Allergy Therapeutics' Peanut Allergy Study Gives Positive Primary Results
August 03 2021 - 4:18AM
Dow Jones News
By Anthony O. Goriainoff
Allergy Therapeutics PLC said Tuesday that a study evaluating
biomarkers from peanut-allergic patients gave positive primary
results.
The U.K. biotechnology company said that its peanut allergy
vaccine candidate, which is based on virus-like particles, had a
successful primary outcome, and that the results confirmed the
peanut vaccine candidate's hypoallergic potential.
The company said the results were encouraging and provided
strong support for the human translation of the pre-clinical
results, as well as strong confidence in the data to be generated
in its planned Phase I study, which is due to start on the first
quarter of 2022.
The results further strengthen the company's Investigational New
Drug application with the U.S. Food and Drug Administration, which
it expects to submit later in the year.
"Through our collaboration with Imperial College London and the
dedication of our clinical and R&D teams at Allergy
Therapeutics, we are another step closer to offering a potentially
transformative treatment option for one of the most dangerous
allergies," Chief Executive Manuel Llobet said.
Shares at 0750 GMT were up 1.40 pence, or 5.2%, at 28.25
pence.
Write to Anthony O. Goriainoff at
anthony.orunagoriainoff@dowjones.com
(END) Dow Jones Newswires
August 03, 2021 04:07 ET (08:07 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
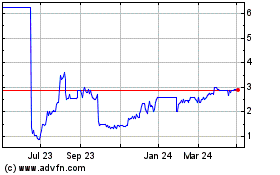
Allergy Therapeutics (LSE:AGY)
Historical Stock Chart
From Mar 2024 to Apr 2024
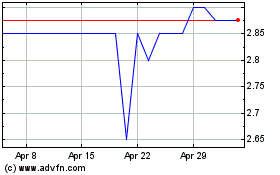
Allergy Therapeutics (LSE:AGY)
Historical Stock Chart
From Apr 2023 to Apr 2024