
1. SUBSCRIPTION AGREEMENT This Subscription Agreement (this “Agreement”) is dated as of December 22, 2023 (the “Effective Date”), among Elicio Therapeutics, Inc., a Delaware corporation (the “Company”), and each purchaser identified on the signature pages hereto (each a “Purchaser” and collectively the “Purchasers”). WHEREAS, the Company and the Purchasers are executing and delivering this Agreement in reliance upon the exemption from securities registration afforded by Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), and Rule 506 of Regulation D as promulgated by the United States Securities and Exchange Commission (the “Commission”) under the Securities Act. WHEREAS, each Purchaser wishes to purchase, and the Company wishes to sell, upon the terms and conditions stated in this Agreement, at the Closing (as defined below) that aggregate number of shares of Common Stock (as defined below) set forth opposite such Purchaser’s name on Exhibit A (the “Shares”). NOW, THEREFORE, in consideration of the mutual covenants contained in this Agreement, and for other good and valuable consideration the receipt and adequacy of which are hereby acknowledged, the Company and each Purchaser agree as follows: 1. DEFINITIONS 1.1 Definitions. In addition to the terms defined elsewhere in this Agreement, the following terms have the meanings set forth in this Section 1.1: “Closing” means the closing of the purchase and sale of the Shares on the Closing Date pursuant to Section 2.1 of this Agreement. “Closing Date” means December 22, 2023. “Common Stock” means the common stock of the Company, $0.01 par value per share, and any other class of securities into which such securities may hereafter be reclassified or changed into. “Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder. “GAAP” means U.S. generally accepted accounting principles consistently applied. “Governmental Entity” shall mean any national, federal, state, county, municipal, local or foreign government, or any political subdivision, court, body, agency or regulatory authority thereof, and any person exercising executive, legislative, judicial, regulatory, taxing or administrative functions of or pertaining to any of the foregoing. “Material Adverse Effect” means a circumstance that (i) could reasonably be expected to have a material adverse effect on the performance of this Agreement or the consummation of any

2 of the transactions contemplated hereby or (ii) could reasonably be expected to have a material adverse effect on the condition (financial or otherwise), prospects, earnings, business or properties of the Company. “Registration Statement” means a registration statement or registration statements of the Company filed under the Securities Act pursuant to Section 4 hereof. “Rule 144” means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same effect as such Rule. “Share Purchase Price” means $5.81 per share. “Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO of the Exchange Act, but shall be deemed to not include the location and/or reservation of borrowable shares of Common Stock. “Trading Day” means a day on which the Common Stock is traded on a Trading Market. “Trading Market” means the following markets or exchanges on which (and if) the Common Stock is listed or quoted for trading on the date in question: the NYSE American; The Nasdaq Capital Market; The Nasdaq Global Market; The Nasdaq Global Select Market; or the New York Stock Exchange. “Transaction Documents” means this Agreement and any other documents or agreements executed and delivered to the Purchasers in connection with the transactions contemplated hereunder. 2. PURCHASE AND SALE 2.1 Closing. (a) At the Closing, upon the terms set forth herein, the Company hereby agrees to issue and sell to each Purchaser, and each Purchaser agrees to purchase from the Company, severally and not jointly, the Shares set forth opposite such Purchaser’s name on Exhibit A hereto, at a purchase price equal to the Share Purchase Price per share of Common Stock. (b) At the Closing, each Purchaser shall deliver to the Company via wire transfer immediately available funds equal to its aggregate purchase price set forth opposite such Purchaser’s name on Exhibit A hereto and the Company shall deliver to each Purchaser its respective Shares and the other items set forth in Section 2.2 of this Agreement deliverable at the Closing on the Closing Date. The Closing shall occur at 10:00 a.m. (New York City Time) on the Closing Date or such other time and location as the parties shall mutually agree. 2.2 Deliveries; Closing Conditions. (a) At the Closing, the Company will deliver or cause to be delivered to each Purchaser certificate(s) or book-entry shares representing the Common Stock, purchased by such

3 Purchaser, registered in the Purchaser’s name. Such delivery shall be against payment of the purchase price therefor by the Purchaser by wire transfer of immediately available funds to the Company in accordance with the Company’s written wiring instructions. (b) The respective obligations of the Company, on the one hand, and the Purchasers, on the other hand, hereunder in connection with the Closing are subject to the following conditions being met: (i) the accuracy in all material respects on the Closing Date of the representations and warranties contained herein (unless made as of a specified date therein) of the Company (with respect to the obligations of the Purchasers) and the Purchasers (with respect to the obligations of the Company); (ii) all obligations, covenants and agreements of the Company (with respect to the obligations of the Purchasers) and the Purchasers (with respect to the obligations of the Company) required to be performed at or prior to the Closing Date shall have been performed in all material respects; (iii) if requested by a Purchaser, evidence of the issuance of the Shares to be purchased by such Purchaser pursuant to this Agreement from the Company’s transfer agent; (iv) Purchasers shall have received a certificate of the Secretary of the Company, dated as of the Closing Date and certifying (i) the Company’s Amended and Restated Certificate of Incorporation, as amended; (ii) the Company’s Amended and Restated Bylaws; (iii) resolutions of the Board of Directors (and/or an authorized committee thereof) approving the Transaction Documents and the transactions contemplated thereby, in form and substance reasonably satisfactory to the Purchasers; (v) Purchasers shall have received a certificate signed by the Chief Executive Officer of the Company, dated as of the Closing Date in form and substance reasonably satisfactory to the Purchasers; (vi) a certificate evidencing the good standing of the Company in Delaware issued by the Secretary of State of Delaware, as of a date within five business days of the Closing Date; and (vii) Purchasers shall have received an opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., counsel for the Company, dated as of the Closing Date, in a form reasonably satisfactory to the Purchasers. 3. REPRESENTATIONS AND WARRANTIES 3.1 Representations and Warranties of the Company. Assuming the accuracy of the representations and warranties of the Purchasers set forth in Section 3.2 of this Agreement and except as set forth in the SEC Reports (defined below), which disclosures serve to qualify these representations and warranties in their entirety, the Company represents and warrants to the Purchasers that the statements contained in this Section 3.1 are true and correct as of the date of the Closing Date:

4 (a) The Company was not and is not an Ineligible Issuer (as defined in Rule 405), without taking account of any determination by the Commission pursuant to Rule 405 that it is not necessary that the Company be considered an Ineligible Issuer. (b) The Company has been duly incorporated and is validly existing as a corporation in good standing under the laws of the jurisdiction in which it is chartered or organized with full corporate power and authority to own or lease, as the case may be, and to operate its properties and conduct its business, and to execute and deliver the Transaction Documents, to be dated as of the Closing Date. The Company is duly qualified to do business as a foreign corporation and is in good standing under the laws of each jurisdiction which requires such qualification except where the failure to be so qualified or to be in good standing would not result in a Material Adverse Effect. The Company has no subsidiaries other than Elicio Operating Company, Inc., Elicio Securities Corporation, and Angion PTY LTD. (c) As of the date hereof, the authorized capital stock of the Company consists of 310,000,000 shares of capital stock, of which 300,000,000 are designated as Common Stock and 10,000,000 are designated as preferred stock, $0.01 par value per share. As of September 30, 2023: (i) 8,384,723 shares of Common Stock were issued and outstanding; (ii) no shares of preferred stock were issued and outstanding; (iii) 1,310,934 shares of Common Stock were issuable (and such number was reserved for issuance) upon exercise of options to purchase Common Stock outstanding as of such date; and (iv) 148,764 shares of Common Stock were issuable (and such number was reserved for issuance) upon exercise of warrants to purchase Common Stock outstanding as of such date. (d) The outstanding shares of Common Stock have been duly and validly authorized and issued and are fully paid and nonassessable; the Shares have been duly and validly authorized and, when issued and delivered to and paid for by the Purchasers pursuant to this Agreement, will be fully paid and nonassessable; the certificates for the securities are in valid form; the holders of outstanding shares of capital stock of the Company are not entitled to preemptive or other rights to subscribe for the Shares, except for any such rights as have been effectively waived or complied with; and, except as set forth in the SEC Reports or except as set forth in Section 3.1(c) above, no options, warrants or other rights to purchase, agreements or other obligations to issue, or rights to convert any obligations into or exchange any securities for, shares of capital stock of or ownership interests in the Company are outstanding. (e) This Agreement has been duly authorized, executed and delivered by the Company. (f) The Company is not and, after giving effect to the offering and sale of the Shares and the application of the proceeds as described in Section 5.4 of this Agreement, will not be an “investment company” as defined in the Investment Company Act of 1940, as amended. (g) No consent, approval, authorization, filing with or order of any court or governmental agency or body is required in connection with the transactions contemplated herein, except as may be required under the Securities Act, blue sky laws of any jurisdiction in connection with the purchase of the Shares by the Purchasers.

5 (h) Neither the issue and sale of the Shares, nor the consummation of any other of the transactions contemplated by any Transaction Document nor the fulfillment of the terms thereof, will conflict with, result in a breach or violation of, or imposition of any lien, charge or encumbrance upon any property or assets of the Company or its subsidiaries pursuant to, (i) the charter or by-laws of the Company, (ii) the terms of any indenture, contract, lease, mortgage, deed of trust, note agreement, loan agreement or other agreement, obligation, condition, covenant or instrument to which the Company is a party or bound or to which its property is subject, or (iii) any statute, law, rule, regulation, judgment, order or decree applicable to the Company of any court, regulatory body, administrative agency, governmental body, arbitrator or other authority having jurisdiction over the Company or any of its properties (the items listed in subclause (iii), collectively, “Applicable Laws”). (i) The Company’s Common Stock is registered under Section 12 of the Exchange Act. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof, since January 1, 2023 (the foregoing materials, including the exhibits thereto and documents incorporated by reference therein, being collectively referred to herein as the “SEC Reports”) on a timely basis or has received a valid waiver or extension of such time of filing and has filed any such SEC Reports prior to the expiration of any such extension. As of their respective dates, the SEC Reports complied in all material respects with the requirements of the Exchange Act and, in each case, to the rules promulgated thereunder, as applicable, and none of the SEC Reports, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. (j) The financial statements and the related notes of the Company included in the SEC Reports comply in all material respects with applicable accounting requirements and the rules and regulations of the Commission with respect thereto as in effect at the time of filing. Such financial statements have been prepared in accordance with GAAP, except as may be otherwise specified in such financial statements or the notes thereto and except that unaudited financial statements may not contain all footnotes required by GAAP, and fairly present in all material respects the consolidated financial position of the Company as of and for the dates thereof and the consolidated results of operations and cash flows for the periods then ended, subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments. Except as set forth in the financial statements of the Company included in the SEC Reports filed prior to the date hereof, the Company has not incurred any liabilities, contingent or otherwise, except those incurred in the ordinary course of business, consistent (as to amount and nature) with past practices since the date of such financial statements, none of which, individually or in the aggregate, have had or would reasonably be expected to have a Material Adverse Effect. (k) No action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company or its property is pending or, to the knowledge of the Company, threatened that is likely to have a Material Adverse Effect, whether or not arising from transactions in the ordinary course of business. (l) The Company and its subsidiaries have good and marketable title to all real properties and all personal properties other tangible properties and assets owned by them, in each

6 case free from Liens and defects, except such as would not have or would not reasonably be expected to have a Material Adverse Effect. The Company and its subsidiaries hold any leased real or personal property under valid, subsisting and enforceable leases with which the Company and its subsidiaries are in compliance and with no exceptions, except such as would not have or would not reasonably be expected to have a Material Adverse Effect. (m) The Company is not in violation or default of (i) any provision of its charter or bylaws, (ii) the terms of any indenture, contract, lease, mortgage, deed of trust, note agreement, loan agreement or other agreement, obligation, condition, covenant or instrument to which it is a party or bound or to which its property is subject, or (iii) any Applicable Laws, except in the case of clauses (ii) and (iii), as would not reasonably be expected to have a Material Adverse Effect. (n) Baker Tilly US, LLP, who have certified certain financial statements of the Company and delivered their report with respect to the audited financial statements included in the SEC Reports, are independent public accountants with respect to the Company within the meaning of the Securities Act and the applicable published rules and regulations thereunder. (o) There are no transfer taxes or other similar fees or charges under Federal law or the laws of any state, or any political subdivision thereof, required to be paid in connection with the execution and delivery of this Agreement, or the issuance by the Company or sale by the Company of the Shares. (p) The Company has timely filed all tax returns that are required to be filed or has requested extensions thereof (except in any case in which the failure so to file would not have a Material Adverse Effect, whether or not arising from transactions in the ordinary course of business) and has paid all taxes required to be paid by it and any other assessment, fine or penalty levied against it, to the extent that any of the foregoing is due and payable, except for any such assessment, fine or penalty that is currently being contested in good faith or as would not have a Material Adverse Effect. (q) No labor dispute with the employees of the Company or its subsidiaries exists or, to the knowledge of the Company, is imminent, and the Company is not aware of any existing or imminent labor disturbance by the employees of any of its or its subsidiaries’ principal suppliers, manufacturers, customers or contractors, which, in either case, would result in a Material Adverse Effect. (r) The Company is insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as the Company reasonably believes are prudent and customary in the businesses in which it is engaged; all policies of insurance and fidelity or surety bonds insuring the Company or its business, assets, employees, officers and directors are in full force and effect; the Company is in compliance with the terms of such policies and instruments in all material respects; and there are no claims by the Company under any such policy or instrument as to which any insurance company is denying liability or defending under a reservation of rights clause; the Company has not been refused any insurance coverage sought or applied for; and the Company has no reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be

7 necessary to continue its business at a cost that would not have a Material Adverse Effect, whether or not arising in the ordinary course of business. (s) The Company possesses all licenses, certificates, permits and other authorizations issued by all applicable authorities necessary to conduct its business, and the Company has not received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit which, singly or in the aggregate, if the subject of an unfavorable decision, ruling or finding, would have a Material Adverse Effect. (t) Except as described in the SEC Reports, the Company: (A) is and at all times has been in material compliance with all statutes, rules or regulations of the U.S. Food and Drug Administration (the “FDA”) and other comparable Governmental Entities applicable to the ownership, testing, development, manufacture, packaging, processing, use, distribution, marketing, labeling, promotion, sale, offer for sale, storage, import, export or disposal of any product under development, manufactured or distributed by the Company (“Product Laws”); (B) has not received any FDA Form 483, notice of adverse finding, warning letter, untitled letter or other correspondence or written notice from the FDA or any other Governmental Entity alleging or asserting material noncompliance with any Product Laws or any licenses, certificates, approvals, clearances, exemptions, authorizations, permits and supplements or amendments thereto required by any such Product Laws (“Authorizations”); (C) possesses all material Authorizations and such Authorizations are valid and in full force and effect and the Company is not in material violation of any term of any such Authorizations; (D) has not received written notice of any claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action from the FDA or any other Governmental Entity or third party alleging that any product operation or activity is in material violation of any Product Laws or Authorizations and has no knowledge that the FDA or any other Governmental Entity or third party is considering any such claim, litigation, arbitration, action, suit, investigation or proceeding; (E) has not received notice that the FDA or any other Governmental Entity has taken, is taking or intends to take action to limit, suspend, modify or revoke any material Authorizations and has no knowledge that the FDA or any other Governmental Entity is considering such action; and (F) has filed, obtained, maintained or submitted all material reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments as required by any Product Laws or Authorizations and that all such reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments were materially complete and correct on the date filed (or were corrected or supplemented by a subsequent submission). (u) The Company has operated and currently is in compliance with all applicable health care laws, rules and regulations to the extent they apply to the Company and its current activities (except where such failure to operate or non-compliance would not, singly or in the aggregate, result in a Material Adverse Effect), including, without limitation, (i) the Federal, Food, Drug and Cosmetic Act (21 U.S.C. §§ 301 et seq.); (ii) all applicable federal, state, local and all applicable foreign healthcare related fraud and abuse laws, including, without limitation, the federal Anti- kickback Statute (42 U.S.C. § 1320a-7b(b)), the U.S. Physician Payments Sunshine Act (42 U.S.C. § 1320a-7h), the civil False Claims Act (31 U.S.C. §§ 3729 et seq.), the criminal False Claims Law (42 U.S.C. § 1320a-7b(a)), all criminal laws relating to healthcare fraud and abuse, including but not limited to 18 U.S.C. Sections 286 and 287, the healthcare fraud criminal provisions under the U.S. Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) (42 U.S.C.

8 Section 1320d et seq.), the exclusion laws (42 U.S.C. § 1320a-7), and the civil monetary penalties law (42 U.S.C. § 1320a-7a); (iii) HIPAA, as amended by the Health Information Technology for Economic Clinical Health Act (42 U.S.C. Section 17921 et seq.); (iv) the regulations promulgated pursuant to such laws; and (v) any other similar local, state, federal, or foreign laws (collectively, the “Health Care Laws”). Neither the Company, nor to the Company’s knowledge, any of its officers, directors, employees or agents have engaged in activities which are, as applicable, cause for false claims liability, civil penalties, or mandatory or permissive exclusion from Medicare, Medicaid, or any other state or federal healthcare program. The Company has not received written notice or other correspondence of any claim, action, suit, audit, survey, proceeding, hearing, enforcement, investigation, arbitration or other action (“Action”) from any court or arbitrator or Governmental Entity or third party alleging that any product operation or activity is in violation of any Health Care Laws, and, to the Company’s knowledge, no such claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action is threatened. The Company is not a party to and does not have any ongoing reporting obligations pursuant to any corporate integrity agreement, deferred prosecution agreement, monitoring agreement, consent decree, settlement order, plan of correction or similar agreement imposed by any Governmental Entity. Additionally, neither the Company, nor to the Company’s knowledge, any of its employees, officers or directors, has been excluded, suspended, disqualified, or debarred from participation in any U.S. state or federal health care program or human clinical research or, to the knowledge of the Company, is subject to a governmental inquiry, investigation, proceeding, or other similar action that could reasonably be expected to result in debarment, suspension, disqualification, or exclusion. (v) The nonclinical studies and clinical trials conducted by or, to the Company’s knowledge, on behalf of the Company were and, if still ongoing, are being conducted in all material respects in accordance with experimental protocols, procedures and controls pursuant to accepted professional scientific standards and all Authorizations and Product Laws, including, without limitation, the Federal Food, Drug and Cosmetic Act and the rules and regulations promulgated thereunder (collectively, “FFDCA”); the descriptions of the results of such nonclinical studies and clinical trials contained in the SEC Reports are, to the Company’s knowledge, accurate and complete in all material respects and fairly present the data derived from such nonclinical studies and clinical trials; except to the extent disclosed in the SEC Reports, the Company is not aware of any nonclinical studies or clinical trials, the results of which the Company believes reasonably call into question any study or trial results described or referred to in the SEC Reports when viewed in the context in which such results are described; and, except to the extent disclosed in the SEC Reports, the Company has not received any written notices or other correspondence from the FDA or any other Governmental Entity requiring the termination or suspension of any studies or clinical trials conducted by or on behalf of the Company, other than ordinary course communications with respect to modifications in connection with the design and implementation of such studies or trials, copies of which communications have been made available to you. (w) The Company owns or has valid, binding and enforceable licenses or other rights under the patents, patent applications, licenses, inventions, copyrights, know-how (including trade secrets and other unpatented and/or unpatentable proprietary or confidential information, systems or procedures), trademarks, service marks, trade names or other intellectual property necessary for, or used in the conduct, or the proposed conduct, of the business of the Company (collectively, the “Intellectual Property”) to the knowledge of the Company; the patents, trademarks, and copyrights, if any, included within the Intellectual Property are valid, enforceable, and subsisting;

9 other than as disclosed in the SEC Reports, to the knowledge of the Company (A) the Company is not obligated to pay a material royalty, grant a license to, or provide other material consideration to any third party in connection with the Intellectual Property, (B) the Company has not received any notice of any claim of infringement, misappropriation or conflict with any asserted rights of others with respect to any of the Company’s drug candidates, services, processes or Intellectual Property, (C) neither the sale nor use of any of the discoveries, inventions, drug candidates, services or processes of the Company referred to in the SEC Reports do or will, to the knowledge of the Company, infringe, misappropriate or violate any right or valid patent claim of any third party, (D) none of the technology employed by the Company has been obtained or is being used by the Company in material violation of any contractual obligation binding on the Company or, to the Company’s knowledge, upon any of its officers, directors or employees or otherwise in violation of the rights of any persons, (E) no third party has any ownership right in or to any Intellectual Property that is owned by the Company, other than any co-owner of any patent constituting Intellectual Property who is listed on the records of the U.S. Patent and Trademark Office (the “USPTO”) and any co-owner of any patent application constituting Intellectual Property who is named in such patent application, and, to the knowledge of the Company, no third party has any ownership right in or to any Intellectual Property in any field of use that is exclusively licensed to the Company, other than any licensor to the Company of such Intellectual Property, (F) there is no material infringement by third parties of any Intellectual Property, (G) there is no pending or, to the Company’s knowledge, threatened action, suit, proceeding or claim by others challenging the Company’s rights in or to any Intellectual Property, and (H) there is no pending or, to the Company’s knowledge, threatened action, suit, proceeding or claim by others challenging the validity or scope of any Intellectual Property. The Company is in compliance in all material respects with the terms of each agreement pursuant to which Intellectual Property has been licensed to the Company, and all such agreements are in full force and effect. (x) All patents and patent applications necessary for, or used in the conduct, or the proposed conduct, of the business of the Company and owned by or licensed to the Company or under which the Company has rights have, to the knowledge of the Company, been duly and properly filed and maintained; to the knowledge of the Company, the parties prosecuting such patent applications have complied with their duty of candor and disclosure to the USPTO in connection with such applications; and the Company is not aware of any facts required to be disclosed to the USPTO that were not disclosed to the USPTO and which would preclude the grant of a patent in connection with any such application or would reasonably be expected to form the basis of a finding of invalidity with respect to any patents that have issued with respect to such applications. To the Company’s knowledge, all patents and patent applications owned by the Company and filed with the USPTO or any foreign or international patent authority (the “Company Patent Rights”) and all patents and patent applications in-licensed by the Company and filed with the USPTO or any foreign or international patent authority (the “In-licensed Patent Rights”) have been duly and properly filed; the Company believes it has complied with its duty of candor and disclosure to the USPTO for the Company Patent Rights and, to the Company’s knowledge, the licensors of the In-licensed Patent Rights have complied with their duty of candor and disclosure to the USPTO for the In-licensed Patent Rights. (y) [Reserved]

10 (z) Except as disclosed in the SEC Reports, the Company maintains a system of internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with generally accepted accounting principles and to maintain asset accountability; (iii) access to assets is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. Except as disclosed in the SEC Reports, (i) the Company’s internal controls over financial reporting are effective and (ii) the Company is not aware of any material weakness in its internal controls over financial reporting. (aa) The Company maintains “disclosure controls and procedures” (as such term is defined in Rule 13a-15(e) under the Exchange Act); such disclosure controls and procedures are effective. (bb) The Company has not taken, directly or indirectly, any action designed to or that would constitute or that might reasonably be expected to cause or result in, under the Exchange Act or otherwise, stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of the Shares. (cc) Except as described in the SEC Reports or would not, singly or in the aggregate, result in a Material Adverse Effect, (A) neither the Company nor any subsidiary has violated or is in violation of any Applicable Laws relating to pollution or protection of human health, the environment (including, without limitation, ambient air, surface water, groundwater, land surface or subsurface strata) or wildlife, including, without limitation, laws and regulations relating to the release or threatened release of chemicals, pollutants, contaminants, wastes, toxic substances, hazardous substances, petroleum or petroleum products, asbestos-containing materials or mold (collectively, “Hazardous Materials”) or to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials (collectively, “Environmental Laws”), (B) the Company and its subsidiaries have all permits, authorizations and approvals required for their operations under any applicable Environmental Laws and are each in compliance with their requirements, (C) there are no pending or, to the knowledge of the Company threatened, administrative, regulatory or judicial Actions relating to any Environmental Law against the Company or any subsidiary and (D) to the Company’s knowledge, there are no events or circumstances that would reasonably be expected to form the basis of an order for clean- up or remediation, or an Action by any private party or Governmental Entity, against or affecting the Company or any subsidiary relating to Hazardous Materials or any Environmental Laws. (dd) None of the following events has occurred or exists: (i) a failure to fulfill the obligations, if any, under the minimum funding standards of Section 302 of the United States Employee Retirement Income Security Act of 1974, as amended (“ERISA”), and the regulations and published interpretations thereunder with respect to a Plan that is required to be funded, determined without regard to any waiver of such obligations or extension of any amortization period; (ii) an audit or investigation by the Internal Revenue Service, the U.S. Department of Labor, the Pension Benefit Guaranty Corporation or any other federal or state governmental agency or any foreign regulatory agency with respect to the employment or compensation of employees by any of the Company that would reasonably be expected to have a Material Adverse

11 Effect; (iii) any breach of any contractual obligation, or any violation of law or applicable qualification standards, with respect to the employment or compensation of employees by the Company that would reasonably be expected to have a Material Adverse Effect; or (iv) a non- exempt prohibited transaction, within the meaning of Section 406 of ERISA or Section 4975 of the Code with respect to any Plan that would reasonably be expected to have a Material Adverse Effect. None of the following events has occurred or is reasonably likely to occur: (i) a material increase in the aggregate amount of contributions required to be made to all Plans in the current fiscal year of the Company compared to the amount of such contributions made in the most recently completed fiscal year of the Company; (ii) a material increase in the “accumulated post- retirement benefit obligations” (within the meaning of Statement of Financial Accounting Standards 106) of the Company as compared to the amount of such obligations in the most recently completed fiscal year of the Company; (iii) any event or condition giving rise to a liability under Title IV of ERISA that would reasonably be expected to have a Material Adverse Effect; or (iv) the filing of a claim by one or more employees or former employees of the Company related to their employment that would reasonably be expected to have a Material Adverse Effect. For purposes of this paragraph, the term “Plan” means a plan (within the meaning of Section 3(3) of ERISA) subject to Title IV of ERISA with respect to which the Company may have any liability. (ee) There is and has been no failure on the part of the Company and any of the Company’s directors or officers, in their capacities as such, to comply in all material respects with any provision of the Sarbanes-Oxley Act of 2002 and the rules and regulations promulgated in connection therewith (the “Sarbanes-Oxley Act”), including Section 402 relating to loans. (ff) None of the Company, its subsidiaries or, to the knowledge of the Company, any director, officer, agent, employee, affiliate or other person acting on behalf of the Company or its subsidiaries is aware of or has taken any action, directly or indirectly, that would result in a violation by such persons of the Foreign Corrupt Practices Act of 1977, as amended, and the rules and regulations thereunder (the “FCPA”), including, without limitation, making use of the mails or any means or instrumentality of interstate commerce corruptly in furtherance of an offer, payment, promise to pay or authorization of the payment of any money, or other property, gift, promise to give, or authorization of the giving of anything of value to any “foreign official” (as such term is defined in the FCPA) or any foreign political party or official thereof or any candidate for foreign political office, in contravention of the FCPA and the Company has and, to the knowledge of the Company, its affiliates have conducted their businesses in compliance with the FCPA and have instituted and maintain policies and procedures designed to ensure, and which are reasonably expected to continue to ensure, continued compliance therewith. (gg) The operations of the Company and its subsidiaries are and have been conducted at all times in compliance with applicable financial recordkeeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, the money laundering statutes of all applicable jurisdictions, the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued, administered or enforced by any Governmental Entity (collectively, the “Money Laundering Laws”); and no Action by or before any Governmental Entity involving the Company or its subsidiaries with respect to the Money Laundering Laws is pending or, to the knowledge of the Company, threatened.

12 (hh) None of the Company, its subsidiaries or, to the knowledge of the Company, any director, officer, agent, employee, affiliate or representative of the Company or its subsidiaries is an individual or entity (“Person”) currently the subject or target of any sanctions administered or enforced by the United States Government (including, without limitation, the U.S. Department of the Treasury’s Office of Foreign Assets Control), the United Nations Security Council, the European Union, His Majesty’s Treasury, or other relevant sanctions authority (collectively, “Sanctions”); and the Company will not directly or indirectly use the proceeds of the sale of the Shares, or lend, contribute or otherwise make available such proceeds to any subsidiary, joint venture partners or other Person, to fund any activities of or business with any Person, or in any country or territory, that, at the time of such funding, is the subject of Sanctions or in any other manner that will result in a violation by any Person (including any Person participating in the transaction, whether as an agent, advisor, investor or otherwise) of Sanctions. (ii) None of the Company, its subsidiaries or, to the knowledge of the Company, any director, officer, agent, employee, affiliate or representative of the Company, is a Person that is, or is 50% or more owned or otherwise controlled by a Person that is: (i) the subject of any Sanctions; or (ii) located, organized or resident in a country or territory that is, or whose government is, the subject of Sanctions that broadly prohibit dealings with that country or territory (collectively, “Sanctioned Jurisdictions” and each, a “Sanctioned Jurisdiction”). (jj) Neither the Company nor any of its subsidiaries has engaged in any dealings or transactions with or for the benefit of a Sanctioned Person, or with or in a Sanctioned Jurisdiction, in the preceding 3 years, nor does the Company have any plans to increase its dealings or transactions with Sanctioned Persons, or with or in Sanctioned Jurisdictions. (kk) The Common Stock is listed on The Nasdaq Global Select Market. The Company has taken no action designed to, or likely to have the effect of, terminating the registration of the Common Stock under the Exchange Act or delisting the Common Stock from The Nasdaq Global Select Market, nor has the Company received any notification that the Commission or The Nasdaq Global Select Market is contemplating terminating such registration or listing. To the Company’s knowledge, it is in compliance with all applicable listing requirements of The Nasdaq Global Select Market. The Company is not aware of any circumstance that would cause the Shares being issued hereunder to not be approved for listing by The Nasdaq Global Select Market. (ll) Neither the Company, nor any of the Company’s affiliates or any other person acting on the Company’s behalf, has directly or indirectly engaged in any form of general solicitation or general advertising with respect to the Shares, nor have any of such persons made any offers or sales of any security of the Company, or any of the Company’s affiliates or solicited any offers to buy any security of the Company, or any of the Company’s or any affiliates under circumstances that would require registration of the Shares under the Securities Act or any other securities laws or cause this offering of Shares to be integrated with any prior offering of securities of the Company for purposes of the Securities Act in any manner that would affect the validity of the private placement exemption under the Securities Act for the offer and sale of the Shares hereunder. (mm) No “bad actor” disqualifying event described in Rule 506(d)(1)(i)-(viii) of the 1933 Act (a “Disqualification Event”) is applicable to the Company or, to the Company’s knowledge,

13 any Company Covered Person (as defined below), except for a Disqualification Event as to which Rule 506(d)(2)(ii–iv) or (d)(3), is applicable. “Company Covered Person” means, with respect to the Company as an “issuer” for purposes of Rule 506 promulgated under the 1933 Act, any person listed in the first paragraph of Rule 506(d)(1). (nn) The Company shall file a Form D with respect to the Shares as required under Regulation D and, to the extent the Form D is not publicly available on the Commission’s EDGAR reporting system, to provide a copy thereof to each Purchaser promptly after such filing. The Company, on or before the Closing Date, shall take such action as the Company shall reasonably determine is necessary in order to obtain an exemption for or to qualify the Shares for sale and issuance to the Purchasers at the Closing pursuant to this Agreement under applicable securities or blue sky laws of the states of the United States (or to obtain an exemption from such qualification), and, if requested by a Purchaser, shall provide evidence of any material action so taken to such Purchaser on or prior to the Closing Date. The Company shall make all filings and reports relating to the offer and sale of the Shares required under applicable securities or blue sky laws of the states of the United States following the Closing Date. (oo) Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2, no registration under the Securities Act is required for the offer and sale of the Shares by the Company to the Purchasers as contemplated hereby.1 3.2 Representations, Warranties and Covenants of the Purchasers. Each Purchaser, for itself and for no other Purchaser, hereby represents, warrants and covenants to the Company as of the Closing: (a) Purchaser represents and warrants that: (i) Purchaser has all requisite legal and corporate or other power and capacity and has taken all requisite corporate or other action to execute and deliver this Agreement, to purchase the Shares and to carry out and perform all of its obligations under this Agreement; and (ii) this Agreement constitutes the legal, valid and binding obligation of the Purchaser, enforceable against the Purchaser in accordance with its terms, except as limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance or other similar laws relating to or affecting the enforcement of creditors’ rights generally. (b) At the time such Purchaser was offered the Shares, it was, and as of the date hereof it is: (i) an “accredited investor” as defined in Rule 501(a)(1), (a)(2), (a)(3), (a)(7) or (a)(8) under the Securities Act or (ii) a “qualified institutional buyer” as defined in Rule 144A(a) under the Securities Act. Such Purchaser is not required to be registered as a broker-dealer under Section 15 of the Exchange Act. Such Purchaser has the authority and is duly and legally qualified to purchase and own the Shares. Such Purchaser is aware of the Company’s business affairs and financial condition and has had access to and has acquired sufficient information about the Company to reach an informed and knowledgeable decision to acquire the Shares. Purchaser has such business and financial experience as is required to give it the capacity to protect its own interests in connection with the purchase of the Shares and such Purchaser is able to bear the economic risk of an investment in the Shares and, at the present time, is able to afford a complete 1 Note to Draft: Investment Company rep is covered in Section 3.1(f).

14 loss of such investment. Such Purchaser acknowledges that it has had the opportunity to review the Company’s filings with the Commission and has been afforded (i) the opportunity to ask such questions as it has deemed necessary of, and to receive answers from, representatives of the Company concerning the terms and conditions of the offering of the Shares and the merits and risks of investing in the Shares and (ii) the opportunity to obtain such additional information that the Company possesses or can acquire without unreasonable effort or expense that is necessary to make an informed investment decision with respect to the investment. Such Purchaser is not a member of the Financial Industry Regulatory Authority or an “associated person” (as such term is defined under the FINRA rules and regulations). (c) Each Purchaser is purchasing the Shares for its own account, for investment purposes only, and not with a present view to, or for, resale, distribution or fractionalization thereof, in whole or in part, within the meaning of the Securities Act. Each Purchaser understands and acknowledges that the Shares are “restricted securities” and understands that its acquisition of the Shares has not been registered under the Securities Act or registered or qualified under any state securities law in reliance on specific exemptions therefrom, which exemptions may depend upon, among other things, the bona fide nature of each Purchaser’s investment intent as expressed herein. Each Purchaser will not, directly or indirectly, offer, sell, transfer or otherwise dispose of (or solicit any offers to buy, purchase or otherwise acquire or take a pledge of) the Shares except in compliance with the Securities Act and the rules and regulations promulgated thereunder. (d) Each Purchaser represents and acknowledges that it has not been solicited to offer to purchase or to purchase any Shares by means of any general solicitation or advertising within the meaning of Regulation D under the Securities Act. (e) Each Purchaser represents that it is not a person of the type described in Section 506(d) of Regulation D under the Securities Act that would disqualify the Company from engaging in a transaction pursuant to Section 506 of Regulation D under the Securities Act. (f) Each Purchaser understands that the Shares being offered and sold to it in reliance on specific exemptions from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the truth and accuracy of, and each Purchaser’s compliance with, the representations, warranties, agreements, acknowledgements and understandings of each Purchaser set forth herein in order to determine the availability of such exemptions and the eligibility of each Purchaser to acquire the Shares. Each Purchaser further acknowledges and understands that the Shares may not be resold or otherwise transferred except in a transaction registered under the Securities Act or unless an exemption from such registration is available. (g) Dispositions. (i) Each Purchaser will not, prior to the effectiveness of a Resale Registration Statement (as defined below), if then prohibited by law or regulation: (i) sell, offer to sell, solicit offers to buy, dispose of, loan or grant any right with respect to (collectively, a “Disposition”) the Shares; or (ii) engage in any hedging or other transaction which is designed or could reasonably be expected to lead to or result in a Disposition of the Shares by the Purchaser or an affiliate.

15 (ii) As of the Closing Date, each Purchaser has not directly or indirectly, nor has any person acting on behalf of or pursuant to any understanding with the Purchaser, engaged in any purchases or sales of the Company’s securities (including, without limitation, any Short Sales involving the Company’s securities) since the time that the Purchaser was first contacted by the Company or any other person regarding the transactions contemplated hereby. Each Purchaser covenants that neither it nor any person acting on its behalf or pursuant to any understanding with it will engage in any purchases or sales of the Company’s securities (including, without limitation, any Short Sales involving the Company’s securities) prior to the time that the transactions contemplated by this Agreement are publicly disclosed. (h) Each Purchaser has independently evaluated the merits of its decision to purchase Shares pursuant to this Agreement. Each Purchaser understands that nothing in this Agreement or any other materials presented to such Purchaser in connection with the purchase and sale of the Shares constitutes legal, tax or investment advice. (i) Each Purchaser will hold in confidence all information concerning this Agreement and the sale and issuance of the Shares until the Company has made a public announcement concerning this Agreement and the sale and issuance of the Shares, which shall be made not later than 5:30 pm New York time on the fourth Trading Day immediately after the signing of this Agreement. (j) Each Purchaser understands that no United States federal or state agency or any other government or governmental agency has passed upon or made any recommendation or endorsement of the Shares. (k) Legend. (i) Each Purchaser understands that the Shares shall bear a restrictive legend in substantially the following form (and a stop transfer order may be placed against transfer of the certificates for the Shares): “THE SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR THE SECURITIES LAWS OF ANY STATE OF THE UNITED STATES OR IN ANY OTHER JURISDICTION. THE SECURITIES REPRESENTED HEREBY MAY NOT BE OFFERED, SOLD OR TRANSFERRED IN THE ABSENCE OF AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER APPLICABLE SECURITIES LAWS UNLESS OFFERED, SOLD OR TRANSFERRED PURSUANT TO AN AVAILABLE EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THOSE LAWS.” (ii) The Company shall, at its sole expense, upon appropriate notice from any Purchaser stating that the Shares have been sold pursuant to an effective Registration Statement, timely prepare and deliver certificates or book-entry shares representing the shares to be delivered to a transferee pursuant to the Registration Statement, which certificates or book- entry shares shall be free of any restrictive legends and in such denominations and registered in

16 such names as such Purchaser may request. Further, the Company shall, at its sole expense, cause its legal counsel or other counsel satisfactory to the transfer agent: (i) while the Registration Statement is effective, to issue to the transfer agent a “blanket” legal opinion to allow sales without restriction pursuant to the effective Registration Statement, and (ii) provide all other opinions as may reasonably be required by the transfer agent in connection with the removal of legends. A Purchaser may request that the Company remove, and the Company agrees to authorize the removal of, any legend from such shares, following the delivery by a Purchaser to the Company or the Company’s transfer agent of a legended certificate representing such shares: (i) following any sale of such shares pursuant to Rule 144, (ii) if such shares are eligible for sale under Rule 144(b)(1), or (iii) following the time that the Registration Statement is declared effective. If a legend removal request is made pursuant to the foregoing, the Company will, no later than three business days following the delivery by a Purchaser to the Company or the Company’s transfer agent of a legended certificate representing such shares (or a request for legend removal, in the case of shares issued in book-entry form), deliver or cause to be delivered to such Purchaser a certificate representing such shares that is free from all restrictive legends or an equivalent book- entry position, as requested by the Purchaser. Certificates for shares free from all restrictive legends may be transmitted by the Company’s transfer agent to the Purchasers by crediting the account of the Purchaser’s prime broker with the Depository Trust Company (“DTC”) as directed by such Purchaser. The Company warrants that the shares shall otherwise be freely transferable on the books and records of the Company as and to the extent provided in this Agreement. If a Purchaser effects a transfer of the shares in accordance with Section 3.2(k)(ii), the Company shall permit the transfer and shall promptly instruct its transfer agent to issue one or more certificates or credit shares to the applicable balance accounts at DTC in such name and in such denominations as specified by such Purchaser to effect such transfer. Each Purchaser hereby agrees that the removal of the restrictive legend pursuant to this Section 3.2(k)(ii) is predicated upon the Company’s reliance that such Purchaser will sell any such shares pursuant to either the registration requirements of the Securities Act, including any applicable prospectus delivery requirements, or an exemption therefrom. (l) If Purchaser is not a United States person (as defined by Section 7701(a)(30) of the Code), Purchaser hereby represents that it has satisfied itself as to the full observance of the laws of its jurisdiction in connection with any invitation to subscribe for the Shares or any use of this Agreement, including (a) the legal requirements within its jurisdiction for the purchase of the Shares, (b) any foreign exchange restrictions applicable to such purchase or acquisition, (c) any government or other consents that may need to be obtained, and (d) the income tax and other tax consequences, if any, that may be relevant to the purchase, holding, redemption, sale or transfer of the Shares. The Purchaser’s subscription and payment for and continued beneficial ownership of the Shares will not violate any applicable securities or other laws of the Purchaser’s jurisdiction. (m) Such Purchaser understands that no United States federal or state agency or any other governmental or state agency has passed on or made recommendations or endorsement of the Shares or the suitability of the investment in the Shares nor have such authorities passed upon or endorsed the merits of the offering of the Shares pursuant to this Agreement. (n) The execution, delivery and performance of this Agreement and the consummation by such Purchaser of the transactions contemplated hereby and thereby or relating hereto or thereto do not and will not (i) result in a violation of such Purchaser’s charter documents,

17 bylaws or other organizational documents, if applicable, (ii) conflict with nor constitute a default (or an event which with notice or lapse of time or both would become a default) under any agreement to which such Purchaser is a party, nor (iii) result in a violation of any law, rule, or regulation, or any order, judgment or decree of any court or governmental agency applicable to such Purchaser or its properties (except for such conflicts, defaults and violations as would not, individually or in the aggregate, have a material adverse effect on such Purchaser). Such Purchaser is not required to obtain any consent, authorization or order of, or make any filing or registration with, any court or governmental agency in order for it to execute, deliver or perform any of its obligations under this Agreement nor to purchase the Shares in accordance with the terms hereof, provided that for purposes of the representation made in this sentence, such Purchaser is assuming and relying upon the accuracy of the relevant representations and agreements of the Company herein. 4. REGISTRATION RIGHTS 4.1 Definitions. For the purpose of this Section 4: (a) the term “Resale Registration Statement” shall mean any registration statement required to be filed by Section 4.2 below, and shall include any preliminary prospectus, final prospectus, exhibit or amendment included in or relating to such registration statements; and (b) the term “Registrable Shares” means the Shares; provided, however, that a security shall cease to be a Registrable Share upon the earliest to occur of the following: (i) a Resale Registration Statement registering such security under the Securities Act has been declared or becomes effective and such security has been sold or otherwise transferred by the holder thereof pursuant to and in a manner contemplated by such effective Resale Registration Statement, (ii) such security is sold pursuant to Rule 144 under circumstances in which any legend borne by such security relating to restrictions on transferability thereof, under the Securities Act or otherwise, is removed by the Company, (iii) such security is eligible to be sold pursuant to Rule 144 without condition or restriction, including without any limitation as to volume of sales, and without the Holder complying with any method of sale requirements or notice requirements under Rule 144, or (iv) such security shall cease to be outstanding following its issuance. 4.2 Registration Procedures and Expenses. The Company shall: (a) use best efforts to file a Resale Registration Statement (the “Mandatory Registration Statement”) with the Commission on or before March 31, 2024 (the “Filing Date”) to register the applicable Registrable Shares on Form S-3 under the Securities Act (providing for shelf registration of such Registrable Shares under Commission Rule 415); (b) use its commercially reasonable efforts to cause each Mandatory Registration Statement to be declared effective within 30 days following each Filing Date (or, in the event the staff of the Commission (the “Staff”) reviews and has written comments to any Mandatory Registration Statement, within 90 days following the receipt of such written comments) (the earlier of the foregoing or the applicable date set forth in Section 4.2(h), the “Effectiveness Date”), such efforts to include, without limiting the generality of the foregoing, preparing and

18 filing with the Commission any financial statements or other information that is required to be filed prior to the effectiveness of such Mandatory Registration Statement; (c) notwithstanding anything contained in this Agreement to the contrary, in the event that the Commission limits the amount of Registrable Shares or otherwise requires a reduction in the number of Registrable Shares that may be included and sold by the Purchasers in a Mandatory Registration Statement (in each case, subject to Section 4.3), then the Company shall prepare and file (i) within 20 business days of the first date or time that such excluded Registrable Shares may then be included in a Resale Registration Statement if the Commission shall have notified the Company that certain Registrable Shares were not eligible for inclusion in such Resale Registration Statement or (ii) in all other cases, within 30 days following the date that the Company becomes aware that such additional Resale Registration Statement is required (the “Additional Filing Date”), a Resale Registration Statement (any such Resale Registration Statement registering such excluded Registrable Shares, an “Additional Registration Statement” and, together with the Mandatory Registration Statement, a “Resale Registration Statement”) to register any Registrable Shares that have been excluded (or, if applicable, the maximum number of such excluded Registrable Shares that the Company is permitted to register for resale on such Additional Registration Statement consistent with Commission guidance), if any, from being registered on the Mandatory Registration Statement; (d) use its commercially reasonable efforts to cause any such Additional Registration Statement to be declared effective as promptly as practicable following the Additional Filing Date, such efforts to include, without limiting the generality of the foregoing, preparing and filing with the Commission any financial statements or other information that is required to be filed prior to the effectiveness of any such Additional Registration Statement; (e) prepare and file with the Commission such amendments and supplements to such Resale Registration Statements and the prospectus used in connection therewith as may be necessary to keep such Resale Registration Statements continuously effective and free from any material misstatement or omission to state a material fact therein until termination of such obligation as provided in Section 4.6 below, subject to the Company’s right to suspend pursuant to Section 4.5; (f) furnish to the Purchasers such number of copies of prospectuses in conformity with the requirements of the Securities Act and such other documents as the Purchasers may reasonably request, in order to facilitate the public sale or other disposition of all or any of the Registrable Shares by the Purchasers; (g) file such documents as may be required of the Company for normal securities law clearance for the resale of the Registrable Shares in such states of the United States as may be reasonably requested by the Purchasers and use its commercially reasonable efforts to maintain such blue sky qualifications during the period the Company is required to maintain effectiveness of the Resale Registration Statements; provided, however, that the Company shall not be required in connection with this Section 4.2(g) to qualify as a foreign corporation or execute a general consent to service of process in any jurisdiction in which it is not now so qualified or has not so consented;

19 (h) upon notification by the Commission that a Resale Registration Statement will not be reviewed or is not subject to further review by the Commission, the Company shall within five business days following the date of such notification request acceleration of such Resale Registration Statement (with the requested effectiveness date to be not more than two business days later); (i) upon notification by the Commission that that a Resale Registration Statement has been declared effective by the Commission, the Company shall file the final prospectus under Rule 424 of the Securities Act (“Rule 424”) within the applicable time period prescribed by Rule 424; (j) advise the Purchasers promptly: (i) of the effectiveness of a Resale Registration Statement or any post- effective amendments thereto; (ii) of any request by the Commission for amendments to a Resale Registration Statement or amendments to the prospectus or for additional information relating thereto; (iii) of the issuance by the Commission of any stop order suspending the effectiveness of a Resale Registration Statement under the Securities Act or of the suspension by any state securities commission of the qualification of the Registrable Shares for offering or sale in any jurisdiction, or the initiation of any proceeding for any of the preceding purposes; and (iv) of the existence of any fact and the happening of any event that makes any statement of a material fact made in a Resale Registration Statement, the prospectus and amendment or supplement thereto, or any document incorporated by reference therein, untrue, or that requires the making of any additions to or changes in a Resale Registration Statement or the prospectus in order to make the statements therein not misleading; (k) cause all Registrable Shares to be listed on each securities exchange, if any, on which equity securities by the Company are then listed; (l) bear all expenses in connection with the procedures in paragraphs (a) through (l) of this Section 4.2 and the registration of the Registrable Shares on such Resale Registration Statement and the satisfaction of the blue sky laws of such states; and (m) if (i) the initial Resale Registration Statement covering the Registrable Shares is not filed with the Commission on or prior to the Filing Date, (ii) the initial Resale Registration Statement or any other Resale Registration Statement, as applicable, is not declared effective by the Commission (or otherwise does not become effective) for any reason on or prior to the applicable Effectiveness Date, (iii) after its Effectiveness Date, (A) such Registration Statement ceases for any reason (including without limitation by reason of a stop order, or the Company’s failure to update the Resale Registration Statement), to remain continuously effective as to all Registrable Shares for which it is required to be effective, or (B) the Purchasers are not permitted to utilize the prospectus therein to resell such Registrable Shares or (iv) after the Filing Date, and only in the event a Resale Registration Statement is not effective or available to sell all

20 Registrable Shares, the Company fails to file with the Commission any required reports under Section 13 or 15(d) of the Exchange Act such that it is not in compliance with Rule 144(c)(1), as a result of which the Purchasers who are not affiliates are unable to sell Registrable Shares without restriction under Rule 144 (any such failure or breach in clauses (i) through (iv) above being referred to as an “Event,” and, for purposes of clauses (i), (ii), (iii) or (iv), the date on which such Event occurs, being referred to as an “Event Date”), then, in addition to any other rights the Purchasers may have hereunder or under applicable law on each such Event Date and on each monthly anniversary of each such Event Date (if the applicable Event shall not have been cured by such date) until the applicable Event is cured, the Company will pay to each Purchaser an amount in cash, as liquidated damages and not as a penalty (“Liquidated Damages”), equal to 1% of the aggregate purchase price paid by such Purchaser pursuant to this Agreement for any Registrable Shares held by such Purchaser on the Event Date. The Liquidated Damages pursuant to the terms hereof shall apply on a daily pro rata basis for any portion of a month prior to the cure of an Event, except in the case of the first Event Date. Such payments shall constitute the Purchasers’ exclusive monetary remedy for such events, but shall not affect the right of the Purchasers to seek injunctive relief. Such payments shall be made to each Purchaser in cash no later than five (5) Business Days after the date payable (such applicable date, the “Payment Date”). Interest shall accrue on the amount of Liquidated Damages that are not be paid by the Payment Date at the rate of 1% per month, accruing daily from the date such Liquidated Damages are due until such amount, plus interest thereon, is paid in full. Notwithstanding any other provision herein, with respect to a Purchaser (i) the Filing Date and each Effectiveness Date for a Resale Registration Statement shall be extended, without default by or Liquidated Damages payable by the Company to such Holder hereunder if the Company’s failure to make such filing or obtain such effectiveness results from the failure of such Purchaser to timely provide the Company with information requested by the Company and necessary to complete a Resale Registration Statement in accordance with the requirements of the Securities Act (in which case any such deadline would be extended with respect to all Registrable Shares held by such Purchaser until such time as the Purchaser provides such requested information), it being understood that the failure of such Purchaser to timely provide such information to the Company shall not affect the rights of other Purchasers herein, and (ii) in no event shall the aggregate amount of Liquidated Damages (or interest thereon) paid under this Agreement to any Purchaser exceed, in the aggregate, 5% of the aggregate purchase price of the Shares purchased by such Purchaser under this Agreement. 4.3 Rule 415; Cutback. If at any time the Staff takes the position that the offering of some or all of the Registrable Shares in a Registration Statement is not eligible to be made on a delayed or continuous basis under the provisions of Rule 415 under the Securities Act or requires any Purchaser to be named as an “underwriter,” the Company shall (in consultation with legal counsel to Purchaser) use its commercially reasonable efforts to persuade the Commission that the offering contemplated by the Registration Statement is a valid secondary offering and not an offering “by or on behalf of the issuer” as defined in Rule 415 and that none of the Purchasers is an “underwriter.” In the event that, despite the Company’s commercially reasonable efforts and compliance with the terms of this Section 4.3, the Staff refuses to alter its position, the Company shall (i) remove from the Registration Statement such portion of the Registrable Shares (the “Cut Back Shares”) and/or (ii) agree to such restrictions and limitations on the registration and resale of the Registrable Shares as the Staff may require to assure the Company’s compliance with the requirements of Rule 415

21 (collectively, the “SEC Restrictions”); provided, however, that the Company shall not agree to name any Purchaser as an “underwriter” in such Registration Statement without the prior written consent of such Purchaser. Any cutback imposed on the Purchasers pursuant to this Section 4.3 shall be allocated among the Purchasers on a pro rata basis, unless the SEC Restrictions otherwise require or provide or the Purchasers holding a majority of the Registrable Shares otherwise agree. No liquidated damages shall accrue as to any Cut Back Shares until such date as the Company is able to effect the registration of such Cut Back Shares in accordance with any SEC Restrictions (such date, the “Restriction Termination Date” of such Cut Back Shares). From and after the Restriction Termination Date applicable to any Cut Back Shares, all of the provisions of this Section 4 shall again be applicable to such Cut Back Shares; provided, however, that (x) the Filing Deadline for the Registration Statement including such Cut Back Shares shall be 10 business days after such Restriction Termination Date, and (y) the Effectiveness Deadline with respect to such Cut Back Shares shall be the earlier of (A) the fifth (5th) Business Day after the Commission informs the Company (orally or in writing, whichever is earlier) that no review of such Resale Registration Statement will be made or that the Commission has no further comments on such Resale Registration Statement and (B) 30th day immediately after the Restriction Termination Date (or the 90th day immediately after the Restriction Termination Date if the Commission reviews such Resale Registration Statement). 4.4 Indemnification. (a) The Company agrees to indemnify and hold harmless the Purchasers, and the partners, members, managers, officers, directors, trustees, advisors, employees and agents of the Purchasers and each person, if any, who controls the Purchasers within the meaning of the Securities Act or the Exchange Act, from and against any losses, claims, damages or liabilities to which they may become subject (under the Securities Act or otherwise) insofar as such losses, claims, damages or liabilities (or actions or proceedings in respect thereof) arise out of, or are based upon, any material breach of this Agreement by the Company or any untrue statement or alleged untrue statement of a material fact contained in a Resale Registration Statement or any omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading or arise out of any failure by the Company to fulfill any undertaking included in a Resale Registration Statement and the Company will, as incurred, reimburse the Purchasers, and their partners, members, officers, directors or controlling Persons for any legal or other expenses reasonably incurred in investigating, defending or preparing to defend any such action, proceeding or claim; provided, however, that the Company shall not be liable in any such case to the extent that such loss, claim, damage or liability (collectively, “Loss”) arises out of, or is based upon: (i) an untrue statement or omission or alleged untrue statement or omission made in such Resale Registration Statement in reliance upon and in conformity with written information furnished to the Company by or on behalf of the Purchasers, or their partners, members, officers, directors or controlling persons specifically for use in preparation of a Resale Registration Statement; or (ii) any breach of this Agreement by the Purchasers; provided further, however, that the Company shall not be liable to the Purchasers (or any partner, member, officer, director or controlling Person of the Purchasers) to the extent that any such Loss is caused by an untrue statement or omission or alleged untrue statement or omission made in any preliminary prospectus if either (i) (A) any Purchaser failed to send or deliver a copy of the final prospectus with or prior to, or any Purchaser failed to confirm that a final prospectus was deemed to be delivered prior to

22 (in accordance with Rule 172 of the Securities Act), the delivery of written confirmation of the sale by a Purchaser to the Person asserting the claim from which such Loss resulted and (B) the final prospectus corrected such untrue statement or omission, (ii) (X) such untrue statement or omission is corrected in an amendment or supplement to the prospectus and (Y) having previously been furnished by or on behalf of the Company with copies of the prospectus as so amended or supplemented or notified by the Company that such amended or supplemented prospectus has been filed with the Commission, in accordance with Rule 172 of the Securities Act, any Purchaser thereafter fails to deliver such prospectus as so amended or supplemented, with or prior to or a Purchaser fails to confirm that the prospectus as so amended or supplemented was deemed to be delivered prior to (in accordance with Rule 172 of the Securities Act), the delivery of written confirmation of the sale by a Purchaser to the person asserting the claim from which such Loss resulted or (iii) a Purchaser sold Registrable Shares in violation of such Purchasers’ covenant contained in Section 3.2 of this Agreement. (b) The Purchasers agree, severally and not jointly, to indemnify and hold harmless the Company (and each Person, if any, who controls the Company within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act, each officer of the Company who signs a Resale Registration Statement and each director of the Company), from and against any Losses to which the Company (or any such officer, director or controlling person) may become subject (under the Securities Act or otherwise), insofar as such Losses (or actions or proceedings in respect thereof) arise out of, or are based upon, any material breach of this Agreement by the Purchasers or untrue statement or alleged untrue statement of a material fact contained in a Resale Registration Statement (or any omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading in each case, on the effective date thereof), if, and only to the extent, such untrue statement or omission or alleged untrue statement or omission was made in reliance upon and in conformity with written information furnished by or on behalf of the Purchasers specifically for use in preparation of a Resale Registration Statement, and the Purchasers, severally and not jointly, will reimburse the Company (and each of its officers, directors or controlling persons) for any legal or other expenses reasonably incurred in investigating, defending or preparing to defend any such action, proceeding or claim; provided, however, that in no event shall any indemnity under this Section 4.4(b) be greater in amount than the dollar amount of the proceeds received by the Purchasers upon the sale of such Registrable Shares (net of all expenses paid by such Purchaser in connection with any claim relating to this Section 4.4(b) and the amount of any damages such Purchaser has otherwise been required to pay by reason of such untrue statement or omission). (c) Promptly after receipt by any indemnified person of a notice of a claim or the beginning of any action in respect of which indemnity is to be sought against an indemnifying person pursuant to this Section 4.4, such indemnified person shall notify the indemnifying person in writing of such claim or of the commencement of such action, and, subject to the provisions hereinafter stated, in case any such action shall be brought against an indemnified person and such indemnifying person shall have been notified thereof, such indemnifying person shall be entitled to participate therein, and, to the extent that it shall wish, to assume the defense thereof, with counsel reasonably satisfactory to such indemnified person. After notice from the indemnifying person to such indemnified person of its election to assume the defense thereof, such indemnifying person shall not be liable to such indemnified person for any legal expenses subsequently incurred

23 by such indemnified person in connection with the defense thereof; provided, however, that if there exists or shall exist a conflict of interest that would make it inappropriate in the reasonable judgment of the indemnified person for the same counsel to represent both the indemnified person and such indemnifying person or any affiliate or associate thereof, the indemnified person shall be entitled to retain its own counsel at the expense of such indemnifying person; provided, further, that no indemnifying person shall be responsible for the fees and expense of more than one separate counsel for all indemnified parties. The indemnifying party shall not settle an action without the consent of the indemnified party, which consent shall not be unreasonably withheld. (d) If the indemnification provided for in this Section 4.4 is held by a court of competent jurisdiction to be unavailable to an indemnified party with respect to any Losses referred to herein, the indemnifying party, in lieu of indemnifying such indemnified party thereunder, shall to the extent permitted by applicable law contribute to the amount paid or payable by such indemnified party as a result of such Loss in such proportion as is appropriate to reflect the relative fault of the indemnifying party on the one hand and of the indemnified party on the other, as well as any other relevant equitable considerations; provided, that in no event shall any contribution by an indemnifying party hereunder be greater in amount than the dollar amount of the proceeds received by such indemnifying party upon the sale of such Registrable Shares. 4.5 Prospectus Suspension. Each Purchaser acknowledges that there may be times when the Company must suspend the use of the prospectus forming a part of a Resale Registration Statement until such time as an amendment to a Resale Registration Statement has been filed by the Company and declared effective by the Commission, or until such time as the Company has filed an appropriate report with the Commission pursuant to the Exchange Act. Each Purchaser hereby covenants that it will not sell any Registrable Shares pursuant to said prospectus during the period commencing at the time at which the Company gives the Purchasers notice of the suspension of the use of said prospectus and ending at the time the Company gives the Purchasers notice that the Purchasers may thereafter effect sales pursuant to said prospectus; provided, that such suspension periods shall in no event exceed 30 consecutive trading days or 60 total trading days in any 12 month period (any such suspension, an “Allowed Delay”) and that, in the good faith judgment of the Board, the Company would, in the absence of such delay or suspension hereunder, be required under state or federal securities laws to disclose any corporate development, a potentially significant transaction or event involving the Company, or any negotiations, discussions, or proposals directly relating thereto, in either case the disclosure of which would reasonably be expected to have a Material Adverse Effect upon the Company or its stockholders. The Company shall use commercially reasonable efforts to terminate an Allowed Delay as promptly as practicable and shall provide prompt written notice to Purchasers whose Registrable Shares are included in the Resale Registration Statement of the termination of an Allowed Delay and take such other reasonable actions to permit registered sales of Registrable Shares as contemplated hereby. 4.6 Termination of Obligations. The obligations of the Company pursuant to Section 4.2 hereof shall cease and terminate, with respect to any Registrable Shares, upon the earlier to occur of (a) such time such Registrable Shares have been resold, or (b) such time as such Registrable Shares no longer remain Registrable Shares pursuant to Section 4.1(b) hereof.

24 4.7 Reporting Requirements. (a) With a view to making available the benefits of certain rules and regulations of the Commission that may at any time permit the sale of the Shares to the public without registration or pursuant to a registration statement on Form S-3, the Company agrees to: (i) make and keep public information available, as those terms are understood and defined in Rule 144; (ii) file with the Commission in a timely manner all reports and other documents required of the Company under the Securities Act and the Exchange Act; and (iii) so long as a Purchaser owns Registrable Shares, to furnish to such Purchaser upon request (A) a written statement by the Company as to whether it is in compliance with the reporting requirements of Rule 144, the Securities Act and the Exchange Act, or whether it is qualified as a registrant whose securities may be resold pursuant to Commission Form S-3, (B) a copy of the most recent annual or quarterly report of the Company and such other reports and documents so filed by the Company and (C) such other information as may be reasonably requested to permit the Purchaser to sell such securities pursuant to Rule 144. 4.8 Blue Sky. The Company shall obtain and maintain all necessary blue sky law permits and qualifications, or secured exemptions therefrom, required by any state for the offer and sale of Registrable Shares; provided, however, that the Company shall not be obligated to file any general consent to service of process or to qualify as a foreign corporation or as a dealer in securities in any jurisdiction in which it is not so qualified or to subject itself to taxation in respect of doing business in any jurisdiction in which it is not otherwise so subject. 5. OTHER AGREEMENTS OF THE PARTIES 5.1 Securities Laws Disclosure; Publicity. The Company shall: (a) issue a press release disclosing the material terms of the transactions contemplated hereby promptly following the execution and delivery hereof (the “Press Release”), and (b) by 5:30 p.m. (New York City time) on the fourth Trading Day following the date hereof, file a Current Report on Form 8-K disclosing the material terms of the transactions contemplated hereby (the “Form 8-K”). From and after the issuance of the Press Release, no Purchaser shall be in possession of any material, non- public information received from the Company or any of their respective officers, directors or employees that is not disclosed in the Press Release. 5.2 Non-Public Information. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, the Company covenants and agrees that neither it nor any other person acting on its behalf will provide any Purchaser or its agents or counsel with any information that the Company believes constitutes material non-public information. 6. MISCELLANEOUS 6.1 Termination. This Agreement may be terminated by any Purchaser, as to such Purchaser’s obligations hereunder only and without any effect whatsoever on the obligations

25 between the Company and the other Purchasers, by written notice to the other parties, if the Closing has not been consummated within ten calendar days from the Effective Date through no fault of such Purchaser; provided, however, that no such termination will affect the right of any party to sue for any breach by the other party (or parties). 6.2 Fees and Expenses. Each party shall pay the fees and expenses of its advisers, counsel, accountants and other experts, if any, and all other expenses incurred by such party incident to the negotiation, preparation, execution, delivery and performance of this Agreement. Notwithstanding the foregoing, the Company shall pay all transfer agent fees, stamp taxes and other taxes and duties levied in connection with the delivery of any Shares to the Purchasers. 6.3 Entire Agreement. The Transaction Documents, together with the exhibits and schedules thereto, contain the entire understanding of the parties with respect to the subject matter hereof and supersede all prior agreements and understandings, oral or written, with respect to such subject matter, which the parties acknowledge have been merged into such documents, exhibits and schedules. 6.4 Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed given and effective upon actual receipt via mail, courier or confirmed email by the party to whom such notice is required to be given. The address for such notices and communications shall be as set forth on the signature pages attached hereto. 6.5 Amendments; Waivers. No provision of this Agreement may be waived or amended except in a written instrument signed, in the case of an amendment, by (a) the Company and (b) Purchasers holding at least a majority of the Shares then-held by a Purchaser or, in the case of a waiver, by the party against whom enforcement of any such waived provision is sought. No waiver of any default with respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any party to exercise any right hereunder in any manner impair the exercise of any such right. 6.6 Headings. The headings herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any of the provisions hereof. 6.7 Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their permitted successors and assigns. The Company may not assign this Agreement or any rights or obligations hereunder without the prior written consent of each Purchaser (other than by merger). The Purchasers may not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Company (other than by merger). 6.8 Third-Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns and is not for the benefit of, nor may any provision hereof be enforced by, any other person. 6.9 Governing Law. All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of

26 conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement and any other Transaction Documents (whether brought against a party hereto or its respective affiliates, directors, officers, shareholders, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the State of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the State of New York for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein (including with respect to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law. If either party shall commence an action or proceeding to enforce any provisions of the Transaction Documents, then the prevailing party in such action or proceeding shall be reimbursed by the other party for its reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. 6.10 Execution. This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that the parties need not sign the same counterpart. In the event that any signature on this Agreement is delivered by facsimile transmission or by e-mail delivery of a “.pdf” format data file, such signature shall create a legally valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or “.pdf” signature page were an original thereof. 6.11 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable. 6.12 Rescission and Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) any of the other Transaction Documents, whenever any Purchaser exercises a right, election, demand or option under a Transaction Document and the Company does not timely perform its related obligations within the periods therein provided, then such Purchaser may rescind or withdraw, in its sole discretion from

27 time to time upon written notice to the Company, any relevant notice, demand or election in whole or in part without prejudice to its future actions and rights. 6.13 Replacement of Securities. If any certificate or instrument evidencing any Shares is mutilated, lost, stolen or destroyed, the Company shall issue or cause to be issued in exchange and substitution for and upon cancellation thereof (in the case of mutilation), or in lieu of and substitution therefor, a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company of such loss, theft or destruction and customary and reasonable indemnity or bond, if requested. The applicant for a new certificate or instrument under such circumstances shall also pay any reasonable third-party costs (including customary indemnity) associated with the issuance of such replacement Shares. 6.14 Independent Nature of Purchasers’ Obligations and Rights. The obligations of each Purchaser under any Transaction Document are several and not joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance or non- performance of the obligations of any other Purchaser under any Transaction Document. Nothing contained herein or in any other Transaction Document, and no action taken by any Purchaser pursuant thereto, shall be deemed to constitute the Purchasers as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by the Transaction Documents. Each Purchaser shall be entitled to independently protect and enforce its rights, including without limitation, the rights arising out of this Agreement or out of the other Transaction Documents, and it shall not be necessary for any other Purchaser to be joined as an additional party in any proceeding for such purpose. 6.15 Construction. The parties agree that each of them and/or their respective counsel has reviewed and had an opportunity to revise the Transaction Documents and, therefore, the normal rule of construction to the effect that any ambiguities are to be resolved against the drafting party shall not be employed in the interpretation of the Transaction Documents or any amendments hereto. 6.16 WAIVER OF JURY TRIAL. IN ANY ACTION, SUIT, OR PROCEEDING IN ANY JURISDICTION BROUGHT BY ANY PARTY AGAINST ANY OTHER PARTY, THE PARTIES EACH KNOWINGLY AND INTENTIONALLY, TO THE GREATEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY ABSOLUTELY, UNCONDITIONALLY, IRREVOCABLY AND EXPRESSLY WAIVES FOREVER TRIAL BY JURY. [Remainder of page intentionally left blank.]

28 IN WITNESS WHEREOF, the parties hereto have caused this Subscription Agreement to be duly executed by their respective authorized signatories as of the date first indicated above. ELICIO THERAPEUTICS, INC. /s/ Robert Connelly Name: Robert Connelly Title: Chief Executive Officer Address for Notice: 451 D Street, 5th Floor Boston, Massachusetts 02210 Email: brian.piekos@elicio.com; megan.filoon@elicio.com Attention: Chief Financial Officer; General Counsel, Secretary and Compliance Officer With a copy to (which shall not constitute notice): Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. One Financial Center Boston, MA 02111 Email: wchicks@mintz.com; dabagliebter@mintz.com Attention: William Hicks, Esq. and Daniel Bagliebter, Esq.

29 PURCHASERS: GKCC, LLC By: /s/ Yekaterina Chudnovsky Name: Yekaterina Chudnovsky Title: Manager Address:501 Silverside Road, Suite 87AVA Wilmington, DE 19809 Email: yekatie@gmail.com With copy to: Orrick, Herrington and Sutcliffe LLP 222 Berkeley St #2000 Boston, MA 02116 Email: sthau@orrick.com; avanderlaan@orrick.com Attention: Stephen Thau, Esq.; Albert Vanderlaan, Esq.
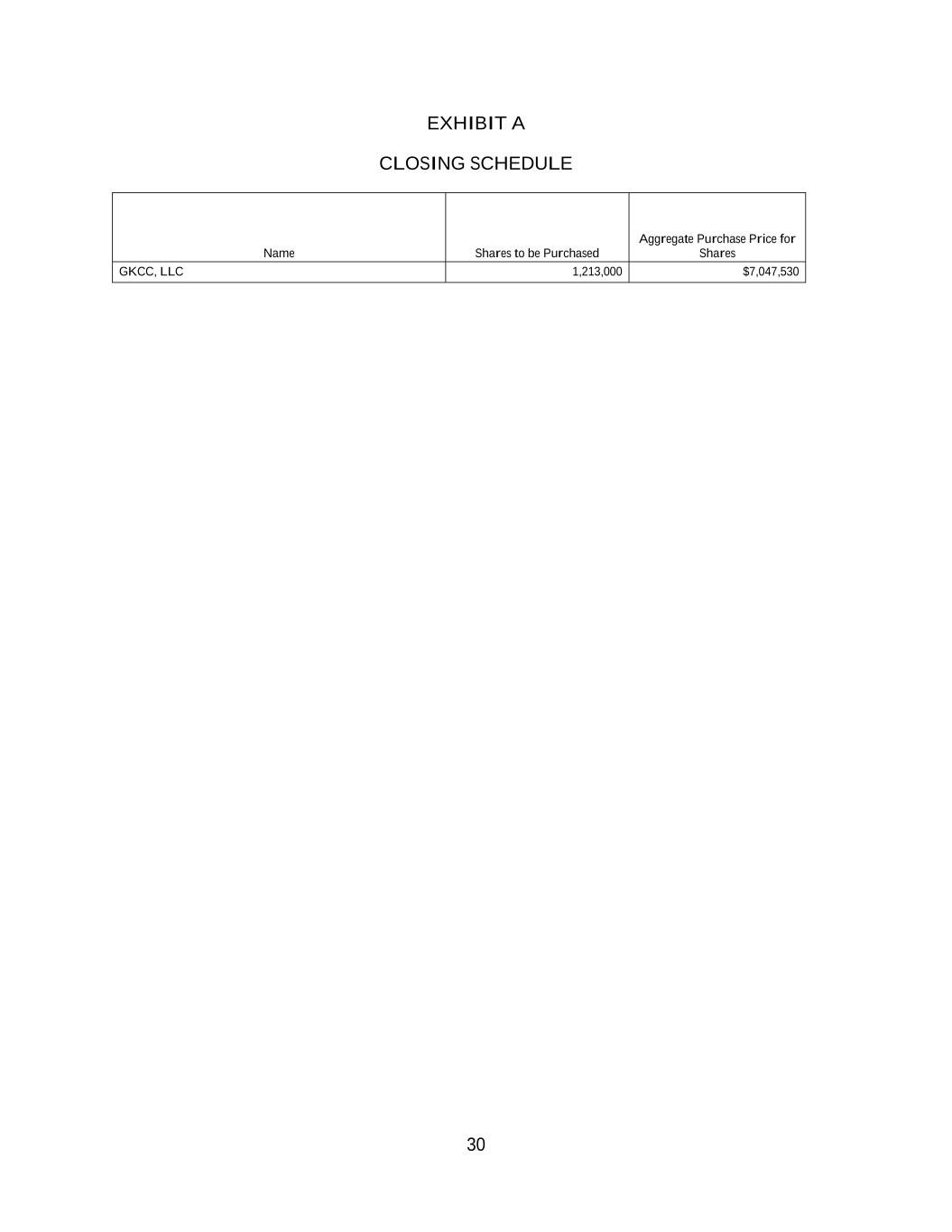
30 EXHIBIT A CLOSING SCHEDULE Name Shares to be Purchased Aggregate Purchase Price for Shares GKCC, LLC 1,213,000 $7,047,530