UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the Month of February 2024
Commission File Number: 001-38104
IMMURON LIMITED
(Name of Registrant)
Level 3, 62 Lygon Street, Carlton South,
Victoria, 3053, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
Indicate
by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information
to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes
☐ No ☒
If “Yes”
is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-
IMMURON LIMITED
EXPLANATORY NOTE
Immuron Limited (the “Company”) published
one announcement (the “Public Notices”) to the Australian Securities Exchange on February 21, 2024 titled:
| |
- |
“Presentation to Australian Biologics Festival 2024” |
A copy of the Public Notice is attached as an exhibit to this report
on Form 6-K.
This report on Form 6-K (including the exhibit
hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”) and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, except as shall be
expressly set forth by specific reference in such filing.
EXHIBITS
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
IMMURON LIMITED |
| |
|
|
| |
 |
| |
|
|
| Date: February 21, 2024 |
By: |
/s/ Phillip Hains |
| |
|
Phillip Hains |
| |
|
Company Secretary |
3
Exhibit 99.1

Australian
Biologics Festival 2024
Melbourne,
Australia, February 21, 2024: Immuron Limited (ASX: IMC; NASDAQ: IMRN), an Australian based and globally integrated biopharmaceutical
company is pleased to advise our Research & Development Manager, Joanne Casey, PhD will be presenting at the Australian Biologics
Festival 2024 on Wednesday, 21st February in Melbourne, Australia.
A
copy of the presentation being made is included below.
Authorised for release by the Board of Immuron Limited.
-
- - END - - -
COMPANY CONTACT:
Steven Lydeamore
Chief Executive Officer
Ph: +61 (0)3 9824 5254
info@immuron.com
About
Travelan®
Travelan®
is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting traveller’s diarrhoea,
a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a highly purified
tabletised preparation of hyperimmune bovine antibodies and other factors, which when taken with meals bind to diarrhoea-causing bacteria
and prevent colonisation and the pathology associated with traveller’s diarrhoea. In Australia, Travelan® is a listed medicine
on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Traveller’s Diarrhoea,
reduce the risk of minor gastrointestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product
(NPN 80046016) and is indicated to reduce the risk of Traveller’s Diarrhoea. In the U.S., Travelan® is sold as a dietary supplement
for digestive tract protection.
About
Traveller’s Diarrhoea
Traveller’s
Diarrhoea is a gastrointestinal infection with symptoms that include loose, watery (and occasionally bloody) stools, abdominal cramping,
bloating, and fever, Enteropathogenic bacteria are responsible for most cases, with enterotoxigenic Escherichia coli (ETEC) playing
a dominant causative role. Campylobacter spp. are also responsible for a significant proportion of cases. The more serious infections
with Salmonella spp. the bacillary dysentery organisms belonging to Shigella spp. and Vibrio spp. (the causative agent of cholera) are
often confused with Traveller’s Diarrhoea as they may be contracted while travelling and initial symptoms are often indistinguishable.
About
Immuron
Immuron
Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercialising orally delivered
targeted polyclonal antibodies for the treatment of infectious diseases.
For more
information visit: http://www.immuron.com
FORWARD-LOOKING
STATEMENTS:
This
press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933
and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any
statements relating to our growth strategy and product development programs and any other statements that are not historical facts.
Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that
could negatively affect our business, operating results, financial condition and stock value. Factors that could cause actual
results to differ materially from those currently anticipated include: risks relating to our growth strategy; our ability to obtain,
perform under and maintain financing and strategic agreements and relationships; risks relating to the results of research and
development activities; risks relating to the timing of starting and completing clinical trials; uncertainties relating to
preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key
personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent
and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any
obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect
any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as
required by law.


1 2 ND AUSTRALIAN BIOLOGICS FESTIVAL 2024 NASDAQ: IMRN ASX: IMC NAVIGATING NEW HORIZONS: TRAVELAN ® MARKET EXPANSION INTO THE USA STRATEGIC COLLABORATIONS, PARTNERSHIPS AND CLINICAL TRIALS JERRY KANELLOS, PhD Chief Scientific Officer Presented by: JOANNE CASEY, PhD R&D Manager

Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections. Words such as “anticipates,” “expects,” “ intends,” “ plans,” “ believes,” “ seeks,” “estimates,” “guidance” and s imilar expressions are intended to identify forward - looking statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain r i sks and uncertainties, some of which are beyond Immuron’s control, including those r i sks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ f rom those expressed or forecasted in the forward - looking statements. The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made. Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, c i rcumstances or unanticipated events occurring after the date of this presentation except as required by law or by any appropriate regulatory authority. FY 2024 results in this presentation are subject to audit review. 2 SAFE HARBOR STATEMENT

TOPICS 3 Introduction • Immuron’s Pipeline Technology • Travelan – commercial product Partnering with US Department of Defense (DoD) • History of collaborations • DoD funding Commercial pathway of Travelan® in the US • FDA clinical pathway to BLA • Scale - up Product Development • Current status and future

Immuron Ltd is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Company Overview » Platform Technology: capable of producing highly specific orally active immunoglobulins to any enteric pathogen » Two commercially available oral immunotherapeutic products – Travelan® and Protectyn® » Three pipeline assets in four clinical programs Dual listed (ASX:IMC) (NASDAQ:IMRN) 4

5 PLATFORM TECHNOLOGY Bovine colostrum is the first milk of cows after calving. It is rich in immunoglobulins, lactoferrin, lysozyme, lactoperoxidase, growth factors and bioactive peptides. Colostrum has higher levels of protein, fat, vitamins, and minerals when compared to milk. This enables full development of the newborn calf in addition to immunity against several pathogens. * Immuron’s proprietary technology platform combines the natural human nutrition & health benefits of bovine colostrum with a novel class of specifically targeted oral polyclonal antibodies that offer delivery within the gastrointestinal (“GI”) tract and can be used to target viruses or bacteria and neutralize the toxins they produce at mucosal surfaces. STEP 1 Development of Highly Specific Vaccines STEP 2 Isolation of Hyperimmune antibody - rich bovine colostrum STEP 3 Oral Antimicrobial therapeutics without drawbacks of antibiotics FINAL PRODUCT Toxin Neutralization + Clearance of targeted gut pathogens * Gomes et. al., NFS Journal, Volume 25, November 2021, pages 1 - 11, https://doi.org/10.1016/j.nfs.2021.10.001

6 INTELLECTUAL PROPERTY Due to the practical nature of the technology used to produce our products, the company retains a significant amount of know - how and other unregistered intellectual property which presents significant hurdles to competitors in producing the same products. The company has a policy of actively in - licensing and filing upon new technology that relates to all relevant business objectives. Immuron currently has a suite of patents either approved or pending that cover the full spectrum of its Technology Platform. METHODS AND COMPOSITIONS FOR THE TREATMENT AND/OR PROPHYLAXIS OF CLOSTRIDIUM DIFFICILE ASSOCIATED DISEASE Granted (Expiry: April 17, 2034): Australia, New Zealand, Canada, USA, Belgium, France, Germany, Italy, Netherlands, Spain, Switzerland, United Kingdom Pending: China COMPOSITION AND METHOD FOR THE TREATMENT AND PREVENTION OF ENTERIC BACTERIAL INFECTIONS Granted (Expiry: February 25, 2028): USA Granted (Expiry: March 4, 2024): Australia, Canada, India, New Zealand, Austria, Denmark, Finland, France, Germany, Greece, Spain, Sweden, United Kingdom

7 BILLION DOLLAR MARKET - INDUSTRY OVERVIEW Billion Dollar Market Travellers’ diarrhoea treatment market is large and growing at a CAGR of ~7% Industry tailwinds Travel picking up significantly following COVID lockdowns Frequent Symptoms 30% - 70% of travelers experience travellers’ diarrhoea 1 • There are no current reliable vaccines for prevention of Travellers’ diarrhoea 1 • Enterotoxigenic Escherichia coli (ETEC) is the leading cause of Travellers’ diarrhoea 1 • Travelan® is a hyperimmune bovine colostrum produced by immunization of cows during gestation with a vaccine consisting of antigens derived from 13 different ETEC strains known to cause Travelers’ diarrhea • Travelan® is broadly cross - reactive with other ETEC strains not included in the vaccine and other gram - negative bacteria ( Shigella, Vibrio cholera, Campylobacter spp .) 2,3 1 Centers for Disease Control and Prevention CDC.gov 2024 2 Sears et al., Clin. Vaccine Immunol. 2017 https://doi.org/10.1128/cvi.00186 - 16 3 Islam et al., PLOS one 2023 https://doi.org/10.1371/journal.pone.0294021

8 PLOS ONE RESEARCH ARTICLE Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model ( Macaca mulatta ). Abstract Infectious diarrhea is a World Health Organization public health priority area due to the lack of effective vaccines and an accelerating global antimicrobial resistance crisis. New strategies are urgently needed such as immunoprophylactic for prevention of diarrheal diseases. Hyperimmune bovine colostrum (HBC) is an established and effective prophylactic for infectious diarrhea. The commercial HBC product, Travelan® (Immuron Ltd, Australia) targets multiple strains of enterotoxigenic Escherichia coli (ETEC) is highly effective in preventing diarrhea in human clinical studies. Although Travelan® targets ETEC, preliminary studies suggested cross - reactivity with other Gram - negative enteric pathogens including Shigella and Salmonella species. For this study we selected an invasive diarrheal/dysentery - causing enteric pathogen, Shigella , to evaluate the effectiveness of Travelan®, both in vitro and in vivo . Here we demonstrate broad cross - reactivity of Travelan® with all four Shigella spp . ( S . flexneri , S . sonnei , S . dysenteriae and S . boydii) and important virulence factor Shigella antigens. Naïve juvenile rhesus macaques (NJRM) were randomized, 8 dosed with Travelan® and 4 with a placebo intragastrically twice daily over 6 days. All NJRM were challenged with S . flexneri 2a strain 2457T on the 4th day of treatment and monitored for diarrheal symptoms. All placebo - treated NJRM displayed acute dysentery symptoms within 24 – 36 hours of challenge. Two Travelan® - treated NJRM displayed dysentery symptoms and six animals remained healthy and symptom - free post challenge; resulting in 75% efficacy of prevention of shigellosis (p = 0.014). These results strongly indicate that Travelan® is functionally cross - reactive and an effective prophylactic for shigellosis. This has positive implications for the prophylactic use of Travelan® for protection against both ETEC and Shigella spp . diarrheal infections. Future refinement and expansion of pathogens recognized by HBC including Travelan® could revolutionize current management of gastrointestinal infections and outbreaks in travelers’ including military, peacekeepers, humanitarian workers and in populations living in endemic regions of the world. Islam et al., PLOS one 2023 https://doi.org/10.1371/journal.pone.0294021

9 » Travelan® is a pasteurized, lactose - reduced, low - fat, high - protein colostrum powder which contains over 80% proteins by weight. » Travelan® is enriched with anti - Enterotoxigenic E.coli (ETEC) antibodies (35 - 45% w/w), which target ETEC in the gastrointestinal tract » Travelan® is a biological product intended to prevent Travellers’ Diarrhoea without major alterations to the microbiome, unlike antibiotics » Travelan® is the world’s first listed medicine (2004) on the Australian Register for Therapeutic goods (TGA) indicated to reduce the risk of Travellers’ Diarrhoea (AUST L 106709) » Travelan® has been marketed in Canada since 2013 as a natural health product (NPN 80046016) indicated to reduce the risk of TD » Travelan® has been marketed since 2015 in the US as a dietary supplement for digestive tract protection TRAVELAN® Australian Packaging USA Packaging

TRAVELAN DISTRIBUTION CAPABILITY • Retail network includes over 3,500 pharmacies • Online sales of both Travelan® and Protectyn® • Protectyn® is distributed by Osborne Health Supplies USA • Travelan® is sold to travel medicine clinics and on our own amazon.com shopfront (launched July 2023) Canada • We re - launched in January 2024 International • We are evaluating options to enter international markets through distributors Key Commentary Australia Shandex Ontario, Canada Distributes to all provinces of Canada Immuron Ltd Vancouver, BC, Canada Immuron Inc. New York, USA RJW Logistics Chicago, USA Distributes to all states of USA Immuron Ltd Melbourne, Australia Distributes to all states of Australia and to distributors in USA and Canada Established Developing Canada USA Australia Retail Pharmacy B2B E - commerce 10
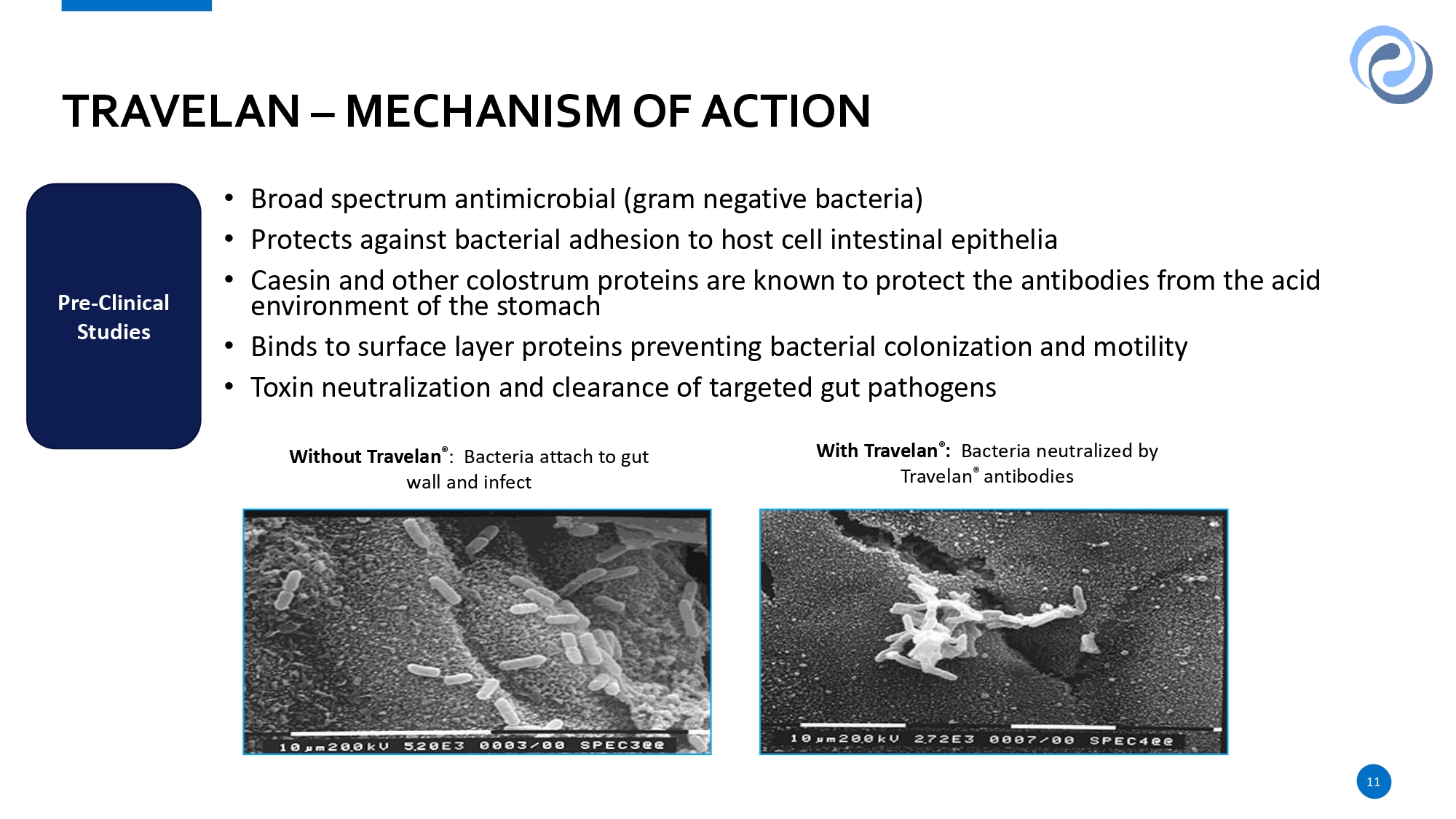
• Broad spectrum antimicrobial (gram negative bacteria) • Protects against bacterial adhesion to host cell intestinal epithelia • Caesin and other colostrum proteins are known to protect the antibodies from the acid environment of the stomach • Binds to surface layer proteins preventing bacterial colonization and motility • Toxin neutralization and clearance of targeted gut pathogens TRAVELAN – MECHANISM OF ACTION Pre - Clinical Studies Without Travelan ® : Bacteria attach to gut wall and infect With Travelan ® : Bacteria neutralized by Travelan ® antibodies 11

Vaccine » Manufacture involves proprietary shearing technology developed to harvest cell surface antigens from 13 Enterotoxigenic E. coli (ETEC) strains known to cause Travelers’ diarrhea » Approved by performing a risk assessment for use in food - producing animals with the Australian Pesticides and Veterinary Medicines Authority (APVMA) » Manufactured under Good Manufacturing Practice (GMP) in a facility licensed by the APVMA Colostrum » Colostrum is collected from cattle on dairy farms using GMP - compliant collection and Manufacturing methods, using Australian Dairy Industry approved proceduresand FDA guidelines » Manufacturing Process: i) pasteurization, ii) separation of fat, iii) ultrafiltration to reduce lactose and concentrate the product, and iv) spray - drying to produce a stable colostrum powder milled to 100 microns » No chemical additives are used in the manufacturing process » Colostrum Product analyzed for safety under Codex Alimentarius and FDA regulations for human consumption 12 TRAVELAN® MANUFACTURING
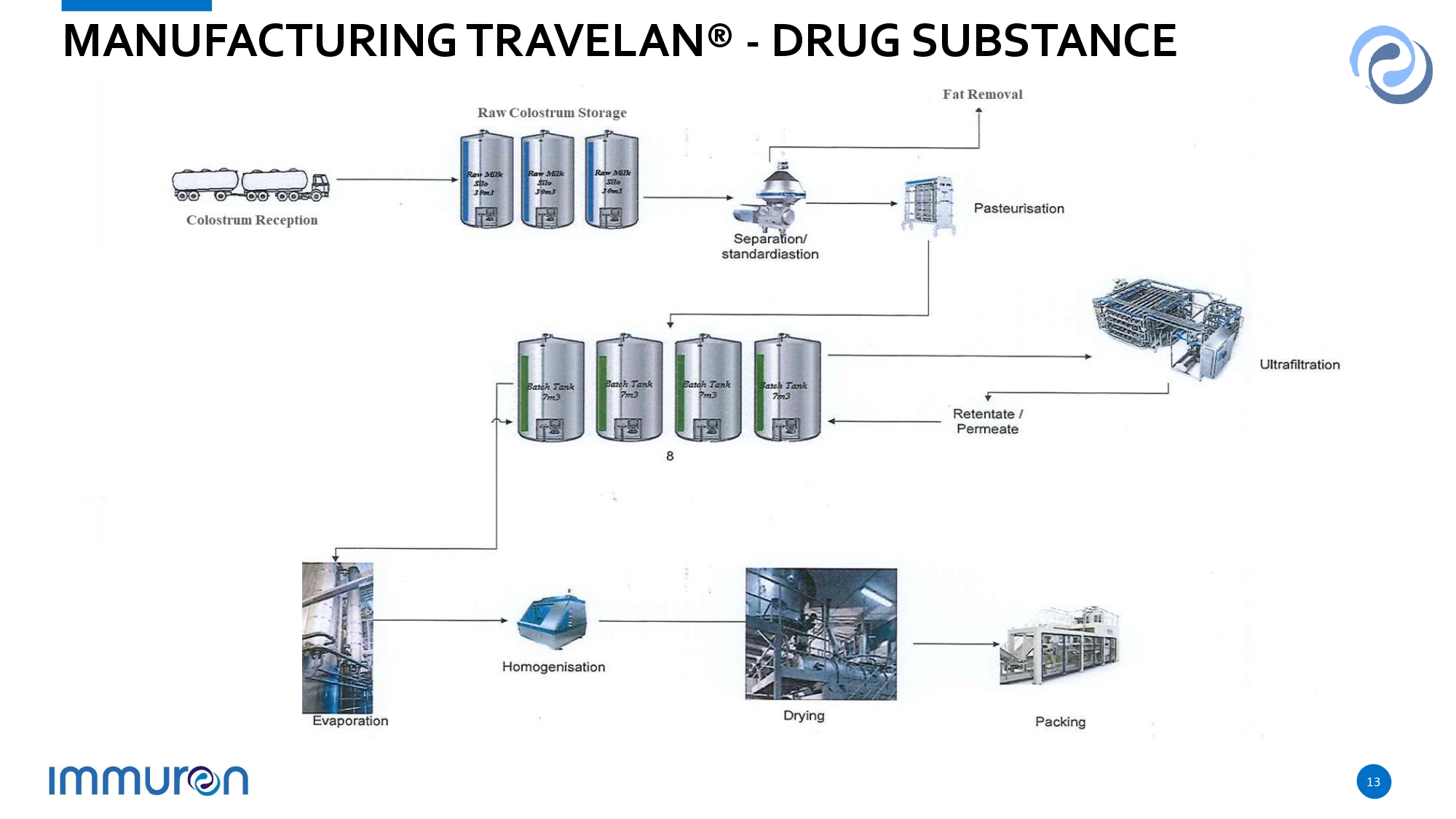
MANUFACTURING TRAVELAN® - DRUG SUBSTANCE 13
» Tablets packaged in unit - dose blister packs containing 200 mg of bovine colostrum formulated as a 700 mg solid oral dose tablet with non - novel excipients » All excipients are within regulations of United States Pharmacopeia and National Formulary » Product specifications include i) IgG content, ii) antibody ETEC titer, iii) metal content iv) microbiological sterility analysis » Current FDA CMC requirements for Phase 2 Biologics (CBER) Stability data » 25 o C - 48 month (real - time) » 40 o C / 75% RH – 12 month (real - time) » Long term - 20 o C/4 o C (real - time) 14 MANUFACTURING – TRAVELAN® DRUG PRODUCT

15 » Randomised, double - blind, placebo - controlled human infection model studies » Travelan or placebo 200 - 400 mg 3 times daily for 7 days » Challenge with ETEC 2 days after dosing TRAVELAN ® SAFETY AND EFFICACY 84% to over 90% prophylactic efficacy in the Travelan treatment arm 1 Otto W, Najnigier B, Stelmasiak T, Robins - Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol. 2011 Jul;46(7 - 8):862 - 8. doi: 10.3109/00365521.2011.574726. PMID: 21526980; PMCID: PMC3154584.

• Travelan® is well tolerated and has been administered orally in over 500 subjects in clinical trials with doses up to 4.8 g per day for 28 days with no treatment related Serious adverse events (SAEs) • Clinically evaluated in Children ( 6 to 19 years of age) • Pharmacovigilance has reported no product related SAEs to date • Reported Adverse Events (AEs) are mild in severity (abdominal discomfort, bloating, constipation, flatulence, nausea and occasionally diarrhea (usually related to lactose intolerance) 16 TRAVELAN® SAFETY

NEW HORIZONS EXPANSION INTO THE US 17 1. Collaboration with the US Department of Defense (DOD) 2. FDA Clinical Pathway to approval of a listed medicine 3. Immuron distribution and expansion plans

TRAVELERS’ DIARRHEA AND THE US MILITARY 18

• The literature indicates that overall, ~50% of US travelers to remote or less developed countries seek pre - travel guidance. Advice from physicians, travel clinics, pharmacists, or other source. • Physicians’ standard protocol for a patient traveling to a high - risk area is the prescription of a 5 - day course of an antibiotic (typically ciprofloxacin or azithromycin) for the patient to fill and take if symptoms develop (e . g . , diarrhea, fever) . • Concerned travelers are prescribed antibiotics to be taken prophylactically TRAVELERS’ DIARRHEA – CURRENT MANAGEMENT IN THE US 19
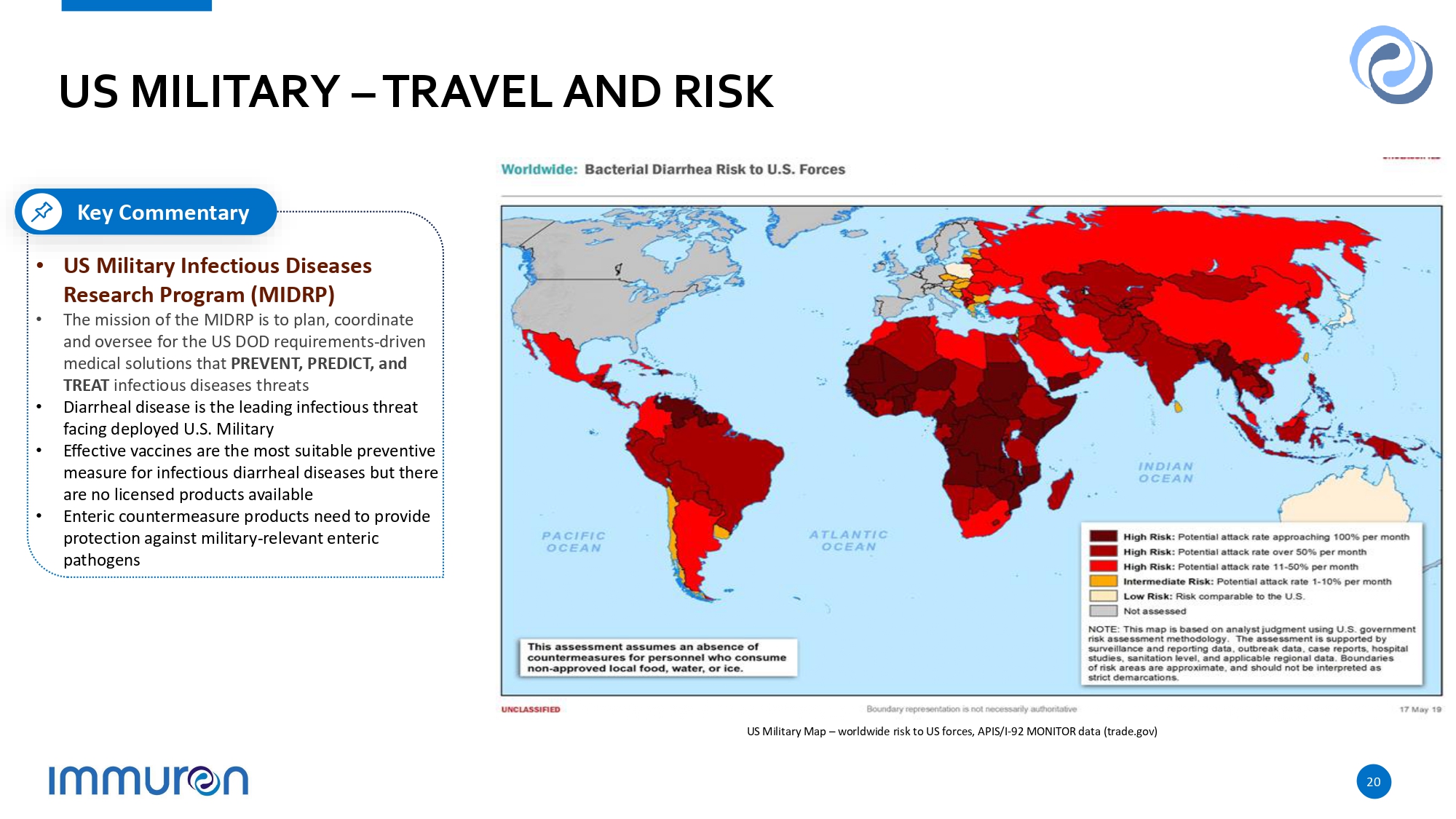
US MILITARY – TRAVEL AND RISK US Military Map – worldwide risk to US forces, APIS/I - 92 MONITOR data (trade.gov) Key Commentary • US Military Infectious Diseases Research Program (MIDRP) • The mission of the MIDRP is to plan, coordinate and oversee for the US DOD requirements - driven medical solutions that PREVENT, PREDICT, and TREAT infectious diseases threats • Diarrheal disease is the leading infectious threat facing deployed U.S. Military • Effective vaccines are the most suitable preventive measure for infectious diarrheal diseases but there are no licensed products available • Enteric countermeasure products need to provide protection against military - relevant enteric pathogens 20
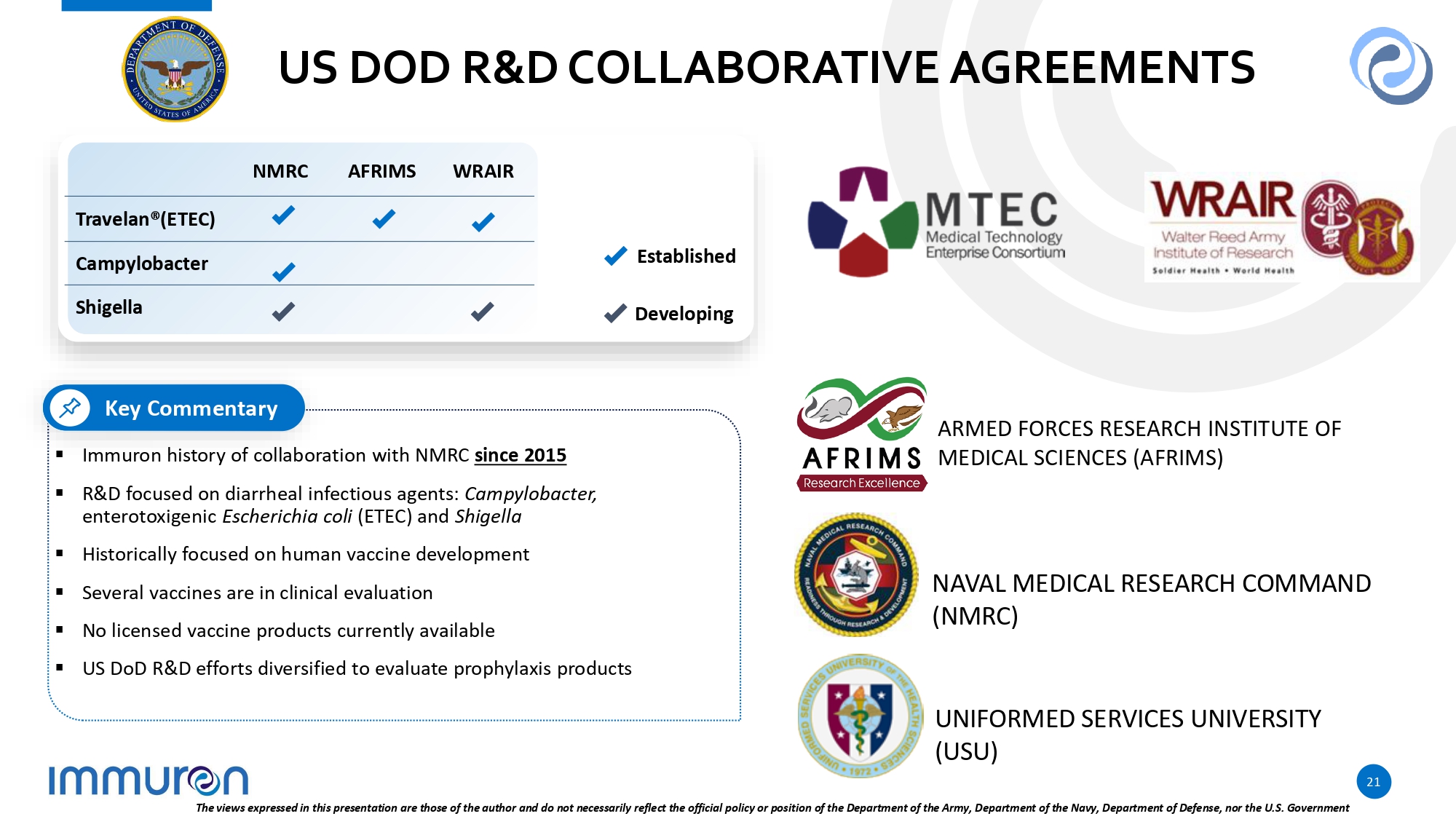
US DOD R&D COLLABORATIVE AGREEMENTS ▪ Immuron history of collaboration with NMRC since 2015 ▪ R&D focused on diarrheal infectious agents: Campylobacter, enterotoxigenic Escherichia coli (ETEC) and Shigella ▪ Historically focused on human vaccine development ▪ Several vaccines are in clinical evaluation ▪ No licensed vaccine products currently available ▪ US DoD R&D efforts diversified to evaluate prophylaxis products Key Commentary Established Developing WRAIR AFRIMS NMRC Travelan®(ETEC) Campylobacter Shigella ARMED FORCES RESEARCH INSTITUTE OF MEDICAL SCIENCES (AFRIMS) NAVAL MEDICAL RESEARCH COMMAND (NMRC) UNIFORMED SERVICES UNIVERSITY (USU) 21 The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government
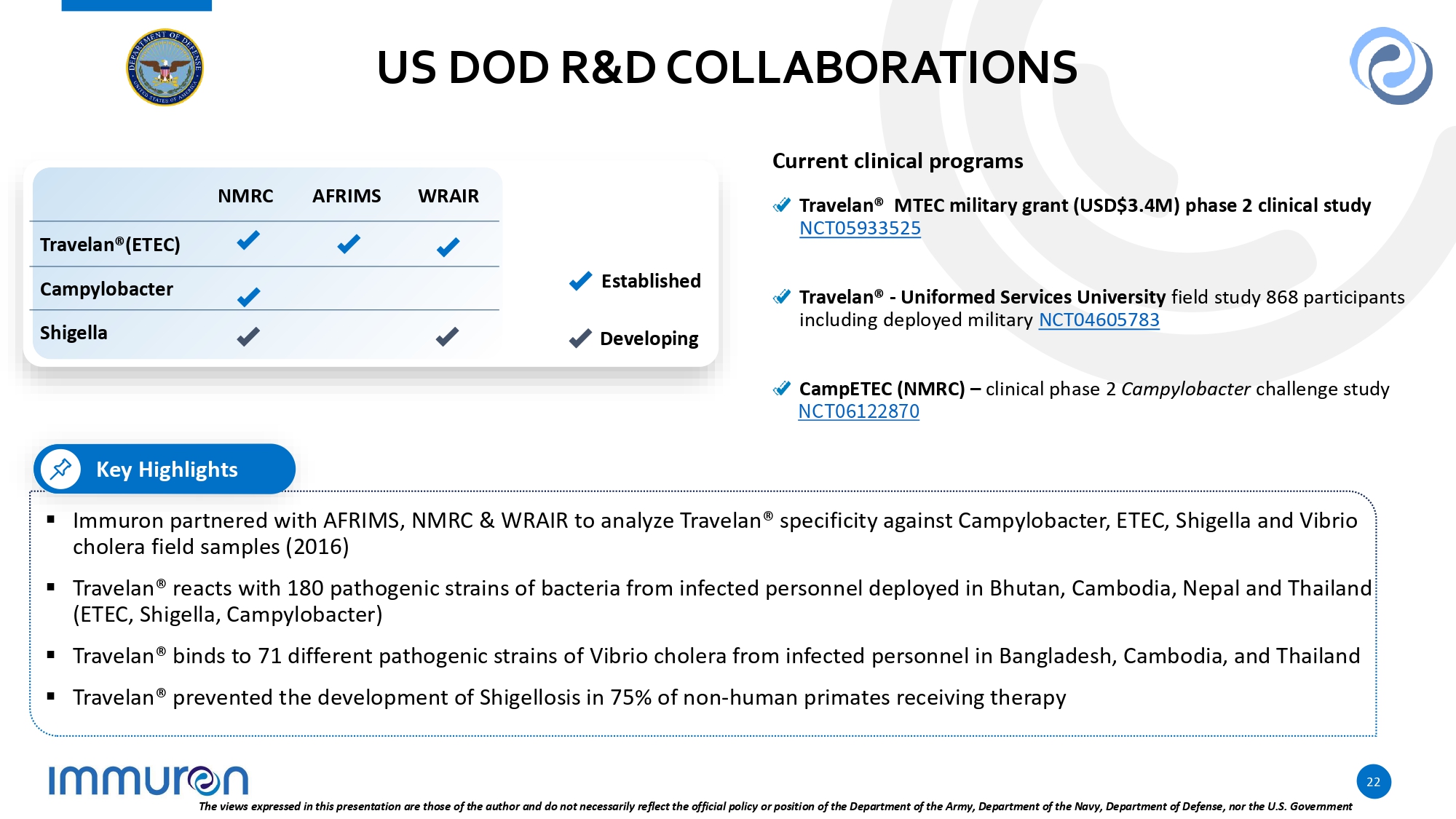
US DOD R&D COLLABORATIONS Key Highlights ▪ Immuron partnered with AFRIMS, NMRC & WRAIR to analyze Travelan® specificity against Campylobacter, ETEC, Shigella and Vibrio cholera field samples (2016) ▪ Travelan® reacts with 180 pathogenic strains of bacteria from infected personnel deployed in Bhutan, Cambodia, Nepal and Thailand (ETEC, Shigella, Campylobacter) ▪ Travelan® binds to 71 different pathogenic strains of Vibrio cholera from infected personnel in Bangladesh, Cambodia, and Thailand ▪ Travelan® prevented the development of Shigellosis in 75% of non - human primates receiving therapy Current clinical programs Travelan® MTEC military grant (USD$3.4M) phase 2 clinical study NCT05933525 Travelan® - Uniformed Services University field study 868 participants including deployed military NCT04605783 Established Developing CampETEC (NMRC) – clinical phase 2 Campylobacter challenge study NCT06122870 WRAIR AFRIMS NMRC Travelan®(ETEC) Campylobacter Shigella 22 The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government
23 PARTNERING WITH US DEPARTMENT OF DEFENSE • Join funding consortiums like MTEC which the U.S. Army Medical Research and Development Command (USAMRDC) and other DoD agencies offer funding opportunities to members through solicitations called Requests for Prototype Proposals (RPPs): https://mtec - sc.org/how - to - join/ • Register on the various U.S. Government / DoD grant funding platforms as a Nontraditional Defense Contractor : http://uscode.house.gov/view.xhtml?req=granuleid:USC - prelim - title10 - section3014&num=0&edition=prelim • The System for Award Management (SAM.gov) is an official website of the U.S. Government . https://sam.gov/content/home • There is no cost to use SAM.gov. You can use this site to: Register to do business with the U.S. Government Search for contract opportunities Access publicly available awards • Understand the terms and processes , e.g. • Technology Readiness Levels: https://mtec - sc.org/wp - content/uploads/2016/12/TRL - definitions.pdf • Grant application and approval process : https://research.uga.edu/docs/units/dsc/MTEC - Proposal - Preparation - Guide.pdf • Connect with DOD leadership at USAMRDC and others like the US Military Infectious Diseases Research Program (MIDRP); key decision - making bodies for future solicitations; membership of MTEC facilitates this Co MTEC Membership Note: In order to respond to any solicitation on this site, you must be a member of the MTEC Consortium . Solicitations include awards from: Military Infectious Diseases Research Program (MIDRP) Combat Casualty Care Research Program (CCCRP) Military Operational Medicine Research Program (MOMRP) Clinical and Rehabilitative Medicine Research Program (CRMRP) Medical Simulation and Information Sciences Research Program (MSISRP) The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government
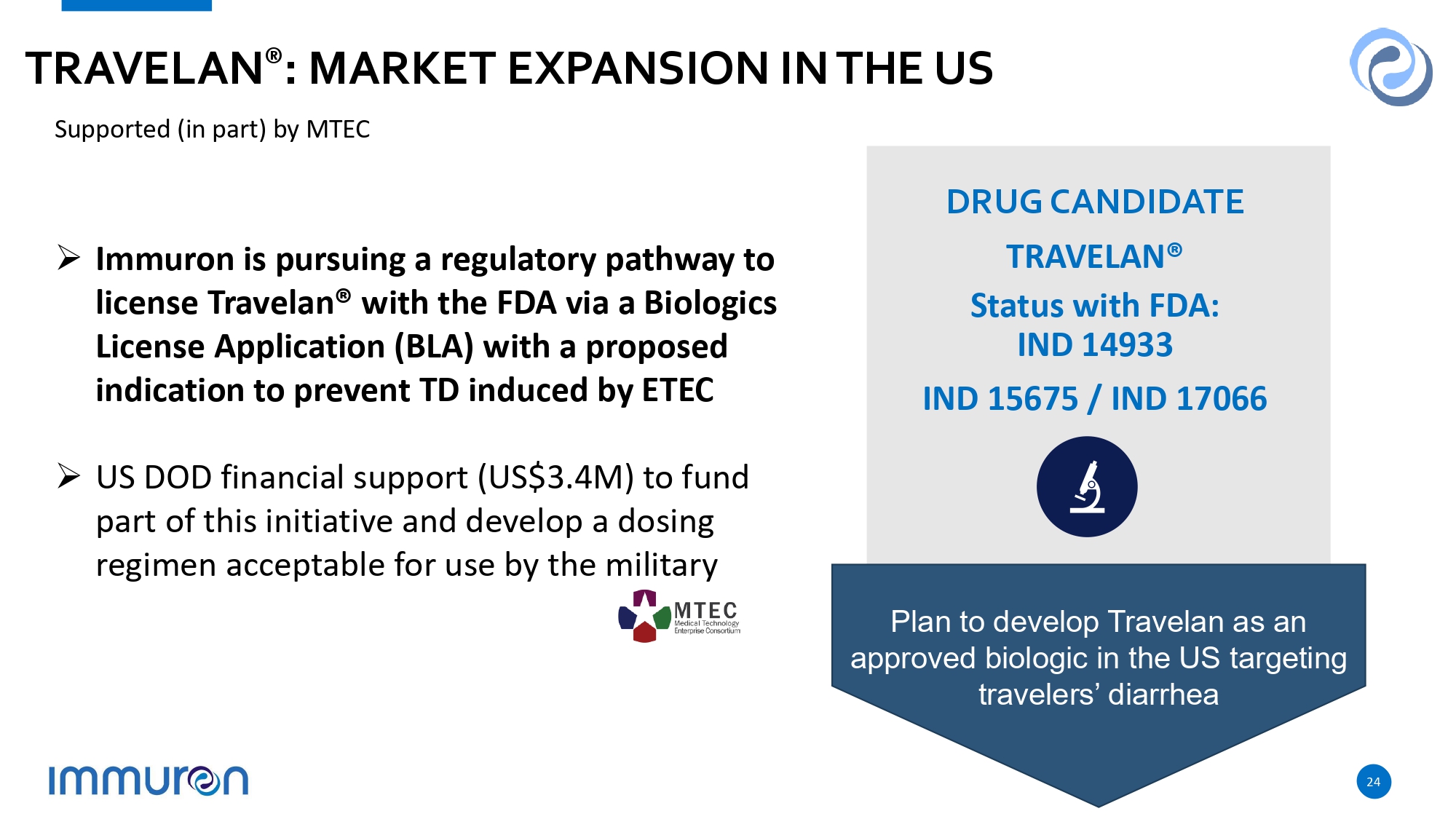
24 TRAVELAN ® : MARKET EXPANSION IN THE US DRUG CANDIDATE TRAVELAN® Status with FDA: IND 14933 IND 15675 / IND 17066 » US DOD financial support (US$3.4M) to fund part of this initiative and develop a dosing regimen acceptable for use by the military Plan to develop Travelan as an approved biologic in the US targeting travelers’ diarrhea » Immuron is pursuing a regulatory pathway to license Travelan® with the FDA via a Biologics License Application (BLA) with a proposed indication to prevent TD induced by ETEC Supported (in part) by MTEC
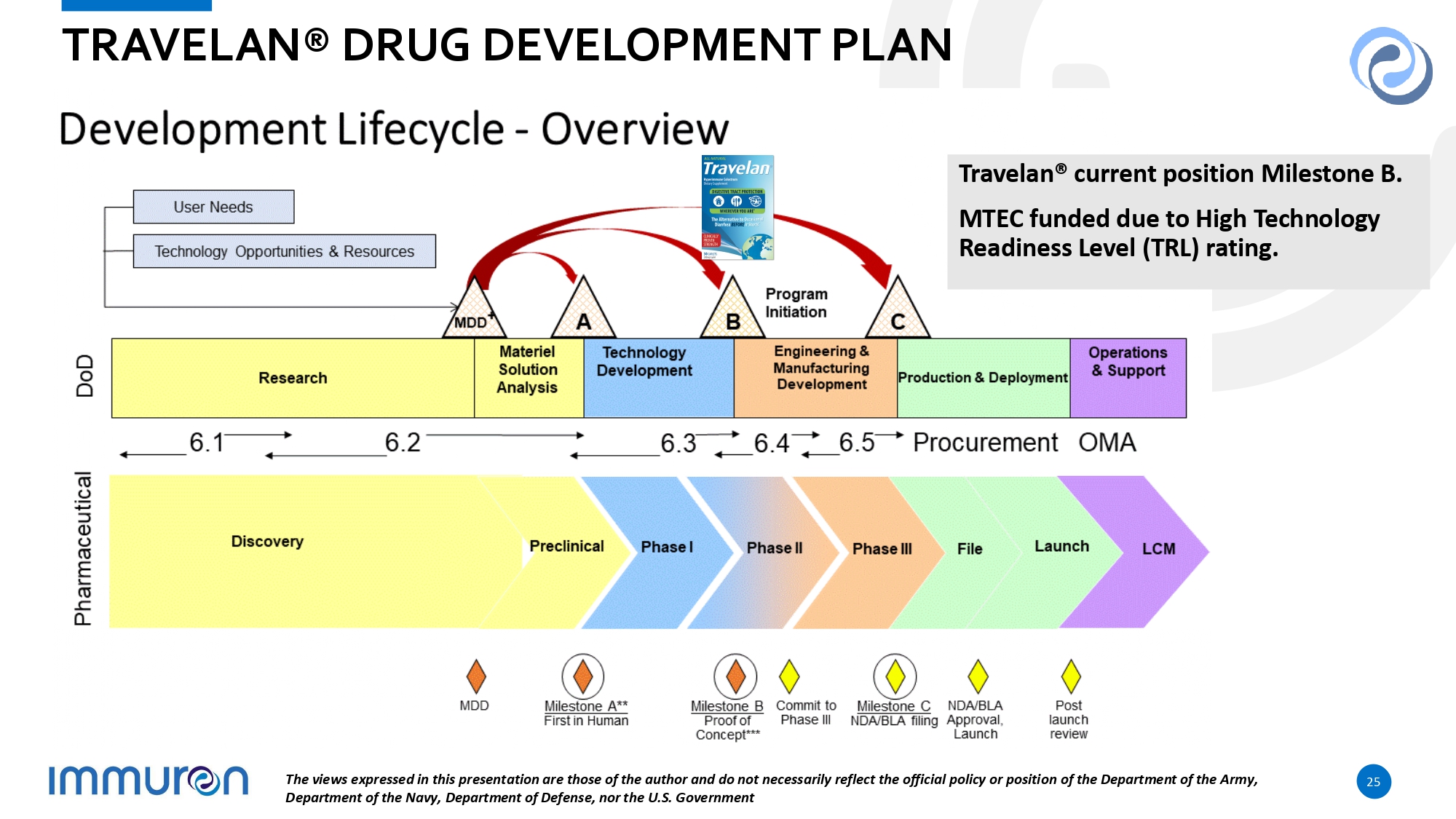
25 TRAVELAN® DRUG DEVELOPMENT PLAN Travelan® current position Milestone B. MTEC funded due to High Technology Readiness Level (TRL) rating. The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government
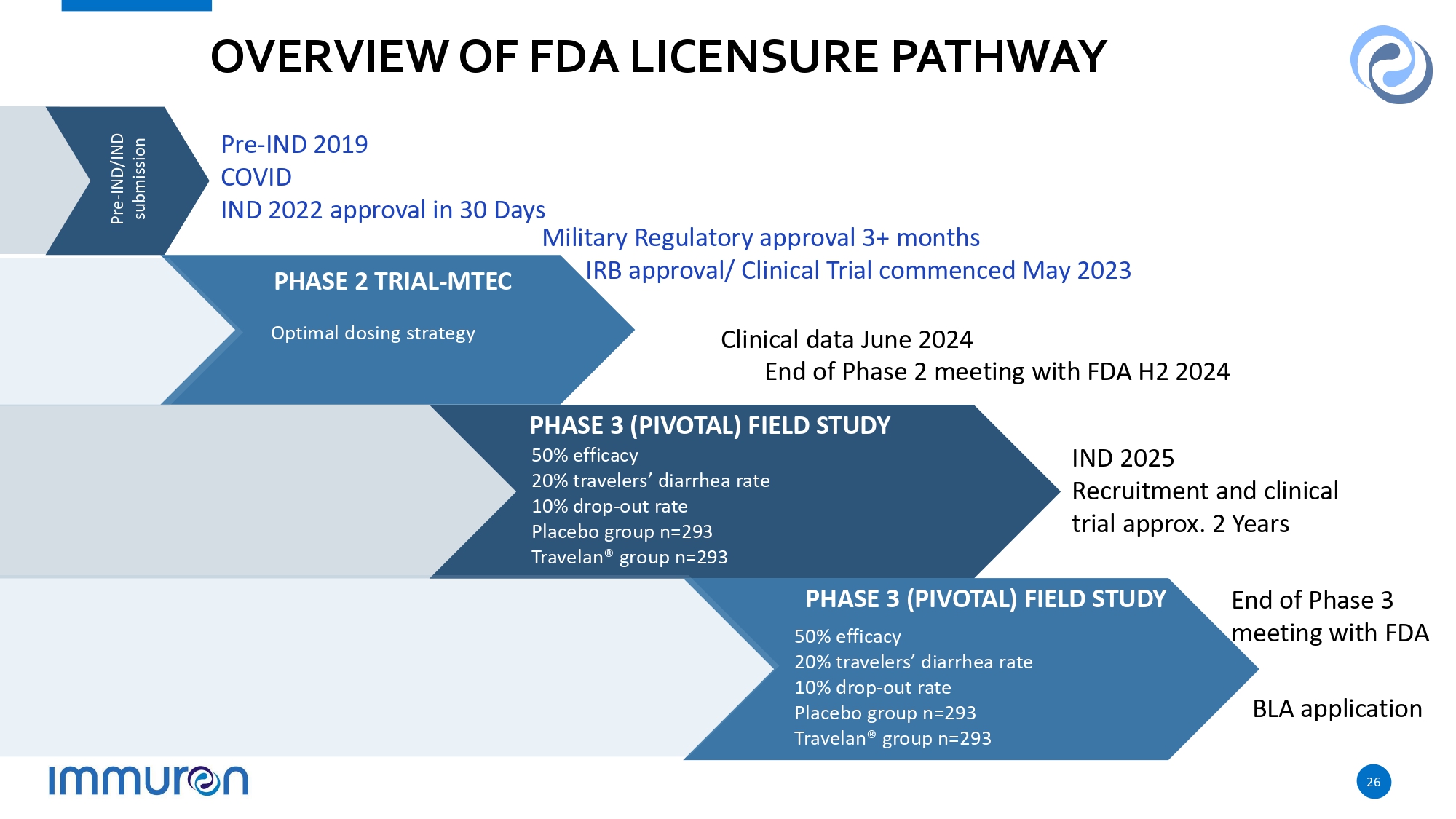
OVERVIEW OF FDA LICENSURE PATHWAY PHASE 3 (PIVOTAL) FIELD STUDY 50% efficacy 20% travelers’ diarrhea rate 10% drop - out rate Placebo group n=293 Travelan® group n=293 Pre - IND/IND submission PHASE 3 (PIVOTAL) FIELD STUDY 50% efficacy 20% travelers’ diarrhea rate 10% drop - out rate Placebo group n=293 Travelan® group n=293 Optimal dosing strategy PHASE 2 TRIAL - MTEC Clinical data June 2024 End of Phase 2 meeting with FDA H2 2024 26 IND 2025 Recruitment and clinical trial approx. 2 Years Pre - IND 2019 COVID IND 2022 approval in 30 Days Military Regulatory approval 3+ months IRB approval/ Clinical Trial commenced May 2023 BLA application End of Phase 3 meeting with FDA

x Immuron is the sole proprietary of the Travelan® product and associated intellectual property x Strong US DoD collaboration x Immuron has an international distribution network to ensure prompt delivery to deployed US military if necessary x All Immuron’s commercial operations involve suitably, qualified cGMP service providers that meet the regulatory requirements of the company’s Quality Management Systems 27 COMPANY COMPETENCIES & MANUFACTURING AND DISTRIBUTION EXPANSION PLANS

COMPETITOR MARKET ANALYSIS (US) 28 Average cost: 2 - week trip Dosing Indication Drug PRESENTLY, THERE IS NO FDA - APPROVED DRUG TO PREVENT TRAVELERS’ DIARRHEA $29.57 – 30 caplets 5 3 caps (200 mg) TID Dietary supplement TRAVELAN® FDA - APPROVED DRUG TREATMENTS FOR DIARRHEA $20.94 1 2 tabs QID Relief for heartburn, nausea, indigestion, upset stomach, and diarrhea PEPTO BISMOL $22.00 2 (2x24 caplets) 2 tabs (2 mg) Decrease the frequency of diarrhea in TD, gastroenteritis, inflammatory bowel disease, and short bowel syndrome IMMODIUM $59.92 3 (2x 5 - day supply) 500 mg Bacterial infections CIPROFLOXACIN $778.10 4 (without insurance) 3 caps (200 mg) TID Treatment of travelers’ diarrhea RIFAXIMIN 1. Amazon.com 2. Amazon.com 3. Amazon.com (Cipro) 4. Amazon.com (Xifaxan) 5. Amazon.com International Society of Travel Medicine, 2017 guidelines for treating travelers’ diarrhea included: • Antibiotics should NOT be used routinely, except in patients at high risk of complications • Rifaximin recommended when antibiotic prophylaxis is indicated • Fluoroquinolones not recommended for prophylaxis • Insufficient evidence to recommend prebiotics or probiotics
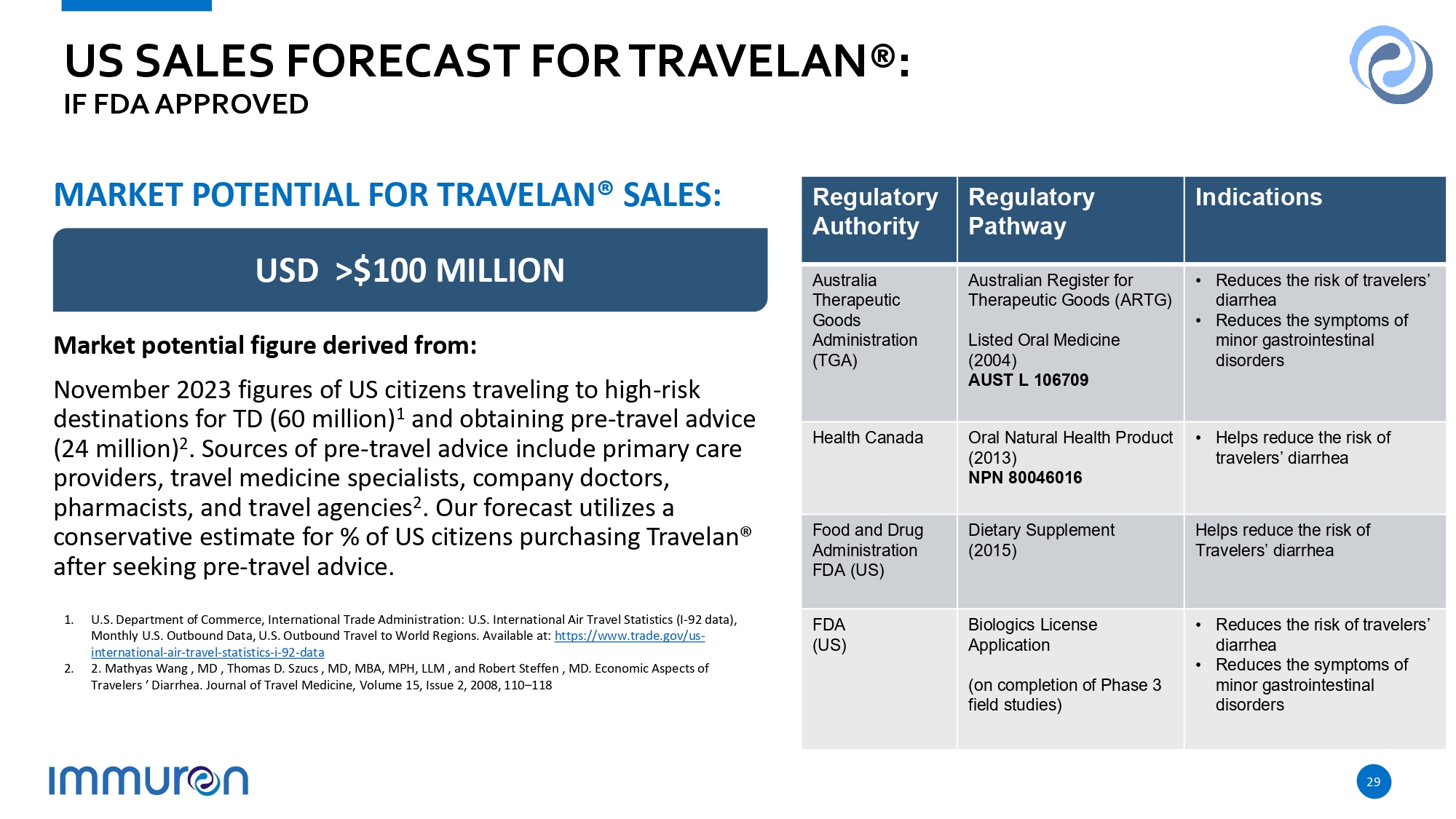
US SALES FORECAST FOR TRAVELAN®: IF FDA APPROVED 1. U.S. Department of Commerce, International Trade Administration: U.S. International Air Travel Statistics (I - 92 data), Monthly U.S. Outbound Data, U.S. Outbound Travel to World Regions. Available at: https://www.trade.gov/us - international - air - travel - statistics - i - 92 - data 2. 2. Mathyas Wang , MD , Thomas D. Szucs , MD, MBA, MPH, LLM , and Robert Steffen , MD. Economic Aspects of Travelers ’ Diarrhea. Journal of Travel Medicine, Volume 15, Issue 2, 2008, 110 – 118 MARKET POTENTIAL FOR TRAVELAN® SALES: USD >$100 MILLION Market potential figure derived from: November 2023 figures of US citizens traveling to high - risk destinations for TD (60 million) 1 and obtaining pre - travel advice (24 million) 2 . Sources of pre - travel advice include primary care providers, travel medicine specialists, company doctors, pharmacists, and travel agencies 2 . Our forecast utilizes a conservative estimate for % of US citizens purchasing Travelan® after seeking pre - travel advice. 29 Indications Regulatory Pathway Regulatory Authority • Reduces the risk of travelers’ diarrhea • Reduces the symptoms of minor gastrointestinal disorders Australian Register for Therapeutic Goods (ARTG) Listed Oral Medicine (2004) AUST L 106709 Australia Therapeutic Goods Administration (TGA) • Helps reduce the risk of travelers’ diarrhea Oral Natural Health Product (2013) NPN 80046016 Health Canada Helps reduce the risk of Travelers’ diarrhea Dietary Supplement (2015) Food and Drug Administration FDA (US) • Reduces the risk of travelers’ diarrhea • Reduces the symptoms of minor gastrointestinal disorders Biologics License Application (on completion of Phase 3 field studies) FDA (US)

THANK YOU Immuron team NAVAL MEDICAL RESEARCH COMMAND UNIFORMED SERVICES UNIVERSITY ARMED FORCES RESEARCH INSTITUTE OF MEDICAL SCIENCES Funding support Collaborators: 30
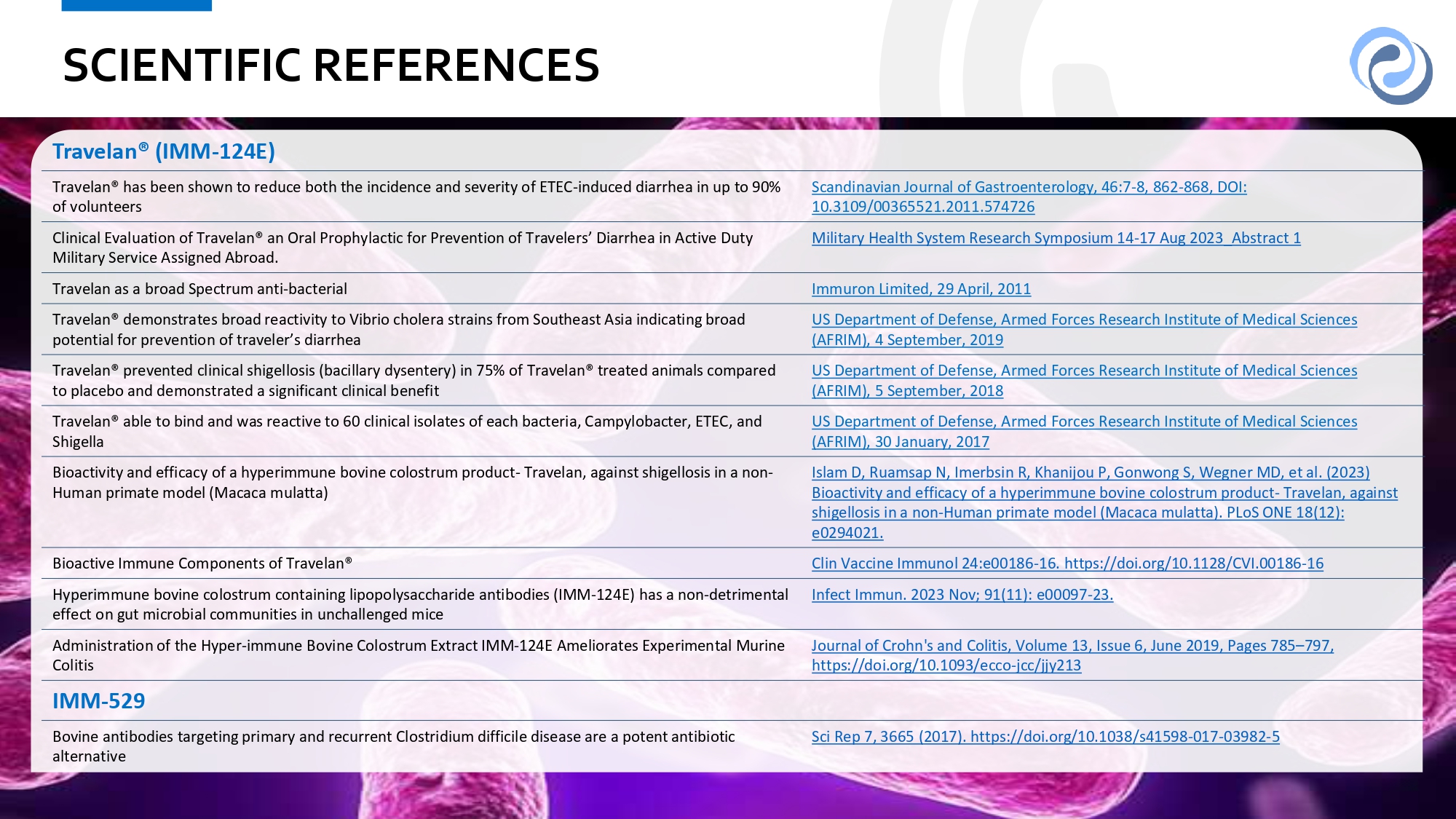
3 1 31 SCIENTIFIC REFERENCES Travelan® (IMM - 124E) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Military Health System Research Symposium 14 - 17 Aug 2023_Abstract 1 Clinical Evaluation of Travelan® an Oral Prophylactic for Prevention of Travelers’ Diarrhea in Active Duty Military Service Assigned Abroad. Immuron Limited, 29 April, 2011 Travelan as a broad Spectrum anti - bacterial US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam D, Ruamsap N, Imerbsin R, Khanijou P, Gonwong S, Wegner MD, et al. (2023) Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta). PLoS ONE 18(12): e0294021. Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta) Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 Bioactive Immune Components of Travelan® Infect Immun. 2023 Nov; 91(11): e00097 - 23. Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn's and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative
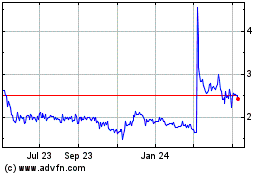
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Apr 2023 to Apr 2024