false 0001419041 0001419041 2023-07-28 2023-07-28
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 28, 2023
FORTE BIOSCIENCES, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-38052 |
|
26-1243872 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 3060 Pegasus Park Dr. Building 6 Dallas, Texas |
|
75247 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (310) 618-6994
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.001 par value |
|
FBRX |
|
The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 1.01. |
Entry into a Material Definitive Agreement. |
Securities Purchase Agreement
On July 28, 2023, Forte Biosciences, Inc. (the “Company”) entered into a Securities Purchase Agreement (the “Purchase Agreement”) for a private placement (the “Private Placement”) with certain qualified institutional buyers, institutional accredited investors and certain executive officers, senior management, and members of the Board of Directors of the Company (the “Board”) (each, a “Purchaser” and collectively, the “Purchasers”). Pursuant to the Purchase Agreement, the Company agreed to sell to the Purchasers (i) 15,166,957 shares of the Company’s common stock, par value $0.001 per share (the “Shares”), at a purchase price of $1.006 per Share, and (ii) 9,689,293 pre-funded warrants (the “Pre-Funded Warrants”) to purchase Common Stock (the “Warrant Shares” and together with the Shares and the Pre-Funded Warrants, the “Securities”), at a purchase price of $1.005 per Pre-Funded Warrant. The Pre-Funded Warrants will have an exercise price of $0.001 per share of Common Stock, be immediately exercisable and remain exercisable until exercised in full. The holders of Pre-Funded Warrants may not exercise a Pre-Funded Warrant if the holder, together with its affiliates, would beneficially own more than 9.99% of the number of shares of Common Stock outstanding immediately after giving effect to such exercise. The holders of Pre-Funded Warrants may increase or decrease such percentages not in excess of 19.99% by providing at least 61 days’ prior notice to the Company. The Purchase Agreement also provides certain Purchasers a participation right in future offerings of the Company’s equity securities.
The Private Placement closed on July 31, 2023, subject to the satisfaction of customary closing conditions. The gross proceeds of the Private Placement were approximately $25 million, before deducting offering expenses payable by the Company. The Company intends to use the net proceeds from the Private Placement to fund working capital and other general corporate purposes.
Certain executive officers, senior management, and Board members of the Company participated in the Private Placement, purchasing approximately $1.16 million of Securities in the aggregate at a purchase price of $1.01 per share, representing the consolidated closing bid price of the Company’s common stock immediately preceding the time the Company entered into the Purchase Agreement pursuant to NASDAQ Rule 5635. The participation of these executive officers and Board members in the Private Placement was disclosed to, and approved by, the Board and the audit committee of the Board.
The foregoing description of the Purchase Agreement and the Pre-Funded Warrants does not purport to be complete and is qualified in its entirety by reference to the complete text of the Purchase Agreement and the form of the Pre-Funded Warrant, which are attached hereto as Exhibits 10.1 and 4.1, respectively, to this Current Report on Form 8-K and are hereby incorporated by reference into this Item 1.01.
Registration Rights
In connection with the Private Placement, the Company and the Purchasers entered into a Registration Rights Agreement, dated July 28, 2023 (the “Registration Rights Agreement”), providing for the registration for resale of the Securities (including the Warrant Shares) that are not then registered on an effective registration statement, pursuant to a registration statement (the “Registration Statement”) to be filed with the Securities and Exchange Commission (the “SEC”) within thirty (30) days following the closing of the Private Placement. The Company has agreed to use its best efforts to cause the Registration Statement to be declared effective as soon as possible, but in no event later than 60 days after the closing of the Private Placement (or 90 days in the event of a full review of the Registration Statement by the SEC), and to keep the Registration Statement continuously effective from the date on which the SEC declares the Registration Statement to be effective until such date that all Registrable Securities (as such term is defined in the Registration Rights Agreement) covered by the Registration Statement have been publicly sold by the Purchasers or otherwise shall have ceased to be Registrable Securities under the Registration Rights Agreement.
The Company has granted the Purchasers customary indemnification rights in connection with the Registration Rights Agreement. The Purchasers have also granted the Company customary indemnification rights in connection with the Registration Rights Agreement.
The foregoing description of the Registration Rights Agreement does not purport to be complete and is qualified in its entirety by reference to the Registration Rights Agreement, a copy of which is filed as Exhibit 10.2 hereto and incorporated by reference into this Item 1.01.
Amendment No. 2 to Preferred Stock Rights Agreement
As previously disclosed, on July 11, 2022, the Board authorized and declared a dividend distribution of one right (each, a “Right”) for each outstanding share of common stock of the Company to stockholders of record as of the close of business on July 21, 2022. In connection with the distribution of the Rights, the Company entered into a Preferred Stock Rights Agreement, dated as of July 12, 2022, between the Company and Computershare Trust Company, N.A., as rights agent, as amended on June 26, 2023 by that certain Amendment No. 1 to the Rights Agreement between the Company and Computershare Trust Company, N.A., as rights agent (the “Rights Agreement”).
On July 28, 2023, the Company entered into Amendment No. 2 to the Rights Agreement between the Company and Computershare Trust Company, N.A., as rights agent (the “Amendment”), which amends the Rights Agreement. The Amendment prevents the approval, execution, delivery or performance of the Purchase Agreement or the Pre-Funded Warrants, or the consummation of any of the transactions contemplated by the Purchase Agreement or the Pre-Funded Warrants, including any issuance of the Shares pursuant to the terms of the Purchase Agreement or the Pre-Funded Warrants, from, among other things, (i) causing or permitting the Rights to be exercised or exchanged, or (ii) causing any Purchaser or any of their respective affiliates to be deemed an Acquiring Person (as defined in the Rights Agreement) for any purpose under the Rights Agreement.
The Rights are in all respects subject to and governed by the provisions of the Rights Agreement, as amended by the Amendment. The description of the Rights Agreement is qualified in its entirety by reference to the full text of the Rights Agreement, a copy of which has been previously filed by the Company. The description of the Amendment is qualified in its entirety by reference to the full text of the Amendment, which is attached hereto as Exhibit 4.2 and incorporated herein by reference.
Item 3.02. Unregistered Sales of Equity Securities.
The information contained above under Item 1.01 is hereby incorporated by reference in response to this Item 3.02 of this Current Report on Form 8-K.
The Company will sell the securities to “accredited investors,” as that term is defined in the Securities Act of 1933, as amended (the “Securities Act”), in reliance on the exemption from registration afforded by Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated under the Securities Act and corresponding provisions of state securities or “blue sky” laws. The Purchasers represented that they are acquiring the securities for investment only and not with a view towards the resale or distribution thereof in violation of the Securities Act. Accordingly, the securities have not been registered under the Securities Act and such securities may not be offered or sold in the United States absent registration or an exemption from registration under the Securities Act and any applicable state securities laws.
Neither this Current Report on Form 8-K, nor any exhibit attached hereto, is an offer to sell or the solicitation of an offer to buy the Securities described herein.
Item 3.03. Material Modifications to Rights of Security Holders.
The information set forth under the caption “Amendment No. 2 to Preferred Stock Rights Agreement” in the section titled “Item 1.01. Entry into a Material Definitive Agreement” is incorporated herein by reference.
Item 7.01. Regulation FD Disclosure.
On August 1, 2023, the Company issued a press release announcing, among other things, the Private Placement and preclinical data on FB-102. The press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated into this Item 7.01 by reference.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act, except as expressly set forth by specific reference in such filing.
Item 8.01. Other Events.
The information set forth under the caption “Amendment No. 2 to Preferred Stock Rights Agreement” in the section titled “Item 1.01. Entry into a Material Definitive Agreement” is incorporated by reference.
The Company has updated its corporate presentation that it uses when meeting with investors, analysts and others to, among other matters, include additional preclinical data related to FB-102. A copy of the Company’s updated corporate presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K.
Forward Looking Statements
This report contains certain forward-looking statements regarding the business of the Company that are not a description of historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding the use of proceeds of the offering; the completion of the offering; and the registration of securities issued in the offering. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, those associated with market conditions; satisfaction of customary closing conditions in the Private Placement; and risks related to the Company’s estimates regarding future expenses, capital requirements and need for additional financing.
Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, including under the caption “Item 1A. Risk Factors” and in the Company’s subsequent Quarterly Report on Form 10-Q filed on May 15, 2023, and elsewhere in the Company’s reports and other documents that the Company has filed, or will file, with the SEC from time to time that are available at www.sec.gov.
You are cautioned not to place undue reliance on forward-looking statements which are current only as of the date hereof. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
|
|
| Exhibit |
|
Description |
| 4.1 |
|
Form of Pre-Funded Warrant |
| 4.2 |
|
Amendment No. 2 to Preferred Stock Rights Agreement, dated as of June 26, 2023, by and between Forte Biosciences, Inc. and Computershare Trust Company, N.A., as rights agent. |
| 10.1 |
|
Securities Purchase Agreement, dated July 28, 2023, by and among the Company and the Purchasers |
| 10.2 |
|
Registration Rights Agreement, dated July 28, 2023, by and among the Company and the Purchasers |
| 99.1 |
|
Press Release dated August 1, 2023 |
| 99.2 |
|
Corporate Presentation dated August 1, 2023 |
| 104 |
|
The cover page of this Current Report on Form 8-K, formatted in inline XBRL |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
FORTE BIOSCIENCES, INC. |
|
|
|
|
| Date: August 1, 2023 |
|
|
|
By: |
|
/s/ Antony Riley |
|
|
|
|
|
|
Antony Riley Chief Financial Officer |
Exhibit 4.1
THESE SECURITIES AND THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES ARE BEING OFFERED TO INVESTORS WITHOUT REGISTRATION WITH THE UNITED STATES
SECURITIES AND EXCHANGE COMMISSION UNDER THE SECURITIES ACT IN RELIANCE UPON REGULATION D PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (“THE SECURITIES ACT”). TRANSFER OF THESE SECURITIES AND THE SECURITIES ISSUABLE UPON
EXERCISE OF THESE SECURITIES IS PROHIBITED, EXCEPT PURSUANT TO REGISTRATION UNDER THE SECURITIES ACT, OR PURSUANT TO AVAILABLE EXEMPTION FROM REGISTRATION. HEDGING TRANSACTIONS MAY NOT BE CONDUCTED UNLESS IN COMPLIANCE WITH THE SECURITIES ACT.
FORTE BIOSCIENCES, INC.
FORM OF PRE-FUNDED WARRANT TO PURCHASE COMMON STOCK
Number of Shares: [ ]
(subject to adjustment)
|
|
|
| Warrant No. |
|
Original Issue Date: [ ], 2023 |
Forte Biosciences, Inc. , a Delaware corporation (the “Company”), hereby certifies that, for good and
valuable consideration, the receipt and sufficiency of which are hereby acknowledged, [ ] or its registered assigns (the “Holder”), is entitled, subject to the terms set forth below, to purchase from the
Company up to a total of [ ] shares of common stock, $0.001 par value per share (the “Common Stock”), of the Company (each such share, a “Warrant Share” and all such shares, the
“Warrant Shares”) at an exercise price per share equal to $0.001 per share (as adjusted from time to time as provided in Section 9 herein, the “Exercise Price”), upon surrender of this
Warrant to Purchase Common Stock (including any Warrants to Purchase Common Stock issued in exchange, transfer or replacement hereof, the “Warrant”) at any time and from time to time on or after the date hereof (the
“Original Issue Date”), subject to the following terms and conditions:
1. Definitions. For purposes of this Warrant, the
following terms shall have the following meanings:
(a) “Affiliate” means any Person directly or indirectly controlled by, controlling or under
common control with, a Holder, but only for so long as such control shall continue. For purposes of this definition, “control” (including, with correlative meanings, “controlled by”, “controlling” and “under common
control with”) means, with respect to a Person, possession, direct or indirect, of (a) the power to direct or cause direction of the management and policies of such Person (whether through ownership of securities or partnership or other
ownership interests, by contract or otherwise), or (b) at least 50% of the voting securities (whether directly or pursuant to any option, warrant or other similar arrangement) or other comparable equity interests.
(b) “Commission” means the United States Securities and Exchange Commission.
(c) “Closing Sale Price” means, for any security as of any date, the last trade price for such security on the Principal Trading Market for
such security, as reported by Bloomberg Financial Markets, or, if such Principal Trading Market begins to operate on an extended hours basis and does not designate the last trade price, then the last trade price of such security prior to 4:00 P.M.,
New York City time, as reported by Bloomberg Financial Markets, or if the foregoing do not apply, the last trade price of such security in the over-the-counter market on
the electronic bulletin board for such security as reported by Bloomberg Financial Markets, or if no last trade price is reported for such security by Bloomberg Financial Markets, the average of the bid and ask prices, of any market makers for such
security as reported in the OTC Link or “pink sheets” by OTC Markets Group Inc. (formerly OTC Markets Inc.) as of 4:00 P.M., New York City time on such date. If the Closing Sale Price cannot be calculated for a security on a particular
date on any of the foregoing bases, the Closing Sale Price of such security on such date shall be the fair market value as mutually determined by the Company and the Holder. If the Company and the Holder are unable to agree upon the fair market
value of such security, then the Board of Directors of the Company shall use its good
faith judgment to determine the fair market value. The Board of Directors’ determination shall be binding upon all parties absent demonstrable error. All such determinations shall be
appropriately adjusted for any stock dividend, stock split, stock combination or other similar transaction during the applicable calculation period.
(d)
“Principal Trading Market” means the national securities exchange or other trading market on which the Common Stock is primarily listed on and quoted for trading, which, as of the Original Issue Date, shall be the Nasdaq Capital
Market.
(e) “Securities Act” means the Securities Act of 1933, as amended.
(f) “Securities Purchase Agreement” means that certain Securities Purchase Agreement dated as of July 28, 2023.
(g) “Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, for the Company’s primary
trading market or quotation system with respect to the Common Stock that is in effect on the date of delivery of an applicable Exercise Notice, which as of the Original Issuance Date was “T+2”.
(g) “Trading Day” means any weekday on which the Principal Trading Market is normally open for trading.
(h) “Transfer Agent” means Computershare Trust Company, N.A., the Company’s transfer agent and registrar for the Common Stock, and any
successor appointed in such capacity, or if none, the Company.
2. Issuance of Securities; Registration of Warrants. The Warrant, as initially
issued by the Company, is offered and sold pursuant to the Securities Purchase Agreement. Accordingly, as of the Original Issue Date, the Warrant and the Warrant Shares are “restricted securities” under Rule 144 promulgated under the
Securities Act. The Company shall register ownership of this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder (which shall include the initial Holder
or, as the case may be, any assignee to which this Warrant is assigned hereunder) from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any
distribution to the Holder, and for all other purposes, absent actual notice to the contrary. This Warrant may be, at the option of the Holder, either (x) represented by an original Warrant certificate or (y) issued by book-entry
registration in the Warrant Register. For the avoidance of doubt, any Warrant issued by book-entry registration in the Warrant Register shall nonetheless be subject to the terms and conditions of such Warrant certificate to the same extent as if
such Warrant were represented by an original Warrant certificate.
3. Registration of Transfers. Subject to compliance with all applicable
securities laws, the Company shall, or will cause its Transfer Agent to, register the transfer of all or any portion of this Warrant in the Warrant Register, upon surrender of this Warrant, and payment for all applicable transfer taxes (if any).
Notwithstanding the foregoing, no surrender of a Warrant shall be required if such Warrant is represented by book-entry registration in the Warrant Register. Upon any such registration or transfer, a new warrant to purchase Common Stock in
substantially the form of this Warrant (any such new warrant, a “New Warrant”) evidencing the portion of this Warrant so transferred shall be issued to the transferee, and a New Warrant evidencing the remaining portion of this
Warrant not so transferred, if any, shall be issued to the transferring Holder. The acceptance of the New Warrant by the transferee thereof shall be deemed the acceptance by such transferee of all of the rights and obligations in respect of the New
Warrant that the Holder has in respect of this Warrant. The Company shall, or will cause its Transfer Agent to, prepare, issue and deliver at the Company’s own expense any New Warrant under this Section 3. Until due
presentment for registration of transfer, the Company may treat the registered Holder hereof as the owner and holder for all purposes, and the Company shall not be affected by any notice to the contrary.
4. Exercise and Duration of Warrants.
(a) All or any
part of this Warrant shall be exercisable by the registered Holder in any manner permitted by this Warrant at any time and from time to time on or after the Original Issue Date.
(b) The Holder may exercise this Warrant by delivering to the Company (i) an exercise notice, in the form attached as Schedule 1 hereto (the
“Exercise Notice”), completed and duly signed, and (ii) payment of the Exercise Price
(other than in the case of a cashless exercise) for the number of Warrant Shares as to which this Warrant is being exercised (which may take the form of a “cashless exercise” if so
indicated in the Exercise Notice pursuant to Section 10 below), and the date on which the last of such items is delivered to the Company (as determined in accordance with the notice provisions hereof) is an
“Exercise Date.” The Holder shall not be required to deliver the original Warrant in order to effect an exercise hereunder nor shall any medallion guarantee (or other type of guarantee or notarization) of any Exercise Notice be
required. Execution and delivery of the Exercise Notice shall have the same effect as cancellation of the original Warrant and issuance of a New Warrant evidencing the right to purchase the remaining number of Warrant Shares, if any. The aggregate
exercise price of this Warrant, except for the Exercise Price, was pre-funded to the Company on or before the Original Issue Date, and consequently no additional consideration (other than the Exercise Price)
shall be required by to be paid by the Holder to effect any exercise of this Warrant. The Holder shall not be entitled to the return or refund of all, or any portion, of such pre-funded exercise price under
any circumstance or for any reason whatsoever. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares
hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof. The aggregate exercise price of this Warrant, except for the Exercise Price, was pre-funded to the Company on or before the Original Issue Date, and consequently no additional consideration (other than the Exercise Price) shall be required by to be paid by the Holder to effect any exercise of
this Warrant.
5. Delivery of Warrant Shares.
(a)
Upon exercise of this Warrant, the Company shall promptly (but in no event later than the number of Trading Days comprising the Standard Settlement Period ), credit such aggregate number of shares of Common Stock to which the Holder is entitled
pursuant to such exercise to the Holder’s or its designee’s balance account with The Depository Trust Company (“DTC”) through its Deposit/Withdrawal At Custodian system, or if the Transfer Agent is not participating in the
Fast Automated Securities Transfer Program (the “FAST Program”) or if the certificates are required to bear a legend regarding restriction on transferability, issue and dispatch by overnight courier to the address as specified in
the Exercise Notice, a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of shares of Common Stock to which the Holder is entitled pursuant to such exercise. The Company agrees to
maintain a transfer agent that is a participant in the FAST program so long as the Warrant remains outstanding and exercisable. The Holder, or any natural person or legal entity (each, a “Person”) so designated by the Holder to receive
Warrant Shares, shall be deemed to have become the holder of record of such Warrant Shares as of the Exercise Date, irrespective of the date such Warrant Shares are credited to the Holder’s DTC account or the date of delivery of the
certificates evidencing such Warrant Shares, as the case may be.
(b) If within the Standard Settlement Period after the Exercise Date, the Company fails
to deliver to the Holder or its designee the required number of Warrant Shares in the manner required pursuant to Section 5(a) or fails to credit the Holder’s or its designee’s balance account with DTC for such number of Warrant
Shares to which the Holder is entitled (other than a failure caused by incorrect or incomplete information provided by the Holder to the Company), and if after such number of Trading Days comprising the Standard Settlement Period and prior to the
receipt of such Warrant Shares, the Holder purchases (in an open market transaction or otherwise) shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such
exercise (a “Buy-In”), then the Company shall, within two (2) Trading Days after the Holder’s request and in the Holder’s sole discretion, either (1) pay in cash to the
Holder an amount equal to the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased, at which point the Company’s obligation to issue such Warrant Shares shall terminate or
(2) promptly honor its obligation to deliver to the Holder or its designee such Warrant Shares or credit the Holder’s or its designee’s balance account with DTC for such Warrant Shares and pay cash to the Holder in an amount equal to
the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased in the Buy-In, less the product of (A) the number of shares of Common
Stock purchased in the Buy-In, times (B) the Closing Sale Price of a share of Common Stock on the Exercise Date.
(c) To the extent permitted by law and subject to Section 5(b), the Company’s obligations to issue and deliver Warrant Shares in accordance with and
subject to the terms hereof (including the limitations set forth in Section 11 below) are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any
waiver or consent with respect to any provision hereof, the recovery of any judgment against any Person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or
termination, or any breach or alleged breach by the Holder or any other Person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other Person, and irrespective of any other circumstance that might
otherwise limit such obligation of the Company to the Holder in connection with the issuance of Warrant Shares. Subject to Section 5(b), nothing herein shall limit the Holder’s right to pursue any other remedies available to it hereunder,
at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common Stock in book-entry form upon exercise of the Warrant as
required pursuant to the terms hereof.
6. Charges, Taxes and Expenses. Issuance and delivery of shares of Common Stock in book-entry form upon
exercise of this Warrant shall be made without charge to the Holder for any issue or transfer tax, transfer agent fee or other incidental tax or expense (excluding any applicable stamp duties) in respect of the issuance of such shares, all of which
taxes and expenses shall be paid by the Company; provided, however, that the Company shall not be required to pay any tax that may be payable in respect of any transfer involved in the registration of any Warrant Shares or the Warrants in a
name other than that of the Holder or an Affiliate thereof. The Holder shall be responsible for all other tax liability that may arise as a result of holding or transferring this Warrant or receiving Warrant Shares upon exercise hereof.
7. Replacement of Warrant. If this Warrant is mutilated, lost, stolen or destroyed, the Company shall issue or cause to be issued in exchange and
substitution for and upon cancellation hereof, or in lieu of and substitution for this Warrant, a New Warrant, but only upon receipt of evidence reasonably satisfactory to the Company of such loss, theft or destruction (in such case) and, in each
case, a customary and reasonable contractual indemnity, if requested by the Company. If a New Warrant is requested as a result of a mutilation of this Warrant, then the Holder shall deliver such mutilated Warrant to the Company as a condition
precedent to the Company’s obligation to issue the New Warrant.
8. Reservation of Warrant Shares. The Company covenants that it will, at all
times while this Warrant is outstanding, reserve and keep available out of the aggregate of its authorized but unissued and otherwise unreserved Common Stock, solely for the purpose of enabling it to issue Warrant Shares upon exercise of this
Warrant as herein provided, the number of Warrant Shares that are initially issuable and deliverable upon the exercise of this entire Warrant, free from preemptive rights or any other Purchase Rights (as defined below) of persons other than the
Holder (taking into account the adjustments and restrictions of Section 9). The failure of the Company to reserve and keep available out of the aggregate of its authorized but unissued and otherwise unreserved Common Stock
a sufficient number of shares of Common Stock to enable it to issue Warrant Shares upon exercise of this Warrant as herein provided is referred to herein as an “” Authorized Share Failure.” The Company covenants that all
Warrant Shares so issuable and deliverable shall, upon issuance and the payment of the applicable Exercise Price in accordance with the terms hereof, be duly and validly authorized, issued and fully paid and
non-assessable. The Company will take all such action as may be necessary to assure that such shares of Common Stock may be issued as provided herein without violation of any applicable law or regulation, of
any requirements of any securities exchange or automated quotation system upon which the Common Stock may be listed or of any contract to which the Company or any of its subsidiaries is bound. The Company further covenants that it will not, without
the prior written consent of the Holder, take any actions to increase the par value of the Common Stock at any time while this Warrant is outstanding. In furtherance of the Company’s obligations set forth in this Section 8, as soon as
practicable after the date of the occurrence of an Authorized Share Failure, but in no event later than ninety (90) days after the occurrence of such Authorized Share Failure, the Company shall hold a meeting of its stockholders for the
approval of an increase in the number of authorized shares of Common Stock. In connection with such meeting, the Company shall provide each stockholder with a proxy statement and shall use its reasonable best efforts to solicit its
stockholders’ approval of such increase in authorized shares of Common Stock and to cause its board of directors to recommend to the stockholders that they approve such proposal. Notwithstanding the foregoing, if any such time of an Authorized
Share Failure, the Company is able to obtain the written consent of a majority of the shares of its issued and outstanding shares of Common Stock to approve the increase in the number of authorized shares of Common Stock, the Company may satisfy
this obligation by obtaining such consent and submitting for filing with the SEC a definitive Information Statement on Schedule 14C, and such obligation shall be deemed satisfied on the 21st calendar day after such filing is accepted.
9. Certain Adjustments. The Exercise Price and number of Warrant Shares issuable upon exercise of
this Warrant are subject to adjustment from time to time as set forth in this Section 9.
(a) Stock Dividends and Splits.
If the Company, at any time while this Warrant is outstanding, (i) pays a stock dividend on its Common Stock or otherwise makes a distribution on any class of capital stock payable in shares of Common Stock, (ii) subdivides its outstanding
shares of Common Stock into a larger number of shares of Common Stock, (iii) combines its outstanding shares of Common Stock into a smaller number of shares of Common Stock or (iv) issues by reclassification of shares of capital stock any
additional shares of Common Stock of the Company, then in each such case the Exercise Price shall be multiplied by a fraction, the numerator of which shall be the number of shares of Common Stock outstanding immediately before such event and the
denominator of which shall be the number of shares of Common Stock outstanding immediately after such event. Any adjustment made pursuant to clause (i) of this paragraph shall become effective immediately after the record date for the
determination of stockholders entitled to receive such dividend or distribution, provided, however, that if such record date shall have been fixed and such dividend is not fully paid on the date fixed therefor, the Exercise Price shall be recomputed
accordingly as of the close of business on such record date and thereafter the Exercise Price shall be adjusted pursuant to this paragraph as of the time of actual payment of such dividends. Any adjustment pursuant to clause (ii) or
(iv) of this paragraph shall become effective immediately after the effective date of such subdivision or combination or reclassification.
(b)
Pro Rata Distributions. If on or after the Original Issue Date, the Company shall declare or make any dividend or other pro rata distribution of its assets (or rights to acquire its assets) (including, without limitation, by way of return of
capital) to holders of shares of Common Stock (including, without limitation, any distribution of cash, stock or other securities, property, options, evidence of indebtedness or any other assets by way of a dividend, spin off, reclassification,
corporate rearrangement, scheme of arrangement or other similar transaction, but, for the avoidance of doubt, excluding any distribution of shares of Common Stock subject to Section 9(a), any distribution of Purchase Rights subject to
Section 9(c) and any Fundamental Transaction (as defined below) subject to Section 9(d)) (a “Distribution”) then, in each such case, upon any exercise of this Warrant that occurs after the record date fixed for
determination of stockholders entitled to receive such distribution, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of shares of
Common Stock acquirable upon such exercise of this Warrant (without regard to any limitations or restrictions on exercise of this Warrant, including without limitation, the Maximum Percentage (as defined below)) immediately before the date on which
a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, that to the extent that the
Holder’s right to participate in any such Distribution would result in the Holder (including its Affiliates or members of a Section 13(d) group) exceeding the Maximum Percentage, then the Holder shall not be entitled to participate in such
Distribution to such extent (and shall not be entitled to beneficial ownership of such shares of Common Stock as a result of such Distribution (and beneficial ownership) to such extent) and the portion of such Distribution shall be held in abeyance
for the benefit of the Holder until such time or times as (all or a portion) its right thereto would not result in the Holder (including Affiliates and any member of a Schedule 13(d) group) exceeding the Maximum Percentage, at which time or times
the Holder shall be granted (all or such portion of) such Distribution (and any Distributions declared or made on such initial Distribution or on any subsequent Distribution held similarly in abeyance) to the same extent as if there had been no such
limitation).
(c) Purchase Rights. If at any time on or after the Original Issue Date, the Company grants, issues or sells any Options, Convertible
Securities or rights to purchase stock, warrants, securities or other property, in each case pro rata to the record holders of any class of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire,
upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to
any limitations or restrictions on exercise of this Warrant, including without limitation, the Maximum Percentage) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record
is taken, the date as of which the record holders of Common Stock are to be determined for the grant, issuance or sale of such Purchase Rights (provided, that to the extent that the Holder’s right to participate in any such Purchase Right would
result in the Holder (including any Affiliates or members of a Schedule 13(d) group) exceeding the Maximum Percentage, then the Holder shall not be entitled to participate in such Purchase Right to such extent (and shall not be entitled to
beneficial ownership of such Common
Stock as a result of such Purchase Right (and beneficial ownership) to such extent) and such Purchase Right to such extent shall be held in abeyance for the benefit of the Holder until such time
or times as its right thereto would not result in the Holder (including Affiliates or members of a Schedule 13(d) group) exceeding the Maximum Percentage, at which time or times the Holder shall be granted such right (and any Purchase Right granted,
issued or sold on such initial Purchase Right or on any subsequent Purchase Right to be held similarly in abeyance) to the same extent as if there had been no such limitation). As used in this Section 9(c), (i) “Options”
means any rights, warrants or options to subscribe for or purchase shares of Common Stock or Convertible Securities and (ii) “Convertible Securities” mean any capital stock, debt, securities or other contractual rights (other
than Options) directly or indirectly convertible into or exercisable or exchangeable for shares of Common Stock.
(c) Fundamental Transactions. If,
at any time while this Warrant is outstanding (i) the Company effects any merger or consolidation of the Company with or into another Person, in which the Company is not the surviving entity and in which the stockholders of the Company
immediately prior to such merger or consolidation do not own, directly or indirectly, at least 50% of the voting power of the surviving entity immediately after such merger or consolidation, (ii) the Company effects any sale to another Person
of all or substantially all of its assets in one transaction or a series of related transactions, (iii) pursuant to any tender offer or exchange offer (whether by the Company or another Person), holders of capital stock who tender shares
representing more than 50% of the voting power of the capital stock of the Company and the Company or such other Person, as applicable, accepts such tender for payment, (iv) the Company consummates a stock purchase agreement or other business
combination (including, without limitation, a reorganization, recapitalization, spin-off or scheme of arrangement) with another Person whereby such other Person acquires more than the 50% of the voting power
of the capital stock of the Company (except for any such transaction in which the stockholders of the Company immediately prior to such transaction maintain, in substantially the same proportions, the voting power of such Person immediately after
the transaction), provided, however, that the forgoing shall not include transactions for which the primary purpose is raising capital or (v) the Company effects any reclassification of the Common Stock or any compulsory share exchange pursuant
to which the Common Stock is effectively converted into or exchanged for other securities, cash or property (other than as a result of a subdivision or combination of shares of Common Stock covered by Section 9(a) above)
(in any such case, a “Fundamental Transaction”), then following such Fundamental Transaction the Holder shall have the right to receive, upon exercise of this Warrant, the same amount and kind of securities, cash or property as it
would have been entitled to receive upon the occurrence of such Fundamental Transaction if it had been, immediately prior to such Fundamental Transaction, the holder of the number of Warrant Shares then issuable upon exercise in full of this Warrant
without regard to any limitations on exercise contained herein (the “Alternate Consideration”). The Company shall not effect any Fundamental Transaction in which the Company is not the surviving entity unless (i) the Alternate
Consideration is solely cash and the Company provides for the simultaneous “cashless exercise” of this Warrant pursuant to Section 10 below or (ii) prior to or simultaneously with the consummation thereof, any successor to
the Company, surviving entity or other Person (including any purchaser of assets of the Company) shall assume the obligation to deliver to the Holder such Alternate Consideration as, in accordance with the foregoing provisions, the Holder may be
entitled to receive, and the other obligations under this Warrant. The provisions of this paragraph (c) shall similarly apply to subsequent transactions analogous of a Fundamental Transaction type.
(d) Number of Warrant Shares. Simultaneously with any adjustment to the Exercise Price pursuant to Section 9, the number of
Warrant Shares that may be purchased upon exercise of this Warrant shall be increased or decreased proportionately, so that after such adjustment the aggregate Exercise Price payable hereunder for the increased or decreased number of Warrant Shares
shall be the same as the aggregate Exercise Price in effect immediately prior to such adjustment. Notwithstanding the foregoing, in no event may the Exercise Price be adjusted below the par value of the Common Stock then in effect.
(e) Calculations. All calculations under this Section 9 shall be made to the nearest
one-tenth of one cent or the nearest share, as applicable.
(f) Notice of Adjustments. Upon the occurrence
of each adjustment pursuant to this Section 9, the Company at its expense will, at the written request of the Holder, promptly compute such adjustment, in good faith, in accordance with the terms of this Warrant and prepare
a certificate setting forth such adjustment, including a statement of the adjusted Exercise Price and adjusted number or type of Warrant Shares or other securities issuable upon exercise of this Warrant (as applicable), describing the transactions
giving rise to such adjustments and showing in detail the
facts upon which such adjustment is based. Upon written request, the Company will promptly deliver a copy of each such certificate to the Holder and to the Company’s transfer agent.
(g) Notice of Corporate Events. If, while this Warrant is outstanding, the Company (i) declares a dividend or any other pro rata distribution of
cash, securities or other property in respect of its Common Stock, including, without limitation, any granting of rights or warrants to subscribe for or purchase any capital stock of the Company or any subsidiary, (ii) authorizes or approves,
enters into any agreement contemplating or solicits stockholder approval for any Fundamental Transaction or (iii) authorizes the voluntary dissolution, liquidation or winding up of the affairs of the Company, then, except if such notice and the
contents thereof shall be deemed to constitute material non-public information, the Company shall deliver to the Holder a notice of such transaction at least ten (10) days prior to the applicable record
or effective date on which a Person would need to hold Common Stock in order to participate in or vote with respect to such transaction; provided, however, that the failure to deliver such notice or any defect therein shall not affect the
validity of the corporate action required to be described in such notice. In the event such notice and the contents thereof shall be deemed to constitute material non-public information, the Company shall (on
the same time frame set forth in the immediately prior sentence) offer the Holder the ability to sign a confidentiality agreement related thereto sufficient to allow the Holder to receive such notice, and the Company shall deliver such notice
immediately upon execution of such confidentiality agreement. If the holder does not sign the confidentiality agreement, then the Holder shall not receive such notice. In addition, if while this Warrant is outstanding, the Company authorizes or
approves, enters into any agreement contemplating or solicits stockholder approval for any Fundamental Transaction contemplated by Section 9(d), other than a Fundamental Transaction under clause (iii) of
Section 9(d), the Company shall deliver to the Holder a notice of such Fundamental Transaction at least thirty (30) days prior to the date such Fundamental Transaction is consummated. Holder agrees to maintain any information disclosed
pursuant to this Section 9(g) in confidence until such information is publicly available, and shall comply with applicable law with respect to trading in the Company’s securities following receipt any such information.
10. Payment of Exercise Price. Notwithstanding anything contained herein to the contrary, the Holder may, in its sole discretion, satisfy its
obligation to pay the Exercise Price through a “cashless exercise”, in which event the Company shall issue to the Holder the number of Warrant Shares in an exchange of securities effected pursuant to Section 3(a)(9) of the Securities
Act, as determined as follows:
X = Y [(A-B)/A]
where:
“X” equals the number of Warrant Shares to be
issued to the Holder;
“Y” equals the total number of Warrant Shares with respect to which this Warrant is then being exercised;
“A” equals the Closing Sale Price of the shares of Common Stock (as reported by Bloomberg Financial Markets) as of the Trading Day on the date
immediately preceding the Exercise Date; and
“B” equals the Exercise Price then in effect for the applicable Warrant Shares at the time of such
exercise.
For purposes of Rule 144 promulgated under the Securities Act, it is intended, understood and acknowledged that the Warrant Shares issued in a
“cashless exercise” transaction shall be deemed to have been acquired by the Holder, and the holding period for the Warrant Shares shall be deemed to have commenced, on the date this Warrant was originally issued (provided that the
Commission continues to take the position that such treatment is proper at the time of such exercise). In the event that a registration statement registering the issuance of Warrant Shares is, for any reason, not effective at the time of exercise of
this Warrant, then the Warrant may only be exercised through a cashless exercise, as set forth in this Section 10. Except as set forth in Section 5(b) (Buy-In remedy) and Section 12 (payment of
cash in lieu of fractional shares), in no event will the exercise of this Warrant be settled in cash.
11. Limitations on Exercise.
(a) Any Holder of Warrants shall be prohibited from exercising the Holder’s Warrants
if, immediately prior to or following such exercise (or portion of such exercise thereof), the Holder, together with its Affiliates and any member of a Section 13(d) group, beneficially owns or would beneficially own as determined in accordance
with Section 13(d) of the U.S. Securities Exchange Act of 1934, as amended, and the rules thereunder (the “Exchange Act”) more than 9.99% (the “Beneficial Ownership Limitation”) of the issued and outstanding
Common Stock or any other class of equity security (other than an exempted security) of the Company that is registered pursuant to Section 12 of the Exchange Act. For purposes of calculating beneficial ownership, the aggregate number of shares
of Common Stock beneficially owned by the Holder, together with its affiliates and any member of the Section 13(d) group, shall include the number of shares of Common Stock issuable upon exercise of the relevant Warrants with respect to which
the determination is being made, but shall exclude the number of shares of Common Stock which are issuable upon (i) conversion of the remaining, unconverted Warrants beneficially owned by a Holder, together with its Affiliates and any member of
a Section 13(d) group, and (ii) exercise or conversion of the unexercised or unconverted portion of any other securities of the Company beneficially owned by such Holder, together with its Affiliates and any member of a Section 13(d)
group (including, without limitation, any convertible notes, convertible stock or warrants) that are subject to a limitation on conversion or exercise analogous to the limitation contained herein. For purposes of this Section 11(a), beneficial
ownership and whether a Holder is a member of a Section 13(d) group shall be calculated and determined in accordance with Section 13(d) of the Exchange Act and the rules promulgated thereunder, it being acknowledged and agreed that the
Holder is solely responsible for any schedules required to be filed in accordance therewith. For purposes of the Warrants, in determining the number of outstanding shares of Common Stock, a Holder of Warrants may rely on the number of outstanding
shares of Common Stock as reflected in (1) the Company’s most recent Form 10-K, Form 10-Q, Current Report on Form 8-K
or other public filing with the Securities and Exchange Commission, as the case may be, (2) a more recent public announcement by the Company or (3) any other notice by the Company or the Company’s transfer agent setting forth the
number of shares of Common Stock outstanding (the “Reported Outstanding Share Number”). If the Company receives an Exercise Notice from the Holder at a time when the actual number of outstanding shares of Common Stock is less than
the Reported Outstanding Share Number, the Company shall (i) notify the Holder in writing of the number of shares of Common Stock then outstanding and, to the extent that such Exercise Notice would otherwise cause the Holder’s beneficial
ownership, as determined pursuant to this Section 11(a), to exceed the Beneficial Ownership Limitation, the Holder must notify the Company of a reduced number of Warrant Shares to be purchased pursuant to such Exercise Notice (the number of
shares by which such purchase is reduced, the “Reduction Shares”) and (ii) as soon as reasonably practicable, the Company shall return to the Holder any exercise price paid by the Holder for the Reduction Shares. For any reason
at any time, upon the written or oral request of a Holder of Warrants, the Company shall within one (1) Business Day confirm to such Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of
Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder, together with its Affiliates and any member of a Section 13(d) group, since the date as of
which the Reported Outstanding Share Number was reported. In the event that the issuance of Common Stock to the Holder upon exercise of this Warrant results in the Holder, together with its Affiliates and any member of a Section 13(d) group,
being deemed to beneficially own, in the aggregate, more than the Beneficial Ownership Limitation of the number of outstanding shares of Common Stock (as determined under Section 13(d) of the Exchange Act), the number of shares so issued by
which the Holder’s, together with its Affiliates and any member of a Section 13(d) group, aggregate beneficial ownership exceeds the Beneficial Ownership Limitation (the “Excess Shares”) shall be deemed null and void and
shall be cancelled ab initio and any portion of this Warrant so exercised shall be reinstated, and the Holder shall not have the power to vote or to transfer the Excess Shares. As soon as reasonably practicable after the issuance of the Excess
Shares has been deemed null and void, the Company shall return to the Holder the exercise price paid by the Holder for the Excess Shares. By written notice to the Company, a Holder of Warrants may from time to time increase or decrease the
Beneficial Ownership Limitation to any other percentage not in excess of 19.99% (the “Maximum Percentage”) specified in such notice; provided that any increase in the Beneficial Ownership Limitation will not be effective until the
sixty-first (61st) day after such notice is delivered to the Company.
12. No Fractional Shares. No fractional Warrant Shares will be issued in
connection with any exercise of this Warrant. In lieu of any fractional shares that would otherwise be issuable, the number of Warrant Shares to be issued shall be rounded down to the next whole number and the Company shall pay the Holder in cash
the fair market value (based on the Closing Sale Price) for any such fractional shares.
13. Notices. Any and all notices or other communications
or deliveries hereunder (including, without limitation, any Exercise Notice) shall be in writing and shall be deemed given and effective on the earliest of (i) the date of
transmission, if such notice or communication is delivered via facsimile or confirmed e-mail at the facsimile number or
e-mail address specified in the books and records of the Transfer Agent prior to 5:30 P.M., New York City time, on a Trading Day, (ii) the next Trading Day after the date of transmission, if such notice
or communication is delivered via facsimile or confirmed e-mail at the facsimile number or e-mail address specified in the books and records of the Transfer Agent on a
day that is not a Trading Day or later than 5:30 P.M., New York City time, on any Trading Day, (iii) the Trading Day following the date of mailing, if sent by nationally recognized overnight courier service specifying next business day
delivery, or (iv) upon actual receipt by the Person to whom such notice is required to be given, if by hand delivery.
14. Warrant Agent. The
Company shall initially serve as warrant agent under this Warrant. Upon thirty (30) days’ notice to the Holder, the Company may appoint a new warrant agent. Any corporation into which the Company or any new warrant agent may be merged or
any corporation resulting from any consolidation to which the Company or any new warrant agent shall be a party or any corporation to which the Company or any new warrant agent transfers substantially all of its corporate trust or shareholders
services business shall be a successor warrant agent under this Warrant without any further act. Any such successor warrant agent shall promptly cause notice of its succession as warrant agent to be mailed (by first class mail, postage prepaid) to
the Holder at the Holder’s last address as shown on the Warrant Register.
15. Miscellaneous.
(a) No Rights as a Stockholder. Except as otherwise set forth in this Warrant, the Holder, solely in such Person’s capacity as a holder of this
Warrant, shall not be entitled to vote or receive dividends or be deemed the holder of share capital of the Company for any purpose, nor shall anything contained in this Warrant be construed to confer upon the Holder, solely in such Person’s
capacity as the Holder of this Warrant, any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification of stock, consolidation,
merger, amalgamation, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights, or otherwise, prior to the issuance to the Holder of the Warrant Shares which such Person is then entitled to receive upon the due
exercise of this Warrant. In addition, nothing contained in this Warrant shall be construed as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a stockholder of the Company, whether
such liabilities are asserted by the Company or by creditors of the Company.
(b) Authorized Shares. (i) Except and to the extent as waived or
consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate or articles of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or
sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all
such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (a) not increase the par value of any Warrant
Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (b) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and non-assessable Warrant Shares upon the exercise of this Warrant, and (c) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having
jurisdiction thereof as may be necessary to enable the Company to perform its obligations under this Warrant.
(ii) Before taking any action which would
result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public
regulatory body or bodies having jurisdiction thereof.
(c) Successors and Assigns. Subject to the restrictions on transfer set forth in this
Warrant and compliance with applicable securities laws, this Warrant may be assigned by the Holder. This Warrant may not be assigned by the Company without the written consent of the Holder, except to a successor in the event of a Fundamental
Transaction. This Warrant shall be binding on and inure to the benefit of the Company and the Holder and their respective successors and assigns. Subject to the preceding sentence, nothing in this Warrant shall be construed to give to any Person
other than the Company and the Holder any legal or equitable right, remedy or cause of action
under this Warrant. This Warrant may be amended only in writing signed by the Company and the Holder, or their successors and assigns.
(d) Amendment and Waiver. Except as otherwise provided herein, the provisions of the Warrants may be amended and the Company may take any action herein
prohibited, or omit to perform any act herein required to be performed by it, only if the Company has obtained the written consent of the Holder.
(e)
Acceptance. Receipt of this Warrant by the Holder shall constitute acceptance of and agreement to all of the terms and conditions contained herein.
(f) Governing Law; Jurisdiction. ALL QUESTIONS CONCERNING THE CONSTRUCTION, VALIDITY, ENFORCEMENT AND INTERPRETATION OF THIS WARRANT SHALL BE GOVERNED
BY AND CONSTRUED AND ENFORCED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK WITHOUT REGARD TO THE PRINCIPLES OF CONFLICTS OF LAW THEREOF. EACH OF THE COMPANY AND THE HOLDER HEREBY IRREVOCABLY SUBMITS TO THE EXCLUSIVE JURISDICTION OF THE STATE
AND FEDERAL COURTS SITTING IN THE CITY OF NEW YORK, BOROUGH OF MANHATTAN, FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION HEREWITH OR WITH ANY TRANSACTION CONTEMPLATED HEREBY OR DISCUSSED HEREIN (INCLUDING WITH RESPECT TO THE
ENFORCEMENT OF ANY OF THE TRANSACTION DOCUMENTS), AND HEREBY IRREVOCABLY WAIVES, AND AGREES NOT TO ASSERT IN ANY SUIT, ACTION OR PROCEEDING, ANY CLAIM THAT IT IS NOT PERSONALLY SUBJECT TO THE JURISDICTION OF ANY SUCH COURT. EACH OF THE COMPANY AND
THE HOLDER HEREBY IRREVOCABLY WAIVES PERSONAL SERVICE OF PROCESS AND CONSENTS TO PROCESS BEING SERVED IN ANY SUCH SUIT, ACTION OR PROCEEDING BY MAILING A COPY THEREOF VIA REGISTERED OR CERTIFIED MAIL OR OVERNIGHT DELIVERY (WITH EVIDENCE OF DELIVERY)
TO SUCH PERSON AT THE ADDRESS IN EFFECT FOR NOTICES TO IT AND AGREES THAT SUCH SERVICE SHALL CONSTITUTE GOOD AND SUFFICIENT SERVICE OF PROCESS AND NOTICE THEREOF. NOTHING CONTAINED HEREIN SHALL BE DEEMED TO LIMIT IN ANY WAY ANY RIGHT TO SERVE
PROCESS IN ANY MANNER PERMITTED BY LAW. EACH OF THE COMPANY AND THE HOLDER HEREBY WAIVES ALL RIGHTS TO A TRIAL BY JURY.
(g) Headings. The headings
herein are for convenience only, do not constitute a part of this Warrant and shall not be deemed to limit or affect any of the provisions hereof.
(h)
Severability. In case any one or more of the provisions of this Warrant shall be invalid or unenforceable in any respect, the validity and enforceability of the remaining terms and provisions of this Warrant shall not in any way be affected
or impaired thereby, and the Company and the Holder will attempt in good faith to agree upon a valid and enforceable provision which shall be a commercially reasonable substitute therefor, and upon so agreeing, shall incorporate such substitute
provision in this Warrant.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
IN WITNESS WHEREOF, the Company has caused this Warrant to be duly executed by its authorized officer as of
the date first indicated above.
|
|
|
| By: |
|
|
| Name: |
|
Paul Wagner |
| Title: |
|
Chief Executive Officer |
SCHEDULE 1
FORM OF EXERCISE NOTICE
[To be
executed by the Holder to purchase shares of Common Stock under the Warrant]
Ladies and Gentlemen:
(1) The undersigned is the Holder of Warrant No. __ (the “Warrant”) issued by Forte Biosciences, Inc. , a Delaware corporation (the
“Company”). Capitalized terms used herein and not otherwise defined herein have the respective meanings set forth in the Warrant.
(2)
The undersigned hereby exercises its right to purchase Warrant Shares pursuant to the Warrant.
(3) The Holder intends that payment of the Exercise Price
shall be made as (check one):
| |
☐ |
“Cashless Exercise” under Section 10 of the Warrant |
(4) If the Holder has elected a Cash Exercise, the Holder shall pay the sum of $ in immediately available funds to the Company in accordance with the terms of
the Warrant.
(5) Pursuant to this Exercise Notice, the Company shall deliver to the Holder Warrant Shares determined in accordance with the terms of the
Warrant.
(6) By its delivery of this Exercise Notice, the undersigned represents and warrants to the Company that in giving effect to the exercise
evidenced hereby the Holder will not beneficially own in excess of the number of shares of Common Stock (as determined in accordance with Section 13(d) of the Securities Exchange Act of 1934, as amended) permitted to be owned under
Section 11(a) of the Warrant to which this notice relates.
|
|
|
|
|
| Dated: |
|
|
|
|
|
|
|
| Name of Holder: |
|
|
|
|
|
|
|
| By: |
|
|
|
|
| Name: |
|
|
|
|
| Title: |
|
|
|
|
(Signature must conform in all respects to name of Holder as specified on the face of the Warrant)
Exhibit 4.2
AMENDMENT NO. 2 TO
PREFERRED STOCK RIGHTS AGREEMENT
This AMENDMENT NO. 2 TO PREFERRED STOCK RIGHTS AGREEMENT (this “Amendment”) is dated as of July 28, 2023
(the “Effective Date”), and amends that certain Preferred Stock Rights Agreement, dated as of July 12, 2022, as amended by that certain Amendment No. 1 dated as of June 26, 2023 (the “Rights
Agreement”), by and between Forte Biosciences, Inc., a Delaware corporation (the “Company”), and Computershare Trust Company, N.A., a federally chartered trust company, as rights agent (the “Rights Agent”).
Capitalized terms used in this Amendment and not otherwise defined herein shall have the respective meanings given to them in the Rights Agreement.
RECITALS
WHEREAS, the board of directors of the Company (the “Board”) has determined that it is in the best interests
of the Company and its stockholders to raise additional capital for the Company through the sale and issuance by the Company to certain institutional and accredited investors and certain members of the Board and Company’s management (the
“Purchasers”) of shares of the Common Stock and pre-funded warrants (“Pre-Funded Warrants”) to purchase shares of Common Stock (the
“Warrant Shares”), pursuant to that certain securities purchase agreement (the “Securities Purchase Agreement”) to be entered into as of the Effective Date by and among the Company and each Purchaser and the Pre-Funded Warrants to be issued to the Purchasers, as applicable, pursuant to the terms and subject to the conditions set forth in the Securities Purchase Agreement;
WHEREAS, in accordance with Section 27 of the Rights Agreement, for so long as the Rights are redeemable, the Company may
in its sole discretion supplement or amend the Rights Agreement in any respect without the approval of any holders of Rights Certificates, Preferred Stock or Common Stock, and the Rights Agent must, if the Company so directs, execute such supplement
or amendment;
WHEREAS, the Rights are currently redeemable;
WHEREAS, the board of directors of the Company has determined that this Amendment is advisable, fair to and in the best
interests of the Company and its stockholders;
WHEREAS, the Company has delivered to the Rights Agent a certificate
stating that this Amendment complies with Section 27 of the Rights Agreement; and
WHEREAS, the Rights Agent is
directed to join in this Amendment.
AGREEMENT
NOW, THEREFORE, in consideration of the premises and the mutual agreements set forth herein, the parties hereto hereby agree
as follows:
1. Amendment of the Rights Agreement.
| |
A. |
Section 1 of the Rights Agreement is hereby amended by adding the following additional definitions:
|
| |
(hhh) |
“Purchaser” has the meaning ascribed to such term in the Securities Purchase Agreement.
|
| |
(iii) |
“Pre-Funded Warrants” has the meaning ascribed to such
term in the Securities Purchase Agreement. |
(jjj) “Securities Purchase Agreement”
means the Securities Purchase Agreement, dated as of July 28, 2023, by and among the Company and each Purchaser.
B. The following is added as a new
Section 38 of the Rights Agreement:
“Section 38. Exception for Securities Purchase Agreement.
Notwithstanding anything to the contrary in this Agreement, none of the approval, execution, delivery or performance of the Securities Purchase Agreement or the Pre-Funded Warrants, or the consummation of any
of the transactions contemplated by the Securities Purchase Agreement or the Pre-Funded Warrants, including any issuance or acquisition of shares of Common Stock pursuant to the terms of the Securities
Purchase Agreement or the Pre-Funded Warrants, shall (a) result in a Stock Acquisition Date, a Distribution Date or in any way permit any Rights to be exercised pursuant to Section 7, or otherwise,
for consideration or exchanged pursuant to Section 24; (b) constitute a Section 11(a)(ii) Event or a Section 13 Event; (c) cause any of Purchaser or any of their respective Affiliates or Associates (each, a “Purchaser
Person”) to be deemed to be an “Acquiring Person” for any purpose in this Agreement (it being understood that other future actions by any Purchaser Person could still result in such Purchaser Person being an Acquiring Person for
purposes of the Agreement); or (d) cause any officer, director or employee of any Purchaser Person to be deemed to be, solely by reason of such Person’s status or authority as such, the “Beneficial Owner” of or to
“Beneficially Own” any securities that are “Beneficially Owned” by a Purchaser Person, including in a fiduciary capacity. Nothing in this Agreement shall be construed to give any holder of Rights or any other Person any legal or
equitable rights, remedy or claim under this Agreement in connection with the execution, delivery or performance of the Securities Purchase Agreement or the Pre-Funded Warrants, or the consummation of any of
the transactions contemplated by the Securities Purchase Agreement or the Pre-Funded Warrants.”
2. No Other Amendment; Effect of Amendment. Except as and to the extent expressly modified by this Amendment, the
Rights Agreement and the exhibits thereto remain in full force and effect in all respects without any modification. This Amendment will be deemed an amendment to the Rights Agreement and will become effective on the Effective Date. All references to
the Rights Agreement shall, from and after the Effective Date, be deemed to be references to the Rights Agreement as amended hereby. In the event of a conflict or an inconsistency between this Amendment and the Rights Agreement and the exhibits
thereto, the provisions of this Amendment will govern.
3. Counterparts. This Amendment may be executed in any
number of counterparts and each of such counterparts will for all purposes be deemed to be an original, and all such counterparts will together constitute one and the same instrument, it being understood that all parties hereto need not sign the
same counterpart. A signature to this Amendment executed and/or transmitted electronically (including by fax and .pdf) will have the same authority, effect, and enforceability as an original signature. No party hereto may raise the use of such
electronic transmission to deliver a signature, or the fact that any signature or agreement or instrument was transmitted or communicated through such electronic transmission, as a defense to the formation of a contract, and each party hereto
forever waives any such defense, except to the extent that such defense relates to lack of authenticity.
4. Severability. If any term, provision, covenant or restriction of this Amendment is held by a court of competent
jurisdiction or other authority to be invalid, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions of this Amendment will remain in full force and effect and will in no way be affected, impaired or
invalidated; provided, however, that if any such excluded term, provision, covenant or restriction adversely affects the rights, immunities, duties or obligations of the Rights Agent, then the Rights Agent shall be entitled
to resign immediately.
5. Descriptive Headings. The descriptive headings of the several sections of this
Amendment are inserted for convenience only and will not control or affect the meaning or construction of any of the provisions hereof.
6. Further Assurances. Each of the parties to this Amendment will reasonably cooperate and take such action as may
be reasonably requested by the other party in order to carry out the provisions and purposes of this Amendment, the Rights Agreement and the transactions contemplated hereunder and thereunder.
7. Governing Law. This Amendment, and all claims or causes of action (whether in contract or in tort or otherwise, or
whether at law (including at common law or by statute) or in equity) that may be based on, arise out of or relate to this
-2-
Amendment, or the negotiation, execution, performance or subject matter of this Amendment, will be governed by and construed in accordance with the laws of the State of Delaware.
[Signature page follows.]
-3-
IN WITNESS WHEREOF, the parties hereto have caused this Amendment to be duly executed as of
the day and year first written above.
|
|
|
|
|
| By: |
|
/s/ Paul
A. Wagner |
| Name: |
|
Paul A. Wagner, Ph.D. |
| Title: |
|
Chief Executive Officer |
|
|
|
|
| COMPUTERSHARE TRUST COMPANY, N.A. |
|
|
|
|
|
| By: |
|
/s/ Kathy Heagerty |
| Name: |
|
Kathy Heagerty |
| Title: |
|
Manager, Client Management |
[Signature Page to Amendment No. 2 to Preferred Stock Rights Agreement]
Exhibit 10.1
SECURITIES PURCHASE AGREEMENT
BY AND AMONG
FORTE
BIOSCIENCES, INC.,
AND
THE PURCHASERS
JULY 28, 2023
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
|
| 1. |
|
Definitions |
|
|
1 |
|
|
|
|
| 2. |
|
Purchase and Sale of Common Stock |
|
|
5 |
|
|
|
2.1 |
|
Purchase and Sale |
|
|
5 |
|
|
|
2.2 |
|
Closing |
|
|
5 |
|
|
|
|
| 3. |
|
Representations and Warranties of the Company |
|
|
6 |
|
|
|
3.1 |
|
Organization and Power |
|
|
6 |
|
|
|
3.2 |
|
Capitalization |
|
|
6 |
|
|
|
3.3 |
|
Registration Rights |
|
|
6 |
|
|
|
3.4 |
|
Authorization |
|
|
7 |
|
|
|
3.5 |
|
Valid Issuance |
|
|
7 |
|
|
|
3.6 |
|
No Conflict |
|
|
7 |
|
|
|
3.7 |
|
Consents |
|
|
8 |
|
|
|
3.8 |
|
SEC Filings; Financial Statements |
|
|
8 |
|
|
|
3.9 |
|
Absence of Changes |
|
|
9 |
|
|
|
3.10 |
|
Absence of Litigation |
|
|
9 |
|
|
|
3.11 |
|
Compliance with Law; Permits |
|
|
9 |
|
|
|
3.12 |
|
Intellectual Property |
|
|
10 |
|
|
|
3.13 |
|
Employee Benefits |
|
|
10 |
|
|
|
3.14 |
|
Taxes |
|
|
11 |
|
|
|
3.15 |
|
Environmental Laws |
|
|
11 |
|
|
|
3.16 |
|
Title |
|
|
12 |
|
|
|
3.17 |
|
Insurance |
|
|
12 |
|
|
|
3.18 |
|
Nasdaq Stock Market |
|
|
12 |
|
|
|
3.19 |
|
Sarbanes-Oxley Act |
|
|
12 |
|
|
|
3.20 |
|
Regulatory Compliance |
|
|
13 |
|
|
|
3.21 |
|
Accounting Controls and Disclosure Controls and Procedures |
|
|
13 |
|
|
|
3.22 |
|
Price Stabilization of Common Stock |
|
|
13 |
|
|
|
3.23 |
|
Investment Company Act |
|
|
13 |
|
|
|
3.24 |
|
General Solicitation; No Integration or Aggregation |
|
|
13 |
|
|
|
3.25 |
|
Brokers and Finders |
|
|
14 |
|
|
|
3.26 |
|
Reliance by the Purchasers |
|
|
14 |
|
|
|
3.27 |
|
No Disqualification Events |
|
|
14 |
|
|
|
3.28 |
|
No Additional Agreements |
|
|
14 |
|
|
|
3.29 |
|
Anti-Bribery and Anti-Money Laundering Laws |
|
|
14 |
|
|
|
3.30 |
|
Company IT Systems; Cybersecurity |
|
|
15 |
|
|
|
3.31 |
|
Transactions with Affiliates and Employees |
|
|
15 |
|
|
|
3.32 |
|
Shell Company Status |
|
|
15 |
|
|
|
3.33 |
|
Regulation D |
|
|
15 |
|
|
|
3.34 |
|
Independent Public Accountants |
|
|
16 |
|
|
|
3.35 |
|
Stock Plans |
|
|
16 |
|
|
|
3.36 |
|
Changes |
|
|
16 |
|
|
|
3.37 |
|
Healthcare Regulatory Proceedings |
|
|
16 |
|
|
|
3.38 |
|
Regulatory Matters |
|
|
17 |
|
i
|
|
|
|
|
|
|
|
|
|
|
3.39 |
|
No Other Representations or Warranties |
|
|
17 |
|
|
|
|
| 4. |
|
Representations and Warranties of Each Purchaser |
|
|
18 |
|
|
|
4.1 |
|
Organization |
|
|
18 |
|
|
|
4.2 |
|
Authorization |
|
|
18 |
|
|
|
4.3 |
|
No Conflict |
|
|
18 |
|
|
|
4.4 |
|
Consents |
|
|
18 |
|
|
|
4.5 |
|
Residency |
|
|
19 |
|
|
|
4.6 |
|
Brokers and Finders |
|
|
19 |
|
|
|
4.7 |
|
Investment Representations and Warranties |
|
|
19 |
|
|
|
4.8 |
|
Intent |
|
|
19 |
|
|
|
4.9 |
|
Investment Experience; Ability to Protect Its Own Interests and Bear Economic
Risks |
|
|
20 |
|
|
|
4.10 |
|
Tax Advisors |
|
|
20 |
|
|
|
4.11 |
|
Securities Not Registered; Legends |
|
|
20 |
|
|
|
4.12 |
|
Reliance by the Company |
|
|
21 |
|
|
|
4.13 |
|
No General Solicitation |
|
|
21 |
|
|
|
4.14 |
|
No Reliance |
|
|
21 |
|
|
|
4.15 |
|
Access to Information |
|
|
22 |
|
|
|
4.16 |
|
Certain Trading Activities |
|
|
22 |
|
|
|
4.17 |
|
Disqualification Event |
|
|
22 |
|
|
|
|
| 5. |
|
Covenants |
|
|
23 |
|
|
|
5.1 |
|
Further Assurances |
|
|
23 |
|
|
|
5.2 |
|
Listing |
|
|
23 |
|
|
|
5.3 |
|
Disclosure of Transactions |
|
|
23 |
|
|
|
5.4 |
|
Integration |
|
|
24 |
|
|
|
5.5 |
|
Pledge of Securities |
|
|
24 |
|
|
|
5.6 |
|
Subsequent Equity Sales |
|
|
24 |
|
|
|
5.7 |
|
Reservation of Common Stock |
|
|
25 |
|
|
|
5.8 |
|
Use of Proceeds |
|
|
25 |
|
|
|
5.9 |
|
Removal of Legends |
|
|
25 |
|
|
|
5.10 |
|
Participation Rights |
|
|
25 |
|
|
|
|
| 6. |
|
Conditions of Closing |
|
|
28 |
|
|
|
6.1 |
|
Conditions to the Obligation of the Purchasers |
|
|
28 |
|
|
|
6.2 |
|
Conditions to the Obligation of the Company |
|
|
29 |
|
|
|
|
| 7. |
|
Termination |
|
|
30 |
|
|
|
7.1 |
|
Conditions of Termination |
|
|
30 |
|
|
|
|
| 8. |
|
Miscellaneous Provisions |
|
|
31 |
|
|
|
8.1 |
|
Public Statements or Releases |
|
|
31 |
|
|
|
8.2 |
|
Interpretation |
|
|
31 |
|
|
|
8.3 |
|
Notices |
|
|
31 |
|
|
|
8.4 |
|
Severability |
|
|
32 |
|
|
|
8.5 |
|
Governing Law; Submission to Jurisdiction; Venue; Waiver of Trial by Jury |
|
|
32 |
|
ii
|
|
|
|
|
|
|
|
|
| |
|
8.6 |
|
Waiver |
|
|
33 |
|
|
|
8.7 |
|
Expenses |
|
|
33 |
|
|
|
8.8 |
|
Assignment |
|
|
33 |
|
|
|
8.9 |
|
Confidential Information |
|
|
33 |
|
|
|
8.10 |
|
Third Parties |
|
|
34 |
|
|
|
8.11 |
|
Independent Nature of Purchasers’ Obligations and Right |
|
|
34 |
|
|
|
8.12 |
|
Counterparts |
|
|
34 |
|
|
|
8.13 |
|
Entire Agreement; Amendments |
|
|
35 |
|
|
|
8.14 |
|
Survival |
|
|
35 |
|
|
|
8.15 |
|
Mutual Drafting |
|
|
35 |
|
|
|
8.16 |
|
Additional Matters |
|
|
35 |
|
|
|
| Exhibits |
|
|
|
|
|
|
| Exhibit A Purchasers |
|
|
A-1 |
|
|
|
| Exhibit B Form of Pre-Funded Warrant |
|
|
B-1 |
|
|
|
| Exhibit C Form of Registration Rights Agreement |
|
|
C-1 |
|
iii
This SECURITIES PURCHASE AGREEMENT (this “Agreement”) is
dated as of July 28, 2023, by and among Forte Biosciences, Inc., a Delaware corporation (the “Company”), and the individuals and entities listed on Exhibit A attached to this Agreement (each, a
“Purchaser” and together, the “Purchasers”).
WHEREAS, the Company and the Purchasers
are executing and delivering this Agreement in reliance upon the exemption from securities registration afforded by Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), and Rule 506 of Regulation D
promulgated by the United States Securities and Exchange Commission (the “SEC”) under the Securities Act;
WHEREAS, the Company desires to issue and sell to the Purchasers, and each Purchaser desires to purchase from the Company,
severally and not jointly, upon the terms and subject to the conditions stated in this Agreement, shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”), and/or
pre-funded warrants to purchase Common Stock in the form attached hereto as Exhibit B (the “Pre-Funded Warrants”); and
WHEREAS, contemporaneously with the sale of the Shares (as defined below) and the
Pre-Funded Warrants, the parties hereto will execute and deliver a Registration Rights Agreement, substantially in the form attached hereto as Exhibit C, pursuant to which the Company will agree to
provide certain registration rights in respect of the Shares and the Warrant Shares (as defined below) under the Securities Act and applicable state securities laws.
NOW THEREFORE, in consideration of the mutual agreements, representations, warranties and covenants herein contained, the
Company and each Purchaser, severally and not jointly, agree as follows:
1. Definitions. As
used in this Agreement, the following terms shall have the following respective meanings:
“2023 SEC
Reports” shall mean (a) the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and (b) any Quarterly Reports on Form
10-Q or any Current Reports on Form 8-K filed or furnished (as applicable) by the Company after December 31, 2022, together in each case with any documents
incorporated by reference therein or exhibits thereto.
“Affiliate” shall mean, with respect to any
Person, any other Person that, directly or indirectly through one or more intermediates, controls, is controlled by or is under common control with such Person.
“Agreement” has the meaning set forth in the recitals hereof.
“Amended and Restated Bylaws” shall mean the Bylaws of the Company, as currently in effect.
“Amended and Restated Certificate of Incorporation” shall mean the Certificate of Incorporation of the
Company, as currently in effect.
“ATM Floor Price” has the meaning set forth in
Section 5.10(c) hereof.
“ATM Target Amount” has the meaning set forth in
Section 5.10(c) hereof.
“Benefit Plan” or “Benefit Plans”
shall mean employee benefit plans as defined in Section 3(3) of ERISA and all other employee benefit practices or arrangements, including, without limitation, any such practices or arrangements providing severance pay, sick leave, vacation pay,
salary continuation for disability, retirement benefits, deferred compensation, bonus pay, incentive pay, stock options or other stock-based compensation, hospitalization insurance, medical insurance, life insurance, scholarships or tuition
reimbursements, maintained by the Company or to which the Company is obligated to contribute for employees or former employees.
“Board of Directors” shall mean the board of directors of the Company.
“Closing” has the meaning set forth in Section 2.2 hereof.
“Closing Date” shall mean July 31, 2023 or such other date as mutually agreed by the Company and the
Purchasers, but in no event later than the third (3rd) business day following the date hereof.
“Code” shall mean the Internal Revenue Code of 1986, as amended.
“Common Stock” has the meaning set forth in the recitals hereof.
“Common Stock Equivalents” shall mean any securities of the Company which would entitle the holder thereof to
acquire at any time Common Stock, including, without limitation, any debt, preferred stock, rights, options, warrants or other instrument that is at any time convertible into or exchangeable for, or otherwise entitles the holder thereof to receive,
Common Stock.
“Company” has the meaning set forth in the recitals hereof.
“Company IT Systems” has the meaning set forth in Section 3.30 hereof.
“Covered Person” has the meaning set forth in Section 3.27 hereof.
“Disclosure Document” has the meaning set forth in Section 5.3 hereof.
“Disqualification Event” has the meaning set forth in Section 3.27 hereof.
“Drug Regulatory Agency” shall mean the FDA or other comparable governmental authority responsible for
regulation of the research, development, testing, manufacturing, processing, storage, labeling, sale, marketing, advertising, distribution and importation or exportation of drug products and drug product candidates.
“Environmental Laws” has the meaning set forth in Section 3.15 hereof.
“ERISA” shall mean the Employee Retirement Income Security Act of 1974, as amended.
“Exchange Act” shall mean the Securities Exchange Act of 1934, as amended, and all of the rules and
regulations promulgated thereunder.
2
“Expected Sale Date” has the meaning set forth in
Section 5.10(a) hereof.
“Financial Statements” has the meaning set forth in
Section 3.8(b) hereof.
“GAAP” has the meaning set forth in
Section 3.8(b) hereof.
“Governmental Authorizations” has the meaning set forth
in Section 3.11 hereof.
“Intellectual Property” has the meaning set forth in
Section 3.12(a) hereof.
“Material Adverse Effect” shall mean any change,
event, circumstance, development, condition, occurrence or effect that, individually or in the aggregate, (a) was, is, or would reasonably be expected to be, materially adverse to the business, financial condition, prospects, assets,
liabilities, stockholders’ equity or results of operations of the Company and its subsidiaries, taken as a whole, or (b) materially delays or materially impairs the ability of the Company to comply, or prevents the Company from complying,
with its obligations under this Agreement or with respect to the Closing or would reasonably be expected to do so.
“Offer Notice” has the meaning set forth in Section 5.10(a) hereof.
“Participation Period” has the meaning set forth in Section 5.10(a) hereof.
“Person” shall mean an individual, partnership, corporation, limited liability company, business trust, joint
stock company, trust, unincorporated association, joint venture or any other entity or organization.
“Pre-Funded Warrants” has the meaning set forth in the recitals hereof.
“Purchaser” and “Purchasers” have the meanings set forth in the recitals hereof.
“Purchaser Adverse Effect” has the meaning set forth in Section 4.3 hereof.
“Registration Rights Agreement” has the meaning set forth in Section 6.1(j) hereof.
“Rule 144” shall mean Rule 144 promulgated by the SEC pursuant to the Securities Act, as such Rule may
be amended from time to time, or any similar rule or regulation hereafter adopted by the SEC having substantially the same effect as such Rule.
“SEC” has the meaning set forth in the recitals hereof.
“SEC Reports” has the meaning set forth in Section 3.8(a) hereof.
“Securities” shall mean the Shares, the Pre-Funded Warrants and the
Warrant Shares.
“Securities Act” has the meaning set forth in the recitals hereof.
“Shares” shall mean the shares of Common Stock issued or issuable to each Purchaser pursuant to this
Agreement, but excluding the Warrant Shares.
3
“Short Sales” include, without limitation, (i) all
“short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, whether or not against the box, and all types of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales,
swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) and similar arrangements (including on a total return basis), and (ii) sales and other transactions through
non-U.S. broker dealers or non-U.S. regulated brokers (but shall not be deemed to include the location and/or reservation of borrowable shares of Common Stock).
“Subscription Amount” shall mean the aggregate amount to be paid for the Shares and Pre-Funded Warrants purchased hereunder as indicated under the heading “Subscription Amount” on Exhibit A.
“Tax Returns” shall mean returns, reports, information statements and other documentation (including any
additional or supporting material) filed or maintained, or required to be filed or maintained, in connection with the calculation, determination, assessment or collection of any Tax and shall include any amended returns required as a result of
examination adjustments made by the Internal Revenue Service or other Tax authority.
“Tax” or
“Taxes” shall mean (a) any and all federal, state, local, non-U.S. and other taxes, levies, fees, imposts, duties and charges of whatever kind (including any interest, penalties or
additions to the tax imposed in connection therewith or with respect thereto), whether or not imposed on the Company, including, without limitation, taxes imposed on, or measured by, income, franchise, profits or gross receipts, and also ad valorem,
value added, sales, use, service, real or personal property, capital stock, license, payroll, withholding, employment, social security, workers’ compensation, unemployment compensation, utility, severance, production, excise, stamp, occupation,
premium, windfall profits, transfer and gains taxes and customs duties, and (b) any liability for any amounts of the type described in clause (a) of another Person as a transferee or successor, by contract (including any Tax Sharing
Agreement), under Treasury Regulations Section 1.1502-6 or analogous state, local or non-U.S. law, as a result of being a party or as a result of any express or
implied obligation to indemnify any other Person, or otherwise, in each case, whether disputed or not.
“Tax
Sharing Agreement” shall mean any Tax allocation agreement, Tax indemnification agreement, Tax sharing agreement or similar contract or arrangement, whether or not written.
“Transaction Agreements” shall mean this Agreement, the Registration Rights Agreement and the Pre-Funded Warrants.
“Transfer Agent” shall mean, with respect to the
Common Stock, Computershare Trust Company, N.A., or such other financial institution that provides transfer agent services as proposed by the Company and consented to by the Purchasers, which consent shall not be unreasonably withheld.
“Warrant Shares” shall mean the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants.
“Willful Breach” has the meaning set forth in
Section 7 hereof.
4
2. Purchase and Sale of Common Stock.
2.1 Purchase and Sale. On the Closing Date, upon the terms and subject to the conditions set
forth herein, the Company agrees to sell, and the Purchasers, severally and not jointly, agree to purchase, up to an aggregate of $25,010,000 of Shares and/or Pre-Funded Warrants. The purchase price per share
of Common Stock shall be $1.006 per share, or such higher amount as set forth on Exhibit A (the “Purchase Price”). Each Purchaser who so elects to purchase Pre-Funded Warrants in lieu
of Shares shall deliver to the Company at least one business day prior to the Closing Date a calculation of the number of Pre-Funded Warrants to be delivered in lieu of Shares. Subject to and upon the terms
and conditions set forth in this Agreement, at the Closing, the Company shall issue and sell to each Purchaser, and each Purchaser, severally and not jointly, shall purchase from the Company, (a) that number of Shares equal to the dollar amount
set forth opposite such Purchaser’s name on Exhibit A under the heading “Aggregate Purchase Price” divided by the Purchase Price and/or (b) a Pre-Funded Warrant to purchase the
number of Warrant Shares set forth opposite such purchasers name as indicated on Exhibit A under the heading “Warrant Shares”, if any, at a purchase price equal to $1.005 and an exercise price equal to $0.001 per Warrant Share.
Exhibit A to this Agreement shall be updated to reflect the number of Shares and Warrant Shares purchased at Closing in accordance with a Purchaser’s election to purchase
Pre-Funded Warrants pursuant to this Section 2.1.
2.2 Closing. Subject to the satisfaction or waiver of the conditions set forth in
Section 6 of this Agreement, the closing of the purchase and sale of the Securities (the “Closing”) shall occur remotely via the exchange of documents and signatures on the Closing Date. At the Closing, the
Securities shall be issued and registered in the name of such Purchaser, or in such nominee name(s) as designated by such Purchaser, representing the number of Shares to be purchased by such Purchaser at such Closing as set forth in Exhibit
A, and, if applicable, a Pre-Funded Warrant, in each case against payment to the Company of the purchase price therefor in full by wire transfer to the Company of immediately available funds, at or prior
to the Closing, in accordance with wire instructions provided by the Company to the Purchasers at least three business days prior to the Closing, to an account to be designated by the Company. On the Closing Date, the Company will issue the Shares
in book-entry form, free and clear of all restrictive and other legends (except as expressly provided in Section 4.11 hereof) and shall provide evidence of such issuance from the Company’s Transfer Agent as of the
Closing Date to each Purchaser. For each Purchaser of Pre-Funded Warrants pursuant to Section 2.1 the Company shall deliver a Pre-Funded Warrant registered in the
name of such Purchaser to purchase up to a number of shares of Common Stock equal to the portion of the Purchaser’s Subscription Amount applicable to Pre-Funded Warrants divided by $1.005, with an
exercise price equal to $0.001 per Warrant Share, subject to adjustment as provided therein. The failure of the Closing to occur on the Closing Date shall not terminate this Agreement or otherwise relieve any party of any of its obligations
hereunder.
5
3. Representations and Warranties of the
Company. The Company hereby represents and warrants to each of the Purchasers that the statements contained in this Section 3 are true and correct as of the date hereof and the Closing Date (except for the
representations and warranties that speak as of a specific date, which shall be made as of such date):
3.1 Organization and Power. The Company is a corporation duly organized, validly existing and
in good standing under the laws of the State of Delaware, has the requisite power and authority to own, lease and operate its properties and to carry on its business as now conducted and is qualified to do business in each jurisdiction in which the
character of its properties or the nature of its business requires such qualification, except where such failure to be in good standing or to have such power and authority or to so qualify would not reasonably be expected to have a Material Adverse
Effect. As of the date hereof, the Company has two subsidiaries which are set forth on Exhibit 21.1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2022. Each of the
Company’s subsidiaries is duly incorporated and validly existing and in good standing under the laws of the jurisdiction of its incorporation and has the requisite power and authority to carry on their business as now conducted and to own or
lease its properties. Each of the Company’s subsidiaries is duly qualified to do business as foreign corporations and is in good standing in each jurisdiction in which such qualification is required unless the failure to so qualify has not had
and would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect.
3.2 Capitalization. The authorized capital stock of the Company consists of 200,000,000 shares
of Common Stock and 10,000,000 shares of preferred stock, par value $0.001 per share. The Company’s disclosure of its issued and outstanding capital stock in the 2023 SEC Reports containing such disclosure was accurate in all material respects
as of the date indicated in such 2023 SEC Reports (except for subsequent issuances, if any, pursuant to this Agreement or pursuant to reservations, agreements or employee benefit plans, in each case, referred to in the 2023 SEC Reports or pursuant
to the exercise of convertible securities or options or the vesting of restricted stock units referred to in the 2023 SEC Reports). All of the issued and outstanding shares of Common Stock have been duly authorized and validly issued and are fully
paid and non-assessable, and outstanding warrants have been issued in material compliance with all applicable securities laws. None of the outstanding shares of capital stock of the Company were issued in
violation of any preemptive or other similar rights of any securityholder of the Company which have not been waived. Other than as disclosed in the SEC Reports and the transactions contemplated by the Transaction Agreements, there are no outstanding
options, warrants, rights (including conversion or preemptive rights and rights of first refusal or similar rights) or agreements, orally or in writing, to purchase or acquire from the Company any shares of Common Stock or preferred stock, or any
securities convertible into or exchangeable for shares of Common Stock or preferred stock.
3.3 Registration Rights. Except as set forth in the Transaction Agreements or as disclosed in
the 2023 SEC Reports, the Company is presently not under any obligation, and has not granted any rights, to register under the Securities Act any of the Company’s presently outstanding securities or any of its securities that may hereafter be
issued that have not expired or been satisfied.
6
3.4 Authorization. The Company has all
requisite corporate power and authority to enter into the Transaction Agreements and to carry out and perform its obligations under the terms of the Transaction Agreements. All corporate action on the part of the Company, its officers, directors and
stockholders necessary for the authorization, issuance and sale of the Securities, the authorization, execution, delivery and performance of the Transaction Agreements and the consummation of the transactions contemplated herein has been taken. This
Agreement has been duly executed and delivered by the Company and assuming the due authorization, execution and delivery by each Purchaser and that this Agreement constitutes the legal, valid and binding agreement of each Purchaser, this Agreement
constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, except as such enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium and similar laws
relating to or affecting creditors generally or by general equity principles (regardless of whether such enforceability is considered in a proceeding in equity or at law). Upon their respective execution by the Company and the other parties thereto
and assuming that they constitute legal, valid and binding agreements of the other parties thereto, the Registration Rights Agreement and Pre-Funded Warrants will constitute legal, valid and binding
obligations of the Company, enforceable against the Company in accordance with their terms, except as such enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium and similar laws relating to or affecting creditors
generally or by general equity principles (regardless of whether such enforceability is considered in a proceeding in equity or at law).
3.5 Valid Issuance. The Shares being purchased by the Purchasers hereunder, upon issuance
pursuant to the terms hereof, against full payment therefor in accordance with the terms of this Agreement, will be duly and validly issued, fully paid and non-assessable and will be issued free and clear of
any liens or other restrictions (other than those under applicable state and federal securities laws). The Warrant Shares have been duly and validly authorized and reserved for issuance and, upon exercise of the
Pre-Funded Warrants in accordance with their terms, including the payment of any exercise price therefor, will be validly issued, fully paid and nonassessable and will be issued free and clear of any liens or
other restrictions (other than those under applicable state and federal securities laws). Subject to the accuracy of the representations and warranties made by the Purchasers in Section 4 hereof, the offer and sale of the
Securities to the Purchasers is and will be in compliance with applicable exemptions from (i) the registration and prospectus delivery requirements of the Securities Act and (ii) the registration and qualification requirements of
applicable securities laws of the states of the United States.
3.6 No Conflict. The
execution, delivery and performance of the Transaction Agreements by the Company, the issuance of the Shares and Pre-Funded Warrants, the reservation for issuance and issuance of the Warrant Shares and the
consummation of the other transactions contemplated hereby will not (i) conflict with or result in any violation of, breach or (with or without notice or lapse of time, or both) under, conflict with, or give rise to a right of termination,
cancellation or acceleration of any obligation, a change of control right or to a loss of a material benefit under any provision of the Amended and Restated Certificate of Incorporation or Amended and Restated Bylaws or the charter or bylaws of any
subsidiary, (ii) conflict with or result in a violation of or default (with or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation or acceleration of any obligation, a change of control right or
to a loss of a benefit under any agreement or instrument, credit facility, franchise, license, judgment, order, statute, law, ordinance, rule or regulations, applicable to the Company or any subsidiary or
7
their properties or assets, or (iii) result in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental authority
to which the Company is subject (including federal and state securities laws and regulations) and the rules and regulations of any self-regulatory organization to which the Company or its securities are subject (including, without limitation, the
rules and regulations of Nasdaq), or by which any property or asset of the Company is bound or affected, except, in the case of clauses (ii) and (iii), as would not, individually or in the aggregate, be reasonably expected to have a
Material Adverse Effect.
3.7 Consents. Assuming the accuracy of the representations and
warranties of the Purchasers, no consent, approval, authorization, filing with or order of or registration with, any court or governmental agency or body, or of or with any self-regulatory organization or
other non-governmental regulatory authority (including, without limitation, Nasdaq), or approval of the stockholders of the Company is required in connection with the transactions contemplated
herein, except such as (a) have been or will be obtained or made under the Securities Act or the Exchange Act, (b) the filing of any requisite notices and/or application(s) to the Nasdaq Capital Market for the issuance and sale of the
Securities and the listing of the Shares and the Warrant Shares for trading or quotation, as the case may be, thereon in the time and manner required thereby, (c) are required to consummate the transactions contemplated by the Transaction
Agreements or (d) may be required under the securities, or blue sky, laws of any state jurisdiction in connection with the offer and sale of the Securities by the Company in the manner contemplated herein.
3.8 SEC Filings; Financial Statements.
(a) The Company’s Common Stock is registered under Section 12 of the Exchange Act. The
Company has filed or furnished, as applicable, all forms, statements, certifications, reports and documents (the foregoing materials, including the exhibits thereto and documents incorporated by reference therein) required to be filed or furnished
by it with the SEC under the Exchange Act or the Securities Act for the two years preceding the date hereof (the “SEC Reports”) on a timely basis or has received a valid extension of such time of filing and has filed any such SEC
Reports prior to the expiration of any such extension. As of the time it was filed with the SEC (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing), each of the SEC Reports complied in all
material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case may be) and, as of the time they were filed, none of the SEC Reports contained any untrue statement of a material fact or omitted to state a
material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. As used in this Section 3.8, the term
“file” and variations thereof shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC. The Company satisfies the registrant requirements for the use
of a registration statement on Form S-3 to register the Shares and the Warrant Shares for resale by the Purchasers under the Securities Act.
(b) The financial statements of the Company included in the SEC Reports, together with the related
notes and schedules (collectively, the “Financial Statements”), fairly present in all material respects the financial position of the Company as of the dates indicated, and the results of its operations and cash flows for the
periods therein specified, all in accordance with United States generally accepted accounting principles (“GAAP”) (except as
8
otherwise noted therein, and in the case of unaudited financial statements, as permitted by Form 10-Q of the SEC, and except that the unaudited financial
statements may not contain footnotes and are subject to normal and recurring year-end adjustments) applied on a consistent basis unless otherwise noted therein throughout the periods therein specified. Except
as set forth in the Financial Statements filed prior to the date hereof, the Company has not incurred any liabilities, contingent or otherwise, except those incurred in the ordinary course of business, consistent (as to amount and nature) with past
practices since the date of such Financial Statements, none of which, individually or in the aggregate, have had or would reasonably be expected to have a Material Adverse Effect.
3.9 Absence of Changes. Except as otherwise stated or disclosed in the 2023 SEC Reports filed
at least one business day prior to the date hereof, between December 31, 2022 and the date of this Agreement, (a) the Company has conducted its business only in the ordinary course of business (except for the execution and performance of
this Agreement and the discussions, negotiations and transactions related thereto) and (b) there has not been any Material Adverse Effect.
3.10 Absence of Litigation. Except as disclosed in the 2023 SEC Reports, there is no action,
suit, proceeding, arbitration, claim, investigation or inquiry pending or, to the Company’s knowledge, threatened in writing by or before any federal, state, local or foreign governmental or regulatory commission, board, body, authority or
agency, or before or by any self-regulatory organization or other non-governmental regulatory authority (including, without limitation, the Nasdaq) against the Company or any subsidiary or, to the
Company’s knowledge, any of their respective directors or officers, or any of their respective properties, which, individually or in the aggregate, has had or would reasonably be expected to have a Material Adverse Effect, nor are there any
orders, writs, injunctions, judgments or decrees outstanding of any court or government agency or instrumentality and binding upon the Company that have had or would reasonably be expected to have a Material Adverse Effect. Neither the Company, nor
to the knowledge of the Company, any director or officer thereof, is, or within the last ten years has been, the subject of any action involving a claim of violation of or liability under federal or state securities laws relating to the Company or a
claim of breach of fiduciary duty relating to the Company.
3.11 Compliance with Law;
Permits. None of the Company and its subsidiaries is in violation of, and has received any notices of violations with respect to, any laws, statutes, ordinances, rules or regulations of any governmental body, court or government agency or
instrumentality, except for violations which, individually or in the aggregate, have not had and would not reasonably be expected to have a Material Adverse Effect. The Company has all required licenses, permits, certificates and other
authorizations (collectively, “Governmental Authorizations”) from such federal, state or local government or governmental agency, department or body that are currently necessary for the operation of the business of the Company and
its subsidiaries as currently conducted, except where the failure to possess currently such Governmental Authorizations has not had and is not reasonably expected to have a Material Adverse Effect. None of the Company or its subsidiaries has
received any written notice regarding any revocation or material modification of any such Governmental Authorization, which, individually or in the aggregate, if the subject of an unfavorable decision, ruling or finding, has or would reasonably be
expected to result in a Material Adverse Effect.
9
3.12 Intellectual Property. The Company and
its subsidiaries own or possess valid rights, or can acquire on commercially reasonable terms sufficient legal rights to use all patents, patent applications, proprietary information, trademarks (both registered and unregistered), service marks,
trade names, trademark registrations, service mark registrations, copyrights, licenses, know-how, inventions, software, works of authorships, trademarks, formulae, Internet domain names and other intellectual
property (including trade secrets and other unpatented and/or unpatentable proprietary or confidential information, systems or procedures) (collectively, the “Intellectual Property”), necessary for the conduct of their respective
businesses as conducted as of the date hereof or as described to be conducted in the SEC Reports; the Company and its subsidiaries have not received any written notice of any claim of infringement or conflict which asserted Intellectual Property
rights of others; to the knowledge of the Company, the Company and its subsidiaries’ respective businesses as now conducted do not give rise to any infringement of, any misappropriation of, or other violation of, any valid and enforceable
Intellectual Property of any other person; all licenses for the use of the Intellectual Property Rights described in the SEC Reports are valid, binding upon, and enforceable by or against the parties thereto in accordance with its terms; the Company
has no reason to believe that the licensors under such licenses and other agreements do not have and/or did not have all requisite power and authority to grant the rights to the Intellectual Property purported to be granted thereby; the Company has
complied in all material respects with, and is not in material breach nor has received any asserted or threatened claim of breach of any Intellectual Property license, and the Company has no knowledge of any breach or anticipated breach by any other
person to any Intellectual Property license; there are no pending, or to the Company’s knowledge, threatened judicial proceedings or interference proceedings against the Company or its subsidiaries challenging the Company’s rights in or to
or the validity of the scope of any of the Company’s Intellectual Property; to the Company’s knowledge, no other entity or individual has any right or claim in any of the Company’s or its subsidiaries’ Intellectual Property by
virtue of any contract, license or other agreement entered into between such entity or individual and the Company or by any non-contractual obligation, other than by written licenses granted by the Company,
except for such right or claim that would not reasonably be expected to, individually or in the aggregate, have a Material Adverse Effect; the Company and its subsidiaries have not received any written notice of any claim challenging the rights of
the Company in or to any Intellectual Property owned, licensed or optioned by the Company or its subsidiaries which claim, if the subject of an unfavorable decision would result in a Material Adverse Effect; the Company has taken all reasonable
steps to protect, maintain and safeguard its Intellectual Property, including the execution of appropriate nondisclosure and confidentiality agreements.
3.13 Employee Benefits. Except as would not be reasonably likely to result in a Material
Adverse Effect, each Benefit Plan has been established and administered in accordance with its terms and in compliance with the applicable provisions of ERISA, the Code, the Patient Protection and Affordable Care Act of 2010, as amended, and other
applicable laws, rules and regulations. The Company is in compliance with all applicable federal, state and local laws, rules and regulations regarding employment, except for any failures to comply that are not reasonably likely, individually or in
the aggregate, to have a Material Adverse Effect. There is no labor dispute, strike or work stoppage against the Company pending or, to the knowledge of the Company, threatened which may interfere with the business activities of the Company, except
where such dispute, strike or work stoppage is not reasonably likely, individually or in the aggregate, to have a Material Adverse Effect.
10
3.14 Taxes. Each of the Company and its
subsidiaries has filed all federal income Tax Returns and other material Tax Returns required to have been filed under applicable law (or extensions have been duly obtained), and all such Tax Returns are true, complete and correct in all material
respects. Each of the Company and its subsidiaries has paid all Taxes required to have been paid by it (whether or not shown due on any Tax Return). Each of the Company and its subsidiaries has complied in all material respects with all applicable
laws relating to the payment and withholding of Taxes. The Company is not a party to any Tax Sharing Agreement. There are no Liens on any of the assets of the Company with respect to Taxes. No assessment on Taxes is pending, proposed, in progress,
or threatened against the Company. All Tax deficiencies which have been proposed, asserted or assessed against the Company have been fully paid or finally settled. The Company has not received a notice of a claim by any Tax authority in any
jurisdiction where the Company does not file tax returns that the Company is or may be subject to taxation by, or required to file tax returns with, that jurisdiction, and, to the knowledge of the Company, there is no basis for any such claim to be
made. The Company has not constituted a “distributing corporation” or a “controlled corporation” under Section 355 of the Code. The Company is not, nor has it ever been, a “United States real property holding
corporation” within the meaning of Section 897(c)(2) of the Code. The Company has not been included in an affiliated group (as defined in Section 1504 of the Code or any similar group under a corresponding or similar provision of state, local,
or non-U.S. law) and is not liable for the Taxes of any other Person (i) under Treasury Regulations Section 1.1502-6 (or any corresponding or similar provision of state, local or non-U.S. law), or (ii) as a transferee or successor, by assumption or
operation of law, or by Contract or otherwise. The charges, accruals and reserves on the books of the Company in respect of any income and corporation tax liability for any years not finally determined are adequate to meet any assessments or
reassessments for additional income tax for any years not finally determined, except to the extent of any inadequacy that would not result in a Material Adverse Effect.
3.15 Environmental Laws. Each of the Company and its subsidiaries (i) is in compliance
with any and all applicable foreign, federal, state and local laws and regulations relating to the protection of human health and safety, the environment or hazardous or toxic substances or wastes, pollutants or contaminants (“Environmental
Laws”), (ii) has received all permits and other Governmental Authorizations required under applicable Environmental Laws to conduct its business and (iii) is in compliance with all terms and conditions of any such permit, license
or approval, except where such noncompliance with Environmental Laws, failure to receive required permits, licenses or other approvals or failure to comply with the terms and conditions of such permits, licenses or approvals would not, individually
or in the aggregate, reasonably be expected to have a Material Adverse Effect. The Company has not received since January 1, 2021, any written notice or other communication (in writing or otherwise), whether from a governmental authority or
other Person, that alleges that the Company, any subsidiary or any of their respective properties, assets and operations is not in compliance with any Environmental Law and, to the knowledge of the Company, there are no circumstances that may
prevent or interfere with the Company’s compliance with any Environmental Law in the future, except where such failure to comply would not reasonably be expected to have a Material Adverse Effect. To the knowledge of the Company: (i) no
current or (during the time a prior property was leased or controlled by the Company or its subsidiaries) prior property leased or controlled by the Company or its subsidiaries has received since January 1, 2021, any written notice or other
communication relating to property owned or leased at any time by the Company or its
11
subsidiaries, whether from a governmental authority, or other Person, that alleges that such current or prior owner or the Company or its subsidiaries is not in compliance with or violated any
Environmental Law relating to such property and (ii) the Company has no material liability under any Environmental Law.
3.16 Title. Each of the Company and its subsidiaries has good and marketable title to all
personal property owned by it that is material to the business of the Company, free and clear of all liens, encumbrances and defects except such as do not materially affect the value of such property and do not interfere with the use made and
proposed to be made of such property by the Company or its subsidiaries, as the case may be. Any real property and buildings held under lease by the Company or its subsidiaries is held under valid, subsisting and enforceable leases with such
exceptions as are not material and do not interfere with the use made and proposed to be made of such property and buildings by the Company or its subsidiaries, as the case may be. The Company does not own any real property.
3.17 Insurance. The Company carries or is entitled to the benefits of insurance in such amounts
and covering such risks that is customary for comparably situated companies and is adequate for the conduct of its business and the value of its properties and assets, and each of such insurance policies is in full force and effect and the Company
is in compliance in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers, since January 1, 2022, the Company has not received any notice or other communication regarding any
actual or possible: (i) cancellation or invalidation of any insurance policy or (ii) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy.
3.18 Nasdaq Stock Market. The issued and outstanding shares of Common Stock are registered
pursuant to Section 12(b) of the Exchange Act and are listed for trading on the Nasdaq Capital Market under the symbol “FBRX”. There is no suit, action, proceeding or investigation pending or, to the knowledge of the Company,
threatened against the Company by Nasdaq or the SEC, respectively, to prohibit or terminate the listing of the Common Stock on the Nasdaq Capital Market or to deregister the Common Stock under the Exchange Act. The Company has not, in the previous
twelve (12) months, received (i) written notice from the Nasdaq Stock Market LLC that the Company is not in compliance with the listing or maintenance requirements of the Nasdaq Stock Market LLC that would result in immediate delisting or
(ii) any notification, staff delisting determination, or public reprimand letter that requires a public announcement by the Company of any noncompliance or deficiency with respect to such listing or maintenance requirements. The Company is in
compliance with all listing and maintenance requirements of the Nasdaq Stock Market LLC on the date hereof. The Company has taken no action as of the date hereof that is designed to terminate the registration of the Common Stock under the Exchange
Act nor has the Company received any notification that the Commission is contemplating terminating such registration.
3.19 Sarbanes-Oxley Act. The Company is, and since January 1, 2021 has been, in compliance in
all material respects with applicable requirements of the Sarbanes-Oxley Act of 2002 and applicable rules and regulations promulgated by the SEC thereunder. Except as disclosed in the SEC Reports, the Company is not aware of any material weaknesses
in its internal control over financial reporting.
12
3.20 Regulatory Compliance. The preclinical
tests and other studies used to support regulatory approval (collectively, “studies”) being conducted by the Company are being conducted in all material respects in accordance with the approved protocols and applicable laws and
regulations.
3.21 Accounting Controls and Disclosure Controls and Procedures. The Company
maintains a system of internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that is sufficient to provide
reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including policies and procedures sufficient to provide reasonable assurance
(i) that the Company maintains records that in reasonable detail accurately and fairly reflect the Company’s transactions and dispositions of assets, (ii) that transactions are recorded as necessary to permit preparation of financial
statements in accordance with GAAP, (iii) that receipts and expenditures are made only in accordance with authorizations of management and the Board of Directors and (iv) regarding prevention or timely detection of the unauthorized
acquisition, use or disposition of the Company’s assets that could have a material effect on the Company’s financial statements. Except as disclosed in the Company’s SEC Reports filed prior to the date hereof, the Company has not
identified any material weaknesses in the design or operation of the Company’s internal control over financial reporting. The Company’s “disclosure controls and procedures” (as defined in Rules
13a-15(e) and 15d-15(e) of the Exchange Act) are designed to provide reasonable assurance that all information (both financial and
non-financial) required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the
rules and forms of the SEC, and that all such information is accumulated and communicated to the Company’s management as appropriate to allow timely decisions regarding required disclosure.
3.22 Price Stabilization of Common Stock. Neither the Company nor any subsidiary nor any of
their respective directors or officers, affiliates or controlling persons has taken, nor will it take, directly or indirectly, any action designed to stabilize or manipulate the price of the Common Stock to facilitate the sale or resale of the
Securities.
3.23 Investment Company Act. Neither the Company nor any of its subsidiaries
is, and immediately after receipt of payment for the Securities hereunder and the application of the net proceeds from such issuance and sale, none of the Company nor any of its subsidiaries will be, an “investment company” or an entity
“controlled” by an “investment company,” within the meaning of the Investment Company Act of 1940, as amended.
3.24 General Solicitation; No Integration or Aggregation. Neither the Company nor any other
person or entity authorized by the Company to act on its behalf has engaged in a general solicitation or general advertising (within the meaning of Regulation D of the Securities Act) of investors with respect to offers or sales of Common Stock
pursuant to this Agreement. The Company has not, directly or indirectly, sold, offered for sale, solicited offers to buy or otherwise negotiated in respect of, any security (as defined in the Securities Act) which, to its knowledge, is or will be
(i) integrated with the Securities sold pursuant to this Agreement for purposes of the Securities Act or (ii) aggregated with prior offerings by the Company for the purposes of the rules and regulations of the Nasdaq Capital Market.
13
3.25 Brokers and Finders. Neither the Company
nor any other Person authorized by the Company to act on its behalf has retained, utilized or been represented by any broker or finder in connection with the transactions contemplated by this Agreement.
3.26 Reliance by the Purchasers. The Company acknowledges that each of the Purchasers will rely
upon the truth and accuracy of, and the Company’s compliance with, the representations, warranties, agreements, acknowledgements and understandings of the Company set forth herein.
3.27 No Disqualification Events. No “bad actor” disqualifying event described in Rule
506(d)(1)(i)-(viii) of the Securities Act (a “Disqualification Event”) is applicable to the Company or, to the knowledge of the Company, any Covered Person (as defined below), except for a Disqualification Event as to which Rule
506(d)(2)(ii–iv) or (d)(3), is applicable. “Covered Person” means, with respect to the Company as an “issuer” for purposes of Rule 506 promulgated under the Securities Act, any person listed in the first paragraph of
Rule 506(d)(1). The Company is not aware of any Person (other than any Covered Person) that has been or will be paid (directly or indirectly) remuneration for solicitation of purchasers in connection with the sale of the Securities pursuant to this
Agreement.
3.28 No Additional Agreements. The Company does not have any agreement or
understanding with any Purchaser with respect to the transactions contemplated by the Transaction Agreements other than as specified in the Transaction Agreements.
3.29 Anti-Bribery and Anti-Money Laundering Laws. Each of the Company, its subsidiaries and any
of their respective officers, directors, supervisors, managers, agents, or employees are and have at all times been in compliance with and its participation in the offering will not violate: (A) anti-bribery and anti-corruption laws,
including but not limited to, any applicable law, rule, or regulation of any locality, including but not limited to any law, rule, or regulation promulgated to implement the OECD Convention on Combating Bribery of Foreign Public Officials in
International Business Transactions, signed December 17, 1997, including the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.K. Bribery Act 2010, or any other law, rule or regulation of similar purposes and scope (collectively,
the “Anti-Bribery and Anti-Corruption Laws”) or (B) anti-money laundering laws, including, but not limited to, applicable federal, state, international, foreign or other laws, regulations or government guidance regarding anti-money
laundering, including, without limitation, Title 18 US. Code sections 1956 and 1957, the Patriot Act, the Bank Secrecy Act, and international anti-money laundering principles or procedures by an intergovernmental group or organization, such as the
Financial Action Task Force on Money Laundering, of which the United States is a member and with which designation the United States representative to the group or organization continues to concur, all as amended, and any executive order, directive,
or regulation pursuant to the authority of any of the foregoing, or any orders or licenses issued thereunder (collectively, the “Money Laundering Laws”). No action, suit or proceeding by or before any court or governmental agency,
authority or body or any arbitrator or non-governmental authority involving the Company or any subsidiary with respect to the Anti-Bribery and Anti-Corruption Laws or Money Laundering Laws is pending
or, to the Company’s knowledge, threatened.
14
3.30 Company IT Systems; Cybersecurity. The
Company and its subsidiaries own or have a valid right to access and use all computer systems, networks, hardware, software, databases, websites, and equipment used to process, store, maintain and operate data, information, and functions used in
connection with the business of the Company and its subsidiaries (the “Company IT Systems”), except as would not, individually or in the aggregate, have a Material Adverse Effect. The Company IT Systems are adequate for, and operate
and perform in all material respects as required in connection with, the operation of the business of the Company and its subsidiaries as currently conducted, except as would not, individually or in the aggregate, have a Material Adverse Effect. The
Company and its subsidiaries have implemented commercially reasonable backup, security and disaster recovery technology consistent in all material respects with applicable regulatory standards and customary industry practices. Except as would not
reasonably be expected to have a Material Adverse Effect, (a) there has been no security breach or other compromise of or relating to the Company IT Systems; (b) the Company has not been notified of, and has no knowledge of any event or
condition that would reasonably be expected to result in, any such security breach or other compromise of the Company IT Systems; (c) the Company and its subsidiaries have implemented policies and procedures with respect to the Company IT
Systems that are reasonably consistent with industry standards and practices, or as required by applicable regulatory standards; and (d) the Company and its subsidiaries are presently in material compliance with all applicable laws or statutes,
judgments, orders, rules and regulations of any court or arbitrator or governmental or regulatory authority and contractual obligations relating to the privacy and security of the Company IT Systems and to the protection of the Company IT Systems
from unauthorized use, access, misappropriation or modification.
3.31 Transactions with
Affiliates and Employees. Except for the transactions contemplated by the Transaction Agreements, no relationship, direct or indirect, exists between or among the Company, on the one hand, and the directors, officers, stockholders, customers or
suppliers of the Company, on the other hand, that is required to be described in the SEC Reports that is not so described.
3.32 Shell Company Status. The Company is not currently, and has never been, an issuer
identified in Rule 144(i)(1) under the Securities Act.
3.33 Regulation D. Assuming the
accuracy of the Purchasers in Section 4 hereof, no registration under the Securities Act is required for the offer and sale of the Securities by the Company to the Purchasers hereunder. The Company shall file a Form D with respect to the
Securities as required under Regulation D and, to the extent the Form D is not publicly available on the Commission’s EDGAR reporting system, to provide a copy thereof to each Purchaser promptly after such filing. The Company, on or before the
Closing Date, shall take such action as the Company shall reasonably determine is necessary in order to obtain an exemption for or to qualify the Securities for sale to the Purchasers at the Closing, pursuant to this Agreement under applicable
securities or blue sky laws of the states of the United States (or to obtain an exemption from such qualification), and, if requested by a Purchaser, shall provide evidence of any material action so taken to such Purchaser on or prior to the Closing
Date. The Company shall make all filings and reports relating to the offer and sale of the Securities required under applicable securities or blue sky laws of the states of the United States following the Closing.
15
3.34 Independent Public Accountants. Mayer,
Hoffman & McCann P.C., who have certified certain financial statements of the Company and delivered their report with respect to the audited financial statements included in the SEC Reports, are independent public accountants with respect to the
Company within the meaning of the Securities Act and the applicable published rules and regulations thereunder.
3.35 Stock Plans. Each stock option granted under any equity incentive plan of the Company
(each, a “Stock Plan”) was granted with a per share exercise price no less than the fair market value per share of Common Stock on the grant date of such option, and no such grant involved any “back-dating” or similar
practice with respect to the effective date of such grant, each such option (i) was granted in compliance with applicable law and with the applicable Stock Plan(s), (ii) was duly approved by the board of directors (or a duly authorized
committee thereof or by an executive officer pursuant to Section 157(c) of the Delaware General Corporation Law) of the Company and (iii) has been properly accounted for in the Company’s financial statements in accordance with U.S.
generally accepted accounting principles and disclosed in the Company’s filings with the Commission.
3.36 Changes. Since the date of the latest audited financial statements included within the
2023 SEC Reports, except as specifically disclosed in a subsequent 2023 SEC Reports filed prior to the date hereof (i) there has been no event, occurrence or development that has had or that could reasonably be expected to have a Material
Adverse Effect; (ii) the Company has not incurred any liabilities (contingent or otherwise) other than (A) trade payables and accrued expenses incurred in the ordinary course of business consistent with past practice and
(B) liabilities not required to be reflected in the Company’s financial statements pursuant to GAAP or disclosed in filings made with the Commission; (iii) the Company has not altered its method of accounting or changed its principal
registered public accounting firm; (iv) the Company has not declared or made any dividend or distribution of cash or other property to its stockholders or purchased, redeemed or made any agreements to purchase or redeem any shares of its
capital stock; (v) there has not been any material damage, destruction or loss, whether or not covered by insurance, to any assets or properties of the Company; (vi) there has not been any waiver, not in the ordinary course of business, by
the Company of a material right or of a material debt owed to it; (vii) there has not been any satisfaction or discharge of a material lien, claim or encumbrance or payment of any obligation by the Company, except in the ordinary course of
business; (viii) there has not been any change or amendment to the Amended and Restated Certificate of Incorporation or Amended and Restated Bylaws of the Company, or termination of or material amendment to any material contract;
(ix) there has not been any material labor difficulties or, to the Company’s Knowledge, labor union organizing activities with respect to employees of the Company; (j) there has not been any material transaction entered into by the
Company other than in the ordinary course of business; and (k) there has not been a loss of the services of any executive officer (as defined in Rule 405 under the Securities Act) of the Company.
3.37 Healthcare Regulatory Proceedings. There is no legal or governmental proceeding to which
the Company or any of its subsidiaries is a party or of which any property or assets of the Company or any of its subsidiaries is the subject, including any proceeding before the U.S. Department of Health and Human Services (“HHS”),
U.S. state boards of pharmacy or comparable federal, state, local or foreign governmental bodies; and to the Company’s knowledge after reasonable investigation and due diligence inquiry, no such proceedings are threatened by
16
governmental or regulatory authorities or threatened by others. The Company is in material compliance with all applicable federal, state, local and foreign laws, regulations, orders and decrees
governing its business as prescribed by the HHS, or any other federal, state or foreign agencies or bodies engaged in the regulation of the Company’s business. Neither the Company nor any of its subsidiaries is a party to any corporate
integrity agreements, monitoring agreements, consent decrees, settlement orders, or similar agreements with or imposed by any governmental or regulatory authority. Additionally, neither the Company, any of its subsidiaries nor any of their
respective employees, officers, directors, or, to the Company’s knowledge, any agents has been excluded, suspended or debarred from participation in any U.S. federal health care program or human clinical research or, to the knowledge of the
Company, is subject to a governmental inquiry, investigation, proceeding, or other similar action that could reasonably be expected to result in debarment, suspension, or exclusion.
3.38 Regulatory Matters. The Company has not received any correspondence or notice from any
court or arbitrator or federal, state, local, or foreign governmental or regulatory authority, alleging or asserting noncompliance with the Health Care Laws (as defined below). The Company and its directors, officers, employees and, to the
Company’s knowledge, agents are and have been in material compliance with all applicable health care laws, including without limitation, the Federal Food, Drug, and Cosmetic Act (21 U.S.C. §301 et seq.), the Controlled Substances Act (21
U.S.C. § 801 et seq.), the federal Anti- Kickback Statute (42 U.S.C. § 1320a-7b(b)), the civil False Claims Act (31 U.S.C. § 3729 et seq.), the criminal False Claims Law (42 U.S.C. § 1320a-7b(a)), the Civil Monetary Penalties Law (42 U.S.C. § 1320a-7a), the Health Insurance Portability and Accountability Act of 1996 (42 U.S.C. § 1320d et seq.),
as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (42 U.S.C. § 17921 et seq.) the exclusions law (42 U.S.C. § 1320a-7), Medicare (Title XVIII of the Social
Security Act), Medicaid (Title XIX of the Social Security Act), and the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, and the regulation promulgated pursuant to such laws,
and comparable state laws, and all other local, state, federal, national, supranational, and foreign laws relating to development of pharmaceuticals or related services, or billing or reimbursement therefor, including without limitation, such laws
regarding health care fraud and abuse, kickbacks, self-referrals, false claims, and the regulation of the Company (collectively, “Health Care Laws”); provided, however, “Health Care Laws” shall not include Privacy Laws.
Neither the Company nor any of its officers, directors, employees, or, to the Company’s knowledge, any agents has been or is currently excluded from participation in the Medicare and Medicaid programs or any other state or federal health care
program.
3.39 No Other Representations or Warranties. Except for the representations and
warranties of the Company expressly set forth in this Article III, with respect to the transactions contemplated by this Agreement, the Company (i) expressly disclaims any representations or warranties of any kind or nature, express or implied,
including with respect to the condition, value or quality of the Company or any of the assets or properties of the Company, and (ii) specifically disclaims any representation or warranty of merchantability, usage, suitability or fitness for any
particular purpose with respect to any of the assets or properties of the Company. Notwithstanding the foregoing, in making the decision to invest in the Securities, the Purchasers will rely, and the
17
Company agrees that the Purchasers may rely, on the information that has been provided in writing to Purchasers by the Company or on behalf of the Company, including the SEC Reports.
4. Representations and Warranties of Each Purchaser. Each Purchaser, severally for itself and
not jointly with any other Purchaser, represents and warrants to the Company that the statements contained in this Section 4 are true and correct as of the date hereof and the Closing Date:
4.1 Organization. Such Purchaser is duly organized, validly existing and in good standing under
the laws of the jurisdiction of its organization and has the requisite power and authority to own, lease and operate its properties and to carry on its business as now conducted.
4.2 Authorization. Such Purchaser has all requisite corporate or similar power and authority to
enter into this Agreement and the other Transaction Agreements to which it will be a party and to carry out and perform its obligations hereunder and thereunder. All corporate, member or partnership action on the part of such Purchaser or its
stockholders, members or partners necessary for the authorization, execution, delivery and performance of this Agreement and the other Transaction Agreements to which it will be a party and the consummation of the other transactions contemplated
herein has been taken. The signature of the Purchaser on this Agreement is genuine and the signatory to this Agreement, if the Purchaser is an individual, has the legal competence and capacity to execute the same or, if the Purchaser is not an
individual, the signatory has been duly authorized to execute the same on behalf of the Purchaser. Assuming this Agreement constitutes the legal and binding agreement of the Company, this Agreement constitutes a legal, valid and binding obligation
of such Purchaser, enforceable against such Purchaser in accordance with its terms, except as such enforceability may be limited or otherwise affected by bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium and/or similar laws
relating to or affecting the rights of creditors generally or by general equity principles (regardless of whether such enforceability is considered in a proceeding in equity or at law).
4.3 No Conflict. The execution, delivery and performance of the Transaction Agreements by such
Purchaser, the purchase of the Securities in accordance with their terms and the consummation by such Purchaser of the other transactions contemplated hereby will not conflict with or result in any violation of, breach or default by such Purchaser
(with or without notice or lapse of time, or both) under, conflict with, or give rise to a right of termination, cancellation or acceleration of any obligation, a change of control right or to a loss of a material benefit under (i) any
provision of the organizational documents of such Purchaser, including, without limitation, its incorporation or formation papers, bylaws, indenture of trust or partnership or operating agreement, as may be applicable or (ii) any agreement or
instrument, undertaking, credit facility, franchise, license, judgment, order, ruling, statute, law, ordinance, rule or regulations, applicable to such Purchaser or its respective properties or assets, except, in the case of clause (ii), as would
not, individually or in the aggregate, be reasonably expected to materially delay or hinder the ability of such Purchaser to perform its obligations under the Transaction Agreements (such delay or hindrance, a “Purchaser Adverse
Effect”).
4.4 Consents. All consents, approvals, orders and authorizations
required on the part of such Purchaser in connection with the execution, delivery or performance of this Agreement, the issuance of the Securities and the consummation of the other transactions
18
contemplated herein have been obtained or made, other than such consents, approvals, orders and authorizations the failure of which to make or obtain, individually or in the aggregate, would not
reasonably be expected to have a Purchaser Adverse Effect.
4.5 Residency. Such
Purchaser’s residence (if an individual) or offices in which its investment decision with respect to the Securities was made (if an entity) are located at the address immediately below such Purchaser’s name on Exhibit A.
4.6 Brokers and Finders. Such Purchaser has not retained, utilized or been represented by any
broker or finder in connection with the transactions contemplated by this Agreement whose fees the Company would be required to pay.
4.7 Investment Representations and Warranties. Each Purchaser hereby represents and warrants
that, it (i) as of the date hereof is, and on each date on which it exercises the Pre-Funded Warrants, will be, if an entity, a “qualified institutional buyer” (as defined in Rule 144A under the
Securities Act) or an institutional “accredited investor” as that term is defined in Rule 501(a) under Regulation D promulgated pursuant to the Securities Act; or (ii) if an individual, is an “accredited investor” as that
term is defined in Rule 501(a) of Regulation D of the Securities Act and has such knowledge and experience in financial and business matters as to be able to protect its own interests in connection with an investment in the Securities. Each
Purchaser further represents and warrants that (x) it is capable of evaluating the merits and risk of such investment, and (y) that it has not been organized for the purpose of acquiring the Securities and is an “institutional
account” as defined by FINRA Rule 4512(c). Such Purchaser understands and agrees that the offering and sale of the Securities has not been registered under the Securities Act or any applicable state securities laws and is being made in reliance
upon federal and state exemptions for transactions not involving a public offering which depend upon, among other things, the bona fide nature of the investment intent and the accuracy of such Purchaser’s representations as expressed herein.
4.8 Intent. Purchaser is purchasing the Shares and, if applicable, Pre-Funded Warrants, and, upon the exercise of the Pre-Funded Warrants, will acquire the Warrant Shares issuable upon exercise thereof, solely for investment purposes, for
such Purchaser’s own account and not for the account of others, and not with a view towards, or for offer or sale in connection with, any distribution or dissemination thereof; provided, however, that by making the representations herein, such
Purchaser does not agree to hold any of the Securities for any minimum or other specific term and reserves the right to dispose of the Securities at any time in accordance with or pursuant to a registration statement or an exemption under the
Securities Act. Notwithstanding the foregoing, if such Purchaser is purchasing the Securities as a fiduciary or agent for one or more investor accounts, such Purchaser has full investment discretion with respect to each such account, and the full
power and authority to make the acknowledgements, representations and agreements herein on behalf of each owner of each such account. Each Purchaser has no present arrangement to sell the Securities to or through any person or entity. Each Purchaser
understands that the Securities must be held indefinitely unless such Securities are resold pursuant to a registration statement under the Securities Act or an exemption from registration is available.
19
4.9 Investment Experience; Ability to Protect Its
Own Interests and Bear Economic Risks. Each Purchaser, or the Purchaser’s professional advisors, have such knowledge and experience in finance, securities, taxation, investments and other business matters as to be capable of evaluating the
merits and risks of investments of the kind described in this Agreement, and the Purchaser has had an opportunity to seek, and has sought, such accounting, legal, business and tax advice as such Purchaser has considered necessary to make an informed
investment decision. By reason of the business and financial experience of such Purchaser or such Purchaser’s professional advisors (who are not affiliated with or compensated in any way by the Company or any of its affiliates or selling
agents), such Purchaser can protect such Purchaser’s own interests in connection with the transactions described in this Agreement. Each Purchaser acknowledges that such Purchaser (i) is a sophisticated investor, experienced in investing
in private placements of equity securities and capable of evaluating investment risks independently, both in general and with regard to all transactions and investment strategies involving a security or securities and (ii) has exercised
independent judgment in evaluating its participation in the purchase of the Securities. Each Purchaser acknowledges that such Purchaser is aware that there are substantial risks incident to the purchase and ownership of the Securities, including
those set forth in the Company’s filings with the SEC. Alone, or together with any professional advisor(s), such Purchaser has adequately analyzed and fully considered the risks of an investment in the Securities and determined that the
Securities are a suitable investment for the Purchaser. Each Purchaser is, at this time and in the foreseeable future, able to afford the loss of such Purchaser’s entire investment in the Securities and such Purchaser acknowledges specifically
that a possibility of total loss exists.
4.10 Tax Advisors. Such Purchaser has had
the opportunity to review with such Purchaser’s own tax advisors the federal, state and local tax consequences of its purchase of the Securities set forth opposite such Purchaser’s name on Exhibit A, where applicable, and the
transactions contemplated by this Agreement. Such Purchaser acknowledges that Purchaser shall be responsible for any of such Purchaser’s tax liabilities that may arise as a result of the transactions contemplated by this Agreement, and that the
Company and any of its agents have not provided any tax advice or any other representation or guarantee regarding the tax consequences of the transactions contemplated by the Agreement.
4.11 Securities Not Registered; Legends. Such Purchaser acknowledges and agrees that the
Securities are being offered in a transaction not involving any public offering within the meaning of the Securities Act, and such Purchaser understands that the Securities have not been registered under the Securities Act, by reason of their
issuance by the Company in a transaction exempt from the registration requirements of the Securities Act, and that the Securities must continue to be held and may not be offered, resold, transferred, pledged or otherwise disposed of by such
Purchaser unless a subsequent disposition thereof is registered under the Securities Act or is exempt from such registration and in each case in accordance with any applicable securities laws of any state of the United States. Such Purchaser
understands that the exemptions from registration afforded by Rule 144 (the provisions of which are known to it) promulgated under the Securities Act depend on the satisfaction of various conditions including, but not limited to, the time and manner
of sale, the holding period and on requirements relating to the Company which are outside of such Purchaser’s control and which the Company may not be able to satisfy, and that, if applicable, Rule 144 may afford the basis for sales only in
limited amounts. Such Purchaser acknowledges and agrees that it has been advised to consult legal counsel prior to making any offer, resale, transfer, pledge or disposition of any of the Securities. Such Purchaser acknowledges
20
that no federal or state agency has passed upon or endorsed the merits of the offering of the Shares or made any findings or determination as to the fairness of this investment.
Each Purchaser understands that the Securities may bear one or more legends in the following form:
“THESE SECURITIES [AND THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES] ARE BEING OFFERED TO INVESTORS WITHOUT
REGISTRATION WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (“THE SECURITIES ACT”) IN RELIANCE UPON REGULATION D PROMULGATED UNDER THE SECURITIES ACT. TRANSFER OF THESE SECURITIES
[AND THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES] IS PROHIBITED, EXCEPT PURSUANT TO REGISTRATION UNDER THE SECURITIES ACT, OR PURSUANT TO AVAILABLE EXEMPTION FROM REGISTRATION. HEDGING TRANSACTIONS MAY NOT BE CONDUCTED UNLESS IN
COMPLIANCE WITH THE SECURITIES ACT.”
In addition, stock certificates representing the Securities may contain a
legend regarding affiliate status of the Purchaser, if applicable.
4.12 Reliance by the
Company. Such Purchaser acknowledges that the Company will rely upon the truth and accuracy of, and the Purchaser’s compliance with, the representations, warranties, agreements, acknowledgements and understandings of such Purchaser set
forth herein.
4.13 No General Solicitation. The Purchaser acknowledges and agrees that the
Purchaser is purchasing the Securities directly from the Company. Purchaser became aware of this offering of the Securities directly from the Company as a result of a pre-existing, substantive relationship
with the Company, and/or its advisors (including, without limitation, attorneys, accountants, bankers, consultants and financial advisors), agents, control persons, representatives, affiliates, directors, officers, managers, members, and/or
employees, and/or the representatives of such persons. The Securities were offered to Purchaser solely by direct contact between Purchaser and the Company, and/or its representatives. Purchaser did not become aware of this offering of the
Securities, nor were the Securities offered to Purchaser, by any other means, and none of the Company and/or its representatives acted as investment advisor, broker or dealer to Purchaser. The Purchaser is not purchasing the Securities as a result
of any advertisement, article, notice or other communication regarding the Securities published in any newspaper, magazine or similar media or broadcast over television, radio or the internet or presented at any seminar, to the Purchaser’s
knowledge, or any other general solicitation or general advertisement, including any of the methods described in Section 502(c) of Regulation D under the Securities Act.
4.14 No Reliance. The Purchaser further acknowledges that there have not been and
Purchaser hereby agrees that it is not relying on and has not relied on, any statements, representations, warranties, covenants or agreements made to the Purchaser by or on behalf of the Company, any of its affiliates or any control persons,
officers, directors, employees, partners, agents or representatives of any of the foregoing or any other person or entity, expressly or by
21
implication, other than the SEC Reports and those representations, warranties and covenants of the Company expressly set forth in this Agreement.
4.15 Access to Information. In making its decision to purchase the Securities, Purchaser has
relied solely upon independent investigation made by Purchaser and upon the representations, warranties and covenants set forth herein. The Purchaser acknowledges and agrees that the Purchaser has received such information as the Purchaser deems
necessary in order to make an investment decision with respect to the Securities, including, with respect to the Company. Without limiting the generality of the foregoing, each Purchaser acknowledges that such Purchaser has reviewed the 2023 SEC
Reports filed prior to the date hereof. The Purchaser acknowledges and agrees that the Purchaser and the Purchaser’s professional advisor(s), if any, have had the opportunity to ask such questions, receive such answers and obtain such
information as the Purchaser and such Purchaser’s professional advisor(s), if any, have deemed necessary to make an investment decision with respect to the Securities and that the Purchaser has independently made his, her or its own analysis
and decision to invest in the Company.
4.16 Certain Trading Activities. Other than
consummating the transaction contemplated hereby, the Purchaser has not, nor has any Person acting on behalf of or pursuant to any understanding with such Purchaser, directly or indirectly executed any purchases or sales, including Short Sales, of
the securities of the Company during the period commencing as of the time that such Purchaser was first contacted by the Company or any other Person regarding the transaction contemplated hereby and ending immediately prior to the date hereof.
Notwithstanding the foregoing, in the case of a Purchaser that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Purchaser’s assets and the portfolio managers have no direct knowledge of
the investment decisions made by the portfolio managers managing other portions of such Purchaser’s assets, the representation set forth above shall only apply with respect to the portion of the assets managed by the portfolio manager that made
the investment decision to purchase the Securities covered by this Agreement. Other than to other Persons party to this Agreement, such Purchaser has maintained the confidentiality of all disclosures made to it in connection with this transaction
(including the existence and terms of this transaction), other than to a such Purchaser’s outside attorney, accountant, auditor, or investment advisor only to the extent necessary to permit evaluation of the investment, and the performance of
the necessary or required tax, accounting, financial, legal, or administrative tasks and services and other than as may be required by law. Notwithstanding the foregoing, for avoidance of doubt, nothing contained herein shall constitute a
representation or warranty, or preclude any actions, with respect to the identification of the availability of, or securing of, available shares to borrow in order to effect Short Sales or similar transactions in the future.
4.17 Disqualification Event. To the extent the Purchaser is one of the covered persons
identified in Rule 506(d)(1), the Purchaser represents that no disqualifying event described in Rule 506(d)(1)(i-viii) of the Securities Act (a “Disqualification Event”) is applicable to the
Purchaser or any of its Rule 506(d) Related Parties (as defined below), except, if applicable, for a Disqualification Event as to which Rule 506(d)(2)(ii) or (iii) or (d)(3) is applicable. The Purchaser hereby agrees that it shall notify the
Company promptly in writing in the event a Disqualification Event becomes applicable to the Purchaser or any of its Rule 506(d) Related Parties, except, if applicable, for a Disqualification Event as to which Rule 506(d)(2)(ii) or (iii) or
(d)(3) is applicable. For purposes of this Section, “Rule 506(d) Related Party” shall mean a person or
22
entity that is a beneficial owner of the Purchaser’s securities for purposes of Rule 506(d) of the Securities Act.
5. Covenants.
5.1 Further Assurances. Each party agrees to cooperate with each other and their respective
officers, employees, attorneys, accountants and other agents, and, generally, do such other reasonable acts and things in good faith as may be necessary to effectuate the intents and purposes of this Agreement, subject to the terms and conditions
hereof and compliance with applicable law, including taking reasonable action to facilitate the filing of any document or the taking of reasonable action to assist the other parties hereto in complying with the terms hereof. The Purchaser
acknowledges that the Company will rely on the acknowledgments, understandings, agreements, representations and warranties contained in this Agreement. Prior to the Closing, the Purchaser agrees to promptly notify the Company if any of the
acknowledgments, understandings, agreements, representations and warranties set forth in Section 4 of this Agreement are no longer accurate.
5.2 Listing. The Company shall use commercially reasonable efforts to prepare and file the
Notification Form: Listing of Additional Shares covering all of the Shares and Warrant Shares in the manner required by the Nasdaq Capital Market. The Company shall use commercially reasonable efforts to maintain the listing of its Common Stock on
the Nasdaq Capital Market and comply with the Company’s reporting, filing and other obligations under the bylaws or rules of such market or exchange.
5.3 Disclosure of Transactions. The Company shall, on or prior to the fourth (4th) business day immediately following the date hereof, file with the SEC a Current Report on Form 8-K (the “Disclosure Document”) disclosing
all material terms of the transactions contemplated hereby, by the other Transaction Agreements (and including as exhibits to such Current Report on Form 8-K the material Transaction Agreements (including,
without limitation, this Agreement, the form-of Pre-Funded Warrant and the Registration Rights Agreement)). Upon the filing of the Disclosure Document, to the knowledge
of the Company, no Purchaser shall be in possession of any material, non-public information received from the Company or any of its officers, directors, or employees or agents, that is not disclosed in the
Disclosure Document unless otherwise specifically agreed in writing by such Purchaser. In addition, effective upon the filing of the Disclosure Document, the Company acknowledges and agrees that any and all confidentiality or similar obligations
under this Agreement, or an agreement entered into in connection with the transactions contemplated by the Transaction Agreements, whether written or oral, between the Company, any of its subsidiaries or any of their respective officers, directors,
agents, employees or affiliates, on the one hand, and any of the Purchasers or any of their respective officers, directors, agents, employees or investment advisers, on the other hand, shall terminate unless otherwise specifically agreed in writing
by such Purchaser. Notwithstanding anything in this Agreement to the contrary, the Company shall not publicly disclose the name of any Purchaser or any of its affiliates or advisers, or include the name of any Purchaser or any of its affiliates or
advisers in any press release or filing with the SEC (other than any registration statement contemplated by the Registration Rights Agreement) or any regulatory agency, without the prior written consent of such Purchaser, except (i) as required
by the federal securities law in connection with (A) any registration statement contemplated by the Registration Rights Agreement and (B)
23
the filing of final Transaction Agreements (including signature pages thereto) with the SEC or pursuant to other routine proceedings of regulatory authorities, or (ii) to the extent such
disclosure is required by law, at the request of the staff of the SEC or regulatory agency or under the regulations of the Nasdaq Capital Market, in which case the Company will provide the Purchaser with prior written notice (including by e-mail) of such disclosure under this clause (ii).
5.4 Integration. The Company has not sold, offered for sale or solicited offers to buy and
shall not, and shall use its commercially reasonable efforts to ensure that no Affiliate of the Company shall, sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2 of the
Securities Act) that will be integrated with the offer or sale of the Securities in a manner that would require the registration under the Securities Act of the sale of the Securities to the Purchasers, or that will be integrated with the offer or
sale of the Securities for purposes of the rules and regulations of the Nasdaq Capital Market such that it would require stockholder approval prior to the closing of such other transaction unless stockholder approval is obtained before the closing
of such subsequent transaction.
5.5 Pledge of Securities. The Company acknowledges and
agrees that the Securities may be pledged by a Purchaser in its sole discretion in connection with a bona fide margin agreement or other loan or financing arrangement that is secured by the Securities. The pledge of Securities shall not be deemed to
be a transfer, sale or assignment of the Securities hereunder, and no Purchaser effecting a pledge of Securities shall be required to provide the Company with any notice thereof or otherwise make any delivery to the Company pursuant to this
Agreement. The Company hereby agrees to execute and deliver such documentation as a pledgee of the Securities may reasonably request in connection with a pledge of the Securities to such pledgee by a Purchaser; provided that any and all costs to
effect the pledge of the Securities are borne by the pledgor and/or pledgee and not the Company. Notwithstanding the foregoing, any Purchaser that is subject to the Company’s Insider Trading Policy must comply with such policy as it may pertain
to any pledges of Securities.
5.6 Subsequent Equity Sales. From the date hereof until 90
days after the Closing Date, without the consent of the Purchasers of at least a majority in interest of the Securities then held by Purchasers, the Company shall not (a) issue shares of Common Stock or Common Stock Equivalents, or
(b) file with the SEC a registration statement under the Securities Act relating to any shares of Common Stock or Common Stock Equivalents. Notwithstanding the foregoing, the provisions of this Section 5.6 shall not
apply to (i) the issuance of the Securities hereunder, (ii) the transactions contemplated by the Registration Rights Agreement, (iii) the issuance of Common Stock upon the exercise of any options or warrants outstanding on the date
hereof, (iv) the issuance of Common Stock or Common Stock Equivalents to employees, directors or consultants pursuant to (a) any stock option or equity incentive or employee stock purchase plan in effect on the date hereof, or (b) any
compensation agreements, (v) the issuance of Common Stock in connection with acquisitions or strategic transactions, provided that any such issuance shall only be to a Person which is, itself or through its subsidiaries, an operating company in
a business synergistic with the business of the Company, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities
and (vi) the filing of a registration statement on Form S-8; provided that the aggregate number of shares of Common Stock issued in accordance with
24
clause (v) of this Section 5.6 do not exceed 5% of the number of shares of Common Stock outstanding immediately after the issuance and sale of the Securities.
5.7 Reservation of Common Stock. As of the date hereof, the Company has reserved and the
Company shall continue to reserve and keep available at all times, free of preemptive rights, a sufficient number of shares of Common Stock for the purpose of enabling the Company to issue all of the Warrant Shares upon conversion of any Pre-Funded Warrant.
5.8 Use of Proceeds. The Company
shall use the proceeds from the sale of the Securities for working capital and general corporate purposes.
5.9 Removal of Legends.
(a) In connection with any sale, assignment, transfer or other disposition of the Shares or Warrant
Shares by a Purchaser pursuant to Rule 144 or pursuant to any other exemption under the Securities Act such that the purchaser acquires freely tradable shares and upon compliance by the Purchaser with the requirements of this Agreement, if requested
by the Purchaser by notice to the Company, the Company shall request the Transfer Agent to remove any restrictive legends related to the book entry account holding such shares and make a new, unlegended entry for such book entry shares sold or
disposed of without restrictive legends within two (2) business days of any such request therefor from such Purchaser, provided that the Company has timely received from the Purchaser customary representations and other documentation reasonably
acceptable to the Company in connection therewith. The Company shall be responsible for the fees of its Transfer Agent, its legal counsel and all DTC fees associated with such legend removal.
(b) Subject to receipt from the Purchaser by the Company and the Transfer Agent of customary
representations and other documentation reasonably acceptable to the Company and the Transfer Agent in connection therewith, upon the earliest of such time as the Shares or Warrant Shares (i) have been registered under the Securities Act
pursuant to an effective registration statement, (ii) have been sold pursuant to Rule 144, or (iii) are eligible for resale under Rule 144(b)(1) or any successor provision, the Company shall, in accordance with the provisions of this
Section 5.9(b) and within two (2) business days of any request therefor from a Purchaser accompanied by such customary and reasonably acceptable documentation referred to above, (A) deliver to the Transfer Agent
irrevocable instructions that the Transfer Agent shall make a new, unlegended entry for such book entry shares, and (B) cause its counsel to deliver to the Transfer Agent one or more opinions to the effect that the removal of such legends in
such circumstances may be effected under the Securities Act if required by the Transfer Agent to effect the removal of the legend in accordance with the provisions of this Agreement. Any shares subject to legend removal under this
Section 5.9 may be transmitted by the Transfer Agent to the Purchaser by crediting the account of the Purchaser’s prime broker with the DTC System as directed by such Purchaser. The Company shall be responsible for the
fees of its Transfer Agent and all DTC fees associated with such issuance
5.10 Participation
Rights.
(a) Non-Underwritten Offerings.
25
(i) If, during the period from the Closing Date through and including the
ten (10) year anniversary of the Closing Date (the “Participation Period”), the Company proposes to offer and sell any New Securities (as defined below) in an offering that is conducted pursuant to an exemption from
registration under the Securities Act, or in an offering that is registered under the Securities Act that is not conducted as a firm-commitment underwritten offering, then, subject to compliance with all applicable securities laws and regulations,
each Purchaser listed on Schedule A shall have the right to purchase, on the same terms, including the price per security, and subject to the same conditions, as are applicable to the other investors in such offering, that amount of New Securities
being offered for sale in such offering equal to their Pro Rata Share (as set forth on Schedule A) of the total amount of New Securities offered for sale in such offering up to the Maximum Participation Amount (as set forth on Schedule A).
(ii) If the Company proposes to conduct an offering with respect to which the Purchasers would have rights to purchase New
Securities pursuant to this Section 5.10(a), the Company shall give written notice (the “Offer Notice”) to the Purchasers at least three (3) Business Days prior to the commencement of the offering of the New Securities,
stating (i) its bona fide intention to offer such New Securities, (ii) the number, type and material terms of such New Securities to be offered, (iii) the price and terms, if any, upon which it proposes to offer such New Securities,
(iv) each Purchaser’s Pro Rata Share and (v) the estimated date and time at which the Company expects to enter into a definitive agreement for the sale of the New Securities (the “Expected Sale Date”). If the
information contained in the Offer Notice constitutes material non-public information (as defined under the applicable securities laws), the Company shall deliver such Offer Notice only to the individuals
identified on the Schedule of Purchasers and shall not communicate the information to anyone else acting on behalf of such Purchasers without the consent of such individuals.
(iii) If a Purchaser desires to exercise its rights under this Section 5.10(a) to participate in such offering, then such
Purchaser must provide a written notice to the Company by not later than 6:00 p.m. (New York City time) on the day immediately preceding the Expected Sale Date set forth in the Offer Notice, stating the amount of such Purchaser’s elected
participation. If the Company receives no such notice from such Purchaser within the time period set forth herein, such Purchaser shall be deemed to have notified the Company that it does not elect to purchase any New Securities in connection with
such offering and the Company shall be free to sell such securities in the offering; provided, however, that if the Expected Sale Date shall be delayed by for any reason, the Company shall promptly inform the Purchasers of such delay and
Purchasers’ rights in this Section 5.10(a)(iii) shall be revived with an ability to provide written notice by not later than 6:00 p.m. (New York City time) on the day immediately preceding the new Expected Sale Date.
(b) Underwritten Offerings. If, during the Participation Period, the Company proposes to offer
and sell any New Securities in a firm commitment underwritten offering registered under the Securities Act, then, subject to compliance with all applicable securities laws and regulations, the Company will use its commercially reasonable efforts to
cause the managing underwriter(s) of such offering to contact the Purchasers about potentially participating in such offering and to provide to the Purchasers, on the same terms, including the price per security, and subject to the same conditions,
as are applicable to the public in such offering, the opportunity to purchase that amount of New Securities being offered for sale in such offering equal to their respective Pro Rata Share of the total amount of New Securities offered for
26
sale in such offering (excluding securities issuable to the underwriter(s) of the offering upon exercise of a customary overallotment or other option to purchase additional shares).
(c) Sales of Equity under ATMs or Equity Lines. If during the Participation Period, the Company
intends to sell New Securities using any “at-the-market sales” facility or equity line, then with respect to each activation, the Company shall provide
Purchasers with an Offer Notice in accordance with Section 5.10(a) above, specifying the target amount intended to be sold (the “ATM Target Amount”) and the lowest price per share (the “ATM Floor
Price”) at which such New Securities may be sold under such activation. Using the same notice mechanism as set forth in Section 5.10(a) above, Purchasers shall have the right to purchase its Pro Rata Amount of the ATM Target Amount at
the ATM Floor Price, and the Company shall be free to sell the remainder of the Target Amount at or above the ATM Floor Price.
(d) General Terms Applicable to Participation Rights.
(i) Notwithstanding anything to the contrary in this Section 5.10 and unless otherwise agreed by the Purchasers, in the
event the Company determines to abandon a proposed offering regarding which the Company or any underwriter have provided notice to the Purchasers pursuant to this Section 5.10, the Company shall, or shall cause the managing underwriter(s), to
confirm such abandonment to the Purchasers in the same manner and on the same day as such abandonment is communicated to other potential investors. If, by the tenth (10th) Business Day following delivery of notice of the offering to the Purchasers
pursuant to this Section 5.10, no public disclosure regarding a transaction with respect to the applicable offering has been made, such offering shall be deemed to have been abandoned and the Purchasers shall be deemed to not be in possession
of any material non-public information (as defined under applicable securities laws) with respect to the proposed offering, unless the Company advises the Purchasers that the offering has not been abandoned.
The Company understands and confirms that the Purchasers may rely on this Section 5.10(d)(i) when effecting transactions in securities of the Company.
(ii) Subject to compliance with all applicable securities laws and regulations, the Purchasers may apportion any New
Securities to be purchased pursuant to their rights in this Section 5.10 in such proportion as they deem appropriate among themselves and any of their respective Affiliates (as defined below).
(iii) The rights of the Purchasers under this Section 5.10 to purchase New Securities in an offering will be conditioned
upon the completion of such offering.
(iv) The rights of the Purchasers described in this Section 5.10 will
terminate and be of no further force or effect upon the earlier to occur of the following: (i) with respect to any such Purchaser, at such time as each such Purchaser and/or its Affiliates has purchased its Maximum Participation Amount (as set
forth on Schedule A); and (ii) with respect to all Purchasers, the ten (10) year anniversary of the Closing Date.
(v) The Company and the Purchasers hereby acknowledge that nothing in this Section 5.10 constitutes an offer or the
commitment by any Person (as defined below) to purchase any New Securities in any offering.
27
For purposes of this Section 5.10, “New Securities” means,
collectively, equity securities of the Company, whether or not currently authorized, as well as options or warrants to purchase such equity securities, or securities of any type whatsoever that are convertible or exchangeable into or exercisable for
such equity securities, other than (i) any shares of capital stock or options to purchase shares of capital stock, or other equity-based awards (including restricted stock units), issued or granted to employees (or prospective employees who
have accepted an offer of employment), directors or consultants of the Company or any of its subsidiaries, pursuant to any Company stock-based compensation plan or arrangement; (ii) any securities issued by the Company upon the exercise,
exchange or conversion of any securities that are exercisable or exchangeable for, or convertible into, shares of capital stock and are outstanding as of the date if this Agreement or issued pursuant to this Agreement, provided that such exercise,
exchange or conversion is effected pursuant to the terms of such securities as in effect on the date of this Agreement or as provided in this Agreement; (iii) any securities issued by the Company as full or partial consideration in connection
with a merger, acquisition, consolidation or purchase of all or substantially all of the securities or assets of a corporation or other entity approved by the Board, (iv) any securities issued by the Company in connection with a transaction
with an unaffiliated third party approved by the Board that includes a bona fide commercial relationship with the Company (including any joint venture, marketing or distribution arrangement, strategic alliance, collaboration agreement or corporate
partnering, intellectual property license agreement or acquisition agreement with the Company) and (v) any securities issued by the Company to banks, equipment lessors or other financial institutions, or to real property lessors, pursuant to a
debt financing, equipment leasing or real property leasing transaction approved by the Board.
6. Conditions of Closing.
6.1 Conditions to the Obligation of the Purchasers. The several obligations of each Purchaser
to consummate the transactions to be consummated at the Closing, and to purchase and pay for the Securities being purchased by it at the Closing pursuant to this Agreement, are subject to the satisfaction or waiver in writing of the following
conditions precedent:
(a) Representations and Warranties. The representations and
warranties of the Company contained herein shall be true and correct on and as of the Closing Date with the same force and effect as though made on and as of the Closing Date (it being understood and agreed by each Purchaser that for purposes of
this Section 6.1(a), in the case of any representation and warranty of the Company contained herein (i) which is not hereinabove qualified by application thereto of a materiality standard, such representation and
warranty need be true and correct only in all material respects; provided that (x) the representations and warranties of the Company contained in Sections 3.1, 3.4 and 3.5 shall be true and correct in all respects, and
(y) the representations and warranties of the Company contained in Section 3.2 shall be true and correct in all respects, other than for de minimis inaccuracies, or (ii) which is made as of a specific date, such
representation and warranty need be true and correct only as of such specific date) and consummation of the Closing shall constitute a reaffirmation by the Company of each of the representations and warranties of the Company contained in this
Agreement as of the Closing Date.
(b) Performance. The Company shall have performed in all
material respects all obligations and conditions herein required to be performed or observed by the Company on or prior to the Closing Date.
28
(c) No Injunction. The purchase of and
payment for the Securities by each Purchaser shall not be prohibited or enjoined by any law or governmental or court order or regulation and no such prohibition shall have been threatened in writing.
(d) Consents. The Company shall have obtained the consents, permits, approvals, registrations
and waivers necessary for the consummation of the purchase and sale of the Securities.
(e) Transfer Agent. The Company shall have furnished all required materials to the Transfer
Agent to reflect the issuance of the Shares at the Closing.
(f) Adverse Changes. Since the
date hereof, no event or series of events shall have occurred that has had or would reasonably be expected to have a Material Adverse Effect.
(g) Opinion of Company Counsel. The Company shall have delivered to the Purchasers the opinion
of Wilson Sonsini Goodrich & Rosati, P.C., dated as of the Closing Date in customary form and substance to be reasonably agreed upon with the Purchasers.
(h) Compliance Certificate. The Chief Executive Officer of the Company shall have delivered to
the Purchasers at the Closing Date a certificate certifying that the conditions specified in Sections 6.1(a) (Representations and Warranties), 6.1(b) (Performance), 6.1(c) (No Injunction) and 6.1(k) (Listing Requirements)
of this Agreement have been fulfilled.
(i) Secretary’s Certificate. The Secretary of
the Company shall have delivered to the Purchasers at the Closing Date a certificate certifying (i) the Amended and Restated Certificate of Incorporation; (ii) the Amended and Restated Bylaws; and (iii) resolutions of the
Company’s Board of Directors (or an authorized committee thereof) approving this Agreement and the transactions contemplated by this Agreement.
(j) Registration Rights Agreement. The Company shall have executed and delivered the
Registration Rights Agreement in the form attached hereto as Exhibit C (the “Registration Rights Agreement”) to the Purchasers.
(k) Listing Requirements. The Common Stock shall be listed on the Nasdaq Capital Market and
shall not have been suspended, as of the Closing Date, by the SEC or the Nasdaq Capital Market from trading thereon nor shall suspension by the SEC or the Nasdaq Capital Market have been threatened, as of the Closing Date, either (i) in writing
by the SEC or the Nasdaq Capital Market or (ii) by falling below the minimum listing maintenance requirements of the Nasdaq Capital Market (with a reasonable prospect of delisting occurring after giving effect to all applicable notice, appeal,
compliance and hearing periods); and the Company shall have filed with Nasdaq a Notification Form: Listing of Additional Shares for the listing of the Shares and Warrant Shares and shall have received confirmation from Nasdaq that it has completed
its review of such form with no objections to the transactions contemplated herein.
6.2 Conditions to the Obligation of the Company. The obligation of the Company to consummate
the transactions to be consummated at the Closing, and to issue and sell
29
to each Purchaser the Common Stock to be purchased by it at the Closing pursuant to this Agreement, is subject to the satisfaction or waiver in writing of the following conditions precedent:
(a) Representations and Warranties. The representations and warranties contained herein of each
Purchaser shall be true and correct on and as of the Closing Date, with the same force and effect as though made on and as of the Closing Date (it being understood and agreed by the Company that, in the case of any representation and warranty of a
Purchaser contained herein which is not hereinabove qualified by application thereto of a materiality standard, such representation and warranty need be true and correct only in all material respects; provided that the representations and warranties
of a Purchaser contained in Sections 4.1 and 4.2 shall be true and correct in all respects) and consummation of the Closing shall constitute a reaffirmation by the Purchaser of each of the representations, warranties, covenants and
agreements of the Purchaser contained in this Agreement as of the Closing Date.
(b) Performance. Each Purchaser shall have performed in all material respects all obligations
and conditions herein required to be performed or observed by such Purchaser on or prior to the Closing Date.
(c) Injunction. The purchase of and payment for the Securities by each Purchaser shall not be
prohibited or enjoined by any law or governmental or court order or regulation.
(d) Registration Rights Agreement. Each Purchaser shall have executed and delivered the
Registration Rights Agreement to the Company in the form attached as Exhibit C.
(e) Payment. The Company shall have received payment, by wire transfer of immediately available
funds, in the full amount of the purchase price for the number of Securities being purchased by each Purchaser at the Closing as set forth in Exhibit A.
7. Termination.
7.1 Conditions of Termination. This Agreement shall terminate and be void and of no further
force and effect, and all obligations of the parties hereunder shall terminate without any further liability on the part of any party in respect thereof, upon the earlier to occur of (a) the mutual written agreement of the Company and each of
the Purchasers, (b) if, on the Closing Date, any of the conditions of Closing set forth in Section 6 have not been satisfied as of the time required hereunder to be so satisfied or waived by the party entitled to grant
such waiver, or are not capable of being satisfied and, as a result thereof, the transactions contemplated by this Agreement will not be and are not consummated, or (c) if the Closing has not occurred on or before August 11, 2023, other
than solely as a result of a Willful Breach of a Purchaser’s obligations hereunder; provided, however, that nothing herein shall relieve any party to this Agreement of any liability for common law fraud or for any Willful Breach of any
representation, warranty, covenant, obligation or other provision contained in this Agreement and each party will be entitled to any remedies at law or in equity to recover losses, liabilities or damages arising from any such Willful Breach.
“Willful Breach” means a deliberate act or deliberate failure to act, taken with the actual knowledge that such act or failure to act would result in or constitute a material breach of this Agreement.
30
8. Miscellaneous Provisions.
8.1 Public Statements or Releases; Use of Name and Logo.
(a) Except as set forth in Section 3, neither the Company nor any Purchaser shall make any public
announcement with respect to the existence or terms of this Agreement or the transactions provided for herein without the prior approval of the other parties. Notwithstanding the foregoing, and subject to compliance with Section 3, nothing in
this Section 8.1 shall prevent any party from making any public announcement it considers necessary in order to satisfy its obligations under the law, including applicable securities laws, or under the rules of any national securities exchange.
(b) The Company grants the Purchasers permission to use the Company and its subsidiaries’
names and logos in marketing materials, limited to customary tombstone announcements, of the Purchasers and their respective Affiliates.
8.2 Interpretation. The words “hereof,” “herein” and “hereunder”
and words of similar import when used in this Agreement will refer to this Agreement as a whole and not to any particular provision of this Agreement, and section and subsection references are to this Agreement unless otherwise specified. The
headings in this Agreement are included for convenience of reference only and will not limit or otherwise affect the meaning or interpretation of this Agreement. Whenever the words “include,” “includes” or “including”
are used in this Agreement, they will be deemed to be followed by the words “without limitation.” The phrases “the date of this Agreement,” “the date hereof” and terms of similar import, unless the context otherwise
requires, will be deemed to refer to the date set forth in the first paragraph of this Agreement. The meanings given to terms defined herein will be equally applicable to both the singular and plural forms of such terms. All matters to be agreed to
by any party hereto must be agreed to in writing by such party unless otherwise indicated herein. References to agreements, policies, standards, guidelines or instruments, or to statutes or regulations, are to such agreements, policies, standards,
guidelines or instruments, or statutes or regulations, as amended or supplemented from time to time (or to successors thereto).
8.3 Notices. Any notices or other communications required or permitted to be given hereunder
shall be in writing and shall be deemed to be given (a) when delivered if personally delivered to the party for whom it is intended, (b) when delivered, if sent by electronic mail or facsimile with receipt confirmed during normal business
hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day, (c) three (3) days after having been sent by certified or registered mail, return-receipt requested and postage prepaid, or
(d) one (1) business day after deposit with a nationally recognized overnight courier, freight prepaid, specifying next business day delivery, with written verification of receipt:
(a) If to the Company, addressed as follows:
Forte Biosciences, Inc.
3060
Pegasus Park Drive, Building 6
Dallas, Texas 75247
31
Attention: Paul Wagner, Chief Executive Officer
Email: pwagner@fortebiorx.com
with a copy to (which shall not constitute notice):
Wilson Sonsini Goodrich & Rosati, P.C.
12235 El Camino Real
San
Diego, CA 92130
Attention: Dan Koeppen
Email: dkoeppen@wsgr.com
(b) If to any Purchaser, at its address set forth on Exhibit A or to such e-mail address, facsimile number or address as subsequently modified by written notice given in accordance with this Section 8.3.
Any Person may change the address to which notices and communications to it are to be addressed by notification as provided
for herein.
8.4 Severability. If any part or provision of this Agreement is held
unenforceable or in conflict with the applicable laws or regulations of any jurisdiction, the invalid or unenforceable part or provisions shall be replaced with a provision which accomplishes, to the extent possible, the original business purpose of
such part or provision in a valid and enforceable manner, and the remainder of this Agreement shall remain binding upon the parties hereto.
8.5 Governing Law; Submission to Jurisdiction; Venue; Waiver of Trial by Jury.
(a) This Agreement shall be governed by, and construed in accordance with, the laws of the State of
New York without regard to choice of laws or conflicts of laws provisions thereof that would require the application of the laws of any other jurisdiction, except to the extent that mandatory principles of Delaware law may apply.
(b) The Company and each of the Purchasers hereby irrevocably and unconditionally:
(i) submits for itself and its property in any legal action or proceeding relating solely to this
Agreement or the transactions contemplated hereby, to the general jurisdiction of the any state court or United States Federal court sitting in the City of New York in the State of New York;
(ii) consents that any such action or proceeding may be brought in such courts, and waives any
objection that it may now or hereafter have to the venue of any such action or proceeding in any such court or that such action or proceeding was brought in an inconvenient court and agrees not to plead or claim the same to the extent permitted by
applicable law;
(iii) agrees that service of process in any such action or proceeding may be
effected by mailing a copy thereof by registered or certified mail (or any substantially similar form of mail), postage prepaid, to the party, as the case may be, at its address set forth in Section 8.3 or at such other
address of which the other party shall have been notified pursuant thereto;
32
(iv) agrees that nothing herein shall affect the
right to effect service of process in any other manner permitted by law or shall limit the right to sue in any other jurisdiction for recognition and enforcement of any judgment or if jurisdiction in the courts referenced in the foregoing clause
(i) are not available despite the intentions of the parties hereto;
(v) agrees that final
judgment in any such suit, action or proceeding brought in such a court may be enforced in the courts of any jurisdiction to which such party is subject by a suit upon such judgment, provided that service of process is effected upon such party in
the manner specified herein or as otherwise permitted by law;
(vi) agrees that to the extent that
such party has or hereafter may acquire any immunity from jurisdiction of any court or from any legal process with respect to itself or its property, such party hereby irrevocably waives such immunity in respect of its obligations under this
Agreement, to the extent permitted by law; and
(vii) irrevocably and unconditionally waives trial
by jury in any legal action or proceeding in relation to this Agreement.
8.6 Waiver. No
waiver of any term, provision or condition of this Agreement, whether by conduct or otherwise, in any one or more instances, shall be deemed to be, or be construed as, a further or continuing waiver of any such term, provision or condition or as a
waiver of any other term, provision or condition of this Agreement.
8.7 Expenses. Each
party shall pay its own out-of-pocket fees and expenses, including the fees and expenses of attorneys, accountants and consultants employed by such party, incurred in
connection with the proposed investment in the Securities, the negotiation of the Transaction Agreements and the consummation of the transactions contemplated thereby.
8.8 Assignment. None of the parties may assign its rights or obligations under this Agreement
or designate another person (i) to perform all or part of its obligations under this Agreement or (ii) to have all or part of its rights and benefits under this Agreement, in each case without the prior written consent of (x) the
Company, in the case of a Purchaser, and (y) the Purchasers, in the case of the Company, provided that a Purchaser may, without the prior consent of the Company, assign some or all of its rights to purchase the Securities hereunder to any of
its affiliates or to any other investment funds or accounts managed or advised by the investment manager who acts on behalf of such Purchaser (provided each such assignee agrees to be bound by the terms of this Agreement and makes the same
representations and warranties set forth in Section 4 hereof). In the event of any assignment in accordance with the terms of this Agreement, the assignee shall specifically assume and be bound by the provisions of this
Agreement by executing a writing agreeing to be bound by and subject to the provisions of this Agreement and shall deliver an executed counterpart signature page to this Agreement and, notwithstanding such assumption or agreement to be bound hereby
by an assignee, no such assignment shall relieve any party assigning any interest hereunder from its obligations or liability pursuant to this Agreement.
8.9 Confidential Information.
(a) Each Purchaser covenants that until such time as the transactions contemplated by this Agreement
are publicly disclosed by the Company, such Purchaser will
33
maintain the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction), other than to such Purchaser’s outside
attorney, accountant, auditor or investment advisor only to the extent necessary to permit evaluation of the investment, and the performance of the necessary or required tax, accounting, financial, legal, or administrative tasks and services and
other than as may be required by law.
(b) The Company may request from the Purchasers such
additional information as the Company may deem necessary to evaluate the eligibility of the Purchaser to acquire the Securities, and the Purchaser shall promptly provide such information as may reasonably be requested to the extent readily
available; provided, that the Company agrees to keep any such information provided by the Purchaser confidential, except (i) as required by the federal securities laws, rules or regulations and (ii) to the extent such disclosure is
required by other laws, rules or regulations, at the request of the staff of the SEC or regulatory agency or under the regulations of Nasdaq. The Purchaser acknowledges that the Company may file a copy of this Agreement and the Registration Rights
Agreement with the SEC as exhibits to a periodic report or a registration statement of the Company.
8.10 Third Parties. Nothing in this Agreement, express or implied, is intended to confer on any
Person other than the parties to this Agreement any rights, remedies, claims, benefits, obligations or liabilities under or by reason of this Agreement, and no Person that is not a party to this Agreement (including, without limitation, any partner,
member, shareholder, director, officer, employee or other beneficial owner of any party to this Agreement, in its own capacity as such or in bringing a derivative action on behalf of a party to this Agreement) shall have any standing as a third
party beneficiary with respect to this Agreement or the transactions contemplated hereby.
8.11 Independent Nature of Purchasers’ Obligations and Right. The
obligations of each Purchaser under this Agreement are several and not joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance obligations of any other Purchaser under this Agreement.
Nothing contained herein, and no action taken by any Purchaser pursuant hereto, shall be deemed to constitute the Purchasers as, and the Company acknowledges that the Purchasers do not so constitute, a partnership, an association, a joint venture or
any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group, and the Company will not assert any such claim with respect to such obligations or the transactions contemplated by this Agreement.
The Company acknowledges and each Purchaser confirms that it has independently participated in the negotiation of the transaction contemplated hereby with the advice of its own counsel and advisors. Each Purchaser also acknowledges that Wilson
Sonsini Goodrich & Rosati, P.C. has rendered legal advice to the Company and not such Purchaser. Each Purchaser shall be entitled to independently protect and enforce its rights, including, without limitation, the rights arising out of this
Agreement, and it shall not be necessary for any other Purchaser to be joined as an additional party in any proceeding for such purpose. The Company has elected to provide all Purchasers with the same terms and Transaction Agreements for the
convenience of the Company and not because it was required or requested to do so by any Purchaser.
8.12 Counterparts. This Agreement may be signed in any number of counterparts, each of which
shall be an original, but all of which together shall constitute one instrument.
34
8.13 Entire Agreement; Amendments. This
Agreement and the other Transaction Agreements constitute the entire agreement between the parties hereto respecting the subject matter hereof and supersedes all prior agreements, negotiations, understandings, representations and statements
respecting the subject matter hereof, whether written or oral. Except as set forth in Section 2.1, no amendment, modification, alteration, or change in any of the terms of this Agreement shall be valid or binding upon the
parties hereto unless made in writing and duly executed by the Company and the Purchasers of at least a majority in interest of the Securities then held by the Purchasers. Notwithstanding the foregoing, this Agreement may not be amended and the
observance of any term of this Agreement may not be waived with respect to any Purchaser without the written consent of such Purchaser unless such amendment or waiver applies to all Purchasers in the same fashion and provided that the consent of
each Purchaser is required for the waiver of any of the conditions set forth in Section 6.1(d), 6.1(f), or 6.1(k). The Company, on the one hand, and each Purchaser, on the other hand, may by an instrument
signed in writing by such parties waive the performance, compliance or satisfaction by such Purchaser or the Company, respectively, with any term or provision hereof or any condition hereto to be performed, complied with or satisfied by such
Purchaser or the Company, respectively.
8.14 Survival. The covenants, representations and
warranties made by each party hereto contained in this Agreement shall survive the Closing and the delivery of the Shares in accordance with their respective terms. Each Purchaser shall be responsible only for its own representations, warranties,
agreements and covenants hereunder.
8.15 Mutual Drafting. This Agreement is the
joint product of each Purchaser and the Company and each provision hereof has been subject to the mutual consultation, negotiation and agreement of such parties and shall not be construed for or against any party hereto.
8.16 Additional Matters. For the avoidance of doubt, the parties acknowledge and confirm that
the terms and conditions of the Securities were determined as a result of arm’s-length negotiations.
[Remainder of Page Intentionally Left Blank.]
35
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of
the day and year first above written.
|
|
|
| COMPANY: |
|
| FORTE BIOSCIENCES, INC. |
|
|
| By: |
|
/s/ Paul Wagner |
|
|
Name: Paul Wagner |
|
|
Title: Chief Executive Officer |
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of
the day and year first above written.
PURCHASER:
EXHIBIT B
FORM OF PRE-FUNDED WARRANT
Filed as Exhibit 4.1 to Registrant’s Current Report on Form 8-K, filed with the SEC on August 1, 2023.
B-1
EXHIBIT C
FORM OF REGISTRATION RIGHTS AGREEMENT
Filed as Exhibit 10.2 to Registrant’s Current Report on Form 8-K, filed with the SEC on August 1, 2023.
C-1
Exhibit 10.2
FORM OF REGISTRATION RIGHTS AGREEMENT
This REGISTRATION RIGHTS AGREEMENT (the “Agreement”) is made as of July 28, 2023 by and among Forte
Biosciences, Inc., a corporation organized and existing under the laws of the State of Delaware (the “Company”), the several purchasers signatory hereto (each, a “Purchaser” and collectively, the
“Purchasers”).
RECITALS
WHEREAS, the Company and the Purchasers are parties to a Securities Purchase Agreement, dated as of July 28, 2023 (the “Purchase
Agreement”), pursuant to which the Purchasers are purchasing shares of capital stock and/or pre-funded warrants of the Company; and
WHEREAS, in connection with the consummation of the transactions contemplated by the Purchase Agreement, and pursuant to the terms of the
Purchase Agreement, the parties desire to enter into this Agreement in order to grant certain rights to the Purchasers as set forth below.
NOW, THEREFORE, in consideration of the covenants and promises set forth herein, and for other good and valuable consideration, the receipt
and sufficiency of which are hereby acknowledged, the parties hereby agree as follows:
AGREEMENT
1. Certain Definitions. Unless the context otherwise requires, the following terms, for all purposes of this Agreement, shall
have the meanings specified in this Section 1. Capitalized terms used and not otherwise defined herein that are defined in the Purchase Agreement shall have the meanings given such terms in the Purchase Agreement.
“Agreement” has the meaning set forth in the recitals.
“Allowed Delay” has the meaning set forth in Section 2.1(b)(ii).
“Board” means the board of directors of the Company.
“Business Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States
or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close; provided, however, for clarification, commercial banks shall not be deemed to be authorized or required by law
to remain closed due to “stay at home”, “shelter-in-place”, “non-essential employee”
or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in the
State of New York are generally are open for use by customers on such day.
“Common Stock” means shares of the common
stock, par value $0.001 per share, of the Company.
“Company” has the meaning set forth in the recitals.
“Effectiveness Deadline” means, with respect to the Shelf Registration Statement or New Registration Statement, the
sixtieth (60th) calendar day following the Closing Date (or, in the event the SEC reviews and
has written comments to the Shelf Registration Statement or the New Registration Statement, the ninetieth (90th) calendar day following the Closing Date); provided, however, that if
the Company is notified by the SEC (either orally or in writing, whichever is earlier) that the Shelf Registration Statement or the New Registration Statement will not be reviewed or is no longer subject to further review and comments, the
Effectiveness Deadline as to such Shelf Registration Statement shall be the fifth (5th) Business Day following the date on which the Company is so notified if such date precedes the dates otherwise required above; provided, further,
that if the Effectiveness Deadline falls on a Saturday, Sunday or other day that the SEC is closed for business, the Effectiveness Deadline shall be extended to the next Business Day on which the SEC is open for business; provided,
further, that if the SEC is closed for operations due to a government shutdown or lapse in appropriations, the Effectiveness Deadline shall be extended by the same amount of days that the SEC remains closed for operations.
“Effectiveness Period” has the meaning set forth in Section 2.1(b)(i).
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC
promulgated thereunder.
“Filing Deadline” has the meaning set forth in Section 2.1(a).
“FINRA” means the Financial Industry Regulatory Authority.
“Form S-3” means such form under the Securities Act as in effect
on the date hereof or any successor or similar registration form under the Securities Act subsequently adopted by the SEC that permits inclusion or incorporation of substantial information by reference to other documents filed by the Company with
the SEC.
“Free Writing Prospectus” means an issuer free writing prospectus, as defined in Rule 433 under the
Securities Act, relating to an offer of Registrable Securities.
“Holder” means any Purchaser or its Permitted Assignee
owning or having the right to acquire Registrable Securities.
“Losses” has the meaning set forth in Section 2.5(a).
“New Registration Statement” has the meaning set forth in Section 2.1(a).
“Participating Holder” means with respect to any registration, any Holder of Registrable Securities covered by the applicable
Registration Statement.
“Permitted Assignee” means (a) with respect to a partnership, its partners, former partners
or any affiliate, including employees of such partnership or affiliate, (b) with respect to a corporation, its stockholders in accordance with their interest in the corporation, (c) with respect to a limited liability company, its members
or former members in accordance with their interest in the limited liability company, (d) with respect to an individual party, any Family Member of such party, (e) an entity or trust that is controlled by, controls, or is under common
control with a transferor, or (f) a party to this Agreement.”
“Pre-Funded
Warrants” means the Pre-Funded Warrants issued pursuant to the Purchase Agreement.
“Prospectus” means the prospectus included in any Registration Statement (including a prospectus that discloses information
previously omitted from a prospectus filed as part of an effective Shelf Registration
-2-
Statement in reliance upon Rule 430A or Rule 430B promulgated under the Securities Act), all amendments and supplements to such prospectus,
including pre- and post-effective amendments to such Registration Statement, and all other material incorporated by reference in such prospectus.
“Register,” “registered” and “registration” refer to a registration effected by preparing
and filing a registration statement in compliance with the Securities Act, and the declaration or ordering of effectiveness of such registration statement or document.
“Registrable Securities” means (i) the Shares, (ii) the Warrant Shares, (iii) any Common Stock issued as a
dividend or other distribution with respect to, or in exchange for or in replacement of, Shares or Warrant Shares. Notwithstanding the foregoing, Securities or any such Common Stock, as applicable, shall cease to be Registrable Securities for all
purposes hereunder upon the earliest to occur of the following: (a) the sale by any Person of such Securities or any such Common Stock, as applicable, either pursuant to a registration statement under the Securities Act or under Rule 144
(or any similar provision then in effect) (in which case, only such Securities or any such Common Stock, as applicable, sold shall cease to be Registrable Securities) other than to a Permitted Assignee, (b) such Securities shall have been
otherwise transferred (other than to a Permitted Assignee), new certificates for such Securities not bearing a legend restricting further transfer shall have been delivered by Company and subsequent public distribution of such Securities shall not
require registration under the Securities Act, or (c) such Securities cease to be outstanding.
“Registration
Statement” means any registration statement of the Company that covers the resale of any of the Registrable Securities pursuant to the provisions of this Agreement filed with, or to be filed with, the SEC under the rules and
regulations promulgated under the Securities Act, including the related Prospectus, amendments and supplements to such registration statement, including pre- and post-effective amendments, and all
exhibits and all material incorporated by reference or deemed to be incorporated by reference in such registration statement.
“Remainder Registration Statement” has the meaning set forth in Section 2.1(a).
“Required Holders” means the Holders holding a majority of the Registrable Securities outstanding from time to time.
“Rule 144” means Rule 144 as promulgated by the SEC under the Securities Act, as such rule may be amended from time to
time, or any similar successor rule that may be promulgated by the SEC having substantially the same effect as such Rule.
“SEC” means the Securities and Exchange Commission or any other federal agency at the time administering the Securities Act.
“SEC Guidance” means any publicly-available written or oral guidance, comments, requirements or requests of the SEC
staff under the Securities Act; provided, that any such oral guidance, comments, requirements or requests are reduced to writing by the SEC.
“Securities” means the Shares and Warrant Shares issued pursuant to the Purchase Agreement.
“Securities Act” means the Securities Act of 1933, as amended, or any similar successor federal statute and the
rules and regulations thereunder, all as the same shall be in effect from time to time.
“Shares” means the shares
of Common Stock issued or issuable to the Purchasers pursuant to the Purchase Agreement.
-3-
“Shelf Registration Statement” has the meaning set forth in
Section 2.1(a).
“Transaction Agreements” means this Agreement, the Purchase Agreement and the Pre-Funded Warrants, all exhibits and schedules thereto and hereto and any other documents or agreement executed in connection with the transactions contemplated hereunder or thereunder.
“Warrant Shares” means the shares of Common Stock issued or issuable upon the exercise of
Pre-Funded Warrants.
2. Registration Rights.
2.1 Shelf Registration.
(a) Registration Statements. On or prior to thirty (30) days following the Closing Date (the
“Filing Deadline”), the Company shall prepare and file with the SEC a Registration Statement on Form S-3 (or, if Form S-3 is not then available to the
Company, on such form of registration statement as is then available to effect a registration for resale of the Registrable Securities), subject to the provisions of Section 2.1(c), for the resale of the Registrable Securities pursuant to an
offering to be made on a continuous basis pursuant to Rule 415 under the Securities Act (the “Shelf Registration Statement”). Such Shelf Registration Statement shall, subject to the limitations of Form
S-3, include the aggregate amount of Registrable Securities to be registered therein and shall contain (except if otherwise required pursuant to written comments received from the SEC upon a review of such
Shelf Registration Statement) the “Plan of Distribution” substantially in the form of Annex A (which may be modified to respond to comments, if any, provided by the SEC). To the extent the staff of the SEC does not permit all of the
Registrable Securities to be registered on the Shelf Registration Statement filed pursuant to this Section 2.1(a) or for any other reason any Registrable Securities are not then included in a Registration Statement filed under this
Agreement, the Company shall (i) inform each of the Participating Holders thereof and use its reasonable best efforts to file amendments to the Shelf Registration Statement as required by the SEC and/or (ii) withdraw the Shelf Registration
Statement and file a new registration statement (a “New Registration Statement”), in either case covering the maximum number of Registrable Securities permitted to be registered by the SEC, on Form
S-3 or such other form available to register for resale the Registrable Securities as a secondary offering. Notwithstanding any other provision of this Agreement, if any SEC Guidance sets forth a limitation of
the number of Registrable Securities permitted to be registered on a particular Registration Statement as a secondary offering, unless otherwise directed in writing by a Holder as to its Registrable Securities, the number of Registrable Securities
to be registered on such Registration Statement will first be reduced by Registrable Securities not acquired pursuant to the Purchase Agreement (whether pursuant to registration rights or otherwise), and second by Registrable Securities acquired
pursuant to the Purchase Agreement (applied, in the case that some Securities may be registered, to the Holders on a pro rata basis based on the total number of unregistered Securities held by such Holders, subject to a determination by the SEC that
certain Holders must be reduced first based on the number of Securities held by such Holders). In the event the Company amends the Shelf Registration Statement or files a New Registration Statement, as the case may be, under clauses (i) or
(ii) above, the Company will use its reasonable best efforts to file with the SEC, as promptly as allowed by the SEC or SEC Guidance provided to the Company or to registrants of securities in general, one or more Registration Statements on Form
S-3 or such other form available to register for resale those Registrable Securities that were not registered for resale on the Shelf Registration Statement, as amended, or the New Registration Statement (the
“Remainder Registration Statement”) and use commercially reasonable efforts to have such Remainder Registration Statement(s) to become effective as promptly as practicable after the filing thereof, but in any event no later than
sixty (60) calendar days after the filing of such Remainder Registration Statement (the “Additional Effectiveness Deadline”); provided, however, that if the Company is notified by the SEC (either orally or in writing, whichever is
earlier) that the Remainder Registration Statement will not
-4-
be reviewed or is no longer subject to further review and comments, the Additional Effectiveness Deadline as to such Remainder Registration Statement shall be the fifth (5th) Business Day
following the date on which the Company is so notified if such date precedes the dates otherwise required above; provided, further, that if the Additional Effectiveness Deadline falls on a Saturday, Sunday or other day that the SEC is closed for
business, the Additional Effectiveness Deadline shall be extended to the next Business Day on which the SEC is open for business. In no event shall any Participating Holder be identified as a statutory underwriter in the Registration Statement
unless in response to a comment or request from the staff of the SEC or another regulatory agency; provided, however, that if the SEC requests that a Participating Holder be identified as a statutory underwriter in the Registration
Statement, such Holder will have an opportunity to withdraw from the Registration Statement.
(b) Effectiveness.
(i) The Company shall use reasonable best efforts to have the Shelf Registration Statement or New Registration Statement declared effective
as soon as practicable but in no event later than the Effectiveness Deadline (including filing with the SEC a request for acceleration of effectiveness in accordance with Rule 461 promulgated under the Securities Act), and shall use its reasonable
best efforts to keep the Shelf Registration Statement or New Registration Statement continuously effective under the Securities Act until the earlier of (A) such time as all of the Registrable Securities covered by such Registration Statement
have been publicly sold by the Holders (other than to Permitted Assignees), or (B) the date that all the Shares and the Warrant Shares cease to be Registrable Securities (the “Effectiveness Period”); provided, that, the
Company will not be obligated to update the Registration Statement and no sales may be made under the applicable Registration Statement during any Allowed Delay of which the Holders have received notice. The Company shall notify the Participating
Holders of the effectiveness of a Registration Statement by e-mail as promptly as practicable, and shall, if requested provide the Participating Holders with copies of the final Prospectus to be used in
connection with the sale or other disposition of the securities covered thereby.
(ii) On not more than two occasions and for not more
than forty-five (45) consecutive days or for a total of not more than ninety (90) days, in each case in any twelve (12) month period, the Company may suspend the use of any Prospectus included in any Registration Statement
contemplated by this Section 2 if (A) the negotiation or consummation of a transaction by the Company is pending or an event has occurred, which negotiation, consummation or event, the Board reasonably believes, upon the advice of legal
counsel, would require additional disclosure by the Company in the Registration Statement of material information that the Company has a bona fide business purpose for keeping confidential and the
non-disclosure of which in the Registration Statement would be expected, in the reasonable determination of the Board, upon the advice of legal counsel, to cause the Registration Statement to fail to comply
with applicable disclosure requirements, or (B) the Company determines in good faith, upon advice of legal counsel, that such suspension is necessary to amend or supplement the Registration Statement or the related Prospectus so that such
Registration Statement or Prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the case of the Prospectus in light of the
circumstances under which they were made, not misleading (an “Allowed Delay”); provided, that the Company shall promptly (1) notify each Participating Holder in writing of the commencement of an Allowed Delay, but shall
not (without the prior written consent of a Participating Holder) disclose to such Participating Holder any material non-public information giving rise to an Allowed Delay, (2) advise the
Participating Holders in writing to cease all sales under such Registration Statement until the end of the Allowed Delay and (3) use commercially reasonable efforts to terminate an Allowed Delay as promptly as practicable.
-5-
(c) In the event that Form
S-3 is not available for the registration of the resale of Registrable Securities hereunder, the Company shall (i) register the resale of the Registrable Securities on another appropriate form reasonably
acceptable to the Holders and (ii) undertake to register the Registrable Securities on Form S-3 promptly after such form is available; provided, that the Company shall
maintain the effectiveness of the Registration Statement then in effect until such time as a Registration Statement on Form S-3 covering the Registrable Securities has been declared effective by the
SEC.
2.2 Expenses. The Company will pay all expenses associated with each Registration Statement, including
filing and printing fees, the Company’s counsel and accounting fees and expenses, costs associated with clearing the Registrable Securities for sale under applicable state securities laws and listing fees, but excluding discounts, commissions,
fees of underwriters, selling brokers, dealer managers or similar securities industry professionals with respect to the Registrable Securities being sold.
2.3 Company Obligations. The Company will use reasonable best efforts to effect the registration of the
Registrable Securities in accordance with the terms hereof, and pursuant thereto the Company will:
(a) prepare the
required Registration Statement including all exhibits and financial statements required under the Securities Act to be filed therewith, and provide copies to and permit each Participating Holder to review each Registration Statement and all
amendments and supplements thereto prior to their filing with the SEC and a reasonable opportunity to furnish comments thereon (it being acknowledged and agreed that if a Participating Holder does not object to or comment on the aforementioned
documents, then the Participating Holder shall be deemed to have consented to and approved the use of such documents);
(b) if the Registration Statement is subject to review by the Commission, use its commercially reasonable efforts to
promptly respond to all comments, diligently pursue resolution of any comments to the satisfaction of the Commission and file all amendments and supplements to such Registration Statement as may be required to respond to comments from the Commission
and otherwise to enable such Registration Statement to be declared effective;
(c) file with the SEC a Registration
Statement relating to the Registrable Securities including all exhibits and financial statements required by the SEC to be filed therewith, and use reasonable best efforts to cause such Registration Statement to become effective under the Securities
Act;
(d) prepare and file with the SEC such amendments and post-effective amendments to such Registration
Statement and the related Prospectus as may be necessary to keep such Registration Statement effective for the Effectiveness Period and to comply with the provisions of the Securities Act and the Exchange Act with respect to the distribution of all
of the Registrable Securities covered thereby;
(e) (i) notify the Participating Holders by e-mail as promptly as practicable after any Registration Statement is declared effective or any post-effective amendment to a Registration Statement is declared effective, and shall simultaneously provide the
Participating Holders with copies of any related Prospectus to be used in connection with the sale or other disposition of the securities covered thereby (provided, that the Company will not have any obligation to provide any document
pursuant to this clause that is available on the EDGAR system), (ii) promptly notify the Participating Holders by e-mail no later than one (1) trading day following the date (A) of the issuance by
the SEC of any stop order suspending the effectiveness of such Registration Statement covering any or all of the Registrable Securities or any order by the SEC preventing or suspending the use of any preliminary or final Prospectus or the initiation
of any proceedings for such purposes, (B) of the receipt by the Company of any notification with respect to the suspension of the qualification of the Registrable Securities for offering or sale in any jurisdiction and (C) of the receipt
by the Company of any notification with respect to the initiation or threatening of any proceeding for the suspension
-6-
of the qualification of the Registrable Securities for offering or sale in any jurisdiction, provided, that, notwithstanding anything to the contrary set forth herein, the Company shall not (and
shall not have an obligation to), when so advising Participating Holders of such events, provide the Participating Holders with any material nonpublic information regarding the Company;
(f) promptly notify the Participating Holders by e-mail, at any time prior to
the end of the Effectiveness Period, upon discovery that, or upon the happening of any event as a result of which, the Prospectus includes an untrue statement of a material fact or omits to state any material fact required to be stated therein or
necessary to make the statements therein not misleading in light of the circumstances then existing (provided that such notice shall not, without the prior written consent of a Participating Holder, disclose to such Participating Holder any
material nonpublic information regarding the Company), and promptly prepare, file with the SEC and furnish to such holder a supplement to or an amendment of such Prospectus as may be necessary so that such Prospectus shall not include an untrue
statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein not misleading in light of the circumstances then existing;
(g) promptly incorporate in a Prospectus supplement, Free Writing Prospectus or post-effective amendment to the
applicable Registration Statement such information as the Participating Holders reasonably request to be included therein relating to the plan of distribution with respect to such Registrable Securities, and make all required filings of such
Prospectus supplement, Free Writing Prospectus or post-effective amendment as soon as reasonably practicable after being notified of the matters to be incorporated in such Prospectus supplement, Free Writing Prospectus or post-effective amendment;
(h) furnish to each Participating Holder whose Registrable Securities are included in any Registration Statement
(i) promptly after the same is prepared and filed with the SEC, if requested by the Participating Holder, one (1) copy of any Registration Statement and any amendment thereto, each preliminary prospectus and Prospectus and each amendment
or supplement thereto, and each letter written by or on behalf of the Company to the SEC or the staff of the SEC, and each item of correspondence from the SEC or the staff of the SEC, in each case relating to such Registration Statement (other than
any portion thereof which contains information for which the Company has sought confidential treatment), and (ii) such number of copies of a Prospectus, including a preliminary prospectus, and all amendments and supplements thereto and such
other documents as such Participating Holder may reasonably request in order to facilitate the disposition of the Registrable Securities owned by such Participating Holder that are covered by such Registration Statement;
(i) on or prior to the date on which the Registration Statement is declared effective, use its reasonable best efforts
to register or qualify, or cooperate with the Participating Holders and their respective counsel, in connection with the registration or qualification (or exemption from the registration or qualification) of such Registrable Securities for offer and
sale under the applicable state securities or “Blue Sky” laws of those jurisdictions within the United States as any Participating Holder or their respective counsel reasonably request in writing and do any and all other acts or things
reasonably necessary or advisable to keep such registration or qualification (or exemption therefrom) in effect during the Effectiveness Period, provided that the Company shall not be required to qualify generally to do business or
as a dealer in securities in any jurisdiction where it is not then so qualified or to take any action which would subject it to taxation or general service of process in any such jurisdiction where it is not then so subject;
(j) within two (2) Business Days after a Registration Statement which covers Registrable Securities is ordered
effective by the SEC, the Company shall deliver to the transfer agent for such Registrable Securities (with copies to the Participating Holder whose Registrable Securities are included in
-7-
such Registration Statement) confirmation that such Registration Statement has been declared effective by the SEC;
(k) cooperate with each Participating Holder participating in the disposition of such Registrable Securities and their
respective counsel in connection with any filings required to be made with FINRA or any other securities regulatory authority;
(l) otherwise use reasonable best efforts to comply with all applicable rules and regulations of the SEC under the
Securities Act and the Exchange Act, including, without limitation, Rule 172 under the Securities Act, file any final Prospectus, including any supplement or amendment thereof, with the SEC pursuant to Rule 424 under the Securities Act, promptly
inform the Participating Holders in writing if, at any time during the Effectiveness Period, the Company does not satisfy the conditions specified in Rule 172 and, as a result thereof, the Participating Holders are required to deliver a Prospectus
in connection with any disposition of Registrable Securities and take such other actions as may be reasonably necessary to facilitate the registration of the Registrable Securities hereunder; and make available to its security holders, as soon as
reasonably practicable, an earnings statement covering satisfying the provisions of Section 11(a) of the Securities Act;
(m) use commercially reasonable efforts to maintain the listing of all Registrable Securities on each securities
exchange on which the Common Stock is then listed or quoted and on each inter-dealer quotation system on which any of the Common Stock is then quoted;
(n) with a view to making available to the Purchasers the benefits of Rule 144 (or its successor rule) and any
other rule or regulation of the SEC that may at any time permit the Purchasers to sell shares of Common Stock to the public without registration, the Company covenants and agrees to: (i) make and keep public information available, as those
terms are understood and defined in Rule 144, until the earlier of (A) the date as all of the Registrable Securities shall have been otherwise transferred (other than to a Permitted Assignee), new certificates for such Securities not
bearing a legend restricting further transfer shall have been delivered by Company and subsequent public distribution of such Securities shall not require registration under the Securities Act or (B) such date as all of the Registrable
Securities shall have been resold; (ii) file with the SEC in a timely manner all reports and other documents required of the Company under the Exchange Act; and (iii) furnish to each Purchaser upon request, as long as such Purchaser owns
any Registrable Securities, (A) a written statement by the Company that it has complied with the reporting requirements of the Exchange Act, (B) a copy of the Company’s most recent Annual Report
on Form 10-K or Quarterly Report on Form 10-Q, and (C) such other information as may be reasonably requested in order to avail such
Purchaser of any rule or regulation of the SEC that permits the selling of any such Registrable Securities without registration;
(o) use commercially reasonable efforts to obtain all other approvals, consents, exemptions or authorizations from such
governmental agencies or authorities as may be necessary to enable the Holders and underwriters to consummate the disposition of Registrable Securities;
(p) use its commercially reasonable efforts to cause or maintain the shares of Common Stock to be quoted or listed on
Nasdaq;
(q) cooperate with the Holders of Registrable Securities being offered pursuant to the Registration
Statement to issue and deliver, or cause its transfer agent to issue and deliver, certificates or evidence of book-entry positions representing Registrable Securities to be offered pursuant to the Registration Statement within a reasonable time
after the delivery of certificates or evidence of book-entry positions representing the Registrable Securities to the transfer agent or the Company, as applicable, and enable such
-8-
certificates or positions to be in such denominations or amounts as the Holders may reasonably request and registered in such names as the Holders may request; and
(r) use its commercially reasonable efforts to avoid the issuance of, or, if issued, obtain the withdrawal of
(i) any order stopping or suspending the effectiveness of a Registration Statement or suspending or preventing the use of any related prospectus, or (ii) any suspension of the qualification (or exemption from qualification) of any of the
Registrable Securities for sale in any jurisdiction, at the earliest practicable moment.
2.4 Obligations of the
Purchasers.
(a) Notwithstanding any other provision of the Agreement, no Holder of Registrable Securities may
include any of its Registrable Securities in the Registration Statement pursuant to this Agreement unless the Holder furnishes to the Company a completed and signed selling stockholder questionnaire in customary form that contains such information
regarding Purchaser, the securities of the Company held by Purchaser and the intended method of disposition of the Registrable Securities as shall be reasonably requested by the Company to effect the registration of the Registrable Securities, at
least ten (10) Business Days prior to the first anticipated filing date of any Registration Statement if such Purchaser elects to have any of its Registrable Securities included in the Registration Statement. Each Holder who intends to include
any of its Registrable Securities in the Registration Statement shall promptly furnish the Company in writing such other information as the Company may reasonably request in writing. Each Holder acknowledges and agrees that the information in the
selling shareholder questionnaire or request for further information as described in this Section 2.4(a) will be used by the Company in the preparation of the Registration Statement and hereby consents to the inclusion of such information in
the Registration Statement. The Company shall not be obligated to file more than one post-effective amendment or supplement in any sixty (60) day period following the date such Registration Statement is declared effective for the purposes of
naming Holders as selling security holders who are not named in such Registration Statement at the time of effectiveness.
(b) Each Purchaser, by its acceptance of the Registrable Securities agrees to cooperate with the Company as reasonably
requested by the Company in connection with the preparation and filing of a Registration Statement hereunder, unless such Purchaser has notified the Company in writing of its election to exclude all of its Registrable Securities from such
Registration Statement. The Company may require each selling Holder to furnish to the Company a certified statement as to (i) the number of shares of Common Stock beneficially owned by such Holder and any affiliate thereof, (ii) any FINRA
affiliations, (iii) any natural persons who have the power to vote or dispose of the Common Stock and (iv) any other information as may be requested by the SEC, FINRA or any state securities commission.
(c) Each Purchaser agrees that, upon receipt of any notice from the Company of either (i) the commencement of an
Allowed Delay pursuant to Section 2.1(b)(ii) or (ii) the happening of any event of the kind described in Section 2.3(d) and Section 2.3(e) hereof, such Purchaser will promptly discontinue disposition of Registrable
Securities pursuant to the Registration Statement covering such Registrable Securities, until the Purchaser is advised by the Company that such dispositions may again be made and/or the use of the applicable Prospectus (as it may have been
supplemented or amended) may be resumed.
2.5 Indemnification.
(a) Indemnification by the Company. The Company will indemnify and hold harmless each Participating Holder who
sells Registrable Securities covered by such Registration Statement and its officers, directors, members, employees, shareholders, managers, partners, and agents, successors and assigns, and each other person, if any, who controls such Participating
Holder within the meaning of the Securities Act,
-9-
against any losses, claims, damages, liabilities and expense (including reasonable attorney fees) (collectively, “Losses”), actually incurred, joint or several, to which they may
become subject under the Securities Act or otherwise, insofar as such Losses (or actions in respect thereof) arise out of or are based upon: (i) any untrue statement or alleged untrue statement of any material fact contained in any Registration
Statement, any preliminary Prospectus or final Prospectus, or any amendment or supplement thereof or arising out of or relating to any omission or alleged omission to state therein a material fact required to be stated therein or necessary to make
the statements therein (in the case of any Prospectus or form of prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading; or (ii) any violation by the Company or its agents of any rule or
regulation promulgated under the Securities Act applicable to the Company or its agents and relating to action or inaction required of the Company in connection with such registration; and will reimburse such Participating Holder who sells
Registrable Securities covered by such Registration Statement, and each such officer, director, employee, agent or member and each such controlling person for any legal or other expenses reasonably incurred by them in connection with investigating
or defending any such Loss or action; provided, however, that the Company will not be liable in any such case to the extent that any such Losses arise out of or are based upon (x) an untrue statement or alleged untrue statement or
omission or alleged omission so made in reliance upon or in conformity with information furnished by such Purchaser or any such controlling person in writing specifically for use in such Registration Statement or Prospectus (preliminary, final or
summary) or any amendment or supplement thereto or to the extent that such information relates to such Holder or such Holder’s proposed method of distribution of Registrable Securities and was reviewed and approved in writing by such Holder
expressly for use in the Registration Statement, such Prospectus or such form of Prospectus or in any amendment or supplement thereto (it being understood that each Holder has approved Annex A hereto for this purpose), (y) the use by a
Holder of an outdated or defective Prospectus after the Company has notified such Holder in writing that such Prospectus is outdated or defective or (z) a Purchaser’s (or any other indemnified Person’s) failure to send or give a copy
of the Prospectus or supplement (as then amended or supplemented), if required, pursuant to Rule 172 under the Securities Act (or any successor rule) to the Persons asserting an untrue statement or alleged untrue statement or omission or alleged
omission at or prior to the written confirmation of the sale of Registrable Securities to such Person if such statement or omission was corrected in such Prospectus or supplement.
(b) Indemnification by the Participating Holders. Each Purchaser agrees, severally but not jointly with any
other Purchaser, to indemnify and hold harmless, to the fullest extent permitted by law, the Company, its directors, officers, employees, stockholders, agents, and each person who controls the Company (within the meaning of the Securities Act and
the Exchange Act) against any Losses (i) arising out of, based on, or resulting from any untrue statement or alleged untrue statement of a material fact or any omission or alleged omission of a material fact required to be stated in any
Registration Statement or Prospectus (preliminary, final or summary) or any amendment or supplement thereto or necessary to make the statements therein (in the case of any Prospectus or form of prospectus or supplement thereto, in light of the
circumstances under which they were made) not misleading, to the extent, but only to the extent that such untrue statement or alleged untrue statement or omission or alleged omission is contained in any information furnished in writing by such
Purchaser to the Company specifically for inclusion in such Registration Statement or Prospectus or amendment or supplement thereto, or a document incorporated by reference into any of the foregoing; or to the extent that such information relates to
such Holder or such Holder’s proposed method of distribution of Registrable Securities and was reviewed and approved in writing by such Holder expressly for use in a Registration Statement (it being understood that the Holder has approved Annex
A hereto for this purpose), such Prospectus or such form of Prospectus or in any amendment or supplement thereto or (ii) related to the use by such Holder of an outdated or defective Prospectus after the Company has notified such Holder in
writing that the Prospectus is outdated or defective. In no event shall the liability of any selling Holder hereunder be greater in amount than the dollar amount of the net proceeds received by
-10-
such Holder upon the sale of the Registrable Securities included in the Registration Statement giving rise to such indemnification obligation.
(c) Conduct of Indemnification Proceedings. Any Person entitled to indemnification hereunder shall (i) give
prompt written notice to the indemnifying party of any claim with respect to which it seeks indemnification and (ii) permit such indemnifying party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified
party (provided, however, that such indemnified party shall, at the expense of the indemnified party, be entitled to counsel of its own choosing to monitor such defense); provided that, any Person entitled to
indemnification hereunder shall have the right to employ separate counsel and to participate in the defense of such claim, but the fees and expenses of such counsel shall be at the expense of such Person unless (A) the indemnifying party has
agreed to pay such fees or expenses, or (B) the indemnifying party shall have failed to assume the defense of such claim and employ counsel reasonably satisfactory to such person or (C) in the reasonable judgment of counsel to any such
Person, a conflict of interest exists between such person and the indemnifying party with respect to such claims (in which case, if the Person notifies the indemnifying party in writing that such Person elects to employ separate counsel at the
expense of the indemnifying party, the indemnifying party shall not have the right to assume the defense of such claim on behalf of such Person); and provided, further, that the failure of any indemnified party to give notice as
provided herein shall not relieve the indemnifying party of its obligations hereunder, except to the extent that such failure to give notice shall materially adversely affect the indemnifying party in the defense of any such claim or litigation. It
is understood that the indemnifying party shall not, in connection with any proceeding in the same jurisdiction, be liable for fees or expenses of more than one separate firm of attorneys at any time for all such indemnified parties. No indemnifying
party will, except with the consent of the indemnified party, consent to entry of any judgment or enter into any settlement that does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a
release from all liability in respect of such claim or litigation. The indemnification provided for under this Agreement shall remain in full force and effect regardless of any investigation made by or on behalf of the indemnified party, or any
officer, director, shareholder, manager, partners, employee, agent, affiliate, or controlling person of such indemnified party and shall survive the transfer of the Securities.
(d) Contribution. If for any reason the indemnification provided for in the preceding paragraphs (a) and
(b) is unavailable to an indemnified party or insufficient to hold it harmless, other than as expressly specified therein, then the indemnifying party shall contribute to the amount paid or payable by the indemnified party as a result of such
loss, claim, damage or liability in such proportion as is appropriate to reflect the relative fault of the indemnified party and the indemnifying party, as well as any other relevant equitable considerations. No Person guilty of fraudulent
misrepresentation within the meaning of Section 11(f) of the Securities Act shall be entitled to contribution from any Person not guilty of such fraudulent misrepresentation. In no event shall the contribution obligation of a Holder be
greater in amount than the dollar amount of the net proceeds received by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
3. Miscellaneous.
3.1 Governing Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the
internal laws of the State of New York without regard to the choice of law principles thereof. Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the state and federal courts located in the State of New York for the
purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on each party hereto
anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. Each of the parties hereto irrevocably consents to the jurisdiction of any such court in any such suit, action or proceeding and to the
-11-
laying of venue in such court. Each of the parties hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably
waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
3.2 Assignments and Transfers by Purchasers. The provisions of this Agreement shall be binding upon and inure to
the benefit of the Purchasers and their respective successors and assigns. A Holder may transfer or assign, in whole or from time to time in part, to one or more persons its rights hereunder in connection with the transfer of Registrable Securities
by such Holder to such person, provided that such Holder complies with all laws applicable thereto, and the provisions of the Purchase Agreement, and provides written notice of assignment to the Company promptly after such assignment is
effected, and such person agrees in writing to be bound by all of the provisions contained herein.
3.3 Assignments and Transfers by the Company. This Agreement may not be assigned by the Company (whether by
operation of law or otherwise) without the prior written consent of the Required Holders, provided, however, that in the event that the Company is a party to a merger, consolidation, share exchange or similar business combination
transaction in which the Common Stock is converted into the equity securities of another Person, from and after the effective time of such transaction, such Person shall, by virtue of such transaction, be deemed to have assumed the
obligations of the Company hereunder, the term “Company” shall be deemed to refer to such Person and the term “Registrable Securities” shall be deemed to include the securities received by the Holders in connection with such
transaction unless such securities are otherwise freely tradable (without restriction, including manner of sale, current information requirements or volume limitations pursuant to Rule 144) by the Holders after giving effect to such transaction.
3.4 Entire Agreement; Amendment. This Agreement and the other Transaction Agreements constitute the full
and entire understanding and agreement between the parties with regard to the subjects hereof and thereof. Any previous agreements among the parties relative to the specific subject matter hereof are superseded by this Agreement. This Agreement may
be amended only by a writing signed by the Company and the Required Holders. The Company may take any action herein prohibited, or omit to perform any act herein required to be performed by it, only if the Company shall have obtained the written
consent to such amendment, action or omission to act of the Required Holders. Notwithstanding the foregoing, this Agreement may not be amended and the observance of any term hereof may not be waived with respect to any Holder without the written
consent of such Holder if such amendment or waiver on its face materially and adversely affects the rights of such Holder under this Agreement in a manner that is different than the other Holders. The Holders acknowledge that by the operation of
this Section 3.4, the Required Holders may have the right and power to diminish or eliminate all rights of the Holders under this Agreement.
3.5 No Inconsistent Agreements. The Company has not entered, as of the date hereof, and shall not enter, on or
after the date of this Agreement, into any agreement with respect to its securities that would have the effect of impairing the rights granted to the Holders in this Agreement or otherwise conflicts with the provisions hereof.
3.6 Independent Nature of Holders’ Obligations and Rights. The obligations of each Holder hereunder are
several and not joint with the obligations of any other Holder hereunder, and no Holder shall be responsible in any way for the performance of the obligations of any other Holder hereunder. Nothing contained herein or in any other agreement or
document delivered at any closing, and no action taken by any Holder pursuant hereto or thereto, shall be deemed to constitute the Holders as a partnership, an association, a joint venture or any other kind of group or entity, or create a
presumption that the Holders are in any way acting in concert or as a group or entity with respect to such obligations or the transactions contemplated by this Agreement or any other matters and the Company acknowledges that the Holders are not
acting in concert or as a group, and the Company shall not assert any such claim, with respect to such obligations or
-12-
transactions. Except as expressly provided herein, each Holder shall be entitled to protect and enforce its rights, including without limitation the rights arising out of this Agreement, and it
shall not be necessary for any other Holder to be joined as an additional party in any proceeding for such purpose. The use of a single agreement with respect to the obligations of the Company contained herein was solely in the control of the
Company, not the action or decision of any Holder, and was done solely for the convenience of the Company and not because it was required or requested to do so by any Holder. Except as expressly provided herein, it is expressly understood and agreed
that each provision contained in this Agreement is between the Company and a Holder, solely, and not between the Company and the Holders collectively and not between and among Holders.
3.7 Notices. All notices and other communications provided for or permitted hereunder shall be made as set forth
in Section 8.3 of the Purchase Agreement.
3.8 Third Parties. This Agreement does not create any
rights, claims or benefits inuring to any person that is not a party hereto nor create or establish any third party beneficiary hereto; provided, that the indemnified parties are intended third party beneficiaries of Section 2.5.
3.9 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of
competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated,
and the parties hereto shall use their reasonable best efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby
stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or
unenforceable.
3.10 Headings. The headings herein are for convenience only, do not constitute a part of
this Agreement and shall not be deemed to limit or affect any of the provisions hereof.
3.11 Counterparts.
This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to each other
party, it being understood that the parties need not sign the same counterpart. In the event that any signature is delivered by facsimile transmission or by e-mail delivery of a “.pdf”
format data file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or “.pdf” signature page were an
original thereof.
3.12 Delays or Omissions. It is agreed that no delay or omission to exercise any right,
power or remedy accruing to any party upon any breach or default of any other party under this Agreement shall impair any such right, power or remedy, nor shall it be construed to be a waiver of any such breach or default, or any acquiescence
therein, or of any similar breach or default thereafter occurring; nor shall any waiver of any single breach or default be deemed a waiver of any other breach or default theretofore or thereafter occurring. It is further agreed that any waiver,
permit, consent or approval of any kind or character of any breach or default under this Agreement, or any waiver of any provisions or conditions of this Agreement must be in writing and shall be effective only to the extent specifically set forth
in writing, and that all remedies, either under this Agreement, by law or otherwise, shall be cumulative and not alternative.
3.13 Consents. Any permission, consent, or approval of any kind or character under this Agreement shall be in
writing and shall be effective only to the extent specifically set forth in such writing.
-13-
3.14 SPECIFIC PERFORMANCE. THE PARTIES HERETO AGREE THAT
IRREPARABLE DAMAGE WOULD OCCUR IN THE EVENT THAT ANY OF THE PROVISIONS OF THIS AGREEMENT WERE NOT PERFORMED IN ACCORDANCE WITH ITS SPECIFIC INTENT OR WERE OTHERWISE BREACHED. IT IS ACCORDINGLY AGREED THAT THE PARTIES SHALL BE ENTITLED TO AN
INJUNCTION OR INJUNCTIONS, WITHOUT BOND, TO PREVENT OR CURE BREACHES OF THE PROVISIONS OF THIS AGREEMENT AND TO ENFORCE SPECIFICALLY THE TERMS AND PROVISIONS HEREOF, THIS BEING IN ADDITION TO ANY OTHER REMEDY TO WHICH THEY MAY BE ENTITLED BY
LAW OR EQUITY, AND ANY PARTY SUED FOR BREACH OF THIS AGREEMENT EXPRESSLY WAIVES ANY DEFENSE THAT A REMEDY IN DAMAGES WOULD BE ADEQUATE.
3.15 Construction of Agreement. No provision of this Agreement shall be construed against either party as the
drafter thereof.
3.16 Section References. Unless otherwise stated, any reference
contained herein to a Section or subsection refers to the provisions of this Agreement.
3.17 Variations of
Pronouns. All pronouns and all variations thereof shall be deemed to refer to the masculine, feminine, or neuter, singular or plural, as the context in which they are used may require.
[Remainder of Page Intentionally Left Blank; Signature Pages Follow]
-14-
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized
officers to execute this Agreement as of the day and year first written above.
|
|
|
| COMPANY: |
|
|
| By: |
|
/s/ Paul Wagner |
| Name: |
|
Paul Wagner |
| Title: |
|
Chief Executive Officer |
[Signature Page to Registration Rights Agreement]
-15-
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized
officers to execute this Agreement as of the day and year first written above.
[Signature Page to Registration Rights Agreement]
-16-
Annex A
PLAN OF DISTRIBUTION
The
selling stockholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling shares of common stock or interests in shares
of common stock received after the date of this prospectus from a selling stockholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their shares of common
stock or interests in shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at
prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The selling
stockholders may use any one or more of the following methods when disposing of shares or interests therein:
· ordinary brokerage transactions and transactions in which the broker-dealer solicits
purchasers;
· block trades in which the broker-dealer will attempt to sell the
shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
· purchases by a broker-dealer as principal and resale by the broker-dealer for its
account;
· an exchange distribution in accordance with the rules of the applicable
exchange;
· privately negotiated transactions;
· short sales;
· through the writing or settlement of options or other hedging transactions, whether
through an options exchange or otherwise;
· broker-dealers may agree with the
selling stockholders to sell a specified number of such shares at a stipulated price per share;
· a combination of any such methods of sale; and
· any other method permitted by applicable law.
The selling stockholders may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by
them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule
424(b)(3) or other applicable provision of the Securities Act of 1933, as amended (the “Securities Act”), amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders
under this prospectus. The selling stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling stockholders for purposes of this
prospectus.
-17-
In connection with the sale of our common stock or interests therein, the selling
stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The selling stockholders may also
sell shares of our common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option
or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus,
which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the selling stockholders from the sale of the common stock offered by them will be the purchase price of the common
stock less discounts or commissions, if any. Each of the selling stockholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly
or through agents. We will not receive any of the proceeds from this offering. Upon any exercise of the warrants by payment of cash, however, we will receive the exercise price of the warrants.
The selling stockholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the
Securities Act, provided that they meet the criteria and conform to the requirements of that rule, or another available exemption from the registration requirements of the Securities Act.
The selling stockholders and any underwriters, broker-dealers or agents that participate in the sale of the common stock or interests therein
may be “underwriters” within the meaning of Section 2(a)(11) of the Securities Act. Any discounts, commissions, concessions or profit they earn on any resale of the shares may be underwriting discounts and commissions under the
Securities Act. Selling stockholders who are “underwriters” within the meaning of Section 2(a)(11) of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act.
To the extent required, the shares of our common stock to be sold, the names of the selling stockholders, the respective purchase prices and
public offering prices, the names of any agents, dealer or underwriter, and any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective
amendment to the registration statement that includes this prospectus.
In order to comply with the securities laws of some states, if
applicable, the common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common stock may not be sold unless it has been registered or qualified for sale or an exemption
from registration or qualification requirements is available and is complied with.
We have advised the selling stockholders that the
anti-manipulation rules of Regulation M under the Securities Exchange Act of 1934, as amended, may apply to sales of shares in the market and to the activities of the selling stockholders and their affiliates. In addition, to the extent applicable,
we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling stockholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The selling stockholders
may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the selling stockholders against liabilities, including liabilities under the Securities Act and state securities
laws, relating to the registration of the shares offered by this prospectus.
-18-
We have agreed with the selling stockholders to use reasonable best efforts to cause the
registration statement of which this prospectus constitutes a part to become effective and to remain continuously effective until the earlier of (1) such time as all of the shares covered by this prospectus have been disposed of pursuant to and
in accordance with such registration statement or (2) the date that all the shares covered by this prospectus cease to be Registrable Securities.
-19-
Exhibit 99.1

FORTE BIOSCIENCES, INC. ANNOUNCES $25 MILLION FINANCING AND R&D UPDATE FOR FB-102
-FB-102 Has Demonstrated Potentially Best in
Class Activity including Superiority to Standard of Care in GvHD
-Proof of Concept Pre-clinical Data in Additional Indications
with Large Market Potential Underscores Meaningful Opportunity
-$25 Million Financing Highlights Significant Support from Top Tier
Institutional Investors for FB-102 Potential
DALLAS, TX – AUGUST 1, 2023 – Forte
Biosciences, Inc. (www.fortebiorx.com) (NASDAQ: FBRX), a biopharmaceutical company focused on autoimmune diseases, today announced key R&D updates for FB-102 and the closing of financing to
support the advancement of FB-102.
“FB-102 had demonstrated
potentially best in class activity including in graft versus host disease (GvHD), an indication for which there is a very high unmet medical need and potentially abbreviated clinical development pathway. We plan to advance FB-102 into the clinic in 2024. Beyond GvHD, based on extensive proof of concept data, we believe FB-102 has considerable opportunity in a variety of indications with large
markets.” said Paul Wagner, Ph.D., Chairman and Chief Executive Officer of Forte Biosciences. “Our financing by top-tier institutional investors highlights the meaningful potential for FB-102. We are deeply appreciative of the support from preeminent institutional biotechnology investors including Alger, BVF Partners, Farallon Capital Management, Perceptive Advisors and Tybourne Capital
Management.”
FB-102 R&D Updates
Forte’s wholly owned antibody, FB-102, targets CD-122, a subunit of the IL-2 and IL-15 receptor and mediates NK and CD8+ T cells, which have been implicated in a variety of autoimmune and immune related diseases.
FB-102 has demonstrated potentially best in class activity including in validated models of GvHD with superiority to
standard of care both as a single agent and in combination with standard of care.
In a validated preclinical model of aggressive acute GvHD using
transplanted human donor PBMCs, FB-102 reported 90% survival versus 0% survival on vehicle control and compared to 60% survival on ruxolitinib. In combination with ruxolitinib, the only FDA-approved treatment for acute GvHD, FB-102 reported 90% survival compared to 0% survival on vehicle control and 30% survival for ruxolitinib alone with extended duration of
dosing compared to the single agent study.
Additionally, in a mouse model of chronic GvHD conducted by Forte, anti-CD-122 treatment has shown superior activity compared to placebo or ruxolitinib.
Published preclinical PoC
data also support the therapeutic potential of anti-CD122 in multiple autoimmune diseases anti-CD-122 including in vitiligo, celiac disease, alopecia areata, Type 1
diabetes and solid organ transplant.
About Forte
Forte Biosciences, Inc. is a biopharmaceutical company that is advancing its product candidate,
FB-102, which is a proprietary molecule with potentially broad autoimmune applications including in such indications as graft-versus-host disease, vitiligo and alopecia areata.
Forward Looking Statements
Forte
cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,”
“should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,”
“predicts,” “potential” or “continue” or the negatives of these terms or other similar expressions. These statements are based on the Company’s current beliefs and expectations. Forward looking statements include
statements regarding the use of proceeds of the offering; our plans to advance FB-102 into clinical trials and the expected timelines related thereto; our projections regarding the market size for FB-102; the therapeutic potential of FB-102; and Forte’s plans to develop and potentially commercialize its product candidates, including
FB-102. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without
limitation: risks associated with market conditions; risks related to the Company’s estimates regarding future expenses, capital requirements and need for additional financing; uncertainties associated with the clinical development and
regulatory approval of Forte’s product candidates, including potential delays in the commencement, enrollment and completion of clinical trials; the risk that results from early-preclinical studies may not be predictive of results from
later-stage studies or clinical trials; Forte’s ability to successfully enter into collaborations, and to fulfill its obligations under any such collaboration agreements; the clinical utility, potential benefits and market acceptance of
Forte’s product candidates; Forte’s commercialization, marketing and manufacturing capabilities and strategy; developments and projections relating to Forte’s competitors and its industry; the impact of government laws and
regulations; Forte’s ability to protect its intellectual property position; Forte’s estimates regarding future revenue, expenses, capital requirements and need for additional financing; and the impact of global events on the Company, the
Company’s industry or the economy generally. Information on these and additional risks, uncertainties, and other information affecting Forte’s business and operating results is contained in Forte’s Annual Report on Form 10-K for the year ended December 31, 2022 as filed with the Securities and Exchange Commission on March 31, 2023, in the Company’s subsequent Quarterly Report on Form 10-Q filed on May 15, 2023, and in its other filings with the Securities and Exchange Commission. All forward-looking statements in this press release are current only as of the date hereof and, except as
required by applicable law, Forte undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking
statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
Contact:
LifeSci Advisors
Mike Moyer, Managing Director
mmoyer@lifesciadvisors.com
Forte Biosciences, Inc.
Paul Wagner, CEO
investors@fortebiorx.com

Exhibit 99.2 FORTE BIOSCIENCES CORPORATE OVERVIEW CORPORATE PRESENTATION
AUGUST 1, 2023

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS ¡ Certain
statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation
Act of 1995, known as the PSLRA. These include statements regarding management’s intention, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking
statement can be guaranteed, and actual results may differ materially from those projected. Forte Biosciences, Inc. (“we”, the “Company” or “Forte”) undertakes no obligation to publicly update any forward-looking
statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,”
“projects,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,”
“guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. ¡ Such forward-looking statements are based on our expectations and involve
risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, risks relating to the business and prospects of the Company;
Forte’s plans to develop and potentially commercialize its product candidates, including FB-102; the risk that results from early-preclinical studies may not be predictive of results from later-stage studies or clinical trials; the timing of
initiation of Forte’s planned clinical trials; the timing of the availability of data from Forte’s clinical trials; the timing of any planned investigational new drug application or new drug application; Forte’s plans to research,
develop and commercialize its current and future product candidates; Forte’s projections of the size of the market for FB-102; Forte’s ability to successfully enter into collaborations, and to fulfill its obligations under any such
collaboration agreements; the clinical utility, potential benefits and market acceptance of Forte’s product candidates; Forte’s commercialization, marketing and manufacturing capabilities and strategy; developments and projections
relating to Forte’s competitors and its industry; the impact of government laws and regulations; Forte’s ability to protect its intellectual property position; Forte’s estimates regarding future revenue, expenses, capital
requirements and need for additional financing; and the impact of global events on the Company, the Company’s industry or the economy generally. ¡ We have based these forward-looking statements largely on our current expectations and
projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs, and these statements represent our views as of the date of this presentation. We may not
actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Forward-looking statements are inherently subject to risks and
uncertainties, some of which cannot be predicted or quantified. Information regarding certain risks, uncertainties and assumptions may be found in our filings with the Securities and Exchange Commission, including under the caption “Risk
Factors” and elsewhere in our Annual Report on Form 10-K for the year ending December 31, 2022 and subsequent filings with the Securities and Exchange Commission. New risk factors emerge from time to time and it is not possible for our
management team to predict all risk factors or assess the impact of all factors on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any
forward-looking statements. While we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our
views as of any date subsequent to the date of this presentation. 2

EXPERIENCED MANAGEMENT Forte’s management has extensive experience
in manufacturing, quality, regulatory and clinical development Paul Wagner, Ph.D., CFA – CEO Hubert Chen, M.D. – Chief Scientific Officer Tony Riley – Chief Financial Officer Chris Roenfeldt, PMP – Chief Operating Officer
Steven Ruhl – Chief Technical Officer 5

FB-102 PROGRAM OVERVIEW

FB-102 OVERVIEW + • CD122 is a subunit of IL-2/IL-15 receptors
which are key regulators of NK, CD 8 T cells and Tregs + • FB-102 (Forte’s anti-CD122 antibody) is designed to mediate NK and CD 8 T cells specifically • Significant amount of PoC preclinical data supports activity of CD-122
blockade, including in GvHD and vitiligo • FB-102 has demonstrated significant preclinical activity in validated GvHD models • Expect FB-102 to be in the clinic in early 2024 • We believe FB-102 for the treatment of GvHD addresses
unmet need and could allow for abbreviated development path 5

ANTI-CD122: HIGHLIGHTS OF PRECLINICAL DATA

ANTI-CD122 BLOCKADE HAS DEMONSTRATED EFFICACY IN MULTIPLE ANIMAL MODELS
Disease Species Outcome Reference GVHD Mouse Prolonged survival JN Bio patent, 2015 Vitiligo Mouse Enhanced repigmentation Sci Transl Med, 2018 Alopecia areata Mouse Prevented fur loss Nature Med, 2014 Type 1 diabetes Mouse Delayed disease onset JCI
Insight, 2018 Celiac disease Mouse Improved IL-15-induced PNAS, 2009 mucosal damage Skin and kidney transplant Mouse/Monkey Prolonged graft survival in J Clin Invest, 2018 rejection combination with CTLA-4 7

ANTI-CD122 IN A HUMANIZED MOUSE MODEL OF ACUTE GVHD: AMELIORATED BODY
WEIGHT LOSS AND PROLONGED SURVIVAL Humanized mouse model of graft vs host disease Aggressive, rapid onset of GVHD: significant weight loss and mortality within 2 weeks Immunodeficient Transfer of peripheral (NOG) mice blood mononuclear cells Day 10
Anti-CD122 Control Results Survival 66% 0% US9,028,830 8

CD-122 BLOCKADE PRODUCES SIGNIFICANT REPIGMENTATION IN A MOUSE VITILIGO
MODEL Richmond et al. Sci Transl Med. 2018 July 18; 10(450) Following 8 weeks of anti-CD122 treatment (weeks 12-20), the vitiligo mice demonstrated a statistically significant change in tail pigmentation (p=0.0001)
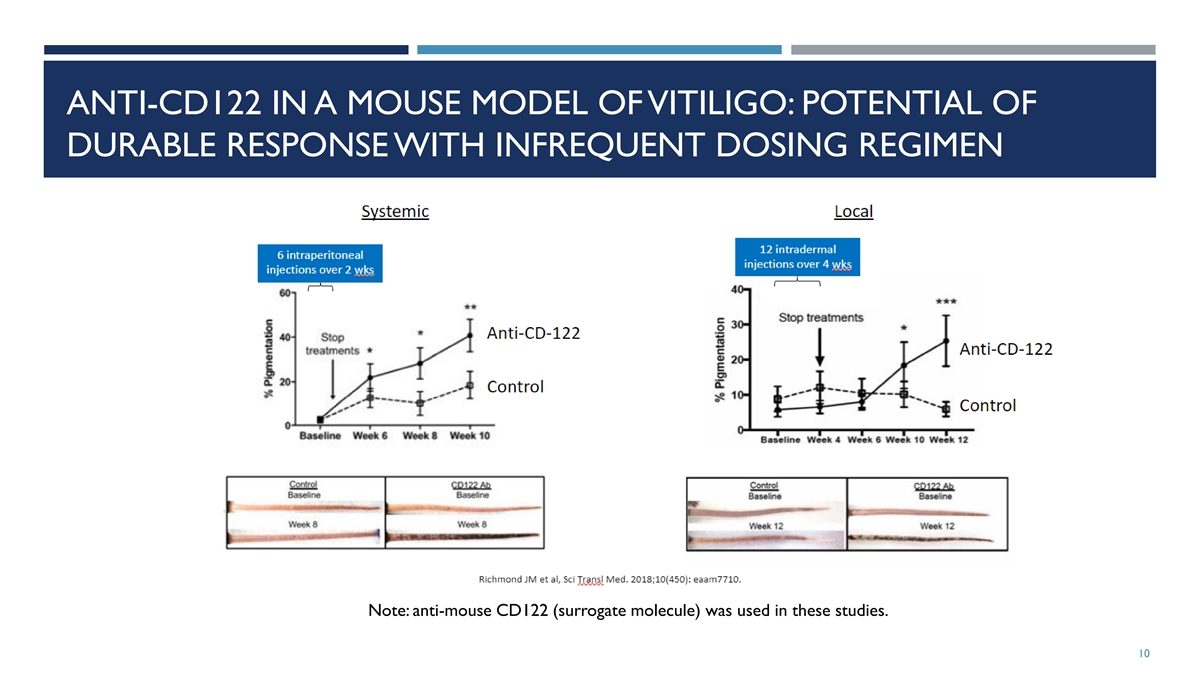
ANTI-CD122 IN A MOUSE MODEL OF VITILIGO: POTENTIAL OF DURABLE RESPONSE
WITH INFREQUENT DOSING REGIMEN Note: anti-mouse CD122 (surrogate molecule) was used in these studies. 10

ANTI-CD122 AS AN EFFECTIVE PROPHYLAXIS AGAINST FUR LOSS IN A MOUSE
MODEL OF ALOPECIA AREATA Anti-CD122 C3H/HeJ mice were treated systemically from the time of grafting with anti-CD122. Xing et al. Nature Medicine Vol 20, No. 9, p 1043 Note: anti-mouse CD122 (surrogate molecule) was used in these studies.

FB-102 DEVELOPMENT STRATEGY: PRIORITIZATION OF INDICATIONS

POTENTIAL INDICATIONS OF ANTI-CD122: GRAFT-VS-HOST DISEASE (GVHD) AND
VITILIGO AS FIRST-TIER CHOICES Strength of existing data & Timeline/resource to Commercial opportunity w/ Overall score Indication mechanism of action proof-of-concept in patients projected target product profile (product of 3 subscores) Acute
GVHD 3 2 2 12 Vitiligo 3 2 2 12 Chronic GVHD 2 2 2 8 Kidney transplant rejection 3 1 2 6 Alopecia areata 1 3 1 3 Celiac disease (refractory) 1 1 2 2 Type 1 diabetes 2 1 1 2 Eosinophilic esophagitis 1 1 2 2 IBD 1 2 1 2 (ulcerative colitis) NASH 1 1 1
1 Each category scored from 1 (low) to 3 (high) 13

GRAFT VS HOST DISEASE (GVHD): A SERIOUS COMPLICATION OF ALLOGENEIC STEM
CELL TRANSPLANTATION Cause: donor immune cells attack host tissues Classification Acute (~5K US prevalence - NIH) - Occurs in up to 50% of recipients. - Onset typically within 3 months of transplant - Usually combination or organs involved: skin
(rash), GI tract (vomiting, diarrhea), liver (jaundice) Chronic (~14K US prevalence - NIH) - Develops in up to 40% of recipients. - In addition to skin, GI tract and liver, may involve lungs, mucosal surfaces (eyes, mouth, GU tract), muscle, joints
(connective tissue) https://www.lls.org/booklet/graft-versus-host-disease 14

CD8 AND NK CELLS IN THE PATHOGENESIS OF ACUTE GVHD: POTENTIAL OF
ANTI-CD122 ANTAGONISM WITH FB102 Anti-CD122: blocking key cellular mediators of GVHD IFNg IL-2 Inflammation & apoptosis Devetten MP, Biol Blood Marrow Transplant. 2004;10:815. Simonetta F, Front Immunol. 2017;8:465. Khandelwal P, Biol Blood
Marrow Transplant. 2020;26:1. 15

LARGE UNMET NEED IN VITILIGO AND ALOPECIA AREATA (COMBINED $6 BILLION
MARKET BY 2026) Vitiligo Vitiligo is an autoimmune disease of the skin mediated primarily by NK and CD8+ T cells that kill melanocytes and create white spots. In the US there are approximately 2 m people with vitiligo. The global vitiligo treatment
market size was valued at $1.2 billion in 2018 and is projected to reach $1.9 billion by 2026, exhibiting a CAGR of 5.8% (Fortune Business Insights) Alopecia Areata (AA) AA is an autoimmune disease in which immune cells attack and damage hair
follicles and is mediated primarily by CD8+ T cells and NK cells The global alopecia treatment market was valued at $2.7 billion in 2018, and is projected to reach $3.9 billion by 2026, registering a CAGR of 4.6% from 2019 to 2026 (Allied Mkt
Research) While JAK inhibitors have demonstrated efficacy in AA and vitiligo, regulatory scrutiny of the JAK class including black box warnings has dampened enthusiasm for this class and as a result there remains a significant unmet need for safe
and effective therapies for treating AA and vitiligo

FB-102 IN GRAFT VS HOST DISEASE

ACUTE GVHD: TREATMENT PARADIGM AND DEVELOPMENT PIPELINE
https://www.lls.org/booklet/graft-versus-host-disease https://www.jakafi.com/pdf/prescribing-information.pdf 18

POTENTIAL INDICATIONS AND CLINICAL SUCCESS CRITERIA OF ANTI-CD122 IN
ACUTE GVHD Corticosteroids First-line Goal: improve Day 28 ORR vs. Anti- Second-line CD122 ruxolitinib monotherapy Anti- Goal: achieve Day 28 ORR >60% Third-line CD122 after ruxolitinib failure
https://www.lls.org/booklet/graft-versus-host-disease https://www.jakafi.com/pdf/prescribing-information.pdf 19

APPROVAL OF RUXOLITINIB IN ACUTE GVHD WAS BASED ON THE RESULTS FROM AN
OPEN-LABEL, SINGLE-ARM STUDY Efficacy is based on Day 28 overall response rate as defined by CIBMTR criteria. https://www.jakafi.com/pdf/prescribing-information.pdf 20

CHRONIC GVHD: TREATMENT PARADIGM AND DEVELOPMENT PIPELINE
https://www.lls.org/booklet/graft-versus-host-disease https://www.rxabbvie.com/pdf/imbruvica_pi.pdf https://www.jakafi.com/pdf/prescribing-information.pdf https://products.sanofi.us/rezurock/rezurock.pdf 21

POTENTIAL INDICATIONS AND CLINICAL SUCCESS CRITERIA OF ANTI-CD122 IN
CHRONIC GVHD Corticosteroids First-line Second-line Goal: improve Month 6 ORR Anti- (esp. CR) vs. standard of care. CD122 Third-line Anti- Fourth-line https://www.lls.org/booklet/graft-versus-host-disease Goal: achieve Month 6 ORR >70% CD122
https://www.rxabbvie.com/pdf/imbruvica_pi.pdf https://www.jakafi.com/pdf/prescribing-information.pdf https://products.sanofi.us/rezurock/rezurock.pdf 22

APPROVAL OF REZUROCK IN CHRONIC GVHD WAS BASED ON THE RESULTS FROM AN
OPEN-LABEL, SINGLE-ARM STUDY Efficacy is based on overall response rate after 6 months of treatment as defined by the 2014 NIH response criteria. https://products.sanofi.us/rezurock/rezurock.pdf 23

KADMON PURCHASED BY SANOFI FOR $1.9B TO FURTHER STRENGTHEN GROWTH OF
TRANSPLANT BUSINESS 24

FB-102 GRAFT VS HOST DISEASE PRECLINICAL DATA

CHRONIC GVHD STUDY WITH ANTI-CD122 AND RUXOLITINIB End of Treatment
start study 40 50 60 Day 1 10 20 30 Vehicle FB650 (100 mpk IP every other day) Ruxolitinib (60 mpk PO twice daily) N=16-18 per group 26

CHRONIC GVHD STUDY RESULTS: GVHD SCORE Treatment start 3 Vehicle
Vehicle 2 Anti-CD122 Ruxolitinib Ruxolitinib 1 0 Anti-CD122 30 34 38 42 46 50 54 58 62 66 Days after transplant Mean (SEM), n=16-18 per group Day 60 results: P<0.01 for Vehicle vs Anti-CD122, P<0.05 for Ruxolitinib vs Anti-CD122 27 GVHD
Score

GENERATING PRECLINICAL EFFICACY DATA IN A HUMANIZED MOUSE MODEL OF
ACUTE GVHD Humanized mouse model of graft vs host disease 28

DOSE-RANGING INVESTIGATION OF FB102 IN A HUMANIZED MOUSE MODEL OF ACUTE
GVHD: THERAPEUTIC MODE Irradiation & End of PBMC transplantation treatment 1 2 3 4 5 6 8 10 12 13 14 Day 7 9 11 FB102 Once-daily IP administration Start dosing on Day 5, (75, 125, 175 mpk) once daily Vehicle Once-daily IP administration
Twice-daily oral administration Ruxolitinib Start dosing on Day 5, (60 mpk) twice daily Twice-daily oral administration Vehicle N=10 per cohort 29
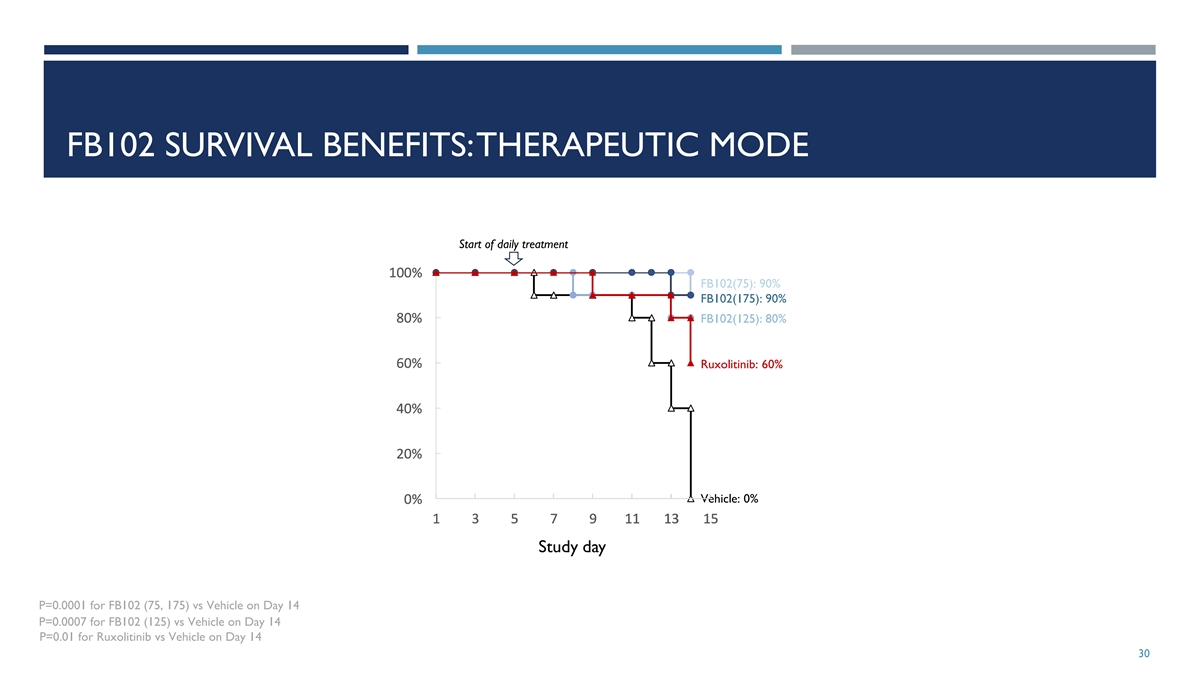
FB102 SURVIVAL BENEFITS: THERAPEUTIC MODE Start of daily treatment 1 1
1 1 10 0 0 0 00 0 0 0 0% % % % % FB102(75): 90% FB102(175): 90% 8 8 8 8 80 0 0 0 0% % % % % FB102(125): 80% 6 6 6 6 60 0 0 0 0% % % % % Ruxolitinib: 60% 4 4 4 4 40 0 0 0 0% % % % % 2 2 2 2 20 0 0 0 0% % % % % Vehicle: 0% 0 0 0 0 0% % % % % 1 1 1 1 1
3 3 3 3 3 5 5 5 5 5 7 7 7 7 7 9 9 9 9 9 1 1 1 1 11 1 1 1 1 1 1 1 1 13 3 3 3 3 1 1 1 1 15 5 5 5 5 Study day P=0.0001 for FB102 (75, 175) vs Vehicle on Day 14 P=0.0007 for FB102 (125) vs Vehicle on Day 14 P=0.01 for Ruxolitinib vs Vehicle on Day 14
30

MONO VS COMBINATION THERAPIES WITH FB102, RUXOLITINIB OR
CORTICOSTEROIDS Irradiation & End of study PBMC injection (D18) Day 1 5 8 12 15 18 Ruxolitinib Twice-daily oral (60 mpk) FB102+Ruxolitinib Once-daily IP + twice-daily oral (75 mpk+60 mpk) Placebo Once-daily IP + twice-daily oral (Combination of
vehicles) N=10 per cohort 31

FB102 SURVIVAL BENEFITS: COMBINATION WITH RUXOLITINIB VS MONOTHERAPY
Start of daily treatment 100% 1 10 00 0% % FB102+Rux: 90% 8 8 80 0 0% % % 6 6 60 0 0% % % 4 4 40 0 0% % % Ruxolitinib: 30% 2 2 20 0 0% % % Vehicle: 0% 0 0 0% % % 1 1 1 3 3 3 5 5 5 7 7 7 9 9 9 1 1 11 1 1 1 1 13 3 3 1 1 15 5 5 1 1 17 7 7 Study day
P=0.0001 for FB102+Rux vs Vehicle on Day 18 P=0.02 for FB102+Rux vs Ruxolitinib Mono on Day 18 32

TRANSLATION OF HUMANIZED ACUTE GVHD MOUSE FINDINGS INTO PATIENT
RESPONSE: PROMISING FB102 RESULTS Magnitude of FB102 preclinical efficacy correlates with positive clinical response. Preclinical Data Indication/ Clinical Data Company Candidate Mechanism (survival in humanized Phase (Day 28 ORR) GVHD model)
Second/Third-line Forte Biosciences FB102 Anti-CD122 90% (vs 0% for control) TBD Preclinical Second-line 1 4 Incyte Ruxolitinib JAK 1/2 inhibition 90% (vs 0% for control) 62% Commercial First-line 2 5 Equillium/Ono Itolizumab Anti-CD6 50% (vs 10%
for control) >50% Phase 3 First-line 3 Incyte Itacitinib JAK 1 inhibition 20% (vs 0% for control) N.S. vs PBO Terminated 1. Huarte E et al. Immunotherapy. 2021;13:977. 2. Ng CT et al. Blood. 2019;134 (Supp 1):5063. 3. Courtois J et al. Bone
Marrow Transplant. 2021;56:2672. Day 60 results shown. 4. Zeiser R et al. N Engl J Med. 2020;382:1800. 5. Equillium Corporate Presentation, September 2022. 6. Zeiser R et al. Lancet Haematol. 2022;9:e14. 33

FB-102 OVERVIEW + • CD122 is a subunit of IL-2/IL-15 receptors
which are key regulators of NK, CD 8 T cells and Tregs + • FB-102 (Forte’s anti-CD122 antibody) is designed to mediate NK and CD 8 T cells specifically • Significant amount of PoC preclinical data supports activity of CD-122
blockade, including in GvHD and vitiligo • FB-102 has demonstrated significant preclinical activity in validated GvHD models • Expect FB-102 to be in the clinic in early 2024 • We believe FB-102 for the treatment of GvHD addresses
unmet need and could allow for abbreviated development path 34
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Forte Biosciences (NASDAQ:FBRX)
Historical Stock Chart
From Apr 2024 to May 2024
Forte Biosciences (NASDAQ:FBRX)
Historical Stock Chart
From May 2023 to May 2024