true
Amended to update share information
0001402328
0001402328
2023-01-01
2023-09-30
0001402328
dei:BusinessContactMember
2023-01-01
2023-09-30
0001402328
2023-09-30
0001402328
2022-12-31
0001402328
us-gaap:SeriesBPreferredStockMember
2023-09-30
0001402328
us-gaap:SeriesBPreferredStockMember
2022-12-31
0001402328
2023-07-01
2023-09-30
0001402328
2022-07-01
2022-09-30
0001402328
2022-01-01
2022-09-30
0001402328
2021-12-31
0001402328
2022-09-30
0001402328
us-gaap:CommonStockMember
2023-06-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001402328
us-gaap:PreferredStockMember
2023-06-30
0001402328
us-gaap:ComprehensiveIncomeMember
2023-06-30
0001402328
us-gaap:RetainedEarningsMember
2023-06-30
0001402328
2023-06-30
0001402328
us-gaap:CommonStockMember
2022-12-31
0001402328
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001402328
us-gaap:PreferredStockMember
2022-12-31
0001402328
us-gaap:ComprehensiveIncomeMember
2022-12-31
0001402328
us-gaap:RetainedEarningsMember
2022-12-31
0001402328
us-gaap:CommonStockMember
2022-06-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001402328
us-gaap:PreferredStockMember
2022-06-30
0001402328
us-gaap:ComprehensiveIncomeMember
2022-06-30
0001402328
us-gaap:RetainedEarningsMember
2022-06-30
0001402328
2022-06-30
0001402328
us-gaap:CommonStockMember
2021-12-31
0001402328
us-gaap:AdditionalPaidInCapitalMember
2021-12-31
0001402328
us-gaap:PreferredStockMember
2021-12-31
0001402328
us-gaap:ComprehensiveIncomeMember
2021-12-31
0001402328
us-gaap:RetainedEarningsMember
2021-12-31
0001402328
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2023-07-01
2023-09-30
0001402328
us-gaap:PreferredStockMember
2023-07-01
2023-09-30
0001402328
us-gaap:ComprehensiveIncomeMember
2023-07-01
2023-09-30
0001402328
us-gaap:RetainedEarningsMember
2023-07-01
2023-09-30
0001402328
us-gaap:CommonStockMember
2023-01-01
2023-09-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-09-30
0001402328
us-gaap:PreferredStockMember
2023-01-01
2023-09-30
0001402328
us-gaap:ComprehensiveIncomeMember
2023-01-01
2023-09-30
0001402328
us-gaap:RetainedEarningsMember
2023-01-01
2023-09-30
0001402328
us-gaap:CommonStockMember
2022-07-01
2022-09-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2022-07-01
2022-09-30
0001402328
us-gaap:PreferredStockMember
2022-07-01
2022-09-30
0001402328
us-gaap:ComprehensiveIncomeMember
2022-07-01
2022-09-30
0001402328
us-gaap:RetainedEarningsMember
2022-07-01
2022-09-30
0001402328
us-gaap:CommonStockMember
2022-01-01
2022-09-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-09-30
0001402328
us-gaap:PreferredStockMember
2022-01-01
2022-09-30
0001402328
us-gaap:ComprehensiveIncomeMember
2022-01-01
2022-09-30
0001402328
us-gaap:RetainedEarningsMember
2022-01-01
2022-09-30
0001402328
us-gaap:CommonStockMember
2023-09-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001402328
us-gaap:PreferredStockMember
2023-09-30
0001402328
us-gaap:ComprehensiveIncomeMember
2023-09-30
0001402328
us-gaap:RetainedEarningsMember
2023-09-30
0001402328
us-gaap:CommonStockMember
2022-09-30
0001402328
us-gaap:AdditionalPaidInCapitalMember
2022-09-30
0001402328
us-gaap:PreferredStockMember
2022-09-30
0001402328
us-gaap:ComprehensiveIncomeMember
2022-09-30
0001402328
us-gaap:RetainedEarningsMember
2022-09-30
0001402328
us-gaap:SalesRevenueNetMember
us-gaap:ProductConcentrationRiskMember
SBFM:GenericPharmaceuticalsMember
2023-01-01
2023-09-30
0001402328
us-gaap:SalesRevenueNetMember
us-gaap:ProductConcentrationRiskMember
SBFM:OTCProductsMember
2023-01-01
2023-09-30
0001402328
SBFM:SingleHealthcareFocusedInstitutionalInvestorMember
2023-05-15
2023-05-16
0001402328
SBFM:SingleHealthcareFocusedInstitutionalInvestorMember
us-gaap:CommonStockMember
2023-05-15
2023-05-16
0001402328
SBFM:SingleHealthcareFocusedInstitutionalInvestorMember
SBFM:MayPreFundedWarrantsMember
2023-05-15
2023-05-16
0001402328
SBFM:SingleHealthcareFocusedInstitutionalInvestorMember
SBFM:MayPreFundedWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:NoraPharmaMember
2022-10-19
2022-10-20
0001402328
SBFM:NoraPharmaMember
SBFM:MalekChamounMember
2022-10-20
0001402328
SBFM:NoraPharmaMember
SBFM:MalekChamounMember
2023-01-01
2023-09-30
0001402328
SBFM:NoraPharmaMember
SBFM:MalekChamounMember
2023-09-30
0001402328
SBFM:NoraPharmaMember
2022-10-20
0001402328
2022-01-01
2022-12-31
0001402328
2021-01-01
2021-12-31
0001402328
SBFM:NoraPharmaMember
2022-01-01
2022-12-31
0001402328
SBFM:NoraPharmaMember
2021-01-01
2021-12-31
0001402328
SBFM:FirstReverseStockSplitMember
2022-02-08
2022-02-09
0001402328
srt:DirectorMember
2023-09-30
0001402328
us-gaap:SeriesBPreferredStockMember
srt:ChiefExecutiveOfficerMember
2022-12-31
0001402328
SBFM:PublicOfferingMember
2022-02-16
2022-02-17
0001402328
us-gaap:CommonStockMember
SBFM:PublicOfferingMember
2022-02-16
2022-02-17
0001402328
SBFM:TradeableWarrantsMember
SBFM:PublicOfferingMember
2022-02-16
2022-02-17
0001402328
us-gaap:SeriesBPreferredStockMember
2022-02-21
2022-02-22
0001402328
SBFM:TradeableWarrantsMember
SBFM:PublicOfferingMember
2022-02-22
0001402328
us-gaap:PrivatePlacementMember
2022-03-13
2022-03-14
0001402328
us-gaap:PrivatePlacementMember
SBFM:CommonStockMemberAndInvestorWarrantsMember
2022-03-13
2022-03-14
0001402328
us-gaap:PrivatePlacementMember
2022-04-27
2022-04-28
0001402328
us-gaap:PrivatePlacementMember
SBFM:CommonStockMemberAndAprilWarrantsMember
2022-04-27
2022-04-28
0001402328
SBFM:NoraPharmaIncMember
2022-10-19
2022-10-20
0001402328
2023-01-19
0001402328
us-gaap:CommonStockMember
2023-01-01
2023-09-30
0001402328
SBFM:StockIssuedForWarrantExercisesMember
2022-01-01
2022-12-31
0001402328
SBFM:StockIssuedForWarrantExercisesMember
2023-01-01
2023-06-30
0001402328
SBFM:WarrantsExercisedMember
2022-01-01
2022-12-31
0001402328
SBFM:WarrantsExercisedMember
2023-01-01
2023-06-30
0001402328
us-gaap:CommonStockMember
2023-07-01
2023-07-31
0001402328
SBFM:PreFundedWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:PreFundedWarrantsMember
2023-09-30
0001402328
SBFM:TradeableWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:TradeableWarrantsMember
2023-09-30
0001402328
SBFM:InvestorWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:InvestorWarrantsMember
2023-09-30
0001402328
SBFM:AprilWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:AprilWarrantsMember
2023-09-30
0001402328
SBFM:MayPreFundedWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:MayPreFundedWarrantsMember
2023-09-30
0001402328
SBFM:MayInvestorWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:MayInvestorWarrantsMember
2023-09-30
0001402328
SBFM:AllWarrantsMember
2023-01-01
2023-09-30
0001402328
SBFM:CompanyPublicOfferingMember
SBFM:TradeableWarrantsMember
2022-02-01
2022-02-28
0001402328
SBFM:TwoPrivatePlacementsMember
SBFM:InvestorWarrantsMember
2022-03-01
2022-03-31
0001402328
SBFM:TwoPrivatePlacementsMember
SBFM:AprilWarrantsMember
2022-04-01
2022-04-30
0001402328
SBFM:TwoPrivatePlacementsMember
SBFM:MayInvestorWarrantsMember
2023-05-01
2023-05-31
0001402328
SBFM:PreFundedWarrantsMember
2022-03-01
2022-03-31
0001402328
SBFM:PreFundedWarrantsMember
2022-04-01
2022-04-30
0001402328
SBFM:PreFundedWarrantMember
2023-05-01
2023-05-31
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:CAD
Table
of Contents
As filed with the Securities and Exchange
Commission on February 9, 2024
Registration No. 333-276817
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1/A
(Amendment No. 1)
REGISTRATION STATEMENT UNDER THE SECURITIES
ACT OF 1933
Sunshine Biopharma,
Inc.
(Exact name of registrant as specified in its charter)
| Colorado |
|
8731 |
|
20-5566275 |
|
(State or other jurisdiction of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
1177
Avenue of the Americas, 5th Floor
New York, NY 10036
332-216-1147
(Address, including zip code and telephone number,
including
area code, of registrant’s principal executive
offices)
Dr. Steve N. Slilaty
1177 Avenue of the Americas, 5th
Floor
New York, NY 10036
332-216-1147
(Name, address, including zip code and telephone
number, including area code, of agent for service)
Copies to:
|
Gregory Sichenzia, Esq.
Jeff Cahlon, Esq.
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas, 31st Floor
New York, New York 10036
212-930-9700 |
|
Anthony W. Basch, Esq.
J. Britton Williston, Esq.
Chenxi Lu, Esq.
Kaufman & Canoles, P.C.
1021 E. Cary St., Suite 1400
Richmond, Virginia 23219
804-771-5700 |
Approximate date of commencement of proposed sale
to the public: As soon as practicable after the effective date of the registration statement.
If any of the securities being registered on this
form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☒
If this form is filed to register additional securities
for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number
of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| |
Large accelerated filer ☐ |
Accelerated filer ☐ |
| |
Non-accelerated filer ☒ |
Smaller reporting company ☒ |
| |
|
Emerging growth company ☐ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such
date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states
that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of
1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting
pursuant to said Section 8(a), may determine.
The information in this prospectus
is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange
Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities
in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION |
DATED
FEBRUARY 9, 2024 |
39,215,687 Units, Each
Unit Consisting of One Share of Common Stock or One Pre-Funded Warrant to Purchase One Share of Common Stock, one-tenth of a Series A
Warrant to Purchase one Share of Common Stock and two-tenths of a Series B Warrant to Purchase one Share of Common Stock
11,764,706 Shares of
Common Stock Underlying the Series A and Series B Warrants

Sunshine
Biopharma, Inc. is offering, on a firm commitment, underwritten basis, 39,215,687 units (the
“Units”), each Unit consisting of one share of our common stock, $0.001 par value
per share, one-tenth (1/10) of a Series A warrant (“Series A Warrant”) to purchase
one share of common stock and two-tenths (2/10) of a Series B warrant (“Series B Warrant”)
to purchase one share of common stock, at an assumed public offering price of $0.255 per
Unit, which was the last reported sale price of our common stock on The Nasdaq Capital Market,
or Nasdaq, on February 6, 2024.
The Units have no stand-alone rights and will
not be certificated or issued as stand-alone securities. Each Series A Warrant offered hereby is immediately exercisable on the date
of issuance at an exercise price of $3.825 (assuming an offering price of $0.255 per Unit) per share of common stock, or pursuant to
alternate cashless exercise option, and will expire two-and-a-half years from the closing date of this public offering. Each Series B
Warrant offered hereby is immediately exercisable on the date of issuance at an exercise price of $4.335 (assuming an offering price
of $0.255 per Unit) per share of common stock, and will expire five years from the closing date of this public offering.
Under the alternate cashless exercise option of the
Series A Warrants, beginning on the date of the Warrant Stockholder Approval (described below), the holder of the Series A Warrant, has
the right to receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would
be issuable upon a cash exercise of the Series A Warrant and (y) 2.0. In addition, beginning on the date of the Warrant Stockholder
Approval, the Series A Warrants and Series B Warrants will contain a reset of the exercise price to a price equal to the lesser of (i)
the then exercise price and (ii) lowest volume weighted average price for the five trading days immediately preceding and immediately
following the date we effect a reverse stock split in the future with a proportionate adjustment to the number of shares underlying the
Series A Warrants and Series B Warrants. Finally, beginning on the date of the Warrant Stockholder Approval, with certain exceptions,
the Series B Warrants will provide for an adjustment to the exercise price and number of shares underlying the Series B Warrants upon
our issuance of our common stock or common stock equivalents at a price per share that is less than the exercise price of the Series B
Warrant.
The alternate cashless exercise option included in the Series A Warrants
and the other adjustment provisions described in the above paragraph included in the Series A Warrants and Series B Warrants will be available
only upon receipt of such stockholder approval as may be required by the applicable rules and regulations of the Nasdaq Capital Market
to permit the alternate cashless exercise of the Series A Warrants and the other adjustment provisions described in the above paragraph
included in the Series A Warrants and Series B Warrants (the “Warrant Stockholder Approval”). In the event that we are unable
to obtain the Warrant Stockholder Approval, the Series A Warrants will not be exercisable using the alternate cashless exercise option
and the other adjustment provisions described in the above paragraph included in the Series A Warrants and Series B Warrants will not
be effective, and therefore the Series A Warrants and Series B Warrants may have substantially less value. See the Risk Factor on page
15 relating to the Series A Warrants and Series B Warrants and Warrant Stockholder Approval, and see the section entitled “Warrant
Stockholder Approval” on page 40 for additional details regarding the Warrant Stockholder Approval.
We are also offering to each purchaser of Units
that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding common stock immediately
following the consummation of this offering, the opportunity to purchase Units consisting of one pre-funded warrant (in lieu of one share
of common stock, each a “Pre-Funded Warrant”), one-tenth (1/10) of a Series A Warrant and two-tenths (2/10) of a Series B
Warrant. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded
Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such
limit may be increased to up to 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise.
Each Pre-Funded Warrant will be exercisable for one share of common stock. The purchase price of each Unit including a Pre-Funded Warrant
will be equal to the price per Unit including one share of common stock, minus $0.001, and the remaining exercise price of each Pre-Funded
Warrant will equal $0.001 per share. The Pre-Funded Warrants will be immediately exercisable (subject to the beneficial ownership cap)
and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Unit including a Pre-Funded Warrant
we sell (without regard to any limitation on exercise set forth therein), the number of Units including a share of common stock we are
offering will be decreased on a one-for-one basis.
This prospectus also includes the shares of common
stock issuable upon exercise of the Series A Warrants, Series B Warrants, and the Pre-Funded Warrants.
The common stock and Pre-Funded Warrants can each
be purchased in this offering only with the accompanying Series A Warrants and Series B Warrants that are part of a Unit, but the components
of the Units will be immediately separable and will be issued separately in this offering. See “Description of Capital Stock”
in this prospectus for more information.
Our
common stock is listed on The Nasdaq Capital Market, or Nasdaq, under the symbol “SBFM.”
The last reported sale price of our common stock on Nasdaq on February 6, 2024 was $0.255
per share. There is no established public trading market for the Series A Warrants, Series
B Warrants, or the Pre-Funded Warrants, and we do not intend to list the Series A Warrants,
Series B Warrants, or the Pre-Funded Warrants on any national securities exchange or trading
system. Without an active trading market, the liquidity of the Series A Warrants, Series
B Warrants, and the Pre-Funded Warrants will be limited.
The final public offering price of the Units will
be determined through negotiation between us and the underwriter, based upon a number of factors, including our history and our prospects,
the industry in which we operate, our past and present operating results, the previous experience of our executive officers and the general
condition of the securities markets at the time of this offering.
We
have granted Aegis Capital Corp., as underwriter, an option, exercisable for 45 days from
the closing date of this offering, to purchase up to 5,882,353 additional shares of common
stock and/or Pre-Funded Warrants, representing 15% of the shares of common stock and/or Pre-Funded
Warrants sold in the offering, and/or up to 588,235 Series A Warrants, representing 15% of
the Series A Warrants sold in the offering, and/or up to 1,176,470 Series B Warrants, representing
15% of the Series B Warrants sold in the offering. The underwriter may exercise the over-allotment
option with respect to shares of common stock only, Pre-Funded Warrants only, Series A Warrants
only, Series B Warrants only, or any combination thereof.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
Investing in our securities involves a high
degree of risk. See “Risk Factors” beginning on page 4 of this prospectus for a discussion of information that should be
considered in connection with an investment in our securities.
| | |
Per
Unit | | |
Total | |
| Public offering price | |
$ | | | |
$ | | |
| Underwriting discounts and commissions (8.0%)(1) | |
$ | | | |
$ | | |
| Proceeds before expenses | |
$ | | | |
$ | | |
| (1) |
Does not include a non-accountable expense allowance equal to 1.0% of the public offering price. See “Underwriting” for a description of compensation payable to the underwriter. |
The underwriter expects to
deliver our securities to purchasers in the offering on or about , 2024.
Aegis Capital Corp.
The date of this prospectus is ,
2024
TABLE OF CONTENTS
You should rely only on the information
contained in this prospectus, as supplemented and amended. We have not authorized anyone to provide you with information that is
different. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus may only
be accurate on the date of this prospectus. We take no responsibility for, and can provide no assurance as to the reliability of,
any other information that others may give you. Neither we nor the underwriter is making an offer to sell or seeking offers to buy
these securities in any jurisdiction where, or to any person to whom, the offer or sale is not permitted. The information contained
in this prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this
prospectus or of any sale of our securities. Our business, financial condition, results of operations and future growth prospects
may have changed since those dates.
For investors outside the United States: We have
not, and the underwriter has not, done anything that would permit this offering or possession or distribution of this prospectus in any
jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into
possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and
the distribution of this prospectus outside the United States.
PROSPECTUS SUMMARY
This summary highlights certain information
about us and this offering contained elsewhere in this prospectus. Because it is only a summary, it does not contain all of the information
that you should consider before investing in our securities and it is qualified in its entirety by, and should be read in conjunction
with, the more detailed information appearing elsewhere in this prospectus. Before you decide to invest in our securities, you should
read the entire prospectus carefully, including “Risk Factors” beginning on page 4, and the financial statements and related
notes included in this prospectus.
As used in this prospectus and unless otherwise
indicated, the terms “we,” “us,” “our,” “Sunshine Biopharma,” or the “Company”
refer to Sunshine Biopharma, Inc. and its wholly owned subsidiaries.
Overview
We are a pharmaceutical company offering and researching
life-saving medicines in a wide variety of therapeutic areas, including oncology and antivirals. In addition to pursuing our own drug
development program, we operate two wholly owned subsidiaries: (i) Nora Pharma Inc. (“Nora Pharma”), a Canadian corporation
with a portfolio consisting of 51 generic prescription drugs on the market in Canada and 32 additional drugs scheduled to be launched
in Canada in 2024 and 2025, and (ii) Sunshine Biopharma Canada Inc. (“Sunshine Canada”), a Canadian corporation which develops
and sells nonprescription over-the-counter (“OTC”) products.
Corporate Information
Our principal executive offices are located at
1177 Avenue of the Americas, 5th Floor, New York, NY 10036, and our telephone number is 332-216-1147. Our website address is www.sunshinebiopharma.com.
Information on our website is not part of this prospectus.
THE OFFERING
| Units offered |
|
39,215,687 Units(1)
on a firm commitment basis. Each Unit will consist of one share of common stock (or Pre-Funded Warrant to purchase one share
of our common stock in lieu thereof), one-tenth (1/10) of a Series A Warrant to purchase one share of common stock and two-tenths
(2/10) of a Series B Warrant to purchase one share of common stock. The Units have no stand-alone rights and will not be certificated
or issued as stand-alone securities. The shares of common stock and Pre-Funded Warrants, if any, can each be purchased in this offering
only with the accompanying Series A Warrants and Series B Warrants as part of Units (other than pursuant to the underwriter’s
option to purchase additional shares of Common Stock and/or Pre-Funded Warrants and/or Series A Warrants and/or Series B Warrants),
but the components of the Units will be immediately separable and will be issued separately in this offering. |
| |
|
|
| Series A Warrants and Series B Warrants offered |
|
3,921,569 Series A Warrants and 7,843,137 Series B Warrants. Each Unit includes one share of common
stock, one-tenth (1/10) of a Series A Warrant and two-tenths (2/10) of a Series B Warrant. Each Series A Warrant is exercisable at
a price of $3.825 per share (assuming an offering price of $0.255 per Unit), or pursuant to an alternate cashless exercise option,
and each Series B Warrant is exercisable at a price of $4.335 per share (assuming an offering price of $0.255 per Unit). The Series
A Warrants and Series B Warrants will be immediately exercisable and will expire two-and-a-half years (with respect to the Series
A Warrants) or five years (with respect to the Series B Warrants) from the closing date of this public offering. See “Description
of Capital Stock—Series A Warrants and Series B Warrants Offered in this Offering.” |
| |
|
|
|
Pre-Funded Warrants offered
|
|
We are also offering to certain purchasers whose
purchase of Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties,
beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following
the consummation of this offering, the opportunity to purchase, if such purchasers so choose, in lieu of Units including shares of Common
Stock, Units including Pre-Funded Warrants in lieu of shares of common stock that would otherwise result in any such purchaser’s
beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. The purchase price
of each Unit including a Pre-Funded Warrant will be equal to the price at which a Unit is sold to the public in this offering, minus $0.001,
and the exercise price of each Pre-Funded Warrant will be $0.001 per share.
Each Pre-Funded Warrant will be exercisable for
one share of our common stock and will be exercisable at any time after its original issuance until exercised in full, provided that the
purchaser will be prohibited from exercising Pre-Funded Warrants for shares of our common stock if, as a result of such exercise, the
purchaser, together with its affiliates and certain related parties, would own more than 4.99% of the total number of shares of our common
stock then issued and outstanding. However, any holder may increase such percentage to any other percentage not in excess of 9.99%, provided
that any increase in such percentage shall not be effective until 61 days after such notice to us.
This prospectus also relates to the offering
of the common stock issuable upon exercise of the Pre-Funded Warrants. See “Description of Capital Stock—Pre-Funded Warrants Offered in this Offering.” |
| Common stock outstanding before this offering(2) |
|
28,024,290 shares |
| |
|
|
| Common stock outstanding after this offering |
|
67,239,977 shares |
| |
|
|
|
Over-allotment option |
|
The underwriter has
a 45-day option to purchase up to an additional 15% of the total number of shares of common stock and/or Pre-Funded Warrants and/or Series
A Warrants and/or Series B Warrants. |
| |
|
|
| Use of proceeds |
|
We intend to use the net
proceeds of this offering for general corporate purposes, including working capital. We may also use a portion of the net proceeds
to acquire or invest in businesses, technologies, and products that are complementary to our own, although we have no current
binding agreements with respect to any acquisitions as of the date of this prospectus. See “Use of
Proceeds.” |
| |
|
|
| Risk factors |
|
Investing in our securities is highly speculative and involves a high degree of risk. You should carefully consider the information set forth in the “Risk Factors” section beginning on page 4 before deciding to invest in our securities. |
| |
|
|
| Listing |
|
Our common stock is listed on Nasdaq under the symbol “SBFM.” There is no established public trading market for the Series A Warrants, Series B Warrants, or Pre-Funded Warrants, and we do not intend to list the Series A Warrants, Series B Warrants, or the Pre-Funded Warrants on any national securities exchange or trading system. |
| (1) |
Based on assumed public offering price of $0.255 per Unit. |
| |
|
|
(2) |
Based on shares of common stock outstanding
on February 8, 2024, and excludes: |
| |
|
|
|
| |
|
· |
23,395,046 shares issuable upon exercise of outstanding warrants with a weighted average exercise price of $1.94; and |
| |
|
|
|
| |
|
· |
30,000
outstanding shares of Series B Preferred Stock, which are not convertible into common stock. |
Unless otherwise indicated, all information in
this prospectus assumes no exercise by the underwriter its over-allotment option, no exercise of any Series A Warrants or Series B Warrants
issued in this offering, and no sale of any Pre-Funded Warrants in this offering.
RISK FACTORS
Investing in our securities includes a high
degree of risk. Prior to making a decision about investing in our securities, you should consider carefully the specific factors discussed
below, together with all of the other information contained in this prospectus. Our business, financial condition, results of operations
and prospects could be materially and adversely affected by these risks.
Risks Related to Our Business
We have incurred losses and may never achieve profitability.
We have an accumulated deficit of $62,655,634
as of September 30, 2023. We incurred a net loss of $3,256,020 for the nine months ended September 30, 2023 and a net loss of $26,744,440
for the year ended December 31, 2022. We may never achieve profitability.
We are subject to the significant risks
associated with the generic pharmaceutical business.
Since our acquisition of Nora Pharma in October
2022, we have generated revenues primarily through sales of generic pharmaceutical products in Canada, and we expect this to remain the
case for the foreseeable future. Generic pharmaceuticals are, as a general matter, significantly less profitable than innovative medicines,
In recent years, the generic pharmaceutical business
has experienced increased volatility in volumes due in large part to global supply chain issues and the COVID-19 pandemic. In 2022, the
global economy was continuing to recover from the impacts of the COVID-19 pandemic and also began experiencing additional macroeconomic
pressures such as rising inflation and disruptions to the global supply chain, in part resulting from the ongoing conflict between Russia
and Ukraine. We may experience supply discontinuities due to macroeconomic issues, regulatory actions, including sanctions and trade restrictions,
labor disturbances and approval delays, which may impact our ability to timely meet demand in certain instances. These adverse market
forces have a direct impact on our overall performance. Any such disruptions could have a material adverse impact on our business and
our results of operation and financial condition.
Other risks associated with our generic pharmaceutical
business include:
| · |
Current macroeconomic conditions are becoming increasingly less stable due to the war in Ukraine, and tensions in the Far East.
Destabilized macroeconomics conditions pose a serious threat to supply chains around the world including those for the generic
pharmaceutical business. Nearly all of Nora Pharma’s generic drugs are manufactured outside Canada and the United States and
could experience disruptions which would adversely affect Nora Pharma’s main source of revenue. |
| |
|
| · |
Supply chains discontinuities due to
other issues, including unforeseen regulatory actions, economic sanctions, trade restrictions, labor disturbances and approval delays,
may impact the Company’s ability to timely meet customer demand in certain instances. These adverse market forces would have a direct
impact on Nora Pharma’s ability to achieve its sales projections. |
| |
|
| · |
A significant portion of Nora Pharma’s
revenues are derived from relatively few key customers, and any financial difficulties experienced by a single key customer, or any delay
in receiving payments from such a customer, could have a material adverse effect on Nora Pharma’s business, financial condition,
and results of operations. |
| |
|
| · |
If Nora Pharma encounters difficulties
in executing launches of new products, it may not be able to offset the increasing price erosion on existing products resulting from pricing
pressures and accelerated generics approvals for competitors. Such unsuccessful launches can be caused by many factors, including, delays
in regulatory approvals, lack of operational or clinical readiness or patent litigation. Failure or delays to execute launches of new
generic products could have a material adverse effect on Nora Pharma’s business and its ability to realize projected sales. |
Sales of our generic products may be adversely
affected by the drug regulatory environment in Canada.
Currently we sell our generic drugs only in Canada.
Our net sales may be affected by fluctuations in the buying patterns of our customers resulting from government lead pricing pressures
and other factors. Our generic sales in Canada are done via retail pharmacies, pharmacy channels, distributors, and wholesalers. Pricing
pressures in Canada represent the highest risk due to ongoing and unresolved negotiations between the pharmaceutical industry and the
federal government. These together with the fact that a significant portion of our revenues is derived from relatively few key customers,
any financial difficulties experienced by a single key customer, or any delay in receiving payments from such a customer, could have a
material adverse effect on our business, financial condition, and results of operations.
Our revenues and profits from generic products may decline as
a result of competition from other pharmaceutical companies and changes in regulatory policy.
Our generic drugs face intense competition. Prices
of generic drugs may, and often do, decline, sometimes dramatically, especially as additional generic pharmaceutical companies receive
approvals and enter the market for a given product and competition intensifies. Consequently, our ability to sustain our sales and profitability
on any given product over time is affected by the number of companies selling such product, including new market entrants, and the timing
of their approvals.
Furthermore, brand pharmaceutical companies continue
to manage products in a challenging environment through marketing agreements with payers, pharmacy benefits managers and generic manufacturers.
For example, brand companies often sell or license their own generic versions of their products, either directly or through other generic
pharmaceutical companies (so-called “authorized generics”). No significant regulatory approvals are required for
authorized generics, and brand companies do not face any other significant barriers to entry into such market. Brand companies may seek
to delay introductions of generic equivalents through a variety of commercial and regulatory tactics. These actions may increase the costs
and risks of our efforts to introduce generic products and may delay or prevent such introduction altogether.
We may experience delays in launching of our new generic products.
If we cannot execute timely launches of new products,
we may not be able to offset the increasing price erosion on existing products resulting from pricing pressures and accelerated generics
approvals for competing products. Such unsuccessful launches can be caused by many factors, including delays in regulatory approvals,
lack of operational or clinical readiness or patent litigation. Failure or delays to execute launches of new generic products could have
a material adverse effect on our business, financial condition, and results of operations.
We may not receive required regulatory approval for any of our
non-generic pharmaceutical product candidates.
We have not received approval for any of our proprietary
(non-generic) drug development operations product candidates from the FDA. Any compounds that we discover or in-license will require extensive
and costly development, preclinical testing and clinical trials prior to seeking regulatory approval for commercial sales. Our most advanced
product candidate, K1.1 mRNA, and our potential Covid-19 treatments in development, may never be approved for commercial sale. We have
not made any filings to date with the FDA or other regulatory bodies in other jurisdictions. The time required to attain product sales
and profitability is lengthy and highly uncertain. If we fail to obtain required regulatory approvals for our pharmaceutical product candidates,
our business will be materially harmed.
As we have no approved non-generic pharmaceutical products on
the market, we do not expect to generate significant revenues from non-generic pharmaceutical product sales in the foreseeable future,
if at all.
To date, we have no approved non-generic pharmaceutical
products on the market and have generated product revenues solely from our OTC supplements operations and generic pharmaceutical product
sales. We have funded our operations primarily from sales of our securities. We have not received, and do not expect to receive for at
least the next three to four years, if at all, any revenues from the commercialization of our non-generic pharmaceutical product candidates.
To obtain revenues from sales of such pharmaceutical product candidates, we must succeed, either alone or with third parties, in developing,
obtaining regulatory approval for manufacturing, marketing and distributing drugs with commercial potential. We may never succeed in these
activities, and we may not generate sufficient revenues to continue our business operations or achieve profitability.
We will require additional funding to satisfy
our future capital needs, which may not be available.
We may require significant additional funding
in large part due to our research and development expenses, future preclinical and clinical testing costs, and the absence of significant
revenues in the near future. We do not know whether additional financing will be available to us on favorable terms or at all. If
we cannot raise additional funds, we may be required to reduce our capital expenditures, scale back product development programs, reduce
our workforce and license to others products or technologies that we may otherwise be able to commercialize. We are currently unable to
project when or whether our operations will generate positive cash flows from operations.
Any additional equity securities we issue or issuances
of debt we may enter into or undertake may have rights, preferences or privileges senior to those of existing holders of common stock.
To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights
to our technologies or product candidates or grant licenses on terms that are not favorable to us.
The FDA may change its approval policies
or requirements, or apply interpretations to its policies or requirements, in a manner that could delay or prevent commercialization of
K1.1mRNA or our potential Covid-19 treatment in development.
Regulatory requirements may change in a manner
that requires us to conduct additional clinical trials, which may delay or prevent commercialization of our K1.1 mRNA and potential Covid-19
treatment in development. We cannot provide any assurance that the FDA will not require us to repeat existing studies or conduct new or
unforeseen experiments in order to demonstrate the safety and efficacy of any product candidate before considering the approval of such
product candidate.
The product candidate we are developing
for the treatment of Covid-19 may not be granted an emergency use authorization by the FDA. If we do not receive such authorization, or
if, once granted, it is terminated, we will be required to pursue the drug approval process, which is lengthy and expensive.
Subject to completing and receiving favorable
results for clinical trials, we intend to seek emergency use authorization, or EUA, for a potential Covid-19 treatment, which would allow
us to market and sell such product candidate without the need to pursue the lengthy and expensive drug approval process. The FDA may issue
an EUA during a public health emergency if it determines that the potential benefits of a product outweigh the potential risks and if
other regulatory criteria are met. In addition, the FDA may revoke an EUA where it is determined that the underlying health emergency
no longer exists or warrants such authorization. We may not receive EUA for any Covid-19 treatment product candidate. In addition, even
if we do receive EUA for any product candidate, we cannot predict how long such EUA will remain in place. If we fail to receive an EUA
for any Covid-19 product candidate, or such EUA is granted but subsequently terminated, our business, financial condition and results
of operations could be adversely affected.
Our business would be materially harmed
if we fail to obtain FDA approval for our pharmaceutical product candidates.
We anticipate that our ability to generate significant
product revenues from our drug development business will depend on the successful development and commercialization of K1.1 mRNA or our
potential Covid-19 treatment in development. The FDA may not approve in a timely manner, or at all, any of our drug candidates. If we
are unable to submit a new drug application, or NDA for our product candidates, we will be unable to commercialize such products and our
business will be materially harmed. The FDA can and does reject NDAs, and often requires additional clinical trials, even when product
candidates performed well or achieved favorable results in large-scale Phase III clinical trials. The FDA imposes substantial requirements
on the introduction of pharmaceutical products through lengthy and detailed laboratory and clinical testing procedures, sampling activities
and other costly and time-consuming procedures. Satisfaction of these requirements typically takes several years and may vary substantially
based upon the type and complexity of the pharmaceutical product. Our product candidates are novel compounds or new chemical entities,
which may further increase the time required for satisfactory testing procedures.
Data obtained from preclinical and clinical activities
are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. In addition, delays or rejections
may be encountered based on changes in, or additions to, regulatory policies for drug approval during product development and regulatory
review. Government regulation may delay or prevent the commencement of clinical trials or marketing of our product candidates, impose
costly procedures upon our activities and provide an advantage to our competitors with greater financial resources or more experience
in regulatory affairs. The FDA may not approve our product candidates for clinical trials or marketing on a timely basis or at all. Delayed
or failed approvals would adversely affect the marketing of our product candidates and our liquidity and capital resources.
Drug products and their manufacturers are subject
to continual regulatory review after the product receives FDA approval. Later discovery of previously unknown problems with a product
or manufacturer may result in additional clinical testing requirements or restrictions on such product or manufacturer, including withdrawal
of the product from the market. Failure to comply with applicable regulatory requirements can, among other things, result in fines, injunctions
and civil penalties, suspensions or withdrawals of regulatory approvals, product recalls, operating restrictions or shutdown and criminal
prosecution. We may lack sufficient resources and expertise to address these and other regulatory issues as they arise.
We may be sued or become a party to litigation,
which could require significant management time and attention and result in significant legal expenses and may result in an unfavorable
outcome which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
We may be forced to incur costs and expenses in
connection with defending ourselves with respect to litigation and the payment of any settlement or judgment in connection therewith if
there is an unfavorable outcome. The expense of defending litigation may be significant. The amount of time to resolve lawsuits is unpredictable
and defending ourselves may divert management’s attention from the day-to-day operations of our business, which could adversely
affect our business, results of operations and cash flows. In addition, an unfavorable outcome in any such litigation could have a material
adverse effect on our business, results of operations and cash flows.
If we are unable to attract and retain qualified
scientific, technical, and key management personnel, or if our key executive, Dr. Steve N. Slilaty, discontinues his employment with us,
it may delay our research and development efforts.
We rely on the services of Dr. Slilaty for
strategic and operational management, as well as for scientific and/or medical expertise in the development of our products. The
loss of Dr. Slilaty would result in a significant negative impact on our ability to implement our business plan. The loss of Dr.
Slilaty will also significantly delay or prevent the achievement of our business objectives.
Our business exposes us to potential product
liability risks, and we may be unable to acquire and maintain sufficient insurance to provide adequate coverage against potential liabilities.
Our business exposes us to potential product liability
risks that are inherent in the testing, manufacturing and marketing of pharmaceutical products and OTC supplements. The use of our product
candidates in clinical trials also exposes us to the possibility of product liability claims and possible adverse publicity. These risks
will increase to the extent our pharmaceutical product candidates receive regulatory approval and are commercialized. We currently have
product liability insurance for our generic drugs, and we plan to obtain product liability insurance in connection with our OTC supplements
and future clinical trials of our pharmaceutical product candidates in the near future. However, our current and future product liability
insurance, once obtained, may not provide adequate coverage against potential liabilities. On occasion, juries have awarded large
judgments in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series
of claims brought against us would decrease our cash reserves and could cause our stock price to fall significantly.
We face regulation and risks related to
hazardous materials and environmental laws, violations of which may subject us to claims for damages or fines that could materially affect
our business, cash flows, financial condition and results of operations.
Our research and development activities involve
the use of controlled and/or hazardous materials and chemicals. The risk of accidental contamination or injury from these materials cannot
be completely eliminated. In the event of an accident, we could be held liable for any damages or fines that result, and the liability
could have a material adverse effect on our business, financial condition, and results of operations. We are also subject to federal,
state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products.
If we fail to comply with these laws and regulations or with the conditions attached to our operating licenses, the licenses could be
revoked, and we could be subjected to criminal sanctions and substantial liability or be required to suspend or modify our operations.
In addition, we may have to incur significant costs to comply with future environmental laws and regulations. We do not currently have
a pollution and remediation insurance policy.
Third party manufacturers may not be able
to manufacture our pharmaceutical product candidates, which would prevent us from commercializing our product candidates.
If any of our pharmaceutical product candidates
is approved by the FDA or other regulatory agencies for commercial sale, we will need third parties to manufacture the product in larger
quantities. If we are able to reach an agreement with any collaborator or third party manufacturer in the future, of which there can be
no assurance due to factors beyond our control, these collaborators and/or third party manufacturers may not be able to increase their
manufacturing capacity for any of our product candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing
may require additional validation studies, which the FDA must review and approve. If we are unable to increase the manufacturing capacity
for a product candidate successfully, the regulatory approval or commercial launch of that product candidate may be delayed or there may
be a shortage in the supply of the product candidate. Our product candidates require precise, high-quality manufacturing. The failure
of collaborators or third-party manufacturers to achieve and maintain these high manufacturing standards, including the incidence of manufacturing
errors, could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost
overruns or other problems that could seriously harm our business.
If we are unable to establish sales and
marketing capabilities for our pharmaceutical product candidates or enter into agreements with third parties to sell and market any such
products we may develop, we may be unable to generate revenues from our pharmaceutical business.
We do not currently have product sales and marketing
capabilities for our pharmaceutical operations. If we receive regulatory approval to commence commercial sales of any of our pharmaceutical
product candidates, we will have to establish a sales and marketing organization with appropriate technical expertise and distribution
capabilities or make arrangements with third parties to perform these services in other jurisdictions. If we receive approval in applicable
jurisdictions to commercialize K1.1 mRNA for the treatment of liver cancer indication, we intend to engage additional pharmaceutical or
health care companies with existing distribution systems and direct sales organizations to assist us in North America and throughout the
world. We may not be able to negotiate favorable distribution partnering arrangements, if at all. To the extent we enter into co-promotion
or other licensing arrangements, any revenues we receive will depend on the efforts of third parties and will not be under our control.
If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, our
ability to generate product revenues, and become profitable, would be severely limited.
Even if we obtain required US and foreign regulatory
approvals, as applicable, factors that may inhibit our efforts to commercialize our pharmaceutical product candidates without strategic
partners or licensees include:
| |
· |
difficulty recruiting and retaining adequate numbers of effective sales and marketing personnel; |
| |
· |
the inability of sales personnel to obtain access to, or persuade adequate numbers of, physicians to prescribe our products; |
| |
· |
the lack of complementary products to be offered
by sales personnel, which may put us at a competitive disadvantage against
companies with broader product lines; and |
| |
· |
unforeseen costs associated with creating an independent sales and marketing organization. |
Even if we successfully develop and
obtain approval for our proprietary drug product candidates, our business will not be profitable if such products do not achieve and maintain
market acceptance.
Even if our proprietary drug product candidates
are approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of our approved product candidates
by physicians, healthcare professionals, patients and third-party payors, and our resulting profitability and growth, will depend on a
number of factors, including:
| |
· |
our ability to provide acceptable evidence of safety and efficacy; |
| |
· |
relative convenience and ease of administration; |
| |
· |
the prevalence and severity of any adverse side effects; |
| |
· |
the availability of alternative treatments; |
| |
· |
the details of FDA labeling requirements, including the scope of approved indications and any safety warnings; |
| |
· |
pricing and cost effectiveness; |
| |
· |
the effectiveness of our or our collaborators' sales and marketing strategy; |
| |
· |
our ability to obtain sufficient third-party insurance coverage or reimbursement; and |
| |
· |
our ability to have the product listed on insurance company formularies. |
If our proprietary drug product candidates achieve
market acceptance, we may not maintain that market acceptance over time if new products or technologies are introduced that are received
more favorably or are more cost effective. Complications may also arise, such as development of new know-how or new medical or therapeutic
capabilities by other parties that render our product obsolete.
Because the results of preclinical studies
for our preclinical product candidates are not necessarily predictive of future results, our pharmaceutical product candidates may not
have favorable results in later clinical trials or ultimately receive regulatory approval.
Our proprietary drug product candidates have not
been tested in clinical trials. Positive results from preclinical studies are no assurance that later clinical trials will succeed. Preclinical
studies are not designed to establish the clinical efficacy of our preclinical product candidates. We will be required to demonstrate
through clinical trials that our product candidates are safe and effective for use before we can seek regulatory approvals for commercial
sale. There is typically an extremely high rate of failure as product candidates proceed through clinical trials. If our product
candidates fail to demonstrate sufficient safety and efficacy in any clinical trial, we would experience potentially significant delays
in, or be required to abandon, development of that product candidate. This would adversely affect our ability to generate revenues
and may damage our reputation in the industry and in the investment community.
The future clinical testing of our proprietary
drug product candidates could be delayed, resulting in increased costs to us and a delay in our ability to generate revenues.
Our proprietary drug product candidates will require
additional preclinical testing and extensive clinical trials prior to submitting a regulatory application for commercial sales. We do
not know whether clinical trials will begin on time, if at all. Delays in the commencement of clinical testing could significantly
increase our product development costs and delay product commercialization. In addition, many of the factors that may cause, or lead to,
a delay in the commencement of clinical trials may also ultimately lead to denial of regulatory approval of a product candidate. Each
of these results would adversely affect our ability to generate revenues.
The commencement of clinical trials can be delayed
for a variety of reasons, including delays in:
| |
· |
demonstrating sufficient safety to obtain regulatory approval to commence a clinical trial; |
| |
· |
reaching agreement on acceptable terms with prospective research organizations and trial sites; |
| |
· |
manufacturing sufficient quantities of a product candidate; |
| |
· |
obtaining institutional review board approvals to conduct clinical trials at prospective sites; and |
| |
· |
procuring adequate financing to fund the work. |
In addition, the commencement of clinical trials
may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population,
the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease,
and the eligibility criteria for the clinical trial. If we are unable to enroll a sufficient number of evaluable patients, the clinical
trials for our product candidates could be delayed until sufficient numbers are achieved.
We face or will face significant competition
from other biotechnology, pharmaceutical and OTC supplements companies, and our operating results will suffer if we fail to compete effectively.
Most of our pharmaceutical company competitors,
such as Merck, Bristol-Myers Squibb, Pfizer, Amgen, and others, are large pharmaceutical companies with substantially greater financial,
technical, and human resources than we have. The biotechnology and pharmaceutical industries are intensely competitive and subject to
rapid and significant technological change. The drugs that we are attempting to develop will compete with existing therapies if we receive
marketing approval. Because of their significant resources, our competitors may be able to use discovery technologies and techniques,
or partnerships with collaborators, to develop competing products that are more effective or less costly than the product candidate we
are developing. This may render our technology or product candidate obsolete and noncompetitive. Academic institutions, government agencies,
and other public and private research organizations may seek patent protection with respect to potentially competitive products or technologies
and may establish exclusive collaborative or licensing relationships with our competitors.
Our competitors may succeed in obtaining FDA or
other regulatory approvals for product candidates more rapidly than us. Companies that complete clinical trials, obtain required regulatory
agency approvals and commence commercial sale of their drugs before we do may achieve a significant competitive advantage, including certain
FDA marketing exclusivity rights that would delay or prevent our ability to market certain products. Any approved drugs resulting from
our research and development efforts, or from our joint efforts with our existing or future collaborative partners, might not be able
to compete successfully with our competitors' existing or future products.
We also face competition in our OTC supplements
business. The business of marketing OTC supplements is highly competitive. This market segment includes numerous manufacturers, marketers,
and retailers that actively compete for the business of consumers both in the United States and abroad. The market is highly sensitive
to the introduction of new products, which may rapidly capture a significant share of the market. Sales of similar products by competitors
may materially and adversely affect our business, financial condition, and results of operations.
The market for our potential Covid-19 treatment
in development could be adversely affected if the Covid-19 disease outbreak subsides.
Disease outbreaks are unpredictable. In the event
that the Covid-19 outbreak subsides, or Covid-19 is substantially eradicated, there may be reduced demand or need for our potential Covid-19
treatment in development, which may have a negative effect on the market for such treatment, even if it is approved.
The Covid-19 pandemic has significantly
impacted worldwide economic conditions and could have a material adverse effect on our operations and business.
While we have been able to continue to operate,
the global Covid-19 pandemic has caused disruptions in supply chains, affecting production and sales across a range of industries. While
the disruptions are currently expected to be temporary, there is considerable uncertainty around the duration and the impact of these
disruptions.
The extent of the impact of Covid-19 on our operational
and financial performance will depend on the on-going and future impact on our customers, vendors, service providers, and availability
of labor as well as the potential impact of future expanded local, state, or federal restrictions – all of which are uncertain and
are difficult to predict.
Because our proprietary drug product candidates
and our development and collaboration efforts depend on our intellectual property rights, adverse events affecting our intellectual property
rights will harm our ability to commercialize products.
Our success will depend to a large degree on our
own and our licensors’ ability to obtain and defend patents for each party's respective technologies and the compounds and other
products, if any, resulting from the application of such technologies. The patent positions of pharmaceutical and biotechnology companies
can be highly uncertain and involve complex legal and technical questions. No consistent policy regarding the breadth of claims allowed
in biotechnology patents has emerged to date. Accordingly, we cannot predict the breadth of claims that will be allowed or maintained,
after challenge, in our or other companies' patents.
The degree of future protection for our proprietary
rights is uncertain, and we cannot ensure that:
| |
· |
we were the first to make the inventions covered by each of our pending patent applications; |
| |
· |
we were the first to file patent applications for these inventions; |
| |
· |
others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
| |
· |
any patents issued to us or our collaborators
will provide a basis for commercially viable products, will provide us with
any competitive advantages or will not be challenged
by third parties; |
| |
· |
our pending patent applications will result in issued patents; |
| |
· |
we will develop additional proprietary technologies that are patentable; |
| |
· |
the patents of others will not have a negative effect on our ability to do business; or |
| |
· |
our issued patents will have sufficient useful life remaining for commercial viability of our product candidate. |
If we cannot maintain the confidentiality of our
technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or
protect our proprietary information will be impaired. In addition, some of the technology we have developed or licensed relies on inventions
developed using U.S. and other governments’ resources. Under applicable law, the U.S. government has the right to require us to
grant a nonexclusive, partially exclusive or exclusive license for such technology to a responsible applicant or applicants, upon terms
that are reasonable under the circumstances, if the government determines that such action is necessary.
Confidentiality agreements with employees
and others may not adequately prevent disclosure of trade secrets and other proprietary information and may not adequately protect our
intellectual property.
We rely on trade secrets to protect our technology,
particularly when we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. In
order to protect our proprietary technology and processes, we rely in part on confidentiality and intellectual property assignment agreements
with our employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not
effectively prevent disclosure of confidential information nor result in the effective assignment to us of intellectual property and may
not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches of the agreements.
In addition, others may independently discover our trade secrets and proprietary information, and in such case we could not assert any
trade secret rights against such party. Enforcing a claim that a party illegally obtained and is using our trade secrets is difficult,
expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect
trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary
rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
The implementation of our business plan
may result in a period of rapid growth that will impose a significant burden on our current administrative and operational resources.
Our ability to effectively manage our growth will
require us to substantially expand the capabilities of our administrative and operational resources by attracting, training, managing,
and retaining additional qualified personnel, including additional members of management, technicians, and others. To successfully develop
our products, we will need to manage operating, producing, marketing and selling our products. There can be no assurances that we will
be able to do so. Our failure to successfully manage our growth will have a negative impact on our anticipated results of operations.
A significant or prolonged economic downturn
could have a material adverse effect on our results of operations.
A significant or prolonged economic downturn may
adversely affect the disposable income of many consumers and may lower demand for our OTC supplement products. Any decline in economic
conditions could negatively impact our business. A significant decline in consumer demand, even if only due in part to general economic
conditions, could have a material adverse effect on our revenues and profit margins.
The failure of our service providers and
suppliers to supply quality services and materials in sufficient quantities, at a favorable price, and in a timely fashion could adversely
affect the results of our operations.
Our outside manufacturer buys raw materials for
our OTC supplements business from a limited number of suppliers. The loss of any of our major suppliers or of any supplier who, through
our contract manufacturer, provides us materials that are hard to obtain elsewhere at the same quality could adversely affect our business
operations. Although we believe we could establish alternate manufacturers and sources for most of our raw materials, any delay in locating
and establishing relationships with other sources could result in shortages of products we manufacture from such raw materials, with a
resulting loss of sales and customers. In certain situations, we may need to alter our products or with our customer’s consent to
substitute different materials from alternative sources.
A shortage of raw materials or an unexpected interruption
of supply could also result in higher prices for those materials. We have experienced increases in various raw material costs, transportation
costs and the cost of petroleum-based raw materials and packaging supplies used in our business. Increasing cost pricing pressures on
raw materials and other products have continued throughout fiscal 2020 as a result of limited supplies of various ingredients, the effects
of higher labor and transportation costs, and impact of Covid-19. We expect these upward pressures to continue through fiscal 2021. Although
we may be able to raise our prices in response to significant increases in the cost of raw materials, we may not be able to raise prices
sufficiently or quickly enough to offset the negative effects such cost increases could have on our results of operations or financial
condition.
There can be no assurance suppliers will provide
the quality raw materials we need in the quantities requested or at a price we are willing to pay. Because we do not control the actual
production of these raw materials, we are also subject to delays caused by interruption in production of materials including but not limited
to those resulting from conditions outside of our control, such as pandemics, weather, transportation interruptions, strikes, terrorism,
natural disasters, and other catastrophic events.
Our OTC supplements business is subject
to the effects of adverse publicity, which could negatively affect our sales and revenues.
Our business can be affected by adverse publicity
or negative public perception about us, our competitors, our products, or our industry or competitors generally. Adverse publicity may
include publicity about the OTC supplements industry generally, the efficacy, safety and quality of OTC supplements and other health care
products or ingredients in general or our products or ingredients specifically, and regulatory investigations, regardless of whether these
investigations involve us or the business practices or products of our competitors, or our customers. Any adverse publicity or negative
public perception could have a material adverse effect on our business, financial condition and results of operations. Our business, financial
condition and results of operations could be adversely affected if any of our products or any similar products distributed by other companies
are alleged to be or are proved to be harmful to consumers or to have unanticipated and unwanted health consequences.
Our manufacturing and third-party fulfillment
activities are subject to certain risks.
Our OTC supplements products are manufactured
at third party manufacturing facilities in Canada. As a result, we are dependent on the uninterrupted and efficient operation of these
facilities. Such manufacturing operations, and those of its suppliers, are subject to power failures, blackouts, border shutdowns, telecommunications
failures, computer viruses, cybersecurity vulnerabilities, human error, breakdown, failure or substandard performance of our facilities,
our equipment, the improper installation or operation of equipment, terrorism, pandemics (including Covid-19), natural or other disasters,
intentional acts of violence, and the need to comply with the requirements or directives of governmental agencies, including the FDA.
The occurrence of these or any other operational problems at such facilities may have a material adverse effect on our business, financial
condition and results of operations.
Risks Related to This Offering and Our Common
Stock
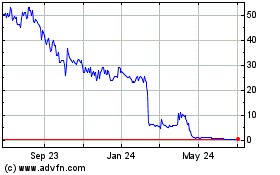
There is a limited market for our common
stock, and investors may find it difficult to buy and sell our shares.
Our common stock has been traded on the Nasdaq
Capital Market since February 2022 and previously traded on the over-the-counter market. There is no assurance an active trading market
for our common stock will be sustained or that we will remain eligible for continued listing on the Nasdaq Capital Market.
If we are unable to continue to meet the
listing requirements of Nasdaq, our common stock will be delisted.
Our common stock currently trades on Nasdaq, where
it is subject to various listing requirements. On March 24, 2023, the Company received a notification letter from Nasdaq’s Listing
Qualifications Department notifying the Company that, because the closing bid price for the Company’s common stock listed on Nasdaq
was below $1.00 for 30 consecutive trading days, the Company no longer meets the minimum bid price requirement for continued listing under
Nasdaq Marketplace Rule 5550(a)(2), requiring a minimum bid price of $1.00 per share. On September 21, 2023, the Company received another
notification letter from Nasdaq advising that Nasdaq’s staff has determined that the Company is eligible for an extension of an
additional 180 calendar day period, or until March 18, 2024, to cure the bid price deficiency. We have obtained shareholder approval for
and intend to complete a reverse stock split prior to March 18, 2024 to regain compliance with this rule. If we are unable to achieve
and maintain compliance with such listing standards or other Nasdaq listing requirements in the future, we could be subject to suspension
and delisting proceedings. A delisting of our common stock and our inability to list on another national securities market could negatively
impact us by: (i) reducing the liquidity and market price of our common stock; (ii) reducing the number of investors willing to hold or
acquire our common stock, which could negatively impact our ability to raise equity financing; (iii) limiting our ability to use certain
registration statements to offer and sell freely tradable securities, thereby limiting our ability to access the public capital markets;
and (iv) impairing our ability to provide equity incentives to our employees.
We do not intend to pay dividends on our
common stock for the foreseeable future.
We have paid no dividends on our common stock
to date, and we do not anticipate paying any dividends to holders of our common stock in the foreseeable future. While our future dividend
policy will be based on the operating results and capital needs of the business, we currently anticipate that we will retain any earnings
to finance our future expansion and for the implementation of our business plan. Investors should take note of the fact that a lack of
a dividend can further affect the market value of our common stock and could significantly affect the value of any investment in the Company.
Our articles of incorporation allow for
our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights
of the holders of our common stock.
Our board of directors has the authority to fix
and determine the relative rights and preferences of preferred stock. Our board of directors has the authority to issue up to 30,000,000
shares of our preferred stock without further stockholder approval. 1,000,000 shares of preferred stock are designated Series B Preferred
Stock. 10,000 shares of Series B Preferred Stock are outstanding and held by our chief executive officer. Our board of directors could
authorize the creation of additional series of preferred stock that would grant to holders of preferred stock the right to our assets
upon liquidation, or the right to receive dividend payments before dividends are distributed to the holders of common stock. In addition,
subject to the rules of any securities exchange on which our stock is then listed, our board of directors could authorize the creation
of additional series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock,
which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders.
The Series A Warrants, Series B Warrants,
and Pre-Funded Warrants will not be listed or quoted on any exchange.
There is no established public trading market
for the Series A Warrants Series B Warrants, or Pre-Funded Warrants being offered in this offering, and we do not expect a market to develop.
In addition, we do not intend to apply to list the Series A Warrants, Series B Warrants, or Pre-Funded Warrants on any national securities
exchange or other nationally recognized trading system, including Nasdaq. Without an active market, the liquidity of the Series A Warrants,
Series B Warrants, and Pre-Funded Warrants will be limited.
Except as otherwise provided in the Series
A Warrants, Series B Warrants, and Pre-Funded Warrants, holders of Series A Warrants, Series B Warrants, and Pre-Funded Warrants purchased
in this offering will have no rights as stockholders until such holders exercise their Series A Warrants, Series B Warrants, or Pre-Funded
Warrants and acquire our common stock.
Except as otherwise provided in the Series A Warrants,
Series B Warrants, and Pre-Funded Warrants, until holders of Warrants or Pre-Funded Warrants acquire our common stock upon exercise of
the Series A Warrants, Series B Warrants, or Pre-Funded Warrants, holders of Series A Warrants, Series B Warrants, and Pre-Funded Warrants
will have no rights with respect to our common stock underlying such Series A Warrants, Series B Warrants, and Pre-Funded Warrants. Upon
exercise of the Series A Warrants, Series B Warrants, and Pre-Funded Warrants, the holders will be entitled to exercise the rights of
a holder of our common stock only as to matters for which the record date occurs after the exercise date.
Additional stock offerings in the future
may dilute then-existing shareholders’ percentage ownership of the Company.
Given our plans and expectations that we will
need additional capital and personnel, we anticipate that we will need to issue additional shares of common stock or securities convertible
or exercisable for shares of common stock, including convertible preferred stock, convertible notes, stock options or warrants. The issuance
of additional securities in the future will dilute the percentage ownership of then current stockholders.
Provisions of the Series A Warrants and
Series B Warrants offered pursuant to this prospectus could discourage an acquisition of us by a third-party.
Certain provisions of the Series A Warrants and
Series B Warrants offered pursuant to this prospectus could make it more difficult or expensive for a third-party to acquire us. The Series
A Warrants and Series B Warrants prohibit us from engaging in certain transactions constituting “fundamental transactions”
unless, among other things, the surviving entity assumes our obligations under the Series A Warrants and Series B Warrants. These and
other provisions of the Series A Warrants and Series B Warrants could prevent or deter a third-party from acquiring us even where the
acquisition could be beneficial to you.
The Series A Warrants and Series B Warrants
may have an adverse effect on the market price of our common stock and make it more difficult to effect a business combination.
To the extent we issue shares of common stock
to effect a future business combination, the potential for the issuance of a substantial number of additional shares of common stock upon
exercise of the Series A Warrants and Series B Warrants could make us a less attractive acquisition vehicle in the eyes of a target business.
Such Series A Warrants and Series B Warrants, when exercised, will increase the number of issued and outstanding shares of common stock
and reduce the value of the shares issued to complete the business combination. Accordingly, the Series A Warrants and Series B Warrants
may make it more difficult to effectuate a business combination or increase the cost of acquiring a target business. Additionally, the
sale, or even the possibility of a sale, of the shares of common stock underlying the Series A Warrants and Series B Warrants could have
an adverse effect on the market price for our securities or on our ability to obtain future financing. If and to the extent the Series
A Warrants and Series B Warrants are exercised, you may experience dilution to your holdings.
We will likely not receive any additional funds
upon the exercise of the Series A Warrants.
If we receive the Warrant Stockholder Approval, the
Series A Warrants may be exercised by way of an alternative cashless exercise, meaning that the holder may not pay a cash purchase price
upon exercise, but instead would receive upon such exercise the net number of shares of our common stock determined according to the formula
set forth in the Series A Warrants. Accordingly, we will likely not receive any additional funds upon the exercise of the Series A Warrants.
Certain beneficial provisions in the Series
A Warrants and Series B Warrants will not be effective until we are able to receive stockholder approval of such provisions, and if we
are unable to obtain such approval the Warrant will have significantly less value.
Under Nasdaq listing rules, the alternative cashless
exercise option in the Series A Warrants and certain anti-dilution provisions in the Series B Warrants will not be effective until, and
unless, we obtain the approval of our stockholders. While we intend to promptly seek stockholder approval, there is no guarantee that
the Warrant Stockholder Approval will ever be obtained. If we are unable to obtain the Warrant Stockholder Approval, the foregoing provisions
will not become effective and the Series A Warrants and Series B Warrants will have substantially less value. In addition, we will incur
substantial cost, and management will devote substantial time and attention, in attempting to obtain the Warrant Stockholder Approval.
Management will have broad discretion as
to the use of the proceeds from this offering and may not use the proceeds effectively.
Our management will have broad discretion in the
application of the net proceeds from this offering and could spend the proceeds in ways that may not improve our results of operations
or enhance the value of our common stock. Our failure to apply these funds effectively could have a material adverse effect on our business
and cause the price of our common stock to decline.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements
within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities
Exchange Act of 1934, or the Exchange Act. Forward-looking statements give current expectations or forecasts of future events or our future
financial or operating performance. We may, in some cases, use words such as “anticipate,” “believe,” “could,”
“estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,”
“project,” “should,” “will,” “would” or the negative of those terms, and similar expressions
that convey uncertainty of future events or outcomes to identify these forward-looking statements.
These forward-looking statements reflect our management’s
beliefs and views with respect to future events, are based on estimates and assumptions as of the date of this prospectus and are subject
to risks and uncertainties, many of which are beyond our control, that could cause our actual results to differ materially from those
in these forward-looking statements. We discuss many of these risks in greater detail in this prospectus under “Risk Factors.”
Moreover, new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact
of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially
from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on
these forward-looking statements.
We undertake no obligation to publicly update
any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by
applicable laws or regulations.
USE OF PROCEEDS
We estimate that the net proceeds from the
sale of the securities we are offering will be approximately $8.5 million (or approximately $9.8 million if the underwriter exercises
in full its over-allotment option), after deducting the estimated underwriting discounts and commissions and estimated offering costs
payable by us.
We intend to use the net proceeds from this offering
for general corporate purposes, including working capital. We may also use a portion of the net proceeds to acquire or invest in businesses,
technologies, and products that are complementary to our own, although we have no current binding agreements with respect to any acquisitions
as of the date of this prospectus.
This expected use of the net proceeds from this
offering represents our intentions based upon our current plans and business conditions. Pending our use of the net proceeds from this
offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade,
interest bearing instruments and U.S. government securities.
MARKET FOR COMMON STOCK AND RELATED STOCKHOLDER
MATTERS
Our common stock is listed on the Nasdaq Capital
Market under the symbol “SBFM.”
As of February
6, 2024, there were approximately 149 holders of record of our common stock.
Equity Compensation Plan Information
The following table sets forth information regarding
our equity compensation plans as of December 31, 2023.
| Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average
exercise price of outstanding options, warrants and rights |
Number
of securities remaining available for future issuance under equity compensation plans |
| Equity
compensation plans approved by security holders(1) |
-- |
-- |
3,320,988 |
| Equity
compensation plans not approved by security holders |
-- |
-- |
-- |
(1) Represents our 2023 Equity
Incentive Plan.
Dividend Policy
We have not paid any dividends since our incorporation
and do not anticipate paying any dividends in the foreseeable future. At present, our policy is to retain earnings, if any, to develop
and market our products. Our payment of dividends in the future will depend upon, among other factors, our earnings, capital requirements,
and operating financial conditions.
CAPITALIZATION
The following table sets forth our cash and our
capitalization as of September 30, 2023, on:
| |
· |
an actual basis; and |
| |
|
|
| |
· |
on an as adjusted basis
to give effect to the sale by us of 39,215,687 Units in this offering, at the assumed public offering price of $0.255 per Unit, after
deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read this table in conjunction with
“Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our financial statements
for the period ended September 30, 2023, and the related notes thereto, included in this prospectus.
| |
|
As of September 30, 2023 |
|
| |
|
Actual |
|
|
As adjusted |
|
| Cash and cash equivalents |
|
$ |
18,846,140 |
|
|
$ |
27,296,140 |
|
| Total liabilities |
|
|
5,686,801 |
|
|
|
5,686,801 |
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
| Series B Preferred Stock, $0.10 par value: 1,000,000 shares authorized; 10,000 shares issued and outstanding |
|
|
1,000 |
|
|
|
1,000 |
|
| Common Stock, $0.001 par value: 3,000,000,000 shares authorized; 25,678,290 shares issued and
outstanding, actual; 64,893,977 shares issued and outstanding, as adjusted |
|
|
25,678 |
|
|
|
64,894 |
|
| Capital paid in excess of par value |
|
|
84,387,890 |
|
|
|
92,798,674 |
|
| Accumulated comprehensive income |
|
|
204,549 |
|
|
|
204,549 |
|
| Accumulated (deficit) |
|
|
(62,655,634 |
) |
|
|
(62,655,634 |
) |
| Total stockholders’ equity |
|
|
21,963,483 |
|
|
|
30,413,483 |
|
The above table is based on 25,678,290 shares
of common stock outstanding as of September 30, 2023, and excludes 23,395,046 shares issuable upon exercise of outstanding warrants with
a weighted average exercise price of $1.94.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion highlights the principal
factors that have affected our financial condition and results of operations as well as our liquidity and capital resources for the periods
described. This discussion should be read in conjunction with our financial statements and the related notes included in this prospectus.
This discussion contains forward-looking statements. Please see “Cautionary Note Regarding Forward-Looking Statements” for
a discussion of the uncertainties, risks and assumptions associated with these forward-looking statements.
Results of Operations
Comparison of results
of operations for the three months ended September 30, 2023 and 2022
During the three months
ended September 30, 2023, we generated $5,957,668 in sales, compared to $132,808 for the three months ended September 30, 2022, an increase
of $5,824,860. The increase is attributable to sales generated by our wholly owned subsidiary, Nora Pharma, which we acquired in October
2022. The direct cost for generating these sales was $3,967,412 (66.6%) for the three months ended September 30, 2023, compared to $65,783
(49.5%) for the three months ended September 30, 2022. The increase in the cost of goods sold in 2023 is due to increased cost of manufacturing
of the generic prescription drugs sold by Nora Pharma. Our gross profit grew to $1,990,256 for the three months ended September 30, 2023,
compared to $67,025 for the three months ended September 30, 2022.
General and administrative
expenses during the three-month period ended September 30, 2023, were $2,769,730, compared to $1,785,005 during the three-month period
ended September 30, 2022, an increase of $984,725. This increase was the result of increased overhead associated with being a Nasdaq listed
company and expenses related to Nora Pharma operations. Specifically, we incurred increased costs in consulting ($58,929), office ($467,397),
salaries ($549,377) and taxes ($52,586). Overall, we incurred a loss of $779,474 from our operations for the three months ended September
30, 2023, compared to a loss of $1,717,980 from our operations in the three-month period ended September 30, 2022.
In addition, we had net
interest income of $168,904 during the three months ended September 30, 2023, compared to a net interest income of approximately $260,936
during the three months ended September 30, 2022, as a result of interest earned on cash on hand.
As a result, we incurred a net loss of $651,482
($0.04 per share) for the three months ended September 30, 2023, compared to a net loss of $1,457,019 ($0.08 per share) for the three-month
period ended September 30, 2022.
Comparison of results of operations for
the nine months ended September 30, 2023 and 2022
During the nine months ended September 30, 2023,
we generated revenues of $16,412,586, compared to revenues of $405,760 for the nine months ended September 30, 2022, an increase of $16,006,826.
The increase is attributable to sales generated by our recently acquired wholly owned subsidiary, Nora Pharma. The direct cost for generating
these revenues was $10,641,461 (64.8%) for the nine months ended September 30, 2023, compared to $200,311 (49.4%) for the nine months
ended September 30, 2022. The increase in the cost of goods sold in 2023 is due to increased cost of manufacturing of the generic prescription
drugs sold by Nora Pharma. Our gross profit increased to $5,771,125 for the nine months ended September 30, 2023, compared to a gross
profit of $205,449 for the same period in 2022.
General and administrative expenses during the
nine-month period ended September 30, 2023, were $9,369,203 compared to $3,842,589 during the nine-month period ended September 30, 2022,
an increase of $5,526,614. This increase was the result of increased overhead associated with being a Nasdaq listed company and expenses
related to Nora Pharma operations. Specifically, we incurred increased costs in accounting ($63,608), consulting ($475,817), office costs
($972,328), research and development ($269,407), salaries ($3,239,801) and taxes ($212,953). Overall, we incurred a loss of $3,598,078
from our operations in the nine-month period ended September 30, 2023, compared to a loss from operations of $3,637,140 in the similar
period of 2022.
In addition, we had net interest income of $517,163
during the nine months ended September 30, 2023, compared to a net interest income of $394,118 during the nine months ended September
30, 2022, as a result of interest earned on cash on hand.
As a result, we incurred a net loss of $3,256,020
($0.12 per share) for the nine-month period ended September 30, 2023, compared to a net loss of $3,232,125 ($0.26 per share) for the nine-month
period ended September 30, 2022.
Comparison of Results
of Operations for the fiscal years ended December 31, 2022 and 2021
During our fiscal year
ended December 31, 2022, we generated revenues of $4,345,603, compared to revenues of $228,426, in 2021. The increase was the result of
our acquisition of Nora Pharma in October 2022, which accounted for $3,803,106 of these revenues. The cost of sales in 2022 and 2021 for
generating these revenues was $2,649,028 and $117,830, respectively.
General and administrative
expenses for our fiscal year ended December 31, 2022, were $28,697,325, compared to $2,550,730 during our fiscal year ended December 31,
2021, an increase of $26,146,595. The increase was largely a result of goodwill impairment of $18,326,719 and costs and expenses relating
to the Nora Pharma acquisition.
We also incurred $39,412
in interest expense and $0 in losses from debt conversion in 2022, compared to $328,818 in interest expense and $9,726,485 in losses from
debt conversion in 2021. The decrease in interest expense and losses from debt conversion in 2022 was due to our repayment of all outstanding
debt in 2022.
As a result, we incurred
a net loss of $26,511,136 for the year ended December 31, 2022, compared to a net loss of $12,436,447 for the year ended December 31,
2021.
Liquidity and Capital Resources
As of September 30, 2023, we had cash or cash
equivalents of $18,846,140.
Net cash used in operating activities was $6,085,435
during the nine months ended September 30, 2023, compared to $3,001,746 during the nine-month period ended September 30, 2022. The increase
was a result of the addition of Nora Pharma’s operations.
Cash flows used in investing activities were $386,920
for the nine months ended September 30, 2023, compared to $0 for the nine months ended September 30, 2022. The increase was the result
of cash invested in Nora Pharma.
Cash flows provided by financing activities were
$3,456,106 during the nine months ended September 30, 2023, compared to $41,561,363 during the nine months ended September 30, 2022. The
decrease was primarily as a result of one offering made during the nine months ended September 30, 2023, compared to three offerings completed
in February, March, and April 2022, and to a lesser extent due to our repurchase of a total of $540,629 in common stock in the first and
third quarter of 2023.
As of December 31, 2022, we had cash and cash
equivalents of $21,826,437.
On February 17, 2022, we completed an underwritten
public offering of common stock and warrants for gross proceeds of $8 million. We received net proceeds of approximately $6.8 million
from the offering.
On March 14, 2022, we completed a private placement
of common stock and warrants for gross proceeds of $8 million. We received net proceeds of approximately $6.8 million from the private
placement.
On April 28, 2022, we completed a private placement
of common stock and warrants for gross proceeds of approximately $19.5 million. We received net proceeds of approximately $16.8 million
from the private placement.
During the fiscal year ended December 31, 2022,
we received aggregate proceeds of $13,193,177 in connection with warrant exercises.
During the year ended December 31, 2021, we issued
a total of 559,144 shares of our common stock valued at $12,705,214 for the conversion of outstanding notes payable, reducing the debt
by $2,867,243 and interest payable by $127,986 and generating a loss on conversion of $9,726,485.
During the year ended December 31, 2021, we did
not sell any of our capital stock for cash; however, we entered into the following new debt arrangements:
| · | On January 12, 2021, we issued a note in the
principal amount of $150,000 with interest accruing at 5% per year, due January 12, 2023. The note was convertible after 180 days from
issuance into common stock at a price of $0.30 per share. This note was converted to common stock on December 20, 2021. |
| | | |
| · | On January 27, 2021, we issued a note in the
principal amount of $300,000 with interest accruing at 5% per year, due January 27, 2023. The note was convertible after 180 days from
issuance into common stock at a price equal to $0.50 per share. This note was converted to common stock on December 20, 2021. |
| | | |
| · | On February 12, 2021, we issued a note in the
principal amount of $700,000 with interest accruing at 5% per year, due February 12, 2023. The note was convertible after 180 days from
issuance into common stock at a price of $0.60 per share. This note was converted to common stock on December 20, 2021. |
| | | |
| · | On April 5, 2021, we issued a note in the principal
amount of $330,000 with interest accruing at 10% per year, due January 5, 2022. The note was convertible after 180 days from issuance
into common stock at a price 35% below market value. On October 13, 2021, the noteholder converted $330,000 in principal and $16,500 in
accrued interest into 26,250 shares of common stock leaving a principal balance of $0. We repaid this note. |
| | | |
| · | On April 20, 2021, we issued a note in the principal
amount of $500,000 with interest accruing at 5% per year, due April 20, 2023. The note was convertible after 180 days from issuance into
common stock at a price of $0.30 per share. We repaid this note following the closing of our public offering in February 2022. |
| | | |
| · | On July 6, 2021, we issued a note in the principal
amount of $900,000 with interest accruing at 5% per year, due July 6, 2023. The note was convertible after 180 days from issuance into
common stock at a price of $0.30 per share. We repaid this note following the closing of our public offering in February 2022. In connection
with this debt financing, we agreed to allow the lender, who is also the holder of a note dated November 25, 2020, to convert a total
of $240,000 in principal into 120,000 shares of common stock leaving a principal balance of $10,000 and accrued interest of $7,750. On
July 6, 2021, we paid off the remaining principal balance of this note and received forgiveness of the accrued interest. |
| | | |
| · | On August 18, 2021, we issued a note in the principal
amount of $500,000 with interest accruing at 5% per year, due August 18, 2023. The note is convertible after 180 days from issuance into
common stock at a price equal to $0.30 per share. We repaid this note following the closing of our public offering in February 2022. |
Cash flows used in investing activities were $14,619,390
during the year ended December 31, 2022, compared to $0 during our fiscal year ended December 31, 2021. The reason for the increase was
due to the acquisition of Nora Pharma. Net cash flows provided by financing activities were $39,465,107 in 2022 compared to $2,904,675
in 2021. The increase was primarily a result of the three (3) rounds of financing which took place in February, March, and April 2022.
Net cash used in operations was $5,248,358 in 2022, compared to $1,829,128 in 2021. The reason for the increase was the acquisition of
Nora Pharma.
We are not generating adequate revenues from our
operations to fully implement our business plan as set forth herein. On February 17, 2022, we received net proceeds of approximately $6.8
million from the sale of common stock and warrants in an underwritten public offering. On March 14, 2022, we received net proceeds of
approximately $6.8 million from the sale of common stock and warrants in a private placement. On April 28, 2022, we received net proceeds
of approximately $16.8 million from the sale of common stock and warrants in a private placement. On May 16, 2023, we received net proceeds
of approximately $4.1 million from the sale of common stock and warrants in a private placement. We believe our existing cash will be
sufficient to fund our operations, including general and administrative expenses, research and development activities, and the generic
pharmaceuticals sales business, for the next 18 to 24 months. There is no assurance our estimates will be accurate.
Management estimates that we will need additional
capital in the amount of approximately $30 million for expansion of our drug development activities and generic pharmaceuticals operations,
including possibly a Phase I clinical trial. Additional capital may not be available on terms acceptable to us, or at all. Currently,
we do not have any committed arrangements for financing and can provide no assurance that we will be able to obtain financing when required.
No assurance can be given that we will obtain access to capital markets in the future or that financing, adequate to satisfy the cash
requirements of implementing our business will be available on acceptable terms. Our inability to obtain acceptable financing could have
an adverse effect upon the results of our operations and financial condition.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition
and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally
accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the
amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis,
we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the
circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not
readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Leases
We follow the guidance in ASC 842 “Accounting
for Leases,” as amended, which requires us to evaluate the lease agreements we enter into to determine whether they represent operating
or capital leases at the inception of the lease.
Our
wholly owned subsidiary, Nora Pharma, currently occupies a 23,500 square foot facility located at 1565 Boulevard Lionel-Boulet,
Varennes, Quebec, Canada, J3X 1P7 pursuant to a lease agreement that expires in January 2030, with an option to extend for 5 years.
This site is composed of 18,500 square feet of warehouse space and 5,000 square feet of executive office space. The facility houses
all administrative, marketing, quality control, regulatory affairs, and other operations personal, as well as a Health Canada
licensed warehouse space. We pay a monthly rent of $27,250 CAD (approximately $19,900 USD), including taxes.
Recently Adopted Accounting Standards
In February 2020, the FASB issued ASU 2020-02,
Financial Instruments-Credit Losses (Topic 326) and Leases (Topic 842) - Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting
Bulletin No. 119 and Update to SEC Section on Effective Date Related to Accounting Standards Update No. 2016-02, Leases (Topic 842) which
amends the effective date of the original pronouncement for smaller reporting companies. ASU 2016-13 and its amendments will be effective
for the Company for interim and annual periods in fiscal years beginning after December 15, 2022. The Company believes the adoption will
modify the way the Company analyzes financial instruments, but it does not anticipate a material impact on results of operations. The
Company is in the process of determining the effects adoption will have on its consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt
– Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity
(Subtopic 815 – 40), (“ASU 2020-06”). ASU 2020-06 simplifies the accounting for certain financial instruments with characteristics
of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU2020-06 amendments
are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted,
but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. The Company is
evaluating the impact of this guidance on its unaudited consolidated financial statements.
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet
arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition,
revenues or expenses, results of operations, liquidity, capital expenditures or capital resources and would be considered material to
investors.
BUSINESS
History
We were incorporated in the State of Colorado
on August 31, 2006, and on October 15, 2009, we acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition.
Sunshine Biopharma, Inc. held an exclusive license
to a new anticancer drug bearing the laboratory name, Adva-27a (the “License Agreement”). Upon completion of the reverse acquisition
transaction, we changed our name to Sunshine Biopharma, Inc. and began operating as a pharmaceutical company.
In December 2015, we acquired all worldwide issued
(US Patent Number 8,236,935, and 10,272,065) and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 for the Adva-27a anticancer
compound and terminated the License Agreement.
In early 2020, we initiated a new R&D project
focused on the development of a treatment for COVID-19 and on May 22, 2020, we filed a provisional patent application in the United States
for the new coronavirus treatment. The patent application covers composition subject matter pertaining to small molecules for inhibition
of the main Coronavirus protease, Mpro. On April 30, 2021, we filed a PCT application containing new research results and extending coverage
to include the Coronavirus Papain-Like protease, PLpro.
In June 2021, we initiated another R&D project
in which we set out to determine if certain mRNA molecules can be used as anticancer agents. The data obtained for mRNA molecules bearing
the laboratory name K1.1 became the subject of a new patent application filed in April 2022.
In October 2022, we acquired Nora Pharma, a Canadian
generic pharmaceuticals company based in the greater Montreal area. Nora Pharma has 41 employees and operates in a 23,500 square foot
facility certified by Health Canada. Nora Pharma currently sells 51 generic prescription drugs in Canada. The consolidated financial statements
contained in this prospectus include the results of operations of Nora Pharma and Sunshine Canada.
Generic Prescription Drugs on the Market
As a result of the acquisition of Nora Pharma
we now have the following generic prescription drugs on the market in Canada:
| Drug |
Action/Indication |
Reference Brand |
| Alendronate |
Osteoporosis |
Fosamax® |
| Amlodipine |
Cardiovascular |
Norvasc® |
| Apixaban |
Cardiovascular |
Eliquis® |
| Atorvastatin |
Cardiovascular |
Lipitor® |
| Azithromycin |
Antibacterial |
Zithromax® |
| Candesartan |
Hypertension |
Atacand® |
| Candesartan HCTZ |
Hypertension |
Atacand Plus® |
| Celecoxib |
Anti-inflammatory |
Celebrex® |
| Cetirizine |
Allergy |
Reactine® |
| Ciprofloxacin |
Antibiotic |
Cipro® |
| Citalopram |
Central nervous system |
Celexa® |
| Clindamycin |
Antibiotic |
Dalacin® |
| Clopidogrel |
Cardiovascular |
Plavix® |
| Dapagliflozin |
Diabetes |
Forxiga® |
| Donepezil |
Central nervous system |
Aricept® |
| Duloxetine |
Central nervous system |
Cymbalta® |
| Dutasteride |
Urology |
Avodart® |
| Escitalopram |
Central nervous system |
Cipralex® |
| Ezetimibe |
Cardiovascular |
Ezetrol® |
| Finasteride |
Urology |
Proscar® |
| Flecainide |
Cardiovascular |
Tambocor® |
| Fluconazole |
Antifungal |
Diflucan® |
| Fluoxetine |
Central nervous system |
Prozac® |
| Hydroxychloroquine |
Antimalarial |
Plaquenil® |
| Lacosamide |
Central nervous system |
Vimpat® |
| Letrozole |
Oncology |
Femara® |
| Levetiracetam |
Central nervous system |
Keppra® |
| Mirtazapine |
Central nervous system |
Remeron® |
| Metformin |
Diabetes |
Glucophage® |
| Montelukast |
Allergy |
Singulair® |
| Olmesartan |
Cardiovascular |
Olmetec® |
| Olmesartan HCTZ |
Cardiovascular |
Olmetec Plus® |
| Pantoprazole |
Gastroenterology |
Pantoloc® |
| Paroxetine |
Central nervous system |
Paxil® |
| Perindopril |
Cardiovascular |
Coversyl® |
| Pravastatin |
Cardiovascular |
Pravachol® |
| Pregabalin |
Central nervous system |
Lyrica® |
| Quetiapine |
Central nervous system |
Seroquel® |
| Quetiapine XR |
Central nervous system |
Seroquel XR® |
| Ramipril |
Cardiovascular |
Altace® |
| Rizatriptan ODT |
Central nervous system |
Maxalt® ODT |
| Rosuvastatin |
Cardiovascular |
Crestor® |
| Sertraline |
Central nervous system |
Zoloft® |
| Sildenafil |
Urology |
Viagra® |
| Tadalafil |
Urology |
Cialis® |
| Telmisartan |
Cardiovascular |
Micardis® |
| Telmisartan HCTZ |
Cardiovascular |
Micardis Plus® |
| Topiramate |
Anticonvulsant |
Topamax® |
| Tramadol Acetaminophen |
Central nervous system |
Tramacet® |
| Zolmitriptan |
Central nervous system |
Zomig® |
| Zopiclone |
Central nervous system |
Imovane® |
Generic Prescription Drugs Pipeline
In addition to the 51 drugs on the market, we
currently have the following roster of generic prescription drugs scheduled to be launched in 2024 and 2025:
| Generic Drugs |
Therapeutic Area(s) |
Development Stage |
Launch Date |
| Group A (2 Products) |
Cardiovascular, CNS* |
Under manufacturing |
2024Q1 |
| Group B (6 Products) |
Oncology, Gastroenterology, CNS* |
Under regulatory review |
2024Q2 |
| Group C (3 Products) |
Central Nervous System, Diabetes, CNS* |
Under regulatory review |
2024Q3 |
| Group D (5 Products) |
Cardiovascular, Urology, Endocrinology |
Under regulatory review |
2024Q4 |
| Group E (16 Products) |
Cardiovascular, Oncology, Anti-infectives, Anti-
inflammatory, Diabetes, Gastroenterology, CNS* |
Soon to be under regulatory
review |
2025 |
* Central Nervous System
We believe the addition of these products to our
existing portfolio will strengthen our presence in the Canadian generic drugs marketplace and provide us with greater access to pharmacies
as we become more of a go-to supplier for every-day and specialty medicines.
Proprietary Drugs in Development
We are currently developing the following drug
candidates:
| Proprietary Drugs |
Therapeutic Area |
Development Stage |
Launch Date |
| Adva-27a (Small Molecule) |
Oncology (Pancreatic Cancer) |
Paused (See below) |
TBD* |
| K1.1 (mRNA LNP) |
Oncology (Liver Cancer) |
Preclinical |
TBD* |
| SBFM-PL4 (Small Molecule) |
Antiviral (COVID-19) |
Preclinical |
TBD* |
* To be determined
Adva-27a Anticancer Drug
Adva-27a is a small molecule designed for the
treatment of aggressive forms of cancer. A Topoisomerase II inhibitor, Adva-27a has been shown to be effective at destroying Multidrug
Resistant Cancer cells including Pancreatic Cancer cells, Breast Cancer cells, Small-Cell Lung Cancer cells and Uterine Sarcoma cells
(Published in ANTICANCER RESEARCH, Volume 32, Pages 4423-4432, October 2012). We are the direct owner of all patents pertaining to Adva-27a
including U.S. Patents Number 8,236,935 and 10,272,065.
In December 2022, we entered into a research agreement
with the Jewish General Hospital (“JGH”), to conduct the IND-enabling studies of Adva-27a (the “Research Agreement”).
In August 2023, we were informed by the JGH that the lab results on testing of the Adva-27a molecule were not favorable. After conclusion
of an internal review of the lab results on November 2, 2023, we provided notice to JGH of termination of the Research Agreement. We have
now paused the IND-enabling studies of Adva-27a pending a review of the possibility of chemical modification of the compound to address
the suboptimal performance of the molecule in certain studies.
K1.1 Anticancer mRNA
In June 2021, we initiated a new research project
in which we set out to determine if certain mRNA molecules can be used as anti-cancer agents. The data collected to date have shown that
a selected group of mRNA molecules are capable of destroying cancer cells in vitro including multidrug resistant breast cancer cells (MCF-7/MDR),
ovarian adenocarcinoma cells (OVCAR-3), and pancreatic cancer cells (SUIT-2). Studies using non-transformed (normal) human cells (HMEC
cells) showed that these mRNA molecules had little cytotoxic effects. These new mRNA molecules, bearing the laboratory name K1.1, are
readily adaptable for delivery into patients using the mRNA vaccine technology. In April 2022, we filed a provisional patent application
in the United States covering the subject mRNA molecules.
We recently concluded an agreement with a specialized
partner for the purposes of formulating our K1.1 mRNA molecules into lipid nanoparticles, ready for use to conduct studies in xenograft
mice. Such xenograft, and other, studies are currently underway.
SBFM-PL4 Coronavirus Treatment
The initial genome expression products following
infection by Betacoronavirus, the causative agent of COVID-19, are two large polyproteins, referred to as pp1a and pp1ab. These two polyproteins
are cleaved at 15 specific sites by two virus encoded proteases, called Mpro and PLpro, to generate 16 different non-structural proteins
essential for viral replication. Mpro and PLpro represent attractive anti-viral drug development targets as they play a central role in
the early stages of viral replication. PLpro is of particular interest as a therapeutic target in that, in addition to processing essential
viral proteins, it is also responsible for suppression of the human immune system making the virus more life-threatening. PLpro is present
only in Betacoronaviruses, the subgroup of Coronaviruses represented by the highly pathogenic SARS-CoV, MERS-CoV, and SARS-CoV-2.
Our Anti-Coronavirus research effort has been
focused on developing an inhibitor of PLpro and, on May 22, 2020, we filed a patent application in the United States covering composition
subject matter pertaining to small molecules for inhibition of the Coronavirus PLpro as well as Mpro.
In February 2022, we expanded our PLpro inhibitors
research effort by entering into a research agreement with the University of Arizona for the purposes of conducting research focused on
determining the in vivo safety, pharmacokinetics, and dose selection properties of three University of Arizona owned PLpro inhibitors,
to be followed by efficacy testing in mice infected with SARS-CoV-2 (the “Research Project”). Under the agreement, the University
of Arizona granted the Company a first option to negotiate a commercial, royalty-bearing license for all intellectual property developed
by University of Arizona under the Research Project. In addition, the Company and the University of Arizona entered into an option agreement
(the “Option Agreement”) whereby the Company was granted a first option to negotiate a royalty-bearing commercial license
for the underlying technology of the Research Project. On September 13, 2022, we exercised our options, and on February 24, 2023, we entered
into an exclusive worldwide license agreement with the University of Arizona for all of the technology related to the Research Project.
We have recently expanded our objective to include
the development of an injectable candidate of first-in-class PLpro inhibitor to treat SARS-CoV2 and potentially SARS-CoV and MERS-CoV
infection in patients who could not use Paxlovid, Molnupiravir, or Remdesivir, due to concerns about drug interaction and possible ‘rebound’
infections and other side effects.
Intellectual Property
We are the sole owner of all worldwide rights
pertaining to Adva-27a. These patent rights are covered by PCT/FR2007/000697 and PCT/CA2014/000029. The patent applications filed under
these two PCT's have been issued in the United States (US Patent Number 8,236,935 and 10,272,065), Europe, and Canada.
On May 22, 2020, we filed a provisional patent
application in the United States for a new treatment for Coronavirus infections. Our patent application covers composition subject matter
pertaining to small molecules for inhibition of the main Coronavirus protease, Mpro, an enzyme that is essential for viral replication.
The patent application has a priority date of May 22, 2020. On April 30, 2021, we filed a PCT application containing new research results
and extending coverage to include the Coronavirus Papain-Like protease, PLpro. The priority date of May 22, 2020, has been maintained
in the newly filed PCT application.
On April 20, 2022, we filed a provisional patent
application in the United States covering mRNA molecules capable of destroying cancer cells in vitro. The patent application contains
composition and utility subject matter pertaining to the structure and sequence of the relevant mRNA molecules.
Our wholly owned subsidiary, Nora Pharma, owns
180 Drug Identification Numbers (“DIN’s”) issued by Health Canada for prescription drugs currently on the market in
Canada. These DIN’s were secured through in-licenses or cross-licenses from international manufacturers of generic pharmaceutical
products.
In addition, we are the owner of two Natural Product
Numbers (“NPN’s”) issued by Health Canada: NPN 80089663 authorizes us to manufacture and sell our in-house developed
OTC product, Essential 9™, and NPN 80093432 authorizes us to manufacture and sell the OTC product, Calcium-Vitamin D under the brand
name Essential Calcium-Vitamin D™.
Manufacturing
Our generic drugs are
manufactured by several different international partners under long-term contracts.
We currently do not have
any proprietary drugs on the market. Research quantities of our proprietary drug candidates are currently manufactured at the University
of Arizona located in Tucson, Arizona (Anti-Coronavirus compounds), WuXi App Tech located in Hong Kong, China (Adva-27a compound), and
Arranta Bio MA LLC located in Watertown, Massachusetts (K1.1 mRNA).
Our OTC products are
manufactured under contract by INOV Pharma Inc. located in Montreal, Canada.
Marketing and Sales
Our generic drugs are
currently being sold across Canada. All of our generic drug sales are conducted by Nora Pharma’s sales representatives based in
key Provinces across Canada. In addition, a segment of our marketing team offers human resources and commercial assistance to pharmacies
and pharmacy owners by providing experienced pharmacists and technical assistant recruitment services as well as training and education
support.
Our OTC products are
currently sold in the U.S. and Canada through Amazon.com and Amazon.ca, respectively. Our personnel together with outside consultants
develop and place ads on Google, YouTube, Amazon, and other media outlets. The same team manages our accounts with Amazon.
Government Regulations
All of our business operations,
including our generic drugs, proprietary drugs, and OTC products operations, are subject to extensive and frequently changing federal,
state, provincial and local laws and regulations.
In the United States,
the Federal Government agency responsible for regulating prescription drugs and nonprescription OTC supplements is the U.S. Food and Drug
Administration (“FDA”). The Canadian counterpart to the FDA is Health Canada. Though the FDA and Health Canada have generally
similar requirements for drugs and OTC supplements to be approved or allowed to be marketed, approval in one jurisdiction does not automatically
result in approval in the other. In Canada, prescription drugs and nonprescription OTC supplements are authorized through the issuance
by Health Canada of a Drug Identification Number (DIN) for the former and a Natural Product Number (NPN) for the latter. In the United
States, the marketing of OTC supplements does not require prior approval from the FDA, provided that the ingredients are known to the
FDA. In both the U.S. and Canada, the ingredients, manufacturing processes and facilities for all drugs and OTC supplements must meet
the guidelines for Good Manufacturing Practices (“GMP”). Moreover, all drug manufacturers must perform a series of tests,
both during and after production, to show that every drug or supplement batch made meets the regulatory requirements for that product.
Our generic prescription
medicines are produced following the same Good Manufacturing Practices (GMP) guidelines as for brand-name drugs. Prescription drugs dossiers
are filed with Health Canada in order to obtain a manufacturing Notice of Compliance (NOC) and a Drug Identification Number (DIN). The
same grant the applicant marketing authorization in Canada. In the case of Nora Pharma’s products, Nora Pharma secures cross-licenses
from supply partners holding NOC’s and in turn applies to Health Canada to obtain DIN’s issued in Nora Pharma’s name
in order to commercialize in Canada. In Canada, the pan-Canadian Pharmaceutical Alliance (pCPA), an alliance of the provincial, territorial
and federal governments that collaborates on a range of public drug plan initiatives to increase and manage access to clinically effective
and affordable drug treatments, determines generic drugs pricing based on a percentage of the brand-name reference products.
In the area of proprietary
drug development where our Anti-Coronavirus and Anti-Cancer compounds fall, we will be subject to significant regulations in the U.S.
in order to obtain approval of the FDA to offer our products for sale. The approximate procedure for obtaining FDA approval involves
an initial filing of an IND application following which the FDA would review and allow for the drug developer to proceed with Phase I
clinical trials. Following completion of Phase I, the results are filed with the FDA and a request is made to proceed to Phase II. Similarly,
following completion of Phase II the data are filed with the FDA and a request is made to proceed to Phase III. Following completion
of Phase III, a new drug application, or NDA is submitted and a request is made for marketing approval. Depending on various issues and
considerations, the FDA could provide “emergency use authorization” or limited approval for “compassionate-use”
if the drug treats terminally ill patients with limited other treatment options available. As of the date of the filing of this prospectus,
we have not made any filings with the FDA or other regulatory bodies in other jurisdictions. We anticipate filing an initial IND application
for an anti-Covid-19 compound within approximately one year and filing an initial IND for our anti-cancer compound within approximately
two years. We have however had discussions with clinicians and as a result we believe that the FDA and Health Canada are likely to
grant us a so-called “fast-track” process on the basis of the ongoing Covid-19 pandemic and the terminal nature of the cancer
type we are planning to treat. There are no assurances this will occur.
In connection with OTC
supplements, the FDA regulates the formulation, manufacturing, packaging, storage, labeling, promotion, distribution, and sale of such
products, while the Federal Trade Commission (“FTC”) regulates marketing and advertising claims. In August 2007, a rule issued
by the FDA went into effect requiring companies that manufacture, package, label, distribute or hold OTC supplements to meet certain GMP
requirements to ensure such products are of the quality specified and are properly packaged and labeled. We are committed to meeting or
exceeding the standards set by the FDA and the FTC and we believe we are currently operating within both the FDA and FTC mandates.
Employees
As of the date of this
prospectus we have a total of 46 employees, composed of our management team (5)
and employees of Nora Pharma (41).
Presently, our proprietary
drug development activities are subcontracted out to specialized service providers in the U.S. and Canada. We also use consultants for
various other activities including legal, marketing, accounting, and IT.
Labors laws in Quebec
provide for certain guaranteed minimum entitlements, including minimum wages, maternity leave, medical leave, employee termination conditions,
etc. Moreover, the Province of Quebec has various language laws governing language use. These laws require corporate operations carried
out in the Province of Quebec to be conducted to a large extent, and some cases entirely, in French. We and our Canadian subsidiaries
operating in the Province of Quebec are fully compliant with these laws.
Competition
The Canadian generic
pharmaceuticals market is valued at approximately $7.2 billion CAD (approximately $5.3 billion USD). Generic pharmaceutical companies
produce and deliver more than 70% of the prescribed medicines with high quality at affordable prices. There are more than 35 active generic
players in the market, of which, the top 3 hold approximately 50% share of the market. Nora Pharma is relatively new in this space but
has demonstrated one of the fastest year-over-year sales increase amongst its peers.
Our Anti-Coronavirus
drug development project is in direct competition with several companies in the U.S. that have developed effective vaccines or treatment
options for Covid-19. The companies focused on treatments include Pfizer, Merck, Gilead, Eli Lilly, and Regeneron. Today two leading vaccines
(Pfizer’s, and Moderna’s) and two antibody treatments (Regeneron’s, and Eli Lilly’s) are in use. Gilead’s
Remdesivir, an antiviral injectable, was approved by the FDA for treatment of Covid-19 in October 2020. In addition, in December 2021,
Pfizer received Emergency Use Authorization (“EUA”), for its antiviral pill, Paxlovid, and, in the same month, the FDA granted
Merck EUA for its antiviral pill, Molnupiravir. While the approved vaccines, pills and injectable treatments are effective, we believe
that additional treatment options such as the one we are developing which targets a different part of the virus could potentially form
an important component of the range of anti-coronavirus treatment options available to attending physicians.
In the area of anticancer
drug development, we compete with large publicly and privately held companies engaged in developing new cancer therapies. There are numerous
other entities engaged in oncology therapeutics development that have greater resources than the resources presently available to us.
Nearly all major pharmaceutical companies including Merck, Amgen, Roche, Pfizer, Bristol-Myers Squibb and Novartis, to name a few, have
on-going anticancer drug development programs and some of the drugs they may develop could be in direct competition with our own. In addition,
a number of smaller companies are working in the area of cancer therapy and could develop drugs that may be in competition with ours.
Similarly, our OTC products
fall directly within a very crowded and highly competitive product sector. As of the date of this prospectus, we believe Essential 9™
is the only Essential Amino Acid product that comprises all 9 essential amino acids in capsule form. We believe this may provide us with
a competitive advantage, at least for the near future but there are no assurances that this will occur.
Properties
Our principal place of business is located at
1177 Avenue of the Americas, 5th Floor, New York, NY 10036, pursuant to a month-to-month arrangement and a pay-per-use plan. Our minimum
monthly rent is $289.00.
Our wholly owned subsidiary, Nora Pharma, currently
occupies a 23,500 square foot facility located at 1565 Boulevard Lionel-Boulet, Varennes, Quebec, Canada, J3X 1P7 pursuant to a lease
agreement that expires in January 2030, with an option to extend for 5 years. This site is composed of 18,500 square feet of warehouse
space and 5,000 square feet of executive office space. The facility houses all administrative, marketing, quality control, regulatory
affairs, and other operations personal, as well as a Health Canada licensed warehouse space. We pay a monthly rent of $27,250 CAD (approximately
$19,900 USD), including taxes.
Legal Proceedings
We are not party to, and our property is not the subject of, any material
legal proceedings.
MANAGEMENT
Directors and Executive Officers
The following table and biographical summaries
set forth information, including principal occupation and business experience about our directors and executive officers:
| Name |
|
Age |
|
Position(s) |
| |
|
|
|
|
| Dr. Steve N. Slilaty |
|
71 |
|
President, Chief Executive Officer and Chairman |
| |
|
|
|
|
| Dr. Abderrazzak Merzouki |
|
59 |
|
Chief Science Officer and Director |
| |
|
|
|
|
| Camille Sebaaly |
|
62 |
|
Chief Financial Officer and Secretary |
| |
|
|
|
|
| Dr. Rabi Kiderchah |
|
51 |
|
Director |
| |
|
|
|
|
| David Natan |
|
70 |
|
Director |
| |
|
|
|
|
| Dr. Andrew Keller |
|
70 |
|
Director |
| |
|
|
|
|
| Malek Chamoun |
|
39 |
|
Chief Development Officer and President of Nora Pharma Inc. |
| |
|
|
|
|
| Marc Beaudoin |
|
57 |
|
Chief Operating Officer |
Dr. Steve N. Slilaty was appointed
as our chief executive officer and chairman of our board of directors on October 15, 2009. Dr. Slilaty is an accomplished scientist
and business executive. His scientific publications are widely cited. Sunshine Biopharma is the third in a line of biotechnology companies
that Dr. Slilaty founded and managed. The first, Quantum Biotechnologies Inc. later known as Qbiogene Inc., was founded
in 1991 and is now a member of a family of companies owned by MP Biomedicals, one of the largest international suppliers of biotechnology
reagents and other research products. The second company which Dr. Slilaty founded, Genomics One Corporation, conducted an
initial public offering of its capital stock in 1999 and, on the basis of its ownership of Dr. Slilaty’s patented TrueBlue Technology,
Genomics One became one of the key participants in the Human Genome Project and reached a market capitalization of $1 billion in
2000. Formerly, Dr. Slilaty was a research team leader at the Biotechnology Research Institute (Montreal), a division of the National
Research Council of Canada. Dr. Slilaty is one of the pioneers of Gene Therapy having developed the first gene delivery system applicable
to humans in 1983 [Science 220: 725-727 (1983)]. Dr. Slilaty's other distinguished scientific career accomplishment was
the discovery of a new class of enzymes, the S24 Family of Proteases (IUBMB Enzyme: EC 3.4.21.88) [Proc. Natl. Acad. Sci. U.S.A. 84:
3987-3991 (1987)]. In addition, Dr. Slilaty (i) developed the first site-directed mutagenesis system applicable to double-stranded
DNA [Analyt. Biochem. 185: 194-200 (1990)], (ii) cloned the gene for the first yeast-lytic enzyme (lytic b-1,3-glucanase)
[J. Biol. Chem. 266: 1058-1063 (1991)], (iii) developed a new molecular strategy for increasing the rate of enzyme reactions
[Protein Engineering 4: 919-922 (1991)], and (iv) constructed a powerful new cloning system for genomic sequencing (TrueBlue®
Technology) [Gene 213: 83-91 (1998)]. Most recently, Dr. Slilaty, in collaboration with Institut National des Sciences Appliquée
(France), State University of New York at Binghamton (USA) and École Polytechnique, Université de Montréal (Canada),
designed, patented, and advanced the development the first, and currently the only known anticancer compound (Adva-27a) capable of destroying
multidrug resistant cancer cells [Anticancer Res. 32: 4423 (2011) and US Patent Numbers: 8,236,935 and 10,272,065]. These
and other works of Dr. Slilaty are cited in research papers, editorials, review articles and textbooks. Dr. Slilaty is the author of 18
original research papers and 10 issued and pending. These and other works of Dr. Slilaty are cited in research papers, editorials, review
articles and textbooks. Dr. Slilaty received his Ph.D. degree in Molecular Biology from the University of Arizona in 1983 and Bachelor
of Science degree in Genetics and Biochemistry from Cornell University in 1976. Dr. Slilaty has received research grants from the NIH
and NSF and he is the recipient of the 1981 University of Arizona Foundation award for Meritorious Performance in Teaching. Dr. Slilaty’s
scientific knowledge and experience qualifies him to serve on our board of directors.
Dr. Abderrazzak Merzouki has served
as a director since February 2016 and as chief science officer since January 2024. He served as chief operating officer from February
2016 to January 2024. In addition to his positions with our Company since January 2016 he has been self-employed as a consultant in the
fields of biotechnology and pharmacology. From July 2007 through December 2016, Dr. Merzouki worked at the Institute of Biomedical Engineering
in the Department of Chemical Engineering at Ecole Polytechnique de Montreal, where he taught and acted as a senior scientist involved
in the research and development of plasmid and siRNA-based therapies. Dr. Merzouki is a molecular biologist and an immunologist with extensive
experience in the area of gene therapy where he performed several preclinical studies for pharmaceutical companies involving the use of
adenoviral vectors for cancer therapy and plasmid vectors for the treatment of peripheral arterial occlusions. Dr. Merzouki also
has extensive expertise in the design of expression vectors, and production and purification of recombinant proteins. He developed technologies
for production of biogeneric therapeutic proteins for the treatment of various diseases including cancer, diabetes, hepatitis and multiple
sclerosis. Dr. Merzouki obtained his Ph.D. in Virology and Immunology from Institut Armand-Frappier in Quebec and received his post-doctoral
training at the University of British Columbia and the BC Center for Excellence in HIV/AIDS research. Dr. Merzouki has over 30 publications
and 70 communications in various, highly respected scientific journals in the field of cellular and molecular biology. Dr. Merzouki’s
scientific knowledge and experience qualifies him to serve on our board of directors.
Camille Sebaaly was appointed as
our chief financial officer, secretary and a director of our Company on October 15, 2009. He resigned as a director of the Company in
October 2021. Since 2001, Mr. Sebaaly has been self-employed as a business consultant, primarily in the biotechnology and biopharmaceutical
sectors. He held a number of senior executive positions in various areas including financial management, business development, project
management and finance. As an executive and an entrepreneur, he combines expertise in strategic planning and finance with strong skills
in business development and deal structure and negotiations. In addition, Mr. Sebaaly worked in operations, general management, investor
relations, marketing and business development with emphasis on international business and marketing of advanced technologies including
hydrogen generation and energy saving. In the area of marketing, Mr. Sebaaly has evaluated market demands and opportunities, created strategic
marketing and business development plans, designed marketing communications and launched market penetration programs. Mr. Sebaaly graduated
from State University of New York at Buffalo with an Electrical and Computer Engineering Degree in 1987.
Dr. Rabi Kiderchah has served as
a director of the Company since October 2021. Dr. Kiderchah is a licensed physician in Canada. From 2000 until August 2021, he was working
at Argenteuil Hospital, Lachute, Quebec, Canada, as an emergency room physician. He has also worked as what is referred to in Canada as
a “medecins depanneurs”, working in rural areas where there are not enough ER doctors. Since August 2011 he has worked at
Rabi Kiderchah Medecin Inc. as a freelance physician in the Quebec, Canada area. He received a Bachelor of Science degree in 1994 and
an MD degree in 1998 from the University of Montreal. Dr. Kiderchah’s medical and scientific knowledge and experience qualifies
him to serve on our board of directors.
David Natan has served as a director
of the Company since February 2022. He currently serves as CEO of Natan & Associates, LLC, a consulting firm offering CFO services
to public and private companies since 2007. From February 2010 to May 2020, Mr. Natan served as CEO of ForceField Energy, Inc. (OTCMKTS:
FNRG), a company focused on LED lighting products. From February 2002 to November 2007, Mr. Natan served as CFO of PharmaNet Development
Group, Inc., a drug development company, and, from June 1995 to February 2002, as CFO and VP of Global Technovations, Inc., a manufacturer
and marketer of speaker components. Prior to that, Mr. Natan served in various roles with Deloitte & Touche LLP. From April 2020 through
June 2023, Mr. Natan was Executive Vice President and Chief Financial Officer for Airborne Motorworks, Inc., Spokane, WA, a privately-held
aerospace transportation company. Mr. Natan currently serves as a member of the Board of Directors and Chair of the Audit Committee of
NetBrands, Inc. (OTC: NBND), a distributor of snack products, since February 2021; and serves as a member of the Board of Directors and
Chair of the Audit Committee of Titan Pharmaceuticals Inc. (NASDAQ: TTNP) a pharmaceutical company, since August 2022. Additionally, in
November 2023, Mr. Natan was appointed to the board of Directors and Audit Committee Chair of Minim Inc. (NASDAQ: MINM). Mr. Natan holds
a B.A. in Economics from Boston University. Mr. Natan’s experience as a business executive and as a director and chairperson of
audit committees for public companies qualifies him to serve on our board of directors.
Dr. Andrew M. Keller has served
as a director of the Company since February 2022. From 2016 through November 2019, Dr. Keller was the Chief Medical Officer at the Western
Connecticut Medical Group, Bethel CT, a multispecialty organization. He was employed by this group beginning in 1989, and in 2003 became
Chief – Section of Cardiovascular Diseases. In 2014 he was appointed Chief Medical Informatics Officer. Previously, Dr. Keller was
an Assistant Professor of Medicine/Radiology at Columbia University, The College of Physicians and Surgeons, NY, NY. Dr. Keller retired
as a practicing physician in 2019. Upon his retirement as a practicing physician Dr. Keller enrolled as a full time student at Quinnipiac
University College of Law, where he graduated with a Juris Doctor degree in 2023. In July 2023, Dr. Keller passed the Bar exam and was
admitted to practice law in the State of Connecticut in November 2023. Since November 2023 he has been employed at the Law Office of Robin
P. Keller LLC, Norwalk, CT advocating for the educational needs of disabled children with medically complex diagnoses. Dr. Keller received
a Doctor of Medicine degree in 1979 from The Ohio State University and a Bachelor of Arts degree in Physics, Magna Cum Laude from Ithaca
College in 1975. Dr. Keller’s medical, scientific and legal knowledge and experience qualify him to serve on our board of directors.
Malek Chamoun was appointed as our
Chief Development Officer in January 2024. In addition, he is President of Nora Pharma, Inc., our wholly owned subsidiary that we acquired
in October 2022. In 2017 he founded Nora Pharma, where he has been the President and CEO since inception. Mr. Chamoun received a bachelor’s
degree in business administration from Hautes Études Commerciales, Montreal, Quebec, Canada in 2008 and became a licensed CPA in
Canada in 2012. He devotes all of his business time to Nora Pharma’s affairs.
Marc Beaudoin was appointed as our
Chief Operating Officer in January 2024. Mr. Beaudoin was the sole owner of M.A. Beaudoin Consulting Group Inc., a privately held business
strategy consulting company in the Canadian pharmaceutical and biopharmaceutical sectors since 2016. From January 2018 through February
2019, he was employed by the KDA Group, Inc., a publicly held Canadian healthcare company, as the COO of KDA Group and CEO of its Canadian
generic pharmaceutical division, Pharapar. From 2006 to 2016, he held several executive positions at Sandoz Canada in various areas including
Marketing and Communications, Strategic Planning, Business Development & Portfolio Management. As an executive and an entrepreneur,
he combines expertise in strategic planning with operational and commercial execution. Mr. Beaudoin obtained his MBA from Sherbrooke University
in 2018. He also holds multiple certifications (including a fellowship) from the Association for Supply Chain Management.
Board of Directors Term of Office
Directors are elected at our annual meeting of
shareholders and serve for one year until the next annual meeting of shareholders or until their successors are elected and qualified.
Director Independence
Our independent directors consist of Dr. Kidershah,
Mr. Natan and Dr. Keller.
Committees of our Board of Directors
The Company has established an audit committee,
a compensation committee, and a corporate governance and nominating committee of our board of directors, each of which is comprised of
each of our independent directors.
No Family Relationships
There is no family relationship between any director
and executive officer or among any directors or executive officers.
Involvement in Certain Legal Proceedings
Our directors and executive officers have not
been involved in any of the following events during the past ten years:
| |
1. |
any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| |
|
|
| |
2. |
any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| |
3. |
being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| |
|
|
| |
4. |
being found by a court of competent jurisdiction in a civil action, the SEC or the CFTC to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| |
|
|
| |
5. |
being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| |
|
|
| |
6. |
being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Code of Ethics
We have adopted a Code of Ethics that applies
to our principal executive officer, principal financial officer, and principal accounting officer. Our Code of Ethics is available on
our website at www.sunshinebiopharma.com.
Executive Compensation
The following table sets forth compensation information
for services rendered by our executive officers in all capacities during the last two completed fiscal years.
| Name and Principal Position |
|
Year |
|
Salary
($) |
|
Bonus
($) |
|
Stock
Awards
($) |
|
All Other Compensation
($) |
|
Total
($) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Dr. Steve N. Slilaty |
|
2022 |
|
360,000 |
|
10,000 |
|
– |
|
– |
|
370,000 |
|
| Chief Executive Officer and Director |
|
2023 |
|
378,000 |
|
182,000 |
|
– |
|
– |
|
560,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Camille Sebaaly |
|
2022 |
|
300,000 |
|
630,000 |
|
– |
|
– |
|
930,000 |
|
| Chief Financial Officer |
|
2023 |
|
300,000 |
|
420,000 |
|
– |
|
– |
|
720,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dr. Abderrazzak Merzouki |
|
2022 |
|
240,000 |
|
245,000 |
|
– |
|
– |
|
485,000 |
|
| Chief Operating Officer and Director |
|
2023 |
|
240,000 |
|
– |
|
– |
|
– |
|
240,000 |
|
Employment Agreements
On April 8, 2022, we entered into
an employment agreement with Dr. Steve N. Slilaty, our Chief Executive Officer. Pursuant to the employment agreement, Dr. Slilaty will
continue to serve as our CEO and will be paid a base annual salary of $360,000 (which will increase annually at the rate of the Consumer
Price Index or 5%, whichever is higher). The employment agreement has a term of four years and will renew automatically for a term of
an additional three years. In the event the employment agreement is terminated by the Company without cause, the Company will pay Dr.
Slilaty $10 million. Upon expiration of the employment agreement, the Company will pay Dr. Slilaty $2 million.
Outstanding Equity Awards at 2023 Fiscal Year-End
We did not have any outstanding equity awards
as of December 31, 2023.
Director Compensation
The following table sets forth
compensation we paid to our directors during the year ended December 31, 2023.
| Name |
|
Fees Earned or Paid in Cash ($) |
|
Stock Awards |
|
Option Awards |
|
All Other Compensation |
|
Total ($) |
| Dr. Rabi Kiderchah |
|
80,000 |
|
– |
|
– |
|
– |
|
80,000 |
| Mr. David Natan |
|
80,000 |
|
– |
|
– |
|
– |
|
80,000 |
| Dr. Abderrazzak Merzouki |
|
80,000 |
|
– |
|
– |
|
– |
|
80,000 |
| Dr. Andrew Keller |
|
80,000 |
|
– |
|
– |
|
– |
|
80,000 |
| Dr. Steve N. Slilaty |
|
80,000 |
|
– |
|
– |
|
– |
|
80,000 |
TRANSACTIONS WITH RELATED PERSONS
A note payable dated December 31, 2019, held by
our chief executive officer, having a face value of $128,269 and accruing interest at 12% was due December 31, 2020. On December 31, 2020,
we renewed the note together with accrued interest of $15,392 for a 12-month period. The new note had a face value of $143,661, accrued
interest at 12% per year, and had a maturity date of December 31, 2021. On August 24, 2021, we paid off the entire principal balance of
this note, together with accrued interest of $12,929 by making a cash payment of $156,590.
On February 22, 2022, we redeemed 990,000 shares
of Series B Preferred Stock held by Dr. Steve Slilaty, our CEO, at a redemption price equal to the stated value of $0.10 per share.
On February 8, 2024, the Company issued and sold 20,000 shares
of Series B Preferred Stock to Dr. Steve Slilaty for a purchase price equal to the stated value of $0.10 per share.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND MANAGEMENT
The
following table sets forth certain information regarding the ownership of common stock as
of February 8, 2024, by (i) each of our directors, (ii) each of our executive officers,
(iii) all of our directors and executive officers as a group, and (iv) any person or group
as those terms are used in Section 13(d)(3) of the Exchange Act, believed by us to beneficially
own more than 5% of our common stock. Unless otherwise indicated, all shares are owned directly
and the indicated person has sole voting and investment power. The percentages listed are
based upon 28,024,290 common shares issued and outstanding and 30,000 Series B Preferred
shares outstanding as of February 8, 2024. Unless otherwise indicated, the address of
each holder is c/o Sunshine Biopharma, Inc., 1177 Avenue of the Americas, 5th Floor, New
York, NY 10036.
| Title of Class |
|
Name and Address of Beneficial Owner |
|
Amount and Nature of Beneficial Ownership |
|
|
Percent of Class |
|
| |
|
|
|
|
|
|
|
|
| Common |
|
Dr. Steve N. Slilaty(1) |
|
|
3,821,024 |
(3) |
|
|
13.7% |
|
| Preferred |
|
|
|
|
30,000 |
(2) |
|
|
100% |
|
| |
|
|
|
|
|
|
|
|
|
|
| Common |
|
Camille Sebaaly(1) |
|
|
174,465 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
|
| Common |
|
Dr. Abderrazzak Merzouki(1) |
|
|
116,720 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
|
| Common |
Dr. Andrew Keller(1) |
|
|
0 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
| Common |
David Natan(1) |
|
|
0 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
| Common |
Dr. Rabi Kiderchah(1) |
|
|
1,625 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
| Common |
|
Malek Chamoun(1) |
|
|
3,700,000 |
(3) |
|
|
13.2% |
|
| |
|
|
|
|
|
|
|
|
|
|
| Common |
|
Marc Beaudoin(1) |
|
|
0 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Common |
|
All Officers and Directors as Group (8 persons): |
|
|
4,113,834 |
(2)(3) |
|
|
14.7% |
|
___________________
* Less than 1%.
| |
(1) |
Officer and/or director of our Company. |
| |
(2) |
Includes 30,000
shares of the Company’s Series B Preferred Stock. Each share of Series B Preferred Stock is entitled to 1,000 votes. |
| |
(3) |
Includes 3,700,000 common shares owned by Malek Chamoun, the President of Nora Pharma Inc., a company acquired by the Company in October 2022. Dr. Slilaty controls the voting of Mr. Chamoun’s shares through a voting agreement between Mr. Chamoun and Dr. Slilaty dated October 20, 2022. |
DESCRIPTION OF CAPITAL STOCK
General
Our authorized capital stock consists of 3,000,000,000
shares of common stock, par value of $0.001 per share, and 30,000,000 shares of preferred stock, par value $0.10 per share. 1,000,000
shares of our preferred stock are designated as Series B Preferred Stock.
As of February 8, 2024, there were 28,024,290
shares of our common stock, outstanding, which does not include:
| · | 23,395,046 shares issuable upon exercise of outstanding
warrants with a weighted average exercise price of $1.94; and |
| | | |
| · | 30,000
outstanding shares of
Series B Preferred Stock, which are not convertible into common stock. |
Common Stock
Holders of our common stock are entitled to one
vote for each share on all matters submitted to a stockholder vote. Holders of common stock do not have cumulative voting rights. Therefore,
holders of a majority of the voting power of our stockholders for the election of directors can elect all of the directors. Holders of
one-third of the voting power of the Company’s stockholders, outstanding and entitled to vote, represented in person or by proxy,
are necessary to constitute a quorum at any meeting of stockholders. A vote by the holders of a majority of the voting power of the Company’s
stockholders is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to the Company’s
certificate of incorporation.
Holders of our common stock are entitled to share
in all dividends that the board of directors, in its discretion, declares from legally available funds. In the event of a liquidation,
dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment
of liabilities and after providing for each class of stock, if any, having preference over the common stock. The Company’s common
stock has no pre-emptive rights, no conversion rights and there are no withdrawal provisions applicable to the Company’s common
stock.
Pre-Funded Warrants to be issued in this offering
The following summary of certain terms and provisions
of the Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by the provisions
of, the Pre-Funded Warrant. Prospective investors should carefully review the terms and provisions of the form of Pre-Funded Warrant for
a complete description of the terms and conditions of the Pre-Funded Warrants.
The term “pre-funded” refers to the
fact that the purchase price of our common stock in this offering includes almost the entire exercise price that will be paid under the
Pre-Funded Warrants, except for a nominal remaining exercise price of $0.001. The purpose of the Pre-Funded Warrants is to enable investors
that may have restrictions on their ability to beneficially own more than 4.99% (or, upon election of the holder, 9.99%) of our outstanding
shares of common stock following the consummation of this offering the opportunity to make an investment in the Company without triggering
their ownership restrictions, by receiving Pre-Funded Warrants in lieu of our common stock which would result in such ownership of more
than 4.99% (or 9.99%), and receive the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at such
nominal price at a later date.
Duration. The Pre-Funded Warrants offered
hereby will entitle the holders thereof to purchase our shares of common stock at a nominal exercise price of $0.001 per share, commencing
immediately on the date of issuance. There is no expiration date for the Pre-Funded Warrants.
Exercise Limitation. A holder will not
have the right to exercise any portion of the Pre-Funded Warrant if the holder (together with its affiliates) would beneficially own in
excess of 4.99% (or, upon election of the holder, 9.99%) of the number of our shares of common stock outstanding immediately after giving
effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. However, any
holder may increase or decrease such percentage (up to 9.99%), provided that any increase will not be effective until the 61st day after
such election. It is the responsibility of the holder to determine whether any exercise would exceed the exercise limitation.
Exercise Price. The Pre-Funded Warrants
will have an exercise price of $0.001 per share. The exercise price is subject to appropriate adjustment in the event of certain stock
dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also
upon any distributions of assets, including cash, stock or other property to our shareholders.
Transferability. Subject to applicable
laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Absence of Trading Market. There is no
established trading market for the Pre-Funded Warrants and we do not expect a market to develop. In addition, we do not intend to apply
for the listing of the Pre-Funded Warrants on any national securities exchange or other trading market. Without an active trading market,
the liquidity of the Pre-Funded Warrants will be limited.
Fundamental Transactions. In the event
of a fundamental transaction, generally including any reorganization, recapitalization or reclassification of our common stock, the sale,
transfer or other disposition of all or substantially all of our properties or assets, our consolidation, merger, amalgamation or arrangement
with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial
owner of 50% of the voting power represented by our outstanding common stock, the holder will have the right to receive, for each share
of common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction,
the number of shares of the successor or acquiring corporation or of us if we are the surviving corporation, and any additional consideration
receivable as a result of such fundamental transaction by a holder of the number of shares for which the Pre-Funded Warrant was exercisable
immediately prior to such fundamental transaction. The holders of the Pre-Funded Warrants may also
require us to purchase the Pre-Funded Warrants from the holders by paying to each holder an amount equal to the Black Scholes value of
the remaining unexercised portion of the Pre-Funded Warrants on the date of the fundamental transaction.
No Rights as a Shareholder. Except as otherwise
provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of our shares of common stock, the holder of Pre-Funded
Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises
the Pre-Funded Warrant.
Warrant Stockholder Approval
Under Nasdaq listing rules, the alternative
cashless exercise option (described below) in the Series A Warrants, certain anti-dilution provisions in the Series B Warrants (described
below), and the reverse stock split provision in both Series A Warrants and Series B Warrants (each described below) will not be effective
until, and unless, we obtain the approval of our stockholders. While we intend to promptly seek stockholder approval, there is no guarantee
that the Warrant Stockholder Approval will ever be obtained. If we are unable to obtain the Warrant Stockholder Approval, the foregoing
provisions will not become effective and the Series A Warrants and Series B Warrants will have substantially less value. In addition,
we will incur substantial cost, and management will devote substantial time and attention, in attempting to obtain the Warrant Stockholder
Approval.
Series A Warrants and Series B Warrants to
be issued in this offering
The following summary of certain terms and provisions
of the Series A Warrants and Series B Warrants included in the Units and Pre-offered hereby is not complete and is subject to, and qualified
in its entirety by the provisions of the forms of Series A Warrant and Series B Warrant, which are filed as an exhibit to the registration
statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the
forms of Series A Warrant and Series B Warrant.
Exercisability. The Series A Warrants and
Series B Warrants are exercisable immediately and at any time up to the date that is two-and-a-half years (with respect to the Series
A Warrants) or five years (with respect to the Series B Warrants) after their original issuance. The Series A Warrants and Series B Warrants
will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any
time a registration statement registering the issuance of the shares of common stock underlying the Series A Warrants and Series B Warrants
under the Securities Act is effective and available for the issuance of such shares, by payment in full in immediately available funds
for the number of shares of common stock purchased upon such exercise. If a registration statement registering the issuance of the shares
of common stock underlying the Series A Warrants or Series B Warrants under the Securities Act is not effective, the holder may elect
to exercise the Series A Warrants or Series B Warrants through a cashless exercise, in which case the holder would receive upon such exercise
the net number of shares of common stock determined according to the formula set forth in the warrant. No fractional shares of common
stock will be issued in connection with the exercise of Series A Warrants or Series B Warrants. In lieu of fractional shares, we will
pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
On or after receipt of the Warrant Stockholder Approval,
a holder may also effect an “alternative cashless exercise” at any time while the Series A Warrants are outstanding. In such
event, the aggregate number of shares issuable in such alternative cashless exercise will be equal to the number of Series A Warrants
being exercised multiplied by two.
Exercise Limitation. A holder will not
have the right to exercise any portion of the Series A Warrants or Series B Warrants if the holder (together with its affiliates) would
beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise,
as such percentage ownership is determined in accordance with the terms of the Series A Warrants and Series B Warrants. However, any holder
may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage
shall not be effective until 61 days following notice from the holder to us.
Exercise
Price. The exercise price per whole share of common stock purchasable upon exercise of
the Series A Warrants is $3.825, and the exercise price per whole share of common stock purchasable
upon exercise of the Series B Warrants is $4.335. The exercise price is subject to appropriate
adjustment in the event of certain stock dividends and distributions, stock splits, stock
combinations, reclassifications or similar events affecting our common stock and also upon
any distributions of assets, including cash, stock or other property to our stockholders.
Subsequent Financing. In addition, conditioned
upon the receipt of the Warrant Stockholder Approval, and subject to certain exemptions, if we sell, enter into an agreement to sell,
or grant any option to purchase, or sell, enter into an agreement to sell, or grant any right to reprice, or otherwise dispose of or
issue (or announce any offer, sale, grant or any option to purchase or other disposition) any shares of common stock, at an effective
price per share less than the exercise price of the Series B Warrants then in effect, the exercise price of the Series B Warrants will
be reduced to such price (subject to a floor of $[*] prior to the Warrant Stockholder Approval), and the number of shares issuable
upon exercise will be proportionately adjusted such that the aggregate exercise price will remain unchanged.
Reverse Stock Split. Conditioned upon the receipt
of the Warrant Stockholder Approval, if at any time on or after the date of issuance there occurs any share split, share dividend, share
combination recapitalization or other similar transaction involving our common stock and the lowest daily volume weighted average price
during the period commencing five consecutive trading days immediately preceding and the five consecutive trading days immediately following
such event is less than the exercise price of the Series A Warrants or Series B Warrants then in effect, then the exercise price of the
Series A Warrants and Series B Warrants will be reduced to the lowest daily volume weighted average price during such period and the number
of shares issuable upon exercise will be proportionately adjusted such that the aggregate price will remain unchanged.
Transferability. Subject to applicable
laws, the Series A Warrants and Series B Warrants may be offered for sale, sold, transferred or assigned without our consent.
Warrant Agent. The Series A Warrants
and Series B Warrants will be issued in registered form under a warrant agency agreement between Equiniti Trust Company, as warrant agent,
and us. The Series A Warrants and Series B Warrants will initially be represented only by one or more global warrants deposited with
the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a
nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the event
of a fundamental transaction, as described in the Series A Warrants and Series B Warrants and generally including any reorganization,
recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our
properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common
stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the
holders of the Series A Warrants and Series B Warrants will be entitled to receive upon exercise of the Series A Warrants and Series
B Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Series
A Warrants and Series B Warrants immediately prior to such fundamental transaction. The holders of
the Series A Warrants and Series B Warrants may also require us to purchase the Series A Warrants and Series B Warrants from the holders
by paying to each holder an amount equal to the Black Scholes value of the remaining unexercised portion of the Series A Warrants and
Series B Warrants on the date of the fundamental transaction.
Rights as a Stockholder. Except as otherwise
provided in the Series A Warrants or Series B Warrants or by virtue of such holder’s ownership of shares of our common stock, the
holder of a Series A Warrants or Series B Warrants does not have the rights or privileges of a holder of our common stock, including any
voting rights, until the holder exercises the Series A Warrant or Series B Warrants.
Governing Law. The Series A Warrants, Series
B Warrants, and the warrant agency agreement are governed by New York law.
Reverse Stock Split. The Company shall
effect a reverse stock split within seven (7) business days after the date that is the earlier of the date on which (x) the first meeting
of stockholders to obtain Warrant Stockholder Approval is held or (y) the items to be approved under the Warrant Stockholder Approval
have been approved in accordance with the applicable laws and corporate governing documents of the Company (the “First Reverse Split
Date”). No reverse stock split shall be effectuated before the First Reverse Split Date, except if the consent has been obtained
from a purchasers of the majority of the Units.
Blank Check Preferred Stock
Our articles of incorporation authorize the
issuance of 30,000,000 shares of preferred stock, par value $0.10 per share, in one or more series, subject to any limitations
prescribed by law, without further vote or action by the stockholders. Each such series of preferred stock shall have such number of
shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be
determined by our board of directors, which may include, among others, dividend rights, voting rights, liquidation preferences,
conversion rights and preemptive rights.
Series B Preferred Stock
1,000,000 shares of our authorized preferred
stock have been designated Series B Preferred Stock. 30,000 shares of Series B Preferred Stock are outstanding and held by our
chief executive officer, Dr. Steve N. Slilaty.
The Series B Preferred Stock votes together with
the common stock on all matters submitted to a vote of the Company’s stockholders. Each share of Series B Preferred Stock entitles
the holder to 1,000 votes.
Upon any liquidation or dissolution of the Company,
the Series B Preferred Stock will be entitled to a payment equal to the stated value of $0.10 per share, prior to any payments being made
with respect to the common stock. The Series B Preferred Stock is not redeemable by the Company and is entitled to dividends when, as
and if declared by the board of directors in its sole discretion.
UNDERWRITING
Aegis Capital Corp., or
Aegis, is acting as the underwriter of the offering. We have entered into an underwriting agreement dated ,
2024 with the underwriter. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter,
and the underwriter has agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page
of this prospectus, the number of Units including a share of common stock, and the number of Units including a Pre-Funded Warrants, listed
next to its name in the following table:
| Underwriter |
|
Number
of Units including Common Stock |
|
Number
of
Units including Pre-Funded Warrant |
| Aegis Capital Corp. |
|
|
|
- |
| Total |
|
|
|
|
The underwriter is committed to purchase all the
Units offered by us, other than those covered by the over-allotment option described below, if they purchase any Units. The obligations
of the underwriter may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant
to the underwriting agreement, the underwriter’s obligations are subject to customary conditions, representations and warranties
contained in the underwriting agreement, such as receipt by the underwriter of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriter against
specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriter may be required to
make in respect thereof.
The underwriter is offering the Units, shares
of common stock, Pre-Funded Warrants, Series A Warrants and Series B Warrants subject to prior sale, when, as and if issued to and accepted
by it, subject to approval of legal matters by its counsel and other conditions specified in the underwriting agreement. The underwriter
reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-allotment Option
We have granted to the underwriter an Over-Allotment
Option exercisable not later than 45 days after the closing date of this offering to purchase up to a number of additional shares of Common
Stock and/or Pre-Funded Warrants and/or Series A Warrants and/or Series B Warrants equal to 15% of the number of securities sold in this
offering at the applicable public offering price listed on the cover of this prospectus, less the underwriting discounts and commissions.
The underwriter may exercise its Over-Allotment Option, if any, made in connection with this offering. If any additional shares of Common
Stock and/or Pre-Funded Warrants and/or Series A Warrants and/or Series B Warrants are purchased, the underwriter will offer these securities
on the same terms as those on which the other securities are being offered.
Discounts, Commissions and Reimbursement
The following table shows the public offering
price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the
underwriter of its over-allotment option.
| |
|
Per
Unit including Common Stock |
|
|
Per
Unit including Pre-Funded Warrant |
|
|
Total
with no Over-Allotment |
|
|
Total
with Over-Allotment |
|
| Public offering price |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Underwriting discounts and commissions (8.0%) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Non-accountable expense allowance (1.0%)(1) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Proceeds, before expenses, to us |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
(1) We have agreed to pay a non-accountable expense
allowance to the underwriter equal to 1.0% of the gross proceeds received in this offering.
The underwriter proposes to offer the Units to
the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriter may offer some of the
Units to other securities dealers at such price less a concession not in excess of $
per Unit. If all of the Units offered by us are not sold at the public offering price, the underwriter may change the offering price and
other selling terms by means of a supplement to this prospectus.
We have also agreed to pay $150,000 of the underwriter’s
legal expenses relating to the offering.
We estimate that the total expenses of the
offering payable by us, excluding the discount and non-accountable expense allowance, will be approximately $650,000.
Discretionary Accounts
The underwriter does not intend to confirm sales
of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Pursuant to “lock-up” agreements,
our executive officers and directors and shareholders holding at least five percent (5%) of the outstanding shares of common stock have
agreed, subject to limited exceptions, without the prior written consent of the underwriter not to directly or indirectly offer to sell,
sell, pledge or otherwise transfer or dispose of any of shares of (or enter into any transaction or device that is designed to, or could
be expected to, result in the transfer or disposition by any person at any time in the future of) our common stock, enter into any swap
or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of
shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments
thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable
for common stock or any of our other securities or publicly disclose the intention to do any of the foregoing, for a period of 90 days
from the closing date of this offering.
Company Standstill
We have agreed that without the prior written
consent of the underwriter, we will not, for a period of ninety (90) days after the closing of this offering, subject to certain exceptions,
(a) offer, sell, issue, or otherwise transfer or dispose of, directly or indirectly, any equity of the Company or any securities convertible
into or exercisable or exchangeable for equity of the Company; (b) file or caused to be filed any registration statement with the Commission
relating to the offering of any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of
the Company; or (c) enter into any agreement or announce the intention to effect any of the actions described in subsections (a) or (b)
hereof.
Right of First Refusal
We have granted the underwriter a right of first
refusal, for a period of three years from the consummation of this offering, to act as sole book-runner, sole manager, sole placement
agent, sole agent, sole book-runner, sole book-running manger and/or sole underwriter, at the underwriter’s sole discretion, for
each and every future public and private equity or debt offering or debt refinancing, including all equity linked financings (each, a
“Subject Transaction”), during such three year period, of the Company, or any successor to or subsidiary of the Company, on
terms and conditions customary to the underwriter for such Subject Transactions.
Tail Financing
In addition, we have agreed to pay the above cash
compensation to the extent that any fund which the underwriter contacted or introduced to us during the term of our engagement agreement
with the underwriter dated January 23, 2024, provides financing or capital in any public or private offering or capital raising transaction
during the three-month period following the closing of this offering or expiration or termination of our engagement letter with the underwriter
dated January 23, 2024.
Electronic Offer, Sale and Distribution of
Securities
A prospectus in electronic format may be made
available on the websites maintained by one or more of the underwriter or selling group members. The underwriter may agree to allocate
a number of securities to underwriter and selling group members for sale to its online brokerage account holders. Internet distributions
will be allocated by the underwriter and selling group members that will make internet distributions on the same basis as other allocations.
Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into,
this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should
not be relied upon by investors.
Stabilization
In connection with this offering, the underwriter
may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover
positions created by short sales.
Stabilizing transactions permit bids to purchase
shares so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding
a decline in the market price of the shares while the offering is in progress.
Over-allotment transactions involve sales by the
underwriter of shares in excess of the number of shares the underwriter is obligated to purchase. This creates a syndicate short position
which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted
by the underwriter is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position,
the number of shares involved is greater than the number of shares in the over-allotment option. The underwriter may close out any short
position by exercising its over-allotment option and/or purchasing shares in the open market.
Syndicate covering transactions involve purchases
of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the
source of shares to close out the short position, the underwriter will consider, among other things, the price of shares available for
purchase in the open market as compared with the price at which they may purchase shares through exercise of the over-allotment option.
If the underwriter sells more shares than could be covered by exercise of the over-allotment option and, therefore, has a naked short
position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created
if the underwriter is concerned that after pricing there could be downward pressure on the price of the shares in the open market that
could adversely affect investors who purchase in the offering.
Penalty bids permit the underwriter to reclaim
a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or
syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering
transactions and penalty bids may have the effect of raising or maintaining the market price of our shares of common stock or preventing
or retarding a decline in the market price of our shares of common stock. As a result, the price of our common stock in the open market
may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter make any representation
or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may
be affected in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive market making
In connection with this offering, the underwriter
and selling group members may engage in passive market making transactions in our common stock on the Nasdaq Capital Market in accordance
with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending
through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent
bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then
be lowered when specified purchase limits are exceeded.
Other
Relationships
The underwriter
and its affiliates have in the past and may in the future provide various investment banking, commercial banking and other financial
services for us and our affiliates for which they have in the past and may in the future receive customary fees. In February 2022, Aegis
served as the underwriter in connection with a public offering of units, with each unit consisting of one share of common stock and two
warrants, each warrant exercisable for one share of common stock, pursuant to an underwriting agreement between Aegis and us containing
standard terms. Aegis received an underwriting discount of 8%, and a non-accountable expense allowance equal to 1% of the gross proceeds
of the public offering. In March 2022, Aegis acted as the placement agent in connection with a private placement for the Company’s
common stock or pre-funded warrants and warrants exercisable for common stock. Aegis was paid a commission equal to 10% of the gross
proceeds received by the Company in the private placement and 2% of the gross proceeds as a non-accountable expense allowance. The Company
paid Aegis certain fees and expenses including attorney fees. In April 2022, Aegis acted as the placement agent in connection with the
private placement for the Company’s common stock or pre-funded warrants and warrants exercisable for common stock. Aegis was paid
a commission equal to 10% of the gross proceeds received by the Company, and a non-accountable expense allowance equal to 2% of the gross
proceeds, and will receive 5% of the proceeds from any exercise of warrants, payable on exercise. In May 2023, Aegis acted as the placement
agent in connection with the private placement for the Company’s common stock, pre-funded warrants, each exercisable to purchase
one share of common stock, and warrants, each exercisable to purchase one share of common stock. Aegis was paid a commission equal to
10% of the gross proceeds received by the Company, and a non-accountable expense allowance equal to 2% of the gross proceeds, and will
receive 10% of the proceeds from any exercise of warrants, payable on exercise.
Offer restrictions outside the United States
Other than in the United States, no action has
been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction
where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly,
nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities
be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and
regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe
any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell
or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation
is unlawful.
LEGAL MATTERS
We are being represented by Sichenzia Ross Ference
Carmel LLP, New York, New York, with respect to certain legal matters as to United States federal securities and New York State law. The
enforceability of the pre-funded warrants, Series A Warrants, and Series B Warrants will be passed upon for us by Sichenzia Ross Ference
Carmel LLP, New York, New York. The validity of the securities being offered by this prospectus, including the shares, Units, Pre-Funded
Warrants, Series A Warrants, Series B Warrants, and shares underlying the Pre-Funded Warrants, Series A Warrants, and Series B Warrants,
will be passed upon for us by Andrew I. Telsey, P.C., Englewood, Colorado. Certain legal matters in connection with this offering have
been passed upon for the underwriter by Kaufman & Canoles, P.C., Richmond, Virginia.
EXPERTS
The consolidated financial statements of Sunshine
Biopharma, Inc. at December 31, 2022 and 2021, and for each of the two years in the period ended December 31, 2022, included in this prospectus
have been audited by B F Borgers CPA PC, independent registered public accounting firm, as set forth in their report thereon, appearing
therein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus, which constitutes a part of the
registration statement on Form S-1 that we have filed with the SEC under the Securities Act, does not contain all of the information in
the registration statement and its exhibits. For further information with respect to us and the securities offered by this prospectus,
you should refer to the registration statement and the exhibits filed as part of that document. Statements contained in this prospectus
as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you
to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified
in all respects by this reference.
We are subject to the reporting requirements of
the Exchange Act, and file annual, quarterly and current reports, and other information with the SEC. The SEC maintains an Internet site
that contains these reports and other information filed electronically by us with the SEC, which are available on the SEC’s website
at http://www.sec.gov. We also maintain a website at https://sunshinebiopharma.com, at which you may access these materials free of charge
as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or
that can be accessed through, our website is not part of this prospectus.
Sunshine
Biopharma, Inc.
CONSOLIDATED
FINANCIAL STATEMENTS
At September
30, 2023 and December 31, 2022
TABLE OF
CONTENTS
CONSOLIDATED
FINANCIAL STATEMENTS
With Independent
Accountant’s Audit Report
At December
31, 2022 and 2021
Sunshine
Biopharma, Inc.
Consolidated
Balance Sheets
| | |
| | |
| |
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| | |
(Unaudited) | | |
| |
| ASSETS | |
| | | |
| | |
| Current Assets: | |
| | | |
| | |
| Cash and cash
equivalents | |
$ | 18,846,140 | | |
$ | 21,826,437 | |
| Accounts receivable | |
| 2,034,119 | | |
| 1,912,153 | |
| Inventory | |
| 4,517,044 | | |
| 3,289,945 | |
| Prepaid
expenses | |
| 37,556 | | |
| 283,799 | |
| Total Current Assets | |
| 25,434,859 | | |
| 27,312,334 | |
| | |
| | | |
| | |
| Property and equipment | |
| 334,922 | | |
| 394,249 | |
| Intangible assets | |
| 1,216,207 | | |
| 776,856 | |
| Right-of-use-asset | |
| 664,296 | | |
| 760,409 | |
| TOTAL
ASSETS | |
$ | 27,650,284 | | |
$ | 29,243,848 | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable and accrued
expenses | |
$ | 2,220,870 | | |
$ | 2,802,797 | |
| Earnout payable | |
| 2,547,831 | | |
| 3,632,000 | |
| Income tax payable | |
| 201,541 | | |
| 373,191 | |
| Right-of-use-liability | |
| 117,840 | | |
| 123,026 | |
| Total Current Liabilities | |
| 5,088,082 | | |
| 6,931,014 | |
| | |
| | | |
| | |
| Long-Term Liabilities: | |
| | | |
| | |
| Deferred tax liability | |
| 43,032 | | |
| 43,032 | |
| Right-of-use-liability | |
| 555,687 | | |
| 642,232 | |
| Total Long-Term Liabilities | |
| 598,719 | | |
| 685,264 | |
| TOTAL
LIABILITIES | |
| 5,686,801 | | |
| 7,616,278 | |
| | |
| | | |
| | |
| SHAREHOLDERS' EQUITY | |
| | | |
| | |
| Preferred
Stock, Series B $0.10 par value per share; 1,000,000 shares authorized; 10,000 Shares issued and outstanding | |
| 1,000 | | |
| 1,000 | |
| Common
Stock, $0.001 par value per share; 3,000,000,000 shares authorized; 25,678,290 and 22,585,632 shares issued and outstanding as of
September 30, 2023 and December 31, 2022, respectively | |
| 25,678 | | |
| 22,585 | |
| Capital
paid in excess of par value | |
| 84,387,890 | | |
| 80,841,752 | |
| Accumulated
comprehensive income | |
| 204,549 | | |
| 161,847 | |
| Accumulated
(Deficit) | |
| (62,655,634 | ) | |
| (59,399,614 | ) |
| TOTAL
SHAREHOLDERS' EQUITY | |
| 21,963,483 | | |
| 21,627,570 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES AND SHAREHOLDERS' EQUITY | |
$ | 27,650,284 | | |
$ | 29,243,848 | |
The
accompanying notes are an integral part of these unaudited financial statements
Sunshine
Biopharma, Inc.
Consolidated
Statements of Operations and Comprehensive Loss (Unaudited)
| | |
| | | |
| | | |
| | | |
| | |
| | |
3
Months Ended September 30, | | |
9
Months Ended September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
| | |
| | |
| | |
| |
| Sales | |
$ | 5,957,668 | | |
$ | 132,808 | | |
$ | 16,412,586 | | |
$ | 405,760 | |
| Cost of sales | |
| 3,967,412 | | |
| 65,783 | | |
| 10,641,461 | | |
| 200,311 | |
| Gross profit | |
| 1,990,256 | | |
| 67,025 | | |
| 5,771,125 | | |
| 205,449 | |
| | |
| | | |
| | | |
| | | |
| | |
| General and Administrative Expenses: | |
| | | |
| | | |
| | | |
| | |
| Accounting | |
| 56,350 | | |
| 122,913 | | |
| 301,381 | | |
| 237,773 | |
| Consulting | |
| 221,781 | | |
| 162,852 | | |
| 745,850 | | |
| 270,033 | |
| Director fees | |
| 100,000 | | |
| 100,000 | | |
| 300,000 | | |
| 200,000 | |
| Legal | |
| 133,302 | | |
| 146,467 | | |
| 392,874 | | |
| 403,386 | |
| Marketing | |
| 241,897 | | |
| 217,666 | | |
| 502,987 | | |
| 400,386 | |
| Office | |
| 544,215 | | |
| 76,818 | | |
| 1,422,058 | | |
| 449,730 | |
| R&D | |
| 238,012 | | |
| 362,500 | | |
| 1,039,502 | | |
| 770,095 | |
| Salaries | |
| 1,144,377 | | |
| 595,000 | | |
| 4,344,801 | | |
| 1,105,000 | |
| Taxes | |
| 52,586 | | |
| – | | |
| 212,953 | | |
| – | |
| Depreciation | |
| 37,210 | | |
| 789 | | |
| 106,797 | | |
| 6,186 | |
| Total General and Administrative
Expenses: | |
| 2,769,730 | | |
| 1,785,005 | | |
| 9,369,203 | | |
| 3,842,589 | |
| | |
| | | |
| | | |
| | | |
| | |
| (Loss) from operations | |
| (779,474 | ) | |
| (1,717,980 | ) | |
| (3,598,078 | ) | |
| (3,637,140 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other Income (Expense): | |
| | | |
| | | |
| | | |
| | |
| Foreign exchange | |
| 40 | | |
| 25 | | |
| (206 | ) | |
| 45 | |
| Interest income | |
| 207,431 | | |
| 260,938 | | |
| 624,361 | | |
| 406,984 | |
| Debt release | |
| – | | |
| – | | |
| – | | |
| 10,852 | |
| Interest
expense | |
| (38,527 | ) | |
| (2 | ) | |
| (107,198 | ) | |
| (12,866 | ) |
| Total Other Income (Expense) | |
| 168,944 | | |
| 260,961 | | |
| 516,957 | | |
| 405,015 | |
| | |
| | | |
| | | |
| | | |
| | |
| Provision for income taxes | |
| (40,952 | ) | |
| – | | |
| (174,899 | ) | |
| – | |
| Net (Loss) | |
$ | (651,482 | ) | |
$ | (1,457,019 | ) | |
$ | (3,256,020 | ) | |
$ | (3,232,125 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Gain (Loss) from foreign
exchange translation | |
| (460,507 | ) | |
| (45,126 | ) | |
| 42,702 | | |
| (56,764 | ) |
| Comprehensive (Loss) | |
$ | (1,111,989 | ) | |
$ | (1,502,145 | ) | |
$ | (3,213,318 | ) | |
$ | (3,288,889 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Basic (Loss) per common share | |
$ | (0.04 | ) | |
$ | (0.08 | ) | |
$ | (0.133 | ) | |
$ | (0.26 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted Average Common
Shares Outstanding (Basic and Diluted) | |
| 25,690,449 | | |
| 18,885,632 | | |
| 24,507,122 | | |
| 12,789,733 | |
The
accompanying notes are an integral part of these unaudited financial statements
Sunshine
Biopharma, Inc.
Consolidated
Statements of Cash Flows (Unaudited)
| | |
| | |
| |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Cash Flows From Operating Activities: | |
| | | |
| | |
| Net (Loss) | |
$ | (3,256,020 | ) | |
$ | (3,232,125 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 106,794 | | |
| 6,186 | |
| Foreign exchange | |
| (374 | ) | |
| 45 | |
| Debt release | |
| – | | |
| (10,852 | ) |
| Accounts receivable | |
| (118,482 | ) | |
| 7,776 | |
| Inventory | |
| (1,221,112 | ) | |
| (163,991 | ) |
| Prepaid expenses | |
| 247,977 | | |
| 2,235 | |
| Accounts payable and accrued expenses | |
| (587,973 | ) | |
| 437,267 | |
| Income tax payable | |
| (172,076 | ) | |
| – | |
| Interest payable | |
| (1,084,169 | ) | |
| (48,287 | ) |
| Net Cash Flows (Used) in Operations | |
| (6,085,435 | ) | |
| (3,001,746 | ) |
| | |
| | | |
| | |
| Cash Flows From Investing Activities: | |
| | | |
| | |
| Reduction in Right-of-use asset | |
| 97,498 | | |
| – | |
| Purchase of intangible assets | |
| (19,804 | ) | |
| – | |
| Purchase of equipment | |
| (464,614 | ) | |
| – | |
| Net Cash Flows (Used) in Investing Activities | |
| (386,920 | ) | |
| – | |
| | |
| | | |
| | |
| Cash Flows From Financing Activities: | |
| | | |
| | |
| Common stock issued | |
| 4,089,218 | | |
| 43,560,363 | |
| Exercise of warrants | |
| 1,156 | | |
| – | |
| Purchase of treasury stock | |
| (541,143 | ) | |
| (99,000 | ) |
| Lease liability | |
| (93,125 | ) | |
| – | |
| Payments of notes payable | |
| – | | |
| (1,900,000 | ) |
| Net Cash Flows Provided by Financing Activities | |
| 3,456,106 | | |
| 41,561,363 | |
| | |
| | | |
| | |
| Cash and Cash Equivalents at Beginning of Period | |
| 21,826,437 | | |
| 2,045,167 | |
| Net increase (decrease) in cash and cash equivalents | |
| (3,016,249 | ) | |
| 38,559,617 | |
| Effect of exchange rate changes on cash | |
| – | | |
| (105,617 | ) |
| Foreign currency translation adjustment | |
| 35,952 | | |
| 56,764 | |
| Cash and Cash Equivalents at End of Period | |
$ | 18,846,140 | | |
$ | 40,555,931 | |
| | |
| | | |
| | |
| Supplementary Disclosure of Cash Flow Information: | |
| – | | |
| – | |
| Cash paid for interest | |
$ | – | | |
$ | 61,151 | |
| Cash paid for income taxes | |
$ | – | | |
$ | – | |
The
accompanying notes are an integral part of these unaudited financial statements
Sunshine
Biopharma, Inc.
Consolidated
Statement of Shareholders' Equity (Unaudited)
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
Number
Of Common Shares | | |
Common | | |
Capital
Paid in Excess of Par | | |
Number
Of Preferred Shares | | |
Preferred | | |
Comprehensive | | |
Accumulated | | |
| |
| | |
Issued | | |
Stock | | |
Value | | |
Issued | | |
Stock | | |
Income | | |
Deficit | | |
Total | |
| Three Month Period Ended September
30, 2023 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
at June 30, 2023 | |
| 25,746,302 | | |
$ | 25,746 | | |
$ | 84,422,143 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | 665,056 | | |
$ | (62,004,152 | ) | |
$ | 23,109,793 | |
| Repurchase
stock | |
| (68,012 | ) | |
| (68 | ) | |
| (34,253 | ) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Net (loss) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (460,507 | ) | |
| (651,482 | ) | |
| (1,111,989 | ) |
| Balance
at September 30, 2023 | |
| 25,678,290 | | |
$ | 25,678 | | |
$ | 84,387,890 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | 204,549 | | |
$ | (62,655,634 | ) | |
$ | 21,963,483 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Nine Month Period Ended September
30, 2023 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
December 31, 2022 | |
| 22,585,632 | | |
$ | 22,585 | | |
$ | 80,841,752 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | 161,847 | | |
$ | (59,399,614 | ) | |
$ | 21,627,570 | |
| Repurchase
of common stock | |
| (513,723 | ) | |
| (514 | ) | |
| (540,629 | ) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Common
stock and pre-funded warrants issued in a private offering | |
| 2,450,000 | | |
| 2,451 | | |
| 4,086,767 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 4,089,218 | |
| Exercise
of warrants | |
| 1,156,381 | | |
| 1,156 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 1,156 | |
| Net (loss) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 42,702 | | |
| (3,256,020 | ) | |
| (3,213,318 | ) |
| Balance
at September 30, 2023 | |
| 25,678,290 | | |
$ | 25,678 | | |
$ | 84,387,890 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | 204,549 | | |
$ | (62,655,634 | ) | |
$ | 21,963,483 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Three Month Period Ended September
30, 2022 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
at June 30, 2022 | |
| 18,885,632 | | |
$ | 18,886 | | |
$ | 76,331,451 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | (34,777 | ) | |
$ | (34,430,280 | ) | |
$ | 41,886,280 | |
| Net (loss) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (45,126 | ) | |
| (1,457,019 | ) | |
| (1,502,145 | ) |
| Balance
at September 30, 2022 | |
| 18,885,632 | | |
$ | 18,886 | | |
$ | 76,331,451 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | (79,903 | ) | |
$ | (35,887,299 | ) | |
$ | 40,384,135 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Nine Month Period Ended September
30, 2022 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
December 31, 2021 | |
| 2,595,620 | | |
$ | 2,596 | | |
$ | 32,787,379 | | |
| 1,000,000 | | |
$ | 100,000 | | |
$ | (23,139 | ) | |
$ | (32,655,174 | ) | |
$ | 211,662 | |
| Common
stock and pre-funded warrants issued in public offering | |
| 6,656,526 | | |
| 6,657 | | |
| 30,360,528 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 30,367,185 | |
| Exercise
of warrants | |
| 9,633,486 | | |
| 9,633 | | |
| 13,183,544 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 13,193,177 | |
| Preferred
stock purchased from related party | |
| – | | |
| – | | |
| – | | |
| (990,000 | ) | |
| (99,000 | ) | |
| – | | |
| – | | |
| (99,000 | ) |
| Net (loss) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (56,764 | ) | |
| (3,232,125 | ) | |
| (3,288,889 | ) |
| Balance
at September 30, 2022 | |
| 18,885,632 | | |
$ | 18,886 | | |
$ | 76,331,451 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | (79,903 | ) | |
$ | (35,887,299 | ) | |
$ | 40,384,135 | |
The
accompanying notes are an integral part of these unaudited financial statements
Sunshine
Biopharma, Inc.
Notes to
Unaudited Consolidated Financial Statements
For the
Nine Months Ended September 30, 2023 and 2022
Note
1 – Description of Business
The
Company was originally incorporated under the name Mountain West Business Solutions, Inc. on August 31, 2006, in the State of Colorado.
Effective October 15, 2009, the Company acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition. Upon
completion of the reverse acquisition transaction, the Company changed its name to Sunshine Biopharma, Inc. and began operating as a
pharmaceutical company.
In
addition to conducting its own drug development activities, Sunshine Biopharma operates two wholly owned subsidiaries: (i) Nora Pharma
Inc. (“Nora Pharma”), a Canadian corporation with a portfolio of pharmaceutical products consisting of 51 generic prescription
drugs on the market in Canada, and (ii) Sunshine Biopharma Canada Inc. (“Sunshine Canada”), a Canadian corporation which
develops and sells nonprescription over-the-counter (“OTC”) products. In addition to the 51 generic prescription drugs currently
on the market in Canada, the Company has 32 additional generic prescription drugs scheduled to be launched in 2024 and 2025 in Canada.
The
Company has determined that it has two reportable segments:
| |
· |
Prescription
Generic Pharmaceuticals (“Generic Pharmaceuticals”) |
| |
· |
Nonprescription
Over-The-Counter Products (“OTC Products) |
Through December 31, 2022 and as of September
30, 2023, sales from the Generic Pharmaceuticals segment represented approximately 97% of total revenues of the Company while the remaining
approximately 3% was generated from the sale of OTC Products. Based on these results, the Company deems segmentation reporting to be immaterial
at September 30, 2023.
The Company is not subject to material customer
concentration risks as it sells its products directly to pharmacies in several Canadian Provinces. Provincial governments in Canada reimburse
patients for their prescription drugs expenditures to various degrees under drug reimbursement programs, making generic drugs prices
highly dependent on government regulations which may change over time. The most recent negotiations between the pan-Canadian Pharmaceutical
Alliance and the Canadian Generic Pharmaceutical Association have resulted in updated generic pricing for certain products which took
effect on October 1, 2023. The updated prices are valid for three years and the agreement contains an option to extend for an additional
two years.
In
addition, the Company is engaged in the development of the following proprietary drugs:
| · | Adva-27a,
a small chemotherapy molecule for treatment of pancreatic cancer (IND-enabling studies were paused on November 2, 2023 due to unfavorable
results. See Note 13 – Subsequent Events) |
| · | K1.1
mRNA, a lipid nano-particle (LNP) targeted for liver cancer |
| · | SBFM-PL4,
a protease inhibitor for treatment of Coronavirus infections |
Note
2 – Basis of Presentation
The
unaudited financial statements of the Company for the nine months periods ended September 30, 2023 and 2022 have been prepared in accordance
with accounting principles generally accepted in the United States of America for interim financial information and pursuant to the requirements
for reporting on Form 10-Q and Regulation S-X. Accordingly, they do not include all the information and footnotes required by accounting
principles generally accepted in the United States of America for complete financial statements. However, such information reflects all
adjustments (consisting solely of normal recurring adjustments), which are, in the opinion of management, necessary for the fair presentation
of the financial position and the results of operations. Results shown for interim periods are not necessarily indicative of the results
to be obtained for a full fiscal year. The balance sheet information as of December 31, 2022, was derived from the audited financial statements
included in the Company's financial statements as of and for the year ended December 31, 2022, included in the Company’s Annual
Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on April 4, 2023. These financial statements
should be read in conjunction with that report.
Note
3 – Private Placement
On
May 16, 2023, the Company completed a private placement pursuant to a securities purchase agreement with a single institutional investor for gross proceeds of approximately $5 million, before deducting fees to the placement agent and other offering
expenses payable by the Company. The net proceeds received by the Company were $4,089,218.
In
connection with the private placement, the Company issued (i) 2,450,000
shares of common stock, (ii) 3,502,381
pre-funded warrants (the “May Pre-Funded Warrants”),
and (iii) investor warrants (the “May Investor Warrants”) to purchase up to 11,904,762 shares of common stock at
$0.59 per share. Each share of common stock and accompanying two May Investor Warrants were sold together at a combined offering price
of $0.84 and each May Pre-Funded Warrant and accompanying two May Investor Warrants were sold together at a combined offering price of
$0.839. The May Pre-Funded Warrants are immediately exercisable at a nominal exercise price of $0.001, and may be exercised at any time
until all of the May Pre-Funded Warrants are exercised in full. The May Investor Warrants which have an exercise price of $0.59 per share
(subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the date of issuance.
As of September 30, 2023, a total of 1,156,381
May Pre-Funded Warrants and no May Investor Warrants have been exercised. The net proceeds received from the exercise of May Pre-Funded
Warrants were $1,156.
Note
4 – Acquisition of Nora Pharma Inc.
On
October 20, 2022, the Company acquired all of the issued and outstanding shares of Nora Pharma Inc. The purchase price for the
shares was $18,860,637
(USD), $14,346,637
of which was paid in cash and the remainder was paid through the issuance of 3,700,000
shares of the Company’s common stock valued at $4,514,000
or $1.22 per share. Nora Pharma sells generic pharmaceutical products in Canada. Nora Pharma’s operations are authorized by a
Drug Establishment License issued by Health Canada.
The
following table summarizes the allocation of the purchase price as of October 20, 2022, the acquisition date using Nora Pharma’s
balance sheet assets and liabilities:
| Schedule of allocation of purchase price | |
| |
| Accounts receivable | |
$ | 1,358,121 | |
| Inventory | |
| 3,181,916 | |
| Intangible assets | |
| 659,571 | |
| Equipment & furniture | |
| 210,503 | |
| Other assets | |
| 1,105,093 | |
| Total assets | |
| 6,515,204 | |
| Liabilities assumed | |
| (5,981,286 | ) |
| Net assets | |
| 533,918 | |
| Goodwill | |
| 18,326,719 | |
| Total Consideration | |
$ | 18,860,637 | |
The
value of the 3,700,000 common shares issued as part of the consideration paid for Nora Pharma was determined based on the closing market
price of the Company’s common shares on the acquisition date, October 20, 2022 ($1.22 per share).
The
Company impaired 100% of the goodwill amount in 2022 and plans to depreciate the intangible assets as detailed in Note 5 below.
As
part of the consideration paid for Nora Pharma, the Company agreed to a $5,000,000 CAD ($3,632,000 USD) earnout amount payable to Mr.
Malek Chamoun, the Seller of Nora Pharma. The earnout is payable in the form of twenty (20) payments of $250,000 CAD for every $1,000,000
CAD increase in gross sales (as defined in the Purchase Agreement) above Nora Pharma’s June 30, 2022 gross sales, provided that
his employment with the Company is not terminated pursuant to the Company’s employment agreement with him. The total earnout amount
of $3,632,000 has been recorded as a salary payable. During the nine-month period ended September 30, 2023, the Company paid an earn-out
amount of $1,084,169 leaving a balance earn-out to be paid of $2,547,831 at September 30, 2023.
The
unaudited financial information in the table below summarizes the combined results of operations of the Company and Nora Pharma for the
years ended December 31, 2022 and 2021, on a pro forma basis, as though the two companies had been combined as of January 1, 2021. The
unaudited pro forma financial information does not purport to be indicative of the Company's combined results of operations which would
have been obtained had the acquisition taken place on January 1, 2021, nor should it be taken as indicative of future consolidated results
of operations:
| Schedule of Pro Forma results from acquisition | |
| | |
| |
| Pro Forma Results
From Acquisition | |
December
31,
2022 | | |
December
31,
2021 | |
| Total revenues | |
$ | 14,758,115 | | |
$ | 7,927,165 | |
| Net (loss) from operations | |
$ | (26,192,503 | ) | |
$ | (2,224,253 | ) |
| Net (loss) | |
$ | (26,164,764 | ) | |
$ | (12,289,655 | ) |
| Basic and fully diluted (loss) per share | |
$ | (1.74 | ) | |
$ | (4.70 | ) |
| Weighted average number of shares outstanding | |
| 15,056,097 | | |
| 2,612,061 | |
Note
5 – Intangible Assets
Intangible
assets, net, consisted of the following at September 30, 2023:
| Schedule of intangible assets | |
| | |
| Balance June 30, 2023 | |
$ | 1,233,570 | |
| Dossier fee additions | |
| 13,905 | |
| Balance at September 30, 2023 | |
| 1,247,475 | |
| Less accumulated amortization | |
| (31,268 | ) |
| Finite-lived intangible
assets, net, at September 30, 2023 | |
$ | 1,216,207 | |
| | |
| | |
| Balance December 31, 2022 | |
$ | 776,856 | |
| Dossier fee additions | |
| 470,619 | |
| Balance at September 30, 2023 | |
| 1,247,475 | |
| Less accumulated amortization | |
| (31,268 | ) |
| Finite-lived intangible
assets, net, at September 30, 2023 | |
$ | 1,216,207 | |
Amortization
expense for the three months period ended September 30, 2023, and the nine months period ended September 30, 2023, amounted to $10,797
and $26,746, respectively.
As
of September 30, 2023, estimated amortization expense of the Company’s intangible assets for each of the next five years is as follows:
| Schedule of estimated amortization expense | |
| | |
| 2024 | |
$ | 55,418 | |
| 2025 | |
| 55,418 | |
| 2026 | |
| 54,240 | |
| 2027 | |
| 15,599 | |
| 2028 | |
| 7,370 | |
Note
6 – Reverse Stock Splits
Effective
February 9, 2022, the Company completed a 1 for 200 reverse split of its common stock. The Company had previously completed two 20 to
1 reverse stock splits, one in 2019 and the other in 2020. The Company’s financial statements reflect all three reverse stock splits
on a retroactive basis for all periods presented and for all references to common stock, unless specifically stated otherwise.
Note
7 – Capital Stock
The
Company’s authorized capital is comprised of 3,000,000,000
shares of common stock, par value $0.001,
and 30,000,000
shares of preferred stock, $0.10
par value. As of December 31, 2022 and September 30, 2023, the Company had authorized 1,000,000
shares of Series B Preferred Stock. The Series B Preferred Stock is non-convertible, non-redeemable and non-retractable. It has
superior liquidation rights to the common stock at $0.10 per share and gives the holder the right to 1,000 votes per share. As of
September 30, 2023 and December 31, 2022, 10,000
shares of Series B Preferred Stock are outstanding and held by the Company’s chief executive officer.
On
February 17, 2022, the Company completed a public offering and received net proceeds of $6,833,071 from the offering. Pursuant to the
public offering, the Company issued and sold an aggregate of 1,882,353 shares of common stock and 4,102,200 warrants to purchase shares
of common stock (the “Tradeable Warrants”).
On
February 22, 2022, the Company redeemed 990,000
shares of Series B Preferred Stock from the CEO of the Company at a redemption price equal to the stated value of $0.10 per share.
The remaining 10,000 shares of Series B Preferred Stock could not be voted pursuant to a warrant agent agreement relating to the
Tradeable Warrants (the “Warrant Agent Agreement”). On October 12, 2023, the Company held a special meeting of the
holders of the outstanding Tradeable Warrants in which the holders of the majority of the outstanding Tradeable Warrants approved an
amendment to the Warrant Agent Agreement to eliminate the provision that prohibited the Company’s CEO from exercising his
voting rights under the Series B Preferred Stock, as well as to lower the exercise price of the Tradeable Warrants to $0.11. The
Company entered into the amendment to the Warrant Agent Agreement on October 18, 2023.
On
March 14, 2022, the Company completed a private placement and received net proceeds of $6,781,199. In connection with this private placement,
the Company issued (i) 2,301,353 shares of its common stock together with investor warrants (“Investor Warrants”) to
purchase up to 2,301,353 shares of common stock, and (ii) 1,302,251 pre-funded warrants (“Pre-Funded Warrants”) with
each Pre-Funded Warrant exercisable for one share of common stock, together with Investor Warrants to purchase up to 1,302,251 shares
of common stock. Each share of common stock and accompanying Investor Warrant was sold together at a combined offering price of $2.22
and each Pre-Funded Warrant and accompanying Investor Warrant were sold together at a combined offering price of $2.219. The Pre-Funded
Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded
Warrants are exercised in full. The Investor Warrants have an exercise price of $2.22 per share (subject to adjustment as set forth in
the warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On
April 28, 2022, the Company completed another private placement and received net proceeds of $16,752,915. In connection with this private
placement, the Company issued (i) 2,472,820 shares of its common stock together with warrants (“April Warrants”) to
purchase up to 4,945,640 shares of common stock, and (ii) 2,390,025 pre-funded warrants (“Pre-Funded Warrants”)
with each Pre-Funded Warrant exercisable for one share of common stock, together with April Warrants to purchase up to 4,780,050 shares
of common stock. Each share of common stock and accompanying two April Warrants were sold together at a combined offering price of $4.01
and each Pre-Funded Warrant and accompanying two April Warrants were sold together at a combined offering price of $4.009. The Pre-Funded
Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded
Warrants are exercised in full. The April Warrants have an exercise price of $3.76 per share (subject to adjustment as set forth in the
warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On
October 20, 2022, the Company issued 3,700,000 shares of common stock as part of the acquisition of Nora Pharma. These shares were valued
at $4,514,000, or $1.22 per share.
On
January 19, 2023, the Company announced a stock repurchase program of up to $2 million (“Stock Repurchase
Program”). During the six months ended June 30, 2023, the Company repurchased a total of 445,711 shares of common stock at an average
price of $1.1371 per share for a total cost of $506,822. The 445,711 repurchased common shares were cancelled and returned to treasury
reducing the number of issued and outstanding shares from 22,585,632 to 22,139,921.
On
May 16, 2023, the Company completed a private placement pursuant to a securities purchase agreement with a single institutional investor
for gross proceeds of approximately $5
million, before deducting fees to the placement agent and other
offering expenses payable by the Company. The net proceeds received by the Company were $4,089,218.
In connection with the private placement, the Company issued (i) 2,450,000
shares of common stock, (ii) 3,502,381
pre-funded warrants (the “May Pre-Funded Warrants”),
and (iii) investor warrants (the “May Investor Warrants”) to purchase up to 11,904,762 shares of common stock at
$0.59 per share. Each share of common stock and accompanying two May Investor Warrants were sold together at a combined offering price
of $0.84 and each May Pre-Funded Warrant and accompanying two May Investor Warrants were sold together at a combined offering price of
$0.839. The May Pre-Funded Warrants are immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time
until all of the May Pre-Funded Warrants are exercised in full. The May Investor Warrants which have an exercise price of $0.59 per share
(subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the date of issuance.
In
2022 and the first six months of 2023, the Company issued a total of 10,789,867 shares of common stock in connection with warrant exercises
for aggregate net proceeds of $13,194,335.
In
July 2023, the Company repurchased a total of 68,012 shares of common stock on the open market under the Stock Repurchase Program announced
on January 19, 2023, at an average price of $0.5046 per share for a total cost of $34,321. In October 2023, the 68,012 repurchased common
shares were cancelled and returned to treasury reducing the number of issued and outstanding shares from 25,746,302 to 25,678,290.
As
of September 30, 2023 and December 31, 2022, the Company has a total of 25,678,290 and 22,585,632 shares of common stock issued and outstanding,
respectively.
The
Company has declared no dividends since inception.
Note
8 – Warrants
The
Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10 or ASC 815-40. Under ASC 480-10, warrants
are considered a liability if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number
of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to
determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement
for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are
measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after
the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification
under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed
to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP standard. Equity-classified
warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
In
2022 and during the first nine months of 2023, the Company completed four financing events, and in connection therewith, it
issued warrants as follows:
| Schedule of warrants issued with financing |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise Price |
|
Expiry Date |
| Pre-Funded Warrants |
|
3,692,276 |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
4,102,200 |
|
$2.22* |
|
February 2027 |
| Investor Warrants |
|
3,603,604 |
|
$2.22 |
|
March 2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April 2027 |
| May Pre-Funded Warrants |
|
3,502,381 |
|
$0.001 |
|
Unlimited |
| May Investor Warrants |
|
11,904,762 |
|
$0.59 |
|
November 2028 |
As
of September 30, 2023, all of the Pre-Funded Warrants and a total of 3,138,507 Tradeable Warrants, 2,802,703 Investor Warrants, and 1,156,381
May Pre-Funded Warrants were exercised resulting in aggregate proceeds of $13,194,335 received by the Company.
The Company’s
outstanding warrants at September 30, 2023 consisted of the following:
| Schedule of outstanding warrants |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise Price |
|
Expiry Date |
| Pre-Funded Warrants |
|
None |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
963,693 |
|
$2.22* |
|
February 2027 |
| Investor Warrants |
|
800,901 |
|
$2.22 |
|
March 2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April 2027 |
| May Pre-Funded Warrants |
|
2,346,000 |
|
$0.001 |
|
Unlimited |
| May Investor Warrants |
|
11,904,762 |
|
$0.59 |
|
November 2028 |
Note
9 – Net Loss Per Common Share
Basic
net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during
the period, without consideration for common stock equivalents.
Diluted
net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during
the period, taking into consideration common stock equivalents.
In
February 2022, the Company issued 4,102,200 Tradeable Warrants pursuant to the Company’s Public Offering. In March and April 2022,
the Company issued 3,603,604 Investor Warrants and 9,725,690 April Warrants pursuant to two private placements. In May 2023, the Company
issued 11,904,762 May Investor Warrants pursuant to two private placements. As of September 30, 2023, 3,138,507 Tradeable Warrants and
2,802,703 Investor Warrants were exercised, leaving 963,693 Tradeable Warrants, 800,901 Investor Warrants, 9,725,690 April Warrants,
and 11,904,762 May Investor Warrants outstanding. These warrants are dilutive and were included in the diluted earnings per share.
In
March and April 2022, the Company issued and sold Pre-Funded Warrants to purchase an aggregate of 3,692,276 shares of common stock at
a nominal exercise price of $0.001 per share. During the nine months ended September 30, 2023, all of these warrants were exercised and
therefore had no remaining dilutive effect.
In
May 2023, the Company issued and sold May Pre-Funded Warrants to purchase an aggregate of 3,502,381
shares of common stock at a nominal exercise price of $0.001 per
share. During the nine months ended September 30, 2023, 1,156,381
of these warrants were exercised leaving 2,346,000
outstanding. These warrants were not included in the calculation
of weighted average outstanding shares as they would be ant-dilutive.
Note
10 – Lease
The
Company has obligations as a lessee for office space with initial non-cancellable terms in excess of one year. The Company classified
the lease as an operating lease. The lease contains a renewal option for a period of five years. Because the Company is certain to exercise
the renewal option, the optional period is included in determining the lease term, and associated payments under the renewal option are
included in the lease payments. The Company’s lease does not include termination options for either party to the lease or restrictive
financial or other covenants. Payments due under the lease contract include fixed payments plus a variable Payment. The Company’s
office space lease requires it to make variable payments for the Company’s proportionate share of building’s property taxes,
insurance, and common area maintenance. These variable lease payments are not included in lease payments used to determine lease liability
and are recognized as variable costs when incurred.
Amounts
reported on the balance sheet as of September 30, 2023 were as follows:
| |
Schedule of lease information |
|
|
Operating lease ROU asset |
$664,296 |
|
Operating Lease liability - Short-term |
$117,840 |
|
Operating lease liability - Long-term |
$555,687 |
|
Remaining lease term |
6 years 3 months |
|
Discount rate |
6% |
Amounts
disclosed for ROU assets obtained in exchange for lease obligations and reductions of ROU assets resulting from reductions of lease obligations
include amounts reduced from the carrying amount of ROU assets resulting from deferred rent.
Maturities
of lease liabilities under non-cancellable operating leases at September 30, 2023 are as follows:
| Schedule of maturities of lease liabilities |
|
|
| 2023 |
$ |
30,124 |
| 2024 |
|
116,090 |
| 2025 |
|
116,277 |
| 2026 |
|
110,134 |
| 2027 |
|
103,736 |
| Thereafter |
|
197,166 |
Note
11 – Management and Director Compensation
The
Company paid its officers cash compensation totaling $245,000 and $362,500 and $1,290,000 and $770,095 for the three and nine-month periods
ended September 30, 2023 and 2022, respectively.
The
Company paid its directors cash compensation totaling $100,000 and $300,000 and $100,000 and $200,000 for the three and nine-month periods
ended September 30, 2023 and 2022, respectively.
Note
12 – Income Taxes
In
calculating the provision for income taxes on an interim basis, the Company uses an estimate of the annual effective tax rate based upon
currently known facts and circumstances and applies that rate to its year-to-date earnings or losses. The Company’s effective tax
rate is based on expected income and statutory tax rates and takes into consideration permanent differences between financial statement
and tax return income applicable to the Company in the various jurisdictions in which the Company operates. The effect of discrete items,
such as changes in estimates, changes in rates or tax status, and unusual or infrequently occurring events, is recognized in the interim
period in which the discrete item occurs. The accounting estimates used to compute the provision for income taxes may change as new events
occur, additional information is obtained or as the result of new judicial interpretations or regulatory or tax law changes.
The Company’s
interim effective tax rate, inclusive of discrete items, for the nine-month periods ended September 30, 2023 and 2022 was 26.83%.
Note
13 – Subsequent Events
On
October 12, 2023, the Company held a special meeting of the holders of its outstanding Tradeable Warrants in which the holders of the
majority of the outstanding Tradeable Warrants approved an amendment to the Warrant Agent Agreement to (i) reduce the exercise price
of the Tradeable Warrants to $0.11, subject to further adjustment as provided therein, and (ii) eliminate the provision that prohibits
the Company’s CEO from exercising his voting rights under his Series B Preferred Stock.
In
December 2022, the Company had entered into a research agreement with the Jewish General Hospital (“JGH”), Montreal, Canada
to conduct IND-enabling studies of the Company’s anticancer drug candidate, Adva-27a (the “Research Agreement”). In
August 2023, the Company was advised by JGH that the lab results on testing of the Adva-27a molecule were not favorable. After conclusion
of an internal review of the lab results on November 2, 2023, the Company provided notice of termination of the Research Agreement, which
will become effective on December 2, 2023, pursuant to the terms of the Research Agreement. The Company has now paused the IND-enabling
studies of Adva-27a pending a review of the possibility of chemical modification of the compound to address the suboptimal performance
of the molecule in certain studies.
Report
of Independent Registered Public Accounting Firm
To
the shareholders and the board of directors of Sunshine Biopharma, Inc.:
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of Sunshine Biopharma, Inc. (the "Company") as of December 31, 2022
and 2021, the related consolidated statements of operations and comprehensive income (loss), shareholders' equity, and cash flows for
each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the "financial statements").
In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December
31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022,
in conformity with accounting principles generally accepted in the United States.
Basis
for Opinion
These
financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's
financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or
fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides
a reasonable basis for our opinion.
Critical
Audit Matters
Critical
audit matters are matters arising from the current period audit of the financial statements that were communicated or are required to
be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements
and (2) involved especially challenging, subjective, or complex judgments.
We
determined that there are no critical audit matters.
/s/ BF
Borgers CPA PC (PCAOB ID 5041)
BF
Borgers CPA PC
We
have served as the Company's auditor since 2013
Lakewood,
CO
March
31, 2023
PCAOB ID 5041
Sunshine
Biopharma, Inc.
Consolidated Balance Sheets
| | |
| | | |
| | |
| | |
December 31, | | |
December 31, | |
| | |
2022 | | |
2021 | |
| ASSETS | |
| | | |
| | |
| | |
| | | |
| | |
| Current Assets: | |
| | | |
| | |
| Cash and cash
equivalents | |
$ | 21,826,437 | | |
$ | 2,045,167 | |
| Accounts receivable | |
| 1,912,153 | | |
| 7,798 | |
| Inventory | |
| 3,289,945 | | |
| 105,650 | |
| Prepaid expenses | |
| 283,799 | | |
| 29,625 | |
| Deposits | |
| – | | |
| 7,590 | |
| Total Current Assets | |
| 27,312,334 | | |
| 2,195,830 | |
| | |
| | | |
| | |
| Property and equipment | |
| 394,249 | | |
| 7,061 | |
| Intangible assets | |
| 776,856 | | |
| – | |
| Right-of-use-asset | |
| 760,409 | | |
| – | |
| | |
| | | |
| | |
| TOTAL ASSETS | |
$ | 29,243,848 | | |
$ | 2,202,891 | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable & accrued
expenses | |
$ | 2,802,796 | | |
$ | 42,942 | |
| Earn-out payable | |
| 3,632,000 | | |
| – | |
| Interest payable | |
| – | | |
| 48,287 | |
| Income tax payable | |
| 373,191 | | |
| – | |
| Current
portion - Right-of-use-liability | |
| 123,026 | | |
| – | |
| Total Current Liabilities | |
| 6,931,014 | | |
| 91,229 | |
| | |
| | | |
| | |
| Long-Term Liabilities: | |
| | | |
| | |
| Notes payable | |
| – | | |
| 1,900,000 | |
| Right-of-use-liability | |
| 642,232 | | |
| – | |
| Deferred tax liability | |
| 43,032 | | |
| – | |
| Total Long-Term Liabilities | |
| 685,264 | | |
| 1,900,000 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES | |
| 7,616,278 | | |
| 1,991,229 | |
| | |
| | | |
| | |
| SHAREHOLDERS' EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| Preferred Stock, Series
B $0.10
par value per share; 1,000,000
shares authorized; 10,000
and 1,000,000
shares issued and outstanding as of December 31, 2022 and December
31, 2021, respectively | |
| 1,000 | | |
| 100,000 | |
| Common Stock, $0.001
par value per share; 3,000,000,000
shares authorized; 22,585,632
and 2,591,240
shares issued and outstanding as of December 31, 2022 and December
31, 2021, respectively | |
| 22,585 | | |
| 2,591 | |
| Capital paid in excess of par value | |
| 80,841,752 | | |
| 32,787,384 | |
| Accumulated comprehensive
income | |
| 161,847 | | |
| (23,139 | ) |
| Accumulated
(Deficit) | |
| (59,399,614 | ) | |
| (32,655,174 | ) |
| | |
| | | |
| | |
| TOTAL SHAREHOLDERS' EQUITY | |
| 21,627,570 | | |
| 211,662 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES AND
SHAREHOLDERS' EQUITY | |
$ | 29,243,848 | | |
$ | 2,202,891 | |
See
Accompanying Notes to These Consolidated Financial Statements.
Sunshine
Biopharma, Inc.
Consolidated Statements of Operations and Comprehensive Loss
| | |
| | | |
| | |
| | |
December 31, | | |
December 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Revenue | |
$ | 4,345,603 | | |
$ | 228,426 | |
| Cost of sales | |
| 2,649,028 | | |
| 117,830 | |
| Gross profit | |
| 1,696,575 | | |
| 110,596 | |
| | |
| | | |
| | |
| General and Administrative Expenses: | |
| | | |
| | |
| Accounting | |
| 341,139 | | |
| 118,423 | |
| Consulting | |
| 842,894 | | |
| 50,873 | |
| Directors Fees | |
| 300,000 | | |
| – | |
| Legal | |
| 565,265 | | |
| 232,616 | |
| Marketing | |
| 578,085 | | |
| – | |
| Office | |
| 796,007 | | |
| 248,561 | |
| R&D | |
| 811,858 | | |
| 672,209 | |
| Salaries | |
| 6,054,962 | | |
| 1,215,307 | |
| Taxes | |
| 55,233 | | |
| – | |
| Depreciation
and amortization | |
| 25,163 | | |
| 12,741 | |
| Goodwill impairment | |
| 18,326,719 | | |
| – | |
| Total General and Administrative
Expenses | |
| 28,697,325 | | |
| 2,550,730 | |
| | |
| | | |
| | |
| (Loss) from operations | |
| (27,000,750 | ) | |
| (2,440,134 | ) |
| | |
| | | |
| | |
| Other Income (Expenses): | |
| | | |
| | |
| Loss on debt conversions | |
| – | | |
| (9,726,485 | ) |
| Foreign exchange | |
| (476 | ) | |
| 50 | |
| Interest income | |
| 518,650 | | |
| – | |
| Interest expense | |
| (39,412 | ) | |
| (328,818 | ) |
| Debt forgiveness | |
| 10,852 | | |
| 51,031 | |
| Interest
forgiveness | |
| – | | |
| 7,909 | |
| Total Other Income (Expenses) | |
| 489,614 | | |
| (9,996,313 | ) |
| | |
| | | |
| | |
| Net (loss) before income taxes | |
| (26,511,136 | ) | |
| (12,436,447 | ) |
| Provision
for income taxes | |
| 233,304 | | |
| – | |
| Net (Loss) | |
| (26,744,440 | ) | |
| (12,436,447 | ) |
| Comprehensive income (loss): | |
| | | |
| | |
| Gain
(Loss) from foreign exchange translation | |
| 184,986 | | |
| (20,268 | ) |
| Comprehensive (Loss) | |
$ | (26,559,454 | ) | |
$ | (12,456,715 | ) |
| | |
| | | |
| | |
| Basic and diluted (Loss)
per common share | |
$ | (1.76 | ) | |
$ | (4.76 | ) |
| | |
| | | |
| | |
| Weighted average common shares outstanding
(Basic & Diluted) | |
| 15,180,868 | | |
| 2,612,061 | |
See
Accompanying Notes to These Consolidated Financial Statements.
Sunshine
Biopharma, Inc.
Consolidated Statements of Cash Flows
| | |
| | | |
| | |
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| Cash Flows From Operating
Activities: | |
| | |
| |
| Net
(Loss) | |
$ | (26,744,440 | ) | |
$ | (12,436,447 | ) |
| Adjustments to reconcile net
loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation
and amortization | |
| 25,163 | | |
| 12,741 | |
| Goodwilll impairment | |
| 18,326,719 | | |
| – | |
| Foreign
exchange (gain) | |
| 548 | | |
| (50 | ) |
| Stock issued
for services | |
| – | | |
| 918,000 | |
| Debt release | |
| (10,852 | ) | |
| – | |
| Loss on
debt conversion | |
| – | | |
| 9,726,485 | |
| Gain on
interest and debt forgiveness | |
| – | | |
| 58,940 | |
| Changes in operating assets
and liabilities: | |
| | | |
| | |
| Accounts
receivable | |
| (524,486 | ) | |
| (5,882 | ) |
| Inventory | |
| 42,983 | | |
| (81,879 | ) |
| Prepaid
expenses | |
| 82,846 | | |
| (26,847 | ) |
| Accounts
payable & accrued expenses | |
| 3,359,141 | | |
| (18,156 | ) |
| Deferred
tax liability | |
| 3,628 | | |
| – | |
| Income
tax payable | |
| 238,679 | | |
| – | |
| Interest
payable | |
| (48,287 | ) | |
| 23,967 | |
| Net
Cash Flows (Used) in Operations | |
| (5,248,358 | ) | |
| (1,829,128 | ) |
| | |
| | | |
| | |
| Cash Flows
From Investing Activities: | |
| | | |
| | |
| Reduction
in Right of use asset | |
| 33,379 | | |
| – | |
| Nora Pharma
Inc. acquisition | |
| (14,346,637 | ) | |
| – | |
| Cash from
Nora Pharma Inc. acquisition | |
| (1,135 | ) | |
| – | |
| Purchase
of intangible assets | |
| (111,015 | ) | |
| – | |
| Purchase
of equipment | |
| (193,982 | ) | |
| – | |
| Net
Cash Flows (Used) in Investing Activities | |
| (14,619,390 | ) | |
| – | |
| | |
| | | |
| | |
| Cash Flows
From Financing Activities: | |
| | | |
| | |
| Proceeds
public and private offerings of common stock, net | |
| 30,367,185 | | |
| – | |
| Warrant
exercises | |
| 13,193,177 | | |
| – | |
| Purchase of preferred shares | |
| (99,000 | ) | |
| – | |
| Reduction
in lease liability | |
| (31,924 | ) | |
| – | |
| Payoff
of Nora Pharma Inc.’s debt | |
| (2,064,331 | ) | |
| – | |
| Proceeds
from notes payable | |
| – | | |
| 3,318,500 | |
| Note payable
used to pay fees | |
| – | | |
| 61,500 | |
| Payments
of notes payable | |
| (1,900,000 | ) | |
| (475,325 | ) |
| Net
Cash Flows Provided by Financing Activities | |
| 39,465,107 | | |
| 2,904,675 | |
| | |
| | | |
| | |
| Cash and
Cash Equivalents at Beginning of Period | |
| 2,045,167 | | |
| 989,888 | |
| Net Increase (Decrease) in
cash and cash equivalents | |
| 19,597,359 | | |
| 1,075,547 | |
| Effect of exchange rate changes
on cash | |
| (1,075 | ) | |
| – | |
| Foreign
currency translation adjustment | |
| 184,986 | | |
| (20,268 | ) |
| Cash
and Cash Equivalents at End of Period | |
$ | 21,826,437 | | |
$ | 2,045,167 | |
| | |
| | | |
| | |
| Supplementary
Disclosure of Cash Flow Information: | |
| – | | |
| – | |
| Cash
paid for interest | |
$ | 48,287 | | |
$ | 38,117 | |
| Cash
paid for income taxes | |
$ | – | | |
$ | – | |
| Stock
issued for note conversions | |
$ | – | | |
$ | 12,705,214 | |
| Stock
issued for acquisition of Nora Pharma, Inc. | |
$ | 4,514,000 | | |
$ | – | |
See
Accompanying Notes to These Consolidated Financial Statements.
Sunshine
Biopharma, Inc.
Consolidated Statement of Shareholders' Equity
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Number Of Common Shares | | |
Common | | |
Capital Paid in Excess of Par | | |
Number Of Preferred Shares | | |
Preferred | | |
Compre-
hensive | | |
Accumulated | | |
| |
| | |
Issued | | |
Stock | | |
Value | | |
Issued | | |
Stock | | |
Income | | |
Deficit | | |
Total | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance December
31, 2020 | |
| 1,732,096 | | |
$ | 1,732 | | |
$ | 19,165,029 | | |
| 1,000,000 | | |
$ | 100,000 | | |
$ | (2,871 | ) | |
$ | (20,218,727 | ) | |
$ | (954,837 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common
stock issued for the reduction of debt and payment of interest | |
| 559,144 | | |
| 559 | | |
| 12,704,655 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 12,705,214 | |
| Common
stock issued for services | |
| 300,000 | | |
| 300 | | |
| 917,700 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 918,000 | |
| Net (loss) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (20,268 | ) | |
| (12,436,447 | ) | |
| (12,456,715 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
at December 31, 2021 | |
| 2,591,240 | | |
$ | 2,591 | | |
$ | 32,787,384 | | |
| 1,000,000 | | |
$ | 100,000 | | |
$ | (23,139 | ) | |
$ | (32,655,174 | ) | |
| 211,662 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Fractional
shares issued for reverse stock split | |
| 4,380 | | |
| 4 | | |
| (4 | ) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Common
stock and warrants issued in offerings | |
| 6,656,526 | | |
| 6,657 | | |
| 30,360,528 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 30,367,185 | |
| Exercise of warrants | |
| 9,633,486 | | |
| 9,633 | | |
| 13,183,544 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 13,193,177 | |
| Preferred
stock purchased from related party | |
| – | | |
| – | | |
| – | | |
| (990,000 | ) | |
| (99,000 | ) | |
| – | | |
| – | | |
| (99,000 | ) |
| Common
stock issued as part of Nora Pharma Inc. acquisition | |
| 3,700,000 | | |
| 3,700 | | |
| 4,510,300 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 4,514,000 | |
| Net
(loss) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 184,986 | | |
| (26,744,440 | ) | |
| (26,559,454 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
at December 31, 2022 | |
| 22,585,632 | | |
$ | 22,585 | | |
$ | 80,841,752 | | |
| 10,000 | | |
$ | 1,000 | | |
$ | 161,847 | | |
$ | (59,399,614 | ) | |
$ | 21,627,570 | |
See
Accompanying Notes to These Consolidated Financial Statements.
Sunshine
Biopharma, Inc.
Notes
to Consolidated Financial Statements
December
31, 2022 and 2021
Note
1 – Description of Business
Sunshine
Biopharma, Inc. (the “Company”) was originally incorporated under the name Mountain West Business Solutions, Inc. on August
31, 2006, in the State of Colorado.
Effective
October 15, 2009, the Company acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition. Sunshine Biopharma,
Inc. held an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a (the “License Agreement”).
Upon completion of the reverse acquisition transaction, the Company changed its name to Sunshine Biopharma, Inc. and began operating
as a pharmaceutical company focusing on the development of the licensed Adva-27a anticancer drug. In December 2015, the Company acquired
all rights to Adva-27a by purchasing PCT/FR2007/000697 and PCT/CA2014/000029 and terminated the License Agreement.
On
May 22, 2020, the Company filed a provisional patent application in the United States for a new treatment for Coronavirus infections.
The Company’s patent application covers composition subject matter pertaining to small molecules for inhibition of the main Coronavirus
protease, Mpro, an enzyme that is essential for viral replication. The patent application has a priority date of May 22, 2020. On April
30, 2021, the Company filed a PCT application containing new research results and extending coverage to include the Coronavirus Papain-Like
protease, PLpro. The priority date of May 22, 2020 has been maintained in the newly filed PCT application. The Company’s lead Anti-Coronavirus
compound arising from these patents bears the laboratory name SBFM-PL4.
On
April 20, 2022, the Company filed a provisional patent application in the United States covering mRNA molecules capable of destroying
cancer cells in vitro. The patent application contains composition and utility subject matter pertaining to the structure and sequence
of such mRNA molecules.
On
February 18, 2022, the Company entered into a research agreement (the “SRA”) with the University of Arizona for the purposes
of conducting research focused on determining the in vivo safety, pharmacokinetics, and dose selection properties of three University
of Arizona owned PLpro inhibitors, to be followed by efficacy testing in mice infected with SARS-CoV-2 (the “Research Project”).
Under the SRA, the University of Arizona granted the Company a first option to negotiate a commercial, royalty-bearing license for all
intellectual property developed by University of Arizona personnel under the Research Project. In addition, the Company and the University
of Arizona entered into an Option Agreement whereby the Company was granted a first option to negotiate a royalty-bearing commercial
license for the underlying technology of the Research Project. Encouraged by the results to date, the Company submitted a Notice of Option
Exercise to the University of Arizona on September 13, 2022.
On October 20,
2022, the Company acquired Nora Pharma Inc. (“Nora Pharma”), a Canadian generic pharmaceuticals company. Based in the greater
Montreal area, Nora Pharma has 37 employees and operates in a 15,000 square foot facility certified by Health Canada. Nora Pharma currently
offers 60 products, including 49 generic prescription drugs, and 11 OTC products. Nora Pharma sales were $10.7 million USD during its
fiscal year ended June 30, 2022. The consolidated financial statements contained in this report include the results of operations of
Nora Pharma from October 20, 2022 through December 31, 2022.
Note
2 – Summary of Significant Accounting Policies
This
summary of significant accounting policies is presented to assist the reader in understanding the Company's financial statements. The
consolidated financial statements and notes are representations of the Company's management, which is responsible for their integrity
and objectivity. These accounting policies conform to Generally Accepted Accounting Principles and have been consistently applied in
the preparation of the financial statements.
IMPACT
OF CORONAVIRUS (COVID-19) PANDEMIC
In
March 2020, the World Health Organization declared Coronavirus and its associated disease, COVID-19, a global pandemic. Conditions surrounding
the Coronavirus outbreak are continuing to evolve and government authorities around the world have and continue to implement various
measures to mitigate the spread of the virus. The outbreak and related mitigation measures have had and will continue to have a material
adverse impact on the world economies and the Company's business activities. It is not possible for the Company to predict the duration
or magnitude of the adverse conditions of the outbreak and their effects on the Company’s business or ability to raise funds. No
adjustments have been made to the amounts reported in the Company's financial statements as a result of this matter.
PRINCIPLES
OF CONSOLIDATION
The
accompanying consolidated financial statements include the accounts of the Company and its subsidiaries, all wholly owned. All intercompany
accounts and transactions have been eliminated in consolidation.
USE
OF ESTIMATES
The
preparation of financial statements in conformity with US Generally Accepted Accounting Principles requires management to make estimates
and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the
date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The more significant
estimates and assumptions made by management are valuation of equity instruments, depreciation of property and equipment, and deferred
tax asset valuation. Actual results could differ from those estimates as the current economic environment has increased the degree of
uncertainty inherent in these estimates and assumptions.
TRADE
ACCOUNTS RECEIVABLE AND ALLOWANCE FOR DOUBTFUL ACCOUNTS
Trade accounts
receivable are stated at net realizable value. The majority of customers are not extended credit and therefore time to maturity for receivables
is short. On a periodic basis, management evaluates its trade accounts receivable and determines whether to record an allowance for doubtful
accounts or if any accounts should be written off based on a past history of write-offs, collections and current credit conditions. A
receivable is considered past due if the Company has not received payments based on agreed-upon terms. The Company generally does not
require any security or collateral to support its receivables.
INVENTORY
VALUATION
Inventory
is valued at the lower of cost and net realizable value. Cost is determined using the first in, first out method. Net realizable value
is the estimated selling price in the ordinary course of business, less the costs of completion and costs necessary to make the sale.
The cost of inventory includes the purchase price and other costs directly attributable to the acquisition of finished goods.
CASH
AND CASH EQUIVALENTS
For
the Balance Sheets and Statements of Cash Flows, all highly liquid investments with maturity of 90 days or less are considered to be
cash equivalents. The Company had a cash balance of $21,826,437
and $2,045,167
as of December 31, 2022 and December 31, 2021, respectively. At
times such cash balances may be in excess of the FDIC limit of $250,000 in the U.S. or the equivalent in Canada.
PROPERTY
AND EQUIPMENT
Property
and equipment are reviewed for recoverability when events or changes in circumstances indicate that its carrying value may exceed future
undiscounted cash inflows. As of December 31, 2022 and 2021, the Company had not identified any such impairment. Repairs and maintenance
are charged to operations when incurred and improvements and renewals are capitalized.
Property
and equipment are stated at cost. Depreciation is calculated according to the following methods at the following annual rates and period
for financial reporting purposes and accelerated methods for tax purposes. Their estimated useful lives are as follows:
| Schedule of
estimated useful lives |
|
|
| Office Equipment: |
Straight-line and Declining balance
method |
5-7
Years / 20% |
| Computer Equipment: |
Declining balance method |
55% |
| Laboratory Equipment: |
Straight-line method |
5
Years |
| Vehicles: |
Straight-line and Declining balance method |
5
Years / 30% |
INTANGIBLE
ASSETS
Intangible
assets are amortized over their estimated useful lives according to the following methods at the following annual rates and period:
| Licenses: |
Straight-line
method |
5
Years |
| Website: |
Declining balance method |
55% |
Intangible
assets are tested for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable.
The carrying amount of a long-lived asset is not recoverable when it exceeds the sum of the undiscounted cash flows expected to result
from its use and eventual disposal. In such a case, an impairment loss must be recognized and is equivalent to the excess of the carrying
amount of a long-lived asset over its fair value.
INTELLECTUAL
PROPERTY RIGHTS - PATENTS
The
cost of patents acquired is capitalized and is amortized over the remaining life of the patents.
The
Company evaluates recoverability of identifiable intangible assets whenever events or changes in circumstances indicate that intangible
assets carrying amount may not be recoverable. Such circumstances include but are not limited to: (1) a significant decrease in the market
value of an asset, (2) a significant adverse change in the extent or manner in which an asset is used, or (3) an accumulation of cost
significantly in excess of the amount originally expected for the acquisition of an asset. The Company measures the carrying amount of
such assets against the estimated undiscounted future cash flows associated with it.
BASIC
AND DILUTED NET GAIN (LOSS) PER SHARE
The
Company computes loss per share in accordance with ASC 260, Earnings per Share. ASC 260 requires presentation of both basic and diluted
earnings per share (“EPS”) on the face of the income statement.
Basic
net income (loss) per share is calculated by dividing net (loss) by the weighted-average common shares outstanding. Diluted net income
per share is calculated by dividing net income by the weighted-average common shares outstanding during the period using the treasury
stock method or the two-class method, whichever is more dilutive. As the Company incurred net losses for the year ended December 31,
2022, no
potentially dilutive securities were included in the calculation
of diluted earnings per share as the impact would have been anti-dilutive.
INCOME
TAXES
In
accordance with ASC 740 – Income Taxes, the provision for income taxes is computed using the asset and liability method.
The liability method measures deferred income taxes by applying enacted statutory rates in effect at the balance sheet date to the differences
between the tax basis of assets and liabilities and their reported amounts on the financial statements. The resulting deferred tax assets
or liabilities have been adjusted to reflect changes in tax laws as they occur. A valuation allowance is provided when it is more likely
than not that a deferred tax asset will not be realized.
The
Company expects to recognize the financial statement benefit of an uncertain tax position only after considering the probability that
a tax authority would sustain the position in an examination. For tax positions meeting a "more-likely-than-not" threshold, the
amount to be recognized in the financial statements will be the benefit expected to be realized upon settlement with the tax authority.
For tax positions not meeting the threshold, no financial statement benefit is recognized. As of December 31, 2022 the Company had no
uncertain tax positions. The Company recognizes interest and penalties, if any, related to uncertain tax positions as general and administrative
expenses. The Company currently has no federal or state tax examinations nor has it had any federal or state examinations since its inception.
To date, the Company has not incurred any interest or tax penalties.
For
Canadian and US tax purposes, the Company’s 2019 through 2021 tax years remain open for examination by the tax authorities under
the normal three-year statute of limitations.
FUNCTIONAL
CURRENCY
The
U.S. dollar is the functional currency of the Company which is operating in the United States. The functional currency for the Company's
Canadian subsidiaries is the Canadian dollar.
The
Company translates its Canadian subsidiaries' financial statements into U.S. dollars as follows:
| |
· |
Assets and liabilities are
translated at the exchange rate in effect as of the financial statement date. |
| |
· |
Income statement accounts
are translated using the weighted average exchange rate for the period. |
The
Company includes translation adjustments from currency exchange and the effect of exchange rate changes on intercompany transactions
of a long-term investment nature as a separate component of shareholders’ equity. There are currently no transactions of a long-term
investment nature, nor any gains or losses from non U.S. currency transactions.
CONCENTRATION
OF CREDIT RISKS
Financial
instruments that potentially subject the Company to concentrations of credit risk consist principally of cash equivalents and trade receivables.
The Company places its cash equivalents with high credit quality financial institutions.
FINANCIAL
INSTRUMENTS AND FAIR VALUE OF FINANCIAL INSTRUMENTS
The
Company applies the provisions of accounting guidance, FASB Topic ASC 825, Financial Instruments. ASC 825 requires all entities to disclose
the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet, for which it
is practicable to estimate fair value, and defines fair value of a financial instrument as the amount at which the instrument could be
exchanged in a current transaction between willing parties. As of December 31, 2022 and 2021, the fair value of cash, accounts receivable
and notes receivable, accounts payable, accrued expenses, and other payables approximated carrying value due to the short maturity of
the instruments, quoted market prices or interest rates which fluctuate with market rates.
The
Company defines fair value as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction
between market participants at the measurement date. The Company applies the following fair value hierarchy, which prioritizes the inputs
used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is
available and significant to the fair value measurement. The hierarchy gives the highest priority to unadjusted quoted prices in active
markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements).
| |
· |
Level 1 – Level 1
inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability
to access at the measurement date. |
| |
|
|
| |
· |
Level 2 – Level 2
inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly
or indirectly. If the asset or liability has a specified (contractual) term, a Level 2 input must be observable for substantially
the full term of the asset or liability. |
| |
|
|
| |
· |
Level 3 – Level 3
inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability
at the measurement date. |
The
carrying value of financial assets and liabilities recorded at fair value is measured on a recurring or nonrecurring basis. Financial
assets and liabilities measured on a non-recurring basis are those that are adjusted to fair value when a significant event occurs. The
Company had no financial assets or liabilities carried and measured on a nonrecurring basis during the reporting periods. Financial assets
and liabilities measured on a recurring basis are those that are adjusted to fair value each time a financial statement is prepared.
NOTES
PAYABLE
Borrowings
are recognized initially at fair value, net of transaction costs incurred. Borrowings are subsequently carried at amortized cost; any
difference between the proceeds (net of transaction costs) and the redemption value is recognized in the income statement over the period
of the borrowings using the effective interest method.
ACCOUNTING
FOR DERIVATIVES LIABILITIES
The
Company evaluates stock options, stock warrants or other contracts to determine if those contracts or embedded components of those contracts
qualify as derivatives to be separately accounted for under the relevant sections of ASC Topic 815-40, Derivative Instruments and Hedging:
Contracts in Entity’s Own Equity. The result of this accounting treatment could be that the fair value of a financial instrument
is classified as a derivative instrument and is marked-to-market at each balance sheet date and recorded as a liability. In the event
that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operations as other income or
other expense.
Upon
conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value
is reclassified to equity. Financial instruments that are initially classified as equity that become subject to reclassification under
ASC Topic 815-40 are reclassified to a liability account at the fair value of the instrument on the reclassification date. The Company
determined that none of the Company’s financial instruments meet the criteria for derivative accounting as of December 31, 2022
and 2021.
EQUITY
INSTRUMENTS ISSUED TO EMPLOYEES OR NON-EMPLOYEES FOR ACQUIRING GOODS OR SERVICES
The
stock-based compensation expense for both employee and non-employee awards is generally recognized on a straight-line basis over the
requisite service period of the award. The Company accounts for stock-based compensation to employees in conformity with the provisions
of ASC Topic 718, Stock Based Compensation. Stock-based compensation to employees consisting of stock option grants and restricted shares
are recognized in the statement of operations based on their fair values at the date of grant. The Company accounts for equity instruments
issued to non-employees in accordance with the provisions of ASC Topic 718, based upon the fair-value of the underlying instrument.
REVENUE
RECOGNITION
The Company
generates sales from three revenue streams: (1) Generic Drugs, (2) OTC Supplements, and (3) Commissions Income.
In
Canada, governmental regulations require that companies recognize revenues upon completion of the work by issuing an invoice and remitting
the applicable sales taxes (GST and QST) to the appropriate government agency. The Company’s wholly owned Canadian subsidiaries'
revenue recognition policy is in compliance with these local regulations.
The
Company recognizes revenues for product sales and commissions when title and risk of loss has passed to the customer, which is typically
upon delivery to the customer, when estimated rebates are reasonably determinable, and when collectability is reasonably assured.
Trade
sales and commissions are accounted for when persuasive evidence of an arrangement exists, the goods have been received by the client,
the price is fixed or determinable and collection is reasonably assured.
LEASES
The
Company recognizes and measures its leases in accordance with FASB ASC 842, Leases. The Company is a lessee in a non-cancellable operating
lease for office space. The Company determines if an arrangement is a lease, or contains a lease, at inception of a contract and when
the terms of an existing contract are changed. The Company recognizes a lease liability and a right-of-use (ROU) asset at the commencement
date. The lease liability is initially and subsequently recognized based on the present value of its future lease payments. Variable
payments are included in the future lease payments when those variable payments depend on an index or a rate. The discount rate is the
implicit rate if it is readily determinable or otherwise the Company uses its incremental borrowing rate. The implicit rates of the Company's
lease are not readily determinable and accordingly, the Company uses its incremental borrowing rate based on the information available
at the commencement date for all leases. The Company’s incremental borrowing rate for a lease is the 6% interest it would have
to pay on a collateralized basis to borrow an amount equal to the lease payments under similar terms and in a similar economic environment.
The ROU asset is subsequently measured throughout the lease term at the remaining amount (i.e. present value of the remaining lease
payments), plus unamortized initial direct costs, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of
lease incentives received, and any impairment recognized. Lease cost for lease payments is recognized on a straight-line basis over the
lease term.
The
Company has elected, for all underlying classes of assets, not to recognize ROU assets and lease liabilities for short-term leases that
have a lease term of 12 months or less at lease commencement, and do not include an option to purchase the underlying asset that the
Company is reasonably certain to exercise. The Company recognizes the lease cost associated with its short-term leases on a straight-line
basis over the lease term.
Under
the available practical expedient, we account for the lease and non-lease components as a single lease component for all classes of underlying
assets as both a lessee and lessor. Further, we elected a short-term lease exception policy on all classes of underlying assets, permitting
us to not apply the recognition requirements of this standard to short-term leases (i.e. leases with terms of 12 months or less).
LEGAL
FEES
During
the years ended December 31, 2022 and 2021, the legal fees incurred were related to services provided to the Company in connection with
the Securities and Exchange Commission requirements and other regulatory and contracts matters.
RECENTLY
ISSUED ACCOUNTING PRONOUNCEMENTS
The
Company has implemented all new accounting pronouncements that are in effect and that may impact its financial statements and does not
believe that there are any other new pronouncements that have been issued that might have a material impact on its financial position
or results of operations.
Note
3 – Acquisition of Nora Pharma Inc.
On October 20, 2022 the Company acquired all of
the issued and outstanding shares of Nora Pharma Inc. (“Nora” Pharma), a Canadian privately held company. The purchase price
for the shares was $18,860,637 which was paid in cash ($14,346,637) and by the issuance of 3,700,000 shares of the Company’s common
stock valued at $4,514,000 or $1.22 per share. Nora Pharma is a certified company offering generic pharmaceutical products in Canada.
Nora Pharma’s operations are authorized by a Drug Establishment License issued by Health Canada. Nora Pharma is also registered
with the FDA.
The following table summarizes the allocation of the purchase price as of October 20, 2022, the acquisition date using Nora
Pharma’s balance sheet assets and liabilities:
| Schedule of allocation of the purchase price | |
| | |
| Accounts receivable | |
$ | 1,358,121 | |
| Inventory | |
| 3,181,916 | |
| Intangible assets | |
| 659,571 | |
| Equipment & furniture | |
| 210,503 | |
| Other assets | |
| 1,105,093 | |
| Total assets | |
| 6,515,204 | |
| | |
| | |
| Liabilities
assumed | |
| (5,981,286 | ) |
| | |
| | |
| Net assets | |
| 533,918 | |
| | |
| | |
| Goodwill | |
| 18,326,719 | |
| | |
| | |
| Total
Consideration | |
$ | 18,860,637 | |
Management
has determined that going forward it is in the best interest of the Company to impair 100% of the goodwill in the
current, 2022 fiscal year. The Company will review the value of the intangible and other assets on an annual basis and make
adjustments to the carrying amounts as necessary.
The
fair value of the 3,700,000 common shares issued as part of the consideration paid for Nora Pharma was determined on the basis of
the closing market price of the Company’s common shares on the acquisition date, October 20, 2022 ($1.22 per
share).
The
fair value of the financial assets acquired includes receivables, Inventory, furniture, fixtures, and processing equipment, and right
to use assets was $5,858,369.
The
unaudited financial information in the table below summarizes the combined results of operations of the Company (Sunshine Biopharma and
Nora Pharma) for the years ended December 31, 2022 and 2021, on a pro forma basis, as though the companies had been combined as of January
1, 2021. The unaudited pro forma financial information does not purport to be indicative of the Company's combined results of operations
which would actually have been obtained had the acquisition taken place on January 1, 2021, nor should it be taken as indicative of future
consolidated results of operations.
| Pro
Forma results from acquisition | |
December
31,
2022 | | |
December
31,
2021 | |
| Total
revenues | |
$ | 14,758,115 | | |
$ | 7,927,165 | |
| Net (loss)
from operations | |
$ | (26,192,503 | ) | |
$ | (2,224,253 | ) |
| Net (loss) | |
$ | (26,164,764 | ) | |
$ | (12,289,655 | ) |
| | |
| | | |
| | |
| Basic and fully
(loss) per share | |
$ | (1.74 | ) | |
$ | (4.70 | ) |
| Weighted average shares outstanding | |
| 15,056,097 | | |
| 2,612,061 | |
In
addition, the Company paid off Nora Pharma’s debt by making cash payments totaling $2,064,331 directly to Nora Pharma creditors
at or before closing in order to secure creditor consent for the acquisition transaction.
Note 4 – Earnout
As part of the Nora Pharma acquisition the Company
agreed to an earnout of $5,000,000 CAD ($3,632,000 USD) payable to Mr. Chamoun, the Seller. The earnout is payable in the form of twenty
(20) payments of $250,000 CAD for every $1,000,000 CAD increase in gross sales (as defined in the Purchase Agreement) above Nora Pharma’s
June 30, 2022 gross sales, provided that his employment with the Company is not terminated pursuant to the Company’s Employment
Agreement with him. The total earnout amount of $3,632,000 has been recorded as a salary payable.
Note 5 – Goodwill and Intangible Assets
As
result of the Nora Pharma acquisition the Company now has goodwill of $18,226,881 and
intangible assets of $659,571
on its balance sheet. Management has determined that it is in the best interest of the Company to (i) impair 100% of the goodwill in
the current, 2022 fiscal year, and (ii) review the intangible assets for amortization or possible partial of full impairment on an
annual basis.
Note
6 – Patents and Other Intellectual Property
The following
is a list of the patents and other intellectual property held by the Company at December 31, 2022:
In
December 2015, the Company acquired all worldwide issued (US Patent Number 8,236,935, and US Patent Number 10,272,065) and pending patents
under PCT/FR2007/000697 and PCT/CA2014/000029 for the Adva-27a anticancer compound.
On
May 22, 2020, the Company filed a provisional patent application in the United States for a new treatment for Coronavirus infections.
The Company’s patent application covers composition subject matter pertaining to small molecules for inhibition of the main Coronavirus
protease, Mpro, an enzyme that is essential for viral replication. The patent application has a priority date of May 22, 2020. On April
30, 2021, the Company filed a PCT application containing new research results and extending coverage to include the Coronavirus Papain-Like
protease, PLpro. The priority date of May 22, 2020 has been maintained in the newly filed PCT application.
On
April 20, 2022, the Company filed a provisional patent application in the United States covering mRNA molecules capable of destroying
cancer cells in vitro. The patent application contains composition and utility subject matter pertaining to the structure and sequence
of such mRNA molecules.
In addition, the
Company owns 152 DIN’s issued by Health Canada for prescription drugs currently on the market in Canada. These DIN’s were
secured through in-licenses or cross-licenses from international manufacturers of generic pharmaceutical products.
The Company also
owns two NPN’s issued by Health Canada: (i) NPN 80089663 authorizes us to manufacture and sell our in-house developed OTC supplement,
Essential 9™, and (ii) NPN 80093432 authorizes us to manufacture and sell the OTC supplement, Calcium-Vitamin D under the brand
name Essential Calcium-Vitamin D™.
Note
7 – Reverse Stock Splits
Effective
February 9, 2022, the Company completed a 1
for 200 reverse split of its common stock. The
Company had previously completed two 20 to 1 reverse stock splits, one in 2019 and the other in 2020.
The Company’s
financial statements reflects all three reverse stock splits on a retroactive basis for all periods presented and for all references
to common stock, unless specifically stated otherwise.
Note
8 – Capital Stock
The Company’s
authorized capital is comprised of 3,000,000,000
shares of $0.001
par value common stock and 30,000,000
shares of $0.10
par value preferred stock, to have such rights
and preferences as the Directors of the Company have or may assign from time to time. Out of the authorized Preferred Stock, the Company
had previously designated 850,000 shares as Series “A” Preferred Stock (“Series A”). At December 31, 2019, the
Company had no issued and outstanding shares of Series A. On June 17, 2020, the Company filed an amendment to its Articles of Incorporation
(the “Amendment”) eliminating the Series A shares and the designation thereof, which shares were returned to the status of
undesignated shares of Preferred Stock. In addition, the Amendment increased the number of authorized Series B Preferred Shares from
five hundred thousand (500,000) to one million (1,000,000) shares. The Series B Preferred Stock is non-convertible, non-redeemable and
non-retractable. It has superior liquidation rights to the common stock at $0.10 per share and gives the holder the right to 1,000 votes
per share. As of December 31, 2021, there were 1,000,000
shares of the Series B Preferred Stock held by
the CEO of the Company.
On
February 17, 2022, the Company’s public offering closed and the Company received net proceeds of $6,833,071
from the offering. Pursuant to the public offering, the Company
issued and sold an aggregate of 1,882,353
shares of common stock and 4,102,200
warrants to purchase shares of common stock (the “Tradeable
Warrants”) (including 337,494 Tradeable Warrants resulting from partial exercise of the overallotment option granted to the underwriter).
On
February 22, 2022, the Company redeemed 990,000
shares of Series B Preferred Stock from the CEO of the Company
at a redemption price equal to the stated value of $0.10 per share.
On
March 14, 2022, the Company completed a private placement and received net proceeds of $6,781,199.
In connection with this private placement, the Company issued (i) 2,301,353
shares of its common stock together with investor warrants (“Investor
Warrants”) to purchase up to 2,301,353
shares of common stock, and (ii) 1,302,251
pre-funded warrants (“Pre-Funded Warrants”) with each
Pre-Funded Warrant exercisable for one share of common stock, together with Investor Warrants to purchase up to 1,302,251 shares of common
stock. Each share of common stock and accompanying Investor Warrant was sold together at a combined offering price of $2.22 and each
Pre-Funded Warrant and accompanying Investor Warrant were sold together at a combined offering price of $2.219. The Pre-Funded Warrants
were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded Warrants
are exercised in full. The Investor Warrants have an exercise price of $2.22 per share (subject to adjustment as set forth in the warrant),
are exercisable upon issuance and will expire five years from the date of issuance.
On
April 28, 2022, the Company completed another private placement and received net proceeds of $16,752,915.
In connection with this private placement, the Company issued (i) 2,472,820 shares
of its common stock together with warrants (“April Warrants”) to purchase up to 4,945,640 shares
of common stock, and (ii) 2,390,025 pre-funded
warrants (“Pre-Funded Warrants”) with each Pre-Funded Warrant exercisable for one share of common stock, together with
April Warrants to purchase up to 4,780,050 shares of common stock. Each share of common stock and accompanying two April Warrants
were sold together at a combined offering price of $4.01 and each Pre-Funded Warrant and accompanying two April Warrants were sold
together at a combined offering price of $4.009. The Pre-Funded Warrants were immediately exercisable, at a nominal exercise price
of $0.001, and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The April Warrants have an
exercise price of $3.76
per share (subject to adjustment as set forth in the warrant), are exercisable upon issuance and will expire five years from the
date of issuance.
On October 20, 2022, the Company issued 3,700,000
shares of Common Stock as part of the acquisition of Nora Pharma. These shares were valued at $4,514,000, or $1.22 per share.
During
the fiscal year ended December 31, 2021, the Company issued an aggregate of 559,144
shares of its Common Stock valued at $12,705,214
in connection with the conversion of $2,867,243
in debt and interest of $127,986
resulting in a loss of $9,726,485
on conversion. In addition, the Company issued 300,000
shares of its Common Stock valued at $918,000
as compensation to its directors. In total, 859,114
shares of Common Stock were issued during the fiscal year ended
December 31, 2021.
Through December
31, 2022 and December 31, 2021, the Company has issued and outstanding a total of 22,585,632
and 2,591,240
shares of Common Stock, respectively.
The
Company has declared no dividends since inception.
Note
9 – Warrants
The
Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10 or ASC 815-40. Under ASC 480-10, warrants
are considered a liability if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number
of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to
determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement
for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are
measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after
the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification
under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed
to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP standard. Equity-classified
warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
During
the fiscal year ended December 31, 2022, the Company completed three financing events, and in connection therewith, it issued warrants
as follows:
| Warrants issued
with financing |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise
Price |
|
Expiry
Date |
| Pre-Funded Warrants |
|
3,692,276 |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
4,102,200 |
|
$2.22* |
|
February
2027 |
| Investor Warrants |
|
3,603,604 |
|
$2.22 |
|
March
2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April
2027 |
| * |
The
Tradeable Warrants had an initial exercise price of $4.25, subject to adjustment. Upon the closing of the Company’s private
placement on March 14, 2022, the exercise price of the Tradeable Warrants was reduced to $2.22, in accordance with the terms thereof. |
During
the fiscal year ended December 31, 2022, all of the Pre-Funded Warrants and a total of 3,138,507
Tradeable Warrants were exercised resulting in aggregate proceeds
of $6,971,178
received by the Company. In addition, during the fiscal year ended
December 31, 2022, a total of 2,802,703
Investor Warrants were exercised resulting in aggregate proceeds
of $6,222,001
received by the Company.
The Company’s
outstanding warrants at December 31, 2022 consisted of the following:
| Schedule of outstanding warrants |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise
Price |
|
Expiry
Date |
| Pre-Funded Warrants |
|
None |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
963,693 |
|
$2.22 |
|
February
2027 |
| Investor Warrants |
|
800,901 |
|
$2.22 |
|
March
2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April
2027 |
At
December 30, 2022, the final trading day of the year, the closing price of the Company’s common stock was $0.64 per share, a value
well below the exercise price of these warrants.
Note
10 – Earnings Per Share
The
following table sets forth the computation of basic and diluted net income per share for the years ended December 31:
| Schedule of
earnings per share computation | |
| | | |
| | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Net gain (loss)
attributable to common stock | |
$ | (26,744,440 | ) | |
$ | (12,436,447 | ) |
| Basic weighted average outstanding
shares of common stock | |
| 15,180,868 | | |
| 2,612,061 | |
| Dilutive common share equivalents | |
| 0 | | |
| 0 | |
| Dilutive weighted average outstanding
shares of common stock | |
| 15,180,868 | | |
| 2,612,061 | |
| Net gain (loss) per share attributable
to common stock | |
$ | (1.76 | ) | |
$ | (4.76 | ) |
Note
11 – Income Taxes
The
components of the provision for income taxes were as follows:
| Provision for income taxes | |
| |
| Current: | |
| |
| Federal | |
$ | – | |
| State | |
| – | |
| Foreign | |
| 139,856 | |
| Deferred: | |
| | |
| Federal | |
| – | |
| State | |
| – | |
| Foreign | |
| 3,628 | |
| Total | |
$ | 143,484 | |
The
components of the net deferred tax assets were as follows:
| Components
of net deferred tax assets | |
| |
| Deferred Tax Assets: | |
| |
| Net
Operating Loss, Credits and Carryforwards | |
$ | 4,323,025 | |
| Fixed Assets | |
| 98,957 | |
| Intangibles | |
| 1,021,230 | |
| Research and
Development | |
| 90,000 | |
| Other DTA | |
| 161,719 | |
| Lease Liability | |
| 202,793 | |
| Valuation
Allowance | |
| (5,596,431 | ) |
| Total
Deferred Tax Assets | |
| 301,293 | |
| | |
| | |
| Deferred Tax
Liabilities: | |
| | |
| Fixed Assets | |
| – | |
| Intangibles | |
| (142,817 | ) |
| Right-of-Use
Asset | |
| (201,508 | ) |
| Total
Deferred Tax Liabilities | |
| (344,325 | ) |
| | |
| | |
| Net
Deferred Tax Liability | |
$ | (43,032 | ) |
Note
12 – Notes Payable
As
of December 31, 2022 and December 31, 2021, the Company had $0
and $1,900,000,
respectively in notes payable outstanding. At December 31, 2022 and December 31, 2021, total accrued interest on Notes Payable was $0
and $48,287,
respectively.
The
Company’s Notes Payable at December 31, 2021 consisted of the following:
On
April 20, 2021, the Company received monies in exchange for a Note Payable having a Face Value of $500,000
with interest accruing at 5%
due April
20, 2023. The Note was convertible after 180 days from issuance
into common stock at a price equal to $0.30 per share. On February 17, 2022, the Company paid off the entire principal balance of this
Note, together with accrued interest of $20,753
by making cash payment of $520,753.
On
July 6, 2021, the Company received monies in exchange for a Note Payable having a Face Value of $900,000
with interest accruing at 5%,
due July
6, 2023. The Note was convertible after 180 days from issuance
into common stock at a price equal to $0.30 per share. On February 17, 2022, the Company paid off the entire principal balance of this
Note, together with accrued interest of $27,863
by making cash payment of $927,863.
On
August 18, 2021, the Company received monies in exchange for a Note Payable having a Face Value of $500,000
with interest accruing at 5%,
due August
18, 2023. The Note was convertible after 180 days from issuance
into common stock at a price equal to $0.30 per share. On February 17, 2022, the Company paid off the entire principal balance of this
Note, together with accrued of $12,534
by making cash payment of $512,534.
At
December 31, 2022 and December 31, 2021, total accrued interest on Notes Payable was $-0-
and $48,287,
respectively.
Note
13 – Notes Payable - Related Party
A
Note Payable dated December 31, 2019 held by the CEO of the Company having a Face Value of $128,269
and accruing interest at 12%
was due December
31, 2020. On December 31, 2020, the Company renewed the Note together
with accrued interest of $15,392
for a 12-month period. The new Note has a face Value of $143,661,
accrues interest at 12%
per annum, and has a maturity date of December
31, 2021. On August 24, 2021, the Company paid off the entire principal
balance of this Note, together with accrued interest of $12,929
by issuing cash payment of $156,590.
Note
14 – Lease
The
Company has obligations as a lessee for office space with initial non-cancellable terms in excess of one year. The Company classified
the lease as an operating lease. The lease contains a renewal option for a period of five years. Because the Company is certain to exercise
the renewal option, the optional period is included in determining the lease term, and associated payments under the renewal option are
included in the lease payments. The Company’s lease does not include termination options for either party to the lease or restrictive
financial or other covenants. Payments due under the lease contract include fixed payments plus a variable Payment. The Company’s
office space lease requires it to make variable payments for the Company’s proportionate share of building’s property taxes,
insurance, and common area maintenance. These variable lease payments are not included in lease payments used to determine lease liability
and are recognized as variable costs when incurred.
Amounts
reported on the balance sheet as of December 31, 2022 were as follows:
| |
Lease information |
|
| |
Operating lease ROU asset |
$760,409 |
| |
Operating Lease liability - Short-term |
$123,026 |
| |
Operating lease liability - Long-term |
$642,232 |
| |
Remaining lease term |
7
years |
| |
Discount rate |
6% |
Amounts
disclosed for ROU assets obtained in exchange for lease obligations and reductions of ROU assets resulting from reductions of lease obligations
include amounts reduced from the carrying amount of ROU assets resulting from deferred rent.
Maturities
of lease liabilities under non-cancellable operating leases at December 31, 2022 are as follows:
| Maturities
of lease liabilities |
|
| 2023 |
$123,026 |
| 2024 |
$115,879 |
| 2025 |
$116,066 |
| 2026 |
$109,934 |
| 2027 |
$103,547 |
| Thereafter |
$196,807 |
Note
15 – Management and Director Compensation
The
Company paid its officers cash compensation totaling $1,785,000
and $297,307
for the years ended December 31, 2022 and 2021, respectively. Of
these amounts attributable to the Company’s CEO, $60,000
and $110,000,
respectively was paid to Advanomics Corporation, a company controlled by the CEO of the Company. In addition, the Company issued 300,000
shares of common stock valued at $918,000
to its officers during year ended December 31, 2021. The value
of these shares was based upon the closing price of the Company’s common stock of $3.06 on the issuance date.
The
Company paid its directors cash compensation totaling $300,000
and $0 for
the years ended December 31, 2022 and 2021, respectively.
Note
16 – Subsequent Events
On January 19,
2023, the Company announced a stock repurchase program of up to $2 million. As of the date of this report, the Company has repurchased
a total of 445,711 shares of Common Stock at an average price of $1.1371 per share for a total cost of $506,822. As of the date of this
report, the repurchased shares have not been returned to treasury.
39,215,687 Units, Each
Unit Consisting of One Share of Common Stock or One Pre-Funded Warrant to Purchase One Share of Common Stock, one-tenth of a Series A
Warrant to Purchase one Share of Common Stock and two-tenths of a Series B Warrant to Purchase one Share of Common Stock
11,764,706 Shares
of Common Stock Underlying the Series A and Series B Warrants

PROSPECTUS
Aegis Capital Corp.
,
2024
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses
paid or payable by us in connection with the sale of the securities being registered, other than underwriting discounts and commissions.
All amounts shown are estimates except for the Securities and Exchange Commission, or SEC, registration fee, the Nasdaq listing fee, and
the FINRA filing fee.
| Expense | |
Amount
Paid or
to be Paid | |
| SEC registration fee | |
$ | 10,015 | |
| FINRA filing fee | |
| 10,678 | |
| Legal fees and expenses | |
| 450,000 | |
| Accounting fees and expenses | |
| 20,000 | |
| Miscellaneous expenses | |
| 10,000 | |
| Expense reimbursement to underwriter | |
| 150,000 | |
| Total | |
$ | 650,693 | |
Item 14. Indemnification of Directors
and Officers.
Section 7-108-402 of the Colorado Business Corporation
Act (the “CBCA”) provides, generally, that the articles of incorporation may contain a provision eliminating or limiting the
personal liability of a director to the corporation or its shareholders for monetary damages for breach of fiduciary duty as a director,
except that any such provision shall not eliminate or limit the liability of a director for (i) any breach of the director’s duty
of loyalty to the corporation or its shareholders, (ii) acts or omissions not in good faith or which involve intentional misconduct or
a knowing violation of law, (iii) acts specified in Section 7-108-403 of the CBCA, or (iv) any transaction from which the director directly
or indirectly derived an improper personal benefit.
Section 7-109-102(1) of the CBCA permits
indemnification of a director of a Colorado corporation, in the case of a third party action, if the director (a) conducted himself
or herself in good faith, (b) reasonably believed that (i) in the case of conduct in his or her official capacity, his or her
conduct was in the corporation’s best interest, or (ii) in all other cases, his or her conduct was not opposed to the corporation’s
best interest, and (c) in the case of any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful.
Section 7-109-103 further provides for mandatory indemnification of directors and officers who are successful on the merits or otherwise
in litigation.
Section 7-109-102(4) of the CBCA limits
the indemnification that a corporation may provide to its directors in two key respects. A corporation may not indemnify a director
in a derivative action in which the director is held liable to the corporation, or in any proceeding in which the director is held liable
on the basis of his improper receipt of a personal benefit. Sections 7-109-104 of the CBCA permits a corporation to advance expenses
to a director, and Section 7-109-107(1)(c) of the CBCA permits a corporation to indemnify and advance litigation expenses to
officers, employees and agents who are not directors to a greater extent than directors if consistent with law and provided for by the
bylaws, a resolution of directors or shareholders, or a contract between the corporation and the officer, employee or agent.
Our bylaws include provisions that require the
company to indemnify our directors or officers against monetary damages for actions taken as a director or officer of our Company. We
are also expressly authorized to carry directors’ and officers’ insurance to protect our directors, officers, employees and
agents for certain liabilities. Our articles of incorporation do not contain any limiting language regarding director immunity from liability.
Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have
been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is
therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant
of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit
or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will,
unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction
the question whether such indemnification by us is against public policy as expressed hereby in the Securities Act and we will be governed
by the final adjudication of such issue.
Item 15. Recent Sales of Unregistered
Securities.
On February 12, 2021, we issued a note in the
principal amount of $700,000, convertible after 180 days from issuance into common stock at a price equal to $0.60 per share. This note
was converted to common stock on December 20, 2021.
On April 5, 2021, we issued a note in the
principal amount of $330,000. The note was convertible after 180 days from issuance into common stock at a price equal to the lower of
$60.00 or 35% below market. On October 13, 2021, the noteholder converted $330,000 in principal and $16,500 in accrued interest into 26,250
shares of common stock leaving a principal balance of $0.
On April 20, 2021, we issued a note in the principal
amount of $500,000. The note is convertible after 180 days from issuance into common stock at a price equal to $0.30 per share.
On April 22, 2021, a note holder converted a total
of $11,028 in principal and $4,472 in accrued interest into 77,500 shares of common stock.
On July 6, 2021, the Company issued a note in
the principal amount of $900,000, convertible after 180 days from issuance into common stock at a price of $0.30 per share. In connection
with this debt financing, the Company agreed to allow the lender, who is also the holder of a note dated November 25, 2020 (the “November
Note”), to convert a total of $240,000 in principal amount of the November Note into 120,000 shares of common stock leaving a principal
balance of $10,000 and accrued interest of $7,750.
On August 18, 2021, the Company issued a issued
a note in the principal amount of $500,000, convertible after 180 days from issuance into common stock at a price of $0.30 per share.
During the nine months ended September 30, 2021,
the Company issued a total of 518,370 shares of common stock valued at $11,981,072 for the conversion of outstanding notes, reducing the
debt by $1,233,028 and interest payable by $38,201 and generating a loss on conversion of $10,709,843.
During the nine months ended September 30, 2021,
the Company issued 300,000 shares of common stock to its officers and directors as compensation for their services to the Company.
On December 20, 2021, the Company
issued 14,524 shares of common stock upon conversion of $1,361,000 in convertible debt.
On March 10, 2022, the Company
entered into a securities purchase agreement with certain accredited investors for a private placement for the Company’s common
stock or pre-funded warrants and warrants exercisable for common stock. Pursuant to the purchase agreement, the Company sold (i) 2,301,353
shares of its common stock together with warrants to purchase up to 2,301,353 shares of common stock, and (ii) 1,302,251 pre-funded warrants
with each pre-funded warrant exercisable for one share of common stock, together with warrants to purchase up to 1,302,251 shares of common
stock. Each share of common stock and accompanying warrant were sold together at a combined offering price of $2.22, and each pre-funded
warrant and accompanying warrant were sold together at a combined offering price of $2.219. The pre-funded warrants were immediately exercisable,
at a nominal exercise price of $0.001, and may be exercised at any time until all of the pre-funded warrants are exercised in full. The
warrants had an initial exercise price of $2.22 per share, were exercisable upon issuance and will expire five years from the date of
issuance. Aegis Capital Corp. acted as the placement agent in connection with the private placement. Aegis was paid a commission equal
to 10% of the gross proceeds received by the Company in the private placement and 2% of the gross proceeds as a non-accountable expense
allowance. The Company paid Aegis $100,000 for fees and expenses including attorney fees.
On April 25, 2022, the
Company entered into a securities purchase agreement with certain accredited investors for a private placement for the Company’s
common stock or pre-funded warrants, and warrants exercisable for common stock. Pursuant to the purchase agreement, the Company sold (i)
2,472,820 shares of its common stock, (ii) warrants to purchase up to 9,725,690 shares of common stock, and (iii) 2,390,025 pre-Funded
warrants with each pre-funded warrant exercisable for one share of common stock. Each share of common stock and accompanying warrants
were sold together at a combined offering price of $4.01, and each pre-funded warrant and accompanying two warrants were sold together
at a combined offering price of $4.009. The pre-funded warrants were immediately exercisable, at a nominal exercise price of $0.001, and
may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrants had an initial exercise price of
$3.76 per share (subject to adjustment as set forth therein), were exercisable upon issuance and will expire five years from the date
of issuance.
Aegis acted as the placement
agent in connection with the private placement and was paid a commission equal to 10% of the gross proceeds received by the Company, and
a non-accountable expense allowance equal to 2% of the gross proceeds, and will receive 5% of the proceeds from any exercise of warrants,
payable on exercise.
During the three months
ended June 30, 2022, the Company issued 2,802,703 shares of common stock upon exercise of warrants with an exercise price of $2.22, and
3,692,276 shares of common stock upon exercise of pre-funded warrants with an exercise price of $0.001.
Effective October 20,
2022, the Company entered into a share purchase agreement with Malek Chamoun and Nora Pharma, wherein the Company acquired all of the
issued and outstanding shares of Nora Pharma in consideration of a purchase price of $30,000,000 CAD (approximately $21,900,000 USD),
which was paid as follows: (a) $5,000,000 CAD (approximately $3,650,000 USD) by issuance of 3,700,000 shares of the Company’s common
stock; (b) $20,000,000 CAD (approximately $14,600,000 USD) in cash, which was subject to customary adjustments in accordance with the
Purchase Agreement; and (c) 5,000,000 CAD (approximately $3,650,000 USD) in the form of earn-out payable to Mr. Chamoun once earned in
a maximum of twenty (20) payments of $250,000 CAD for every $1,000,000 CAD increase in gross sales (as defined in the Purchase Agreement)
above Nora Pharma’s June 30, 2022 gross sales, provided that his employment with the Company is not terminated pursuant to the Company’s
employment agreement with him.
On May 12, 2023, the
Company entered into a securities purchase agreement with a certain accredited investor for a private placement for the Company’s
common stock, pre-funded warrants, each exercisable to purchase one share of common stock, and warrants, each exercisable to purchase
one share of common stock. Pursuant to the purchase agreement, the Company sold (i) 2,450,000 shares of its common stock, (ii) warrants
to purchase up to 11,904,762 shares of common stock, and (iii) 3,502,381 pre-funded warrants. Each share of common stock and accompanying
two warrants were sold together at a combined offering price of $0.84, and each pre-funded warrant and accompanying two warrants were
sold together at a combined offering price of $0.839. The pre-funded warrants are immediately exercisable, at a nominal exercise price
of $0.001, and may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrants have an initial exercise
price of $0.59 per share (subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years
from the date of issuance.
Aegis acted as the placement
agent in connection with the private placement and was paid a commission equal to 10% of the gross proceeds received by the Company, and
a non-accountable expense allowance equal to 2% of the gross proceeds, and will receive 10% of the proceeds from any exercise of warrants,
payable on exercise.
On February 8, 2024, the Company issued and
sold 20,000 shares of Series B Preferred Stock to Dr. Steve Slilaty for a purchase price equal to the stated value of $0.10 per share.
In connection with the foregoing, we relied upon
the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended, for transactions not involving
a public offering.
Item 16. Exhibits and Financial Statement
Schedules.
| 1.1 |
|
Form of Underwriting Agreement (filed herewith) |
| 3.1 |
|
Articles
of Incorporation (2) |
| 3.2 |
|
Certificate
of Amendment to Articles of Incorporation filed November 2, 2009 (3) |
| 3.3 |
|
Statement
of Share and Equity Capital Exchange (4) |
| 3.4 |
|
Articles
of Amendment to Articles of Incorporation filed July 13, 2010 (4) |
| 3.5 |
|
Articles
of Amendment to Articles of Incorporation filed May 27, 2015 (5) |
| 3.6 |
|
Articles
of Amendment to Articles of Incorporation (6) |
| 3.7 |
|
Articles
of Amendment to Articles of Incorporation (7) |
| 3.8 |
|
Bylaws
(14) |
| 4.1 |
|
Description
of Registrant’s Securities (16) |
| 5.1 |
|
Opinion of Andrew I. Telsey, P.C. (filed herewith) |
| 5.2 |
|
Opinion of Sichenzia Ross Ference Carmel LLP (filed herewith) |
| 10.1 |
|
Patent
Purchase Agreement with Advanomics Corporation (8) |
| 10.2 |
|
Second
Patent Purchase Agreement with Advanomics Corporation (9) |
| 10.3 |
|
Amendment
No. 1 to Patent Purchase Agreement with Advanomics Corporation dated October 8, 2016, including Secured Convertible Promissory Note
(10) |
| 10.4 |
|
Amendment
No. 1 to Patent Purchase Agreement with Advanomics Corporation dated December 28, 2016, including Secured Convertible Promissory
Note (10) |
| 10.5 |
|
Form
of Warrant, dated February 17, 2024 (1) |
| 10.6 |
|
Warrant
Agent Agreement between the Company and Equiniti , dated February 17, 2022 (1) |
| 10.7 |
|
Sponsored
Research Agreement, dated October 6, 2020, between the Company and the University of Georgia Research Foundation, Inc.
(11) * |
| 10.8 |
|
Research
Agreement between the Company and Arizona Board of Regents on behalf of the University of Arizona (12) |
| 10.9 |
|
Form of Warrant, dated March 14, 2022 (15) |
| 10.10 |
|
Form
of Amendment to Warrant, dated March 24, 2022 (17) |
| 10.11 |
|
Employment
Agreement between Sunshine Biopharma, Inc. and Dr. Steve Slilaty (18) |
| 10.12 |
|
Form
of Warrant, dated April 28, 2022 (19) |
| 10.13 |
|
Share
Purchase Agreement between Sunshine Biopharma, Inc., Malek Chamoun and Nora Pharma Inc. (20) |
| 10.14 |
|
Employment
Agreement between Sunshine Biopharma, Inc., Nora Pharma Inc. and Malek Chamoun (20) |
| 10.15 |
|
License
Agreement between the Company and the University of Arizona (22) ** |
| 10.16 |
|
Form of Warrant, dated May 16, 2023 (22) |
| 10.17 |
|
Amendment No. 1 to Warrant Agent Agreement, dated October 18, 2023 (23) |
_______________________
| |
* |
Portions of the exhibit have been omitted. |
| (1) |
Incorporated by reference to 8-K filed with the SEC on February 17, 2022 |
| (2) |
Incorporated by reference to SB-2 filed with the SEC on October 19, 2007. |
| (3) |
Incorporated by reference to 8-K filed with the SEC on November 6, 2009. |
| (4) |
Incorporated by reference to 10-Q filed with the SEC on August 4, 2010. |
| (5) |
Incorporated by reference to 8-K filed with the SEC on June 1, 2015. |
| (6) |
Incorporated by reference to 8-K filed with the SEC on June 24, 2020. |
| (7) |
Incorporated by reference to 8-K filed February 9, 2022. |
| (8) |
Incorporated by reference to 8-K filed with the SEC on October 9, 2015. |
| (9) |
Incorporated by reference to 8-K filed with the SEC on December 28, 2015. |
| (10) |
Incorporated by reference to 8-K filed with the SEC on March 14, 2016. |
| (11) |
Incorporated by reference to S-1/A filed with the SEC on January 24, 2022. |
| (12) |
Incorporated by reference to 8-K filed with the SEC on February 25, 2022. |
| (13) |
Incorporated by reference to 10-K filed with the SEC on May 1, 2020. |
| (14) |
Incorporated by reference to 8-K filed with the SEC on April 19, 2023. |
| (15) |
Incorporated by reference to 8-K filed with the SEC on March 15, 2022. |
| (16) |
Incorporated by reference to 10-K filed with the SEC on March 21, 2022. |
| (17) |
Incorporated by reference to 8-K filed with the SEC on March 24, 2022. |
| (18) |
Incorporated by reference to 8-K filed with the SEC on April 8, 2022. |
| (19) |
Incorporated by reference to 8-K filed with the SEC on April 28, 2022. |
| (20) |
Incorporated by reference to 8-K filed with the SEC on October 20, 2022. |
| (21) |
Incorporated by reference to 8-K filed with the SEC on February 28, 2023. |
| (22) |
Incorporated by reference to 8-K filed with the SEC on May 16, 2023. |
| (23) |
Incorporated by reference to 8-K filed with the SEC on October 20, 2023. |
| (24) |
Incorporated by reference to S-8 filed with the SEC on January 8, 2024. |
| (25) |
Incorporated by reference to 10-K filed with the SEC on April 4, 2023. |
(b) Financial statement schedule.
None.
Item 17. Undertakings.
| (a) |
The undersigned registrant hereby undertakes: |
| |
|
| (1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement: |
| |
|
| (i) |
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| |
|
| (ii) |
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| |
|
| (iii) |
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) |
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
|
| (3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| |
|
| (4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| |
|
| (i) |
If the registrant is relying on Rule 430B (§230.430B of this chapter): |
| |
|
| (A) |
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) (§230.424(b)(3) of this chapter) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| |
|
| (B) |
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) (§230.424(b)(2), (b)(5), or (b)(7) of this chapter) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) (§230.415(a)(1)(i), (vii), or (x) of this chapter) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or |
| (ii) |
If the registrant is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| |
|
| (5) |
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
| |
|
| |
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
|
| (i) |
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter); |
| |
|
| (ii) |
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| |
|
| (iii) |
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| |
|
| (iv) |
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| |
|
| (b) |
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue |
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned,
thereunto duly authorized in the City of New York, State of New York, on February 9, 2024.
| |
SUNSHINE BIOPHARMA, INC. |
| |
|
|
| |
|
|
| |
By: |
/s/ Dr. Steve N. Slilaty |
| |
|
Dr. Steve N. Slilaty |
| |
|
Chief Executive Officer |
Pursuant to the requirements
of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates
indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Dr. Steve N. Slilaty |
|
Chief Executive Officer and Director |
|
February 9, 2024 |
| Dr. Steve N. Slilaty |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/ Camille Sebaaly * |
|
Chief Financial Officer |
|
February 9, 2024 |
| Camille Sebaaly |
|
(Principal Financial and Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Dr. Abderrazzak Merzouki * |
|
Director |
|
February 9, 2024 |
| Dr. Abderrazzak Merzouki |
|
|
|
|
| |
|
|
|
|
| /s/ David
Natan * |
|
Director |
|
February 9, 2024 |
| David Natan |
|
|
|
|
| |
|
|
|
|
| /s/ Dr. Andrew
Keller * |
|
Director |
|
February 9, 2024 |
| Dr. Andrew Keller |
|
|
|
|
| |
|
|
|
|
| /s/ Dr. Rabi Kiderchah * |
|
Director |
|
February 9, 2024 |
| Dr. Rabi Kiderchah |
|
|
|
|
* By /s/ Dr. Steve N. Slilaty
Attorney-in-fact
Exhibit 1.1
UNDERWRITING AGREEMENT
[●], 2024
Aegis Capital Corp.
1345 Avenue of the Americas, 27th Floor
New York, NY 10105
Ladies and Gentlemen:
Sunshine Biopharma, Inc., a Colorado corporation
(the “Company”), agrees, subject to the terms and conditions in this agreement (this “Agreement”),
to issue and sell to Aegis Capital Corp. (the “Underwriter”) an aggregate of [●] of the Company’s
units (each, a “Closing Unit”), with each Closing Unit consisting of either: (A) one (1) share of Common Stock,
$0.001 par value per share (the “Closing Shares”) of the Company (the “Common Stock”)
and one-tenth (1/10th) of a Series A warrant to purchase one (1) share of Common Stock at a per Share exercise price of $[●] (representing
1500.0% of the per Closing Common Unit (as defined below) offering price; and two-tenths (2/10th) of a Series B warrant to purchase one
(1) share of Common Stock at a per Share exercise price of $[●] (representing 1700.0% of the per Closing Common Unit (as defined
below) offering price (each, a “Closing Common Unit”); or (B) one pre-funded warrant (each, a “Pre-funded
Warrant”) to purchase one (1) share of Common Stock at an exercise price of $0.001 and one-tenth (1/10th) of a Series A
warrant to purchase one (1) share of Common Stock at a per-Share exercise price of $[●] (representing 1500.0% of the per Closing
Common Unit offering price; and two-tenths (2/10th) of a Series B warrant to purchase one (1) share of Common Stock at a per-Share exercise
price of $[●] (representing 1700.0% of the per Closing Common Unit offering price (each, a “Closing Pre-funded Unit”).
The shares of Common Stock referred to in this Section are hereinafter referred to as the “Closing Shares”;
the Warrants referred to in this Section are hereinafter referred to as the “Closing Warrants”; and the Pre-funded
Warrants referred to in this Section are hereinafter referred to as the “Closing Pre-funded Warrants.” No Closing
Common Units will be certificated, and the Closing Shares and the Closing Warrants comprising the Closing Common Units will be separated
immediately upon issuance. No Closing Pre-funded Units will be certificated, and the Closing Pre-funded Warrants and the Closing Warrants
comprising the Closing Pre-funded Units will be separated immediately upon issuance. At the option of the Underwriter, the Company agrees,
subject to the terms and conditions herein, to issue and sell additional Option Securities (as defined in Section 4.2 hereof). The Closing
Units and the Option Securities are herein referred to collectively as the “Securities”. The number of Closing
Units and Option Securities to be purchased by the Underwriter is set forth opposite its name in Schedule 4.1.2 hereto. Aegis Capital
Corp. has agreed to act as the Underwriter in connection with the offering and sale of the Securities.
1.1.
“Affiliate” has the meaning set forth in Rule 405 under the Securities Act.
1.2.
“Applicable Time” means Eastern Time on the date hereof.
1.3.
“Bona Fide Electronic Road Show” means a “bona fide electronic road show” (as defined in
Rule 433(h)(5) under the Securities Act) that the Company has made available without restriction by “graphic means” (as defined
in Rule 405 under the Securities Act) to any person.
1.4.
“Business Day” means a day on which the Nasdaq Capital Market is open for trading and on which banks
in New York are open for business and not permitted by law or executive order to be closed.
1.5.
“Commission” means the United States Securities and Exchange Commission.
1.6.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated
thereunder.
1.7.
“Exempt Issuance” means securities issued (i) under the Company’s current or future equity incentive
plans or issued to employees, directors, consultants or officers as compensation or consideration in the ordinary course of business,
including any issuance of options (and the underlying shares of Common Stock) in exchange for options issued under the Company’s
equity incentive plans, (ii) issued pursuant to agreements, options, restricted share units or convertible securities existing as of the
date hereof provided the terms are not modified, (iii) issued pursuant to acquisitions or strategic transactions (whether by merger, consolidation,
purchase of equity, purchase of assets, reorganization or otherwise) approved by a majority of the disinterested directors of the Company,
provided that such securities are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights
that require or permit the filing of any registration statement in connection therewith during the Standstill Period, and provided that
any such issuance shall only be to a Person (or to the equityholders of a Person) which is, itself or through its subsidiaries, an operating
company or an owner of an asset in a business synergistic with the business of the Company and shall provide to the Company additional
benefits in addition to the investment of funds, but shall not include a transaction in which the Company is issuing securities primarily
for the purpose of raising capital or to an entity whose primary business is investing in securities.
1.8.
“Final Prospectus” means the prospectus in the form first filed with the Commission pursuant to and within
the time limits described in Rule 424(b) under the Securities Act.
1.9.
“Free Writing Prospectus” has the meaning set forth in Rule 405 under the Securities Act.
1.10.
“Indebtedness” means (a) any liabilities for borrowed money or amounts owed in excess of $100,000 in
aggregate (other than trade accounts payable incurred in the ordinary course of business), (b) all guaranties, endorsements and other
contingent obligations in respect of indebtedness of others, whether or not the same are or should be reflected in the Company’s
consolidated balance sheet (or the notes thereto), except guaranties by endorsement of negotiable instruments for deposit or collection
or similar transactions in the ordinary course of business; and (c) the present value of any lease payments in excess of $100,000 in aggregate
due under leases required to be capitalized in accordance with GAAP.
1.11.
“Investment Company Act” means the Investment Company Act of 1940, as amended, and the rules and regulations
promulgated thereunder.
1.12.
“Issuer Free Writing Prospectus” means an “issuer free writing prospectus” (as defined in
Rule 433(h)(1) under the Securities Act).
1.13.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association,
joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any
kind.
1.14.
“Preliminary Prospectus” means any preliminary prospectus included in the Registration Statement prior
to the time at which the Commission declared the Registration Statement effective.
1.15.
“Pricing Disclosure Package” means the Preliminary Prospectus collectively with this Agreement (including
documents attached hereto or incorporated by reference herein) and the documents and pricing information set forth in Schedule 1.15 hereto.
1.16.
“Prospectus Delivery Period” means such period of time after the first date of the public offering of
the Units as in the opinion of counsel for the Underwriter a prospectus relating to the Units is required by law to be delivered (or required
to be delivered but for Rule 172 under the Securities Act) in connection with sales of the Units by the Underwriter or dealer.
1.17.
“Registration Statement” means (a) the registration statement on Form S-1 (File No. 333-276817), including
a prospectus, registering the offer and sale of the Closing Units under the Securities Act as amended at the time the Commission declared
it effective, including each of the exhibits, financial statements and schedules thereto, (b) any Rule 430A Information, and (c) any Rule
462(b) Registration Statement, including in each case any documents incorporated by reference therein.
1.18.
“Rule 430A Information” means the information deemed, pursuant to Rule 430A under the Securities Act,
to be part of the Registration Statement at the time the Commission declared the Registration Statement effective.
1.19.
“Rule 462(b) Registration Statement” means an abbreviated registration statement to register the offer
and sale of additional Units pursuant to Rule 462(b) under the Securities Act.
1.20.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002, as amended, and the rules and regulations
promulgated thereunder.
1.21.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated
thereunder.
1.22.
“Standstill Period” has the meaning set forth in Section 5.11.1 hereof.
1.23.
“Testing-the-Waters Communication” means any oral or Written Communication with potential investors undertaken
in reliance on Section 5(d) of the Securities Act and Rule 163B thereunder.
1.24.
“U.S. Company Counsel” means, with respect to New York law and federal securities law, Sichenzia Ross
Ference Carmel LLP, with office at 1185 Avenue of the Americas, 31st Floor New York, New York 10036, and with respect to Colorado law,
Andrew I. Telsey, P.C.
1.25.
“Written Communication” has the meaning set forth in Rule 405 under the Securities Act.
1.26.
“Written Testing-the-Waters Communications” means any Testing-the-Waters Communication that is a Written
Communication.
| 2. | Representations and Warranties of the Company. The Company hereby represents and warrants
to, and agrees with, the Underwriter that the following matters are true and accurate and do not contain any untrue statement of a material
fact or omit to state a material fact required to be stated therein or necessary to make the statements therein not misleading. Any certificate
signed by an officer of the Company and delivered to the Underwriter or to counsel for the Underwriter shall be deemed to be a representation
and warranty by the Company to the Underwriter as to the matters set forth therein. |
2.1.
Registration Statement. The Company has prepared and filed the Registration Statement with the Commission under the
Securities Act. The Commission has declared the Registration Statement effective under the Securities Act and the Company has not as of
the date of this Agreement filed a post-effective amendment to the Registration Statement. The Commission has not issued any order suspending
the effectiveness of the Registration Statement or any order preventing or suspending the use of the Registration Statement, the Final
Prospectus, any Preliminary Prospectus, any Issuer Free Writing Prospectus or any Testing-the-Waters Communication, and no proceedings
for such purpose or pursuant to Section 8A of the Securities Act have been initiated, are pending before or, to the Company’s knowledge,
threatened by the Commission.
2.1.1.
The Registration Statement, at the time it became effective, did not contain, and any post-effective amendment thereto, as of the
effective date of such amendment, will not contain, any untrue statement of a material fact or omit to state a material fact required
to be stated therein or necessary to make the statements therein not misleading; provided that the Company makes no representation
or warranty with respect to any statements or omissions made in reliance upon and in conformity with information relating to the Underwriter
furnished to the Company in writing by the Underwriter expressly for use in the Registration Statement (including any post-effective amendment
thereto), the Pricing Disclosure Package, the Final Prospectus (including any amendments or supplements thereto), any Preliminary Prospectus,
any Issuer Free Writing Prospectus or any Testing-the-Waters Communication, it being understood and agreed that the only such information
furnished by the Underwriter consists of the information under “Discretionary Accounts,” “Electronic Offer, Sale and
Distribution of Securities,” “Stabilization,” and “Passive Market Making” in the Underwriting section of
the Registration Statement. (collectively, the “Underwriter Information”).
2.1.2.
Each of the Registration Statement and any post-effective amendment thereto, at the time it became effective and at the date hereof,
complied and will comply in all material respects with the Securities Act.
2.2.
Pricing Disclosure Package. The Pricing Disclosure Package, as of the Applicable Time, did not, and as of the Closing
Date (as defined below) and as of any Additional Closing Date (as defined below), as the case may be, will not, contain any untrue statement
of a material fact or omit to state a material fact necessary in order to make the statements therein, in the light of the circumstances
under which they were made, not misleading; provided that the Company makes no representation or warranty with respect to any statements
or omissions made in reliance upon and in conformity with the Underwriter Information.
2.3.
Final Prospectus.
2.3.1.
Each of the Final Prospectus and any amendments or supplements thereto, as of its date, as of the time it is filed with the Commission
pursuant to Rule 424(b) under the Securities Act, as of the Closing Date and as of any Additional Closing Date, as the case may be, will
not contain any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements therein,
in the light of the circumstances under which they were made, not misleading; provided that the Company makes no representation
or warranty with respect to any statements or omissions made in reliance upon and in conformity with the Underwriter Information.
2.3.2.
Each of the Final Prospectus and any amendments or supplements thereto, at the time it is filed with the Commission pursuant to
Rule 424(b) under the Securities Act, as of the Closing Date and as of any Additional Closing Date, as the case may be, will comply in
all material respects with the Securities Act.
2.4.
Preliminary Prospectuses.
2.4.1.
Each Preliminary Prospectus, when considered together with any amendments or supplements thereto, as of the time it was filed with
the Commission pursuant to Rule 424(a) under the Securities Act, if any, did not contain any untrue statement of a material fact or omit
to state a material fact necessary in order to make the statements therein, in the light of the circumstances under which they were made,
not misleading; provided that the Company makes no representation or warranty with respect to any statements or omissions made
in reliance upon and in conformity with the Underwriter Information.
2.4.2.
Each Preliminary Prospectus, when considered together with any amendments or supplements thereto, at the time it was filed with
the Commission pursuant to Rule 424(a) under the Securities Act, if any, complied in all material respects with the Securities Act.
2.5.
Issuer Free Writing Prospectuses.
2.5.1.
Each Issuer Free Writing Prospectus, when considered together with the Preliminary Prospectus accompanying, or delivered prior
to the delivery of, such Issuer Free Writing Prospectus, did not, as of the date of such Issuer Free Writing Prospectus, and will not,
as of the Closing Date and as of any Additional Closing Date, as the case may be, contain any untrue statement of a material fact or omit
to state a material fact necessary in order to make the statements therein, in the light of the circumstances under which they were made,
not misleading; provided that the Company makes no representation or warranty with respect to any statements or omissions made
in reliance upon and in conformity with the Underwriter Information.
2.5.2.
Each Issuer Free Writing Prospectus, at the time of filing with the Commission, complied or will comply in all material respects
with the Securities Act.
2.5.3.
The Company has filed, or will file, with the Commission, within the time period specified in Rule 433(d) under the Securities
Act, any Free Writing Prospectus it is required to file pursuant to Rule 433(d) under the Securities Act. The Company has made available
any Bona Fide Electronic Road Show used by it in compliance with Rule 433(d)(8)(ii) under the Securities Act such that no filing of any
“road show” (as defined in Rule 433(h) under the Securities Act) (“Road Show”) is required in connection
with the offering of the Units.
2.5.4.
Except for the Issuer Free Writing Prospectuses, if any, set forth in Schedule 2.5.4 hereto and electronic road shows, if any,
each furnished to the Underwriter before first use, the Company has not used, authorized the use of, referred to or participated in the
planning for use of, and will not, without the prior consent of the Underwriter, use, authorize the use of, refer to or participate in
the planning for use of, any Free Writing Prospectus.
2.6.
Testing-the-Waters Communications. The Company has not (x) alone engaged in any Testing-the-Waters Communication
other than Testing-the-Waters Communications with the consent of the Underwriter or any underwriter that the Company has previously identified
to the Underwriter with entities that are qualified institutional buyers within the meaning of Rule 144A under the Act or institutions
that are accredited investors within the meaning of Rule 501 under the Act, and (y) authorized anyone other than the Underwriter, or any
underwriter that the Company has previously identified to the Underwriter, to engage in Testing-the-Waters Communications.
2.7.
No Other Disclosure Materials. Other than the Registration Statement, the Pricing Disclosure Package, the Final Prospectus
and the Road Show, the Company (including its agents and representatives, other than the Underwriter or any underwriter that the Company
has previously identified to the Underwriter, as to which no representation or warranty is given) has not, directly or indirectly, distributed,
prepared, used, authorized, approved or referred to, and will not distribute, prepare, use, authorize, approve or refer to, any offering
material in connection with the offering and sale of the Units.
2.8.
Reserved.
2.9.
Smaller Reporting Company. From the time of initial filing of the Registration Statement with the Commission (or,
if earlier, the first date on which the Company engaged directly or through any person authorized to act on its behalf in any Testing-the-Waters
Communication) through the date hereof, the Company has been and is a “smaller reporting company,” as defined in Rule 12b-2
under the Exchange Act.
2.10.
Due Authorization. The Company has full right, power and authority to execute and deliver this Agreement and to perform
its obligations hereunder; and all action required to be taken for the due and proper authorization, execution and delivery by it of this
Agreement and the consummation by it of the transactions contemplated hereby has been duly and validly taken.
2.11.
Underwriting Agreement. This Agreement has been duly authorized, executed and delivered by the Company and, assuming
the due authorization, execution and delivery by the other parties hereto, constitutes the legal, valid and binding obligation of the
Company, enforceable against the Company in accordance with its terms, except as (i) the enforcement may be limited by bankruptcy, insolvency,
fraudulent transfer, reorganization, moratorium or other similar laws relating to or affecting the rights and remedies of creditors generally
or by general equitable principles (whether considered in a proceeding at law or in equity) relating to enforceability and (ii) rights
to indemnification and contribution hereunder may be limited by applicable law and public policy considerations.
2.12.
No Material Adverse Change. Except as otherwise disclosed in the Registration Statement, the Pricing Disclosure Package
and the Final Prospectus (in each case exclusive of any amendment or supplement thereto), since the date of the most recent financial
statements included or incorporated by reference in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus:
(i) there has been no material adverse change, or any development that could result in a material adverse change, in or affecting the
condition (financial or otherwise), earnings, business, properties, management, financial position, stockholders’ equity, or results
of operations, whether or not arising from transactions in the ordinary course of business, of the Company and its subsidiaries, considered
as a whole; (ii) there has been no change in the share capital (other than (A) the issuance of Shares upon the exercise or settlement
(including any “net” or “cashless” exercises or settlements) of stock options, restricted stock units or warrants
described as outstanding, (B) the grant of options and awards under existing equity incentive plans, or (C) the repurchase of shares of
Common Stock by the Company, which were issued pursuant to the early exercise of stock options by option holders and are subject to repurchase
by the Company, in each case, as described in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus), or
material change in the short-term debt or long-term debt of the Company or any of its subsidiaries, considered as a whole; and (iii) the
Company and its subsidiaries, considered as a whole, have not incurred any material liability or obligation, indirect, direct or contingent
(whether or not in the ordinary course of business); nor entered into any transaction or agreement (whether or not in the ordinary course
of business) that is material to the Company and its subsidiaries, considered as a whole; and (iv) there has been no dividend or distribution
of any kind declared, set aside for payment, paid or made by the Company or, except for dividends paid to the Company or other subsidiaries
of the Company, any of its subsidiaries on any class of capital stock or repurchase or redemption by the Company or any of its subsidiaries
of any class of capital stock.
2.13.
Organization and Good Standing of the Company and its Subsidiaries. The Company and each of its subsidiaries have
been duly incorporated and are validly existing and in good standing under the laws of their respective jurisdictions of organization,
are duly qualified to do business and are in good standing in each jurisdiction in which their respective ownership or lease of property
or the conduct of their respective businesses requires such qualification, and have all power and authority (corporate and other) necessary
to own, lease or hold their respective properties and to conduct the businesses in which they are engaged as described in the Registration
Statement, the Pricing Disclosure Package and the Final Prospectus, except where the failure to be in good standing, to be so qualified
or to have such power or authority could not, individually or in the aggregate, have a material adverse effect on the condition (financial
or otherwise), earnings, business, properties, management, financial position, stockholders’ equity, or results of operations of
the Company and its subsidiaries, considered as a whole, or adversely affect the performance by the Company of its obligations under this
Agreement (a “Material Adverse Effect”).
2.14.
Capitalization. The capitalization of the Company is as set forth in the Registration Statement, the Pricing Disclosure
Package and the Final Prospectus under the heading “Capitalization”. All of the outstanding capital stock of the Company has
been duly authorized and validly issued and is fully paid and non-assessable. The Securities have been duly authorized and, when issued
and paid for as contemplated herein, will be validly issued, fully paid and non-assessable; the holders thereof are not and will not be
subject to personal liability by reason of being such holders; the Securities are not and will not be subject to the preemptive rights
of any holders of any security of the Company or similar contractual rights granted by the Company; and all corporate action required
to be taken for the authorization, issuance and sale of the Securities has been duly and validly taken. None of the outstanding shares
of Common Stock of the Company were issued in violation of any preemptive rights, rights of first refusal or other similar rights to subscribe
for or purchase securities of the Company. Except for the right of participation granted pursuant to the Company’s private placement
that closed on May 16, 2023 or as disclosed in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus, there
are no authorized or outstanding options, warrants, preemptive rights, rights of first refusal or other rights to acquire, or instruments
convertible into or exchangeable or exercisable for, any Shares of, or other equity interest in, the Company or any of its subsidiaries.
All of the outstanding shares of, or other equity interest in, each of the Company’s subsidiaries (i) have been duly authorized
and validly issued, (ii) are fully paid and non-assessable, and (iii) are owned by the Company, directly or through the Company’s
subsidiaries, free and clear of any security interest, mortgage, pledge, lien, encumbrance, charge, claim or restriction on voting or
transfer (collectively, “Liens”).
2.15.
Common Stock Incentive Plans. With respect to the Common Stock options (the “Stock Options”)
granted pursuant to the Common Stock-based compensation plans of the Company and its subsidiaries (the “Company Common Stock
Incentive Plans”), (i) each Stock Option intended to qualify as an “incentive stock option” under Section 422
of the Internal Revenue Code of 1986, as amended (the “Code”), so qualifies, (ii) each grant of a Stock Option
was duly authorized by all necessary corporate action, including, as applicable, approval by the board of directors of the Company (or
a duly constituted and authorized committee thereof) and any required stockholders approval by the necessary number of votes or written
consents, and the award agreement governing such grant (if any), to the Company’s knowledge, was duly executed and delivered by
each party thereto, (iii) each such grant was made in all material respects in accordance with the terms of the Company Common Stock Incentive
Plans, and (iv) each such grant was properly accounted for in accordance with United States generally accepted accounting principles applied
on a consistent basis during the periods involved (“GAAP”) in the financial statements (including the related
notes) of the Company.
2.16.
No Violation or Default. Neither the Company nor any of its subsidiaries is: (i) in violation of its charter, by-laws
or similar organizational documents; (ii) in default, and no event has occurred that, with notice or lapse of time or both, would constitute
such a default, in the due performance or observance of any term, covenant, condition or other obligation contained in any indenture,
mortgage, deed of trust, loan agreement, contract, undertaking or other agreement or instrument to which the Company or any of its subsidiaries
is a party or by which the Company or any of its subsidiaries is bound or to which any property, right or asset of the Company or any
of its subsidiaries is subject; or (iii) in violation of any law or statute applicable to the Company or any of its subsidiaries or any
judgment, order, rule or regulation of any court or arbitrator or governmental or regulatory authority having jurisdiction over the Company
or any of its subsidiaries, or any of their respective properties or assets, except, in the case of clauses (ii) and (iii) above, for
any such default or violation that would not, individually or in the aggregate, have a Material Adverse Effect.
2.17.
No Conflicts. None of (i) the execution, delivery and performance of this Agreement by the Company, (ii) the issuance,
sale and delivery of the Closing Units or the Option Securities, (iii) the application of the proceeds of the offering as described under
“Use of Proceeds” in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus, or (iv) the consummation
of the transactions contemplated herein will: (x) result in any violation of the terms or provisions of the charter, by-laws or similar
organizational documents of the Company or any of its subsidiaries; (y) conflict with, result in a breach or violation of, or require
the approval of stockholders, members or partners or any approval or consent of any persons under, any of the terms or provisions of,
constitute a default under, result in the termination, modification, or acceleration of, or result in the creation or imposition of any
lien, charge or encumbrance upon any property, right or asset of the Company or any of its subsidiaries pursuant to, any indenture, mortgage,
deed of trust, loan agreement, note agreement, contract, undertaking or other agreement, obligation, condition, covenant or instrument
to which the Company or any of its subsidiaries is a party or by which the Company or any of its subsidiaries is bound or to which any
property, right or asset of the Company or any of its subsidiaries is subject; or (z) result in the violation of any law, statute, judgment,
order, rule, decree or regulation applicable to the Company or any of its subsidiaries of any court, arbitrator, governmental or regulatory
authority, agency or body having jurisdiction over the Company or any of its subsidiaries or any of their respective properties or assets.
2.18.
No Consents Required. No consent, approval, authorization, order, filing, registration, license or qualification
of or with any court, arbitrator, or governmental or regulatory authority, agency, or body is required for (i) the execution, delivery
and performance by the Company of this Agreement; (ii) the issuance, sale and delivery of the Securities; or (iii) the consummation of
the transactions contemplated herein, except for such consents, approvals, authorizations, orders, filings, registrations or qualifications
as (x) have already been obtained or made and are still in full force and effect, (y) may be required by FINRA and the Nasdaq Capital
Market, and (z) may be required under applicable federal or state securities laws in connection with the purchase, distribution and resale
of the Securities by the Underwriter.
2.19.
Independent Accountants. BF Borgers CPA PC, which expressed its opinion with respect to the financial statements
(which term as used in this Agreement includes the related notes thereto) and supporting schedules included in the Registration Statement,
the Pricing Disclosure Package and the Final Prospectus, is an independent registered public accounting firm with respect to the Company
and its subsidiaries within the meaning of the rules and regulations of the Commission and the Public Company Accounting Oversight Board
and as required by the Securities Act.
2.20.
Financial Statements and Other Financial Data. The financial statements (including the related notes thereto), together
with the supporting schedules, included in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus comply
in all material respects with the applicable requirements of the Securities Act and present fairly the consolidated financial position
of the entities to which they relate as of and at the dates indicated and the results of their operations and cash flows for the periods
specified. Such financial statements, notes and schedules have been prepared in conformity with GAAP applied on a consistent basis throughout
the periods involved, except as may be expressly stated in the notes thereto and except, in the case of unaudited interim financial statements,
subject to normal year end audit adjustments and the exclusion of certain footnotes as permitted by the applicable rules of the Commission.
The financial data set forth in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus under the captions
“Capitalization” present fairly the information set forth therein on a basis consistent with that of the financial statements
included in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus.
2.21.
Statistical and Market-Related Data. The statistical and market-related data included in the Registration Statement,
the Pricing Disclosure Package and the Final Prospectus are based on or derived from sources that the Company reasonably and in good faith
believes to be accurate and reliable in all material respects.
2.22.
Forward-Looking Statements. No forward-looking statement (within the meaning of Section 27A of the Securities Act
and Section 21E of the Exchange Act) included in the Registration Statement, the Pricing Disclosure Package or the Final Prospectus has
been made or reaffirmed without a reasonable basis or has been disclosed other than in good faith.
2.23.
Legal Proceedings. Except as disclosed in the Registration Statement, the Pricing Disclosure Package and the Final
Prospectus, (i) there are no legal, governmental or regulatory investigations, actions, demands, claims, suits, arbitrations, inquiries
or proceedings (collectively, “Actions”) pending to which the Company or any of its subsidiaries is or may be
a party or to which any property, right or asset of the Company or any of its subsidiaries is or may be the subject that, individually
or in the aggregate, if determined adversely to the Company or any of its subsidiaries, could have a Material Adverse Effect; and (ii)
to the knowledge of the Company, no such Actions are threatened or contemplated by any governmental or regulatory authority or by others.
2.24.
Labor Disputes. No labor disturbance by or dispute with the employees of the Company or any of its subsidiaries exists
or, to the knowledge of the Company, is threatened or contemplated that could, individually or in the aggregate, have a Material Adverse
Effect.
2.25.
Intellectual Property Rights. (i) The Company and its subsidiaries own or have the right to use all patents, patent
applications, trademarks, service marks, trade names, and other source indicators and registrations and applications for registration
thereof, domain name registrations, copyrights and registrations and applications for registration thereof, technology and know-how, trade
secrets, and all other intellectual property and related proprietary rights (collectively, “Intellectual Property Rights”)
necessary to conduct their respective businesses; (ii) other than as disclosed in the Prospectus, neither the Company nor any of its subsidiaries
has received any notice of infringement, misappropriation or other conflict with (and neither the Company nor any of its subsidiaries
is otherwise aware of any infringement, misappropriation or other conflict with) the Intellectual Property Rights of any other person,
except for such infringement, misappropriation or other conflict as could not have a Material Adverse Effect; and (iii) to the knowledge
of the Company, the Intellectual Property Rights of the Company and its subsidiaries are not being infringed, misappropriated or otherwise
violated by any person.
2.26.
Licenses and Permits. (i) The Company and its subsidiaries possess such valid and current certificates, authorizations,
approvals, licenses and permits (collectively, “Authorizations”) issued by, and have made all declarations,
amendments, supplements and filings with, the appropriate state, federal or foreign regulatory agencies or bodies necessary to own, lease
and operate their respective properties and to conduct their respective businesses as set forth in the Registration Statement, the Pricing
Disclosure Package and the Final Prospectus; (ii) all such Authorizations are valid and in full force and effect and the Company and its
subsidiaries are in compliance with the terms and conditions of all such Authorizations; and (iii) neither the Company nor any of its
subsidiaries has received notice of any revocation, termination or modification of, or non-compliance with, any such Authorization or
has any reason to believe that any such Authorization will not be renewed in the ordinary course, except where, in the case of clauses
(i), (ii) and (iii), the failure to possess, make or obtain such Authorizations (by possession, declaration or filing) could not, individually
or in the aggregate, have a Material Adverse Effect.
2.27.
Title to Property. The Company and its subsidiaries have good and marketable title to, or have valid and enforceable
rights to lease or otherwise use, all items of real property and personal property (other than with respect to Intellectual Property Rights,
which is addressed exclusively in Section 2.25) that are material to the respective businesses of the Company and its subsidiaries, in
each case, free and clear of all liens, encumbrances, claims, and defects and imperfections of title, except such liens, encumbrances,
claims, defects and imperfections as (i) are disclosed in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus,
or (ii) do not materially affect the value of such property and do not materially interfere with the use made or proposed to be made of
such property by the Company and its subsidiaries. The Company and its subsidiaries have good and marketable title to, or have valid and
enforceable rights to lease or otherwise use, all items of real and personal property that are material to the respective businesses of
the Company and its subsidiaries, in each case, free and clear of all liens, encumbrances, claims and defects and imperfections of title,
except such liens, encumbrances, claims, defects and imperfections as (i) are disclosed in the Registration Statement, the Pricing Disclosure
Package and the Final Prospectus, or (ii) do not materially affect the value of such property and do not materially interfere with the
use made or proposed to be made of such property by the Company and its subsidiaries. All items of real and personal property held under
lease by the Company and its subsidiaries are held under valid, subsisting and enforceable leases, with such exceptions as do not materially
interfere with the use made or proposed to be made of such property by the Company and its subsidiaries.
2.28.
Taxes. The Company and each of its subsidiaries have filed all federal, state, local and foreign tax returns required
to be filed through the date hereof or have timely requested extensions thereof and have paid all taxes required to be paid thereon (except
as currently being contested in good faith and for which reserves required by GAAP have been created in the financial statements of the
Company). The charges, accruals and reserves in respect of any income and other tax liability in the financial statements of the Company
referred to in Section 2.20 are adequate, in accordance with GAAP principles, to meet any assessments for any taxes of the Company accruing
through the end of the last period specified in such financial statements.
2.29.
Solvency. Based on the consolidated financial condition of the Company as of the Closing Date, after giving effect
to the receipt by the Company of the proceeds from the sale of the Closing Units hereunder, (i) the fair saleable value of the Company’s
assets exceeds the amount that will be required to be paid on or in respect of the Company’s existing debts and other liabilities
(including known contingent liabilities) as they mature, (ii) the Company’s assets do not constitute unreasonably small capital
to carry on its business as now conducted and as proposed to be conducted including its capital needs taking into account the particular
capital requirements of the business conducted by the Company, consolidated and projected capital requirements and capital availability
thereof, and (iii) the current cash flow of the Company, together with the proceeds the Company would receive, were it to liquidate all
of its assets, after taking into account all anticipated uses of the cash, would be sufficient to pay all amounts on or in respect of
its liabilities when such amounts are required to be paid. The Company does not intend to incur debts beyond its ability to pay such debts
as they mature (taking into account the timing and amounts of cash to be payable on or in respect of its debt). The Company has no knowledge
of any facts or circumstances which lead it to believe that it will file for reorganization or liquidation under the bankruptcy or reorganization
laws of any jurisdiction within one year from the Closing Date. The Registration Statement sets forth as of the date hereof all outstanding
secured and unsecured Indebtedness of the Company or any Subsidiary, or for which the Company or any Subsidiary has commitments. Neither
the Company nor any Subsidiary is in default with respect to any Indebtedness.
2.30.
Investment Company Act. Neither the Company nor any of its subsidiaries is or, after giving effect to the offer and
sale of the Securities and the application of the proceeds therefrom as described under “Use of Proceeds” in the Registration
Statement, the Pricing Disclosure Package and the Final Prospectus, will be required to register as an “investment company”
(as defined in the Investment Company Act).
2.31.
Insurance. The Company and its subsidiaries are insured by recognized, financially sound institutions in such amounts,
with such amounts, with such deductibles and covering such losses and risks as the Company reasonably believes to be adequate for the
conduct of their respective businesses and the value of their respective properties and as is prudent and customary for companies engaged
in similar businesses in similar industries. All insurance policies and fidelity or surety bonds insuring the Company and its subsidiaries
or their respective businesses, assets, employees, officers and directors are in full force and effect; the Company and its subsidiaries
are in compliance with the terms of such policies in all material respects; neither the Company nor any of its subsidiaries has received
notice from any insurer or agent of such insurer that capital improvements or other expenditures are required to be made in order to continue
such insurance; and neither the Company nor any of its subsidiaries has been refused any insurance coverage sought or applied for. There
are no claims by the Company or any of its subsidiaries under any such policy as to which any insurer is denying liability or defending
under a reservation of rights clause; and neither the Company nor any of its subsidiaries has any reason to believe that it will not be
able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as
may be necessary to continue its business at a cost that could not have a Material Adverse Effect.
2.32.
No Stabilization or Manipulation. None of the Company, nor its Affiliates, or, to the knowledge of the Company, any
person acting on its or any of their behalf (other than the Underwriter, as to which no representation or warranty is given) has taken,
directly or indirectly, any action designed to or that has constituted or that could reasonably be expected to cause or result in the
stabilization or manipulation of the price of any securities of the Company. The Company acknowledges that the Underwriter may engage
in passive market making transactions in the Common Stock on the Nasdaq Capital Market (the “Exchange”) in accordance
with Regulation M under the Exchange Act (“Regulation M”).
2.33.
Compliance with the Sarbanes-Oxley Act. The Company and, to the knowledge of the Company, its officers and directors,
in their capacities as such, are and have been in compliance with all applicable provisions of the Sarbanes-Oxley Act.
2.34.
Accounting Controls. The Company and its subsidiaries maintain systems of “internal control over financial
reporting” (as defined in Rule 13a-15(f) of the Exchange Act) that comply with the requirements of the Exchange Act and have been
designed by, or under the supervision of, their principal executive and principal financial officers, or persons performing similar functions,
to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with GAAP. The Company and its subsidiaries maintain internal accounting controls sufficient to provide reasonable
assurance that (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions
are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability;
(iii) access to assets is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded
accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to
any differences. Other than as disclosed in the Registration Statement, the Company maintains a system of internal control over financial
reporting and the Company is not aware of any other material weaknesses in its internal control over financial reporting (whether or not
remediated). Other than as disclosed in the Registration Statement, since the date of the most recent balance sheet included in the Registration
Statement, the Pricing Disclosure Package and the Final Prospectus, (x) the Company’s auditors and the board of directors of the
Company have not been advised of (A) any new significant deficiencies or material weaknesses in the design or operation of the internal
control over financial reporting of the Company and its subsidiaries which could adversely affect the Company’s ability to record,
process, summarize, and report financial data; or (B) any fraud, whether or not material, that involves management or other employees
who have a role in the internal control over financial reporting of the Company or its subsidiaries; and (y) there have been no significant
changes in the internal control over financial reporting of the Company or its subsidiaries or in other factors that could significantly
affect, such internal control over financial reporting, including any corrective actions with regard to significant deficiencies or material
weaknesses, since the respective dates as of which information is given in the Registration Statement, the Pricing Disclosure Package
and the Final Prospectus.
2.35.
Disclosure Controls and Procedures. The Company and its subsidiaries have established and maintain disclosure controls
and procedures (as such term is defined in Rule 13a-15(e) under the Exchange Act) that are designed to comply with the requirements of
the Exchange Act; such disclosure controls and procedures are designed to ensure that information required to be disclosed by the Company
and its subsidiaries in the reports they file or submit under the Exchange Act is recorded, processed, summarized and reported within
the time periods specified in the Commission’s rules and forms, including controls and procedures designed to ensure that such information
is accumulated and communicated to the Company’s management as appropriate to allow timely decisions regarding required disclosure;
and such disclosure controls and procedures are effective to perform the functions for which they were established.
2.36.
Compliance with Environmental Laws. The Company and each of its subsidiaries (i) are, and at all times prior hereto
were, in compliance with all Environmental Laws (as defined below) applicable to such entity, which compliance includes, without limitation,
obtaining, maintaining and complying with all permits and authorizations and approvals required by Environmental Laws to conduct their
respective businesses; and (ii) have not received notice or otherwise have knowledge of any actual or alleged violation of Environmental
Laws, or of any actual or potential liability for or other obligation concerning the presence, disposal or release of hazardous or toxic
substances or wastes, pollutants or contaminants, and, except as described in the Registration Statement, the Pricing Disclosure Package
and the Final Prospectus, (x) there are no proceedings that are pending, or known to be contemplated, against the Company or any of its
subsidiaries under Environmental Laws, other than such proceedings regarding which would not, individually or in the aggregate, have a
Material Adverse Effect; (y) to the knowledge of the Company, none of the Company or any of its subsidiaries is aware of any issues regarding
compliance with Environmental Laws, including any pending or proposed Environmental Laws, or liabilities or other obligations under Environmental
Laws or concerning hazardous or toxic substances or wastes, pollutants or contaminants, that could reasonably be expected to have a material
effect on the capital expenditures, earnings or competitive position of the Company and its subsidiaries; and (z) none of the Company
or any of its subsidiaries anticipates material capital expenditures relating to Environmental Laws. As used herein, the term “Environmental
Laws” means any laws, regulations, ordinances, rules, orders, judgments, decrees, permits or other legal requirements of
any governmental authority, including, without limitation, any international, foreign, national, state, provincial, regional, or local
authority, relating to pollution, the protection of human health or safety, the environment, or natural resources, or to the use, handling,
storage, manufacturing, transportation, treatment, discharge, disposal or release of hazardous or toxic substances or wastes, pollutants
or contaminants.
2.37.
ERISA. Each “employee benefit plan” (within the meaning of Section 3(3) of the Employee Retirement Security
Act of 1974, as amended (“ERISA”)) for which the Company or any member of its “Controlled Group”
(defined as any organization which is a member of a controlled group of corporations within the meaning of Section 414 of the Code) would
have any liability (each, a “Plan”) complies in form with the requirements of all applicable statutes, rules
and regulations including ERISA and the Code, and has been maintained and administered in substantial compliance with its terms and with
the requirements of all applicable statutes, rules and regulations including ERISA and the Code; (ii) with respect to each Plan subject
to Title IV of ERISA or Section 302 of ERISA or Section 412 and 430 of the Code (A) no “reportable event” (within the meaning
of Section 4043(c) of ERISA) has occurred or is reasonably expected to occur, (B) no failure to satisfy the minimum funding standard (within
the meaning of Section 302 of ERISA or Section 412 and 430 of the Code), whether or not waived, has occurred or is reasonably expected
to occur, (C) the fair market value of the assets under each Plan (excluding for these purposes accrued but unpaid contributions) exceeds
the present value of all benefits accrued under such Plan (determined based on those assumptions used to fund such Plan) and (D) neither
the Company or any member of its Controlled Group has incurred, or reasonably expects to incur, any liability under Title IV of ERISA
(other than contributions to the Plan or premiums to the Pension Benefit Guaranty Corporation in the ordinary course and without default)
in respect of a Plan (including a “multiemployer plan”, within the meaning of Section 4001(a)(3) of ERISA); (iii) each Plan
that is intended to be qualified under Section 401(a) of the Code is so qualified and nothing has occurred, whether by action or by failure
to act, which would cause the loss of such qualification; and (iv) no prohibited transaction, within the meaning of Section 406 of ERISA
or Section 4975 of the Code, has occurred with respect to any Plan excluding transactions to which a statutory or administrative prohibited
transaction exemption applies.
2.38.
FDA. As to each product subject to the jurisdiction of the U.S. Food and Drug Administration (“FDA”)
under the Federal Food, Drug and Cosmetic Act, as amended, and the regulations thereunder (“FDCA”) that is manufactured,
packaged, labeled, tested, distributed, sold, and/or marketed by the Company or any of its Subsidiaries (each such product, a “Pharmaceutical
Product”), such Pharmaceutical Product is being manufactured, packaged, labeled, tested, distributed, sold and/or marketed
by the Company in compliance with all applicable requirements under FDCA and similar laws, rules and regulations relating to registration,
investigational use, premarket clearance, licensure, or application approval, good manufacturing practices, good laboratory practices,
good clinical practices, product listing, quotas, labeling, advertising, record keeping and filing of reports, except where the failure
to be in compliance would not have a Material Adverse Effect. There is no pending, completed or, to the Company's knowledge, threatened,
action (including any lawsuit, arbitration, or legal or administrative or regulatory proceeding, charge, complaint, or investigation)
against the Company or any of its Subsidiaries, and none of the Company or any of its Subsidiaries has received any notice, warning letter
or other communication from the FDA or any other governmental entity, which (i) contests the premarket clearance, licensure, registration,
or approval of, the uses of, the distribution of, the manufacturing or packaging of, the testing of, the sale of, or the labeling and
promotion of any Pharmaceutical Product, (ii) withdraws its approval of, requests the recall, suspension, or seizure of, or withdraws
or orders the withdrawal of advertising or sales promotional materials relating to, any Pharmaceutical Product, (iii) imposes a clinical
hold on any clinical investigation by the Company or any of its Subsidiaries, (iv) enjoins production at any facility of the Company or
any of its Subsidiaries, (v) enters or proposes to enter into a consent decree of permanent injunction with the Company or any of its
Subsidiaries, or (vi) otherwise alleges any violation of any laws, rules or regulations by the Company or any of its Subsidiaries, and
which, either individually or in the aggregate, would have a Material Adverse Effect. The properties, business and operations of the Company
have been and are being conducted in all material respects in accordance with all applicable laws, rules and regulations of the FDA. The
Company has not been informed by the FDA that the FDA will prohibit the marketing, sale, license or use in the United States of any product
proposed to be developed, produced or marketed by the Company nor has the FDA expressed any concern as to approving or clearing for marketing
any product being developed or proposed to be developed by the Company.
2.39.
Related Party Transactions. Except as disclosed in the Registration Statement, the Pricing Disclosure Package and
the Final Prospectus, no relationship, direct or indirect, exists between or among the Company or any of its subsidiaries, on the one
hand, and the directors, officers, stockholders, other Affiliates, customers or suppliers of the Company or any of its subsidiaries, on
the other hand, that would be required by the Securities Act to be described in the Registration Statement, the Pricing Disclosure Package
and the Final Prospectus.
2.40.
No Unlawful Contributions or Other Payments. Neither the Company nor any of its subsidiaries, nor any director, officer
of the Company, nor, to the knowledge of the Company, any agent, employee, Affiliate or other person associated with or acting on behalf
of the Company or any of its subsidiaries has (i) used any corporate funds for any unlawful contribution, gift, entertainment or other
unlawful expense relating to political activity; (ii) made any direct or indirect unlawful payment to any foreign or domestic government
or regulatory official or employee; (iii) made any bribe, rebate, payoff, influence payment, kickback or other unlawful payment; or (iv)
violated or is in violation of any provision of (y) the Foreign Corrupt Practices Act of 1977, as amended, and the rules and regulations
thereunder (the “FCPA”), or (z) any non-U.S. anti-bribery or anti-corruption statute or regulation. The Company
and its subsidiaries have instituted and maintain and enforce policies and procedures designed to promote and ensure compliance with all
applicable anti-bribery and anti-corruption laws.
2.41.
Compliance with Anti-Money Laundering Laws. The operations of the Company and its subsidiaries are and have been
conducted at all times in compliance with all applicable financial recordkeeping and reporting requirements, including those of the Currency
and Foreign Transactions Reporting Act of 1970, as amended, the applicable anti-money laundering statutes of all jurisdictions where the
Company or any of its subsidiaries conduct business, the rules and regulations thereunder and any related or similar rules, regulations
or guidelines issued, administered or enforced by any governmental agency (collectively, the “Anti-Money Laundering Laws”);
and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company
or any of its subsidiaries with respect to the Anti-Money Laundering Laws is pending or, to the knowledge of the Company, threatened.
2.42.
Compliance with OFAC. Neither the Company nor any of its subsidiaries nor any director, officer of the Company, nor,
to the knowledge of the Company, any agent, employee or Affiliate of the Company or any of its subsidiaries is an individual or entity
(an “OFAC Person”), or is owned or controlled by an OFAC Person, that is currently the subject or target of
any sanctions administered or enforced by the U.S. government (including, without limitation, the Office of Foreign Assets Control of
the U.S. Treasury Department (“OFAC”) or the U.S. Department of State and including, without limitation, the
designation as a “specially designated national” or “blocked person”), the United Nations Security Council, the
European Union, His Majesty’s Treasury, or other relevant sanctions authority (collectively, “Sanctions”),
nor is the Company or any of its subsidiaries located, organized or resident in a country or territory that is the subject or the target
of Sanctions, including, without limitation, Crimea, Cuba, Iran, North Korea, Sudan and Syria (each, a “Sanctioned Country”);
and the Company will not directly or indirectly use the proceeds of the offering, or lend, contribute or otherwise make available such
proceeds to any subsidiary, joint venture partner or other OFAC Person (i) to fund or facilitate any activities of or business with any
OFAC Person that, at the time of such funding or facilitation, is the subject or the target of Sanctions, (ii) to fund or facilitate any
activities or business in any Sanctioned Country or (iii) in any other manner that will result in a violation by any OFAC Person (including
any OFAC Person participating in the transaction, whether as underwriter, advisor, investor or otherwise) of Sanctions. Since the Company’s
inception, the Company and its subsidiaries have not knowingly engaged in and are not now knowingly engaged in any dealings or transactions
with any OFAC Person that at the time of the dealing or transaction is or was the subject or the target of Sanctions or with any Sanctioned
Country.
2.43.
No Registration Rights. Except as described in the Registration Statement, the Pricing Disclosure Package and the
Final Prospectus, there are no contracts, agreements or understandings between the Company or any of its subsidiaries, on the one hand,
and any person, on the other hand, granting such person any rights to require the Company or any of its subsidiaries to file a registration
statement under the Securities Act with respect to any securities of the Company or any of its subsidiaries owned or to be owned by such
person or to require the Company or any of its subsidiaries to include such securities in any securities to be registered pursuant to
any registration statement to be filed by the Company or any of its subsidiaries under the Securities Act.
2.44.
Subsidiaries. The subsidiaries of the Company shall be referred to hereinafter each as a “Subsidiary”
and collectively as “Subsidiaries.” The Subsidiaries of the Company are as set forth in Exhibit 23.1 to the
Registration Statement and such Subsidiaries are the only “significant subsidiaries” (as defined under Rule 1.02(w) of Regulation
S-X under the Securities Act) of the Company (the “Significant Subsidiaries”).
2.45.
No Restrictions on Subsidiaries. Except as disclosed in the Registration Statement, the Pricing Disclosure Package
and the Final Prospectus, no Subsidiary of the Company is currently prohibited, directly or indirectly, from paying any dividends to the
Company, from making any other distribution on such Subsidiary’s share capital or similar ownership interest, from repaying to the
Company any loans or advances to such Subsidiary from the Company or from transferring any of such Subsidiary’s properties or assets
to the Company or any other Subsidiary of the Company.
2.46.
Exchange Listing. The Common Stock is listed on the Exchange, and the Company has taken no action designed to, or
likely to have the effect of, delisting the Common Stock from the Exchange, nor has the Company received any notification that the Exchange
is contemplating terminating such listing, except as described in the Registration Statement, the Disclosure Package and the Prospectus.
2.47.
Exchange Act Registration. The Common Stock is registered pursuant to Section 12(b) under the Exchange Act. The Company
has taken no action designed to, or likely to have the effect of, terminating the registration of the Common Stock under the Exchange
Act, nor has the Company received any notification that the Commission is contemplating terminating such registration.
2.48.
Prior Securities Transactions. No securities of the Company have been sold by the Company or by or on behalf of,
or for the benefit of, any person or persons controlling, controlled by or under common control with the Company, except as disclosed
in the Registration Statement, the Disclosure Package and the Prospectus.
2.49.
Application of Takeover Protections. The Company and the Board of Directors have taken all necessary action, if any,
in order to render inapplicable any control share acquisition, business combination, poison pill (including any distribution under a rights
agreement) or other similar anti-takeover provision under the Company’s certificate of incorporation (or similar charter documents)
or the laws of its state of incorporation that is or could become applicable as a result of the Underwriter and the Company fulfilling
their obligations or exercising their rights hereunder (including documents incorporated herein by reference or attached hereto).
2.50.
D&O Questionnaires. To the Company’s knowledge, all information contained in the questionnaires (the “Questionnaires”)
completed by each of the Company’s directors, officers and beneficial holders of 5% or more of the Company’s Common Stock
immediately prior to the Offering as supplemented by all information concerning the Company’s directors, officers and principal
stockholders as described in the Registration Statement, the Disclosure Package and the Prospectus, provided to the Underwriter is true
and correct in all material respects and the Company has not become aware of any information which would cause the information disclosed
in the Questionnaires to become inaccurate and incorrect in any material respect.
2.51.
No Integrated Offering. Neither the Company, nor any of its Affiliates, nor any Person acting on its or their behalf
has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances
that would cause this offering of the Closing Units to be integrated with prior offerings by the Company for purposes of any applicable
stockholder approval provisions of any Trading Market on which any of the securities of the Company are listed or designated.
2.52.
Litigation; Governmental Proceedings. There is no material action, suit, proceeding, inquiry, arbitration, investigation,
litigation or governmental proceeding pending or, to the Company’s knowledge, threatened against, or involving the Company, any
of its Subsidiaries or, to the Company’s knowledge, any executive officer or director which has not been disclosed in the Registration
Statement, the Disclosure Package and the Prospectus which is required to be disclosed.
2.53.
FINRA Matters.
2.53.1.
No Broker’s Fees. Except as disclosed in the Registration Statement, the Pricing Disclosure Package and the
Final Prospectus, neither the Company nor any of its subsidiaries is a party to any contract, agreement or understanding with any person
(other than this Agreement) that would give rise to a valid claim against any of them or the Underwriter for a brokerage commission, finder’s
fee or like payment in connection with the offering and sale of the Securities.
2.53.2.
Payments Within Six (6) Months. Except as described in the Registration Statement, the Disclosure Package and the
Prospectus, the Company has not made any direct or indirect payments (in cash, securities or otherwise) to: (i) any person, as a finder’s
fee, consulting fee or otherwise, in consideration of such person raising capital for the Company or introducing to the Company persons
who raised or provided capital to the Company; (ii) any FINRA member; or (iii) any person or entity that has any direct or indirect affiliation
or association with any FINRA member, within the six (6) months prior to the initial filing of the Registration Statement, other than
the payment to the Underwriter as provided hereunder in connection with the Offering.
2.53.3.
Use of Proceeds. None of the net proceeds of the Offering will be paid by the Company to any participating FINRA
member or its affiliates, except as specifically authorized herein.
2.53.4.
FINRA Affiliation. There is no (i) officer or director of the Company, (ii) to the Company’s knowledge, any
beneficial owner of 10% or more of any class of the Company’s securities or (iii) to the Company’s knowledge, any beneficial
owner of the Company’s unregistered equity securities which were acquired during the 180-day period immediately preceding the filing
of the Registration Statement that is an affiliate or associated person of a FINRA member participating in the Offering (as determined
in accordance with the rules and regulations of FINRA).
2.53.5.
Information. All information provided by the Company in its FINRA questionnaire to Underwriter counsel specifically
for use by Underwriter counsel in connection with its Public Offering System filings (and related disclosure) with FINRA is true, correct
and complete in all material respects.
| 3. | Representations and Warranties of the Underwriter. The Underwriter represents and warrants
to, and agrees with, the Company: |
3.1.
No Testing-the-Waters Communications. The Underwriter has not (i) alone engaged in any Testing-the-Waters Communication
and (ii) authorized anyone to engage in Testing-the-Waters Communications. The Underwriter has not distributed, or authorized anyone else
to distribute, any Written Testing-the-Waters Communications.
4.1.
Agreements to Sell and Purchase. On the basis of the representations, warranties and covenants herein and subject
to the conditions herein and any adjustments made in accordance with Section 4.3 hereof,
4.1.1.
The Company agrees to issue and sell the Closing Units to the Underwriter; and
4.1.2.
The Underwriter agrees to purchase from the Company the number of Closing Units set forth opposite the Underwriter’s name
in Schedule 4.1.2 hereto, subject to such adjustments as the Underwriter in its sole discretion shall make to eliminate any sales or purchases
of fractional Shares.
4.1.3.
The Closing Units are to be offered initially to the public at the offering price set forth on the cover page of the Final Prospectus
(the “Public Offering Price”). The purchase price per Closing Unit to be paid by the Underwriter to the Company
shall be $[●] per Unit (the “Purchase Price”), which represents the Public Offering Price less an underwriting
discount of 8.0% and a non-accountable expense allowance of 1.0%.
4.1.4.
Payment for the Closing Units (the “Closing Units Payment”) shall be made by wire transfer in immediately
available funds to the accounts specified by the Company to the Underwriter at the offices of Kaufman & Canoles, P.C. at 10:00 a.m.,
ET, on [●], 2024 or at such other place on the same or such other date and time, not later than the fifth (5th) Business Day thereafter,
as the Underwriter and the Company may agree upon in writing (the “Closing Date”). The Closing Units Payment
shall be made against delivery of the Closing Units to be purchased on the Closing Date to the Underwriter with any transfer taxes, stamp
duties and other similar taxes payable in connection with the sale of the Closing Units duly paid by the Company.
4.2.
Over-Allotment Option.
4.2.1.
On the basis of the representations, warranties and covenants herein and subject to the conditions herein, the Underwriter is hereby
granted an option (the “Over-Allotment Option”) to purchase, in the aggregate, up to [●] additional shares
of Common Stock, representing 15.0% of the Closing Shares and Pre-funded Warrants sold in the offering from the Company (the “Option
Shares”) and/or up to [●] Series A Warrants to purchase an aggregate of an additional [●] shares of Common Stock,
representing 15.0% of the Closing Series A Warrants sold in the offering from the Company; and/or [●] Series B Warrants to purchase
an aggregate of an additional [●] shares of Common Stock, representing 15.0% of the Closing Series B Warrants sold in the offering
from the Company (the “Option Warrants”). The purchase price to be paid per Option Share shall be equal to the
purchase price per Closing Unit set forth in Section 4.1 hereof (less $0.01 attributable to each whole Option Warrant included in the
Closing Unit) and the purchase price to be paid per Option Warrant shall be equal to $0.01 per Option Warrant. The Over-allotment Option
is, at the Underwriter’s sole discretion, for Option Shares and Option Warrants together, solely Option Shares, solely Option Warrants,
or any combination thereof (each, an “Option Security” and collectively, the “Option Securities”).
The Closing Units and the Option Securities are collectively referred to as the “Securities”. The Securities
and the shares of Common Stock issuable upon exercise of the Pre-funded Warrants and the Warrants (the “Underlying Shares”),
are collectively referred to as the “Public Securities.” The Public Securities shall be issued directly by the
Company and shall have the rights and privileges described in the Registration Statement, the Pricing Disclosure Package and the Prospectus.
The Closing Warrants and the Option Warrants, if any, shall be issued pursuant to, and shall have the rights and privileges set forth
in, the form of Warrant, and the Closing Pre-funded Warrants shall be issued pursuant to, and shall have the rights and privileges set
forth in, the form of Pre-funded Warrant. The offering and sale of the Public Securities is herein referred to as the “Offering”.
4.2.2.
upon an exercise of the Over-Allotment Option and subject to the terms and conditions herein, the Company agrees to issue and sell
the Option Securities to the Underwriter;
4.2.3.
The Underwriter may exercise the Over-Allotment Option at any time in whole, or from time to time in part, on or before the forty-fifth
(45th) day following the date of the Final Prospectus, by written notice from the Underwriter to the Company (the “Over-Allotment
Exercise Notice”). The Underwriter must give the Over-Allotment Exercise Notice to the Company at least two (2) Business
Days prior to the Closing Date or the applicable Additional Closing Date, as the case may be. The Underwriter may cancel any exercise
of the Over-Allotment Option at any time prior to the Closing Date or the applicable Additional Closing Date, as the case may be, by giving
written notice of such cancellation to the Company.
4.2.4.
The Over-Allotment Exercise Notice shall set forth each of the following:
4.2.4.1
the aggregate number of Option Securities as to which the Over-Allotment Option is being exercised.
4.2.4.2
the Over-Allotment Option Purchase Price.
4.2.4.3
the names and denominations in which the Option Securities are to be registered.
4.2.4.4
the applicable Additional Closing Date, which may be the same date and time as the Closing Date but shall not be earlier than the
Closing Date nor later than the tenth (10th) full Business Day after the date of the Over-Allotment Exercise Notice.
4.2.5.
Payment for the Option Securities (the “Option Securities Payment”) shall be made by wire transfer in
immediately available funds to the accounts specified by the Company to the Underwriter at the offices of Kaufman & Canoles, P.C.
at 10:00 a.m. ET on the date specified in the corresponding Over-Allotment Exercise Notice, or at such other place on the same or such
other date and time, not later than the fifth Business Day thereafter, as the Underwriter and the Company may agree upon in writing (an
“Additional Closing Date”). The Option Securities Payment shall be made against delivery to the Underwriter
for the respective accounts of the Underwriter of the Option Securities to be purchased on any Additional Closing Date, with any transfer
taxes, stamp duties and other similar taxes payable in connection with the sale of the Option Securities duly paid by the Company. Delivery
of the Option Securities shall be made through the facilities of DTC unless the Underwriter shall otherwise instruct.
4.3.
Public Offering. The Company understands that the Underwriter intends to make a public offering of the Units as soon
after the effectiveness of this Agreement as in the judgment of the Underwriter is advisable, and initially to offer the Units on the
terms set forth in the Final Prospectus. The Company acknowledges and agrees that the Underwriter may offer and sell Units to or through
any Affiliate of the Underwriter.
| 5. | Covenants of the Company. The Company hereby covenants and agrees with the Underwriter as
follows: |
5.1.
Filings with the Commission. The Company will:
5.1.1.
prepare and file the Final Prospectus (in a form approved by the Underwriter and containing the Rule 430A Information) with the
Commission in accordance with and within the time periods specified by Rules 424(b) and 430A under the Securities Act.
5.1.2.
file any Issuer Free Writing Prospectus with the Commission to the extent required by Rule 433 under the Securities Act.
5.1.3.
file with the Commission such reports as may be required by Rule 463 under the Securities Act.
5.2.
Notice to the Underwriter. The Company will advise the Underwriter promptly, and confirm such advice in writing:
5.2.1.
when the Registration Statement has become effective.
5.2.2.
when the Final Prospectus has been filed with the Commission.
5.2.3.
when any amendment to the Registration Statement has been filed or becomes effective.
5.2.4.
when any Rule 462(b) Registration Statement has been filed with the Commission.
5.2.5.
when any supplement to the Final Prospectus, any Issuer Free Writing Prospectus, any Written Testing-the-Waters Communication or
any amendment to the Final Prospectus has been filed or distributed.
5.2.6.
of (x) any request by the Commission for any amendment to the Registration Statement or any amendment or supplement to the Final
Prospectus, (y) the receipt of any comments from the Commission relating to the Registration Statement or (z) any other request by the
Commission for any additional information, including, but not limited to, any request for information concerning any Testing-the-Waters
Communication.
5.2.7.
of (x) the issuance by the Commission of any order suspending the effectiveness of the Registration Statement or preventing or
suspending the use of the Registration Statement, the Pricing Disclosure Package, the Final Prospectus, any Preliminary Prospectus, any
Issuer Free Writing Prospectus or any Written Testing-the-Waters Communication or (y) the initiation or, to the knowledge of the Company,
threatening of any proceeding for that purpose or pursuant to Section 8A of the Securities Act.
5.2.8.
of the occurrence of any event or development within the Prospectus Delivery Period as a result of which, the Final Prospectus,
the Pricing Disclosure Package, any Issuer Free Writing Prospectus or any Written Testing-the-Waters Communication as then amended or
supplemented would include any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements
therein, in the light of the circumstances existing when the Final Prospectus, the Pricing Disclosure Package, any such Issuer Free Writing
Prospectus or any such Written Testing-the-Waters Communication is delivered to a purchaser, not misleading.
5.2.9.
of the issuance by any governmental or regulatory authority or any order preventing or suspending the use of any of the Registration
Statement, the Pricing Disclosure Package, the Final Prospectus, any Preliminary Prospectus, any Issuer Free Writing Prospectus or any
Testing-the-Waters Communication or the initiation or threatening for that purpose.
5.2.10.
of the receipt by the Company of any notice with respect to any suspension of the qualification of the Units for offer and sale
in any jurisdiction or the initiation or, to the knowledge of the Company, threatening of any proceeding for such purpose.
5.3.
Ongoing Compliance.
5.3.1.
If during the Prospectus Delivery Period:
5.3.1.1
any event or development shall occur or condition shall exist as a result of which the Final Prospectus as then amended or supplemented
would include any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements therein,
in the light of the circumstances existing when the Final Prospectus is delivered to a purchaser, not misleading, the Company will, as
soon as reasonably possible, notify the Underwriter thereof and forthwith prepare and, subject to Section 5.4 hereof, file with the Commission
and furnish, at its own expense, to the Underwriter and to such dealers as the Underwriter may designate such amendments or supplements
to the Final Prospectus as may be necessary so that the statements in the Final Prospectus as so amended or supplemented will not, in
the light of the circumstances existing when the Final Prospectus is delivered to a purchaser, be misleading; or
5.3.1.2
it is necessary to amend or supplement the Final Prospectus to comply with applicable law, the Company will, as soon as reasonably
possible, notify the Underwriter thereof and forthwith prepare and, subject to Section 5.4 hereof, file with the Commission and furnish,
at its own expense, to the Underwriter and to such dealers as the Underwriter may designate such amendments or supplements to the Final
Prospectus as may be necessary so that the Final Prospectus will comply with applicable law; and
5.3.2.
if at any time prior to the Closing Date or any Additional Closing Date, as the case may be:
5.3.2.1
any event or development shall occur or condition shall exist as a result of which the Pricing Disclosure Package as then amended
or supplemented would include any untrue statement of a material fact or omit to state any material fact necessary in order to make the
statements therein, in the light of the circumstances existing when the Pricing Disclosure Package is delivered to a purchaser, not misleading,
the Company will immediately notify the Underwriter thereof and forthwith prepare and, subject to Section 5.4 hereof, file with the Commission
(to the extent required) and furnish, at its own expense, to the Underwriter and to such dealers as the Underwriter may designate such
amendments or supplements to the Pricing Disclosure Package as may be necessary so that the statements in the Pricing Disclosure Package
as so amended or supplemented will not, in the light of the circumstances existing when the Pricing Disclosure Package is delivered to
a purchaser, be misleading; or
5.3.2.2
it is necessary to amend or supplement the Pricing Disclosure Package to comply with applicable law, the Company will immediately
notify the Underwriter thereof and forthwith prepare and, subject to Section 5.4 hereof, file with the Commission (to the extent required)
and furnish, at its own expense, to the Underwriter and to such dealers as the Underwriter may designate such amendments or supplements
to the Pricing Disclosure Package as may be necessary so that the Pricing Disclosure Package will comply with applicable law.
5.4.
Amendments, Supplements and Issuer Free Writing Prospectuses. Before (i) using, authorizing, approving, referring
to, distributing or filing any Issuer Free Writing Prospectus, (ii) filing (x) any Rule 462(b) Registration Statement or (y) any amendment
or supplement to the Registration Statement or the Final Prospectus, or (iii) distributing any amendment or supplement to the Pricing
Disclosure Package or the Final Prospectus, the Company will furnish to the Underwriter and counsel for the Underwriter a copy of the
proposed Issuer Free Writing Prospectus, Rule 462(b) Registration Statement or other amendment or supplement for review and will not use,
authorize, refer to, distribute or file any such Issuer Free Writing Prospectus or Rule 462(b) Registration Statement, or file or distribute
any such proposed amendment or supplement (A) to which the Underwriter objects in a timely manner and (B) which is not in compliance with
the Securities Act. The Company will, pursuant to reasonable procedures developed in good faith, retain copies of each Issuer Free Writing
Prospectus that is not filed with the Commission in accordance with Rule 433 under the Securities Act.
5.5.
Delivery of Copies. The Company will, upon request of the Underwriter, deliver, without charge, (i) to the Underwriter,
three signed copies of the Registration Statement as originally filed and each amendment thereto, in each case, including all exhibits
and consents filed therewith; and (ii) to each Underwriter (A) a conformed copy of the Registration Statement as originally filed and
each amendment thereto (without exhibits and consents) and (B) during the Prospectus Delivery Period, as many copies of the Final Prospectus
(including all amendments and supplements thereto and each Issuer Free Writing Prospectus) as the Underwriter may reasonably request.
5.6.
Blue Sky Compliance. The Company will use its best efforts, with the Underwriter’s cooperation, if necessary,
to qualify or register (or to obtain exemptions from qualifying or registering) the Units for offer and sale under the securities or Blue
Sky laws of such jurisdictions as the Underwriter shall reasonably request and will use its reasonable best efforts, with the Underwriter’s
cooperation, if necessary, to continue such qualifications, registrations and exemptions in effect so long as required for the distribution
of the Units; provided that the Company shall not be required to (i) qualify as a foreign corporation or other entity or as a dealer
in securities in any such jurisdiction where it would not otherwise be required to so qualify, (ii) file any general consent to service
of process in any such jurisdiction or (iii) subject itself to taxation in any such jurisdiction if it is not otherwise so subject.
5.7.
Earning Statement. The Company will make generally available to its security holders and the Underwriter as soon
as practicable an earning statement that satisfies the provisions of Section 11(a) of the Securities Act and Rule 158 under the Securities
Act covering a period of at least 12 months beginning after the “effective date” (as defined in Rule 158 under the Securities
Act) of the Registration Statement; provided that the Company will be deemed to have furnished such statement to its security holders
and the Underwriter to the extent it is filed on the Commission’s Electronic Data Gathering, Analysis and Retrieval system (“EDGAR”).
5.8.
Stockholder Approval. The Company shall (a) hold a special meeting of stockholders (which may also be at the annual
meeting of stockholders) at the earliest practicable date after the date hereof, or (b) obtain by written consent the Stockholder Approval,
but in no event later than twenty (20) days (which time period, for the avoidance of doubt, does not include the time required to file
and mail an information statement on Schedule 14C with respect to such written consent as required under the Exchange Act), after the
Closing Date for the purpose of obtaining Stockholder Approval (as defined below), if required to effect the purpose thereof, with the
recommendation of the Board that such proposal be approved, and the Company shall, if applicable, solicit proxies from its stockholders
in connection therewith in the same manner as all other management proposals in such proxy statement and all management-appointed proxyholders
shall vote their proxies in favor of such proposal. The Company shall use its reasonable best efforts to obtain such Stockholder Approval,
and officers, directors and stockholders subject to Lock-Up Agreement pursuant to Section 8.9 shall, if applicable, cast their proxies
in favor of such proposal. If the Company does not obtain Stockholder Approval at the first meeting or by written consent, the Company
shall call a meeting every twenty (20) days thereafter to seek Stockholder Approval until the earlier of the date Stockholder Approval
is obtained or the Warrants are no longer outstanding. “Stockholder Approval” has the meaning set forth in the
Warrants.
5.9.
Reverse Stock Split. The Company shall effect a reverse stock split within seven (7) business days after the date
that is the earlier of the date on which (x) the first meeting of stockholders to obtain Stockholder Approval is held or (y) the items
to be approved under the Stockholder Approval have been approved in accordance with the applicable laws and corporate governing documents
of the Company (including, if applicable and without limitation, the filing and mailing of an information statement on Schedule 14C (the
“First Reverse Split Date”). No reverse stock split shall be effectuated before the First Reverse Split Date,
except if the consent has been obtained from a purchasers of a majority of the Closing Units.
5.10.
Use of Proceeds. The Company shall apply the net proceeds from the sale of the Closing Units and the Option Securities
in the manner described under the caption “Use of Proceeds” in the Registration Statement, the Pricing Disclosure Package
and the Final Prospectus.
5.11.
Clear Market.
5.11.1.
For a period of ninety (90) days after the Closing Date (the “Standstill Period”), the Company will not
(x) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any
option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, or file with the Commission a registration
statement under the Securities Act relating to, any shares of Common Stock or any securities convertible into or exercisable or exchangeable
for shares of Common Stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (y) enter into
any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the shares of Common
Stock or any such other securities, whether any such transaction described in clause (x) or (y) above is to be settled by delivery of
shares of Common Stock or such other securities, in cash or otherwise, without the prior written consent of the Underwriter.
5.11.2.
The restrictions contained in Section 5.11.1 hereof shall not apply to: (A) the Units, (B) any shares of Common Stock issued under
Company Common Stock Incentive Plans or warrants issued by the Company, in each case, described as outstanding in the Registration Statement,
the Pricing Disclosure Package and the Final Prospectus, (C) any options and other awards granted under a Company Common Stock Incentive
Plan as described in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus, provided that such securities
are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights that require or permit any
registration statement in connection therewith to be filed publicly or declared effective during the Standstill Period (D) the amendment
of a Company Common Stock Incentive Plan as described in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus,
(E) the filing by the Company of any registration statement on Form S-8 or a successor form thereto relating to a Company Common Stock
Incentive Plan described in the Registration Statement, the Pricing Disclosure Package and the Final Prospectus and (F) shares of Common
Stock or other securities issued pursuant to acquisitions or strategic transactions (whether by merger, consolidation, purchase of equity,
purchase of assets, reorganization or otherwise) approved by a majority of the disinterested directors of the Company, provided that such
securities are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights that require or
permit the filing of any registration statement in connection therewith during the Standstill Period, and provided that any such issuance
shall only be to a Person (or to the equityholders of a Person) which is, itself or through its subsidiaries, an operating company or
an owner of an asset in a business synergistic with the business of the Company and shall provide to the Company additional benefits in
addition to the investment of funds, but shall not include a transaction in which the Company is issuing securities primarily for the
purpose of raising capital or to an entity whose primary business is investing in securities; provided, however, that any
such shares of Common Stock or other securities issued or granted pursuant to clauses (B), (C) and (F) during the Standstill Period shall
not be saleable in the public market until the expiration of the Standstill Period.
5.11.3.
If the Underwriter, in its sole discretion, agrees to release or waive the restrictions set forth in any Lock-Up Agreement as described
in Section 8.9 and provides the Company with notice of the impending release or waiver substantially in the form of Exhibit 5.10.3.1
hereto at least three (3) Business Days before the effective date of the release or waiver, then the Company agrees to announce the impending
release or waiver by a press release substantially in the form of Exhibit 5.10.3.2 hereto through a major news service at
least two (2) Business Days before the effective date of the release or waiver.
5.11.4.
For a period of ninety (90) days after the date of the Closing Date, the Company shall be prohibited from effecting or entering
into an agreement to effect any issuance by the Company or any of its Subsidiaries of Common Stock or Common Stock Equivalents (or a combination
of units thereof) involving a Variable Rate Transaction. “Variable Rate Transaction” means a transaction in
which the Company (i) issues or sells any debt or equity securities that are convertible into, exchangeable or exercisable for, or include
the right to receive, additional shares of Common Stock either (A) at a conversion price, exercise price or exchange rate or other price
that is based upon, and/or varies with, the trading prices of or quotations for the Common Stock at any time after the initial issuance
of such debt or equity securities or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date
after the initial issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly
related to the business of the Company or the market for the Common Stock or (ii) enters into, or effects a transaction under, any agreement,
including, but not limited to, an equity line of credit or an “at-the-market offering”, whereby the Company may issue securities
at a future determined price regardless of whether shares pursuant to such agreement have actually been issued and regardless of whether
such agreement is subsequently canceled. The Underwriter shall be entitled to obtain injunctive relief against the Company to preclude
any such issuance, which remedy shall be in addition to any right to collect damages.
5.11.5.
Notwithstanding the foregoing, this Section 5.11 shall not apply to an Exempt Issuance, except that no Variable Rate Transaction
shall be an Exempt Issuance.
5.12.
No Stabilization or Manipulation. None of the Company, its Affiliates or any person acting on its or any of their
behalf (other than the Underwriter, as to which no covenant is given) will take, directly or indirectly, any action designed to or that
constitutes or that could reasonably be expected to cause or result in the stabilization or manipulation of the price of any securities
of the Company. The Company acknowledges that the Underwriter may engage in passive market making transactions in the Common Stock on
the Exchange in accordance with Regulation M.
5.13.
Investment Company Act. The Company shall not invest, or otherwise use the proceeds received by the Company from
the sale of the Closing Units or the Option Securities in such a manner as would require the Company or any of its subsidiaries to register
as an “investment company” (as defined in the Investment Company Act) under the Investment Company Act.
5.14.
Transfer Agent. For the period of two years from the date of this Agreement, the Company shall engage and maintain,
at its expense, a registrar and transfer agent for the Common Stock.
5.15.
Reports. For the period of two years from the date of this Agreement, the Company will furnish to the Underwriter,
as soon as they are available, copies of all reports or other communications (financial or other) furnished to holders of the Common Stock,
and copies of any reports and financial statements furnished to or filed with the Commission; provided that the Company will be
deemed to have furnished such reports and financial statements to the Underwriter to the extent they are filed on the Commission’s
Electronic Data Gathering, Analysis and Retrieval system.
5.16.
Right of First Refusal. The Company agrees that, if, for the period ending three (3) years after the Closing Date,
the Company or any of its subsidiaries: (a) decides to finance or refinance any indebtedness, the Underwriter (or any affiliate designated
by the Underwriter) shall have the right to act as sole book-runner, sole manager, sole placement agent or sole agent with respect to
such financing or refinancing; or (b) decides to raise funds by means of a public offering (including at-the-market facility) or a private
placement or any other capital raising financing of equity, equity-linked or debt securities, the Underwriter (or any affiliate designated
by the Underwriter) shall have the right to act as sole book-running manager, sole underwriter or sole placement agent for such financing.
If the Underwriter or one of its affiliates decides to accept any such engagement, the agreement governing such engagement (each, a “Subsequent
Transaction Agreement”) will contain, among other things, provisions for customary fees for transactions of similar size
and nature, but in no event will the fees be less than those outlined herein, and the provisions of this Agreement, including indemnification,
that are appropriate to such a transaction. Notwithstanding the foregoing, the decision to accept the Company’s engagement under
this Section 5.16 shall be made by the Underwriter or one of its affiliates, by a written notice to the Company, within ten (10) days
of the receipt of the Company’s notification of its financing needs, including a detailed term sheet. The Underwriter’s determination
of whether in any case to exercise its right of first refusal will be strictly limited to the terms on such term sheet, and any waiver
of such right of first refusal shall apply only to such specific terms. If the Underwriter waives its right of first refusal, any deviation
from such terms (including without limitation after the launch of a subsequent transaction) shall void the waiver and require the Company
to seek a new waiver from the right of first refusal on the terms set forth in this Section 5.16.
| 6. | Covenants of the Underwriter. The Underwriter hereby covenants and agrees with the Company
as follows: |
6.1.
Underwriter Free Writing Prospectus. The Underwriter has not used, authorized the use of, referred to or participated
in the planning for use of, and will not use, authorize the use of, refer to or participate in the planning for use of, any Free Writing
Prospectus (which term includes use of any written information furnished to the Commission by the Company and not incorporated by reference
into the Registration Statement and any press release issued by the Company) other than (i) a Free Writing Prospectus that contains no
“issuer information” filed or required to be filed pursuant to Rule 433(d) under the Securities Act (“Issuer Information”)
that was not included in the Preliminary Prospectus or a previously filed Issuer Free Writing Prospectus, (ii) any Issuer Free Writing
Prospectus listed in Schedule 2.5.4 hereto or prepared pursuant to Section 2.5.4 or Section 5.4 hereof (including any electronic road
show), or (iii) any Free Writing Prospectus prepared by the Underwriter and approved by the Company in advance in writing.
6.2.
Section 8A Proceedings. The Underwriter is not subject to any pending proceeding under Section 8A of the Securities
Act with respect to the offering of the Units and will promptly notify the Company if any such proceeding against it is initiated during
the Prospectus Delivery Period.
7.1.
Company Expenses. The Company hereby agrees to pay on the Closing Date all expenses incident to the performance of
the obligations of the Company under this Agreement including, but not limited to: (a) all filing fees and expenses relating to the registration
of the Units with the Commission; (b) all filing fees and expenses associated with the review of the offering of the Units by FINRA; (c)
all fees and expenses relating to the listing of the Shares and the Series A tradable Warrants and the Series B tradable Warrants on the
Exchange (to the extent relevant) or on such other stock exchanges as the Company and the Underwriter together determine; (d) all fees,
expenses and disbursements relating to the registration or qualification of the Units under the “blue sky” securities laws
of such states and other jurisdictions as the Underwriter may reasonably designate (including, without limitation, all filing and registration
fees, and the reasonable fees and disbursements of the Company’s “blue sky” counsel, which will be Underwriter’s
counsel) unless such filings are not required in connection with the Company’s proposed Exchange listing; (e) all fees, expenses
and disbursements relating to the registration, qualification or exemption of the Securities under the securities laws of such foreign
jurisdictions as the Underwriter may reasonably designate; (f) the costs of all mailing and printing of the underwriting documents, the
Registration Statement, Pricing Disclosure Package, the Final Prospectus, any Preliminary Prospectus, any Issuer Free Writing Prospectus
or any Testing-the-Waters Communication and all amendments, supplements and exhibits thereto as the Underwriter may reasonably deem necessary;
(g) the costs of preparing, printing and delivering certificates representing the Shares; (h) fees and expenses of the transfer agent
for the Shares; (i) transfer and/or stamp taxes, if any, payable upon the transfer of securities from the Company to the Underwriter;
(j) the fees and expenses of the Company’s accountants; and (k) reasonable legal fees and disbursements for the Underwriter’s
counsel. The total amount payable by the Company pursuant to (k) to the Underwriter shall not exceed $150,000. The Underwriter may deduct
from the net proceeds of the Offering payable to the Company on the Closing Date the expenses set forth herein to be paid by the Company
to the Underwriter. Except as provided for in this Agreement, the Underwriter shall bear the costs and expenses incurred by them in connection
with the sale of the Units and the transactions contemplated thereby.
7.2.
Non-accountable Expenses. On the Closing Date, the Company shall pay to the Underwriter, by deduction from the net
proceeds of the Offering a non-accountable expense allowance equal to one percent (1.0%) of the gross proceeds received by the Company
from the sale of the Closing Units, provided, however, that in the event that the Offering is terminated, the Company agrees to reimburse
the Underwriter pursuant to Section 12 hereof.
7.3.
Underwriter Expenses. Except to the extent otherwise provided in this Section 7 or Section 9 hereof, the Underwriter
will pay all of its own costs and expenses, including the fees and expenses of their counsel, any stock transfer taxes on resale of any
of the Shares held by them, and any advertising expenses connected with any offers they may make.
7.4.
Company Reimbursement. The provisions of this Section 7 shall not affect any agreement that the Company may make
for the sharing of such costs and expenses.
| 8. | Conditions of the Obligations of the Underwriter. The obligations of the Underwriter to
purchase the Closing Units as provided herein on the Closing Date or the Option Securities as provided herein on any Additional Closing
Date, as the case may be, shall be subject to the timely performance by the Company of its covenants and other obligations hereunder,
and to each of the following additional conditions: |
8.1.
Registration Compliance; No Stop Order.
8.1.1.
The Registration Statement and any post-effective amendment thereto shall have become effective, no stop order suspending the effectiveness
of the Registration Statement or any post-effective amendment thereto shall be in effect, and no proceeding for such purpose or pursuant
to Section 8A of the Securities Act shall be pending before or threatened by the Commission.
8.1.2.
The Company shall have filed the Final Prospectus and each Issuer Free Writing Prospectus with the Commission in accordance with
and within the time periods prescribed by Section 5.1 hereof.
8.1.3.
The Company shall have (A) disclosed to the Underwriter all requests by the Commission for additional information relating to the
offer and sale of the Units and (B) complied with such requests to the reasonable satisfaction of the Underwriter.
8.2.
Representations and Warranties. The representations and warranties of the Company contained herein shall be true
and correct on the date hereof and on and as of the Closing Date or any Additional Closing Date, as the case may be, and the statements
of the Company and its officers made in any certificates delivered pursuant to this Agreement shall be true and correct on and as of the
Closing Date or any Additional Closing Date, as the case may be.
8.3.
Auditor Comfort Letters. On the date of this Agreement and on the Closing Date or any Additional Closing Date, as
the case may be, BF Borgers CPA PC shall have furnished to the Underwriter, at the request of the Company, letters, dated the respective
dates of delivery thereof and addressed to the Underwriter, in form and substance reasonably satisfactory to the Underwriter, containing
statements and information of the type customarily included in accountants’ “comfort letters” to underwriters with respect
to the financial statements and certain financial information contained in each of the Registration Statement, the Pricing Disclosure
Package and the Final Prospectus; provided that the letter delivered on the Closing Date or any Additional Closing Date, as the
case may be, shall use a “cut-off” date no more than two business days prior to the Closing Date or such Additional Closing
Date, as the case may be.
8.4.
No Material Adverse Change. No event or condition of a type described in Section 2.12 hereof shall have occurred
or shall exist, which event or condition is not described in each of the Pricing Disclosure Package and the Final Prospectus (in each
case, exclusive of any amendment or supplement thereto), the effect of which in the judgment of the Underwriter makes it impracticable
or inadvisable to proceed with the offering, sale or delivery of the Units on the Closing Date or any Additional Closing Date, as the
case may be, in the manner and on the terms contemplated by this Agreement, the Pricing Disclosure Package and the Final Prospectus (in
each case, exclusive of any amendment or supplement thereto).
8.5.
Opinion and Negative Assurance Letter of Counsel to the Company. U.S. Company Counsel shall each have furnished to
the Underwriter, at the request of the Company, its (i) written opinion, addressed to the Underwriter and dated the Closing Date or any
Additional Closing Date, as the case may be, and (ii) negative assurance letter, addressed to the Underwriter and dated the Closing Date
or any Additional Closing Date, as the case may be, in each case, in a form reasonably satisfactory to the Underwriter.
8.6.
Officer’s Certificate. The Underwriter shall have received as of the Closing Date or any Additional Closing
Date as the case may be, a certificate of an executive officer of the Company who has specific knowledge of the Company’s financial
matters and is satisfactory to the Underwriter, (i) confirming that such officer has carefully reviewed the Registration Statement, the
Pricing Disclosure Package, the Final Prospectus, each Issuer Free Writing Prospectus and each Written Testing-the-Waters Communication
and, to the knowledge of such officer, the representations set forth in Sections 2.1.2, 2.2, 2.3.1, 2.4.1, 2.5.1, and 2.6, hereof are
true and correct on and as of the Closing Date or any Additional Closing Date, as the case may be; and (ii) confirming that all of the
other representations and warranties of the Company in this Agreement are true and correct on and as of the Closing Date or any Additional
Closing Date, as the case may be, and that the Company has complied with all agreements and covenants and satisfied all other conditions
on its part to be performed or satisfied hereunder at or prior to the Closing Date or any Additional Closing Date, as the case may be.
8.7.
No Legal Impediment to Issuance and Sale. No action shall have been taken and no statute, rule, regulation or order
shall have been enacted, adopted or issued by any federal, state or foreign governmental or regulatory authority that would, as of the
Closing Date or any Additional Closing Date, as the case may be, prevent the issuance, sale or delivery of the Closing Units or the Option
Securities by the Company; and no injunction or order of any federal, state or foreign court shall have been issued that would, as of
the Closing Date or any Additional Closing Date, as the case may be, prevent the issuance, sale or delivery of the Closing Units or the
Option Securities.
8.8.
Good Standing. The Underwriter shall have received on and as of the Closing Date and any Additional Closing Date,
as the case may be, satisfactory evidence of the good standing of the Company and its Significant Subsidiaries in its jurisdiction of
incorporation, in writing from the appropriate governmental authorities of such jurisdiction.
8.9.
Lock-Up Agreements. The Lock-Up Agreements substantially in the form of Exhibit 8.9 hereto executed
by the officers, directors and certain stockholders of the Company relating to sales and certain other dispositions of shares of Common
Stock or certain other securities, delivered to the Underwriter on or before the date hereof, shall be in full force and effect on the
Closing Date or any Additional Closing Date, as the case may be.
8.10.
Exchange Listing. On the Closing Date or any Additional Closing Date, as the case may be, the Shares shall have been
approved for listing on the Exchange, subject to notice of issuance.
8.11.
Additional Documents. On or prior to the Closing Date or any Additional Closing Date, as the case may be, the Underwriter
and its counsel shall have received such information, certificates and other additional documents from the Company as they may reasonably
require in order to evidence the accuracy of any of the representations and warranties, or the satisfaction of any of the covenants, closing
conditions or other obligations, contained in this Agreement.
All opinions, letters, certificates
and other documents delivered pursuant to this Agreement will be deemed to be in compliance with the provisions hereof only if they are
reasonably satisfactory in form and substance to counsel for the Underwriter.
If any condition specified
in this Section 8 is not satisfied when and as required to be satisfied, this Agreement and all obligations of the Underwriter hereunder
may be terminated by the Underwriter by notice to the Company at any time on or prior to the Closing Date or any Additional Closing Date,
as the case may be, which termination shall be without liability on the part of any party to any other party, except that the Company
shall continue to be liable for the payment of expenses under Section 7 and Section 12 hereof and except that the provisions of Section
9 and Section 10 hereof shall at all times be effective and shall survive any such termination.
9.1.
Indemnification of the Underwriter by the Company. The Company agrees to indemnify and hold harmless the Underwriter,
its Affiliates, directors, officers, employees and agents and each person, if any, who controls the Underwriter within the meaning of
Section 15 of the Securities Act or Section 20 of the Exchange Act from and against any and all losses, claims, damages and liabilities
(including, without limitation, all reasonable legal fees and other expenses incurred in connection with any suit, action or proceeding
or any claim asserted, as such fees and expenses are incurred), joint or several, that arise out of or are based upon (i) any untrue statement
or alleged untrue statement of a material fact contained in the Registration Statement (or any amendment or supplement thereto), or the
omission or alleged omission therefrom of a material fact required to be stated therein or necessary in order to make the statements therein
not misleading, or (ii) any untrue statement or alleged untrue statement of a material fact contained in any Pricing Disclosure Package
(including any Pricing Disclosure Package that has subsequently been amended), the Final Prospectus (or any amendment or supplement thereto),
any Preliminary Prospectus, any Issuer Information, any Issuer Free Writing Prospectus, any Written Testing-the-Waters Communication or
any Road Show, or the omission or alleged omission therefrom of a material fact necessary in order to make the statements therein, in
the light of the circumstances under which they were made, not misleading, in each case, except insofar as such losses, claims, damages
or liabilities arise out of, or are based upon, any untrue statement or omission or alleged untrue statement or omission made in reliance
upon and in conformity with the Underwriter Information. The indemnity agreement set forth in this Section 9.1 shall be in addition to
any liabilities that the Company may otherwise have.
9.2.
Indemnification of the Company by the Underwriter. The Underwriter agrees to indemnify and hold harmless the Company,
its directors, each officer who signed the Registration Statement and each person, if any, who controls the Company within the meaning
of Section 15 of the Securities Act or Section 20 of the Exchange Act from and against any and all losses, claims, damages and liabilities
(including, without limitation, all reasonable legal fees and other expenses incurred in connection with any suit, action or proceeding
or any claim asserted, as such fees and expenses are incurred), joint or several, to the same extent as the indemnity set forth in Section
9.1 hereof; provided, however, that the Underwriter shall be liable only to the extent that any untrue statement or omission or alleged
untrue statement or omission was made in the Registration Statement (or any amendment or supplement thereto), any Pricing Disclosure Package
(including any Pricing Disclosure Package that has subsequently been amended), the Final Prospectus (or any amendment or supplement thereto),
any Preliminary Prospectus, any Issuer Information, any Issuer Free Writing Prospectus, any Written Testing-the-Waters Communication or
any Road Show in reliance upon, and in conformity with, the Underwriter Information relating to the Underwriter. The indemnity agreement
set forth in this Section 9 shall be in addition to any liabilities that the Underwriter may otherwise have.
9.3.
Notifications and Other Indemnification Procedures. If any suit, action, proceeding (including any governmental or
regulatory investigation), claim or demand shall be brought or asserted against any person in respect of which indemnification may be
sought pursuant to any of the preceding subsections of this Section 9, such person (the “Indemnified Person”)
shall promptly notify the person against whom such indemnification may be sought (the “Indemnifying Person”)
in writing; provided that the failure to notify the Indemnifying Person shall not relieve it from any liability that it may have
under any of the preceding subsections of this Section 9 except to the extent that it has been materially prejudiced by such failure;
and provided, further, that the failure to notify the Indemnifying Person shall not relieve it from any liability that it may have
to an Indemnified Person otherwise than under any of the preceding subsections of this Section 9. If any such proceeding shall be brought
or asserted against an Indemnified Person and it shall have notified the Indemnifying Person thereof, the Indemnifying Person shall retain
counsel reasonably satisfactory to the Indemnified Person (who shall not, without the consent of the Indemnified Person, be counsel to
the Indemnifying Person) to represent the Indemnified Person in such proceeding and shall pay the reasonable and documented fees and expenses
of such counsel related to such proceeding, as incurred. In any such proceeding, any Indemnified Person shall have the right to retain
its own counsel, but the fees and expenses of such counsel shall be at the expense of such Indemnified Person unless (i) the Indemnifying
Person and the Indemnified Person shall have mutually agreed to the contrary; (ii) the Indemnifying Person has failed within a reasonable
time to retain counsel reasonably satisfactory to the Indemnified Person; (iii) the Indemnified Person shall have reasonably concluded
that there may be legal defenses available to it that are different from or in addition to those available to the Indemnifying Person;
or (iv) the named parties in any such proceeding (including any impleaded parties) include both the Indemnifying Person and the Indemnified
Person and representation of both parties by the same counsel would be inappropriate due to actual or potential differing interest between
them. It is understood and agreed that the Indemnifying Person shall not, in connection with any proceeding or related proceedings in
the same jurisdiction, be liable for the fees and expenses of more than one separate firm (in addition to any local counsel) for all Indemnified
Persons, and that all such fees and expenses shall be paid or reimbursed as they are incurred. Any such separate firm for (i) the Underwriter,
its Affiliates, directors, officers, employees and agents and each person, if any, who controls the Underwriter within the meaning of
Section 15 of the Securities Act or Section 20 of the Exchange Act shall be designated in writing by the Underwriter; and (ii) the Company,
its directors, its officers who signed the Registration Statement and each person, if any, who controls the Company within the meaning
of Section 15 of the Securities Act or Section 20 of the Exchange Act shall be designated in writing by the Company.
9.4.
Settlements. The Indemnifying Person under this Section 9 shall not be liable for any settlement of any proceeding
effected without its written consent, which consent may not be unreasonably withheld, but if settled with such consent or if there be
a final judgment for the plaintiff, the Indemnifying Person agrees to indemnify the Indemnified Person from and against any loss, claim,
damage, liability or expense by reason of such settlement or judgment. Notwithstanding the foregoing sentence, if at any time an Indemnified
Person shall have requested an Indemnifying Person to reimburse the Indemnified Person for any reasonably incurred and documented fees
and expenses of counsel as contemplated by this Section 9, the Indemnifying Person agrees that it shall be liable for any settlement of
any proceeding effected without its written consent if (i) such settlement is entered into more than forty-five (45) days after receipt
by such Indemnifying Person of the aforesaid request, (ii) such Indemnifying Person shall not have reimbursed the Indemnified Person in
accordance with such request, or shall not have disputed in good faith the Indemnified Person’s entitlement to such reimbursement,
prior to the date of such settlement and (iii) such Indemnified Person shall have given the Indemnifying Person at least forty-five (45)
days’ prior notice of its intention to settle. No Indemnifying Person shall, without the prior written consent of the Indemnified
Person effect any settlement, compromise or consent to the entry of judgment in any pending or threatened action, suit or proceeding in
respect of which any Indemnified Person is or could have been a party and indemnity was or could have been sought hereunder by such Indemnified
Person, unless such settlement, compromise or consent (x) includes an unconditional release of such Indemnified Person, in form and substance
reasonably satisfactory to such Indemnified Person, from and against all liability on claims that are the subject matter of such action,
suit or proceeding and (y) does not include any statements as to or any findings of fault, culpability or failure to act by or on behalf
of any Indemnified Person.
10.1.
To the extent the indemnification provided for in Section 9 hereof is unavailable to or insufficient to hold harmless an Indemnified
Person in respect of any losses, claims, damages, liabilities or expenses referred to therein, then each Indemnifying Person, in lieu
of indemnifying such Indemnified Person thereunder, shall contribute to the aggregate amount paid or payable by such Indemnified Person,
as incurred, as a result of any losses, claims, damages, liabilities or expenses referred to therein (i) in such proportion as is appropriate
to reflect the relative benefits received by the Company, on the one hand, and the Underwriter, on the other hand, from the offering of
the Units pursuant to this Agreement or (ii) if the allocation provided by clause (i) above is not permitted by applicable law, in such
proportion as is appropriate to reflect not only the relative benefits referred to in clause (i) above but also the relative fault of
the Company, on the one hand, and the Underwriter, on the other hand, in connection with the statements or omissions that resulted in
such losses, claims, damages, liabilities or expenses, as well as any other relevant equitable considerations. The relative benefits received
by the Company, on the one hand, and the Underwriter, on the other hand, in connection with the offering of the Units pursuant to this
Agreement shall be deemed to be in the same respective proportions as the total net proceeds from the offering of the Units pursuant to
this Agreement (before deducting expenses) received by the Company, on the one hand, and the total underwriting discounts and commissions
received by the Underwriter, on the other hand, in each case as set forth in the table on the cover of the Final Prospectus bear to the
aggregate initial offering price of the Units. The relative fault of the Company, on the one hand, and the Underwriter, on the other hand,
shall be determined by reference to, among other things, whether the untrue or alleged untrue statement of a material fact or omission
or alleged omission to state a material fact relates to information supplied by the Company, on the one hand, or the Underwriter, on the
other hand, and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such statement
or omission.
10.2.
The amount paid or payable by a party as a result of the losses, claims, damages, liabilities and expenses referred to above shall
be deemed to include, subject to the limitations set forth in Section 9 hereof, all reasonable legal or other fees or expenses incurred
by such party in connection with investigating or defending any action or claim. The provisions set forth in Section 9 hereof with respect
to notice of commencement of any action shall apply if a claim for contribution is to be made under this Section 10; provided,
however, that no additional notice shall be required with respect to any action for which notice has been given under Section 9 hereof
for purposes of indemnification.
10.3.
The Company and the Underwriter agree that it would not be just and equitable if contribution pursuant to this Section 10 was determined
by pro rata allocation or by any other method of allocation which does not take account of the equitable considerations referred to in
this Section 10.
10.4.
Notwithstanding the provisions of this Section 10, the Underwriter shall not be required to contribute any amount in excess of
the amount by which the total discounts and commissions received by the Underwriter in connection with the Units distributed by it exceeds
the amount of any damages the Underwriter has otherwise paid or become liable to pay by reason of any untrue or alleged untrue statement
or omission or alleged omission. No person guilty of fraudulent misrepresentation (within the meaning of Section 11 of the Securities
Act) shall be entitled to contribution from any person who was not guilty of such fraudulent misrepresentation.
10.5.
For purposes of this Section 10, each director, officer, employee and agent of the Underwriter and each person, if any, who controls
the Underwriter within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act shall have the same rights to
contribution as the Underwriter, and each director and officer of the Company who signed the Registration Statement, and each person,
if any, who controls the Company with the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act, shall have the
same rights to contribution as the Company.
10.6.
The remedies provided for in Section 9 and Section 10 hereof are not exclusive and shall not limit any rights or remedies which
may otherwise be available to any Indemnified Person at law or in equity.
11.1.
Prior to the delivery of and payment for the Units on the Closing Date or any Additional Closing Date, as the case may be, this
Agreement may be terminated by the Underwriter in the absolute discretion of the Underwriter by notice given to the Company if after the
execution and delivery of this Agreement: (i) trading or quotation of any securities issued or guaranteed by the Company shall have been
suspended or materially limited on any securities exchange, quotation system or in the over-the-counter market; (ii) trading in securities
generally on any of the New York Stock Exchange, the Nasdaq Stock Exchange or the over-the-counter market shall have been suspended or
materially limited; (iii) a general banking moratorium on commercial banking activities shall have been declared by federal or New York
state authorities; (iv) there shall have occurred a material disruption in commercial banking or securities settlement, payment or clearance
services in the United States; (v) there shall have occurred any outbreak or escalation of national or international hostilities or any
crisis or calamity, or any change in the United States or international financial markets, or any substantial change or development involving
a prospective substantial change in general economic, financial or political conditions in the United States or internationally, as in
the judgment of the Underwriter is material and adverse and makes it impracticable or inadvisable to proceed with the offering, sale or
delivery of the Units on the Closing Date or any Additional Closing Date, as the case may be, in the manner and on the terms described
in the Pricing Disclosure Package or to enforce contracts for the sale of securities; or (vi) the Company or any of its subsidiaries shall
have sustained a loss by strike, fire, flood, earthquake, accident or other calamity of such character as in the judgment of the Underwriter
may interfere materially with the conduct of the business and operations of the Company and its subsidiaries, considered as one entity,
regardless of whether or not such loss shall have been insured.
11.2.
Any termination pursuant to this Section 11 shall be without liability on the part of: (x) the Company to the Underwriter, except
that the Company shall continue to be liable for the payment of expenses under Section 7; (y) the Underwriter to the Company; or (z) any
party hereto to any other party except that the provisions of Section 9, Section 10 and this Section 11 hereof shall at all times be effective
and shall survive any such termination. In the event this Agreement is terminated pursuant to this Section 11, the Underwriter shall be
entitled to cash compensation of 8.0% of the gross proceeds with respect to any public or private offering or other financing or capital
raising transaction of any kind (“Tail Financing”) to the extent that such financing or capital is provided
to the Company by investors the Underwriter has introduced to and/or contacted on behalf of the Company through an in-person, electronic
or telephonic communication or investors that Aegis had “wall-crossed” in connection with this Offering (or any entity under
common management or having a common investment advisor), if such Tail Financing is consummated at any time within the three (3) months
period beginning on the Closing, expiration or termination of this Agreement.
| 12. | Reimbursement of the Underwriter’s Expenses. If (a) the Company fails to deliver the
Units to the Underwriter for any reason at the Closing Date or any Additional Closing Date, as the case may be, in accordance with this
Agreement or (b) the Underwriter declines to purchase the Units for any reason permitted under this Agreement, then the Company agrees
to reimburse the Underwriter for all reasonable out-of-pocket costs and expenses (including the reasonable and documented fees and expenses
of counsel to the Underwriter) incurred by the Underwriter in connection with this Agreement and the applicable offering contemplated
hereby. |
| | | |
| 13. | Representations and Indemnities to Survive Delivery. The respective indemnities, rights
of contribution, agreements, representations, warranties and other statements of the Company and the Underwriter set forth in or made
pursuant to this Agreement or made by or on behalf of the Company or the Underwriter pursuant to this Agreement or any certificate delivered
pursuant hereto shall remain in full force and effect, regardless of any investigation made by or on behalf of the Underwriter, the Company
or any of their respective officers or directors or any controlling person, as the case may be, and shall survive delivery of and payment
for the Units sold hereunder and any termination of this Agreement. |
| | | |
| 14. | Notices. All notices, requests, consents, claims, demands, waivers and other communications
under this Agreement shall be in writing and shall be deemed to have been duly given (i) when delivered by hand (with written confirmation
of receipt), (ii) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested), (iii) on the
date sent by facsimile (with confirmation of transmission) or email of a PDF document if sent during normal business hours of the recipient,
and on the next Business Day if sent after normal business hours of the recipient, or (iv) on the third day after the date mailed, by
certified or registered mail (in each case, return receipt requested, postage pre-paid). Such communications must be sent to the respective
parties at the following addresses (or at such other address for a party as shall be specified in a notice given in accordance with this
Section 14): |
| | | |
| If to the Underwriter: |
|
Aegis Capital Corp.
1345 Avenue of the Americas, 27th Floor New York,
NY 10105
Email: reide@aegiscap.com
Attention: Robert Eide |
| |
|
| with a copy to: |
|
Kaufman & Canoles, P.C.
Two James Center, 14th Floor, 1021 E. Cary St., Richmond,
VA 23219
Email:awbasch@kaufcan.com
Attention:Anthony Basch |
| |
|
| If to the Company: |
|
Sunshine Biopharma, Inc.
1177 Avenue of the Americas, 5th Floor
New York, NY 10036
Email:steve.slilaty@sunshinebiopharma.com
Attention: Dr. Steve Slilaty |
| |
|
|
| with copy to: |
|
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas, 31st Floor
New York, New York 10036
Email:jcahlon@srfc.law
Attention:Jeff Cahlon |
| |
|
|
Any party hereto may change
the address or facsimile number for receipt of communications by giving written notice to the others in accordance with this Section 14.
| 15. | Successors. This Agreement shall inure solely to the benefit of and be binding upon the
Underwriter, the Company and the other indemnified parties referred to in Section 9 and Section 10 hereof, and in each case their respective
successors. Nothing in this Agreement is intended, or shall be construed, to give any other person or entity any legal or equitable right,
benefit, remedy or claim under, or in respect of or by virtue of, this Agreement or any provision contained herein. The term “successors,”
as used herein, shall not include any purchaser of the Units from the Underwriter merely by reason of such purchase. |
| | | |
| 16. | Partial Unenforceability. The invalidity or unenforceability of any Section, paragraph or
provision of this Agreement shall not affect the validity or enforceability of any other Section, paragraph or provision hereof. If any
Section, paragraph or provision of this Agreement is for any reason determined to be invalid or unenforceable, there shall be deemed to
be made such minor changes (and only such minor changes) as are necessary to make it valid and enforceable. |
| | | |
| 17. | Governing Law. This Agreement and any claim, controversy or dispute arising under or related
to this Agreement, whether sounding in contract, tort or statute, shall be governed by and construed in accordance with the internal laws
of the State of New York applicable to agreements made and to be performed in such state (including its statute of limitations), without
giving effect to the conflict of laws provisions thereof to the extent such principles or rules would require or permit the application
of the laws of any jurisdiction other than those of the State of New York. |
| | | |
| 18. | Consent to Jurisdiction. No legal suit, action or proceeding arising out of or relating
to this Agreement or the transactions contemplated hereby (each, a “Related Proceeding”) may be commenced, prosecuted
or continued in any court other than the courts of the State of New York located in the City and County of New York or in the United States
District Court for the Southern District of New York, which courts (collectively, the “Specified Courts”) shall
have jurisdiction over the adjudication of any Related Proceeding, and the parties to this Agreement hereby irrevocably consent to the
exclusive jurisdiction the Specified Courts and personal service of process with respect thereto. The parties to this Agreement hereby
irrevocably waive any objection to the laying of venue of any Related Proceeding in the Specified Courts and irrevocably waive and agree
not to plead or claim in any Specified Court that any Related Proceeding brought in any Specified Court has been brought in an inconvenient
forum. |
| | | |
| 19. | Equitable Remedies. Each party to this Agreement acknowledges and agrees that (a) a breach
or threatened breach by the Company of any of its obligations under Section 5.11 or Section 5.16 would give rise to irreparable harm to
the Underwriter for which monetary damages would not be an adequate remedy and (b) if a breach or a threatened breach by the Company of
any such obligations occurs, the Underwriter will, in addition to any and all other rights and remedies that may be available to such
party at law, at equity, or otherwise in respect of such breach, be entitled to equitable relief, including a temporary restraining order,
an injunction, specific performance of the terms of Section 5.11 or Section 5.16 and any other relief that may be available from a court
of competent jurisdiction, without any requirement to (i) post a bond or other security, or (ii) prove actual damages or that monetary
damages will not afford an adequate remedy. Each party to this Agreement agrees that such party shall not oppose or otherwise challenge
the existence of irreparable harm, the appropriateness of equitable relief or the entry by a court of competent jurisdiction of an order
granting equitable relief, in either case, consistent with the terms of this Section 19. |
| | | |
| 20. | Waiver of Jury Trial. The parties to this Agreement hereby irrevocably waive, to the fullest
extent permitted by applicable law, any and all right to trial by jury in any Related Proceeding. |
| | | |
| 21. | No Fiduciary Relationship. The Company acknowledges and agrees that: (i) the purchase and
sale of the Units pursuant to this Agreement, including the determination of the offering price of the Units and any related discounts
and commissions, is an arm’s-length commercial transaction between the Company, on the one hand, and the Underwriter, on the other
hand; (ii) in connection with each transaction contemplated hereby and the process leading to such transaction the Underwriter is and
has been acting solely as a principal and is not the agent or fiduciary of the Company or its Affiliates, stockholders, members, partners,
creditors or employees or any other party; (iii) the Underwriter has not assumed and will not assume an advisory or fiduciary responsibility
in favor of the Company with respect to any of the transactions contemplated hereby or the process leading thereto (irrespective of whether
the Underwriter has advised or is currently advising the Company on other matters) or any other obligation to the Company except the obligations
expressly set forth in this Agreement; (iv) the Underwriter and its respective Affiliates may be engaged in a broad range of transactions
that involve interests that differ from those of the Company, and the Underwriter has no obligation to disclose any of such interests
by virtue of any fiduciary or advisory relationship; and (v) the Underwriter has not provided any legal, accounting, regulatory or tax
advice in any jurisdiction with respect to the offering contemplated hereby, and the Company has consulted its own legal, accounting,
regulatory and tax advisors to the extent they deemed appropriate. The Company waives and releases, to the full extent permitted by applicable
law, any claims it may have against the Underwriter arising from an alleged breach of fiduciary duty in connection with the offering of
the Units or any matters leading up to the offering of the Units. |
| 22. | Compliance with the USA Patriot Act. In accordance with the requirements of the USA Patriot
Act (Title III of Pub. L. 107-56 (signed into law October 26, 2001)), the Underwriter is required to obtain, verify and record information
that identifies their respective clients, including the Company, which information may include the name and address of its clients, as
well as other information that will allow the Underwriter to properly identify their respective clients. |
| | | |
| 23. | Entire Agreement. This Agreement, together with any contemporaneous written agreements and
any prior written agreements (to the extent not superseded by this Agreement) that relate to the offering of the Units, represents the
entire agreement among the Company and the Underwriter with respect to the preparation of the Registration Statement, the Pricing Disclosure
Package, the Final Prospectus, each Preliminary Prospectus, each Issuer Free Writing Prospectus, each Testing-the-Waters Communication
and each Road Show, the purchase and sale of the Units and the conduct of the offering contemplated hereby. |
| | | |
| 24. | Amendments or Waivers. No amendment or waiver of any provision of this Agreement, nor any
consent or approval to any departure therefrom, shall in any event be effective unless the same shall be in writing and signed by all
the parties hereto. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not
expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after the waiver.
No failure to exercise, or delay in exercising, any right, remedy, power or privilege arising from this Agreement shall operate or be
construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any
other or further exercise of any other right, remedy, power or privilege. |
| | | |
| 25. | Section Headings. The headings herein are included for convenience of reference only and
are not intended to be part of, or to affect the meaning or interpretation of, this Agreement. |
| | | |
| 26. | Counterparts. This Agreement may be executed in counterparts, each of which will be deemed
an original, but all of which together will be deemed to be one and the same agreement. Counterparts may be delivered via facsimile, email
(including PDF or any electronic signature complying with the U.S. federal ESIGN Act of 2000) or other transmission method, and any counterpart
so delivered will be deemed to have been duly and validly delivered and be valid and effective for all purposes. |
| | | |
| 27. | Recognition of the U.S. Special Resolution Regimes. |
27.1.
In the event that the Underwriter is a Covered Entity (as defined below) becomes subject to a proceeding under a U.S. Special Resolution
Regime, the transfer from the Underwriter of this Agreement, and any interest and obligation in or under this Agreement, will be effective
to the same extent as the transfer would be effective under the U.S. Special Resolution Regime (as defined below) if this Agreement, and
any such interest and obligation, were governed by the laws of the United States or a state of the United States.
27.2.
In the event that the Underwriter is a Covered Entity or a BHC Act Affiliate (as defined below) of the Underwriter becomes subject
to a proceeding under a U.S. Special Resolution Regime, Default Rights (as defined below) under this Agreement that may be exercised against
the Underwriter is permitted to be exercised to no greater extent than such Default Rights could be exercised under the U.S. Special Resolution
Regime if this Agreement were governed by the laws of the United States or a state of the United States.
27.3.
As used in this section:
27.3.1.
“BHC Act Affiliate” has the meaning assigned to the term “affiliate” in, and shall be interpreted
in accordance with, 12 U.S.C. § 1841(k).
27.3.2.
“Covered Entity” means any of the following:
27.3.2.1
a “covered entity” as that term is defined in, and interpreted in accordance with, 12 C.F.R. § 252.82(b);
27.3.2.2
a “covered bank” as that term is defined in, and interpreted in accordance with, 12 C.F.R. § 47.3(b); or
27.3.2.3
a “covered FSI” as that term is defined in, and interpreted in accordance with, 12 C.F.R. § 382.2(b).
27.3.3.
“Default Right” has the meaning assigned to that term in, and shall be interpreted in accordance with,
12 C.F.R. §§ 252.81, 47.2 or 382.1, as applicable.
27.3.4.
“U.S. Special Resolution Regime” means each of (i) the Federal Deposit Insurance Act and the regulations
promulgated thereunder and (ii) Title II of the Dodd-Frank Wall Street Reform and Consumer Protection Act and the regulations promulgated
thereunder.
[SBFM Underwriting Agreement Signature Page
Follows]
[SBFM Underwriting Agreement Signature Page]
If the foregoing is in accordance
with your understanding, please indicate your acceptance of this Agreement by signing in the space provided below.
|
Very truly yours,
SUNSHINE BIOPHARMA, INC. |
| |
|
| By: |
|
|
| Name: |
|
Dr. Steve Slilaty |
| Title: |
|
Chief Executive Officer |
Confirmed and accepted as of the date first above
written:
AEGIS CAPITAL CORP.
| |
|
|
| By: |
|
|
| Name: |
|
Robert Eide |
| Title: |
|
Chief Executive Officer |
SCHEDULE 1.15
Pricing Disclosure Package
Number of Closing Shares:
[●]
Number of Units containing Firm Shares
(“Common Units”) [●]
Number of Units containing Pre-funded
Warrants (“Pre-funded Units”) [●]
Number of Option Shares: [●]
Number of Option Common Warrants: [●]
Number of Option Series A Common Warrants: [●]
Public Offering Price per Common Unit: $[●]
Public Offering Price per Pre-Funded Unit: $[●]
Exercise Price per Pre-funded Warrant: $0.001
Exercise price per Series A Common Warrant
per whole share: $[●]
Exercise price per Series B Common Warrant
per whole share: $[●]
Underwriting Discount per Common Unit
and per Pre-Funded Unit: $[●]
Non-accountable expense allowance per
Common Unit and per Pre-Funded Unit: $[●]
Purchase Price per Option Share: $[●]
Purchase Price per Option Pre-funded Warrant: $[●]
Purchase Price per full Option Common
Warrant: $0.01
SCHEDULE 2.5.4
Free Writing Prospectuses
SCHEDULE 2.44
Significant Subsidiaries
| |
|
|
| Significant Subsidiaries |
|
Place of Incorporation |
| [●] |
|
[●] |
| |
|
| |
|
|
SCHEDULE 4.1.2
Closing Securities
| Underwriter |
|
Number of Closing Units to Be Purchased |
|
Number of Option Securities to Be Purchased if the Maximum Over-Allotment Option Is Exercised |
| Aegis Capital Corp. |
|
[●] |
|
[●] |
| Total: |
|
[●] |
|
[●] |
EXHIBIT 5.10.3.1
Form of Lock-Up Waiver
[●], 202[●]
[Waiver Recipient Name and Address]
Re: Lock-Up Agreement Waiver
Ladies and Gentlemen:
[Pursuant to Section 8.9 of the Underwriting Agreement,
dated [●], 2024 (the “Underwriting Agreement”), among Sunshine Biopharma, Inc., a Colorado corporation
(the “Company”), and Aegis Capital Corp. (the “Underwriter”), and the Lock-Up Agreement,
dated [●], 2024 (the “Lock-Up Agreement”), between you and the Underwriter relating to the Company’s
shares of Common Stock, $0.001 par value per share (the “Share”), the Underwriter hereby gives its consent to
allow you to sell up to [●] Share [solely from and including [DATE] to and including [DATE]].]
[Pursuant to Section 5.11 of the Underwriting
Agreement, the Underwriter hereby gives its consent to allow the Company to issue and sell up to [●] Shares pursuant to an offering
of the Shares to commence prior to the expiration of the Lock-Up Period as defined in the Underwriting Agreement[, provided that such
offering closes on or prior to [●]].]
AEGIS CAPITAL CORP.
| |
|
|
|
| |
By: |
|
|
| |
Name: |
|
Robert Eide |
| |
Title: |
|
Chief Executive Officer |
EXHIBIT 5.10.3.2
Form of Lock-Up Waiver Press Release
Sunshine Biopharma, Inc.
[Date]
Sunshine Biopharma, Inc., a Colorado corporation
(the “Company”) announced today that Aegis Capital Corp., acting as the Underwriter in the Company’s recent public offering
of the Company’s Units consisting of one (1) share of Common Stock; and one-tenth (1/10th) of a Series A warrant to purchase one
(1) share of Common Stock at a per-Share exercise price of $[●] (representing 1500.0% of the per Closing Common Unit offering price;
and two-tenths (2/10th) of a Series B warrant to purchase one (1) share of Common Stock at a per-Share exercise price of $[●] (representing
1700.0% of the per Closing Common Unit offering price, is [waiving] [releasing] a lock-up restriction with respect to the Company’s
shares of Common Stock held by [certain officers or directors] [an officer or director] of the Company. The [waiver] [release] will take
effect on [Date], and the Shares may be sold on or after such date.
This press release is not an offer or sale
of the securities in the United States or in any other jurisdiction where such offer or sale is prohibited, and such securities may not
be offered or sold in the United States absent registration or an exemption from registration under the Securities Act of 1933, as amended.
EXHIBIT 8.9
Form of Lock-Up Agreement
Exhibit 5.1
| Andrew I. Telsey, P.C. Attorney at Law |
|
6198 S. Moline Court, Englewood, Colorado 80111
Telephone: 303/521-7447 • E-Mail: andrew@telseylaw.com |
February 9, 2024
Board of Directors
Sunshine Biopharma, Inc.
| Re: |
Sunshine Biopharma, Inc. |
| |
Form S-1/ Registration Statement
and Related Prospectus |
| |
SEC file No. 333-276817 |
Gentlemen:
We have acted as counsel to Sunshine Biopharma,
Inc., a Colorado corporation (the “Company”), in connection with the filing by the Company of a Registration Statement
on Form S-1 (File No. 333-276817) initially filed with the Securities and Exchange Commission (the “SEC”) on February
1, 2024 and amended on February 8, 2024 (as so amended, the “Registration Statement”) under the Securities Act of 1933,
as amended (the “Securities Act”). The Registration Statement relates to proposed issuance and sale by the Company
of (i) 39,215,687 units (the “Closing Units”), with each Closing Unit consisting of either (A) one share of the Company’s
common stock, $0.001 par value (“Common Stock”) (collectively, the “Closing Shares”) and one-tenth
(1/10) of a Series A warrant (“Series A Warrant”) to purchase one share of Common Stock and two-tenths (2/10) of a
Series B warrant to purchase one share of Common Stock (“Series B Warrant” and, together with the Series A Warrant,
the “Warrants”), or (B) one pre-funded warrant (each, a “Pre-funded Warrant”) to purchase one share
of Common Stock at an exercise price of $0.001 until such time as the Pre-Funded Warrant is exercised in full subject to adjustment as
provided in the Pre-funded Warrant and one-tenth (1/10) of a Series A Warrant to purchase one share of Common Stock and two-tenths (2/10)
of a Series B Warrant to purchase one share of Common Stock.. The Units, the Shares, the Warrants, the shares underlying the Warrants
(the “Warrant Shares”), the Pre-Funded Warrants and shares underlying the Pre-Funded Warrants (the “Pre-Funded Warrant
Shares”) are referred to herein, collectively, as the “Securities.” The proposed maximum aggregate offering price
of the Securities is $67,850,000. The Securities are to be sold by the Company pursuant to an underwriting agreement by and between the
Company and Aegis Capital Corp., as the underwriter (the “Underwriting Agreement”).
You have requested our opinion as to the matters
set forth below in connection with the issuance of the Securities. For purposes of rendering that opinion, we have examined: (i) the Registration
Statement, (ii) the form of Underwriting Agreement, (iii) the Warrant Agency Agreement; (iv) the form of Series A Warrant, (v) the form
of Series B Warrant, (vi) the form of Pre-Funded Warrant, (vii) the Articles of Incorporation of the Company, as amended and in effect
as of the date hereof (the “Charter”), (viii) the Company’s Amended and Restated Bylaws (the “Bylaws”),
(ix) the Company’s stock ledger, and (x) the corporate action of the Company’s Board of Directors which, among other things,
authorizes the issuance of the Securities. We have also reviewed such matters of law as we have deemed necessary to render the opinion
expressed herein.
For the purposes of this opinion letter, we have
assumed that each document submitted to us is accurate and complete, that each such document that is an original is authentic, the conformity
to the original or final versions of the documents submitted to us as copies or drafts, including without limitation, the Charter, the
Bylaws, the Underwriting Agreement, the Warrant Agency Agreement and the forms of the Warrants and Pre-Funded Warrants, and that all signatures
on each such document are genuine. We have also assumed the legal capacity of natural persons and have made such other assumptions as
are customary in opinion letters of this kind. We have not verified any of those assumptions or any of the other assumptions contained
herein.
Our opinions set forth below in numbered paragraph
1, numbered paragraph 2, numbered paragraph 3 and numbered paragraph 4 are limited to the Colorado Business Corporation Act. Each of our
opinions set forth below is subject to the application of equitable principles and considerations of public policy.
Based upon and subject to the foregoing, it is
our opinion that:
1. The Closing Units
have been duly authorized for issuance by the Company. The Closing Units, if and when issued, delivered and paid for as described in the
prospectus related to the Registration Statement (the “Prospectus”) and pursuant to the Underwriting Agreement, will
constitute valid and binding obligations of the Company, enforceable against the Company in accordance with their terms, subject to the
effects of bankruptcy, insolvency, fraudulent transfer, reorganization, receivership, moratorium and other laws affecting the rights and
remedies of creditors generally and to the exercise of judicial discretion in accordance with general principles of equity, whether applied
by a court of law or equity.
2. The Closing Shares
have been duly authorized for issuance by the Company and, when issued, delivered and paid for as described in the Prospectus and pursuant
to the Underwriting Agreement, will be validly issued, fully-paid and non-assessable.
3. The Warrants and Pre-Funded
Warrants have been duly authorized for issuance by the Company.
4. The Warrant Shares
and Pre-Funded Warrant Shares have been duly authorized for issuance by the Company and, when issued and delivered by the Company against
payment therefor, upon exercise of the Warrants or Pre-Funded Warrants, as applicable, in accordance with the terms therein and the terms
of the Warrants or Pre-Funded Warrants, as applicable, will be validly issued, fully-paid and non-assessable.
The opinions set forth above are subject to the
following additional assumptions:
(a) the Registration
Statement and any amendment thereto (including any post-effective amendment) will have become effective under the Securities Act, and
such effectiveness shall not have been terminated, suspended or rescinded;
(b) any Prospectus required
by applicable law will have been delivered and filed as required by such laws;
(c) all Securities offered
pursuant to the Registration Statement will be (i) issued and sold in the manner provided in the Registration Statement and the Prospectus,
(ii) issued and sold only upon payment of the consideration fixed therefor in accordance with the Underwriting Agreement, the Warrant
Agency Agreement and, if applicable, the Securities themselves, and there will not have occurred any change in law or fact affecting the
validity of the opinion rendered herein with respect thereto between the date hereof and the date of such issuance and (iii) duly noted
in the Company’s stock or warrant ledger, as applicable, upon their issuance;
(d) the Company will
have sufficient authorized and unissued shares of Common Stock at the time of each issuance of a Warrant Share or Pre-Funded Warrant Share
upon the exercise of a Warrant or Pre-Funded Warrant, as applicable, and each such Warrant Share or Pre-Funded Warrant Share, as applicable,
as well as the Shares, will be noted in the Company’s stock ledger upon issuance;
(e) the Company’s
Board of Directors shall have approved the issuance of the Closing Units, the final number of Securities to be issued and the price to
be paid therefor pursuant to the Underwriting Agreement and the Warrant Agency Agreement, as applicable, and
(f)
to the extent that the obligations of the Company under any Warrant Agency Agreement or other agreement pursuant to which any Securities
offered pursuant to the Registration Statement are to be issued or governed, including any amendment or supplement thereto, may be dependent
upon such matters, (i) each party to any such agreement other than the Company (including any applicable warrant agent or other party
acting in a similar capacity with respect to any Securities) will be duly organized, validly existing and in good standing under the laws
of its jurisdiction of organization; that each such other party will be duly qualified to engage in the activities contemplated thereby;
(ii) each such agreement and the applicable Securities will have been duly authorized, executed and delivered by each such other party
and will constitute the valid and binding obligations of each such other party, enforceable against each such other party in accordance
with their terms; (iii) each such other party will be in compliance, with respect to acting in any capacity contemplated by any such agreement,
with all applicable laws and regulations; and (iv) each such other party will have the requisite organizational and legal power and authority
to perform its obligations under each such agreement.
We hereby consent to the filing of this opinion
letter with the SEC as Exhibit 5.1 to the Registration Statement. We also consent to the reference to our Firm under the caption “Legal
Matters” in the Registration Statement and in the Prospectus. In giving our consent, we do not thereby admit that we are experts
with respect to any part of the Registration Statement, the Prospectus or any prospectus supplement within the meaning of the term “expert”,
as used in Section 11 of the Securities Act or the rules and regulations promulgated thereunder, nor do we admit that we are in the category
of persons whose consent is required under Section 7 of the Securities Act or the rules and regulations thereunder. We assume no obligation
to update or supplement our opinion to reflect any changes of law or fact that may occur.
Yours truly,
ANDREW I. TELSEY, P.C.
/s/ ANDREW I. TELSEY
Exhibit 5.2

February 9, 2024
Sunshine Biopharma, Inc.
1177 Avenue of the Americas, 5th Floor
New York, NY 10036
Ladies and Gentlemen:
We have acted as counsel to Sunshine Biopharma,
Inc., a Colorado corporation (the “Company”), in connection with the Registration Statement on Form S-1 (File No. 333-276817)
(such registration statement, as amended through the date hereof, the “Registration Statement”) filed by the Company
with the Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933, as amended (the “Securities
Act”), relating to the proposed offer and sale by the Company (the “Offering”) of units (each, a “Closing
Unit”), with each Closing Unit consisting of either (A) one share of the Company’s common stock (“Common Stock”)
(collectively, the “Closing Shares”) and one-tenth (1/10) of a Series A warrant (“Series A Warrant”)
to purchase one share of Common Stock and two-tenths (2/10) of a Series B warrant to purchase one share of Common Stock (“Series
B Warrant” and, together with the Series A Warrant, the “Warrants”), or (B) one pre-funded warrant (each,
a “Pre-funded Warrant”) to purchase one share of Common Stock at an exercise price of $0.001 until such time as the
Pre-Funded Warrant is exercised in full subject to adjustment as provided in the Pre-funded Warrant and one-tenth (1/10) of a Series A
Warrant to purchase one share of Common Stock and two-tenths (2/10) of a Series B Warrant to purchase one share of Common Stock. The term
“Closing Units” also includes any additional Closing Units (and the underlying securities) that may be issued by the Company
pursuant to Rule 462(b) under the Securities Act in connection with the Offering. The proposed maximum aggregate offering price of the
Closing Units is $67,850,000.
This opinion letter is being furnished in accordance
with the requirements of Item 601(b)(5) of Regulation S-K under the Securities Act.
We have reviewed originals or copies of the Registration
Statement, the prospectus contained in the Registration Statement (the “Prospectus”), the articles of incorporation
and bylaws of the Company, as amended through the date hereof (the “Organizational Documents”), the form of warrant
agent agreement (the “Warrant Agent Agreement”) proposed to be entered into by the Company and Equiniti Trust Company,
as warrant agent (the “Warrant Agent”), the form of Series A Warrant that is filed as an exhibit to the Registration
Statement (the “Series A Warrant Agreement”), and the form of Series B Warrant that is filed as an exhibit to the Registration
Statement (the “Series B Warrant Agreement”, together with the Warrant Agent Agreement, the Series A Warrant Agreement
and the Series B Warrant Agreement, the “Transaction Documents”), and such other corporate records, agreements and
documents of the Company, certificates or comparable documents of public officials and officers of the Company and have made such other
investigations as we have deemed necessary as a basis for the opinions set forth below.
In rendering the opinion set forth below, we have
assumed:
| |
a. |
the genuineness of all signatures; |
| |
b. |
the legal capacity of natural persons; |
| |
c. |
the authenticity of all documents submitted to us as originals; |
| |
d. |
the conformity to original documents of all documents submitted to us as duplicates or conformed copies; |
| |
e. |
as to matters of fact, the truthfulness of the representations made in certificates or comparable documents of public officials and officers of the Company; |

| |
f. |
the board of directors of the Company or a duly constituted and acting committee of such board of directors will have taken all action necessary to set the public offering price of the Closing Units; |
| |
g. |
with respect to the issuance of the Common Stock, the amount of valid consideration paid in respect of such Common Stock will equal or exceed the par value of such Common Stock; and |
| |
h. |
neither the execution and delivery by the Company of the Transaction Documents nor the performance by the Company of its obligations thereunder, including the issuance and sale of the Closing Units, Closing Shares, Warrants, Pre-funded Warrants, and the shares of the Company’s common stock underlying the Warrants and Pre-funded Warrants: (i) conflicts or will conflict with the Organizational Documents, (ii) constitutes or will constitute a violation of, or a default under, any lease, indenture, instrument or other agreement to which the Company or its property is subject, (iii) contravenes or will contravene any order or decree of any governmental authority to which the Company or its property is subject, or (iv) violates or will violate any law, rule or regulation to which the Company or its property is subject (except that we do not make the assumption set forth in this clause (iv) with respect to the Opined-on Law (as defined below). |
We have not independently established the validity of the foregoing
assumptions.
Our opinion is limited to the laws of the State
of New York (the “Opined-on Law”) and we do not express any opinion herein concerning any other law. This opinion letter
speaks only as of its date.
Based upon the foregoing, and subject to the qualifications,
assumptions and limitations stated herein, we are of the opinion that:
| |
1. |
The Warrants included in the Closing Units, when the Closing Units are issued and sold by the Company in the manner contemplated in the Registration Statement and Prospectus against payment therefor, and assuming the due authorization, execution and delivery of such Warrants by the Warrant Agent, will constitute the legal, valid, and binding obligations of the Company, enforceable against the Company in accordance with their terms, under the laws of the State of New York, subject to applicable bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium and similar laws affecting creditors’ rights and remedies generally, and subject, as to enforceability, to general principles of equity, including principles of commercial reasonableness, good faith and fair dealing (regardless of whether enforcement is sought in a proceeding at law or in equity). |
| |
2. |
The Pre-Funded Warrants included in the Closing Units, when the Closing Units are issued and sold by the Company in the manner contemplated in the Registration Statement and Prospectus against payment therefor, and assuming the due authorization, execution and delivery of such Pre-Funded Warrants by the Warrant Agent, will constitute the legal, valid, and binding obligations of the Company, enforceable against the Company in accordance with their terms, under the laws of the State of New York, subject to applicable bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium and similar laws affecting creditors’ rights and remedies generally, and subject, as to enforceability, to general principles of equity, including principles of commercial reasonableness, good faith and fair dealing (regardless of whether enforcement is sought in a proceeding at law or in equity). |
We hereby consent to the filing of this opinion
letter as Exhibit 5.2 to the Registration Statement and to the use of our name under the caption “Legal Matters” in the Prospectus.
In giving such consent, we do not hereby admit that we are within the category of persons whose consent is required under Section 7 of
the Securities Act, and the rules and regulations of the Commission promulgated thereunder.
Very truly yours,
/s/ Sichenzia Ross Ference Carmel LLP
Sichenzia Ross Ference Carmel LLP
Exhibit 10.19
WARRANT AGENT AGREEMENT
This WARRANT AGENT AGREEMENT
(this “Warrant Agreement”) dated as of February __, 2024 (the “Issuance Date”) is between Sunshine
Biopharma, Inc., a Colorado corporation (the “Company”), and Equiniti Trust Company (the “Warrant Agent”).
WHEREAS, pursuant to the terms
of that certain Underwriting Agreement (“Underwriting Agreement”), dated February __, 2024, by and among the Company
and Aegis Capital Corp., as the underwriter set forth therein (the “Underwriter”), the Company is engaged in a public
offering of units (the “Units”), with each Unit consisting of one (1) share of common stock (the “Shares”),
par value $0.001 per share (the “Common Stock”) of the Company, one-tenth of one Series A warrant (the “Series
A Warrants”) to purchase one share of Common Stock and two-tenths of a Series B warrant (the “Series B Warrants,” and
collectively with the Series A Warrants, the “Warrants”) to purchase one (1) share of Common Stock; or (ii) one pre-funded
warrant (the “Pre-Funded Warrants”) to purchase one share of common stock, one-tenth of one Series A Warrant, and two-tenths
of one Series B Warrant;
WHEREAS, the Company has filed
with the Securities and Exchange Commission (the “Commission”) a Registration Statement on Form S-1 (File No. 333-276817)
(as the same may be amended from time to time, the “Registration Statement”), for the registration under the Securities
Act of 1933, as amended (the “Securities Act”), of the Units, Shares, Pre-funded Warrants, and shares underlying Pre-Funded
Warrants and Warrants, and such Registration Statement was declared effective on February __, 2024; and
WHEREAS, the Company desires
the Warrant Agent to act on behalf of the Company, and the Warrant Agent is willing to so act, in accordance with the terms set forth
in this Warrant Agreement in connection with the issuance, registration, transfer, exchange and exercise of the Warrants;
WHEREAS, the Company desires
to provide for the provisions of the Warrants, the terms upon which they shall be issued and exercised, and the respective rights, limitation
of rights, and immunities of the Company, the Warrant Agent, and the holders of the Warrants; and
WHEREAS, all acts and things
have been done and performed which are necessary to make the Warrants the valid, binding and legal obligations of the Company, and to
authorize the execution and delivery of this Warrant Agreement.
NOW, THEREFORE, in consideration
of the mutual agreements herein contained, the parties hereto agree as follows:
1. Appointment of Warrant Agent. The Company
hereby appoints the Warrant Agent to act as agent for the Company with respect to the Warrants, and the Warrant Agent hereby accepts such
appointment and agrees to perform the same in accordance with the express terms and conditions set forth in this Warrant Agreement (and
no implied terms or conditions).
2. Warrants.
2.1. Form of Warrants.
The Warrants shall be registered securities and shall be evidenced by a global warrant (“Global Warrant”) in the forms
of Exhibit A-1 with respect to the Series A Warrants) and Exhibit A-2 (with respect to the Series B Warrants) to this Warrant
Agreement, which shall be deposited on behalf of the Company with a custodian for The Depository Trust Company (“DTC”)
and registered in the name of Cede & Co., a nominee of DTC. The terms of the Global Warrant are incorporated herein by reference.
If DTC subsequently ceases to make its book-entry settlement system available for the Warrants, the Company may instruct the Warrant Agent
regarding making other arrangements for book-entry settlement. In the event that the Warrants are not eligible for, or it is no longer
necessary to have the Warrants available in, book-entry form, the Company may instruct the Warrant Agent to provide written instructions
to DTC to deliver to the Warrant Agent for cancellation the Global Warrant, and the Company shall instruct the Warrant Agent to deliver
to DTC separate certificates evidencing Warrants (“Definitive Certificates” and, together with the Global Warrant,
“Warrant Certificates”) registered as requested through the DTC system.
2.2. Issuance and Registration
of Warrants.
2.2.1. Warrant Register.
The Warrant Agent shall maintain books (“Warrant Register”) for the registration of original issuance and the registration
of transfer of the Warrants. Any Person in whose name ownership of a beneficial interest in the Warrants evidenced by a Global Certificate
is recorded in the records maintained by DTC or its nominee shall be deemed to the “beneficial owner” thereof; provided, that
all such beneficial interests shall be held through a Participant of DTC.
2.2.2. Issuance of Warrants.
Upon the initial issuance of the Warrants, the Warrant Agent shall issue the Global Warrant and deliver the Warrants in the DTC book-entry
settlement system in accordance with written instructions delivered to the Warrant Agent by the Company. Ownership of security entitlements
in the Warrants shall be shown on, and the transfer of such ownership shall be effected through, records maintained (i) by DTC and (ii)
by institutions that have accounts with DTC (each, a “Participant”).
2.2.3. Beneficial Owner;
Holder. Prior to due presentment for registration of transfer of any Warrant, the Company and the Warrant Agent may deem and treat
the person in whose name that Warrant shall be registered on the Warrant Register (the “Holder,” which shall include
a Holder’s transferees, successors and assigns and a “Holder” shall include, if the Warrants are held in “street
name,” a Participant, any designee appointed by such Participant and each “beneficial owner” of such Warrants) as the
absolute owner of such Warrant for purposes of any exercise thereof, and for all other purposes, and neither the Company nor the Warrant
Agent shall be affected by any notice to the contrary. Notwithstanding the foregoing, nothing herein shall prevent the Company, the Warrant
Agent or any agent of the Company or the Warrant Agent from giving effect to any written certification, proxy or other authorization furnished
by DTC governing the exercise of the rights of a holder of a beneficial interest in any Warrant. The rights of beneficial owners in a
Warrant evidenced by the Global Warrant shall be exercised by the Holder or a Participant through the DTC system, except to the extent
set forth herein or in the Global Warrant.
2.2.4. Delivery of Warrant
Certificate. A Holder has the right to elect at any time or from time to time a Warrant Exchange (as defined below) pursuant to a
Warrant Certificate Request Notice (as defined below). Upon written notice by a Holder to the Warrant Agent for the exchange of some or
all of such Holder’s Global Warrants for a Warrant Certificate evidencing the same number of Warrants, which request shall be in
the form attached hereto as Exhibit B (a “Warrant Certificate Request Notice” and the date of delivery of such
Warrant Certificate Request Notice by the Holder, the “Warrant Certificate Request Notice Date” and the deemed surrender
upon delivery by the Holder of a number of Global Warrants for the same number of Warrants evidenced by a Warrant Certificate, a “Warrant
Exchange”), the Warrant Agent shall promptly effect the Warrant Exchange and shall promptly issue and deliver to the Holder
a Warrant Certificate for such number of Warrants in the name set forth in the Warrant Certificate Request Notice. Such Warrant Certificate
shall be dated the date of issuance of the Warrant Certificate, shall include the initial exercise date of the Warrants, shall be executed
by an authorized signatory of the Company and shall be reasonably acceptable in all respects to such Holder. In connection with a Warrant
Exchange, the Company agrees to deliver, or to direct the Warrant Agent to deliver, the Warrant Certificate to the Holder within three
(3) Trading Days of the Warrant Certificate Request Notice pursuant to the delivery instructions in the Warrant Certificate Request Notice
(“Warrant Certificate Delivery Date”). If the Company fails for any reason to deliver to the Holder the Warrant Certificate
subject to the Warrant Certificate Request Notice by the Warrant Certificate Delivery Date, the Company shall pay to the Holder, in cash,
as liquidated damages and not as a penalty, for each $1,000 of shares of Common Stock issuable upon exercise of the Warrants (the “Warrant
Shares”) evidenced by such Warrant Certificate (based on the VWAP (as defined in the Warrants) of the Common Stock on the Warrant
Certificate Request Notice Date), $10 per Trading Day for each Trading Day after such Warrant Certificate Delivery Date until such Warrant
Certificate is delivered or, prior to delivery of such Warrant Certificate, the Holder rescinds such Warrant Exchange. The Company covenants
and agrees that, upon the date of delivery of the Warrant Certificate Request Notice, the Holder shall be deemed to be the holder of the
Warrant Certificate and, notwithstanding anything to the contrary set forth herein, the Warrant Certificate shall be deemed for all purposes
to contain all of the terms and conditions of the Warrants evidenced by such Warrant Certificate and the terms of this Agreement.
2.2.5. Execution. The
Warrant Certificates shall be executed on behalf of the Company by any authorized officer of the Company (an “Authorized Officer”),
which need not be the same authorized signatory for all of the Warrant Certificates, either manually or by facsimile signature. The Warrant
Certificates shall be countersigned by an authorized signatory of the Warrant Agent, which need not be the same signatory for all of the
Warrant Certificates, and no Warrant Certificate shall be valid for any purpose unless so countersigned. In case any Authorized Officer
of the Company that signed any of the Warrant Certificates ceases to be an Authorized Officer of the Company before countersignature by
the Warrant Agent and issuance and delivery by the Company, such Warrant Certificates, nevertheless, may be countersigned by the Warrant
Agent, issued and delivered with the same force and effect as though the person who signed such Warrant Certificates had not ceased to
be such officer of the Company; and any Warrant Certificate may be signed on behalf of the Company by any person who, at the actual date
of the execution of such Warrant Certificate, shall be an Authorized Officer of the Company authorized to sign such Warrant Certificate,
although at the date of the execution of this Warrant Agreement any such person was not such an Authorized Officer.
2.2.6. Registration of Transfer.
At any time at or prior to the Expiration Date (as defined below), a transfer of any Warrants may be registered and any Warrant Certificate
or Warrant Certificates may be split up, combined or exchanged for another Warrant Certificate or Warrant Certificates evidencing the
same number of Warrants as the Warrant Certificate or Warrant Certificates surrendered. Any Holder desiring to register the transfer of
Warrants or to split up, combine or exchange any Warrant Certificate shall make such request in writing delivered to the Warrant Agent,
and shall surrender to the Warrant Agent the Warrant Certificate or Warrant Certificates evidencing the Warrants the transfer of which
is to be registered or that is or are to be split up, combined or exchanged and, in the case of registration of transfer, shall provide
a signature guarantee. Thereupon, the Warrant Agent shall countersign and deliver to the person entitled thereto a Warrant Certificate
or Warrant Certificates, as the case may be, as so requested. The Company and the Warrant Agent may require payment, by the Holder requesting
a registration of transfer of Warrants or a split-up, combination or exchange of a Warrant Certificate (but, for purposes of clarity,
not upon the exercise of the Warrants and issuance of Warrant Shares to the Holder), of a sum sufficient to cover any tax or governmental
charge that may be imposed in connection with such registration of transfer, split-up, combination or exchange, together with reimbursement
to the Company and the Warrant Agent of all reasonable expenses incidental thereto. All such fees and expenses shall be paid by the Company,
and not by any Holder.
2.2.7. Loss, Theft and Mutilation
of Warrant Certificates. Upon receipt by the Company and the Warrant Agent of evidence reasonably satisfactory to them of the loss,
theft, destruction or mutilation of a Warrant Certificate, and, in case of loss, theft or destruction, of indemnity or security in customary
form and amount, and reimbursement to the Company and the Warrant Agent of all reasonable expenses incidental thereto, and upon surrender
to the Warrant Agent and cancellation of the Warrant Certificate if mutilated, the Warrant Agent shall, on behalf of the Company, countersign
and deliver a new Warrant Certificate of like tenor to the Holder in lieu of the Warrant Certificate so lost, stolen, destroyed or mutilated.
The Warrant Agent may charge the Holder an administrative fee for processing the replacement of lost Warrant Certificates,. The Warrant
Agent may receive compensation from the surety companies or surety agents for administrative services provided to them.
2.2.8. Proxies. The Holder
of a Warrant may grant proxies or otherwise authorize any person, including the Participants and beneficial holders that may own interests
through the Participants, to take any action that a Holder is entitled to take under this Agreement or the Warrants; provided,
however, that at all times that Warrants are evidenced by a Global Warrant, exercise of those Warrants shall be effected on their
behalf by Participants through DTC in accordance the procedures administered by DTC.
3. Terms and Exercise of Warrants.
3.1. Exercise Price.
Each Warrant shall entitle the Holder, subject to the provisions of the applicable Warrant Certificate and of this Warrant Agreement,
to purchase from the Company the number of shares of Common Stock stated therein, at the price of $___ per whole share, with respect to
the Series A Warrants, or $__ with respect to the Series B Warrants, subject to the subsequent adjustments provided in the Global Warrant.
The term “Exercise Price” as used in this Warrant Agreement refers to the price per share at which shares of Common
Stock may be purchased at the time a Warrant is exercised.
3.2. Duration of Warrants.
A Warrant may be exercised only during the period (“Exercise Period”) commencing on the date of issuance and ending
on the Termination Date. For purposes of this Warrant Agreement, the “Termination Date” shall have the
meaning set forth in the Global Warrant. Each Warrant not exercised on or before the Termination Date shall cease to be exercisable at
the close of business on the Termination Date.
3.3. Exercise of Warrants.
3.3.1. Exercise. Subject
to the provisions of the Global Warrant, a Holder (or a Participant or a designee of a Participant acting on behalf of a Holder) may exercise
Warrants by delivering to the Warrant Agent during the Exercise Period a notice of exercise of the Warrants to be exercised (i) in the
form attached to the Global Warrant or (ii) via an electronic warrant exercise through the DTC system (each, an “Election to
Purchase”); provided, that if a Holder exercises a Warrant later than 5:00 P.M., New York City time on any Trading Day or at
any time on a day that is not a Trading Day, the Warrant will be deemed exercised as of opening of trading on the next Trading Day. All
other requirements for the exercise of a Warrant shall be as set forth in the Warrant. All exercise funds shall be delivered to the Warrant
Agent for the processing of the exercises.
3.3.2. The Warrant Agent shall,
by 5:00 p.m., New York City time, on the Trading Day following the Exercise Date of any Warrant, advise the Company, the transfer agent
and registrar for the Company’s Common Stock, in respect of (i) the number of Warrant Shares indicated on the Notice of Exercise
as issuable upon such exercise with respect to such exercised Warrants, (ii) the instructions of the Holder or Participant, as the case
may be, provided to the Warrant Agent with respect to the delivery of the Warrant Shares and the number of Warrants that remain outstanding
after such exercise and (iii) such other information as the Company or such transfer agent and registrar shall reasonably request. The
Company shall issue the Warrant Shares in compliance with the terms of the Warrant.
3.3.3. Valid Issuance.
All Warrant Shares issued by the Company upon the proper exercise of a Warrant in conformity with this Warrant Agreement shall be validly
issued, fully paid and non-assessable.
3.3.4. No Fractional Exercise.
Notwithstanding any provision contained in this Warrant Agreement to the contrary, no fractional shares or scrip representing fractional
shares shall be issued upon the exercise of the Warrant. As to any fraction of a share which the Holder would otherwise be entitled to
purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an
amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
3.3.5. No Transfer Taxes.
The Company shall not be required to pay any stamp or other tax or governmental charge required to be paid in connection with any transfer
involved in the issue of the Warrant Shares upon the exercise of Warrants; and in the event that any such transfer is involved, the Company
shall not be required to issue or deliver any Warrant Shares until such tax or other charge shall have been paid or it has been established
to the Company’s satisfaction that no such tax or other charge is due.
3.3.6. Date of Issuance.
The Company will treat an exercising Holder as a beneficial owner of the Warrant Shares as of the Exercise Date, and for purposes of Regulation
SHO, a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing this Warrant held in book-entry form
through DTC shall be deemed to have exercised its interest in this Warrant upon instructing its broker that is a DTC participant to exercise
its interest in this Warrant, except that, if the Exercise Date is a date when the stock transfer books of the Company are closed, such
person shall be deemed to have become the holder of such shares at the open of business on the next succeeding date on which the stock
transfer books are open.
4. Adjustments. Upon every adjustment of
the Exercise Price or the number of Warrant Shares issuable upon exercise of a Warrant, the Company shall give written notice thereof
to the Warrant Agent, which notice shall state the Exercise Price resulting from such adjustment and the increase or decrease, if any,
in the number of Warrant Shares purchasable at such price upon the exercise of a Warrant, setting forth in reasonable detail the method
of calculation and the facts upon which such calculation is based. Upon the occurrence of any event specified in Section 3 of the Warrant,
then, in any such event, the Company shall give written notice to the Warrant Agent. Failure to give such notice, or any defect therein,
shall not affect the legality or validity of such event. The Warrant Agent shall be entitled to rely conclusively on, and shall be fully
protected in relying on, any certificate, notice or instructions provided by the Company with respect to any adjustment of the Exercise
Price or the number of shares issuable upon exercise of a Warrant, or any related matter, and the Warrant Agent shall not be liable for
any action taken, suffered or omitted to be taken by it in accordance with any such certificate, notice or instructions or pursuant to
this Warrant Agreement. The Warrant Agent shall not be deemed to have knowledge of any such adjustment unless and until it shall have
received written notice thereof from the Company.
5. Restrictive Legends; Fractional Warrants.
In the event that a Warrant Certificate surrendered for transfer bears a restrictive legend, the Warrant Agent shall not register that
transfer until the Warrant Agent has received an opinion of counsel for the Company stating that such transfer may be made and indicating
whether the Warrants must also bear a restrictive legend upon that transfer. The Warrant Agent shall not be required to effect any registration
of transfer or exchange which will result in the transfer of or delivery of a Warrant Certificate for a fraction of a Warrant.
6. Other Provisions Relating to Rights of Holders
of Warrants.
6.1. No Rights as Stockholder.
Except as otherwise specifically provided herein, a Holder, solely in its capacity as a holder of Warrants, shall not be entitled to vote
or receive dividends or be deemed the holder of share capital of the Company for any purpose, nor shall anything contained in this Warrant
Agreement be construed to confer upon a Holder, solely in its capacity as the registered holder of Warrants, any of the rights of a stockholder
of the Company or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification
of share capital, consolidation, merger, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights
or rights to participate in new issues of shares, or otherwise, prior to the issuance to the Holder of the Warrant Shares which it is
then entitled to receive upon the due exercise of Warrants.
6.2. Reserved.
6.3 Reservation of Common
Stock. The Company shall at all times reserve and keep available a number of its authorized but unissued shares of Common Stock that
will be sufficient to permit the exercise in full of all outstanding Warrants issued pursuant to this Warrant Agreement.
7. Concerning the Warrant Agent and Other Matters.
7.1. Any instructions given
to the Warrant Agent orally, as permitted by any provision of this Warrant Agreement, shall be confirmed in writing by the Company as
soon as practicable. The Warrant Agent shall not be liable or responsible and shall be fully authorized and protected for acting, or failing
to act, in accordance with any oral instructions which do not conform with the written confirmation received in accordance with this Section
7.1.
7.2. (a) Whether or not any
Warrants are exercised, for the Warrant Agent’s services as agent for the Company hereunder, the Company shall pay to the Warrant
Agent such fees as may be separately agreed between the Company and Warrant Agent and the Warrant Agent’s out of pocket expenses
in connection with this Warrant Agreement, including, without limitation, the fees and expenses of the Warrant Agent’s counsel.
While the Warrant Agent endeavors to maintain out-of-pocket charges (both internal and external) at competitive rates, these charges may
not reflect actual out-of-pocket costs, and may include handling charges to cover internal processing and use of the Warrant Agent’s
billing systems. (b) All amounts owed by the Company to the Warrant Agent under this Warrant Agreement are due within 30 days of the invoice
date. Delinquent payments are subject to a late payment charge of one and one-half percent (1.5%) per month commencing 45 days from the
invoice date. The Company agrees to reimburse the Warrant Agent for any attorney’s fees and any other costs associated with collecting
delinquent payments. (c) No provision of this Warrant Agreement shall require Warrant Agent to expend or risk its own funds or otherwise
incur any financial liability in the performance of any of its duties under this Warrant Agreement or in the exercise of its rights.
7.3. As agent for the Company
hereunder the Warrant Agent: (a) shall have no duties or obligations other than those specifically set forth herein or as may subsequently
be agreed to in writing by the Warrant Agent and the Company; (b) shall be regarded as making no representations and having no responsibilities
as to the validity, sufficiency, value, or genuineness of the Warrants or any Warrant Shares; (c) shall not be obligated to take any legal
action hereunder; if, however, the Warrant Agent determines to take any legal action hereunder, and where the taking of such action might,
in its judgment, subject or expose it to any expense or liability it shall not be required to act unless it has been furnished with an
indemnity reasonably satisfactory to it; (d) may rely on and shall be fully authorized and protected in acting or failing to act upon
any certificate, instrument, opinion, notice, letter, telegram, telex, facsimile transmission or other document or security delivered
to the Warrant Agent and believed by it to be genuine and to have been signed by the proper party or parties; (e) shall not be liable
or responsible for any recital or statement contained in the Registration Statement or any other documents relating thereto; (f) shall
not be liable or responsible for any failure on the part of the Company to comply with any of its covenants and obligations relating to
the Warrants, including without limitation obligations under applicable securities laws; (g) may rely on and shall be fully authorized
and protected in acting or failing to act upon the written, telephonic or oral instructions with respect to any matter relating to its
duties as Warrant Agent covered by this Warrant Agreement (or supplementing or qualifying any such actions) of officers of the Company,
and is hereby authorized and directed to accept instructions with respect to the performance of its duties hereunder from the Company
or counsel to the Company, and may apply to the Company, for advice or instructions in connection with the Warrant Agent’s duties
hereunder, and the Warrant Agent shall not be liable for any delay in acting while waiting for those instructions; any applications by
the Warrant Agent for written instructions from the Company may, at the option of the Agent, set forth in writing any action proposed
to be taken or omitted by the Warrant Agent under this Warrant Agreement and the date on or after which such action shall be taken or
such omission shall be effective; the Warrant Agent shall not be liable for any action taken by, or omission of, the Warrant Agent in
accordance with a proposal included in such application on or after the date specified in such application (which date shall not be less
than five business days after the date such application is sent to the Company, unless the Company shall have consented in writing to
any earlier date) unless prior to taking any such action, the Warrant Agent shall have received written instructions in response to such
application specifying the action to be taken or omitted; (h) may consult with counsel satisfactory to the Warrant Agent, including its
in-house counsel, and the advice of such counsel shall be full and complete authorization and protection in respect of any action taken,
suffered, or omitted by it hereunder in good faith and in accordance with the advice of such counsel; (i) may perform any of its duties
hereunder either directly or by or through nominees, correspondents, designees, or subagents, and it shall not be liable or responsible
for any misconduct or negligence on the part of any nominee, correspondent, designee, or subagent appointed with reasonable care by it
in connection with this Warrant Agreement; (j) is not authorized, and shall have no obligation, to pay any brokers, dealers, or soliciting
fees to any person; and (k) shall not be required hereunder to comply with the laws or regulations of any country other than the United
States of America or any political subdivision thereof.
7.4. (a) In the absence of
gross negligence or willful or illegal misconduct on its part, as defined in Section 7.5, the Warrant Agent shall not be liable for any
action taken, suffered, or omitted by it or for any error of judgment made by it in the performance of its duties under this Warrant Agreement.
Anything in this Warrant Agreement to the contrary notwithstanding, in no event shall Warrant Agent be liable for special, indirect, incidental,
consequential or punitive losses or damages of any kind whatsoever (including but not limited to lost profits), even if the Warrant Agent
has been advised of the possibility of such losses or damages and regardless of the form of action. Any liability of the Warrant Agent
will be limited in the aggregate to one year’s fees paid by the Company hereunder. The Warrant Agent shall not be liable for any
failures, delays or losses, arising directly or indirectly out of conditions beyond its reasonable control including, but not limited
to, acts of government, exchange or market ruling, suspension of trading, work stoppages or labor disputes, fires, civil disobedience,
riots, rebellions, storms, electrical or mechanical failure, computer hardware or software failure, communications facilities failures
including telephone failure, war, terrorism, insurrection, earthquakes, floods, acts of God or similar occurrences. (b) In the event any
question or dispute arises with respect to the proper interpretation of the Warrants or the Warrant Agent’s duties under this Warrant
Agreement or the rights of the Company or of any Holder, the Warrant Agent shall not be required to act and shall not be held liable or
responsible for its refusal to act until the question or dispute has been judicially settled (and, if appropriate, it may file a suit
in interpleader or for a declaratory judgment for such purpose) by final judgment rendered by a court of competent jurisdiction, binding
on all persons interested in the matter which is no longer subject to review or appeal, or settled by a written document in form and substance
satisfactory to Warrant Agent and executed by the Company and each such Holder. In addition, the Warrant Agent may require for such purpose,
but shall not be obligated to require, the execution of such written settlement by all the Holders and all other persons that may have
an interest in the settlement.
7.5. The Company covenants
to indemnify the Warrant Agent and hold it harmless from and against any loss, liability, claim or expense (“Loss”)
arising out of or in connection with the Warrant Agent’s duties under this Warrant Agreement, including the costs and expenses of
defending itself against any Loss, unless such Loss shall have been determined by a court of competent jurisdiction to be a result of
the Warrant Agent’s gross negligence or willful misconduct.
7.6. Unless terminated earlier
by the parties hereto, this Agreement shall terminate 90 days after the earlier of the Expiration Date and the date on which no Warrants
remain outstanding (the “Termination Date”). On the business day following the Termination Date, the Agent shall deliver
to the Company any entitlements, if any, held by the Warrant Agent under this Warrant Agreement. The Agent’s right to be reimbursed
for fees, charges and out-of-pocket expenses as provided in this Section 8 shall survive the termination of this Warrant Agreement.
7.7. If any provision of this
Warrant Agreement shall be held illegal, invalid, or unenforceable by any court, this Warrant Agreement shall be construed and enforced
as if such provision had not been contained herein and shall be deemed an Agreement among the parties to it to the full extent permitted
by applicable law.
7.8. The Company represents
and warrants that: (a) it is duly incorporated and validly existing under the laws of its jurisdiction of incorporation; (b) the offer
and sale of the Warrants and the execution, delivery and performance of all transactions contemplated thereby (including this Warrant
Agreement) have been duly authorized by all necessary corporate action and will not result in a breach of or constitute a default under
the articles of association, bylaws or any similar document of the Company or any indenture, agreement or instrument to which it is a
party or is bound; (c) this Warrant Agreement has been duly executed and delivered by the Company and constitutes the legal, valid, binding
and enforceable obligation of the Company; (d) the Warrants will comply in all material respects with all applicable requirements of law;
and (e) to the best of its knowledge, there is no litigation pending or threatened as of the date hereof in connection with the offering
of the Warrants.
7.9. In the event of inconsistency
between this Warrant Agreement and the descriptions in the Warrant Certificate, as it may from time to time be amended, the terms of the
Warrant Certificate shall control.
7.10. Set forth in Exhibit
C hereto is a list of the names and specimen signatures of the persons authorized to act for the Company under this Warrant Agreement
(the “Authorized Representatives”). The Company shall, from time to time, certify to you the names and signatures of
any other persons authorized to act for the Company under this Warrant Agreement.
7.11. Except as expressly
set forth elsewhere in this Warrant Agreement, all notices, instructions and communications under this Agreement shall be in writing,
shall be effective upon receipt and shall be addressed, if to the Company, to its address set forth beneath its signature to this Agreement,
or, if to the Warrant Agent, to Equiniti Trust Company, 1110 Centre Pointe Curve, Suite 101, Mendota Heights, Minnesota 55120, or to such
other address of which a party hereto has notified the other party.
7.12. (a) This Warrant Agreement
shall be governed by and construed in accordance with the laws of the State of New York. All actions and proceedings relating to or arising
from, directly or indirectly, this Warrant Agreement may be litigated in courts located within the Borough of Manhattan in the City and
State of New York. The Company hereby submits to the personal jurisdiction of such courts and consents that any service of process may
be made by certified or registered mail, return receipt requested, directed to the Company at its address last specified for notices hereunder.
Each of the parties hereto hereby waives the right to a trial by jury in any action or proceeding arising out of or relating to this Warrant
Agreement. (b) This Warrant Agreement shall inure to the benefit of and be binding upon the successors and assigns of the parties hereto.
This Warrant Agreement may not be assigned, or otherwise transferred, in whole or in part, by either party without the prior written consent
of the other party, which the other party will not unreasonably withhold, condition or delay; except that (i) consent is not required
for an assignment or delegation of duties by Warrant Agent to any affiliate of Warrant Agent and (ii) any reorganization, merger, consolidation,
sale of assets or other form of business combination by Warrant Agent or the Company shall not be deemed to constitute an assignment of
this Warrant Agreement. (c) No provision of this Warrant Agreement may be amended, modified or waived, except in a written document signed
by both parties. The Company and the Warrant Agent may amend or supplement this Warrant Agreement without the consent of any Holder for
the purpose of curing any ambiguity, or curing, correcting or supplementing any defective provision contained herein or adding or changing
any other provisions with respect to matters or questions arising under this Agreement as the parties may deem necessary or desirable
and that the parties determine, in good faith, shall not adversely affect the interest of the Holders. All other amendments and supplements
shall require the vote or written consent of Holders of at least 50.1% of the then outstanding Warrants, provided that adjustments may
be made to the Warrant terms and rights in accordance with Section 4 without the consent of the Holders.
7.13. Payment of Taxes.
The Company will from time to time promptly pay all taxes and charges that may be imposed upon the Company or the Warrant Agent in respect
of the issuance or delivery of Warrant Shares upon the exercise of Warrants, but the Company may require the Holders to pay any transfer
taxes in respect of the Warrants or such shares. The Warrant Agent may refrain from registering any transfer of Warrants or any delivery
of any Warrant Shares unless or until the persons requesting the registration or issuance shall have paid to the Warrant Agent for the
account of the Company the amount of such tax or charge, if any, or shall have established to the reasonable satisfaction of the Company
and the Warrant Agent that such tax or charge, if any, has been paid.
7.14. Resignation of Warrant
Agent.
7.14.1. Appointment of Successor
Warrant Agent. The Warrant Agent, or any successor to it hereafter appointed, may resign its duties and be discharged from all further
duties and liabilities hereunder after giving thirty (30) days’ notice in writing to the Company, or such shorter period of time
agreed to by the Company. The Company may terminate the services of the Warrant Agent, or any successor Warrant Agent, after giving thirty
(30) days’ notice in writing to the Warrant Agent or successor Warrant Agent, or such shorter period of time as agreed. If the office
of the Warrant Agent becomes vacant by resignation, termination or incapacity to act or otherwise, the Company shall appoint in writing
a successor Warrant Agent in place of the Warrant Agent. If the Company shall fail to make such appointment within a period of 30 days
after it has been notified in writing of such resignation or incapacity by the Warrant Agent, then the Warrant Agent or any Holder may
apply to any court of competent jurisdiction for the appointment of a successor Warrant Agent at the Company’s cost. Pending appointment
of a successor to such Warrant Agent, either by the Company or by such a court, the duties of the Warrant Agent shall be carried out by
the Company. Any successor Warrant Agent (but not including the initial Warrant Agent), whether appointed by the Company or by such court,
shall be a person organized and existing under the laws of any state of the United States of America, in good standing, and authorized
under such laws to exercise corporate trust powers and subject to supervision or examination by federal or state authority. After appointment,
any successor Warrant Agent shall be vested with all the authority, powers, rights, immunities, duties, and obligations of its predecessor
Warrant Agent with like effect as if originally named as Warrant Agent hereunder, without any further act or deed, and except for executing
and delivering documents as provided in the sentence that follows, the predecessor Warrant Agent shall have no further duties, obligations,
responsibilities or liabilities hereunder, but shall be entitled to all rights that survive the termination of this Warrant Agreement
and the resignation or removal of the Warrant Agent, including but not limited to its right to indemnity hereunder. If for any reason
it becomes necessary or appropriate or at the request of the Company, the predecessor Warrant Agent shall execute and deliver, at the
expense of the Company, an instrument transferring to such successor Warrant Agent all the authority, powers, and rights of such predecessor
Warrant Agent hereunder; and upon request of any successor Warrant Agent the Company shall make, execute, acknowledge, and deliver any
and all instruments in writing for more fully and effectually vesting in and confirming to such successor Warrant Agent all such authority,
powers, rights, immunities, duties, and obligations.
7.14.2. Notice of Successor
Warrant Agent. In the event a successor Warrant Agent shall be appointed, the Company shall give notice thereof to the predecessor
Warrant Agent and the transfer agent for the Common Stock not later than the effective date of any such appointment.
7.14.3. Merger or Consolidation
of Warrant Agent. Any person into which the Warrant Agent may be merged or converted or with which it may be consolidated or any person
resulting from any merger, conversion or consolidation to which the Warrant Agent shall be a party or any person succeeding to the shareowner
services business of the Warrant Agent or any successor Warrant Agent shall be the successor Warrant Agent under this Warrant Agreement,
without any further act or deed. For purposes of this Warrant Agreement, “person” shall mean any individual, firm, corporation,
partnership, limited liability company, joint venture, association, trust or other entity, and shall include any successor (by merger
or otherwise) thereof or thereto.
8. Miscellaneous Provisions.
8.1. Persons Having Rights
under this Warrant Agreement. Nothing in this Warrant Agreement expressed and nothing that may be implied from any of the provisions
hereof is intended, or shall be construed, to confer upon, or give to, any person or corporation other than (i) the parties hereto, and
(ii) the Holders (including, without limitation, all “beneficial holders”) of the Warrants ) any right, remedy, or claim under
or by reason of this Warrant Agreement or of any covenant, condition, stipulation, promise, or agreement hereof.
8.2. Examination of the
Warrant Agreement. A copy of this Warrant Agreement shall be available at all reasonable times at the office of the Warrant Agent
designated for such purpose for inspection by any Holder. Prior to such inspection, the Warrant Agent may require any such holder to provide
reasonable evidence of its interest in the Warrants.
8.3. Counterparts.
This Warrant Agreement may be executed in any number of original, facsimile or electronic counterparts and each of such counterparts shall
for all purposes be deemed to be an original, and all such counterparts shall together constitute but one and the same instrument.
8.4. Effect of Headings.
The Section headings herein are for convenience only and are not part of this Warrant Agreement and shall not affect the interpretation
thereof.
9. Certain Definitions. As used herein,
the following terms shall have the following meanings:
(a) “Trading Day” means any
day on which the Common Stock is traded on the Trading Market, or, if the Trading Market is not the principal trading market for the Common
Stock, then on the principal securities exchange or securities market in the United States on which the Common Stock is then traded.
(b) “Trading Market” means
NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market or the New York Stock Exchange.
[Signature Page Follows]
IN WITNESS WHEREOF, this Warrant Agent Agreement has been duly executed
by the parties hereto as of the day and year first above written.
| |
SUNSHINE BIOPHARMA, INC. |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
| |
EQUINITI TRUST COMPANY |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
Exhibit 10.20
PRE-FUNDED WARRANT TO PURCHASE COMMON STOCK
SUNSHINE BIOPHARMA, INC.
| Warrant Shares: [●] |
Initial Exercise Date: [●], 2024 |
| |
Issue Date: [●], 2024 |
THIS PRE-FUNDED WARRANT
TO PURCHASE COMMON STOCK (the “Warrant”) certifies that, for value received, [●] or its assigns (the
“Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter
set forth, at any time until this Warrant is exercised in full (the “Termination Date”) but not thereafter,
to subscribe for and purchase from Sunshine Biopharma, Inc., a Colorado corporation (the “Company”), up to [●]
shares (as subject to adjustment hereunder, the “Warrant Shares”) of Common Stock. The purchase price of one
(1) share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2.2. This Warrant shall initially
be issued and maintained in the form of a security held in book-entry form and the Depository Trust Company or its nominee (“DTC”)
shall initially be the sole registered holder of this Warrant, subject to a Holder’s right to elect to receive a Warrant in certificated
form pursuant to the terms of the Warrant Agent Agreement, in which case this sentence shall not apply.
| 1. | Definitions. In addition to the terms defined elsewhere in this Warrant or in the Underwriting
Agreement dated [●], 2024, the following terms have the meanings indicated in this Section 1: |
1.1.
“Affiliate” means any Person that, directly or indirectly through one or more intermediaries, controls
or is controlled by or is under common control with a Person, as such terms are used in and construed under Rule 405 under the Securities
Act.
1.2.
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies:
(a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the
nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based
on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market,
the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c)
if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on
the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price
per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined
by an independent appraiser selected in good faith by the Holders of a majority in interest of the Warrants then outstanding and reasonably
acceptable to the Company, the fees and expenses of which shall be paid by the Company.
1.3.
“Board of Directors” means the board of directors of the Company.
1.4.
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The
City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks
shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”,
“non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the
direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial
banks in The City of New York generally are open for use by customers on such day.
1.5.
“Commission” means the United States Securities and Exchange Commission.
1.6.
“Common Stock” means the common stock of the Company, $0.001 par value per share, and any other class
of securities into which such securities may hereafter be reclassified or changed.
1.7.
“Common Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle
the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant
or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof
to receive, Common Stock.
1.8.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated
thereunder.
1.9.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association,
joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any
kind.
1.10.
“Registration Statement” means the Company’s registration statement on Form S-1 (File No. 333-276817).
1.11.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated
thereunder.
1.12.
“Subsidiary” means any subsidiary of the Company and shall, where applicable, also include any direct
or indirect subsidiary of the Company formed or acquired after the date hereof.
1.13.
“Trading Day” means a day on which the Common Stock is traded on a Trading Market.
1.14.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed
or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global
Select Market, the New York Stock Exchange, OTCQB or OTCQX (or any successors to any of the foregoing).
1.15.
“Transfer Agent” means Equiniti, the current transfer agent of the Company, with a mailing address of
1110 Centre Pointe Curve, Suite 101, Mendota Heights, Minnesota 55120 and an email address of, and any successor transfer agent of the
Company.
1.16.
“Underwriting Agreement” means the underwriting agreement, dated as of [●], 2024, between the Company
and Aegis Capital Corp., as amended, modified or supplemented from time to time in accordance with its terms.
1.17.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies:
(a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for
such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg
L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not
a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX
as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are
then reported on the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most
recent bid price per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock
as determined by an independent appraiser selected in good faith by the holders of a majority in interest of the Warrants then outstanding
and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
1.18.
“Warrant Agent Agreement” means that certain Warrant Agent agreement, dated on or about the Initial Exercise
Date, between the Company and the Warrant Agent.
1.19.
“Warrant Agent” means the Transfer Agent and any successor warrant agent of the Company.
1.20.
“Warrants” means this Warrant and other Common Stock purchase warrants issued by the Company pursuant
to the Registration Statement.
2.1.
Exercise of Warrant. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part,
at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company of a duly
executed PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise substantially in the form attached hereto as Exhibit
2.1 (the “Notice of Exercise”). Within the earlier of (i) two (2) Trading Days and (ii) the number of
Trading Days comprising the Standard Settlement Period (as defined in Section 2.4.1 herein) following the date of exercise as aforesaid,
the Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice of Exercise by wire transfer
or cashier’s check drawn on a United States bank unless the cashless exercise procedure specified in Section 2.3 below is specified
in the applicable Notice of Exercise. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other
type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding anything herein to the contrary, the Holder
shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available
hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation
within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this
Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering
the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased.
The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company
shall deliver any objection to any Notice of Exercise within one (1) Trading Day of receipt of such notice. The Holder and any assignee,
by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a
portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than
the amount stated on the face hereof.
Notwithstanding
the foregoing in this Section 2.1, a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing this
Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions), shall effect exercises
made pursuant to this Section 2.1 by delivering to DTC (or such other clearing corporation, as applicable) the appropriate instruction
form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other clearing corporation, as applicable),
subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agent Agreement,
in which case this sentence shall not apply.
2.2.
Exercise Price. The aggregate exercise price of this Warrant, except for a nominal exercise price of $0.001 per Warrant
Share, was pre-funded to the Company on or prior to the Initial Exercise Date and, consequently, no additional consideration (other than
the nominal exercise price of $0.001 per Warrant Share) shall be required to be paid by the Holder to any Person to effect any exercise
of this Warrant. The Holder shall not be entitled to the return or refund of all, or any portion, of such pre-paid aggregate exercise
price under any circumstance or for any reason whatsoever, including in the event this Warrant shall not have been exercised prior to
the Termination Date. The remaining unpaid exercise price per share of Common Stock under this Warrant shall be $0.001, subject to adjustment
hereunder (the “Exercise Price”).
2.3.
Cashless Exercise. This Warrant may also be exercised, in whole or in part, at such time by means of a “cashless
exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing
[(A-B) (X)] by (A), where:
| (A) = | as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice
of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2.1 hereof on a day that is not a Trading
Day or (2) both executed and delivered pursuant to Section 2.1 hereof on a Trading Day prior to the opening of “regular trading
hours” (as defined in Rule 600(b) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) at
the option of the Holder, either (y) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or
(z) the Bid Price of the Common Stock on the principal Trading Market as reported by Bloomberg L.P. as of the time of the Holder’s
execution of the applicable Notice of Exercise if such Notice of Exercise is executed during “regular trading hours” on a
Trading Day and is delivered within two (2) hours thereafter (including until two (2) hours after the close of “regular trading
hours” on a Trading Day) pursuant to Section 2.1 hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if the
date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2.1 hereof
after the close of “regular trading hours” on such Trading Day; |
| | | |
| (B) = | the Exercise Price of this Warrant, as adjusted hereunder; and |
| | | |
| (X) = | the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the
terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
If Warrant Shares
are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act,
the Warrant Shares shall take on the registered characteristics of the Warrants being exercised, and the holding period of the Warrant
Shares being issued may be tacked on to the holding period of this Warrant. Assuming (i) the Holder is not an Affiliate of the Company,
and (ii) all of the applicable conditions of Rule 144 promulgated under the Securities Act with respect to Holder and the Warrant Shares
are met in the case of such a cashless exercise, the Company agrees that the Company will cause the removal of the legend from such Warrant
Shares (including by delivering an opinion of the Company’s counsel to the Company’s transfer agent at its own expense to
ensure the foregoing), and the Company agrees that the Holder is under no obligation to sell the Warrant Shares issuable upon the exercise
of the Warrant prior to removing the legend. The Company agrees not to take any position contrary to this Section 2.3.
2.4.
Mechanics of Exercise.
2.4.1.
Delivery of Warrant Shares upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted
by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository
Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant
in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale
of the Warrant Shares by Holder or (B) the Warrant Shares are eligible for resale by the Holder without volume or manner-of-sale limitations
pursuant to Rule 144 (assuming cashless exercise of the Warrants), and otherwise by physical delivery of a certificate, for the number
of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise
by the date that is the earliest of (i) two (2) Trading Days after the delivery to the Company of the Notice of Exercise, (ii) one (1)
Trading Day after delivery of the aggregate Exercise Price to the Company and (iii) the number of Trading Days comprising the Standard
Settlement Period after the delivery to the Company of the Notice of Exercise (such date, the “Warrant Share Delivery Date”).
Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of
the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares,
provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of
(i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period following delivery of the Notice
of Exercise. Notwithstanding anything herein to the contrary, upon delivery of the Notice of Exercise, the Holder shall be deemed for
purposes of Regulation SHO under the Exchange Act to have become the holder of the Warrant Shares irrespective of the date of delivery
of the Warrant Shares. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise
by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each
$1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise),
$10 per Trading Day (increasing to $20 per Trading Day on the third (3rd) Trading Day after the Warrant Share Delivery Date) for each
Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. The Company
agrees to maintain a Transfer Agent that is a participant in the FAST program so long as this Warrant remains outstanding and exercisable.
As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a number of
Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery of the
Notice of Exercise. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to 12:00 p.m. (New
York City time) on the Initial Exercise Date, which may be delivered at any time after the time of execution of the Underwriting Agreement,
the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial Exercise Date
and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of the aggregate
Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date.
2.4.2.
Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at
the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the
Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which
new Warrant shall in all other respects be identical with this Warrant.
2.4.3.
Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares
pursuant to Section 2.4.1 by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
2.4.4.
Compensation for Buy-In on Failure to Timely Deliver Warrant Shares upon Exercise. In addition to any other rights
available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with
the provisions of Section 2.4.1 above pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the
Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise
purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated
receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to the Holder the amount,
if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock
so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver
to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation
was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares
for which such exercise was not honored and return any amount received by the Company in respect of the Exercise Price for those Warrant
Shares (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would
have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the Holder purchases
Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant to purchase
shares of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately
preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating
the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing
herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without
limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares
of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
2.4.5.
No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon
the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise,
the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the Exercise Price or round up to the next whole share.
2.4.6.
Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue
or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be
paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by
the Holder; provided, however, that, in the event that Warrant Shares are to be issued in a name other than the name of
the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto as Exhibit 2.4.6
duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any
transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise
and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day
electronic delivery of the Warrant Shares.
2.4.7.
Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely
exercise of this Warrant, pursuant to the terms hereof.
2.5.
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall
not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect
to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates,
and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution
Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of
the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties
shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is
being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, unexercised
portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion
of the unexercised or unconverted portion of any other securities of the Company (including, without limitation, any other Common Stock
Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the
Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2.5,
beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated
thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance
with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith.
To the extent that the limitation contained in this Section 2.5 applies, the determination of whether this Warrant is exercisable (in
relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant
is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be the Holder’s
determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates
and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial Ownership Limitation,
and the Company shall have no obligation to verify or confirm the accuracy of such determination. In addition, a determination as to any
group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations
promulgated thereunder. For purposes of this Section 2.5, in determining the number of outstanding shares of Common Stock, a Holder may
rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent periodic or annual report
filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a more recent written notice
by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the written or oral request
of a Holder, the Company shall within one (1) Trading Day confirm orally and in writing to the Holder the number of shares of Common Stock
then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion
or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution Parties since the date
as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation”
shall be 4.99% (or, upon election by a Holder prior to the issuance of any Warrants, 9.99%) of the number of shares of Common Stock outstanding
immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon notice
to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2.5, provided that the Beneficial
Ownership Limitation in no event exceeds 9.99% of the number of shares of Common Stock outstanding immediately after giving effect to
the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2.5 shall continue
to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice is
delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity
with the terms of this Section 2.5 to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended
Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such
limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
3.1.
Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend
or otherwise makes a distribution or distributions on shares of Common Stock or any other equity or equity equivalent securities payable
in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise
of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way
of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares
of Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of
which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such
event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number
of Shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant
shall remain unchanged. Any adjustment made pursuant to this Section 3.1 shall become effective immediately after the record date for
the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective
date in the case of a subdivision, combination or re-classification.
3.2.
Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3.1 above, if at any time the Company
grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all
(or substantially all) of the record holders of any class of shares of Common Stock (the “Purchase Rights”),
then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the
Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant
(without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately
before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the
date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights
(provided, however, that, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder
exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent
(or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to
such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding
the Beneficial Ownership Limitation).
3.3.
Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any
dividend or other distribution of its assets (or rights to acquire its assets) to all (or substantially all) holders of shares of Common
Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property
or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction)
(a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall
be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held
the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise
hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for
such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined
for the participation in such Distribution (provided, however, that, to the extent that the Holder’s right to participate in any
such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate
in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to
such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as
its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation). To the extent that this Warrant has not
been partially or completely exercised at the time of such Distribution, such portion of the Distribution shall be held in abeyance for
the benefit of the Holder until the Holder has exercised this Warrant.
3.4.
Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly,
in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company (and
all of its subsidiaries taken as a whole), directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance
or other disposition of all or substantially all of its assets in one or a series of related transactions, (iii) any, direct or indirect,
purchase offer, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common
Stock are permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders
of 50% or more of the outstanding Common Stock or 50% or more of the voting power of the common equity of the Company, (iv) the Company,
directly or indirectly, in one or more related transactions effects any reclassification, reorganization or recapitalization of the Common
Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities,
cash or property, or (v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase
agreement or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme
of arrangement) with another Person or group of Persons whereby such other Person or group acquires 50% or more of the outstanding shares
of Common Stock or 50% or more of the voting power of the common equity of the Company (each a “Fundamental Transaction”),
then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have
been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without
regard to any limitation in Section 2.5 on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring
corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”)
receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable
immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2.5 on the exercise of this Warrant). For
purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration
based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the
Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any
different components of the Alternate Consideration.
If holders of Common Stock are given any choice as to the securities, cash or property
to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives
upon any exercise of this Warrant following such Fundamental Transaction. Notwithstanding anything to the contrary, in the event of a
Fundamental Transaction, the Company or any Successor Entity (as defined below) shall, at the Holder’s option, exercisable at any
time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction (or, if later, the date of the public
announcement of the applicable Fundamental Transaction), purchase this Warrant from the Holder by paying to the Holder an amount of cash
equal to the Black Scholes Value (as defined below) of the remaining unexercised portion of this Warrant on the date of the consummation
of such Fundamental Transaction; provided, however, that, if the Fundamental Transaction is not within the Company’s control, including
not approved by the Company’s Board of Directors, the Holder shall only be entitled to receive from the Company or any Successor
Entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of this
Warrant, that is being offered and paid to the holders of Common Stock of the Company in connection with the Fundamental Transaction,
whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of Common Stock are given
the choice to receive from among alternative forms of consideration in connection with the Fundamental Transaction; provided, further,
that if holders of Common Stock of the Company are not offered or paid any consideration in such Fundamental Transaction, such holders
of Common Stock will be deemed to have received common stock/shares of the Successor Entity (which Entity may be the Company following
such Fundamental Transaction) in such Fundamental Transaction. “Black Scholes Value” means the value of this
Warrant based on the Black-Scholes Option Pricing Model obtained from the “OV” function on Bloomberg, L.P. (“Bloomberg”)
determined as of the day of consummation of the applicable contemplated Fundamental Transaction for pricing purposes and reflecting (A)
a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement
of the applicable contemplated Fundamental Transaction and the Termination Date, (B) an expected volatility equal to the greater of (1)
100% and (2) the 100 day volatility as obtained from the HVT function on Bloomberg (determined utilizing a 365 day annualization factor)
as of the Trading Day immediately following the public announcement of the applicable contemplated Fundamental Transaction, (C) the underlying
price per share used in such calculation shall be the greater of (i) the sum of the price per share being offered in cash, if any, plus
the value of any non-cash consideration, if any, being offered in such Fundamental Transaction and (ii) the highest VWAP during the period
beginning on the Trading Day immediately preceding the public announcement of the applicable contemplated Fundamental Transaction (or
the consummation of the applicable Fundamental Transaction, if earlier) and ending on the Trading Day of the Holder’s request pursuant
to this Section 3.4 and (D) a remaining option time equal to the time between the date of the public announcement of the applicable contemplated
Fundamental Transaction and the Termination Date and (E) a zero cost of borrow. The payment of the Black Scholes Value will be made by
wire transfer of immediately available funds (or such other consideration) within the later of (i) five (5) Business Days after the Holder’s
election and (ii) the date of consummation of the Fundamental Transaction. The Company shall cause any successor entity in a Fundamental
Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in writing all of the
obligations of the Company under this Warrant in accordance with the provisions of this Section 3.4 pursuant to written agreements in
form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay) prior to such Fundamental
Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security of the Successor Entity
evidenced by a written instrument substantially similar in form and substance to this Warrant that is exercisable for a corresponding
number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common Stock acquirable
and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior to such Fundamental
Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock (but taking into account
the relative value of the shares of Common Stock prior to such Fundamental Transaction and the value of such shares of capital stock,
such number of shares of capital stock and such exercise price being for the purpose of protecting the economic value of this Warrant
immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in form and substance to
the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the term “Company”
under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction, each and every provision of
this Warrant referring to the “Company” shall refer instead to each of the Company and the Successor Entity or Successor Entities,
jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company, may exercise every right
and power of the Company prior thereto and the Successor Entity or Successor Entities shall assume all of the obligations of the Company
prior thereto under this Warrant with the same effect as if the Company and such Successor Entity or Successor Entities, jointly and severally,
had been named as the Company herein. For the avoidance of doubt, the Holder shall be entitled to the benefits of the provisions of this
Section 3.4 regardless of (i) whether the Company has sufficient authorized shares of Common Stock for the issuance of Warrant Shares
and/or (ii) whether a Fundamental Transaction occurs prior to the Initial Exercise Date.
3.5.
Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a
share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as
of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
3.6.
Notice to Holder.
3.6.1.
Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3,
the Company shall promptly deliver to the Holder by email a notice setting forth the Exercise Price after such adjustment and any resulting
adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
3.6.2.
Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever
form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock,
(C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares
of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with
any reclassification of the Common Stock, any consolidation or merger to which the Company (or any of its Subsidiaries) is a party, any
sale or transfer of all or substantially all of its assets, or any compulsory share exchange whereby the Common Stock is converted into
other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding
up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by email to the Holder at its last email
address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective
date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution,
redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be
entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification,
consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected
that holders of the Common Stock of record shall be entitled to exchange their shares of Common Stock for securities, cash or other property
deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver
such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified
in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding
the Company or any of the Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report
on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the
effective date of the event triggering such notice except as may otherwise be expressly set forth herein.
4.1.
Transferability. Subject to compliance with any applicable securities laws, this Warrant and all rights hereunder
are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent,
together with a written assignment of this Warrant substantially in the form attached hereto as Exhibit 2.4.6 duly executed by
the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such
surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee
or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the
assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. Notwithstanding
anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company unless the Holder
has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company within three (3) Trading Days
of the date on which the Holder delivers an assignment form to the Company assigning this Warrant in full. The Warrant, if properly assigned
in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
4.2.
New Warrants. If this Warrant is not held in global form through DTC (or any successor depositary), this Warrant
may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written
notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject
to compliance with Section 4.1, as to any transfer which may be involved in such division or combination, the Company shall execute and
deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All
Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this Warrant
except as to the number of Warrant Shares issuable pursuant thereto.
4.3.
Warrant Register. The Warrant Agent shall register this Warrant, upon records to be maintained by the Warrant Agent
for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company
and the Warrant Agent may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise
hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
5.1.
No Rights as Stockholder until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting
rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2.4.1, except as
expressly set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise”
pursuant to Section 2.3 or to receive cash payments pursuant to Section 2.4.1 and Section 2.4.4 herein, in no event shall the Company
be required to net cash settle an exercise of this Warrant.
5.2.
Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence
reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant
Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant,
shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the
Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant
or stock certificate.
5.3.
Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of
any right required or granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the
next succeeding Business Day.
5.4.
Authorized Shares.
5.4.1.
Reservation of Authorized and Unissued Shares. The Company covenants that, during the period the Warrant is outstanding,
it will reserve from its authorized and unissued Common Stock a sufficient
number of shares of Common Stock to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this
Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged
with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take
all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of
any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company
covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon
exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized,
validly issued, fully paid and nonassessable (which means that no further sums are required to be paid by the holders thereof in connection
with the issue thereof) and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than
taxes in respect of any transfer occurring contemporaneously with such issue).
5.4.2.
Noncircumvention. Except and to the extent as waived or consented to by the Holder, the Company shall not by any
action, including, without limitation, amending its certificate of incorporation or through any reorganization, transfer of assets, consolidation,
merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of
any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of
all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without
limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable
therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate
in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant
and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body
having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
5.4.3.
Authorizations, Exemptions and Consents. Before taking any action that would result in an adjustment in the number
of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or
exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
5.5.
Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Warrant
shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles
of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the
transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers,
shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City
of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of
New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated
hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that
it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient
venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any
such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery)
to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient
service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any
other manner permitted by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant,
the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees
and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. Notwithstanding
the foregoing, nothing in this paragraph shall limit or restrict the federal district court in which a Holder may bring a claim under
the federal securities laws.
5.6.
Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not
registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities
laws.
5.7.
Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part
of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies, notwithstanding
the fact that the right to exercise this Warrant terminates on the Termination Date. No provision of this Warrant shall be construed as
a waiver by the Holder of any rights which the Holder may have under the federal securities laws and the rules and regulations of the
Commission thereunder. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with
any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as
shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of
appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights,
powers or remedies hereunder.
5.8.
Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including,
without limitation, any Notice of Exercise, shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized
overnight courier service, addressed to the Company, at 1177 Avenue of the Americas, 5th Floor, New York, NY 10036, Attention: Steve Slilaty,
Chief Executive Officer, email address: steve.slilaty@sunshinebiopharma.com, or such other email address or address as the Company may
specify for such purposes by notice to the Holders. Any and all notices or other communications or deliveries to be provided by the Company
hereunder shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service addressed
to each Holder at the e-mail address or address of such Holder appearing on the books of the Company. Any notice or other communication
or deliveries hereunder shall be deemed given and effective on the earliest of (i) the time of transmission, if such notice or communication
is delivered via e-mail at the e-mail address set forth in this Section 5.8 prior to 5:30 p.m. (New York City time) on any date, (ii)
the next Trading Day after the time of transmission, if such notice or communication is delivered via e-mail at the e-mail address set
forth in this Section 5.8 on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (iii) the
second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon actual
receipt by the party to whom such notice is required to be given. To the extent that any notice provided hereunder constitutes, or contains,
material, non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice with the
Commission pursuant to a Current Report on Form 8-K.
5.9.
Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise
this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability
of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the
Company or by creditors of the Company.
5.10.
Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of
damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not
be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive
and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
5.11.
Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced
hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted
assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and
shall be enforceable by the Holder or holder of Warrant Shares.
5.12.
Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the
Company, on the one hand, and the Holder, on the other hand.
5.13.
Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective
and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision
shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining
provisions of this Warrant.
5.14.
Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose,
be deemed a part of this Warrant.
5.15.
Warrant Agent Agreement. If this Warrant is held in global form through DTC (or any successor depositary), this Warrant
is issued subject to the Warrant Agent Agreement. To the extent any provision of this Warrant conflicts with the express provisions of
the Warrant Agent Agreement, the provisions of this Warrant shall govern and be controlling.
********************
[SBFM Investor Pre-Funded Registered Warrant
Signature Page Follows]
[SBFM Investor Pre-Funded Registered Warrant
Signature Page]
IN WITNESS WHEREOF, the Company
has caused this Pre-Funded Registered Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
SUNSHINE BIOPHARMA, INC.
By:______________________________
Name:Steve Slilaty
Its:Chief Executive Officer
Exhibit 2.1
NOTICE OF EXERCISE
To:SUNSHINE
BIOPHARMA, INC.
(1)
The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant
(only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes,
if any.
(2)
Payment shall take the form of (check applicable box):
[ ]in lawful money
of the United States.
[ ]if permitted the cancellation of
such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2.3, to exercise this Warrant with
respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2.3.
(3)
Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following
DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE
OF HOLDER]
| Name of Investing Entity: |
|
| Signature of Authorized Signatory of Investing Entity: |
|
| Name of Authorized Signatory: |
|
| Title of Authorized Signatory: |
|
| Date: |
|
Exhibit 2.4.6
ASSIGNMENT FORM
(To assign the foregoing Warrant,
execute this form and supply required information. Do not use this form to exercise the Warrant to purchase shares of Common Stock.)
FOR VALUE RECEIVED, the foregoing
Warrant and all rights evidenced thereby are hereby assigned to
| Name: |
|
| Address: |
|
| Phone Number: |
|
| Email Address: |
|
| Date: |
|
| Holder’s Signature: |
|
| Holder’s Address: |
|
Exhibit 10.21
SERIES A WARRANT TO PURCHASE COMMON STOCK
SUNSHINE BIOPHARMA, INC.
| Warrant Shares: [●] |
Initial Exercise Date: [●], 2024 |
|
CUSIP:
ISIN: |
Issue Date: [●], 2024 |
THIS WARRANT TO PURCHASE
COMMON STOCK (the “Warrant”) certifies that, for value received, [●] or its assigns (the “Holder”)
is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after
the Initial Exercise Date and on or prior to 5:00 p.m. (New York City time) on [●], 2026 (the “Termination Date”)
but not thereafter, to subscribe for and purchase from Sunshine Biopharma, Inc., a Colorado corporation (the “Company”),
up to [●] shares (as subject to adjustment hereunder, the “Warrant Shares”) of Common Stock. The purchase
price of one (1) share of Common Stock under this Series A Warrant (this “Warrant”) shall be equal to the Exercise
Price, as defined in Section 2.2. This Warrant shall initially be issued and maintained in the form of a security held in book-entry
form and the Depository Trust Company or its nominee (“DTC”) shall initially be the sole registered holder of
this Warrant, subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant
Agent Agreement, in which case this sentence shall not apply.
| 1. | Definitions. In addition to the terms defined elsewhere in this Warrant or in the Underwriting
Agreement dated [●], 2024, the following terms have the meanings indicated in this Section 1: |
1.1.
“Affiliate” means any Person that, directly or indirectly through one or more intermediaries, controls
or is controlled by or is under common control with a Person, as such terms are used in and construed under Rule 405 under the Securities
Act.
1.2.
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies:
(a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the
nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based
on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market,
the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c)
if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on
the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price
per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined
by an independent appraiser selected in good faith by the Holders of a majority in interest of the Warrants then outstanding and reasonably
acceptable to the Company, the fees and expenses of which shall be paid by the Company.
1.3.
“Board of Directors” means the board of directors of the Company.
1.4.
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The
City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks
shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”,
“non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the
direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial
banks in The City of New York generally are open for use by customers on such day.
1.5.
“Commission” means the United States Securities and Exchange Commission.
1.6.
“Common Stock” means the common stock of the Company, $0.001 par value per share, and any other class
of securities into which such securities may hereafter be reclassified or changed.
1.7.
“Common Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle
the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant
or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof
to receive, Common Stock.
1.8.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated
thereunder.
1.9.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association,
joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any
kind.
1.10.
“Registration Statement” means the Company’s registration statement on Form S-1 (File No. 333-276817).
1.11.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated
thereunder.
1.12.
“Stockholder Approval” means such approval as may be required by the applicable rules and regulations
of the Nasdaq Capital Market (or any successor entity) from the stockholders of the Company, or board of directors in lieu thereof, with
respect to issuance of all of the Warrants and the Warrant Shares upon the exercise thereof, including without limitation:
1.12.1.
to give full effect to alternative cashless exercises pursuant to Section 2.3 hereof.
1.12.2.
to consent to any adjustment to the exercise price or number of shares of Common Stock underlying the Warrants in the event of
a Share Combination Event pursuant to Section 3.10.
1.12.3.
to consent to the voluntary adjustment, from time to time, of the exercise price of any and all currently outstanding warrants
pursuant to Section 3.11.
1.13.
“Subsidiary” means any subsidiary of the Company and shall, where applicable, also include any direct
or indirect subsidiary of the Company formed or acquired after the date hereof.
1.14.
“Trading Day” means a day on which the Common Stock is traded on a Trading Market.
1.15.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed
or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global
Select Market, the New York Stock Exchange, OTCQB or OTCQX (or any successors to any of the foregoing).
1.16.
“Transfer Agent” means Equiniti, the current transfer agent of the Company, with a mailing address of
1110 Centre Pointe Curve, Suite 101, Mendota Heights, Minnesota 55120 and an email address of issuerservices@equiniti.com, and any successor
transfer agent of the Company.
1.17.
“Underwriting Agreement” means the underwriting agreement, dated as of [●], 2024, between the Company
and Aegis Capital Corp., as amended, modified or supplemented from time to time in accordance with its terms.
1.18.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies:
(a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for
such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg
L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not
a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX
as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are
then reported on the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most
recent bid price per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock
as determined by an independent appraiser selected in good faith by the holders of a majority in interest of the Warrants then outstanding
and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
1.19.
“Warrant Agent Agreement” means that certain Warrant Agent agreement, dated on or about the Initial Exercise
Date, between the Company and the Warrant Agent.
1.20.
“Warrant Agent” means the Transfer Agent and any successor warrant agent of the Company.
1.21.
“Warrants” means this Warrant and other Common Stock purchase warrants issued by the Company pursuant
to the Registration Statement.
2.1.
Exercise of Warrant. Subject to the provisions of Section 2.5 herein, exercise of the purchase rights represented
by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination
Date by delivery to the Company of a duly executed PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise substantially
in the form attached hereto as Exhibit 2.1 (the “Notice of Exercise”). Within the earlier of (i)
two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period (as defined in Section 2.4.1 herein)
following the date of exercise as aforesaid, the Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in
the applicable Notice of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise
procedure specified in Section 2.3 below is specified in the applicable Notice of Exercise. For the avoidance of doubt, any reference
to cashless exercise herein shall include a reference to alternative cashless exercise. No ink-original Notice of Exercise shall be required,
nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding
anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder
has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall
surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise
is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares
available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal
to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant
Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1) Trading
Day of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of
the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available
for purchase hereunder at any given time may be less than the amount stated on the face hereof.
Notwithstanding
the foregoing in this Section 2.1, a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing
this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions), shall effect
exercises made pursuant to this Section 2.1 by delivering to DTC (or such other clearing corporation, as applicable) the appropriate
instruction form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other clearing corporation,
as applicable), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant
Agent Agreement, in which case this sentence shall not apply.
2.2.
Exercise Price. The exercise price per Warrant Share shall be $[●], subject to adjustment hereunder (the “Exercise
Price”).
2.3.
Cashless Exercise. If at the time of exercise hereof there is no effective registration statement registering, or
the prospectus contained therein is not available for the issuance of the Warrant Shares to the Holder or the resale of the Warrant Shares
by the Holder, then this Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise”
in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by
(A), where:
| (A) = | as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice
of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2.1
hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2.1
hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b) of Regulation NMS promulgated
under the federal securities laws) on such Trading Day, (ii) at the option of the Holder, either (y) the VWAP on the Trading Day immediately
preceding the date of the applicable Notice of Exercise or (z) the Bid Price of the Common Stock on the principal Trading Market as reported
by Bloomberg L.P. as of the time of the Holder’s execution of the applicable Notice of Exercise if such Notice of Exercise is executed
during “regular trading hours” on a Trading Day and is delivered within two (2) hours thereafter (including until two (2)
hours after the close of “regular trading hours” on a Trading Day) pursuant to Section 2.1
hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and
such Notice of Exercise is both executed and delivered pursuant to Section 2.1
hereof after the close of “regular trading hours” on such Trading Day; |
| | | |
| (B) = | the Exercise Price of this Warrant, as adjusted hereunder; and |
| | | |
| (X) = | the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the
terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
If Warrant Shares
are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act,
the Warrant Shares shall take on the registered characteristics of the Warrants being exercised, and the holding period of the Warrant
Shares being issued may be tacked on to the holding period of this Warrant. Assuming (i) the Holder is not an Affiliate of the Company,
and (ii) all of the applicable conditions of Rule 144 promulgated under the Securities Act with respect to Holder and the Warrant Shares
are met in the case of such a cashless exercise, the Company agrees that the Company will cause the removal of the legend from such Warrant
Shares (including by delivering an opinion of the Company’s counsel to the Company’s transfer agent at its own expense to
ensure the foregoing), and the Company agrees that the Holder is under no obligation to sell the Warrant Shares issuable upon the exercise
of the Warrant prior to removing the legend. The Company agrees not to take any position contrary to this Section 2.3.
The Holder may also
effect an “alternative cashless exercise” following the Stockholder Approval Date. In such event, the aggregate number of
Warrant Shares issuable in such alternative cashless exercise pursuant to any given Notice of Exercise electing to effect an alternative
cashless exercise shall equal the product of (i) the aggregate number of Warrant Shares that would be issuable upon exercise of this Warrant
in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise, multiplied
by (ii) 2.0. Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via
cashless exercise pursuant to this Section 2.3 (including an alternative
cashless exercise pursuant to this paragraph). Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant
shall be automatically exercised via cashless exercise pursuant to this Section 2.3.
2.4.
Mechanics of Exercise.
2.4.1.
Delivery of Warrant Shares upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted
by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository
Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant
in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale
of the Warrant Shares by Holder or (B) the Warrant Shares are eligible for resale by the Holder without volume or manner-of-sale limitations
pursuant to Rule 144 (assuming cashless exercise of the Warrants), and otherwise by physical delivery of a certificate, for the number
of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise
by the date that is the earliest of (i) two (2) Trading Days after the delivery to the Company of the Notice of Exercise, (ii) one (1)
Trading Day after delivery of the aggregate Exercise Price to the Company and (iii) the number of Trading Days comprising the Standard
Settlement Period after the delivery to the Company of the Notice of Exercise (such date, the “Warrant Share Delivery Date”).
Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of
the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares,
provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of
(i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period following delivery of the Notice
of Exercise. Notwithstanding anything herein to the contrary, upon delivery of the Notice of Exercise, the Holder shall be deemed for
purposes of Regulation SHO under the Exchange Act to have become the holder of the Warrant Shares irrespective of the date of delivery
of the Warrant Shares. If the Company fails for any reason (other than the failure of the Holder to timely deliver the aggregate Exercise
Price, unless the Warrant is validly exercised by means of a cashless exercise) to deliver to the Holder the Warrant Shares subject to
a Notice of Exercise by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as
a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable
Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the third (3rd) Trading Day after the Warrant Share Delivery
Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise.
The Company agrees to maintain a Transfer Agent that is a participant in the FAST program so long as this Warrant remains outstanding
and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed
in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of
delivery of the Notice of Exercise. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to
12:00 p.m. (New York City time) on the Initial Exercise Date, which may be delivered at any time after the time of execution of the Underwriting
Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial
Exercise Date and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of
the aggregate Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date.
2.4.2.
Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at
the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the
Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which
new Warrant shall in all other respects be identical with this Warrant.
2.4.3.
Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares
pursuant to Section 2.4.1 by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise; provided,
however, that the Holder shall be required to return any Warrant Shares subject to any such rescinded exercise notice concurrently with
the return to Holder of the aggregate Exercise Price paid to the Company for such Warrant Shares and the restoration of Holder’s
right to acquire such Warrant Shares pursuant to this Warrant (including, issuance of a replacement warrant certificate evidencing such
restored right).
2.4.4.
Compensation for Buy-In on Failure to Timely Deliver Warrant Shares upon Exercise. In addition to any other rights
available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with
the provisions of Section 2.4.1 above pursuant to an exercise on or before the Warrant Share Delivery Date (other than the failure
of the Holder to timely deliver the aggregate Exercise Price, unless the Warrant is validly exercised by means of a cashless exercise),
and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s
brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which
the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to
the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the
shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was
required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to
such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent
number of Warrant Shares for which such exercise was not honored and return any amount received by the Company in respect of the Exercise
Price for those Warrant Shares (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of
Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example,
if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise
of shares of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately
preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating
the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing
herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without
limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares
of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
2.4.5.
No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon
the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise,
the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the Exercise Price or round up to the next whole share.
2.4.6.
Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue
or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be
paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by
the Holder; provided, however, that, in the event that Warrant Shares are to be issued in a name other than the name of
the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto as Exhibit 2.4.6
duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any
transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise
and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day
electronic delivery of the Warrant Shares.
2.4.7.
Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely
exercise of this Warrant, pursuant to the terms hereof.
2.5.
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall
not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect
to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates,
and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution
Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of
the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties
shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is
being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, unexercised
portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion
of the unexercised or unconverted portion of any other securities of the Company (including, without limitation, any other Common Stock
Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the
Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2.5,
beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated
thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance
with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith.
To the extent that the limitation contained in this Section 2.5 applies, the determination of whether this Warrant is exercisable
(in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this
Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be
the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together
with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial
Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination. In addition, a
determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and
the rules and regulations promulgated thereunder. For purposes of this Section 2.5, in determining the number of outstanding shares
of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent
periodic or annual report filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a
more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the
written or oral request of a Holder, the Company shall within one (1) Trading Day confirm orally and in writing to the Holder the number
of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving
effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution
Parties since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership
Limitation” shall be 4.99% (or, upon election by a Holder prior to the issuance of any Warrants, 9.99%) of the number of
shares of Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of
this Warrant. The Holder, upon notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this
Section 2.5, provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of Common Stock
outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder
and the provisions of this Section 2.5 shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective
until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented
in a manner otherwise than in strict conformity with the terms of this Section 2.5 to correct this paragraph (or any portion hereof)
which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements
necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor
holder of this Warrant.
3.1.
Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend
or otherwise makes a distribution or distributions on shares of Common Stock or any other equity or equity equivalent securities payable
in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise
of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way
of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares
of Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of
which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such
event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number
of Shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant
shall remain unchanged. Any adjustment made pursuant to this Section 3.1 shall become effective
immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become
effective immediately after the effective date in the case of a subdivision, combination or re-classification.
3.2.
Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3.1 above, if at any time the
Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata
to all (or substantially all) of the record holders of any class of shares of Common Stock (the “Purchase Rights”),
then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the
Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant
(without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately
before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the
date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights
(provided, however, that, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder
exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent
(or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to
such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding
the Beneficial Ownership Limitation).
3.3.
Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any
dividend or other distribution of its assets (or rights to acquire its assets) to all (or substantially all) holders of shares of Common
Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property
or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction)
(a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall
be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held
the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise
hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for
such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined
for the participation in such Distribution (provided, however, that, to the extent that the Holder’s right to participate in any
such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate
in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to
such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as
its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation). To the extent that this Warrant has not
been partially or completely exercised at the time of such Distribution, such portion of the Distribution shall be held in abeyance for
the benefit of the Holder until the Holder has exercised this Warrant.
3.4.
Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly,
in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company or
any Subsidiary, directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all
or substantially all of its assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer
or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell,
tender or exchange their shares for other securities, cash or property and has been accepted by the holders of 50% or more of the outstanding
Common Stock or 50% or more of the voting power of the common equity of the Company, (iv) the Company, directly or indirectly, in one
or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share
exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v)
the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business
combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another
Person or group of Persons whereby such other Person or group acquires 50% or more of the outstanding shares of Common Stock or 50% or
more of the voting power of the common equity of the Company (each a “Fundamental Transaction”), then, upon
any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable
upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to
any limitation in Section 2.5 on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring
corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”)
receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable
immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2.5 on the exercise of this Warrant).
For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate
Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction,
and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value
of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash
or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration
it receives upon any exercise of this Warrant following such Fundamental Transaction. Notwithstanding anything to the contrary, in the
event of a Fundamental Transaction, the Company or any Successor Entity (as defined below) shall, at the Holder’s option, exercisable
at any time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction (or, if later, the date of the
public announcement of the applicable Fundamental Transaction), purchase this Warrant from the Holder by paying to the Holder an amount
of cash equal to the Black Scholes Value (as defined below) of the remaining unexercised portion of this Warrant on the date of the consummation
of such Fundamental Transaction; provided, however, that, if the Fundamental Transaction is not within the Company’s control, including
not approved by the Company’s Board of Directors, the Holder shall only be entitled to receive from the Company or any Successor
Entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of this
Warrant, that is being offered and paid to the holders of Common Stock of the Company in connection with the Fundamental Transaction,
whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of Common Stock are given
the choice to receive from among alternative forms of consideration in connection with the Fundamental Transaction; provided, further,
that if holders of Common Stock of the Company are not offered or paid any consideration in such Fundamental Transaction, such holders
of Common Stock will be deemed to have received common stock/shares of the Successor Entity (which Entity may be the Company following
such Fundamental Transaction) in such Fundamental Transaction. “Black Scholes Value” means the value of this
Warrant based on the Black-Scholes Option Pricing Model obtained from the “OV” function on Bloomberg, L.P. (“Bloomberg”)
determined as of the day of consummation of the applicable contemplated Fundamental Transaction for pricing purposes and reflecting (A)
a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement
of the applicable contemplated Fundamental Transaction and the Termination Date, (B) an expected volatility equal to the greater of (1)
100% and (2) the 100 day volatility as obtained from the HVT function on Bloomberg (determined utilizing a 365 day annualization factor)
as of the Trading Day immediately following the public announcement of the applicable contemplated Fundamental Transaction, (C) the underlying
price per share used in such calculation shall be the greater of (i) the sum of the price per share being offered in cash, if any, plus
the value of any non-cash consideration, if any, being offered in such Fundamental Transaction and (ii) the highest VWAP during the period
beginning on the Trading Day immediately preceding the public announcement of the applicable contemplated Fundamental Transaction (or
the consummation of the applicable Fundamental Transaction, if earlier) and ending on the Trading Day of the Holder’s request pursuant
to this Section 3.7 and (D) a remaining option time equal to the time between the date of the public announcement of the applicable
contemplated Fundamental Transaction and the Termination Date and (E) a zero cost of borrow.
The payment of the Black Scholes Value will
be made by wire transfer of immediately available funds (or such other consideration) within the later of (i) five (5) Business Days after
the Holder’s election and (ii) the date of consummation of the Fundamental Transaction. The Company shall cause any successor entity
in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in
writing all of the obligations of the Company under this Warrant in accordance with the provisions of this Section 3.7 pursuant to
written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay)
prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security
of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant that is exercisable
for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common
Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior
to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock
(but taking into account the relative value of the shares of Common Stock prior to such Fundamental Transaction and the value of such
shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic
value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in
form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the
term “Company” under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction,
each and every provision of this Warrant referring to the “Company” shall refer instead to each of the Company and the Successor
Entity or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company,
may exercise every right and power of the Company prior thereto and the Successor Entity or Successor Entities shall assume all of the
obligations of the Company prior thereto under this Warrant with the same effect as if the Company and such Successor Entity or Successor
Entities, jointly and severally, had been named as the Company herein. For the avoidance of doubt, the Holder shall be entitled to the
benefits of the provisions of this Section 3.7 regardless of (i) whether the Company has sufficient authorized shares of Common Stock
for the issuance of Warrant Shares and/or (ii) whether a Fundamental Transaction occurs prior to the Initial Exercise Date.
3.5.
Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th
of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding
as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
3.6.
Notice to Holder.
3.6.1.
Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3,
the Company shall promptly deliver to the Holder by email a notice setting forth the Exercise Price after such adjustment and any resulting
adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
3.6.2.
Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever
form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock,
(C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares
of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with
any reclassification of the Common Stock, any consolidation or merger to which the Company (or any of its Subsidiaries) is a party, any
sale or transfer of all or substantially all of its assets, or any compulsory share exchange whereby the Common Stock is converted into
other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding
up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by email to the Holder at its last email
address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective
date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution,
redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be
entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification,
consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected
that holders of the Common Stock of record shall be entitled to exchange their shares of Common Stock for securities, cash or other property
deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver
such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified
in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding
the Company or any of the Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report
on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the
effective date of the event triggering such notice except as may otherwise be expressly set forth herein.
3.7.
Share Combination Event Adjustment. In addition to the adjustments set forth in Section 3.1 above, if at any
time and from time to time on or after the Issuance Date there occurs any share split, share dividend, share combination recapitalization
or other similar transaction involving the Common Stock (each, a “Share Combination Event”, and such date thereof,
the “Share Combination Event Date”) and the lowest VWAP during the period commencing Five (5) consecutive Trading
Days immediately preceding and the Five (5) consecutive Trading Days immediately following the Share Combination Event Date (the “Event
Market Price”) (provided if the Share Combination Event is effective after close of Trading on the primary Trading Market,
then commencing on the next Trading Day which period shall be the “Share Combination Adjustment Period”) is
less than the Exercise Price then in effect (after giving effect to the adjustment in clause 3.1 above), then at the close of trading
on the primary Trading Market on the last day of the Share Combination Adjustment Period, the Exercise Price then in effect on such Fifth
(5th) Trading Day shall be reduced (but in no event increased) to the Event Market Price and the number of Warrant Shares issuable hereunder
shall be increased such that the aggregate Exercise Price of this Warrant on the Issuance Date for the Warrant Shares then outstanding
shall remain unchanged. For the avoidance of doubt, if the adjustment in the immediately preceding sentence would otherwise result in
an increase in the Exercise Price hereunder, no adjustment shall be made, and if this Warrant is exercised, on any given Exercise Date
during the Share Combination Adjustment Period, solely with respect to such portion of this Warrant exercised on such applicable Exercise
Date, such applicable Share Combination Adjustment Period shall be deemed to have ended on, and included, the Trading Day immediately
prior to such Exercise Date and the Event Market Price on such applicable Exercise Date will be the lowest VWAP of the Common Stock immediately
during such the Share Combination Adjustment Period prior to such Exercise Date and ending on, and including the Trading Day immediately
prior to such Exercise Date.
3.8.
Voluntary Adjustment by Company. Subject to the rules and regulations of the Trading Market and the consent of the
Holders of a majority in interest of the Warrants then outstanding, the Company may at any time during the term of this Warrant reduce
the then current Exercise Price to any amount and for any period of time deemed appropriate by the Board of Directors.
3.9.
Stockholder Approval. The Company shall seek Stockholder Approval in the time period and the manner provided in the
Underwriting Agreement.
3.10.
Reserved.
4.1.
Transferability. Subject to compliance with any applicable securities laws, this Warrant and all rights hereunder
(including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the
principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially in the form
attached hereto as Exhibit 2.4.6 duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer
taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver
a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified
in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned,
and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically
surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this
Warrant to the Company within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning
this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant
Shares without having a new Warrant issued.
4.2.
New Warrants. If this Warrant is not held in global form through DTC (or any successor depositary), this Warrant
may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written
notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject
to compliance with Section 4.1, as to any transfer which may be involved in such division or combination, the Company shall execute
and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice.
All Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this
Warrant except as to the number of Warrant Shares issuable pursuant thereto.
4.3.
Warrant Register. The Warrant Agent shall register this Warrant, upon records to be maintained by the Warrant Agent
for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company
and the Warrant Agent may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise
hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
5.1.
No Rights as Stockholder until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting
rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2.4.1, except
as expressly set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise”
pursuant to Section 2.3 or to receive cash payments pursuant to Section 2.4.1 and Section 2.4.4 herein,
in no event shall the Company be required to net cash settle an exercise of this Warrant.
5.2.
Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence
reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant
Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant,
shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the
Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant
or stock certificate.
5.3.
Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of
any right required or granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the
next succeeding Business Day.
5.4.
Authorized Shares.
5.4.1.
Reservation of Authorized and Unissued Shares. The Company covenants that, during the period the Warrant is outstanding,
it will reserve from its authorized and unissued Common Stock a sufficient
number of shares of Common Stock to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this
Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged
with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take
all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of
any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company
covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon
exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized,
validly issued, fully paid and nonassessable (which means that no further sums are required to be paid by the holders thereof in connection
with the issue thereof) and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than
taxes in respect of any transfer occurring contemporaneously with such issue).
5.4.2.
Noncircumvention. Except and to the extent as waived or consented to by the Holders of a majority in interest of
the Warrants then outstanding, the Company shall not by any action, including, without limitation, amending its certificate of incorporation
or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary
action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith
assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights
of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not
increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in
par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully
paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such
authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the
Company to perform its obligations under this Warrant.
5.4.3.
Authorizations, Exemptions and Consents. Before taking any action that would result in an adjustment in the number
of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or
exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
5.5.
Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Warrant
shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles
of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the
transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers,
shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City
of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of
New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated
hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that
it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient
venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any
such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery)
to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient
service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any
other manner permitted by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant,
the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees
and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. Notwithstanding
the foregoing, nothing in this paragraph shall limit or restrict the federal district court in which a Holder may bring a claim under
the federal securities laws.
5.6.
Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not
registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities
laws.
5.7.
Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part
of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies, notwithstanding
the fact that the right to exercise this Warrant terminates on the Termination Date. No provision of this Warrant shall be construed as
a waiver by the Holder of any rights which the Holder may have under the federal securities laws and the rules and regulations of the
Commission thereunder. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with
any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as
shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of
appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights,
powers or remedies hereunder.
5.8.
Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including,
without limitation, any Notice of Exercise, shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized
overnight courier service, addressed to the Company, at 1177 Avenue of the Americas, 5th Floor, New York, NY 10036, Attention: Steve Slilaty,
Chief Executive Officer, email address: steve.slilaty@sunshinebiopharma.com, or such other email address or address as the Company may
specify for such purposes by notice to the Holders. Any and all notices or other communications or deliveries to be provided by the Company
hereunder shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service addressed
to each Holder at the e-mail address or address of such Holder appearing on the books of the Company. Any notice or other communication
or deliveries hereunder shall be deemed given and effective on the earliest of (i) the time of transmission, if such notice or communication
is delivered via e-mail at the e-mail address set forth in this Section 5.8 prior to 5:30 p.m. (New York City time) on any date,
(ii) the next Trading Day after the time of transmission, if such notice or communication is delivered via e-mail at the e-mail address
set forth in this Section 5.8 on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day,
(iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon
actual receipt by the party to whom such notice is required to be given. To the extent that any notice provided hereunder constitutes,
or contains, material, non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice
with the Commission pursuant to a Current Report on Form 8-K.
5.9.
Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise
this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability
of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the
Company or by creditors of the Company.
5.10.
Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of
damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not
be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive
and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
5.11.
Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced
hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted
assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and
shall be enforceable by the Holder or holder of Warrant Shares.
5.12.
Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the
Company, on the one hand, and the Holders of a majority in interest of the Warrants then outstanding, on the other hand.
5.13.
Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective
and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision
shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining
provisions of this Warrant.
5.14.
Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose,
be deemed a part of this Warrant.
5.15.
Warrant Agent Agreement. If this Warrant is held in global form through DTC (or any successor depositary), this Warrant
is issued subject to the Warrant Agent Agreement. To the extent any provision of this Warrant conflicts with the express provisions of
the Warrant Agent Agreement, the provisions of this Warrant shall govern and be controlling.
********************
[SBFM Investor Registered Series A Warrant Signature
Page Follows]
[SBFM Investor Registered Series A Warrant Signature
Page]
IN WITNESS WHEREOF, the Company
has caused this Registered Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
SUNSHINE BIOPHARMA, INC.
By:______________________________
Name:Steve Slilaty
Its:Chief Executive Officer
Exhibit 2.1
NOTICE OF EXERCISE
To:SUNSHINE
BIOPHARMA, INC.
(1)
The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant
(only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes,
if any.
(2)
Payment shall take the form of (check applicable box):
[ ]in lawful money
of the United States.
[ ]if permitted the cancellation of
such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2.3, to exercise this Warrant
with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2.3.
[ ]if permitted the cancellation of
such number of Warrant Shares as is necessary, in accordance with the provisions of subsection 2.3, to exercise this Warrant pursuant
to the “alternative cashless exercise” procedure set forth in subsection 2.3.
(3)
Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following
DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE
OF HOLDER]
| Name of Investing Entity: |
|
| Signature of Authorized Signatory of Investing Entity: |
|
| Name of Authorized Signatory: |
|
| Title of Authorized Signatory: |
|
| Date: |
|
Exhibit 2.4.6
ASSIGNMENT FORM
(To assign the foregoing Warrant,
execute this form and supply required information. Do not use this form to exercise the Warrant to purchase shares of Common Stock.)
FOR VALUE RECEIVED, the foregoing
Warrant and all rights evidenced thereby are hereby assigned to
| Name: |
|
| Address: |
|
| Phone Number: |
|
| Email Address: |
|
| Date: |
|
| Holder’s Signature: |
|
| Holder’s Address: |
|
Exhibit 10.22
SERIES B WARRANT TO PURCHASE COMMON STOCK
SUNSHINE BIOPHARMA, INC.
| Warrant Shares: [●] |
Initial Exercise Date: [●], 2024 |
|
CUSIP:
ISIN: |
Issue Date: [●], 2024 |
THIS WARRANT TO PURCHASE
COMMON STOCK (the “Warrant”) certifies that, for value received, [●] or its assigns (the “Holder”)
is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after
the Initial Exercise Date and on or prior to 5:00 p.m. (New York City time) on [●], 2029 (the “Termination Date”)
but not thereafter, to subscribe for and purchase from Sunshine Biopharma, Inc., a Colorado corporation (the “Company”),
up to [●] shares (as subject to adjustment hereunder, the “Warrant Shares”) of Common Stock. The purchase
price of one (1) share of Common Stock under this Series B Warrant (this “Warrant”) shall be equal to the Exercise
Price, as defined in Section 2.2. This Warrant shall initially be issued and maintained in the form of a security held in book-entry
form and the Depository Trust Company or its nominee (“DTC”) shall initially be the sole registered holder of
this Warrant, subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant
Agent Agreement, in which case this sentence shall not apply.
| 1. | Definitions. In addition to the terms defined elsewhere in this Warrant or in the Underwriting
Agreement dated [●], 2024, the following terms have the meanings indicated in this Section 1: |
1.1.
“Affiliate” means any Person that, directly or indirectly through one or more intermediaries, controls
or is controlled by or is under common control with a Person, as such terms are used in and construed under Rule 405 under the Securities
Act.
1.2.
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies:
(a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the
nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based
on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market,
the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c)
if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on
the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price
per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined
by an independent appraiser selected in good faith by the Holders of a majority in interest of the Warrants then outstanding and reasonably
acceptable to the Company, the fees and expenses of which shall be paid by the Company.
1.3.
“Board of Directors” means the board of directors of the Company.
1.4.
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The
City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks
shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”,
“non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the
direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial
banks in The City of New York generally are open for use by customers on such day.
1.5.
“Commission” means the United States Securities and Exchange Commission.
1.6.
“Common Stock” means the common stock of the Company, $0.001 par value per share, and any other class
of securities into which such securities may hereafter be reclassified or changed.
1.7.
“Common Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle
the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant
or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof
to receive, Common Stock.
1.8.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated
thereunder.
1.9.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association,
joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any
kind.
1.10.
“Registration Statement” means the Company’s registration statement on Form S-1 (File No. 333-276817).
1.11.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated
thereunder.
1.12.
“Stockholder Approval” means such approval as may be required by the applicable rules and regulations
of the Nasdaq Capital Market (or any successor entity) from the stockholders of the Company, or board of directors in lieu thereof, with
respect to issuance of all of the Warrants and the Warrant Shares upon the exercise thereof, including without limitation:
1.12.1.
to render inapplicable clause (i) of the definition of the Floor Price in Section 3.2
hereof.
1.12.2.
to give full effect to the adjustment in the exercise price and number of Warrant Shares following a Dilutive Issuance pursuant
to Section 3.2.
1.12.3.
to consent to any adjustment to the exercise price or number of shares of Common Stock underlying the Warrants in the event of
a Share Combination Event pursuant to Section 3.10.
1.12.4.
to consent to the voluntary adjustment, from time to time, of the exercise price of any and all currently outstanding warrants
pursuant to Section 3.11.
1.13.
“Subsidiary” means any subsidiary of the Company and shall, where applicable, also include any direct
or indirect subsidiary of the Company formed or acquired after the date hereof.
1.14.
“Trading Day” means a day on which the Common Stock is traded on a Trading Market.
1.15.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed
or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global
Select Market, the New York Stock Exchange, OTCQB or OTCQX (or any successors to any of the foregoing).
1.16.
“Transfer Agent” means Equiniti, the current transfer agent of the Company, with a mailing address of
1110 Centre Pointe Curve, Suite 101, Mendota Heights, Minnesota 55120 and an email address of issuerservices@equiniti.com, and any successor
transfer agent of the Company.
1.17.
“Underwriting Agreement” means the underwriting agreement, dated as of [●], 2024, between the Company
and Aegis Capital Corp., as amended, modified or supplemented from time to time in accordance with its terms.
1.18.
“Variable Rate Transaction” means a transaction in which the Company (i) issues or sells any debt or
equity securities that are convertible into, exchangeable or exercisable for, or include the right to receive, additional shares of Common
Stock either (A) at a conversion price, exercise price or exchange rate or other price that is based upon, and/or varies with, the trading
prices of or quotations for the shares of Common Stock at any time after the initial issuance of such debt or equity securities or (B)
with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such debt
or equity security or upon the occurrence of specified or contingent events directly or indirectly related to the business of the Company
or the market for the shares of Common Stock or (ii) enters into, or effects a transaction under, any agreement, including, but not limited
to, an equity line of credit, whereby the Company may issue securities at a future determined price.
1.19.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies:
(a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for
such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg
L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not
a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX
as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are
then reported on the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most
recent bid price per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock
as determined by an independent appraiser selected in good faith by the holders of a majority in interest of the Warrants then outstanding
and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
1.20.
“Warrant Agent Agreement” means that certain Warrant Agent agreement, dated on or about the Initial Exercise
Date, between the Company and the Warrant Agent.
1.21.
“Warrant Agent” means the Transfer Agent and any successor warrant agent of the Company.
1.22.
“Warrants” means this Warrant and other Common Stock purchase warrants issued by the Company pursuant
to the Registration Statement.
2.1.
Exercise of Warrant. Subject to the provisions of Section 2.5 herein, exercise of the purchase rights represented
by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination
Date by delivery to the Company of a duly executed PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise substantially
in the form attached hereto as Exhibit 2.1 (the “Notice of Exercise”). Within the earlier of (i)
two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period (as defined in Section 2.4.1 herein)
following the date of exercise as aforesaid, the Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in
the applicable Notice of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise
procedure specified in Section 2.3 below is specified in the applicable Notice of Exercise. No ink-original Notice of Exercise shall
be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding
anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder
has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall
surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise
is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares
available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal
to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant
Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1) Trading
Day of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of
the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available
for purchase hereunder at any given time may be less than the amount stated on the face hereof.
Notwithstanding
the foregoing in this Section 2.1, a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing
this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions), shall effect
exercises made pursuant to this Section 2.1 by delivering to DTC (or such other clearing corporation, as applicable) the appropriate
instruction form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other clearing corporation,
as applicable), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant
Agent Agreement, in which case this sentence shall not apply.
2.2.
Exercise Price. The exercise price per Warrant Share shall be $[●], subject to adjustment hereunder (the “Exercise
Price”).
2.3.
Cashless Exercise. If at the time of exercise hereof there is no effective registration statement registering, or
the prospectus contained therein is not available for the issuance of the Warrant Shares to the Holder or the resale of the Warrant Shares
by the Holder, then this Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise”
in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by
(A), where:
| (A) = | as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice
of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2.1
hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2.1
hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b) of Regulation NMS promulgated
under the federal securities laws) on such Trading Day, (ii) at the option of the Holder, either (y) the VWAP on the Trading Day immediately
preceding the date of the applicable Notice of Exercise or (z) the Bid Price of the Common Stock on the principal Trading Market as reported
by Bloomberg L.P. as of the time of the Holder’s execution of the applicable Notice of Exercise if such Notice of Exercise is executed
during “regular trading hours” on a Trading Day and is delivered within two (2) hours thereafter (including until two (2)
hours after the close of “regular trading hours” on a Trading Day) pursuant to Section 2.1
hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and
such Notice of Exercise is both executed and delivered pursuant to Section 2.1
hereof after the close of “regular trading hours” on such Trading Day; |
| | | |
| (B) = | the Exercise Price of this Warrant, as adjusted hereunder; and |
| | | |
| (X) = | the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the
terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
If Warrant Shares
are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act,
the Warrant Shares shall take on the registered characteristics of the Warrants being exercised, and the holding period of the Warrant
Shares being issued may be tacked on to the holding period of this Warrant. Assuming (i) the Holder is not an Affiliate of the Company,
and (ii) all of the applicable conditions of Rule 144 promulgated under the Securities Act with respect to Holder and the Warrant Shares
are met in the case of such a cashless exercise, the Company agrees that the Company will cause the removal of the legend from such Warrant
Shares (including by delivering an opinion of the Company’s counsel to the Company’s transfer agent at its own expense to
ensure the foregoing), and the Company agrees that the Holder is under no obligation to sell the Warrant Shares issuable upon the exercise
of the Warrant prior to removing the legend. The Company agrees not to take any position contrary to this Section 2.3.
Notwithstanding
anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant
to this Section 2.3.
2.4.
Mechanics of Exercise.
2.4.1.
Delivery of Warrant Shares upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted
by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository
Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant
in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale
of the Warrant Shares by Holder or (B) the Warrant Shares are eligible for resale by the Holder without volume or manner-of-sale limitations
pursuant to Rule 144 (assuming cashless exercise of the Warrants), and otherwise by physical delivery of a certificate, for the number
of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise
by the date that is the earliest of (i) two (2) Trading Days after the delivery to the Company of the Notice of Exercise, (ii) one (1)
Trading Day after delivery of the aggregate Exercise Price to the Company and (iii) the number of Trading Days comprising the Standard
Settlement Period after the delivery to the Company of the Notice of Exercise (such date, the “Warrant Share Delivery Date”).
Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of
the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares,
provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of
(i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period following delivery of the Notice
of Exercise. Notwithstanding anything herein to the contrary, upon delivery of the Notice of Exercise, the Holder shall be deemed for
purposes of Regulation SHO under the Exchange Act to have become the holder of the Warrant Shares irrespective of the date of delivery
of the Warrant Shares. If the Company fails for any reason (other than the failure of the Holder to timely deliver the aggregate Exercise
Price, unless the Warrant is validly exercised by means of a cashless exercise) to deliver to the Holder the Warrant Shares subject to
a Notice of Exercise by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as
a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable
Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the third (3rd) Trading Day after the Warrant Share Delivery
Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise.
The Company agrees to maintain a Transfer Agent that is a participant in the FAST program so long as this Warrant remains outstanding
and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed
in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of
delivery of the Notice of Exercise. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to
12:00 p.m. (New York City time) on the Initial Exercise Date, which may be delivered at any time after the time of execution of the Underwriting
Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial
Exercise Date and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of
the aggregate Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date.
2.4.2.
Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at
the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the
Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which
new Warrant shall in all other respects be identical with this Warrant.
2.4.3.
Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares
pursuant to Section 2.4.1 by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise; provided,
however, that the Holder shall be required to return any Warrant Shares subject to any such rescinded exercise notice concurrently with
the return to Holder of the aggregate Exercise Price paid to the Company for such Warrant Shares and the restoration of Holder’s
right to acquire such Warrant Shares pursuant to this Warrant (including, issuance of a replacement warrant certificate evidencing such
restored right).
2.4.4.
Compensation for Buy-In on Failure to Timely Deliver Warrant Shares upon Exercise. In addition to any other rights
available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with
the provisions of Section 2.4.1 above pursuant to an exercise on or before the Warrant Share Delivery Date (other than the failure
of the Holder to timely deliver the aggregate Exercise Price, unless the Warrant is validly exercised by means of a cashless exercise),
and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s
brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which
the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to
the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the
shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was
required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to
such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent
number of Warrant Shares for which such exercise was not honored and return any amount received by the Company in respect of the Exercise
Price for those Warrant Shares (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of
Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example,
if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise
of shares of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately
preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating
the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing
herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without
limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares
of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
2.4.5.
No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon
the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise,
the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the Exercise Price or round up to the next whole share.
2.4.6.
Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue
or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be
paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by
the Holder; provided, however, that, in the event that Warrant Shares are to be issued in a name other than the name of
the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto as Exhibit 2.4.6
duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any
transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise
and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day
electronic delivery of the Warrant Shares.
2.4.7.
Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely
exercise of this Warrant, pursuant to the terms hereof.
2.5.
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall
not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect
to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates,
and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution
Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of
the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties
shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is
being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, unexercised
portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion
of the unexercised or unconverted portion of any other securities of the Company (including, without limitation, any other Common Stock
Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the
Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2.5,
beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated
thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance
with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith.
To the extent that the limitation contained in this Section 2.5 applies, the determination of whether this Warrant is exercisable
(in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this
Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be
the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together
with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial
Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination. In addition, a
determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and
the rules and regulations promulgated thereunder. For purposes of this Section 2.5, in determining the number of outstanding shares
of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent
periodic or annual report filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a
more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the
written or oral request of a Holder, the Company shall within one (1) Trading Day confirm orally and in writing to the Holder the number
of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving
effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution
Parties since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership
Limitation” shall be 4.99% (or, upon election by a Holder prior to the issuance of any Warrants, 9.99%) of the number of
shares of Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of
this Warrant. The Holder, upon notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this
Section 2.5, provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of Common Stock
outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder
and the provisions of this Section 2.5 shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective
until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented
in a manner otherwise than in strict conformity with the terms of this Section 2.5 to correct this paragraph (or any portion hereof)
which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements
necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor
holder of this Warrant.
3.1.
Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend
or otherwise makes a distribution or distributions on shares of Common Stock or any other equity or equity equivalent securities payable
in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise
of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way
of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares
of Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of
which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such
event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number
of Shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant
shall remain unchanged. Any adjustment made pursuant to this Section 3.1 shall become effective
immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become
effective immediately after the effective date in the case of a subdivision, combination or re-classification.
3.2.
Subsequent Equity Sales. If the Company or any Subsidiary thereof, as applicable, at any time while this Warrant
is outstanding, shall sell, enter into an agreement to sell, or grant any option to purchase, or sell, enter into an agreement to sell,
or grant any right to reprice, or otherwise dispose of or issue (or announce any offer, sale, grant or any option to purchase or other
disposition) any shares of Common Stock or Common Stock Equivalents, at an effective price per share less than the Exercise Price then
in effect (such lower price, the “Base Share Price” and such issuances collectively, a “Dilutive
Issuance”) (it being understood and agreed that if the holder of the shares of Common Stock or share of Common Stock Equivalents
or such other securities so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion,
exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance,
be entitled to receive shares of Common Stock at an effective price per share that is less than the Exercise Price, such issuance shall
be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance at such effective price), then simultaneously
with the consummation (or, if earlier, the announcement (provided such Dilutive Issuance occurs)) of each Dilutive Issuance the Exercise
Price shall be reduced and only reduced to equal the Base Share Price, provided that the Base Share Price shall not be less than (i) $[●]
or (ii) in the event of Stockholder Approval, the price of the Dilutive Issuance (the “Floor Price”) (subject
to adjustment for reverse and forward stock splits, recapitalizations and similar transactions following the date of the Underwriting
Agreement). Notwithstanding the foregoing, (i) no adjustments shall be made, paid or issued under this Section 3.2 in respect of an Exempt
Issuance and (ii) if one or more Dilutive Issuances occurred prior to the Stockholder Approval being obtained and the reduction of the
Exercise Price was limited by clause (i) of the definition of Floor Price, once the Stockholder Approval is obtained, the Exercise Price
will automatically be reduced to equal the greater of (x) the lowest Base Share Price with respect to any Dilutive Issuance that occurred
prior to the Stockholder Approval being obtained, and (y) the price determined by reference to clause (ii) of the definition of Floor
Price. The Company shall notify the Holder, in writing, no later than the Trading Day following the issuance or deemed issuance of any
shares of Common Stock or share of Common Stock Equivalents subject to this Section 3(b), indicating therein the applicable issuance price,
or applicable reset price, exchange price, conversion price and other pricing terms (such notice, the “Dilutive Issuance Notice”).
For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 3(b), upon the
occurrence of any Dilutive Issuance, the Holder is entitled to receive a number of Warrant Shares based upon the Base Share Price regardless
of whether the Holder accurately refers to the Base Share Price in the Notice of Exercise. If the Company enters into a Variable Rate
Transaction, the Company shall be deemed to have issued shares of Common Stock or share of Common Stock Equivalents at the lowest possible
price, conversion price or exercise price at which such securities may be issued, converted or exercised.
3.3.
Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3.1 above, if at any time the
Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata
to all (or substantially all) of the record holders of any class of shares of Common Stock (the “Purchase Rights”),
then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the
Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant
(without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately
before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the
date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights
(provided, however, that, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder
exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent
(or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to
such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding
the Beneficial Ownership Limitation).
3.4.
Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any
dividend or other distribution of its assets (or rights to acquire its assets) to all (or substantially all) holders of shares of Common
Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property
or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction)
(a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall
be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held
the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise
hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for
such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined
for the participation in such Distribution (provided, however, that, to the extent that the Holder’s right to participate in any
such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate
in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to
such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as
its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation). To the extent that this Warrant has not
been partially or completely exercised at the time of such Distribution, such portion of the Distribution shall be held in abeyance for
the benefit of the Holder until the Holder has exercised this Warrant.
3.5.
Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly,
in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company or
any Subsidiary, directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all
or substantially all of its assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer
or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell,
tender or exchange their shares for other securities, cash or property and has been accepted by the holders of 50% or more of the outstanding
Common Stock or 50% or more of the voting power of the common equity of the Company, (iv) the Company, directly or indirectly, in one
or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share
exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v)
the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business
combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another
Person or group of Persons whereby such other Person or group acquires 50% or more of the outstanding shares of Common Stock or 50% or
more of the voting power of the common equity of the Company (each a “Fundamental Transaction”), then, upon
any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable
upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to
any limitation in Section 2.5 on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring
corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”)
receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable
immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2.5 on the exercise of this Warrant).
For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate
Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction,
and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value
of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash
or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration
it receives upon any exercise of this Warrant following such Fundamental Transaction.
Notwithstanding anything to the contrary, in the
event of a Fundamental Transaction, the Company or any Successor Entity (as defined below) shall, at the Holder’s option, exercisable
at any time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction (or, if later, the date of the
public announcement of the applicable Fundamental Transaction), purchase this Warrant from the Holder by paying to the Holder an amount
of cash equal to the Black Scholes Value (as defined below) of the remaining unexercised portion of this Warrant on the date of the consummation
of such Fundamental Transaction; provided, however, that, if the Fundamental Transaction is not within the Company’s control, including
not approved by the Company’s Board of Directors, the Holder shall only be entitled to receive from the Company or any Successor
Entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of this
Warrant, that is being offered and paid to the holders of Common Stock of the Company in connection with the Fundamental Transaction,
whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of Common Stock are given
the choice to receive from among alternative forms of consideration in connection with the Fundamental Transaction; provided, further,
that if holders of Common Stock of the Company are not offered or paid any consideration in such Fundamental Transaction, such holders
of Common Stock will be deemed to have received common stock/shares of the Successor Entity (which Entity may be the Company following
such Fundamental Transaction) in such Fundamental Transaction. “Black Scholes Value” means the value of this
Warrant based on the Black-Scholes Option Pricing Model obtained from the “OV” function on Bloomberg, L.P. (“Bloomberg”)
determined as of the day of consummation of the applicable contemplated Fundamental Transaction for pricing purposes and reflecting (A)
a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement
of the applicable contemplated Fundamental Transaction and the Termination Date, (B) an expected volatility equal to the greater of (1)
100% and (2) the 100 day volatility as obtained from the HVT function on Bloomberg (determined utilizing a 365 day annualization factor)
as of the Trading Day immediately following the public announcement of the applicable contemplated Fundamental Transaction, (C) the underlying
price per share used in such calculation shall be the greater of (i) the sum of the price per share being offered in cash, if any, plus
the value of any non-cash consideration, if any, being offered in such Fundamental Transaction and (ii) the highest VWAP during the period
beginning on the Trading Day immediately preceding the public announcement of the applicable contemplated Fundamental Transaction (or
the consummation of the applicable Fundamental Transaction, if earlier) and ending on the Trading Day of the Holder’s request pursuant
to this Section 3.7 and (D) a remaining option time equal to the time between the date of the public announcement of the applicable
contemplated Fundamental Transaction and the Termination Date and (E) a zero cost of borrow. The payment of the Black Scholes Value will
be made by wire transfer of immediately available funds (or such other consideration) within the later of (i) five (5) Business Days after
the Holder’s election and (ii) the date of consummation of the Fundamental Transaction. The Company shall cause any successor entity
in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in
writing all of the obligations of the Company under this Warrant in accordance with the provisions of this Section 3.7 pursuant to
written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay)
prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security
of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant that is exercisable
for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common
Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior
to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock
(but taking into account the relative value of the shares of Common Stock prior to such Fundamental Transaction and the value of such
shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic
value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in
form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the
term “Company” under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction,
each and every provision of this Warrant referring to the “Company” shall refer instead to each of the Company and the Successor
Entity or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company,
may exercise every right and power of the Company prior thereto and the Successor Entity or Successor Entities shall assume all of the
obligations of the Company prior thereto under this Warrant with the same effect as if the Company and such Successor Entity or Successor
Entities, jointly and severally, had been named as the Company herein. For the avoidance of doubt, the Holder shall be entitled to the
benefits of the provisions of this Section 3.7 regardless of (i) whether the Company has sufficient authorized shares of Common Stock
for the issuance of Warrant Shares and/or (ii) whether a Fundamental Transaction occurs prior to the Initial Exercise Date.
3.6.
Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th
of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding
as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
3.7.
Notice to Holder.
3.7.1.
Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3,
the Company shall promptly deliver to the Holder by email a notice setting forth the Exercise Price after such adjustment and any resulting
adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
3.7.2.
Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever
form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock,
(C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares
of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with
any reclassification of the Common Stock, any consolidation or merger to which the Company (or any of its Subsidiaries) is a party, any
sale or transfer of all or substantially all of its assets, or any compulsory share exchange whereby the Common Stock is converted into
other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding
up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by email to the Holder at its last email
address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective
date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution,
redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be
entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification,
consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected
that holders of the Common Stock of record shall be entitled to exchange their shares of Common Stock for securities, cash or other property
deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver
such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified
in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding
the Company or any of the Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report
on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the
effective date of the event triggering such notice except as may otherwise be expressly set forth herein.
3.8.
Share Combination Event Adjustment. In addition to the adjustments set forth in Section 3.1 above, if at any
time and from time to time on or after the Issuance Date there occurs any share split, share dividend, share combination recapitalization
or other similar transaction involving the Common Stock (each, a “Share Combination Event”, and such date thereof,
the “Share Combination Event Date”) and the lowest VWAP during the period commencing Five (5) consecutive Trading
Days immediately preceding and the Five (5) consecutive Trading Days immediately following the Share Combination Event Date (the “Event
Market Price”) (provided if the Share Combination Event is effective after close of Trading on the primary Trading Market,
then commencing on the next Trading Day which period shall be the “Share Combination Adjustment Period”) is
less than the Exercise Price then in effect (after giving effect to the adjustment in clause 3.1 above), then at the close of trading
on the primary Trading Market on the last day of the Share Combination Adjustment Period, the Exercise Price then in effect on such Fifth
(5th) Trading Day shall be reduced (but in no event increased) to the Event Market Price and the number of Warrant Shares issuable hereunder
shall be increased such that the aggregate Exercise Price of this Warrant on the Issuance Date for the Warrant Shares then outstanding
shall remain unchanged. For the avoidance of doubt, if the adjustment in the immediately preceding sentence would otherwise result in
an increase in the Exercise Price hereunder, no adjustment shall be made, and if this Warrant is exercised, on any given Exercise Date
during the Share Combination Adjustment Period, solely with respect to such portion of this Warrant exercised on such applicable Exercise
Date, such applicable Share Combination Adjustment Period shall be deemed to have ended on, and included, the Trading Day immediately
prior to such Exercise Date and the Event Market Price on such applicable Exercise Date will be the lowest VWAP of the Common Stock immediately
during such the Share Combination Adjustment Period prior to such Exercise Date and ending on, and including the Trading Day immediately
prior to such Exercise Date.
3.9.
Voluntary Adjustment by Company. Subject to the rules and regulations of the Trading Market and the consent of the
Holders of a majority in interest of the Warrants then outstanding, the Company may at any time during the term of this Warrant reduce
the then current Exercise Price to any amount and for any period of time deemed appropriate by the Board of Directors.
3.10.
Stockholder Approval. The Company shall seek Stockholder Approval (which may also be at the annual meeting of stockholders)
within the time period and the manner provided in the Underwriting Agreement.
3.11.
Reserved.
4.1.
Transferability. Subject to compliance with any applicable securities laws, this Warrant and all rights hereunder
(including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the
principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially in the form
attached hereto as Exhibit 2.4.6 duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer
taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver
a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified
in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned,
and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically
surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this
Warrant to the Company within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning
this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant
Shares without having a new Warrant issued.
4.2.
New Warrants. If this Warrant is not held in global form through DTC (or any successor depositary), this Warrant
may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written
notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject
to compliance with Section 4.1, as to any transfer which may be involved in such division or combination, the Company shall execute
and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice.
All Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this
Warrant except as to the number of Warrant Shares issuable pursuant thereto.
4.3.
Warrant Register. The Warrant Agent shall register this Warrant, upon records to be maintained by the Warrant Agent
for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company
and the Warrant Agent may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise
hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
5.1.
No Rights as Stockholder until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting
rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2.4.1, except
as expressly set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise”
pursuant to Section 2.3 or to receive cash payments pursuant to Section 2.4.1 and Section 2.4.4 herein,
in no event shall the Company be required to net cash settle an exercise of this Warrant.
5.2.
Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence
reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant
Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant,
shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the
Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant
or stock certificate.
5.3.
Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of
any right required or granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the
next succeeding Business Day.
5.4.
Authorized Shares.
5.4.1.
Reservation of Authorized and Unissued Shares. The Company covenants that, during the period the Warrant is outstanding,
it will reserve from its authorized and unissued Common Stock a sufficient
number of shares of Common Stock to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this
Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged
with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take
all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of
any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company
covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon
exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized,
validly issued, fully paid and nonassessable (which means that no further sums are required to be paid by the holders thereof in connection
with the issue thereof) and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than
taxes in respect of any transfer occurring contemporaneously with such issue).
5.4.2.
Noncircumvention. Except and to the extent as waived or consented to by the Holders of a majority in interest of
the Warrants then outstanding, the Company shall not by any action, including, without limitation, amending its certificate of incorporation
or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary
action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith
assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights
of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not
increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in
par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully
paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such
authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the
Company to perform its obligations under this Warrant.
5.4.3.
Authorizations, Exemptions and Consents. Before taking any action that would result in an adjustment in the number
of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or
exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
5.5.
Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Warrant
shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles
of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the
transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers,
shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City
of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of
New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated
hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that
it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient
venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any
such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery)
to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient
service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any
other manner permitted by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant,
the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees
and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. Notwithstanding
the foregoing, nothing in this paragraph shall limit or restrict the federal district court in which a Holder may bring a claim under
the federal securities laws.
5.6.
Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not
registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities
laws.
5.7.
Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part
of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies, notwithstanding
the fact that the right to exercise this Warrant terminates on the Termination Date. No provision of this Warrant shall be construed as
a waiver by the Holder of any rights which the Holder may have under the federal securities laws and the rules and regulations of the
Commission thereunder. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with
any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as
shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of
appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights,
powers or remedies hereunder.
5.8.
Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including,
without limitation, any Notice of Exercise, shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized
overnight courier service, addressed to the Company, at 1177 Avenue of the Americas, 5th Floor, New York, NY 10036, Attention: Steve Slilaty,
Chief Executive Officer, email address: steve.slilaty@sunshinebiopharma.com, or such other email address or address as the Company may
specify for such purposes by notice to the Holders. Any and all notices or other communications or deliveries to be provided by the Company
hereunder shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service addressed
to each Holder at the e-mail address or address of such Holder appearing on the books of the Company. Any notice or other communication
or deliveries hereunder shall be deemed given and effective on the earliest of (i) the time of transmission, if such notice or communication
is delivered via e-mail at the e-mail address set forth in this Section 5.8 prior to 5:30 p.m. (New York City time) on any date,
(ii) the next Trading Day after the time of transmission, if such notice or communication is delivered via e-mail at the e-mail address
set forth in this Section 5.8 on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day,
(iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon
actual receipt by the party to whom such notice is required to be given. To the extent that any notice provided hereunder constitutes,
or contains, material, non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice
with the Commission pursuant to a Current Report on Form 8-K.
5.9.
Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise
this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability
of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the
Company or by creditors of the Company.
5.10.
Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of
damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not
be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive
and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
5.11.
Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced
hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted
assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and
shall be enforceable by the Holder or holder of Warrant Shares.
5.12.
Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the
Company, on the one hand, and the Holders of a majority in interest of the Warrants then outstanding, on the other hand.
5.13.
Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective
and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision
shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining
provisions of this Warrant.
5.14.
Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose,
be deemed a part of this Warrant.
5.15.
Warrant Agent Agreement. If this Warrant is held in global form through DTC (or any successor depositary), this Warrant
is issued subject to the Warrant Agent Agreement. To the extent any provision of this Warrant conflicts with the express provisions of
the Warrant Agent Agreement, the provisions of this Warrant shall govern and be controlling.
********************
[SBFM Investor Registered Series B Warrant Signature
Page Follows]
[SBFM Investor Registered Series B Warrant Signature
Page]
IN WITNESS WHEREOF, the Company
has caused this Registered Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
SUNSHINE BIOPHARMA, INC.
By:______________________________
Name:Steve Slilaty
Its:Chief Executive Officer
Exhibit 2.1
NOTICE OF EXERCISE
To:SUNSHINE
BIOPHARMA, INC.
(1)
The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant
(only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes,
if any.
(2)
Payment shall take the form of (check applicable box):
[ ]in lawful money
of the United States.
[ ]if permitted the cancellation of
such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2.3, to exercise this Warrant
with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2.3.
(3)
Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following
DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE
OF HOLDER]
| Name of Investing Entity: |
|
| Signature of Authorized Signatory of Investing Entity: |
|
| Name of Authorized Signatory: |
|
| Title of Authorized Signatory: |
|
| Date: |
|
Exhibit 2.4.6
ASSIGNMENT FORM
(To assign the foregoing Warrant,
execute this form and supply required information. Do not use this form to exercise the Warrant to purchase shares of Common Stock.)
FOR VALUE RECEIVED, the foregoing
Warrant and all rights evidenced thereby are hereby assigned to
| Name: |
|
| Address: |
|
| Phone Number: |
|
| Email Address: |
|
| Date: |
|
| Holder’s Signature: |
|
| Holder’s Address: |
|
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
We hereby consent to the inclusion in this Registration Statement of
Sunshine Biopharma Inc. on Form S-1/A, of our report dated March 31, 2023, on the financial statements of Sunshine Biopharma Inc. for the
years ended December 31, 2022 and 2021.
In addition, we consent to the reference to us
under the heading “Experts” in the Registration Statement.
/s/ B.F. Borger CPA PC
Certified Public Accountants
Denver, Colorado
February 9, 2024
v3.24.0.1
| X |
- DefinitionDescription of changes contained within amended document.
| Name: |
dei_AmendmentDescription |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe date the document was made available and submitted, in YYYY-MM-DD format. The date of submission, date of acceptance by the recipient, and the document effective date are all potentially different.
| Name: |
dei_DocumentCreationDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Balance Sheets (Unaudited) - USD ($)
|
Sep. 30, 2023 |
Dec. 31, 2022 |
| Current Assets: |
|
|
| Cash and cash equivalents |
$ 18,846,140
|
$ 21,826,437
|
| Accounts receivable |
2,034,119
|
1,912,153
|
| Inventory |
4,517,044
|
3,289,945
|
| Prepaid expenses |
37,556
|
283,799
|
| Total Current Assets |
25,434,859
|
27,312,334
|
| Property and equipment |
334,922
|
394,249
|
| Intangible assets |
1,216,207
|
776,856
|
| Right-of-use-asset |
664,296
|
760,409
|
| TOTAL ASSETS |
27,650,284
|
29,243,848
|
| Current Liabilities: |
|
|
| Accounts payable and accrued expenses |
2,220,870
|
2,802,797
|
| Earnout payable |
2,547,831
|
3,632,000
|
| Income tax payable |
201,541
|
373,191
|
| Right-of-use-liability |
117,840
|
123,026
|
| Total Current Liabilities |
5,088,082
|
6,931,014
|
| Long-Term Liabilities: |
|
|
| Deferred tax liability |
43,032
|
43,032
|
| Right-of-use-liability |
555,687
|
642,232
|
| Total Long-Term Liabilities |
598,719
|
685,264
|
| TOTAL LIABILITIES |
5,686,801
|
7,616,278
|
| SHAREHOLDERS' EQUITY |
|
|
| Preferred Stock, Series B $0.10 par value per share; 1,000,000 shares authorized; 10,000 Shares issued and outstanding |
1,000
|
1,000
|
| Common Stock, $0.001 par value per share; 3,000,000,000 shares authorized; 25,678,290 and 22,585,632 shares issued and outstanding as of September 30, 2023 and December 31, 2022, respectively |
25,678
|
22,585
|
| Capital paid in excess of par value |
84,387,890
|
80,841,752
|
| Accumulated comprehensive income |
204,549
|
161,847
|
| Accumulated (Deficit) |
(62,655,634)
|
(59,399,614)
|
| TOTAL SHAREHOLDERS' EQUITY |
21,963,483
|
21,627,570
|
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY |
$ 27,650,284
|
$ 29,243,848
|
| X |
- References
+ Details
| Name: |
SBFM_EarnoutPayable |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deferred tax asset, of deferred tax liability attributable to taxable differences, with jurisdictional netting, and liabilities classified as noncurrent and other.
| Name: |
us-gaap_DeferredTaxAndOtherLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph ((a)(1),(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of obligation due after one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 23: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Balance Sheets (Unaudited) (Parenthetical) - $ / shares
|
Sep. 30, 2023 |
Dec. 31, 2022 |
| Common stock, par value |
$ 0.001
|
$ 0.001
|
| Common stock, shares authorized |
3,000,000,000
|
3,000,000,000
|
| Common stock, shares issued |
25,678,290
|
22,585,632
|
| Common stock, shares outstanding |
25,678,290
|
22,585,632
|
| Series B Preferred Stock [Member] |
|
|
| Preferred stock, par value |
$ 0.10
|
$ 0.10
|
| Preferred stock, shares authorized |
1,000,000
|
1,000,000
|
| Preferred stock, shares issued |
10,000
|
10,000
|
| Preferred stock, shares outstanding |
10,000
|
10,000
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesBPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Consolidated Statements of Operations and Comprehensive Loss (Unaudited) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Income Statement [Abstract] |
|
|
|
|
| Sales |
$ 5,957,668
|
$ 132,808
|
$ 16,412,586
|
$ 405,760
|
| Cost of sales |
3,967,412
|
65,783
|
10,641,461
|
200,311
|
| Gross profit |
1,990,256
|
67,025
|
5,771,125
|
205,449
|
| General and Administrative Expenses: |
|
|
|
|
| Accounting |
56,350
|
122,913
|
301,381
|
237,773
|
| Consulting |
221,781
|
162,852
|
745,850
|
270,033
|
| Director fees |
100,000
|
100,000
|
300,000
|
200,000
|
| Legal |
133,302
|
146,467
|
392,874
|
403,386
|
| Marketing |
241,897
|
217,666
|
502,987
|
400,386
|
| Office |
544,215
|
76,818
|
1,422,058
|
449,730
|
| R&D |
238,012
|
362,500
|
1,039,502
|
770,095
|
| Salaries |
1,144,377
|
595,000
|
4,344,801
|
1,105,000
|
| Taxes |
52,586
|
0
|
212,953
|
0
|
| Depreciation |
37,210
|
789
|
106,797
|
6,186
|
| Total General and Administrative Expenses: |
2,769,730
|
1,785,005
|
9,369,203
|
3,842,589
|
| (Loss) from operations |
(779,474)
|
(1,717,980)
|
(3,598,078)
|
(3,637,140)
|
| Other Income (Expense): |
|
|
|
|
| Foreign exchange |
40
|
25
|
(206)
|
45
|
| Interest income |
207,431
|
260,938
|
624,361
|
406,984
|
| Debt release |
0
|
0
|
0
|
10,852
|
| Interest expense |
(38,527)
|
(2)
|
(107,198)
|
(12,866)
|
| Total Other Income (Expense) |
168,944
|
260,961
|
516,957
|
405,015
|
| Net (loss) before income taxes |
(610,530)
|
(1,457,019)
|
(3,081,121)
|
(3,232,125)
|
| Provision for income taxes |
(40,952)
|
0
|
(174,899)
|
0
|
| Net (Loss) |
(651,482)
|
(1,457,019)
|
(3,256,020)
|
(3,232,125)
|
| Gain (Loss) from foreign exchange translation |
(460,507)
|
(45,126)
|
42,702
|
(56,764)
|
| Comprehensive (Loss) |
$ (1,111,989)
|
$ (1,502,145)
|
$ (3,213,318)
|
$ (3,288,889)
|
| Basic (Loss) per common share |
$ 0.04
|
$ 0.08
|
$ 0.133
|
$ 0.26
|
| X |
- References
+ Details
| Name: |
SBFM_ConsultingExpense |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_OfficeExpense |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_Taxes |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GeneralAndAdministrativeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income, amortization of premium and accretion of discount, on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), investment in debt security measured at amortized cost (held-to-maturity) and investment in debt security measured at fair value with change in fair value recognized in net income (trading); classified as operating.
| Name: |
us-gaap_InterestIncomeDebtSecuritiesOperating |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense provided in the period for legal costs incurred on or before the balance sheet date pertaining to resolved, pending or threatened litigation, including arbitration and mediation proceedings. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_LegalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionExpenditures for planning and executing the conception, pricing, promotion, and distribution of ideas, goods, and services. Costs of public relations and corporate promotions are typically considered to be marketing costs. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_MarketingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNoninterest expense related to directors' fees which are fees paid by an Entity to its directors. Directors' fees may be paid in addition to salary and other benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.14)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NoninterestExpenseDirectorsFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before tax, after reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionA fee charged for services from professionals such as doctors, lawyers and accountants. The term is often expanded to include other professions, for example, pharmacists charging to maintain a medicinal profile of a client or customer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (k)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
| Name: |
us-gaap_ProfessionalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for salary and wage arising from service rendered by nonofficer employee. Excludes allocated cost, labor-related nonsalary expense, and direct and overhead labor cost included in cost of good and service sold. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SalariesAndWages |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Statements of Operations and Comprehensive Loss (Unaudited) (Parenthetical) - shares
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Income Statement [Abstract] |
|
|
|
|
| Weighted average common shares outstanding (Basic) |
25,690,449
|
18,885,632
|
24,507,122
|
12,789,733
|
| Weighted average common shares outstanding (Diluted) |
25,690,449
|
18,885,632
|
24,507,122
|
12,789,733
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Statements of Cash Flows (Unaudited) - USD ($)
|
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Cash Flows From Operating Activities: |
|
|
| Net (Loss) |
$ (3,256,020)
|
$ (3,232,125)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Depreciation and amortization |
106,794
|
6,186
|
| Foreign exchange |
(374)
|
45
|
| Debt release |
0
|
(10,852)
|
| Accounts receivable |
(118,482)
|
7,776
|
| Inventory |
(1,221,112)
|
(163,991)
|
| Prepaid expenses |
247,977
|
2,235
|
| Accounts payable and accrued expenses |
(587,973)
|
437,267
|
| Income tax payable |
(172,076)
|
0
|
| Interest payable |
(1,084,169)
|
(48,287)
|
| Net Cash Flows (Used) in Operations |
(6,085,435)
|
(3,001,746)
|
| Cash Flows From Investing Activities: |
|
|
| Reduction in Right-of-use asset |
97,498
|
0
|
| Purchase of intangible assets |
(19,804)
|
0
|
| Purchase of equipment |
(464,614)
|
0
|
| Net Cash Flows (Used) in Investing Activities |
(386,920)
|
0
|
| Cash Flows From Financing Activities: |
|
|
| Common stock issued |
4,089,218
|
43,560,363
|
| Exercise of warrants |
1,156
|
0
|
| Purchase of treasury stock |
(541,143)
|
(99,000)
|
| Lease liability |
(93,125)
|
0
|
| Payments of notes payable |
0
|
(1,900,000)
|
| Net Cash Flows Provided by Financing Activities |
3,456,106
|
41,561,363
|
| Cash and Cash Equivalents at Beginning of Period |
21,826,437
|
2,045,167
|
| Net increase (decrease) in cash and cash equivalents |
(3,016,249)
|
38,559,617
|
| Effect of exchange rate changes on cash |
0
|
(105,617)
|
| Foreign currency translation adjustment |
35,952
|
56,764
|
| Cash and Cash Equivalents at End of Period |
18,846,140
|
40,555,931
|
| Supplementary Disclosure of Cash Flow Information: |
|
|
| Cash paid for interest |
0
|
61,151
|
| Cash paid for income taxes |
$ 0
|
$ 0
|
| X |
- References
+ Details
| Name: |
SBFM_PaymentForLeaseLiability |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_PurchaseOfTreasuryStock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_ReductionInRightOfUseAsset |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe current period expense charged against earnings on long-lived, physical assets not used in production, and which are not intended for resale, to allocate or recognize the cost of such assets over their useful lives; or to record the reduction in book value of an intangible asset over the benefit period of such asset; or to reflect consumption during the period of an asset that is not used in production. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_DepreciationAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in interest payable, which represents the amount owed to note holders, bond holders, and other parties for interest earned on loans or credit extended to the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInterestPayableNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to acquire asset without physical form usually arising from contractual or other legal rights, excluding goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for a borrowing supported by a written promise to pay an obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of net unrealized gain (loss) related to the change in fair value of foreign currency exchange rate derivatives designated as cash flow hedging instruments. Recorded in accumulated other comprehensive income to the extent that the cash flow hedge is determined to be effective. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 815
-SubTopic 10
-Section 50
-Paragraph 4C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480434/815-10-50-4C
| Name: |
us-gaap_UnrealizedGainLossOnForeignCurrencyDerivativesNetBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Statement of Shareholders' Equity (Unaudited) - USD ($)
|
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Preferred Stock [Member] |
Comprehensive Income [Member] |
Retained Earnings [Member] |
Total |
| Beginning balance, value at Dec. 31, 2021 |
$ 2,596
|
$ 32,787,379
|
$ 100,000
|
$ (23,139)
|
$ (32,655,174)
|
$ 211,662
|
| Shares, Outstanding, Beginning Balance at Dec. 31, 2021 |
2,595,620
|
|
1,000,000
|
|
|
|
| Common stock and pre-funded warrants issued in public offering |
$ 6,657
|
30,360,528
|
|
|
|
30,367,185
|
| [custom:CommonStockAndPrefundedWarrantsIssuedInPrivateOfferingShares] |
6,656,526
|
|
|
|
|
|
| Exercise of warrants |
$ 9,633
|
13,183,544
|
|
|
|
13,193,177
|
| Stock Issued During Period, Shares, Conversion of Convertible Securities |
9,633,486
|
|
|
|
|
|
| Preferred stock purchased from related party |
|
|
$ (99,000)
|
|
|
(99,000)
|
| Stock Repurchased During Period, Shares |
|
|
(990,000)
|
|
|
|
| Net (loss) |
|
|
|
(56,764)
|
(3,232,125)
|
(3,288,889)
|
| Ending balance, value at Sep. 30, 2022 |
$ 18,886
|
76,331,451
|
$ 1,000
|
(79,903)
|
(35,887,299)
|
40,384,135
|
| Shares, Outstanding, Ending Balance at Sep. 30, 2022 |
18,885,632
|
|
10,000
|
|
|
|
| Beginning balance, value at Jun. 30, 2022 |
$ 18,886
|
76,331,451
|
$ 1,000
|
(34,777)
|
(34,430,280)
|
41,886,280
|
| Shares, Outstanding, Beginning Balance at Jun. 30, 2022 |
18,885,632
|
|
10,000
|
|
|
|
| Net (loss) |
|
|
|
(45,126)
|
(1,457,019)
|
(1,502,145)
|
| Ending balance, value at Sep. 30, 2022 |
$ 18,886
|
76,331,451
|
$ 1,000
|
(79,903)
|
(35,887,299)
|
40,384,135
|
| Shares, Outstanding, Ending Balance at Sep. 30, 2022 |
18,885,632
|
|
10,000
|
|
|
|
| Beginning balance, value at Dec. 31, 2022 |
$ 22,585
|
80,841,752
|
$ 1,000
|
161,847
|
(59,399,614)
|
21,627,570
|
| Shares, Outstanding, Beginning Balance at Dec. 31, 2022 |
22,585,632
|
|
10,000
|
|
|
|
| Repurchase of common stock |
$ (514)
|
(540,629)
|
|
|
|
|
| [custom:RepurchaseOfCommonStockShares] |
(513,723)
|
|
|
|
|
|
| Common stock and pre-funded warrants issued in a private offering |
$ 2,451
|
4,086,767
|
|
|
|
4,089,218
|
| Stock Issued During Period, Shares, New Issues |
2,450,000
|
|
|
|
|
|
| Exercise of warrants |
$ 1,156
|
|
|
|
|
1,156
|
| Stock Issued During Period, Shares, Conversion of Convertible Securities |
1,156,381
|
|
|
|
|
|
| Stock Repurchased During Period, Shares |
445,711
|
|
|
|
|
|
| Net (loss) |
|
|
|
42,702
|
(3,256,020)
|
(3,213,318)
|
| Ending balance, value at Sep. 30, 2023 |
$ 25,678
|
84,387,890
|
$ 1,000
|
204,549
|
(62,655,634)
|
21,963,483
|
| Shares, Outstanding, Ending Balance at Sep. 30, 2023 |
25,678,290
|
|
10,000
|
|
|
|
| Beginning balance, value at Jun. 30, 2023 |
$ 25,746
|
84,422,143
|
$ 1,000
|
665,056
|
(62,004,152)
|
23,109,793
|
| Shares, Outstanding, Beginning Balance at Jun. 30, 2023 |
25,746,302
|
|
10,000
|
|
|
|
| Repurchase stock |
$ (68)
|
(34,253)
|
|
|
|
|
| [custom:RepurchaseStockShares] |
(68,012)
|
|
|
|
|
|
| Net (loss) |
|
|
|
(460,507)
|
(651,482)
|
(1,111,989)
|
| Ending balance, value at Sep. 30, 2023 |
$ 25,678
|
$ 84,387,890
|
$ 1,000
|
$ 204,549
|
$ (62,655,634)
|
$ 21,963,483
|
| Shares, Outstanding, Ending Balance at Sep. 30, 2023 |
25,678,290
|
|
10,000
|
|
|
|
| X |
- References
+ Details
| Name: |
SBFM_CommonStockAndPrefundedWarrantsIssuedInPrivateOfferingShares |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_CommonStockAndPrefundedWarrantsIssuedInPublicOffering |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_RepurchaseOfCommonStock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_RepurchaseOfCommonStockShares |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_RepurchaseStock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_RepurchaseStockShares |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of stock that has been repurchased during the period and has not been retired and is not held in treasury. Some state laws may mandate the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Description of Business
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Description of Business |
Note
1 – Description of Business
The
Company was originally incorporated under the name Mountain West Business Solutions, Inc. on August 31, 2006, in the State of Colorado.
Effective October 15, 2009, the Company acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition. Upon
completion of the reverse acquisition transaction, the Company changed its name to Sunshine Biopharma, Inc. and began operating as a
pharmaceutical company.
In
addition to conducting its own drug development activities, Sunshine Biopharma operates two wholly owned subsidiaries: (i) Nora Pharma
Inc. (“Nora Pharma”), a Canadian corporation with a portfolio of pharmaceutical products consisting of 51 generic prescription
drugs on the market in Canada, and (ii) Sunshine Biopharma Canada Inc. (“Sunshine Canada”), a Canadian corporation which
develops and sells nonprescription over-the-counter (“OTC”) products. In addition to the 51 generic prescription drugs currently
on the market in Canada, the Company has 32 additional generic prescription drugs scheduled to be launched in 2024 and 2025 in Canada.
The
Company has determined that it has two reportable segments:
| |
· |
Prescription
Generic Pharmaceuticals (“Generic Pharmaceuticals”) |
| |
· |
Nonprescription
Over-The-Counter Products (“OTC Products) |
Through December 31, 2022 and as of September
30, 2023, sales from the Generic Pharmaceuticals segment represented approximately 97% of total revenues of the Company while the remaining
approximately 3% was generated from the sale of OTC Products. Based on these results, the Company deems segmentation reporting to be immaterial
at September 30, 2023.
The Company is not subject to material customer
concentration risks as it sells its products directly to pharmacies in several Canadian Provinces. Provincial governments in Canada reimburse
patients for their prescription drugs expenditures to various degrees under drug reimbursement programs, making generic drugs prices
highly dependent on government regulations which may change over time. The most recent negotiations between the pan-Canadian Pharmaceutical
Alliance and the Canadian Generic Pharmaceutical Association have resulted in updated generic pricing for certain products which took
effect on October 1, 2023. The updated prices are valid for three years and the agreement contains an option to extend for an additional
two years.
In
addition, the Company is engaged in the development of the following proprietary drugs:
| · | Adva-27a,
a small chemotherapy molecule for treatment of pancreatic cancer (IND-enabling studies were paused on November 2, 2023 due to unfavorable
results. See Note 13 – Subsequent Events) |
| · | K1.1
mRNA, a lipid nano-particle (LNP) targeted for liver cancer |
| · | SBFM-PL4,
a protease inhibitor for treatment of Coronavirus infections |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Basis of Presentation
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation |
Note
2 – Basis of Presentation
The
unaudited financial statements of the Company for the nine months periods ended September 30, 2023 and 2022 have been prepared in accordance
with accounting principles generally accepted in the United States of America for interim financial information and pursuant to the requirements
for reporting on Form 10-Q and Regulation S-X. Accordingly, they do not include all the information and footnotes required by accounting
principles generally accepted in the United States of America for complete financial statements. However, such information reflects all
adjustments (consisting solely of normal recurring adjustments), which are, in the opinion of management, necessary for the fair presentation
of the financial position and the results of operations. Results shown for interim periods are not necessarily indicative of the results
to be obtained for a full fiscal year. The balance sheet information as of December 31, 2022, was derived from the audited financial statements
included in the Company's financial statements as of and for the year ended December 31, 2022, included in the Company’s Annual
Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on April 4, 2023. These financial statements
should be read in conjunction with that report.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Private Placement
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Private Placement |
|
| Private Placement |
Note
3 – Private Placement
On
May 16, 2023, the Company completed a private placement pursuant to a securities purchase agreement with a single institutional investor for gross proceeds of approximately $5 million, before deducting fees to the placement agent and other offering
expenses payable by the Company. The net proceeds received by the Company were $4,089,218.
In
connection with the private placement, the Company issued (i) 2,450,000
shares of common stock, (ii) 3,502,381
pre-funded warrants (the “May Pre-Funded Warrants”),
and (iii) investor warrants (the “May Investor Warrants”) to purchase up to 11,904,762 shares of common stock at
$0.59 per share. Each share of common stock and accompanying two May Investor Warrants were sold together at a combined offering price
of $0.84 and each May Pre-Funded Warrant and accompanying two May Investor Warrants were sold together at a combined offering price of
$0.839. The May Pre-Funded Warrants are immediately exercisable at a nominal exercise price of $0.001, and may be exercised at any time
until all of the May Pre-Funded Warrants are exercised in full. The May Investor Warrants which have an exercise price of $0.59 per share
(subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the date of issuance.
As of September 30, 2023, a total of 1,156,381
May Pre-Funded Warrants and no May Investor Warrants have been exercised. The net proceeds received from the exercise of May Pre-Funded
Warrants were $1,156.
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosurePrivatePlacementAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_PrivatePlacementTextBlock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Acquisition of Nora Pharma Inc.
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Business Combination and Asset Acquisition [Abstract] |
|
| Acquisition of Nora Pharma Inc. |
Note
4 – Acquisition of Nora Pharma Inc.
On
October 20, 2022, the Company acquired all of the issued and outstanding shares of Nora Pharma Inc. The purchase price for the
shares was $18,860,637
(USD), $14,346,637
of which was paid in cash and the remainder was paid through the issuance of 3,700,000
shares of the Company’s common stock valued at $4,514,000
or $1.22 per share. Nora Pharma sells generic pharmaceutical products in Canada. Nora Pharma’s operations are authorized by a
Drug Establishment License issued by Health Canada.
The
following table summarizes the allocation of the purchase price as of October 20, 2022, the acquisition date using Nora Pharma’s
balance sheet assets and liabilities:
| Schedule of allocation of purchase price | |
| |
| Accounts receivable | |
$ | 1,358,121 | |
| Inventory | |
| 3,181,916 | |
| Intangible assets | |
| 659,571 | |
| Equipment & furniture | |
| 210,503 | |
| Other assets | |
| 1,105,093 | |
| Total assets | |
| 6,515,204 | |
| Liabilities assumed | |
| (5,981,286 | ) |
| Net assets | |
| 533,918 | |
| Goodwill | |
| 18,326,719 | |
| Total Consideration | |
$ | 18,860,637 | |
The
value of the 3,700,000 common shares issued as part of the consideration paid for Nora Pharma was determined based on the closing market
price of the Company’s common shares on the acquisition date, October 20, 2022 ($1.22 per share).
The
Company impaired 100% of the goodwill amount in 2022 and plans to depreciate the intangible assets as detailed in Note 5 below.
As
part of the consideration paid for Nora Pharma, the Company agreed to a $5,000,000 CAD ($3,632,000 USD) earnout amount payable to Mr.
Malek Chamoun, the Seller of Nora Pharma. The earnout is payable in the form of twenty (20) payments of $250,000 CAD for every $1,000,000
CAD increase in gross sales (as defined in the Purchase Agreement) above Nora Pharma’s June 30, 2022 gross sales, provided that
his employment with the Company is not terminated pursuant to the Company’s employment agreement with him. The total earnout amount
of $3,632,000 has been recorded as a salary payable. During the nine-month period ended September 30, 2023, the Company paid an earn-out
amount of $1,084,169 leaving a balance earn-out to be paid of $2,547,831 at September 30, 2023.
The
unaudited financial information in the table below summarizes the combined results of operations of the Company and Nora Pharma for the
years ended December 31, 2022 and 2021, on a pro forma basis, as though the two companies had been combined as of January 1, 2021. The
unaudited pro forma financial information does not purport to be indicative of the Company's combined results of operations which would
have been obtained had the acquisition taken place on January 1, 2021, nor should it be taken as indicative of future consolidated results
of operations:
| Schedule of Pro Forma results from acquisition | |
| | |
| |
| Pro Forma Results
From Acquisition | |
December
31,
2022 | | |
December
31,
2021 | |
| Total revenues | |
$ | 14,758,115 | | |
$ | 7,927,165 | |
| Net (loss) from operations | |
$ | (26,192,503 | ) | |
$ | (2,224,253 | ) |
| Net (loss) | |
$ | (26,164,764 | ) | |
$ | (12,289,655 | ) |
| Basic and fully diluted (loss) per share | |
$ | (1.74 | ) | |
$ | (4.70 | ) |
| Weighted average number of shares outstanding | |
| 15,056,097 | | |
| 2,612,061 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_BusinessCombinationAndAssetAcquisitionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for business combinations, including leverage buyout transactions (as applicable), and divestitures. This may include a description of a business combination or divestiture (or series of individually immaterial business combinations or divestitures) completed during the period, including background, timing, and assets and liabilities recognized and reclassified or sold. This element does not include fixed asset sales and plant closings. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 805
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//805/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205-20/tableOfContent
| Name: |
us-gaap_MergersAcquisitionsAndDispositionsDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Intangible Assets
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Goodwill and Intangible Assets Disclosure [Abstract] |
|
| Intangible Assets |
Note
5 – Intangible Assets
Intangible
assets, net, consisted of the following at September 30, 2023:
| Schedule of intangible assets | |
| | |
| Balance June 30, 2023 | |
$ | 1,233,570 | |
| Dossier fee additions | |
| 13,905 | |
| Balance at September 30, 2023 | |
| 1,247,475 | |
| Less accumulated amortization | |
| (31,268 | ) |
| Finite-lived intangible
assets, net, at September 30, 2023 | |
$ | 1,216,207 | |
| | |
| | |
| Balance December 31, 2022 | |
$ | 776,856 | |
| Dossier fee additions | |
| 470,619 | |
| Balance at September 30, 2023 | |
| 1,247,475 | |
| Less accumulated amortization | |
| (31,268 | ) |
| Finite-lived intangible
assets, net, at September 30, 2023 | |
$ | 1,216,207 | |
Amortization
expense for the three months period ended September 30, 2023, and the nine months period ended September 30, 2023, amounted to $10,797
and $26,746, respectively.
As
of September 30, 2023, estimated amortization expense of the Company’s intangible assets for each of the next five years is as follows:
| Schedule of estimated amortization expense | |
| | |
| 2024 | |
$ | 55,418 | |
| 2025 | |
| 55,418 | |
| 2026 | |
| 54,240 | |
| 2027 | |
| 15,599 | |
| 2028 | |
| 7,370 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all or part of the information related to intangible assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350-30/tableOfContent
| Name: |
us-gaap_IntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Reverse Stock Splits
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Reverse Stock Splits |
|
| Reverse Stock Splits |
Note
6 – Reverse Stock Splits
Effective
February 9, 2022, the Company completed a 1 for 200 reverse split of its common stock. The Company had previously completed two 20 to
1 reverse stock splits, one in 2019 and the other in 2020. The Company’s financial statements reflect all three reverse stock splits
on a retroactive basis for all periods presented and for all references to common stock, unless specifically stated otherwise.
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureReverseStockSplitsAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_ReverseStockSplitTextBlock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Capital Stock
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Equity [Abstract] |
|
| Capital Stock |
Note
7 – Capital Stock
The
Company’s authorized capital is comprised of 3,000,000,000
shares of common stock, par value $0.001,
and 30,000,000
shares of preferred stock, $0.10
par value. As of December 31, 2022 and September 30, 2023, the Company had authorized 1,000,000
shares of Series B Preferred Stock. The Series B Preferred Stock is non-convertible, non-redeemable and non-retractable. It has
superior liquidation rights to the common stock at $0.10 per share and gives the holder the right to 1,000 votes per share. As of
September 30, 2023 and December 31, 2022, 10,000
shares of Series B Preferred Stock are outstanding and held by the Company’s chief executive officer.
On
February 17, 2022, the Company completed a public offering and received net proceeds of $6,833,071 from the offering. Pursuant to the
public offering, the Company issued and sold an aggregate of 1,882,353 shares of common stock and 4,102,200 warrants to purchase shares
of common stock (the “Tradeable Warrants”).
On
February 22, 2022, the Company redeemed 990,000
shares of Series B Preferred Stock from the CEO of the Company at a redemption price equal to the stated value of $0.10 per share.
The remaining 10,000 shares of Series B Preferred Stock could not be voted pursuant to a warrant agent agreement relating to the
Tradeable Warrants (the “Warrant Agent Agreement”). On October 12, 2023, the Company held a special meeting of the
holders of the outstanding Tradeable Warrants in which the holders of the majority of the outstanding Tradeable Warrants approved an
amendment to the Warrant Agent Agreement to eliminate the provision that prohibited the Company’s CEO from exercising his
voting rights under the Series B Preferred Stock, as well as to lower the exercise price of the Tradeable Warrants to $0.11. The
Company entered into the amendment to the Warrant Agent Agreement on October 18, 2023.
On
March 14, 2022, the Company completed a private placement and received net proceeds of $6,781,199. In connection with this private placement,
the Company issued (i) 2,301,353 shares of its common stock together with investor warrants (“Investor Warrants”) to
purchase up to 2,301,353 shares of common stock, and (ii) 1,302,251 pre-funded warrants (“Pre-Funded Warrants”) with
each Pre-Funded Warrant exercisable for one share of common stock, together with Investor Warrants to purchase up to 1,302,251 shares
of common stock. Each share of common stock and accompanying Investor Warrant was sold together at a combined offering price of $2.22
and each Pre-Funded Warrant and accompanying Investor Warrant were sold together at a combined offering price of $2.219. The Pre-Funded
Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded
Warrants are exercised in full. The Investor Warrants have an exercise price of $2.22 per share (subject to adjustment as set forth in
the warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On
April 28, 2022, the Company completed another private placement and received net proceeds of $16,752,915. In connection with this private
placement, the Company issued (i) 2,472,820 shares of its common stock together with warrants (“April Warrants”) to
purchase up to 4,945,640 shares of common stock, and (ii) 2,390,025 pre-funded warrants (“Pre-Funded Warrants”)
with each Pre-Funded Warrant exercisable for one share of common stock, together with April Warrants to purchase up to 4,780,050 shares
of common stock. Each share of common stock and accompanying two April Warrants were sold together at a combined offering price of $4.01
and each Pre-Funded Warrant and accompanying two April Warrants were sold together at a combined offering price of $4.009. The Pre-Funded
Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded
Warrants are exercised in full. The April Warrants have an exercise price of $3.76 per share (subject to adjustment as set forth in the
warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On
October 20, 2022, the Company issued 3,700,000 shares of common stock as part of the acquisition of Nora Pharma. These shares were valued
at $4,514,000, or $1.22 per share.
On
January 19, 2023, the Company announced a stock repurchase program of up to $2 million (“Stock Repurchase
Program”). During the six months ended June 30, 2023, the Company repurchased a total of 445,711 shares of common stock at an average
price of $1.1371 per share for a total cost of $506,822. The 445,711 repurchased common shares were cancelled and returned to treasury
reducing the number of issued and outstanding shares from 22,585,632 to 22,139,921.
On
May 16, 2023, the Company completed a private placement pursuant to a securities purchase agreement with a single institutional investor
for gross proceeds of approximately $5
million, before deducting fees to the placement agent and other
offering expenses payable by the Company. The net proceeds received by the Company were $4,089,218.
In connection with the private placement, the Company issued (i) 2,450,000
shares of common stock, (ii) 3,502,381
pre-funded warrants (the “May Pre-Funded Warrants”),
and (iii) investor warrants (the “May Investor Warrants”) to purchase up to 11,904,762 shares of common stock at
$0.59 per share. Each share of common stock and accompanying two May Investor Warrants were sold together at a combined offering price
of $0.84 and each May Pre-Funded Warrant and accompanying two May Investor Warrants were sold together at a combined offering price of
$0.839. The May Pre-Funded Warrants are immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time
until all of the May Pre-Funded Warrants are exercised in full. The May Investor Warrants which have an exercise price of $0.59 per share
(subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the date of issuance.
In
2022 and the first six months of 2023, the Company issued a total of 10,789,867 shares of common stock in connection with warrant exercises
for aggregate net proceeds of $13,194,335.
In
July 2023, the Company repurchased a total of 68,012 shares of common stock on the open market under the Stock Repurchase Program announced
on January 19, 2023, at an average price of $0.5046 per share for a total cost of $34,321. In October 2023, the 68,012 repurchased common
shares were cancelled and returned to treasury reducing the number of issued and outstanding shares from 25,746,302 to 25,678,290.
As
of September 30, 2023 and December 31, 2022, the Company has a total of 25,678,290 and 22,585,632 shares of common stock issued and outstanding,
respectively.
The
Company has declared no dividends since inception.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Warrants
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Warrants |
|
| Warrants |
Note
8 – Warrants
The
Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10 or ASC 815-40. Under ASC 480-10, warrants
are considered a liability if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number
of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to
determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement
for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are
measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after
the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification
under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed
to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP standard. Equity-classified
warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
In
2022 and during the first nine months of 2023, the Company completed four financing events, and in connection therewith, it
issued warrants as follows:
| Schedule of warrants issued with financing |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise Price |
|
Expiry Date |
| Pre-Funded Warrants |
|
3,692,276 |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
4,102,200 |
|
$2.22* |
|
February 2027 |
| Investor Warrants |
|
3,603,604 |
|
$2.22 |
|
March 2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April 2027 |
| May Pre-Funded Warrants |
|
3,502,381 |
|
$0.001 |
|
Unlimited |
| May Investor Warrants |
|
11,904,762 |
|
$0.59 |
|
November 2028 |
| * |
The
Tradeable Warrants had an initial exercise price of $4.25, subject to adjustment. Upon the closing of the Company's private placement
on March 14, 2022, the exercise price of the Tradeable Warrants was reduced to $2.22, in accordance with the terms thereof. |
As
of September 30, 2023, all of the Pre-Funded Warrants and a total of 3,138,507 Tradeable Warrants, 2,802,703 Investor Warrants, and 1,156,381
May Pre-Funded Warrants were exercised resulting in aggregate proceeds of $13,194,335 received by the Company.
The Company’s
outstanding warrants at September 30, 2023 consisted of the following:
| Schedule of outstanding warrants |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise Price |
|
Expiry Date |
| Pre-Funded Warrants |
|
None |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
963,693 |
|
$2.22* |
|
February 2027 |
| Investor Warrants |
|
800,901 |
|
$2.22 |
|
March 2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April 2027 |
| May Pre-Funded Warrants |
|
2,346,000 |
|
$0.001 |
|
Unlimited |
| May Investor Warrants |
|
11,904,762 |
|
$0.59 |
|
November 2028 |
| * |
On
October 12, 2023, the Company held a special meeting of the holders of its outstanding Tradeable Warrants in which a majority of the
holders approved an amendment to the Warrant Agent Agreement to reduce the exercise price of the Tradeable Warrants to $0.11 per warrant.
The amendment was executed on October 18, 2023. |
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureWarrantsAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantsDisclosureTextBlock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Net Loss Per Common Share
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Earnings Per Share [Abstract] |
|
| Net Loss Per Common Share |
Note
9 – Net Loss Per Common Share
Basic
net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during
the period, without consideration for common stock equivalents.
Diluted
net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during
the period, taking into consideration common stock equivalents.
In
February 2022, the Company issued 4,102,200 Tradeable Warrants pursuant to the Company’s Public Offering. In March and April 2022,
the Company issued 3,603,604 Investor Warrants and 9,725,690 April Warrants pursuant to two private placements. In May 2023, the Company
issued 11,904,762 May Investor Warrants pursuant to two private placements. As of September 30, 2023, 3,138,507 Tradeable Warrants and
2,802,703 Investor Warrants were exercised, leaving 963,693 Tradeable Warrants, 800,901 Investor Warrants, 9,725,690 April Warrants,
and 11,904,762 May Investor Warrants outstanding. These warrants are dilutive and were included in the diluted earnings per share.
In
March and April 2022, the Company issued and sold Pre-Funded Warrants to purchase an aggregate of 3,692,276 shares of common stock at
a nominal exercise price of $0.001 per share. During the nine months ended September 30, 2023, all of these warrants were exercised and
therefore had no remaining dilutive effect.
In
May 2023, the Company issued and sold May Pre-Funded Warrants to purchase an aggregate of 3,502,381
shares of common stock at a nominal exercise price of $0.001 per
share. During the nine months ended September 30, 2023, 1,156,381
of these warrants were exercised leaving 2,346,000
outstanding. These warrants were not included in the calculation
of weighted average outstanding shares as they would be ant-dilutive.
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Lease
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Lease |
|
| Lease |
Note
10 – Lease
The
Company has obligations as a lessee for office space with initial non-cancellable terms in excess of one year. The Company classified
the lease as an operating lease. The lease contains a renewal option for a period of five years. Because the Company is certain to exercise
the renewal option, the optional period is included in determining the lease term, and associated payments under the renewal option are
included in the lease payments. The Company’s lease does not include termination options for either party to the lease or restrictive
financial or other covenants. Payments due under the lease contract include fixed payments plus a variable Payment. The Company’s
office space lease requires it to make variable payments for the Company’s proportionate share of building’s property taxes,
insurance, and common area maintenance. These variable lease payments are not included in lease payments used to determine lease liability
and are recognized as variable costs when incurred.
Amounts
reported on the balance sheet as of September 30, 2023 were as follows:
| |
Schedule of lease information |
|
|
Operating lease ROU asset |
$664,296 |
|
Operating Lease liability - Short-term |
$117,840 |
|
Operating lease liability - Long-term |
$555,687 |
|
Remaining lease term |
6 years 3 months |
|
Discount rate |
6% |
Amounts
disclosed for ROU assets obtained in exchange for lease obligations and reductions of ROU assets resulting from reductions of lease obligations
include amounts reduced from the carrying amount of ROU assets resulting from deferred rent.
Maturities
of lease liabilities under non-cancellable operating leases at September 30, 2023 are as follows:
| Schedule of maturities of lease liabilities |
|
|
| 2023 |
$ |
30,124 |
| 2024 |
|
116,090 |
| 2025 |
|
116,277 |
| 2026 |
|
110,134 |
| 2027 |
|
103,736 |
| Thereafter |
|
197,166 |
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureLeaseAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Management and Director Compensation
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Management And Director Compensation |
|
| Management and Director Compensation |
Note
11 – Management and Director Compensation
The
Company paid its officers cash compensation totaling $245,000 and $362,500 and $1,290,000 and $770,095 for the three and nine-month periods
ended September 30, 2023 and 2022, respectively.
The
Company paid its directors cash compensation totaling $100,000 and $300,000 and $100,000 and $200,000 for the three and nine-month periods
ended September 30, 2023 and 2022, respectively.
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureManagementAndDirectorCompensationAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_ManagementCompensationAndDirectorFeesTextBlock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Income Taxes
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Note
12 – Income Taxes
In
calculating the provision for income taxes on an interim basis, the Company uses an estimate of the annual effective tax rate based upon
currently known facts and circumstances and applies that rate to its year-to-date earnings or losses. The Company’s effective tax
rate is based on expected income and statutory tax rates and takes into consideration permanent differences between financial statement
and tax return income applicable to the Company in the various jurisdictions in which the Company operates. The effect of discrete items,
such as changes in estimates, changes in rates or tax status, and unusual or infrequently occurring events, is recognized in the interim
period in which the discrete item occurs. The accounting estimates used to compute the provision for income taxes may change as new events
occur, additional information is obtained or as the result of new judicial interpretations or regulatory or tax law changes.
The Company’s
interim effective tax rate, inclusive of discrete items, for the nine-month periods ended September 30, 2023 and 2022 was 26.83%.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Subsequent Events
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
Note
13 – Subsequent Events
On
October 12, 2023, the Company held a special meeting of the holders of its outstanding Tradeable Warrants in which the holders of the
majority of the outstanding Tradeable Warrants approved an amendment to the Warrant Agent Agreement to (i) reduce the exercise price
of the Tradeable Warrants to $0.11, subject to further adjustment as provided therein, and (ii) eliminate the provision that prohibits
the Company’s CEO from exercising his voting rights under his Series B Preferred Stock.
In
December 2022, the Company had entered into a research agreement with the Jewish General Hospital (“JGH”), Montreal, Canada
to conduct IND-enabling studies of the Company’s anticancer drug candidate, Adva-27a (the “Research Agreement”). In
August 2023, the Company was advised by JGH that the lab results on testing of the Adva-27a molecule were not favorable. After conclusion
of an internal review of the lab results on November 2, 2023, the Company provided notice of termination of the Research Agreement, which
will become effective on December 2, 2023, pursuant to the terms of the Research Agreement. The Company has now paused the IND-enabling
studies of Adva-27a pending a review of the possibility of chemical modification of the compound to address the suboptimal performance
of the molecule in certain studies.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Acquisition of Nora Pharma Inc. (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Business Combination and Asset Acquisition [Abstract] |
|
| Schedule of allocation of purchase price |
| Schedule of allocation of purchase price | |
| |
| Accounts receivable | |
$ | 1,358,121 | |
| Inventory | |
| 3,181,916 | |
| Intangible assets | |
| 659,571 | |
| Equipment & furniture | |
| 210,503 | |
| Other assets | |
| 1,105,093 | |
| Total assets | |
| 6,515,204 | |
| Liabilities assumed | |
| (5,981,286 | ) |
| Net assets | |
| 533,918 | |
| Goodwill | |
| 18,326,719 | |
| Total Consideration | |
$ | 18,860,637 | |
|
| Schedule of Pro Forma results from acquisition |
| Schedule of Pro Forma results from acquisition | |
| | |
| |
| Pro Forma Results
From Acquisition | |
December
31,
2022 | | |
December
31,
2021 | |
| Total revenues | |
$ | 14,758,115 | | |
$ | 7,927,165 | |
| Net (loss) from operations | |
$ | (26,192,503 | ) | |
$ | (2,224,253 | ) |
| Net (loss) | |
$ | (26,164,764 | ) | |
$ | (12,289,655 | ) |
| Basic and fully diluted (loss) per share | |
$ | (1.74 | ) | |
$ | (4.70 | ) |
| Weighted average number of shares outstanding | |
| 15,056,097 | | |
| 2,612,061 | |
|
| X |
- DefinitionTabular disclosure of pro forma results of operations for a material business acquisition or series of individually immaterial business acquisitions that are material in the aggregate. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-SubTopic 10
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479328/805-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(3)
-SubTopic 10
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479328/805-10-50-2
| Name: |
us-gaap_BusinessAcquisitionProFormaInformationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_BusinessCombinationAndAssetAcquisitionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of transactions that are recognized separately from the acquisition of assets and assumptions of liabilities in the business combination. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479328/805-10-50-2
| Name: |
us-gaap_BusinessCombinationSeparatelyRecognizedTransactionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Intangible Assets (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Goodwill and Intangible Assets Disclosure [Abstract] |
|
| Schedule of intangible assets |
| Schedule of intangible assets | |
| | |
| Balance June 30, 2023 | |
$ | 1,233,570 | |
| Dossier fee additions | |
| 13,905 | |
| Balance at September 30, 2023 | |
| 1,247,475 | |
| Less accumulated amortization | |
| (31,268 | ) |
| Finite-lived intangible
assets, net, at September 30, 2023 | |
$ | 1,216,207 | |
| | |
| | |
| Balance December 31, 2022 | |
$ | 776,856 | |
| Dossier fee additions | |
| 470,619 | |
| Balance at September 30, 2023 | |
| 1,247,475 | |
| Less accumulated amortization | |
| (31,268 | ) |
| Finite-lived intangible
assets, net, at September 30, 2023 | |
$ | 1,216,207 | |
|
| Schedule of estimated amortization expense |
| Schedule of estimated amortization expense | |
| | |
| 2024 | |
$ | 55,418 | |
| 2025 | |
| 55,418 | |
| 2026 | |
| 54,240 | |
| 2027 | |
| 15,599 | |
| 2028 | |
| 7,370 | |
|
| X |
- DefinitionTabular disclosure of amortization expense of assets, excluding financial assets, that lack physical substance, having a limited useful life.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance with a finite life, by either major class or business segment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Warrants (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Warrants |
|
| Schedule of warrants issued with financing |
| Schedule of warrants issued with financing |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise Price |
|
Expiry Date |
| Pre-Funded Warrants |
|
3,692,276 |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
4,102,200 |
|
$2.22* |
|
February 2027 |
| Investor Warrants |
|
3,603,604 |
|
$2.22 |
|
March 2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April 2027 |
| May Pre-Funded Warrants |
|
3,502,381 |
|
$0.001 |
|
Unlimited |
| May Investor Warrants |
|
11,904,762 |
|
$0.59 |
|
November 2028 |
| * |
The
Tradeable Warrants had an initial exercise price of $4.25, subject to adjustment. Upon the closing of the Company's private placement
on March 14, 2022, the exercise price of the Tradeable Warrants was reduced to $2.22, in accordance with the terms thereof. |
|
| Schedule of outstanding warrants |
| Schedule of outstanding warrants |
|
|
|
|
|
|
| Type |
|
Number |
|
Exercise Price |
|
Expiry Date |
| Pre-Funded Warrants |
|
None |
|
$0.001 |
|
Unlimited |
| Tradeable Warrants |
|
963,693 |
|
$2.22* |
|
February 2027 |
| Investor Warrants |
|
800,901 |
|
$2.22 |
|
March 2027 |
| April Warrants |
|
9,725,690 |
|
$3.76 |
|
April 2027 |
| May Pre-Funded Warrants |
|
2,346,000 |
|
$0.001 |
|
Unlimited |
| May Investor Warrants |
|
11,904,762 |
|
$0.59 |
|
November 2028 |
| * |
On
October 12, 2023, the Company held a special meeting of the holders of its outstanding Tradeable Warrants in which a majority of the
holders approved an amendment to the Warrant Agent Agreement to reduce the exercise price of the Tradeable Warrants to $0.11 per warrant.
The amendment was executed on October 18, 2023. |
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureWarrantsAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantsIssuedWithFinancingTableTextBlock |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of warrants or rights issued. Warrants and rights outstanding are derivative securities that give the holder the right to purchase securities (usually equity) from the issuer at a specific price within a certain time frame. Warrants are often included in a new debt issue to entice investors by a higher return potential. The main difference between warrants and call options is that warrants are issued and guaranteed by the company, whereas options are exchange instruments and are not issued by the company. Also, the lifetime of a warrant is often measured in years, while the lifetime of a typical option is measured in months. Disclose the title of issue of securities called for by warrants and rights outstanding, the aggregate amount of securities called for by warrants and rights outstanding, the date from which the warrants or rights are exercisable, and the price at which the warrant or right is exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
| Name: |
us-gaap_ScheduleOfStockholdersEquityNoteWarrantsOrRightsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Lease (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Lease |
|
| Schedule of lease information |
| |
Schedule of lease information |
|
|
Operating lease ROU asset |
$664,296 |
|
Operating Lease liability - Short-term |
$117,840 |
|
Operating lease liability - Long-term |
$555,687 |
|
Remaining lease term |
6 years 3 months |
|
Discount rate |
6% |
|
| Schedule of maturities of lease liabilities |
| Schedule of maturities of lease liabilities |
|
|
| 2023 |
$ |
30,124 |
| 2024 |
|
116,090 |
| 2025 |
|
116,277 |
| 2026 |
|
110,134 |
| 2027 |
|
103,736 |
| Thereafter |
|
197,166 |
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureLeaseAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_SalesRevenueNetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_ProductConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=SBFM_GenericPharmaceuticalsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=SBFM_OTCProductsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Private Placement (Details Narrative) - USD ($)
|
|
9 Months Ended |
May 16, 2023 |
Sep. 30, 2023 |
| Common Stock [Member] |
|
|
| Securities Financing Transaction [Line Items] |
|
|
| Stock Issued During Period, Shares, New Issues |
|
2,450,000
|
| Single Healthcare Focused Institutional Investor [Member] |
|
|
| Securities Financing Transaction [Line Items] |
|
|
| Gross proceeds from private placement |
$ 5,000,000
|
|
| Net proceeds from private placement |
$ 4,089,218
|
|
| Single Healthcare Focused Institutional Investor [Member] | May Pre Funded Warrants [Member] |
|
|
| Securities Financing Transaction [Line Items] |
|
|
| Net proceeds from private placement |
|
$ 1,156
|
| Stock Issued During Period, Shares, New Issues |
3,502,381
|
1,156,381
|
| Single Healthcare Focused Institutional Investor [Member] | Common Stock [Member] |
|
|
| Securities Financing Transaction [Line Items] |
|
|
| Stock Issued During Period, Shares, New Issues |
2,450,000
|
|
| X |
- References
+ Details
| Name: |
SBFM_GrossProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's raising of capital via private rather than public placement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SecuritiesFinancingTransactionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=SBFM_SingleHealthcareFocusedInstitutionalInvestorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Acquisition of Nora Pharma Inc. (Details) - Nora Pharma [Member]
|
Oct. 20, 2022
USD ($)
|
| Business Acquisition [Line Items] |
|
| Business combination, account receivable |
$ 1,358,121
|
| Business combination, inventory |
3,181,916
|
| Business combination, intangible assets |
659,571
|
| Business combination, equipment and furniture |
210,503
|
| Business combination, other assets |
1,105,093
|
| Business combination, total assets |
6,515,204
|
| Business combination, liabilities assumed |
(5,981,286)
|
| Business combination, net assets |
533,918
|
| Business combination, goodwill |
18,326,719
|
| Business combination, total consideration |
$ 18,860,637
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-5
| Name: |
us-gaap_BusinessAcquisitionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of assets acquired at the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount due from customers or clients for goods or services, including trade receivables, that have been delivered or sold in the normal course of business, and amounts due from others, including related parties expected to be converted to cash, sold or exchanged within one year or the normal operating cycle, if longer, acquired at the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedCurrentAssetsReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of identifiable intangible assets recognized as of the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedIntangibles |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of inventory recognized as of the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedInventory |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities assumed at the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount recognized as of the acquisition date for the identifiable assets acquired in excess of (less than) the aggregate liabilities assumed. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of assets expected to be realized or consumed after one year or the normal operating cycle, if longer, acquired at the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedNoncurrentAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of property, plant, and equipment recognized as of the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount recognized for assets, including goodwill, in excess of (less than) the aggregate liabilities assumed. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredGoodwillAndLiabilitiesAssumedNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated impairment loss of an asset representing future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482598/350-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Goodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=SBFM_NoraPharmaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Acquisition of Nora Pharma Inc. (Details - Pro Forma results) - USD ($)
|
12 Months Ended |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Business Acquisition [Line Items] |
|
|
| Total revenues |
$ 14,758,115
|
$ 7,927,165
|
| Net (loss) from operations |
(26,192,503)
|
(2,224,253)
|
| Net (loss) |
$ (26,164,764)
|
$ (12,289,655)
|
| Nora Pharma [Member] |
|
|
| Business Acquisition [Line Items] |
|
|
| Basic (loss) per share |
$ (1.74)
|
$ (4.70)
|
| Fully diluted (loss) per share |
$ (1.74)
|
$ (4.70)
|
| Weighted average number of shares outstanding, Basic |
15,056,097
|
2,612,061
|
| Weighted average number of shares outstanding, Diluted |
15,056,097
|
2,612,061
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-5
| Name: |
us-gaap_BusinessAcquisitionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of pro forma income from continuing operations as if the business combination had been completed at the beginning of a period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479328/805-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479328/805-10-50-2
| Name: |
us-gaap_BusinessAcquisitionsProFormaIncomeLossFromContinuingOperationsBeforeChangesInAccountingAndExtraordinaryItemsNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=SBFM_NoraPharmaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Acquisition of Nora Pharma Inc. (Details Narrative)
|
|
9 Months Ended |
|
|
|
Oct. 20, 2022
USD ($)
shares
|
Sep. 30, 2023
USD ($)
|
Dec. 31, 2022
USD ($)
|
Oct. 20, 2022
CAD ($)
|
| Business Acquisition [Line Items] |
|
|
|
|
| Earnout payable |
|
$ 2,547,831
|
$ 3,632,000
|
|
| Nora Pharma [Member] |
|
|
|
|
| Business Acquisition [Line Items] |
|
|
|
|
| Purchase price of shares |
$ 18,860,637
|
|
|
|
| Payments to acquire shares |
$ 14,346,637
|
|
|
|
| Stock issued for acquisition | shares |
3,700,000
|
|
|
|
| Stock issued for acquisition, value |
$ 4,514,000
|
|
|
|
| Nora Pharma [Member] | Malek Chamoun [Member] |
|
|
|
|
| Business Acquisition [Line Items] |
|
|
|
|
| Earnout payable |
$ 3,632,000
|
2,547,831
|
|
$ 5,000,000
|
| Payment of earnout liability |
|
$ 1,084,169
|
|
|
| X |
- References
+ Details
| Name: |
SBFM_EarnoutPayable |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
SBFM_PaymentOfEarnoutLiability |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479907/805-20-50-5
| Name: |
us-gaap_BusinessAcquisitionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of consideration transferred, consisting of acquisition-date fair value of assets transferred by the acquirer, liabilities incurred by the acquirer, and equity interest issued by the acquirer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 8
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479637/805-30-30-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479581/805-30-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 7
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479637/805-30-30-7
| Name: |
us-gaap_BusinessCombinationConsiderationTransferred1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of business during the period. The cash portion only of the acquisition price. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479581/805-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireBusinessesGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued during the period pursuant to acquisitions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesAcquisitions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued pursuant to acquisitions during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueAcquisitions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=SBFM_NoraPharmaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=SBFM_MalekChamounMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Intangible Assets (Details)
|
3 Months Ended |
9 Months Ended |
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2023
USD ($)
|
| Goodwill and Intangible Assets Disclosure [Abstract] |
|
|
| Finite lived intangible assets, beginning balance |
$ 1,233,570
|
$ 776,856
|
| Dossier fee additions |
13,905
|
470,619
|
| Finite lived intangible assets, ending balance |
1,247,475
|
1,247,475
|
| Less accumulated amortization |
(31,268)
|
(31,268)
|
| Finite lived intangible assets, net |
$ 1,216,207
|
$ 1,216,207
|
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 928
-SubTopic 340
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483147/928-340-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in carrying value of assets, excluding financial assets and goodwill, lacking physical substance with a finite life.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsPeriodIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the reverse stock split arrangement. Also provide the retroactive effect given by the reverse split that occurs after the balance sheet date but before the release of financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB Topic 4.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityReverseStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=SBFM_FirstReverseStockSplitMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Capital Stock (Details Narrative) - USD ($)
|
|
|
|
|
|
|
1 Months Ended |
6 Months Ended |
9 Months Ended |
12 Months Ended |
|
May 16, 2023 |
Oct. 20, 2022 |
Apr. 28, 2022 |
Mar. 14, 2022 |
Feb. 22, 2022 |
Feb. 17, 2022 |
Jul. 31, 2023 |
Jun. 30, 2023 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Jan. 19, 2023 |
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock, shares authorized |
|
|
|
|
|
|
|
|
|
3,000,000,000
|
|
3,000,000,000
|
|
| Common stock, par value |
|
|
|
|
|
|
|
|
|
$ 0.001
|
|
$ 0.001
|
|
| Stock repurchase program amount |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,000,000
|
| Aggregate net proceeds |
|
|
|
|
|
|
|
|
|
$ 1,156
|
$ 0
|
|
|
| Common stock, shares outstanding |
|
|
|
|
|
|
|
|
|
25,678,290
|
|
22,585,632
|
|
| Dividends |
|
|
|
|
|
|
|
|
|
$ 0
|
|
|
|
| Stock Issued For Warrant Exercises [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares issued, shares |
|
|
|
|
|
|
|
|
10,789,867
|
|
|
10,789,867
|
|
| Warrants Exercised [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Aggregate net proceeds |
|
|
|
|
|
|
|
|
$ 13,194,335
|
|
|
$ 13,194,335
|
|
| Nora Pharma Inc [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued for acquisition, shares |
|
|
3,700,000
|
|
|
|
|
|
|
|
|
|
|
| Stock issued for acquisition, value |
|
|
$ 4,514,000
|
|
|
|
|
|
|
|
|
|
|
| Private Placement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net proceeds issuance of private placement |
|
|
|
$ 16,752,915
|
$ 6,781,199
|
|
|
|
|
|
|
|
|
| Single Healthcare Focused Institutional Investor [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| [custom:GrossProceedsFromIssuanceOfPrivatePlacement] |
|
$ 5,000,000
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from Issuance of Private Placement |
|
$ 4,089,218
|
|
|
|
|
|
|
|
|
|
|
|
| Tradeable Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
|
|
|
|
|
4,102,200
|
|
|
|
| Exercise price of warrants |
[1],[2] |
|
|
|
|
|
|
|
|
$ 2.22
|
|
|
|
| Common Stock Member And Investor Warrants [Member] | Private Placement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued new, shares |
|
|
|
|
2,301,353
|
|
|
|
|
|
|
|
|
| Common Stock Member And April Warrants [Member] | Private Placement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued new, shares |
|
|
|
2,472,820
|
|
|
|
|
|
|
|
|
|
| Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Payment for stock repurchased |
|
|
|
|
|
|
|
|
|
$ 506,822
|
|
|
|
| May Pre Funded Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
|
|
|
|
|
3,502,381
|
|
|
|
| Exercise price of warrants |
|
|
|
|
|
|
|
|
|
$ 0.001
|
|
|
|
| May Pre Funded Warrants [Member] | Single Healthcare Focused Institutional Investor [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued new, shares |
|
3,502,381
|
|
|
|
|
|
|
|
1,156,381
|
|
|
|
| Proceeds from Issuance of Private Placement |
|
|
|
|
|
|
|
|
|
$ 1,156
|
|
|
|
| Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued new, shares |
|
|
|
|
|
|
|
|
|
2,450,000
|
|
|
|
| Stock repurchased, shares |
|
|
|
|
|
|
|
68,012
|
|
445,711
|
|
|
|
| Payment for stock repurchased |
|
|
|
|
|
|
|
$ 34,321
|
|
|
|
|
|
| Common stock, shares outstanding |
|
|
|
|
|
|
|
|
|
25,678,290
|
|
22,585,632
|
|
| Common Stock [Member] | Single Healthcare Focused Institutional Investor [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued new, shares |
|
2,450,000
|
|
|
|
|
|
|
|
|
|
|
|
| Public Offering [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net proceeds issuance of private placement |
|
|
|
|
|
|
$ 6,833,071
|
|
|
|
|
|
|
| Public Offering [Member] | Tradeable Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
|
|
4,102,200
|
|
|
|
|
|
|
| Exercise price of warrants |
|
|
|
|
|
$ 0.11
|
|
|
|
|
|
|
|
| Public Offering [Member] | Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued new, shares |
|
|
|
|
|
|
1,882,353
|
|
|
|
|
|
|
| Series B Preferred Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock, shares authorized |
|
|
|
|
|
|
|
|
|
1,000,000
|
|
1,000,000
|
|
| Preferred stock, par value |
|
|
|
|
|
|
|
|
|
$ 0.10
|
|
$ 0.10
|
|
| Preferred stock, shares issued |
|
|
|
|
|
|
|
|
|
10,000
|
|
10,000
|
|
| Number of shares redeemed |
|
|
|
|
|
990,000
|
|
|
|
|
|
|
|
| Director [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock, shares authorized |
|
|
|
|
|
|
|
|
|
30,000,000
|
|
|
|
| Preferred stock, par value |
|
|
|
|
|
|
|
|
|
$ 0.10
|
|
|
|
| Chief Executive Officer [Member] | Series B Preferred Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock, shares issued |
|
|
|
|
|
|
|
|
|
|
|
10,000
|
|
|
|
| X |
- References
+ Details
| Name: |
SBFM_GrossProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantsIssuedShares |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of paid and unpaid cash, stock, and paid-in-kind (PIK) dividends declared, for example, but not limited to, common and preferred stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-SubTopic 405
-Topic 942
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481071/942-405-45-2
| Name: |
us-gaap_Dividends |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to reacquire common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsForRepurchaseOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's raising of capital via private rather than public placement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued during the period pursuant to acquisitions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesAcquisitions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued pursuant to acquisitions during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueAcquisitions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of stock bought back by the entity at the exercise price or redemption price. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockRedeemedOrCalledDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of stock repurchase plan authorized.
| Name: |
us-gaap_StockRepurchaseProgramAuthorizedAmount1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=SBFM_StockIssuedForWarrantExercisesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=SBFM_WarrantsExercisedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=SBFM_NoraPharmaIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=SBFM_SingleHealthcareFocusedInstitutionalInvestorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_TradeableWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_CommonStockMemberAndInvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_CommonStockMemberAndAprilWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=SBFM_PublicOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesBPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_DirectorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefExecutiveOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Warrants (Details - Warrants issued with financing)
|
9 Months Ended |
|
Sep. 30, 2023
$ / shares
shares
|
|---|
| Pre Funded Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants | shares |
3,692,276
|
|
| Exercise price | $ / shares |
$ 0.001
|
|
| Expiry date |
Unlimited
|
|
| Tradeable Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants | shares |
4,102,200
|
|
| Exercise price | $ / shares |
$ 2.22
|
[1],[2] |
| Expiry date |
February 2027
|
|
| Investor Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants | shares |
3,603,604
|
|
| Exercise price | $ / shares |
$ 2.22
|
|
| Expiry date |
March 2027
|
|
| April Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants | shares |
9,725,690
|
|
| Exercise price | $ / shares |
$ 3.76
|
|
| Expiry date |
April 2027
|
|
| May Pre Funded Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants | shares |
3,502,381
|
|
| Exercise price | $ / shares |
$ 0.001
|
|
| Expiry date |
Unlimited
|
|
| May Investor Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants | shares |
11,904,762
|
|
| Exercise price | $ / shares |
$ 0.59
|
|
| Expiry date |
November 2028
|
|
|
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantExpirationDate |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantsIssuedShares |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_TradeableWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_InvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_AprilWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayInvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Warrants (Details - Warrants outstanding)
|
9 Months Ended |
|
Sep. 30, 2023
$ / shares
shares
|
|---|
| Pre Funded Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants outstanding | shares |
0
|
|
| Exercise price | $ / shares |
$ 0.001
|
|
| Expiry date |
Unlimited
|
|
| Tradeable Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants outstanding | shares |
963,693
|
|
| Exercise price | $ / shares |
$ 2.22
|
[1],[2] |
| Expiry date |
February 2027
|
|
| Investor Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants outstanding | shares |
800,901
|
|
| Exercise price | $ / shares |
$ 2.22
|
|
| Expiry date |
March 2027
|
|
| April Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants outstanding | shares |
9,725,690
|
|
| Exercise price | $ / shares |
$ 3.76
|
|
| Expiry date |
April 2027
|
|
| May Pre Funded Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants outstanding | shares |
2,346,000
|
|
| Exercise price | $ / shares |
$ 0.001
|
|
| Expiry date |
Unlimited
|
|
| May Investor Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of warrants outstanding | shares |
11,904,762
|
|
| Exercise price | $ / shares |
$ 0.59
|
|
| Expiry date |
November 2028
|
|
|
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantExpirationDate |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_TradeableWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_InvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_AprilWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayInvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Warrants (Details Narrative) - USD ($)
|
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Proceeds from Warrant Exercises |
$ 1,156
|
$ 0
|
| Tradeable Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Warrants exercised |
3,138,507
|
|
| Investor Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Warrants exercised |
2,802,703
|
|
| May Pre Funded Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Warrants exercised |
1,156,381
|
|
| All Warrants [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Proceeds from Warrant Exercises |
$ 13,194,335
|
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-option equity instruments exercised by participants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(2)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_TradeableWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_InvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_AllWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Net Loss Per Common Share (Details Narrative) - shares
|
1 Months Ended |
9 Months Ended |
May 31, 2023 |
Apr. 30, 2022 |
Mar. 31, 2022 |
Feb. 28, 2022 |
Sep. 30, 2023 |
| Tradeable Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
4,102,200
|
| Warrant exercised |
|
|
|
|
3,138,507
|
| Warrants outstanding, shares |
|
|
|
|
963,693
|
| Investor Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
3,603,604
|
| Warrant exercised |
|
|
|
|
2,802,703
|
| Warrants outstanding, shares |
|
|
|
|
800,901
|
| April Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
9,725,690
|
| Warrants outstanding, shares |
|
|
|
|
9,725,690
|
| May Investor Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
11,904,762
|
| Warrants outstanding, shares |
|
|
|
|
11,904,762
|
| Pre Funded Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
3,692,276
|
| Warrant exercised |
|
3,692,276
|
3,692,276
|
|
1,156,381
|
| Warrants outstanding, shares |
|
|
|
|
0
|
| Pre Funded Warrant [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
3,502,381
|
|
|
|
|
| May Pre Funded Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
|
3,502,381
|
| Warrant exercised |
|
|
|
|
1,156,381
|
| Warrants outstanding, shares |
|
|
|
|
2,346,000
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share, Amount |
|
|
|
|
2,346,000
|
| Company Public Offering [Member] | Tradeable Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
|
4,102,200
|
|
| Two Private Placements [Member] | Investor Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
|
3,603,604
|
|
|
| Two Private Placements [Member] | April Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
|
9,725,690
|
|
|
|
| Two Private Placements [Member] | May Investor Warrants [Member] |
|
|
|
|
|
| Securities Financing Transaction [Line Items] |
|
|
|
|
|
| Warrants issued, shares |
11,904,762
|
|
|
|
|
| X |
- References
+ Details
| Name: |
SBFM_WarrantsIssuedShares |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SecuritiesFinancingTransactionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-option equity instruments exercised by participants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(2)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_TradeableWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_InvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_AprilWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayInvestorWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_PreFundedWarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=SBFM_MayPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=SBFM_CompanyPublicOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=SBFM_TwoPrivatePlacementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
SBFM_DisclosureLeaseAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_LesseeOperatingLeaseRemainingLeaseTerm1 |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
SBFM_DisclosureLeaseAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
SBFM_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFour |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease having initial or remaining lease term in excess of one year to be paid in remainder of current fiscal year. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Management and Director Compensation (Details Narrative) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Management And Director Compensation |
|
|
|
|
| Officers cash compensation |
$ 245,000
|
$ 362,500
|
$ 1,290,000
|
$ 770,095
|
| Directors cash compensation |
$ 100,000
|
$ 100,000
|
$ 300,000
|
$ 200,000
|
| X |
- References
+ Details
| Name: |
SBFM_DisclosureManagementAndDirectorCompensationAbstract |
| Namespace Prefix: |
SBFM_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNoninterest expense related to directors' fees which are fees paid by an Entity to its directors. Directors' fees may be paid in addition to salary and other benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.14)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NoninterestExpenseDirectorsFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for salary and wage arising from service rendered by nonofficer and officer employees. Excludes allocated cost, labor-related nonsalary expense, and direct and overhead labor cost included in cost of good and service sold.
| Name: |
us-gaap_SalariesWagesAndOfficersCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.0.1
Sunshine Biopharma (NASDAQ:SBFM)
Historical Stock Chart
From Mar 2024 to Apr 2024
Sunshine Biopharma (NASDAQ:SBFM)
Historical Stock Chart
From Apr 2023 to Apr 2024