AbCellera Posts Positive Results For Advanced Covid-19 Treatment Trial
March 10 2021 - 12:32PM
Dow Jones News
By Kimberly Chin
AbCellera Biologics Inc. reported positive results for an
advanced trial of its Covid-19 combination treatment, as the
company seeks to expand its antibody therapy globally.
The Vancouver, Canada-based biotechnology firm, alongside its
partner Eli Lilly & Co., said their bamlanivimab (LY-CoV555)
treatment in combination with etesevimab reduced hospitalizations
and deaths in Covid-19 positive patients at high risk by 87%
compared with a placebo, the company said.
This was the second Phase 3 trial of the combination treatment
with positive results, AbCellera said.
The study's results support the use of its combination antibody
therapy, adding to its safety and efficacy profile, the company
said. AbCellera's Covid-19 treatment was granted emergency-use
authorization by the U.S. Food and Drug Administration and given a
positive scientific opinion by the European Medicine Agency's
committee that evaluates such treatments for human use.
AbCellera's shares rose 5.3% to $32.28 Wednesday.
Write to Kimberly Chin at kimberly.chin@wsj.com
(END) Dow Jones Newswires
March 10, 2021 12:17 ET (17:17 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
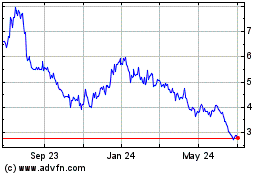
AbCellera Biologics (NASDAQ:ABCL)
Historical Stock Chart
From Mar 2024 to Apr 2024
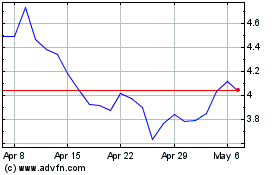
AbCellera Biologics (NASDAQ:ABCL)
Historical Stock Chart
From Apr 2023 to Apr 2024