Roivant's Dermavant Gets FDA OK of Vtama for Plaque Psoriasis
May 24 2022 - 6:49AM
Dow Jones News
By Colin Kellaher
Roivant Sciences Ltd.'s Dermavant Sciences unit on Tuesday said
it received U.S. Food and Drug Administration approval for its
Vtama cream 1% for the topical treatment of adults with the chronic
inflammatory disease plaque psoriasis.
The pharmaceutical company said Vtama is the first and only
FDA-approved steroid-free topical medication in its class, and the
first topical novel chemical entity launched for psoriasis in the
U.S. in 25 years.
Dermavant said it is ready for a June launch, with product and
sample manufacturing runs completed and a fully staffed commercial
team.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
May 24, 2022 06:34 ET (10:34 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
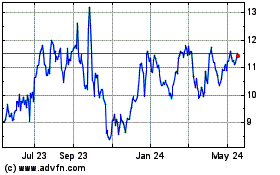
Roivant Sciences (NASDAQ:ROIV)
Historical Stock Chart
From Mar 2024 to Apr 2024
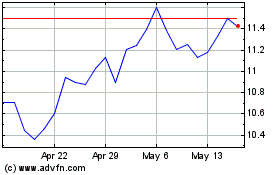
Roivant Sciences (NASDAQ:ROIV)
Historical Stock Chart
From Apr 2023 to Apr 2024