NEW
YORK, July 5, 2024 /PRNewswire/ -- The
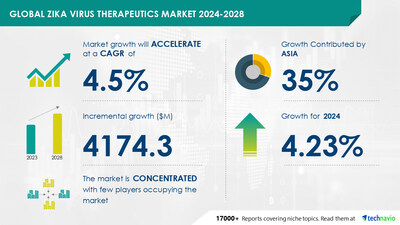
global zika virus therapeutics market size is estimated
to grow by USD 4.17 billion from
2024-2028, according to Technavio. The market is estimated to grow
at a CAGR of 4.5% during the forecast
period. Availability of serology kit for qualitative
diagnosis of zika virus infection is driving market
growth, with a trend towards increasing efforts by
government and private organizations to facilitate vaccine
development. However, asymptomatic nature of the infection
poses a challenge. Key market players include Aurobindo
Pharma Ltd., Bharat Biotech Ltd., BioVaxys Technology Corp.,
Emergent BioSolutions Inc., GeoVax Labs Inc., Granules India Ltd.,
Inovio Pharmaceuticals Inc., Johnson and Johnson Services Inc.,
Merck and Co. Inc., Moderna Inc., Perrigo Co. Plc, Sun
Pharmaceutical Industries Ltd., Takeda Pharmaceutical Co. Ltd., and
Valneva SE.
Get a detailed analysis on regions, market
segments, customer landscape, and companies - Click for the
snapshot of this report
|
Forecast
period
|
2024-2028
|
|
Base Year
|
2023
|
|
Historic
Data
|
2018 - 2022
|
|
Segment
Covered
|
Product (Acetaminophen
and Other NSAIDs) and Geography (North America, Europe, Asia, and
Rest of World (ROW))
|
|
Region
Covered
|
North America, Europe,
Asia, and Rest of World (ROW)
|
|
Key companies
profiled
|
Aurobindo Pharma Ltd.,
Bharat Biotech Ltd., BioVaxys Technology Corp., Emergent
BioSolutions Inc., GeoVax Labs Inc., Granules India Ltd., Inovio
Pharmaceuticals Inc., Johnson and Johnson Services Inc., Merck and
Co. Inc., Moderna Inc., Perrigo Co. Plc, Sun Pharmaceutical
Industries Ltd., Takeda Pharmaceutical Co. Ltd., and Valneva
SE
|
Key Market Trends Fueling Growth
The Zika virus market is currently focused on the development of
vaccines to combat the disease, as there is no approved treatment
or vaccine available. The World Health Organization (WHO) and the
United Nations International Children's Emergency Fund (UNICEF) are
collaborating with various organizations to advance vaccine
research. For instance, the National Institute of Allergy and
Infectious Diseases (NIAID) is conducting Phase I trials for a Zika
virus inactivated purified vaccine (ZPIV) in adults and for
combination vaccines. The NIAID and the Walter Reed Army Institute
of Research (WRAIR) are also collaborating on a ZPIV accelerated
vaccination schedule study. Private organizations, such as Bharat
Biotech Ltd. And Takeda, are also developing ZPIVs against Zika
virus infection. Inactivated vaccines eliminate the disease-causing
part of the virus while maintaining antigenicity. They are safe in
pregnancy and have been licensed for the prevention of viral
diseases like Japanese encephalitis and tick-borne encephalitis.
However, they require multiple doses and adjuvants for optimal
immune response. Moderna recently announced the ongoing Phase 2
clinical trial of its mRNA-1893 candidate in the US and
Puerto Rico, in collaboration with
BARDA. The initial findings from the Phase 1 study showed that both
10 ug and 30 ug dose levels effectively seroconverted participants
and were generally well-tolerated. These developments are expected
to positively impact the growth of the Zika virus therapeutics
market in the coming years.
The Zika Virus Therapeutics Market is experiencing significant
growth due to the ongoing global health crisis. Key trends include
the exploration of caspase-8 as a potential therapeutic target for
inhibiting viral replication and targeting host factors. However,
the emergence of drug-resistant strains poses a challenge,
requiring the development of broader efficacy therapeutic
approaches. Repurposing existing drugs and advancing through the
drug development process is a priority, focusing on safety and
efficacy in clinical trials. Market segmentation includes oral and
injection antiviral medications, vaccines, and treatment modalities
such as supportive care, symptomatic relief, and medical
intervention for severe cases. Ongoing preclinical studies on
related viruses and novel drug targets offer promising
opportunities. Driving factors include public health concerns,
neurological complications, and commercial opportunities. Key
players are focusing on patient recruitment, safety profile,
optimal dosing regimens, and effectiveness in human trials.
Approval and commercialization, raw materials, and diagnostic
testing are also crucial aspects of the market. Treatment options
range from hospital care to homecare and clinic settings, with
vaccines and antiviral medications being the primary focus. Market
growth is expected to continue as the world seeks effective
solutions for Zika virus infections.
Discover 360° analysis of this market. For
complete information, schedule your consultation- Book
Here!
Market Challenges
- The asymptomatic nature of Zika virus infection poses
challenges to the growth of the Zika Virus Therapeutics Market.
Approximately 80% of the infected population is asymptomatic,
leading to underdiagnosis and decreased incidence rates. This
uncertainty complicates cohort studies and modeling, making it
difficult to determine transmission dynamics and control
interventions. Pregnant women, who are at higher risk, are
monitored through RT-PCR and serology tests, but an estimated 19%
of infected women go undiagnosed. To improve diagnosis rates,
routine testing of the susceptible population is necessary. The
lack of clear symptoms hinders the development and implementation
of effective therapeutic options, limiting market expansion.
- The Zika virus therapeutic market faces significant challenges
due to the mosquito-borne nature of the disease, primarily
transmitted by Aedes mosquitoes. The clinical manifestations of
Zika virus infection include fever, rash, malaise, headache,
arthralgia, and pruritic maculopapular rashes. In severe cases,
Zika virus can cause birth defects, such as microcephaly, optic
neuropathy, congenital glaucoma, and other congenital disabilities.
Comorbidities and epidemics can further complicate treatment.
Current options for managing Zika virus include symptomatic relief
with acetaminophen. However, there is a pressing need for potential
treatments, with a small molecule inhibitor called Emricasan
showing promise in laboratory studies due to its antiviral activity
against the Zika virus and its ability to target a host protein
involved in liver disease. The drug market is eagerly awaiting the
development of effective therapeutics to mitigate the impact of
Zika virus infection, particularly in preventing severe birth
defects transmitted through sexual contact from the mother to the
fetus.
For more insights on driver and
challenges - Download a Sample Report
Segment Overview
This zika virus therapeutics market report extensively covers
market segmentation by
- Product
- 1.1 Acetaminophen
- 1.2 Other NSAIDs
- Geography
- 2.1 North America
- 2.2 Europe
- 2.3 Asia
- 2.4 Rest of World (ROW)
1.1 Acetaminophen- The Zika Virus Therapeutics
Market refers to the sales and production of drugs used to treat
Zika virus infections. Key players in this market include Merck
& Co., Teva Pharmaceutical Industries, and Sanofi. These
companies develop and manufacture antiviral medications to prevent
and treat Zika virus symptoms. The market's growth is driven by the
increasing number of Zika virus cases worldwide and the need for
effective treatments. Companies invest in research and development
to bring new therapies to market and expand their product
offerings.
For more information on market segmentation with
geographical analysis including forecast (2024-2028) and historic
data (2018 - 2022) - Download a Sample Report
Research Analysis
The Zika virus infection is a mosquito-borne disease transmitted
primarily by Aedes mosquitoes. The virus can cause severe birth
defects, including microcephaly, in fetuses when a pregnant woman
is infected. Zika virus can also be transmitted through sexual
contact. The drug market for potential treatments against Zika
virus is growing due to the ongoing epidemic. Small molecule
inhibitors are being explored as possible therapeutics. Dengue,
Malaria, Typhoid, and Pneumonia are other diseases transmitted by
Aedes mosquitoes. Clinical manifestations of Zika virus infection
include rash, malaise, headache, low-grade fever, and arthralgia.
Acetaminophen is commonly used to alleviate symptoms. Nature
Biomedical Engineering published a study on potential treatments
for Zika virus. Roche, TIB Molbiol Group, DiaSorin, and Quest
Diagnostics are among the companies involved in the development of
diagnostic tests for Zika virus.
Market Research Overview
The Zika virus therapeutics market refers to the development of
potential treatments for Zika virus infection, which is primarily
transmitted by Aedes mosquitoes and can cause severe birth defects,
including microcephaly, during pregnancy. Several drug market
opportunities exist for antiviral medications, small molecule
inhibitors, and vaccines. Emricasan, a small molecule inhibitor
with antiviral activity against Zika virus, has shown promise in
laboratory studies by targeting host proteins such as caspase-8 and
inhibiting viral replication. However, challenges include the
development of drug-resistant strains, safety and efficacy in
clinical trials, and broader efficacy against related viruses like
Dengue, Malaria, Typhoid, and Pneumonia. Repurposing existing drugs
and advances in technology, such as computational modeling and
genomic analysis, are driving the drug development process. Market
segmentation includes oral, injection, antiviral medications,
vaccines, and various treatment modalities such as hospital,
homecare, clinic, and medical intervention for severe cases.
Symptomatic relief, supportive care, diagnostic testing, and
follow-up care are also essential components of the market. Driving
factors include public health concerns, neurological complications,
commercial opportunities, reimbursement policies, and advances in
technology. Ethical considerations and patient recruitment
difficulties are significant challenges. Zika virus outbreaks and
testing, as well as gaps in understanding Zika virus biology and
transmission dynamics, highlight the need for effective drug
targets and treatment strategies. The NCBI provides valuable
resources for research on Zika virus and related viruses.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Product
-
- Acetaminophen
- Other NSAIDs
- Geography
-
- North America
- Europe
- Asia
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory
company. Their research and analysis focuses on emerging market
trends and provides actionable insights to help businesses identify
market opportunities and develop effective strategies to optimize
their market positions.
With over 500 specialized analysts, Technavio's report library
consists of more than 17,000 reports and counting, covering 800
technologies, spanning across 50 countries. Their client base
consists of enterprises of all sizes, including more than 100
Fortune 500 companies. This growing client base relies on
Technavio's comprehensive coverage, extensive research, and
actionable market insights to identify opportunities in existing
and potential markets and assess their competitive positions within
changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/zika-virus-therapeutics-market-size-is-set-to-grow-by-usd-4-17-billion-from-2024-2028--availability-of-serology-kit-for-qualitative-diagnosis-of-zika-virus-infection-to-boost-the-market-growth-technavio-302189587.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/zika-virus-therapeutics-market-size-is-set-to-grow-by-usd-4-17-billion-from-2024-2028--availability-of-serology-kit-for-qualitative-diagnosis-of-zika-virus-infection-to-boost-the-market-growth-technavio-302189587.html
SOURCE Technavio