Filed Pursuant to Rule 424(b)(5)
Registration No. 333-225517
PROSPECTUS SUPPLEMENT
(To Prospectus dated June 29, 2018)

BRAINSTORM CELL THERAPEUTICS INC.
Up to $45,000,000
Common Stock
This prospectus supplement and the accompanying
prospectus relate to the offer and sale from time to time of shares of our common stock, par value $0.00005 per share, having an
aggregate offering price of up to $45,000,000. Shares of our common stock to which this prospectus supplement relates may be offered
over a period of time and from time to time through SVB Leerink LLC and Raymond James & Associates, Inc., as our distribution
agents, which we refer to as our Distribution Agents, for sale to the public in accordance with the terms of an Amended and Restated
Distribution Agreement we have entered into with the Distribution Agents. Sales of shares of our common stock, if any, may be made
in transactions that are deemed to be “at-the-market offerings” as defined in Rule 415 under the Securities Act of
1933, as amended, or the Securities Act, including sales made directly on or through the Nasdaq Capital Market, sales made to or
through a market maker other than on an exchange, in transactions at market prices prevailing at the time of sale or at prices
related to such market prices, or any other method permitted by law. Under the terms of the Amended and Restated Distribution Agreement,
we may also sell our common stock to either of the Distribution Agents as principals for their own accounts at prices agreed upon
at the time of sale. If we sell our common stock to the Distribution Agents as principals, we will enter into a separate terms
agreement with the Distribution Agents. The Distribution Agents are not required to sell any specific dollar amount or number of
securities, but will act as the Distribution Agents using commercially reasonable efforts consistent with their normal trading
and sales practice, on mutually agreed upon terms between us and the Distribution Agents. There is no arrangement for funds to
be received in any escrow, trust or similar arrangement.
We previously sold 2,446,641 shares of
common stock for gross proceeds of approximately $23.11 million of common stock under our Distribution Agreement we previously
entered into with Raymond James & Associates, Inc. pursuant to a prospectus supplement dated March 6, 2020. We may offer and
sell up to an additional $45,000,000 of common stock through the Distribution Agents pursuant to this prospectus supplement. Our
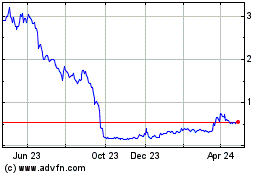
common stock is listed on the Nasdaq Capital Market under the symbol “BCLI.” On September 24, 2020, the last reported
sale price of our common stock on the Nasdaq Capital Market was $14.19 per share.
We will pay the Distribution Agents a
commission rate equal to 3.0% of the gross sales price of all shares sold by them as the Distribution Agents under the Amended
and Restated Distribution Agreement. In connection with the sale of our common stock on our behalf, the Distribution Agents will
be deemed to be “underwriters” within the meaning of the Securities Act and the compensation of the Distribution Agents
will be deemed to be underwriting commissions. We have also agreed to provide rights of indemnification and contribution to the
Distribution Agents with respect to certain liabilities, including liabilities under the Securities Act.
Investing in our common stock involves
a high degree of risk. Before deciding whether to invest in our common stock, you should review carefully the risks and uncertainties
that are described in the “Risk Factors” section beginning on page S-10 of this prospectus supplement, and in the documents
incorporated by reference herein, including our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2019
as well as the risks and uncertainties described in the other documents incorporated herein by reference.
Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
supplement and the accompanying prospectus are truthful or complete. Any representation to the contrary is a criminal offense.
|
SVB Leerink
|
Raymond James
|
The date of this prospectus supplement is
September 25, 2020
TABLE OF CONTENTS
Prospectus Supplement
ABOUT THIS PROSPECTUS SUPPLEMENT
On June 8, 2018, we filed with the Securities
and Exchange Commission (the “SEC”) a registration statement on Form S-3 (File No. 333-225517) using a shelf registration
process relating to the securities described in this prospectus supplement, which registration statement was declared effective
by the SEC on June 29, 2018. Under this shelf registration process, we may offer and sell, either individually or in combination,
in one or more offerings, common stock, warrants and units, for total gross proceeds of up to $100 million.
This document consists of two parts. The
first part is the prospectus supplement, which describes the specific terms of this offering. The second part is the accompanying
prospectus, which provides more general information, some of which may not apply to the securities offered by this prospectus supplement.
We urge you to read carefully this prospectus
supplement, the accompanying prospectus and any free writing prospectuses we have authorized for use in connection with this offering,
together with information incorporated by reference in this prospectus supplement and the accompanying prospectus, before investing
in any of the securities being offered under this prospectus supplement. You should rely only on the information contained in,
or incorporated by reference into, this prospectus supplement and the accompanying prospectus, along with the information contained
in any free writing prospectuses we have authorized for use in connection with this offering. We have not authorized anyone to
provide you with different or additional information. This prospectus supplement is an offer to sell only the securities offered
hereby, but only under circumstances and in jurisdictions where it is lawful to do so.
The information appearing in this prospectus
supplement, the accompanying prospectus or any related free writing prospectus is accurate only as of the date on the front of
the document and any information we have incorporated by reference is accurate only as of the date of the document incorporated
by reference, regardless of the time of delivery of this prospectus supplement, the accompanying prospectus or any related free
writing prospectus, or any sale of a security. Our business, financial condition, results of operations and prospects may have
changed since those dates.
This prospectus supplement and any free
writing prospectus that we have authorized for use in connection with this offering may add, update or change the information contained
in the accompanying prospectus and the documents incorporated by reference in this prospectus supplement and the accompanying prospectus.
To the extent that any statement that we make in this prospectus supplement or any related free writing prospectus is inconsistent
with statements made in the accompanying prospectus or any documents incorporated by reference into this prospectus supplement
or the related free writing prospectus, as the case may be, you should rely on the information in this prospectus supplement or
the related free writing prospectus. If any statement in one of these documents is inconsistent with a statement in another document
having a later date - for example, a document incorporated by reference in the accompanying prospectus - the statement in the document
having the later date modifies or supersedes the earlier statement.
We further note that the representations,
warranties and covenants made by us in the Amended and Restated Distribution Agreement or any other agreement that is filed as
an exhibit to any document that is incorporated by reference in this prospectus supplement and in the accompanying prospectus were
accurate only as of the date when made. Moreover, such representations, warranties and covenants should not be relied on as accurately
representing the current state of our affairs.
In this prospectus supplement, unless
otherwise expressly stated or the context otherwise requires, the terms “we,” “us,” “our,”
“Brainstorm” and the “Company” refer to Brainstorm Cell Therapeutics Inc. and our subsidiaries on a combined
basis, except that in the description of the securities offered, these terms refer solely to Brainstorm Cell Therapeutics Inc.
and not to any of our subsidiaries.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current
reports, proxy statements and other information with the SEC. We file these documents with the SEC electronically. You can access
the electronic versions of these filings on the SEC’s internet website found at http://www.sec.gov.
This prospectus supplement and the accompanying
prospectus omit some information contained in the registration statement in accordance with SEC rules and regulations. You should
review the information and exhibits included in the registration statement for further information about us and the securities
offered by us. Statements in this prospectus supplement and the accompanying prospectus concerning any document filed as an exhibit
to the registration statement or otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference
to these filings. You should review the complete document to evaluate these statements.
INCORPORATION OF CERTAIN DOCUMENTS BY
REFERENCE
The SEC’s rules allow us to “incorporate
by reference” information into this prospectus supplement and the accompanying prospectus, which means that we can disclose
important information to you by referring you to another document filed separately with the SEC. The information incorporated by
reference is deemed to be part of this prospectus supplement and the accompanying prospectus, and later information that we file
with the SEC will automatically update and supersede that information. Any statement contained in a previously filed document incorporated
by reference shall be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement
contained in this prospectus supplement modifies or replaces that statement.
We incorporate by reference, as of their
respective dates of filing, the documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c),
14 or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) between the date of this prospectus
supplement and the termination of this offering of the securities described in this prospectus supplement. We are not, however,
incorporating by reference any documents or portions thereof, whether specifically listed below or filed in the future, that are
not deemed “filed” with the SEC.
|
|
·
|
Our Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on February 18, 2020;
|
|
|
|
|
|
|
·
|
Our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2020 and June 30, 2020, filed with the SEC on May 7, 2020 and August 5, 2020;
|
|
|
·
|
Our Current Reports on Form 8-K filed with the SEC on March 6, 2020, March 13, 2020, March 31, 2020, April 3, 2020, June 26, 2020 and September 3, 2020; and
|
|
|
·
|
The description of our common stock contained in our
registration statement on Form 8-A, filed with the SEC on September 24, 2014, including any amendment or report filed for the
purpose of updating such description.
|
You may request a free copy of any of
the documents incorporated by reference in this prospectus supplement by writing or telephoning us at the following address:
Brainstorm
Cell Therapeutics Inc.
1325 Avenue of Americas, 28th Floor
New
York, NY 10019
Attention: Chief Executive Officer
(201) 488-0460
These filings and reports can also be
found on our website, located at http://www.brainstorm-cell.com, by following the links to “Investor Relations”
and “SEC Filings.”
The information contained on (or accessible
through) our website does not constitute a part of this prospectus supplement.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus supplement,
the accompanying prospectus, any free writing prospectus and the documents incorporated herein and therein by reference may contain
forward-looking statements within the meaning of the federal securities laws. These forward-looking statements are intended to
qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. All statements
other than statements of historical fact included in this prospectus supplement, the accompanying prospectus, any free writing
prospectus or the documents incorporated herein or therein by reference, are forward looking statements. The words “believe,”
“may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,”
“anticipate,” “intend,” “expect,” “plan” and similar words are intended to identify
estimates and forward-looking statements.
Our forward-looking
statements are based on our current assumptions and expectations about future events and trends, which affect or may affect our
business, strategy, operations, financial performance or prospects. Although we believe that these estimates and forward-looking
statements are based upon reasonable assumptions, they are subject to numerous known and unknown risks and uncertainties and are
made in light of information currently available to us. Many important factors may materially and adversely affect the assumptions
and expectations described in the forward-looking statements. You should read this prospectus supplement, the accompanying prospectus,
any free writing prospectus, and the documents we incorporate by reference herein and therein, completely and with the understanding
that our actual future results may be materially different and worse than what we expect.
Moreover, we operate
in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for us to predict
all risk factors and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor,
or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
We qualify all of our forward-looking statements by these cautionary statements.
The following factors,
among others, could cause our financial and operational performance to differ materially from that expressed in such forward-looking
statements:
|
|
·
|
our need to raise additional capital;
|
|
|
·
|
our ability to continue as a going concern;
|
|
|
·
|
regulatory approval of our NurOwn® treatment candidate;
|
|
|
·
|
the success of our product development programs and research;
|
|
|
·
|
regulatory and personnel issues;
|
|
|
·
|
development of a global market for our services;
|
|
|
·
|
the ability to secure and maintain research institutions to conduct our clinical trials;
|
|
|
·
|
the ability to generate significant revenue;
|
|
|
·
|
the ability of our NurOwn® treatment candidate to achieve broad acceptance as a treatment option for Amyotrophic Lateral Sclerosis (“ALS”, also known as Lou Gehrig’s disease) or other neurodegenerative diseases;
|
|
|
·
|
our ability to manufacture and commercialize our NurOwn® treatment candidate;
|
|
|
·
|
obtaining patents that provide meaningful protection;
|
|
|
·
|
competition and market developments;
|
|
|
·
|
our ability to protect our intellectual property from infringement by third parties;
|
|
|
·
|
health reform legislation;
|
|
|
·
|
demand for our services;
|
|
|
·
|
currency exchange rates;
|
|
|
·
|
product liability claims and litigation;
|
|
|
·
|
disruptions in our business (supply chains, workforce availability, disruptions in
partnered medical centers in the United States and Europe, travel restrictions) due to the novel strain of coronavirus, SARS-CoV-2
(“COVID-19”) outbreak, including our clinical development activities; and
|
|
|
·
|
other risks and uncertainties detailed from time to time in our SEC filings.
|
Estimates and forward-looking statements
speak only as of the date they were made, and, except to the extent required by law, we undertake no obligation to update or to
review any estimate and/or forward-looking statement because of new information, future events or other factors. Estimates and
forward-looking statements involve risks and uncertainties and are not guarantees of future performance. As a result of the risks
and uncertainties described herein and in our other SEC filings, the results and outcomes set forth in the forward-looking statements
discussed in this prospectus supplement, the accompanying prospectus, any free writing prospectus, and the documents incorporated
by reference herein and therein, might not occur and our future results and our performance may differ materially from those expressed
in these forward-looking statements due to, but not limited to, the factors mentioned above. Because of these uncertainties, you
should not place undue reliance on these forward-looking statements when making an investment decision.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights
selected information contained or incorporated by reference in this prospectus supplement or the accompanying prospectus and may
not contain all the information that you need to consider in making your investment decision. To understand this offering fully,
you should carefully read this prospectus supplement, the accompanying prospectus, any free writing prospectuses we have authorized
for use in connection with this offering and the documents incorporated by reference herein and therein carefully. In particular,
you should carefully read the sections titled “Risk Factors” in this prospectus supplement and in the accompanying
prospectus and the documents identified in the section “Incorporation of Certain Documents by Reference.”
Overview
Company Overview
|
|
·
|
Brainstorm Cell Therapeutics Inc. is a leading biotechnology company committed to the development
and commercialization of best-in-class autologous cellular therapies for the treatment of neurodegenerative diseases including:
Amyotrophic Lateral Sclerosis (“ALS”, also known as Lou Gehrig’s disease); Progressive Multiple Sclerosis (“PMS”);
Alzheimer's disease ("AD"); and other neurodegenerative diseases.
|
|
|
·
|
NurOwn® leverages innovative and proprietary cell culture methods to induce autologous bone marrow-derived mesenchymal
stem cells ("MSCs") to secrete high levels of neurotrophic factors ("NTFs"), modulate neuroinflammatory and
neurodegenerative disease processes, promote neuronal survival and improve neurological function.
|
|
|
·
|
Our wholly owned Israeli subsidiary, Brainstorm Cell Therapeutics Ltd. (“Israeli Subsidiary”), holds exclusive
rights to commercialize NurOwn® technology through a licensing agreement with Ramot at Tel Aviv University Ltd. (“Ramot”),
the technology transfer company of Tel Aviv University, Israel.
|
|
|
·
|
The Israeli Subsidiary was granted approval by the Israeli Ministry of Health (“MoH”)
to treat ALS patients with NurOwn® under the Hospital Exemption Pathway (“HE”).
|
|
|
·
|
NurOwn® has a strong and comprehensive intellectual property portfolio.
|
|
|
·
|
NurOwn® was granted Fast Track designation by the U.S. Food and Drug Administration (“FDA”)
and Orphan Drug status by the FDA and the European Medicines Agency (“EMA”) for ALS.
|
|
|
·
|
We have been granted Micro, Small and Medium-Sized Enterprise (“SME”) status by the
European Medicines Agency's (“EMA”) SME office.
|
|
|
·
|
We currently employ 43 employees in the United States and in Israel. Most of the senior management
team is based in the United States, and all of Brainstorm’s ongoing clinical trial sites for ALS and PMS are in the United
States. The clinical trial sites for Brainstorm's AD trial will be in Europe. Brainstorm’s R&D center is located in Petach
Tikva, Israel.
|
|
|
·
|
The outbreak of COVID-19 disease and its impact:
|
The outbreak
of COVID-19 disease has currently impacted and may continue to adversely impact our business, including our preclinical studies
and clinical trials. In December 2019, a novel strain of coronavirus, surfaced in Wuhan, China. Since then, COVID-19 has spread
to multiple countries, including the United States, Europe and Israel, where the Company conducts its operations, as well as its
clinical trials for NurOwn®. In response to the spread of COVID-19 and to ensure safety of employees and continuity of business
operations, we closed our offices in the United States and limited the number of employees working in our offices in Israel, with
our administrative employees continuing their work remotely. Though we limited the number of staff in any given research and development
laboratory, our research and development laboratory in Israel and manufacturing sites in U.S. remain open.
As of the
date of this prospectus supplement, our U.S. Phase 2 PMS clinical trial has continued with slight delays in the pace of enrollment
due to site access restrictions related to the global COVID-19 pandemic. Scheduled March and April 2020 new patient enrollments
were deferred to May 2020 due to site closures related to COVID-19. As of June 2020, all the trial sites were prepared to continue
with the trial and as of August 4, 2020, all 20 study participants have been enrolled in the study. We are currently collecting
the clinical and biomarker data from treated patients. Dosing of all participants is expected to be completed in the fourth quarter
of 2020. The Phase 3 ALS clinical trial continued to provide necessary treatments to study participants despite severe constraints
in the affected healthcare institutions due to COVID-19. As of July 2, 2020, the study completed dosing of all the participants
in the Phase 3 ALS trial. The Phase 3 ALS trial is expected to generate top-line data by the end of November 2020. We recently
announced a new clinical program focused on the development of NurOwn® as a treatment for AD. As part of the newly announced
program, we are planning a multi-national Phase 2 clinical trial in Europe to evaluate the safety and efficacy of NurOwn® treatment
in patients with prodromal to mild AD.
Recent Developments
|
|
·
|
We have made significant progress in the past 12 months advancing the NurOwn® ALS Phase 3 clinical
trial at all 6 U.S. investigative sites (Mass General Hospital, UMass, Mayo Clinic, CPMC, Cedars Sinai and UC Irvine). This clinical
trial builds upon promising efficacy seen in three prior early-stage ALS clinical trials, including a U.S. randomized placebo-controlled
Phase 2 trial. Enrollment for NurOwn® ALS Phase 3 trial was completed in October 2019 and dosing of all participants in the
trial was completed as of July 2, 2020. The trial is expected to generate top-line data by the end of November 2020 to support
an FDA Biologics License Applications (“BLA”) filing.
|
|
|
·
|
On April 3, 2020, we announced that our wholly owned subsidiary, Brainstorm Cell Therapeutics Ltd.,
has been awarded a non-dilutive grant of approximately $1.5 million by the Israel Innovation Authority. The grant enables us to
continue development of advanced cellular manufacturing capabilities, furthers the development of MSC-derived exosomes as a novel
therapeutic platform, and will ultimately enable Brainstorm to expand its therapeutic pipeline in neurodegenerative disorders.
As of June 30, 2020, we have received $583,000 out of the $1.5 million awarded.
|
|
|
·
|
On July 1, 2020, we received a non-dilutive bonus payment of $700,000 from California Institute
for Regenerative Medicine (“CIRM”) for treating more California participants than originally proposed in our Phase 3 ALS trial.
|
|
|
·
|
On July 2, 2020, we announced the completion of all dosing of participants in its NurOwn® Phase
3 ALS clinical trial.
|
|
|
·
|
On July 8, 2020, we hosted a Key Opinion Leader webinar to discuss NurOwn® Phase 2 AD Program.
The webinar featured presentations by two lead investigators in our planned European Phase 2 trial: Philip Scheltens, M.D., Ph.D.,
Professor of Cognitive Neurology and Director of the Alzheimer Centre at the VU University Medical Center in Amsterdam, Netherlands;
and Bruno Dubois, M.D., Ph.D., Professor of Neurology at the Neurological Institute of the Salpétrière University
Hospital in Paris, France.
|
|
|
·
|
On July 23, 2020, we announced a groundbreaking pre-clinical study of NurOwn® derived exosome-based
treatment for COVID-19 Acute Respiratory Disease Syndrome. Intratracheal administration of exosomes extracted from MSC’s
using NurOwn® technology resulted in statistically significant improvement in multiple lung parameters in a mouse model. With
this study, we have successfully completed our first milestone in developing an innovative exosome-based platform-technology for
the treatment severe COVID-19 infection.
|
|
|
·
|
On August 19, 2020, we announced the publication of a manuscript titled, “Effects of MSC-NTF
cells on T and B regulatory cell function in ALS” in the journal Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration.
|
|
|
·
|
On August 25, 2020, we announced the acceptance of a clinical abstract documenting an association
between magnetic resonance imaging (“MRI”) measures and functional improvement in patients with PMS. The data was presented as a
poster on September 11-13 at the eighth joint virtual meeting of the Americas Committee for Treatment and Research in Multiple
Sclerosis (“ACTRIMS”) and the European Committee for Treatment and Research in Multiple Sclerosis (“ECTRIMS”).
|
|
|
·
|
On September 2, 2020, we announced the appointment of Anthony P. Waclawski, Ph.D. as Executive
Vice President, Global Head of Regulatory Affairs to further strengthen its regulatory expertise and capabilities as ALS phase
3 clinical trial nears completion in Q4. Dr. Waclawski is an industry veteran and a recognized leader in regulatory affairs with
over 35 years of multinational experience in the FDA regulatory approval process, including BLAs, New Drug Applications (“NDAs”),
and FDA Advisory Committees.
|
|
|
·
|
On
September 16, 2020, we announced that the Japanese Patent Office (“JPO”) has granted Brainstorm's Japanese Patent,
number: 6,753,887, titled: “Methods of Generating Mesenchymal Stem Cells which Secrete Neurotrophic Factors.”
|
Prior ATM Facility
On March 6, 2020, we entered into a
Distribution Agreement (the “Prior ATM”) with Raymond James & Associates, Inc. (the “Agent”)
providing for the sale, from time to time through the Agent, of shares of Common Stock having an aggregate offering amount of
up to $50,000,000. We filed a prospectus supplement on March 6, 2020, with the “SEC” in connection with the Prior
ATM. During the quarter ended June 30, 2020, we sold an aggregate of 1,162,527 shares of Common Stock pursuant to the Prior
ATM at an average price of $6.57 per share, raising gross proceeds of approximately $7.64 million. Since its inception on
March 6, 2020 through the quarter ended June 30, 2020, we sold an aggregate of 1,499,014 shares of Common Stock at an average
price of $6.27 per share, raising gross proceeds of approximately $9.40 million. From July 1, 2020 through September 24, 2020,
the Company sold an aggregate of 947,627 additional shares of Common Stock pursuant to the Prior ATM, at an average price of
$14.48 per share, raising additional gross proceeds of approximately $13.71 million.
Corporate Information
We are incorporated under the laws of the
State of Delaware. Our principal executive offices are located at 1325 Avenue of Americas, 28th Floor, New York, NY
10019, and our telephone number is (201) 488-0460. We also maintain offices at 12 N State Route 17, Suite 201, Paramus, NJ 07652,
and in Petach Tikva, Israel. We maintain an Internet website at http://www.brainstorm-cell.com. The information contained
on (or accessible through) our website is not incorporated into this prospectus supplement or the accompanying prospectus.
THE OFFERING
|
Securities offered
|
Shares of common stock, par value $0.00005
per share, having an aggregate gross sales price of up to $45,000,000.
|
|
|
|
|
Manner of offering
|
“At the market” offerings that
may be made from time to time through SVB Leerink LLC and Raymond James & Associates, Inc. as sales agents. See “Plan
of Distribution.”
|
|
|
|
|
Use of proceeds
|
We intend to use the proceeds of this offering
to fund our operations, which includes, but is not limited to, advancing the Company's clinical programs, commercial production
of the investigational therapeutic NurOwn® (whether for ALS or other indications), regulatory, pre-marketing and commercialization
preparation activities of NurOwn® for ALS, working capital and general corporate purposes.
|
|
|
|
|
Nasdaq Capital Market symbol
|
“BCLI”
|
|
Dividend policy
|
We have not previously paid cash dividends
on our common stock. It is our current intention to invest our cash flow and earnings in the growth of our business and, therefore,
we do not plan to pay cash dividends for the foreseeable future. Investors should not purchase our common stock with the expectation
of receiving cash dividends.
|
|
|
|
|
Risk factors
|
This investment involves a high
degree of risk. See “Risk Factors” beginning on page S-10 of this prospectus supplement and other information included
or incorporated by reference herein, as well as the risks and uncertainties described in the other documents we file with the
SEC, for a discussion of factors you should carefully consider before deciding to invest in our common stock.
|
Unless otherwise indicated, the number of shares of our common
stock to be outstanding immediately after this offering as shown above is based on 29,669,855 shares of common stock outstanding
as of June 30, 2020 but excluding the following as of such date:
|
|
·
|
1,894,340 shares of common stock issuable upon the exercise of share options outstanding as of June 30, 2020 at a weighted average exercise price of $4.13 per share;
|
|
|
·
|
4,724,868 shares of common stock issuable upon the exercise of warrants outstanding as of June 30, 2020 at a weighted average exercise price of $6.29 per share; and
|
|
|
·
|
1,393,410 shares of common stock reserved for future issuance under our equity incentive plans as of June 30, 2020.
|
RISK FACTORS
An
investment in our common stock involves a high degree of risk. Before you invest in our common stock, you should carefully consider
the risk factors set forth below, those risk factors related to us and our business described in “Item 1A. Risk Factors”
in Part I of our most recent Annual Report on Form 10-K, as amended, our most recent Quarterly Report on Form 10-Q, any subsequently
filed Quarterly Reports on Form 10-Q and any subsequently filed Current Reports on Form 8-K, which are incorporated herein by reference,
and those risk factors that may be included in any applicable prospectus supplement, together with all of the other information
included in this prospectus supplement, the accompanying base prospectus and the documents we incorporate by reference, in evaluating
an investment in our common stock. If any of the risks discussed in the foregoing documents were to occur, our business, financial
condition, results of operations and cash flows could be materially adversely affected. Please read “Special Note Regarding
Forward-Looking Statements.”
Risks Relating to this Offering and an Investment in Our
Common Stock
The number of shares of our common
stock available for future sale could adversely affect the market price of our common stock.
We cannot predict
whether future issuances of shares of our common stock or the availability of shares for resale in the open market will decrease
the market price per share of our common stock. We may sell shares of our common stock under this prospectus supplement with an
aggregate gross offering price of up to $45,000,000. We may also sell additional shares of our common stock in the future under
the prospectus which accompanies this prospectus supplement or in other offerings or other acquisitions we may undertake. Sales
of substantial amounts of shares of our common stock in the public market, or the perception that such sales might occur, could
adversely affect the market price of our common stock.
Our management will have broad discretion
as to the use of proceeds from this offering. You may not agree with the manner in which we use the proceeds and our use of those
proceeds may not yield a favorable return on investment.
We intend to use
the net proceeds of this offering to advance the Company’s clinical programs and for working capital and general corporate
purposes. We have not designated the amount of net proceeds we will use for any particular purpose and our management will retain
broad discretion to allocate the net proceeds of this offering. The net proceeds may be applied in ways with which some investors
in this offering may not agree. Moreover, our management may use the net proceeds for corporate purposes that may not increase
our market value or make us more profitable. In addition, it may take us some time to effectively deploy the net proceeds from
this offering. Until the net proceeds are effectively deployed, our return on equity and earnings per share may be negatively impacted.
Management’s failure to use the net proceeds of this offering effectively could have an adverse effect on our business, financial
condition and results of operations.
Share ownership by our officers
and directors and certain agreements may make it more difficult for third parties to acquire us or effectuate a change of control
that might be viewed favorably by other stockholders.
As of August 31,
2020, our executive officers and directors beneficially owned, directly or indirectly, in the aggregate, approximately 22.1% of
our common stock. As a result, if our executive officers and directors were to oppose a third party’s acquisition proposal
for, or a change in control of, the Company, our executive officers and directors may have sufficient voting power to be able to
block or at least delay such an acquisition or change in control from taking place, even if other stockholders would support such
a sale or change of control. In addition, a number of our executive officers have change of control agreements which could increase
the costs and, therefore, lessen the attractiveness of an acquisition of the Company to a potential acquiring party.
We may sell additional shares of
common stock in the future which could result in dilution to our stockholders.
As of August
31, 2020, a total of approximately 68.4 million authorized but unissued shares of our common stock are available for future
sale and issuance by action of our board of directors alone, including sales of up to $45,000,000 in value of shares of our
common stock under this prospectus supplement. Accordingly, if we were to sell additional shares in the future, our
stockholders could suffer dilution in their investment in their shares of our common stock and in their percentage ownership
of the Company.
We may issue additional equity securities,
or engage in other transactions which could dilute our book value or affect the priority of our common stock, which may adversely
affect the market price of our common stock.
Our board of directors
may determine from time to time to raise additional capital by issuing additional shares of our common stock or other securities.
In addition, we may issue additional securities in connection with future acquisitions we may make. We are not restricted from
issuing additional shares of common stock, including securities that are convertible into or exchangeable for, or that represent
the right to receive, common stock. We cannot predict or estimate the amount, timing, or nature of any future offerings or issuances
of additional stock in connection with acquisitions, or the prices at which such offerings may be affected. Such offerings could
be dilutive to common stockholders. New investors also may have rights, preferences and privileges that are senior to, and that
adversely affect, our then-current common stockholders. Additionally, if we raise additional capital by making additional offerings
of debt or securities, upon liquidation of the Company, holders of our debt securities, and lenders with respect to other borrowings,
will receive distributions of our available assets prior to the holders of our common stock. Additional equity offerings may dilute
the holdings of our existing stockholders or reduce the market price of our common stock, or both. Holders of our common stock
are not entitled to preemptive rights or other protections against dilution.
A failure to maintain effective
internal control over financial reporting could have a material adverse effect on our business and stock prices.
Although we are not
required to obtain or include in our annual reports on Form 10-K an attestation report from our independent registered accountants
with respect to the effectiveness of our internal control over financial reporting, like all other public companies, our Chief
Executive Officer and our Chief Financial Officer are required, annually, to assess, and disclose their findings in our annual
reports on Form 10-K with respect to, the effectiveness of our internal control over financial reporting in a manner that meets
the requirements of Section 404(a) of the Sarbanes-Oxley Act. The rules governing the standards that must be met for our Chief
Executive and Chief Financial Officers to assess and report on the effectiveness of our internal control over financial reporting
are complex and require significant documentation, testing and possible remediation, which could significantly increase our operating
expenses.
Additionally, if
we are unable to maintain the effectiveness of our internal control over financial reporting in the future, we may be unable to
report our financial results accurately and on a timely basis. In such an event, investors and clients may lose confidence in the
accuracy and completeness of our financial statements, as a result of which our liquidity, access to capital markets, and perceptions
of our creditworthiness could be adversely affected and the market prices of our common stock could decline. In addition, we could
become subject to investigations by the Nasdaq Capital Market, the SEC or other regulatory authorities, which could require us
to expend additional financial and management resources. As a result, an inability to maintain the effectiveness of our internal
control over financial reporting in the future could have a material adverse effect on our business, financial condition, results
of operations and prospects.
If securities or industry analysts
do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could
decline.
The trading market
for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our
business. If one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our
business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish
reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
The shares of our common stock offered
under this prospectus supplement and the accompanying base prospectus may be sold in "at-the-market" offerings, and investors
who buy shares at different times will likely pay different prices.
Investors who purchase
shares under this prospectus supplement and the accompanying base prospectus at different times will likely pay different prices,
and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary
the timing, prices, and numbers of shares sold, and to determine the minimum sales price for shares sold. Investors may experience
declines in the value of their shares as a result of share sales made in connection with "at-the-market" offerings at
prices lower than the prices they paid.
The actual number of shares we will
issue under the distribution agreement, at any one time or in total, is uncertain.
Subject to certain
limitations in the Amended and Restated Distribution Agreement and compliance with applicable law, we and the Distribution Agents
may mutually agree to sell shares of our common stock under a transaction acceptance at any time throughout the term of the Amended
and Restated Distribution Agreement. The number of shares that are sold by the Distribution Agents after agreement on the terms
of the transaction acceptance will fluctuate based on the market price of the shares of our common stock during the sales period
and limits we set with the Distribution Agents. Because the price per share of each share sold will fluctuate based on the market
price of our shares of common stock during the sales period, it is not possible to predict the number of shares that will ultimately
be issued.
We do not anticipate paying any cash
dividends on our common stock in the foreseeable future. As a result, you will need to sell your shares of common stock to receive
any income or realize a return on your investment.
To date, we have not
paid any cash dividends on our common stock. We do not anticipate paying any cash dividends on our common stock in the foreseeable
future. We cannot assure you that we would, at any time, generate sufficient surplus cash that would be available for distribution
to the holders of our common stock as a dividend.
USE OF PROCEEDS
We may issue and
sell shares of our common stock having aggregate sale proceeds of up to $45,000,000 from time to time. The amount of proceeds from
this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold. There
can be no assurance that we will be able to sell any shares under or fully utilize the Amended and Restated Distribution Agreement
with the Distribution Agents as a source of financing. Because there is no minimum offering amount required as a condition to close
this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time.
We intend to use
the proceeds of this offering to fund our operations, which includes, but is not limited to advancing the Company’s clinical
programs, commercial production of the investigational therapeutic NurOwn® (whether for ALS or other indications), regulatory,
pre-marketing and commercialization preparation activities of NurOwn® for ALS, working capital and general corporate purposes.
Our expected use of the net proceeds from this offering is based upon our present plans and business condition. As of the date
of this prospectus supplement, we cannot predict with certainty all of the particular uses for the net proceeds to be received
upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing
of our actual use of proceeds will vary depending on numerous factors, including the factors described under the heading “Risk
Factors” beginning on page S-10 and under the heading “Risk Factors” in the prospectus accompanying this prospectus
supplement and our other SEC filings. As a result, management will retain broad discretion over the allocation of the net proceeds
from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds.
DILUTION
If you invest in this
offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price
per share and the as-adjusted net tangible book value per share of our common stock after giving effect to this offering. We calculate
net tangible book value per share by dividing the net tangible book value, which is tangible assets less total liabilities, by
the number of outstanding shares of our common stock. Dilution represents the difference between the amount per share paid by purchasers
of shares in this offering and the net tangible book value per share of our common stock immediately after giving effect to this
offering. Our net tangible book value as of June 30, 2020 was approximately ($9.82 million) or ($0.33) per share.
After giving effect
to the sale of our common stock pursuant to this prospectus supplement and accompanying prospectus in the aggregate amount of $45.0
million at an assumed offering price of $15.09 per share (the last reported sale price of our common stock on September 18, 2020),
and after deducting commissions and estimated aggregate offering expenses payable by us, our net tangible book value as of June
30, 2020 would have been $53.47 million, or $1.64 per share of common stock. This represents an immediate increase in the net tangible
book value of $1.31 per share to our existing stockholders and an immediate dilution in net tangible book value of $13 per share
to investors participating in this offering. The following table illustrates this per share dilution:
|
Assumed offering price per share
|
|
|
|
|
|
$
|
15.09
|
|
|
Net tangible book value per share as of June 30, 2020
|
|
$
|
0.33
|
|
|
|
|
|
|
Increase in net tangible book value per share attributable to this offering
|
|
$
|
1.31
|
|
|
|
|
|
|
As-adjusted net tangible book value per share as of June 30, 2020 after giving effect to this offering
|
|
|
|
|
|
$
|
1.64
|
|
|
Dilution per share to new investors purchasing shares in this offering
|
|
|
|
|
|
$
|
13.00
|
|
The table above assumes for illustrative
purposes that an aggregate of 2,982,107 shares of our common stock are sold pursuant to this prospectus supplement and the accompanying
prospectus at a price of $15.09 per share (the last reported sale price of our common stock on September 18, 2020), for aggregate
gross proceeds of $45.0 million. The shares sold in this offering, if any, will be sold from time to time at various prices. An
increase of $1.00 per share in the price at which the shares are sold from the assumed offering price to $16.09 per share, assuming
all of our common stock in the aggregate amount of $45.0 million is sold at that price, would result in an adjusted net tangible
book value per share after the offering of $1.65 per share and would increase the dilution in net tangible book value per share
to new investors in this offering to $13.96 per share, after deducting underwriting commissions and estimated aggregate offering
expenses payable by us. A decrease of $1.00 per share in the price at which the shares are sold from the assumed offering price
to $14.09 per share, assuming all of our common stock in the aggregate amount of $45.0 million is sold at that price, would result
in an adjusted net tangible book value per share after the offering of $1.63 per share and would decrease the dilution in net tangible
book value per share to new investors in this offering to $12.04 per share, after deducting underwriting commissions and estimated
aggregate offering expenses payable by us. This information is supplied for illustrative purposes only.
The above discussion and table are based on 29,669,855 shares
of our common stock issued and outstanding as of June 30, 2020 and excludes the following:
|
|
·
|
1,894,340 shares of common stock issuable upon the exercise of share options outstanding as of June 30, 2020 at a weighted average exercise price of $4.13 per share;
|
|
|
·
|
4,724,868 shares of common stock issuable upon the exercise of warrants outstanding as of June 30, 2020 at a weighted average exercise price of $6.29 per share; and
|
|
|
·
|
1,393,410 shares of common stock reserved for future issuance under our equity incentive plans as of June 30, 2020.
|
DIVIDEND POLICY
We have not declared
or paid any cash dividends on our capital stock since our inception. We currently intend to retain future earnings, if any, to
finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future.
Payment of future dividends, if any, will be at the discretion of our board of directors and will depend on our financial condition,
results of operations, capital requirements, restrictions contained in current or future financing instruments, provisions of applicable
law and other factors the board deems relevant.
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS
FOR
NON-U.S. HOLDERS OF COMMON STOCK
The following is a general discussion of certain material U.S.
federal income tax consequences of the ownership and disposition of our common stock by a beneficial owner that is a "Non-U.S.
Holder." A Non-U.S. Holder means a beneficial owner of our common stock that, for U.S. federal income tax purposes, is:
|
|
·
|
a non-resident alien individual;
|
|
|
·
|
a foreign corporation or any other foreign organization taxable
as a corporation for U.S. federal income tax purposes; or
|
|
|
·
|
a foreign estate or trust, the income of which is not subject to U.S. federal
income tax on a net income basis.
|
If an entity that is
classified as a partnership for U.S. federal income tax purposes holds our common stock, the U.S. federal income tax treatment
of a partner will generally depend on the status of the partner and the activities of the partnership. Partnerships holding our
common stock and partners in such partnerships are urged to consult their own tax advisers regarding the particular U.S. federal
income tax consequences of holding and disposing of our common stock.
This discussion is based on the Internal
Revenue Code of 1986, as amended (the "Code"), and administrative pronouncements, judicial decisions and final, temporary
and proposed Treasury Regulations, any of which may be changed subsequent to the date of this prospectus supplement, possibly retroactively,
so as to result in U.S. federal income tax consequences different from those discussed below. We have not sought an opinion of
counsel with respect to the statements made and conclusions reached in this discussion. This discussion does not address all aspects
of U.S. federal income taxation that may be relevant to Non-U.S. Holders in light of their particular circumstances, including
alternative minimum tax and Medicare contribution tax consequences. It does not address any tax consequences arising under U.S.
federal estate or gift tax laws or under the laws of any state, local or foreign jurisdiction and is limited to Non-U.S. Holders
that hold our common stock as a capital asset within the meaning of Section 1221 of the Code.
This discussion also does not consider any
specific facts or circumstances that may apply to a Non-U.S. holder and does not address the special tax rules
applicable to particular Non-U.S. holders, such as:
|
|
·
|
Non-U.S. Holders that own, or have owned, actually or constructively, more than 5% of our common stock,
|
|
|
·
|
tax-exempt, governmental or international organizations;
|
|
|
·
|
financial institutions;
|
|
|
·
|
brokers or dealers in securities;
|
|
|
·
|
regulated investment companies;
|
|
|
·
|
“controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate
earnings to avoid U.S. federal income tax;
|
|
|
·
|
“qualified foreign pension funds” as defined in Section 897(I)(2) of the Code and entities all of the interests
of which are held by qualified foreign pension funds;
|
|
|
·
|
persons that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated
investment;
|
|
|
·
|
persons subject to special tax accounting rules as a result of any item of gross income with respect to our common stock being
taken into account in an applicable financial statement; and
|
|
|
·
|
former citizens or long-term residents of the United States.
|
You are urged to consult your tax adviser
with respect to the particular tax consequences to you of owning and disposing of our common stock, including the consequences
under the laws of any state, local or foreign jurisdiction.
Dividends
In general, any distribution we make to
a Non-U.S. Holder with respect to our common stock that constitutes a dividend for U.S. federal income tax purposes will be subject
to withholding tax at a rate of 30% of the gross amount, unless the Non-U.S. Holder is eligible for a reduced rate of withholding
tax under an applicable income tax treaty and the Non-U.S. Holder provides certification of its eligibility for such reduced rate
on a properly completed applicable Internal Revenue Service ("IRS") Form W-8. A Non-U.S. Holder that is eligible for
a reduced rate of withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by filing
an appropriate claim for refund with the IRS. A distribution will constitute a dividend under U.S. federal income tax principles
to the extent of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes. Any distribution
not constituting a dividend will reduce the Non-U.S. Holder's adjusted basis in our common stock and, to the extent it exceeds
the holder's adjusted basis, will be treated as gain from the sale or exchange of such shares.
If a Non-U.S. Holder is engaged in a trade
or business in the United States, and if dividends paid to the Non-U.S. Holder are effectively connected with the conduct of this
trade or business (and, if an applicable income tax treaty provides, the dividend is attributable to a permanent establishment
or fixed base maintained by the Non-U.S. Holder in the United States), the Non-U.S. Holder will not be subject to the withholding
tax discussed above if certain certification requirements are satisfied but instead will generally be taxed in the same manner
as a U.S. person who holds our common stock. A Non-U.S. Holder can generally satisfy the certification requirements by delivering
a properly executed IRS Form W-8ECI to claim an exemption from the withholding tax to the withholding agent. A corporate Non-U.S.
Holder receiving effectively connected dividends may also be subject to an additional "branch profits tax" imposed at
a rate of 30% (or a lower treaty rate if applicable). A Non-U.S. Holder may obtain a refund of any excess amounts withheld under
these rules if the Non-U.S. Holder is eligible for a reduced rate of United States withholding tax and an appropriate claim for
refund is timely filed with the IRS.
Gain on Disposition of Common
Stock
Subject to the discussion below regarding
backup withholding, a Non-U.S. Holder will generally not be subject to U.S. federal income tax on the gain realized on a sale or
other disposition of our common stock unless:
• the gain is effectively connected with a trade
or business of the Non-U.S. Holder in the United States and, if an applicable income tax treaty provides, the gain is attributable
to a permanent establishment maintained by the Non-U.S. Holder in the United States;
• the Non-U.S. Holder is an individual present
in the U.S. for an aggregate of 183 days or more during the taxable year of the disposition and meets certain other conditions;
or
• We are or were a "United States real property
holding corporation," as defined in the Code, at any time within the five-year period preceding the disposition or the Non-U.S.
Holder's holding period, whichever period is shorter, and our common stock constitutes a "United States real property interest".
If the gain is described in the first bullet
point above, the Non-U.S. Holder will generally be taxed in the same manner as a U.S. person on the net gain from the disposition
of the common stock, unless an applicable tax treaty provides otherwise. A corporate Non-U.S. Holder whose gain from dispositions
of our common stock may be effectively connected with its conduct of a trade or business in the United States may also be subject
to an additional "branch profits tax" imposed at a rate of 30% (or a lower treaty rate if applicable). Non-U.S. Holders
whose gain from dispositions of our common stock may be effectively connected with a trade or business in the United States are
urged to consult their own tax advisers with respect to the U.S. federal income tax consequences of the ownership and disposition
of our common stock, including the possible imposition of a branch profits tax in the case of a corporate Non-U.S. Holder.
If the gain is described in the second bullet
point above, the Non-U.S. Holder will generally be taxed at a flat rate of 30% (or a lower treaty rate if applicable) on its gain
derived from the sale or other disposition, which may be offset by U.S.-source losses from sales or exchanges of other capital
assets recognized during the year.
With respect to the third bullet point,
we believe that we are not, and we do not anticipate becoming, a United States real property holding corporation. However, if we
were to become a United States real property holding corporation, any gain recognized on a disposition of our common stock by a
Non-U.S. Holder that did not own (actually or constructively) more than 5% of our common stock at any time during the shorter of
the period the Non-U.S. Holder held the shares or the five-year period ending on the date of disposition would not be subject to
U.S. federal income tax, provided that our common stock is "regularly traded on an established securities market".
Information Reporting Requirements
and Backup Withholding
Information returns will be filed with the
IRS in connection with payments of dividends and the proceeds from a sale or other disposition of our common stock. These information
reporting requirements apply regardless of whether withholding was reduced or eliminated by an applicable tax treaty or withholding
was not required because the dividends were effectively connected with a trade or business in the United States conducted by the
Non-U.S. Holder. Copies of such information returns may also be made available to the tax authorities of the country in which a
Non-U.S. Holder resides under the provisions of an applicable income tax treaty or other agreement with such tax authorities.
U.S. backup withholding will generally apply
to the payment of dividends to Non-U.S. Holders unless such Non-U.S. Holders furnish to the payor a Form W-8BEN or Form W-8BEN-E
(or other applicable form) or otherwise establish an exemption. Backup withholding is not an additional tax. The amount of any
backup withholding from a payment to a Non-U.S. Holder will generally be allowed as a credit against such holder's U.S. federal
income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the
IRS.
You are urged to consult your tax adviser
regarding the application of backup withholding and information reporting in your particular circumstances.
Withholdable Payments to Foreign
Financial Entities and Certain Other Foreign Entities
The Foreign Account Tax Compliance Act ("FATCA")
imposes a 30% U.S. withholding tax on certain payments, including dividends on our common stock, made to a foreign entity if such
entity fails to satisfy certain disclosure and reporting rules that generally require (i) in the case of a foreign financial institution,
that the entity identify and provide information in respect of financial accounts with such entity held (directly or indirectly)
by U.S. persons and U.S.-owned foreign entities, and (ii) in the case of a non-financial foreign entity, that the entity identify
and provide information in respect of substantial U.S. owners of such entity. While existing Treasury Regulations would also require
withholding on payments of gross proceeds of the sale or other disposition of our common stock, recently proposed Treasury Regulations
eliminate this requirement, and the Proposed Regulations may be relied upon by taxpayers until they are finalized. Various other
requirements and exceptions are provided under FATCA and additional requirements and exceptions may be provided in subsequent guidance.
In addition, the United States has entered into (and may enter into additional) intergovernmental agreements ("IGAs")
with foreign governments relating to the implementation of, and information sharing under, FATCA. IGAs may alter one or more of
the FATCA information reporting rules.
You should consult your tax adviser regarding
the possible impact of these rules on your investment in our common stock, and the entities through which you hold our common stock,
including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of this
30% withholding tax under FATCA.
PLAN OF DISTRIBUTION
We have entered into an Amended and Restated
Distribution Agreement under which we may issue and sell our common stock having an aggregate sales price of up to $45,000,000
from time to time through or to SVB Leerink and Raymond James together the Distribution Agents. Sales of our common stock, if any,
may be made by means of ordinary brokers' transactions on the Nasdaq Capital Market or otherwise at market prices, in block transactions
or as otherwise agreed with the Distribution Agents.
The Distribution Agents, as sales agents,
are not required to sell any specific number or dollar amount of common stock but will use commercially reasonable efforts, consistent
with their normal trading and sales practices, to solicit offers to purchase the common stock upon entering into a transaction
notice with us that will specify the number of common stock to be sold and such other matters as may be agreed upon by us and the
Distribution Agents. Subject to the terms and conditions of the Amended and Restated Distribution Agreement, the Distribution Agents
will use commercially reasonable efforts, consistent with their normal trading and sales practices, to sell on our behalf all of
the designated common stock pursuant to the terms agreed to with us, which terms will include the number of common stock to be
offered and any minimum price below which sales may not be made. The obligation of the Distribution Agents under the Amended and
Restated Distribution Agreement to sell shares pursuant to any transaction notice is subject to a number of conditions, which the
Distribution Agents reserves the right to waive in their sole discretion.
The Distribution Agents, in their capacity
as sales agents, may arrange for or make sales at the market in the existing trading market for our common stock, including sales
made to or through a market maker or through an electronic communications network, or in any other manner that may be deemed to
be an “at-the-market” offering as defined in Rule 415 promulgated under the Securities Act. If agreed to in a separate
terms agreement, we may also sell common stock to each of the Distribution Agents as principals, at a purchase price agreed upon
by the Distribution Agents and us. We or the Distribution Agents may suspend the offering of common stock upon notice and subject
to other conditions.
We will pay the Distribution Agents a commission
equal to 3.0% of the gross sales price of any such shares sold through them as sales agents, or purchased by them as principals,
as set forth in the Amended and Restated Distribution Agreement. The remaining sales proceeds, after deducting any transaction
fees imposed by any governmental or self-regulatory organization in connection with the sales, will equal our net proceeds for
the sale of the common stock. We also have agreed to reimburse the Distribution Agents for all fees and expenses of counsel for
the Distribution Agents, of up to $50,000, as provided in the Amended and Restated Distribution Agreement.
Settlement for sales of common stock will
occur on the second business day following the date on which any sales are made in return for payment of the net proceeds to us.
There is no arrangement for funds to be received in an escrow, trust or similar arrangement. We will report at least quarterly
the number of shares of common stock sold through the Distribution Agents, as agents, in at-the-market offerings and the gross
and net proceeds to us.
For certain periods before and after any sale transactions under the Amended and Restated Distribution Agreement, we will not offer to sell, sell, contract to sell, grant any option to sell or dispose
of, directly or indirectly, any of our common stock or any securities convertible into or exercisable, redeemable or exchangeable
for such shares or (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences
of ownership of such shares, whether any such transaction described in clause (i) or, without the prior written consent of the Distribution Agents, other
than the shares to be sold pursuant to the Amended and Restated Distribution Agreement and shares issued upon the exercise or conversion
of any of our securities, convertible securities, options or rights outstanding at the beginning of such period or any grants of
options or awards or securities issued pursuant to existing stock-based compensation plans.
EXPERTS
The financial statements of the Company
as included in our Annual Report on Form 10-K for the year ended December 31, 2019, are incorporated herein by reference in reliance
on the report of Brightman, Almagor Zohar & Co., Certified Public Accountants, a firm in the Deloitte Global Network, an independent
registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
LEGAL MATTERS
The validity of the securities being offered
hereby will be passed upon by our legal counsel, Goodwin Procter LLP, Boston, Massachusetts. Certain legal matters will be passed
upon for the Distribution Agents by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, N.Y.
PROSPECTUS
BRAINSTORM CELL
THERAPEUTICS INC.
$100,000,000
Common Stock
Warrants
Units
This prospectus relates to common stock, warrants and units
that we may sell from time to time in one or more offerings up to a total dollar amount of $100,000,000 on terms to be determined
at the time of sale. We will provide specific terms of these securities in supplements to this prospectus. You should read this
prospectus and any supplement carefully before you invest. This prospectus may not be used to offer and sell securities unless
accompanied by a prospectus supplement for those securities.
Our common stock is traded on the Nasdaq Capital Market under
the symbol “BCLI.” On June 7, 2018, the last reported sales price of our common stock was $4.71 per share.
These securities may be sold directly by us, through dealers
or agents designated from time to time, to or through underwriters or through a combination of these methods. See “Plan of
Distribution” in this prospectus. We may also describe the plan of distribution for any particular offering of these securities
in any applicable prospectus supplement. If any agents, underwriters or dealers are involved in the sale of any securities in respect
of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements with them in a prospectus
supplement. The net proceeds we expect to receive from any such sale will also be included in a prospectus supplement.
Investing in our securities involves a high degree of risk.
Beginning on page 2, we discuss several “Risk Factors” that you should consider before investing in our securities.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
The date of this prospectus is June 29,
2018.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we
filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process. Under this shelf
registration process, we may sell any combination of the securities described in this prospectus in one or more offerings up to
a total dollar amount of $100,000,000. This prospectus provides you with a general description of the securities we may offer.
Each time we sell securities, we will provide a prospectus supplement that will contain specific information about the securities
being offered and the terms of that offering. The prospectus supplement may also add to, update or change information contained
in this prospectus. You should read both this prospectus and any prospectus supplement together with the additional information
described under the heading “Where You Can Find More Information” carefully before making an investment decision.
You should rely only on the information contained or incorporated
by reference in this prospectus or any applicable prospectus supplement. We have not authorized any other person to provide you
with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are
not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted.
You should not assume that the information appearing in this
prospectus or any applicable prospectus supplement is accurate as of any date other than the date on the front cover of this prospectus
or the applicable prospectus supplement, or that the information contained in any document incorporated by reference is accurate
as of any date other than the date of the document incorporated by reference, regardless of the time of delivery of this prospectus
or any prospectus supplement or any sale of a security. Our business, financial condition, results of operations and prospects
may have changed since such dates.
Unless the context otherwise requires, the terms “Brainstorm,”
“the Company,” “our company,” “we,” “us,” “our” and similar names refer
collectively to Brainstorm Cell Therapeutics Inc. and its subsidiaries.
ABOUT BRAINSTORM CELL THERAPEUTICS INC.
Brainstorm Cell Therapeutics Inc. is a biotechnology company
committed to bring innovative Central Nervous System (“CNS”) adult stem cell therapies to the market to improve the
lives of patients with debilitating neurodegenerative diseases. As a leader in CNS regenerative cellular medicines, Brainstorm
is leveraging NurOwn®, its proprietary autologous mesenchymal stem cell platform technology, a strong and expanded IP portfolio,
as well as manufacturing and commercialization capabilities, to address growing unmet medical needs across a broad range of neurodegenerative
disorders, such as Amyotrophic Lateral Sclerosis (“ALS”, also known as Lou Gehrig’s disease), Multiple Sclerosis
(“MS”), Parkinson’s disease (“PD”) and Autism Spectrum Disorders (“ASD”). The NurOwn®
proprietary technology is fully licensed to and developed by Brainstorm Cell Therapeutics Ltd., our wholly-owned subsidiary (the
“Israeli Subsidiary”).
NurOwn® technology is based on an innovative
manufacturing protocol, which induces the differentiation of bone marrow-derived mesenchymal stem cells (“MSC”) into
cells capable of releasing high levels of multiple neurotrophic factors (“MSC-NTF” cells) for neuroprotection while
maintaining the immunodulatory effects of MSC cells. These factors are known to be critical for the growth, survival and differentiation
of neurons, they include: glial-derived neurotrophic factor (“GDNF”); brain-derived neurotrophic factor (“BDNF”);
vascular endothelial growth factor (“VEGF”); and hepatocyte growth factor (“HGF”). GDNF is one of
the most potent survival factors known for peripheral neurons. VEGF and HGF have been demonstrated to have important neuro-protective
effects in ALS and in other neurodegenerative diseases.
Our approach to the treatment of neurodegenerative
diseases with autologous adult stem cells includes a multi-step process that includes: harvesting of undifferentiated stem cells
from the patient's own bone marrow; processing of cells at the manufacturing site and cryopreservation to enable multiple treatments
from a single bone marrow sample; and intrathecal (“IT”) injection of MSC-NTF cells into the same patient by standard
lumbar puncture. This procedure does not require hospitalization and has been shown to be safe and well tolerated in multiple CNS
clinical trials to date. The ongoing US Phase 3 ALS study is evaluating the therapeutic potential of repeated dosing (every
2 months). The proprietary technology and manufacturing processing of NurOwn® (MSC-NTF cells) for clinical use is conducted
in full compliance with current Good Manufacturing Practice (“cGMP”).
The NurOwn® Transplantation Process includes:
|
|
·
|
Bone marrow aspiration from patient;
|
|
|
·
|
Isolation and propagation of the mesenchymal stem cells (MSC);
|
|
|
·
|
Cryopreservation of MSC;
|
|
|
·
|
Thawing and differentiation of the MSC into neurotrophic-factor secreting (MSC-NTF; NurOwn®) cells; and
|
|
|
·
|
Autologous transplantation into the patient’s cerebrospinal fluid by IT injection (lumbar puncture).
|
The ability to induce differentiation of
autologous adult mesenchymal stem cells into MSC-NTF cells before transplantation is unique to NurOwn®, making
it the first-of-its-kind for the treatment of neurodegenerative diseases.
The specialized MSC-NTF cells secrete multiple
neurotrophic factors that may lead to:
|
|
·
|
Protection of existing motor neurons;
|
|
|
·
|
Promotion of motor neuron repair; and
|
|
|
·
|
Re-establishment of functional nerve-muscle interaction.
|
The NurOwn® approach is autologous,
using the patient’s own bone-marrow derived stem cells for “self-transplantation”. In autologous transplantation,
there is no risk of rejection or introduction of donor antigens and no need for treatment with immunosuppressive agents, which
can cause severe and/or long-term side effects. In addition, the use of adult stem cells is free of ethical controversies associated
with the use of embryonic-derived stem cells in some countries.
NurOwn® is currently in a Phase 3 late
stage clinical development program for the treatment of ALS. It has been granted Fast Track designation by the U.S. Food and Drug
Administration (“FDA”) for this indication, and has been granted Orphan Status, which provides the potential for an
extended period of exclusivity, in both the United States and in Europe. We have completed two early stage clinical trials of NurOwn®
in patients with ALS at the Hadassah Medical Center (“Hadassah”) in Jerusalem as well as a Phase 2 double-blind,
placebo-controlled, clinical study at three prestigious US medical centers, all highly experienced in the management and investigation
of ALS.
We are incorporated under the laws of the State of Delaware.
Our principal executive offices are located at 1745 Broadway, 17th Floor, New York, NY 10019, and our telephone number is (201) 488-0460.
We maintain a website at http://www.brainstorm-cell.com. The information on our website is not incorporated by reference
into this prospectus and should not be considered to be part of this prospectus.
RISK FACTORS
Investing in our securities involves significant risks. Please
see the risk factors under the heading “Risk Factors” in our most recent Annual Report on Form 10-K, as revised or
supplemented by our Quarterly Reports on Form 10-Q filed with the SEC since the filing of our most recent Annual Report on Form
10-K, each of which are on file with the SEC and are incorporated by reference in this prospectus. Before making an investment
decision, you should carefully consider these risks as well as other information we include or incorporate by reference in this
prospectus and any prospectus supplement. The risks and uncertainties we have described are not the only ones facing our company.
Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business
operations.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements
and other information with the SEC. You may read and copy any materials we have filed with the SEC at the SEC’s Public Reference
Room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by
calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements,
and other information regarding issuers that file electronically with the SEC. Our SEC filings are also available to you on the
SEC’s Internet site at www.sec.gov.
This prospectus is part of a registration statement that we
filed with the SEC. This prospectus does not contain all of the information included in the registration statement, including certain
exhibits and schedules. You can obtain a copy of the registration statement and exhibits from the SEC at the address listed above
or from the SEC’s Internet site.
Our Internet address is www.brainstorm-cell.com. The
information on our Internet website is not incorporated by reference in this prospectus or any prospectus supplement.
SPECIAL NOTE REGARDING FORWARD-LOOKING
INFORMATION
This prospectus and each prospectus supplement includes and
incorporates forward-looking statements within the meaning of the federal securities laws. These forward-looking statements are
based on management’s beliefs and assumptions. In addition, other written or oral statements that constitute forward-looking
statements are based on current expectations, estimates and projections about the industry and markets in which we operate and
statements may be made by or on our behalf. Words such as “may,” “will,” “should,” “could,”
“expects,” “hopes,” “anticipates,” “believes,” “intends,” “plans,”
“estimates,” “predicts,” “likely,” “potential,” or “continue” or the
negative of any of these terms or similar words and expressions are intended to identify such forward-looking statements. These
statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult
to predict. There are a number of important factors that could cause our actual results to differ materially from those indicated
by such forward-looking statements. Forward-looking statements include, but are not limited to, statements about potential
future business operations and performance, including statements regarding the market potential for treatment of neurodegenerative
disorders such as ALS, the sufficiency of our existing capital resources for continuing operations in 2018, the safety and clinical
effectiveness of our NurOwn® technology, our clinical trials of NurOwn® and its related clinical development, and our ability
to develop collaborations and partnerships to support our business plan. These statements reflect our views with respect to future
events as of the date of this prospectus and any accompanying prospectus supplement and are based on assumptions and subject to
risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These
forward-looking statements represent our estimates and assumptions only as of the date of this prospectus and any accompanying
prospectus supplement and, except as required by law, we undertake no obligation to update or review publicly any forward-looking
statements, whether as a result of new information, future events or otherwise after the date of this prospectus and any accompanying
prospectus supplement. We anticipate that subsequent events and developments will cause our views to change. We have included important
factors in the cautionary statements included or incorporated in this prospectus and any accompanying prospectus supplement, particularly
under the heading “Risk Factors,” that we believe could cause actual results or events to differ materially from the
forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions,
merger, dispositions, joint ventures or investments we may undertake. We qualify all of our forward-looking statements by these
cautionary statements.
INCORPORATION OF CERTAIN INFORMATION
BY REFERENCE
The SEC allows us to “incorporate” into this prospectus
information and reports that we file with the SEC. This means that we can disclose important information to you by referring to
other documents that contain that information. Any information that we incorporate by reference is considered part of this prospectus.
The documents and reports that we list below are incorporated by reference into this prospectus, other than any portion of any
such documents that are not deemed “filed” under the Exchange Act in accordance with the Exchange Act and applicable
SEC rules.
In addition, all documents and reports which we file pursuant
to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the termination of
the offering made hereby are incorporated by reference in this prospectus as of the respective filing dates of these documents
and reports.
We have filed the following documents with the SEC. These documents
are incorporated herein by reference as of their respective dates of filing:
|
|
(2)
|
Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018;
|
|
|
(4)
|
All of our filings pursuant to the Exchange Act after the date of filing the initial registration statement and prior to the effectiveness of the registration statement; and
|
|
|
(5)
|
The description of our common stock contained in our Registration Statement on Form 8-A filed on September 24, 2014, including any amendments or reports filed for the purpose of updating such description.
|
You may request a copy of these documents, which will be provided
to you at no cost, by writing or telephoning us at:
Brainstorm
Cell Therapeutics Inc.
1745 Broadway, 17th Floor
New
York, NY 10019
Attention: Chief Executive Officer
(201) 488-0460
Statements contained in documents that we file with the SEC
and that are incorporated by reference in this prospectus will automatically update and supersede information contained in this
prospectus, including information in previously filed documents or reports that have been incorporated by reference in this prospectus,
to the extent the new information differs from or is inconsistent with the old information. Any statement so modified or superseded
will not be deemed to be a part of this prospectus or any prospectus supplement, except as so modified or superseded. Because information
that we later file with the SEC will update and supersede previously incorporated information, you should look at all of the SEC
filings that we incorporate by reference to determine if any of the statements in this prospectus or any prospectus supplement
or in any documents previously incorporated by reference have been modified or superseded.
USE OF PROCEEDS
We currently intend to use the estimated net proceeds from the
sale of these securities for general corporate purposes, which may include the following:
|
|
·
|
the research, development and clinical trials for our treatments;
|
|
|
·
|
pursuing growth initiatives;
|
|
|
·
|
any other purpose that we may specify in any prospectus supplement.
|
We have not yet determined the amount of net proceeds to be
used specifically for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility
in applying the net proceeds from the sale of these securities. Pending any use, as described above, we intend to invest the net
proceeds in high-quality, short-term, interest-bearing securities. Our plans to use the estimated net proceeds from the sale of
these securities may change, and if they do, we will update this information in a prospectus supplement.
THE SECURITIES WE MAY OFFER
The descriptions of the securities contained in this prospectus,
together with the applicable prospectus supplements, summarize the material terms and provisions of the various types of securities
that we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular terms of
the securities offered by that prospectus supplement. If we so indicate in the applicable prospectus supplement, the terms of the
securities may differ from the terms we have summarized below. We will also include in the prospectus supplement information, where
applicable, about material United States federal income tax considerations relating to the securities, and the securities exchange,
if any, on which the securities will be listed.
We may sell from time to time, in one or more offerings:
|
|
·
|
warrants to purchase common stock or units;
|
|
|
·
|
units comprised of common stock and warrants; or
|
|
|
·
|
any combination of the foregoing securities.
|
In this prospectus, we refer to the common stock, warrants and
units collectively as “securities.” The total dollar amount of all securities that we may issue will not exceed $100,000,000.
This prospectus may not be used to consummate a sale of securities
unless it is accompanied by a prospectus supplement.
DESCRIPTION OF COMMON STOCK
The following is a summary of all material characteristics of
our common stock as set forth in our certificate of incorporation and bylaws. The summary does not purport to be complete and is
qualified in its entirety by reference to our certificate of incorporation and bylaws, and, to the extent applicable, to the provisions
of the Delaware General Corporation Law.
We are authorized to issue 100,000,000 shares of common
stock, $0.00005 par value. As of June 7, 2018, there were 20,249,526 shares of our common stock issued and
outstanding, held by approximately 43 record holders.
The holders of common stock are entitled to one vote per share
on all matters to be voted upon by stockholders, including the election of directors. The holders of common stock do
not have any cumulative voting, conversion, redemption or preemptive rights. The holders of common stock are entitled
to receive ratably dividends as may be declared from time to time by our Board of Directors out of funds legally available for
that purpose. In the event of our liquidation, dissolution, or winding up, the holders of common stock are entitled
to share ratably in our assets available for distribution to such holders. All issued and outstanding shares of common
stock are fully paid and non-assessable.
Anti-Takeover Provisions of Delaware Law
We are subject to Section 203 of the Delaware General Corporation
Law, which prohibits a publicly-held Delaware corporation from engaging in a “business combination,” except under certain
circumstances, with an “interested stockholder” for a period of three years following the date such person became an
“interested stockholder” unless:
|
|
·
|
before such person became an interested stockholder, the board of directors of the corporation approved either the business combination or the transaction that resulted in the interested stockholder becoming an interested stockholder;
|
|
|
·
|
upon the consummation of the transaction that resulted in the interested stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding shares held by directors who also are officers of the corporation and shares held by employee stock plans; or
|
|
|
·
|
at or following the time such person became an interested stockholder, the business combination is approved by the board of directors of the corporation and authorized at a meeting of stockholders by the affirmative vote of the holders of 66 2/3% of the outstanding voting stock of the corporation which is not owned by the interested stockholder.
|
The term “interested stockholder” generally is defined
as a person who, together with affiliates and associates, owns, or, within the three years prior to the determination of interested
stockholder status, owned, 15% or more of a corporation’s outstanding voting stock. The term “business combination”
includes mergers, asset or stock sales and other similar transactions resulting in a financial benefit to an interested stockholder.
Section 203 makes it more difficult for an “interested stockholder” to effect various business combinations with a
corporation for a three-year period. The existence of this provision would be expected to have an anti-takeover effect with respect
to transactions not approved in advance by our Board of Directors, including discouraging attempts that might result in a premium
over the market price for the shares of common stock held by stockholders.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American
Stock Transfer & Trust Company LLC.
Nasdaq Capital Market
Our common stock is traded on the Nasdaq Capital Market under
the trading symbol “BCLI.”
DESCRIPTION OF WARRANTS
We may issue warrants for the purchase of common stock or units.
Warrants may be issued independently or together with common stock or units, and the warrants may be attached to or separate from
such securities. We may issue warrants directly or under a warrant agreement to be entered into between us and a warrant agent.
We will name any warrant agent in the applicable prospectus supplement. Any warrant agent will act solely as our agent in connection
with the warrants of a particular series and will not assume any obligation or relationship of agency or trust for or with any
holders or beneficial owners of warrants.
The following is a description of the general terms and provisions
of any warrants we may issue and may not contain all the information that is important to you. You can access complete information
by referring to the applicable prospectus supplement. In the applicable prospectus supplement, we will describe the terms of the
warrants and any applicable warrant agreement, including, where applicable, the following:
|
|
·
|
the
title of the warrants;
|
|
|
·
|
the
offering price and aggregate number of warrants offered;
|
|
|
·
|
the
designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security;
|
|
|
·
|
the
date on and after which the warrants and the related securities will be separately transferable;
|
|
|
·
|
any
information with respect to book-entry procedures;
|
|
|
·
|
in
the case of warrants to purchase common stock or units, the number of shares of common stock or units, as the case may be, purchasable
upon the exercise of one warrant and the price at which these securities may be purchased upon such exercise;
|
|
|
·
|
the
effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants;
|
|
|
·
|
the
terms of any rights to redeem or call the warrants;
|
|
|
·
|
any
provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants;
|
|
|
·
|
the
dates on which the right to exercise the warrants will commence and expire;
|
|
|
·
|
the
manner in which the warrant agreement and warrants may be modified;
|
|
|
·
|
a
discussion of any material U.S. federal income tax considerations of holding or exercising the warrants;
|
|
|
·
|
the
terms of the securities issuable upon exercise of the warrants; and
|
|
|
·
|
any
other specific terms, preferences, rights or limitations of or restrictions on the warrants.
|
DESCRIPTION OF UNITS
The following description, together with the additional information
we include in any applicable prospectus supplement, summarizes the material terms and provisions of the units that we may offer
under this prospectus. Units may be offered independently or together with common stock and warrants offered by any prospectus
supplement, and may be attached to or separate from those securities.
While the terms we have summarized below will generally apply
to any future units that we may offer under this prospectus, we will describe the particular terms of any series of units that
we may offer in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement
may differ from the terms described below.
We will incorporate by reference into the registration statement
of which this prospectus is a part the form of unit agreement, including a form of unit certificate, if any, that describes the
terms of the series of units we are offering before the issuance of the related series of units. The following summaries of material
provisions of the units and the unit agreements are subject to, and qualified in their entirety by reference to, all the provisions
of the unit agreement applicable to a particular series of units. We urge you to read the applicable prospectus supplements related
to the units that we sell under this prospectus, as well as the complete unit agreements that contain the terms of the units.
We may issue units, in one or more series, consisting of common
stock and warrants. Each unit will be issued so that the holder of the unit is also the holder of each security included in the
unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement
under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at
any time, or at any time before a specified date.
We will describe in the applicable prospectus supplement the
terms of the series of units, including the following:
|
|
·
|
the
title of the units;
|
|
|
·
|
the
aggregate number of units;
|
|
|
·
|
the
price or prices at which the units will be issued;
|
|
|
·
|
the
designation and terms of the units and of the securities comprising the units, including whether and under what circumstances
those securities may be held or transferred separately;
|
|
|
·
|
the
effect of any merger, consolidation, sale or other transfer of our business on the units and the applicable unit agreement;
|
|
|
·
|
the
name and address of any unit agent;
|
|
|
·
|
the
terms of the governing unit agreement;
|
|
|
·
|
any
applicable material U.S. Federal income tax consequences; and
|
|
|
·
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities
comprising the units.
|
The provisions described in this section, as well as those described
under “Description of Common Stock,” and “Description of Warrants,” will apply to each unit and to the
common stock and warrants included in each unit, respectively.
PLAN OF DISTRIBUTION
We may sell the securities being offered hereby in one or more
of the following ways from time to time:
|
|
·
|
through
agents to the public or to investors;
|
|
|
·
|
to
one or more underwriters or dealers for resale to the public or to investors;
|
|
|
·
|
in
“at the market offerings,” within the meaning of Rule 415(a)(4) of the Securities Act, to or through a market maker
or into an existing trading market, or an exchange or otherwise; or
|
|
|
·
|
through
a combination of any of these methods of sale.
|
The securities that we distribute by any of these methods may
be sold, in one or more transactions, at:
|
|
·
|
a
fixed price or prices, which may be changed;
|
|
|
·
|
market
prices prevailing at the time of sale;
|
|
|
·
|
prices
related to prevailing market prices; or
|
We will set forth in a prospectus supplement the terms of the
offering of our securities, including:
|
|
·
|
the
name or names of any agents or underwriters;
|
|
|
·
|
the
purchase price of our securities being offered and the proceeds we will receive from the sale;
|
|
|
·
|
any
over-allotment options under which underwriters may purchase additional securities from us;
|
|
|
·
|
any
agency fees or underwriting discounts and commissions and other items constituting agents’ or underwriters’ compensation;
|
|
|
·
|
any
public offering price;
|
|
|
·
|
any
discounts or concessions allowed or reallowed or paid to dealers; and
|
|
|
·
|
any
securities exchanges on which such securities may be listed.
|
Underwriters
Underwriters, dealers and agents that participate in the distribution
of the securities may be underwriters as defined in the Securities Act and any discounts or commissions they receive from us and
any profit on their resale of the securities may be treated as underwriting discounts and commissions under the Securities Act.
We will identify in the applicable prospectus supplement any underwriters, dealers or agents and will describe their compensation.
In no event will the aggregate value of compensation received or to be received by Financial Industry Regulatory Authority members
or independent broker-dealers exceed 8% for the sale of the securities registered hereunder. We may have agreements with the underwriters,
dealers and agents to indemnify them against specified civil liabilities, including liabilities under the Securities Act. Underwriters,
dealers and agents may engage in transactions with or perform services for us or our subsidiaries in the ordinary course of their
businesses.
If we use underwriters for a sale of securities, the underwriters
will acquire the securities for their own account. The underwriters may resell the securities in one or more transactions, including
negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. The obligations
of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement.
The underwriters will be obligated to purchase all the securities offered if they purchase any of the securities offered. We may
change from time to time any initial public offering price and any discounts or concessions the underwriters allow or reallow or
pay to dealers. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement
naming the underwriters the nature of any such relationship.
If indicated in the applicable prospectus supplement, we will
authorize underwriters or other persons acting as our agents to solicit offers by particular institutions to purchase securities
from us at the public offering price set forth in such prospectus supplement pursuant to delayed delivery contracts providing for
payment and delivery on the date or dates stated in such prospectus supplement. Each delayed delivery contract will be for an amount
no less than, and the aggregate principal amounts of securities sold under delayed delivery contracts shall be not less nor more
than, the respective amounts stated in the applicable prospectus supplement. Institutions with which such contracts, when authorized,
may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable
institutions and others, but will in all cases be subject to our approval. The obligations of any purchaser under any such contract
will be subject to the conditions that (a) the purchase of the securities shall not at the time of delivery be prohibited
under the laws of any jurisdiction in the United States to which the purchaser is subject, and (b) if the securities are being
sold to underwriters, we shall have sold to the underwriters the total principal amount of the securities less the principal amount
thereof covered by the contracts. The underwriters and such other agents will not have any responsibility in respect of the validity
or performance of such contracts.
Agents
We may designate agents who agree to use their reasonable efforts
to solicit purchases for the period of their appointment or to sell securities on a continuing basis.
Direct Sales
We may also sell securities directly to one or more purchasers
without using underwriters or agents. We may also make direct sales through subscription rights distributed to our shareholders
on a pro rata basis, which may or may not be transferable. In any distribution of subscription rights to shareholders, if all of
the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may
engage the services of one or more underwriters, dealers or agents, including standby underwriters, to sell the unsubscribed securities
to third parties.
Trading Markets and Listing of Securities
Unless otherwise specified in the applicable prospectus supplement,
each class or series of securities will be a new issue with no established trading market, other than our common stock, which is
listed on the Nasdaq Capital Market. We may elect to list any other class or series of securities on any exchange, but we are not
obligated to do so. It is possible that one or more underwriters may make a market in a class or series of securities, but the
underwriters will not be obligated to do so and may discontinue any market making at any time without notice. We cannot give any
assurance as to the liquidity of the trading market for any of the securities.
Stabilization Activities
In connection with an offering, an underwriter may purchase
and sell securities in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover
positions created by short sales. Shorts sales involve the sale by the underwriters of a greater number of securities than they
are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’
option to purchase additional securities from us, if any, in the offering. If the underwriters have an over-allotment option to
purchase additional securities from us, the underwriters may close out any covered short position by either exercising their over-allotment
option or purchasing securities in the open market. In determining the source of securities to close out the covered short position,
the underwriters may consider, among other things, the price of securities available for purchase in the open market as compared
to the price at which they may purchase securities through the over-allotment option. “Naked” short sales are any sales
in excess of such option or where the underwriters do not have an over-allotment option. The underwriters must close out any naked
short position by purchasing securities in the open market. A naked short position is more likely to be created if the underwriters
are concerned that there may be downward pressure on the price of the securities in the open market after pricing that could adversely
affect investors who purchase in the offering.
Accordingly, to cover these short sales positions or to otherwise
stabilize or maintain the price of the securities, the underwriters may bid for or purchase securities in the open market and may
impose penalty bids. If penalty bids are imposed, selling concessions allowed to syndicate members or other broker-dealers participating
in the offering are reclaimed if securities previously distributed in the offering are repurchased, whether in connection with
stabilization transactions or otherwise. The effect of these transactions may be to stabilize or maintain the market price of the
securities at a level above that which might otherwise prevail in the open market. The impositions of a penalty bid may also effect
the price of the securities to the extent that it discourages resale of the securities. The magnitude or effect of any stabilization
or other transactions is uncertain. These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced,
may be discontinued at any time.
EXPERTS
The consolidated financial statements as of and for the years
ended December 31, 2017 and 2016, incorporated in this prospectus by reference from the Company’s Annual Report on Form
10-K filed on March 8, 2018 for the year ended December 31, 2017, have been audited by Brightman Almagor Zohar &
Co., a member of Deloitte Touche Tohmatsu Limited, an independent registered public accounting firm, as stated in their report
(which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph regarding the
Company's ability to continue as a going concern), which is incorporated herein by reference, and has been so incorporated in reliance
upon the report of such firm given upon their authority as experts in accounting and auditing.
LEGAL MATTERS
Validity of the securities offered by this prospectus will be
passed upon for us by BRL Law Group LLC, Boston, Massachusetts. As of June 8, 2018, Thomas B. Rosedale, the Managing
Member of BRL Law Group LLC, beneficially and of record owns 69,090 shares of our common stock.

BRAINSTORM
CELL THERAPEUTICS INC.
Up to
$45,000,000
Common
Stock
PROSPECTUS
SUPPLEMENT
|
SVB Leerink
|
Raymond James
|
September 25, 2020
Brainstorm Cell Therapeu... (NASDAQ:BCLI)
Historical Stock Chart
From Mar 2024 to Apr 2024
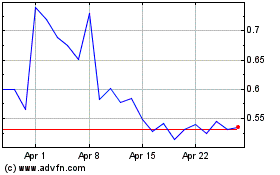
Brainstorm Cell Therapeu... (NASDAQ:BCLI)
Historical Stock Chart
From Apr 2023 to Apr 2024