UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer Pursuant to Rule
13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the Month of August, 2023
Commission File Number: 001-37353
BIONDVAX PHARMACEUTICALS LTD.
(Translation of registrant’s name into English)
Kiryat Hadassah, Building 1, JBP
Jerusalem, Israel 911200
(Address of principal executive office)
Indicate by check mark whether the registrant files
or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
EXPLANATORY
NOTE
BiondVax
Pharmaceuticals Ltd. (the “Company”) has made available an updated presentation about its business, a copy of which
is furnished herewith as Exhibit 99.1 and incorporated by reference. The presentation includes, among others, additional information
about the in vivo proof of concept conducted with the Company’s inhaled anti-COVID-19 nanoAb and the value proposition of the Company’s
anti-IL-17 nanoAb. The new updates in the Presentation are not an admission as to the materiality of any information therein.
The information contained in the presentation is summary information that should be considered in the context of the Company’s
filings with the Securities and Exchange Commission and other public announcements the Company may make by press release or otherwise
from time to time.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
BiondVax Pharmaceuticals Ltd. |
| |
|
|
| Date: August 23, 2023 |
By: |
/s/ Amir Reichman |
| |
|
Amir Reichman |
| |
|
Chief Executive Officer |
2
Exhibit 99.1

N o n c o n f i d e n t i al p r e s e n t at i o n | A u g u s t 22, 2023

This communication contains forward - looking statements within the meaning of the Private Litigation Reform Act of 1995 . Words such as “expect,” “believe,” “intend,” “plan,” “continue,” “may,” “will,” “anticipate,” and similar expressions are intended to identify forward - looking statements . All statements, other than statements of historical facts, included in this communication regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward - looking statements . Examples of such statements include, but are not limited to, statements regarding the potential of nanobodies ; statements regarding the commercial potential of our Contract Development and Manufacturing Organization (CDMO) and the capabilities and potential of our manufacturing facility in Jerusalem . These forward - looking statements reflect management’s current views with respect to certain current and future events and are subject to various risks, uncertainties and assumptions that could cause the results to differ materially from those expected b y the management of BiondVax Pharmaceuticals Ltd . Risks and uncertainties include, but are not limited to, the risk that the potential of the nanobodies will not be met, including commercial potential ; the risk that the commercial potential of the CDMO will not be met ; the financial projections contained in this communication will not be accurate ; [we may not receive regulatory approval of BiondVax’s manufacturing facility, if at all or when required] ; the lease for the manufacturing facility will not be renewed when the current term expires ; it may be difficult to find qualified employees and sub - contractors to operate the manufacturing facility ; the manufacturing facility will not be able to be used for a wide variety of applications and other vaccine and treatment technologies ; and drug development involves a lengthy and expensive process with uncertain outcomes . More detailed information about the risks and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Company's Annual Report on Form 10 - K filed with the Securities and Exchange Commission on April 17 , 2023 . BiondVax undertakes no obligation to revise or update any forward - looking statement for any reason . SAFE HARBOR STATEMENT

Amir Reichman MSc, MBA | CEO Senior leadership positions at GSK Vaccines, Belgium; Global pharmaceutical engineering & supply chain leadership, including large capital expenditure projects building vaccines manufacturing sites in Belgium, Italy, Germany, Hungary and USA. Dr. Tamar Ben - Yedidia PhD | Chief Science Officer (CSO) Co - invented and guided BiondVax’s previous recombinant protein vaccine candidate through 8 clinical trials including pivotal Phase 3 in 12,000+ participants. PhD (Weizmann Institute of Science). Elad Mark BSc(Eng), MBA | Chief Operating Officer (COO) Principal process engineer with biotechnology industry experience encompassing diverse project stages including feasibility studies, conceptual and detailed design, commissioning, qualification, and process validation. Led scale - up, tech transfer, manufacturing of recombinant proteins in China, mAbs for Novartis Singapore. Dr. Dalit Weinstein Fischer PhD | VP Technical R&D Extensive cGMP operational experience in biological process development and production . Prior to joining BiondVax, Mr . Mark led Novartis’s $ 800 million investment in a biologics facility in Singapore focused on drug substance manufacturing based on cell culture technology . PhD Molecular Genetics and Microbiology (Hadassah M . S) EXECUTIVE LEADERSHIP : DEEP PHARMA EXPERIENCE

20+ years of recombinant protein process development from bench to Phase 3; cGMP manufacturing from preclinical through Phase 3 clinical trials; Startups and big pharma leadership, in USA, Israel, Europe, China, and Singapore; Decades of accumulated knowledge in process development, analytical method development, QA and QC, targeting commercial scale economics and viability. Barry Cohen, BSc Technical R&D Downstream Process Team Leader Production processes development and protein purification from laboratory to GMP conditions. Navah Figov, MSc Technical R&D Downstream Process Team Leader Expertise in downstream process including both developing purification and scale - up processes for GMP pharmaceutical manufacturing Merav Kamensky, MSc Head of Quality Control Biopharma QC and biological analytical method qualification experience Dr. Oded Ovadia, PhD Director of Analytical Methods & Preclinical Trials Experienced in analytical development of biosimilar and innovative biomolecules Dr. Tehila Sonnenfeld, PhD Director of Production Extensive experience in aseptic production under GMP conditions Alona Tal, MSc & Dr. Naama Adi Hen, PhD Quality Assurance Sr. Managers Maxim Leykin BSc (Eng) Head of Engineering Engineering project management and maintenance leadership with Omrix, Intel, SodaStream Zohar Gadri, MSc Technical R&D Upstream Process Team Leader Production processes development and protein purification from laboratory to GMP conditions T OP TIER LEADERSHIP TEAM

NeuroDerm Ltd (Senior Scientist), Novartis Vaccines USA (R&D and Global Supply chain), GSK Vaccines Belgium (Global Supply Chain and Global Engineering) Amir Reichman, MBA CEO BioLineRx (CEO, Director), OurCrowd (Partner), Clil Medical (CEO), Vital Spark (CEO), Kitov Pharmaceuticals (Co - founder, Director) Morris C. Laster, MD Director Gamida Cell Ltd. (Nasdaq: GMDA) (President, CEO, Director), Denali Ventures LLC (VP) Yael Margolin, PhD External Director Biodar (CEO), Rodar (Founder) Avner Rotman, PhD Director Aentib Group (Managing Director). Founder, director, chairman, and/or investor in over 20 biotech companies Mark Germain Chairman Bristol Myers Squibb (NYSE: BMY) (Senior Vice President, Corporate Strategy) Samuel Moed Director Experienced in Wall Street investment banking; Capacity Funding LLC (Principal) Adi Raviv, MBA External Director Glaxo SmithKline (NYSE: GSK) Global Vaccines (Senior Vice President Finance and CFO), Gavi (Advisor for COVAX) Jay Green External Director North America Israel I NTERNATIONALLY RECOGNIZED B OARD OF D IRECTORS

MAX PLANCK & BIONDVAX COLLABORATION Designed to create significant clinical and commercial advantages • World - class science & access to leading scientists • NanoAb platform for development of promising potent therapeutics • Patents covering NanoAbs & their manufacturing MAX PLANCK & UMG 1 BIONDVAX • Recombinant protein drug development experience from lab to Phase 3 clinical trial • Manufacturing, quality, international regulatory experience • GMP biologics manufacturing facility • Best in class equipped labs • Top - tier big pharma & biotech leadership expertise 1. Max Planck Institute for Multidisciplinary Sciences and the University Medical Center Göttingen (UMG) PROF. DR. DIRK GÖRLICH • Director of Max Planck Institute for Multidisciplinary Sciences • Winner of inaugural World Laureates Association (WLA) Prize in Life Sciences or Medicine PROF. DR. MATTHIAS DOBBELSTEIN • Fellow at Max Planck Institute for Multidisciplinary Sciences • UMG Head of Department

THE TECHNOLOGY : N ANO A BS * Also trademarked by ABLYNX N.V., a wholly owned subsidiary of Sanofi, as Nanobody. BiondVax has no affiliation with and is not endorsed by Sanofi. HU M AN A N T I BO D Y A L P A C A - D ERI V E D N A N O A B Alpaca - derived antibody (V H H) fragments (NanoAbs)*

PLATFORM VALUE PROPOSITION NanoAbs ’ unique physicochemical attributes can generate multiple crucial advantages vs human monoclonal antibodies (mAbs) Manufacturing • X10 active pharmaceutical ingredient (API) per gram of manufactured protein vs. mAbs • Faster and lower cost production in yeast (pichia) vs mammalian cells R&D • Quicker antibody discovery and optimization due to massive libraries • De - risked pipeline development leveraging approved mAb targets Product • Hyper - thermostable = longer shelf life, easier storage & distribution • Superior specificity & affinity to target potentially enables lower dose, fewer adverse events, lower cost • Adaptable half life Patient Safety & Convenience • Multiple, easier routes of administration • Lower immunogenicity • Fewer contraindications • Potentially safer & lower dose

ME CH A N I S M OF A CT I ON COMPOSITION O F M A TTE R COMME R CI A L ض ض Validated by existing but sub - optimal mAb therapies Well understood Assessing safety & efficacy of alpaca - derived NanoAbs TBD + Solid demand for available mAbs, and underserved populations NanoAbs feature a favorable path to market compared to risks associated with traditional drug development S O UR C E O F R I S K MOL E CU L A R T A R G ET NANO AB VALIDATED THERAPEUTIC USE First commercial VHH - antibody is blood disorder therapy Caplacizuma – by Ablynx, a company acquired by Sanofi in 2018 for $4.8B DERISKED DRUG DEVELOPMENT

PIPELINE MOLECULAR TARGETS Psoriasis, PSA, Atopic Dermatitis, HS Asthma, Atopic Dermatitis Wet AMD IL - 17A IL - 17F IL - 17A/F IL - 4Ra IL - 13 TSLP VEGF - A ANG - 2 HETERODIMER COVID - 19 RBD • Single compound targeting IL - 17A and IL - 17F and IL - 17AF • Novel local use • Novel and larger target population for biologicals • Asthma ~ quick time to POC • Antibodies targeting IL - 13 and TSLP, have large commercial potential • Targets well - validated • Development competition limited • Large commercial opportunity • Strong in vivo data for inhaled therapeutic and prophylactic

Clinical Phase 1/2 Toxicology In - Vitro/In vivo proof - of - concept Manufacturing Process & Analytical Method Development Drug discovery (Max Planck) Molecular Target (by priority) Indication Clones generated VHH antibody fragment selected Alpacas immunized Ready for partnering RBD COVID - 19 therapeutic Ready for partnering RBD COVID - 19 prophylactic Est. H2’24 Est. H1’24 IL - 17A, F, AF Psoriasis, PSA, Atopic Dermatitis, HS Est. 2025 IL - 4Ra Asthma, Atopic Dermatitis Est. 2025 IL - 13 Est. 2025 TSLP TBD VEGF - A Wet AMD TBD ANG - 2 PIPELINE DEVELOPMENT STATUS & PLANS Q 3 2 0 2 3 Est. = Estimated timing
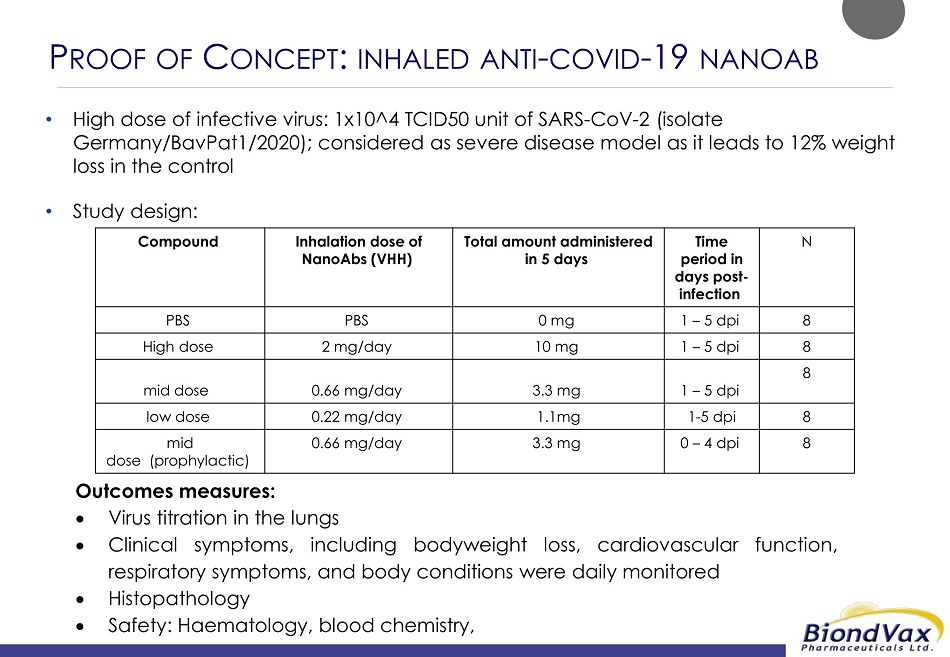
P ROOF OF C ONCEPT : INHALED ANTI - COVID - 19 NANOAB • High dose of infective virus: 1x10^4 TCID50 unit of SARS - CoV - 2 (isolate Germany/BavPat1/2020); considered as severe disease model as it leads to 12% weight loss in the control • Study design: N Time period in days post - infection Total amount administered in 5 days Inhalation dose of NanoAbs (VHH) Compound 8 1 – 5 dpi 0 mg PBS PBS 8 1 – 5 dpi 10 mg 2 mg/day High dose 8 1 – 5 dpi 3.3 mg 0.66 mg/day mid dose 8 1 - 5 dpi 1.1mg 0.22 mg/day low dose 8 0 – 4 dpi 3.3 mg 0.66 mg/day mid dose (prophylactic) Outcomes measures: Virus titration in the lungs Clinical symptoms, including bodyweight loss, cardiovascular function, respiratory symptoms, and body conditions were daily monitored Histopathology Safety: Haematology, blood chemistry,

INHALED ANTI - COVID - 19 NANOAB PROTECTED HAMSTERS FROM SEVERE DISEASE Weight loss of infected animals. Data are shown as mean “ SD. Statistical analysis using ANOVA with Šídák's multiple comparisons test with statistically significant difference between control and experimental group on days 3 - 6 Day 3 post - infection: Control vs. Prophylactic mid dose: ***0.0004 Prophylactic mid dose vs. Therapeutic mid dose: * 0.0452 Prophylactic mid dose vs. Therapeutic low dose: **0.0057 Day 4 post - infection: Control vs. Therapeutic high dose: *0.0425 Control vs. Prophylactic mid dose: ***0.0002 Prophylactic mid dose vs. Therapeutic low dose: **0.0063 Day 5 post - infection: Control vs. Therapeutic high dose: *0.0219 Control vs. Prophylactic mid dose: ****<0.0001 Control vs. Therapeutic mid dose: **0.0041 Control vs. Therapeutic low dose: **0.0017 Therapeutic high dose vs. Prophylactic mid dose: *0.0161 Prophylactic mid dose vs. Therapeutic mid dose: **0.0037 Prophylactic mid dose vs. Therapeutic low dose: ***0.0002 Day 6 post - infection: Control vs. Therapeutic high dose: ***0.0005 Control vs. Prophylactic mid dose: ****<0.0001 Control vs. Therapeutic mid dose: ****<0.0001 Control vs. Therapeutic low dose: ***0.0003 Prophylactic mid dose vs. Therapeutic low dose: *0.0400

INHALED ANTI - COVID - 19 NANOAB VIRTUALLY ELIMINATED VIRAL LOAD IN THE LUNGS Virus quantification, a measure of viral titer per gram lungs by: TCID50 (left) & PCR (RNA copies, right) Over a week after infection, compared to placebo group, hamsters treated with BiondVax’s NanoAb had over 30 times lower SARS - COV - 2 viral titers in their lungs 10 1 10 2 10 3 10 4 10 5 10 6 ٓٓٓٓ Titer [TCID 50 /g] limit of detection ٓٓٓٓ ٓٓٓٓ ٓٓٓٓ * p<0.05; ** p<0.01; ***p<0.001, ****p<0.0001 9 8 7 6 5 4 3 2 1 SARS - CoV - 2 RNA copies/ml BALF ٓٓ ٓٓٓٓ ٓٓ ٓ detection limit

NEXT NANOAB: ANTI - I NTERLEUKIN 17 A/F Large and growing psoriasis market with underserved needs The opportunity: • Current biologics and new oral treatments are systemic, not topical • Recently developed systemic treatments target mostly severe psoriasis, which is 15% of the psoriatic patients (due to costs and risk vs. benefit for patients) • Mild to moderate psoriatic patients need a local treatment that is highly efficacious, specific and hence safe to use. 8.6M US patients + another 8M EU €9B Biologics market in 2020 growing at 7% CAGR €4 - 5B Market estimate for IL - 17s Abs in 2026 Current standards of care: • Topical (steroids, vitamin D analogues, retinoids) • Phototherapy • Systemic • Traditional systemics (e.g. methotrexate, acitretin, and ciclosporin) • Injected biologics (mAbs) • Oral (including Otezla, Xeljanz, Sotyktu)

W HY AN ANTI - IL - 17 NANO A B ? • IL - 17 as a target achieved a high score by L.E.K consulting triaging over 800 potential targets for nanoAbs: • Relative development risk: Medium to low • Strong scientific evidence for efficacy by mAbs • Time to PoC: 1Y for pre - clinical, 2Y for clinical PoC • Small size Phase 3 trial: 350 - 400 patients • Time to market: 4 - 5Y • BiondVax aims to develop ID or topical formulation to increase patient compliance • Target population: mild to moderate patients (85% of patients) • While IL - 17A and F as targets have been validated by different mAbs, our single NanoAb can target IL - 17 forms A, F, and AF complex • High affinity of the selected NanoAb (at the range of pM) • Multiple indications: Psoriasis, HS, PSA & Ankylosing Spondylitis • Easier, lower cost manufacturing as compared to Bimekizumab • Patent submitted BiondVax’s anti - IL - 17 NanoAb exhibits several key advantages IL - 17 plays a key role in the development of psoriatic lesions, which are in essence an acute, local (dermis) flareup of a chronic underlying disease. It allows for local (rather than systemic) administration of anti – IL - 17 drugs to stop the cytokine cascade.

ANTI - IL - 17 NANOAB FOR PSORIASIS M O L EC UL A R T A RG ET M E C HANI S M OF A CT I ON COMPOS I T I ON O F M A TTE R COMME R CI A L Attractive market potential, proven target, short time to POC Validated by existing therapies: Cosentyx, Siliq, Taltz and bimekizumab Moonlake’s (MLTX) Sonelokimab Nanobody “demonstrated rapid & durable clinical response” in Phase 2b trial Solid demand for available mAbs with massive underserved populations ض ض + TBD Well understood BiondVax’s Advantage BiondVax MoonLake (Nasdaq: MLTX) ✓ Simpler to manufacture 1 NanoAb 2 nanobodies to target IL - 17A and IL - 17F Composition of matter ✓ Address 85% of population Mild to moderate patients Moderate to severe patients Target Pop ✓ Anticipated painless, side effects, safer, less Directly to lesion (intradermal) Systemic (subcutaneous) Administration MoonLake: Hasn’t announced Phase 3 plans; BiondVax moving quickly Preclinical Phase 2b Development stage

GMP MANUFACTURING AND R & D LABORATORIES • Analytical methods development combined with best - in - class QC capabilities and equipment • Labs for manufacturing process development and scale up allow for implementation of quality by design and design of experiment principles • GMP suites for up stream fermentation, down stream purification, media and buffer preparations, formulation and aseptic automated filling of PFS & vials • Designed to meet FDA and EMA regulatory standards • Single - use equipment enables: • Adaptable manufacturing processes for a pipeline of different products • Quicker lead times • Faster time - to - market for new products Industry standard aseptic facility: Labs, clean rooms, warehouse, offices BiondVax’s 1850m 2 GMP Biologics Manufacturing Facility | Jerusalem

CDMO SERVICES De - risking R&D investments by leveraging internal capabilities While NanoAb pipeline development is our core focus, we offer CDMO services, leveraging our cGMP manufacturing facility, aseptic fermentation and fill and finish suites, R&D and QC laboratories, and experienced professionals. Our CDMO services may support: • Pharmaceutical and biotech companies • Alternative protein food tech companies • Process development, analytics and manufacturing for clinical trials A SEP TI C G MP M A N U F A C T U R I N G SU I TES STA TE - O F - TH E - A R T R & D A N D Q C L A B O R A T O R I E S P HA RM A C MC E XP E RIE N CE More info biondvax.com/cdmo

SELECT FINANCIALS & CAP TABLE • $7.6M cash as of June 30, 2023 ADS OUTSTANDING CAP TABLE As of July 31, 2023 3,651,927 Ordinary ADS 675,000 Pre - funded ADS 4,3 26 , 927 Primary shares out 4,700 $5 Warrants (Expire 20 Dec 2023) 1,600,000 $5 Warrants (Expire 22 Dec 2025) 277,448 ESOP BVXV 20 €24M European Investment Bank (EIB) loan payable Dec 31, 2027.

B I ON DV A X . C OM Contact: Joshua Phillipson, Investor Relations j.phillipson@biondvax.com +972.8.930.2529 N A SD A Q : BV XV | A ug us t 2023
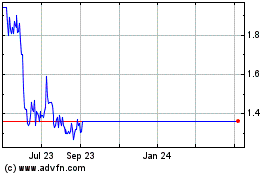
BiondVax Pharmaceuticals (NASDAQ:BVXV)
Historical Stock Chart
From Mar 2024 to Apr 2024
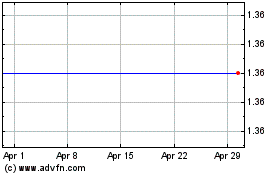
BiondVax Pharmaceuticals (NASDAQ:BVXV)
Historical Stock Chart
From Apr 2023 to Apr 2024