UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the Month of November 2023
Commission File Number: 001-38104
IMMURON LIMITED
(Name of Registrant)
Level 3, 62 Lygon Street, Carlton South,
Victoria, 3053, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form
40-F ☐
Indicate by check mark whether by furnishing the information contained
in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities
Exchange Act of 1934.
Yes ☐ No
☒
If “Yes” is marked, indicate below the file number assigned
to the registrant in connection with Rule 12g3-2(b): 82-
IMMURON LIMITED
EXPLANATORY NOTE
Immuron Limited (the “Company”) published
one announcement (the “Public Notices”) to the Australian Securities Exchange on November 28, 2023 titled:
| |
- |
“Investor Webinar Presentation” |
A copy of the Public Notice is attached as an exhibit to this report
on Form 6-K.
This report on Form 6-K (including the exhibit
hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”) and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, except as shall be
expressly set forth by specific reference in such filing.
EXHIBITS
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
IMMURON LIMITED |
| |
|
|
| |
 |
| |
|
|
| Date: November 28, 2023 |
By: |
/s/ Phillip Hains |
| |
|
Phillip Hains |
| |
|
Company Secretary |
3
Exhibit 99.1

Investor
Webinar Presentation
Melbourne, Australia, November 28, 2023: Immuron
Limited (ASX: IMC; NASDAQ: IMRN), an Australian based and globally integrated biopharmaceutical company is pleased to invite shareholders
to attend an investor webinar “Coffee Microcaps Morning Meeting”, to be held on Tuesday 30th November 2023, 9:00am ADEST.
CEO, Steven Lydeamore will provide an overview
and update on the business and upcoming milestones.
Following the presentation, attendees will have
the opportunity to ask questions directly to Mr Lydeamore during a moderated Q & A session.
This webinar can be viewed live via zoom &
you register via the link below.
Zoom: https://us02web.zoom.us/webinar/register/WN_73dRq8CsQK6Ahor0F8UzgA#/registration
Authorised for release by the Board of Immuron
Limited.
- - - END - - -
|
COMPANY CONTACT:
Steven Lydeamore
Chief Executive Officer
Ph: +61 (0)3 9824 5254
info@immuron.com |
|
|
About Travelan®
Travelan®
is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting traveller’s diarrhoea,
a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce.
Travelan® is a highly purified tabletised preparation of hyperimmune bovine antibodies and other factors, which when taken
with meals bind to diarrhoea-causing bacteria and prevent colonisation and the pathology associated with traveller’s diarrhoea.
In Australia, Travelan® is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated
to reduce the risk of Traveller’s Diarrhoea, reduce the risk of minor gastrointestinal disorders and is antimicrobial. In Canada,
Travelan® is a licensed natural health product (NPN 80046016) and is indicated to reduce the risk of Traveller’s
Diarrhoea. In the U.S., Travelan® is sold as a dietary supplement for digestive tract protection.
About Traveller’s Diarrhoea
Traveller’s Diarrhoea
is a gastrointestinal infection with symptoms that include loose, watery (and occasionally bloody) stools, abdominal cramping, bloating,
and fever, Enteropathogenic bacteria are responsible for most cases, with enterotoxigenic Escherichia coli (ETEC) playing a dominant
causative role. Campylobacter spp. are also responsible for a significant proportion of cases. The more serious infections with Salmonella
spp. the bacillary dysentery organisms belonging to Shigella spp. and Vibrio spp. (the causative agent of cholera) are often confused
with Traveller’s Diarrhoea as they may be contracted while travelling and initial symptoms are often indistinguishable.


About Immuron
Immuron Limited (ASX:
IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercialising orally delivered targeted polyclonal
antibodies for the treatment of infectious diseases.
For more information
visit: http://www.immuron.com
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934,
each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development
programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations
and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock
value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth
strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the
results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties
relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key
personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and
intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation
or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in
our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.


1 1 COFFEE MICROCAPS 30 NOVEMBER , 2023 NASDAQ: IMRN ASX: IMC Steven Lydeamore - CEO

2 2 Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections. Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “guidance” and similar expressions are intended to identify forward - looking statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain risks and uncertainties, some of which are beyond Immuron’s control, including those risks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ from those expressed or forecasted in the forward - looking statements. The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made. Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, circumstances or unanticipated events occurring after the date of this presentation except as required by law or by any appropriate regulatory authority. SAFE HARBOR STATEMENT FY2024 results in this presentation are subject to audit review.
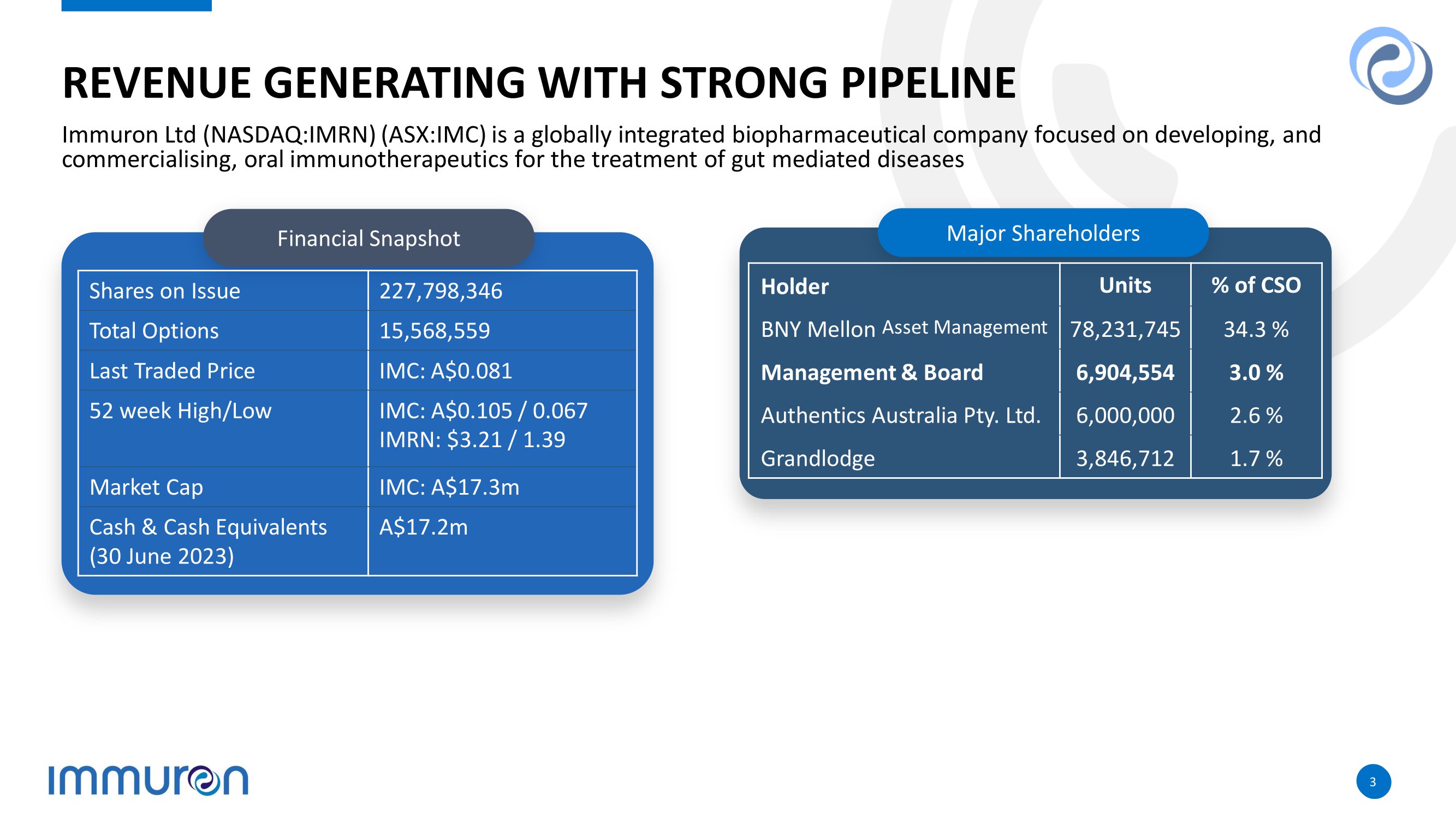
3 3 REVENUE GENERATING WITH STRONG PIPELINE Immuron Ltd (NASDAQ:IMRN) (ASX:IMC) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Financial Snapshot 227,798,346 Shares on Issue 15,568,559 Total Options IMC: A$0.0 81 Last Traded Price IMC: A$0.105 / 0.067 IMRN: $3.21 / 1.39 52 week High/Low IMC: A$17.3m Market Cap A$17.2m Cash & Cash Equivalents (30 June 2023) Major Shareholders % of CSO Units Holder 34.3 % 78,231,745 BNY Mellon Asset Management 3.0 % 6,904,554 Management & Board 2.6 % 6,000,000 Authentics Australia Pty. Ltd. 1.7 % 3,846,712 Grandlodge

4 4 REVENUE GENERATING WITH STRONG PIPELINE Immuron Ltd (NASDAQ:IMRN) (ASX:IMC) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Status at Coffee Microcaps - 30 November 2023 Status at Coffee Microcaps - 2 May 2023 FY23 revenue of A$1.80 million, up 136% 1Q FY24 revenue of A$1.57 million Travelan® is now the #2 SKU and fastest growing in Australian pharmacy antidiarrheal category 1 Australian sales YTD FY24 have exceeded full year FY23 FY23 YTD April revenue of A$1.78 million, up 233% on pcp Travelan® successfully launched in USA on amazon.com Safety stock established for all products, all markets Travelan® set up for launch in USA on amazon.com pending establishing safety stock Completed recruitment and in - patient phase 1H 2024 Anticipated top line results and clinical study report Travelan® (IMM - 124E) IND filed and received FDA authorization FDA removed IND Clinical Hold Institutional Review Board Approval 1H 2024 anticipated clinical trial initiation and completion of in - patient phase CampETEC toxicology study report completed and submitted to the FDA Travelan® Uniformed Health Services University clinical trial reaches 50% of 868 patients Travelan® Uniformed Health Services University clinical trial reaches 35% of 868 patients December 2023 Anticipated cGMP manufacture 1H 2024 Anticipated FDA pre - IND submission IMM - 529 ( clostridioides difficile) solid dose active formulation development completed 1. IQVIA Australia Pharmacy Scan – Antidiarrheal segment, value sales 13 weeks to 21 October 2023
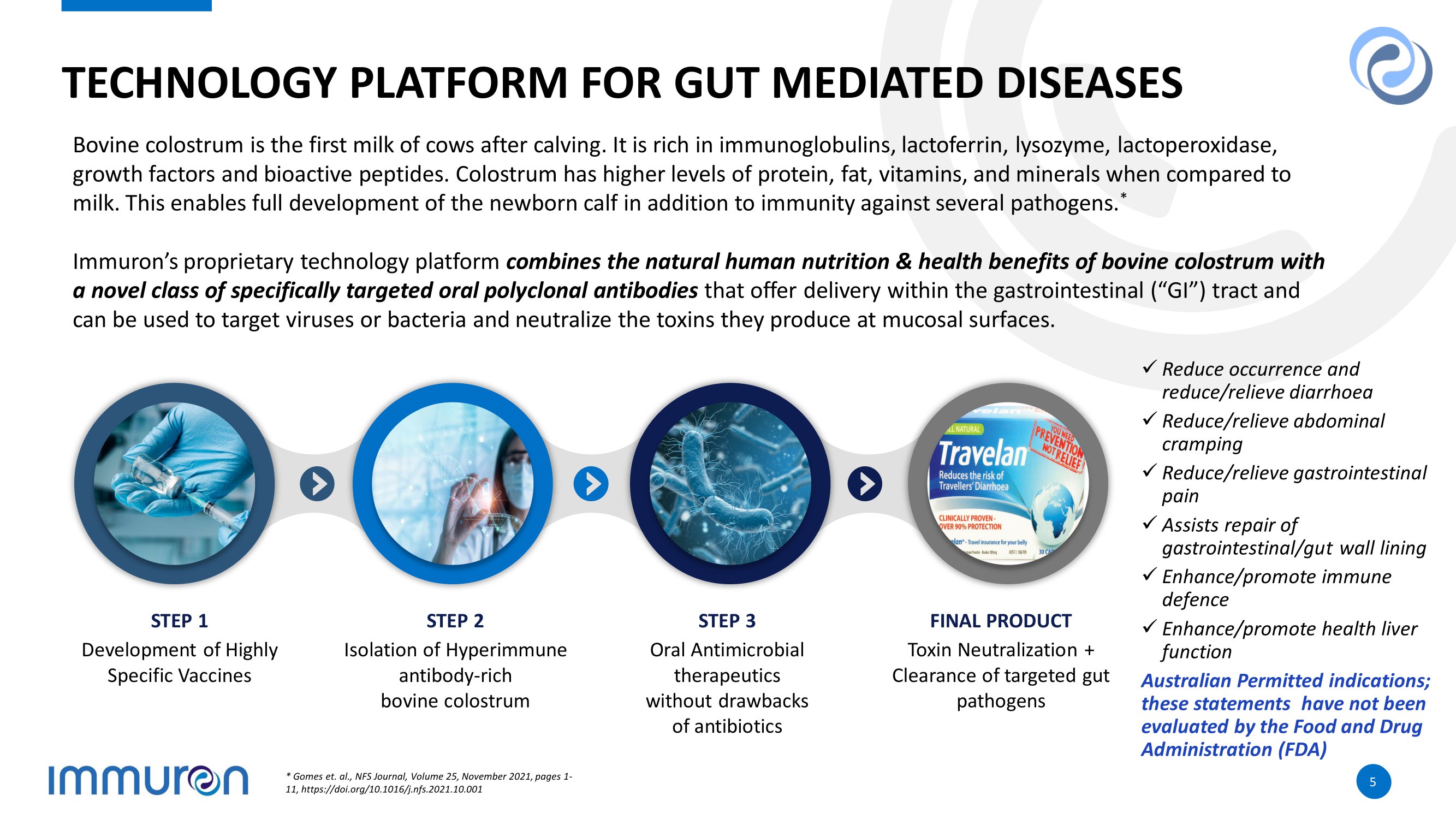
5 5 TECHNOLOGY PLATFORM FOR GUT MEDIATED DISEASES Bovine colostrum is the first milk of cows after calving. It is rich in immunoglobulins, lactoferrin, lysozyme, lactoperoxida se, growth factors and bioactive peptides. Colostrum has higher levels of protein, fat, vitamins, and minerals when compared to milk. This enables full development of the newborn calf in addition to immunity against several pathogens. * Immuron’s proprietary technology platform combines the natural human nutrition & health benefits of bovine colostrum with a novel class of specifically targeted oral polyclonal antibodies that offer delivery within the gastrointestinal (“GI”) tract and can be used to target viruses or bacteria and neutralize the toxins they produce at mucosal surfaces. Development of Highly Specific Vaccines STEP 1 Isolation of Hyperimmune antibody - rich bovine colostrum STEP 2 Oral Antimicrobial therapeutics without drawbacks of antibiotics STEP 3 Toxin Neutralization + Clearance of targeted gut pathogens FINAL PRODUCT x Reduce occurrence and reduce/relieve diarrhoea x Reduce/relieve abdominal cramping x Reduce/relieve gastrointestinal pain x Assists repair of gastrointestinal/gut wall lining x Enhance/promote immune defence x Enhance/promote health liver function Australian Permitted indications; these statements have not been evaluated by the Food and Drug Administration (FDA) * Gomes et. al., NFS Journal, Volume 25, November 2021, pages 1 - 11, https://doi.org/10.1016/j.nfs.2021.10.001

6 6 STRONG SALES GROWTH IN ATTRACTIVE MARKET Industry tailwinds Travel picking up significantly following COVID lockdowns $83m Based on US annual travel numbers and a penetration rate of 15%, the market potential is estimated at $83m 5 $50m Based on EU travel numbers and a penetration rate of 15%, the market potential is estimated at $50m 5 Frequent 30% - 70% of travelers experience TD 4 Traveller’s Diarrhoea (TD) Market is large and growing ~7% 3 CAGR 1. https://www.abs.gov.au/statistics/industry/tourism - and - transport/overseas - arrivals - and - departures - australia/latest - release 2. https://www.trade.gov/sites/default/files/2023 - 09/US - Outbound - to - World - Regions.xlsx 3. Research Reports World, 28 May 2021: Global Traveler’s Diarrhea Therapeutics Industry Research Report, Growth Trends and C omp etitive Analysis 2021 - 2027 4. Centers for Disease Control and Prevention Yellow Book 5. IMC Company Report - Travelan Market Analysis 2019 Chief Commercial Officer has 20+ year’s experience with local and global (Asia, UK) commercial leadership roles with GSK and P&G Net Sales FY23: A$1.16 million 1QFY24: A$1.35 million 229% higher than pre - pandemic period 1QFY20 Net Sales FY23: A$0.64 million 1QFY24: A$0.21 million 9% lower than pre - pandemic period 1QFY20 Australia 12 months to July 2023 short term resident returns 77% of those in 2019 1 USA Total departures June 2023 99.4% of June 2019 2
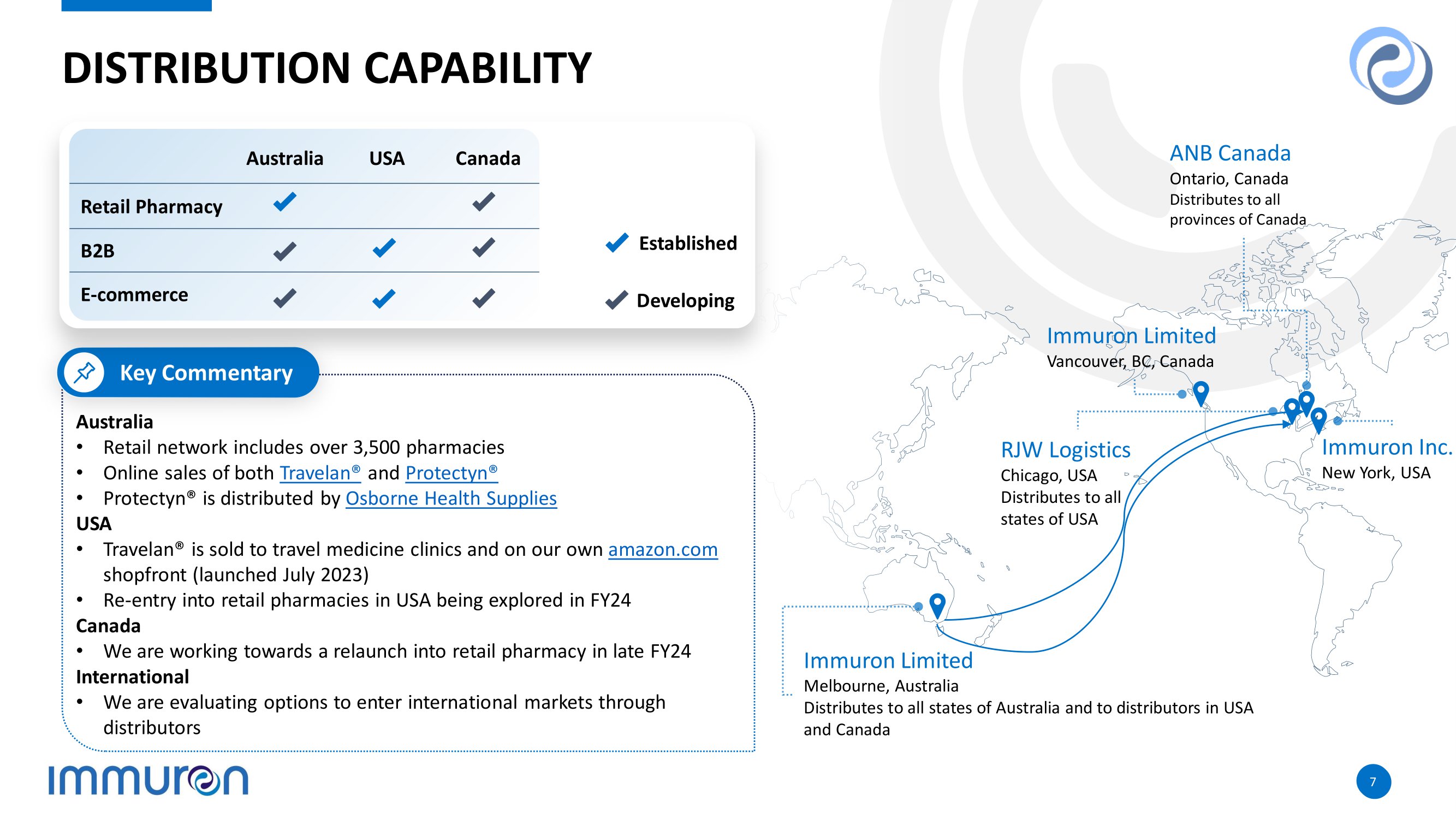
7 7 DISTRIBUTION CAPABILITY Australia • Retail network includes over 3,500 pharmacies • Online sales of both Travelan® and Protectyn® • Protectyn® is distributed by Osborne Health Supplies USA • Travelan® is sold to travel medicine clinics and on our own amazon.com shopfront (launched July 2023) • Re - entry into retail pharmacies in USA being explored in FY24 Canada • We are working towards a relaunch into retail pharmacy in late FY24 International • We are evaluating options to enter international markets through distributors Key Commentary ANB Canada Ontario, Canada Distributes to all provinces of Canada Immuron Limited Vancouver, BC, Canada Immuron Inc. New York, USA RJW Logistics Chicago, USA Distributes to all states of USA Immuron Limited Melbourne, Australia Distributes to all states of Australia and to distributors in USA and Canada Established Developing Canada USA Australia Retail Pharmacy B2B E - commerce
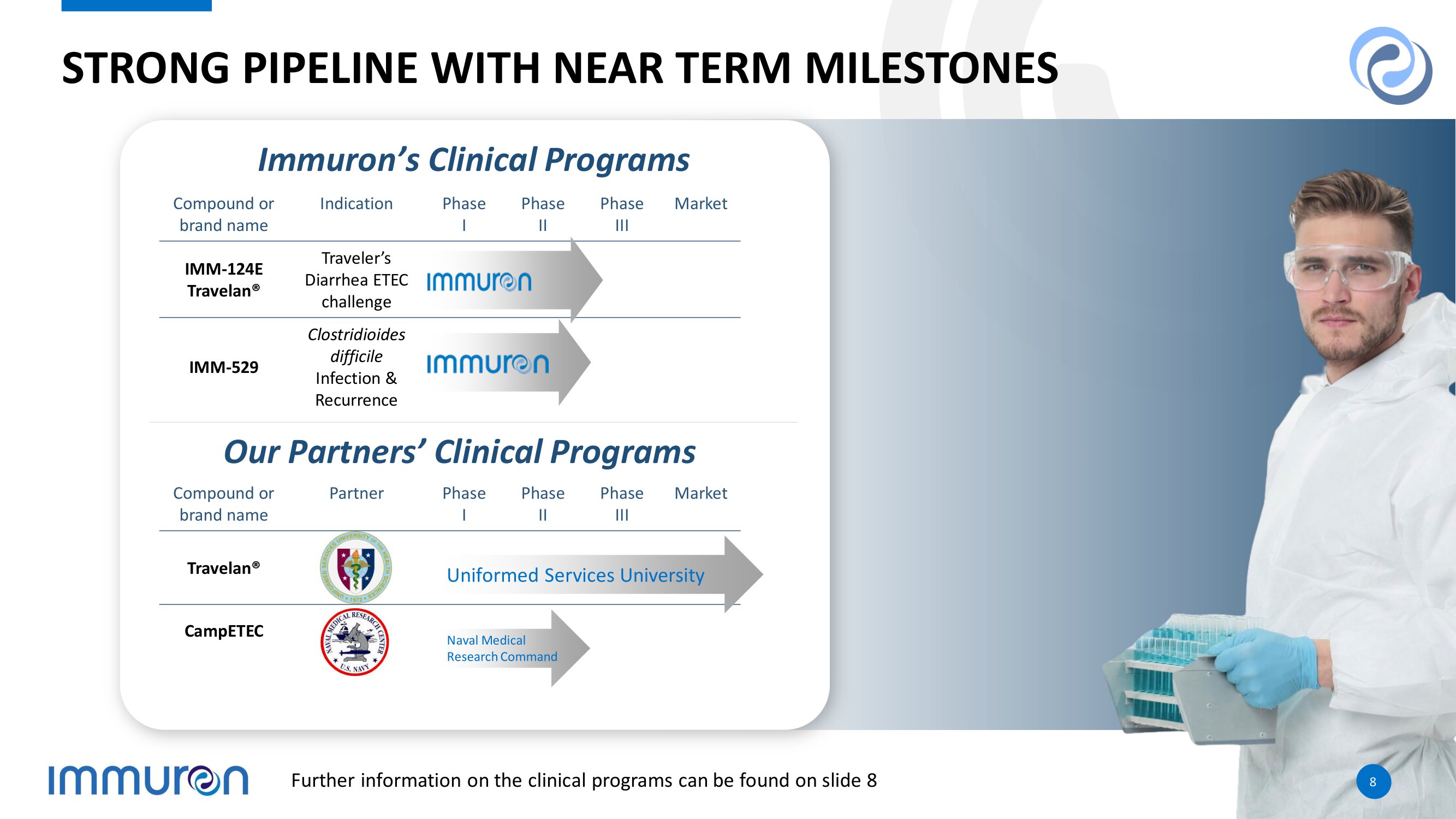
8 8 STRONG PIPELINE WITH NEAR TERM MILESTONES 8 8 Market Phase III Phase II Phase I Partner Compound or brand name Travelan® CampETEC Market Phase III Phase II Phase I Indication Compound or brand name Traveler’s Diarrhea ETEC challenge IMM - 124E Travelan® Clostridioides difficile Infection & Recurrence IMM - 529 Uniformed Services University Naval Medical Research Command Immuron’s Clinical Programs Our Partners’ Clinical Programs Further information on the clinical programs can be found on slide 8
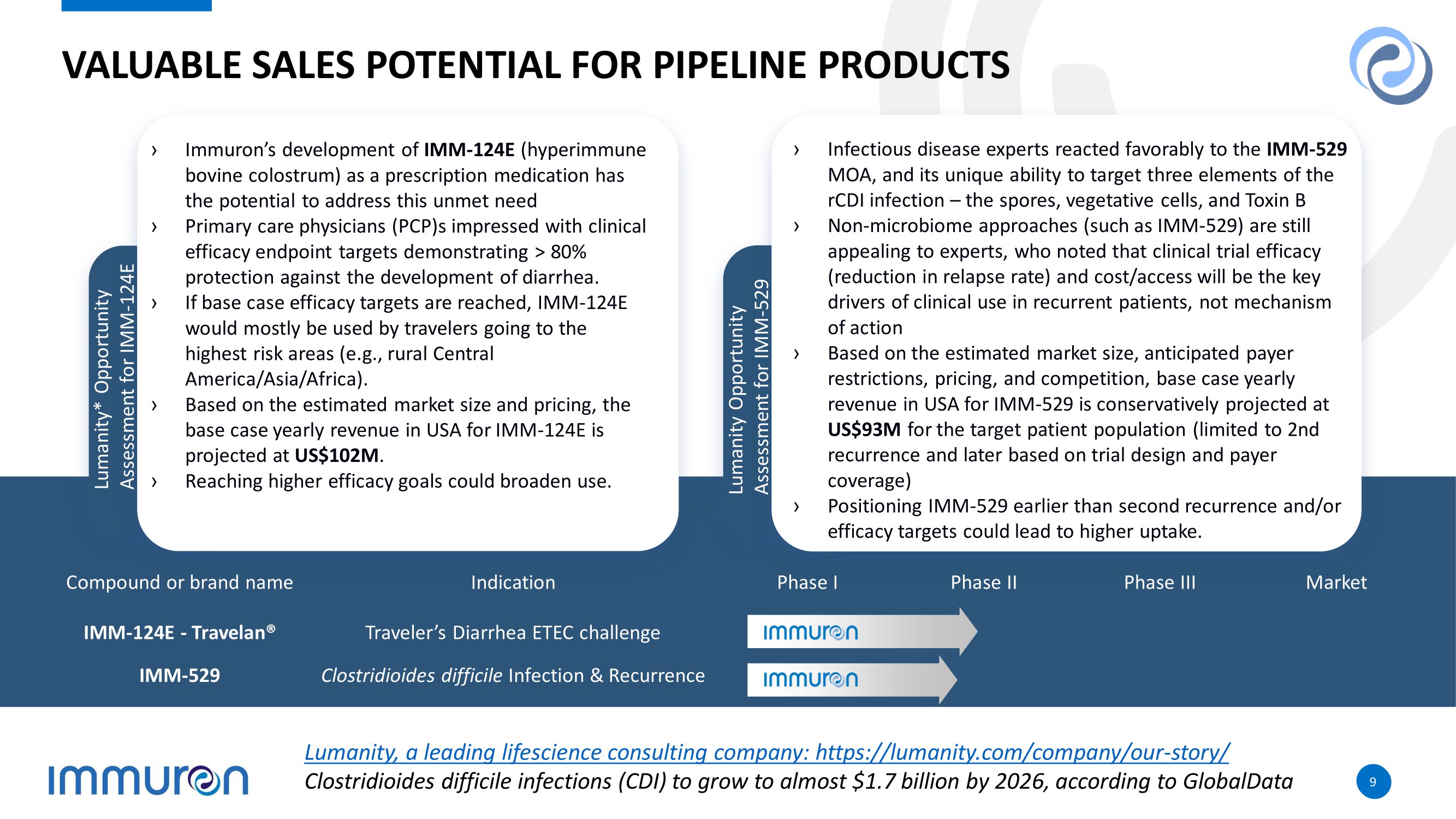
9 9 VALUABLE SALES POTENTIAL FOR PIPELINE PRODUCTS Corporate › Immuron’s development of IMM - 124E (hyperimmune bovine colostrum) as a prescription medication has the potential to address this unmet need › Primary care physicians (PCP)s impressed with clinical efficacy endpoint targets demonstrating > 80% protection against the development of diarrhea. › If base case efficacy targets are reached, IMM - 124E would mostly be used by travelers going to the highest risk areas (e.g., rural Central America/Asia/Africa). › Based on the estimated market size and pricing, the base case yearly revenue in USA for IMM - 124E is projected at US$102M . › Reaching higher efficacy goals could broaden use. Lumanity* Opportunity Assessment for IMM - 124E Corporate › Infectious disease experts reacted favorably to the IMM - 529 MOA, and its unique ability to target three elements of the rCDI infection – the spores, vegetative cells, and Toxin B › Non - microbiome approaches (such as IMM - 529) are still appealing to experts, who noted that clinical trial efficacy (reduction in relapse rate) and cost/access will be the key drivers of clinical use in recurrent patients, not mechanism of action › Based on the estimated market size, anticipated payer restrictions, pricing, and competition, base case yearly revenue in USA for IMM - 529 is conservatively projected at US$93M for the target patient population (limited to 2nd recurrence and later based on trial design and payer coverage) › Positioning IMM - 529 earlier than second recurrence and/or efficacy targets could lead to higher uptake. Lumanity Opportunity Assessment for IMM - 529 Market Phase III Phase II Phase I Indication Compound or brand name Traveler’s Diarrhea ETEC challenge IMM - 124E - Travelan® Clostridioides difficile Infection & Recurrence IMM - 529 Lumanity, a leading lifescience consulting company: https://lumanity.com/company/our - story/ Clostridioides difficile infections (CDI) to grow to almost $1.7 billion by 2026, according to GlobalData
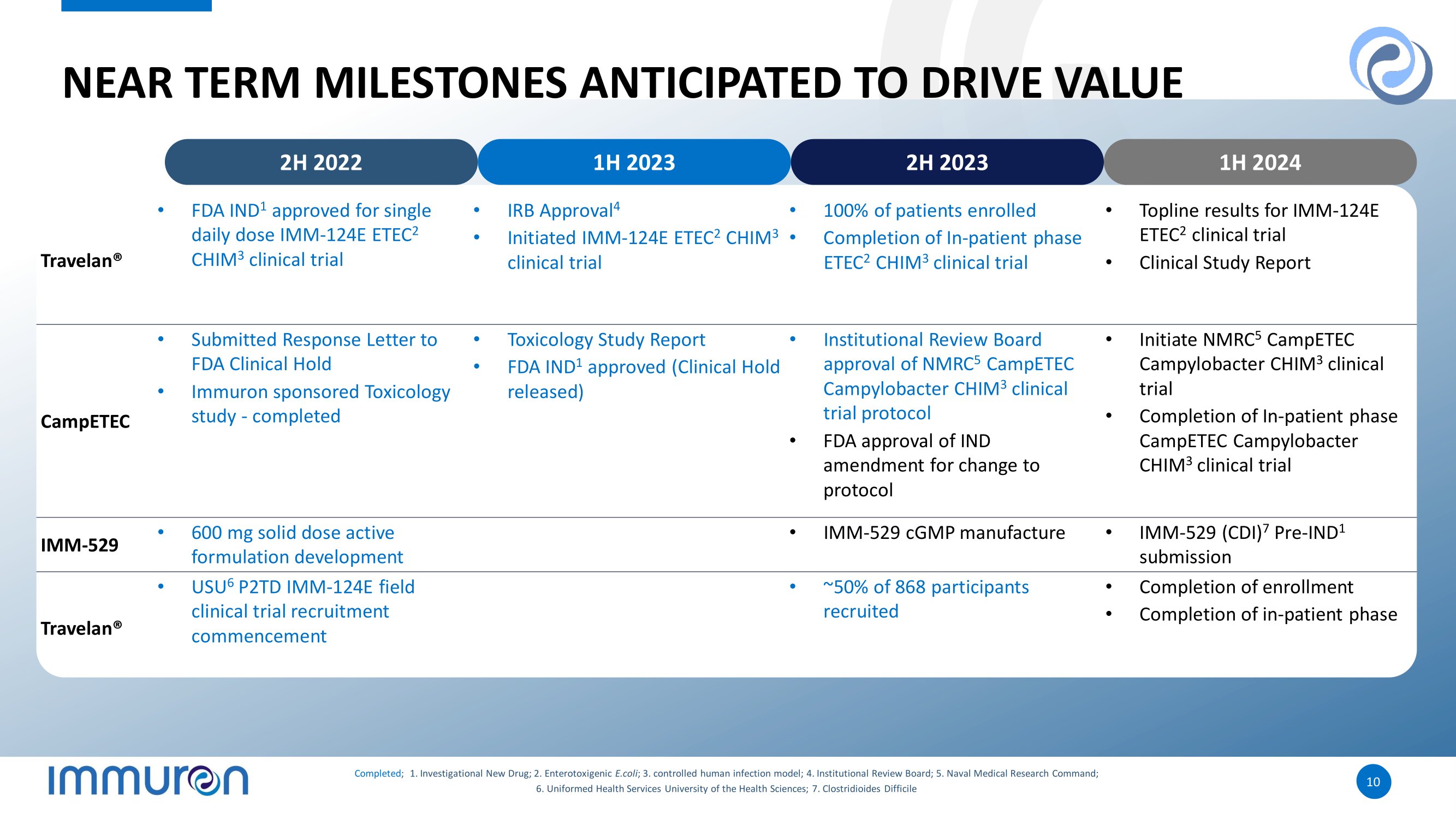
10 10 NEAR TERM MILESTONES ANTICIPATED TO DRIVE VALUE • Topline results for IMM - 124E ETEC 2 clinical trial • Clinical Study Report • 100% of patients enrolled • Completion of In - patient phase ETEC 2 CHIM 3 clinical trial • IRB Approval 4 • Initiated IMM - 124E ETEC 2 CHIM 3 clinical trial • FDA IND 1 approved for single daily dose IMM - 124E ETEC 2 CHIM 3 clinical trial Travelan® • Initiate NMRC 5 CampETEC Campylobacter CHIM 3 clinical trial • Completion of In - patient phase CampETEC Campylobacter CHIM 3 clinical trial • Institutional Review Board approval of NMRC 5 CampETEC Campylobacter CHIM 3 clinical trial protocol • FDA approval of IND amendment for change to protocol • Toxicology Study Report • FDA IND 1 approved (Clinical Hold released) • Submitted Response Letter to FDA Clinical Hold • Immuron sponsored Toxicology study - completed CampETEC • IMM - 529 (CDI) 7 Pre - IND 1 submission • IMM - 529 cGMP manufacture • 600 mg solid dose active formulation development IMM - 529 • Completion of enrollment • Completion of in - patient phase • ~50% of 868 participants recruited • USU 6 P2TD IMM - 124E field clinical trial recruitment commencement Travelan ® 2H 2022 1H 2023 2H 2023 1H 2024 Completed ; 1. Investigational New Drug; 2. Enterotoxigenic E.coli ; 3. controlled human infection model; 4. Institutional Review Board; 5. Naval Medical Research Command; 6. Uniformed Health Services University of the Health Sciences; 7. Clostridioides Difficile

11 11 SCIENTIFIC REFERENCES Travelan® (IMM - 124E) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Military Health System Research Symposium 14 - 17 Aug 2023_Abstract 1 Clinical Evaluation of Travelan® an Oral Prophylactic for Prevention of Travelers’ Diarrhea in Active Duty Military Service Assigned Abroad. Immuron Limited, 29 April, 2011 Travelan as a broad Spectrum anti - bacterial US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam et al., 2020. Submitted to mSphere, American Society for Microbiology Efficacy of hyperimmune bovine colostrum against shigellosis in rhesus macaque (Macaca mulatta), and bioactivity of HBC against common enteric pathogens Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 Bioactive Immune Components of Travelan® Infect Immun. 2023 Nov; 91(11): e00097 - 23. Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn’s and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative

12 12 EMAIL: STEVE@IMMURON.COM PHONE: AUSTRALIA: +613 8892 4854 STEVEN LYDEAMORE CHIEF EXECUTIVE OFFICER IMMURON LIMITED CONTACT INFORMATION:
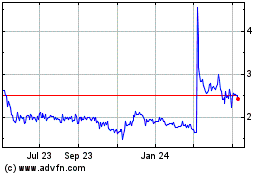
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Apr 2023 to Apr 2024