false 0001556263 0001556263 2023-12-06 2023-12-06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 6, 2023
Syros Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-37813 |
|
45-3772460 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
| 35 CambridgePark Drive Cambridge, Massachusetts |
|
02140 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (617) 744-1340
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| |
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered or to be registered pursuant to Section 12(b) of the Act.
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.001 par value |
|
SYRS |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. |
Regulation FD Disclosure. |
On December 6, 2023, Syros Pharmaceuticals, Inc. (the “Company”) will hold a conference call and webcast in which the Company’s management will review a slide presentation describing initial randomized data from its ongoing SELECT-AML-1 Phase 2 trial evaluating tamibarotene in combination with venetoclax and azacitidine in newly diagnosed, unfit patients with acute myeloid leukemia and RARA gene overexpression. This slide presentation is attached as Exhibit 99.1 to this Form 8-K and incorporated herein by reference. The information responsive to Item 7.01 of this Form 8-K and Exhibit 99.1 hereto shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such a filing.
On December 6, 2023, the Company issued a press release announcing the initial randomized data from the ongoing SELECT-AML-1 trial. The press release issued in connection with this announcement is attached as Exhibit 99.2 to this Form 8-K and incorporated herein by reference.
| Item 9.01 |
Financial Statements and Exhibits. |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
SYROS PHARMACEUTICALS, INC. |
|
|
|
|
| Date: December 6, 2023 |
|
|
|
By: |
|
/s/ Jason Haas |
|
|
|
|
|
|
Jason Haas Chief Financial Officer |

SELECT-AML-1: Initial Randomized Data
Evaluating Tamibarotene in Newly Diagnosed AML Patients Ineligible for Standard Induction Therapy December 6, 2023 Exhibit 99.1

Forward-looking statements This
presentation contains forward-looking statements (including within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended) concerning Syros and other matters, such as
Syros’ clinical development plans, including with respect to tamibarotene, Syros’ ability to deliver benefit to patients and value to stockholders, the timing and impact of upcoming clinical data readouts, and the sufficiency of
Syros’ capital resources to fund its operating expenses and capital expenditure requirements into 2025. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial
condition, or otherwise, based on management’s current beliefs, as well as assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and
depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,”
“likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions. Statements that are not historical facts are forward-looking statements. Forward-looking statements are
based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various
factors, including, without limitation, Syros’ ability to: advance the development of its programs, including tamibarotene, under the timelines it projects in current and future clinical trials; demonstrate in any current and future clinical
trials the requisite safety, efficacy and combinability of its drug candidates; sustain the response rates and durability of response seen to date with its drug candidates; successfully develop a companion diagnostic test to identify patients with
the RARA biomarker; obtain and maintain patent protection for its drug candidates and the freedom to operate under third party intellectual property; obtain and maintain necessary regulatory approvals; identify, enter into out-licensing arrangements
with third parties; manage competition; manage expenses; raise the substantial additional capital needed to achieve its business objectives; attract and retain qualified personnel; and successfully execute on its business strategies. The
foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk
factors included in Syros’ Annual Report on Form 10-K for the year ended December 31, 2022 and Syros’ Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, each of which is on file with the Securities and Exchange
Commission (SEC). Except as required by applicable law, Syros undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or
otherwise.
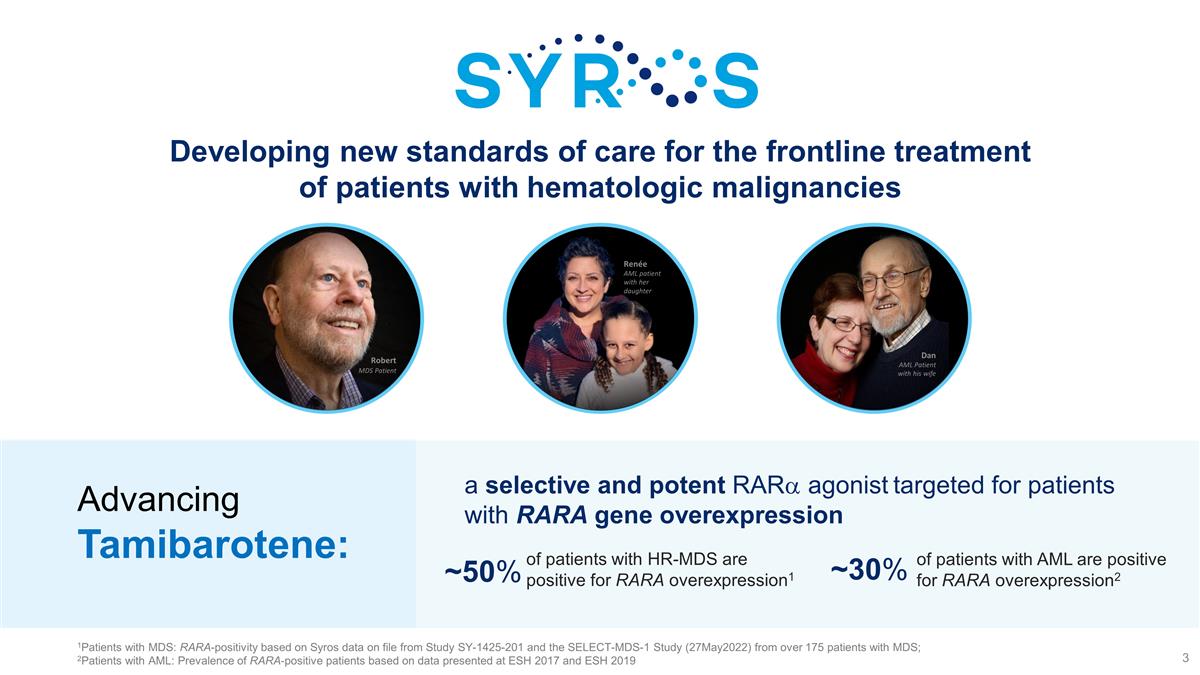
Developing new standards of care for
the frontline treatment of patients with hematologic malignancies Robert MDS Patient Dan AML Patient with his wife Renée AML patient with her daughter of patients with HR-MDS are positive for RARA overexpression1 ~50% ~30% of patients with AML
are positive for RARA overexpression2 a selective and potent RARa agonist targeted for patients with RARA gene overexpression Advancing Tamibarotene: 1Patients with MDS: RARA-positivity based on Syros data on file from Study SY-1425-201
and the SELECT-MDS-1 Study (27May2022) from over 175 patients with MDS; 2Patients with AML: Prevalence of RARA-positive patients based on data presented at ESH 2017 and ESH 2019
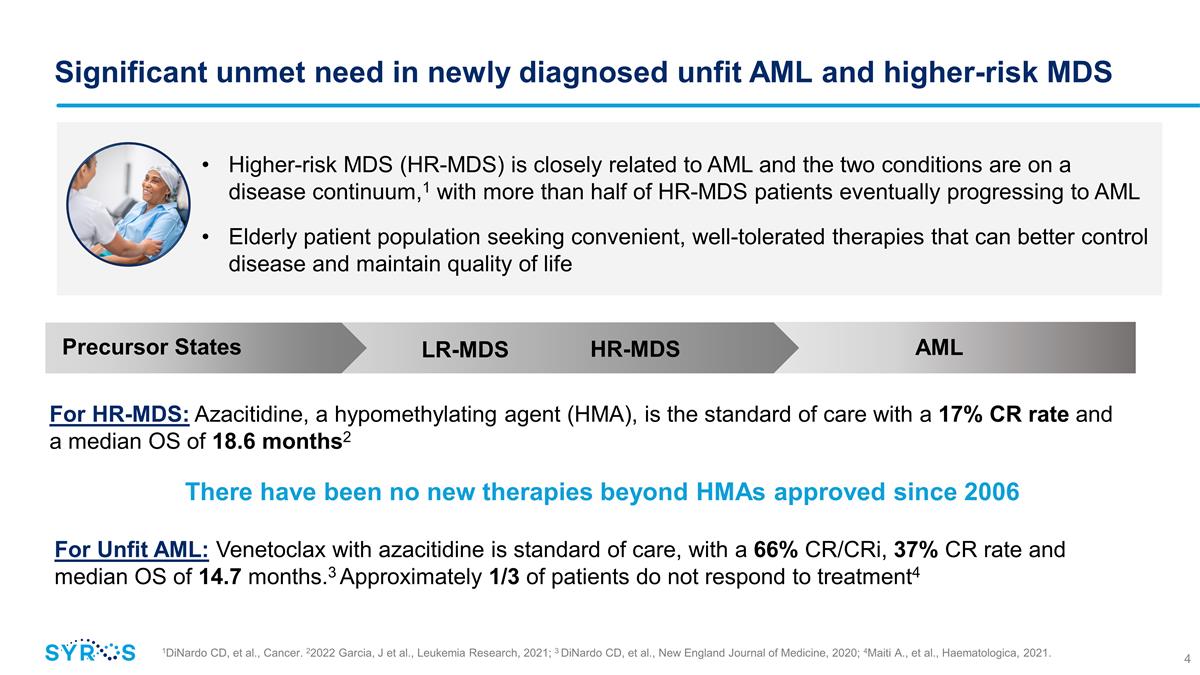
Significant unmet need in newly
diagnosed unfit AML and higher-risk MDS 1DiNardo CD, et al., Cancer. 22022 Garcia, J et al., Leukemia Research, 2021; 3 DiNardo CD, et al., New England Journal of Medicine, 2020; 4Maiti A., et al., Haematologica, 2021. Higher-risk MDS (HR-MDS) is
closely related to AML and the two conditions are on a disease continuum,1 with more than half of HR-MDS patients eventually progressing to AML Elderly patient population seeking convenient, well-tolerated therapies that can better control disease
and maintain quality of life Precursor States AML HR-MDS LR-MDS For HR-MDS: Azacitidine, a hypomethylating agent (HMA), is the standard of care with a 17% CR rate and a median OS of 18.6 months2 There have been no new therapies beyond HMAs approved
since 2006 For Unfit AML: Venetoclax with azacitidine is standard of care, with a 66% CR/CRi, 37% CR rate and median OS of 14.7 months.3 Approximately 1/3 of patients do not respond to treatment4
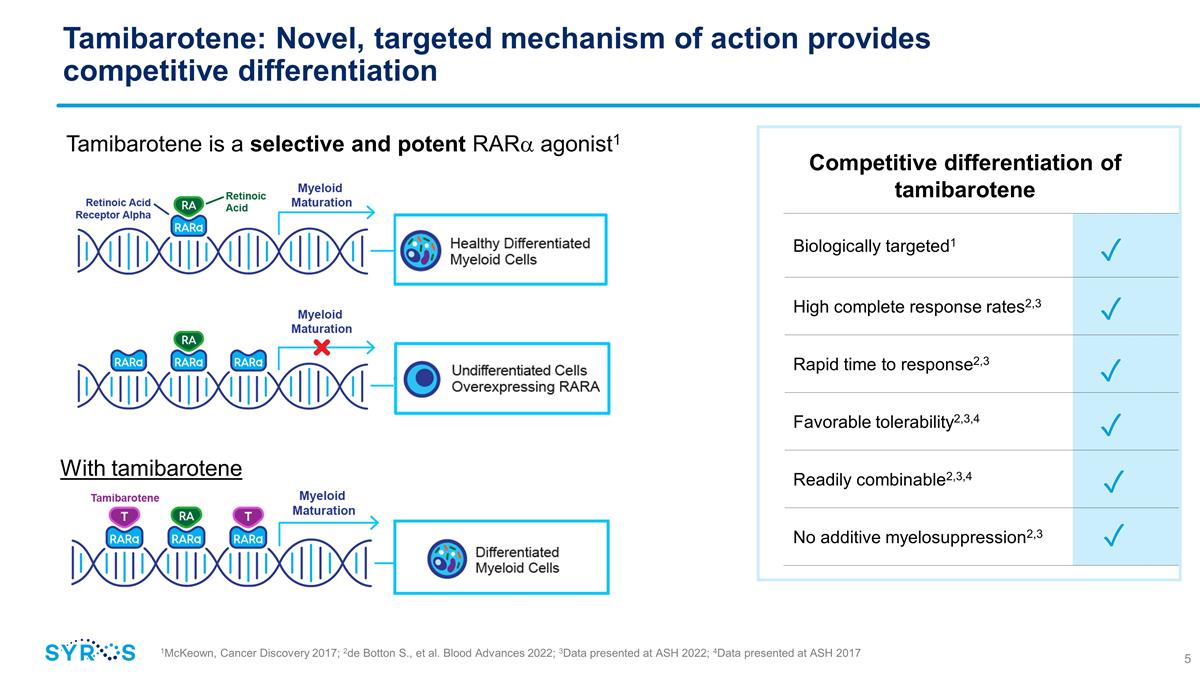
Biologically targeted1 High complete
response rates2,3 Rapid time to response2,3 Favorable tolerability2,3,4 Readily combinable2,3,4 No additive myelosuppression2,3 Tamibarotene: Novel, targeted mechanism of action provides competitive differentiation Tamibarotene is a
selective and potent RARa agonist1 ✓ ✓ ✓ ✓ ✓ ✓ 1McKeown, Cancer Discovery 2017; 2Data presented at ASH 2017; 3de Botton S., et al. Blood Advances 2022; 4Data presented at ASH 2022 Competitive differentiation
of tamibarotene With tamibarotene 1McKeown, Cancer Discovery 2017; 2de Botton S., et al. Blood Advances 2022; 3Data presented at ASH 2022; 4Data presented at ASH 2017
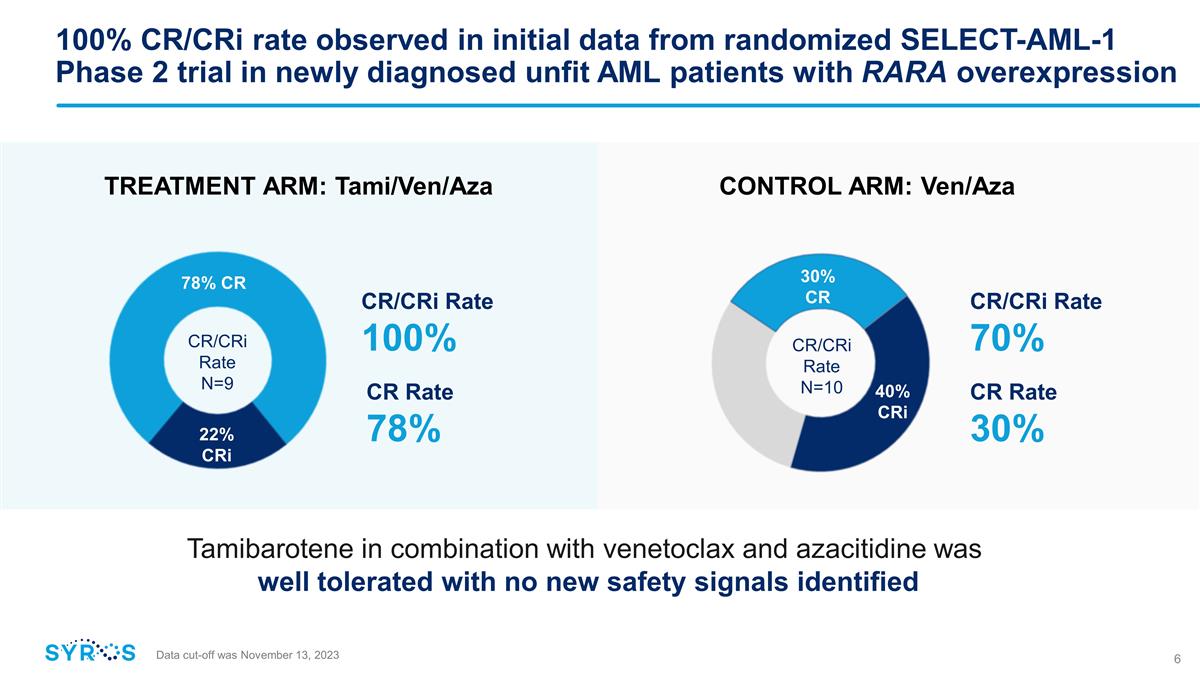
100% CR/CRi rate observed in
initial data from randomized SELECT-AML-1 Phase 2 trial in newly diagnosed unfit AML patients with RARA overexpression CR/CRi Rate 70% CR/CRi Rate 100% CONTROL ARM: Ven/Aza TREATMENT ARM: Tami/Ven/Aza Tamibarotene in combination with
venetoclax and azacitidine was well tolerated with no new safety signals identified 22% CRi 78% CR CR Rate 78% CR/CRi Rate N=9 30% CR 40% CRi CR/CRi Rate N=10 CR Rate 30% Data cut-off was November 13, 2023
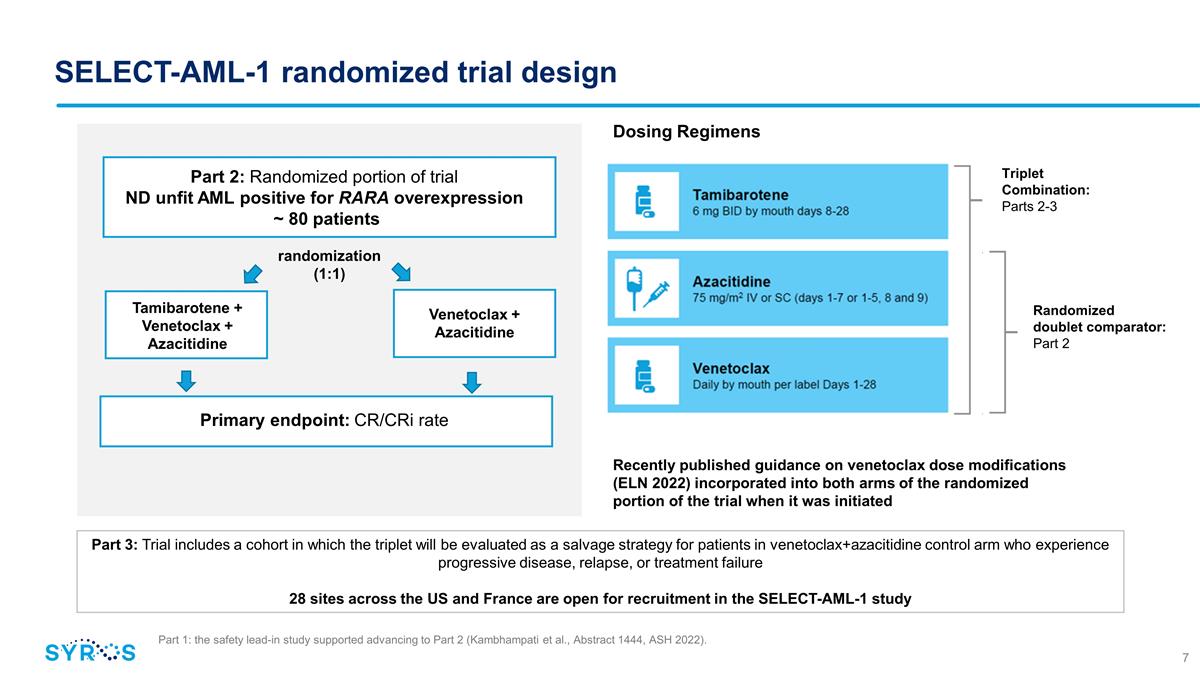
SELECT-AML-1 randomized trial design
Part 3: Trial includes a cohort in which the triplet will be evaluated as a salvage strategy for patients in venetoclax+azacitidine control arm who experience progressive disease, relapse, or treatment failure 28 sites across the US and
France are open for recruitment in the SELECT-AML-1 study Dosing Regimens Recently published guidance on venetoclax dose modifications (ELN 2022) incorporated into both arms of the randomized portion of the trial when it was initiated Part
2: Randomized portion of trial ND unfit AML positive for RARA overexpression ~ 80 patients Venetoclax + Azacitidine randomization (1:1) Tamibarotene + Venetoclax + Azacitidine Primary endpoint: CR/CRi rate Triplet Combination: Parts 2-3 Randomized
doublet comparator: Part 2 Part 1: the safety lead-in study supported advancing to Part 2 (Kambhampati et al., Abstract 1444, ASH 2022).
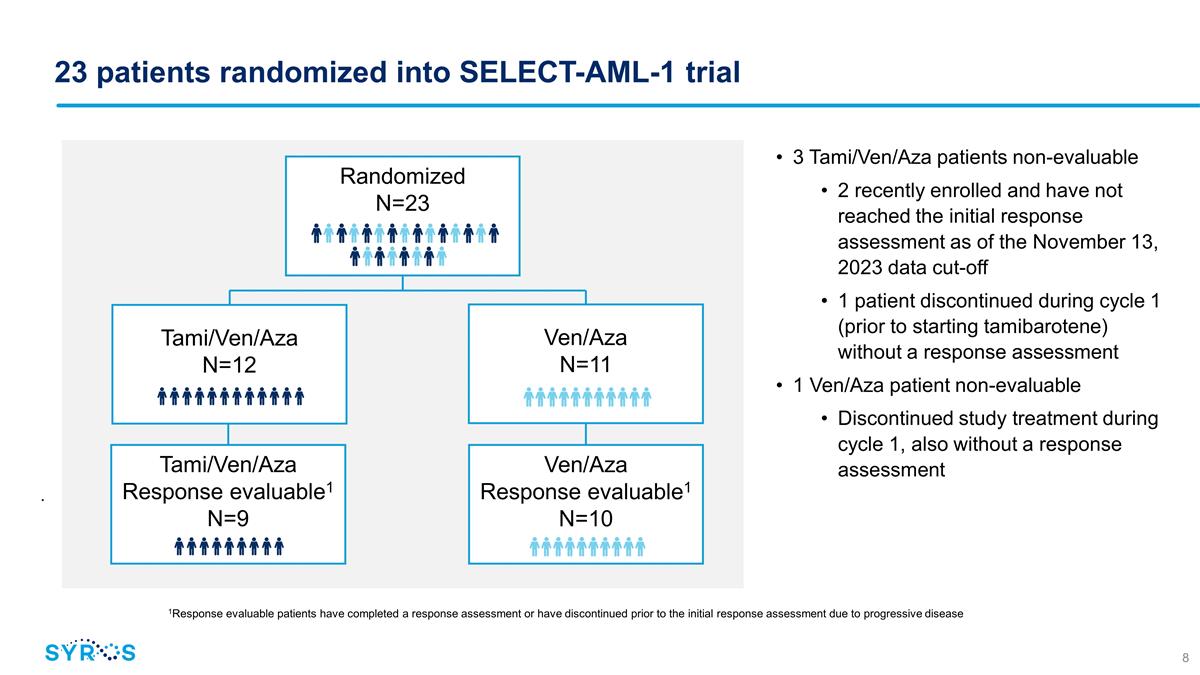
23 patients randomized
into SELECT-AML-1 trial . 3 Tami/Ven/Aza patients non-evaluable 2 recently enrolled and have not reached the initial response assessment as of the November 13, 2023 data cut-off 1 patient discontinued during cycle 1 (prior to
starting tamibarotene) without a response assessment 1 Ven/Aza patient non-evaluable Discontinued study treatment during cycle 1, also without a response assessment Randomized N=23 Tami/Ven/Aza N=12 Ven/Aza N=11
Tami/Ven/Aza Response evaluable1 N=9 Ven/Aza Response evaluable1 N=10 1Response evaluable patients have completed a response assessment or have discontinued prior to the initial response assessment due to progressive disease

Enrolled patients representative of
elderly unfit AML patient population and demonstrated balanced randomization across the two arms Characteristic Tami/Ven/Aza N=12 Ven/Aza N=11 Median age, years (range) 77 (66, 85) 76 (69, 84) Age group, years, n (%)
≤75 4 (33) 5 (45) >75 8 (67) 6 (55) Sex, n (%) Male 5 (42) 5 (45) Female 7 (58) 6 (55) Diagnosis, n (%) De novo AML 9 (75) 8 (73)
Secondary AML 3 (25) 3 (27) Baseline bone marrow blasts ≤30% 5 (42) 4 (36) ELN risk status, n (%) Favorable 3 (25) 2 (18) Intermediate 5 (42) 4
(36) Adverse 3 (25) 5 (45) Missing 1 (8) 0 (0)
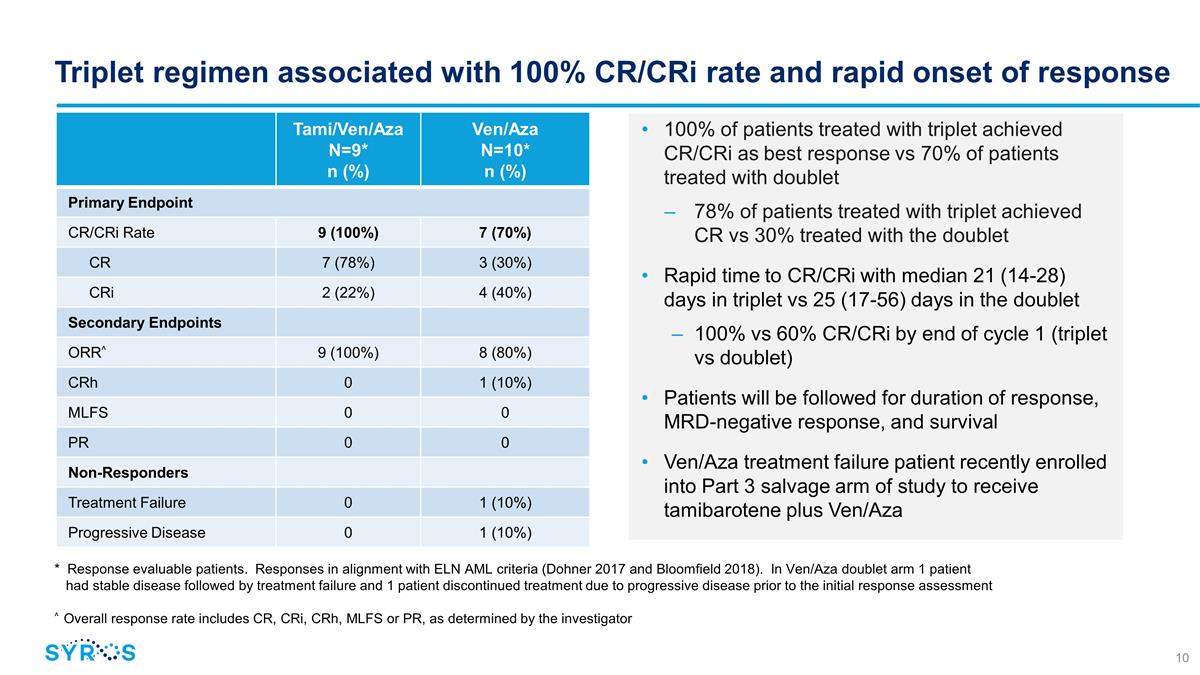
Triplet regimen associated with
100% CR/CRi rate and rapid onset of response Tami/Ven/Aza N=9* n (%) Ven/Aza N=10* n (%) Primary Endpoint CR/CRi Rate 9 (100%) 7 (70%) CR 7 (78%) 3 (30%) CRi 2 (22%) 4 (40%) Secondary Endpoints ORR^ 9 (100%) 8 (80%) CRh 0 1 (10%) MLFS 0 0 PR 0 0
Non-Responders Treatment Failure 0 1 (10%) Progressive Disease 0 1 (10%) 100% of patients treated with triplet achieved CR/CRi as best response vs 70% of patients treated with doublet 78% of patients treated with triplet achieved CR vs 30% treated
with the doublet Rapid time to CR/CRi with median 21 (14-28) days in triplet vs 25 (17-56) days in the doublet 100% vs 60% CR/CRi by end of cycle 1 (triplet vs doublet) Patients will be followed for duration of response, MRD-negative response,
and survival Ven/Aza treatment failure patient recently enrolled into Part 3 salvage arm of study to receive tamibarotene plus Ven/Aza * Response evaluable patients. Responses in alignment with ELN AML criteria (Dohner 2017 and
Bloomfield 2018). In Ven/Aza doublet arm 1 patient had stable disease followed by treatment failure and 1 patient discontinued treatment due to progressive disease prior to the initial response assessment ^ Overall response rate includes CR, CRi,
CRh, MLFS or PR, as determined by the investigator
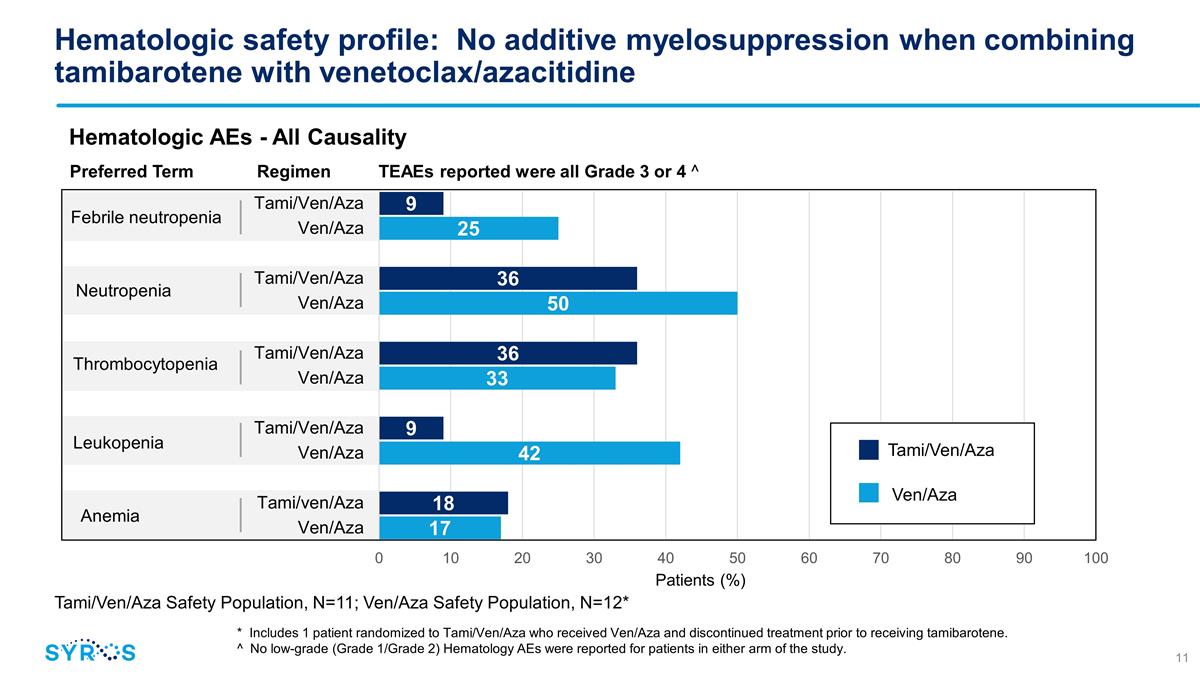
Hematologic safety profile:
No additive myelosuppression when combining tamibarotene with venetoclax/azacitidine Febrile neutropenia Neutropenia Thrombocytopenia Leukopenia Anemia Hematologic AEs - All Causality Tami/Ven/Aza Safety Population, N=11; Ven/Aza Safety Population,
N=12* * Includes 1 patient randomized to Tami/Ven/Aza who received Ven/Aza and discontinued treatment prior to receiving tamibarotene. ^ No low-grade (Grade 1/Grade 2) Hematology AEs were reported for patients in either arm of the study. Preferred
Term Regimen TEAEs reported were all Grade 3 or 4 ^ Tami/Ven/Aza Ven/Aza
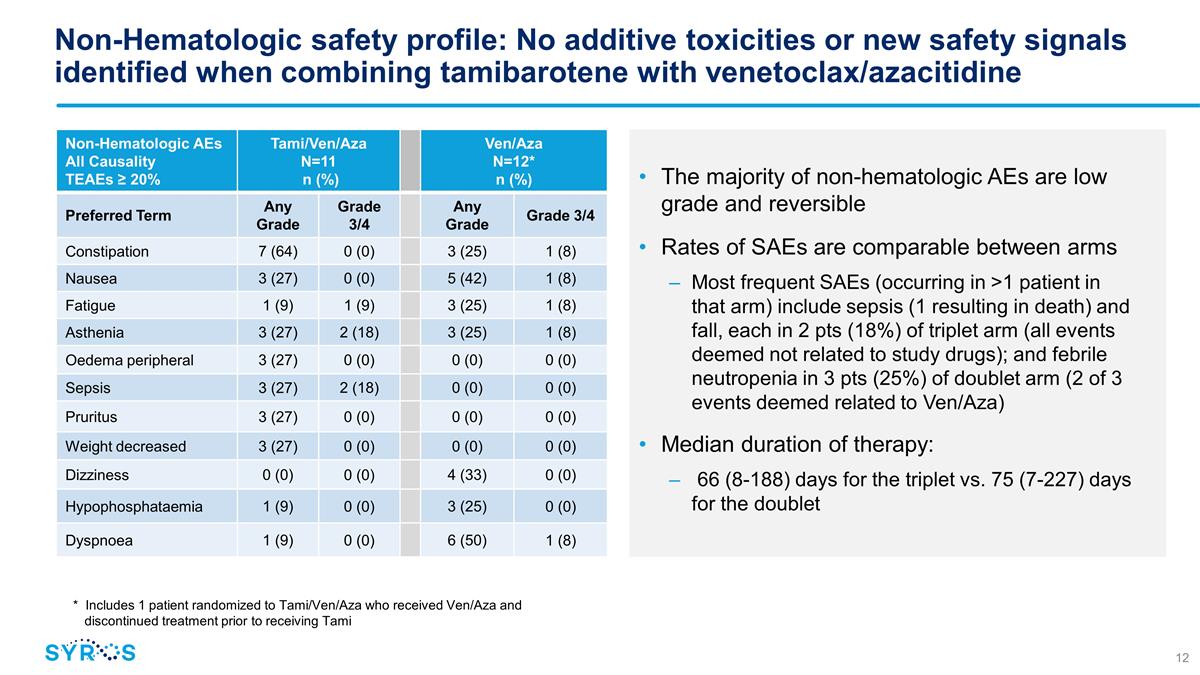
Non-Hematologic safety profile: No
additive toxicities or new safety signals identified when combining tamibarotene with venetoclax/azacitidine The majority of non-hematologic AEs are low grade and reversible Rates of SAEs are comparable between arms Most frequent
SAEs (occurring in >1 patient in that arm) include sepsis (1 resulting in death) and fall, each in 2 pts (18%) of triplet arm (all events deemed not related to study drugs); and febrile neutropenia in 3 pts (25%) of doublet arm (2 of 3 events
deemed related to Ven/Aza) Median duration of therapy: 66 (8-188) days for the triplet vs. 75 (7-227) days for the doublet Non-Hematologic AEs All Causality TEAEs ≥ 20% Tami/Ven/Aza N=11 n (%) Ven/Aza N=12* n (%) Preferred Term Any Grade Grade
3/4 Any Grade Grade 3/4 Constipation 7 (64) 0 (0) 3 (25) 1 (8) Nausea 3 (27) 0 (0) 5 (42) 1 (8) Fatigue 1 (9) 1 (9) 3 (25) 1 (8) Asthenia 3 (27) 2 (18) 3 (25) 1 (8) Oedema peripheral 3 (27) 0 (0) 0 (0) 0 (0) Sepsis 3 (27) 2 (18) 0 (0) 0 (0) Pruritus
3 (27) 0 (0) 0 (0) 0 (0) Weight decreased 3 (27) 0 (0) 0 (0) 0 (0) Dizziness 0 (0) 0 (0) 4 (33) 0 (0) Hypophosphataemia 1 (9) 0 (0) 3 (25) 0 (0) Dyspnoea 1 (9) 0 (0) 6 (50) 1 (8) * Includes 1 patient randomized to Tami/Ven/Aza who received Ven/Aza
and discontinued treatment prior to receiving Tami
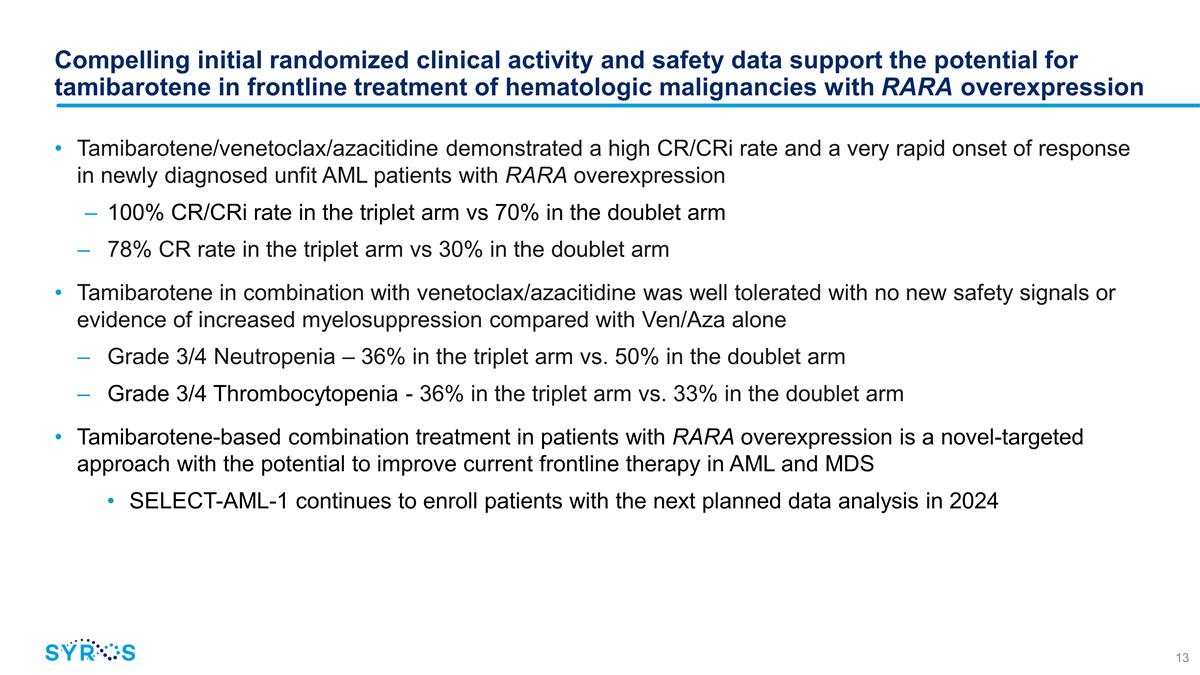
Compelling initial randomized
clinical activity and safety data support the potential for tamibarotene in frontline treatment of hematologic malignancies with RARA overexpression Tamibarotene/venetoclax/azacitidine demonstrated a high CR/CRi rate and a very rapid onset of
response in newly diagnosed unfit AML patients with RARA overexpression 100% CR/CRi rate in the triplet arm vs 70% in the doublet arm 78% CR rate in the triplet arm vs 30% in the doublet arm Tamibarotene in combination with
venetoclax/azacitidine was well tolerated with no new safety signals or evidence of increased myelosuppression compared with Ven/Aza alone Grade 3/4 Neutropenia – 36% in the triplet arm vs. 50% in the doublet arm Grade 3/4 Thrombocytopenia -
36% in the triplet arm vs. 33% in the doublet arm Tamibarotene-based combination treatment in patients with RARA overexpression is a novel-targeted approach with the potential to improve current frontline therapy in AML and MDS
SELECT-AML-1 continues to enroll patients with the next planned data analysis in 2024
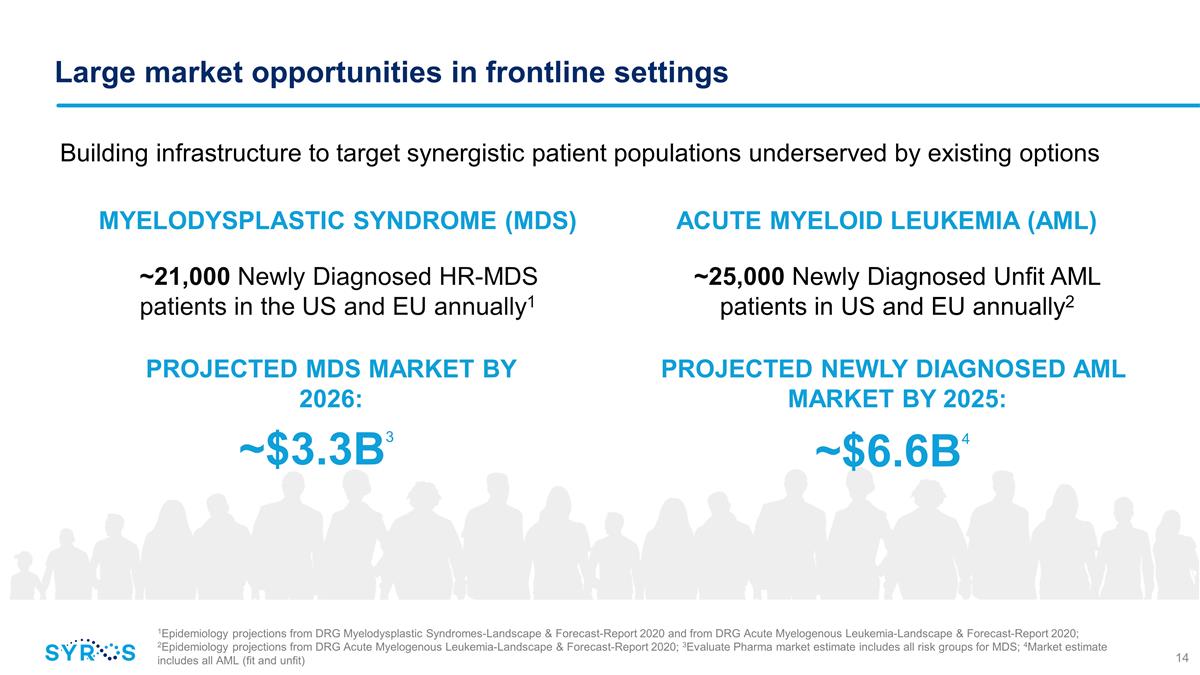
Large market opportunities in
frontline settings ~25,000 Newly Diagnosed Unfit AML patients in US and EU annually2 Building infrastructure to target synergistic patient populations underserved by existing options ~21,000 Newly Diagnosed HR-MDS patients in the US and EU annually1
~$3.3B3 MYELODYSPLASTIC SYNDROME (MDS) ACUTE MYELOID LEUKEMIA (AML) PROJECTED MDS MARKET BY 2026: ~$6.6B4 PROJECTED NEWLY DIAGNOSED AML MARKET BY 2025: 1Epidemiology projections from DRG Myelodysplastic Syndromes-Landscape & Forecast-Report 2020
and from DRG Acute Myelogenous Leukemia-Landscape & Forecast-Report 2020; 2Epidemiology projections from DRG Acute Myelogenous Leukemia-Landscape & Forecast-Report 2020; 3Evaluate Pharma market estimate includes all risk groups for
MDS; 4Market estimate includes all AML (fit and unfit)
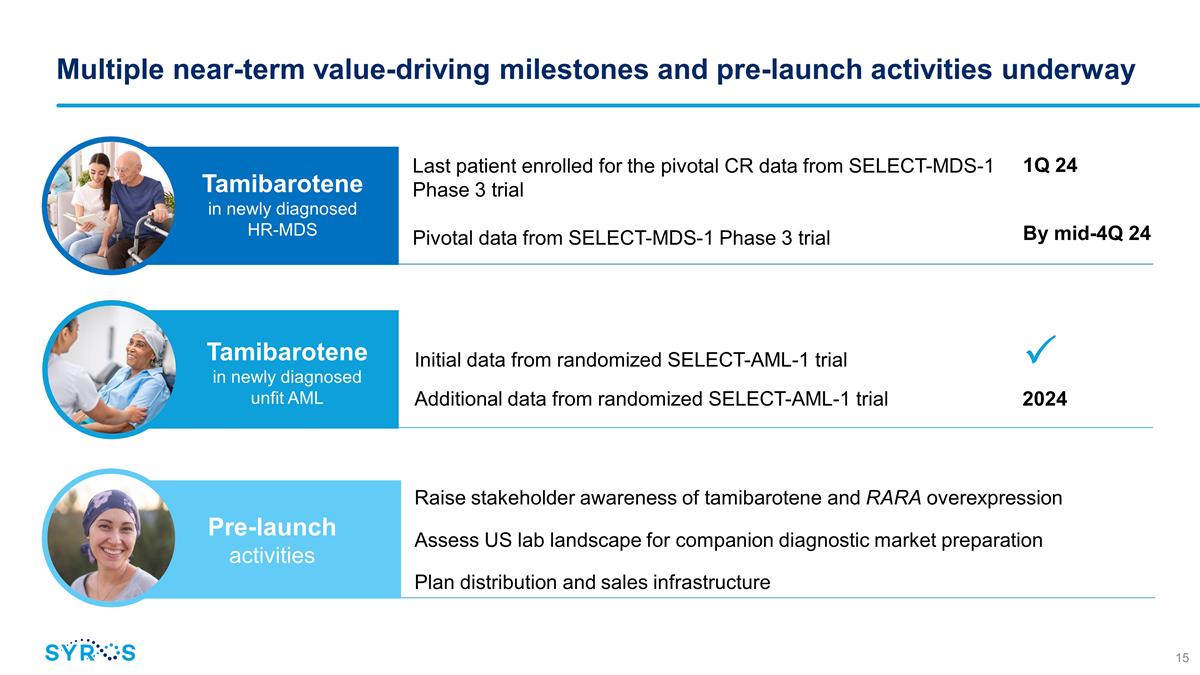
Multiple near-term value-driving
milestones and pre-launch activities underway Last patient enrolled for the pivotal CR data from SELECT-MDS-1 Phase 3 trial Pivotal data from SELECT-MDS-1 Phase 3 trial Initial data from randomized SELECT-AML-1 trial
Additional data from randomized SELECT-AML-1 trial 2024 1Q 24 By mid-4Q 24 Tamibarotene in newly diagnosed HR-MDS Tamibarotene in newly diagnosed unfit AML Raise stakeholder awareness
of tamibarotene and RARA overexpression Assess US lab landscape for companion diagnostic market preparation Plan distribution and sales infrastructure Pre-launch activities ��

Exhibit 99.2
Syros Announces Encouraging Initial Data from Randomized
SELECT-AML-1 Phase 2 Clinical Trial Evaluating Tamibarotene in Combination with Venetoclax and Azacitidine
— 100% CR/CRi Rate in Patients Treated with Tamibarotene, Venetoclax and Azacitidine Compared to 70% in Patients
Randomized to Treatment with Venetoclax and Azacitidine Alone —
— Triplet Regimen Continues to Demonstrate Favorable
Tolerability —
— Additional Data Expected in 2024 —
— Management to Host Conference Call at 8:30 a.m. ET Today —
CAMBRIDGE, Mass., December 6, 2023 – Syros Pharmaceuticals (NASDAQ: SYRS), a biopharmaceutical company committed to advancing new standards of care
for the frontline treatment of hematologic malignancies, today announced strong and encouraging initial data from its ongoing SELECT-AML-1 Phase 2 trial evaluating
tamibarotene, an oral, selective, retinoic acid receptor alpha (RARα) agonist, in combination with venetoclax and azacitidine in newly diagnosed, unfit patients with acute myeloid leukemia (AML) and RARA gene overexpression.
“I am highly encouraged by the initial data from the randomized portion of
SELECT-AML-1,” said Thomas Cluzeau, MD, PhD, Head of Hematology at Nice University Hospital, Côte d’Azur University in France. “Despite the recent
advances in treatment for unfit AML patients, there remains a substantial need for options that offer higher response rates and improved overall survival, particularly for the one-third of patients who do not
respond to existing standard-of-care. I believe tamibarotene may offer a significant therapeutic advance for the treatment of AML and I am eager to continue enrolling
patients in the ongoing SELECT-AML-1 trial.”
“These data
highlight the potential of tamibarotene to be a cornerstone therapy for newly diagnosed, unfit AML patients with RARA overexpression, further demonstrating its differentiated product profile and validating our biologically targeted
approach,” said David A. Roth, M.D., Chief Medical Officer of Syros. “These results — the first from a randomized, controlled study — demonstrate the potential impact of adding tamibarotene to the
standard-of-care, venetoclax and azacitidine and, importantly, are consistent with prior experience. Across multiple clinical trials, we have observed
tamibarotene’s ability to rapidly deliver clinically relevant activity, with a well-tolerated safety profile, including in a combination setting. We look forward to advancing our comprehensive clinical development program for tamibarotene, with
additional data from SELECT-AML-1 and pivotal complete response data from our
SELECT-MDS-1 trial in higher-risk myelodysplastic syndrome with RARA overexpression expected next year, as we work to deliver profound benefit to patients with
hematologic malignancies.”
Initial Data from SELECT-AML-1 Phase 2
Trial
SELECT-AML-1 is evaluating the safety and efficacy of
tamibarotene in combination with venetoclax and azacitidine compared to venetoclax and azacitidine in approximately 80 patients randomized 1:1. The trial is also evaluating the triplet regimen as a salvage strategy in patients in the control arm who
do not respond to venetoclax and azacitidine. The primary endpoint of the trial is complete response rate (CR)/complete response with incomplete hematologic recovery (CRi). In December 2022, Syros reported data from the safety lead-in portion of SELECT-AML-1, in which five of six response evaluable patients (83%) achieved CR/CRi.
As of November 13, 2023, 23 newly diagnosed unfit AML patients positive for RARA overexpression had enrolled in the randomized portion of the
trial, including 19 who were evaluable for response. The median age of the patients for the triplet arm was 77 (ranging from 66-85) and the median age of the patients for the doublet arm was 76 (ranging from 69-84).
Clinical Activity Data
| |
• |
|
The primary endpoint (CR/CRi rate), defined in alignment with ELN AML criteria (Dohner 2017 and Bloomfield 2018),
was 100% among response evaluable patients (nine of nine) treated with the combination of tamibarotene, venetoclax and azacitidine, as compared to 70% of patients (seven of ten) treated with the control (venetoclax and azacitidine alone).
|
| |
• |
|
Seven of the nine response evaluable patients (78%) treated with the combination of tamibarotene, venetoclax and
azacitidine achieved a CR and two patients (22%) achieved a CRi. |
| |
• |
|
Three of the ten response evaluable patients (30%) treated with the control achieved a CR and four patients (40%)
achieved a CRi. |
| |
• |
|
Median time to CR/CRi response was 21 days (ranging from 14-28) among
patients treated with the combination of tamibarotene, venetoclax and azacitidine, as compared to 25 days (ranging from 17-56) among patients treated with the control, with the CR/CRi being reached by 100% of
patients in the triplet arm by the end of cycle one, compared with 60% of patients in the doublet control arm. |
Safety Data
| |
• |
|
Consistent with prior clinical experience from the safety lead-in portion
of this study, tamibarotene administered in combination with approved doses of venetoclax and azacitidine was generally well tolerated, and the overall safety profile demonstrated no additive toxicities or new safety signals, or evidence of
increased myelosuppression compared to treatment with the doublet combination of venetoclax and azacitidine. The majority of non-hematologic adverse events (AEs) were
low-grade and reversible, and rates of serious adverse events (SAEs) were comparable between the study arms. |
| |
• |
|
Median duration of treatment was 66 days (ranging from 8-188) among
patients treated with the combination of tamibarotene, venetoclax and azacitidine, and 75 days (ranging from 7-227) for patients treated with the control. Patients will be followed for duration of response,
minimal residual disease (MRD)-negative response, and survival. |
Syros continues to enroll patients in SELECT-AML-1 and anticipates reporting updated data from the trial in 2024.
Syros is also evaluating
tamibarotene in combination with azacitidine in the SELECT-MDS-1 Phase 3 clinical trial in newly diagnosed higher-risk myelodysplastic syndrome patients with RARA
gene overexpression. Syros expects to complete patient enrollment in SELECT-MDS-1 in the first quarter of 2024 and to report pivotal CR data by the middle of the fourth
quarter of 2024.
Conference Call and Webcast
Syros
will host a conference call today at 8:30 a.m. ET to discuss these data. To access the live conference call, please dial (888) 259-6580 (domestic) or (416) 764-8624
(international) and refer to conference ID 19696416. A webcast of the call will also be available on the Investors & Media section of the Syros website at www.syros.com. An archived replay of the webcast will be available for approximately
30 days following the presentation.
Upcoming Investor Conference
Syros will also present at the JMP Securities Hematology and Oncology Summit today. Management will participate in a fireside chat at 11:00 a.m. ET. To access
the live webcast and subsequent archived recording of the event, please visit the Investors & Media section of the Syros website at www.syros.com.
About Syros Pharmaceuticals
Syros is committed to
developing new standards of care for the frontline treatment of patients with hematologic malignancies. Driven by the motivation to help patients with blood disorders that have largely eluded other targeted approaches, Syros is developing
tamibarotene, an oral selective RARα agonist in frontline patients with higher-risk myelodysplastic syndrome and acute myeloid leukemia with RARA gene overexpression. For more information,
visit www.syros.com and follow us on Twitter (@SyrosPharma) and LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, including without
limitation statements regarding Syros’ clinical development plans, including with respect to the progression of its clinical trials involving tamibarotene, the timing and impact of upcoming clinical data readouts, the timing to complete patient
enrollment in SELECT-MDS-1, and the therapeutic potential of tamibarotene. The words “anticipate,” “believe,” “continue,”
“could,” “estimate,” “expect,” “hope,” “intend,”
“may,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “would,” and similar expressions are intended
to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking
statements as a result of various important factors, including Syros’ ability to: advance the development of its programs under the timelines it projects in current and future clinical trials; demonstrate in any current and future clinical
trials the requisite safety, efficacy and combinability of its drug candidates; sustain the response rates and durability of response seen to date with its drug candidates; successfully develop a companion diagnostic test to identify patients with
the RARA biomarker; obtain and maintain patent protection for its drug candidates and the freedom to operate under third party intellectual property; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration
agreements with third parties; manage competition; manage expenses; raise the substantial additional capital needed to achieve its business objectives; attract and retain qualified personnel; and successfully execute on its business strategies;
risks described under the caption “Risk Factors” in Syros’ Annual Report on Form 10-K for the year ended December 31, 2022 and Quarterly Report on Form
10-Q for the quarter ended September 30, 2023, each of which is on file with the Securities and Exchange Commission; and risks described in other filings that Syros makes with the Securities and Exchange
Commission in the future.
Syros Contact
Karen
Hunady
Director of Corporate Communications & Investor Relations
1-857-327-7321
khunady@syros.com
Investor Relations
Hannah Deresiewicz
Stern Investor Relations, Inc.
212-362-1200
hannah.deresiewicz@sternir.com
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
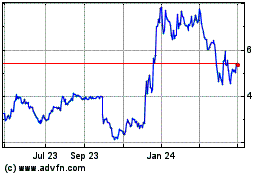
Syros Pharmaceuticals (NASDAQ:SYRS)
Historical Stock Chart
From Mar 2024 to Apr 2024
Syros Pharmaceuticals (NASDAQ:SYRS)
Historical Stock Chart
From Apr 2023 to Apr 2024