to certain conditions and limitations, including the right
of the underwriters of an offering to limit the number of shares of our common stock included in any such registration under certain circumstances. We are generally required to pay all expenses incurred in connection with registrations effected in
connection with the following rights, excluding underwriting discounts and commissions. The registration rights described below shall not apply to shares of common stock that are eligible to be sold by persons who are not affiliates of the Company
(as defined in Rule 144 of the Securities Act), and have not been affiliates of the Company during the preceding three months, pursuant to Rule 144(b)(1) under the Securities Act.
Demand Rights
. Any holder or holders who collectively hold registrable securities representing at least 40% of the registrable
securities then outstanding shall have the right, exercisable by written notice, to have us prepare and file a registration statement under the Securities Act covering the registrable securities that are the subject of such request; provided, that
we are not obligated to prepare and file a registration statement if neither Form S-3
17
nor another short form registration statement is available to us, unless the registrable securities that are the subject of such request have an expected aggregate offering price to the public of
at least $1,000,000. Subject to the foregoing, the holders shall be permitted one demand registration. In addition, under certain circumstances, the underwriters, if any, may limit the number of shares of our common stock included in any such
registration, and we may postpone or suspend the filing or effectiveness of such registration.
Piggyback Rights
. If at
any time we propose to register our common stock under the Securities Act, other than in a registration statement relating solely to sales of securities to participants in a dividend reinvestment plan, or Form S-4 or S-8 or any successor form or in
connection with an acquisition or exchange offer or an offering of securities solely to our existing stockholders or employees, we are required to (i) give prompt written notice to all holders of registrable securities of our intention to
effect such a registration and (ii) include in such registration all registrable securities which are permitted under applicable securities laws to be included in the form of registration statement we select and with respect to which we have
received written requests for inclusion therein within 30 days after the receipt of our notice. We shall have the right to postpone or withdraw any such registration without obligation to any stockholder. In addition, under certain circumstances,
the underwriters, if any, may limit the number of shares of our common stock included in any such registration.
Transactions with
Principal Stockholders
On July 7, 2011, we issued 51,789 shares of our common stock to Legg Mason, one of the
former beneficial owners of more than five percent of our common stock, upon the net exercise of previously issued warrants to purchase shares of our common stock.
On July 27, 2011, we issued 51,756 shares of our common stock to SMALLCAP, one of the former beneficial owners of more than five percent of our common stock, upon the net exercise of previously
issued warrants to purchase shares of our common stock.
On September 1, 2011, we issued 2,789 shares of our common stock
to Pieter Muntendam, one of our former executive officers, upon the net exercise of previously issued warrants to purchase shares of our common stock.
On November 29, 2011, we issued 150,538 shares of our common stock to Stelios Papadopoulos, one of our directors, upon the net exercise of previously issued warrants to purchase shares of our common
stock.
On May 21, 2012, we issued 25,939 shares of our common stock to NewcoGen — PE LLC upon the net exercise of
previously issued warrants to purchase shares of our common stock.
On August 20, 2012, we issued 21,537 shares of our
common stock to NewcoGen — Elan LLC upon the net exercise of previously issued warrants to purchase shares of our common stock.
On June 11, 2013, we issued 326,860 shares of our common stock to Gilde Europe Food & Agribusiness Fund B.V upon the net exercise of previously issued warrants to purchase shares of our
common stock.
Agreements with Directors and Executive Officers
Please see “Executive Compensation” in our Definitive Proxy Statement on Schedule 14A filed on April 24, 2014 for
additional information regarding compensation of our executive officers and directors.
We have entered into agreements with
our named executive officers. For information regarding these agreements, please refer to the section entitled “Executive Compensation — Narrative Disclosure to Summary Compensation Table” in our Definitive Proxy Statement on Schedule
14A filed on April 24, 2014. On December 17, 2013, we terminated the consulting agreement with our Executive Chairman, Stéphane Bancel, with retroactive effect to November 1, 2013. Mr. Bancel continues to serve as the
Chairman of our Board of
18
Directors. For information regarding this agreement, please refer to the section entitled “Board of Directors and Corporate Governance — Director Compensation” in our Definitive
Proxy Statement on Schedule 14A filed on April 24, 2014.
Our restated certificate of incorporation and restated bylaws
provide that we will indemnify our directors and officers to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with our directors and executive officers. These agreements provide that we will,
among other things, indemnify and advance expenses to our directors and officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any
action by us arising out of such person’s services as our director or officer, or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and
retain qualified persons as directors and officers.
Policy for Approval of Related Person Transactions
Pursuant to the written charter of our audit committee, the audit committee is responsible for reviewing and approving, prior to our entry
into any such transaction, all transactions in which we are a participant and in which any parties related to us, including our executive officers, our directors, beneficial owners of more than five percent of our securities, immediate family
members of the foregoing persons and any other persons whom our Board of Directors determines may be considered related parties, has or will have a direct or indirect material interest.
In reviewing and approving such transactions, the audit committee shall obtain, or shall direct our management to obtain on its behalf,
all information that the committee believes to be relevant and important to a review of the transaction prior to its approval. Following receipt of the necessary information, a discussion shall be held of the relevant factors if deemed to be
necessary by the committee prior to approval. If a discussion is not deemed to be necessary, approval may be given by written consent of the committee. This approval authority may also be delegated to the chairman of the audit committee in some
circumstances. No related party transaction shall be entered into prior to the completion of these procedures.
The audit
committee or its chairman, as the case may be, shall approve only those related party transactions that are determined to be in, or not inconsistent with, the best interests of us and our stockholders, taking into account all available facts and
circumstances as the committee or the chairman determines in good faith to be necessary. These facts and circumstances will typically include, but not be limited to, the benefits of the transaction to us; the impact on a director’s independence
in the event the related party is a director, an immediate family member of a director or an entity in which a director is a partner, stockholder or executive officer; the availability of other sources for comparable products or services; the terms
of the transaction; and the terms of comparable transactions that would be available to unrelated third parties or to employees generally. No member of the audit committee shall participate in any review, consideration or approval of any related
party transaction with respect to which the member or any of his or her immediate family members is the related party.
DESCRIPTION OF SECURITIES TO BE REGISTERED (COMMON STOCK)
We are authorized to issue 100,000,000 shares of Common
Stock, par value $0.001 per share. On June 30, 2014, we had 34,417,249 shares of Common Stock outstanding and approximately 33 stockholders of record. The number of shares of Common Stock outstanding as of June 30, 2014 excludes the
following:
|
|
•
|
|
3,042,661 shares of our common stock issuable upon exercise of stock options outstanding under our stock plans, at a weighted average exercise price of
$3.10 per share;
|
|
|
•
|
|
864,555 shares of common stock issuable upon the exercise of outstanding warrants, with a weighted-average exercise price of $1.04 per share;
|
|
|
•
|
|
927,619 shares of our common stock available for future grant or issuance pursuant to our stock plan and employee stock purchase plan.
|
19
The following summary of certain provisions of our common stock does not purport to be
complete. You should refer to our restated certificate of incorporation and our restated bylaws, both of which are included as exhibits to the registration statement of which this prospectus is a part. The summary below is also qualified by
provisions of applicable law.
General
Holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, and do not have cumulative voting rights. Subject to preferences that
may be applicable to any outstanding shares of preferred stock, holders of common stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by our board of directors out of funds legally available for
dividend payments. All shares of common stock outstanding as of the date of this prospectus and, upon issuance and sale, all shares of common stock that may be offered pursuant to this prospectus, will be fully paid and nonassessable. The holders of
common stock have no preferences or rights of conversion, exchange, pre-emption or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. In the event of any liquidation, dissolution or
winding-up of our affairs, holders of common stock will be entitled to share ratably in our assets that are remaining after payment or provision for payment of all of our debts and obligations and after liquidation payments to holders of outstanding
shares of preferred stock, if any.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
NASDAQ Capital Market
Our common stock is listed for quotation on The NASDAQ Capital Market under the symbol “BGMD.”
CERTAIN PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF
INCORPORATION AND BYLAWS
Anti-Takeover Provisions of our Certificate of Incorporation and Bylaws
In addition to the board of directors’ ability to issue shares of preferred stock, our restated certificate of incorporation and restated bylaws contain other provisions that are intended to enhance
the likelihood of continuity and stability in the composition of the board of directors and which may have the effect of delaying, deferring or preventing a future takeover or change in control of our company unless such takeover or change in
control is approved by our board of directors.
These provisions, summarized below, are expected to discourage coercive
takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our
potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement
of their terms.
Classified board of directors; removal of directors for cause.
Our restated certificate of
incorporation and restated bylaws provide for our board of directors to be divided into three classes serving staggered terms. At each annual meeting of stockholders, directors elected to succeed those directors whose terms expire will be elected
for a three-year term of office. All directors elected to our classified board of directors will serve until the election and qualification of their respective successors or their earlier resignation or removal. The board of
20
directors is authorized to create new directorships and to fill such positions so created and is permitted to specify the class to which any such new position is assigned. The person filling such
position would serve for the term applicable to that class. The board of directors (or its remaining members, even if less than a quorum) is also empowered to fill vacancies on the board of directors occurring for any reason for the remainder of the
term of the class of directors in which the vacancy occurred. Members of the board of directors may only be removed for cause and only by the affirmative vote of 80% of our outstanding voting stock. These provisions are likely to increase the time
required for stockholders to change the composition of the board of directors. For example, in general, at least two annual meetings will be necessary for stockholders to effect a change in a majority of the members of the board of directors. The
provision for a classified board could prevent a party who acquires control of a majority of our outstanding common stock from obtaining control of our board of directors until our second annual stockholders meeting following the date the acquirer
obtains the controlling stock interest. The classified board provision could have the effect of discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us and could increase the likelihood that
incumbent directors will retain their positions.
Advance notice provisions for stockholder proposals.
Our restated
bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors, as well as procedures for including
proposed nominations at special meetings at which directors are to be elected. Stockholders at our annual meeting may only consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of
our board or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given to our secretary timely written notice, in proper form, of the stockholder’s intention to
bring that business before the meeting, and who has complied with the procedures and requirements set forth in the bylaws. Although our bylaws do not give our board of directors the power to approve or disapprove stockholder nominations of
candidates or proposals regarding other business to be conducted at a special or annual meeting, our bylaws may have the effect of precluding the conduct of some business at a meeting if the proper procedures are not followed or may discourage or
defer a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of us.
Special meetings of stockholders.
Special meetings of the stockholders may be called only by our board of directors pursuant to a resolution adopted by a majority of the total number of authorized
directors. Stockholders are not permitted to call a special meeting or to require our board of directors to call a special meeting.
No stockholder action by written consent.
Our restated certificate of incorporation and restated bylaws do not permit our stockholders to act by written consent. As a result, any action to be
effected by our stockholders must be effected at a duly called annual or special meeting of the stockholders.
Super-majority stockholder vote required for certain actions.
The Delaware General Corporation Law provides generally that the
affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless the corporation’s certificate of incorporation or bylaws, as the case may be,
requires a greater percentage. Our restated certificate of incorporation requires the affirmative vote of the holders of at least 80% of our outstanding voting stock to amend or repeal certain provisions of our restated certificate of incorporation.
This 80% stockholder vote would be in addition to any separate class vote that might in the future be required pursuant to the terms of any preferred stock that might then be outstanding. In addition, an 80% vote is also required for any amendment
to, or repeal of, our restated bylaws by the stockholders. Our restated bylaws may be amended or repealed by a vote of a majority of the total number of authorized directors.
Provisions of Delaware Law Governing Business Combinations
We are subject
to the “business combination” provisions of Section 203 of the Delaware General Corporation Law. In general, such provisions prohibit a publicly held Delaware corporation from engaging in any
21
“business combination” transactions with any “interested stockholder” for a period of three years after the date on which the person became an “interested
stockholder,” unless:
|
|
•
|
|
prior to such date, the board of directors approved either the “business combination” or the transaction which resulted in the
“interested stockholder” obtaining such status; or
|
|
|
•
|
|
upon consummation of the transaction which resulted in the stockholder becoming an “interested stockholder,” the “interested
stockholder” owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the
“interested stockholder”) those shares owned by (a) persons who are directors and also officers and (b) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held
subject to the plan will be tendered in a tender or exchange offer; or
|
|
|
•
|
|
at or subsequent to such time the “business combination” is approved by the board of directors and authorized at an annual or special meeting
of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the “interested stockholder.”
|
A “business combination” is defined to include mergers, asset sales and other transactions resulting in financial benefit to a
stockholder. In general, an “interested stockholder” is a person who, together with affiliates and associates, owns 15% or more of a corporation’s voting stock or within three years did own 15% or more of a corporation’s voting
stock. The statute could prohibit or delay mergers or other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us.
Limitations on Liability and Indemnification of Officers and Directors
Our
restated certificate of incorporation limits the liability of our officers and directors to the fullest extent permitted by the Delaware General Corporation Law, and our restated certificate of incorporation and restated bylaws provide that we will
indemnify our officers and directors to the fullest extent permitted by such law. We have also entered into indemnification agreements with our officers and directors and expect to enter into a similar agreement with any new officers and directors.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION
FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons of the registrant pursuant to the foregoing provisions, or
otherwise, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
LEGAL MATTERS
The validity of the common stock offered in this prospectus was passed upon by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., Boston, Massachusetts.
EXPERTS
The consolidated financial statements incorporated in this prospectus by reference from the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2013 have been audited by
Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report, which is incorporated
22
herein by reference (which report expresses an unqualified opinion on the consolidated financial statements and includes an explanatory paragraph relating to the substantial doubt about the
Company’s ability to continue as a going concern). Such financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and file annual, quarterly and current
reports, proxy statements and other information with the SEC. You may read and copy these reports, proxy statements and other information at the SEC’s public reference facilities at 100 F Street, N.E., Room 1580, Washington, D.C.
20549. You can request copies of these documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference facilities. SEC filings are also
available at the SEC’s website at http://www.sec.gov
.
Our common stock is listed on The NASDAQ Capital Market, and you can read and inspect our filings at the offices of the Financial Industry Regulatory Authority at 1735 K Street,
Washington, D.C. 20006.
We have filed a registration statement, of which this prospectus is a part, covering the securities
offered hereby. As allowed by SEC rules, this prospectus does not include all of the information contained in the registration statement and the included exhibits, financial statements and schedules. You are referred to the registration statement,
the included exhibits, financial statements and schedules for further information. This prospectus is qualified in its entirety by such other information.
We also maintain a website at http://www.bg-medicine.com, through which you can access our SEC filings. The information set forth on our website is not part of this prospectus.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can
disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we
filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and the
registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (Commission File
No. 001-33827).
|
|
•
|
|
our Annual Report on Form 10-K for the fiscal year ended December 31, 2013 filed on March 27, 2014;
|
|
|
•
|
|
our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 filed on May 14, 2014;
|
|
|
•
|
|
our Current Reports on Form 8-K filed on January 27, 2014, March 12, 2014, March 20, 2014, April 3,
2014, May 14, 2014 and June 5, 2014 (other than the portions of those reports not deemed to be filed);
|
|
|
•
|
|
the portions of our Definitive Proxy Statement on Schedule 14A filed on April 24, 2014 that are deemed “filed” with the SEC under the
Exchange Act; and
|
|
|
•
|
|
the description of our common stock contained in our Registration Statement on Form 8-A filed on December 3, 2010, including any amendment or
report filed for the purpose of updating such description.
|
23
Any statement contained in a document incorporated or deemed to be incorporated by reference
in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
You may request, orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be
provided to you at no cost, by contacting: Investor Relations, BG Medicine, Inc., 880 Winter Street, Suite 210, Waltham, Massachusetts 02451, (781) 890-1199, email address:
investor@bg-medicine.com.
In
addition, copies of any or all of the documents incorporated herein by reference may be accessed at our website at http://www.bg-medicine.com.
You should rely only on information contained in, or incorporated by reference into, this prospectus and any prospectus supplement. We have not authorized anyone to provide you with information different
from that contained in this prospectus or incorporated by reference in this prospectus.
24
BG MEDICINE, INC.
4,106,071 shares of Common Stock
The date of this prospectus is , 2014
PART II INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, payable by the Company in connection with the registration and sale of the Common Stock being registered. All amounts are estimates except the SEC
registration fee.
|
|
|
|
|
|
|
|
|
Amount
to be paid
($)
|
|
|
SEC registration fee
|
|
$
|
963.32
|
*
|
|
Printing expense
|
|
|
5,000.00
|
|
|
Legal fees and expenses
|
|
|
50,000.00
|
|
|
Accounting fees and expenses
|
|
|
20,000.00
|
|
|
Miscellaneous Fees
|
|
|
2,000.00
|
|
|
Total
|
|
$
|
77,963.32
|
|
Item 14.
Indemnification of Directors and Officers.
Our restated certificate of incorporation and restated bylaws provide that each
person who was or is made a party or is threatened to be made a party to or is otherwise involved (including, without limitation, as a witness) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of
the fact that he or she is or was on or our directors or officers or is or was serving at our request as a director, officer, or trustee of another corporation, or of a partnership, joint venture, trust or other enterprise, including service with
respect to an employee benefit plan, whether the basis of such proceeding is alleged action in an official capacity as a director, officer or trustee or in any other capacity while serving as a director, officer or trustee, shall be indemnified and
held harmless by us to the fullest extent authorized by the Delaware General Corporation Law against all expense, liability and loss (including attorneys’ fees, judgments, fines, ERISA excise taxes or penalties and amounts paid in settlement)
reasonably incurred or suffered by such.
Section 145 of the Delaware General Corporation Law permits a corporation to
indemnify any director or officer of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by
reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, and,
with respect to any criminal action or proceeding, if he or she had no reason to believe his or her conduct was unlawful. In a derivative action (
i.e
., one brought by or on behalf of the corporation), indemnification may be provided only for
expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not
opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was
brought shall determine that the defendant is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
Pursuant to Section 102(b)(7) of the Delaware General Corporation Law, Article NINTH of our restated certificate of incorporation eliminates the liability of a director to us or our stockholders
for monetary damages for such a breach of fiduciary duty as a director, except for liabilities arising:
|
|
•
|
|
from any breach of the director’s duty of loyalty to us or our stockholders;
|
|
|
•
|
|
from acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
|
II-1
|
|
•
|
|
under Section 174 of the Delaware General Corporation Law; and
|
|
|
•
|
|
from any transaction from which the director derived an improper personal benefit.
|
We carry insurance policies insuring our directors and officers against certain liabilities that they may incur in their capacity as
directors and officers. In addition, we have entered into indemnification agreements with our directors and officers.
Any
underwriting agreements that we may enter into will likely provide for the indemnification of us, our controlling persons, our directors and certain of our officers by the underwriters against certain liabilities, including liabilities under the
Securities Act of 1933, as amended.
The foregoing discussion of our certificate of incorporation, bylaws, indemnification
agreements, and Delaware law is not intended to be exhaustive and is qualified in its entirety by such certificate of incorporation, bylaws, indemnification agreements, or law.
Item 15. Recent Sales of Unregistered Securities
Since
January 1, 2011, we offered, issued and/or sold the following securities, which were not registered under the Securities Act of 1933, as amended:
|
|
1.
|
On February 9, 2011, in connection with the closing of our initial public offering, we issued 3,304 shares of the Common Stock to one accredited investor upon the
automatic net exercise of warrants to purchase shares of the Common Stock.
|
|
|
2.
|
On February 9, 2011, in connection with the completion of our initial public offering, we issued an aggregate of 908,651 shares of the Common Stock to 14 existing,
accredited investors upon the automatic conversion of the unpaid principal amount and the accrued but unpaid interest on certain outstanding bridge notes, as of February 9, 2011, based on the initial public offering price of $7.00 per share.
The outstanding bridge notes with an aggregate principal amount of $6.0 million were issued in three $2.0 million tranches in March 2010, September 2010 and November 2010 and carried an interest rate of 12% per year.
|
|
|
3.
|
On July 7, 2011, we issued 51,789 shares of the Common Stock to Legg Mason upon the net exercise of previously issued warrants to purchase shares of the Common
Stock, without any additional consideration received by us. We originally issued the warrants in 2010 in connection with certain private placement transactions.
|
|
|
4.
|
On July 27, 2011, we issued 51,756 shares of the Common Stock to SMALLCAP upon the net exercise of previously issued warrants to purchase shares of the Common
Stock, without any additional consideration received by us. We originally issued the warrants in 2010 in connection with certain private placement transactions.
|
|
|
5.
|
On September 1, 2011, we issued 2,789 shares of the Common Stock to Pieter Muntendam upon the net exercise of previously issued warrants to purchase shares of the
Common Stock.
|
|
|
6.
|
On November 29, 2011, we issued 150,538 shares of the Common Stock to Stelios Papadopoulos upon the net exercise of previously issued warrants to purchase shares
of the Common Stock, without additional consideration received by us. We originally issued the warrants in 2004, 2005, 2006, 2008 and 2010 in connection with certain private placement transactions.
|
|
|
7.
|
On February 10, 2012, we issued to (i) an affiliate of General Electric Capital Corporation a warrant to purchase up to 24,438 shares of the Common Stock and
(ii) Comerica Bank a warrant to purchase up to 12,219 shares of the Common Stock, in each case, at an exercise price equal to $6.82 per share, pursuant to the terms of our loan agreement with these parties.
|
II-2
|
|
8.
|
On May 21, 2012, we issued 25,939 shares of Common Stock to NewcoGen — PE LLC upon the net exercise of previously issued warrants to purchase shares of Common
Stock, without additional consideration received by us. We originally issued the warrants in 2006, 2008 and 2010 in connection with certain private placement transactions.
|
|
|
9.
|
On August 20, 2012, we issued 21,537 shares of Common Stock to NewcoGen — Elan LLC upon the net exercise of previously issued warrants to purchase shares of
Common Stock, without additional consideration received by us. We originally issued the warrants in 2006 and 2010 in connection with certain private placement transactions.
|
|
|
10.
|
On January 24, 2013, we entered into a common stock purchase agreement with Aspire Capital Fund, LLC, or Aspire Capital, which provides that, upon the terms and
subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $12 million of shares of the Company’s Common Stock over the 24-month term of the agreement. Under the agreement, we
issued 132,743 shares of Common Stock to Aspire Capital as commitment shares on that date.
|
|
|
11.
|
On May 8, 2013, we issued an aggregate of 110,401 additional warrants to purchase common stock to an affiliate of General Electric Capital Corporation and to
Comerica Bank exercisable at $1.70 per share and adjusted the warrant price of previously issued warrants to $1.70 per share.
|
These issuances and the issuances of the underlying warrants referenced above were made in reliance on Section 4(a)(2) of the Securities Act, or Regulation D promulgated thereunder, as sales not
involving a public offering. No underwriters were involved in the foregoing sales of securities. The recipients of securities in each of the above-referenced transactions represented their intentions to acquire the securities for investment purposes
only and not with a view to, or for sale in connection with, any distribution thereof and appropriate legends were affixed to the instruments representing such securities issued in such transactions. All recipients either received adequate
information about us or had, through their relationship with us, adequate access to such information.
Item 16. Exhibits and Financial
Statement Schedules.
See Exhibit Index following the signature page to this Registration Statement.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in
which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any
prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or
events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the
estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum
aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration
statement;
II-3
(2) That, for the purpose of determining any liability under the Securities Act of 1933,
each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain
unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of
1933 to any purchaser: each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A,
shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration
statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first
use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers, and
controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the
Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the
registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the
matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such
issue.
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Post-Effective Amendment No. 1 to
the Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Waltham, Massachusetts, on July 7, 2014.
|
|
|
|
|
BG MEDICINE, INC.
|
|
|
|
|
By
|
|
/s/ Paul R. Sohmer, M.D.
|
|
|
|
Paul R. Sohmer, M.D.
President and Chief Executive Officer
|
POWER OF ATTORNEY
We, the undersigned officers and directors of BG Medicine, Inc., hereby severally constitute and appoint Paul R. Sohmer, M.D., and Stephen P. Hall, and each of them singly (with full power to each of them
to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution in each of them for him and in his name, place and stead, and in any and all capacities, to sign any and all amendments (including
post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, and generally to do all such things in our name and
behalf in our capacities as officers and directors to enable BG Medicine, Inc. to comply with the provisions of the Securities Act of 1933, as amended, and all requirements of the Securities and Exchange Commission, hereby ratifying and confirming
our signatures as they may be signed by our said attorneys, or any of them, to said registration statement and any and all amendments thereto.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed below by the following persons in the capacities and on the dates indicated.
|
|
|
|
|
|
|
|
|
Signatures
|
|
Title
|
|
Date
|
|
|
|
|
|
|
By:
|
|
/s/ Paul R. Sohmer, M.D.
Paul R. Sohmer, M.D.
|
|
President and Chief Executive Officer (principal executive officer) and Director
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Stephen P. Hall
Stephen P. Hall
|
|
Executive Vice President, Chief Financial Officer and Treasurer (principal financial officer and principal accounting officer)
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Noubar B. Afeyan, Ph.D.
Noubar B. Afeyan, Ph.D.
|
|
Director
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Harrison M. Bains
Harrison M. Bains
|
|
Director
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Stéphane Bancel
Stéphane Bancel
|
|
Chairman of the Board of Directors
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Timothy Harris, Ph.D., D.Sc.
Timothy Harris, Ph.D., D.Sc.
|
|
Director
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Stelios Papadopoulos, Ph.D.
Stelios Papadopoulos, Ph.D.
|
|
Director
|
|
July 7, 2014
|
|
|
|
|
|
|
By:
|
|
/s/ Brian Posner
Brian Posner
|
|
Director
|
|
July 7, 2014
|
II-5
EXHIBIT INDEX
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference
herein from
Form or
Schedule
|
|
Filing Date
|
|
SEC File/
Reg. Number
|
|
3.1
|
|
Restated Certificate of Incorporation of the Registrant
|
|
|
|
Form 8-K
(Exhibit 3.1)
|
|
2/11/11
|
|
001-33827
|
|
|
|
|
|
|
|
|
3.2
|
|
Restated Bylaws of the Registrant
|
|
|
|
Form 8-K
(Exhibit 3.2)
|
|
2/11/11
|
|
001-33827
|
|
|
|
|
|
|
|
|
4.1
|
|
Form of Common Stock Certificate
|
|
|
|
Amendment No. 5 to
Form S-1
(Exhibit 4.1)
|
|
11/22/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.2
|
|
Fourth Amended and Restated Investor Rights Agreement, dated as of July 10, 2008
|
|
|
|
Form S-1
(Exhibit 4.2)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.3
|
|
Form of Common Stock Warrant issued to General Electric Capital Corporation
|
|
|
|
Form S-1
(Exhibit 4.4)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.4
|
|
Form of Common Stock Bridge Financing Warrant, together with a schedule of warrant holders
|
|
|
|
Form S-1
(Exhibit 4.5)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.5
|
|
Warrant issued to Silicon Valley Bank, dated November 9, 2007
|
|
|
|
Form S-1
(Exhibit 4.6)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.6
|
|
Warrant issued to Silicon Valley Bank, dated March 28, 2008
|
|
|
|
Form S-1
(Exhibit 4.7)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.7
|
|
Form of 2010 Common Stock Bridge Warrant, together with a schedule of warrant holders
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 4.8)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
4.8.1
|
|
Warrant issued to GE Capital Equity Investments, Inc., dated as of February 10, 2012
|
|
|
|
Form 8-K
(Exhibit 10.5)
|
|
2/16/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
4.8.2
|
|
Amendment No. 1 to Warrant by and between the Registrant and GE Capital Equity Investments, Inc., dated as of May 8, 2013
|
|
|
|
Form 8-K
(Exhibit 10.2)
|
|
5/9/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
4.9.1
|
|
Warrant issued to Comerica Bank, dated as of February 10, 2012
|
|
|
|
Form 8-K
(Exhibit 10.6)
|
|
2/16/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
4.9.2
|
|
Amendment No. 1 to Warrant by and between the Registrant and Comerica Bank, dated as of May 8, 2013
|
|
|
|
Form 8-K
(Exhibit 10.3)
|
|
5/9/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
4.10
|
|
Form of Senior Indenture
|
|
|
|
Form S-3
(Exhibit 4.8)
|
|
5/25/12
|
|
333-181699
|
|
|
|
|
|
|
|
|
4.11
|
|
Form of Subordinated Indenture
|
|
|
|
Form S-3
(Exhibit 4.9)
|
|
5/25/12
|
|
333-181699
|
|
|
|
|
|
|
|
|
4.12
|
|
Registration Rights Agreement, dated as of January 24, 2013, by and between the Registrant and Aspire Capital Fund, LLC
|
|
|
|
Form 8-K
(Exhibit 4.1)
|
|
1/24/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
5.1
|
|
Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C.
|
|
|
|
Form S-1
(Exhibit 5.1)
|
|
4/29/13
|
|
333-188211
|
|
|
|
|
|
|
|
|
|
|
Lease Agreements
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1
|
|
Second Amendment to Lease, Sublease, and Assignment, Assumption and Amendment of Sublease by and between the Registrant and 610 Lincoln LLC, dated as of May 19,
2009
|
|
|
|
Form S-1
(Exhibit 10.1)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.1.1
|
|
Lease by and between the Registrant and Waltham Winter Street 880 LP, dated June 10, 2013
|
|
|
|
Form 10-Q
(Exhibit 10.6)
|
|
8/9/13
|
|
001-33827
|
II-6
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference
herein from
Form or
Schedule
|
|
Filing Date
|
|
SEC File/
Reg. Number
|
|
10.2
|
|
Sublease Agreement by and between the Registrant and GPC Biotech, dated as of April 14, 2005, as amended
|
|
|
|
Form S-1
(Exhibit 10.2)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
|
|
Loan Agreements
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3.1
|
|
Loan and Security Agreement by and among the Registrant, General Electric Capital Corporation as Agent, the Lenders and the Guarantors, dated as of February 10,
2012
|
|
|
|
Form 8-K
(Exhibit 10.1)
|
|
2/16/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.3.2
|
|
First Amendment to Loan and Security Agreement by and between the Registrant and General Electric Capital Corporation, dated May 8, 2013
|
|
|
|
Form 8-K
(Exhibit 10.1)
|
|
5/9/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.4
|
|
Promissory Note issued by the Registrant to General Electric Capital Corporation, dated as of February 10, 2012
|
|
|
|
Form 8-K
(Exhibit 10.2)
|
|
2/16/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.5
|
|
Promissory Note issued by the Registrant to Comerica Bank, dated as of February 10, 2012
|
|
|
|
Form 8-K
(Exhibit 10.3)
|
|
2/16/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.6
|
|
Pledge Agreement by and between the Registrant and General Electric Capital Corporation, dated as of February 10, 2012
|
|
|
|
Form 8-K
(Exhibit 10.4)
|
|
2/16/12
|
|
001-33827
|
|
|
|
|
|
|
|
Agreements with Respect to Collaborations, Licenses, Research and Development
|
|
|
|
|
|
|
|
|
|
|
10.7.1+
|
|
License and Distribution Agreement by and between the Registrant and Abbott Laboratories, dated as of November 11, 2009
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.4)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.7.2+
|
|
First Amendment to License and Distribution Agreement by and between the Registrant and Abbott Laboratories, dated as of February 3, 2010
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.4.1)
|
|
3/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.8.1+
|
|
Product License and Collaboration Agreement, Licensing Addendum No. 1 and Licensing Addendum No. 2 by and between the Registrant and ACS Biomarker B.V., dated as of May 4,
2007
|
|
|
|
Amendment No. 1 to
Form S-1
(Exhibit 10.5)
|
|
2/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.8.2+
|
|
Sublicense Agreement between the Registrant and ACS Biomarker B.V. dated July 11, 2012
|
|
|
|
Form 10-Q
(Exhibit 10.2)
|
|
11/13/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.9+
|
|
Strategic Agreement by and between the Registrant and Humana Inc., dated as of May 25, 2007, as amended May 12, 2008 and August 12, 2009
|
|
|
|
Amendment No. 1 to
Form S-1
(Exhibit 10.6)
|
|
2/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.10+
|
|
Participation Agreement by and between the Registrant and Philips Medical Systems Nederland B.V., dated as of December 22, 2006
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.8)
|
|
3/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.11.1+
|
|
Participation Agreement by and between the Registrant and AstraZeneca AB, dated as of November 24, 2006, as amended August 20, 2007
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.9)
|
|
3/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.11.2+
|
|
Amendment to the Participation Agreement by and between the Registrant and AstraZeneca AB, dated November 23, 2010
|
|
|
|
Form 10-K
(Exhibit 10.11.2)
|
|
3/30/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.12.1+
|
|
Participation Agreement by and between the Registrant and Merck & Co., Inc., dated as of July 28, 2006, as amended October 10, 2006 and June 14, 2007
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.10)
|
|
3/12/10
|
|
333-164574
|
II-7
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference
herein from
Form or
Schedule
|
|
Filing Date
|
|
SEC File/
Reg. Number
|
|
10.12.2+
|
|
Amendment to the Participation Agreement by and between the Registrant and Merck Sharp & Dohme Corp. (formerly Merck & Co., Inc.), dated as of May 28, 2010
|
|
|
|
Form 10-K
(Exhibit 10.12.2)
|
|
3/30/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.13+
|
|
Participation Agreement by and between the Registrant and Abbott Laboratories, dated as of March 28, 2008
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.11)
|
|
3/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.14.1
|
|
Participation Agreement by and between the Registrant and Takeda Pharmaceutical Company Limited, dated as of March 31, 2008
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.12)
|
|
3/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.14.2+
|
|
Amendment to the Participation Agreement by and between the Registrant and Takeda Pharmaceutical Company Limited, dated as of January 5, 2011
|
|
|
|
Form 10-K
(Exhibit 10.14.2)
|
|
3/30/12
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.15+
|
|
Supply Agreement by and between the Registrant and Corgenix Medical Corporation, dated as of March 20, 2009
|
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 10.13)
|
|
3/12/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.16+
|
|
License and Supply Agreement by and between the Registrant and Laboratory Corporation of America Holdings, dated as of May 13, 2010
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.14)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.17+
|
|
License and Distribution Agreement by and between the Registrant and Inverness Medical Innovations, Inc. (predecessor to Alere Inc.), dated as of March 19, 2010
|
|
|
|
Amendment No. 4 to
Form S-1
(Exhibit 10.15)
|
|
11/8/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.18+
|
|
License and Distribution Agreement by and between the Registrant and bioMérieux SA, dated as of May 29, 2010
|
|
|
|
Amendment No. 4 to
Form S-1
(Exhibit 10.16)
|
|
11/8/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.19+
|
|
License and Distribution Agreement by and between the Registrant and Siemens Healthcare Diagnostics Inc., dated as of December 14, 2010
|
|
|
|
Amendment No. 9 to
Form S-1
(Exhibit 10.17)
|
|
2/3/11
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.20+
|
|
Supply Agreement by and between the Registrant and Health Diagnostic Laboratory, Inc., dated as of March 15, 2011
|
|
|
|
Amendment No. 1 to
Form 10-Q
(Exhibit 10.1)
|
|
10/4/11
|
|
001-33827
|
|
|
|
|
|
|
|
|
|
|
Agreements with Executive Officers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.21*
|
|
Amended and Restated Change of Control Cash Severance Agreement by and between the Registrant and Pieter Muntendam, dated as of August 1, 2007
|
|
|
|
Form S-1
(Exhibit 10.11)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.22*
|
|
Amended and Restated Change of Control Cash Severance Agreement by and between the Registrant and Neal Gordon, dated as of December 22, 2008
|
|
|
|
Form S-1
(Exhibit 10.12)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.23*
|
|
Amended and Restated Cash Severance Agreement by and between the Registrant and Aram Adourian, dated as of August 1, 2007
|
|
|
|
Amendment No. 4 to
Form S-1
(Exhibit 10.23)
|
|
11/8/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.24*
|
|
Amended and Restated Cash Severance Agreement by and between the Registrant and Anastasia Rader, dated as of August 1, 2007
|
|
|
|
Amendment No. 4 to
Form S-1
(Exhibit 10.24)
|
|
11/8/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.25*
|
|
Form of Indemnification Agreement between the Registrant and its Directors and Executive Officers
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.21)
|
|
8/31/10
|
|
333-164574
|
II-8
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference
herein from
Form or
Schedule
|
|
Filing Date
|
|
SEC File/
Reg. Number
|
|
10.26*
|
|
Severance Agreement and Release by and between the Registrant and William Densel, dated as of March 26, 2013
|
|
|
|
Form 10-Q
(Exhibit 10.1)
|
|
5/10/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.27*
|
|
Severance Agreement and Release by and between the Registrant and Neal Gordon, effective as of January 10, 2013
|
|
|
|
Form 10-K
(Exhibit 10.33)
|
|
3/18/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.28*
|
|
Consulting Agreement by and between the Registrant and Neal Gordon, effective as of January 1, 2013
|
|
|
|
Form 10-K
(Exhibit 10.34)
|
|
3/18/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.29*
|
|
Side Letter Agreement by and between the Registrant and Aram Adourian, effective as of October 4, 2012
|
|
|
|
Form 10-K
(Exhibit 10.35)
|
|
3/18/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.30*
|
|
Separation and Release Agreement by and between the Registrant and Charles H. Abdalian, Jr., effective as of November 13, 2013
|
|
|
|
Form 10-K
(Exhibit 10.30)
|
|
3/27/14
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.31*
|
|
Salary Modification Agreement by and between the Registrant and Anastasia Rader, effective as of January 7, 2013
|
|
|
|
Form 10-K
(Exhibit 10.37)
|
|
3/18/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.32*
|
|
Employment Agreement by and between the Registrant and Paul Sohmer, M.D., dated May 8, 2013
|
|
|
|
Form 10-Q
(Exhibit 10.4)
|
|
8/9/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.33*
|
|
Employment Agreement by and between the Registrant and Stephen P. Hall, dated November 13, 2013
|
|
|
|
Form 10-K
(Exhibit 10.33)
|
|
3/27/14
|
|
001-33827
|
|
|
|
|
|
|
|
|
|
|
Equity Compensation Plans
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.34*
|
|
2001 Stock Option and Incentive Plan, as amended
|
|
|
|
Form S-1
(Exhibit 10.15)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.35*
|
|
Form of Incentive Stock Option Agreement under the 2001 Stock Option and Incentive Plan
|
|
|
|
Form S-1
(Exhibit 10.16)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.36*
|
|
Form of Non-Qualified Stock Option Agreement under the 2001 Stock Option and Incentive Plan
|
|
|
|
Form S-1
(Exhibit 10.17)
|
|
1/29/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.37*
|
|
Non-Qualified Stock Option Agreement by and between the Registrant and Paul Sohmer, M.D., dated May 8, 2013
|
|
|
|
Form 10-Q
(Exhibit 10.5)
|
|
8/9/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
10.38*
|
|
2010 Employee, Director and Consultant Stock Plan
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.25)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.39*
|
|
Form of Stock Option Agreement under the 2010 Employee, Director and Consultant Stock Plan
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.26)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.40*
|
|
Form of Restricted Stock Agreement under the 2010 Employee, Director and Consultant Stock Plan
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.27)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.41*
|
|
2010 Employee Stock Purchase Plan
|
|
|
|
Amendment No. 3 to
Form S-1
(Exhibit 10.28)
|
|
8/31/10
|
|
333-164574
|
|
|
|
|
|
|
|
|
10.42*
|
|
Non-Employee Director Compensation Policy
|
|
|
|
Form 10-K
(Exhibit 10.36)
|
|
3/30/12
|
|
001-33827
|
II-9
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference
herein from
Form or
Schedule
|
|
Filing Date
|
|
SEC File/
Reg. Number
|
|
|
|
Agreements with Investors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.43
|
|
Common Stock Purchase Agreement, dated as of January 24, 2013, by and between the Registrant and Aspire Capital Fund, LLC
|
|
|
|
Form 8-K
(Exhibit 10.1)
|
|
1/24/13
|
|
001-33827
|
|
|
|
|
|
|
|
|
|
|
Other Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21.1
|
|
Subsidiaries of the Registrant
|
|
|
|
Form 10-K
|
|
3/27/14
|
|
001-33827
|
|
|
|
|
|
|
|
|
23.1
|
|
Consent of Deloitte & Touche LLP
|
|
X
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.2
|
|
Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. (included in Exhibit 5.1 to the initial registration statement)
|
|
|
|
Form S-1
(Exhibit 5.1)
|
|
4/29/13
|
|
333-188211
|
|
|
|
|
|
|
|
|
24.1
|
|
Powers of Attorney (included on the signature page hereto)
|
|
X
|
|
|
|
|
|
|
|
(*)
|
Management contract or compensatory plan or arrangement.
|
|
(+)
|
Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions of this Exhibit, which portions have been omitted and filed
separately with the Securities and Exchange Commission as part of an application for confidential treatment pursuant to the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended, as applicable.
|
II-10
BG Medicine (CE) (USOTC:BGMD)
Historical Stock Chart
From Oct 2024 to Nov 2024
BG Medicine (CE) (USOTC:BGMD)
Historical Stock Chart
From Nov 2023 to Nov 2024