As
filed with the Securities and Exchange Commission on July 17, 2023
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933
NanoVibronix,
Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
3842 |
|
01-0801232 |
(State
or other jurisdiction
of
incorporation or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
525
Executive Blvd.
Elmsford,
New York 10523
(914)
233-3004
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Brian
Murphy
Chief
Executive Officer
NanoVibronix,
Inc.
525
Executive Blvd.
Elmsford,
New York
(914)
233-3004
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Rick
A. Werner, Esq.
Jayun
Koo, Esq.
Haynes
and Boone, LLP
30
Rockefeller Plaza, 26th Floor
New
York, New York 10112
(212)
659-7300
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this registration statement is declared effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer |
☐ |
|
Accelerated
filer |
☐ |
| |
|
|
|
|
| Non-accelerated
filer |
☒ |
|
Smaller
reporting company |
☒ |
| |
|
|
|
|
| |
|
|
Emerging
growth company |
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date
as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does
it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject
to Completion, dated July 17, 2023
Preliminary
Prospectus

NanoVibronix,
Inc.
Up
to Shares of Common Stock
Prefunded
Warrants to purchase up to
Shares of Common Stock
Common
Warrants to purchase up to Shares of Common Stock
Shares
of Common Stock underlying Prefunded Warrants and Common Warrants
Placement
Agent Warrants to Purchase up to Shares of Common Stock
Shares
of Common Stock Underlying the Placement Agent Warrants
We
are offering shares of common stock, together with warrants to
purchase shares of common stock, each a Common Warrant, at an
assumed combined public offering price of $ per share and Common Warrant, which
is equal to the closing price per share of our common stock on The Nasdaq Capital Market (“Nasdaq”), on ,
2023 (and the shares issuable from time to time upon exercise of the Common Warrants), pursuant to this prospectus. The shares of common
stock and Common Warrants will be separately issued but must be purchased together in this offering. Each share of common stock
is being offered together with a Common Warrant to purchase share of common stock. Each Common Warrant will have an exercise price
of $ per share, (representing
% of the price at which a share of common stock and accompanying Common Warrant are sold to the public in this offering), will be exercisable
upon issuance and will expire years from the date of issuance.
We
are also offering prefunded warrants, or Prefunded Warrants, to purchase up to an aggregate of
shares of common stock to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together
with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of
our outstanding common stock following the consummation of this offering in lieu of the shares of our common stock that would result
in ownership in excess of 4.99% (or, at the election of the purchaser, 9.99%). Each Prefunded Warrant will be exercisable for
one share of common stock at an exercise price of $0.0001 per share. Each Prefunded Warrant is being offered together with
the same Common Warrant described above being offered with each share of common stock. The assumed combined public offering price
for each such Prefunded Warrant, together with the Common Warrant, is $
which is equal to the closing price of our common stock on Nasdaq on , 2023, less
the $0.0001 per share exercise price of each such Prefunded Warrant. Each Prefunded Warrant will be exercisable
upon issuance and will expire when exercised in full. The Prefunded Warrants and Common Warrants are immediately separable and
will be issued separately in this offering, but must be purchased together in this offering. For each Prefunded Warrant we sell,
the number of shares of common stock we are offering will be decreased on a one-for-one basis. This offering also relates to the shares
of common stock issuable upon the exercise of the Prefunded Warrants and the Common Warrants.
This
offering will terminate on , unless we decide to terminate the offering (which
we may do at any time in our discretion) prior to that date. We will have one closing for all the securities purchased in this offering.
The combined public offering price per share (or Prefunded Warrant) and Common Warrant will be fixed for the duration of this
offering.
We
have engaged , or the placement agent to act as our exclusive placement agent in connection with this offering. The placement agent has
agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is
not purchasing or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale
of any specific number or dollar amount of securities. We have agreed to pay to the placement agent the placement agent fees set forth
in the table below, which assumes that we sell all of the securities offered by this prospectus. There is no arrangement for funds to
be received in escrow, trust or similar arrangement. There is no minimum offering requirement. We will bear all costs associated with
the offering. See “Plan of Distribution” on page 17 of this prospectus for more information regarding these arrangements.
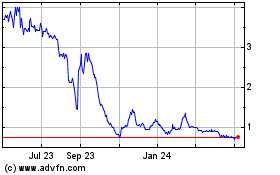
Our
common stock is listed on Nasdaq under the symbol “NAOV.” The closing price of our common stock on Nasdaq on July 14,
2023 was $3.35 per share.
All share, Common Warrant, and Prefunded Warrant
numbers are based on an assumed combined public offering price of $ per share and the accompanying Common Warrant and $ per Prefunded
Warrant and the accompanying Common Warrant. The actual combined public offering price per share and Common Warrant and the actual combined
public offering price per Prefunded Warrant and Common Warrant will be determined through negotiation among us, the placement agent
and the investors in the offering based on market conditions at the time of pricing, and may be at a discount to the current market price
of our common stock. Therefore, the recent market price per share of common stock used throughout this prospectus as an assumed combined
public offering price may not be indicative of the final offering price. There is no established trading market for the Prefunded
Warrants or the Common Warrants, and we do not expect a market to develop. We do not intend to apply for a listing of the Prefunded
Warrants or the Common Warrants on any securities exchange or other nationally recognized trading system. Without an active trading
market, the liquidity of the Prefunded Warrants and the Common Warrants will be limited.
You
should read this prospectus, together with additional information described under the headings “Information Incorporated by Reference”
and “Where You Can Find More Information,” carefully before you invest in any of our securities.
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page 5 of this prospectus for a discussion
of risks that should be considered in connection with an investment in our securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed
upon the adequacy or the accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| | |
Per Share and
Accompanying
Common
Warrant | | |
Per Pre-
Funded
Warrant and
Accompanying
Common
Warrant | | |
Total | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | |
| Placement agent’s fees(1) | |
$ | | | |
$ | | | |
$ | | |
| Proceeds to us, before expenses(2) | |
$ | | | |
$ | | | |
$ | | |
(1)
We have also agreed to pay the placement agent a management fee of % of the aggregate gross proceeds raised
in this offering and to reimburse the placement agent for certain of its offering-related expenses. In addition, we have agreed to issue
the placement agent or its designees warrants, or the placement agent warrants, to purchase a number of shares of common stock
equal to % of the shares of common stock sold in this offering (including the shares of common stock issuable
upon the exercise of the Prefunded Warrants), at an exercise price of $ per share, which represents
% of the public offering price per share of common stock and accompanying Common Warrant. See “Plan
of Distribution” for a description of the compensation to be received by the placement agent.
(2)
Because there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual
public offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less
than the total maximum offering amounts set forth above. For more information, see “Plan of Distribution”.
Delivery
of the securities offered hereby is expected to be made on or about , 2023, subject to satisfaction of customary closing conditions.
The
date of this prospectus is , 2023
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
The
registration statement of which this prospectus forms a part that we filed with the Securities and Exchange Commission (the “SEC”)
includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with
the SEC, together with the additional information described under the headings “Where You Can Find Additional Information”
and “Information Incorporated by Reference” before making your investment decision. You should rely only on the information
provided in or incorporated by reference in this prospectus, in any prospectus supplement or in a related free writing prospectus, or
documents to which we otherwise refer you. In addition, this prospectus contains summaries of certain provisions contained in some of
the documents described herein, but reference is made to the actual documents for complete information.
This
prospectus includes important information about us, the securities being offered and other information you should know before investing
in our securities. You should not assume that the information contained in this prospectus is accurate on any date subsequent to the
date set forth on the front cover of this prospectus, even though this prospectus is delivered or securities are sold or otherwise disposed
of on a later date. It is important for you to read and consider all information contained in this prospectus in making your investment
decision. All of the summaries in this prospectus are qualified in their entirety by the actual documents. Copies of some of the documents
referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which
this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find
Additional Information.”
We
have not, and the placement agent has not, authorized anyone to provide any information or to make any representations other than those
contained or incorporated by reference in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which
we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that
others may give you. The information contained in this prospectus or incorporated by reference in this prospectus or contained in any
applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of our securities.
Our business, financial condition, results of operations and prospects may have changed since that date.
For
investors outside the United States: We have not, and the placement agent has not, done anything that would permit this offering or possession
or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons
outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating
to, the offering of the securities and the distribution of this prospectus outside the United States.
Unless
otherwise indicated, information contained in this prospectus or incorporated by reference in this prospectus concerning our industry,
including our general expectations and market opportunity, is based on information from our own management estimates and research, as
well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are
derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which
we believe to be reasonable. In addition, assumptions and estimates of our and our industry’s future performance are necessarily
uncertain due to a variety of factors, including those described in “Risk Factors” beginning on page 5 of this prospectus.
These and other factors could cause our future performance to differ materially from our assumptions and estimates.
This
prospectus is an offer to sell only the securities offered hereby, and only under circumstances and in jurisdictions where it is lawful
to do so. We are not, and the placement agent is not, making an offer to sell these securities in any state or jurisdiction where the
offer or sale is not permitted.
PROSPECTUS
SUMMARY
This
summary provides an overview of selected information contained in other parts of this prospectus or incorporated by reference into this
prospectus from our filings with the SEC and does not contain all of the information you should consider before investing in our securities.
You should carefully read the prospectus and the information incorporated by reference herein in their entirety before investing in our
securities, including the information discussed under “Risk Factors” and our financial statements and notes thereto that
are included elsewhere in this prospectus or incorporated herein by reference. Some of the statements in this prospectus constitute forward-looking
statements that involve risks and uncertainties. See information set forth under the section “Special Note Regarding Forward-Looking
Statements.” As used in this prospectus, unless the context otherwise indicates, the terms “we,” “our,”
“us,” or the “Company” refer to NanoVibronix, Inc., a Delaware corporation, and its subsidiaries taken as a whole.
Overview
We
are a medical device company focusing on noninvasive biological response-activating devices that target wound healing and pain therapy
and can be administered at home, without the assistance of medical professionals. Our primary products, which are in various stages of
clinical and market development, currently consist of:
| |
● |
UroShield™,
an ultrasound-based product that is designed to prevent bacterial colonization and biofilm in urinary catheters, increase antibiotic
efficacy and decrease pain and discomfort associated with urinary catheter use. |
| |
|
|
| |
● |
PainShield™,
a patch-based therapeutic ultrasound technology to treat pain, muscle spasm and joint contractures by delivering a localized ultrasound
effect to treat pain and induce soft tissue healing in a targeted area; and |
| |
|
|
| |
● |
WoundShield™,
a patch-based therapeutic ultrasound device intended to facilitate tissue regeneration and wound healing by using ultrasound to increase
local capillary perfusion and tissue oxygenation. |
Each
of our PainShield, UroShield, and WoundShield products employs a small, disposable transducer that transmits low frequency, low intensity
ultrasound acoustic waves that seek to repair and regenerate tissue, musculoskeletal and vascular structures, and decrease biofilm formation
on urinary catheters and associated urinary tract infections. Through their size, effectiveness and ease of use, these products are intended
to eliminate the need for technicians and medical personnel to manually administer ultrasound treatment through large transducers, thereby
promoting patient independence and enabling more cost-effective home-based care.
PainShield
is currently cleared for marketing in the United States by the U.S. Food and Drug Administration although to date there has not been
a significant sales and marketing effort. All three of our products have CE Mark approval in the European Union, and a certificate allowing
us to sell PainShield, UroShield and WoundShield in Israel. We are able to sell PainShield, UroShield and WoundShield in India and Ecuador
based on our CE Mark. We have consummated sales of PainShield and UroShield in the relevant markets, although to date sales have been
minimal; WoundShield has not generated significant revenue to date. Outside of the United States we generally apply, through our distributor,
for approval in a particular country for a particular product only when we have a distributor in place with respect to such product.
Intellectual
Property – Patents
We
seek patent protection for our inventions not only to differentiate our products and technologies, but also to develop opportunities
for licensing and securing our rights to profits therefrom. With the aim of optimizing commercial and regulatory success, our proprietary
technology and innovative applications thereof are protected by a variety of patent claims. We believe that our granted patents and pending
applications collectively protect our technology, both in terms of our existing products, as well as our anticipated pipeline of new
offerings.
Our
patent portfolio includes at least the following issued patents, as well as a number of corresponding foreign patents in relevant jurisdictions:
(1) U.S. Patent No. 7,393,501 to “Method, Apparatus and System for Treating Biofilms Associated With Catheters” (expiring
on December 19, 2023); (2) U.S. Patent No. 7,829,029 to “Acoustic Add-On Device for Biofilm Prevention in Urinary Catheter”
(expiring on October 27, 2025); (3) U.S. Patent No. 9,028,748 to “System and Method for Surface Acoustic Wave Treatment of Medical
Devices” (expiring on July 11, 2030); and (4) U.S. Patent No. 9,585,977 directed to “System and Method for Surface
Acoustic Waves Treatment of Skin” (expiring on August 20, 2033). These patents cover a wide range of embodiments and applications
of our proprietary surface acoustic wave (SAW) technology, including our commercialized Painshield, Painshield Plus, Woundshield and
Uroshield devices. Specifically, the patents provide for methods of generating surface acoustic waves on surfaces of indwelling medical
devices and to topical and urological applications therefor, for alleviating pain and for wound healing, and for preventing formation
of bacterial biofilms on catheters. Pending patent applications related to Uroshield devices are directed to Multiple Frequency Surface
Acoustic Waves for Internal Medical Device and System, Device, and Method for Mitigating Bacterial Biofilms Associated with Indwelling
Medical Devices which covers the next generation of Uroshield devices operating at multiple frequencies and devices which are compatible
in portable and wireless systems. Another pending patent application related to Uroshield devices is directed to System, Device, and
Method for Mitigating Bacterial Biofilms Associated with Indwelling Medical Devices (US patent application US 17/646,715 filed on
December 31, 2021).
Pending
patent applications related to Painshield, Painshield Plus, Woundshield devices are directed to Transdermal Patch of a Portable Ultrasound-Generating
System for Improved Delivery of Therapeutic Agents and Associated Methods of Treatment (US patent application 17/025,969 filed on
September 18, 2020); Portable Ultrasound System and Methods of Treating Facial Skin by Application of Surface Acoustic Waves (US
patent application 17/646,753 filed on January 3, 2022) and Improved Injection Needle Assembly (US patent application 17/646,804
filed on December 31, 2021).
Nasdaq
Minimum Stockholders’ Equity Requirement
On
May 23, 2023, we received a letter from the Listing Qualifications Department of Nasdaq indicating that we no longer comply with the
minimum stockholders’ equity requirement under Nasdaq Listing Rule 5550(b)(1) for continued listing on Nasdaq because our stockholders’
equity of approximately $2.2 million as reported in our Quarterly Report on Form 10-Q for the period ended March 31, 2023, is below the
required minimum of $2.5 million, and as of May 22, 2023, we did not meet the alternative compliance standards relating to the market
value of listed securities of $35 million or net income from continuing operations of $500,000 in the most recently completed fiscal
year or in two of the last three most recently completed fiscal years.
In
accordance with Nasdaq Listing Rules, we had 45 calendar days, or until July 7, 2023, to submit a plan to regain compliance. On
July 7, 2023 we submitted our plan to regain compliance with the Nasdaq minimum stockholders’ equity standard. If our plan
is accepted, Nasdaq may grant us an extension of up to 180 calendar days from the date of the notification letter to evidence compliance. However, there
can be no assurance that our plan will be accepted or that if it is, we will be able to regain compliance. If our plan to regain compliance
with the minimum stockholders’ equity standard is not accepted or if it is accepted but we do not regain compliance by the end
of the extension granted by Nasdaq, or we fail to satisfy another Nasdaq requirement for continued listing, Nasdaq staff could provide
notice that our common stock will become subject to delisting. In such event, Nasdaq rules permit us to appeal the decision to reject
its proposed compliance plan or any delisting determination to a Nasdaq Hearings Panel. Accordingly, there can be no guarantee that we
will be able to maintain our Nasdaq listing.
Mio-Guard
Agreement
On
June 14, 2023 we announced that we entered into a distribution agreement with Mio-Guard, LLC (“Mio-Guard”) for the sale and
distribution of our PainShield MD product. Under the terms of the multi-year agreement, Mio-Guard has the exclusive right to sell and
distribute the PainShield MD product to its customers throughout the United States in the areas of athletic team sports and sports medicine.
CMS
Reimbursement
In
addition to the need to obtain regulatory approvals, we anticipate that sales volumes and prices of our UroShield and PainShield, products
will depend in large part on the availability of insurance coverage and reimbursement from third party payers. Third party payers include
governmental programs such as Medicare and Medicaid in the United States, private insurance plans and workers’ compensation plans.
We do not currently have reimbursement codes for use of WoundShield in any of the markets in which we have regulatory authority to sell
WoundShield. Of the markets in which we have regulatory authority to sell PainShield, prior to January 2020, we only had reimbursement
codes in the United States (i.e., CPT codes) for clinical use only. Effective as of January 2020, the U.S. Centers for Medicare and Medicaid
Services (“CMS”) approved our PainShield™ for reimbursement for Medicare beneficiaries on a national basis. We have
been actively taking steps to work toward having CMS assign a reimbursement value, including conducting additional longevity testing
by an independent laboratory and launching a direct-to-consumer rental program for PainShield™, as we were denied reimbursement
in September 2022 due to a lack of “life-cycle” testing. We have recently provided CMS with additional data and continue
to work with qualified legal representation to achieve our goal. The latest CMS application included PainShield products
and supplies. PainShield™ currently is subject to reimbursement under certain workers’ compensation plans and Veterans Administration
facilities.
Protrade
Proceeding
On
February 26, 2021, Protrade Systems, Inc. (“Protrade”) filed a Request for Arbitration (the “Request”) with the
International Court of Arbitration (the “ICA”) of the International Chamber of Commerce alleging that we were in breach of
an Exclusive Distribution Agreement dated March 7, 2019 (the “Distribution Agreement”) between Protrade and the Company.
Protrade alleges, in part, that we breached the Distribution Agreement by discontinuing the manufacture of the DV0057 Painshield MD device
in favor of an updated 10-100-001 Painshield MD device. Protrade claims damages estimated at $3 million.
On
March 15, 2022, the arbitrator issued a final award, which, determined that (i) we had the right to terminate the Distribution Agreement;
(ii) we did not breach the duty of good faith and fair dealing with regard to the Distribution Agreement; and (iii) we did not breach
any confidentiality obligations to Protrade.
Nevertheless the arbitrator determined that we did not comply with the obligation to supply Protrade with a year’s supply of patches,
and awarded Protrade $1,500,250, which consists of $1,432,000 for “lost profits” and $68,250 as reimbursement of
arbitration costs, on the grounds that we allegedly failed to supply Protrade with certain patches utilized by users of DV0057 Painshield
MD device. The arbitrator based the decision on the testimony of Protrade’s president who asserted that a user would use in excess
of 33 patches per each device. We believe that the number of patches per device alleged by Protrade is grossly inflated, and that these
claims were not properly raised before the arbitrator. Accordingly, on April 13, 2022, we
submitted an application for the correction of the award which the arbitrator denied on June 22, 2022.
On
April 5, 2022, Protrade filed a Petition with the Supreme Court of New York Nassau County seeking to confirm the Award. On April 13,
2022, we submitted an application to the ICA seeking to correct an error in the award based on the evidence that we only sold 2-3 reusable
patches per device contrary to the 33 reusable patches claimed by Protrade. The same arbitrator who issued the award, denied the application.
On
July 22, 2022, we filed a cross-motion seeking to vacate arbitration award on the grounds that the arbitrator exceeded her authority,
that the award was procured by fraud, and that the arbitrator failed to follow procedures established by New York law. In particular,
we averred in our motion that Protrade’s witness made false statements in arbitration, and that the arbitrator resolved a claim
that was never raised by Protrade and that has no factual basis.
On
October 3, 2022, the court issued a decision granting Protrade its petition to confirm the Award and
denying the cross-motion.
On
November 9, 2022, we filed a motion to re-argue and renew our cross-motion to vacate the arbitration decision based on newer information
that was not available during the initial hearing. On the same day, we also filed a notice of appeal with the Appellate Division, Second
Department. On March 21, 2023, the Court denied the motion to re-argue and renew. We filed a notice of appeal of this decision with the
Appellate Division, Second Department on April 5, 2023.
On
July 10, 2023, we filed our appeal with the Appellate Division, Second Department. We intend to continue to vigorously pursue our opposition
to the award in all appropriate fora.
Corporate
Information
We
were organized in the State of Delaware on October 20, 2003. Our principal executive offices are located at 525 Executive Boulevard,
Elmsford, New York 10523. Our telephone number is (914) 233-3004. Our website address is www.nanovibronix.com. Information accessed through
our website is not incorporated into this prospectus and is not a part of this prospectus.
THE OFFERING
| Common
Stock to be Offered |
|
shares
based on the sale of our common stock at an assumed combined public offering price of $
per share of common stock and accompanying Common Warrant, which is the closing price of
our common stock on Nasdaq on ,
2023, and assuming no sale of any Prefunded Warrants. |
| |
|
|
Prefunded
Warrants
to be Offered |
|
We
are also offering to certain purchasers whose purchase of shares of common stock in this
offering would otherwise result in the purchaser, together with its affiliates and certain
related parties, beneficially owning more than 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding common stock immediately following the consummation of this offering,
the opportunity to purchase, if such purchasers so choose, Prefunded Warrants to purchase
shares of common stock, in lieu of shares of common stock that would otherwise result in
any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the
purchaser, 9.99%) of our outstanding common stock. Each Prefunded Warrant will be
exercisable for one share of our common stock. The purchase price of each Prefunded
Warrant and accompanying Common Warrant will equal the price at which the share of common
stock and accompanying Common Warrant are being sold to the public in this offering, minus
$0.0001, and the exercise price of each Prefunded Warrant will be $0.0001
per share. The Prefunded Warrants will be exercisable immediately and may be exercised
at any time until all of the Prefunded Warrants are exercised in full.
This
offering also relates to the shares of common stock issuable upon exercise of the Prefunded Warrants sold in this offering.
For each Prefunded Warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one
basis. Because we will issue a Common Warrant for each share of our common stock and for each Prefunded Warrant sold in this
offering, the number of Common Warrants sold in this offering will not change as a result of a change in the mix of the shares of
our common stock and Prefunded Warrants sold. |
| |
|
|
| Common
Warrants to be Offered |
|
Each
share of our common stock and each Prefunded Warrant to purchase one share of our
common stock is being sold together with a Common Warrant to purchase
share of our common stock. Each Common Warrant will have an exercise price of $ per share
(representing % of the price at which a share of common stock and accompanying Common
Warrant are sold to the public in this offering), will be immediately exercisable and
will expire on the anniversary of the original
issuance date.
The
shares of common stock and Prefunded Warrants, and the accompanying Common Warrants, as the case may be, can only be purchased
together in this offering but will be issued separately and will be immediately separable upon issuance. This prospectus also relates
to the offering of the shares of common stock issuable upon exercise of the Common Warrants. |
| Lock-up
Agreements |
|
We
and all of our executive officers and directors will enter into lock-up agreements with the placement agent. Under these agreements,
we and each of these persons may not, without the prior written approval of the placement agent, offer, sell, contract to
sell or otherwise dispose of or hedge common stock or securities convertible into or exchangeable for common stock, subject to certain
exceptions. The restrictions contained in these agreements will be in effect for a period of days
after the date of the closing of this offering. For more information, see “Plan of Distribution.” |
| |
|
|
| Placement Agent Warrants |
|
We
have agreed to issue to the placement agent or its designees placement agent warrants to
purchase up to % of the aggregate number of shares of common stock sold in this offering
(including the shares of common stock issuable upon the exercise of the Prefunded Warrants)
at an exercise price equal to % of the combined public offering price per share and accompanying
Common Warrant to be sold in this offering. The placement agent warrants will be exercisable
upon issuance and will expire years from the commencement of sales under this offering.
|
| |
|
|
| Common
Stock Outstanding After This Offering(1) |
|
shares (assuming
we sell only shares of common stock and no Prefunded Warrants and assuming no exercise
of the Common Warrants). |
| |
|
|
| Use
of Proceeds |
|
We
estimate that the net proceeds from this offering will be approximately $
million, based on an assumed combined public offering price of $ per share of common stock
and accompanying Common Warrant which was the closing price of our common stock on Nasdaq
on , 2023, after deducting the
placement agent fees and estimated offering expenses payable by us, and assuming we sell
only shares of common stock and no Prefunded Warrants and excluding the proceeds,
if any, from the exercise of the Common Warrants in this offering.
We
currently intend to use the net proceeds from the offering for general corporate purposes, including funding of our development programs,
commercial planning and sales and marketing expenses, potential strategic acquisitions, general and administrative expenses
and working capital. See “Use of Proceeds” beginning on page 10. |
| Risk
Factors |
|
See
“Risk Factors” beginning on page 5 of this prospectus and other information included and incorporated by reference in this
prospectus for a discussion of the risk factors you should consider carefully when making an investment decision. |
| |
|
|
| Nasdaq
Symbol |
|
Our
common stock is listed on Nasdaq under the symbol “NAOV.” There is no established trading market for the Common Warrants
or the Prefunded Warrants, and we do not expect a trading market to develop. We do not intend to list the Common Warrants
or the Prefunded Warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the
Common Warrants and the Prefunded Warrants will be extremely limited. |
(1)
The number of shares of our common stock to be outstanding immediately after the closing of this offering is based on 1,662,377
shares of common stock outstanding as of July 7, 2023 and, unless otherwise indicated, excludes, as of that date:
| |
● |
142,160
shares of common stock issuable upon the exercise of stock options issued under the 2014 Long-Term Incentive Plan (the “2014
Plan”), at a weighted-average exercise price of $25.31 per share; |
| |
● |
86,375 shares of common stock available for issuance under the 2014 Plan; |
| |
● |
78,252 shares of common stock issuable upon the exercise of warrants outstanding at a weighted average exercise
price of $58.10 per share; and |
| |
● |
up to shares of common stock issuable upon the exercise
of the placement agent warrants at an exercise price of $ per share to be issued to the placement
agent or its designees as compensation in connection with this offering. |
Except
as otherwise indicated, the information in this prospectus assumes: (i) no sale of the Prefunded Warrants in this offering, which,
if sold, would reduce the number of shares of common stock that we are offering on an one-for-one basis; (ii) no exercise of any Common
Warrants issued in this offering; (iii) no exercise of the warrants to be issued to the placement agent or its designees in connection
with this offering; and (iv) no exercise of the options or warrants described above.
RISK
FACTORS
An
investment in our securities involves certain risks. You should not invest unless you are able to bear the complete loss of your investment.
You should carefully consider the risks described below and discussed under the section entitled “Risk Factors” in our most
recent Annual Report on Form 10-K which is incorporated herein by reference, together with other information in this prospectus and the
information and documents incorporated by reference in this prospectus, including our future reports on Form 10-K and 10-Q. Our business,
business prospects, financial condition or results of operations could be seriously harmed as a result of these risks, which could cause
the trading price of our common stock to decline, resulting in a loss of all or part of your investment. Additional risks and uncertainties
not presently known to us or that we currently deem immaterial also may materially and adversely affect our business, financial condition
and results of operations. Please also read carefully the section below entitled “Special Note Regarding Forward-Looking Statements.”
Our
financial statements have been prepared on a going concern basis, and do not include adjustments that might be necessary if we are unable
to continue as a going concern. Management has substantial doubt about our ability to continue as a going concern.
Our
unaudited condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization
of assets and the satisfaction of liabilities in the normal course of business. During the three months ended March 31, 2023, our cash
used in operations was $1.2 million leaving a cash balance of approximately $1.5 million as of March 31, 2023. Because we do not have
sufficient resources to fund our operations for the next twelve months from the date of this filing, management has substantial doubt
about our ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the
recoverability and classification of asset amounts or the classification of liabilities that might be necessary should we be unable to
continue as a going concern.
We
will need to raise additional capital to finance its losses and negative cash flows from operations and may continue to be dependent
on additional capital raising as long as our products do not reach commercial profitability. There are no assurances that we would be
able to raise additional capital on terms favorable to it. If we are unsuccessful in commercializing our products and raising capital,
we will need to reduce activities, curtail, or cease operations.
If
we fail to comply with the continued listing requirements of Nasdaq, our common stock may be delisted and the price of our common stock
and our ability to access the capital markets could be negatively impacted.
Our
common stock is currently listed for trading on Nasdaq. We must satisfy Nasdaq’s continued listing requirements, including, among
other things, a minimum stockholders’ equity of $2.5 million and a minimum closing bid price of $1.00 per share or risk
delisting, which would have a material adverse effect on our business. A delisting of our common stock from Nasdaq could materially reduce
the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting
could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in
the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities.
On
May 23, 2023, we received a letter from the Listing Qualifications Department of Nasdaq indicating that we no longer comply with the
minimum stockholders’ equity requirement under Nasdaq Listing Rule 5550(b)(1) for continued listing on Nasdaq because our stockholders’
equity of approximately $2.2 million as reported in our Quarterly Report on Form 10-Q for the period ended March 31, 2023, is below the
required minimum of $2.5 million, and as of May 22, 2023, we did not meet the alternative compliance standards relating to the market
value of listed securities of $35 million or net income from continuing operations of $500,000 in the most recently completed fiscal
year or in two of the last three most recently completed fiscal years.
In
accordance with Nasdaq Listing Rules, we had 45 calendar days, or until July 7, 2023, to submit a plan to regain compliance. On
July 7, 2023 we submitted our plan to regain compliance with the Nasdaq minimum stockholders’ equity standard. If our plan is accepted,
Nasdaq may grant us an extension of up to 180 calendar days from the date of the notification letter to evidence compliance.
However,
there can be no assurance that our plan will be accepted or that if it is, we will be able to regain compliance. If our plan to regain
compliance with the minimum stockholders’ equity standard is not accepted or if it is accepted but we do not regain compliance
by the end of the extension granted by Nasdaq, or we fail to satisfy another Nasdaq requirement for continued listing, Nasdaq staff could
provide notice that our common stock will become subject to delisting.
There is no assurance that we can regain compliance with such minimum listing requirements. If our common stock were delisted from Nasdaq,
trading of our common stock would most likely take place on an over-the-counter market established for unlisted securities, such as the
OTCQB or the Pink Market maintained by OTC Markets Group Inc. An investor would likely find it less convenient to sell, or to obtain
accurate quotations in seeking to buy, our common stock on an over-the-counter market, and many investors would likely not buy or sell
our common stock due to difficulty in accessing over-the-counter markets, policies preventing them from trading in securities not listed
on a national exchange or other reasons. In addition, as a delisted security, our common stock would be subject to SEC rules as a “penny
stock,” which impose additional disclosure requirements on broker-dealers. The regulations relating to penny stocks, coupled with
the typically higher cost per trade to the investor of penny stocks due to factors such as broker commissions generally representing
a higher percentage of the price of a penny stock than of a higher-priced stock, would further limit the ability of investors to trade
in our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable
to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business
development opportunities. For these reasons and others, delisting would adversely affect the liquidity, trading volume and price of
our common stock, causing the value of an investment in us to decrease and having an adverse effect on our business, financial condition
and results of operations, including our ability to attract and retain qualified employees and to raise capital.
We
have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our
management will have broad discretion in the application of the net proceeds, including for any of the purposes described in the section
of this prospectus entitled “Use of Proceeds.” You will be relying on the judgment of our management with regard to
the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net
proceeds are being used appropriately. The failure by our management to apply these funds effectively could result in financial losses
that could have a material adverse effect on our business, cause the price of our securities to decline and delay the development of
our product candidates. Pending the application of these funds, we may invest the net proceeds from this offering in a manner that does
not produce income or that loses value.
You
will experience immediate and substantial dilution in the net tangible book value of the shares you purchase in this offering and may
experience additional dilution in the future.
The
combined public offering price per share of common stock and related Common Warrant, and the combined public offering price of each Prefunded
Warrant and related Common Warrant, will be substantially higher than the as adjusted net tangible book value per share of our common
stock after giving effect to this offering.
Assuming
the sale of shares of our common stock and Common Warrants to purchase up to shares
of common stock at an assumed combined public offering price of $ per share and related Common Warrant,
the closing sale price per share of our common stock on Nasdaq on , 2023, assuming no sale of any
Prefunded Warrants in this offering, no exercise of the Common Warrants being offered in this offering and after deducting the
placement agent fees and commissions and estimated offering expenses payable by us, you will incur immediate dilution of approximately
$ per share. As a result of the dilution in net tangible book value to investors purchasing securities
in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of
the liquidation of our company. See the section entitled “Dilution” below for a more detailed discussion of the dilution
you will incur if you participate in this offering. To the extent shares are issued under outstanding options and warrants at exercise
prices lower than the public offering price of our common stock in this offering, including the shares underlying the Prefunded
Warrants, holders will incur further dilution.
Purchasers
who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers
that purchase without the benefit of a securities purchase agreement.
In
addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that
enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue
a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the
securities purchase agreement including: (i) timely delivery of shares; (ii) agreement to not enter into variable rate financings for
year from closing, subject to certain exceptions; (iii) agreement to not enter into any financings for days from closing;
and (iv) indemnification for breach of contract.
There
may be future sales of our securities or other dilution of our equity, which may adversely affect the market price of our common stock.
Even
with the proceeds from this offering, we expect we will need to raise additional capital, potentially shortly after this offering. In
order to raise additional capital in the future, we may offer additional shares of common stock or other securities convertible into
or exchangeable for our common stock. We are generally not restricted from issuing additional securities, including shares of common
stock, securities that are convertible into or exchangeable for, or that represent the right to receive, common stock or substantially
similar securities. The issuance of securities in future offerings may cause further dilution to our stockholders, including investors
in this offering. We may not be able to sell shares or other securities in any other offering at a price per share that is equal to or
greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future
could have rights superior to existing stockholders. The price per share at which we sell additional shares of common stock or securities
convertible into shares of common stock in future transactions may be higher or lower than the price per share in this offering. You
will also incur dilution upon exercise of any outstanding stock options, warrants or upon the issuance of shares of common stock under
our stock incentive programs. In addition, the sale of shares of common stock in this offering and any future sales of a substantial
number of shares of common stock in the public market, or the perception that such sales may occur, could adversely affect the price
of our common stock. We cannot predict the effect, if any, that market sales of those shares of common stock or the availability of those
shares for sale will have on the market price of our common stock.
There
is no public market for the Common Warrants or Prefunded Warrants being offered by us in this offering.
There
is no established public trading market for the Common Warrants or the Prefunded Warrants, and we do not expect a market to develop.
In addition, we do not intend to apply to list the Common Warrants or Prefunded Warrants on any national securities exchange or
other nationally recognized trading system. Without an active market, the liquidity of the Common Warrants and Prefunded Warrants
will be limited.
The
Common Warrants and Prefunded Warrants are speculative in nature.
The
Common Warrants and Prefunded Warrants offered hereby do not confer any rights of share of common stock ownership on their holders,
such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a
fixed price. Specifically, commencing on the date of issuance, holders of the Common Warrants may acquire the shares of common stock
issuable upon exercise of such warrants at an exercise price of $ per share of
common stock, and holders of the Prefunded Warrants may acquire the shares of common stock issuable upon exercise of such warrants
at an exercise price of $0.0001 per share of common stock. Moreover, following this offering, the market value of the Common Warrants is uncertain and there can be no assurance that the market value of the Common Warrants,
if any, will equal or exceed their public offering prices. There can be no assurance that the market price of the shares of
common stock will ever equal or exceed the exercise price of the Common Warrants, and consequently, whether it will ever be profitable
for holders of Common Warrants to exercise the Common Warrants.
Holders
of the Prefunded Warrants and the Common Warrants offered hereby will have no rights as common stockholders with respect to the
shares our common stock underlying the warrants until such holders exercise their warrants and acquire our common stock, except as otherwise
provided in the Prefunded Warrants and the Common Warrants.
Until
holders of the Common Warrants and the Prefunded Warrants acquire shares of our common stock upon exercise thereof, such holders
will have no rights with respect to the shares of our common stock underlying such warrants, except to the extent that holders of such
Common Warrants and Prefunded Warrants will have certain rights to participate in distributions or dividends paid on our common
stock as set forth in the Common Warrants and the Prefunded Warrants. Upon exercise of the Common Warrants and the Prefunded
Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date
occurs after the exercise date.
This
is a best efforts offering, with no minimum amount of securities is required to be sold, and we may not raise the amount of capital we
believe is required for our business plans, including our near-term business plans.
The
placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement
agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar
amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering.
Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement
agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above.
We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and
investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to support our
continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required
for our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable
to us.
A
more active, liquid trading market for our common stock may not develop, and the price of our common stock may fluctuate significantly.
Historically,
the market price of our common stock has fluctuated over a wide range. There has been relatively limited trading volume in
the market for our common stock, and a more active, liquid public trading market may not develop or may not be sustained. Limited liquidity
in the trading market for our common stock may adversely affect a stockholder’s ability to sell its shares of common stock at the
time it wishes to sell them or at a price that it considers acceptable. If a more active, liquid public trading market does not develop
we may be limited in our ability to raise capital by selling shares of common stock and our ability to acquire other companies or assets
by using shares of our common stock as consideration. In addition, if there is a thin trading market or “float” for our stock,
the market price for our common stock may fluctuate significantly more than the stock market as a whole. Without a large float, our common
stock would be less liquid than the stock of companies with broader public ownership and, as a result, the trading prices of our common
stock may be more volatile and it would be harder for a stockholder to liquidate any investment in our common stock. Furthermore, the
stock market is subject to significant price and volume fluctuations, and the price of our common stock could fluctuate widely in response
to several factors, including:
| |
● |
our
quarterly or annual operating results; |
| |
● |
changes
in our earnings estimates; |
| |
● |
investment
recommendations by securities analysts following our business or our industry; |
| |
● |
additions
or departures of key personnel; |
| |
● |
changes
in the business, earnings estimates or market perceptions of our competitors; |
| |
● |
our
failure to achieve operating results consistent with securities analysts’ projections; |
| |
● |
changes
in industry, general market or economic conditions; and |
| |
● |
announcements
of legislative or regulatory changes. |
The
stock market has experienced extreme price and volume fluctuations in recent years that have significantly affected the quoted prices
of the securities of many companies, including companies in the medical device industry. The changes often appear to occur without regard
to specific operating performance. The price of our common stock could fluctuate based upon factors that have little or nothing to do
with us and these fluctuations could materially reduce our stock price.
We
have never declared or paid any cash dividends on our common stock and, accordingly, stockholders must rely on stock appreciation for
any return on their investment.
We
have never declared or paid any cash dividends on our common stock, and we do not intend to pay any cash dividends on our common stock.
Rather, we currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our
business and for general corporate purposes, and we do not anticipate paying any cash dividends in the foreseeable future. Consequently,
investors must rely on sales of their common stock after price appreciation, which may never occur, as the primary way to realize any
gains on their investment.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the information incorporated by reference in this prospectus contain “forward-looking statements,” which include
information relating to future events, future financial performance, strategies, expectations, competitive environment and regulation.
Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,”
“continue,” “expects,” “anticipates,” “future,” “intends,” “plans,”
“believes,” “estimates,” and similar expressions, as well as statements in future tense, are intended to identify
forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results and may not
be accurate indications of when such performance or results will actually be achieved. Forward-looking statements are based on information
we have when those statements are made or our management’s good faith belief as of that time with respect to future events, and
are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or
suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| |
● |
Our
history of losses and expectation of continued losses. |
| |
|
|
| |
●
|
Global
economic and political instability and conflicts, such as the conflict between Russia and Ukraine, could adversely affect our business,
financial condition or results of operations. |
| |
|
|
| |
● |
Increasing
inflation could adversely affect our business, financial condition, results of operations or cash flows. |
| |
|
|
| |
● |
The
geographic, social and economic impact of COVID-19 on the Company’s business operations. |
| |
|
|
| |
● |
Our
ability to raise funding for, and the timing of, clinical studies and eventual U.S. Food and Drug Administration (“FDA”)
approval of our product candidates. |
| |
|
|
| |
● |
Regulatory
actions that could adversely affect the price of or demand for our approved products. |
| |
|
|
| |
● |
Market
acceptance of existing and new products. |
| |
|
|
| |
● |
Favorable
or unfavorable decisions about our products from government regulators, insurance companies or other third-party payers (including
CMS). |
| |
|
|
| |
● |
Risks
of product liability acclaims and the availability of insurance. |
| |
|
|
| |
● |
Our
ability to generate internal growth. |
| |
|
|
| |
● |
Risks
related to computer system failures and cyber-attacks. |
| |
|
|
| |
● |
Our
ability to obtain regulatory approval in foreign jurisdictions. |
| |
|
|
| |
● |
Uncertainty
regarding the success of our clinical trials for our products in development. |
| |
|
|
| |
● |
Risks
related to our operations in Israel, including political, economic and military instability. |
| |
|
|
| |
● |
The
price of our securities is volatile with limited trading volume. |
| |
|
|
| |
● |
Our
ability to regain compliance with the continued listing requirements of Nasdaq and the risk that our common stock will be delisted
if we cannot do so. |
| |
● |
Our
ability to maintain effective internal control over financial reporting and to remedy identified material weaknesses. |
| |
|
|
| |
● |
We
are a “smaller reporting company” and have reduced disclosure obligations that may make our stock less attractive to
investors. |
| |
|
|
| |
● |
Our
intellectual property portfolio and our ability to protect our intellectual property rights. |
| |
|
|
| |
● |
Our
ability to recruit and retain qualified regulatory and research and development personnel. |
| |
|
|
| |
● |
Unforeseen
changes in healthcare reimbursement for any of our approved products. |
| |
|
|
| |
● |
The
adoption of health policy changes and health care reform. |
| |
● |
Lack
of financial resources to adequately support our operations. |
| |
|
|
| |
● |
Difficulties
in maintaining commercial scale manufacturing capacity and capability. |
| |
|
|
| |
● |
Changes
in our relationship with key collaborators. |
| |
|
|
| |
● |
Changes
in the market valuation or earnings of our competitors or companies viewed as similar to us. |
| |
|
|
| |
● |
Our
failure to comply with regulatory guidelines. |
| |
|
|
| |
● |
Uncertainty
in industry demand and patient wellness behavior. |
| |
|
|
| |
● |
General
economic conditions and market conditions in the medical device industry. |
| |
|
|
| |
● |
Future
sales of large blocks of our common stock, which may adversely impact our stock price. |
| |
|
|
| |
● |
Depth
of the trading market in our common stock. |
You
should read this prospectus and any related free-writing prospectus and the documents incorporated by reference in this prospectus with
the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different
from what we expect. The forward-looking statements contained or incorporated by reference in this prospectus are expressly qualified
in their entirety by this cautionary statement. We do not undertake any obligation to publicly update any forward-looking statement to
reflect events or circumstances after the date on which any such statement is made or to reflect the occurrence of unanticipated events.
USE
OF PROCEEDS
We
estimate that we will receive net proceeds of approximately $ million from
the sale of the securities offered by us in this offering, assuming a combined public offering price of $
per share of common stock and accompanying Common Warrant, the closing price per share of our common stock on Nasdaq on ,
2023, after deducting the placement agent fees and estimated offering expenses payable by us and assuming no sale of any Prefunded
Warrants offered in this offering. However, because this is a best efforts offering with no minimum number of securities or amount
of proceeds as a condition to closing, the actual offering amount, the placement agent’s fees and net proceeds to us are not presently
determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus, and we may not sell
all or any of the securities we are offering. As a result, we may receive significantly less in net proceeds.
We
intend to use the net proceeds from this offering for general corporate purposes, including funding of our development programs, commercial
planning and sales and marketing expenses, potential strategic acquisitions, general and administrative expenses and working capital.
The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our development and
commercialization efforts, the status of and results from our clinical trials, whether or not we enter into strategic collaborations
or partnerships, and our operating costs and expenditures. In addition, while we have not entered into any binding agreements or commitments
relating to any significant transaction as of the date of this prospectus that we expect to use the net proceeds from this offering,
we may use a portion of the net proceeds to pursue acquisitions, joint ventures and other strategic transactions.
A
$1.00 increase or decrease in the assumed combined public offering price of $ per common stock and accompanying Common Warrant would
increase or decrease the net proceeds from this offering by approximately $ million, assuming that the number of shares of common stock
and Common Warrants offered by us, as set forth on the cover page of this prospectus, remains the same and assuming no sale of Prefunded
Warrants and after deducting the estimated placement agent fees and estimated offering expenses payable by us. An increase or decrease
of 100,000 in the number of shares of common stock and accompanying Common Warrants offered by us would increase or decrease our proceeds
by approximately $ million, assuming the assumed combined public offering price of $ per common stock and accompanying Common Warrant
remains the same, and after deducting placement agent fees and estimated offering expenses payable by us.
Our
management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways
that do not improve our results of operations or enhance the value of our common stock. Accordingly, you will be relying on the judgment
of our management on the use of net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether
the proceeds are being used appropriately. Our failure to apply these funds effectively could have a material adverse effect on our business
and cause the price of our common stock to decline.
Pending
other uses, we intend to invest the proceeds to us in investment-grade, interest-bearing securities such as money market funds, certificates
of deposit, or direct or guaranteed obligations of the U.S. government, or hold as cash. We cannot predict whether the proceeds invested
will yield a favorable, or any, return.
DILUTION
If
you invest in our securities in this offering, your interest will be diluted immediately to the extent of the difference between the
public offering price paid by the purchasers in this offering and the as adjusted net tangible book value per shares of common stock
after this offering.
Our
net tangible book value of our common stock as of March 31, 2023, was approximately $2.2 million or approximately $1.33
per share of our common stock, based upon 1,662,330 shares of our common stock outstanding as of that date. Net tangible book value per
share is determined by dividing our total tangible assets, less total liabilities, by the number of shares of our common stock outstanding
as of March 31, 2023. Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers
in this offering and the net tangible book value per share of our common stock immediately after this offering.
After
giving effect to the sale by us in this offering of shares of our common stock and accompanying Common
Warrants in this offering at an assumed combined public offering price of $ per share and Common
Warrant, based on the closing price of our common stock on Nasdaq on , 2023, assuming no sale of
any Prefunded Warrants in this offering, no exercise of any of the Common Warrants being offered in this offering, including the
warrants issued to the placement agent or its designees, and after deducting the placement agent fees and estimated offering expenses
payable by us, our as adjusted net tangible book value as of March 31, 2023 would have been approximately $
million, or approximately $ per share of common stock. This represents an immediate increase in net
tangible book value of approximately $ per share of common stock to our existing security holders
and an immediate dilution in as adjusted net tangible book value of approximately $ per share to
purchasers of our securities in this offering, as illustrated by the following table:
| Assumed public offering price per share and accompanying Common Warrant | |
| | | |
$ | | |
| Historical net tangible book value per share as of March 31, 2023 | |
$ | 1.33 | | |
| | |
| Increase in net tangible book value per share attributable to this offering | |
$ | | | |
| | |
| As adjusted net tangible book value per share after giving effect to this offering as of March 31, 2023 | |
| | | |
$ | | |
| Dilution per share to investors participating in this offering | |
| | | |
$ | | |
A
$0.10 increase in the assumed combined public offering price of $ per share and Common Warrant would increase our as adjusted net tangible
book value after this offering by $ million, or $ per share, and the dilution per share to investors purchasing securities in this offering
would be approximately $ per share, assuming that the maximum number of securities offered by us, as set forth on the cover page of this
prospectus, remains the same and after deducting the placement agent fees and estimated offering expenses payable by us. Similarly, a
$0.10 decrease in the assumed combined public offering price of $ per share and Common Warrant would decrease our as adjusted net tangible
book value after this offering by $ million, or $ per share, and the dilution per share to investors purchasing securities in this offering
would be $ per share, assuming that the maximum number of securities offered by us, as set forth on the cover page of this prospectus,
remains the same and after deducting the placement agent fees and estimated offering expenses payable by us.
We
may also increase or decrease the number of securities we are offering from the assumed maximum number of securities set forth above.
An increase of 100,000 shares from the assumed maximum number of shares set forth on the cover page of this prospectus would increase
our as adjusted net tangible book value after this offering by $ million, or $ per share, and the dilution per share to investors purchasing
securities in this offering would be approximately $ per share, assuming that the assumed public offering price remains the same and
after deducting the placement agent fees and estimated offering expenses payable by us. Similarly, a decrease of 100,000 shares from
the assumed maximum number of shares set forth on the cover page of this prospectus would decrease our as adjusted net tangible book
value after this offering by $ million, or $ per share, and the dilution per share to investors purchasing securities in this offering
would be approximately $ per share, assuming that the assumed public offering price remains the same and after deducting the placement
agent fees and estimated offering expenses payable by us.
The
information discussed above is illustrative only and will adjust based on the actual combined public offering price, the actual number
of securities that we offer in this offering, and other terms of this offering determined at pricing. The foregoing discussion and table
assumes no sale of Prefunded Warrants, which if sold, would reduce the number of shares of common stock that we are offering on
a one-for-one basis and does not take into account further dilution to investors in this offering that could occur upon the exercise
of outstanding options and warrants having a per share exercise price less than the public offering price per share and Common Warrant
in this offering.
The
discussion and table above are based on 1,662,330 shares of common stock outstanding as of March 31, 2023, which excludes, unless otherwise
indicated, as of that date:
| |
● |
142,160 shares of common stock issuable upon the exercise of stock options issued under the 2014 Long-Term Incentive Plan (the
“2014 Plan”), at a weighted-average exercise price of $25.31 per share; |
| |
● |
262,976 shares of common stock available for issuance under the 2014 Plan; |
| |
● |
78,252 shares of common stock issuable upon the exercise of warrants outstanding at a weighted average exercise price of $58.26
per share; and |
| |
● |
up to shares of common stock issuable upon the exercise of warrants at an exercise price of $ per share to be issued to the placement
agent or its designees as compensation in connection with this offering. |
To
the extent that options or warrants outstanding as of March 31, 2023 have been or may be exercised or we issue other shares, investors
purchasing securities in this offering may experience further dilution. In addition, we may seek to raise additional capital in the future
through the sale of equity or convertible debt securities. To the extent we raise additional capital through the sale of equity or convertible
debt securities, the issuance of such securities could result in further dilution to our stockholders.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
The
following is a summary of the material terms of our common stock. For additional information about our authorized capital, including
our common stock and our outstanding warrants to purchase common stock, we refer you to our amended and restated certificate of incorporation
and amended and restated bylaws that are currently in effect, which are included herein as Exhibit 3.2 and Exhibit 3.3, respectively,
and our filings with the SEC that are incorporated by reference in this prospectus, including our Annual Report on Form 10-K for the
year ended December 31, 2022. For instructions on how to find copies of these documents, please read “Where You Can Find Additional
Information” and “Incorporation of Certain Information by Reference.”
Common
Stock
The
holders of common stock are entitled to one vote per share. Our certificate of incorporation does not provide for cumulative voting.
All of our directors hold office for one-year terms until the election and qualification of their successors. The holders of our common
stock are entitled to receive ratably such dividends, if any, as may be declared by the board of directors out of legally available funds.
Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets that are legally
available for distribution. The holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights,
preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders
of any series of preferred stock, which may be designated solely by action of the board of directors and issued in the future.
The
transfer agent and registrar for our common stock is VStock Transfer, LLC. The transfer agent’s address is 18 Lafayette Place,
Woodmere, New York 11598. Our common stock is listed on Nasdaq under the symbol “NAOV.”
Common
Warrants
The
following summary of certain terms and provisions of the Common Warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the Common Warrant, the form of which will be filed as an exhibit to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of
Common Warrant for a complete description of the terms and conditions of the Common Warrants.
Duration,
Exercise Price and Form
Each
Common Warrant offered hereby will have an exercise price equal to $ per share. The Common
Warrants will be immediately exercisable and may be exercised until the year anniversary
of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate
adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise
price. The Common Warrants will be issued separately from the common stock or the Prefunded Warrants, as the case may be, and
may be transferred separately immediately thereafter. The Common Warrants will be issued in certificated form only.
Exercisability
The
Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise
notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of
a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s
warrants to the extent that the holder would own more than 4.99% of the outstanding common stock (or at the election of a holder prior
to the date of issuance, 9.99%) immediately after exercise, except that upon at least 61 days’ prior notice from the holder to
us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of
the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is
determined in accordance with the terms of the Common Warrants.
Cashless
Exercise
If
at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available
for the issuance of the underlying shares to the holder, in lieu of making the cash payment otherwise contemplated to be made to us upon
such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole
or in part) the net number of shares of common stock determined according to a formula set forth in the Common Warrant.
Fundamental
Transactions
In
the event of a fundamental transaction, as described in the Common Warrants and generally including any reorganization, recapitalization
or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets,
our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person
or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Common
Warrants will be entitled to receive upon exercise of the Common Warrants the kind and amount of securities, cash or other property that
the holders would have received had they exercised the Common Warrants immediately prior to such fundamental transaction. In addition,
in certain circumstances, upon a fundamental transaction, the holder of a Common Warrant will have the right to require us to repurchase
its Common Warrants at the Black-Scholes value; provided, however, that, if the fundamental transaction is not within our control, including
not approved by our Board, then the holder will only be entitled to receive the same type or form of consideration (and in the same proportion),
at the Black-Scholes value of the unexercised portion of the Common Warrant that is being offered and paid to the holders of our common
stock in connection with the fundamental transaction.
Transferability
Subject
to applicable laws, a Common Warrant may be transferred at the option of the holder upon surrender of the Common Warrant to us together
with the appropriate instruments of transfer.
Fractional
Shares
No
fractional shares of common stock will be issued upon the exercise of the Common Warrants. Rather, the number of shares of common stock
to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such
final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading
Market
There
is no established trading market for the Common Warrants, and we do not expect a market to develop. We do not intend to apply for a listing
of the Common Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the
liquidity of the Common Warrants will be limited. The common stock issuable upon exercise of the Common Warrants is currently listed
on Nasdaq.
Rights
as a Stockholder
Except
as otherwise provided in the Common Warrants or by virtue of the holders’ ownership of shares of common stock, the holders of the
Common Warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until such
Common Warrant holders exercise their Common Warrants.
Waivers
and Amendments
No
term of the Common Warrants may be amended or waived without the written consent of the majority of the holders of the Common Warrants
purchased in this offering.
Prefunded
Warrants
The
following summary of certain terms and provisions of the Prefunded Warrants that are being offered hereby is not complete and
is subject to, and qualified in its entirety by, the provisions of the Prefunded Warrant, the form of which will be filed as an
exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms
and provisions of the form of Prefunded Warrant for a complete description of the terms and conditions of the Prefunded
Warrants.
Duration,
Exercise Price and Form
The
Prefunded Warrants offered hereby will have an exercise price of $0.0001 per share. The Prefunded Warrants will
be immediately exercisable and may be exercised at any time after their original issuance until such Prefunded Warrants are exercised
in full. The exercise price and number of shares of common stock issuable upon exercise are subject to appropriate adjustment in the
event of share dividends, share splits, reorganizations or similar events affecting our shares of common stock. The Prefunded
Warrants and Common Warrants are immediately separable and will be issued separately in this offering, but must be purchased together
in this offering. The Prefunded Warrants will be issued in certificated form only.
Exercisability
The Prefunded Warrants will be
exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment
in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed
below). A holder (together with its affiliates) may not exercise any portion of the Prefunded Warrant to the extent that the holder
would own more than 4.99% (or at the election of a holder prior to the date of issuance, 9.99%) of the outstanding common stock immediately
after exercise; provided, however, that upon 61 days’ notice to us, the holder may increase or decrease such beneficial
ownership limitation, provided that in no event shall the beneficial ownership limitation exceed 9.99% and any increase in the beneficial
ownership limitation will not be effective until 61 days following notice of such increase from the holder to us.
Cashless Exercise
At the time a holder exercises its Prefunded
Warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common
stock determined according to a formula set forth in the Prefunded Warrant.
Fundamental Transactions
In the event of a fundamental transaction,
as described in the Prefunded Warrants and generally including any reorganization, recapitalization or reclassification of our
common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger
with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial
owner of 50% of the voting power represented by our outstanding common stock, the holders of the Prefunded Warrants will be entitled
to receive upon exercise of the Prefunded Warrants the kind and amount of securities, cash or other property that the holders
would have received had they exercised the Prefunded Warrants immediately prior to such fundamental transaction.
Transferability
Subject to applicable laws, a Prefunded
Warrant may be transferred at the option of the holder upon surrender of the Prefunded Warrant to us together with the appropriate
instruments of transfer.
Fractional Shares
No fractional shares of common stock
will be issued upon the exercise of the Prefunded Warrants. Rather, the number of shares of common stock to be issued will, at
our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in
an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading market
for the Prefunded Warrants, and we do not expect a market to develop. We do not intend to apply for a listing of the Prefunded
Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity
of the Prefunded Warrants will be limited. The common stock issuable upon exercise of the Prefunded Warrants is currently
listed on Nasdaq.
Rights as a Stockholder
Except as otherwise provided in the Prefunded
Warrants or by virtue of the holders’ ownership of shares of common stock, the holders of Prefunded Warrants do not
have the rights or privileges of holders of our shares of common stock, including any voting rights, until such Prefunded Warrant
holders exercise their warrants.
Waivers
and Amendments
No
term of the Prefunded Warrants may be amended or waived without the written consent of the majority of the holders of the Prefunded Warrants
purchased in this offering.
Placement
Agent Warrants
We
have also agreed to issue to the placement agent (or its designees) placement agent warrants to purchase up to shares of common stock.
The placement agent warrants will be exercisable immediately and will have substantially the same terms as the Common Warrants described
above, except that the placement agent warrants will have an exercise price of $ per share (representing % of the offering price per
share and accompanying Common Warrant) and a termination date that will be five years from the commencement of the sales pursuant
to this offering. See “Plan of Distribution” below.
Delaware Anti-Takeover Law, Provisions of our Certificate
of Incorporation and Bylaws
Delaware Anti-Takeover
Law
We are subject to Section 203
of the Delaware General Corporation Law. Section 203 generally prohibits a public Delaware corporation from engaging in a “business
combination” with an “interested stockholder” for a period of three years after the date of the transaction in which
the person became an interested stockholder, unless:
| |
● |
prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| |
|
|
| |
● |
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (i) shares owned by persons who are directors and also officers and (ii) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| |
|
|
| |
● |
on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business
combination to include:
| |
● |
any merger or consolidation involving the corporation and the interested stockholder; |
| |
|
|
| |
● |
any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| |
|
|
| |
● |
subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| |
|
|
| |
● |
any transaction involving the corporation that has the
effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested
stockholder; or |
| |
|
|
| |
● |
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines
an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of the
corporation and any entity or person affiliated with, or controlling, or controlled by, the entity or person. The term “owner”
is broadly defined to include any person that, individually, with or through that person’s affiliates or associates, among other
things, beneficially owns the stock, or has the right to acquire the stock, whether or not the right is immediately exercisable, under
any agreement or understanding or upon the exercise of warrants or options or otherwise or has the right to vote the stock under any agreement
or understanding, or has an agreement or understanding with the beneficial owner of the stock for the purpose of acquiring, holding, voting
or disposing of the stock.
The restrictions in Section 203
do not apply to corporations that have elected, in the manner provided in Section 203, not to be subject to Section 203 of the Delaware
General Corporation Law or, with certain exceptions, which do not have a class of voting stock that is listed on a national securities
exchange or held of record by more than 2,000 stockholders. Our certificate of incorporation and bylaws do not opt out of Section 203.
Section 203 could delay or prohibit
mergers or other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us even
though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Certificate of Incorporation and Bylaws
Provisions of our certificate
of incorporation and bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our
management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our
stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common
stock. Among other things, our certificate of incorporation and bylaws:
| |
● |
permit our board of directors to issue up to 11,000,000 shares of preferred stock, without further action by the stockholders, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in control; |
| |
● |
provide that the authorized number of directors may be changed only by resolution of a majority of the total number of authorized directors whether or not there exist any vacancies in previously authorized directorships (the “Whole Board”); |
| |
|
|
| |
● |
provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| |
|
|
| |
● |
do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); |
| |
|
|
| |
● |
provide that special meetings of our stockholders may be called only by a resolution adopted by a majority of the Whole Board; and |
| |
|
|
| |
● |
set forth an advance notice procedure with regard to the nomination, other than by or at the direction of our board of directors, of candidates for election as directors and with regard to business to be brought before a meeting of stockholders. |
PLAN OF DISTRIBUTION
Pursuant to an engagement agreement,
dated , 2023 (as amended, the “Placement Agent Agreement”),
we have engaged to act as our exclusive placement
agent to solicit offers to purchase the securities offered pursuant to this prospectus on a reasonable best efforts basis. The Placement
Agent Agreement does not give rise to any commitment by the placement agent to purchase any of our securities, and the placement agent
will have no authority to bind us by virtue of the Placement Agent Agreement. The placement agent is not purchasing or selling any of
the securities offered by us under this prospectus, nor is it required to arrange for the purchase or sale of any specific number or
dollar amount of securities. The placement agent has agreed to use reasonable best efforts to arrange for the sale of the securities
by us. Therefore, we may not sell all of the shares of common stock, Prefunded Warrants and Common Warrants being offered. The
terms of this offering are subject to market conditions and negotiations between us, the placement agent and prospective investors.
The placement agent does not guarantee that it will be able to raise new capital in any prospective offering. The placement agent may
engage sub-agents or selected dealers to assist with the offering.
We will enter into a securities
purchase agreement directly with institutional investors, at such investor’s option, which purchase our securities in this
offering. Investors which do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with
the purchase of our securities in this offering. In addition to rights and remedies available to all purchasers in this offering
under federal securities and state law, the purchasers which enter into a securities purchase agreement will also be able to bring
claims of breach of contract against us. The ability to pursue a claim for breach of contract is material to larger purchasers in
this offering as a means to enforce the following covenants uniquely available to them under the securities purchase agreement: (i)
a covenant to not enter into variable rate financings for a period of
following
the closing of the offering, subject to an exception; and (ii) a covenant to not enter into any equity financings
for days from closing of the
offering, subject to certain exceptions. The nature of the representations, warranties and covenants in the securities purchase
agreements shall include:
| |
● |
standard issuer representations and warranties on matters such as organization, qualification, authorization, no conflict, no governmental filings required, current in SEC filings, no litigation, labor or other compliance issues, environmental, intellectual property and title matters and compliance with various laws such as the Foreign Corrupt Practices Act; and |
| |
● |
covenants
regarding matters such as registration of warrant shares, no integration with other offerings, filing of an 8-K to disclose entering
into these securities purchase agreements, no stockholder rights plans, no material nonpublic information, use of proceeds,
indemnification of purchasers, reservation and listing of shares of common stock, and no subsequent equity sales for
days. |
We will deliver the securities being issued to the
investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. We expect to deliver
the securities being offered pursuant to this prospectus on or about , 2023. There is no minimum number of securities or amount of proceeds
that is a condition to closing of this offering.
Fees and Expenses
We have agreed to pay the placement agent a total
cash fee equal to % of the aggregate gross proceeds raised in the offering and a management fee equal to % of the gross proceeds raised
in this offering. We will reimburse the placement agent a nonaccountable expense allowance of $ , its legal fees and expenses in an amount
up to $ and its clearing expense in an amount up to $15,950 in connection with this offering. We estimate the total offering expenses
of this offering that will be payable by us, excluding the placement agent fees and expenses, will be approximately $ million.
Placement Agent Warrants
In addition, we have agreed to issue
to the placement agent or its designees warrants, or the placement agent warrants, to purchase up to %
of the aggregate number of shares of common stock sold in this offering (including shares underlying any Prefunded Warrants),
at an exercise price equal to % of the public offering price per share and accompanying Common Warrant
to be sold in this offering. The placement agent warrants will be exercisable upon issuance and will expire years
from the commencement of sales under this offering.
If at the time of exercise there is no effective registration
statement registering, or the prospectus contained therein is not available for the resale of warrant shares by the holders of the placement
agent warrants, then the placement agent warrants may be exercised, in whole or in part, at such time by means of a “cashless exercise”
in which the holders shall be entitled to receive a number of warrant shares as calculated in the placement agent warrants.
The placement agent warrants provide for customary
anti-dilution provisions (for share dividends, splits and recapitalizations and the like) consistent with FINRA Rule 5110.
Tail
In the event that any investors that
were contacted by the placement agent or were introduced to the Company by the placement agent during the term of our engagement agreement
with the placement agent provide any capital to us in a public or private offering or capital-raising transaction within 12 months following
the termination or expiration of our engagement agreement with the placement agent, we shall pay the placement agent the cash and warrant
compensation provided above on the gross proceeds from such investors. The placement agent will only be entitled to such fee to
the extent that the parties are directly introduced to us by the placement agent, in accordance with FINRA Rule 2010.
Lock-Up Agreements
Our officers and directors,
representing beneficial ownership of % of our outstanding shares of common stock, have
agreed with the placement agent to be subject to a lock-up period of
days following the closing of this offering. This means that, during the applicable lock-up period, such persons may not offer for
sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose
of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for,
shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these
lock-up restrictions. We have also agreed to similar lock-up restrictions on the issuance and sale of our securities for
days following the closing of this offering, although we will be
permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. The lock-up period
is subject to an additional extension to accommodate for our reports of financial results or material news releases. The placement
agent may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
In addition, subject to certain exceptions,
we have agreed to not issue any securities that are subject to a price reset based on the trading prices of our common stock or upon
a specified or contingent event in the future, or enter into any agreement to issue securities at a future determined price for a period
of year following the closing date of this offering.
Indemnification
We have agreed to indemnify the placement agent against
certain liabilities, including certain liabilities under the Securities Act, or to contribute to payments that the placement agent may
be required to make in respect of those liabilities.
In addition, we will indemnify the
purchasers of securities in this offering against liabilities arising out of or relating to (i) any breach of any of the representations,
warranties, covenants or agreements made by us in the securities purchase agreement or related documents or (ii) any action instituted
against a purchaser by a third party (other than a third party who is affiliated with such purchaser) with respect to the securities
purchase agreement or related documents and the transactions contemplated thereby, subject to certain exceptions
Regulation M Compliance
The placement
agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any fees received by
it and any profit realized on the sale of our securities offered hereby by it while acting as principal might be deemed to be underwriting
discounts or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities
Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations
may limit the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement
agent may not (i) engage in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities
or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed
their participation in the distribution.
Other Relationships
The placement agent and its affiliates have engaged,
and may in the future engage, in investment banking transactions and other commercial dealings in the ordinary course of business with
us or our affiliates. The placement agent has received, or may in the future receive, customary fees and commissions for these transactions.
In addition, in the ordinary course of their business
activities, the placement agent and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities
(or related derivative securities) for their own account and for the accounts of their customers. Such investments and securities activities
may involve securities and/or instruments of ours or our affiliates. The placement agent and its affiliates may also make investment recommendations
and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend
to clients that they acquire, long and/or short positions in such securities and instruments.
The placement agent acted as the placement agent in
connection with several registered offerings in the past three years and it received compensation for each such offering. However, except
as disclosed in this prospectus, we have no present arrangements with the placement agent for any further services.
Electronic Distribution
A prospectus in electronic format may be made available
on a website maintained by the placement agent and the placement agent may distribute prospectuses electronically. Other than the prospectus
in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus
forms a part, has not been approved and/or endorsed by us or the placement agent and should not be relied upon by investors.
Transfer Agent
The transfer agent and registrar for our common stock
is VStock Transfer, LLC.
Nasdaq listing
Our shares of common stock are listed
on Nasdaq under the symbol “NAOV.”
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference”
information that we file with it into this prospectus, which means that we can disclose important information to you by referring you
to those documents. The information incorporated by reference is an important part of this prospectus. The information incorporated by
reference is considered to be a part of this prospectus, and information that we file later with the SEC will automatically update and
supersede information contained in this prospectus.
We incorporate by reference the documents listed below and any future filings
made with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act made after the date of the initial registration statement
of which this prospectus forms a part and prior to effectiveness of the registration statement and subsequent to the date of this prospectus
until the termination of the offering of the securities described in this prospectus (other than information in such filings that was
“furnished,” under applicable SEC rules, rather than “filed”). We incorporate by reference the following documents
or information that we have filed with the SEC:
| |
● |
Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on April 17, 2023; |
| |
|
|
| |
● |
Our
Definitive Proxy Statement on Schedule
14A, filed with the SEC on May 1, 2023; |
| |
|
|
| |
● |
Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, filed with the SEC on May 15, 2023; |
| |
|
|
| |
● |
Our Current Report on Form 8-K, filed with the SEC on February 8, 2023; |
| |
|
|
| |
● |
Our Current Report on Form 8-K, filed with the SEC on March 3, 2023; |
| |
|
|
| |
● |
Our Current Report on Form 8-K, filed with the SEC on April 19, 2023; |
| |
|
|
| |
● |
Our Current Report on Form 8-K, filed with the SEC
on May 25, 2023;
|
| |
● |
Our
Current Report on Form
8-K, filed with the SEC on June 21, 2023; and |
| |
● |
The description of our common stock contained in our Registration Statement on Form 8-A, filed on October 19, 2017 pursuant to Section 12(b) of the Exchange Act, which incorporates by reference the description of the shares of our common stock contained in the “Description of Securities” filed as Exhibit 4.15 to our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on April 17, 2023, and any amendment or report filed with the SEC for purposes of updating such description. |
In addition, all other reports subsequently filed
by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, prior to the termination of the
offering (excluding any information furnished rather than filed) shall be deemed to be incorporated by reference into this prospectus.
Notwithstanding the statements in the preceding paragraphs,
no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the
SEC pursuant to the Securities Exchange Act of 1934, as amended shall be incorporated by reference into this prospectus.
Any statement contained in this prospectus or contained
in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded
to the extent that a statement contained in this prospectus or any subsequently filed supplement to this prospectus, or document deemed
to be incorporated by reference into this prospectus, modifies or supersedes such statement. Any statements so modified or superseded
shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may request a copy of these filings at no cost,
by writing or telephoning us at the following address:
Stephen Brown
Chief Financial Officer
NanoVibronix, Inc.
525 Executive Blvd.
Elmsford, New York
(914) 233-3004
You may also access these filings on our website at
www.nanovibronix.com. You should rely only on the information incorporated by reference or provided in this prospectus. We have not authorized
anyone else to provide different or additional information on our behalf. An offer of these securities is not being made in any jurisdiction
where the offer or sale is not permitted. You should not assume that the information in this prospectus is accurate as of any date other
than the date of those respective documents.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of a registration statement
we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits
to the registration statement.
For further information with respect to us and the
securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a
part of the registration statement. You should rely only on the information contained in this prospectus or incorporated by reference
into this prospectus. We have not authorized anyone else to provide you with different information. We are not making an offer of these
securities in any jurisdiction where the offer is not permitted. You should assume that the information contained in this prospectus,
or any document incorporated by reference in this prospectus, is accurate only as of the date of those respective documents, regardless
of the time of delivery of this prospectus or any sale of our securities.
We file annual, quarterly and current reports, proxy
statements and other information with the SEC. Our SEC filings are available to the public from commercial document retrieval services
and over the Internet at the SEC’s website at http://www.sec.gov.
We maintain a website at www.nanovibronix.com. You
may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports
filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably
practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed
through, our website is not incorporated by reference into, and is not part of, this prospectus.
LEGAL MATTERS
The validity of the securities being
offered hereby and certain other legal matters will be passed upon for us by Haynes and Boone, LLP, New York, New York.
is acting as counsel to
the placement agent in connection with this offering.
EXPERTS
The consolidated financial statements
of NanoVibronix, Inc. and its subsidiary as of December 31, 2022, and December 31, 2021, and for each of the two years in the period ended
December 31, 2022, included in the Annual Report on Form 10-K for the year ended December 31, 2022, and incorporated in this prospectus
by reference, have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company’s
ability to continue as a going concern as described in Note 2 to the financial statements) of Marcum LLP, an independent registered public
accounting firm, given on the authority of said firm as experts in auditing and accounting.

NANOVIBRONIX, INC.
Up
to Shares of Common Stock
Prefunded
Warrants to purchase up to
Shares of Common Stock
Common
Warrants to purchase up to
Shares of Common Stock
Shares
of Common Stock underlying Prefunded Warrants and Common Warrants
Placement
Agent Warrants to Purchase up to
Shares of Common Stock
Shares of Common Stock Underlying the Placement
Agent Warrants
| |
Preliminary Prospectus
, 2023
|
|
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the estimated costs
and expenses payable by the registrant expected to be incurred in connection with the sale and distribution of the securities being registered
hereby (other than placement agent fees). All of such costs and expenses are estimates, except for the SEC registration fee and the Financial
Industry Regulatory Authority (“FINRA”) filing fee.
| | |
Amount to be Paid | |
| SEC registration fee | |
$ | 1,153.66 | |
| FINRA filing fee | |
$ | 2,070.32 | |
| Printing fees and expenses | |
$ | * | |
| Legal fees and expenses | |
$ | * | |
| Transfer agent and registrar fees and expenses | |
$ | * | |
| Accounting fees and expenses | |
$ | * | |
| Miscellaneous fees and expenses | |
$ | * | |
| | |
| | |
| Total | |
$ | * | |
* To be filed by amendment.
Item 14. Indemnification of Directors and Officers
Section 145 of the General Corporation
Law of the State of Delaware provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are,
may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit
or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was
a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer,
employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement
actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith
and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to
any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative
action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably
incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in
a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification
will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation
unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought
determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our certificate of incorporation
and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the
provisions of the General Corporation Law of the State of Delaware, as amended from time to time, subject to any permissible expansion
or limitation of such indemnification, as may be set forth in any stockholders’ or directors’ resolution or by contract. Any
repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation
on the liability of any of our directors or officers existing as of the time of such repeal or modification.
We are also permitted to apply
for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the
General Corporation Law of the State of Delaware would permit indemnification.
Item 15. Recent Sales of Unregistered Securities.
Bespoke
Agreement
On
February 11, 2019, we entered into a consulting agreement with Bespoke Growth Partners, Inc. (“Bespoke”), pursuant to
which, amongst other things, Bespoke was entitled to receive up to 32,500 shares of common stock of the Company, of which 13,750
shares were issued on the date of signing. On August 5, 2020, we issued an additional 18,750 shares of common stock, valued at
approximately $566,000, or $30.20 per share, to Bespoke under the Agreement. Such issuance was undertaken in reliance upon the
exemption from the registration requirements of the Securities Act of 1933, as amended, (the “Securities Act”), pursuant to Section 4(a)(2) thereof and Rule 506 of Regulation D
promulgated thereunder.
August
2020 Underwriter Warrants
On
August 24, 2020, we entered into an underwriting agreement with H.C. Wainwright & Co., LLC (“Wainwright”), pursuant
to which Wainwright acted as the sole book-running manager for an underwritten public offering of our securities. As partial
compensation for Wainwright’s services as underwriter in the offering, on August 27, 2020, we issued to Wainwright warrants to
purchase 16,993 shares of common stock, equal to 7.5% of the aggregate number of shares of common stock placed in the offering (the
“August 2020 Wainwright Warrants”). The August 2020 Wainwright Warrants expire on August 24, 2025 and have an exercise
price of $18.75 per share (equal to 125% of the offering price per share).
The
August 2020 Wainwright Warrants were issued in reliance on the exemptions from registration requirements of the Securities Act
provided by Section 4(a)(2) under the Securities Act and Regulation D promulgated thereunder.
September
2020 Underwriter Warrants
On
September 22, 2020, we entered into an underwriting agreement with Wainwright pursuant to which Wainwright acted as the sole
book-running manager for an underwritten public offering. As partial compensation for Wainwright’s services as underwriter
in the offering, on September 25, 2020, we issued to Wainwright warrants to purchase 6,731 shares of common stock, equal to 7.5% of
the aggregate number of shares of common stock placed in the offering (the “September 2020 Wainwright Warrants”). The
September 2020 Wainwright Warrants expire on September 22, 2025, and have an exercise price
of $25.00 per share (equal to 125% of the offering price per share).
The
September 2020 Wainwright Warrants were issued in reliance on the exemptions from registration requirements of the Securities Act
provided by Section 4(a)(2) under the Securities Act and Regulation D promulgated thereunder.
December
2020 Private Placement
On
December 2, 2020, we entered into a Securities Purchase Agreement with certain institutional and accredited investors, pursuant to
which we issued and sold to such investors in a private placement on December 7, 2020, an aggregate of (i) 295,715 shares of the our
common stock, at an offering price of $14.00 per share and (ii) pre-funded warrants to purchase up to 132,858 shares of common stock
(the “December 2020 Pre-funded Warrants”), at a purchase price of $13.98 per Pre-funded Warrant, for gross proceeds of
approximately $6.0 million, and net proceeds of approximately $5.4 million.
The
December 2020 Pre-funded Warrants have an exercise price of $0.02 per share. The December 2020 Pre-funded Warrants were immediately exercisable
and may be exercised at any time after their original issuance until such December 2020 Pre-funded Warrants are exercised in full. A
holder of a December 2020 Pre-funded Warrant may not exercise any portion of such holder’s December 2020 Pre-funded Warrants to
the extent that the holder, together with its affiliates, would beneficially own more than 4.99% (or, at the election of the holder,
9.99%) of the Company’s outstanding shares of common stock immediately after exercise, except that upon at least 61 days’
prior notice from the holder to the Company, the holder may increase the beneficial ownership limitation to up to 9.99% of the number
of shares of common stock outstanding immediately after giving effect to the exercise.
Wainwright
acted as the exclusive placement agent for the December 2020 Private Placement. As partial compensation for Wainwright’s services as placement agent for the private placement, we issued to Wainwright,
or its designees, warrants to purchase up to 32,143 shares of common stock, equal to 7.5% of the aggregate number of shares of
common stock and shares of common stock issuable upon the exercise of the December 2020 Pre-funded Warrants placed in the private placement (the “December 2020 Wainwright Warrants”). The December 2020 Wainwright Warrants are exercisable
immediately, have a term of five years from the date of the December 2020 Securities Purchase Agreement and an exercise price of
$17.50 per share (equal to 125% of the offering price per share).
The
securities issued in the private placement and the December 2020 Wainwright Warrants, and the shares of common stock issuable upon
the exercise of the December 2020 Pre-funded Warrants and the December 2020 Wainwright Warrants were offered pursuant to the
exemption from registration requirements of the Securities Act provided in Section 4(a)(2) under the Securities Act of 1933, as
amended, and Rule 506(b) promulgated thereunder.
January 2021 Letter Agreements
On January 21, 2021, we entered into
letter agreements (the “Letter Agreements”) with certain existing accredited investors to exercise certain outstanding warrants
(the “Existing Warrants”) to purchase up to an aggregate of 60,299 shares of the Company’s common stock at an
exercise price per share of $23.30 (the “Exercise”). Certain of the Existing Warrants (the “Registered Existing
Warrants”) and the shares of common stock underlying the Registered Existing Warrants have been registered pursuant to a registration
statement on Form S-3 (File No. 333-251264) and a registration statement on Form S-1 (File No. 333-218871). In consideration for the
exercise of the Existing Warrants for cash, the exercising holders received new unregistered warrants to purchase up to an aggregate
of 60,299 shares of common stock (the “New Warrants”) at an exercise price of $20.80 per share and with an
exercise period of seven years from the initial closing date.
The New Warrants were issued in reliance on
the exemption from registration requirements of the Securities Act provided by Section 4(a)(2) of the Securities Act and Rule 506 of
Regulation D promulgated thereunder.
MTSG Agreement
On January 1, 2022, we entered
into an International Marketing, Sales and Clinical Management Agreement (the “MTSG Agreement”) with MedTech Solutions Group,
LLC (“MTSG”). We appointed MTSG to provide international marketing, sales and clinical management expertise for commercialization
of our products in certain countries in Europe (excluding England, Scotland, Wales, Rep. of Ireland, Northern Ireland, Malta and Turkey),
Africa, Asia, Central and South America, and North America (Excluding the United States and Canada) as well as Israel and Palestine.
The MTSG Agreement provided that
MTSG or its designees be entitled to additional consideration in the form of annual grants of stock warrants at our discretion
for meeting revenue targets set forth in the MTSG Agreement. On June 14, 2022, MTSG was awarded warrants to purchase up to 1,250
shares of common stock, with an exercise price of $20.00 per share, that were exercisable at any time after the date
that is six months from the date of issuance and had an expiration date of June 14, 2029.
These securities were issued in reliance
upon exemptions from registration afforded by Section 4(a)(2) of the Securities Act of 1933 and Rule 506 of Regulation D promulgated
thereunder.
On September 30, 2022 the MTSG Agreement was terminated, and all warrants
issued to MTSG thereunder were cancelled as of that date.
November 2022 Placement Agent Warrants
On December 1, 2022, we closed a registered
direct offering of our shares of common stock. As partial compensation for Wainwright’s services as placement agent for the offering,
we issued to Wainwright or its designees, warrants to purchase up to 18,000 shares of common stock, equal to 7.5% of the aggregate
number securities in the offering (the “November 2022 Placement Agent Warrants”). The November Placement Agent
Warrants expire on November 29, 2027, and have an exercise price of $12.50 per share of common stock (equal to 125%
of the offering price per share of common stock).
The November 2022 Placement Agent Warrants
were issued in reliance on the exemptions from registration requirements of the Securities Act provided by Section 4(a)(2) under the
Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
| |
(a) |
The Exhibit Index is hereby incorporated herein by reference. |
| |
|
|
| |
(b) |
All schedules have been omitted because they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto. |
Item 17. Undertakings.
(1) The undersigned registrant hereby undertakes:
(a) To file, during any period
in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required
by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus
any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof)
which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding
the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed
that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more
than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in
the effective registration statement; and
(iii) To include any material information
with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information
in the registration statement provided, however, that paragraphs (a)(1)(i), (ii), and (iii) of this section do not apply if the information
required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission
by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)) that are incorporated
by reference in the registration statement, or, as to a registration statement.
provided, however, that paragraphs
(a)(1)(i), (ii), and (iii) of this section do not apply if the information required to be included in a post-effective amendment by those
paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d)
of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)) that are incorporated by reference in the registration statement.
(b) That, for the purpose of determining
any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating
to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof.
(c) To remove from registration
by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(d) That, for
the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b)
as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than
prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date
it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that
is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement
or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first
use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement
or made in any such document immediately prior to such date of first use.
(e) That,
for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the
securities, the undersigned registrant hereby undertakes that in a primary offering of securities of the undersigned registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or
prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter);
(ii) Any free writing prospectus
relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free
writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided
by or on behalf of the undersigned registrant; and
(iv) Any other communication that
is an offer in the offering made by the undersigned registrant to the purchaser.
(2) The undersigned registrant hereby undertakes that,
for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section
13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant
to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
(3) The undersigned registrant hereby undertakes that:
(a) For purposes of determining
any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement
in reliance on Rule 430A and contained in a form of prospectus filed by the undersigned registrant pursuant to Rule 424(b)(1) or (4) or
497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(b) For the purpose of determining
any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
(4) Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions,
or otherwise, the registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other
than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the
successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the
securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in
the Securities Act and will be governed by the final adjudication of such issue.
(5) The undersigned registrant hereby undertakes that
for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section
13(a) or section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant
to section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
EXHIBIT INDEX
| Exhibit No. |
|
Description |
| |
|
|
| 3.1 |
|
Amended and Restated Certificate of Incorporation (as presently in effect) (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on April 17, 2015) |
| |
|
|
| 3.2 |
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to Amendment No. 3 to the Registration Statement on Form S-1 filed with the Securities and Exchange Commission on April 30, 2014) |
| |
|
|
| 3.3 |
|
Amendment to the Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on November 3, 2021). |
| |
|
|
| 3.4 |
|
Certificate of Amendment of Certificate of Incorporation (creating the Series C Preferred Stock) (incorporated by reference to Exhibit 3.3 to Amendment No. 3 to the Registration Statement on Form S-1 filed with the Securities and Exchange Commission on April 30, 2014) |
| |
|
|
| 3.5 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series D Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on November 7, 2017) |
| |
|
|
| 3.6 |
|
Certificate of Designation, Preferences, Rights and Limitations of Series E Preferred Stock (incorporated by reference to Exhibit 4.1 to the Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 19, 2019) |
| |
|
|
|
3.7 |
|
Certificate of Amendment of the Amended and Restated Certificate of Designation (incorporated herein by reference to Exhibit 3.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 21, 2019) |
| |
|
|
| 3.8 |
|
Certificate of Amendment of Certificate of Incorporation (incorporated by reference to Exhibit 3.7 to the Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 15, 2021) |
| |
|
|
|
3.9 |
|
Amendment to the Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on November 3, 2021) |
| |
|
|
| 3.10 |
|
Certificate of Designation, Preferences, Rights and Limitations of Series F Preferred Stock (incorporated by reference to Exhibit 3.1 to the Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2022) |
| |
|
|
| 3.11 |
|
Certificate of Amendment of Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Current Report filed with the Securities and Exchange Commission on February 8, 2023) |
| |
|
|
| 4.1 |
|
Form of Common Stock Certificate (incorporated by reference to Exhibit 4.2 to Amendment No. 1 to the Registration Statement on Form S-1 filed with the Securities and Exchange Commission on March 6, 2014) |
| 4.2 |
|
Form of May 10 and May 15, 2019 Warrants (incorporated by reference to Exhibit 4.1 to the Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 20, 2019) |
| |
|
|
| 4.3 |
|
Form of Warrant (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on June 26, 2019) |
| |
|
|
| 4.4 |
|
Form of Preferred Warrant (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2019) |
| |
|
|
| 4.5 |
|
Form of Common Warrant (incorporated by reference to Exhibit 4.3 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2019) |
| |
|
|
| 4.6 |
|
Form of Warrant Amendment (incorporated by reference to Exhibit 4.10 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 20, 2020) |
| |
|
|
| 4.7 |
|
Form of Underwriter Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on August 26, 2020). |
| |
|
|
| 4.8 |
|
Form of Underwriter Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on September 24, 2020). |
| |
|
|
| 4.9 |
|
Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 7, 2020). |
| |
|
|
|
4.10 |
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 7, 2020). |
| |
|
|
| 4.11 |
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 1, 2020). |
| |
|
|
| 4.12^ |
|
Form of Pre-Funded Warrant |
| |
|
|
| 4.13^ |
|
Form of Common Warrant |
| |
|
|
| 4.14^ |
|
Form of Placement Agent Warrant |
| |
|
|
| 5.1^ |
|
Opinion of Haynes and Boone, LLP |
| |
|
|
| 10.1+ |
|
NanoVibronix, Inc. 2004 Global Share Option Plan (incorporated by reference to Exhibit 10.14 to Amendment No. 1 to the Registration Statement on Form S-1 filed with the Securities and Exchange Commission on March 6, 2014) |
| |
|
|
| 10.2+ |
|
Form of Indemnification Agreement between NanoVibronix, Inc. and certain of its officers and directors (incorporated by reference to Exhibit 10.16 to Amendment No. 1 to the Registration Statement on Form S-1 filed with the Securities and Exchange Commission on March 6, 2014) |
| |
|
|
| 10.3+ |
|
NanoVibronix, Inc. 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.27 to Amendment No. 3 to the Registration Statement on Form S-1 filed with the Securities and Exchange Commission on April 30, 2014) |
| |
|
|
| 10.4+ |
|
Letter Agreement, dated March 25, 2015, by and between NanoVibronix, Inc. and Martin Goldstein (incorporated by reference to Exhibit 10.39 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 30, 2015) |
| 10.5+ |
|
Form of Incentive Stock Option Award Agreement under the 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.40 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 30, 2015) |
| |
|
|
| 10.6+ |
|
Form of Nonqualified Stock Option Award Agreement under the 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.41 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 30, 2015) |
| |
|
|
| 10.7+ |
|
Form of Restricted Stock Award Agreement under the 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.42 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 30, 2015) |
| |
|
|
| 10.8+ |
|
Form of 3(i) Award Agreement under the Israeli Appendix to the 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.43 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 30, 2015) |
| |
|
|
| 10.9+ |
|
Form of 102 Award Agreement under the Israeli Appendix to the 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.44 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 30, 2015) |
| |
|
|
| 10.10+ |
|
Employment Agreement, dated October 13, 2016, by and between NanoVibronix, Inc. and Brian Murphy (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on October 19, 2016) |
| |
|
|
| 10.11 |
|
Form of Amendment to Warrant to Purchase Common Stock, effective as of January 27, 2017 (incorporated by reference to Exhibit 10.46 to the Annual Report on Form 10-K filed with the Securities Exchange Commission on March 31, 2017) |
| |
|
|
| 10.12 |
|
Form of Convertible Promissory Note (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 7, 2017) |
| |
|
|
| 10.13 |
|
Form of Warrant to Purchase Common Stock (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 7, 2017) |
| |
|
|
| 10.14 |
|
Warrant to Purchase Common Stock, dated March 23, 2017, by and between NanoVibronix, Inc. and an individual investor (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 27, 2017) |
| |
|
|
| 10.15+ |
|
First Amendment to Nonqualified Stock Option Agreement, dated March 30, 2017, between NanoVibronix, Inc. and Ira A. Greenstein (incorporated by reference to Exhibit 10.51 to the Annual Report on Form 10-K filed with the Securities Exchange Commission on March 31, 2017) |
| |
|
|
| 10.16+ |
|
First Amendment to Nonqualified Stock Option Agreement, dated March 30, 2017, between NanoVibronix, Inc. and Ira A. Greenstein (incorporated by reference to Exhibit 10.52 to the Annual Report on Form 10-K filed with the Securities Exchange Commission on March 31, 2017) |
| |
|
|
| 10.17+ |
|
Offer Letter, dated October 14, 2016, between NanoVibronix, Inc. and Christopher M. Fashek (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on October 19, 2016) |
| 10.18+ |
|
Nonqualified Stock Option Agreement, dated October 14, 2016, between NanoVibronix, Inc. and Christopher M. Fashek (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on October 19, 2016) |
| |
|
|
| 10.19 |
|
Form of Convertible Promissory Note (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 5, 2017) |
| |
|
|
| 10.20 |
|
Form of Warrant to Purchase Common Stock (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 5, 2017) |
| |
|
|
| 10.21 |
|
Form of Letter Agreement, dated September 7, 2017, between NanoVibronix, Inc. and holders of the 2017 Notes (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K/A filed with the Securities and Exchange Commission on September 14, 2017) |
| |
|
|
| 10.22 |
|
Form of Warrant (incorporated by reference to Exhibit 10.39 to the Annual Report on Form 10-K/A filed with the Securities and Exchange Commission on May 13, 2019) |
| |
|
|
| 10.23 |
|
Securities Purchase Agreement, dated as of June 21, 2019, by and among the Company and each investor identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on June 26, 2019) |
| |
|
|
| 10.24 |
|
Securities Purchase Agreement, dated as of July 31, 2019, by and among the Company and each investor identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2019) |
| |
|
|
| 10.25 |
|
Securities Purchase Agreement, dated as of July 31, 2019, by and among the Company and each investor identified on the signature pages thereto (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2019) |
| |
|
|
| 10.26 |
|
Form of Note (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on June 26, 2020). |
| |
|
|
| 10.27 |
|
Form of Warrant (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on June 26, 2020). |
| 10.28 |
|
Note with Cross River Bank (SBA-Payroll Protection Program loan) dated May 14, 2020 (incorporated by reference to Exhibit 10.3 to the Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 19, 2020). |
| |
|
|
| 10.29+ |
|
Employment Agreement, dated as of October 5, 2020, between NanoVibronix, Inc. and Stephen Brown (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on October 8, 2020). |
| |
|
|
| 10.30+ |
|
Option Cancellation and Release Agreement, dated November 2, 2020, by and between NanoVibronix, Inc. and Brian Murphy (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2020). |
| |
|
|
| 10.31+ |
|
Option Cancellation and Release Agreement, dated November 2, 2020, by and between NanoVibronix, Inc. and Christopher Fashek (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2020). |
| |
|
|
| 10.32+ |
|
Option Cancellation and Release Agreement, dated November 2, 2020, by and between NanoVibronix, Inc. and Martin Goldstein (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2020). |
| |
|
|
| 10.33+ |
|
Option Cancellation and Release Agreement, dated November 2, 2020, by and between NanoVibronix, Inc. and Michael Ferguson (incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2020). |
| |
|
|
| 10.34+ |
|
Option Cancellation and Release Agreement, dated November 2, 2020, by and between NanoVibronix, Inc. and Stephen Brown (incorporated by reference to Exhibit 10.5 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2020). |
| |
|
|
| 10.35+ |
|
Option Cancellation and Release Agreement, dated November 2, 2020, by and between NanoVibronix, Inc. and Thomas Mika (incorporated by reference to Exhibit 10.6 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2020). |
| |
|
|
| 10.36 |
|
Form of Securities Purchase Agreement, dated December 2, 2020 (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 7, 2020). |
| |
|
|
| 10.37 |
|
Form of Registration Rights Agreement, dated December 2, 2020 (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 7, 2020). |
| |
|
|
| 10.38# |
|
Amended and Restated Distribution Agreement for “Private Labeled” Products dated December 10, 2020 by and between NanoVibronix, Inc. and Ultra Pain Products Inc (incorporated by reference to Exhibit 10.58 to the Annual Report on Form 10-K filed with the Securities and Exchange Commission on April 15, 2021). |
| |
|
|
| 10.39+ |
|
Second Amendment to the NanoVibronix, Inc. 2014 Long-Term Incentive Plan. (incorporated by reference to Annex A to the Company’s definitive proxy statement on Schedule 14A filed with the SEC on April 30, 2019). |
| |
|
|
|
10.40+ |
|
Third Amendment to the Nanovibronix, Inc. 2014 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 30, 2021). |
|
* |
|
Filed herewith |
| ** |
|
Furnished herewith |
| ^ |
|
To be filed by amendment. |
| + |
|
Management contract or compensatory plan or arrangement. |
| # |
|
Certain portions of this exhibit have been redacted pursuant to Item 601(b)(10) of Regulation S-K. The omitted information is (i) not material and (ii) would likely cause competitive harm to the Company if publicly disclosed. |
SIGNATURES
Pursuant to the requirements of the Securities
Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, duly authorized, in City
of Elmsford, State of New York, on July 17, 2023.
| |
NANOVIBRONIX, INC. |
| |
|
|
| |
By: |
/s/ Brain Murphy |
| |
Name: |
Brian
Murphy |
| |
Title: |
Chief Executive Officer |
Power of Attorney
KNOW ALL BY THESE PRESENTS, that
each person whose signature appears below constitutes and appoints Brian Murphy and Stephen Brown as his or her true and lawful attorneys-in-fact
and agents, with the full power of substitution, for him or her and in his or her name, place or stead, in any and all capacities, to
sign any and all amendments to this registration statement (including post-effective amendments), and to sign any registration statement
for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under
the Securities Act, and all post-effective amendments thereto, and to file the same, with exhibits thereto and other documents in connection
therewith, with the SEC, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform
each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he
or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their or her substitute
or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of
the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Brian Murphy |
|
Chief Executive Officer and Director |
|
|
| Brian Murphy |
|
(Principal Executive Officer) |
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Stephen Brown |
|
Chief Financial Officer |
|
|
| Stephen Brown |
|
(Principal Financial and Accounting Officer) |
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Christopher Fashek |
|
Chairman of the Board of Directors |
|
|
| Christopher Fashek |
|
|
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Martin Goldstein |
|
|
|
|
| Martin Goldstein |
|
Director |
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Harold Jacob, M.D. |
|
|
|
|
| Harold Jacob, M.D. |
|
Director |
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Michael Ferguson |
|
|
|
|
| Michael Ferguson |
|
Director |
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Thomas R. Mika |
|
|
|
|
| Thomas R. Mika |
|
Director |
|
July
17, 2023 |
| |
|
|
|
|
| /s/ Aurora Cassirer |
|
Director |
|
July
17, 2023 |
| Aurora Cassirer |
|
|
|
|
| |
|
|
|
|
| /s/ Maria Schroeder |
|
Director |
|
July
17, 2023 |
| Maria Schroeder |
|
|
|
|
Exhibit
23.1
Independent
Registered Public Accounting Firm’s Consent
We
consent to the incorporation by reference in this Registration Statement of NanoVibronix, Inc. on Form S-1 of our report dated April
17, 2023, which includes an explanatory paragraph as to the Company’s ability to continue as a going concern, with respect to our
audits of the consolidated financial statements of NanoVibronix, Inc. as of December 31, 2022 and 2021 and for each of the two years
in the period ended December 31, 2022 appearing in the Annual Report on Form 10-K of NanoVibronix, Inc. for the year ended December 31,
2022. We also consent to the reference to our firm under the heading “Experts” in the Prospectus, which is part of this Registration
Statement.
/s/
Marcum llp
Marcum
llp
New
York, NY
July
17, 2023
EXHIBIT
107
EX-FILING
FEES
Calculation
of Filing Fee Tables
S-1
(Form
Type)
NANOVIBRONIX,
INC.
(Exact
Name of Registrant as Specified in its Charter)
Registrant
Name in English, if applicable
(Translation
of Registrant’s Name into English)
Table
1: Newly Registered and Carry Forward Securities
| | |
Security
Type | |
Security
Class Title | |
Fee Calculation or
Carry Forward
Rule | |
Amount
Registered | | |
Proposed
Maximum
Offering Price | | |
Maximum Aggregate Offering
Price(1) | | |
Fee
Rate | | |
Amount
of
Registration
Fee (2)
|
|
| Fees
to Be Paid | |
| |
| |
| |
| | | |
| | | |
| | | |
| | |
|
|
|
|
| | |
| |
| |
| |
| | | |
| | | |
| | | |
| | |
|
|
|
|
| | |
Equity | |
Common
Stock, $0.001 par value per share (2)(3) | |
457(o) | |
| – | | |
| – | | |
$ | 5,000,000.00 | | |
$ | 0.00011020 | | |
$ |
551.00 |
|
| | |
Other | |
Warrants
to purchase Common Stock (4) | |
457(g) | |
| – | | |
| – | | |
| – | | |
| – | | |
$ |
0.00 |
|
| | |
Equity | |
Common
Stock issuable upon exercise of Warrants to purchase Common Stock (2) | |
457(o) | |
| – | | |
| – | | |
$ | 5,000,000.00 | | |
$ | 0.00011020 | | |
$ |
551.00 |
|
| | |
Other | |
Prefunded
Warrants to purchase Common Stock (3)(4) | |
457(g) | |
| – | | |
| – | | |
| – | | |
| – | | |
$ |
0.00 |
|
| | |
Equity | |
Common
Stock issuable upon exercise of the Pre-Funded Warrants (2)(3) | |
457(o) | |
| – | | |
| – | | |
| – | | |
| – | | |
$ |
0.00 |
|
| | |
Other | |
Placement
Agent Warrants to purchase shares of Common Stock (4) | |
457(g) | |
| – | | |
| – | | |
| – | | |
| – | | |
$ |
0.00 |
|
| | |
Equity | |
Common
Stock issuable upon exercise of the Placement Agent Warrants (2) | |
457(o) | |
| – | | |
| – | | |
$ | 468,750 | | |
$ | 0.00011020 | | |
$ |
51.66 |
|
| TOTAL
OFFERING AMOUNTS | |
| |
| |
| |
| | | |
| | | |
$ | 10,468,750.00 | | |
| | | |
$ |
1,153.66 |
|
| TOTAL
FEES PREVIOUSLY PAID | |
| |
| |
| |
| | | |
| | | |
| | | |
| | | |
|
– |
|
| TOTAL
FEE OFFSETS | |
| |
| |
| |
| | | |
| | | |
| | | |
| | | |
|
– |
|
| NET
FEES DUE | |
| |
| |
| |
| | | |
| | | |
| | | |
| | | |
$ |
1,153.66 |
|
| |
(1) |
Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the
“Securities Act”). |
| |
|
|
| |
(2) |
Pursuant
to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of additional securities
as may be issuable to prevent dilution resulting from stock splits, dividends or similar transactions. |
| |
|
|
| |
(3) |
The proposed
maximum aggregate offering price of the common stock will be reduced on a dollar-for-dollar basis based on the offering price of
any prefunded warrants issued in the offering, and the proposed maximum aggregate offering price of the prefunded warrants to be
issued in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any common stock issued in the
offering. Accordingly, the proposed maximum aggregate offering price of the common stock and pre-funded warrants (including the common
stock issuable upon exercise of the pre-funded warrants), if any, is $5,000,000.00. |
| |
|
|
| |
(4) |
Pursuant
to Rule 457(g) under the Securities Act, no separate registration fee is required for the warrants because the warrants are being
registered in the same registration statement as the common stock issuable upon exercise of such warrants. |
NanoVibronix (NASDAQ:NAOV)
Historical Stock Chart
From Apr 2024 to May 2024
NanoVibronix (NASDAQ:NAOV)
Historical Stock Chart
From May 2023 to May 2024