Meiji Seika Pharma Receives Approval for Adding Domestic Manufacturing Sites in Japan for KOSTAIVE®, a Self-amplifying mRNA Vaccine Against COVID-19
January 31 2025 - 3:00AM
Business Wire
Meiji Seika Pharma Co., Ltd. (Headquarters: Tokyo, Japan,
President and Representative Director: Daikichiro Kobayashi)
announced today that it has received approval for a partial
amendment to the manufacturing and marketing approval of “KOSTAIVE®
for Intramuscular Injection,” a self-amplifying mRNA vaccine for
protection against COVID-19, to include domestic manufacturing
sites in Japan.
Meiji Seika Pharma and ARCALIS Co., Ltd. (Headquarters:
Minami-soma City, Fukushima Prefecture; President: Satoshi
Takamatsu) have been working together to establish an
infrastructure that enables the integrated production of
pharmaceuticals, from active ingredients to final products, within
Japan. With this approval, ARCALIS’s Minami-soma facilities and
Meiji Seika Pharmatech Co., Ltd., a subsidiary of Meiji Seika
Pharma, have been added as manufacturing sites. The technology
transfer was managed and implemented by CSL Seqirus, the global
licensor of KOSTAIVE®. As a result, domestically produced products,
with active pharmaceutical ingredients manufactured at ARCALIS's
Minami-soma facilities and formulated at Meiji Seika Pharmatech,
are now able to be shipped for commercial use in Japan.
“We are pleased with the approval of domestic manufacturing
sites in Japan for KOSTAIVE®,” said Dr. Pad Chivukula, COO of
Arcturus Therapeutics. “As a joint venture partner with ARCALIS,
Arcturus is delighted to support this effort through the continued
transfer of our next-generation technologies, including LUNAR®
lipid nanoparticle formulation processes and STARR® sa-mRNA drug
substance manufacturing.”
About sa-mRNA
mRNA vaccines help protect against infectious diseases by
providing a blueprint for cells in the body to make a protein to
help our immune systems recognize and fight the disease. Unlike
standard mRNA vaccines, self-amplifying mRNA vaccines instruct the
body to make more mRNA and protein to boost the immune
response.
About Meiji Seika Pharma
Meiji Seika Pharma, since it launched penicillin in 1946, has
been providing efficacious and high-quality pharmaceutical products
including therapeutics and vaccines for infectious diseases,
therapeutics for central nervous system diseases and generic drugs,
to meet various medical needs.
(https://www.meiji.com/global/pharmaceuticals/)
About Arcturus
Therapeutics
Arcturus Therapeutics Holdings Inc., founded in 2013, is a
commercial mRNA Medicines and vaccines Company focused on the
development of infectious disease vaccines and opportunities within
liver and respiratory rare diseases. (https://arcturusrx.com/)
About CSL Seqirus
CSL Seqirus, a subsidiary of CSL Limited, is one of the world's
largest suppliers of influenza vaccines. The company has
state-of-the-art manufacturing facilities in the U.S., U.K., and
Australia, and leading research and development capabilities.
(https://www.cslseqirus.com)
About ARCALIS
ARCALIS, Inc. is a joint venture company between Axcelead Inc.
and Arcturus Therapeutics Inc., which owns a group of world-class
drug-discovery and healthcare platform companies. The company
provides drug discovery support, as well as a contract development
and manufacturing business (CDMO business), for mRNA medicines and
vaccines. (https://corp.arcalis.co.jp/en)
View source
version on businesswire.com: https://www.businesswire.com/news/home/20250131312981/en/
Meiji Seika Pharma Public Relations Sayoko Taga
pr-pharma@meiji.com Arcturus Therapeutics Public Relations &
Investor Relations Neda Safarzadeh VP, Head of IR/PR/Marketing
(858) 900-2682 IR@ArcturusRx.com
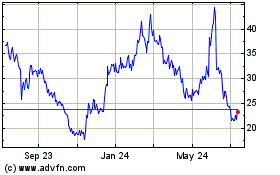
Arcturus Therapeutics (NASDAQ:ARCT)
Historical Stock Chart
From Jan 2025 to Feb 2025
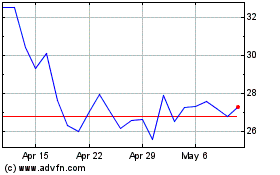
Arcturus Therapeutics (NASDAQ:ARCT)
Historical Stock Chart
From Feb 2024 to Feb 2025