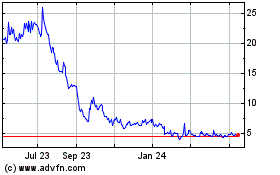
false
0001571934
0001571934
2023-10-31
2023-10-31
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): October 31, 2023
Synaptogenix, Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
001-40458 |
46-1585656 |
(State or other jurisdiction
of incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
1185
Avenue of the Americas, 3rd
Floor
New York, New
York 10036
(Address of principal executive offices and zip code)
Registrant’s telephone number, including
area code: (973) 242-0005
(Former Name or Former Address, if Changed Since
Last Report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General
Instruction A.2. below):
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which
registered |
| Common Stock, $0.0001 par value per share |
|
SNPX |
|
The Nasdaq Stock Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company. x
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 1.01 Entry into a Material Definitive
Agreement.
On October 31, 2023, Synaptogenix,
Inc. (the “Company”) entered into a share purchase agreement (the “Purchase Agreement”) with Cannasoul Analytics
Ltd. (“Cannasoul”), pursuant to which the Company agreed to purchase from Cannasoul (i) 12,737 shares of Cannasoul’s
Series A preferred shares, no par value per share (the “Preferred Shares”), at a price of $44.1550 per Preferred Share and
(ii) a convertible preferred note in an aggregate amount of up to $1,437,598.49 (the “Initial Convertible Note”). Additionally,
the Company agreed to purchase up to four additional convertible preferred notes in a total amount of up to approximately $2,000,000
(or approximately $500,000 per convertible preferred note), subject to Cannasoul achieving certain revenue and expense goals (the “Milestones”)
over the next four quarters (the “Milestone Convertible Notes”) as set forth in the Purchase Agreement. The Company’s
purchase of the Preferred Notes, the Initial Convertible Notes and the Milestone Convertible Notes is herein referred to as the “Investment.”
If Cannasoul fails to achieve a Milestone, the Company will not be obligated to purchase the applicable Milestone Convertible Note. If
Cannasoul achieves a Milestone and the Company fails to purchase the applicable Milestone Convertible Note, Cannasoul will have the right
to convert all the Company’s Preferred Shares into Cannasoul’s ordinary shares and the Company will lose certain board appointment
rights and certain rights in Cannasoul’s subsidiaries, each as further described below.
Dr. David (Dedi) Meiri, founder
of Cannasoul, heads the Laboratory of Cancer Biology and Cannabinoid Research at the Technion – Israel Institute of Technology
(“Technion”), where he develops cannabinoids for various indications. Pursuant to a license agreement between Cannasoul,
Dr. Meiri and Technion, Cannasoul has a right of first look with respect to each new cannabinoid indication developed in Dr. Meiri’s
laboratory and not otherwise funded by a third party. Whenever Cannasoul exercises this right of first look, it creates a new subsidiary
to carry out the development of such indication (each, a “Project Subsidiary”). In connection with the Investment, the Company
received certain rights in existing Project Subsidiaries. Additionally, Cannasoul granted the Company a right of first look to invest
in any new Project Subsidiaries as well as certain preemptive and veto rights in connection with such Project Subsidiaries. Pursuant
to a collaboration agreement entered into by the Company and Cannasoul on October 31, 2023 (the “Collaboration Agreement”),
the parties agreed to form a joint research committee with equal representation from the Company and Cannasoul (the “JRC”).
The JRC will evaluate the technology and compounds developed by Dr. Meiri in his laboratory, assess the feasibility of developing and
commercializing each compound and, for those compounds with respect to which the Company exercises its right of first look, collaborate
on the strategy for clinical development of such compounds.
In connection with the Investment, Cannasoul adopted
amended and restated articles of incorporation (the “Cannasoul Charter”). Pursuant to the Cannasoul Charter, the Company has
a number of rights as investor, including but not limited to (i) the right to appoint and dismiss three of the seven members of Cannasoul’s
board of directors and veto power with respect to a fourth member, (ii) preemptive rights to participate pro rata in any pre-initial public
offering financings by Cannasoul, (iii) rights of first refusal with respect to transfers of Cannasoul ordinary shares by other investors,
(iv) rights of co-sale with respect to proposed sales or transfers of Cannasoul ordinary shares by certain key investors, (v) veto rights
with respect to certain major transactions, any amendment to the Cannasoul Charter, approval of Cannasoul’s budget and other items.
Also on October 31, 2023, the
Company entered into an investor rights agreement (the “Investor Rights Agreement”) with Cannasoul, the founders of Cannasoul
and certain investors in Cannasoul (the Company and the other investors collectively, the “Investors”). Pursuant to the Investor
Rights Agreement, if Cannasoul ever completes an initial public offering in the United States, the Investors may, subject to conditions
set forth in the Investor Rights Agreement, request that Cannasoul file a registration statement with the U.S. Securities and Exchange
Commission covering the resale of the ordinary shares underlying the Preferred Shares.
The Purchase Agreement contains
certain representations and warranties, covenants and indemnities customary for similar transactions. The representations, warranties
and covenants contained in the Purchase Agreement were made solely for the benefit of the parties to the Purchase Agreement and may be
subject to limitations agreed upon by the contracting parties.
The foregoing descriptions of
the Purchase Agreement, the Collaboration Agreement and the Investor Rights Agreement do not purport to be complete and are qualified
in their entirety by reference to the full text of the Purchase Agreement, the Collaboration Agreement and the Investor Rights Agreement,
forms of which are filed as Exhibits 10.1, 10.2 and 10.3, respectively, to this Current Report on Form 8-K and incorporated herein by
reference.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit |
Description |
| |
|
| 10.1 |
Share Purchase Agreement, dated October 31, 2023, by
and between Synaptogenix, Inc. and Cannasoul Analytics Ltd. |
| 10.2 |
Collaboration Agreement, dated October 31, 2023, by
and between Synaptogenix, Inc. and Cannasoul Analytics Ltd. |
| 10.3 |
Investor Rights Agreement, dated October 31, 2023,
by and between Synaptogenix, Inc., Cannasoul Analytics Ltd. and other investors party thereto. |
| 104 |
Cover Page Interactive Data File (embedded within the
Inline XBRL document) |
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| Date: November 6, 2023 |
By: |
/s/
Robert Weinstein |
| |
Name: |
Robert Weinstein |
| |
Title: |
Chief Financial Officer |
Exhibit 10.1
Execution
Copy
SHARE PURCHASE AGREEMENT
This Share Purchase Agreement (this “Agreement”)
is made as of October 31, 2023, by and among Cannasoul Analytics Ltd., a company duly incorporated under the laws of the State of
Israel, registration number 515782894, having its principal offices at 9 Tarshish Street, Caesarea, Israel (the “Company”),
and the Investor set forth in Schedule A (the “Investor”). Each of the Company and the Investors individually
referred hereinafter as a “Party” and jointly as the “Parties”.
WHEREAS,
the Company is engaged in the provision of analytical services to the medical cannabis industry and is involved, including via the Company’s
subsidiaries, in various research and development projects in the cannabis field (the “Business Activity”); and
WHEREAS,
the Board of Directors of the Company (the “Board”) has determined that it is in the best interests of the Company
to raise capital by means of (a) the issuance of up to 12,784 of the Company’s Series A Preferred Shares, with no par
value each (“Preferred Shares”), representing 5% of the Company’s issued and outstanding share capital (the “Issued
Shares” or “Purchased Shares”), to the Investor, at a price per share equal to US$44.0215 (the “PPS”),
reflecting Company pre-money valuation of US$ 12,000,000 on a Fully Diluted Basis, against the payment of an aggregate purchase price
of US$ 562,770.86 (the “Investment Amount”), and (b) the issuance of a series of convertible notes (each, a “Convertible
Note”) in an aggregate amount of up to US$ 3,437,242.74 (the “Convertible Note Amount”) convertible into
78,081 Preferred Shares in the form attached hereto as Schedule B (the “Convertible Note”), as more fully
set forth in this Agreement; and
WHEREAS,
the Investor desires to purchase and the Company desires to issue and sell to the Investors the Purchased Shares and the Convertible Note
pursuant to the terms and conditions more fully set forth in this Agreement.
NOW,
THEREFORE, in consideration of the mutual promises and covenants set forth herein, the Parties hereby agree as follows:
1. The
Transaction.
1.1. Issuance
and Purchase of Shares. Subject to the terms and conditions hereof, the Investor shall purchase from the Company and the Company
shall issue and allot to the Investor the Purchased Shares, comprising five (5) per cent of the Company’s issued and outstanding
share capital, each Purchased Share at the PPS, against the payment of the Investment Amount.
1.2. Issuance
of the Initial Convertible Note. Subject to the terms and conditions hereof, at the Closing, the Investor shall purchase from
the Company and the Company shall issue to the Investor a Convertible Note against payment of an amount of $1,437,229.14 (the “Initial
Convertible Note”).
1.3. Issuance
of Subsequent Convertible Notes. Following the Closing, and subject to the meeting of the milestones set out in Schedule
1.3 attached hereto (each, a “Milestone”), the Investor shall transfer the amounts set out in Schedule 1.3
on the dates set out in Schedule 1.3 against issuance by the Company of additional Convertible Notes in the amounts transferred by the
Investor. The Company shall provide confirmation in writing to the Investor upon the successful fulfillment of each Milestone immediately
upon the completion of the relevant quarter. In the event that any of the Milestones are not met by the Company, or the Company does not
provide notification that the Milestone has been met by the seventh day following the end of the relevant quarter, then the Investor shall
no longer be obligated to make the payment due with respect to such Milestone or any subsequent Milestone and the Company shall not issue
a Convertible Note to the Investor with respect to such Milestone with respect to which payment was not transferred by the Investor. In
the event that a Milestone is met and the Investor does not make the payment in respect of such Milestone in accordance with Schedule
1.3 (a "Milestone Default"), then the Company shall be entitled, without any further action necessary on the part of
the Investor, to convert all Preferred Shares held by the Investor or Preferred Shares into which any outstanding Convertible Notes are
to be converted, into Ordinary Shares and further the Investor shall only be entitled to appoint one (1) director to the Board, all
as more fully set out in the Amended Articles. Furthermore, in the event of a Milestone Default, all rights granted to the Investor pursuant
to Schedules 2.1.6 – 2.1.10 shall be cancelled except for the Investor's right to appoint two (2) directors to Psyga Bio in
accordance with the terms of Schedule 2.1.6.
2. Closing.
The purchase of the Issued Shares by the Investor and the issuance of the Issued Shares by the Company shall take place remotely, via
the exchange of documents and signatures on the date hereof, or at such other time and place as the Company and the Investor mutually
agree upon orally or in writing (including via e-email) (the “Closing”). At the Closing, the following transactions
shall occur, which transactions shall be deemed to take place simultaneously and no transaction shall be deemed to have been completed
or any document delivered until all such transactions have been completed and all required documents delivered:
2.1. The
Company shall deliver to the Investor the following documents:
2.1.1. Copies
of the Unanimous Written Resolutions and consents of the Company’s shareholders in the form attached as Schedule 2.1.1A
hereto, by which, inter alia (i) the execution, delivery and performance by the Company of this Agreement, including for avoidance
of doubt the issuance of the Purchased Shares, and all exhibits and appendices attached thereto (collectively, the “Transaction
Documents”) and the transactions contemplated thereunder shall be approved; (ii) the Articles of Association of the Company
shall be replaced with the Amended and Restated Articles of Association attached as Schedule 2.1.1B hereto (the “Amended
Articles”); and (iii) each of the shareholders of the Company shall waive any anti-dilution rights, preemptive rights,
veto rights, rights of first refusal or the like, and any other right to receive shares or other securities of the Company (to the extent
such rights exist), in connection with the transactions contemplated hereunder;
2.1.2. Copies
of Unanimous Written Resolutions of the Board of Directors of the Company (the “Board”) in the form attached as Schedule
2.1.2 hereto, by which, inter alia (i) the execution, delivery and performance by the Company of the Transaction Documents,
and the transactions contemplated hereby and thereby shall be approved; (ii) the sale, issuance and allotment to the Investors of
the Purchased Shares; and (iv) the recommendation to the Shareholders of the Company to approve the replacement of the Articles of
Association of the Company with the Amended Articles;
2.1.3. Validly
executed share certificates covering the Issued Shares, issued in the name of the Investor, in the form attached hereto as Schedule 2.1.3;
2.1.4. A
Compliance Certificate in the form attached hereto as Schedule 2.1.4, duly executed by the Chief Executive Officer
of the Company and dated as of the Closing, confirming and certifying that the representations and warranties set forth in Section 4
of this Agreement are true and correct as of the date hereof and through the Closing, and that the Company has performed and complied
with all of its covenants, agreements, and undertakings set forth herein.
2.1.5. An
Opinion of Counsel of Weksler & Co., counsel to the Company, in the forms attached hereto as Schedule 1.1.1,
and which is dated as of the Closing;
2.1.6. A
letter addressed to the Investor and signed by Psyga Bio Ltd. in the form attached hereto as Schedule 2.1.6;
2.1.7. A
letter addressed to the Investor and signed by Nidra Logic Ltd. in the form attached hereto as Schedule 2.1.7;
2.1.8. A
letter addressed to the Investor and signed by Professor David (Dedi) Meiri in connection with his shares in Cavnox Ltd. in the form attached
hereto as Schedule 2.1.8;
2.1.9. A
letter addressed to the Investor and signed by Cavnox Ltd. in the form attached hereto as Schedule 2.1.9;
2.1.10. A
letter addressed to the Investor and signed by Cannasoul in the form attached hereto as Schedule 2.1.10.
2.1.11. Duly
executed addendums and letter between the Company and its subsidiaries and the Technion Research Development Fund Ltd., in the forms attached
hereto as Schedule 2.1.11(i), Schedule 2.1.11(ii) and Schedule 2.1.11(iii);
2.1.12. At
the Closing, the Company shall receive a duly executed copy of a director appointment letter from the Investor, in the form attached hereto
as Schedule 2.1.12.
2.1.13. The
Company’s budget attached hereto as Schedule 2.1.13 (the “Budget”) shall have been approved by the Board.
2.1.14. The
Initial Convertible Note duly executed by the Company.
2.2. The
Company shall register the allotment of the Issued Shares to the Investor in its shareholders register and deliver a copy to the Investor,
in the form attached as Schedule 2.2 hereto.
2.3. The
Company, the Investor and certain other shareholders of the Company shall execute and deliver the Amended and Restated Investors’
Rights Agreement, a copy of which is attached hereto as Schedule 2.3 (the “Investors’ Rights Agreement”).
2.4. The
Investor and the Company shall execute and deliver a Research and Collaboration Agreement in the form attached as Schedule 2.4
(the “Collaboration Agreement”).
2.5. The
Investor shall transfer the Investment Amount and the Convertible Note Amount to the Company's bank account detailed in Schedule
2.3 hereto, in immediately available funds.
3. [RESERVED].
4. Representations
and Warranties of Company. The Company hereby represents and warrants to the Investor, as of the date hereof and as of the Closing
as follows, subject to the exceptions set forth in the Disclosure Schedule attached hereto as Schedule 4. For the purposes
hereof, unless the context indicates otherwise, all references to the Company this Section 4 shall include the Subsidiaries
(as defined below)) (the “Disclosure Schedule”, section numbers in the Disclosure Schedule correspond to the section
numbers in this Agreement), and acknowledge that the Investor are entering into this Agreement in reliance thereon, as follows:
“Subsidiaries” shall refer
to Cavnox Ltd., Psyga Bio Ltd., MyPlant Bio Ltd., Nidra Logic Ltd. and Cannasoul Lab Services Ltd.
4.1. Organization.
The Company is duly incorporated and validly existing under the laws of the State of Israel and its control and management are in Israel.
The Company has all requisite power and authority to execute to own, lease and operate its properties and assets and to conduct its business
as now being conducted or as currently proposed to be conducted and to execute deliver this Agreement and other agreements contemplated
hereby or which are ancillary hereto and to consummate the transactions contemplated hereby and thereby.
4.2. Share
Capital. The authorized share capital of the Company immediately prior to the Closing shall be 10,090,865 shares divided into:
4.2.1. 10,000,000
Ordinary Shares of which (i) 242,901 are issued and outstanding as of the date of this Agreement, (ii) 16,363 Ordinary Shares
reserved for issuance under the Company’s employees share option plan, of which 7,757 Ordinary Shares are non-promised and unallocated,
and (iii) 90,865 Series A Preferred Shares, none of which shall be outstanding, and are reserved and/or are to be issued according
to the terms of this Agreement;
4.2.2. Except
for the transactions contemplated by this Agreement and/or in accordance with the Amended Articles, there are no other shareholders agreements,
voting rights, preemptive rights, rights of first refusal, convertible securities, outstanding warrants, options or other rights to subscribe
for, purchase or acquire from the Company any share capital of the Company and there are no contracts or binding commitments providing
for the issuance of, or the granting of rights to acquire, any share capital of the Company or under which the Company is, or may become,
obligated to issue any of its securities or to give any other similar rights to the shareholders of the company or to third parties. All
issued and outstanding share capital of the Company has been duly authorized, and is validly issued and outstanding and fully paid and
nonassessable. The Issued Shares, when issued, sold and delivered in accordance with this Agreement, and the Preferred Shares issued upon
conversion of the Convertible Note, when issued, sold and delivered in accordance with the Convertible Note, will be duly authorized,
validly issued, fully paid, nonassessable, free of any preemptive rights, will have the rights, preferences, privileges, and restrictions
set forth in the Amended Articles, will be free and clear of any liens, claims, encumbrances or third party rights of any kind (except
as specified in the Amended Articles) and duly registered in the name of the Investor in the Company's register of shareholders and will
be offered, sold and issued in compliance with all applicable securities laws. The Ordinary Shares issuable upon conversion of the Issued
Shares and the Preferred Shares issuable upon conversion of the Convertible Note have been duly authorized and reserved for issuance by
all necessary corporate action and, upon issuance in accordance with the terms of the Amended Articles, shall be duly and validly issued,
fully paid, non-assessable, free of any preemptive rights, will have the rights, preferences, privileges and restrictions set forth in
the Amended Articles and will be issued in compliance with all applicable securities laws. The Company has not granted any anti-dilution
rights to any of the shareholders or other parties concurrent to this transaction or any other transaction with the Company and no such
rights will be triggered as a result of the closing of this transaction and/or the issuance of the Issued Shares or the Preferred Shares
upon conversion of the Convertible Note.
4.3. Ownership
of Shares. A complete and correct capitalization table of the Company on a Fully Diluted Basis (including, for the avoidance of doubt,
all of the outstanding options, warrants or other rights to purchase shares of the Company's share capital, including the rights to purchase
shares under the Convertible Note) immediately prior to and following the Closing is set forth in Schedule 4.2.2 of
the Disclosure Schedule. The shareholders identified in Schedule 4.2.2 as the shareholders of the Company are the lawful
owners, beneficially and of record, of all of the issued and outstanding share capital of the Company and of all rights thereto (including,
for the avoidance of doubt, all options, warrants and other rights to purchase shares of the Company's share capital), free and clear
of all liens, claims, charges, encumbrances, restrictions, rights, options to purchase, proxies, voting trust and other voting agreements,
calls or commitments of every kind, and none of the said individuals owns any other shares, options or other rights to subscribe for,
purchase or acquire any share capital of the Company from the Company or from each other, save as pursuant to the Amended Articles. For
purposes hereof, “Fully Diluted Basis” means all issued shares, warrants, options, convertible loans, convertible securities
or rights, adjustments, options (whether issued, granted or promised), convertible loans, rights and convertible securities, on an as-if
exercised and as-converted basis (including without limitation, all rights and promises of any kind that could directly or indirectly
result in the acquisition of shares of the Company), adjustments of numbers of issued or deemed issued shares triggered by, or in connection
with, the transaction contemplated hereby (if any) and any anti-dilution adjustments and any unallocated options reserved for grant under
the Company’s share option plan.
4.4. Subsidiaries.
The Company does not own or control, directly or indirectly, any interest, shares or rights in any corporation, association or business
entity or other joint venture, except for as set forth in Schedule 4.4. The Company owns, beneficially and of record,
the issued and outstanding share capital issued to it by the entities specified in Schedule 4.4, and all rights thereto,
free and clear of liens, claims, charges, encumbrances, restrictions, rights, options to purchase, proxies, or other voting agreements.
All issued and outstanding share capital of each of the Subsidiaries was duly authorized, and is validly issued and outstanding and fully
paid and non-assessable. The Subsidiaries are corporations duly organized and validly existing under the laws of the respective states
of their incorporation and have full corporate power and authority to own, lease and operate their respective properties and assets and
to conduct their business as now being conducted and as currently proposed to be conducted. The Subsidiaries are duly qualified to transact
business and, if applicable, are in good standing, in each jurisdiction in which they respectively operate.
4.5. Directors,
Officers. The directors and officers of the Company and the Subsidiaries as of the Closing are set forth in Schedule 4.5.
Other than as set forth in Schedule 4.5, each officer of the Company is currently devoting all of his/her business time
to the conduct of the business of the Company Except as set forth in Schedule 4.5, the Company is not aware that any officer
or employee of the Company is planning to dedicate less than all of his/her business time to the conduct of the business of the Company
in the future. Other than pursuant to the Amended Articles, the Company has no agreement, obligation or commitment with respect to the
election of any individual or individuals to the Board and there is no voting agreement or other arrangement among the Company's shareholders.
All agreements, commitments and understandings, whether written or oral, with respect to any compensation to be provided to any of the
Company's directors or officers have been fully disclosed in writing to the Investors.
4.6. Financial
Statements. The Company has furnished the Investors with its audited and consolidated annual financial statements including the Company's
Subsidiaries, for the period ended December 31, 2021 which are attached hereto as Schedule 4.6 and
a trial balance sheet as at and for the period ending June 30, 2023 which is attached hereto as Schedule 4.6.
The Financial Statements are true and correct in all material respects, are in accordance with the books and records of the Company and
have been prepared in accordance with Israel generally accepted accounting principles (“GAAP”) consistently
applied, and fairly and accurately present in all material respects the financial position of the Company as of such dates and the results
of its operations and cash flows for the periods then ended. Except for obligations and liabilities reflected in the Financial Statements,
the Company has no off balance sheet obligations and liabilities of any nature to, or any financial interest in, any third party or entities,
the purpose or effect of which is to defer, postpone, reduce or otherwise avoid or adjust the recording of expenses incurred by the Company.
All proper and necessary books of account and accounting records have been maintained by the Company, are in its possession and contain
accurate information in accordance with GAAP, consistently applied relating to all transactions to which the Company has been a party.
The Company has no liabilities, debts or obligations, whether accrued, absolute or contingent, matured or unmatured or determined or undeterminable,
exceeding US$20,000 in the aggregate, provided however, that with respect to any employment, advisor and consultant agreements, such amount
will be calculated based on the outstanding amount owed to such consultant or advisor in accordance with the applicable employment, consultant
or advisor agreement, and the Company has not taken any loans from third parties other than the liabilities set forth in Schedule
4.6 attached hereto. The Company is not a guarantor of any debt or obligation of another, nor has the Company given any indemnification,
loan, security or otherwise agreed to become directly or contingently liable for any obligation of any person, and no person has given
any guarantee of, or security for, any obligation of the Company. Since its inception, the Company has been operated only in the usual
and ordinary course of business. Since 30 June 2023, and except as set forth in Schedule 4.6, there has not been:
(a) any
material change in the assets, liabilities, condition (financial or otherwise) or business of the Company;
(b) any
damage, destruction or loss, whether or not covered by insurance, materially and adversely affecting the assets, properties, conditions
(financial or otherwise), operating results or business of the Company;
(c) any
waiver by the Company of a valuable right or of a material debt owed to it;
(d) any
satisfaction or discharge of any material lien, material claim or material encumbrance or payment of any material obligation by the Company,
except in the ordinary course of business and that is not individually or in the aggregate adverse to the assets, properties, condition
(financial or otherwise), operating results or business of the Company;
(e) any
material change or amendment to a material contract or material arrangement by which the Company or any of its respective assets or properties
is bound or subject;
(f) any
material change in any compensation arrangement or agreement with any employee of the Company;
(g) any
loans, guarantees or advances made by the Company to its employees, officers, shareholders or directors other than travel advances made
in the ordinary course of business;
(h) any
sale, transfer or lease of, except in the ordinary course of business, or mortgage or pledge of imposition of lien on, any of the Company’s
assets;
(i) any
change in the accounting methods or accounting principles or practices employed by the Company;
(j) any
other event or condition of any character that would materially adversely affect the assets, properties, condition (financial or otherwise),
operating results or business of the Company as currently being conducted or as currently proposed to be conducted;
(k) any
declaration, setting aside or payment or other distribution in respect of any of the Company’s share capital, or any direct or indirect
redemption, purchase, or other acquisition of any of such shares by the Company;
(l) any
receipt of written notice that there has been a loss of, or material order cancellation by, any major customer of the Company;
(m) any
resignation or termination of employment of any officer or key employee of the Company;
(n) any
other event or condition of any character that would likely materially and adversely affect the assets, properties, financial condition,
operating results or business of the Company, as such business is presently conducted;
(o) any
failure of the Company to pay its debts as they come due; or
(p) any
arrangement or commitment by the Company to do any of the foregoing
4.7. Authorization;
Approvals. All corporate action has been taken or will be taken prior to Closing on the part of the Company necessary for (i) the
authorization, execution, delivery, and performance of all of the Company's obligations under this Agreement, and (ii) the authorization,
issuance, and sale of the Issued Shares and the shares issued upon conversion of the Convertible Note. This Agreement and its ancillary
documents, when executed and delivered by or on behalf of the Company, shall constitute the valid and legally binding obligations of the
Company, legally enforceable against the Company in accordance with their respective terms. Other than the approval from the Israeli Cannabis
Unit (ICU) with respect to the Preferred Shares issuable upon conversion of the Convertible Note, no consent, approval, order, license,
permit, action by, or authorization of or designation, declaration, or filing with any governmental authority on the part of the Company
is required that has not been, or will not have been, obtained by the Company prior to the Applicable Closing in connection with (i) the
valid execution, delivery and performance of this Agreement, and (ii) the offer, sale or issuance of the Issued Shares and the Preferred
Shares issuable upon conversion of the Convertible Note.
4.8. Compliance
and Permits. The Company have complied and is in compliance with all domestic and foreign, laws and regulations applicable to its
business, the violation of which would have a material adverse effect to the assets, liabilities, financial condition or operating results
of the Company. The Company has not received any notice that any violation of the foregoing is being or may be alleged and has no knowledge
of any facts which are reasonably likely to result in such violation. The Company is not in default under (a) its current Articles
of Association, or other governing instruments of the Company (b) any agreement, to which the Company is a party or by which any
of its property is bound, which materially affects the Company’s business, condition (financial or otherwise), affairs, operations
or assets, or (c) any applicable law and regulation or any order, writ, injunction or judgment of any court or any governmental or
official authority, domestic or foreign applicable to the Company. The Company is not aware of any third party being in default under
any agreement with the Company. The Company has all franchises, permits, licenses, and any similar authority necessary for the conduct
of its Business Activity as now being conducted (the "Permits"), and the Company believes it can obtain, without undue
burden or expense, any similar authority for the conduct of its Business Activity as currently planned to be conducted. A true and correct
list of all Permits, and their current status (approved / pending) is set forth in Schedule 4.8.
4.9. No
Breach. Neither the execution and delivery of this Agreement nor compliance by the Company with the terms and provisions thereof,
will conflict with, or result in a breach or violation of, any of the terms, conditions and provisions of: (i) the Company’s
Current Articles of association, as in effect prior to the adoption of the Amended Articles,
(ii), any judgment, order, injunction, decree, or ruling of any court or governmental authority, domestic or foreign with respect to the
Company, (iii) any agreement, contract, lease, license or commitment to which the Company is a party or to which it is subject, or
(iv) applicable law. Such execution, delivery and compliance will not (a) give to others any rights, including rights of termination,
cancellation or acceleration, in or with respect to any agreement, contract or commitment, or to any of the properties of the Company,
or (b) otherwise require the consent or approval of any person or entity, which consent or approval has not heretofore been obtained.
To the Company’s knowledge, no third party is in default under any material agreement, contract or other material instrument, document
or agreement to which the Company is a party.
4.10. Ownership
of Assets; No Indebtedness. The Company has no material assets, other than as provided in Schedule 4.10.
Except as set forth in Schedule 4.10 the Company does not currently lease or license any real property nor does the
Company own any real property. Except as set forth in Schedule 4.10 the Company owns, or holds under
lease, all real and personal property, whether tangible or intangible, used by it in its business. All property owned by the Company is
so owned free and clear of all mortgages, pledges, liens, licenses, leases security interests, encumbrances or charges other than those
identified on Schedule 4.10 and except for statutory liens for the payment of current taxes that are not yet delinquent
and encumbrances and liens that arise in the ordinary course of business and do not materially impair the Company’s ownership or
use of such property or assets. The Company has not assumed, guaranteed, endorsed or otherwise become directly or contingently liable
for any indebtedness of any other person or entity (including, without limitation, liability by way of agreement, contingent or otherwise,
to purchase, to provide funds for payment, to supply funds to or otherwise invest in the debtor, or otherwise to assure the creditor against
loss).
4.11. Intellectual
Property and Other Intangible Assets.
4.11.1. For
purposes of this Agreement, the term “Intellectual Property” shall mean and refer to all (i) United States and other
patents and patent applications, and any divisional, continuation, continuation in part, reissue, renewal or re-examination patent issuing
therefrom (including any foreign counterparts), (ii) copyrights and registrations thereof, (iii) mask works and registrations
and applications for registration thereof, (iv) computer software, including any and all software implementations of algorithms,
models and methodologies, whether in source code or object code, (v) databases and compilations, including any and all data and collections
of data, whether machine readable or otherwise, (vi) technology supporting any Internet site(s) operated by or on behalf of
the Company, (vii) trade secrets and other confidential business information, whether patentable or unpatentable and whether or not
reduced to practice, know-how, technology, proprietary processes, techniques, methodologies, formulae, algorithms, models, modules, user
interfaces, research and development information, copyrightable works, financial, marketing and business data, pricing and cost information,
business and marketing plans and customer and supplier lists and information, inventions, source code, object code, and, with respect
to all of the foregoing, related confidential documentation, (viii) trademarks, service marks, trade names, domain names and applications
and registrations therefor, (ix) all documentation, including user manuals and training materials relating to any of the foregoing
and descriptions, flow-charts and other work product used to design, plan, organize and develop any of the foregoing and (x) other
proprietary rights relating to the foregoing.
4.11.2. Schedule
4.11 identifies each (a) patent, trademark, copyright, domain name or registration which has been issued to the Company with
respect to any of the Company’s Intellectual Property; (b) pending patent, trademark or copyright application or application
for registration which the Company has made with respect to any of the Company’s Intellectual Property; (c) each trade name
or unregistered trademark used by the Company; and (d) license, agreement, or other permission pursuant to which the Company has
received from or granted to any third party with respect to any of the Company’s Intellectual Property and the terms thereof. The
Company owns and has developed, or has obtained the right to use, free and clear of all liens, claims and restrictions, all Intellectual
Property used or required for use in the conduct of the Business Activity as now conducted and as currently proposed to be conducted,
without infringing upon, misappropriating or violating (i) to the knowledge of the Company, any third party's patent or patent applications;
and (ii) any other right, lien, or claim of others. Save for standard off-the-shelf software licenses, the Company is not obligated
or under any liability whatsoever to make any payments by way of royalties, fees or otherwise to any owner or licensee of, or other claimant
to any Intellectual Property or any other intangible asset, with respect to the use thereof or in connection with the conduct of its Business
Activity as now conducted or as currently proposed to be conducted or otherwise.
4.11.3. The
Company has taken security measures to protect the secrecy, confidentiality and value of all the Intellectual Property, which measures
are reasonable and customary in the industry in which the Company operates.
4.11.4. The
Company has not violated or by conducting its Business Activity as proposed, would not violate, infringe or misappropriate, to the Company'
knowledge (i) any third party's patent or patent applications, or (ii) any other proprietary rights of any other person or entity.
Neither the Company’s founders nor, to the knowledge of the Company, any of the Company's employees is obligated under any contract
(including licenses, covenants or commitments of any nature) or other agreement, or subject to any judgment, decree or order of any court
or administrative agency, that would interfere with the use of the Company’s founders’ or such employee's best efforts to
promote the interests of the Company or that would conflict with the Company's Business Activity as conducted and as currently proposed
to be conducted.
4.11.5. There
are no outstanding licenses, or agreements of any kind relating to the Company's Intellectual Property necessary for the Company’s
Business Activity as now conducted, nor is the Company bound by or a party to any licenses or agreements of any kind with respect to the
Intellectual Property of any other person or entity (including without limitation any software or other material that is distributed as
“free software”, “open source software” or under a similar licensing or distribution model). The Company is not
aware of any Intellectual Property owned by any third party which is needed by the Company to conduct its Business Activity as currently
conducted or proposed to be conducted.
4.11.6. No
source code of any of the Company's proprietary software has been licensed or otherwise provided or disclosed to another person or entity,
and the Company does not have any duty or obligation (whether present, contingent, or otherwise) to license or otherwise provide the source
code for any of the Company's proprietary software to any person or entity.
4.11.7. All
of the Company’s employees, officers, directors or consultants and service providers involved in the development of Intellectual
Property have entered into written agreements with the Company assigning to the Company all rights in the Intellectual Property developed
in the course of their employment by, or provision of services to, the Company. No Intellectual Property necessary to enable the activities
of the business of the Company as now being conducted or as presently proposed to be conducted is owned by any current (or past), consultant
or employee, director or shareholder of the Company. Any and all Intellectual Property of any kind which has been developed as of the
date hereof by any employee or consultant of the Company is the property solely of the Company. No funding or facilities of an authority
or a university, college, other educational institution or research center was used in the development of any of its Intellectual Property.
The Company is not or has not been a member or promoter of, or contributor to, any industry standards bodies, patent pooling organizations
or similar organizations that could require or obligate the Company to grant or offer to any person any license or right to any of its
owned Intellectual Property. To the Company’s best knowledge, none of its Intellectual Property is subject to any proceeding or
outstanding governmental order or settlement agreement or stipulation that (a) restricts in any manner the use, transfer or licensing
thereof, or the making, using, sale, or offering for sale of the Company’s products or services by the Company or (b) may affect
the validity, use or enforceability of such Intellectual Property right. Except as set forth in Schedule 4.11, to the Company’s
knowledge, the Company’s employees are not and were not, during the period in which they were developing any of the Employee IP
(as defined below), employed by or engaged as a consultant with any academic, military, security forces, medical institution or similar
governmental institution. Employee IP shall mean all Intellectual Property developed by any employee of the Company prior to or following
the incorporation of the Company for the benefit of or in relation to the provisions of services to or furthering the business of the
Company.
4.11.8. The
Intellectual Property owned by the Company is free and clear of all liens, encumbrances or other restrictions and no item of such Intellectual
Property is subject to any outstanding injunction, judgment, order, decree, ruling or charge. No action, suit, proceeding, hearing, investigation,
charge, complaint, claim or demand is pending (or, to the best knowledge of the Company, is threatened) against the Company or the Company’s
founders, to the best knowledge of the Company, against any person or entity owning the Intellectual Property licensed to the Company,
orally or in writing, that challenges the legality, validity, enforceability, or ownership of, or the right of the Company to use, any
one or more items of the Intellectual Property owned or licensed by the Company. The Company has not agreed to indemnify any person or
entity for or against any interference, infringement, misappropriation, or other conflict with respect to any one or more items of the
Intellectual Property.
4.12. Taxes.
The Company has duly and timely filed all tax returns and reports (including information returns and reports) as required by applicable
law. Each such return or report was true and complete in all material respects when filed. None of such returns or reports has been audited
by any taxing authority and the Company has not been advised that any of such returns or reports will be audited. There is no pending
(or threatened by written notice delivered to the Company prior to the date hereof) dispute, examination, audit, claim or other action
concerning any tax or tax return of the Company claimed or raised by any tax authority. Any and all taxes and other charges due by the
Company to any local or foreign tax authorities (including, without limitation, those due in respect of the properties, income, franchises,
licenses, sales or payrolls) have been timely paid. The Company has never had any tax deficiency proposed or assessed against it and has
not executed any waiver of any statute of limitations on the assessment or collection of any tax or governmental charge The Company has
not incurred any taxes, assessments or governmental charges other than in the ordinary course of business, and the Company has made adequate
provisions on its books of account for all taxes, assessments and governmental charges with respect to their business, properties and
operations. The Company has not made any elections under applicable laws or regulations (other than elections that related solely to methods
of accounting, depreciation or amortization) that would have an adverse effect on the Company, its financial condition, its business as
presently conducted or currently proposed to be conducted or any of its properties or assets. The Company is not currently liable for
any tax due (whether income tax, capital gains tax, or otherwise). The Company has withheld or collected from each payment made to each
of its employees and/or service providers, the amount of all taxes required to be withheld or collected therefrom and have paid the same
to the proper tax receiving officers or authorized depositories, all to the extent regarded under applicable law.
4.13. Agreements.
Schedule 4.12 contains a true and complete list of all material contracts, licenses, commitments or undertakings, written
or oral, to which the Company is a party or by which its property is bound. (“Material Contracts”). Each of
such Material Contracts is in full force and effect and are the legal, valid and binding obligations of the Company enforceable against
it in accordance with its terms and no notice of termination of any such contract has been received or served by the Company, and neither
the Company nor, to the best knowledge of the Company, any other party thereto is in breach thereof. Except as set forth on Schedule
4.12 hereto, the Company does not have any: (i) employment or consulting contracts, deferred compensation agreements
or bonus, incentive, profit-sharing, or pension plans currently in force and effect, or any understanding with respect to any of the foregoing;
(ii) contracts with any affiliate of the Company or any of its officers or directors; (iii) contracts with any labor union or
association representing any employee of the Company; (iv) contracts for the sale of any of the assets of the Company other than
in the ordinary course of business or for the grant to any person of any preferential rights to purchase any of its assets; (v) contracts
containing covenants of the Company not to compete in any line of business or with any person in any geographical area or covenants of
any other person not to compete with the Company in any line of business or in any geographical area; (vi) contracts relating to
the acquisition by the Company of any operating business or the share capital of any other person; (vii) contracts relating to the
borrowing of money; (viii) any other contracts that involve more than US$30,000 in the aggregate or US$20,000 annually or require
performance by any party more than one year from the date hereof. None of the Material Contracts is currently being renegotiated and the
validity and effectiveness of all Material Contracts will not be materially adversely affected by the transactions contemplated by this
Agreement or any ancillary documents hereto. None of the Material Contracts may be terminated in accordance with its terms as a result
of the transactions contemplated by this Agreement and any ancillary documents hereto and the Company has not received any notice of termination
of any Material Contract.
4.14. Employment.
4.14.1. Except
as listed on Schedule 4.13 the Company has no employment or consulting contracts, deferred compensation agreements
or bonus, incentive, profit-sharing, or pension plans currently in force and effect, or any understanding with respect to any of the
foregoing. The Company has complied with all applicable employment laws policies, procedures and agreements relating to employment, terms
and conditions of employment and to the proper withholding and remission to the proper tax and other authorities of all sums required
to be withheld from employees or persons deemed to be employees under applicable laws respecting such withholding.
4.14.2. To
the Company’s knowledge, none of the Company’s employees are obligated under any contract (including licenses, covenants or
commitments of any nature) or other agreement, or subject to any judgment, decree or order of any court or administrative agency, that
would interfere with the use of such employee’s best efforts to promote the interests of the Company or that would conflict with
the Company's business as conducted and as currently proposed to be conducted.
4.14.3. There
have been no notifications provided to the Company regarding pending claims by any current or former employees or consultants for compensation
on termination of employment or engagement with the Company. The Company does not have any unsatisfied obligations of any nature to any
of its former employees or consultants (including those currently engaged through current contractors or suppliers of the Company) and
their termination was in compliance with all applicable legal requirements and agreements and they are owed nothing from the Company with
respect to their employment or engagement period and/or termination or otherwise.
4.14.4. To
the Company’s knowledge (after reasonable inquiry), there is no unfair labor practice charge or complaint, or other proceeding,
against the Company pending or threatened before any governmental entity and the Company has not received any notice of any such charge,
complaint or other proceeding.
4.14.5. All
previous and current consultants of the Company have been appropriately classified as such and the Company is under the reasonable opinion
that such consultants are not entitled to any rights, privileges or other entitlements applicable to “employees” under the
applicable labor or employee law or governmental regulations.
4.15. Litigation.
There is no civil, criminal or arbitration proceeding, governmental inquiry or investigation pending or currently threatened against the
Company, or any directors, officers, shareholders, consultants or employees of the Company in their capacity as such before any court,
arbitration board or tribunal or administrative or other governmental agency, and, to the Company’s best knowledge, there is no
basis for the foregoing. Neither the Company nor any of its officers are a party to the provisions of any order, writ, injunction, judgment
or decree of any court or governmental agency or instrumentality. There is no action, suit, proceeding or investigation initiated by the
Company currently pending or that the Company intends to initiate.
4.16. No
Public Offer. Neither the Company nor anyone acting on its behalf has offered securities of the Company or any part thereof or any
similar securities for issuance or sale to, or solicited any offer to acquire any of the same from, anyone so as to make issuance and
sale of the Issued Shares hereunder or the Preferred Shares issued upon conversion of the Convertible Note not exempt from the registration
requirements of Section 5 of the Securities Act of 1933, as amended (the "Securities Act") or the Israeli Securities
Law, 1968. None of the shares of the Company's share capital issued and outstanding has been offered or sold in such a manner as to make
the issuance and sale of such shares not exempt from such registration requirements, and all such share capital has been offered and sold
in compliance with all applicable securities laws.
4.17. Brokers.
Except as set forth in Schedule 4.17, no agent, broker, investment banker, person or firm acting in a similar capacity on
behalf of or under the authority of the Company is or will be entitled to any broker's or finder's fee or any other commission or similar
fee, directly or indirectly, on account of any action taken by the Company in connection with any of the transactions contemplated under
the Agreement.
4.18. Powers
of Attorney. There are no outstanding powers of attorney executed by or on behalf of the Company or on behalf of any of the Company's
Subsidiaries.
4.19. Government
Funding. The Company has not received any grant or other support or benefits (including, without limitation, tax benefits) from any
Israeli or foreign government entity.
4.20. Records.
The minutes of the Company which have been provided to the Investors contain accurate and complete copies of the minutes of every meeting
and/or written resolution of the Company's shareholders and the Board (and any committee thereof). No resolutions have been passed, enacted,
consented to or adopted by the directors (or any committee thereof) or shareholders of the Company, except for those contained in the
Company's minute books. The corporate records of the Company are complete and accurate in all material respects.
4.21. Interested
Party Transactions. Except as set forth in Schedule 4.21, no officer, director or shareholder of the Company, or
any affiliate of any such person or entity or the Company, has or has had, either directly, or to the best of the Company's knowledge,
indirectly, (a) an interest in any person or entity which (i) furnishes or sells services or products which are furnished or
sold or are proposed to be furnished or sold by the Company, or (ii) purchases from or sells or furnishes to the Company any goods
or services, or (b) has a beneficial interest in any contract or agreement to which the Company is a party or by which it may be
bound or affected. There are no existing arrangements or proposed transactions between the Company and any officer, director, or holder
of more than two percent (2%) of the share capital of the Company, or to the Company's best knowledge, any affiliate or associate of any
such person (“Interested Party Transactions”). No employee (including the company’s founder) shareholder, officer,
or director of the Company is indebted to the Company, nor is the Company indebted (or committed to make loans or extend or guarantee
credit) to any of them.
4.22. Privacy.
The Company is in compliance with any and all legal requirements, orders or other requirement relating to privacy, data protection, export
and the collection and use of personal information in applicable jurisdictions (“Privacy Laws”). The Company
has not received any written notice of any claims or been charged with the violation of any Privacy Laws. To the Company’s best
knowledge, no person has gained unauthorized access to or made any unauthorized use of any personally identifiable information maintained
by the Company. The Company has reasonable security measures in place to protect personally identifiable information from use by any other
person that is unlawful or that would violate Privacy Laws and no material changes have occurred with respect to the treatment of personal
information by the Company since its incorporation.
4.23. Insurance.
Schedule 4.23 hereto sets forth a true, complete and accurate list of all insurance policies which the Company currently
maintains with respect to its assets, liabilities, employees, officers, directors or other representatives (the “Insurance Policy/ies”).
The Insurance Policies are in full force and effect and will not lapse or be subject to suspension, modification, revocation, cancellation,
termination or non-renewal by reason of the execution, delivery or performance of the Transaction Documents. The Company has paid all
premiums or other payments due under each Insurance Policy and has otherwise performed in all material respects all of its respective
obligations thereunder. The Company has not received any notice that any Insurance Policy is not in full force and effect and no claims
have been submitted by the Company under any Insurance Policy.
4.24. Full
Disclosure. No representation or warranty in this Agreement (including the schedules attached hereto) nor any certificate made or
delivered in connection herewith contains any untrue statement of a material fact or omits to state a material fact necessary to make
the statements herein or therein not misleading, in view of the circumstances in which they were made. There is no material fact or information
relating to the business, financial condition, operations, or assets of the Company (excluding, for the avoidance of doubt, any general
facts or information regarding the industry in which the Company operates), known to the Company as of the Closing, that has not been
disclosed to the Investors by the Company.
5. Representations
and Warranties of the Investor. The Investor represents and warrants to the Company, and acknowledge that the Company is entering
into this Agreement in reliance thereon, as follows:
5.1. Enforceability.
The Investor has all necessary authority to execute and deliver this Agreement. This Agreement, when executed and delivered by the Investors,
will constitute the valid, binding and enforceable obligations of the Investor, except (i) as limited by laws relating to the availability
of specific performance, injunctive relief, or other equitable remedies, and (ii) to the extent the indemnification provisions contained
in this Agreement may be limited by applicable federal or state securities laws.
5.2. Experience.
The Investor is an experienced investor, particularly in the securities of companies in the development stage. The Investor is either
an “accredited investor” as defined in Rule 501 of Regulation D promulgated under the Securities Act of 1933 as amended
(the “Securities Act”) or is not a “US Person”. The Investor acknowledges that it can bear the economic
risk of his investment, and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits
and risks of the investment in the Issued Shares and has the capacity to protect its own interests. Moreover, the Investor acknowledges
that due to the inherent risk involved in such investment, the Investor's investment may be substantially or totally lost.
5.3. No
Public Market. The Investors understand that the Issued Shares and the Preferred Shares issued upon conversion of the Convertible
Note have not been registered under the Securities Act and no public market now exists for any of the securities issued by the Company
and that the Company has made no assurances that a public market will ever exist for the Company’s securities.
5.4. Disclosure
of Information. The Investors further represent that it has had an opportunity to ask questions and receive answers from the Company
regarding the terms and conditions of the offering of the Issued Shares and the business, properties, prospects and financial condition
of the Company.
5.5. Nothing
in this Section 5 shall be deemed or construed so as to derogate from the representations and warranties of the Company in Section 4
above.
6. Conditions
Precedent to Closing by the Investor. The obligations of the Investor to purchase the Issued Shares, to invest in the Convertible
Note and to transfer the funds at the Closing are subject to the fulfillment at or before the Closing of the following conditions precedent,
any one or more of which may be waived in whole or in part by the Investor, which waiver shall be at the sole discretion of the Investor:
6.1. Representations
and Warranties. The representations and warranties made by the Company in this Agreement shall have been true and correct as of the
Closing in all respects.
6.2. Covenants.
All covenants, agreements, and conditions contained in this Agreement to be performed or complied with by the Company prior to the Closing
shall have been performed or complied with by the Company prior to or at the Closing.
6.3. Consents, etc.
The Company shall have secured all permits, consents and authorizations that shall be necessary or required lawfully to consummate this
Agreement and to issue the Issued Shares to the Investor at the Closing.
6.4. Delivery
of Documents. All of the documents to be executed and delivered by the Company shall be in the respective form attached to this Agreement
or, if no such forms are attached, in a form satisfactory to the Investor and their respective counsel, in their reasonable discretion,
and shall have been delivered to the Investor or to counsel to the Investor.
6.5. No
Injunction. No injunction, judgment, order, decree, statute, law, ordinance, rule or regulation, entered, enacted, promulgated,
enforced or issued by any court or other authority of competent jurisdiction or other similar legal restraint or prohibition preventing,
enjoining, restraining, prohibiting or making illegal the consummation of this Agreement or any of the transactions contemplated hereby
shall be in effect on the Applicable Closing.
7. Conditions
Precedent to Closing by the Company. The Company’s obligations to sell and issue the Issued Shares and issue the Convertible
Note at the Closing to the Investor are subject to the fulfillment at or before the Closing of the conditions that (a) all covenants,
agreements and conditions contained in this Agreement to be performed, or complied with, by the Investor prior to or at the Closing shall
have been performed or complied with by the Investor prior to or at the Closing, including, without limitation, the payment of the Investment
Amount in immediately available US Dollar funds to the account designated by the Company, (b) the representations and warranties
made by the Investor in this Agreement shall be true and correct as of the date of the Closing, which conditions may be waived in whole
or in part by the Company, and which waiver shall be at the sole discretion of the Company.
8. Covenants.
8.1. Approval
of Investor by Israeli Medical Cannabis Unit. As soon as practical following the signing of this Agreement, the Company shall submit
an application to the Israeli Medical Cannabis Unit of the Ministry of Health (“Yakar”) requesting that the
Investor be entitled to hold more than five (5) percent of the issued and outstanding share capital of the Company (the “Yakar
Approval”). The Company and the Investor shall cooperate and each shall take all reasonable actions necessary to expedite the
receipt of the Yakar Approval.
8.2. Expenses.
The Company shall reimburse the Investor, subject to and upon the occurrence of the Closing, for its legal fees and other expenses actually
incurred with respect to the transaction set forth in this Agreement; provided, however, that such reimbursement does not
exceed forty thousand US Dollars ($40,000) plus Value Added Tax.
9. [RESERVED].
10. Miscellaneous
10.1. Governing
Law; Jurisdiction. This Agreement shall be governed by and construed in accordance with the laws of the State of Israel, without regard
to any applicable principles of conflicts of laws. Any dispute arising under or in relation to this Agreement shall be resolved exclusively
in the competent court located in Tel Aviv, Israel, and each of the Parties irrevocably submits to the exclusive jurisdiction of
such court.
10.2. Successors
and Assigns; Assignment. Except as otherwise expressly limited herein, the provisions hereof shall inure to the benefit of, and be
binding upon, the successors, assigns, heirs, executors, and administrators of the parties hereto. None of the rights, privileges, or
obligations set forth in, arising under, or created by this Agreement may be assigned or transferred by the Company except to a successor
to substantially all of the business and assets of the Company who has assumed in writing all obligations under this Agreement. Subject
to Section 10.3 below, none of the rights, privileges, or obligations set forth in, arising under, or created by this Agreement
may be assigned or transferred without the prior consent in writing of the Company, except that the Investor shall be entitled (at its
sole discretion) to assign their rights to invest the Issued Shares either to the person controlling 100% of the Investor (if the Investor
is a legal entity) or to any legal entity wholly owned by the Investor, in each case without the need to obtain the consent of the Company
or any other third party, by providing a written notice to the Company with respect to such assignment prior to the date of the Closing.
10.3. Transfer
of Rights. The Investor shall have the right to transfer its rights and obligations under this Agreement (including the Issued Shares)
in accordance with the Amended Articles.
10.4. Entire
Agreement; Amendment and Waiver. This Agreement and the schedules hereto constitute the full and entire understanding and agreement
between the Parties with regard to the subject matters hereof and thereof and any other written or oral agreement relating to the subject
matter hereof existing between the Parties is expressly canceled. Any term of this Agreement may be amended only with the written consent
of the Company and the Investor. The observance of any term hereof may be waived (either prospectively or retroactively and either generally
or in a particular instance) only with the written consent of the Party against such waiver is sought.
10.5. Confidentiality;
Press Release. Each Party shall keep this Agreement in strict confidence and shall not disclose the existence of this Agreement and/or
the terms hereof to any third party and/or issue any press release or make a public statement with respect to any of the foregoing, and/or
use the name of the other Parties, without the prior written consent of the other Parties, except for a disclosure on a need to know basis
(including to such Party's advisors) or as required by law, regulation or by any governmental authority. Notwithstanding the above, it
is agreed that, upon the Closing, the parties shall issue a press release in connection with the transactions contemplated hereby in a
mutually agreed form and any information included therein shall be able to be used by either party in any future press release or public
statement.
10.6. Notices, etc.
All notices and other communications required or permitted hereunder to be given to a Party to this Agreement shall be in writing (email
will be deemed as writing) and shall be addressed to such Party’s address as set forth below or at such other address as the Party
shall have furnished to each other Party in writing in accordance with this provision:
if to the Investor:
With a copy (which shall not constitute notice) to:
Amar, Reiter, Jeanne, Shochatovitch & Co., Lawyers
Technology Park,The Tower main building ("HaMigdal"), 6th floor,
2 Agudat Sport Hapoel Rd. Jerusalem 9695801 Israel
Attention: Daniel Chinn, Esq.
Email: danielc@ayr.co.il
if to the Company
Info@Cannasoul.co.il
5 Tarshish St., Caesarea, Israel, P.O.B. 3150
With a copy (which shall
not constitute notice)
to:
Any
notice sent in accordance with this Section shall be deemed received (i) if sent via email, within two (2) business days,
upon confirmation of receipt, or (ii) if delivered in person or by courier service, upon delivery, provided however
that any notices sent in accordance with sub-sections (ii) shall be also sent via email.
10.7. Delays
or Omissions. No delay or omission to exercise any right, power, or remedy accruing to any Party upon any breach or default under
this Agreement, shall be deemed a waiver of any other breach or default theretofore or thereafter occurring. Any waiver, permit, consent,
or approval of any kind or character on the part of any Party of any breach or default under this Agreement, or any waiver on the part
of any Party of any provisions or conditions of this Agreement, must be in writing and shall be effective only to the extent specifically
set forth in such writing. Except as stated in this Agreement, all remedies, either under this Agreement or by law or otherwise afforded
to any of the Parties, shall be cumulative and not alternative.
10.8. Severability.
If any provision of this Agreement is held by a court of competent jurisdiction to be unenforceable under applicable law, then such provision
shall be excluded from this Agreement and the remainder of this Agreement shall be interpreted as if such provision were so excluded and
shall be enforceable in accordance with its terms; provided, however, that in such event this Agreement shall be interpreted so as to
give effect, to the greatest extent consistent with and permitted by applicable law, to the meaning and intention of the excluded provision
as determined by such court of competent jurisdiction.
10.9. Counterparts.
This Agreement may be executed in any number of counterparts, each of which shall be deemed an original and enforceable against the Parties
actually executing such counterpart, and all of which together shall constitute one and the same instrument. The exchange of a fully executed
Agreement (in counterparts or otherwise) by electronic transmission (including in PDF format) or by facsimile shall be sufficient to immediately
bind the Parties to the terms and conditions of this Agreement.
10.10. Titles
and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing
or interpreting this Agreement.
[Signature Page Follows]
[Signature Page to Share
Purchase Agreement]
IN WITNESS WHEREOF the Parties have signed this Agreement as of the
date first hereinabove set forth.
| Company |
|
| |
|
| Cannasoul Analytics Ltd. |
|
| |
|
| By: |
/s/ Shmuel Mandel |
|
| Name: |
Shmuel Mandel |
|
| Title: |
Chief Executive Officer |
|
| |
|
| Investor |
|
| |
|
| Synaptogenix, Inc. |
|
| |
|
| By: |
/s/ Alan Tuchman |
|
| Name: |
Alan Tuchman |
|
| Title: |
Chief Executive Officer |
|
Exhibit 10.2
COLLABORATION AGREEMENT
This
Collaboration Agreement (“Agreement”) is effective as of the date of last signature below (“Effective Date”)
and is by and between Synaptogenix, Inc., a Delaware corporation with its principal place of business at 1185 Avenue Of The
Americas, 3rd Floor, New York, NY 10036, United States ("Synaptogenix"), and Cannasoul Analytics Ltd., a company duly
incorporated under the laws of the State of Israel, registration number 515782894, having its principal offices at 9 Tarshish Street,
Caesarea, Israel (“Cannasoul”). Synaptogenix and Cannasoul are sometimes referred to herein individually as a
“Party” and collectively as the “Parties”.
BACKGROUND
| A. | Cannasoul is a drug discovery company developing therapeutics derived from natural compounds. |
| B. | Synaptogenix is a clinical-stage biotech company focused on discovery and development of therapeutics
for neurodegenerative diseases and developmental disorders. |
| C. | The Parties entered into that certain Share Purchase Agreement of even date herewith (“Investment
Agreement”) under which Synaptogenix will invest up to $4,000,000 in Cannasoul, all in accordance with the terms thereof. |
| D. | The Parties wish to work together to identify compounds for development, and to develop such identified
compounds, all in accordance with the terms of this Agreement. |
NOW, THEREFORE, the Parties
agree as follows:
ARTICLE 1
Definitions
In this Agreement, the below
terms and expressions shall have the following meanings, and such meanings shall apply equally to both the singular and plural forms of
the terms defined:
1.1 “Affiliate”
means, with respect to an entity, any other person or entity, directly or indirectly through one or more intermediaries, controlled by,
controlling, or under common control with such entity, whether now or in the future, with “control” meaning (a) direct
or indirect beneficial ownership of more than 50% of the voting stock or other ownership interest of, or more than 50% interest in the
income of, the applicable person or entity, or (b) the possession, directly or indirectly, of the power to direct the management
or policies of the applicable person or entity, whether through the ownership of voting securities or other equity rights, by contract
relating to voting rights or corporate governance, or otherwise. Notwithstanding the above, Synaptogenix and Cannasoul shall not be deemed
for the purposes of this Agreement to be Affiliates.
1.2 “Applicable
Laws” means all applicable laws, statutes, rules, regulations and other pronouncements having the effect of law of any federal,
national, multinational, state, provincial, county, city or other political subdivision, agency or other body, domestic or foreign, including
any applicable rules, regulations, guidelines, or other requirements of Governmental Authorities that may be in effect from time to time.
1.3 “Background
Intellectual Property” means any and all Intellectual Property: (a) that is Controlled by a Party or any of its Affiliates
at the Effective Date; and (b) that is Controlled by a Party or any of its Affiliates after the Effective Date and during the Term
as a result of activities conducted outside the framework of this Agreement; and (c) Improvements thereto.
1.4 “Cannasoul
Inventions” means all Inventions made solely by or on behalf of Cannasoul and its Affiliates.
1.5 “Control”
or “Controlled” means, with respect to Patents and Know-How, that a Party owns or has a license to use, practice grant
access to, license or sublicense such Patents or Know-How, as provided herein without violating the terms of any agreement or other arrangement
with any Third Party and without violating Applicable Laws.
1.6 “Executive
Officer” of each Party means Alan Tuchman for Synaptogenix and Shmuel Mandel for Cannasoul.
1.7 “Governmental
Authority” means all agencies, commissions, officials, courts and other governmental and regulatory authorities and instrumentalities
having jurisdiction over a Party and/or the Parties, including within the Territory, and all states or other political subdivisions thereof
and supranational bodies applicable thereto, and includes regulatory authorities.
1.8 “Improvement”
means any Invention that constitutes an improvement, modification, enhancement, or derivative to or of any Intellectual Property that
is Controlled by a Party.
1.9 “Intellectual
Property” means all Patents, rights to inventions, copyrights, design rights, trademarks, trade secrets, Know-How, and all other
intellectual property (whether registered or unregistered) and all applications and rights to apply for any of the foregoing, anywhere
in the world
1.10 “Invention”
means any invention or discovery, and all Know-How, whether or not patentable, that is conceived, discovered or generated, in whole or
in part, by or on behalf of a Party or its Affiliates or subcontractors under this Agreement.
1.11 “Joint
Inventions” means all Inventions created, conceived, discovered, developed, generated, invented, made or reduced to practice
jointly by or on behalf of Cannasoul and Synaptogenix, including in each case with or without any Third Party.
1.12 “Know-How”
means any tangible and intangible information and data, and materials, discoveries, improvements, inventions, compositions of matter,
cell lines, assays, sequences, processes, methods, knowledge, protocols, formulas, utility, formulations, inventions (whether patentable
or not), know-how and trade secrets, in each case that either Party treats as confidential or proprietary information and that is not
generally known to the public, and excluding any of the foregoing to the extent claimed in any Patents.
1.13 “Patents”
means any provisional and non-provisional patents and patent applications, together with all certificates of invention and applications
for certificates of invention, additions, divisions, continuations, continuations-in-part, substitutions, and reissues claiming priority
thereto, as well as any re-examinations, extensions, registrations, patent term extensions, supplemental protection certificates, renewals
and the like with respect to any of the foregoing and all foreign counterparts thereof.
1.14 “Synaptogenix
Inventions” means all Inventions made solely by or on behalf of Synaptogenix and its Affiliates.
1.15 “Technion”
means Technion ± Israel Institute of Technology and/or The Technion Research and Development Foundation Ltd.
1.16 “Technion
License Agreement” means that certain agreement signed among the Technion, Cannasoul and Dr. David Meiri and dated February 15,
2018, as amended from time to time.
1.17 “Third
Party” means any person other than Synaptogenix, Cannasoul, or their respective Affiliates.
ARTICLE 2
COLLABORATION
2.1 Goal.
The goal of the Parties’ collaboration under this Agreement is to (a) discuss and evaluate technology and compounds developed
by Prof. Meiri at his lab at the Technion, which are the subject of the Technion License Agreement (the “Technion Compounds”),
(b) assess the feasibility of developing and commercializing the Technion Compounds, and (c) collaborate on the strategy for
clinical development of certain Technion Compounds.
2.2 Research
Plans. For each Technion Compound identified to Cannasoul from the Technion in accordance with the Technion License Agreement, Cannasoul
will promptly inform the JRC thereof, and the JRC will evaluate the applicable Technion Compound and in case the JRC determines that Cannasoul
and Synaptogenix should collaborate in the research and/or commercialization of such Technion Compound then the JRC will prepare a written
research plan describing the Parties’ activities with respect to their research of such Technion Compound (each a “Research
Plan”). Each Research Plan will identify the Technion Compound being evaluated, the process for evaluation, successful end points
(if known), and such other scientific, technical and operational provisions as the Parties may agree. The Parties will perform the research
and evaluation activities set forth in the Research Plan. Each Party may share a proposed update to the Research Plan with the JRC to
review, discuss, and determine whether to approve of such update. No update to the Research Plan will be effective unless approved by
the JRC. Once approved by the JRC, such updated Research Plan will become effective and replace the prior Research Plan. In the event
of any conflict between the terms of the Research Plan and the terms of this Agreement, the terms of this Agreement will control.
2.3 Clinical
Strategy. The Parties may agree from time to time for Synaptogenix to provide clinical trial support, strategy and consulting services
to Cannasoul in connection with the clinical development of certain technologies, all as will be detailed in the Research Plan. Synaptogenix
will invoice Cannasoul for such services at a rate to be agreed by the Parties in the framework of the Research Plan. Cannasoul will
pay invoices within thirty (30) days of receipt.
2.4 Research
Costs and Expenses. Except as otherwise set forth herein or as otherwise agreed by the Parties in writing, each Party is responsible
for its own costs and expenses incurred in performing the Research Plan.
2.5 Cannasoul
Research Activities. Cannasoul will perform all activities allocated to it under the Research Plan and prepare and deliver to Synaptogenix
all deliverables, data and reports in accordance with the applicable Research Plan (“Cannasoul Research Activities”).
2.6 Synaptogenix
Research Activities. Synaptogenix will perform all activities allocated to it under the Research Plan (“Synaptogenix Research
Activities,” and together with the Cannasoul Research Activities, the “Research Activities”).
2.7 Research
Records and Reports.
(a) Records.
During the Term and for three (3) years thereafter, the Parties will maintain written and electronic records of all Research Activities
performed by such Party, including the Research Results, in sufficient detail and in good scientific manner, appropriate for scientific,
patent, and regulatory purposes and in compliance with Applicable Law, which records will be complete and properly reflect all work done
and results achieved in the performance of its Research Activities by or on behalf of such Party.
(b) Research
Reports. Each Party will keep the other Party reasonably informed on the status, progress, and results of its activities under the
Research Plan by providing the other Party, through the JRC, with a report containing summaries of the Research Results each Calendar
Quarter in the format specified in the Research Plan, which report will (a) include summaries of the Research Results generated by
such Party since the last quarterly report provided to the other Party, (b) an update on such Party’s progress under the Research
Plan with respect to the performance of the Cannasoul Research Activities or Synaptogenix Research Activities, and (c) a summary
report or presentation in the form to be agreed to by the JRC. Upon the written request of either Party, the other Party will grant such
Party access, or otherwise provide to such Party, any applicable databases or records in order for such Party to review the raw data underlying
any Research Results.
(c) Conduct
of Research. Each Party will perform its Research Activities diligently and in good scientific manner, in compliance with all Applicable
Laws, including cGMP, GLP, and GCP, as applicable. Each Party will devote the efforts of suitably qualified and trained employees and
research assistants capable of carrying out the Research Activities set forth under the Research Plan to a professional workmanlike standard.
(d) Materials.
To facilitate the conduct of the Research Activities or the performance of other activities under this Agreement, each Party may provide
those materials described in the Research Plan (collectively, the “Materials”), in each case, in the quantities and
the manner set forth in the Research Plan, for the performance of the Research Activities thereunder. Except as otherwise set forth in
this Agreement, all such Materials will remain the sole property of the supplying Party, will be used only in the performance of obligations
or exercise of rights under this Agreement expressly in accordance with the applicable Research Plan or other written agreement by the
Parties, will be subject to any limitations specified in writing by the supplying Party in connection with such provision and use, may
not be transferred or permitted to be transferred to any Third Party without the supplying Party’s prior written consent (except
as expressly permitted under the applicable Research Plan), and will not be used in research or testing involving human subjects, unless
expressly agreed by the supplying Party. Each Party acknowledges that the other Party is providing the Materials for investigational use
only in laboratory animals or in in vitro experiments as further set forth in the Research Plan. THE MATERIALS ARE PROVIDED “AS
IS” AND WITHOUT ANY REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF
FITNESS FOR ANY PARTICULAR PURPOSE OR ANY WARRANTY THAT THE USE OF THE MATERIALS WILL NOT INFRINGE OR VIOLATE ANY PATENT OR OTHER PROPRIETARY
RIGHTS OF ANY THIRD PARTY.
ARTICLE 3
GOVERNANCE
3.1 Alliance
Managers. Promptly after the Effective Date, each Party will appoint a representative to act as its alliance manager under this Agreement
(each, an “Alliance Manager”) by providing written notification to the other Party. The Alliance Managers will be primarily
responsible for facilitating the flow of information and otherwise promoting communication, coordination, and collaboration between the
Parties under this Agreement. The Alliance Managers will have the right to attend all meetings of the JRC as non-voting members, and will
bring matters to the attention of the JRC if the Alliance Manager reasonably believes that such matter warrants such attention. Each Party
may replace its Alliance Manager at any time upon written notice to the other Party.
3.2 Joint
Research Committee.
3.2.1 Formation
of the JRC. Within thirty (30) days after the Effective Date, Synaptogenix and Cannasoul will establish a joint research committee
(“JRC”), which shall be composed of an equal number of representatives of Cannasoul and Synaptogenix (each of whom
must have appropriate technical credentials, experience, knowledge, and authority for such role), and which will have the responsibilities
set forth in this ARTICLE 3. The JRC will have no responsibility and authority other than that which responsibility and authority
is expressly set forth in this ARTICLE 3.
3.2.2 Membership.
Each Party may replace its JRC representative(s) at any time upon notice to the other Party. Synaptogenix will designate one of its
JRC members as one of the co-chairpersons of the JRC and Cannasoul will designate one of its members as the other co-chairperson of the
JRC. For each Calendar Year, the co-chairpersons will alternate serving in the role of “lead co-chairperson” for the JRC.
The lead co-chairperson or his or her designee, in collaboration with the Alliance Managers, will be responsible for calling meetings,
preparing and circulating an agenda in advance of each meeting, and preparing and issuing minutes of each meeting within thirty (30) days
thereafter. Such minutes will be finalized upon endorsement by all JRC members.
3.2.3 Meetings.
The JRC will hold meetings at such times as it elects to do so, but in no event will such meetings be held less frequently than once each
Calendar Quarter, unless otherwise agreed by the Parties. Meetings of the JRC may be held by audio or video teleconference; provided,
however, that at least one JRC meeting per year will be held in-person (alternating offices) unless otherwise agreed by the Parties. The
Alliance Manager of each Party will attend each meeting of the JRC as a non-voting participant. Each Party will be responsible for all
of its own expenses of participating in any JRC meeting.
3.2.4 Meeting
Agendas. Each Party will disclose to the other Party the proposed agenda items along with appropriate information at least five (5) business
days in advance of each meeting of the JRC; provided that under exigent circumstances requiring JRC input, a Party may provide its agenda
items to the other Party within a lesser period of time in advance of the meeting, or may propose that there not be a specific agenda
for a particular meeting, so long as such other Party consents to such later addition of such agenda items or the absence of a specific
agenda for such JRC meeting.
3.2.5 Non-Member
Attendance. Each Party may from time to time invite a reasonable number of participants, in addition to its representatives, to attend
the JRC meetings in a non-voting capacity; provided that if either Party intends to have any Third Party (including any consultant) attend
such a meeting, then such Party will provide at least five days prior written notice to the other Party and obtain the other Party’s
approval for such Third Party to attend such meeting, which approval will not be unreasonably withheld or delayed. Such Party will ensure
that such Third Party is bound by confidentiality and non-use obligations consistent with the terms of this Agreement.
3.2.6 Specific
Responsibilities of the JRC. The responsibilities of the JRC will be to:
(a) oversee
the execution of the Research Plan;
(b) coordinate
the Research Activities and facilitate communications between the Parties with respect to the Research Activities;
(c) review,
discuss, and determine whether to approve updates to the Research Plan;
(d) review
and discuss the results of performance of the Research Activities under the Research Plan, including all reports provided by the Parties,
and the anticipated timeline for initiating and completing all activities set forth in the Research Plan;
(e) perform
such other functions as appropriate to further the purposes of this Agreement, as expressly set forth in this Agreement, or allocated
to it by the Parties’ written agreement.
3.3 Decision-Making.
3.3.1 General
Decision-Making Process. A quorum for a meeting of the JRC will require the presence of at least one representative from each Party
and no action taken at any JRC meeting will be effective unless at least one representative of each Party is participating. All decisions
within the authority of the JRC will be made by consensus, with each Party’s representatives collectively having one vote.
3.3.2 Decisions
of the JRC. The JRC will use good faith efforts to promptly resolve any such matter for which it has authority. If, after the use
of good faith efforts, the JRC is unable to resolve any such matter or any other disagreement between the Parties that may be referred
to the JRC, then a Party may refer such matter to the Party’s respective Executive Officer for resolution as set forth below.
3.3.3 Resolution
of Disputes. If a Party makes an election under Section 3.3.2 to refer for resolution by the Executive Officers a matter as to
which the JRC cannot reach a consensus decision, then the JRC will submit in writing the respective positions of the Parties to their
respective Executive Officers. The Executive Officers will use good faith efforts to resolve any such matter so referred to them as soon
as practicable but in any event within fifteen (15) days after such matter is referred to them, and any final decision that the Executive
Officers jointly agree to in writing will be conclusive and binding on the Parties as a decision of the JRC.
3.4 Limitations
of Committee Authority and Decision-Making. The JRC will only have the powers expressly assigned to it in this ARTICLE 3 and
elsewhere in this Agreement and will not have the authority to: (a) modify or amend the terms and conditions of this Agreement; (b) waive
or determine either Party’s compliance with the terms and conditions of under this Agreement; or (c) decide any issue in a
manner that would conflict with the express terms and conditions of this Agreement. In addition, notwithstanding anything to the contrary
set forth in this Agreement, without the other Party’s prior written consent, neither Party (in the exercise of a Party’s
final decision-making authority), the JRC, nor a Party’s Executive Officer, in each case, may make a decision that could reasonably
be expected to require the other Party to take any action that such other Party reasonably believes would (i) require such other
Party to violate any Applicable Law, the requirements of any regulatory authority, or any agreement with any Third Party entered into
by such other Party or (ii) require such other Party to infringe or misappropriate any Intellectual Property of any Third Party.
ARTICLE 4
Confidentiality
4.1 Confidentiality
Obligations. All information disclosed by one Party to the other Party pursuant to this Agreement shall be “Confidential
Information” of the disclosing Party for all purposes hereunder. Each Party agrees that, for the Term and for five (5) years
thereafter, such Party shall, and shall ensure that its and its Affiliates’ officers, directors, employees and agents shall, keep
completely confidential (using at least the same standard of care as it uses to protect proprietary or confidential information of its
own, but in no event less than reasonable care) and not reverse-engineer, publish or otherwise disclose, and not use for any purpose except
as expressly permitted hereunder, any Confidential Information of the other Party. The foregoing obligations shall not apply to any information
disclosed by a Party hereunder to the extent that the receiving Party can demonstrate with competent evidence that such information:
(a) was
already known to the receiving Party or its Affiliate, other than under an obligation of confidentiality, at the time of disclosure;
(b) was
generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
(c) became
generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission
of the receiving Party or its Affiliate in breach of this Agreement;
(d) is
developed by the receiving Party independently from, and without use of or reliance upon the disclosing Party’s Confidential Information,
as evidenced by the receiving Party’s records; or
(e) was
subsequently lawfully disclosed on a non-confidential basis to the receiving Party or its Affiliate by a Third Party other than in contravention
of a confidentiality obligation of such Third Party to the disclosing Party.
4.2 Authorized
Disclosure. A Party may disclose the other Party’s Confidential Information to the extent such disclosure is required by valid
court order or legal process, provided that, to the extent consistent with such order or process, such Party gives the other Party advance
notice of such required disclosure, limits the disclosure to that actually required, and uses commercially reasonable efforts to cooperate
in the other Party’s attempts to obtain a protective order or confidential treatment of the information required to be disclosed
at the other Party’s request and expense.
4.3 Confidentiality
of Agreement Terms. The Parties acknowledge that the terms of this Agreement shall be treated confidentially as Confidential Information
of each Party. Notwithstanding the foregoing, such terms may be disclosed by a Party in confidence to investment bankers, investors, and
potential investors or acquirers, solely in the context of a potential transaction and for the limited purpose of evaluating such potential
transaction. In addition, a copy of this Agreement may be filed by a Party with the Securities and Exchange Commission and/or any applicable
securities exchange as required by Applicable Law. In connection with any such filing, such Party shall endeavor to obtain confidential
treatment of economic and trade secret information. In any event, the Parties agree to take all reasonable action to avoid disclosure
of Confidential Information except as permitted hereunder.
4.4 Publicity.
Neither Party shall originate any publicity, news release or public announcement, written or oral relating to this Agreement, including
its existence or the negotiations without the prior written approval of the other Party, which approval shall not be unreasonably withheld,
except as required by Applicable Law or the rules of a stock exchange or an authorized department of the government provided that
in such case each Party shall use commercially reasonable efforts to enable the other Party to review such publication in advance and
in writing.
ARTICLE 5
INTELLECTUAL
PROPERTY
5.1 Inventorship.
All determinations of inventorship under this Agreement will be in accordance with U.S. patent law.
5.2 Ownership
of Materials. Materials that are Controlled by a Party are proprietary to such Party, and such Party will retain all rights, title,
and interests in and to such Materials and all Intellectual Property rights therein, including all Patents relating to such Materials.
5.3 Ownership
of Technology.
5.3.1 Synaptogenix
Ownership. Synaptogenix will solely own all rights, title, and interest in and to Synaptogenix Inventions and to Background Intellectual
Property Controlled by Synaptogenix.
5.3.2 Cannasoul
Ownership. Cannasoul will solely own all rights, title, and interest in and to Cannasoul Inventions and to Background Intellectual
Property Controlled by Cannasoul.
5.3.3 Joint
Ownership. The Parties will jointly own all Joint Inventions, and, subject to the terms and conditions set forth in this Agreement,
each Party is entitled to practice Joint Inventions for all purposes on a worldwide basis and to license such Joint Inventions through
multiple tiers without consent of the other Party (where consent is required by Applicable Law, such consent is deemed hereby granted)
and without a duty of accounting to the other Party. Each Party will grant and hereby does grant to the other Party all further permissions,
consents, and waivers with respect to, and all licenses under, the Joint Inventions, throughout the world, necessary to provide the other
Party with full rights of use and exploitation of Joint Inventions. Without limitation, each Party will cooperate with the other Party
if the Parties determine to apply for U.S. or foreign patent protection for any Joint Inventions and will obtain the cooperation of the
individual inventors of any such Inventions.
5.4 No
Rights or Licenses. Neither Party grants to the other Party any rights or licenses in or to any Intellectual Property rights, whether
by implication, estoppel, or otherwise, in connection with this Agreement.
ARTICLE 6
Term;
Termination
6.1 Term
and Expiration. The term of this Agreement shall commence upon the Effective Date and shall terminate on the fifth (5th)
anniversary thereof unless terminated earlier as permitted herein (“Term”).
6.2 Termination.
Synaptogenix may terminate this Agreement upon thirty (30) days’ notice to Cannasoul. Each Party may terminate this Agreement upon
notice if the other Party materially breaches any provision hereof and does not cure such breach within thirty (30) days of receipt of
notice of such breach by the other Party.
6.3 Surviving
Obligations. Notwithstanding anything to the contrary herein, ARTICLE 5, Section 6.2 and ARTICLE 8 shall survive termination
or expiration of this Agreement. Unless explicitly stipulated to the contrary in this Agreement, expiration or termination of this Agreement
shall not relieve the Parties of any liability which accrued hereunder prior to the effective date of such expiration or termination nor
preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of
this Agreement.
ARTICLE 7
WARRANTIES
7.1 Mutual
Representations and Warranties. Each Party hereby represents and warrants to the other Party, as of the Effective Date, that:
7.1.1 Good
Standing. Each Party is duly organized, validly existing, and in good standing under the Applicable Laws of the jurisdiction of its
incorporation or organization;
7.1.2 Corporate
Power and Authority. Each Party (a) has the requisite power and authority and the legal right to enter into this Agreement and
to perform its obligations hereunder and (b) has taken all requisite action on its part to authorize the execution and delivery of
this Agreement and the performance of its obligations hereunder;
7.1.3 Binding
Obligation. This Agreement has been duly executed and delivered on behalf of each Party, and constitutes a legal, valid and binding
obligation, enforceable against each Party in accordance with the terms hereof; and
7.1.4 No
Conflict. The execution, delivery, and performance of this Agreement by each Party will not constitute a default under or conflict
with any agreement, instrument or understanding to which either entity is a party or by which either entity is bound, or violate any Applicable
Law of any Governmental Authority or administrative or other agency having jurisdiction over either Party.
7.2 Additional
Representations and Warranties of Cannasoul. Cannasoul hereby represents and warrants to Synaptogenix that Cannasoul has a right of
first look from the Technion Research and Development Foundation Ltd. to negotiate the use the Technion Technologies as contemplated herein,
as detailed in the Technion License Agreement.
ARTICLE 8
Indemnification, Insurance
8.1 Indemnification
by Cannasoul. Cannasoul will indemnify, defend, and hold harmless Synaptogenix and its Affiliates, and each of their respective directors,
officers, employees, and agents (collectively “Synaptogenix Indemnitees”), from and against all claims, demands, actions,
or other proceedings brought by any Third Party (each a “Claim”) against any of the Synaptogenix Indemnitees, and shall
pay all losses, liabilities, damages, and expenses, including reasonable attorneys’ fees and costs payable to such Third Party pursuant
to such Claim (collectively, “Liabilities”), to the extent resulting from any arising out of:
8.1.1 the
conduct of the Cannasoul Research Activities by or on behalf of Cannasoul (including by an Affiliate or subcontractor of Cannasoul);
8.1.2 the
material breach of any representation, warranty, or covenant under this Agreement by or on behalf of Cannasoul or any of its Affiliates;
or
8.1.3 the
gross negligence, recklessness, or wrongful intentional acts or omissions of any Cannasoul Indemnitees in the course of performing activities
under this Agreement.
8.2 Indemnification
by Synaptogenix. Synaptogenix will indemnify, defend, and hold harmless Cannasoul and its Affiliates and each of their respective
directors, officers, employees, and agents (collectively “Cannasoul Indemnitees”), from and against all Claims brought
against any of the Cannasoul Indemnitees, and shall pay all Liabilities payable to such Third Party pursuant to such Claim, to the extent
resulting from:
8.2.1 the
conduct of the Synaptogenix Research Activities by or on behalf of Synaptogenix (including by an Affiliate or subcontractor of Synaptogenix);
8.2.2 the
material breach of any representation, warranty, or covenant under this Agreement by or on behalf of Synaptogenix or any of its Affiliates;
or
8.2.3 the
gross negligence, recklessness, or wrongful intentional acts or omissions of any Synaptogenix Indemnitees in the course of performing
activities under this Agreement.
8.3 Indemnification
Procedure.
8.3.1 Notice.
If either Party is seeking indemnification under Section 8.1 or Section 8.2 (the “Indemnified Party”), then
it will promptly inform the other Party (the “Indemnifying Party”) of the Claim giving rise to the obligation to indemnify
pursuant to such Section as soon as reasonably practicable after receiving notice of the Claim, provided, however,
that no delay or failure on the part of the Indemnified Party in notifying the Indemnifying Party will relieve the Indemnifying Party
from any obligation hereunder unless (and then only to the extent that) such delay or failure is prejudicial to or otherwise adversely
affects the Indemnifying Party.
8.3.2 Control.
The Indemnifying Party will have the right, exercisable by notice to the Indemnified Party within thirty (30) days after receipt of notice
from the Indemnified Party of the commencement of or assertion of the Claim, to assume the direction and control of the defense, litigation,
settlement, appeal, or other disposition of such Claim for which it is obligated to indemnify the Indemnified Party (including the right
to settle the Claim solely for monetary consideration) with counsel selected by the Indemnifying Party and reasonably acceptable to the
Indemnified Party. During such time as the Indemnifying Party is controlling the defense of such Claim, the Indemnified Party will cooperate
with the Indemnifying Party, and will cause its Affiliates and agents to cooperate upon request of the Indemnifying Party, in the defense
or prosecution of the Claim, including by furnishing such records, information, and testimony and attending such conferences, discovery
proceedings, hearings, trials or appeals as may reasonably be requested by the Indemnifying Party. In the event that the Indemnifying
Party does not notify the Indemnified Party of the Indemnifying Party’s intent to defend any Claim within thirty (30) days after
notice thereof, the Indemnified Party may (without further notice to the Indemnifying Party) undertake the defense thereof with counsel
of its choice and at the Indemnifying Party’s expense (including reasonable, out-of-pocket attorneys’ fees and costs and expenses
of enforcement or defense). The Indemnifying Party or the Indemnified Party, as the case may be, will have the right to participate (including
the right to conduct discovery, interview and examine witnesses and participate in all settlement conferences), but not control, at its
own expense and with counsel of its choice, in the defense of any Claim that has been assumed by the other Party.
8.3.3 Settlement.
Notwithstanding any provision to the contrary in this Agreement, the Indemnifying Party will not enter into any settlement, consent judgment,
or other voluntary final disposition of any Claim that admits any wrongdoing or fault by any Synaptogenix Indemnitees or Cannasoul Indemnitees,
or imposes on any Synaptogenix Indemnitees or Cannasoul Indemnitees any payment or other liability, without the prior written consent
of such Indemnified Party.
8.4 Mitigation
of Loss. Each Indemnified Party will take and will procure that its Affiliates take all such reasonable steps and action as are reasonably
necessary or as the Indemnifying Party may reasonably require in order to mitigate any claims (or potential losses or damages) under this
ARTICLE 8. Nothing in this Agreement will or will be deemed to relieve any Party of any common law or other duty to mitigate any
losses incurred by it.
ARTICLE 9
MISCELLANEOUS
9.1 Entire
Agreement; Amendments. This Agreement and the attachments hereto and the Research Plans, contain the entire understanding and agreement
of the Parties with respect to the subject matter hereof and cancel and supersede any and all prior negotiations, correspondence, understandings
and agreements between the Parties, whether oral or written, regarding such subject matter. No waiver, modification or amendment of any
provision of this Agreement shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party.
9.2 Assignments.
Except as expressly provided herein, neither this Agreement nor any rights and obligations hereunder shall be assignable by a Party without
the prior written consent of the other Party; provided, however, that a Party may assign this Agreement in its entirety to any Affiliate
or to any successor in interest by way of merger, acquisition or sale of all or substantially all of its assets to which this Agreement
relates, provided that such successor agrees in writing to be bound by the terms of this Agreement as if it were the assigning Party.
This Agreement shall be binding upon the successors and permitted assigns of the Parties. Any assignment not in accordance with this Section 8.2
shall be void.
9.3 Notices.
Any notice, waiver or consent required or permitted to be given under this Agreement shall be in writing and shall be deemed to have been
sufficiently given if delivered by international courier service (signature required) to the other Party at its address shown below or
such other address notified by such other Party from time to time.
If to Cannasoul, addressed to:
Cannasoul Analytics Ltd.
9 Tarshish Street, Caesarea
Israel
Attn: Dr. Shmuel Mandel, CEO
If to Synaptogenix, addressed to:
Synaptogenix Bioscience, Inc.
1185 Avenue Of The Americas, 3rd Floor
New York, NY 10036
United States,
Attn: Robert Weinstein, CEO
9.4 Waiver.
A waiver by either Party of any of the terms and conditions of this Agreement in any instance shall not be deemed or construed to be a
waiver of such term or condition for the future, or of any subsequent breach hereof. All rights, remedies, undertakings, obligations and
agreements contained in this Agreement shall be cumulative and none of them shall be in limitation of any other remedy, right, undertaking,
obligation or agreement of either Party.
9.5 Severability.
Should any provision of this Agreement be or become invalid, ineffective or unenforceable as a whole or in part, the validity, effectiveness
and enforceability of the remaining provisions shall not be affected thereby. Any such invalid, ineffective or unenforceable provision
shall be deemed replaced by such valid, effective and enforceable provision as comes closest to the economic intent and the purpose of
such invalid, ineffective or unenforceable provision. The aforesaid shall apply mutatis mutandis to any gap in this Agreement.
9.6 Governing
Law. This Agreement shall be governed by and construed in accordance with the laws of the State of New York, United States, without
regard to its choice of law provisions, including to matters of validity, construction, effect and performance. Matters relating to the
inventorship, initial ownership, scope, validity, enforceability or infringement of any Intellectual Property rights in a particular country
shall be determined in accordance with the law applicable in the country in which such Intellectual Property rights have been issued or
otherwise exist. Any dispute, controversy or claim arising from or in connection with this Agreement or its validity shall be submitted
exclusively to the courts located in New York, New York, and each Party hereby submits to the personal jurisdiction of such courts.
9.7 Mutual
Cooperation. Each of the Parties shall use its commercially reasonable efforts to take, or cause to be taken, all action or do or
cause to be done, and to assist and cooperate with each other Party in doing, all things necessary, proper or advisable to consummate
and make effective, in the most expeditious manner practicable, the transactions contemplated by this Agreement (in each case, to the
extent that the same is within the control of such Party).
9.8 Third
Party Beneficiaries. All rights, benefits and remedies under this Agreement are solely intended for the benefit of Synaptogenix and
Cannasoul. No Third Party shall have any rights whatsoever to (a) enforce any obligation contained in this Agreement; (b) seek
a benefit or remedy for any breach of this Agreement; or (c) take any other action relating to this Agreement under any legal theory,
including but not limited to, actions in contract, tort (including but not limited to negligence, gross negligence and strict liability),
or as a defense, setoff or counterclaim to any action or claim brought or made by the Parties.
9.9 Language.
This Agreement and all other communications under or in connection with this Agreement shall be written and executed in the English language.
Any translation into any other language shall not be an official version thereof, and in the event of any conflict in interpretation between
the English version and such translation, the English version shall prevail.
9.10 Counterparts.
This Agreement may be executed simultaneously in any number of counterparts, any one of which need not contain the signature of more than
one Party but all such counterparts taken together shall constitute one and the same agreement.
9.11 Headings.
The headings in this Agreement are for convenience of reference only and shall not limit or otherwise affect the meaning hereof.
[The remainder of this page has been intentionally
left blank]
IN WITNESS WHEREOF, the Parties
have caused this Agreement to be executed by their respective duly authorized representatives effective as of the Effective Date.
| |
SYNAPTOGENIX, INC. |
| |
|
| |
By: |
/s/ Alan Tuchman |
| |
|
| |
Name: |
Alan Tuchman |
| |
|
| |
Title: |
Chief Executive Officer |
| |
|
| |
|
| |
CANNASOUL ANALYTICS LTD. |
| |
|
| |
By: |
/s/ Shmuel Mandel |
| |
|
| |
Name: |
Shmuel Mandel |
| |
|
| |
Title: |
Chief Executive Officer |
Exhibit 10.3
CANNASOUL ANALYTICS LTD.
AMENDED AND RESTATED INVESTORS' RIGHTS AGREEMENT
THIS
AMENDED AND RESTATED INVESTORS’ RIGHTS AGREEMENT (the “Agreement”) is made as of the 24th day of
October 2023, by and among Cannasoul Analytics Ltd.., Reg. No. 515782894, a company incorporated under the laws of the
State of Israel (the “Company”), the founders of the Company listed on Schedule A attached hereto (the “Founders”),
and the investors listed on Schedule B attached hereto (each, an “Investor” and collectively, the “Investors”,
and together with the Founders, the “Shareholders”).
RECITALS
WHEREAS, certain of the Investors
(the “Existing Investors”) hold shares of the Company’s Ordinary Shares and possess registration rights, and
other rights pursuant to that certain Investors’ Rights Agreement dated as of 1 July 2018, by and among the Company, Professor
David (Dedi) Meiri (the “Founder”) and such Existing Investors (the “Prior Agreement”); and
WHEREAS, certain of the Investors
(the “Additional Existing Investors”) hold shares of the Company’s Ordinary Shares and possess registration
rights, information rights, and other rights pursuant to that certain Share Purchase Agreement dated as of 1 July 2019, by and among
the Company and such Additional Existing Investors (the “Prior SPA”); and
WHEREAS, the parties to the
Prior Agreement and the Prior SPA desire to amend and restate the Prior Agreement in its entirety and to terminate certain provision
so the Prior SPA and to accept the rights created pursuant to this Agreement in lieu of the rights granted to them under the Prior Agreement
and certain rights granted to them under the Prior SPA; and
WHEREAS, certain of the Investors
are parties to that certain Series A Preferred Stock Purchase Agreement of even date herewith by and among the Company and such
Investors (the “Series A Agreement”), under which certain of the Company’s and such Investors’ obligations
are conditioned upon the execution and delivery of this Agreement by such Investors, the Existing Investors and the Additional Existing
Investors, and the Company.
WHEREAS, in order to induce
the Investors to invest funds in the Company pursuant to the Series A Agreement, the Company, the Founder the Existing Investors
and the Additional Existing Investors agree that this Agreement shall govern, inter alia, certain rights of the Shareholders to
cause the Company to register Ordinary Shares (as defined below) issued or issuable to them and certain other matters as set forth herein.
NOW, THEREFORE, in consideration
of the foregoing recitals, the mutual promises hereinafter set forth, and other good and valuable consideration, the receipt and sufficiency
of which are hereby acknowledged, the Existing Investors hereby agree that the Prior Agreement shall be amended and restated and replaced
in its entirety by this Agreement, and the parties further hereby agree as follows:
| 1.1 | Definitions.
For purposes of this Agreement: |
| (i) | The term “Act” means the
US Securities Act of 1933, as amended. |
| (ii) | The term “Articles” means
the Company’s Amended and Restated Articles of Association in effect as of the date
of the Closing (as such term is defined in the Series A Agreement), as amended from
time to time. |
| (iii) | The term “Board” means
the Company’s board of directors. |
| (iv) | The term “Business Day”
shall have the meaning set forth in the Articles. |
| (v) | The terms “Form S-3”
and “Form F-3” mean such form under the Act as in effect on the date
hereof or any registration form under the Act subsequently adopted by the SEC that permits
inclusion or incorporation of substantial information by reference to other documents filed
by the Company with the SEC. |
| (vi) | The term “Holder” means
any holder of Registrable Securities who is a party to this Agreement, or any permitted assignee
thereof in accordance with Section 1.12 hereof to whom rights have been duly assigned
in accordance thereof. |
| (vii) | The term “Initial Offering”
means the Company’s first underwritten public offering of its Ordinary Shares pursuant
to an effective registration statement under the Act or under equivalent securities law of
another jurisdiction which such other jurisdiction shall be acceptable to holders of majority
of the Company’s Preferred Shares on an as converted basis. |
| (viii) | The term “Major Holder”
shall mean any Investor who holds at least five (5) per cent of the issued and outstanding
share capital of the Company. |
| (ix) | The term “Ordinary Shares”
shall have the meaning set forth in the Articles. |
| (x) | The term “Preferred Shares”
shall have the meaning set forth in the Articles. |
| (xi) | The term “register,” “registered,”
and “registration” refer to a registration effected by preparing and filing
a registration statement or similar document in compliance with the Act, and the declaration
or ordering of effectiveness of such registration statement or document. |
| (xii) | The term “Registrable Securities”
means (i) the Ordinary Shares issuable or issued upon conversion of the Preferred Shares,
(ii) any Ordinary Shares held by an Investor or issuable or issued upon conversion of
any other securities of the Company held by an Investor, (iii) any Ordinary Shares issued
as (or issuable upon the conversion or exercise of any warrant, right or other security that
is issued as) a dividend, share split or other distribution with respect to, or in exchange
for or conversion into, or in replacement of, the shares referenced in (i) above in
each case in connection with any merger, consolidation, reclassification, recapitalization
or similar event, excluding in all cases, however, (a) any Registrable Securities sold
by a person in a transaction in which his rights under this Section 1 are not duly assigned,
and (b) any securities that may be sold pursuant to Rule 144 under the Act (or
similar rule). |
| (xiii) | The number of shares of "Registrable
Securities then outstanding" shall be determined by the number of Ordinary Shares
outstanding, which are Registrable Securities, calculated on an as-converted basis. |
| (xiv) | The term “1934 Act” means
the US federal Securities Exchange Act of 1934, as amended. |
| 1.2 | Request
for Registration. |
(i) Subject
to the conditions of this Section 1.2, if at any time after six (6) months of the effective date of an Initial Offering and
ending five (5) years thereafter, but subject to Section 1.13 and to the terms of any “lock-up agreement”
entered into between the underwriters of the Company’s Initial Offering and a Holder (unless waived by such underwriters), the
Company shall receive a written request from the Holders of at least 40% of the Registrable Securities then outstanding (the “Initiating
Holders”) that the Company file a registration statement under the Act covering the registration of Registrable Securities,
provided that the anticipated offer price would exceed $5,000,000, then the Company shall, within twenty (20) days of the receipt thereof,
give written notice of such request to all Holders of Registrable Securities, and subject to the limitations of this Section 1.2,
use commercially reasonable efforts to effect, as soon as practicable, and in any event within sixty (60) days after the date such request
is given by the Initiating Holders, the registration under the Act of all Registrable Securities that such Holders request to be registered
in a written request received by the Company within twenty (20) days of the mailing of the Company’s notice pursuant to this Section 1.2(i).
(ii) If
the Initiating Holders intend to distribute the Registrable Securities covered by their request by means of an underwriting, they shall
so advise the Company as a part of their request made pursuant to this Section 1.2 and the Company shall include such information
in the written notice referred to in Section 1.2(i). The underwriter(s) will be selected by the Board of Directors and shall
be reasonably acceptable to a majority in interest of the Initiating Holders In such event the right of any Holder to include its Registrable
Securities in such registration shall be conditioned upon such Holder’s participation in such underwriting and the inclusion of
such Holder’s Registrable Securities in the underwriting (unless otherwise mutually agreed by a majority in interest of the Initiating
Holders and such Holder) to the extent provided herein. All Holders proposing to distribute their securities through such underwriting
shall enter into an underwriting agreement in customary form with the underwriter or underwriters selected for such underwriting by the
Company and reasonably acceptable to a majority in interest of the Initiating Holders (which underwriter or underwriters shall be reasonably
acceptable to the Company).
(iii) Notwithstanding
any other provision of this Agreement, if the underwriter advises the Company that marketing factors require a limitation of the number
of securities underwritten (including Registrable Securities), then the Company shall so advise all Holders of Registrable Securities
that would otherwise be underwritten pursuant hereto, and the number of shares that may be included in the underwriting shall be allocated
to the Holders of such Registrable Securities on a pro rata basis based on the number of Registrable Securities then held by each such
Holder out of the number of Registrable Securities then held by all such Holders (including the Initiating Holders). Any Registrable
Securities excluded or withdrawn from such underwriting shall be withdrawn from the registration.
(iv) The
Company shall not be required to effect a registration pursuant to this Section 1.2:
(b) after the Company
has effected two (2) registrations pursuant to this Section 1.2 and such registrations have been declared or ordered effective
(a registration shall be counted as “effected” for purposes of this Section 1.2 even if the Initiating Holders (i) withdrew
their request for such registration (except, if at the time of such withdrawal, the Holders shall have learned of a material adverse
change in the condition business, or prospects of the Company from that known to the Holders at the time of their request and have withdrawn
the request with reasonable promptness after learning of such information), (ii) elected not to pay the registration expenses therefor,
and (iii) forfeited their right to one demand registration statement hereunder, in which case such withdrawn registration statement
shall be counted as “effected” for purposes of this Section 1.2); provided that a registration shall not be counted
as “effected” if, fewer than fifty percent (50%) of the total number of Registrable Securities that Holders have requested
to be included in such registration statement are actually included;
(c) during the period
starting with the date sixty (60) days prior to the Company’s good faith estimate of the date of the filing of, and ending
on a date one hundred eighty (180) days following the effective date of, a Company-initiated registration subject to Section 1.3
below, provided that the Company is actively employing in good faith all commercially reasonable efforts to cause such registration
statement to become effective;
(d)
if the Initiating Holders propose to dispose of shares of Registrable Securities that may be immediately registered under the Act pursuant
to a request made pursuant to Subsection 1.4; or
(e) if the Company
shall furnish to the Holders requesting a registration statement pursuant to this Section 1.2, a certificate signed by the Chairman
of the Board stating that in the good faith judgment of the Board, it would be seriously detrimental to the Company and its shareholders
for such registration statement to be effected at such time (or, if a registration statement has been filed, it would be seriously detrimental
to the Company or its shareholders for such registration statement to become effective or remain effective for as long as such registration
statement otherwise would be required to remain effective), in which event the Company shall have the right to defer such filing for
a period of not more than ninety (90) days after receipt of the request of the Initiating Holders, provided that such right to delay
a request shall be exercised by the Company not more than once in any twelve (12)-month period.
1.3 Company
Registration. Other than in connection with a request for registration pursuant to Section 1.2 or 1.4 of this Agreement,
if (but without any obligation to do so) the Company proposes to register (including for this purpose a registration effected by the
Company for shareholders other than the Holders) any of its shares or other securities under the Act in connection with the public offering
of such securities (other than a registration relating solely to the sale of securities to participants in an employee benefit plan,
a registration relating to a corporate reorganization or other transaction under Rule 145 of the Act, a registration on any form
that does not include substantially the same information as would be required to be included in a registration statement covering the
sale of the Registrable Securities, or a registration in which the only Ordinary Shares being registered are Ordinary Shares issuable
upon conversion of debt securities that are also being registered), the Company shall, at such time, promptly give each Holder written
notice of such registration. Upon the written request of each Holder given within twenty (20) days after mailing of such notice by the
Company in accordance with Section 4.6, the Company shall, subject to the provisions of Section 1.3(i), cause to be registered
under the Act all of the Registrable Securities that each such Holder has requested to be included in such registration on the terms
set forth in this Agreement. Prior to the commencement of any “road show,” any Holder shall have the right to withdraw its
request for inclusion of its Registrable Securities in any registration by giving written notice to the Company of its request to withdraw
and such withdrawal shall be irrevocable and, after making such withdrawal, such Holder shall no longer have any right to include Registrable
Securities in the registration under this Section 1.3 as to which such withdrawal was made.
(i) Right
to Terminate Registration. The Company shall have the right to terminate or withdraw any registration or offering initiated by it
under this Section 1.3 prior to the effectiveness of such registration whether or not any Holder has elected to include Registrable
Securities in such registration or offering. The expenses of such withdrawn registration shall be borne by the Company in accordance
with Section 1.7 hereof.
(ii) Underwriting
Requirements. In connection with any offering involving an underwriting of shares of the Company’s share capital, the Company
shall not be required under this Section 1.3 to include any of the Holders’ securities in such underwriting unless the Holders
accept the terms of the underwriting, in customary form, as agreed upon between the Company and the underwriters selected by it, and
enter into such underwriting agreement, and then only in such quantity as the underwriters determine in their sole discretion will not
materially and adversely jeopardize the success of the offering by the Company. Notwithstanding any other provision of this Agreement,
if the total number of securities, including Registrable Securities, requested by Holders to be included in such offering exceeds the
number of securities sold other than by the Company that the underwriters determine in their sole discretion could materially and adversely
jeopardize the success of the offering, then the Company shall be required to include in the offering only that number of such securities
that the underwriters determine in their sole discretion will not materially and adversely jeopardize the success of the offering, the
securities so included to be apportioned as follows: (i) first, to the Company, and (ii) second, among the Holders of Registrable
Securities held by the Investors requested to be included in such offering, on a pro rata basis relative to their holding among the Registrable
Securities;. Any Registrable Shares excluded or withdrawn from such underwriting shall be excluded and withdrawn from the registration.
(iii) For
purposes of the preceding section concerning apportionment, for any shareholder that is a Holder of Registrable Securities that is a
partnership or corporation, the partners, retired partners and shareholders of such Holder, or the estates and family members of any
such partners and retired partners and any trusts for the benefit of any of the foregoing persons or any entity that controls or is controlled
by or is under common control or management with the respective Holder, shall be deemed to be a single “Holder,” as applicable
and any pro rata reduction with respect to such “Holder” shall be based upon the aggregate amount of Registrable Securities
owned by all such related entities and individuals.
(iv) Not
a Demand Registration. Registration pursuant to this Section 1.3 shall not be deemed a demand registration as described
in Section 1.2 or Section 1.4.
1.4 Form S-3
or Form F-3 Registration. In case the Company shall receive from the Holders of at least 10% of the Registrable Securities then
outstanding (the “F-3 Holders”), a written request or requests that the Company effect a registration on Form S-3
or Form F-3, or any similar registration statement under relevant non-U.S. securities laws, and any related qualification or compliance
with respect to all or a part of the Registrable Securities owned by such F-3 Holder(s), the Company shall:
(i) within
fifteen (15) business days after the date such request is received, give written notice of the proposed registration, and any related
qualification or compliance, to all other Holders; and
(ii) use
commercially reasonable efforts to effect, as soon as practicable, such registration and all such qualifications and compliances as may
be so requested and as would permit or facilitate the sale and distribution of all or such portion of such Holders’ Registrable
Securities as are specified in such request, together with all or such portion of the Registrable Securities of any other Holders joining
in such request as are specified in a written request given within twenty (20) days after receipt of such written notice from the Company,
provided, however, that the Company shall not be obligated to effect any such registration, qualification or compliance, pursuant to
this section 1.4, in case of any of sub-sections 1.4(iii)(a) through (e) below.
(iii) All
written requests from any Holder to effect a registration on Form F-3 or Form S-3 pursuant to this Section 1.41.4
shall indicate whether such Holder intends to effect the offering promptly following effectiveness of the registration statement or whether
such Holder intends for the registration statement to remain effective so that such Holder may affect the offering on a delayed basis
(a “Shelf Request”). In the event a Form F-3 or Form S-3 is filed pursuant to a Shelf Request, upon a written
request (a “Form F-3 Demand Notice”) from any Holder entitled to sell securities pursuant to such Form F-3
or Form S-3 without filing a post-effective amendment, that the Company effect an offering with respect to Registrable Securities
(a “Takedown”), the Company will, as soon as practicable, (x) deliver a notice relating to the proposed Takedown
to all other Holders who are named or are entitled to be named as a selling shareholder in such Form F-3 or Form S-3 without
filing a post-effective amendment thereto and (y) promptly (and in any event not later than ten (10) Business Days after receiving
such request) supplement the prospectus to an effective shelf registration statement as would permit or facilitate the sale and distribution
of all or such portion of the Registrable Securities as are specified in such Form F-3 Demand Notice together with the Registrable
Securities requested to be included in such Takedown by any other Holders who notify the Company in writing within ten (10) Business
Days after receipt of such notice from the Company; except that the Company shall not be obligated to effect any such Takedown, pursuant
to this section 1.4:
(a) if Form S-3
or Form F-3 is not available for such offering by the Holders;
(b) if the Company
has filed two (2) Form S-3 or Form F-3 registration statements or two (2) Takedowns, as applicable, pursuant to this
Section 1.3 in any 12 (twelve) month period and such registrations have been declared or ordered effective;
(c) if
the Company shall furnish to the F-3 Holders a certificate signed by the Chairman of the Board stating that in the good faith judgment
of the Board, it would be seriously detrimental to the Company and its shareholders for such Form S-3 or Form F-3 Registration
or Takedown, as applicable, to be effected at such time, in which event the Company shall have the right to defer the filing of
the Form S-3 or Form F-3 registration statement or the Takedown, as applicable, for a period of not more than ninety (90) days
after receipt of the request of the F-3 Holder(s) under this Section 1.4; provided, however, that the Company shall
not utilize this right more than once in any twelve month period;
(d) during
the period that is thirty (30) days before the Company’s good faith estimate of the date of filing of, and ending on a date that
is ninety (90) days after the effective date of, a registration under the Act pursuant to Section 1.2 or this Section 1.4,
or a Takedown, as applicable, or in which the F-3 Holders had an opportunity to participate pursuant to the provisions of Section 1.3,
provided that the Company is actively employing in good faith commercially reasonable efforts to cause such registration statement to
become effective;
(e) in
any particular jurisdiction in which the Company would be required to qualify to do business or to execute a general consent to service
of process in effecting such registration, qualification or compliance; or
(f) if
the Holders participating in such registration propose to sell Registrable Securities and such other securities (if any) or if the Registrable
Securities requested to be offered pursuant to such Takedown, as applicable, shall be at an aggregate price to the public (net of any
underwriters’ discounts or commissions) of less than one million US Dollars ($1,000,000).
(iv) Subject
to the foregoing, the Company shall file a registration statement or prospectus supplement covering the Registrable Securities and other
securities so requested to be registered or sold (as applicable) as soon as practicable after receipt of the request or requests of the
Holders. Registrations or Takedowns effected pursuant to this Section 1.4 shall not be counted as requests for registration effected
pursuant to Section 1.2.
1.5 [Reserved].
1.6 Obligations
of the Company. Whenever required under this Section 1 to effect the registration of any Registrable Securities, the Company
shall, as expeditiously as reasonably possible:
(i) prepare
and file with the SEC a registration statement with respect to such Registrable Securities and use commercially reasonable efforts to
cause such registration statement to become effective, and, upon the request of the Holders of a majority of the Registrable Securities
registered thereunder, use commercially reasonable efforts to keep such registration statement effective for a period of up to one hundred
and twenty (120) days or, if earlier, until the distribution contemplated in the Registration Statement has been completed;
(ii) prepare
and file with the SEC such amendments and supplements to such registration statement and the prospectus used in connection with such
registration statement as may be necessary to comply with the provisions of the Act with respect to the disposition of all securities
covered by such registration statement;
(iii) furnish
to the Holders such number of copies of a prospectus, including a preliminary prospectus, in conformity with the requirements of the
Act, and such other documents as the Holders may reasonably request in order to facilitate the disposition of the Registrable Securities
owned by them that are included in such registration;
(iv) use
commercially reasonable efforts to register and qualify the securities covered by such registration statement under such other securities
or Blue Sky laws of such jurisdictions as shall be reasonably requested by the Holders, provided that the Company shall not be required
in connection therewith or as a condition thereto to qualify to do business or to file a general consent to service of process in any
such states or jurisdictions;
(v) in
the event of any underwritten public offering, enter into and perform its obligations under an underwriting agreement, in usual and customary
form, with the managing underwriter(s) of such offering;
(vi) notify
each Holder of Registrable Securities covered by such registration statement at any time when a prospectus relating thereto is required
to be delivered under the Act or the happening of any event as a result of which the prospectus included in such registration statement,
as then in effect, includes an untrue statement of a material fact or omits to state a material fact required to be stated therein or
necessary to make the statements therein not misleading in the light of the circumstances then existing;
(vii) notify
each Holder of Registrable Securities covered by such registration statement, promptly after the Company shall receive notice thereof,
of the time when such registration statement becomes effective or when any amendment or supplement or any prospectus forming a part of
such registration has been filed.
(viii) notify
each Holder of Registrable Securities covered by such registration statement, promptly of any request by the SEC for the amending or
supplementing of such registration statement or prospectus for additional information.
(ix) advise
each Holder whose Registrable Securities are included in such registration statement promptly after the Company shall receive notice
or otherwise obtain knowledge of the issuance of any order by the SEC suspending the effectiveness of such registration statement or
amendment thereto or of the initiation or threatening of any proceeding for that purpose; and promptly use its commercially reasonable
efforts to prevent the issuance of any stop order or to obtain its withdrawal promptly if a stop order should be issued.
(x) use
its commercially reasonable efforts to furnish, at the request of any Holder requesting registration of Registrable Securities pursuant
to this Agreement on the date that such Registrable Securities are delivered to the underwriters for sale in connection with a registration
pursuant to this Agreement, or if such securities are being sold through underwriters, on the date that the registration statement with
respect to such securities becomes effective, and subject to each such requesting Holder to whom the letter below is addressed providing
a customary representation letter to the independent registered public accounting firm of the Company in form and substance reasonably
satisfactory to such accountants, (i) an opinion, dated such date, of the counsel representing the Company for the purposes of such
registration, in form and substance as is customarily given to underwriters in an underwritten public offering, addressed to the underwriters,
if any, provided that the delivery of any “10b-5 statement” and opinion may be conditioned on the prior or concurrent delivery
of a letter pursuant to subsection (ii) below and (ii) a letter dated such date, from the independent certified public accountants
of the Company, in form and substance as is customarily given by independent certified public accountants to underwriters in an underwritten
public offering (to the extent deliverable in accordance with their professional standards), addressed to the underwriters, if any; provided,
that the Company shall only be required to comply with this clause (x) in connection with an underwritten offering.
(xi) use
its commercially reasonable efforts to cause all such Registrable Securities registered pursuant hereunder to be listed on each securities
exchange on which similar securities issued by the Company are then listed; and
(xii) provide
a transfer agent and registrar for all Registrable Securities registered pursuant hereunder and provide a CUSIP number for all such Registrable
Securities, in each case not later than the effective date of such registration.
1.7 Information
from Holder. It shall be a condition precedent to the obligations of the Company to take any action pursuant to this Section 1
with respect to the Registrable Securities of any selling Holder that such Holder shall promptly furnish to the Company such information
regarding itself, the Registrable Securities held by it, and the intended method of disposition of such securities as is reasonably required
to timely effect the registration of such Holder’s Registrable Securities.
1.8 Expenses
of Registration. All expenses, other than Selling Expenses (as defined below), incurred in connection with registrations, filings
or qualifications pursuant to Sections 1.2, 1.3 and 1.4, including (without limitation) all registration, filing and qualification
fees, printers’ and accounting fees, fees and disbursements of counsel for the Company and the reasonable fees and disbursements
of one counsel for the selling Holders selected by a majority in interest of the Holders participating in such registration, shall be
borne by the Company. Notwithstanding the foregoing, the Company shall not be required to pay for any expenses of any registration proceeding
begun pursuant to Section 1.2 or Section 1.4 if the registration request is subsequently withdrawn at the request of the Holders
of a majority of the Registrable Securities to be registered (in which case all participating Holders shall bear such expenses pro rata
based upon the number of Registrable Securities that were to be requested in the withdrawn registration), unless, in the case of a registration
requested under Section 1.2, the Holders of a majority of the Registrable Securities agree to forfeit their right to each of the
two demands registration pursuant to Section 1.2, provided, however, that if at the time of such withdrawal, the Holders
have learned of a material adverse change in the condition, business, or prospects of the Company from that known to the Holders at the
time of their request and have withdrawn the request with reasonable promptness following disclosure by the Company of such material
adverse change, then the Holders shall not be required to pay any of such expenses and shall retain their rights pursuant to Section 1.2
or 1.4 to the extent that such registration shall not constitute the use of a demand registration. "Selling Expenses"
shall mean underwriting discounts, applicable stock transfer taxes and selling commissions applicable to the sale of Registrable Securities.
All Selling Expenses relating to Registrable Securities registered pursuant to this Section 1 shall be borne and paid by the Holders
pro rata on the basis of the number of Registrable Securities registered on their behalf.
1.9 Delay
of Registration. No Holder shall have any right to obtain or seek an injunction restraining or otherwise delaying any such registration
as the result of any controversy that might arise with respect to the interpretation or implementation of this Section 1.
1.10 Indemnification.
In the event any Registrable Securities are included in a registration statement under this Section 1:
(i) To
the extent permitted by law, the Company will indemnify and hold harmless each selling Holder, the partners or officers, directors and
shareholders of each selling Holder, legal counsel and accountants for each selling Holder, any underwriter (as defined in the Act) for
such selling Holder and each person, if any, who controls such selling Holder or underwriter within the meaning of the Act or the 1934
Act, against any losses, claims, damages or liabilities (joint or several) to which they may become subject under the Act, the 1934 Act
or any state securities laws, insofar as such losses, claims, damages, or liabilities (or actions in respect thereof) arise out of or
are based upon any of the following statements, omissions or violations (collectively a “Violation”): (i) any
untrue statement or alleged untrue statement of a material fact contained in such registration statement, including any preliminary prospectus
or final prospectus contained therein or any amendments or supplements thereto, (ii) the omission or alleged omission to state therein
a material fact required to be stated therein, or necessary to make the statements therein not misleading, or (iii) any violation
or alleged violation by the Company of the Act, the 1934 Act, any state securities laws or any rule or regulation promulgated under
the Act, the 1934 Act or any state securities laws, in connection with the offering covered by such registration statement; and the Company
will reimburse each such selling Holder, underwriter or controlling person for any legal or other expenses reasonably incurred by them
in connection with investigating or defending any such loss, claim, damage, liability or action; provided, however, that
the indemnity agreement contained in this subsection l.10(i) shall not apply to amounts paid in settlement of any such loss,
claim, damage, liability or action if such settlement is effected without the consent of the Company (which consent shall not be unreasonably
withheld), nor shall the Company be liable in any such case for any such loss, claim, damage, liability or action to the extent that
it arises out of or is based upon a Violation that occurs solely in reliance upon and in conformity with written information furnished
expressly for use in connection with such registration by any such Holder, underwriter, controlling person or other aforementioned Person
expressly for use in connection with such registration; provided further, however, that the foregoing indemnity agreement with
respect to any preliminary prospectus shall not inure to the benefit of any Holder or underwriter, or any person controlling such Holder
or underwriter, from whom the person asserting any such losses, claims, damages or liabilities purchased shares in the offering, if a
copy of the prospectus (as then amended or supplemented if the Company shall have furnished any amendments or supplements thereto) was
not sent or given by or on behalf of such Holder or underwriter to such person, if required by law so to have been delivered, at or prior
to the written confirmation of the sale of the shares to such person, and if the prospectus (as so amended or supplemented) would have
cured the defect giving rise to such loss, claim, damage or liability.
(ii) To
the extent permitted by law, each selling Holder will indemnify and hold harmless the Company, each of its directors, each of its officers
who has signed the registration statement, each person, if any, who controls the Company within the meaning of the Act, legal counsel
and accountants for the Company, any underwriter, any other Holder selling securities in such registration statement and any controlling
person of any such underwriter or other Holder or any of such other Holder’s partners, directors or officers or any Person who
controls such Holder within the meaning of the Act or the 1934 Act, against any losses, claims, damages or liabilities (joint or several)
to which any of the foregoing persons may become subject, under the Act, the 1934 Act or any other federal or state laws or the equivalent
securities exchange law of another jurisdiction, insofar as such losses, claims, damages or liabilities (or actions in respect thereto)
arise out of or are based upon any Violation, in each case to the extent (and only to the extent) that such Violation occurs in reliance
upon and in conformity with written information furnished by such Holder expressly for use in connection with such registration and has
not been corrected in a subsequent writing prior to or concurrently with the sale of Registrable Securities to the person asserting the
claim; and each such Holder will reimburse any person intended to be indemnified pursuant to this subsection 1.10(ii), for any legal
or other expenses reasonably incurred by the Company or any such person in connection with investigating or defending any such loss,
claim, damage, liability or action; provided, however, that the indemnity agreement contained in this subsection 1.10(ii) shall
not apply to amounts paid in settlement of any such loss, claim, damage, liability or action if such settlement is effected without the
consent of the Holder (which consent shall not be unreasonably withheld), and provided further that in no event shall any indemnity under
this subsection 1.10(ii) exceed the proceeds from the offering received by such Holder (net of any Selling Expenses paid by
such Holder), except in the case of fraud or willful misconduct by such Holder.
(iii) Promptly
after receipt by an indemnified party under this Section 1.10 of notice of the commencement of any action (including any governmental
action), involving the subject matter of the foregoing indemnity provisions, such indemnified party will, if a claim in respect thereof
is to be made against any indemnifying party under this Section 1.10, deliver to the indemnifying party a written notice of the
commencement thereof and the indemnifying party shall have the right to participate in, and, to the extent the indemnifying party so
desires, jointly with any other indemnifying party similarly noticed, to assume the defense thereof with counsel mutually and reasonably
satisfactory to the parties; provided, however, that an indemnified party shall have the right to retain its own counsel, with the fees
and expenses to be paid by the indemnifying party, if representation of such indemnified party by the counsel retained by the indemnifying
party would be inappropriate due to actual or potential differing interests between such indemnified party and any other party represented
by such counsel in such proceeding. The failure to deliver written notice to the indemnifying party within a reasonable time of the commencement
of any such action, if prejudicial to its ability to defend such action, shall relieve such indemnifying party of any liability to the
indemnified party under this Section 1.10, but the omission so to deliver written notice to the indemnifying party will not relieve
it of any liability that it may have to any indemnified party otherwise than under this Section 1.10.
(iv) If
the indemnification provided for in this Section 1.10 either (i) is held by a court of competent jurisdiction to be unavailable
to an indemnified party with respect to any loss, liability, claim, damage or expense referred to herein (by the entry of a final judgment
or decree by a court of competent jurisdiction and the expiration of time to appeal or the denial of the last right of appeal), or (ii) contribution
under the Act may be required on the part of any such indemnified party in circumstances for which indemnification is provided under
this Section 1.10, then, in each such case, the Company and the indemnifying party, in lieu of indemnifying such indemnified
party hereunder to which they may be subject (after contribution from others), shall contribute in such proportion so that such Holder
is responsible for the portion represented by the percentage that the public offering price of its Registrable Securities offered by
and sold under the registration statement bears to the public offering price of all securities offered by and sold under such registration
statement, and the Company and other selling Holders are responsible for the remaining portion; provided, however, that,
in any such case: (A) no such Holder will be required to contribute any amount in excess of the public offering price of all such
Registrable Securities offered and sold by such Holder pursuant to such registration statement; (B) no person or entity guilty of
fraudulent misrepresentation (within the meaning of Section 11(f) of the Act) will be entitled to contribution from any person
or entity who was not guilty of such fraudulent misrepresentation; and (C) no Holder shall be required to contribute any amount
in excess of the amount such Holder would have been required to indemnify if indemnification had been applicable in accordance with its
terms, except in the case of willful misconduct or fraud by such Holder.
(v) Notwithstanding
the foregoing, to the extent that the provisions on indemnification and contribution contained in the underwriting agreement entered
into in connection with the underwritten public offering are in conflict with the foregoing provisions, the provisions in the underwriting
agreement shall control.
(vi) The
obligations of the Company and Holders under this Section 1.10 shall survive the completion of any offering of Registrable Securities
in a registration statement under this Section 1, and otherwise.
1.11 Reports
Under Securities Exchange Act of 1934. In the event the Company becomes subject to reporting under the 1934 Act, then with a view
to making available to the Holders the benefits of Rule 144 promulgated under the Act (“SEC Rule 144”)
and any other rule or regulation of the SEC that may at any time permit a Holder to sell securities of the Company to the public
without registration or pursuant to a registration on Form S-3 or Form F-3, the Company agrees to:
(i) make
and keep public information available, as those terms are understood and defined in SEC Rule 144, at all times after the effective
date of the Initial Offering;
(ii) use
commercially reasonable efforts to file with the SEC in a timely manner all reports and other documents required of the Company under
the Act and the 1934 Act (at any time after the Company has become subject to such reporting requirements); and
(iii) furnish
to any Holder, so long as the Holder owns any Registrable Securities, forthwith upon request (i) to the extent accurate, a
written statement by the Company that it has complied with the reporting requirements of SEC Rule 144 (at any time after ninety
(90) days after the effective date of the first registration statement filed by the Company for the Initial Offering), the Act and the
1934 Act (at any time after it has become subject to such reporting requirements), or that it qualifies as a registrant whose securities
may be resold pursuant to Form S-3 or Form F-3 (at any time after it so qualifies), and (ii) such other information as
may be reasonably requested in availing any Holder of any rule or regulation of the SEC that permits the selling of any such securities
without registration (at any time after the Company has become subject to the Reporting Requirements under the 1934 Act) or pursuant
to such form (at any time after the Company so qualifies to use such form).
1.12 Assignment
of Registration Rights. The rights to cause the Company to register Registrable Securities pursuant to this Agreement may be assigned
(but only with all related obligations) by a Holder to a transferee or assignee of such securities, provided that such transfer
or assignment is made pursuant to the provisions of the Articles and any provisions and agreements relating to transfer of securities,
and provided further that: (a) the Company is, as promptly as practicable after such transfer, furnished with written notice
of the name and address of such transferee or assignee and the securities with respect to which such registration rights are being assigned;
(b) such transferee or assignee agrees in a written instrument delivered and reasonably satisfactory to the Company to be bound
by and subject to the terms and conditions of this Agreement, including without limitation the provisions of Section 1.14 below;
and (c) such assignment shall be effective only if immediately following such transfer the further disposition of such securities
by the transferee or assignee is restricted under the Act. Except as set forth in this Section1.12, none of the rights, privileges,
or obligations set forth in, arising under, or created by this Agreement may be assigned or transferred without the prior consent in
writing of the Company. Except as otherwise expressly limited herein, the provisions hereof shall inure to the benefit of, and are binding
upon, the successors, assigns, heirs, executors, and administrators of the parties hereto.
1.13 Limitations
on Subsequent Registration Rights. From and after the date of this Agreement, the Company shall not, without the prior written consent
of the Holders of at least a majority of the Registrable Securities, excluding, for the avoidance of doubt, the Ordinary Registerable
Securities, enter into any agreement with any holder or prospective holder of any securities of the Company that would provide to such
holder the right (a) to include such securities in any registration on other than either a pro rata basis with respect to the Registrable
Securities or on a subordinate basis after all Holders have had the opportunity to include in the registration ad offering all shares
of Registrable Securities that they wish to include, or (b) to demand registration of their securities. Provided that, no such agreements
shall be entered even if approved in accordance with this clause 1.13, if such agreement alters or adversely affects the rights of any
Holder under this Agreement in a manner which is different than or disproportional to the manner in which it affects all other Holders,
unless such Holder has provided its prior written consent.
1.14 “Market
Stand-Off” Agreement. Each Holder hereby agrees that it will not, without the prior written consent of the managing underwriter,
during the period commencing on the date of the final prospectus relating to an Initial Offering, and ending on the date specified by
the Company and the managing underwriter (such period not to exceed one hundred eighty (l80) days in the case of an Initial Offering
or ninety (90) days in any subsequent offering, unless otherwise requested by the Company or the underwriter to accommodate regulatory
restrictions) (i) lend, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract
to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of Ordinary
Shares or any securities convertible into or exercisable or exchangeable for Ordinary Shares (held immediately before the effective date
of the registration statement for such offering), or (ii) enter into any swap or other arrangement that transfers to another, in
whole or in part, any of the economic consequences of ownership of such securities, whether any such transaction described in clause (i) or
(ii) above is to be settled by delivery of shares of Ordinary Shares or such other securities, in cash or otherwise (the “Lock-Up”).
The foregoing provisions of this Section 1.14 shall only be applicable to the Holders if all officers and directors, and, in case
of an Initial Offering, all holders of at least 1% (one percent) of the Company's voting securities enter into similar agreements. The
foregoing provisions of this Section shall not apply to the sale of any shares to an underwriter pursuant to an underwriting agreement.
The underwriters in connection with the Company’s registration are intended third party beneficiaries of this Section 1.14
and shall have the right, power and authority to enforce the provisions hereof as though they were a party hereto. In addition, at the
underwriters’ request, each Holder shall enter into a lock-up agreement in customary form reflecting the foregoing. The obligations
described in this Section 1.13 shall not apply to a registration relating solely to employee benefit plans on Form S-8
or similar forms that may be promulgated in the future, or a registration relating solely to a Commission Rule 145 transaction on
Form F-4 or similar forms that may be promulgated in the future. In order to enforce the foregoing covenant, the Company may impose
stop-transfer instructions with respect to the Registrable Securities of each Holder (and the shares or securities of every other person
subject to the foregoing restriction) until the end of such one hundred and eighty (180) or ninety (90) day period. In addition to the
foregoing, no Holder that would be required to sign an agreement restricting its ability to transfer pursuant to this section shall distribute
shares to its shareholders, partners or members pursuant to Sections 1.2 and 1.3 until such time as such Holder has signed
such an agreement required pursuant hereto. Any discretionary waiver or termination of the restrictions listed in this Section 1.13
by the Company or the underwriters shall apply pro rata to all Shareholders, based on the number of shares subject to such restrictions.
2. Restrictions
on Transfer.
2.1 The
Ordinary Shares, Preferred Shares and the Registrable Securities shall not be sold, pledged, or otherwise transferred, and the Company
shall not recognize and shall issue stop-transfer instructions to its transfer agent with respect to any such sale, pledge, or transfer,
except upon the conditions specified in this Agreement, which conditions are intended to ensure compliance with the provisions of the
Act. A transferring Holder will cause any proposed purchaser, pledgee, or transferee of the Ordinary Shares, Preferred Shares and the
Registrable Securities held by such Holder to agree to take and hold such securities subject to the provisions and upon the conditions
specified in this Agreement.
2.2 Each
certificate, instrument, or book entry representing (i) the Ordinary Shares, (ii) the Preferred Shares, (iii) the Registrable
Securities, and (iv) any other securities issued in respect of the securities referenced in clauses (i), (ii) and (iii), upon
any stock split, stock dividend, recapitalization, merger, consolidation, or similar event, shall (unless otherwise permitted by the
provisions of Subsection 2.3 be notated with a legend substantially in the following form:
THE SECURITIES REPRESENTED HEREBY HAVE BEEN ACQUIRED
FOR INVESTMENT AND HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933. SUCH SHARES MAY NOT BE SOLD, PLEDGED, OR TRANSFERRED
IN THE ABSENCE OF SUCH REGISTRATION OR A VALID EXEMPTION FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS OF SAID ACT.
THE SECURITIES REPRESENTED HEREBY MAY BE
TRANSFERRED ONLY IN ACCORDANCE WITH THE TERMS OF AN INVESTORS' RIGHTS AGREEMENT BY AND AMONG THE COMPANY AND THE OTHER PARTIES THERETO,
A COPY OF WHICH IS ON FILE WITH THE COMPANY.
The Holders consent to the Company making a notation
in its records and giving instructions to any transfer agent of the Registrable Securities in order to implement the restrictions on
transfer set forth in this Section 2.
2.3 The
holder of such Registrable Securities, by acceptance of ownership thereof, agrees to comply in all respects with the provisions of this
Section 2. Before any proposed sale, pledge, or transfer of any Restricted Securities, unless there is in effect a registration
statement under the Act covering the proposed transaction, the Holder thereof shall give notice to the Company of such Holder’s
intention to effect such sale, pledge, or transfer. Each such notice shall describe the manner and circumstances of the proposed sale,
pledge, or transfer in sufficient detail and, if reasonably requested by the Company, shall be accompanied at such Holder’s expense
by either (i) a written opinion of legal counsel who shall, and whose legal opinion shall, be reasonably satisfactory to the Company,
addressed to the Company, to the effect that the proposed transaction may be effected without registration under the Act; (ii) a
“no action” letter from the Securities and Exchange Commission to the effect that the proposed sale, pledge, or transfer
of such Registrable Securities without registration will not result in a recommendation by the staff of the Securities and Exchange Commission
that action be taken with respect thereto; or (iii) any other evidence reasonably satisfactory to counsel to the Company to the
effect that the proposed sale, pledge, or transfer of the Registrable Securities may be effected without registration under the Act,
whereupon the Holder of such Registrable Securities shall be entitled to sell, pledge, or transfer such Registrable Securities in accordance
with the terms of the notice given by the Holder to the Company. The Company will not require such a legal opinion or “no action”
letter (x) in any transaction in compliance with SEC Rule 144; or (y) in any transaction in which such Holder distributes
Registrable Securities to an affiliate of such Holder for no consideration; provided that each transferee agrees in writing to
be subject to the terms of this Section 2. Each certificate, instrument, or book entry representing the Registrable Securities
transferred as above provided shall be notated with, except if such transfer is made pursuant to SEC Rule 144, the appropriate restrictive
legend set forth in Subsection 2.2, except that such certificate instrument, or book entry shall not be notated with such restrictive
legend if, in the opinion of counsel for such Holder and the Company, such legend is not required in order to establish compliance with
any provisions of the Act.
2.4 Termination
of Registration Rights. The right of any Holder to request registration or inclusion of Registrable Securities in any registration
pursuant to Section 1 shall terminate upon the earliest to occur of:
(i) the
closing of a Merger and Acquisition, as such term is defined in the Articles; and
(ii) such
time as Rule 144 or another similar exemption under the Act is available for the sale of all of such Holder’s Registrable
Securities without limitation during a three-month period without registration.
(iii) the
5th anniversary of the Initial Offering.
| 3. | Affirmative Covenants. Until
the consummation of an Initial Offering, the Company undertakes towards the Investors, as
follows: |
3.1 Delivery
of Financial Information. The Company will furnish the following reports to each Major Holder, provided that the Board has
not reasonably determined that such Major Holder is a competitor of the Company (it being clarified, that the mere ownership of securities
by a venture capital fund in companies which may be deemed to be a competitor of the Company, shall not, as of itself, cause the venture
capital fund to be deemed a competitor):
(i) As
soon as practicable after the end of each fiscal year and in any event within one-hundred and twenty (120) days thereafter, the consolidated
balance sheet and statement of shareholder equity of the Company as of the end of such fiscal year, and the consolidated statements of
income and cash flow for the fiscal year then ended, all in reasonable detail, stating in each case in comparative form the figures of
the preceding fiscal year, United States dollar denominated, audited and certified by independent public accounting firm of which is
one of (or is affiliated with one of) the "Big Four" US accounting firms, in each case in English and accompanied by an opinion
in English of such firm which opinion shall state that such balance sheet and statements of shareholder equity, income and cash flow
have been prepared in accordance with International Financial Reporting Standards (“IFRS”) applied on a basis consistent
with that of the preceding fiscal year, and present fairly and accurately the financial position of the Company as of their date, and
that the audit by such accountants in connection with such financial statements has been made in accordance with United States generally
accepted auditing standards.
(ii) As
soon as practicable, and in any event: (A) within thirty (30) days after the end of the first, second, third and fourth quarters
of each fiscal year of the Company, un-audited consolidated balance sheets of the Company as of the end of such quarter and consolidated
statements of income and cash flow of the Company for such quarter and for the portion of the fiscal year ending with such period, in
each case in English and in reasonable detail, stating in each case in comparative form the figures for the corresponding period of the
preceding fiscal year and United States dollar denominated, prepared in accordance with IFRS, except that such statements may (i) be
subject to year-end audit adjustment, and (ii) not contain notes that may be required in accordance with IFRS.
(iii) As
soon as practicable, and in any event within thirty (30) days after the end of each month, a monthly report in form and substance determined
from time to time by the Company’s Board of Directors, in English.
(iv) As
soon as practicable, and in any event at least thirty (30) days before each fiscal year, a comprehensive operation budget, forecasting
the Company’s revenues, expenses and cash position on a monthly basis for the upcoming fiscal year.
(v) upon
request from a Major Holder, a statement showing the number of shares of each class and series of capital stock and securities convertible
into or exercisable for shares of capital stock outstanding at the end of the period, the Ordinary Shares issuable upon conversion or
exercise of any outstanding securities convertible or exercisable for Ordinary Shares and the exchange ratio or exercise price applicable
thereto, and the number of shares of issued options andoptions not yet issued but reserved for issuance, if any, all in sufficient detail
as to permit such Major Holder to calculate its respective percentage equity ownership in the Company.
(vi) Such
other information that any Major Holder may from time to time reasonably request, without derogating from such Major Holder’s statutory
rights.
provided,
however, that in each of the above clauses the Company shall not be obligated to provide any information pursuant to this
Section 3.1 that it deems in good faith to be (i) a trade secret or similar confidential or personal information of the
Company or any affiliate thereof (ii) the disclosure of such information would be reasonably expected to adversely affect the attorney-client
privilege between the Company and its counsel or cause the Company to violate a confidentiality undertaking with a third party; (iii) the
disclosure of such information would be reasonably expected to create a conflict of interests or potential conflict of interests between
the Company and such Major Holder or any affiliate thereof, if so determined by the Board.
(vii) Notwithstanding
anything else herein to the contrary, the Company may cease providing the information set forth in this Section 3.1 during the period
starting with the date sixty (60) days before the Company’s good-faith estimate of the date of filing of a registration statement,
if it reasonably concludes it must do so to comply with the Securities and Exchange Commission rules applicable to such registration
statement and related offering; provided that the Company’s covenants under this Section 3.1 shall be reinstated at such time
as the Company is no longer actively employing its commercially reasonable efforts to cause such registration statement to become effective.
3.2 Inspection
and Visitation. The Company will permit each Major Holder and its representatives, provided that the Board has not reasonably determined
that such Major Holder is a competitor of the Company (it being clarified, that the mere ownership of securities by a venture capital
fund in companies which may be deemed to be a competitor of the Company, shall not, as of itself, cause the venture capital fund to be
deemed a competitor), at such Major Holder’s expense, access, during normal business hours and upon reasonable notice, to visit
and inspect any of the properties of the Company, to examine its books and records and to discuss its affairs, finances and accounts
with the Company's officers; except that the Company shall not be obligated pursuant to this Section 3.2 to provide access to any
information that it reasonably considers to be (i) a trade secret or confidential information (unless covered by an enforceable
confidentiality agreement, in form acceptable to the Company) or (ii) the disclosure of which, would be reasonably expected to adversely
affect the attorney-client privilege between the Company and its counsel or cause the Company to violate a confidentiality undertaking
with a third party; or (iii) where the Board determines in good faith that the disclosure of such information would be reasonably
expected to create a conflict of interests or potential conflict of interests between the Company and such Major Holder or any affiliate
thereof, provided that to the extent that such conflict may be removed by replacing the applicable Major Holder representative and/or
limiting access to certain representatives of the Major Holder, then the Company shall notify the Major Holder of such option and the
conditions to such disclosure and provide to a substitute representative of the Major Holder with information pursuant to the terms of
this Section 3.2 This Section shall not limit any rights that the Major Holders may have under applicable law. Such authorized
representatives that are not employed by such Major Holder may, at the Company’s request, be required to execute confidentiality
agreements prior to being granted access as aforesaid; and if employed by such Major Holder shall be bound by the confidentiality undertaking
towards the Company pursuant to Section 3.5.
3.3 Assignment
of Information and Inspection Covenants. The rights under Section 3.1 and 3.2 are transferable (but only with
all related obligations) to any transferee of Registrable Securities that (i) after such transfer, holds at least such number of
Registrable Securities (subject to appropriate adjustment for share splits, share dividends, combinations and other recapitalizations)
such that it would be defined as a Major Holder under the Articles, or (ii) is a Permitted Transferee of a Holder.
3.4 Termination
of Information and Inspection Covenants. The covenants set forth in Sections 3.1 and 3.2 hereof shall terminate and be of no
further force or effect upon (i) immediately prior to the closing of an Initial Offering, (ii) immediately prior to the closing
of a Merger and Acquisition, or (iii) when the Company first becomes subject to the periodic reporting requirements of Sections 12(g) or
15(d) of the 1934 Act, whichever event shall occur first.
3.5 Confidentiality.
Each Holder agrees that any confidential or proprietary information relating to or obtained from the Company (including, without limitations,
information provided to it under this Agreement (including notice of the Company’s intention to file a registration statement))
will not be disclosed, divulged or used to any other person or for any other purpose (other than to monitor its investment in the Company)
(except to its officers and its legal, financial and technical advisors to the extent necessary to obtain their services in connection
with monitoring its investment in the Company), without the prior written consent of the Company; provided that, in connection
with periodic non-public reports to its shareholders, partners, attorneys, accountants, consultants, and other professionals bound by
legal or ethical confidentiality obligations toward the Holder (collectively, “Partners”), each Holder may, without first
obtaining such written consent (but only after informing such Partners that such information is confidential and directing such Partners
to maintain the confidentiality of such information), make general standard statements, not containing technical or other confidential
information, regarding the nature and progress of the Company's business; and provided further, that each Holder may provide summary
information regarding the Company's financial information in its reports to their respective Partners (but only after informing such
Partners that such information is confidential and directing such shareholders or partners to maintain the confidentiality of such information),
but may not annex to such reports the full financial information to be provided under this Agreement by the Company. For the avoidance
of doubt, with respect to a certain Shareholder, Confidential Information shall not include any information which: (1) was in the
public domain prior to the time of disclosure by the Company; (2) enters the public domain after disclosure by the Company to such
Shareholder through no action or inaction of such Shareholder; (3) is already in the possession of such Shareholder free of any
obligation of confidentiality at the time of disclosure by the Company without a breach of any obligation of confidentiality (by such
Shareholder or by any third party from whom such information have been obtained); or (4) is required by law to be disclosed by such
Shareholder; provided that such Shareholder shall use all commercially reasonable efforts to maintain the confidentiality of such disclosed
information and further provided that such Shareholder promptly as practicable notifies the Company of such disclosure and takes reasonable
steps to minimize the extent of any such required disclosure and to obtain confidential treatment for any information so disclosed. The
obligations of the Holders under this Section 3.5 shall survive any termination of this Agreement in accordance with the terms
hereof.
3.6 Accounting.
The Company will maintain and cause each of its subsidiaries to maintain a system of accounting established and administered in accordance
with IFRS consistently applied and will set aside on its books and cause each of its subsidiaries to set aside on its books all such
proper reserves as shall be required by IFRS.
3.7 ESOP
Vesting. Unless otherwise determined by the Board in accordance with the Articles, options granted under the Company's 2019 Employee
Share Option Plan (or any other option plan adopted thereafter by the Board) to Company's employees, directors or consultants shall vest
according to a standard four (4) year vesting schedule with a one (1) year cliff and quarterly vesting thereafter. All options
holders shall be required to execute a proxy in a form prescribed by the Company. The Company shall not grant any options which include
provisions allowing for acceleration of unvested options upon certain events without the consent of the Board, including the consent
of one of Preferred Directors (as defined in the Articles).
3.8 FCPA.
The Company covenants that it shall not (and shall not permit any of its subsidiaries or affiliates or any of its or their respective
directors, officers, managers, employees, independent contractors, representatives or agents to) promise, authorize or make any payment
to, or otherwise contribute any item of value to, directly or indirectly, to any third party, including any Non-U.S. Official (as such
term is defined in the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”)), in each case, in violation
of the FCPA, the U.K. Bribery Act, or any other applicable anti-bribery or anti-corruption law. The Company further covenants that it
shall (and shall cause each of its subsidiaries and affiliates to) cease all of its or their respective activities, as well as remediate
any actions taken by the Company, its subsidiaries or affiliates, or any of their respective directors, officers, managers, employees,
independent contractors, representatives or agents in violation of the FCPA, the U.K. Bribery Act, or any other applicable anti-bribery
or anti-corruption law. The Company further covenants that it shall (and shall cause each of its subsidiaries and affiliates to) maintain
systems of internal controls (including, but not limited to, accounting systems, purchasing systems and billing systems) to ensure compliance
with the FCPA, the U.K. Bribery Act, or any other applicable anti-bribery or anti-corruption law. Upon request, the Company agrees to
provide responsive information and/or certifications concerning its compliance with applicable anti-corruption laws. The Company shall
promptly notify each Investor if the Company becomes aware of any Enforcement Action (as defined in the Purchase Agreement). The Company
shall and shall cause any direct or indirect subsidiary or entity controlled by it, whether now in existence or formed in the future,
to comply with the FCPA. The Company shall use its best efforts to cause any direct or indirect subsidiary, whether now in existence
or formed in the future, to comply in all material respects with all applicable laws.
3.9 US
Tax Covenants. With respect to each Investor who is a Major Holder, the Company represents, warrants and covenants to such Investor
as follows:
(i) US
Tax Classification. The Company represents, warrants and agrees that the Company has not made any election to have the Company treated
as an entity other than an association taxable as a corporation for United States federal income tax purposes on Internal Revenue Service
Form 8832, in the manner described under Section 301.7701-3(c) of the United States Treasury Regulations. Without
the consent of the Investor, the Company shall not make any election to be treated as a partnership for United States federal income
tax purposes, and the Company shall not take any other action or reporting position that would be inconsistent with the treatment of
the Company as a corporation for United States federal income tax purposes.
(ii) Assistance
with Tax Filings and Reporting. If the Company determines that the Investor is subject to any tax filing or reporting obligation
in any jurisdiction as a result of the activities of the Company, the Company shall notify the Investor of such determination. At the
Investor’s request, the Company shall provide such information as may reasonably requested to enable the Investor to comply with
its tax reporting and compliance obligations attributable to its investment in the Company (including, without limitation, any
reporting obligations under Sections 6038 and 6038B of the U.S. Internal Revenue Code of 1986, as amended (the “Code”)).
If the Company is required to deduct and withhold taxes on any payment to the Investor, at the written request of the Investor the Company
will use commercially reasonable efforts to assist the Investor in obtaining any available reduced rate of, exemption from, or refund
of such tax (including the obtaining of a valid certificate issued by the applicable tax authority prescribing such reduced rate or exemption),
pursuant to any applicable tax treaty or applicable law, provided the Investor timely provides the Company with all necessary forms and
information to establish a reduced rate of, exemption from, or refund of such tax.
(iii) PFIC.
The Company shall, at the Investor’s request, provide the Investor with information reasonably required by the Investor in
order for the Investor to determine whether or not the Company (or any direct or indirect subsidiary of the Company (each, a “Subsidiary”)
is “passive foreign investment company” within the meaning of Section 1297 of the Code (a “PFIC”).
In addition to the foregoing information, the Company shall promptly provide the Investor with such other information requested
by the Investor to the extent the Investor deems such information reasonably necessary for the Company (or any of Subsidiary) to be treated
as a “qualified electing fund” (within the meaning of Section 1295 of the Code) with respect to any beneficial owner
of the Investor that is a United States person (within the meaning of Section 7701(a)(30) of the Code).
(iv) CFC.
The Company shall use its commercially reasonable efforts to minimize the likelihood that the Company will be classified as a “controlled
foreign corporation” (a “CFC”) within the meaning of Section 957 of the Code. The Company shall also provide
to the Investor such information as may be reasonably required in order to enable the Investor to verify the CFC status of each such
entity and to determine, with respect to any such entity determined to be a CFC, whether the Investor (or any beneficial owner thereof)
is a United States shareholder (with the meaning of 951(a) of the Code) with respect to such CFC. At the request of the Investor,
the Company shall provide the Investor with reasonable access to information with respect to the Company or any Subsidiary (including
the books and records thereof) as may be available to the Company to enable the Investor (and each beneficial owner thereof) to comply
with all tax filing and payment obligations (and any other information reporting obligations) arising out of the Investor’s ownership
of an interest (directly or indirectly) in the Company or such Subsidiary. Without limiting the generality of the foregoing, with respect
to any such entity that is a CFC, the Company shall provide the Investor, upon request, with such information as may be necessary (A) to
calculate the earnings and profits of such CFC for purposes of Sections 952 or 1248 of the Code or otherwise; and (B) to determine
the extent to which such CFC’s income is required to be included in the gross income of the Investor (or any beneficial owner thereof)
as “subpart F income” (within the meaning of Section 952 of the Code), “global intangible low-tax income”
(within the meaning of Section 951A of the Code) or otherwise.
Notwithstanding anything herein to the contrary,
the provisions of this Section 3.10 and its subsections may not be waived on behalf of any Major Holder who is a US Taxpayer without
the written consent of such Major Holder.
3.10 Nidra
Logic. The Company agrees and undertakes towards the Investors that it shall not enter into any investment agreement, whether by
way of equity, convertible securities, debt or other instrument with Nidra Logic Ltd. unless such transaction is approved by the Board
including the approval of a majority of the Preferred Directors. The above shall not include the investment of an amount of up to $170,000
into Nidra Logic Ltd. by way of SAFE or similar convertible equity.
4.1 Successors
and Assigns. Except as otherwise provided herein, the terms and conditions of this Agreement shall inure to the benefit of and are
binding upon the respective successors and permitted assigns of the parties (including transferees of any shares of Registrable Securities).
Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective
successors and assigns any rights, remedies, obligations, or liabilities under or by reason of this Agreement, except as expressly provided
in this Agreement.
4.2 Effect
of Change in Company’s Capital Structure. If, from time to time, there is any stock dividend, stock split or other change in
the character or amount of any of the outstanding shares of the Company, then in such event any and all new, substituted or additional
securities to which a shareholder is entitled by reason of the shareholder’s ownership of the shares of the Company shall be immediately
subject to the rights and obligations set forth in this Agreement, with the same force and effect as the shares subject to such rights
immediately before such event.
4.3 Governing
Law. This Agreement shall be governed by and construed exclusively under the laws of the State of Israel, without regard to the conflict
of laws provisions thereof, and the competent courts in Tel-Aviv-Jaffa shall have sole jurisdiction over this Agreement and each of the
parties hereby submits irrevocably to the exclusive jurisdiction of such courts. Each of the parties hereto (i) consents to submit
itself to the exclusive jurisdiction of the abovementioned courts in the event any dispute arises out of this Agreement or the transactions
contemplated by this Agreement, (ii) agrees that it shall not attempt to deny or defeat such jurisdiction by motion or other request
for leave from the abovementioned court, (iii) agrees that it shall not bring any action relating to this Agreement or the transactions
contemplated by this Agreement in any court other than the abovementioned court, and (iv) irrevocably consents to service of process
in the manner provided by Section 4.6 or as otherwise provided by applicable law.
4.4 Counterparts.
This Agreement may be executed in two or more counterparts (including by signature by electronic means (such as Docusign or the like),
or by e-mail and PDF files), each of which shall be deemed an original, but all of which together shall constitute one and the same instrument,
it being understood that all parties need not sign the same counterpart. The exchange of an executed Agreement (in counterparts or otherwise)
or by electronic delivery (in .pdf format or the like) shall be sufficient to bind the parties to the terms and conditions of this Agreement,
as an original.
4.5 Titles
and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing
or interpreting this Agreement.
4.6 Notices.
Any notice required or permitted to be given to a party pursuant to the provisions of this Agreement will be in writing and will be effective
and deemed given to such party under this Agreement on the earliest of the following: (a) the date of personal delivery; (b) one
(1) business day after transmission by facsimile or Email (provided that no notification of failure to deliver was received), addressed
to the other party at its Email address, with confirmation of transmission; (c) one (1) Business Day after deposit with a return
receipt express courier for deliveries within Israel, or three (3) Business Days after such deposit for deliveries outside of Israel;
or (d) three (3) Business Days after deposit in the mail by registered or certified mail (return receipt requested) for deliveries
within Israel. All notices not delivered personally or by Email will be sent with postage and/or other charges prepaid and properly addressed
to the party to be notified at:
If to the Company:
Israel
Attention:
E-mail:
With mandatory copy (which shall not constitute notice) to:
Israel
Attention: Adv.
Email:
If
to the Holders: to the addresses set forth in the Company’s register of shareholders.
With mandatory copy (which shall not constitute notice) to:
Amar, Reiter, Jeanne, Shochatovitch &
Co. (AYR)
Malha Technology Park, The Tower (“HaMigdal”),
2 Agudat Hapoel Street, Jerusalem, 9695801, Israel
Attention: Adv. Daniel Chinn
Email:
DanielC@ayr.co.il
or at such other address as such other party
may designate by ten (10) days advance written notice to the other parties hereto. All notices for delivery outside Israel will
be sent by Email or by express courier. Any notice given hereunder to more than one person will be deemed to have been given, for purposes
of counting time periods hereunder, on the date effectively given to the last party required to be given such notice.
4.7 Expenses.
If any action at law or in equity is necessary to enforce or interpret the terms of this Agreement, the prevailing party shall be entitled
to reasonable attorneys’ fees, costs and necessary disbursements in addition to any other relief to which such party may be entitled.
4.8 Entire
Agreement: Amendments and Waivers.
This Agreement (including the Exhibits hereto,
if any), constitutes the full and entire understanding and agreement among the parties with regard to the subjects hereof and thereof.
Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally or in a particular
instance and either retroactively or prospectively), only with the written consent of the Company and the holders of a majority of the
Registrable Securities including the written consent of the holders of at least a majority of the Registrable Securities held by holders
of Preferred Shares; provided, however, that in the event that such amendment or waiver adversely affects the specific obligations and/or
rights or preferences of a certain Holder or group of Holders or certain class or series of shares in a manner which is different than
or disproportional to the manner in which it affects all other Holders, such amendment or waiver shall also require the written consent
of holders of a majority of the Registrable Securities held by such Holder or such group, class or series, as the case may be; it being
clarified that an amendment or waiver that by its terms is applied to all classes of shares but has a different or disproportionate economic
effect on different classes of shares, or a different or disproportionate effect on different shareholders due to the number of shares
held by them, shall, notwithstanding the different or disproportionate effect, be still considered as not having a different or disproportional
effect on any specific Holder or group of Holders class or series of shares. Any amendment or waiver effected in accordance with this
paragraph shall be binding upon each Holder of the Company and each future holder of shares, and the Company. For the avoidance of doubt
and without derogating from any other provision contained herein (including without limitation Section 1.13), granting by the Company
of registration rights and/or similar rights equal to or on parity with those of the Holders to holders of a new class(es) of preferred
shares shall not be deemed an amendment pursuant to which the rights or preferences of any Holder(s) are adversely altered.
4.9 Termination
of Prior Agreements. By signature hereon of the relevant parties, Section 9 of the Share Purchase Agreement signed by the Company
and certain of its shareholders dated 1 July 2019, are hereby terminated and cancelled.
4.10 Severability.
If one or more provisions of this Agreement are held to be unenforceable under applicable law, such provision shall be excluded from
this Agreement and the balance of the Agreement shall be interpreted as if such provision were so excluded and shall be enforceable in
accordance with its terms; provided, however, that in such event this Agreement shall be interpreted so as to give effect to the greatest
extent consistent with and permitted by applicable law, to the meaning and intention of the excluded provision as determined by such
court of competent jurisdiction.
4.11 Delays
or Omissions. No delay or omission to exercise any right, power, or remedy accruing to any party upon any breach or default under
this Agreement, shall be deemed a waiver of any other breach or default theretofore or thereafter occurring. Any waiver, permit, consent,
or approval of any kind or character on the part of any party of any breach or default under this Agreement, or any waiver on the part
of any party of any provisions or conditions of this Agreement, must be in writing and shall be effective only to the extent specifically
set forth in such writing.
4.12 Aggregation
of Shares. All shares of Registrable Securities held or acquired by entities or persons who are Permitted Transferees of each other
shall be aggregated together for the purpose of determining the availability of any rights under this Agreement and for the calculation
of the pro rata shares of such affiliated entities.
4.13 Holder
Assignees. Any rights conferred upon each Holder or any of
its affiliates, may be transferred or assigned, free of any restriction, to their Permitted Transferees (as defined in the Articles)
holding shares in the Company.
[Signature Pages Immediately Follow]
IN
WITNESS WHEREOF, the parties hereto have executed this Investors’ Rights Agreement as of the date first written above.
| COMPANY: |
|
| |
|
| |
|
| CANNASOUL ANALYTICS LTD. |
|
| |
|
| |
|
| By: |
/s/Shmuel Mandel |
|
| |
|
| Name: Shmuel Mandel |
|
| |
|
| Title: Chief Executive Officer |
|
IN
WITNESS WHEREOF, the parties hereto have executed this Investors’ Rights Agreement as of the date first written above.
INVESTORS:
| |
SYNAPTOGENIX, INC. |
| |
|
| |
By: |
/s/ Alan Tuchaman |
| |
|
| |
Name: Alan Tuchman |
| |
|
| |
Title: Chief Executive Officer |
v3.23.3
Cover
|
Oct. 31, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 31, 2023
|
| Entity File Number |
001-40458
|
| Entity Registrant Name |
Synaptogenix, Inc.
|
| Entity Central Index Key |
0001571934
|
| Entity Tax Identification Number |
46-1585656
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
1185
Avenue of the Americas
|
| Entity Address, Address Line Two |
3rd
Floor
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10036
|
| City Area Code |
973
|
| Local Phone Number |
242-0005
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
SNPX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Synaptogenix (NASDAQ:SNPX)
Historical Stock Chart
From Mar 2024 to Apr 2024
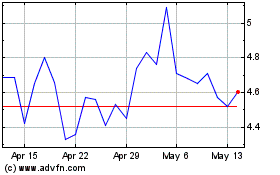
Synaptogenix (NASDAQ:SNPX)
Historical Stock Chart
From Apr 2023 to Apr 2024