UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10
(Amendment #4)
GENERAL
FORM OF REGISTRATION OF SECURITIES
Pursuant
to Section 12(b) or (g) of the Securities Exchange Act of 1934
NOVELSTEM
INTERNATIONAL CORP.
(Exact
name of registrant as specified in its charter)
| Florida |
|
65-0385686 |
(State
or other jurisdiction
of
incorporation or organization) |
|
(I.R.S.
Employer
Identification
No.) |
| 2255
Glades Road, Suite 221A, Boca Raton, FL |
|
33431 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
| Registrant’s
telephone number, including area code |
(410)
654-3315 |
Securities
to be registered pursuant to Section 12(b) of the Act:
| Title
of each class to be so registered |
|
Name
of each exchange on which each class is to be registered |
| None |
|
None |
Securities
to be registered pursuant to Section 12(g) of the Act:
| Common
Stock, par value $0.01 per share |
| (Title
of Class) |
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filed, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer ☐ |
Accelerated
filer ☐ |
| Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
Emerging
growth company ☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Item
1. Business.
NovelStem International Corp.
(“NovelStem” or the “Company”) is a development stage biotechnology holding company focused on the
stem cell-based technology developed by its affiliate, NewStem Ltd, an Israeli biotech company (“NewStem”), in which
the Company owns an approximate 31% equity interest. NovelStem was formed in January 1993 as Big Entertainment, Inc. Thereafter,
the Company changed its name to Hollywood.Com Inc. and, later to Hollywood Media Corp. (“Hollywood Media”).
In 2018, the Company shifted
its business focus from media to cutting edge biotech when it acquired a substantial ownership interest in NewStem, and changed its name
to NovelStem. As a significant shareholder in NewStem, and the substantial commitment of our management and financial resources to NewStem,
including the fact that our Executive Chairman, Jan Loeb, is also the Chairman of NewStem, we have the ability to exert significant influence
over the management and operations of NewStem resulting in NewStem functioning as a minority operating subsidiary of the Company. Since
his appointment in July 2018, Mr. Loeb has acted in an executive capacity on behalf of the Company and has served in a de facto
leadership role. In September 2022, the Board appointed Mr. Loeb as Executive Chairman of NovelStem in order to ratify Mr. Loeb’s
position and clarify his executive role. On January 13, 2023, the Board appointed Mr. Loeb as President. With respect to NewStem, Mr.
Loeb, as the Chairman, calls and presides over the meetings of NewStem’s Board of Directors. Additionally, Mr. Loeb leverages his
financial expertise by guiding NewStem’s financial and strategic planning, including the raising and deployment of capital, developing
and modifying NewStem’s business plan and budget and by participating in the negotiation of NewStem’s material contracts
as required. NewStem does not currently have an appointed Chief Financial Officer and, as such, Mr. Loeb serves as the de facto
Chief Financial Officer and Chief Strategic Officer of NewStem
NovelStem depends entirely on
earnings and cash from its investments in NewStem and our 50% equity interest in a legacy joint venture named NetCo Partners (“NetCo”
or “NetCo Partners”). The Company’s principal operations coincide with those of NewStem. We have not received
any dividend payments or other distributions from NewStem in the fiscal years ended December 31, 2021 and 2020 or the nine months
ended September 30, 2022. We received distributions of earnings from NetCo of $21,290 and $9,375, respectively, for the fiscal
years ended December 31, 2021 and 2020. We have not received any distributions of earnings from NetCo during the nine months ended
September 30, 2022.
NewStem
NewStem
is a development stage Israeli biotech limited liability company focused on human Pluripotent Stem Cells (hPSCs) in general, and Haploid
human Pluripotent Stem Cells (HhPSCs), in particular. These cells have the potential to change the face of medical research as they play
a pivotal role in cancer research, regenerative medicine and disease therapy. NewStem established a discovery bio-platform based on haploid
human embryonic stem cell technology for genome-wide screenings and is currently using this platform for the discovery and development
of oncology drugs based on synthetic lethal interaction and developing a personalized diagnostic for early detection of chemotherapy
resistance. NewStem has incurred losses since inception and has not generated any revenues to date. NewStem filed an FDA Pre-Submission
and received a CE Mark from the European Medicines Agency (EMA) for its in vitro diagnostic device (IVDD). NewStem does not have an
FDA approved medical device. The NewStem Software Diagnostic Device (NSDD) is CE marked under EU regulation as an “other”
IVD under Directive 98/79/EC since March 2022.
NewStem performs genome-wide
genetic screening to identify synthetic lethal interactions with common cancer-related mutations. The first step in the process is to
create a model with relevant cancer-related mutations in HhPSCs, where, subsequently, a library targeting approximately 18,000 coding
genes is induced. At the end of this step, each cell has two mutations, one in the cancer related gene and the other in a coding gene.
A genome-wide genetic screening is performed, both on normal HhPSCs and genomic modified HhPSCs to which a cancer-related mutation was
inserted. The goal of such screens is to identify mutations that in combination with a cancer-related mutation will kill the cells. Following
bioinformatic analysis of the genetic screening results, novel targets are identified and validated, first in HhPSCs and then cancer
models (tumor organoids and PDX). NewStem has validated several targets in HhPSCs and will move next to validation in cancer models.
To identify novel targets for drug development, NewStem performs genome-wide genetic screening. The validation process requires additional
experiments that corroborate the results in independent experiments that corroborate the results in independent experiments that are
performed on haploid human embryonic stem cells and cancer models. For validated targets, artificial intelligence (AI) based
drug discovery will be performed following by hit to lead process and ADMET that will support the transition to clinical trials.
In reference to AI-based drug
discovery, AI can assist in structure-based drug discovery by predicting the 3D protein structure and the chemical environment of the
target protein site, thus helping to predict the effect of a compound on the target along with safety considerations before their synthesis
or production and, accordingly, accelerates the drug development process.
In reference
to the hit to lead process- this is the iterative process of lead improvement. It is the stage where a hit, typically a small molecule
identified in a high throughput screen, is chemically modified into a lead molecule following improvements in activity against the target.
In reference
to ADMET, this is the five-letter acronym for absorption, distribution, metabolism, excretion, and toxicity that describes pharmacokinetics.
ADMET plays key roles in drug discovery and development. A high-quality drug candidate should not only have sufficient efficacy against
the therapeutic target, but also show appropriate ADMET properties at a therapeutic dose.
NewStem
possesses pioneering intellectual property, reagents and experience related to the isolation and differentiation of HhPSCs and hPSCs,
their genetic manipulation, immunogenicity, tumorigenicity and their unique capacity in disease modeling.
We
believe that NewStem is currently the only company worldwide to develop products based on this innovative proprietary technology. These
products refer to the medical device platform that provides information to oncologists regarding the presence of mutations in the patient’s
tumor profile which may confer resistance to different anti-cancer drugs and to anticancer drugs that target tumors with specific mutations
based on a synthetic-lethal interaction approach.
NewStem’s
technology solutions are derived from an exclusive, worldwide license from Yissum Research Development Company, Hebrew University’s
technology transfer company (“Yissum”) and The New York Stem Cells Foundation, based on the findings and inventions of Prof.
Nissim Benvenisty, Director of the Azrieli Center for Stem Cells and Genetic Research, The Hebrew University of Jerusalem (the “License”).
The License provides NewStem an exclusive worldwide license to make commercial use of the License and to develop, manufacture, market,
distribute or sell a product in the field of therapeutics, diagnostics, screening, development and testing. In consideration for the
grant of the License, NewStem is obligated to pay royalties of up to 3% of net sales and up to 12% of “Sublicense Consideration”
(as defined in the License).
NovelStem
was the original seed investor in NewStem providing $2 million in July 2018 and another $2 million over the next two and a half years.
We currently own a 30.99% equity interest in NewStem. The remaining equity interests in NewStem are owned by Yissum and Professor Benvenisty,
each of whom owns a 30.99% equity interest, Illumina Cambridge LTD, which owns a 5.39% equity interest, and management and a number of
other shareholders who own collectively approximately 1.64%. Currently, our President and Executive Chairman, Jan Loeb, is also
the Chairman of the Board of NewStem. Professor Benvenisty and a representative of Yissum occupy the other two Board seats.
Pursuant
to NewStem’s Articles of Association, investors (including NovelStem) are granted certain rights and are subject to certain restrictions
with respect to their equity interests in NewStem. NovelStem has preemptive rights to purchase additional shares issued by NewStem up
to its pro-rata share of all outstanding shares of NewStem held by all shareholders of NewStem, until the consummation of either an initial
public offering or a liquidation event. Such pro-rata share may be increased into an over-allotment if other shareholders decline to
exercise their preemptive rights. The Board of Directors of NewStem may make capital calls on NovelStem and the other shareholders, in
respect of any sum unpaid in respect of shares held by such shareholder. All shareholders holding at least 10% of the outstanding shares,
including NovelStem, may exercise a right of first refusal on all sales of shares of NewStem other than transfers to certain permitted
transferees. NovelStem and other shareholders have a co-sale right to sell their shares in place of those that would be issued and sold
by NewStem’s founder. The shares of NewStem are subject to a drag-along right, compelling all shares to be sold in the event that
a transaction meant to sell all shares of NewStem is approved by shareholders holding at least 65% of the vote of all shares of NewStem.
Competition
The
technologies underlying NewStem’s products are subject to rapid and profound technological change. Competition intensifies as technical
advances in each field are made and become more widely known. We can give no assurance that others will not develop services, products,
or processes with significant advantages over the products, services, and processes that NewStem offers or is seeking to develop. Any
such occurrence could have a material and adverse effect on NewStem’s and our business, results of operations and financial condition.
NewStem
plans to enhance and broaden its product offerings in response to changing customer demands and competitive pressure and technologies.
The success of any new product offering or enhancement to an existing product will depend on numerous factors, including the ability
to:
| - |
Properly
identify and anticipate physician and patient needs; |
| - |
Develop
and introduce new products or product enhancements in a timely manner; |
| - |
Adequately
protect intellectual property and avoid infringing upon the intellectual property rights of third parties; |
| - |
Demonstrate
the safety and efficacy of new products; and |
| - |
Obtain
the necessary regulatory clearances or approvals for new products or product enhancements. |
Government
Regulation
In
the United States, pharmaceutical products are subject to extensive regulation by the Federal Food and Drug Administration and Cosmetic
Act or the FDA. The FDA and other federal and state statutes and regulations, govern, among other things, the research, development,
testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and
reporting, sampling, and import and export of pharmaceutical products. The FDA has very broad enforcement authority and failure to abide
by applicable regulatory requirements can result in administrative or judicial sanctions being imposed on NewStem, including warning
letters, refusals of government contracts, clinical holds, civil penalties, injunctions, restitution, disgorgement of profits, recall
or seizure of products, total or partial suspension of production or distribution, withdrawal of approval, refusal to approve pending
applications, and criminal prosecution.
FDA
Approval Process
NewStem’s
therapeutic product candidates are expected to be regulated by the FDA as drugs. No manufacturer may market a new drug until it has submitted
a New Drug Application, or NDA, to the FDA, and the FDA has approved it.
The
testing and approval process requires substantial time, effort and financial resources, and NewStem’s product candidates may not
be approved on a timely basis, if at all. The time and expense required to perform the clinical testing necessary to obtain FDA approval
for regulated products can frequently exceed the time and expense of the research and development initially required to create the product.
The results of preclinical studies and initial clinical trials of NewStem’s product candidates are not necessarily predictive of
the results from large-scale clinical trials, and clinical trials may be subject to additional costs, delays or modifications due to
a number of factors, including difficulty in obtaining enough patients, investigators or product candidate supply. Failure by NewStem
to obtain, or any delay in obtaining, regulatory approvals or in complying with requirements could adversely affect the commercialization
of product candidates and NewStem’s (and, therefore, the Company’s) ability to receive product or royalty revenues.
The diagnostic product
(NSDD) will be considered a medical device. A Pre-Submission (Pre-Sub) regarding the NSDD was submitted to FDA in March 2022, and the
FDA’s written feedback was received in May 2022. The FDA requested that the presented intended use and pivotal clinical testing
design be modified. NewStem still needs to present to the FDA a Supplement to the Pre-Sub, presenting such modifications, and asking
it to confirm that the de novo route is indeed applicable to the device. Once an agreement is reached with the FDA, the device will be
subjected to a retrospective pivotal clinical testing that will be followed by the de novo submission to the FDA.
Other
Regulatory Requirements
After
approval, drug products are subject to extensive continuing regulation by the FDA, which include obligations to manufacture products
in accordance with Good Manufacturing Practice, or GMP, maintain and provide to the FDA updated safety and efficacy information, report
adverse experiences with the product, keep certain records and submit periodic reports, obtain FDA approval of certain manufacturing
or labeling changes, and comply with FDA promotion and advertising requirements and restrictions. Failure by NewStem to meet these obligations
can result in various adverse consequences, both voluntary and FDA-imposed, including product recalls, withdrawal of approval, restrictions
on marketing, and the imposition of civil fines and criminal penalties against the NDA holder. In addition, later discovery of previously
unknown safety or efficacy issues may result in restrictions on the product, manufacturer or NDA holder.
Outside
the United States, NewStem’s ability to market a product is contingent upon receiving marketing authorization from the appropriate
regulatory authorities. The requirements governing marketing authorization, pricing and reimbursement vary widely from jurisdiction to
jurisdiction. At present, foreign marketing authorizations are applied for at a national level, although within the European Union registration
procedures are available to companies wishing to market a product in more than one European Union member state.
NewStem
is also subject to various environmental, health and safety regulations including those governing laboratory procedures and the handling,
use, storage, treatment, and disposal of hazardous materials. From time to time, and in the future, NewStem’s operations may involve
the use of hazardous materials.
NetCo
Partners
In
June 1995, we and C.P. Group Inc. (“C.P. Group”), formed the joint venture, NetCo Partners. NetCo owns the entertainment
property, “Net Force”, about a division of the FBI investigating crimes and adventures involving the internet and the digital
world.
NovelStem
and C.P. Group each own 50% of the ownership interest in NetCo Partners. NetCo
Partners owns all rights in all media to the Net Force property including film, television, and video games.
In
1997, NetCo Partners licensed to Putnam Berkley the rights to publish the first six Net Force books in North America, which books were
written and published. This agreement was subsequently renewed in December 2001 for four more books that were created and published.
There was also a series of books targeted to the young adult market, Net Force Explorer, also published by Putnam Berkley. Net
Force books have so far been published in mass market paperback format. The first book in the series was adapted as a four-hour mini-series
on the ABC television network.
In
2019, NetCo Partners entered into a new publishing agreement with HarperCollins. Three novels and two Net Force novellas have been published
under that agreement. Through its interest in NetCo Partners, NovelStem receives distributions of its 50% share of proceeds generated
from the rights to Net Force.
Competition
Competition
in the publishing and video game industries is intense. Many new products and services are regularly introduced in each major industry
segment (console, mobile and PC), but only a relatively small number of “hit” titles account for a significant portion of
total revenue in each segment. NetCo Partners’ competitors range from established interactive entertainment companies and diversified
media companies to emerging start-ups, and we expect new competitors to continue to emerge throughout the world.
See
Item 8 – Legal Proceedings for information concerning proceedings related to NetCo Partners.
Employees
We do not currently have any
employees; however, the Company relies on consultants to perform the duties that would be performed by employees.
Item
1A. Risk Factors
Our
business is subject to certain risks, including those described below. If any of the events described in the following risk factors actually
occurs then our business, results of operations and financial condition could be materially adversely affected. More detailed information
concerning these risks is contained in other sections of this registration statement, including “Business” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations.”
Risks
Relating to our Business
We
are a holding company the principal assets of which are illiquid, ownership interests in NewStem and NetCo Partners.
Our
Company’s primary assets are equity interests in NewStem and NetCo Partners. Our President and Executive Chairman, Jan Loeb,
is also the Chairman of NewStem and through this shared management structure along with our 30.99% ownership interest in NewStem, we
are able to exert significant influence over the operations of NewStem. Additionally, we are a 50% partner in NetCo and through our ownership
interest, are able to exert significant influence over this entity and its operations.
We
conduct no other business and, as a result, we depend entirely upon earnings and cash flow from NewStem and NetCo Partners. If we decide
in the future to pay dividends, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt
of dividends or other payments from our operating subsidiaries.
We may be deemed an investment company, which could impose on
us burdensome compliance requirements.
The Investment Company Act of 1940, as amended
(the “Investment Company Act”), requires companies to register as an investment company if they are engaged primarily in
the business of investing, reinvesting, owning, holding, or trading securities. Section 3(a)(1)(A) of the Investment Company Act defines
an investment company as any issuer that is or holds itself out as being engaged primarily in the business of investing, reinvesting
or trading in securities. In addition, Section 3(a)(1)(C) of the Investment Company Act defines an investment company as any issuer that
is engaged or proposes to engage in the business of investing, reinvesting, owning, holding or trading in securities and owns or proposes
to acquire investment securities having a value exceeding 40% of the value of the issuer’s total assets (exclusive of U.S. government
securities and cash items) on an unconsolidated basis. We are not in the business of investing, reinvesting, owning, holding or trading
securities and we do not believe that we would meet the definition of an “investment company” under the Investment Company
Act, as further discussed below.
Pursuant to Rule 3a-1 under the Investment Company
Act, an entity will not be considered an investment company if, on a consolidated basis with its wholly owned subsidiaries, less than
45% of its total assets exclusive of U.S. government securities and cash items) are composed of assets that are investment securities
(the “Asset Test”), and less than 45% of its income is derived from investment securities (the “Income Test”).
Rule 3a-1 also provides that securities issued by a company (i) that is “controlled primarily” by the issuer, (ii) through
which the issuer engages in a business other than that of investing, reinvesting, owning, holding, or trading in securities, and (iii)
that is not, itself, an investment company will not be deemed investment securities for purposes of the Asset and Income Tests. In order
for a company to be presumed to be “controlled primarily” by the issuer, the issuer must at a minimum control at least 25%
of the voting securities of the company, and the degree of the issuer’s control must be greater than that of any other person.
While we believe that our ownership of NewStem
would otherwise meet the Asset and Income Tests, we also believe that New Stem is “controlled primarily” by NovelStem since
maintaining greater control over NewStem than any other person as a result of numerous factors including: our [ %] equity interest in
NewStem; the fact that our Executive Chairman of the Board, Jan Loeb, is also the Chairman of the Board of NewStem; rights conferred
on NovelStem under NewStem’s governing documents and by contract; NovelStem’s status as the principal source of investment
capital for NewStem; NewStem’s dependency on NovelStem’s management, operational, administrative, financial and other resources.
As a result, we do not believe our shares in NewStem constitute investment securities for purposes of Rule 3a-1.
Accordingly, since (1) NovelStem is not in the
business of investing, reinvesting, owning, holding or trading securities and (2) we believe that on a consolidated basis less than 45%
of our total assets (exclusive of U.S. government securities and cash items) are composed of, and less than 45% of our income is derived
from, assets that could be considered investment securities, we do not believe that we should be deemed to be an investment company.
However, if the Securities and Exchange Commission deems us to be an investment company, or if we were ever to lose primary control of
NewStem or otherwise cause our equity interests in NewStem to trigger the Asset or Income Tests, we may have imposed upon us additional
burdensome requirements, including having to register as an investment company, adopting a specific form of corporation structure and
having to comply with certain reporting, record keeping, voting, proxy, and disclosure requirements. Such additional requirements
would require us to incur additional costs and could have an adverse effect on our results of operations and our ability to effectively
carry out our business plan.
Our
investments in NewStem and NetCo are illiquid.
Our
shares in NewStem and our ownership interest in NetCo Partners are illiquid and have extremely limited liquidity rights. The transferability
of these interests is restricted under federal and state securities laws and the governing documents of each of NewStem and NetCo Partners.
We
depend on our executive officers and consultants and other key individuals along with the executive officers and key individuals
of NewStem to continue the implementation of our long-term business strategy and
could be harmed by the loss of their services and our inability to make up for such loss with qualified replacements.
We
believe that our continued growth and future success will depend in large part on the skills of our management team and the management
teams of NewStem and NetCo Partners and our and our partners’ respective abilities to motivate and retain these individuals and
other key individuals. Jan Loeb, our President and Executive Chairman, is also the Chairman of NewStem, and therefore has the
shared responsibility of growing the business and operations of NewStem. The loss of any of their service could reduce our ability to
successfully implement our long-term business strategy which may result in a loss of revenue, and the value of our common stock could
be materially adversely affected. Leadership changes will occur from time to time and we cannot predict whether significant resignations
will occur or whether NewStem will be able to recruit additional qualified personnel. We believe these management teams possess valuable
knowledge about our, NewStem’s and NetCo’s respective industries and that their knowledge and relationships would be very
difficult to replicate. The loss of key personnel, or the inability to recruit and retain qualified and talented personnel in the future,
could have an adverse effect on the respective businesses of NewStem and NetCo, and, consequently, our business, financial condition
and/or operating results.
We
and NewStem have limited operating histories and have generated no revenue to date.
We
and NewStem have a limited operating history and do not have a meaningful historical record of sales and revenues, nor do we or NewStem
have an established business track record. While we believe that we have the opportunity to be successful, there can be no assurance
that we will be successful in accomplishing our business initiatives, or that we will be able to achieve any significant levels of revenues
or net income.
We
have identified material weaknesses in our internal control and procedures and internal control over financial reporting. If not
remediated, our failure to establish and maintain effective disclosure controls and procedures and internal control over financial reporting
could result in material misstatements in our financial statements and a failure to meet our reporting and financial obligations, each
of which could have a material adverse effect on our financial condition and the trading price of our common stock.
Maintaining
effective internal control over financial reporting and effective disclosure controls and procedures are necessary for us to produce
reliable financial statements. We have re-evaluated our internal control over financial reporting and our disclosure controls and
procedures and concluded that they were not effective as of September 30, 2022 and we concluded there was a material weakness
in the design of our internal control over financial reporting.
A
material weakness is defined as a deficiency, or a combination of deficiencies, in internal control over financial reporting such that
there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or
detected on a timely basis.
NewStem
may be unable to respond effectively to technological changes in its industry, which could reduce the demand for its products and services.
NewStem’s
future business success will depend upon its ability to maintain and enhance its product portfolio with respect to advances in technological
improvements for certain products that meet customer needs and market conditions in a cost-effective and timely manner. NewStem may not
be successful in gaining access to new products that successfully compete or are able to anticipate customer needs and preferences, and
customers may not accept one or more of its products. If NewStem fails to keep pace with evolving technological innovations or fails
to modify its products and services in response to customers’ needs or preferences, then NewStem’s and our business, financial
condition and results of operations could be adversely affected.
Rapid
technological change could cause products to become obsolete, and if NewStem does not enhance its product offerings through research
and development efforts, it may be unable to effectively compete.
The
technologies underlying NewStem’s products are subject to rapid and profound technological change. Competition intensifies as technical
advances in each field are made and become more widely known. We can give no assurance that others will not develop services, products,
or processes with significant advantages over the products, services, and processes that NewStem offers or is seeking to develop. Any
such occurrence could have a material and adverse effect on NewStem’s and our business, results of operations and financial condition.
NewStem
plans to enhance and broaden its product offerings in response to changing customer demands and competitive pressure and technologies.
The success of any new product offering or enhancement to an existing product will depend on numerous factors, including the ability
to:
| - |
Properly
identify and anticipate physician and patient needs; |
| - |
Develop
and introduce new products or product enhancements in a timely manner; |
| - |
Adequately
protect intellectual property and avoid infringing upon the intellectual property rights of third parties; |
| - |
Demonstrate
the safety and efficacy of new products; and |
| - |
Obtain
the necessary regulatory clearances or approvals for new products or product enhancements. |
If
NewStem does not develop and, when necessary, obtain regulatory clearance or approval for new products or product enhancements in time
to meet market demand, or if there is insufficient demand for these products or enhancements, its results of operations will suffer.
NewStem’s research and development efforts may require a substantial investment of time and resources before it is adequately able
to determine the commercial viability of a new product, technology, material or other innovation. In addition, even if NewStem is able
to successfully develop enhancements or new generations of its products, these enhancements or new generations of products may not produce
sales in excess of the costs of development, and they may be quickly rendered obsolete by changing customer preferences or the introduction
by competitors of products embodying new technologies or features.
Our
ongoing viability as a company depends on NewStem’s ability to successfully develop and commercialize its products.
NewStem
is principally focused on utilizing proprietary hPSCs and HhPSCs in the development of diagnostic and therapeutic products in oncology. NewStem must develop diagnostics and therapeutics successfully test them for safety and efficacy in the targeted patient population
and manufacture the finished drugs on a commercial scale to meet regulatory standards and receive regulatory approvals. The development
and commercialization process is both time-consuming and costly, and involves a high degree of business risk. The results of pre-clinical
and clinical testing of product candidates are uncertain, and there can be no assurance that NewStem will be able to obtain regulatory
approvals of its product candidates. If obtained, regulatory approval may take longer or be more expensive than anticipated. Furthermore,
even if regulatory approvals are obtained, NewStem’s products may not perform as we expect and NewStem may not be able to successfully
and profitably produce and market any products. Delays in any part of the process or our inability to obtain regulatory approval of such
products could adversely affect NewStem’s and, therefore, NovelStem’s future operating results by restricting (or even prohibiting)
the introduction and sale of such products.
The
value of our investment in NetCo Partners and our ability to receive distributions may be affected by disputes between the Company and
C.P. Group, our partner in NetCo Partners.
The
Company and C.P. Group each own a 50% interest in NetCo Partners. The joint venture agreement governing NetCo Partners provides for mutual
decision making among the Company and C.P. Group generally (subject to exceptions) and arbitration in the event any controversy or disagreement
arises. The Company and C.P. Group are currently in arbitration as to ongoing scope and the operation of NetCo Partners. If we are unable
to resolve such dispute in a manner favorable to the Company, our investment in NetCo Partners and our ability to continue to receive
distributions from our interest in NetCo Partners could have an adverse effect on our business, financial condition or operating
results.
NetCo
Partners’ business is intensely competitive and “hit” driven. NetCo Partners may not deliver “hit” products
and services, or consumers may prefer a competitors’ products or services over NetCo Partners.
Competition
in the publishing and video game industries is intense. Many new products and services are regularly introduced in each major industry
segment (console, mobile and PC), but only a relatively small number of “hit” titles account for a significant portion of
total revenue in each segment. NetCo Partners’ competitors range from established interactive entertainment companies and diversified
media companies to emerging start-ups, and we expect new competitors to continue to emerge throughout the world. If NetCo Partner’s
competitors develop and market more successful and engaging products or services, offer competitive products or services at lower price
points, or if NetCo Partners does not develop high-quality, well-received and engaging products and services, NetCo Partners’ and
our revenue, margins, and profitability will decline.
If
NetCo Partners fails to develop relationships with new creative talent, its business could be adversely affected.
NetCo
Partners’ business, in particular the trade publishing and media portions of the business, is highly dependent on maintaining strong
relationships with the authors, illustrators and other creative talent who produce the products and services that are sold to its customers.
Any overall weakening of these relationships, or the failure to develop successful new relationships, could have an adverse impact on
NetCo Partners’ and the Company’s business and financial performance.
Risks
relating to our common stock
Because
our holding company structure creates restrictions on the payment of dividends, our ability to pay dividends is limited.
We
are a holding company whose primary assets are our ownership of equity interests in NewStem and NetCo Partner. We conduct no other
business and, as a result, we depend entirely upon NewStem’s and NetCo Partners’ earnings and cash flow. If we decide
in the future to pay dividends, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt
of dividends or other payments from NewStem or NetCo. NewStem and/or NetCo may be restricted in their ability to pay dividends,
make distributions or otherwise transfer funds to us prior to the satisfaction of other obligations, including the payment of operating
expenses or debt service, appropriation to reserves prescribed by laws and regulations, covering losses in previous years, restrictions
on the conversion of local currency into U.S. dollars or other hard currency, completion of relevant procedures with governmental authorities
or banks and other regulatory restrictions. We do not presently have any intention to declare or pay dividends in the future. You should
not purchase shares of our common stock in anticipation of receiving dividends in future periods.
Because
we do not intend to pay any cash dividends on our common stock, our shareholders will not be able to receive a return on their shares
unless they sell them.
We
intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends
on our common stock in the foreseeable future. Unless we pay dividends, our shareholders will not be able to receive a return on their
shares unless they sell them. Shareholders may never be able to sell shares when desired. Before you invest in our securities, you should
be aware that there are various risks. You should consider carefully these risk factors, together with all of the other information included
in this annual report before you decide to purchase our securities. If any of the following risks and uncertainties develop into actual
events, our business, financial condition or results of operations could be materially adversely affected.
We
are an emerging growth company and the reduced disclosure requirements applicable to emerging growth companies could make our common
stock less attractive to investors.
We
are an emerging growth company. Under the JOBS Act, emerging growth companies can take advantage of certain exemptions from various reporting
requirements that are applicable to other public companies including, without limitation, reduced disclosure obligations regarding executive
compensation in our periodic reports and proxy statements, exemptions from the requirements of holding a non-binding advisory shareholder
vote on executive compensation and golden parachute payments, exemption from the requirement of auditor attestation in the assessment
of our internal control over financial reporting and exemption from any requirement that may be adopted by the Public Company Accounting
Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information
about our audit and the financial statements (auditor discussion and analysis). As a result of the foregoing, the information that we
provide shareholders may be different than what is available with respect to other public companies.
In
addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period
provided in Section 7(a)(2)(B) of the Securities Act of 1933for complying with new or revised accounting standards. We plan to elect
to use the extended period for compliance and, as a result, our financial statements may not be comparable to companies that comply with
public company effective dates.
Reporting
requirement under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and compliance with
the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), including establishing and maintaining acceptable internal
controls over financial reporting, are costly and may increase substantially.
The
rules and regulations of the SEC require a public company to prepare and file periodic reports under the Exchange Act, which will require that the Company engage legal, accounting, auditing and other professional
services. The engagement of such services is costly. Additionally, the Sarbanes-Oxley Act requires, among other things, that we design, implement and maintain adequate internal controls and procedures over financial reporting.
The costs of complying with the Sarbanes-Oxley Act and the limited technically qualified personnel we have may make it difficult for
us to design, implement and maintain adequate internal controls over financial reporting. In the event that we fail to maintain an effective
system of internal controls or discover material weaknesses in our internal controls, we may not be able to produce reliable financial
reports or report fraud, which may harm our overall financial condition and result in loss of investor confidence and a decline in our
share price.
As
a public company, we will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Act of
2010 and other applicable securities rules and regulations. Despite recent reforms made possible by the JOBS Act, compliance with these
rules and regulations will nonetheless increase our legal and financial compliance costs, make some activities more difficult, time-consuming
or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.”
The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and operating
results.
We
are working with our legal, accounting and financial advisors to identify those areas in which changes should be made to our financial
and management control systems to manage our growth and our obligations as a public company. These areas include corporate governance,
corporate control, disclosure controls and procedures and financial reporting and accounting systems. We have made, and will continue
to make, changes in these and other areas. However, we anticipate that the expenses that will be required in order to adequately prepare
for being a public company could be material. We estimate that the aggregate cost of increased legal services; accounting and audit functions;
personnel, such as a chief financial officer familiar with the obligations of public company reporting; consultants to design and implement
internal controls; and financial printing alone will be a few hundred thousand dollars per year and could be several hundred thousand
dollars per year. In addition, we may incur additional expenses related to director compensation and/or premiums for directors’
and officers’ liability insurance, the costs of which we cannot estimate at this time. We may also incur additional expenses associated
with investor relations and similar functions, the cost of which we also cannot estimate at this time. However, these additional expenses
individually, or in the aggregate, may also be material.
In
addition, being a public company could make it more difficult or more costly for us to obtain certain types of insurance, including directors’
and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher
costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain
qualified persons to serve on our board of directors, our board committees or as executive officers.
The
increased costs associated with operating as a public company may decrease our net income or increase our net loss and may cause us to
reduce costs in other areas of our business or increase the prices of our products or services to offset the effect of such increased
costs. Additionally, if these requirements divert our management’s attention from other business concerns, they could have a material
adverse effect on our business, financial condition and results of operations.
There
is a very limited trading market for our common stock and investors are not assured of the opportunity to sell their stock, should they
desire to do so.
Our
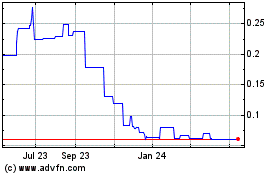
common stock is currently quoted on the OTC Pink Market. However, our stock has traded in very limited quantities in the past.
We believe a significant factor in the limited market is our limited capitalization and liquidity, results of operation and the characterization
of our stock as a “penny stock.” We hope to remedy our financial condition and results of operation in the future. This,
in turn, may assist us in obtaining listing of our stock on other exchanges. However, there is no assurance that any of these objectives
will be met or that the market will ever increase to a point where investors could sell their stock at a desirable price, should they
desire to do so.
The
price of our common stock could be highly volatile.
Our
shares of common stock are quoted on the OTC Pink Market. It is likely that our common stock will be subject to price volatility, low
volumes of trades and large spreads in bid and ask prices quoted by market makers. Due to the low volume of shares traded on any trading
day, persons buying or selling in relatively small quantities may easily influence prices of our common stock. This low volume of trades
could also cause the price of our stock to fluctuate greatly, with large percentage changes in price occurring in any trading day session.
Holders of our common stock may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due
to low volume trading. If high spreads between the bid and ask prices of our common stock exist at the time of a purchase, the stock
would have to appreciate substantially on a relative percentage basis for an investor to recoup their investment. Broad market fluctuations
and general economic and political conditions may also adversely affect the market price of our common stock. No assurance can be given
that an active market in our common stock will be sustained. If an active market does not continue, holders of our common stock may be
unable to readily sell the shares they hold or may not be able to sell their shares at all.
We
may be deemed an investment company, which could impose on us burdensome compliance requirements.
The
Investment Company Act of 1940, as amended (the “Investment Company Act”), requires companies to register as an investment
company if they are engaged primarily in the business of investing, reinvesting, owning, holding, or trading securities. Generally, companies
may be deemed investment companies under the Investment Company Act if they are viewed as engaging in the business of investing in securities
or they own investment securities having a value exceeding 40% of certain assets. We are not in the business of investing, reinvesting,
owning, holding or trading securities. However, if the Securities and Exchange Commission deems us to be an investment company, we may
have imposed upon us additional burdensome requirements, including having to register as an investment company, adopting a specific form
of corporation structure and having to comply with certain reporting, record keeping, voting, proxy, and disclosure requirements.
Such additional requirements would require us to incur additional costs and have an adverse effect on our results of operations and our
ability to effectively carry out our business plan.
Item
2. Financial Information.
Management’s
Discussion and Analysis of the Results of Operations
Statements
in the following discussion and throughout this registration statement that are not historical in nature are “forward-looking statements.”
You can identify forward-looking statements by the use of words such as “expect,” “anticipate,” “estimate,”
“may,” “will,” “should,” “intend,” “believe,” and similar expressions. Although
we believe the expectations reflected in these forward-looking statements are reasonable, such statements are inherently subject to risk
and we can give no assurances that our expectations will prove to be correct. Actual results could differ from those described in this
registration statement because of numerous factors, many of which are beyond our control. These factors include, without limitation,
those described under Item 1A “Risk Factors” We undertake no obligation to update these forward-looking statements to reflect
events or circumstances after the date of this registration statement or to reflect actual outcomes.
The
following discussion of our financial condition and results of operations should be read in conjunction with our financial
statements and the related notes thereto and other financial information appearing elsewhere in this Form 10.
Overview
We are a development stage company
and reported net losses of $1,346,000 and $545,000 for the years ended December 31, 2021 and 2020, respectively, and $877,000 and
$736,000 for the nine months ended September 30, 2022 and 2021, respectively. We had current assets of $37,000 and current liabilities
of $193,00 as of December 31, 2021. As of December 31, 2020, our current assets and current liabilities were $121,000 and $69,000, respectively.
As of September 30, 2022, our current assets and current liabilities were $66,000 and $126,000, respectively. We
have prepared our financial statements for the years ended December 31, 2021 and 2020 and the nine months ended September
30, 2022 assuming that we will continue as a going concern. Our continuation as a going concern is dependent upon improving our profitability
and the continuing financial support from our shareholders. Our sources of capital in the past have included the sale of equity securities,
which include common stock sold in private transactions, large alternative minimum tax refunds, and short-term debt.
Results
of Operations.
Comparison
of the twelve months ended December 31, 2021 and December 31, 2020
The
following table sets forth certain operational data for the twelve months ended December 31, 2021, compared to the twelve months ended
December 31, 2020:
| | |
Twelve Months Ended December 31, | |
| |
| | |
2021 | | |
2020 | | |
Change | |
| Operating expenses: | |
| | | |
| | | |
| | |
| General and administrative expenses | |
$ | 222,769 | | |
$ | 150,708 | | |
$ | 72,061 | |
| Stock compensation expense | |
| 272,766 | | |
| 79,587 | | |
| 193,179 | |
| Total operating expenses | |
| 495,535 | | |
| 230,295 | | |
| 265,240 | |
| Loss from operations | |
| (495,535 | ) | |
| (230,295 | ) | |
| 265,240 | |
| Interest (income) expense, net | |
| 6,825 | | |
| (16,514 | ) | |
| 23,339 | |
| Net loss before equity in net loss of equity method investees | |
| (502,360 | ) | |
| (213,781 | ) | |
| (288,579 | ) |
| Equity in net loss of equity method investees | |
| (843,268 | ) | |
| (331,658 | ) | |
| (511,610 | ) |
| Net loss | |
$ | (1,345,628 | ) | |
$ | (545,439 | ) | |
$ | (800,189 | ) |
Revenue.
During
the twelve months ended December 31, 2021, and 2020, the Company had no revenue, therefore no customers accounted for 10% or more
of our total net revenues.
Cost
of Revenue.
Cost
of Revenue for the twelve months ended December 31, 2021 and 2020 was $0 and $0, respectively. We had no revenues during those periods.
Gross
Profit.
Consequently,
we achieved a gross profit of $0 and $0 for the twelve months ended December 31, 2021 and 2020, respectively.
General
and Administrative (“G&A”) and Stock Compensation Expenses
We
incurred G&A expenses of $223,000 and $151,000 for the twelve months ended December 31, 2021 and 2020, respectively.
The increase in G&A included increases in professional fees of $58,000, insurance premiums of $6,340 and investor relation costs
of $4,100.
We incurred stock compensation
expense of $273,000 and $80,000 for the twelve months ended December 31, 2021 and 2020, respectively. The increase of $193,000 in
stock compensation expense is attributable to more stock options being granted during the twelve months ended December 31, 2021 compared
to the previous twelve months.
Other
Income (Expense), net.
We
have generated other income of $0 and $17,000 for the twelve months ended December 31, 2021 and 2020, respectively. This other income
generated during the twelve months ended December 31, 2020 is comprised of interest earned related to previous years alternative minimum
tax refunds. Additionally, we incurred interest expense related to financing arrangements of $7,000 and $1,000, respectively, for the
twelve months ended December 31, 2021 and 2020.
Income
Tax Expense.
Our
income tax expenses for the twelve months ended December 31, 2021 and 2020 were $0 and $0, respectively.
Equity
in net loss of equity method investees.
We
reported net loss from equity method investees of $843,000 and $332,000, respectively, for the twelve months ended December 31, 2021
and 2020. The net loss from equity method investees reported for the twelve months ended December 31, 2021 consisted of income allocated
from NetCo of $21,000 and a net loss allocated from NewStem of $864,000. The net loss from equity method investees reported for the twelve
months ended December 31, 2020 consisted of net income from NetCo of $9,400 and net loss of $341,000 from NewStem.
Net
Loss
The
net loss for the twelve months ended December 31, 2021 was $1,346,000 compared to a loss of $545,000 for the twelve months ended December
31, 2020. The difference relates to the increase in loss from equity method investees and the increased G&A and stock compensation
expenses previously discussed.
Comparison of the nine
months ended September 30, 2022 and September 30, 2021
The following table sets forth
certain operational data for the nine and three months ended September 30, 2022, compared to the nine and three
months ended September 30, 2021:
| | |
Nine Months Ended September 30 | | |
| | |
Three Months Ended September 30 | | |
| |
| | |
2022 | | |
2021 | | |
Change | | |
2022 | | |
2021 | | |
Change | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| G&A expenses | |
$ | 376,247 | | |
$ | 136,047 | | |
$ | 240,200 | | |
$ | 131,060 | | |
$ | 60,133 | | |
$ | 70,927 | |
| Contra expenses - legal fees | |
| (310,000 | ) | |
| - | | |
| (310,000 | ) | |
| - | | |
| - | | |
| - | |
| Stock compensation expense | |
| 198,494 | | |
| 229,891 | | |
| (31,397 | ) | |
| 75,150 | | |
| 73,045 | | |
| 2,105 | |
| Total operating expenses | |
| 264,741 | | |
| 365,938 | | |
| (101,197 | ) | |
| 206,210 | | |
| 133,178 | | |
| 73,032 | |
| Loss from operations | |
| (264,741 | ) | |
| (365,938 | ) | |
| (101,197 | ) | |
| (206,210 | ) | |
| (133,178 | ) | |
| 73,032 | |
| Interest expense | |
| 5,542 | | |
| 4,417 | | |
| 1,125 | | |
| 3,530 | | |
| 2,205 | | |
| 1,325 | |
| Net loss before equity in net | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| loss of equity method investees | |
| (270,283 | ) | |
| (370,355 | ) | |
| 100,072 | | |
| (209,740 | ) | |
| (135,383 | ) | |
| (74,357 | ) |
| Equity in net loss of equity method investees | |
| (606,736 | ) | |
| (365,543 | ) | |
| (241,193 | ) | |
| (85,532 | ) | |
| (196,333 | ) | |
| 110,801 | |
| Net loss | |
$ | (877,019 | ) | |
$ | (735,898 | ) | |
$ | (141,121 | ) | |
$ | (295,272 | ) | |
$ | (331,716 | ) | |
$ | 36,444 | |
Revenue.
During the nine and three
months ended September 30, 2022, and 2021, the Company had no revenue, therefore no customers accounted for 10% or more of
our total net revenues.
Revenue for the nine and three months ended
September 30. 2022 and September 30, 2021 were $0 and $0, respectively.
Cost of Revenue.
Cost of Revenue for the nine
and three months ended September 30, 2022 and 2021 was $0 and $0, respectively, as we had no Revenue
Gross Profit (Loss).
We achieved a gross profit of
$0 for the nine and three months ended September 30, 2022 and 2021.
General and Administrative
Expenses (“G&A”) and Stock Compensation Expense
The Company incurs G&A
expenses primarily related to professional fees and insurance. We incurred G&A expenses of $376,000 and $138,000 for the nine months
ended September 30, 2022 and 2021, respectively, and $131,000 and $60,000 for the three months ended September 30, 2022 and 2021, respectively.
Specifically, professional fees increased by $199,000 in the nine months ended September 30, 2022 as compared to the nine months ended
September 30, 2021 and $57,000 in the three months ended September 30, 2022 as compared to the three months ended September 30, 2021.
Insurance costs increased by $34,000 in the nine months ended September 30, 2022 as compared to the same period in 2021 and $12,000 in
the three months ended September 30, 2022 as compared to the three months ended September 30, 2021. Our increase in G&A expenses
relates primarily to professional fees incurred in the audit of our financial statements for the years ended December 31, 2021 and 2020
and, in the preparation, and filing of our Form 10 registration statement.
Stock compensation expense
decreased by $31,000 in the nine months ended September 30, 2022 as compared to the nine months ended September 30, 2021 due to a smaller
number of options awarded in the current period as compared to the prior period.
During the nine months ended
September 30, 2022 we recorded a contra expense of $310,000 which is comprised of funds from a litigation funding agreement. This agreement
was signed during the first quarter of 2022 with Omni Bridgeway to fund our arbitration against our 50% joint venture partner in NetCo
Partners, C.P. Group. This is a nonrecourse agreement, and the Company has no obligation to repay any funds received under the agreement.
In the event of a favorable outcome, Omni Bridgeway would recover disbursed funding as part of their investment return.
As part of that funding arrangement,
Omni Bridgeway agreed to reimburse NovelStem $310,000 which was comprised of $140,000 for reimbursement of previously incurred legal
expenses and $170,000 for working capital needs including previously incurred general and administrative costs. There was no contra expense
in the nine months ended September 30, 2021.
Other Expenses, net.
We incurred interest expense
related to financing arrangements of $6,000 and $4,000, respectively, for the nine months ended September
30, 2022 and 2021 and $4,000 and $2,000, respectively for the three months ended September 30, 2022 and 2021.
Income
Tax Expense.
Our
income tax expenses for the six and three months ended September 30, 2022 and 2021 was $0 and $0, respectively.
Equity
in net loss of equity method investees.
We
reported net losses from equity method investees in all periods presented. The net losses
reported for the three and nine months ended September 30, 2022 were fully comprised of net
losses from NewStem. The net losses reported for the nine months ended September 30, 2021
included net income of $9,000 from NetCo which was offset by net loss of $375,000 from NewStem.
Net
Loss
The
net loss for the nine months ended September 30, 2022 was $877,000 compared
to a loss of $736,000 for the nine months ended September 30, 2021.
The difference relates to the increase in loss from equity method investees reduced by the
$310,000 in contra expenses and lower stock compensation expenses somewhat offset by higher
general and administrative expenses including increased professional fees.
The net loss for the three
months ended September 30, 2022 was $295,000 compared to a loss of $332,000 for the three months ended September 30, 2021. The difference
relates to the decrease in loss from equity method investees and the higher general and administrative expenses including increased professional
fees.
Liquidity
and Capital Resources
We
have never paid dividends on our common stock. Our present policy is to apply cash to investments in product development, acquisitions
or expansion; consequently, we do not expect to pay dividends on common stock in the foreseeable future.
We
expect to incur significantly greater expenses in the near future as we expand our business or enter into strategic partnerships. We
also expect our general and administrative expenses to increase as we expand our finance and administrative staff, add infrastructure,
and incur additional costs related to being a reporting act company, including directors’ and officers’ insurance and increased
professional fees.
The
Company will need to obtain additional funds to continue its operations. Management’s plans with regard to these matters include
additional financing and fundraising until its equity investment in NewStem is profitable. Although management continues to pursue these
plans, there is no assurance that the Company will be successful in obtaining sufficient cash from financing on terms acceptable to the
Company, or that NewStem will become profitable.
In
May 2022, the Company entered into an agreement with Jan Loeb, our President and Executive Chairman and Jerry Wolasky, a member
of the Board, which was amended in July 2022, to borrow up to an aggregate of $600,000 for working capital needs. This agreement provides
for funding through January 31, 2024, provides for interest at a rate of 8% per annum and matures the earlier of January 31, 2024 or
20 months from the date of the first funded amount unless the lenders agree to extend the due date at that time. As of the date of this
registration statement, the Company has drawn down $200,000 under the aforementioned agreement.
Net
Cash Provided By (Used In) Operating Activities.
For
the year ended December 31, 2021, net cash used in operating activities was $181,000, which consisted primarily of a net loss of $1,345,000,
offset by noncash equity in loss of equity method investees of $865,000 and stock-based compensation of $273,000 and an increase
in accrued liabilities and other payables of $24,000.
For
the year ended December 31, 2020, net cash generated from operating activities was $419,000, which consisted primarily of a net loss
of $545,000, offset by a non-cash equity loss of NewStem Ltd. of $341,000 and non-cash equity income allocated from
NetCo of $9,000, stock-based compensation of $80,000, a $509,000 decrease in current assets and an increase in accrued liabilities and
other payables of $35,000. The decrease in current assets related primarily to the collection of a refund receivable of a federal alternative
minimum tax refund.
For
the nine months ended September 30, 2022, net cash used in operating activities was $57,000 which consisted
primarily of a net loss of $877,000, offset by a non-cash equity loss of NewStem Ltd. of $607,000 and stock-based compensation
of $198,000 and a net increase in operating liabilities over operating assets of $15,000.
Net
Cash Used In Investing Activities.
For
the year ended December 31, 2021, no net cash was used in investing activities. For the year ended December 31, 2020 $1,000,000 was used
in investing activities as we paid milestone payments to NewStem Ltd. for additional investment.
For
the nine months ended September 30, 2022 and 2021, no net cash was used in investing activities.
Net
Cash Provided By Financing Activities.
For
the year ended December 31, 2021, net cash provided by financing activities was $100,000 consisting of short-term borrowings from
a director.
For
the year ended December 31, 2020, net cash provided by financing activities was $600,000 consisting of proceeds from the sale of stock.
For the nine months ended
September 30, 2022, net cash provided by financing activities was $65,000 consisting of the funding of new related party debt and the
retirement of previous related party debt.
For the nine months ended
September 30, 2021, net cash provided by financing activities was $100,000 consisting of the funding of related party debt.
Off-Balance
Sheet Arrangements
We
are not party to any off-balance sheet transactions. We have no guarantees or obligations other than those which arise out of normal
business operations.
Contractual
Obligations and Commercial Commitments
As
of December 31, 2021, we did not have contractual obligations and commercial commitments.
Item
3. Properties.
Our
corporate office is located at 2255 Glades Road, Boca Raton, FL 33431. We believe that our facilities are adequate for our current operations.
Item
4. Security Ownership of Certain Beneficial Owners and Management.
The
following table sets forth certain information with respect to the beneficial ownership of our common stock, as of June 28, 2023,
for each person known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock, each of our directors
and all directors as a group. The Company has no executive officers. Except as indicated in footnotes to this table, we believe that
the shareholders named in this table will have sole voting and investment power with respect to all shares of common stock shown to be
beneficially owned by them, based on information provided to us by such shareholders.
Security
Ownership of Certain Beneficial Owners and Management
| Name and Address of beneficial owner (6) | |
Amount and nature of beneficial ownership | | |
Percent of total common equity (1) | |
| Christine Jenkins | |
| -- | | |
| -- | |
| Michael Sosnowik | |
| 2,770,270 | | |
| 5.9 | % |
| Stephen Gans | |
| 5,537,978 | | |
| 11.8 | % |
| Jan Loeb | |
| 7,570,673 | (2)(3)(4) | |
| 15.1 | % |
| Jerry Wolasky | |
| 10,122,973 | (3)(4) | |
| 21.5 | % |
| Tracy Clifford | |
| 1,100,000 | (4) | |
| 2.3 | % |
| Eric Richman | |
| 754,054 | (3)(4) | |
| 1.5 | % |
| Mitchell Rubenstein | |
| 2,958,108 | (4)(5) | |
| 6.1 | % |
| David Seltzer | |
| 4,028,378 | (3)(4) | |
| 8.6 | % |
| All directors and officers as a group (seven persons) | |
| 26,484,186 | | |
| 49.4 | % |
(1)
Applicable percentage ownership is based on 46,881,475 shares of common stock outstanding as of June 28, 2023, together with securities
exercisable or convertible into shares of common stock within 60 days of June 28, 2023. Beneficial ownership is determined in
accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to
securities. Shares of common stock that a person has the right to acquire beneficial ownership of upon the exercise or conversion of
options, convertible stock, warrants or other securities that are currently exercisable or convertible or that will become exercisable
or convertible within 60 days of June 28, 2023, are deemed to be beneficially owned by the person holding such securities for
the purpose of computing the number of shares beneficially owned and percentage of ownership of such person, but are not treated as outstanding
for the purpose of computing the percentage ownership of any other person.
(2)
Includes 1,108,108 held in an IRA and 874,528 held as Trustee for the Steinberg Family Trust. Includes warrants to purchase 2.25 million
shares of common stock at an exercise price of $0.13 per share and options to purchase 1.10 million shares of common
stock at an exercise price of $0.10 per share.
(3)
Includes options to purchase 150,000 shares of common stock at an exercise price of $0.10 per share.
(4)
Director.
(5)
Includes options and warrants to purchase 1,850,000 shares of common stock at an exercise price of $0.10 per share.
(6)
The address of each person is c/o NovelStem International Corp. 2255 Glades Road, Suite 221A, Boca Raton, FL 33431.
Item
5. Directors and Executive Officers.
Biographical
and certain other information concerning the Company’s officers and directors is set forth below. There are no familial
relationships among any of our officers or directors. Except as indicated below, none of our directors is a director in any other
reporting companies. None of our officers or directors has been affiliated with any company that has filed for bankruptcy within
the last ten years except that Jan Loeb has previously been affiliated with Kid Brands, Inc., which filed for bankruptcy in June 2014.
We are not aware of any proceedings to which any of our officers or directors, or any associate of any such officer or
director is a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries. Unless
otherwise indicated, there are no arrangements or understandings between any officer and any other person pursuant to which such person
was selected as an officer.
| |
Jan
Loeb – President and Executive Chairman – 63 Mr. Loeb has more than 40 years of business, money management
and investment banking experience. He has served as Chairman of our Board since July 2018 and on September 29, 2022 was appointed
as Executive Chairman. On January 13, 2023, Mr. Loeb was appointed President of the Company. He has been the Managing Member
of Leap Tide Capital Management LLC since 2007 and has served as President and CEO of Acorn Energy, Inc. since January 2016 and as
a Director since August 2015. He has been a Director of Keweenaw Land Association, Ltd. From 2005 to 2007, Mr. Loeb was President
of Leap Tide’s predecessor, formerly known as AmTrust Capital Management Inc. He served as a Portfolio Manager of Chesapeake
Partners from February 2004 to January 2005 and as Managing Director at Jefferies & Company, Inc. from 2002 to 2004. From 1994
to 2001, he served as Managing Director at Dresdner Kleinwort Wasserstein, Inc. (formerly Wasserstein Perella & Co., Inc.). Mr.
Loeb was a Lead Director of American Pacific Corporation from 2013 to 2014 and a Director from 1997 to 2014. He also served as an
Independent Director of Pernix Therapeutics Holdings Inc. (formerly, Golf Trust of America, Inc.) from 2006 to 2011 and as a Director
of TAT Technologies, Ltd. from 2009 to 2016. |
| |
|
| |
Christine Jenkins – Vice President and
Chief Financial Officer – 59
Ms. Jenkins has over thirty-five years of experience in public accounting, including audit, consulting and corporate tax. Ms. Jenkins
is currently serving as a consultant providing audit and accounting consultation to publicly-traded and large privately held companies.
From 2010 to 2018 Ms. Jenkins was an audit partner with Cherry Bekaert, LLP. Prior to Cherry Bekaert, from 1995 to 2010, Ms. Jenkins
was a partner in a local accounting firm in Atlanta, GA. Prior experience included audit and tax positions in public accounting firms. |
| |
|
| |
Mitchell
Rubenstein – Director – 68 Mr. Rubenstein co-founded and served as Chairman of HMC from its inception to June 2018,
during which period the company returned approximately $37 million to shareholders in the form of dividends and share repurchases,
including a tender offer. He founded Syfy Channel and numerous other media and digital businesses. |
| |
|
| |
Eric
Richman – Director -61 Mr. Richman is a life science executive with significant leadership, operational and strategic experience
from over 25 years in the field. He is currently The CEO of Gain Therapeutics and was a Venture Partner at Brace Pharma Capital and
serves on the boards of LabConnect, F2G (board observer) and previously ADMA Biologics (NASDAQ: ADMA). Previously he served as President
& CEO of PharmAthene and prior to that was part of the founding team at MedImmune, responsible for the U.S. launch of its first
commercial product and an integral part of the global launch teams for other products. He began his career at HealthCare Ventures,
a life-sciences focused VC firm and formerly was a Director of Lev Pharmaceuticals (sold to Viropharma) and American Bank (sold to
Congressional Bancshares) and served as CEO of Tyrogenex (sold to Betta Pharma). |
| |
David
Seltzer – Director – 62 Mr. Seltzer is the CEO and Founder of Reliable 1 Laboratories LLC, a distributor of OTC medications
and nutritional supplements to independent pharmacies, longterm care pharmacies, hospitals and government organizations. He is also
a minority owner and Director at Leading Pharma LLC, a generic manufacturer of prescription drugs, having previously served as President
and CEO and later Chairman of Hi-Tech Pharmacal Co., Inc., which was acquired by Akorn, Inc. for $640 million in 2014. |
| |
|
| |
Jerry
Wolasky – Director – 64 Mr. Wolasky has over 35 years’ experience in the wholesale Pharmaceutical business,
most recently for the past 15 years in his current role as President of HealthSource Distributors LLC. He previously served in executive
positions of increasing responsibility for AmerisourceBergen, and its predecessor company, Bergen Brunswig. |
| |
|
| |
Tracy
Clifford – Director -53 Ms. Clifford has over twenty years of experience in accounting and finance, including mergers and
acquisitions of public companies. Ms. Clifford is the CFO of Acorn Energy, Inc. and COO of its operating subsidiary Omnimetrix Inc.
and since 2015 she has served as a contract CFO and COO for several clients, participated on advisory boards and worked on numerous
project engagements. Ms. Clifford previously served as CFO, Principal Accounting Officer, Corporate Controller and Secretary for
a publicly traded pharmaceutical company and a publicly-traded REIT from 1999 to 2015. Ms. Clifford’s prior experience included
accounting leadership positions at United Healthcare, the North Broward Hospital District and the audit team of Deloitte & Touche. |
Changes
in control
There
are no arrangements which may at a subsequent date result in a change in control of the Company
Item
6. Executive Compensation.
Prior to being appointed as our Chief Financial Officer
on September 29, 2022 and Vice President on January 13, 2023, beginning in March 2022, Ms. Jenkins served as our outside consultant
providing certain financial services. Ms. Jenkins is paid on an hourly basis. Mr. Loeb was appointed as Executive Chairman on September
29, 2022 and President on January 13, 2023 and does not receive any compensation for his role as an officer of the Company.
The
Company pays compensation to its directors pursuant to the NovelStem International Corp. Equity Incentive Plan (the “Plan”).
The
Plan provides for the grant to officers, directors, third party contractors and other future key employees of options to purchase
shares of common stock. Under the Plan, the Company is authorized to issue up to 7,000,000 shares of common stock as equity awards under
the Plan. Awards may be made in the form of options, stock appreciation rights (“SARs”), restricted stock or restricted stock
units, or stock bonus awards in respect of the Company’s common stock of the Company. Grants to any single participant or non-executive
director during any calendar year may not exceed 1,000,000 shares.
The
purchase price may be paid in cash or at the end of the option term, if the option is “in-the-money”, it is automatically
exercised “net”. In a net exercise of an option, the Company does not require a payment of the exercise price of the option
from the optionee but reduces the number of shares of common stock issued upon the exercise of the option by the smallest number of whole
shares that has an aggregate fair market value equal to or in excess of the aggregate exercise price for the option shares covered by
the option exercised. Each option is exercisable to one share of the Company’s common stock.
Options
awarded under the Plan shall be awarded at an exercise price of not less than the fair market value of a share of our common stock as
of the grant date and shall vest and become exercisable after a period not to exceed seven (7) years. SARs awarded under the Plan shall
have a strike price per share of common stock of not less than the fair market value of a share of our common stock, provided that, in
the case of a SAR granted in tandem with an option, the strike price shall not be less than the exercise price of the related option.
A SAR granted in tandem with an option shall become exercisable and shall expire according to the same vesting schedule and expiration
provisions as the corresponding option, such date not to exceed seven (7) years of the grant date.
In
the event of the termination of an employee, third
party service provider, officer or Director’s service on the Board of the Company for any reason other than for cause, all of the
Options which are then vested may be exercised within 18 months of such termination, provided that, in no event shall this extension
period continue beyond the expiration of the term of the option(s). In addition, any such extension shall be applicable only to the extent
that such option or options are vested and exercisable according to the terms of the Plan and any applicable option agreement.
Any unvested options are immediately terminated on the effective date of the termination. In the event of termination of an employee,
third party service provider, officer or Director’s service for cause, all options are forfeited and deemed cancelled and
no longer exercisable as of the date of termination.
Director
Compensation For The Fiscal Year Ended December 31, 2021
| Name | |
Fees
earned or paid in cash ($) | | |
Stock
Awards ($) | | |
Option
Awards ($) (1) | | |
Non-equity
incentive plan compensation ($) | | |
Nonqualified
deferred compensation earnings ($) | | |
All
other compensation ($) | | |
Total
($) | |
| Jan Loeb | |
| - | | |
| - | | |
| 14,500 | | |
| - | | |
| - | | |
| - | | |
| 14,500 | |
| Mitchell Rubenstein | |
| - | | |
| - | | |
| 14,500 | | |
| - | | |
| - | | |
| - | | |
| 14,500 | |
| Eric Richman | |
| - | | |
| - | | |
| 14,500 | | |
| - | | |
| - | | |
| - | | |
| 14,500 | |
| David Seltzer | |
| - | | |
| - | | |
| 14,500 | | |
| - | | |
| - | | |
| - | | |
| 14,500 | |
| Jerry Wolasky | |
| - | | |
| - | | |
| 14,500 | | |
| - | | |
| - | | |
| - | | |
| 14,500 | |
| Tracy Clifford | |
| - | | |
| - | | |
| 14,500 | | |
| - | | |
| - | | |
| - | | |
| 14,500 | |
| Directors
as a Group | |
| | | |
| | | |
| 87,000 | | |
| | | |
| | | |
| | | |
| 87,000 | |
| |
(1) |
Grant
date fair value computed in accordance with FASB ASC Topic 718 |
Compensation
Committee Interlocks and Insider Participation
We
do not have a compensation committee or persons participating in deliberations concerning executive officer compensation, as there was
no executive officer compensation paid.
Item
7. Certain Relationships and Related Transactions, and Director Independence.
Jan
Loeb, our President and Executive Chairman of the Board, is also the Chairman of the Board of NewStem.
In
June 2018, four directors participated in a financing conducted by the Company and purchased shares of our common stock as follows:
| | |
Shares | | |
Aggregate
Purchase Price | |
| Jan
Loeb | |
| 1,000,000 | | |
$ | 100,000 | |
| Jerry
Wolasky | |
| 5,000,000 | | |
| 500,000 | |
| Mitchell
Rubenstein | |
| 1,000,000 | | |
| 100,000 | |
| David
Seltzer | |
| 3,000,000 | | |
| 300,000 | |
| Total | |
| 10,000,000 | | |
$ | 1,000,000 | |
On
June 29, 2018, we issued five-year warrants to purchase up to 750,000 shares of common stock with an exercise price of $0.10 per share
to Mitchell Rubenstein in consideration for services rendered to the Company. These warrants expire on June 28, 2023.
On
June 29, 2018, we issued five-year warrants to purchase up to 2,250,000 shares of common stock with an exercise price of $0.13 per share
to Jan Loeb in consideration for services rendered to the Company. These warrants expire on June 28, 2023.
In
June 2020, three directors participated in a financing conducted by the Company and purchased shares of common stock as follows:
| | |
Shares | | |
Aggregate Purchase Price | |
| Jan Loeb | |
| 1,000,000 | | |
$ | 100,000 | |
| Jerry Wolasky | |
| 4,000,000 | | |
| 400,000 | |
| Eric Richman | |
| 500,000 | | |
| 50,000 | |
| Total | |
| 5,500,000 | | |
$ | 550,000 | |
On
November 15, 2021, in connection with the June 2018 and June 2020 financings, the Company issued 1,729,729 shares of common stock to
directors as a result of certain contingent assets not being realized, as required by the governing financing documents. A summary
of the shares issued follows:
| | |
Shares | |
| Jan Loeb | |
| 270,270 | |
| Mitchell Rubenstein | |
| 108,108 | |
| Jerry Wolasky | |
| 972,973 | |
| David Seltzer | |
| 324,324 | |
| Eric Richman | |
| 54,054 | |
| Total | |
| 1,729,729 | |
On
April 12, 2021, the Company entered into a promissory note (the “Note”) with Stephen Gans for $100,000. The Note accrued
interest at 8% per annum and matured on April 12, 2022. The proceeds of this Note were used to pay operating expenses of the Company
including directors and officer insurance premiums. Interest expense accrued related this this Note was $5,752 for the year ended December
31, 2021. The Note and all accrued interest were paid in full on February 16, 2022.
In
May 2022, the Company entered into an agreement Jan Loeb and Jerry Wolasky, a member of the Board, which was amended in July 2022, to
borrow up to an aggregate of $600,000 for working capital needs. This agreement provides for funding through January 31, 2024, provides
for interest at a rate of 8% per annum and matures the earlier of January 31, 2024 or 20 months from the date of the first funded amount
unless the lenders agree to extend the due date at that time. As of the date of this registration statement, the Company has drawn down
$200,000 under the aforementioned agreement.
Except
as disclosed herein, no director, executive officer, shareholder holding at least 5% of shares of our common stock, or any family member
thereof, had any material interest, direct or indirect, in any transaction, or proposed transaction since January 1, 2019, in which the
amount involved in the transaction exceeds the lesser of $120,000 or one percent of the average of our total assets at the year-end for
the last two completed fiscal years.
Review,
Approval or Ratification of Transactions with Related Persons
The
Board conducts an appropriate review of and oversees all related party transactions on a continuing basis and reviews potential conflict
of interest situations where appropriate. The Board has adopted formal standards to apply when it reviews, approves or ratifies any related
party transaction. In addition, the Board applies the following standards to such reviews: (i) all related party transactions must be
fair and reasonable and on terms comparable to those reasonably expected to be agreed to with independent third parties for the same
goods and/or services at the time they are authorized by the Board and (ii) all related party transactions should be authorized, approved
or ratified by the affirmative vote of a majority of the directors who have no interest, either directly or indirectly, in any such related
party transaction.
Director
Independence.
We
have determined that, under the criteria established by NASDAQ and by our board of directors, Tracy Clifford, Eric Richman,
Mitchell Rubenstein and David Seltzer are independent.
Item
8. Legal Proceedings.
As noted above, NetCo owns
all rights to the “Tom Clancy’s Net Force” intellectual property in all media, including film, television, and video
games. As part of the joint venture, NetCo has published more than a dozen books and had an ABC miniseries.
After
Tom Clancy passed away in 2013, his estate and business partners refused to cooperate in exploiting the intellectual property.
After trying to amicably resolve the dispute, the Company initiated arbitration proceedings with the American Arbitration Association.
The Company’s arbitration demand asserts claims for breach of the joint venture agreement and breach of fiduciary duty. Both
claims arise from C.P. Group’s failure to make reasonable, good faith efforts to exploit the full array of media rights relating
to Net Force. The Company’s goal is to maximize the total potential value of the NetCo intellectual property across video
games, streaming, digital media, merchandising and other ancillary markets. The Company believes that the value of the intellectual
property is significant.
The arbitration evidentiary
hearing concluded on October 20, 2022, and the arbitrator ordered the parties to submit post-hearing briefs. The briefs were
filed January 26, 2023. It is unknown as to how long the arbitrator will take to render his decision.
To fund efforts to maximize the value of NetCo,
NovelStem has secured non-recourse litigation funding.
Item
9. Market Price of and Dividends on the Registrant’s Common Equity and Related Shareholder Matters.
Market
information
There
is no established public trading market in our common stock, and a regular trading market may not develop, or if developed, may not be
sustained. Our securities are currently quoted on the OTC Markets Pink under the symbol “NSTM”. The following reflect
inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| | |
High | | |
Low | |
| Fiscal 2023 | |
| | | |
| | |
| Quarter
ended 6/30/2023 (through June 28, 2023) | |
$ | 0.24 | | |
$ | 0.18 | |
| Quarter
ended 3/31/2023 | |
$ | 0.20 | | |
$ | 0.15 | |
| Fiscal 2022 | |
| | | |
| | |
Quarter ended 12/31/2022
| |
$ | 0.21 | | |
$ | 0.11 | |
| Quarter ended 9/30/2022 | |
$ | 0.28 | | |
$ | 0.14 | |
| Quarter ended 6/30/2022 | |
$ | 0.33 | | |
$ | 0.18 | |
| Quarter ended 3/31/2022 | |
$ | 0.30 | | |
$ | 0.13 | |
| | |
| | | |
| | |
| Fiscal 2021 | |
| | | |
| | |
| Quarter ended 12/31/2021 | |
$ | 0.31 | | |
$ | 0.20 | |
| Quarter ended 9/30/2021 | |
$ | 0.35 | | |
$ | 0.19 | |
| Quarter ended 6/30/2021 | |
$ | 0.35 | | |
$ | 0.23 | |
| Quarter ended 3/31/2021 | |
$ | 0.30 | | |
$ | 0.16 | |
| | |
| | | |
| | |
| Fiscal 2020 | |
| | | |
| | |
| Quarter ended 12/31/2020 | |
$ | 0.20 | | |
$ | 0.05 | |
| Quarter ended 9/30/2020 | |
$ | 0.09 | | |
$ | 0.05 | |
| Quarter ended 6/30/2020 | |
$ | 0.13 | | |
$ | 0.07 | |
| Quarter ended 3/31/2020 | |
$ | 0.13 | | |
$ | 0.08 | |
Holders
As
of June 28, 2023 there were 46,881,475 shares of common stock outstanding held by approximately 90 record holders.
Dividends
We
have never paid cash dividends on any of our capital stock and currently intend to retain our future earnings, if any, to fund the development
and growth of our business. We do not expect to pay any dividends on any of our capital stock in the foreseeable future.
Securities
authorized for issuance under equity compensation plans.
Equity
Compensation Plan Information
| Plan
category | |
Number
of securities to be issued upon exercise of outstanding options, warrants and rights | | |
Weighted-average
exercise price of outstanding options, warrants and rights | | |
Number
of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |
| | |
(a) | | |
(b) | | |
(c) | |
| Equity compensation plans
approved by security holders | |
| | | |
| | | |
| | |
| Equity compensation
plans not approved by security holders | |
| 8,400,000 | | |
$ | 0.13 | | |
| 1,600,000 | |
| Total | |
| 8,400,000 | | |
$ | 0.13 | | |
| 1,600,000 | |
Item
10. Recent Sales of Unregistered Securities.
In
June and July 2018, we issued 19 million shares to ten investors for an aggregate purchase price of $1.9 million. Jan Loeb, Jerry
Wolasky, Mitchell Rubenstein, and David Seltzer, members of our Board of Directors, participated in the financing.
On
June 29, 2018, we issued five-year warrants to purchase up to 750,000 shares of common stock with an exercise price of $0.10 per share
to Mitchell Rubenstein in consideration for services rendered to the Company. These warrants expire on June 28, 2023.
On
June 29, 2018, we issued five-year warrants to purchase up to 2,250,000 shares of common stock with an exercise price of $0.13 per share
to Jan Loeb in consideration for services rendered to the Company. These warrants expire on June 28, 2023.
In
2019 we issued options to purchase up to 300,000 shares of common stock at an exercise price of $0.10 per share to members of our Board
pursuant to the Plan in consideration for their services on our Board.
On
December 11, 2019, we issued 2,500,000 shares of common stock to Stephen Gans for a purchase price of $.10 per share.
On
June 25, 2020, we issued 6,000,000 shares of common stock in consideration for a purchase price of $0.10 per share to three existing
shareholders. Jan Loeb, Jerry Wolasky and Eric Richman, members of our Board of Directors, participated in the financing.
In
2020 we issued options to purchase up to 3,700,000 shares of common stock at an exercise price of $0.10 per share to members of our Board
pursuant to the Plan in consideration for their services on our Board.
On
November 15, 2021, in a noncash transaction, the Company issued approximately 3,000,000 shares of common stock to certain
existing shareholders. The subscription agreements governing the original investments in June 2018 and June 2020
provided for the issuance of additional shares if certain contingent assets were not realized. It was determined during the year
ended December 31, 2021 that the contingent asset would not be realized and such additional shares were issued for no
additional consideration.
In
2022 we issued options to purchase up to 1,100,000 shares of common stock at an exercise price of $0.29 per share to members of our Board
pursuant to the Plan in consideration for their services on our Board.
None
of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. We believe that
the offers, sales, and issuances of the above securities were exempt from registration under the Securities Act by virtue of Section 4(a)(2)
of the Securities Act or Regulation D promulgated thereunder as transactions by an issuer not involving any public offering, or in reliance
on Rule 701 promulgated under Section 3(b) of the Securities Act because the transactions were pursuant to compensatory benefit
plans or contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions
represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any
distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had
adequate information about us or had adequate access, through their relationships with us, to information about us.
Item
11. Description of Registrant’s Securities to be Registered.
The
following description summarizes the material terms of our capital stock as of the date of this registration statement. Because it is
only a summary, it does not contain all the information that may be important to you. For a complete description of our capital stock,
you should refer to our Articles of Incorporation and our Bylaws, and to the provisions of applicable Florida law.
Common
Stock
We
are authorized to issue up to 100,000,000 shares of our common stock, par value $0.01. Each share of common stock entitles the holder
to one (1) vote on each matter submitted to a vote of our shareholders, including the election of directors. There is no cumulative voting.
Our shareholders are entitled to receive ratably such dividends, if any, as may be declared from time to time by the Board of Directors.
Shareholders have no preemptive, conversion or other subscription rights. There are no redemption or sinking fund provisions related
to our common stock. In the event of liquidation, dissolution or winding up of the Company, our shareholders are entitled to share ratably
in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding.
Preferred
Stock
We
are authorized to issue up to 1,000,000 shares of preferred stock, par value $0.01, in one or more classes or series. The Board of Directors
is authorized to fix whether or not each class or series is to have voting rights and what the preferences and relative, participating
or other special rights may be. The Board of Directors is authorized to determine the dividend rate and the manner and timing pursuant
to which dividends are paid. No preferred stock is currently outstanding.
Options
We
are authorized to up to 7 million shares of common stock pursuant to the Plan. As of the date of this registration statement, we have
issued options to purchase up to an aggregate of 5.4 million shares of common stock with exercise prices ranging from $0.10 to $0.29
per share. Of the foregoing, 4.3 million options have vested and 1.1 million options will vest on January 1, 2023. The options
expire seven years after the grant date.
Warrants
0n
June 29, 2018, we issued five year warrants to purchase up to 750,000 shares of common stock with an exercise price of $0.10 per
share. These warrants expire on June 28, 2023.
On
June 29, 2018, we issued five year warrants to purchase up to 2,250,000 shares of common stock with an exercise price of $0.13
per share. These warrants expire on June 28, 2023.
Item
12. Indemnification of Directors and Officers.
The
Company has authority under Section 607.0850 of the Florida Business Corporation Act (the “FBCA”) to indemnify its directors
and officers to the extent provided for in the FBCA against liability which a director or officer may incur in his or her capacity as
such. The Company’s Articles of Incorporation, as amended, provide that the Company shall indemnify its officers and directors
to the fullest extent not prohibited by law.
The
Company has entered into agreements with each of its directors wherein it agreed to indemnify each of them to the fullest extent permitted
by law, and the Company may from time to time enter into other agreements with such persons regarding such indemnification.
The
provisions of the FBCA that authorize indemnification do not eliminate the duty of care of a director, and in appropriate circumstances,
equitable remedies such as injunctive or other forms of non-monetary relief will remain available under Florida law. In addition, the
FBCA does not permit indemnification of a director or officer under certain circumstances, including in the event that a judgment or
other final adjudication establishes that his or her actions or omissions were material to the cause of action so adjudicated and constitute:
(a) violations of criminal laws, unless the director or officer had reasonable cause to believe his conduct was lawful or had no reasonable
cause to believe his conduct was unlawful, (b) deriving an improper personal benefit from a transaction, (c) in the case of a director,
a circumstance under which the director has liability for voting for or assenting to an unlawful distribution, and (d) willful misconduct
or conscious disregard for the best interests of the Company in a proceeding by or in the right of the Company to procure a judgment
in its favor or in a proceeding by or in the right of a shareholder.
The
Company has obtained and currently intends to maintain in effect directors’ and officers’ liability insurance policies providing
customary coverage for its directors and officers against losses and liabilities incurred by them in their capacities as directors and
officers of the Company.
The
above discussion of the Company’s Articles of Incorporation, indemnification agreements, and Florida laws, is only a general summary
and is respectively qualified in its entirety by such documents and laws.
Item
13. Financial Statements and Supplementary Data.
The
information required by this item may be found beginning on page F-1 of this Form 10.
Item
14. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item
15. Financial Statements and Exhibits.
| |
(a) |
Financial
Statements. |
The
following financial statements are filed as part of this registration statement:
NOVELSTEM
INTERNATIONAL CORP.
Years
Ended December 31, 2021 and 2020
Index
to Audited Financial Statements
NOVELSTEM INTERNATIONAL CORP.
Nine Months Ended September 30, 2022 and 2021
Index to Unaudited Condensed
Financial Statements
NEWSTEM,
LTD.
Years
Ended December 31, 2021 and 2020
Index
to Audited Financial Statements
NEWSTEM, LTD
Nine Months Ended September 30, 2022 and 2021
Index to Unaudited Condensed Financial Statements
Report
of Independent Registered Public Accounting Firm
To
the Board of Directors and Shareholders
NovelStem
International Corp.
Boca
Raton, Florida
Opinion
on the Financial Statements
We
have audited the accompanying balance sheets of NovelStem International Corp. (the “Company”) as of December 31, 2021 and
2020, and the related statements of operations, shareholders’ equity, and cash flows for each of the years in the two-year period
ended December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion,
the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and
2020, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity
with accounting principles generally accepted in the United States of America.
Restatement
of Previously Issued Financial Statements
As
discussed in Note 8, the accompanying financial statements referred to in the previous paragraph have been restated for a correction
of an error.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,
we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe our audits provide
a reasonable basis for our opinion.
/s/
Cherry Bekaert LLP
We
have served as the Company’s auditor since 2021.
Fort
Lauderdale, Florida
August
1, 2022 (except for the effect of the restatement disclosed in Notes 3 and 8
as
to which the date is October 11, 2022).
NOVELSTEM
INTERNATIONAL CORP.
BALANCE SHEETS
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 8,666 | | |
$ | 89,594 | |
| Prepaid expenses | |
| 28,316 | | |
| 31,235 | |
| Total current assets | |
| 36,982 | | |
| 120,829 | |
| Investment in Netco Partners | |
| 137,011 | | |
| 137,011 | |
| Investment in NewStem, Ltd | |
| 2,435,155 | | |
| 3,299,713 | |
| Total assets | |
$ | 2,609,148 | | |
$ | 3,557,553 | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 49,777 | | |
$ | 29,071 | |
| Note payable | |
| 100,000 | | |
| - | |
| Accrued expenses | |
| 43,425 | | |
| 39,674 | |
| Total current liabilities | |
| 193,202 | | |
| 68,745 | |
| Commitments and contingencies (see Note 7) | |
| | | |
| | |
| Shareholders’ equity: | |
| | | |
| | |
| Common stock, $.01 par value, 100,000,000 shares authorized, 50,316,672 and 47,316,674 shares issued, respectively, and 46,881,475 and 43,881,477 shares outstanding, respectively, as of December 31, 2021 and 2020 | |
| 468,815 | | |
| 438,815 | |
| Additional paid-in capital | |
| 290,321,665 | | |
| 290,078,899 | |
| Accumulated deficit | |
| (288,174,780 | ) | |
| (286,829,152 | ) |
| Treasury stock, at cost, 3,435,197 shares at December 31, 2021 and 2020 | |
| (199,754 | ) | |
| (199,754 | ) |
| Total shareholders’ equity | |
| 2,415,946 | | |
| 3,488,808 | |
| Total liabilities and shareholders’ equity | |
$ | 2,609,148 | | |
$ | 3,557,553 | |
The
accompanying notes are an integral part of these financial statements.
NOVELSTEM
INTERNATIONAL CORP.
STATEMENTS
OF OPERATIONS
| | |
Year Ended
December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| Operating expenses: | |
| | | |
| | |
| General and administrative expenses | |
$ | 222,769 | | |
$ | 150,708 | |
| Stock compensation expense | |
| 272,766 | | |
| 79,587 | |
| Total operating expenses | |
| 495,535 | | |
| 230,295 | |
| Loss from operations | |
| (495,535 | ) | |
| (230,295 | ) |
| Interest (income) expense | |
| 6,825 | | |
| (16,514 | ) |
| Loss before income taxes | |
| (502,360 | ) | |
| (213,781 | ) |
| Provision for income tax | |
| - | | |
| - | |
| Net loss before equity in net loss of equity method investees | |
| (502,360 | ) | |
| (213,781 | ) |
| Equity in net loss of equity method investees | |
| (843,268 | ) | |
| (331,658 | ) |
| Net loss | |
$ | (1,345,628 | ) | |
$ | (545,439 | ) |
| | |
| | | |
| | |
| Basic and diluted net loss per share: | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.03 | ) | |
$ | (0.01 | ) |
| Weighted average number of shares outstanding – basic | |
| 44,259,559 | | |
| 40,988,326 | |
| Weighted average number of shares outstanding – diluted | |
| 44,259,559 | | |
| 40,988,326 | |
The
accompanying notes are an integral part of these financial statements.
NOVELSTEM
INTERNATIONAL CORP.
STATEMENTS
OF SHAREHOLDERS’ EQUITY
| | |
| | |
| | |
| | |
| | |
Number | | |
| | |
| |
| | |
| | |
| | |
Additional | | |
| | |
of | | |
| | |
Total | |
| | |
Number of | | |
Common | | |
Paid-In | | |
Accumulated | | |
Treasury | | |
Treasury | | |
Shareholders’ | |
| | |
Shares | | |
Stock | | |
Capital | | |
Deficit | | |
Shares | | |
Stock | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, January 1, 2020 | |
| 37,881,477 | | |
$ | 378,815 | | |
$ | 289,459,312 | | |
$ | (286,283,713 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 3,354,660 | |
| Net loss, restated | |
| - | | |
| - | | |
| - | | |
| (545,439 | ) | |
| - | | |
| - | | |
| (545,439 | ) |
| Proceeds from sale of stock | |
| 6,000,000 | | |
| 60,000 | | |
| 540,000 | | |
| - | | |
| - | | |
| - | | |
| 600,000 | |
| Stock option compensation | |
| - | | |
| - | | |
| 79,587 | | |
| - | | |
| | | |
| | | |
| 79,587 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance December 31, 2020, Restated | |
| 43,881,477 | | |
| 438,815 | | |
| 290,078,899 | | |
| (286,829,152 | ) | |
| 3,435,197 | | |
| (199,754 | ) | |
| 3,488,808 | |
| Net loss, restated | |
| - | | |
| - | | |
| - | | |
| (1,345,628 | ) | |
| - | | |
| - | | |
| (1,345,628 | ) |
| Stock issued | |
| 2,999,998 | | |
| 30,000 | | |
| (30,000 | ) | |
| - | | |
| - | | |
| - | | |
| - | |
| Stock option compensation | |
| - | | |
| - | | |
| 272,766 | | |
| - | | |
| - | | |
| - | | |
| 272,766 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, December 31, 2021, Restated | |
| 46,881,475 | | |
$ | 468,815 | | |
$ | 290,321,665 | | |
$ | (288,174,780 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 2,415,946 | |
The
accompanying notes are an integral part of these financial statements.
NOVELSTEM
INTERNATIONAL CORP.
STATEMENTS
OF CASH FLOWS
| | |
Year Ended | |
| | |
December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (1,345,628 | ) | |
$ | (545,439 | ) |
| Equity in loss of equity method investees | |
| 864,558 | | |
| 331,658 | |
| Distribution from NetCo | |
| - | | |
| 9,375 | |
| Stock-based compensation | |
| 272,766 | | |
| 79,587 | |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Current assets and other assets | |
| 2,919 | | |
| 508,866 | |
| Accounts payable and accrued expenses | |
| 24,457 | | |
| 35,291 | |
| Net cash provided from operating activities | |
| (180,928 | ) | |
| 419,338 | |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Investment in NewStem, Ltd. | |
| - | | |
| (1,000,000 | ) |
| Net cash from investing activities | |
| - | | |
| (1,000,000 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from issuance of notes payable | |
| 100,000 | | |
| - | |
| Proceeds from the sale of stock | |
| - | | |
| 600,000 | |
| Net cash from financing activities | |
| 100,000 | | |
| 600,000 | |
| | |
| | | |
| | |
| Net change in cash | |
| (80,928 | ) | |
| 19,338 | |
| Cash at the beginning of the year | |
| 89,594 | | |
| 70,256 | |
| Cash at the end of the year | |
$ | 8,666 | | |
$ | 89,594 | |
| | |
| | | |
| | |
| Supplemental cash flow information: | |
| | | |
| | |
| Cash received during the year for: | |
| | | |
| | |
| Alternative minimum tax refund | |
$ | - | | |
$ | 518,819 | |
| Cash paid during the year for: | |
| | | |
| | |
| Interest | |
$ | 1,073 | | |
$ | 1,081 | |
The
accompanying notes are an integral part of these financial statements.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
NOTE
1—NATURE OF OPERATIONS
Description
of Business
NovelStem
International Corp. (“NovelStem” or the “Company”) is a holding company whose principal assets are a 31.5% equity
interest in NewStem Ltd, an Israeli biotech company (“NewStem”), and a 50% ownership interest in NetCo Partners
(“NetCo”). NovelStem was formerly known as Hollywood Media Corp. The Company was incorporated in the State of Florida on
January 22, 1993 and changed its name to NovelStem International Corp. in September 2018 as a result of its business focus shift from
a media business to cutting edge biotech.
NewStem
focuses on the development and commercialization of diagnostic technology that can predict patients’ anti-cancer drug resistance,
allowing for targeted cancer treatments and the potential to reduce resistance to chemotherapy.
NetCo
is a legacy media business interest which owns “Net Force”, a book publishing franchise.
Liquidity
and Management’s Plans
Since
inception, the Company has accumulated a deficit of approximately $288,000,000. The accumulated deficit of the Company subsequent to
its business focus shift and name change in September 2018 is approximately $1,513,000 which is comprised primarily of allocated losses
from equity method investments and general and administrative costs incurred by the Company.
The
Company will need to obtain additional funds to continue its operations. Management’s plans with regard to these matters include
additional financing and fundraising until its equity investment in NewStem is profitable. Although management continues to pursue these
plans, there is no assurance that the Company will be successful in obtaining sufficient cash from financing on terms acceptable to the
Company, or that NewStem will become profitable (see Note 3).
In
the period subsequent to these financial statements, the Company entered into a financing agreement to borrow up to $600,000 for working
capital needs (see Note 9). Following this financing, the Company believes that its cash resources are sufficient for the operations
of the next 12 months.
NOTE
2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The Financial Accounting Standards Board (“FASB”) has established the FASB Accounting Standards Codification (“ASC”)
as the single source of authoritative GAAP.
Use
of Estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect certain
reported amounts and disclosures. Accordingly, actual results could differ from those estimates.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
Cash
and Cash Equivalents
Cash
and cash equivalents include certain investments in highly liquid debt instruments with original maturities of three months or less at
the date of purchase. The Company had no cash equivalents as of either year end presented.
Equity
Investments
Investee
companies that are not consolidated, but over which the Company exercises significant influence, are accounted for under the equity method
of accounting. Whether or not the Company exercises significant influence with respect to an Investee depends on an evaluation of several
factors, including, among others, representation on the Investee company’s board of directors and ownership level, which is generally
a 20% to 50% interest in the voting securities of the Investee company. Under the equity method of accounting, an Investee company’s
accounts are not reflected within the Company’s Balance Sheets or Statements of Operations; however, the Company’s share
of the earnings or losses of the Investee company is reflected in the caption “Equity in net income (loss) of affiliates”
in the Statements of Operations. The Company’s carrying value in an equity method Investee company is reflected in the caption
“Investment in Investee company’ in the Company’s Balance Sheets.
When
the Company’s carrying value in an equity method Investee company is reduced to zero, no further losses are recorded in the Company’s
financial statements unless the Company guarantied obligations of the Investee company or has committed additional funding. When the
Investee company subsequently reports income, the Company will not record its share of such income until it equals the amount of its
share of losses not previously recognized.
The
Company reviews equity investments for impairment on an annual basis, or earlier if events or changes in circumstances indicate that
the carrying amounts might not be recoverable.
The
Company holds a minority investment in an entity, NewStem, Ltd (“NewStem”) which is accounted for pursuant to the equity
method of accounting. Additionally, the Company is a 50% owner in NetCo Partners (NetCo” which is accounted for pursuant to the
equity method of accounting. See Note 3.
Treasury
Stock
Shares
of common stock repurchased are recorded at cost as treasury stock.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
Stock-Based
Compensation
The
Company accounts for stock-based awards in accordance with applicable accounting principles, which requires compensation expense related
to share-based transactions to be measured and recognized in the financial statements based on a determination of the fair value of the
stock options. The grant date fair value is determined using the Black-Scholes-Merton (“Black-Scholes”) pricing model. For
all stock options, the Company recognizes expense over on an accelerated basis over the requisite service period (generally the vesting
period of the equity grant). The Company’s option pricing model requires the input of highly subjective assumptions, including
the expected stock price volatility, expected term, and forfeiture rate. Any changes in these highly subjective assumptions significantly
impact stock-based compensation expense.
Options
awarded to purchase shares of common stock issued to non-employees in exchange for services are accounted for as variable awards in accordance
with applicable accounting principles. Such options are valued using the Black-Scholes option pricing model.
In
the event of the termination of an employee, third party service provider, officer or Director’s service on the Board of the Company
for any reason other than for cause, all of the Options which are then vested may be exercised within 18 months of such termination,
provided that, in no event shall this extension period continue beyond the expiration of the term of the option(s). In addition, any
such extension shall be applicable only to the extent that such option or options are vested and exercisable according to the terms of
the Plan and this Agreement. Any unvested options are immediately terminated on the effective date of the termination. In the event of
termination of an employee, third party service provider, officer or Director’s service for cause, all Options are forfeited and
deemed cancelled and no longer exercisable on the date of termination.
See
Note 5 for the assumptions used to calculate the fair value of stock-based compensation. Upon the exercise of options, it is the Company’s
policy to issue new shares rather than utilizing treasury shares.
Income
Taxes
Deferred
income taxes are determined using the asset and liability method in accordance with Accounting Standards Codification (“ASC”)
Topic 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the
financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income taxes are measured
using enacted tax rates expected to apply to taxable income in years in which such temporary differences are expected to be recovered
or settled. The effect of a change in tax rates on deferred income taxes is recognized in the statement of operations of the period that
includes the enactment date. In addition, a valuation allowance is established to reduce any deferred tax asset for which it is determined
that it is more likely than not that some portion of the deferred tax asset will not be realized.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
Basic
and Diluted Net Loss Per Share
Basic
net income per share is computed by dividing the net income by the weighted average number of shares outstanding during the year, excluding
treasury stock. Diluted net income per share is computed by dividing the net income by the weighted average number of shares outstanding
plus the dilutive potential of common shares which would result from the exercise of stock options and warrants. The dilutive effects
of stock options and warrants are excluded from the computation of diluted net income per share if the effect of doing so would be antidilutive.
The
following data represents the amounts used in computing earnings per share and the effect on net income (loss) and the weighted average
number of shares of dilutive potential common stock:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| Net loss available to common shareholders | |
$ | (1,345,628 | ) | |
$ | (545,439 | ) |
| | |
| | | |
| | |
| Weighted average shares outstanding: | |
| | | |
| | |
| -Basic | |
| 44,259,559 | | |
| 40,988,326 | |
| Add: Warrants | |
| - | | |
| - | |
| Add: Stock options | |
| - | | |
| - | |
| -Diluted | |
| 44,259,559 | | |
| 40,988,326 | |
| | |
| | | |
| | |
| Basic and diluted net loss per share | |
$ | (0.03 | ) | |
$ | (0.01 | ) |
NOTE
3—EQUITY METHOD INVESTMENTS
Investment
in NewStem, Ltd.
In
2018, the Company entered into a Share Purchase Agreement with NewStem and other related parties to provide aggregate funding of up to
$4,000,000 to NewStem Ltd. This funding was to be provided through the sale of up to 50,000 common shares of NewStem, Ltd. to the Company
representing 33% of New Stem Ltd.’s outstanding shares. In 2018, the Company purchased 25,000 shares of NewStem, Ltd. for $2,000,000
acquiring an ownership interest of 20%. The Company made additional investments in 2019 and 2020 purchasing 12,500 shares each year for
a $1,000,000 investment each year resulting in an ownership interest of 31.51% and 33.33%, respectively, as of December 31, 2021 and
2020.
The
Company accounts for its investment in NewStem under the equity method. At December 31, 2021 and 2020, the carrying value of the investment
in NewStem exceeded the underlying net assets of NewStem by $2,435,155 and $3,299,713, respectively. The excess relates to identified
intangible assets including license agreements, specialized work force (goodwill) and two separate projects of in process research and
development (“IPR&D”) related to stem cell-based diagnostics and therapeutics for cancer chemotherapies.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
The
Company assesses its investment in NewStem for impairment on an annual basis.
NewStem
is in the development stage and has incurred losses since its inception and has yet to generate any revenues. NewStem will need to obtain
additional funds to continue its operations. NewStem management’s plans with regard to these matters include continued development,
marketing and licensing of its products, as well as seeking additional financing arrangements. Although management continues to pursue
these plans, there is no assurance that the Company will be successful in obtaining sufficient cash from sales of products or financing
on terms acceptable to the Company. NewStem management reports fundraising activities in April 2022 that provide additional funding of
approximately $1,600,000.
The
following table represents the Company’s investment in NewStem Ltd.:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| Investment in NewStem, Ltd., beginning | |
$ | 3,299,713 | | |
$ | 2,640,746 | |
| Allocation of net loss from NewStem, Ltd. | |
| (864,558 | ) | |
| (341,033 | ) |
| Purchase of NewStem, Ltd shares | |
| - | | |
| 1,000,000 | |
| Investment in NewStem, Ltd., ending | |
$ | 2,435,155 | | |
$ | 3,299,713 | |
The
results of operations and financial position of the Company’s investment in NewStem Ltd. are summarized below:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| Condensed income statement information: | |
| | | |
| | |
| Net sales | |
$ | - | | |
$ | - | |
| Gross margin | |
$ | - | | |
$ | - | |
| Net loss | |
$ | (2,630,000 | ) | |
$ | (1,224,000 | ) |
| Company’s allocation of net loss from NewStem, Ltd. | |
$ | (864,558 | ) | |
$ | (341,033 | ) |
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| Condensed balance sheet information: | |
| | | |
| | |
| Current assets | |
$ | 1,425,000 | | |
$ | 1,599,000 | |
| Non-current assets | |
$ | 41,000 | | |
$ | 14,000 | |
| Current liabilities | |
$ | 227,000 | | |
$ | 69,000 | |
| Non-current liabilities | |
$ | 134,000 | | |
$ | - | |
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
Investment
in NetCo Partners
NovelStem
owns a 50% interest in NetCo Partners, a joint venture that owns the Net Force publishing franchise, NetCo Partners. The Company accounts
for its investment in NetCo Partners under the equity method and recognizes nominal royalties from this arrangement. The Company assesses
its investment in NetCo for impairment on an annual basis.
The
following table represents the Company’s investment in NetCo Partners:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| Investment in NetCo Partners, beginning | |
$ | 137,011 | | |
$ | 137,011 | |
| Allocation of net income from Netco Partners | |
| 21,290 | | |
| 9,375 | |
| Distribution from NetCo Partners | |
| (21,290 | ) | |
| (9,375 | ) |
| Investment in NetCo Partners, ending | |
$ | 137,011 | | |
$ | 137,011 | |
The
results of operations and financial position of the Company’s investment in NetCo Partners are summarized below:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| Condensed income statement information: | |
| | | |
| | |
| Net sales | |
$ | 42,580 | | |
$ | 18,750 | |
| Gross margin | |
$ | - | | |
$ | - | |
| Net income | |
$ | 42,580 | | |
$ | 18,750 | |
| Company’s allocation of net income from Netco Partners | |
$ | 21,290 | | |
$ | 9,375 | |
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| Condensed balance sheet information: | |
| | |
| |
| Current assets | |
$ | 13,475 | | |
$ | 28,580 | |
| Non-current assets | |
$ | 272,799 | | |
$ | 272,799 | |
| Current liabilities | |
$ | 12,252 | | |
$ | 27,357 | |
| Non-current liabilities | |
$ | - | | |
$ | - | |
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
NOTE
4—NOTE PAYABLE
On
April 12, 2021, the Company entered into a promissory note (the “Note”) with a related party (individual) for $100,000. The
Note accrues interest at 8% per annum and matured on April 12, 2022. The proceeds of this Note were used to pay operating expenses of
the Company including directors and officer insurance premiums. Interest expense accrued related this this Note was $5,752 for the year
ended December 31, 2021. The Note and all accrued interest were paid in full on February 16, 2022.
NOTE
5—EQUITY
(a)
General
At
December 31, 2021 and 2020 the Company had issued and outstanding 46,881,475 and 43,881,477, respectively, shares of its common stock,
par value $0.01 per share. Holders of outstanding common stock are entitled to receive dividends when, as and if declared by the Board
and to share ratably in the assets of the Company legally available for distribution in the event of a liquidation, dissolution or winding
up of the Company.
On
November 15, 2021, in a noncash transaction, the Company issued approximately 3,000,000 shares of common stock to existing holders of
subscription agreements dated June 2020. These subscription agreements provided for the issuance of additional shares if certain contingent
assets were not realized. It was determined during the year ended December 31, 2021 that the contingent asset would not be realized and
the shares were issued.
(b)
Summary Employee Option Information
The
Company’s stock option plans provide for the grant to officers, directors, third party contractors and other future key employees
of options to purchase shares of common stock. The purchase price may be paid in cash or at the end of the option term, if the option
is “in-the-money”, it is automatically exercised “net”. In a net exercise of an option, the Company does not
require a payment of the exercise price of the option from the optionee but reduces the number of shares of common stock issued upon
the exercise of the option by the smallest number of whole shares that has an aggregate fair market value equal to or in excess of the
aggregate exercise price for the option shares covered by the option exercised. Each option is exercisable to one share of the Company’s
common stock. Most options expire within six years from the date of the grant and generally vest on the first anniversary date of their
issuance. Pursuant to the Equity Incentive Plan the Company’s board of directors approved on November 12, 2018, an aggregate of
4,300,000 options have been issued to directors and investor relations professionals.
The
Company utilized the Black-Scholes option-pricing model to estimate fair value, utilizing the following assumptions for the respective
years (all in weighted averages):
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| Risk-free interest rate | |
| 1.6 | % | |
| 0.9 | % |
| Expected term, in years | |
| 6 | | |
| 6 | |
| Expected volatility | |
| 140.2 | % | |
| 171.9 | % |
| Expected dividend yield | |
| 0 | % | |
| 0 | % |
| Determined weighted average grant date fair value per option | |
$ | - | | |
$ | 0.09 | |
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
The
expected term of the options represents an estimate of the length of time until the expected date of exercising the options. Options
granted have a maximum life of 6 years. With respect to determining expected exercise behavior, the Company has grouped its option grants
into certain groups in order to track exercise behavior and establish historical rates. The Company estimated volatility by considering
historical stock volatility over the expected term of the option. The risk-free interest rates are based on the U.S. Treasury yields
for a period consistent with the expected term. The dividend yield of 0% is based on the Company’s history and expectation of dividend
payout. The Company has not paid and does not anticipate paying of dividends in the near future.
(c)
Summary Option Information
A
summary of the Company’s option plans as of December 31, 2021 and 2020, as well as changes during each of the years then ended,
is presented below:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
Number | | |
Weighted | | |
Number | | |
Weighted | |
| | |
of | | |
Average | | |
of | | |
Average | |
| | |
Options | | |
Exercise | | |
Options | | |
Exercise | |
| | |
(in shares) | | |
Price | | |
(in shares) | | |
Price | |
| Outstanding at beginning of year | |
| 4,300,000 | | |
| 0.10 | | |
| 600,000 | | |
| 0.10 | |
| Granted | |
| - | | |
| - | | |
| 3,700,000 | | |
| 0.10 | |
| Outstanding at end of year | |
| 4,300,000 | | |
| 0.10 | | |
| 4,300,000 | | |
| 0.10 | |
| Exercisable at end of year | |
| 4,300,000 | | |
| 0.10 | | |
| 600,000 | | |
| 0.10 | |
Stock-based
compensation expense was approximately $273,000 and $80,000 in the years ending December 31, 2021 and 2020, respectively.
The
total compensation cost related to non-vested awards not yet recognized was approximately $273,000 as of December 31, 2020. All awards
were vested as of December 31, 2021.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
(d)
Warrants
The
Company has issued warrants at exercise prices equal to or greater than market value of the Company’s common stock at the date
of issuance. A summary of warrant activity follows:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
Number of | | |
Weighted | | |
Number | | |
Weighted | |
| | |
shares | | |
Average | | |
of | | |
Average | |
| | |
underlying | | |
Exercise | | |
Options | | |
Exercise | |
| | |
warrants | | |
Price | | |
(in shares) | | |
Price | |
| Outstanding at beginning of year | |
| 3,000,000 | | |
| 0.12 | | |
| 3,000,000 | | |
| 0.12 | |
| Granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Forfeited or expired | |
| - | | |
| - | | |
| - | | |
| - | |
| Outstanding at end of year | |
| 3,000,000 | | |
| 0.12 | | |
| 3,000,000 | | |
| 0.12 | |
The
warrants outstanding at December 31, 2021 have a weighted average remaining contractual life of approximately 1.5 years.
NOTE
6—INCOME TAXES
For
the years ended December 31, 2021 and 2020, the Company incurred net operating losses and, accordingly, no provision for income taxes
has been recorded. In addition, no benefit for income taxes has been recorded due to the uncertainty of the realization of any tax assets.
At December 31, 2021 and 2020, the Company had approximately $118,000,000 and $141,000,000, respectively of net operating losses subject
to IRC Section 382 limitations, of which $6,200,000 and $6,000,000, respectively, were available for carryforward after the consideration
of IRC Section 382 limitations. State of Florida net operating losses available for carryforward approximate the federal net operating
loss carryforward amounts.
The
federal and state net operating losses expire beginning in 2021. Approximately $23,000,000 and $3,000,000, respectively, of federal and
state losses expired in December 2021. The Company has approximately $1,635,000 in federal and state losses that do not expire. The remaining
losses expire from 2022 through 2036. The majority of these expiring losses are further limited by IRC section 382 as shown in the deferred
tax table below. All such deferred tax assets have been offset with a full valuation allowance.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
The
Company’s income tax provision differs from the expense that would result from applying statutory rates to income before taxes.
A reconciliation of the provision (benefit) for income taxes with amounts determined by applying the statutory U.S. federal income tax
rate to income before income taxes is as follows:
| | |
Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| Computed tax at the federal statutory rate of 21% | |
$ | (303,582 | ) | |
$ | (114,542 | ) |
| Penalties | |
| - | | |
| 11 | |
| State income taxes, net of federal income tax benefit | |
| (13,191 | ) | |
| (4,977 | ) |
| Change in federal valuation allowance | |
| 316,773 | | |
| 119,508 | |
| Total provision for income tax | |
$ | - | | |
$ | - | |
Deferred
income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial
reporting purposes and the amounts used for income tax purposes. Deferred tax assets as of December 31, 2021 and 2020 consist of the
following:
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| | |
Restated | | |
Restated | |
| Outside tax basis difference in equity investments | |
$ | 1,700,000 | | |
$ | 1,700,000 | |
| Federal and state net operating loss carryforwards available after consideration of IRC Section 382 limitations | |
| 1,676,585 | | |
| 1,594,884 | |
| Charitable contribution carryforward | |
| - | | |
| 47,329 | |
| General business credit | |
| 41,551 | | |
| 41,551 | |
| Stock compensation | |
| 99,103 | | |
| 26,820 | |
| Total deferred tax assets | |
| 3,517,239 | | |
| 3,410,584 | |
| Federal and state net operating loss carryforwards subject to IRC Section 382 limitations | |
| 28,425,763 | | |
| 33,470,874 | |
| Less valuation allowance for net operating loss limitations | |
| (28,425,763 | ) | |
| (33,470,874 | ) |
| Valuation allowance | |
| (2,482,298 | ) | |
| (2,008,207 | ) |
| Subtotal deferred tax assets | |
| 1,034,941 | | |
| 1,402,377 | |
| Deferred tax liability, equity method intangible assets | |
| (1,034,941 | ) | |
| (1,402,377 | ) |
| Net deferred tax assets | |
$ | - | | |
$ | - | |
Management
has evaluated all tax positions that could have a significant effect on the combined financial statements and determined the Companies
had no significant uncertain income tax positions at December 31, 2021 and 2020.
During
the year ended December 31, 2020, the Company received a refund of 2019 alternative minimum taxes in the amount of $518,819.
NOVELSTEM
INTERNATIONAL CORP.
Notes
to Financial Statements
NOTE
7—COMMITMENTS AND CONTINGENCIES
The
Company is the claimant in an arbitration proceeding against their 50% partner in NetCo Partners. The Company initiated the arbitration
proceeding in an effort to maximize the total potential value to be derived from fully utilizing the NetCo Partners intellectual property
across publishing, entertainment, digital media, merchandising and other ancillary markets. Arbitration proceedings for the joint owners
of NetCo Partners are scheduled during 2022.
NOTE
8 – CORRECTION OF ERROR
The
accompanying financial statements have been restated to reflect additional losses from the Company’s equity method investment in
NewStem. The previously issued financial statements as of and for the years ended December 31, 2021 and 2020, contained an error whereby
the Company suspended and deferred the recording of allocated losses from NewStem of $843,268 and $35,462, respectively. The Company
had recorded losses to offset its memo investment account to zero, but failed to record losses to offset the financial basis differential
maintained in intangible asset memo accounts.
The
following is a summary of the restatements:
| | |
December 31, 2021 | |
| | |
Originally Reported | | |
Adjustment | | |
Restated | |
| Total current assets | |
$ | 36,982 | | |
$ | - | | |
$ | 36,982 | |
| Investment in NewStem, Ltd | |
| 3,335,175 | | |
| (900,020 | ) | |
| 2,435,155 | |
| Total assets | |
| 3,509,168 | | |
| (900,020 | ) | |
| 2,609,148 | |
| Total current liabilities | |
| 193,202 | | |
| - | | |
| 193,202 | |
| Stockholders’ equity | |
| 3,315,966 | | |
| (900,020 | ) | |
| 2,415,946 | |
| | |
December 31, 2020 | |
| | |
Originally Reported | | |
Adjustment | | |
Restated | |
| Total current assets | |
$ | 120,829 | | |
$ | - | | |
$ | 120,829 | |
| Investment in NewStem, Ltd | |
| 3,335,175 | | |
| (35,462 | ) | |
| 3,299,713 | |
| Total assets | |
| 3,593,015 | | |
| (35,462 | ) | |
| 3,557,553 | |
| Total current liabilities | |
| 68,745 | | |
| - | | |
| 68,745 | |
| Stockholders’ equity | |
| 3,524,270 | | |
| (35,462 | ) | |
| 3,488,808 | |
| | |
Year Ended December 31, 2021 | |
| | |
Originally Reported | | |
Adjustment | | |
Restated | |
| Loss before income taxes | |
$ | (502,360 | ) | |
$ | - | | |
$ | (502,360 | ) |
| Provision for income tax | |
| - | | |
| - | | |
| - | |
| Net loss before equity in net loss of equity method investees | |
| (502,360 | ) | |
| - | | |
| (502,360 | ) |
| Equity in net income (loss) of equity method investees | |
| 21,290 | | |
| (864,558 | ) | |
| (843,268 | ) |
| Net loss | |
| (481,070 | ) | |
| (864,558 | ) | |
| (1,345,628 | ) |
| | |
Year Ended December 31, 2020 | |
| | |
Originally Reported | | |
Adjustment | | |
Restated | |
| Loss before income taxes | |
$ | (213,781 | ) | |
$ | - | | |
$ | (213,781 | ) |
| Provision for income tax | |
| - | | |
| - | | |
| - | |
| Net loss before equity in net loss of equity method investees | |
| (213,781 | ) | |
| - | | |
| (213,781 | ) |
| Equity in net loss of equity method investees | |
| (296,196 | ) | |
| (35,462 | ) | |
| (331,658 | ) |
| Net loss | |
| (509,977 | ) | |
| (35,462 | ) | |
| (545,439 | ) |
NOTE
9—SUBSEQUENT EVENTS
The
Company granted 1,100,000 stock options to directors in January 2022.
On
February 11, 2022, the Company entered into a nonrecourse litigation funding agreement (the “Agreement”) with Omni Bridgeway
(Fund 4) Invt. 3 L.P. (“Omni”) related to an ongoing arbitration proceeding disclosed in Note 7. The Agreement provides for
Omni to fund all costs related to the arbitration up to $1,000,000 in exchange for an assignment of a certain portion of rights to and
interest in claims related to this arbitration. The agreement provides for specific calculations of the portion of any claims collected
to be received by Omni with the remainder collectible by the Company.
In
May 2022, the Company entered into a finance agreement with two individuals who are shareholders and directors, which was amended in
July 2022, to borrow up to $600,000 for working capital needs. This agreement provides for funding through January 31, 2024, provides
for interest at a rate of 8% per annum and matures the earlier of January 31, 2024 or 20 months from the date of the first funded amount
unless the shareholders agree to extend the due date at that time. The Company received advances of $100,000 pursuant to this agreement
in May 2022, subsequent to the date of these financial statements.
NOVELSTEM
INTERNATIONAL CORP.
CONDENSED BALANCE SHEETS
| | |
2022 | | |
2021 | |
| | |
As
of | |
| | |
September
30, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
(Unaudited) | | |
| |
| | |
| | |
| |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 16,892 | | |
$ | 8,666 | |
| Prepaid
expenses | |
| 48,940 | | |
| 28,316 | |
| Total current assets | |
| 65,832 | | |
| 36,982 | |
| Investment in Netco Partners | |
| 137,011 | | |
| 137,011 | |
| Investment
in NewStem Ltd | |
| 1,828,419 | | |
| 2,435,155 | |
| Total
assets | |
$ | 2,031,262 | | |
$ | 2,609,148 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDERS’
EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 61,460 | | |
$ | 49,777 | |
| Note payable | |
| - | | |
| 100,000 | |
| Accrued expenses | |
| 64,172 | | |
| 43,425 | |
| Total current liabilities | |
| 125,632 | | |
| 193,202 | |
| | |
| | | |
| | |
| Long-term
note payable, including accrued interest | |
| 168,209 | | |
| - | |
| Total
liabilities | |
| 293,841 | | |
| 193,202 | |
| | |
| | | |
| | |
| Commitments and contingencies
(see Note 7) | |
| - | | |
| | |
| Shareholders’ equity: | |
| | | |
| | |
| Common stock, $.01 par value, 100,000,000 shares authorized, 50,316,672 shares issued, and 46,881,475 shares outstanding as of
September 30, 2022 and December 31, 2021 | |
| 468,815 | | |
| 468,815 | |
| Additional paid-in capital | |
| 290,520,159 | | |
| 290,321,665 | |
| Accumulated deficit | |
| (289,051,799 | ) | |
| (288,174,780 | ) |
| Treasury stock, at cost, 3,435,197 shares as of September 30, 2022 and December 31, 2021 | |
| (199,754 | ) | |
| (199,754 | ) |
| Total shareholders’ equity | |
| 1,737,421 | | |
| 2,415,946 | |
| Total
liabilities and shareholders’ equity | |
$ | 2,031,262 | | |
$ | 2,609,148 | |
The
accompanying notes are an integral part of these condensed financial statements.
NOVELSTEM
INTERNATIONAL CORP.
CONDENSED
STATEMENTS OF OPERATIONS
(UNAUDITED)
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
Nine
Months Ended | | |
Three
Months Ended | |
| | |
September
30, | | |
September
30, | |
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
| | |
| | |
| | |
| |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| General and administrative expenses | |
$ | 376,247 | | |
$ | 136,047 | | |
$ | 131,060 | | |
$ | 60,133 | |
| Contra expenses - legal fees and administrative costs (Note 8) | |
| (310,000 | ) | |
| - | | |
| - | | |
| - | |
| Stock compensation expense | |
| 198,494 | | |
| 229,891 | | |
| 75,150 | | |
| 73,045 | |
| Total
operating expenses | |
| 264,741 | | |
| 365,938 | | |
| 206,210 | | |
| 133,178 | |
| Loss from operations | |
| (264,741 | ) | |
| (365,938 | ) | |
| (206,210 | ) | |
| (133,178 | ) |
| Interest
expense | |
| 5,542 | | |
| 4,417 | | |
| 3,530 | | |
| 2,205 | |
| Provision
for income tax | |
| - | | |
| - | | |
| - | | |
| - | |
| Loss before equity in net loss of equity method investees | |
| (270,283 | ) | |
| (370,355 | ) | |
| (209,740 | ) | |
| (135,383 | ) |
| Equity
in net loss of equity method investees | |
| (606,736 | ) | |
| (365,543 | ) | |
| (85,532 | ) | |
| (196,333 | ) |
| Net loss | |
$ | (877,019 | ) | |
$ | (735,898 | ) | |
$ | (295,272 | ) | |
$ | (331,716 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted net loss
per share: | |
| | | |
| | | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.02 | ) | |
$ | (0.02 | ) | |
$ | (0.01 | ) | |
$ | (0.01 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average number
of shares outstanding – basic and diluted | |
| 46,881,475 | | |
| 43,881,477 | | |
| 46,881,475 | | |
| 43,881,477 | |
The
accompanying notes are an integral part of these unaudited condensed financial statements.
NOVELSTEM
INTERNATIONAL CORP.
CONDENSED
STATEMENTS OF SHAREHOLDERS’ EQUITY
(UNAUDITED)
For
the Three and Nine Months Ended September 30, 2022:
| | |
Shares | | |
Stock | | |
Capital | | |
Deficit | | |
Shares | | |
Stock | | |
Equity | |
| | |
| | |
| | |
Additional | | |
| | |
Number
of | | |
| | |
Total | |
| | |
Number
of | | |
Common | | |
Paid-In | | |
Accumulated | | |
Treasury | | |
Treasury | | |
Shareholders’ | |
| | |
Shares | | |
Stock | | |
Capital | | |
Deficit | | |
Shares | | |
Stock | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, January 1, 2022 | |
| 46,881,475 | | |
$ | 468,815 | | |
$ | 290,321,665 | | |
$ | (288,174,780 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 2,415,946 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (217,464 | ) | |
| - | | |
| - | | |
| (217,464 | ) |
| Stock
option compensation | |
| - | | |
| - | | |
| 49,011 | | |
| - | | |
| - | | |
| - | | |
| 49,011 | |
| Balance, March 31, 2022 | |
| 46,881,475 | | |
$ | 468,815 | | |
$ | 290,370,676 | | |
$ | (288,392,244 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 2,247,493 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (364,283 | ) | |
| - | | |
| - | | |
| (364,283 | ) |
| Stock
option compensation | |
| - | | |
| - | | |
| 74,333 | | |
| - | | |
| - | | |
| - | | |
| 74,333 | |
| Balance, June 30, 2022 | |
| 46,881,475 | | |
$ | 468,815 | | |
$ | 290,445,009 | | |
$ | (288,756,527 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 1,957,543 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (295,272 | ) | |
| - | | |
| - | | |
| (295,272 | ) |
| Stock
option compensation | |
| - | | |
| - | | |
| 75,150 | | |
| - | | |
| - | | |
| - | | |
| 75,150 | |
| Balance, September 30,
2022 | |
| 46,881,475 | | |
$ | 468,815 | | |
$ | 290,520,159 | | |
$ | (289,051,799 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 1,737,421 | |
For
the Three and Nine Months Ended September 30, 2021:
| | |
| | |
| | |
Additional | | |
| | |
Number
of | | |
| | |
Total | |
| | |
Number
of | | |
Common | | |
Paid-In | | |
Accumulated | | |
Treasury | | |
Treasury | | |
Shareholders’ | |
| | |
Shares | | |
Stock | | |
Capital | | |
Deficit | | |
Shares | | |
Stock | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, January 1, 2021 | |
| 43,881,477 | | |
$ | 438,815 | | |
$ | 290,078,899 | | |
$ | (286,829,152 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 3,488,808 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (212,593 | ) | |
| - | | |
| - | | |
| (212,593 | ) |
| Stock
option compensation | |
| - | | |
| - | | |
| 83,400 | | |
| - | | |
| - | | |
| - | | |
| 83,400 | |
| Balance March 31, 2021 | |
| 43,881,477 | | |
| 438,815 | | |
| 290,162,299 | | |
| (287,041,745 | ) | |
| 3,435,197 | | |
| (199,754 | ) | |
| 3,359,615 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (191,588 | ) | |
| - | | |
| - | | |
| (191,588 | ) |
| Stock
option compensation | |
| - | | |
| - | | |
| 73,446 | | |
| - | | |
| - | | |
| - | | |
| 73,446 | |
| Balance, June 30, 2021 | |
| 43,881,477 | | |
| 438,815 | | |
| 290,235,745 | | |
| (287,233,333 | ) | |
| 3,435,197 | | |
| (199,754 | ) | |
| 3,241,473 | |
| Beginning balance, value | |
| 43,881,477 | | |
| 438,815 | | |
| 290,235,745 | | |
| (287,233,333 | ) | |
| 3,435,197 | | |
| (199,754 | ) | |
| 3,241,473 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (331,716 | ) | |
| - | | |
| - | | |
| (331,716 | ) |
| Net income (loss) | |
| - | | |
| - | | |
| - | | |
| (331,716 | ) | |
| - | | |
| - | | |
| (331,716 | ) |
| Stock
option compensation | |
| - | | |
| - | | |
| 73,045 | | |
| - | | |
| - | | |
| - | | |
| 73,045 | |
| Balance, September 30,
2021 | |
| 43,881,477 | | |
$ | 438,815 | | |
$ | 290,308,790 | | |
$ | (287,565,049 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 2,982,802 | |
| Ending balance, value | |
| 43,881,477 | | |
$ | 438,815 | | |
$ | 290,308,790 | | |
$ | (287,565,049 | ) | |
| 3,435,197 | | |
$ | (199,754 | ) | |
$ | 2,982,802 | |
The
accompanying notes are an integral part of these condensed financial statements.
NOVELSTEM
INTERNATIONAL CORP.
CONDENSED
STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | |
2022 | | |
2021 | |
| | |
Nine
Months Ended | |
| | |
September
30, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Cash flows from operating
activities: | |
| | | |
| | |
| Net loss | |
$ | (877,019 | ) | |
$ | (735,898 | ) |
| Equity in loss of equity method investees | |
| 606,736 | | |
| 365,543 | |
| Distribution from NetCo Partners | |
| - | | |
| 9,290 | |
| Stock-based compensation | |
| 198,494 | | |
| 229,891 | |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Current assets and other assets | |
| (20,624 | ) | |
| 2,547 | |
| Accounts payable and accrued expenses | |
| 35,639 | | |
| 1,655 | |
| Net cash
from operating activities | |
| (56,774 | ) | |
| (126,972 | ) |
| | |
| | | |
| | |
| Cash flows from financing
activities: | |
| | | |
| | |
| Proceeds from (repayment of) short-term note payable | |
$ | (100,000 | ) | |
$ | 100,000 | |
| Proceeds
from long-term notes payable | |
| 165,000 | | |
| - | |
| Net cash from financing activities | |
| 65,000 | | |
| 100,000 | |
| | |
| | | |
| | |
| Net change in cash | |
| 8,226 | | |
| (26,972 | ) |
| Cash
at the beginning of the period | |
| 8,666 | | |
| 89,594 | |
| Cash
at the end of the period | |
$ | 16,892 | | |
$ | 62,622 | |
| | |
| | | |
| | |
| Supplemental cash flow information: | |
| | | |
| | |
| Cash paid during the period
for: | |
| | | |
| | |
| Interest | |
$ | 8,085 | | |
$ | 4,417 | |
The
accompanying notes are an integral part of these condensed financial statements.
NOVELSTEM
INTERNATIONAL CORP.
NOTES
TO CONDENSED FINANCIAL STATEMENTS
(Unaudited)
NOTE
1—NATURE OF OPERATIONS
Description
of Business
NovelStem
International Corp. (“NovelStem” or the “Company”) is a holding company whose principal assets are a 30.99% equity
interest in NewStem Ltd, an Israeli biotech company (“NewStem”), and a 50% equity interest in NetCo Partners (“NetCo”).
NovelStem was formerly known as Hollywood Media Corp. The Company was incorporated in the State of Florida on January 22, 1993 and changed
its name to NovelStem International Corp. in September 2018 as a result of its business focus shift from a media business to biotech.
NewStem
focuses on the development and commercialization of diagnostic technology that can predict patients’ anti-cancer drug resistance,
allowing for targeted cancer treatments and the potential to reduce resistance to chemotherapy. NewStem is collaborating with life sciences companies for the development
of drugs and reagents. NetCo is a legacy media business interest
which owns “Net Force”, a book publishing franchise.
Liquidity
and Management’s Plans
Since
inception, the Company has accumulated a deficit of approximately $289,000,000. The accumulated deficit of the Company subsequent to
its business focus shift and name change in September 2018 is approximately $2,390,000 which is comprised primarily of allocated losses
from equity method investments and general and administrative costs incurred by the Company.
The
Company will need to obtain additional funds to continue its operations. Management’s plans with regard to these matters include
additional financing and fundraising until its equity investment in NewStem is profitable. Although management continues to pursue these
plans, there is no assurance that the Company will be successful in obtaining sufficient cash from financing on terms acceptable to the
Company, or that NewStem will become profitable.
The
Company entered into a financing agreement with related parties to borrow up to $600,000
for working capital needs (see Note 4). Following this financing, the Company believes that its cash resources are sufficient for
the operations of the next twelve months.
NOTE
2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of
America (“GAAP”). The Financial Accounting Standards Board (“FASB”) has established the FASB Accounting Standards
Codification (“ASC”) as the single source of authoritative GAAP.
The
accompanying unaudited condensed financial statements included in this report have been prepared by the Company pursuant to the
rules and regulations of the U.S. Securities and Exchange Commission (“SEC”) for interim reporting and include all
adjustments (consisting only of normal recurring adjustments) that are, in the opinion of management, necessary for a fair
presentation. These condensed financial statements have not been audited. The results of operations for the nine-month periods ended
September 30, 2022 and 2021 are not necessarily indicative of the operating results for the full year.
Certain
information and footnote disclosures normally included in condensed financial statements prepared in accordance with GAAP have been
condensed or omitted pursuant to such rules and regulations for interim reporting. The Company believes that the disclosures
contained herein are adequate to make the information presented not misleading. These condensed financial statements should be read
in conjunction with the Company’s Form 10 filed with the Securities and Exchange Commission on August 2, 2022 for the years
ended December 31, 2021 and 2020.
Equity
Investments
Investee
companies that are not consolidated, but over which the Company exercises significant influence, are accounted for under the equity method
of accounting. Whether or not the Company exercises significant influence with respect to an investee depends on an evaluation of several
factors, including, among others, representation on the investee company’s board of directors and ownership level, which is generally
a 20% to 50% interest in the voting securities of the investee company. Under the equity method of accounting, an investee company’s
accounts are not reflected within the Company’s balance sheets or statements of operations; however, the Company’s share
of the earnings or losses of the investee company is reflected in the caption “Equity in net income (loss) of investee company”
in the statements of operations. The Company’s carrying value in an equity method investee company is reflected in the caption
“Investment in investee company’ in the Company’s balance sheets.
The
Company reviews equity investments for impairment on an annual basis, or earlier if events or changes in circumstances indicate that
the carrying amounts might not be recoverable.
The
Company holds a minority investment in an entity, NewStem, which is accounted for pursuant to the equity
method of accounting. Additionally, the Company is a 50% partner in NetCo (which is accounted for pursuant
to the equity method of accounting. See Note 3.
Basic
and Diluted Net Loss Per Share
Basic
net loss per share is computed by dividing the net loss by the weighted average number of shares outstanding during the period,
excluding treasury stock. Diluted net income (loss) per share is computed by dividing the net income (loss) by the weighted average
number of shares outstanding plus the dilutive potential of common shares which would result from the exercise of stock options and
warrants. The dilutive effects of stock options and warrants are excluded from the computation of diluted net income (loss) per
share if the effect of doing so would be antidilutive.
The
following data represents the amounts used in computing earnings per share and the effect on net income (loss) and the weighted average
number of shares of dilutive potential common stock (unaudited):
SCHEDULE OF WEIGHTED AVERAGE NUMBER OF SHARES OF DILUTIVE
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
Nine
Months Ended
September
30, | | |
Three
Months Ended
September
30, | |
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| Net loss available
to common shareholders | |
$ | (877,019 | ) | |
$ | (735,898 | ) | |
$ | (295,272 | ) | |
$ | (331,716 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding: | |
| | | |
| | | |
| | | |
| | |
| -Basic | |
| 46,881,475 | | |
| 43,881,477 | | |
| 46,881,475 | | |
| 43,881,477 | |
| Add: Warrants | |
| - | | |
| - | | |
| - | | |
| - | |
| Add:
Stock options | |
| - | | |
| - | | |
| - | | |
| - | |
| -Diluted | |
| 46,881,475 | | |
| 43,881,477 | | |
| 46,881,475 | | |
| 43,881,477 | |
| | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted net loss
per share | |
$ | (0.02 | ) | |
$ | (0.02 | ) | |
$ | (0.01 | ) | |
$ | (0.01 | ) |
NOTE
3—EQUITY METHOD INVESTMENTS
Investment
in NewStem
In
2018, the Company entered into a Share Purchase Agreement with NewStem and other related parties to provide aggregate funding of up
to $4,000,000
to NewStem. This funding was to be provided through the sale of up to 50,000
common shares of NewStem to the Company representing 33%
of New Stem’s outstanding shares. In 2018, the Company purchased 25,000
shares of NewStem for $2,000,000
acquiring an ownership interest of 20%.
The Company made additional investments in 2019 and 2020 purchasing 12,500
shares each year for a $1,000,000
investment each year resulting in an ownership interest of 31.51%
as of September 30, 2021. The Company’s ownership interest is 30.99%
as of September 30, 2022 as a result of the issuance of additional NewStem shares to new investors.
The
Company accounts for its investment in NewStem under the equity method. At September 30, 2022 and December 31, 2021, the carrying value
of the investment in NewStem exceeded the underlying net assets of NewStem by $1,828,419 and $2,435,155, respectively. The excess relates
to identified intangible assets including license agreements, specialized work force (goodwill) and two separate projects of in process
research and development (“IPR&D”) related to stem cell-based diagnostics and therapeutics for cancer chemotherapies.
The
Company assesses its investment in NewStem for impairment on an annual basis.
NewStem
is in the development stage and has incurred losses since its inception and has yet to generate any revenues. NewStem will need to obtain
additional funds to continue its operations. NewStem management’s plans with regard to these matters include continued development,
marketing and licensing of its products, as well as seeking additional financing arrangements. Although management continues to pursue
these plans, there is no assurance that the Company will be successful in obtaining sufficient cash from sales of products or financing
on terms acceptable to the Company. NewStem entered into a share purchase agreement with two investors for the purchase of 2,647 ordinary
shares for total consideration of $800,000. Based on NewStem’s agreement with one of its other shareholders, NewStem is entitled
to a matching investment (“the matching investment”) which will bring the total funding to $1,600,000. As of September 30,
2022, the matching investment has not yet been received.
The
following table represents the Company’s investment in NewStem:
SCHEDULE OF INVESTMENTS
| | |
Nine Months Ended
September 30, 2022 | | |
Year Ended
December 31, 2021 | |
| | |
(Unaudited) | | |
| |
| Investment in
NewStem, beginning | |
$ | 2,435,155 | | |
$ | 3,299,713 | |
| Allocation
of net loss from NewStem | |
| (606,736 | ) | |
| (864,558 | ) |
| Investment
in NewStem, ending | |
$ | 1,828,419 | | |
$ | 2,435,155 | |
The
results of operations of the Company’s investment in NewStem is summarized below (unaudited):
SCHEDULE
OF OPERATIONS AND FINANCIAL POSITION INVESTMENT
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
Nine
Months Ended
September
30, | | |
Three
Months Ended
September
30, | |
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
| | |
| | |
| | |
| |
| Condensed income statement
information: | |
| | | |
| | | |
| | | |
| | |
| Net
sales | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Gross
margin | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Net
loss | |
$ | (1,935,000 | ) | |
$ | (1,132,000 | ) | |
$ | (276,000 | ) | |
$ | (589,000 | ) |
| Company’s allocation
of net loss from
NewStem | |
$ | (606,736 | ) | |
$ | (374,833 | ) | |
$ | (85,532 | ) | |
$ | (196,333 | ) |
The financial position of the Company’s investment in NewStem is
summarized below:
| | |
September
30, 2022 | | |
December
31, 2021 | |
| | |
As
of | |
| | |
September
30, 2022 | | |
December
31, 2021 | |
| | |
(Unaudited) | | |
| |
| Condensed balance sheet information: | |
| | | |
| | |
| Current
assets | |
$ | 573,000 | | |
$ | 1,425,000 | |
| Non-current
assets | |
$ | 27,000 | | |
$ | 41,000 | |
| Current
liabilities | |
$ | 104,000 | | |
$ | 227,000 | |
| Non-current
liabilities | |
$ | 112,000 | | |
$ | 134,000 | |
Investment
in NetCo
NovelStem
owns a 50% interest in NetCo, a joint venture that owns the Net Force publishing franchise. The Company accounts
for its investment in NetCo under the equity method and recognizes nominal royalties from this arrangement. The Company assesses
its investment in NetCo for impairment on an annual basis.
The
following table represents the Company’s investment in NetCo:
SCHEDULE OF INVESTMENTS
| | |
Nine Months Ended
September 30, 2022 | | |
Year Ended
December 31, 2021 | |
| | |
(Unaudited) | | |
| |
| Investment in
NetCo, beginning | |
$ | 137,011 | | |
$ | 137,011 | |
| Allocation of net income from
Netco | |
| - | | |
| 21,290 | |
| Distribution
from NetCo | |
| - | | |
| (21,290 | ) |
| Investment
in NetCo, ending | |
$ | 137,011 | | |
$ | 137,011 | |
The
results of operations of the Company’s investment in NetCo is summarized below (unaudited):
SCHEDULE
OF OPERATIONS AND FINANCIAL POSITION INVESTMENT
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
Nine
Months Ended
September
30, | | |
Three
Months Ended
September
30, | |
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| | |
| | |
| | |
| | |
| |
| Condensed income statement
information: | |
| | | |
| | | |
| | | |
| | |
| Net
sales | |
$ | - | | |
$ | 18,580 | | |
$ | - | | |
$ | - | |
| Gross
margin | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Net
income | |
$ | - | | |
$ | 18,580 | | |
$ | - | | |
$ | - | |
| Company’s allocation
of net income from
NetCo | |
$ | - | | |
$ | 9,290 | | |
$ | - | | |
$ | - | |
The
financial position of the Company’s investment in NetCo is summarized below:
| | |
September
30, 2022 | | |
December
31, 2021 | |
| | |
As
of | |
| | |
September
30, 2022 | | |
December
31, 2021 | |
| | |
(Unaudited) | | |
| |
| Condensed balance sheet information: | |
| | | |
| | |
| Current
assets | |
$ | 1,223 | | |
$ | 13,475 | |
| Non-current
assets | |
$ | 272,799 | | |
$ | 272,799 | |
| Current
liabilities | |
$ | - | | |
$ | 12,252 | |
| Non-current
liabilities | |
$ | - | | |
$ | - | |
NOTE
4—NOTES PAYABLE RELATED PARTIES
On
April 12, 2021, the Company entered into a promissory note (the “Note”) with a related party (individual) for $100,000.
The Note accrued interest at 8%
per annum and matured on April
12, 2022. The proceeds of this Note were used to pay operating expenses of the Company. Interest expense related to this Note
was $1,198
for the nine months ended September 30, 2022. The Note and accrued interest of $6,752 were paid in full on February 16,
2022.
In
May 2022, the Company entered into long-term notes payable in the form of finance agreements with two individuals who are related parties, which were amended in July 2022, to borrow up to $600,000
for working capital needs. One of the individuals is a director and shareholder, the other is our
Executive Chairman who is also a shareholder. These agreements provide
for funding through January 31, 2024, provide for interest at a rate of 8%
per annum and mature the earlier of January
31, 2024 or twenty months from the date of the
first funded amount (May 2022) unless the shareholders agree to extend the due date at that time. The balance outstanding on these notes
was $165,000 as
of September 30, 2022. Related interest accrued and payable at maturity is $3,209
at September 30, 2022.
NOTE
5—EQUITY
(a)
General
At
September 30, 2022 and December 31, 2021, the Company had issued and outstanding 46,881,475 shares of its common stock, par value $0.01
per share. Holders of outstanding common stock are entitled to receive dividends when, as and if declared by the Board and to share ratably
in the assets of the Company legally available for distribution in the event of a liquidation, dissolution or winding up of the Company.
(b)
Summary Employee Option Information
The
Company’s stock option plan provides for the grant to officers, directors, third party contractors and other future key employees
of options to purchase shares of common stock. The purchase price may be paid in cash or at the end of the option term. If the option
is “in-the-money”, it is automatically exercised “net”. In a net exercise of an option, the Company does not
require a payment of the exercise price of the option from the optionee but reduces the number of shares of common stock issued upon
the exercise of the option by the smallest number of whole shares that has an aggregate fair market value equal to or in excess of the
aggregate exercise price for the option shares covered by the option exercised. Each option is exercisable to one share of the Company’s
common stock. Most options expire within six years from the date of the grant and generally vest on the first anniversary date of their
issuance. Pursuant to the Equity Incentive Plan the Company’s board of directors approved on November 12, 2018, an aggregate of
5,400,000 options have been issued to directors and investor relations professionals.
The
Company utilized the Black-Scholes option-pricing model to estimate fair value, utilizing the following assumptions for the respective
periods (all in weighted averages):
SCHEDULE OF FAIR VALUE OF OPTION USING VALUATION ASSUMPTIONS
| | |
Nine
Months Ended
September
30, | |
| | |
2022 | | |
2021 | |
| Risk-free interest
rate | |
| 1.5 | % | |
| 1.6 | % |
| Expected term of options,
in years | |
| 3.9 | | |
| 6 | |
| Expected annual volatility | |
| 185.8 | % | |
| 140.2 | % |
| Expected dividend yield | |
| 0 | % | |
| 0 | % |
| Determined weighted average grant date fair value per
option | |
$ | 0.27 | | |
$ | - | |
The
expected term of the options represents an estimate of the length of time until the expected date of exercising the options. Options
granted have a maximum life of 6 years. With respect to determining expected exercise behavior, the Company has grouped its option grants
into certain groups to track exercise behavior and establish historical rates. The Company estimated volatility by considering
historical stock volatility over the expected term of the option. The risk-free interest rates are based on the U.S. Treasury yields
for a period consistent with the expected term. The dividend yield of 0% is based on the Company’s history and expectation of dividend
payout. The Company has not paid and does not anticipate paying dividends in the near future.
(c)
Summary Option Information
A
summary of the Company’s option plans for the nine months ended September 30, 2022, is presented below (unaudited):
SCHEDULE OF STOCK OPTION ACTIVITIES
| | |
Number | | |
Weighted | |
| | |
of | | |
Average | |
| | |
Options | | |
Exercise | |
| | |
(in
shares) | | |
Price | |
| Outstanding, December 31, 2021 | |
| 4,300,000 | | |
$ | 0.10 | |
| Granted | |
| 1,100,000 | | |
| 0.29 | |
| Outstanding, September 30, 2022 | |
| 5,400,000 | | |
$ | 0.14 | |
| Exercisable, September 30, 2022 | |
| 4,300,000 | | |
$ | 0.14 | |
Stock-based
compensation expense was approximately $198,000 and $75,000 in the nine months and three months ended September 30, 2022, respectively.
Stock-based compensation expense was approximately $230,000 and $73,000 for the nine months and three months ended September 30, 2021,
respectively.
The
total compensation cost related to non-vested awards not yet recognized was approximately $100,000
as of September 30, 2022. As of September 30,
2022, 1,100,000
options were unvested. These options vest one
year from their grant date which is January 2023.
(d)
Warrants
The
Company has issued warrants at exercise prices equal to or greater than the market value of the Company’s common stock at the
date of issuance. A summary of warrant activity follows (unaudited):
SUMMARY
OF WARRANTS ACTIVITY
| | |
Number of | | |
Weighted | |
| | |
shares | | |
Average | |
| | |
underlying | | |
Exercise | |
| | |
warrants | | |
Price | |
| Outstanding, December 31, 2021 | |
| 3,000,000 | | |
$ | 0.12 | |
| Granted | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | |
| Forfeited
or expired | |
| - | | |
| - | |
| Outstanding, September 30, 2022 | |
| 3,000,000 | | |
$ | 0.12 | |
The
warrants outstanding at September 30, 2022 have a weighted average remaining contractual life of approximately nine months.
NOTE
6—INCOME TAXES
The
Company’s income tax provision differs from the expense that would result from applying statutory rates to income (loss)
before taxes. A reconciliation of the provision (benefit) for income taxes with amounts determined by applying the statutory U.S.
federal income tax rate to income before income taxes is as follows (unaudited):
SCHEDULE
OF INCOME BEFORE INCOME TAX
| | |
| | | |
| | |
| | |
Nine
Months Ended
September
30, | |
| | |
2022 | | |
2021 | |
| Computed tax at
the federal statutory rate of 21% | |
$ | (184,174 | ) | |
$ | (176,854 | ) |
| State income taxes, net of
federal income tax benefit | |
| (38,106 | ) | |
| (36,591 | ) |
| Change
in federal valuation allowance | |
| 222,280 | | |
| 213,445 | |
| Total
provision for income tax | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| | |
Three
Months Ended
September
30, | |
| | |
2022 | | |
2021 | |
| Computed tax at
the federal statutory rate of 21% | |
$ | (62,007 | ) | |
$ | (69,660 | ) |
| State income taxes, net of
federal income tax benefit | |
| (12,829 | ) | |
| (14,413 | ) |
| Change
in federal valuation allowance | |
| 74,836 | | |
| 84,073 | |
| Total
provision for income tax | |
$ | - | | |
$ | - | |
NOTE
7—COMMITMENTS AND CONTINGENCIES
The
Company is the claimant in an arbitration proceeding against their 50%
partner in NetCo. The Company initiated the arbitration proceeding in an effort to maximize the total potential value to be derived
from fully utilizing the NetCo intellectual property across publishing, entertainment, digital media, merchandising and other
ancillary markets. Arbitration hearings were held at the end of July 2022. During the proceedings the arbitrator put the arbitration
on hold and asked the parties to try and negotiate a settlement. Arbitration proceedings were concluded in October 2022 and legal
counsel for both parties are in the process of filing final briefs which are due to the arbitrator in January 2023. It is unknown as
to how long the arbitrator will take to render his decision.
NOTE
8—LITIGATION FUNDING AGREEMENT
On
February 11, 2022, the Company entered into a nonrecourse litigation funding agreement (the “Agreement”) with Omni Bridgeway
(Fund 4) Invt. 3 L.P. (“Omni”) related to an ongoing arbitration proceeding disclosed in Note 7. The Agreement provides for
Omni to fund all costs related to the arbitration up to $1,000,000 in exchange for an assignment of a certain portion of rights to and
interest in claims related to this arbitration. The agreement provides for specific calculations of the portion of any claims collected
to be received by Omni with the remainder collectible by the Company. During the nine months ended September 30, 2022, the Company received
$310,000 pursuant to this agreement for the reimbursement of legal costs and working capital expenditures, including previously incurred
general and administrative costs.
NOTE
9—SUBSEQUENT EVENTS
As
disclosed in Note 4, the Company has in place notes payable with related parties. The Company received total advances of $35,000 related
to these agreements in October 2022, subsequent to the date of these financial statements. To date, the Company has received advances of $200,000 pursuant to these agreements.
In
November 2022, these notes payable were amended to increase the interest rate to 10% per annum on all future advances.
 |
|
| |
|
| |
Somekh
Chaikin
KPMG
Millennium Tower
17
Ha’arba’a Street, PO Box 609
Tel
Aviv 61006, Israel
+972
3 684 8000
Report
of Independent Registered Public Accounting Firm
To
the Shareholders and the Board of Directors of NewStem Ltd.
Opinion
on the Financial Statements
We
have audited the accompanying balance sheets of NewStem Ltd. as of December 31, 2021 and 2020, the related statements of operations,
changes in shareholders’ equity and cash flows for each of the years in the two-year period ended December 31, 2021, and the
related notes (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material
respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash
flows for each of the years in the two-year period ended December 31, 2021, in conformity with U.S. generally accepted accounting
principles.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these
financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight
Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to
obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits
provide a reasonable basis for our opinion.
Critical
Audit Matters
Critical
audit matters are matters arising from the current period audit of the financial statements that were communicated or required to
be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements
and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
Somekh
Chaikin
Member
Firm of KPMG International
We
have served as the Company’s auditor since 2021.
Tel
Aviv, Israel
July
17, 2022
KPMG
Somekh Chaikin, an Israeli partnership and a member firm of the KPMG global organization of independent member firms affiliated with
KPMG International Limited, a private English company limited by guarantee |
NewStem
Ltd.
Balance
Sheets as of December 31,
| | |
| | |
2021 | | |
2020 | |
| | |
Note | | |
US$ thousands | | |
US$ thousands | |
| Assets | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| Current assets | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
| 3 | | |
| 601 | | |
| 790 | |
| Marketable securities | |
| 7 | | |
| - | | |
| 778 | |
| Prepaid share-based payment | |
| 8C | | |
| 771 | | |
| - | |
| Other accounts receivable | |
| 4 | | |
| 53 | | |
| 31 | |
| | |
| | | |
| | | |
| | |
| Total current assets | |
| | | |
| 1,425 | | |
| 1,599 | |
| | |
| | | |
| | | |
| | |
| Non-current assets | |
| | | |
| | | |
| | |
| Property and equipment, net | |
| 5 | | |
| 41 | | |
| 14 | |
| | |
| | | |
| | | |
| | |
| Total assets | |
| | | |
| 1,466 | | |
| 1,613 | |
| | |
| | | |
| | | |
| | |
| Liabilities and shareholders’ equity | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| Current liabilities | |
| | | |
| | | |
| | |
| Accrued expenses and other payables | |
| 6 | | |
| 127 | | |
| 69 | |
| Other liabilities | |
| 9B | | |
| 100 | | |
| - | |
| | |
| | | |
| | | |
| | |
| Total Current liabilities | |
| | | |
| 227 | | |
| 69 | |
| | |
| | | |
| | | |
| | |
| Non-current liabilities | |
| | | |
| | | |
| | |
| Convertible financial instrument | |
| 8E | | |
| 134 | | |
| - | |
| | |
| | | |
| | | |
| | |
| Total liabilities | |
| | | |
| 361 | | |
| 69 | |
| | |
| | | |
| | | |
| | |
| Shareholders’ equity | |
| 8 | | |
| | | |
| | |
| Ordinary shares | |
| | | |
| * | | |
| * | |
| Additional paid-in capital | |
| | | |
| 6,734 | | |
| 4,543 | |
| Accumulated deficit | |
| | | |
| (5,629 | ) | |
| (2,999 | ) |
| | |
| | | |
| | | |
| | |
| Total shareholders’ equity | |
| | | |
| 1,105 | | |
| 1,544 | |
| | |
| | | |
| | | |
| | |
| Total liabilities and shareholders’ equity | |
| | | |
| 1,466 | | |
| 1,613 | |
| |
|
| Ayelet
Dilion Mashiah |
|
| CEO
|
|
Date
of approval of the financial statements: July 17, 2022.
*
Represents an amount lower than $1 thousands.
The
accompanying notes are an integral part of the financial statements.
NewStem
Ltd.
Statements
of Operations for the Year Ended December 31
| | |
| | |
2021 | | |
2020 | |
| | |
Note | | |
US$ thousands | | |
US$ thousands | |
| Operating expenses | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| Research and development expenses | |
| | | |
| 2,451 | | |
| 1,100 | |
| Less – Grants received | |
| | | |
| (90 | ) | |
| (200 | ) |
| Research and development expenses, net | |
| | | |
| 2,361 | | |
| 900 | |
| | |
| | | |
| | | |
| | |
| General and administrative expenses | |
| | | |
| 264 | | |
| 331 | |
| | |
| | | |
| | | |
| | |
| Operating loss | |
| | | |
| 2,625 | | |
| 1,231 | |
| | |
| | | |
| | | |
| | |
| Financial expenses )income(, net | |
| 10 | | |
| 5 | | |
| (7 | ) |
| | |
| | | |
| | | |
| | |
| Loss for the year | |
| | | |
| 2,630 | | |
| 1,224 | |
The
accompanying notes are an integral part of the financial statements.
NewStem
Ltd.
Statements
of Changes in Shareholders’ Equity
| | |
| | |
| | |
Additional | | |
| | |
| |
| | |
| | |
| | |
paid-in | | |
Accumulated | | |
| |
| | |
Ordinary shares | | |
capital | | |
deficit | | |
Total | |
| | |
Number of shares | | |
US$ thousands | | |
US$ thousands | | |
US$ thousands | | |
US$ thousands | |
| Balance as of January 1, 2020 | |
| 137,500 | | |
| * | | |
| 3,224 | | |
| (1,775 | ) | |
| 1,449 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of ordinary shares | |
| 12,500 | | |
| * | | |
| 1,000 | | |
| - | | |
| 1,000 | |
| Stock based compensation | |
| - | | |
| - | | |
| 319 | | |
| - | | |
| 319 | |
| Loss for the year | |
| - | | |
| - | | |
| - | | |
| (1,224 | ) | |
| (1,224 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance as of December 31, 2020 | |
| 150,000 | | |
| * | | |
| 4,543 | | |
| (2,999 | ) | |
| 1,544 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of ordinary shares in exchange of services | |
| 8,696 | | |
| * | | |
| 1,952 | | |
| - | | |
| 1,952 | |
| Stock based compensation | |
| - | | |
| - | | |
| 239 | | |
| - | | |
| 239 | |
| Loss for the year | |
| - | | |
| - | | |
| - | | |
| (2,630 | ) | |
| (2,630 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance as of December 31, 2021 | |
| 158,696 | | |
| * | | |
| 6,734 | | |
| (5,629 | ) | |
| 1,105 | |
*
Represents an amount less than $1 thousands.
The
accompanying notes are an integral part of the financial statements.
NewStem
Ltd.
Statements
of Cash Flows for the year ended December 31
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Cash flows from operating activities | |
| | | |
| | |
| | |
| | | |
| | |
| Loss for the year | |
| (2,630 | ) | |
| (1,224 | ) |
| | |
| | | |
| | |
| Adjustments required to reconcile loss to net cash used in operating | |
| | | |
| | |
| activities: | |
| | | |
| | |
| | |
| | | |
| | |
| Depreciation | |
| 12 | | |
| 6 | |
| Revaluation of marketable securities | |
| 4 | | |
| (7 | ) |
| Stock based compensation | |
| 1,420 | | |
| 319 | |
| Increase in other accounts receivable | |
| (22 | ) | |
| (9 | ) |
| Increase in other liabilities | |
| 100 | | |
| - | |
| Accrued expenses and other payables | |
| 58 | | |
| 27 | |
| | |
| | | |
| | |
| Net cash used in operating activities | |
| (1,058 | ) | |
| (888 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds from the sale of marketable securities | |
| 774 | | |
| - | |
| Purchase of property and equipment | |
| (39 | ) | |
| (9 | ) |
| | |
| | | |
| | |
| Net cash provided by (used in) investing activities | |
| 735 | | |
| (9 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds from a convertible financial instrument | |
| 134 | | |
| - | |
| Issuance of shares | |
| - | | |
| 1,000 | |
| | |
| | | |
| | |
| Net cash provided by financing activities | |
| 134 | | |
| 1,000 | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| Net (decrease) increase in cash and cash equivalents | |
| (189 | ) | |
| 103 | |
| | |
| | | |
| | |
| Cash and cash equivalents at the beginning of the year | |
| 790 | | |
| 687 | |
| | |
| | | |
| | |
| Cash and cash equivalents at the end of the year | |
| 601 | | |
| 790 | |
The
accompanying notes are an integral part of the financial statements.
NewStem
Ltd.
Notes
to the Financial Statements for the year ended December 31, 2021
Note
1 - General
| |
A. |
NewStem
Ltd. (“the Company”) was incorporated in September 2016 under the laws of the State of Israel and commenced its business
operations in July 2018. |
| |
|
|
| |
B. |
The
Company is a development stage company utilizing its pioneering intellectual property related to haploid human embryonic stem cells
for the development of personalized diagnostics and therapeutics for genetic and epigenetic diseases. |
| |
|
|
| |
C. |
Since
inception, the Company has accumulated a deficit of $5,629 thousand. |
| |
|
The
Company will need to obtain additional funds to continue its operations. Management’s plans with regard to these matters include
continued development, marketing and licensing of its products, as well as seeking additional financing arrangements. Although management
continues to pursue these plans, there is no assurance that the Company will be successful in obtaining sufficient cash from sales
of products or financing on terms acceptable to the Company. Following the fund-raising mentioned in note 13, the Company believes
that its cash resources are sufficient for the operations of the next 12 months. |
| |
|
|
| |
D. |
Definitions |
In
these financial statements –
| |
1. |
The
Company – NewStem Ltd. |
| |
2. |
Related
Party – Within its meaning in ASC 850, “Related Party Transactions”. |
Note
2 - Significant Accounting Policies
The
significant accounting policies applied on a consistent basis are as follows:
The
financial statements are prepared in accordance with accounting principles generally accepted in the United States (“US GAAP”).
| |
B. |
Presentation
of financial information |
The
currency of the primary economic environment in which the Company conducts its operations is the U.S. dollar. Accordingly, the Company
uses the U.S. dollar as its functional and reporting currency.
The
preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates
and assumptions regarding transactions or matters the final effect of which on the financial statements cannot be accurately determined
at the time of their preparation. Even though the estimates and assumptions are based on management’s best judgment, the final
effect of such transactions or matters may be different from the estimates and assumptions made in their respect.
As
applicable to these financial statements, the most significant estimates and assumptions relate to stock-based compensation.
| |
D. |
Cash
and cash equivalents |
Cash
and cash equivalents include short-term bank deposits with an original maturity not exceeding three months, that is not restricted for
use.
| |
E. |
Property
and equipment |
Property
and equipment are stated at cost. Depreciation is computed by using the straight-line method, over the assets’ estimated useful
life.
The
annual depreciation rate for Software and Computers is 33%.
Estimates
of the depreciation method, useful life and residual value are reviewed at least at the end of each reporting year and adjusted as
necessary.
Long-lived
assets, including definite life intangible assets, held and used by the Company, are reviewed for impairment whenever events or changes
in circumstance indicate that the carrying amount of the assets may not be recoverable. No such impairment was recorded in 2021 or 2020.
| |
F. |
Concentrations
of credit risk |
Financial
instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, and
marketable securities.
Cash
and cash equivalents are invested in a major bank in Israel. Management believes that the financial institution that hold the Company’s
investments are financially sound and, accordingly, a minimal credit risk exists with respect to these investments.
The
marketable securities consisted of mutual fund investment which invest in highly rated debentures. Management is of the opinion that
the credit risk in respect to these securities is insignificant. The marketable securities were sold during 2021.
The
Company has no off-balance-sheet concentration of credit risk such as foreign exchange contracts, option contracts or other foreign hedging
arrangements.
Pursuant
to Section 14 of the Severance Compensation Law, 1963 (“Section 14”), the Company’s employees, covered by this section,
are entitled only to monthly deposits, at a rate of 8.33% of their monthly salary, made in their name with insurance companies and/or
pension funds. Payments in accordance with Section 14 release the Company from any liability for future severance payments in respect
of those employees. Deposits under Section 14 are not recorded as an asset in the Company’s balance sheet. As of December 31, 2021
and 2020, all of the Company’s employees are included under Section 14.
Marketable
securities are recorded at fair value. Changes in fair value of the securities are reported as financial income or expenses in the statement
of operations.
| |
I. |
Research
and development costs |
Research
and development expenses consist mainly of labor costs. Costs are expensed as incurred.
A
grant received is presented as an offset from research and development expenses. See also Note 2M.
Deferred
income taxes are determined using the asset and liability method in accordance with Accounting Standards Codification (“ASC”)
Topic 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the
financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income taxes are measured
using enacted tax rates expected to apply to taxable income in years in which such temporary differences are expected to be recovered
or settled. The effect of a change in tax rates on deferred income taxes is recognized in the statement of operations of the period that
includes the enactment date. In addition, a valuation allowance is established to reduce any deferred tax asset for which it is determined
that it is more likely than not that some portion of the deferred tax asset will not be realized.
| |
K. |
Fair
value of financial instruments |
The
following methods and assumptions were used by the Company in estimating its fair value disclosures for financial instruments:
The
carrying amounts of cash and cash equivalents, trade receivables, other accounts receivable, trade payables and other liabilities approximate
their fair value due to the short-term maturity of such instruments.
The
Company adopted ASC 820 Fair Value Measurements (“ASC 820”) which clarifies that fair value is an exit price, representing
the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants.
As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in
pricing an asset or a liability. As a basis for considering such assumptions, ASC 820 establishes a three-tier value hierarchy, which
prioritizes the inputs used in the valuation methodologies in measuring fair value:
| |
Level
1 |
- |
Observable
inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| |
|
|
|
| |
Level
2 |
- |
Other
inputs that are directly or indirectly observable in the marketplace. |
| |
|
|
|
| |
Level
3 |
- |
Unobservable
inputs which are supported by little or no market activity. |
The
fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when
measuring fair value.
| |
L. |
Stock-based
compensation |
The
Company accounts for its stock options grants under the fair value recognition provisions of ASC Topic 718. The Company currently uses
the straight-line amortization method for recognizing share option compensation costs. The Company recognizes compensation cost for an
award with only service conditions that has a graded vesting schedule on a straight-line basis over the requisite service period for
the entire award, provided that the cumulative amount of compensation cost recognized at any date at least equals the portion of the
grant-date value of such award that is vested at that date.
The
Company records prepaid share-based payment as an asset in cases where a fully vested equity award was granted but the services have
not been fully received, as required by ASC 718-10 stock compensation.
The
Company receives from time-to-time grants from various sources to fund certain research and development activities. To date, the grants’
terms have stated that if such research and development activities are not successful, the Company would not be obligated to refund any
payment previously received. Given such terms, since the financial risk associated with the research and development remains with the
grantor, the Company does not recognize a liability associated with such funding.
Grants
that do not include a specific deliverable in the terms are offset from research and development expenses.
| |
N. |
Recently
Issued Accounting Pronouncements |
In
February 2016, the FASB issued ASU No. 2016-02, Leases, which would require lessees to put all leases on their balance sheets, whether
operating or financing, while continuing to recognize the expenses on their income statements in a manner similar to current practice.
The guidance states that a lessee would recognize a lease liability for the obligation to make lease payments and a right-to-use asset
for the right to use the underlying asset for the lease term. In June 2020, the FASB issued ASU No. 2020-05, Revenue from Contracts with
Customers (Topic 606) and Leases (Topic 842): Effective Dates for Certain Entities, which defers the effective date of ASU 2016-02 for
non-public entities to fiscal years beginning after December 15,2021, and interim periods within fiscal years beginning after December
15, 2022. The guidance will be effective for the Company beginning January 1, 2022, and interim periods in fiscal years beginning January
1, 2023. The Company is currently evaluating the effect that ASU 2016-02 will have on its consolidated financial statements and related
disclosures.
Note
3 - Cash and Cash Equivalents
The
Company’s cash and cash equivalents balance at December 31, 2021 and 2020, is denominated in the following currencies:
| | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| US Dollars | |
| 371 | | |
| 769 | |
| New Israeli Shekels | |
| 97 | | |
| 21 | |
| Great British Pound | |
| 133 | | |
| - | |
| | |
| | | |
| | |
| | |
| 601 | | |
| 790 | |
Note
4 - Other Accounts Receivable
| | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Government institutions | |
| 52 | | |
| 22 | |
| Prepaid expenses | |
| 1 | | |
| 9 | |
| | |
| | | |
| | |
| | |
| 53 | | |
| 31 | |
Note
5 - Property and Equipment, net
| | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Cost: | |
| | | |
| | |
| Software and Computers | |
| 62 | | |
| 23 | |
| | |
| | | |
| | |
| Accumulated depreciation: | |
| | | |
| | |
| Software and Computers | |
| 21 | | |
| 9 | |
| | |
| | | |
| | |
| Depreciated cost | |
| 41 | | |
| 14 | |
Note
6 – Accounts payable
| | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Employees and payroll accruals | |
| 104 | | |
| 62 | |
| Accrued expenses and other payables | |
| 23 | | |
| 7 | |
| | |
| | | |
| | |
| | |
| 127 | | |
| 69 | |
Note
7 - Marketable Securities
The
marketable securities as of December 31, 2020 are an investment in a mutual fund, which invests in dollar-linked channels. During 2021,
the Company had sold its marketable securities.
Note
8 - Share Capital
Composition:
| | |
As of December 31, 2021 |
|
| | |
| | |
Issued and |
|
| | |
Authorized | | |
fully paid |
|
| | |
Number of shares |
|
| | |
| | | |
|
|
|
| Ordinary shares NIS 0.01 par value (“Ordinary Shares”) | |
| 1,000,000 | | |
|
158,696 |
|
| | |
As of December 31, 2020 |
|
| | |
| | |
Issued and |
|
| | |
Authorized | | |
fully paid |
|
| | |
Number of shares |
|
| | |
| | | |
|
|
|
| Ordinary shares
NIS 0.01 par value | |
| 1,000,000 | | |
|
150,000 |
|
| |
A. |
In
2016, the Company issued to its founders 100,000 Ordinary Shares. |
| |
|
|
| |
B. |
On
June 2018, the Company entered into an investment agreement for the issuance of 50,000 Ordinary Shares, representing 33% of the Company’s
issued and outstanding shares for a total consideration of $4,000 thousands. In 2018, the Company issued to its investors 25,000
Ordinary Shares for a total amount of $2,000 thousands. The remainder of the investment in the amount of $2,000 thousands was subject
to two equal tranches milestones. During 2019 the Company issued additional 12,500 Ordinary Shares for a total amount of $1,000 thousands. |
| |
|
|
| |
|
In
2020, the Company met all milestones set in the investment agreement. As such, the 3rd and last investment tranche $1,000 thousands
was paid during 2020 and an additional 12,500 Ordinary Shares were issued. |
| |
|
|
| |
C. |
In
September 2021, the Company signed a service agreement with a third-party in which such third party committed to provide the Company
certain services in exchange to 5% (fully diluted) of the Company’s Ordinary Shares amounting to 8,696 Ordinary Shares. The
Company recognized the transaction based on the fair value of the shares at $1,952 thousands. However, as part of the services was
rendered in 2022, the Company recognized a balance of approximately $771 thousands as Prepaid share-based payment. |
| |
|
|
| |
D. |
Stock
option plan: |
In
2018 the Company adopted a stock option plan for its employees, service providers and officers, pursuant to which, and to a resolution
of the Company’s board of directors dated October 31, 2018, the Company reserved for issuance 6,250 Ordinary Shares.
In
June 2021, the Company increased its reserved stock option plan to 13,654 Ordinary Shares.
The
contractual life of the share option is 10 years from the respective date of grant. Share options to employees, service providers
and officers granted under the stock option plan shall be vesting in installments, gradually over a period of 4 years from the grant
date.
Below
is a summary of employee option activity under the Company’s equity incentive plan during the current year:
| | |
Year ended December 31, 2021 | |
| | |
| | |
| | |
Weighted | | |
| |
| | |
| | |
Weighted | | |
average | | |
Aggregate | |
| | |
| | |
average | | |
remaining | | |
intrinsic | |
| | |
Number of | | |
exercise price | | |
contractual | | |
value | |
| | |
options | | |
US$ | | |
term (years) | | |
US$ thousands | |
| Outstanding at the | |
| | | |
| | | |
| | | |
| | |
| Beginning of the year | |
| 7,636 | | |
| 51.85 | | |
| | | |
| | |
| Granted | |
| 5,509 | | |
| 278.00 | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Outstanding at the end of the year | |
| 13,145 | | |
| 146.63 | | |
| 8.25 | | |
| 1,475 | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercisable at the end of the year | |
| 6,332 | | |
| 46.05 | | |
| 7.23 | | |
| 1,260 | |
| |
1. |
The
aggregate intrinsic value represents the total intrinsic value (the difference between the Company’s stock fair value on December
31, 2021 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders
had all option holders exercised their options on December 31, 2021. |
| |
|
|
| |
2. |
Fair
value measurement: |
The
fair value of each option granted during 2021 and 2020 was estimated on the date of grant, using the Binomial model taking into account
the following assumptions:
| | |
2021 | | |
2020 | |
| Dividend yield | |
| 0 | % | |
| 0 | % |
| Expected volatility | |
| 76 | % | |
| 89 | % |
| Weighted average risk-free interest | |
| 1.5 | % | |
| 1.36 | % |
| Expected life | |
| 10 years | | |
| 10 years | |
| | |
| | | |
| | |
Expected
volatility was calculated based on market benchmarks.
Since
the Company’s shares are not publicly traded and its shares are rarely traded privately, expected volatility is estimated based
on the average historical volatility of similar entities with publicly traded shares.
The
expected option term represents the period that the Company’s share options are expected to be outstanding. Since
the options were granted to executives, the assumption is that the option will be exercised close to the expiration date. The
risk-free interest rate is based on the yield from U.S. Federal Reserve rates. The Company has historically not paid dividends and has
no plans to pay dividends in the foreseeable future.
| |
D. |
Stock
option plan (cont’d): |
| |
3. |
The
following table sets forth the total stock-based compensation expense resulting from stock options included in the statements of
operations. |
| | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Research and development | |
| 185 | | |
| 220 | |
| General and administrative | |
| 54 | | |
| 99 | |
| | |
| | | |
| | |
| Total stock-based compensation expense | |
| 239 | | |
| 319 | |
| |
E. |
Convertible
Financial Instruments |
In
November 2021, the Company signed a Simple Agreement for Future Equity (“SAFE”) with an investor in the amount of 100 thousand
Great British Pound (“GBP”) (approximately US$134 thousands). According to the agreement, the SAFE does not bear interest
and is convertible to the Company’s ordinary shares, as follows:
| |
(a) |
In
the event of a financing round of at least 1 million GBP, the SAFE will be automatically converted into ordinary shares at the price
determined in such round; |
| |
(b) |
In
the event that the financing round is below 1 million GBP, the SAFE may be converted into ordinary shares at the price determined
in such round, at the discretion of the investor; |
| |
(c) |
If
no financing round occurs, the SAFE amount shall automatically be converted into ordinary shares at the earlier of: (a) an M&A
transaction – using the price per share determined in such transaction, or (b) 36 months after the date of the agreement, at
the fair market value of an ordinary share at that time. |
The
SAFE was treated for accounting purposes as a liability, since this arrangement is settled in a variable number of shares and the investor
is not exposed to the changes in the fair value of the shares during the period from the transfer of funds until conversion.
The
convertible financial instrument is presented at fair value. Due to the proximity of the agreement to the balance sheet date, it was
determined that there was no change to the fair value since receipt of the proceeds, and the convertible financial instrument was presented
at $134 thousands. The convertible financial instrument is considered a Level 3 fair value measurement.
Note
9 - Commitments and Contingent Liabilities
As
part of the Company’s research and development efforts, the Company received licenses to use intellectual property developed by
Yissum Research and Development Company of the Hebrew University of Jerusalem (“Yissum”) and New York Stem Cell Foundation
(“NYSCF”). During 2017, Yissum and NYSCF granted the Company an exclusive license to make commercial use of that intellectual
property, in order to develop, manufacture, market, distribute or sell products, subject to certain terms and events. In consideration
for the grant of the license, the Company shall pay Yissum and NYSCF royalties at a rate of up to 3% of the net sales and sublicense
fees at a rate of up to 12% of sublicense consideration, subject to certain terms, as set forth in the agreement. As of December 31,
2021, the Company has yet to incur revenues, therefore no provision was recorded for these commitments in the financial statements.
During
December 2021, the Company received a prepayment of $100 thousand as part of a research agreement with a third-party, which was finalized
in 2022. The research agreement determines that the Company will use its intellectual property to further develop know-how that will
allow the third party to use such developed know-how for its commercial purposes. The total consideration for the research, to be paid
over two years, amounts to $500 thousand. The third party shall pay the Company royalties of up to 3.5% from any sales that include the
Company’s developed know-how, and additional royalties for any sublicense, as set forth in the research agreement.
Note
10 - Financial Expenses (Income), net
| | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Bank commissions | |
| 1 | | |
| 1 | |
| Revaluation of marketable securities to market value | |
| 4 | | |
| (7 | ) |
| Currency exchange differences | |
| - | | |
| (1 | ) |
| | |
| | | |
| | |
| | |
| 5 | | |
| (7 | ) |
Note
11 - Related Parties
The
Company engaged with its shareholders to receive consulting services and lab renting.
Transactions
| | |
Year ended | | |
Year ended | |
| | |
December 31 | | |
December 31 | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Research and development expenses | |
| 309 | | |
| 329 | |
The
Company leases a laboratory from a shareholder. The lease is for an initial three-year term expiring in June 2021. The Company extended
the lease until June 2022 and it has an option to further extend the term for an additional one-year period. Each party shall be entitled
to terminate the agreement within 30 days’ notice.
The
lease has been classified as an operating lease and the expenses are included in the data presented above. Total lease cost associated
with this lease for the year ended December 31, 2021 and 2020 was US$48 thousand and US$35 thousand, respectively.
Note
12 - Taxes on Income
| |
A. |
The
Company is incorporated in Israel and is subject to Israeli taxation. |
| |
|
|
| |
B. |
The
Israeli corporate income tax rate was 23% in 2021 and 2020. |
| |
|
The
main reconciling items from the statutory tax rate of the Company to the effective tax rate (0%) is the change in valuation allowance
(see note 12D) and non-deductible expenses. |
| |
|
|
| |
C. |
Net
operating loss carried forward |
As
of December 31, 2021, the Company has net operating tax losses carried forward indefinitely of approximately $3.1 million, (December
31, 2020 - $2.1 million).
The
tax effects of temporary differences that give rise to significant components of the Company’s deferred tax assets and liabilities
are as follows:
| | |
December 31, | |
| | |
2021 | | |
2020 | |
| | |
US$ thousands | | |
US$ thousands | |
| Deferred tax assets: | |
| | | |
| | |
| Net operating losses | |
| 719 | | |
| 474 | |
| Research and development credit carried forward | |
| 230 | | |
| 163 | |
| Provision for employees ‘benefits | |
| 4 | | |
| 10 | |
| | |
| 953 | | |
| 647 | |
| | |
| | | |
| | |
| Less valuation allowance | |
| (953 | ) | |
| (647 | ) |
| | |
| | | |
| | |
| Net deferred tax assets | |
| - | | |
| - | |
| |
D. |
Deferred
income taxes (cont’d) |
The
Company has provided a full valuation allowance in respect of deferred tax assets resulting from the tax loss carried forward. Management
currently believes that, since the Company has a history of losses, it is more likely than not that the deferred tax assets related to
the loss carried forward and other temporary differences will not be realized in the foreseeable future.
Note
13 – Subsequent Events
On
April 30, 2022, the Company signed a share purchase agreement with two investors for the purchase of 2,647 Ordinary Shares of the Company
(par value ILS 0.01) for a total consideration of US$800 thousands. Based on the Company’s agreement with one of its shareholders,
that shareholder is committed to invest a matching amount which will bring the total funding to US$1,600 thousands.
NewStem
Ltd.
Condensed
Interim Balance Sheets as of
(Unaudited)
| | |
September
30 | | |
December
31 | |
| | |
2022 | | |
2021 | |
| | |
US$
thousands | | |
US$
thousands | |
| Assets | |
| | | |
| | |
| | |
| | | |
| | |
| Current
assets | |
| | | |
| | |
| Cash and cash
equivalents | |
| 519 | | |
| 601 | |
| Prepaid share-based payment | |
| - | | |
| 771 | |
| Other
accounts receivable | |
| 54 | | |
| 53 | |
| Total
current assets | |
| 573 | | |
| 1,425 | |
| | |
| | | |
| | |
| Non-current
assets | |
| | | |
| | |
| Property
and equipment, net | |
| 27 | | |
| 41 | |
| | |
| | | |
| | |
| Total
assets | |
| 600 | | |
| 1,466 | |
| | |
| | | |
| | |
| Liabilities
and shareholders’ equity | |
| | | |
| | |
| | |
| | | |
| | |
| Current
liabilities | |
| | | |
| | |
| Accrued expenses and other
payables | |
| 87 | | |
| 127 | |
| Other
liabilities | |
| 17 | | |
| 100 | |
| Total
current liabilities | |
| 104 | | |
| 227 | |
| | |
| | | |
| | |
| Non-current
liabilities | |
| | | |
| | |
| Convertible
financial instrument | |
| 112 | | |
| 134 | |
| Total
liabilities | |
| 216 | | |
| 361 | |
| | |
| | | |
| | |
| Shareholders’
equity | |
| | | |
| | |
| Ordinary shares | |
| *
| | |
| *
| |
| Additional paid-in capital | |
| 7,948 | | |
| 6,734 | |
| Accumulated
deficit | |
| (7,564 | ) | |
| (5,629 | ) |
| Total
shareholders’ equity | |
| 384 | | |
| 1,105 | |
| | |
| | | |
| | |
| Total
liabilities and shareholders’ equity | |
| 600 | | |
| 1,466 | |
/s/
Ayelet Dilion Mashiah |
|
| Ayelet
Dilion Mashiah |
|
| CEO
|
|
Date
of approval of the financial statements: November 2, 2022
*
Represents an amount lower than $1 thousand.
The
accompanying notes are an integral part of the condensed interim financial statements.
NewStem
Ltd.
Condensed
Interim Statements of Operations for the
(Unaudited)
| | |
Nine-month | | |
Nine-month | |
| | |
period
ended | | |
period
ended | |
| | |
September
30, | | |
September
30, | |
| | |
2022 | | |
2021 | |
| | |
US$
thousands | | |
US$
thousands | |
| Operating
expenses | |
| | | |
| | |
| | |
| | | |
| | |
| Research and development
expenses | |
| 1,917 | | |
| 1,055 | |
| Less
– grants and participations received | |
| (183 | ) | |
| (90 | ) |
| Research and development expenses,
net | |
| 1,734 | | |
| 965 | |
| | |
| | | |
| | |
| General
and administrative expenses | |
| 210 | | |
| 163 | |
| | |
| | | |
| | |
| Operating
loss | |
| 1,944 | | |
| 1,128 | |
| | |
| | | |
| | |
| Financial
(income) expenses, net | |
| (9 | ) | |
| 4 | |
| | |
| | | |
| | |
| Loss
for the period | |
| 1,935 | | |
| 1,132 | |
The
accompanying notes are an integral part of the condensed interim financial statements.
NewStem
Ltd.
Condensed
Interim Statements of Changes in Shareholders’ Equity
(Unaudited)
| | |
| | |
| | |
Additional | | |
| | |
| |
| | |
| | |
| | |
paid-in | | |
Accumulated | | |
| |
| | |
Ordinary
shares | | |
capital | | |
deficit | | |
Total | |
| | |
Number
of shares | | |
US$
thousands | | |
US$
thousands | | |
US$
thousands | | |
US$
thousands | |
| For the
nine - month period ended | |
| | |
| | |
| | |
| | |
| |
| September
30, 2022 | |
| | |
| | |
| | |
| | |
| |
| | |
| | |
| | |
| | |
| | |
| |
| Balance
as of January 1, 2022 | |
| 158,696 | | |
| * | | |
| 6,734 | | |
| (5,629 | ) | |
| 1,105 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance
of ordinary shares, net | |
| 2,647 | | |
| *
| | |
| 800 | | |
| - | | |
| 800 | |
| Share based compensation | |
| - | | |
| - | | |
| 414 | | |
| - | | |
| 414 | |
| Loss
for the period | |
| - | | |
| - | | |
| - | | |
| (1,935 | ) | |
| (1,935 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as at September 30, 2022 | |
| 161,343 | | |
| * | | |
| 7,948 | | |
| (7,564 | ) | |
| 384 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| For the
nine - month period ended | |
| | | |
| | | |
| | | |
| | | |
| | |
| September
30, 2021 | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of January 1, 2021 | |
| 150,000 | | |
| *
| | |
| 4,543 | | |
| (2,999 | ) | |
| 1,544 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance
of ordinary shares in exchange | |
| | | |
| | | |
| | | |
| | | |
| | |
| of
services, net | |
| 8,696 | | |
| * | | |
| 1,952 | | |
| - | | |
| 1,952 | |
| Share based compensation | |
| - | | |
| - | | |
| 154 | | |
| - | | |
| 154 | |
| Loss
for the period | |
| - | | |
| - | | |
| - | | |
| (1,132 | ) | |
| (1,132 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of September 30, 2021 | |
| 158,696 | | |
| *
| | |
| 6,649 | | |
| (4,131 | ) | |
| 2,518 | |
*
Represents an amount less than $1 thousand.
The
accompanying notes are an integral part of the condensed interim financial statements.
NewStem
Ltd.
Condensed
Interim Statements of Cash Flows for the
(Unaudited)
| | |
Nine-month | | |
Nine-month | |
| | |
period
ended | | |
period
ended | |
| | |
September
30, | | |
September
30, | |
| | |
2022 | | |
2021 | |
| | |
US$
thousands | | |
US$
thousands | |
| Cash flows
from operating activities | |
| | | |
| | |
| | |
| | | |
| | |
| Loss for the period | |
| (1,935 | ) | |
| (1,132 | ) |
| | |
| | | |
| | |
| Adjustments required to reconcile
loss for the period to net cash used | |
| | | |
| | |
| in operating activities: | |
| | | |
| | |
| | |
| | | |
| | |
| Depreciation | |
| 14 | | |
| 8 | |
| Revaluation of marketable
securities | |
| - | | |
| 4 | |
| Revaluation of convertible
financial instrument | |
| (22 | ) | |
| - | |
| Share based compensation | |
| 1,185 | | |
| 336 | |
| Increase in other accounts
receivable | |
| (1 | ) | |
| (47 | ) |
| Increase (decrease) in accrued
expenses and other payables | |
| (40 | ) | |
| 11 | |
| Decrease
in other liabilities | |
| (83 | ) | |
| - | |
| | |
| | | |
| | |
| Net
cash used in operating activities | |
| (882 | ) | |
| (820 | ) |
| | |
| | | |
| | |
| Cash flows
from investing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds from the sale of
marketable securities | |
| - | | |
| 774 | |
| Purchase
of property and equipment | |
| - | | |
| (26 | ) |
| | |
| | | |
| | |
| Net
cash provided by investing activities | |
| - | | |
| 748 | |
| | |
| | | |
| | |
| Cash flows
from financing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds
from issuance of ordinary shares, net | |
| 800 | | |
| - | |
| | |
| | | |
| | |
| Net
cash provided by financing activities | |
| 800 | | |
| - | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| Net
decrease in cash and cash equivalents | |
| (82 | ) | |
| (72 | ) |
| | |
| | | |
| | |
| Cash
and cash equivalents at the beginning of the period | |
| 601 | | |
| 790 | |
| | |
| | | |
| | |
| Cash
and cash equivalents at the end of the period | |
| 519 | | |
| 718 | |
The
accompanying notes are an integral part of the condensed interim financial statements.
NewStem
Ltd.
Notes
to the Condensed Interim Financial Statements as of September 30, 2022
Note
1 - General
| |
A. |
NewStem
Ltd. (“the Company”) was incorporated in September 2016 under the laws of the State of Israel and commenced its business
operations in July 2018. |
| |
|
|
| |
B. |
The
Company is a development stage company utilizing its pioneering intellectual property related to haploid human embryonic stem cells
for the development of personalized diagnostics and therapeutics for genetic and epigenetic diseases. |
| |
|
|
| |
C. |
Since
inception, the Company has accumulated losses of $7,564 thousand. |
| |
|
The
Company will need to obtain additional funds to continue its operations. Management’s plans with regard to these matters include
continued development, marketing and licensing of its products, as well as seeking additional financing arrangements. Although management
continues to pursue these plans, there is no assurance that the Company will be successful in obtaining sufficient cash from sales
of products or financing on terms acceptable to the Company. |
| |
|
|
| |
|
The
company currently has agreements in place and draft agreements that will provide cash resources that should suffice for the operations
of the next 12 months. If the draft agreements do not materialize to final agreements, the Company may incur difficulties to continue
to operate its business and that may raise doubt about its ability to continue as a going concern. The interim financial statements
do not include any adjustments that might result from the outcome of these uncertainties. |
| |
|
|
| |
D. |
Definitions |
| |
|
|
| |
|
In
these financial statements – |
| |
1. |
The
Company – NewStem Ltd. |
| |
2. |
Related
Party – Within its meaning in ASC 850, “Related Party Transactions”. |
Note
2 - Basis of Presentation
The
accompanying condensed interim balance sheet as of September 30, 2022, and the condensed interim statements of operations, changes in
shareholders’ equity and cash flows for the nine-month period ended September 30, 2022 are unaudited. These unaudited condensed
interim financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America.
The
unaudited condensed interim financial statements contain all adjustments which, in the opinion of management, are necessary to present
fairly, the financial information included therein. It is suggested that these condensed interim financial statements be read in conjunction
with the audited financial statements and accompanying notes included in the Company’s report for the year ended December 31, 2021
(“the 2021 Financial Statements”). Results for the interim periods presented are not necessarily indicative of the results
to be expected for the full year.
The
accounting principles used in the preparation of the interim statements are consistent with those used in the preparation of the 2021
Financial Statements, except as follows:
The
Company signed a development agreement with a third-party. As part of the agreement, which is not part of the Company’s ordinary
activities, the Company receives reimbursement for its research and development expenses and will receive subsequent royalties from any
future sales that include the Company’s developed intellectual property. The Company has implemented ASC 808 “Collaborative
Arrangements” (“ASC 808”) and based on ASC 808 guidance has applied an accounting policy according to which such reimbursements
are offset from its research and development expenses.
NewStem
Ltd.
Notes
to the Condensed Interim Financial Statements as of September 30, 2022
Note
3 - Related Parties
The
Company engaged with its shareholders to receive consulting services and lab renting.
Transactions
| | |
Nine-month | | |
Nine-month | |
| | |
period
ended | | |
period
ended | |
| | |
September
30, | | |
September
30, | |
| | |
2022 | | |
2021 | |
| | |
US$
thousands | | |
US$
thousands | |
| Research
and development expenses | |
| 267 | | |
| 237 | |
The
Company leases a laboratory from a shareholder. The lease is for an initial three-year term expiring in June 2021. The Company extended
the lease until June 2023. Each party shall be entitled to terminate the agreement within 30 days’ notice.
The
lease has been classified as an operating lease and the expenses are included in the data presented above. Total lease cost associated
with this lease for the nine-month period ended September 30, 2022 and 2021 was US$33 thousand and US$33 thousand, respectively.
Note
4 - Events during the period
| |
A. |
Further
to what is stated in Note 8C of the 2021 Financial Statements, the service agreement with a third-party came to its end in March
2022. Accordingly, the prepaid share-based payment balance, was fully recognized as an expense in the Statement of Operations. |
| |
|
|
| |
B. |
On
April 30, 2022, the Company signed a share purchase agreement with two investors for the purchase of 2,647 Ordinary Shares of the
Company (par value ILS 0.01) for a total consideration of US$800 thousands. Based on the Company’s agreement with
one of its other shareholders, the Company is entitled to a matching investment (“the matching investment”) which will
bring the total funding to US$1,600 thousands. As of September 30, 2022, the matching investment has not yet been received. |
(b)
Exhibits.
SIGNATURES
Pursuant
to the requirements of Section 12 of the Securities Exchange Act of 1934, the registrant has duly caused this registration statement
to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
NOVELSTEM
INTERNATIONAL CORP. |
| |
|
|
| Date:
June 28, 2023 |
By:
|
/s/
Jan Loeb |
| |
Name: |
Jan
Loeb |
| |
Title: |
President
and Executive Chairman |
NovelStem (PK) (USOTC:NSTM)
Historical Stock Chart
From Mar 2024 to Apr 2024
NovelStem (PK) (USOTC:NSTM)
Historical Stock Chart
From Apr 2023 to Apr 2024