Filed Pursuant to General Instruction II.L of Form F-10
File No. 333-259994
This prospectus supplement (the "Prospectus Supplement"), together with the short form base shelf prospectus dated July 5, 2021 to which it relates, as amended or supplemented (the "Base Shelf Prospectus"), and each document deemed to be incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus, as amended or supplemented (collectively, the "Prospectus"), constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and only by persons permitted to sell such securities. No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise.
Information has been incorporated by reference in the Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of Cybin Inc. at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9, telephone 1-866-292-4601, and are also available electronically at www.sedar.com and www.sec.gov/edgar.
PROSPECTUS SUPPLEMENT
(To the Short Form Base Shelf Prospectus dated July 5, 2021)

CYBIN INC.
U.S.$35,000,000
Common Shares
Cybin Inc. ("Cybin" or the "Corporation") is hereby qualifying the distribution (the "Offering") of common shares in the capital of the Corporation (the "Common Shares"), having an aggregate sale price of up to U.S.$35,000,000 (or the equivalent in United States dollars determined using the daily exchange rate posted by the Bank of Canada on the date the Common Shares are sold), see "Plan of Distribution" and "Description of the Common Shares".
The issued and outstanding Common Shares are listed in Canada on the Neo Exchange Inc. ("NEO") and in the United States on the NYSE American LLC ("NYSE American"), in each case under the trading symbol "CYBN". On August 5, 2022, the last trading day prior to the filing of this Prospectus Supplement, the closing prices of the Common Shares listed on the NEO and NYSE American were $0.95 and U.S.$0.73, respectively.
The Corporation has entered into an "at-the-market equity" distribution agreement dated August 8, 2022 (the "Distribution Agreement") with Cantor Fitzgerald Canada Corporation (the "Canadian Agent") and Cantor Fitzgerald & Co. (the "US Agent", together with the Canadian Agent, the "Agents") pursuant to which the Corporation may distribute Common Shares from time to time through the Agents, as agents, in accordance with the terms of the Distribution Agreement, see "Plan of Distribution". Sales of Common Shares, if any, under the Prospectus are anticipated to be made in transactions that are deemed to be "at-the-market distributions" as defined in National Instrument 44-102 - Shelf Distributions ("NI 44-102") and an "at-the-market offering" as defined in Rule 415(a)(4) under the United States Securities Act of 1933, as amended (the "U.S. Securities Act"), in privately negotiated transactions and/or any other method permitted by applicable law including sales made directly on the NEO by the Canadian Agent, and on NYSE American by the US Agent, or any other recognized marketplace upon which the Common Shares are listed or quoted or where the Common Shares are traded in Canada or the United States. The Common Shares will be distributed at the market prices prevailing at the time of the sale. As a result, prices at which Common Shares are sold may vary as between purchasers and during the period of any distribution. There is no minimum amount of funds that must be raised under the Offering. This means that the Offering may terminate after raising only a portion of the Offering amount set out above, or none at all. The Canadian Agent is not registered as a broker-dealer in the United States and, accordingly, will only sell Common Shares on marketplaces in Canada. The US Agent is not registered as an investment dealer in any Canadian jurisdiction and, accordingly, will only sell Common Shares on marketplaces in the United States. See "Plan of Distribution".
The Offering is being made concurrently in Canada under the terms of the Prospectus and in the United States under the Corporation's Registration Statement on Form F-10 (File No. 333-259994) (the "Registration Statement"), filed with the United States Securities and Exchange Commission (the "SEC"), of which this Prospectus Supplement forms a part.
The Corporation will pay the Agents compensation for their services in acting as agent in connection with the sale of Common Shares pursuant to the Distribution Agreement equal to 3% of the gross sale price per Common Share sold (the "Commission").
The Corporation has applied to list the Common Shares distributed hereunder on the NEO and NYSE American has authorized the listing of the Common Shares to be offered in the Offering. Listing will be subject to the Corporation fulfilling all listing requirements of the NEO and NYSE American.
An investment in the Common Shares is highly speculative and involves significant risks that you should consider before purchasing Common Shares. See the "Risk Factors" section in this Prospectus Supplement, including in the documents incorporated by reference.
The net proceeds that the Corporation will receive from sales of the Common Shares will vary depending on the number of shares actually sold and the offering price for such shares, but will not exceed U.S.$33,950,000 in the aggregate. See "Use of Proceeds" for how the net proceeds, if any, from sales under this Prospectus Supplement will be used.
The Corporation is permitted, under the multi-jurisdictional disclosure system adopted by the United States and Canada ("MJDS"), to prepare this Prospectus Supplement and the accompanying Base Shelf Prospectus in accordance with Canadian disclosure requirements. Purchasers of Common Shares should be aware that such requirements are different from those of the United States. Financial statements included or incorporated by reference herein have been prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, and may not be comparable to financial statements of United States companies, which are prepared under United States generally accepted accounting principles, or "US GAAP". Such financial statements are subject to the standards of the Public Company Accounting Oversight Board (United States) and the SEC independence standards.
This Prospectus Supplement and the Base Shelf Prospectus do not contain all of the information set forth in the Registration Statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC, or the schedules or exhibits that are part of the Registration Statement. Investors in the United States should refer to the Registration Statement and the exhibits thereto for further information with respect to the Corporation and the Common Shares.
Purchasers of Common Shares should be aware that the acquisition of Common Shares may have tax consequences both in the United States and in Canada. Such consequences for purchasers who are resident in, or citizens of, the United States or who are resident in Canada may not be described fully herein. Purchasers of Common Shares should read the tax discussion contained in this Prospectus Supplement and consult their own tax advisors. See "Certain Canadian Income Tax Considerations" and "Certain United States Federal Income Tax Considerations".
The enforcement by investors of civil liabilities under United States federal securities laws may be affected adversely by the fact that the Corporation is incorporated under the laws of Canada, certain of the officers and directors are not residents of the United States, that some or all of the Agents or experts named in this Prospectus Supplement and in the accompanying Base Shelf Prospectus are not residents of the United States, and that a substantial portion of the assets of the Corporation and such persons are located outside the United States. See "Enforcement of Civil Liabilities".
NEITHER THE SEC NOR ANY STATE SECURITIES COMMISSION OR REGULATOR HAS APPROVED OR DISAPPROVED THE COMMON SHARES NOR PASSED UPON THE ACCURACY OR ADEQUACY OF THE PROSPECTUS AND THIS PROSPECTUS SUPPLEMENT. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
In connection with the sale of the Common Shares on the Corporation's behalf, the Agents may be deemed to be an "underwriter" within the meaning of Section 2(a)(11) of the U.S. Securities Act, and the compensation of the Agents may be deemed to be underwriting commissions or discounts. The Corporation has agreed to provide indemnification and contribution to the Agents against certain liabilities, including liabilities under the U.S. Securities Act.
As sales agents, the Agents will not engage in any prohibited transactions to stabilize or maintain the price of the Common Shares. Neither the Agents nor any person or company acting jointly or in concert with either of the Agents may, in connection with the distribution, enter into any transaction that is intended to stabilize or maintain the market price of the Common Shares distributed under this Prospectus Supplement, including selling an aggregate number or principal amount of Common Shares that would result in the Agents creating an over-allocation position in the Common Shares.
Each of Douglas Drysdale, Michael Palfreyman, Alex Nivorozhkin and Brett Greene, officers of the Corporation, resides outside of Canada. They have each appointed Maxims CS Inc., Suite 1800, 181 Bay Street, Toronto, Ontario, M5J 2T9, as agent for service of process in Ontario. Prospective purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
Except as otherwise indicated, references to "Canadian dollars" or "$" are to the currency of Canada. Certain totals, subtotals and percentages may not precisely reconcile due to rounding.
The Corporation's head and registered office is located at 100 King Street West, Suite 5600, Toronto, ON M5X 1C9.
Cantor
TABLE OF CONTENTS
Prospectus Supplement
Base Shelf Prospectus
ABOUT THIS PROSPECTUS SUPPLEMENT
This document consists of two parts. The first part is this Prospectus Supplement, which describes certain terms of the Common Shares that the Corporation is offering and also adds to and updates certain information contained in the Base Shelf Prospectus and the documents incorporated by reference therein. The second part, the Base Shelf Prospectus, gives more general information, some of which may not apply to the Common Shares offered hereunder. Defined terms or abbreviations used in this Prospectus Supplement that are not defined herein have the meanings ascribed thereto in the Base Shelf Prospectus. Investors should rely only on the information contained or incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus. The Corporation has not, and the Agents have not, authorized anyone to provide investors with different or additional information. The Corporation is not, and the Agents are not, making an offer to sell Common Shares in any jurisdiction where the offer or sale is not permitted. Investors should not assume that the information appearing in this Prospectus Supplement, the Base Shelf Prospectus or any documents incorporated by reference herein or therein, is accurate as of any date other than the date indicated in those documents, as the Corporation's business, operating results, financial condition and prospects may have changed since such date. Before you invest, you should carefully read this Prospectus Supplement, the accompanying Base Shelf Prospectus and all information incorporated by reference herein and therein. These documents contain information you should consider when making your investment decision.
The Corporation filed the Base Shelf Prospectus with the securities commissions in all Canadian provinces and territories (the "Canadian Qualifying Jurisdictions") in order to qualify the offering of the securities described in the Base Shelf Prospectus in accordance with National Instrument 44-102 Shelf Distributions. The Ontario Securities Commission issued a receipt dated July 5, 2021 in respect of the final Base Shelf Prospectus as the principal regulatory authority under Multilateral Instrument 11-102 Passport System, and each of the other commissions in the Canadian Qualifying Jurisdictions is deemed to have issued a receipt under National Policy 11-202 Process for Prospectus Review in Multiple Jurisdictions.
The Base Shelf Prospectus also forms part of the Registration Statement that the Corporation filed with the SEC on October 1, 2021 under the U.S. Securities Act utilizing the MJDS. The Registration Statement became effective under the U.S. Securities Act on October 8, 2021. The Registration Statement includes the Base Shelf Prospectus with certain modifications and deletions permitted by Form F-10 and the rules and regulations of the SEC. This Prospectus Supplement is being filed by the Corporation with the SEC in accordance with General Instruction II.L of Form F-10.
Unless otherwise indicated or the context otherwise requires, all references in this Prospectus Supplement to "Cybin" or the "Corporation", except as otherwise indicated or as the context otherwise indicates, mean Cybin Inc. and its subsidiaries and associated corporations.
This Prospectus Supplement is deemed to be incorporated by reference in the Base Shelf Prospectus solely for the purposes of the Offering. Other documents are also incorporated or deemed to be incorporated by reference in this Prospectus Supplement and in the Base Shelf Prospectus. See "Documents Incorporated by Reference".
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus Supplement, and in certain documents incorporated by reference herein, constitute "forward-looking information" and "forward-looking statements," within the meaning of applicable securities laws. All statements other than statements of historical fact, including, without limitation, those regarding the Corporation's future financial position and results of operations, strategy, plans, objectives, goals and targets, future developments in the markets where the Corporation participates or is seeking to participate, and any statements preceded by, followed by or that include the words "believe", "expect", "aim", "intend", "plan", "continue", "will", "may", "would", "anticipate", "estimate", "forecast", "predict", "project", "seek", "should", "objective", "assumes" or similar expressions or the negative thereof, are forward-looking statements.
These statements are not historical facts but instead represent only the Corporation's expectations, estimates and projections regarding future events. These statements are not guarantees of future performance and involve assumptions, risks and uncertainties that are difficult to predict. Therefore, actual results may differ materially from what is expressed, implied or forecasted in such forward-looking statements. Additional factors that could cause actual results, performance or achievements to differ materially include, but are not limited to, those discussed under "Risk Factors" in the Annual Information Form (as defined herein) and in this Prospectus Supplement and in other documents incorporated by reference herein. Management provides forward-looking statements because it believes they provide useful information to readers when considering their investment objectives and cautions readers that the information may not be appropriate for other purposes. Consequently, all of the forward-looking statements made in this Prospectus Supplement and in documents incorporated by reference herein are qualified by these cautionary statements and other cautionary statements or factors contained herein, and there can be no assurance that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Corporation. These forward-looking statements are made as of the date of this Prospectus Supplement and the Corporation assumes no obligation to update or revise them to reflect subsequent information, events or circumstances or otherwise, except as required by law.
The forward-looking statements in this Prospectus Supplement and in documents incorporated by reference herein are based on numerous assumptions regarding the Corporation's present and future business strategies and the environment in which the Corporation will operate in the future, including assumptions regarding business and operating strategies, and the Corporation's ability to operate on a profitable basis.
Some of the risks which could affect future results and could cause results to differ materially from those expressed in the forward-looking statements contained herein include: the net proceeds, if any, from sales under this Prospectus Supplement; the Corporation's use of proceeds and business objectives and milestones and the anticipated timing of execution, see "Use of Proceeds"; novel coronavirus "COVID-19"; limited operating history; achieving publicly announced milestones; speculative nature of investment risk; early stage of the industry and product development; regulatory risks and uncertainties; plans for growth; limited products; limited marketing and sales capabilities; no assurance of commercial success; no profits or significant revenues; reliance on third parties for clinical development activities; risks related to third party relationships; reliance on contract manufacturers; safety and efficacy of products; clinical testing and commercializing products; completion of clinical trials; commercial grade product manufacturing; nature of regulatory approvals; unfavourable publicity or consumer perception; social media; biotechnology and pharmaceutical market competition; reliance on key executives and scientists; employee misconduct; business expansion and growth; negative results of external clinical trials or studies; product liability; enforcing contracts; product recalls; distribution and supply chain interruption; difficulty to forecast; promoting the brand; product viability; success of quality control systems; reliance on key inputs; liability arising from fraudulent or illegal activity; operating risk and insurance coverage; costs of operating as public company; management of growth; conflicts of interest; foreign operations; cybersecurity and privacy risk; environmental regulation and risks; decriminalisation of psychedelics; forward-looking statements may prove to be inaccurate; effects of inflation; political and economic conditions; application and interpretation of tax laws; enforcement of civil liabilities; Risks Related to Intellectual Property: trademark protection; trade secrets; patent law reform; patent litigation and intellectual property; protection of intellectual property; third-party licences; Financial and Accounting Risks: substantial number of authorized but unissued Common Shares; dilution; negative cash flow from operating activities; additional capital requirements; lack of significant product revenue; estimates or judgments relating to critical accounting policies; inadequate internal controls; Risks related to the Common Shares: market for the Common Shares; significant sales of Common Shares; volatile market price for the Common Shares; tax issues; no dividends; Risks related to the Offering: an investment in the Common Shares is highly speculative; completion of the Offering; negative operating cash flow and going concern; discretion in the use of proceeds; potential dilution; trading market; significant sales of Common Shares; positive return not guaranteed.
Although the forward-looking statements are based upon what management currently believes to be reasonable assumptions, the Corporation cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. In particular, the Corporation has made assumptions regarding, among other things:
• substantial fluctuation of losses from quarter to quarter and year to year due to numerous external risk factors, and anticipation that the Corporation will continue to incur significant losses in the future;
• uncertainty as to the Corporation's ability to raise additional funding to support operations;
• the Corporation's ability to access additional funding;
• the fluctuation of foreign exchange rates;
• the duration of COVID-19 and the extent of its economic and social impact;
• the risks associated with the development of the Corporation's product candidates which are at early stages of development;
• reliance upon industry publications as the Corporation's primary sources for third-party industry data and forecasts;
• reliance on third parties to plan, conduct and monitor the Corporation's preclinical studies and clinical trials;
• reliance on third party contract manufacturers to deliver quality clinical and preclinical materials;
• the Corporation's product candidates may fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or may not otherwise produce positive results;
• risks related to filing investigational new drug applications to commence clinical trials and to continue clinical trials if approved;
• the risks of delays and inability to complete clinical trials due to difficulties enrolling patients;
• competition from other biotechnology and pharmaceutical companies;
• the Corporation's reliance on the capabilities and experience of the Corporation's key executives and scientists and the resulting loss of any of these individuals;
• the Corporation's ability to fully realize the benefits of acquisitions;
• the Corporation's ability to adequately protect the Corporation's intellectual property and trade secrets;
• the risk of patent-related or other litigation; and
• the risk of unforeseen changes to the laws or regulations in the United States, Canada, the United Kingdom, Ireland and other jurisdictions in which the Corporation operates.
Drug development involves long lead times, is very expensive and involves many variables of uncertainty. Anticipated timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation. Every patient treated on future studies can change those assumptions either positively (to indicate a faster timeline to new drug applications and other approvals) or negatively (to indicate a slower timeline to new drug applications and other approvals). This Prospectus Supplement and the documents incorporated by reference herein contain certain forward-looking statements regarding anticipated or possible drug development timelines. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assumes the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Corporation's development efforts to date.
In addition to the factors set out above and those identified under the heading "Risk Factors" in the Annual Information Form and in this Prospectus Supplement, other factors not currently viewed as material could cause actual results to differ materially from those described in the forward-looking statements. Although the Corporation has attempted to identify important risks and factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors and risks that cause actions, events or results not to be anticipated, estimated or intended. Accordingly, readers should not place any undue reliance on forward-looking statements.
Many of these factors are beyond the Corporation's ability to control or predict. These factors are not intended to represent a complete list of the general or specific factors that may affect the Corporation. The Corporation may note additional factors elsewhere in this Prospectus Supplement and in any documents incorporated by reference herein. All forward-looking statements speak only as of the date made. All subsequent written and oral forward-looking statements attributable to the Corporation, or persons acting on the Corporation's behalf, are expressly qualified in their entirety by the cautionary statements. Except as required by law, the Corporation undertakes no obligation to update any forward-looking statement.
The forward-looking statements contained in this Prospectus Supplement and the documents incorporated by reference herein are expressly qualified in their entirety by the foregoing cautionary statement. Investors should read this entire Prospectus, including the Annual Information Form, the documents incorporated by reference herein, and each applicable Prospectus Supplement, and consult their own professional advisers to ascertain and assess the income tax and legal risks and other aspects associated with holding securities of the Corporation.
DOCUMENTS INCORPORATED BY REFERENCE
This Prospectus Supplement is deemed to be incorporated by reference in the Base Shelf Prospectus solely for the purpose of the Offering.
As at the date hereof, the following documents of the Corporation filed with the securities commissions or similar authorities in Canada, and filed with, or furnished to, the SEC, are specifically incorporated by reference into, and form an integral part of, this Prospectus Supplement:
1. annual information form of the Corporation dated June 20, 2022 (the "Annual Information Form") for the year ended March 31, 2022;
2. the audited consolidated financial statements of the Corporation and the notes thereto as at and for the fiscal year ended March 31, 2022, together with the auditor's report thereon;
3. management's discussion and analysis of the Corporation for the year ended March 31, 2022;
4. the Corporation's unaudited interim condensed consolidated financial statements for the three months ended June 30, 2022, and related notes thereto (the "Interim Financial Statements");
5. the management's discussion and analysis for the three ended June 30, 2022 (the "Interim MD&A"); and
6. management information circular of the Corporation dated July 13, 2022 relating to an annual meeting of shareholders of the Corporation to be held on August 15, 2022.
Any document of the type referred to in Section 11.1 of Form 44-101F1 - Short Form Prospectus Distributions filed by the Corporation with a securities commission or similar regulatory authority in Canada subsequent to the date of this Prospectus Supplement and prior to the termination of this distribution shall be deemed to be incorporated by reference in the Prospectus Supplement for the purposes of the Offering. In addition, if the Corporation disseminates a news release in respect of previously undisclosed information that, in the Corporation's determination, constitutes a "material fact" (as such term is defined under applicable Canadian securities laws), the Corporation will identify such news release as a "designated news release" for the purposes of the Prospectus in writing on the face page of the version of such news release that the Corporation files on SEDAR (any such news release, a "Designated News Release"), and any such Designated News Release shall be deemed to be incorporated by reference into the Prospectus only for the purposes of the Offering. These documents will be available through the internet on the Corporation's SEDAR profile, which can be accessed at www.sedar.com. In addition, any other report on Form 6-K or 40-F or the exhibits thereto filed or furnished, as applicable, by the Corporation with the SEC, under the United States Securities Exchange Act of 1934, as amended (the "Exchange Act"), from the date of this Prospectus Supplement and prior to the termination or completion of the Offering shall be deemed to be incorporated by reference as exhibits to the Registration Statement of which this Prospectus Supplement and accompanying Base Shelf Prospectus forms a part, but in the case of any report on Form 6-K, only if and to the extent expressly so provided in any such report. The Corporation's current reports on Form 6-K and annual reports on Form 40-F are available on EDGAR at www.sec.gov.
Any statement contained in this Prospectus Supplement or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the purposes of the Offering to the extent that a statement contained herein or in any subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus Supplement or Base Shelf Prospectus modifies or supersedes that statement. Any statement so modified or superseded shall not constitute a part of this Prospectus Supplement except as so modified or superseded. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
References to the Corporation's website in any documents that are incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus do not incorporate by reference the information on such website into this Prospectus Supplement and the Base Shelf Prospectus and the Corporation disclaims any such incorporation by reference. Neither the Corporation nor the Agents have provided or otherwise authorized any other person to provide investors with information other than that contained or incorporated by reference in this Prospectus Supplement or accompanying Base Shelf Prospectus, and neither the Corporation nor the Agents take any responsibility for other information that others may give you. If an investor is provided with different or inconsistent information, he or she should not rely on it.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
In addition to the documents specified in this Prospectus Supplement and in the accompanying Base Shelf Prospectus under "Documents Incorporated by Reference", the following documents have been or will be filed with the SEC as part of the Registration Statement: (i) the Distribution Agreement; (ii) powers of attorney from certain of the Corporation's directors and officers (included on the signature page of the Registration Statement); (iii) the consent of Zeifmans LLP; and (iv) the consent of the Corporation's Canadian counsel, Aird & Berlis LLP.
ADDITIONAL INFORMATION
The Corporation has filed with the SEC the Registration Statement under the U.S. Securities Act with respect to the Common Shares offered under this Prospectus Supplement. This Prospectus Supplement, the accompanying Base Shelf Prospectus and the documents incorporated by reference herein and therein, which form a part of the Registration Statement, do not contain all of the information set forth in the Registration Statement, certain parts of which are contained in the exhibits to the Registration Statement as permitted by the rules and regulations of the SEC. Information omitted from this Prospectus Supplement or the Base Shelf Prospectus but contained in the Registration Statement is available on EDGAR under the Corporation's profile at www.sec.gov. Reference is also made to the Registration Statement and the exhibits thereto for further information with respect to the Corporation, the Offering and the Common Shares. Statements contained in this Prospectus Supplement as to the contents of certain documents are not necessarily complete and, in each instance, reference is made to the copy of the document filed as an exhibit to the Registration Statement. Each such statement is qualified in its entirety by such reference.
The Corporation is required to file with the various securities commissions or similar authorities in all of the provinces and territories of Canada, annual and quarterly reports, material change reports and other information. The Corporation is also an SEC registrant subject to the informational requirements of the Exchange Act and, accordingly, files with, or furnishes to, the SEC certain reports and other information. Under MJDS, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Corporation's officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
THE CORPORATION
This summary does not contain all the information that may be important to you in deciding whether to invest in the Common Shares. You should read the entire Prospectus, including the section entitled "Risk Factors", the applicable Prospectus Supplement, and the documents incorporated by reference herein, including the Annual Information Form, before making such decision.
Summary of the Business
The Corporation is a biotechnology company focused on advancing pharmaceutical therapies, delivery mechanisms, novel compounds and protocols as potential therapies for various psychiatric and neurological conditions. The Corporation is developing technologies and delivery systems aiming to improve the pharmacokinetics of its psychedelic molecules while retaining the therapeutics benefit. The new molecules and delivery systems are expected to be studied through clinical trials to confirm safety and efficacy.
The Corporation has historically had two business segments: (a) Serenity Life Sciences Inc. and Cybin US Holdings Inc. ("Cybin U.S.") that focus on the research and development of psychedelic pharmaceutical products; and (b) Natures Journey Inc. ("Natures Journey") that focused on consumer mental wellness, including non-psychedelic nutraceutical products (the "Product Line") and consumer mental wellness. In November 2021, the Corporation decided to not proceed with the Natures Journey business segment in order to prioritize its research and development of psychedelic pharmaceutical products.
Psychedelics
The Corporation is conducting research and development of psychedelic therapeutics that aim to address unmet mental health conditions by leveraging proprietary drug discovery platforms, novel formulation approaches, innovative drug delivery systems, and optimized treatment regimens. This comprehensive strategy is predicated on structural modifications of known and well understood tryptamine derivatives to improve their pharmacokinetic properties without altering their respective pharmacology.
Across the various research and development programs, the Corporation is researching and developing a wide array of novel, synthetic psychedelic API intended to be delivered through innovative drug delivery systems including sublingual films,1 orally disintegrating tablets ("ODT")2 and via inhalation.
The Corporation intends to apply for regulatory approval for products targeting major depressive disorder ("MDD"), alcohol use disorder ("AUD") and various anxiety disorders.3 The Corporation is also developing products that may have the potential to address neuroinflammation.4
Non-Psychedelics
In November 2021, the Corporation decided to not proceed with the Natures Journey business segment, including the Product Line in order to prioritize its progression of its research and development of psychedelic pharmaceutical products. As of the date hereof, the Corporation has not begun operations nor generated any revenue from the sale of the Product Line and it does not expect any revenues from the Product Line going forward.
For additional information in respect of the Corporation and its operation, please see the Corporation's Annual Information Form incorporated by reference into this Prospectus Supplement.
Recent Developments
There have been no material developments in the business of the Corporation since August 7, 2022, the date of the Corporation's most recently issued Interim Financial Statements and Interim MD&A.
___________________________________________
Intercorporate Relationships
As at the date of this Prospectus Supplement, the Corporation's corporate structure includes the following material wholly-owned subsidiaries:

Note:
(1) The shareholders of Adelia Therapeutics Inc. ("Adelia") hold certain non-voting securities of Cybin U.S. issued in connection with the acquisition of Adelia on December 14, 2020 (the "Adelia Transaction"). For additional information in respect of the Adelia Transaction, please see the Corporation's Annual Information Form incorporated by reference in this Prospectus Supplement.
DESCRIPTION OF THE COMMON SHARES
The Corporation is authorized to issue an unlimited number of Common Shares and an unlimited number of preferred shares. As at August 8, 2022, the Corporation had 166,120,171 Common Shares and nil preferred shares issued and outstanding. For a summary of certain material attributes and characteristics of the Common Shares, see "Description of the Securities Being Distributed - Common Shares" in the Base Shelf Prospectus.
CONSOLIDATED CAPITALIZATION
There have been no material changes in the Corporation's share or loan capitalization on a consolidated basis since the date of the Interim Financial Statements. As a result of the Offering, the shareholder's equity of the Corporation will increase by the amount of the net proceeds of the Offering and the number of issued and outstanding Common Shares will increase by the number of Common Shares actually distributed under the Offering.
USE OF PROCEEDS
The net proceeds from the Offering are not determinable in light of the nature of the distribution. The net proceeds of any given distribution of Common Shares through the Agents in an "at-the-market distribution" or "at-the-market offering" will represent the gross proceeds after deducting the applicable compensation payable to the Agents under the Distribution Agreement and the expenses of the distribution. The proceeds actually received by the Corporation will depend on the number of Common Shares actually sold and the offering price of such Common Shares. See "Plan of Distribution".
Assuming net proceeds of the maximum of U.S.$33,950,000 on or before the expiry of the Prospectus on August 5, 2023, the Corporation intends to use the net proceeds of the Offering for: (i) growth opportunities, and (ii) working capital initiatives. The Corporation believes it is prudent, particularly at the Corporation's stage of development and the competitive industry landscape, to secure capital for general corporate and working capital purposes to ensure that the Corporation maintains sufficient liquidity and capital resources in the near to medium term.
At any given time, the Corporation may be engaged in discussions and activities in respect of potential growth initiatives and other strategic opportunities that are complementary or accretive to the Corporation's business, which may include acquisitions or other investments. As of the date of this Prospectus Supplement, the Corporation has not identified any specific investments or projects, nor any probable or significant acquisitions it wishes to undertake; however, it is important for the Corporation to have funds available to quickly and opportunistically pursue such opportunities as they arise. To the extent the Corporation requires additional capital, it may raise funds through debt and equity financing in the future.
The use of the net proceeds of the Offering to fund potential growth initiatives and other strategic opportunities is subject to change due to the influence of many evolving variables, including those as described under "Stage of Development" in the Interim MD&A and in the Annual Information Form, any changes in legislation and regulations, applications for licenses and the receipt of required licenses, renewals and other regulatory approvals. As a result, the Corporation cannot provide definitive details with respect to timing or specific uses of the net proceeds of the Offering. Such decisions will depend on market and competitive factors, as described herein, as they evolve over time. See "Cautionary Statement Regarding Forward-Looking Statements" and "Risk Factors".
There may be circumstances where for sound business reasons, the Corporation reallocates the use of proceeds depending on the amount of proceeds raised, the time periods in which the proceeds are raised, developments in relation to potential growth initiatives and other strategic opportunities or unforeseen events, see "Risk Factors - Risks Related to the Offering - Discretion in the Use of Proceeds".
Since inception, the Corporation has financed its operations primarily from the issuance of equity and interest income on funds available for investment. To date, the Corporation has raised approximately $120,000,000 in gross proceeds through private placement and prospectus offerings. The Corporation has experienced operating losses and cash outflows from operations since incorporation and will require ongoing financing to continue its research and development activities. As the Corporation has not yet achieved profitability, there are uncertainties regarding its ability to continue as a going concern. The Corporation has not earned any revenue or reached successful commercialization of any products. The Corporation's success is dependent upon the ability to finance its cash requirements to continue its activities. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained, or at all. To the extent that the Corporation has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Corporation will be required to raise additional funds through the issuance of additional equity securities, through loan financing, or other means, such as through partnerships with other companies and research and development reimbursements. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained. See "Risk Factors - Risks Related to an Offering - Negative Operating Cash Flow and Going Concern".
Until applied, the net proceeds will be held as cash balances in the Corporation's bank account or invested in certificates of deposit and other instruments issued by banks or obligations of or guaranteed by the Government of Canada or any province thereof in accordance with the Corporation's investment policies.
For detailed information in respect of the Corporation's business objectives and milestones, sources and use of capital, and the application of proceeds from prior offerings by the Corporation, prospective purchasers should carefully consider the information described in the Base Shelf Prospectus, Annual Information Form and the Interim MD&A, to which there has been no material changes since the date of the applicable document.
PLAN OF DISTRIBUTION
The Corporation has entered into the Distribution Agreement with the Agents under which the Corporation may issue and sell from time to time Common Shares having an aggregate sale price of up to U.S.$35,000,000 (or the equivalent in United States dollars determined using the daily exchange rate posted by the Bank of Canada on the date the Common Shares are sold) in each of the provinces and territories of Canada and in the United States pursuant to placement notices delivered by the Corporation to the Agents from time to time in accordance with the terms of the Distribution Agreement. Sales of Common Shares, if any, will be made in transactions that are deemed to be "at-the-market distributions" as defined in NI 44-102 and an "at-the-market offering" as defined in Rule 415(a)(4) under the U.S. Securities Act, in privately negotiated transactions and/or any other method permitted by applicable law including sales made by the Agents directly on the NEO, NYSE American or any other trading market for the Common Shares in Canada or the United States. Subject to the pricing parameters in a placement notice, the Common Shares will be distributed at the market prices prevailing at the time of the sale. As a result, prices may vary as between purchasers and during the period of distribution. The Corporation cannot predict the number of Common Shares that the Corporation may sell under the Distribution Agreement on the NEO, NYSE American or any other trading market for the Common Shares in Canada, or if any Common Shares will be sold.
The Agents are not required to sell any specific number or dollar amount of Common Shares, but will use its commercially reasonable efforts to sell the Common Shares pursuant to the terms and conditions of the Distribution Agreement. There is no minimum amount of funds that must be raised under the Offering. This means that the Offering may terminate after only raising a small portion of the offering amount set out above, or none at all.
The Agents will offer the Common Shares subject to the terms and conditions of the Distribution Agreement from time to time or as otherwise agreed upon by the Corporation and the Agents. Subject to the terms and conditions of the Distribution Agreement, the Agents will use their commercially reasonable efforts to sell, on the Corporation's behalf, all of the Common Shares requested to be sold by the Corporation. The Corporation may instruct the Agents not to sell Common Shares if the sales cannot be achieved at or above the price designated by the Corporation in a particular placement notice.
Neither the Corporation nor the Agents may suspend the Offering upon proper notice to the other party. The Corporation and the Agents each have the right, by giving written notice as specified in the Distribution Agreement, to terminate the Distribution Agreement in each party's sole discretion at any time. Pursuant to the Distribution Agreement, the Offering will terminate upon the earliest of (i) the termination of the Distribution Agreement as provided for therein, (ii) the date on which the aggregate gross proceeds from sales of Common Shares pursuant to the Distributions Agreement equal U.S.$35,000,000 and (iii) August 5, 2023.
The Corporation will pay the Agents the Commission for their services in acting as agent in connection with the sale of Common Shares pursuant to the Distribution Agreement in an amount equal to 3% of the gross sale price per Common Share sold (the "Commission"). Provided, however, that the Corporation shall not be obligated to pay the Agents any Commission on any sale of Common Shares that it is not possible to settle due to (i) a suspension or material limitation in trading in securities generally on the NEO or NYSE American, (ii) a material disruption in securities settlement or clearance services in Canada or the United States, (iii) failure by the applicable Agent to comply with its obligations under the terms of the Distribution Agreement; or (iv) if the Corporation and the Agents agree, pursuant to the terms of the Distribution Agreement, that no sale of Common shares will take place. The sales proceeds remaining after payment of the Commission and after deducting any expenses payable by the Corporation and any transaction or filing fees imposed by any governmental, regulatory, or self-regulatory organization in connection with the sales, will equal the net proceeds to the Corporation from the sale of such Common Shares.
The applicable Agent or Agents will provide written confirmation to the Corporation no later than the opening of the trading day immediately following the trading day on which it has made sales of the Common Shares under the Distribution Agreement. Each confirmation will include the number of Common Shares sold on such day (including the number of Common Shares sold on the NEO and or NYSE American, or on any other marketplace in Canada or the United States), the average price of the Common Shares sold on such day (including the average price of Common Shares sold on NEO, NYSE American or on any other marketplace in Canada or the United States), the gross proceeds, the Commission payable by the Corporation to the Agents with respect to such sales and the net proceeds payable to the Corporation. The Agents will also assist the Corporation with such other periodic reporting as may be reasonably requested by the Corporation with respect to the sales of Common Shares.
The Corporation will disclose the number and average price of the Common Shares sold under this Prospectus Supplement, as well as the gross proceeds, Commission and net proceeds from sales hereunder in the Corporation's annual and interim financial statements and related management discussion & analysis, annual information forms and annual reports on Form 40-F, filed on SEDAR and filed or furnished, as applicable, with the SEC on EDGAR, for any quarters or annual periods in which sales of Common Shares occur.
Settlement for sales of Common Shares will occur, unless otherwise specified in an applicable placement notice, on the second trading day on the applicable exchange following the date on which any sales were made in return for payment of the net proceeds to the Corporation. There is no arrangement for funds to be received in an escrow, trust or similar arrangement. Sales of Common Shares in the United States will be settled through the facilities of The Depository Trust Corporation or by such other means as the Corporation and the Agents may agree upon and sales of Common Shares in Canada will be settled through the facilities of The Canadian Depository for Securities or by such other means as the Corporation and the Agents may agree.
The Canadian Agent is not registered as a broker-dealer in the United States and, accordingly, will only sell Common Shares on marketplaces in Canada. The US Agent is not registered as an investment dealer in any Canadian jurisdiction and, accordingly, will only sell Common Shares on marketplaces in the United States.
The Offering is being made in Canada under the terms of this Prospectus Supplement and concurrently in the United States under the terms of the Corporation's Registration Statement filed with the SEC of which this Prospectus Supplement forms a part. In connection with the sale of the Common Shares on the Corporation's behalf, the Agents may be deemed to be an "underwriter" within the meaning of Section 2(a)(11) of the U.S. Securities Act, and the compensation of the Agents may be deemed to be underwriting commissions or discounts. The Corporation has agreed to provide indemnification and contribution to the Agents against certain liabilities, including liabilities under the U.S. Securities Act.
The Corporation has agreed to pay the reasonable fees, disbursements and expenses of counsel to the Agents in connection with the Offering, subject to the terms of the Distribution Agreement and any other agreement in writing between the Corporation and the Agents. No agent, underwriter or dealer involved in the distribution of Common Shares under the Offering, no affiliate of such an agent, underwriter or dealer and no person or company acting jointly or in concert with such an agent, underwriter or dealer has over-allotted, or will over-allot, securities in connection with such distribution or effected, or will affect any other transactions that are intended to stabilize or maintain the market price of the Common Shares.
The total expenses related to the commencement of the Offering to be paid by the Corporation, excluding the Commission payable to the Agents under the Distribution Agreement, are estimated to be approximately up to U.S.$300,000.
Each of the Agents and its affiliates have in the past provided and may in the future provide various investment banking, commercial banking and other financial services for the Corporation and its affiliates, for which services they have received and may in the future receive customary fees. To the extent required by Regulation M, the Agents will not engage in any market making activities involving the Common Shares while the Offering is ongoing under this Prospectus Supplement.
The Corporation has applied to list the Common Shares offered by this Prospectus Supplement on the NEO and NYSE American has authorized the listing of the Common Shares to be offered in the Offering. Listing will be subject to the Corporation fulfilling all of the listing requirements of the NEO and NYSE American.
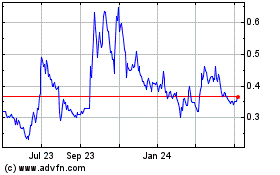
TRADING PRICE AND VOLUME
The Common Shares are listed for trading on the NEO and NYSE American under the symbol "CYBN". The following table sets forth the reported monthly range of high and low prices per Common Share and total monthly volumes traded on the NEO and NYSE American for each of the months indicated during the 12-month period prior to the date of this Prospectus Supplement.
NEO Price Range
|
| |
| Month |
|
High ($)(1) |
|
Low ($)(1) |
|
Volume(1) |
| |
|
|
|
|
|
|
| August 2021 |
|
3.99 |
|
2.15 |
|
11,652,282 |
| |
|
|
|
|
|
|
| September 2021 |
|
3.55 |
|
2.54 |
|
9,933,259 |
| |
|
|
|
|
|
|
| October 2021 |
|
2.79 |
|
2.24 |
|
5,973,696 |
| |
|
|
|
|
|
|
| November 2021 |
|
2.94 |
|
1.65 |
|
14,160,868 |
| |
|
|
|
|
|
|
| December 2021 |
|
1.94 |
|
1.35 |
|
6,191,101 |
| |
|
|
|
|
|
|
| January 2022 |
|
1.54 |
|
1.21 |
|
3,636,976 |
| |
|
|
|
|
|
|
| February 2022 |
|
1.46 |
|
1.11 |
|
2,935,312 |
| |
|
|
|
|
|
|
| March 2022 |
|
1.20 |
|
0.95 |
|
3,883,026 |
| |
|
|
|
|
|
|
| April 2022 |
|
1.12 |
|
0.68 |
|
1,784,193 |
| |
|
|
|
|
|
|
| May 2022 |
|
0.54 |
|
0.50 |
|
3,017,959 |
| |
|
|
|
|
|
|
| June 2022 |
|
1.06 |
|
0.67 |
|
2,662,940 |
| |
|
|
|
|
|
|
| July 2022 |
|
0.79 |
|
0.69 |
|
1,697,807 |
| |
|
|
|
|
|
|
| August (1 - 5), 2022 |
|
0.95 |
|
0.78 |
|
744,487 |
Note:
(1) Source: TMX Money as of the date of this Prospectus Supplement.
On August 5, 2022, being the last day on which the Common Shares traded prior to the date of this Prospectus Supplement, the closing price of the Common Shares as reported on the NEO was $0.95.
| NYSE American Price Range |
| |
| Month |
|
High (U.S.$)(1) |
|
Low (U.S.$)(1) |
|
Volume(1) |
| |
|
|
|
|
|
|
| August 2021(2) |
|
3.38 |
|
1.68 |
|
27,050,719 |
| |
|
|
|
|
|
|
| September 2021 |
|
2.86 |
|
1.98 |
|
23,267,531 |
| |
|
|
|
|
|
|
| October 2021 |
|
2.29 |
|
1.81 |
|
26,186,831 |
| |
|
|
|
|
|
|
| November 2021 |
|
2.355 |
|
1.29 |
|
57,337,568 |
| |
|
|
|
|
|
|
| December 2021 |
|
1.54 |
|
1.05 |
|
32,155,939 |
| |
|
|
|
|
|
|
| January 2022 |
|
1.22 |
|
0.82 |
|
19,882,525 |
| |
|
|
|
|
|
|
| February 2022 |
|
1.16 |
|
0.862 |
|
13,843,580 |
| |
|
|
|
|
|
|
| March 2022 |
|
0.95 |
|
0.70 |
|
14,441,487 |
| |
|
|
|
|
|
|
| April 2022 |
|
0.88 |
|
0.42 |
|
15,659,793 |
| |
|
|
|
|
|
|
| May 2022 |
|
0.71 |
|
0.3903 |
|
14,762,190 |
| |
|
|
|
|
|
|
| June 2022 |
|
0.86 |
|
0.5301 |
|
12,694,019 |
| |
|
|
|
|
|
|
| July 2022 |
|
0.65 |
|
0.50 |
|
11,863,781 |
| |
|
|
|
|
|
|
| August (1 - 5), 2022 |
|
0.73 |
|
0.58 |
|
5,173,572 |
Notes:
(1) Source: NYSE American as of the date of this Prospectus Supplement.
(2) Prior to August 5, 2021, the Common Shares were quoted on the OTCQB® Venture Market under the symbol "CLXPF" (the "OTCQB"). The OTCQB quotation ceased when the Common Shares were listed on NYSE American on August 5, 2021.
On August 5, 2022, being the last day on which the Common Shares traded prior to the date of this Prospectus Supplement, the closing price of the Common Shares as reported on NYSE American was U.S.$0.73.
PRIOR SALES
The following table sets forth the details regarding all issuances of Common Shares, including issuances of all securities convertible or exchangeable into Common Shares, during the 12-month period before the date of this Prospectus Supplement:
| Date of Issuance |
|
Number of
Securities Issued |
|
Type |
|
Issuance / Exercise
Price Per Security |
| August 12, 2021(1) |
|
100,000 |
|
Common Shares |
|
$0.75 |
| August 12, 2021(1) |
|
255,887 |
|
Common Shares |
|
$0.64 |
| August 16, 2021 |
|
215,000 |
|
Options |
|
$2.48 |
| August 17, 2021(2) |
|
18,788.5 |
|
Exchangeable Securities |
|
$33.70(4) |
| August 18, 2021 |
|
300,000 |
|
Options |
|
$2.48 |
| August 31, 2021(2) |
|
9,392.6 |
|
Exchangeable Securities |
|
$33.80(5) |
| September 10, 2021(1) |
|
191,540 |
|
Common Shares |
|
$0.75 |
| September 16, 2021(3) |
|
125,000 |
|
Common Shares |
|
$0.64 |
| September 16, 2021(1) |
|
370,000 |
|
Common Shares |
|
$0.75 |
| September 27, 2021 |
|
585,000 |
|
Options |
|
$3.15 |
| September 27, 2021 |
|
195,000(6) |
|
Options |
|
$2.87 |
| September 30, 2021 |
|
450,000 |
|
Options |
|
$3.15 |
| September 30, 2021 |
|
740,000(7) |
|
Options |
|
$2.78 |
| October 13, 2021(1) |
|
18,000 |
|
Common Shares |
|
$0.25 |
| October 21, 2021(3) |
|
325,000 |
|
Common Shares |
|
$0.25 |
| October 29, 2021(3) |
|
150,000 |
|
Common Shares |
|
$1.36 |
| October 29, 2021(1) |
|
5,500 |
|
Common Shares |
|
$0.25 |
| November 16, 2021(3) |
|
175,000 |
|
Common Shares |
|
$1.39 |
| November 16, 2021(1) |
|
1,000,000 |
|
Common Shares |
|
$0.25 |
| November 18, 2021(2) |
|
28,903 |
|
Exchangeable Securities |
|
$24.40(8) |
| November 23, 2021(3) |
|
88,300 |
|
Common Shares |
|
$1.39 |
| November 29, 2021(2) |
|
31,721.5 |
|
Exchangeable Securities |
|
$19.80(9) |
| November 30, 2021(3) |
|
300,000 |
|
Common Shares |
|
$1.39 |
| December 16, 2021(1) |
|
150,000 |
|
Common Shares |
|
$0.25 |
| December 16, 2021(3) |
|
62,500 |
|
Common Shares |
|
$0.64 |
| December 31, 2021 |
|
40,000 |
|
Options |
|
$3.15 |
| December 31, 2021 |
|
1,250,000 |
|
Options |
|
$1.50 |
| January 6, 2022(2) |
|
15,611.4 |
|
Exchangeable Securities |
|
$15.10(10) |
| January 21, 2022(11) |
|
586,800 |
|
Common Shares |
|
$1.25 |
| February 14, 2022(2) |
|
41,028.2 |
|
Exchangeable Securities |
|
$13.43(12) |
| February 15, 2022(1) |
|
27,585 |
|
Common Shares |
|
$0.64 |
| February 18, 2022(2) |
|
17,239.5 |
|
Exchangeable Securities |
|
$13.50(13) |
| February 28, 2022(3) |
|
37,500 |
|
Common Shares |
|
$0.64 |
| March 4, 2022(11) |
|
1,254,360 |
|
Common Shares |
|
$1.13 |
| March 4, 2022 |
|
1,075,600 |
|
Options |
|
$1.13 |
| March 4, 2022 |
|
60,000 |
|
Options |
|
$3.15 |
| March 8, 2022 |
|
400,000 |
|
Options |
|
$1.02 |
| March 18, 2022(1) |
|
100,000 |
|
Common Shares |
|
$0.25 |
| March 25, 2022(2) |
|
90,546 |
|
Exchangeable Securities |
|
$10.00(14) |
| April 1, 2022(2) |
|
22,428.3 |
|
Exchangeable Securities |
|
$10.20(15) |
| May 5, 2022(15) |
|
380,230 |
|
Common Shares |
|
$0.68 |
| May 24, 2022(1) |
|
500,000 |
|
Common Shares |
|
$0.25 |
| June 14, 2022(1) |
|
500,000 |
|
Common Shares |
|
$0.25 |
| June 22, 2022(1) |
|
99,638 |
|
Common Shares |
|
$0.64 |
| June 22, 2022(2) |
|
456.50 |
|
Exchangeable Securities |
|
$10.20(16) |
| June 24, 2022(2) |
|
266,933.1 |
|
Exchangeable Securities |
|
$7.62(17) |
| June 27, 2022(2) |
|
37,366.2 |
|
Exchangeable Securities |
|
$7.50(18) |
| June 30, 2022 |
|
65,000 |
|
Options |
|
$1.00 |
| June 30, 2022 |
|
500,000 |
|
Options |
|
$0.90 |
Notes:
(1) Common Shares issued on exercise of warrants to purchase Common Shares (each, a "Warrant").
(2) Represents Class B common shares in the capital of Cybin U.S. ("Class B Shares") issued in connection with the Adelia Transaction to Adelia shareholders. The Class B Shares are exchangeable at the holder's option for Common Shares on the basis of 10 Common Shares for 1 Class B Share, subject to customary adjustments.
(3) Common Shares issued on exercise of options to purchase Common Shares granted pursuant to the Corporation's equity incentive plan (each, an "Option").
(4) Price per Class B Share of Cybin U.S., which are exchangeable for 187,886 Common Shares, resulting in an effective issue price of $3.37 per Common Share.
(5) Price per Class B Share of Cybin U.S., which are exchangeable for 93,926 Common Shares, resulting in an effective issue price of $3.38 per Common Share.
(6) As the result of the termination of an employee of the Corporation, 20,000 options expired on October 21, 2021.
(7) On March 4, 2022, 20,000 options were cancelled pursuant to an acknowledgement agreement.
(8) Price per Class B Share of Cybin U.S., which are exchangeable for 289,030 Common Shares, resulting in an effective issue price of $2.44 per Common Share.
(9) Price per Class B Share of Cybin U.S., which are exchangeable for 317,215 Common Shares, resulting in an effective issue price of $1.98 per Common Share.
(10) Price per Class B Share of Cybin U.S., which are exchangeable for 156,114 Common Shares, resulting in an effective issue price of $1.51 per Common Share.
(11) Common Shares issued in exchange for Class B Shares.
(12) Price per Class B Share of Cybin U.S., which are exchangeable for 410,282 Common Shares, resulting in an effective issue price of $1.34 per Common Share.
(13) Price per Class B Share of Cybin U.S., which are exchangeable for 172,395 Common Shares, resulting in an effective issue price of $1.35 per Common Share.
(14) Price per Class B Share of Cybin U.S., which are exchangeable for 905,460 Common Shares, resulting in an effective issue price of $1.00 per Common Share.
(15) Price per Class B Share of Cybin U.S., which are exchangeable for 224,283 Common Shares, resulting in an effective issue price of $1.02 per Common Share.
(16) Price per Class B Share of Cybin U.S., which are exchangeable for 4,560 Common Shares, resulting in an effective issue price of $1.02 per Common Share.
(17) Price per Class B Share of Cybin U.S., which are exchangeable for 2,669,331 Common Shares, resulting in an effective issue price of $0.762 per Common Share.
(18) Price per Class B Share of Cybin U.S., which are exchangeable for 373,662 Common Shares, resulting in an effective issue price of $0.75 per Common Share.
CERTAIN CANADIAN INCOME TAX CONSIDERATIONS
The following is, as at the date of this short form prospectus, a summary of the principal Canadian federal income tax considerations under the Tax Act generally applicable to an investor who acquires Common Shares as beneficial owner pursuant to the Offering and who, for the purposes of the Tax Act and at all relevant times, deals at arm's length with the Corporation and the Agents, is not affiliated with the Corporation or the Agents, and who acquires and holds the Common Shares as capital property (a "Holder"). Generally, the Common Shares will be considered to be capital property to a Holder thereof provided that the Holder does not use the Common Shares in the course of carrying on a business of trading or dealing in securities and such Holder has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder (i) that is a "financial institution" for the purposes of the mark-to-market rules contained in the Tax Act; (ii) that is a "specified financial institution" as defined in the Tax Act; (iii), an interest in which would be a "tax shelter investment" as defined in the Tax Act; (iv) that has made a functional currency reporting election under section 261 of the Tax Act; (v) that has entered into or will enter into a "derivative forward agreement" or "synthetic disposition arrangement", as those terms are defined in the Tax Act, with respect to the Common Shares; or (vi) that receives dividends on the Common Shares under or as part of a "dividend rental arrangement", as defined in the Tax Act. Such Holders should consult their own tax advisors with respect to an investment in Common Shares.
Additional considerations, not discussed herein, may be applicable to a Holder that is a corporation resident in Canada, and is, or becomes, or does not deal at arm's length for purposes of the Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or event or series of transactions or events that includes the acquisition of Common Shares, controlled by a non-resident person, or group of non-resident persons not dealing with each other at arm's length, for purposes of the foreign affiliate dumping rules in section 212.3 of the Tax Act. Such Holders should consult their own tax advisors.
This summary is based upon the current provisions of the Tax Act and the regulations thereunder in force as of the date hereof and counsel's understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency (the "CRA"). This summary takes into account all specific proposals to amend the Tax Act and the regulations thereunder publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the "Tax Proposals") and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. This summary does not otherwise take into account any changes in law or in the administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial decision or action, nor does it take into account or consider other federal or any provincial, territorial or foreign income tax considerations, which considerations may differ significantly from the Canadian federal income tax considerations discussed in this summary.
This summary is not exhaustive of all possible Canadian federal income tax considerations applicable to a Holder in respect of the transactions described herein. The income or other tax consequences will vary depending on the particular circumstances of the Holder, including the province or provinces in which the Holder resides or carries on business. Accordingly, this summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice or representations to any particular Holder. Moreover, no advance income tax ruling has been applied for or obtained from the CRA to confirm the tax consequences of any of the transactions described herein. Holders should consult their own legal and tax advisors for advice with respect to the tax consequences of an investment in the Common Shares based on their particular circumstances.
Currency Conversion
Subject to certain exceptions that are not discussed herein, for the purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of Common Shares (including dividends, adjusted cost base and proceeds of disposition) must be expressed in Canadian dollars. Amounts denominated in U.S. dollars must generally be converted into Canadian dollars based on the single daily exchange rate as quoted by the Bank of Canada on the date such amounts arise or such other rate of exchange as is acceptable to the CRA.
Holders Resident in Canada
The following portion of this summary is generally applicable to a Holder who at all relevant times, for purposes of the Tax Act, is or is deemed to be resident in Canada (a "Resident Holder").
Certain Resident Holders whose Common Shares might not constitute capital property may make, in certain circumstances, an irrevocable election permitted by subsection 39(4) of the Tax Act to deem the Common Shares, and every other "Canadian security" as defined in the Tax Act, held by such persons in the taxation year of the election and each subsequent taxation year to be capital property. Resident Holders should consult their own tax advisors regarding this election.
Dividends
Dividends received or deemed to be received on the Common Shares will be included in computing a Resident Holder's income. In the case of an individual (other than certain trusts), such dividends will be subject to the gross-up and dividend tax credit rules normally applicable in respect of "taxable dividends" received from "taxable Canadian corporations" (each as defined in the Tax Act). An enhanced gross-up and dividend tax credit will be available to individuals in respect of "eligible dividends" designated by the Corporation to the Resident Holder in accordance with the provisions of the Tax Act. There may be limitations on the ability of the Corporation to designate dividends as eligible dividends.
Dividends received or deemed to be received on a Common Share by a Resident Holder that is a corporation will be included in computing the corporation's income and will generally be deductible in computing its taxable income. In certain circumstances, subsection 55(2) of the Tax Act will treat a taxable dividend received (or deemed to be received) by a Resident Holder that is a corporation as proceeds of disposition or a capital gain. Resident Holders that are corporations should consult their own tax advisors in this regard.
A Resident Holder that is a "private corporation" (as defined in the Tax Act) or a "subject corporation" (as defined in subsection 186(3) of the Tax Act) may be liable to pay an additional tax (refundable in certain circumstances) under Part IV of the Tax Act on dividends received or deemed to be received on the Common Shares to the extent such dividends are deductible in computing the Resident Holder's taxable income. Resident Holders that are corporations should consult their own tax advisors regarding their particular circumstances.
Dispositions of Common Shares
Upon a disposition (or a deemed disposition) of a Common Share (other than a disposition to the Corporation that is not a sale in the open market in the manner in which shares would normally be purchased by any member of the public in an open market), a Resident Holder generally will realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of such share, net of any reasonable costs of disposition, are greater (or are less) than the adjusted cost base of such share to the Resident Holder immediately before the disposition or deemed disposition. The adjusted cost base to a Resident Holder of a Common Share will be determined by averaging the cost of that Common Share with the adjusted cost base (determined immediately before the acquisition of the Common Share) of all other Common Shares held as capital property at that time by the Resident Holder. For a description of the treatment of capital gains and capital losses, see "Certain Canadian Income Tax Considerations -Holders Resident in Canada - Capital Gain / Loss" below.
Capital Gain / Loss
Generally, a Resident Holder is required to include in computing its income for a taxation year one-half of the amount of any capital gain (a "taxable capital gain") realized in the year. Subject to and in accordance with the provisions of the Tax Act, a Resident Holder is required to deduct one-half of the amount of any capital loss (an "allowable capital loss") realized in a taxation year from taxable capital gains realized in the year by such Resident Holder. Allowable capital losses in excess of taxable capital gains for the taxation year of disposition may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any following taxation year against net taxable capital gains realized in such years to the extent and under the circumstances described in the Tax Act.
The amount of any capital loss realized on the disposition or deemed disposition of Common Shares by a Resident Holder that is a corporation may be reduced by the amount of dividends received or deemed to have been received by it on such shares or shares substituted for such shares to the extent and in the circumstances specified by the Tax Act. Similar rules may apply where a Common Share is owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary. Resident Holders to whom these rules may be relevant should consult their own tax advisors.
A Resident Holder that is, throughout the relevant taxation year, a "Canadian-controlled private corporation" or "CCPC" (as defined in the Tax Act) will be subject to an additional tax (refundable in certain circumstances) in respect of its "aggregate investment income" (as defined in the Tax Act) for the year, which will include taxable capital gains. Draft legislation released in the 2022 Canadian federal budget extends liability for the additional tax payable by a "Canadian-controlled private corporation" to a "substantive CCPC" (as defined in a draft amendment to the Tax Act). Resident Holders that are "Canadian-controlled private corporations" or "substantive CCPCs" should consult their own tax advisors regarding their particular circumstances.
Minimum Tax
Capital gains realized and dividends received (or deemed to be received) by a Resident Holder that is an individual or a trust, other than certain specified trusts, may give rise to minimum tax under the Tax Act. Resident Holders that are individuals should consult their own tax advisors in this regard.
Holders Not Resident in Canada
The following portion of this summary is generally applicable to a Holder who at all relevant times, for purposes of the Tax Act, (i) is not, and is not deemed to be, resident in Canada; and (ii) does not use or hold and is not deemed to use or hold its Common Shares in, or in the course of carrying on, a business in Canada (a "Non-Resident Holder"). Special rules, which are not discussed in this summary, may apply to a Non-Resident Holder that is an insurer carrying on business in Canada and elsewhere or that is an "authorized foreign bank" (as defined in the Tax Act). Such Non-Resident Holders should consult their own tax advisors.
The term "US Holder," for the purposes of this summary, means a Non-Resident Holder who, for purposes of the Canada-United States Tax Convention (1980), as amended (the "Canada-US Tax Treaty"), is at all relevant times a resident of the United States and is a "qualifying person" within the meaning of the Canada-US Tax Treaty eligible for the full benefits of the Canada-US Tax Treaty. In some circumstances, persons deriving amounts through fiscally transparent entities (including limited liability companies) may be entitled to benefits under the Canada-US Tax Treaty. Non-Resident Holders are urged to consult their own tax advisors to determine their entitlement to benefits under the Canada-US Tax Treaty and related compliance requirements based on their particular circumstances.
Dividends
Dividends paid or credited or deemed under the Tax Act to be paid or credited by the Corporation to a Non-Resident Holder on the Common Shares will generally be subject to Canadian withholding tax at the rate of 25% on the gross amount of the dividend, unless such rate is reduced by the terms of an applicable income tax treaty or convention. Under the Canada-US Tax Treaty, the withholding rate on any such dividend beneficially owned by a Non-Resident Holder that is a US Holder or otherwise entitled to the relevant benefits of such treaty is generally reduced to 15%, and to 5% if such Non-Resident Holder is a company that beneficially owns at least 10% of the voting stock of the Corporation. Non-Resident Holders should consult their own tax advisors to determine their entitlement to benefits under any applicable tax treaty or convention based on their particular circumstances.
Dispositions of Common Shares
A Non-Resident Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a Common Share unless the Common Share constitutes (or is deemed to constitute) "taxable Canadian property" to the Non-Resident Holder thereof for purposes of the Tax Act at the time of disposition, and is not "treaty-protected property" (as defined in the Tax Act) of the Non-Resident Holder at the time of the disposition.
Provided the Common Shares are listed on a "designated stock exchange", as defined in the Tax Act (which currently includes the NEO), at the time of disposition, the Common Shares generally will not constitute taxable Canadian property of a Non-Resident Holder at that time, unless at any time during the 60 month period immediately preceding the disposition the following two conditions are met concurrently: (i) the Non-Resident Holder, persons with whom the Non-Resident Holder did not deal at arm's length, partnerships in which the Non-Resident Holder or any such non-arm's length person holds a membership interest (either directly or indirectly through one or more partnerships), or the Non-Resident Holder together with all such persons and partnerships, owned 25% or more of the issued shares of any class or series of shares of the Corporation; and (ii) more than 50% of the fair market value of the Common Shares was derived directly or indirectly from one or any combination of real or immovable property situated in Canada, Canadian resource properties (as defined in the Tax Act), timber resource properties (as defined in the Tax Act) or options, interests or for civil law rights in such property, whether or not such property exists. Notwithstanding the foregoing, a Common Share may be deemed to be "taxable Canadian property" in certain other circumstances.
The Common Shares of a US Holder will generally constitute "treaty-protected property" for purposes of the Tax Act at any given time unless the value of the Common Shares is derived principally from real property situated in Canada at that time. For this purpose, "real property" has the meaning that term has under the laws of Canada and includes any option or similar right in respect thereof and in any case, includes usufruct of real property, rights to explore for or to exploit mineral deposits, sources and other natural resources and rights to amounts computed by reference to the amount or value of production from such resources.
Non-Resident Holders should consult their own tax advisors as to whether their Common Shares constitute "taxable Canadian property".
If the Common Shares are "taxable Canadian property" to a Non-Resident Holder and such Non-Resident Holder is not exempt from tax under the Tax Act in respect of the disposition of such Common Shares pursuant to an applicable income tax treaty or convention, the tax consequences as described above under the headings "Certain Canadian Income Tax Considerations - Holders Resident in Canada - Dispositions of Common Shares" and "Certain Canadian Income Tax Considerations - Holders Resident in Canada - Capital Gain / Loss" will generally apply. Such Non-Resident Holders should consult their own tax advisors.
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership and disposition of Common Shares acquired pursuant to this Offering. This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from or relating to the acquisition, ownership and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership and disposition of Common Shares. In addition, except as specifically set forth below, this summary does not discuss applicable income tax reporting requirements. Each prospective U.S. Holder should consult its own tax advisors regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
No ruling from the Internal Revenue Service (the "IRS") has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, or contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the conclusions described in this summary.
Scope of this Summary
Authorities
This summary is based on the U.S. Internal Revenue Code of 1986, as amended (the "Code"), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the current provisions of the Canada-United States Tax Convention (1980) (the "Canada-U.S. Tax Convention"), and U.S. court decisions that are applicable, and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis, which could affect the U.S. federal income tax considerations described in this summary. Except as provided herein, this summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term "U.S. Holder" means a beneficial owner of Common Shares acquired pursuant to this Offering that is for U.S. federal income tax purposes:
-
An individual who is a citizen or resident of the United States;
-
a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia;
-
an estate whose income is subject to U.S. federal income taxation regardless of its source; or
- a trust that (1) is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
Non-U.S. Holders
For purposes of this summary, a "non-U.S. Holder" is a beneficial owner of Common Shares that is not a U.S. Holder or an entity classified as a partnership for U.S. federal income tax purposes. This summary does not address the U.S. federal, state or local tax consequences to non-U.S. Holders arising from or relating to the acquisition, ownership and disposition of Common Shares. Accordingly, a non-U.S. Holder should consult its own tax advisors regarding the U.S. federal, state or local and non-U.S. tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership and disposition of Common Shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to U.S. Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) have a "functional currency" other than the U.S. dollar; (e) own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other integrated transaction; (f) acquire Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); (h) are subject to the alternative minimum tax; (i) are subject to special tax accounting rules with respect to Common Shares; (j) are partnerships or other "pass-through" entities (and partners or other owners thereof); (k) are S corporations (and shareholders thereof); (l) are U.S. expatriates or former long-term residents of the United States subject to Section 877 or 877A of the Code; (m) hold Common Shares in connection with a trade or business, permanent establishment, or fixed base outside the United States; or (n) own or have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the outstanding shares of the Corporation. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisors regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership (or other "pass-through" entity) for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity or arrangement and the partners (or other owners or participants) of such entity or arrangement generally will depend on the activities of the entity or arrangement and the status of such partners (or owners or participants). This summary does not address the tax consequences to any such partner (or owner or participant). Partners (or other owners or participants) of entities or arrangements that are classified as partnerships or as "pass-through" entities for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership and disposition of Common Shares.
Passive Foreign Investment Company Rules
PFIC Status
If the Corporation was to constitute a "passive foreign investment company" under the meaning of Section 1297 of the Code (a "PFIC", as defined below) for any year during a U.S. Holder's holding period, then certain potentially adverse rules would affect the U.S. federal income tax consequences to a U.S. Holder as a result of the acquisition, ownership and disposition of Common Shares. Based on current business plans and financial expectations, the Corporation expects to be a PFIC for its current tax year and may be a PFIC in future tax years. No opinion of legal counsel or ruling from the IRS concerning the status of the Corporation as a PFIC has been obtained or is currently planned to be requested. The determination of whether any corporation was, or will be, a PFIC for a tax year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. In addition, whether any corporation will be a PFIC for any tax year depends on the assets and income of such corporation over the course of each such tax year and, as a result, cannot be predicted with certainty as of the date of this document. Accordingly, there can be no assurance that the IRS will not challenge any determination made by the Corporation (or any subsidiary of the Corporation) concerning its PFIC status. Each U.S. Holder should consult its own tax advisors regarding the PFIC status of the Corporation and each subsidiary of the Corporation.
In any year in which the Corporation is classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form 8621 annually.
The Corporation generally will be a PFIC if, for a tax year, (a) 75% or more of the gross income of the Corporation is passive income (the "PFIC income test") or (b) 50% or more of the value of the Corporation's assets either produce passive income or are held for the production of passive income, based on the quarterly average of the fair market value of such assets (the "PFIC asset test"). "Gross income" generally includes all sales revenues less the cost of goods sold, plus income from investments and from incidental or outside operations or sources, and "passive income" generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions. Active business gains arising from the sale of commodities generally are excluded from passive income if substantially all of a foreign corporation's commodities are stock in trade or inventory, depreciable property used in a trade or business, or supplies regularly used or consumed in the ordinary course of its trade or business, and certain other requirements are satisfied.
For purposes of the PFIC income test and PFIC asset test described above, if the Corporation owns, directly or indirectly, 25% or more of the total value of the outstanding shares of another corporation, the Corporation will be treated as if the Corporation (a) held a proportionate share of the assets of such other corporation and (b) received directly a proportionate share of the income of such other corporation. In addition, for purposes of the PFIC income test and PFIC asset test described above, and assuming certain other requirements are met, "passive income" does not include certain interest, dividends, rents, or royalties that are received or accrued by the Corporation from certain "related persons" (as defined in Section 954(d)(3) of the Code) also organized in Canada, to the extent such items are properly allocable to the income of such related person that is not passive income.
Under certain attribution rules, if the Corporation is a PFIC, U.S. Holders will generally be deemed to own their proportionate share of the Corporation's direct or indirect equity interest in any company that is also a PFIC (a "Subsidiary PFIC"), and will generally be subject to U.S. federal income tax on their proportionate share of (a) any "excess distributions," as described below, on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by the Corporation or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax under the PFIC rules even if no distributions are received and no redemptions or other dispositions of Common Shares are made.
Default PFIC Rules Under Section 1291 of the Code
If the Corporation is a PFIC for any tax year during which a U.S. Holder owns Common Shares, the U.S. federal income tax consequences to such U.S. Holder of the acquisition, ownership, and disposition of Common Shares will depend on whether and when such U.S. Holder makes an election to treat the Corporation and each Subsidiary PFIC, if any, as a "qualified electing fund" or "QEF" under Section 1295 of the Code (a "QEF Election") or makes a mark-to-market election under Section 1296 of the Code (a "Mark-to-Market Election"). A U.S. Holder that does not make either a QEF Election or a Mark-to-Market Election will be referred to in this summary as a "Non-Electing U.S. Holder".
A Non-Electing U.S. Holder will be subject to the rules of Section 1291 of the Code (described below) with respect to: (a) any gain recognized on the sale or other taxable disposition of Common Shares; and (b) any "excess distribution" received on the Common Shares. A distribution generally will be an "excess distribution" to the extent that such distribution (together with all other distributions received in the current tax year) exceeds 125% of the average distributions received during the three preceding tax years (or during a U.S. Holder's holding period for the Common Shares, if shorter).
Under Section 1291 of the Code, any gain recognized on the sale or other taxable disposition of Common Shares (including an indirect disposition of the stock of any Subsidiary PFIC), and any "excess distribution" received on Common Shares or with respect to the stock of a Subsidiary PFIC, must be ratably allocated to each day in a Non-Electing U.S. Holder's holding period for the respective Common Shares. The amount of any such gain or excess distribution allocated to the tax year of disposition or distribution of the excess distribution and to years before the entity became a PFIC, if any, would be taxed as ordinary income (and not eligible for certain preferred rates). The amounts allocated to any other tax year would be subject to U.S. federal income tax at the highest tax rate applicable to ordinary income in each such year, and an interest charge would be imposed on the tax liability for each such year, calculated as if such tax liability had been due in each such year. A Non-Electing U.S. Holder that is not a corporation must treat any such interest paid as "personal interest," which is not deductible.
If the Corporation is a PFIC for any tax year during which a Non-Electing U.S. Holder holds Common Shares, the Corporation will continue to be treated as a PFIC with respect to such Non-Electing U.S. Holder, regardless of whether the Corporation ceases to be a PFIC in one or more subsequent tax years. A Non-Electing U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above), but not loss, as if such Common Shares were sold on the last day of the last tax year for which the Corporation was a PFIC.
QEF Election
A U.S. Holder that makes a timely and effective QEF Election for the first tax year in which the holding period of its Common Shares begins generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to its Common Shares. A U.S. Holder that makes a timely and effective QEF Election will be subject to U.S. federal income tax on such U.S. Holder's pro rata share of (a) the net capital gain of the Corporation, which will be taxed as long-term capital gain to such U.S. Holder, and (b) the ordinary earnings of the Corporation, which will be taxed as ordinary income to such U.S. Holder. Generally, "net capital gain" is the excess of (a) net long-term capital gain over (b) net short-term capital loss, and "ordinary earnings" are the excess of (a) "earnings and profits" over (b) net capital gain. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on such amounts for each tax year in which the Corporation is a PFIC, regardless of whether such amounts are actually distributed to such U.S. Holder by the Corporation. However, for any tax year in which the Corporation is a PFIC and has no net income or gain, U.S. Holders that have made a QEF Election would not have any income inclusions as a result of the QEF Election. If a U.S. Holder that made a QEF Election has an income inclusion, such a U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as "personal interest," which is not deductible.
A U.S. Holder that makes a timely and effective QEF Election with respect to the Corporation generally (a) may receive a tax-free distribution from the Corporation to the extent that such distribution represents "earnings and profits" of the Corporation that were previously included in income by the U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder's tax basis in the Common Shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, a U.S. Holder that makes a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of Common Shares.
The procedure for making a QEF Election, and the U.S. federal income tax consequences of making a QEF Election, will depend on whether such QEF Election is timely. A QEF Election will be treated as "timely" if such QEF Election is made for the first year in the U.S. Holder's holding period for the Common Shares in which the Corporation was a PFIC. A U.S. Holder may make a timely QEF Election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such year. If a U.S. Holder does not make a timely and effective QEF Election for the first year in the U.S. Holder's holding period for the Common Shares, the U.S. Holder may still be able to make a timely and effective QEF Election in a subsequent year if such U.S. Holder meets certain requirements and makes a "purging" election to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above) as if such Common Shares were sold for their fair market value on the day the QEF Election is effective. If a U.S. Holder makes a QEF Election but does not make a "purging" election to recognize gain as discussed in the preceding sentence, then such U.S. Holder shall be subject to the QEF Election rules and shall continue to be subject to tax under the rules of Section 1291 discussed above with respect to its Common Shares. If a U.S. Holder owns PFIC stock indirectly through another PFIC, separate QEF Elections must be made for the PFIC in which the U.S. Holder is a direct shareholder and the Subsidiary PFIC for the QEF rules to apply to both PFICs.
A QEF Election will apply to the tax year for which such QEF Election is timely made and to all subsequent tax years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent tax year, the Corporation ceases to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those tax years in which the Corporation is not a PFIC. Accordingly, if the Corporation becomes a PFIC in another subsequent tax year, the QEF Election will be effective and the U.S. Holder will be subject to the QEF rules described above during any subsequent tax year in which the Corporation qualifies as a PFIC.
For each tax year that the Corporation qualifies as a PFIC, the Corporation: (a) intends to make publicly available to U.S. Holders, upon their written request, a "PFIC Annual Information Statement" for the Corporation as described in Treasury Regulation Section 1.1295-1(g) (or any successor Treasury Regulation) and (b) upon written request, intends to use commercially reasonable efforts to provide such additional information that such U.S. Holder is reasonably required to obtain in connection with maintaining such QEF Election with regard to the Corporation. The Corporation may elect to provide such information on the Corporation's website. However, U.S. Holders should be aware that the Corporation can provide no assurances that the Corporation will provide any such information relating to any Subsidiary PFIC and as a result, a QEF Election may not be available with respect to any Subsidiary PFIC. Because the Corporation may own shares in one or more Subsidiary PFICs at any time, U.S. Holders will continue to be subject to the rules discussed above with respect to the taxation of gains and excess distributions with respect to any Subsidiary PFIC for which the U.S. Holders do not obtain such required information. Each U.S. Holder should consult its own tax advisors regarding the availability of, and procedure for making, a QEF Election with respect to the Corporation and any Subsidiary PFIC.
A U.S. Holder makes a QEF Election by attaching a completed IRS Form 8621, including a PFIC Annual Information Statement, to a timely filed United States federal income tax return. However, if the Corporation does not provide the required information with regard to the Corporation or any of its Subsidiary PFICs, U.S. Holders will not be able to make a QEF Election for such entity and will continue to be subject to the rules of Section 1291 of the Code discussed above that apply to Non-Electing U.S. Holders with respect to the taxation of gains and excess distributions.
Mark-to-Market Election
A U.S. Holder may make a Mark-to-Market Election only if the Common Shares are marketable stock. The Common Shares generally will be "marketable stock" if the Common Shares are regularly traded on (a) a national securities exchange that is registered with the SEC, (b) the national market system established pursuant to section 11A of the Exchange Act, or (c) a foreign securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such foreign exchange has trading volume, listing, financial disclosure, and surveillance requirements, and meets other requirements and the laws of the country in which such foreign exchange is located, together with the rules of such foreign exchange, ensure that such requirements are actually enforced and (ii) the rules of such foreign exchange effectively promote active trading of listed stocks. If such stock is traded on such a qualified exchange or other market, such stock generally will be "regularly traded" for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Each U.S. Holder should consult its own tax advisor in this matter.
A U.S. Holder that makes a Mark-to-Market Election with respect to its Common Shares generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to such Common Shares. However, if a U.S. Holder does not make a Mark-to-Market Election beginning in the first tax year of such U.S. Holder's holding period for the Common Shares for which the Corporation is a PFIC and such U.S. Holder has not made a timely QEF Election, the rules of Section 1291 of the Code discussed above will apply to certain dispositions of, and distributions on, the Common Shares.
A U.S. Holder that makes a Mark-to-Market Election will include in ordinary income, for each tax year in which the Corporation is a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the Common Shares, as of the close of such tax year over (b) such U.S. Holder's adjusted tax basis in such Common Shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the excess, if any, of (a) such U.S. Holder's adjusted tax basis in the Common Shares, over (b) the fair market value of such Common Shares (but only to the extent of the net amount of previously included income as a result of the Mark-to-Market Election for prior tax years).
A U.S. Holder that makes a Mark-to-Market Election generally also will adjust such U.S. Holder's tax basis in the Common Shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of Common Shares, a U.S. Holder that makes a Mark-to-Market Election will recognize ordinary income or ordinary loss (not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior tax years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior tax years). Losses that exceed this limitation are subject to the rules generally applicable to losses provided in the Code and Treasury Regulations.
A U.S. Holder makes a Mark-to-Market Election by attaching a completed IRS Form 8621 to a timely filed United States federal income tax return. A Mark-to-Market Election applies to the tax year in which such Mark-to-Market Election is made and to each subsequent tax year, unless the Common Shares cease to be "marketable stock" or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisors regarding the availability of, and procedure for making, a Mark-to-Market Election.
Although a U.S. Holder may be eligible to make a Mark-to-Market Election with respect to the Common Shares, no such election may be made with respect to the stock of any Subsidiary PFIC that a U.S. Holder is treated as owning, because such stock is not marketable. Hence, the Mark-to-Market Election will not be effective to avoid the application of the default rules of Section 1291 of the Code described above with respect to deemed dispositions of Subsidiary PFIC stock or excess distributions from a Subsidiary PFIC to its shareholder.
Other PFIC Rules
Under Section 1291(f) of the Code, the IRS has issued proposed Treasury Regulations that, subject to certain exceptions, would cause a U.S. Holder that had not made a timely QEF Election to recognize gain (but not loss) upon certain transfers of Common Shares that would otherwise be tax-deferred (e.g., gifts and exchanges pursuant to corporate reorganizations). However, the specific U.S. federal income tax consequences to a U.S. Holder may vary based on the manner in which Common Shares are transferred.
Certain additional adverse rules may apply with respect to a U.S. Holder if the Corporation is a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example, under Section 1298(b)(6) of the Code, a U.S. Holder that uses Common Shares as security for a loan will, except as may be provided in Treasury Regulations, be treated as having made a taxable disposition of such Common Shares.
In addition, a U.S. Holder who acquires Common Shares from a decedent will not receive a "step up" in tax basis of such Common Shares to fair market value unless such decedent had a timely and effective QEF Election in place.
Special rules also apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult with its own tax advisors regarding the availability of the foreign tax credit with respect to distributions by a PFIC.
The PFIC rules are complex, and each U.S. Holder should consult its own tax advisors regarding the PFIC rules and how the PFIC rules may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares.
General Rules Applicable to the Ownership and Disposition of Common Shares
The following discussion is subject, in its entirety, to the rules described above under the heading "Passive Foreign Investment Company Rules".
Distributions on Common Shares
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to an Common Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the current or accumulated "earnings and profits" of the Corporation, as computed for U.S. federal income tax purposes. A dividend generally will be taxed to a U.S. Holder at ordinary income tax rates if the Corporation is a PFIC for the tax year of such distribution or the preceding tax year. To the extent that a distribution exceeds the current and accumulated "earnings and profits" of the Corporation, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder's tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares. (See "Sale or Other Taxable Disposition of Common Shares" below). However, the Corporation does not intend to maintain the calculations of its earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder therefore should assume that any distribution by the Corporation with respect to the Common Shares will constitute ordinary dividend income. Dividends received on Common Shares by corporate U.S. Holders generally will not be eligible for the "dividends received deduction". Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Canada-U.S. Tax Convention or the Common Shares are readily tradable on a United States securities market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions are satisfied, including that the Corporation not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisors regarding the application of such rules.
Sale or Other Taxable Disposition of Common Shares
Upon the sale or other taxable disposition of Common Shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the U.S. dollar value of cash received plus the fair market value of any property received and such U.S. Holder's tax basis in such Common Shares sold or otherwise disposed of. A U.S. Holder's tax basis in Common Shares generally will be such U.S. Holder's U.S. dollar cost for such Common Shares. Gain or loss recognized on such sale or other disposition generally will be long-term capital gain or loss if, at the time of the sale or other disposition, the Common Shares have been held for more than one year.
Preferential tax rates currently apply to long-term capital gain of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gain of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
Additional Considerations
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or on the sale, exchange or other taxable disposition of Common Shares, generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a tax basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on Common Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax. Generally, a credit will reduce a U.S. Holder's U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder's income that is subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. The foreign tax credit rules are complex, and involve the application of rules that depend on a U.S. Holder's particular circumstances. Accordingly, each U.S. Holder should consult its own U.S. tax advisors regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a non-U.S. entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult their own tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the U.S. or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of, Common Shares will generally be subject to information reporting and backup withholding tax if a U.S. Holder (a) fails to furnish such U.S. Holder's correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder's U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisors regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN LIGHT OF THEIR OWN PARTICULAR CIRCUMSTANCES.
RISK FACTORS
An investment in the Common Shares is highly speculative and subject to a number of risks. Before deciding whether to invest, investors should consider carefully the risks factors set forth below and in the documents incorporated by reference in the Base Shelf Prospectus and this Prospectus Supplement (including those discussed under the heading "Risk Factors" in the Base Shelf Prospectus and the Corporation's most recent annual information form, annual management discussion & analysis and interim management discussion & analysis) and all of the other information in the Prospectus Supplement (including, without limitation, the documents incorporated by reference). The risks described in this Prospectus Supplement are not the only risks that affect the Corporation. Additional risks and uncertainties that the Corporation is unaware of, or that the Corporation currently deems not to be material, may also become important factors that affect the Corporation. If any such risks actually occur, the Corporation's business, financial condition or results of operations could be materially adversely affected, with the result that the trading price of the Common Shares could decline and investors could lose all or part of their investment.
Risk Factors Related to the Offering
"At-the-Market" Offerings
Investors who purchase Common Shares in this Offering at different times will likely pay different prices, and so may experience different outcomes in their investment results. The Corporation will have discretion, subject to market demand, to vary the timing, prices, and numbers of Common Shares sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their Common Shares as a result of sales made at prices lower than the prices they paid.
An Investment in the Common Shares is Highly Speculative
An investment in the Common Shares and the Corporation's prospects generally are speculative due to the risky nature of its business and the present stage of its development. Investors may lose their entire investment. There is no assurance that risk management steps taken will avoid future loss due to the occurrence of the risks described or incorporated by reference herein, or other unforeseen risks. If any of such risks actually occur, then the Corporation's business, financial condition and operating results could be adversely affected. Investors should carefully consider all risks and consult with their professional advisors to assess any investment in the Corporation.
Negative Operating Cash Flow and Going Concern
The Corporation has had negative cash flow from operating activities since inception. Drug development involves long lead times, is very expensive and involves many variables of uncertainty. As such, significant capital investment will be required to achieve the Corporation's existing plans. The Corporation's net losses have had and will continue to have an adverse effect on, among other things, shareholder equity, total assets and working capital. The Corporation expects that losses may fluctuate from quarter to quarter and year to year, and that such fluctuations may be substantial based on the stage of development of its principal programs. The Corporation cannot predict when it will become profitable, if at all. Accordingly, the Corporation may be required to obtain additional financing in order to meet its future cash commitments.
The threat of the Corporation's ability to continue as a going concern will be removed only when, in the opinion of the Corporation's auditor, the Corporation's revenues have reached a level that is able to sustain its business operations. If the Corporation is unable to obtain additional financing from outside sources and eventually generate enough revenues, the Corporation may be forced to sell a portion or all of the Corporation's assets, or curtail or discontinue the Corporation's operations. If any of these events happen, shareholders could lose all or part of their investment. The Corporation's financial statements do not include any adjustments to the Corporation's recorded assets or liabilities that might be necessary if the Corporation becomes unable to continue as a going concern. See "Risk Factors - Risks Related to the Offering - Potential Need for Additional Financing".
Discretion in the Use of Proceeds
The Corporation will have discretion concerning the use of the net proceeds of the Offering as well as the timing of their expenditures, and may apply the net proceeds of the Offering in ways other than as described under "Use of Proceeds". As a result, an investor will be relying on the judgment of management for the application of the net proceeds of the Offering. Management may use the net proceeds of the Offering in ways that an investor may not consider desirable. The results and the effectiveness of the application of the net proceeds are uncertain. If the net proceeds are not applied effectively, the Corporation's business, prospects, financial position, financial condition or results of operations may suffer.
Potential Need for Additional Financing
The continued development of the Corporation will require additional financing. The Corporation's activities do have scope for flexibility in terms of the amount and timing of expenditures, and expenditures may be adjusted accordingly. However, further operations will require additional capital and will depend on the Corporation's ability to obtain financing through debt, equity or other means. The Corporation's ability to meet its obligations and maintain operations may be contingent upon successful completion of additional financing arrangements. There is no assurance that the Corporation will be successful in obtaining the required financing in the future or that such financing will be available on terms acceptable to the Corporation. In addition, any future financing may also be dilutive to existing shareholders of the Corporation. See "Risk Factors - Risks Related to the Offering - Negative Operating Cash Flow and Going Concern" and "Risk Factors - Risks Related to the Offering - Potential Dilution".
No Certainty of Net Proceeds from the Offering
There is no certainty that U.S.$35,000,000, or any amount, will be raised under the Offering. The Agents have agreed to use commercially reasonable efforts to sell the Common Shares when and to the extent requested by the Corporation, but the Corporation is not required to request the sale of the maximum amount offered or any amount and, if the Corporation requests a sale, the Corporation and the Agents are not obligated to purchase any Common Shares that are not sold. As a result of the Offering being made on a commercially reasonable efforts basis with no minimum, and only as requested by the Corporation, the Corporation may raise substantially less than the maximum total offering amount or nothing at all.
Potential Dilution
The Corporation's articles of incorporation and by-laws allow it to issue an unlimited number of Common Shares for such consideration and on such terms and conditions as established by the board of directors of the Corporation, in many cases, without the approval of the Corporation's shareholders. With any additional issuance of Common Shares, investors will suffer dilution to their voting power and the Corporation may experience dilution in its earnings per share. "Risk Factors - Risks Related to an Offering - Potential Need for Additional Financing".
Trading Market
The Corporation cannot assure that a market will continue to develop or be sustained for the Common Shares. If a market does not continue to develop or is not sustained, it may be difficult for purchasers to sell Common Shares at an attractive price or at all. The Corporation cannot predict the prices at which the Common Shares will trade.
Significant Sales of Common Shares
Significant sales of Common Shares, or the availability of such securities for sale, could adversely affect the prevailing market prices for the Common Shares. A decline in the market prices of the Common Shares could impair the Corporation's ability to raise additional capital through the sale of securities should it desire to do so.
Positive Return Not Guaranteed
There is no guarantee that the Common Shares will earn any positive return in the short term or long term. A holding of Common Shares is highly speculative and involves a high degree of risk and should be undertaken only by holders whose financial resources are sufficient to enable them to assume such risks and who have no need for immediate liquidity in their investment. A holding of Common Shares is appropriate only for holders who have the capacity to absorb a loss of some or all of their holdings.
Application and Interpretation of Tax Laws
The Corporation is subject to direct and indirect taxes in various foreign jurisdictions. The amount of tax that the Corporation pays, directly or indirectly, is subject to the interpretation of applicable tax laws in the jurisdictions of operations in which the Corporation has interests. The Corporation has taken and will continue to take tax positions based on the application and interpretation of tax laws, but tax accounting often involves complex matters and judgment is required in determining the Corporation's foreign provisions for taxes and other tax liabilities. There can be no assurance that a taxing authority will not have a different interpretation of the law and assess the Corporation, or the operations in which the Corporation has interests, with additional taxes. Further, the Corporation's future effective tax rates could be impacted by changes in tax laws or regulations, and changing interpretation of existing laws or regulations. Both domestic and international tax laws, and interpretation of the tax laws, are subject to change as a result of changes in fiscal policy, changes in legislation, evolution of regulation and court rulings. The application of these tax laws and related regulations is subject to legal and factual interpretation, judgment and uncertainty.
Enforcement of Civil Liabilities
Certain of the Corporation's subsidiaries and assets are located outside of Canada. Accordingly, it may be difficult for investors to enforce within Canada any judgments obtained against the Corporation, including judgments predicated upon the civil liability provisions of applicable Canadian securities laws or otherwise. Consequently, investors may be effectively prevented from pursuing remedies against the Corporation under Canadian securities laws or otherwise.
The Corporation has subsidiaries incorporated in the United States and Ireland. It may not be possible for shareholders to effect service of process outside of Canada against the directors and officers of the Corporation who are not resident in Canada. In the event a judgment is obtained in a Canadian court against one or more of such persons for violations of Canadian securities laws or otherwise, it may not be possible to enforce such judgment against persons not resident in Canada. Additionally, it may be difficult for an investor, or any other person or entity, to assert Canadian securities law or other claims in original actions instituted in the United States and Ireland. Courts in such jurisdictions may refuse to hear a claim based on a violation of Canadian securities laws or otherwise on the grounds that such jurisdiction is not the most appropriate forum to bring such a claim. Even if a foreign court agrees to hear a claim, it may determine that the local law, and not Canadian law, is applicable to the claim. If Canadian law is found to be applicable, the content of applicable Canadian law must be proven as a fact, which can be a time consuming and costly process. Certain matters of procedure will also be governed by foreign law. See the section entitled "Enforcement of Civil Liabilities."
The Corporation expects to be a "passive foreign investment company", which may have adverse U.S. federal income tax consequences for U.S. investors
Based on current business plans and financial expectations, the Corporation expects to be a PFIC for its current tax year and may be a PFIC in future tax years. If the Corporation is a PFIC for any year during a U.S. taxpayer's holding period of Common Shares, then such U.S. taxpayer generally will be required to treat any gain realized upon a disposition of the Common Shares or any so-called excess "distribution" received on its Common Shares as ordinary income, and to pay an interest charge on a portion of such gain or distribution. In certain circumstances, the sum of the tax and the interest charge may exceed the total amount of proceeds realized on the disposition, or the amount of excess distribution received, by the U.S. taxpayer. Subject to certain limitations, these tax consequences may be mitigated if a U.S. taxpayer makes a timely and effective QEF Election (as defined below) or a Mark-to-Market Election (as defined below). Subject to certain limitations, such elections may be made with respect to the Common Shares. A U.S. taxpayer who makes a timely and effective QEF Election generally must report on a current basis its share of the Corporation's net capital gain and ordinary earnings for any year in which the Corporation is a PFIC, whether or not the Corporation distributes any amounts to its shareholders. A U.S. taxpayer who makes the Mark-to-Market Election generally must include as ordinary income each year the excess of the fair market value of the Common Shares over the taxpayer's basis therein. This paragraph is qualified in its entirety by the discussion above under the heading "Certain United States Federal Income Tax Considerations - Passive Foreign Investment Company Rules." Each potential investor who is a U.S. taxpayer should consult its own tax advisor regarding the tax consequences of the PFIC rules and the acquisition, ownership, and disposition of the Common Shares.
EXPERTS
The matters referred to under "Certain Canadian Income Tax Considerations" and "Certain United States Federal Income Tax Considerations", as well as certain other legal matters relating to the issue and sale of the Common Shares, will be passed upon on behalf of the Corporation by Aird & Berlis LLP, with respect to Canadian legal matters, and by Dorsey & Whitney LLP, with respect to United States legal matters, and certain legal matters on behalf of the Agents by Bennett Jones LLP, with respect to Canadian legal matters, and Mintz, Levin, Cohn, Ferris, Glovsky, and Popeo, P.C., with respect to United States legal matters.
As of the date of this Prospectus Supplement, the partners and associates of Aird & Berlis LLP and Bennett Jones LLP, beneficially owned, directly or indirectly, less than 1% of the outstanding securities of the Corporation.
AUDITORS, TRANSFER AGENT AND REGISTRAR
Zeifmans LLP, Chartered Professional Accountants, are the auditors of the Corporation and have confirmed that they are independent of the Corporation within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation. Neither Zeifmans LLP nor any designated professional thereof, had any registered or beneficial interest in any securities or other property of the Corporation at the time they audited the relevant financial statements incorporated by reference in this Prospectus Supplement or at any time thereafter.
The registrar and transfer agent of the Common Shares is Odyssey Trust Company at its principal office in Calgary, Alberta.
EXEMPTIONS
Pursuant to a decision of the Autorité des marchés financiers dated June 10, 2021, the Corporation was granted a permanent exemption from the requirement to translate into French this Prospectus Supplement, the Base Shelf Prospectus as well as the documents incorporated by reference herein and therein. The exemption was granted on the condition that the Base Shelf Prospectus and any Prospectus Supplement (other than in relation to an "at-the-market distribution") be translated into French if the Corporation offers securities to Québec purchasers in connection with an offering other than in relation to an "at-the-market distribution".
ENFORCEMENT OF CIVIL LIABILITIES
The Corporation is a corporation existing under the Business Corporations Act (Ontario). Other than Douglas Drysdale, Michael Palfreyman, Alex Nivorozhkin and Brett Greene, all of the directors and officers, and all of the experts named in this Prospectus Supplement or the accompanying Base Shelf Prospectus, are residents of Canada or otherwise reside outside the United States, and all or a substantial portion of their assets, and a majority of the Corporation's assets, are located outside the United States. The Corporation has appointed an agent for service of process in the United States, but it may be difficult for holders of the Common Shares who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for holders of the Common Shares who reside in the United States to realize upon judgments of courts of the United States predicated upon the Corporation's civil liability and the civil liability of its directors, officers and experts under the United States federal securities laws or the securities or "Blue Sky" laws of any state within the United States.
The Corporation has been advised by its Canadian counsel, Aird & Berlis LLP, that there is doubt as to the enforceability in Canada by a court in original actions, or in actions to enforce judgments of United States courts, of civil liabilities predicated solely upon United States federal securities laws or any such state securities or "Blue Sky" laws of any state within the United States.
The Corporation filed with the SEC, concurrently with the Registration Statement, an appointment of agent for service of process on Form F-X. Under the Form F-X, the Corporation appointed C T Corporation System as the agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving the Corporation in a United States court arising out of, related to, or concerning the offering of Common Shares under this Prospectus Supplement and the accompanying Base Shelf Prospectus.
SHORT FORM BASE SHELF PROSPECTUS

CYBIN INC.
$125,000,000
Common Shares
Warrants
Units
Debt Securities
Subscription Receipts
This short form base shelf prospectus (“Prospectus”) relates to the offering for sale from time to time (each, an “Offering”) by Cybin Inc. (the “Corporation” or “Cybin”) during the 25-month period that this Prospectus, including any of amendments thereto, remains valid, of up to $125,000,000 in the aggregate of: (i) common shares (“Common Shares”) of the Corporation; (ii) warrants (“Warrants”) to purchase other Securities (as defined below) of the Corporation; (iii) units (“Units”) comprising of one or more of the other Securities, (iv) senior and subordinated unsecured debt securities (collectively, “Debt Securities”), including debt securities convertible or exchangeable into other securities of the Corporation, and (v) subscription receipts (“Subscription Receipts” and together with the Common Shares, Warrants, Units and Debt Securities, collectively referred to herein as the “Securities”). The Securities may be offered separately or together, in amounts, at prices and on terms determined based on market conditions at the time of the sale and as set forth in an accompanying prospectus supplement (“Prospectus Supplement”).
All shelf information permitted under applicable laws to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus, except in cases where an exemption from such delivery requirements is available. Each Prospectus Supplement containing the specific terms of any Securities will be incorporated by reference into this Prospectus for the purposes of securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains.
The specific terms of any Securities offered will be described in a Prospectus Supplement, including: (i) in the case of Common Shares, the number of Common Shares offered, the offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event the offering is a non-fixed price distribution) and any other specific terms; (ii) in the case of Warrants, the number of Warrants being offered, the offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event the offering is a non-fixed price distribution), the designation, number and terms of the other Securities purchasable upon exercise of the Warrants, and any procedures that will result in the adjustment of those numbers, the exercise price, the dates and periods of exercise and any other specific terms; (iii) in the case of Units, the number of Units offered, the offering price, the designation, number and terms of the other Securities comprising the Units, and any other specific terms; (iv) in the case of Debt Securities, the specific designation of the Debt Securities, whether such Debt Securities are senior or subordinate, the aggregate principal amount of the Debt Securities being offered, the currency or currency unit in which the Debt Securities may be purchased, authorized denominations, any limit on the aggregate principal amount of the Debt Securities of the series being offered, the issue and delivery date, the maturity date, the offering price (at par, at a discount or at a premium), the interest rate or method of determining the interest rate, the interest payment date(s), any conversion or exchange rights that are attached to the Debt Securities, any redemption provisions, any repayment provisions and any other specific terms; and (v) in the case of Subscription Receipts, the number of Subscription Receipts being offered, the offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event the offering is a non-fixed price distribution), the terms, conditions and procedures for the conversion of the Subscription Receipts into other Securities, the designation, number and terms of such other Securities, and any other specific terms. A Prospectus Supplement relating to a particular Offering of Securities may include terms pertaining to the Securities being offered thereunder that are not within the terms and parameters described in this Prospectus.
The Securities may be sold through underwriters or dealers, directly by us pursuant to applicable statutory exemptions, or through designated agents from time to time. See “Plan of Distribution”. The Prospectus Supplement relating to a particular Offering of Securities will identify each underwriter, dealer or agent, as the case may be, engaged by the Corporation in connection with the offering and sale of the Securities, and will set forth the terms of the Offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection with the Offering, the method of distribution of the Securities, the initial issue price (in the event that the offering is a fixed price distribution), the net proceeds to us and any other material terms of the plan of distribution.
The Securities may be sold from time to time in one or more transactions at a fixed price or prices or at non-fixed prices. This Prospectus may qualify an “at-the-market distribution”, as defined in National Instrument 44-102 – Shelf Distributions (“NI 44-102”). If offered on a non-fixed price basis, the Securities may be offered at market prices prevailing at the time of sale, at prices determined by reference to the prevailing price of a specified security in a specified market or at prices to be negotiated with purchasers including sales in transactions that are deemed to be “at-the-market distributions”, including sales made directly on the Neo Exchange Inc. (the “NEO”) or other existing trading markets for the Securities, and as set forth in an accompanying Prospectus Supplement, in which case the compensation payable to an underwriter, dealer or agent in connection with any such sale will be decreased by the
amount, if any, by which the aggregate price paid for the Securities by the purchasers is less than the gross proceeds paid by the underwriter, dealer or agent to the Corporation. The price at which the Securities will be offered and sold may vary from purchaser to purchaser and during the period of distribution. See “Plan of Distribution”.
This Prospectus does not qualify the issuance of Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to one or more underlying interests including, for example, an equity or debt security, a statistical measure of economic or financial performance including, but not limited to, any currency, consumer price or mortgage index, or the price or value of one or more commodities, indices or other items, or any other item or formula, or any combination or basket of the foregoing items. For greater certainty, this Prospectus may qualify for issuance Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to published rates of a central banking authority or one or more financial institutions, such as a prime rate or bankers’ acceptance rate, or to recognized market benchmark interest rates such as the London Inter-Bank Offered Rate (LIBOR), Euro Inter-Bank Offered Rate (EURIBOR) or a United States federal funds rate.
No underwriter or dealer involved in an “at-the-market distribution” under this Prospectus, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot securities in connection with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the offered Securities or securities of the same class as the Securities distributed under the “at-the-market distribution”, including selling an aggregate number or principal amount of Securities that would result in the underwriter creating an over-allocation position in the Securities.
In connection with any Offering of the Securities, subject to applicable laws and other than an “at-the-market distribution”, the underwriters or agents may over-allot or effect transactions that stabilize or maintain the market price of the offered Securities at a level above that which might otherwise prevail on the open market. Such transactions, if commenced, may be interrupted or discontinued at any time. See “Plan of Distribution”.
The Common Shares are listed on the NEO under the trading symbol “CYBN”, and in the United States on the OTCQB under the trading symbol “CLXPF”. On July 2, 2021, the last trading day prior to the filing of this Prospectus, the closing prices of the Common Shares listed on the NEO and the OTCQB were $2.60 and US$2.12, respectively.
Unless specified in the applicable Prospectus Supplement, there is no market through which the Subscription Receipts, Warrants, Units and Debt Securities may be sold and purchasers may not be able to resell the Subscription Receipts, Warrants, Units and Debt Securities purchased under this Prospectus and the Prospectus Supplement. This may affect the pricing of the Subscription Receipts, Warrants, Units and Debt Securities in the secondary market, the transparency and availability of trading prices, the liquidity of the Subscription Receipts, Warrants, Units and Debt Securities and the extent of issuer regulation. See “Risk Factors”.
Prospective investors should be aware that the purchase of Securities may have tax consequences that may not be fully described in this Prospectus or in any Prospectus Supplement, and should carefully review the tax discussion, if any, in the applicable Prospectus Supplement and in any event consult with a tax advisor.
An investment in the Securities is subject to a number of risks, including those risks described in this Prospectus and documents incorporated by reference into this Prospectus. See “Risk Factors” in this Prospectus and in the Corporation’s Annual Information Form and Annual MD&A (each as defined herein) incorporated by reference herein.
No person is authorized by the Corporation to provide any information or to make any representation other than as contained in this Prospectus in connection with the issue and sale of the Securities offered hereunder.
No underwriter has been involved in the preparation of this Prospectus or performed any review of the contents hereof.
Each of Douglas Drysdale, Michael Palfreyman, Alex Nivorozhkin and Brett Green, officers of the Corporation, resides outside of Canada. They have each appointed Maxims CS Inc., Suite 1800, 181 Bay Street, Toronto, Ontario, M5J 2T9, as agent for service of process in Ontario. Prospective purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process, see “Risk Factors – Risks Related to an Offering – Enforcement of Civil Liabilities”.
In this Prospectus, references to the “Corporation”, “Cybin”, “we”, “us” and “our” refer to Cybin Inc. and/or, as applicable, one or more of its subsidiaries. The Corporation’s registered and head office is located at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9.
The Corporation currently has two business segments: (a) Serenity Life Sciences Inc. (“Serenity Life”) and Cybin US Holdings Inc. (“Cybin U.S.”) that focus on the research, development and commercialization of psychedelic-inspired regulated medicines; and (b) Natures Journey Inc. (“Natures Journey”) that focuses on consumer mental wellness, including, but not limited to, non-psychedelic mushroom nutraceutical products. Like most life sciences and pharmaceutical companies, Serenity Life’s and Cybin U.S.’s (psychedelic) business is focused on research and development, see “Use of Proceeds”. Currently the Corporation plans to conduct research and development on synthesized API from pharmaceutical manufacturers in Jamaica. No product will be commercialized prior to applicable legal or regulatory approval.
The Canadian and United States federal governments regulate drugs through the Controlled Drugs and Substances Act (Canada) (the “CDSA”) and the Controlled Substances Act (21 U.S.C. § 811) (the “CSA”), respectively, which place controlled substances in a schedule. Under the CDSA, psilocybin is currently a Schedule III drug. Under the CSA, psilocybin is currently a Schedule I drug.
Unlike in Canada and the United States, psilocybin mushrooms are not an illegal drug under Jamaica’s Dangerous Drugs Act, 1948. The Corporation’s activity in relation to the sponsored research of psilocybin mushrooms, botanicals and other related fungi is limited to the jurisdiction of Jamaica. The Corporation’s future business activities in Jamaica involve the import of psychedelic and pharmaceutical based medicines (derived from mushrooms) for the purposes of research and development and clinical trials in Jamaica.
ii
In both Canada and the United States, the applicable federal government is responsible for regulating, among other things, the approval, import, sale and marketing of drugs, including any psychedelic substances, whether natural or novel. Health Canada, and the Food and Drug Administration (“FDA”) in the United States, have not approved psilocybin as a drug for any indication. It is illegal to possess such substances without a prescription. The Corporation does not directly engage in any activities that would trigger the need to comply with any federal laws related to psychedelic substances. See “Regulatory Overview – Research and Development”.
The Corporation does not deal with psychedelic substances except in jurisdictions where such activity is not illegal and then only within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Corporation initially intends to sponsor and work with licensed third parties in Jamaica to conduct any clinical trials and research relating to psychedelics and currently does not handle controlled or restricted substances under the CDSA or CSA. If the Corporation were to conduct this work without reliance on third parties, it would need to obtain the required licenses, approvals and authorizations from Health Canada, the FDA or other applicable regulatory bodies. The Corporation does not have any direct or indirect involvement with illegal selling, production or distribution of any substances in jurisdictions in which it operates.
Natural health products (“NHPs”), prescription drugs, and non-prescription drugs are all classified and regulated under the federal Food and Drugs Act (Canada) (the “Canadian FDA”). Labelling, marketing and selling of any NHPs must comply with the Canadian FDA, including by ensuring that the Corporation’s products are not packaged or marketed in a manner that is misleading or deceptive to a consumer. In the United States, foods, drugs and dietary supplements are subject to extensive regulation. The Federal Food, Drug, and Cosmetic Act and the Dietary Supplement Health and Education Act of 1994 generally govern, among other things, the research, development, testing, manufacturing, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of foods, drugs and dietary supplements. See “Regulatory Overview – United States”.
The Corporation’s operations are conducted in strict compliance with local laws where such activities are permissible and do not require any specific legal or regulatory approvals.
Given the early stage of its prescription drug product development, the Corporation can make no assurance that its research and development program will result in regulatory approval or commercially viable products. To achieve profitable operations, the Corporation, alone or with others, must successfully develop, gain regulatory approval for, and market its future products. The Corporation currently has no products that have been approved by Health Canada, the Ministry of Health (Jamaica), the FDA, or any similar regulatory authority. To obtain regulatory approvals for its prescription drug product candidates being developed and to achieve commercial success, clinical trials must demonstrate that the prescription drug product candidate are safe for human use and that they demonstrate efficacy. See “Risk Factors” herein and “Risk Factors” in the Annual Information Form.
Certain statements throughout this Prospectus regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms have not been evaluated by Health Canada, the FDA or other similar regulatory authorities, nor has the efficacy of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms been confirmed by approved research. There is no assurance that psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms can be used to diagnose, treat, cure or prevent any disease or condition and robust scientific research and clinical trials are needed. There are multiple risk factors regarding the ability to successfully commercially scale a chemically synthesized process to obtain psilocybin and other analogues, including, but not limited to, regulatory changes or other changes in law, and risks related to drug development. See “Risk Factors” herein and “Risk Factors” in the Annual Information Form.
The Corporation oversees and monitors compliance with applicable laws in each jurisdiction in which it operates. In addition to the Corporation’s senior executives and the employees responsible for overseeing compliance, the Corporation has local regulatory/compliance counsel engaged in every jurisdiction in which it operates. See “Compliance Program”. Additionally, the Corporation has received legal opinions or advice in each jurisdiction where it currently operates regarding (a) compliance with applicable regulatory frameworks and (b) potential exposure and implications arising from applicable laws in jurisdictions where the Corporation has operations or intends to operate.
For these reasons, the Corporation may be (a) subject to heightened scrutiny by regulators, stock exchanges, clearing agencies and other authorities, (b) susceptible to regulatory changes or other changes in law, and (c) subject to risks related to drug development, among other things. There are a number of risks associated with the business of the Corporation. See “Risk Factors” herein and “Risk Factors” in the Annual Information Form.
An investment in the Securities is highly speculative and involves a high degree of risk that should be considered by potential purchasers. An investment in the Securities is suitable only for those purchasers who are willing to risk a loss of some or all of their investment and who can afford to lose some or all of their investment. A prospective purchaser should therefore review this Prospectus and the documents incorporated by reference herein in their entirety, including the Annual Information Form, and carefully consider the risk factors described under the section “Risk Factors” in this Prospectus, prior to investing in the Securities. See “Cautionary Note Regarding Forward-Looking Information” and “Risk Factors”.
iii
GENERAL MATTERS
Investors should rely only on the information contained in or incorporated by reference into this Prospectus or any applicable Prospectus Supplement. The Corporation has not authorized anyone to provide investors with different information. Information contained on the Corporation’s website shall not be deemed to be a part of this Prospectus or incorporated by reference herein or in any applicable Prospectus Supplement and may not be relied upon by prospective investors for the purpose of determining whether to invest in the Securities qualified for distribution under this Prospectus. The Corporation is not making an offer of these Securities in any jurisdiction where the offer is not permitted. Investors should not assume that the information contained in this Prospectus is accurate as of any date other than the date on the front of this Prospectus or the date of the relevant document incorporated by reference. The Corporation’s business, operating results, financial condition and prospects may have changed since that date.
The information contained on www.cybin.com is not intended to be included in or incorporated by reference herein, and prospective purchasers should not rely on such information when deciding whether or not to invest in any Securities.
In this Prospectus, unless stated otherwise or the context requires otherwise, all dollar amounts are expressed in Canadian dollars.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus, and in certain documents incorporated by reference in this Prospectus, constitute “forward-looking information” and “forward-looking statements”. All statements other than statements of historical fact contained in this Prospectus and in documents incorporated by reference in this Prospectus, including, without limitation, those regarding the Corporation’s future financial position and results of operations, strategy, plans, objectives, goals and targets, future developments in the markets where the Corporation participates or is seeking to participate, and any statements preceded by, followed by or that include the words “believe”, “expect”, “aim”, “intend”, “plan”, “continue”, “will”, “may”, “would”, “anticipate”, “estimate”, “forecast”, “predict”, “project”, “seek”, “should”, “objective”, “assumes” or similar expressions or the negative thereof, are forward-looking statements.
These statements are not historical facts but instead represent only the Corporation’s expectations, estimates and projections regarding future events. These statements are not guarantees of future performance and involve assumptions, risks and uncertainties that are difficult to predict. Therefore, actual results may differ materially from what is expressed, implied or forecasted in such forward-looking statements. Additional factors that could cause actual results, performance or achievements to differ materially include, but are not limited to, those discussed under “Risk Factors” in the Annual Information Form and in this Prospectus and in other documents incorporated by reference in this Prospectus. Management provides forward-looking statements because it believes they provide useful information to readers when considering their investment objectives and cautions readers that the information may not be appropriate for other purposes. Consequently, all of the forward-looking statements made in this Prospectus and in documents incorporated by reference in this Prospectus are qualified by these cautionary statements and other cautionary statements or factors contained herein, and there can be no assurance that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Corporation. These forward-looking statements are made as of the date of this Prospectus and the Corporation assumes no obligation to update or revise them to reflect subsequent information, events or circumstances or otherwise, except as required by law.
The forward-looking statements in this Prospectus and in documents incorporated by reference in this Prospectus are based on numerous assumptions regarding the Corporation’s present and future business strategies and the environment in which the Corporation will operate in the future, including assumptions regarding business and operating strategies, and the Corporation’s ability to operate on a profitable basis.
1
Some of the risks which could affect future results and could cause results to differ materially from those expressed in the forward-looking statements contained herein include: novel coronavirus “COVID-19”; limited operating history; achieving publicly announced milestones; speculative nature of investment risk; early stage of the industry and product development; regulatory risks and uncertainties; Jamaican operations; emerging market risk; plans for growth; limited products; limited marketing and sales capabilities; no assurance of commercial success; no profits or significant revenues; reliance on third parties for clinical development activities; risks related to third party relationships; reliance on contract manufacturers; safety and efficacy of products; clinical testing and commercializing products; completion of clinical trials; commercial grade product manufacturing; nature of regulatory approvals; unfavourable publicity or consumer perception; social media; biotechnology and pharmaceutical market competition; reliance on key executives and scientists; employee misconduct; business expansion and growth; negative results of external clinical trials or studies; product liability; enforcing contracts; product recalls; distribution and supply chain interruption; difficulty to forecast; promoting the brand; product viability; success of quality control systems; reliance on key inputs; liability arising from fraudulent or illegal activity; operating risk and insurance coverage; costs of operating as public company; management of growth; conflicts of interest; foreign operations; cybersecurity and privacy risk; environmental regulation and risks; Risks Related to Intellectual Property: trademark protection; trade secrets; patent law reform; patent litigation and intellectual property; protection of intellectual property; third-party licences; Financial and Accounting Risks: substantial number of authorized but unissued common shares; dilution; negative cash flow from operating activities; additional capital requirements; lack of significant product revenue; estimates or judgments relating to critical accounting policies; Risks related to the Common Shares: market for the Common Shares; significant sales of Common Shares; volatile market price for the Common Shares; tax issues; no dividends; Risks related to an Offering and the Corporation: an investment in the Securities is speculative; completion of an Offering; receipt of all regulatory and stock exchange approvals in respect of an Offering; forward-looking statements may prove to be inaccurate; potential dilution; potential need for additional financing; negative operating cash flow and going concern; discretion over the use of proceeds; management of growth; the Common Shares are subject to market price volatility; no history of payment of cash dividends; limited operating history as a public company; and risks relating to research and development objectives and milestones.
Although the forward-looking statements contained in this Prospectus are based upon what management currently believes to be reasonable assumptions, the Corporation cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. In particular, the Corporation has made assumptions regarding, among other things:
| |
• |
|
substantial fluctuation of losses from quarter to quarter and year to year due to numerous external risk factors, and anticipation that we will continue to incur significant losses in the future;
|
| |
• |
|
uncertainty as to the Corporation’s ability to raise additional funding to support operations;
|
| |
• |
|
the Corporation’s ability to access additional funding;
|
| |
• |
|
the fluctuation of foreign exchange rates;
|
| |
• |
|
the duration of COVID-19 and the extent of its economic and social impact;
|
| |
• |
|
the risks associated with the development of the Corporation’s product candidates which are at early stages of development;
|
| |
• |
|
reliance upon industry publications as the Corporation’s primary sources for third-party industry data and forecasts;
|
| |
• |
|
reliance on third parties to plan, conduct and monitor the Corporation’s preclinical studies and clinical trials;
|
| |
• |
|
reliance on third party contract manufacturers to deliver quality clinical and preclinical materials;
|
| |
• |
|
the Corporation’s product candidates may fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or may not otherwise produce positive results;
|
2
| |
• |
|
risks related to filing investigational new drug applications to commence clinical trials and to continue clinical trials if approved;
|
| |
• |
|
the risks of delays and inability to complete clinical trials due to difficulties enrolling patients;
|
| |
• |
|
competition from other biotechnology and pharmaceutical companies;
|
| |
• |
|
the Corporation’s reliance on the capabilities and experience of the Corporation’s key executives and scientists and the resulting loss of any of these individuals;
|
| |
• |
|
the Corporation’s ability to fully realize the benefits of acquisitions;
|
| |
• |
|
the Corporation’s ability to adequately protect the Corporation’s intellectual property and trade secrets;
|
| |
• |
|
the risk of patent-related or other litigation; and
|
| |
• |
|
the risk of unforeseen changes to the laws or regulations in the United States, Jamaica and Canada and other jurisdictions in which the Corporation operates.
|
Drug development involves long lead times, is very expensive and involves many variables of uncertainty. Anticipated timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation. Every patient treated on future studies can change those assumptions either positively (to indicate a faster timeline to new drug applications and other approvals) or negatively (to indicate a slower timeline to new drug applications and other approvals). This Prospectus and the documents incorporated by reference herein contain certain forward-looking statements regarding anticipated or possible drug development timelines. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assumes the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Corporation’s development efforts to date.
In addition to the factors set out above and those identified under the heading “Risk Factors” in the Annual Information Form and in this Prospectus, other factors not currently viewed as material could cause actual results to differ materially from those described in the forward-looking statements. Although the Corporation has attempted to identify important risks and factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors and risks that cause actions, events or results not to be anticipated, estimated or intended. Accordingly, readers should not place any undue reliance on forward-looking statements.
Many of these factors are beyond the Corporation’s ability to control or predict. These factors are not intended to represent a complete list of the general or specific factors that may affect the Corporation. The Corporation may note additional factors elsewhere in this Prospectus and in any documents incorporated by reference into this Prospectus. All forward-looking statements speak only as of the date made. All subsequent written and oral forward-looking statements attributable to the Corporation, or persons acting on the Corporation’s behalf, are expressly qualified in their entirety by the cautionary statements. Except as required by law, the Corporation undertakes no obligation to update any forward-looking statement.
The forward-looking statements contained in this Prospectus and the documents incorporated by reference herein are expressly qualified in their entirety by the foregoing cautionary statement. Investors should read this entire Prospectus, including the Annual Information Form, the documents incorporated by reference herein, and each applicable Prospectus Supplement, and consult their own professional advisers to ascertain and assess the income tax and legal risks and other aspects associated with holding Securities.
3
EXEMPTION
Pursuant to a decision of the Autorité des marchés financiers dated June 10, 2021, the Corporation was granted a permanent exemption from the requirement to translate into French this Prospectus as well as the documents incorporated by reference therein and any Prospectus Supplement to be filed in relation to an “at-the-market distribution”. This exemption is granted on the condition that this Prospectus and any Prospectus Supplement (other than in relation to an “at-the-market distribution”) be translated into French if the Corporation offers Securities to Québec purchasers in connection with an Offering of Securities other than in relation to an “at-the-market distribution”.
TRADEMARKS AND SERVICE MARKS
This prospectus includes trademarks, trade names and service marks which are protected under applicable intellectual property laws for use in connection with the operation of our business, and which are the property of the Corporation. All other trade names, trademarks or service marks appearing in this prospectus that are not identified as marks owned by us are the property of their respective owners. Solely for convenience, trademarks, service marks and trade names referred to in this prospectus may be listed without the ®, (TM) and (sm) symbols, however, we will assert, to the fullest extent under applicable law, our applicable rights in these trademarks, service marks and trade names.
MARKETING MATERIALS
Any template version of marketing materials (as such terms are defined in National Instrument 41-101 – General Prospectus Requirements) that are utilized in connection with the distribution of Securities will be filed under the Corporation’s profile on www.sedar.com (SEDAR). In the event that such marketing materials are filed after the date of the applicable Prospectus Supplement for the offering and before termination of the distribution of such Securities, such filed versions of the marketing materials will be deemed to be incorporated by reference into the applicable Prospectus Supplement for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains.
MARKET AND INDUSTRY DATA
Market and industry data contained and incorporated by reference in this Prospectus or any applicable Prospectus Supplement concerning economic and industry trends is based upon good faith estimates of our management or derived from information provided by industry sources. The Corporation believes that such market and industry data is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of such information and we have not independently verified the assumptions upon which projections of future trends are based. While the Corporation is not aware of any misstatements regarding the industry data presented herein, the Corporation’s estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under “Cautionary Note Regarding Forward-Looking Information” and “Risk Factors” in this Prospectus.
4
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of the Corporation at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9, telephone (908) 764-8385, and are also available electronically on SEDAR.
The following documents of the Corporation filed with the securities commissions or similar authorities in Canada are incorporated by reference in this Prospectus:
Any document of the type referred to in Section 11.1 of Form 44-101F1 – Short Form Prospectus Distributions filed by the Corporation with a securities commission or similar regulatory authority in Canada after the date of this Prospectus and prior to 25 months from the date hereof shall be deemed to be incorporated by reference in this Prospectus.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded for the purposes of this Prospectus to the extent that a statement contained herein or in any subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus modifies or supersedes that statement. Any statement so modified or superseded shall not constitute a part of this Prospectus except as so modified or superseded. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
Upon filing of a new annual information form and related annual financial statements with, and where required, accepted by, the applicable securities regulatory authorities during the currency of this Prospectus, the previous annual information form, including all amendments thereto, the previous annual financial statements and all interim financial statements (including any interim period management’s discussion and analysis related thereto), material change reports and management information circulars filed prior to the commencement of the fiscal year in which the new annual information form is filed, shall be deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of Securities hereunder.
A Prospectus Supplement containing the specific terms of any Securities offered thereunder will be delivered to purchasers of such Securities together with this Prospectus to the extent required under applicable securities laws and will be deemed to be incorporated by reference into this Prospectus as of the date of such Prospectus Supplement solely for the purposes of the Securities offered hereunder and thereunder.
5
THE CORPORATION
This summary does not contain all the information that may be important to you in deciding whether to invest in the Securities. You should read the entire Prospectus, including the section entitled “Risk Factors”, the applicable Prospectus Supplement, and the documents incorporated by reference herein, including the Annual Information Form, before making such decision.
Summary of the Business
The Corporation is a biotechnology company focused on advancing pharmaceutical therapies, delivery mechanisms, novel compounds and protocols as potential therapies for various psychiatric and neurological conditions. The Corporation is developing technologies and delivery systems aiming to improve the pharmacokinetics of its psychedelic molecules while retaining the therapeutics benefit. The new molecules and delivery systems are expected to be studied through clinical trials to confirm safety and efficacy.
The Corporation believes that there is presently a sizeable legal market for psychedelic pharmaceutical and nutraceutical products and, further, believes that there is a promising prospect for a strong, legal psychedelic pharmaceutical and nutraceutical industry to emerge globally. In particular, although the legal market for psychedelic pharmaceutical products is presently limited, globally, and in some jurisdictions it is still in its early stages, the Corporation believes that , in time, owing to generally increased acceptance and regulation of psychedelic-based treatments, this will give way to the emergence of numerous and sizable opportunities for market participants, including the Corporation.
Psychedelics are progressively emerging as potential alternative candidates for conventional therapies for individuals suffering from elusive maladies like post-traumatic stress disorder (“PTSD”), addiction, anxiety, and depression.1 For example, in August of 2020, as a result of the efforts of TheraPsil, a non-profit coalition that advocates for a legal, Special Access Programme access to psilocybin therapy for palliative care of Canadians, four Canadians with incurable cancer were approved by the Canadian federal Minister of Health, to use psilocybin therapy in the treatment of their end-of-life distress.2
As of the date of this Prospectus, certain synthetic psychoactive tryptamines and phenthylamines are being researched as candidates for the treatment of several psychiatric conditions, such as PTSD and depression.3 In 2018 and 2019, for example, the FDA granted breakthrough therapy designation for psilocybin for use as a candidate in the treatment of Major Depressive Disorder (“MDD”).4 At present, treatments for such conditions are limited in effectiveness, with some traditional treatment methods posing a heightened risk of complications. By contrast, the Corporation expects that psychoactive compounds, such as psilocybin, may in time also emerge as a safer and healthier medical treatment alternative for various ailments.
| 1 |
https://www.baystreet.ca/stockstowatch/7145/Magic-Mushroom-Market-Set-to-Grow-10-Feet-Tall.
|
| 2 |
https://www.forbes.com/sites/davidcarpenter/2020/08/08/four-terminally-ill-canadians-gain-legal-right-to-use-magic-mushrooms-for-end-of-life-distress/#3194f50a2bdf.
|
| 3 |
https://www.healtheuropa.eu/worlds-first-magic-mushroom-nasal-spray-for-ptsd-and-depression/95434/.
|
| 4 |
https://www.biopharmaglobal.com/2019/11/26/usona-institute-receives-fda-breakthrough-therapy-designation-for-psilocybin-for-the-treatment-of-major-depressive-disorder/.
|
6
The Corporation’s target market is focused on psychedelic pharmaceutical and non-psychedelic products. The Corporation views its synthetic psychedelic substances as boosters for the brain that can potentially rebuild pathways and break negative patterns all while looking at non-psychedelic medical extracts as the next wave of nutraceuticals that can potentially optimize overall health.5
The Corporation currently has two business segments: (a) Serenity Life and Cybin U.S. that focus on the research and development of psychedelic pharmaceutical products; and (b) Natures Journey that focuses on consumer mental wellness, including non-psychedelic nutraceutical products.
For additional information in respect of the Corporation and its operation, please see the Corporation’s Annual Information Form incorporated by reference into this Prospectus.
Inter-Corporate Relationships
As at the date of this Prospectus, the Corporation’s corporate structure includes the following material wholly-owned subsidiaries:

Note: (1) The Adelia Shareholders (defined below) hold certain non-voting securities of Cybin US, see “Select Recent Developments” below.
| 5 |
Certain statements regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms have not been evaluated by Health Canada, the FDA or other similar regulatory authorities, nor has the efficacy of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms been confirmed by approved research. There is no assurance that psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms can be used to diagnose, treat, cure or prevent any disease or condition and robust scientific research and clinical trials are needed. There are multiple risk factors regarding the ability to successfully commercially scale a chemically synthesized process to obtain psilocybin and other analogues.
|
7
COVID-19 Pandemic
On March 11, 2020, the World Health Organization declared the outbreak of COVID-19 a pandemic. Since the outbreak of COVID-19, the Corporation has focused its efforts on safeguarding the health and well-being of its employees, consultants and community members. To help slow the spread of COVID-19, the Corporation’s employees have been working remotely, where possible, and abiding by local and national guidance put in place in Canada, the United States, and Jamaica related to social distancing and restrictions on travel outside of the home. The Corporation has and will continue to abide by the protocols within Canada, the United States, and Jamaica regarding the performance of work activities. The duration and the immediate and eventual impact of the COVID-19 pandemic remains unknown. In particular, it is not possible to reliably estimate the length and severity of these developments and the impact on the financial results and condition of the Corporation. To date, a number of businesses have suspended or scaled back their operations and development as cases of COVID-19 have been confirmed, for precautionary purposes or as governments have declared a state of emergency or taken other actions. In the event that the operations or development of the Corporation are suspended or scaled back, or if the Corporation’s supply chains are disrupted, such events may have a material adverse effect on the Corporation. The breadth of the impact of the COVID-19 pandemic on investors, businesses, the global economy and financial and commodity markets may also have a material adverse effect on the Corporation.
For additional information see “Risk Factors – Risks Related to the Business of the Corporation – Novel Coronavirus COVID-19”.
Select Recent Developments
On December 4, 2020, the Corporation entered into a contribution agreement (the “Contribution Agreement”) with Cybin Corp., Cybin U.S. and all of the shareholders (the “Adelia Shareholders”) of Adelia Therapeutics Inc. (“Adelia”) whereby Cybin U.S. agreed to purchase from the Adelia Shareholders all of the issued and outstanding Adelia shares in exchange for the Class B Shares. The Adelia Transaction closed on December 14, 2020.
Pursuant to the Contribution Agreement and the support agreement entered into among Cybin U.S. and the Adelia Shareholders (the “Support Agreement”), the Adelia Shareholders received non-voting Class B common shares in the capital of Cybin U.S. (each a “Class B Share”), which are exchangeable for Common Shares, on a 10 Common Shares for 1 Class B Share basis, at the option of the holder thereof, subject to customary adjustments. The Class B Shares issued to the Adelia Shareholders on the closing of the Adelia Transaction are exchangeable for a total of 8,688,330 Common Shares. The aggregate value of the Class B Shares to be issued to the Adelia Shareholders on the closing of the Adelia Transaction was $10,773,529.50.
Under the Contribution Agreement, the Adelia Shareholders are also entitled to Class B Shares upon the occurrence of certain milestones as set out in the Contribution Agreement, which are also exchangeable for Common Shares on a 10 Common Shares for 1 Class B Share basis. The total value of the Class B Shares issuable pursuant to the milestones is up to $9,388,045.50 assuming all milestones are met prior to the applicable deadlines. In March, 2021, pursuant to the terms of the Contribution Agreement, an aggregate of 42,247.3 Class B Shares were issued to the Adelia Shareholders in satisfaction of $686,306.31 due to them upon meeting the certain relevant milestone.
No Class B Shares are exchangeable prior to the first anniversary of closing of the Adelia Transaction, and not more than: (i) 33 1/3% of the Class B Shares will be exchangeable prior to the second anniversary of the Adelia Transaction; (ii) 66 2/3% of the Class B Shares will be exchangeable prior to the third anniversary of the Adelia Transaction; and (iii) thereafter, 100% of the Class B Shares will be exchangeable. The Class B Shares issued to the Adelia Shareholders are exchangeable for a total of 511,631 Common Shares, resulting in an effective issue price of $1.99 per Common Share.
8
REGULATORY OVERVIEW
A summary of the applicable regulatory framework for the Corporation’s various business segments and proposed business activity are set forth below.
| |
|
|
|
|
|
Business Segment
|
|
Current/Proposed
Location of Operation
|
|
Summary of Applicable Regulatory Frameworks
|
| Serenity Life & Cybin U.S.(1) |
|
Canada, United Kingdom, United States & Jamaica |
|
The Canadian and United States federal governments regulate drugs through the CDSA and the CSA, respectively, which place controlled substances in a schedule.(2)
Under the CDSA, psilocybin is currently a Schedule III drug.(3)
Under the CSA, psilocybin is currently a Schedule I drug.(4)
Misuse of Drugs Regulations 2001 and the Misuse of Drugs Regulations 2001
|
| |
|
|
| Natures Journey(5) |
|
Canada and the United States |
|
Food and Drugs Act (Canada)
Dietary Supplement Health and Education Act of 1994
The Federal Food, Drug, and Cosmetic Act
|
| |
|
|
| Jamaica Research & Development |
|
Jamaica |
|
Psilocybin mushrooms are not an illegal drug under Jamaica’s Dangerous Drugs Act, 1948.(6)
The Corporation’s activity in relation to the sponsored research of psilocybin mushrooms, botanicals and other related fungi is limited to the jurisdiction of Jamaica.
|
Notes:
| (1) |
Business segment focuses on the research, development and commercialization of psychedelic-inspired regulation medicines.
|
| (2) |
In both Canada and the United States, the applicable federal government is responsible for regulating, among other things, the approval, import, sale and marketing of drugs, including any psychedelic substances, whether natural or novel. Health Canada and the FDA have not approved psilocybin as a drug for any indication. It is illegal to possess such substances without a prescription. The Corporation does not directly engage in any activities that would trigger the need to comply with any federal laws related to psychedelic substances. See “Regulatory Overview – Research and Development”.
|
| (3) |
For further information on the Canadian regulatory framework, see “Regulatory Overview – Canada – Psychedelics.”
|
| (4) |
For further information on the United States regulatory framework, see “Regulatory Overview – United States.”
|
| (5) |
Business segment focuses on consumer mental wellness, including non-psychedelic mushroom nutraceutical products.
|
| (6) |
Psilocybin mushrooms do not fall within the definition of a dangerous drug under the Dangerous Drugs Act, 1948 in Jamaica. For further information on the Jamaica regulatory framework, see “Regulatory Overview – Jamaica.”
|
9
Canada
Psychedelics
In Canada, oversight of healthcare is divided between the federal and provincial governments. The federal government is responsible for regulating, among other things, the approval, import, sale, and marketing of drugs such as psilocybin and other psychedelic substances, whether natural or novel. The provincial/territorial level of government has authority over the delivery of health care services, including regulating health facilities, administering health insurance plans such as the Ontario Health Insurance Plan, distributing prescription drugs within the province, and regulating health professionals such as doctors, psychologists, psychotherapists and nurse practitioners. Regulation is generally overseen by various colleges formed for that purpose, such as the College of Physicians and Surgeons of Ontario.
Certain psychoactive compounds, such as psilocybin, are considered controlled substances under Schedule III of the CDSA. In order to conduct any scientific research, including pre-clinical and clinical trials, using psychoactive compounds listed as controlled substances under the CDSA, an exemption under Section 56 of the CDSA (“Section 56 Exemption”) is required. This exemption allows the holder to possess and use the controlled substance without being subject to the restrictions set out in the CDSA. The Corporation has not applied for a Section 56 Exemption from Health Canada.
The possession, sale or distribution of controlled substances is prohibited unless specifically permitted by the government. A party may seek government approval for a Section 56 Exemption to allow for the possession, transport or production of a controlled substance for medical or scientific purposes. Products that contain a controlled substance such as psilocybin cannot be made, transported or sold without proper authorization from the government. A party can apply for a Dealer’s Licence under the Food and Drug Regulations (Part J). In order to qualify as a licensed dealer, a party must meet all regulatory requirements mandated by the regulations including having compliant facilities, compliant materials and staff that meet the qualifications under the regulations of a senior person in charge and a qualified person in charge. Assuming compliance with all relevant laws (Controlled Drugs and Substances Act, Food and Drugs Regulations) and subject to any restrictions placed on the licence by Health Canada, an entity with a Dealer’s Licence may produce, assemble, sell, provide, transport, send, deliver, import or export a restricted drug (as listed in Part J in the Food and Drugs Regulations – which includes psilocybin and psilocin) (see s. J.01.009 (1) of the Food and Drug Regulations).
The Corporation intends to sponsor and work with licensed third parties to conduct any clinical trials and research and does not handle controlled substances. If the Corporation were to conduct this work without the reliance on third parties, it would need to obtain additional licences and approvals described above.
Non-Psychedelics
NHPs, prescription drugs, and non-prescription drugs are all classified and regulated under the Canadian FDA.
The product safety, quality, manufacturing, packaging, labeling, storage, importation, advertising, distribution, sale and clinical trials of NHPs, drugs, cosmetics and foods are subject to regulation primarily under the Canadian FDA and associated regulations, including the Food and Drug Regulations, Cosmetic Regulations and the Natural Health Products Regulations, and related Health Canada guidance documents and policies (collectively, the “Canadian Regulations”). In addition, drugs and NHPs are regulated under the federal Controlled Drugs and Substances Act if the product is considered a “controlled substance” or a “precursor,” as defined in that statute or in related regulatory provisions.
Health Canada is primarily responsible for administering the Canadian Regulations.
Health Canada and the Canadian Regulations also set out requirements for establishment and site licences, market authorization for drugs and NHP licences. Each NHP must have a product licence or a Homeopathic
10
Medicine Number (“DIN-HM”) issued by Health Canada before it can be sold in Canada. Health Canada assigns a natural health product number (“NPN”) to each NHP once Health Canada issues the licence for that NHP. The Canadian Regulations require that all drugs and NHPs be manufactured, packaged, labeled, imported, distributed and stored under Canadian Good Manufacturing Practices (“GMP”) or the equivalent thereto, and that all premises used for manufacturing, packaging, labeling and importing drugs and NHPs have a site licence (NHPs) or establishment licence (drugs), which requires GMP compliance. The Canadian Regulations also set out requirements for labeling, packaging, clinical trials and adverse reaction reporting.
Health Canada and the Canadian Regulations, among other things, govern the manufacture, formulation, packaging, labeling, advertising and sale of NHPs and drugs, and regulate what may be represented on labels and in promotional materials regarding the claimed properties of products. The Canadian Regulations also require NHPs and drugs sold in Canada to affix a label showing specified information, such as the proper and common name of the medicinal and non- medicinal ingredients and their source, the name and address of the manufacturer/product licence holder, its lot number, adequate directions for use, a quantitative list of its medical ingredients and its expiration date. In addition, the Canadian Regulations require labeling to bear evidence of the marketing authorization as evidenced by the designation drug identification number, DIN-HM or NPN, followed by an eight-digit number assigned to the product and issued by Health Canada.
The Corporation’s expected nutraceutical products will be considered “food” and, as such, will be principally regulated under the Canadian FDA and the Canadian Regulations. The Corporation must ensure that the labelling, marketing and selling of any of its products comply with the Canadian FDA, including by ensuring that the Corporation’s products are not packaged or marketed in a manner that is misleading or deceptive to a consumer.
See “Description of the Business—Research and Development” in the Corporation’s Annual Information Form for additional information concerning the regulation applicable to the process required before prescription drug product candidates may be marketed in Canada.
United States
The FDA and other federal, state, local and foreign regulatory agencies impose substantial requirements upon the clinical development, approval, labeling, manufacture, marketing and distribution of drug products. These agencies regulate, among other things, research and development activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, advertising and promotion of any prescription drug product candidates or commercial products. The regulatory approval process is generally lengthy and expensive, with no guarantee of a positive result. Moreover, failure to comply with applicable FDA or other requirements may result in civil or criminal penalties, recall or seizure of products, injunctive relief including partial or total suspension of production, or withdrawal of a product from the market. The Corporation intends to file an investigational new drug application (“IND”) with the FDA in the first half of 2021.6
Psilocybin, psilocin, dimethyltryptamine, and 5-Methoxy-N-N-dimethyltryptamine are strictly controlled under the CSA as Schedule I substances. Schedule I substances by definition have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. Schedule I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements and criteria for importation. Anyone wishing to conduct research on
| 6 |
The Corporation has not yet held a pre-IND meeting with the FDA, in preparation for the filing of an IND application for the Sublingual Film. The Corporation has assumed that the FDA will grant such a Pre-IND meeting and that it will be able to complete the IND approval process; however, there is no guarantee that any such IND application will be accepted or granted by FDA. The Corporation has contracted with IntelgenX to develop a sublingual film formulation of psilocybin. IntelgenX has produced multiple formulation types but the final formulation has not been selected. Successful completion of formulation is necessary before clinical trials supplies can be provided to investigators.
|
11
substances listed in Schedule I under the CSA must register with the U.S. Drug Enforcement Administration (“DEA”), and obtain DEA approval of the research proposal.
See “Description of the Business – Research and Development” in the Corporation’s Annual Information Form for additional information concerning the regulation applicable to the process required before prescription drug product candidates may be marketed in the United States.
The FDA also regulates the formulation, manufacturing, preparation, packaging, labeling, holding, and distribution of foods, drugs and dietary supplements under the Federal Food, Drug and Cosmetic Act (“FFDCA”) and the Dietary Supplement Health and Education Act of 1994 (“DSHEA”). “Dietary supplements” are defined as vitamins, minerals, herbs, other botanicals, amino acids and other dietary substances for human use to supplement the diet, as well as concentrates, metabolites, constituents, extracts or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were on the market prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. New dietary ingredients (i.e., not marketed in the U.S. prior to October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been “present in the food supply as an article used for food” without being “chemically altered.” A new dietary ingredient notification must provide the FDA with evidence of a “history of use or other evidence of safety” establishing that use of the dietary ingredient, when used under the conditions recommended or suggested in the labeling of the dietary supplement, “will reasonably be expected to be safe.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. There can be no assurance that the FDA will accept the evidence of safety for any new dietary ingredients that the Corporation may want to market, and the FDA’s refusal to accept such evidence could prevent the marketing of such dietary ingredients.
The DSHEA revised the provisions of the FFDCA concerning the composition and labeling of dietary supplement ingredients and products. Under the DSHEA, dietary supplement labeling must include the statement of identity (name of the dietary supplement), the net quantity of contents statement (amount of the dietary supplement), the nutrition labeling, the ingredient list, and the name and place of business of the manufacturer, packer, or distributor. The DSHEA also states that dietary supplements may display “statements of nutritional support,” provided certain requirements are met. Such statements must be submitted to the FDA within 30 days of first use in marketing and must be accompanied by a label disclosure that “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.” Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect body structure, function or well-being, but may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat, or prevent a disease. Any statement of nutritional support the Corporation makes in labeling must possess scientific evidence substantiating that the statement is truthful and not misleading. If the FDA were to determine that a particular statement of nutritional support was an unacceptable drug claim or an unauthorized version of a health claim about disease risk reduction for a food product, or if the FDA were to determine that a particular claim was not adequately supported by existing scientific data or was false or misleading, the Corporation would be prevented from using that claim. In addition, the FDA deems promotional and internet materials as labeling; therefore, the Corporation’s promotional and internet materials must comply with FDA requirements and could be the subject of regulatory action by the FDA, or by the Federal Trade Commission (the “FTC”) if that agency or other governmental authorities, reviewing the materials as advertising, considers the materials false and misleading.
U.S. laws also require recordkeeping and reporting to the FDA of all serious adverse events involving dietary supplements products. The Corporation will need to comply with such recordkeeping and reporting requirements, and implement procedures governing adverse event identification, investigation and reporting. As a result of reported adverse events, health and safety risks or violations of applicable laws and regulations, the Corporation may from time to time elect, or be required, to recall, withdraw or remove a product from a market, either temporarily or permanently.
12
The Corporation’s expected nutraceutical products will be considered “food” and must be labeled as such. Within the U.S., this category of products is subject to the federal Nutrition, Labeling and Education Act (“NLEA”), and regulations promulgated under the NLEA. The NLEA regulates health claims, ingredient labeling and nutrient content claims characterizing the level of a nutrient in the product. The ingredients in conventional foods must either be generally recognized as safe by experts for the purposes to which they are put in foods, or be approved as food additives under FDA regulations. If the Corporation’s expected nutraceutical products were regulated as foods, it would be required to comply with the Federal Food Safety & Modernization Act and applicable regulations. The Corporation would be required to provide foreign supplier certifications evidencing the Corporation’s compliance with FDA requirements.
The FDA has broad authority to enforce the provisions of the FFDCA applicable to foods, drugs, dietary supplements, and cosmetics, including powers to issue a public warning letter to a company, to publicize information about illegal or harmful products, to request a recall of products from the market, and to request the United States Department of Justice to initiate a seizure action, an injunction action, or a criminal prosecution in the U. S. courts. The Corporation could be subject to fines and penalties, including under administrative, civil and criminal laws for violating U.S. laws and regulations, and the Corporation’s expected nutraceutical products could be banned or subject to recall from the marketplace. The Corporation could also be subject to possible business and consumer claims under applicable statutory, product liability and common laws.
The FTC will exercise jurisdiction over the advertising of the Corporation’s expected nutraceutical products in the United States. The FTC has in the past instituted enforcement actions against several dietary supplement and food companies and against manufacturers of dietary supplement products, including for false and misleading advertising, label claims or product promotional claims. In addition, the FTC has increased its scrutiny of the use of testimonials, which the Corporation may utilize, as well as the role of endorsements and product clinical studies. The Corporation cannot be sure that the FTC, or comparable foreign agencies, will not question the Corporation’s advertising, product claims, promotional materials or other operations in the future. The FTC has broad authority to enforce its laws and regulations, including the ability to institute enforcement actions that could result in recall actions, consent decrees, injunctions, and civil and criminal penalties by the companies involved. Failure to comply with the FTC’s laws and regulations could impair the Corporation’s ability to market the Corporation’s expected nutraceutical products.
The Corporation will also be subject to regulation under various state and local laws, ordinances and regulations that include provisions governing, among other things, the registration, formulation, manufacturing, packaging, labeling, advertising, sale and distribution of foods and dietary supplements. In addition, in the future, the Corporation may become subject to additional laws or regulations administered by the FDA or by other federal, state, local or foreign governmental authorities, to the repeal of laws or regulations that the Corporation considers favorable, or to more stringent interpretations of current laws or regulations. In the future, the Corporation believes that the dietary supplement industry will likely face increased scrutiny from federal, state and local governmental authorities. It is difficult to predict the effect future laws, regulations, repeals or interpretations will have on the Corporation’s business. However, such changes could require the reformulation of products, recalls or discontinuance of products, additional administrative requirements, revised or additional labeling, increased scientific substantiation or other requirements. Any such changes could have a material adverse effect on the Corporation’s business or financial performance.
Jamaica
Psilocybin mushrooms do not fall within the definition of a dangerous drug under the Dangerous Drugs Act (the “DDA”) in Jamaica. The Corporation’s future business activities in Jamaica involve the import of psychedelic and pharmaceutical based medicines (derived from mushrooms) for the purposes of conducting research and development as well as testing on human subjects i.e., clinical trials in Jamaica. It is intended that the clinical trials will be conducted by the University of West Indies (“UWI”) and the Corporation will act as a sponsor (the “Clinical Trials”).
13
The process of conducting clinical trials in Jamaica is governed by the Ministry of Health, Jamaica Guidelines for the Conduct of Research on Human Subjects (the “Guidelines”). The Corporation and the UWI would be required to ensure that the clinical trials are being conducted in accordance with these Guidelines. The Guidelines provide that prior to conducting research on human subjects, all researchers (i.e., academics, scientists, students, and investigators) are required to prepare a research protocol/proposal.
Research protocols should be submitted to the Medical Officer of Health in the parish where the proposed research is to be conducted, for evaluation of the ethical and scientific merits. Where the site of the proposed research includes a hospital, the Senior Medical Officer of the facility should also receive a copy of the research protocol, and his/her approval to conduct the study should be obtained.
The regulation of the sale, manufacturing, importation and distribution of drugs in Jamaica is largely governed by the Food & Drugs Act, 1964 (the “Jamaica FDA”) and the Food and Drugs Regulations, 1975 (the “Regulations”). Section 4 of the Jamaica FDA prohibits the importation of any drug into Jamaica unless it conforms to the law of the country in which it was manufactured or produced and is accompanied by a certificate declaring that the drug does not contravene any known laws of that country and that its sale therein for consumption or use by or for man or animal, as the case may be, would not constitute a violation of the laws of that country.
Regulation 40 stipulates that, a person shall not sell, manufacture, import or distribute a drug unless that drug has been registered with the MOH. The Regulations further state that a permit must be obtained from the Ministry of Health Jamaica (“MOH”) for the sale, manufacturing, importation and distribution of drugs into Jamaica. Additionally, Regulation 65 states that a person shall not import, sell, advertise for sale, or manufacture a new drug in Jamaica unless that person has obtained a licence from the MOH.
Failure to comply with section 4 of the Jamaica FDA shall result in such person being guilty of an offence and liable to a fine not exceeding J$1,000,000 (approximately US$6,711) or to imprisonment with or without hard labour for a term not exceeding twelve months. Where a person committing an offence under the Jamaica FDA is a corporation, the chairman, president, the officers and every director thereof concerned in the management of such corporation, shall also be guilty of the same offence unless he/she proves that the act or omission constituting the offence took place without his/her knowledge or that he/she exercised all due diligence to prevent the commission thereof.
Regulation 87 provides that any person who fails to comply with the Regulations shall be guilty of an offence and shall be liable to a fine not exceeding J$2,000 (approximately US$13) or to imprisonment for a term not exceeding twelve months.
In the event that the Clinical Trials include the preparation and manufacture of precursor chemicals, then the Precursor Chemicals Act (the “PCA”) may be applicable to the Clinical Trials. As per section 6 of the PCA, any person who proposes to engage in any prescribed activity shall apply to the Pharmaceutical & Regulatory Affairs Department of the MOH for a licence to engage in such prescribed activity.
Section 23 under the PCA stipulates that any person who engages in any prescribed activity without obtaining the requisite licence shall be guilty of an offence and liable to a fine not exceeding J$3,000,000 (approximately US$20,134) or to imprisonment for a term not exceeding three years or to both such fine and imprisonment.
As of the date of this Prospectus, the Corporation’s sponsorship of the Clinical Trials has not commenced. The Corporation has submitted its application to the Institutional Review Board and Ethics Committee of the Ministry of Health Jamaica (“Jamaica IRB”) and is awaiting comments. Once such comments are settled, if any, the Corporation will begin its sponsorship of the Clinical Trials subject to applicable laws. The Corporation is unable to apply for an import licence for its sponsored Clinical Trial materials until it receives final Jamaica IRB approval. Once received, the Corporation will apply to obtain an import licence and any other required licences.
14
United Kingdom
In the UK, there are two main “layers” of regulation with which products containing controlled substances must comply. These are: (i) controlled drugs legislation, which applies to all products irrespective of the type of product, and (ii) the regulatory framework applicable to a specific category of products, in this case, pharmaceuticals and food/food supplements.
The main UK controlled drugs legislation is the Misuse of Drugs Act 1971 (“MDA”) and the Misuse of Drugs Regulations 2001 (“MDR”), each as amended. The MDA sets out the penalties for unlawful production, possession and supply of controlled drugs based on three classes of risk (A, B and C). The MDR sets out the permitted uses of controlled drugs based on which Schedule (1 to 5) they fall within.
In the United Kingdom, “Fungus (of any kind) which contains psilocin or an ester of psilocin” is controlled as a Class A drug under the MDA and Schedule 1 drug under the MDR. As psilocybin is a phosphate ester of psilocin, even if it were isolated from psilocin, it would still fulfil this definition.
In the United Kingdom, Class A drugs are deemed to be the most dangerous, and so carry the harshest punishments for unlawful manufacture, production, possession and supply. Schedule 1 drugs can only be lawfully manufactured, produced, possessed and supplied under a Home Office licence. Whilst exemptions do exist, none are applicable to the API.
Licensing Requirements
The Corporation obtains acceptable psychedelic agent psilocybin (“API”) from the pharmaceutical ingredient provider who is based in the United States. The API itself is expected to be manufactured and packaged in FDA-registered facilities in the United Kingdom. The API is expected to be sent directly to the Corporation’s partners for research and development purposes in the United States, Canada and Jamaica.
Although the facilities in the UK are currently FDA-registered, this would not be sufficient to ensure the existence of valid marketing activities at this site. As mentioned above, in order to produce, possess and supply the API, the UK-based facility must also hold a domestic licence issued by the Home Office covering the manufacture, production, possession and supply of a controlled substance, as well as an export licence for each API shipment. The export application must include details of the importer and any import licence required by the local authorities in the United States.
All premises that are licensed in connection with the possession, supply, manufacture and/or production of controlled drugs are required to adhere to detailed security standards.7
Typically, when controlled drugs are being transported between licensees, responsibility for their security remains with the owner and does not transfer to either the courier or the customer until the drugs arrive at their destination and are signed for. However, where a third party is involved in the transit and/or storage of controlled drugs, even if they are not the legal owners, this party also carries responsibility for their security by virtue of being ‘in possession’ of them. Under the Home Office guidance, each organisation involved in the movement of controlled drugs should have a standard operating procedure covering their responsibilities, record keeping, reconciliation and reporting of thefts/losses.8
| 7 |
Home Office guidance; Security guidance for all existing or prospective Home Office Controlled Drug Licensees and/or Precursor Chemical Licensees or Registrants; 2020; https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/ 857591/Security_Guidance_for_all_Businesses_and_Other_Organisations_v1.4_Jan_2020.pdf.
|
| 8 |
Home Office guidance; Guidelines for Standard Operating Procedures (SOPs); https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/480572/StandardOpProcedure.pdf.
|
15
Pharmaceutical Products
Products are regulated as “medicinal products” under UK legislation (the Human Medicines Regulations 2012) if (i) they are presented as a substance or combination of substances having properties for treating or preventing disease in human beings having a medicinal effect (e.g., in marketing claims) or (ii) have a medicinal effect (i.e., even if no claims are made about the product).
A product has a “medicinal effect” if it has a pharmacological, immunological or metabolic effect on the body that restores, corrects or modifies a physiological function. Whether this is the case for a specific product will depend on factors such as the concentration of the psilocybin/psilocin and the mode of action of any psilocybin/psilocin absorbed in the body.
If a product is a medicinal product, a marketing authorisation for the product is required before the product can be placed on the market in the UK. The process for obtaining a marketing authorisation involves submitting pre-clinical and clinical data as well as quality and manufacturing information in the form of a common technical document. In addition to a marketing authorisation for the product itself, companies carrying out activities involving medicinal products, such as manufacturing, distribution and wholesaling, need to meet defined standards (GMP) and/or Good Distribution Practice (GDP) and to hold a related licence from the UK Medicines and Healthcare products Regulatory Agency (“MHRA”).
As mentioned above, once the API has been made in the UK, it is expected to be sent directly to the Corporation’s partners for research and development purposes in the United States, Canada or Jamaica. How the API is subsequently processed will determine the licences that the UK-based facility must hold. In particular:
| |
• |
|
If the API is just one ‘ingredient’ of the investigational medicinal product (“IMP”) which is used in the clinical trial then the UK-based facility must register with the MHRA and provide the MHRA with 60 days’ notice of the intended start of manufacture/distribution, and comply with GMP and Good Distribution Practice for active substances.
|
| |
• |
|
Conversely, if the API will itself constitute the IMP, the manufacturer must hold a Manufacturer’s Authorisations for IMPs licence (“MIA(IMP)”). In this scenario, an MIA(IMP) would be required regardless of whether the IMP is for use in the UK, another EEA Member State or a third country (such as the United States, Canada or Jamaica).
|
Some products fall on the borderline between medicines and another category such as medical devices, cosmetics or food supplements. The regulatory status of the product will be determined by i) the actual effect of the product on the body and ii) any claims made about the effect of the product. Where a product is potentially both a medicinal product and another category of product, the legal position in the UK (and EU) is that it will be regulated as a medicinal product.
Food/Food Supplements
| |
• |
|
Functional foods and nutraceuticals must comply with general UK food laws.
|
| |
• |
|
Ordinarily, food and food ingredients do not need to be pre-authorised before they can be placed on the market. However, “novel foods”, which are foods that have not been consumed to a significant degree by humans in the UK or EU before 15 May 1997 do require pre-authorisation under the EU Novel Foods Regulation (EU) 2015/2283, which has been retained in UK law post-Brexit. Whilst psychedelic mushrooms may have been consumed in the past, the same cannot be said for isolated psilocybin or psilocin. For this reason, it is likely that any food item containing isolated psilocybin and/or psilocin that is not considered to be a medicinal product would fulfil the definition of a ‘novel food’.
|
| |
• |
|
To place a novel food on the market in the UK, it must be authorised in advance (either under an EU authorisation if granted pre-1 January 2021, or after this date under a Great Britain authorisation for
|
16
| |
England, Scotland and Wales and under an EU authorisation for Northern Ireland). Under the updated EU Novel Foods Regulation, novel foods authorisations are now generic and not applicant-specific as they were under the previous novel foods legislation. As such, in principle, once authorised, anyone can place the authorised novel food on the UK market provided that it complies with the terms of the authorisation which include conditions of use, specifications and labelling requirements.
|
| |
• |
|
Since novel food applications are a material investment, companies are using two routes to try to protect their assets: drafting the application narrowly and as specific as possible to their own product, making it more challenging for other companies to produce an ingredient that meets the conditions of the authorisation; and if the application relies on newly developed scientific evidence which is designated by the applicant as proprietary in the application, and accepted as such in the application process, that proprietary evidence will be protected by a 5-year period of exclusivity for the applicant for that novel ingredient.
|
| |
• |
|
In broad terms, the information required in the application dossier includes: a description of the production process; the detailed composition of the novel food; scientific evidence demonstrating that the novel food does not pose a safety risk to human health; and the proposed conditions of intended use and labelling requirements. The responsibility to obtain a novel foods authorisation would be that of the person who intended to commercialise the product, and not the manufacturer of the psilocybin/psilocin itself.
|
In addition to novel foods legislation, the person who intends to commercialise the product in the UK would also have to comply with the full body of food legislation, which includes food labelling and food hygiene requirements.
Research and Development
The Corporation is focused on development of psychedelic medicines and other products, through research and development of novel chemical compounds and delivery mechanisms and study of such compounds in clinical environments around the world including, but not limited to research and studies to be conducted with the UWI and, its affiliate, the Caribbean Institute for Health Research. The Corporation anticipates growing its pipeline of psychedelic pharmaceutical products inspired medicines through its internal research, development, proprietary discovery programs, mergers and acquisitions, joint ventures and collaborative development agreements. For the time being, the Corporation maintains intellectual property generated by its R&D programs through patent filings and as trade secrets. The Corporation anticipates that as these programs mature more patent applications will be filed and more details about these programs will be disclosed at such time.
As a result of COVID-19, UWI has implemented certain facility procedures and is utilizing technology in an effort to mitigate the effects of the pandemic, specifically by moving patient interactions to remote status wherever possible. The Corporation cannot guarantee that the continued effects of COVID-19 will not impact patient recruiting for clinical trials and institutional processes at UWI or other institutions involved in pharmaceutical product development.
Psychedelics are a class of drug whose primary action is to trigger psychedelic experiences via serotonin receptor agonism, causing thought, visual and auditory changes, and altered state of consciousness. Major psychedelic drugs include mescaline, LSD, psilocybin, and dimethyl tryptamine (“DMT”). Psilocybin is a naturally occurring psychedelic prodrug compound produced by more than 200 species of mushrooms, collectively known as psilocybin mushrooms. The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. As a prodrug, psilocybin is quickly converted by the body to psilocin, which has mind-altering effects.
The pharmacokinetics, pharmacology and human metabolism of psilocybin are well known and well characterized. In conjunction with psychotherapy, psilocybin has been utilized broadly in phase II clinical trials.
17
Psilocybin found in certain species of mushrooms is a non-habit forming naturally occurring psychedelic compound. Once ingested, psilocybin is rapidly metabolized to psilocin, which then acts on serotonin receptors in the brain. The Corporation intends to research and sponsor clinical trials on the efficacy of chemically synthesized psilocybin as it relates to the following indications9:
| |
• |
|
mental health (depression, PTSD, anxiety and attention deficit hyperactivity disorder); and
|
| |
• |
|
addiction (alcohol, drugs and cigarettes).
|
In late 2019, management of Cybin Corp. commenced research and development on the delivery of synthetic psilocybin and other psychedelics through mechanisms such as sublingual film delivery. Cybin has filed a patent application for such delivery mechanism.
In partnership with UWI, the Corporation plans to conduct research and development of synthetic psilocybin. The Corporation’s activity in relation to the intended research of psilocybin mushrooms, botanicals and other related fungi is limited to the jurisdiction of Jamaica and the Corporation does not deal with psychedelic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks in order to identify and develop treatments for medical conditions and does not have any direct or indirect involvement with illegal selling, production or distribution of any substances in jurisdictions in which it operates. The Corporation’s Jamaica team is composed of business consultants, legal counsel and local post-doctoral research students. As of the date of this Prospectus, the Corporation’s sponsorship of the clinical trials has not commenced. The Corporation has submitted its application to the Jamaica IRB and has received conditional approval and is awaiting final approval. Once such comments are settled, if any, the Corporation will begin its sponsorship of the clinical trials subject to applicable laws. The Corporation is unable to apply for an import license for its sponsored clinical trial materials until it receives final Jamaica IRB approval, once received, the Corporation will apply to obtain an import license.
Research and development is led by the Corporation’s North American Chief Research and Development Officer, Dr. Michael G. Palfreyman. Dr. Palfreyman, who holds a PhD in Neuroscience and Neuropharmacology from the University of Nottingham, United Kingdom, is an accomplished pharmaceutical industry veteran responsible for more than 30 successful clinical programs.
The Corporation has also retained Stosic and Associates, a leading government relations firm, to work with high level pharmaceutical, institutional and government relations individuals to progress the acceptance of psychedelics in Canada for medical use.
The Corporation’s research and development must be conducted in strict compliance with the regulations of federal, state, local and regulatory agencies in Canada and the United States, and the equivalent regulatory agencies in the other jurisdictions in which the Corporation operates, including Jamaica. These regulatory authorities regulate, among other things, the research, manufacture, promotion and distribution of drugs in specific jurisdictions under applicable laws and regulations. It is important to note, that unlike in Canada and the United States, psilocybin mushrooms are not an illegal drug under Jamaica’s DDA. Accordingly, conducting
| 9 |
Certain statements regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms have not been evaluated by Health Canada, the FDA or other similar regulatory authorities, nor has the efficacy of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms been confirmed by approved research. There is no assurance that psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds, nutraceutical products or functional mushrooms can be used to diagnose, treat, cure or prevent any disease or condition and robust scientific research and clinical trials are needed. There are multiple risk factors regarding the ability to successfully commercially scale a chemically synthesized process to obtain psilocybin and other analogues.
|
18
research on psilocybin mushrooms does not contravene the laws of Jamaica and does not require any permit or authorization from Jamaican regulatory authorities.
Canada
Psychedelics
The process required before a prescription drug product candidate may be marketed in Canada generally involves:
| |
• |
|
Chemical and Biological Research – Laboratory tests are carried out on tissue cultures and with a variety of small animals to determine the effects of the drug. If the results are promising, the manufacturer will proceed to the next step of development.
|
| |
• |
|
Pre-Clinical Development – Animals are given the drug in varying amounts over differing periods of time. If it can be shown that the drug causes no serious or unexpected harm at the doses required to have an effect, the manufacturer will proceed to clinical trials.
|
| |
• |
|
Clinical Trials – Phase I – The first administration in humans is to test if people can tolerate the drug. If this testing is to take place in Canada, the manufacturer must prepare a clinical trial application for the Therapeutic Products Directorate of Health Canada (the “TPD”). This includes the results of the first two steps and a proposal for testing in humans. If the information is sufficient, the Health Products and Food Branch of Health Canada (the “HPFB”) grants permission to start testing the drug, generally first on healthy volunteers.
|
| |
• |
|
Clinical Trials – Phase II – Phase II trials are carried out on people with the target condition, who are usually otherwise healthy, with no other medical condition. Trials carried out in Canada must be approved by the TPD. In phase II, the objective of the trials is to continue to gather information on the safety of the drug and begin to determine its effectiveness.
|
| |
• |
|
Clinical Trials – Phase III – If the results from phase II show promise, the manufacturer provides an updated clinical trial application to the TPD for phase III trials. The objectives of phase III include determining whether the drug can be shown to be effective, and have an acceptable side effect profile, in people who better represent the general population. Further information will also be obtained on how the drug should be used, the optimal dosage regimen and the possible side effects.
|
| |
• |
|
New Drug Submission – If the results from phase III continue to be favourable, the drug manufacturer can submit a new drug submission (“NDS”) to the TPD. A drug manufacturer can submit an NDS regardless of whether the clinical trials were carried out in Canada. The TPD reviews all the information gathered during the development of the drug and assesses the risks and benefits of the drug. If it is judged that, for a specific patient population and specific conditions of use, the benefits of the drug outweigh the known risks, the HPFB will approve the drug by issuing a notice of compliance.
|
United States
The process required before a prescription drug product candidate may be marketed in the United States generally involves:
| |
• |
|
completion of extensive non-clinical laboratory tests, animal studies and formulation studies, all performed in accordance with the FDA’s Good Laboratory, Good Clinical and/or Manufacturing Practice regulations;
|
| |
• |
|
submission to the FDA of an IND, which must become effective before human clinical trials may begin;
|
| |
• |
|
approval by an institutional review board or independent ethics committee at each clinical trial site before each trial may be initiated;
|
19
| |
• |
|
for some products, performance of adequate and well-controlled human clinical trials in accordance with the FDA’s regulations, including Good Clinical Practices, to establish the safety and efficacy of the prescription drug product candidate for each proposed indication;
|
| |
• |
|
submission to the FDA of a New Drug Application (“NDA”); and
|
| |
• |
|
FDA review and approval of the NDA prior to any commercial marketing, sale or shipment of the drug.
|
The testing and approval process requires substantial time, effort and financial resources, and the Corporation cannot be certain that any approvals for its prescription drug product candidates will be granted on a timely basis, if at all.
Non-clinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals and other animal studies. The results of non-clinical tests, together with manufacturing information and analytical data, are submitted as part of an IND to the FDA. Some non-clinical testing may continue even after an IND is submitted. The IND also includes one or more protocols for the initial clinical trial or trials and an investigator’s brochure. An IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions relating to the proposed clinical trials as outlined in the IND and places the clinical trial on a clinical hold. In such cases, the IND sponsor and the FDA must resolve any outstanding concerns or questions before any clinical trials can begin. Clinical trial holds also may be imposed at any time before or during studies due to safety concerns or non-compliance with regulatory requirements.
An independent institutional review board (“IRB”), at each of the clinical centers proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center. An IRB considers, among other things, whether the risks to individuals participating in the trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the consent form signed by the trial participants and must monitor the study until completed. The FDA, the IRB, or the sponsor may suspend or discontinue a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trials to public registries.
The FDA offers a number of regulatory mechanisms that provide expedited or accelerated approval procedures for selected drugs and indications which are designed to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These include programs such as Breakthrough Therapy designations, Fast Track designations, Priority Review and Accelerated Approval, which the Corporation may need to rely upon in order to receive timely approval or to be competitive.
The Corporation may plan to seek orphan drug designation for certain indications qualified for such designation. The U.S., E.U. and other jurisdictions may grant orphan drug designation to drugs intended to treat a “rare disease or condition,” which, in the U.S., is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or 200,000 or more individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the United States for this type of disease or condition will be recovered from sales of the product. In the E.U., orphan drug designation can be granted if: the disease is life threatening or chronically debilitating and affects no more than 50 in 100,000 persons in the E.U.; without incentive it is unlikely that the drug would generate sufficient return to justify the necessary investment; and no satisfactory method of treatment for the condition exists or, if it does, the new drug will provide a significant benefit to those affected by the condition. Orphan drug designation must be requested before submitting an NDA. If a product that has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the U.S. and 10 years in the E.U. Orphan drug designation does not prevent competitors from developing or
20
marketing different drugs for the same indication or the same drug for different indications. After orphan drug designation is granted, the identity of the therapeutic agent and its potential orphan use are publicly disclosed. Orphan drug designation does not convey an advantage in, or shorten the duration of, the development, review and approval process. However, this designation provides an exemption from marketing and authorization (NDA) fees.
Drugs manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, reporting of adverse experiences with the product, and complying with promotion and advertising requirements. The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-market testing, including phase IV clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. In addition, drug manufacturers and their subcontractors involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including current Good Manufacturing Practices, which impose certain procedural and documentation requirements. Failure to comply with statutory and regulatory requirements may subject a manufacturer to legal or regulatory action, such as warning letters, suspension of manufacturing, product seizures, injunctions, civil penalties or criminal prosecution. There is also a continuing, annual prescription drug product program user fee.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information, requirements for post-market studies or clinical trials to assess new safety risks, or imposition of distribution or other restrictions under a risk evaluation and mitigation strategy.
Controlled Substances
The CSA and its implementing regulations establish a “closed system” of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA. The DEA is responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
Facilities that manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s). For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized.
The DEA inspects all manufacturing facilities to review security, recordkeeping, reporting and handling prior to issuing a controlled substance registration. The specific security requirements vary by the type of business activity and the schedule and quantity of controlled substances handled. The most stringent requirements apply to manufacturers of Schedule I and Schedule II substances. Required security measures commonly include background checks on employees and physical control of controlled substances through storage in approved vaults, safes and cages, and through use of alarm systems and surveillance cameras. Once registered, manufacturing facilities must maintain records documenting the manufacture, receipt and distribution of all controlled substances. Manufacturers must submit periodic reports to the DEA of the distribution of Schedule I and II controlled substances, Schedule III narcotic substances, and other designated substances. Registrants must also report any controlled substance thefts or significant losses, and must obtain authorization to destroy or dispose of controlled substances. Imports of Schedule I and II controlled substances for commercial purposes are
21
generally restricted to substances not already available from a domestic supplier or where there is not adequate competition among domestic suppliers. In addition to an importer or exporter registration, importers and exporters must obtain a permit for every import or export of a Schedule I and II substance or Schedule III, IV and V narcotic, and submit import or export declarations for Schedule III, IV and V non-narcotics.
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within Schedules I and II that may be manufactured or produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. The quotas apply equally to the manufacturing of the active pharmaceutical ingredient and production of dosage forms. The DEA may adjust aggregate production quotas a few times per year, and individual manufacturing or procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments for individual companies.
Individual U.S. states also establish and maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. State authorities, including boards of pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on the Corporation’s business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
Patent Cooperation Treaty
The Patent Cooperation Treaty (the “PCT”) facilitates filing for patent recognition in multiple jurisdictions simultaneously using a single uniform patent application. 193 countries, including Canada and the United States have ratified the PCT.
Ultimately, patents are still granted in each country individually. As such, the PCT procedure consists of two phases: filing of an international application, and national evaluation under the patent laws in force in each country where a patent is sought.
Within 12 months of filing a provisional patent application at the United States Patent and Trademark Office, the Corporation may elect to file a regular utility patent application in the United States in tandem with filing a PCT application with the World Intellectual Property Office, in each case claiming priority to the provisional patent application. Within 30 months of the provisional filing date, deadlines begin for a PCT application to enter the national phase in desired jurisdictions globally, such as Canada (30 months) and Europe (31 months), in each case claiming priority to the provisional patent application.
While the Corporation is focused on programs using psychedelic-inspired compounds, the Corporation does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Corporation is exploring drug development within approved laboratory clinical trial settings conducted within approved regulatory frameworks. Though highly speculative, should any prescription drug product be developed by the Corporation (which, if it does occur, would not be for several years), such drug product will not be commercialized prior to receipt of applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended use(s) is successfully developed. The Corporation may also employ non-prescription drugs, where appropriate.
22
COMPLIANCE PROGRAM
The Corporation oversees and monitors compliance with applicable laws in each jurisdiction in which it operates. In addition to the Corporation’s senior executives and the employees responsible for overseeing compliance, the Corporation has local counsel engaged in every jurisdiction in which it operates and has received legal opinions or advice in each of these jurisdictions regarding (a) compliance with applicable regulatory frameworks, and (b) potential exposure to, and implications arising from, applicable laws in jurisdictions in which the Corporation has operations or intends to operate.
The Corporation works with third parties who require regulatory licensing to handle scheduled drugs. The Corporation continuously updates its compliance and channel programs to maintain regulatory standards set for drug development. The Corporation also works with clinical research organizations who maintain batch records and data storage for the Corporation’s clinical programs.
Additionally, the Corporation has established a Medical & Clinical Advisory Team, a Research, Clinical and Regulatory Team and a Government Relations and Communications Team with cross-functional expertise in business, neuroscience, pharmaceuticals, mental health and psychedelics to advise management.
In conjunction with the Corporation’s human resources and operations departments, the Corporation oversees and implements training on the Corporation’s protocols. The Corporation will continue to work closely with external counsel and other compliance experts, and is evaluating the engagement of one or more independent third party providers to further develop, enhance and improve its compliance and risk management and mitigation processes and procedures in furtherance of continued compliance with the laws of the jurisdictions in which the Corporation operates.
The programs currently in place include monitoring by executives of the Corporation to ensure that operations conform to and comply with required laws, regulations and operating procedures. The Corporation is currently in compliance with the laws and regulations in all jurisdictions and the related licencing framework applicable to its business activities.
The Corporation and, to its knowledge, each of its third-party researchers, suppliers and manufacturers have not received any non-compliance, citations or notices of violation which may have an impact on the Corporation’s licences, business activities or operations.
The Corporation conducts due diligence on third-party researchers, medical professionals, clinics, cultivators, processors and others as applicable, with whom it engages. Such due diligence includes but is not limited to the review of necessary licenses and the regulatory framework enacted in the jurisdiction of operation. Further, the Corporation generally obtains, under its contractual arrangements, representations and warranties from such third parties pertaining to compliance with applicable licensing requirements and the regulatory framework enacted in the jurisdiction of operation.
CONSOLIDATED CAPITALIZATION
Since the date of the Annual Financial Statements, there has been no material change to the share and loan capital of the Corporation.
The applicable Prospectus Supplement will describe any material changes, and the effect of such material changes, on the share and loan capitalization of the Corporation that will result from the issuance of Securities pursuant to each Prospectus Supplement.
23
USE OF PROCEEDS
The net proceeds from any Offering of Securities and the proposed use of those proceeds will be set forth in the applicable Prospectus Supplement relating to that Offering of Securities. Notwithstanding, the Corporation’s management has broad discretion in the application of proceeds of an Offering of Securities. On the basis of results obtained or for other sound business reasons, the Corporation may re-allocate funds as required. Accordingly, the Corporation’s actual use of proceeds may vary significantly from any proposed use of proceeds disclosed in any applicable Prospectus Supplement. See “Risk Factors – Risks Related to an Offering – Discretion over the Use of Proceeds”.
Sources and Uses of Capital
The Corporation had cash and cash equivalents on hand as at May 31, 2021 of approximately $58,409,485 including the remaining net proceeds from the Corporation’s bought deal short form prospectus offering for aggregate gross proceeds of $34,303,500 (the “February Offering”) that was completed on February 4, 2021. The Corporation’s current financial resources are sufficient to meet its short-term liquidity requirements and to fund its operations for at least the coming 12 months, exclusive of any additional proceeds to be raised through an Offering of Securities. The Corporation’s expectation is based on significant assumptions and is subject to significant risk, see “Cautionary Note Regarding Forward Looking Information” and “Risk Factors”. As at May 31, 2021, the Corporation anticipates that it will require approximately $39,417,732 to continue operations over the next 12 months, including funding for the Corporations business objectives and milestones.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Program(1)
|
|
Anticipated
12 Month Use
of Funds(2) |
|
|
Use of Proceeds
Disclosure in
February 2021
Prospectus(3) |
|
|
Amounts
Remaining to be
Applied From
Proceeds(4) |
|
|
Psilocybin Program
|
|
$ |
3,791,727 |
|
|
$ |
3,837,600 |
|
|
$ |
2,841,727 |
|
|
Deuterated Tryptamines Preclinical Programs
|
|
$ |
5,662,888 |
|
|
$ |
11,920,000 |
|
|
$ |
10,862,888 |
|
|
Phenethylamine Preclinical Development Program
|
|
$ |
2,375,141 |
|
|
$ |
2,600,000 |
|
|
$ |
2,375,141 |
|
|
Nutraceutical Products
|
|
$ |
500,000 |
|
|
$ |
500,000 |
|
|
$ |
500,000 |
|
|
Technology
|
|
$ |
4,187,704 |
|
|
$ |
6,425,000 |
|
|
$ |
6,187,764 |
|
|
Other, including General and Administrative
|
|
$ |
22,900,272 |
|
|
$ |
10,778,000 |
|
|
($ |
69,709 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$ |
39,417,732 |
|
|
$ |
36,060,600 |
|
|
$ |
22,697,751 |
|
Notes:
| (1) |
Please see the Corporation’s Annual MD&A for a description of the Corporation’s Programs. All milestones are subject to receipt of all necessary approvals, including the academic and scientific organizations with which the Corporation is working.
|
| (2) |
Certain amounts have been converted from USD to CAD at an exchange rate of 1.2575:1.
|
| (3) |
Represent proceeds from private placements previously disclosed in the Listing Statement, as well proceeds disclosed in the February Offering prospectus.
|
For detailed information in respect of the Corporation’s business objectives and milestones, and the application of proceeds from prior offerings by the Corporation, prospective purchasers of Securities should carefully consider the information described in the interim and annual management’s discussion and analysis of the Corporation, and the documents incorporated by reference herein, including the applicable Prospectus Supplement.
The expected uses of capital represents the Corporation’s current intentions based upon its present plans and business condition, which could change in the future as its plans and business conditions evolve. The amounts and timing of the actual use of available capital will depend on multiple factors and there may be circumstances where, for sound business reasons, a reallocation of capital, or termination of a program objective, may be necessary in order for the Corporation to achieve its program objectives. The Corporation may also require
24
additional funds in order to fulfill its expenditure requirements to meet existing and any new business objectives, and the Corporation expects to either issue additional securities or incur debt to do so. The material factors or assumptions used to develop the estimated amounts for the 12 months period disclosed above are included in the “Cautionary Note Regarding Forward-Looking Information” section above. The actual amount that the Corporation spends in connection with each of the identified uses and programs will depend on a number of factors, including those listed under “Risk Factors” in, or incorporated by reference in, this Prospectus.
Certain COVID-19 related risks could delay or slow the implementation of the certain of the Corporation’s planned programs resulting in additional costs for the Corporation. The extent to which COVID-19 may impact the Corporation’s business activities will depend on future developments, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions, business disruptions, and the effectiveness of actions taken in Canada, the United States, Jamaica, and other countries to contain and treat the disease. As these events are highly uncertain, the Corporation cannot determine their potential impact on operations at this time. The COVID-19 pandemic may negatively impact the Corporation’s business through disruption of supply and manufacturing, which would influence the amount and timing of planned expenditure. For example, prolonged disruptions in the supply of goods and services relied on by the Corporation to develop its products or restrictions resulting from government regulations that impact the Corporation’s ability to conduct its studies and clinic trials, may adversely impact the Corporation’s business. See “The Corporation – COVID-19 Pandemic” and “Risk Factors – Risks Related to the Business of the Corporation – Novel Coronavirus COVID-19”.
Negative Cash Flow From Operations
Since inception, the Corporation has financed its operations primarily from the issuance of equity and interest income on funds available for investment. To date, the Corporation has raised approximately $90,000,000 in gross proceeds through private placement and prospectus offerings. The Corporation has experienced operating losses and cash outflows from operations since incorporation and will require ongoing financing to continue its research and development activates. As the Corporation has not yet achieved profitability, there are uncertainties regarding its ability to continue as a going concern. The Corporation has not earned any revenue or reached successful commercialization of any products. The Corporation’s success is dependent upon the ability to finance its cash requirements to continue its activities. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained, or at all. To the extent that the Corporation has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Corporation will be required to raise additional funds through the issuance of additional equity securities, through loan financing, or other means, such as through partnerships with other companies and research and development reimbursements. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained. See “Risk Factors – Risks Related to an Offering – Negative Operating Cash Flow and Going Concern”.
DIVIDEND POLICY
The Corporation has never declared nor paid dividends on the Common Shares. Currently, the Corporation intends to retain its future earnings, if any, to fund the development and growth of its business, and the Corporation does not anticipate declaring or paying any dividends on the Common Shares in the near future, although the Corporation reserves the right to pay dividends if and when it is determined to be advisable by the board of directors of the Corporation. As a result, shareholders will have to rely on capital appreciation, if any, to earn a return on investment in the Common Shares in the foreseeable future. See “Risk Factors – Risks Related to an Offering – Speculative Nature of Investment Risk”.
25
DESCRIPTION OF SECURITIES BEING DISTRIBUTED
The following is a brief summary of certain general terms and provisions of the Securities that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Securities as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such Offering of Securities, and the extent to which the general terms and provisions described below may apply to such Securities will be described in the applicable Prospectus Supplement.
Common Shares
The Corporation is authorized to issue an unlimited number of Common Shares and an unlimited number of preferred shares. As at July 2, 2021, the Corporation had 148,413,013 Common Shares and nil preferred shares issued and outstanding.
Each Common Share entitles the holder thereof to one vote at meetings of shareholders of the Corporation other than meetings of the holders of another class of shares. Each holder of Common Shares is also entitled to receive dividends if, as and when declared by the board of directors of the Corporation. Holders of Common Shares are entitled to participate in any distribution of the Corporation’s net assets upon liquidation, dissolution or winding-up on an equal basis per share. There are no pre-emptive, redemption, retraction, purchase or conversion rights attaching to the Common Shares.
Common Shares may be sold separately or together with certain other Securities under this Prospectus. Common Shares may also be issuable on conversion, exchange, exercise or maturity of certain other Securities qualified for issuance under this Prospectus.
Warrants
Warrants may be offered separately or together with other Securities, as the case may be. Each series of Warrants may be issued under a separate warrant indenture or warrant agency agreement to be entered into between the Corporation and one or more banks or trust companies acting as Warrant agent or may be issued as stand-alone contracts. The applicable Prospectus Supplement will include details of the Warrant agreements, if any, governing the Warrants being offered. The Warrant agent, if any, will be expected to act solely as the agent of the Corporation and will not assume a relationship of agency with any holders of Warrant certificates or beneficial owners of Warrants. The following sets forth certain general terms and provisions of the Warrants that may be offered under this Prospectus. The specific terms of the Warrants, and the extent to which the general terms described in this section apply to those Warrants, will be set forth in the applicable Prospectus Supplement.
A copy of any warrant indenture or any warrant agency agreement relating to an offering of Warrants will be filed by the Corporation with the relevant securities regulatory authorities in Canada after it has been entered into by the Corporation.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Warrants being offered thereby, which may include, without limitation, the following (where applicable):
| |
• |
|
the designation of the Warrants;
|
| |
• |
|
the aggregate number of Warrants offered and the offering price;
|
| |
• |
|
the designation, number and terms of the other Securities purchasable upon exercise of the Warrants, and procedures that will result in the adjustment of those numbers;
|
| |
• |
|
the exercise price of the Warrants;
|
| |
• |
|
the dates or periods during which the Warrants are exercisable;
|
| |
• |
|
the designation and terms of any securities with which the Warrants are issued;
|
26
| |
• |
|
if the Warrants are issued as a unit with another Security, the date on and after which the Warrants and the other Security will be separately transferable;
|
| |
• |
|
any minimum or maximum amount of Warrants that may be exercised at any one time;
|
| |
• |
|
whether such Warrants will be listed on any securities exchange;
|
| |
• |
|
any terms, procedures and limitations relating to the transferability, exchange or exercise of the Warrants;
|
| |
• |
|
certain material Canadian tax consequences of owning the Warrants; and
|
| |
• |
|
any other material terms and conditions of the Warrants.
|
Units
The Corporation may issue Units comprised of one or more of the other Securities described herein in any combination. Each Unit may be issued so that the holder of the Unit is also the holder of each Security included in the Unit; thus, the holder of a Unit may have the rights and obligations of a holder of each included Security. Any Unit agreement under which a Unit may be issued may provide that the Securities included in the Unit may not be held or transferred separately at any time or at any time before a specified date.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Units being offered thereby, which may include, without limitation, the following (where applicable):
| |
• |
|
the designation, number and terms of the Units and of the Securities comprising the Units, including whether and under what circumstances those Securities may be held or transferred separately;
|
| |
• |
|
any provisions for the issuance, payment, settlement, transfer or exchange of the Units or of the Securities comprising the Units;
|
| |
• |
|
certain material Canadian tax consequences of owning the Securities comprising the Units; and
|
| |
• |
|
any other material terms and conditions of the Units.
|
Debt Securities
Debt Securities will be senior or subordinated unsecured indebtedness of the Corporation as described in the relevant Prospectus Supplement. If the Debt Securities are senior indebtedness, they will rank equally and rateably with all other unsecured indebtedness of the Corporation, from time to time issued and outstanding, which is not subordinated.
If the Debt Securities are subordinated indebtedness, they will rank equally and rateably with all other subordinated Debt Securities from time to time issued and outstanding. In the event of the insolvency or winding-up of the Corporation, the subordinated Debt Securities will be subordinated and postponed in right of payment to the prior payment in full of all other liabilities and indebtedness of the Corporation, other than indebtedness that, by its terms, ranks equally with, or subordinate to, such subordinated Debt Securities.
Any convertible or exchangeable Debt Securities will be convertible or exchangeable only for other securities of the Corporation.
In conformity with applicable laws of Canada, for all bonds and notes of companies that are publicly offered, the Debt Securities will be governed by a document called an “indenture”. There will be a separate indenture for the senior Debt Securities and the subordinated Debt Securities. An indenture is a contract between a financial institution, acting on your behalf as trustee of the Debt Securities offered, and us. The trustee has two main roles. First, subject to some limitations on the extent to which the trustee can act on your behalf, the trustee can enforce your rights against us if we default on our obligations under the indenture. Second, the trustee performs certain
27
administrative duties for us. The aggregate principal amount of Debt Securities that may be issued under each indenture is unlimited. A copy of the form of each indenture to be entered into in connection with offerings of Debt Securities will be filed with the applicable securities regulatory authorities in Canada when it is entered into. A copy of any indenture or supplement thereto entered into by us will be filed with securities regulatory authorities and will be available on our profile on SEDAR.
This Prospectus does not qualify for issuance Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to one or more underlying interests including, for example, an equity or debt security, a statistical measure of economic or financial performance including, but not limited to, any currency, consumer price or mortgage index, or the price or value of one or more commodities, indices or other items, or any other item or formula, or any combination or basket of the foregoing items. For greater certainty, this Prospectus may qualify for issuance Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to published rates of a central banking authority or one or more financial institutions, such as a prime rate or bankers’ acceptance rate, or to recognized market benchmark interest rates such as LIBOR, EURIBOR or a United States federal funds rate.
Selected provisions of the Debt Securities and the indenture(s) under which such Debt Securities will be issued are summarized below. This summary is not complete. The statements made in this Prospectus relating to any indenture and Debt Securities to be issued thereunder are summaries of certain anticipated provisions thereof and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable indenture. The indentures will not limit the amount of Debt Securities that we may issue thereunder. We may issue Debt Securities from time to time under an indenture in one or more series by entering into supplemental indentures or by our board of directors or a duly authorized committee authorizing the issuance. The Debt Securities of a series need not be issued at the same time, bear interest at the same rate or mature on the same date.
The Prospectus Supplement for a particular series of Debt Securities will disclose the specific terms of such Debt Securities, including the price or prices at which the Debt Securities to be offered will be issued. The terms and provisions of any Debt Securities offered under a Prospectus Supplement may differ from the terms described below, and may not be subject to or contain any or all of such terms. Those terms may include some or all of the following:
| |
• |
|
the designation, aggregate principal amount and authorized denominations of such Debt Securities;
|
| |
• |
|
the indenture under which such Debt Securities will be issued and the trustee(s) thereunder;
|
| |
• |
|
the currency or currency units for which the Debt Securities may be purchased and the currency or currency unit in which the principal and any interest is payable (in either case, if other than Canadian dollars);
|
| |
• |
|
whether such Debt Securities are senior or subordinated and, if subordinated, the applicable subordination provisions;
|
| |
• |
|
the percentage of the principal amount at which such Debt Securities will be issued;
|
| |
• |
|
the date or dates on which such Debt Securities will mature;
|
| |
• |
|
the rate or rates per annum at which such Debt Securities will bear interest (if any), or the method of determination of such rates (if any);
|
| |
• |
|
the dates on which any such interest will be payable and the record dates for such payments;
|
| |
• |
|
any redemption term or terms under which such Debt Securities may be defeased;
|
| |
• |
|
whether such Debt Securities are to be issued in registered form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof;
|
| |
• |
|
the place or places where principal, premium and interest will be payable;
|
28
| |
• |
|
the designation and terms of any other Securities with which the Debt Securities will be offered, if any, and the principal amount of Debt Securities that will be offered with each Security;
|
| |
• |
|
the securities exchange(s) on which such series of Debt Securities will be listed, if any;
|
| |
• |
|
any terms relating to the modification, amendment or waiver of any terms of such Debt Securities or the applicable indenture;
|
| |
• |
|
any change in the right of the trustee or the holders to declare the principal, premium and interest with respect to such series of debt securities to be due and payable;
|
| |
• |
|
any limit upon the aggregate principal amount of the Debt Securities of such series that may be authenticated and delivered under the indenture;
|
| |
• |
|
if other than the Corporation or the trustee, the identity of each registrar and/or paying agent;
|
| |
• |
|
if Debt Securities are issued as a Unit with another Security, the date on and after which the Debt Securities and other Security will be separately transferable;
|
| |
• |
|
if Debt Securities are to be issued upon the exercise of Warrants, the time, manner and place for such Securities to be authenticated and delivered;
|
| |
• |
|
if Debt Securities are to be convertible or exchangeable into other securities of the Corporation, the terms and procedures for the conversion or exchange of the Debt Securities into other securities; and
|
| |
• |
|
any other specific terms of the Debt Securities of such series, including any events of default or covenants.
|
Subscription Receipts
Subscription Receipts may be offered separately or together with other Securities, as the case may be. The Subscription Receipts may be issued under a subscription receipt agreement.
The applicable Prospectus Supplement will include details of any subscription receipt agreement covering the Subscription Receipts being offered. A copy of any subscription receipt agreement relating to an offering of Subscription Receipts will be filed by the Corporation with the relevant securities regulatory authorities in Canada after the Corporation has entered into it. The specific terms of the Subscription Receipts, and the extent to which the general terms described in this section apply to those Subscription Receipts, will be set forth in the applicable Prospectus Supplement. This description may include, without limitation, the following (where applicable):
| |
• |
|
the number of Subscription Receipts;
|
| |
• |
|
the price at which the Subscription Receipts will be offered;
|
| |
• |
|
the terms, conditions and procedures for the conversion of the Subscription Receipts into other Securities;
|
| |
• |
|
the designation, number and terms of the other Securities that may be exchanged upon conversion of each Subscription Receipt;
|
| |
• |
|
the designation, number and terms of other Securities with which the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security;
|
| |
• |
|
terms applicable to the gross or net proceeds from the sale of the Subscription Receipts plus any interest earned thereon;
|
| |
• |
|
certain material Canadian tax consequences of owning the Subscription Receipts; and
|
| |
• |
|
any other material terms and conditions of the Subscription Receipts.
|
29
PLAN OF DISTRIBUTION
General
The Corporation may from time to time during the 25-month period that this Prospectus, including any amendments and supplements hereto, remains valid, offer for sale and sell up to an aggregate of $125,000,000 in Securities hereunder.
The Securities may be sold by us (i) directly pursuant to applicable statutory exemptions, (ii) to or through underwriters or dealers, or (iii) through designated agents. The Prospectus Supplement relating to a particular Offering of Securities will identify any underwriter, dealer or agent engaged in connection with the offering and sale of such Securities, and will set forth the terms of the offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection with the offering, the method of distribution of the Securities, the purchase price of the Securities (or the manner of determination thereof if offered on a non-fixed price basis), the net proceeds to us and any other material terms of the plan of distribution (including sales in transactions that are deemed to be “at-the-market distributions” as defined in NI 44-102). Any initial offering price and discounts, concessions or commissions allowed or re-allowed or paid to underwriters, dealers or agents may be changed from time to time. Only underwriters named in the Prospectus Supplement are deemed to be underwriters in connection with our Securities offered by that Prospectus Supplement.
The Securities may be sold from time to time in one or more transactions at a fixed price or prices or at non-fixed prices. If offered on a non-fixed price basis, the Securities may be offered at market prices prevailing at the time of sale, at prices determined by reference to the prevailing price of a specified security in a specified market or at prices to be negotiated with purchasers including sales in transactions that are deemed to be “at-the-market” distributions, including sales made directly on the NEO or other existing trading markets for the Securities, in which case the compensation payable to an underwriter, dealer or agent in connection with any such sale will be decreased by the amount, if any, by which the aggregate price paid for the Securities by the purchasers is less than the gross proceeds paid by the underwriter, dealer or agent to the Corporation. The price at which the Securities will be offered and sold may vary from purchaser to purchaser and during the period of distribution.
Sales of Securities under an “at-the-market distribution”, if any, will be made pursuant to an accompanying Prospectus Supplement. Sales of Securities under any “at-the-market” program will be made in transactions that are “at-the-market distributions” as defined in NI 44-102. The volume and timing of any “at-the-market distributions” will be determined at the Corporation’s sole discretion.
No underwriter or dealer involved in an “at-the-market distribution” under this Prospectus, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot securities in connection with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the offered Securities or securities of the same class as the Securities distributed under the “at-the-market distribution”, including selling an aggregate number or principal amount of Securities that would result in the underwriter creating an over-allocation position in the Securities.
In connection with the sale of the Securities, underwriters, dealers or agents may receive compensation from the Corporation including in the form of underwriters’, dealers’ or agents’ fees, commissions or concessions.
Underwriters, dealers and agents that participate in the distribution of the Securities may be deemed to be underwriters for the purposes of applicable Canadian securities legislation and any such compensation that they receive from the Corporation and any profit that they make on the resale of the Securities, may be deemed to be underwriting commissions.
Underwriters, dealers or agents who participate in the distribution of the Securities may be entitled, under agreements to be entered into with the Corporation to indemnification by the Corporation against certain
30
liabilities, including liabilities under Canadian securities legislation, or to contribution with respect to payments, which such underwriters, dealers or agents may be required to make in respect thereof. Such underwriters, dealers and agents may be customers of, engage in transactions with, or perform services for, the Corporation in the ordinary course of business.
In connection with any Offering of Securities, subject to applicable laws and other than an “at-the-market distribution”, the underwriters, dealers or agents, as the case may be, may over-allot or effect transactions which stabilize, maintain or otherwise affect the market price of the offered Securities at a level other than those which otherwise might prevail on the open market. Such transactions may be commenced, interrupted or discontinued at any time.
Unless specified in the applicable Prospectus Supplement, there is no market through which the Subscription Receipts, Warrants, Units and Debt Securities may be sold and purchasers may not be able to resell the Subscription Receipts, Warrants, Units and Debt Securities purchased under this Prospectus and the Prospectus Supplement. This may affect the pricing of the Subscription Receipts, Warrants, Units and Debt Securities in the secondary market, the transparency and availability of trading prices, the liquidity of the Subscription Receipts, Warrants, Units and Debt Securities and the extent of issuer regulation. See “Risk Factors”.
Offerings in the United States
The Securities have not been, and will not be, registered under the U.S. Securities Act or any state securities laws and, subject to certain exceptions, may not be offered or sold or otherwise transferred or disposed of in the United States absent registration or pursuant to an applicable exemption from registration under the U.S. Securities Act and applicable state securities laws. In addition, until 40 days after the commencement of an Offering of Securities under any applicable Prospectus Supplement, an offer or sale of Securities within the United States by any dealer (whether or not participating in the Offering of Securities) may violate the registration requirements of the U.S. Securities Act if such offer is made otherwise than in reliance on an exemption from the registration requirements of the U.S. Securities Act.
RISK FACTORS
An investment in the Securities involves a high degree of risk and must be considered speculative due to the nature of the Corporation’s business and present stage of development. Before making an investment decision, prospective purchasers of Securities should carefully consider the information described in this Prospectus and the documents incorporated by reference herein, including the applicable Prospectus Supplement. There are certain risks inherent in an investment in the Securities, including the factors described below and under the heading “Risk Factors” in the Annual Information Form and under the heading “Risks and Uncertainties” in the Annual MD&A, and any other risk factors described herein or in a document incorporated by reference herein, which investors should carefully consider before investing. Additional risk factors relating to a specific Offering of Securities will be described in the applicable Prospectus Supplement. Some of the factors described herein, in the documents incorporated by reference herein, and/or the applicable Prospectus Supplement are interrelated and, consequently, investors should treat such risk factors as a whole. If any of the risk factors described herein, in the Annual Information Form, in another document incorporated by reference herein or in the applicable Prospectus Supplement occur, it could have a material adverse effect on the business, financial condition and results of operations of the Corporation. Additional risks and uncertainties of which the Corporation currently is unaware or that are unknown or that it currently deems to be immaterial could have a material adverse effect on the Corporation’s business, financial condition and results of operation. The Corporation cannot assure purchasers that it will successfully address any or all of these risks. There is no assurance that any risk management steps taken will avoid future loss due to the occurrence of the risks described herein, in the Annual Information Form, in the other documents incorporated by reference herein or in the applicable Prospectus Supplement or other unforeseen risks.
31
Risks Related to the Business of the Corporation
Novel Coronavirus “COVID-19”
The outbreak of the novel strain of coronavirus, specifically identified as “COVID-19”, has resulted in governments worldwide enacting emergency measures to combat the spread of the virus. These measures, including the implementation of travel bans, self-imposed quarantine periods and social distancing, have caused material disruption to businesses globally resulting in an economic slowdown. Global equity markets have experienced significant volatility and weakness. Governments and central banks have reacted with significant monetary and fiscal interventions designed to stabilize economic conditions. The duration and impact of the COVID-19 outbreak is unknown at this time, as is the efficacy of the government and central bank interventions. It is not possible to reliably estimate the length and severity of these developments and the impact on the financial results and condition of the Corporation and its operating subsidiaries in future periods. However, depending on the length and severity of the pandemic, COVID-19 could impact the Corporation’s operations, could cause delays relating to approval from Health Canada, the FDA and equivalent organizations in other countries, could postpone research activities, and could impair the Corporation’s ability to raise funds depending on COVID-19s effect on capital markets.
The rapid development of the COVID-19 pandemic and the measures being taken by governments and private parties to respond to it are extremely fluid. While the Corporation has continuously sought to assess the potential impact of the pandemic on its operations, any assessment is subject to extreme uncertainty as to probability, severity and duration. The Corporation has attempted to assess the impact of the pandemic by identifying risks in the following principle areas:
| |
• |
|
Mandatory Closure. In response to the pandemic, many provinces, states and localities have implemented mandatory shut-downs of business to prevent the spread of COVID-19. In the locations where the Corporation operates or conducts research activity, these activities have been deemed an “essential service”, and thus not subject to the mandatory closures applicable to nonessential businesses. The Corporation’s ability to generate revenue and meet its milestones could be materially impacted by any shut down of operations or services.
|
| |
• |
|
Research and Development Disruptions. The Corporation relies on a third parties for its research and development activities. If these third parties are unable to continue operating due to mandatory closures or other effects of the pandemic, it may negatively impact the Corporation’s ability to meet its milestones and may significantly delay development. At this time, the Corporation has not experienced any significant disruptions.
|
| |
• |
|
Staffing Disruption. The Corporation is, for the time being, implementing among its staff where feasible “social distancing” measures recommended by local authorities. The Corporation has cancelled nonessential travel by employees, implemented remote meetings where possible, and permitted all staff who can work remotely to do so. For those whose duties require them to work on-site, measures have been implemented to reduce infection risk, such as reducing contact with patients, mandating additional cleaning and hand disinfection and providing masks and gloves to certain personnel. Nevertheless, despite such measures, the Corporation may find it difficult to ensure that its operations remain staffed due to employees falling ill with COVID-19, becoming subject to quarantine, or deciding not to come to come to work on their own volition to avoid infection.
|
The Corporation is actively addressing the risk to business continuity represented by each of the above factors through the implementation of a broad range of measures throughout its structure and is re-assessing its response to the COVID-19 pandemic on an ongoing basis. The above risks individually or collectively may have a material impact on the Corporation’s ability to generate revenue.
The Corporation has sufficient cash on hand raised via equity financings to fund its operations for the next 12-months and meet its working capital requirements. It is anticipated that the long-term goals of the Corporation
32
will require additional capital contributions via debt or equity financings. In the event that the impact of COVID-19 worsens and negatively affects capital markets generally, there is a risk that the Corporation may not be able to secure funding for these long-term objectives. See “The Corporation – COVID-19 Pandemic”.
Regulatory Risks and Uncertainties
In Canada, certain psychedelic drugs, including psilocybin, are classified as Schedule III drugs under the CDSA and as such, medical and recreational use is illegal under Canadian federal laws. In the United States, certain psychedelic drugs, including psilocybin, are classified as Schedule I drugs under the CSA and the Controlled Substances Import and Export Act and as such, medical and recreational use is illegal under the U.S. federal laws. There is no guarantee that psychedelic drugs or psychedelic inspired drugs will ever be approved as medicines in any jurisdiction in which the Corporation operates. All activities involving such substances by or on behalf of the Corporation are conducted in accordance with applicable federal, provincial, state and local laws. Further, all facilities engaged with such substances by or on behalf of the Corporation do so under current licences and permits issued by appropriate federal, provincial and local governmental agencies. While the Corporation is focused on programs using psychedelic inspired compounds, the Corporation does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates and does not intend to have any such involvement. However, the laws and regulations generally applicable to the industry in which the Corporation is involved in may change in ways currently unforeseen. Any amendment to or replacement of existing laws or regulations, including the classification or re-classification of the substances the Corporation is developing or working with, which are matters beyond the Corporation’s control, may cause the Corporation’s business, financial condition, results of operations and prospects to be adversely affected or may cause the Corporation to incur significant costs in complying with such changes or it may be unable to comply therewith. A violation of any applicable laws and regulations of the jurisdictions in which the Corporation operates could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings initiated by either government entities in the jurisdictions in which the Corporation operates, or private citizens or criminal charges.
The loss of the necessary licences and permits for Schedule III drugs could have an adverse effect on the Corporation’s operations.
The psychedelic drug industry is a fairly new industry and the Corporation cannot predict the impact of the ever-evolving compliance regime in respect of this industry. Similarly, the Corporation cannot predict the time required to secure all appropriate regulatory approvals for future products, or the extent of testing and documentation that may, from time to time, be required by governmental authorities. The impact of compliance regimes, any delays in obtaining, or failure to obtain regulatory approvals may significantly delay or impact the development of markets, its business and products, and sales initiatives and could have a material adverse effect on the business, financial condition and operating results of the Corporation.
The success of the Corporation’s business is dependent on the reform of controlled substances laws pertaining to psilocybin. If controlled substances laws are not favourably reformed in Canada, the United States, and other global jurisdictions, including Jamaica, the commercial opportunity that the Corporation is pursuing may be highly limited.
The Corporation makes no medical, treatment or health benefit claims about the Corporation’s proposed products. The FDA, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocybin, DMT, psilocybin analogues, or other psychedelic compounds or nutraceutical products. The efficacy of such products have not been confirmed by approved research. There is no assurance that the use of psilocybin, DMT, psilocybin analogues, or other psychedelic compounds or nutraceuticals can diagnose, treat, cure or prevent any disease or condition. Vigorous scientific research and clinical trials are needed. The Corporation has not conducted clinical trials for the use of its proposed products. Any references to quality, consistency, efficacy and safety of potential products do not imply that the Corporation verified such in clinical trials or that the
33
Corporation will complete such trials. If the Corporation cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on the Corporation’s performance and operations.
The FDA has broad authority to enforce the provisions of the FFDCA applicable to foods, drugs, dietary supplements, and cosmetics, including powers to issue a public warning letter to a company, to publicize information about illegal or harmful products, to request a recall of products from the market, and to request the United States Department of Justice to initiate a seizure action, an injunction action, or a criminal prosecution in the U. S. courts. The Corporation could be subject to fines and penalties, including under administrative, civil and criminal laws for violating U.S. laws and regulations, and the Corporation’s products could be banned or subject to recall from the marketplace. The Corporation could also be subject to possible business and consumer claims under applicable statutory, product liability and common laws.
Risks Related to an Offering
Speculative Nature of Investment Risk
An investment in the Securities carries a high degree of risk and should be considered as a speculative investment. The Corporation has no history of earnings, limited cash reserves, limited operating history, has not paid dividends, and is unlikely to pay dividends in the immediate or near future.
Negative Operating Cash Flow and Going Concern
The Corporation has negative cash flow from operating activities and has historically incurred net losses. There is no assurance that sufficient revenues will be generated in the near future. To the extent that the Corporation has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Corporation will be required to raise additional funds through the issuance of additional equity securities or through loan financing. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained, or at all. The Corporation’s ability to successfully raise additional capital and maintain liquidity may by impaired by factors outside of its control, such as a shift in consumer attitudes towards certain therapeutic methods or a downturn in the economy.
Any inclusion in the Corporation’s financial statements of a going concern opinion may negatively impact the Corporation’s ability to raise future financing and achieve future revenue. The threat of the Corporation’s ability to continue as a going concern will be removed only when, in the opinion of the Corporation’s auditor, the Corporation’s revenues have reached a level that is able to sustain its business operations. If the Corporation is unable to obtain additional financing from outside sources and eventually generate enough revenues, the Corporation may be forced to sell a portion or all of the Corporation’s assets, or curtail or discontinue the Corporation’s operations. If any of these events happen, you could lose all or part of your investment. The Corporation’s financial statements do not include any adjustments to the Corporation’s recorded assets or liabilities that might be necessary if the Corporation becomes unable to continue as a going concern. See “Risk Factors – Risks Related to an Offering – Potential Need for Additional Financing”.
Discretion over the Use of Proceeds
While detailed information regarding the use of proceeds from the sale of the Securities will be described in the applicable Prospectus Supplement, the Corporation will have broad discretion over the use of net proceeds from an offering by the Corporation of its Securities. There may be circumstances where, for sound business reasons, a reallocation of funds may be deemed prudent or necessary. In such circumstances, the net proceeds will be reallocated at the Corporation’s sole discretion.
Management will have discretion concerning the use of proceeds ascribed in the applicable Prospectus Supplement as well as the timing of their expenditures. As a result, an investor will be relying on the judgment of management for the application of the proceeds. Management may use the net proceeds described in a Prospectus
34
Supplement in ways that an investor may not consider desirable. The results and the effectiveness of the application of the proceeds are uncertain. If the proceeds are not applied effectively, the Corporation’s results of operations may suffer. See “Use of Proceeds”.
Potential Need for Additional Financing
The continued development of the Corporation will require additional financing. The Corporation’s activities do have scope for flexibility in terms of the amount and timing of expenditures, and expenditures may be adjusted accordingly. However, further operations will require additional capital and will depend on the Corporation’s ability to obtain financing through debt, equity or other means. The Corporation’s ability to meet its obligations and maintain operations may be contingent upon successful completion of additional financing arrangements. There is no assurance that the Corporation will be successful in obtaining the required financing in the future or that such financing will be available on terms acceptable to the Corporation. In addition, any future financing may also be dilutive to existing shareholders of the Corporation. See “Risk Factors – Risks Related to an Offering – Negative Operating Cash Flow and Going Concern” and “– Potential Dilution”.
Volatile Market Price of Corporation’s Common Shares
The securities market in Canada has recently experienced a high level of price and volume volatility, and the market prices of securities of many companies have experienced wide fluctuations in price which have not necessarily been related to the operating performance, underlying asset values or prospects of such companies. There can be no assurance that continual fluctuations in price will not occur. It may be anticipated that any market for the Common Shares will be subject to market trends generally, notwithstanding any potential success of the Corporation. The value of the Common Shares distributed hereunder will be affected by such volatility.
The volatility of the Common Shares may affect the ability of holders to sell the Common Shares at an advantageous price or at all. Market price fluctuations in the Common Shares may be adversely affected by a variety of factors relating to the Corporation’s business, including fluctuations in the Corporation’s operating and financial results, such results failing to meet the expectations of securities analysts or investors and downward revisions in securities analysis’ estimates in connection therewith, sales of additional Common Shares, governmental regulatory action, adverse change in general market conditions or economic trends, acquisitions, dispositions or other material public announcements by the Corporation or its competitors, along with a variety of additional factors, including, without limitation, those set forth under the heading “Cautionary Note Regarding Forward-Looking Information”. In addition, the market price for securities on stock markets, including the Neo is subject to significant price and trading fluctuations. These fluctuations have resulted in volatility in the market prices of securities that often has been unrelated or disproportionate to changes in operating performance. These broad market fluctuations may materially adversely affect the market price of the Corporation.
Additionally, the value of the Common Shares is subject to market value fluctuations based upon factors that influence the Corporation’s operations, such as legislative or regulatory developments, competition, technological change and changes in interest rates or foreign exchange rates. There can be no assurance that the market price of the Common Shares will not experience significant fluctuations in the future, including fluctuations that are unrelated to the Corporation’s performance. As at the date of this Prospectus, only the Common Shares are listed on a securities exchange and may be purchased in the secondary market.
Potential Dilution
The Corporation’s articles of incorporation and by-laws allow it to issue an unlimited number of Common Shares for such consideration and on such terms and conditions as established by the board of directors of the Corporation, in many cases, without the approval of the Corporation’s shareholders. The Corporation cannot predict the size of future issuances of Common Shares or other Securities or the effect that future issuances and sales of Common Shares or other Securities will have on the market price of our Securities. Issuances of a
35
substantial number of additional Securities, or the perception that such issuances could occur, may adversely affect prevailing market prices for the Common Shares. With any additional issuance of Common Shares, investors will suffer dilution to their voting power and the Corporation may experience dilution in its earnings per share. “Risk Factors – Risks Related to an Offering – Potential Need for Additional Financing”.
Market for Securities
There is currently no market through which the Securities, other than the Common Shares, may be sold and, unless otherwise specified in the applicable Prospectus Supplement, such unlisted Securities may not be listed on any securities or stock exchange or any automated dealer quotation system. As a consequence, purchasers may not be able to resell such unlisted Securities purchased under this Prospectus. This may affect the pricing of our Securities, other than our Common Shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these Securities and the extent of issuer regulation. There can be no assurance that an active trading market for our Securities, other than our Common Shares, will develop or, if developed, that any such market, including for our Common Shares, will be sustained.
Enforcement of Civil Liabilities
Certain of our subsidiaries and assets are located outside of Canada. Accordingly, it may be difficult for investors to enforce within Canada any judgments obtained against the Corporation, including judgments predicated upon the civil liability provisions of applicable Canadian securities laws or otherwise. Consequently, investors may be effectively prevented from pursuing remedies against the Corporation under Canadian securities laws or otherwise.
The Corporation has subsidiaries incorporated in the United States. It may not be possible for shareholders to effect service of process outside of Canada against the directors and officers of the Corporation who are not resident in Canada. In the event a judgment is obtained in a Canadian court against one or more of such persons for violations of Canadian securities laws or otherwise, it may not be possible to enforce such judgment against persons not resident in Canada. Additionally, it may be difficult for an investor, or any other person or entity, to assert Canadian securities law or other claims in original actions instituted in the United States. Courts in such jurisdiction may refuse to hear a claim based on a violation of Canadian securities laws or otherwise on the grounds that such jurisdiction is not the most appropriate forum to bring such a claim. Even if a foreign court agrees to hear a claim, it may determine that the local law, and not Canadian law, is applicable to the claim. If Canadian law is found to be applicable, the content of applicable Canadian law must be proven as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by foreign law.
CERTAIN FEDERAL INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement will include a general summary of certain Canadian federal income tax consequences which may be applicable to a purchaser of Securities offered thereunder. Investors should read the tax discussion in any Prospectus Supplement with respect to a particular offering and consult their own tax advisors with respect to their own particular circumstances.
LEGAL MATTERS
Certain legal matters in connection with the offering of the Securities will be passed upon by Aird & Berlis LLP on behalf of the Corporation. As at the date of this Prospectus, the designated professionals of Aird & Berlis LLP, as a group, beneficially own, directly or indirectly, less than one percent of the securities of the Corporation.
36
AUDITORS, TRANSFER AGENT AND REGISTRAR
Zeifmans LLP, Chartered Professional Accountants, are the auditors of the Corporation and have confirmed that they are independent of the Company within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation. Neither Zeifmans LLP nor any designated professional thereof, had any registered or beneficial interest in any securities or other property of the Corporation at the time they prepared the relevant financial statements incorporated by reference in this Prospectus or at any time thereafter
The registrar and transfer agent of the Common Shares is Odyssey Trust Company at its principal office in Calgary, Alberta.
37
Cybin (AMEX:CYBN)
Historical Stock Chart
From Mar 2024 to Apr 2024
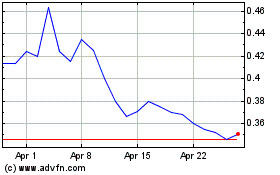
Cybin (AMEX:CYBN)
Historical Stock Chart
From Apr 2023 to Apr 2024