
Courageous Innovation Clinical Showcase February 21, 2024 Dedicated to Bringing Game-Changing Gene & Cell Therapies and Vaccines to Market and Working Even Harder to Provide Access to Patients Globally

Forward Looking Statements 2 This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, which are subject to risks and uncertainties. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements include, but are not limited to, statements regarding our clinical development activities and related anticipated timelines. Such statements are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from our current expectations. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Exchange Commission (SEC), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual reports that we file with the SEC. Any forward-looking statements that we make in this presentation speak only as of the date of this presentation. Except as required by law, we assume no obligation to update forward-looking statements contained in this presentation whether as a result of new information, future events, or otherwise, after the date of this presentation.

3 Agenda Opening: Dr. Shankar Musunuri, Chairman, CEO & Co-founder, Ocugen Modifier Gene Therapy Platform & Program Update: Dr. Arun Upadhyay, CSO & Head of R&D, Ocugen Modifier Gene Therapy Market Potential: Mike Shine, SVP Commercial, Ocugen Q&A 10-minute Break Panel: Modifier Gene Therapy & OCU400 in the Clinic Moderated by Swayampakula Ramakanth, PhD, Managing Director of Equity Research at H.C. Wainwright • Dr. Lejla Vajzovic, Associate Professor of Ophthalmology with Tenure, Director of Duke Vitreoretinal Fellowship Program at Duke Eye Center and Duke University School of Medicine • Dr. Byron Lam, Professor of Ophthalmology, Dr. Mark J. Daily, Endowed Chair in Ophthalmology at the University of Miami • Dr. Neena Haider, inventor of modifier gene therapy, CEO & CSO Shifa Precision, faculty at Harvard Medical School OCU400 • Phase 1/2 clinical trial participant who has completed 12 months of therapy

4 We’re Here to Make an Impact Through Courageous Innovation Founded 2013 Headquarters Malvern, PA Employees ~61 (U.S.) Ticker Symbol OCGN R&D Center Hyderabad, India Company Overview Our Values • Respect • Integrity • Teamwork • Accountability

Through Courageous Innovation, We are Leveraging Our First-in-Class Platforms to Address Serious Unmet Medical Needs Modifier Gene Therapy Platform First-in-Class • Therapeutic Focus: inherited retinal diseases and larger blindness diseases with unmet need • Differentiator: master gene regulator; gene-agnostic approach • Pipeline: o OCU400 (Ph1/2): RP* & LCA**; Orphan drug designation from FDA/EMA, RMAT from FDA ⎼ Ph3 target: March/April 2024 o OCU410 (Ph1/2): dry AMD o OCU410ST (Ph1/2): Stargardt; Orphan drug designation from FDA Inhalation Vaccines Platform First-in-Class • Therapeutic Focus: Flu and COVID-19 • Differentiator: inhalation for improved durability and transmission control • Pipeline: o OCU500 (Preclin): COVID-19 vaccine (NIH/NIAID Nextgen Collaboration – Ph1 planned for early 2024) o OCU510 (Preclin): flu quadrivalent o OCU520 (Preclin): COVID-19 + flu combo Regenerative Cell Therapy Platform First-in-Class • Therapeutic Focus: articular cartilage lesions • Differentiator: 3-D scaffold • Pipeline: o NeoCart (Ph3): articular cartilage defects in the knee o Ph3 target: 2H2024 o Completed cGMP facility construction o RMAT designation from FDA *RP, retinitis pigmentosa **LCA, Leber congenital amaurosis 5

6 Entering a Year of Execution; New $7 PT; Reiterate Buy Raising Price Target to $8 Based On Clinical Progress Execution of High Value Gene Therapies Will Increase Valuation Key Gene Therapy Milestones Achieved in 2023 • First gene therapy program to get alignment with FDA for broad RP indication in Ph3 • Initiated dosing in OCU410 Ph1/2 clinical trial (dry AMD/GA) • Initiated dosing in OCU410ST Ph1/2 clinical trial (Stargardt) Key Gene Therapy Target Milestones for 2024 • Initiate OCU400 Ph3 clinical trial and recruit efficiently – in line with 2026 BLA approval target • Continue to provide OCU400 Ph3 clinical updates • Provide preliminary safety/efficacy updates from OCU410 Ph1/2 clinical trial in GA patients • Provide preliminary safety/efficacy updates from OCU410ST Ph1/2 clinical trial in Stargardt patients • Finalize big Pharma partner for OCU400 – to maximize value for patients and shareholders

Ocugen’s Gene Therapy – Fundamentally Changing Gene Therapy 20th Century Disruptive Biotechnologies • Penicillin • Polio vaccine • Recombinant DNA • Production of monoclonal antibodies • Genetically engineered bacteria 21st Century Biotechnology Innovations • Human Genome fully sequenced • Gene and cell therapies/CRISPR • First commercial gene therapies • mRNA vaccines Ocugen’s Modifier Gene Therapy (MGT) – First Broad Mutation Agnostic and Multifactorial Gene Therapy • Current gene therapy/CRISPR: Costly, mutation-specific and typically addresses ultra rare patient groups • Ocugen MGT: Potential to treat broad cohorts of patients with inherited retinal diseases with a single therapy • Being studied in diseases that affect millions (dry AMD) as a potential one-time therapy for life 7

Ocugen’s Modifier Gene Therapy is a First-in-Class Platform Technology Designed to Restore Homeostasis and Preserve Vision Modifier Gene Therapy Platform First-in-Class • Therapeutic Focus: Inherited retinal diseases and larger blindness diseases with unmet need • Differentiator: “Master Gene Regulator”; gene-agnostic approach o OCU400 (NR2E3) has been shown in humans (Ph 1/2) to improve vision in patients with RHO gene mutations. o OCU410 (RORA) has been shown to beneficially modify the four pathways which directly contribute to dry Age-Related Macular Degeneration (dry AMD) in multiple animal models • Pipeline: o OCU400 (Ph1/2): RP* & LCA**; Orphan drug designation from FDA/EMA and RMAT from FDA ⎼ Ph3 start target: March/April 2024 o OCU410 (Ph1/2) underway: GA o OCU410ST (Ph1/2) underway: Stargardt Disease o Received orphan drug designation from FDA *RP, retinitis pigmentosa **LCA, Leber congenital amaurosis 8

Retinitis Pigmentosa: Inherited Retinal Disease with a High Unmet Need 110,000 RP Retinitis Pigmentosa (RP) • RP is a group of rare, genetic disorders that involve a breakdown and loss of cells in the retina • RP is associated with mutations in more than 100 genes Leber Congenital Amaurosis (LCA) • LCA is a group of inherited retinal diseases characterized by severe vision impairment or blindness at birth, caused by degeneration and/or dysfunction of photoreceptors • LCA is associated with mutations in one of more than two dozen genes Disease Overview Prevalence 15,000 LCA 1.5m RP 180,000 LCA Patient Experience Available Treatment Options and Goals Currently Approved Treatments: Luxturna® • Limited for use in patients with the RPE65 mutation only o U.S. Prevalence of RPE65: 1,000 – 2,000 o Global Prevalence of RPE65: 6,000 Treatment Goals • Stabilization of vision is crucial for patients with RP and LCA due to the progressive and degenerative nature of these diseases • Preservation of remaining vision, slowing disease progression, or improving the vision can significantly impact patients’ quality of life Retinitis Pigmentosa Leber Congenital Amaurosis 9

OCU400: A Novel Gene-Agnostic Modifier Gene Therapy for RP and LCA 1 FDA & EMA granted expanded Orphan Drug Designations for all RP and LCA mutations FDA granted RMAT designation for RP indication associated with NR2E3 and RHO mutations OCU400 addresses shortcomings of current gene therapy approaches • Broad-spectrum, gene-agnostic approach to genetically diverse inherited retinal diseases • Consists of human Nuclear Hormone Receptor gene, NR2E3, with potential to preserve/improve/restore retina function • Being developed as one-time, curative therapy with a single sub-retinal injection Phase 3 trial to initiate in March/April 2024 • FDA has agreed to trial design and primary endpoint o Favorable design based on Ph 1/2 results • First gene agnostic / multi mutation Ph 3 RP trial • Ph3 study duration- 1 yr. from patient dosing 10

• OCU400 continued to be generally safe and well-tolerated in subjects across different mutations and dose levels • Efficacy measurements suggest positive trends in Best-Corrected Visual Acuity (BCVA) and Multi-Luminance Mobility Testing (MLMT), and Low-Luminance Visual Acuity (LLVA) among treated eyes • 89% (16/18) of subjects demonstrated preservation or improvement in the treated eye either on BCVA or LLVA or MLMT scores from baseline • 78% (14/18) of subjects demonstrated stabilization or improvement in treated eyes in MLMT scores from baseline • 80% (8/10) of RHO mutation subjects experienced either stabilization or increase in MLMT scores from baseline • Treatment effect in RHO patients supports the gene-agnostic mechanism of action of OCU400 Ph 1/2 Safety and Efficacy Summary 11

A PHASE 3, MULTI-CENTER, RANDOMIZED STUDY TO ASSESS THE EFFICACY, SAFETY AND TOLERABILITY OF SUBRETINAL OCU400 GENE THERAPY FOR THE TREATMENT OF RETINITIS PIGMENTOSA

RP and LCA—Unmet need and Treatment Benefit Target 8 • IRDs, such as RP and LCA, are a group of heterogenous genetic disorders that affect the retina, the light-sensitive tissue at the back of the eye • They often lead to a gradual loss of vision over time and can ultimately result in blindness • Stabilization of vision is crucial for patients with RP and LCA due to the progressive and degenerative nature of these diseases • Preservation of remaining vision, slowing disease progression, or improving the vision can significantly impact patients’ quality of life. Such outcomes not only can enhance the quality of life for affected individuals but also provide hope that future treatments that could ultimately lead to vision restoration. • Comprehensive care, early diagnosis, and access to emerging therapies are essential components of a strategy to stabilize vision in RP and LCA patients 13
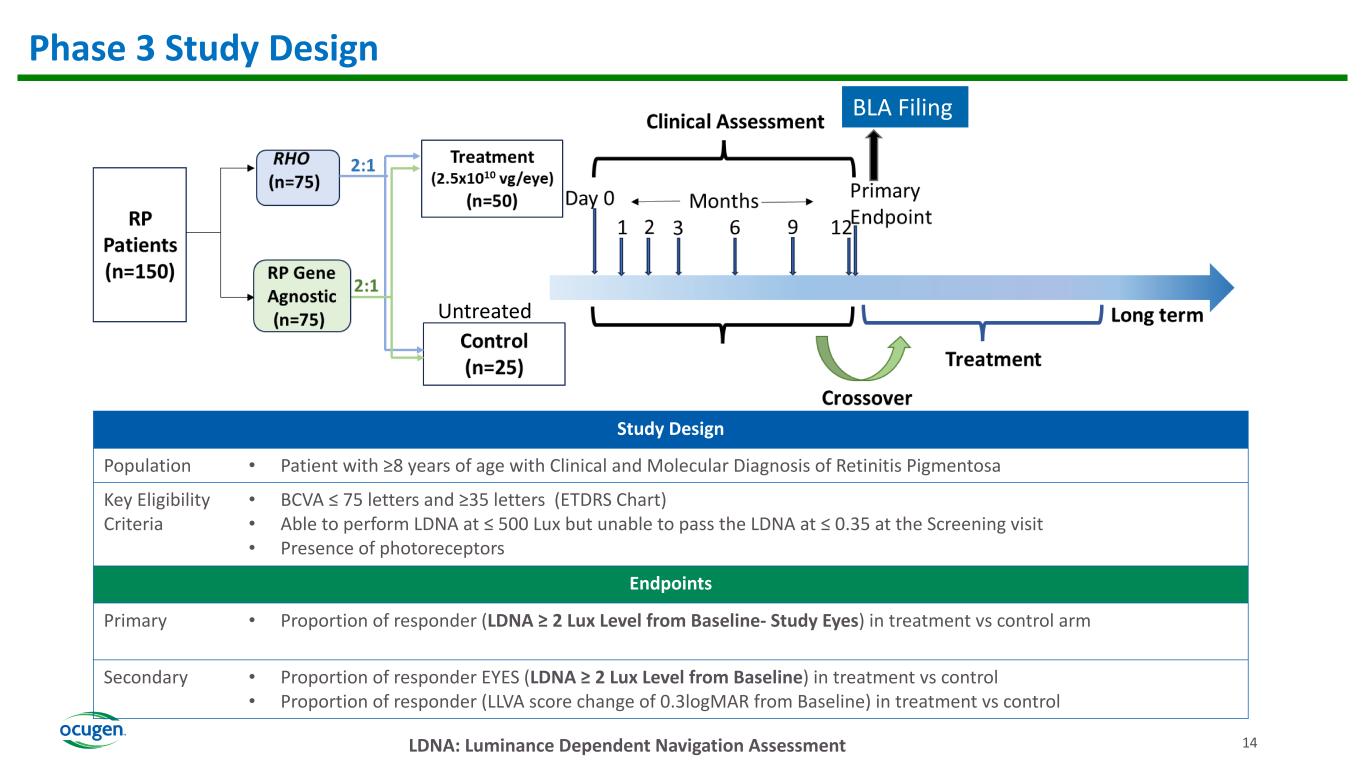
14 Phase 3 Study Design LDNA: Luminance Dependent Navigation Assessment Study Design Population • Patient with ≥8 years of age with Clinical and Molecular Diagnosis of Retinitis Pigmentosa Key Eligibility Criteria • BCVA ≤ 75 letters and ≥35 letters (ETDRS Chart) • Able to perform LDNA at ≤ 500 Lux but unable to pass the LDNA at ≤ 0.35 at the Screening visit • Presence of photoreceptors Endpoints Primary • Proportion of responder (LDNA ≥ 2 Lux Level from Baseline- Study Eyes) in treatment vs control arm Secondary • Proportion of responder EYES (LDNA ≥ 2 Lux Level from Baseline) in treatment vs control • Proportion of responder (LLVA score change of 0.3logMAR from Baseline) in treatment vs control BLA Filing Untreated 14

15 Phase 3 Study: Statistical Considerations Sample Size (N=150); Two Arm ( RHO and Gene Agnostic) Population • Each arm will enroll 75 participants • In each arm, participants (N=75) will be randomized 2:1 to the Treatment group (2.5 x 1010 vg/eye of OCU400) and Untreated Control group • This sample size is needed to achieve the targeted level of statistical power in each arm (RHO and Gene Agnostic). Assumptions • The response rates are expected to be: • 0.50 for the active treatment (OCU400) based on Phase 1/2 Study results in Intent-to- Treat population • and 0.1 for untreated control based on prior studies and to account for patient random positive response Power • >90% Hypothesis • The Primary efficacy hypothesis is that the response rate is higher with treatment group compared to control in the RHO subgroup • The conditional efficacy hypothesis is that the response rate is higher with treatment compared to control in the Gene Agnostic subgroup. 15

Phase 1/2 Patients Population Meeting “Intent to Treat Population” Criteria for Phase 3 Study* *RHO +AR-NR2E3 Subjects (- Adverse Events, Sentinel); and Ceiling Effect (RHO) Subjects; ^ ceiling effect (AR-NR2E3) # AR-NR2E3 Subjects: Baseline MLMT at 5 Lux level; 1Lux level improvement resulted in ceiling effect on old scale on 0-6 Lux levels Improvement: LLVA: ≥ 5 letters change MLMT: ≥1 change in Lux Level Stabilization: LLVA: ± 4 letters change MLMT: 0 change in Lux Level LDNA: Luminance Dependent Navigation Assessment (Updated Mobility Course) • Wide range of light intensity (0.04-500 Lux) and Lux Levels (0-9) • Uniform correlation between Lux level and Lux intensity ^ # # 16

OCU400: Demonstrate Gene Agnostic Effect in RHO Patients Showed stabilization or improvement on both the parameters from Baseline Stabilization: LLVA: ± 4 letters change MLMT: 0 change in Lux Level Improvement: LLVA: ≥ 5 letters change MLMT: ≥1 change in Lux Level 17*Primary Endpoint: Proportion of responder (LDNA ≥ 2 Lux Level from Baseline- Study Eyes) in treatment vs control arm More than 50% ITT RHO Patients Meet Responder Criteria LDNA: Luminance Dependent Navigation Assessment (Updated Mobility Course) • Wide range of light intensity (0.04-500 Lux) and Lux Levels (0-9) • Uniform correlation between Lux level and Lux intensity

RP LCA Adult & Pediatric • Both FDA & EMA granted broad orphan drug designation for RP & LCA • Received RMAT designation from FDA RHO NR2E3 CEP290 Indication Expect to pursue LCA indication in 2H 2024 Treatment of retinitis pigmentosa (RP) In December 2023, received alignment from FDA on key aspects of the Phase 3 clinical trial design to assess the safety and efficacy of OCU400 to establish the gene-agnostic effect of OCU400 in patients with RP (broad indication) OCU400: Expected Pathway to Clinical Development & Potential Approval 18

OCU400 RP – U.S. & EU Market Potential • First five years projected penetration 3% - 15% • 5-year revenue: $47B projection* 0 2 4 6 8 10 12 14 16 18 20 2026 2027 2028 2029 2030 $ Bi lli on s Year Revenue 19 *Internal modelling based on: 1) Wong, C.H., Li, D., Wang, N. et al. The estimated annual financial impact of gene therapy in the United States. Gene Ther 30, 761–773 (2023) 2)Gong J, Cheung S, Fasso-Opie A, Galvin O, Moniz LS, Earle D, Durham T, Menzo J, Li N, Duffy S, Dolgin J, Shearman MS, Fiorani C, Banhazi J, Daly A. The Impact of Inherited Retinal Diseases in the United States of America (US) and Canada from a Cost-of-Illness Perspective. Clin Ophthalmol. 2021;15:2855-2866 3) Manlong Xu, Yi Zhai, Ian M. MacDonald; Visual Field Progression in Retinitis Pigmentosa. Invest. Ophthalmol. Vis. Sci. 2020;61(6):56. • Price assumption U.S.: $750K per dose ($1.5M per patient) • Price assumption EU: $600K per dose ($1.2M per patient)

OCU400 RP – U.S. & EU Market Potential • First five years projected penetration 3% - 15% • 5-year revenue: $47B projection • Downside: $30B* 0 2 4 6 8 10 12 14 16 18 20 2026 2027 2028 2029 2030 $ Bi lli on s YearRevenue Revenue Downside 20 Downside assumptions: • Price assumption U.S.: $425K per dose ($850k per patient) • Price assumption EU: $375K per dose ($750k per patient)

OCU410 GA Market Potential • First five years projected penetration U.S.: 2% - 8% and EU: 1% - 4% • First 5-years cumulative revenue projection: $75B* 0 5 10 15 20 25 2027 2028 2029 2030 2031 $ Bi lli on s Year Revenue• Price assumption U.S.: $225K per dose ($450k per patient) • Price assumption EU: $200K per dose ($400K per patient) *Internal modelling based on: 1) Sarda SP, Heyes A, Bektas M, Thakur T, Chao W, Intorcia M, Wronski S, Jones DL. Humanistic and Economic Burden of Geographic Atrophy: A Systematic Literature Review. Clin Ophthalmol. 2021;15:4629-4644 2) Nabin Paudel, Laura Brady, Petia Stratieva, Avril Daly; Socioeconomic burden of advanced Age-related Macular Degeneration (AMD) in the United States of America (USA), Germany and Bulgaria. Invest. Ophthalmol. Vis. Sci. 2023;64(8):1746. 21

22 Ocugen™ Vision Fully integrated, patient-centric biotech company focused on vaccines in support of public health and gene and cell therapies targeting unmet medical needs through Courageous Innovation

23 Dr. Ramakanth is a Managing Director of Equity Research at H.C. Wainwright whose research focuses on the healthcare sector. His research covers companies operating in oncology, wound health, medical devices, spine health and AI Drug Discovery. Dr. Ramakanth possesses over twenty years of experience as a Life Sciences Equity Analyst as well as seven years of biotechnology industry experience. Prior to joining H.C. Wainwright, Dr. Ramakanth worked as a Junior Analyst at both Jefferies and Merrill Lynch covering large cap pharmaceuticals. Dr. Ramakanth was also a Junior Analyst at both First Albany and Rodman & Renshaw where he focused on biotechnology companies. He also conducted bench research and directed a preclinical drug development group at Regeneron Pharmaceuticals, Inc. His educational background includes a Ph.D. in Pharmacology/Toxicology from the University of Utah, an MBA from Rutgers University, an M.S. in Pharmaceutics from Auburn University, and a B. Pharm (Hons.) from BITS, India. Dr. Ramakanth also completed a post-doctoral fellowship at the University of Texas MD Anderson Cancer Center. Swayampakula Ramakanth, PhD

Neena Haider, PhD Dr. Haider is the founder of Shifa Precision, a world-renowned geneticist, and visionary scientist who has brought multiple gene therapies to the clinic. She has served/serves in numerous leadership roles and steering committees at institutions including Harvard Medical School, National Institute of Health (NIH), the U.S. Congress, National Science Foundation (NSF) and NASA. Dr. Haider is an internationally recognized scientist who has authored approximately 50 research articles, reviews, and book chapters. Her work has been cited over 3,000 times and appears in textbooks in other countries. Dr. Haider understands the needs and gaps in the current healthcare system and built Shifa Precision as a solution to actualize personalized precision medicine and developed precision therapies to achieve better health outcomes for all. Dr. Haider has a strong interest and commitment to the education of young scientists, supporting and creating a diverse and inclusive environment, and to mentor and nurture the growth of young students. She has chaired a task force on mentoring, invited as a speaker for a workshop on resilience for the BIPOC community, and developed a Positive Mindful Wellness workshop and Science of Mindfulness course at HMS. Dr. Haider continues to teach at Harvard Medical School and to train young scholars how to become mindful innovators.

Byron L. Lam, MD Dr. Lam is the Mark J. Daily Professor at the Bascom Palmer Eye Institute, University of Miami Miller School of Medicine. Dr. Lam is a clinical scientist with expertise and translational research experience in the areas of hereditary retinal disease, neuro- ophthalmology, and ophthalmic epidemiology. His research focus includes gene therapy, clinical visual function testing, ophthalmic molecular imaging, and biomarkers. In addition to clinical studies, Dr. Lam has performed numerous clinical trials including Leber hereditary optic neuropathy and hereditary retinal disease. Dr. Lam serves on the editorial board of the American Journal of Ophthalmology. He is the author of the textbook Electrophysiology of Vision: Clinical Testing and Applications.

Lejla Vajzovic, MD, FASRS Dr. Vajzovic is a vitreoretinal surgeon and tenured Associate Professor of Ophthalmology at Duke University School of Medicine with expertise in adult and pediatric retinal diseases and surgery. She serves as a principal investigator for numerous national clinical trials in early to late stages of development. Her research interests span from pediatric to adult retinal diseases such as dry and wet age-related macular degeneration, diabetic retinopathy and venous occlusive diseases, as well as vitreoretinal surgical diseases. She is a co-director of the Duke Pediatric Retina and Optic Nerve Center, and directs the Duke Center for Artificial and Regenerative Vision, where she performs gene-therapy delivery, and devices implantation to restore vision to individuals with total blindness. An influential educator, she organizes and directs several highly successful national and international courses, including the first-of-its-kind Advances in Pediatric Retina Course at Duke and the international Duke fellows and general Advances in Vitreous Surgery Course. She is director of prestigious Duke Vitreoretinal Surgical Fellowship and director of Duke Eye Center’s Continuing Medical Education program. In addition, she serves as a Retina Society American Academy of Ophthalmology (AAO) Council Representative and American Society of Retina Specialist (ASRS) Research and Safety in Therapeutics Committee Member. She is elected member of the Retina Society, Macula Society and Club Jules Gonin Society.
