Report of Foreign Issuer Pursuant to Rule 13a-16 or 15d-16 (6-k)
November 01 2022 - 7:22AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN
PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of November 2022.
Commission File Number: 001-39071
ADC Therapeutics SA
(Exact name of registrant as specified in its
charter)
Biopôle
Route de la Corniche
3B
1066 Epalinges
Switzerland
(Address of principal executive office)
Indicate by check
mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
Indicate by check mark if the registrant
is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant
is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐

ADC Therapeutics Appoints Kristen Harrington-Smith
as Chief Commercial Officer and Peter Graham as Chief Legal Officer
LAUSANNE, Switzerland,
November 1, 2022 – ADC Therapeutics SA (NYSE: ADCT) today announced the appointment of Kristen Harrington-Smith as Chief Commercial
Officer, effective November 17, 2022. The Company also announced the appointment of Peter
Graham as Chief Legal Officer, effective November 1, 2022. Both Ms. Harrington-Smith and Mr. Graham will serve on the Company’s
leadership team.
Kristen Harrington-Smith appointed
as Chief Commercial Officer
Ms.
Harrington-Smith is a seasoned leader with over 20 years of experience in the pharmaceutical industry. Most recently, she has served
as Chief Commercial Officer of Immunogen where she has been responsible for building the commercial organization and preparing for the
launch of its first commercial product. Prior to that, she served as Vice President and Head, US Hematology at Novartis Pharmaceuticals,
where she led the teams responsible for a portfolio of therapies in both malignant and non-malignant hematologic diseases including
diffuse large B-cell lymphoma (DLBCL), acute myeloid leukemia, chronic myeloid leukemia, and myelodysplastic syndrome. In previous roles
of increasing seniority at Novartis, Ms. Harrington-Smith was Vice President and Head, US CAR-T, responsible for the commercial launch
of Kymriah®, the first CAR-T cell therapy for both DLBCL and acute lymphoblastic leukemia, building the management, sales, marketing,
and market access teams, and supporting the launch of Gilenya® for the treatment of multiple sclerosis. Ms. Harrington-Smith received
an MBA from the Kenan-Flagler Business School at the University of North Carolina and a
BA from Williams College.
Peter Graham appointed as Chief Legal
Officer
Mr. Graham is a
senior legal executive with over 25 years of legal, compliance and executive management experience primarily in publicly traded biotechnology,
pharmaceutical and medical device companies. From 2015 until its sale to Halozyme Therapeutics, Inc. in 2022, Mr. Graham served as Executive
Vice President, General Counsel, Chief Compliance Officer, Human Resources and Secretary for Antares Pharma, Inc., a commercial-stage
specialty pharmaceutical and combination product company. Previously, he served as Executive Vice President, General Counsel, Chief Compliance
Officer and Global Human Resources at Delcath Systems, Inc., a combination products company focused on cancers of the liver. Earlier
in his career, Mr. Graham held various legal and compliance executive leadership roles at ACIST Medical Systems, Inc., E-Z-EM, Inc.,
and AngioDynamics, Inc. Mr. Graham received his J.D. from Yeshiva University’s Benjamin N. Cardozo School of Law and his BA in
Political Science from the University of Wisconsin-Madison.
About ADC Therapeutics
ADC Therapeutics
(NYSE: ADCT) is a commercial-stage biotechnology company improving the lives of those affected by cancer with its next-generation, targeted
antibody drug conjugates (ADCs). The Company is advancing its proprietary PBD-based ADC technology to transform the treatment paradigm
for patients with hematologic malignancies and solid tumors.
ADC Therapeutics’
CD19-directed ADC ZYNLONTA (loncastuximab tesirine-lpyl) is approved by the FDA for the treatment of relapsed or refractory diffuse large
b-cell lymphoma after two or more lines of systemic therapy. ZYNLONTA is also in development in combination with other agents. Cami (camidanlumab
tesirine) is being evaluated in a pivotal Phase 2 trial for relapsed or refractory Hodgkin lymphoma and in a Phase 1b clinical trial
for various advanced solid tumors. In addition to ZYNLONTA and Cami, ADC Therapeutics has multiple ADCs in ongoing clinical and preclinical
development.
ADC Therapeutics
is based in Lausanne (Biopôle), Switzerland and has operations in London, the San Francisco Bay Area and New Jersey.
ZYNLONTA® is a registered trademark
of ADC Therapeutics SA.
INCORPORATION
BY REFERENCE
This Report on
Form 6-K shall be deemed to be incorporated by reference into the registration statements on Form F-3 (Registration Nos. 333-256807, 333-267293
and 333-267295) of ADC Therapeutics SA and to be a part thereof from the date on which this report is filed, to the extent not superseded
by documents or reports subsequently filed or furnished.
SIGNATURE
Pursuant to the
requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
| |
ADC Therapeutics SA |
| Date: November 1, 2022 |
|
| |
|
| |
By: |
/s/ Michael Forer |
| |
Name: |
Michael Forer |
| |
Title: |
Executive Vice President and General Counsel |
ADC Therapeutics (NYSE:ADCT)
Historical Stock Chart
From Aug 2024 to Sep 2024
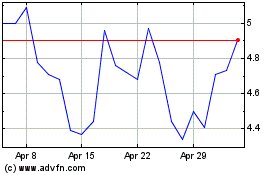
ADC Therapeutics (NYSE:ADCT)
Historical Stock Chart
From Sep 2023 to Sep 2024