
Investor Presentation

Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Biohaven Ltd. (the “Company”) and our planned and ongoing trials for our BHV-2100, troriluzole, BHV- 5500, BHV-1100, Taldefgrobep Alfa, BHV-8000, BHV-7000, and BHV-7010 development programs, the timing of and the availability of data from our clinical trials, the timing and our decisions to proceed with our planned regulatory filings, the timing of and our ability to obtain regulatory approvals for our product candidates, the clinical potential utility of our product candidates, alone and as compared to other existing potential treatment options, and the potential advancement of our early phase programs including ARM™, MATE™, MODE™, TRPM3, UC1MT, TYK2/JAK1, and Kv7. The use of certain words, including “continue”, “plan”, “will”, “believe”, “may”, “expect”, “anticipate” and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of our development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the Food and Drug Administration; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven's product candidates; the potential for Biohaven's product candidates to be first in class or best in class therapies; and the effectiveness and safety of Biohaven's product candidates. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the Company’s filings with the Securities and Exchange Commission, including within the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. This presentation also contains market data and other information based on industry publications, reports by market research firms or published independent sources. Some market data and information is also based on the Company’s good faith estimates, which are derived from management’s knowledge of its industry and such independent sources referred to above. Biohaven | Investor Presentation2 SEPTEMBER 2023

Pfizer Acquisition of CGRP Platform $13B

HIGH VALUE PLATFORMS INNOVATIVE PORTFOLIO PROVEN BUSINESS FORMULA Pursuing novel paths of science to transform the treatment of neurological and neuropsychiatric diseases In-house scientific expertise to enable a broad therapeutic portfolio addressing patient needs with intention Formula for continued growth built upon past success of experienced team and a resilient focus on creating value for patients and shareholders

PRECLINICAL PHASE 1 PHASE 2 PHASE 3 FILED ION CHANNEL: Kv7 ACTIVATOR Kv7 ION CHANNEL: TRPM3 INHIBITOR TRPM3 INFLAMMATORY: TYK2/JAK1 INHIBITOR TYK2/JAK1 GLUTAMATE PLATFORM Troriluzole MYOSTATIN PLATFORM BISPECIFIC TARGETED CELL THERAPY CD-38 DISCOVERY RESEARCH IgG Degrader IgA Degrader Next-Gen ADC Platform BHV-4157 | Obsessive-Compulsive Disorder BHV-2000 | Spinal Muscular Atrophy BHV-1100 | Multiple Myeloma BHV-7000 | Epilepsy, Bipolar Disorder BHV-1300 | Immune-Mediated Diseases BHV-7010 | Epilepsy, Mood Disorders BHV-2100 | Chronic Pain Disorders IgA Nephropathy Oncology BHV-8000 | Neuroinflammatory Disorders BHV-2000 | Metabolic Disorders SEPTEMBER 2023Biohaven | Investor Presentation5

Kv7 Platform Is Broadly Applicable to Hyperexcitability Disorders Beyond Epilepsy Kv7.1: cardiac Kv7.2: CNS BHV-7000 activator Kv7.3: CNS BHV-7000 activator Kv7.4: smooth muscle and inner ear Kv7.5: vascular tissue, neurons, skeletal muscle Stria Vascularis Organ of Corti Vascular tissue Gastrointestinal tract Adventitia Media Intima Inner Ear Cardiac Muscle Neuron Axon initial segment Node of Ranvier Synapse Serosa Muscle layer Mucosa Submucosa 5 FAMILY SUBTYPES Primary localizations: Bladder Source: Adapted from Soldovieri et al. Physiology. 2011;26(5):365-376. SEPTEMBER 2023Biohaven | Investor Presentation6

Significant Unmet Needs Remain for the 3.5 Million Patients Living with Epilepsy in the US After starting an ASM, 80% of patients experience burdensome adverse events, which can include: • Somnolence • Dizziness • Cognitive dysfunction • Mood disturbances 80% 1/3 people are treatment refractory despite availability of anti-seizure medications (ASMs), surgery, and diet modifications Devinsky et al. Nat Rev Dis Primers. 2018;4:18024; Kanner, Bicchi. JAMA. 2022;327(13):1269-1281. SEPTEMBER 2023Biohaven | Investor Presentation7

BHV-7000 is chemically stable to photo-oxidation BHV-7000 is selective for Kv7 over GABAA receptors a BHV-7000 has a wide therapeutic index preclinically a 1st and 2nd Gen Kv7 Activators Show Clinical Anti-seizure POC, But Off-target Activities, Opportunity for 3rd Gen Kv7 Differentiation XEN1101 • XEN1101 and ezogabine significantly greater GABAA receptor allosteric activators than BHV-7000 in vitroa • GABAA receptor activation: somnolence, dizziness, fatigue, diplopia Ezogabine XEN1101 BHV-7000 G A B A A A ct iv at io n a (% B as el in e) 0 20 40 60 GABAA Posi ive Allosteric Modulation % In cr ea se fr om b as el in e 10 µM BHV-7000 10 µM EZO 10 µM XEN1101 ✱✱ ✱✱ 0 20 40 60 GABAA Positive Allosteric Modulation % In cr ea se fr om b as el in e 10 µM BHV-7000 10 µM EZO 10 µM XEN1101 ✱✱ ✱✱ ** ** ** significantly different from BHV-7000, all tested at 10 µM EZOGABINE • Unstable when exposed to light • Label warnings for skin discoloration • Black box warning for retinal abnormalities/vision loss FDA Drug Safety Communication: Potiga (ezogabine) [04-26-2013] BHV-7000 • Potent activator of Kv7 channels • Effective and well-tolerated in preclinical seizure assays Th er ap eu tic In de x Ezogabineb XEN1101b BHV-7000a 10 20 30 40 50 Kv7 Biohaven | Investor Presentation8 a. Biohaven data on file (2022). b. Calculated as ratio of TD50 (rotarod) to ED50 (MES seizure assay) data presented by Xenon at Epilepsy Foundation Pipeline Conference, San Francisco (Feb 2018). Preclinical results are consolidated from separate reports and graphed together. Data presented is not the result of any head-to-head clinical trials and does not mean or suggest that BHV-7000 is clinically more safe and effective. SEPTEMBER 2023

Rat Rotarod BHV-7000: First Kv7.2/7.3 Activator in Clinical Development Designed Specifically to Exclude GABAA Receptor Activation No impact on motor function observed with BHV-7000 across effective dose range Ti m e to fa ll - R ot ar od Vehicle 0.3 1 3 10 30 0 50 100 150 200 250 BHV-7000 (mg/kg) Presented at 2023 American Society for Experimental Neurotherapeutics (ASENT) Annual Meeting March 13-15, 2023. EC50, half maximal effective concentration; EZO, ezogabine 100 80 60 50 40 27 20 4 0 XEN1101 Ezogabine Rotarod MES m g/ kg * Adapted from https://www.xenon-pharma.com/wp- content/uploads/2018/05/XEN1101_EILAT_15May2018_FINAL_YPG_web.pdf BHV-7000 Ezogabine *Mouse ED50 or TD50 (Mean 95% CI) SEPTEMBER 2023Biohaven | Investor Presentation9

BHV-7000 Exhibits Highly Differentiated Preclinical Profile Biohaven | Investor Presentation10 Wide Therapeutic Index Kv7.2/7.3 Activator GABAA Activity “dialed-out” Ezogabine XEN1101 BHV-7000 Activator, clinical and preclinical anti-seizure activity Activator, clinical and preclinical anti-seizure activity Activator, clinical and preclinical anti-seizure activity GABAA activity present GABAA activity present Negligible GABAA activity <3x reporteda,b >40xb<5x reporteda Kv7 a. Calculated as ratio of TD50 (rotarod) to ED50 (MES seizure assay) data presented by Xenon at Epilepsy Foundation Pipeline Conference, San Francisco (Feb 2018). b. Biohaven data on file (2022). Preclinical results are consolidated from separate reports and not a result from head-to-head comparisons Data presented is not the result of any head-to-head clinical trials and does not mean or suggest that BHV-7000 is clinically more safe and effective. SEPTEMBER 2023

BHV-7000: Well-Tolerated Across Phase 1 SAD/MAD Cohorts MedDRA System Organ Class Placebo (N=15) n (%) BHV-7000 (N=46) n (%) Nervous system disorders 1 (6.7) 7 (15.2) Gastrointestinal disorders 1 (6.7) 6 (13.0) Musculoskeletal disorders 0 5 (10.9) Infections 0 2 (4.3) Investigations 1 (6.7) 2 (4.3) Respiratory disorders 0 2 (4.3) Skin disorders 0 2 (4.3) Eye disorders 0 1 (2.2) General disorders 0 1 (2.2) Procedural complications 1 (6.7) 1 (2.2) Psychiatric disorders 0 1 (2.2) Renal disorders 1 (6.7) 1 (2.2) SAFETY AND TOLERABILITY No SAEs No severe TEAEs, 1 moderate TEAE, remaining TEAEs mild by severity DOSING SAD: single doses up to 100 mg MAD: multiple doses up to 40 mg daily x15 days Exposures exceeded EC50 in MES preclinical seizure model SEPTEMBER 2023Biohaven | Investor Presentation11

BHV-7000: Phase 1 SAD/MAD CNS TEAEs by Dose and Cohort CNS AEsa Placebo N=10 4 mg N=6 10 mg N=6 25 mg (Fasted) N=6 25 mg (Fed) N=6 50 mg N=6 100 mg N=5 BHV-7000 Overall N=29 Headache 0 0 1 (16.7) 1 (16.7) 0 1 (16.7) 0 3 (10.3) Dizziness 0 0 1 (16.7) 0 0 0 0 1 (3.4) Myoclonus 0 0 0 1 (16.7) 0 0 0 1 (3.4) CNS AEsa Placebo N=5 10 mg N=5 25 mg N=6 40 mg N=6 BHV-7000 Overall N=17 Headache 1 (20.0) 0 0 3 (50.0)1 3 (17.6) Single Ascending Dose Multiple Ascending Dose aMedDRA® Preferred Term within the System Organ Class of “Nervous System Disorders” aMedDRA® Preferred Term within the System Organ Class of “Nervous System Disorders” SEPTEMBER 2023Biohaven | Investor Presentation12 1. Incidents of headache were classified as mild

BHV-7000: Not Associated with CNS AEs Typical of Other ASMs 80% 80% of patients will experience an AE after starting an ASM1 Challenges with Existing ASMs GABAA pathway activated by other ASMs is associated with AEs such as somnolence and dizziness2 Several ASMs cause behavioral (irritability, anger, aggression) or psychiatric (depressive mood, anxiety, psychosis) AEs3,4 Pooled CNS AEsa,5 BHV-7000 MAD Pooled N=17 Somnolence 0% Headache 18% Balance disorder 0% Dizziness 0% Memory impairment 0% Sensory disturbance 0% Speech disorder 0% Xen1101 MAD Pooled N=18 39% 39% 17% 17% 28% 11% 33% aMedDRA® Preferred Term within the System Organ Class of “Nervous System Disorders” Data presented is not the result of any head-to-head clinical trials and does not mean or suggest that BHV-7000 is clinically more safe and effective.5 Devinsky et al. Nat Rev Dis Primers. 2018;4:18024. 2. Abou-Khalil. Continuum (Minneap Minn). 2022;28(2):500-535. 3. Steinhoff et al. Epilepsy Behav. 2021;123:108270. 4. Chen et al. Epilepsy Behav. 2017;76:24-31. 5. 73rd Annual American Epilepsy Society Meeting 2019, Abstract #3.31. Poster presented November 25, 2019. AE, adverse event; ASM, anti-seizure medication; CNS, central nervous system; GABA, gamma-aminobutyric acid; MAD, multiple ascending dose; MedDRA, Medical Dictionary for Regulatory Activities SEPTEMBER 2023Biohaven | Investor Presentation13

BHV-7000: Summary and Clinical Program Status • Potent, selective activator of Kv7.2/Kv7.3 potassium channels • Structurally/pharmacologically distinct from other K+ channel activators • Designed to “dial out” GABAA receptor activation, improve tolerability • Potent in MES epilepsy model without adverse neurobehavior or motor effects • Well-tolerated in Phase 1 SAD/MAD study • EEG study; initial Phase 1 results confirm target engagement at both low and high doses of BHV-7000, full results expected YE 2023 • Once daily dosing with extended-release formulation There is a missing piece in epilepsy treatment for better-tolerated, efficacious anti-seizure medications Phase 1 EEG Phase 2/3 Focal EpilepsyPhase 1 SAD/MAD CNS, central nervous system; EEG, electroencephalography; GABA, gamma-aminobutyric acid; MAD, multiple ascending dose; MES, maximal electroshock seizure; SAD, single ascending dose SEPTEMBER 202314 Biohaven | Investor Presentation
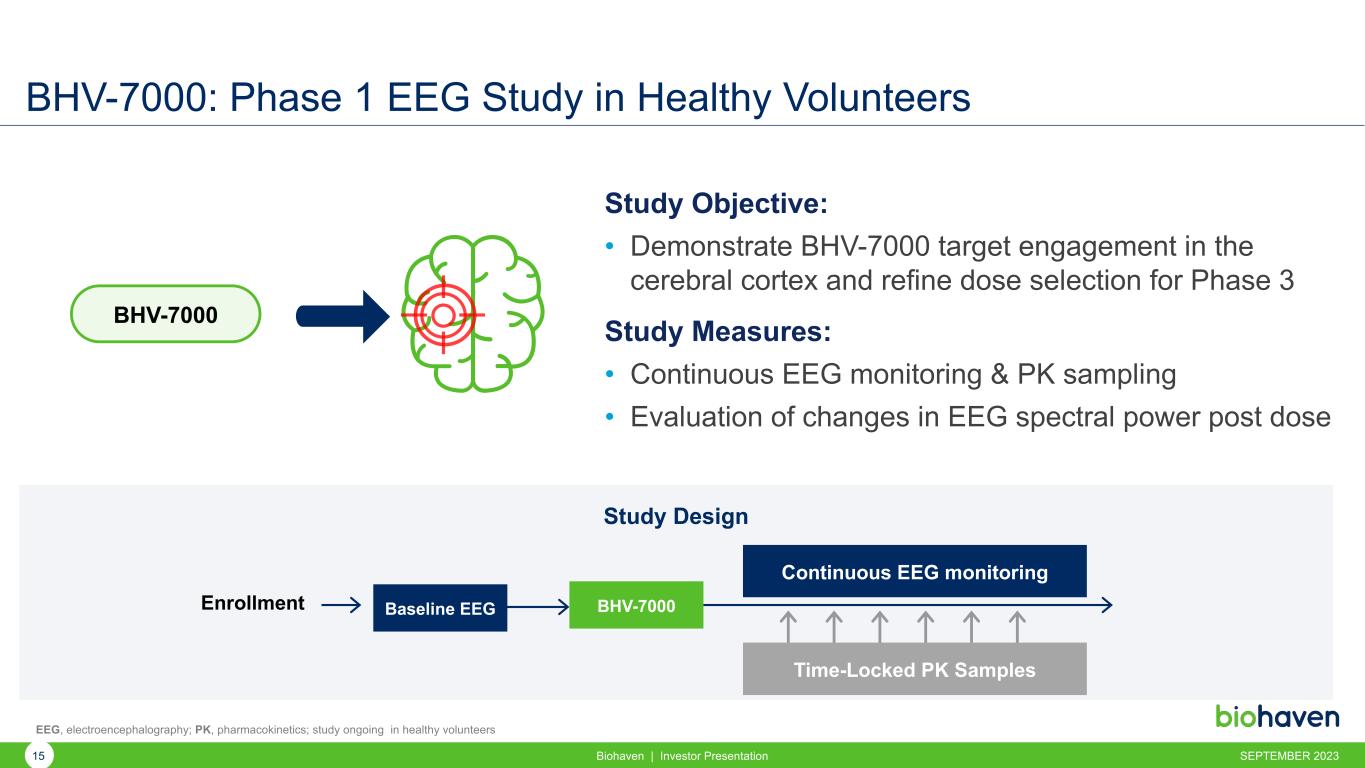
BHV-7000: Phase 1 EEG Study in Healthy Volunteers Study Objective: • Demonstrate BHV-7000 target engagement in the cerebral cortex and refine dose selection for Phase 3 Study Measures: • Continuous EEG monitoring & PK sampling • Evaluation of changes in EEG spectral power post dose Study Design Baseline EEG Continuous EEG monitoring Enrollment Time-Locked PK Samples BHV-7000 BHV-7000 EEG, electroencephalography; PK, pharmacokinetics; study ongoing in healthy volunteers SEPTEMBER 202315 Biohaven | Investor Presentation

Increases Observed in EEG Spectral Power Across Beta and Gamma Bands After a 10 mg Dose of BHV-7000 in Subjects With Drug Concentrations >EC50 PREDOSE POST DOSE G AM M A BE TA AL PH A TH ET A D EL TA FR E Q U N E C Y B A N D S E E G S P E C TR A L P O W E R BHV-7000: Spectral Power Changes Observed After 10 mg Dose with Concentrations ≥ EC50 SEPTEMBER 2023Biohaven | Investor Presentation16 *EC50 based on preclinical maximal electroshock seizure (MES) models No Changes Observed in EEG Spectral Power Across all Frequency Bands After a 10 mg Dose of BHV-7000 in Subjects With Drug Concentrations <EC50 PREDOSE POST DOSE G AM M A BE TA AL PH A TH ET A D EL TA FR EQ U N EC Y BA N D S EEG SPEC TR AL PO W ER HOT OFF THE PRESS

BHV-7000: Increases in Spectral Power Were Observed in all Frequency Bands and all Brain Regions After 50 mg Dose SEPTEMBER 2023Biohaven | Investor Presentation17 HOT OFF THE PRESS Increases Observed in EEG Spectral Power Across All Frequency Bands After a 50 mg Dose of BHV-7000 Demonstrating Central Nervous System Target Engagement G AM M A BE TA AL PH A TH ET A D EL TA FR EQ U N EC Y BA N D S EEG SPEC TR AL PO W ER PREDOSE POST DOSE

BHV-7000: Mean Predicted Concentration vs. Time Profiles of BHV-7000 Extended Release (ER) For 25 mg ER, 50 mg ER, 75 mg ER Once Daily Dosing at Steady State SEPTEMBER 2023Biohaven | Investor Presentation18 *EC20 and EC50 values from preclinical maximal electroshock seizure (MES) model M ea n Pr ed ic te d BH V- 70 00 C on ce nt ra tio n at S te ad y St at e Time 25 mg ER QD 50 mg ER QD 75 mg ER QD Mean Predicted BHV-7000 Concentration MES EC50 MES EC20

BHV-7000: Phase 3 Trials in Focal Epilepsy Two multicenter, international, placebo-controlled, double-blind studies to evaluate the efficacy of BHV-7000 in adolescents and adults with refractory focal epilepsy Randomization 1:1:1 Double-Blind Phase 8 and 12 weeks Extension Study 1+ years Placebo 25 mg Continue Dose Level Key Inclusion Criteria: • 12-75 years old • Refractory focal epilepsy Primary Endpoint: median percent change (US), ≥50% responder rate (EU) Secondary Endpoint: QOLIE-31, Seizure Freedom QOLIE-31, Quality of Life in Epilepsy Inventory; Planned study design for Phase 3 trials SEPTEMBER 2023 Biohaven | Investor Presentation 19 Screening / Observation Phase Randomization 1:1:1 Placeb 75 mg 50 mg Continue Dose Level To evaluate safety, tolerability, and efficacy of BHV-7000 50 mg

Kv7 PLATFORM Summary Proprietary Chemical Library of Novel Kv7 Activators In-house synthesis with differentiated pharmacological and structural profiles and potential for multiple indications Kv7.2/7.3 Activation Clinically validated mechanism of action in epilepsy BHV-7000: Potential Best-in-Class with Differentiation Preclinical data suggests wide therapeutic index in the clinic by dialing out GABA risk (somnolence, sedation, dizziness) BHV-7000 Series COM Patent Protection covered until 2039 (excl extensions) Once-daily, Extended-Release Formulation Identified Status Update BHV-7000 well tolerated in Phase 1 SAD/MAD study CNS target engagement confirmed in EEG biomarker study Phase 3 study initiation in Epilepsy & Bipolar Disorder expected YE 2023 SEPTEMBER 2023Biohaven | Investor Presentation20

Bipolar Disorder Affects 11 Million Adults in the US While characterized by mania, patients largely suffer from depression, yet few effective options for BPD and maintenance treatment1-4 ~50% of patients are medication nonadherent; discontinuations commonly due to poor tolerability4,5~50% No new mood stabilizer approved in last 20 years excluding antipsychotics6 • Lamotrigine - last novel mood stabilizer approved; utility primarily in maintenance, limited efficacy in acute depressive episodes • Serious AEs observed with current mood stabilizers (thyroid/renal function issues, liver tox, thrombocytopenia, rash, SJS3,9) • Risks of metabolic dysfunction, weight gain, and cognitive slowing • Adherence issues lead to ineffective treatment and risk of relapse5,7,8 20 years 1. Tondo et al Curr Neuropharmacol. 2017;15(3):353-358. 2. Miller et al. J Affect Disord. 2014;169(Suppl 1):S3-11. 3. Carvalho et al. N Engl J Med. 2020;383(1):58-66. 4. McIntyre, Calabrese. Curr Med Res Opin. 2019;35(11):1993-2005. 5. Jawad et al. Ther Adv Psychopharmacol. 2018;8(12):349-363. 6. Rhee et al. Am J Psychiatry. 2020;177(8):706-715. 7. Fung et al. J Affect Disord. 2019;257:17-22. 8. Marzani, Neff. Am Fam Physician. 2021;103(4):227-239. 9. Bobo. Mayo Clin Proc. 2017;92(10):1532-1551. AE, adverse event; BD, bipolar disorder SEPTEMBER 2023Biohaven | Investor Presentation21

Compelling Evidence for Kv7 Activation in Bipolar Disorder Treatment Overlapping molecular, cellular mechanism in bipolar disorder ü ANK-3: highly implicated gene in bipolar disorder; codes for a protein that anchors Kv7 channels to the cell membrane1 ü Kv7.2/7.3 channels among most dysregulated proteins in bipolar brain tissue2 ü Bipolar patients exhibit several relevant epigenetic changes linked to Kv73 Preclinical evidence for manic and depressive poles ü Mice who upregulate Kv7 are resilient to stress-induced depressive effects4 ü Kv7 activators reverse & prevent pathologic hyperactivity in depression and mania models5,6 ü Kv7 mutations cause transdiagnostic mood disturbances including hyperactivity, insomnia, anxiety, and cognitive dysfunction1 1. Judy et al. Front Genet. 2013;4:87. 2. Kristensen et al. J Neurochem. 2012;3:373-382. 3. Kaminsky et al. Bipolar Disord. 2015;2:150-159. 4. Friedman et al. Nat Commun. 2016;24(7):11671. 5. Dencker et al. Behav Brain Res. 2010;1:78-83. 6. Redrobe et al. Behav Brain Res. 2009;198(2):481-485. ANK-3, ankyrin 3 SEPTEMBER 2023Biohaven | Investor Presentation22

BHV-7000: Demonstrates Potential for Clinical Translation of Kv7 Activation in Bipolar Disorder Kv 7. 2 Kv 7. 3 BHV-7000 Retigabine1 Increased M-current Reduced neuronal hyperactivity Lamotrigine2 Lithium3 indirect BHV-7000 shares mechanistic overlap with cornerstone bipolar treatments indirect Retigabine vs placebo; MDD measured via MADRS and fMRI4,5 ~7 decrease vs placebo with retigabine in depression improvement fMRI study: Retigabine effects mediated by the limbic system Medications such as antipsychotics and lithium have established efficacy in unipolar and bipolar depression Ezogabine Placebo -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 C ha ng e in V S ac tiv at io n to re w ar d an tic ip at io n (o ut co m e > ba se lin e) 0 1 2 3 4 5 -20 -15 -10 -5 0 C ha ng e in M AD R S sc or e Weeks 1. Friedman et al. Nat Commun. 2016; 24;7:11671. 2. Friedman et al. Science. 2014;344(6181):313-319. 3. Kristensen et al. J Neurochem. 2012;3:373-382. 4. Amann et al. J Clin Psychopharmacol. 2006;26(5):534-536. 5. Costi et al. Am J Psychiatry. 2021;178(5):437-446. fMRI, functional magnetic resonance imaging; GSK3B, Glycogen Synthase Kinase 3 Beta; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder Robust Preliminary Acute Efficacy in MDD Likely To Translate to Bipolar Disorder SEPTEMBER 2023Biohaven | Investor Presentation23

BHV-7000: Potentially Addresses Key Unmet Needs in Bipolar Disorder by Reducing Stress-Related Hyperactivity While Enhancing Resilience • Patients change / discontinue medication after ~2 months • >50% of patients discontinue at 6 months due to intolerance1 ü Favorable safety and tolerability over current mood stabilizers and antipsychotics • 1st line mood stabilizers require titration or frequent laboratory monitoring, burdening both prescribers and patients3 ü No titration / safety laboratory monitoring anticipated • Minimal efficacy • Widely prescribed antidepressants carry “switching” risk ü Novel mechanism - potential for robust antidepressant effects without “switching” risk 1. Jawad et al. Ther Adv Psychopharmacol. 2018;8(12):349-363. 2. Vieta et al. Nat Rev Dis Primers. 2018;4:18008. 3. Yatham et al. Bipolar Disord. 2018;20(2):97-170. SEPTEMBER 2023Biohaven | Investor Presentation24 BHV-7000Currently Approved Products

BHV-7000: Potential to Overcome Challenges With Existing Therapies Potential for best-in-category tolerability and safety • Low burden to patients and providers, enabling safer, easier long-term treatment • No expected long-term metabolic side effects, no “switching” risk, no titration, and no drug monitoring Lithium Valproate SSRI Antipsychotics Lamotrigine Metabolic AEs Hepatic AEs Renal AEs Rash / SJS Sexual SE Sedation / Cognitive AE Drug monitoring Switching risk Titration SJS, Stevens-Johnson Syndrome Patient Burden SEPTEMBER 2023Biohaven | Investor Presentation25

TRPM3: A Novel Peripheral Target for Neuropathic Pain Oral class terminated for hyperthermia No new molecules in this class despite promising Multiple TRPA1 programs terminated Conflicting data for pro- and anti-nociceptive role; remains in active drug development Promising as a novel target: broader expression than TRPV1 and TRPA1 in peripheral neurons; unlikely to affect body temperature homeostasis Peripheral sensory neuron Pain sensation CGRP X ? ? TRPV1 TRPA1 TRPM8 TRPM3 See Koivisto et al. Nat Rev Drug Discov. 2022;21(1):41-59 for background on TRP channel drug development. 2021: Nobel Prize in Physiology or Medicine awarded to David Julius and Ardem Patapoutian for their discovery of the cellular sensors of temperature and pressure SEPTEMBER 2023Biohaven | Investor Presentation26

TRPM3 (BHV-2100) Reduces Pain in Preclinical Models of Chemotherapy and Diabetic Neuropathy Encouraging evidence of pain reduction without the sedation observed with high dose tramadol/gabapentin Biohaven | Investor Presentation27 BHV-2100 5 mg/kg BHV-2100 25 mg/kg Tramadol 5 mg/kg BHV-2100 1 mg/kg Vehicle BHV-2100 1 mg/kg BHV-2100 25 mg/kg Pregabalin 30 mg/kg Gabapentin 150 mg/kg BHV-2100 5 mg/kg Vehicle DISCOVERY RESEARCH Drug administered 7 days after diabetic induction with STZ in rats Drug administered 6 days after oxaliplatin in mice Source: Biohaven data on file SEPTEMBER 2023

Molecular Characteristics Molecular characteristics predict convenient, safe molecule with optimal target product profile for a daily oral medicine BHV-2100: A Versatile Agent for Treatment of Multiple Pain Conditions Unmet Need in Neuropathic Pain Disorders >50% of patients with common neuropathic pain disorders (e.g., diabetic peripheral neuropathy) are inadequately controlled even with 2+ medications to attempt to control pain Preclinical Data Preclinical data shows potent reversal of pain in multiple translatable animal models Selective and Potent Selective and potent inhibition of TRPM3 provides a novel, non- opioid approach to neuropathic pain treatment Selectivity within TRP family, avoids potential class liabilities such as hyperthermia Investigational New Drug filing planned for 2H 2023 SEPTEMBER 2023Biohaven | Investor Presentation28

TYK2 (BHV-8000) Overview First-in-Class Oral Brain-Penetrant TYK2/JAK1 Inhibitor Uniquely potent, TYK2/JAK1 selective, brain penetrant inhibitor Breaks the Cycle of Neuroinflammation Reduces inflammatory impacts of microglia, astrocytes, & infiltrating T-lymphocytes Potential in Multiple Neuroinflammatory Disorders Strong evidence supports efficacy in Parkinson’s disease, Alzheimer’s disease, Multiple Sclerosis and other neuroinflammatory diseases BHV-8000 Series COM Patent Protection covered until 2037 (excl extensions) Favorable PK/PD and Selectivity Profile Avoids class risks associated with JAK2/3 inhibition Encouraging Results from Ongoing FIH Phase 1 Clinical Trial Multiple cohorts dosed in SAD portion of SAD/MAD study Projected therapeutic concentrations achieved BHV-8000 well tolerated, only mild adverse events reported SEPTEMBER 2023Biohaven | Investor Presentation29

BHV-8000: TYK2/JAK1 in Neuroinflammatory Disease • Dual inhibition of TYK2 and JAK1 can effectively block Th17 cell generation, Type I IFN signaling, and inflammation • JAK inhibitors are more efficacious anti-inflammatory agents than anti-TNFs JAK2JAK3JAK1 IFNγ IL-6 TYK2 Type I IFN IL-23 → IL-17 IL-12 IL-2,IL-4, IL-7 IL-9, IL-15, IL-21 Erythropoietin, GH, leptin, thrombopoietin, prolactin, GM-CSF Adapted from Gonciarz et al. Immunotherapy 2021;13(13):1135-1150. GH, growth hormone; GM-CSF, granulocyte macrophage colony stimulating factor; IFN, interferon; IL, interleukin; JAK, Janus kinase; STAT, signal transducer and activator of transcription proteins; Th, T helper cell; TNF, tumor necrosis factor; TYK, tyrosine kinase SEPTEMBER 2023Biohaven | Investor Presentation30

Cellular Drivers In Neuroinflammation: Predominant TYK2/JAK1 Effects TYK2/JAK1 cytokines • IFN-g • IL-b • TNF downstream of IFN-g • IL-8 • GM-CSF, MCP-1 Microglia are the resident macrophages of the CNS, playing an important role in neuroinflammation, repair and maintenance Microglia • IFN-g • IL-12 • TNF • IL-8 Abnormal astrocytic activity may exacerbate inflammatory reactions and contribute to tissue damage Astrocytes Lymphocytes, other leukocytes • IL-23 • IL-17 downstream of IL-23 • IL-2, IL-4 Strong evidence for Th17 lymphocyte involvement in the pathogenesis of multiple sclerosis and Parkinson’s disease SEPTEMBER 2023Biohaven | Investor Presentation31

BHV-8000: Summary Selectivity is a differentiator • Selective inhibition of TYK2/JAK1 provides potential for best-in-class immunomodulation in neuroinflammatory disorders • Selectivity for TYK2/JAK1 mitigates non-selective JAK class liabilities, largely related to JAK2 and JAK3 inhibition, and offers potential to improve benefit-risk for the highly selective BHV-8000 dual kinase inhibitor Potential in multiple neuroinflammatory disorders • Complements other approaches directly addressing neurodegeneration such as amyloid, α-synuclein, tau, and mitochondrial targeting therapies • Strong evidence supports potential efficacy in Parkinson’s disease, Alzheimer’s disease, and further neuroinflammatory diseases • Phase 1 initiated May 2023; multiple cohorts dosed in SAD portion of SAD/MAD study • Phase 2 in Parkinson’s disease anticipated to begin in 2024 • Partner (Highlightll Pharmaceuticals) anticipates initiating a study in Alzheimer’s disease in China in 2024 Clinical trials underway and anticipated in 2024 SEPTEMBER 2023Biohaven | Investor Presentation32
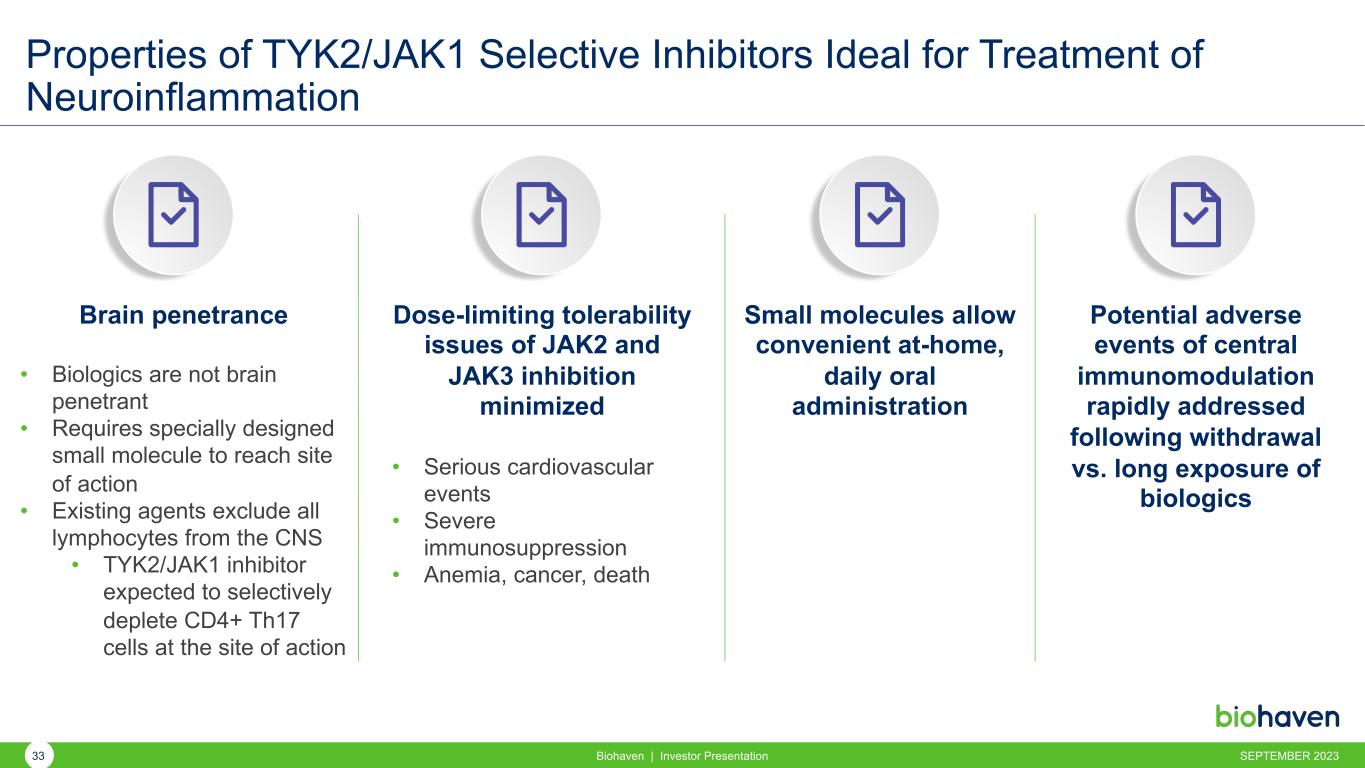
Properties of TYK2/JAK1 Selective Inhibitors Ideal for Treatment of Neuroinflammation Brain penetrance • Biologics are not brain penetrant • Requires specially designed small molecule to reach site of action • Existing agents exclude all lymphocytes from the CNS • TYK2/JAK1 inhibitor expected to selectively deplete CD4+ Th17 cells at the site of action Dose-limiting tolerability issues of JAK2 and JAK3 inhibition minimized • Serious cardiovascular events • Severe immunosuppression • Anemia, cancer, death Small molecules allow convenient at-home, daily oral administration Potential adverse events of central immunomodulation rapidly addressed following withdrawal vs. long exposure of biologics SEPTEMBER 2023Biohaven | Investor Presentation33

BHV-8000: Targets Both Axes of Neuroinflammation in Parkinson’s Disease Dopaminergic neuron 1. a-syn aggregates in dopaminergic neurons Microglia 5. Microglia present α-syn to T cells with MHCII Th1/Th17 cells 3. Microglia release chemokines to recruit T cells to CNS 6. T cells release IFN-γ, which binds to its receptor on microglia SUBSTANTIA NIGRA 4. T cells migrate from periphery to CNS 2. Aggregated a-syn taken up by microglia Th1/Th17 cells 7. Microglia release inflammatory cytokines that damage dopaminergic neurons CD4+ T cell ENTERIC NERVOUS SYSTEM AND PERIPHERY 1. a-syn aggregates in enteric neurons 2. Aggregated a-syn is taken up by M1 macrophages M1 Macrophage 2. a-syn is presented with MHCII to CD4+ T cells Enteric neuron 3. CD4+ T cell that binds a-syn loaded MHCII is activated and clonally expands 1. Allen Reish, Standaert. J Parkinsons Dis. 2015;5(1):1-19. 2. Fu et al. J Neuroinflammation. 2022;19(1):98. α-syn, alpha-synuclein; CD4, cluster of differentiation 4; CNS, central nervous system; IFN-γ, interferon-gamma; IL, interleukin; JAK, Janus kinase; M1, classically activated; MHC, major histocompatibility complex; Th, T helper cell; TYK, tyrosine kinase TYK2/JAK2 INHIBITION OF PARKINSON’S NEUROINFLAMMATORY CASCADE TYK2/JAK1 inhibitors reduce Th17 cell activation and expansion by inhibiting IL-23 signaling and reduce microglial activation by inhibiting IFN-γ signaling triggered by pathogenic α-synuclein aggregates1,2 SEPTEMBER 2023Biohaven | Investor Presentation34

• Multiple sclerosis is an inflammatory disease in which humoral immune and cell-mediated immune responses target CNS antigens • IL-17A-defective mice are highly resistant to induction of EAE • PKM2 activators mediate potent inhibitory effects in EAE model due to Th17 cell effects • In a meta-analyses of literature, TNF-a, IL-15, IL-12, IL-23/IL-17, and IFNg were elevated in or predictors of MS patients vs. controls • Secukinemab (IL-17A) demonstrates an effect in relapsing remitting MS • Brain penetrant TYK2/JAK1 kinase inhibitors reduce Th17 cells (IL-17 and IL-23) and target IL-12 signal transduction • BHV-8000 is ideally suited to reducing neuroinflammation in MS TYK2/JAK1 Inflammatory Pathways in Multiple Sclerosis X X X X McGinley et al, Immunity 52:342-356, Palle et al, Med Sci, 5:23, 2017; Bai et al, Frontiers in Neuroscience, 10.3389, Oct 4, 2019, Havrdova, Multiple Sclerosis Journal, 18_509, 2012; Figure from Nat Rev Neurosci. 2002 Apr;3(4):291-301. doi: 10.1038/nrn784. SEPTEMBER 2023Biohaven | Investor Presentation35

TYK2/JAK1 Inhibition Reduces Several Key Cytokines Driving Alzheimer’s Disease (AD) Pathology Neuroinflammation is a key event in AD pathogenesis, suggesting that a combination of anti-amyloid β (Aβ)/tau and anti-inflammation therapies is necessary1,2 Neuroinflammation Aβ plaque deposition + tau accumulation Microglia activation and release of cytokines Synapse removal + cognitive decline • Cytokines and chemokines affect tau phosphorylation and amyloid β (Aβ) deposition • Cytokines released include IL-1b, IL-6, TNF-α, and IFN- γ • Aβ deposition also induces expression TYK2 and JAK1 • TYK2 is the most prominent gene altered by Aβ3 • Concomitant presence of Aβ, tau, and microglial activation abnormalities dramatically increases odds of cognitive symptoms2 Aβ plaque Microglia Tau Neuron Inflammatory cytokines Adapted from Neher. Immunity. 2022;55(5):821-823. 1. Domingues et al. Curr Alzheimer Res. 2017;14(8):870-882. 2. Pascoal et al. Nat Med. 2021;27(9):1592-1599. 3. Nevado-l et al. Cells. 2019;8:825. SEPTEMBER 2023Biohaven | Investor Presentation36

Biohaven | Investor Presentation37 OBSESSIVE COMPULSIVE DISORDER (OCD) • Compelling mechanistic rationale for cortico-striatal glutamate abnormalities in OCD patients • Strong PoC in Phase 2 • Well-characterized in 1,000+ patients • Two Phase 3 studies ongoing; enrollment completion anticipated YE 2023 SEPTEMBER 2023 OCD Summary Glutamate Platform TRORILUZOLE

Troriluzole in OCD: Framing the Unmet Need OCD Affects 1.2% of the US population, but only 1 in 6 people with OCD are treated with a pharmaceutical medication Limited ongoing investigations in the treatment of OCD, predicting a paucity of new approved agents in the near future Ketamine shown to have short-term efficacy; associated with stigma and undesirable side effects (e.g., hallucinations, dissociative symptoms) Rapastinel also acts on glutamate system, shown in pilot study to reduce symptoms of OCD, anxiety, and depression within hours (but lasted less than 1 week) SSRIs are the only medication approved for OCD Take weeks to months to take effect Often need higher doses than for antidepressants, increasing dose-related side effects / do not work for everyone GLUTAMATE SEPTEMBER 2023Biohaven | Investor Presentation38

BHV-4157 Troriluzole Treated OCD Patients: Strong Signal Observed in Phase 2 POC Supports Advancement to Phase 3 STUDY BHV-4157-202 Patients with moderate-to-severe OCD (Y-BOCS score ≥ 19) and inadequate response to standard of care SAMPLE SIZE 226 subjects RANDOMIZATION 1:1 DOSE Troriluzole 200 mg QD vs Placebo QD (in patients on standard of care) PRIMARY OUTCOME Y-BOCS, precedented outcome measure accepted by FDA Table 1: Troriluzole Effect on OCD in Phase 2/3 Trial1 Y-BOCS Total Change from Baseline Week 4 (N=115a, 111b) 8 (N=108a, 96b) 12 (N=102a, 99b) a. Placeboa -2.9 -3.6 -4.9 b. Troriluzoleb -3.4 -5.1* -5.9 p-value 0.451 0.041 0.220 1. BHV-4157-202 Final Unblinded Analysis YBOCS Total Change from Baseline by Week LSMeans from MMRM Model MITT Data Set Table 2: Troriluzole Effect on Patients with Severe OCD1 Y-BOCS Total Change from Baseline Week 4 (N=47c, 49d) 8 (N=45c, 42d) 12 (N=43c, 44d) a. Placeboc -3.5 -3.1 -4.6 b. Troriluzoled -4.1 -6.0* -7.0 p-value 0.584 0.035 0.084 1. Patients at baseline with median Y-BOCS total scores > 26 (severe OCD symptoms). * p < 0.05 versus placebo Y-BOCS, Yale-Brown Obsessive Compulsive Scale (FDA accepted outcome measure) GLUTAMATE Biohaven | Investor Presentation39 SEPTEMBER 2023

Myostatin Platform TALDEFGROBEP ALFA BHV-2000 Non-Clinical • Well characterized in over 20 animal studies for safety and models of disease • Includes juvenile animals permitting the safety down to 2 years of age Clinical • In prior studies, 359 participants received taldefgrobep: 179 healthy participants and 180 participants with Duchenne Muscular Dystrophy 5-12 years old • Administration by subcutaneous injections in the arm, thigh, and abdomen • Demonstrated dose-dependent suppression of free serum myostatin • MRI and DXA data was consistent with a positive beneficial effect on muscle health • Generally safe and well-tolerated SEPTEMBER 2023Biohaven | Investor Presentation40

Myostatin and Activin A are Potent Muscle Regulators SEPTEMBER 2023Biohaven | Investor Presentation41 Blocking Myostatin and Activin A Leads to Muscle Hypertrophy • Myostatin is a secreted protein belonging to the TGF-ß superfamily of signaling molecules • Myostatin signals by binding initially to the activin type 2 receptors, ActRIIA and ActRIIB, which then engages the activin type 1 receptors, ALK4 and ALK5 • Genetic and pharmacological studies in multiple species, including humans, have shown that myostatin normally acts to block skeletal muscle growth • The function of myostatin in muscle is partially redundant with that of the related protein activin A Myostatin (GDF-8) is naturally expressed by skeletal muscle and actively inhibits skeletal muscle growth Myostatin Negatively Regulates Muscle Growth
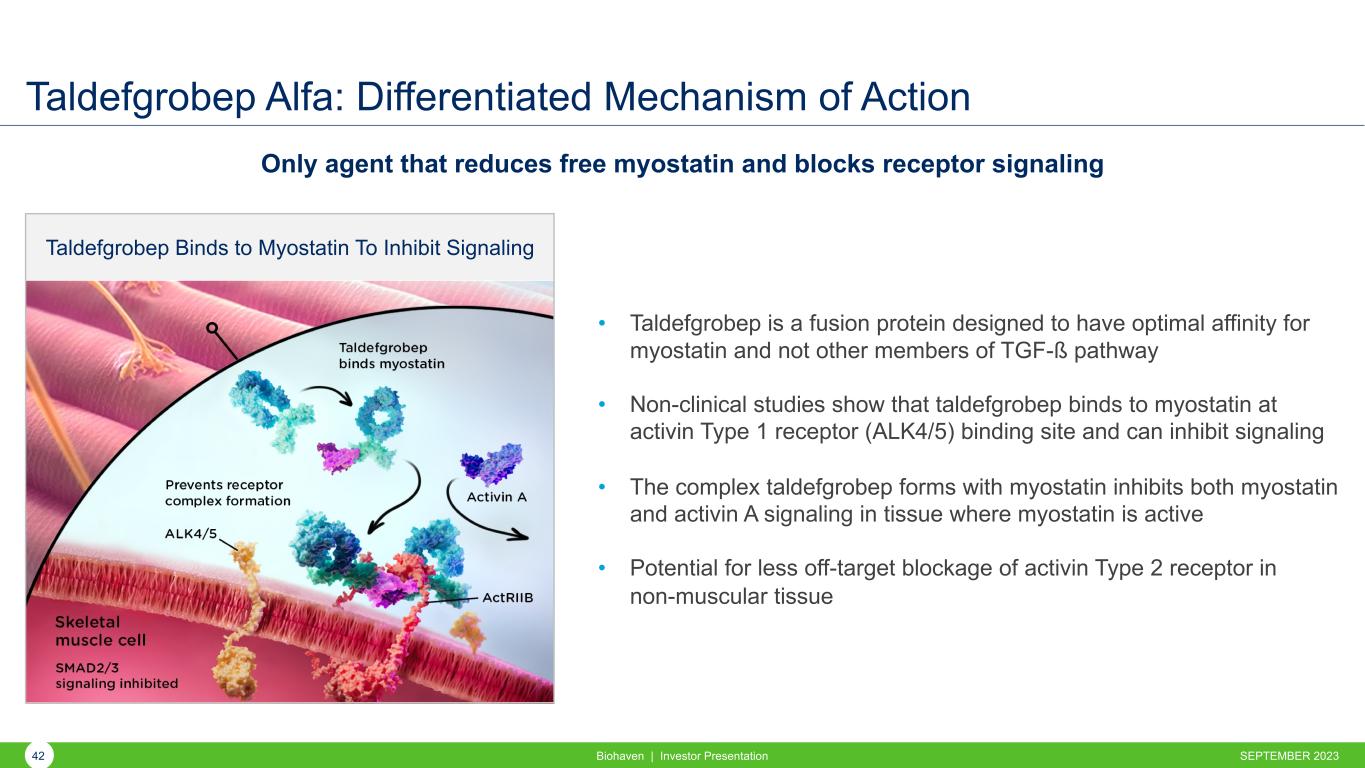
Taldefgrobep Alfa: Differentiated Mechanism of Action SEPTEMBER 2023Biohaven | Investor Presentation42 • Taldefgrobep is a fusion protein designed to have optimal affinity for myostatin and not other members of TGF-ß pathway • Non-clinical studies show that taldefgrobep binds to myostatin at activin Type 1 receptor (ALK4/5) binding site and can inhibit signaling • The complex taldefgrobep forms with myostatin inhibits both myostatin and activin A signaling in tissue where myostatin is active • Potential for less off-target blockage of activin Type 2 receptor in non-muscular tissue Only agent that reduces free myostatin and blocks receptor signaling Taldefgrobep Binds to Myostatin To Inhibit Signaling

Myostatin Free and Drug Bound Levels Taldefgrobep Alfa: Activity Confirmed in Human Studies SEPTEMBER 2023Biohaven | Investor Presentation43 • Healthy volunteers showed dose dependent increases in exposure and lowering of free myostatin when administered subcutaneously on a weekly basis for 4 weeks • Accumulation of the taldefgrobep/myostatin complex drives competitive inhibition of free myostatin and activin A binding • Participants demonstrated an increase in lean skeletal tissue (MRI) and increase in lean body mass along with a reduction of intramuscular fat volume (DXA) Fr ee a nd B ou nd M yo st at in an d M yo st at in C om pl ex % C ha ng e fr om B as el in e Study Day Myostatin Drug Complex, 45mg Q1W Myostatin, 45mg Q1W Placebo Dosing period Decrease in Intramuscular Fat In tr am us cu la r F at V ol um e (M ea n % C ha ng e fr om B as el in e) Day 29 Day 57 Increased Lean Body Mass Le an B od y M as s (M ea n % C ha ng e fr om B as el in e) Day 15 Day 29 Day 57

Myostatin: Strong Scientific Rationale in Spinal Muscular Atrophy SEPTEMBER 2023Biohaven | Investor Presentation44 COMBINATION THERAPY STUDIES OF SMN UPREGULATION IN SMA DISEASE MOUSE MODEL DEMONSTRATED: ü Improved life span and strength, along with improved muscle function ü Increased nerve branching, size of post-synaptic area, innervated neuromuscular junctions, enlarged sensory neurons in DRG • SMA is a neurodegenerative disease; patients retain intact muscle as a target for improvements of function • Disease modifying therapies approved and widely accessible and effective in SMA patients • Disease area has well established validated clinical endpoints with proven regulatory path for approval SMN, survival motor neuron; DRG, Dorsal root ganglia

Phase 3 RESILIENT Study Overview RESILIENT is a randomized, placebo-controlled trial testing the effectiveness and safety of taldefgrobep as an adjunctive treatment Taldefgrobep, or a placebo, will be given while the participant is: • Already taking a stable dose of nusinersen and/or • Already taking a stable dose of risdiplam and/or • Have a history of onasemnogene abeparvovec-xioi PRIMARY OBJECTIVE Change in the 32 item Motor Function Measure (MFM-32) total score between Baseline and Week 48 48-Week, Double-Blind, Placebo-Controlled Study in Pediatric and Adult Participants With Spinal Muscular Atrophy Study Design • Estimated enrollment: 180 participants • 2:1 randomization: taldefgrobep alfa vs placebo Double-Blind Phase 48 weeks Screening ≤ 6 weeks Optional Open-Label Extension Phase 48 weeks Safety Follow-Up 8 weeks R Double- Blind Phase 48 weeks Taldefgrobep Alfa Weight-based 35 mg or 50 mg weekly, SC Matching Placebo + + SMN Upregulator Stable regimen of nusinersen and/or risdiplam and/or history of treatment with onasemnogene abeparvovec- xioi SMN Upregulator Stable regimen of nusinersen and/or risdiplam and/or history of treatment with onasemnogene abeparvovec- xioi Taldefgrobep Alfa Weight-based 35 mg or 50 mg weekly, SC + SMN Upregulator Stable regimen of nusinersen and/or risdiplam and/or history of treatment with onasemnogene abeparvovec-xioi Randomization RESILIENT Phase 3 Study Design SEPTEMBER 2023Biohaven | Investor Presentation45

RESILIENT Study Population • We include a broad population given high unmet need across SMA population, and changing treatment paradigms • Field has evolved with disease modifying therapies and widespread newborn screening, early treatment, and potentially combinations of therapies • Shift to focus more on functional status rather than SMA Type; treated patients are achieving milestones they would not have otherwise • Approximately 180 participants with SMA are expected to enter the treatment phase RESILIENT is not restricted nor limited to patients based on ambulatory status, background therapy, or classification of SMA SEPTEMBER 2023Biohaven | Investor Presentation46

Obesity is a Public Health Crisis • Obesity is a DISEASE of excess and/or abnormal adipose tissue § Cardio-metabolic risk is closely correlated with visceral adiposity • By 2030, it is estimated that 1 billion people worldwide will be living with obesity, including ~50% of American adults1 § Obesity and related comorbid disease costs the US healthcare system ~175 billion USD annually2 § A small proportion of eligible individuals are currently being treated with anti-obesity medications (AOMs)3 • Treatment of obesity is an area of critical unmet medical need 1, The World Obesity Federation. World Obesity Atlas 2022. https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022; Accessed 17-NOV-2022. 2, CDC. Adult obesity facts. https://www.gov/obesity/data/adult.html; Accessed 13-NOV-2022. 3. Saxon DR, et. al., Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009-2015. dPrimeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond). 2011 Jul;35(7):971-81. doi: 10.1038/ijo.2010.216. Epub 2010 Oct 26. PMID: 20975726. Endocrine • Diabetes mellitus • Cushing syndrome • Hypothyroidism • Subfertility GI • Hiatus hernia • Gallbladder disease • Inguinal hernia Carcinoma • Breast • Colorectal • Endometrial Musculoskeletal • Osteoarthritis • Back pain Cardiovascular • Sudden death • Cardiomyopathy • Hypertension • Ischemic heart disease • Peripheral vascular disease • Deep vein thrombosis • Pulmonary embolism Respiratory • Restrictive lung disease • Obstructive sleep apnea • Obesity hypoventilation syndrome • Difficult intubation Genitourinary • Menstrual problems • Female incontinence • Renal calculi Complications of Obesity4 SEPTEMBER 2023Biohaven | Investor Presentation47

Entering a New Era of Hope and Opportunity for Adults Living with Obesity • This is a time of rapid change and renewed excitement in the weight management space • Highly potent anti-obesity medications (AOMs) and combination therapies are approaching efficacy outcomes comparable to bariatric surgery • Competition in the weight loss space is intensifying but opportunities for disruption exist 0% 5% 10% 15% 20% 25% 30% 35% Intensive Lifestyle Intervention ILI + older medications (eg, orlistat NAL/BUP, PHEN,TPM, liraglutide) Semaglutide 2.4mg Tirzepatide Laparoscopic Band Sleeve Gastrectomy Roux-en-Y gastric bypass Biliopancreatic Diversion Total body weight reduction by most common intervention SEPTEMBER 2023Biohaven | Investor Presentation48

Comparative Efficacy Outcomes in Adults Living with Overweight and Obesity • In the clinic, anti-myostatin therapies have repeatedly demonstrated the ability to increase lean mass, reduce fat mass, and improve glucose metabolism across diverse patient populations • Improvements in body composition are optimized by those agents that can target both myostatin and activin A signaling Drug Dosing Δ Total Body Weight Δ Total Fat Mass Δ Lean Body Mass Δ A1C Phentermine/ topiramate n=1,469 PO once daily -7.8% to -9.8% NA NA -0.4% Naltrexone/ bupropion n=1,161 1-2 PO twice daily -5.4% -11.7% NA -0.6% Bimagrumab n=37 IV Q4W -6.5% -20.5% +3.6% -0.76% Semaglutide 2.4 n=1,306 SC QW -14.9% -19.3% -9.7% -1.6% Tirzepatide n=1,896 SC QW -20.9% -33.9% -10.9% -2.3% Sleeve Gastrectomy n=85 NA -26.4% -40.3% -16.5% to -19.5% -2.67% Qsymia USPI; Greenway FL, et. al. COR-I. Lancet. 2010(9741):595-605; Contrave USPI (32/650mg); Heymsfield SB, et. al. JAMA. 2021; Wilding JPH, et. al. STEP1. NEJM 2021;384(11):989-1002; Wilding JPH, et. al. STEP 1 Body Composition. J Endocr Soc. 2021;5(1):A16-17; Wegovy USPI (STEP2); Jastreboff AM, et. al. SURMOUNT1. NEJM. 2022;387(3):205-16; Mounjaro USPI (15mg); Sylivris A. et. al. Obes Rev. 2022;23(7):e13422; Maimoun L. et. al. Surg Obes Relat Dis. 2019;15(11):1965-73; Zhang H-W, et. al. Gastric Bypass in Chinese w/ DM and obesity. Ann Transl Med. 2020;8(6):372-82; The Phase 2 bimagrumab study was conducted in adults living with obesity and Type-2 DM, while the Phase 3 phentermine/topiramate, naltrexone/bupropion, semaglutide, and tirzepatide studies were conducted in adults with overweight or obesity but no history of T2DM (unless otherwise specified). The time to analysis varies by asset, phentermine/topiramate (1 year), naltrexone/bupropion (56 weeks), bimagrumab (48 weeks), semaglutide (68 weeks), tirzepatide (72 weeks), sleeve gastrectomy (1 year). Notably, change in HbA1c data have been standardized (all representative of change seen in adults with overweight/obesity plus T2DM). In the tirzepatide (15mg) and semaglutide (1mg) studies, conducted in non-diabetic adults with overweight/obesity, the mean change in HbA1c was -0.52 and -0.52%, respectively; Represents cumulative mean change in fat mass across all tirzepatide dose levels. Abati E, Manini A, Comi GP, et. al. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79(7):374; Lee S-J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest. 2021;131(9):e148372 AM, morning; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not available; PO, oral; QW, once weekly; TC, total cholesterol; TG, total glucose. SEPTEMBER 2023Biohaven | Investor Presentation49

Inhibiting Myostatin Signaling Demonstrates Important Physical and Metabolic Change Myostatin as a Pharmacologic Target Improved bone mineral density Increased basal metabolic rate Improved insulin sensitivity Reduction in intramuscular fat Reduction in intrahepatic fat Reduction in total body fat mass Reduction in visceral fat Increased lean mass • Clinically, taldefgrobep has been generally safe and well-tolerated with low rates of GI and musculoskeletal complaints • In healthy adults, taldefgrobep generated significant improvements in body composition relative to placebo SEPTEMBER 2023Biohaven | Investor Presentation50

Advancing Taldefgrobep in Obesity • Leveraging available pre-clinical and early clinical data allows for significant acceleration of development timelines • Pre-IND meeting for obesity completed with FDA • Proof-of-concept trial in adults living with overweight and obesity NOVEL MECHANISM TARGETING BODY COMPOSITION Potential for combination with GLP-1 class SEPTEMBER 2023Biohaven | Investor Presentation51

Myostatin Platform TALDEFGROBEP ALFA BHV-2000 Novel Mechanism of Blocking Myostatin and Activin A Signaling • Human data showing potent reduction in free myostatin and accumulation of myostatin-taldefgrobep complex • Short duration clinical studies demonstrated improvement in lean body mass and loss of adipose tissue Advanced Development Program • Large preclinical and clinical safety package licensed from BMS • Existing database includes pediatric and adult clinical data • Generally safe and well tolerated in multiple clinical studies Spinal Muscular Atrophy (SMA) • Single Pivotal Study launched in mid-2022 • Orphan Drug obtained in the US & EU; Fast-Track Designation in US • Global Study; Enrollment Completed in 4Q 2023 Development Opportunities • Attractive opportunity for metabolic disorders including obesity • Additional neuromuscular, bone, and metabolic indications being evaluated SEPTEMBER 2023Biohaven | Investor Presentation52

DEGRADER PLATFORM Overview A Pipeline of Therapies Potential to support numerous clinical candidates spanning across a wide range of indications by targeting pathogenic proteins and antibodies Potential First-in-Class Targeted Protein Degradation MOA Provides unique advantages, e.g. accelerated path from discovery to clinic BHV-1300 First-in-human MOA for efficient removal of pathogenic IgG with proven mechanism for autoimmune disorders Galactose Deficient IgA1 Degradation Novel antibody-based degrader for treatment of IgA nephropathy Disease-Specific, Autoantibody-Targeted Degraders Selective removal of autoantibodies implicated in multiple immune driven degenerative disorders SEPTEMBER 2023Biohaven | Investor Presentation53 Highly competitive safety, manufacturable and PD profile

MoDE™ Degraders: Multiple Asset Opportunities and Efficient Timelines Key Value Inflection Points IgG and IgA antibodies are the first targets for Biohaven’s powerful degradation platform PROGRAM 2023 2024 2025 2026 2027 Discovery targets Realization of value inflection points will be project specific and aligned with cohesive portfolio strategy AutoAb, autoantibody; Ig, immunoglobulin; IND, Investigational New Drug; MoDE™, molecular degrader of extracellular proteins; RA, rheumatoid arthritis IgG RA, autoimmune IND Filing (2H) IgA IgA nephropathy IND Filing (1H) Undisclosed AutoAb #1 IND Filing Undisclosed AutoAb #2 IND Filing SEPTEMBER 2023Biohaven | Investor Presentation54

• Harnesses the body’s own machinery for degrading proteins • Extracellular protein targets are eliminated via the asialoglycoprotein receptor (ASGPR) • Protein targets are degraded via endolysosomal proteolysis * formerly BH 2640 Bispecific Platform: IgG Degradation via MoDE™ SEPTEMBER 2023Biohaven | Investor Presentation55 Molecular Degraders of Extracellular Proteins (MoDE): small molecules bind extracellular target proteins and cause them to be removed from the body through the liver Binds liver (ASGPR) Binds protein target Pathogenic target protein and MoDEs in circulation

A First-in-Class Mechanism: Hepatic ASGPR Receptor Harnessed for Efficient and Safe Removal of Circulating Pathogenic Targets SEPTEMBER 2023Biohaven | Investor Presentation56 MoDE™ binds circulating target and efficiently delivers it to ASGPR on hepatocytes • Internalized target is rapidly degraded in lysosomes • Degree of target degradation is precisely controlled ASGPR receptors are rapidly recycled. Optimized safety and efficacy is achieved through experimental balancing of relative affinities for ASGPR and target protein. subcutaneous or intravenous injection MoDEs™ are administered via *Stylistic representation ASGPR, asialoglycoprotein receptor; MoDE™, molecular degrader of extracellular proteins 2 3 4 1 Symbol Legend Degradation Target HepatocyteBifunctional MoDE™ Degrader Asialoglycoprotein Receptor (ASGPR)*

Hepatic ASGPR Receptor Harnessed for Efficient and Safe Removal of Pathogenic Targets • High capacity ASGPR hepatocellular receptors internalize plasma proteins with specific motifs • Bispecific ASGPR-binders with target-binder effectively removes pathogenic target from the circulation • IgG may be more rapidly removed from the circulation than FcRN inhibitory antibody or antibody fragments, without causing hypoalbuminemia or dyslipidemia • Improved, dialable potency (deeper IgG/IgA reductions possible) • Improved pharmacodynamics (faster onset of action) • Improved safety profile (fewer side-effects, rapid drug elimination) SEPTEMBER 2023Biohaven | Investor Presentation57 BHV-1300: A highly optimized Biohaven ASGPR binder advancing as drug candidate ü Balances liver removal of unbound to target-bound drug ü Optimizes safety vs efficacy ü Improves kinetics of target removal ü Suitable Target Product Profile for a rapid onset medication with weekly or less frequent SC administration

HepG2 cells internalize IgG-BHV-1300 complexes In vitro BHV-1300 Mediates Removal, Uptake and Degradation of IgG SEPTEMBER 2023Biohaven | Investor Presentation58 1 10 100 1000 0 20000 40000 60000 80000 nM M FI Uptake of IgG in HepG2 EC50 110 BHV-1300 125nM BHV-1300 Untreated Uptake of IgG in HepG2 1 10 100 1000 10000 0 20000 40000 60000 80000 nM M FI Uptake of IgG in HepG2 EC50 99 BHV-1300 1 10 100 1000 0 20000 40000 60000 80000 nM M FI Uptake of IgG in HepG2 EC50 110 BHV-1300

BHV-1300 human IgG depletion in mice, single dose 12 mg/kg BHV-1300 Selected for Remarkable Efficiency in Removal of Exogenously Administered Human IgG in Mouse Screen Approximately 40% IgG removal achieved in 2 hours with a molar ratio of drug-to-target = 1.0 SEPTEMBER 2023Biohaven | Investor Presentation59 0 2 4 6 8 10 0 1×109 2×109 3×109 4×109 5×109 Hours Ig G p g/ m l BHV-1300 human IgG depletion in mice, single dose 12 mg/kg BHV1300 SD, 12 mg/kg +IgG IgG

IgG is Specifically Depleted: IgA and IgM Levels Remain Unchanged SEPTEMBER 2023Biohaven | Investor Presentation60 0 100 200 300 400 0 100 200 300 Hours Ig M % T 0t IgM % change by group 0 mg/kg 10 mg/kg 30 mg/kg 75 mg/kg 150 mg/kg 250 mg/kg • Preliminary BHVN data and literature consistent with an absence of effects on serum albumin, LDL, HDL or triglycerides, and specificity for targeted IgG species • Remarkable drug efficiency in mouse given exogenous IgG recapitulated in monkeys with endogenous IgG. Molar ratio of approximately 1.0 allows 60% IgG lowering following a single dose BHV-1300 administered IV to cynomolgus macaques 0 5 10 15 0 50 100 150 200 Days % Ig G T0 IgG 0 5 10 15 0 50 100 150 200 Days Ig A % T 0t IgA 0 5 10 15 0 50 100 150 200 Days Ig M % T 0t IgMIgG % change by group IgA % change by group IgM % change by group Days

BHV-1300 Administered by SC, IM or IV Routes has Comparable Bioavailability SEPTEMBER 2023Biohaven | Investor Presentation61 Potentially allows at- home, self administration with subcutaneous injection 0 10 20 30 10 100 1000 10000 100000 Hours ng /m l BHV-1300 levels in plasma after a SD of 10 mg/kg in the presence of human IgG BHV-1300 IV 10 mg/kg BHV-1300 SQ 10 mg/kg BHV1300 IM 10 mg/kg F SQ ~ 99% F IM ~ 93% BHV-1300 levels in plasma after a single dose of 10 mg/kg in the presence of human IgG
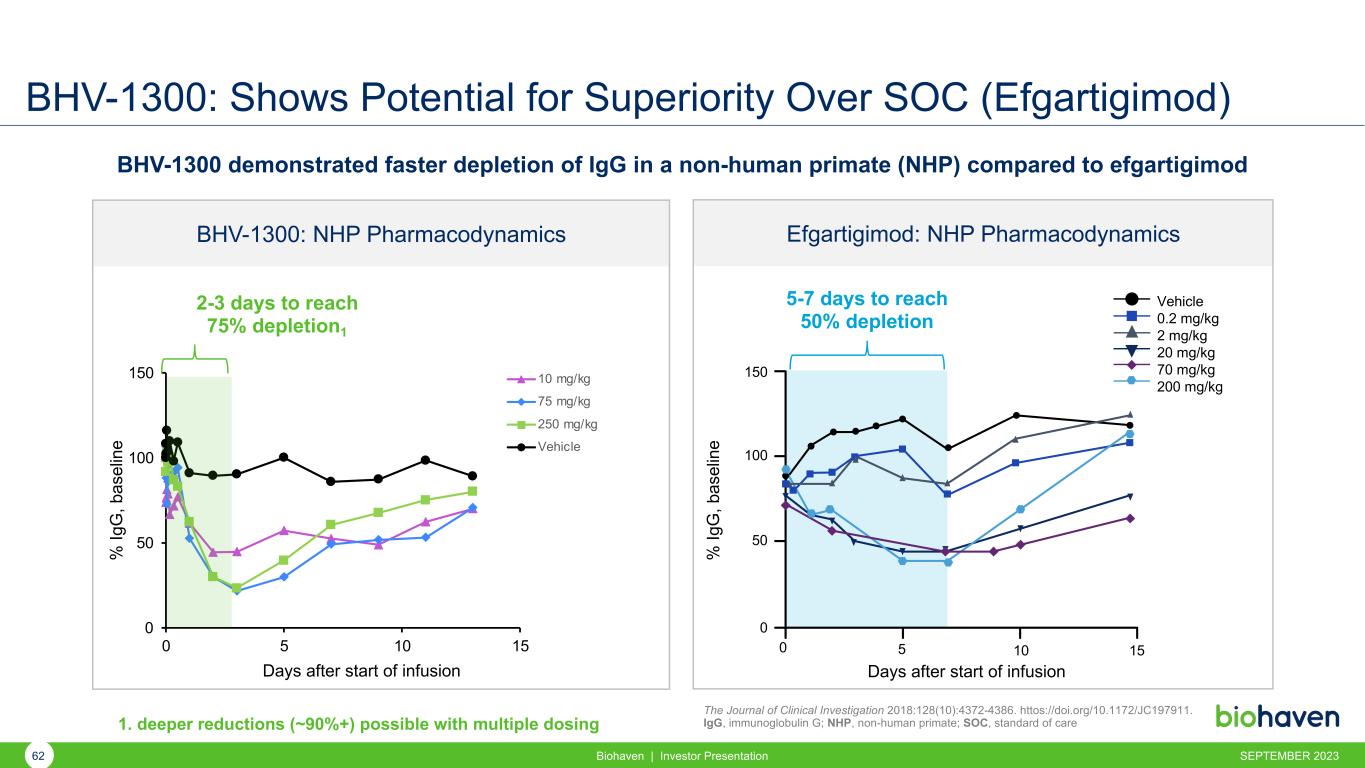
BHV-1300: Shows Potential for Superiority Over SOC (Efgartigimod) Biohaven | Investor Presentation The Journal of Clinical Investigation 2018:128(10):4372-4386. httos://doi.org/10.1172/JC197911. IgG, immunoglobulin G; NHP, non-human primate; SOC, standard of care BHV-1300 demonstrated faster depletion of IgG in a non-human primate (NHP) compared to efgartigimod 5-7 days to reach 50% depletion 0 5 10 15 0 50 100 150 Days after start of infusion Vehicle 0.2 mg/kg 2 mg/kg 20 mg/kg 70 mg/kg 200 mg/kg Efgartigimod: NHP Pharmacodynamics % Ig G , b as el in e 0 50 100 150 0 5 10 15 10 mg/kg 75 mg/kg 250 mg/kg Vehicle BHV-1300: NHP Pharmacodynamics % Ig G , b as el in e Days after start of infusion 2-3 days to reach 75% depletion1 SEPTEMBER 202362 1. deeper reductions (~90%+) possible with multiple dosing

BHV-1300: Repeat dosing allows for deep reductions of over 90% -200 200 400 Day 0 ↓ Day 8 ↓ Day 15 ↓ 0 50 100 150 Hours % c ha ng e / T 0 IgG: % by group:1:200K Control BHV1300, 75mg/kg BHV1300, 250mg/kg BHV1300, 375mg/kg BHV1300, 500mg/kg Day -7 0 Dose BHV-1300: NHP Pharmacodynamics Combined results of multiple experiments suggests repeat dosing of BHV-1300 in cynomolgus can result in over 90% lowering of IgG Biohaven | Investor Presentation Dose 1 Dose 2 Dose 3 Dose 4 Timeframe 63 0 Time SEPTEMBER 2023

BHV-1300: Specific and Rapid Pathogenic Target Removal • Mechanism not expected to cause hypoalbuminemia or dyslipidemia • Improved and optimizable potency for target removal • Deeper target removal when required • Improved pharmacodynamics with faster onset of action than FcRn inhibition • Improved safety profile expected (fewer side effects, rapid drug elimination) FcRn, neonatal Fc receptor; Ig, immunoglobulin; MoDE™, molecular degrader of extracellular proteins BHV-1300 can specifically remove target IgG from circulation faster than FcRn inhibitory antibodies, antibody fragments, or immunosuppressants HepatocyteBHVN degrader SEPTEMBER 2023Biohaven | Investor Presentation64

BHV-1300 DIFFERENTIATED FROM COMPETITORS in FcRn class such as efgartigimod and nipocalimab with a unique MOA, faster onset, and predicted to have improved safety PHASE 2 STUDY Start in 2024 BHV-1300: Has Potential to Add Significant Value Across Rare And Common Diseases With a Differentiated Profile from FcRn Class Rheumatoid Arthritis Systemic Lupus Erythematosus Primary Sjögren’s Lupus Nephritis Comparative programs from FcRn class Generalized Myasthenia Gravis Chronic Inflammatory Demyelinating Polyneuropathy Pemphigus Warm Autoimmune Hemolytic Anemia Immune Thrombocytopenia R ar e D is ea se s C om m on D iseases SEPTEMBER 2023Biohaven | Investor Presentation65

Rheumatoid Arthritis Is a Heterogenous Autoimmune Disorder Marked by Autoantibodies of Various Classes 1. Adapted from Van Delft and Huizinga, An overview of autoantibodies in rheumatoid arthritis; J Autoimmun 2020. 2. UpToDate accessed Jan 2023. At risk Rheumatoid Arthritis Transition to symptomatic RA Pre-symptomatic RA Symptomatic RA H E A L T H Y A R T H R A L G I AS T A R T A U T O I M M U N I T Y Infections / changes microbiome Expansion, epitope spreading etc. Pathogenic autoantibodies Childhood infections / generation autoreactive B cells Lifestyle and environmental factors (smoking, weight, diet, contraceptives) Genetic susceptibility / hormonal factors(HLA, (fe)male hormones, menopause) Without effective and early intervention, inflammation and joint destruction lead to loss of physical function and extremely poor QoL Additional health risks include elevated risk for cardiovascular disease, osteoporosis, and certain types of cancer (e.g., lymphoma) Autoantibodies can start to accumulate 10 years prior to clinical arthritis1 THE ACR/EULAR DIAGNOSTIC CRITERIA INCLUDE2: • 3+ joints involved • Acute phase biomarkers of inflammation including elevated ESR and CRP • Symptom duration • Presence of RF and ACPA IgG+ ACPA in ~70% of RA population with increasing evidence they may be pathogenic in RA SEPTEMBER 2023Biohaven | Investor Presentation66

• Rheumatoid Factor (RF) antibodies are primarily IgM but form immune complexes with IgG • Anti-citrillunated protein antibodies (ACPAs) including anti- cyclic citrullinated peptide-2 (anti-CCP2) are primarily IgG, but some IgA and IgM species exist • All immune complexes can cause damage in joints, connective tissue in many organs, and bone • Comorbidities from cardiovascular to malignancy need to be closely monitored • In patients who are not in remission, status every 4-12 weeks needed to tightly control severity of flares and progression • An IgG degrader could remove a major component of these immune complexes without lowering B cell counts Exact Etiology of RA is Multifactorial With Environmental, Genetic, and T Cell Components, However Autoantibody Presence is a Main Feature Figure adapted from Malmstrom and Gronwall, The parallel worlds of ACPA-positive and RF-positive B cells; Nature Rev Rheum 2018. SEPTEMBER 2023Biohaven | Investor Presentation67

Preclinical Studies Show the Gd-IgA1–Specific MoDE™ Selectively Degrades the Gd-IgA1 Present in IgAN Ab, antibody; EC50, half maximal effective concentration Gd, galactose-deficient; Ig, immunoglobulin; IgAN, IgA nephropathy; MoDE™, molecular degrader of extracellular proteins • Reduces only pathogenic Gd-IgA1 • Spares IgA, limiting immune impacts EC50 Galactose deficient IgA1 0.84 Total IgA 33 Ab binder Anti–Gd-IgA1 antibody BH 3845 Antibody-based MoDE™ removes Gd-IgA1 At low concentrations, this Gd-IgA1–specific MoDE™ selectively degrades Gd-IgA1 and spares total IgA, limiting the impact on the immune system Gd-IgA1–Specific MoDE™ Gd-IgA1–Specific MoDE™ Selectively Degrades Gd-IgA1 SEPTEMBER 2023Biohaven | Investor Presentation68 40X galactose deficient IgA1 total IgA

Greatly Reduced Half-life Compared to Controls Treatment Half-life (h) Antigen-MoDE™ <2 Antigen (Neg. Control) 27.5 PBS 29.7 Study Design Leveraging Known AutoAb Epitope–MoDE™ Shows Rapid Reduction of Pathogenic AutoAb Day -1 0 1 2 3 4 Antibody injection Blood draw Drug injection 0 1 2 3 4 0 20 40 60 80 100 Antigen-MoDE™ (1 mpk) Antigen (1 mpk eq) PBS Days post treatment % a nt ib od y re m ai ni ng AutoAb, autoantibody; MoDE™, molecular degrader of extracellular proteins; PBS, phosphate-buffered saline Rapid, potent depletion with antibody half-life reduced by at least 15-fold Nearly Complete AutoAb Degradation in 4 Hours SEPTEMBER 2023Biohaven | Investor Presentation69

Summary: Biohaven MoDE™ Extracellular Degraders Provide Optionality Subcutaneous or intravenous injection ü Numerous extracellular and circulating targets are involved in pathology and make excellent targets ü IND 2H 2023 for lead program BHV-1300 which has “pipeline in a product” potential • BHV-1300 has optimized chemistry with differentiated mechanism of action compared to standard of care, as well as to other novel agents in development ü Additional programs in development exploring targeting specific autoantibodies and Gd-IgA1 ü Once a target is identified, approximately ~1 year to degrader candidate • Extracellular degrader as fast as 1.5-3 years to IND versus 6-10 years for typical small molecule program Select ligand for valid target, conjugate Dev, discovery tox combined, pharmacology in parallel Bifunctional molecule SEPTEMBER 2023Biohaven | Investor Presentation70

Bispecific Platform: CD38 Targeted Cell Therapy for Multiple Myeloma CD38 Antibody Recruiting Molecule (ARM™) • Modular bispecific molecules with two moieties, each designed for non-covalent binding to a specific target • Redirect endogenous antibodies to target cancerous or virally infected cells for immune destruction Biohaven | Investor Presentation71 Key Potential Advantage Over Biologics ü Lower manufacturing cost ü More versatile — smaller and tunable ü Faster and less expensive to develop ü Better safety and efficacy • Non-immunogenic; better dosing • Enhanced PK properties • Reduced NK cell fratricide compared to daratumumab ARM BHV-1100 CD38 Targeting Therapy Mult iple Mye lom a Cell Multipl e Myelo ma Cell Multiple Myeloma CellNK Cell BHV-1100 + CIML NK cell CD38 ARM™ Binds to CD38 receptor on the surface of multiple myeloma cells SEPTEMBER 2023
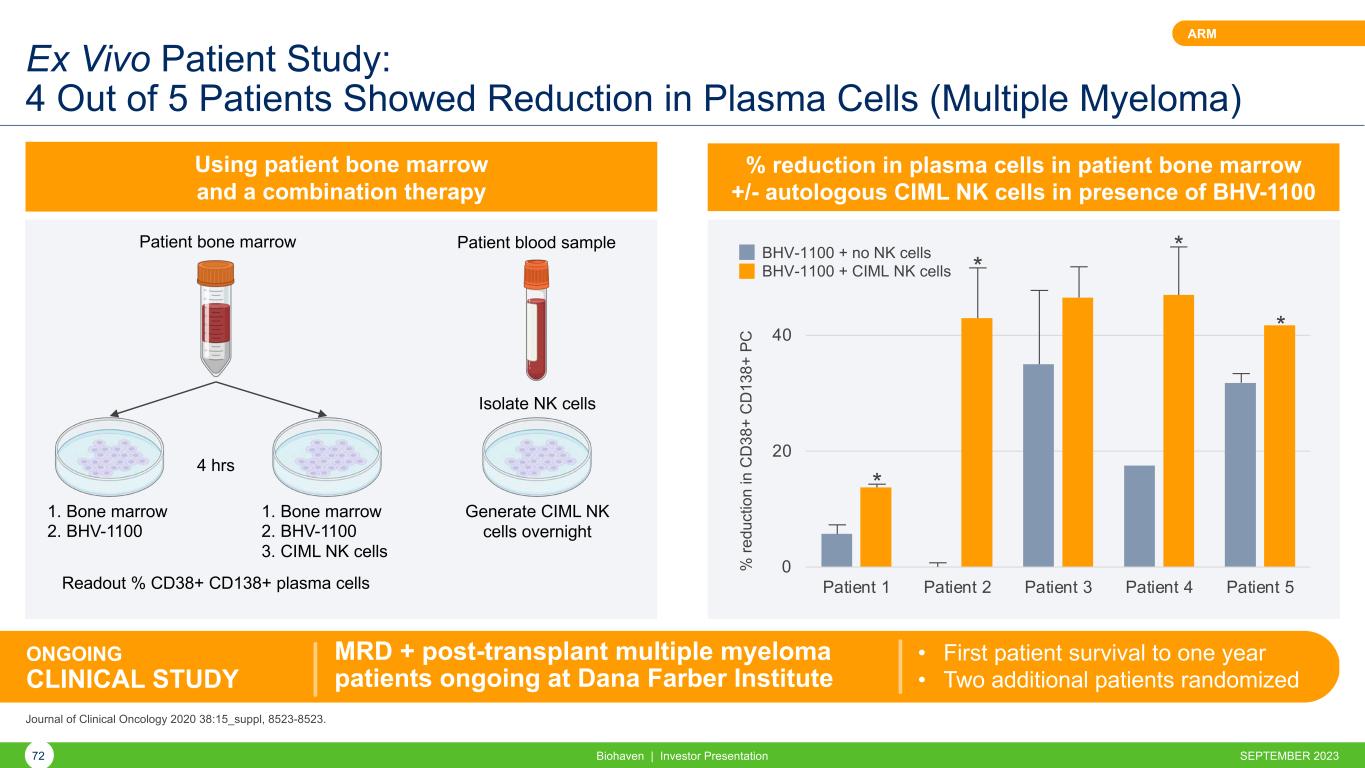
Ex Vivo Patient Study: 4 Out of 5 Patients Showed Reduction in Plasma Cells (Multiple Myeloma) Biohaven | Investor Presentation72 ARM Using patient bone marrow and a combination therapy % reduction in plasma cells in patient bone marrow +/- autologous CIML NK cells in presence of BHV-1100 0 20 40 Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 % re du ct io n in C D 38 + C D 13 8+ P C * * * * Patient blood samplePatient bone marrow Isolate NK cells 1. Bone marrow 2. BHV-1100 4 hrs 1. Bone marrow 2. BHV-1100 3. CIML NK cells Readout % CD38+ CD138+ plasma cells Generate CIML NK cells overnight ONGOING CLINICAL STUDY MRD + post-transplant multiple myeloma patients ongoing at Dana Farber Institute • First patient survival to one year • Two additional patients randomized Journal of Clinical Oncology 2020 38:15_suppl, 8523-8523. BHV-1100 + no NK cells BHV-1100 + CIML NK cells SEPTEMBER 2023

Second-generation ADC-linker technologiesBiohaven third-generation technology DISCOVERY RESEARCH Bispecific Platform: Advancing Next-Generation, Site-Specific Antibody Drug Conjugates (ADCs) Potential for best-in-class A single residue per heavy chain is available for conjugation • Controlled DAR* ratio is critical to therapeutic index • MATE tech precisely defines DAR* Improved linker stability should yield wider therapeutic index • Improved safety: less systemic, untargeted payload • Improved efficacy: targeted payload delivered to tumor Uses native antibody: potentially improved CMC vs. current tech N N N N O O O Payloadn n O O O Payload N H Click chemistry to engineered antibody Biohaven | Investor Presentation73 *DAR = Drug antibody ratio 0 0.1 0.2 0.3 0.4 0.5 1 2 3 4 5 6 Fr ac tio n La be lle d Residue Peptide Mapping (typical cartoon) 0 0.2 0.4 0.6 0.8 1 1 2 3 4 5 6 Fr ac tio n La be lle d Residue Peptide Mapping (typical cartoon) SEPTEMBER 2023

Biohaven’s Next-Generation Site-Specific ADCs ü ADAPTABLE Complements and improves multiple existing ADC payload-linker technologies ü STABLE Improved ADC plasma stability with controlled DAR potentially improves therapeutic index ü EFFECTIVE Improved efficacy in mouse tumor model also suggests potential for increased therapeutic index ü MULTIPURPOSE Conjugates IgG1, 2 & 4 and manufacturable: Single step conjugation with predictable good yields, low aggregation ü NOVEL IP filed globally in key markets CONJUGATION CHEMISTRY SUPERIOR TO INDUSTRY STANDARD maleimide and lipophilic click chemistry Attachment to two specific lysines provides stable and consistent drug antibody ratio (DAR) SEPTEMBER 2023Biohaven | Investor Presentation74

• Nearly all existing methods involve extensive antibody manipulation or engineering • Potential impact on activity, clearance, immunogenicity, and COGs • Drug linkage can reverse over time, “leaking” free payload Challenges of Alternate ADC Protein Engineering and Chemistry N Payload S O O N Payload O O DAR, drug antibody ratio • High DAR species can cause CMC issues like aggregation, in vivo instability leading to toxicity • Heterogeneity complicates CMC, may compromise efficacy Kadcyla® 0 20 40 60 80 100 0 1 2 3 4 5 6 7 8 % T ot al A b Number of Drugs/Antibody Conjugation through lysine residues 0 20 40 60 80 100 0 1 2 3 4 5 6 7 8 % T ot al A b Number of Drugs/Antibody Conjugation through reduced inter-chain disulfide bonds 0 20 40 60 80 100 0 1 2 3 4 5 6 7 8 % T ot al A b Number of Drugs/Antibody Conjugation through engineered cysteine residues DAR 2–4 Millions >100 One Adcetris® SEPTEMBER 2023Biohaven | Investor Presentation75

N N N N O Payload Click chemistry to engineered antibody Undesirably large, lipophilic linkage Biohaven chemistry Stable, physicochemically benign amide linkage O Payload N H Potential “Best-in-class” Site-specific ADCs N PayloadS O O Industry standard maleimide Poorly stable linkage IMPROVED LINKER STABILITY predicted to improve therapeutic index ü Improved safety: Reduced untargeted payload in systemic circulation driving toxicity ü Improved efficacy: Increased targeted payload reaches tumor, higher doses possible USES NATIVE ANTIBODY Likely improved CMC vs. current site-specific technologies ADCs prepared based on Adcetris® SEPTEMBER 2023Biohaven | Investor Presentation76

Time, h Mouse Time, h % C ha ng e Human Time, h MouseHuman % C ha ng e RT 37C RT 37C Time, h BH3973: Improved Plasma Stability Over Adcetris® • ADC toxicity/tolerability directly relates to free payload • Enhanced stability reduces free payload, and potentially allows for higher drug concentration at targeted tumor site for same tolerability Temperature, Conc. 37C, High 37C, Low RT, High RT, Low Adcetris® Biohaven BH3973 SEPTEMBER 2023Biohaven | Investor Presentation77

Adcetris® and BH3973 Demonstrate Comparable PK • BH3973 half-life 7-8 days across all doses • Adcetris® half-life 5-6 days across all doses BH3973 Adcetris® SEPTEMBER 2023Biohaven | Investor Presentation78
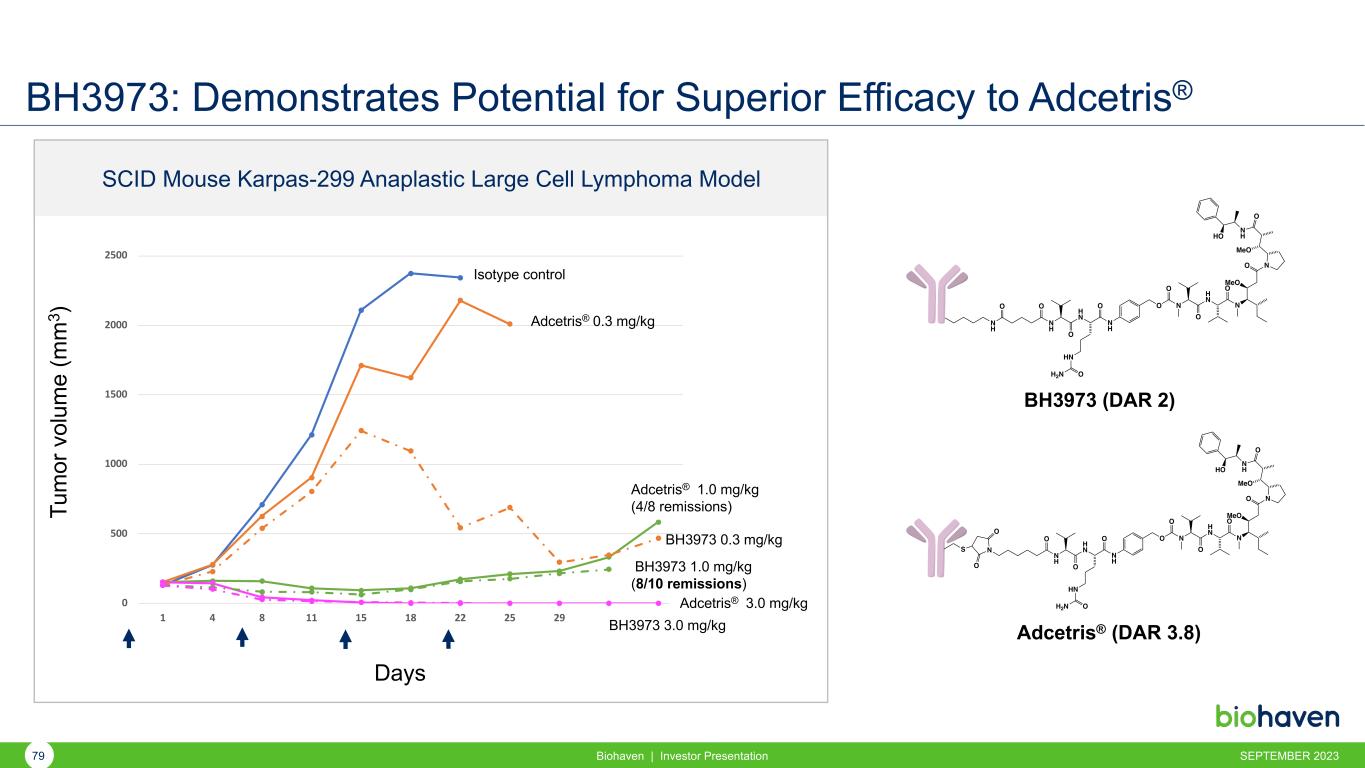
BH3973: Demonstrates Potential for Superior Efficacy to Adcetris® Biohaven | Investor Presentation 0 500 1000 1500 2000 2500 1 4 8 11 15 18 22 25 29 32 37BH3973 3.0 mg/kg Adcetris® 0.3 mg/kg BH3973 0.3 mg/kg Tu m or v ol um e (m m 3 ) Days Isotype control Adcetris® 3.0 mg/kg BH3973 1.0 mg/kg (8/10 remissions) N H N H O H N O HN H2N O N H O O N O O H N O N O MeO NO MeO N H O HO BH3973 (DAR 2) Adcetris® (DAR 3.8) N H O H N O HN H2N O N H O O N O H N O N O MeO NO MeO N H O HO NS O O SCID Mouse Karpas-299 Anaplastic Large Cell Lymphoma Model Adcetris® 1.0 mg/kg (4/8 remissions) SEPTEMBER 202379

BH3973: Improves Survival in Preclinical Model Compared to Adcetris® With Half the Payload Biohaven | Investor Presentation 0 5 10 15 20 25 30 35 40 45 50 55 60 0 10 20 30 40 50 60 70 80 90 100 Effect of Treatment on Survival (Dose @ 0.3 mg/kg + Controls) Study Day Su rv iv al (% ) No Treatment BH3973 0.3 mg/kg Vehicle Adcetris 0.3 mg/kg 0 5 10 15 20 25 30 35 40 45 50 55 60 0 10 20 30 40 50 60 70 80 90 100 Study Day Su rv iv al (% ) Effect of Treatment on Survival (Dose @ 1.0 mg/kg + Controls) No Treatment BH3973 1.0 mg/kg Vehicle Adcetris 1.0 mg/kg Effect of Treatment on Survival (Dose @ 0.3 mg/kg + Controls) Effect of Treatment on Survival (Dose @ 1.0 mg/kg + Controls) SEPTEMBER 202380

BH3973: Summary üExisting, highly effective ADC formats such as Adcetris® and optimized warheads may potentially be enhanced with improved safety, efficacy, manufacturability and patent life üDifferentiated in vivo efficacy and safety results of BH3973 compared to Adcetris® üBroad patent coverage BIOHAVEN’S ADC TECHNOLOGY IS AN IDEAL ADD-ON TO IN-HOUSE DEVELOPED UNIQUE ANTIBODIES, BISPECIFICS Even competitor molecules SEPTEMBER 2023Biohaven | Investor Presentation81

Capitalization Considerations CGRP royalties: Pfizer will make royalty payments in low- to mid-teens% in respect of annual U.S. net sales of rimegepant and zavegepant >$5.25B, subject to annual cap ($400M/year)3 ~$349.0 million2 SHARE O/S CASH & MARKETABLE SECURITIES POTENTIAL ROYALTIES 1. Excludes outstanding options. 2. As of July 27, 2023. 3. Cap reached if aggregate annual U.S. net sales of rimegepant and zavegepant amount to $8.15B. Royalty payments would be in respect of years ended on or before 12/31/40. Biohaven | Investor Presentation82 68.3 million1 SEPTEMBER 2023

INDICATIONS 1H 2023 2H 2023 2024 BHV-7000 Kv7 Channel Activator Focal Epilepsy Bipolar Disorder BHV-7010 Kv7 Channel Activator Epilepsy and Mood Disorders BHV-2100 TRPM3 Chronic Pain Disorders BHV-8000 TYK2/JAK1 Neuroinflammatory Disorders Troriluzole NCE Prodrug of Riluzole Obsessive-Compulsive Disorder Taldefgrobep alfa Anti-Myostatin Adnectin Spinal Muscular Atrophy Metabolic Disorders BHV-1300 IgG Degrader Immune-Mediated Diseases Gd-IgA1 Degrader IgA Nephropathy Submit IND Anticipated Near-Term Milestones SEPTEMBER 2023Biohaven | Investor Presentation83 Milestone achieved Complete Enrollment Initiate Phase 3EEG Biomarker DataPhase 1 Topline Initiate Phase 3 Submit IND Initiate Phase 1 Initiate Phase 2 — PD Complete Enrollment Initiate Phase 3* * Planning in progress | PD, Parkinson’s disease Submit IND / Start Phase 1 Initiate Phase 2 File IND