
42nd Annual J.P. Morgan Healthcare Conference Bob Duggan Dr. Maky Zanganeh Chairman & CEO CEO & President

Forward Looking Statement Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. 2 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Any statements in this presentation about the Company’s future expectations, plans and prospects, including but not limited to, statements about the clinical and preclinical development of the Company’s product candidates, entry into and actions related to the Company’s partnership with Akeso Inc., the therapeutic potential of the Company’s product candidates, the potential commercialization of the Company’s product candidates, the timing of initiation, completion and availability of data from clinical trials, the potential submission of applications for marketing approvals, potential acquisitions and other statements containing the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "would," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the results of our evaluation of the underlying data in connection with the development and commercialization activities for ivonescimab, the outcome of discussions with regulatory authorities, including the Food and Drug Administration, the uncertainties inherent in the initiation of future clinical trials, availability and timing of data from ongoing and future clinical trials, the results of such trials, and their success, and global public health crises that may affect timing and status of our clinical trials and operations, whether preliminary results from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials or preclinical studies will be indicative of the results of later clinical trials, whether business development opportunities to expand the Company’s pipeline of drug candidates, including without limitation, through potential acquisitions of, and/or collaborations with, other entities occur, expectations for regulatory approvals, laws and regulations affecting government contracts and funding awards, availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements and other factors discussed in the "Risk Factors" section of filings that the Company makes with the Securities and Exchange Commission. Any change to our ongoing trials could cause delays, affect our future expenses, and add uncertainty to our commercialization efforts, as well as to affect the likelihood of the successful completion of clinical development of ivonescimab. Accordingly, the audience should not place undue reliance on forward-looking statements or information. In addition, any forward-looking statements included in this presentation represent the Company’s views only as of the date of this presentation and should not be relied upon as representing the Company’s views as of any subsequent date. The Company specifically disclaims any obligation to update any forward-looking statements included in this presentation.
Company Details Focus ONCOLOGY Partnership Akeso Inc. Summit License Territories United States, Canada, Europe, Japan Chief Executive Officers Bob Duggan Chairman & CEO Dr. Maky Zanganeh CEO & President NASDAQ SMMT Market Cap $1.83B* Cash $186M* Employees 111† Offices Miami, FL Menlo Park, CA Oxford, UK …Improve quality of life, increase potential duration of life, and resolve serious medical healthcare needs… MISSION Unmatched high-speed execution, proven track record FOCUSED ON PATIENTS FIRST LEADERSHIP Lead Compound: Ivonescimab Only Phase III PD-1/VEGF Bispecific Antibody in Summit’s License Territories* 2024 Focus Execute on Phase III clinical trials Expand clinical development plan Summit Therapeutics *As of December 31, 2023; †As of January 8, 2024 3 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. *There are no known PD-1-based bispecific antibodies approved by the U.S. Food and Drug Administration (“FDA”) or the European Medicines Agency (“EMA”).

Partnership with An Aligned Mission Bringing ivonescimab to patients around the world Summit is actively recruiting two Phase III NSCLC clinical trials LEADING BIOPHARMA COMPANY IN CHINA Michelle Xia, Ph.D. Co-Founder, Chairwoman, President and CEO Over 2,800 Employees End-to-end In-house Capabilities INNOVATOR1,2 World’s first marketed PD-1 bispecific (cadonilimab)3 3 commercial drugs in China 120+ worldwide clinical trials* 30+ drug candidates 19 clinical-stage candidates 6+ bispecific antibodies 1.Akeso Press Release (2022-12-06), 2. Akeso website accessed on 12.1.23, 3. https://scrip.citeline.com/SC146649/China-Approves- Worlds-First-Bispecific-IO-Drug-Amid-PD-1L1-Glut. Accessed 1.4.24. *Including ISTs with Akeso products. NSCLC: Non-small Cell Lung Cancer Globally, 1,600+ patients treated with ivonescimab across all trials to date 4 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies.

Shaping the Path to Become a Commercial Entity SITC: Society for Immunotherapy of Cancer; MOA: Mechanism of Action; ASCO: American Society of Clinical Oncology; NSCLC: Non-small Cell Lung Cancer; EORTC: European Organisation for Research and Treatment of Cancer; NCI: National Cancer Institute; AACR: American Association for Cancer Research; IST: Investigator Sponsored Trials Multiple Meetings First Patient In First Patient In Accepted IST Proposals (ivonescimab) Partnership Consummated Q1 2023 Q2 2023 Q3 2023 Q4 2023 Raised $500M MOA (Poster) EORTC-NCI-AACR MOA (Poster) Phase II Data (Article) NSCLC Phase II Data (Poster) 5 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. T H E L AN C E T SITC

Ivonescimab: Mechanism of Action
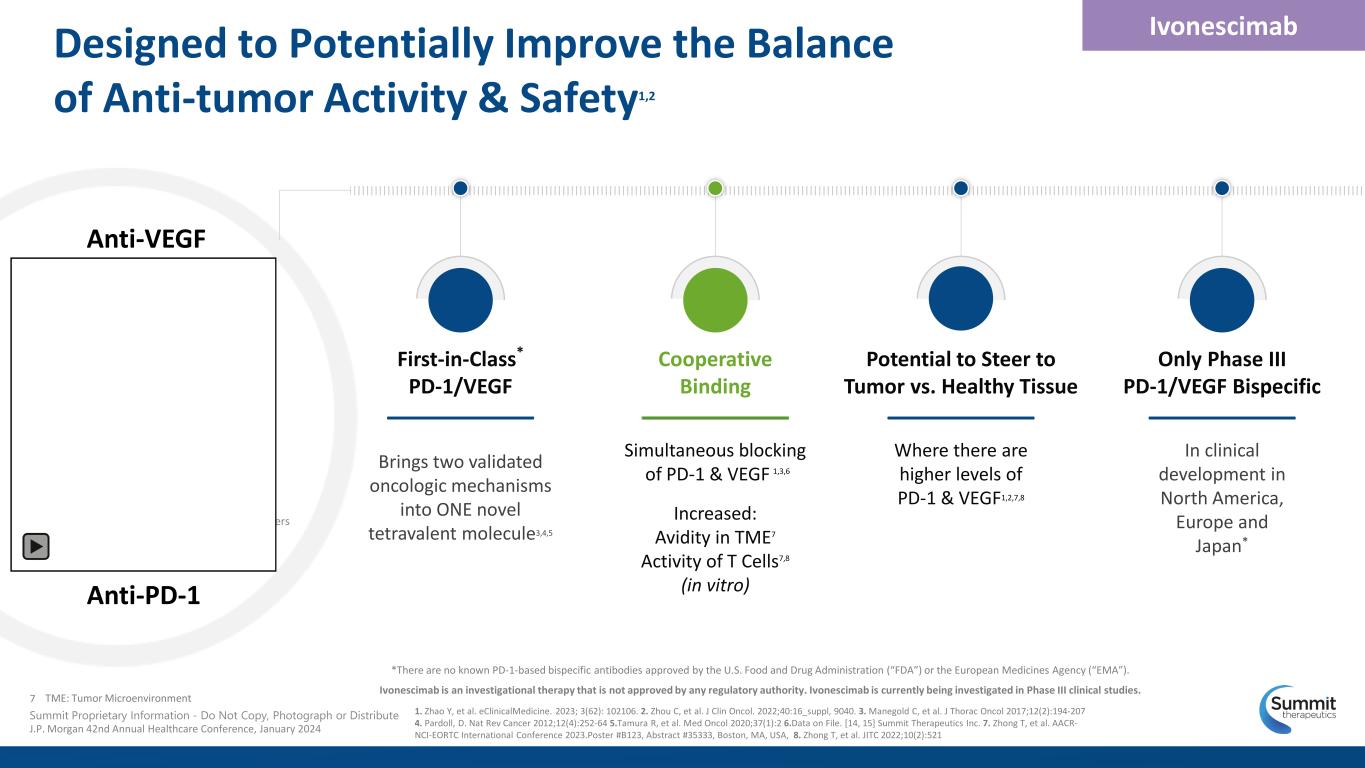
Designed to Potentially Improve the Balance of Anti-tumor Activity & Safety1,2 *There are no known PD-1-based bispecific antibodies approved by the U.S. Food and Drug Administration (“FDA”) or the European Medicines Agency (“EMA”). Ivonescimab is an investigational therapy that is not approved by any regulatory authority. Ivonescimab is currently being investigated in Phase III clinical studies. Ivonescimab First-in-Class* PD-1/VEGF Brings two validated oncologic mechanisms into ONE novel tetravalent molecule3,4,5 Potential to Steer to Tumor vs. Healthy Tissue Where there are higher levels of PD-1 & VEGF1,2,7,8 Only Phase III PD-1/VEGF Bispecific In clinical development in North America, Europe and Japan* Cooperative Binding Simultaneous blocking of PD-1 & VEGF 1,3,6 Increased: Avidity in TME7 Activity of T Cells7,8 (in vitro) 7 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 1. Zhao Y, et al. eClinicalMedicine. 2023; 3(62): 102106. 2. Zhou C, et al. J Clin Oncol. 2022;40:16_suppl, 9040. 3. Manegold C, et al. J Thorac Oncol 2017;12(2):194-207 4. Pardoll, D. Nat Rev Cancer 2012;12(4):252-64 5.Tamura R, et al. Med Oncol 2020;37(1):2 6.Data on File. [14, 15] Summit Therapeutics Inc. 7. Zhong T, et al. AACR- NCI-EORTC International Conference 2023.Poster #B123, Abstract #35333, Boston, MA, USA, 8. Zhong T, et al. JITC 2022;10(2):521 TME: Tumor Microenvironment Anti-VEGF Anti-PD-1 Linkers

VEGF Dimer PD-1 Receptor in T Cell Increased Avidity in TME VEGF increases affinity to PD-1 by >18X4 PD-1 increases affinity to VEGF by >4X4 (in vitro) Enhanced Activity of T Cells VEGF dimer leads to potential interconnection of ivonescimab molecules, which may increase activity of T cells4,5 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. Ivonescimab is currently being investigated in Phase III clinical studies Simultaneous blocking of PD-1 & VEGF1,2,3 Ivonescimab 8 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Cooperative Binding Greater Than the Sum of Its Parts 1. Zhao Y. et al., eClinicalMedicine. 2023; 3(62): 102106.,; 2. Manegold C, et al. J Thorac Oncol 2017;12(2):194-207 ; 3. Data on File. [14, 15] Summit Therapeutics Inc.; 4. Zhong T, et al. AACR-NCI-EORTC International Conference 2023.Poster #B123,Abstract #35333, Boston, MA, USA,; 5. Zhong T, et al. JITC 2022;10(2):521.

Ivonescimab Clinical Data

AK112-201 Cohort 1: Squamous only; ivonescimab + chemo Phase II, N=63 ORR† 67% DCR† 95% mDOR† 12.8m mPFS [95% CI] 11.1m [9.5 – 16.3] KEYNOTE-407 China Extension4 pembrolizumab + chemo; randomized Phase III, N=65 KEYNOTE-407 Global5 pembrolizumab + chemo; randomized Phase III, N=278 ORR† 80% 63% DCR† 91% 86% mDOR 7.1m 8.8m mPFS [95% CI] 8.3m [6.2 – 10.5] 8.0m [6.3 – 8.4] Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. Data generated and analyzed by Akeso. Percent Changes from Baseline in Target Lesions Sum of Diameters (N=60) ORR: Overall Response Rate, DCR: Disease Control Rate; mDOR: median Duration of Response, mPFS: median Progression Free Survival, DCO: data cutoff, NSCLC: Non-small Cell Lung Cancer, 1L: First Line, CI: Confidence Interval, SQ: Squamous, mFU: median follow-up, chemo: chemotherapy, m: month 1. Zhang L, et al., ASCO 2023 poster #9087 2. Akeso Press Release, January 7, 2024 3. Data on File 8. Summit Therapeutics Inc. 4. Cheng et. al. JTO Clin Res Rep (2021) 5. Paz-Ares, et. al. Journal of Thoracic Onc (2020) 1L Adv/Metastatic Squamous NSCLC1,2,3 AK112-201 includes 10 mg/kg dosing (16%) and 20 mg/kg dosing (84%) of ivonescimab 10 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Established Standard of Care Presentation exclusively for purposes of evaluating the landscape for ivonescimab † Includes subjects with at least one post-baseline tumor assessment, ORR based on confirmed BOR; N=60 evaluable for response Phase II Ivonescimab + Chemo Median follow up 21.0 months (DCO:10/10/2023)

OS: overall survival, DCO: data cutoff NSCLC: Non-small Cell Lung Cancer, 1L: First Line, CI: Confidence Interval, SQ: Squamous, pembro: pembrolizumab, chemo: chemotherapy, NE: Not Established, NR Not Reached, TRAEs: Treatment Related Adverse Events Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. Data generated and analyzed by Akeso. KEYNOTE-407 China Extension4 pembrolizumab + chemo; randomized Phase III, N=65 KEYNOTE-407 Global5 pembrolizumab + chemo; randomized Phase III, N=278 mOS 30.1m [18.2 – NR] 17.2m [14.4 – 19.7) 12m OS 78.5% 64.7% 24m OS 56.9% 36.0% 1. Zhang L, et al., ASCO 2023 poster #9087 2. Akeso Press Release, January 7, 2024 3. Data on File 8. Summit Therapeutics Inc. 4. Cheng, et. al. JTO Clin Res Rep (2021) 5. Novello, et. al. J Clin Oncol 41, no. 11 (2023) 1L Adv/Metastatic Squamous NSCLC1,2,3 Phase II Ivonescimab + Chemo Median follow up 21.0 months (DCO:10/10/2023) 11 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 AK112-201 Cohort 1: Squamous only ivonescimab + chemo Phase II, N=63 mOS Not Reached [22.5 – NE] 12m OS 85.6% 24m OS 64.8% AK112-201 includes 10 mg/kg dosing (16%) and 20 mg/kg dosing (84%) of ivonescimab Established Standard of Care Presentation exclusively for purposes of evaluating the landscape for ivonescimab

MARIPOSA-24 amivantamab + chemo; randomized Phase III, N=131 KEYNOTE-7895 pembrolizumab + chemo; randomized Phase III, N=245 KEYNOTE-7895 placebo + chemo; randomized Phase III, N=247 ORR† [95% CI] 64.1% [55 – 72] 29.0% [23 – 35] 27.1% [22 – 33] mDOR 6.9m 6.3m 5.6m mPFS [95% CI] 6.3m [5.6 – 8.4] 5.6m [5.5 – 5.8] 5.5m [5.4 – 5.6] AK112-201 Cohort 2 (EGFR+) ivonescimab + chemo Phase II, N=19 ORR† [95% CI] 68.4% [43 – 87] mDOR† 8.7m mPFS [95% CI] 8.5m [5.5 – 13.3] 1. Akeso Press Release, January 7, 2024 2. Data on File 9. Summit Therapeutics Inc. 3. Zhang L, et al., ASCO 2023 poster #9087 4. Passaro A, et al. Ann Onc. 2023. 5. Yang, Oral LBA9000, ASCO 2023 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. Data generated and analyzed by Akeso. ORR: Overall Response Rate, mDOR: median Duration of Response, mPFS: median Progression Free Survival, DCO: data cutoff, NSCLC: Non-small Cell Lung Cancer, 1L: First Line, CI: Confidence Interval, chemo: chemotherapy, m: month 2L+ EGFR-TKI Progressors1,2,3 Phase II Ivonescimab + Chemo Median follow up 25.8 months (DCO:10/10/2023) Percent Changes from Baseline in Target Lesions Sum of Diameters Full Analysis Set (N=19) † Includes subjects with at least one post-baseline tumor assessment, ORR based on confirmed BOR 12 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 AK112-201 includes 10 mg/kg dosing (53%) and 20 mg/kg dosing (47%) of ivonescimab Relevant Phase III Studies Not Currently Approved in this Setting Presentation exclusively for purposes of evaluating the landscape for ivonescimab

MARIPOSA-24 amivantamab + chemo; randomized Phase III, N=131 KEYNOTE-7895 pembrolizumab + chemo; randomized Phase III, N=245 KEYNOTE-7895 placebo + chemo; randomized Phase III, N=247 mOS NR 15.9m [13.7 – 18.8] 14.7m [12.7 – 17.1] 12m OS NR 62% 59% AK112-201 Cohort 2 (EGFR+) ivonescimab + chemo Phase II, N=19 mOS 22.5m [10.4 – NE] 12m OS 74% mOS: median Overall Survival, OS: Overall Survival, chemo: chemotherapy, m: month, NR: not reached, NE: not established, TRAEs: Treatment Related Adverse Events Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. Data generated and analyzed by Akeso. Overall Survival (OS) for EGFR-TKI Progressors (N=19) 2L+ EGFR-TKI Progressors1,2,3 Phase II Ivonescimab + Chemo Median follow up 25.8 months (DCO:10/10/2023) 13 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Relevant Phase III Studies Not Currently Approved in this Setting Presentation exclusively for purposes of evaluating the landscape for ivonescimab 1. Akeso Press Release, January 7, 2024 2. Data on File 9. Summit Therapeutics Inc. 3. Zhang L, et al., ASCO 2023 poster #9087 4. Passaro A, et al. Ann Onc. 2023. 5. Yang, Oral LBA9000, ASCO 2023 AK112-201 includes 10 mg/kg dosing (53%) and 20 mg/kg dosing (47%) of ivonescimab

Ivonescimab Phase II Safety Data in Key NSCLC Settings Ivonescimab Demonstrated a Tolerable Safety Profile DCO: 10/10/2023 14 Number (%) Grade >3 TRAE 28 (44.4) TRAE leading to discontinuation of ivonescimab 7 (11.1) TRAE leading to death 0 Number (%) Grade >3 TRAE 7 (36.8) TRAE leading to discontinuation of ivonescimab 0 TRAE leading to death 0 Data generated based on Akeso sponsored studies. Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. Top Treatment-Emergent Adverse Events All TEAE 62 (98.4) Anemia 39 (61.9) Neutropenia 28 (44.4) Leukopenia 26 (41.3) Top Treatment-Emergent Adverse Events All TEAE 19 (100.0) Anemia 13 (68.4) Neutropenia 12 (63.2) ALT increased 12 (63.2) Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 1L Squamous Ivonescimab + Chemotherapy (n=63) 2L+ EGFRm Ivonescimab + Chemotherapy (n=19) 1. Data on File 4. Summit Therapeutics Inc.

Ivonescimab Opportunity

There are no PD-1/VEGF bispecific antibodies approved or in Phase III in Summit’s license territories Approved Anti PD-(L)1 Therapies Approved Anti-VEGF Therapies There are 50+ Approved Indications for PD-(L)1 & VEGF Therapies Data from cancer.gov updated 2023 16 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Primary Peritoneal Cancer Glioblastoma Esophageal Cancer Melanoma Basel Cell Carcinoma Cervical Cancer Biliary Tract Carcinoma Classic Hodgkin Lymphoma Colorectal Cancer Cutaneous Squamous Cell Cancer Endometrial Carcinoma Gastric Cancer Gastroesophageal Junction Cancer Head & Neck Squamous Cell Cancer Hepatocellular Carcinoma Malignant Pleural Mesothelioma Merkel Cell Carcinoma Nasopharyngeal Carcinoma Non-Small Cell Lung Cancer Primary Mediastinal Large B-Cell Lymphoma Renal Cell Carcinoma Small Cell Lung Cancer Urothelial Carcinoma Triple-Negative Breast Cancer NSCLC (Non-Squamous) Ovarian Cancer Pancreatic Cancer Thyroid Cancer MSI-H or dMMR Colorectal Cancer Peritoneal Mesothelioma Pericardial Mesothelioma Testicular Mesothelioma MSI-H or dMMR Endometrial Carcinoma MSI-H or dMMR Gastric Cancer TMB-H Cholangiocarcinoma TMB-H Gallbladder Cancer TMB-H Melanoma TMB-H Colorectal Cancer TMB-H Gastric Cancer Epithelial Ovarian Fallopian Tube Alveolar Soft Part Sarcoma Approved Anti PD-(L)1 & Anti-VEGF Therapies

Focusing On High Unmet Needs – Near Term * UK, Germany, France, Italy, Spain 1 American Cancer Society: www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (Accessed Jan 2024); World Health Organization: International Agency for Research on Cancer, Globocan data by country (UK, Spain, France, Italy, Germany); Japan National Cancer Registry.; 2 Represents 2L+ EGFRm patients in above jurisdictions; ~14k patients in US; Sources: American Cancer Society; Zhang, et al; Oncotarget (2016); Uhlig, et al. JAMA Netw Open (2019); Ganti, et al. JAMA Oncol (2021); Japan National Cancer Registry; Decision Resources Group; AZ Epidemiology Data (June 2022).; 3 Represents 1L SQ-NSCLC patients in above jurisdictions; ~30k patients in US; Sources: Sekine I, et al. Cancer Sci (2020); Uhlig, et al. JAMA Netw Open (2019); Ganti et al. JAMA Oncol. (2021); Decision Resources Group; AZ Epidemiology Data (June 2022). ~600,0001 Lung Cancer Patients in the US, Europe 5*, Japan ~40k Potential Patients to Help Treat2 ~80k Potential Patients to Help Treat3 17 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024

Trial Indication Histology/Population Regimen Phase III NSCLC EGFRm+ 2L+ Advanced or Metastatic Combo ivonescimab + chemo vs. placebo + chemo NSCLC Squamous 1L Metastatic Combo ivonescimab + chemo vs. pembro + chemo Indication Regimen Phase I Phase II Phase III NSCLC: 2L EGFRm+ Randomized: Combo (chemo) vs. chemo NSCLC: 1L PD-L1 TPS>1% Randomized: Monotherapy vs. pembro (PD-1) NSCLC: 1L Squamous Randomized: Combo (chemo) vs. tislelizumab (PD-1) + chemo NSCLC: 1L Squamous Randomized: Combo (chemo) vs. pembro (PD-1) + chemo Advanced Solid Tumors Monotherapy NSCLC Combo (chemo) NSCLC Monotherapy GYN Tumors Monotherapy Ovarian Cancer Combination (PARPi) NSCLC Monotherapy & Combo (chemo) CRC Combo (CD47 + chemo) HCC Monotherapy NSCLC Combo (PD-1 / CTLA-4 bsAb + chemo) HNSCC Combo (CD47) Advanced Solid Tumors** Combo (CD47, CD47 + chemo, chemo) TNBC Comb (chemo, CD47 + chemo) NSCLC Combo (CD73 + chemo) Advanced Solid Tumors Monotherapy ES-SCLC Combo (chemo) Ivonescimab Global Oncology Clinical Trials These ivonescimab clinical trials are being conducted in China and/or Australia and are fully sponsored and managed by Akeso. NSCLC: Non-Small-cell Lung Cancer, EGFRm+: Epidermal Growth Factor Receptor mutant positives, Combo: Combination, Chemo: Chemotherapy, pembro: pembrolizumab, CRC: Colorectal Cancer, HCC: Hepatocellular Carcinoma, HNSCC: Head & Neck Squamous Cell Carcinoma, BTC: Biliary Tract Cancer, TNBC: Triple Negative Breast Cancer, ES-SCLC: Extensive Stage Small Cell Lung Cancer, PD-1: Programmed Cell Death Protein 1, PARPi: poly(ADP-ribose) polymerase inhibitors Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. **Includes Gastric, BTC, Pancreatic, NSCLC Same Subset Patient Population 1,600+ Patients Treated with Ivonescimab 19 Clinical Trials 4 Phase III 13 Phase II 2 Phase I 7 Dedicated Trials Outside NSCLC 18 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 Same Subset Patient Population

Ivonescimab: Expected 2024 Key Catalysts

Ivonescimab Phase III Trials – Expected 2024 Short-Term Catalysts Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. CDE: Centre for Drug Evaluation *NDA Filing by Akeso with the CDE for Marketing Approval in China, 2023 Head-to-Head vs. Pembrolizumab Same Subset Patient Population 20 Summit Proprietary Information - Do Not Copy, Photograph or Distribute J.P. Morgan 42nd Annual Healthcare Conference, January 2024 AK112-301 CDE Decision Expected* & Topline Data AK112-303 Interim Analysis Randomized Phase III Trial vs. Pembrolizumab Last Patient In H1 H2

Video

Q&A