Form 8-K - Current report
February 29 2024 - 8:30AM
Edgar (US Regulatory)
false
0001659617
0001659617
2024-02-29
2024-02-29
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
DATE OF REPORT (DATE OF EARLIEST EVENT REPORTED): February 29, 2024
MOLECULIN BIOTECH, INC.
(Exact Name of Registrant as Specified in its Charter)
|
Delaware
|
001-37758
|
47-4671997
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(Commission File No.)
|
(I.R.S. Employer Identification
No.)
|
5300 Memorial Drive, Suite 950, Houston, TX 77007
(Address of principal executive offices and zip code)
(713) 300-5160
(Registrant’s telephone number, including area code)
(Former name or former address, if changed from last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
☐
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
☐
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
☐
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-14(c))
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading Symbol (s)
|
Name of each exchange on which registered
|
|
Common Stock, par value $.001 per share
|
MBRX
|
The NASDAQ Stock Market LLC
|
|
Item 7.01
|
Regulation FD Disclosure
|
On February 29, 2024, Moleculin Biotech, Inc. (the “Company”), held a virtual investor call with the Company’s Chairman and Chief Executive Officer, Walter Klemp. The call can be viewed via the following link: https://www.virtualinvestorco.com/wtm-mbrx-part3.
A copy of the script is attached to this report as Exhibit 99.1 and is incorporated by reference herein.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, is being furnished and shall not be “filed” for the purpose of the Securities Exchange Act of 1934, as amended (“Exchange Act”), nor shall it be incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended (“Securities Act”), unless specifically identified therein as being incorporated by reference.
|
Item 9.01
|
Financial Statements and Exhibits.
|
|
Exhibit
No.
|
Description
|
| |
|
|
99.1
|
|
| |
|
|
104
|
Cover page Interactive Data File (formatted as Inline XBRL document)
|
SIGNATURE
Pursuant to the requirements of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
MOLECULIN BIOTECH, INC.
|
|
| |
|
|
|
| |
|
|
|
| |
Date:
|
February 29, 2024
|
|
| |
|
|
|
| |
By:
|
/s/ Jonathan P. Foster
|
|
| |
|
Jonathan P. Foster
|
|
Exhibit 99.1
Virtual Investor: What this Means – Three Part Series
Participants:
| |
●
|
Wally Klemp, Chairman and Chief Executive Officer
|
Moleculin Bio (Nasdaq: MBRX)
Jenene Introduction:
| |
●
|
Welcome back for another Virtual Investor – What This Means Segment. My name is Jenene Thomas, CEO of JTC IR, and I will be today’s moderator.
|
Today we are featuring Moleculin Biotech and I am pleased to be joined by Walter Klemp, Chairman and Chief Executive Officer of the Company.
Before we get started, I just want to inform our audience that Moleculin Bio is listed on the Nasdaq and trades under the ticker M B R X. During today’s discussion, the Company will be making forward-looking statements and actual results could differ materially from these forward-looking statements. Some of the factors that could cause actual results to differ materially from these contemplated by such forward-looking statements are discussed in the periodic reports Moleculin files with the Securities and Exchange Commission. These documents are available in the Investors section of the Company's website and on the Securities and Exchange Commission's website. We encourage you to review these documents carefully.
Moderated Questions – Part 3
Jenene Introduction: So, we are thrilled to kick off the final Part 3 of our What This Means Series with Walter Klemp, CEO of Moleculin Biotech. If you missed Parts 1 or 2, we encourage you to watch them at your convenience by going to [www.virtualinvestorco.com]. Wally, when we finished Part 2, we left off with an understanding of just how strong Management believes Annamycin’s performance is in its current AML clinical trial, especially relative to the competition. In wrapping up this series, we’re hoping to now dig deeper into how you intend to win approval for Annamycin and hopefully attract the kinds of partnerships that eventually lead to a liquidity event for Moleculin shareholders.
Before we get started, I just want to inform our audience that Moleculin Bio is listed on the Nasdaq and trades under the ticker M B R X. During today’s discussion, the Company will be making forward-looking statements and actual results could differ materially from these forward-looking statements. Some of the factors that could cause actual results to differ materially from these contemplated by such forward-looking statements are discussed in the periodic reports Moleculin files with the Securities and Exchange Commission. These documents are available in the Investors section of the Company's website and on the Securities and Exchange Commission's website. We encourage you to review these documents carefully.
| |
1.
|
Q: To start with, what’s the overall pathway and timeline you envision for Annamycin approval in the AML indication?
|
| |
a.
|
Jenene, our intended pathway is designed to track with the multiple approvals we’ve recently seen for targeted therapies directed toward relapsed and refractory AML patients. Just like Annamycin, these other drugs showed promising CR data in early-stage trials and then proposed a simple single-arm pivotal trial design with Complete Response as the primary efficacy endpoint. Most of these trials were in the range of 140 to 200 patients with protocols that were discussed in advance with the FDA and the EMA.
|
| |
b.
|
This gives us what we believe is a solid roadmap for an accelerated approval pathway for Annamycin. With this approach, we believe we can receive approval to begin selling Annamycin based on the results of our next pivotal clinical trial. We expect that to be a single-arm open-label trial in the range of 100 to 200 patients. This means a relatively quick approval trial that we think we can begin by year-end and complete within 18 months of initiation. Because it’s open-label, it also means we can report progress as the trial unfolds on a regular basis rather than having to wait for a big reveal two years from now.
|
| |
c.
|
The way accelerated approvals typically work in this case is that, if Annamycin is approved based on the pivotal trial I just described, we would have to agree to conduct a confirmatory Phase 3 trial after new drug approval. But by that time, Annamycin is already generating revenue and may have resulted in the kind of larger pharma partnership that constitutes an effective exit opportunity for Moleculin shareholders.
|
| |
2.
|
Q: You sound pretty confident in your belief that Annamycin will succeed in winning approval. Why is that?
|
| |
a.
|
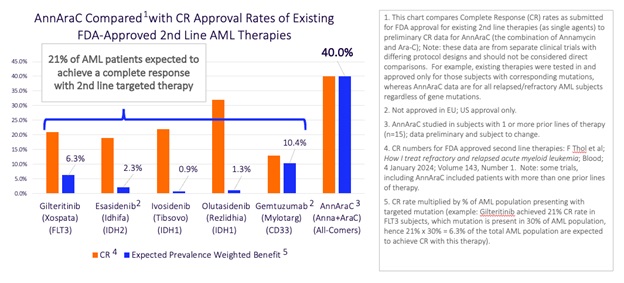
Well, when you look at the data we shared in Part 2 of this series (and we’ll show it again here), you can see that we are already generating a higher CR/CRi level than any of the prior 2nd line drug approvals.
|
| |
b.
|
And when you then weight those performance numbers with the percentage of the AML population that each therapy is designed to address, AnnAraC has the potential to help twice as many patients as all of the targeted therapies combined!
|
| |
c.
|
But, to be frank, we believe we are being conservative here. Before this trial even began, we told investors that we expected results in 2nd line or better patients to be stronger than 3rd line and worse. That’s just common sense, since with every passing month and every additional therapy, AML patients’ chances for success go down.
|
| |
d.
|
Well, we are seeing that happen and once the database review is complete, we believe a look at the stratified results for 2nd line subjects will reveal an even stronger performance than we are showing here. The point is that we believe our numbers in our pivotal trial will likely be better than the All-Comers results we are currently generating and that’s why we are so confident in our belief that Annamycin will be approved.
|
| |
3.
|
Q: So, can you walk us through what you expect to happen and when as this accelerated approval process unfolds?
|
| |
a.
|
Sure. To begin with, we need to close out the 2nd line patient portion of our current clinical trial. And, I’d like to clarify something in that regard. We designed this trial deliberately so that it could be stratified by the number of prior lines of therapy each subject has had. Our target is to enroll a total of 28 patients although we could choose to close the trial short of that number. The idea was to have approximately a third of the subjects come in as 2nd line, another third as 3rd line or greater and the final third as 1st line patients. But since our intent is to structure our pivotal trial focused on 2nd line patients only, as soon as we have enough 2nd line patients, we can go to the FDA and EMA with that data and negotiate our pivotal trial design. In other words, we don’t need to wait to fill out the cohorts of 1st line and 3rd line patients before starting the Pivotal Trial process.
|
| |
b.
|
And, as of the end of January, we’ve now recruited enough 2nd line patients to meet our goal and by the end of this month, we should have the necessary 2nd line patient data. Based on this, we have already started the internal process of requesting a meeting with FDA and we expect to have the results from that meeting late this summer or early fall. Our goal is to conduct the Pivotal Approval trial in both the US and the EU to facilitate approvals in both jurisdictions and to increase access to high-recruiting sites.
|
| |
4.
|
Q: Given this approach, why are you enrolling 1st line patients in the current trial?
|
| |
a.
|
Remember that the accelerated approval process requires a follow-on confirmatory Phase 3 trial after new drug approval. By definition, such Phase 3 trials must be 2-arm trials, comparing AnnAraC (again, that’s the combination of Annamycin and Ara-C or Cytarabine) to an existing standard of care. Since it would be unwieldy to position against another 2nd line therapy since they are all targeted therapies, a better trial design may be to position against first line therapy. But we can’t be sure about that until we actually generate some performance data in 1st line patients.
|
| |
5.
|
Q: OK, given all this, what do you see as communication milestones for investors over the coming months and quarters?
|
| |
a.
|
Well, thinking sequentially here, we want to host an “R&D Day” with key opinion leaders from around the world to discuss these results and weigh in on their clinical significance and we are targeting to host that sometime in March or April, depending on scheduling.
|
| |
b.
|
We expect to be filing our request for an End of Phase 2 Meeting with the FDA about this same time but keep in mind that there are established timelines for their response and our eventual conclusion of the pivotal trial design process. That probably translates into our ability to signal FDA feedback sometime in Q3.
|
| |
c.
|
Although we don’t think it is likely, if it turns out we can’t reach agreement with the FDA on this pivotal trial design, we always have the option of just continuing our development in the EU but, again, we don’t think that is likely.
|
| |
d.
|
If all of this falls into place, we should be starting the pivotal trial before the end of this year or early in Q1 of ’25.
|
| |
e.
|
As we have in the past, we will periodically communicate progress against this targeted timeline to the investing public.
|
| |
6.
|
Q: This has been extremely informative, Wally. Are there any final thoughts you want to leave with investors?
|
| |
a.
|
As I have said repeatedly in recent months, I consider our current share value to be significantly lower than it should be given the significance of the data that are now coming out and I think I’ve demonstrated in this What This Means series why I believe that. Remember, the last major advancements in AML resulted in Jazz Pharma paying $1.5 billion for Vyxeos, a drug that marginally increased the CR rate for 1st line patients only in AML, and Venetoclax that is now generating $2 billion in annual revenue for AbbVie.
|
| |
b.
|
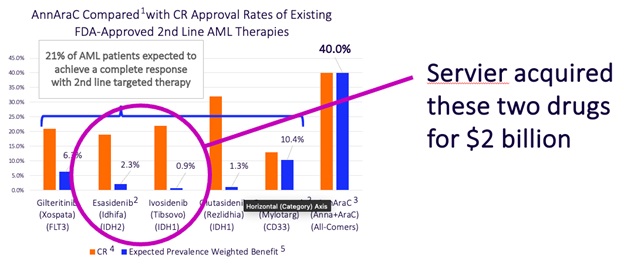
More recently, Servier, a regional pharma company based in France, paid $2 billion for the company that created two of the targeted therapies recently approved for 2nd line AML therapy. Those two drugs combined are expected to achieve CRs in a little over 3% of 2nd line AML patients. Our preliminary data support our belief that Annamycin could produce CRs in over 10 times as many patients!
|
| |
c.
|
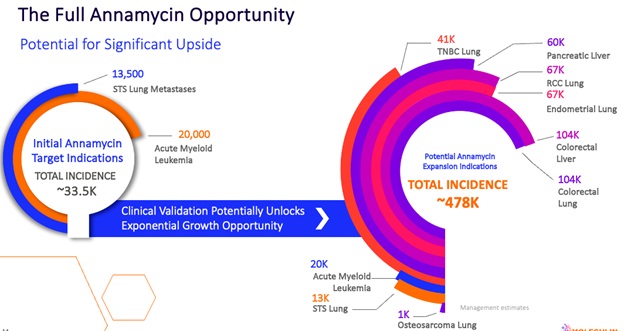
But the opportunity is really bigger than that. We haven’t focused on it in this series, but Annamycin has the potential for use in a much wider range of indications. Our ongoing MB-107 clinical trial in soft tissue sarcoma is demonstrating activity in that indication as well and our preclinical data points to similar opportunities in enough other indications that we believe the ultimate opportunity for Annamycin is 10 times greater than it is for drugs like Vyxeos.
|
| |
d.
|
We believe the ultimate value of Annamycin will be expressed in billions and we believe we are now on the verge of demonstrating that to the world.
|
Jenene Closing:
| |
●
|
With that, this concludes the Virtual Investor What this Means segment with Moleculin. I would like to thank Wally Klemp, Chairman and CEO of Moleculin for joining us today.
|
| |
●
|
I would also like to thank our viewers for your time and attention. As a reminder, you can access the webcast replay from today’s event at: www.virtualinvestorco.com.
|
v3.24.0.1
Document And Entity Information
|
Feb. 29, 2024 |
| Document Information [Line Items] |
|
| Entity, Registrant Name |
MOLECULIN BIOTECH, INC.
|
| Document, Type |
8-K
|
| Document, Period End Date |
Feb. 29, 2024
|
| Entity, Incorporation, State or Country Code |
DE
|
| Entity, File Number |
001-37758
|
| Entity, Tax Identification Number |
47-4671997
|
| Entity, Address, Address Line One |
5300 Memorial Drive, Suite 950
|
| Entity, Address, City or Town |
Houston
|
| Entity, Address, State or Province |
TX
|
| Entity, Address, Postal Zip Code |
77007
|
| City Area Code |
713
|
| Local Phone Number |
300-5160
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity, Emerging Growth Company |
false
|
| Title of 12(b) Security |
Common Stock
|
| Trading Symbol |
MBRX
|
| Security Exchange Name |
NASDAQ
|
| Amendment Flag |
false
|
| Entity, Central Index Key |
0001659617
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Moleculin Biotech (NASDAQ:MBRX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Moleculin Biotech (NASDAQ:MBRX)
Historical Stock Chart
From Apr 2023 to Apr 2024