Revance Says FDA Accepts sBLA for Daxxify to Treat Cervical Dystonia
January 06 2023 - 6:49PM
Dow Jones News
By Stephen Nakrosis
Revance Therapeutics, Inc. said the U.S. Food and Drug
Administration will review the company's supplemental biologics
license application for Daxxify as a treatment for cervical
dystonia in adults.
The company said Daxxify, or DaxibotulinumtoxinA-lanm, is an
injection to treat cervical dystonia, a chronic and debilitating
neurologic condition affecting neck muscles.
Mark J. Foley, the company's chief executive, said "positive
results from our ASPEN Phase 3 clinical program demonstrate the
potential of Daxxify to bring sustained symptom relief to cervical
dystonia patients, along with the potential for reduced frequency
of annual injections."
The company said Daxxify was approved by the FDA for temporary
improvement of moderate to severe glabellar lines in adults.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
January 06, 2023 18:34 ET (23:34 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
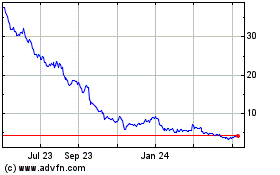
Revance Therapeutics (NASDAQ:RVNC)
Historical Stock Chart
From Mar 2024 to Apr 2024
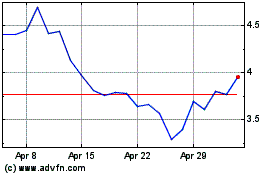
Revance Therapeutics (NASDAQ:RVNC)
Historical Stock Chart
From Apr 2023 to Apr 2024