Filed Pursuant to Rule 424(b)(3)
Registration No. 333-267053
PROSPECTUS

Waldencast plc
Up to 29,533,282
Class A Ordinary Shares (for issuance)
Up to 121,120,063
Class A Ordinary Shares (for resale)
Up to 18,033,332
Warrants to Purchase Class A Ordinary Shares (for resale)
This prospectus relates to the issuance by us
of up to 29,533,282 Class A ordinary shares, par value $0.0001 (“Class A ordinary shares”), consisting of: (i) Class A ordinary
shares issuable upon the exercise of the private placement warrants (as defined below) and (ii) Class A ordinary shares issuable upon
the exercise of the public warrants (as defined below).
This prospectus also relates to the resale
by certain of the selling holders named in this prospectus and their pledgees, donees, transferees, assignees and successors (the “Selling
Holders”) of: (1) up to 121,120,063 Class A ordinary shares, consisting of (i) 8,545,000 Class A ordinary shares converted from
the founder shares (as defined below) held in the aggregate by Burwell Mountain PTC LLC, as trustee of Burwell Mountain Trust (collectively,
“Burwell”), Dynamo Master Fund and Waldencast Ventures LP (each, a member of the Waldencast Long-Term Capital LLC, a Cayman
Islands limited liability company (the “Sponsor”)), originally issued in a private placement to the Sponsor, for approximately
$0.0035 per share, prior to our initial public offering (as defined below); (ii) 80,000 Class A ordinary shares converted from the founder
shares held by the Investor Directors (as defined below), issued in a private placement to the Investor Directors, for approximately
$0.0035 per share, prior to our initial public offering; (iii) 20,000 Class A ordinary shares issued to Aaron Chatterley, one of our
independent directors, in a private placement exempt from registration pursuant to Section 4(a)(2) of the Securities Act, and Rule 506
of Regulation D promulgated thereunder, in connection with the consummation of the Business Combination (as defined below), for no consideration;
(iv) 28,237,506 Class A ordinary shares issued in connection with the Business Combination as the equity portion of the merger consideration
pursuant to the Obagi Merger Agreement (as defined below) at an acquiror share value of $10.00 per share; (v) 21,104,225 Class A ordinary
shares issuable in exchange for 21,104,225 Waldencast LP Common Units (as defined below) (and the redemption by Waldencast of an equivalent
number of Class B ordinary shares, par value $0.0001 per share (“Class B ordinary shares”) held by such holder for no additional
consideration) issued in connection with the Business Combination as the equity portion of the transaction consideration pursuant to
the Milk Equity Purchase Agreement (as defined below) at a common unit value of $10.00 per unit; (vi) 11,800,000 Class A ordinary shares
issued in the PIPE Investments (as defined below) pursuant to the Subscription Agreements (as defined below), at a purchase price of
$10.00 per share at the closing of the Business Combination; (vii) 33,300,000 Class A ordinary shares issued pursuant the Forward Purchase
Agreements (as defined below), which Forward Purchase Securities (as defined below) were issued at a purchase price of $10.00 per Forward
Purchase Security at the closing of the Business Combination; and (viii) 18,033,332 Class A ordinary shares issuable upon the exercise
of the private placement warrants and (2) up to 18,033,332 private placement warrants, which private placement warrants were issued in
a private placement at the time of our initial public offering, at a purchase price of $1.50 per private placement warrant.
This prospectus provides you with a general description
of such securities and the general manner in which we and the Selling Holders may offer or sell the securities. More specific terms of
any securities that we and the Selling Holders may offer or sell may be provided in a prospectus supplement that describes, among other
things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also
add, update or change information contained in this prospectus.
The Class A ordinary shares being offered for resale pursuant to this
prospectus by the Selling Holders represent approximately 82.5% of shares outstanding of the Company as of July 27, 2022 (after giving
effect to the issuance of shares upon exercise of outstanding public warrants and private placement warrants). Of such shares, 102,366,731
Class A ordinary shares, or approximately 70.0% of shares outstanding of the Company as of July 27, 2022 (after giving effect to the issuance
of shares upon exercise of outstanding public warrants and private placement warrants), are subject to Lock-Up agreements pursuant to
which certain of the Selling Holders have agreed not to transfer, assign or sell during the respective Lock-Up Periods (as defined below),
which range from six months to twelve months from the closing of the Business Combination on June 27, 2022, subject to certain exceptions
as described below. Nonetheless, given the substantial number of Class A ordinary shares being registered for potential resale by Selling
Holders pursuant to this prospectus, the sale of shares by the Selling Holders, or the perception in the market that the Selling Holders
of a large number of shares intend to sell shares, could increase the volatility of the market price of our Class A ordinary shares or
result in a significant decline in the public trading price of our Class A ordinary shares. Even if our trading price is significantly
below $10.00, the offering price for the Legacy units (as defined below) offered in our initial public offering, the per share price of
the shares issued in the PIPE Investment and the per Forward Purchase Security price of the Forward Purchase Security issued pursuant
to the Forward Purchase Agreements, certain of the Selling Holders, including Burwell, Dynamo Master Fund and Waldencast Ventures LP,
may still have an incentive to sell our Class A ordinary shares because they hold founder shares that were originally purchased by the
Sponsor at prices lower than the public investors or the current trading price of our Class A ordinary shares. For example, based on the
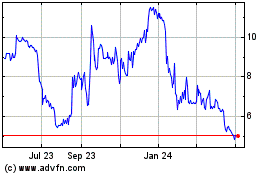
closing price of our Class A ordinary shares of $8.22 as of October 12, 2022, the holders of the 8,625,000 Class A ordinary shares
converted from the founder shares would experience a potential profit of up to approximately $8.22 per share, or up to approximately
$70.9 million in the aggregate.
We will not receive any proceeds from the
sale of the Class A ordinary shares or warrants by the Selling Holders pursuant to this prospectus. We also will not receive any
proceeds from the issuance of the Class A ordinary shares by us pursuant to this prospectus, except with respect to amounts received
by us upon exercise of the warrants to the extent such warrants are exercised for cash. We believe the likelihood that warrant holders
will exercise their warrants, and therefore the amount of cash proceeds that we would receive, is dependent upon the trading price of
our Class A ordinary shares. As of the date of this prospectus, our warrants are “out-of-the money,” which means that the
trading price of Class A ordinary shares underlying our warrants is below the $11.50 exercise price of the warrants. For so long as the
warrants remain “out-of-the money,” we do not expect warrant holders to exercise their warrants and, therefore, we do not
expect to receive cash proceeds from any such exercise. See the risk factor entitled “There is no guarantee that the warrants
will ever be in the money, and they may expire worthless” for more information.
We will pay the expenses, other than underwriting
discounts and commissions, associated with the sale of securities pursuant to this prospectus. Our registration of the securities covered
by this prospectus does not mean that either we or the Selling Holders will issue, offer or sell, as applicable, any of the securities.
The Selling Holders may offer and sell the securities covered by this prospectus in a number of different ways and at varying prices.
We provide more information in the section entitled “Plan of Distribution.”
You should read this prospectus and any prospectus
supplement or amendment carefully before you invest in our securities.
Our Class A ordinary shares and warrants are traded on The Nasdaq
Stock Market LLC (“Nasdaq”) under the symbols “WALD” and “WALDW,” respectively. On October 12, 2022,
the closing price of our Class A ordinary shares was $8.22 per share and the closing price of our warrants was $0.62 per warrant.
Investing in our securities involves risks. See
“Risk Factors” beginning on page 10 and in any applicable prospectus supplement.
None of the U.S. Securities and Exchange Commission
or any state securities commission has approved or disapproved of the securities or determined if this prospectus is accurate or adequate.
Any representation to the contrary is a criminal offense.
The date of this prospectus
is October 13, 2022.
TABLE OF CONTENTS
About
this Prospectus
This prospectus is part of a
registration statement on Form F-1 that we filed with the SEC using a “shelf” registration process. Under this shelf registration
process, we and the Selling Holders may, from time to time, issue, offer and sell, as applicable, any combination of the securities described
in this prospectus in one or more offerings. We may use the shelf registration statement to issue up to an aggregate of 29,533,282 Class
A ordinary shares upon exercise of the public warrants and private placement warrants. The Selling Holders may use the shelf registration
statement to sell up to an aggregate of 121,120,063 Class A ordinary shares and up to 18,033,332 warrants from time to time through any
means described in the section entitled “Plan of Distribution.” More specific terms of any securities that the Selling
Holders offer and sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices
of the Class A ordinary shares and/or warrants being offered and the terms of the offering.
A prospectus supplement may
also add, update or change information included in this prospectus. Any statement contained in this prospectus will be deemed to be modified
or superseded for purposes of this prospectus to the extent that a statement contained in such prospectus supplement modifies or supersedes
such statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement
so superseded will be deemed not to constitute a part of this prospectus. You should rely only on the information contained in this prospectus,
any applicable prospectus supplement or any related free writing prospectus. See “Where You Can Find More Information.”
Neither we nor the Selling
Holders have authorized anyone to provide any information or to make any representations other than those contained in this prospectus,
any accompanying prospectus supplement or any free writing prospectus we have prepared. We and the Selling Holders take no responsibility
for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer
to sell only the securities offered hereby and only under circumstances and in jurisdictions where it is lawful to do so. No dealer, salesperson
or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus
supplement or any related free writing prospectus. This prospectus is not an offer to sell securities, and it is not soliciting an offer
to buy securities, in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this
prospectus or any prospectus supplement is accurate only as of the date on the front of those documents only, regardless of the time of
delivery of this prospectus or any applicable prospectus supplement, or any sale of a security. Our business, financial condition, results
of operations and prospects may have changed since those dates.
This prospectus contains
summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for
complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred
to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this
prospectus is a part, and you may obtain copies of those documents as described below under “Where You Can Find More Information.”
On July 27, 2022 (the “Closing
Date”), we consummated the previously announced business combination with Obagi Global Holdings Limited, a Cayman Islands exempted
company limited by shares (“Obagi”), and Milk Makeup LLC, a Delaware limited liability company (“Milk”). Pursuant
to the Agreement and Plan of Merger, dated as of November 15, 2021 (the “Obagi Merger Agreement”), by and among us, Obagi
Merger Sub, Inc., a Cayman Islands exempted company limited by shares and our indirect subsidiary (“Merger Sub”), and Obagi,
Merger Sub merged with and into Obagi, with Obagi surviving as our indirect subsidiary (the “Obagi Merger”). Pursuant to the
Equity Purchase Agreement, dated as of November 15, 2021 (the “Milk Equity Purchase Agreement” and, together with the Obagi
Merger Agreement, the “Transaction Agreements”), by and among us, Obagi Holdco 1 Limited, a limited company incorporated under
the laws of Jersey (“Holdco 1”), Waldencast Partners LP, a Cayman Islands exempted limited partnership (“Waldencast
LP” and together with Holdco 1, the “Milk Purchasers”), Milk, certain former members of Milk (the “Milk Members”),
and Shareholder Representative Services LLC, a Colorado limited liability company, solely in its capacity as representative of the Milk
Members (the “Equityholder Representative”), the Milk Purchasers acquired from the Milk Members, and the Milk Members sold
to the Milk Purchasers, all of the issued and outstanding membership units of Milk (the “Milk Membership Units”) in exchange
for the Milk Cash Consideration (as defined in the Milk Equity Purchase Agreement), the Milk Equity Consideration (as defined in the Milk
Equity Purchase Agreement), which consists of partnership units of Waldencast LP (“Waldencast LP Common Units”) exchangeable
for our Class A ordinary shares, and our Class B ordinary shares (the “Milk Transaction”).
On July 26, 2022, prior to
the Closing Date, with the approval of our shareholders, and in accordance with the Companies Act (As Revised) of the Cayman Islands (“Cayman
Act”), the Companies (Jersey) Law 1991, as amended (the “Jersey Companies Law”), and our amended and restated memorandum
and articles of association (the “Constitutional Document”), we effected a deregistration under the Cayman Act and a domestication
under Part 18C of the Jersey Companies Law, pursuant to which our jurisdiction of incorporation was changed from the Cayman Islands to
Jersey and we changed our name from “Waldencast Acquisition Corp.” to “Waldencast plc” (the “Domestication”
and, collectively with the Obagi Merger, the Milk Transaction and the other transactions contemplated by the Transaction Agreements, the
“Business Combination”).
The Jersey Financial Services
Commission (the “JFSC”) has given, and has not withdrawn, its consent under Article 4 of the Control of Borrowing (Jersey)
Order 1958 to the issue of the warrants.
The JFSC is protected by
the Control of Borrowing (Jersey) Law 1947, as amended, against liability arising from the discharge of its functions under that Law.
A copy of this prospectus
has been delivered to the Jersey Registrar of Companies (the “Jersey Registrar”) in accordance with Article 5 of the Companies
(General Provisions) (Jersey) Order 2002 and the Jersey Registrar has given, and has not withdrawn, his consent to its circulation.
It must be directly understood
that, in giving these consents, neither the Jersey Registrar nor the JFSC takes any responsibility for the financial soundness of the
Company or for the correctness of any statements made, or opinions expressed, with regard to it.
The JFSC is protected by
the Control of Borrowing (Jersey) Law 1947, as amended, against liability arising from the discharge of its functions under that law.
The directors of the Company
have taken all reasonable care to ensure that the facts stated in this prospectus are true and accurate in all material respects, and
that there are no other facts the omission of which would make misleading any statement in the document, whether or facts or of opinion.
The directors accept responsibility accordingly.
It should be remembered that
the price of securities and the income from them can go down as well as up.
If you are in any doubt about
the contents of this prospectus, you should consult your stockbroker, bank manager, solicitor, accountant or financial adviser.
Unless the context indicates
otherwise, references to the “Company,” “Waldencast,” “we,” “us” and “our”
refer, prior to the Business Combination, to Waldencast Acquisition Corp., and, following the Business Combination, to Waldencast plc,
including its subsidiaries.
Trademarks
This document contains references
to trademarks and service marks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus
may appear without the ® or ™ symbols, but such references are not intended to indicate,
in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and
trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship
with, or endorsement or sponsorship of us by, any other companies.
Selected
Definitions
Unless stated in this prospectus
or the context otherwise requires, references to:
| ● | “affiliate”
or “Affiliate” means, with respect to any specified Person, any Person that, directly or indirectly, controls, is controlled
by, or is under common control with, such specified Person, whether through one or more intermediaries or otherwise. The term “control”
(including the terms “controlling,” “controlled by” and “under common control with”) means the possession,
directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the
ownership of voting securities, by contract or otherwise; |
| ● | “Beauty FPA Investor” means the Sponsor, in its
capacity as managing member of Beauty Ventures; |
| ● | “Beauty Ventures” means Beauty Ventures LLC,
a Cayman Islands limited liability company, that is managed by the Sponsor; |
| ● | “Board” means our board of directors; |
| ● | “Business Combination” means the Obagi Merger,
the Milk Transaction, the other transactions contemplated by the Transaction Agreements and the Domestication; |
| ● | “Cedarwalk” means Cedarwalk Skincare Ltd., a
Cayman Islands exempted company limited by shares; |
| ● | “Class A ordinary shares” means our Class A
ordinary shares, par value $0.0001 per share; |
| ● | “Class B ordinary shares” means our Class B
ordinary shares, par value $0.0001 per share; |
| ● | “Closing Date” means the date of the Obagi Closing
and the date of the Milk Closing, together; |
| ● | “Code”
means the U.S. Internal Revenue Code of 1986, as amended; |
| ● | “Constitutional Document” means our memorandum
and articles of association; |
| ● | “Continental” means Continental Stock Transfer
& Trust Company; |
| ● | “COVID-19” means SARS-CoV-2 or COVID-19, and
any evolutions or mutations thereof or related or associated epidemics, pandemics or disease outbreaks; |
| ● | “Dollars” or “$” means lawful money
of the U.S.; |
| ● | “Domestication” means the domestication by way
of continuance of Waldencast as a Jersey public limited company and deregistration in the Cayman Islands in accordance with Part 18C
of the Jersey Companies Law and the Cayman Islands Companies Act; |
| ● | “DTC” means The Depository Trust Company; |
| ● | “Exchange Act” means the Securities Exchange
Act of 1934, as amended; |
| ● | “Forward Purchaser” means each of Burwell, Dynamo
Master Fund, and Beauty Ventures; |
| ● | “Forward Purchase Agreements” means the Third-Party
Forward Purchase Agreement and the Sponsor Forward Purchase Agreement, together; |
| ● | “Forward Purchase Securities” means the Class
A ordinary shares and warrants issued pursuant to the Forward Purchase Agreements; |
| |
● |
“founder shares” means the Waldencast Acquisition Corp. Class B ordinary shares purchased by the Sponsor in a private placement prior to the initial public offering, and the Class A ordinary shares issued upon the conversion thereof; |
| ● | “GAAP” means generally accepted accounting principles in the U.S.; |
| ● | “Governmental Authority” means any federal, state,
provincial, municipal, local or foreign government, governmental authority, regulatory or administrative agency, governmental commission,
department, board, bureau, agency or instrumentality, court or tribunal, or arbitrator; |
| ● | “Holdco 1” means Obagi Holdco 1 Limited, a private
limited company incorporated under the laws of Jersey; |
| ● | “Holdco 2” means Obagi Holdco 2 Limited, a private
limited company incorporated under the laws of Jersey; |
| ● | “initial public offering” means our initial public
offering that was consummated on March 18, 2021; |
| ● | “Investor Directors” means Sarah Brown, Juliette
Hickman, Lindsay Pattison and Zach Werner; |
| ● | “Investor Rights Agreement” means the Investor
Rights Agreement, dated July 27, 2022, entered into by and among us, Cedarwalk, the Sponsor and CWC Skincare Ltd., the guarantor of Cedarwalk’s
obligations thereunder; |
| ● | “IRS” means the U.S. Internal Revenue Service; |
| ● | “Jersey Companies Law” means the Companies (Jersey)
Law 1991, as amended; |
| ● | “JOBS Act” means the Jumpstart Our Business Startups
Act of 2012; |
| ● | “Legacy units” means the units of Waldencast
Acquisition Corp., each unit representing one Class A ordinary share and one-third of one redeemable warrant to acquire one Class A ordinary
share; |
| ● | “Lock-Up Agreements” means the Obagi Lock-Up
Agreement and the Milk Lock-Up Agreement, together; |
| ● | “Merger Sub” means Obagi Merger Sub, Inc., a
Cayman Islands exempted company limited by shares; |
| ● | “Milk” means Milk Makeup LLC, a Delaware limited
liability company; |
| ● | “Milk Closing” means the closing of the transactions
contemplated by the Milk Equity Purchase Agreement; |
| ● | “Milk Equity Purchase Agreement” means the Equity
Purchase Agreement, dated as of November 15, 2021, by and among us, Waldencast LP, Holdco 1, Milk, the Milk Members and the Equityholder
Representative; |
| ● | “Milk Lock-Up Agreement” means each of the lock-up
agreements, dated as of July 27, 2022, entered into by each of the Milk Members; |
| ● | “Milk Members” means the preferred and common
members of Milk; |
| ● | “Milk Membership Units” mean the issued and outstanding
membership units of Milk; |
| ● | “Milk Transaction” means the Milk Purchasers’
acquisition from the Milk Members, and the Milk Members’ sale to the Milk Purchasers, of the Milk Membership Units in exchange
for the Milk Cash Consideration (as defined in the Milk Equity Purchase Agreement), the Milk Equity Consideration (as defined in the
Milk Equity Purchase Agreement), which consists of Waldencast LP Common Units exchangeable for our Class A ordinary shares, and our Class
B ordinary shares; |
| ● | “Nasdaq” means The Nasdaq Stock Market LLC; |
| ● | “Obagi” means Obagi Global Holdings Limited,
a Cayman Islands exempted company limited by shares; |
| ● | “Obagi Closing” means the closing of the transactions
contemplated by the Obagi Merger Agreement; |
| ● | “Obagi Common Stock” means the shares in the
capital of Obagi of par value US $0.50 each per share; |
| ● | “Obagi Holdco” means Obagi Holdings Company Limited,
a Cayman Islands exempted company limited by shares; |
| ● | “Obagi Hong Kong” means Obagi Hong Kong Limited; |
| ● | “Obagi Lock-Up Agreement” means each of the lock-up
agreements, dated as of July 27, 2022, entered into by each of the Obagi Shareholders; |
| ● | “Obagi Merger” means the merger of Merger Sub
with and into Obagi, with Obagi surviving the merger as a wholly owned subsidiary of Holdco 2; |
| ● | “Obagi Merger Agreement” means that certain Agreement
and Plan of Merger, dated as of November 15, 2021, by and among us, Merger Sub and Obagi; |
| ● | “Obagi Shareholders” means the shareholders of
Obagi; |
| ● | “Obagi Worldwide” means Obagi Cosmeceuticals
LLC, a Delaware limited liability company, and Obagi Holdings Company Limited, a Cayman Islands exempted company limited by shares; |
| ● | “ordinary shares” means our Class A ordinary
shares and our Class B ordinary shares, collectively; |
| ● | “Person” means any individual, firm, corporation,
partnership, exempted limited partnership, limited liability company, exempted company, incorporated or unincorporated association, joint
venture, joint stock company, Governmental Authority or instrumentality or other entity of any kind; |
| ● | “PFIC” means a passive foreign investment company; |
| ● | “PIPE Investment” means the purchase of our shares
pursuant to the PIPE Subscription Agreements; |
| ● | “PIPE Investors” means those certain investors
participating in the purchase of our shares pursuant to the PIPE Subscription Agreements; |
| |
● |
“private placement warrants” means those certain private placement warrants issued (i) in connection with the consummation of our initial public offering in a private placement to our Sponsor; and/or (ii) in connection with the consummation of our Business Combination in a private placement (A) pursuant to the Forward Purchase Agreements and (B) as a result of the conversion of $1,500,000 working capital loans, at the price of $1.50 per warrant; |
| ● | “public warrants” means the redeemable warrants
(including those underlying the units) that were offered and sold by us in our initial public offering and registered pursuant to the
initial public offering registration statement or our redeemable warrants issued as a matter of law upon the conversion thereof at the
time of the Domestication, as context requires; |
| ● | “Registration Rights Agreement” means the Amended
and Restated Registration Rights Agreement, dated as of July 27, 2022, entered into by and among us, the Sponsor and certain of our shareholders,
Obagi and Milk and certain of their respective affiliates; |
| ● | “Sarbanes-Oxley Act” means the Sarbanes-Oxley
Act of 2002, as amended; |
| ● | “SEC” means the U.S. Securities and Exchange
Commission; |
| ● | “Securities Act” means the Securities Act of
1933, as amended; |
| ● | “Sponsor” means Waldencast Long-Term Capital
LLC, a Cayman Islands limited liability company; |
| |
● |
“Stockholder Support Agreement” means that certain Support Agreement, dated November 15, 2021, by and among us, Cedarwalk and Obagi, as amended and modified from time to time; |
| ● | “Transaction Agreements” means the Obagi Merger
Agreement together with the Milk Equity Purchase Agreement; |
| ● | “Transactions” means the Obagi Merger together
with the Milk Transaction; |
| ● | “transfer agent” means Continental, acting as
transfer agent; |
| ● | “U.S. Holder”
means a beneficial owner of our Class A ordinary shares who or that is, for U.S. federal income tax purposes: (a) an individual citizen
or resident of the U.S., (b) a corporation (or other entity that is treated as a corporation for U.S. federal income tax purposes) that
is created or organized (or treated as created or organized) in or under the laws of the U.S. or any state thereof or the District of
Columbia, (c) an estate whose income is subject to U.S. federal income tax regardless of its source, or (d) a trust if (i) a U.S. court
can exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all
substantial decisions of the trust or (ii) it has a valid election in place to be treated as a U.S. person; |
| ● | “Waldencast” and the “Registrant”
mean Waldencast Acquisition Corp., a Cayman Islands exempted company limited by shares, prior to the Domestication, and Waldencast plc,
a public limited company incorporated under the laws of Jersey, after the Domestication; |
| ● | “Waldencast LP” means Waldencast Partners LP,
a Cayman Islands exempted limited partnership; |
| |
● |
“Waldencast LP Common Units” means limited partnership units of Waldencast LP that, in the case of such units issued as part of, or in respect of, the Milk Equity Consideration (as defined in the Milk Equity Purchase Agreement), are redeemable at the option of the holder of such units and, if such option is exercised, exchangeable for Class A ordinary shares or cash in accordance with the terms of the Amended and Restated Waldencast Partners LP Agreement; |
| ● | “warrants” means the public warrants and the
private placement warrants; and |
| ● | “Working Capital Loans” means any loan made to
Waldencast by any of the Sponsor, an affiliate of the Sponsor, or any of Waldencast’s officers or directors, and evidenced by a
promissory note, for the purpose of financing costs incurred in connection with a Business Combination. |
Unless otherwise stated in
this prospectus or the context otherwise requires, all references in this prospectus to our warrants include such Class A ordinary shares
underlying the warrants.
Cautionary
Note Regarding Forward-Looking Statements
This prospectus contains
statements that are forward-looking and as such are not historical facts. This includes, without limitation, statements regarding our
financial position, business strategy and the plans and objectives of management for future operations. These statements constitute forecasts
and forward-looking statements, and are not guarantees of performance. Such statements can be identified by the fact that they do not
relate strictly to historical or current facts. When used in this prospectus, words such as “anticipate,” “believe,”
“continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,”
“plan,” “possible,” “potential,” “predict,” “project,” “should,”
“strive,” “would” and similar expressions may identify forward-looking statements, but the absence of these words
does not mean that a statement is not forward-looking. When we discuss our strategies or plans, we are making forecasts or forward-looking
statements. Such statements are based on the beliefs of, as well as assumptions made by and information currently available to, our management.
Forward-looking statements
in this prospectus and in any document incorporated by reference in this prospectus may include, for example, statements about:
| |
● |
the inability to recognize the anticipated benefits of the transactions with Obagi and Milk; |
| |
● |
changes in general economic conditions, including as a result of the COVID-19 pandemic; |
| |
● |
the ability to continue to meet Nasdaq’s listing standards; |
| |
● |
volatility of our securities due to a variety of factors, including our inability to implement its business plans or meet or exceed its financial projections and changes; |
| |
● |
the ability to implement business plans, forecasts, and other expectations, and identify and realize additional opportunities; |
| |
● |
the ability of Waldencast to implement its strategic
initiatives and continue to innovate Obagi’s and Milk’s existing products and anticipate and respond to market trends
and changes in consumer preferences; and |
| ● | other factors detailed in the section entitled “Risk
Factors.” |
The forward-looking statements
contained in this prospectus and in any document incorporated by reference in this prospectus are based on current expectations and beliefs
concerning future developments and their potential effects on us. There can be no assurance that future developments affecting us will
be those that we have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond
our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied
by these forward-looking statements. These risks and uncertainties include, but are not limited to, those factors described in the section
entitled “Risk Factors” beginning on page 10 of this prospectus. Should one or more of these risks or uncertainties
materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these
forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new
information, future events or otherwise, except as may be required under applicable securities laws.
Prospectus
Summary
This summary highlights, and
is qualified in its entirety by, the more detailed information and financial statements included elsewhere in this prospectus. This summary
does not contain all of the information that may be important to you in making your investment decision. You should read this entire prospectus
carefully, including our financial statements and the related notes included in this prospectus and the information set forth under the
headings “Risk Factors,” “Waldencast’s Management’s Discussion and Analysis of Financial Condition and Results
of Operations,” “Obagi’s Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and “Milk’s Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding
to invest in our Class A ordinary shares or warrants.
Overview
Waldencast
Founded by Michel Brousset
and Hind Sebti, our ambition is to build a global best-in-class beauty and wellness operating platform by developing, acquiring, accelerating,
and scaling conscious, high-growth purpose-driven brands. Our vision is fundamentally underpinned by our brand-led business model that
ensures proximity to our customers, business agility and market responsiveness, while maintaining each brand’s distinct DNA. The
first step in realizing our vision was the business combination with Obagi and Milk. As part of the Waldencast platform, our brands will
benefit from the operational scale of a multi-brand platform; the expertise in managing global beauty brands at scale; a balanced portfolio
to mitigate category fluctuations; asset light efficiency; and the market responsiveness and speed of entrepreneurial indie brands.
We were incorporated on December
8, 2020 as a Cayman Islands exempted company and a blank check company solely for the purpose of effecting the Business Combination, which
was consummated on July 27, 2022. On July 26, 2022, with the approval of our shareholders, and in accordance with the Cayman Act, the
Jersey Companies Law and the Constitutional Document, we effected the Domestication, pursuant to which our jurisdiction of incorporation
was changed from the Cayman Islands to Jersey and our name was changed from Waldencast Acquisition Corp. to Waldencast plc, a
public limited company incorporated under the laws of Jersey. Upon the closing of the Business Combination, we acquired the businesses
of Obagi and Milk, which are now indirect subsidiaries of Waldencast.
Our Professional Skincare Segment: Obagi
Our professional skincare
segment consists of the Obagi business. Obagi is currently headquartered in Long Beach, California, but will begin operating out of new
headquarters in Houston, Texas in the third quarter of 2022. Obagi is a pioneer of the professional skincare category and its products
are rooted in research and skin biology. Obagi develops, markets and sells innovative skin health products in more than 60 countries
around the world. The Obagi® collection of products includes the following brands, with more than 200 products sold throughout
the medical, spa and retail channels: Obagi Medical®, Obagi Clinical®, Obagi Professional™ and
Skintrinsiq™. While the product portfolio consists predominantly of cosmetic and over-the-counter (“OTC”) drug products,
Obagi does offer prescription-strength drug products, which require approval from the U.S. Food and Drug Administration (the “FDA”)
prior to marketing. We have not sought or obtained FDA pre-market approval or foreign regulatory authorities’ authorization for
any Obagi products, including the Skintrinsiq device, which we believe does not require marketing authorization from the FDA. These prescription-strength
products include the Obagi Nu-Derm® System and related products, some of which contain a 4% concentration of the ingredient
hydroquinone (“HQ”). These products are marketed as prescription-use only drugs but have not received marketing authorization
from the FDA or other regulatory authorities. The FDA has historically utilized a risk-based enforcement approach with respect to drugs
marketed without the required New Drug Application (“NDA”) in accordance with a Compliance Policy Guide (“CPG”)
it issued in 2006 and subsequently amended in 2011, in which the FDA announced a drug safety initiative to remove unapproved drugs from
the market, and established enforcement priorities and a policy of enforcement discretion with respect to marketed unapproved products.
We believe Obagi’s prescription-only HQ products do not fall within the categories of unapproved drugs for which the FDA has indicated
it prioritizes enforcement. We have not received any communications from the FDA or any similar regulatory authorities regarding its
HQ or any of its other products. However, whether due to safety concerns or otherwise, in the future the FDA may choose to pursue an
enforcement action against us and determine that Obagi HQ products should be removed from the market until we obtain FDA approval of
the required NDA. Although Obagi prescription-only HQ products are made with 4% HQ, the FDA has historically expressed concerns regarding
the safety of 2% HQ products sold on an OTC basis. In addition, the CARES Act implemented a number of changes to regulation of OTC drugs,
one of which prohibited the sale of HQ (at any concentration level) from being marked in the U.S. as an OTC drug without FDA approval
effective September 2020. On April 19, 2022, the FDA announced that it had issued warning letters to 12 companies for continuing to sell
2% HQ products on an OTC basis in violation of the CARES Act. The FDA’s announcement also cited reports describing serious side
effects associated with the use of skin lightening products containing HQ, including reports of skin rashes, facial swelling, and ochronosis
(discoloration of the skin). The FDA’s safety concerns regarding these lower-concentration OTC HQ products could prompt the FDA
to assert that Obagi’s higher-concentration, prescription-only HQ products represent a higher priority for enforcement pursuant
to the active CPG. In addition, Obagi’s prescription-only products are not currently available in pharmacies. Certain states, including
Massachusetts, Montana, New Hampshire, New York and Texas, prohibit physicians from dispensing prescription products without a pharmacy
or other license or authorization, permitting dispensing of such products only in certain limited circumstances. For these states we
offer alternate products under our Obagi Nu-Derm Fx® and Obagi-C Fx product lines that contain the skin brightening ingredient arbutin
rather than 4% HQ. Further, we are aware that the state of Texas and Puerto Rico, as well as certain credit card authorization vendors,
have taken action against physician customers who sell Obagi’s prescription products to patients over the Internet. Most of these
physicians ceased selling the prescription products online, offering them only in office to patients, and/or chose to sell Obagi’s
alternate arbutin products online instead. These actions have not had a material impact on our or Obagi’s sales or net revenue.
The Obagi Nu-Derm System and related products accounted for approximately 24.5% and 32.6% of our net revenue for the years ended December
31, 2021 and 2020, respectively, and 31.9% and 27.1% of our net revenue for the six months ended June 30, 2022 and 2021, respectively.
For further details, see the section entitled “Our Business—Information About Obagi — The Skincare Market —
Obagi Medical.”
Our “Clean”
Makeup Segment: Milk
Our “clean”
makeup segment consists of the Milk business. Milk is a leading, award-winning clean prestige makeup brand with unique products, a dedicated
following among Gen-Z consumers and an emerging global presence. Milk has achieved significant growth thus far but believes even more
significant growth opportunities remain in terms of building awareness, product and category expansion, channel expansion and regional
expansion.
We believe that Milk’s
inclusive brand values, “clean” product philosophy and commitments to sustainability and philanthropy are at the zeitgeist
of what will motivate the next generation of beauty consumers around the world, and that these values and product attributes will only
become more relevant. We believe that Milk’s ability to authentically connect with youth culture while developing unique, effective
and easy to use products that are also 100% vegan, clean and cruelty-free sets Milk apart from other brands.
Milk was launched in
2016 with the goal of building a global movement to challenge and broaden the definition of beauty. Community and self-expression are
at the heart of everything Milk does, believing that it’s not how you wear your makeup, it’s what you do in it that matters.
This ethos is captured in Milk’s brand signature, “Live Your Look.”
Background
Domestication and
Business Combination
On the Closing Date, we consummated
the previously announced business combination with Obagi and Milk.
Pursuant to the Obagi Merger
Agreement, by and among us, Merger Sub, and Obagi, Merger Sub merged with and into Obagi, with Obagi surviving as our indirect subsidiary.
Pursuant to the Milk Equity
Purchase Agreement, by and among us, Holdco Purchaser, Waldencast LP, Milk, the Milk Members, and the Equityholder Representative, the
Milk Purchasers acquired from the Milk Members, and the Milk Members sold to the Milk Purchasers, the Milk Membership Units in exchange
for the Milk Cash Consideration (as defined in the Milk Equity Purchase Agreement), the Milk Equity Consideration (as defined in the Milk
Equity Purchase Agreement), which consists of Waldencast LP Common Units exchangeable for our Class A ordinary shares, and our Class B
ordinary shares.
On July 26, 2022, prior to
the Closing Date, with the approval of our shareholders, and in accordance with the Cayman Act, the Jersey Companies Law, and the Constitutional
Document, we effected a deregistration under the Cayman Act and a domestication under Part 18C of the Jersey Companies Law (by means of
filing a memorandum and articles of association with the Registrar of Companies in Jersey), pursuant to which our jurisdiction of incorporation
was changed from the Cayman Islands to Jersey and we changed our name from “Waldencast Acquisition Corp.” to “Waldencast
plc.”
In connection with the Domestication:
(i) each of the then issued and outstanding Waldencast Acquisition Corp. Class A ordinary shares, par value $0.0001 per share, converted
automatically, on a one-for-one basis, into our Class A ordinary shares, par value $0.0001 per share, (ii) each of the then issued and
outstanding Waldencast Acquisition Corp. Class B ordinary shares, par value $0.0001 per share, converted automatically, on a one-for-one
basis, into our Class A ordinary shares, (iii) each of the then issued and outstanding Waldencast Acquisition Corp. warrants converted
automatically, on a one-for-one basis, into our warrants, pursuant to the Warrant Agreement, dated March 15, 2021 (the “Warrant
Agreement”), between us and Continental Stock Transfer & Trust Company, as warrant agent (the “Warrant Agent”),
and (iv) each of the then issued and outstanding Waldencast Acquisition Corp. units were cancelled and the holders thereof were entitled,
on a one-for-one basis, to one Class A ordinary share and one-third of one warrant.
The Business Combination
was consummated on July 27, 2022. The transaction was unanimously approved by our Board and was approved at the extraordinary general
meeting of our shareholders held on July 25, 2022 (the “Extraordinary General Meeting”). Our shareholders also voted to approve
all other proposals presented at the Extraordinary General Meeting. As a result of the Business Combination, Obagi and Milk have become
indirect subsidiaries of the Company.
The foregoing description
of the Business Combination does not purport to be complete and is qualified in its entirety by the full text of the Obagi Merger Agreement,
which is incorporated by reference hereto as Exhibit 2.1, and the full text of the Milk Equity Purchase Agreement, which is incorporated
herein by reference hereto as Exhibit 2.2.
PIPE Investments &
Forward Purchase Agreements
In connection with the execution
of the Transaction Agreements, we entered into certain subscription agreements, executed on or prior to November 14, 2021 (the “Initial
Subscription Agreements”), pursuant to which certain investors (the “Initial PIPE Investors”) agreed to purchase, in
the aggregate, 10,500,000 Class A ordinary shares at $10.00 per share for an aggregate commitment amount of $105.0 million (the “Initial
PIPE Investment”).
The Transaction Agreements
provided that we could enter into additional subscription agreements with investors to participate in the purchase of our shares after
November 15, 2021 but prior to the Closing Date. On June 14, 2022, we entered into subsequent subscription agreements (the “June
Subsequent Subscription Agreements”) with certain investors (collectively, the “June Subsequent PIPE Investors”) on
the same terms as the Initial PIPE Investors, pursuant to which the June Subsequent PIPE Investors collectively subscribed for 800,000
Class A ordinary shares for an aggregate purchase price equal to $8.0 million (the “June Subsequent PIPE Investment”).
On July 15, 2022, we further
entered into subsequent subscription agreements (the “July Subsequent Subscription Agreements” and together with the Initial
Subscription Agreements and the June Subsequent Subscription Agreements, the “PIPE Subscription Agreements”) with certain
investors (collectively, the “July Subsequent PIPE Investors” and, together with the Initial PIPE Investors and the June Subsequent
PIPE Investors, the “PIPE Investors”) on the same terms as the Initial PIPE Investors and the June Subsequent PIPE Investors.
Pursuant to, and on the terms and subject to the conditions of the applicable July Subsequent Subscription Agreement, the July Subsequent
PIPE Investors collectively subscribed for 500,000 Class A ordinary shares for an aggregate purchase price equal to $5,000,000 (the “July
Subsequent PIPE Investment” and together with the Initial PIPE Investment and the June Subsequent PIPE Investment, the “PIPE
Investment”).
In connection with our initial
public offering; (i) on February 22, 2021, we, the Sponsor and Dynamo Master Fund (a member of the Sponsor) entered into a Forward Purchase
Agreement (the “Sponsor Forward Purchase Agreement”), which was subsequently amended by the assignment and assumption agreement
entered into by and between the Sponsor and Burwell on December 20, 2021, under which the Sponsor assigned, and Burwell assumed, all of
the Sponsor’s rights and benefits under the Sponsor Forward Purchase Agreement, pursuant to which, Burwell and Dynamo Master Fund
committed to subscribe for and purchase 16,000,000 Class A ordinary shares and 5,333,333 warrants for an aggregate commitment amount of
$160.0 million; and (ii) we and Beauty Ventures LLC (“Beauty Ventures” and, together with Dynamo Master Fund and Burwell,
the “Forward Purchasers”) entered into a Forward Purchase Agreement on March 1, 2021 (“the Third-Party Forward Purchase
Agreement” and, together with the Sponsor Forward Purchase Agreement, the “Forward Purchase Agreements”), pursuant to
which Beauty Ventures committed to subscribe for and purchase 17,300,000 Class A ordinary shares and up to 5,766,666 warrants for an aggregate
commitment amount of $173.0 million. Members of our Sponsor or their affiliates will begin to receive a twenty percent (20%) performance
fee allocation on the return of the forward purchase securities in excess of the hurdle rate, calculated on the total return generated
from forward purchase securities (whether by dividend, transfer or increase in value as measured from date of issuance), when the return
of such securities (less the expenses of Beauty Ventures) underlying the Third-Party Forward Purchase Agreement exceeds a hurdle rate
of five percent (5%) accrued annually until the fifth anniversary of the issuance of such securities. In the event of a transfer and subsequent
sale of any forward purchase securities prior to such fifth anniversary, the performance fee for the period between such transfer and
such fifth anniversary will be calculated based on the proceeds generated by such sale.
We granted the PIPE Investors
and the Forward Purchasers certain registration rights in connection with the PIPE Subscription Agreements and the Forward Purchase Agreements.
Lock-Up Restrictions
Pursuant to a Letter Agreement,
dated March 15, 2021, between us and our initial shareholders (as amended, the “Letter Agreement”), such shareholders have
agreed not to transfer, assign or sell any of their founder shares until the earlier to occur of: (A) July 27, 2023 (one year after the
completion of our initial business combination); and (B) subsequent to our initial business combination (x) if the last reported sale
price of our Class A ordinary shares equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share dividends, rights
issuances, consolidations, reorganizations, recapitalizations and other similar transactions) for any 20 trading days within any 30-trading
day period commencing at least 150 days after our initial business combination or (y) the date on which we complete a liquidation, merger,
share exchange, reorganization or other similar transaction that results in all of our public shareholders having the right to exchange
their ordinary shares for cash, securities or other property (except with respect to permitted transferees). Any permitted transferees
would be subject to the same restrictions and other agreements of our initial shareholders with respect to any founder shares.
In addition, pursuant to
the Sponsor Forward Purchase Agreement, Burwell and Dynamo Master Fund agreed not to transfer, assign or sell any of their respective
Forward Purchase Securities until the earlier to occur of: (A) July 27, 2023 (one year after the completion of our initial business combination);
and (B) subsequent to our initial business combination (x) if the last reported sale price of our Class A ordinary shares equals or exceeds
$12.00 per share (as adjusted for share sub-divisions, share dividends, rights issuances, consolidations, reorganizations, recapitalizations
and other similar transactions) for any 20 trading days within any 30-trading day period commencing at least 150 days after our initial
business combination or (y) the date on which we complete a liquidation, merger, share exchange, reorganization or other similar transaction
that results in all of our public shareholders having the right to exchange their ordinary shares for cash, securities or other property
(except with respect to permitted transferees). Any permitted transferees would be subject to the same restrictions and other agreements
as a purchaser under the Sponsor Forward Purchase Agreement with respect to any such Forward Purchase Securities.
Further, pursuant to the
Transaction Agreements, at the Closing Date, certain of the Obagi Shareholders entered into lock-up agreements (the “Obagi Lock-Up
Agreement”) and certain of the Milk Members entered into lock-up agreements (the “Milk Lock-Up Agreement” and together
with the Obagi Lock-Up Agreement, the “Lock-Up Agreements”), pursuant to which they agreed not to transfer, assign or sell
during the respective Lock-Up Period (as defined below), (I) in the case of any of our Class A ordinary shares and the Waldencast
LP Common Units, as applicable, received as consideration in connection with the Business Combination, until the earlier of (A) one year
after the Closing and (B) (x) if the last reported sale price of our Class A ordinary shares equals or exceeds $12.00 per
share (as adjusted for share sub-divisions, share dividends, rights issuances, reorganizations, recapitalizations and the like) for any
20 trading days within any 30-trading day period commencing at least 150 days after the date of the Closing or (y) the date on which we
complete a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of our shareholders having
the right to exchange their Class A ordinary shares for cash, securities or other property; and (II) in the event that a certain
portion of the Obagi Cash Consideration (as defined therein) or the Milk Cash Consideration (as defined therein) is paid in our equity
of Waldencast as a result of the occurrence of certain events set forth in the Obagi Merger Agreement and the Milk Equity Purchase Agreement,
as applicable, such equity of Waldencast received by Obagi or Milk, for the same period as set forth in clause (I) above, provided that
solely for the purpose of this clause (II), the term “one-year” in clause (I)(A) shall be replaced with the term “six
months.”
Corporate Information
We were incorporated as
a Cayman Islands exempted company on December 8, 2020 under the name Waldencast Acquisition Corp. Upon the closing of the Business Combination,
with the approval of our shareholders, and in accordance with the Cayman Act, the Jersey Companies Law, and the Constitutional Document,
we effected a deregistration under the Cayman Act and a domestication under Part 18C of the Jersey Companies Law (by means of filing
a memorandum and articles of association with the Registrar of Companies in Jersey), pursuant to which our jurisdiction of incorporation
was changed from the Cayman Islands to Jersey and we changed our name from Waldencast Acquisition Corp. to Waldencast plc, a
public limited company incorporated under the laws of Jersey. Our Class A ordinary shares and our warrants are listed on Nasdaq
under the symbols “WALD” and “WALDW,” respectively. Our registered office is 2nd Floor Sir Walter
Raleigh House, 48-50 Esplanade, St. Helier, Jersey JE2 3QB and our principal executive office is 10 Bank Street, Suite 560, White Plains,
NY 10606, and our telephone number is (917) 546-6828. Our register of members is kept at our registered office. Our secretary is Maples
Company Secretary (Jersey) Limited of 2nd Floor, Sir Walter Raleigh House, 48-50 Esplanade, St Helier, JE2 3QB, Jersey. Maples
Company Secretary (Jersey) Limited is regulated to conduct trust company business by the JFSC pursuant to the Financial Services (Jersey)
Law 1998. Our website address is www.waldencast.com. Our website and the information contained on, or that can be accessed
through, our website is not deemed to be incorporated by reference in, and is not considered part of, this prospectus.
Emerging Growth Company
We are an “emerging
growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act, and it may take advantage of certain
exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including,
but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced
disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements
of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously
approved.
Further, Section 102(b)(1)
of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until
private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class
of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS
Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging
growth companies but any such election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which
means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging
growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison
of our financial statements with certain other public companies difficult or impossible because of the potential differences in accounting
standards used.
We will remain an emerging
growth company until the earlier of: (1) the last day of the fiscal year (a) following the fifth anniversary of the closing of our
initial public offering, (b) in which we have total annual gross revenue of at least $1,070.0 million or (c) in which we are deemed
to be a large accelerated filer, which means the market value of its common equity that is held by non-affiliates exceeds $700.0 million
as of the end of the prior fiscal year’s second fiscal quarter; and (2) the date on which we have issued more than $1,000 million
in non-convertible debt securities during the prior three-year period. References herein to “emerging growth company” shall
have the meaning associated with it in the JOBS Act.
Foreign Private Issuer
We are a “foreign private
issuer” under SEC rules and will report under the Exchange Act as a non-U.S. company with “foreign private issuer” status
and will be subject to the reporting requirements under the Exchange Act applicable to foreign private issuers. This means that, even
after e no longer qualify as an “emerging growth company,” as long as we qualify as a “foreign private issuer”
under the Exchange Act, we will be exempt from certain provisions of and intend to take advantage of certain exemptions from the Exchange
Act that are applicable to U.S. public companies. Such exemptions include the sections of the Exchange Act regulating the solicitation
of proxies, consents or authorizations in respect of a security registered under the Exchange Act and the sections of the Exchange Act
requiring insiders to file public reports of their stock ownership.
Additionally, we will not
be required to file our annual report on Form 20-F until 120 days after the end of each fiscal year and we will furnish reports on Form
6-K to the SEC regarding certain information required to be publicly disclosed by us in Jersey or that is distributed or required to be
distributed by us to our shareholders. Further, based on our foreign private issuer status, we will not be required to file periodic reports
and financial statements with the SEC as frequently or as promptly as a U.S. company whose securities are registered under the Exchange
Act. We will also not be required to comply with Regulation FD, which addresses certain restrictions on the selective disclosure of material
information. In addition, among other matters, our officers, directors and principal shareholders will be exempt from the reporting and
“short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect
to their purchases and sales of our ordinary shares.
We may take advantage of
these reporting exemptions until such time as it is no longer a “foreign private issuer.” We could lose our status as a “foreign
private issuer” under current SEC rules and regulations if more than 50% of our outstanding voting securities become directly or
indirectly held of record by U.S. holders and any one of the following is true: (i) the majority of our directors or executive officers
are U.S. citizens or residents; (ii) more than 50% of our assets are located in the U.S.; or (iii) our business is administered
principally in the U.S.
We may choose to take advantage
of some but not all of these reduced burdens. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly,
the information contained in this prospectus may be different from the information you receive from our competitors that are public companies,
or other public companies in which you have made an investment.
Risk Factors
You should consider carefully
the risks and uncertainties described in this prospectus before investing in our securities. These risks are discussed more fully in the
section titled “Risk Factors” following this summary. If any of these risks actually occur, our business, financial
condition or results of operations would likely be materially adversely affected. These risks include, but are not limited to, the following:
Risks Related
to Our Professional Skincare Segment: Obagi
| ● | The
loss of a significant customer in our Obagi segment could materially and adversely affect
our business, financial condition and results of operations. |
| ● | Our revenues and financial results depend significantly on
sales of our Obagi Nu-Derm products. If we are unable to manufacture or sell the Nu-Derm products in sufficient quantities and in a timely
manner, or maintain physician and/or patient acceptance of Nu-Derm products, our business will be materially and adversely impacted. |
| |
● |
We are dependent on third parties to manufacture products for our
Obagi segment which entails several risks we would not face if we manufactured the products ourselves. |
| |
|
|
| |
● |
We rely on third parties to distribute Obagi products and the failure
of these parties to provide their services on a timely basis or to comply with our quality standards and controls could materially
and adversely affect our business |
| |
● |
The regulatory approval
processes of the FDA and comparable foreign authorities for drugs are lengthy, time-consuming and inherently unpredictable, and if
we are required to seek and obtain any regulatory approvals that may be required for our Obagi products, we may be unable to obtain
or maintain such regulatory approvals, which would substantially harm our business. |
| |
● |
Our Obagi products containing the active ingredient,
hydroquinone, are marketed as prescription-use only drugs but have not received required premarket authorization from the FDA or
other regulatory authorities, and the FDA could require us to remove these products from the market until we obtain approval of the
required NDA, and we could be found to be marketing and selling these products in violation of the law. |
| |
● |
Our Obagi products may cause adverse events or
side effects, or could be associated with safety issues, that could result in recalls, withdrawals, or regulatory enforcement action.
For example, the FDA has historically expressed concerns regarding the safety of HQ products, including risks for potentially serious
side effects, including skin rashes, facial swelling, skin discoloration, carcinogenicity and reproductive toxicity. |
| |
● |
Failure to obtain regulatory approvals or to comply
with regulations in foreign jurisdictions would prevent us from marketing our Obagi products internationally. |
Risks Related
to Our “Clean” Makeup Segment: Milk
| |
● |
Our
Milk segment has a history of net losses and may experience future losses. |
| |
● |
The loss of a significant reseller could materially and adversely affect our Milk segment’s business, financial condition and results of operations. |
| |
● |
We rely on a number
of third-party suppliers, distributors and other vendors, and they may not continue to produce products or provide services that
are consistent with our standards or applicable regulatory requirements, which could harm our brand. |
| |
● |
A negative reputation event to any of our Milk
segment’s retail partners or beauty industry could cause a decline in our net revenues or a reduction in our earnings. |
| ● | We have growing operations in China, which exposes us to
risks inherent in doing business in that country. |
| ● | We are involved, and may become involved in the future, in
disputes and other legal or regulatory proceedings, including an ongoing legal proceeding involving our founders, that, if adversely
decided or settled, could materially and adversely affect our business, financial condition and results of operations. |
General Business Risks and Risks Related
to Our Financial Condition and Operations
| ● | We may make investments into or acquire other companies,
which could divert our management’s attention, result in dilution to our shareholders and otherwise disrupt our operations, and
we may have difficulty integrating any such acquisitions successfully or realizing the anticipated benefits therefrom, any of which could
have an adverse effect on our business, financial condition and results of operations. |
| ● | We may face risks related to companies in the beauty and
skincare industries. |
| ● | We face intense
competition, in some cases from companies that have significantly greater resources than
we do, which could limit our ability to generate sales and/or render our products obsolete.
If we are unable to compete effectively, our results will suffer. |
| |
● |
A disruption in our operations could materially
and adversely affect our business.
|
| |
● |
Our new product introductions may not
be as successful as we anticipate.
|
| |
● |
Global or regional conditions may
adversely affect our business. |
| ● | Your rights and responsibilities as a shareholder will be
governed by Jersey law, which differs in some material respects with respect to the rights and responsibilities of shareholders of U.S.
companies. |
| ● | Our only material asset is our indirect interest in Waldencast
LP, and we are accordingly dependent upon distributions from Waldencast LP to pay dividends, taxes and other expenses. |
| |
● |
Any damage to our reputation or brands may materially
and adversely affect our business, financial condition and results of operations. |
Risks Related to Ownership of our Class A ordinary shares
and warrants
| ● | We are currently an emerging growth company within the meaning
of the Securities Act, and to the extent we have taken advantage of certain exemptions from disclosure requirements available to emerging
growth companies, this could make our securities less attractive to investors and may make it more difficult to compare our performance
with other public companies. |
The
Offering
We are registering the issuance
by us of up to 29,533,282 Class A ordinary shares that may be issued upon exercise of warrants to purchase Class A ordinary shares,
including the public warrants and the private placement warrants. We are also registering the resale by the Selling Holders or their permitted
transferees of (i) up to 121,120,063 Class A ordinary shares and (ii) up to 18,033,332 warrants. Any investment in the securities offered
hereby is speculative and involves a high degree of risk. You should carefully consider the information set forth under “Risk
Factors” on page 10 of this prospectus.
The following information
is as of August 15, 2022 and does not give effect to issuances of our Class A ordinary shares or warrants after such date, or the exercise
of warrants after such date.
Issuance of Class A Ordinary Shares
Class A ordinary shares to be issued upon exercise of all
public warrants and private placement warrant |
|
29,533,282 Class A ordinary shares. |
| |
|
|
Class A ordinary shares outstanding prior to exercise of all
public warrants and private placement warrants |
|
86,460,560 Class A ordinary shares. |
| |
|
|
| Use of proceeds |
|
We will receive up to an aggregate of approximately $339.6 million from the exercise of all public
warrants and private placement warrants assuming the exercise in full of all such warrants for cash. Unless we inform you otherwise
in a prospectus supplement or free writing prospectus, to the extent we elect the exercise of such warrants for cash, we intend to
use the net proceeds from such exercise for general corporate purposes. To the extent the warrants are exercised on a “cashless”
basis, we will receive no proceeds. |
| |
|
|
| |
|
We believe the likelihood that warrant holders will exercise their warrants, and therefore the
amount of cash proceeds that we would receive, is dependent upon the trading price of our Class A ordinary shares. Our warrants are
currently out-of-the money, which means that the trading price of the Class A ordinary shares underlying our warrants is below the
$11.50 exercise price of the warrants. For so long as the warrants remain “out-of-the money,” we do not expect warrant
holders to exercise their warrants and, therefore, we do not expect to receive cash proceeds from any such exercise. See the risk
factor entitled “There is no guarantee our warrants will ever be in the money, and they may expire worthless”
for more information. |
Resale of Class A Ordinary Shares and Warrants
| Class A ordinary shares offered by the Selling Holders |
121,120,063 Class A ordinary shares. |
| |
|
| Warrants offered by the Selling Holders |
18,033,332 warrants. |
| |
|
| Exercise price for warrants |
$11.50 |
| |
|
| Redemption |
The warrants are redeemable in certain circumstances. See “Description of Share Capital—Redeemable Warrants” for further discussion. |
| |
|
| Use of proceeds |
We will not receive any proceeds from the sale of the Class A ordinary
shares and warrants to be offered by the Selling Holders. With respect to Class A ordinary shares underlying the warrants, we will
not receive any proceeds from such shares except with respect to amounts received by us upon exercise of such warrants to the extent
such warrants are exercised for cash. We believe the likelihood that warrant holders will exercise their warrants, and therefore
the amount of cash proceeds that we would receive, is dependent upon the trading price of our Class A ordinary shares. Our warrants
are currently out-of-the money, which means that the trading price of the Class A ordinary shares underlying our warrants is below
the $11.50 exercise price of the warrants. For so long as the warrants remain “out-of-the money,” we do not expect warrant
holders to exercise their warrants and, therefore, we do not expect to receive cash proceeds from any such exercise. See the risk
factor entitled “There is no guarantee our warrants will ever be in the money, and they may expire worthless” for
more information. |
| |
|
| Lock-up agreements |
Certain securities that are owned by the Selling Holders are subject to the lock-up provisions pursuant to the Letter Agreement, the Sponsor Forward Purchase Agreement or the Lock-Up Agreements, as applicable, which provide for certain restrictions on transfer until the termination of applicable lock-up periods. See the Letter Agreement, the Sponsor Forward Purchase Agreement and the Form of Lock-Up Agreement, which are filed as exhibits to the registration statement of which this prospectus forms a part. |
| |
|
| Risk factors |
See the section titled “Risk Factors” beginning on page 10 of this prospectus and other information included in this prospectus for a discussion of factors that you should consider carefully before deciding to invest in our Class A ordinary shares and warrants. |
| |
|
| Nasdaq symbol for our Class A ordinary shares |
“WALD.” |
| |
|
| Nasdaq symbol for our warrants |
“WALDW.” |
Risk
Factors
An investment in
our Class A ordinary shares and warrants involves a high degree of risk. You should consider carefully the following risks, together
with the financial and other information contained in this prospectus, including “Waldencast’s Management’s
Discussion and Analysis of Financial Condition and Results of Operations,” “Obagi’s Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and “Milk’s Management’s Discussion and
Analysis of Financial Condition and Results of Operations,” before you decide to purchase our Class A ordinary shares or
warrants. If any of the following risks actually occur, our business, financial condition and operating results could be materially
and adversely affected. In that case, the market price of our Class A ordinary shares and warrants could decline and you may lose
all or a part of your investment. The risks discussed below are not the only risks we face. Additional risks or uncertainties not
currently known to us, or that we currently deem immaterial, may also have a material adverse effect on our business, financial
condition and operating results. See “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to Our Professional Skincare Segment: Obagi
The loss of a
significant customer in our Obagi segment could materially and adversely affect our business, financial condition and results of operations.
Our Obagi products are sold in the U.S. to healthcare professionals through an authorized wholesale distributor, Boxout Health. Under this
model, we sell the products to Boxout Health, which then sells the products through to our physician customers when they order them.
As a result, Boxout Health accounted for approximately 34.8% and 54.1% of Obagi’s net revenue in the years ended December 31, 2021 and
2020, respectively, and approximately 32.7% and 41.0% of its net revenue for the six months ended June 30, 2022 and 2021,
respectively. Our agreement with Boxout Health does not contain any minimum purchase requirements on its part. Accordingly, we do
not have any guarantees regarding the quantity of each of our products that Boxout Health will order each quarter. We provide Boxout
Health with forecasts of demand for our products from our physician customers, however, our forecasts may not always accurately
assess demand for one or more products during any given quarter, which many affect subsequent orders for the affected products from
them. Our e-commerce partners and international distributors purchase our products directly from us. One of our distributors in
Southeast Asia, Gevie, Inc. together with its affiliate, Lemed, Inc. (collectively, the “SA Distributor”), accounted for
approximately 28.0% and 8.1% of Obagi’s net revenue in the years ended December 31, 2021 and 2020, respectively, and 38.4% and 23.9% of
its net revenue for the six months ended June 30, 2022 and 2021, respectively. Our agreement with the SA Distributor grants the SA
Distributor a non-exclusive right to distribute our products in Vietnam and South Korea, contains minimum purchase requirements and
has a term that expires on December 31, 2026. In January 2022, we executed an amendment with the SA Distributor to expand the
countries within Southeast Asia in which it may distribute our products. Accordingly, our sales to such distributor may comprise an
even greater proportion of our net revenue in the future. We are currently in discussions with the SA Distributor to expand the
countries in Southeast Asia in which it may distribute our products. In the event that we do grant the SA Distributor the right to
distribute products in additional countries, our sales to such distributor may comprise an even greater proportion of our net
revenue in the future.
Our revenues and financial
results depend significantly on sales of our Obagi Nu-Derm products. If we are unable to manufacture or sell the Nu-Derm products in sufficient
quantities and in a timely manner, or maintain physician and/or patient acceptance of Nu-Derm products, our business will be materially
and adversely impacted.
To date, a substantial
portion of Obagi’s revenues have resulted from sales of our principal product line, the Obagi Nu-Derm System and related
products. Nu-Derm products accounted for approximately 24.5% and 32.6% of Obagi’s net revenue for the years ended December 31,
2021 and 2020, respectively, and 31.9% and 27.1% of its net revenue for the six months ended June 30, 2022 and 2021, respectively. Although
we currently offer other products such as Obagi-C Rx, Professional-C, ELASTIderm, CLENZIderm, Blue Peel products and our Obagi Clinical
line, and intend to introduce additional new products, we still expect sales of our Obagi Nu-Derm System and related products to account
for a substantial portion of our sales for the foreseeable future. Because our business is highly dependent on Nu-Derm products, factors
adversely affecting the pricing of, or demand for, these products could have a material and adverse effect on our business.
Sales of our Obagi Nu-Derm
products also experience seasonality. We believe this is due to variability in patient compliance that relates to several factors such
as a tendency to travel and/or engage in other disruptive activities during the summer months. Additionally, our commercial success depends
in large part on our ability to sustain market acceptance of the Nu-Derm System. If existing users of our products determine that our
products do not satisfy their requirements, if our competitors develop a product that is perceived by patients or physicians to better
satisfy their respective requirements, or if state or federal regulations or enforcement actions prohibit sales of the Nu-Derm System,
individual products within the system, or any related products, sales of these products may decline, and our total net revenue may correspondingly
decline. We cannot assure you that we will be able to continue to manufacture these products in commercial quantities at acceptable costs.
Our inability to do so would adversely affect our operating results and cause our business to suffer.
We are dependent on third parties to
manufacture products for our Obagi segment which entails several risks we would not face if we manufactured the products ourselves
We currently outsource
all our product manufacturing to third-party contract manufacturers for our Obagi segment. We have two or more qualified manufacturers
for some of our key products, however, certain products, including some of our sun protection products, are currently supplied by a single
source. In the event that such a sole source supplier or any of our other third-party manufacturers terminates its supply arrangement
with us, experiences financial difficulties, encounters regulatory or quality assurance issues, experiences a significant disruption
in supply of raw materials or components for our products or suffers any damage to its facilities, we may experience delays in securing
sufficient amounts of our products, which could harm our business, reputation and relationships with customers.
Bausch Health Companies Inc.
(“Bausch Health”), which formerly owned the business of Obagi, is our only supplier and manufacturer of tretinoin. We have
a contract with Bausch Health that has an initial termination date in 2027. While there are several other manufacturers of generic tretinoin,
the termination of this agreement or any loss of services under the agreement could be difficult for us to replace upon the same favorable
terms.
We expect to continue
to rely on third parties to produce materials required for clinical trials and for the commercial production of our products in our Obagi
segment. However, there are a limited number of third-party manufacturers that operate under the FDA’s current Good Manufacturing
Practices (“cGMPs”) regulations and that have the necessary expertise and capacity to manufacture our products. As a result,
for the six months ended June 30, 2022, Obagi purchased approximately 31.0% and 8.4% of its products from G.S. Cosmeceutical USA Inc.
(“G.S. Cosmeceutical”) and Swiss America CDMO LLC (“Swiss”), respectively. In the year ended December 31, 2021,
Obagi purchased approximately 44.3% and 5.4% of its products from G.S. Cosmeceutical and Swiss, respectively. In the year ended December
31, 2020, Obagi purchased approximately 50.0% and 12.0% of its products from G.S. Cosmeceutical and Swiss, respectively. In the event
one of these suppliers terminates its arrangement with us, it may be difficult for us to locate alternate manufacturers for our anticipated
future needs. If we are unable to arrange for third-party manufacturing of our products, or to do so on commercially reasonable terms,
we may not be able to complete development of, market and sell our new products.
Further, our third-party
contract manufacturers may:
| |
● |
have
economic or business interests or goals that are inconsistent with ours; |
| |
● |
take
actions contrary to our instructions, requests, policies or objectives; |
| |
● |
be
unable or unwilling to fulfill their obligations to comply with applicable regulations, including those regarding the safety and
quality of products and ingredients and good manufacturing practices; |
| |
● |
have
financial difficulties; |
| |
● |
encounter
raw material or labor shortages; |
| |
● |
encounter
increases in raw material or labor costs that may affect our procurement costs; |
The occurrence of any
of these events, alone or together, could have a material adverse effect on our business, financial condition and results of operations.
In addition, such problems may require us to find new manufacturers or other third-party service providers, and there can be no assurance
that we would be successful in finding alternatives that third-party suppliers, manufacturers or distributors meeting our standards of
innovation and quality.
Reliance on third-party
manufacturers also entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third
party for regulatory compliance and quality assurance. To the extent that any of our third-party manufacturers fails to comply with regulatory
requirements or encounters quality assurance issues, we may experience an interruption in the supply of products, which could impair
our customer relationships and adversely affect our business, financial condition and results of operations. In addition, reliance on
third-party manufacturers subjects us to the possibility of breach of the manufacturing agreement by the third party, and the possibility
of termination or non-renewal of the agreement by the third party. Dependence upon third parties for the manufacture of our products
may also reduce our profit margins or the sale of our products, and may limit our ability to develop and deliver products on a timely
and competitive basis.
We rely on third
parties to distribute Obagi products and the failure of these parties to provide their services on a timely basis or to comply with our
quality standards and controls could materially and adversely affect our business
We have outsourced the
distribution of our products to Boxout Health for our physician-dispensed sales as well as to third-party logistics providers, who store
and distribute our products to our international distributors and retail and spa customers. We currently rely exclusively on third-party
distributors to promote, market and sell our products outside of North America. The failure of one or more of these entities to provide
the expected services on a timely basis, or at all, or at the prices we expect, or the costs and disruption incurred in changing these
outsourced functions to being performed under our management and direct control or that of a third party, may have a material adverse
effect on our business, financial condition and results of operations.
Although we provide our
international distributors with training and promotional materials for Obagi products, we do not directly control their marketing and
sales efforts; we must rely on them to register our products in their respective territories and comply with all local laws and regulations
with respect to the registration, promotion and sale of our products, as well as the Foreign Corrupt Practices Act. The failure of one
or more of these distributors to comply with applicable laws could have a material and adverse effect on our business and reputation.
In addition, our distributors may engage in other activities or employ practices that may harm our reputation, disclose our confidential
information to competitors or third parties or be acquired by, work with or come under the control of our competitors. If any of these
events were to occur, our business, financial condition and results of operations could be materially impaired. We are not party to long-term
contracts with any of these parties, and upon expiration of these existing agreements, we may not be able to renegotiate the terms on
a commercially reasonable basis, or at all.
We have appointed Obagi
Hong Kong, a subsidiary of Cedarwalk, the former shareholder of Obagi, as the exclusive distributor of our Obagi products in the China
Region. In addition, we have licensed our trademarks and product formulas to Obagi Hong Kong for commercialization in the China Region.
As we continue to expand our international business, we may enter into similar arrangements with other third parties. The failure of
Obagi Hong Kong, or any other third-party distributor or licensee to comply with our quality standards and other controls, could materially
and adversely affect our financial condition and operating results. Our licensees or others may dispute the scope of their rights under
any of these licenses. Our licensees under these licenses may breach the terms of their respective agreements. Loss or breach of any
of these licenses for any reason could materially and adversely affect our financial condition and operating results. Any dispute with
a licensee could be complex, expensive and time-consuming, and an outcome adverse to us could materially and adversely affect our business
and impair our ability to commercialize our licensed products.
Laws, regulations, enforcement trends or
changes in existing regulations governing the introduction, marketing and sale of our OTC drug, device and cosmetic products to consumers
could harm our business.
Our Obagi segment products
are subject to regulation by the FDA, the Federal Trade Commission (the “FTC”) and comparable state, local and foreign regulatory
authorities, including the European Commission, and over time, the regulatory landscape for our products has become more complex with
increasingly strict requirements. If the laws and regulations governing our products continue to change, we may find it necessary to
alter some of the ways we have traditionally marketed our products to stay in compliance with applicable regulations, and this could
add to the costs of our operations and have a material adverse effect on our business. To the extent federal, state, local or foreign
regulatory requirements regarding consumer protection, or the ingredients, claims or safety of our products continue to change in the
future, such changes could require us to reformulate or discontinue certain products, revise the product packaging or labeling, or adjust
operations and systems, any of which could result in, among other things, increased costs, delays in product launches, product returns
or recalls and lower net sales, and therefore could have a material adverse effect on our business, financial condition and results of
operations. Noncompliance with applicable regulations could result in enforcement action by the FDA or other regulatory authorities within
or outside the U.S., including, but not limited to, product seizures, injunctions, product recalls, and criminal or civil monetary penalties,
all of which could have a material adverse effect on our business, financial condition and results of operations.
In
the U.S., with the exception of color additives, the FDA does not currently require premarket approval for or premarket review of products
intended to be sold as cosmetics. However, the FDA may in the future require premarket approval, clearance or registration/notification
of cosmetic products, establishments or manufacturing facilities. The statutory and regulatory requirements applicable to drugs are extensive
and require significant resources and time to ensure compliance. The Federal Food, Drug, and Cosmetic Act (“FDCA”) defines
cosmetics, in relevant part, as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise
applied to the human body . . . for cleansing, beautifying, promoting attractiveness, or altering the appearance.” The term “drug,”
in contrast, is defined by reference to its intended use, as “articles intended for use in the diagnosis, cure, mitigation, treatment,
or prevention of disease” and “articles (other than food) intended to affect the structure or any function of the body of
man or other animals.” Therefore, almost any ingested or topical or injectable product that, through its label or labeling (including
Internet websites, promotional pamphlets, and other marketing material), is claimed to be beneficial for such uses will be regulated
by the FDA as a drug. This definition also includes components of drugs, such as active pharmaceutical ingredients. Drug products must
generally either receive premarket approval from the FDA or conform to a “monograph” for a particular drug category, as established
by the FDA’s OTC Drug Review process, which has been subject to recent reforms pursuant to the Coronavirus Aid, Relief, and Economic
Security Act (“CARES Act”).
In the European Union (the “E.U.”), cosmetics are subject
to notification through the Cosmetic Products Notification Portal by the company responsible for placing them on the E.U. market. A cosmetic
is defined as “any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair,
nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly
to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours.”
Consequently, a product is notably considered to be a cosmetic if it is presented as protecting the skin, maintaining the skin in good
condition or improving the appearance of the skin, provided that it is not a medicinal product due to its composition or intended use.
In the E.U., the composition of a cosmetic may not be such that it has a significant effect on the body through a pharmacological, immunological
or metabolic mode of action. No test has been determined yet for the significance of the effect. Indeed, by contrast, a medicinal product
is defined as “any substance or combination of substances presented as having properties for treating or preventing disease in human
beings; or any substance or combination of substances which may be used in or administered to human beings either with a view to restoring,
correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical
diagnosis.”
We
market certain products, such as eyelash serums and chemical peels, as cosmetics (i.e., not pursuant to an FDA approval or OTC monograph),
but the FDA may disagree with our determination that these products do not require FDA premarket review and approval. We also market
the Skintrisiq device for use in connection with certain of our cosmetic and OTC skin care products. We believe that, based on its intended
use, the Skintrinsiq device does not meet the FDCA’s definition of a medical device and have not sought FDA premarket review of
this product. However, the FDA may disagree with our determination and subject the Skintrinsiq device to medical device regulations.
Similar risks may apply in foreign jurisdictions where we market our products.
If
any of our products we intend to sell as cosmetics or for use with our cosmetic products were to be regulated as drugs or medical devices,
we might be required to conduct, among other things, clinical trials to demonstrate the safety and efficacy of these products and/or
submit applications to the FDA in order to obtain required marketing authorizations for such products, and we may be required to cease
distribution of or recall these products. We may not have sufficient resources to conduct any required clinical trials or to ensure compliance
with the manufacturing requirements applicable to drugs or medical devices. If the FDA or any other regulatory authorities determine
that any of our products intended to be sold as cosmetics or for use with our cosmetic products should be classified and regulated as
drug products or medical devices, or were to ban or restrict the use of certain ingredients in such cosmetic products, and we are unable
to comply with applicable requirements for those products, we may be unable to continue to market those products and may be subject to
enforcement action.
In many countries, including
the U.S., E.U., Canada, Australia and Japan, where HQ is regulated as a drug and requires a prescription, we have not sought nor obtained
regulatory approval to distribute our HQ products in these countries, and instead offer our Nu-Derm Fx and Obagi-C Fx solutions, which
contain the skin brightening agent arbutin, for these markets. The effects of arbutin on the skin could be attributed to their gradual
hydrolysis and release of HQ. In the E,U., the safety of alpha- and beta-arbutin has been previously assessed by the European Commission’s
Scientific Committee on Consumer Products (SCCS) in 2015 and 2008 respectively, which concluded that the use of alpha-arbutin is safe
for consumers in cosmetic products in a concentration up to 2% in face creams and up to 0.5% in body lotions, and the use of beta-arbutin
is safe for consumers in cosmetic products in a concentration up to 7% in face creams provided that the contamination of hydroquinone
in the cosmetic formulations remain below 1 ppm. Nevertheless, the SCCS highlighted in both opinions that a potential combined use of
HQ releasing substances in cosmetic products has not been evaluated. HQ is listed in Annex II to the Cosmetic Regulation, which means
that, with certain exceptions not applicable to us, UQ is a prohibited cosmetic ingredient in the EU. Recently, concerns have been raised
within the European Commission on the HQ content, its release, as well as on the aggregate exposure from cosmetic products containing
alphaarbutin and/or beta-arbutin. This led to additional consultation with the SCCS and resulted in the identification of a number of
issues in the previous submissions, in particular the stability and dermal absorption of alpha-arbutin and/or beta-arbutin, the release
rate of HQ and the aggregate exposure calculation from cosmetics exposure. Following this, a call for data was launched from July 2020
to April 2021 during which interested parties were asked to contribute with data/information relevant to the stability of alpha- and
beta-arbutin, their dermal absorption, the HQ release rate (including biotransformation) and the aggregate exposure. In its preliminary
opinion on the safety of alpha-arbutin and beta-arbutin in cosmetic products dated March 15-16, 2022, the SCCS considered that it cannot
conclude on the safety of alpha-arbutin (when used in face creams up to a maximum concentration of 2% and in body lotions up to a maximum
concentration of 0.5%) or beta-arbutin (when used in face cream up to a maximum concentration of 7%) because not all relevant scientific
data which are required for the safety assessment, e.g. data on the degradation/metabolism of arbutin when exposed to the skin microbiome/enzymes
and the release and final fate of HQ, are available. This preliminary opinion is open for comments until May 27, 2022, on the basis of
which the SCCS will issue a final, safety opinion which may include specific recommendations on the use of arbutin in cosmetic products.
Scientific opinions of the SCCS that consider a substance to be unsafe for cosmetic products, or only safe under certain circumstances,
are typically followed by a decision of the European Commission to amend the Cosmetics Regulation to add the substance to the list of
substances prohibited in cosmetic products (annex II) or to add the substance to the list of substances that can only be used under certain
circumstances (annex III). This means that, depending on the outcome of the SCCS’s safety assessment, it cannot be excluded that
the use of alpha- and/or beta-arbutin in certain cosmetic products will be banned or restricted in the E.U. per future E.U. legislation.
Usually in cases where cosmetics products that are already on the market, suddenly become non-compliant due to a legislative modification,
a transition regime will be imposed, allowing manufacturers a certain amount of time to comply with the rules. In addition, Obagi’s
arbutin products are permitted to be sold in the Asia-Pacific region countries in which Obagi distributes such products.
Any
inquiry into the regulatory status of our cosmetics and related products, including the Skintrinsiq device, and any related interruption
in the marketing and sale of these products could damage our reputation and image in the marketplace. In recent years, the FDA has issued
warning letters to several cosmetic companies alleging improper claims regarding their cosmetic products. The FDA has also historically
taken action against manufacturers of some light-emitting products used to alter or improve appearance on the grounds that those products
are medical devices that require clearance or approval. If the FDA or any other regulatory authorities determine that we have made inappropriate
drug or medical device claims regarding our products, we could receive a warning or untitled letter, be required to modify our product
claims or take other actions to satisfy the FDA or any other regulatory authorities. In addition, plaintiffs’ lawyers have filed
class action lawsuits against cosmetic companies after receipt of these types of FDA warning letters. There can be no assurance that
we will not be subject to state, federal or foreign government actions or class action lawsuits, which could harm our business, financial
condition and results of operations.
In
addition, we sell products, such as sunscreens, that are subject to the FDA’s OTC drug monograph regulatory requirements. The FDA
regulates the formulation, manufacturing, packaging and labeling of OTC drug products. Certain of our products, such as some of our sunscreen
and acne drug products, are regulated pursuant to the FDA’s OTC drug monographs that specify acceptable active drug ingredients
and acceptable product claims that are generally recognized as safe and effective for particular uses. If any of these products that
are marketed as OTC drugs pursuant to an OTC monograph are not in compliance with the applicable FDA monograph, we may be required to
reformulate the product, stop making claims relating to such product or stop selling the product until we are able to obtain costly and
time-consuming FDA approvals. We are also required to submit adverse event reports to the FDA for our OTC drug products, and failure
to comply with this requirement may subject us to FDA regulatory action. Moreover, the FDA’s process for establishing, amending,
and finalizing monographs has recently been reformed pursuant to the CARES Act. If these reforms affect the regulatory requirements to
which we or our products are subject, or if we do not comply, we could be subject to enforcement action, which could materially adversely
affect our business.
The regulatory approval processes of
the FDA and comparable foreign authorities for drugs are lengthy, time-consuming and inherently unpredictable, and if we are required
to seek and obtain any regulatory approvals that may be required for our Obagi products, we may be unable to obtain or maintain such
regulatory approvals, which would substantially harm our business.
Any Obagi products that
are regulated by the FDA as drugs must generally obtain premarket approval from the FDA, unless subject to the OTC monograph process
or subject to other limited exceptions. The FDA approves new drugs through the NDA or Abbreviated New Drug Application (“ANDA”)
processes before they may be legally marketed in the U.S. In the NDA process, an applicant must generally demonstrate through well-controlled
clinical trials that a drug is safe and effective for its intended uses. The Hatch-Waxman Act established the ANDA process, which is
an abbreviated FDA approval procedure for drugs that are shown to be bioequivalent to proprietary drugs previously approved by the FDA
through its NDA process. Premarket applications for generic drugs are termed “abbreviated” because such applications generally
do not include preclinical and clinical data to demonstrate safety and effectiveness. Instead, an ANDA applicant must demonstrate that
its product is bioequivalent to the innovator drug. In certain situations, an applicant may obtain ANDA approval of a generic drug with
a strength or dosage form that differs from a referenced innovator drug pursuant to the filing and approval of an ANDA Suitability Petition.
Similar requirements apply in foreign jurisdictions.
Certain of our Obagi
products, including our tretinoin-based products, are marketed pursuant to an ANDA held by Bausch Health or dispensed under the category
of unlicensed medicines in the United Kingdom (the “UK”). However, we have not sought or obtained FDA premarket approval
or foreign regulatory authorities’ authorization for any of our products. The time required to obtain approval by the FDA and comparable
foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous
factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations or the type
and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development
and may vary among jurisdictions.
The
FDA or any foreign regulatory authorities can delay, limit or deny approval or require us to conduct additional nonclinical or clinical
testing or abandon a program for, among others, the following reasons:
| ● | the
FDA or comparable foreign regulatory authorities may disagree with the design or implementation
of any clinical trials we may be required to conduct; |
| | | |
| ● | we
may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory
authorities that a product is safe and effective for its proposed indication or bioequivalent
to a listed drug; |
| ● | the
results of clinical trials may not meet the level of statistical significance required by
the FDA or comparable foreign regulatory authorities for approval; |
| ● | serious
and unexpected drug-related side effects experienced by participants in our clinical trials
or by individuals using drugs similar to our products; |
| ● | we
may be unable to demonstrate that a product’s clinical and other benefits outweigh
its safety risks; |
| ● | the
FDA or comparable foreign regulatory authorities may disagree with our interpretation of
data from preclinical studies or clinical trials; |
| ● | the
data collected from clinical trials may not be acceptable or sufficient to support the submission
of an NDA, ANDA or other submission or to obtain regulatory approval in the U.S. or elsewhere,
and we may be required to conduct additional clinical studies; |
| ● | the
FDA’s or the applicable foreign regulatory authority may disagree regarding the formulation,
labeling and/or the specifications of our products; |
| ● | the
FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes
or facilities of third-party manufacturers with which we contract for clinical and commercial
supplies; and |
| ● | the
approval policies or regulations of the FDA or comparable foreign regulatory authorities
may significantly change in a manner rendering our clinical data insufficient for any approvals. |
The
lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory
approvals to market our products, which could significantly harm our business, results of operations and prospects.
Our
Obagi products containing the active ingredient, hydroquinone, are marketed as prescription-use only drugs but have not received required
premarket authorization from the FDA or other regulatory authorities, and the FDA could require us to remove these products from the
market until we obtain approval of the required NDA, and we could be found to be marketing and selling these products in violation of
the law.
We
market our Obagi products that contain HQ on a prescription-only (i.e., non-OTC) basis, and we have not sought nor obtained premarket
approval from the FDA to market these products in the U.S., nor have we sought marketing authorizations in other jurisdictions. Although,
to date, neither the FDA nor any other regulators have taken action against us for selling our prescription HQ products in the U.S. and
in other jurisdictions without marketing approval, there can be no assurance the FDA or any other regulatory authorities will not take
enforcement action against us, or otherwise require us to obtain premarket approval or similar authorization of our prescription HQ products,
and we may be required to suspend marketing of our prescription HQ products unless and until such products are approved.
Based
on the historical evolution of the legal and regulatory framework applicable to drugs in the U.S., the FDA acknowledges that there are
some drugs on the market that lack required FDA approval for marketing. The FDA has historically utilized a risk-based enforcement approach
with respect to drugs marketed without required approvals. In 2003, the FDA issued a CPG, which was finalized in 2006 and subsequently
amended in 2011, in which it announced a drug safety initiative to remove unapproved drugs from the market and established enforcement
priorities and a policy of enforcement discretion with respect to marketed unapproved products. Under this policy, the FDA indicated
that it intended to give higher priority to enforcement actions involving unapproved drug products in certain categories, including drugs
with potential safety risks and ineffective dugs that could be used in lieu of effective treatments. Although this CPG was withdrawn
and the drug safety initiative was terminated on the basis of a Federal Register notice in 2020, a subsequent Federal Register notice
in May 2021 withdrew the prior notice terminating the program and the CPG, and the FDA indicated that it plans to continue to prioritize
enforcement based on its existing general approach, which involves risk-based prioritization in light of all the facts of a given circumstance,
and issue new guidance on this topic.
We believe our prescription-only
HQ products do not fall within the previously established categories of unapproved drugs for which the FDA has indicated it prioritizes
enforcement. We have not received any communications from the FDA or any similar regulatory authority regarding these or any of our other
products. However, in the future the FDA may pursue an enforcement action against us and determine that our HQ products should be removed
from the market until we obtain approval of an NDA. For example, although our prescription-only HQ products are made with 4% HQ, the
FDA has expressed concerns regarding the safety of 2% HQ products marketed OTC. In addition, the CARES Act implemented a number of changes
to regulation of OTC drugs, one of which prohibited the sale of HQ (at any concentration level) from being marked in the U.S. as an OTC
drug without FDA approval effective September 2020. On April 19, 2022, the FDA announced that it had issued warning letters to 12 companies
for continuing to sell 2% HQ products on an OTC basis in violation of the CARES Act. The FDA’s announcement also cited reports
describing serious side effects associated with the use of skin lightening products containing HQ, including reports of skin rashes,
facial swelling, and skin discoloration. See the section entitled “—Risks Related to Our Professional Skincare Segment:
Obagi—Our products may cause adverse events or side effects, or could be associated with safety issues, that could result in recalls,
withdrawals, or regulatory enforcement action. For example, the FDA has historically expressed concerns regarding the safety of HQ products,
including risks for potentially serious side effects, including skin rashes, facial swelling, skin discoloration, carcinogenicity and
reproductive toxicity.” If the FDA determines that our prescription-only HQ products present the same concerns
as OTC 2% HQ products, the FDA could determine that our HQ products also represent a high priority for enforcement of the requirements
for an NDA.
If
we are required to seek FDA approval or foreign authorities’ authorization of these products, our attention and resources will
be dedicated to the clinical development and regulatory approval processes, which will be time-consuming and very expensive. We may also
not successfully obtain such approvals or may be delayed in obtaining such approvals if one of our competitors obtains approval and non-patent
marketing exclusivity for the same uses for which we intend to seek approvals. In addition, if we are determined to be marketing our
prescription HQ products unlawfully, or if patients experience adverse events from using our prescription HQ products, we may be required
to recall or cease distribution of these products and may be subject to product liability claims or enforcement action. If we are required
to suspend or cease marketing of our prescription HQ products for any reason, our business would be materially adversely affected.
In
addition, even if we obtain regulatory approvals for any of our prescription HQ products, such approvals will require the submission
of reports to regulatory authorities and surveillance to monitor the safety and efficacy of the product, may contain significant limitations
related to use restrictions for specified age groups, warnings, precautions or contraindications, and may include burdensome post-approval
study or risk management requirements. In addition, if the FDA or foreign regulatory authorities approve our products, the manufacturing
processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping
for our product candidates will be subject to extensive and ongoing regulatory requirements, any of which may materially increase our
costs and limit our ability to maintain profitability.
Our
Obagi products may cause adverse events or side effects, or could be associated with safety issues, that could result in recalls, withdrawals,
or regulatory enforcement action. For example, the FDA has historically expressed concerns regarding the safety of HQ products, including
risks for potentially serious side effects, including skin rashes, facial swelling, skin discoloration, carcinogenicity and reproductive
toxicity.
Adverse
events or other undesirable side effects caused by our Obagi products could cause us or regulatory authorities to issue warnings about
our products or could lead to recalls or regulatory enforcement action. For example, our HQ products could be subject to enforcement
action and/or recalls based on the FDA’s concerns regarding OTC HQ-based products. Specifically, in August 2006, the FDA issued
a proposed rule that cited certain preclinical evidence suggesting that HQ may be a carcinogen, if orally administered, may present fertility
risks and may be related to a skin condition called ochronosis, which results in the darkening and thickening of the skin and the appearance
of small bumps and grayish-brown spots, after use of concentrations as low as 1 to 2 percent. The FDA also concluded that it could not
rule out the potential carcinogenic risk from topically applied HQ. Accordingly, the FDA recommended that additional studies be conducted
to determine if there is a risk to humans from the use of HQ. The FDA nominated HQ for further study by the National Toxicology Program
(the “NTP”), and in December 2009, the NTP Board of Scientific Counselors approved the nomination.
On
April 19, 2022, the FDA announced that it had issued warning letters to 12 companies for continuing to sell 2% HQ products on an OTC
basis in violation of the CARES Act. The FDA’s announcement also cited reports describing serious side effects associated with
the use of skin lightening products containing HQ, including reports of skin rashes, facial swelling, and skin discoloration.
Our
Obagi Nu-Derm Clear, Blender and Sunfader products, and our Obagi-C Rx C-Clarifying Serum and Obagi-C Rx C-Night Therapy Cream products,
which are part of our Obagi-C Rx Systems, contain HQ at 4% concentration. Until the completion of the NTP studies, the FDA recommended
classifying OTC skin-bleaching drug products, including HQ, as not generally recognized as safe and effective (“GRASE”),
as misbranded, and as new drugs within the meaning of the FDCA, meaning that such products would need to be approved through the NDA
process in order to be legally marketed in the U.S.
Although
this proposed rule was never finalized, in March 2020, Congress passed the CARES Act, which among other things, amended the FDCA to incorporate
FDA’s proposed rulemaking with respect to OTC drugs into final OTC monograph determinations. In particular, the CARES Act deemed
any OTC drugs that were identified as not GRASE in the FDA’s most recent proposed rulemaking for such OTC drugs to be “new
drugs” and misbranded within the meaning of the FDCA, meaning that as of September 23, 2020, such drugs required an approved drug
application before they could be lawfully marketed. As a result, products containing HQ were prohibited from being marketed in the U.S.
as OTC drug products without an approved NDA. Subsequently, in April 2022, the FDA issued the aforementioned warning letters to 12 companies
for selling 2% HQ products on an OTC basis, citing violations of the applicable CARES Act provisions.
While the legal framework
with respect to HQ products marketed OTC does not directly affect the regulatory status of our prescription-only HQ products, the FDA’s
cited concerns regarding the safety of HQ in OTC products at concentrations as low as 1% or 2% could nevertheless trigger regulatory
scrutiny of our prescription-only HQ products. To the extent that the FDA were to determine that our prescription-use only HQ products
present safety concerns, the FDA could determine that the products should be recalled, and such determination could trigger the FDA to
require marketing authorization for these products based on the FDA’s established enforcement priorities for drugs marketed without
an approved NDA. See the section entitled “—Risks Related to Our Professional Skincare Segment: Obagi—Our
products containing the active ingredient, hydroquinone, are marketed as prescription-use only drugs but have not received required premarket
authorization from the FDA or other regulatory authorities, and the FDA could require us to remove these products from the market until
we obtain approval of the required NDA, and we could be found to be marketing and selling these products in violation of the law.”
If
our Obagi products are associated with undesirable side effects or adverse events, a number of potentially significant negative consequences
could result, including, but not limited to:
| ● | regulatory
authorities may suspend, limit or withdraw approvals of such product (to the extent subject
to such approvals), or seek an injunction against its manufacture or distribution; |
| ● | regulatory
authorities may require warnings or issue safety alerts, Dear Healthcare Provider letters,
press releases or other communications containing warnings or other safety information about
the product; |
| ● | we
may be required to change the way the product is administered or conduct clinical trials; |
| ● | we
may be subject to fines, injunctions or the imposition of criminal penalties; |
| ● | we
could be sued and held liable for harm caused to patients; and |
| ● | our
reputation may suffer. |
Any
of these events could seriously harm our business.
Regulations
could prohibit physicians from dispensing our prescription-only products directly or through their e-commerce websites.
In
our primary market, the U.S., we market our prescription-only products and systems directly to physicians to dispense in their offices.
All such products and systems we sell are dispensed by physicians directly to their patients in their offices. Although several of our
systems contain prescription-strength HQ products and we sell different strengths of tretinoin, all of which require a prescription,
our products are not currently available in pharmacies. Certain states, including Massachusetts, Montana, New Hampshire, New York and
Texas, prohibit physicians from dispensing prescription products without a pharmacy or other license or authorization, permitting physicians
dispensing of such products only in certain limited circumstances for “immediate need” until a patient can get to a pharmacy
or in rural areas with a small population. For these states we offer alternate products under our Obagi Nu-Derm Fx and Obagi-C Fx product
lines that contain the skin brightening ingredient arbutin rather than 4% HQ. We are aware that the State of Texas and Puerto Rico, as
well as certain credit card authorization vendors, that have taken action against physician customers who sell our prescription products
to patients over the Internet, questioning whether these practices are consistent with such states’ pharmacy licensure and physician
dispensing rules and requiring them to either obtain the proper licenses or to cease selling our prescription products through their
e-commerce sites. Most of these physicians ceased selling the prescription products online, offering them only in office to patients,
and/or chose to sell our alternate arbutin products online instead. These actions did not have a material impact on our sales or net
revenue. However, in the future there may be additional states that take similar enforcement actions against our customers. In the event
that occurs, affected customers may be unable to continue selling our prescription-strength products over the Internet or at all, or
be required to incur additional costs to obtain the required licensure to be able to dispense the products, which may discourage such
customers from continuing to purchase them. Moreover, in the event state regulations change or the interpretation of existing regulations
change that limit or prohibit the ability of physicians to dispense prescription products directly to patients in their offices, or limit
or prevent our ability to distribute products directly through physicians, patients may be unable to obtain our prescription-strength
products, as they are not currently available in pharmacies, which would have a material effect on our business and on both customers’
and patients’ ability to purchase HQ products.
Our
ability to commercially distribute our products and our business may be significantly harmed if we or our contract manufacturers fail
to comply with applicable laws and regulations.
We
do not currently have the infrastructure or internal capability to manufacture our products. We rely, and expect to continue to rely,
on third-party manufacturers for the production of our products. The facilities used by our contract manufacturers are generally subject
to regulation under the FDCA and FDA implementing regulations, and comparable regulatory frameworks in foreign markets. The FDA and foreign
authorities may inspect the facilities of our third-party manufacturers periodically to determine if we and our third-party manufacturers
are complying with applicable provisions of the FDCA, FDA and foreign regulations.
Manufacturing
facilities for drug products are required to comply with the FDA’s cGMPs and with similar requirements outside the U.S., which
require manufacturers to maintain, among other things, stringent vendor qualifications, ingredient identification procedures, manufacturing
controls and record keeping processes. With respect to cosmetic products, the FDA has not promulgated mandatory cGMP regulations. However,
adherence to recommended cGMPs can reduce the risk that the FDA finds such products have been rendered adulterated or misbranded in violation
of applicable law. The FDA’s draft guidance on cosmetic cGMPs, most recently updated in June 2013, provides recommendations related
to process documentation, recordkeeping, building and facility design, equipment maintenance and personnel. The FDA also recommends that
manufacturers maintain product complaint and recall files and voluntarily report adverse events to the agency. In addition, FDA regulations
prohibit or otherwise restrict the use of certain ingredients in cosmetic products. Similar or stricter requirements may apply in foreign
jurisdictions. For instance, in the E.U., cosmetic products must be manufactured in compliance with good manufacturing practice and E.U.
regulations equally prohibit or otherwise restrict the use of certain ingredients in cosmetic products, including tretinoin.
We
rely on third parties to manufacture our products in accordance with our specifications and in compliance with applicable laws and regulations,
including the cosmetic cGMP guidelines in the FDA’s draft guidance and applicable cGMP or similar requirements for drug products.
Compliance with these standards can increase the cost of manufacturing our products as we work with our vendors to assure they are qualified
and in compliance. For our tretinoin-based products, which we distribute pursuant to an ANDA held by Bausch Health or which we dispense
under the category of unlicensed medicines in the United Kingdom, we also rely on our contract manufacturers to maintain appropriate
regulatory clearances or approvals, or otherwise qualify for exemptions from FDA premarket review requirements for such products.
We
do not have complete control over all aspects of the manufacturing process of, and are dependent on, our contract manufacturing partners
for compliance with cGMP and similar regulations. Our contract manufacturing partners may be found in violation of applicable requirements,
which could have a material adverse effect on us and our business. For example, G.S. Cosmeceutical, which manufactures our prescription
HQ products, has been subject to a number of FDA inspections in recent years in which the FDA identified a number of violations, including
most recently following an inspection ended in August 2019. Although we believe G.S. Cosmeceutical has resolved the observations identified
by the FDA during the August 2019 inspection, there is no assurance that the FDA will not identify further deficiencies in future inspections.
If we or our contract manufacturers fail to comply with these applicable standards, laws, and regulations, it could lead to customer
complaints, adverse events, product withdrawal or recall, or increase the likelihood that our products are rendered adulterated or misbranded,
any of which could result in negative publicity, remedial costs, or regulatory enforcement that could impact our ability to continue
selling certain products. In addition, reliance on third-party manufacturers entails additional risks, including:
| ● | the
failure of the third party to manufacture our products on schedule, or at all, including
if our third-party contractors give greater priority to the supply of other products over
our products or otherwise do not satisfactorily perform according to the terms of the agreements
between us and them; |
| ● | the
reduction or termination of production or deliveries by suppliers, or the raising of prices
or renegotiation of terms; |
| ● | the
termination or nonrenewal of arrangements or agreements by our third-party contractors at
a time that is costly or inconvenient for us; |
| ● | the
breach by the third-party contractors of our agreements with them; |
| ● | the
failure of third-party contractors to comply with applicable regulatory requirements; and |
| ● | the
failure of the third party to manufacture our products according to our specifications. |
Our
failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed
on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or
recalls of product candidates or drugs, operating restrictions and criminal prosecutions, any of which could significantly and adversely
affect supplies of our product candidates or drugs and harm our business and results of operations.
Failure to obtain regulatory approvals
or to comply with regulations in foreign jurisdictions would prevent us from marketing our Obagi products internationally.
We
market our products outside of the U.S. To market our products in many non-U.S. jurisdictions, we must obtain separate regulatory approvals
and comply with numerous and varying regulatory requirements. In some countries, we do not have to obtain prior regulatory approval but
do have to comply with other regulatory restrictions on the manufacture, importation, distribution, marketing and sale of our products.
We may be unable to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market.
The approval procedure varies among countries and can involve additional testing and data review. The time required to obtain approval
in non-U.S. jurisdictions may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all
of the risks associated with obtaining FDA approval. In addition, many countries from time to time evaluate the regulatory status of
various products and ingredients. We may not obtain foreign regulatory approvals on a timely basis, if at all, or may choose not to implement
a country’s labeling requirements if to do so would have a negative impact on our international or domestic operations. If any
of our products receives FDA approval, such approvals do not ensure approval by regulatory agencies in other countries, and approval
by one foreign regulatory authority does not ensure approval by regulatory agencies in other foreign countries or by the FDA. The failure
to obtain any required approvals could materially harm our business.
In
the United Kingdom, certain of our products may be deemed medicinal products and therefore subject to regulation by the Medicines and
Healthcare products Regulatory Agency (“MHRA”) under the medicines regime. We have not obtained a marketing authorization
for such products in the United Kingdom, however the UK’s Human Medicines Regulations 2012 allow for supply of medicinal products
which have not been authorized for marketing to patients with special needs at the request of the healthcare professional responsible
for the treatment of individual patients. Our tretinoin-based products are currently supplied in the United Kingdom under the category
of an unlicensed medicine or “special.” Unlicensed medicines should not, however, be supplied where an equivalent licensed
medicinal product can meet special needs of the patient. The responsibility for deciding whether an individual patient has “special
needs,” which a licensed product cannot meet, is a matter for the healthcare professional. Examples of “special needs”
include an intolerance or allergy to a particular ingredient, or an inability to ingest solid oral dosage forms. The MHRA has a wide
range of enforcement powers and failure to comply with regulatory restrictions or obtain regulatory approvals if required could harm
our business. If the MHRA were to decide that our products do not meet the “specials”’ requirements, we may need to
cease supply of these products and obtain a marketing authorization in the United Kingdom.
In
addition, if foreign regulatory authorities were to ban or restrict the use of certain ingredients in cosmetic products, and we are unable
to comply with the applicable requirements and regulations for those products, we may be unable to continue to market those products
and may be subject to enforcement action. For instance, in certain countries we offer our Nu-Derm Fx and Obagi-C Fx solutions, which
contain the skin brightening agent arbutin. In the E.U., the safety of alpha- and beta-arbutin is currently being assessed by the SCCS.
See “—Laws, regulations, enforcement trends or changes in existing regulations governing the introduction, marketing and
sale of our OTC drug, device and cosmetic products to consumers could harm our business.” If the European Commission were to
decide to restrict or ban the use of alpha- and/or beta-arbutin in certain cosmetic products, we may be unable to continue marketing
Nu-Derm Fx and Obagi-C Fx solutions as cosmetics in the EU.
If
we fail to comply with governmental regulations, we could face substantial penalties and our business, financial condition and results
of operations could be adversely affected.
The
healthcare industry in and outside the U.S. is heavily regulated and closely scrutinized by federal, state, local and foreign authorities.
Although our offerings are not currently covered by any commercial third-party payor or government healthcare program, our business activities
may nonetheless be subject to regulation and enforcement by the U.S. Department of Justice, the Department of Health and Human Services
and other federal, state and foreign governmental authorities. Federal, state and foreign laws and regulations that may affect our ability
to conduct business include, without limitation:
| ● | the
federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from
knowingly and willfully soliciting, offering, receiving or paying any remuneration, directly
or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral
of an individual for, or the purchase, lease, order, or arranging for or recommending the
purchase, lease or order of, any item or service, for which payment may be made, in whole
or in part, under federal healthcare programs such as Medicare and Medicaid. A person or
entity does not need to have actual knowledge of the statute or specific intent to violate
it in order to have committed a violation; |
| | | |
| ● | the federal civil false claims laws, including, without limitation,
the federal False Claims Act, which can be enforced through “qui tam,” or whistleblower actions, by private citizens, on behalf
of the federal government, and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly
presenting, or causing to be presented, false or fraudulent claims for payment of government funds, or knowingly making or using or causing
to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly and improperly avoiding,
decreasing or concealing an obligation to pay money to the federal government. In addition, the government may assert that a claim including
items and services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes
of the civil False Claims Act; |
| | | |
| ● | the
Civil Monetary Penalties Law, which prohibits, among other things, an individual or entity
from offering remuneration to a federal healthcare program beneficiary that the individual
or entity knows or should know is likely to influence the beneficiary to order or receive
healthcare items or services from a particular provider any item or service for which payment
may be made by the federal healthcare program; |
| | | |
| ● | the
criminal healthcare fraud provisions of HIPAA and related rules that prohibit knowingly and
willfully executing a scheme or artifice to defraud any healthcare benefit program or falsifying,
concealing or covering up a material fact or making any material false, fictitious or fraudulent
statement in connection with the delivery of or payment for healthcare benefits, items or
services. Similar to the federal Anti-Kickback Statute, a person or entity does not need
to have actual knowledge of the statute or specific intent to violate it to have committed
a violation; |
| | | |
| ● | the
federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices,
biologicals, and medical supplies for which payment is available under Medicare, Medicaid
or the Children’s Health Insurance Program to report annually to the Centers for Medicare
& Medicaid Services under the Open Payments Program, information related to payments
or other transfers of value made to teaching hospitals, physicians (as defined by statute)
and certain non-physician practitioners including physician assistants and nurse practitioners,
as well as ownership and investment interests held by physicians and their immediate family
members; |
| | | |
| ● | federal
consumer protection and unfair competition laws, which broadly regulate platform activities
and activities that potentially harm consumers; and |
| | | |
| ● | state
and foreign law equivalents of each of the above federal laws, such as anti-kickback, self-referral
and false claims laws which may apply to items or services reimbursed by any third-party
payor, including commercial insurers, and self-pay patients. |
Because
of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our
business activities could be subject to challenge under one or more of such laws. Achieving and sustaining compliance with these laws
may prove costly. The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have
not been fully interpreted by regulatory authorities or the courts, and their provisions are sometimes complex and open to a variety
of interpretations. Failure to comply with these laws and other laws can result in significant penalties, including, without limitation,
administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations, integrity
oversight and reporting obligations and exclusion from participation in federal, state and foreign healthcare programs and imprisonment.
Our failure to accurately anticipate the application of these laws and regulations to our business or any other failure to comply with
regulatory requirements could create liability for us and negatively affect our business. In addition, any action against us for violation
of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our
management’s attention from the operation of our business and result in adverse publicity, or otherwise result in a material adverse
impact on our business, results of operations, financial condition, cash flows and/or reputation.
We
and our manufacturers and suppliers license certain product and device technologies from third parties. If these licenses are breached,
terminated or disputed, our ability to commercialize products dependent on these technologies and patents may be compromised.
We have licensed certain
products and proprietary technology for various products, including our SUZANOBAGIMD® products, Nu-Cil™
Eyelash Enhancing Serum and Skintrinsiq device. If one or more of our licenses that we have with the parties who own these formulas or
technologies terminate, or if we violate the terms of our licenses or otherwise lose our rights to these products or technology, we may
be unable to continue developing and selling our products that are covered by these licenses. Our licensors or others may dispute the
scope of our rights under any of these licenses. The licensors under these licenses may breach the terms of their respective agreements
or fail to prevent infringement of the licensed formulas or technology by third parties. Loss of any of these licenses for any reason
could materially and adversely affect our financial condition and operating results.
Further,
we purchase products from manufacturers and suppliers who have licensed patent rights to use and sell these products from third-party
licensors, and if any dispute arises as to these licensed rights, the third-party licensors may bring legal actions against us, our respective
licensees, suppliers, customers or collaborators, and claim damages and seek to enjoin the manufacturing and marketing of such products.
In
addition, if we determine that our products do not incorporate the patented technology that we have licensed from third parties, or that
one or more of the patents that we have licensed are not valid, we may dispute our obligation to pay royalties to our licensors. Any
dispute with a licensor could be complex, expensive and time-consuming and an outcome adverse to us could materially and adversely affect
our business and impair our ability to commercialize our patent-licensed products.
We
received a Paycheck Protection Program loan, and our application for such loan could in the future be determined to have been impermissible
or could result in damage to our reputation.
In
May 2020, we applied for and received an unsecured $6.8 million loan under the Paycheck Protection Program (the “PPP Loan”).
In June 2021, the PPP Loan was fully forgiven. The Paycheck Protection Program was established under the CARES Act, and is administered
by the U.S. Small Business Administration (the “SBA”).
Our
receipt of the PPP Loan or the forgiveness of the PPP Loan could result in adverse publicity. In addition, if we are later determined
to have been ineligible to receive the PPP Loan or loan forgiveness, we may be subject to significant penalties, including significant
civil, criminal and administrative penalties, we could be required to repay the PPP Loan in its entirety, and our reputation could suffer.
A review or audit by the SBA or other government entity or claims under the U.S. False Claims Act could consume significant financial
and management resources.
Risks
Related to Our “Clean” Makeup Segment: Milk
Our Milk segment’s growth and
profitability is dependent on a number of factors, and its historical growth may not be indicative of its future growth.
Our Milk segment’s
historical growth should not be considered as indicative of our future performance. We may not be successful in executing our growth
strategy, and even if we achieve our strategic plan, we may not be able to achieve or sustain profitability. In future periods, our revenue
could decline, or grow more slowly than we expect. We also may incur significant losses in the future for a number of reasons, including
the following risks and the other risks described in this prospectus, and we may encounter unforeseen expenses, difficulties, complications,
delays and other unknown factors:
| ● | we
may lose one or more significant retailers, or sales of our products through these retailers
may decrease; |
| ● | the
ability of our third-party suppliers to produce our products and of our distributors to distribute
our products could be disrupted; |
| ● | our
products may be the subject of regulatory actions, including, but not limited to, actions
by the FDA, the FTC and the Consumer Product Safety Commission (“CPSC”) in the
U.S.; |
| ● | we
may be unable to introduce new products that appeal to consumers or otherwise successfully
compete with our competitors in the cosmetics industry; |
| ● | we
may be unsuccessful in enhancing the recognition and reputation of our brand, and our brand
may be damaged as a result of, among other reasons, our failure, or alleged failure, to comply
with applicable ethical, social, product, labor or environmental standards; |
| ● | we
may experience service interruptions, data corruption, cyber-based attacks or network security
breaches which result in the disruption of our operating systems or the loss of confidential
information of our consumers; |
| ● | we
may be unable to retain key members of our senior management team or attract and retain other
qualified personnel; and |
| ● | we
may be affected by any adverse economic conditions in the U.S. or internationally. |
Our Milk segment has a history of net
losses and may experience future losses.
Our Milk segment has
a history of net losses and may not be able to establish profitable operations. It reported net losses of $7.8 million, $12.9 million
and $11.0 million during the fiscal years ended December 31, 2021, 2020 and 2019, respectively, with the exception of the six months
ended June 30, 2022 and 2021, during which it reported net income of $5.0 million and $2.0 million, respectively. We may incur additional
operating losses in the future. Furthermore, our strategic plan will require a significant investment in product development, sales,
marketing, personnel, technology and administrative programs, which may not result in the accelerated net sales growth that we anticipate.
As a result, there can be no assurance that we will ever generate substantial net sales or achieve or sustain profitability.
The loss of a significant reseller could
materially and adversely affect our Milk segment’s business, financial condition and results of operations.
We sell the majority
of our Milk products in the U.S. through Sephora. In North America, Sephora accounted for approximately 69%, 56% and 78% of Milk’s
total net sales during the years ended December 31, 2021, 2020 and 2019, respectively, and 74% and 63% of Milk’s total net sales
during the six months ended June 30, 2022 and 2021, respectively. Any disruption to Sephora’s ability to properly receive, deliver,
service, promote or market our brand and products would negatively impact us. We also sell Milk products direct to consumers through
our website, milkmakeup.com. The loss of our relationship with Sephora or any of our other distributors could have a material and adverse
impact on our future operating results for our Milk segment.
Our retailers generally are not under
any obligation to purchase our Milk segment products, and business challenges at one or more of these retailers, could adversely affect
our results of operations.
As is typical in the beauty
industry, our business with retailers is based primarily upon discrete sales orders, and we do not have contracts requiring retailers
to make firm purchases from us. Accordingly, retailers could reduce their purchasing levels or cease buying products from us at any time
and for any reason. If we lose a significant reseller or if sales of our Milk segment products to a significant retailer materially decrease,
it could have a material adverse effect on our business, financial condition and results of operations.
Because a high percentage
of our sales are made through our retailers, our results are subject to risks relating to the general business performance of our retailers,
with significant exposure to Sephora. Factors that adversely affect our retailers’ businesses may also have a material adverse
effect on our business, financial condition and results of operations. These factors may include:
| |
● |
any
reduction in consumer traffic and demand at our retailers as a result of economic downturns, pandemics or other health crises, changes
in consumer preferences or reputational damage as a result of, among other developments, data privacy breaches, regulatory investigations
or employee misconduct; |
| |
● |
any
credit risks associated with the financial condition of our retailers; and |
| |
● |
the
effect of consolidation or weakness in the retail industry or at certain retailers, including store closures and the resulting uncertainty. |
We maintain supply relationships for
certain key components, and our business and operating results could be harmed if supply is restricted or ends, or the price of raw materials
used in our manufacturing process increases.
We are dependent on a limited
number of suppliers for certain components that are integral to our finished products. If these or other suppliers encounter financial,
operating or other difficulties or if our relationship with them changes, we may be unable to quickly establish or qualify replacement
sources of supply and we could face production interruptions, delays and inefficiencies. In addition, technology changes by our vendors
could disrupt access to required manufacturing capacity or require expensive, time-consuming development efforts to adapt and integrate
new equipment or processes. Our growth may exceed the capacity of one or more of these suppliers to produce the needed equipment and
materials in sufficient quantities to support our growth. Any one of these factors could harm our business and growth prospects.
A negative reputation event to any of
our Milk segment’s retail partners or the beauty industry could cause a decline in our net revenues or a reduction in our earnings.
Our Milk segment is dependent
on our retail partnerships, especially with Sephora, to deliver our results. Any negative impact to their reputation that could cause
a reduction in consumer traffic would negatively impact us. Furthermore, we operate in a single category, or subset of categories, within
beauty. Any event which causes the entire industry to be negatively perceived would also potentially negatively impact us.
In
order to deepen our market penetration and raise awareness of our Milk brand and products, we have increased the amount we spend on marketing
activities, which may not ultimately prove successful or an effective use of our resources.
To increase awareness
of our Milk segment products and services domestically and internationally, we have increased the amount we spend and anticipate spending
in the future on marketing activities. Our marketing efforts and costs are significant and include national and regional campaigns involving
outdoor media, social media, additional placements and alliances with strategic partners. We attempt to structure our advertising/marketing
campaigns in ways we believe most likely to increase brand awareness and adoption; however, there is no assurance our campaigns will
achieve the returns on advertising spend desired or successfully increase brand or product awareness sufficiently to sustain or increase
our growth goals, which could have an adverse effect on our gross margin and business overall.
We rely on a number of third-party suppliers,
distributors and other vendors, and they may not continue to produce products or provide services that are consistent with our standards
or applicable regulatory requirements, which could harm our brand, cause consumer dissatisfaction and require us to find alternative
suppliers of our products.
We use multiple third-party
suppliers based in the U.S. and overseas to source substantially all of our products. Certain of these third-party suppliers manufacture
components and packaging while other third-party suppliers will fill and assemble the product. We engage our third-party suppliers on
a purchase order basis and are not party to long-term contracts with any of them. The ability of these third parties to supply our products
may be affected by competing orders placed by other persons and the demands of those persons. If we experience significant increases
in demand or need to replace a significant number of existing suppliers, there can be no assurance that additional supply capacity will
be available when required on terms that are acceptable to us, or at all, or that any supplier will allocate sufficient capacity to us
in order to meet our requirements.
In addition, quality
control problems or supply chain issues, such as the use of ingredients and delivery of products that do not meet our quality control
standards and specifications or comply with applicable laws or regulations, including the Controlled Substances Act and the FDCA, could
harm our business. These quality control or supply chain problems could result in regulatory action, such as restrictions on importation,
legal prohibitions on the sale of products or other penalties, or result in products of inferior quality or product stock outages or
shortages, harming our sales and creating inventory write-downs for unusable products.
Further, our third-party
suppliers and distributors may:
| |
● |
have economic or business interests or goals that
are inconsistent with ours; |
| |
● |
take actions contrary to our instructions, requests,
policies or objectives; |
| |
● |
be unable or unwilling to fulfill their obligations
under relevant purchase orders, including obligations to meet our production deadlines, quality standards, pricing guidelines and
product specifications, or to comply with applicable regulations, including those regarding the safety and quality of products and
ingredients and good manufacturing practices; |
| |
● |
have financial difficulties; |
| |
● |
encounter raw material or labor shortages; |
| |
● |
encounter increases in raw material or labor costs
which may affect our procurement costs; |
| |
● |
disclose our confidential information or intellectual
property to competitors or third parties; |
| |
● |
engage in activities or employ practices that may
harm our reputation; and |
| |
● |
work with, be acquired by, or come under control
of our competitors. |
The occurrence of any
of these events, alone or together, could have a material adverse effect on our business, financial condition and results of operations.
In addition, such problems may require us to find new third-party suppliers or distributors, and there can be no assurance that we would
be successful in finding third-party suppliers or distributors meeting our standards of innovation and quality.
The management and oversight
of the engagement and activities of our third-party suppliers and distributors requires substantial time, effort and expense of our employees,
and we may be unable to successfully manage and oversee the activities of our third-party suppliers and distributors. If we experience
any supply chain disruptions caused by our inability to locate suitable third-party suppliers, or if our raw material suppliers experience
problems with product quality or disruptions or delivery of the raw materials or components used to make such products, our business,
financial condition and results of operations could be materially and adversely affected.
We rely heavily on our third-party agency
and direct sales forces to sell our Milk segment products in the U.S. and internationally, and any failure to train and maintain our
third-party agency and direct sales forces could harm our business.
Our ability to sell our
Milk segment products and generate revenues depends in part upon our third-party agency and direct sales forces within the U.S. and internationally.
We do not have any long-term employment contracts with our third-party agency and direct sales forces and the loss of the services provided
by these key personnel may harm our business. In order to provide more comprehensive sales and service coverage, we continue to increase
the size of our sales force to pursue growth opportunities within and outside of our existing geographic markets. To adequately train
new representatives to successfully market and sell our products and for them to establish strong customer relationships takes time.
As a result, if we are unable to retain our third-party agency and direct sales personnel or quickly replace them with individuals of
equivalent technical expertise and qualifications, if we are unable to successfully instill technical expertise in new and existing sales
representatives, if we fail to establish and maintain strong relationships with our customers or if our efforts at specializing our selling
techniques do not prove successful and cost-effective, our net revenues, our gross margin and ability to maintain market share could
be materially harmed.
We
have growing operations in China, which exposes us to risks inherent in doing business in that country.
We
currently source components in China and do not have substantial alternatives to those suppliers. We also utilize warehouse services
provided by our third-party distributors. With the rapid development of the Chinese economy, the cost of labor has increased and may
continue to increase in the future. Our results of operations will be materially and adversely affected if our labor costs, or the labor
costs of our suppliers, increase significantly. In addition, we and our suppliers may not be able to find a sufficient number of qualified
workers due to the intensely competitive and fluid market for skilled labor in China. Furthermore, pursuant to Chinese labor laws, employers
in China are subject to various requirements when signing labor contracts, paying remuneration, determining the term of employees’
probation and unilaterally terminating labor contracts. These labor laws and related regulations impose liabilities on employers and
may significantly increase the costs of workforce reductions. If we decide to change or reduce our workforce, these labor laws could
limit or restrict our ability to make such changes in a timely, favorable and effective manner. Additionally, the Chinese government
may impose additional regulations regarding ingredients and composition of cosmetics and these regulations may affect our products. Any
of these events may materially and adversely affect our business, financial condition and results of operations.
Operating in China exposes
us to political, legal and economic risks. In particular, the political, legal and economic climate in China, both nationally and regionally,
is fluid and unpredictable. Our ability to operate in China may be adversely affected by changes in U.S. and Chinese laws and regulations
such as those related to, among other things, taxation, import and export tariffs, environmental regulations, land use rights, intellectual
property, currency controls, network security, employee benefits, hygiene supervision and other matters. In addition, we or our suppliers
may not obtain or retain the requisite legal permits to continue to operate in China, and costs or operational limitations may be imposed
in connection with obtaining and complying with such permits. In addition, Chinese trade regulations are in a state of flux, and we may
become subject to other forms of taxation, tariffs and duties in China. Furthermore, the third parties we rely on in China may disclose
our confidential information or intellectual property to competitors or third parties, which could result in the illegal distribution
and sale of counterfeit versions of our products. If any of these events occur, our business, financial condition and results of operations
could be materially and adversely affected. See the section entitled “—Risks Related to Our “Clean” Makeup
Segment: Milk—Recent and potential additional tariffs imposed by the U.S. government or a global trade war could increase
the cost of our products, which could materially and adversely affect our business, financial condition and results of operations”
for further discussion of risks related to tariffs between the U.S. and China.
Recent and potential additional tariffs
imposed by the U.S. government or a global trade war could increase the cost of our products, which could materially and adversely affect
our business, financial condition and results of operations.
The U.S. government has
imposed increased tariffs on certain imports from China, some of which cover products that we import from that country. We currently
source important components for our products from third-party suppliers in China, and, as such, current tariffs may increase our cost
of goods, which may result in lower gross margin on certain of our products. In any case, increased tariffs on imports from China could
materially and adversely affect our business, financial condition and results of operations. In retaliation for the current U.S. tariffs,
China has implemented tariffs on a wide range of American products. There is also a concern that the imposition of additional tariffs
by the U.S. could result in the adoption of tariffs by other countries as well, leading to a global trade war. Trade restrictions implemented
by the U.S. or other countries in connection with a global trade war could materially and adversely affect our business, financial condition
and results of operations.
Our Milk facilities are subject to regulation
under the Federal Food, Drug and Cosmetic Act and FDA implementing regulations.
Our facilities and those
of our third-party manufacturers are subject to regulation under the FDCA and FDA implementing regulations. The FDA may inspect all of
our or our third-party manufacturers’ facilities periodically to determine if such facilities comply with the FDCA and FDA regulations.
In addition, our facilities for manufacturing OTC drug products must comply with the FDA’s cGMP that require our third-party manufacturers
to maintain, among other things, good manufacturing processes, including stringent vendor qualifications, ingredient identification,
manufacturing controls and record keeping.
Our operations could
be harmed if regulatory authorities make determinations that we, or our vendors, are not in compliance with these regulations. If the
FDA finds a violation of cGMPs, it may enjoin the operations of our third-party manufacturers, seize product, restrict the importation
of goods, and impose administrative, civil or criminal penalties. If we or our third-party manufacturers fail to comply with applicable
regulatory requirements, we could be required to take costly corrective actions, including suspending manufacturing operations, changing
product formulations, suspending sales, or initiating product recalls. In addition, compliance with these regulations has increased and
may further increase the cost of manufacturing certain of our products to ensure and maintain compliance. Any of these outcomes could
have a material adverse effect on our business, financial condition and results of operations.
We are involved, and may become involved
in the future, in disputes and other legal or regulatory proceedings, including an ongoing legal proceeding involving our founders, that,
if adversely decided or settled, could materially and adversely affect our business, financial condition and results of operations.
We are, and may in the
future become, party to litigation, regulatory proceedings or other disputes. In general, claims made by or against us in disputes and
other legal or regulatory proceedings can be expensive and time-consuming to bring or defend against, requiring us to expend significant
resources and divert the efforts and attention of our management and other personnel from our business operations. These potential claims
include, but are not limited to, personal injury claims, class action lawsuits, intellectual property claims, employment litigation and
regulatory investigations and causes of action relating to the advertising and promotional claims about our products. Any adverse determination
against us in these proceedings, or even the allegations contained in the claims, regardless of whether they are ultimately found to
be without merit, may also result in settlements, injunctions or damages that could have a material adverse effect on our business, financial
condition and results of operations.
As an example, a lawsuit
was commenced in New York state court on June 27, 2016, captioned Joseph v. Rassi, et al., Case No. 510914/2016 (N.Y. Sup. Ct. Oct. 6,
2016) in which the plaintiff has asserted claims against founders Mazdack Rassi, Erez Shternlicht and Moishe Mana, as well as against
Legs Media LLC, Milk Agency, LLC, Milk Makeup Holdings, LLC, Milk Makeup Management, LLC, Milk Studios, LLC, Milk and Scott Sassa. Plaintiff
alleges that Rassi, Shternlicht, and Mana breached the duty they owed to Legs Media by misappropriating its “corporate opportunity”
related to the Milk Makeup concept for their own benefit. There is a parallel proceeding in Delaware Court of Chancery, captioned Mana
v. Joseph, Civil Action No. 12715 (Del. Ch. Sept. 2, 2016) in which Rassi, Mana, Shternlicht, Legs Media, LLC, Milk Studios, LLC, Milk,
and Milk Agency, LLC have asserted various claims against Joseph and, among other things, are seeking declaratory judgment that Joseph
has no ownership interest in Milk Studios, LLC or Milk. The Delaware litigation is currently stayed pending developments in the New York
litigation. On April 13, 2022, Joseph filed a Motion for Injunctive Relief and to Compel Emergency Discovery seeking an order directing
the defendants to produce certain documents related to the Milk Transaction and enjoining the parties to the Milk Transaction from completing
the Milk Transaction until twenty-one (21) days after the defendants produce requested documents, allegedly to provide Joseph with sufficient
time to determine whether to seek additional relief, including potentially a permanent injunction. Plaintiff’s application for
an order to show cause was rejected, for failure to pay the requisite fee to the court, before the motion was heard. It was never refiled,
and has now been obviated by the consummation of the Business Combination.
Although our management
believes these allegations are without merit and the possibility of a material impact on its financial conditions or results of operation
is remote, and we intend to defend vigorously against such allegations, any adverse determination against the Company, Milk’s founders,
Milk or its affiliates in these proceedings, or even the allegations contained in the claims, regardless of whether they are ultimately
found to be without merit, may result in distractions to management, reputational harm, injunctive relief, settlement costs, damages
and/or defense costs that could adversely affect our business operations and financial condition.
Risks Related to Marketing Activities
Use of social media may materially and
adversely affect our reputation or subject us to fines or other penalties.
We rely to a large extent
on our online presence to reach consumers, and we, along with our retailers, offer consumers the opportunity to rate and comment on our
products on e-commerce websites. Negative commentary or false statements regarding us or our products may be posted on these e-commerce
websites or social media platforms and may harm our reputation or business. Our target consumers often value readily available information
and often act on such information without further investigation and without regard to its accuracy. The harm may be immediate without
affording us an opportunity for redress or correction. In addition, we may face claims relating to information that is published or made
available through the interactive features of our e-commerce website. For example, we may receive third-party complaints that the comments
or other content posted by users on our platforms infringe third-party intellectual property rights or otherwise infringe the legal rights
of others. While the Communications Decency Act and Digital Millennium Copyright Act generally protect online service providers from
claims of copyright infringement or other legal liability for the self-directed activities of its users, if it were determined that we
did not meet the relevant safe harbor requirements under either law, we could be exposed to claims related to advertising practices,
defamation, intellectual property rights, rights of publicity and privacy, and personal injury torts. We could incur significant costs
investigating and defending such claims and, if we are found liable, significant damages. If any of these events occur, our business,
financial condition and results of operations could be materially and adversely affected.
We also use third-party
social media platforms as marketing tools. For example, we maintain Snapchat, Facebook, TikTok, Instagram and YouTube accounts. As e-commerce
and social media platforms continue to rapidly evolve, we must continue to maintain a presence on these platforms and establish a presence
on new or emerging popular social media platforms. If we are unable to cost-effectively use social media platforms as marketing tools,
our ability to acquire new consumers and our financial condition may suffer. Furthermore, as laws and regulations rapidly evolve to govern
the use of these platforms and devices, the failure by us, our employees or third parties acting at our direction to abide by applicable
laws and regulations in the use of these platforms and devices could subject us to regulatory investigations, class action lawsuits,
liability, fines or other penalties and could have a material adverse effect on our business, financial condition and results of operations.
In addition, an increase
in the use of social media for product promotion and marketing may cause an increase in the burden on us to monitor compliance of such
materials and increase the risk that such materials could contain problematic product or marketing claims in violation of applicable
regulations.
Risks
Related to Information Technology and Cybersecurity
We
are increasingly dependent on information technology, and if we are unable to protect against service interruptions, data corruption,
cyber-based attacks or network security breaches, our operations could be disrupted.
We rely on information
technology networks and systems to market and sell our Obagi and Milk products, to process electronic and financial information, to assist
with sales tracking and reporting, to manage a variety of business processes and activities and to comply with regulatory, legal and
tax requirements. We are increasingly dependent on a variety of information systems to effectively process consumer orders from our e-commerce
business. We depend on our information technology infrastructure for digital marketing activities and for electronic communications among
our personnel, retailers, customers, consumers, distributors and suppliers around the world. These information technology systems, some
of which are managed by third parties, may be susceptible to damage, disruptions or shutdowns due to failures during the process of upgrading
or replacing software, databases or components, power outages, hardware failures, computer viruses, attacks by computer hackers, telecommunication
failures, user errors or catastrophic events. Any material disruption of our systems, or the systems of our third-party service providers,
could disrupt our ability to track, record and analyze the products that we sell and could negatively impact our operations, shipment
of goods, ability to process financial information and transactions and our ability to receive and process provider and e-commerce orders
or engage in normal business activities. If our information technology systems suffer damage, disruption or shutdown, we may incur substantial
cost in repairing or replacing these systems, and if we do not effectively resolve the issues in a timely manner, our business, financial
condition and results of operations may be materially and adversely affected, and we could experience delays in reporting our financial
results.
Our e-commerce operations
are important to our business. Our e-commerce websites serve as an effective extension of our marketing strategies by introducing potential
new consumers to our brands, product offerings, retailers and enhanced content. Due to the importance of our e-commerce operations, we
are vulnerable to website downtime and other technical failures. Our failure to successfully respond to these risks in a timely manner
could reduce e-commerce sales and damage the reputation of our brands.
We
must successfully maintain and upgrade our information technology systems, and our failure to do so could have a material adverse effect
on our business, financial condition and results of operations.
We
have identified the need to expand and improve our information technology systems and personnel to support expected future growth. These
types of activities subject us to inherent costs and risks associated with replacing and changing these systems, including impairment
of our ability to leverage our e-commerce channels, fulfill provider and customer orders, potential disruption of our internal control
structure, substantial capital expenditures, additional administration and operating expenses, acquisition and retention of sufficiently
skilled personnel to implement and operate the new systems, demands on management time and other risks and costs of delays or difficulties
in transitioning to or integrating new systems into our current systems. These implementations, modifications and upgrades may not result
in productivity improvements at a level that outweighs the costs of implementation, or at all. In addition, difficulties with implementing
new technology systems, delays in our timeline for planned improvements, significant system failures, or our inability to successfully
modify our information systems to respond to changes in our business needs may cause disruptions in our business operations and have
a material adverse effect on our business, financial condition and results of operations.
If
we fail to adopt new technologies or adapt our e-commerce website and systems to changing consumer requirements or emerging industry
standards, our business may be materially and adversely affected.
To remain competitive,
we must continue to enhance and improve the responsiveness, functionality and features of our information technology networks and systems,
including our e-commerce websites. Our competitors are continually innovating and introducing new products to increase their consumer
base and enhance user experience. As a result, to attract and retain consumers and compete in the skincare and beauty markets, we must
continue to invest resources to enhance our information technology and improve our existing products and services for our consumers.
The Internet and the online retail industry are characterized by rapid technological evolution, changes in consumer requirements and
preferences, frequent introductions of new products and services embodying new technologies and the emergence of new industry standards
and practices, any of which could render our existing technologies and systems obsolete. Our success will depend, in part, on our ability
to identify, develop, acquire or license leading technologies useful in our business, and respond to technological advances and emerging
industry standards and practices in a cost-effective and timely way. The development of our e-commerce websites and other proprietary
technology entails significant technical and business risks. There can be no assurance that we will be able to properly implement or
use new technologies effectively or adapt our e-commerce website and systems to meet consumer requirements or emerging industry standards.
If we are unable to adapt in a cost-effective and timely manner in response to changing market conditions or consumer requirements, whether
for technical, legal, financial or other reasons, our business, financial condition and results of operations may be materially and adversely
affected.
Failure
to protect sensitive information of our consumers and information technology systems against security breaches could damage our reputation
and brand and substantially harm our business, financial condition and results of operations.
We
collect, maintain, transmit and store data about our consumers, suppliers and others, including personal data and financial information,
consumer payment information, as well as other confidential and proprietary information important to our business.
We
have in place technical and organizational measures to maintain the security and safety of critical proprietary, personal, employee,
provider and financial data that we continue to maintain and upgrade to industry standards. However, advances in technology, the pernicious
ingenuity of criminals, new exposures via cryptography, acts or omissions by our employees, contractors or service providers or other
events or developments could result in a compromise or breach in the security of confidential or personal data. We and our service providers
may not be able to prevent third parties, including criminals, competitors or others, from breaking into or altering our systems, disrupting
business operations or communications infrastructure through denial-of-service attacks, attempting to gain access to our systems, information
or monetary funds through phishing or social engineering campaigns, installing viruses or malicious software (including ransomware) on
our e-commerce website or devices used by our employees or contractors, or carrying out other activity intended to disrupt our systems
or gain access to confidential or sensitive information in our or our service providers’ systems. We are not aware of any breach
or compromise of the personal data of consumers, but we have been subject to attacks in the past (phishing, denial of service, etc.),
and we cannot guarantee that our security measures will be sufficient to prevent a material breach or compromise in the future.
Furthermore,
such third parties may engage in various other illegal activities using such information, including credit card fraud or identity theft,
which may cause additional harm to us, our consumers and our brand. We may also be vulnerable to error or malfeasance by our own employees
or other insiders. Third parties may attempt to fraudulently induce our or our service providers’ employees to misdirect funds
or to disclose information in order to gain access to personal data we maintain about our consumers or website users. In addition, we
have limited control or influence over the security policies or measures adopted by third-party retailers of online payment services
through which some of our consumers may elect to make payment for purchases at our e-commerce website. Contracted third-party delivery
service providers may also violate their confidentiality or data processing obligations and disclose or use information about our consumers
inadvertently or illegally.
If
a material security breach were to occur, our reputation and brand could be damaged, and we could be required to expend significant capital
and other resources to alleviate problems caused by such breach, including exposure to litigation or regulatory action and a risk of
loss and possible liability. Actual or anticipated attacks may cause us to incur increasing costs, including costs to deploy additional
personnel and protection technologies, train employees and engage third-party experts and consultants. Any compromise or breach of our
security measures, or those of our third-party service providers, may violate applicable privacy, data security, financial, cyber and
other laws and cause significant legal and financial exposure, adverse publicity, and a loss of confidence in our security measures,
all of which could have a material adverse effect on our business, financial condition and results of operations. We may be subject to
post-breach review of the adequacy of our privacy and security controls by regulators and other third parties, which could result in
post-breach regulatory investigation, fines and consumer litigation as well as regulatory oversight, at significant expense and risking
reputational harm.
Furthermore, we are subject
to diverse laws and regulations in the U.S., the E.U., and other international jurisdictions that require notification to affected individuals
in the event of a breach involving personal information. These required notifications can be time-consuming and costly. The interpretation,
standards and enforcement practices relating to these laws and regulations may change in the future. Furthermore, failure to comply with
these laws and regulations could subject us to regulatory scrutiny and additional liability, including injunctions, fines and/or other
proceedings. Although we maintain relevant insurance, we cannot be certain that our insurance coverage will be adequate for all breach-related
liabilities or that insurance will continue to be available to us on economically reasonable terms, or at all. We may need to devote
significant resources to protect against security breaches or to address problems caused by breaches, diverting resources from the growth
and expansion of our business.
Payment methods used on our e-commerce
websites subject us to third-party payment processing-related risks.
We accept payments from
our consumers using a variety of methods, including online payments with credit cards and debit cards issued by major banks, payments
made with gift cards processed by third-party retailers and payments through third-party online payment platforms such as PayPal, Afterpay
and Apple Pay. We also rely on third parties to provide payment processing services. For certain payment methods, including credit and
debit cards, we pay interchange and other fees, which may increase over time and raise our operating costs and lower our profit margins.
We may also be subject to fraud and other illegal activities in connection with the various payment methods we offer, including online
payment options and gift cards. Transactions on our e-commerce websites are card-not-present transactions, so they present a greater
risk of fraud. Criminals are using increasingly sophisticated methods to engage in illegal activities such as unauthorized use of credit
or debit cards and bank account information. Requirements relating to consumer authentication and fraud detection with respect to online
sales are complex. We may ultimately be held liable for the unauthorized use of a cardholder’s card number in an illegal activity
and be required by card issuers to pay charge-back fees. Charge-backs result not only in our loss of fees earned with respect to the
payment, but also leave us liable for the underlying money transfer amount. If our charge-back rate becomes excessive, card associations
also may require us to pay fines or refuse to process our transactions. In addition, we may be subject to additional fraud risk if third-party
service providers or our employees fraudulently use consumer information for their own gain or facilitate the fraudulent use of such
information. Overall, we may have little recourse if we process a criminally fraudulent transaction.
We
are subject to payment card association operating rules, certification requirements and various rules, regulations and requirements governing
electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. As our business
changes, we may also be subject to different rules under existing standards, which may require new assessments that involve costs above
what we currently pay for compliance. If we fail to comply with the rules or requirements of any provider of a payment method we accept,
or if the volume of fraud in our transactions limits or terminates our rights to use payment methods we currently accept, or if a data
breach occurs relating to our payment systems, among other things, we may be subject to fines and higher transaction fees and lose our
ability to accept credit and debit card payments from our consumers, process electronic funds transfers or facilitate other types of
online payments, and our reputation and our business, financial condition and results of operations could be materially and adversely
affected.
Our business is subject to complex and
evolving U.S. and foreign laws and regulations regarding privacy and data protection. Many of these laws and regulations are subject
to change and uncertain interpretation, and any failure or perceived failure to comply could result in claims, changes to our business
practices, monetary penalties or increased costs of operations, or otherwise could harm our business.
We are subject to a variety
of laws and regulations in the U.S. and abroad regarding privacy and data protection, some of which can be enforced by private parties
or government entities and some of which provide for significant penalties for noncompliance. Such laws and regulations govern the collection,
use, disclosure, retention, and security of personal information, such as information that we may collect in connection with clinical
trials. Implementation standards, interpretations and enforcement practices are likely to remain uncertain for the foreseeable future,
and we cannot yet determine the impact future laws, regulations, standards, or perception of their requirements may have on our business.
This evolution may create uncertainty in our businesses, affect our ability to operate in certain jurisdictions or to collect, store,
transfer, use and share personal information, necessitate the acceptance of more onerous obligations in our contracts, result in liability
or impose additional costs on us. The cost of compliance with these laws, regulations and standards is high and is likely to increase
in the future. Any failure or perceived failure by us to comply with federal, state or foreign laws or regulation, our internal policies
and procedures or our contracts governing our processing of personal information could result in negative publicity, claims by third
parties, government investigations and enforcement actions, including injunctions, fines and/or criminal penalties if we knowingly obtain
or disclose individually identifiable health information from a covered entity in a manner that is not authorized or permitted by HIPAA
or applicable state laws, any of which could have a material adverse effect on our operations, financial performance and business.
In the U.S., numerous
federal and state laws and regulations, including federal and state health information privacy laws, state data breach notification laws,
and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use,
disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators
and third-party providers. For example, the California Consumer Privacy Act (the “CCPA”), which went into effect on January
1, 2020, creates individual privacy rights for California consumers, including the right to opt out of certain disclosures of personal
information, increases the privacy and security obligations of entities handling certain personal information, and also establishes significant
penalties for noncompliance. The CCPA also provides for a private right of action for data breaches, which is expected to increase data
breach litigation. The CCPA may increase our compliance costs and potential liability. Additionally, in November 2020, California voters
passed the California Privacy Rights Act (the “CPRA”). The CPRA, which is expected to take effect on January 1, 2023, significantly
expands the CCPA, including by introducing additional obligations such as data minimization and storage limitations, granting additional
rights to consumers such as correction of personal information and additional opt-out rights and creating a new entity, the California
Privacy Protection Agency, to implement and enforce the law. The CPRA may require us to modify our data collection or processing practices
and policies, cause us to incur substantial costs and expenses to comply, and increase our potential exposure to regulatory enforcement
and/or litigation.
Other U.S. states have
also enacted or are considering enacting stricter data privacy laws. For example, on March 2, 2021, Virginia enacted the Virginia Consumer
Data Protection Act, a comprehensive privacy statute that is similar to the CCPA and CPRA.
Further, the FTC and
many state Attorneys General continue to enforce federal and state consumer protection laws against companies for online collection,
use, dissemination and security practices that appear to be unfair or deceptive. For example, according to the FTC, violating consumers’
privacy rights or failing to take appropriate steps to keep consumers’ personal information secure can constitute unfair acts or
practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s
data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the
size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
We are also or may become
subject to rapidly evolving data protection laws, rules and regulations in foreign jurisdictions. For example, the EU’s General
Data Protection Regulation 2016/679 (the “GDPR”) governs certain collection and other processing activities involving personal
data about data subjects in the European Economic Area (“EEA”). Among other things, the GDPR imposes requirements regarding
the security of personal data and the rights of data subjects to access and delete personal data, requires having lawful bases on which
personal data can be processed, includes requirements relating to the consent of individuals to whom the personal data relates, requires
detailed notices for clinical trial participants and investigators and regulates transfers of personal data from the EEA to third countries
that have not been found to provide adequate protection to such personal data, including the U.S. In addition, the GDPR imposes substantial
administrative fines for breaches and violations ranging from €10.0 million to €20.0 million or 2% to 4% of our annual global
revenue, whichever is higher. The GDPR also confers a private right of action on data subjects to lodge complaints with supervisory authorities,
seek judicial remedies (including data subject-led class actions and injunctions) and obtain compensation for damages resulting from
violations of the GDPR. Further, following the UK’s withdrawal from the E.U. on January 31, 2021 (“Brexit”), organizations
have been subject to the UK General Data Protection Regulation (“UK GDPR”), which, together with the amended UK Data Protection
Act 2018, transposed the GDPR in UK national law. The UK GDPR imposes separate and additional fines to the GDPR, ranging from £8.7
million to £17.5 million or 2% to 4% of total worldwide annual revenue, whichever is higher.
We are also subject to
E.U. and UK rules with respect to cross-border transfers of personal data out of the EEA and the UK to third countries. Recent legal
developments in the E.U. and the UK have created complexity and uncertainty regarding transfers of personal data. In July 2020, the Court
of Justice of the European Union (“CJEU”) in Schrems II invalidated the E.U.-U.S. Privacy Shield Framework,
a mechanism for the transfer of personal data from the EEA to the U.S., and made clear that reliance on standard contractual clauses
may not be sufficient in all circumstances, in which organizations may be required to take supplementary measures. On June 4, 2021, the
European Commission published a new set of modular standard contractual clauses which are designed to take into account the CJEU’s
judgement in Schrems II and must be used for all new contracts entered into, and new processing operations undertaken,
as of September 2021. The new standard contractual clauses only apply to the transfer of personal data outside of the EEA and not the
UK. Although the European Commission adopted an adequacy decision with respect to the UK in June 2021, allowing the flow of personal
data from the EEA to the UK to continue, this decision will be regularly reviewed and may be revoked if the UK diverges from its current
adequate data protection laws following Brexit. Furthermore, the UK Information Commissioner’s Office has consulted on, and is
developing, its own international data transfer requirements, including its own specific international data transfer agreement and a
UK addendum to the standard contractual clauses. We are accordingly monitoring these developments, but we may, in addition to other impacts,
experience additional costs associated with increased compliance burdens and be required to engage in new contract negotiations with
third parties that aid in processing personal data on our behalf. As supervisory authorities issue further guidance on personal data
export mechanisms, including circumstances where the standard contractual clauses cannot be used, and/or start taking enforcement action,
we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer
personal data between and among countries and regions in which we operate, it could affect the manner in which we operate our business
and could harm our business, financial condition and results of operations. The relationship between the UK and the E.U. in relation
to certain aspects of data protection laws remains unclear and it is unclear how UK data protection laws and regulations will develop
in the medium to longer term.
Regulators in the EEA
and the UK are increasingly focusing on compliance with requirements in the online behavioral advertising ecosystem. National laws in
the EEA that implement the ePrivacy Directive are likely to be replaced by the ePrivacy Regulation, which will significantly increase
fines for noncompliance, although it will not have effect in the UK as a result of Brexit. This again introduces the possibility that
we will be subject to separate and additional legal regimes with respect to eprivacy, which may result in further costs and may necessitate
changes to our business practices. The GDPR and UK GDPR requires opt-in, informed consent for the placement of cookies on a customer’s
device, and imposes conditions on obtaining valid consent (e.g., a prohibition on prechecked consents). Increased regulation of cookies
may lead to broader restrictions and impairments on our online activities and may negatively impact our effects to understand our customers,
and there has been a notable rise in enforcement activity from supervisory authorities across the EEA in relation to cookies-related
violations.
Compliance with existing,
not yet effective, and proposed privacy and data protection laws and regulations can be costly and can delay or impede our ability to
market and sell our products, affect our ability to conduct business through websites and mobile applications we and our partners may
operate, require us to modify or amend our information practices and policies, change and limit the way we use consumer information in
operating our business, increase our operating costs, or require significant management time and attention. Failure to comply could result
in negative publicity or subject us to inquiries or investigations, claims or other remedies, including significant fines and penalties,
or demands that we modify or cease existing business practices. We may also face civil claims, including representative actions and other
class action type litigation (where individuals have suffered harm), potentially amounting to significant compensation or damages liabilities,
as well as associated costs, and diversion of internal resources. Any of the foregoing could have a material adverse effect on our business,
financial condition and results of operations.
Risks
Related to Conducting Business Internationally
International
sales and operations comprise an increasingly significant portion of our business, which exposes us to foreign operational, political
and other risks that may harm our business.
We
generate an increasing share of our revenue from international sales and maintain international operations, including supply and distribution
chains that are, and will continue to be, an increasingly significant part of our business. Since our growth strategy depends in part
on our ability to penetrate international markets and increase the localization of our products and services, we expect to continue to
increase our sales and presence outside the U.S., particularly in markets we believe to have high-growth potential. The substantial up-front
investment required, the lack of consumer awareness of our products in certain jurisdictions outside of the U.S., differences in consumer
preferences and trends between the U.S. and other jurisdictions, the risk of inadequate intellectual property protections and differences
in packaging, labeling and related laws, rules and regulations are all substantial matters that need to be evaluated prior to doing business
in new jurisdictions, and which make the success of our international efforts uncertain.
Moreover,
our international operations expose us to other risks and uncertainties that are customarily encountered in non-U.S. operations and that
may have a material effect on our results of operations and business as a whole, including:
| ● | local
political and economic instability; |
| | | |
| ● | increased
expense of developing, testing and making localized versions of our products; |
| | | |
| ● | difficulties
in hiring and retaining employees; |
| | | |
| ● | differing
employment practices and laws and labor disruptions; |
| | | |
| ● | pandemics,
such as the COVID-19 pandemic, and natural disasters; |
| | | |
| ● | difficulties
in managing international operations, including any travel restrictions imposed on us or
our customers, such as those imposed in response to the COVID-19 pandemic; |
| | | |
| ● | fluctuations
in currency exchange rates; |
| | | |
| ● | foreign
exchange controls that could make it difficult to repatriate earnings and cash; |
| | | |
| ● | import
and export controls, license requirements and restrictions; |
| | | |
| ● | controlling
production volume and quality of the manufacturing process; |
| | | |
| ● | acts
of terrorism and acts of war; |
| | | |
| ● | general
geopolitical instability and the responses to it, such as the possibility of economic sanctions,
trade restrictions and changes in tariffs (e.g., recent economic sanctions implemented by
the U.S. against China and Russia and tariffs imposed by the U.S. and China); |
| | | |
| ● | interruptions
and limitations in telecommunication services; |
| | | |
| ● | product
or material transportation delays or disruption, including as a result of customs clearance,
violence, protests, police and military actions, or natural disasters; |
| | | |
| ● | risks
of noncompliance by our employees, contractors, or partners or agents with, and burdens of complying with, a wide variety of extraterritorial,
regional and local laws, including competition laws and anti-bribery laws such as the U.S. Foreign Corrupt Practices Act (the “FCPA”)
and the UK Bribery Act 2010, despite our compliance efforts and activities; |
| ● | the
impact of government-led initiatives to encourage the purchase or support of domestic vendors,
which can affect the willingness of customers to purchase products from, or collaborate to
promote interoperability of products with, companies whose headquarters or primary operations
are not domestic; |
| | | |
| ● | an
inability to obtain or maintain adequate intellectual property protection for our brand and
products; |
| | | |
| ● | longer
payment cycles and greater difficulty in accounts receivable collection; |
| | | |
| ● | a
legal system subject to undue influence or corruption; |
| | | |
| ● | a
business culture in which illegal sales practices may be prevalent; and |
| | | |
| ● | potential
adverse tax consequences. |
If
any of the risks outlined above materialize in the future, we could experience production delays and lost or delayed revenues, among
other potential negative consequences that could materially impact our international operations and adversely affect our business as
a whole.
Adverse
economic conditions in the U.S., Europe or any of the other countries in which we may conduct business could negatively affect our business,
financial condition and results of operations.
Consumer spending on
skincare and cosmetic products is influenced by general economic conditions and the availability of discretionary income. Adverse economic
conditions in the U.S., Europe or any of the other jurisdictions in which we do significant business, or periods of inflation or high
energy prices, may contribute to higher unemployment levels, decreased consumer spending, reduced credit availability and declining consumer
confidence and demand, each of which poses a risk to our business. A decrease in consumer spending or in consumer confidence and demand
for our products could have a significant negative impact on our net sales and profitability, including our operating margins and return
on invested capital. These economic conditions could cause some of our retailers or suppliers to experience cash flow or credit problems
and impair their financial condition, which could disrupt our business and adversely affect product orders, payment patterns and default
rates and increase our bad debt expense.
Legal,
political and economic conditions surrounding the exit of the United Kingdom from the E.U. are a source of instability and uncertainty.
On
January 31, 2020, the United Kingdom formally withdrew from the E.U. Uncertainties regarding trade arrangements between the United Kingdom
and the E.U resulting from Brexit could result in increased costs or otherwise adversely impact our operations in the E.U. and the United
Kingdom. We distribute our products to our E.U. and United Kingdom based retailers. Depending on tariffs and trade regulation negotiations,
we may be forced to acquire duplicate arrangements in the E.U. either temporarily or permanently, which may increase our costs in the
E.U. and the United Kingdom.
Further,
following Brexit, the United Kingdom’s data protection framework has become separate and independent from the E.U. In particular,
the United Kingdom has transposed the E.U.’s GDPR into UK domestic law, which imposes separate and additional fines for noncompliance
(i.e., ranging from £8.7 million to £17.5 million or 2% to 4% of total worldwide annual revenue, whichever is higher). Thus,
if a regulatory issue arose in both the E.U. and the United Kingdom (e.g., a personal data breach that affected both E.U. and
United Kingdom data subjects ), then we would be at risk of receiving administrative fines for any noncompliance from supervisory authorities
in the E.U., as well as the UK Information Commissioner’s Office.
In
addition, the longer term economic, legal, political, regulatory and social framework to be put in place between the United Kingdom and
the E.U. following Brexit remains unclear and may continue to have a material and adverse effect on global economic conditions and the
stability of global financial markets and may significantly reduce global market liquidity and restrict the ability of key market participants
to operate in certain financial markets. Any of these factors could depress economic activity and restrict our access to capital, which
could materially and adversely affect our business, financial condition and results of operations.
We are subject to the U.K. Bribery Act,
the U.S. Foreign Corrupt Practices Act and other anti-corruption laws and anti-money laundering laws. Failure to comply with these laws
could subject us to penalties and other adverse consequence.
Our operations are subject
to anti-corruption laws, including the Bribery Act, the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the
U.S. Travel Act, and other anti-corruption laws and anti-money laundering laws that apply in countries where we conduct activities. The
Bribery Act, the FCPA and these other anti-corruption laws generally prohibit us and our employees, agents, representatives, business
partners, and third-party intermediaries from authorizing, promising, offering, or providing, directly or indirectly, improper or prohibited
payments, or anything else of value, to recipients in the public or private sector in order to obtain or retain business or gain some
other business advantage. These laws have been enforced aggressively in recent years and are interpreted broadly. Under the Bribery Act,
we may also be liable for failing to prevent a person associated with us from committing a bribery offense. Additionally, we are required
to comply with all applicable economic and financial sanctions and trade embargoes, and export/import control laws.
We have international
activities and frequently use third parties to sell our products and conduct our business abroad. We, our employees, agents, representatives,
business partners or third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies
or state-owned or affiliated entities. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries,
our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize such activities. While we have
mechanisms to identify high-risk individuals and entities before contracting with them, we will be operating in a number of jurisdictions
that pose a high risk of potential Bribery Act or FCPA violations. We cannot assure you that all of our employees, agents, representatives,
business partners or third-party intermediaries will not take actions that violate applicable law, for which we may be ultimately held
responsible. As we increase our international sales and business, our risks under these laws may increase.
Some of these anti-corruption
laws also require that we keep accurate books and records and maintain internal controls and compliance procedures reasonably designed
to prevent any corrupt conduct. While we will have policies and procedures to address compliance with those laws, we cannot assure you
that none of our employees, agents, representatives, business partners or third-party intermediaries will take actions that violate our
policies and applicable law, for which we may be ultimately held responsible. In addition, we cannot predict the nature, scope or effect
of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be
administered or interpreted.
Any violations of these
anti-corruption laws, or even allegations of such violations, can lead to an investigation and/or enforcement action, which could disrupt
our operations, involve significant management distraction and lead to significant costs and expenses, including legal fees. If we, or
our employees or agents acting on our behalf, are found to have engaged in practices that violate these laws and regulations, we could
suffer severe fines and penalties, profit disgorgement, injunctions on future conduct, securities litigation, bans on transacting government
business, delisting from securities exchanges and other consequences that may have a material adverse effect on our business, financial
condition and results of operations. In addition, our brand and reputation, our sales activities or our stock price could be adversely
affected if we become the subject of any negative publicity related to actual or potential violations of anti-corruption, anti-bribery
or trade control laws and regulations.
Risks
Related to Evolving Laws and Regulations and Compliance with Laws and Regulations
New
laws, regulations, enforcement trends or changes in existing regulations governing the introduction, marketing and sale of our products
to consumers could harm our business.
There
has been an increase in regulatory activity and activism in the U.S. and abroad, and the regulatory landscape is becoming more complex
with increasingly strict requirements. If this trend continues, we may find it necessary to alter some of the ways we have traditionally
manufactured and marketed our products in order to stay in compliance with a changing regulatory landscape, and this could add to the
costs of our operations and have an adverse impact on our business. To the extent federal, state, local or foreign regulatory changes
regarding consumer protection, or the ingredients, claims or safety of our products, occurs in the future, they could require us to reformulate
or discontinue certain of our products, revise the product packaging or labeling, or adjust operations and systems, any of which could
result in, among other things, increased costs, delays in product launches, product returns or recalls and lower net sales, and therefore
could have a material adverse effect on our business, financial condition and results of operations. Noncompliance with applicable regulations
could result in enforcement action by regulatory authorities within or outside the U.S., including, but not limited to, product seizures,
injunctions, product recalls and criminal or civil monetary penalties, all of which could have a material adverse effect on our business,
financial condition and results of operations.
In
the U.S., with the exception of color additives, the FDA does not currently require pre-market approval for products intended to be sold
as cosmetics. However, the FDA may in the future require pre-market approval, clearance or registration/notification of cosmetic products,
establishments or manufacturing facilities. Moreover, such products could also be regulated as both drugs and cosmetics simultaneously,
as the categories are not mutually exclusive. The statutory and regulatory requirements applicable to drugs are extensive and require
significant resources and time to ensure compliance. For example, if any of our products intended to be sold as cosmetics were to be
regulated as drugs, we might be required to conduct, among other things, clinical trials to demonstrate the safety and efficacy of these
products. We may not have sufficient resources to conduct any required clinical trials or to ensure compliance with the manufacturing
requirements applicable to drugs. If the FDA determines that any of our products intended to be sold as cosmetics should be classified
and regulated as drug products and we are unable to comply with applicable drug requirements, we may be unable to continue to market
those products. Any inquiry into the regulatory status of our cosmetics and any related interruption in the marketing and sale of these
products could damage our reputation and image in the marketplace.
In
recent years, the FDA has issued warning letters to several cosmetic companies alleging improper claims regarding their cosmetic products.
If the FDA determines that we have made inappropriate drug claims regarding our products intended to be sold as cosmetics, we could receive
a warning or untitled letter, be required to modify our product claims or take other actions to satisfy the FDA. In addition, plaintiffs’
lawyers have filed class action lawsuits against cosmetic companies after receipt of these types of FDA warning letters. There can be
no assurance that we will not be subject to state and federal government actions or class action lawsuits, which could harm our business,
financial condition and results of operations.
Additional
state and federal requirements may be imposed on consumer products as well as cosmetics, cosmetic ingredients or the labeling and packaging
of products intended for use as cosmetics. For example, several lawmakers are currently focused on giving the FDA additional authority
to regulate cosmetics and their ingredients. This increased authority could require the FDA to impose increased testing and manufacturing
requirements on cosmetic manufacturers or cosmetics or their ingredients before they may be marketed. We are unable to ascertain what,
if any, impact any increased statutory or regulatory requirements may have on our business.
Our
products are also subject to regulation by the CPSC in the U.S. under the provisions of the Consumer Product Safety Act, as amended by
the Consumer Product Safety Improvement Act of 2008. These statutes and the related regulations ban from the market consumer products
that fail to comply with applicable product safety laws, regulations and standards. The CPSC has the authority to require the recall,
repair, replacement or refund of any such banned products or products that otherwise create a substantial risk of injury, and may seek
penalties for regulatory noncompliance under certain circumstances. The CPSC also requires manufacturers of consumer products to report
certain types of information to the CPSC regarding products that fail to comply with applicable regulations. Certain state laws also
address the safety of consumer products, and mandate reporting requirements, and noncompliance may result in penalties or other regulatory
action.
Government
regulations and private party actions relating to the marketing and advertising of our products and services may restrict, inhibit or
delay our ability to sell our products and harm our business, financial condition and results of operations.
Governmental
Authorities regulate advertising and product claims regarding the performance and benefits of our products. These regulatory authorities
typically require a reasonable basis to support any marketing claims. What constitutes a reasonable basis for substantiation can vary
widely from market to market, and there is no assurance that the efforts that we undertake to support our claims will be deemed adequate
for any particular product or claim. A significant area of risk for such activities relates to improper or unsubstantiated claims about
our products and their use or safety. If we are unable to show adequate substantiation for our product claims, or our promotional materials
make claims that exceed the scope of allowed claims for the classification of the specific product, the FDA, the FTC or other regulatory
authorities could take enforcement action or impose penalties, such as monetary consumer redress, requiring us to revise our marketing
materials, amend our claims or stop selling certain products, all of which could harm our business, financial condition and results of
operations. Any regulatory action or penalty could lead to private party actions, or private parties could seek to challenge our claims
even in the absence of formal regulatory actions, which could harm our business, financial condition and results of operations.
Government
regulation of the Internet and e-commerce is evolving, and unfavorable changes or failure by us to comply with these regulations could
substantially harm our business, financial condition and results of operations.
We are subject to general
business regulations and laws as well as regulations and laws specifically governing the Internet and e-commerce. Existing and future
regulations and laws could impede the growth of the Internet, e-commerce or mobile commerce. These regulations and laws may involve taxes,
tariffs, privacy and data security, anti-spam, content protection, electronic contracts and communications, consumer protection, social
media marketing, third-party cookies, web beacons and similar technology for online behavioral advertising and gift cards. It is not
clear how existing laws governing issues such as property ownership, sales and other taxes and consumer privacy apply to the Internet,
as the vast majority of these laws were adopted prior to the advent of the Internet and do not contemplate or address the unique issues
raised by the Internet or e-commerce. It is possible that general business regulations and laws, or those specifically governing the
Internet or e-commerce, may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and may conflict
with other rules or our practices. We cannot be sure that our practices have complied, comply or will comply fully with all such laws
and regulations. Any failure, or perceived failure, by us to comply with any of these laws or regulations could result in damage to our
reputation, a loss in business and proceedings or actions against us by governmental entities or others. Any such proceeding or action
could hurt our reputation, force us to spend significant amounts in defense of these proceedings, distract our management, increase our
costs of doing business, decrease the use of our website by consumers and suppliers and may result in the imposition of monetary liability.
We may also be contractually liable to indemnify and hold harmless third parties from the costs or consequences of noncompliance with
any such laws or regulations. In addition, it is possible that governments of one or more countries may seek to censor content available
on our website or may even attempt to completely block access to our website. Adverse legal or regulatory developments could substantially
harm our business. In particular, in the event that we are restricted, in whole or in part, from operating in one or more countries,
our ability to retain or increase our consumer base may be adversely affected, and we may not be able to maintain or grow our net sales
and expand our business as anticipated. See the section entitled “—Risks Related to Information Technology and Cybersecurity—Our
business is subject to complex and evolving U.S. and foreign laws and regulations regarding privacy and data protection. Many of these
laws and regulations are subject to change and uncertain interpretation, and any failure or perceived failure to comply could result
in claims, changes to our business practices, monetary penalties or increased costs of operations, or otherwise could harm our business”
for a discussion of the risks our business faces related to changes in privacy and data protection laws.
Changes in laws or regulations, or how
such laws or regulations are interpreted or applied, or a failure to comply with any laws and regulations, may adversely affect our business
and results of operations.
Our business is subject
to regulation by various federal, state, local and foreign governments. In certain jurisdictions, these regulatory requirements may be
more stringent than those in the U.S. Noncompliance with applicable regulations or requirements could subject us to investigations, sanctions,
enforcement actions, disgorgement of profits, fines, damages, civil and criminal penalties, injunctions or other collateral consequences.
If any governmental sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, operating
results, and financial condition could be materially adversely affected. In addition, responding to any action will likely result in
a significant diversion of management’s attention and resources and an increase in professional fees. Enforcement actions and sanctions
could harm our business, reputation, operating results and financial condition.
Further, we use a number
of third parties to perform services or act on our behalf. It may be the case that one or more of those third parties may violate applicable
federal, state, local, and international laws, including, but not limited to, those related to corruption, bribery, economic and financial
sanctions and trade embargoes and export/import controls. Despite the significant challenges in asserting and maintaining control and
compliance by these third parties, we may be held fully liable for third parties’ actions as fully as if they were a direct employee
of ours. Such liabilities may create harm to our reputation, inhibit our plans for expansion, or lead to extensive liability either to
private parties or government regulators, which could adversely impact our business, results of operations, and financial condition.
Additionally, Governmental
Authorities regulate advertising and product claims regarding the performance and benefits of our products. These regulatory authorities
typically require a reasonable basis to support any marketing claims. What constitutes a reasonable basis for substantiation can vary
widely from market to market, and there is no assurance that the efforts that we undertake to support our claims will be deemed adequate
for any particular product or claim. A significant area of risk for such activities relates to improper or unsubstantiated claims about
our products and their use or safety. If we are unable to show adequate substantiation for our product claims, or our promotional materials
make claims that exceed the scope of allowed claims for the classification of the specific product, the FDA, the FTC or other regulatory
authorities could take enforcement action or impose penalties, such as monetary consumer redress, requiring us to revise our marketing
materials, amend our claims or stop selling certain products, all of which could harm our business, financial condition and results of
operations. Any regulatory action or penalty could lead to private party actions, or private parties could seek to challenge our claims
even in the absence of formal regulatory actions, which could harm our business, financial condition and results of operations.
General Business Risks and Risks Related
to Our Financial Condition and Operations
We
may make investments into or acquire other companies, which could divert our management’s attention, result in dilution to our
shareholders and otherwise disrupt our operations, and we may have difficulty integrating any such acquisitions successfully or realizing
the anticipated benefits therefrom, any of which could have an adverse effect on our business, financial condition and results of operations.
As
part of our business strategy, we may seek to acquire or invest in businesses that we believe could complement or expand our existing
and future offerings or otherwise offer growth opportunities. The success of any attempts to grow our business through acquisitions to
complement our business depends in part on the availability of, our ability to identify and our ability to engage and pursue suitable
acquisition candidates. We may not be able to find suitable acquisition candidates, and we may not be able to complete acquisitions on
favorable terms, if at all. If we do complete future acquisitions, we cannot assure you that they will ultimately strengthen our competitive
position or that they will be viewed positively by customers, financial markets or investors.
The
pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating
and pursuing suitable acquisitions, whether or not they are consummated, and the costs incurred likely would not be recoverable. In addition,
we have limited experience in acquiring other businesses and may have difficulty integrating acquired businesses or assets, or otherwise
realizing any of the anticipated benefits of acquisitions. If we acquire additional businesses, we may not be able to integrate the acquired
operations and technologies successfully, or effectively manage the combined business following the acquisition. Integration may prove
to be difficult due to the necessity of integrating personnel with disparate business backgrounds, different geographical locations and
who may be accustomed to different corporate cultures. Additionally, with multiple business combinations, we could face additional risks,
including additional burdens and costs with respect to possible multiple negotiations and due diligence investigations (if there are
multiple sellers) and the additional risks associated with the subsequent assimilation of the operations and services or products of
multiple acquired companies with different businesses in a single operating business.
We
also may not achieve the anticipated benefits from any acquired business due to a number of factors, including:
| ● | inability
to integrate or benefit from acquired technologies or services in a profitable manner; |
| | | |
| ● | unanticipated
costs or liabilities, including legal liabilities, associated with any such acquisition; |
| | | |
| ● | difficulty
converting the customers of an acquired business into our current and future offerings; |
| | | |
| ● | diversion
of management’s attention or resources from other business concerns; |
| | | |
| ● | adverse
effects on our existing business relationships with customers, members or strategic partners
as a result of the acquisition; |
| | | |
| ● | complexities
associated with managing the geographic separation of acquired businesses and consolidating
multiple physical locations; |
| | | |
| ● | coordination
of product development and sales and marketing functions; |
| | | |
| ● | the
potential loss of key employees; |
| | | |
| ● | acquisition
targets not having as robust internal controls over financial reporting as would be expected
of a public company; |
| | | |
| ● | becoming
subject to new regulations as a result of an acquisition, including if we acquire a business
serving customers in a regulated industry or acquire a business with customers or operations
in a country in which we do not already operate; |
| | | |
| ● | possible
cash flow interruption or loss of revenue as a result of transitional matters; and |
| | | |
| ● | use
of substantial portions of our available cash to consummate an acquisition. |
We
may issue equity securities or incur indebtedness to pay for any such acquisition or investment, which could adversely affect our business,
financial condition or results of operations. Any such issuances of additional capital stock may cause shareholders to experience significant
dilution of their ownership interests and the per share value of our ordinary shares to decline.
We
may face risks related to companies in the beauty and skincare industries.
We
may be subject to, and possibly adversely affected by, the following risks:
| ● | an
inability to compete effectively in a highly competitive environment with many incumbents
having substantially greater resources, which may give them the ability to spend more aggressively
than us on advertising, promotions and/or marketing activities and have more flexibility
than us to respond to changing business and economic conditions; |
| | | |
| ● | an
inability to manage rapid change, increasing consumer expectations and growth; |
| ● | an
inability to build strong brand identity and improve customer satisfaction and loyalty; |
| | | |
| ● | decreases
in consumer spending or in retailer and consumer confidence and demand for skincare and beauty
products; |
| | | |
| ● | a
reliance on proprietary technology to provide services and to manage our operations, and
the failure of this technology to operate effectively, or our failure to use such technology
effectively; |
| | | |
| ● | an
inability to deal with our customers’ privacy concerns; |
| | | |
| ● | an
inability to attract and retain physician customers for our Obagi products or customers for
our Milk products; |
| | | |
| ● | an
inability to license or enforce intellectual property rights on which our business may depend; |
| | | |
| ● | any
significant disruption in our computer systems or those of third parties that we would utilize
in our operations; |
| | | |
| ● | an
inability by us, or a refusal by third parties, to license content to us upon acceptable
terms; |
| | | |
| ● | potential
liability for negligence, copyright, trademark infringement or state consumer fraud or other
claims based on the nature and content of materials that we may distribute; |
| | | |
| ● | competition
for advertising revenue; |
| | | |
| ● | competition
for the discretionary spending of consumers, which may intensify in part due to the current
inflationary environment, advances in technology and changes in consumer expectations and
behavior; |
| | | |
| ● | competition
for consumer recognition and market share with products that have achieved significant national
and international brand name recognition and consumer loyalty; |
| | | |
| ● | disruption
or failure of our networks, systems or technology as a result of computer viruses, “cyber-attacks,”
misappropriation of data or other malfeasance, as well as outages, natural disasters, terrorist
attacks, accidental releases of information or similar events; |
| | | |
| ● | an
inability to obtain necessary hardware, software and operational support; |
| | | |
| ● | reliance
on third-party manufacturers, distributors, vendors or other service providers; |
| | | |
| ● | changes
in consumer preferences, volatility in the prices of raw materials and competition in the
markets in which we operate; |
| | | |
| ● | increased
and evolving oversight by federal, state, local and/or foreign regulatory authorities, which
may require us to reformulate or discontinue certain products or revise product packaging,
labeling or promotional claims; and |
| | | |
| ● | an
inability to obtain or retain licenses on which our business may depend. |
Any of the foregoing
could have an adverse impact on our operations. Additionally, see the sections entitled “—Risks Related to Our Professional
Skincare Segment: Obagi” and “—Risks Related to Our “Clean” Makeup Segment: Milk” for
a discussion of the risks our Obagi and Milk segments, respectively, face related to companies in their respective industries.
We face intense competition, in some
cases from companies that have significantly greater resources than we do, which could limit our ability to generate sales and/or render
our products obsolete. If we are unable to compete effectively, our results will suffer.
The markets for aesthetic
and therapeutic skin health products and cosmetic brands are highly competitive and we expect the intensity of competition to increase
in the future. We also expect to encounter increased competition as we enter new markets and/or distribution channels, attempt to penetrate
existing markets with new products and expand into new distribution channels. We may not be able to compete effectively in these markets,
may face significant pricing pressure from our competitors and may lose existing market share to our competitors.
Competition in the cosmetics
industry is based on the introduction of new products, pricing of products, quality of products, quality of packaging, brand awareness,
perceived value and quality, innovation, distribution and in-store presence and visibility, promotional activities, advertising, editorials,
e-commerce and mobile commerce initiatives and other activities. We must compete with a high volume of new product introductions and
existing products by diverse companies across several different distribution channels.
Many multinational consumer
companies have greater financial, technical or marketing resources, longer operating histories, greater brand recognition or larger customer
bases than we do and may be able to respond more effectively to changing business and economic conditions than we can. Our competitors
may attempt to gain market share by offering products at prices at or below the prices at which our products are typically offered, including
through the use of large percentage discounts. Competitive pricing may require us to reduce our prices, which would decrease our profitability
or result in lost sales. Our competitors, many of whom have greater resources than we do, may be better able to withstand these price
reductions and lost sales. Our competitors may also leverage their scale for advantageous in-store support at retailers or for advantages
in procuring raw materials or using up capacity at third-party manufacturers or warehouses that we cannot replicate given our size.
Our competitors’
greater financial resources enable them, among other things, to make greater research and development investments and spread their research
and development costs, as well as their marketing and promotional costs, over a broader revenue base. It is also possible that developments
by our competitors could make our products or technologies less competitive or obsolete. The treatment of skin conditions and the enhancement
of the appearance of skin are especially the subjects of active research and development by many potential competitors, including major
pharmaceutical companies and specialized biotechnology firms, such as those listed above, as well as universities and other research
institutions. Competitive advances may also include the potential development of new laser or radio frequency therapies to treat hyperpigmentation
and photodamaged skin. While we intend to expand our technological capabilities to remain competitive, research and development by others
may result in the introduction of new products by competitors that represent substantial improvements over our existing products. If
that occurs, sales of our existing products could decline rapidly. Similarly, if we fail to make sufficient investments in research and
development programs, our current and planned products could be surpassed by more effective or advanced products developed by our competitors.
It is difficult for us
to predict the timing and scale of our competitors’ activities or whether new competitors will emerge in the cosmetics industry
and/or in aesthetic and therapeutic skin health products. For example, in recent years, numerous online, “indie” and influencer-backed
cosmetics companies have emerged and garnered significant followings. In addition, further technological breakthroughs, including new
and enhanced technologies that increase competition in the online retail market, new product offerings by competitors and the strength
and success of our competitors’ marketing programs may impede our growth and the implementation of our business strategy.
Our ability to compete
also depends on the continued strength of our Obagi and Milk brands and products, the success of our marketing, innovation and execution
strategies, the continued diversity of our product offerings, the successful management of new product introductions and innovations,
strong operational execution, including in order fulfillment, and our success in entering new markets and expanding our business in existing
geographies. If we are unable to continue to compete effectively, it could have a material adverse effect on our business, financial
condition and results of operations.
If we fail to manage our inventory effectively,
our results of operations, financial condition and liquidity may be materially and adversely affected.
Our business requires
us to manage a large volume of inventory effectively. We depend on our forecasts of demand for, and popularity of, various products to
make purchase decisions and to manage our inventory of stock-keeping units. Demand for products, however, can change significantly between
the time inventory or components are ordered and the date of sale. Demand may be affected by new product launches, changes in customer
and consumer preferences or spending patterns, changes in product cycles and pricing, product defects and promotions, and our customers
may not purchase products in the quantities that we expect. It may be difficult to accurately forecast demand and determine appropriate
levels of product or components. Our ability to accurately forecast demand may be further hindered in the future as we expand the percentage
of our sales made outside of the U.S. because we depend on our international distributors to provide us with forecasts for demand for
our products in their respective territories.
If we or our distributors
overestimate demand, we may be required to write-off inventories and increase our reserves for product returns. If we or our distributors
underestimate demand, we may not have sufficient inventory of products to ship to our customers. Our Obagi products have expiration dates
that generally range from 24 to 36 months from the date of manufacture. We establish reserves for potentially excess, dated or otherwise
impaired inventories. However, we may not be able to accurately estimate the reserve requirement that will be needed in the future. Although
our estimates are reviewed quarterly for reasonableness, our product return activity could differ significantly from our estimates. Judgment
is required in estimating these reserves and we rely on data from third parties, including, but not limited to, distributor forecasts
and independent market research reports. The actual amounts could be different from our estimates, and differences are accounted for
in the period in which they become known. If we determine that the actual amounts exceed our reserve amounts, we will record a charge
to earnings to approximate the difference. A material reduction in earnings resulting from a charge would have a material adverse effect
on our net income, results of operations and financial condition.
A disruption in our operations could
materially and adversely affect our business.
As a company engaged
in distribution on a global scale, our operations, including those of our third-party suppliers, brokers and delivery service providers,
are subject to the risks inherent in such activities, including industrial accidents, environmental events, strikes and other labor disputes,
disruptions in information systems, product quality control, safety, licensing requirements and other regulatory issues, as well as natural
disasters, pandemics (such as the COVID-19 pandemic), border disputes, acts of terrorism and other external factors over which we and
our third-party suppliers, brokers and delivery service providers have no control. The loss of, or damage to, the manufacturing facilities
or distribution centers of our third-party suppliers, brokers and delivery service providers could materially and adversely affect our
business, financial condition and results of operations.
We depend heavily on
contracted third-party delivery service providers to deliver our products to our distribution facilities and logistics retailers, and
from there to our distributors and retailers. Interruptions to or failures in these delivery services could prevent the timely or successful
delivery of our products.
These interruptions or
failures may be due to unforeseen events that are beyond our control or the control of our third-party delivery service providers, such
as inclement weather, natural disasters or labor unrest. If our products are not delivered on time or are delivered in a damaged state,
customers and retailers may refuse to accept our products and have less confidence in our services.
Our ability to meet the
needs of our customers depends on the proper operation of our third-party distribution facilities, where most of our inventory that is
not in transit is housed. Our insurance coverage may not be sufficient to cover the full extent of any loss or damage to our inventory
or distribution facilities, and any loss, damage or disruption of the facilities, or loss or damage of the inventory stored there, could
materially and adversely affect our business, financial condition and results of operations.
See the sections entitled
“—Risks Related to Our Professional Skincare Segment: Obagi— We are dependent on third parties to manufacture products
for our Obagi segment which entails several risks we would not face if we manufactured the products ourselves” and “—Risks
Related to Our “Clean” Makeup Segment: Milk—We rely on a number of third-party suppliers, distributors and other vendors,
and they may not continue to produce products or provide services that are consistent with our standards or applicable regulatory requirements,
which could harm our brand, cause consumer dissatisfaction and require us to find alternative suppliers of our products” and
““—Risks Related to Our “Clean” Makeup Segment: Milk—We rely heavily on our third-party agency
and direct sales forces to sell our Milk segment products in the U.S. and internationally, and any failure to train and maintain our
third-party agency and direct sales forces could harm our business” for a discussion of Obagi segment’s and Milk segment’s
reliance on third parties.
Our new product introductions may not
be as successful as we anticipate.
We have an established
process for the development, evaluation and validation of our new product concepts. Nonetheless, each new product launch involves risks,
as well as the possibility of unexpected consequences. For example, the acceptance of new product launches and sales to our customers
may not be as high as we anticipate, due to lack of acceptance of the products themselves or their price, or limited effectiveness of
our marketing strategies. In addition, our ability to launch new products may be limited by delays or difficulties affecting the ability
of our suppliers or manufacturers to timely manufacture, distribute and ship new products or displays for new products. Sales of new
products may be affected by inventory management by our customers, and we may experience product shortages. We may also experience a
decrease in sales of certain existing products as a result of newly launched products. Any of these occurrences could delay or impede
our ability to achieve our sales objectives, which could have a material adverse effect on our business, financial condition and results
of operations.
As part of our ongoing
business strategy, we expect we will continue to develop new products, potentially in new categories, expand our Obagi brand further
into the consumer and spa channels, build and execute a global e-commerce platform and expand our presence in key large international
markets. Any expansion into new product categories, channels or markets may prove to be an operational and financial constraint that
inhibits our ability to successfully accomplish such expansion. Our inability to introduce successful products in our traditional categories
or in adjacent categories and channels could limit our future growth and have a material adverse effect on our business, financial condition
and results of operations.
If we fail to generate sufficient cash
flow from our operations, we will be unable to continue to develop and commercialize new products.
Waldencast expects capital
and operating expenditures to increase over the next several years as we expand the infrastructure, distribution channels and our commercialization,
research and development and manufacturing activities of our Obagi and Milk businesses. Waldencast believes that net cash provided by
operating activities and existing cash and cash equivalents, including proceeds received from the Credit Agreement, dated as of June
24, 2022, by and among Waldencast Finco Limited, a wholly-owned subsidiary of Waldencast, Waldencast Partner LP, as the parent guarantor,
the lenders party thereto and JPMorgan Chase Bank, N.A., as administrative agent (the “2022 Credit Agreement”), will be sufficient
to fund our operations for the foreseeable future. However, our present and future funding requirements will depend on many factors,
including, among other things:
| |
● |
the
level of research and development investment required to maintain and improve our competitive position; |
| |
● |
the success of our product
sales and related collections; |
| |
● |
our need or decision
to acquire or license complementary businesses, products or technologies; |
| |
● |
costs relating to the
expansion of our distribution channels; |
| |
● |
costs relating to the
expansion of the sales force, management and operational support; |
| |
● |
competing technological
and market developments; and |
| |
● |
costs relating to changes
in regulatory policies or laws that affect our operations. |
If our net cash provided by operating activities
and existing cash and cash equivalents are not sufficient to fund our operations in the future, Waldencast may need to draw down on the
revolving loan facility under the 2022 Credit Agreement or raise additional funds, and Waldencast cannot be certain that such funds will
be available on acceptable terms when needed, if at all. If Waldencast is required to draw down on the revolving loan facility under
the 2022 Credit Agreement, our ability to in-license new technologies, develop future products or expand the pipeline of our products
could all be negatively impacted, which would have an adverse effect on our ability to grow our business and remain competitive in the
markets in which we operate.
We
are subject to risks related to our dependency on our directors and officers and on key personnel, employees and independent contractors
of Obagi and Milk, including highly skilled technical experts, as well as risks related to attracting, retaining and developing human
capital in a highly competitive market.
Our
operations are dependent upon a relatively small group of individuals and in particular, Michel Brousset and Hind Sebti. We believe that
our success depends on the continued service of our directors and officers, at least until we have completed our initial business combination.
The unexpected loss of the services of one or more of our directors or officers could have a detrimental effect on us.
Additionally, our success
and future growth depend upon the continued services of Obagi’s and Milk’s management teams and other key employees and independent
contractors, including highly skilled technical experts. From time to time, there may be changes in our management team resulting from
the hiring or departure of executives, key employees and independent contractors, which could disrupt our business. The loss of one or
more members of our, Obagi’s or Milk’s management teams, key employees or independent contractors could harm our business,
and we may not be able to find adequate replacements. We may not be able to retain the services of any members of our, Obagi’s
or Milk’s senior management teams, key employees or independent contractors, including highly skilled experts. If we lose the services
of one or more of these individuals, finding a replacement could be difficult, may take an extended period of time and could significantly
impede the achievement of our business objectives. This may have a material adverse effect on our results of operations and financial
condition.
Our success depends on
our continued ability to attract, retain and motivate highly qualified management, business development, sales and marketing, product
development and other personnel. We may have difficulty recruiting and retaining such qualified personnel due to current market conditions
and the existence of many similar competitive job openings. The failure to attract and retain qualified personnel could have a significant
negative impact on our future product sales and business results.
In
addition, prospective and existing employees and independent contractors often consider the value of the equity awards they receive in
connection with their employment. If the perceived value of our equity awards declines, experiences significant volatility or increases
such that prospective employees or independent contractors believe there is limited or less upside to the value of such equity awards,
it may adversely affect our ability to recruit and retain key employees and independent contractors. If we fail to attract new personnel
or fail to retain and motivate our current personnel, our business and future growth prospects would be harmed.
Although
we currently maintain directors’ and officers’ liability insurance coverage, such coverage may not be sufficient to cover
the types or extent of claims or loss that may be incurred or received. Our insurance coverage may not be sufficient to protect us from
any loss now or in the future and we may not be able to successfully claim our losses under our current insurance policy on a timely
basis, or at all. Our inability to obtain and maintain appropriate insurance coverage could cause a substantial business disruption,
adverse reputational impact and regulatory scrutiny.
If
we incur any loss that is not covered by our directors’ and officers’ liability insurance policy, or the compensated amount
is significantly less than our actual loss, our business, financial condition and results of operations could be materially and adversely
affected.
See the sections entitled
“—General Business Risks and Risks Related to Our Financial Condition and Operations—The design, development, manufacture
and sale of our products involve the risk of product liability and other claims by consumers and other third parties, and our insurance
may be insufficient to cover any such claims” and “—General Business Risks and Risks Related to Our Financial
Condition and Operations—We may be required to recall products and may face product liability claims, either of which could result
in unexpected costs and damage our reputation” for a discussion of our liability insurance.
The design, development, manufacture
and sale of our products involve the risk of product liability and other claims by consumers and other third parties, and our insurance
may be insufficient to cover any such claims.
The design, development,
manufacture and sale of our products involves an inherent risk of product liability claims and the associated adverse publicity. We regularly
monitor the use of our products for trends or increases in reports of adverse events or product complaints. In some, but not all, cases,
an increase in adverse event reports may be an indication that there has been a change in a product’s specifications or efficacy.
Such changes could lead to a recall of the product in question or, in some cases, increases in product liability claims related to the
product in question. Although we maintain general liability and product liability insurance, the scope and limits of such insurance may
not be sufficient to cover the types or extent of liability claims or loss, including, without limitation, liability claims or loss relating
to product liability that may be incurred or received. There also may be product liability risks for which we do not maintain or procure
insurance coverage or for which the insurance coverage may not cover or be adequate. If we incur any product liability loss that is not
covered by our general liability or product liability insurance policies, or any net insurance recovery is less than our actual loss,
then there may be a material adverse effect on our business, results of operations, financial condition and cash flows.
We could also be subject
to a variety of other types of claims, proceedings, investigations and litigation initiated by government agencies or third parties.
These include compliance matters, product regulation or safety, taxes, employee benefit plans, employment discrimination, health and
safety, environmental, antitrust, customs, import/export, government contract compliance, financial controls or reporting, intellectual
property, allegations of misrepresentation, false claims or false statements, commercial claims, claims regarding promotion of our products
and services, shareholder derivative suits or other similar matters. Negative publicity, whether accurate or inaccurate, about the efficacy,
safety or side effects of our products or product categories, whether involving us or a competitor, could materially reduce market acceptance
of our products, cause consumers to seek alternatives to our products, result in product withdrawals and cause our stock price to decline.
Negative publicity could also result in an increased number of product liability claims, whether or not these claims have a basis in
scientific fact. Any such claims, proceedings, investigations or litigation, regardless of the merits, might result in substantial costs,
restrictions on product use or sales, or otherwise injure our business. Resulting lawsuits may divert our management from pursuing our
business strategy and may be costly to defend. In addition, if we are held liable in any of these resulting lawsuits, we may incur substantial
liabilities and may be forced to limit or forgo further commercialization of those products.
Although we maintain
general liability and product liability insurance in amounts that we believe are reasonably adequate to insulate us from potential claims,
the scope and limits of such insurance may not be sufficient to cover the types or extent of liability claims or loss, including, without
limitation, liability claims or loss relating to product liability that may be incurred or received. There also may be product liability
risks for which we do not maintain or procure insurance coverage or for which the insurance coverage may not respond. If we incur any
product liability loss that is not covered by our general liability or product liability insurance policies, or any net insurance recovery
is less than our actual loss, our business, financial condition and results of operations could be materially and adversely affected.
In addition, our inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against
potential product liability claims could prevent or inhibit the commercial production and sale of our products, which would adversely
affect our business.
We may be required to recall products
and may face product liability claims, either of which could result in unexpected costs and damage our reputation.
We sell products for
human use. Our products intended for use as cosmetics are not generally subject to pre-market approval or registration processes, so
we cannot rely upon a government safety panel to qualify or approve our products for use. A product may be safe for the general population
when used as directed but could cause an adverse reaction for a person who has a health condition or allergies, or who is taking a prescription
medication. If we discover that any of our products are causing adverse reactions, we could suffer adverse publicity or regulatory/government
sanctions.
Potential product liability
risks may arise from the testing, manufacture and sale of our products, including that the products fail to meet quality or manufacturing
specifications, contain contaminants, include inadequate instructions as to their proper use, include inadequate warnings concerning
side effects and interactions with other substances or for persons with health conditions or allergies, or cause adverse reactions or
side effects. Product liability claims could increase our costs, and adversely affect our business, financial condition and results of
operations. As we continue to offer an increasing number of new products, our product liability risk may increase. It may be necessary
for us to recall products that do not meet approved specifications or because of the side effects resulting from the use of our products,
which would result in adverse publicity, potentially significant costs in connection with the recall and could have a material adverse
effect on our business, financial condition and results of operations.
In addition, plaintiffs
in the past have received substantial damage awards from other cosmetic and drug companies based upon claims for injuries allegedly caused
by the use of their products. Although we currently maintain general liability and product liability insurance, any claims brought against
us may exceed our existing or future insurance policy coverage or limits. In addition, there may be liability risks, including, without
limitation, product liability risks, for which we do not maintain or procure insurance coverage or for which the insurance coverage may
not cover or be adequate. To the extent that any judgment against us that is in excess of our policy limits or is not covered by our
insurance policies, the judgment would have to be paid from our cash reserves, which would reduce our capital resources. In addition,
we may be required to pay higher premiums and accept higher deductibles in order to secure adequate insurance coverage in the future.
Further, we may not have sufficient capital resources to pay a judgment, in which case our creditors could levy against our assets. Any
product liability claim or series of claims brought against us could harm our business significantly, particularly if a claim were to
result in adverse publicity or damage awards that are in excess of our insurance policy limits or not covered, in whole or in part, by
our insurance policies.
We
may be exposed to unknown or contingent liabilities and may be required to subsequently take write-downs or write-offs, restructuring
and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and the
price of our securities, which could cause you to lose some or all of your investment.
We
cannot assure you that the due diligence conducted in relation to Obagi and Milk has identified all material issues or risks associated
with Obagi and Milk, their businesses or the industries in which they compete.
Furthermore,
we cannot assure you that factors outside of Obagi’s, Milk’s and our control will not later arise. As a result of these factors,
we may be exposed to liabilities and incur additional costs and expenses and we may be forced to later write down or write off assets,
restructure our operations, or incur impairment or other charges that could result in our reporting losses. Even if our due diligence
has identified certain risks, unexpected risks may arise and previously known risks may materialize in a manner not consistent with our
preliminary risk analysis. If any of these risks materialize, this could have a material adverse effect on our financial condition and
results of operations and could contribute to negative market perceptions about our securities. Additionally, we have no indemnification
rights against Obagi or Obagi Shareholders under the Obagi Merger Agreement or against Milk or the Milk Members under the Milk Equity
Purchase Agreement.
Accordingly,
our shareholders or warrant holders could suffer a reduction in the value of their securities. Such shareholders and warrant holders
are unlikely to have a remedy for such reduction in value unless they are able to successfully claim that the reduction was due to the
breach by our directors or officers of a duty of care or other fiduciary duty owed to them, or if they are able to successfully bring
a private claim under securities laws that the registration statement or proxy statement/prospectus relating to the Business Combination
contained an actionable material misstatement or material omission.
Our inability to anticipate and respond
to market trends and changes in consumer preferences could adversely affect our financial results.
Our continued success
depends on our ability to anticipate and react in a timely and cost-effective manner to changes in consumer preferences for skincare
and cosmetic products. We must continually work to develop, manufacture and market new products and create product line extensions for
existing and emerging or new distribution channels. The development of new products and line extensions requires significant commitments
of personnel and financial resources, and we cannot assure you that these products will be commercially successful. We reevaluate our
development efforts regularly to assess whether our efforts to develop a particular new product or technology are progressing at a rate
that justifies our continued expenditures. On the basis of these reevaluations, we have abandoned in the past, and may abandon in the
future, our efforts on a particular product or technology. If we fail to take any product or technology from the development stage to
market on a timely basis or fail to gain successful market adoption of such product, we may incur significant expenses without a near-term
or any financial return.
In addition, we must
continually monitor attitudes toward our industry and product lines, as well as where and how consumers shop to satisfy changing consumer
preferences. To remain competitive, we must maintain and enhance the recognition of our brands in existing and new distribution channels,
achieve a favorable mix of products among these channels, successfully manage our inventories, and modernize and refine our approach
as to how and where we market and sell our products. We recognize that consumer preferences cannot be predicted with certainty and can
change rapidly, driven by the use of digital and social media by consumers and the speed by which information and opinions are shared.
If we are unable to anticipate and respond to sudden challenges that we may face in the marketplace, trends in the market for our products
and changing consumer demands and sentiment, our financial results will suffer. In addition, from time to time, sales growth or profitability
may be concentrated in a relatively small number of products, channels or countries. If such a situation persists or a number of our
products, channels or countries fail to perform as expected, there could be a material adverse effect on our business.
In key markets, such
as the U.S., we have seen a shift in consumer preference to the online channel, which accelerated in response to the COVID-19 pandemic.
During this time, we saw our sales becoming increasingly dependent on key e-commerce retailers, which could result in an increased risk
related to the concentration of our customers. A severe, adverse impact on the business operations of our customers could have a corresponding
material adverse effect on us.
Our success depends, in part, on the
quality, efficacy and safety of our products.
Any loss of confidence
on the part of consumers in the ingredients used in our products, whether related to product contamination or product safety or quality
failures, actual or perceived, or inclusion of prohibited ingredients, could tarnish the image of our brand and could cause consumers
to choose other products. Allegations of contamination or other adverse effects on product safety or suitability for use by a particular
consumer, even if untrue, may require us to expend significant time and resources responding to such allegations and could, from time
to time, result in a recall of a product from any or all of the markets in which the affected product was distributed. Any such issues
or recalls could negatively affect our profitability and brand image.
If our products are found
to be, or perceived to be, defective or unsafe, or if they otherwise fail to meet consumers’ expectations, our relationships with
consumers could suffer, the appeal of our brand could be diminished, we may need to recall some of our products and/or become subject
to regulatory action and we could lose sales or market share or become subject to boycotts or liability claims. In addition, third parties
may sell counterfeit versions of some of our products. These counterfeit products may pose safety risks, may fail to meet consumers’
expectations, and may have a negative impact on our business. Any of these outcomes could result in a material adverse effect on our
business, financial condition and results of operations.
Our failure to successfully in-license
or acquire additional products and technologies would impair our ability to grow.
We intend to in-license,
acquire, develop and market new products and technologies. Because we have limited internal research capabilities, our business model
depends in part on our ability to license patents, products and/or technologies from third parties. The success of this strategy also
depends upon our ability and the ability of our third-party formulators to formulate products under such licenses, as well as our ability
to manufacture, market and sell such licensed products.
We may not be able to
successfully identify any new products to in-license, acquire or internally develop. Moreover, negotiating and implementing an economically
viable acquisition is a lengthy and complex process. Other companies, including those with substantially greater financial, marketing
and sales resources, may compete with us for the acquisition of products. We may not be able to acquire or in-license the rights to such
products on terms that we find acceptable, or at all. As a result, our ability to grow our business or increase our profits could be
adversely impacted.
Demand for our products may not increase
as rapidly as we anticipate due to a variety of factors, including a weakness in general economic conditions.
Consumer spending habits
are affected by, among other things, prevailing economic conditions, levels of employment, salaries and wage rates, consumer confidence
and consumer perception of economic conditions. A general slowdown in the U.S. economy and certain international economies, continuing
inflationary pressure on the price of consumer goods, or an uncertain economic outlook would adversely affect consumer spending habits,
which may, among other things, result in reduced consumer spending, including on cosmetics products, which would have a material adverse
effect on our sales and operating results.
The
historical financial results of Obagi and Milk and unaudited pro forma financial information included elsewhere in this prospectus may
not be indicative of our actual financial position or results of operations would have been.
The
historical financial results of Obagi and Milk included in this prospectus do not reflect the financial condition, results of operations
or cash flows each would have achieved as a standalone company during the periods presented or those we will achieve in the future. This
is primarily the result of the following factors: (i) we have incurred and will incur additional ongoing costs as a result of the Business
Combination, including costs related to public company reporting, investor relations and compliance with the Sarbanes-Oxley Act;
and (ii) our capital structure is different from that reflected in Obagi’s and Milk’s historical financial statements. Our
financial condition and future results of operations could be materially different from amounts reflected in its historical financial
statements included elsewhere in this prospectus, so it may be difficult for investors to compare our future results to historical results
or to evaluate its relative performance or trends in its business.
Similarly,
the unaudited pro forma financial information is presented for illustrative purposes only and has been prepared based on a number of
assumptions and does not reflect the costs of any integration activities or cost savings or synergies that may be achieved as a result
of the Business Combination. The unaudited pro forma financial statements are not necessarily indicative of what the combined company’s
balance sheet or statement of operations actually would have been had the Business Combination been completed as of the dates indicated,
nor do they purport to project the future financial position or operating results of the combined company. The actual financial position
and results of operations may differ significantly from the pro forma amounts reflected herein due to a variety of factors. See the section
entitled “Unaudited Pro Forma Condensed Combined Financial Information.”
Our operating results have fluctuated
in the past and we expect our future quarterly and annual operating results to fluctuate for a variety of reasons.
Our quarterly results
of operations have varied in the past and are likely to vary significantly in the future due to a number of factors, many of which are
outside of our control, including:
| |
● |
demand for and market acceptance of our products; |
| |
● |
the development of new competitive products by others; |
| |
● |
changes in regulatory classifications of our products; |
| |
● |
changes in physician or patient acceptance of the use of our physician-dispensed
products; |
| |
● |
changes in treatment practices of physicians who currently prescribe
our products; |
| |
● |
reduced demand for our Obagi products
during the summer months due to variability of patient compliance resulting from travel and other disruptive activities,
particularly during July and August; |
| |
● |
delays between our expenditures to acquire new product lines or
expand into new distribution channels and the generation of revenues from those new products or distribution channels; |
| |
● |
capital investments and expenditures to support strategic initiatives; |
| |
● |
the timing, release and competitiveness of our products; |
| |
● |
increases in the cost of raw materials used to manufacture our products; |
| |
● |
the mix of products that we sell during any time period; |
| |
● |
the amount and timing of operating expenses; |
| |
● |
increased price competition; |
| |
● |
our ability to achieve and sustain a level of liquidity sufficient
to grow and support our business and operations; |
| |
● |
legal costs and settlement expenses associated with litigation and
related reimbursements from insurance carriers, if any; |
| |
● |
changes in the regulatory environment; |
| |
● |
legal costs and potential penalties related to regulatory matters;
and |
| |
● |
adverse changes in the level of economic activity in the U.S. and
other major regions in which we do business, including any economic downturn caused by the COVID-19 pandemic and other factors outside
our control. |
| |
● |
limited visibility into, and difficulty predicting from quarter
to quarter, the level of activity in our customers’ practices; |
| |
● |
changes in the technology or advertising landscape that increase
costs for consumer reach, engagement, acquisition or conversion; |
| |
● |
changes in geographic, channel, or product mix; |
| |
● |
weakness in consumer spending as a result of a slowdown in the global,
U.S. or other economies; |
| |
● |
higher manufacturing costs; |
| |
● |
competition in general and competitive developments in the market; |
| |
● |
changes in relationships with our customers and distributors, including
timing of orders; |
| |
● |
changes in the timing of when revenues are recognized, including
as a result of the timing of receipt of product orders and shipments, the introduction of new products, product offerings or promotions,
modifications to our terms and conditions or as a result of new accounting pronouncements or changes to critical accounting estimates; |
| |
● |
fluctuations in currency exchange rates against the U.S. dollar; |
| |
● |
our inability to scale, suspend or reduce production based on variations
in product demand; |
| |
● |
seasonal fluctuations in demand; |
| |
● |
success of or changes to our marketing programs from quarter to
quarter; |
| |
● |
increased advertising or marketing efforts or aggressive price competition
from competitors; |
| |
● |
changes to our effective tax rate; |
| |
● |
unanticipated delays and disruptions in the manufacturing process
caused by insufficient capacity or availability of raw materials, turnover in the labor force or the introduction of new production
processes, power outages or natural or other disasters beyond our control; |
| |
● |
underutilization of manufacturing facilities; |
| |
● |
major changes in available technology or the preferences of customers
may cause our current product offerings to become less competitive or obsolete; |
| |
● |
costs and expenditures in connection with litigation; |
| |
● |
costs and expenditures in connection with the establishment of treatment
planning and fabrication facilities in international locations; |
| |
● |
costs and expenditures in connection with the hiring and deployment
of direct sales force personnel; |
| |
● |
unanticipated delays in our receipt of customer records for any
reason; |
| |
● |
disruptions to our business due to political, economic or other
social instability or any governmental regulatory or similar actions, including the impact of a pandemic such as the COVID-19 pandemic,
any of which results in changes in consumer spending habits or consumers being unable or unwilling to visit spas, as well as any
impact on workforce absenteeism; |
| |
● |
inaccurate forecasting of net sales, production and other operating
costs; |
| |
● |
investments in research and development to develop new products
and enhancements; and |
| |
● |
timing of industry tradeshows. |
To respond to these and
other factors, we may make business decisions that adversely affect our operating results such as modifications to our pricing policy,
promotions, development efforts, product releases, business structure or operations. Most of our expenses, such as employee compensation,
are relatively fixed in the short term. Moreover, our expense levels are based, in part, on our expectations regarding future revenue
levels. As a result, if our net revenues for a particular period fall below expectations, we may be unable to adjust spending quickly
enough to offset any shortfall in net revenues. Due to these and other factors, we believe that quarter-to-quarter comparisons of our
operating results may not be meaningful. You should not rely on our results for any one quarter as an indication of our future performance.
Due to the factors summarized
above, we do not believe that period-to-period comparisons of our results of operations are necessarily meaningful and should not necessarily
be relied upon to predict future results of operations. In addition, due to the reduced demand for Obagi products during the summer months
as discussed above, Obagi’s results of operations for the third quarter have historically been lower than our results of operations
for the second quarter. While Obagi enjoyed greater revenues in the third quarter of 2021 as compared to the preceding second quarter
due to product launches in July and August of 2021, we generally anticipate to see some seasonality in our product sales. It is also
possible that in future periods, our results of operations will not meet the expectations of investors or analysts, or any published
reports or analyses regarding our company. In that event, the price of our common stock could decline, perhaps substantially.
Public health emergencies, epidemics
or pandemics, such as COVID-19, or other external factors beyond our control, have had, and could in the future have, an adverse impact
on our operations and financial condition. We may experience difficulty sourcing, manufacturing or supplying our products, which would
in turn decrease our momentum and net sales and profit potential.
Public health emergencies,
epidemics or pandemics have had, and could in the future have, an adverse impact on our operations and financial condition. The COVID-19
pandemic has resulted, and other infectious diseases could result, in a widespread health crisis that has, and could continue to, adversely
affect the economies and financial markets worldwide, business operations and the conduct of commerce generally, which could be materially
and adversely affect our business, including the Obagi or Milk segments of our business. The extent of such impact will depend on future
developments, which are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of
COVID-19, the development of a more infectious or lethal mutation of the COVID-19 virus, and the actions to contain COVID-19 or treat
its impact, among others.
The disruptions posed
by COVID-19 have continued, and other matters of global concern may continue, for an extensive period of time, and if our Obagi or Milk
segments is unable to recover from business disruptions due to COVID-19 or other matters of global concern on a timely basis, our financial
condition and results of operations may be materially adversely affected. Although the impact of COVID-19 on Obagi’s manufacturing
and supply chain to date has not been material, temporary or permanent closures of Obagi’s direct and indirect suppliers could
result in adverse effects to its supply chain and has resulted in and could continue to result in adverse effects to Milk’s supply
chain, and other supply chain disruptions could occur, which might adversely affect the ability of Obagi and Milk to procure sufficient
inventory to support customer orders. Our business, including the Obagi and Milk business segments, may also incur additional costs due
to delays caused by COVID-19, which could adversely affect our financial condition and results of operations.
The Obagi segment’s
net revenue was materially and adversely affected in the first two quarters of 2020, as many of physician customers were required to
close their practices for several weeks, which greatly diminished demand for our Obagi products. Although some of these customers have
now established websites to be able to provide products to their patients through online sales, any re-implementation of similar restrictions
could have a material impact on our future net revenue.
In addition, the COVID-19
pandemic has caused our Obagi segment to modify its business practices, including accelerating the launch of a redesigned website to
enable us to sell our own products online, creating an e-commerce platform to enable our physician customers to sell Obagi products online
to their patients, designing a Door-Step Delivery Program for our customers’ patients, and investing in our Helping Hands program
to provide free hand sanitizer to healthcare professionals. We may take further actions as may be required by government authorities
or as we determine are in the best interests of our employees, customers and their patients, any of which may require unanticipated investments
in management time and money.
The impact of COVID-19
on the global supply chain has caused a number of challenges that are in many cases beyond our control. To name a few: availability of
raw materials and components, production and transport delays, loss of productivity in warehousing and shipping, reduction in our customers’
ability to ship from their warehouses to stores, and reductions in stores’ ability to properly offer advice and service and to
supply our products and service our gondolas in the same way that was done prior to COVID-19. This impact instore is particularly challenging
for prestige cosmetics where consumers are paying a premium price in order to be able to access advice and samples among other things
that justify paying more instead of purchasing a product in a self-serve environment
Global
or regional conditions may adversely affect our business.
Consumer spending on
skincare and cosmetic products is influenced by general economic conditions and the availability of discretionary income. Virtually all
of our products are purchased based on consumer choice due to the fact that our products are generally considered cosmetic in nature
and not covered by health insurance policies. As a result, they are typically paid for directly by the customer out of disposable income
and are not subject to reimbursement by third-party payors such as health insurers. A decrease in consumer spending or in consumer confidence
and demand for our products could have a significant negative impact on our net sales and profitability, including our operating margins
and return on invested capital. These economic conditions could cause some of our retailers or suppliers to experience cash flow or credit
problems and impair their financial condition, which could disrupt our business and adversely affect product orders, payment patterns
and default rates and increase our bad debt expense. If consumers reassess their spending choices, the demand for our products
could decline significantly. If demand declines significantly in our major markets, such as North America, Asia and the Middle East,
it would have a material adverse effect on our sales and profitability.
Adverse
changes in global or regional economic conditions periodically occur, including recession or slowing growth, changes, or uncertainty
in fiscal, monetary or trade policy, higher interest rates, tighter credit, inflation, lower capital expenditures by businesses, increases
in unemployment and lower consumer confidence and spending. Adverse changes in economic conditions can harm global business and adversely
affect our business. Such adverse changes could result from geopolitical and security issues, such as armed conflict and civil or military
unrest, political instability, human rights concerns and terrorist activity, catastrophic events such as natural disasters and public
health issues (including the COVID-19 pandemic), supply chain interruptions, new or revised export, import or doing-business regulations,
including trade sanctions and tariffs or other global or regional occurrences.
In
particular, in response to Russia’s invasion of Ukraine, the North Atlantic Treaty Organization deployed additional military forces
to eastern Europe, and the United States, the European Union, and several other countries are imposing far-reaching sanctions and export
control restrictions on Russian entities and individuals, including the removal of certain financial institutions from the Society for
Worldwide Interbank Financial Telecommunication payment system. Although the length and impact of the ongoing military conflict in Ukraine
is highly unpredictable, the conflict could lead to market disruptions, including significant volatility in commodity prices, credit
and capital markets, as well as supply chain interruptions. Additionally, Russian military actions and the resulting sanctions could
adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets, particularly
if current or new sanctions continue for an extended period of time or if geopolitical tensions result in expanded military operations
on a global scale. In addition, the invasion of Ukraine by Russia, and the impact of sanctions against Russia and the potential for retaliatory
acts from Russia, could result in increased cyberattacks against U.S. companies.
Additionally,
tensions between the United States and China have led to increased tariffs and trade restrictions. The United States has imposed economic
sanctions on certain Chinese individuals and entities and restrictions on the export of U.S.-regulated products and technology to certain
Chinese technology companies. These and other global and regional conditions may adversely impact our business.
Any
of the foregoing factors may have the effect of heightening many of the other risks described in the “Risk Factors” section
of this prospectus.
We may not be able to successfully implement
our growth strategy.
Our future growth, profitability
and cash flows depend upon our ability to successfully implement our business strategy, which, in turn, is dependent upon a number of
key initiatives, including our ability to:
| |
● |
predict our growth and manage our financial
investments in additional headcount and operational resources appropriately to deliver our targeted goals
|
| |
● |
grow
awareness, relevance and trial of our brand and products; |
| |
● |
source,
launch and execute our innovation plans with excellence; |
| |
● |
maintain
a regular supply of our core existing products and execute effective go-to-market strategies to grow them; |
| |
● |
maintain and further
strengthen our existing relationships and develop new relationships with physician customers, internal distributors and retail partners
in each market where we operate; |
| |
|
|
| |
● |
Launch innovative new
products that meet the constantly changing preferences of consumers |
| |
● |
maintain and enhance
our reputation as a provider of high-quality products; |
| |
● |
Expand our ecommerce
websites and digital platforms |
| |
● |
maintain the ability
to sell our products within our retail partners and operate and ship from our own platforms without interruption; |
| |
● |
enhance the productivity
of our brand within our points of distribution; |
| |
● |
maintain and enhance
our digital platforms and capabilities; |
| |
● |
execute our go-to-market
strategies effectively; |
| |
● |
protect our key talent
from leaving; |
| |
● |
ensure that we are able
to sell our products with attractive margins that deliver profit; |
| |
● |
achieve our growth targets
with the financial investments outlined in our plans; and |
| |
● |
predict our growth and
manage our financial investments appropriately in order to deliver our targets. |
There can be no assurance
that we can successfully achieve any or all of the above initiatives in the manner or time period that we expect. Further, achieving
these objectives will require investments that may result in short-term cost increases with net sales materializing on a longer-term
horizon and therefore may be dilutive to our earnings. We cannot provide any assurance that we will realize, in full or in part, the
anticipated benefits we expect our strategy will achieve. The failure to realize those benefits could have a material adverse effect
on our business, financial condition and results of operations.
Impairment of our significant intangible assets may reduce
our profitability.
The costs of our goodwill,
acquired product rights, distribution rights, and trademarks are recorded as intangible assets and all, except for goodwill, are amortized
over the period that we expect to benefit from the applicable assets. Under GAAP, we review our goodwill and long-lived asset group for
impairment when events or changes in circumstances indicate the carrying value may not be recoverable. Additionally, goodwill is required
to be tested for impairment at least annually. The qualitative and quantitative analysis used to test goodwill are dependent upon various
assumptions and reflect management’s best estimates. Changes in certain assumptions including revenue growth rates, discount rates,
earnings multiples and future cash flows may cause a change in circumstances indicating that the carrying value of goodwill or the asset
group may be impaired. We may be required to record a significant charge to earnings in the financial statements during the period in
which any impairment of goodwill or asset group is determined.
We evaluate periodically
the recoverability and the amortization period of our intangible assets. Some factors we consider important in assessing whether or not
impairment exists include performance relative to expected historical or projected future operating results, significant changes in the
manner of our use of the assets or the strategy for our overall business, and significant negative industry or economic trends. These
factors, assumptions, and changes in them could result in an impairment of our long-lived assets. Any impairment of our intangible assets
may reduce our profitability and have a material adverse effect on our results of operations and financial condition.
We
have identified a material weakness in our internal control over financial reporting. If we are unable to develop and maintain an effective
system of internal control over financial reporting, we may not be able to accurately report its financial results in a timely manner,
which may adversely affect investor confidence in us and materially and adversely affect our business and operating results.
In
connection with the preparation of our financial statements as of September 30, 2021, we reevaluated the classification of our Class
A ordinary shares subject to possible redemption. After consultation with our advisors and discussion with our independent registered
public accounting firm, our management and our audit committee concluded that the previously issued financial statements as of March
18, 2021, March 31, 2021 and June 30, 2021 and for the periods from January 1, 2021 through March 31, 2021, and the three months and
six months ended June 30, 2021 should be restated to report all Class A ordinary shares subject to possible redemption as temporary equity.
In addition, we identified a deficiency in our accounting for warrants, which resulted in the restatement of our audited opening balance
sheet as of March 18, 2021. We determined that these deficiencies constituted a material weakness in accounting for complex financial
instruments.
A
material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is
a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented, or detected
and corrected on a timely basis. Based on the material weakness as described above, our management has concluded that our internal control
over financial reporting was not effective as of June 30, 2022.
Effective
internal controls are necessary for us to provide reliable financial reports and prevent fraud. We have taken a number of measures to
remediate the material weaknesses, and continue to evaluate steps to remediate the material weakness. However, these remediation measures
may be time consuming and costly and there is no assurance that these initiatives will ultimately have the intended effects. If we are
unable to remediate our material weakness in a timely manner or we identify additional material weaknesses, we may be unable to provide
required financial information in a timely and reliable manner and we may incorrectly report financial information. If our financial
statements are not filed on a timely basis, we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory
authorities. Failure to timely file would cause us to be ineligible to utilize short form registration statements on Form F-3 or Form
F-4, which may impair our ability to obtain capital in a timely fashion, to execute our business strategies or issue shares to effect
an acquisition. If any of these events were to occur, it could have a material adverse effect on our business.
In
addition, the existence of material weaknesses or a significant deficiency in internal control over financial reporting could adversely
affect our reputation or investor perceptions of us, which could have a negative effect on the trading price of our securities.
We
can provide no assurance that the measures we have taken and plans to take in the future will remediate the material weakness identified
or that any additional material weaknesses or restatements of financial results will not arise in the future due to a failure to implement
and maintain adequate internal control over financial reporting. In addition, even if we are successful in strengthening its controls
and procedures, in the future those controls and procedures may not be adequate to prevent or identify irregularities or errors or to
facilitate the fair presentation of its financial statements.
We may face litigation and other risks as
a result of the material weakness in our internal control over financial reporting.
As a result of such material
weakness describe above, and other matters raised or that may in the future be raised by the SEC, we face potential litigation or other
disputes which may include, among others, claims invoking the federal and state securities laws, contractual claims or other claims arising
from the material weakness in our internal control over financial reporting and the preparation of our financial statements. As of the
date of this prospectus, we have no knowledge of any such litigation or dispute. However, we can provide no assurance that such litigation
or dispute will not arise in the future. Any such litigation or dispute, whether successful or not, could have a material adverse effect
on our business, results of operations and financial condition.
Our
warrants are accounted for as liabilities and the changes in value of our warrants could have a material effect on our financial results.
On
April 12, 2021, the Acting Director of the Division of Corporation Finance and Acting Chief Accountant of the SEC together issued a statement
regarding the accounting and reporting considerations for warrants issued by SPACs entitled “Staff Statement on Accounting and
Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”)” (the “SEC
Statement”). Specifically, the SEC Statement focused on certain settlement terms and provisions related to certain tender offers
following a Business Combination, which terms are similar to those contained in the warrant agreement governing our warrants. As a result
of the SEC Statement, we reevaluated the accounting treatment of our 11,500,000 public warrants and 5,933,333 private placement warrants
and determined to classify the warrants as derivative liabilities measured at fair value, with changes in fair value each period reported
in earnings.
As
a result, included on our balance sheet as of June 30, 2022 contained elsewhere in this prospectus are derivative liabilities related
to embedded features contained within our warrants. ASC Topic 815, Derivatives and Hedging (“ASC 815”), provides
for the remeasurement of the fair value of such derivatives at each balance sheet date, with a resulting non-cash gain or loss related
to the change in the fair value being recognized in earnings in the statement of operations. As a result of the recurring fair value
measurement, our financial statements and results of operations may fluctuate quarterly based on factors which are outside of our control.
Due to the recurring fair value measurement, we expect that we will recognize non-cash gains or losses on our warrants each reporting
period and that the amount of such gains or losses could be material.
Foreign
currency exchange rate fluctuations and restrictions on the repatriation of cash could adversely affect our results of operations, financial
position and cash flows.
Our business is exposed
to fluctuations in exchange rates. Although our reporting currency is the U.S. dollar, we operate in different geographical areas and
transact in a range of currencies in addition to the U.S. dollar, such as the British pound, the Canadian dollar, the Chinese yuan and
the E.U. euro. As a result, movements in exchange rates may cause our revenue and expenses to fluctuate, impacting our profitability,
financial position and cash flows. Future business operations and opportunities, including any continued expansion of our business outside
the U.S., may further increase the risk that cash flows resulting from these activities may be adversely affected by changes in currency
exchange rates. In the event we are unable to offset these risks, there may be a material adverse impact on our business and operations.
In appropriate circumstances where we are unable to naturally offset our exposure to these currency risks, we may enter into derivative
transactions to reduce such exposures. Even where we implement hedging strategies to mitigate foreign currency risk, these strategies
might not eliminate our exposure to foreign exchange rate fluctuations and involve costs and risks of their own, such as ongoing management
time and expertise, external costs to implement the strategies and potential accounting implications. Nevertheless, exchange rate fluctuations
may either increase or decrease our revenues and expenses as reported in U.S. dollars. Moreover, foreign governments may restrict transfers
of cash out of the country and control exchange rates. There can be no assurance that we will be able to repatriate earnings generated,
or cash held, by us and our subsidiaries due to exchange control restrictions or the requirements to hold cash locally to meet regulatory
solvency requirements. This could have a material adverse effect on our business, financial condition and results of operations.
Your
rights and responsibilities as a shareholder are governed by Jersey law, which differs in some material respects with respect to the
rights and responsibilities of shareholders of U.S. companies.
We
are organized under the laws of the Bailiwick of Jersey, Channel Islands, a British crown dependency that is an island located off the
coast of Normandy, France. Jersey is not a member of the EU. Jersey legislation regarding companies is largely based on English corporate
law principles. The rights and responsibilities of the holders of our Class A ordinary shares are governed by the Constitutional Document
and by Jersey law, including the provisions of the Jersey Companies Law. These rights and responsibilities differ in some material respects
from the rights and responsibilities of shareholders in U.S. corporations.
In
particular, Jersey law significantly limits the circumstances under which shareholders of companies may bring derivative actions and,
in most cases, only the corporation may be the proper claimant or plaintiff for the purposes of maintaining proceedings in respect of
any wrongful act committed against it. Neither an individual nor any group of shareholders has any right of action in such circumstances.
Jersey law also does not afford appraisal rights to dissenting shareholders in the form typically available to shareholders of a U.S.
corporation. However, there can be no assurance that Jersey law will not change in the future or that it will serve to protect the investors
in a similar fashion afforded under corporate law principles in the U.S., which could adversely affect the rights of investors.
It
may be difficult to enforce a U.S. judgment against us or our directors and officers outside the U.S., or to assert U.S. securities law
claims outside the U.S.
Investors
may have difficulties pursuing an original action brought in a court in a jurisdiction outside the U.S., including Jersey, for liabilities
under the securities laws of the U.S. The U.S. and Jersey currently do not have a treaty providing for the reciprocal recognition and
enforcement of judgments (as opposed to arbitration awards) in civil and commercial matters. Consequently, a final judgment for payment
rendered by any federal or state court in the U.S. based on civil liability, whether or not predicated solely upon U.S. federal securities
laws, would not automatically be recognized and is not directly enforceable in Jersey. Rather, a judgment of a U.S. court constitutes
a cause of action which may be enforced by Jersey courts provided that:
| ● | the
applicable U.S. courts had jurisdiction over the case, as recognized under Jersey law; |
| | | |
| ● | the
judgment is given on the merits and is final, conclusive and non-appealable; |
| ● | the
judgment relates to the payment of a sum of money, not being taxes, fines or similar governmental
penalties; |
| | | |
| ● | the
defendant is not immune under the principles of public international law; |
| | | |
| ● | the
same matters at issue in the case were not previously the subject of a judgment or disposition
in a separate court; |
| | | |
| ● | the
judgment was not obtained by fraud; and |
| | | |
| ● | the
recognition and enforcement of the judgment is not contrary to public policy in Jersey. |
Subject
to the foregoing, investors may be able to enforce in Jersey judgments in civil and commercial matters that have been obtained from U.S.
federal or state courts. However, it is doubtful that an original action based on U.S. federal or state securities laws could be brought
before Jersey courts. In addition, a plaintiff who is not resident in Jersey may be required to provide a security bond in advance to
cover the potential of the expected costs of any case initiated in Jersey.
Our
only material asset is our indirect interest in Waldencast LP, and we are accordingly dependent upon distributions from Waldencast LP
to pay dividends, taxes and other expenses.
We
are a holding company with no material assets other than indirect equity interests in Waldencast LP. As such, we do not have any independent
means of generating revenue or cash flow, and our ability to pay taxes and operating expenses or declare and pay dividends in the future,
if any, will be dependent upon the results of operations and cash flows of Waldencast LP and its subsidiaries. We intend to cause Waldencast
LP to make distributions to its members, including Holdco 1, in an amount at least sufficient to allow for the payment of all applicable
taxes, and to pay the corporate and other overhead expenses of us and Holdco 1. There can be no assurance, however, that Waldencast LP
and its subsidiaries will generate sufficient cash flow to distribute funds to Holdco 1, or that applicable legal and contractual restrictions,
including negative covenants in Waldencast LP’s debt instruments, will permit such distributions. It could materially and adversely
affect our liquidity and financial condition if Waldencast LP is restricted from, or otherwise unable to, distribute sufficient cash
to us.
Any
legal proceedings, investigations or claims against us could be costly and time-consuming to defend and could harm our reputation regardless
of the outcome. In addition, our business and operations could be negatively affected if they become subject to any securities litigation
or shareholder activism, which could cause us to incur significant expense, hinder execution of business and growth strategy and impact
our share price.
We
are and may in the future become subject to legal proceedings, investigations and claims, including claims that arise in the ordinary
course of business, such as claims by customers, claims or investigations brought by regulators or employment claims made by our current
or former employees and independent contractors.
We
are not currently a party to any pending or, to our knowledge, threatened litigation that will have a significant effect on our financial
position or profitability. Any litigation, investigation or claim, whether meritorious or not, could harm our reputation, will increase
our costs and may divert management’s attention, time and resources, which may in turn harm our business, financial condition and
results of operations. Insurance might not cover such claims, might not provide sufficient payments to cover all the costs to resolve
one or more such claims and might not continue to be available on terms acceptable to us. A claim brought against us for which we are
uninsured or underinsured could result in unanticipated costs, potentially harming our business, financial position and results of operations.
In
the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has
often been brought against that company. Shareholder activism, which could take many forms or arise in a variety of situations, as well
as the frequency of lawsuits against Special Purpose Acquisition Company (“SPAC”) sponsors, has been increasing recently,
especially in the context of SPAC business combinations. Volatility in the share price of our Class A ordinary shares or other reasons
may in the future cause us to become the target of securities litigation or shareholder activism. Securities litigation and shareholder
activism, including potential proxy contests, could result in substantial costs and divert management’s and our Board’s attention
and resources from our business. Additionally, such securities litigation and shareholder activism could give rise to perceived uncertainties
as to our future, adversely affect its relationships with suppliers and service providers and make it more difficult to attract and retain
qualified personnel. Also, we may be required to incur significant legal fees and other expenses related to any securities litigation
and activist shareholder matters. Further, our share price could be subject to significant fluctuation or otherwise be adversely affected
by the events, risks and uncertainties of any securities litigation and shareholder activism.
See the sections entitled
and “—Risks Related to Our “Clean” Makeup Segment: Milk—We are involved, and may become involved
in the future, in disputes and other legal or regulatory proceedings, including an ongoing legal proceeding involving our founders, that,
if adversely decided or settled, could materially and adversely affect our business, financial condition and results of operations”
for a discussion of the risks our Milk segment faces related to both potential and ongoing legal proceedings.
Any damage to our reputation or brands
may materially and adversely affect our business, financial condition and results of operations.
We believe that developing
and maintaining our brands is critical and that our financial success is directly dependent on consumer perception of our brands. Furthermore,
the importance of brand recognition may become even greater as competitors offer more products similar to ours.
Many factors, some of
which are beyond our control, are important to maintaining our reputation and brands. These factors include our ability to comply with
ethical, social, product, labor and environmental standards. Any actual or perceived failure in compliance with such standards could
damage our reputation and brands. The growth of our brands depends largely on our ability to provide a high-quality consumer experience,
which in turn depends on our ability to bring innovative, effective products to the market at competitive prices that respond to consumer
demands and preferences. If we are unable to preserve our reputation, enhance our brand recognition or increase positive awareness of
our products, it may be difficult for us to maintain and grow our consumer base, and our business, financial condition and results of
operations may be materially and adversely affected.
Our Milk segment has
relatively low brand awareness among consumers when compared to other cosmetics brands and maintaining and enhancing the recognition
and reputation of our brand is critical to our business and future growth. Many factors, some of which are beyond our control, are important
to maintaining our reputation and brand. These factors include our ability to comply with ethical, social, product, labor and environmental
standards. Any actual or perceived failure in compliance with such standards could damage our reputation and brand.
The growth of our Milk
brand especially depends largely on our ability to provide a high-quality consumer experience, which in turn depends on our ability to
bring innovative products to the market at competitive prices that respond to consumer demands and preferences. Additional factors affecting
our consumer experience include a reliable and user-friendly website interface and mobile applications for our consumers to browse and
purchase products on our e-commerce website. If we are unable to preserve our reputation, enhance our brand recognition or increase positive
awareness of our products and Internet platforms, it may be difficult for us to maintain and grow our consumer base, and our business,
financial condition and results of operations may be materially and adversely affected.
The success of our brands
may also suffer if our marketing plans or product initiatives do not have the desired impact on our brands’ image or our ability
to attract consumers. Further, our brand value could diminish significantly due to a number of factors, including consumer perception
that we have acted in an irresponsible manner, adverse publicity about our products, our failure to maintain the quality of our products,
product contamination, the failure of our products to deliver consistently positive consumer experiences, or the products becoming unavailable
to consumers.
The
obligations associated with being the publicly traded entity in the “Up-C” structure involve significant expenses and will
require significant resources and management attention, which may divert from our business operations.
As
the publicly traded entity in the Up-C structure, we are subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley
Act. The Exchange Act requires the filing of annual, quarterly and current reports with respect to a public company’s
business and financial condition. The Sarbanes-Oxley Act requires, among other things, that a public company establish and
maintain effective internal control over financial reporting. As a result, we will incur significant legal, accounting and other expenses
that Obagi and Milk did not previously incur. Our entire management team and many of our other employees will need to devote substantial
time to compliance, and may not effectively or efficiently manage our transition into a public company. We will bear all of the internal
and external costs of preparing and distributing periodic public reports in compliance with our obligations under the securities laws.
In
addition, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act, and
the related rules and regulations implemented by the SEC and Nasdaq, have increased legal and financial compliance costs and will make
some compliance activities more time-consuming. If our efforts to comply with new laws, regulations and standards differ from the activities
intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings
against us, and our business may be harmed. In the future, it may be more expensive or more difficult for us to obtain director and officer
liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These
factors could also make it more difficult for us to attract and retain qualified people to serve on our Board, our Board committees or
as executive officers.
Risks Related to our Intellectual Property
Our success depends on our ability to
operate our business without infringing, misappropriating or otherwise violating the trademarks, patents, copyrights and other proprietary
rights of third parties.
Our commercial success
depends in part on our ability to operate without infringing, misappropriating or otherwise violating the trademarks, patents, copyrights,
trade secrets and other proprietary rights of others. We cannot be certain that the conduct of our business does not and will not infringe,
misappropriate or otherwise violate such rights. From time to time, we receive allegations of trademark or patent infringement, and third
parties have filed claims against us with allegations of intellectual property infringement. In addition, third parties may involve us
in intellectual property disputes as part of a business model or strategy to gain competitive advantage.
To the extent we gain
greater visibility and market exposure as a public company or otherwise, we may also face a greater risk of being the subject of such
claims and litigation. For these and other reasons, third parties may allege that our products or activities infringe, misappropriate
or otherwise violate their trademark, patent, copyright or other proprietary rights. Defending against allegations and litigation could
be expensive, occupy significant amounts of time, divert management’s attention from other business concerns and have an adverse
impact on our ability to bring products to market. In addition, if we are found to infringe, misappropriate or otherwise violate third-party
trademark, patent, copyright or other proprietary rights, our ability to use our brands to the fullest extent may be limited, we may
need to obtain a license, which may not be available on commercially reasonable terms, or at all, or we may need to redesign or rebrand
our marketing strategies or products, which may not be possible.
If we are unable to protect our proprietary
rights, we may not be able to compete effectively.
Our success depends significantly
on our ability to protect our proprietary rights to the formulas and technologies used for our products. We rely primarily on maintaining
the confidentiality of our trade secrets and the protection of trade secret laws, as well as a combination of patent, copyright, trademark
and trade dress (including common law trademark and trade dress) laws, and nondisclosure, confidentiality and other contractual restrictions,
to protect our proprietary formulas and technologies. However, these legal means afford only limited protection and may not adequately
protect our rights or permit us to gain or keep any competitive advantage. For example, our trade secrets may be misappropriated by current
or former employees, contractors or parties with whom we partner, or may be inadvertently disclosed or obtained by breach of a confidentiality
agreement or other confidentiality obligation. Although we have taken steps to protect our intellectual property and proprietary formulas
and technologies, including entering into confidentiality agreements and intellectual property assignment agreements with our employees,
consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other
proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. Further, the parties with
whom we enter into confidentiality and intellectual property assignment agreements could dispute the ownership of intellectual property
developed under these agreements. In addition, the laws of some foreign countries may not protect our intellectual property rights to
the same extent as the laws of the U.S.
Risks
Related to Ownership of our Class A ordinary shares and warrants
The
price of our Class A ordinary shares and warrants may be volatile.
The
price of our Class A ordinary shares, as well as our warrants, may fluctuate due to a variety of factors, including:
| ● | changes
in the industries in which we and our customers operate; |
| | | |
| ● | developments
involving our competitors; |
| | | |
| ● | changes
in laws and regulations affecting its business; |
| | | |
| ● | variations
in its operating performance and the performance of its competitors in general; |
| | | |
| ● | actual
or anticipated fluctuations in our quarterly or annual operating results; |
| | | |
| ● | publication
of research reports by securities analysts about us or our competitors or our industry; |
| | | |
| ● | the
public’s reaction to our press releases, our other public announcements and our filings
with the SEC; |
| | | |
| ● | actions
by stockholders, including the sale by Beauty Ventures of any of their shares of our common
stock; |
| | | |
| ● | additions
and departures of key personnel; |
| | | |
| ● | commencement
of, or involvement in, litigation involving the combined company; |
| | | |
| ● | changes
in its capital structure, such as future issuances of securities or the incurrence of additional
debt; |
| |
● |
the volume of our ordinary shares available for
public sale, including sales pursuant to this prospectus, or the perception that such sales could occur; and |
| ● | general
economic and political conditions, such as the effects of the COVID-19 outbreak, recessions,
interest rates, local and national elections, fuel prices, international currency fluctuations,
corruption, political instability and acts of war or terrorism. |
These
market and industry factors may materially reduce the market price of our Class A ordinary shares and warrants regardless of our operating
performance.
We
may issue additional Class A ordinary shares or other equity securities without your approval, which would dilute your ownership interests
and may depress the market price of our Class A ordinary shares and warrants.
Our
directors, employees and consultants and our subsidiaries may be granted equity awards under the Waldencast 2022 Incentive Award Plan.
You will experience additional dilution when those equity awards and purchase rights become vested and settled or exercisable, as applicable,
for our Class A ordinary shares.
Further,
we may issue Class A ordinary shares or other equity securities of equal or senior rank in the future in connection with, among
other things, future acquisitions or repayment of outstanding indebtedness, without shareholder approval, in a number of circumstances.
The
issuance of additional Class A ordinary shares or other equity securities will significantly dilute the equity interests of existing
holders of our securities and may adversely affect prevailing market prices for our Class A ordinary shares or warrants.
Warrants
will be exercisable for our Class A ordinary shares, which would increase the number of shares eligible for future resale in the public
market and result in dilution to our shareholders, including our non-redeeming shareholders.
Outstanding warrants
to purchase an aggregate of 29,533,282 Class A ordinary shares will become exercisable in accordance with the terms of the Warrant Agreement
governing those securities. These warrants will become exercisable at any time commencing on the later of 30 days after the completion
of the Business Combination and 12 months from the closing of our public offering. The exercise price of these warrants will be $11.50
per share. To the extent such warrants are exercised, additional Class A ordinary shares will be issued, which will result in dilution
to our existing Class A ordinary shareholders and increase the number of shares eligible for resale in the public market. Sales of substantial
numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of
our Class A ordinary shares. However, there is no guarantee that the public warrants will ever be in the money prior to their expiration,
and as such, the warrants may expire worthless. The trading price of our Class A ordinary shares is currently below the $11.50 exercise
price. For so long as the warrants remain “out-of-the money,” we do not expect warrant holders to exercise their warrants.
In
addition, our shareholders who exercise redemption rights with respect to their public shares will retain their public warrants, which
may be exercised by such redeeming shareholders for our Class A ordinary shares. Such exercises of our warrants held by redeeming shareholders
will result in dilution to our non-redeeming shareholders.
We
may amend the terms of the warrants in a manner that may be adverse to holders with the approval by the holders of at least 65% of the
then outstanding public warrants.
The
warrants were issued in registered form under the Warrant Agreement between the Warrant Agent and Waldencast. The Warrant Agreement provides
that (a) the terms of the warrants may be amended without the consent of any holder for the purpose of (i) curing any ambiguity or correct
any mistake, including to conform the provisions of the warrant agreement to the description of the terms of the warrants and the warrant
agreement set forth in this prospectus, or defective provision or (ii) adding or changing any provisions with respect to matters or questions
arising under the Warrant Agreement as the parties to the warrant agreement may deem necessary or desirable and that the parties deem
to not adversely affect the rights of the registered holders of the warrants under the warrant agreement and (b) all other modifications
or amendments require the vote or written consent of at least 65% of the then outstanding public warrants; provided that any amendment
that solely affects the terms of the private placement warrants or any provision of the warrant agreement solely with respect to the
private placement warrants will also require at least 65% of the then outstanding private placement warrants.
Accordingly,
we may amend the terms of the public warrants in a manner adverse to a holder if holders of at least 65% of the then outstanding public
warrants approve of such amendment. Although our ability to amend the terms of the public warrants with the consent of at least 65% of
the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the
exercise price of the warrants, shorten the exercise period or decrease the number of our Class A ordinary shares purchasable upon exercise
of a warrant.
We
may redeem your unexpired warrants prior to their exercise at a time that is disadvantageous to you, thereby making your warrants worthless.
We
have the ability to redeem the outstanding warrants at any time after they become exercisable and prior to their expiration, at a price
of $0.01 per warrant if, among other things, the last reported sale price of our Class A ordinary shares for any 20 trading days within
a 30-trading day period ending on the third trading day prior to the date on which we send the notice of redemption to the warrant holders
equals or exceeds $18.00 per share (as adjusted for share splits, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations
and the like). Redemption of the outstanding warrants as described above could force you to: (1) exercise your warrants and pay the exercise
price therefor at a time when it may be disadvantageous for you to do so; (2) sell your warrants at the then current market price when
you might otherwise wish to hold your warrants; or (3) accept the nominal redemption price which, at the time the outstanding warrants
are called for redemption, we expect would be substantially less than the market value of your warrants. None of the private placement
warrants will be redeemable by us (subject to limited exceptions) so long as they are held by the Sponsor or its permitted transferees.
In
addition, we have the ability to redeem the outstanding warrants at any time after they become exercisable and prior to their expiration,
at a price of $0.10 per warrant if, among other things, the last reported sale price of our Class A ordinary shares for any 20 trading
days within a 30-trading day period ending on the third trading day prior to the date on which we send the notice of redemption to the
warrant holders equals or exceeds $10.00 per share (as adjusted for share splits, share dividends, rights issuances, subdivisions, reorganizations,
recapitalizations and the like). In such a case, the holders will be able to exercise their warrants prior to redemption for a number
of our Class A ordinary shares determined based on the redemption date and the fair market value of our Class A ordinary shares. The
value received upon exercise of the warrants (1) may be less than the value the holders would have received if they had exercised their
warrants at a later time where the underlying share price is higher and (2) may not compensate the holders for the value of the warrants,
including because the number of ordinary shares received is capped at 0.361 Class A ordinary shares per warrant (subject to adjustment)
irrespective of the remaining life of the warrants.
We
currently intend to retain our future earnings, if any, to finance the further development and expansion of its business and do not intend
to pay cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board and
will depend on our financial condition, results of operations, capital requirements and future agreements and financing instruments,
business prospects and such other factors as our Board deems relevant.
There
is no guarantee that the warrants will ever be in the money, and they may expire worthless.
The exercise price for
the warrants is $11.50 per Class A ordinary share. Trading prices of the Class A ordinary shares during the past month have not exceeded
the $11.50 threshold for the warrants to be in-the-money. We believe the likelihood that warrant holders will exercise their warrants
is dependent upon the trading price of our Class A ordinary shares, which is currently below the $11.50 exercise price. For so long as
the warrants remain “out-of-the money,” we do not expect warrant holders to exercise their warrants. There is no guarantee
that the warrants will ever be in the money prior to their expiration, and as such, the warrants may expire worthless.
We
do not intend to pay cash dividends for the foreseeable future.
We
currently intend to retain our future earnings, if any, to finance the further development and expansion of our business and do not intend
to pay cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board and
will depend on our financial condition, results of operations, capital requirements, restrictions contained in any future agreements
and financing instruments, business prospects and such other factors as our Board deems relevant.
If
analysts do not publish research about our business or if they publish inaccurate or unfavorable research, our stock price and trading
volume could decline.
The
trading market for our Class A ordinary shares and warrants will depend in part on the research and reports that analysts publish about
our business. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade our securities or
publish inaccurate or unfavorable research about our business, the price of our Class A ordinary shares and/or warrants could decline.
If few analysts cover us, the demand for our securities could decrease and our stock price and trading volume may decline. Similar results
may occur if one or more of these analysts stop covering us in the future or fail to publish reports on us regularly.
A
market for our securities may not develop or be sustained, which would adversely affect the liquidity and price of our securities.
An
active trading market for our securities may never develop or, if developed, may not be sustained, which would have an adverse effect
on the price and trading volume of our securities. You may be unable to sell your securities unless a market can be established and sustained.
Members of the Sponsor, their affiliates
and Cedarwalk will continue to have significant ownership of us after the Business Combination.
Immediately following the
consummation of the Business Transaction, (a) members of the Sponsor and their affiliates owned a combined ownership interest of 40.8%,
comprised of the following: (i) Burwell held an ownership interest of 8.1% of the fully diluted Class A ordinary shares, (ii) Dynamo Master
Fund held an ownership interest of 13.5% of the fully diluted Class A ordinary shares, (iii) Waldencast Ventures held an ownership interest
of 3.5% of the fully diluted Class A ordinary shares, and (iv) Beauty FPA Investor held an ownership interest of 15.7% of the fully diluted
Class A ordinary shares; and (b) Cedarwalk held an ownership interest of 24.5% of the fully diluted Class A ordinary shares.
As a result of such ownership,
members of the Sponsor, their affiliates and Cedarwalk exercise significant influence over all matters requiring shareholder approval,
including the election and removal of directors, appointment and removal of officers, any amendment of our Constitutional Document, and
any approval of significant corporate transactions. Additionally, the Sponsor, their affiliates and Cedarwalk’s interests may differ
from those of other shareholders. As a result, the concentration of voting power with members of the Sponsor, their affiliates and Cedarwalk
may have an adverse effect on the price of our securities.
Future resales of our Class A ordinary
shares, including the shares being registered for resale pursuant to this prospectus, may cause the market price of our securities to
drop significantly, even if our business is doing well.
Subject to certain exceptions,
(i) the initial shareholders, subject to the Letter Agreement and (ii) Burwell and Dynamo Master Fund, subject to the Sponsor Forward
Purchase Agreement, agreed not to transfer, assign or sell any of their founder shares and forward purchase agreement securities, as applicable,
until the earlier to occur of: (A) one year after the completion of our initial business combination; and (B) subsequent to our initial
business combination (x) if the last reported sale price of our Class A ordinary shares equals or exceeds $12.00 per share (as adjusted
for share sub-divisions, share dividends, rights issuances, consolidations, reorganizations, recapitalizations and other similar transactions)
for any 20 trading days within any 30-trading day period commencing at least 150 days after our initial business combination or (y) the
date on which we complete a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of our
public shareholders having the right to exchange their ordinary shares for cash, securities or other property (except with respect to
permitted transferees).
Further,
certain of the Obagi Shareholders and certain of the Milk Members entered into Lock-Up Agreements pursuant to which they are contractually
restricted from selling or transferring any of their shares of our securities during the respective Lock-Up Period (as defined below),
(I) in the case of any Class A ordinary shares and the Waldencast LP Common Units, as applicable, received as consideration in connection
with the Business Combination, until the earlier of (A) one year after the Closing Date and (B) (x) if the last reported sale price of
our Class A ordinary shares equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share dividends, rights issuances,
reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days
after the date of the Closing Date or (y) the date on which we complete a liquidation, merger, share exchange, reorganization or other
similar transaction that results in all of our shareholders having the right to exchange their Class A ordinary shares for cash, securities
or other property and (II) that certain portion of the Obagi Cash Consideration (as defined in the Obagi Merger Agreement) or the Milk
Cash Consideration (as defined in the Milk Equity Purchase Agreement) paid in equity of Waldencast as a result of the occurrence of certain
events set forth in the Obagi Merger Agreement and the Milk Equity Purchase Agreement, as applicable, such equity of Waldencast received
by Obagi or Milk for the same period as set forth in clause (I) above, provided that solely for the purpose of this clause (II), the
term “one-year” in clause (I)(A) shall be replaced with the term “six months.”
However,
following the expiration of each Lock-Up Period, the applicable shareholders will not be restricted from selling our Class A ordinary
shares held by them, other than by applicable securities laws. Additionally, the PIPE Investors are not restricted from selling any of
their Class A ordinary shares, other than by applicable securities laws. As such, sales of a substantial number of our Class A ordinary
shares in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number
of shares intend to sell shares, could reduce the market price of our Class A ordinary shares.
As
restrictions on resale end and registration statements are available for use, the sale or possibility of sale of these shares could have
the effect of increasing the volatility in our share price or the market price of our Class A ordinary shares could decline if the holders
of currently restricted shares sell them or are perceived by the market as intending to sell them.
Sales of our Class A ordinary shares,
or the perception of such sales, including by the Selling Holders pursuant to this prospectus, in the public market or otherwise could
cause the market price for our Class A ordinary shares to decline and certain of the Selling Holders may still receive significant proceeds.
The sale of our Class
A ordinary shares in the public market or otherwise, including sales pursuant to this prospectus, or the perception that such sales could
occur, could harm the prevailing market price of our Class A ordinary shares. These sales, or the possibility that these sales may occur,
also might make it more difficult for us to sell equity securities in the future at a time and at a price that it deems appropriate.
Burwell, Dynamo Master Fund and Waldencast Ventures LP, Cedarwalk and certain Milk Members that collectively beneficially own in excess
of 65% of the Company’s outstanding shares in the aggregate will be able to resell their shares for so long as the registration
statement of which this prospectus forms a part is available for use (following the expiration of the applicable lock-up periods). Resales
of our Class A ordinary shares may cause the market price of our securities to drop significantly, regardless of the performance of our
business.
The Class A ordinary
shares being offered for resale under this prospectus, assuming the exercise in full of the Company’s public and private placement
warrants, would represent approximately 82.5% of shares outstanding of the Company, based on the outstanding shares of the Company as
of July 27, 2022 and after giving effect to all such issuances. Of such shares, approximately 70.0% of shares outstanding of the Company
as of July 27, 2022 (after giving effect to the issuance of shares upon exercise of outstanding public warrants and private placement
warrants), are subject to Lock-Up agreements pursuant to which certain of the Selling Holders have agreed not to transfer, assign or
sell during the respective Lock-Up Periods, which range from six months to twelve months from the closing of the Business Combination
on June 27, 2022, subject to certain exceptions. Nonetheless, given the substantial number of Class A ordinary shares being registered
for potential resale by the Selling Holders pursuant to this prospectus, the sale of shares by various selling securityholders, or the
perception in the market that the shareholders of a large number of shares intend to sell shares, could increase the volatility of the
market price of our Class A ordinary shares or result in a significant decline in the public trading price of our Class A ordinary shares.
See “—Risks Related to Ownership of our Class A ordinary shares and warrants— Future resales of our Class A ordinary
shares, including the shares being registered for resale pursuant to this prospectus, may cause the market price of our securities to
drop significantly, even if our business is doing well.”
As of the date of this prospectus,
the market price of our Class A ordinary shares is below $10.00 per share, which was (i) the price per Legacy Unit sold in the initial
public offering, (ii) the per share price of the 11,800,000 Class A ordinary shares issued in the PIPE Investments, (iii) the per Forward
Purchase Security price of the 33,300,000 Class A ordinary shares and 11,100,000 warrants issued pursuant the Forward Purchase Agreements,
and (iv) the per share value of the consideration issued pursuant to the Obagi Merger Agreement and the per unit value of the consideration
issued pursuant to the Milk Equity Purchase Agreement, respectively. However, certain of our Selling Holders hold Class A ordinary shares
that were originally purchased by the Sponsor in a private placement prior to the initial public offering at prices that may be significantly
below the trading price of our Class A ordinary shares and the sale of which would result in such Selling Holders realizing a significant
gain. These shares represent 5.9% of the total number of shares outstanding as of July 27, 2022. Even if our trading price is significantly
below $10.00, such holders, including Burwell, Dynamo Master Fund and Waldencast Ventures LP, may still have an incentive to sell our
Class A ordinary shares because they hold founder shares that were originally purchased by the Sponsor at prices lower than the public
investors or the current trading price of our Class A ordinary shares. For example, based on the closing price of our Class A ordinary
shares of $8.22 as of October 12, 2022, the holders of the 8,625,000 Class A ordinary shares converted from the founder shares would experience
a potential profit of up to approximately $8.22 per share, or up to approximately $70.9 million in the aggregate. Public holders of our
Class A ordinary shares may not experience a similar rate of return on their shares.
We
are currently an emerging growth company within the meaning of the Securities Act, and to the extent we have taken advantage of
certain exemptions from disclosure requirements available to emerging growth companies, this could make our securities less attractive
to investors and may make it more difficult to compare our performance with other public companies.
We
are currently an “emerging growth company” within the meaning of the Securities Act, as modified by the JOBS Act,
and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that
are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements
of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and
proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder
approval of any golden parachute payments not previously approved. As a result, our shareholders may not have access to certain information
they may deem important. We cannot predict whether investors will find our securities less attractive because we will rely on these exemptions.
If some investors find our securities less attractive as a result of our reliance on these exemptions, the trading prices of our securities
may be lower than they otherwise would be, there may be a less active trading market for our securities and the trading prices of our
securities may be more volatile.
Further,
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting
standards until private companies (that is, those that have not had a Securities Act registration statement declared effective
or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial
accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply
with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. We have elected not
to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application
dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies
adopt the new or revised standard. This may make comparison of our financial statements with another public company, which is neither
an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible
because of the potential differences in accounting standards used.
If
we cease to be an emerging growth company, we will no longer be able to take advantage of certain exemptions from reporting, and, absent
other exemptions or relief available from the SEC, we will also be required to comply with the auditor attestation requirements of Section
404 of the Sarbanes-Oxley Act. We will incur additional expenses in connection with such compliance and our management will need to devote
additional time and effort to implement and comply with such requirements.
As
a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information
with the SEC than a U.S. company. This may limit the information available to holders of the ordinary shares.
We
are a foreign private issuer, as such term is defined in Rule 405 under the Securities Act, however, under Rule 405, the
determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed
second fiscal quarter and, accordingly, the next determination will be made with respect to us on June 30, 2023.
As
a foreign private issuer, we are not subject to all of the disclosure requirements applicable to public companies organized within the
U.S. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural
requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange
Act, including the U.S. proxy rules under Section 14 of the Exchange Act (including the requirement applicable to emerging growth companies
to disclose the compensation of our Chief Executive Officer and the other two most highly compensated executive officers on an individual,
rather than an aggregate, basis). In addition, our officers and directors are exempt from the reporting and “short-swing”
profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities.
Moreover, while we submit quarterly interim consolidated financial data to the SEC under cover of the SEC’s Form 6-K, we are not
required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies and are
not required to file quarterly reports on Form 10-Q or current reports on Form 6-K under the Exchange Act. We also are exempt from
the requirements to obtain shareholder approval for certain issuances of securities, including shareholder approval of share option plans.
In
addition, as a foreign private issuer, we are exempt from the provisions of Regulation FD, which prohibits issuers from making selective
disclosure of material nonpublic information.
Furthermore,
our ordinary shares are not listed and we do not currently intend to list our ordinary shares on any market in the Bailiwick of Jersey,
our home country. As a result, we are not subject to the reporting and other requirements of companies listed in the Bailiwick of Jersey.
For instance, we are not required to publish quarterly or semi-annual financial statements. Accordingly, there may be less publicly available
information concerning our business than there would be if we were a U.S. public company and you may not be afforded the same protections
or information that would be made available to you were you investing in a U.S. domestic issuer.
As
a foreign private issuer, we are permitted to rely on exemptions from certain stock exchange corporate governance standards. As a result,
our shareholders may be afforded less protection than shareholders of companies that are subject to all corporate governance requirements
of the Exchange Act and U.S. stock exchanges.
As
a foreign private issuer, we have the option to follow certain home country corporate governance practices rather than those of the U.S.
stock exchanges, provided that we disclose the requirements we are not following and describe the home country practices we are following.
Any
foreign private issuer exemptions we avail ourselves of in the future may reduce the scope of information and protection to which you
are otherwise entitled as an investor. As a result, there may be less publicly available information concerning our business than there
would be if we were a U.S. public company and you may not be afforded the same protections or information that would be made available
to you were you investing in a U.S. domestic issuer.
We
may lose our foreign private issuer status in the future, which could result in significant additional cost and expense.
In
order to maintain our current status as a foreign private issuer, either (a) more than 50% of our outstanding voting securities must
be either directly or indirectly owned of record by non-residents of the U.S. or (b)(i) a majority of our executive officers or directors
may not be U.S. citizens or residents, (ii) more than 50% of our assets cannot be located in the U.S. and (iii) our business must be
administered principally outside the U.S. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign
private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as a
U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports
and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available
to a foreign private issuer. For example, the annual report on Form 10-K requires domestic issuers to disclose executive compensation
information on an individual basis with specific disclosure regarding the domestic compensation philosophy, objectives, annual total
compensation (base salary, bonus, and equity compensation) and potential payments in connection with change in control, retirement, death
or disability, while the annual report on Form 20-F permits foreign private issuers to disclose compensation information on an aggregate
basis. We would also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors, and principal shareholders
will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. We may also be required
to modify certain of our policies to comply with good governance practices associated with U.S. domestic issuers. The additional requirements
that we would become subject to and any modification of our policies if we were to lose our foreign private issuer status could lead
us to incur significant additional legal, accounting and other expenses. In addition, we may lose our ability to rely upon exemptions
from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
Jersey
law and our Constitutional Document contain certain provisions, including anti-takeover provisions that limit the ability of stockholders
to take certain actions and could delay or discourage takeover attempts that stockholders may consider favorable.
The
Constitutional Document and Jersey law contain provisions that could have the effect of rendering more difficult, delaying, or preventing
an acquisition that shareholders may consider favorable, including transactions in which shareholders might otherwise receive a premium
for their shares. These provisions could also limit the price that investors might be willing to pay in the future for our ordinary shares,
and therefore depress the trading price of our Class A ordinary shares. These provisions could also make it difficult for shareholders
to take certain actions, including electing directors who are not nominated by the current members of our Board or taking other corporate
actions, including effecting changes in our management. Among other things, the Constitutional Document includes provisions regarding:
| ● | providing
for a classified board of directors with staggered, three-year terms; |
| | | |
| ● | the
ability of our Board to issue shares of preferred stock, and to determine the price and other
terms of those shares, including preferences and voting rights, without stockholder approval,
which could be used to significantly dilute the ownership of a hostile acquirer; |
| | | |
| ● | our
Board will have the exclusive right to elect directors to fill a vacancy created by the expansion
of our Board or the resignation, death or removal of a director, which will prevent shareholders
from being able to fill vacancies on our Board; and |
| | | |
| ● | the
limitation of the liability of, and the indemnification of, our directors and officers. |
These
provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our Board or management.
Our
Constitutional Document limits liability of our non-employee directors, Cedarwalk and the Sponsor and their respective affiliates and
representatives’ liability to us for breach of fiduciary duty and could also prevent us from benefiting from corporate opportunities
that might otherwise have been available to us.
Our
Constitutional Document provides that, to the fullest extent permitted by law, and other than corporate opportunities that are expressly
presented to one of our directors or officers in his or her capacity as such, our non-employee directors, Cedarwalk and the Sponsor and
their respective affiliates and representatives:
| ● | will
not have any fiduciary duty to refrain from (i) engaging in and possessing interests in other
business ventures of every type and description, including those engaged in the same or similar
business activities or lines of business in which we or any of our subsidiaries now engages
or proposes to engage or (ii) competing with us or any of our affiliates, subsidiaries or
representatives, on its own account, or in partnership with, or as an employee, officer,
director or shareholder of any other Person (other than us or any of our subsidiaries); |
| ● | will have no duty to communicate or present such transaction
or matter to us or any of our subsidiaries, as the case may be; and |
| ● | will not be liable to us or our shareholders or to any of
our subsidiaries for breach of any duty (fiduciary, contractual or otherwise) as a shareholder or director of us by reason of the fact
that such Person, directly or indirectly, pursues or acquires such opportunity for itself, herself or himself, directs such opportunity
to another Person or does not present such opportunity to us or any of our subsidiaries, affiliates or representatives. |
Risks Related to Taxation
Any disparity between
the U.S. corporate tax rate and the U.S. tax rate applicable to non-corporate Members of Waldencast LP may complicate our ability to maintain
its intended capital structure, which could impose transaction costs on it and require management attention.
Waldencast LP is treated
as a partnership for U.S. federal income tax purposes and, as such, generally is not subject to U.S. federal income tax. Instead, its
taxable income is generally allocated to its members, including Holdco 1. If and when Waldencast LP generates taxable income, it will
generally make cash distributions, or tax distributions, to each of its members, including Holdco 1, based on each member’s allocable
share of net taxable income (calculated under certain assumptions) multiplied by an assumed tax rate. The assumed tax rate for this purpose
will be the highest effective marginal combined federal, state, and local income tax rate applicable to an individual or corporate member
(whichever is higher). In the event of any disparity between the tax rates applicable to corporate and non-corporate taxpayers, Holdco
1 could receive tax distributions from Waldencast LP in excess of its actual tax liability, which could result in it accumulating cash
in excess of its tax liability. This would complicate our ability to maintain certain aspects of our capital structure. Such cash, if
retained, could cause the value of a Waldencast LP Common Unit to deviate from the value of a Class A ordinary share. In addition, such
cash, if used to purchase additional Waldencast LP Common Units, could result in deviation from the one-to-one relationship between our
Class A ordinary shares outstanding and Waldencast LP Common Units unless a corresponding number of additional Class A ordinary shares
are distributed as a stock dividend. We may, if permitted under our debt agreements, choose to pay dividends to all holders of our Class
A ordinary shares with any excess cash. These considerations could have unintended impacts on the pricing of our Class A ordinary shares
and may impose transaction costs and require management efforts to address on a recurring basis. To the extent that we do not distribute
such excess cash as dividends on our Class A ordinary shares and instead, for example, hold such cash balances or lend them to Waldencast
LP, holders of Waldencast LP Common Units during a period in which we hold such cash balances could benefit from the value attributable
to such cash balances as a result of redeeming or exchanging their Waldencast LP Common Units and obtaining ownership of our Class A ordinary
shares (or a cash payment based on the value of our Class A ordinary shares). In such case, these holders of Waldencast LP Common Units
could receive disproportionate value for their Waldencast LP Common Units exchanged during this time frame.
Failure to comply
with applicable transfer pricing and similar regulations could harm our business and financial results.
In many countries, including
the US., we are subject to transfer pricing and other tax regulations designed to ensure that appropriate levels of income are reported
as earned in each jurisdiction and are taxed accordingly. Although we believe that we are in substantial compliance with all applicable
regulations and restrictions, we are subject to the risk that Governmental Authorities could audit our transfer pricing and related practices
and assert that additional taxes are owed. In the event that the audits or assessments are concluded adversely to us, we may or may not
be able to offset or mitigate the consolidated effect.
We may be a passive
foreign investment company (“PFIC”), which could result in adverse U.S. federal income tax consequences to U.S. Holders.
If we are a PFIC for any
taxable year, or portion thereof, that is included in the holding period of a U.S. Holder (as defined herein), such U.S. Holder may be
subject to certain adverse U.S. federal income tax consequences and may be subject to additional reporting requirements. We are not expected
to be treated as a PFIC for the taxable year ending on December 31, 2022, or the foreseeable future. However, the facts on which any determination
of PFIC status are based may not be known until the close of each taxable year in question, and, in the case of our prior taxable year
ended December 31, 2021, until as late as the close of the taxable year ending on December 31, 2023.
Please see the section entitled
“U.S. Federal Income Tax Considerations—PFIC Considerations” for a more detailed discussion with respect to our
PFIC status. U.S. Holders are urged to consult their tax advisors regarding the possible application of the PFIC rules to holders of our
Class A ordinary shares.
We may be treated
as a corporation resident in the U.S. for U.S. federal income tax purposes.
A corporation is generally
considered a tax resident in the jurisdiction of its organization or incorporation. Thus, as a corporation incorporated under the laws
of Jersey, we should generally be classified as a non-U.S. corporation (and therefore as a non-U.S. tax resident) for U.S. federal income
tax purposes. In certain circumstances, however, under Section 7874 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”),
a corporation organized outside the U.S. will be treated as a U.S. corporation (and, therefore, as a U.S. tax resident).
Based on the rules in effect
currently, we do not expect to be treated as a U.S. corporation for U.S. federal income tax purposes by virtue of Section 7874. Nevertheless,
because the Section 7874 rules and exceptions are complex, subject to factual and legal uncertainties, and may change in the future (possibly
with retroactive effect), there can be no assurance that we will not be treated as a U.S. corporation for U.S. federal income tax purposes.
In addition, it is possible that a future acquisition of the stock or assets of a U.S. corporation could result in our being treated as
a U.S. corporation.
We continue to operate
so as to be treated exclusively as a resident of Jersey for tax purposes, but the tax authorities of other jurisdictions may treat us
as also being a resident of, or as having a taxable presence in, another jurisdiction for tax purposes.
Our residence for tax purposes
(including, for the avoidance of doubt, withholding tax and tax treaty eligibility purposes) is exclusively in Jersey and we have no taxable
presence in the form of a fixed place of business or permanent establishment in any other jurisdiction.
Because we are incorporated
under Jersey law and have our registered office in Jersey, we are considered to be resident in Jersey for Jersey tax purposes. In addition,
we maintain our management, organizational and operational structures in such a manner that we should not be regarded as a tax resident
of any other jurisdiction either for domestic law purposes or for the purposes of any applicable tax treaty (notably any applicable tax
treaty with Jersey) and should be deemed resident only in Jersey and that we should not have a fixed place of business or permanent establishment
outside Jersey.
However, the determination
of our tax residence, which primarily depends upon our place of effective management, as well as the characterization of fixed places
of business or permanent establishments outside our jurisdiction of incorporation, are questions of fact based on all circumstances. Because
such determinations are highly fact-sensitive, no assurance can be given regarding their outcome.
A failure to maintain exclusive
tax residence in Jersey or not to maintain a fixed place of business or permanent establishment or other taxable presence outside Jersey
could result in significant adverse tax consequences to us (including but not limited to tax consequences relating to corporate taxes,
taxes on the supplies of goods and services (for example, value added taxes) and payroll and employment taxes). A failure to maintain
exclusive tax residence in Jersey could also result in significant adverse tax consequences for shareholders. The impact of this risk
would differ based on the views taken by each relevant tax authority and, in respect of the taxation of shareholders, on their specific
situation.
We may in the future amend
our management, organizational and operational structures in such a manner that we may be regarded as a tax resident of another jurisdiction
either for domestic law purposes or for the purposes of any applicable tax treaty. A change in tax residence could result in significant
adverse tax consequences to us and our shareholders, and we may opt to make such changes without consideration of the tax consequences
for shareholders.
Higher volatility and longer
expected terms result in an increase to stock-based compensation determined at the date of grant. Future stock-based compensation cost
and unrecognized stock-based compensation will increase to the extent that we grant additional equity awards to employees, or we assume
unvested equity awards in connection with acquisitions. If there are any modifications or cancellations of the underlying unvested securities,
we may be required to accelerate any remaining unearned stock-based compensation cost or incur incremental cost.
The expected stock price
volatility for common stock was estimated by taking the average historic price volatility for industry peers based on daily price observations
over a period equivalent to the expected term of the stock option grants. Industry peers consist of several public companies in our industry
which are of similar size, complexity and stage of development. The risk-free interest rate for the expected term of the option is based
on the U.S. Treasury implied yield at the date of grant. The weighted-average expected term is determined based on the simplified method,
which results in an expected term based on the midpoint between the vesting date and contractual term of an option. The simplified method
was chosen because we have limited historical option exercise experience.
If factors change and we
employ different assumptions, stock-based compensation cost on future awards may differ significantly. No stock-based compensation expense
as been realized in current or prior periods.
Use
of Proceeds
All of the securities
offered by the Selling Holders pursuant to this prospectus will be sold by the Selling Holders for their respective accounts. We will
not receive any of the proceeds from these sales. We could receive up to an aggregate of approximately $339.6 million from the exercise
of all warrants assuming the exercise in full of all such warrants for cash. Unless we inform you otherwise in a prospectus supplement
or free writing prospectus, to the extent we elect the exercise of such warrants for cash, we intend to use the net proceeds from such
exercise for general corporate purposes. We believe the likelihood that warrant holders will exercise their warrants, and therefore the
amount of cash proceeds that we would receive, is dependent upon the trading price of our Class A ordinary shares. As of the date of
this prospectus, our warrants are “out-of-the money,” which means that the trading price of the Class A ordinary shares underlying
our warrants is below the $11.50 exercise price of the warrants. For so long as the warrants remain “out-of-the money,” we
do not expect warrant holders to exercise their warrants and, therefore, we do not expect to receive cash proceeds from any such exercise.
See the risk factor entitled “There is no guarantee that the warrants will ever be in the money, and they may expire worthless”
for more information.
The Selling Holders will
pay any underwriting commissions and discounts, and expenses incurred by the Selling Holders for brokerage, marketing costs, or legal
services (other than those detailed below). We will bear the costs, fees and expenses incurred in effecting the registration of the securities
covered by this prospectus, including all registration and filing fees, securities or blue sky law compliance fees, Nasdaq listing fees
and expenses of our counsel and our independent registered public accounting firm, and fees and expenses of one legal counsel.
Dividend
Policy
We have not paid any cash
dividends on our Class A ordinary shares to date and do not intend to pay cash dividends in the foreseeable future. The payment of cash
dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements and general financial condition.
The payment of any cash dividends is within the discretion of our Board. Further, our ability to declare dividends may be limited by the
terms of financing or other agreements we or our subsidiaries enter into from time to time.
Unaudited
Pro Forma Condensed Combined Financial Information
Defined
terms included below have the same meaning as terms defined and included elsewhere in this prospectus.
Introduction:
The
unaudited pro forma condensed combined financial information is prepared in accordance with Article 11 of Regulation S-X as amended by
the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.” The unaudited
pro forma condensed combined financial information presents the pro forma effects of the acquisition of Milk and Obagi by Waldencast resulting
in reorganization into an umbrella partnership C corporation structure (or “Up-C” structure), and other agreements entered
into as part of the Transaction Agreements.
Waldencast
was a blank check company incorporated in the Cayman Islands on December 8, 2020 formed for the purpose of effecting a merger, share
exchange, asset acquisition, share purchase, reorganization or similar business combination. On March 18, 2021, Waldencast consummated
the initial public offering of 34,500,000 units, inclusive of the partial exercise by the underwriters of the over-allotment option, at
$10.00 per unit, generating gross proceeds of $345.0 million. Each unit consisted of one Class A ordinary share and one-third
of one public warrant. Each public warrant is exercisable for one Class A ordinary share at a price of $11.50 per share. Simultaneously
with the closing of the initial public offering, Waldencast consummated the private sale of 5,933,333 private placement warrants at a
price of $1.50 per private placement warrant to the Sponsor, generating gross proceeds to Waldencast of $8.9 million. The private
placement warrants are identical to the warrants sold as part of the units in Waldencast’s initial public offering except that,
so long as they are held by the Sponsor or its permitted transferees: (1) they will not be redeemable by Waldencast; (2) they (including
the shares issuable upon exercise of these warrants) may not, subject to certain limited exceptions, be transferred, assigned or sold
by the Sponsor until 30 days after the completion of Waldencast’s initial business combination; (3) they may be exercised by the
holders on a cashless basis; and (4) they (including the shares issuable upon exercise of these warrants) are entitled to registration
rights. Upon the closing of the initial public offering and the sale of the private placement warrants (inclusive of the partial exercise
by the underwriters of the over-allotment option), $345.0 million ($10.00 per unit) of the net proceeds of the initial public offering
and a portion of the proceeds from the sale of the private placement warrants was placed in the trust account established for the benefit
of Waldencast’s public shareholders. As of Closing, immediately prior to the effect of redemptions, there was approximately $345.8 million
held in the trust account.
Milk
is a clean prestige makeup brand that develops and sells cruelty-free, paraben-free, and 100% vegan cosmetics, skincare and other beauty
products. It generates revenue from the sale of products to retailers, as well as sales direct to consumer via its online website. Its
three most popular products are the Hydro Grip Primer, Kush Mascara and the Mini Hydro Grip.
Obagi
is a global skincare products company that develops, markets and sells innovative skin health products in more than 60 countries around
the world. Every product Obagi develops stems from a deep understanding of the skin and how healthy skin functions. Its product portfolio
is designed to prevent or improve common, visible skin concerns such as fine lines and wrinkles, elasticity, photodamage, hyperpigmentation,
acne, oxidative stress, environmental damage and hydration.
Waldencast is a Jersey
corporation that will be subject to U.S. taxation on its share of the U.S. effectively connected income earned from its partnership investment,
which owns both Obagi and Milk. The organizational structure following the completion of the Business Combination is commonly referred
to as an Up-C structure. This organizational structure will allow the Milk Members to retain an equity ownership in the form of Common
Units in Waldencast LP, which owns Obagi and Milk. The Milk Members may exchange Waldencast LP Common Units (together with the cancellation
of an equal number of shares of voting, Class B ordinary shares) into Class A ordinary shares. There is no tax receivable agreement
in place for such exchange of Common Units. The Waldencast public shareholders will continue to hold Class A ordinary shares in
the company, which, upon consummation of the Business Combination, was renamed Waldencast plc. The parties agreed to structure the Business
Combination in this manner for tax and other business purposes, and we do not believe that our Up-C organizational structure will give
rise to any significant business or strategic benefit or detriment. See the section entitled “Risk Factors—General Business
Risks and Risks Related to Our Financial Condition and Operations” for additional information on our organizational structure.
The
unaudited pro forma condensed combined balance sheet combines the Milk unaudited historical condensed balance sheet as of June 30, 2022,
the Obagi unaudited historical condensed consolidated balance sheet as of June 30, 2022, and the Waldencast unaudited historical condensed
balance sheet as of June 30, 2022, giving effect to the Business Combination as if it had been consummated on June 30, 2022. The unaudited
pro forma condensed combined statement of operations for the six months ended June 30, 2022 and the year ended December 31, 2021
presents the pro forma effect of the Business Combination as if completed on January 1, 2021.
We
refer to the unaudited pro forma condensed combined balance sheet and the unaudited pro forma condensed combined statement of operations
as the “pro forma financial statements.”
The
pro forma financial statements are not necessarily indicative of what the combined company’s balance sheet or statement of operations
actually would have been had the Business Combination been completed as of the dates indicated, nor do they purport to project the future
financial position or operating results of the combined company. The actual financial position and results of operations may differ significantly
from the pro forma amounts reflected herein due to a variety of factors. The pro forma financial information is presented for illustrative
purposes only and does not reflect the costs of any integration activities or cost savings or synergies that may be achieved as a result
of the Business Combination.
The
unaudited pro forma condensed combined financial statements were prepared using the acquisition method of accounting under the provisions
of ASC Topic 805, Business Combinations (“ASC 805”) on the basis of Waldencast as the accounting acquirer and Obagi
and Milk as the accounting acquirees. Waldencast has been determined to be the accounting acquirer based on evaluation of the following
factors (prior to the Closing Date):
| ● | The owners of Waldencast have the largest voting interest
in the combined company; |
| ● | The Sponsor and its affiliates nominated the majority of
the initial members who will serve on the board of directors of Waldencast (Obagi nominated 1 director, and Milk nominated 0 directors);
and |
| ● | Waldencast’s existing management holds executive management
roles for the post-combination company whilst Obagi and Milk management team members reported into the current Waldencast executive team. |
The
factors discussed above support the conclusion that Waldencast acquired control of Obagi and Milk and is the accounting acquirer. Therefore,
the Business Combination constitutes a change in control and was accounted for using the acquisition method. Under the acquisition method
of accounting, the purchase price was allocated to the tangible and identifiable intangible assets acquired and liabilities assumed of
Obagi and Milk, based on their estimated acquisition-date fair values. These estimates were determined through established and generally
accepted valuation techniques.
The
following tables represent the pro forma ownership of the combined company which reflects the cumulative effect of actual redemptions,
the consummation of the Business Combination and the consummation of the related transactions.
The
following summarizes the pro forma ownership of Class A ordinary shares, excluding potential Class A ordinary shares from dilutive
securities, following the Business Combination:
| | |
Shares | | |
Ownership % (10) | |
| Waldencast Public Shareholders(1) | |
| 4,478,054 | | |
| 4.2 | % |
| Burwell(2) | |
| 7,848,333 | | |
| 7.3 | % |
| Dynamo Master Fund(3) | |
| 13,848,333 | | |
| 12.9 | % |
| Waldencast Ventures(4) | |
| 2,848,334 | | |
| 2.6 | % |
| Beauty FPA Investor(5) | |
| 17,300,000 | | |
| 16.1 | % |
| Investor Directors(6) | |
| 100,000 | | |
| 0.1 | % |
| PIPE Investors(7) | |
| 11,800,000 | | |
| 11.0 | % |
| Cumulative Waldencast shareholders | |
| 58,223,054 | | |
| 54.1 | % |
| Former Obagi Owners interest in Waldencast(8) | |
| 28,237,506 | | |
| 26.3 | % |
| Former Milk Owners interest in Waldencast(9) | |
| 21,104,225 | | |
| 19.6 | % |
| Total | |
| 107,564,785 | | |
| 100.0 | % |
| 1) | 30,021,946 Class A ordinary shares were redeemed in
connection with the Business Combination. |
| 2) | Includes 5,000,000 Class A ordinary shares acquired
pursuant to the Sponsor Forward Purchase Agreement for an investment of $50.0 million by Burwell, in exchange for a portion of the
Forward Purchase Amount and 2,848,333 Class A ordinary shares issued upon conversion of the Class B ordinary shares. Class A
ordinary shares were issued upon the automatic conversion of the Class B ordinary shares concurrently with the consummation of the
Business Combination. |
| 3) | Includes 11,000,000 Class A ordinary shares acquired
pursuant to the Sponsor Forward Purchase Agreement for an investment of $110.0 million by Dynamo Master Fund (a member of our Sponsor),
in exchange for a portion of the Forward Purchase Amount and 2,848,333 Class A ordinary shares issued upon conversion of the existing
Class B ordinary shares. Class A ordinary shares were issued upon the automatic conversion of the Class B ordinary shares
concurrently with the consummation of the Business Combination. |
| 4) | Class A ordinary shares issued upon conversion of the
Class B ordinary shares. Class A ordinary shares were issued upon the automatic conversion of the Class B ordinary shares
concurrently with the consummation of the Business Combination. |
| 5) | 17,300,000 Class A ordinary shares acquired pursuant
to the Third-Party Forward Purchase Agreement for an investment of $173.0 million by Beauty Ventures in exchange for a portion of
the Forward Purchase Amount. |
| 6) | 100,000 Class A ordinary shares held by the Investor
Directors, issued upon conversion of the Class B ordinary shares. Class A ordinary shares were issued upon the automatic conversion
of the Class B ordinary shares concurrently with the consummation of the Business Combination. |
| 7) | Represents the private placement pursuant to which Waldencast
entered into Subscription Agreements with certain PIPE Investors whereby such investors have agreed to subscribe for Class A ordinary
shares at a purchase price of $10.00 per share. The PIPE Investors participating in the PIPE Investment, purchased an aggregate of 11,800,000
Class A ordinary shares. |
| 8) | Represents Obagi owners’ interest in 28,237,506 Class A
ordinary shares. |
| 9) | Represents the Milk Members’ noncontrolling economic
interest in Waldencast LP Common Units, which are redeemable at the option of the holder of such units, and if such option is exercised,
are exchangeable, at the option of Waldencast, for Class A ordinary shares on a 1 for 1 basis or cash (together with the cancellation
of an equal number of shares of voting, Class B ordinary shares). |
| 10) | Percentage totals may not foot due to rounding. |
The
following summarizes the pro forma ownership of Class A ordinary shares, on a fully dilutive basis, following the Business Combination:
| | |
Shares | | |
Ownership % (8) | |
| Waldencast Public Shareholders (1) | |
| 15,978,004 | | |
| 10.9 | % |
| Burwell Mountain Trust(2) | |
| 11,826,110 | | |
| 8.1 | % |
| Dynamo Master Fund(3) | |
| 19,826,109 | | |
| 13.5 | % |
| Waldencast Ventures(4) | |
| 5,159,447 | | |
| 3.5 | % |
| Beauty FPA Investor(5) | |
| 23,066,666 | | |
| 15.7 | % |
| Investor Directors | |
| 100,000 | | |
| 0.2 | % |
| PIPE Investors | |
| 11,800,000 | | |
| 8.0 | % |
| Cumulative Waldencast shareholders | |
| 87,756,336 | | |
| 59.8 | % |
| Former Obagi Owners interest in Waldencast(6) | |
| 35,934,428 | | |
| 24.5 | % |
| Former Milk Owners interest in Waldencast(7) | |
| 23,142,337 | | |
| 15.8 | % |
| Total | |
| 146,833,101 | | |
| 100.0 | % |
| 1) | Includes the impact of the exercise of 11,499,950 of public
warrants. |
| 2) | Includes the exercise of (i) 1,977,777 private placement
warrants and the exercise of 333,333 Working Capital Loan warrants, which are indirectly owned by Burwell Mountain PTC LLC, as the trustee
of Burwell Mountain Trust (a member of the Sponsor), and (ii) 1,666,667 Sponsor FPA Warrants. |
| 3) | Includes the exercise of (i) 1,977,777 private placement
warrants and the exercise of 333,333 Working Capital Loan warrants, which are indirectly owned by Dynamo Master Fund as a member of the
Sponsor, and (ii) 3,666,666 Sponsor FPA Warrants. |
| 4) | Includes the exercise of 1,977,779 private placement warrants
and the exercise of 333,334 Working Capital Loan warrants, which are indirectly owned by Waldencast Ventures as a member of the Sponsor. |
| 5) | Includes the exercise of 5,766,666 Beauty FPA Warrants. |
| 6) | Includes
the exercise of rollover equity awards, consisting of 5,906,630 stock options, and 1,790,292 restricted stock units. |
| 7) | Includes the exercise of rollover equity awards, consisting
of 1,887,264 stock appreciation rights and 150,848 options. |
| 8) | Percentage totals may not foot due to rounding. |
The
following unaudited pro forma condensed combined balance sheet as of June 30, 2022 and the unaudited pro forma condensed combined statements
of operations for the six months ended June 30, 2022 and year ended December 31, 2021 are based on the historical financial statements
of Waldencast, Obagi, and Milk. The unaudited pro forma adjustments are based on information currently available, and assumptions and
estimates underlying the unaudited pro forma adjustments are described in the accompanying notes. Actual results may differ materially
from the assumptions used to present the accompanying unaudited pro forma condensed combined financial information.
The
assumptions and estimates underlying the unaudited adjustments to the unaudited pro forma condensed combined financial information is
described in the accompanying notes, which should be read in conjunction with, the following:
| ● | Waldencast’s unaudited interim condensed financial
statements and related notes as of June 30, 2022 and for the six months ended June 30, 2022 and 2021, included elsewhere in this prospectus. |
| ● | Waldencast’s
audited financial statements and related notes as of and for the year ended December 31, 2021 included elsewhere in this prospectus. |
| ● | Milk’s and Obagi’s unaudited consolidated financial
statements and related notes as of June 30, 2022 and for the six months ended June 30, 2022 and 2021 included elsewhere in this prospectus. |
| ● | Milk’s
and Obagi’s audited financial statements and related notes as of and for the year ended December 31, 2021 included elsewhere
in this prospectus. |
| ● | “Waldencast’s Management’s Discussion
and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. |
| ● | “Milk’s Management’s Discussion and
Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. |
| ● | “Obagi’s Management Discussion and Analysis
of Financial Condition and Results of Operations” included elsewhere in this prospectus. |
Unaudited Pro Forma
Condensed Combined Balance Sheet
As of June 30, 2022
(in thousands, except
per share data)
| | |
As of June 30, 2022 | | |
| | |
Transaction Accounting Adjustments | | |
| | |
As of
June 30,
2022 | |
| | |
Waldencast
(Historical) | | |
Obagi Global
Holdings
Limited
(Adjusted, Note 2) | | |
Milk
Makeup
LLC
(Historical) | | |
Reclassification
Adjustments | | |
Debt
Refinance | | |
| | |
Business
Combination
adjustments | | |
| | |
Pro Forma
Combined | |
| ASSETS | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Current assets: | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 100 | | |
$ | 6,242 | | |
$ | 2,666 | | |
$ | - | | |
| 45,944 | | |
| L | | |
$ | 345,313 | | |
| A | | |
$ | 44,172 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (76,199 | ) | |
| B | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 118,000 | | |
| C | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 333,000 | | |
| D | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (429,975 | ) | |
| E | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 103 | | |
| J | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (300,905 | ) | |
| M | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (117 | ) | |
| N | | |
| | |
| Restricted cash | |
| . | | |
| 650 | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 650 | |
| Accounts and note receivable, net | |
| - | | |
| 75,165 | | |
| 6,390 | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 81,555 | |
| Inventories | |
| - | | |
| 20,789 | | |
| 24,835 | | |
| - | | |
| | | |
| | | |
| 18,950 | | |
| E | | |
| 64,574 | |
| Prepaid expenses | |
| 282 | | |
| 8,086 | | |
| - | | |
| 840 | | |
| | | |
| | | |
| (283 | ) | |
| B | | |
| 8,925 | |
| Other current assets | |
| - | | |
| 373 | | |
| - | | |
| - | | |
| 278 | | |
| L | | |
| - | | |
| | | |
| 651 | |
| Prepaid expenses and other current assets | |
| - | | |
| - | | |
| 453 | | |
| (453 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Prepaid supplier | |
| - | | |
| - | | |
| 387 | | |
| (387 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Total current assets, net | |
| 382 | | |
| 111,305 | | |
| 34,731 | | |
| - | | |
| 46,222 | | |
| | | |
| 7,887 | | |
| | | |
| 200,527 | |
| Investment held in Trust Account | |
| 345,313 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (345,313 | ) | |
| A | | |
| - | |
| Property, plant equipment, net | |
| - | | |
| 3,640 | | |
| 5,938 | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 9,578 | |
| Intangible assets, net | |
| - | | |
| 70,096 | | |
| - | | |
| - | | |
| | | |
| | | |
| 533,404 | | |
| E | | |
| 603,500 | |
| Goodwill | |
| - | | |
| 44,489 | | |
| - | | |
| - | | |
| | | |
| | | |
| 321,206 | | |
| E | | |
| 365,695 | |
| Other assets | |
| - | | |
| 1,274 | | |
| - | | |
| - | | |
| 832 | | |
| L | | |
| - | | |
| | | |
| 2,106 | |
| Due from officers | |
| - | | |
| - | | |
| 780 | | |
| - | | |
| | | |
| | | |
| (780 | ) | |
| J | | |
| - | |
| Total assets | |
$ | 345,695 | | |
$ | 230,804 | | |
$ | 41,449 | | |
$ | - | | |
$ | 47,054 | | |
| | | |
$ | 516,404 | | |
| | | |
$ | 1,181,406 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accounts payable | |
$ | 1,039 | | |
$ | 18,323 | | |
$ | 6,590 | | |
$ | - | | |
| | | |
| | | |
$ | (5,077 | ) | |
| B | | |
$ | 20,875 | |
| Current portion of long-term debt, net | |
| - | | |
| 22,603 | | |
| | | |
| - | | |
| (14,824 | ) | |
| L | | |
| - | | |
| | | |
| 7,779 | |
| Tenant allowance liability - current | |
| - | | |
| - | | |
| 161 | | |
| (161 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Accrued expenses and other current liabilities | |
| - | | |
| - | | |
| 6,328 | | |
| (6,328 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Line of credit | |
| - | | |
| - | | |
| 1,500 | | |
| - | | |
| (1,500 | ) | |
| L | | |
| | | |
| | | |
| - | |
| Due to related party | |
| 155 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 155 | |
| Other current liabilities | |
| - | | |
| 14,417 | | |
| - | | |
| 6,489 | | |
| | | |
| | | |
| - | | |
| | | |
| 20,906 | |
| Total current liabilities | |
| 1,194 | | |
| 55,343 | | |
| 14,579 | | |
| - | | |
| (16,324 | ) | |
| | | |
| (5,077 | ) | |
| | | |
| 49,715 | |
| Warrant liabilities | |
| 12,552 | | |
| - | | |
| 148 | | |
| (148 | ) | |
| | | |
| | | |
| 1,500 | | |
| K | | |
| 13,904 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (148 | ) | |
| N | | |
| | |
| Deferred rent - non-current | |
| - | | |
| - | | |
| 1,172 | | |
| (1,172 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Tenant allowance liability - non-current | |
| - | | |
| - | | |
| 978 | | |
| (978 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Long-term debt, net | |
| - | | |
| 100,764 | | |
| - | | |
| - | | |
| 73,689 | | |
| L | | |
| | | |
| | | |
| 174,453 | |
| Deferred income tax liabilities | |
| - | | |
| 548 | | |
| - | | |
| - | | |
| (2,423 | ) | |
| L | | |
| 72,255 | | |
| E | | |
| 70,380 | |
| Other liabilities | |
| - | | |
| 619 | | |
| - | | |
| 16,099 | | |
| | | |
| | | |
| (13,801 | ) | |
| B | | |
| 2,917 | |
| Deferred legal fees | |
| 13,801 | | |
| - | | |
| - | | |
| (13,801 | ) | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Forward purchase agreement liabilities | |
| 7,992 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (7,992 | ) | |
| D | | |
| - | |
| Working Capital Promissory Note – related party | |
| 2,100 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (1,500 | ) | |
| K | | |
| 600 | |
| Deferred underwriters’ discount | |
| 12,075 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (12,075 | ) | |
| B | | |
| - | |
| Total liabilities | |
| 49,714 | | |
| 157,274 | | |
| 16,877 | | |
| - | | |
| 54,942 | | |
| | | |
| 33,162 | | |
| | | |
| 311,969 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| COMMITMENTS AND CONTINGENCIES | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Class A ordinary shares subject to possible redemption | |
| 345,313 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (345,313 | ) | |
| F | | |
| - | |
| Redeemable Series A preferred units | |
| - | | |
| - | | |
| 44,319 | | |
| - | | |
| | | |
| | | |
| (44,319 | ) | |
| F | | |
| - | |
| Redeemable Series B preferred units | |
| - | | |
| - | | |
| 26,227 | | |
| - | | |
| | | |
| | | |
| (26,227 | ) | |
| F | | |
| - | |
| Redeemable Series C preferred units | |
| - | | |
| - | | |
| 46,373 | | |
| - | | |
| | | |
| | | |
| (46,373 | ) | |
| F | | |
| - | |
| Redeemable Series D preferred units | |
| - | | |
| - | | |
| 30,237 | | |
| - | | |
| | | |
| | | |
| (30,237 | ) | |
| F | | |
| - | |
| SHAREHOLDERS’ EQUITY | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common units | |
| - | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Common stock | |
| - | | |
| 4,000 | | |
| - | | |
| - | | |
| | | |
| | | |
| (4,000 | ) | |
| H | | |
| - | |
| Preference shares | |
| - | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Class A ordinary shares | |
| - | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| 1 | | |
| C | | |
| 6 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 5 | | |
| D | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 3 | | |
| E | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| F | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (3 | ) | |
| M | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| O | | |
| | |
| Class B ordinary shares | |
| 1 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (1 | ) | |
| H | | |
| - | |
| Class B non-economic voting shares | |
| - | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| 1 | | |
| E | | |
| 1 | |
| Additional paid-in capital | |
| - | | |
| 72,822 | | |
| - | | |
| - | | |
| | | |
| | | |
| 117,999 | | |
| C | | |
| 802,629 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 340,987 | | |
| D | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 371,325 | | |
| E | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 492,469 | | |
| F | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (125,159 | ) | |
| G | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 4,001 | | |
| H | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (170,410 | ) | |
| I | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (677 | ) | |
| J | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (300,902 | ) | |
| M | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 174 | | |
| O | | |
| | |
| Accumulated deficit | |
| (49,333 | ) | |
| (2,575 | ) | |
| (122,584 | ) | |
| - | | |
| (7,888 | ) | |
| L | | |
| (45,528 | ) | |
| B | | |
| (102,892 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 125,159 | | |
| G | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 31 | | |
| N | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (174 | ) | |
| O | | |
| | |
| Accumulated other comprehensive income (loss) | |
| - | | |
| (717 | ) | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| (717 | ) |
| Total shareholders’ equity | |
| (49,332 | ) | |
| 73,530 | | |
| (122,584 | ) | |
| - | | |
| (7,888 | ) | |
| | | |
| 805,301 | | |
| | | |
| 699,027 | |
| Noncontrolling interest | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| | | |
| 170,410 | | |
| I | | |
| 170,410 | |
| Total Equity | |
| (49,332 | ) | |
| 73,530 | | |
| (122,584 | ) | |
| - | | |
| (7,888 | ) | |
| | | |
| 975,711 | | |
| | | |
| 869,437 | |
| Total liabilities and shareholders’ equity | |
$ | 345,695 | | |
$ | 230,804 | | |
$ | 41,449 | | |
$ | - | | |
| 47,054 | | |
| | | |
$ | 516,404 | | |
| | | |
$ | 1,181,406 | |
Unaudited Pro Forma
Condensed Combined Statement of Operations
For the six months
ended June 30, 2022
(in thousands, except
per share data)
| | |
For the Six Months Ended
June 30, 2022 | | |
| | |
Transaction Accounting
Adjustments | | |
| | |
For the
Six Months
Ended
June 30,
2022 | |
| | |
Waldencast
(Historical) | | |
Obagi
Global
Holdings
Limited
(Adjusted,
Note 2) | | |
Milk
Makeup
LLC
(Historical) | | |
Reclassification Adjustments | | |
Debt
Refinance | | |
| | |
Business
Combination
adjustments | | |
| | |
Pro Forma
Combined | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Net revenue | |
$ | - | | |
$ | 99,654 | | |
$ | 38,548 | | |
$ |
- | | |
| | | |
| | | |
$ | - | | |
| | | |
$ | 138,202 | |
| Cost of goods sold (exclusive of depreciation and amortization
shown separately below) | |
| - | | |
| 22,781 | | |
| 13,365 | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 36,146 | |
| Formation and operating costs | |
| 8,401 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (8,401 | ) | |
| BB | | |
| - | |
| Selling, general and administrative | |
| - | | |
| 54,520 | | |
| 18,815 | | |
| - | | |
| | | |
| | | |
| (6,139 | ) | |
| BB | | |
| 69,913 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 2,717 | | |
| FF | | |
| | |
| Research and development | |
| - | | |
| 3,262 | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 3,262 | |
| Depreciation and amortization | |
| - | | |
| 7,076 | | |
| 1,169 | | |
| - | | |
| | | |
| | | |
| 6,357 | | |
| GG | | |
| 14,602 | |
| Income/(loss) from operations | |
| (8,401 | ) | |
| 12,015 | | |
| 5,199 | | |
| - | | |
| - | | |
| | | |
| 5,466 | | |
| | | |
| 14,279 | |
| Interest income on operating account | |
| (1 | ) | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| (1 | ) |
| Interest income on marketable securities held in Trust
Account | |
| (261 | ) | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| 261 | | |
| AA | | |
| - | |
| Change in fair value of forward purchase agreement
liabilities | |
| (5,328 | ) | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| 5,328 | | |
| HH | | |
| - | |
| Change in fair value of warrant liabilities | |
| (8,602 | ) | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| (8,602 | ) |
| Interest expense, net | |
| - | | |
| 5,719 | | |
| 21 | | |
| - | | |
| 177 | | |
| II | | |
| | | |
| | | |
| 5,917 | |
| Other (income)/expense, net | |
| - | | |
| (74 | ) | |
| 217 | | |
| - | | |
| | | |
| | | |
| (314 | ) | |
| EE | | |
| (171 | ) |
| Total other
(income)/expense, net | |
| (14,192 | ) | |
| 5,645 | | |
| 238 | | |
| - | | |
| 177 | | |
| | | |
| 5,275 | | |
| | | |
| (2,857 | ) |
| Income/(loss)
before income taxes | |
| 5,791 | | |
| 6,370 | | |
| 4,961 | | |
| - | | |
| (177 | ) | |
| | | |
| 191 | | |
| | | |
| 17,136 | |
| Income tax expense (benefit) | |
| - | | |
| (43 | ) | |
| - | | |
| - | | |
| 1,344 | | |
| II | | |
| (1,069 | ) | |
| CC | | |
| 232 | |
| Net income (loss) | |
$ | 5,791 | | |
$ | 6,413 | | |
$ | 4,961 | | |
$ | - | | |
| (1,521 | ) | |
| | | |
$ | 1,260 | | |
| | | |
$ | 16,904 | |
| Net income attributable to noncontrolling interest | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 3,313 | | |
| DD | | |
| 3,313 | |
| Net income attributable to controlling interest | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| | | |
| 13,591 | |
| Earnings per share (Note 6) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income per Class A ordinary share - Basic | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
$ | 0.16 | |
| Weighted average Class A ordinary share outstanding
- Basic | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 86,460,560 | |
| Net income per Class A ordinary share - Diluted | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 0.15 | |
| Weighted average Class A ordinary share outstanding
- Diluted | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 111,475,855 | |
Unaudited Pro Forma
Condensed Combined Statement of Operations
For the year ended
December 31, 2021
(in thousands, except
per share data)
| | |
For the Year Ended
December 31, 2021 | | |
| | |
Transaction Accounting Adjustments | | |
| | |
For the
Year
Ended
December 31,
2021 | |
| | |
Waldencast
Acquisition
Corp.
(Historical) | | |
Obagi
Global
Holdings
Limited
(Adjusted,
Note 2) | | |
Milk
Makeup
LLC
(Historical) | | |
Reclassification Adjustments | | |
Debt
Refinance | | |
| | |
Business
Combination
adjustments | | |
| | |
Pro Forma
Combined | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Net revenue | |
$ | - | | |
$ | 178,681 | | |
$ | 47,076 | | |
$ | - | | |
| | | |
| | | |
$ | -
| | |
| | | |
$ | 225,757 | |
| Cost of goods sold (exclusive of depreciation and amortization
shown separately below) | |
| - | | |
| 42,310 | | |
| 21,781 | | |
| - | | |
| | | |
| | | |
| 18,950 | | |
| GG | | |
| 83,041 | |
| Formation and operating costs | |
| 9,133 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 9,133 | |
| Selling, general and administrative | |
| - | | |
| 94,457 | | |
| 30,764 | | |
| - | | |
| | | |
| | | |
| 60,742 | | |
| BB | | |
| 191,813 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 5,676 | | |
| FF | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 174 | | |
| JJ | | |
| | |
| Research and development | |
| - | | |
| 6,991 | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 6,991 | |
| Depreciation and amortization | |
| - | | |
| 13,580 | | |
| 1,975 | | |
| - | | |
| | | |
| | | |
| 12,744 | | |
| GG | | |
| 28,299 | |
| Income/(loss) from operations | |
| (9,133 | ) | |
| 21,343 | | |
| (7,444 | ) | |
| - | | |
| - | | |
| | | |
| (98,286 | ) | |
| | | |
| (93,520 | ) |
| Interest income on operating account | |
| (1 | ) | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| (1 | ) |
| Interest income on marketable securities held in Trust
Account | |
| (52 | ) | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| 52 | | |
| AA | | |
| - | |
| Offering expenses related to warrant issuance | |
| 719 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 719 | |
| Change in fair value of forward purchase agreement
liabilities | |
| 1,665 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| (1,665 | ) | |
| HH | | |
| - | |
| Change in fair value of warrant liabilities | |
| 2,964 | | |
| - | | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| 2,964 | |
| Loss on Extinguishment of Debt | |
| - | | |
| 2,317 | | |
| - | | |
| - | | |
| 10,311 | | |
| II | | |
| - | | |
| | | |
| 12,628 | |
| Gain on PPP Loan forgiveness | |
| - | | |
| (6,824 | ) | |
| - | | |
| - | | |
| | | |
| | | |
| - | | |
| | | |
| (6,824 | ) |
| Interest expense, net | |
| - | | |
| 11,158 | | |
| 18 | | |
| - | | |
| 993 | | |
| II | | |
| - | | |
| | | |
| 12,169 | |
| Other (income)/expense, net | |
| - | | |
| 194 | | |
| 385 | | |
| - | | |
| | | |
| | | |
| (1,421 | ) | |
| EE | | |
| (842 | ) |
| Total other
(income)/expense, net | |
| 5,295 | | |
| 6,845 | | |
| 403 | | |
| - | | |
| 11,304 | | |
| | | |
| (3,034 | ) | |
| | | |
| 20,813 | |
| Income/(loss)
before income taxes | |
| (14,428 | ) | |
| 14,498 | | |
| (7,847 | ) | |
| - | | |
| (11,304 | ) | |
| | | |
| (95,252 | ) | |
| | | |
| (114,333 | ) |
| Income tax expense (benefit) | |
| - | | |
| 11,131 | | |
| - | | |
| - | | |
| 199 | | |
| II | | |
| (18,329 | ) | |
| CC | | |
| (6,999 | ) |
| Net (loss) income | |
$ | (14,428 | ) | |
$ | 3,367 | | |
$ | (7,847 | ) | |
$ | - | | |
| (11,503 | ) | |
| | | |
$ | (76,923 | ) | |
| | | |
$ | (107,334 | ) |
| Net loss attributable to noncontrolling interest | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (19,107 | ) | |
| DD | | |
| (19,107 | ) |
| Net loss attributable to controlling interest | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| | | |
| (88,227 | ) |
| Earnings per share (Note 6) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss per Class A ordinary share - Basic and Diluted | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
$ | (1.02 | ) |
| Weighted average Class A ordinary shares outstanding
- Basic and Diluted | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 86,460,560 | |
NOTES TO UNAUDITED
PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 1. Basis of pro
forma presentation
The
unaudited pro forma condensed combined financial statements have been prepared assuming the Business Combination is accounted for using
the acquisition method of accounting with Waldencast as the acquiring entity. Under the acquisition method of accounting, Waldencast’s
assets and liabilities will retain their carrying values and the assets and liabilities associated with Milk and Obagi will be recorded
at their fair values measured as of the acquisition date. The excess of the purchase price over the estimated fair values of the net assets
acquired, if applicable, will be recorded as goodwill. The acquisition method of accounting is based on ASC 805 and uses the fair value
concepts defined in ASC Topic 820, Fair Value Measurements (“ASC 820”). In general, ASC 805 requires, among other things,
that assets acquired, and liabilities assumed be recognized at their fair values as of the acquisition date by Waldencast, who was determined
to be the accounting acquirer.
ASC
820 defines fair value, establishes a framework for measuring fair value, and sets forth a fair value hierarchy that prioritizes and ranks
the level of observability of inputs used to develop the fair value measurements. Fair value is defined in ASC 820 as “the price
that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement
date.” This is an exit price concept for the valuation of the asset or liability. In addition, market participants are assumed to
be buyers and sellers in the principal (or the most advantageous) market for the asset or liability. Fair value measurements for a non-financial
asset assume the highest and best use by these market participants. Many of these fair value measurements can be highly subjective, and
it is possible that other professionals applying reasonable judgment to the same facts and circumstances, could develop and support a
range of alternative estimated amounts.
The
transaction accounting adjustments represent management’s estimates based on information available as of the date of the filing
of the condensed combined financial information and do not reflect possible adjustments related to restructuring or integration activities
that have to be determined.
The
accompanying unaudited pro forma condensed combined financial information was prepared using the acquisition method of accounting in accordance
with ASC 805 and are based on certain currently available information and certain assumptions and methodologies that Waldencast believes
are reasonable under the circumstances. The unaudited condensed transaction accounting adjustments, which are described in the accompanying
notes, may be revised as additional information becomes available. Therefore, it is likely that the actual adjustments will differ from
the transaction accounting adjustments and it is possible the differences may be material. Waldencast believes that its assumptions and
methodologies provide a reasonable basis for presenting all of the significant effects of the Business Combination based on information
available to management at this time and that the transaction accounting adjustments give appropriate effect to those assumptions and
are properly applied in the unaudited pro forma condensed combined financial information.
The
historical financial statements have been adjusted in the unaudited pro forma condensed combined financial information to reflect transaction
accounting adjustments in connection with the Business Combination as well as the distribution by Obagi to Cedarwalk of all of the issued
and outstanding shares of capital stock of Obagi Hong Kong and certain related assets prior to the Closing of the Business Combination
(the “Obagi China Distribution”).
The
unaudited pro forma condensed combined balance sheet as of June 30, 2022, assumes that the Business Combination occurred on June 30, 2022.
The unaudited pro forma condensed combined statements of operations for the period for the six months ended June 30, 2022 and year ended
December 31, 2021, present pro forma effect to the Business Combination as if it had been completed on January 1, 2021. These
periods are presented on the basis of Waldencast being the accounting acquirer. The pro forma basic and diluted loss per share amounts
presented in the unaudited pro forma condensed combined statement of operations are based upon the number of Class A ordinary shares
outstanding, assuming the Business Combination and related transactions occurred on January 1, 2021.
Note 2. Description
of the Business Combination
Pursuant
to the Transaction Agreements, Obagi owners received a combination of cash and Class A ordinary shares in Waldencast and Milk owners
received a combination of cash, partnership interest of Waldencast LP and Class B ordinary shares. The Business Combination was structured
as an Up-C transaction, whereby the Milk shareholders as the Milk Members retained common units in Waldencast LP, which hold the net assets
of Milk and Obagi. The Milk Members received approximately 19.6% of the economic interests in Waldencast LP.
The
transaction occurred as follows: Obagi merged into Holdco 1 and became an indirect subsidiary of Waldencast, Milk was acquired by Waldencast
through Waldencast LP and Holdco 1 acquiring the Milk Membership Units (organized as an “Up-C”) through a subsidiary, and
the Obagi assets was contributed to the Milk Up-C structure.
Below is a
diagram of the final structure of the transaction:

Obagi China Distribution
In
connection with the Obagi Merger, Obagi carved out its businesses located in China—Obagi Shanghai Cosmeceuticals Co. Ltd., Obagi
Xi’an Pharmaceuticals Technology Co., Ltd., and Obagi Hong Kong Limited (the “Obagi China Business”)—through the
Obagi China Distribution. The adjusted Obagi results include adjustments to eliminate the assets, liabilities and operations of the Obagi
China Business that was distributed to Cedarwalk.
Below
is a summary of the carve-out adjustments recorded against the historical financial statements of Obagi to eliminate the assets, liabilities
and operations of the Obagi China Businesses:
Condensed Consolidated
Balance Sheet as of June 30, 2022
| ($ in thousands, except share data) | |
Obagi Global Holdings
Limited (Historical) | | |
China
Carve-Out
Adjustments | | |
Obagi Global Holdings
Limited (Adjusted) | |
| ASSETS | |
| | |
| | |
| |
| Current assets: | |
| | |
| | |
| |
| Cash and cash equivalents | |
$ | 6,742 | | |
$ | (500 | ) | |
$ | 6,242 | |
| Restricted cash | |
| 650 | | |
| - | | |
| 650 | |
| Accounts and note receivable, net | |
| 77,665 | | |
| (2,500 | ) | |
| 75,165 | |
| Inventories | |
| 27,586 | | |
| (6,797 | ) | |
| 20,789 | |
| Prepaid expenses | |
| 8,086 | | |
| - | | |
| 8,086 | |
| Other current assets | |
| 374 | | |
| - | | |
| 374 | |
| Total current assets | |
| 121,103 | | |
| (9,797 | ) | |
| 111,306 | |
| Property, plant equipment, net | |
| 3,777 | | |
| (138 | ) | |
| 3,639 | |
| Intangible assets, net | |
| 73,069 | | |
| (2,973 | ) | |
| 70,096 | |
| Goodwill | |
| 44,489 | | |
| - | | |
| 44,489 | |
| Other assets | |
| 1,274 | | |
| - | | |
| 1,274 | |
| Total assets | |
$ | 243,712 | | |
$ | (12,908 | ) | |
$ | 230,804 | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | | |
| | |
| Accounts payable | |
$ | 18,323 | | |
$ | - | | |
$ | 18,323 | |
| Current portion of long-term debt, net | |
| 22,603 | | |
| - | | |
| 22,603 | |
| Other current liabilities | |
| 14,417 | | |
| - | | |
| 14,417 | |
| Total current liabilities | |
| 55,343 | | |
| - | | |
| 55,343 | |
| Long-term debt, net | |
| 100,764 | | |
| - | | |
| 100,764 | |
| Deferred income tax liabilities | |
| 548 | | |
| - | | |
| 548 | |
| Other liabilities | |
| 619 | | |
| - | | |
| 619 | |
| Total liabilities | |
| 157,274 | | |
| - | | |
| 157,274 | |
| Shareholder’s equity | |
| | | |
| | | |
| | |
| Common stock, 25,000,000 shares authorized; $0.50 par value; 8,000,002 shares issued and outstanding as of June 30, 2022 and December 31, 2021 | |
| 4,000 | | |
| - | | |
| 4,000 | |
| Additional paid-in capital | |
| 100,113 | | |
| (27,291 | ) | |
| 72,822 | |
| Accumulated deficit | |
| (17,713 | ) | |
| 15,138 | | |
| (2,575 | ) |
| Accumulated other comprehensive income (loss) | |
| 38 | | |
| (755 | ) | |
| (717 | ) |
| Total shareholders’ equity (deficit) | |
| 86,438 | | |
| (12,908 | ) | |
| 73,530 | |
| TOTAL LIABILITIES AND SHAREHOLDER’S EQUITY (DEFICIT) | |
$ | 243,712 | | |
$ | (12,908 | ) | |
$ | 230,804 | |
Condensed Consolidated
Statements of Operations for the six months ended June 30, 2022
| ($ in thousands) |
|
Obagi Global Holdings
Limited (Historical) |
|
|
China
Carve-Out
Adjustments |
|
|
Obagi Global Holdings
Limited (Adjusted) |
|
| Net revenue |
|
$ |
106,440 |
|
|
$ |
(6,786 |
) |
|
$ |
99,654 |
|
| Cost of goods sold (exclusive of depreciation and amortization shown separately below) |
|
|
24,701 |
|
|
|
(1,920 |
) |
|
|
22,781 |
|
| Selling, general and administrative |
|
|
68,418 |
|
|
|
(13,898 |
) |
|
|
54,520 |
|
| Research and development |
|
|
3,262 |
|
|
|
- |
|
|
|
3,262 |
|
| Depreciation and amortization |
|
|
7,369 |
|
|
|
(293 |
) |
|
|
7,076 |
|
| Total operating expenses |
|
|
103,750 |
|
|
|
(16,111 |
) |
|
|
87,639 |
|
| Operating (loss) income |
|
|
2,690 |
|
|
|
9,325 |
|
|
|
12,015 |
|
| Interest expense |
|
|
5,719 |
|
|
|
- |
|
|
|
5,719 |
|
| Other expense, net |
|
|
(74 |
) |
|
|
- |
|
|
|
(74 |
) |
| (Loss)/Income before income taxes |
|
|
(2,955 |
) |
|
|
9,325 |
|
|
|
6,370 |
|
| Income tax expense (benefit) |
|
|
(40 |
) |
|
|
(3 |
) |
|
|
(43 |
) |
| Net (loss)/income |
|
$ |
(2,915 |
) |
|
$ |
9,328 |
|
|
$ |
6,413 |
|
Condensed Consolidated
Statements of Operations for the year ended December 31, 2021
| ($ in thousands) |
|
Obagi Global Holdings
Limited (Historical) |
|
|
China
Carve-Out
Adjustments |
|
|
Obagi Global Holdings
Limited (Adjusted) |
|
| Net revenue |
|
$ |
206,069 |
|
|
$ |
(27,388 |
) |
|
$ |
178,681 |
|
| Cost of goods sold (exclusive of depreciation and amortization shown separately below) |
|
|
48,708 |
|
|
|
(6,398 |
) |
|
|
42,310 |
|
| Selling, general and administrative |
|
|
118,243 |
|
|
|
(23,786 |
) |
|
|
94,457 |
|
| Research and development |
|
|
6,991 |
|
|
|
- |
|
|
|
6,991 |
|
| Depreciation and amortization |
|
|
14,053 |
|
|
|
(473 |
) |
|
|
13,580 |
|
| Total operating expenses |
|
|
187,995 |
|
|
|
(30,657 |
) |
|
|
157,338 |
|
| Operating income |
|
|
18,074 |
|
|
|
3,269 |
|
|
|
21,343 |
|
| Interest expense |
|
|
11,156 |
|
|
|
2 |
|
|
|
11,158 |
|
| Loss on Extinguishment of Debt |
|
|
2,317 |
|
|
|
- |
|
|
|
2,317 |
|
| Gain on PPP Loan forgiveness |
|
|
(6,824 |
) |
|
|
- |
|
|
|
(6,824 |
) |
| Other expense, net |
|
|
194 |
|
|
|
- |
|
|
|
194 |
|
| Income before income taxes |
|
|
11,231 |
|
|
|
3,267 |
|
|
|
14,498 |
|
| Income tax expense (benefit) |
|
|
11,301 |
|
|
|
(170 |
) |
|
|
11,131 |
|
| Net (loss) income |
|
$ |
(70 |
) |
|
$ |
3,437 |
|
|
$ |
3,367 |
|
2022 Credit Agreement
On
June 24, 2022, Waldencast entered into the 2022 Credit Agreement with a syndicate of lenders led by JPMorgan Chase Bank, N.A., as
administrative agent. The 2022 Credit Agreement provided the combined company with a term loan of $175.0 million (the “Term
Loan”) and a revolving credit facility of $11.1 million with a borrowing capacity up to $50.0 million (the “Revolver”),
for a total outstanding balance of $186.1 million at the Closing Date.
The
Term Loan and the Revolver will mature on July 26, 2026. Borrowings under the Term Loan were used to, among other things, repay outstanding
amounts under, and terminate, the existing credit facilities of Obagi and Milk.
Waldencast
is currently evaluating the accounting for the debt refinancing but has assumed that debt extinguishment accounting is applied for pro
forma purposes. Depending on the completion of such analysis, there may be changes in the amount of estimated debt extinguishment gains
or losses. However, such changes are not expected to be material. Refer to adjustments (L) and (II) for the impact of the 2022 Credit
Agreement on the Unaudited Pro Forma Condensed Combined Balance Sheet as of June 30, 2022 and Unaudited Pro Forma Condensed Combined Statement
of Operations for the six months ended June 30, 2022 and year ended December 31, 2021.
Note 3. Accounting
Policies
Management
has performed an initial review of the accounting policies of each entity to conform the accounting policies to those of Waldencast, the
accounting acquirer. In doing so, management identified presentation differences that would have an impact on the unaudited pro forma
condensed combined financial information and recorded necessary adjustments. In addition, adjustments were made to the historical presentation
of the statement of operations and balance sheets of Obagi and Milk to conform to the financial statement presentation Waldencast.
Management
will perform a comprehensive review of accounting policies for each entity and conform them to the accounting policies of Waldencast as
the accounting acquirer. As a result of the review, management may identify differences between the accounting policies of the entities
which, when conformed, could have a material impact on the financial statements of the post-combination company.
Note 4. Adjustments
to Pro Forma Condensed Combined Balance Sheet
Explanations
of the adjustments to the pro forma balance sheet are as follows:
| (A) | Reflects the reclassification of $345.3 million of cash
and cash equivalents and investments held in the trust account that became available for transaction consideration, transaction expenses,
underwriting commission, redemption of Waldencast public shares, and the operating activities of Waldencast following the Business Combination. |
| (B) | Reflects the payment of $76.2 million in estimated transaction
costs, net of the relief of prepaid transaction costs of $0.3 million, out of the total estimated transaction costs of approximately
$88.5 million, comprised of advisory, banking, printing, legal, and accounting fees and equity issuance costs that are expensed
as a part of the Business Combination. Of the $76.2 million of payment in estimated transactions costs, $18.9 million was previously
accrued, $12.1 million was recorded as deferred underwriting and the remaining balance of $45.5 million was expensed through
Accumulated Deficit, all of which were partially offset by the relief of prepaid transaction costs of $0.3 million. |
| (C) | Reflects the gross cash proceeds of $118.0 million generated
from the PIPE Financing through the issuance of 11,800,000 shares of Class A ordinary shares to the PIPE Investors and allocated
to Class A ordinary shares and Additional paid-in-capital using a par value of $0.0001 per share and a purchase price of $10.00
per share, respectively. |
| (D) | Reflects the gross proceeds from the Sponsor and Beauty Ventures
in the amount of $160.0 million and $173.0 million, respectively and allocated to Class A ordinary shares and Additional
paid-in capital using a par value of $0.0001 per shares and a purchase of $10.00 and the settlement of Waldencast’s related Forward
Purchase Agreements liability of $13.3 million through the issuance of Class A shares. |
| (E) | Represents the purchase price allocation adjustments resulting
from the Business Combination. The calculation of the purchase price and allocation to assets acquired and liabilities assumed is preliminary.
The preliminary allocation to assets and liabilities is based on estimates, assumptions, valuations, and other studies that have not
progressed to a stage where there is sufficient information to make a definitive calculation. Accordingly, the purchase price allocation
reflected in the unaudited transaction accounting adjustments will remain preliminary until Waldencast determines the final purchase
price and the fair values of assets acquired and liabilities assumed. The final determination of the purchase price and related allocation
is anticipated to be completed as soon as practicable after the completion of the Business Combination. Potential differences may include,
but are not limited to, changes in allocation to intangible assets and change in fair value of financing receivable, deferred tax liability,
and property and equipment. Equity consideration was calculated using a $8.72 share price as of the Closing Date. |
The
following summarizes the purchase price accounting pro forma adjustments made to the unaudited pro forma condensed combined balance sheet
as of June 30, 2022, net of reversals of any historical amounts:
| ($ in thousands) |
|
Milk |
|
|
Obagi |
|
|
Total |
|
| Cash consideration |
|
$ |
112,500 |
|
|
$ |
317,475 |
|
|
$ |
429,975 |
|
| Inventory fair value |
|
|
9,263 |
|
|
|
9,687 |
|
|
|
18,950 |
|
| Intangibles |
|
|
141,500 |
|
|
|
391,904 |
|
|
|
533,404 |
|
| Deferred Tax Liability |
|
|
- |
|
|
|
72,255 |
|
|
|
72,255 |
|
| Goodwill |
|
|
136,382 |
|
|
|
184,824 |
|
|
|
321,206 |
|
| Additional paid-in-capital |
|
|
174,644 |
|
|
|
196,681 |
|
|
|
371,325 |
|
Obagi Purchase
Price Allocation
The
following is a preliminary estimate of the fair value of consideration for Obagi and a preliminary purchase price allocation in connection
with the Business Combination.
| ($ in thousands) | |
| |
| Equity consideration | |
$ | 246,231 | |
| Cash consideration to Sellers | |
| 327,500 | |
| Cash consideration reduction related to Conditional Consent, Waiver & Acknowledgment | |
| (10,025 | ) |
| Fair value of vested roll-over equity awards | |
| 26,544 | |
| Repayment of debt | |
| 123,367 | |
| Total purchase consideration | |
$ | 713,617 | |
| Cash and cash equivalents | |
| 6,242 | |
| Restricted cash | |
| 650 | |
| Accounts and notes receivable, net | |
| 75,165 | |
| Inventories | |
| 30,475 | |
| Prepaid expenses | |
| 8,086 | |
| Other current assets | |
| 374 | |
| Property, plant and equipment, net | |
| 3,777 | |
| Intangible assets, net | |
| 462,000 | |
| Goodwill | |
| 229,313 | |
| Deferred income taxes | |
| - | |
| Other assets | |
| 1,274 | |
| Accounts payable | |
| (18,323 | ) |
| Other current liabilities | |
| (14,417 | ) |
| Current portion of long-term debt, net | |
| - | |
| Long-term debt, net | |
| - | |
| Deferred tax liability | |
| (70,380 | ) |
| Other liabilities | |
| (619 | ) |
| Fair value of net assets acquired from Obagi | |
$ | 713,617 | |
Purchase
Consideration: The estimated value of the Obagi rollover equity awards attributable to pre-combination service as of the
Closing Date of $26.5 million was included in the total purchase consideration. As part of the Obagi China Distribution, Waldencast
and Cedarwalk entered into the Conditional Consent, Waiver and Acknowledgment Agreement whereby the parties agreed that
any inventory on hand purchased by Obagi on behalf of the Obagi China Businesses would be settled at close via a reduction in
the cash consideration paid to the Obagi sellers by Waldencast. The reduction in the cash consideration amounted to $10.0
million as of the Closing Date.
Inventories: Obagi’s
inventory is mainly comprised of finished goods, as Obagi leverages contract manufacturers to source, manufacture and package its
products. Obagi carries minimal work-in-progress inventory as (i) an assortment of finished goods that are grouped together
by third-party packagers and sold as kits, and (ii) certain raw materials are purchased on behalf of some of its contract manufacturers.
The fair value of the finished goods was determined using the comparative sales method, which utilizes the actual or expected selling
prices of finished goods to customers as a basis for determining fair market values of those finished goods.
Intangible
Assets: The following describes intangible assets that may be identified that met either the separability criterion or the
contractual-legal criterion described in ASC 805, and the anticipated valuation approach. The trademarks represent the over-arching
name of all Obagi’s products and is valued using a relief from royalty method. Formulations developed by Obagi differentiate
them from others in the market and are valued using the relief from royalty method. The customer lists represent the relationships
that Obagi has with partners, resulting in distribution covering over 60 countries around the world, also valued using a relief from royalty
method. The licensing agreements give Obagi the right to use certain IP assets of Rohto, as well as allowing Rohto to use the Obagi
name for products they manufacture and distribute in Japan for which Obagi receives a royalty. The licensing agreements are valued
using the discounted cash flow method.
| ($ in thousands, except for weighted average useful life) | |
Weighted
average
useful life
(years) | | |
Fair value | | |
Amortization
expense
for the
six months
ended
June 30,
2022 | | |
Amortization
expense
for the
year ended
December 31,
2021 | |
| Trade name/trademark | |
| Indefinite | | |
$ | 297,000 | | |
$ | - | | |
$ | - | |
| Licensing agreements | |
| 10 | | |
| 132,000 | | |
| 6,600 | | |
| 13,200 | |
| Formulations | |
| 10 | | |
| 10,000 | | |
| 500 | | |
| 1,000 | |
| Customer / distributor relationships | |
| 10 | | |
| 23,000 | | |
| 1,150 | | |
| 2,300 | |
| Reversal of historical balance and amortization | |
| N/A | | |
| (70,096 | ) | |
| (6,756 | ) | |
| (13,469 | ) |
| Total Adjustment | |
| | | |
$ | 391,904 | | |
$ | 1,494 | | |
$ | 3,031 | |
Deferred
Tax Liability: This adjustment reflects the establishment of deferred tax liabilities on the Obagi fair value purchase
accounting adjustments, resulting in an ending deferred tax liability balance of $70.4 million. Note that this deferred tax liability
is a sufficient source of income to realize the historical Obagi deferred tax assets and these adjustments include the
release of the historical Obagi valuation allowance of $14.3 million. The unaudited pro forma condensed combined financial information
does not reflect the income tax effects of the Milk pro forma adjustments as any change in the deferred tax balance would be offset
by an increase in the valuation allowance given that Milk has incurred cumulative losses in recent years.
Goodwill: Goodwill
represents Obagi’s assembled workforce, as well as the future economic benefits arising from the results of the Business Combination
that will enhance Obagi products available to both new and existing customers, and increase its competitive position. The pro forma
adjustment of $184.8 million reflects the goodwill generated from the Obagi Merger of $229.3 million, net of the elimination
of historical Obagi goodwill of $44.5 million.
Milk Purchase Price
Allocation
The
following is a preliminary estimate of the fair value of consideration for Milk transferred and a preliminary purchase price allocation
in connection with the Business Combination.
| ($ in thousands) | |
| |
| Equity consideration | |
$ | 184,029 | |
| Cash consideration to Sellers | |
| 112,500 | |
| Fair value of vested roll-over equity awards | |
| 5,912 | |
| Equity based compensation treated as consideration (Acceleration of Awards) | |
| 8,599 | |
| Repayment of debt | |
| 1,500 | |
| Total purchase consideration | |
$ | 312,540 | |
| Cash | |
| 2,769 | |
| Accounts receivable, net | |
| 6,390 | |
| Inventories | |
| 34,098 | |
| Prepaid expenses and other current assets | |
| 453 | |
| Prepaid supplier | |
| 387 | |
| Property, plant equipment, net | |
| 5,938 | |
| Intangible assets, net | |
| 141,500 | |
| Goodwill | |
| 136,382 | |
| Accounts payable | |
| (6,590 | ) |
| Tenant allowance liability - current | |
| (161 | ) |
| Accrued expenses and other current liabilities | |
| (6,328 | ) |
| Line of credit | |
| - | |
| Warrant liabilities | |
| (148 | ) |
| Deferred rent - non-current | |
| (1,172 | ) |
| Tenant allowance liability - non-current | |
| (978 | ) |
| Fair value of net assets acquired from Milk | |
$ | 312,540 | |
Purchase
Consideration: The equity consideration of $184.0 million reflects the fair value of the Milk Members’ noncontrolling
economic interest in Waldencast LP Common Units, which will be redeemable at the option of the holder of such units, and if such
option is exercised, will be exchangeable, at the option of Waldencast, for an equal number of Class A ordinary shares or cash
(together with the cancellation of an equal number of shares of voting, Class B ordinary shares) into Class A ordinary
shares on a 1-for-1 basis. $8.6 million of the total purchase consideration relates to the acceleration of equity awards for
certain Milk executives. Of that amount, $1.7 million relates to equity awards with a performance component based on Milk’s
value at the Closing Date. The $1.7 million was based on the achievement of performance objectives that resulted in 33% of the objective
achieved. As such, 33% of the awards were accelerated. The remaining 67% of the performance awards were forfeited.
Inventories: Milk
utilizes contract manufacturers to source, manufacture, and package its products. Thus, Milk primarily holds finished goods inventory.
The fair value of the finished goods was determined using the comparative sales method, which utilizes the actual or expected selling
prices of finished goods to customers as a basis for determining fair market values of those finished goods.
Intangible
Assets: The following describes intangible assets that may be identified that met either the separability criterion or the
contractual-legal criterion described in ASC 805, and the anticipated valuation approach. The trademarks represent Milk’s increasing
brand presence in the cosmetics and skincare market and is valued using a relief from royalty method. Formulations developed by Milk
represent their proprietary formulas in their products, and they are upgraded every 3-5 years. The formulations are valued using
the relief from royalty method. The customer/distributor relationships mainly consisted of Milk’s relationship with Sephora,
its key distributor. Sales directly to Sephora accounted for approximately 74% of gross revenue and sales indirectly to Sephora through
major retailer distribution arrangements accounted for approximately 17% of gross revenue for the six months ended June 30, 2022.
The customer/distributor relationships are valued using the distributor method.
The
unaudited pro forma condensed combined financial information does not reflect the income tax effects of the Milk pro forma adjustments
as any change in the deferred tax balance would be offset by an increase in the valuation allowance given that Milk has incurred cumulative
losses in recent years.
| ($ in thousands, except for weighted average useful life) |
|
Weighted
average
useful life
(years) |
|
|
Fair value |
|
|
Amortization
expense
for the
six months
ended
June 30,
2022 |
|
|
Amortization
expense
for the
year ended
December 31,
2021 |
|
| Trade name/trademark |
|
|
15 |
|
|
$ |
126,000 |
|
|
$ |
4,200 |
|
|
$ |
8,400 |
|
| Formulations |
|
|
6 |
|
|
|
1,500 |
|
|
|
125 |
|
|
|
250 |
|
| Customer / distributor relationships |
|
|
13 |
|
|
|
14,000 |
|
|
|
538 |
|
|
|
1,077 |
|
| Reversal of historical balance and amortization |
|
|
N/A |
|
|
|
- |
|
|
|
- |
|
|
|
(14 |
) |
| Total |
|
|
|
|
|
$ |
141,500 |
|
|
$ |
4,863 |
|
|
$ |
9,713 |
|
Goodwill: Goodwill
of $136.4 million represents Milk’s assembled workforce, as well as the future economic benefits arising from the results of
the business combination that will enhance Milk’s products available to both new and existing customers, and increase Milk’s
competitive position.
| (F) | Reflects the reclassification of $345.3 million of Class A
ordinary shares subject to possible redemption from temporary equity to permanent equity and $147.2 million related to the settlement
of Milk Preferred Units into additional paid in capital of the combined company. |
| (G) | Represents the elimination of the historical accumulated
deficit of Obagi and Milk of $2.6 million and $122.6 million, respectively, into additional paid in capital of the combined
company as part of the purchase accounting adjustments. |
| (H) | Reflects the settlement of Obagi’s historical equity
into additional paid in capital of $4.0 million. |
| (I) | Represents the transaction accounting adjustments to record
the noncontrolling interest in Milk of $170.4 million, which was calculated based on the noncontrolling interest percentage of 19.6%
multiplied by the pro forma net assets of the combined company. |
| (J) | Represents the stock settlement of amounts due from an officer
of $0.7 million, which reduced the equity consideration to Milk by 67,778 Class A ordinary shares, and cash settlement of amounts due
from other officers of $0.1 million. |
| (K) | Represents the conversion of the Working Capital Loan into
warrants. |
| (L) | Represents the proceeds from the 2022 Credit Agreement, net
of unamortized debt issuance costs, repayment of historical Obagi and Milk debts, elimination of historical Obagi debt issuance costs
and payment of payment of exit fees from the extinguishment of historical Obagi debt as further shown below: |
| ($ in thousands) | |
Total | |
| New debt | |
| 186,117 | |
| New debt issuance costs (contra-liability) | |
| (3,884 | ) |
| New debt issuance costs (asset)(1) | |
| (1,110 | ) |
| Repayment of historical Obagi debt(2) | |
| (127,351 | ) |
| Obagi debt exit fee(2) | |
| (6,327 | ) |
| Repayment of historical Milk Debt | |
| (1,500 | ) |
| Impact to Cash and Cash Equivalents | |
| 45,944 | |
| Elimination of historical Obagi debt issuance costs(3) | |
| (3,984 | ) |
| Obagi debt exit fee(2) | |
| (6,327 | ) |
| Deferred tax liability | |
| 2,423 | |
| Total Impact to Accumulated Deficit | |
| (7,888 | ) |
| | |
Current | | |
Long-term | | |
Total | |
| New debt issuance costs (asset)(1) | |
| 278 | | |
| 832 | | |
| 1,110 | |
| Total impact to Assets | |
| 278 | | |
| 832 | | |
| 1,110 | |
| | |
Current | | |
Long-term | | |
Total | |
| Repayment of historical Obagi debt | |
| (25,125 | ) | |
| (102,226 | ) | |
| (127,351 | ) |
| Elimination of historical Obagi debt issuance costs(3) | |
| 2,522 | | |
| 1,462 | | |
| 3,984 | |
| Deferred tax liability | |
| - | | |
| (2,423 | ) | |
| (2,423 | ) |
| Repayment of historical Milk debt | |
| (1,500 | ) | |
| - | | |
| (1,500 | ) |
| New debt | |
| 8,750 | | |
| 177,367 | | |
| 186,117 | |
| New debt issuance costs (contra-liability) | |
| (971 | ) | |
| (2,913 | ) | |
| (3,884 | ) |
| Total impact to liabilities | |
| (16,324 | ) | |
| 71,266 | | |
| 54,942 | |
| (1) | The new debt issuance costs related to the Revolver of approximately
$1.1 million are classified as a deferred asset. |
| (2) | As of the Closing Date, the proceeds from the new debt of
were used to 1) repay Obagi’s historical debt, which had an outstanding balance of $128.8 million 2) pay accrued interest expense
of $0.8 million, and 3) pay an exit fee of $6.3 million, which is reflected as part of Loss on Extinguishment of Debt on the Unaudited
Pro Forma Condensed Combined Statement of Operations for the year ended December 31, 2021, represented by adjustment (II). |
| (3) | The elimination of historical Obagi debt issuance costs is
reflected as part of Loss on Extinguishment of Debt on the Unaudited Pro Forma Condensed Combined Statement of Operations for the year
ended December 31, 2021, represented by adjustment (II). |
| (M) | Reflects the redemption of 30,021,946 public shares for aggregate
redemption payments of $300.9 million allocated to Class A ordinary shares and Additional Paid-In Capital using a par value $0.0001 per
share and a redemption price of $10.02 per share, respectively. |
| (N) | Reflects the cash settlement of Milk’s warrants. |
| (O) | Reflects the issuance of additional 20,000 Class A ordinary
shares to investor directors at the Closing Date. |
Note 5. Adjustments
to Pro Forma Condensed Combined Statements of Operations
Explanations of the adjustments
to the pro forma statement of operations are as follows:
| (AA) | Represents the elimination of investment income related to
the marketable securities held in the trust account. |
| (BB) | Reflects the total estimated transaction costs expensed as
a part of the Business Combination in the statement of operations for the year ended December 31, 2021. Transaction costs are reflected
as if incurred on January 1, 2021, the date the Business Combination is deemed to have occurred for the purposes of the unaudited
pro forma condensed statement of operations. |
| (CC) | For the six month period ended June 30, 2022, this adjustment
reflects the estimated income tax effect of the pro forma adjustments. The tax effect of the pro forma adjustments was calculated using
the historical statutory rates in effect for the periods presented. The tax effect does not reflect the income tax effects of the Milk
pro forma adjustments as any deferred tax effect would be offset by an increase in the valuation allowance given that Milk has incurred
cumulative losses in recent years. Transaction costs expected to be incurred in connection with the Business Combination have not been
assessed for deductibility for income tax purposes and accordingly are assumed to be nondeductible for pro forma purposes. For the year
ended December 31, 2021, this adjustment reflects (1) the removal of the 2021 Obagi valuation allowance of $14.3 million
that was recorded in 2021 and will be removed as a proforma adjustment due to the company being able realize its deferred tax assets
after including purchase accounting deferred tax liabilities, and (2) the estimated income tax effect of the pro forma adjustments. The
tax effect of the pro-forma adjustments was calculated using the historical statutory rates in effect for the periods presented. The
tax effect does not reflect the income tax effects of the Milk pro forma adjustments as any deferred tax effect would be offset by an
increase in the valuation allowance given that Milk has incurred cumulative losses in recent years. Transaction costs incurred in connection with the Business Combination have not been assessed for deductibility for income tax purposes and
accordingly are assumed to be nondeductible for pro forma purposes. |
| (DD) | Reflects noncontrolling interest in Milk. For the six months
ended June 30, 2022, net income attributable to noncontrolling interest was $3.3 million. For the year ended December 31, 2021,
net loss attributable to noncontrolling interest was $19.1 million. |
| (EE) | Obagi Worldwide entered into the IP License Agreement with
Obagi Hong Kong, which requires Obagi Hong Kong to pay Obagi a royalty of 5.5% on gross sales of licensed products and services, less
taxes and refunded returns. This adjustment reflects royalty income of $0.3 million and $1.4 million that would have been received
for the six months ended June 30, 2022 and year ended December 31, 2021, respectively. |
| (FF) | Reflects the incremental fair value of unvested equity awards
that will roll over into equity awards of Waldencast. The fair value was measured and recorded using the Black-Scholes model using the
assumed stock price of Waldencast shares at Closing of $8.72 and the exercise price of the original awards converted based on the contractual
exchange ratios for the rollover. |
| (GG) | Reflects the incremental amortization expense and cost of
goods sold related to the fair value adjustments for intangible assets and inventory for both Milk and Obagi. Refer to the tables below
show the breakout of each of these adjustments: |
| | |
Intangible Assets Adjustments | |
| ($ in thousands) | |
Amortization
expense
for the
six months
ended
June 30,
2022 | | |
Amortization
expense
for the
year ended
December 31,
2021 | |
| Obagi | |
| | |
| |
| Trade name/trademark | |
$ | - | | |
$ | - | |
| Licensing agreements | |
| 6,600 | | |
| 13,200 | |
| Formulations | |
| 500 | | |
| 1,000 | |
| Customer / distributor relationships | |
| 1,150 | | |
| 2,300 | |
| Reversal of historical amortization of intangible assets | |
| (6,756 | ) | |
| (13,469 | ) |
| Milk | |
| | | |
| | |
| Trade name/trademark | |
| 4,200 | | |
| 8,400 | |
| Formulations | |
| 125 | | |
| 250 | |
| Customer / distributor relationships | |
| 538 | | |
| 1,077 | |
| Reversal of historical amortization of intangible assets | |
| - | | |
| (14 | ) |
| Total | |
$ | 6,357 | | |
$ | 12,744 | |
| | |
Inventory Adjustments | |
| ($ in thousands) | |
Cost of goods
sold for the
six months
ended
June 30,
2022 | | |
Cost of goods
sold for the
year ended
December 31,
2021 | |
| Milk | |
$ | - | | |
$ | 9,263 | |
| Obagi | |
$ | - | | |
$ | 9,687 | |
| Total | |
$ | - | | |
$ | 18,950 | |
| (HH) | Represents the elimination of the fair value adjustment as
Waldencast’s Forward Purchase Agreements liability will be settled in connection with the Business Combination. |
| (II) | Represents differences in interest expense and amortization
of debt issuance costs and a one-time charge to Loss on Extinguishment of Debt on the Unaudited Pro Forma Condensed Combined Statement
of Operations for the year ended December 31, 2021, as a result of incurring new debt under the 2022 Credit Agreement and extinguishing
historical Obagi debt under the 2021 Credit Agreement. The interest rate of the new debt is 5.9%, which is the sum of the Term SOFR rate
of 2.3%, plus 0.1% (“Adjusted Term SOFR Rate”) and the Applicable Rate of 3.5%, as defined in the 2022 Credit Agreement. |
| ($ in thousands) | |
Interest
expense
for the
six months
ended
June 30,
2022 | | |
Interest
expense
for the
year ended
December 31,
2021 | |
| Elimination of historical Obagi interest expense and amortization of debt issuance costs | |
$ | (5,719 | ) | |
$ | (11,158 | ) |
| Interest expense and amortization of debt issuance costs on new debt | |
| 5,896 | | |
| 12,151 | |
| Income tax expense attributed to the elimination of historical Obagi interest expense and amortization of debt issuance costs | |
| 1,344 | | |
| 2,622 | |
| Transaction Accounting Adjustment | |
$ | 1,521 | | |
$ | 3,615 | |
A
0.125% change in the floating rate would result in the following additional expense by period:
| Borrowing Type | |
Total outstanding
principal
as of
Closing | | |
Change in floating
rate | | |
Interest expense
for the
year ended
December 31,
2021 | | |
Interest expense
for the
six months ended
June 30,
2022 | |
| Term Loan | |
| 175,000 | | |
| 0.125 | % | |
| 219 | | |
| 55 | |
| Revolver | |
| 11,117 | | |
| 0.125 | % | |
| 14 | | |
| 3 | |
| (JJ) | Reflects the compensation cost associated with the issuance
of additional 20,000 Class A ordinary shares to investor directors at the Closing Date. |
Note 6. Pro Forma
Earnings Per Share Information
As
a result of the Business Combination, for the six months ended June 30, 2022, the pro forma basic number of shares is reflective of 86,460,560
Class A ordinary shares outstanding. The diluted number of shares is reflective of 111,475,855 Class A ordinary shares after
giving effect to the conversion of Class B ordinary shares (and exchange of the related noncontrolling interests) and certain dilutive
stock compensation awards. For the year ended December 31, 2021, both the pro forma basic and diluted number of shares are reflective
of 86,460,560 Class A ordinary shares outstanding because all potentially dilutive securities are antidilutive due to the pro forma
net loss.
| (in thousands, except share data) |
|
Six Months
Ended
June 30,
2022 |
|
|
Year Ended
December 31,
2021 |
|
| Pro forma net income/(loss) attributable to Waldencast |
|
$ |
13,591 |
|
|
$ |
(88,227 |
) |
| Weighted average shares of Class A ordinary shares outstanding - basic |
|
|
86,460,560 |
|
|
|
86,460,560 |
|
| Net income/(loss) per share - basic |
|
$ |
0.16 |
|
|
$ |
(1.02 |
) |
| (in thousands, except share data) |
|
Six Months
Ended
June 30,
2022 |
|
|
Year Ended
December 31,
2021 |
|
| Pro forma net income/(loss) attributable to Waldencast |
|
$ |
13,591 |
|
|
$ |
(88,227 |
) |
| Plus: additional income, net of estimated taxes, upon conversion of noncontrolling interests and Class B non-economic voting shares |
|
|
3,313 |
|
|
|
- |
|
| Pro forma net income/(loss) for dilutive purposes |
|
$ |
16,904 |
|
|
$ |
(88,227 |
) |
| Weighted average pro forma shares of Class A ordinary shares outstanding |
|
|
86,460,560 |
|
|
|
86,460,560 |
|
| Plus: additional shares upon conversion of noncontrolling interests and Class B non-economic voting shares |
|
|
21,104,225 |
|
|
|
- |
|
| Plus: dilutive effect of stock compensation awards |
|
|
3,911,070 |
|
|
|
- |
|
| Weighted average pro forma shares of Class A ordinary shares outstanding - diluted |
|
|
111,475,855 |
|
|
|
86,460,560 |
|
| Net income/(loss) per share - diluted |
|
$ |
0.15 |
|
|
$ |
(1.02 |
) |
Earnings
per share (“EPS”) exclude warrants that would be anti-dilutive to pro forma EPS. Below is a summary of anti-dilutive instruments
that were excluded from the pro forma EPS for the six months ended June 30, 2022 and year ended December 31, 2021:
| | |
Six Months
Ended
June 30,
2022 | | |
Year Ended
December 31,
2021 | |
| Public Warrants (IPO) | |
| 11,499,950 | | |
| 11,499,950 | |
| Private Placement Warrants (Founder) | |
| 5,933,333 | | |
| 5,933,333 | |
| Warrants (Sponsor Forward Purchase Agreement) | |
| 5,333,333 | | |
| 5,333,333 | |
| Warrants (3rd Party Forward Purchase Agreement) | |
| 5,766,666 | | |
| 5,766,666 | |
| Warrants (Working Capital Loan)(1) | |
| 1,000,000 | | |
| 1,000,000 | |
| (1) | During the year ended December 31, 2021, certain Waldencast
officers and directors loaned Waldencast funds in the form of promissory notes for working capital needs. At the Closing Date, the notes
were converted into warrants at a price of $1.50 per warrant. Such warrants will be identical to the Private Placement Warrants. The
warrants have been reflected in the pro forma financial statements (See adjustment K above). The warrants are anti-dilutive at Closing
and do not impact dilutive EPS. |
SELECTED UNAUDITED
PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The
following summary unaudited pro forma condensed combined financial data (the “summary pro forma data”) gives effect to the
Business Combination contemplated by the Business Combination Agreement described in the section entitled “Unaudited Pro Forma
Condensed Combined Financial Information.”
The
summary unaudited pro forma condensed combined balance sheet data as of June 30, 2022 gives pro forma effect to the Business Combination
as if it had occurred on June 30, 2022. The summary unaudited pro forma condensed combined statement of operations data for the six
months ended June 30, 2022 and for the year ended December 31, 2021 gives pro forma effect to the Business Combination as if it had
occurred on January 1, 2021.
The
summary pro forma data have been derived from, and should be read in conjunction with, the unaudited pro forma condensed combined financial
information of the combined company appearing elsewhere in this Form F-1 and in our definitive proxy statement/final prospectus dated
July 7, 2022, and filed by us with the SEC on July 7, 2022 (including the accompanying notes). The unaudited pro forma condensed combined
financial information is based upon, and should be read in conjunction with, the historical financial statements of Waldencast, Obagi
and Milk and related notes included in this Form F-1 and in our definitive proxy statement/final prospectus dated July 7, 2022, and
filed by us with the SEC on July 7, 2022. The summary pro forma data have been presented for informational purposes only and are not necessarily
indicative of what the combined company’s financial position or results of operations actually would have been had the Business
Combination been completed as of the dates indicated. In addition, the summary pro forma data do not purport to project the future financial
position or operating results of the combined company.
The
following table presents summary pro forma data after giving effect to the Business Combination:
| (in thousands, except share and per share
data) | |
Pro Forma
Combined | |
| | |
| |
| Summary Unaudited Pro Forma Condensed Combined Statement of Operations Six
Months June 30, 2022 | |
| |
| Revenue | |
| 138,202 | |
| Net income | |
| 16,904 | |
| Pro forma adjusted EBITDA | |
| 32,448 | |
| Net income per share - basic | |
| 0.16 | |
| Weighted-average Class A ordinary shares outstanding - basic | |
| 86,460,560 | |
| Net income per share - diluted | |
| 0.15 | |
| Weighted-average Class A ordinary shares outstanding - diluted | |
| 111,475,855 | |
| | |
| | |
| Summary Unaudited Pro Forma Condensed Combined Statement of Operations Year
Ended December 31, 2021 | |
| | |
| Revenue | |
| 225,757 | |
| Net loss | |
| (107,334 | ) |
| Pro forma adjusted EBITDA | |
| 38,772 | |
| Net loss per share - basic and diluted | |
| (1.02 | ) |
| Weighted-average Class A ordinary shares outstanding - basic and diluted | |
| 86,460,560 | |
| | |
| | |
| Summary Unaudited Pro Forma Condensed Combined Balance Sheet as of June 30,
2022 | |
| | |
| Total assets | |
| 1,181,406 | |
| Total liabilities | |
| 311,969 | |
| Total equity | |
| 869,437 | |
We
believe that pro forma adjusted EBITDA is useful to investors as a means of evaluating operating performance and reflects the EBITDA of
Waldencast, Obagi, and Milk on a combined basis. Pro forma adjusted EBITDA is a non-GAAP measure, which is an addition to, and not a substitute
for or superior to, measures of financial performance prepared in accordance with GAAP and should not be considered as alternatives to
performance measures derived in accordance with GAAP. Non-GAAP financial measures as used by us may not be comparable to similarly titled
amounts used by other companies.
We
believe these non-GAAP financial measures:
| |
● |
reflect the ongoing business of the combined company in a manner that allows for meaningful period-to-period comparison and analysis of trends in its business, as they exclude certain non-recurring income and expense that do not occur regularly as part of the normal activities; |
| |
● |
provide useful information in understanding and evaluating the underlying sustainable performance of the combined business across periods; and |
| |
● |
provide a normalized view of the operating performance of the combined business by excluding items that are either noncash or infrequently occurring in nature. |
Pro
forma adjusted EBITDA is one of the primary measures management of the combined company will use for planning and budgeting processes,
and to monitor and evaluate financial and operating results.
Pro
forma adjusted EBITDA gives effect to the Business Combination (including the Obagi China Distribution) as if it had been consummated
on January 1, 2021.
The
table below presents our pro forma adjusted EBITDA reconciled to our net Income/(loss), the closest GAAP measure for the period indicated:
| (in thousands) | |
Six months
ended
June 30,
2022 | | |
Year ended
December 31,
2021 | |
| Pro Forma Net Income (Loss) | |
$ | 16,904 | | |
$ | (107,334 | ) |
| Adjusted For: | |
| | | |
| | |
| Interest expense, net | |
| 5,916 | | |
| 12,168 | |
| Income tax benefit | |
| 232 | | |
| (6,999 | ) |
| Depreciation and amortization | |
| 14,602 | | |
| 28,299 | |
| Transaction costs(1) | |
| - | | |
| 76,434 | |
| Loss on extinguishment of debt | |
| - | | |
| 12,628 | |
| Gain on PPP loan forgiveness | |
| - | | |
| (6,824 | ) |
| Stock-based compensation expense | |
| 2,848 | | |
| 5,927 | |
| Inventory fair value adjustment(2) | |
| - | | |
| 18,950 | |
| Change in fair value of warrant liabilities | |
| (8,602 | ) | |
| 2,964 | |
| Restructuring costs(3) | |
| 289 | | |
| 1,972 | |
| Loss on disposal of assets | |
| 24 | | |
| 166 | |
| Foreign currency transaction loss | |
| 235 | | |
| 421 | |
| Pro Forma Adjusted EBITDA | |
$ | 32,448 | | |
$ | 38,772 | |
| (1) | Includes mainly professional service fees in connection with
the Business Combination. |
| (2) | Reflects non-cash, non-recurring fair value inventory step-up
adjustment as part of the purchase accounting in connection with the Business Combination. |
| (3) | Reflects one-time expenses in connection with realignment
of Obagi’s organizational structure to support new strategies and relocate corporate headquarters. |
Waldencast’s
Management’s Discussion and Analysis of
Financial Condition and Results of Operations
References
in this prospectus to “we,” “us” or the “Company” refer to Waldencast plc. References to our “management”
or our “management team” refer to our officers and directors, and references to the “Sponsor” refer to Waldencast
Long-Term Capital LLC. The following discussion and analysis of our financial condition and results of operations should be read in conjunction
with the financial statements and the notes thereto contained elsewhere in this prospectus. Certain information contained in the discussion
and analysis set forth below includes forward-looking statements that involve risks and uncertainties.
Overview
We
were a blank check company incorporated in the Cayman Islands on December 8, 2020 formed for the purpose of effecting a merger, share
exchange, asset acquisition, share purchase, reorganization or similar business combination. We effectuated the Business Combination using
cash derived from the proceeds of the initial public offering and the sale of the private placement warrants, our shares, debt or a combination
of cash, shares and debt.
We
completed our initial public offering on March 18, 2021 and completed the Business Combination on July 27, 2022 as described below.
Recent Developments
On
July 27, 2022, subsequent to the fiscal quarter ended June 30, 2022, the fiscal quarter to which the accompanying financial statements
relate, we consummated the Business Combination with (i) Obagi and Merger Sub pursuant to the Obagi Merger Agreement, by and among us,
Obagi and Merger Sub; and (ii), Milk, Holdco 1, Waldencast LP, the Milk Members, and the Equityholder Representative, pursuant to the
Milk Equity Purchase Agreement by and among us, Holdco 1, Waldencast LP, Milk, the Milk Members and the Equityholder Representative.
Upon
the consummation of the Business Combination: (i) Merger Sub merged with and into Obagi and the separate corporate existence of
Merger Sub ceased, with Obagi surviving as our indirect subsidiary; (ii) in connection with the Obagi Merger, among other things, each
outstanding ordinary share of Obagi as of immediately prior to the effective time of the Obagi Merger (other than in respect of Excluded
Shares (as defined in the Obagi Merger Agreement)) was cancelled and converted into the right to receive (a) an amount in cash equal to
the quotient obtained by dividing (1) the Obagi Cash Consideration (as defined in the Obagi Merger Agreement) by (2) the number of Aggregate
Fully Diluted Company Common Shares (as defined in the Obagi Merger Agreement), and (b) a number of Class A ordinary shares equal to the
quotient obtained by dividing (1) the Obagi Stock Consideration (as defined in the Obagi Merger Agreement) by (2) the number of Aggregate
Fully Diluted Company Common Shares; (iii) the Milk Purchasers acquired from the Milk Members and the Milk Members sold to the Milk Purchasers
the Milk Membership Units in exchange for the Milk Cash Consideration (as defined in the Milk Equity Purchase Agreement), and the Milk
Equity Consideration (as defined in the Milk Equity Purchase Agreement), which consist of Waldencast LP Common Units exchangeable for
our Class A ordinary shares, and our Class B ordinary shares; and (iv) in connection with the Milk Transaction, among other things, (a)
Holdco 1 purchased from the Milk Members a percentage of the Milk Membership Units in exchange for (1) the Milk Cash Consideration and
(2) a number of our Class B ordinary shares equal to the Milk Equity Consideration and (b) Waldencast LP purchased from the Milk Members
the remainder of the outstanding Milk Membership Units in exchange for the Milk Equity Consideration.
Immediately
following consummation of the Business Combination, (i) Holdco 1 contributed its equity interest in (a) Milk to Waldencast LP in exchange
for limited partnership units in Waldencast LP and (b) Holdco 2 in exchange for limited partnership units in Waldencast LP. The combined
company is organized in an “Up-C” structure, in which the equity interests of Obagi and Milk are held by Waldencast LP. We,
in turn, hold our interests in Obagi and Milk through Waldencast LP and Holdco 1.
Prior
to the consummation of the Business Combination, following the approval of our shareholders, and in accordance with the Cayman Act, the
Jersey Companies Law and our Constitutional Document, we effected a deregistration under the Cayman Act and a domestication under Part
18C of the Jersey Companies Law (by means of filing a memorandum and articles of association with the Registrar of Companies in Jersey),
pursuant to which our jurisdiction of incorporation was changed from the Cayman Islands to Jersey. Upon the effective time of the Domestication,
we were renamed “Waldencast plc.”
In
connection with the Domestication, (i) each of the then issued and outstanding Waldencast Acquisition Corp. Class
A ordinary shares, par value $0.0001 per share, was converted automatically, on a one-for-one basis, into a Class A ordinary share,
(ii) each of the then issued and outstanding Waldencast Acquisition Corp. Class B ordinary
shares, par value $0.0001 per share, was converted automatically, on a one-for-one basis, into a Class A ordinary share, (iii) each
then issued and outstanding Waldencast Acquisition Corp. warrant was converted automatically
into a warrant, pursuant to the Warrant Agreement, dated March 15, 2021, between us and Continental Stock Transfer & Trust Company,
as warrant agent, and (iv) each then issued and outstanding Waldencast Acquisition Corp. unit
was cancelled and the holders thereof were entitled to one Class A ordinary share and one-third of one warrant.
As
previously disclosed, in connection with our initial public offering; (i) on February 22, 2021, we, the Sponsor and Dynamo Master Fund
(a member of the Sponsor) entered into the Sponsor Forward Purchase Agreement, which was subsequently amended by the assignment and assumption
agreement entered into by and between the Sponsor and Burwell on December 20, 2021, under which the Sponsor assigned, and Burwell assumed,
all of the Sponsor’s rights and benefits under the Sponsor Forward Purchase Agreement, pursuant to which, Burwell and Dynamo Master
Fund committed to subscribe for and purchase 16,000,000 Class A ordinary shares and 5,333,333 warrants for an aggregate commitment amount
of the Sponsor FPA Investment; and (ii) the Company and Beauty Ventures LLC entered into the Third-Party Forward Purchase Agreement, pursuant
to which Beauty Ventures committed to subscribe for and purchase 17,300,000 Class A ordinary shares and up to 5,766,666 warrants for an
aggregate commitment amount of $173,000,000 (together with the Sponsor FPA Investment, the “FPA Investments”). The FPA Investments
were consummated substantially concurrently with the consummation of the Business Combination.
As
previously disclosed, on November 14, 2021, concurrently with the execution of the Transaction Agreements, we entered into the Initial
Subscription Agreements, executed on or prior to November 14, 2021, pursuant to which the Initial PIPE Investors agreed to purchase, in
the aggregate, the Initial PIPE Investment, consisting of 10,500,000 Class A ordinary shares at $10.00 per share for an aggregate
commitment amount of $105.0 million. The Transaction Agreements provided that we could enter into additional subscription agreements with
investors to participate in the purchase of our shares after November 15, 2021 but prior to the Closing Date. On June 14, 2022, we entered
into the June Subsequent Subscription Agreements with the June Subsequent PIPE Investors on the same terms as the Initial PIPE Investors,
pursuant to which the June Subsequent PIPE Investors collectively subscribed for the June Subsequent PIPE Investment, consisting of 800,000
shares of Class A ordinary shares for an aggregate purchase price equal to $8.0 million. On July 15, 2022, we entered into the July Subsequent
Subscription Agreements the July Subsequent PIPE Investors on the same terms as the Initial PIPE Investors and the June Subsequent PIPE
Investors. Pursuant to, and on the terms and subject to the conditions of the applicable July Subsequent Subscription Agreement, the July
Subsequent PIPE Investors collectively subscribed for 500,000 Class A ordinary shares for an aggregate purchase price equal to $5,000,000.
Results of Operations
As
of June 30, 2022, we had neither engaged in any operations nor generated any operating revenues. Our only activities through June 30,
2022 were organizational activities and those necessary to prepare for the initial public offering,
the search for a prospective initial business combination, and the negotiation and execution of the Business Combination. We did not generate
any operating revenues until after the completion of the Business Combination. Following the consummation
of the initial public offering, we generated non-operating income in the form of
interest income on cash and cash equivalents from the net proceeds derived from the initial public offering and the sale of the private
placement warrants. We expect that we will incur increased expenses as a result of being a public company (for legal, financial reporting,
accounting and auditing compliance).
For
the three months ended June 30, 2022, we had a net income of $2,767,058, which consisted of a non-cash change in fair value of warrant
derivative liabilities and Forward Purchase Agreement liabilities of $4,243,333 and $2,664,000, respectively, and interest income from
operating bank account of $126, and interest income on marketable securities held in the trust account
established at the consummation of our initial public offering at J.P. Morgan Chase Bank, N.A. and maintained by Continental, acting as
trustee (the “Trust Account”) of $257,125, offset by operating costs of $4,397,526.
For
the six months ended June 30, 2022, we had a net income of $5,789,973, which consisted of a non-cash change in fair value of warrant derivative
liabilities and Forward Purchase Agreement liabilities of $8,601,666 and $5,328,000, respectively, and interest income from operating
bank account of 565, and interest income on marketable securities held in the Trust Account of $260,745, offset by operating costs of
$8,401,003.
For
the three months ended June 30, 2021, we had a net loss of $1,551,508, which included a loss from operations of $201,567, a loss from
the change in fair value of warrant liabilities and Forward Purchase Agreement liabilities of $697,334 and 666,000, respectively, offset
by interest income from operating bank account of $311 and interest income on marketable securities held in the Trust Account of $13,082.
For
the six months ended June 30, 2021, we had a net loss of $2,735,465, which included a loss from operations of $319,082, offering cost
expense allocated to warrants of $719,201, a loss from the change in fair value of warrant liabilities and Forward Purchase Agreement
liabilities of $1,046,000 and $666,000, respectively, offset by interest income from operating bank account of $442 and interest income
on marketable securities held in the Trust Account of $14,376.
Liquidity and Capital Resources
On
March 18, 2021, we consummated the initial public offering of 34,500,000 Legacy Units at
$10.00 per Legacy Unit, generating gross proceeds of $345,000,000, which is discussed in Note 3 to the unaudited condensed financial statements.
Simultaneously with the closing of our initial public offering, we consummated the sale of
5,933,333 private placement warrants, at a price of $1.50 per private placement warrant, which is discussed in Note 4 to the unaudited
condensed financial statements.
Following
the initial public offering and the sale of the private placement warrants, a total of $345,000,000
was placed in the Trust Account. We incurred $20,169,599 in transaction costs, including $6,900,000 of underwriting fees, $12,075,000
of deferred underwriting fees and $1,194,599 of other costs.
As
of June 30, 2022, cash used in operating activities was $2,004,041. Net income of $5,265,534 was affected by non-cash changes in the deferred
legal fees of $6,139,534, offset by the fair value of warrant derivative liabilities, and Forward Purchase Agreement liabilities of $8,601,666
and $5,328,000, respectively, and interest earned on marketable securities held in the Trust Account of $260,745. Changes in current assets
and liabilities provided $781,302 of cash for operating activities.
As
of June 30, 2022, we had marketable securities held in the Trust Account of $345,312,792. We used substantially all of the funds held
in the Trust Account, including any amounts representing interest earned on the Trust Account, which interest shall be net of taxes payable
and excluding deferred underwriting commissions, to complete our Business Combination. We may withdraw interest from the Trust Account
to pay taxes, if any. Through June 30, 2022, we did not withdraw any interest earned on the Trust Account to pay our taxes. To the extent
that our share capital or debt is used, in whole or in part, as consideration to complete a business combination (including the Business
Combination), the remaining proceeds held in the Trust Account will be used as working capital to finance the operations of the target
business or businesses, make other acquisitions and pursue our growth strategies.
As
of June 30, 2022, we had cash in an operating bank account, outside of the Trust Account, with $99,727 available for working capital
needs. As of June 30, 2022, we had a working capital deficit of $811,657. All remaining funds held in the Trust Account were generally
unavailable for our use, prior to an initial business combination, and were restricted for use either in a business combination, to redeem
Class A ordinary shares or with respect to the interest earned, to be withdrawn for the payment of taxes. As of June 30, 2022, none of
the amount in the Trust Account was withdrawn as described above.
On
October 28, 2021, we drew down the entire balance of the Working Capital Promissory Note (as defined below) initially available, and the
Sponsor deposited $1,500,000 in our operating bank account. In addition, we issued working capital promissory notes to the Sponsor
on (i) May 20, 2022, for up to $600,000 (“May Working Capital Note”) and (ii) July 15, 2022, for up to $450,000 (“July
Working Capital Note” and, together with May Working Capital Note, the “Non-Convertible Working Capital Notes”), in
each case, for working capital purposes. As of July 27, 2022, we had a total aggregate principal amount of $1,050,000 in outstanding borrowings
under the Non-Convertible Working Capital Notes. In connection with the closing of Business Combination, the aggregate outstanding balance
under the Non-Convertible Working Capital Notes of $1,050,000 was repaid to the Sponsor. Borrowings under the Non-Convertible Working
Capital Notes are no longer available.
As
of July 27, 2022, substantial doubt about our ability to continue as going concern was alleviated due to closing of the Business Combination.
There has not been a material change in our liquidity or capital resources since the Closing Date. See the section titled “Unaudited
Pro Forma Condensed Combined Financial Information” for information relating to the impact of the closing of the Business Combination
on our financial position as of the Closing Date.
The sale of our Class
A ordinary shares in the public market or otherwise, including sales pursuant to this prospectus, or the perception that such sales could
occur, could harm the prevailing market price of our Class A ordinary shares. These sales, or the possibility that these sales may occur,
also might make it more difficult for us to sell equity securities in the future at a time and at a price that it deems appropriate.
Immediately following the consummation of the Business Transaction, (a) members of the Sponsor and their affiliates owned a combined
ownership interest of 40.8%, comprised of the following: (i) Burwell held an ownership interest of 8.1% of the fully diluted Class A
ordinary shares, (ii) Dynamo Master Fund held an ownership interest of 13.5% of the fully diluted Class A ordinary shares, (iii) Waldencast
Ventures held an ownership interest of 3.5% of the fully diluted Class A ordinary shares, and (iv) Beauty FPA Investor held an ownership
interest of 15.7% of the fully diluted Class A ordinary shares; and (b) Cedarwalk held an ownership interest of 24.5% of the fully diluted
Class A ordinary shares. Such holders will be able to resell their shares for so long as the registration statement of which this prospectus
forms a part is available for use (following the expiration of the applicable lock-up periods). Resales of our Class A ordinary shares
may cause the market price of our securities to drop significantly, regardless of the performance of our business.
The Class A ordinary
shares being offered for resale under this prospectus, assuming the exercise in full of the Company’s public and private placement
warrants, would represent approximately 82.5% of shares outstanding of the Company, based on the outstanding shares of the Company as
of July 27, 2022 and after giving effect to all such issuances. As such, sales of a substantial number of Class A ordinary shares in
the public market could occur at any time. Given the substantial number of Class A ordinary shares being registered for potential resale
by the Selling Holders pursuant to this prospectus, the sale of shares by various selling securityholders, or the perception in the market
that the shareholders of a large number of shares intend to sell shares, could increase the volatility of the market price of our Class
A ordinary shares or result in a significant decline in the public trading price of our Class A ordinary shares.
While we could potentially
receive up to an aggregate of $339,632,743 in gross proceeds from the exercise of the warrants, assuming the exercise in full of all
of the public and private placement warrants, no assurances can be made that the holders will elect to exercise any or all of such warrants
and, accordingly, no assurance that we will receive any proceeds from the exercise of the warrants. Further, we believe the likelihood
that warrant holders will exercise their warrants, and therefore the amount of cash proceeds that we would receive, is dependent upon
the trading price of our Class A ordinary shares. However, as of the date of this prospectus, our warrants are “out-of-the money,”
which means that the trading price of the Class A ordinary shares underlying our warrants is below the $11.50 exercise price of the warrants.
For so long as the warrants remain “out-of-the money,” we do not expect warrant holders to exercise their warrants and, accordingly,
no assurance that we will receive any proceeds from the exercise of the warrants.
As of the Closing Date,
we did not anticipate, nor did we rely on, receiving any proceeds from the exercise of the warrants to meet our future capital needs.
We believe that the proceeds of the Business Combination and related transactions, including the proceeds from the PIPE Investment and
the Forward Purchase Securities, along with the rest of our current balance of cash and cash equivalents and our forecasted cash from
operations, will be adequate to fund operations, manufacturing, research and development, administration as well as sales and marketing
costs for at least the next 12 months. Our foreseeable cash needs, in addition to our recurring operating expenses, include our expected
capital expenditures to support expansion of our infrastructure and workforce, leases for office space, and minimum contractual obligations.
Our future capital requirements
will depend on many factors, including our growth rate, the timing and extent of spending to support research and development efforts,
the expansion of sales and marketing activities, the introduction of new and enhanced product and service offerings, and the cost of
any future acquisitions of technology or businesses. In the event that additional financing is required from outside sources, we may
be unable to raise the funds on acceptable terms, if at all. We may seek additional opportunist capital through equity and/or debt financings
depending on market conditions. If we are required to raise additional funds by issuing equity securities, dilution to public shareholders
would result. Any equity securities issued may also provide for rights, preferences or privileges senior to those of holders of our Class
A ordinary shares. If we raise funds by issuing debt securities, such debt securities would have rights, preferences and privileges senior
to those of holders of our Class A ordinary shares. The terms of debt securities or borrowings could impose significant restrictions
on our operations. The credit market and financial services industry have in the past, and may in the future, experience periods of uncertainty
that could impact the availability and cost of equity and debt financing.
Off-Balance Sheet Arrangements
We
have no obligations, assets or liabilities, which would be considered off-balance sheet arrangements as of June 30, 2022 and December
31, 2021. We do not participate in transactions that create relationships with unconsolidated entities or financial partnerships, often
referred to as variable interest entities, which would have been established for the purpose of facilitating off-balance sheet arrangements.
We have not entered into any off-balance sheet financing arrangements, established any special purpose entities, guaranteed any debt or
commitments of other entities, or purchased any non-financial assets.
Contractual Obligations
We
do not have any long-term debt, capital lease obligations, operating lease obligations or long-term liabilities, other than an agreement
to pay the Sponsor a monthly fee of $10,000 for office space administrative and support services provided to us. We began incurring these
fees on March 15, 2021, but ceased to incur these fees following the completion of the Business Combination.
Critical Accounting Policies
This
management’s discussion and analysis of our financial condition and results of operations is based on our unaudited condensed financial
statements, which have been prepared in accordance with GAAP. The preparation of our unaudited condensed financial statements requires
us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of
contingent assets and liabilities in our unaudited condensed financial statements. On an ongoing basis, we evaluate our estimates and
judgments, including those related to fair value of financial instruments and accrued expenses. We base our estimates on historical experience,
known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form
the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual
results may differ from these estimates under different assumptions or conditions. We have identified the following as our critical accounting
policies:
Class A Ordinary
Shares Subject to Possible Redemption
Prior
to the consummation of the Business Combination, we accounted for our ordinary shares subject to possible redemption in accordance with
the guidance in ASC Topic 480, Distinguishing Liabilities from Equity. Ordinary shares subject to mandatory redemption are classified
as a liability instrument and are measured at fair value. Conditionally redeemable ordinary shares (including ordinary shares that features
redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not
solely within our control) are classified as temporary equity. At all other times, ordinary shares are classified as a component of shareholders’
deficit. Our ordinary shares feature certain redemption rights that are considered to be outside of our control and subject to occurrence
of uncertain future events. As of June 30, 2022 and December 31, 2021, 34,500,000 shares of Class A ordinary shares subject to possible
redemption are presented at redemption value as temporary equity, outside of the shareholders’ deficit section of our balance sheets.
Warrant Liabilities
and Forward Purchase Agreements
We
account for the warrants issued in connection with our initial public offering in accordance
with ASC 815, under which the warrants do not meet the criteria for equity classification and must be recorded as liabilities. The warrants
meet the definition of a derivative as contemplated in ASC 815, and therefore the warrants are measured at fair value at inception and
at each reporting date in accordance with ASC 820 with changes in fair value recognized in the unaudited condensed statements of operations
in the period of change.
We
account for the Forward Purchase Agreements in accordance with ASC 815-40 as a derivative liability. These liabilities are subject to
re-measurement at each balance sheet date, with changes in fair value recognized in the unaudited condensed statements of operations.
Conversion Feature
of Working Capital Promissory Note
On
August 18, 2021, we issued the Working Capital Promissory Note (as defined below) to the Sponsor. The Working Capital Promissory Note
was issued in order to finance certain transaction costs in connection with the Business Combination. At the lender’s discretion,
it may elect to convert up to $1,500,000 of the unpaid principal balance of the Working Capital Promissory Note into warrants, at a price
of $1.50 per warrant, with each whole warrant exercisable for one of our Class A ordinary shares upon the consummation of an initial business
combination. This embedded conversion feature is subject to remeasurement at each balance sheet date until exercised, and any change in
fair value is recognized in our unaudited condensed statements of operations. The value of the conversion features was considered de minimis
both as of June 30, 2022, and December 31, 2021.
Net Income (Loss)
Per Ordinary Shares
Net
income (loss) per share is computed by dividing net income (loss) by the weighted-average number of shares of ordinary shares outstanding
during the period.
Our
unaudited condensed statements of operations include a presentation of net income (loss) per share for ordinary shares subject to possible
redemption and apply the two-class method in calculating net loss per share. Basic and diluted net income (loss) per ordinary share for
Class A redeemable ordinary shares is calculated by dividing the allocable interest income earned on the Trust Account, net of applicable
franchise and income taxes, by the weighted average number of Class A ordinary shares subject to possible redemption outstanding since
original issuance. Basic and diluted net income (loss) per share for Class A and Class B non-redeemable ordinary shares is calculated
by dividing the net income (loss), adjusted for income (loss) attributable to Class A redeemable ordinary shares, by the weighted average
number of Class A and Class B non-redeemable ordinary shares outstanding for the period. Class B non-redeemable ordinary shares include
the founder shares as these shares do not have any redemption features and do not participate in the income earned on the Trust Account.
Recent Accounting
Pronouncements
In
August 2020, the FASB issued Accounting Standard Update No. 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20)
and Derivatives and Hedging-Contracts in an Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and
Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by
removing major separation models required under current GAAP. ASU 2020-06 also removes certain settlement conditions that are required
for equity-linked contracts to qualify for scope exception, and it simplifies the diluted earnings per share calculation in certain areas.
We adopted ASU 2020-06 on January 1, 2021. Adoption of the ASU did not impact our financial position, results of operations or cash flows.
We
do not believe that any other recently issued, but not yet effective, accounting pronouncements, which, if currently adopted, would have
a material impact on our unaudited condensed financial statements.
JOBS Act
On
April 5, 2012, the JOBS Act was signed into law. The JOBS Act contains provisions that, among other things, relax certain reporting requirements
for qualifying public companies. We qualify as an “emerging growth company” under the JOBS Act and are allowed to comply with
new or revised accounting pronouncements based on the effective date for private (not publicly traded) companies. We elected to delay
the adoption of new or revised accounting standards, and as a result, we may not comply with new or revised accounting standards on the
relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result, our unaudited condensed
financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company
effective dates.
As an “emerging growth
company”, we are not required to, among other things, (i) provide an auditor’s attestation report on our system of internal
controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging
growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may
be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information
about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation related
items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median
employee compensation. These exemptions will apply for a period of five years following the completion of our initial
public offering or until we are no longer an “emerging growth company,” whichever is earlier.
Obagi’s
Management’s Discussion and Analysis of
Financial Condition and Results of Operations
The
following discussion and analysis of the financial condition and results of operations of Obagi should be read together with its respective
audited financial statements as of December 31, 2021 and 2020, and for the years ended December 31, 2021, 2020 and 2019, as well as unaudited
condensed interim consolidated financial statements as of June 30, 2022, and December 31, 2021 and for the six months ended June 30, 2022
and 2021, in each case together with related notes thereto, included elsewhere in this prospectus. The discussion and analysis should
also be read together with the sections entitled “Our Business—Information About Obagi” and “Unaudited Pro Forma
Condensed Combined Financial Information.” Except where the context otherwise requires, the terms “we,” “us”
and “our” in this discussion and analysis refer to Obagi prior to the Business Combination and to Obagi following the consummation
of the Business Combination. The following discussion contains forward-looking statements that reflect future plans, estimates, beliefs
and expected performance. The forward-looking statements are dependent upon events, risks and uncertainties that may be outside of Obagi’s
control. Our actual results may differ significantly from those projected in the forward-looking statements. Factors that might cause
future results to differ materially from those projected in the forward-looking statements include, but are not limited to, those discussed
in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” included elsewhere
in this prospectus. Certain amounts may not foot due to rounding.
Overview
We are a global skincare
company that develops, markets, and sells innovative skin health products in more than 60 countries around the world. With a 33-year legacy,
today we exist to create the future of skin health so that every face is cared for everywhere. With our unique position rooted in the
aesthetic physician community, we offer transformative solutions at various stages of the skincare journey to help consumers greet the
future with confidence. Our product portfolio is designed to prevent or improve common, visible skin concerns such as fine lines and wrinkles,
elasticity, photodamage, hyperpigmentation, acne, oxidative stress, environmental damage, and hydration.
Our product portfolio today
includes more than 200 cosmetic, OTC and prescription products sold through three distinct brands—Obagi Medical, Obagi Clinical
and Obagi Professional—as well as the Skintrinsiq device.
In the United States, we
sell our Obagi Medical systems and related products through our direct sales force to dermatologists, plastic surgeons and other physicians
who are focused on aesthetic and therapeutic skincare, including physicians on site at medical spas. The medical professionals we
sell to then dispense our products in-office directly to their patients, a distribution method commonly referred to as the “physician-dispensed”
channel. We sell our Obagi Clinical products through retail channels, our Obagi Professional line will be sold to licensed aestheticians
and spas, and the Skintrinsiq device is sold through the physician-dispensed channel as well as to aestheticians and spas.
Internationally we sell our
products through distribution partners across Central America, Europe, the Middle East, and Asia. We also advance our development objectives
through product and license agreements with third parties. These agreements may include patent and technology licenses, product licenses
and new product collaboration agreements. We compete in Japan through a strategic licensing agreement with a Japanese pharmaceutical manufacturer
and distributor that sells a series of OTC and cosmetic products under the Obagi brand name in the Japanese skincare retail channels.
Recent Events
Impact of COVID-19 Pandemic
In March 2020, the World
Health Organization declared the novel strain of COVID-19, a global pandemic and recommended containment and mitigation measures worldwide.
COVID-19 has disrupted everyday life and markets worldwide, leading to significant business and supply-chain disruption, as well as broad-based
changes in supply and demand. While the quarantine, social distancing and other regulatory measures instituted or recommended in response
to COVID-19 are expected to be temporary, the duration of the business disruptions, and related financial impact, cannot be estimated
at this time.
The direct impact of COVID-19
on our business, beyond disruptions to normal business operations, resulted from the closure of physician customers’ practices for
several weeks, which greatly diminished demand for our products. Although some of these customers have now established websites to be
able to provide products to their patients through online sales, any re-implementation of similar restrictions could have a material impact
on our future net revenue.
In addition, the COVID-19
pandemic caused us to modify our business practices, including accelerating the launch of a redesigned website to enable us to sell our
own products online, creating an e-commerce platform to enable our physician customers to sell Obagi products online to their patients,
designing a “Door-Step Delivery Program” for our customers’ patients, and investing in our “Helping Hands”
program to provide free hand sanitizer to healthcare professionals. To help ensure the health and safety of our employees, we also created
COVID-19 response teams to provide support to employees to address new challenges as a result of COVID-19 and transitioned to a hybrid
work environment where employees are in the office on a part-time basis. We may take further actions as may be required by Governmental
Authorities or as we determine is in the best interests of our employees, customers and their patients, any of which may require unanticipated
investments in management time and money.
While the impact of COVID-19
on our manufacturing and supply chains, sales and marketing, and commercial and clinical trial operations has not been material to date,
the impact of COVID-19 over the long-term is uncertain and cannot be predicted with confidence.
The extent of the continued
impact of the COVID-19 pandemic on our operational and financial performance will depend on various future developments, including the
duration and spread of outbreaks, the timing and nature of actions taken to respond to COVID-19, and the resulting economic consequences
and the impact on our customers, consumers and employees, all of which is uncertain at this time.
Indebtedness
In December 2018, we entered
into a credit agreement (the “2018 Credit Agreement”) with a syndicate of lenders including Wells Fargo Bank, National Association
as administrative agent for the lenders. The 2018 Credit Agreement included a term loan of $90.0 million (the “2018 Term Loan”)
and a revolving credit facility with borrowing capacity of up to $35.0 million (the “2018 Revolving Credit Facility”). In
March 2020, we increased the 2018 Revolving Credit Facility by an additional $10.0 million, to $45.0 million, to help us manage our capital
needs in response to COVID-19.
In March 2021, we replaced
the 2018 Credit Agreement for a new financing agreement with a syndicate of lenders, including TCW Asset Management Company LLC as administrative
agent for the lenders (the “2021 Credit Agreement”). The 2021 Credit Agreement included a term loan of $110.0 million (the
“2021 Term Loan”) and a revolving credit facility with borrowing capacity of up to $40.0 million (the “2021 Revolving
Credit Facility”).
We also received the PPP
Loan in May 2020 in the amount of $6.8 million. The PPP was established as part of the CARES Act and provided for loans to qualifying
businesses for amounts up to 2.5 times the average monthly payroll expenses of the qualifying business. We were able to utilize the full
amount to protect our employees as part of our initiatives to respond to COVID-19. We used the proceeds for purposes consistent with the
PPP, and in June 2021, the full amount of the PPP Loan was forgiven, inclusive of accrued interest of $0.1 million. We recognized a gain
on forgiveness of the PPP Loan of $6.8 million in the second quarter of 2021.
Business Combination
In accounting for the Business
Combination, Waldencast was deemed to be the accounting acquirer and will continue as the SEC registrant. We were the accounting acquiree,
however we were considered the predecessor entity for purposes of financial reporting. Accordingly, our financial statements for previous
periods will be disclosed in Waldencast’s future periodic reports filed with the SEC. Under the acquisition method of accounting,
Waldencast’s assets and liabilities retained their carrying values and the assets and liabilities associated with us and Milk was
recorded at their fair values measured as of the acquisition date, which created a new basis of accounting. The excess of the purchase
price over the estimated fair values of the net assets acquired was recorded as goodwill. The fair value measurement period will remain
open upon the consummation of the Business Combination while Waldencast awaits further information and analysis to determine the acquisition
date fair values of certain acquired assets and assumed liabilities. Consequently, our basis of accounting, including our assets and liabilities
and results of operations for periods after the consummation of the Business Combination will not be comparable to those reflected in
the audited consolidated financial statements included elsewhere in this prospectus, under the section “Unaudited Pro Forma Condensed
Combined Financial Information.”
Immediately prior to the
closing of the Business Combination, we carved out and distributed 100% of the Obagi China Business to our shareholder, Cedarwalk, pursuant
to the Obagi China Distribution. Following the Obagi China Distribution, the Obagi China Business will continue to be held by Cedarwalk,
which also owns approximately 24.5% of the fully diluted Class A ordinary shares as of the closing of the Business Combination. In connection
with the Obagi China Distribution, Obagi Hong Kong Limited entered into the Supply Agreement (as defined below) and the Intellectual Property
License Agreement (as defined below) with us, under which it will pay us royalties based on its sales of Obagi branded products in the
China Region. Following the close of the Business Combination our future results will not be comparable to the historical financial results
of Obagi, which include the operating results of the Obagi China Business. Please refer to “Unaudited Pro Forma Condensed Combined
Financial Information” for the depiction of the impact on our historical financial statements of disposing of the Obagi China
Business and combining with Waldencast and Milk.
Non-GAAP Financial Measures
In addition to our results
of operations and measures of performance determined in accordance with GAAP, we believe that certain non-GAAP financial measures are
useful in evaluating and comparing our financial and operational performance over multiple periods, identifying trends affecting our business,
formulating business plans, and making strategic decisions.
Adjusted gross profit and
Adjusted EBITDA are key performance measures that our management uses to assess our financial performance and for internal planning and
forecasting purposes. We believe these non-GAAP measures are also useful to investors and other interested parties in evaluating our operating
performance, as they are similar to measures reported by our public competitors.
Adjusted gross profit and
Adjusted EBITDA are not intended to be substitutes for any GAAP financial measures and, as calculated, may not be comparable to other
similarly titled measures of performance of other companies in other industries or within the same industry.
Adjusted gross profit reflects
GAAP net revenue less cost of goods sold (exclusive of depreciation and amortization expense). Adjusted gross profit is reconciled to
GAAP gross profit, which is not presented on the consolidated statements of operations and comprehensive (loss) income but represents
the most comparable GAAP metric. GAAP gross profit reflects GAAP net revenue less cost of goods sold (exclusive of depreciation and amortization
expense) and less certain intangible asset amortization expense pertaining to cost of goods sold.
We define and calculate Adjusted
EBITDA as GAAP net income (loss) before interest income or expense, income tax expense, depreciation and amortization, and further adjusted
for the following items: one-time restructuring and transaction-related costs as described in the reconciliation below.
Adjusted gross profit and
Adjusted EBITDA exclude certain expenses that are required in accordance with GAAP because they are non-core to our regular business,
such as transaction-related costs; non-cash expenses, such as depreciation and amortization; or other expenses that are not related to
our underlying business performance, such as interest expense. There are limitations to non-GAAP financial measures because they exclude
charges and credits that are required to be included in GAAP financial presentation. The items excluded from GAAP financial measures such
as net income/loss to arrive at non-GAAP financial measures are significant components for understanding and assessing our financial performance.
Non-GAAP financial measures should be considered together with, and not alternatives to, financial measures prepared in accordance with
GAAP.
Adjusted Gross Profit
The table below presents
our Adjusted gross profit reconciled to GAAP gross profit, as explained above.
| | |
Six Months Ended
June 30, | |
| (in thousands) | |
2022 | | |
2021 | |
| Net revenue | |
$ | 106,440 | | |
$ | 94,204 | |
| Cost of goods sold (exclusive of depreciation and amortization expense) | |
| 24,701 | | |
| 23,463 | |
| Amortization expense (1) | |
| 2,446 | | |
| 2,446 | |
| Gross profit | |
$ | 79,293 | | |
$ | 68,295 | |
| Add: Amortization expense (1) | |
| 2,446 | | |
| 2,446 | |
| Adjusted gross profit | |
$ | 81,739 | | |
$ | 70,741 | |
| | |
Year Ended
December 31, | |
| (in thousands) | |
2021 | | |
2020 | | |
2019 | |
| Net revenue | |
$ | 206,069 | | |
$ | 84,145 | | |
$ | 117,085 | |
| Cost of goods sold (exclusive of depreciation and amortization expense) | |
| 48,708 | | |
| 19,969 | | |
| 26,687 | |
| Amortization expense (1) | |
| 4,889 | | |
| 4,890 | | |
| 4,871 | |
| Gross profit | |
$ | 152,472 | | |
$ | 59,286 | | |
$ | 85,527 | |
| Add: Amortization expense (1) | |
| 4,889 | | |
| 4,890 | | |
| 4,871 | |
| Adjusted gross profit | |
$ | 157,361 | | |
$ | 64,176 | | |
$ | 90,398 | |
| |
(1) |
Includes supply agreement and developed technology intangible asset amortization expense that pertains to cost of goods sold. |
Adjusted EBITDA
The table below presents
our Adjusted EBITDA reconciled to our net (loss) income, the closest GAAP measure for the periods indicated:
| | |
Six Months Ended
June 30, | |
| (in thousands) | |
2022 | | |
2021 | |
| Net (loss) income | |
$ | (2,915 | ) | |
$ | 12,946 | |
| Adjusted for: | |
| | | |
| | |
| Interest expense | |
| 5,719 | | |
| 5,041 | |
| Income tax (benefit) expense | |
| (40 | ) | |
| 1,948 | |
| Depreciation and amortization | |
| 7,369 | | |
| 6,936 | |
| Restructuring cost(1)(2) | |
| 289 | | |
| - | |
| Transaction related costs(1) (3) | |
| 4,555 | | |
| - | |
| Loss on extinguishment of debt | |
| - | | |
| 2,317 | |
| Adjusted EBITDA | |
$ | 14,977 | | |
$ | 29,188 | |
| | |
Year ended December 31, | |
| (in thousands) | |
2021 | | |
2020 | | |
2019 | |
| Net (loss) income | |
$ | (70 | ) | |
$ | (9,171 | ) | |
$ | 5,820 | |
| Adjusted for: | |
| | | |
| | | |
| | |
| Interest expense | |
| 11,156 | | |
| 6,281 | | |
| 6,834 | |
| Income tax expense (benefit) | |
| 11,301 | | |
| (5,094 | ) | |
| (1,589 | ) |
| Depreciation and amortization | |
| 14,053 | | |
| 13,426 | | |
| 12,940 | |
| Restructuring cost(1)(2) | |
| 1,972 | | |
| - | | |
| - | |
| Transaction related costs(1) (3) | |
| 5,244 | | |
| - | | |
| - | |
| Loss on extinguishment of debt | |
| 2,317 | | |
| - | | |
| - | |
| Gain on PPP Loan forgiveness | |
| (6,824 | ) | |
| - | | |
| - | |
| Adjusted EBITDA | |
$ | 39,149 | | |
$ | 5,442 | | |
$ | 24,005 | |
| (1) |
We did not incur any non-core restructuring or transaction costs; therefore, no adjustments were made for these amounts for the six months ended June 30, 2021 or the years ended December 31, 2020 and 2019. |
| (2) |
Includes certain one-time costs incurred to realign the company’s organizational structure to support new strategies and relocate corporate headquarters. |
| (3) |
Includes mainly advisory, consulting, accounting and legal expenses in connection with the Business Combination (see “Business Combination” section within). |
Components of Results of
Operations
Net Revenue
Net revenue is generated
primarily from two sources: (1) product sales and (2) royalties.
Net Product Sales
We generate product sales
revenue from sales of our products to distributors and directly to retailers. We recognize revenue for product sales at a point in time,
when transfer of control has passed to the customer, based on the terms of the sale. In the United States, we sell the majority our products
to healthcare professionals through an authorized wholesale distributor, Boxout Health. Under this model, we sell the products to Boxout
Health, which then sells the products through to our physician customers when they order them. We recognize revenue for product sales
to our U.S. physician customers upon transfer of control to Boxout. As a result, Boxout Health accounted for approximately 34.8%, 54.1%,
and 61.0% of our net revenue during the years ended December 31, 2021, 2020, and 2019, respectively. For the six months ended June 30,
2022 and 2021, sales to Boxout accounted for 32.7% and 41.0% of our net revenue, respectively. Product sales revenue is recognized net
of provisions for estimated discounts and allowances, chargebacks, distribution fees, returns, and rebates. Chargebacks are a result of
promotional product discounts that we provide to physicians on sales from Boxout. When Boxout reports the chargebacks to us, we reimburse
Boxout for the discounts taken. Product sales revenue primarily includes revenue from our two sales channels: Obagi Medical and Obagi
Clinical. In addition, in July 2021 we launched our Skintrinsiq device.
Royalties
We also generate royalty
revenue from the sale of products in Japan through a strategic licensing agreement with a Japanese pharmaceutical manufacturer and distributor
that sells a series of OTC and cosmetic products under the Obagi brand name in the Japanese retail skincare channels. Revenue for royalty
income is recognized in the period that corresponds to the related Rohto net sales and is presented within the Obagi Medical sales channel.
Our royalty revenue from skin health systems and products in Japan was approximately $5.7 million, or 2.8% of our net revenue, $5.9 million,
or 7.0% of our net revenue, and $6.3 million, or 5.4% of our net revenue, for the years ended December 31, 2021, 2020, and 2019, respectively.
For the six months ended June 30, 2022 and 2021, our royalty revenue from skin health systems and products in Japan was approximately
$2.5 million, or 2.3% of our net revenue, and $3.2 million, or 3.4% of our net revenue, respectively.
Cost of Goods Sold (exclusive of depreciation
and amortization shown separately below)
Cost of goods sold consists
primarily of expenses related to inventory, including when inventory is sold or written down. We expect that cost of revenue will increase
in absolute dollars as our revenue grows and will vary from period-to-period as a percentage of revenue.
Selling, General, and Administrative
Selling, general and administrative
costs include expenses we incur in our normal course of business relating to salaries, bonuses and benefits, marketing, office supplies,
computer and technology, rent and utilities, insurance, legal and professional fees, city, state and property taxes, and advertising expenses.
We expect that selling, general and administrative expenses will increase in absolute dollars as we continue to invest in building and
maintaining our customer base, growing our business, enhancing our brand awareness, hiring additional personnel and upgrading and expanding
our systems, processes, and controls to support the growth in our business, as well as due to the increased compliance and reporting requirements
we will have as a public company.
In connection with the Business
Combination, we expect to incur incremental ongoing and one-time expenses. Our incremental ongoing costs include additional selling, general
and administrative expenses including legal, consulting, regulatory, accounting, insurance, investor relations and other expenses. The
Sarbanes-Oxley Act, as well as rules adopted by the SEC and national securities exchanges, require public companies to implement specified
corporate governance practices that are not currently applicable to us as a private company. These additional rules and regulations will
increase our legal, regulatory, financial and insurance compliance costs. We expect any one-time expenses incurred in connection with
the Business Combination to include professional fees, consulting fees and certain filing and listing fees.
Research and Development
Substantially all research
and development expenses are related to new product development and design improvements in current products.
Research and development
costs primarily consist of employee-related costs, including salaries, bonuses, and benefits. We plan to continue to invest in personnel
to support our research and development efforts. As a result, we expect that research and development expenses will increase in absolute
dollars for the foreseeable future as we continue to invest to support these activities.
Depreciation and Amortization
Depreciation and amortization
expenses are related to our equipment and intangible assets. Cost of goods sold is presented on the consolidated statements of operations
and comprehensive (loss) income exclusive of depreciation and amortization expense.
Results of Operations
Comparison of Six Months Ended June 30,
2022 to Six Months Ended June 30, 2021
The following tables summarize
our consolidated statements of operations data, in dollars and as a percentage of total revenue, for the six months ended June 30, 2022
and 2021.
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Net revenue | |
$ | 106,440 | | |
| 94,204 | | |
| 12,236 | | |
| 13.0 | % |
| Cost of goods sold (exclusive of depreciation and amortization expense) | |
| 24,701 | | |
| 23,463 | | |
| 1,238 | | |
| 5.3 | % |
| Selling, general and administrative | |
| 68,418 | | |
| 45,698 | | |
| 22,720 | | |
| 49.7 | % |
| Research and development | |
| 3,262 | | |
| 2,534 | | |
| 728 | | |
| 28.7 | % |
| Depreciation and amortization | |
| 7,369 | | |
| 6,936 | | |
| 433 | | |
| 6.2 | % |
| Total operating expenses | |
| 103,750 | | |
| 78,631 | | |
| 25,119 | | |
| 31.9 | % |
| Operating income | |
| 2,690 | | |
| 15,573 | | |
| (12,883 | ) | |
| (82.7 | )% |
| Interest expense | |
| 5,719 | | |
| 5,041 | | |
| 678 | | |
| 13.4 | % |
| Loss on extinguishment of debt | |
| - | | |
| 2,317 | | |
| (2,317 | ) | |
| (100.0 | )% |
| Gain on PPP loan forgiveness | |
| - | | |
| (6,824 | ) | |
| 6,824 | | |
| (100.0 | )% |
| Other (income) expense, net | |
| (74 | ) | |
| 145 | | |
| (220 | ) | |
| (150.7 | )% |
| (Loss) Income before income taxes | |
| (2,955 | ) | |
| 14,894 | | |
| (17,849 | ) | |
| (119.8 | )% |
| Income tax (benefit) expense | |
| (40 | ) | |
| 1,948 | | |
| (1,988 | ) | |
| (102.1 | )% |
| Net (loss) income | |
| (2,915 | ) | |
| 12,946 | | |
| (15,861 | ) | |
| (122.5 | )% |
Net Revenue
The following tables provide
our revenue by sales channel, as well as by revenue source and geographic region (based on the location of the end customer), for the
periods presented.
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Sales Channel | |
| | |
| | |
| | |
| |
| Medical | |
$ | 85,354 | | |
$ | 91,186 | | |
$ | (5,832 | ) | |
| (6.4 | )% |
| as a percentage of total revenue | |
| 80.2 | % | |
| 96.8 | % | |
| | | |
| | |
| Clinical | |
$ | 20,477 | | |
$ | 2,330 | | |
$ | 18,147 | | |
| 778.8 | % |
| as a percentage of total revenue | |
| 19.2 | % | |
| 2.5 | % | |
| | | |
| | |
| Other | |
$ | 609 | | |
$ | 688 | | |
$ | (79 | ) | |
| (11.5 | )% |
| as a percentage of total revenue | |
| 0.6 | % | |
| 0.7 | % | |
| | | |
| | |
| Total | |
$ | 106,440 | | |
$ | 94,204 | | |
$ | 12,236 | | |
| 13.0 | % |
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Source and Region | |
| | |
| | |
| | |
| |
| North America | |
$ | 46,615 | | |
$ | 46,008 | | |
$ | 607 | | |
| 1.3 | % |
| as a percentage of total revenue | |
| 43.8 | % | |
| 48.8 | % | |
| | | |
| | |
| Asia Pacific | |
$ | 50,275 | | |
$ | 36,041 | | |
$ | 14,234 | | |
| 39.5 | % |
| as a percentage of total revenue | |
| 47.2 | % | |
| 38.3 | % | |
| | | |
| | |
| Rest of the World | |
$ | 7,086 | | |
$ | 8,926 | | |
$ | (1,840 | ) | |
| (20.6 | )% |
| as a percentage of total revenue | |
| 6.7 | % | |
| 9.5 | % | |
| | | |
| | |
| Net Product Sales | |
$ | 103,976 | | |
$ | 90,975 | | |
$ | 13,001 | | |
| 14.3 | % |
| as a percentage of total revenue | |
| 97.7 | % | |
| 96.6 | % | |
| | | |
| | |
| Asia Pacific Royalties | |
$ | 2,464 | | |
$ | 3,229 | | |
$ | (765 | ) | |
| (23.7 | )% |
| as a percentage of total revenue | |
| 2.3 | % | |
| 3.4 | % | |
| | | |
| | |
| Total | |
$ | 106,440 | | |
$ | 94,204 | | |
$ | 12,236 | | |
| 13.0 | % |
Net revenue increased by
$12.2 million, or 13.0%, for the six months ended June 30, 2022, compared to the same period in 2021, primarily related to an increase
in net product sales of $13.0 million.
The increase in net product
sales was primarily attributable to a $14.2 million increase in our Asia Pacific region resulting from the addition of new customer accounts,
expansion into new territories and increased international distributor sales online, in-store and through physicians’ offices in
existing territories, including increases of $18.1 million in the Clinical channel, partially offset by a $5.8 million decrease in the
Medical channel due to product mix.
The increase in product sales
in our Asia Pacific region are partially offset by a $1.8 million decrease in product sales in our Rest of the World region, driven by:
(1) a $0.7 million decrease in sales in the United Kingdom during the six months ended June 30, 2022 compared to the same period in 2021
as a result of increased sales in 2021 from increased business coming out of COVID-19 restrictions that were in place throughout 2020;
(2) a $0.6 million decrease in sales as a result of international sanctions on Russia and the war in Ukraine; and (3) a $0.5 million decrease
in sales in Guatemala and Costa Rica as a result of COVID-19 business closures.
Royalty revenue decreased
by $0.8 million, or 23.7%, for the six months ended June 30, 2022 compared to the same period in 2021 due to a general decrease in consumer
spending in Japan during the first half of 2022, resulting from the impacts of a COVID-19 outbreak in Japan during the same period.
Cost of Goods Sold (exclusive of depreciation
and amortization)
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Cost of goods sold (exclusive of depreciation and amortization) | |
$ | 24,701 | | |
$ | 23,463 | | |
$ | 1,238 | | |
| 5.3 | % |
| as a percentage of total revenue | |
| 23.2 | % | |
| 24.9 | % | |
| | | |
| | |
Cost of goods sold (exclusive
of depreciation and amortization) for the six months ended June 30, 2022 increased by $1.2 million, or 5.3%, compared to same period in
2021. The increase was primarily driven by increased sales associated with the partial economic recovery from COVID-19 that increased
the cost of goods sold by $0.8 million and increased freight costs by $0.4 million. The increase in costs of goods sold was partially
offset by favorable lower cost product mixes for the six months ended June 30, 2022, $0.6 million cost saving measures within specific
product mixes, an increase of $0.3 million in product returns, and overall favorable exchange rate fluctuations.
Selling, General and Administrative
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Selling, general and administrative | |
$ | 68,418 | | |
$ | 45,698 | | |
$ | 22,720 | | |
| 49.7 | % |
| as a percentage of total revenue | |
| 64.3 | % | |
| 48.5 | % | |
| | | |
| | |
Selling, general and administrative
expenses increased by $22.7 million, or 49.7%, for the six months ended June 30, 2022 compared to the same period in 2021, which was attributable
to an increase of $16.3 million in selling expenses and $6.4 million in general and administrative expense.
The increase in selling expenses
was primarily driven by (a) a $6.6 million increase in additional fees paid and promotional products provided to distributors; (b) a $3.6
million increase in marketing, advertising, and promotions primarily related to increased marketing in China, new product launches, and
website development; and (c) a $2.1 million increase in travel and entertainment expenses from resumed sales meetings and conferences
following a partial economic recovery from COVID-19.
The increase in general and
administrative expenses was primarily driven by a $4.5 million increase in fees resulting from the Business Combination and a $0.7 million
increase in wages and salaries and employee benefits due to general increases in salaries and wages as the economy partially recovered.
Research and Development
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Research and development | |
$ | 3,262 | | |
$ | 2,534 | | |
$ | 728 | | |
| 28.7 | % |
| as a percentage of total revenue | |
| 3.1 | % | |
| 2.7 | % | |
| | | |
| | |
Research and development
expenses increased by $0.7 million, or 28.8%, for the six months ended June 30, 2022 compared to the same period in 2021, primarily due
to an increase in regulatory compliance spending and product development costs.
Depreciation and Amortization
| | |
Six
Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Depreciation and amortization | |
$ | 7,369 | | |
$ | 6,936 | | |
$ | 433 | | |
| 6.2 | % |
| as a percentage of total revenue | |
| 6.9 | % | |
| 7.4 | % | |
| | | |
| | |
Depreciation and amortization
expenses remained relatively consistent for the six months ended June 30, 2022 when compared to the same period in 2021.
Interest Expense
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Interest expense | |
$ | 5,719 | | |
$ | 5,041 | | |
$ | 678 | | |
| 13.4 | % |
| as a percentage of total revenue | |
| 5.4 | % | |
| 5.4 | % | |
| | | |
| | |
Interest expense increased
by $0.7 million, or 13.5%, for the six months ended June 30, 2022 compared to the same period in 2021. The increase was primarily due
to refinancing of our debt in March 2021 at higher interest rates.
Loss on Extinguishment of Debt
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Loss on extinguishment of debt | |
$ | - | | |
$ | 2,317 | | |
$ | (2,317 | ) | |
| (100.0 | )% |
| as a percentage of total revenue | |
| - | | |
| 2.5 | % | |
| | | |
| | |
The loss on extinguishment
of debt for the six months ended June 30, 2021 is primarily related to the write-off of previously deferred financing costs due to the
refinancing of our debt in March 2021.
Gain on PPP Loan Forgiveness
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs. 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Gain on PPP Loan forgiveness | |
$ | - | | |
$ | (6,824 | ) | |
$ | 6,824 | | |
| (100.0 | )% |
| as a percentage of total revenue | |
| - | | |
| (7.2 | )% | |
| | | |
| | |
For the six months ended
June 30, 2021, we recognized a gain of $6.8 million associated with the forgiveness of the PPP Loan borrowed in 2020.
Income Tax (Benefit) Expense
| | |
Six
Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Income tax (benefit) expense | |
$ | (40 | ) | |
$ | 1,948 | | |
$ | (1,988 | ) | |
| (102.1 | )% |
| Effective tax rate | |
| | | |
| | | |
| | | |
| | |
For the six months ended
June 30, 2022, an income tax benefit of $0.04 million was recognized, with a corresponding effective tax rate of 1.4%, compared to an
income tax expenses of $1.9 million, with a corresponding effective tax rate of 13.1% recognized for the six months ended June 30, 2021.
The decrease in the effective tax rate for the six months ended June 30, 2022 when compared to the six months ended June 30, 2021 is primarily
attributable to the change in valuation allowance and the change in pre-tax earnings during the comparable periods.
Comparison of the Years Ended December
31, 2021 and 2020
The following tables summarize
our consolidated statements of operations data, in dollars and as a percentage of total revenue, for the fiscal year ended December 31,
2021 and 2020.
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Net revenue | |
$ | 206,069 | | |
$ | 84,145 | | |
$ | 121,924 | | |
| 144.9 | % |
| Cost of goods sold (exclusive of depreciation and amortization expense) | |
| 48,708 | | |
| 19,969 | | |
| 28,739 | | |
| 143.9 | % |
| Selling, general and administrative | |
| 118,243 | | |
| 54,794 | | |
| 63,449 | | |
| 115.8 | % |
| Research and development | |
| 6,991 | | |
| 3,929 | | |
| 3,062 | | |
| 77.9 | % |
| Depreciation and amortization | |
| 14,053 | | |
| 13,426 | | |
| 627 | | |
| 4.7 | % |
| Total operating expenses | |
| 187,995 | | |
| 92,118 | | |
| 95,877 | | |
| 104.3 | % |
| Operating income (loss) | |
| 18,074 | | |
| (7,973 | ) | |
| 26,047 | | |
| (326.7 | )% |
| Interest expense | |
| 11,156 | | |
| 6,281 | | |
| 4,875 | | |
| 77.6 | % |
| Loss on extinguishment of debt | |
| 2,317 | | |
| - | | |
| 2,317 | | |
| 100 | % |
| Gain on PPP Loan forgiveness (Note 8) | |
| (6,824 | ) | |
| - | | |
| (6,824 | ) | |
| (100 | )% |
| Other expense – net | |
| 194 | | |
| 11 | | |
| 183 | | |
| n.m. | |
| Income (loss) before income taxes | |
| 11,231 | | |
| (14,265 | ) | |
| 25,496 | | |
| (178.7 | )% |
| Income tax expense (benefit) | |
| 11,301 | | |
| (5,094 | ) | |
| 16,395 | | |
| (321.8 | )% |
| Net (loss) income | |
| (70 | ) | |
| (9,171 | ) | |
| 9,101 | | |
| (99.2 | )% |
n.m. = not meaningful
Net Revenue
The following tables provide
our revenue by sales channel, as well as by revenue source and geographic region (based on the location of the end customer), for the
periods presented.
| | |
Year Ended
December 31, | | |
2021 vs 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Sales Channel | |
| | |
| | |
| | |
| |
| Medical | |
$ | 198,592 | | |
$ | 82,532 | | |
$ | 116,060 | | |
| 140.6 | % |
| as a percentage of total revenue | |
| 96.4 | % | |
| 98.1 | % | |
| | | |
| | |
| Clinical | |
$ | 6,268 | | |
$ | 1,613 | | |
$ | 4,655 | | |
| 288.6 | % |
| as a percentage of total revenue | |
| 3.0 | % | |
| 1.9 | % | |
| | | |
| | |
| Other | |
$ | 1,209 | | |
$ | - | | |
$ | 1,209 | | |
| 100.0 | % |
| as a percentage of total revenue | |
| 0.6 | % | |
| - | | |
| | | |
| | |
| Total | |
$ | 206,069 | | |
$ | 84,145 | | |
$ | 121,924 | | |
| 144.9 | % |
| | |
Year Ended
December 31, | | |
2021 vs 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Source and Region | |
| | |
| | |
| | |
| |
| North America | |
$ | 92,771 | | |
$ | 55,389 | | |
$ | 37,382 | | |
| 67.5 | % |
| as a percentage of total revenue | |
| 45.1 | % | |
| 65.8 | % | |
| | | |
| | |
| Asia Pacific | |
$ | 91,908 | | |
$ | 16,696 | | |
$ | 75,212 | | |
| 450.5 | % |
| as a percentage of total revenue | |
| 44.6 | % | |
| 19.8 | % | |
| | | |
| | |
| Rest of the World | |
$ | 15,678 | | |
$ | 6,156 | | |
$ | 9,522 | | |
| 154.7 | % |
| as a percentage of total revenue | |
| 7.6 | % | |
| 7.3 | % | |
| | | |
| | |
| Net Product Sales | |
$ | 200,357 | | |
$ | 78,241 | | |
$ | 122,116 | | |
| 156.1 | % |
| as a percentage of total revenue | |
| 97.2 | % | |
| 93.0 | % | |
| | | |
| | |
| Asia Pacific Royalties | |
$ | 5,712 | | |
$ | 5,904 | | |
$ | (192 | ) | |
| (3.3 | )% |
| as a percentage of total revenue | |
| 2.8 | % | |
| 7.0 | % | |
| | | |
| | |
| Total | |
$ | 206,069 | | |
$ | 84,145 | | |
$ | 121,924 | | |
| 144.9 | % |
Net revenue increased by
$121.9 million, or 144.9%, for the year ended December 31, 2021, compared to the same period in 2020, primarily due to increased product
sales of $122.1 million. The increase in product sales was primarily attributable to a $131.0 million increase resulting from the impact
of partial economic recovery from COVID-19, sales of a newly launched skincare device, and increased wholesale and international distributor
revenue, primarily in our North America and Asia Pacific regions, including $125.0 million, $4.6 million, and $1.4 million increases to
our Medical, Clinical, and Other sales channels, respectively. A further increase of $3.6 million was a result of increased online sales
as we continued to develop our website capabilities, and which primarily impacted our Medical sales channel. These increases were partially
offset by $12.8 million in additional sales discounts and allowances, distribution service fees, and sales returns impacting the Medical
sales channel due to the increase in sales volumes resulting from the economic recovery from COVID-19.
Royalty revenue remained
relatively consistent for the year ended December 31, 2021 compared to the same period in 2020.
Cost of Goods Sold (exclusive of depreciation
and amortization)
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Cost of goods sold (exclusive of depreciation and amortization) | |
$ | 48,708 | | |
$ | 19,969 | | |
$ | 28,739 | | |
| 143.9 | % |
| as a percentage of total revenue | |
| 23.6 | % | |
| 23.7 | % | |
| | | |
| | |
Cost of goods sold (exclusive
of depreciation and amortization) for the year ended December 31, 2021 increased by $28.7 million, or 143.9%, compared to same period
in 2020, which was primarily driven by increased sales associated with the partial economic recovery from COVID-19 that increased the
cost of goods sold by $29.3 million and increased freight costs by $0.9 million. The increase in costs of goods sold was partially offset
by a decrease of $1.5 million for a favorable product pricing arrangement for a specific product with one of our vendors and an increase
of $0.6 million in product returns.
Selling, General and Administrative
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Selling, general and administrative | |
$ | 118,243 | | |
$ | 54,794 | | |
$ | 63,449 | | |
| 115.8 | % |
| as a percentage of total revenue | |
| 57.4 | % | |
| 65.1 | % | |
| | | |
| | |
Selling, general and administrative
expenses increased by $63.4 million or 115.8% for the year ended December 31, 2021 compared to the same period in 2020, which was attributable
to an increase of $55.2 million in selling expenses and $8.2 million in general and administrative expense.
The increase in selling expenses
was primarily driven by (a) a $35.6 million increase in additional fees paid to distributors; (b) a $0.7 million increase in credit card
fees from increased sales volume; (c) an $11.0 million increase in marketing, advertising, and promotions primarily related to increased
marketing in China, new product launches, and website development, partially offset by a $1.3 million decrease in celebrity endorsements;
(d) a $4.7 million increase in salaries and wages due to increased headcount for new product marketing initiatives, increased commissions
as sales increased, and providing more employee rewards; and (e) a $1.4 million increase due to additional border taxes resulting from
increased sales in China.
The increase in general and
administrative expenses was primarily driven by a $5.2 million increase in fees resulting from the Business Combination, a $2.3 million
increase in wages and salaries and employees benefits due to general increases in salaries and wages as the economy partially recovered,
and a $0.5 million increase in consulting fees.
Research and Development
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Research and development | |
$ | 6,991 | | |
$ | 3,929 | | |
$ | 3,062 | | |
| 77.9 | % |
| as a percentage of total revenue | |
| 3.4 | % | |
| 4.7 | % | |
| | | |
| | |
Research and development
expenses increased by $3.1 million or 77.9% for the year ended December 31, 2021 compared to the same period in 2020 primarily due to
a $2.1 million increase in regulatory compliance spending in fiscal year 2021 and a $0.5 million increase related to product development.
Depreciation and Amortization
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Depreciation and amortization | |
$ | 14,053 | | |
$ | 13,426 | | |
$ | 627 | | |
| 4.7 | % |
| as a percentage of total revenue | |
| 6.8 | % | |
| 16.0 | % | |
| | | |
| | |
Depreciation and amortization
expenses remained relatively consistent for the year ended December 31, 2021 when compared to the same period in 2020.
Interest Expense
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Interest expense | |
$ | 11,156 | | |
$ | 6,281 | | |
$ | 4,875 | | |
| 77.6 | % |
| as a percentage of total revenue | |
| 5.4 | % | |
| 7.5 | % | |
| | | |
| | |
Interest expense increased
by $4.9 million or 77.6% for the year ended December 31, 2021 compared to 2020. The increase was primarily due to refinancing of our debt
in March 2021 at higher interest rates as well as additional borrowings incurred from the bank in the latter half of the 2020 fiscal year.
Loss on Extinguishment of Debt
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Loss on extinguishment of debt | |
$ | 2,317 | | |
$ | - | | |
$ | 2,317 | | |
| 100.0 | % |
| as a percentage of total revenue | |
| 1.1 | % | |
| - | | |
| | | |
| | |
The loss on extinguishment
of debt for the year ended December 31, 2021 is primarily related to the write-off of previously deferred financing costs due to the refinancing
of our debt in March 2021.
Gain on PPP Loan Forgiveness
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Gain on PPP Loan forgiveness | |
$ | (6,824 | ) | |
$ | - | | |
$ | (6,824 | ) | |
| (100.0 | )% |
| as a percentage of total revenue | |
| 3.3 | % | |
| - | | |
| | | |
| | |
For the year ended December
31, 2021, we recognized a gain of $6.8 million associated with the forgiveness of the PPP Loan borrowed in 2020.
Income Tax Expense (Benefit)
| | |
Year Ended
December 31, | | |
2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Income tax expense (benefit) | |
$ | 11,301 | | |
$ | (5,094 | ) | |
$ | 16,395 | | |
| (321.8 | )% |
| Effective tax rate | |
| 100.6 | % | |
| 35.6 | % | |
| | | |
| | |
For the year ended December
31, 2021, income tax expense of $11.3 million was recognized, with a corresponding effective tax rate of 100.6%, compared to an income
tax benefit of $5.1 million recognized for the year ended December 31, 2020, with a corresponding effective tax rate of 35.6%. The increase
in the effective tax rate for the year ended December 31, 2021 when compared to the year ended December 31, 2020 was primarily attributable
to the valuation allowance of $14.3 million recognized to account for the portion of the deferred tax asset that is more likely than not
to be realized due to a cumulative loss incurred in our U.S. subsidiary over the three-year period ended December 31, 2021 (see Note 13
to our consolidated financial statements included in Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022,
and filed with the SEC on July 7, 2022 for more information). This was partially offset by the change in the mix of pre-tax earnings in
tax jurisdictions with different statutory tax rates during the comparable periods as well as the nontaxable gain on the PPP Loan forgiveness.
Comparison of the Years
Ended December 31, 2020 and 2019
The following tables summarize
our consolidated statements of operations data, in dollars and as a percentage of total revenue, for the fiscal year ended December 31,
2020 and 2019.
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Net revenue | |
$ | 84,145 | | |
$ | 117,085 | | |
$ | (32,940 | ) | |
| (28.1 | )% |
| Cost of goods sold (exclusive of depreciation and amortization expense) | |
| 19,969 | | |
| 26,687 | | |
| (6,718 | ) | |
| (25.2 | )% |
| Selling, general and administrative | |
| 54,794 | | |
| 62,762 | | |
| (7,968 | ) | |
| (12.7 | )% |
| Research and development | |
| 3,929 | | |
| 3,484 | | |
| 445 | | |
| 12.8 | % |
| Depreciation and amortization | |
| 13,426 | | |
| 12,940 | | |
| 486 | | |
| 3.8 | % |
| Total operating expenses | |
| 92,118 | | |
| 105,873 | | |
| (13,755 | ) | |
| (13.0 | )% |
| Operating income (loss) | |
| (7,973 | ) | |
| 11,212 | | |
| (19,185 | ) | |
| (171.1 | )% |
| Interest expense | |
| 6,281 | | |
| 6,834 | | |
| (553 | ) | |
| (8.1 | )% |
| Other expense – net | |
| 11 | | |
| 147 | | |
| (136 | ) | |
| n.m. | |
| (Loss) income before income taxes | |
| (14,265 | ) | |
| 4,231 | | |
| (18,496 | ) | |
| (437.2 | )% |
| Income tax benefit | |
| (5,094 | ) | |
| (1,589 | ) | |
| (3,505 | ) | |
| 220.6 | % |
| Net (loss) income | |
| (9,171 | ) | |
| 5,820 | | |
| (14,991 | ) | |
| (257.6 | )% |
n.m. = not meaningful
Net Revenue
The following tables provide
our revenue by sales channel, as well as by revenue source and geographic region (based on the location of the end customer), for the
periods presented.
| | |
Year Ended
December 31, | | |
2020 vs 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Sales Channel | |
| | |
| | |
| | |
| |
| Medical | |
$ | 82,532 | | |
$ | 114,585 | | |
$ | (32,053 | ) | |
| (28.0 | )% |
| as a percentage of total revenue | |
| 98.1 | % | |
| 97.9 | % | |
| | | |
| | |
| Clinical | |
$ | 1,613 | | |
$ | 2,500 | | |
$ | (887 | ) | |
| (35.5 | )% |
| as a percentage of total revenue | |
| 1.9 | % | |
| 2.1 | % | |
| | | |
| | |
| Total | |
$ | 84,145 | | |
$ | 117,085 | | |
$ | (32,940 | ) | |
| (28.1 | )% |
| | |
Year Ended
December 31, | | |
2020 vs 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Source and Region | |
| | |
| | |
| | |
| |
| North America | |
$ | 55,389 | | |
$ | 85,025 | | |
$ | (29,636 | ) | |
| (34.9 | )% |
| as a percentage of total revenue | |
| 65.8 | % | |
| 72.6 | % | |
| | | |
| | |
| Asia Pacific | |
$ | 16,696 | | |
$ | 20,496 | | |
$ | (3,800 | ) | |
| (18.5 | )% |
| as a percentage of total revenue | |
| 19.8 | % | |
| 17.5 | % | |
| | | |
| | |
| Rest of the World | |
$ | 6,156 | | |
$ | 5,225 | | |
$ | 931 | | |
| 17.8 | % |
| as a percentage of total revenue | |
| 7.3 | % | |
| 4.5 | % | |
| | | |
| | |
| Net Product Sales | |
$ | 78,241 | | |
$ | 110,746 | | |
$ | (32,505 | ) | |
| (29.4 | )% |
| as a percentage of total revenue | |
| 93.0 | % | |
| 94.6 | % | |
| | | |
| | |
| Asia Pacific Royalties | |
$ | 5,904 | | |
$ | 6,339 | | |
$ | (435 | ) | |
| (6.9 | )% |
| as a percentage of total revenue | |
| 7.0 | % | |
| 5.4 | % | |
| | | |
| | |
| Total | |
$ | 84,145 | | |
$ | 117,085 | | |
$ | (32,940 | ) | |
| (28.1 | )% |
Net revenue decreased by
$32.9 million, or 28.1%, for the year ended December 31, 2020, compared to the same period in 2019, due primarily to a decrease of $40.5
million in product sales, including $38.3 million and $2.2 million decreases in the Medical and Clinical sales channels, respectively.
The decrease in product sales was primarily attributable to the effects of COVID-19 in our North America and Asia Pacific geographic regions,
which impacted all product categories as a result of the closure of physician offices as well as lower demand for skincare products. The
decrease in product sales also reduced the number of physician chargebacks by $5.3 million, impacting the Medical sales channel. This
decrease was partially offset by (a) $3.0 million in the Medical sales channel from the discontinuance of prompt pay discounts for certain
customers and rebates for international distributors; (b) an increase in net revenue of $0.9 million in the Medical sales channel primarily
due to increased market share in Europe, which was slightly offset by decreased market share in Africa and South America; and (c) by an
increase of $0.7 million in the Medical sales channel for a favorable product pricing arrangement as a result of dual sourced products.
Royalty revenue remained
relatively consistent for the year ended December 31, 2020 when compared to the same period in 2019.
Cost of Goods Sold (exclusive of depreciation
and amortization)
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Cost of goods sold (exclusive of depreciation and amortization) | |
$ | 19,969 | | |
$ | 26,687 | | |
$ | (6,718 | ) | |
| (25.2 | )% |
| as a percentage of total revenue | |
| 23.7 | % | |
| 22.8 | % | |
| | | |
| | |
Cost of goods sold for the
year ended December 31, 2020 decreased by $6.7 million, or 25.2%, compared to same period in 2019 due to the decline in sales caused primarily
by the impact of COVID-19. Additionally, freight and storage costs decreased by $1.0 million and $0.2 million, respectively, as a result
of bringing in less inventory and reduced warehouse activity resulting from the decline in sales activity caused by COVID-19.
Selling, General and Administrative
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Selling, general and administrative | |
$ | 54,794 | | |
$ | 62,762 | | |
$ | (7,968 | ) | |
| (12.7 | )% |
| as a percentage of total revenue | |
| 65.1 | % | |
| 53.6 | % | |
| | | |
| | |
Selling, general and administrative
expenses decreased by $8.0 million or 12.7% for the year ended December 31, 2020 compared to the same period in 2019, which was attributable
to a decrease of $5.9 million in selling expenses and a decrease of $2.2 million in general and administrative expenses.
The decrease in selling expenses
was primarily driven by (a) a $2.6 million decrease in other professional fees and expenses primarily from decreased distribution service
fees resulting from reduced sales; (b) a $2.5 million decrease in travel and entertainment expenses from suspended travel and events because
of COVID-19; (c) a $0.9 million decrease in salaries and wages due to reduced sales commissions as a result of the reduction in sales
and a decrease in severance payouts; and (d) a $0.3 million decrease in employee benefits primarily due to decreased hiring costs because
hiring was put on hold during COVID-19.
The decrease in general and
administrative expenses was primarily due to (a) a $2.7 million decrease in legal services following the completion in 2019 of arbitration
in connection with our claims that a company and its previous owners had violated the terms of a settlement agreement; (b) a $0.4 million
decrease in travel and entertainment expenses due to suspended travel because of COVID-19, partially offset by a $1.1 million increase
in salaries and wages resulting from promotions and increases in employee bonuses; a $0.3 million increase in employee benefits related
to relocation and housing costs; (c) a $0.2 million increase in audit services for initial public offering readiness and tax advisory
services; and (d) a $0.1 million increase in insurance premiums.
Research and Development
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Research and development | |
$ | 3,929 | | |
$ | 3,484 | | |
$ | 445 | | |
| 12.8 | % |
| as a percentage of total revenue | |
| 4.7 | % | |
| 3.0 | % | |
| | | |
| | |
Research and development
expenses remained relatively consistent for the year ended December 31, 2020 when compared to the same period in 2019.
Depreciation and Amortization
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Depreciation and amortization | |
$ | 13,426 | | |
$ | 12,940 | | |
$ | 486 | | |
| 3.8 | % |
| as a percentage of total revenue | |
| 16.0 | % | |
| 11.1 | % | |
| | | |
| | |
Depreciation and amortization
expenses remained relatively consistent for the year ended December 31, 2020 when compared to the same period in 2019.
Interest Expense
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Interest expense | |
$ | 6,281 | | |
$ | 6,834 | | |
$ | (553 | ) | |
| (8.1 | )% |
| as a percentage of total revenue | |
| 7.5 | % | |
| 5.8 | % | |
| | | |
| | |
Interest expense remained
relatively consistent for the year ended December 31, 2020 when compared to the same period in 2019.
Income Tax Benefit
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Income tax benefit | |
$ | (5,094 | ) | |
$ | (1,589 | ) | |
$ | (3,505 | ) | |
| 220.6 | % |
| Effective tax rate | |
| 35.6 | % | |
| (37.6 | )% | |
| | | |
| | |
For the year ended December
31, 2020, an income tax benefit of $5.1 million was recognized, with a corresponding effective tax rate of 35.6%, compared to an income
tax benefit of $1.6 million recognized for the year ended December 31, 2019, with a corresponding effective tax rate of 37.6%. The change
was primarily due to a larger loss position in the U.S.-based operations in the year ended December 31, 2020.
Liquidity and Capital Resources
We measure liquidity in terms
of our ability to fund the cash requirements of our business operations, including working capital needs, capital expenditures, contractual
obligations, debt service, acquisitions, and other commitments with cash flows from operations and other sources of funding. Our principal
sources of capital and liquidity are our borrowings from banks and cash flows from operations. In response to the loss in demand for our
products and drop in revenue early on in the pandemic, we drew on our 2018 Revolving Credit Facility, and increased our borrowing capacity
by $10.0 million. We also secured a $6.8 million PPP Loan, which was used in accordance with the program and for which forgiveness was
obtained in the second quarter of 2021. Overall, these financing activities resulted in net cash inflow of approximately $16.3 million
in 2020 (excluding $2.0 million of dividends to our shareholder). In March 2021, we were able to access additional credit by replacing
the 2018 Credit Agreement with the 2021 Credit Agreement from a new syndicate of lenders. The 2021 Credit Agreement included a term loan
of $110.0 million and a revolving credit facility with borrowing capacity of up to $40.0 million. Obagi recorded a loss on extinguishment
of the 2018 Credit Agreement of $2.3 million to loss on extinguishment of debt in the accompanying condensed consolidated statement of
operations and comprehensive (loss) income during the six months ended June 30, 2021, which consisted of expensing unamortized debt issuance
costs. We do not anticipate the need for additional credit and are current with all scheduled payments on the term loan under the 2021
Credit Agreement.
We expect capital and operating
expenditures to increase over the next several years as we expand our infrastructure, distribution channels and our commercialization,
clinical trial, research and development and manufacturing activities. We believe that net cash provided by our operating activities and
existing cash and cash equivalents, including access to credit facilities, will be sufficient to fund our operations for the foreseeable
future. The outstanding balance under the 2021 Credit Agreement was settled upon the completion of the Business Combination, which occurred
on July 27, 2022.
If our net cash provided
by operating activities and existing cash and cash equivalents are not sufficient to fund our operations in the future, we may need to
seek additional credit or raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms
when needed, if at all. If we are required to seek additional credit, our ability to in-license new technologies, develop future products
or expand our pipeline of products could all be negatively impacted, which would have an adverse effect on our ability to grow our business
and remain competitive in our marketplace. Further, we may decide to raise additional proceeds by issuing equity securities or securities
that are convertible into our equity. If we sell such securities, investors may be materially diluted as a result of such offerings. In
addition, if we raise additional funds through collaboration, licensing or other similar arrangements, we may be required to relinquish
potentially valuable rights to our future products or proprietary technologies, or grant licenses on terms that are not favorable to us.
If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future
opportunities or respond to competitive pressures or unanticipated customer requirements.
As of June 30, 2022, we had
cash and cash equivalents of $6.7 million.
2021 Credit Agreement
In March 2021, we replaced
the 2018 Credit Agreement with the 2021 Credit Agreement with a new syndicate of lenders, including TCW Asset Management Company LLC as
administrative agent for the lenders. The 2021 Credit Agreement includes the 2021 Term Loan of $110.0 million and the 2021 Revolving Credit
Facility with borrowing capacity of up to $40.0 million.
Both the 2021 Term Loan and
the 2021 Revolving Credit Facility mature in March 2026. The 2021 Credit Agreement interest rate is calculated based on London Inter-Bank
Offered Rate (“LIBOR”) plus applicable margin, as determined by our leverage ratios, and are subject to LIBOR succession provisions.
If LIBOR becomes unavailable, the parties will establish an alternate index rate that gives due consideration to the then prevailing market
convention for determining a rate of interest for leveraged syndicated loans in the United States. In connection with the issuance of
the 2021 Credit Agreement, we incurred $6.4 million of debt issuance costs.
Obagi is a holding company
with no material operations of its own that conducts substantially all of its activities through its wholly owned subsidiaries. Obagi
has no cash and, as a result, all expenditures and obligations of Obagi are allocated to and paid by its subsidiaries. Obagi Cosmeceuticals,
LLC is the borrower under the 2021 Credit Agreement and the since extinguished 2018 Credit Agreement. The 2021 Credit Agreement limits
Obagi and its wholly owned subsidiaries’ ability to declare dividends or make other distributions. Due to these restrictions, substantially
all of the net assets of Obagi’s subsidiaries are restricted. These restrictions have not and are not reasonably likely to have
an impact on the ability of Obagi to meet its cash obligations. See Note 18 to our audited consolidated financial statements included
in our definitive proxy statement/final prospectus dated July 7, 2022, and filed with the SEC on July 7, 2022 for additional information.
As of June 30, 2022 and December
31, 2021, we had an unpaid principal amount of $107.8 million and 109.2 million, respectively, and unamortized debt issuance costs of
$4.0 million and $3.9 million, respectively on the 2021 Term Loan. The interest rate on the 2021 Term Loan was 8.50% and there is no accrued
interest as of June 30, 2022 or December 31, 2021. The outstanding balance under the 2021 Credit Agreement was settled upon the completion
of the Business Combination, which occurred on July 27, 2022.
As of June 30, 2022, the
current portion of the 2021 Term Loan and 2021 Revolving Credit Facility was $4.1 million and $21.0 million, respectively. The current
portion of the unamortized debt issuance costs on the 2021 Term Loan and 2021 Revolving Credit Facility was $1.1 million and $1.4 million,
respectively. The interest rate on the outstanding balance is 8.50%.
As of December 31, 2021,
the current portion of the 2021 Term Loan and 2021 Revolving Credit Facility was $2.8 million and $15.0 million, respectively. The current
portion of the unamortized debt issuance costs on the 2021 Term Loan and 2021 Revolving Credit Facility was $0.9 million and $1.4 million,
respectively. The interest rate on the outstanding balance was 8.50%.
The entire balance was settled
upon the close of the business combination on July 27, 2022.
2018 Credit Agreement
In December 2018, we entered
into the 2018 Credit Agreement with a syndicate of lenders, including Wells Fargo Bank, National Association as administrative agent for
the lenders (the “Syndicate of Banks”). The 2018 Credit Agreement included the 2018 Term Loan of $90.0 million and the 2018
Revolving Credit Facility with borrowing capacity of up to $35.0 million. Both the 2018 Term Loan and the 2018 Revolving Credit Facility
were due to mature in December 2023. In connection with the issuance of the 2018 Credit Agreement, we incurred $2.9 million of debt issuance
costs. The 2018 Credit Agreement was secured by the assets of Obagi. Both the 2018 Term Loan and the 2018 Revolving Credit Facility carried
an interest rate comprised of LIBOR plus applicable margin, as determined by the Company’s leverage ratios, and are subject to LIBOR
succession provisions.
In December 2019, the 2018
Credit Agreement was amended to revise the definition of consolidated earnings before interest, taxes, depreciation, and amortization
(“Consolidated EBITDA”) to allow for certain additional adjustments, in relation to the debt covenants.
In March 2020, an increase
in the commitment on the 2018 Revolving Credit Facility was approved by the Syndicate of Banks for an additional $10.0 million, to $45.0
million.
In November 2020, the 2018
Credit Agreement was amended to waive the event of default, adjust the “Applicable Margin Rates”, and revise the maximum percentages
allowed for the consolidated total leverage ratio (as defined in the 2018 Credit Agreement) as well as the minimum percentages allowed
for the consolidated fixed charge coverage ratio (as defined in the 2018 Credit Agreement). In addition, the amendment revised the 2018
Credit Agreement to include minimum Consolidated EBITDA levels and minimum liquidity levels through the end of fiscal year 2021. Prior
to this amendment, we had not satisfied our debt covenants, which would have represented a default under our 2018 Credit Agreement. However,
subsequent to the amendment and as of the date of the financial statements, we were in compliance with all financial and non-financial
debt and other contractual covenants.
As of December 31, 2020 and
2019, we had an unpaid principal amount of $71.6 million and $81.0 million, respectively, and unamortized debt issuance costs of $2.5
million and $2.3 million, respectively, on the 2018 Term Loan. The interest rate on the 2018 Term Loan was 5.50% and 5.30% as of December
31, 2020 and 2019, respectively.
As of December 31, 2020 and
2019, we had an outstanding balance of $39.0 million and $19.0 million on our 2018 Revolving Credit Facility, respectively. The 2018 Revolving
Credit Facility had an interest rate of 5.50%.
PPP Loan
In May 2020, we received
loan proceeds in the amount of $6.8 million under the PPP from MUFG Union Bank. The PPP Loan accrued interest at a rate of 1.00%. The
loan and accrued interest were forgivable after eight or twenty-four weeks as long as the borrower used the loan proceeds for eligible
purposes, including payroll, benefits, rent and utilities and maintained its payroll levels. We used the proceeds for purposes consistent
with the PPP. In June 2021, we received approval from the Small Business Administration and MUFG Union Bank for forgiveness of the full
amount of the PPP Loan, inclusive of accrued interest of $0.1 million. We recognized a gain on forgiveness of the PPP Loan of $6.8 million
in the second quarter of 2021.
Consolidated Cash Flow Data
Comparison of Six Months Ended June 30, 2022
to Six Months Ended June 30, 2021
| | |
Six Months Ended
June 30, | | |
Six Months Ended
June 30, 2022 vs 2021 | |
| (in thousands, except percentages) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Net cash (used in) provided by operating activities | |
$ | (8,754 | ) | |
$ | 10,528 | | |
$ | (19,282 | ) | |
| (183.1 | )% |
| Net cash used in investing activities | |
| (1,181 | ) | |
| (870 | ) | |
| (311 | ) | |
| (35.7 | )% |
| Net cash provided by financing activities | |
| 3,883 | | |
| 6,962 | | |
| (3,079 | ) | |
| 44.2 | % |
Cash Flows from Operating Activities
Net cash used in and provided
by operating activities decreased by $19.3 million, or 183.1%, for the six months ended June 30, 2022 compared to the same period in 2021,
primarily due to the following:
| |
● |
Lower net income of $15.9 million, primarily resulting from the impact of higher selling, general and administrative expenses due to increased marketing activities following the COVID-19 recovery in existing territories and expansion into new territories, as well as transaction costs related to the Business Combination; |
| |
● |
An increase in inventory of $12.1 million to meet the higher forecasted sales in the Asia Pacific region for the remainder of fiscal year 2022; and |
| |
● |
Decrease in operating activities was partially offset by a $6.8 million PPP loan forgiveness which occurred in the six months ended June 30, 2021. |
Cash Flows from Investing Activities
Net cash used in investing
activities increased by $0.3 million, or 35.7%, for the six months ended June 30, 2022 compared to the same period in 2021, which was
attributable primarily to investments in computer software and website development costs.
Cash Flows from Financing Activities
Net cash provided by financing
activities decreased $3.1 million, or 44.2%, for the six months ended June 30, 2022 compared to the same period in 2021, primarily due
to a $124.0 million decrease in cash borrowed related to the 2021 Credit Agreement, which consists of the 2021 Term Loan and the 2021
Revolving Credit Facility. This decrease was partially offset by $120.2 million of reduced principal payments as a result of the repayment
of the 2018 Term Loan and 2018 Revolving Credit Facility and payments of debt issuance costs related to the 2021 Credit Agreement and
dividend of 0.8 million paid in 2021.
Comparison of the Years Ended December 31,
2021 and 2020
| | |
Year Ended
December 31, 2021 vs. 2020 | |
| (in thousands, except percentages) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Net cash provided by (used in) operating activities | |
$ | 5,070 | | |
$ | (7,251 | ) | |
$ | 12,321 | | |
| (169.9 | )% |
| Net cash used in investing activities | |
| (5,360 | ) | |
| (1,887 | ) | |
| (3,473 | ) | |
| 184.0 | % |
| Net cash provided by financing activities | |
| 5,162 | | |
| 14,319 | | |
| (9,157 | ) | |
| (63.9 | )% |
Cash Flows from Operating Activities
Net cash provided by and
used in operating activities increased by $12.3 million, or 169.9%, for the year ended December 31, 2021 compared to the same period in
2020, primarily due to the following:
| |
- |
Higher net income of $9.1 million resulting from the impact of higher revenues for the year ended December 31, 2021 due to the impact of partial economic recovery from COVID-19, sales of a newly launch skincare device, increased wholesale and international distributor revenue, and increased online sales; |
| |
- |
Higher cash flows from changes in accounts payable, deferred income taxes, and other liabilities of $33.6 million as a result of the timing of payments; and these higher cash flows are partially offset by an: |
| |
o |
Increase in inventory of $6.9 million to meet the higher forecasted sales for the fiscal year 2021; and |
| |
o |
Increase in accounts receivable of $22.8 million due to new customers in China, increased sales, and timing of the payments received from customers. |
Cash Flows from Investing Activities
Net cash used in investing
activities increased by $3.5 million, or 184.0%, for the year ended December 31, 2021 compared to the same period in 2020, which was attributable
primarily to our non-recourse, uncollateralized short-term promissory note of $2.5 million issued to us by a third party (see Note 4 to
our consolidated financial statements included in Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022, and
filed with the SEC on July 7, 2022 for more information) as well as increases in capital expenditures to build out our online sales capabilities
to increase sales and reduce the impact caused by the closure of businesses due to COVID-19.
Cash Flows from Financing Activities
Net cash provided by financing
activities decreased by $9.2 million, or 63.9%, for the year ended December 31, 2021 compared to the same period in 2020, primarily due
to (a) the increases in repayments of our 2018 Revolving Credit Facility of $35.0 million; (b) repayment of the 2018 Term Loan of $63.1
million; (c) payment of debt issuance costs of $5.4 million for the 2021 Credit Agreement; and (d) decreased borrowings from the 2018
Revolving Credit Facility of $9.0 million and the PPP Loan of $6.8 million as a result of partial recovery from COVID-19 as discussed
above. We are able to generate significant cash from our operating activities. This was partially offset by the 2021 Credit Agreement,
which includes term loan proceeds of $110.0 million.
Comparison of the Years Ended December 31,
2020 and 2019
| | |
Year Ended
December 31, | | |
2020 vs. 2019 | |
| (in thousands, except percentages) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Net cash (used in) provided by operating activities | |
$ | (7,251 | ) | |
$ | 3,776 | | |
$ | (11,027 | ) | |
| (292.0 | )% |
| Net cash used in investing activities | |
| (1,887 | ) | |
| (788 | ) | |
| (1,099 | ) | |
| (139.5 | )% |
| Net cash provided by (used in) financing activities | |
| 14,319 | | |
| (6,814 | ) | |
| 21,133 | | |
| 310.1 | % |
Cash Flows from Operating Activities
Net cash used in and provided
by operating activities increased by $11.0 million, or 292.0%, for the year ended December 31, 2020 compared to the same period in 2019,
primarily due to significantly lower cash related operating results of $15.0 million resulting from the impact of lower net revenues during
the first three quarters of fiscal 2020 due to COVID-19 as our customers’ businesses were closed during the first half of 2020,
and lower cash flows from changes in accounts payable, accrued expenses, and other liabilities of $11.7 million as a result of the timing
of payments. This was partially offset by a decrease in inventory of $10.5 million resulting from lower sales because of the impact of
COVID-19 on our business and decrease in accounts receivable due to the timing of payments received from the customers.
Cash Flows from Investing Activities
Net cash used in investing
activities increased by $1.1 million, or 139.5%, for the year ended December 31, 2020 compared to the same period in 2019, which was attributable
primarily to investments in our website to reduce the impact of COVID-19 by introducing online sales capabilities as well as investments
in other equipment related to leasehold improvements and machinery.
Cash Flows from Financing Activities
Net cash provided by and
used in financing activities increased $21.1 million, or 310.1%, for the year ended December 31, 2020 compared to the same period in 2019,
primarily due to proceeds from the PPP Loan of $6.8 million and a decrease in payments to the 2018 Revolving Credit Facility of $34.0
million to meet our operational needs as a result of the impact of COVID-19. This was partially offset by the decrease in the borrowing
from the 2018 Revolving Credit Facility of $18.0 million.
Critical accounting policies,
significant judgments and use of estimates
The preparation of our audited
annual and unaudited condensed interim consolidated financial statements and related notes requires us to make judgments, estimates and
assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets
and liabilities. We based our estimates on historical experience and on various other assumptions that we believe to be reasonable under
the circumstances. We periodically review our estimates and make adjustments when facts and circumstances dictate. To the extent that
there are material differences between these estimates and actual results, our financial condition or results of operations will be affected.
An accounting policy is considered
to be critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time
the estimate is made, and if different estimates that reasonably could have been used, or changes in the accounting estimates that are
reasonably likely to occur periodically, could materially impact our audited consolidated financial statements. We believe that our critical
accounting policies reflect the more significant estimates and assumptions used in the preparation of our audited consolidated financial
statements. The critical accounting policies, judgments and estimates should be read in conjunction with our audited consolidated financial
statements and the notes thereto and other disclosures, included in Waldencast’s definitive proxy statement/final prospectus dated
July 7, 2022, and filed with the SEC on July 7, 2022. For further information, see Note 2 to our audited consolidated financial statements
included in Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022, and filed with the SEC on July 7, 2022.
We believe the following
critical accounting policies, estimates and assumptions may have a material impact on reported financial condition and operating performance
and may involve significant levels of judgment to account for highly uncertain matters or are susceptible to significant change:
Revenue Recognition
We recognize revenue upon
transfer of control of promised products to customers in an amount that reflects the consideration we expect to receive in exchange for
those products. Revenue is recognized net of any taxes collected from customers, which are subsequently remitted to Governmental Authorities.
We primarily recognize revenue from net product sales and royalty income. For the majority of sales, we transfer control, invoice the
customer, and recognize revenue either once the delivery of the product has occurred or once the product has been picked up from the warehouse
by the buyer’s shipping agent. We account for shipping and handling activities as a fulfillment activity instead of a performance
obligation. As such, costs related to shipping and handling are included in selling, general and administrative expenses in the statements
of operations and comprehensive loss (income). We recognize royalty revenue in connection with the Rohto licensing agreement at a point
in time. Based on the licensing agreement, a customer contract with Rohto exists when Rohto sells licensed product to its customer.
Discounts, Allowances, Rebates, and Chargebacks
We offer discounts and other
incentive allowances to customers. The transaction price of product sales includes estimates of these discounts and incentives as variable
consideration. Product sales revenue is recognized net of provisions for estimated discounts and allowances, distribution fees, returns,
and rebates. Provisions for discounts and allowances are estimated based on the most likely amount using contractual sales terms with
customers and historical experience. Accruals for customer rebates are estimated based on the contractual terms and our current evaluation
of our experience.
Services Provided by the Customer
Consideration payable to
a customer for a distinct good or service is treated as a purchase for an amount up to the fair value of such distinct good or service.
When consideration payable for distinct goods or services exceeds the fair value of services provided by the customer, we record those
excess amounts as a reduction of the transaction price in the arrangement. Consideration payable to a customer for non-distinct services,
such as distribution services including packing and shipping, customer service, returns processing and reporting, customer credit, invoicing,
collection and chargeback services, is recorded as a reduction to the transaction price.
Goodwill
Goodwill is calculated as
the excess of the cost of purchased businesses over the fair value of their underlying net assets. We assess goodwill at least annually
as of September 30th for impairment, or more frequently, if certain events or circumstances warrant. We have only one goodwill
reporting unit which we test for impairment at the reporting unit level. We identified our reporting unit by assessing whether the component
of our reporting segment constitutes a business for which discrete financial information is available and management of the reporting
unit regularly reviews the operating results of that component.
When quantitative impairment
testing for goodwill is necessary, it is based upon the fair value of a reporting unit as compared to its carrying value. We make certain
judgments and assumptions in allocating assets and liabilities to determine carrying values for our reporting units. The impairment loss
recognized would be the difference between a reporting unit’s carrying value and fair value in an amount not to exceed the carrying
value of the reporting unit’s goodwill.
Testing goodwill for impairment
requires us to estimate fair values of reporting units using significant estimates and assumptions. The assumptions made will impact the
outcome and ultimate results of the testing. We use industry accepted valuation models and set criteria that are reviewed and approved
by various levels of management and, in certain instances, we engage independent third-party valuation specialists. To determine fair
value of the reporting unit, we used a combination of the income and market approaches, when applicable. We believe the blended use of
both models, when applicable, compensates for the inherent risk associated with either model if used on a stand-alone basis, and this
combination is indicative of the factors a market participant would consider when performing a similar valuation.
Under the income approach,
we determine fair value using a discounted cash flow method, projecting future cash flows of each reporting unit, as well as a terminal
value, and discounting such cash flows at a rate of return that reflects the relative risk of the cash flows. Under the market approach,
when applicable, we utilize information from comparable publicly traded companies with similar operating and investment characteristics
as the reporting units, which creates valuation multiples that are applied to the operating performance of the reporting units being tested,
to value the reporting unit.
The key estimates and factors
used in these approaches include revenue growth rates and profit margins based on our internal forecasts, our specific weighted-average
cost of capital used to discount future cash flows, and comparable market multiples for the industry segment, when applicable, as well
as our historical operating trends. Certain future events and circumstances, including deterioration of market conditions, higher cost
of capital, a decline in actual and expected consumer consumption and demands, could result in changes to these assumptions and judgments.
A revision of these assumptions could cause the fair values of the reporting units to fall below their respective carrying values, resulting
in a non-cash impairment charge. Such charge could have a material effect on the Consolidated Statements of Operations and Balance Sheets.
There were no impairment
charges recorded on goodwill during the years ended December 31, 2021, 2020, and 2019 or during the six months ended June 30, 2022 and
2021. As of June 30, 2022, we did not consider reporting units to be at risk for impairment as we have determined the fair value of our
reporting units significantly exceeds the carrying value.
Stock-based compensation
Stock-based compensation
cost is measured at grant date, based on the fair value of the award, and is generally recognized on a straight-line basis over the requisite
service period for all awards that vest. We estimate the fair value of employee stock-based payment awards subject to service and qualifying
transaction conditions on the date of grant using the Black-Scholes valuation model. The Black-Scholes model requires the use of highly
subjective and complex assumptions, including the option’s expected term and the price volatility of the underlying stock.
We recognize compensation
expense for awards with service and qualifying transaction conditions on a straight-line basis over the requisite service period, which
is generally the award’s vesting period. Compensation expense for employee stock-based awards whose vesting is subject to the fulfillment
of both a service condition and the occurrence of a performance condition is recognized on a graded-vesting basis at the time the achievement
of the performance condition becomes probable. Immediately prior to the consummation of the Business Combination, the qualifying transaction
event became “probable” and previously unrecognized stock-based compensation expense was recognized at the time of close based
on the requisite service period through that date.
Higher volatility and longer
expected terms result in an increase to stock-based compensation determined at the date of grant. Future stock-based compensation cost
and unrecognized stock-based compensation will increase to the extent that we grant additional equity awards to employees, or we assume
unvested equity awards in connection with acquisitions. If there are any modifications or cancellations of the underlying unvested securities,
we may be required to accelerate any remaining unearned stock-based compensation cost or incur incremental cost.
The expected stock price
volatility for common stock was estimated by taking the average historic price volatility for industry peers based on daily price observations
over a period equivalent to the expected term of the stock option grants. Industry peers consist of several public companies in our industry
which are of similar size, complexity and stage of development. The risk-free interest rate for the expected term of the option is based
on the U.S. Treasury implied yield at the date of grant. The weighted-average expected term is determined based on the simplified method,
which results in an expected term based on the midpoint between the vesting date and contractual term of an option. The simplified method
was chosen because we have limited historical option exercise experience.
If factors change and we
employ different assumptions, stock-based compensation cost on future awards may differ significantly. No stock-based compensation expense
as been realized in current or prior periods.
Deferred Tax Assets (and Related Valuation
Allowance)
We recognize net deferred
tax assets to the extent that we believe these assets are more likely than not to be realized. In making such a determination, management
considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected
future taxable income, tax-planning strategies, and results of recent operations. If we determine that deferred tax assets may be able
to be recognized in the future in excess of their net recorded amount, the deferred tax asset valuation allowance would be adjusted, which
would reduce the provision for income taxes. We record uncertain tax positions on the basis of a two-step process whereby (i) management
determines whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position
and (ii) for those tax positions that meet the more-likely-than-not recognition threshold, management recognizes the largest amount of
tax benefit that is more than 50% likely to be realized upon ultimate settlement with the related tax authority.
This requires management
to make judgments and estimates regarding: (i) the timing and amount of the reversal of taxable temporary differences; (ii) expected future
taxable income; and (iii) the impact of tax planning strategies. Future changes to tax rates would also impact the amounts of deferred
tax assets and liabilities and could adversely affect our financial statements. A valuation allowance of $14.8 million and $14.3 million
has been recorded as of June 30, 2022 and December 31, 2021, respectively. No valuation allowance has been recorded as of December 31,
2020 and 2019.
The valuation allowance will
be reduced at such time as management believes it is more-likely-than-not that the deferred tax assets will be realized. The exact timing
and amount of a valuation allowance release are subject to change on the basis of the future level of profitability and changes in tax
law. Release of the valuation allowance would result in the recognition of certain deferred tax assets and a decrease to income tax expense
for the period the release is recorded.
Emerging Growth Company Accounting Election
Section 102(b)(1) of the
JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private
companies are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can choose
not to take advantage of the extended transition period and comply with the requirements that apply to non-emerging growth companies,
and any such election to not take advantage of the extended transition period is irrevocable. We are an “emerging growth company”
as defined in Section 2(a) of the Securities Act, and have irrevocably elected to take advantage of the benefits of this extended transition
period, which means that when a standard is issued or revised and has different application dates for public or private companies, we,
for so long as we remain an emerging growth company, may adopt the new or revised standard at the time private companies are required
to adopt the new or revised standard.
Following the consummation
of the Business Combination, we expect to remain an emerging growth company until the earlier of: (1) the last day of the fiscal year
(a) following the fifth anniversary of the Closing of the Waldencast initial public offering, (b) in which we have total annual revenue
of at least $1,070.0 million, or (c) in which we are deemed to be a large accelerated filter, which means the market value of our common
equity that is held by non-affiliates is equal to or exceeds $700.0 million as of the end of the prior fiscal year’s second fiscal
quarter and our net sales for the year exceed $100.0 million; and (2) the date on which we have issued more than $1,000.0 million in non-convertible
debt securities during the preceding, rolling three year period.
Recent Accounting Pronouncements
See Note 2 to our audited
consolidated financial statements and our interim unaudited condensed consolidated financial statements included elsewhere in this filing
and Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022, and filed with the SEC on July 7, 2022 for more
information regarding recent accounting pronouncements.
Quantitative and Qualitative
Disclosures About Market Risk
We are exposed to market
risks in the ordinary course of our business, which primarily relate to fluctuations in interest rates, foreign exchange and inflation.
Interest Rates
We have interest rate risk
with respect to our indebtedness. As of June 30, 2022, we had an aggregate face value of $128.8 million of outstanding indebtedness all
of which has variable interest rates. A one percent increase or decrease in the annual interest rate on our variable rate borrowings of
$128.8 million would increase or decrease our annual cash interest expense by approximately $1.3 million.
Foreign Exchange Fluctuations
We transact business in multiple
currencies worldwide, of which the most significant currency for the years ended December 31, 2021, 2020 and 2019, and for the six months
ended June 30, 2022 and 2021 was the U.S. dollar. Our international revenue, as well as costs and expenses dominated in foreign currencies,
expose us to the risk of fluctuations in foreign currency exchange rates against the U.S. dollar. As of June 30, 2022, the effect of a
hypothetical 10% change in foreign currency exchange rates would not be material to our financial condition or results of operations.
To date, we have not entered into any hedging arrangements with respect to foreign currency risk. As our international operations grow,
we will continue to reassess our approach to manage our risk relating to fluctuations in currency rates.
Inflation
We do not believe that inflation
has had a material effect on our business, financial condition, or results of operations; however, we continue to monitor the effects
of the global macroeconomic environment, including increasing inflationary pressures. Generally, we have been able to introduce new products
at higher prices, increase prices on select products and implement other operating efficiencies to sufficiently offset cost increases.
Milk’s
Management’s Discussion and Analysis of
Financial Condition and Results of Operations
The following discussion
and analysis of the financial condition and results of operations of Milk should be read together with Milk’s audited financial
statements as of December 31, 2021 and 2020 and for the years ended December 31, 2021, December 31, 2020, and December 31, 2019 along
with our unaudited financial statements as of and for the six months ended June 30, 2022 and 2021, in each case, together with related
notes thereto, included elsewhere in this filing and in Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022,
and filed with the SEC on July 7, 2022. The following discussion contains forward-looking statements that reflect future plans, estimates,
beliefs and expected performance. The forward-looking statements are dependent upon events, risks and uncertainties that may be outside
of our control. Our actual results may differ significantly from those projected in the forward-looking statements. Factors that might
cause future results to differ materially from those projected in the forward-looking statements include, but are not limited to, those
discussed in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” included
elsewhere in this filing and in Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022, and filed with the
SEC on July 7, 2022. Certain amounts may not foot due to rounding. Unless the context otherwise requires, all references in this section
to “Milk”, “we”, “us”, and “our” refer to Milk Makeup LLC and its subsidiaries prior to
the consummation of the Business Combination, which will be the business of Milk Makeup LLC, a wholly owned subsidiary of Waldencast LP,
and its subsidiaries following the consummation of the Business Combination.
Overview
We are a clean prestige makeup
brand that develops and sells cruelty-free, paraben-free, and 100% vegan cosmetics, skincare and other beauty products. We generate revenue
from the sale of cosmetics to retailers, including Sephora in North America, Europe, the Middle East and Australia and Cult Beauty and
Selfridges in the UK, as well as direct to consumer sales via the Milk website.
Business Combination
On November 15, 2021,
Waldencast entered into the Obagi Merger Agreement with Obagi and the Milk Equity Purchase Agreement with Milk in transactions contemplated
by such agreements, pursuant to which each of Obagi and Milk will become a subsidiary of the combined company. On July 27, 2022, the Company
completed the transaction with Waldencast and Obagi.
COVID-19 Pandemic
In the six months ended June
30, 2022, the business is recovering from the COVID-19 pandemic. Store traffic rebounded in the six months ended June 30, 2022 as COVID-19
restrictions were lifted. However, the business was impacted in the six months ended June 30, 2021 and years ended December 31, 2021 and
2020 by the COVID-19 pandemic primarily through a decline in retail traffic. Domestic Sephora stores had the largest reduction in sales
due to Sephora closing its U.S. stores during the rapid spread of the virus beginning in March 2020. As a result, Sephora did not replenish
stores in April 2020 and maintained many closures through July 2020. The reduction of sales in Sephora’s U.S. stores continued after
reopening mainly due to COVID-19 protocols, which contributed to reduced traffic in stores and demand for several product lines.
Key Components of Results
of Operations
Net Sales
We derive product revenue
from the sale of cosmetics to retailers, including off-price retailers, and DTC sales via our website. Product revenue is recognized point
in time upon transfer of control, including passage of title to the customer and transfer of risk of loss related to the products.
Sales directly to Sephora
accounted for approximately 74% and 63% of gross revenue and sales indirectly to Sephora through major retailer distribution arrangements
(i.e., Kohl’s and Sephora inside JCPenney) accounted for approximately 17% and 16% of gross revenue during the six months ended
June 30, 2022 and 2021, respectively. Sales directly to Sephora accounted for approximately 69%, 56% and 78% of gross revenue and sales
indirectly to Sephora through major retailer distribution arrangements accounted for approximately 14%, 7% and 10% of gross revenue during
the year ended December 31, 2021, 2020, and 2019, respectively. We deduct customer credits for damages and returns, promotional discounts,
and expected credits to be issued in the future from gross revenue. Expected credits for damages and returns are based on prior history,
known events, and projections on sales in the current period.
Cost of Goods Sold (exclusive of depreciation
and amortization)
Cost of goods sold includes
the aggregate costs to manufacture our products, including the amounts invoiced by our third-party contract manufacturers for finished
goods, as well as costs related to inbound freight to our distribution center and overhead. Costs of goods sold also includes the effect
of changes in the balance of reserves for excess and obsolete inventory.
Selling, general, and administrative expenses
Selling, general, and administrative
expenses consist primarily of employee-related costs, including salaries, bonuses, fringe benefits, travel and entertainment expenses,
and other related costs associated with administrative services such as legal, accounting, creative, regulatory, and transaction costs
related to the anticipated Business Combination. Selling, general, and administrative expenses also include outbound shipping and handling
costs, product processing costs, facility costs, marketing and digital expenses, costs related to merchandising, and research and development.
Depreciation and amortization
Depreciation and amortization
primarily consist of depreciation of retailer makeup gondolas. During fiscal year 2019, a determination was made to replace a majority
of gondolas at U.S. Sephora stores, and as a result, depreciation was accelerated to reflect the new useful life of the gondolas. Upon
being replaced in 2020, these gondolas were fully depreciated and written off.
Interest expense, net
Interest expense primarily
consists of (i) the $1.3 million discount on the beneficial conversion feature of a secured convertible promissory note to Milk Series
C Preferred Units in 2019; (ii) interest on advances from our line of credit and (iii) interest charged by Sephora on advances related
to the 2020 birthday gift program, offset by interest income from interest on notes issued to employees. For more information, see Note
6 within our annual financial statements contained elsewhere in this prospectus.
Other expense, net
Other expense includes foreign
currency transaction gains/losses, state income taxes, and other miscellaneous expenses.
Results of Operations
The following tables set forth
our results of operations for the periods presented within our financial statements included elsewhere in this prospectus. The period-to-period
comparison of financial results is not necessarily indicative of future results.
Comparison of the six months
ended June 30, 2022 and 2021
The following table summarizes
our statements of operations data:
| | |
Six months ended
June 30, | | |
Change | |
| (In thousands) | |
2022 | | |
2021 | | |
$ | | |
% | |
| Net sales | |
$ | 38,548 | | |
$ | 26,580 | | |
$ | 11,968 | | |
| 45 | % |
| Cost of goods sold (exclusive of depreciation and amortization) | |
| 13,365 | | |
| 11,297 | | |
| 2,068 | | |
| 18 | % |
| Selling, general, and administrative expenses | |
| 18,815 | | |
| 12,415 | | |
| 6,400 | | |
| 52 | % |
| Depreciation and amortization | |
| 1,169 | | |
| 937 | | |
| 232 | | |
| 25 | % |
| Operating income | |
| 5,199 | | |
| 1,931 | | |
| 3,268 | | |
| 169 | % |
| Interest expense, net | |
| 21 | | |
| (25 | ) | |
| 46 | | |
| 184 | % |
| Other expense, net | |
| 217 | | |
| (64 | ) | |
| 281 | | |
| 439 | % |
| Income before provision for income taxes | |
| 4,961 | | |
| 2,020 | | |
| 2,941 | | |
| 146 | % |
| Income tax provision | |
| - | | |
| - | | |
| - | | |
| 0 | % |
| Net income | |
$ | 4,961 | | |
$ | 2,020 | | |
$ | 2,941 | | |
| 146 | % |
| Comprehensive income | |
$ | 4,961 | | |
$ | 2,020 | | |
$ | 2,941 | | |
| 146 | % |
Net sales
Net sales increased $12.0
million, or 45%, to $38.5 million in the six months ended June 30, 2022, from $26.6 million in the six months ended June 30, 2021. The
increase was driven by volume of approximately $11.5 million and favorable average price impact of $1.7 million, partially offset by an
increase in returns and damages of approximately $1.3 million attributed to expanded distribution from new retailers, such as Kohl’s.
Cost of goods sold (exclusive of depreciation
and amortization)
Cost of goods sold increased
$2.1 million, or 18%, to $13.4 million in the six months ended June 30, 2022, from $11.3 million in the six months ended June 30, 2021.
The increase was primarily driven by volume impact of $3.7 million, however, partially offset of by lower average cost impact of $1.6
million.
Selling, general and administrative expense
SG&A expenses increased
$6.4 million, or 52%, to $18.8 million in the six months ended June 30, 2022, from $12.4 million in the six months ended June 30, 2021.
The increase was primarily related to increases in marketing, payroll, and facility costs of $4.8 million, which were driven by the overall
increase in consumer activities impacted by the loosening of COVID-19 pandemic restrictions during the six months ended June 30, 2022.
The increase was further driven by transaction costs of $1.6 million related to the Business Combination during the six months ended June
30, 2022.
Depreciation and amortization
Depreciation and amortization
increased $0. 2 million, or 25%, to $1.2 million in the six months ended June 30, 2022 from $0.9 million in the six months ended June
30, 2021. The increase was concurrent with additions of new gondolas attributed to the opening of new Kohl’s stores during the six
months ended June 30, 2022.
Interest expense, net
Interest expense increased
$46 thousand, or 184%, to $21 thousand in interest expense for the six months ended June 30, 2022 from $25 thousand in interest income
for the six months ended June 30, 2021. The increase was attributed to the increase in fair value of warrant liabilities during the six
months ended June 30, 2022.
Other expense, net
Other expense increased $0.3
million, or 439%, to $0.2 million in the six months ended June 30, 2022 from $0.1 million in other income in the six months ended June
30, 2021. The increase was primarily due to foreign currency transactions.
Comparison of the years
ended December 31, 2021 and 2020
The following table summarizes
our statements of operations data:
| | |
Year ended
December 31, | | |
Change | |
| (In thousands) | |
2021 | | |
2020 | | |
$ | | |
% | |
| Net sales | |
$ | 47,076 | | |
$ | 39,515 | | |
$ | 7,561 | | |
| 19 | % |
| Cost of goods sold (exclusive of depreciation and amortization) | |
| 21,781 | | |
| 23,450 | | |
| (1,669 | ) | |
| (7 | )% |
| Selling, general, and administrative expenses | |
| 30,764 | | |
| 26,559 | | |
| 4,205 | | |
| 16 | % |
| Depreciation and amortization | |
| 1,975 | | |
| 1,746 | | |
| 229 | | |
| 13 | % |
| Operating loss | |
| (7,444 | ) | |
| (12,240 | ) | |
| 4,796 | | |
| (39 | )% |
| Interest expense, net | |
| 18 | | |
| 301 | | |
| (283 | ) | |
| (94 | )% |
| Other expense, net | |
| 385 | | |
| 393 | | |
| (8 | ) | |
| (2 | )% |
| Income before provision for income taxes | |
| (7,847 | ) | |
| (12,934 | ) | |
| 5,087 | | |
| (39 | )% |
| Income tax provision | |
| - | | |
| - | | |
| - | | |
| 0 | % |
| Net loss | |
$ | (7,847 | ) | |
$ | (12,934 | ) | |
$ | 5,087 | | |
| (39 | )% |
| Comprehensive loss | |
$ | (7,847 | ) | |
$ | (12,934 | ) | |
$ | 5,087 | | |
| (39 | )% |
Net sales
Net sales increased $7.6 million,
or 19%, to $47.1 million in the year ended December 31, 2021, from $39.5 million in the year ended December 31, 2020. The increase
was primarily driven by an increase of approximately $13.2 million due to favorable average price, partially offset by a decrease of approximately
$2.2 million due to decrease in volume and an increase in returns and damages of approximately $3.4 million attributed to discontinued
products and the Company’s expected exit from Sephora inside JCPenney during the year ended December 31, 2021.
Cost of goods sold (exclusive of depreciation
and amortization)
Cost of goods sold decreased
$1.7 million, or 7%, to $21.8 million in the year ended December 31, 2021, from $23.5 million in the year ended December
31, 2020. The decrease was primarily driven by a decrease of approximately $1.5 million in inventory write-offs and a decrease of approximately
$0.9 million attributed to decrease in volume, partially offset by an increase of approximately $0.7 million due to an increase in average
cost per unit.
Selling, general and administrative expenses
Selling, general and administrative
expenses increased $4.2 million, or 16%, to $30.8 million in the year ended December 31, 2021, from $26.6 million in the year ended December
31, 2020. The increase in SG&A was primarily related to increases in marketing and payroll costs for a total of $2.7 million. The
increase was further driven by increases in transaction costs of $1.9 million related to the anticipated Business Combination. The increase
in SG&A was partially offset by decreases in fulfilment and logistics costs of $0.4 million attributed to lower e-commerce sales
resulting from the reopening of physical stores.
Depreciation and amortization
Depreciation and amortization
increased $0.2 million, or 13%, to $1.9 million in the year ended December 31, 2021 from $1.7 million in the year ended
December 31, 2020. The increase was primarily driven by an increase of approximately $0.5 million in depreciation expense attributed to
the additions of new gondolas due to opening of new stores during the year ended December 31, 2021, partially offset by a decrease in
amortization expense of $0.3 million as the intangible assets were fully amortized during the year ended December 31, 2021.
Interest expense, net
Interest expense decreased
$0.28 million, or 94%, to $0.02 million in the year ended December 31, 2021 from $0.3 million in the year ended December 31, 2020. The
decrease was attributed to the customer deposits made by Sephora to the Company in 2020 to fund the production of inventory to be used
in a promotional program, for which the Company incurred interest expense during the year ended December 31, 2020 and not during 2021
for the same period, as they were fully settled as of December 31, 2020. The decrease was further attributed to the interest expense
incurred on the line of credit during the year ended December 31, 2020 and not during 2021 for the same period, as the Company did not
draw on the line of credit during the year ended December 31, 2021.
Other expense, net
Other expense decreased $8
thousand, or 2%, to $385 thousand in the year ended December 31, 2021 from $393 thousand in the year ended December 31, 2020.
The decrease was primarily due to foreign currency transactions.
Comparison of Fiscal years
ended December 31, 2020 and 2019
The following table summarizes
our statements of operations data:
| | |
Year ended December 31, | | |
Change | |
| (In
thousands) | |
2020 | | |
2019 | | |
$ | | |
% | |
| Net sales | |
$ | 39,515 | | |
$ | 50,811 | | |
$ | (11,296 | ) | |
| (22 | )% |
| Cost of goods sold (exclusive of depreciation and amortization) | |
| 23,450 | | |
| 23,379 | | |
| 71 | | |
| 0 | % |
| Selling, general, and administrative expenses | |
| 26,559 | | |
| 33,567 | | |
| (7,008 | ) | |
| (21 | )% |
| Depreciation and amortization | |
| 1,746 | | |
| 2,536 | | |
| (790 | ) | |
| (31 | )% |
| Operating loss | |
| (12,240 | ) | |
| (8,671 | ) | |
| (3,569 | ) | |
| 41 | % |
| Interest expense, net | |
| 301 | | |
| 1,369 | | |
| (1,068 | ) | |
| (78 | )% |
| Other expense, net | |
| 393 | | |
| 918 | | |
| (525 | ) | |
| (57 | )% |
| Income before provision for income taxes | |
| (12,934 | ) | |
| (10,958 | ) | |
| (1,976 | ) | |
| 18 | % |
| Income tax provision | |
| — | | |
| — | | |
| — | | |
| 0 | % |
| Net loss | |
$ | (12,934 | ) | |
$ | (10,958 | ) | |
$ | (1,976 | ) | |
| 18 | % |
| Comprehensive loss | |
$ | (12,934 | ) | |
$ | (10,958 | ) | |
$ | (1,976 | ) | |
| 18 | % |
Net sales
Net sales decreased $11.3 million,
or 22%, to $39.5 million in the year ended December 31, 2020 from $50.8 million in the year ended December 31, 2019.
The decrease in net sales was mainly driven by the COVID-19 pandemic, with domestic Sephora stores experiencing the largest reduction
in sales with respect to our products due to the closure of its United States stores as a result of the rapid spread of COVID-19 in March
2020. As a result, Sephora did not replenish stores in April 2020 and maintained many store closures through July 2020. The reduction
of sales in Sephora’s United States stores continued after reopening mainly due to COVID-19 protocols, which contributed to reduced
traffic in stores and demand for several product lines.
Cost of goods sold (exclusive of depreciation
and amortization)
Costs of goods sold increased
$0.1 million, to $23.5 million in the year ended December 31, 2020 from $23.4 million in the year ended December 31,
2019. The increase was primarily driven by the increase in cost of goods sold attributed to sales to off-price retailers of $7.5 million,
partially offset by the decrease in cost of goods sold attributed to a decline in sales to other retailers of $7.5 million. The increase
was also driven by inventory write-offs of $2.0 million that were incurred during the year ended December 31, 2020, which were
partially offset by inventory reserve provision of $1.9 million for the year ended December 31, 2019.
Selling, general and administrative expenses
SG&A expenses decreased
$7.0 million, or 21%, to $26.6 million in the year ended December 31, 2020 from $33.6 million in the year ended December 31,
2019. The decrease was primarily due to reduction in operating expenses as a result of the pandemic. Marketing expenses decreased by $5.9 million,
principally due to the reduction in the number of marketing events and reduced influencer gifting. In addition, payroll expenses decreased
by $1.2 million, as we laid off 30% of our staff in April 2020 due to the COVID-19 pandemic.
Depreciation and amortization
Depreciation and amortization
decreased $0.8 million, or 31%, to $1.7 million in the year ended December 31, 2020 from $2.5 million in the year
ended December 31, 2019. The decrease was primarily driven by $1.1 million in accelerated depreciation related to retailer makeup
gondolas booked in 2019, partially offset by the depreciation expense attributed to the gondolas that were newly installed in 2020.
Interest expense, net
Interest expense decreased
$1.1 million, or 78%, to $0.3 million in the year ended December 31, 2020 from $1.4 million in the year ended December 31,
2019. The decrease was primarily due to the $1.3 million discount on the beneficial conversion feature of a secured convertible promissory
note to Milk Series C Preferred Units given in 2019, which we did not incur in 2020. The decrease was partially offset by interest expense
of $0.2 million primarily attributed to Milk’s line of credit and customer deposits made by Sephora to Milk in 2020 to fund
the production of inventory to be used in a promotional program.
Other expense, net
Other expense decreased $0.5 million,
or 57%, to $0.4 million in the year ended December 31, 2020, from $0.9 million in the year ended December 31, 2019.
The decrease was primarily due to one-time legal and professional fees incurred in 2019 and foreign currency transactions.
Liquidity and Capital Resources
Our liquidity requirements
arise from our working capital needs, our obligations to make scheduled payments of interest on our indebtedness and our need to fund
capital expenditures to support our current operations, including inventories, marketing, and payroll, and to facilitate growth and expansion.
We have financed our operations and expansion by extending our line of credit through April 2023. As of June 30, 2022, we had an accumulated
deficit of $122.6 million. COVID-19, which was declared a global pandemic, had a direct impact on our operations and financial performance
in 2020 and 2021. Management took immediate action to secure financing and increased our cash position in response to these events.
Our primary sources of liquidity
consist of cash totaling $2.7 million, our line of credit financing arrangement with an aggregate outstanding principal limit of
$15.0 million and an outstanding balance of $1.5 million as of June 30, 2022, and additional equity financing in 2020, which is available
for use for working capital and general business purposes, which we believe will be sufficient to provide working capital, make interest
payments and make capital expenditures to support operations and facilitate growth and expansion for the next twelve months. Our principal
uses of cash for the periods presented within our financial statements included elsewhere in this prospectus have been funding our operations.
The following tables summarize
our cash flows for the six months ended June 30, 2022 and 2021 and for the year ended December 31, 2021, 2020, and 2019:
| | |
Six months ended June 30, | |
| (In thousands) | |
2022 | | |
2021 | | |
$ Change | | |
% Change | |
| Net cash (used in) provided by operating activities | |
$ | (1,603 | ) | |
$ | 1,270 | | |
$ | (2,873 | ) | |
| (226 | )% |
| Net cash used in investing activities | |
| (148 | ) | |
| (38 | ) | |
| (110 | ) | |
| 289 | % |
| Net cash provided by financing activities | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
Year ended December 31, | |
| (In thousands) | |
2021 | | |
2020 | | |
$ Change | | |
% Change | |
| Net cash (used in) provided by operating activities | |
$ | (1,740 | ) | |
$ | (4,694 | ) | |
$ | 2,954 | | |
| (63 | )% |
| Net cash used in investing activities | |
| (1,050 | ) | |
| (6,001 | ) | |
| 4,951 | | |
| (83 | )% |
| Net cash provided (used in) by financing activities | |
| - | | |
| 10,000 | | |
| (10,000 | ) | |
| (100 | )% |
| | |
Year ended December 31, | |
| (In thousands) | |
2020 | | |
2019 | | |
$ Change | | |
% Change | |
| Net cash provided by (used in) operating activities | |
$ | (4,694 | ) | |
$ | (20,124 | ) | |
$ | 15,430 | | |
| (77 | )% |
| Net cash used in investing activities | |
| (6,001 | ) | |
| (1,106 | ) | |
| (4,895 | ) | |
| 443 | % |
| Net cash provided by financing activities | |
| 10,000 | | |
| 25,000 | | |
| (15,000 | ) | |
| (60 | )% |
Milk is party to a Loan and
Security Agreement, dated as of October 10, 2019 (as amended, restated, amended and restated, supplemented or otherwise modified
from time to time, the “Credit Agreement”) with Pacific Western Bank, which provides us with a line of credit maturing in
April 2023. The Milk Equity Purchase Agreement requires that, in connection with the closing of the transactions contemplated thereby,
all amounts outstanding under the Credit Agreement be paid off, the Credit Agreement be terminated and all related liens be released in
connection with the closing of the transactions contemplated by the Milk Equity Purchase Agreement.
Operating activities
Cash provided by operating
activities is primarily driven by earnings from operations and changes in net working capital, defined as current assets minus current
liabilities and other liabilities. Changes in net working capital are largely related to changes in account receivable, inventory and
inventory reserves, prepaid and other current assets, accounts payable, and other assets and liabilities.
The decrease in cash used
in operating activities for the six months ended June 30, 2022 as compared to the cash provided by operating activities in the same period
during the previous year was primarily a result of the decrease in changes in net working capital of $6.3 million, partially offset by
changes in net income of $3.0 million and changes in non-cash items of $0.4 million.
The decrease in cash used
in operating activities for the year ended December 31, 2021 as compared to the year ended December 31, 2020 was primarily a result of
the decrease in net loss of $5.1 million and increase in changes in non-cash items of $0.6 million, partially offset by the
decrease in changes in net working capital of $2.7 million.
The decrease in cash used
in operating activities for the year ended December 31, 2020 as compared to the year ended December 31, 2019 was primarily a result of
the increase in changes of net working capital of $20.0 million, partially offset by the increase in net loss of $2.0 million and decrease
in changes in non-cash items of $2.6 million.
Investing activities
Cash used in investing activities
for all periods presented is primarily related to capital expenditures. Capital expenditures are largely related to purchases of gondolas
attributed to opening of new stores during the six months ended June 30, 2022 and year ended December 31, 2021 and those that were newly
installed during the year ended December 31, 2020.
Financing Activities
No cash was provided by or
used in financing activities during the six months ended June 30, 2022 and year ended December 31, 2021. Cash provided by our financing
activities in 2020 was $10.0 million due to proceeds received from contributions from members for the purchase of 982,318 authorized
Milk Series D Preferred Units during the year ended December 31, 2020.
Off-Balance Sheet Arrangements
We did not have during the
periods presented, and we do not currently have, any off-balance sheet financing arrangements or any relationships with unconsolidated
entities or financial partnerships, including entities sometimes referred to as structured finance or special purpose entities, that were
established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Critical Accounting Policies
and Estimates
Our management’s discussion
and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance
with U.S. GAAP. The preparation of financial statements in accordance with U.S. GAAP requires us to make estimates and assumptions that
affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial
statements, as well as the reported revenues and expenses during the reported periods. We employ judgment in making our estimates, but
they are based on historical experience, currently available information and various other assumptions that we believe to be reasonable
under the circumstances. These estimates form the basis for making judgments about the carrying values of assets and liabilities that
are not readily available from other sources. Actual results may differ from these estimates, and material changes in these estimates
could occur in the future.
We believe that the following
accounting policies are critical and involve a high degree of judgment. See Note 2 to our annual financial statements appearing elsewhere
in this prospectus for a description of our other significant accounting policies.
Revenue Recognition
We adopted the new revenue
accounting standard, ASC 606, Revenue from Contracts with Customers, under the modified retrospective method to all contracts as of January
1, 2019. There was no significant impact on Milk’s results of operations or financial condition upon adoption of the new standard.
We generate revenue from the
sale of cosmetics to retailers, including off-price retailers, and DTC sales via our website. Our revenue contracts represent a single
performance obligation to sell products to our customers. We recognize revenue at a point in time upon transfer of control, including
passage of title to the customer and transfer of risk of loss related to the products, in an amount that reflects the consideration we
expect to be entitled to.
For sales to retailers, transfer
of control generally passes upon the pickup of goods by the retailer from our distribution center, unless we are responsible for shipping
the goods, in which case transfer of control passes upon delivery to the retailer. For DTC sales, we charge credit cards in advance of
shipment. Transfer of control passes upon delivery to the customer.
For sales to retailers, we
collect cash generally in 15 to 45 days depending on the retailer. We have not to date experienced material issues with collectability.
For DTC sales, we collect cash in advance of shipment. Sales taxes imposed on DTC sales are recorded as a sales tax liability on the balance
sheet and do not impact net sales.
In measuring revenue and determining
the consideration we are entitled to as part of a contract with a customer, we take into account the related elements of variable consideration.
Such elements of variable consideration include product returns and sales incentives, such as volume rebates and discounts, markdowns,
margin adjustments and early-payment discounts. For the sale of goods with a right of return, we only recognize revenue for the consideration
we expect to be entitled to and record a sales return reserve based on prior history, known events, and projections on sales in the current
period. We estimate sales incentives and other variable consideration using the most likely amount method and record a reserve when control
of the related product is transferred to the customer. Under this method, certain forms of variable consideration are based on expected
sell-through results, which requires subjective estimates. These estimates are supported by historical results as well as specific facts
and circumstances related to the current period. A reserve for expected returns or sales incentives reduces accounts receivable on the
balance sheet. The reserve is trued up for actual results on an ongoing basis.
Our contract assets consist
of receivables. Our contractual liabilities consist of cash collections from our customers prior to the delivery of products purchased
for DTC sales. These contractual liabilities have not been material to our financial statements.
Equity Based Compensation
The Milk Appreciation Rights
Plan, the Milk Incentive Rights Plan, and the Milk Enterprise Management Incentive options for our employees and officers provide for
granting of appreciation rights awards, incentive awards, and options at the discretion of the board of directors. Appreciation rights
awards, incentive awards, and options have no voting rights. We measure all awards using fair values as determined by the Black-Scholes
model.
Under the Milk Appreciation
Rights Plan, the total number of units that may be awarded is 2,371,856 common units. Awards granted during 2021 and 2020 totaled 99,000
and 1,385,206 units, respectively. Award terms are 1 years. Awards granted in 2021 vest 25% on vesting commencement date and the remaining
units vest in equal monthly installments over the following thirty-six months, contingent upon continued employment or service. Awards
granted in 2020 vest in four equal 25% installments on the anniversary date of the vesting commencement date, contingent upon continued
employment or service.
Under the Milk Enterprise
Management Incentive, the total number of units that may be awarded is 161,607 common units. Awards granted during 2021 totaled 161,607
units. Award terms are 10 years. Awards granted in 2021 vest 25% on the first anniversary of the vesting commencement date and the remaining
vest in equal monthly installments over the following three years, contingent upon continued employment or service.
The appreciation right and
option awards granted to employees and non-employees were accounted for as equity-classified awards. The awards are issued as equity appreciation
rights or options in accordance with the respective award letter and may be settled in cash or units at the sole option of the Company.
Our use of the Black-Scholes
option-pricing model requires the input of highly subjective assumptions. If factors change and different assumptions are used, our stock-based
compensation expense could be materially different in the future. Key assumptions and estimates associated with Black-Scholes inputs are
as follows:
| |
● |
Fair Value of Common Stock: As our common units are not publicly traded, the fair value was determined by our management, with input from valuation reports prepared by third-party valuation specialists. Stock-based compensation for financial reporting purposes is measured based on updated estimates of fair value when appropriate, such as when additional relevant information related to the estimate becomes available in a valuation report issued as of a subsequent date. |
| |
● |
Expected Dividend Yield: We have not historically declared or paid dividends and do not anticipate that distributions will be made in the near future. As a result, an expected dividend yield of zero percent was used. |
| |
● |
Expected Volatility: The volatility factor for our unit-based options was estimated using publicly available trading data, which was used to estimate our volatility, had we been public. |
| |
● |
Expected Term: Our expected term represents the period that the awards are expected to be outstanding and was determined as a function of contractual terms of the unit-based awards and vesting schedules. We use the simplified method of calculation for estimating expected term. |
| |
● |
Risk-Free Interest Rate: We base the risk-free interest rate used in the Black-Scholes model on implied yield currently available on U.S. Treasury zero-coupon issues with an equivalent remaining term. |
Common Unit Valuations
Given the absence of a public
trading market for our common units, and in accordance with the AICPA Accounting and Valuation Guide, Valuation of Privately-Held Company
Equity Securities Issued as Compensation, our board of directors exercised reasonable judgment and considered numerous and subjective
factors to determine the best estimate of fair value of our common units, including:
| |
● |
Independent third-party valuations of our common units; |
| |
|
|
| |
● |
The prices at which we sold our common units to outside investors in arms-length transactions; |
| |
|
|
| |
● |
Our results of operations, financial position, and capital resources; |
| |
● |
Industry outlook; |
| |
|
|
| |
● |
The lack of marketability of our common units; |
| |
|
|
| |
● |
The fact that the option grants involve illiquid securities in a private company; |
| |
|
|
| |
● |
The likelihood of achieving a liquidity event, such as an initial public offering or a sale of our company, given prevailing market conditions; |
| |
|
|
| |
● |
The history and nature of our business, industry trends, and competitive environment; and |
| |
|
|
| |
● |
General economic outlook including economic growth, inflation and unemployment, interest rate environment, and global economic trends. |
In valuing our common units,
the fair value of our business, or enterprise value, was determined using the market approach. The market approach estimates value based
on a comparison of the subject company to comparable public companies in a similar line of business, the subject company transaction method,
the backsolve method, and secondary transactions of our equity interests. From the comparable companies method, a representative market
value multiple is determined and then applied to the subject company’s financial results to estimate the value of the subject company.
From the subject company transaction method, in this particular instance, an estimate of our market value was determined based on an offer
to purchase the company and the likelihood of said transaction being consummated. The Backsolve Method “backsolves” to a value
of a company based on its shares sold in a recent, arm’s length priced equity round of financing. The market approach also includes
consideration of the transaction price of secondary sales of our equity interests by investors.
Application of these approaches
involves the use of estimates, judgment, and assumptions that may be highly complex and subjective, such as those regarding our expected
future revenue, expenses, and future cash flows, discount rates, market multiples, the selection of comparable companies and the probability
of possible future events. Changes in any or all of these estimates and assumptions or the relationships between those assumptions impact
our valuations as of each valuation date and may have a material impact on the valuation of our common units.
Recently Adopted Accounting Pronouncements
See Note 2 to our annual financial
statements included elsewhere in this prospectus for more information regarding recently issued accounting pronouncements.
Qualitative and Quantitative Disclosures
about Market Risk
We have in the past and may
in the future be exposed to certain market risks, including interest rate, foreign currency exchange and financial instrument risks, in
the ordinary course of our business. Currently, these risks are not material to our financial condition or results of operations, but
they may be in the future. In particular, upon the consummation of the Business Combination, we expect our exposure to foreign currency
translation and transaction risk to increase. See the section of this filing entitled “Obagi’s Management’s Discussion
and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosures About Market Risk.”
Our
Business
INFORMATION ABOUT WALDENCAST
Unless the context otherwise
requires, all references in this section to “we,” “our,” “us,” the “company,” or “Waldencast”
generally refer to Waldencast plc.
General
Founded by Michel Brousset
and Hind Sebti, our ambition is to build a global best-in-class beauty and wellness operating platform by developing, acquiring, accelerating,
and scaling conscious, high-growth purpose-driven brands. Our vision is fundamentally underpinned by our brand-led business model that
ensures proximity to our customers, business agility and market responsiveness, while maintaining each brand’s distinct DNA. The
first step in realizing our vision was the business combination with Obagi and Milk. As part of the Waldencast platform, our brands will
benefit from the operational scale of a multi-brand platform; the expertise in managing global beauty brands at scale; a balanced portfolio
to mitigate category fluctuations; asset light efficiency; and the market responsiveness and speed of entrepreneurial indie brands.
We were incorporated
on December 8, 2020 as a Cayman Islands exempted company and a blank check company solely for the purpose of effecting the Business Combination,
which was consummated on July 27, 2022. On July 26, 2022, with the approval of our shareholders, and in accordance with the Cayman Act,
the Jersey Companies Law and our Constitutional Document, we effected the Domestication, pursuant to which our jurisdiction of incorporation
was changed from the Cayman Islands to Jersey and our name was changed from Waldencast Acquisition Corp. to Waldencast plc.
Facilities
Our executive offices
are at 10 Bank Street, Suite 560, White Plains, NY 10606. The cost for this space is included in the $0.01 million per month fee that
we pay an affiliate of the Sponsor for office space, administrative and support services. We consider our current office space adequate
for our current operations.
Employees
We currently have two
officers and intend to add full-time employees as necessary. Members of our management team are not obligated to devote any specific
number of hours to our matters but they intend to devote as much of their time as they deem necessary to our affairs. Mr. Brousset will
spend on average at least 90% of his monthly average working time providing services to us and Ms. Sebti will spend on average at least
80% of her monthly average working time providing services to us, with their remaining monthly average working time spent providing services
to Waldencast Ventures.
Competition
There is significant
competition from Obagi’s and Milk’s competitors. We cannot assure you that we have the resources or ability to compete effectively.
Information regarding our competition is set forth in the sections entitled “Information about Obagi— Competition”
and “Information about Milk—Competition.”
Legal Proceedings
On February 17, 2022,
we received a demand on behalf of purported shareholder Pedro Petersen (the “Petersen Demand”). In addition, on March 10,
2022, we received a demand on behalf of purported shareholder Matthew Whitfield (the “Whitfield Demand”). On March 16, 2022,
we received a demand on behalf of purported shareholder Jeffrey D. Justice, II (the “Justice Demand” and, together with the
Petersen Demand and the Whitfield Demand, the “Demands”). The Demands allege the proxy statement/prospectus forming part
of the registration statement on Form F-4 that we filed with the SEC on February 14, 2022 omits material information or contains disclosure
deficiencies that prejudice our shareholders’ ability to make a fully informed decision with respect to the Business Combination.
The Demands request that Waldencast disseminate additional disclosures promptly or prior to the shareholder vote with respect to the
proposed Business Combination. We believe these claims lack merit. Additional information regarding our legal proceedings is set forth
in the sections entitled “Information about Obagi—Legal Proceedings” and “Information about Milk—Legal
Proceedings.”
INFORMATION ABOUT OBAGI
Unless the context
otherwise requires, all references in this section to “Obagi,” “we,” “us,” “our” and
the “company” refer to the business of the Company’s professional skincare segment: Obagi.
Obagi Purpose & Ambition
Obagi exists to create the
future of skincare so every face is cared for, everywhere. We offer transformative solutions at every stage of the skincare journey to
help you greet the future with confidence.
Our ambition is to be the
top dermo credentialed brand in the world, driving strong growth through channel and geographic diversification, and to continue to progress
the industry that we pioneered with devices, new indications, and new product categories.
Corporate Information
Obagi launched in 1988 under
the name Worldwide Product Distribution, Inc. and subsequently changed its name to Obagi Medical Products, Inc. in December 1997. In October
2007, the company completed an initial public offering of its common stock and was traded on Nasdaq under the trading symbol “OMPI.”
In April 2013, the company merged with and into Odysseus Acquisition Corp., with the company as the surviving corporation in the merger,
becoming a wholly owned subsidiary of Bausch Health. In November 2017, Bausch Health sold substantially all of the assets of Obagi
Medical Products, Inc. and its subsidiaries to Obagi Holdco, a wholly owned subsidiary of Obagi, and Obagi Cosmeceuticals, a wholly owned
subsidiary of Obagi Holdco.
Overview
Obagi is a global skincare
products company that is rooted in research and skin biology. We develop, market and sell innovative skin health products in more than
60 countries around the world. Every product we develop stems from a deep understanding of the skin and how healthy skin functions. We
believe the result is an unmatched product portfolio designed to prevent or improve the most common, visible skin concerns such as fine
lines and wrinkles, elasticity, photodamage, hyperpigmentation (spots or patches of skin that are darker than surrounding areas of skin),
acne, oxidative stress, environmental damage and hydration.
Centered on our rich history
in selling products to medical professionals who then dispense the products in-office directly to their patients, a distribution model
referred to as the physician-dispensed channel, our portfolio today includes the following three distinct brands, with more than 200
cosmetic, OTC and prescription products and a device sold throughout the medical, spa and retail channels:
| ● | Obagi
Medical®—Products within our flagship brand are designed for professional
recommendation in a clinical setting. This line of professional systems and products is targeted
to consumers looking for the most advanced product formulations in a customized skincare
regimen. None of our products, including our prescription products, have been approved by
the FDA or other similar regulatory authorities. |
| ● | Obagi
Clinical®—Developed to prevent and reduce the early signs of aging.
We built this line for the ‘skin-tellectual’ consumer who knows high-quality
ingredients and is increasingly focused on product efficacy and quality. |
| ● | Obagi
Professional™—A new, curated portfolio of products for use in spas, we designed
this line for the aesthetician and the consumer who understands the connection between wellness
and skin health. |
| ● | Skintrinsiq™
device—Used in facial treatments offered by physicians’ offices, spas and
aestheticians. |
In
the U.S., we sell our Obagi Medical systems and products to dermatologists, plastic surgeons and other physicians who are focused on
aesthetic and therapeutic skincare, including physicians on site at medical spas, through our direct sales force. The medical
professionals we sell to then dispense our products in-office directly to their patients. As of December 2021, we had over 6,000 active
medical provider accounts, which we define as an account that has purchased our products in the last 12 months, and estimate that there
are approximately 5,100 licensed physicians in total practicing under these active accounts. In addition to providing our physician practices
and medical spas with products, we also offer turn-key practice building programs and patient events to help these physicians grow their
practices, as well as in-office materials on our products for their patients. We sell our Obagi Clinical products through retail channels,
the Obagi Professional line to aestheticians and spas, and the Skintrinsiq device through the physician-dispensed channel as well as
to aestheticians and spas.
Internationally we sell our
products through distribution partners across Latin America, Europe, the Middle East and Asia. We also seek to advance our development
objectives through product and license agreements with third parties. These agreements may include patent and technology licenses, product
licenses and new product collaboration agreements. We compete in the Japanese retail skin care markets through a strategic licensing agreement
with a Japanese pharmaceutical manufacturer and distributor that sells a series of OTC and cosmetic products under the Obagi® brand
name in the Japanese retail channels.
The Skincare Market
According to the American
Society of Plastic Surgeons, there were almost 15,600,000 cosmetic procedures performed in the U.S. in 2020, despite the fact many medical
facilities and clinics were shut down or banned elective procedures for lengthy periods due to the COVID-19 pandemic. We believe this
reflects a growing desire and acceptance among the population to seek assistance from physicians to improve their appearance, including
the appearance of their skin. One key driver of this trend is the aging of the “baby boomer” segment of the U.S. population,
as well as the “millennial” segment, whose oldest members are now nearing 40. As a result, the average age of the country’s
population has increased over the last decade. With the older population’s strong desire to reduce the signs of premature aging,
we expect the market opportunity for skincare products to continue to grow. In particular, women tend to demonstrate a higher motivation
than men to improve their personal appearances. The U.S. Census Bureau estimated that the number of women between the ages of 35 and 69,
the primary users of our products, grew 21.7% between 2000 and 2018. In addition, during the recent COVID-19 pandemic, as in person meetings
shifted to video calls and virtual meetings, we believe adult consumers of all ages were progressively seeing themselves more on screen
and began focusing more on their appearances. We believe that these trends will continue to drive an increased market demand for skincare
products.
According to industry sources,
in 2019, there were approximately 11,080 physicians practicing dermatology and 6,900 practicing plastic surgeons in the U.S. Based on
physicians who have opened accounts with us, we believe multi-specialty physicians are also dedicating resources in their practices to
skin care in response to the rapid increase in consumer demand for non-invasive skincare treatments. The physician-dispensed market experienced
a compound annual growth rate of 8.7% over the five-year period from 2014-2019, according to industry sources.
Over the last ten years,
consumers have become increasingly knowledgeable about skincare science, ingredients and formulations. According to industry sources,
consumers are shifting desire for quality over price and looking for higher performance products. Retailers like Sephora, Ulta, and Target
have been expanding brand assortments to capitalize on the fast-growing premium beauty skincare category. Specifically, they are trying
to attract and maintain customers seeking more advanced products than offered by mass brands, but also looking for the purchasing convenience
and accessibility of retail outlets.
In addition, sales of professional
skincare products through spas have increased, generating $492.1 million of sales in 2019, up 4.7% from $470.0 million in 2018, according
to industry sources.
Outside the U.S., the physician-dispensed
skincare market varies by country due to cultural differences and regulatory requirements. Cultural desires for skin with lighter and
more even pigmentation have created large and growing aesthetic skincare demands throughout Asia, particularly Japan, China, Korea and
India. European and certain South American countries such as Brazil also present large skincare markets due to the complementary growth
in cosmetic procedures and willingness on the part of their consumers to spend discretionary income on aesthetic enhancements. Industry
sources estimate that in 2021 the global market for beauty and personal care was approximately $530 billion, with skincare representing
approximately $155 billion. The physician-dispensed skincare market percentage growth was approximately 2.5 times the percentage growth
of the premium skincare market from 2018-2020. The premium skincare market, defined as buyers who prefer luxury brands, accounted for
$63 billion of the total global skincare market, with approximately 47% of premium skincare buyers, versus approximately 20% of non-premium
buyers, using 8-14 skincare products weekly. The global market for skincare is estimated to reach approximately $204 billion by 2025,
with premium skincare expected to grow at a compound annual growth rate of approximately 11% from 2021 through 2025.
Our comprehensive portfolio
of products is designed to meet the needs of users no matter what their skin tone or where they are in their skincare journey.
Obagi Medical
Our flagship brand, launched
in 1988, is among the most respected and most recognized brands in the professional skincare category. Obagi achieved the highest overall
scores from U.S. providers in the 2020 Kline Perception and Satisfaction Survey, receiving the highest performance ratings on most attributes
among large brands including marketing, product portfolio and innovation, and value added services.1 We believe the strength
of Obagi Medical’s reputation lies in the exacting standards and formulas we have developed to support our products including more
than 30 studies across thousands of subjects and all six skin types on the Fitzpatrick scale, which classifies skin type according to
the amount of pigment a person’s skin has and the skin’s reaction to sun exposure. None of these studies have been used to
support an application for marketing approval with the FDA or other similar regulatory authority. Consequently, they were not designed
to fulfil the specific requirements of such regulatory application process and should not be viewed as a substitute for clinical trials
that would be conducted in connection with the application to the FDA or similar regulatory authority. Products within the Obagi Medical
portfolio are developed using formulations developed specifically for the physician-dispensed market and stringent ingredient standards.
Furthermore, Obagi conducts extensive testing to evaluate the performance of all products in the Obagi Medical portfolio, including the
portfolio’s cosmetic, OTC drug and prescription-strength drug product.
Obagi Medical is anchored
by the Obagi Nu-Derm® System, which we believe is the leading prescription-based topical skin health system on the market. The Obagi
Nu-Derm System and related products accounted for 24.5% and 32.6% of our net revenue in the years ended December 31, 2021 and 2020,
respectively, and 31.9% and 27.1% of our net revenue for the six months ended June 30, 2022 and 2021, respectively. While we have earned
a strong reputation for offering premier hyperpigmentation solutions, our portfolio has expanded over time to include a line of Vitamin
C powered antioxidant products, our Professional-C® line, which accounted for 18.0% and 15.7% of our net revenue for the years ended
December 31, 2021 and 2020, respectively, and 18.1% and 23.7% of our net revenue for the six months ended June 30, 2022 and 2021, respectively,
as well as the ELASTIderm® line, which leverages a patented Bi-Mineral Contour Complex™ technology to address elasticity and
firmness in the skin and accounted for 5.0% and 8.0% of our net revenue for the years ended December 31, 2021 and 2020, respectively,
and 6.7% and 5.7% of our net revenue for the six months ended June 30, 2022 and 2021, respectively. Other products within the comprehensive
Obagi Medical franchise include products to address hydration, sun protection and acne. These solutions incorporate a range of individual
prescription and non-prescription therapeutic agents, as well as cosmetic ingredients to address the needs of consumers who seek advanced
skincare in a customized skincare regimen designed by a professional.
| (1) | 2020 Physician Dispensed Skincare US Perception & Satisfaction
Survey, Kline & Company. |

Certain of our products listed
above, including Nu-Derm Clear, Blender® and Sunfader, as well as Obagi-C Rx C-Clarifying Serum and Obagi-C Rx C-Night Therapy Cream,
which are part of the Obagi-C Rx Systems, contain 4% HQ. These products are marketed as prescription-only drugs, however, we have not
sought nor obtained the required premarket approval from the FDA to market these products in the U.S. The FDA has historically utilized
a risk-based enforcement approach with respect to drugs marketed without approval in accordance with its active CPG, issued in 2006 and
subsequently amended in 2011, in which the FDA announced a drug safety initiative to remove unapproved drugs from the market and established
enforcement priorities and a policy of enforcement discretion with respect to marketed unapproved products. While the FDA has expressed
its view that all prescription HQ products should be reviewed and approved by the FDA, we believe our prescription-only HQ products do
not fall within the previously established categories of unapproved drugs for which the FDA has indicated it prioritizes enforcement.
We have not received any communications from the FDA or any similar regulatory authorities regarding our Nu-Derm HQ or any other products.
However, whether due to safety concerns or otherwise, in the future the FDA may choose to pursue an enforcement action against us and
determine that our HQ products should be removed from the market until we obtain approval of an NDA. For example, although our prescription-only
HQ products are made with 4% HQ, the FDA has historically expressed concerns regarding the safety of 2% HQ products, sold on an OTC basis.
In particular, in August 2006, the FDA issued a proposed rule that cited certain preclinical evidence suggesting that HQ may be carcinogenic,
if orally administered, and may be related to a skin condition called ochronosis, which results in the darkening and thickening of the
skin, and the appearance of small bumps and grayish-brown spots, after use of concentrations as low as 1 to 2 percent. The FDA also concluded
that it could not rule out the potential carcinogenic risk from topically applied HQ, and classified OTC skin-bleaching drug products,
including HQ, as not GRASE, as misbranded, and as new drugs within the meaning of the FDCA, meaning that such products would need to be
approved through the NDA process in order to be legally marketed in the U.S. Although this proposed rule was never finalized, in March
2020 the CARES Act was enacted, which among other things deemed any OTC drugs that were identified as not GRASE in the FDA’s most
recent proposed rulemaking for such OTC drugs to be “new drugs” and misbranded within the meaning of the FDCA, meaning that
such drugs could not be marketed without an approved drug application as of September 23, 2020. As a result, products containing HQ are
prohibited from being marketed in the U.S. as OTC drug products without an approved NDA. Subsequently, on April 19, 2022, the FDA announced
that it had issued warning letters to 12 companies for continuing to sell 2% HQ products on an OTC basis, citing violations of the applicable
CARES Act provisions.
The FDA’s announcement
also cited reports describing serious side effects associated with the use of skin lightening products containing HQ, including reports
of skin rashes, facial swelling, and skin discoloration. The FDA’s safety concerns regarding these lower-concentration OTC HQ products
could prompt the FDA to assert that our higher-concentration, prescription-only HQ products represent a higher priority for enforcement
pursuant to the active CPG. In many countries, including the E.U, Canada, Australia and Japan, HQ is regulated as a drug and requires
a prescription. We have not sought nor obtained regulatory approval to distribute our HQ products in these countries, and instead offer
our Nu-Derm Fx and Obagi-C Fx solutions, which contain the skin brightening agent arbutin, for these markets. In the E.U., the European
Commission has expressed concerns on the potential use of (alpha and beta) arbutin in cosmetic products this has led to additional consultation
with the SCCS (Scientific Committee on Consumer Safety), further to which call for data was launched which ended in April 2021. In the
preliminary opinion of the SCCS on the safety of alpha-arbutin and beta-arbutin in cosmetic products dated March 15-16, 2022, which is
open for comments until May 27, 2022, the SCCS considered that it cannot conclude on the safety of alpha-arbutin (when used in face creams
up to a maximum concentration of 2% and in body lotions up to a maximum concentration of 0.5%) or beta-arbutin (when used in face cream
up to a maximum concentration of 7%) because not all relevant scientific data are available. Depending on the outcome of the SCCS’s
safety assessment, it cannot be excluded that the use of alpha and/or beta-arbutin in certain cosmetic products will be banned or restricted
in the E.U. per future E.U. legislation. Usually in cases were cosmetics products that are already on the market, suddenly become non-compliant
due to a legislative modification, a transition regime will be imposed, allowing manufacturers a certain amount of time to comply with
the rules. See the sections “Government Regulation—U.S. Regulation of Drug Products,” “Risk
Factors—Risks Related to Our Professional Skincare Segment: Obagi——Laws, regulations, enforcement trends
or changes in existing regulations governing the introduction, marketing and sale of our OTC drug, device and cosmetic products to consumers
could harm our business,” “Risk Factors—Risks Related to Our Professional Skincare Segment: Obagi—Our
products containing the active ingredient, hydroquinone, are marketed as prescription-use only drugs but have not received required pre-market
authorization from the FDA or other regulatory authorities, and the FDA could require us to remove these products from the market until
we obtain approval of the required NDA, which would require a significant investment of time and money, and we could be found to be marketing
and selling these products in violation of the law” and “Risk Factors—Risks Related to Our Professional
Skincare Segment: Obagi—Failure to obtain regulatory approvals or to comply with regulations in foreign jurisdictions would
prevent us from marketing our Obagi products internationally.” In addition, Obagi’s arbutin products are permitted to
be sold in the Asia-Pacific region countries in which Obagi distributes such products.
Obagi Clinical
Our Obagi Clinical line,
launched in December 2018, is designed to meet the needs of “skin-tellectual” consumers who may not yet regularly visit a
dermatologist or skincare professional. This retail skincare brand leverages the 33-year legacy of Obagi Medical to incorporate clinically
proven ingredients in cosmetic and OTC solutions that maintain healthy, more youthful-looking skin, mitigate environmental damage and
address the emerging signs of skin aging. This line includes the following:

Obagi Clinical products
accounted for 3.0%, 2.1%, 1.9%, 19.2% and 2.5% of our net revenue for the years ended December 31, 2021, 2020, 2019, and for the
six months ended June 30, 2022 and 2021, respectively.
Skintrinsiq Device
In 2021, facial services
saw the greatest increase in demand among minimally invasive procedures. Capitalizing on this trend, Obagi, in collaboration with Theravent,
Inc., was the first professional skincare company to market a professional-use facial device offering consumers an advanced delivery system
for Obagi’s transformational products.
Leveraging our expansive
knowledge as a leader in the skincare market, we began distributing the Skintrinsiq device powered by Obagi InfuseIQ™ technology
to physician’s offices and medical spas in July 2021. The device features a small footprint and easy-to-use interface designed to
minimize training time and maximize time for skin care. Additionally, the Skintrinsiq device offers physicians another opportunity to
consult with consumers about their at-home skincare regimens and creates a mechanism for patient retention. We believe the Skintrinsiq
pricing allows physicians to quickly recoup their initial expense while offering the facial treatments to patients at a reasonable price.
Based on the intended use of the product, we do not believe that the Skintrinsiq device meets the FDA’s definition of a medical
device, and therefore we believe this product is exempt from FDA premarket review requirements. However, the FDA may disagree with our
determination and require us to cease marketing the Skintrinsiq device unless and until we obtain marketing authorization from the FDA.
We are aware that manufacturers of light emitting products that make express acne treatment claims have sought clearance through the 510(k)
pathway and that the FDA has taken action against manufacturers of some light-emitting products used to alter or improve appearance on
the grounds that those products are unapproved medical devices.
The Skintrinsiq device uses
innovative technology to extract debris and impurities from the skin, infuse Obagi skincare products exactly where they are needed most
and then lock them in so they can continue working even after the treatment ends. We have also incorporated an optional simultaneous LED
light to provide red and/or blue light to help meet a wide variety of skin health goals. Customized Skintrinsiq protocols can accelerate
patients’ transformations and take Obagi® skincare results to the next level. By improving results, we believe the Skintrinsiq
device will help attract new consumers to physician practices and medical spas.
Obagi Professional
Launching in early 2022,
the Obagi Professional line was built for the wellness-conscious consumer who understands the overlap between overall health and skin
health. Designed with insights from partnering with more than 1,300 spas across the U.S., this brand franchise contains a curated line
of technologically advanced products suited for use by professional aestheticians within the spa market. The 16 products offered under
the Obagi Professional line can be used in facial treatments and purchased for take home use.
Obagi Science
Innovative Research
& Development
Over the course of our 33-year
legacy, Obagi has amassed more than 80 global patents on product and technology innovations, which we believe sets us apart from our competitors.
As of June 30, 2022, half of Obagi’s products by net revenue are protected by our patents. The table below sets forth a description
of the material patents owned by Obagi Cosmeceuticals and/or Obagi Holdings Company Limited and the jurisdictions in which they are valid:
|
Name of Patent |
|
Jurisdictions |
|
Expiration Date |
| Anti-Aging Treatment Using Copper and Zinc Compositions |
|
Australia, Canada, Czech Republic, France, Germany, Great Britain Hungary, Italy, Japan, Mexico, Poland, South Korea, Spain, Turkey, U,S. |
|
June 2026 |
| Chemical Compositions and Methods of Making Them |
|
France, Germany, Great Britain, Italy, Japan, Mexico, Poland, South Korea, Spain, U.S. |
|
Jan.2026-Feb. 2027 |
| Methods for Lightening Skin Using Arbutin Compositions |
|
U.S. |
|
Nov. 2028 |
| Skin Lightening Compositions Comprising Arbutin |
|
Canada |
|
Nov. 2028 |
| Skin Treatment Compositions |
|
France, Germany, Great Britain, Japan, Mexico, Netherlands, Norway, Poland, South Korea, Spain, Sweden |
|
Nov. 2028-Aug. 2030 |
| Stable Organic Peroxide Compositions |
|
Belgium, Canada, Czech Republic, Estonia, France, Germany Great Britain, Hungary, Ireland, Italy, Japan, Liechtenstein, Luxembourg, Mexico, Monaco, Netherlands, Romania, South Korea, Slovak Republic, Slovenia, Switzerland, U.S. |
|
Mar.-June 2026 |
Although a number of these
patents will expire over the next five years, we do not believe the expiration of such patents will have a material effect on our business
or results of operations because the formulations for the products covered by such patents are still treated as trade secrets, which are
known only by a limited number of need-to-know employees, manufacturers of the products who are bound by strict confidentiality provisions,
and regulatory authorities as required. In addition, we are aware that other solubilized versions of benzoyl peroxide are already currently
available on the market using different technologies than ours and, as a result, the expiration of the related patents will likely not
have an impact on the availability of competitor products.
World Class Research
and Development (R&D) Program
Our R&D program aims
to design products and execute studies that demonstrate the high-quality design of our formulas and powerful performance of our products.
We apply a scientific approach to all of our products, from inception to development and testing.
After formulation, all of
our products are tested for integrity, safety and performance. Bausch Health, the manufacturer of our tretinoin products, holds an ANDA
for such products, meaning the FDA has found such products to be bioequivalent to other tretinoin products approved through the NDA process.
To approve an NDA for a product, the FDA generally requires applicants to demonstrate the safety and efficacy of the product through successful
completion of well-controlled clinical trials usually employing several hundred to a few thousand subjects. None of our other products
are distributed under an NDA or ANDA approved by the FDA. However, the FDA may require us to conduct well-controlled clinical trials to
establish the safety and efficacy of our prescription strength HQ products and to obtain an NDA to continue marketing our HQ products.
See “—Government Regulation- U.S. Regulation of Drug Products” below. Nonetheless, we do conduct thorough testing
of all of products, whether they are cosmetics, OTC drugs or prescription-strength products, to evaluate their safety and performance.
Prior to launch, our products undergo several safety tests, including, but not limited to, human repeat insult patch tests, used to help
predict the likelihood for induced allergic contact dermatitis, comedogenicity tests, to ensure the product does not clog pores, and cumulative
irritation tests. In addition to these safety and tolerability studies, we have conducted more than 30 studies with the leading academic
institutions and key independent experts in dermatology and aesthetic medicine for our products, including, but not limited to, the following:
|
Product |
|
Study |
|
Principal Investigator |
|
Obagi Nu-Derm System
(hydroquinone) |
|
We have conducted 10 studies on our Nu-Derm System that included a total of over 500 patients with Fitzpatrick skin types I-IV to assess (a) how well the system addresses hyperpigmentation and photodamage; (b) how well the system works as compared to other skincare regimens; and (c) how well the system works when used in conjunction with other skincare solutions. |
|
James H. Herndon, Jr., MD
M.L. Sigler, PhD
Marta I. Rendon, MD FAAD
Michael Gold, MD
Suzanne Bruce, MD
David Pariser, MD
Pearl Grimes, MD
Joel Schelssinger, MD
Mitchel P Goldman, MD
Suzanne Bruce, MD |
|
Obagi-C Rx System
(hydroquinone) |
|
We have conducted two studies on the Obagi-C Rx System, including (a) one that involved 30 patients to assess how well the system addresses photodamage and fine lines and wrinkles and (b) an in vitro study that evaluated how well the L-ascorbic acid in the system absorbs into and remains in the skin. |
|
Suzanne Bruce, MD
Paul A. Lehman, MSc |
|
CLENZIderm System
(OTC monograph for topical acne products) |
|
We have conducted 3 studies on our CLENZIderm System that included a total of 90 patients to assess (a) how well the system addresses acne, (b) how well the solubilized BPO in the system enters skin follicles and (c) how well the system works as compared to other acne regimens. |
|
Leonard Swinyer, MD
Suzzanne Bruce, MD
Mary C. Spellman |
None of our HQ studies have
reported serious adverse events (“SAEs”). A number of participants in the Obagi Nu-Derm System studies reported adverse events
(“AEs”), which included dry skin, erythema (superficial reddening of the skin), pruritus (itchy skin) and skin exfoliation.
No participants that received Obagi Nu-Derm discontinued any of the studies due to the occurrence of an AE. Specifically:
| ● | In
the Herdon Study, in which 71 participants of a total of 301 received Obagi Nu-Derm, there
were no severe side effects, SAEs or AEs reported in the Obagi Nu-Derm arm of the
study, however some participants experienced mild erythema, scaling, burning and itching. |
| ● | In
the Rendon study, 21 of the 38 participants that received Obagi Nu-Derm reported AEs that
were related to the trial material, with such AEs including moderate dry skin, redness, itching
and peeling and one reported case of severe dryness and peeling. No SAEs were reported in
this study. |
| ● | In
the Grimes study, 3 of the 20 participants that received Obagi Nu-Derm reported AEs that
were related to the trial material, with such AEs including 2 participants that experienced
mild dry skin, redness, itching and peeling, and 1 participant that experienced moderate
redness. No severe side effects or SAEs were reported in this study. |
| ● | In
the Nu-Derm Botox study and Nu-Derm IPL study, some participants experienced transient dryness,
redness and irritation, but by the end of the studies those levels had returned to baseline.
No participants in these studies reported severe side effects and no SAEs were reported. |
In
the study assessing how the Obagi-C Rx System, our other HQ product, addressed photodamage, of the 30 participants, 16 reported
in the first week AEs that were at least probably related to the trial material, including 5 reported cases of dryness, 2 redness,
2 peeling, 2 milia (tiny white bumps on the skin), 1 rash, 1 pruritus, 1 contact dermatitis and 1 burning session and 5 reported cases
in the second week of dryness and peeling. No SAEs were reported in this study.
In the CLENZIderm studies,
of the 55 participants in these studies, only one participant reported AEs of dryness and peeling. No SAEs were reported in these studies
and no participants that received the CLENZIderm System discontinued any of the studies due to the occurrence of an AE.
We also help to demonstrate
the craftsmanship of our formulas through stability tests, penetration studies, head-to-head comparative studies and consumer perception
studies. We constantly seek new protocols and testing methods that analyze and demonstrate the power of our products. For example, Obagi-commissioned
head-to-head studies have indicated the Obagi Nu-Derm System, Obagi Nu-Cil™ and Hydrate Luxe® products outperform competitors
across certain key attributes and a consumer preference survey commissioned by Obagi in 2019 highlighted consumer preference for Obagi’s
Professional-C Serum 20% over a leading competitor’s product. We continue to expand our collaborations with leading dermatologists
and institutions to coordinate additional studies for new product uses and formulations. In alignment with our value of promoting and
representing diversity and inclusion in skincare, Obagi was the first physician-dispensed skincare brand to design clinical research protocols
to cover all six skin types across the Fitzpatrick scale. Today, we remain focused on that commitment to ensure we deliver on our promise
of providing high-quality products for every stage of the skincare journey, no matter your age or skin tone.
As discussed above, products
that are regulated solely as cosmetic products are not intended to have a pharmacological effect on the body, and our studies are not
intended to suggest or establish such an effect for such products. Further, our studies are substantially smaller in scale (generally
using over 30 but under 200 subjects) and rigor than FDA required studies, including only one phase of testing rather than the three phases
typically required by the FDA. None of these studies have been used to support an application for marketing approval with the FDA or other
similar regulatory authority. Consequently, they were not designed to fulfil the specific requirements of such regulatory application
process and should not be viewed as a substitute for clinical trials that would be conducted in connection with the application to the
FDA or similar regulatory authority.
SKINCLUSION
In May 2019, we launched
our SKINCLUSION® initiative, designed to elevate the global dialogue about diversity and how we can all make conscious choices to
see the beauty in all our differences. Born from the insight that more than 70% of consumers do not see themselves represented in beauty
marketing and advertising, we aimed to change the narrative. Leading with our conviction to provide transformative skincare for every
face, everywhere, we built a communication platform to discuss why representation matters, show how lack of inclusivity stems from unconscious
bias, and inspire other industry leaders to follow suit. Over the last two years, we have continued to progress this initiative by incorporating
more diversity in our model photography, our social media content, and our brand ambassadors. We champion new areas to represent diversity
such as partnering with a woman who became the first model with Down syndrome to represent a skincare brand.
The campaign mirrors Obagi’s
commitment to diversity and inclusion in all aspects of its business – from corporate culture to product development. With ambassadors
ranging from celebrities and renowned dermatologists to a Down Syndrome advocate, role model and model, the global awareness initiative
also includes donations in excess of $0.2 million to support organizations like the International Cultural Diversity Organization and
Project Implicit.
Sales & Marketing
Domestic
In the U.S., we sell our
Obagi Medical systems and related products to physicians, including physicians on site at medical spas, through our direct sales force.
The medical professionals we sell to then dispense our products in-office, directly to their patients, a distribution method commonly
referred to as the “physician-dispensed” channel. We also sell the Skintrinsiq device through the physician-dispensed channel
as well as to licensed aestheticians. We believe that the physician-dispensed distribution model ultimately results in higher patient
satisfaction because it is better suited to the provision of system-based skin care than traditional distribution channels. Our physician
customer base consists primarily of plastic surgeons and dermatologists, but also includes physicians from other practice areas, such
as general practice, family practice, internal medicine, dental and OBGYNs who are adding skin care to their practices.
As of June 30, 2022, we had
a large healthcare professional account base consisting of more than 6,000 active medical provider accounts, including approximately 5,100
licensed physicians, resulting in broad distribution of Obagi products in the majority of dispensing aesthetic clinics. Our sales and
marketing team consisted of 126 sales, marketing and education specialists, including 94 dedicated sales representatives and managers,
as of June 30, 2022. In addition to providing our accounts with products, we also offer turn-key practice building programs and patient
events to help these physicians grow their practices, as well as in-office materials on our products for their patients.
We sell our products to healthcare professionals in the U.S. through
an authorized wholesale distributor, Boxout Health. Under this model, we sell the products to Boxout Health, which then sells the products
through to our physician customers when they order them. As a result, Boxout Health accounted for approximately 34.8% and 54.1% of net
revenue in the years ended December 31, 2021 and 2020, respectively, and for approximately 32.7% and 41.0% of our net revenue for the
six months ended June 30, 2022 and 2021, respectively. Under our agreement with Boxout Health, Boxout Health has been appointed as the
exclusive authorized distributor of our products in the physician-dispensed market in the U.S. Our agreement with Boxout Health does not
contain any minimum purchase requirements for Boxout Health. Accordingly, we do not have any guarantees regarding the quantity of each
of our products that Boxout Health will order each quarter. The agreement has an initial term through October 2023 and will automatically
renew for additional one-year terms unless we and Boxout Health mutually agree in writing not to renew it or unless one party gives the
other party written notice of its intent not to renew the agreement at least 90 days before the end of the then current term. The agreement
may also be terminated if one party materially breaches the agreement and fails to cure such breach within 30 days of receiving written
notice by the non-breaching party or upon liquidation, dissolution or bankruptcy of one of the parties.
We sell our Obagi Clinical
line of products through our online store at www.obagi.com as well as through Target’s online store at www.target.com.
The U.S. market accounted
for approximately 43.7% and 71.9% of our net revenue in the years ended December 31, 2021 and 2020, respectively, and for approximately
43.6% and 48.8% of our net revenue for the six months ended June 30, 2022 and 2021, respectively.
International
International markets accounted
for approximately 56.3% and 35.0% of our net revenue in the years ended December 31, 2021 and 2020, respectively, and for approximately
56.4% and 51.2% of our net revenue for the six months ended June 30, 2022 and 2021, respectively. We address international markets through
37 international distribution partners that have sales and marketing activities in over 60 countries outside of the U.S., and a trademark
and know-how license agreement and a license distribution agreement for the retail drug store channel in Japan. We target distribution
partners who are capable and willing to mirror our sales and distribution model in the U.S. and who have an established business and reputation
with physicians. The products that we sell internationally are generally the same formulations as those sold in the U.S.; however, in
some instances, formulations have been modified to comply with the regulatory requirements of certain countries. These distributors use
a model similar to our business model in the U.S., addressing their territories through direct sales representatives who sell to physicians,
or through alternative distribution channels, depending on regulatory requirements and industry practices. Our distribution agreements
typically grant distributors the right to distribute and sell our products to licensed medical professionals and skincare clinics within
a specified territory, require them to purchase a specified minimum amount of our products each year and have a term of two to five years.
We generally reserve the rights to distribute our products through other channels and e-commerce in such territories. Although we have
a broad base of distributors, our SA Distributor accounted for approximately 28.0% and 8.1% of our net revenue in the years ended December 31,
2021 and 2020, respectively, and for approximately 38.4% and 23.9% of our net revenue for the six months ended June 30, 2022 and 2021,
respectively. Our agreement with the SA Distributor grants the SA Distributor a non-exclusive right to distribute our products in Vietnam
and South Korea, contains minimum purchase requirements and has a term that expires on December 31, 2026. In January 2022, we executed
an amendment with the SA Distributor to expand the countries within Southeast Asia in which it may distribute our products. Accordingly,
our sales to such distributor may comprise an even greater proportion of our net revenue in the future. Net revenue from sales in Europe
and China, each of which are covered by a number of different distributors, accounted for 7.0% and 13.4% of our net revenue, respectively,
in the year ended December 31, 2021. Net revenue from sales in Europe and China accounted for 5.6% and 6.4% of our net revenue, respectively,
for the six months ended June 30, 2022 and approximately 7.5% and 10.5% of our net revenue, respectively, for the six months ended June
30, 2021. Southeast Asia and China are the only geographic regions that we expected to account for more than 10% of our net revenue for
the year ended December 31, 2021. While we will continue to maintain our distributors in Southeast Asia, Obagi Hong Kong will assume responsibility
for all distribution of our products in the China Region following the Closing Date.
We intend to continue expanding
our international presence by entering into strategic relationships in key locations such as Asia, Europe and South America. We believe
that there is potential for significant sales growth of our products in international markets, particularly in Europe and Brazil where
industry sources estimated skincare markets represented approximately $25 billion and $3 billion in 2021, respectively, due to cultural
emphasis on overall skin health and appearance, and the continued development and acceptance of surgical and non-surgical cosmetic procedures
throughout many countries of the world.
Licensing
In Japan, we built an alternative
model to build a presence and brand awareness for our products. For example, in Japan we launched a formal long-term relationship by entering
into a Trademark and Know-How License Agreement with Rohto to market and sell our Obagi-developed products in Japan. Rohto is a Japanese
pharmaceutical manufacturer and distributor. Under our current agreement, Rohto is licensed to manufacture and sell a series of OTC and
cosmetic products developed by it under the Obagi brand name in the Japanese drug store channel, for which it pays us a license fee. In
2008, we expanded that relationship to provide for collaboration on the development of new products and to pursue the higher end department
store channel in Japan. Our strategic partner in Japan has engaged in aggressive direct-to-consumer advertising, which we believe has
raised consumer demand in Japan, creating greater brand awareness in the physician channel to the benefit of our core prescription lines.
Concurrently with the Closing
Date, we entered into the Intellectual Property License Agreement and Supply Agreement with Obagi Hong Kong for the sale of Obagi products
throughout the China Region, including the People’s Republic of China, Hong Kong, Macau and Taiwan. Under these agreements, we will
supply, or cause to be supplied through certain CMOs, Obagi products to Obagi Hong Kong and its Affiliates, and Obagi Hong Kong will purchase
such products, with the exclusive right to distribute and sell such products in the China Region. In return, Obagi Hong Kong will pay
us a royalty of five and a half percent (5.5%) of gross sales of licensed products, subject to certain deductions.
We will continue to look
for credible partners to address new geographies, and to evaluate alternative channel opportunities in Japan and other countries to drive
brand awareness and accelerate overall market penetration.
Strategic Initiatives for
Driving Growth
Our ambition is to be the
top dermo credentialed brand in the world, driving strong growth through channel and geographic diversification. Obagi plans to focus
on three key initiatives to achieve this goal:
| ● | Brand
Expansion—We plan to continue building upon the highly respected reputation of
the Obagi brand by expanding the brand franchises that leverage our medical heritage. In
December 2018 we introduced the Obagi Clinical brand to online consumers and plan
to expand the retail channels where it is available, thereby introducing more consumers to
the Obagi brand. In addition, in July 2021 we launched the Skintrinsiq device and in the
second quarter of 2022 we plan to launch our Obagi Professional brand in an effort to introduce
even more consumers who may not yet be familiar with physician-dispensed products to Obagi.
We have also experienced positive sales increases following the endorsement of our products
by celebrities and popular publications. In particular, we recognize the opportunity to reach
a younger consumer base that is increasingly interested in clinical skincare products. As
these consumers age and begin to seek higher performance skincare to address the progressive
signs of skin aging, they can graduate to the Obagi Medical line. |
| ● | Channel
Expansion—While we continue undertaking significant efforts to further our market
penetration within the physician-dispensed channel, which we estimate is currently at about
40% in the U.S. market, we intend to also focus on continuing to implement our omni-channel
strategy to expand our distribution beyond this market segment. In December 2020, in tandem
with the redesign of our own website, we launched our first e-commerce store to enable us
to sell our Obagi Medical OTC and cosmetic products directly to consumers. Our Obagi Clinical
line allowed us to branch into retail channels for the first time, and we expect our Obagi
Professional line to enable us to start our footprint within the spa and wellness channel.
We have launched the Skintrinsiq device in 70 pilot clinics as of December 2021, with 73%
of our aesthetic practice partners and 80% of initial consumers recommending the device treatments.
We believe the Skintrinsiq device will also allow us to expand offerings within the spa and
wellness channel and to aestheticians who are not affiliated with a physician’s office. |
| ● | International
Growth—Finally, we intend to continue expanding our international presence. As
we continue to enhance our own U.S. online web store, we plan to build and execute a global
e-commerce strategy that will enable us to reach consumers in several international
markets, including some in which we may not have a distribution partner. At the same time,
we will focus on growing our network of third-party distributors in key large international
markets, such as Europe, Brazil, Southeast Asia and the Middle East. We also plan to drive
distribution of our products through strategic relationships in other countries, including
Japan and China. |
Competitive Strengths
We believe we are well-positioned
to achieve our strategic initiatives as a result of the following competitive strengths:
| ● | Market
leading brand—Obagi has been a leader in the physician-dispensed market from inception,
being one of the first companies to offer skincare products in the market over 30 years
ago. Obagi achieved the highest overall scores from U.S. providers in the 2020 Kline Perception
and Satisfaction Survey, receiving the highest performance ratings on most attributes among
large brands including marketing, product portfolio and innovation, and value-added services. |
| ● | Robust
portfolio of high-quality, innovative products—Our product portfolio currently
consists of over 200 products. While some of the competitors in the skincare market also
offer a broad spectrum of products, only one other competitor, ZO Skin Health, offers both
prescription strength and non-prescription products. In addition, many competitors in our
primary market, physician-dispensed, generally have more narrowly focused offerings, addressing
only targeted skincare problems, while our portfolio addresses all of the most common visible
skin disorders. |
| ● | Diversified,
omni-channel market with global geographic coverage—We believe that our diversified,
omni-channel strategy gives us a competitive advantage over many skincare companies that
choose to focus only on the physician-dispensed market. By offering a skincare line developed
specifically for physicians as well as curated separate product lines for the retail, spa
and wellness channels, we believe we are able to expand our brand recognition and meet the
consumer where they are in their skincare journeys, with the eventual goal of graduating
these consumers to the Obagi Medical line. In addition, our global breadth into over 60 countries
positions us well to achieve worldwide brand recognition. Our strategic relationship with
Rohto and agreements with Obagi Hong Kong allow us to penetrate the markets in Japan and
China, which represents a large growing market for skincare, and we intend to continue expanding
our network of distributors and strategic partners. |
| ● | Specialized
and efficient global sales force—Our domestic internal sales force of 95 professional
representatives and education specialists allows us to develop close relationships
with the physicians who dispense our products throughout the U.S. In addition, our global
sales force consists of 27 international distributors who sell our products in over
60 countries throughout the world. We believe the breadth and experience of our internal
and global sales network provide us with a competitive advantage. |
| ● | Demonstrated
financial performance through disciplined execution—While growth initiatives such
as the ones we have undertaken over the last few years require significant investments, we
have been able to achieve our growth through disciplined execution. Our net revenue
has increased from $94.3 million for the year ended December 31, 2018 to net revenue
of over $206.0 million for the year ending December 31, 2021. |
Competition
The market for aesthetic
and therapeutic skin health products is highly competitive and we expect the intensity of competition to increase in the future. We also
expect to encounter increased competition as we enter new markets and/or distribution channels, attempt to penetrate existing markets
with new products and expand into new distribution channels. Our principal competitors are large, well established companies in the fields
of pharmaceuticals, cosmetics, medical devices and health care. Our largest direct competitors include SkinCeuticals, a division of L’Oréal
S.A., SkinMedica, Inc., a division of Allergan, Inc., ZO Skin Health, PCA Skin and EltaMD, each a division of Colgate-Palmolive. Our indirect
competitors for Obagi Medical® products and direct competitors for our Obagi Clinical® products sell skin care products directly
to consumers, and generally consist of large well known cosmetic companies, including but not limited to, La Roche -Posay, Dermalogica,
Murad and dermatologist backed brands, such as Dr. Dennis Gross. In the spa and wellness channel our main competitors will be Dermalogica,
Murad and Eminence. We also face competition from medical device companies offering products to physicians, aestheticians and spa and
wellness centers that are used in facial treatments.
Competitive
factors in our market include:
| ● | product
efficacy, uniqueness, quality, reliability of performance and convenience of use; |
| ● | brand
awareness and recognition; |
| ● | breadth
of product offerings; |
| ● | sales
and marketing capabilities and methods of distribution; |
| ● | resources
devoted to product education and technical support; |
| ● | speed
of introducing new competitive products and existing product upgrades; and |
We face and will continue
to face intense competition. A number of our competitors have greater research and development and marketing capabilities, more diverse
distribution channels and greater financial resources than we do. These competitors may have developed, or could in the future develop,
new technologies that compete with our products or render our products obsolete. We are also likely to encounter increased competition
as we enter new markets and as we attempt to further penetrate existing markets.
Manufacturing
We currently outsource all
our product manufacturing to third-party contract manufacturers. We have two or more qualified manufacturers for some of our key products,
however, certain products, including some of our sun protection products, are currently supplied by a single source. The termination of
our agreement with a single source supplier or any loss or disruption of services under such agreement could be difficult for us to replace
upon the same favorable terms. The transfer of technology required to begin using a new manufacturer is also a lengthy process, which
can take six to 18 months to achieve. We believe our manufacturing processes provide us with a competitive advantage, which we have developed
through years of experience formulating skin care products. For all of our proprietary product concepts, we believe we own the related
manufacturing processes, methods and formulations.
We use FDA-compliant manufacturers
who specialize in the manufacture of prescription and OTC pharmaceutical and/or cosmetic products. These parties manufacture products
pursuant to our specifications. All of these manufacturers are required by law and by our manufacturing standards to comply with current
Good Manufacturing Practice. We pre-qualify and continually monitor our manufacturers for quality and compliance. We also require documentation
of compliance and quality from those manufacturers for whom we act as representative in connection with the promotion and sale of their
products.
Bausch Health, which formerly
owned the business of Obagi, is our only supplier and manufacturer of tretinoin. We have a contract with Bausch Health that has an initial
termination date in 2027. While there are several other manufacturers of generic tretinoin, the termination of this agreement or any loss
of services under the agreement could be difficult for us to replace upon the same favorable terms.
In October 2020 we entered
into development and production agreement with Theravant, Inc., under which Theravant has agreed to work collaboratively with us to develop
and manufacture the Skintrinsiq device exclusively for Obagi. We serve as the exclusive distributor of the device under the agreement.
The initial term of the agreement expires at the end of December 2023.
Intellectual Property
The design of our systems
and products is generally proprietary to us, and we hold over 80 provisional and issued patents worldwide for the composition of many
of these products. Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates,
technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary
rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications
related to our proprietary technology, inventions and improvements that are important to the development of our business when appropriate.
We also rely on trade secrets, trade dress, know-how, continuing technological innovation and in-licensing opportunities to develop and
maintain our proprietary position.
We have pursued an aggressive
trademark registration policy aimed at achieving brand recognition and product differentiation in the market. We own over 1,000 U.S. and
foreign trademark registrations and applications and common law marks.
We have also acquired rights
to market, distribute, sell and, in some cases, make products pursuant to license agreements with other third parties that grant us the
right to use the product formulas, trademarks and other trade secrets; these agreements do not include products that have underlying patents.
For instance, in December 2016, Obagi entered into an exclusive license and distribution agreement with Nextcell Medical Company, under
which we have the exclusive right to market, sell and distribute the SUZANOBAGIMD® line of products in the U.S. and all U.S. Territories.
The initial term of the agreement expires in April 2023.
In September 2020, we entered
into an exclusive license and distribution agreement with Osmotics Cosmeceuticals LLC, pursuant to which we have been granted the exclusive
right to market, sell and distribute worldwide the Obagi Professional line of products, which does not include any products that have
underlying patents. The initial term of the agreement expires in September 2023 and can be renewed by the other party for additional one-year
terms upon 30 days’ prior written notice to Obagi. In addition, in April 2021, Obagi entered into a master supply and distribution
agreement with Maxey Cosmetics LLC (“Maxey”), under which it has been granted the right to market, sell and distribute Obagi
Nu-Cil™ throughout the world, with such right being (i) exclusive in Asia and (ii) shared with Maxey through any of Maxey’s
current channels (except for certain specified major cosmetic retail outlets) in the U.S., Europe, Africa and the Middle East. The agreement
has an initial term through April 2026.
Employees
At Obagi, we are committed
to hiring and nurturing diverse talent and believe this is one of the keys to our current and future success. More than 52% of our employee
base identifies as BIPOC. Additionally, our leadership team consists of a diverse group of professionals, 70% of whom are women, dedicated
to promoting inclusivity. In 2019, our executive leadership team underwent Unconscious Bias training in partnership with Project Implicit
and the Skinclusion initiative to ensure we proactively address subconscious biases.
The team has built a culture
that combines a fast moving and outcome-oriented mindset with a tight knit sense of community, winning both a Top Workplaces USA Award
and a Top Workplace Led by Women Award in 2021. Obagi is mindful of diversity during the recruiting and retaining process and believes
that diversity is key to its culture and long-term success, with 72% of our employee base being women. As of June 30, 2022, we had 185
employees. Our employees include 129 in sales and marketing, 13 in research, development and quality control, 18 in operations and distribution
and 25 in administrative functions. Our employees are all non-unionized, and we believe our relations with our employees are good.
Properties
Obagi’s corporate headquarters
are located in Long Beach, California, where it occupies facilities totaling approximately 28,300 rentable square feet under a lease that
expires in June 2026. Obagi uses these facilities primarily for management, research and development, sales, marketing, operations, finance,
legal, human resources and general administrative teams.
In November 2021 we entered
into a lease for warehouse facilities located in Conroe, Texas that will expire in February 2031. The facilities include approximately
35,000 square feet of warehouse space and 4,200 square feet of office space. In March 2022, we entered into a lease for office space located
in The Woodlands, Texas that will expire in July 2032. The facilities include approximately 16,460 square feet and we anticipate that
we will relocate our corporate headquarters to such space in the third quarter of 2022.
We believe that our office
and warehouse space is adequate for our current needs and should we need additional space, we believe we will be able to obtain additional
space on commercially reasonable terms.
Government Regulation
Our products are subject
to regulation by the FDA the FTC and comparable state, local and foreign regulatory authorities, and over time, the regulatory landscape
for our products has become more complex with increasingly strict requirements. These laws and regulations govern, among other things,
the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, disposal, record-keeping, promotion,
advertising, distribution, marketing, export and import of drugs and cosmetic products.
U.S. Regulation of
Cosmetics Products
The majority of our products
are cosmetics. The FDCA defines cosmetics as articles or components of articles intended for application to the human body to cleanse,
beautify, promote attractiveness, or alter the appearance. The labeling of cosmetic products is subject to the requirements of the FDCA,
the Fair Packaging and Labeling Act, and other laws and regulations. Cosmetics are not subject to pre-market approval by the FDA; however
certain ingredients, such as color additives, must be pre-approved for the specific intended use in the product and are subject to certain
restrictions on their use. The FDA may, by regulation, require warning statements on certain cosmetic products for specified hazards associated
with such products. FDA regulations also prohibit or otherwise restrict the use of certain types of ingredients in cosmetic products.
In addition, the FDA requires
that cosmetic labeling and claims be truthful and not misleading. Moreover, cosmetics may not be marketed or labeled for their use in
treating, preventing, mitigating, or curing diseases or other conditions or in affecting the structure or function of the body, as such
claims would render the product to be a drug and subject to regulation as a drug. The FDA evaluates the “intended use” of
a product to determine whether it is a drug, cosmetic product, or both. The FDA may also consider labeling claims in determining the intended
use of a product. If the FDA considers label claims for cosmetic products to be claims affecting the structure or function of the human
body, or intended for a disease condition, then such products may be regulated as “new drugs” within the meaning of the FDCA,
meaning that such products would generally require premarket review and approval by the FDA to be legally marketed in the U.S. In addition
to FDA requirements, state consumer protection laws and regulations can subject a cosmetics company to a range of requirements and theories
of liability, including similar standards regarding false and misleading product claims, under which state enforcement or class-action
lawsuits may be brought.
We market certain products,
such chemical peels, as cosmetics, with the stronger peels sold only to licensed healthcare providers for professional use only. However,
FDA may disagree with our determination that these products do not require FDA premarket review and approval. Similar risks may apply
in foreign jurisdictions. If any of our products we intend to sell as cosmetics were to be regulated as drugs, we might be required to
conduct, among other things, clinical trials to demonstrate the safety and efficacy of these products and to apply for pre-market approval
of such products from the FDA.
In the U.S., the FDA has
not promulgated regulations establishing current Good Manufacturing Practices for cosmetics. However, we require all third-party manufacturers
to represent and warrant to us that the products they produce for us are made in accordance with cGMPs, including documentation, recordkeeping,
building and facility design, equipment maintenance and personnel requirements.
U.S. Regulation of
Drug Products
In the U.S., the FDA regulates
drugs under the FDCA and its implementing regulations. Our prescription-only products containing hydroquinone are regulated as drugs,
however, to date, we have not sought or obtained required NDAs or other FDA approvals for these products. Based on the historical evolution
of the legal and regulatory framework applicable to drugs in the U.S., the FDA acknowledges that there are some drugs on the market that
lack required FDA approval for marketing. The FDA has historically utilized a risk-based enforcement approach with respect to drugs marketed
without required approvals. In 2003, the FDA issued a Compliance Policy Guide, or CPG, which was finalized in 2006 and subsequently amended
in 2011, in which the FDA announced a drug safety initiative to remove unapproved drugs from the market and established enforcement priorities
and a policy of enforcement discretion with respect to marketed unapproved products. Under this policy, the FDA indicated that it intended
to give higher priority to enforcement actions involving unapproved drug products in certain categories, including drugs with potential
safety risks and ineffective dugs that could be used in lieu of effective treatments. Although this CPG was withdrawn and the drug safety
initiative was terminated on the basis of a Federal Register notice in 2020, a subsequent Federal Register notice in May 2021 withdrew
the prior notice terminating the program and the CPG, and the FDA indicated that it plans to continue to prioritize enforcement based
on its existing general approach, which involves risk-based prioritization in light of all the facts of a given circumstance. We believe
our prescription-only HQ products do not fall within the previously established categories of unapproved drugs for which the FDA has indicated
it prioritizes enforcement. To date, we have not received any communications from the FDA or any similar regulatory authorities regarding
our Nu-Derm HQ or any other products and neither the FDA nor any other regulators have prohibited us from selling our prescription HQ
products in the U.S. or in other jurisdictions. However, there can be no assurance the FDA or any other regulatory authorities will not
take enforcement action against us, or otherwise require us to obtain premarket approval or similar authorization of our prescription
HQ products.
The process of obtaining
regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require
the expenditure of substantial time and financial resources, and generally involves the following:
| ● | completion
of preclinical laboratory tests, animal studies and formulation studies in accordance with
the FDA’s Good Laboratory Practice requirements and other applicable regulations; |
| ● | submission
to the FDA of an Investigational New Drug application requesting authorization from the FDA
to administer an investigational biologic to humans which must become effective before human
clinical trials may begin; |
| ● | approval
by an independent Institutional Review Board or ethics committee at each clinical site before
each trial may be initiated; |
| ● | performance
of adequate and well-controlled human clinical trials in accordance with good clinical practices
(“GCPs”), to establish the safety and efficacy of the proposed drug for its intended
use. Clinical trials generally include the following: |
| ● | Phase
1: The product is initially introduced into healthy human subjects or patients with the target
disease or condition to test the safety, dosage tolerance, absorption, metabolism
and distribution of the investigational product in humans, the side effects associated with
increasing doses, and, if possible, to gain early evidence on effectiveness. |
| ● | Phase
2: The product is administered to a limited patient population with a specified disease or
condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to
identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials
may be conducted to obtain information prior to beginning larger and more expensive Phase
3 clinical trials. |
| ● | Phase
3: The product candidate is administered to an expanded patient population to further evaluate
dosage, to provide statistically significant evidence of clinical efficacy and to further
test for safety, generally at multiple geographically dispersed clinical trial sites. These
clinical trials are intended to establish the overall risk/benefit ratio of the investigational
product and to provide an adequate basis for product approval. |
| ● | In
some cases, the FDA may require, or sponsors may voluntarily pursue, additional clinical
trials after a product is approved to gain more information about the product. These so-called
Phase 4 studies, may be conducted after initial marketing approval, and may be used to gain
additional experience from the treatment of patients in the intended therapeutic indication.
In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a
condition of approval of an NDA |
| ● | preparation
of and submission to the FDA of an NDA after completion of all pivotal trials; |
| ● | a
determination by the FDA within 60 days of its receipt of an NDA to file the application
for review |
| ● | satisfactory
completion of an FDA advisory committee review, if applicable; |
| ● | satisfactory
completion of an FDA inspection of the manufacturing facility or facilities at which the
drug is produced to assess compliance with cGMP requirements to assure that the facilities,
methods and controls are adequate to preserve the drug’s identity, strength, quality
and purity, and of selected clinical investigation sites to assess compliance with GCPs;
and |
| ● | FDA
review and approval of the NDA to permit commercial marketing of the product for particular
indications for use in the U.S. |
Hatch-Waxman Act
Section 505(j) of the FDCA
establishes an abbreviated approval process for a generic version of approved drug products through the submission of an ANDA. Certain
of our products, including tretinoin product, are marketed pursuant to an ANDA held by Bausch Health. An ANDA provides for marketing of
a generic drug product that has the same active ingredients, dosage form, strength, route of administration, labeling, performance characteristics
and intended use, among other things, to a previously approved product.
Over-the-Counter Drug
Products
We currently market certain
non-prescription drug products, including certain products that are intended to treat acne or be used as sunscreens, which are regulated
as OTC drug products by the FDA. Certain OTC drug products are subject to regulation pursuant to the FDA’s “monographs,”
which provide rules applicable to each therapeutic category of non-prescription drug, and establishes conditions, such as active ingredients,
uses (indications), doses, labeling, and testing procedures, under which an OTC drug within that particular category may be GRASE, and
therefore can be marketed without obtaining pre-market approval of an NDA or ANDA. To be legally marketed, among other things, OTC drug
products marketed under an OTC monograph must be manufactured in compliance with the FDA’s cGMP requirements for drug products,
and the failure to maintain compliance with these requirements could lead to FDA enforcement action. Moreover, a failure to comply with
the OTC monograph requirements could lead the FDA to determine that the drug is not GRASE, and thus is a “new drug” requiring
approval in accordance with the NDA process described above.
U.S. Regulation of
Medical Devices
Medical devices are subject
to extensive and rigorous regulation by the FDA and by other federal, state and local authorities. The Federal Food, Drug, and Cosmetic
Act and related regulations govern the conditions of safety, efficacy, clearance, approval, manufacturing, quality system requirements,
labeling, packaging, distribution, storage, recordkeeping, reporting, marketing, advertising, and promotion of medical devices. Unless
an exemption applies, each medical device commercially distributed in the U.S. requires either FDA clearance of a 510(k) premarket notification
or approval of a premarket approval application (a “PMA”). Under the FDCA, medical devices are classified into one of three
classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent
of manufacturer and regulatory control needed to ensure its safety and effectiveness. Class I includes devices with the lowest risk to
the patient and are those for which safety and effectiveness can be assured by adherence to the FDA’s General Controls for medical
devices, which include compliance with the applicable portions of the Quality System Regulation (“QSR”), facility registration
and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising and promotional materials.
Class II devices are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety
and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries
and FDA guidance documents. While most Class I devices are exempt from the 510(k) premarket notification requirement, manufacturers of
most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission
to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a 510(k) premarket notification
is generally known as 510(k) clearance. Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting
or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent
to that of a legally marketed device, are placed in Class III, requiring approval of a PMA. Some pre-amendment devices are unclassified,
but are subject to the FDA’s premarket notification and clearance process in order to be commercially distributed.
After a device is cleared
or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| ● | establishment
registration and device listing with the FDA; |
| ● | QSR
requirements, which require manufacturers, including third-party manufacturers, to follow
stringent design, testing, control, documentation and other quality assurance procedures
during all aspects of the design and manufacturing process; |
| ● | labeling
and marketing regulations, which require that promotion is truthful, not misleading, fairly
balanced and provide adequate directions for use and that all claims are substantiated, and
also prohibit the promotion of products for unapproved or “off-label” uses and
impose other restrictions on labeling; FDA guidance on off-label dissemination of information
and responding to unsolicited requests for information; |
| ● | clearance
or approval of product modifications to 510(k)-cleared devices that could significantly affect
safety or effectiveness or that would constitute a major change in intended use of one of
our cleared devices; |
| ● | medical
device reporting regulations, which require that a manufacturer report to the FDA if a device
it markets may have caused or contributed to a death or serious injury, or has malfunctioned
and the device or a similar device that it markets would be likely to cause or contribute
to a death or serious injury, if the malfunction were to recur; |
| ● | correction,
removal and recall reporting regulations, which require that manufacturers report to the
FDA field corrections and product recalls or removals if undertaken to reduce a risk to health
posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
| ● | complying
with requirements governing Unique Device Identifiers on devices and also requiring the submission
of certain information about each device to the FDA’s Global Unique Device Identification
Database; |
| ● | the
FDA’s recall authority, whereby the agency can order device manufacturers to recall
from the market a product that is in violation of governing laws and regulations; and |
| | | |
| ● | post-market
surveillance activities and regulations, which apply when deemed by the FDA to be necessary
to protect the public health or to provide additional safety and effectiveness data for the
device. |
Manufacturers
of medical device products marketed in the U.S. are required to comply with the applicable portions of the QSR, which cover the methods
and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging,
distribution, installation and servicing of finished devices intended for human use. Device manufacturers are also subject to periodic
scheduled or unscheduled inspections by the FDA. The FDA has broad regulatory compliance and enforcement powers. If the FDA determines
that a manufacturer has failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement
actions, which may result in any of the following sanctions: warning letters, untitled letters, fines, injunctions, consent decrees and
civil penalties; recalls, withdrawals, or administrative detention or seizure of our products; operating restrictions or partial suspension
or total shutdown of production; refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified
products; withdrawing 510(k) clearances or PMA approvals that have already been granted; refusal to grant export or import approvals
for our products; or criminal prosecution.
Foreign
Government Regulation
To
market our products in many non-U.S. jurisdictions we must obtain separate regulatory approvals and comply with numerous and varying
regulatory requirements. In some countries we do not have to obtain prior regulatory approval but do have to comply with other regulatory
restrictions on the manufacture, importation, distribution, marketing and sale of our products. We may be unable to file for regulatory
approvals and may not receive necessary approvals to commercialize our products in any market. The approval procedure varies among countries
and can involve additional testing and data review. The time required to obtain approval in non-U.S. jurisdictions may differ from that
required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval.
In addition, many countries from time to time evaluate the regulatory status of various products and ingredients. We may not obtain foreign
regulatory approvals on a timely basis, if at all, or may choose not to implement a country’s labeling requirements if to do so
would have a negative impact on our international or domestic operations.
For additional information
on the regulatory requirements related to our products, please see “Risk Factors—Risks Related to Evolving Laws and Regulations
and Compliance with Laws and Regulations.”
Legal
Proceedings
From
time to time, Obagi may be involved in various legal proceedings and subject to claims that arise in the ordinary course of business.
Although the results of litigation and claims are inherently unpredictable and uncertain, Obagi is not currently a party to any legal
proceedings the outcome of which, if determined adversely to Obagi, are believed to, either individually or taken together, have a material
adverse effect on its business, operating results, cash flows or financial condition. Regardless of the outcome, litigation has the potential
to have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
INFORMATION
ABOUT MILK
Unless the context
otherwise requires, all references in this section to “we,” “our,” “us,” the “company,”
or “Milk Makeup” generally refer to the Company’s “clean” makeup segment: Milk.

Milk
Makeup’s Dream & Mission
Our
ambition is to build the top global makeup brand of the next generation. Our mission is to serve and empower our community to
live their look by creating effective, easy to use, vegan, clean cruelty-free beauty products.
Company
Overview
Milk
Makeup is a leading, award-winning clean prestige makeup brand with unique products, a strong following among Gen-Z consumers and an
emerging global presence. Since launching in 2016, the brand has achieved strong rank within our distribution at a total brand and key
product level (as of September, Milk, at Sephora, was the #14 makeup brand, #12 in Beauty and Makeup, #21 in online sales and the
overall #2 clean makeup brand), but we believe even more significant growth opportunities remain in terms of building awareness, product
and category expansion, channel expansion and regional expansion.
We
believe that our inclusive brand values, “clean” product philosophy (as described in the section “Values & Commitments
Overview – ‘Clean’ Products” below) and commitments to sustainability and philanthropy are at the zeitgeist
of what will motivate the next generation of beauty consumers around the world, and that these values and product attributes will only
become more relevant. We believe that our ability to authentically connect with youth culture while developing unique, effective and
easy to use products that are also 100% vegan, clean and cruelty-free sets us apart from other brands.
Milk
Makeup was launched in 2016 with the goal of building a global movement to challenge and broaden the definition of beauty. Community
and self-expression are at the heart of everything we do. We believe that it’s not how you wear your makeup, it’s what you
do in it that matters. This ethos is captured in our brand signature, “Live Your Look.”
Born
out of Milk Studios, a creative hub in downtown New York City, Milk Makeup is the brainchild of Milk Studios co-founder and Chief Brand
Officer Mazdack Rassi, co-founder and COO Dianna Ruth, authentic Milk Girl and creative director Georgie Greville, and beauty + fashion
editor and E! News red carpet correspondent Zanna Roberts Rassi. All aspects of the brand are designed in-house at Milk headquarters,
our downtown NYC home.
We
are now the #2 clean brand in Sephora in the U.S., with our Hydro Grip face primer and Kush mascara as category leading best-sellers
(source: sephora.com). We are a “hybrid” brand, offering an innovative selection of both makeup and skincare products. This
allows us to play a broader role in our community’s beauty routine and also positions us for expansion into new categories both
within and outside of makeup and skincare. Our other top products include Lip + Cheek, Sunshine Skin Tint, Matte Bronzer, Vegan Milk
Moisturizer, Kush Brow, Cooling Water and our new Bionic Blush. We currently have strong positions within Sephora in the primer, mascara,
blush and bronzer categories. We have opportunities in large segments of the cosmetics market such as foundation, concealer, brow, liner
and lip color. Outside of cosmetics, we believe that we have untapped opportunities in skincare and potentially other categories such
as haircare, fragrance, bath and shower which, as illustrated in the chart below, are significant markets. We believe that Milk Makeup
has a broad permission set that will allow us to build a cross-category beauty brand.

Source:
Euromonitor International; Beauty & Personal Care, 2022ed, retail value sales, current prices, 2021 fixed ex rates
The brand is currently distributed
online via milkmakeup.com, in omni-channel retail through Sephora (in the U.S. (including Sephora at Kohl’s), Canada, E.U., Middle
East and Australia), online at Cult Beauty (United Kingdom) and Selfridges (United Kingdom) and cross-border to China through the distributor
SuperOrdinary. We have opportunities to accelerate within our existing footprint and grow in both new channels and regions.
Milk
Makeup is anchored by a strong community with significant room for growth as awareness and distribution continue to build. As of November 22,
2021, Milk Makeup had 1,900,000 followers on Instagram, 8,600,000 likes and 122,000,000 hash-tagged video views on TikTok, 653,000 monthly
views on Pinterest, 99,000 followers on Facebook, 89,000 subscribers on YouTube and 62,000 followers on Twitter.
We
have strong awareness among Gen-Z with opportunities to build across all other age groups. According to a recent survey conducted by
Sephora in June 2020 of female participants aged 13-64 who have purchased beauty products for themselves in the past three months, Milk
Makeup has 82% awareness among “Gen-Z” participants, 69% awareness among “Millennial” participants, 56% awareness
among “Gen-X” participants and 49% awareness among “Boomer” participants. This awareness and resonance with the
younger core audience is a key strength of the brand and, we believe, a key differentiator from the large legacy brands. Our plan is
to continuously renew relevance with our younger core demographic while expanding into more mature demographics, who tend to have higher
disposable income, through awareness building and product storytelling. This will create strong future growth potential for the brand.
Milk
Makeup’s strategy to building a top clean make-up brand of the new generation
Our
strategy is based on 3 pillars:
| ● | Grow
our consumer base: our goal is to drive more awareness and trial of our products
by reinvesting in operational efficiencies, increasing marketing spend and leveraging our
owned ecosystem to drive additional awareness, trial and topline growth. Additionally, we
plan on broadening our brand footprint to recruit more Millennials while continuing to build
on our strong position with Gen-Z consumers. |
| | | |
| ● | Brand
expansion: our goal is to expand our make-up assortment and entering into new categories
by accelerating product innovation and building on hero products to create category champions
(products in the top 3 of their respective categories) with global resonance, continuing
to expand and develop our skincare product presence, evolving our product mix to higher margin
products (such as foundation and skincare) and expanding into additional make-up categories
in the future. |
| | | |
| ● | Internationalize:
our goal is to build global brand availability by continuing to utilize, strengthen and maximize
our distribution relationship throughout the Sephora ecosystem, while expanding further into
key markets, such as the United Kingdom, and leveraging our brand success to develop a highly
efficient international direct-to-consumer model. |
Industry
Overview
Milk
Makeup operates within a subsector of the cosmetics industry known as color cosmetics. The global color cosmetics market is valued at
approximately $66.0 billion with an expected CAGR of 7.0% from 2021 to 2023. The below chart shows the color cosmetics market by region
with expected CAGRs.

Source: Euromonitor International;
Beauty & Personal Care, 2021ed, retail value sales, current prices, 2020 fixed ex rates. North America includes the U.S. and Canada.
The
cosmetics industry is comprised primarily of face makeup, eye makeup, lip products, nail products and cosmetics sets/kits. According
to Euromonitor, global sales of face makeup were $24.0 billion in 2020. Face makeup includes foundation, concealer, primers, setting
sprays and powders, highlighters, bronzers, blush and BB/CC creams. Global sales of eye makeup were approximately $16.0 billion in 2020.
Eye products includes mascara, eyeliner, eyeshadow and brow. Global sales of lip products were approximately $14.0 billion in 2020. Lip
products include lipstick, lip liner, lip gloss, lip liner plumper and tinted lip balm. Global sales of nail products were approximately
$5.0 billion in 2020. Nail products include nail polish, nail remover and nail treatments. Global sales of cosmetics sets/kits were approximately
$3.0 billion in 2020. Cosmetics sets/kits are combinations of products that are packaged together and often offered with a promotional
incentive relative to buying the products separately.
The
cosmetics industry is large and fragmented. There are established multi-national competitors such as Unilever P.L.C., Coty Inc., L’Oréal
S.A., The Estée Lauder Companies, Inc., P&G, Revlon Inc., Shiseido Company, Limited, Puig and e.l.f, Beauty Inc., in addition
to independent companies such as Anastasia Beverly Hills, Pat McGrath Labs, Huda Beauty, Ilia, Kosas, Tower 28 and Forma. The top five
competitors in color cosmetics have a 46% market share. However, the next 13 have only a combined 18% market share. We believe that this
affords the opportunity for well positioned, high quality and well run brands to achieve both scale and profitability given the gross
margins in the category.
We
believe there is a clear opportunity for Milk Makeup to gain share within this market. We believe that our advantage lies in our ability
to build a strong community, our agility and speed to market to respond to emerging trends, as well as our ability to connect deeply
with and meet the needs of the next generation of consumers. In addition, we believe that many of the barriers to entry that previously
prevented younger brands from gaining scale, such as the need to have robust R&D capabilities, heavy traditional media investment
and broad distribution are no longer critical to succeeding in the market today due to the availability of high quality third-party manufacturers,
the strength of social media in driving awareness and relevance, and the weight of e-commerce within beauty and the consumer’s
desire for high performance products and a premium brand experience.
Product
Overview
Milk
Makeup offers an exciting portfolio of 330 makeup and skincare stop keeping units (“SKUs”). Our current product assortment
covers only a fraction of the 1,500-7,000 SKUs offered by other leading make-up specialist brands, which we believe highlights the ample
opportunity to continue to innovate and increase our penetration within make-up. We currently have bestselling products at Sephora in
the U.S. in the primer and mascara categories with our Hydro Grip primer and Kush mascara. We also have strong positions in the blush
category with Lip + Cheek and the bronzer category with Matte Bronzer, showcasing the versatility of the brand. These products are part
of a $5.0 billion prestige color cosmetics category in the U.S. alone, much of which we have yet to truly address. We currently have
limited offerings in the foundation and concealer categories as well as brow, liner, eyeshadow and lip color. We also believe that we
can expand further into other beauty categories such as Skincare, Haircare, Bath/Shower and Fragrance. The chart below highlights the
market breakdown and demonstrates where we have both existing strengths and future opportunities.

| (1) | Market
sizes reflect 2021E data for the U.S. market; Euromonitor International; Beauty & Personal
Care and Color Cosmetics in the US, 2022ed, retail value sales, current prices, 2021 fixed
ex rates; |
| (2) | Areas
where management believes product offering expansions or introduction of new categories are
possible; |
| (3) | Primer
refers to EMI’s category BB/CC creams and skin tints refers to EMI’s premium
foundation/ concealer |
Beyond
strong rankings, our products have won multiple prestigious awards including our Hydro Grip Primer, which won Allure’s 2019 Best
of Beauty award and 2020 Reader’s Choice award, and Sunshine Vitamin C + Squalane Face Oil which won Allure’s 2021 Best of
Beauty award.

Sales
and Distribution Strategy
Since
launching in 2016, Milk Makeup has had a strong exclusive partnership with Sephora covering most markets, including the U.S., Canada,
Europe, the Middle East, Australia and New Zealand. The partnership has allowed the brand to quickly rise within the category ranks through
exceptional launch support and visibility both in-store and online. In a crowded and fragmented makeup market, our partnership with Sephora
has allowed us to break through despite limited marketing spend. This partnership has also allowed the brand to internationalize efficiently
due to the synergies within the Sephora ecosystem in terms of common management, terms, and merchandising.

Milk
Makeup has an established distribution footprint across Sephora North America. In 2019, Milk Makeup began expanding internationally into
Cult Beauty and Sephora Europe, then in 2020 into Sephora Middle East, Selfridges and cross-border into China, and finally in 2021 into
Sephora Australia and New Zealand. We believe that we have significant opportunities to increase our sales within our existing footprint,
including gains in productivity and distribution expansion, such as Sephora at Kohl’s, while expanding into high growth international
markets such as China, Russia, Brazil and Mexico. Currently, no single international jurisdiction represents a material portion of our
sales, with no jurisdiction accounting for more than a total of 2% in sales.
Milk
Makeup has entered into a distribution or vendor agreement with Sephora North America, Sephora Canada, Sephora Middle East, Sephora Australia
and New Zealand, respectively. Pursuant to those agreements, we grant Sephora the exclusive right to import and distribute our selected
products within the territories described in the agreements. In addition, those agreements contain a term of exclusivity, from three
months to three years, which can be automatically renewed by either party. Each of these agreements can be terminated without penalty
by either party by giving advanced notice to the other party. None of these agreements with Sephora contain any minimum purchase requirements.
In early 2022, Milk entered into new amendments with respect to the Sephora agreements that updated certain payment terms and extended
the exclusivity of the Milk brand with Sephora through 2023. The full text of these agreements, or forms thereof, are filed as exhibits
to the registration statement of which this prospectus forms a part.
Milk
Makeup is currently present in 1,335 Sephora locations in the U.S. and 82 in Canada, including Sephora inside JCPenney and Sephora at
Kohl’s locations. In Europe, Milk Makeup is currently present through Sephora in France, Germany, Spain, Sweden, Denmark, Italy,
Poland, Portugal, Switzerland, Greece, the Czech Republic and the Balkans. The table below highlights Milk’s current penetration
in Sephora doors worldwide.

Source:
Data provided by Sephora; Sephora doors include Kohl’s and SiJCP.
milkmakeup.com
is a burgeoning direct-to-consumer, e-commerce platform that currently ships across the U.S. In November 2021, we relaunched a new, upgraded
version of our existing site and we intend to increase our investment and expand distribution globally in the coming years. For the six
months ended June 30, 2022, milkmakeup.com accounted for 5% of net sales, and Milk Makeup management currently expects this to increase
substantially by 2024 as a percent of overall net sales.
Outside
of Sephora and milkmakeup.com, Milk Makeup has a presence in Cult Beauty and Selfridges in the United Kingdom and TMall Global in China
(through the distributor SuperOrdinary). We are actively gauging other expansion opportunities across regions and channels.
Competition
Milk
Makeup identifies its main competitors as Unilever P.L.C., Coty Inc., e.l.f. Beauty Inc., L’Oréal S.A., LVMH Moet Hennessy
Louis Vuitton SE, The Estée Lauder Companies Inc., P&G, Revlon Inc. and Shiseido Company, Limited. These companies own both
legacy brands as well as younger previously independent brands. Milk Makeup also competes directly with privately held brands, including
Pat McGrath Labs, Ilia, Kosas, Anastasia Beverly Hills, Huda Beauty, Forma, Saie, Tarte and Tower 28. Ilia, Saie, Kosas and Tarte, which
are also clean beauty brands and have partnerships with Sephora, are the key competitors of Milk Makeup. Among other areas, Milk Makeup
believes that it competes against other cosmetics brands on price, quality of products and packaging, perceived value, innovation, in-store
presence and visibility, and e-commerce and mobile commerce initiatives. Milk Makeup is focused on expanding its market share in the
color cosmetics industry and continuing to be a leader in clean make-up.
Competitive
Strengths
Authentic
Brand with Innovative, Iconic Products
Milk
Makeup is an authentic, culturally relevant brand with an impactful message of empowering our community to live their look and offering
effective, vegan, clean, easy-to-use products. Our cruelty-free, globally compliant formulas allow diverse consumers to experiment with
their color and find a look that they truly love. Our products have won several industry awards and are top sellers across multiple categories.
Milk Makeup products are designed to have unique and innovative packaging, formula and delivery systems and have proven to have strong
market retention and growth, even in an industry that is constantly evolving. We believe that the desire for shared values and effective,
clean products will only grow and will position our brand for considerable future growth.
Engaged,
Desirable Community
Our
community is the heart, soul and inspiration of our brand and a key point of differentiation both in terms of our ability to resonate
with the elusive younger demographic and our content and community capabilities in social media. Our community has been built organically,
with fans and users advocating for our brand and products across various social media platforms and introducing others to Milk Makeup
products. Since launching, Milk Makeup has built an impressive, inclusive and diverse social media presence, with 1,900,000 followers
on Instagram and 8,600,000 likes on TikTok, as of November 22, 2021. These accounts keep our community engaged with the brand, alert
them to new product offerings and allow us to interact directly with our consumers, monitoring any feedback they may have and adjusting
our content and investments in accordance. In an age where social media and influencer marketing provide tangible results, we believe
that our unique and engaged social media presence provides a competitive edge.
Established
Domestic Omni-Channel Platform with Proof Points Internationally
In
the U.S., Milk Makeup has a leading market position among clean brands in Sephora with strong partnership support and a burgeoning direct-to-consumer
presence through milkmakeup.com. Milk Makeup also has a growing presence across Europe, the Middle East, Australia, New Zealand, the
United Kingdom and cross-border in China. Milk Makeup has expanded its international presence over the past several years, including
by increasing its footprint in the UK and throughout various jurisdictions in Europe in 2019, in the Middle East, cross-border China,
Turkey and further in Europe in 2020, and in Australia, New Zealand and even more substantially in Europe in 2021, and we have aggressive
plans to continue expanding our international footprint. We believe there is considerable opportunity to further increase penetration
across all of the markets where we are currently competing while entering large untapped markets including mainland China, Russia, Brazil,
Mexico and South Asia. Currently, outside of North America, international retail sales of Milk Makeup are highly diversified, with no
single foreign country representing more than 3% of revenue.
Strong
Historical Sales Growth with Potential for Future Growth and Gross Margin Expansion
Milk
Makeup historically has experienced strong, double-digit yearly growth while improving product margins. Milk Makeup management expects
year-over-year net sales growth in 2022, despite the impact of COVID 19 on the prestige makeup market. Milk also plans to execute margin
expansion initiatives, including boosting direct-to-consumer sales and introducing higher margin products. See the Improve Operational
Efficiency and Profitability section below for details.
Growth
Opportunities
Grow
Customer Base and Brand Awareness
Milk
Makeup has a significant opportunity to increase our customer base and drive higher brand awareness and first purchase as consumers become
more educated about our high-quality product offering and strong values around inclusivity and social responsibility. Based on a Sephora
online survey that was conducted in June 2020, which surveyed a total of 4,675 women between the ages of 13-64 who had purchased beauty
products for themselves in the past three months in the U.S., only approximately 20% of Sephora’s total beauty shoppers have awareness
of the brand, and only 16% of this category of shopper has made a Milk Makeup product purchase. This total penetration level of 3% of
Sephora U.S. shoppers indicates a significant expansion opportunity that we can utilize and address. Put into context in terms of ranking,
Milk Makeup is the #13 makeup brand today within Sephora U.S. and we believe we can become a strong top 10 brand in every market. Incremental
opportunities also exist with Milk Makeup’s other retail partnerships such as Cult Beauty and Selfridges, and additionally at new
potential retailers.
We
have several strategic initiatives to drive brand awareness. We plan on leveraging the strength of our current media ecosystem (for which
we were recognized with more than 10 separate awards from multiple publications in 2021) and reinvesting in operational efficiencies
to increase and improve marketing spend behind further expanding reach. Further, we plan to create collaborations with non-beauty brands
to expand reach and increase brand appeal to new audiences. These efforts have the ultimate aim of attracting new Millennial customers
while building upon the existing resonance with Gen-Z customers.
Accelerate
Direct-to-Consumer Channel
In
order to build and accelerate a substantial direct-to-consumer business, Milk Makeup has undertaken several initiatives that we believe
will drive increased reach and improved repeat purchase rates and marketing efficiency. These strategic developments will also position
the business with a diversified sales mix between e-commerce and retail sales channels. Our core direct-to-consumer initiatives include
technological improvements (including website enhancement), optimization of the marketing funnel, development of the e-commerce team,
rollout of direct-to-consumer exclusive offerings and building out operations to expand into new markets. Additionally, we plan to leverage
the data-driven, asset-light Waldencast platform and e-commerce expertise, post-Business Combination, to further accelerate direct-to-consumer
growth.
Expand
Distribution to New Geographies and, Over Time, Potential New Retailers Domestically and Internationally
There
are several large and meaningful potential retail partnerships in international markets for Milk Makeup to pursue. As compared to our
current 100% Sephora door penetration in North America, we have less than 50% door penetration in Sephora stores located in Europe, the
Middle East and Australia / New Zealand and have not yet entered Sephora Asia, Latin America or markets like Russia. Entering more Sephora
doors internationally and enhancing this key partnership is a significant focus area. Further, Milk Makeup can potentially expand upon
our Sephora European brick-and-mortar presence to address untapped opportunities with new channels. Additionally, new market opportunities
also exist for Milk Makeup’s direct-to-consumer platform, as currently milkmakeup.com only ships products in the U.S. In alignment
with our strategy to drive brand awareness, we plan to develop international capabilities that will allow it to service international
markets over time. Finally, we have opportunities to expand our retail partner distribution network over time.
Extend
Product Offerings
Milk
Makeup plans to continue leveraging our relevance and appeal to bring to market an expanded product portfolio that both builds on existing
category strengths such as primer and mascara while addressing new categories. As previously noted, we have established positions in
Mascara, Primer, Blush & Bronzer, but also significant opportunity to enter into make-up adjacencies including Foundation / Concealer,
Lash & Brow, Lip Color and eyeshadow. Further, we are exploring expanding further into Skincare and possibly entering other categories
such as Haircare, Body, Bath & Shower and Fragrance over time.
Improve
Operational Efficiency and Profitability
As
Milk Makeup gains scale, there are several initiatives in place to drive improved operational efficiency and profitability. Our new cost-of-goods-sold
framework, featuring annual negotiations and value analysis on hero SKUs as well as gross margin targets for new products, will facilitate
gross margin expansion. Other strategic tactics include annual price increases on key SKUs, further expansion into higher margin categories
(complexion and skincare) and an evolving channel mix that is more accretive. To help address rising operating costs, Milk Makeup will
continue to undergo forecasting and supply chain optimization projects. With these key developments, we believe that we can continue
to scale rapidly and profitably. While the global supply chain disruption is impacting the overall market, we have taken a proactive
approach to protecting ourselves including increasing stock coverage on our hero products.
Leverage
Favorable Industry Tailwinds
Milk
Makeup is well-positioned to capitalize on strong and favorable industry tailwinds. Color cosmetics is a $66.0 billion industry
with an estimated CAGR of 7.0% from 2021-2023. With consumer desires shifting towards for quality clean, natural and vegan products,
over price considerations, we believe that our brand position as an authentic and innovative provider of vegan, clean, cruelty-free products
provides us an advantage in addressing these evolving customer dynamics. Additionally, in accordance with broader consumer sentiment
focusing on environmental and other environmental, social and governance (“ESG”) concerns, we constantly evaluate our packaging
for more sustainable options, further reinforcing our credibility as a desirable brand for consumers searching for those attributes.
We believe that these consumer desires will only increase over time and that they will help to propel our business as we scale globally.
Customers
Our
objective is to build a robust global, omni-channel network connecting our brand and products with potential consumers. Our strategy
is to build best in class owned e-commerce and social platforms while building strong relationships globally with select omni-channel
retailers. Our executives work with executives of major retail accounts on a regular basis, and we believe that we are viewed as an important
supplier to these customers.
Values
& Commitments Overview
We
believe that a key strength and point of differentiation for us with our community are our shared values and the commitments that we
have made to uphold them. Strong ESG scores and values are table stakes for young consumers and mandatory for modern brands. These are
present in everything that we do.
“Clean”
Products
From
day 1, we have always strived to create breakthrough, effective products that are also clean, vegan and cruelty-free. When we launched,
we called our product ethos “cool, clean beauty that works.”
At
Milk Makeup, we use the word “clean” to describe how we formulate our products. We define “clean” using the following
five factors:
| ● | No
Animal Products and Byproducts: All of our products are Leaping Bunny Certified,
which means that Milk Makeup does not test on animals at any stage in its supply chain. Additionally,
our products have no animal derivatives and are 100% vegan. |
| | | |
| ● | “Clean”
Ingredients: We are dedicated to creating natural products, which means our products
will never contain any of the over 2,500 controversial and potentially harmful or irritating
ingredients, including parabens, sulfates, BHA, BPA, plastic microbeads, talc, urea, retinyl
palmitate, mercury or mercury-containing ingredients, resorcinol, formaldehyde, aluminum
salts, and mineral oil. We publish a complete and growing list of ingredients it will never
use in our “Ingredient No List.” |
| ● | Natural
Products: Milk Makeup follows ISO 16128 guidelines, where “natural”
means plant, mineral, and/or microbiologically derived ingredients. We want to bring products
that are as natural as possible to our community, while also not compromising on quality
and performance. We are always striving to improve, and are currently working toward making
new formulas that are over 80% natural. |
| | | |
| ● | Ethically
Sourced Ingredients: We have committed to ethical and responsible sourcing for our
formulas from start to finish. For any products containing mica or palm-derived ingredients,
we only use ethically sourced and sustainable mica and sustainability certified palm-derived
ingredients. Milk Makeup also exclusively works with GMP (Good Manufacturing Practice) compliant
factories. |
Dedication
to More Sustainable Packaging
Since
our launch in 2016, Milk Makeup has focused on creating packaging that is iconic, innovative, easy to use, and pairs perfectly with our
formulas. We are also committed to reducing our overall impact on the environment. We recognize that we have a lot of work to do in this
area and are constantly striving to make our packaging more sustainable, starting with new products and continuing on to shipping and
partnerships. We are focusing on the following 4 sustainability initiatives:
| ● | More
Sustainable Shipping: Starting in January 2021, we redesigned our e-commerce shipping
system and started transporting our products in a new, sustainable shipping box and bag.
Our new shipping box is printed with petroleum-free plant-based inks and its inner bag is
made from 100% post-consumer waste, both of which are made in the U.S. Both the new box and
bag are both 100% recyclable once the adhesive strip on the packaging is removed. |
| | | |
| ● | Environmentally
Friendly In-Store Packaging: Milk Makeup is working to use less plastic, offer more
refills and use more post-consumer resin and recyclable materials on packaging and in-store
merchandising, which includes launching new product display systems that use 63% less plastic
on average than previous displays, introducing refills where possible, and exploring the
use of mono plastic to make recycling easier. |
| | | |
| ● | How2Recycle
Partnership: In the U.S. and Canada, we have partnered with How2Recycle to help
develop labels to communicate clear recycling instructions on our products. |
| | | |
| ● | g2
revolution Partnership: Milk Makeup has partnered with g2 revolution, a specialty
recycling solutions program that helps to develop ways to responsibly dispose of hard to
recycle excess products and components. |
Commitment
to Diversity and Inclusion
Milk
Makeup’s mission is to empower our community to live their look. We believe in beauty for all, which means we are committed to
the importance of diversity, equity and inclusion (“DE&I”). This commitment starts by striving to create a workplace
where everyone can thrive.
We
have made five commitments with respect to DE&I within our teams, consumers and partners and we have, and intend to continue to update
them on our progress with respect to these commitments every six months. These include increasing representation among our teams and
partners, educating, communicating and taking action internally to support our DE&I objectives, giving back 1% of our sales from
our website to The Center NYC and 1% to the Fashion Scholarship Fund and being accountable by providing updates on our progress every
June and December.
Properties
We
strive to be an “asset light” organization. Milk Makeup’s corporate headquarters are located in New York, New York,
where Milk Makeup occupies facilities totaling approximately 17,500 rentable square feet under a lease that expires November 30,
2030. Milk Makeup primarily uses these facilities for corporate offices and as an in-house studio. Additionally, Milk Makeup subleases
from Milk Studios Los Angeles LLC certain space in Los Angeles, CA for a fee of $0.015 million per month. Milk Makeup primarily uses
these facilities for corporate offices and as an in-house studio.
Milk
Makeup is provided fulfillment services by a third-party. Milk and Distribution Corp. (the “Dotcom”) have entered into a
master service agreement on July 13, 2017, as amended on July 10, 2020 (the “Service Agreement”). Pursuant to the Service
Agreement, Dotcom shall provide the fulfillment services to Milk in exchange for the service fees set forth therein. The primary purpose
of this Services Agreement is to receive finished goods and sample products from co-manufacturers, to store goods at a warehouse in New
Jersey, and to distribute and fulfill goods to wholesale and e-commerce customers. Once finished goods are received, they are stored
in a dedicated Milk Makeup location in the warehouse. Other responsibilities of the services provided include inventory management through
cycle counts and physical inventory, and client relations responsible for SLA reporting, collecting forecasts, program planning, end
of day reporting, weekly/monthly meetings and QBRs. Milk may request Dotcom to provide additional services and Dotcom has the right to
increase its fees on each anniversary date of the Service Agreement. The start date of the original Service Agreement is July 13, 2017.
Such Service Agreement shall continue for 36 months and can be automatically renewed for another 12 months unless terminated early by
either party by giving advanced written notice. An amendment to the original Service Agreement was entered into on July 10, 2020 to extend
such Service Agreement to December 31, 2023 unless terminated by either party with 150 days’ notice.
Milk
Makeup does not own any real property.
Employees
Milk
Makeup’s success depends on its employees and the culture that attracts them. Milk Makeup has built a team of industry professionals
focused on promoting beauty for all. Milk Makeup’s culture focuses on creativity, self-expression, inclusion, respect and trust.
As of December 20, 2021, Milk Makeup had 68 full time employees. None of Milk Makeup’s employees are represented by a labor organization
or are a party to any collective bargaining agreement.
Milk
Makeup is dedicated to employing a more diverse and inclusive hiring and interviewing practice resulting in the hiring of more individuals
who identify as BIPOC. Milk Makeup has also partnered with recruitment agencies who represent talent who identify as BIPOC. 38% of Milk
Makeup’s employees identify as BIPOC. Those team members are represented in Vice President, Director, Manager, Generalist, and
Specialist roles across the brand. Milk Makeup considers its relationships with employees to be vital, and is focused on creating a great
place to work through the effective attraction, development, retention of and compensation to human resource talent.
Trademarks,
Patents and Domain Names
Milk
Makeup believes that our intellectual property has substantial value and has contributed significantly to the success of our business.
We rely on a combination of trademark, trade dress, copyright and trade secret protection to protect our brands, formulas and other intellectual
property. Milk Makeup’s primary intellectual property includes our brands and trademark rights, including the Milk Makeup brand,
for which we have established strong common law rights in the U.S. in association with cosmetics. The Milk Makeup brand has significant
consumer recognition. Milk Makeup’s trademarks are valuable assets that reinforce the distinctiveness of our brand and its customers’
favorable perception of our products.
Milk Makeup has trademark registrations
and applications pending for stylizations of the Milk Makeup name in Australia, South Korea, China, and the United Kingdom. In addition,
we have trademarks registered and applications pending throughout the world for our stylized logos in Australia, Bahrain, Brazil, Canada,
China, the E.U., Hong Kong, India, Indonesia, Israel, Japan, Kuwait, Malaysia, Mexico, Qatar, Russia, Saudi Arabia, Singapore, South Korea,
Thailand, the UAE, the United Kingdom and the U.S. From time to time, we apply to register trademarks for our other brands in the U.S.
and other countries. The registrations of these trademarks in the U.S. and foreign jurisdictions are generally effective for terms of
ten years and require periodic renewals, which for our trademark registrations in the U.S., are presently scheduled between 2027 and 2031;
provided that Milk Makeup continues to comply with all applicable renewal requirements, including, where necessary, the continued use
of trademarks in connection with the listed goods. In addition to trademark protection, Milk Makeup owns numerous domain name registrations,
including milkmakeup.com. Milk Makeup does not have any issued patents or pending patent applications.
Seasonality
and Quarterly Results
Milk
Makeup’s business is subject to moderate seasonal fluctuations driven by retail consumer purchasing habits and timing of purchases
by our retail customers. Additionally, the COVID-19 pandemic had an impact on consumer behavior that resulted in temporary changes in
the seasonal fluctuations of Milk Makeup’s business. As a result of moderate seasonal fluctuations, results for any interim period
are not necessarily indicative of the results that may be achieved for the full fiscal year. Additionally, because a significant percentage
of our net sales are currently concentrated in a limited number of customers, a change in the order pattern or product restocking by
one or more of our large retail customers driven by new product launches, new store openings and/or promotions could cause a significant
fluctuation of our quarterly results or impact our liquidity.
Government
Regulation
Our
operations and products are subject to regulation by governmental authorities including by the U.S. FDA, FTC, CPSC, as well as various
other federal, state, local and foreign regulatory authorities. These laws and regulations principally relate to the design, development,
manufacturing, processing, handling, testing, holding, storage, quality, safety, shipment, sale, ingredients, labeling, advertising,
packaging, marketing, and disposal of our products.
Product
Regulations — U.S.
Under
the FDCA, cosmetics (including personal care products) are defined as articles or components of articles that are applied to the human
body and intended to cleanse, beautify, promote attractiveness or alter its appearance, with the exception of “soap” as defined
by FDA. The labeling of cosmetic products is also subject to the requirements of the FDCA, the Fair Packaging and Labeling Act,
the Poison Prevention Packaging Act and other federal, state, and local laws and regulations, including without limitation
FDA regulations, and those relating to consumer protection and consumer disclosures. Cosmetics are not subject to pre-market approval
by the FDA, however, certain ingredients, such as color additives, must be pre-authorized.
The
FTC, FDA and other Governmental Authorities also regulate advertising and product claims regarding the safety, performance and benefits
of our products. These regulatory authorities typically require a safety assessment of the product or ingredient, and reasonable basis
to support any marketing claims. As such, product claims must be adequately substantiated. What constitutes a reasonable basis for substantiation
of claims can vary widely from market to market, and there is no assurance that our efforts to support our claims will be considered
sufficient. The most significant area of risk for such activities relates to improper or unsubstantiated claims about the use and safety
of our products. If the products or any ingredient therein is or is alleged to be adulterated or misbranded, or if the safety or effectiveness
of the products or ingredients has not been adequately substantiated, or if any other product claims lack adequate substantiation or
are alleged to be false or misleading, regulators may take enforcement action or impose penalties, such as monetary consumer redress,
or consumer lawyers can bring claims or an action against us, including, among other things, seeking damages, an amendment of the claims,
and/or injunctive or other equitable relief, such as requiring us to revise our marketing materials, preventing us from making certain
claims about the products or ingredients, stop selling certain products, or requiring us to add a specific warning label or disclaimer
about the products or ingredients. Any of which could harm our business, financial condition and results of operations. Other warnings
or disclaimers may also be mandated pursuant to federal or state laws and regulations. We may also be subject to regulatory action if
the FDA or other regulators determine our products, product ingredients, or operations do not comply with any applicable laws or regulations,
and we could be required to stop selling, withdraw, recall, re-label or re-package any products on the market.
Our
advertising for products is also regulated by the FTC under the Federal Trade Commission Act. Cosmetics and personal care products must
be advertised and promoted truthfully and otherwise in compliance with state consumer protection laws prohibiting false advertising and
unfair or deceptive trade practices. Also, under the federal Lanham Act, competitors and others can initiate litigation relating to advertising
claims. A company that is found to have violated these laws may be subject to significant liability.
Regulatory
authorities monitor cosmetic products’ regulatory compliance through market surveillance and inspection of cosmetics manufacturers
and distributors to ensure, among other things, that the products’ labeling and advertising is not false nor misleading and is
compliant with legal requirements, that the products do not contain false nor misleading labeling or harmful ingredients, that they are
manufactured under sanitary conditions, or pursuant to cGMPs. Inspections also may arise from consumer or competitor complaints filed
with or brought to the attention of regulatory authorities, including FDA or FTC. In the event a regulatory authority or a court identifies
false or misleading labeling, unsanitary conditions, harmful ingredients, or otherwise a failure to comply with cGMPs or legal requirements,
we may be requested or required by a regulatory authority or required by a court, or we may independently decide to conduct a recall
or market withdrawal of our product or to make changes to our manufacturing processes or product formulations, labeling or marketing,
which could result in an insufficient amount of our products in the market, impact our sales and/or harm our reputation. Fines or other
payments may also be required by a regulator or a court. The FDA or other regulators may determine that our products cannot be marketed
as is or do not meet the regulatory requirements, including without limitation with respect to labeling, marketing, or ingredient formulation,
for the classification of product in which they are marketed. The FDA may take the position that we failed to satisfy premarket requirements
for color additives, or that our products contain otherwise impermissible ingredients, in which case some or all of our products may
be deemed adulterated or misbranded in violation of the FDCA.
The
FDA evaluates whether a product is a drug, a cosmetic or both, including the “intended use” of a cosmetic product. If a cosmetic
product is intended for use in the diagnosis, cure, mitigation, treatment or prevention of a disease condition or to affect the structure
or function of the human body, the FDA will regulate the product as a drug. Drug products will then be subject to applicable requirements
under the FDCA. The FDA may also consider labeling claims in determining the intended use of a product. If the FDA considers label claims
for our cosmetic products to be claims affecting the structure or function of the human body, or considers label claims for our cosmetics
products to be disease/treatment claims, those products may be regulated as “new” drugs. If such products were regulated
as “new” drugs by the FDA, it would be necessary to obtain pre-market approval, which includes, among other things, conducting
clinical trials to demonstrate safety and efficacy of our products in order to continue marketing those products. However, we may not
have sufficient resources to conduct any required clinical studies and because clinical trial outcomes are uncertain we may not be able
to demonstrate sufficient efficacy or safety data to resume future marketing of those products. Any of these actions could result in
harm to our reputation or affect our ability to provide sufficient product to the market.
What
constitutes a reasonable basis for substantiation of claims can vary widely from market to market, and there is no assurance that our
efforts to support our claims will be considered sufficient. The most significant area of risk for such activities relates to improper
or unsubstantiated claims about the use and safety of our products. If we cannot adequately support safety or substantiate our product
claims, or if our promotional materials make claims that exceed the scope of allowed claims for the classification of the specific product,
the FDA, FTC or other regulatory authority could take enforcement action or impose penalties, such as monetary consumer redress, requiring
us to revise our marketing materials, amend our claims or stop selling certain products, all of which could harm our business, financial
condition and results of operations. Any of these actions could result in harm to our reputation or affect our ability to provide sufficient
product to the market. The FDA may change the regulations as to any product category, requiring a change in labeling, product formulation
or analytical testing. However, we may not have sufficient resources to conduct any required analytical testing, reformulate the product
or make required label changes, possibly resulting in an inability to continue or resume marketing these products. Any inquiries or investigations
from the FDA, FTC or other foreign regulatory authorities into the regulatory status of our cosmetic products and any subsequent interruption
in the marketing and sale of those products could severely damage our brand and company reputation in the marketplace.
We
are also subject to a number of federal, state and foreign laws and regulations that affect companies conducting business on the Internet,
or advertising on social media, including consumer protection regulations that regulate retailers and govern the promotion and sale of
merchandise, including by third parties. Many of these laws and regulations are still evolving and being tested in courts, and could
be interpreted in ways that could harm our business. These may involve user privacy, data protection, content, intellectual property,
distribution, electronic contracts and other communications, competition, protection of minors, consumer protection, telecommunications,
product liability, taxation, economic or other trade prohibitions or sanctions and online payment services. In particular, we are subject
to federal, state and foreign laws regarding privacy and protection of people’s data. U.S. federal and state and foreign laws and
regulations are constantly evolving and can be subject to significant change. In addition, the application, interpretation and enforcement
of these laws and regulations are often uncertain, and may be interpreted and applied inconsistently from country to country and inconsistently
with our current policies and practices.
We
are also subject to regulation by the CPSC under the Consumer Product Safety Act, as amended by the Consumer Product Safety
Improvement Act of 2008. These statutes and the related regulations ban from the market consumer products that fail to comply with applicable
product safety laws, regulations, and standards. The CPSC has the authority to require the recall, repair, replacement or refund of any
such banned products or products that otherwise create a substantial risk of injury and may seek penalties for regulatory noncompliance
under certain circumstances. CPSC regulations also require manufacturers of consumer products to report to the CPSC certain types of
information regarding products that fail to comply with applicable regulations. Certain state laws also address the safety of consumer
products, and mandate reporting requirements, and noncompliance may result in penalties or other regulatory action.
Segments
Milk
Makeup operates its business as a single operating and reportable segment. For more information regarding segment reporting, see the
section titled “Milk’s Management’s Discussion and Analysis of Financial Condition and Results of Operations”
contained elsewhere in this prospectus.
Legal
Proceedings
Milk
Makeup is, from time to time, subject to, and is presently involved in, litigation and other proceedings. Milk Makeup believes that there
are no pending lawsuits or claims that, individually or in the aggregate, may have a material adverse effect on the business, financial
condition or results of operations.
A lawsuit was commenced in
New York state court on June 27, 2016, captioned Joseph v. Rassi, et al., Case No. 510914/2016 (N.Y. Sup. Ct. Oct. 6, 2016) in which the
plaintiff has asserted claims against founders Mazdack Rassi, Erez Shternlicht and Moishe Mana, as well as against Legs Media LLC, Milk
Agency, LLC, Milk Makeup Holdings, LLC, Milk Makeup Management, LLC, Milk Studios, LLC, Milk and Scott Sassa. Plaintiff alleges that Rassi,
Shternlicht, and Mana breached the duty they owed to Legs Media by misappropriating its “corporate opportunity” related to
the Milk Makeup concept for their own benefit. There is a parallel proceeding in Delaware Court of Chancery, captioned Mana v. Joseph,
Civil Action No. 12715 (Del. Ch. Sept. 2, 2016) in which Rassi, Mana, Shternlicht, Legs Media, LLC, Milk Studios, LLC, Milk, and Milk
Agency, LLC have asserted various claims against Joseph and, among other things, are seeking declaratory judgment that Joseph has no ownership
interest in Milk Studios, LLC or Milk. The Delaware litigation is currently stayed pending developments in the New York litigation. On
April 13, 2022, Joseph filed a Motion for Injunctive Relief and to Compel Emergency Discovery seeking an order directing the defendants
to produce certain documents related to the Milk Transaction and enjoining the parties to the Milk Transaction from completing the Milk
Transaction until twenty-one (21) days after the defendants produce requested documents, allegedly to provide Joseph with sufficient time
to determine whether to seek additional relief, including potentially a permanent injunction. Plaintiff’s application for an order
to show cause was rejected, for failure to pay the requisite fee to the court, before the motion was heard. It was never refiled, and
has now been obviated by the consummation of the Business Combination.
Although
Milk’s management believes these allegations are without merit and the possibility of a material impact on its financial conditions
or results of operation is remote, and Milk intends to defend vigorously against such allegations, any adverse determination against
the founders, Milk or its affiliates in these proceedings, or even the allegations contained in the claims, regardless of whether they
are ultimately found to be without merit, may result in distractions to management, reputational harm, injunctive relief, settlement
costs, damages and/or defense costs that could adversely affect our business operations and financial condition. Because Milk’s
management has concluded that an adverse outcome in this litigation is remote at this time, it has not established a contingency reserve
in its financial statements with respect to such litigation in accordance with applicable GAAP accounting standards.
Additionally,
certain founders of Milk have agreed, pursuant to an amended and restated founder support and indemnification agreement, dated January
24, 2019, subject to the terms and conditions thereto, to provide indemnification to Milk and its representatives for losses that arise
from (i) any claims brought by or on behalf of any purported current or former holder of equity or rights in Milk or its affiliates arising
out of facts and circumstances in existence prior to December 23, 2016, (ii) any claims against Milk by or on behalf of any Milk affiliates
arising out of facts and circumstances in existence prior to December 23, 2016, and (iii) Milk’s maintenance and administration
of benefit plans. The indemnification agreement would cover potential losses related to the previously disclosed outstanding litigation
related to “corporate opportunity.”
Management
The
following table sets forth the names, ages and positions of our current directors and executive officers:
Name
|
|
Age |
|
Position |
| Executive
Officers/Directors |
|
|
|
|
| Michel
Brousset |
|
50 |
|
Chief
Executive Officer and Class III Director |
| Hind
Sebti |
|
42 |
|
Chief
Growth Officer and Chief Operating Officer |
| Non-Employee
Directors |
|
|
|
|
| Felipe
Dutra |
|
57 |
|
Chairman and Class III Director |
| Cristiano
Souza |
|
48 |
|
Class
II Director |
| Sarah
Brown |
|
59 |
|
Class
I Director |
| Juliette
Hickman |
|
48 |
|
Class
II Director |
| Lindsay
Pattison |
|
49 |
|
Class
I Director |
| Zack
Werner |
|
33 |
|
Class
I Director |
| Simon
Dai |
|
30 |
|
Class
III Director |
| Aaron
Chatterley |
|
56 |
|
Class
II Director |
Executive
Officers
Michel
Brousset has served as a director on our Board and our Chief Executive Officer since January 2021. Mr. Brousset has more than
25 years of experience leading, operating and building global brands at L’Oréal (PAR: OR) and Procter & Gamble (NYSE:
PG) where he worked to launch and build iconic brands across multiple geographies. Most recently, Mr. Brousset founded Waldencast Ventures
LP (“Waldencast Ventures”), a holding company and investment vehicle, in 2019 and has been the Chief Executive Officer since
its inception. Waldencast Ventures partners with, creates, incubates and accelerates next-generation and early-stage beauty and wellness
brands. Mr. Brousset has led investments in the current and former Waldencast Ventures portfolio companies.
Prior
to founding Waldencast Ventures, Mr. Brousset was the Group President of L’Oréal’s Consumer Products Division in North
America from July 2016 to April 2019. In this role, Mr. Brousset managed each of the Presidents of key L’Oréal brands and
the Presidents and cross-functional teams of L’Oréal Canada CPD and L’Oréal Caribe, as well as the heads of
supply chain, finance, human resources (“HR”), information technology (“IT”), legal, research and development
(“R&D”) and consumer and market intelligence (“CMI”). As the Group President of L’Oréal’s
Consumer Products Division in North America, Mr. Brousset led multiple strategic initiatives and acquisitions.
Additionally,
Mr. Brousset was the Chief Executive Officer and Managing Director of L’Oréal U.K. & Ireland between July 2013 and July
2016, where he managed a broad portfolio of brands and all the divisions of L’Oréal for the U.K. and Ireland. In addition,
he managed across all functional areas including supply chain, finance, HR, IT, CMI, legal and regulatory. Mr. Brousset also spent nearly
14 years at Procter & Gamble in various marketing and brand management roles across North America and Western Europe.
Mr. Brousset currently serves
as Chairman of the board of directors of Kjaer Weis and as a member of the board of directors of several Waldencast Ventures portfolio
companies. Our Board has implemented guidelines, pursuant to which, unless and until we and Waldencast Ventures merge or otherwise become
affiliated entities, Mr. Brousset will spend on average at least 90% of his monthly average working time providing services to us, such
that a maximum of 10% may be spent to provide services to Waldencast Ventures. Mr. Brousset holds a B.S. in Economics from the Universidad
del Pacífico in Peru and an M.B.A. from the University of North Carolina – Kenan-Flagler Business School.
Hind Sebti has
served as our Chief Operating Officer since February 2021 and Chief Growth Officer since July 2022. Ms. Sebti has more than 20 years of
experience leading and managing beauty brands across multiple categories and stages during her tenures at L’Oréal (PAR: OR)
and Procter & Gamble (NYSE: PG). Ms. Sebti co-founded Waldencast Ventures alongside Mr. Brousset in 2019. Ms. Sebti brings in-depth
knowledge and understanding of the beauty industry as well as consumer insights to identify and invest in the next-generation beauty brands.
Importantly, Ms. Sebti plays a key role in helping portfolio brands scale, leveraging her extensive multi-category and brand management
experience. Since January 2020, Ms. Sebti has also served as Chief Executive Officer of Waldencast Brands, a subsidiary of Waldencast
Ventures, to incubate and commercialize new brands. Specifically, Ms. Sebti leads the brand creation process, with a focus on creative
and operational optimization, through all stages from conception and product development to go-to-market strategy. Our Board has implemented
guidelines, pursuant to which, unless and until we and Waldencast Ventures merge or otherwise become affiliated entities, Ms. Sebti will
spend on average at least 80% of her monthly average working time providing services to us, such that a maximum of 20% may be spent to
provide services to Waldencast Ventures.
Prior
to Waldencast Ventures, Ms. Sebti held various leadership positions at L’Oréal from April 2013 to December 2018. She was
the General Manager for Maybelline and Essie in the United Kingdom from July 2017 to December 2018. She also held the position of General
Manager of professional haircare brands Redken, Pureology and Mizani from September 2015 to July 2017, where she focused on digitalization
and consumer centricity to drive growth. Ms. Sebti began her tenure at L’Oréal as the Marketing Director of L’Oréal
Paris and Consumer Division Category Director. Prior to L’Oréal, Ms. Sebti held various Business Leader and Brand Manager
positions at Procter & Gamble in the U.K., Ireland and France across brands such as Olay Skin Care and Gillette Venus from January
2002 to March 2013. Ms. Sebti serves as a member of the board of directors of Cosmetic Executive Women U.K. and holds a Master’s
Degree in Industrial Engineering from The National Institute of Applied Science of Lyon.
Non-Employee
Directors
Felipe Dutra has
served as a director on our Board since January 2021 and the Chairman of our Board since July 2022. Mr. Dutra served as the Chief Financial
Officer at Anheuser-Busch InBev (Euronext: ABI) (NYSE: BUD) (MEXBOL: ANB) (JSE: ANH) from January 2005 to April 2020. As CFO of AmBev,
Mr. Dutra led large-scale acquisitions with transaction values exceeding $100.0 billion, as well as numerous buyouts of smaller breweries,
and oversaw the initial public offering of Budweiser APAC (HKG: 1876) on the Hong Kong Stock Exchange. In addition to serving as the Chief
Financial Officer, Mr. Dutra also served as the Chief Technology Officer from 2014 until 2020 to lead the company’s adoption of
digital technology and implementation of data analytics. In this capacity, Mr. Dutra drove the creation of AB InBev’s technology
function by building capabilities to enable digital transformation while creating a network of service centers globally.
Mr. Dutra started his career
at Aracruz Celulose, a Brazilian manufacturer of pulp and paper products. Mr. Dutra served as a member of the board of directors of AmBev
(BOVESPA: ABEV) (NYSE: ABEV) from January 2005 to March 2021 and as a member of the board of directors of Grupo Modelo from December 2010
to June 2013 and Budweiser APAC from September 2019 to June 2020. He holds a degree in Economics from Candido Mendes University and an
M.B.A. from Universidade de São Paulo in Brazil.
Cristiano
Souza has served as a director on our Board since January 2021. Mr. Souza is a senior partner at Dynamo Capital LLP (“DCL”).
Based out of the United Kingdom, DCL is the investment advisor of the Dynamo Fund, an investment fund focused on long-term equity investments.
In addition to providing capital, DCL also collaborates with portfolio companies to help find ways to add and maximize value over time.
Mr.
Souza is also a partner at Dynamo Administração de Recursos (“DAR”), a Brazilian investment manager established
in 1993 focused on long-term equity investments in Brazil. Mr. Souza joined DAR in 1994 and was involved in its investing activities
until 2014 when he relocated to the United Kingdom to focus on the investment advisory of Dynamo Fund. Mr. Souza holds a Bachelor’s
degree in Economics from Candido Mendes University in Rio de Janeiro.
Aaron Chatterley has
served as an independent director on our Board since December 2021. Mr. Chatterley founded the web development company SP New Media in
1996, where he served as the Chief Executive Officer until selling the company in 2000. In 2005, Mr. Chatterley co-founded the online
beauty retailer, feelunique, where he served as the Chief Executive Officer until April 2014. Mr. Chatterley led the partial sale of feelunique
to Palamon Capital Partners in December 2012, as well as the sale of feelunique to LVMH/Sephora in September 2021. In addition, since
2016 Mr. Chatterley has served as a Non-Executive Director of Digital Jersey, an economic development agency, and currently serves as
an audit and risk committee member. Mr. Chatterley also serves as an Ambassador for The Prince’s Trust Women Supporting Women, a
youth charity organization.
Sarah
Brown has served as an independent director on our Board since March 2021. Ms. Brown’s work brings together the worlds
of business, philanthropy, non-profit activism, and youth campaigning. She is the Chair of Theirworld, a global children’s charity
dedicated to ending the global education crisis. She also serves as the Executive Chair of the Global Business Coalition for Education.
She is the Chief Executive Officer of the Office of Gordon and Sarah Brown established in 2010 after Gordon Brown’s premiership
ended in the U.K. Ms. Brown also serves as a Non-Executive Director of Harrods Group Holdings Ltd. Ms. Brown is the author of Behind
the Black Door, a personal memoir. She holds a Bachelor’s of Science degree in Psychology from the University of Bristol. Ms. Brown
was awarded fellowship from the Royal College of Obstetricians and Gynecologists and of the Royal College of Pediatrics and Child Health.
Juliette
Hickman has served as an independent director on our Board since March 2021. Ms. Hickman is a former investment analyst and
investor at Capital World Investors, part of The Capital Group Companies. She joined The Capital Group in 1998 and held the role of investment
analyst and investor initially focusing on the Global Beverage industry until 2020. Ms. Hickman has served as an independent director
for Montanya Distillers since 2019 and an independent director for Keurig Dr. Pepper since January 2021. Ms. Hickman holds a Bachelor’s
of Arts degree in Politics and Public Administration from the Nottingham Trent University.
Lindsay
Pattison has served as an independent director on our Board since March 2021. Ms. Pattison has years of experience in the fields
of marketing, advertising and business-transformation. She was appointed in 2018 as the global Chief Client Officer at WPP PLC, a leading
marketing services organization. Previously, Ms. Pattison was GroupM’s, and then WPP’s, Chief Transformation Officer. She
was the Global Chief Executive Officer of Maxus, a WPP media agency, from 2014 until 2021. Her experience also includes roles at Young
and Rubicam and PHD Media, as well as a client-side role with Sony Ericsson. She serves on the board of directors at the communications
company Chime Ltd and at the international design agency Design Bridge. She served twice on the WEF Global Agenda Council on the Future
of Media. As a passionate and vocal campaigner for gender equality, she launched ‘Walk the Talk,’ an initiative to help senior
women at Maxus to thrive and make progress in their careers - a program now adopted globally by WPP. She sits on WPP’s Inclusion
Council and Risk Committee. Ms. Pattison holds a Bachelor’s of Arts in English Literature from the University of Stirling and completed
the TLC Leaders Program, a leadership course delivered by members of the faculty of Harvard Business School.
Zack
Werner has served as an independent director on our Board since March 2021. Mr. Werner founded The Maze Group in 2016, a highly
technical strategic consultancy focused on data architecture and driving growth through digital marketing. Maze partners with private
equity owned and public clients such as LVMH, HelloFresh, JC Penney, General Electric, and Pat McGrath Labs to optimize customer acquisition,
conversion rate, and retention as well as provide strategies around technology platform and infrastructure transformation. The Maze Group
also partners with private equity clients to co-invest in consumer companies. Mr. Werner also started his career at Universal Music Group
from 2011 until 2013, where he focused on digital distribution deals, customer relationship management and integrated marketing systems.
In addition, in 2017, Mr. Werner became an advisor for Stadium Goods, a sneaker and streetwear marketplace, to oversee e-commerce and
growth.
Simon
Dai has served as a director on our Board since the consummation of the Business Combination. Mr. Dai has served on the board
of directors of Obagi since September 2019, including as its Chairman since July 2020, and has led several investments in the healthcare
space. Since January 2020, Mr. Dai has served as the Co-Chairman and Chief Executive Officer of Presbia PLC, a medical device company
focused on the development of the presbyopia-correcting lens, an innovative solution for the common age-related loss of the ability to
read or focus on near objects. He also co-founded Oxford MEStar in October 2013, a spin-out company from the Institute of Biomedical
Engineering of Oxford University specializing in automation solution, serving as its Chief Executive Officer from October 2016 until
August 2020. Previously, Mr. Dai focused on impact investing at Bill & Melinda Gates Foundation, where he was a Liaison Officer based
in Ethiopia. Mr. Dai received a BA in Sociology from Manchester University, an MSc. in Finance from the London School of Economics and
an MBA from the UCLA Anderson School of Management.
Mr.
Dai has been appointed to our Board by Cedarwalk pursuant to the Investor Rights Agreement. For additional information regarding the
Investor Rights Agreement, see the section entitled “Certain Relationships and Related Party Transactions—Obagi—Obagi
China Distribution—Investor Rights Agreement.”
Family
Relationships
There
are no family relationships between any of our executive officers and directors.
Corporate
Governance
Composition
of the Board of Directors
Our
business and affairs are managed under the direction of our Board. As of the date of this prospectus, our Board consists of nine directors.
Subject to the terms of the Investor Rights Agreement, our Constitutional Document provides that the number of directors on our Board
will be fixed by our Board.
When
considering whether directors and director nominees have the experience, qualifications, attributes and skills, taken as a whole, to
enable our Board to satisfy its oversight responsibilities effectively in light of its business and structure, our Board focuses primarily
on each person’s background and experience in order to provide an appropriate mix of experience and skills relevant to the size
and nature of the business.
Pursuant
to our Investor Rights Agreement with Cedarwalk, the Sponsor and CWC Skincare Ltd., the guarantor of Cedarwalk’s obligation thereunder,
we have agreed to take all necessary action to cause our Board to be comprised of one director nominated by Cedarwalk for as long as
Cedarwalk owns 5% of our then outstanding common stock. Mr. Simon Dai has been elected as the initial director nominee of Cedarwalk,
and serves as a Class II director. See the section entitled “Certain Relationships and Related Party Transactions—Obagi—Obagi
China Distribution—Investor Rights Agreement.”
Classified
Board of Directors
In
accordance with the terms of our Constitutional Document, our Board may consist of no less than five, but no more than 15 natural persons,
such number to be set by the Board by resolution from time to time. Our Board is divided into classes of directors that will serve staggered
three-year terms. At each annual meeting of stockholders, a class of directors will be elected for a three-year term to succeed the same
class whose term is then expiring. As a result, only one class of directors will be elected at each annual meeting of our stockholders,
with the other classes continuing for the remainder of their respective three-year terms. Our Board is divided among the three classes
as follows:
| ● | the
Class I directors, which are Lindsay Pattison, Zack Werner and Sarah Brown, and their terms
will expire at the first annual meeting of stockholders to be held after the consummation
of the Business Combination; |
| | | |
| ● | the
Class II directors, which are Aaron Chatterley, Juliette Hickman and Cristiano Souza, and
their terms will expire at the second annual meeting of stockholders to be held after the
consummation of the Business Combination; and |
| | | |
| ● | the
Class III directors, which are Michel Brousset, Felipe Dutra and Simon Dai, and their terms
will expire at the third annual meeting of stockholders to be held after the consummation
of the Business Combination. |
Each
director’s term will continue until the election and qualification of his or her successor, or his or her earlier death, resignation
or removal. Any increase or decrease in the number of directors will be distributed among the three classes so that, as nearly as possible,
each class will consist of one-third of the directors. This classification of our Board may have the effect of delaying or preventing
changes in control our company.
Director
Independence
As
a result of our common stock being listed on the Nasdaq, we are required to comply with the applicable rules of the exchange in determining
whether a director is independent. We believe that each of Ms. Sarah Brown, Ms. Juliette Hickman, Ms. Lindsay Pattison, Mr. Zack Werner
and Mr. Aaron Chatterley qualifies as “independent” as defined under the applicable Nasdaq rules.
Foreign
Private Issuer Status
We
are domesticated as a public limited company under the laws of Jersey. We report under the Exchange Act as a non-U.S. company with foreign
private issuer status. Under Rule 405 of the Securities Act, the determination of foreign private issuer status is made annually on the
last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, we will make our next determination
on June 30, 2023. For so long as we qualify as a foreign private issuer, we will be exempt from certain provisions of the Exchange Act
that are applicable to U.S. domestic public companies, including:
| ● | the
sections of the Exchange Act requiring insiders to file public reports of their stock ownership
and trading activities and imposing liability for insiders who profit from trades made within
a short period of time; |
| | | |
| ● | the
sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations
in respect of a security registered under the Exchange Act; |
| | | |
| ● | the
rules under the Exchange Act requiring the filing with the SEC of an annual report on Form
10-K (although we will file annual reports on a corresponding form for foreign private issuers),
quarterly reports on Form 10-Q containing unaudited financial and other specified information
(although we will file semi-annual financial information on a current reporting form for
foreign private issuers), or current reports on Form 8-K, upon the occurrence of specified
significant events; and |
| | | |
| ● | Regulation
Fair Disclosure or Regulation FD, which regulates selective disclosure of material non-public
information by issuers. |
Accordingly,
there may be less publicly available information concerning our business than there would be if we were a U.S. public company. Additionally,
certain accommodations in the Nasdaq corporate governance standards allow foreign private issuers, such as us, to follow “home
country” corporate governance practices in lieu of the otherwise applicable corporate governance standards. We do not currently
rely on any “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards.
Committees
of the Board of Directors
Our
Board directs the management of our business and affairs, as provided by Jersey law, and conducts its business through meetings of our
Board and standing committees. We have three standing committees — an audit committee, a compensation committee and a nominating
and governance committee.
In
addition, from time to time, special committees may be established under the direction of our Board when it deems it necessary or advisable
to address specific issues. Copies of the charters for each committee are available on our website, waldencast.com, as required by applicable
SEC and Nasdaq rules. The information on or available through our website is not deemed incorporated in this prospectus and does not
form part of this prospectus.
Audit
Committee
The
audit committee’s responsibilities include, among other things:
| ● | assisting
board oversight of (1) the integrity of our financial statements, (2) our compliance with
legal and regulatory requirements, (3) our independent auditor’s qualifications and
independence and (4) the performance of our internal audit function and independent auditors; |
| | | |
| ● | the
appointment, compensation, retention, replacement and oversight of the work of the independent
auditors and any other independent registered public accounting firm engaged by us; |
| | | |
| ● | pre-approving
all audit and non-audit services to be provided by the independent auditors or any other
registered public accounting firm engaged by us, and establishing pre-approval policies and
procedures; |
| | | |
| ● | reviewing
and discussing with the independent auditors all relationships the auditors have with us
in order to evaluate their continued independence; |
| | | |
| ● | monitoring
compliance by the independent auditors with the audit partner rotation requirements contained
in applicable laws and regulations; |
| | | |
| ● | monitoring
our compliance with the employee conflict of interest requirements contained in applicable
laws and regulations; |
| | | |
| ● | obtaining
and reviewing a report from the independent auditors describing (1) all critical accounting
policies and practices to be used; (2) any critical audit matters arising from the current
period audit; (3) all alternative treatments of financial information that have been discussed
by the independent auditors and management, ramifications of the use of such alternative
disclosures and treatments, and the treatment preferred by the independent auditors; (4)
all other material written communications between the independent auditors and management,
such as any management letter and any schedule of unadjusted audit differences; and (5) any
material financial arrangements which do not appear on our financial statements; |
| | | |
| ● | reviewing
the adequacy and effectiveness of our accounting and internal control policies and procedures
on a regular basis; |
| | | |
| ● | obtaining
and reviewing a report, at least annually, from our management, attested to by the independent
auditors, assessing the effectiveness of our internal control over financial reporting and
stating management’s responsibility for establishing and maintaining adequate internal
control over financial reporting prior to its inclusion in our Annual Report on Form 20-F
or Form 10-K, as applicable; |
| | | |
| ● | meeting
to review and discuss our annual audited financial statements and quarterly financial statements
with management and the independent auditor, including reviewing our specific disclosures
under the section entitled “Waldencast’s Management’s Discussion and
Analysis of Financial Condition and Results of Operations”; |
| | | |
| ● | reviewing
and approving any related party transaction required to be disclosed pursuant to Item 404
of Regulation S-K promulgated by the SEC prior to us entering into such transaction; and |
| | | |
| ● | reviewing
with our legal advisors, when appropriate, any legal, regulatory matters, including any matters
(i) that may have a material impact on our financial statements and (ii) involving potential
or ongoing material violations of law or breaches of fiduciary duty by us or any of our directors,
officers, employees, or agents or breaches of fiduciary duty to us. |
The
audit committee consists of Juliette Hickman, Sarah Brown and Zack Werner, with Juliette Hickman serving as chair. Our Board has determined
that each of the members of the audit committee qualifies as independent under the Nasdaq rules applicable to members of our Board generally
and under the Nasdaq rules and Exchange Act Rule 10A-3 specific to audit committee members and that each of the members of the audit
committee meets the requirements for financial sophistication under the applicable Nasdaq rules. In addition, our Board has determined
that Juliette Hickman qualifies as an “audit committee financial expert,” as such term is defined in Item 407(d)(5) of Regulation
S-K.
Compensation
Committee
The
functions of the compensation committee include:
| ● | reviewing
and approving on an annual basis the corporate goals and objectives relevant to our Chief
Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance
in light of such goals and objectives and determining and approving the remuneration (if
any) of our Chief Executive Officer based on such evaluation; |
| | | |
| ● | reviewing
and making recommendations to our Board with respect to the compensation, and any incentive-compensation
and equity-based plans that are subject to board approval of all of our other officers; |
| | | |
| ● | reviewing
our executive compensation policies and plans; |
| | | |
| ● | implementing
and administering our incentive compensation equity-based remuneration plans; |
| | | |
| ● | assisting
management in complying with our proxy statement and annual report disclosure requirements; |
| | | |
| ● | approving
all special perquisites, special cash payments and other special compensation and benefit
arrangements for our officers and employees; |
| | | |
| ● | producing
a report on executive compensation to be included in our annual proxy statement; and |
| | | |
| ● | reviewing,
evaluating and recommending changes, if appropriate, to the remuneration for directors. |
The
compensation committee consists of Lindsay Pattison, Zack Warner and Juliette Hickman, with Lindsay Pattison serving as chair. Our Board
has determined that each of the members of the compensation committee meets the independence requirements under Nasdaq and SEC rules.
Our Board has determined that each member of this committee will also be a “non-employee director” within the meaning of
Rule 16b-3 under the Exchange Act.
The
composition and function of the compensation committee complies with all applicable requirements of the Sarbanes-Oxley Act and all applicable
SEC rules and regulations. We will comply with future requirements to the extent they become applicable.
Nominating
and Corporate Governance Committee
The
functions of the nominating and corporate governance committee include:
| ● | identifying,
screening and reviewing individuals qualified to serve as directors, consistent with criteria
approved by our Board, and recommending to our Board candidates for nomination for appointment
at the annual general meeting or to fill vacancies on our Board; |
| | | |
| ● | developing
and recommending to our Board, and overseeing implementation of, our corporate governance
guidelines; |
| | | |
| ● | coordinating
and overseeing the annual evaluation of our Board, as whole, and management, and evaluating
and reporting to our Board on the performance and effectiveness of our Board and each of
our committees; and |
| | | |
| ● | reviewing
on a regular basis our overall corporate governance and recommending improvements as and
when necessary. |
The
nominating and corporate governance committee consists of Sarah Brown, Zack Werner and Lindsay Pattison, with Sarah Brown serving as
the chair. Our Board has determined that each of the members of the nominating and governance committee meet the independence requirements
under Nasdaq and SEC rules.
The
composition and function of the nominating and governance committee comply with all applicable requirements of the Sarbanes-Oxley Act
and all applicable SEC rules and regulations. We will comply with future requirements to the extent they become applicable.
Code
of Ethics
We
have a code of ethics and business conduct that applies to all of our directors, officers and employees. The code of ethics is available
on our website, waldencast.com. The information on or available through our website is not deemed incorporated in this prospectus and
does not form part of this prospectus. We intend to make any legally required disclosures regarding amendments to, or waivers of, the
provisions of its code of ethics on our website rather than by filing a Current Report on Form 6-K.
Compensation
Committee Interlocks and Insider Participation
None
of the members of the compensation committee is currently, or has been at any time, one of our officers or employees. None of our executive
officers currently serves, or has served during the last year, as a member of the board of directors or compensation committee of any
entity that has one or more executive officers serving as a member of our Board or compensation committee.
Executive
Compensation
Fiscal
Year 2021 Executive Officer and Director Compensation
During
the year ended on December 31, 2021, none of our directors or executive officers had received any cash compensation for services rendered
us. Our directors and executive officers were reimbursed for any out-of-pocket expenses incurred in connection with activities on our
behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. As of December
31, 2021, our officers and directors and their affiliates were entitled to approximately $1.5 million in reimbursable out-of-pocket expenses
including repayment of amounts pursuant to Working Capital Loans.
As
of the date of this prospectus, we are not party to any agreements with our directors or officers that provide for benefits upon termination
of employment.
Our
2022 Incentive Award Plan
Our
2022 Incentive Award Plan (the “2022 Plan”) became effective upon the consummation of the Business Combination. The purpose
of the 2022 Plan is to provide an additional incentive to officers, employees, non-employee directors and consultants of us or our affiliates
whose contributions are essential to our growth and success, in order to strengthen the commitment of such persons to us and our affiliates,
motivate such persons to faithfully and diligently perform their responsibilities, and attract and retain competent and dedicated persons
whose efforts will result in the long term growth and profitability of us and our affiliates.
This
section summarizes certain principal features of the 2022 Plan.
Administration
and Eligibility
Officers,
employees, non-employee directors and consultants of us and our affiliates are eligible to receive awards under the 2022 Plan. The Board
will administer the 2022 Plan unless they appoint a committee of directors to administer certain aspects of the 2022 Plan. The Board
or committee administering the 2022 Plan is referred to herein as the “plan administrator.” Subject to applicable laws and
regulations, the plan administrator is authorized to delegate its administrative authority under the 2022 Plan to an officer of the Company
or other individual or group.
The
plan administrator will have the authority to exercise all powers either specifically granted under the 2022 Plan or necessary and advisable
in the administration of the 2022 Plan, including, without limitation: (i) to select those eligible recipients who will be granted awards;
(ii) to determine whether and to what extent awards are to be granted hereunder to participants; (iii) to determine the number of our
ordinary shares or cash to be covered by each award; (iv) to determine the terms and conditions, not inconsistent with the terms of the
2022 Plan, of each award granted thereunder; (v) to determine the terms and conditions, not inconsistent with the terms of the 2022 Plan,
which govern all written instruments evidencing awards; (vi) to determine the fair market value in accordance with the terms of the 2022
Plan; (vii) to determine the duration and purpose of leaves of absence which may be granted to a participant without constituting termination
of the participant’s employment or service for purposes of awards; (viii) to adopt, alter and repeal such administrative rules,
guidelines and practices governing the 2022 Plan as it will from time to time deem advisable; (ix) to prescribe, amend and rescind rules
and regulations relating to sub-plans established for the purpose of satisfying applicable foreign laws or qualifying for favorable tax
treatment under applicable foreign laws, which rules and regulations may be set forth in an appendix or appendices to the 2022 Plan;
and (x) to construe and interpret the terms and provisions of the 2022 Plan and any award issued under the 2022 Plan (and any award agreement
relating thereto), and to otherwise supervise the administration of the 2022 Plan and to exercise all powers and authorities either specifically
granted under the 2022 Plan or necessary and advisable in the administration of the 2022 Plan.
Shares
Available and Limitation on Awards
A
maximum of 16,134,716 ordinary shares are reserved for issuance under the 2022 Plan (the “Share Reserve”); provided, however,
that the Share Reserve will automatically increase on January 1st of each calendar year (each, an “Evergreen Date”), prior
to the tenth anniversary of the Effective Date (as such term is defined in the 2022 Plan), in an amount equal to the lesser of (i) 3%
of the total number of our ordinary shares issued and outstanding on the December 31st immediately preceding the applicable Evergreen
Date and (ii) a number of our ordinary shares determined by the plan administrator, including zero. Up to the number of our ordinary
shares reserved for issuance under the 2022 Plan as of the Effective Date may be granted as incentive stock options (“ISOs”).
Our
ordinary shares subject to an award under the 2022 Plan that remain unissued upon the cancellation, termination or expiration of the
award will again become available for grant under the 2022 Plan. However, our ordinary shares that are exchanged by a participant or
withheld by us as full or partial payment in connection with any award under the 2022 Plan, as well as any of our ordinary shares exchanged
by a participant or withheld by us to satisfy the tax withholding obligations related to any award, will not be available for subsequent
awards under the 2022 Plan. To the extent an award is paid or settled in cash, the number of our ordinary shares previously subject to
the award will again be available for grants pursuant to the 2022 Plan. To the extent that an award can only be settled in cash, such
award will not be counted against the total number of our ordinary shares available for grant under the 2022 Plan.
No
non-employee director may be paid, issued, or granted in any one calendar year, equity awards (including any awards issued under the
2022 Plan) and any cash fees with respect to such calendar that, in the aggregate, exceed $400,000 in total value (the value of which
will be based on their grant date fair value of such equity awards for our financial reporting purposes).
Equitable
Adjustments
The
2022 Plan provides that, in the event of a merger, consolidation, reclassification, recapitalization, spin-off, spin-out, repurchase,
reorganization, special or extraordinary dividend, combination or exchange of shares, change in corporate structure or a similar corporate
event affecting our ordinary shares (in each case, a “Change in Capitalization”), the plan administrator will make, in its
sole discretion, an equitable substitution or proportionate adjustment in (i) the number of our ordinary shares reserved under the 2022
Plan, (ii) the kind and number of securities subject to, and the exercise price or base price of, any outstanding options and stock appreciation
rights (“SARs”) granted under the 2022 Plan, (iii) the kind, number and purchase price of ordinary shares, or the amount
of cash or amount or type of property, subject to outstanding restricted stock, restricted stock units, stock bonuses and other share-based
awards granted under the 2022 Plan and (iv) the performance goals and performance periods applicable to any awards granted under the
2022 Plan. The plan administrator will make other equitable substitutions or adjustments as it determines in its sole discretion.
In
addition, in the event of a Change in Capitalization (including a change in control, as described below), the plan administrator may
cancel any outstanding awards for the payment of cash or in-kind consideration. However, if the exercise price or base price of any outstanding
award is equal to or greater than the fair market value of our ordinary shares, cash or other property covered by such award, the Board
may cancel the award without the payment of any consideration to the holder.
Awards
Restricted
stock units, which we refer to as “RSUs,” and restricted stock may be granted under the 2022 Plan. The plan administrator
will determine the purchase price, vesting schedule and performance objectives, if any, applicable to the grant of RSUs and restricted
stock. If the restrictions, performance objectives or other conditions determined by the plan administrator are not satisfied, the RSUs
and restricted stock will be forfeited. Subject to the provisions of the 2022 Plan and the applicable individual award agreement, the
plan administrator may provide for the lapse of restrictions in installments or the acceleration or waiver of restrictions (in whole
or part) under certain circumstances as set forth in the applicable individual award agreement, including the attainment of certain performance
goals, a participant’s termination of employment or service, or a participant’s death or disability. The rights of RSU and
restricted stock holders upon a termination of employment or service will be set forth in individual award agreements.
Unless
the applicable award agreement provides otherwise, participants with restricted stock will generally have all of the rights of a shareholder
during the restricted period, including the right to vote and receive dividends declared with respect to such restricted stock, provided
that any dividends declared during the restricted period with respect to such restricted stock will generally only become payable if
the underlying restricted stock vests. During the restricted period, participants with RSUs will generally not have any rights of a shareholder,
but, if the applicable individual award agreement so provides, may be credited with dividend equivalent rights that will be paid at the
time that our ordinary shares in respect of the related RSUs are delivered to the participant.
We
may issue stock options under the 2022 Plan. Options granted under the 2022 Plan may be in the form of non-qualified options or “incentive
stock options” within the meaning of Section 422 of the Code as set forth in the applicable individual option award agreement.
Except as set forth in the applicable award agreement, the exercise price of all options granted under the 2022 Plan will be determined
by the plan administrator, but in no event may the exercise price be less than 100% of the fair market value of our related ordinary
shares on the date of grant. The maximum term of all stock options granted under the 2022 Plan will be determined by the plan administrator,
but may not exceed eleven years. Each stock option will vest and become exercisable (including in the event of the optionee’s termination
of employment or service) at such time and subject to such terms and conditions as determined by the plan administrator in the applicable
individual option agreement.
SARs
may be granted under the 2022 Plan either alone or in conjunction with all or part of any option granted under the 2022 Plan. A free-standing
SAR granted under the 2022 Plan entitles its holder to receive, at the time of exercise, an amount per share equal to the excess of the
fair market value (at the date of exercise) of an ordinary share over the base price of the free-standing SAR. A SAR granted in conjunction
with all or part of an option under the 2022 Plan entitles its holder to receive, at the time of exercise of the SAR and surrender of
the related option, an amount per share equal to the excess of the fair market value (at the date of exercise) of an ordinary share over
the exercise price of the related option. Except as set forth in the applicable award agreement, each SAR will be granted with a base
price that is not less than 100% of the fair market value of our related ordinary shares on the date of grant. The maximum term of all
SARs granted under the 2022 Plan will be determined by the plan administrator, but may not exceed ten years. The plan administrator may
determine to settle the exercise of a SAR in our ordinary shares, cash, or any combination thereof.
Each
free-standing SAR will vest and become exercisable (including in the event of the SAR holder’s termination of employment or service)
at such time and subject to such terms and conditions as determined by the plan administrator in the applicable individual free-standing
SAR agreement. SARs granted in conjunction with all or part of an option will be exercisable at such times and subject to all of the
terms and conditions applicable to the related option.
Other
stock-based awards, valued in whole or in part by reference to, or otherwise based on, our ordinary shares (including dividend equivalents)
may be granted under the 2022 Plan. Any dividend or dividend equivalent awarded under the 2022 Plan will be subject to the same restrictions,
conditions and risks of forfeiture as the underlying awards and will only become payable if the underlying awards vest. The plan administrator
will determine the terms and conditions of such other stock-based awards, including the number of our ordinary shares to be granted pursuant
to such other stock-based awards, the manner in which such other stock-based awards will be settled (e.g., in our ordinary shares or
cash or other property), and the conditions to the vesting and payment of such other stock-based awards (including the achievement of
performance objectives).
Bonuses
payable in fully vested ordinary shares and awards that are payable solely in cash may also be granted under the 2022 Plan.
The
plan administrator may grant equity-based awards and incentives under the 2022 Plan that are subject to the achievement of performance
objectives selected by the plan administrator in its sole discretion, including, without limitation, one or more of the following business
criteria: (i) earnings, including one or more of operating income, net operating income, earnings before or after taxes, earnings before
or after interest, depreciation, amortization, adjusted EBITDA, economic earnings, or extraordinary or special items or book value per
share (which may exclude nonrecurring items); (ii) pre-tax income or after-tax income; (iii) earnings per share (basic or diluted); (iv)
operating profit; (v) revenue, revenue growth or rate of revenue growth; (vi) return on assets (gross or net), return on investment,
return on capital, or return on equity; (vii) returns on sales or revenues; (viii) operating expenses; (ix) stock price appreciation;
(x) cash flow, free cash flow, cash flow return on investment (discounted or otherwise), net cash provided by operations, or cash flow
in excess of cost of capital; (xi) implementation or completion of critical projects or processes; (xii) cumulative earnings per share
growth; (xiii) operating margin or profit margin; (xiv) stock price or total stockholder return; (xv) cost targets, reductions and savings,
productivity and efficiencies; (xvi) strategic business criteria, consisting of one or more objectives based on meeting specified market
penetration, geographic business expansion, customer satisfaction, employee satisfaction, human resources management, supervision of
litigation, and information technology goals, and goals relating to acquisitions, divestitures, joint ventures and similar transactions,
and budget comparisons; (xvii) personal professional objectives, including any of the foregoing performance goals, the implementation
of policies and plans, the negotiation of transactions, the development of long-term business goals, formation of joint ventures, research
or development collaborations, and the completion of other corporate transactions; and (xviii) any combination of, or a specified increase
in, any of the foregoing. However, non-employee directors may not be granted awards during any calendar year that, when aggregated with
such non-employee director’s cash fees during such calendar year, exceed $750,000 in total value.
The
business criteria may be expressed in terms of attaining a specified level of the particular criteria or the attainment of a percentage
increase or decrease in the particular criteria, and may be applied to us or any of our affiliates, or one of our divisions or strategic
business units or a division or strategic business unit of any of our affiliates, or may be applied to our performance relative to a
market index, a group of other companies or a combination thereof, all as determined by the plan administrator. The business criteria
may also be subject to a threshold level of performance below which no payment will be made, levels of performance at which specified
payments will be made, and a maximum level of performance above which no additional payment will be made. The plan administrator will
have the authority to make equitable adjustments to the business criteria, as may be determined by the plan administrator in its sole
discretion including, without limitation, in the event of acquisitions, dispositions or other corporate events.
Certain
Transactions
In
the event that a “change in control” (as such term is defined in the 2022 Plan) occurs, each award granted under the 2022
Plan will continue to operate in accordance with its terms, subject to adjustment (including, without limitation, assumption or conversion
into equivalent awards of the acquirer’s equity) as described above with respect to Changes in Capitalization.
Except
as provided in the applicable award agreement, if (i) a change in control occurs and (ii) either (x) an outstanding award is not assumed
or substituted in connection with such change in control or (y) an outstanding award is assumed or substituted in connection with such
change in control and a participant’s employment or service is terminated without cause or by the participant for good reason (if
applicable) within 12 months following the change in control, then (i) any unvested or unexercisable portion of an award carrying a right
to exercise will become fully vested and exercisable and (ii) the restrictions, deferral limitations, payment conditions and forfeiture
conditions applicable to any other award granted under the 2022 Plan will lapse, the awards will vest in full and any performance conditions
will be deemed to be achieved at target performance levels.
For
purposes of the 2022 Plan, an outstanding award will be considered to be assumed or substituted for if, following the change in control,
the award remains subject to the same terms and conditions that were applicable to the award immediately prior to the change in control
except that, if the award related to our ordinary shares, the award instead confers the right to receive ordinary shares of the acquiring
entity (or such other security or entity as may be determined by the plan administrator, in its sole discretion).
Withholding
Taxes
Each
participant will be required to make arrangements satisfactory to the plan administrator regarding payment of an amount up to the maximum
statutory rates in the participant’s applicable jurisdictions with respect to any award granted under the 2022 Plan, as determined
by us. We have the right, to the extent permitted by law, to deduct any such taxes from any payment of any kind otherwise due to the
participant. With the approval of the plan administrator, the participant may satisfy the foregoing requirement by either electing to
have us withhold from delivery of our ordinary shares, cash or other property, as applicable, or by delivering already owned unrestricted
ordinary shares, in each case, having a value not exceeding the applicable taxes to be withheld and applied to the tax obligations. We
may also use any other method of obtaining the necessary payment or proceeds, as permitted by law, to satisfy our withholding obligation
with respect to any award.
Amendment,
Termination and Clawback Provisions
The
2022 Plan provides the Board with the authority to amend, alter or terminate the 2022 Plan, but no such action may adversely affect the
rights of any participant with respect to outstanding awards without the participant’s consent. The plan administrator may amend
an award, prospectively or retroactively, but no such amendment may adversely affect the rights of any participant without the participant’s
consent. Shareholder approval of any such action will be obtained if required to comply with applicable law.
All
awards will be subject to the provisions of any clawback policy implemented by us to the extent set forth in such clawback policy, and
will be further subject to such deductions and clawbacks as may be required to be made pursuant to any law, government regulation or
stock exchange listing requirement.
Term
No
award will be granted pursuant to the 2022 Plan on or after the tenth anniversary of the effective date, but awards theretofore granted
may extend beyond that date.
Transferability
and Participant Payments
Until
they are fully vested and/or exercisable, awards under the 2022 Plan are generally non-transferrable, subject to the plan administrator’s
consent, and are generally exercisable only by the participant. With regard to tax withholding, exercise price, and purchase price obligations
arising in connection with awards under the 2022 Plan, generally the plan administrator may, in its discretion, accept cash, ordinary
shares that meet specified conditions, or such other consideration as it deems suitable.
Certain
Relationships and Related Party Transactions
Waldencast
Founder
Shares
In
January 2021, the Sponsor purchased 7,187,500 Class B ordinary shares for an aggregate purchase price of $0.025 million, or approximately
$0.0035 per founder share. In February 2021, the Sponsor transferred 20,000 Class B ordinary shares to each of the Investor Directors,
who are our independent directors, resulting in the Sponsor holding 7,107,500 Class B ordinary shares. In March 2021, we effected a share
capitalization resulting in the Sponsor holding an aggregate of 8,545,000 Class B ordinary shares. As such, the Sponsor and the Investor
Directors collectively owned 20% of our issued and outstanding shares upon consummation of our initial public offering.
These
founder shares are identical to our Class A ordinary shares included in the units sold in our initial public offering, except that (i)
only the holders of the founder shares have the right to vote on the election of directors prior to the initial business combination,
(ii) the founder shares are subject to certain transfer restrictions, (iii) the holders of the founder shares have agreed pursuant to
a letter agreement to waive (x) their redemption rights with respect to the founder shares and public shares held by them in connection
with the completion of a business combination, (y) their redemption rights with respect to any founder shares and public shares held
by them in connection with a shareholder vote to amend our Cayman constitutional documents (A) to modify the substance or timing of our
obligation to allow redemption in connection with our initial business combination or to redeem 100% of our public shares if we do not
complete our initial business combination by March 18, 2023, or (B) with respect to any other provision relating to shareholders’
rights or pre-initial business combination activity and (z) their rights to liquidating distributions from the trust account with respect
to the founder shares if Waldencast fails to complete a business combination by March 18, 2023, (iv) the founder shares are automatically
convertible into Class A ordinary shares at the time of the initial business combination and (v) the founder shares are entitled to registration
rights.
In
connection with the Business Combination, upon the Domestication, 8,625,000 founder shares converted automatically, on a one-for-one
basis, into one Class A ordinary share.
Private
Placement Warrants
Simultaneously
with the consummation of our initial public offering, the Sponsor purchased 5,933,333 private placement warrants at a purchase price
of $1.50 per private placement warrant, or $8.9 million in the aggregate. Each private placement warrant entitles the holder to purchase
one Class A ordinary share for $11.50 per share. The private placement warrants may not be redeemed by us so long as they are held by
the Sponsor or its permitted transferees. If the private placement warrants are held by holders other than the Sponsor or its permitted
transferees, the private placement warrants will be redeemable by us and exercisable by the holders on the same basis as the warrants
included in the units that were sold as part of our initial public offering. The Sponsor, or its permitted transferees, has the option
to exercise the private placement warrants on a cashless basis.
The
private placement warrants are identical to the warrants included in the units sold in our initial public offering except that the private
placement warrants: (i) are not redeemable by us, (ii) may be exercised for cash or on a cashless basis so long as they are held by the
Sponsor or any of its permitted transferees and (iii) are entitled to registration rights (including the ordinary shares issuable upon
exercise of the private placement warrants). Additionally, the purchasers have agreed not to transfer, assign or sell any of the private
placement warrants, including our Class A ordinary shares issuable upon exercise of the private placement warrants (except to certain
permitted transferees), until 30 days after the completion of our initial business combination.
In
connection with the Business Combination, upon the Domestication, each of the 5,933,333 private placement warrants converted automatically
into a warrant to acquire one Class A ordinary share pursuant to the Warrant Agreement.
Registration
Rights
The holders of the founder
shares, private placement warrants, (and any Class A ordinary shares issuable upon (i) the exercise of the private placement warrants
and (ii) the conversion of the founder shares) are entitled to registration rights pursuant to a registration rights agreement dated March 15,
2021 (the “Legacy Registration Rights Agreement”) requiring us to register such securities for resale (in the case of the
founder shares, only after conversion to our Class A ordinary shares). The holders of these securities are entitled to make up to
three demands, excluding short form demands, that we register such securities. In addition, the holders have certain “piggy-back”
registration rights with respect to registration statements filed subsequent to the completion of our initial business combination and
rights to require us to register for resale such securities pursuant to Rule 415 under the Securities Act. We will bear the expenses incurred
in connection with the filing of any such registration statements.
We, the Sponsor, the members
of the Sponsor and certain of our shareholders, Obagi and Milk and certain of their respective affiliates entered into an amended and
restated registration rights agreement, dated July 27, 2022 (the “Registration Rights Agreement”), pursuant to which we agreed
to register for resale, pursuant to Rule 415 under the Securities Act, certain of our Class A ordinary shares and our warrants that
are held by the parties thereto from time to time, subject to the restrictions on transfer therein. The Registration Rights Agreement
amended and restated the Legacy Registration Rights Agreement and terminates with respect to any party thereto, on the date that such
party no longer holds any Registrable Securities (as defined therein).
Related Party Note
and Advances
On January 12, 2021,
we issued a promissory note to the Sponsor, pursuant to which we could borrow an aggregate principal amount of $300,000. The note was
non-interest bearing and payable on the completion of the initial public offering. There were no borrowings outstanding under the note
at the closing of the initial public offering.
On August 18, 2021,
we issued a promissory note to the Sponsor, pursuant to which we could borrow up to an aggregate principal amount of $1,500,000 from the
Sponsor (the “Working Capital Promissory Note”). The note was non-interest bearing, unsecured and due and payable in full
on the earlier of (x) March 18, 2023 and (y) the date we consummate our initial business combination. On October 28, 2021, we
drew down the entire available balance of the Working Capital Promissory Note and the Sponsor deposited $1,500,000 in our operating bank
account. As of July 27, 2022, we had a total aggregate principal amount of $1,500,000 in outstanding borrowings under the Convertible
Working Capital Note. In connection with the closing of Business Combination, the Sponsor elected to convert $1,500,000 of the Convertible
Working Capital Note balance into warrants at a price of $1.50 per warrant for a total of 1,000,000 warrants (the “Working Capital
Warrants”). The Working Capital Warrants issuance was exempt from registration pursuant to Section 4(a)(2) of the Securities Act,
and Rule 506 of Regulation D promulgated thereunder. Borrowings under the Convertible Working Capital Note are no longer available.
In addition, we issued the
following working capital promissory notes to the Sponsor (i) the May Working Capital Note and (ii) the July Working Capital Note, in
each case, for working capital purposes. As of July 27, 2022, we had a total aggregate principal amount of $1,050,000 in outstanding borrowings
under the Non-Convertible Working Capital Notes. In connection with the closing of Business Combination, the aggregate outstanding balance
under the Non-Convertible Working Capital Notes of $1,050,000 was repaid to the Sponsor. Borrowings under the Non-Convertible Working
Capital Notes are no longer available.
Administrative Services
Agreement
We entered into an agreement
whereby, commencing on March 15, 2021, through the earlier of the consummation of a business combination or our liquidation, we agreed
to pay the Sponsor a monthly fee of $0.01 million for office space, administrative, financial and support services. We incurred approximately
$160,000 in administrative expenses under the agreement through June 30, 2022, but ceased to incur these fees following the completion
of the Business Combination.
Waiver and Agreement
In connection with the consummation
of the Business Combination, we waived certain provisions of the Letter Agreement and certain other agreements related thereto (collectively,
the “Waiver”), with respect to any securities held by an Insider (as defined in the Letter Agreement) as of or immediately
following the closing of the Business Combination (the “Lock-Up Securities”) that would disallow a pledge by such Insider
of the Lock-up Securities in connection with a transaction for the purpose of financing such Insider’s payment obligations owed
in connection with the closing of the Business Combination.
In connection with such Waiver,
we entered into that certain Waiver and Agreement, dated as of July 25, 2022, by and between us and Burwell (the “Waiver and Agreement”),
to permit (i) a pledge by Burwell of its Lock-Up Securities to be used as a portion of the collateral under a loan to finance Burwell’s
payment obligations under the Sponsor Forward Purchase Agreement in connection with the closing of the Business Combination and (ii) following
an event of default and exercise of remedies by the lender, or collateral agent or other representative of such lender, in accordance
with the definitive documentation governing the loan, foreclosure on, or a sale, assignment, transfer or other disposition of, the Lock-Up
Securities. Pursuant to the terms of the Waiver and Agreement, in the event of a foreclosure, any such lenders or a collateral agent or
other representative of such lenders, will be required to execute a joinder to the Letter Agreement pursuant to which they will be bound
by the transfer restrictions of the Lock-Up Securities in the Letter Agreement (as modified by the Waiver and Agreement) for the duration
of such agreement. We also agreed to provide any such lender, or collateral agent or other representative of such lender, with customary
registration rights in the event of default, foreclosure or other exercise of remedies following the respective Lock-Up Periods (as defined
in the Letter Agreement).
The foregoing description
of the Waiver and Agreement and the transactions contemplated thereby is not complete and is subject to and qualified in its entirety
by reference thereto, a copy of which is filed as Exhibit 10.31 to the registration statement of which this prospectus forms a part and
the terms of which are incorporated by reference herein.
Indemnification Agreements
In connection with the Business
Combination, we entered into indemnification agreements with each of our directors. The indemnification agreements provide, to the fullest
extent permitted under law, indemnification against all expenses, judgments, fines and amounts paid in settlement relating to, arising
out of or resulting from indemnitee’s status as a director, officer, employee, fiduciary or agent of Waldencast or any other corporation,
limited liability company, partnership, joint venture, trust, employee benefit plan or other entity which such person is or was serving
at our request as a director, officer, employee or agent. In addition, the indemnification agreements provide that we will advance, to
the extent not prohibited by law, the expenses incurred by the indemnitee in connection with any proceeding, and such advancement will
be made within thirty (30) days after the receipt by us of a statement requesting such advances from time to time, whether prior to or
after final disposition of any proceeding.
The foregoing description
of the indemnification agreements and the transactions contemplated thereby is not complete and is subject to and qualified in its entirety
by reference thereto, copies of which are incorporated by reference as Exhibits 10.12 to 10.20 to the registration statement of which
this prospectus forms part and the terms of which are incorporated by reference herein.
Director Interests
Pursuant to Article 75 of
the Jersey Companies Law and the Constitutional Document, any director of the Company who has, directly or indirectly, an interest in
a transaction entered into or proposed to be entered into by the Company or by a subsidiary of the Company which to a material extent
conflicts or may conflict with the interests of the Company and of which the director is aware, is required to disclose to the Company
the nature and extent of the director’s interest.
The following directors have
provided the Company with notification of the following interests:
| ● | Felipe
Dutra and his descendants are eligible beneficiaries of Burwell Mountain Trust and should
be regarded as interested accordingly in any transaction involving Burwell Mountain Trust
and its affiliates. |
| ● | Each
of Sarah Brown, Aaron Chatterley, Juliette Hickman, Lindsay Pattison and Zack Werner each
own 20,000 Class A ordinary shares and should be regarded as interested accordingly in any
transaction involving such Class A ordinary shares. |
| ● | Simon
Dai was nominated for appointment to our Board by Cedarwalk, pursuant to the Investor Rights Agreement, and should be regarded as interested
accordingly in any transaction involving Cedarwalk and its affiliates. |
| ● | Michel
Brousset is the chief executive officer of Waldencast Management, LLC, the general partner of Waldencast Ventures, L.P. Waldencast Ventures,
L.P. holds (a) 2,848,334 Class A ordinary shares that converted automatically, on a one-for-one basis, from Class B ordinary shares upon
the consummation of the Business Combination, (b) 1,977,779 Class A ordinary shares issuable upon exercise of the private placement warrants
and (c) 333,334 Class A ordinary shares issuable upon exercise of the Working Capital Loan warrants. Michel Brousset should be regarded
as interested accordingly in any transaction involving such Class A ordinary shares, Waldencast Ventures, L.P. and its affiliates. |
As
a matter of Jersey law, each director of the Company is under a duty to act honestly and in good faith with a view to acting in the best
interests of the Company, regardless of any other directorship such director may hold. Each director is responsible for advising the
board of directors in advance of any potential conflicts of interest.
Obagi
Operational Support
Services Agreement
In January 2018, Obagi Cosmeceuticals entered into an operational support
services agreement with Obagi Holdco, Obagi Hong Kong and Obagi Shanghai, pursuant to which Obagi Cosmeceuticals provides certain services,
including administrative and product related services, to the other signatories party thereto. Under the agreement, which automatically
renewed for a one-year term on January 1, 2021, Obagi Cosmeceuticals receives service fees in an amount equal to the sum of its costs
incurred in the performance of such services plus five percent (5%). Total service fees paid to Obagi Cosmeceuticals were $2.9 million
and $1.2 million for the six months ended June 30, 2022 and 2021, respectively, and $3.0 million and $0.9 million for the years ended
December 31, 2021 and 2020, respectively. The agreement terminated upon consummation of the Business Combination.
Non-Exclusive Marketing
Services Agreement
In August 2019, Obagi Holdco
and Obagi Shanghai entered into a non-exclusive marketing services agreement, pursuant to which Obagi Shanghai provides certain sales
and marketing services Obagi Holdco in the People’s Republic of China. Under the agreement, which automatically renewed for a one-year
term on January 1, 2021, Obagi Shanghai receives service fees in an amount equal to the sum of its costs incurred in the performance
of such services plus five percent (5%). Total service fees paid to Obagi Shanghai were $3.0 million and $1.0 million for the six months
ended June 30, 2022 and 2021, respectively, and $2.6 million and $1.0 million for the years ended December 31, 2021 and 2020, respectively.
The agreement terminated upon consummation of the Business Combination.
Shareholder Loan
In January 2019, Obagi paid
approximately $2.0 million in accrued interest to a shareholder related to a shareholder loan, the principal of which was repaid in 2018
upon refinancing with a third-party syndicate of banks.
Registration with
National Medical Products Administration in China
In June 2020, Cedarwalk paid
approximately $4.1 million to register Obagi’s products with the National Medical Products Administration in China in exchange for
8,000,000 shares of Obagi Common Stock. This non-cash capital contribution was recorded as additional paid-in capital in Obagi’s
consolidated statements of shareholder’s equity for the year ended December 31, 2020.
Stockholder Support
Agreement
In connection with the execution
of the Obagi Merger Agreement, on November 15, 2021, Obagi entered into the Stockholder Support Agreement with Waldencast and Cedarwalk.
Pursuant to the Stockholder Support Agreement, Cedarwalk agreed to, among other things, vote to adopt and approve, upon the effectiveness
of the Registration Statement being declared effective and delivered or otherwise made available to stockholders, the Obagi Merger Agreement
and the transactions contemplated thereby, in each case, subject to the terms and conditions of the Stockholder Support Agreement.
Cedarwalk also agreed not
to (a) sell or otherwise dispose of, or agree to sell or dispose of, directly or indirectly, any shares of Obagi Common Stock held by
Cedarwalk, (b) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences
of ownership of any of such Obagi Common Stock, or (c) publicly announce any intention to effect any transaction specified in clause (a)
or (b).
The Stockholder Support Agreement
will terminate in its entirety, and be of no further force or effect, upon the earliest of (a) the termination of the Obagi Merger Agreement,
(b) the occurrence of the Obagi Closing, (c) the liquidation of Waldencast, and (d) the written agreement of Waldencast, Obagi and Cedarwalk.
Upon such termination of the Stockholder Support Agreement, all obligations of the parties under the Stockholder Support Agreement will
terminate, without any liability or other obligation on the part of any party thereto to any person in respect thereof or the transactions
contemplated thereby, and no party thereto will have any claim against another (and no person will have any rights against such party),
whether under contract, tort or otherwise, with respect to the subject matter thereof; provided, however, that the termination of the
Stockholder Support Agreement will not relieve any party thereto from liability arising in respect of any breach of the Stockholder Support
Agreement prior to such termination.
Obagi China Distribution
In connection with the pre-closing
distribution by Obagi Holdco to Obagi and the distribution by Obagi to Cedarwalk of all of the issued and outstanding shares of capital
stock of Obagi Hong Kong and certain related assets pursuant to distribution agreements, the following agreements were entered into at
the consummation of the Business Combination: (a) that certain Transition Services Agreement, dated as of July 27, 2022, by and among
Obagi Worldwide, certain of Obagi Cosmeceuticals’ Affiliates and Obagi Hong Kong, pursuant to which Obagi Cosmeceuticals and certain
of its Affiliates shall provide transition services to Obagi Hong Kong (the “Transition Services Agreement”), (b) that certain
Intellectual Property License Agreement, dated as of July 27, 2022, by and among Obagi Worldwide and Obagi Hong Kong pursuant to which
Obagi Worldwide will exclusively license intellectual property relating to the Obagi brand to Obagi Hong Kong with respect to the People’s
Republic of China, inclusive of the Hong Kong Special Administrative Region, the Macau Special Administrative Region, and Taiwan (the
“China Region”) (the “Intellectual Property License Agreement”), and (c) that certain Supply Agreement, dated
as of July 27, 2022, by and between Obagi Cosmeceuticals and Obagi Hong Kong pursuant to which Obagi Cosmeceuticals will supply products
to Obagi Hong Kong for distribution and sale in the China Region (the “Supply Agreement”).
In connection with the consummation
of the Business Combination, we entered into that certain Investor Rights Agreement, dated as of July 27, 2022, by and among us, Cedarwalk
and CWC Skincare Ltd, as guarantor of Cedarwalk’s obligations thereunder (the “Investor Rights Agreement” and, together
with the Transition Services Agreement, Intellectual Property License Agreement, and Supply Agreement, the “Obagi China Related
Party Agreements”).
Transition Services Agreement
Pursuant to the Transition
Services Agreement, Obagi Worldwide and certain of its Affiliates will provide to Obagi Hong Kong and its Affiliates certain transition
services to enable Obagi Hong Kong to conduct Obagi-branded business as a going concern in the China Region. Obagi Worldwide and certain
of its Affiliates will provide the transition services set forth under the Transition Services Agreement for up to twelve (12) months
following the Obagi Closing, with an option for Obagi Hong Kong, in its sole discretion, to extend the service period for up to an additional
twelve (12) months solely with respect to certain services relating to research & development. Services will be charged at the reasonable,
fully-loaded costs of providing the services, but such services will be provided at no charge for a certain period of time or amount of
services.
Intellectual Property
License Agreement
Pursuant to the Intellectual
Property License Agreement, Obagi Worldwide will exclusively license intellectual property relating to the Obagi brand to Obagi Hong Kong
with respect to the China Region, and Obagi Worldwide will retain the rights to such intellectual property to conduct the Obagi-branded
business worldwide except for the China Region. The license from Obagi Worldwide to Obagi Hong Kong will include future intellectual property
of Obagi Worldwide relating to the Obagi brand in the worldwide business, including, but not limited to: (i) trademarks; (ii) domain
names; (iii) patents; (iv) trade secrets and know-how; (v) copyrights; and (vi) product specifications and formulas.
The license will be perpetual,
irrevocable and non-transferable, subject to: (x) a limited right of Obagi Worldwide to terminate for an uncured material breach
of a material provision by Obagi Hong Kong that materially and adversely affects Obagi Worldwide or the Obagi brand, in which case Obagi
Worldwide will purchase Obagi Hong Kong at a discount to fair market value based on an independent valuation procedure; (y) the right
of either party to transfer the Intellectual Property License Agreement without consent of the other party to an Affiliate or to a successor
in interest in connection with any merger, business combination or other change of control transaction, or sale of a product or service
line; and (z) a right of Obagi Hong Kong to sublicense to Affiliates, Approved CMOs and other approved third parties. Upon the termination
of the Intellectual Property License Agreement, at the written request of Obagi Worldwide, Obagi Hong Kong shall promptly cease, and shall
cause its sublicensees to promptly cease, all use of the Licensed IP Rights (as defined in the Intellectual Property License Agreement),
subject to a non-exclusive right to use the Licensed IP Rights for a period of up to nine complete calendar months following the effective
date of termination, in a manner consistent with past practice and in compliance with the terms and conditions of the agreement, to sell
off all inventory of China Products (as defined in the Intellectual Property License Agreement) to consumers in the China Region (and
subject to the terms relating to royalties in the Intellectual Property License Agreement).
Pursuant to the terms of
the Intellectual Property License Agreement, Obagi Hong Kong will be obligated to pay Obagi Worldwide a royalty of five and a half percent
(5.5%) of gross sales of licensed products, subject to certain deductions.
Obagi Supply Agreement
Pursuant to the Obagi Supply
Agreement, Obagi Cosmeceuticals will supply, or cause to be supplied through certain CMOs, products to Obagi Hong Kong and its Affiliates,
and Obagi Hong Kong may purchase such products for distribution and sale in the China Region. In addition to supply procurement services,
Obagi Cosmeceuticals and certain of its Affiliates may provide to Obagi Hong Kong and its Affiliates certain other supply-related services,
with the scope of such services and fees for such services to be determined in good faith by the parties every 12 months. The term of
the Obagi Supply Agreement is perpetual, subject to termination for uncured material breach or termination in the event that the Intellectual
Property License Agreement is terminated.
Investor Rights Agreement
Pursuant to the Investor
Rights Agreement, Cedarwalk has the right to nominate one director for election or appointment to the Board for so long as Cedarwalk
owns 5% of the then-outstanding common stock of Waldencast, and such appointee is initially Simon Dai, who serves as a director of Obagi,
Obagi Holdco and Obagi Hong Kong. Upon such termination of the Supply Agreement:
| ● | Obagi
Hong Kong shall promptly refrain from using the Product Information File, the Specifications
and the Confidential Information of Obagi Cosmeceuticals (each as defined in the Supply Agreement); |
| ● | Obagi
Hong Kong shall return to Obagi Cosmeceuticals all documents relating to the Product Information
File, Specifications and Confidential Information of Obagi Cosmeceuticals. The relevant costs
shall be borne by the party who is responsible for the termination or the non-renewal of
the Supply Agreement; and |
| ● | Unless
the Supply Agreement is terminated by Obagi Hong Kong due to Obagi Cosmeceuticals’
breach of its obligations related to the quality of the products, Obagi Cosmeceuticals shall
complete the manufacturing of all products covered by firm orders and deliver them to the
applicable recipient. |
Milk
Milk receives certain administrative
services from affiliated companies related through common ownership. Such amounts are included in rent and management fee expenses and
amounted to $0.06 million and $0.7 million for the years ended December 31, 2021 and 2020, respectively, and $0.06 million each for
the six months ended June 30, 2022 and 2021.
As of June 30, 2022 and December 31,
2021, Milk had loans due from officers in the aggregate amount of $0.8 million.
Beneficial
Ownership of Securities
The following table sets
forth information known to us regarding the beneficial ownership of our Class
A ordinary shares and our Class B ordinary shares as of August 15, 2022:
| ● | each
person known by us to be the beneficial owner of more than 5% of ordinary shares;; |
| ● | each
of our executive officers and directors; and |
| ● | all
our executive officers and directors as a group. |
Beneficial
ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security
if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are
currently exercisable or exercisable within 60 days.
As of August 15, 2022, there
were 107,564,785 ordinary shares outstanding, consisting of 86,460,560 Class A ordinary shares and 21,104,225 Class B ordinary shares.
Unless otherwise indicated,
we believe that all persons named in the table below have sole voting and investment power with respect to all voting shares beneficially
owned by them.
| Name and Address of Beneficial Owner(1) |
|
Class A
ordinary shares |
|
|
% of
Class A
ordinary
shares
outstanding |
|
|
Class B
ordinary shares(7) |
|
|
% of
Combined
Voting
Power(8) |
|
| 5% Holders |
|
|
|
|
|
|
|
|
|
|
|
|
| Waldencast Long-Term Capital LLC (our Sponsor)(2) |
|
|
23,066,666 |
|
|
|
25.0 |
% |
|
|
— |
|
|
|
20.4 |
% |
| Cedarwalk Skincare Ltd(3) |
|
|
28,237,506 |
|
|
|
32.7 |
% |
|
|
— |
|
|
|
26.3 |
% |
| Dynamo Master Fund(4) |
|
|
19,826,109 |
|
|
|
21.4 |
% |
|
|
— |
|
|
|
17.5 |
% |
| Burwell Mountain Trust(5) |
|
|
11,826,110 |
|
|
|
13.1 |
% |
|
|
— |
|
|
|
10.6 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Directors and Executive Officers |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sarah Brown |
|
|
20,000 |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
| Aaron Chatterley |
|
|
20,000 |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
| Juliette Hickman |
|
|
20,000 |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
| Lindsay Pattison |
|
|
20,000 |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
| Zack Werner |
|
|
20,000 |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
| Michel Brousset(6) |
|
|
5,159,447 |
|
|
|
5.8 |
% |
|
|
— |
|
|
|
4.7 |
% |
| Simon Dai |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Hind Sebti |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Cristiano Souza |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Felipe Dutra(5) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| All Waldencast plc directors and executive officers as a group (11 individuals) |
|
|
5,259,447 |
|
|
|
5.9 |
% |
|
|
— |
|
|
|
4.8 |
% |
| (1) |
Unless otherwise noted, the business address for each of those listed in the table above is c/o Waldencast plc, 10 Bank Street, Suite 560, White Plains, NY 10606. |
| (2) |
Reflects securities held directly by Beauty Ventures consisting of
(i) 17,300,000 Class A ordinary shares and (ii) 5,766,666 Class A ordinary shares issuable upon exercise of warrants to be issued to Beauty
Ventures pursuant to the Third-Party Forward Purchase Agreement. Waldencast Long-Term Capital LLC, our Sponsor, is the managing member
of Beauty Ventures. The voting and investment power of our Sponsor is exercised jointly by Waldencast Ventures, LP, Burwell, and Dynamo
Master Fund. Waldencast Ventures, LP is controlled by Michel Brousset. Burwell Mountain PTC LLC is the trustee of Burwell Mountain Trust,
a non-grantor, fully discretionary dynasty trust duly organized under Wyoming law. See footnote 7 for further details. Dynamo Internacional
Gestão de Recursos Ltda., a Brazilian limited company (“Dynamo International”), is the investment manager of Dynamo
Master Fund. Luiz Orenstein, Bruno Hermes da Fonseca Rudge and Luiz Felipe de Almeida Campos are the largest shareholders of Dynamo International
and together they have the power to direct Dynamo Internacional’s business. |
| |
|
| (3) |
Sijue Dai is deemed to have sole voting and dispositive power with regard to 28,237,506 Class A ordinary shares. His business address is c/o Cedarwalk Skincare Limited, Rm 3001-3010, 30/F, China Resource Building, 26 Harbour Road, Wanchai, Hong Kong. |
| |
|
| (4) |
Dynamo International is the investment manager of Dynamo Master Fund.
Luiz Orenstein, Bruno Hermes da Fonseca Rudge and Luiz Felipe de Almeida Campos are the largest shareholders of Dynamo International and
together they have the power to direct Dynamo International’s business. The securities reported by Dynamo International herein reflect
the distribution by the Sponsor of the founder shares and the private placement warrants to its members that occurred in connection with
the Business Combination. |
| |
|
| (5) |
Burwell Mountain PTC LLC, as trustee of Burwell Mountain Trust, has
the sole voting and dispositive power over the shares held on behalf of the Burwell Mountain Trust, a non-grantor, fully discretionary
dynasty trust duly organized under Wyoming law of which Felipe Dutra and his descendants are eligible beneficiaries. Burwell Mountain
PTC LLC is an independent trustee over which Mr. Dutra has no control. The business address of each is 270 W. Pearl Avenue, Suite 103,
Jackson, WY 83001. The securities reported by Burwell Mountain Trust herein reflect the distribution by the Sponsor of the founder shares
and the private placement warrants to its members that occurred in connection with the Business Combination. In connection with the closing
of the Business Combination, Burwell pledged all of the reported securities held by it to be used as a portion of the collateral pursuant
to a loan agreement with customary default provisions, entered into with the lenders party thereto and under which Acquiom Agency Services
LLC (or its successor) is the collateral agent. In the event of a default under the loan agreement, following such securities respective
lock-up periods, the secured parties under the loan agreement through the collateral agent may foreclose upon any and all securities pledged
to them. |
| (6) |
As described above, following the closing of the Business Combination,
the Sponsor distributed the founder shares and the private placement warrants to its members. As such, Waldencast Ventures, LP holds (i)
2,848,334 Class A ordinary shares that converted automatically, on a one-for-one basis, from Class B ordinary shares upon the consummation
of the Business Combination, (ii) 1,977,779 Class A ordinary shares issuable upon exercise of the private placement warrants and (iii)
333,334 Class A ordinary shares issuable upon exercise of the Working Capital Loan warrants. Mr. Brousset is the chief executive officer
of Waldencast Management, LLC, the general partner of Waldencast Ventures, LP. As such, he may be deemed to beneficially own the shares
held by Waldencast Ventures, LP. |
| |
|
| (7) |
Class B ordinary shares are non-economic voting shares and may be exchanged,
together with an equal amount of Waldencast LP Common Units, for Class A ordinary shares. |
| |
|
| (8) |
Includes both Class A ordinary shares and Class B ordinary shares. |
Selling
Holders
This prospectus also relates
to the resale by certain of the Selling Holders of: (1) up to 121,120,063 Class A ordinary shares, consisting of (i) 8,545,000 Class
A ordinary shares converted from the founder shares; (ii) 80,000 Class A ordinary shares converted from the founder shares held by the
Investor Directors; (iii) 20,000 Class A ordinary shares issued to Aaron Chatterley in a private placement exempt from registration pursuant
to Section 4(a)(2) of the Securities Act, and Rule 506 of Regulation D promulgated thereunder, in connection with the consummation of
the Business Combination; (iv) 28,237,506 Class A ordinary shares issued pursuant to the Obagi Merger Agreement; (v) 21,104,225 Class
A ordinary shares issuable in exchange for 21,104,225 Class B ordinary shares pursuant to the Milk Equity Purchase Agreement; (vi) 11,800,000
Class A ordinary shares issued in the PIPE Investments; (vii) 33,300,000 Class A ordinary shares issued pursuant the Forward Purchase
Agreements; and (viii) 18,033,332 Class A ordinary shares issuable in respect of the private placement warrants and (2) up to 18,033,332
private placement warrants.
Following the expiration
of the applicable lock-up restrictions described herein, the sale of all of the securities registered for resale hereunder (and the Class
A ordinary shares issuable upon exercise of our warrants), or the perception that such sales may occur, may cause the market prices of
our securities to decline significantly. Despite such a decline in price, our Sponsor and our holders may still experience a positive
rate of return on the shares purchased by them due to the lower price per share at which its shares were purchased. See “Risk
Factors—General Business Risks and Risks Related to Our Financial Condition and Operations” for more information.
Prior to the initial
public offering, the Sponsor acquired 7,187,500 founder shares in exchange for a capital contribution of $25,000, or approximately $0.0035
per share. Simultaneously with the consummation of the initial public offering, the Sponsor purchased 5,933,333 private placement warrants
at a purchase price of $1.50 per private placement warrant, or $8,900,000 in the aggregate. In February 2021, the Sponsor transferred
20,000 Class B ordinary shares to each of the Investor Directors, at a purchase price of approximately $0.0035 per share, resulting in
the Sponsor holding 7,107,500 Class B ordinary shares. On March 15, 2021, we effected a dividend of 0.2 of a share of Class B ordinary
shares for each share of Class B ordinary shares, resulting in 8,625,000 shares of Class B ordinary shares being issued and outstanding,
of which 8,545,000 were issued to the Sponsor. As a result, the Sponsor and the Investor Directors collectively owned 20% of our issued
and outstanding shares upon consummation of our initial public offering.
Upon the consummation
of the Business Combination, (i) the founder shares were converted from Class B ordinary shares into Class A ordinary shares, (ii) the
PIPE Investors acquired 11,800,000 Class A ordinary shares pursuant to Subscription Agreements at a price of $10.00 per share, (iii)
the Forward Purchasers acquired 33,300,000 Class A ordinary shares and 11,100,000 warrants to purchase one Class A ordinary share, at
a collective price of $10.00 per Forward Purchase Security, (iv) the Sponsor elected to convert $1,500,000 of the Convertible Working
Capital Note balance into warrants at a price of $1.50 per warrant for a total of 1,000,000 warrants, (iv) we entered into the Registration
Rights Agreement with certain parties, (v) we issued 28,237,506 Class A ordinary shares in connection with the Business Combination as
the equity portion of the merger consideration pursuant to the Obagi Merger Agreement, at an acquiror share value of $10.00 per share,
(vi) we issued 21,104,225 Class B ordinary shares in connection with the 21,104,225 Waldencast LP Common Units issued in connection with
the Business Combination as the equity portion of the transaction consideration pursuant to the Milk Equity Purchase Agreement at a common
unit value of $10.00 per unit. The Waldencast LP Common Units are exchangeable for 21,104,225 Class A ordinary shares and, upon such
exchange, we will redeem an equivalent number of Class B ordinary shares held by such holder for no additional consideration; and (v)
we issued 20,000 Class A ordinary shares to Aaron Chatterley, one of our independent directors, in a private placement exempt from registration
pursuant to Section 4(a)(2) of the Securities Act, and Rule 506 of Regulation D promulgated thereunder, in connection with the consummation
of the Business Combination, for no consideration. The founder shares, the Class A ordinary shares acquired in the PIPE Investment, the
Class A ordinary shares acquired pursuant to the Forward Purchase Agreements, the private placement warrants, and the Class A ordinary
shares issuable upon exercise of the private placement warrants are being registered by the registration statement of which this prospectus
forms a part pursuant to the registration rights granted under certain of the Subscription Agreements and the Registration Rights Agreement.
On October 12, 2022, the
last reported sale price of our Class A ordinary shares on Nasdaq was $8.22 per share and the last reported sale price of our warrants
on Nasdaq was $0.62. Even if our trading price is significantly below $10.00, the offering price for the Legacy units offered in our initial
public offering, certain of the Selling Holders, including Burwell, Dynamo Master Fund and Waldencast Ventures LP, may still have an incentive
to sell our Class A ordinary shares because they hold founder shares that were originally purchased by the Sponsor at prices lower than
the public investors or the current trading price of our Class A ordinary shares. For example, based on the closing price of our Class
A ordinary shares of $8.22 as of October 12, 2022, the holders of the 8,625,000 Class A ordinary shares converted from the founder shares
would experience a potential profit of up to approximately $8.22 per share, or up to approximately $70.9 million in the aggregate. Public
holders of our Class A ordinary shares may not experience a similar rate of return on their shares as a result of these variations in
share prices.
The Selling Holders may from
time to time offer and sell any or all of the Class A ordinary shares and warrants set forth below pursuant to this prospectus. When we
refer to the “Selling Holders” in this prospectus, we mean the persons listed in the table and in the footnotes in the table
below (as such table may be amended from time to time by means of an amendment to the registration statement of which this prospectus
is a part or by supplement to this prospectus), and any pledgees, donees, transferees, assignees, successors and others who later come
to hold any of the Selling Holders’ interest in the Class A ordinary shares or warrants after the date of this prospectus such that
registration rights shall apply to those securities.
The following tables are
prepared based on information provided to us by the Selling Holders. It sets forth the name and address of the Selling Holders, the aggregate
number of Class A ordinary shares or warrants, as applicable, that the Selling Holders may offer pursuant to this prospectus, and the
beneficial ownership of the Selling Holders both before and after the offering. We have based percentage ownership prior to this offering
on 86,460,560 Class A ordinary shares and 29,533,282 warrants outstanding, in each case, as of August 3, 2022. In calculating percentages
of Class A ordinary shares owned by a particular Selling Holder, we treated as outstanding the number of Class A ordinary shares issuable
upon exercise of that particular Selling Holder’s warrants, if any, and did not assume the exercise of any other Selling Holder’s
warrants.
We cannot advise you as to
whether the Selling Holders will in fact sell any or all of such Class A ordinary shares or warrants. In addition, the Selling Holders
may sell, transfer or otherwise dispose of, at any time and from time to time, the Class A ordinary shares and warrants in transactions
exempt from the registration requirements of the Securities Act after the date of this prospectus. For purposes of this table, we have
assumed that the Selling Holders will have sold all of the securities covered by this prospectus upon the completion of the offering.
Class A Ordinary Shares
and Warrants
| | |
Beneficial
Ownership of Securities
Before the Offering | | |
Securities to be Sold
in the Offering | | |
Beneficial
Ownership of Securities
After the Offering | |
Name of Selling Shareholder(1) | |
Number of
Class A
Ordinary
Shares | | |
% | | |
Number of
Warrants | | |
% | | |
Number of
Class A
Ordinary
Shares | | |
Number of
Warrants | | |
Number of
Class A
Ordinary
Shares | | |
% | | |
Number of
Warrants | | |
% | |
| Burwell Mountain Trust(2) | |
| 11,826,110 | | |
| 13.08 | | |
| 3,977,777 | | |
| 13.47 | | |
| 11,826,110 | | |
| 3,977,777 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Dynamo Master Fund(3) | |
| 19,826,109 | | |
| 21.45 | | |
| 5,977,776 | | |
| 20.24 | | |
| 19,826,109 | | |
| 5,977,776 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Beauty Ventures LLC(4) | |
| 23,066,666 | | |
| 25.01 | | |
| 5,766,666 | | |
| 19.53 | | |
| 23,066,666 | | |
| 5,766,666 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Waldencast Long-Term Capital LLC(5) | |
| 23,066,666 | | |
| 25.01 | | |
| 5,766,666 | | |
| 19.53 | | |
| 23,066,666 | | |
| 5,766,666 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Waldencast Ventures, LP(6) | |
| 5,159,447 | | |
| 5.81 | | |
| 2,311,113 | | |
| 7.83 | | |
| 5,159,447 | | |
| 2,311,113 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Cedarwalk Skincare Ltd(7) | |
| 28,237,506 | | |
| 32.66 | | |
| — | | |
| — | | |
| 28,237,506 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Sarah Brown | |
| 20,000 | | |
| * | | |
| — | | |
| — | | |
| 20,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Aaron Chatterley | |
| 20,000 | | |
| * | | |
| — | | |
| — | | |
| 20,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Juliette Hickman | |
| 20,000 | | |
| * | | |
| — | | |
| — | | |
| 20,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Lindsay Pattison | |
| 20,000 | | |
| * | | |
| — | | |
| — | | |
| 20,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Zack Werner | |
| 20,000 | | |
| * | | |
| — | | |
| — | | |
| 20,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| ACG Dairy LLC(8) | |
| 2,306,318 | | |
| 2.67 | | |
| — | | |
| — | | |
| 20,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Amorepacific Group(9) | |
| 2,791,641 | | |
| 3.23 | | |
| — | | |
| — | | |
| 2,791,641 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Main Post Growth Capital, L.P.(10) | |
| 6,090,058 | | |
| 7.04 | | |
| — | | |
| — | | |
| 6,090,058 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Dianna Spiridon Ruth (11) | |
| 396,545 | | |
| * | | |
| — | | |
| — | | |
| 396,545 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Georgia Greville Jasper(12) | |
| 396,545 | | |
| * | | |
| — | | |
| — | | |
| 396,545 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Suzanna Roberts Rassi(13) | |
| 396,545 | | |
| * | | |
| — | | |
| — | | |
| 396,545 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Erez Shternlicht(14) | |
| 2,610,803 | | |
| 3.02 | | |
| — | | |
| — | | |
| 2,610,803 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Mazdack Rassi(15) | |
| 2,610,803 | | |
| 3.02 | | |
| — | | |
| — | | |
| 2,610,803 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Moishe Mana(16) | |
| 2,610,803 | | |
| 3.02 | | |
| — | | |
| — | | |
| 2,610,803 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Scott Sassa(17) | |
| 894,164 | | |
| * | | |
| — | | |
| — | | |
| 894,164 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Antarctic Prion Ltd.(18) | |
| 1,000,000 | | |
| 1.16 | | |
| — | | |
| — | | |
| 1,000,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fitpart Capital Opportunities IC Fund(19) | |
| 4,000 | | |
| * | | |
| — | | |
| — | | |
| 4,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fitpart Diversified Strategies Professional Fund LDC(20) | |
| 457,767 | | |
| * | | |
| 277,767 | | |
| * | | |
| 180,000 | | |
| — | | |
| 277,767 | | |
| * | | |
| 277,767 | | |
| * | |
| Fitpart Dynamic Allocation Professional Fund Ltd.(21) | |
| 800,000 | | |
| * | | |
| — | | |
| — | | |
| 800,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fitpart High Yield IC Fund(22) | |
| 8,000 | | |
| * | | |
| — | | |
| — | | |
| 8,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fitpart Next Generation IC Fund(23) | |
| 8,000 | | |
| * | | |
| — | | |
| — | | |
| 8,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Truxt Brazil Long Short(24) | |
| 240,381 | | |
| * | | |
| 62,791 | | |
| * | | |
| 177,590 | | |
| — | | |
| 62,791 | | |
| * | | |
| 62,791 | | |
| * | |
| Truxt Brazil Long Bias(24) | |
| 3,180,498 | | |
| 3.66 | | |
| 448,261 | | |
| 1.52 | | |
| 2,732,237 | | |
| — | | |
| 448,261 | | |
| * | | |
| 448,261 | | |
| 1.52 | |
| Truxt Brazil Macro(24) | |
| 52,174 | | |
| * | | |
| — | | |
| — | | |
| 52,174 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Truxt Brazil Valor(24) | |
| 2,251,799 | | |
| 2.59 | | |
| 343,543 | | |
| 1.16 | | |
| 1,908,256 | | |
| — | | |
| 343,543 | | |
| * | | |
| 343,543 | | |
| 1.16 | |
| Truxt Investments – Equity Long Only Master Fund LLC(24) | |
| 147,105 | | |
| * | | |
| 17,362 | | |
| * | | |
| 129,743 | | |
| — | | |
| 17,362 | | |
| * | | |
| 17,362 | | |
| * | |
| Kosmin Fund Ltd.(25) | |
| 1,000,000 | | |
| 1.16 | | |
| — | | |
| — | | |
| 1,000,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Rohto Pharmaceutical Co., Ltd.(26) | |
| 250,000 | | |
| * | | |
| — | | |
| — | | |
| 250,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| LMC Investments Inc.(27) | |
| 1,000,000 | | |
| 1.16 | | |
| — | | |
| — | | |
| 1,000,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Marcelo Pradez de Faria Stallone(28) | |
| 133,333 | | |
| * | | |
| 33,333 | | |
| * | | |
| 50,000 | | |
| — | | |
| 83,333 | | |
| * | | |
| 33,333 | | |
| * | |
| Strategic Portfolio Management (Bahamas) Fund Ltd.(29) | |
| 200,000 | | |
| * | | |
| — | | |
| — | | |
| 200,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| WPA International LP(30) | |
| 1,000,000 | | |
| 1.16 | | |
| — | | |
| — | | |
| 1,000,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Yisroel Zahler(31) | |
| 500,000 | | |
| * | | |
| — | | |
| — | | |
| 500,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Azulona LLC(32) | |
| 300,000 | | |
| * | | |
| — | | |
| — | | |
| 300,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| LTS Investments Fund LP – Class 27(33) | |
| 400,000 | | |
| * | | |
| — | | |
| — | | |
| 400,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Nexus Limited(34) | |
| 100,000 | | |
| * | | |
| — | | |
| — | | |
| 100,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| (1) | Unless otherwise noted, the business address for each of those listed in the table above is c/o Waldencast
plc, 10 Bank Street, Suite 560, White Plains, NY 10606. |
| (2) | Burwell Mountain PTC LLC, as trustee of Burwell Mountain Trust, has the sole voting and dispositive power
over the shares held on behalf of the Burwell Mountain Trust, a non-grantor, fully discretionary dynasty trust duly organized under Wyoming
law of which Felipe Dutra and his descendants are eligible beneficiaries. Burwell Mountain PTC LLC is an independent trustee over which
Mr. Dutra has no control. The securities reported by Burwell Mountain Trust herein reflect (i) 7,848,333 Class A ordinary shares and (ii)
3,977,777 Class A ordinary shares issuable upon exercise of warrants. In connection with the closing of the Business Combination, Burwell
pledged all of the reported securities held by it to be used as a portion of the collateral pursuant to a loan agreement with customary
default provisions, entered into with the lenders party thereto and under which Acquiom Agency Services LLC (or its successor) is the
collateral agent. In the event of a default under the loan agreement, the secured parties under the loan agreement through the collateral
agent may foreclose upon any and all securities pledged to them. |
| (3) | Dynamo Internacional is the investment manager of Dynamo Master Fund. Luiz Orenstein, Bruno Hermes da
Fonseca Rudge and Luiz Felipe de Almeida Campos are the largest shareholders of Dynamo International and together they have the power
to direct Dynamo Internacional’s business. The securities reported by Dynamo Master Fund herein reflect (i) 13,848,333 Class A ordinary
shares and (ii) 5,977,776 Class A ordinary shares issuable upon exercise of warrants. The business address of Dynamo Master Fund is Avenida
Ataulfo de Paiva, 1235 – 6th Floor – Leblon – Rio de Janeiro – Brazil, 22440-034. |
| (4) | The securities reported by Beauty Ventures LLC herein reflect (i) 17,300,000
Class A ordinary shares and (ii) 5,766,666 Class A ordinary shares issuable upon exercise of warrants. Waldencast Long-Term Capital LLC,
our Sponsor, is the managing member of Beauty Ventures. The voting and investment power of our Sponsor is exercised jointly by Waldencast
Ventures, LP, Burwell, and Dynamo Master Fund. Waldencast Ventures, LP is controlled by Michel Brousset. Burwell Mountain PTC LLC is the
trustee of Burwell Mountain Trust, a non-grantor, fully discretionary dynasty trust duly organized under Wyoming law. Dynamo International,
is the investment manager of Dynamo Master Fund. Luiz Orenstein, Bruno Hermes da Fonseca Rudge and Luiz Felipe de Almeida Campos are the
largest shareholders of Dynamo International and together they have the power to direct Dynamo International’s business. See footnotes
2, 3, 5 and 6 for further details. |
| (5) | Reflects (i) 17,300,000 Class A ordinary shares and (ii) 5,766,666
Class A ordinary shares issuable upon exercise of warrants held directly by Beauty Ventures LLC. Waldencast Long-Term Capital LLC is the
managing member of Beauty Ventures. The voting and investment power of Waldencast Long-Term Capital LLC is exercised jointly by Waldencast
Ventures, LP, Burwell, and Dynamo Master Fund. Waldencast Ventures, LP is controlled by Michel Brousset. Burwell Mountain PTC LLC is the
trustee of Burwell Mountain Trust, a non-grantor, fully discretionary dynasty trust duly organized under Wyoming law. Dynamo International,
is the investment manager of Dynamo Master Fund. Luiz Orenstein, Bruno Hermes da Fonseca Rudge and Luiz Felipe de Almeida Campos are the
largest shareholders of Dynamo International and together they have the power to direct Dynamo International’s business. See footnotes
2, 3, 4 and 6 for further details. |
| (6) | Michel Brousset, our Chief Executive Officer, may be deemed to have sole voting and dispositive power
with regard to (i) 2,848,334 Class A ordinary shares and (ii) 2,311,113 Class A ordinary shares issuable upon exercise of warrants held
by Waldencast Ventures, LP. |
| (7) | Sijue Dai is deemed to have sole voting and dispositive power with regard to 28,237,506 Class A ordinary
shares. His business address is c/o Cedarwalk Skincare Limited, Rm 3001-3010, 30/F, China Resource Building, 26 Harbour Road, Wanchai,
Hong Kong. |
| (8) | The securities reported for ACG Dairy LLC reflect 2,306,318 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such holder
for no additional consideration). Josh Goldin and Julian Steinberg may be deemed to have shared voting and dispositive power over the
shares. The business address of ACG Dairy LLC is c/o Alliance Consumer Growth, LLC, 410 Park Ave., Suite 600, New York, NY 10022. |
| (9) | The securities reported for Amorepacific Group reflect 2,791,641 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such holder
for no additional consideration). The business address of Amorepacific Group is 100, Hangang-daero, Yongsan-gu, Seoul, Korea. |
| (10) | The securities reported for Main Post Growth Capital, L.P. reflect 6,090,058 Class A ordinary shares issuable
in exchange for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by
such holder for no additional consideration). R. Sean Honey may be deemed to have sole voting and dispositive power over the shares. The
business address of Main Post Growth Capital, L.P. is One Embarcadero Center, Suite 3500, San Francisco, CA 94111. |
| (11) | The securities reported for Dianna Spiridon Ruth reflect 396,545 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such holder
for no additional consideration). |
| (12) | The securities reported for Georgia Greville Jasper reflect 396,545 Class A ordinary shares issuable in
exchange for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such
holder for no additional consideration). |
| (13) | The securities reported for Suzanna Roberts Rassi reflect 396,545 Class A ordinary shares issuable in
exchange for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such
holder for no additional consideration). |
| (14) | The securities reported for Erez Shternlicht reflect 2,610,803 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such holder
for no additional consideration). |
| (15) | The securities reported for Mazdack Rassi reflect 2,610,803 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such holder
for no additional consideration). |
| (16) | The securities reported for Moishe Mana reflect 2,610,803 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such holder
for no additional consideration). |
| (17) | The securities reported for Scott Sassa reflect 894,164 Class A ordinary shares issuable in exchange
for Waldencast LP Common Units (and the redemption by Waldencast of an equivalent number of Class B ordinary shares held by such
holder for no additional consideration) held by Scott M. Sassa and/or the Extra-Crummey Trust dated August 25, 2014. |
| (18) | Marco Racy Kheirallah may be deemed to have sole voting and dispositive power with regard to the 1,000,000
Class A ordinary shares. The business address of Antarctic Prion Ltd. is Rua Jeronimo da Veiga, n. 45, cj 141, São Paulo –
SP, Brazil 04536-000. |
| (19) | Miguel da Cunha Gonçalves Prado may be deemed to have sole voting and dispositive power with regard
to the 4,000 Class A ordinary shares. The business address of Fitpart Capital Opportunities IC Fund is Bahamas Financial Centre, 4th
Floor, Shirley and Charlotte Street, P.O. Box CB-13515, Nassau, The Bahamas. |
| (20) | Fernando Antonio Botelho Prado
may be deemed to have sole voting and dispositive power with regard to the (i) 180,000 Class A ordinary shares and (ii) 277,767 Class
A ordinary shares issuable upon exercise of warrants. The business address of Fitpart Diversified Strategies Professional Fund LDC is
Bahamas Financial Centre, 4th Floor, Shirley and Charlotte Street, P.O. Box CB-13515, Nassau, The Bahamas. |
| (21) | Antonio Carlos de Freitas Valle
and Fernando Antonio Botelho Prado may be deemed to have shared voting and dispositive power with regard to the 800,000 Class A ordinary
shares. The business address of Fitpart Dynamic Allocation Professional Fund Ltd. is Bahamas Financial Centre, 4th Floor,
Shirley and Charlotte Street, P.O. Box CB-13515, Nassau, The Bahamas. |
| (22) | Felipe da Cunha Gonçalves
Prado and João da Cunha Gonçalves Prado may be deemed to have shared voting and dispositive power with regard to the 8,000
Class A ordinary shares. The business address of Fitpart High Yield IC Fund is Bahamas Financial Centre, 4th Floor, Shirley
and Charlotte Street, P.O. Box CB-13515, Nassau, The Bahamas. |
| (23) | Antonio da Cunha Gonçalves
Prado may be deemed to have sole voting and dispositive power with regard to the 8,000 Class A ordinary shares. The business address
of Fitpart Next Generation IC Fund is Bahamas Financial Centre, 4th Floor, Shirley and Charlotte Street, P.O. Box CB-13515,
Nassau, The Bahamas. |
| (24) | Truxt Investimentos Ltda. may
be deemed to have voting and dispositive power with regard to (i) 177,590 Class A ordinary shares and 62,791 Class A ordinary shares
issuable upon exercise of warrants held by Truxt Brazil Long Short; (ii) 2,732,237 Class A ordinary shares and 448,261 Class A ordinary
shares issuable upon exercise of warrants held by Truxt Brazil Long Bias; (iii) 52,174 Class A ordinary shares held by Truxt Brazil Macro;
(iv) 1,908,256 Class A ordinary shares and 343,543 Class A ordinary shares issuable upon exercise of warrants held by Truxt Brazil Valor;
and (v) 129,743 Class A ordinary shares and 17,362 Class A ordinary shares issuable upon exercise of warrants held by Truxt Investments
– Equity Long Only Master Fund LLC. José Alberto Tovar Barreto de Melo and Bruno de Godoy Garcia are the controlling persons
of Truxt Investimentos Ltda.and may be deemed to have shared voting and dispositive power with regard to such securities. The business
address of Truxt Investimentos Ltda. is Av. Ataulfo de Paiva, 153, 6 floor, Leblon, Rio de Janeiro, RJ, 22440-032 Brazil. |
| (25) | David Feffer, Daniel Feffer, Jorge Feffer and Ruben Feffer may be deemed
to have shared voting and dispositive power with regard to the 1,000,000 Class A ordinary shares. The business address of Kosmin Fund
Ltd. is Craigmuir Chambers – Road Town, Tortola VG 1110, British Virgin Islands. |
| (26) | The business address of Rohto
Pharmaceutical Co., Ltd. is 29F Grand Front Osaka Tower B, 3-1 Ofuka-cho. Kita-ku, Osaka 530-0011 Japan. |
| (27) | Carlos Eduardo Sanchez may
be deemed to have sole voting and dispositive power with regard to the 1,000,000 Class A ordinary shares. The business address of LMC
Investments Inc. is Goodmans Bay Corporate Centre, 2nd Floor West Bay Street, P.O. Box SP 61567, Nassau, The Bahamas. |
| (28) | The securities reported for
Marcelo Pradez de Faria Stallone reflect (i) 100,000 Class A ordinary shares and (ii) 33,333 Class A ordinary shares issuable upon exercise
of warrants. The business address of Marcelo Pradez de Faria Stallone is Avenida Prefeito Mendes De Morais, 808 AP. 1001, Rio De Janeiro,
CEP 22610-095, Brazil. |
| (29) | Marcelo Pareira Lopes de Medeiros
and Marcelo Pinto Duarte Barbará may be deemed to have shared voting and dispositive power with regard to the 200,000 Class A
ordinary shares. The business address of Strategic Portfolio Management (Bahamas) Fund Ltd is Goodman’s Bay Corporate Centre, 2nd floor,
309 Weste Bay Street, Nassau, Bahamas. |
| (30) | Edward Henrique de Sá
(herein representing WPA International L.P.) may be deemed to have sole voting and dispositive power with regard to the 1,000,000 Class
A ordinary shares. The business address of WPA International LP is Av. Prefeito Waldemar Grubba, 2633, Sala C, Vila Lalau Jaraguá
do Sul, SC Brazil 89256-900. |
| (31) | The business address of Yisroel
Zahler is 15 Holt Drive, Stony Point, NY 10980. |
| (32) | Claudio Moniz Barretto Garcia
may be deemed to have sole voting and dispositive power with regard to the 300,000 Class A ordinary shares. The business address of Azulona
LLC is c/o Katsky Korins LLP, 605 Third Avenue, New York, NY 10158. |
| (33) | LTS Investments GP Ltd. may be deemed to have sole voting
and dispositive power with regard to the 400,000 Class A ordinary shares. The business address of LTS Investments Fund LP – Class
27 is The Bahamas Financial Centre, 4th Floor, Shirley and Charlotte Street, Nassau, The Bahamas, P.O. Box 4801. |
| (34) | Amalia Cristina Spinardi Thompson Motta is the controlling person of Nexus Limited and may be deemed to
have sole voting and dispositive power with regard to the 100,000 Class A ordinary shares. The business address of Nexus Limited is 2nd
Fl., Goodman’s Bay Corporate Centre, West Bay Street, P.O. Box SP-61567, Nassau, The Bahamas. |
Description
of Share Capital
General
The following summary of
the material terms of our securities is not intended to be a complete summary of the rights and preferences of such securities. The descriptions
below are qualified by reference to the actual text of the Constitutional Document and the provisions of applicable law. We strongly urge
you to read the Constitutional Document in its entirety for a complete description of the rights and preferences of our securities. A
copy of the Constitutional Document is incorporated by reference as an exhibit to the registration statement of which this prospectus
forms a part.
Our authorized share capital
is $112,500.00 divided into 1,000,000,000 Class A ordinary shares with a par value of $0.0001 each, 100,000,000 Class B ordinary shares
with a par value of $0.0001 each and 25,000,000 preference shares with a par value of $0.0001 each.
The issued and outstanding
Class A ordinary shares are duly authorized, validly issued, fully paid and non-assessable. As of the date hereof, there were (i) Class
A ordinary shares issued and outstanding, (ii) Class B ordinary shares issued and outstanding, (iii) no Waldencast preferred shares outstanding,
(iv) private placement warrants outstanding and (v) public warrants outstanding.
Class A Ordinary Shares
Voting Rights
Each of our Class A ordinary
shares entitles the holder to one vote on all matters upon which our Class A ordinary shares are entitled to vote.
Dividends
The holders of our Class
A ordinary shares are entitled to such dividends as may be declared by our Board, subject to the Jersey Companies Law and the Constitutional
Document. Dividends and other distributions on issued and outstanding ordinary shares may be paid out of the funds of Waldencast plc lawfully
available for such purpose, subject to any preference of any of our outstanding preferred shares. Dividends and other distributions will
be distributed among the holders of our Class A ordinary shares on a pro rata basis.
Liquidation, Dissolution
and Winding Up
On a return of capital on
winding up or otherwise (other than on conversion, redemption or purchase of our Class A ordinary shares), assets available for distribution
among the holders of our Class A ordinary shares shall be distributed among the holders of our Class A ordinary shares on a pro rata basis.
Variation of Rights
The rights attached to any
class of our shares (unless otherwise provided by the terms of issue of that class), such as voting, dividends and the like, may be varied
only with the sanction of a special resolution passed at a general meeting or by the written consent of the holders of two-thirds of the
shares of that class or with the sanction of a resolution passed by a majority of not less than two thirds of the votes cast at a separate
meeting of the holders of the shares of that class. The rights conferred upon the holders of the shares of any class shall not (unless
otherwise provided by the terms of issue of that class) be deemed to be varied by the creation or issue of further shares ranking in priority
to or pari passu with such previously existing shares.
Transfer of our Class
A Ordinary Shares
Any shareholder may transfer
all or any of his or her Class A ordinary shares by an instrument of transfer in the usual or common form or any other form prescribed
by the designated stock exchange or as otherwise approved by our Board. In addition, the articles of association prohibit the transfer
of our shares in breach of the rules or regulations of the designated stock exchange or any relevant securities laws (including the Exchange
Act).
Appointment and Removal
of Directors
Our Board is divided into
three classes, Class I Directors, Class II Directors and Class III Directors, with only one class of directors being elected in each year
and each class serving a three-year term. Class I directors were elected to an initial one-year term (and three-year terms subsequently),
the Class II directors were elected to an initial two-year term (and three-year terms subsequently) and the Class III directors were elected
to an initial three-year term (and three-year terms subsequently).
Our directors may by ordinary
resolution appoint any person to be a director to fill a vacancy on our Board or as an addition to the existing Board, subject to the
remaining provisions of the Constitutional Document, the Investor Rights Agreement, applicable law and the listing rules of the designated
stock exchange. Any director so appointed shall hold office until the expiration of the terms of such class of directors or until his
earlier death, resignation or removal.
A director may be appointed
or removed from office by a simple majority of the shareholders entitled to vote by ordinary resolution.
The appointment and removal
of directors is subject to the applicable rules of the designated stock exchange and to the provisions of the Investor Rights Agreement.
The detailed procedures of
the nomination of persons proposed to be elected as directors at any of our general meetings are set out in the Constitutional Document.
Indemnification of
Directors and Officers
To the fullest extent permitted
by law, the Constitutional Document provides that our directors and officers shall be indemnified from and against all liability which
they incur in execution of their duty in their respective offices, except liability incurred by reason of such director’s or officer’s
actual fraud, willful neglect or willful default.
Class B Ordinary Shares
Voting Rights
Each of our Class B ordinary
shares entitles the holder to one vote on all matters upon which our Class B ordinary shares are entitled to vote. Holders of our Class
B ordinary shares vote together with holders of our Class A ordinary shares as a single class.
Dividends
Holders of our Class B ordinary
shares are not entitled to dividends in respect of their Class B ordinary shares.
Liquidation, Dissolution
and Winding Up
Upon our liquidation, dissolution
or winding up, the holders of our Class B ordinary shares will not be entitled to receive any of our assets, except to the extent of the
par value of their Class B ordinary shares, pro rata with the distributions to our Class A ordinary shares.
Mergers, Consolidation
or Tender or Exchange Offer
Holders of our Class B ordinary
shares are not entitled to receive consideration in the form of cash or property (other than stock consideration) in the event of a merger,
consolidation or other business combination requiring the approval of our shareholders or a tender or exchange offer to acquire any of
our ordinary shares.
Transfer
Our Class B ordinary shares
are not transferable unless a corresponding number of Waldencast LP Common Units are simultaneously transferred to the same person in
compliance with the restrictions on transfer contained in the Amended and Restated Waldencast Partners LP Agreement.
Issuance
There will be no further
issuances of our Class B ordinary shares.
Exchange
When a Waldencast LP Common
Unit is redeemed at the option of the holder of such Waldencast LP Common Unit, or, if such option is exercised, is exchanged at our option,
a corresponding Class B ordinary share will automatically be surrendered to us and retired for no consideration.
Other Provisions
Holders of our Class B ordinary
shares will not have any pre-emptive or other subscription rights.
Preferred Shares
Our Board may provide for
other classes of shares, including series of preferred shares, out of the authorized but unissued share capital, which could be utilized
for a variety of corporate purposes, including future offerings to raise capital for corporate purposes or for use in employee benefit
plans. Such additional classes of shares shall have such voting powers (full or limited or without voting powers), designations, preferences
and relative, participating, optional or other special rights and qualifications, limitations or restrictions thereof as may be determined
by our Board. If any preferred shares are issued, the rights, preferences and privileges of holders of our ordinary shares will be subject
to, and may be adversely affected by, the rights of the holders of such preferred shares.
Redeemable Warrants
Public Shareholders’
Warrants
Each whole warrant will entitle
the holder to purchase one of our Class A ordinary shares at a price of $11.50 per share, subject to adjustment as discussed below, at
any time commencing on the later of 30 days after the completion of the Business Combination and 12 months from the closing of our initial
public offering on March 18, 2021, except as described below. Pursuant to the Warrant Agreement, a warrant holder may exercise its warrants
only for a whole number of our Class A ordinary shares. This means only a whole warrant may be exercised at a given time by a warrant
holder. No fractional warrants will be issued upon separation of the units and only whole warrants will trade. The warrants will expire
on July 27, 2027, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
We will not be obligated
to deliver any of our Class A ordinary shares pursuant to the exercise of a warrant and will have no obligation to settle such warrant
exercise unless a registration statement under the Securities Act covering the issuance of our Class A ordinary shares issuable
upon exercise of the public warrants is then effective and a current prospectus relating thereto is current, subject to our satisfying
our obligations described below with respect to registration, or a valid exemption from registration is available, including in connection
with a cashless exercise permitted as a result of a notice of redemption described below under “- Redemption of warrants when
the price per Class A ordinary share equals or exceeds $10.00.” No warrant will be exercisable for cash or on a cashless basis,
and we will not be obligated to issue any shares to holders seeking to exercise their warrants, unless the issuance of the shares upon
such exercise is registered or qualified under the securities laws of the state of the exercising holder, or an exemption is available.
In the event that the conditions in the two immediately preceding sentences are not satisfied with respect to a warrant, the holder of
such warrant will not be entitled to exercise such warrant and such warrant may have no value and expire worthless.
During any period in which
our Class A ordinary shares are, at the time of any exercise of a warrant, not listed on a national securities exchange such that they
satisfy the definition of a “covered security” under Section 18(b)(1) of the Securities Act, we may, at our option, require
holders of public warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of
the Securities Act and, in the event we so elect, we will not be required to file or maintain in effect a registration statement, but
will use our commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption
is not available. In such event, each holder would pay the exercise price by surrendering the public warrants for that number of our Class
A ordinary shares equal to the lesser of (A) the quotient obtained by dividing (x) the product of the number of our Class
A ordinary shares underlying the public warrants, multiplied by the excess of the “fair market value” (defined
below) less the exercise price of the public warrants by (y) the fair market value and (B) 0.361. The “fair market
value” as used in the preceding sentence shall mean the volume weighted average price of our Class A ordinary shares for the 10
trading days ending on the trading day prior to the date on which the notice of exercise is received by the Warrant Agent.
Redemption of warrants
when the price per Class A ordinary share equals or exceeds $18.00. Once the public warrants become exercisable, we may redeem
the public warrants (except as described herein with respect to the private placement warrants):
| ● | in
whole and not in part; |
| ● | at
a price of $0.01 per warrant; |
| ● | upon
not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| ● | if,
and only if, the last reported sale price of our Class A ordinary shares for any 20 trading
days within a 30-trading day period ending on the third trading day prior to the date on
which we send the notice of redemption to the warrant holders (which we refer to as the “Reference
Value”) equals or exceeds $18.00 per share (as adjusted for adjustments to the number
of shares issuable upon exercise or the exercise price of a warrant as described under the
heading “—Redeemable Warrants—Public Shareholders’ Warrants—Anti-dilution
Adjustments”). |
We
will not redeem the public warrants as described above unless a registration statement under the Securities Act covering the
issuance of our Class A ordinary shares issuable upon exercise of the public warrants is then effective and a current prospectus relating
to those Class A ordinary shares is available throughout the 30-day redemption period. If and when the public warrants become
redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale
under all applicable state securities laws.
We have established the second
to last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant
premium to the warrant exercise price. If the foregoing conditions are satisfied and we issue a notice of redemption of the public warrants,
each warrant holder will be entitled to exercise his, her or its warrant prior to the scheduled redemption date. However, the price of
our Class A ordinary shares may fall below the $18.00 redemption trigger price (as adjusted for adjustments to the number of shares issuable
upon exercise or the exercise price of a warrant as described under the heading “—Redeemable Warrants—Public
Shareholders’ Warrants—Anti-dilution Adjustments”) as well as the $11.50 (for whole shares) warrant exercise
price after the redemption notice is issued.
Redemption of warrants
when the price per Class A ordinary share equals or exceeds $10.00. Once the public warrants become exercisable,
we may redeem the outstanding warrants:
| ● | in
whole and not in part; |
| ● | at
$0.10 per public warrant upon a minimum of 30 days’ prior written notice of redemption
provided that holders will be able to exercise their warrants on a cashless basis prior to
redemption and receive that number of shares determined by reference to the table below,
based on the redemption date and the “fair market value” of our Class A ordinary
shares (as defined below) except as otherwise described below; |
| ● | if,
and only if, the Reference Value (as defined above under “Redemption of warrants when
the price per Class A ordinary share equals or exceeds $18.00”) equals or exceeds $10.00
per share (as adjusted for adjustments to the number of shares issuable upon exercise or
the exercise price of a warrant as described under the heading “—Redeemable
Warrants—Public Shareholders’ Warrants-Anti-dilution Adjustments”);
and |
| ● | if
the Reference Value is less than $18.00 per share (as adjusted for adjustments to the number
of shares issuable upon exercise or the exercise price of a warrant as described under the
heading “—Redeemable Warrants—Public Shareholders’ Warrants—Anti-dilution
Adjustments”), the private placement warrants must also be concurrently called
for redemption on the same terms as the outstanding public warrants, as described above. |
During the period beginning
on the date the notice of redemption is given, holders may elect to exercise their warrants on a cashless basis. The numbers in the table
below represent the number of our Class A ordinary shares that a warrant holder will receive upon such cashless exercise in connection
with a redemption by us pursuant to this redemption feature, based on the “fair market value” of our Class A ordinary shares
on the corresponding redemption date (assuming holders elect to exercise their warrants and such warrants are not redeemed for $0.10 per
warrant), determined for these purposes based on volume weighted average price of our Class A ordinary shares during the 10 trading days
immediately following the date on which the notice of redemption is sent to the holders of warrants, and the number of months that the
corresponding redemption date precedes the expiration date of the public warrants, each as set forth in the table below. We will provide
our warrant holders with the final fair market value no later than one business day after the 10-trading day period described above ends.
The share prices set forth
in the column headings of the table below will be adjusted as of any date on which the number of shares issuable upon exercise of a warrant
or the exercise price of a warrant is adjusted as set forth under the heading “—Anti-dilution Adjustments” below.
If the number of shares issuable upon exercise of a warrant is adjusted, the adjusted share prices in the column headings will equal the
share prices immediately prior to such adjustment, multiplied by a fraction, the numerator of which is the number of shares deliverable
upon exercise of a warrant immediately prior to such adjustment and the denominator of which is the number of shares deliverable upon
exercise of a warrant as so adjusted. The number of shares in the table below shall be adjusted in the same manner and at the same time
as the number of shares issuable upon exercise of a warrant. If the exercise price of a warrant is adjusted, (a) in the case of an adjustment
pursuant to the fifth paragraph under the heading “—Redeemable Warrants—Public Shareholders’ Warrants—Anti-dilution
Adjustments” below, the adjusted share prices in the column headings will equal the unadjusted share price multiplied
by a fraction, the numerator of which is the higher of the Market Value and the Newly Issued Price as set forth under the heading
“—Anti-dilution Adjustments” and the denominator of which is $10.00 and (b) in the case of an adjustment pursuant
to the second paragraph under the heading “—Anti-dilution Adjustments” below, the adjusted share prices in the
column headings will equal the unadjusted share price less the decrease in the exercise price of a warrant pursuant to
such exercise price adjustment.
| |
|
Fair Market
Value of Class A Ordinary Shares |
|
Redemption
Date (period to expiration of warrants) |
|
≤$10.00 |
|
$11.00 |
|
$12.00 |
|
$13.00 |
|
$14.00 |
|
$15.00 |
|
$16.00 |
|
$17.00 |
|
≥$18.00 |
| 60 months |
|
0.261 |
|
0.281 |
|
0.297 |
|
0.311 |
|
0.324 |
|
0.337 |
|
0.348 |
|
0.358 |
|
0.361 |
| 57 months |
|
0.257 |
|
0.277 |
|
0.294 |
|
0.310 |
|
0.324 |
|
0.337 |
|
0.348 |
|
0.358 |
|
0.361 |
| 54 months |
|
0.252 |
|
0.272 |
|
0.291 |
|
0.307 |
|
0.322 |
|
0.335 |
|
0.347 |
|
0.357 |
|
0.361 |
| 51 months |
|
0.246 |
|
0.268 |
|
0.287 |
|
0.304 |
|
0.320 |
|
0.333 |
|
0.346 |
|
0.357 |
|
0.361 |
| 48 months |
|
0.241 |
|
0.263 |
|
0.283 |
|
0.301 |
|
0.317 |
|
0.332 |
|
0.344 |
|
0.356 |
|
0.361 |
| 45 months |
|
0.235 |
|
0.258 |
|
0.279 |
|
0.298 |
|
0.315 |
|
0.330 |
|
0.343 |
|
0.356 |
|
0.361 |
| 42 months |
|
0.228 |
|
0.252 |
|
0.274 |
|
0.294 |
|
0.312 |
|
0.328 |
|
0.342 |
|
0.355 |
|
0.361 |
| 39 months |
|
0.221 |
|
0.246 |
|
0.269 |
|
0.290 |
|
0.309 |
|
0.325 |
|
0.340 |
|
0.354 |
|
0.361 |
| 36 months |
|
0.213 |
|
0.239 |
|
0.263 |
|
0.285 |
|
0.305 |
|
0.323 |
|
0.339 |
|
0.353 |
|
0.361 |
| 33 months |
|
0.205 |
|
0.232 |
|
0.257 |
|
0.280 |
|
0.301 |
|
0.320 |
|
0.337 |
|
0.352 |
|
0.361 |
| 30 months |
|
0.196 |
|
0.224 |
|
0.250 |
|
0.274 |
|
0.297 |
|
0.316 |
|
0.335 |
|
0.351 |
|
0.361 |
| 27 months |
|
0.185 |
|
0.214 |
|
0.242 |
|
0.268 |
|
0.291 |
|
0.313 |
|
0.332 |
|
0.350 |
|
0.361 |
| 24 months |
|
0.173 |
|
0.204 |
|
0.233 |
|
0.260 |
|
0.285 |
|
0.308 |
|
0.329 |
|
0.348 |
|
0.361 |
| 21 months |
|
0.161 |
|
0.193 |
|
0.223 |
|
0.252 |
|
0.279 |
|
0.304 |
|
0.326 |
|
0.347 |
|
0.361 |
| 18 months |
|
0.146 |
|
0.179 |
|
0.211 |
|
0.242 |
|
0.271 |
|
0.298 |
|
0.322 |
|
0.345 |
|
0.361 |
| 15 months |
|
0.130 |
|
0.164 |
|
0.197 |
|
0.230 |
|
0.262 |
|
0.291 |
|
0.317 |
|
0.342 |
|
0.361 |
| 12 months |
|
0.111 |
|
0.146 |
|
0.181 |
|
0.216 |
|
0.250 |
|
0.282 |
|
0.312 |
|
0.339 |
|
0.361 |
| 9 months |
|
0.090 |
|
0.125 |
|
0.162 |
|
0.199 |
|
0.237 |
|
0.272 |
|
0.305 |
|
0.336 |
|
0.361 |
| 6 months |
|
0.065 |
|
0.099 |
|
0.137 |
|
0.178 |
|
0.219 |
|
0.259 |
|
0.296 |
|
0.331 |
|
0.361 |
| 3 months |
|
0.034 |
|
0.065 |
|
0.104 |
|
0.150 |
|
0.197 |
|
0.243 |
|
0.286 |
|
0.326 |
|
0.361 |
| 0 months |
|
— |
|
— |
|
0.042 |
|
0.115 |
|
0.179 |
|
0.233 |
|
0.281 |
|
0.323 |
|
0.361 |
This redemption feature differs
from the typical warrant redemption features used in many other blank check offerings, which typically only provide for a redemption of
warrants for cash (other than the private placement warrants) when the trading price for our Class A ordinary shares exceeds $18.00 per
share for a specified period of time. This redemption feature is structured to allow for all of the outstanding warrants to be redeemed
when our Class A ordinary shares are trading at or above $10.00 per share, which may be at a time when the trading price of our Class
A ordinary shares is below the exercise price of the public warrants. We have established this redemption feature to provide us with the
flexibility to redeem the public warrants without the public warrants having to reach the $18.00 per share threshold set forth above under
“—Redemption of warrants when the price per Class A ordinary share equals or exceeds $18.00.” Holders choosing
to exercise their warrants in connection with a redemption pursuant to this feature will, in effect, receive a number of shares for their
warrants based on an option pricing model. This redemption right provides us with an additional mechanism by which to redeem all of the
outstanding warrants, and therefore have certainty as to our capital structure as the public warrants would no longer be outstanding and
would have been exercised or redeemed. We will be required to pay the applicable redemption price to warrant holders if we choose to exercise
this redemption right and it will allow us to quickly proceed with a redemption of the public warrants if we determine it is in our best
interest to do so. As such, we would redeem the public warrants in this manner when we believe it is in our best interest to update our
capital structure to remove the public warrants and pay the redemption price to the warrant holders.
As stated above, we can redeem
the public warrants when our Class A ordinary shares are trading at a price starting at $10.00, which is below the exercise price of $11.50,
because it will provide certainty with respect to our capital structure and cash position while providing warrant holders with the opportunity
to exercise their warrants on a cashless basis for the applicable number of shares. If we choose to redeem the public warrants when our
Class A ordinary shares are trading at a price below the exercise price of the public warrants, this could result in the warrant holders
receiving fewer of our Class A ordinary shares than they would have received if they had chosen to wait to exercise their warrants for
our Class A ordinary shares if and when such Class A ordinary shares were trading at a price higher than the exercise price of $11.50.
No fractional Class A ordinary
shares will be issued upon exercise. If, upon exercise, a holder would be entitled to receive a fractional interest in a share, we will
round down to the nearest whole number of the number of our Class A ordinary shares to be issued to the holder. If, at the time of redemption,
the public warrants are exercisable for a security other than our Class A ordinary shares pursuant to the Warrant Agreement (for instance,
if we are not the surviving company in the Business Combination), the public warrants may be exercised for such security. At such time
as the public warrants become exercisable for a security other than our Class A ordinary shares, we (or surviving company) will use our
commercially reasonable efforts to register under the Securities Act the security issuable upon the exercise of the public warrants.
Redemption Procedures.
A holder of a warrant may notify us in writing in the event it elects to be subject to a requirement that such holder will not have the
right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s
affiliates), to the Warrant Agent’s actual knowledge, would beneficially own in excess of 9.8% (or such other amount as a holder
may specify) of our Class A ordinary shares issued and outstanding immediately after giving effect to such exercise.
Anti-dilution Adjustments.
If the number of issued and outstanding Class A ordinary shares is increased by a capitalization or share dividend payable in our Class
A ordinary shares, or by a split-up of our Class A ordinary shares or other similar event, then, on the effective date of such capitalization
or share dividend, split-up or similar event, the number of our Class A ordinary shares issuable on exercise of each warrant will be increased
in proportion to such increase in the issued and outstanding Class A ordinary shares. A rights offering made to all or substantially all
holders of our Class A ordinary shares entitling holders to purchase our Class A ordinary shares at a price less than the “historical
fair market value” (as defined below) will be deemed a share dividend of a number of our Class A ordinary shares equal to the product
of (1) the number of our Class A ordinary shares actually sold in such rights offering (or issuable under any other equity securities
sold in such rights offering that are convertible into or exercisable for our Class A ordinary shares) and (2) one minus the quotient
of (x) the price per Class A ordinary share paid in such rights offering and (y) the historical fair market value. For these purposes,
(1) if the rights offering is for securities convertible into or exercisable for our Class A ordinary shares, in determining the price
payable for our Class A ordinary shares, there will be taken into account any consideration received for such rights, as well as any additional
amount payable upon exercise or conversion and (2) “historical fair market value” means the volume weighted average price
of our Class A ordinary shares during the 10 trading day period ending on the trading day prior to the first date on which our Class A
ordinary shares trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if we, at any
time while the public warrants are outstanding and unexpired, pay to all or substantially all of the holders of our Class A ordinary shares
a dividend or make a distribution in cash, securities or other assets to the holders of our Class A ordinary shares on account of such
Class A ordinary shares (or other securities into which the public warrants are convertible), other than (a) as described above, or (b)
any cash dividends or cash distributions which, when combined on a per share basis with all other cash dividends and cash distributions
paid on our Class A ordinary shares during the 365-day period ending on the date of declaration of such dividend or distribution does
not exceed $0.50 (as adjusted for share sub-divisions, share dividends, rights issuances, consolidations, reorganizations, recapitalizations
and other similar transactions) but only with respect to the amount of the aggregate cash dividends or cash distributions equal to or
less than $0.50 per share, then the warrant exercise price will be decreased, effective immediately after the effective date of such event,
by the amount of cash and/or the fair market value of any securities or other assets paid on each Class A ordinary share in respect of
such event.
If the number of issued and
outstanding Class A ordinary shares is decreased by a consolidation, combination, reverse share sub-division or reclassification of our
Class A ordinary shares or other similar event, then, on the effective date of such consolidation, combination, reverse share sub-division,
reclassification or similar event, the number of our Class A ordinary shares issuable on exercise of each warrant will be decreased in
proportion to such decrease in issued and outstanding Class A ordinary shares.
Whenever the number of our
Class A ordinary shares purchasable upon the exercise of the public warrants is adjusted, as described above, the warrant exercise price
will be adjusted by multiplying the warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which
will be the number of our Class A ordinary shares purchasable upon the exercise of the public warrants immediately prior to such adjustment
and (y) the denominator of which will be the number of our Class A ordinary shares so purchasable immediately thereafter.
In case of any reclassification
or reorganization of the issued and outstanding Class A ordinary shares (other than those described above or that solely affects the par
value of such Class A ordinary shares), or in the case of any merger or consolidation of us with or into another corporation (other than
a merger or consolidation in which we are the continuing corporation and that does not result in any reclassification or reorganization
of our issued and outstanding Class A ordinary shares), or in the case of any sale or conveyance to another corporation or entity of the
assets or other property of us as an entirety or substantially as an entirety in connection with which we are dissolved, the holders of
the public warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified
in the public warrants and in lieu of our Class A ordinary shares immediately theretofore purchasable and receivable upon the exercise
of the rights represented thereby, the kind and amount of shares, stock or other equity securities or property (including cash) receivable
upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the
holder of the public warrants would have received if such holder had exercised their warrants immediately prior to such event. However,
if such holders were entitled to exercise a right of election as to the kind or amount of securities, cash or other assets receivable
upon such merger or consolidation, then the kind and amount of securities, cash or other assets for which each warrant will become exercisable
will be deemed to be the weighted average of the kind and amount received per share by such holders in such merger or consolidation that
affirmatively make such election, and if a tender, exchange or redemption offer has been made to and accepted by such holders under circumstances
in which, upon completion of such tender or exchange offer, the maker thereof, together with members of any group (within the meaning
of Rule 13d-5(b)(1) under the Exchange Act) of which such maker is a part, and together with any affiliate or associate
of such maker (within the meaning of Rule 12b-2 under the Exchange Act) and any members of any such group of which any
such affiliate or associate is a part, own beneficially (within the meaning of Rule 13d-3 under the Exchange Act) more
than 50% of the issued and outstanding Class A ordinary shares, the holder of a warrant will be entitled to receive the highest amount
of cash, securities or other property to which such holder would actually have been entitled as a shareholder if such warrant holder had
exercised the warrant prior to the expiration of such tender or exchange offer, accepted such offer and all of our Class A ordinary shares
held by such holder had been purchased pursuant to such tender or exchange offer, subject to adjustment (from and after the consummation
of such tender or exchange offer) as nearly equivalent as possible to the adjustments provided for in the Warrant Agreement. Additionally,
if less than 70% of the consideration receivable by the holders of our Class A ordinary shares in such a transaction is payable in the
form of ordinary shares in the successor entity that is listed for trading on a national securities exchange or is quoted in an established
over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of
the warrant properly exercises the warrant within 30 days following public disclosure of such transaction, the warrant exercise price
will be reduced as specified in the Warrant Agreement based on the per share consideration minus Black-Scholes Warrant
Value (as defined in the Warrant Agreement) of the warrant.
The warrants are issued in
registered form under a Warrant Agreement between the Warrant Agent and us. The Warrant Agreement provides that (a) the terms of the public
warrants may be amended without the consent of any holder for the purpose of (i) curing any ambiguity or correct any mistake, including
to conform the provisions of the Warrant Agreement to the description of the terms of the public warrants and the Warrant Agreement set
forth in this prospectus, or defective provision or (ii) adding or changing any provisions with respect to matters or questions arising
under the Warrant Agreement as the parties to the Warrant Agreement may deem necessary or desirable and that the parties deem to not adversely
affect the rights of the registered holders of the public warrants and (b) all other modifications or amendments require the vote or written
consent of at least 65% of the then outstanding public warrants and, solely with respect to any amendment to the terms of the private
placement warrants or working capital warrants or any provision of the Warrant Agreement with respect to the private placement warrants,
forward purchase warrants or working capital warrants, at least 65% of the then outstanding private placement warrants or working capital
warrants, respectively. You should review a copy of the Warrant Agreement, which is filed as an exhibit to the registration statement
of which this prospectus forms part, for a complete description of the terms and conditions applicable to the public warrants.
The warrant holders do not
have the rights or privileges of holders of ordinary shares and any voting rights until they exercise their warrants and receive our Class
A ordinary shares. After the issuance of our Class A ordinary shares upon exercise of the public warrants, each holder will be entitled
to one vote for each share held of record on all matters to be voted on by shareholders.
We have agreed that, subject
to applicable law, any action, proceeding or claim against us arising out of or relating in any way to the Warrant Agreement will be brought
and enforced in the courts of the State of New York or the U.S. District Court for the Southern District of New York, and we irrevocably
submit to such jurisdiction, which jurisdiction will be the exclusive forum for any such action, proceeding or claim. This provision applies
to claims under the Securities Act but does not apply to claims under the Exchange Act or any claim for which the
federal district courts of the U.S. are the sole and exclusive forum.
Private Placement
Warrants
So long as they are held
by our Sponsor or its permitted transferees, the private placement warrants will not be transferable, assignable or salable until 30
days after the Closing Date (except, among other limited exceptions, to our directors and officers and other persons or entities affiliated
with our Sponsor) and they will not be redeemable by us (except as described above under “—Redeemable Warrants—Public
Shareholders’ Warrants—Redemption of warrants when the price per Class A ordinary share equals or exceeds $10.00”)
so long as they are held by our Sponsor or its permitted transferees. Our Sponsor, or its permitted transferees, has the option to exercise
the private placement warrants on a cashless basis and have certain registration rights described herein.
Otherwise, the private placement
warrants have terms and provisions that are identical to those of the public warrants. If the private placement warrants are held by holders
other than our Sponsor or its permitted transferees, the private placement warrants will be redeemable by us in all redemption scenarios
and exercisable by the holders on the same basis as the public warrants.
Except as described under
“—Redeemable Warrants—Public Shareholders’ Warrants—Redemption of warrants when the price per Class A
ordinary share equals or exceeds $10.00,” if holders of the private placement warrants elect to exercise them on a cashless
basis, they would pay the exercise price by surrendering his, her or its warrants for that number of our Class A ordinary shares equal
to the quotient obtained by dividing (x) the product of the number of our Class A ordinary shares underlying the public warrants, multiplied
by the excess of the “historical fair market value” (defined below) less the exercise price of the public warrants by (y)
the historical fair market value. For these purposes, the “historical fair market value” shall mean the average last reported
sale price of our Class A ordinary shares for the 10 trading days ending on the third trading day prior to the date on which the notice
of warrant exercise is sent to the Warrant Agent.
Other Jersey, Channel Islands
Law Considerations
Purchase of our Own
Ordinary Shares
As with declaring a dividend,
we may not buy back or redeem our shares unless our directors who are to authorize the buyback or redemption have made a statutory solvency
statement that, immediately following the date on which the buyback or redemption is proposed, we will be able to discharge our liabilities
as they fall due and, having regard to prescribed factors, we will be able to continue to carry on business and discharge our liabilities
as they fall due for the 12 months immediately following the date on which the buyback or redemption is proposed (or until we are dissolved
on a solvent basis, if earlier).
If the above conditions are
met, we may purchase our ordinary shares in the manner described below.
We may purchase on a stock
exchange our own fully paid ordinary shares pursuant to a special resolution of our shareholders.
We may purchase our own fully
paid ordinary shares other than on a stock exchange pursuant to a special resolution of our shareholders, but only if the purchase is
made on the terms of a written purchase contract which has been approved in advance by an ordinary resolution of our shareholders. The
shareholder from whom we propose to purchase or redeem ordinary shares is not entitled to vote in respect of the ordinary shares to be
purchased.
We may fund a redemption
or purchase of our own ordinary shares from any source. We cannot purchase our ordinary shares if, as a result of such purchase, only
redeemable ordinary shares would remain in issue. If authorized by a resolution of our shareholders, any shares that we redeem or purchase
may be held by us as treasury shares. Any shares held by us as treasury shares may be cancelled, sold, transferred for the purposes of
or under an employee share scheme or held without cancelling, selling or transferring them. Shares redeemed or purchased by us are cancelled
where we have not been authorized to hold such shares as treasury shares.
Mandatory Purchases
and Acquisitions
The Jersey Companies Law
provides that where a person has made an offer to acquire a class or all of our outstanding ordinary shares not already held by the person
and has as a result of such offer acquired or contractually agreed to acquire 90% or more of such outstanding ordinary shares, that person
is then entitled (and may be required) to acquire the remaining ordinary shares. In such circumstances, a holder of any such remaining
ordinary shares may apply to the courts of Jersey for an order that the person making such offer not be entitled to purchase the holder’s
ordinary shares or that the person purchase the holder’s ordinary shares on terms different to those under which the person made
such offer.
Other than as described below
under “—U.K. City Code on Takeovers and Mergers,” we are not subject to any regulations under which a shareholder
that acquires a certain level of share ownership is then required to offer to purchase all of our remaining ordinary shares on the same
terms as such shareholder’s prior purchase.
Compromises and Arrangements
Where we and our creditors
or shareholders or a class of either of them propose a compromise or arrangement between us and our creditors or our shareholders or a
class of either of them (as applicable), the courts of Jersey may order a meeting of the creditors or class of creditors or of our shareholders
or class of shareholders (as applicable) to be called in such a manner as the court directs. Any compromise or arrangement approved by
a majority in number present and voting at the meeting representing 75% or more in value of the creditors or 75% or more of the voting
rights of shareholders or class of either of them (as applicable) if sanctioned by the court, is binding upon us and all the creditors,
shareholders or members of the specific class of either of them (as applicable).
Whether our capital is to
be treated as being divided into a single or multiple class(es) of shares is a matter to be determined by the court. The court may in
its discretion treat a single class of shares as multiple classes, or multiple classes of shares as a single class, for the purposes of
the shareholder approval referred to above taking into account all relevant circumstances, which may include circumstances other than
the rights attaching to the shares themselves.
U.K. City Code on
Takeovers and Mergers
The U.K. City Code on Takeovers
and Mergers (the “Takeover Code”) applies, among other things, (i) to an offer for a public company whose registered office
is in the Channel Islands and whose securities are admitted to trading on a regulated market or a multilateral trading facility in the
United Kingdom or any stock exchange in the Channel Islands or the Isle of Man, or (ii) if the company is a public company and is considered
by the Panel on Takeovers and Mergers (the “Takeover Panel”), to have its place of central management and control in the United
Kingdom or the Channel Islands or the Isle of Man (in each case, a “Code Company”). This is known as the “residency
test.” Under the Takeover Code, the Takeover Panel will determine whether we have our place of central management and control in
the United Kingdom, the Channel Islands or the Isle of Man by looking at various factors, including the structure of our Board, the functions
of the directors, and where they are resident.
If at the time of a takeover
offer, the Takeover Panel determines that the residency test is satisfied and we have our place of central management and control in
the United Kingdom, the Channel Islands or the Isle of Man, we would be subject to a number of rules and restrictions, including but
not limited to the following: (i) our ability to enter into deal protection arrangements with a bidder would be extremely limited; (ii)
we might not, without the approval of our shareholders, be able to perform certain actions that could have the effect of frustrating
an offer, such as issuing shares or carrying out acquisitions or disposals; and (iii) we would be obliged to provide equality of information
to all bona fide competing bidders. The Takeover Code also contains certain
rules in respect of mandatory offers for Code Companies. Under Rule 9 of the Takeover Code, if a person:
| ● | acquires
an interest in shares of a Code Company that, when taken together with shares in which persons
acting in concert with such person are interested, carry 30% or more of the voting rights
of the Code Company; or |
| ● | who, together with persons acting in concert with such person, is interested in shares that in the aggregate
carry not less than 30% and not more than 50% of the voting rights in the Code Company, acquires additional interests in shares that increase
the percentage of shares carrying voting rights in which that person is interested, |
the acquirer, and, depending on the circumstances,
its concert parties, would be required (except with the consent of the Takeover Panel) to make a cash offer (or provide a cash alternative)
for the Code Company’s outstanding shares at a price not less than the highest price paid for any interests in the shares by the
acquirer or its concert parties during the previous 12 months.
We have our place of central
management and control in Jersey (one of the Channel Islands). Therefore, based upon our current and intended plans for our directors
and management, for the purposes of the Takeover Code, we believe that the residency test is met. Therefore, the Takeover Code should
apply to us. It is possible that in the future changes in our Board’s composition, changes in the Takeover Panel’s interpretation
of the Takeover Code, or other events may cause the Takeover Code not to apply to us.
Jersey Regulatory
Matters
The JFSC has given, and has
not withdrawn, its consent under Article 2 of the Control of Borrowing (Jersey) Order 1958 to the issue of our ordinary shares. The JFSC
is protected by the Control of Borrowing (Jersey) Law 1947 against any liability arising from the discharge of its functions under that
law.
Transfer Agent and Warrant
Agent
The transfer agent for our
Class A ordinary shares and warrant agent for our warrants is Continental.
U.S.
Federal Income Tax Considerations
The
following is a discussion of U.S. federal income tax considerations generally applicable to the ownership and disposition of Class A ordinary
shares or public warrants by U.S. Holders. This discussion addresses only those holders of Waldencast securities that hold Class A ordinary
shares and warrants as capital assets within the meaning of the Code (generally, property held for investment), and assumes that any distributions
made (or deemed made) by us on our securities and any consideration received (or deemed received) by a holder in consideration for the
sale or other disposition of our securities will be in U.S. dollars. This discussion does not discuss all aspects of U.S. federal income
taxation that may be relevant to holders in light of their particular circumstances or status, including:
| ● | the
Sponsor or our officers or directors; |
| ● | financial
institutions or financial services entities; |
| ● | taxpayers
that are subject to the mark-to-market accounting rules; |
| ● | governments
or agencies or instrumentalities thereof; |
| ● | regulated
investment companies or real estate investment trusts; |
| ● | expatriates
or former long-term residents of the U.S.; |
| ● | persons
that actually or constructively own five percent or more of our voting shares or five percent or more of the total value of any class
of our shares; |
| ● | persons
that acquired our ordinary shares pursuant to an exercise of employee share options, in connection with employee share incentive plans
or otherwise as compensation or in connection with the performance of services; |
| ● | persons
that hold our ordinary shares as part of a straddle, constructive sale, hedging, conversion or other integrated or similar transaction;
and |
| ● | persons
whose functional currency is not the U.S. dollar. |
This
discussion is based on the Code, proposed, temporary and final Treasury Regulations promulgated under the Code, and judicial and administrative
interpretations thereof, all as of the date hereof. All of the foregoing is subject to change, which change could apply retroactively
and could affect the tax considerations described herein. This discussion does not address U.S. federal taxes other than those pertaining
to U.S. federal income taxation (such as estate or gift taxes, the alternative minimum tax or the Medicare tax on investment income),
nor does it address any aspects of U.S. state or local or non-U.S. taxation.
We
have not and do not intend to seek any rulings from the IRS regarding any of the U.S. federal income tax considerations described herein.
There can be no assurance that the IRS will not take positions inconsistent with the considerations discussed below or that any such positions
would not be sustained by a court.
This
discussion does not consider the tax treatment of partnerships or other pass-through entities or persons who hold our securities through
such entities. If a partnership (or any entity or arrangement so characterized for U.S. federal income tax purposes) holds our securities,
the tax treatment of such partnership and a person treated as a partner of such partnership will generally depend on the status of the
partner and the activities of the partnership. Partnerships holding any of our securities and persons that are treated as partners of
such partnerships should consult their tax advisors.
EACH
HOLDER SHOULD CONSULT ITS TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH HOLDER OF THE OWNERSHIP AND DISPOSITION
OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECTS OF U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX LAWS, AS WELL AS ANY APPLICABLE
TAX TREATIES.
As
used herein, a “U.S. Holder” is a beneficial owner of Class A ordinary shares (as the case may be) who or that is, for U.S.
federal income tax purposes:
| 1. | an individual citizen or resident of the U.S., |
| 2. | a corporation (or other entity that is treated as a corporation
for U.S. federal income tax purposes) that is created or organized (or treated as created or organized) in or under the laws of the U.S.
or any state thereof or the District of Columbia, |
| 3. | an estate whose income is subject to U.S. federal income
tax regardless of its source, or |
| 4. | a trust if (i) a U.S. court can exercise primary supervision
over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of the trust
or (ii) it has a valid election in place to be treated as a U.S. person. |
Tax Residence of Waldencast
for U.S. Federal Income Tax Purposes
A
corporation is generally considered for U.S. federal income tax purposes to be a tax resident in the jurisdiction of its organization
or incorporation. Section 7874 of the Code provides an exception to this general rule, under which a non-U.S. incorporated entity may,
in certain circumstances, be treated as a U.S. corporation for U.S. federal income tax purposes. These rules are complex and there is
limited guidance regarding their application.
Based
on the rules in effect currently and at the time of the Business Combination, the relative values of Obagi and Milk, and the terms of
the Business Combination, we do not expect Waldencast, which is incorporated under the laws of Jersey, to be treated as a U.S. corporation
for U.S. federal income tax purposes by virtue of Section 7874 of the Code as a result of the Business Combination. Nevertheless, because
the rules and exceptions under Section 7874 of the Code are complex, subject to factual and legal uncertainties, and may change in the
future (possibly with retroactive effect), there can be no assurance that we will not be treated as a U.S. corporation for U.S. federal
income tax purposes. In addition, it is possible that a future acquisition of the stock or assets of a U.S. corporation could result in
our being treated as a U.S. corporation at the time of the Business Combination.
If
we were to be treated as a U.S. corporation for U.S. federal income tax purposes, we could be subject to liability for additional U.S.
income taxes, and the gross amount of any dividend payments to our non-U.S. shareholders could be subject to 30% U.S. withholding tax,
depending on the application of any income tax treaty that might apply to reduce the withholding tax. If Holdco 1 were to be disregarded,
or Waldencast were otherwise to be treated as a direct partner in Waldencast LP, dividend payments by us could be treated as wholly or
partially U.S.-source for foreign tax credit and other U.S. federal income tax purposes even if Waldencast plc is treated as a non-U.S.
corporation under Section 7874 of the Code.
The
remainder of this discussion assumes that we will not be treated as a U.S. corporation for U.S. federal income tax purposes under Section
7874 of the Code.
U.S. Federal Income
Tax Considerations of the Ownership and Disposition of Our Securities
Taxation of Dividends
and Other Distributions on Class A ordinary shares
Subject
to the PFIC rules discussed below, if we make a distribution of cash or other property to a U.S. Holder of Class A ordinary shares, such
distribution will generally be treated as a dividend for U.S. federal income tax purposes to the extent the distribution is paid out of
our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Such dividends will be taxable
to a corporate U.S. Holder at regular rates and will not be eligible for the dividends-received deduction generally allowed to domestic
corporations in respect of dividends received from other domestic corporations.
Distributions
in excess of such earnings and profits will generally be applied against and reduce the U.S. Holder’s basis in its Class A ordinary
shares (but not below zero) and, to the extent in excess of such basis, will be treated as gain from the sale or exchange of such Class
A ordinary shares. We may not determine our earnings and profits on the basis of U.S. federal income tax principles, however, in which
case any distribution paid by us will be treated as a dividend.
With
respect to non-corporate U.S. Holders, dividends will generally be taxed at preferential long-term capital gains rates only if (i) the
Class A ordinary shares are readily tradable on an established securities market in the U.S. or (ii) we are eligible for the benefits
of an applicable income tax treaty, in each case provided that we are not treated as a PFIC in the taxable year in which the dividend
was paid or in the previous year and certain holding period or other requirements are met. However, it is unclear whether the redemption
rights with respect to the Waldencast Class A ordinary shares may have prevented the holding period of the Waldencast plc Class A ordinary
shares from commencing prior to the termination of such rights. U.S. Holders should consult their tax advisors regarding the availability
of the lower rate for any dividends paid with respect to our Class A ordinary shares.
Possible Constructive
Distributions
The
terms of each warrant provide for an adjustment to the number of shares for which the warrant may be exercised or to the exercise price
of the warrant in certain events. An adjustment which has the effect of preventing dilution generally is not taxable. However, the U.S.
Holders of our warrants would be treated as receiving a constructive distribution from us if, for example, the adjustment increases the
warrant holders’ proportionate interest in our assets or earnings and profits (e.g., through an increase in the number of ordinary
shares that would be obtained upon exercise or through a decrease to the exercise price) as a result of a distribution of cash to the
holders of our ordinary shares which is taxable to the holders of such ordinary shares as a distribution. Such constructive distribution
would be subject to tax as if the U.S. Holders of our warrants received a cash distribution from us equal to the fair market value of
such increased interest.
Taxation on the Disposition
of our Class A ordinary shares and Warrants
Subject
to the PFIC rules discussed below, upon a sale or other taxable disposition of ordinary shares or warrants, a U.S. Holder will generally
recognize capital gain or loss. The amount of gain or loss recognized will generally be equal to the difference between (i) the sum of
the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. Holder’s adjusted tax
basis in such ordinary shares or warrants disposed of. A U.S. Holder’s adjusted tax basis in its ordinary shares or warrants will
generally equal the U.S. Holder’s acquisition cost reduced by any prior distributions treated as a return of capital. See “—Exercise,
Lapse or Redemption of a Warrant” below for a discussion regarding a U.S. Holder’s basis in an ordinary share acquired
pursuant to the exercise of a warrant.
Under
tax law currently in effect, long-term capital gains recognized by non-corporate U.S. Holders are generally subject to U.S. federal income
tax at a reduced rate of tax. Capital gain or loss will constitute long-term capital gain or loss if the U.S. Holder’s holding period
for the ordinary shares or warrants exceeds one year. However, it is unclear whether the redemption rights with respect to the Class A
ordinary shares may have prevented the holding period of the Class A ordinary shares from commencing prior to the termination of such
rights. The deductibility of capital losses is subject to various limitations.
Exercise, Lapse or
Redemption of a Warrant
Subject
to the PFIC rules discussed below and except as discussed below with respect to the cashless exercise of a warrant, a U.S. Holder will
generally not recognize gain or loss upon the exercise of a warrant. An ordinary share acquired pursuant to the exercise of a warrant
for cash will generally have a tax basis equal to the U.S. Holder’s tax basis in the warrant, increased by the amount paid to exercise
the warrant. It is unclear whether a U.S. Holder’s holding period for the ordinary share will commence on the date of exercise of
the warrant or the day following the date of exercise of the warrant; in either case, the holding period will not include the period during
which the U.S. Holder held the warrant. If a warrant is allowed to lapse unexercised, a U.S. Holder will generally recognize a capital
loss equal to such holder’s tax basis in the warrant.
The
tax consequences of a cashless exercise of a warrant are not clear under current U.S. federal income tax law. A cashless exercise may
be tax-free, either because the exercise is not a realization event or because the exercise is treated as a “recapitalization”
within the meaning of Section 368(a)(1)(E) of the Code. Although we expect a U.S. Holder’s cashless exercise of our warrants (including
after we provide notice of our intent to redeem warrants for cash) to be treated as a recapitalization, a cashless exercise could alternatively
be treated as a taxable exchange in which gain or loss would be recognized.
In
either tax-free situation, a U.S. Holder’s tax basis in the ordinary shares received generally would equal the U.S. Holder’s
tax basis in the warrants. If the cashless exercise was not a realization event, it is unclear whether a U.S. Holder’s holding period
for the ordinary shares would be treated as commencing on the date of exercise of the warrant or the day following the date of exercise
of the warrant. If the cashless exercise were treated as a recapitalization, the holding period of the ordinary shares would include the
holding period of the warrants.
It
is also possible that a cashless exercise could be treated as a taxable exchange in which gain or loss would be recognized. In such event,
a U.S. Holder could be deemed to have surrendered warrants with an aggregate fair market value equal to the exercise price for the total
number of warrants to be exercised. The U.S. Holder would recognize capital gain or loss in an amount equal to the difference between
the fair market value of the warrants deemed surrendered and the U.S. Holder’s adjusted tax basis in such warrants. In this case,
a U.S. Holder’s tax basis in the ordinary shares received would equal the sum of the U.S. Holder’s initial investment in the
warrants exercised and the exercise price of such warrants. It is unclear whether a U.S. Holder’s holding period for the ordinary
shares would commence on the date of exercise of the warrants or the day following the date of exercise of the warrants.
Because
of the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any,
of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S.
Holders should consult their tax advisors regarding the tax consequences of a cashless exercise.
Subject
to the PFIC rules described below, if we redeem warrants for cash pursuant to the redemption provisions described in the section of this
prospectus entitled “Description of Share Capital—Redeemable Warrants—Public Shareholders’ Warrants”
or if we purchase warrants in an open market transaction, such redemption or purchase will generally be treated as a taxable disposition
to the U.S. Holder, taxed as described above under “—Taxation on the Disposition of Waldencast plc Class A Ordinary Shares
and Warrants.”
PFIC Considerations
Definition of a PFIC
A
foreign (i.e., non-U.S.) corporation will be a PFIC for U.S. federal income tax purposes if at least 75% of its gross income in a taxable
year of the foreign corporation, including its pro rata share of the gross income of any corporation in which it is considered to own
at least 25% of the shares by value, is passive income. Alternatively, a foreign corporation will be a PFIC if at least 50% of its assets
in a taxable year of the foreign corporation, ordinarily determined based on fair market value and averaged quarterly over the year, including
its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for
the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than
certain rents or royalties derived from the active conduct of a trade or business) and gains from the disposition of passive assets.
Pursuant
to a start-up exception, a corporation will not be a PFIC for the first taxable year the corporation has gross income, if (1) no predecessor
of the foreign corporation was a PFIC; (2) the corporation satisfies the IRS that it will not be a PFIC for either of the first two taxable
years following the start-up year; and (3) the corporation is not in fact a PFIC for either of those years.
PFIC Status of Waldencast
Although
a foreign corporation’s PFIC determination will be made annually, absent certain elections described below, a determination that
Waldencast Acquisition Corp. or Waldencast is or was a PFIC during the holding period of a U.S. Holder will continue to apply to subsequent
years in which a U.S. Holder continues to hold shares in such entity (including a successor entity), whether or not such entity is a PFIC
in those subsequent years. Because, following the Domestication, Waldencast is treated as the successor to Waldencast Acquisition Corp.
for U.S. federal income tax purposes, any Class A ordinary shares received on the exercise of a warrant treated as exchanged for a Waldencast
Acquisition Corp. warrant in the Domestication may, in the absence of certain elections described below, be treated as stock of a PFIC
if Waldencast Acquisition Corp. was treated as a PFIC during the holding period of a U.S. Holder.
Based
on the anticipated assets and income of the combined company and the application of the start-up exception, neither Waldencast Acquisition
Corp. nor its successor Waldencast is currently expected to be treated as a PFIC for our prior taxable year ending on December 31, 2021
(the “Start-Up Year”), the current taxable year ending on December 31, 2022, or the foreseeable future. However, as further
discussed below, the facts on which any determination of PFIC status are based may not be known until the close of each taxable year in
question, and, in the case of the Start-Up Year, until as late as the close of the taxable year ending on December 31. 2023. Additionally,
there is uncertainty regarding the application of the start-up exception.
Although
Waldencast Acquisition Corp. likely met the PFIC income or asset tests for the Start-Up Year, the start-up exception is expected to apply
to prevent such entity from being treated as a PFIC for the Start-Up Year provided that the combined company does not meet either test
in the two taxable years subsequent to the Start-Up Year. Based on the timing of the Business Combination and the anticipated assets and
income of the combined company, Waldencast is not expected to meet either test for the current taxable year or the foreseeable future.
However, the precise facts on which any such determination must be based will not be known until the end of the current taxable year.
Waldencast may be a PFIC for the current taxable year, or become a PFIC in the future, if the composition of its income or assets, or
the market price of our Class A ordinary shares, were to change, regardless of whether our income and asset composition are as expected
or the start-up exception applies. Accordingly, there can be no assurance with respect to the PFIC status of Waldencast Acquisition Corp.
or Waldencast for the Start-Up Year, the current taxable year, or any future taxable year.
Application of PFIC Rules
to Ordinary Shares and Warrants
If
(i) Waldencast Acquisition Corp. or Waldencast is determined to be a PFIC for any taxable year (or portion thereof) that is included in
the holding period of a U.S. Holder and (ii) the U.S. Holder did not make a timely and effective QEF Election (as defined below) for the
first year in its holding period in which Waldencast Acquisition Corp. or Waldencast (as the case may be) is a PFIC (such taxable year
as it relates to each U.S. Holder, the “First PFIC Holding Year”), a QEF Election along with a purging election, or a “mark-to-market”
election, each as described below under “QEF Election, Mark-to-Market Election and Purging Election,” then such holder will
generally be subject to special rules (the “Default PFIC Regime”) with respect to:
| ● | any
gain recognized by the U.S. Holder on the sale or other disposition of its ordinary shares or warrants; and |
| ● | any
“excess distribution” made to the U.S. Holder (generally, any distributions to such U.S. Holder during a taxable year of
the U.S. Holder that are greater than 125% of the average annual distributions received by such U.S. Holder in respect of its ordinary
shares or warrants during the three preceding taxable years of such U.S. Holder or, if shorter, such U.S. Holder’s applicable holding
period). |
Under
the Default PFIC Regime:
| ● | the
U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period for its ordinary
shares (taking into account the relevant holding period of any warrants exercised therefor); |
| ● | the
amount of gain allocated to the U.S. Holder’s taxable year in which the U.S. Holder recognized the gain or received the excess
distribution, or to the period in the U.S. Holder’s holding period before the first day of the first taxable year in which Waldencast
Acquisition Corp. was or Waldencast is a PFIC, will be taxed as ordinary income; |
| ● | the
amount of gain allocated to other taxable years (or portions thereof) of the U.S. Holder and included in such U.S. Holder’s holding
period will be taxed at the highest tax rate in effect for that year and applicable to the U.S. Holder; and |
| ● | an
additional tax equal to the interest charge generally applicable to underpayments of tax will be imposed on the U.S. Holder in respect
of the tax attributable to each such other taxable year of such U.S. Holder. |
A
U.S. Holder that owns (or is deemed to own) shares in a PFIC during any taxable year of the U.S. Holder may be required to file an IRS
Form 8621 (whether or not the U.S. Holder makes one or more of the elections described below with respect to such shares) with such U.S.
Holder’s U.S. federal income tax return and provide such other information as may be required by the U.S. Treasury Department.
ALL
U.S. HOLDERS ARE URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE EFFECTS OF THE PFIC RULES ON THE OWNERSHIP OR DISPOSITION OF CLASS
A ORDINARY SHARES AND WARRANTS, INCLUDING THE IMPACT OF ANY PROPOSED OR FINAL TREASURY REGULATIONS.
QEF Election, Mark-to-Market
Election and Purging Election
In
general, if Waldencast Acquisition Corp or Waldencast is determined to be a PFIC, a U.S. Holder may avoid the Default PFIC Regime with
respect to its ordinary shares by making a timely and effective “qualified electing fund” (“QEF”) election under
Section 1295 of the Code (a “QEF Election”) for such holder’s First PFIC Holding Year. In order to comply with the requirements
of a QEF Election with respect to our Class A ordinary shares, a U.S. Holder must receive a PFIC Annual Information Statement from Waldencast.
If we determine we are a PFIC for any taxable year, we may endeavor to provide to a U.S. Holder such information as the IRS may require,
including a PFIC Annual Information Statement, in order to enable the U.S. Holder to make and maintain a QEF Election. However, there
is no assurance that we will so endeavor, or that we will have timely knowledge of our status as a PFIC in the future or of the required
information to be provided. U.S. Holders should consult their tax advisors with respect to any QEF Election previously made with respect
to our ordinary shares.
A
U.S. Holder may not make a QEF election with respect to its warrants to acquire our ordinary shares. As a result, if a U.S. Holder sells
or otherwise disposes of such warrants (other than upon exercise of such warrants), any gain recognized will generally be subject to the
special tax and interest charge rules treating the gain as an excess distribution, as described above, if we were a PFIC at any time during
the period the U.S. Holder held the warrants. If a U.S. Holder that exercises such warrants properly makes a QEF election with respect
to the newly acquired ordinary shares (or has previously made a QEF election with respect to our ordinary shares), the QEF election will
apply to the newly acquired ordinary shares, but the adverse tax consequences relating to PFIC shares, adjusted to take into account the
current income inclusions resulting from the QEF election, will continue to apply with respect to such newly acquired ordinary shares
(which will generally be deemed to have a holding period for purposes of the PFIC rules that includes the period the U.S. Holder held
the warrants), unless the U.S. Holder makes a purging election. Under one type of purging election, the U.S. Holder will be deemed to
have sold such shares at their fair market value on the last day of the last year in which Waldencast is treated as a PFIC, and any gain
recognized on such deemed sale will be treated as an excess distribution, as described above. As a result of this election, the U.S. Holder
will have additional basis (to the extent of any gain recognized in the deemed sale) and, solely for purposes of the PFIC rules, a new
holding period in such holder’s Class A ordinary shares. U.S. Holders should consult their tax advisors regarding the application
of the purging elections rules to their particular circumstances.
If
a U.S. Holder has made a QEF election with respect to our ordinary shares, and the special tax and interest charge rules do not apply
to such shares (because of a timely QEF election for such holder’s First PFIC Holding Year or a purge of the PFIC taint pursuant
to a purging election, as described above), any gain recognized on the sale of our ordinary shares will generally be taxable as capital
gain and no interest charge will be imposed under the PFIC rules. U.S. Holders of a QEF are currently taxed on their pro rata shares of
its earnings and profits, whether or not distributed. Any subsequent distribution of such earnings and profits that were previously included
in income generally should not be taxable as a dividend to such U.S. Holders. The tax basis of a U.S. Holder’s shares in a QEF will
be increased by amounts that are included in income, and decreased by amounts distributed but not taxed as dividends, under the above
rules. Such U.S. Holder will not be subject to the QEF inclusion regime with respect to such shares for any taxable year of us that ends
within or with a taxable year of the U.S. Holder and in which we are not a PFIC. On the other hand, if the QEF election is not effective
for each of our taxable years in which we are a PFIC and the U.S. Holder holds (or is deemed to hold) our ordinary shares, the PFIC rules
discussed above will continue to apply to such shares unless the holder makes a purging election, as described above, and pays the tax
and interest charge with respect to the gain inherent in such shares attributable to the pre-QEF election period.
The
QEF election is made on a shareholder-by-shareholder basis and, once made, can be revoked only with the consent of the IRS. A U.S. Holder
generally makes a QEF election by attaching a completed IRS Form 8621 (Return by a Shareholder of a Passive Foreign Investment Company
or Qualified Electing Fund), including the information provided in a PFIC Annual Information Statement, to a timely filed U.S. federal
income tax return for the tax year to which the election relates. Retroactive QEF elections generally may be made only by filing a protective
statement with such return and if certain other conditions are met or with the consent of the IRS. U.S. Holders should consult their own
tax advisors regarding the availability and tax consequences of a retroactive QEF election under their particular circumstances.
Alternatively,
if a U.S. Holder, at the close of its taxable year, owns (or is deemed to own) shares in a PFIC that are treated as marketable shares,
the U.S. Holder may make a mark-to-market election with respect to such shares for such taxable year. If the U.S. Holder makes a valid
mark-to-market election for such holder’s First PFIC Holding Year, such holder will generally not be subject to the Default PFIC
Regime in respect of its ordinary shares as long as such shares continue to be treated as marketable shares. Instead, the U.S. Holder
will generally include as ordinary income for each year in its holding period that Waldencast plc or Waldencast is treated as a PFIC the
excess, if any, of the fair market value of its ordinary shares at the end of its taxable year over the adjusted basis in its ordinary
shares. The U.S. Holder also will be allowed to take an ordinary loss in respect of the excess, if any, of the adjusted basis of its Class
A ordinary shares over the fair market value of its ordinary shares at the end of its taxable year (but only to the extent of the net
amount of previously included income as a result of the mark-to-market election). The U.S. Holder’s basis in its ordinary shares
will be adjusted to reflect any such income or loss amounts, and any further gain recognized on a sale or other taxable disposition of
the ordinary shares in a taxable year in which Waldencast is treated as a PFIC will be treated as ordinary income. Special tax rules may
also apply if a U.S. Holder makes a mark-to-market election for a taxable year after such holder’s First PFIC Holding Year.
The
mark-to-market election is available only for stock that is regularly traded on a national securities exchange that is registered with
the Securities and Exchange Commission, including the Nasdaq. U.S. Holders should consult their own tax advisors regarding the availability
and tax consequences of a mark-to-market election in respect of Waldencast plc Class A ordinary shares under their particular circumstances.
If
Waldencast is a PFIC and, at any time, has a foreign subsidiary that is classified as a PFIC, U.S. Holders would be deemed to own a portion
of the shares of such lower-tier PFIC, and could incur liability for the deferred tax and interest charge described above if Waldencast
plc receives a distribution from, or disposes of all or part of Waldencast’s interest in, the lower-tier PFIC or the U.S. Holders
otherwise were deemed to have disposed of an interest in the lower-tier PFIC. A mark-to-market election would not be available with respect
to such lower-tier PFIC. U.S. Holders are urged to consult their own tax advisors regarding the tax issues raised by lower-tier PFICs.
The
rules dealing with PFICs and with the QEF and mark-to-market elections are very complex and are affected by various factors in addition
to those described above. Accordingly, U.S. Holders of our Class A ordinary shares should consult their tax advisors concerning the application
of the PFIC rules to Waldencast plc Class A ordinary shares under their particular circumstances.
THE
RULES DEALING WITH PFICS ARE COMPLEX AND ARE IMPACTED BY VARIOUS FACTORS IN ADDITION TO THOSE DESCRIBED ABOVE. U.S. HOLDERS ARE URGED
TO CONSULT THEIR TAX ADVISORS REGARDING THE CONSEQUENCES TO THEM OF THE PFIC RULES, INCLUDING, WITHOUT LIMITATION, WHETHER A QEF ELECTION,
A MARK-TO-MARKET ELECTION OR ANY OTHER ELECTION IS AVAILABLE AND THE CONSEQUENCES TO THEM OF ANY SUCH ELECTION, AND THE IMPACT OF ANY
PROPOSED OR FINAL PFIC TREASURY REGULATIONS.
Jersey
Tax Considerations
This summary of Jersey taxation
issues can only provide a general overview of this area and it is not a description of all the tax considerations that may be relevant
to a decision to invest in our Class A ordinary shares.
The following summary of
the anticipated treatment of Waldencast and holders of Class A ordinary shares (other than residents of Jersey) is based on Jersey taxation
law and practice as it is understood to apply at the date of this document and may be subject to any changes in Jersey law occurring after
such date. It does not constitute legal or tax advice and does not address all aspects of Jersey tax law and practice (including such
tax law and practice as it applies to any land or building situate in Jersey). Legal advice should be taken with regard to individual
circumstances. Prospective investors in our Class A ordinary shares should consult their professional advisers on the implications of
acquiring, buying, selling or otherwise disposing of Waldencast plc common stock under the laws of any jurisdiction in which they may
be liable to taxation.
Shareholders should note
that tax law and interpretation can change and that, in particular, the levels and basis of, and reliefs from, taxation may change and
may alter the benefits of investment in our Class A ordinary shares.
Any person who is in any
doubt about their tax position or who is subject to taxation in a jurisdiction other than Jersey should consult their own professional
adviser.
Company Residence
Under the Income Tax (Jersey)
Law 1961 (as amended) (“Tax Law”), a company shall be regarded as resident in Jersey if it is incorporated under the Jersey
Companies Law unless:
| ● | its business is centrally managed and controlled outside
Jersey in a country or territory where the highest rate at which any company may be charged to tax on any part of its income is 10% or
higher; and |
| ● | the company is resident for tax purposes in that country
or territory. |
We are resident for tax purposes
in Jersey and subject to tax in Jersey.
Summary
Under current Jersey law,
there are no capital gains, capital transfer, gift, wealth or inheritance taxes, or any death or estate duties. No capital or stamp duty
is levied in Jersey on the issue, conversion, redemption, or transfer of ordinary shares. On the death of an individual holder of ordinary
shares (whether or not such individual was domiciled in Jersey), duty at rates of up to 0.75% of the value of the relevant ordinary shares
may be payable on the registration of any Jersey probate or letters of administration which may be required in order to transfer, convert,
redeem, or make payments in respect of, ordinary shares held by a deceased individual sole shareholder, subject to a cap of £100,000.
Income Tax
The general rate of income
tax under the Tax Law on the profits of companies regarded as resident in Jersey or having a permanent establishment in Jersey is 0% (“zero
tax rating”), though certain exceptions from zero tax rating might apply.
Withholding Tax
For so long as we are subject
to a zero tax rating, or are deemed to be resident for tax purposes in Jersey, no withholding in respect of Jersey taxation will be required
on payments in respect of our Class A ordinary shares to any holder of our Class A ordinary shares not resident in Jersey.
Stamp Duty
In Jersey, no stamp duty
is levied on the issue or transfer of our Class A ordinary shares except that stamp duty is payable on Jersey grants of probate and letters
of administration, which will generally be required to transfer ordinary shares on the death of a holder of such ordinary shares if such
holder was entered as the holder of the shares on the register maintained in Jersey. In the case of a grant of probate or letters of administration,
stamp duty is levied according to the size of the estate (wherever situated in respect of a holder of ordinary shares domiciled in Jersey,
or situated in Jersey in respect of a holder of ordinary shares domiciled outside Jersey) and is payable on a sliding scale at a rate
of up to 0.75% on the value of an estate up to a maximum stamp duty charge of £100,000. The rules for joint holders through a nominee
are different and advice relating to this form of holding should be obtained from a professional adviser.
Jersey does not otherwise
levy taxes upon capital, inheritances, capital gains or gifts nor are there otherwise estate duties.
Goods and Services Tax
Pursuant to the Goods and
Services Tax (Jersey) Law 2007 (“GST Law”), a tax rate which is currently 5% applies to the supply of goods and services,
unless the supply is regarded as exempt or zero rated, or the relevant supplier or recipient of such goods and services is registered
as an “international services entity.”
A company must register for
GST if its turnover is greater than £300,000 in any 12-month period, and will then need to charge GST to its customers. Companies
can also choose to register voluntarily.
A company may apply to be
registered as an International Services Entity (“ISE”) if it mainly serves non-Jersey residents. By virtue of a company being
an ISE, it will not have to register for GST, will not charge GST on its supplies, and will not be charged GST on its purchases.
We will be an ISE within
the meaning of the GST Law, as we satisfy the requirements of the Goods and Services Tax (International Services Entities) (Jersey) Regulations
2008, as amended. As long as we continue to be such an entity, a supply of goods or of a service made by or to us shall not be a taxable
supply for the purposes of the GST Law.
Substance Legislation
With effect from January
1, 2019, Jersey has implemented legislation to meet E.U. demands for companies to have substance in certain circumstances. Broadly, part
of the legislation is intended to apply to holding companies managed and controlled in Jersey. As we are managed and controlled in Jersey,
this legislation may apply to us on this basis.
Plan
of Distribution
We are registering the
issuance by us of up to 29,533,282 Class A ordinary shares. We are also registering the resale, from time to time, by the Selling
Holders or their permitted transferees of up to 121,120,063 Class A ordinary shares and up to 18,033,332 private placement
warrants.
The Selling Holders may offer
and sell, from time to time, their respective Class A ordinary shares or warrants covered by this prospectus. The Selling Holders will
act independently of us in making decisions with respect to the timing, manner and size of each sale. Such sales may be made on one or
more exchanges or in the over-the-counter market or otherwise, at prices and under terms then prevailing or at prices related to the then
current market price or in negotiated transactions. The Selling Holders may offer and sell their securities by one or more of, or a combination
of, the following methods:
| ● | on
Nasdaq, in the over-the-counter market or on any other national securities exchange on which
our securities are listed or traded; |
| ● | through
trading plans entered into by a Selling Holder pursuant to Rule 10b5-1 under the Exchange
Act that are in place at the time of an offering pursuant to this prospectus or any applicable
prospectus supplement hereto that provide for periodic sales of their securities on the basis
of parameters described in such trading plans; |
| ● | directly
to purchasers, including through a specific bidding, auction or other process, or in in privately
negotiated transactions; |
| ● | in
one or more underwritten offerings; |
| ● | in
block trades in which a broker-dealer will attempt to sell the offered securities as agent
but may purchase and resell a portion of the block as principal to facilitate the transaction; |
| ● | through
purchases by a broker-dealer as principal and resale by the broker-dealer for its account
pursuant to this prospectus; |
| ● | in
ordinary brokerage transactions and transactions in which the broker solicits purchasers; |
| ● | through
the writing or settlement of options (including put or call options) or other hedging transactions,
whether the options are listed on an options exchange or otherwise; |
| ● | in
short sales entered into after the effective date of the registration statement of which
this prospectus is a part; |
| ● | through
agreements with broker-dealers to sell a specified number of the securities at a stipulated
price per share or warrant; |
| ● | by
pledge to secured debts and other obligations; |
| ● | to
or through underwriters or agents; |
| ● | in
“at the market” or through market makers or into an existing market for the securities;
or |
| ● | any
other method permitted pursuant to applicable law. |
The
Selling Holders may sell the securities at prices then prevailing, related to the then prevailing market price or at negotiated prices.
The offering price of the securities from time to time will be determined by the Selling Holders and, at the time of the determination,
may be higher or lower than the market price of our securities on Nasdaq or any other exchange or market.
Pursuant to the Waiver, certain
Selling Holders pledged our securities owned by them. If a donee, pledgee, transferee, or other successor-in-interest intends to sell
our securities in connection with a foreclosure or otherwise, we will, to the extent required, promptly file a prospectus supplement or,
if necessary, a post-effective amendment to the registration statement that includes this prospectus, to name specifically such person
as a Selling Holder. The Selling Holders also may transfer our securities in other circumstances, in which case the transferees, pledgees
or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The Selling Holders may also
sell our securities short and deliver the securities to close out their short positions or loan or pledge the securities to broker-dealers
that in turn may sell the securities. The shares may be sold directly or through broker-dealers acting as principal or agent or pursuant
to a distribution by one or more underwriters on a firm commitment or best-efforts basis. The Selling Holders may also enter into hedging
transactions with broker-dealers. In connection with such transactions, broker-dealers of other financial institutions may engage in short
sales of our securities in the course of hedging the positions they assume with the Selling Holders. The Selling Holders may also enter
into options or other transactions with broker-dealers or other financial institutions, which require the delivery to such broker-dealer
or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution
may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction). In connection with an underwritten offering,
underwriters or agents may receive compensation in the form of discounts, concessions or commissions from the Selling Holders or from
purchasers of the offered securities for whom they may act as agents. In addition, underwriters may sell the securities to or through
dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters and/or
commissions from the purchasers for whom they may act as agents. The Selling Holders and any underwriters, dealers or agents participating
in a distribution of the securities may be deemed to be “underwriters” within the meaning of the Securities Act, and any profit
on the sale of the securities by the Selling Holders and any commissions received by broker-dealers may be deemed to be underwriting commissions
under the Securities Act.
The Selling Holders party
to Subscription Agreements or the Registration Rights Agreement have agreed, and the other Selling Holders may agree, to indemnify an
underwriter, broker-dealer or agent against certain liabilities related to the sale of the securities, including liabilities under the
Securities Act.
In order to comply with the
securities laws of certain states, if applicable, the securities must be sold in such jurisdictions only through registered or licensed
brokers or dealers. In addition, in certain states the securities may not be sold unless they have been registered or qualified for sale
in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
The Selling Holders are subject
to the applicable provisions of the Exchange Act and the rules and regulations under the Exchange Act, including Regulation M. This regulation
may limit the timing of purchases and sales of any of the securities offered in this prospectus by the Selling Holders. The anti-manipulation
rules under the Exchange Act may apply to sales of the securities in the market and to the activities of the Selling Holders and their
affiliates. Furthermore, Regulation M may restrict the ability of any person engaged in the distribution of the securities to engage in
market-making activities for the particular securities being distributed for a period of up to five business days before the distribution.
The restrictions may affect the marketability of the securities and the ability of any person or entity to engage in market-making activities
for the securities.
At the time a particular
offer of securities is made, if required, a prospectus supplement or, if necessary, a post-effective amendment to the registration statement
that includes this prospectus, will be distributed that will set forth the number of securities being offered and the terms of the offering,
including the name of any underwriter, dealer or agent, the purchase price paid by any underwriter, any discount, commission and other
item constituting compensation, any discount, commission or concession allowed or reallowed or paid to any dealer, and the proposed selling
price to the public.
To the extent required, this
prospectus may be amended and/or supplemented from time to time to describe a specific plan of distribution. Instead of selling the securities
under this prospectus, the Selling Holders may sell the securities in compliance with the provisions of Rule 144 under the Securities
Act, if available, or pursuant to other available exemptions from the registration requirements of the Securities Act.
Lock-Up Agreements
Certain of our shareholders
are subject to the transfer restrictions in the Letter Agreement, the Sponsor Forward Purchase Agreement and the Lock-Up Agreements, the
forms of which are incorporated by reference herein as Exhibits 10.8, 10.11 and 10.5, respectively, to the registration statement of which
this prospectus is a part.
Expenses
Related to this Offering
The following table sets
forth the fees and expenses payable by us in connection with the sale and distribution of the securities being registered hereby.
| Expenses | |
Amount
| |
| SEC registration fee | |
$ | 126,388.36 | |
| Legal fees and expenses | |
| * | |
| Accounting fees and expenses | |
| * | |
| Transfer agent fees and expenses | |
| * | |
| Miscellaneous expenses | |
| * | |
| Total | |
$ | * | |
| * | These fees are calculated based on the securities offered and the number of issuances and accordingly
cannot be defined at this time. |
Legal
Matters
Skadden, Arps, Slate, Meagher & Flom LLP, New York, New York, and Maples and Calder (Jersey) LLP, Jersey, have passed upon the validity of the securities of Waldencast
plc offered by this prospectus and certain other legal matters related to this prospectus.
Experts
The financial statements
of Waldencast Acquisition Corp. as of December 31, 2021 and 2020, and for the year ended December 31, 2021 and for the period from December
8, 2020 (inception) through December 31, 2020, included in this prospectus have been audited by Marcum LLP, an independent registered
public accounting firm, as stated in their report appearing herein, which includes an explanatory paragraph as to the company’s
ability to continue as a going concern. Such financial statements are included in reliance upon the report of such firm given upon their
authority as experts in accounting and auditing.
The consolidated financial
statements of Obagi Global Holdings Limited as of December 31, 2021 and December 31, 2020 and for each of the three years in the period
ended December 31, 2021 included in this prospectus, have been audited by Deloitte & Touche LLP, an independent registered public
accounting firm, as stated in their report. Such financial statements are included in reliance upon the report of such firm given their
authority as experts in accounting and auditing.
The financial statements
of Milk Makeup LLC as of December 31, 2021, 2020 and 2019, and for each of the three years then ended included in this prospectus have
been included herein in reliance upon the report of WithumSmith+Brown, PC, independent registered public accounting firm, appearing elsewhere
herein, and upon the authority of said firm as experts in accounting and auditing.
Enforceability
of Civil Liability
We are a Jersey public limited
company. You may have difficulty serving legal process within the U.S. upon us. You may also have difficulty enforcing, both in and outside
the U.S., judgments you may obtain in U.S. courts against us in any action, including actions based upon the civil liability provisions
of U.S. federal or state securities laws. Furthermore, there is doubt that the courts of Jersey would enter judgments in original actions
brought in those courts predicated on U.S. federal or state securities laws. However, we may be served with process in the U.S. with
respect to actions against us arising out of or in connection with violation of U.S. federal securities laws relating to offers and sales
of our securities by serving our U.S. agent irrevocably appointed for that purpose.
A judgment of a U.S. court is not directly enforceable in Jersey, but constitutes a cause of action which may be enforced by Jersey courts
provided that:
| ● | the
applicable U.S. courts had jurisdiction over the case, as recognized under Jersey law; |
| ● | the
judgment is given on the merits and is final, conclusive and non-appealable; |
| ● | the
judgment relates to the payment of a sum of money, not being taxes, fines or similar governmental
penalties; |
| ● | the
defendant is not immune under the principles of public international law; |
| ● | the
same matters at issue in the case were not previously the subject of a judgment or disposition
in a separate court; |
| ● | the
judgment was not obtained by fraud; and |
| ● | the
recognition and enforcement of the judgment is not contrary to public policy in Jersey. |
Jersey
courts award compensation for the loss or damage actually sustained by the plaintiff. Although punitive damages are generally unknown
to the Jersey legal system, there is no prohibition on them either by statute or customary law. Whether a particular judgment may be
deemed contrary to Jersey public policy depends on the facts of each case, though judgments found to be exorbitant, unconscionable, or
excessive will generally be deemed as contrary to public policy. Moreover, certain defendants may qualify for protection under
Protection of Trading Interests Act 1980, an act of the U.K. extended to Jersey by the Protection of Trading Interests Act 1980 (Jersey)
Order, 1983. This Act provides that a qualifying defendant is not liable for multiple damages, in excess of that required for actual
compensation. A “qualifying defendant” for these purposes is a citizen of the U.K. and its Colonies (as defined in the Act),
a corporation or other limited liability entity organized under the laws of the U.K., Jersey or other territory for whose international
relations the U.K. is responsible or a person conducting business in Jersey.
Jersey courts cannot enter
into the merits of the foreign judgment and cannot act as a court of appeal or review over the foreign courts. It is doubtful that an
original action based on U.S. federal or state securities laws could be brought before Jersey courts. In addition, a plaintiff who is
not resident in Jersey may be required to provide a security bond in advance to cover the potential of the expected costs of any case
initiated in Jersey.
Where
You Can Find More Information
We have filed with the SEC
a registration statement (including amendments and exhibits to the registration statement) on Form F-1 under the Securities Act. For purposes
of this section, the term registration statement means the original registration statement and any and all amendments including the schedules
and exhibits to the original registration statement or any amendment. This prospectus, which is part of the registration statement, does
not contain all of the information set forth in the registration statement and the exhibits and schedules to the registration statement.
For further information, we refer you to the registration statement and the exhibits and schedules filed as part of the registration statement.
If a document has been filed as an exhibit to the registration statement, we refer you to the copy of the document that has been filed.
Each statement in this prospectus relating to a document filed as an exhibit is qualified in all respects by the filed exhibit.
We are subject to the informational
requirements of the Exchange Act that are applicable to foreign private issuers. Accordingly, we are required to file or furnish reports
and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. The SEC maintains an Internet website
that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are available
to the public through the SEC’s website at http://www.sec.gov.
As a foreign private issuer,
we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and
our executive officers, directors and principal and Selling Holders are exempt from the reporting and short-swing profit recovery provisions
contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file periodic reports and
financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We maintain a corporate website
at www.waldencast.com. Information contained on, or that can be accessed through, our website does not constitute a part of this
prospectus. We have included our website address in this prospectus solely for informational purposes.
FINANCIAL STATEMENTS
WALDENCAST PLC
| |
|
Page |
| Report of Independent Registered Public Accounting Firm |
|
F-2 |
| Financial Statements |
|
|
| Balance Sheets as of December 31, 2021 and December 31, 2020 |
|
F-3 |
| Statements of Operations for the year ended December 31, 2021 and for the period from December 8, 2020 (inception) through December 31, 2020 |
|
F-4 |
| Statements of Changes in Shareholders’ Deficit for the year ended December 31, 2021 and for the period from December 8, 2020 (inception) through December 31, 2020 |
|
F-5 |
| Statements of Cash Flows for the year ended December 31, 2021 and for the period from December 8, 2020 (inception) through December 31, 2020 |
|
F-6 |
| Notes to Financial Statements |
|
F-7 |
| |
|
|
| Condensed Financial Statements (UNAUDITED) |
|
|
| Condensed Balance Sheets as of June 30, 2022 (Unaudited) and December 31, 2021 |
|
F-23 |
| Unaudited Condensed Statements of Operations for the three and six months ended June 30, 2022 and June 30, 2021 |
|
F-24 |
| Unaudited Condensed Statements of Changes in Shareholders’ Deficit for the three and six months ended June 30, 2022 and June 30, 2021 |
|
F-25 |
| Unaudited Condensed Statements of Cash Flows for the three and six months ended June 30, 2022 and June 30, 2021 |
|
F-26 |
| Notes to Unaudited Condensed Financial Statements |
|
F-27 |
OBAGI GLOBAL HOLDINGS
LIMITED
| |
|
Page |
| Report of Independent Registered Public Accounting Firm |
|
F-46 |
| Consolidated Balance Sheets as of December 31, 2021 and December 31, 2020 |
|
F-47 |
| Consolidated Statements of Operations and Comprehensive Income (Loss) for the years ended December 31, 2021, 2020 and 2019 |
|
F-48 |
| Consolidated Statements of Shareholder’s Equity for the years ended December 31, 2021, 2020 and 2019 |
|
F-49 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2021, 2020 and 2019 |
|
F-50 |
| Notes to Consolidated Financial Statements |
|
F-51 |
| |
|
|
| Unaudited Condensed Consolidated Balance Sheets as of June 30, 2022 and December 31, 2021 |
|
F-72 |
| Unaudited
Condensed Consolidated Statements of Operations and Comprehensive Income (Loss) for the three and six months ended June 30, 2022 and
2021 |
|
F-73 |
| Unaudited Condensed Consolidated Statements of Shareholder’s Equity for the three and six months ended June 30, 2022 and 2021 |
|
F-74 |
| Unaudited Condensed Consolidated Statements of Cash Flows for the three and six months ended June 30, 2022 and 2021 |
|
F-75 |
| Notes to Unaudited Condensed Consolidated Financial Statements |
|
F-76 |
MILK MAKEUP LLC
| |
|
Page |
| Independent Auditor’s Report |
|
F-89 |
| Statements of Operations and Comprehensive Loss for the years ended December 31, 2021, 2020 and 2019 |
|
F-91 |
| Balance Sheets as of December 31, 2021 and December 31, 2020 |
|
F-92 |
| Statements of Redeemable Preferred Units and Members’ Equity for the years ended December 31, 2021, 2020 and 2019 |
|
F-93 |
| Statements of Cash Flows for the years ended December 31, 2021, 2020 and 2019 |
|
F-94 |
| Notes to Financial Statements |
|
F-95 |
| |
|
|
| Unaudited Condensed Statements of Operations and Comprehensive Income for the three and six months ended June 30, 2022 and 2021 |
|
F-109 |
|
Condensed Balance Sheets as of June 30, 2022 (unaudited) and December 31, 2021 |
|
F-110 |
| Unaudited Condensed Statements of Redeemable Preferred Units and Members’ Equity for the three and six months ended June 30, 2022 and 2021 |
|
F-111 |
| Unaudited Condensed Statements of Cash Flows for the three and six months ended June 30, 2022 and 2021 |
|
F-112 |
| Notes to Unaudited Condensed Financial Statements |
|
F-113 |
Report of Independent Registered Public Accounting
Firm
To the Shareholders and Board of Directors of
Waldencast Acquisition Corp.
Opinion on the Financial Statements
We have audited the accompanying
balance sheets of Waldencast Acquisition Corp. (the “Company”) as of December 31, 2021 and 2020, the related statements of
operations, changes in shareholders’ deficit and cash flows for the year ended December 31, 2021 and for the period from December
8, 2020 (inception) through December 31, 2020, and the related notes (collectively referred to as the “financial statements”).
In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December
31, 2021 and 2020, and the results of its operations and its cash flows for the year ended December 31, 2021 and for the period from December
8, 2020 (inception) through December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial
statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1 to the financial
statements, the Company’s business plan is dependent on the completion of a business combination. As of the date of this report
there is no guarantee of a successful completion of a business combination, which raises substantial doubt about the Company’s
ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial
statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements
are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial
statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United
States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities
laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits
in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance
about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to
have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required
to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness
of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing
procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures
that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the
financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management,
as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for
our opinion.
/s/ Marcum LLP
Marcum LLP
We have served as the Company’s auditor
since 2020.
New York, NY
March 31, 2022
PCAOB ID Number 688
WALDENCAST ACQUISITION CORP.
BALANCE SHEETS
| | |
December 31,
2021 | | |
December 31,
2020 | |
| Assets | |
| | |
| |
| Current assets: | |
| | |
| |
| Cash | |
$ | 1,503,768 | | |
$ | — | |
| Prepaid expenses – current | |
| 204,821 | | |
| — | |
| Deferred offering costs associated with initial private offering | |
| — | | |
| 166,792 | |
| Total current assets | |
$ | 1,708,589 | | |
$ | 166,792 | |
| Prepaid expenses – non-current portion | |
| 33,050 | | |
| — | |
| Investment held in Trust Account | |
| 345,052,047 | | |
| — | |
| Total assets | |
| 346,793,686 | | |
| 166,792 | |
| | |
| | | |
| | |
| Liabilities, Redeemable Ordinary Shares and Shareholders’ Deficit | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 272,953 | | |
$ | 177,743 | |
| Due to related party | |
| 95,000 | | |
| — | |
| Total current liabilities | |
| 367,953 | | |
| 177,743 | |
| Warrant liabilities | |
| 21,153,666 | | |
| — | |
| Deferred legal fees | |
| 8,186,101 | | |
| — | |
| Forward purchase agreement liabilities | |
| 13,320,000 | | |
| — | |
| Working Capital Promissory Note – related party | |
| 1,500,000 | | |
| — | |
| Deferred underwriters’ discount | |
| 12,075,000 | | |
| — | |
| Total liabilities | |
| 56,602,720 | | |
| 177,743 | |
| | |
| | | |
| | |
| Commitments & Contingencies (Note 6) | |
| | | |
| | |
| Class A ordinary shares subject to possible redemption, 34,500,000 and no shares at redemption value of $10.00 at December 31, 2021 and December 31, 2020, respectively | |
| 345,000,000 | | |
| — | |
| | |
| | | |
| | |
| Shareholders’ deficit: | |
| | | |
| | |
| Preference shares, $0.0001 par value; 5,000,000 shares authorized; none issued and outstanding | |
| — | | |
| — | |
| Class A ordinary shares, $0.0001 par value; 500,000,000 shares authorized; no shares issued and outstanding (excluding 34,500,000 and no shares subject to redemption) at December 31, 2021 and December 31, 2020, respectively | |
| — | | |
| — | |
| Class B ordinary shares, $0.0001 par value; 50,000,000 shares authorized; 8,625,000 and no shares issued and outstanding at December 31, 2021 and December 31, 2020, respectively | |
| 863 | | |
| — | |
| Additional paid-in capital | |
| — | | |
| — | |
| Accumulated deficit | |
| (54,809,897 | ) | |
| (10,951 | ) |
| Total shareholders’ deficit | |
| (54,809,034 | ) | |
| (10,951 | ) |
| Total liabilities, redeemable ordinary shares and shareholders’ deficit | |
$ | 346,793,686 | | |
$ | 166,792 | |
The accompanying notes are an integral part of these financial statements.
WALDENCAST ACQUISITION CORP.
STATEMENTS OF OPERATIONS
| | |
For the
year ended | | |
For the
period from
December 8,
2020
(inception)
through | |
| | |
December 31,
2021 | | |
December 31,
2020 | |
| | |
| | |
| |
| Formation and operating costs | |
$ | 9,133,011 | | |
$ | 10,951 | |
| Loss from operations | |
| (9,133,011 | ) | |
| (10,951 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Interest income on operating account | |
| 1,146 | | |
| — | |
| Interest income on marketable securities held in Trust Account | |
| 52,047 | | |
| — | |
| Offering expenses related to warrant issuance | |
| (719,201 | ) | |
| — | |
| Change in fair value of forward purchase agreement liabilities | |
| (1,665,000 | ) | |
| — | |
| Change in fair value of warrant liabilities | |
| (2,963,666 | ) | |
| — | |
| Total other expense | |
| (5,294,674 | ) | |
| — | |
| | |
| | | |
| | |
| Net loss | |
$ | (14,427,685 | ) | |
$ | (10,951 | ) |
| | |
| | | |
| | |
| Weighted average shares outstanding, Class A ordinary shares subject to possible redemption | |
| 27,316,438 | | |
| — | |
| Basic and diluted net loss per share, Class A ordinary shares subject to possible redemption | |
$ | (0.41 | ) | |
$ | — | |
| Weighted average shares outstanding, Non-redeemable Class B ordinary shares | |
| 8,410,753 | | |
| — | |
| Basic and diluted net loss per share, Non-redeemable Class B ordinary shares | |
$ | (0.41 | ) | |
$ | — | |
The accompanying notes are an integral part of these financial statements.
WALDENCAST ACQUISITION CORP.
STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
| | |
Ordinary Shares | | |
Additional | | |
| | |
Total | |
| | |
Class A | | |
Class B | | |
Paid-In | | |
Accumulated | | |
Shareholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| Balance as of December 8, 2020 (inception) | |
| — | | |
$ | — | | |
| — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (10,951 | ) | |
| (10,951 | ) |
| Balance as of December 31, 2020 | |
| — | | |
$ | — | | |
| — | | |
$ | — | | |
$ | — | | |
$ | (10,951 | ) | |
$ | (10,951 | ) |
| Issuance of Founder Shares | |
| — | | |
| — | | |
| 8,625,000 | | |
| 863 | | |
| 24,137 | | |
| — | | |
| 25,000 | |
| Sale of 34,500,000 Units on March 18, 2021 through the Initial Public Offering, | |
| 34,500,000 | | |
| 3,450 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 3,450 | |
| Sale of 5,933,333 Private Placement Warrants on March 18, 2021 | |
| — | | |
| — | | |
| — | | |
| — | | |
| 8,900,000 | | |
| — | | |
| 8,900,000 | |
| Initial value of private warrant liabilities | |
| — | | |
| — | | |
| — | | |
| — | | |
| (6,230,000 | ) | |
| — | | |
| (6,230,000 | ) |
| Initial value of FPA liabilities | |
| — | | |
| — | | |
| — | | |
| — | | |
| (11,655,000 | ) | |
| — | | |
| (11,655,000 | ) |
| Class A ordinary shares subject to possible redemption | |
| (34,500,000 | ) | |
| (3,450 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| (3,450 | ) |
| Accretion of Class A ordinary shares subject to possible redemption | |
| — | | |
| — | | |
| — | | |
| — | | |
| 8,960,863 | | |
| (40,371,261 | ) | |
| (31,410,398 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (14,427,685 | ) | |
| (14,427,685 | ) |
| Balance as of December 31, 2021 | |
| — | | |
$ | — | | |
| 8,625,000 | | |
$ | 863 | | |
$ | — | | |
$ | (54,809,897 | ) | |
$ | (54,809,034 | ) |
The accompanying notes are an integral part of these financial statements.
WALDENCAST ACQUISITION CORP.
STATEMENTS OF CASH FLOWS
| | |
For the
year ended | | |
For the
period from December 8,
2020 (inception)
through | |
| | |
December 31,
2021 | | |
December 31,
2020 | |
| Cash Flows from Operating Activities: | |
| | |
| |
| Net loss | |
$ | (14,427,685 | ) | |
$ | (10,951 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Interest earned on Trust Account | |
| (52,047 | ) | |
| — | |
| Increase in deferred legal costs | |
| 8,186,101 | | |
| — | |
| Change in fair value of warrant liabilities | |
| 2,963,666 | | |
| — | |
| Change in fair value of forward purchase agreement liabilities | |
| 1,665,000 | | |
| — | |
| Offering costs allocated to warrants | |
| 719,201 | | |
| — | |
| Changes in current assets and current liabilities: | |
| | | |
| | |
| Prepaid assets | |
| (237,871 | ) | |
| — | |
| Accounts payable and accrued expenses | |
| 272,953 | | |
| 10,951 | |
| Due to related party | |
| 95,000 | | |
| — | |
| Net cash used in operating activities | |
| (815,682 | ) | |
| — | |
| | |
| | | |
| | |
| Cash Flows from Investing Activities: | |
| | | |
| | |
| Investment of cash into Trust Account | |
| (345,000,000 | ) | |
| — | |
| Net cash used in investing activities | |
| (345,000,000 | ) | |
| — | |
| | |
| | | |
| | |
| Cash Flows from Financing Activities: | |
| | | |
| | |
| Proceeds from issuance of Founder Shares | |
| 25,000 | | |
| — | |
| Proceeds from Initial Public Offering, net of underwriters’ discount | |
| 338,100,000 | | |
| — | |
| Proceeds from issuance of Private Placement Warrants | |
| 8,900,000 | | |
| — | |
| Proceeds of Working Capital Promissory Note – related party | |
| 1,500,000 | | |
| | |
| Payments of offering costs | |
| (1,205,550 | ) | |
| — | |
| Net cash provided by financing activities | |
| 347,319,450 | | |
| — | |
| | |
| | | |
| | |
| Net Change in Cash | |
| 1,503,768 | | |
| — | |
| Cash – Beginning | |
| — | | |
| — | |
| Cash – Ending | |
$ | 1,503,768 | | |
$ | — | |
| | |
| | | |
| | |
| Supplemental Disclosure of Non-cash Financing Activities: | |
| | | |
| | |
| Initial value of warrant liabilities | |
$ | 18,190,000 | | |
$ | — | |
| Deferred underwriters’ discount payable charged to additional paid-in capital | |
$ | 12,075,000 | | |
$ | — | |
| Initial value of forward purchase agreement liabilities | |
$ | 11,655,000 | | |
$ | — | |
The accompanying notes are an integral part of
these financial statements.
WALDENCAST ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
Note 1 — Organization and Business Operations
Organization and General
Waldencast Acquisition Corp.
(the “Company”) was incorporated in the Cayman Islands on December 8, 2020. The Company was formed for the purpose of entering
into a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar Business Combination with one or more
businesses (a “Business Combination”). The Company is not limited to a particular industry or geographic region for purposes
of consummating a Business Combination. The Company is an early stage and emerging growth company and, as such, the Company is subject
to all of the risks associated with early stage and emerging growth companies.
The Company was formed on December
8, 2020 and remained dormant through December 31, 2020. For the period from December 8, 2020 (inception) through December 31, 2020, there
had been no activity since the formation of the entity and no equity shares were issued. The Company commenced operations on January 12,
2021 when the Founder Shares were issued. All activity since January 12, 2021 relates to the Company’s formation and the initial
public offering (the “Initial Public Offering”) and identifying a target or targets for a Business Combination, as described
below. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest.
The Company will generate non-operating income in the form of interest income on cash and cash equivalents from the proceeds derived from
the Initial Public Offering.
Financing
On March 18, 2021, the Company
consummated the Initial Public Offering of 34,500,000 units (the “Units” and, with respect to the Class A ordinary shares
included in the Units offered, the “Public Shares”), at $10.00 per Unit, generating gross proceeds of $345,000,000, which
is discussed in Note 3.
Simultaneously with the closing
of the Initial Public Offering, the Company completed the private sale of 5,933,333 warrants (the “Private Placement Warrants”),
at a price of $1.50 per Private Placement Warrant, which is discussed in Note 4.
Transaction costs amounted
to $20,169,599, consisting of $6,900,000 of underwriting fee, $12,075,000 of deferred underwriting fee and $1,194,599 of other offering
costs. Of the total transaction costs, $719,201 was reclassified as non-operating expense in the statements of operations with the rest
of the offering costs charged to shareholders’ deficit. The transaction costs were allocated based on a relative fair value basis,
compared to the total offering proceeds, between the fair value of the public warrant liabilities and the Class A ordinary shares.
Trust Account
Following the closing of the
Initial Public Offering on March 18, 2021, an amount of $345,000,000 from the net proceeds of the sale of the Units in the Initial Public
Offering and the sale of the Private Placement Warrants was placed in a trust account (“Trust Account”) which is invested
in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185
days or less or in any open-ended investment company that holds itself out as a money market fund meeting the conditions of Rule 2a-7
of the Investment Company Act, as determined by the Company. Except with respect to interest earned on the funds held in the Trust Account
that may be released to the Company to pay its taxes, if any, the funds held in the Trust Account will not be released from the Trust
Account until the earliest to occur of: (1) the completion of the Company’s initial Business Combination; (2) the redemption of
any Public Shares properly submitted in connection with a shareholder vote to amend the Company’s amended and restated memorandum
and articles of association (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection
with its initial Business Combination or to redeem 100% of its Public Shares if the Company does not complete its initial Business Combination
within 24 months from the closing of the Initial Public Offering or (B) with respect to any other provision relating to shareholders’
rights or pre-initial Business Combination activity; and (3) the redemption of the Company’s Public Shares if the Company has not
completed its initial Business Combination within 24 months from the closing of the Initial Public Offering, subject to applicable law.
The proceeds deposited in the Trust Account could become subject to the claims of the Company’s creditors, if any, which could have
priority over the claims of the Company’s public shareholders.
Initial Business Combination
The Company’s management
has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering, although substantially
all of the net proceeds are intended to be generally applied toward consummating a Business Combination.
The Company’s Business
Combination must be with one or more target businesses that together have a fair market value equal to at least 80% of the balance in
the Trust Account (net of taxes payable) at the time of the signing of an agreement to enter into a Business Combination. However, the
Company will only complete a Business Combination if the post-Business Combination company owns or acquires 50% or more of the outstanding
voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to register
as an investment company under the Investment Company Act. There is no assurance that the Company will be able to successfully effect
a Business Combination.
The Company will provide its
public shareholders with the opportunity to redeem all or a portion of their Public Shares upon the completion of the initial Business
Combination either (i) in connection with a shareholder meeting called to approve the initial Business Combination or (ii) by means of
a tender offer. The decision as to whether the Company will seek shareholder approval of a proposed initial Business Combination or conduct
a tender offer will be made by the Company, solely in its discretion. The shareholders will be entitled to redeem their shares for a pro
rata portion of the amount then on deposit in the Trust Account (initially $10.00 per share, plus any pro rata interest earned on the
funds held in the Trust Account and not previously released to the Company to pay its tax obligations).
The Class A ordinary shares
subject to redemption is recorded at a redemption value and classified as temporary equity upon the completion of the Initial Public Offering,
in accordance with Accounting Standards Codification (“ASC”) Topic 480, “Distinguishing Liabilities from Equity.”
In such case, the Company will proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 either
immediately prior to or upon consummation of a Business Combination and, if the Company seeks shareholder approval, a majority of the
issued and outstanding shares voted are voted in favor of the Business Combination.
The Company will have 24 months
from the closing of the Initial Public Offering (with the ability to extend with shareholder approval) to consummate a Business Combination
(the “Combination Period”). However, if the Company is unable to complete a Business Combination within the Combination Period,
the Company will redeem 100% of the outstanding Public Shares for a pro rata portion of the funds held in the Trust Account, equal to
the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the Trust Account and not previously
released to the Company, divided by the number of then outstanding Public Shares, subject to applicable law and as further described in
the registration statement, and then seek to dissolve and liquidate.
The Company’s Sponsor,
officers and directors have agreed to (i) waive their redemption rights with respect to their Founder Shares, private placement shares
and Public Shares in connection with the completion of the initial Business Combination, (ii) waive their redemption rights with respect
to their Founder Shares and Public Shares in connection with a shareholder vote to approve an amendment to the Company’s amended
and restated memorandum and articles of association, and (iii) waive their rights to liquidating distributions from the Trust Account
with respect to their Founder Shares and private placement shares if the Company fails to complete the initial Business Combination within
the Combination Period.
The Company’s Sponsor
has agreed that it will be liable to the Company if and to the extent any claims by a third party for services rendered or products sold
to the Company, or a prospective target business with which the Company has entered into a written letter of intent, confidentiality or
similar agreement or Business Combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.00
per Public Share and (ii) the actual amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust
Account, if less than $10.00 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability
will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies
held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity
of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act. However,
the Company has not asked its Sponsor to reserve for such indemnification obligations, nor has the Company independently verified whether
its Sponsor has sufficient funds to satisfy its indemnity obligations and believe that the Company’s Sponsor’s only assets
are securities of the Company. Therefore, the Company cannot assure that its Sponsor would be able to satisfy those obligations.
On February 22, 2021, the Sponsor
and Dynamo Master Fund (a member of the Sponsor) entered into a forward purchase agreement (the “Sponsor Forward Purchase Agreement”),
with the Company that provided for the purchase of up to an aggregate of 13,000,000 units, with each unit consisting of one Class A ordinary
share and one-third of one redeemable warrant, for an aggregate purchase price of $130,000,000, or $10.00 per unit, in a private placement
to close substantially concurrently with the closing of the Company’s initial business combination (the “Forward Purchase
Securities”). The Sponsor Forward Purchase Agreement provided that the applicable forward purchase investors may, in their sole
discretion, increase the amount of capital committed under the Sponsor Forward Purchase Agreement up to an amount not to exceed $160,000,000.
On October 20, 2021, the Company received an allocation notice from the Sponsor and Dynamo Master Fund committing to purchase an aggregate
of 16,000,000 units, with each unit consisting of one Class A ordinary share and one-third of one redeemable warrant, for an aggregate
purchase price of $160,000,000, or $10.00 per unit. On December 20, 2021, the Sponsor and Burwell Mountain Trust (a member of the Sponsor)
entered into an assignment and assumption agreement (the “Assignment and Assumption Agreement”). The Assignment and Assumption
Agreement provides for the assignment by the Sponsor and assumption by Burwell Mountain Trust of all of the Sponsor’s rights and
benefits as purchaser under the Sponsor Forward Purchase Agreement, including the right to purchase the Forward Purchase Securities subscribed
for by the Sponsor.
On November 15, 2021, the Company
entered into an Agreement and Plan of Merger (the “Obagi Merger Agreement”), by and among the Company, Obagi Merger Sub, Inc.,
a Cayman Islands exempted company limited by shares and an indirect wholly owned subsidiary of the Company (“Merger Sub”),
and Obagi Global Holdings Limited, a Cayman Islands exempted company limited by shares (“Obagi”). See Note 6 for further discussion.
On November 15, 2021, the Company
entered into an Equity Purchase Agreement (the “Milk Equity Purchase Agreement” and together with the Obagi Merger Agreement,
the “Transaction Agreements”), by and among the Company, Obagi Holdco 1 Limited, a limited company incorporated under the
laws of Jersey (“Holdco Purchaser”), Waldencast Partners LP, a Cayman Islands exempted limited partnership (“Waldencast
LP” and together with Holdco Purchaser, the “Purchasers”), Milk Makeup LLC, a Delaware limited liability company (“Milk”),
certain members of Milk (the “Milk Members”), and Shareholder Representative Services LLC, a Colorado limited liability company,
solely in its capacity as representative of the Milk Members (the “Equityholder Representative”). See Note 6 for further discussion.
Liquidity and Capital Resources
As of December 31, 2021, the
Company had cash in an operating bank account, outside of the Trust Account, of $1,503,768 available for working capital needs. As of
December 31, 2021 the Company had working capital of $1,340,636. All remaining funds held in the Trust Account are generally unavailable
for the Company’s use, prior to an initial Business Combination, and are restricted for use either in a Business Combination, to
redeem Class A ordinary shares or with respect to the interest earned, to be withdrawn for the payment of taxes. As of December 31, 2021,
none of the amount in the Trust Account was withdrawn as described above.
Through December 31, 2021,
the Company’s liquidity needs were satisfied through receipt of $25,000 from the sale of the Founder Shares and the remaining net
proceeds from the Initial Public Offering and the sale of Private Placement Warrants.
On October 28, 2021, the Sponsor
funded the $1,500,000 available under the Working Capital Promissory Note to the Company (see Note 5). The Company anticipates that the
$1,503,768 in its operating bank account as of December 31, 2021, in addition to the subsequent $1,500,000 draw down of the Working Capital
Promissory Note available, will be sufficient to allow the Company to operate for at least the next 12 months from the issuance of the
financial statements, assuming that a Business Combination is not consummated during that time. Until consummation of its Business Combination,
the Company will be using the funds not held in the Trust Account, and any additional Working Capital Loans (as defined in Note 5) from
the initial shareholders, the Company’s officers and directors, or their respective affiliates (which is described in Note 5), for
identifying and evaluating prospective acquisition candidates, performing business due diligence on prospective target businesses, traveling
to and from the offices, plants or similar locations of prospective target businesses, reviewing corporate documents and material agreements
of prospective target businesses, selecting the target business to acquire and structuring, negotiating and consummating the Business
Combination.
The Company does not believe
It will need to raise additional funds in order to meet the expenditures required for operating its business. However, if the Company’s
estimates of the costs of undertaking in-depth due diligence and negotiating Business Combination is less than the actual amount necessary
to do so, the Company may have insufficient funds available to operate its business prior to the Business Combination. Moreover, the Company
will need to raise additional capital through loans from its Sponsor, officers, directors, or third parties. None of the Sponsor, officers
or directors are under any obligation to advance funds to, or to invest in, the Company. If the Company is unable to raise additional
capital, it may be required to take additional measures to conserve liquidity, which could include, but not necessarily be limited to,
curtailing operations, suspending the pursuit of its business plan, and reducing overhead expenses. The Company cannot provide any assurance
that new financing will be available to it on commercially acceptable terms, if at all.
Going Concern
The
Company anticipates that the $1,503,768 of cash held by Waldencast outside of the Trust Account as of December 31, 2021, will
be sufficient to allow the Company to operate for the remainder of the Combination Period (as defined below). Until consummation of its
Business Combination, the Company will be using the funds not held in the Trust Account, and any additional Working Capital Loans from
the initial shareholders, the Company’s officers and directors, or their respective affiliates, or other third parties, for identifying
and evaluating prospective acquisition candidates, performing business due diligence on prospective target businesses, traveling to and
from the offices, plants or similar locations of prospective target businesses, reviewing corporate documents and material agreements
of prospective target businesses, selecting the target business to acquire and structuring, negotiating and consummating the Business
Combination.
The
Company does not believe it will need to raise additional funds in order to meet the expenditures required for operating the business.
However, if the Company’s estimates of the costs of undertaking in-depth due diligence and negotiating business combination is
less than the actual amount necessary to do so, the Company may have insufficient funds available to operate its business prior to the
business combination. Moreover, the Company will need to raise additional capital through loans from its Sponsor, officers, directors,
or third parties. None of the Sponsor, officers or directors are under any obligation to advance funds to, or to invest in, the Company.
If the Company is unable to raise additional capital, it may be required to take additional measures to conserve liquidity, which could
include, but not necessarily be limited to, curtailing operations, suspending the pursuit of its business plan, and reducing overhead
expenses. The Company cannot provide any assurance that new financing will be available to it on commercially acceptable terms, if at
all.
The Company has 24 months from the closing of
the IPO, which occurred on March 18, 2021 (with the ability to extend with shareholder approval) to consummate a business combination
(the “Combination Period”). However, if the Company is unable to complete a Business Combination within the Combination Period,
the Company will redeem 100% of the outstanding public shares for a pro rata portion of the funds held in the Trust Account, equal to
the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the Trust Account and not previously
released to the Company, divided by the number of then outstanding public shares, subject to applicable law and as further described
in the registration statement, and then seek to dissolve and liquidate.
There
is no guarantee that the Company will be able to consummate a Business Combination within Combination Period, which raises
substantial doubt about the Company’s ability to continue as a going concern until the earlier of the consummation of
the Business Combination or the date the Company is required to liquidate. These Financial Statements do
not include any adjustments relating to the recovery of the recorded assets or the classification of the liabilities that
might be necessary should the Company be unable to continue as a going concern.
Risks and Uncertainties
Management continues to evaluate
the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a
negative effect on the Company’s financial position and/or results of its operations, the specific impact is not readily determinable
as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome
of this uncertainty.
Note 2 — Significant Accounting Policies
Basis of Presentation
The accompanying financial
statements of the Company are presented in U.S. dollars in conformity with accounting principles generally accepted in the United States
of America (“GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”).
In the opinion of management, all adjustments (consisting of normal recurring adjustments) have been made that are necessary to present
fairly the financial position, and the results of its operations and its cash flows.
Emerging Growth Company Status
The Company is an “emerging
growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended, (the “Securities Act”), as modified
by the Jumpstart our Business Startups Act of 2012, (the “JOBS Act”), and it may take advantage of certain exemptions from
various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not
limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure
obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding
a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1)
of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until
private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class
of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS
Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging
growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition
period which means that when a standard is issued or revised and it has different application dates for public or private companies,
the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised
standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging
growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because
of the potential differences in accounting standards used.
Use of Estimates
The preparation of financial
statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses
during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all short-term
investments with an original maturity of three months or less when purchased to be cash equivalents. As of December 31, 2021, the Company
had $1,503,768 in cash in its operating bank account, outside of the Trust Account, and had no cash equivalents.
Investment Held in Trust Account
At December 31, 2021, the Trust
Account had $345,052,047 held in marketable securities. As of December 31, 2021, the Company has not withdrawn any of the interest income
from the Trust Account to pay its tax obligations.
Concentration of Credit Risk
Financial instruments that
potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution, which, at times,
may exceed the Federal Depository Insurance Coverage of $250,000. At December 31, 2021, the Company has not experienced losses
on this account.
Class A Ordinary Shares Subject to Possible
Redemption
The Company accounts for
its Class A ordinary shares subject to possible redemption in accordance with the guidance in ASC Topic 480, “Distinguishing
Liabilities from Equity.” Class A ordinary shares subject to mandatory redemption (if any) are classified as a liability
instrument and are measured at fair value. Conditionally redeemable ordinary shares (including ordinary shares that feature
redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events
not solely within the Company’s control) are classified as temporary equity. At all other times, ordinary shares are
classified as shareholders’ deficit. The Company’s Class A ordinary shares feature certain redemption rights that are
considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, all
shares of Class A ordinary shares subject to possible redemption are presented at redemption value as temporary equity, outside of
the shareholders’ deficit section of the Company’s balance sheet.
All
of the Class A ordinary shares sold as part of the Units in the IPO contain a redemption feature which allows for the redemption
of such Public Shares in connection with the Company’s liquidation, if there is a shareholder vote or tender offer in connection
with the Business Combination and in connection with certain amendments to the Company’s amended and restated memorandum and articles
of association (except that in no event may we redeem our Public Shares in an amount that would cause our net tangible assets to be less
than $5,000,001 following such redemptions pursuant to our amended and restated memorandum and articles of association). In accordance
with the SEC and its staff guidance on redeemable equity instruments, which has been codified in ASC 480-10-S99, redemption provisions
not solely within the control of the Company require ordinary shares subject to redemption to be classified outside of permanent equity.
As of December 31, 2021, the
Class A ordinary shares reflected on the balance sheets are reconciled in the following table:
| Gross proceeds | |
$ | 345,000,000 | |
| Less: | |
| | |
| Proceeds allocated to public warrants | |
| (11,960,000 | ) |
| Issuance costs related to Class A ordinary shares | |
| (19,450,398 | ) |
| Plus: | |
| | |
| Accretion of carrying value to redemption value | |
| 31,410,398 | |
| Contingently redeemable Class A ordinary shares | |
$ | 345,000,000 | |
Net Loss per Ordinary Share
The Company applies the two-class
method in calculating earnings per share. The contractual formula utilized to calculate the redemption amount approximates fair value.
The Class feature to redeem at fair value means that there is effectively only one class of stock. Changes in fair value are not considered
a dividend of the purposes of the numerator in the earnings per share calculation. Net loss per ordinary share is computed by
dividing the pro rata net loss between the Class A ordinary shares and the Class B ordinary shares by the weighted average number
of ordinary shares outstanding for each of the periods. The calculation of diluted loss per ordinary share does not consider
the effect of the warrants and rights issued in connection with the Initial Public Offering since the exercise of the warrants and rights
are contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive. The warrants and FPA units
are exercisable for 61,833,333 shares of Class A ordinary shares in the aggregate. Accretion of the carrying value of Class A ordinary
shares to redemption value is excluded from net loss per ordinary share because the redemption value approximates fair value.
| | |
For the
year ended
December 31,
2021 | | |
For the
period from
December 8,
2020
(inception)
through
December 31,
2020 | |
| Ordinary shares subject to possible redemption | |
| | |
| |
| Numerator: | |
| | |
| |
| Net loss allocable to Class A ordinary shares subject to possible redemption | |
$ | (11,115,177 | ) | |
$ | — | |
| Denominator: | |
| | | |
| | |
| Weighted Average Redeemable Class A ordinary shares, Basic and Diluted | |
| 27,316,438 | | |
| — | |
| Basic and Diluted net loss per share, Redeemable Class A ordinary shares | |
$ | (0.41 | ) | |
$ | — | |
| | |
| | | |
| | |
| Non-Redeemable Ordinary shares | |
| | | |
| | |
| Numerator: | |
| | | |
| | |
| Net loss allocable to Class B ordinary shares not subject to redemption | |
$ | (3,312,508 | ) | |
$ | — | |
| Denominator: | |
| | | |
| | |
| Weighted Average Non-Redeemable Ordinary shares, Basic and Diluted | |
| 8,140,753 | | |
| — | |
| Basic and diluted net loss per share, ordinary shares | |
$ | (0.41 | ) | |
$ | — | |
Offering Costs
The Company complies with the
requirements of the ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A–- “Expenses of Offering”.
Offering costs consist principally of professional and registration fees incurred through the balance sheet date that are related to the
Initial Public Offering and that were charged to shareholders’ deficit upon the completion of the Initial Public Offering. Accordingly,
on December 31, 2021, offering costs totaling $20,169,599 have been charged to temporary equity (consisting of $6,900,000 of underwriting
fee, $12,075,000 of deferred underwriting fee and $1,194,599 of other offering costs). Of the total transaction costs, $719,201 was reclassified
as a non-operating expense in the statements of operations with the rest of the offering cost charged to temporary equity. The transaction
costs were allocated based on a relative fair value basis, compared to the total offering proceeds, between the fair value of the public
warrant liabilities and the Class A ordinary shares.
Fair Value of Financial Instruments
The fair value of the Company’s
assets and liabilities, which qualify as financial instruments under the Financial Accounting Standards Board (“FASB”) ASC
820, “Fair Value Measurements and Disclosures,” approximates the carrying amounts represented in the balance sheets.
Derivative Warrant Liabilities
The Company evaluates its financial
instruments, including issued share purchase warrants, to determine if such instruments are derivatives or contain features that qualify
as embedded derivatives, pursuant to ASC 480 and ASC 815-15. The classification of derivative instruments, including whether such instruments
should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period. The Company has determined its public
warrants, private warrants and contingent forward purchase warrants are derivative instruments.
The Company accounts for its
17,433,333 ordinary share warrants issued in connection with its Initial Public Offering (11,500,000) and Private Placement Warrants (5,933,333)
as derivative warrant liabilities in accordance with ASC 815-40. Accordingly, the Company recognizes the warrant instruments as liabilities
at fair value and adjusts the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each
balance sheet date until exercised, and any change in fair value is recognized in the Company’s statements of operations. The fair
value of warrants issued by the Company in connection with its Initial Public Offering and Private Placement Warrants has been estimated
using Monte-Carlo simulations at each measurement date.
FASB ASC 470-20, “Debt with
Conversion and Other Options,” addresses the allocation of proceeds from the issuance of convertible debt into its equity and debt components.
The Company applied this guidance to allocate Initial Public Offering proceeds from the Units between Class A ordinary shares and warrants,
using the residual method by allocating Initial Public Offering proceeds first to fair value of the warrants and contingent forward purchase
units and then the Class A ordinary shares.
Income Taxes
The Company accounts for income
taxes under ASC Topic 740, “Income Taxes,” which requires an asset and liability approach to financial accounting and reporting
for income taxes. Deferred income tax assets and liabilities are computed for differences between the financial statements and tax bases
of assets and liabilities that will result in future taxable or deductible amounts, based on enacted tax laws and rates applicable to
the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce
deferred tax assets to the amount expected to be realized.
ASC Topic 740 prescribes
a recognition threshold and a measurement attribute for the financial statements recognition and measurement of tax positions taken
or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be
sustained upon examination by taxing authorities. The Company’s management determined that the Cayman Islands is the
Company’s major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits
as income tax expense. As of December 31, 2021 and December 31, 2020, there were no unrecognized tax benefits and no amounts accrued for interest and
penalties. The Company is currently not aware of any issues under review that could result in significant payments, accruals or
material deviation from its position.
The Company is considered to
be an exempted Cayman Islands company with no connection to any other taxable jurisdiction and is presently not subject to income taxes
or income tax filing requirements in the Cayman Islands or the United States. As such, the Company’s tax provision was immaterial
for the year ended December 31, 2021 and for the period from December 8, 2020 (inception) through December 31, 2020.
Recent Accounting Standards
In August 2020, the FASB issued
ASU 2020-06, “Debt-Debt with Conversion and Other Options” (Subtopic 470-20) and “Derivatives and Hedging-Contracts
in an Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity”
(“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under
current GAAP. ASU 2020-06 also removes certain settlement conditions that are required for equity-linked contracts to qualify for scope
exception, and it simplifies the diluted earnings per share calculation in certain areas. The Company adopted ASU 2020-06 on January 1,
2021. Adoption of the ASU did not impact the Company’s financial position, results of operations or cash flows.
The Company’s management
does not believe that any other recently issued, but not yet effective, accounting standards if currently adopted would have a material
effect on the accompanying financial statements.
Note 3 — Initial Public Offering
Pursuant to the Initial Public
Offering, the Company sold 34,500,000 Units, at a price of $10.00 per Unit. Each Unit consists of one share of Class A ordinary shares,
par value $0.0001 per share one-third of one redeemable warrant (“Public Warrant”). Each whole Public Warrant entitles the
holder to purchase one share of Class A ordinary shares at a price of $11.50 per share.
Note 4 — Private Placement Warrants
Simultaneously with the closing
of the Initial Public Offering, the Sponsor purchased an aggregate of 5,933,333 Private Placement Warrants at a price of $1.50 per Private
Placement Warrant, for an aggregate price of $8,900,000. Each Private Placement Warrant is exercisable for one Class A ordinary share
at a price of $11.50 per share, subject to adjustment (see Note 6). If the Company does not complete a Business Combination within the
Combination Period, the proceeds from the sale of the Private Placement Warrants held in the Trust Account will be used to fund the redemption
of the Public Shares (subject to the requirements of applicable law) and the Private Placement Warrants will expire worthless. The initial
fair value of the private warrants was recorded as a liability of $6,230,000 with the excess of cash received over initial fair value
of the warrants of $2,670,000 recorded as additional paid-in capital.
Note 5 — Related Party Transactions
Founder Shares
On January 12, 2021, the Company
issued 7,187,500 Founder Shares to the Sponsor for an aggregate purchase price of $25,000. In February 2021, the Sponsor transferred 20,000
Waldencast Class B ordinary shares to each of the Investor Directors, resulting in the Sponsor holding 7,107,500 Waldencast Class B ordinary
shares. On March 15, 2021, the Company effected a dividend of 0.2 of a share of Class B ordinary shares for each share of Class B ordinary
shares, resulting in 8,625,000 shares of Class B ordinary shares being issued and outstanding, of which 8,545,000 are held by the Sponsor.
The Sponsor has agreed, subject
to limited exceptions, not to transfer, assign or sell any of its Class B ordinary shares or Class A ordinary shares received upon conversion
thereof until the earlier of: (A) one year after the completion of a Business Combination and (B) subsequent to a Business Combination,
(x) if the last reported sale price of the Class A ordinary shares equals or exceeds $12.00 per share (as adjusted for share sub-divisions,
share dividends, rights issuances, consolidations, reorganizations, recapitalizations and the like) for any 20 trading days within any
30-trading day period commencing at least 150 days after a Business Combination, or (y) the date on which the Company completes a liquidation,
merger, amalgamation, share exchange, reorganization or other similar transaction that results in all of the Company’s shareholders
having the right to exchange their ordinary shares for cash, securities or other property.
Related Party Loans
On January 12, 2021, the Sponsor
agreed to loan the Company up to $300,000 to be used for the payment of costs related to the Initial Public Offering pursuant to a promissory
note (the “Sponsor Promissory Note”). The Sponsor Promissory Note was non-interest bearing, unsecured and due upon the earlier
of June 30, 2021 and the closing of the Initial Public Offering. The Company had no borrowings under the Sponsor Promissory Note at the
closing of the Initial Public Offering. Borrowings under the Sponsor Promissory Note are no longer available.
Due to Related Party
The balance of $95,000 represents
the amount accrued for the administrative support services provided (defined below) by the Sponsor from date of the Initial Public Offering
to December 31, 2021.
Administrative Support Agreement
Commencing on the date of the
Initial Public Offering, the Company has agreed to pay the Sponsor a total of $10,000 per month for office space and administrative support
services. Upon completion of the initial Business Combination or the Company’s liquidation, the Company will cease paying these
monthly fees. For the year ended December 31, 2021, the Company has recognized $101,740 of administrative service fee, which is included
in formation and operating costs on the statement of operations.
Working Capital Loans
In
order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of
the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required. Such Working Capital
Loans would be evidenced by promissory notes. The notes may be repaid upon completion of a Business Combination, without interest, or,
at the lender’s discretion, up to $1,500,000 of notes may be converted upon completion of a Business Combination into warrants
at a price of $1.50 per warrant. Such warrants would be identical to the Private Placement Warrants. In the event that a business
combination does not close, the Company may use a portion of proceeds held outside of the Trust Account to repay the Working Capital Loans,
but no proceeds held in the Trust Account would be used for such repayment. On August 18, 2021, the Company issued the Working Capital
Promissory Note to the Sponsor for an aggregate amount of up to $1,500,000. The Working Capital Promissory Note is non-interest bearing
and is due and payable in full on the earlier of (i) the date by which we have to complete a business combination and (ii) the
effective date of a business combination. On October 28, 2021, the Company drew down the entire available balance of the Working
Capital Promissory Note and the Sponsor deposited $1,500,000 in the Company’s operating bank account. As of December 31,
2021, the Company had a total aggregate principal amount of $1,500,000 in outstanding borrowings under the Working Capital Loans,
consisting solely of the Working Capital Promissory Note.
Forward Purchase Agreement
The Company entered into
two separate forward purchase agreements as follows. The Sponsor and Dynamo Master Fund (a member of the Sponsor) entered into the
Sponsor Forward Purchase Agreement, dated as of February 22, 2021, with the Company that will provide for the purchase of up to
an aggregate of 13,000,000 units, with each unit consisting of one Class A ordinary share and one-third of one
redeemable warrant, for an aggregate purchase price of $130,000,000, or $10.00 per unit, in a private placement to close
substantially concurrently with the closing of our initial Business Combination. The Sponsor Forward Purchase Agreement provides
that the applicable forward purchase investors may, in their sole discretion, increase the amount of capital committed under the
Sponsor Forward Purchase Agreement up to an amount not to exceed $160,000,000. Beauty Ventures LLC (“Beauty Ventures”)
entered into a forward purchase agreement (the “Beauty Forward Purchase Agreement,” and together with the Sponsor
Forward Purchase Agreement, the “Forward Purchase Agreements” or “FPA”), dated as of March 1, 2021,
with the Company that provides for the purchase of an aggregate of up to 17,300,000 units, with each unit consisting of
one Class A ordinary share and one-third of one redeemable warrant, for an aggregate purchase price of up to
$173,000,000 (subject to the below), or $10.00 per unit, in a private placement to close substantially concurrently with
the closing of the initial Business Combination. To the extent that the amounts available from the Trust Account and other financing
(including the Sponsor Forward Purchase Agreement) are sufficient for the cash requirements in connection with our initial Business
Combination, the Sponsor may, in its sole discretion, as the managing member of Beauty Ventures, reduce its purchase obligation, up
to the full amount, under the Beauty Forward Purchase Agreement. Members of the Sponsor or their affiliates will receive a
performance fee allocation when the return on the securities underlying the Beauty Forward Purchase Agreement exceeds certain
benchmark returns. The obligations under the forward purchase agreements will not depend on whether any Class A ordinary shares
are redeemed by our public shareholders. The forward purchase shares and the forward purchase warrants included in the units being
sold in the Initial Public Offering, respectively, will be identical to the Public Shares and public warrants included in the units
sold in the Initial Public Offering, respectively, except that the holders thereof will have certain registration rights, as
described herein. On October 20, 2021, the Company received (i) an allocation notice from the Sponsor and Dynamo Master Fund
committing to purchase 16,000,000 units, with each unit consisting of one Class A ordinary share and one-third of one redeemable
warrant, for an aggregate purchase price of $160,000,000, or $10.00 per unit and (ii) an allocation notice from Beauty Ventures
committing to purchase 17,300,000 units, with each unit consisting of one Class A ordinary share and one-third of one redeemable
warrant, for an aggregate purchase price of $173,000,000, or $10.00 per unit. On December 20, 2021, the Sponsor and
Burwell Mountain Trust (a member of the Sponsor) entered into an assignment and assumption agreement. The assignment and assumption
agreement provides for the assignment by the Sponsor and assumption by Burwell Mountain Trust of all of the Sponsor’s rights
and benefits as purchaser under the Sponsor Forward Purchase Agreement, including the right to purchase the Forward Purchase
Securities subscribed for by the Sponsor.
Note 6 — Commitments & Contingencies
Registration Rights
The holders of the Founder
Shares, Private Placement Warrants and any warrants that may be issued upon conversion of Working Capital Loans (and any Class A ordinary
shares issuable upon the exercise of the Private Placement Warrants or warrants issued upon conversion of the Working Capital Loans and
upon conversion of the Founder Shares) will be entitled to registration rights pursuant to a registration rights agreement to be signed
prior to or on the effective date of the Initial Public Offering requiring the Company to register such securities for resale. The holders
of these securities will be entitled to make up to three demands, excluding short form demands, that the Company register such securities.
In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent
to the completion of a Business Combination. The Company will bear the expenses incurred in connection with the filing of any such registration
statements.
Underwriters Agreement
On March 18, 2021, pursuant
to the consummation of the IPO, the Company paid a fixed underwriting discount of $0.20 per Unit, or $6,900,000 in
the aggregate. Additionally, a deferred underwriting discount of $0.35 per Unit, or $12,075,000 in the aggregate, will be payable to the
underwriters from the amounts held in the Trust Account solely in the event that the Company completes an initial Business Combination,
subject to the terms of the underwriting agreement.
Obagi Merger Agreement and Related Agreements
On November 15, 2021, the Company
entered into the Obagi Merger Agreement, by and among the Company, Merger Sub and Obagi. The Obagi Merger Agreement provides that, among
other things and upon the terms and subject to the conditions thereof, the following transactions will occur (together with the other
agreements and transactions contemplated by the Obagi Merger Agreement, the “Obagi Transaction”):
| |
(i) |
at the closing of the transactions contemplated by the Obagi Merger Agreement (the “Obagi Closing”),
upon the terms and subject to the conditions of the Obagi Merger Agreement and in accordance with the Companies Act (As Revised) of the
Cayman Islands (“Cayman Act”), Merger Sub will merge with and into Obagi, the separate corporate existence of Merger Sub
will cease and Obagi will be the surviving company and an indirect wholly owned subsidiary of the Company (the “Merger”); |
| |
(ii) |
as a result of the Merger, among other things, each share of common stock of Obagi that is issued
and outstanding immediately prior to the effective time of the Merger (other than in respect of Excluded Shares (as defined in the Obagi
Merger Agreement)) will be cancelled and converted into the right to receive (i) an amount in cash equal to (A) the Obagi Cash Consideration
(as defined in the Obagi Merger Agreement), subject to substitution for Obagi Stock Consideration (as defined in the Obagi Merger Agreement)
based on the amount of cash available to the Company at the Closing, taking into account, among other things, the level of shareholder
redemptions, divided by (B) the number of Aggregate Fully Diluted Company Common Shares (as defined in the Obagi Merger Agreement), and
(ii) a number of shares of Waldencast Common Stock equal to (A) the Obagi Stock Consideration divided by (B) the number of Aggregate
Fully Diluted Company Common Shares; and |
| |
(iii) |
upon the effective time of the Domestication, the Company will immediately be renamed “Waldencast
plc”. |
The Company’s board of
directors has unanimously (i) approved and declared advisable the Obagi Merger Agreement, the Obagi Transaction and the other transactions
contemplated thereby and (ii) resolved to recommend approval of the Obagi Merger Agreement and related matters by the shareholders of
the Company.
Milk Equity Purchase Agreement
On November 15, 2021, the Company
entered into the Transaction Agreements, by and among the Company, Holdco Purchaser, Waldencast LP, Milk, Milk Members, and the Equityholder
Representative.
The Milk Equity Purchase Agreement
provides that, among other things and upon the terms and subject to the conditions thereof, the following transactions will occur (together
with the other agreements and transactions contemplated by the Milk Equity Purchase Agreement, the “Milk Transaction” and,
together with the Obagi Transaction, the “Obagi and Milk Business Combinations”):
| |
(i) |
at the closing of the transactions contemplated by the Milk Equity Purchase Agreement (the “Milk
Closing” and together with the Obagi Closing, the “Closing”), upon the terms and subject to the conditions of the Milk
Equity Purchase Agreement, the Purchasers will acquire from the Milk Members and the Milk Members will sell to the Purchasers all of
the issued and outstanding membership units of Milk in exchange for the Milk Cash Consideration (as defined in the Milk Equity Purchase
Agreement), and the Milk Equity Consideration (as defined in the Milk Equity Purchase Agreement), which consist of partnership units
of Waldencast LP exchangeable for Waldencast Common Stock, and the Domesticated Acquiror Non-Economic Common Stock (as defined in the
Milk Equity Purchase Agreement); |
| |
(ii) |
as a result of the Milk Transaction, among other things, (i) Holdco Purchaser will purchase from
the Milk Members a percentage of the outstanding membership units in exchange for the Milk Cash Consideration and the Domesticated Acquiror
Non-Economic Common Stock equal to the Milk Equity Consideration and (ii) Waldencast LP will purchase from the Milk Members the remainder
of the outstanding membership units in exchange for the Milk Equity Consideration; and |
| |
(iii) |
upon the effective time of the Domestication, the Company will immediately be renamed “Waldencast
plc.” |
Immediately following consummation
of the Milk Transaction, (i) Holdco Purchaser will contribute its equity interest in (a) Milk to Waldencast LP in exchange for limited
partnership units in Waldencast LP and (b) Holdco 2 in exchange for limited partnership units in Waldencast LP. The combined company will
be organized in an “Up-C” structure, in which the equity interests of Obagi and Milk will be held by Waldencast LP. The Company
will in turn hold its interests in Obagi and Milk through Waldencast LP and Holdco Purchaser.
The Board has unanimously (i)
approved and declared advisable the Milk Equity Purchase Agreement, the Milk Transaction and the other transactions contemplated thereby
and (ii) resolved to recommend approval of the Milk Equity Purchase Agreement and related matters by the shareholders of the Company.
Prior to the Closing, subject
to the approval of the Company’s shareholders, and in accordance with the Cayman Act, the Companies (Jersey) Law 1991, as amended
(the “Jersey Companies Law”) and the Company’s amended and restated memorandum and articles of association, the Company
will effect a deregistration under the Cayman Act and a domestication under Part 18C of the Jersey Companies Law (by means of filing a
memorandum and articles of association with the Registrar of Companies in Jersey), pursuant to which the Company’s jurisdiction
of incorporation will be changed from the Cayman Islands to Jersey (the “Domestication”).
In connection with the Domestication,
(i) each of the then issued and outstanding Class A ordinary shares, par value $0.0001 per share, of the Company, will convert automatically,
on a one-for-one basis, into a Waldencast Common Stock (following its Domestication) (the “Waldencast Common Stock”), (ii)
each of the then issued and outstanding Class B ordinary shares, par value $0.0001 per share, of the Company, will convert automatically,
on a one-for-one basis, into a share of Waldencast Common Stock, (iii) each then issued and outstanding warrant of the Company will convert
automatically into a warrant to acquire one share of Waldencast Common Stock (“Domesticated Waldencast Warrant”), pursuant
to the Warrant Agreement, dated March 15, 2021, between the Company and Continental Stock Transfer & Trust Company, as warrant agent,
and (iv) each then issued and outstanding unit of the Company shall be cancelled and will entitle the holder thereof to one share of Waldencast
Common Stock and one-third of one Domesticated Waldencast Warrant.
On November 15, 2021, the Company
entered into a Sponsor Support Agreement (the “Obagi Sponsor Support Agreement”), by and among the Sponsor, Obagi, the Company
and the persons set forth on Schedule I attached thereto (the “Sponsor Persons”), pursuant to which the Sponsor and the Sponsor
Persons agreed to, among other things, vote in favor of the Obagi Merger Agreement and the transactions contemplated thereby, in each
case, subject to the terms and conditions contemplated by the Obagi Sponsor Support Agreement.
On November 15, 2021, the Company
entered into a Sponsor Support Agreement (the “Milk Sponsor Support Agreement”), by and among the Sponsor, the Equityholder
Representative, the Company and the Sponsor Persons, pursuant to which the Sponsor and the Sponsor Persons agreed to, among other things,
vote in favor of the Milk Equity Purchase Agreement and the transactions contemplated thereby, in each case, subject to the terms and
conditions contemplated by the Milk Sponsor Support Agreement.
On November 15, 2021, the Company
also entered the Stockholder Support Agreement, by and among the Company, Obagi and Cedarwalk. Pursuant to the Stockholder Support Agreement,
Cedarwalk agreed to, among other things, within two (2) business days after the proxy statement/prospectus relating to the approval by
the Company shareholders of the Obagi and Milk Business Combinations is declared effective by the SEC and delivered or otherwise made
available to the Company shareholders, execute and deliver a written consent with respect to the outstanding ordinary shares of Obagi
held by Cedarwalk adopting the Obagi Merger Agreement and related transactions and approving the Obagi and Milk Business Combinations.
The consummation of the proposed
Obagi and Milk Business Combinations is subject to certain conditions as further described in the Obagi Merger Agreement and the Milk
Equity Purchase Agreement.
Note 7 — Class A Ordinary Shares Subject
to Possible Redemption
The Company’s Class A
ordinary shares feature certain redemption rights that are considered to be outside of the Company’s control and subject to the
occurrence of uncertain future events. Accordingly, as of December 31, 2021, 34,500,000 shares of Class A ordinary shares subject to possible
redemption are presented at redemption value as temporary equity, outside of the shareholders’ deficit section of the Company’s
balance sheet. The value of these redeemable shares was calculated as the gross proceeds from the sale of the Public Units reduced by
the proceeds allocable to the Public Warrants, issuance costs related to the Public Units and the accretion of the carrying value to the
redemption value. Upon the consummation of the Initial Public Offering, the Company recorded $31,410,398 in accretion.
Note 8 — Shareholder’s Deficit
Preference Shares
— The Company is authorized to issue a total of 5,000,000 preference shares at par value of $0.0001 each. At December 31, 2021,
there were no preference shares issued or outstanding.
Class A Ordinary Shares
— The Company is authorized to issue a total of 500,000,000 Class A ordinary shares at par value of $0.0001 each. At December 31,
2021, there were no shares issued and outstanding (excluding 34,500,000 shares subject to possible redemption).
Class B Ordinary Shares
— The Company is authorized to issue a total of 50,000,000 shares of Class B ordinary shares at par value of $0.0001 each. At December
31, 2021, there were 8,625,000 Class B ordinary shares issued or outstanding.
Only holders of the Class B
ordinary shares will have the right to vote on the election of directors prior to the Business Combination. Holders of Class A ordinary
shares and holders of Class B ordinary shares will vote together as a single class on all matters submitted to a vote of the Company’s
shareholders except as otherwise required by law.
The Class B ordinary shares
will automatically convert into Class A ordinary shares at the time of the completion of the Business Combination, or earlier at the option
of the holder, on a one-for-one basis, subject to adjustment. In the case that additional Class A ordinary shares, or equity-linked securities,
are issued or deemed issued in excess of the amounts issued in the Initial Public Offering and related to the closing of a Business Combination,
the ratio at which Founder Shares will convert into Class A ordinary shares will be adjusted (subject to waiver by holders of a majority
of the Class B ordinary shares) so that the number of Class A ordinary shares issuable upon conversion of all Founder Shares will equal,
in the aggregate, on an as-converted basis, 20% of the sum of the ordinary shares issued and outstanding upon completion of the Initial
Public Offering plus the number of Class A ordinary shares and equity-linked securities issued or deemed issued in connection with a Business
Combination, excluding any Class A ordinary shares or equity-linked securities issued, or to be issued, to any seller in a Business Combination.
Note 9 — Warrants
Public Warrants may only be
exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only whole warrants will
trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination and (b)
12 months from the closing of the Initial Public Offering. The Public Warrants will expire five years after the completion of a Business
Combination or earlier upon redemption or liquidation.
The Company will not be obligated
to deliver any Class A ordinary shares pursuant to the exercise of a Public Warrant and will have no obligation to settle such Public
Warrant exercise unless a registration statement under the Securities Act with respect to the Class A ordinary shares underlying the Public
Warrants is then effective and a prospectus relating thereto is current, subject to the Company satisfying its obligations with respect
to registration. No Public Warrant will be exercisable, and the Company will not be obligated to issue any shares to holders seeking to
exercise their warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of
the state of the exercising holder, or an exemption is available.
The Company has agreed that
as soon as practicable, but in no event later than 15 business days, after the closing of the Company’s Business Combination, the
Company will use its commercially reasonable efforts to file with the SEC a registration statement for the registration, under the Securities
Act, of the Class A ordinary shares issuable upon exercise of the warrants. The Company will use its commercially reasonable efforts to
cause the same to become effective and to maintain the effectiveness of such registration statement, and a current prospectus relating
thereto, until the expiration or redemption of the warrants in accordance with the provisions of the warrant agreement. If a registration
statement covering the Class A ordinary shares issuable upon exercise of the warrants is not effective by the 60th business day after
the closing of a Business Combination, warrant holders may, until such time as there is an effective registration statement and during
any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis”
in accordance with Section 3(a)(9) of the Securities Act or another exemption. Notwithstanding the above, if the Class A ordinary shares
are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition of a “covered
security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of public warrants who exercise
their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event the
Company so elects, the Company will not be required to file or maintain in effect a registration statement, and in the event the Company
does not so elect, it will use its commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to
the extent an exemption is not available.
Once the warrants become exercisable,
the Company may redeem the Public Warrants for redemption:
| ● | in
whole and not in part; |
| ● | at
a price of $0.01 per Public Warrant; |
| ● | upon
not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| ● | if,
and only if, the reported last sale price of the Class A ordinary shares for any 20 trading days within a 30-trading day period ending
on the third trading day prior to the date on which the Company sends the notice of redemption the warrant holders (the “Reference
Value”) equals or exceeds $18.00 per share (as adjusted). |
Once the Public Warrants become
exercisable, the Company may redeem the Public Warrants:
| ● | in
whole and not in part; |
| |
● |
at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of shares determined by reference to the table below, based on the redemption date and the “fair market value” of the Class A ordinary shares; |
| |
● |
if, and only if, the Reference Value equals or exceeds $10.00 per share (as adjusted); and |
| |
● |
if the Reference Value is less than $18.00 per share (as adjusted), the Private Placement Warrants must also be concurrently called for redemption on the same terms as the outstanding Public Warrants, as described above. |
If and when the warrants become
redeemable by the Company, the Company may exercise its redemption right even if it is unable to register or qualify the underlying securities
for sale under all applicable state securities laws.
The exercise price and number
of ordinary shares issuable upon exercise of the Public Warrants may be adjusted in certain circumstances including in the event of a
share dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except as described below,
the Public Warrants will not be adjusted for issuances of ordinary shares at a price below its exercise price. Additionally, in no event
will the Company be required to net cash settle the Public Warrants. If the Company is unable to complete a Business Combination within
the Combination Period and the Company liquidates the funds held in the Trust Account, holders of Public Warrants will not receive any
of such funds with respect to their Public Warrants, nor will they receive any distribution from the Company’s assets held outside
of the Trust Account with respect to such Public Warrants. Accordingly, the Public Warrants may expire worthless.
In addition, if (x) the Company
issues additional Class A ordinary shares or equity-linked securities for capital raising purposes in connection with the closing of a
Business Combination at an issue price or effective issue price of less than $9.20 per Class A ordinary share (with such issue price or
effective issue price to be determined in good faith by the Company’s board of directors and, in the case of any such issuance to
the Sponsor or its affiliates, without taking into account any Founder Shares held by the Sponsor or such affiliates, as applicable, prior
to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent more than 60%
of the total equity proceeds, and interest thereon, available for the funding of a Business Combination, and (z) the volume weighted average
trading price of the Class A ordinary shares during the 20 trading day period starting on the trading day prior to the day on which the
Company consummates a Business Combination (such price, the “Market Value”) is below $9.20 per share, then the exercise price
of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the Newly Issued Price,
and the $10.00 and $18.00 per share redemption trigger prices will be adjusted (to the nearest cent) to be equal to 100% and 180% of the
higher of the Market Value and the Newly Issued Price, respectively.
The Private Placement Warrants
will be identical to the Public Warrants underlying the Units being sold in the Initial Public Offering, except that the Private Placement
Warrants and the Class A ordinary shares issuable upon the exercise of the Private Placement Warrants will not be transferable, assignable
or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally, the Private
Placement Warrants will be exercisable on a cashless basis and be non-redeemable, except as described above, so long as they are held
by the initial purchasers or their permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable
by such holders on the same basis as Public Warrants.
Note 10 — Fair Value Measurements
Fair value is defined as the
price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants
at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value.
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements)
and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level
1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level
2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices
for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| ● | Level
3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions,
such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
The following table presents
information about the Company’s assets that are measured at fair value on a recurring basis at December 31, 2021 and indicates the
fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
| | |
December 31, | | |
Quoted
Prices In
Active
Markets | | |
Significant
Other
Observable
Inputs | | |
Significant
Other
Unobservable
Inputs | |
| | |
2021 | | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Description | |
| | |
| | |
| | |
| |
| Assets: | |
| | |
| | |
| | |
| |
| Marketable Securities held in Trust Account | |
$ | 345,052,047 | | |
$ | 345,052,047 | | |
$ | — | | |
$ | — | |
| Liabilities: | |
| | | |
| | | |
| | | |
| | |
| Forward purchase agreement liabilities | |
| (13,320,000 | ) | |
| — | | |
| — | | |
| (13,320,000 | ) |
| Warrant liabilities | |
| (21,153,666 | ) | |
| (13,915,000 | ) | |
| — | | |
| (7,238,666 | ) |
| | |
$ | 310,578,381 | | |
$ | 331,137,047 | | |
$ | — | | |
$ | (20,558,666 | ) |
The Company utilizes a Monte
Carlo simulation model to value the warrants at each reporting period, with changes in fair value recognized in the statements of operations.
The estimated fair value of the warrant liabilities is determined using Level 3 inputs. Inherent in a binomial options pricing model are
assumptions related to expected share-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates
the volatility of its ordinary shares based on historical volatility that matches the expected remaining life of the warrants. The risk-free
interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining
life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend
rate is based on the historical rate, which the Company anticipates to remain at zero.
The aforementioned warrant
liabilities are not subject to qualified hedge accounting.
The value of the warrant liabilities
was transferred from Level 3 to Level 1 during the period due to the fact that they are now listed on an active market. There were no
other transfers between Levels 1, 2 or 3 during the year ended December 31, 2021.
The following table provides
quantitative information regarding Level 3 fair value measurements:
| | |
At
December 31,
2021 | |
| Share price | |
$ | 10.00 | |
| Strike price | |
$ | 11.50 | |
| Term (in years) | |
| 5.50 | |
| Volatility | |
| 19.0 | % |
| Risk-free rate | |
| 1.31 | % |
| Dividend yield | |
| 0.0 | % |
The
following table presents the changes in the fair value of warrant liabilities:
| | |
Public | | |
Private
Placement | | |
Warrant
Liabilities | |
| Fair value as of December 31, 2020 | |
$ | — | | |
$ | — | | |
$ | — | |
| Initial measurement on March 18, 2021 | |
| 11,960,000 | | |
| 6,230,000 | | |
| 18,190,000 | |
| Change in fair value of warrant liabilities | |
| 1,955,000 | | |
| 1,008,666 | | |
| 2,963,666 | |
| Fair value as of December 31, 2021 | |
$ | 13,915,000 | | |
$ | 7,238,666 | | |
$ | 21,153,666 | |
Prior to their transfer to
Level 1 inputs, the estimated fair value of warrant liabilities is determined using Level 3 inputs. Inherent in a binomial options pricing
model are assumptions related to expected share-price volatility, expected life, risk-free interest rate and dividend yield. The Company
estimates the volatility of its ordinary shares based on historical volatility that matches the expected remaining life of the warrants.
The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected
remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The
dividend rate is based on the historical rate, which the Company anticipates to remain at zero.
The Company has initially classified
the FPA as a liability. This financial instrument is subject to re-measurement at each balance sheet date. With each such re-measurement,
the FPA asset or liability will be adjusted to fair value, with the change in fair value recognized in the Company’s statements
of operations. As such, the Company recorded a $11,655,000 of derivative liabilities related to the FPA as of March 18, 2021. At December
31, 2021, the re-measurement of the derivative associated with the FPA resulted in the following change in the derivative liabilities
– forward purchase agreement.
| | |
FPA
Liabilities | |
| | |
| |
| Derivative liability – forward purchase agreement at March 18, 2021 | |
$ | 11,655,000 | |
| Change in fair value of derivative liability – forward purchase agreement | |
| 1,665,000 | |
| Derivative liability – forward purchase agreement at December 31, 2021 | |
$ | 13,320,000 | |
The following table presents
information about the assumptions used to value the Company’s FPA liabilities classified as Level 3 in the fair value hierarchy
that are measured at fair value on a recurring basis.
| | |
At
December 31,
2021 | |
| Share price | |
$ | 10.00 | |
| Strike price | |
$ | 10.00 | |
| Term (in years) | |
| 5.50 | |
| Volatility | |
| 19.0 | % |
| Risk-free rate | |
| 1.31 | % |
| Dividend yield | |
| 0.0 | % |
Note 11 — Subsequent Events
The Company evaluated subsequent
events and transactions that occurred after the balance sheet date through the date that the financial statements were issued. Based upon
this review, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
WALDENCAST
PLC
(f/k/a WALDENCAST
ACQUISITION CORP.)
CONDENSED BALANCE SHEETS
| | |
June 30,
2022 | | |
December 31,
2021 | |
| | |
(Unaudited) | | |
| |
| Assets | |
| | |
| |
| Current assets: | |
| | |
| |
| Cash | |
$ | 99,727 | | |
$ | 1,503,768 | |
| Prepaid expenses – current | |
| 282,973 | | |
| 204,821 | |
| Total current assets | |
$ | 382,700 | | |
$ | 1,708,589 | |
| Prepaid expenses – non-current portion | |
| — | | |
| 33,050 | |
| Investments held in Trust Account | |
| 345,312,792 | | |
| 345,052,047 | |
| Total assets | |
$ | 345,695,492 | | |
$ | 346,793,686 | |
| | |
| | | |
| | |
| Liabilities, Class A Ordinary Shares Subject to Possible Redemption and Shareholders’ Deficit | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 1,039,314 | | |
$ | 272,953 | |
| Due to related party | |
| 155,043 | | |
| 95,000 | |
| Total current liabilities | |
| 1,194,357 | | |
| 367,953 | |
| Warrant liabilities | |
| 12,552,000 | | |
| 21,153,666 | |
| Deferred legal fees | |
| 13,801,196 | | |
| 8,186,101 | |
| Forward purchase agreement liabilities | |
| 7,992,000 | | |
| 13,320,000 | |
| Working Capital Promissory Notes – related party | |
| 2,100,000 | | |
| 1,500,000 | |
| Deferred underwriters’ discount | |
| 12,075,000 | | |
| 12,075,000 | |
| Total liabilities | |
| 49,714,553 | | |
| 56,602,720 | |
| | |
| | | |
| | |
| Commitments & Contingencies (Note 6) | |
| | | |
| | |
| Class A ordinary shares subject to possible redemption, 34,500,000 shares at redemption value | |
| 345,312,792 | | |
| 345,000,000 | |
| | |
| | | |
| | |
| Shareholders’ Deficit: | |
| | | |
| | |
| Preference shares, $0.0001 par value; 5,000,000 shares authorized; none issued and outstanding | |
| — | | |
| — | |
| Class A ordinary shares, $0.0001 par value; 500,000,000 shares authorized; no shares issued and outstanding (excluding 34,500,000 and no shares subject to redemption) at June 30, 2022 and December 31, 2021 | |
| — | | |
| — | |
| Class B ordinary shares, $0.0001 par value; 50,000,000 shares authorized; 8,625,000 issued and outstanding at June 30, 2022 and December 31, 2021 | |
| 863 | | |
| 863 | |
| Additional paid-in capital | |
| — | | |
| — | |
| Accumulated deficit | |
| (49,332,716 | ) | |
| (54,809,897 | ) |
| Total Shareholders’ Deficit | |
| (49,331,853 | ) | |
| (54,809,034 | ) |
| Total liabilities, Class A ordinary shares subject to possible redemption and Shareholders’ Deficit | |
$ | 345,695,492 | | |
$ | 346,793,686 | |
See accompanying notes to
the unaudited condensed financial statements.
WALDENCAST PLC
(f/k/a WALDENCAST
ACQUISITION CORP.)
CONDENSED STATEMENTS OF OPERATIONS
(UNAUDITED)
| | |
For
the Three Months Ended
June 30, | | |
For
the Six Months Ended
June 30, | |
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| Formation and operating costs | |
$ | 4,397,526 | | |
$ | 201,567 | | |
$ | 8,401,003 | | |
$ | 319,082 | |
| Loss from operations | |
| (4,397,526 | ) | |
| (201,567 | ) | |
| (8,401,003 | ) | |
| (319,082 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Interest income on operating account | |
| 126 | | |
| 311 | | |
| 565 | | |
| 442 | |
| Interest income on marketable securities held in Trust Account | |
| 257,125 | | |
| 13,082 | | |
| 260,745 | | |
| 14,376 | |
| Offering expenses related to warrant issuance | |
| — | | |
| — | | |
| — | | |
| (719,201 | ) |
| Change in fair value of forward purchase agreement liabilities | |
| 2,664,000 | | |
| (666,000 | ) | |
| 5,328,000 | | |
| (666,000 | ) |
| Change in fair value of warrant liabilities | |
| 4,243,333 | | |
| (697,334 | ) | |
| 8,601,666 | | |
| (1,046,000 | ) |
| Total other income (expense), net | |
| 7,164,584 | | |
| (1,349,941 | ) | |
| 14,190,976 | | |
| (2,416,383 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) | |
$ | 2,767,058 | | |
$ | (1,551,508 | ) | |
$ | 5,789,973 | | |
$ | (2,735,465 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding, Class A ordinary shares subject to possible redemption | |
| 34,500,000 | | |
| 34,500,000 | | |
| 34,500,000 | | |
| 19,933,333 | |
| Basic and diluted net income (loss) per share, Class A ordinary shares subject to possible redemption | |
$ | 0.06 | | |
$ | (0.04 | ) | |
$ | 0.13 | | |
$ | (0.10 | ) |
| Weighted average shares outstanding, Non-redeemable Class B ordinary shares | |
| 8,625,000 | | |
| 8,625,000 | | |
| 8,625,000 | | |
| 7,643,056 | |
| Basic and diluted net income (loss) per share, Non-redeemable Class B ordinary shares | |
$ | 0.06 | | |
$ | (0.04 | ) | |
$ | 0.13 | | |
$ | (0.10 | ) |
See accompanying notes to
the unaudited condensed financial statements.
WALDENCAST PLC
(f/k/a WALDENCAST
ACQUISITION CORP.)
CONDENSED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
FOR THE THREE AND SIX MONTHS
ENDED JUNE 30, 2022 AND JUNE 30, 2021
(UNAUDITED)
| | |
Class B
Ordinary Shares | | |
Additional Paid-in | | |
Accumulated | | |
Total
Shareholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| Balance as of December 31, 2021 | |
| 8,625,000 | | |
$ | 863 | | |
$ | — | | |
$ | (54,809,897 | ) | |
$ | (54,809,034 | ) |
| Net income | |
| — | | |
| — | | |
| — | | |
| 3,022,915 | | |
| 3,022,915 | |
| Balance as of March 31, 2022 | |
| 8,625,000 | | |
$ | 863 | | |
$ | — | | |
$ | (51,786,982 | ) | |
$ | (51,786,119 | ) |
| Accretion for Class A Common Stock to redemption value | |
| — | | |
| — | | |
| — | | |
| (312,792 | ) | |
| (312,792 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| 2,767,058 | | |
| 2,767,058 | |
| Balance as of June 30, 2022 | |
| 8,625,000 | | |
$ | 863 | | |
$ | — | | |
$ | (49,332,716 | ) | |
$ | (49,331,853 | ) |
| | |
Class B
Ordinary Shares | | |
Additional Paid-in | | |
Accumulated | | |
Total
Shareholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| Balance as of December 31, 2020 | |
| — | | |
$ | — | | |
$ | — | | |
$ | (10,951 | ) | |
$ | (10,951 | ) |
| Issuance of Founder Shares | |
| 8,625,000 | | |
| 863 | | |
| 24,137 | | |
| — | | |
| 25,000 | |
| Sale of 5,933,333 Private Placement Warrants on March 18, 2021 | |
| — | | |
| — | | |
| 8,900,000 | | |
| — | | |
| 8,900,000 | |
| Initial value of private warrant liabilities | |
| — | | |
| — | | |
| (6,230,000 | ) | |
| — | | |
| (6,230,000 | ) |
| Initial value of FPA liabilities | |
| — | | |
| — | | |
| (11,655,000 | ) | |
| — | | |
| (11,655,000 | ) |
| Accretion of Class A ordinary shares subject to possible redemption | |
| — | | |
| — | | |
| 8,960,863 | | |
| (40,371,261 | ) | |
| (31,410,398 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (1,183,957 | ) | |
| (1,183,957 | ) |
| Balance as of March 31, 2021 | |
| 8,625,000 | | |
$ | 863 | | |
$ | — | | |
$ | (41,566,169 | ) | |
$ | (41,565,306 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (1,551,508 | ) | |
| (1,551,508 | ) |
| Balance as of June 30, 2021 | |
| 8,625,000 | | |
$ | 863 | | |
$ | — | | |
$ | (43,117,677 | ) | |
$ | (43,116,814 | ) |
See accompanying notes to
the unaudited condensed financial statements.
WALDENCAST
PLC
(f/k/a
WALDENCAST ACQUISITION CORP.)
CONDENSED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | |
For the
Six Months Ended
June 30,
2022 | | |
For the
Six Months Ended
June 30,
2021 | |
| Cash Flows from Operating Activities: | |
| | |
| |
| Net income (loss) | |
$ | 5,789,973 | | |
$ | (2,735,465 | ) |
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | |
| | | |
| | |
| Interest earned on Trust Account | |
| (260,745 | ) | |
| (14,376 | ) |
| Increase in deferred legal costs | |
| 5,615,095 | | |
| — | |
| Change in fair value of warrant liabilities | |
| (8,601,666 | ) | |
| 1,046,000 | |
| Change in fair value of forward purchase agreement liabilities | |
| (5,328,000 | ) | |
| 666,000 | |
| Offering costs allocated to warrants | |
| — | | |
| 719,201 | |
| Changes in current assets and current liabilities: | |
| | | |
| | |
| Prepaid assets | |
| (45,102 | ) | |
| (438,615 | ) |
| Accounts payable and accrued expenses | |
| 766,361 | | |
| 70,746 | |
| Due to related party | |
| 60,043 | | |
| 35,000 | |
| Net cash used in operating activities | |
| (2,004,041 | ) | |
| (651,509 | ) |
| | |
| | | |
| | |
| Cash Flows from Investing Activities: | |
| | | |
| | |
| Investment of cash into Trust Account | |
| — | | |
| (345,000,000 | ) |
| Net cash used in investing activities | |
| — | | |
| (345,000,000 | ) |
| | |
| | | |
| | |
| Cash Flows from Financing Activities: | |
| | | |
| | |
| Proceeds from issuance of Founder Shares | |
| — | | |
| 25,000 | |
| Proceeds from Initial Public Offering, net of underwriters’ discount | |
| — | | |
| 338,100,000 | |
| Proceeds from issuance of Private Placement Warrants | |
| — | | |
| 8,900,000 | |
| Proceeds from Working Capital Promissory Note – related party | |
| 600,000 | | |
| — | |
| Payments of offering costs | |
| — | | |
| (521,631 | ) |
| Net cash provided by financing activities | |
| 600,000 | | |
| 346,503,369 | |
| | |
| | | |
| | |
| Net Change in Cash | |
| (1,404,041 | ) | |
| 851,860 | |
| Cash – Beginning | |
| 1,503,768 | | |
| — | |
| Cash – Ending | |
$ | 99,727 | | |
$ | 851,860 | |
| | |
| | | |
| | |
| Supplemental Disclosure of Non-cash Financing Activities: | |
| | | |
| | |
| Initial value of Class A ordinary shares subject to possible redemption | |
$ | — | | |
$ | 298,882,970 | |
| Initial value of warrant liabilities | |
$ | — | | |
$ | 18,190,000 | |
| Initial value of forward purchase agreement liabilities | |
$ | — | | |
$ | 11,655,000 | |
| Change in value of Class A ordinary shares subject to possible redemption | |
$ | 312,792 | | |
$ | (1,999,790 | ) |
| Deferred underwriters’ discount payable charged to additional paid-in capital | |
$ | — | | |
$ | 12,075,000 | |
See accompanying notes to
the unaudited condensed financial statements.
WALDENCAST
PLC
(f/k/a
WALDENCAST ACQUISITION CORP.)
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 —
Organization and Business Operations
Waldencast
plc, formerly known as Waldencast Acquisition Corp. (the “Company”), was incorporated as a Cayman Islands exempted company
on December 8, 2020. The Company was incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase,
reorganization or similar business combination with one or more businesses (a “Business Combination”). As further discussed
below, the Company completed the Obagi and Milk Business Combinations (as defined below) on July 27, 2022.
Obagi and
Milk Business Combinations
On
July 27, 2022, subsequent to the fiscal quarter ended June 30, 2022, the fiscal quarter to which the accompanying financial statements
relate, the Company consummated its initial business combination (the “Obagi and Milk Business Combinations”) with (i) Obagi
Global Holdings Limited, a Cayman Islands exempted company limited by shares (“Obagi”) and Obagi Merger Sub, Inc., a Cayman
Islands exempted company limited by shares and an indirect wholly owned subsidiary of the Company (“Merger Sub”), pursuant
to an Agreement and Plan of Merger dated November 15, 2021 (the “Obagi Merger Agreement”), by and among the Company, Obagi
and Merger Sub; and (ii), Milk Makeup LLC, a Delaware limited liability company (“Milk”), Obagi Holdco 1 Limited, a limited
company incorporated under the laws of Jersey (“Holdco Purchaser”), Waldencast Partners LP, a Cayman Islands exempted limited
partnership (“Waldencast LP” and together with Holdco Purchaser, the “Purchasers”), certain members of Milk (the
“Milk Members”), and Shareholder Representative Services LLC, a Colorado limited liability company, solely in its capacity
as representative of the Milk Members (the “Equityholder Representative”), pursuant to an Equity Purchase Agreement dated
November 15, 2021 (the “Milk Equity Purchase Agreement” and together with the Obagi Merger Agreement, the “Transaction
Agreements”) by and among the Company, Holdco Purchaser, Waldencast LP, Milk, the Milk Members and the Equityholder Representative.
Upon
the consummation of the Obagi and Milk Business Combinations: (i) Merger Sub merged with and into
Obagi (the “Merger”) and the separate corporate existence of Merger Sub ceased, with Obagi surviving as an indirect subsidiary
of the Company; (ii) as a result of the Merger, among other things, each outstanding share in the capital of Obagi of par value US $0.50 each
per share (the “Obagi Common Stock”) as of immediately prior to the effective time of the Merger (the “Obagi Merger
Effective Time”) (other than in respect of Excluded Shares (as defined in the Obagi Merger Agreement)) were cancelled and converted
into the right to receive (a) an amount in cash equal to the quotient obtained by dividing (1) the Obagi Cash Consideration (as defined
in the Obagi Merger Agreement) by (2) the number of Aggregate Fully Diluted Company Common Shares (as defined in the Obagi Merger Agreement),
and (b) a number of Waldencast plc Class A ordinary shares (defined below) equal to the quotient obtained by dividing (1) the Obagi Stock
Consideration (as defined in the Obagi Merger Agreement) by (2) the number of Aggregate Fully Diluted Company Common Shares; (iii) the
Purchasers acquired from the Milk Members and the Milk Members sold to the Purchasers all of the issued and outstanding membership units
of Milk in exchange for the Milk Cash Consideration (as defined in the Milk Equity Purchase Agreement), and the Milk Equity Consideration
(as defined in the Milk Equity Purchase Agreement), which consist of partnership units of Waldencast LP exchangeable for Waldencast plc
Class A ordinary shares (as defined below), and the Class B ordinary shares, par value $0.0001 per share, of the Company (following
the Domestication) (the “Waldencast plc Non-Economic ordinary shares”); and (iv) as a result of the Milk Transaction, among
other things, (a) Holdco Purchaser purchased from the Milk Members a percentage of the outstanding membership units in exchange for (1)
the Milk Cash Consideration and (3) a number of Waldencast plc Non-Economic ordinary shares equal to the Milk Equity Consideration and
(b) Waldencast LP purchased from the Milk Members the remainder of the outstanding membership units in exchange for the Milk Equity Consideration.
Immediately following consummation
of the Obagi and Milk Business Combinations, (i) Holdco Purchaser contributed its equity interest in (a) Milk to Waldencast LP in exchange
for limited partnership units in Waldencast LP and (b) Obagi Holdco 2 Limited, a limited company incorporated under the laws of Jersey
(“Holdco 2”) and an indirect subsidiary of Waldencast plc, in exchange for limited partnership units in Waldencast LP. The
combined company is organized in an “Up-C” structure, in which the equity interests of Obagi and Milk are held by Waldencast
LP. The Company in turn holds its interests in Obagi and Milk through Waldencast LP and Holdco Purchaser.
Prior
to the consummation of the Obagi and Milk Business Combinations, following the approval of the Company’s shareholders, and in accordance
with the Cayman Act, the Companies (Jersey) Law 1991, as amended (the “Jersey Companies Law”) and the Company’s amended
and restated memorandum and articles of association, the Company effected a deregistration under the Cayman Act and a domestication under
Part 18C of the Jersey Companies Law (by means of filing a memorandum and articles of association with the Registrar of Companies in
Jersey), pursuant to which the Company’s jurisdiction of incorporation was changed from the Cayman Islands to Jersey (the “Domestication”).
Upon the effective time of the Domestication, the Company was renamed “Waldencast plc.”
In
connection with the Domestication, (i) each of the then issued and outstanding Class A ordinary shares, par value $0.0001 per share,
of the Company, was converted automatically, on a one-for-one basis, into an ordinary share, par value $0.0001 per share, of the
Company (following its Domestication) (the “Waldencast plc Class A ordinary shares”), (ii) each of the then issued and outstanding
Class B ordinary shares, par value $0.0001 per share, of the Company, was converted automatically, on a one-for-one basis, into
a Waldencast plc Class A ordinary share, (iii) each then issued and outstanding warrant of the Company was converted automatically into
a warrant to acquire one Waldencast plc Class A ordinary share (“Waldencast plc Warrant”), pursuant to the Warrant Agreement,
dated March 15, 2021, between the Company and Continental Stock Transfer & Trust Company, as warrant agent, and (iv) each then issued
and outstanding unit of the Company was cancelled and the holders thereof were entitled to one Waldencast plc Class A ordinary share
and one-third of one Waldencast plc Warrant.
As previously disclosed, in connection
with the Company’s initial public offering (the “Initial Public Offering”); (i) on February 22, 2021, the Company, Waldencast
Long-Term Capital LLC, a Cayman Islands limited liability company (the “Sponsor”) and Dynamo Master Fund (a member of the
Sponsor) entered into a Forward Purchase Agreement (the “Sponsor Forward Purchase Agreement”), which was subsequently amended
by the assignment and assumption agreement entered into by and between the Sponsor and Burwell Mountain PTC LLC, as trustee of Burwell
Mountain Trust (a member of the Sponsor) (collectively, “Burwell”) on December 20, 2021, under which the Sponsor assigned,
and Burwell assumed, all of the Sponsor’s rights and benefits under the Sponsor Forward Purchase Agreement, pursuant to which, Burwell
and Dynamo Master Fund committed to subscribe for and purchase 16,000,000 Waldencast plc Class A ordinary shares and 5,333,333 Waldencast
plc Warrants for an aggregate commitment amount of $160, 000,000 million (the “Sponsor FPA Investment”); and (ii) the Company
and Beauty Ventures LLC (“Beauty Ventures” and, together with Dynamo Master Fund and Burwell, the “Forward Purchasers”)
entered into a Forward Purchase Agreement on March 1, 2021 (“the Third-Party Forward Purchase Agreement” and, together with
the Sponsor Forward Purchase Agreement, the “Forward Purchase Agreements”), pursuant to which Beauty Ventures committed to
subscribe for and purchase up to 17,300,000 Waldencast plc Class A ordinary shares and up to 5,766,666 Waldencast plc Warrants for an
aggregate commitment amount of $173,000,000 (together with the Sponsor FPA Investment, the “FPA Investments”). The FPA Investments
were consummated substantially concurrently with the consummation of the Obagi and Milk Business Combinations.
As previously disclosed, on November
14, 2021, concurrently with the execution of the Transaction Agreements, the Company entered into certain subscription agreements, executed
on or prior to November 14, 2021 (the “Initial Subscription Agreements”), pursuant to which certain investors (the “Initial
PIPE Investors”) agreed to purchase, in the aggregate, 10,500,000 Waldencast plc Class A ordinary shares at $10.00 per
share for an aggregate commitment amount of $105.0 million (the “Initial PIPE Investment”). The Transaction Agreements provided
that Waldencast could enter into additional subscription agreements with investors to participate in the purchase of shares of Waldencast
plc after November 15, 2021 but prior to July 27, 2022 (the “Closing Date”). On June 14, 2022, Waldencast entered into subsequent
subscription agreements (the “June Subsequent Subscription Agreements”) with certain investors (collectively, the “June
Subsequent PIPE Investors”) on the same terms as the Initial PIPE Investors, pursuant to which the June Subsequent PIPE Investors
collectively subscribed for 800,000 shares of Waldencast plc Class A ordinary shares for an aggregate purchase price equal to $8.0 million
(the “June Subsequent PIPE Investment”). On July 15, 2022, Waldencast entered into subsequent subscription agreements (the
“July Subsequent Subscription Agreements” and together with the Initial Subscription Agreements and the June Subsequent Subscription
Agreements, the “PIPE Subscription Agreements”) with certain investors (collectively, the “July Subsequent PIPE Investors”
and, together with the Initial PIPE Investors and the June Subsequent PIPE Investors, the “PIPE Investors”) on the same terms
as the Initial PIPE Investors and the June Subsequent PIPE Investors. Pursuant to, and on the terms and subject to the conditions of the
applicable July Subsequent Subscription Agreement, the July Subsequent PIPE Investors collectively subscribed for 500,000 shares of Waldencast
plc Class A ordinary shares for an aggregate purchase price equal to $5,000,000 (the “July Subsequent PIPE Investment” and
together with the Initial PIPE Investment and the June Subsequent PIPE Investment, the “PIPE Investment”). The PIPE Investment
was consummated substantially concurrently with the consummation of the Obagi and Milk Business Combinations.
Business Prior
to the Obagi and Milk Business Combinations
All activity for the period from
December 8, 2020 (inception) through June 30, 2021 relates to the Company’s formation and the Initial Public Offering, which
is described below, and, subsequent to the Initial Public Offering, the search for a target company for a Business Combination, and the
negotiation and execution of the Obagi and Milk Business Combinations. The Company has selected December 31 as its fiscal year end.
The Company did not generate any operating revenues until after the completion of its initial Business Combination. Following the consummation
of the Initial Public Offering, the Company generated non-operating income in the
form of interest income on cash and cash equivalents from the net proceeds derived from the Initial Public Offering and the sale of the
Private Placement Warrants (defined below).
Financing
On
March 18, 2021, the Company consummated the Initial Public Offering of 34,500,000 units (the “Units” and, with respect
to the Class A ordinary shares included in the Units offered, the “Public Shares”), at $10.00 per Unit, generating gross
proceeds of $345,000,000, which is discussed in Note 3.
Simultaneously
with the closing of the Initial Public Offering, the Company completed the private sale of 5,933,333 warrants (the “Private
Placement Warrants”), at a price of $1.50 per Private Placement Warrant, which is discussed in Note 4.
Transaction
costs amounted to $20,169,599, consisting of $6,900,000 of underwriting fee, $12,075,000 of deferred underwriting fee and $1,194,599 of
other offering costs. Of the total transaction costs, $719,201 was reclassified as non-operating expense in the unaudited condensed
statements of operations with the rest of the offering costs charged to shareholders’ deficit. The transaction costs were allocated
based on a relative fair value basis, compared to the total offering proceeds, between the fair value of the public warrant liabilities
and the Class A ordinary shares.
Trust Account
Following
the closing of the Initial Public Offering on March 18, 2021, an amount of $345,000,000 from the net proceeds of the sale of the
Units in the Initial Public Offering and the sale of the Private Placement Warrants was placed in a trust account (“Trust Account”),
which is invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with
a maturity of 185 days or less or in any open-ended investment company that holds itself out as a money market fund meeting the conditions
of Rule 2a-7 of the Investment Company Act, as determined by the Company. Except with respect to interest earned on the funds held in
the Trust Account that may be released to the Company to pay its taxes, if any, the funds held in the Trust Account will not be released
from the Trust Account until the earliest to occur of: (1) the completion of the Company’s initial Business Combination; (2) the
redemption of any Public Shares properly submitted in connection with a shareholder vote to amend the Company’s amended and restated
memorandum and articles of association (A) to modify the substance or timing of the Company’s obligation to allow redemption in
connection with its initial Business Combination or to redeem 100% of its Public Shares if the Company does not complete its initial
Business Combination within 24 months from the closing of the Initial Public Offering or (B) with respect to any other provision relating
to shareholders’ rights or pre-initial Business Combination activity; and (3) the redemption of the Company’s Public Shares
if the Company has not completed its initial Business Combination within 24 months from the closing of the Initial Public Offering, subject
to applicable law. The proceeds deposited in the Trust Account could become subject to the claims of the Company’s creditors, if
any, which could have priority over the claims of the Company’s public shareholders.
Initial Business
Combination
The
Company’s management had broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering,
although substantially all of the net proceeds were intended to be generally applied toward consummating a Business Combination.
The
Company’s Business Combination was required to be with one or more target businesses that together had a fair market value equal
to at least 80% of the balance in the Trust Account (net of taxes payable) at the time of the signing of an agreement to enter into
a Business Combination. However, the Company would only complete a Business Combination if the post-Business Combination company owned
or acquired 50% or more of the outstanding voting securities of the target or otherwise acquired a controlling interest in the target
sufficient for it not to be required to register as an investment company under the Investment Company Act. There was no assurance that
the Company would be able to successfully effect a Business Combination.
The
Company was required to provide its public shareholders with the opportunity to redeem all or a portion of their Public Shares upon the
completion of the initial Business Combination either (i) in connection with a shareholder meeting called to approve the initial Business
Combination or (ii) by means of a tender offer. The decision as to whether the Company would seek shareholder approval of a proposed initial
Business Combination or conduct a tender offer was made by the Company, solely in its discretion. The shareholders were entitled to redeem
their shares for a pro rata portion of the amount then on deposit in the Trust Account (initially $10.00 per share, plus any pro
rata interest earned on the funds held in the Trust Account and not previously released to the Company to pay its tax obligations).
The
Class A ordinary shares subject to redemption were recorded at redemption value and classified as temporary equity upon the completion
of the Initial Public Offering, in accordance with Accounting Standards Codification (“ASC”) Topic 480, “Distinguishing
Liabilities from Equity.” The Company would only proceed with a Business Combination if the Company has net tangible assets of at
least $5,000,001 either immediately prior to or upon consummation of a Business Combination and, if the Company sought shareholder
approval, a majority of the issued and outstanding shares voted were voted in favor of the Business Combination.
The
Company had 24 months from the closing of the Initial Public Offering (with the ability to extend with shareholder approval) to consummate
a Business Combination (the “Combination Period”). However, if the Company was unable to complete a Business Combination within
the Combination Period, the Company would redeem 100% of the outstanding Public Shares for a pro rata portion of the funds held in
the Trust Account, equal to the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the
Trust Account and not previously released to the Company, divided by the number of then outstanding Public Shares, subject to applicable
law and as further described in the registration statement, and then would seek to dissolve and liquidate.
The
Company’s Sponsor, officers and directors agreed to (i) waive their redemption rights with respect to their Founder Shares, private
placement shares and Public Shares in connection with the completion of the initial Business Combination, (ii) waive their redemption
rights with respect to their Founder Shares and Public Shares in connection with a shareholder vote to approve an amendment to the Company’s
amended and restated memorandum and articles of association, and (iii) waive their rights to liquidating distributions from the Trust
Account with respect to their Founder Shares and private placement shares if the Company failed to complete the initial Business Combination
within the Combination Period.
The
Company’s Sponsor agreed that it would be liable to the Company if and to the extent any claims by a third party for services rendered
or products sold to the Company, or a prospective target business with which the Company entered into a written letter of intent, confidentiality
or similar agreement or Business Combination agreement, reduced the amount of funds in the Trust Account to below the lesser of (i) $10.00 per
Public Share and (ii) the actual amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust Account,
if less than $10.00 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability
would not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies
held in the Trust Account (whether or not such waiver was enforceable) nor would it apply to any claims under the Company’s indemnity
of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act. However,
the Company has not asked its Sponsor to reserve for such indemnification obligations, nor had the Company independently verified whether
its Sponsor had sufficient funds to satisfy its indemnity obligations and believed that the Company’s Sponsor’s only assets
were securities of the Company. Therefore, the Company could not assure that its Sponsor would be able to satisfy those obligations.
On
February 22, 2021, the Sponsor and Dynamo Master Fund (a member of the Sponsor) entered into a forward purchase agreement (the “Sponsor
Forward Purchase Agreement”), with the Company that provided for the purchase of up to an aggregate of 13,000,000 units,
with each unit consisting of one Class A ordinary share and one-third of one redeemable warrant, for an aggregate purchase price of $130,000,000,
or $10.00 per unit, in a private placement to close substantially concurrently with the closing of the Company’s initial Business
Combination (the “Forward Purchase Securities”). The Sponsor Forward Purchase Agreement provided that the applicable forward
purchase investors may, in their sole discretion, increase the amount of capital committed under the Sponsor Forward Purchase Agreement
up to an amount not to exceed $160,000,000. On October 20, 2021, the Company received an allocation notice from the Sponsor and Dynamo
Master Fund committing to purchase an aggregate of 16,000,000 units, with each unit consisting of one Class A ordinary share
and one-third of one redeemable warrant, for an aggregate purchase price of $160,000,000, or $10.00 per unit. On December 20, 2021,
the Sponsor and Burwell Mountain Trust (a member of the Sponsor) entered into an assignment and assumption agreement (the “Assignment
and Assumption Agreement”). The Assignment and Assumption Agreement provides for the assignment by the Sponsor and assumption by
Burwell Mountain Trust of all of the Sponsor’s rights and benefits as purchaser under the Sponsor Forward Purchase Agreement, including
the right to purchase the Forward Purchase Securities subscribed for by the Sponsor.
On
March 1, 2021, the Company and Beauty Ventures LLC (“Beauty Ventures”) entered into a forward purchase agreement (the “Beauty
Forward Purchase Agreement,” and together with the Sponsor Forward Purchase Agreement, the “Forward Purchase Agreements”
or “FPA”), that provided for the purchase of an aggregate of up to 17,300,000 units, with each unit consisting of
one Class A ordinary share and one-third of one redeemable warrant, for an aggregate purchase price of up to $173,000,000 (subject
to the below), or $10.00 per unit, in a private placement to close substantially concurrently with the closing of the initial Business
Combination. To the extent that the amounts available from the Trust Account and other financing (including the Sponsor Forward Purchase
Agreement) were sufficient for the cash requirements in connection with our initial Business Combination, the Sponsor could, in its sole
discretion, as the managing member of Beauty Ventures, reduce its purchase obligation, up to the full amount, under the Beauty Forward
Purchase Agreement.
Liquidity
and Capital Resources
As
of June 30, 2022, the Company had cash in an operating bank account, outside of the Trust Account, with $99,727 available for working
capital needs. As of June 30, 2022, the Company had a working capital deficit of $811,657. All remaining funds held in the Trust Account
are generally unavailable for the Company’s use, prior to an initial Business Combination, and are restricted for use either in
a Business Combination, to redeem Class A ordinary shares or with respect to the interest earned, to be withdrawn for the payment of taxes.
As of June 30, 2022, none of the amount in the Trust Account was withdrawn as described above.
On
October 28, 2021, the Company drew down the entire balance of the Working Capital Promissory Note (as defined below) initially available,
and the Sponsor deposited $1,500,000 in the Company’s operating bank account. In addition, the Company issued working capital
promissory notes to the Sponsor on (i) May 20, 2022, for up to $600,000 (“May Working Capital Note”) and (ii) July 15, 2022,
for up to $450,000 (“July Working Capital Note” and, together with May Working Capital Note, the “Non-Convertible Working
Capital Notes”), in each case, for working capital purposes. As of July 27, 2022, the Company had a total aggregate principal amount
of $1,050,000 in outstanding borrowings under the Non-Convertible Working Capital Notes. In connection with the closing of Business Combination,
the aggregate outstanding balance under the Non-Convertible Working Capital Notes of $1,050,000 was repaid to the Sponsor. Borrowings
under the Non-Convertible Working Capital Notes are no longer available.
As
of July 27, 2022, substantial doubt about Company’s ability to continue as going concern was alleviated due to closing of business
combination.
Risks and
Uncertainties
Management
is continuing to evaluate the impact of the COVID-19 pandemic and the Russia-Ukraine war and has concluded that while it is reasonably
possible that the virus and the war could have a negative effect on the Company’s financial position and/or results of its operations
and/or search for a target company, the specific impact is not readily determinable as of the date of these unaudited condensed financial
statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Additionally,
in February 2022, the Russian Federation and Belarus commenced a military action with the country of Ukraine. As a result of this action,
various nations, including the United States, have instituted economic sanctions against the Russian Federation and Belarus. Further,
the impact of this action and related sanctions on the world economy are not determinable as of the date of these financial statements
and the specific impact on the Company’s financial condition, results of operations, and cash flows is also not determinable as of the
date of these financial statements.
Note
2 — Significant Accounting Policies
Basis of
Presentation
The
accompanying unaudited condensed financial statements of the Company are presented in U.S. dollars in conformity with accounting principles
generally accepted in the United States of America (“GAAP”) and pursuant to the rules and regulations of the SEC. In the opinion
of management, all adjustments (consisting of normal recurring adjustments) have been made that are necessary to present fairly the financial
position, and the results of its operations and its cash flows. The interim results for the three and six months ended June 30, 2022 are
not necessarily indicative of the results to be expected for the year ending December 31, 2022 or for any future interim periods. The
accompanying unaudited condensed financial statements should be read in conjunction with the Company’s audited financial statements
and notes thereto included in the Annual Report on Form 10-K for the year ended December 31, 2021 (the “2021 Annual Report”)
filed by the Company with the Securities and Exchange Commission (“SEC”) on March 31, 2022.
Emerging
Growth Company Status
The
Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities
Act”), as modified by the Jumpstart our Business Startups Act of 2012, (the “JOBS Act”), and it may take advantage of
certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies
including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley
Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from
the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments
not previously approved.
Further,
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting
standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not
have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards.
The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply
to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended
transition period which means that when a standard is issued or revised and it has different application dates for public or private companies,
the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised
standard. This may make comparison of the Company’s unaudited condensed financial statements with another public company which is
neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult
or impossible because of the potential differences in accounting standards used.
Use of Estimates
The
preparation of unaudited condensed financial statements in conformity with GAAP requires management to make estimates and assumptions
that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited
condensed financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ
from those estimates.
Cash and
Cash Equivalents
The
Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents.
The Company had cash of $99,727 and $1,503,768 in its operating bank account, outside of the Trust Account as of June 30, 2022
and December 31, 2021, respectively. The Company did not have any cash equivalents as of June 30, 2022 and December 31, 2021.
Investment
Held in Trust Account
At
June 30, 2022 and December 31, 2021, the Trust Account had $345,312,792 and $345,052,047 of marketable securities,
respectively. The Company accounts for its marketable securities, which are carried at fair value with changes in fair value
included in the unaudited condensed statements of operations. As of June 30, 2022, the Company has not withdrawn any of the interest
income from the Trust Account to pay its tax obligations.
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution,
which, at times, may exceed the Federal Depository Insurance Coverage of $250,000. At June 30, 2022 and December 31, 2021, the
Company has not experienced losses on this account.
Class A Ordinary
Shares Subject to Possible Redemption
The
Company accounts for its Class A ordinary shares subject to possible redemption in accordance with the guidance in ASC Topic 480, “Distinguishing
Liabilities from Equity.” Class A ordinary shares subject to mandatory redemption (if any) are classified as a liability instrument
and are measured at fair value. Conditionally redeemable ordinary shares (including ordinary shares that feature redemption rights that
are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s
control) are classified as temporary equity. At all other times, ordinary shares are classified as a part of shareholders’ deficit.
The Company’s Class A ordinary shares feature certain redemption rights that are considered to be outside of the Company’s
control and subject to the occurrence of uncertain future events. Accordingly, all shares of Class A ordinary shares subject to possible
redemption are presented at redemption value as temporary equity, outside of the shareholders’ deficit section of the Company’s
condensed balance sheets.
All
of the Class A ordinary shares sold as part of the Units in the Initial Public Offering contain a redemption feature which allows for
the redemption of such Public Shares in connection with the Company’s liquidation, if there is a shareholder vote or tender offer
in connection with the Business Combination and in connection with certain amendments to the Company’s amended and restated memorandum
and articles of association (except that in no event may we redeem our Public Shares in an amount that would cause our net tangible assets
to be less than $5,000,001 following such redemptions pursuant to our amended and restated memorandum and articles of association).
In accordance with the SEC and its staff guidance on redeemable equity instruments, which has been codified in ASC 480-10-S99, redemption
provisions not solely within the control of the Company require ordinary shares subject to redemption to be classified outside of permanent
equity.
As
of June 30, 2022 and December 31, 2021, the Class A ordinary shares reflected on the condensed balance sheets are reconciled in the following
table:
| Gross proceeds | |
$ | 345,000,000 | |
| Less: | |
| | |
| Proceeds allocated to public warrants | |
| (11,960,000 | ) |
| Issuance costs related to Class A ordinary shares | |
| (19,450,398 | ) |
| Plus: | |
| | |
| Accretion of carrying value to redemption value | |
| 31,410,398 | |
| Contingently redeemable Class A ordinary shares, December 31, 2021 | |
$ | 345,000,000 | |
| Plus: | |
| | |
| Accretion of carrying value to redemption value | |
| 312,792 | |
| Contingently redeemable Class A ordinary shares, June 30, 2022 | |
$ | 345,312,792 | |
Net Income
(Loss) per Ordinary Share
The Company applies the two-class
method in calculating earnings per share. The contractual formula utilized to calculate the redemption amount approximates fair value.
The Class A feature to redeem at fair value means that there is effectively only one class of stock. Changes in fair value are not considered
a dividend for the purposes of the numerator in the earnings per share calculation. Net income (loss) per ordinary share is computed by
dividing the pro rata net income (loss) between the Class A ordinary shares and the Class B ordinary shares by the weighted average number
of ordinary shares outstanding for each of the periods. The calculation of diluted income (loss) per ordinary share does not consider
the effect of the warrants and rights issued in connection with the Initial Public Offering since the exercise of the warrants and rights
are contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive. The warrants and Forward
Purchase Agreements warrants are exercisable for 61,833,333 shares of Class A ordinary shares in the aggregate. Accretion of
the carrying value of Class A ordinary shares to redemption value is excluded from net income (loss) per ordinary share because the redemption
value approximates fair value.
Reconciliation of Net Income (Loss)
per Ordinary Share
The
Company’s net income (loss) is adjusted for the portion of net income (loss) that is allocable to each class of ordinary shares.
The allocable net income (loss) is calculated by multiplying net income (loss) by the ratio of weighted average number of shares outstanding
attributable to Class A ordinary shares and Class B ordinary shares to the total weighted average number of shares outstanding
for the period. Accretion of the carrying value of Class A ordinary shares to redemption value is excluded from net income (loss)
per ordinary share because the redemption value approximates fair value.
Accordingly, basic and
diluted income (loss) per ordinary share is calculated as follows:
| | |
For the Three Months Ended June 30, | | |
For the Six Months Ended June 30, | |
| | |
2022 | | |
2021 | | |
2022 | | |
2021 | |
| Ordinary shares subject to possible redemption | |
| | |
| | |
| | |
| |
| Numerator: | |
| | |
| | |
| | |
| |
| Net income (loss) allocable to Class A ordinary shares subject to possible redemption | |
$ | 2,213,646 | | |
$ | (1,241,206 | ) | |
$ | 4,631,978 | | |
$ | (1,977,305 | ) |
| Denominator: | |
| | | |
| | | |
| | | |
| | |
| Weighted Average Redeemable Class A ordinary shares, Basic and Diluted | |
| 34,500,000 | | |
| 34,500,000 | | |
| 34,500,000 | | |
| 19,933,333 | |
| Basic and Diluted net income (loss) per share, Redeemable Class A ordinary shares | |
$ | 0.06 | | |
$ | (0.04 | ) | |
$ | 0.13 | | |
$ | (0.10 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-Redeemable Ordinary shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to Class B ordinary shares not subject to redemption | |
$ | 553,412 | | |
$ | (310,302 | ) | |
$ | 1,157,995 | | |
$ | (758,160 | ) |
| Denominator: | |
| | | |
| | | |
| | | |
| | |
| Weighted Average Non-Redeemable Ordinary shares, Basic and Diluted | |
| 8,625,000 | | |
| 8,625,000 | | |
| 8,625,000 | | |
| 7,643,056 | |
| Basic and diluted net income (loss) per share, ordinary shares | |
$ | 0.06 | | |
$ | (0.04 | ) | |
$ | 0.13 | | |
$ | (0.10 | ) |
Offering
Costs
The
Company complies with the requirements of the Financial Accounting Standards Board (“FASB”) ASC 340-10-S99-1 and
SEC Staff Accounting Bulletin (“SAB”) Topic 5A—“Expenses of Offering.” Offering costs consist principally
of professional and registration fees incurred through the balance sheet date that are related to the Initial Public Offering and that
were charged to shareholders’ deficit upon the completion of the Initial Public Offering. Accordingly, as of the date of the IPO,
offering costs totaling $20,169,599 have been charged to temporary equity (consisting of $6,900,000 of underwriting fee, $12,075,000 of
deferred underwriting fee and $1,194,599 of other offering costs). Of the total transaction costs, $719,201 was reclassified
as a non-operating expense in the unaudited condensed statements of operations with the rest of the offering costs charged to temporary
equity. The transaction costs were allocated based on a relative fair value basis, compared to the total offering proceeds, between the
fair value of the public warrant liabilities and the Class A ordinary shares.
Fair Value of Financial
Instruments
The
fair value of the Company’s assets and liabilities, which qualify as financial instruments under the FASB ASC 820, “Fair Value
Measurements and Disclosures,” approximates the carrying amounts represented in the balance sheets.
Derivative
Warrant Liabilities
The
Company evaluates its financial instruments, including issued share purchase warrants, to determine if such instruments are derivatives
or contain features that qualify as embedded derivatives, pursuant to ASC 480 and ASC 815-15. The classification of derivative instruments,
including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
The Company has determined its public warrants, private warrants and forward purchase agreements are derivative instruments.
The
Company accounts for its 17,433,333 ordinary share warrants issued in connection with its Initial Public Offering (11,500,000)
and Private Placement Warrants (5,933,333) as derivative warrant liabilities in accordance with ASC 815-40. Accordingly, the Company recognizes
the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period. The liabilities
are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company’s
unaudited condensed statements of operations. The fair value of warrants issued by the Company in connection with its Initial Public Offering
and Private Placement Warrants has been estimated using Monte-Carlo simulations at each measurement date.
FASB
ASC 470-20, “Debt with Conversion and Other Options,” addresses the allocation of proceeds from the issuance of convertible
debt into its equity and debt components. The Company applied this guidance to allocate Initial Public Offering proceeds from the Units
between Class A ordinary shares and warrants, using the residual method by allocating Initial Public Offering proceeds first to the fair
value of the warrants and contingent forward purchase units and then the Class A ordinary shares.
Income Taxes
The
Company accounts for income taxes under ASC Topic 740, “Income Taxes,” which requires an asset and liability approach to financial
accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed for differences between the financial
statements and tax bases of assets and liabilities that will result in future taxable or deductible amounts, based on enacted tax laws
and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established,
when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC
Topic 740 prescribes a recognition threshold and a measurement attribute for the financial statements recognition and measurement of tax
positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than
not to be sustained upon examination by taxing authorities. The Company’s management determined that the Cayman Islands is the Company’s
major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense.
As of June 30, 2022 and December 31, 2021, there were no unrecognized tax benefits and no amounts accrued for interest and penalties.
The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation
from its position.
The
Company is considered to be an exempted Cayman Islands company with no connection to any other taxable jurisdiction and is presently not
subject to income taxes or income tax filing requirements in the Cayman Islands or the United States. Therefore, there was no tax
provision for the three and six months ended June 30, 2022 and June 30, 2022
Recent Accounting
Standards
In
August 2020, the FASB issued Accounting Standard Update No. 2020-06, “Debt-Debt with Conversion and Other Options” (Subtopic
470-20) and “Derivatives and Hedging-Contracts in an Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments
and Contracts in an Entity’s Own Equity” (“ASU 2020-06”), which simplifies accounting for convertible instruments
by removing major separation models required under current GAAP. ASU 2020-06 also removes certain settlement conditions that are required
for equity-linked contracts to qualify for scope exception, and it simplifies the diluted earnings per share calculation in certain areas.
The Company adopted ASU 2020-06 on January 1, 2021. Adoption of the ASU did not impact the Company’s financial position, results
of operations or cash flows.
The
Company’s management does not believe that there are any other recently issued, but not yet effective, accounting standards which
if currently adopted would have a material effect on the accompanying unaudited condensed financial statements.
Note 3 —
Initial Public Offering
Pursuant
to the Initial Public Offering, the Company sold 34,500,000 Units, at a price of $10.00 per Unit. Each Unit consists
of one share of Class A ordinary shares, par value $0.0001 per share and one-third of one redeemable warrant (“Public Warrant”).
Each whole Public Warrant entitles the holder to purchase one share of Class A ordinary shares at a price of $11.50 per share.
Note 4 —
Private Placement Warrants
Simultaneously
with the closing of the Initial Public Offering, the Sponsor purchased an aggregate of 5,933,333 Private Placement Warrants
at a price of $1.50 per Private Placement Warrant, for an aggregate price of $8,900,000. Each Private Placement Warrant is exercisable
for one Class A ordinary share at a price of $11.50 per share, subject to adjustment (see Note 6). If the Company does not complete
a Business Combination within the Combination Period, the proceeds from the sale of the Private Placement Warrants held in the Trust Account
will be used to fund the redemption of the Public Shares (subject to the requirements of applicable law) and the Private Placement Warrants
will expire worthless. The initial fair value of the Private Placement Warrants was recorded as a liability of $6,230,000 with the
excess of cash received over initial fair value of the warrants of $2,670,000 recorded as additional paid-in capital.
Note 5 —
Related Party Transactions
Founder Shares
On
January 12, 2021, the Company issued 7,187,500 Founder Shares to the Sponsor for an aggregate purchase price of $25,000. In
February 2021, the Sponsor transferred 20,000 Class B ordinary shares to each of Sarah Brown, Juliette Hickman, Lindsay Pattison
and Zachary Werner (the “Investor Directors”), resulting in the Sponsor holding 7,107,500 Class B ordinary shares.
On March 15, 2021, the Company effected a dividend of 0.2 per share of Class B ordinary shares for each share of Class B ordinary
shares resulting in 8,625,000 shares of Class B ordinary shares being issued and outstanding, of which 8,545,000 are
held by the Sponsor.
The
Sponsor has agreed, subject to limited exceptions, not to transfer, assign or sell any of its Class B ordinary shares or Class A ordinary
shares received upon conversion thereof until the earlier of: (A) one year after the completion of a Business Combination and (B) subsequent
to a Business Combination, (x) if the last reported sale price of the Class A ordinary shares equals or exceeds $12.00 per share (as adjusted
for share sub-divisions, share dividends, rights issuances, consolidations, reorganizations, recapitalizations and the like) for any 20
trading days within any 30-trading day period commencing at least 150 days after a Business Combination, or (y) the date on which the
Company completes a liquidation, merger, amalgamation, share exchange, reorganization or other similar transaction that results in all
of the Company’s shareholders having the right to exchange their ordinary shares for cash, securities or other property.
Related Party
Loans
On
January 12, 2021, the Sponsor agreed to loan the Company up to $300,000 to be used for the payment of costs related to the Initial
Public Offering pursuant to a promissory note (the “Sponsor Promissory Note”). The Sponsor Promissory Note was non-interest
bearing, unsecured and due upon the earlier of June 30, 2021 and the closing of the Initial Public Offering. The Company had no borrowings
under the Sponsor Promissory Note at the closing of the Initial Public Offering. Borrowings under the Sponsor Promissory Note are no longer
available.
Due to Related
Party
As
of June 30, 2022 and December 31, 2021, the balance of $155,043 and $95,000 represents the amount accrued for the administrative
support services provided (defined below) by the Sponsor, respectively.
Administrative
Support Agreement
Commencing
on the date of the Initial Public Offering, the Company has agreed to pay the Sponsor a total of $10,000 per month for office space
and administrative support services. Upon completion of the initial Business Combination or the Company’s liquidation, the Company
will cease paying these monthly fees. For the three and six months ended June 30, 2022 and 2021, the Company incurred $30,000 and
$60,000 in such fees. As of June 30, 2022 and December 31, 2021, the Company had an outstanding unpaid balance amounting to $155,043 and
$95,000, respectively.
Working Capital
Loans
In
order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of
the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required. Such Working Capital
Loans would be evidenced by promissory notes. The notes may be repaid upon completion of a Business Combination, without interest, or,
at the lender’s discretion, up to $1,500,000 of notes may be converted upon completion of a Business Combination into warrants
at a price of $1.50 per warrant. Such warrants would be identical to the Private Placement Warrants. In the event that a Business
Combination does not close, the Company may use a portion of proceeds held outside of the Trust Account to repay the Working Capital Loans,
but no proceeds held in the Trust Account would be used for such repayment. On August 18, 2021, the Company issued the Working Capital
Promissory Note to the Sponsor for an aggregate amount of up to $1,500,000, up to $1,500,000 of such note may be convertible into
warrants, at a price of $1.50 per warrant, at the option of the lender (the “Working Capital Promissory Note”). The Working
Capital Promissory Note is non-interest bearing and is due and payable in full on the earlier of (i) the date by which we have to complete
a Business Combination and (ii) the effective date of a Business Combination. On October 28, 2021, the Company drew down the entire available
balance of the Working Capital Promissory Note and the Sponsor deposited $1,500,000 in the Company’s operating bank account.
As of June 30, 2022 and December 31, 2021, the Company had an aggregate principal amount of $1,500,000 in outstanding borrowings
under the Working Capital Loans, consisting solely of the Working Capital Promissory Note.
On May 20, 2022, the Company
entered into a Promissory Note (the “Working Capital Loan”) with its Sponsor. Pursuant to the Working Capital Loan, the Sponsor
has agreed to loan to the Company up to $600,000 to be used for working capital purposes. The loans will not bear any interest, and will
be repayable by the Company to the Sponsor upon the earlier of (i) the date by which the Company has to complete a Business Combination
pursuant to its Amended and Restated Memorandum and Articles of Association (as may be amended from time to time) and (ii) the effective
date of a Business Combination (such earlier date of (i) and (ii), the “Maturity Date”).
The
conversion feature included in the Working Capital Promissory Note is considered an embedded derivate and is remeasured at the end of
each reporting period. The value of the conversion features was considered de minimis both as of June 30, 2022 and December 31, 2021.
Forward
Purchase Agreement
The
Company entered into two separate forward purchase agreements as follows. The Sponsor and Dynamo Master Fund (a member of the Sponsor)
entered into the Sponsor Forward Purchase Agreement, dated as of February 22, 2021, with the Company that will provide for the purchase
of up to an aggregate of 13,000,000 units, with each unit consisting of one Class A ordinary share and one-third of one redeemable
warrant, for an aggregate purchase price of $130,000,000, or $10.00 per unit, in a private placement to close substantially concurrently
with the closing of our initial Business Combination. The Sponsor Forward Purchase Agreement provides that the applicable forward purchase
investors may, in their sole discretion, increase the amount of capital committed under the Sponsor Forward Purchase Agreement up to an
amount not to exceed $160,000,000. Beauty Ventures entered into the Beauty Forward Purchase Agreement, dated as of March 1, 2021, with
the Company that provides for the purchase of an aggregate of up to 17,300,000 units, with each unit consisting of one Class
A ordinary share and one-third of one redeemable warrant, for an aggregate purchase price of up to $173,000,000 (subject to the below),
or $10.00 per unit, in a private placement to close substantially concurrently with the closing of the initial Business Combination.
To the extent that the amounts available from the Trust Account and other financing (including the Sponsor Forward Purchase Agreement)
are sufficient for the cash requirements in connection with our initial Business Combination, the Sponsor may, in its sole discretion,
as the managing member of Beauty Ventures, reduce its purchase obligation, up to the full amount, under the Beauty Forward Purchase Agreement.
Members of the Sponsor or their affiliates will receive a performance fee allocation when the return on the securities underlying the
Beauty Forward Purchase Agreement exceeds certain benchmark returns. The obligations under the Forward Purchase Agreements will not depend
on whether any Class A ordinary shares are redeemed by our public shareholders. The forward purchase shares and the forward purchase warrants
included in the units being sold in the Initial Public Offering, respectively, will be identical to the Public Shares and public warrants
included in the units sold in the Initial Public Offering, respectively, except that the holders thereof will have certain registration
rights, as described herein. On October 20, 2021, the Company received (i) an allocation notice from the Sponsor and Dynamo Master
Fund committing to purchase 16,000,000 units, with each unit consisting of one Class A ordinary share and one-third of one redeemable
warrant, for an aggregate purchase price of $160,000,000, or $10.00 per unit and (ii) an allocation notice from Beauty Ventures committing
to purchase 17,300,000 units, with each unit consisting of one Class A ordinary share and one-third of one redeemable warrant, for an
aggregate purchase price of $173,000,000, or $10.00 per unit. On December 20, 2021, the Sponsor and Burwell Mountain Trust (a member
of the Sponsor) entered into an Assignment and Assumption Agreement. The Assignment and Assumption Agreement provides for the assignment
by the Sponsor and assumption by Burwell Mountain Trust of all of the Sponsor’s rights and benefits as purchaser under the Sponsor
Forward Purchase Agreement, including the right to purchase the Forward Purchase Securities subscribed for by the Sponsor.
Note 6 —
Commitments & Contingencies
Registration
Rights
The
holders of the Founder Shares, Private Placement Warrants and any warrants that may be issued upon conversion of Working Capital Loans
(and any Class A ordinary shares issuable upon the exercise of the Private Placement Warrants or warrants issued upon conversion of the
Working Capital Loans and upon conversion of the Founder Shares) are entitled to registration rights pursuant to a registration rights
agreement to be signed prior to or on the effective date of the Initial Public Offering requiring the Company to register such securities
for resale. The holders of these securities will be entitled to make up to three demands, excluding short form demands, that the Company
register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration
statements filed subsequent to the completion of a Business Combination. The Company will bore the expenses incurred in connection with
the filing of any such registration statements.
Underwriters
Agreement
On
March 18, 2021, pursuant to the consummation of the Initial Public Offering, the Company paid a fixed underwriting discount of $0.20 per
Unit, or $6,900,000 in the aggregate. Additionally, a deferred underwriting discount of $0.35 per Unit, or $12,075,000 in
the aggregate, will be payable to the underwriters from the amounts held in the Trust Account solely in the event that the Company completes
an initial Business Combination, subject to the terms of the underwriting agreement.
Note 7 —
Class A Ordinary Shares Subject to Possible Redemption
The
Company’s Class A ordinary shares feature certain redemption rights that are considered to be outside of the Company’s control
and subject to the occurrence of uncertain future events. Accordingly, as of June 30, 2022 and December 31, 2021, 34,500,000 shares
of Class A ordinary shares subject to possible redemption are presented at redemption value as temporary equity, outside of the shareholders’
deficit section of the Company’s condensed balance sheets. The value of these redeemable shares was calculated as the gross proceeds
from the sale of Waldencast’s public units reduced by the proceeds allocable to the Public Warrants, issuance costs related to Waldencast’s
public units and the accretion of the carrying value to the redemption value. Upon the consummation of the Initial Public Offering, the
Company recorded $31,410,398 in accretion.
Note 8 —
Shareholders’ Deficit
Preference
Shares — The Company is authorized to issue a total of 5,000,000 preference shares at par value of $0.0001 each.
At June 30, 2022 and December 31, 2021, there were no preference shares issued or outstanding.
Class
A Ordinary Shares — The Company is authorized to issue a total of 500,000,000 Class A ordinary shares at
par value of $0.0001 each. At June 30, 2022 and December 31, 2021, there were no shares issued and outstanding (excluding 34,500,000 shares
subject to possible redemption).
Class
B Ordinary Shares — The Company is authorized to issue a total of 50,000,000 shares of Class B ordinary shares
at par value of $0.0001 each. At June 30, 2022 and December 31, 2021, there were 8,625,000 Class B ordinary shares issued
or outstanding.
Only
holders of the Class B ordinary shares will have the right to vote on the election of directors prior to the Business Combination. Holders
of Class A ordinary shares and holders of Class B ordinary shares will vote together as a single class on all matters submitted to a vote
of the Company’s shareholders except as otherwise required by law.
The
Class B ordinary shares will automatically convert into Class A ordinary shares at the time of the completion of the Business Combination,
or earlier at the option of the holder, on a one-for-one basis, subject to adjustment. In the case that additional Class A ordinary shares,
or equity-linked securities, are issued or deemed issued in excess of the amounts issued in the Initial Public Offering and related to
the closing of a Business Combination, the ratio at which Founder Shares will convert into Class A ordinary shares will be adjusted (subject
to waiver by holders of a majority of the Class B ordinary shares) so that the number of Class A ordinary shares issuable upon conversion
of all Founder Shares will equal, in the aggregate, on an as-converted basis, 20% of the sum of the ordinary shares issued and outstanding
upon completion of the Initial Public Offering plus the number of Class A ordinary shares and equity-linked securities issued or deemed
issued in connection with a Business Combination, excluding any Class A ordinary shares or equity-linked securities issued, or to be issued,
to any seller in a Business Combination.
Note 9 —
Warrants
Public
Warrants may only be exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only
whole warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business
Combination and (b) 12 months from the closing of the Initial Public Offering. The Public Warrants will expire five years after
the completion of a Business Combination or earlier upon redemption or liquidation.
The
Company will not be obligated to deliver any Class A ordinary shares pursuant to the exercise of a Public Warrant and will have no obligation
to settle such Public Warrant exercise unless a registration statement under the Securities Act with respect to the Class A ordinary shares
underlying the Public Warrants is then effective and a prospectus relating thereto is current, subject to the Company satisfying its obligations
with respect to registration. No Public Warrant will be exercisable, and the Company will not be obligated to issue any shares to holders
seeking to exercise their warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities
laws of the state of the exercising holder, or an exemption is available.
The
Company has agreed that as soon as practicable, but in no event later than 15 business days, after the closing of the Company’s
Business Combination, the Company will use its commercially reasonable efforts to file with the SEC a registration statement for the registration,
under the Securities Act, of the Class A ordinary shares issuable upon exercise of the warrants. The Company will use its commercially
reasonable efforts to cause the same to become effective and to maintain the effectiveness of such registration statement, and a current
prospectus relating thereto, until the expiration or redemption of the warrants in accordance with the provisions of the warrant agreement.
If a registration statement covering the Class A ordinary shares issuable upon exercise of the warrants is not effective by the 60th business
day after the closing of a Business Combination, warrant holders may, until such time as there is an effective registration statement
and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless
basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption. Notwithstanding the above, if the Class A
ordinary shares are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition
of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of public
warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act
and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, and
in the event the Company does not so elect, it will use its commercially reasonable efforts to register or qualify the shares under applicable
blue sky laws to the extent an exemption is not available.
Once
the warrants become exercisable, the Company may redeem the Public Warrants for redemption:
| |
● |
in whole and not in part; |
| |
● |
at a price of $0.01 per Public Warrant; |
| |
● |
upon not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| |
● |
if, and only if, the reported last sale price of the Class A ordinary shares for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption the warrant holders (the “Reference Value”) equals or exceeds $18.00 per share (as adjusted). |
Once
the Public Warrants become exercisable, the Company may redeem the Public Warrants:
| |
● |
in whole and not in part; |
| |
● |
at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of shares determined by reference to the table below, based on the redemption date and the “fair market value” of the Class A ordinary shares; |
| |
● |
if, and only if, the Reference Value equals or exceeds $10.00 per share (as adjusted); and |
| |
● |
if the Reference Value is less than $18.00 per share (as adjusted), the Private Placement Warrants must also be concurrently called for redemption on the same terms as the outstanding Public Warrants, as described above. |
If
and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register
or qualify the underlying securities for sale under all applicable state securities laws.
The
exercise price and number of ordinary shares issuable upon exercise of the Public Warrants may be adjusted in certain circumstances including
in the event of a share dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except
as described below, the Public Warrants will not be adjusted for issuances of ordinary shares at a price below its exercise price. Additionally,
in no event will the Company be required to net cash settle the Public Warrants. If the Company is unable to complete a Business Combination
within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of Public Warrants will not receive
any of such funds with respect to their Public Warrants, nor will they receive any distribution from the Company’s assets held outside
of the Trust Account with respect to such Public Warrants. Accordingly, the Public Warrants may expire worthless.
In
addition, if (x) the Company issues additional Class A ordinary shares or equity-linked securities for capital raising purposes in connection
with the closing of a Business Combination at an issue price or effective issue price of less than $9.20 per Class A ordinary share (with
such issue price or effective issue price to be determined in good faith by the Company’s board of directors and, in the case of
any such issuance to the Sponsor or its affiliates, without taking into account any Founder Shares held by the Sponsor or such affiliates,
as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent
more than 60% of the total equity proceeds, and interest thereon, available for the funding of a Business Combination, and (z) the volume
weighted average trading price of the Class A ordinary shares during the 20 trading day period starting on the trading day prior to the
day on which the Company consummates a Business Combination (such price, the “Market Value”) is below $9.20 per share, then
the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the
Newly Issued Price, and the $10.00 and $18.00 per share redemption trigger prices will be adjusted (to the nearest cent) to be equal to
100% and 180% of the higher of the Market Value and the Newly Issued Price, respectively.
The
Private Placement Warrants will be identical to the Public Warrants underlying the Units being sold in the Initial Public Offering, except
that the Private Placement Warrants and the Class A ordinary shares issuable upon the exercise of the Private Placement Warrants will
not be transferable, assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions.
Additionally, the Private Placement Warrants will be exercisable on a cashless basis and be non-redeemable, except as described above,
so long as they are held by the initial purchasers or their permitted transferees, the Private Placement Warrants will be redeemable by
the Company and exercisable by such holders on the same basis as Public Warrants.
Note 10 —
Fair Value Measurements
Fair
value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction
between market participants at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs
used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets
or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level
1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| |
● |
Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| |
● |
Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
The
following table presents information about the Company’s assets that are measured at fair value on a recurring basis at June 30,
2022 and December 31, 2021 and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair
value:
| | |
June 30, | | |
Quoted
Prices In
Active
Markets | | |
Significant
Other
Observable
Inputs | | |
Significant
Other
Unobservable
Inputs | |
| | |
2022 | | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Description | |
| | |
| | |
| | |
| |
| Assets: | |
| | |
| | |
| | |
| |
| Marketable Securities held in Trust Account | |
$ | 345,312,792 | | |
$ | 345,312,792 | | |
$ | — | | |
$ | — | |
| Liabilities: | |
| | | |
| | | |
| | | |
| | |
| Forward purchase agreement liabilities | |
| (7,992,000 | ) | |
| — | | |
| — | | |
| (7,992,000 | ) |
| Warrant liabilities | |
$ | (12,552,000 | ) | |
$ | (8,280,000 | ) | |
$ | — | | |
$ | (4,272,000 | ) |
| | |
December 31, | | |
Quoted
Prices In
Active
Markets | | |
Significant
Other
Observable
Inputs | | |
Significant
Other
Unobservable
Inputs | |
| | |
2021 | | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Description | |
| | |
| | |
| | |
| |
| Assets: | |
| | |
| | |
| | |
| |
| Marketable Securities held in Trust Account | |
$ | 345,052,047 | | |
$ | 345,052,047 | | |
$ | — | | |
$ | — | |
| Liabilities: | |
| | | |
| | | |
| | | |
| | |
| Forward purchase agreement liabilities | |
| (13,320,000 | ) | |
| — | | |
| — | | |
| (13,320,000 | ) |
| Warrant liabilities | |
$ | (21,153,666 | ) | |
$ | (13,915,000 | ) | |
$ | — | | |
$ | (7,238,666 | ) |
The
Company utilizes a Monte Carlo simulation model to value its private warrants at each reporting period, with changes in fair value recognized
in the unaudited condensed statements of operations. The estimated fair value of the warrant liabilities is determined using Level 3 inputs.
Inherent in a binomial options pricing model are assumptions related to expected share-price volatility, expected life, risk-free interest
rate and dividend yield. The Company estimates the volatility of its ordinary shares based on historical volatility that matches the expected
remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for
a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their
remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates to remain at zero.
In
connection with the consummation of the Business Combination, the Company waived those certain provisions as contemplated by the Letter
Agreement, dated as of March 15, 2021, by and among the Company, its officers and directors, the Sponsor, and certain members of the Sponsor
(as amended, the “Letter Agreement”), and certain other agreements related thereto (collectively, the “Waiver”),
with respect to any securities held by an Insider (as defined in the Letter Agreement) as of the closing the Business Combination (the
“Lock-Up Securities”) that would disallow a pledge by such Insider of the Lock-up Securities in a transaction for the purpose
of financing such Insider’s payment obligations owed in connection with the closing of the Obagi and Milk Business Combinations.
On
July 25, 2022, the Company entered into that certain Waiver and Agreement by and between the Company and Burwell (the “Waiver and
Agreement”) to permit a pledge by Burwell of its Lock-Up Securities to be used as a portion of the collateral under a loan to finance
Burwell’s payment obligations under the Sponsor Forward Purchase Agreement in connection with the closing of the Obagi and Milk
Business Combinations. Pursuant to the terms of the Waiver and Agreement, in the event of a foreclosure, any such lenders or a collateral
agents will be required to execute a joinder to the Letter Agreement pursuant to which they will be bound by the transfer restrictions
of the Lock-Up Securities (including the foreclosure of or other exercise of remedies under any such loan documentation) in the Letter
Agreement for the duration of such agreement. The Company also agreed to provide any such lender or collateral agent with customary
registration rights in the event of default, foreclosure or other exercise of remedies following the respective Lock-Up Periods (as defined
in the Letter Agreement).
As
described in Note 1, the Company completed the Obagi and Milk Business Combinations on July 27, 2022.
The
aforementioned warrant liabilities are not subject to qualified hedge accounting.
For
the six months ended June 30, 2022 and 2021, there were no transfers between Levels 1, 2 or 3.
The
following table provides quantitative information regarding Level 3 fair value measurements for private warrants and forward purchase
agreements:
| | |
At
June 30,
2022 | | |
At
December 31,
2021 | |
| Starting share price | |
$ | 10.00 | | |
$ | 10.00 | |
| Strike price | |
$ | 11.50 | | |
$ | 11.50 | |
| Term (in years) | |
| 5.08 | | |
| 5.50 | |
| Volatility | |
| 8.50 | % | |
| 19.0 | % |
| Risk-free rate | |
| 3.01 | % | |
| 1.31 | % |
| Dividend yield | |
| 0.0 | % | |
| 0.0 | % |
The
following table presents the changes in the fair value of warrant liabilities:
| | |
Public | | |
Private
Placement | | |
Warrant
Liabilities | |
| Fair value as of December 31, 2020 | |
$ | — | | |
$ | — | | |
$ | — | |
| Initial measurement on March 18, 2021 | |
| 11,960,000 | | |
| 6,230,000 | | |
| 18,190,000 | |
| Change in fair value of warrant liabilities | |
| 1,955,000 | | |
| 1,008,666 | | |
| 2,963,666 | |
| Fair value as of December 31, 2021 | |
$ | 13,915,000 | | |
$ | 7,238,666 | | |
$ | 21,153,666 | |
| Change in fair value | |
| (2,875,000 | ) | |
| (1,483,333 | ) | |
| (4,358,333 | ) |
| Fair value as of March 31, 2022 | |
$ | 11,040,000 | | |
$ | 5,755,333 | | |
$ | 16,795,333 | |
| Change in fair value | |
| (2,760,000 | ) | |
| (1,483,333 | ) | |
| (4,243,333 | ) |
| Fair value as of June 30, 2022 | |
$ | 8,280,000 | | |
$ | 4,272,000 | | |
$ | 12,552,000 | |
Prior
to their transfer to Level 1 inputs, the estimated fair value of warrant liabilities is determined using Level 3 inputs. Inherent in a
binomial options pricing model are assumptions related to expected share-price volatility, expected life, risk-free interest rate and
dividend yield. The Company estimates the volatility of its ordinary shares based on historical volatility that matches the expected remaining
life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity
similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining
contractual term. The dividend rate is based on the historical rate, which the Company anticipates to remain at zero.
The
Company has initially classified the FPA as a liability. This financial instrument is subject to re-measurement at each balance sheet
date. With each such re-measurement, the FPA asset or liability will be adjusted to fair value, with the change in fair value recognized
in the Company’s condensed statements of operations. As such, the Company recorded a $11,655,000 of derivative liabilities
related to the FPA as of March 18, 2021. At December 31, 2021, the re-measurement of the derivative associated with the FPA resulted in
the following change in the derivative liabilities – forward purchase agreement.
| | |
FPA
Liabilities | |
| | |
| |
| Derivative liability – forward purchase agreement at March 18, 2021 | |
$ | 11,655,000 | |
| Change in fair value of derivative liability – forward purchase agreement | |
| 1,665,000 | |
| Derivative liability – forward purchase agreement at December 31, 2021 | |
$ | 13,320,000 | |
| Change in fair value of derivative liability – forward purchase agreement | |
| (2,664,000 | ) |
| Derivative liability – forward purchase agreement at March 31, 2022 | |
$ | 10,656,000 | |
| Change in fair value of derivative liability – forward purchase agreement | |
| (2,664,000 | ) |
| Derivative liability – forward purchase agreement at June 30, 2022 | |
$ | 7,992,000 | |
The
following table presents information about the assumptions used to value the Company’s FPA liabilities classified as Level 3 in
the fair value hierarchy that are measured at fair value on a recurring basis.
| |
|
At
June 30,
2022 |
|
|
At
December 31,
2021 |
|
| |
|
|
|
|
|
|
| Starting share price |
|
$ |
10.00 |
|
|
$ |
10.00 |
|
| Strike price |
|
$ |
11.50 |
|
|
$ |
11.50 |
|
| Term (in years) |
|
|
5.08 |
|
|
|
5.50 |
|
| Volatility |
|
|
8.50 |
% |
|
|
19.0 |
% |
| Risk-free rate |
|
|
3.01 |
% |
|
|
1.31 |
% |
| Dividend yield |
|
|
0.0 |
% |
|
|
0.0 |
% |
Note 11 —
Subsequent Events
The
Company evaluated subsequent events and transactions that occurred after the balance sheet date through the date that the unaudited condensed
financial statements were issued. Based upon this review, the Company did not identify any subsequent events that would have required
adjustment or disclosure in the unaudited condensed financial statements.
On
July 15, 2022, the Company entered into a Promissory Note with its Sponsor pursuant to which the Sponsor has agreed to loan the Company
up to $450,000 to be used for working capital purposes. The loan will not bear any interest and will be repayable upon the earlier of
the date by which the Company has to complete a merger, share exchange, asset acquisition, share purchase, reorganization or similar business
combination with one or more businesses pursuant to its Amended and Restated Memorandum and Articles of Association or the effective date
of a Business Combination.
In
connection with the consummation of the Business Combination, the Company waived those certain provisions as contemplated by the Letter
Agreement, dated as of March 15, 2021, by and among the Company, its officers and directors, the Sponsor, and certain members of the Sponsor
(as amended, the “Letter Agreement”), and certain other agreements related thereto (collectively, the “Waiver”),
with respect to any securities held by an Insider (as defined in the Letter Agreement) as of the closing the Business Combination (the
“Lock-Up Securities”) that would disallow a pledge by such Insider of the Lock-up Securities in a transaction for the purpose
of financing such Insider’s payment obligations owed in connection with the closing of the Obagi and Milk Business Combinations.
On
July 25, 2022, the Company entered into that certain Waiver and Agreement by and between the Company and Burwell (the “Waiver and
Agreement”) to permit a pledge by Burwell of its Lock-Up Securities to be used as a portion of the collateral under a loan to finance
Burwell’s payment obligations under the Sponsor Forward Purchase Agreement in connection with the closing of the Obagi and Milk
Business Combinations. Pursuant to the terms of the Waiver and Agreement, in the event of a foreclosure, any such lenders or a collateral
agents will be required to execute a joinder to the Letter Agreement pursuant to which they will be bound by the transfer restrictions
of the Lock-Up Securities (including the foreclosure of or other exercise of remedies under any such loan documentation) in the Letter
Agreement for the duration of such agreement. The Company also agreed to provide any such lender or collateral agent with customary
registration rights in the event of default, foreclosure or other exercise of remedies following the respective Lock-Up Periods (as defined
in the Letter Agreement).
As
described in Note 1, the Company completed the Obagi and Milk Business Combinations on July 27, 2022.
REPORT OF INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM
To the shareholders and the Board of Directors
of Obagi Global Holdings Limited.
Opinion on the Financial Statements
We have audited the accompanying
consolidated balance sheets of Obagi Global Holdings Limited and subsidiaries (the “Company”) as of December 31, 2021
and 2020, the related consolidated statements of operations and comprehensive income (loss), shareholder’s equity, and cash flows,
for each of the three years in the period ended December 31, 2021, and the related notes (collectively referred to as the “financial
statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company
as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period
ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
We have not audited any
financial statements of the Company for any period subsequent to December 31, 2021. However, as discussed in Note 1 to the financial statements,
the Company may not be in compliance with its debt covenant ratios for the period ending June 30, 2022 or future periods, that could result
in the underlying debt becoming due and payable. If the debt becomes due and payable, the Company does not have sufficient resources to
satisfy its debt obligation which raises substantial doubt about its ability to continue as a going concern. Management’s plans
in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from
the outcome of this uncertainty.
Basis for Opinion
These financial statements
are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements
based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits
in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America.
Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free
of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit
of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control
over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control
over financial reporting. Accordingly, we express no such opinion.
Our audits included performing
procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures
that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the
financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management,
as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for
our opinion.
/s/ Deloitte and Touche LLP
Costa Mesa, California
April 26, 2022 (June 15, 2022 as to going
concern and liquidity described in Note 1)
We have served as the Company’s auditor since
2018.
OBAGI GLOBAL HOLDINGS LIMITED
CONSOLIDATED BALANCE SHEETS
(In thousands of U.S. dollars, except share
and per share data)
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| | |
| | |
| |
| ASSETS | |
| | |
| |
| CURRENT ASSETS: | |
| | |
| |
| Cash and cash equivalents | |
$ | 12,794 | | |
$ | 8,572 | |
| Restricted cash | |
| 650 | | |
| — | |
| Accounts and note receivable, net | |
| 63,367 | | |
| 37,077 | |
| Inventories | |
| 19,339 | | |
| 13,980 | |
| Prepaid expenses | |
| 7,944 | | |
| 5,908 | |
| Other current assets | |
| 335 | | |
| 805 | |
| Total current assets | |
| 104,429 | | |
| 66,342 | |
| | |
| | | |
| | |
| Property and equipment, net | |
| 3,584 | | |
| 1,622 | |
| Intangible assets, net | |
| 79,574 | | |
| 92,094 | |
| Goodwill | |
| 44,489 | | |
| 44,489 | |
| Deferred income tax assets | |
| — | | |
| 10,526 | |
| Other assets | |
| 1,497 | | |
| 3,350 | |
| TOTAL ASSETS | |
$ | 233,573 | | |
$ | 218,423 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDER’S EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payable | |
$ | 11,375 | | |
$ | 2,880 | |
| Other current liabilities | |
| 12,983 | | |
| 8,507 | |
| Current portion of long-term debt, net | |
| 15,382 | | |
| 47,034 | |
| Total current liabilities | |
| 39,740 | | |
| 58,421 | |
| | |
| | | |
| | |
| Long-term debt, net | |
| 103,423 | | |
| 67,863 | |
| Deferred income tax liabilities | |
| 548 | | |
| — | |
| Other liabilities | |
| 572 | | |
| 747 | |
| Total liabilities | |
| 144,283 | | |
| 127,031 | |
| | |
| | | |
| | |
| COMMITMENTS AND CONTINGENCIES (Note 15) | |
| | | |
| | |
| | |
| | | |
| | |
| SHAREHOLDER’S EQUITY: | |
| | | |
| | |
| Common stock, 25,000,000 shares authorized; $0.50 par
value; 8,000,002 shares issued and outstanding as of December 31, 2021, and 2020, respectively | |
| 4,000 | | |
| 4,000 | |
| Additional paid-in capital | |
| 100,113 | | |
| 100,113 | |
| Accumulated deficit | |
| (14,798 | ) | |
| (12,728 | ) |
| Accumulated other comprehensive (loss) income | |
| (25 | ) | |
| 7 | |
| Total shareholder’s equity | |
| 89,290 | | |
| 91,392 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES AND SHAREHOLDER’S EQUITY | |
$ | 233,573 | | |
$ | 218,423 | |
See accompanying notes
to consolidated financial statements.
OBAGI GLOBAL HOLDINGS LIMITED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
(LOSS)
(In thousands of U.S. dollars, except share
and per share data)
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Net Revenue | |
| 206,069 | | |
| 84,145 | | |
| 117,085 | |
| Cost of goods sold (exclusive of depreciation and amortization shown separately below) | |
| 48,708 | | |
| 19,969 | | |
| 26,687 | |
| Selling, general and administrative | |
| 118,243 | | |
| 54,794 | | |
| 62,762 | |
| Research and development | |
| 6,991 | | |
| 3,929 | | |
| 3,484 | |
| Depreciation and amortization | |
| 14,053 | | |
| 13,426 | | |
| 12,940 | |
| Total operating expenses | |
| 187,995 | | |
| 92,118 | | |
| 105,873 | |
| Operating income (loss) | |
| 18,074 | | |
| (7,973 | ) | |
| 11,212 | |
| Interest expense | |
| 11,156 | | |
| 6,281 | | |
| 6,834 | |
| Loss on extinguishment of debt | |
| 2,317 | | |
| — | | |
| — | |
| Gain on PPP Loan forgiveness (Note 9) | |
| (6,824 | ) | |
| — | | |
| — | |
| Other expense, net | |
| 194 | | |
| 11 | | |
| 147 | |
| Income (loss) before income taxes | |
| 11,231 | | |
| (14,265 | ) | |
| 4,231 | |
| Income tax expense (benefit) | |
| 11,301 | | |
| (5,094 | ) | |
| (1,589 | ) |
| Net (loss) income | |
| (70 | ) | |
| (9,171 | ) | |
| 5,820 | |
| Other comprehensive (loss) income—Foreign currency translation adjustments, net of tax | |
| (32 | ) | |
| 16 | | |
| (9 | ) |
| Comprehensive (loss) income | |
$ | (102) | | |
$ | (9,155 | ) | |
$ | 5,811 | |
| | |
| | | |
| | | |
| | |
| Net (loss) income per share of common stock—Basic and diluted | |
$ | (0.01 | ) | |
$ | (1.14 | ) | |
$ | 0.73 | |
| Weighted average shares of common stock outstanding—Basic and diluted (Note 12) | |
| 8,000,002 | | |
| 8,000,002 | | |
| 8,000,002 | |
See accompanying notes
to consolidated financial statements.
OBAGI GLOBAL HOLDINGS LIMITED
CONSOLIDATED STATEMENTS OF SHAREHOLDER’S EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2021, 2020 AND 2019
(In thousands of U.S. dollars, except share
data)
| | |
Common Stock | | |
Additional Paid-In | | |
Accumulated | | |
Accumulated Other Comprehensive | | |
Total Shareholder’s | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
(Loss) Income | | |
Equity | |
| BALANCE—January 1, 2019 (Note 1) | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 96,055 | | |
$ | (5,758 | ) | |
$ | — | | |
$ | 94,297 | |
| Net income | |
| — | | |
| — | | |
| — | | |
| 5,820 | | |
| — | | |
| 5,820 | |
| Foreign currency translation adjustment | |
| — | | |
| — | | |
| — | | |
| — | | |
| (9 | ) | |
| (9 | ) |
| Dividends paid (Note 11) | |
| — | | |
| — | | |
| — | | |
| (1,575 | ) | |
| — | | |
| (1,575 | ) |
| BALANCE—December 31, 2019 | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 96,055 | | |
$ | (1,513 | ) | |
$ | (9 | ) | |
$ | 98,533 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (9,171 | ) | |
| — | | |
| (9,171 | ) |
| Foreign currency translation adjustment | |
| — | | |
| — | | |
| — | | |
| — | | |
| 16 | | |
| 16 | |
| Non-cash contribution of trademarks | |
| — | | |
| — | | |
| 4,058 | | |
| — | | |
| — | | |
| 4,058 | |
| Dividends paid (Note 11) | |
| — | | |
| — | | |
| — | | |
| (2,044 | ) | |
| — | | |
| (2,044 | ) |
| BALANCE—December 31, 2020 | |
| 8,000,002 | | |
| 4,000 | | |
| 100,113 | | |
| (12,728 | ) | |
| 7 | | |
| 91,392 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (70 | ) | |
| — | | |
| (70 | ) |
| Foreign currency translation adjustment | |
| — | | |
| — | | |
| — | | |
| — | | |
| (32 | ) | |
| (32 | ) |
| Dividends paid (Note 11) | |
| — | | |
| — | | |
| — | | |
| (2,000 | ) | |
| — | | |
| (2,000 | ) |
| BALANCE—December 31, 2021 | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 100,113 | | |
$ | (14,798 | ) | |
$ | (25 | ) | |
$ | 89,290 | |
See accompanying notes
to consolidated financial statements.
OBAGI GLOBAL HOLDINGS LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands of U.S. dollars)
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | |
| | |
| | |
| |
| Net (loss) income | |
$ | (70 | ) | |
$ | (9,171 | ) | |
$ | 5,820 | |
| Adjustments to reconcile net (loss) income to net cash provided by (used in) operating activities: | |
| | | |
| | | |
| | |
| Depreciation and amortization | |
| 14,053 | | |
| 13,426 | | |
| 12,940 | |
| Loss on extinguishment of debt | |
| 2,317 | | |
| — | | |
| — | |
| Gain on PPP loan forgiveness | |
| (6,824 | ) | |
| — | | |
| — | |
| Amortization of debt issuance costs | |
| 1,179 | | |
| 798 | | |
| 731 | |
| Loss on disposal of equipment | |
| 52 | | |
| — | | |
| — | |
| Changes in operating assets and liabilities: | |
| | | |
| | | |
| | |
| Accounts receivable | |
| (23,790 | ) | |
| (961 | ) | |
| (7,166 | ) |
| Inventories | |
| (5,359 | ) | |
| 1,593 | | |
| (8,922 | ) |
| Prepaid expenses | |
| (2,654 | ) | |
| (2,150 | ) | |
| (1,722 | ) |
| Other current assets and other assets | |
| 2,323 | | |
| (998 | ) | |
| (1,050 | ) |
| Deferred income taxes | |
| 11,074 | | |
| (4,743 | ) | |
| (1,590 | ) |
| Accrued interest-related party | |
| - | | |
| - | | |
| (1,959 | ) |
| Accounts payable | |
| 8,495 | | |
| (7,523 | ) | |
| 5,222 | |
| Other current liabilities and other liabilities | |
| 4,275 | | |
| 2,478 | | |
| 1,472 | |
| Net cash provided by (used in) operating activities | |
| 5,070 | | |
| (7,251 | ) | |
| 3,776 | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | |
| | | |
| | | |
| | |
| Capital expenditure on intangible assets | |
| (937 | ) | |
| (652 | ) | |
| (788 | ) |
| Advances for note receivable | |
| (2,500 | ) | |
| — | | |
| — | |
| Capital expenditure on property and equipment | |
| (1,923 | ) | |
| (1,235 | ) | |
| — | |
| Net cash used in investing activities | |
| (5,360 | ) | |
| (1,887 | ) | |
| (788 | ) |
| CASH FLOWS FROM FINANCING ACTIVITIES: | |
| | | |
| | | |
| | |
| Payment of dividends | |
| (2,000 | ) | |
| (2,044 | ) | |
| (1,575 | ) |
| Proceeds from term loan | |
| 110,000 | | |
| — | | |
| — | |
| Proceeds from revolving credit facility | |
| 20,000 | | |
| 29,000 | | |
| 47,000 | |
| Repayment of revolving credit facility | |
| (44,000 | ) | |
| (9,000 | ) | |
| (43,000 | ) |
| Repayment of term loan | |
| (72,455 | ) | |
| (9,370 | ) | |
| (9,000 | ) |
| Proceeds from PPP Loan | |
| — | | |
| 6,750 | | |
| — | |
| Payment of debt issuance costs | |
| (6,383 | ) | |
| (1,017 | ) | |
| (239 | ) |
| Net cash provided by (used in) financing activities | |
| 5,162 | | |
| 14,319 | | |
| (6,814 | ) |
| CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | |
| 4,872 | | |
| 5,181 | | |
| (3,826 | ) |
CASH, CASH EQUIVALENTS
AND RESTRICTED CASH—Beginning of year | |
| 8,572 | | |
| 3,391 | | |
| 7,217 | |
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH—End of year | |
| 13,444 | | |
| 8,572 | | |
| 3,391 | |
| SUPPLEMENTAL CASH FLOW DATA: | |
| | | |
| | | |
| | |
| Income taxes paid | |
$ | — | | |
$ | 9 | | |
$ | 50 | |
| Interest paid-related party | |
$ | — | | |
$ | — | | |
$ | 1,959 | |
| Interest paid | |
$ | 10,014 | | |
$ | 5,449 | | |
$ | 6,055 | |
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | |
| | | |
| | | |
| | |
| Capital expenditures in accounts payable | |
$ | 97 | | |
$ | 186 | | |
$ | — | |
| Capital contribution of trademarks | |
$ | — | | |
$ | 4,058 | | |
$ | — | |
See accompanying notes
to consolidated financial statements.
OBAGI GLOBAL HOLDINGS LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2021 AND 2020, AND FOR
THE YEARS ENDED DECEMBER 31, 2021, 2020 AND 2019
(In thousands of U.S. dollars, except share
and per share data)
| 1. | DESCRIPTION
OF BUSINESS |
Obagi Global Holdings
Limited (“Obagi Global”) is a holding company incorporated in the Cayman Islands that conducts all operations through its
wholly owned subsidiaries (collectively the “Company” or “Obagi”). The Company is a global skincare company that
develops, markets, and sells proprietary-topical aesthetic and therapeutic prescription-strength skincare systems and related products
primarily in the physician-dispensed market. Obagi provides cosmetic, over-the-counter (“OTC”) and prescription products designed
to prevent or improve the most common and visible skin disorders in adult skin, including premature aging, photo damage, hyperpigmentation
(irregular or patchy discoloration of the skin), acne, sun damage, facial redness, and soft tissue deficits, such as fine lines and wrinkles.
The Company is headquartered in Long Beach, California.
On November 30,
2020, the Board of Directors approved (i) an increase in the number of the Company’s authorized shares of common stock from
50,000 to 25,000,000, (ii) an issuance of 4,000,000 shares of common stock to ZhongHua Finance Acquisition Fund I, L.P. (“ZhongHua”),
and (iii) a two-for-one stock split of the Company’s issued and outstanding common stock (in combination with the share issuance
to ZhongHua, the “Stock Split”), all of which became effective on December 2, 2020. The Company is held by a single shareholder,
and the share issuance to ZhongHua was deemed akin to a stock split. All share, per share amounts and related shareholder’s equity
balances presented herein have been retroactively adjusted to reflect the impact of the Stock Split.
On July 15, 2021,
ZhongHua transferred its shares to its affiliate, Cedarwalk Skincare Ltd. (“Cedarwalk”), which is now the sole shareholder
of the Company. This transfer between affiliates did not result in any change of control.
Proposed Merger
On November 15,
2021, Waldencast Acquisition Corp. (“Waldencast”) (NASDAQ: WALD), a special purpose acquisition company, entered into an Agreement
and Plan of Merger (the “Merger Agreement”) with the Company and Obagi Merger Sub, Inc., indirect wholly owned subsidiaries
of Waldencast (“Merger Sub”), pursuant to which the Merger Sub will merge with and into the Company (excluding its operations
in the China Region, as defined below), the separate corporate existence of Merger Sub will cease to exist, with the Company surviving
as an indirect wholly owned subsidiary of Waldencast. Concurrently with the Merger Agreement, Waldencast entered into an Equity Purchase
Agreement with Milk Makeup LLC (“Milk”) (together, the transactions contemplated in the Merger Agreement and Equity Purchase
Agreement, the “Business Combination”) to acquire all of the issued and outstanding membership units of Milk. Following the
Business Combination, the combined company will be organized in an “Up-C” structure, in which the equity interests of the
Company and Milk will be held by Waldencast LP. Waldencast plc’s interests in the Company and Milk will be held through its wholly
owned subsidiaries, Holdco 1 and Waldencast LP. The Business Combination is expected to close in the first half of 2022.
Waldencast will acquire
the Company at an enterprise value of approximately $858,000 excluding 100% of the business of Obagi Hong Kong and certain related entities
and assets (the “Obagi China Business”), consisting of approximately $380,000 in cash (subject to the substitution of an amount
of Waldencast stock, in the event of redemptions of Waldencast stock, and such substituted stock would be subject to a lock-up period,
and subject to adjustment for transaction expenses as set forth in the Merger Agreement), and $275,000 in Class A ordinary shares
of the post-combination entity, Waldencast plc (as defined in the Plan of Domestication).
Immediately prior to
the closing of the Business Combination, the Company will carve out and distribute the Note Receivable and its operations in the People’s
Republic of China, inclusive of the Hong Kong Special Administrative Region, the Macau Special Administrative Region, and Taiwan (the
“China Region”) to its shareholder, Cedarwalk.
In connection with
the distribution, and prior to the Business Combination, the Company will enter into an Intellectual Property License Agreement (the
“IP License Agreement”) with the Obagi China Business. Under the IP License Agreement, the Company will exclusively
license intellectual property relating to the Obagi brand to the Obagi China Business, and the Company will retain the rights to
such intellectual property to conduct the Obagi-branded business worldwide except for the China Region. The Obagi China Business
will pay the Company a royalty of five and a half percent (5.5%) of gross sales of licensed products.
In connection with the
distribution, and prior to the Business Combination, the Company will enter into a Global Supply Services Agreement (the “Supply
Agreement”) with the Obagi China Business, pursuant to which the Company will supply, or cause to be supplied through certain CMOs
(as defined in the Supply Agreement), products to the Obagi China Business and its affiliates, for the Obagi China Business’s distribution
and sale in the China Region. The term of the Supply Agreement is perpetual, subject to termination for material breach and failure to
cure or termination in the event that the IP License Agreement is terminated.
In connection with the
distribution, and prior to the Business Combination, the Company will enter into a Transition Services Agreement (the “Transition
Services Agreement”) with the Obagi China Business, for the provision of certain transition services to enable the Obagi China Business
to conduct Obagi-branded business as a going concern in the China Region. The transition services will be provided for an initial term
of up to twelve (12) months, with an option for the Obagi China Business to extend the service period for up to an additional twelve (12)
months solely as to certain research and development services. Services will be charged at the reasonable, fully-loaded costs of providing
the services, but such services will be provided at no charge for a certain period of time or amount of services.
Going Concern and Liquidity
The Company incurred
significant transaction expenses with respect to the Business Combination for the three months ended March 31, 2022. Under the terms of
the Company’s financing agreement with a syndicate of lenders, including TCW Asset Management Company LLC as administrative agent
for the lenders (the “2021 Credit Agreement”), transaction expenses were not considered an adjustment for the maximum leverage
ratio calculation and, therefore, the Company was not in compliance with the maximum leverage ratio for the period ended March 31, 2022.
As a result, on June 10, 2022 the Company entered into an amendment to the 2021 Credit Agreement to retroactively modify the maximum leverage
ratio allowed for the period ended March 31, 2022 that remediated this compliance matter. The Company was in compliance with the 2021
Credit Agreement as of December 31, 2021.
As the closing of the
Business Combination has not yet occurred, the Company continues to incur transaction expenses. As such, a risk exists that, in the event
that the closing of the Business Combination with Waldencast does not occur, the Company may not be in compliance with its debt covenant
ratios for the period ending June 30, 2022 or future periods. In this scenario, the underlying existing debt may become due and payable,
unless a new amendment is negotiated with existing creditors. If the debt becomes due and payable, the Company does not have sufficient
cash liquidity to satisfy its debt obligation. As a result, this condition raises substantial doubt about the Company’s ability
to continue as a going concern for at least one year from the date that these financial statements are issued.
The Company plans to
seek additional amendments in the event of future non-compliance, or to satisfy such obligation from the resources obtained from the successful
completion of the Business Combination. Successful completion of such plans is dependent on factors outside the Company’s control.
The accompanying consolidated
financial statements do not include any adjustments as to recoverability and classification of assets or the amounts and classifications
of liabilities that may result from a going concern as described above.
| 2. | SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation—The
Company has prepared the accompanying consolidated financial statements pursuant to generally accepted accounting principles in the United
States (“U.S. GAAP”) and applicable rules and regulations of the Securities and Exchange Commission (“SEC”).
Emerging Growth
Company—Section 102(b)(1) of the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) exempts emerging
growth companies from being required to comply with new or revised financial accounting standards until private companies (that is,
those that have not had a registration statement declared effective under the Securities Act of 1933, as amended, or do not have a
class of securities registered under the Exchange Act of 1934, as amended) are required to comply with the new or revised financial
accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period
and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The
Company has elected not to opt out of such extended transition period, which means that when a standard is issued or revised, and it
has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or
revised standard at the time private companies adopt the new or revised standard.
This may make comparison
of the Company’s financial statement with another public company that is neither an emerging growth company nor an emerging growth
company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting
standards used.
Principles of Consolidation—The
accompanying consolidated financial statements include the accounts of Obagi Global Holdings Limited and its wholly-owned subsidiaries.
All intercompany accounts and transactions have been eliminated in consolidation.
Significant Risks
and Uncertainties—In March 2020, the World Health Organization declared the novel strain of coronavirus (“COVID-19”),
a global pandemic and recommended containment and mitigation measures worldwide. COVID-19 has disrupted everyday life and markets worldwide,
leading to significant business and supply-chain disruption, as well as broad-based changes in supply and demand. While the quarantine,
social distancing and other regulatory measures instituted or recommended in response to COVID-19 are expected to be temporary, the duration
of the business disruptions, and related financial impact, cannot be estimated at this time.
The extent of the continued
impact of the COVID-19 pandemic on the Company’s operational and financial performance will depend on various future developments,
including the duration and spread of the outbreak and impact on Obagi employees, customers and their patients, all of which is uncertain
at this time. The direct impact of COVID-19 on the business of the Company, beyond disruptions to normal business operations, resulted
from the closure of physician customers’ practices for several weeks, which greatly diminished demand for the Company’s products.
Although some of these customers have now established websites to be able to provide products to their patients through online sales,
any re-implementation of similar restrictions could have a material impact on the Company’s future net revenue.
While the impact of COVID-19
on the Company’s manufacturing and supply chain, sales and marketing, and commercial and clinical trial operations, to-date has
not been material, the impact of COVID-19 over the long-term is uncertain and cannot be predicted with confidence. The extent of the adverse
impact of COVID-19 on the Company’s operations will depend on the extent and severity of the continued spread of COVID-19 globally,
the timing and nature of actions taken to respond to COVID-19 and the resulting economic consequences.
Use of Estimates—The
preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions
that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those
estimates. Significant estimates and assumptions reflected in the financial statements include, but are not limited to the valuation allowance
for deferred tax assets, fair value measurements including valuation of the Company, useful lives of finite-lived intangible assets, stock-based
compensation, and revenue recognition. The Company bases its estimates on historical experience and on assumptions that it believes are
reasonable. Due to the inherent uncertainty involved in making assumptions and estimates, changes in circumstances could result in actual
results differing from those estimates, and such differences could be material to the Company’s consolidated balance sheets, statements
of operations and comprehensive income (loss).
Concentrations of
Credit Risk—Financial instruments that subject the Company to concentrations of credit risk consist primarily of cash and cash
equivalents and accounts receivable. The Company maintains its cash balances in accounts held by major banks and financial institutions
located primarily in the United States and considers such risk to be minimal. Such bank deposits from time to time may be exposed to credit
risk in excess of the Federal Deposit Insurance Corporation insurance limit.
The Company’s accounts
receivable primarily represent amounts due from wholesale distributors and group purchasing organizations located both inside and outside
the U.S. The Company mitigates its credit risks by performing ongoing credit evaluations of its customers’ financial conditions,
requires customer advance payments in certain circumstances and has historically not had receipt of payment exceed 60 days. The Company
generally does not require collateral.
As of December 31, 2021,
three customers accounted for 35%, 33% and 13% of accounts receivable, respectively. As of December 31, 2020, three customers accounted
for 48%, 21% and 17% of accounts receivable, respectively.
During the years ended
December 31, 2021, 2020, and 2019, two vendors exceeded 10% of inventory purchases. In 2021, the Company purchased approximately
45% and 11% of inventory, respectively, from the two vendors. In 2020, the Company purchased approximately 50% and 12% of inventory, respectively,
from the two vendors. In 2019, the Company purchased approximately 49% and 11% of inventory, respectively, from the two vendors. As of
December 31, 2021, one vendor accounted for 40% of accounts payable. As of December 31, 2020, no vendor accounted for more than 10%
of accounts payable.
Restricted Cash - The
Company’s restricted cash represents funds that are not accessible for general purpose cash needs due to contractual limitations.
As of December 31, 2021, the Company has restricted cash of $650. As of December 31, 2020, the Company did not have restricted
cash. The restricted cash balance represents cash in a savings account held by a major bank located in the United States and provides
collateral for corporate credit cards obtained by the Company for its employees. The Company is required to hold the restricted cash in
the bank’s savings account until August 2022. As of December 31, 2021, the Company’s cash, cash equivalents and restricted
cash balance of $13,444 shown on the consolidated statements of cash flows consisted of $650 restricted cash and $12,794 cash and cash
equivalents.
Cash and Cash Equivalents—The
Company considers highly liquid investments with an initial maturity of three months or less to be cash and cash equivalents.
Inventories—The
Company’s products are produced by third party contract manufacturers. Inventories are comprised mainly of finished goods, which
are valued at the lower of cost or net realizable value, using the standard cost method, which approximates actual costs determined on
a first-in, first-out basis. Net realizable value is determined as estimated selling prices in the ordinary course of business, less reasonably
predictable costs of disposal and transportation. The Company evaluates the carrying value of inventories on a regular basis, taking into
account such factors as historical and anticipated future sales compared with quantities on hand, the price the Company expects to obtain
for products in their respective markets compared with historical cost and the remaining shelf life of goods on hand. If potential impairment
is identified, the Company records write-downs of inventories to cost of goods sold.
Leases —The
Company accounts for leases in accordance with Accounting Standards Codification (“ASC”) Topic 840, Leases (“ASC
840”). An arrangement is or contains a lease if there are specified assets and the right to use the specified asset is conveyed
for a period in exchange for consideration. Upon lease inception, the Company determines if an arrangement contains a lease and whether
that lease meets the classification criteria of a capital or operating lease.
Operating leases are
not recognized on the balance sheet. For income statement purposes, the Company recognizes rent expense on a straight-line basis for operating
leases. For capital leases, the Company recognizes interest expense associated with the capital lease liability and amortization expense
associated with the capital lease asset. For capital lease assets and leasehold improvements, the estimated useful lives are limited to
the shorter of the useful life of the asset or the term of the lease. Variable lease payments are recognized in general and administrative
expenses. The Company’s lease agreements do not contain any material residual value guarantees, restrictions or covenants.
Property and Equipment,
Net—Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is computed using the straight-line
method over the estimated useful lives of respective assets. The estimated useful lives of the Company’s assets are as follows:
| | |
Estimated Useful Lives |
| Machinery equipment | |
3 years |
| Capital lease equipment | |
3 years |
| Leasehold improvements | |
Lesser of useful life or term of lease |
| Computer software and website development | |
3 years |
Expenditures for maintenance
and repairs are charged to expense as incurred. When an asset is sold or otherwise disposed of, the cost and associated accumulated depreciation
are removed from the accounts and the resulting gain or loss is recognized in the consolidated statements of operations and comprehensive
income (loss).
Internal Use Software—Internal
use software consists of capitalized software and website development costs and are included in property and equipment, net on the consolidated
balance sheets. The Company accounts for software that is developed for internal use pursuant to ASC Topic 350-40, Intangibles,
Goodwill and Other — Internal-Use Software. Qualifying costs incurred to develop internal-use software are capitalized
when (i) the preliminary project stage is completed, (ii) management has authorized further funding for the completion of the
project and (iii) it is probable that the project will be completed and perform as intended. These capitalized costs include compensation
for employees who develop internal-use software and external costs related to development of internal use software. Capitalization of
these costs ceases once the project is substantially complete and the software is ready for its intended purpose. Internally developed
software is amortized using the straight-line method over an estimated useful life of three years.
Intangible Assets,
Net—Intangible assets consists primarily of trademarks, customer lists, supply agreements, patents and developed technology.
At initial recognition, intangible assets acquired in a business combination are recognized at their fair value as of the date of acquisition.
Following initial recognition, intangible assets are carried at cost less accumulated amortization and impairment losses, if any, and
are amortized on a straight-line basis over the estimated useful life of the asset.
Impairment of Long-Lived
Assets—Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying
amount of an asset may not be recoverable. If circumstances require a long-lived asset or asset group be tested for possible impairment,
for each asset group held for use with indicators of impairment, the Company compares the expected future cash flows generated by the
asset group, which represents the lowest level at which cash flows are identifiable, with its associated net carrying value. If the net
carrying value of the asset group exceeds expected undiscounted cash flows, the excess of the net book value over estimated fair value
is charged to impairment loss. No impairment loss was recognized for the years ended December 31, 2021, 2020, and 2019.
Goodwill—Goodwill
represents the difference between the purchase price and the fair value of assets and liabilities acquired in a business combination.
The Company reviews goodwill for impairment annually on September 30th and also if events or changes in circumstances
indicate the occurrence of a triggering event. The Company reviews goodwill for impairment by initially considering qualitative factors
to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, including goodwill,
as a basis for determining whether it is necessary to perform a quantitative analysis. If it is determined that it is more likely than
not that the fair value of reporting unit is less than its carrying amount, a quantitative analysis is performed to identify goodwill
impairment. No impairment loss was recognized for the years ended December 31, 2021, 2020, and 2019. The carrying amount of Goodwill
as of December 31, 2021 and 2020 was $44,489.
Deferred Issuance
Costs—The Company capitalizes costs related to the issuance of debt instruments, as applicable. Such costs are initially recorded
as a direct deduction from the applicable debt instrument and amortized over the contractual term of the related debt instrument in Interest
expense, net using the straight-line method, which approximates the effective interest method, in the consolidated statements of operations
and comprehensive income (loss).
Accounts
Receivable, Net—Trade accounts receivable are stated at net realizable value. Receivables represent amounts billed to and
currently due from customers that have yet to be collected. The Company maintains an allowance for doubtful accounts, which
represents allowances for customer trade accounts receivable that are estimated to be partially or entirely uncollectible. These
allowances are used to reduce gross trade receivables to their net realizable value. The Company records these allowances based on
estimates related to the following factors: (i) customer-specific allowances, based upon past collection history and identification
of specific customer risk and (ii) formula-based general allowances based upon an aging schedule.
The Company establishes
a reserve for sales returns that reflects an estimate of future customer returns and are accrued upon sale. The estimate of sales returns
is based upon past return history and takes into consideration other factors including, expiration dates, price changes, seasonality,
and possible change in product demand. Actual returns are charged against the accrual.
Rebates are reserves
for promotions that are extended to physicians and are calculated monthly. Rebates are immediately extended to physicians on their sales
invoices and physicians remit payment for the net sales amount due. The reserve is established based on physician sales and promotions
set for the month. Actual rebates are charged against the accrual when paid by the Company.
The Company pays distribution
fees quarterly to its authorized wholesale distributors. The distribution fees are based on a percentage of gross product sales to such
distributor. The collection of accounts receivable from such distributor is offset by the distribution fee.
Revenue Recognition—
Product Sales
The Company generates
product sales revenue from sales of its products to distributors and directly to retailers. Revenue from product sales is recognized at
a point in time, when transfer of control has passed to the customer, based on the terms of the sale.
The Company has determined
that each of its products is distinct and represents a separate performance obligation. The Company has different contracted shipping
terms with different distributor and direct sales customers that dictate when the right to payment, passage of legal title, transfer of
physical possession, and assumption of the risks and rewards occur. Depending on the contract, the company considers transfer of control
to have occurred either once the delivery of the product has occurred or once the product has been picked up from the Company’s
designated warehouse by the customer’s shipping agent. Customers have a right of return for outdated, non-moving, discontinued,
or recalled product, when product does not meet its specifications. However, historically these returns have not been material.
The Company’s contracts
with customers may include multiple performance obligations. For such arrangements, the Company allocates the transaction price to each
performance obligation based on its relative standalone selling price. Standalone selling price is the price at which Obagi would sell
a promised product separately to a customer.
The Company offers discounts
and other incentive allowances to customers. The transaction price of product sales includes estimates of these discounts and incentives
as variable consideration. Product sales revenue is recognized net of provisions for estimated discounts and allowances, distribution
fees, returns, and rebates. Provisions for discounts and allowances are estimated based on the most likely amount using contractual sales
terms with customers and historical experience. Accruals for customer rebates are estimated based on the contractual terms and the Company’s
current evaluation of its experience.
The Company accounts
for shipping and handling activities as fulfillment activities instead of as performance obligations and recognizes these costs as selling,
general and administrative expenses. Costs related to shipping and handling were not material to the financial statements for the years
ended December 31, 2021, 2020 and 2019.
Royalties
The Company also generates
royalty revenues from products sold under the Obagi name by Rohto Pharmaceutical Co, LTD (“Rohto”). Rohto markets its dermatology
and topical medication products in the Japanese markets under the Obagi brand name through a license agreement with the Company. Under
this agreement, the Company receives a royalty based upon a percentage of Rohto’s net sales of those products. As a sales-based
royalty, these royalties are exempt from the variable consideration guidance. As such, the Company’s recognition of revenue includes
in the transaction price only the royalty amount due as Rohto makes subsequent sales of licensed product. The Company recognizes revenue
for licensing at a point in time, upon Rohto’s sales of Obagi-branded products.
Assets Recognized from Costs to Obtain
a Contract with a Customer
The Company recognizes
the incremental costs of obtaining a customer contract as an expense when incurred if the amortization period of the assets that the Company
otherwise would have recognized is one year or less. The incremental costs to obtain contracts primarily relate to sales commission and
sales-based bonuses. Total capitalizable costs to obtain a contract were immaterial during the years presented.
Research and Development—Research
and development costs are expensed as incurred. Substantially all research and development expenses are related to new product development
and design improvements or increased functionality in current products.
Advertising—Advertising
costs are expensed in the period in which they are incurred. Total advertising costs, included in Selling, general and administrative
expense on the consolidated statements of operations and comprehensive income (loss), were $20,379, $9,572, and $9,733 for the years ended
December 31, 2021, 2020, and 2019, respectively.
Fair Value Measurement—The
Company uses valuation approaches that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent
possible. The Company determines fair value based on assumptions that market participants would use in pricing an asset or liability in
the principal or most advantageous market. When considering market participant assumptions in fair value measurements, the following fair
value hierarchy distinguishes between observable and unobservable inputs, which are categorized in one of the following levels:
| ● | Level
1 inputs: Unadjusted quoted prices in active markets for identical assets or liabilities accessible to the reporting entity at the measurement
date. |
| ● | Level
2 inputs: Other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly,
for substantially the full term of the asset or liability. |
| ● | Level
3 inputs: Unobservable inputs for the asset or liability used to measure fair value to the extent that observable inputs are not available,
thereby allowing for situations in which there is little, if any, market activity for the asset or liability at measurement date. |
The fair values of the
Company’s cash, accounts receivable, accounts payable, and all other current liabilities approximate their carrying values because
of the short maturities on these instruments. Additionally, amortized cost, short-term debt and long-term debt approximate fair value
due to the adjusting interest rates of the term loan, which approximate current market rates.
Stock Based Compensation—The
Company measures the cost of share-based awards granted to eligible employees, directors, and consultants based on the grant-date fair
value of the awards. The grant-date fair value of the stock options is calculated using a Black-Scholes option pricing model. The Black-Scholes
pricing model requires the use of subjective assumptions including the option’s expected term, the volatility of the underlying
stock, the fair value of the stock, dividend yield rate and the risk-free rate. The fair value of the restricted stock units (“Restricted
Stock”) is equal to the price of the Company’s common stock on the grant date. The Company has elected to recognize the effect
of forfeitures in the period in which they occur. Share-based awards are classified as equity, unless the underlying shares are classified
as liabilities or the Company is required to settle the awards by transferring cash or other assets. The Company recognizes compensation
expense for awards with service and qualifying transaction conditions on a straight-line basis over the requisite service period, which
is generally the award’s vesting period. Compensation expense for employee stock-based awards whose vesting is subject to the fulfillment
of both a service condition and the occurrence of a performance condition is recognized on a graded-vesting basis at the time the achievement
of the performance condition becomes probable. As of and prior to December 31, 2021, the Company does not expect the occurrence of
a qualifying transaction event to be “probable”, and therefore no expense has been recorded.
Foreign
Currency—The U.S. dollar is the reporting currency as well as the functional currency of the Company’s consolidated
entities operating in the U.S., and certain of its subsidiaries operating outside of the U.S. For transactions entered into in a
currency other than its functional currency, the monetary assets and liabilities are re-measured into U.S. dollars at the current
exchange rate as of the applicable balance sheet date, and all non-monetary assets and liabilities are re-measured at historical
rates. Income and expenses are re-measured at the average exchange rate prevailing during the period. Gains and losses resulting
from the re-measurement of these subsidiaries’ financial statements are included in the consolidated statements of operations
and comprehensive income (loss).
Gains and losses resulting
from foreign exchange transactions and revaluation of monetary assets and liabilities in non-functional currencies are included in other
income (expense) in the consolidated statements of operations and comprehensive income (loss). Net foreign exchange gain (loss) recorded
in the Company’s consolidated statements of operations and comprehensive income (loss) was insignificant for all periods presented.
Income Taxes—The
Company accounts for income taxes using the asset and liability approach. Under this approach, deferred taxes represent the future tax
consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid.
The provision for income
taxes represents income taxes paid or payable for the current period plus the change in deferred taxes during the period. Deferred taxes
result from differences between the financial and tax basis of the Company’s assets and liabilities and are adjusted for changes
in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely
than not that a tax benefit will be realized. The assessment of whether a valuation allowance is required often requires significant judgment
including the long-range forecast of future taxable income and the evaluation of planning initiatives. Adjustments to the deferred tax
valuation allowances are made to earnings in the period when such assessments are made. A valuation allowance of $14,336 has been recorded
as of December 31, 2021. No valuation allowance has been recorded as of December 31, 2020.
The Company accounts
for a tax benefit from an uncertain position in the consolidated financial statements only if it is more likely than not that the position
is sustainable, based solely on its technical merits and consideration of the relevant taxing authority’s widely understood administrative
practices and precedents. If the recognition threshold for the tax position is met, the Company records only the portion of the tax benefit
that is greater than 50% likely to be realized. As of December 31, 2021, and 2020, the Company had no uncertain positions in the
consolidated financial statements.
The Company recognizes
accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no amounts accrued for interest
and penalties as of December 31, 2021 and 2020.
Net Income (Loss)
Per Share—Basic net income (loss) per share attributable to shareholders of common stock is computed by dividing the Company’s
net income (loss) attributable to common shareholders by the weighted-average number of shares of common stock used in the income (loss)
per share calculation during the period. Diluted net income (loss) per share attributable to shareholders of common stock is computed
by giving effect to all potentially dilutive securities.
Segments—The
Company operates as a single operating and reportable segment. Operating segments are defined as components of an enterprise for which
separate discrete financial information is evaluated regularly by the chief operating decision maker (“CODM”) in deciding
how to allocate resources and assess the Company’s financial and operational performance. The Company has determined that its Chief
Executive Officer is the CODM. To date, the Company’s CODM has made such decisions and assessed performance at the Company-level.
Recently Adopted Accounting Standards—
In January 2017, the
FASB issued ASU No. 2017-04, Intangibles – Goodwill and Other (Topic 350). This ASU simplifies the subsequent measurement
of goodwill. FASB eliminated the Step 2 analysis from the goodwill impairment test which is meant to reduce the cost and complexity of
evaluating goodwill for impairment. The new guidance is effective for the Company for the annual reporting period beginning after January 1,
2022, with earlier adoption permitted. The Company early adopted this ASU as of January 1, 2021. The adoption of this pronouncement
did not have a material impact on the Company’s consolidated financial statements or its goodwill impairment measurement.
Recently Issued Accounting Standards, Not
Yet Adopted—
In February 2016,
the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-02, Leases (Topic 842) and since
that date has issued subsequent amendments to the initial guidance intended to clarify certain aspects of the guidance and to
provide certain practical expedients entities can elect upon adoption. ASU 2016-02 amends the guidance for lease accounting to
require lease assets and liabilities to be recognized on the balance sheet, along with additional disclosures regarding key leasing
arrangements. The amendments in this ASU are effective for fiscal years beginning after December 15, 2018, and interim periods
within those fiscal years, and early adoption is permitted. Entities must adopt the standard using a modified retrospective
transition and apply the guidance to the earliest comparative period presented, with certain practical expedients that entities may
elect to apply. In June 2020, the FASB issued ASU 2020-05, Revenue from Contracts with Customers (Topic 606)
and Leases (Topic 842), which defers the effective date for one year for entities in the “all other”
category and public not-for-profit entities that have not yet issued their financial statements reflecting the adoption of Leases.
Topic 842 is effective for the Company for the annual period ending December 31, 2022, and interim periods within the annual
period ending December 31, 2023, with earlier adoption permitted. The Company will adopt Topic 842 during the annual period
ending December 31, 2022 using the modified retrospective method and as a result will not restate prior periods. The Company
intends to elect the practical expedients provided in the new ASUs that (i) allow historical lease classification of existing
leases, (ii) allow the Company to not reassess whether any expired or existing contracts contain leases, and (iii) allow
the Company to not reassess initial direct costs for any existing leases. The Company is currently evaluating its lease agreements
and summarizing key contract terms and financial information associated with each lease agreement in order to assess the impact the
adoption of ASU 2016-02 will have on its consolidated financial statements. Based on the Company’s current lease portfolio,
the Company preliminarily expects Topic 842 to have a material impact on its consolidated balance sheet primarily related to the
recognition of operating lease assets and liabilities. The Company does not expect the adoption to have a material impact on the
Company’s consolidated statement of operations or on its consolidated statement of cash flows.
In February 2016, the
FASB issued ASU No. 2016-13, Financial Statements—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments.
This ASU adds an impairment model known as current expected credit loss that is based on expected losses rather than incurred losses.
It recognizes an allowance as its estimate of expected credit losses, which may result in more timely recognition of such losses. In 2019,
the FASB issued ASU 2019-10, Financial Instruments—Credit Losses (Topic 326), Derivatives and Hedging (Topic 815),
and Leases (Topic 842), which defers the effective date for entities in the “all other” category and public
not-for-profit entities that have not yet issued their financial statements reflecting the adoption of Credit Losses. The amendments in
this ASU are effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years, and early
adoption is permitted. This guidance is effective for the Company for the annual period beginning on January 1, 2023, and interim
periods within the annual period beginning on January 1, 2023. The Company’s adoption of this standard is not expected to have
a material impact on the Company’s consolidated financial statements.
In December 2019, the
FASB issued ASU No. 2019-12, Income Taxes—Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU
2019-12”). ASU 2019-12 simplifies the accounting for income taxes by removing certain exceptions to the general principles in Topic
740. The amendments also improve consistent application of and simplify GAAP for other areas of Topic 740 by clarifying and amending existing
guidance. ASU 2019-12 is effective for the Company for annual period beginning on January 1, 2022, and interim periods within the
annual period beginning on January 1, 2023. The amendments do not create new accounting requirements. The adoption of this standard
is not expected to have a material impact on the Company’s consolidated financial statements.
In March 2020, the FASB
issued ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting.
This ASU provides relief that, if elected, will require less accounting analysis and less accounting recognition for modifications related
to reference rate reform. This update provides optional guidance for a limited period of time to ease the potential burden in accounting
for reference rate reform on financial reporting. The amendments in the update apply only to contracts, hedging relationships, and other
transactions that reference the London Inter-Bank Offered Rate (“LIBOR”) or another reference rate expected to be discontinued
because of reference rate reform. The amendments in this ASU were effective upon issuance and may be applied through December 31,
2022. The Company is implementing a transition plan to modify its debt with interest rates that are influenced by LIBOR. The Company is
continuing to assess ASU 2020-04 and its impact on the Company’s transition away from LIBOR for its debt.
The Company disaggregates
its revenue from contracts with customers by sales channel, as well as by revenue source and geographic region, based on the location
of the end customer, as it believes it best depicts how the nature, amount, timing and uncertainty of its revenue and cash flows are affected
by economic factors. Total revenue based on the disaggregation criteria was as follows (in thousands):
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Revenue by Sales Channel | |
| | | |
| | | |
| | |
| Medical | |
$ | 198,592 | | |
$ | 82,532 | | |
$ | 114,585 | |
| Clinical | |
| 6,268 | | |
| 1,613 | | |
| 2,500 | |
| Other | |
| 1,209 | | |
| — | | |
| — | |
| Total: | |
$ | 206,069 | | |
$ | 84,145 | | |
$ | 117,085 | |
| | |
| | | |
| | | |
| | |
| Revenue by Geographic Region | |
| | | |
| | | |
| | |
| North America | |
$ | 92,771 | | |
$ | 55,389 | | |
$ | 85,025 | |
| Asia Pacific | |
| 91,908 | | |
| 16,696 | | |
| 20,496 | |
| Rest of the World | |
| 15,678 | | |
| 6,156 | | |
| 5,225 | |
| Net product sales | |
$ | 200,357 | | |
$ | 78,241 | | |
$ | 110,746 | |
| Asia Pacific Royalties | |
| 5,712 | | |
| 5,904 | | |
| 6,339 | |
| Total: | |
$ | 206,069 | | |
$ | 84,145 | | |
$ | 117,085 | |
For the year ended December 31,
2021, the countries that accounted for more than 10% of the Company’s total revenues were the United States, Vietnam, and China,
with net product sales amounting to $90,103, $52,446, and $27,364, respectively. For the year ended December 31, 2020, the United
States was the only country that accounted for more than 10% of the Company’s total revenues, amounting to net product sales of
$54,714. For the year ended December 31, 2019, the countries that accounted for more than 10% of the Company’s total revenues
were the United States and Vietnam, with net product sales amounting to $84,143 and $11,638, respectively.
For the year ended December 31,
2021, two customers accounted for 34% and 25%, respectively, of the Company’s revenue. For the year ended December 31, 2020,
one customer accounted for 54% of the Company’s revenue. For the year ended December 31, 2019, two customers accounted for
61% and 10%, respectively, of the Company’s revenue.
Services Provided by the Customer
Consideration payable
to a customer for distinct goods or services is treated as a purchase for an amount up to the fair value of those distinct goods or services.
The Company recognizes consideration payable to its customers as an operating expense for distinct services which include marketing, technology
support, program management, and regulatory compliance. When consideration payable for the distinct services exceeds the fair value of
services provided by the customer, the Company records those excess amounts as a reduction of the transaction price in the arrangement.
These services are provided by certain customers that are distributors. Consideration payable to a customer for non-distinct services,
mainly traditional distribution services such as packing and shipping, is recorded as a reduction to the transaction price. In cases where
the Company cannot reasonably estimate the fair value of the goods or services received from the customer, it will recognize the consideration
payable as a reduction of the transaction price.
The expenses recognized relating to distinct
services provided by customers are as follows (in thousands):
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Selling, general and administrative | |
$ | 34,010 | | |
$ | 3,545 | | |
$ | 5,426 | |
| Research and development | |
| 2,072 | | |
| — | | |
| — | |
| Total | |
$ | 36,082 | | |
$ | 3,545 | | |
$ | 5,426 | |
| 4. | ACCOUNTS AND NOTE RECEIVABLE, NET |
As of December 31,
2021, accounts receivable, net consisted of accounts receivable of $60,929, less allowance for doubtful accounts of $62. As of December 31,
2020, accounts receivable, net consisted of accounts receivable of $37,139, less allowance for doubtful accounts of $62.
The change in the allowance for doubtful accounts
are as follows:
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | |
| Balance at beginning of period | |
| 62 | | |
| — | |
| Provision for bad debts | |
| — | | |
| 62 | |
| Write-off of uncollectible accounts | |
| — | | |
| — | |
| Balance at end of period | |
| 62 | | |
| 62 | |
On July 30, 2021,
and as amended on December 31, 2021, Obagi Cosmeceuticals LLC, a wholly owned subsidiary of the Company, entered into a non-recourse,
uncollateralized short-term promissory note, not in the ordinary course of business, lending a third party $2,500 (the “Note Receivable”).
This Note Receivable matures on April 30, 2022 and carries an interest rate of 1.00% from July 30, 2021 to September 29,
2021 and 8.00% from September 30, 2021 through maturity. The outstanding principal and accrued interest are due upon maturity. In
connection with the proposed merger described in Note 17, the Note Receivable will be carved out and distributed to the Company’s
shareholder, Cedarwalk, in exchange for cash.
The components of inventories were as follows
(in thousands):
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| Work in process | |
$ | 1,619 | | |
$ | 1,937 | |
| Finished goods | |
| 17,720 | | |
| 12,024 | |
| Goods in transit | |
| — | | |
| 19 | |
| Total | |
$ | 19,339 | | |
$ | 13,980 | |
| 6. | PROPERTY
AND EQUIPMENT—NET |
Property and equipment, net consisted of the
following (in thousands):
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| Computer software and website development costs | |
$ | 4,334 | | |
$ | 1,795 | |
| Other property and equipment | |
| 83 | | |
| 93 | |
| Total property and equipment | |
| 4,417 | | |
| 1,888 | |
| Less accumulated depreciation | |
| (833 | ) | |
| (266 | ) |
| Property and equipment, net | |
$ | 3,584 | | |
$ | 1,622 | |
Depreciation expense
for property and equipment for the years ended December 31, 2021, 2020, and 2019 was $584, $118, and $104, respectively. Depreciation
expense during these years pertain to property and equipment utilized as part of the Company’s selling, general and administrative
activities and therefore has not been allocated to cost of goods sold.
Intangible assets, net consisted of the following
as of December 31, 2021 (in thousands):
| | |
Weighted
Average
Useful
Lives (Years) | | |
Gross
Carrying
Amount | | |
Accumulated
Amortization | | |
Net
Carrying
Amount | |
| Trademarks | |
10 | | |
$ | 46,004 | | |
$ | (17,842 | ) | |
$ | 28,162 | |
| Customer lists | |
10 | | |
| 39,370 | | |
| (16,404 | ) | |
| 22,966 | |
| Supply agreement | |
10 | | |
| 25,570 | | |
| (10,654 | ) | |
| 14,916 | |
| Developed technology | |
10 | | |
| 22,863 | | |
| (9,592 | ) | |
| 13,271 | |
| Patents | |
20 | | |
| 270 | | |
| (11 | ) | |
| 259 | |
| Total | |
| | |
$ | 134,077 | | |
$ | (54,503 | ) | |
$ | 79,574 | |
Intangible assets, net consisted of the following
as of December 31, 2020 (in thousands):
| | |
Weighted
Average
Useful
Lives (Years) | | |
Gross
Carrying
Amount | | |
Accumulated
Amortization | | |
Net
Carrying
Amount | |
| Trademarks | |
10 | | |
$ | 45,240 | | |
$ | (13,207 | ) | |
$ | 32,033 | |
| Customer lists | |
10 | | |
| 39,370 | | |
| (12,467 | ) | |
| 26,903 | |
| Supply agreement | |
10 | | |
| 25,570 | | |
| (8,097 | ) | |
| 17,473 | |
| Developed technology | |
10 | | |
| 22,863 | | |
| (7,260 | ) | |
| 15,603 | |
| Patents | |
20 | | |
| 85 | | |
| (3 | ) | |
| 82 | |
| Total | |
| | |
$ | 133,128 | | |
$ | (41,034 | ) | |
$ | 92,094 | |
No impairment loss was
recognized for the years ended December 31, 2021, 2020, and 2019. Amortization expense for the years ended December 31, 2021, 2020,
and 2019, was $13,469, $13,308, and $12,836, respectively. Expected amortization for each of the years between 2022 through 2026, and
thereafter are as follows (in thousands):
| Years Ending December 31 | |
| |
| 2022 | |
$ | 13,508 | |
| 2023 | |
| 13,508 | |
| 2024 | |
| 13,345 | |
| 2025 | |
| 13,303 | |
| 2026 | |
| 13,303 | |
| Thereafter | |
| 12,607 | |
| | |
$ | 79,574 | |
| 8. | OTHER CURRENT LIABILITIES |
The major components of other current liabilities
consisted of the following (in thousands):
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| Accrued salaries and related expenses | |
$ | 6,741 | | |
$ | 5,811 | |
| Accrued marketing expenses | |
| 2,963 | | |
| 33 | |
| Accrued distribution fees | |
| 1,926 | | |
| 2,327 | |
| Accrued interest | |
| — | | |
| 111 | |
| Other | |
| 1,353 | | |
| 225 | |
| Total | |
$ | 12,983 | | |
$ | 8,507 | |
| | |
| |
As of December 31, | |
| | |
Maturity Date | |
2021 | | |
2020 | |
| 2021 Term Loan | |
March 2026 | |
$ | 109,175 | | |
$ | — | |
| 2021 Revolving Credit Facility | |
March 2026 | |
| 15,000 | | |
| — | |
| 2018 Term Loan | |
December 2023 | |
| — | | |
| 71,630 | |
| 2018 Revolving Credit Facility | |
December 2023 | |
| — | | |
| 39,000 | |
| PPP Loan | |
May 2022 | |
| — | | |
| 6,750 | |
| Unamortized debt issuance costs | |
| |
| (5,370 | ) | |
| (2,483 | ) |
| Net carrying amount | |
| |
| 118,805 | | |
| 114,897 | |
| Less: Current portion of long-term debt | |
| |
| (15,382 | ) | |
| (47,034 | ) |
| Total long-term portion | |
| |
$ | 103,423 | | |
$ | 67,863 | |
2021 Credit Agreement
On March 16, 2021, the
Company replaced the 2018 Credit Agreement (described below) for a new financing agreement with a syndicate of lenders, including TCW
Asset Management Company LLC as administrative agent for the lenders. The 2021 Credit Agreement included a term loan of $110,000 (the
“2021 Term Loan”) and a revolving credit facility with borrowing capacity of up to $40,000 (“2021 Revolving Credit Facility”).
Both the 2021 Term Loan and the 2021 Revolving Credit Facility mature on March 16, 2026. The 2021 Term Loan and 2021 Revolving Credit
Facility bear interest at the London Interbank Offered Rate (“LIBOR”) plus an applicable margin, as determined by the Company’s
leverage ratios, and are subject to LIBOR succession provisions. If LIBOR becomes unavailable, the parties will establish an alternate
index rate that gives due consideration to the then prevailing market convention for determining a rate of interest for leveraged syndicated
loans in the United States. In connection with the issuance of the 2021 Credit Agreement, the Company incurred $6,383 of debt issuance
costs. The 2021 Credit Agreement is secured by the assets of the Company.
As of the date these
consolidated financial statements were issued, the Company is in compliance with financial and non-financial debt and other contractual
covenants in the 2021 Credit Agreement.
As of December 31,
2021, the Company had unpaid principal of $109,175, and unamortized debt issuance costs of $3,938 on the 2021 Term Loan. The interest
rate was 8.50% and the accrued interest was $0 as of December 31, 2021. The current portion of the 2021 Term Loan and 2021 Revolving
Credit Facility is $2,750 and $15,000, respectively. The current portion of the unamortized debt issuance costs on the 2021 Term Loan
and 2021 Revolving Credit Facility is and $936 and $1,432, respectively.
2018 Credit Agreement
On December 13, 2018,
the Company entered into a credit agreement (the “2018 Credit Agreement”) with a syndicate of banks, including Wells Fargo
Bank, National Association as administrative agent for the banks (the “Syndicate of Banks”). The 2018 Credit Agreement included
a term loan of $90,000 (the “2018 Term Loan”) and a revolving credit facility with borrowing capacity of up to $35,000 (“2018
Revolving Credit Facility”). Both the 2018 Term Loan and the 2018 Revolving Credit Facility mature on December 13, 2023. In connection
with the issuance of the 2018 Credit Agreement, the Company incurred $2,914 of debt issuance costs. The 2018 Credit Agreement was secured
by the assets of the Company. Both the Term Loan and the Revolving Credit Facility carry an interest rate of LIBOR plus applicable margin,
as determined by the Company’s leverage ratios, and are subject to LIBOR succession provisions. On December 23, 2019, the 2018 Credit
Agreement was amended to revise the definition of Consolidated earnings before interest, taxes, depreciation, and amortization (“Consolidated
EBITDA”) to allow for certain additional adjustments, in relation to the debt covenants.
On March 9, 2020 an increase
in the commitment on the 2018 Revolving Credit Facility was approved by the Syndicate of Banks for an additional $10,000, to $45,000.
On November 9, 2020,
the 2018 Credit Agreement was amended to waive the event of default, adjust the “Applicable Margin Rates” and revise the maximum
percentages allowed for the Consolidated Leverage Ratio as well as the minimum percentages allowed for the Consolidated Fixed Charge Ratio
(as defined in the 2018 Credit Agreement). In addition, the amendment was revised to include minimum consolidated EBITDA levels and minimum
liquidity levels through the end of fiscal year 2021.
As of December 31, 2020,
the Company had unpaid principal of $71,630 and unamortized debt issuance costs of $2,483 on the 2018 Term Loan. The interest rate on
the 2018 Term Loan was 5.50%. Accrued interest was $67 as of December 31, 2020.
The Company recorded
a loss on extinguishment of the 2018 Credit Agreement of $2,317 to loss on extinguishment of debt in the accompanying consolidated statement
of operations and comprehensive income (loss) during the year ended December 31, 2021, which consisted of expensing unamortized debt
issuance costs.
PPP Loan
On May 11, 2020, the
Company received loan proceeds in the amount of $6,750 under the Paycheck Protection Program (“PPP”) from MUFG Union Bank
(the “PPP Loan”). The PPP, established as part of the Coronavirus aid, Relief and Economic Security Act (“CARES Act”),
provided for loans to qualifying businesses for amounts up to 2.5 times of the average monthly payroll expenses of the qualifying business.
The PPP Loan accrued interest at a rate of 1%. The PPP Loan and accrued interest were forgivable after eight or twenty-four weeks as long
as the borrower used the proceeds for eligible purposes, including payroll, benefits, rent and utilities and maintains its payroll levels.
The Company used the proceeds for purposes consistent with the PPP, and in 2021, received approval from MUFG Union Bank and the Small
Business Administration for forgiveness of the full amount of its PPP Loan, inclusive of accrued interest of $74. The Company recognized
a gain on PPP Loan forgiveness of $6,824 for the year ended December 31, 2021.
Scheduled Debt Maturities
Scheduled maturities
under the Company’s 2021 Credit Agreement (excluding unamortized debt issuance costs of $5,370) as of December 31, 2021 are
as follows (in thousands):
| Year Ending December 31, | |
| |
| 2022 | |
$ | 17,750 | |
| 2023 | |
| 5,500 | |
| 2024 | |
| 5,500 | |
| 2025 | |
| 5,500 | |
| 2026 | |
| 89,925 | |
| Total unpaid principal | |
$ | 124,175 | |
| 10. | STOCK BASED COMPENSATION |
On January 26, 2021,
the Company established a Stock Incentive Plan (the “Stock Plan”), under which stock options, stock awards, and restricted
stock units (“Restricted Stock”) of the Company may be granted to eligible employees, directors, and consultants. Under the
Stock Plan, the Company is authorized to issue of a maximum number of 1,500,000 shares of common stock. Incentive stock options must have
an exercise price at or above the fair market value of the stock on the date of the grant. Stock options and Restricted Stock granted
during the year ended December 31, 2021 have service-based and performance-based vesting conditions.
The options vest over
five years, with 25% of options vesting in four equal quarterly installments at the end of each three-month period through the first anniversary
of the grant, and the remaining 75% vesting in a series of five equal annual installments over the five-year period measured from the
grant date. The Restricted Stock vest in five equal annual installments at the end of each year, over the five-year period from the grant
date. Award holders have a ten-year period to exercise the options before they expire. Notwithstanding achievement of the service-based
condition, the options and Restricted Stock do not vest or become exercisable until a qualifying transaction is consummated prior to the
expiration date. A qualifying transaction consists of either a change in control event or an underwritten initial public offering by the
Company of its equity securities on a U.S. or foreign exchange.
The weighted average
fair value per share of the awards granted for stock options during the year is $15.55 and for Restricted Stock is $38.68. The unrecognized
compensation cost as of December 31, 2021 for stock options and Restricted Stock is $9,411 and $12,442, respectively.
Stock option activity for
the year ended December 31, 2021 was as follows:
| | |
Number of
Common
Stock
Options | | |
Weighted
Average
Exercise Price | | |
Weighted
Average
Remaining
Contractual Life | | |
Aggregate
intrinsic
Value
(in thousands) | |
| Outstanding as of January 1, 2021 | |
| — | | |
$ | — | | |
| — | | |
$ | — | |
| Granted | |
| 800,000 | | |
| 41.10 | | |
| 9.1 | | |
| 16,456 | |
| Exercised | |
| — | | |
| — | | |
| — | | |
| — | |
| Forfeited | |
| — | | |
| — | | |
| — | | |
| — | |
| Vested | |
| — | | |
| — | | |
| — | | |
| — | |
| Outstanding as of December 31, 2021 | |
| 800,000 | | |
$ | 41.10 | | |
| 9.1 | | |
$ | 16,456 | |
The fair value of stock
option awards was determined on the grant date using the Black-Scholes valuation model based on the following weighted-average assumptions:
| | |
For the year ended
December 31,
2021 | |
| Risk-free interest rate(1) | |
| 0.68 | % |
| Expected term (years)(2) | |
| 6.2 | |
| Expected stock price volatility(3) | |
| 43.0 | % |
| Dividend yield(4) | |
| N/A | |
| Common stock per share value | |
$ | 38.68 | |
| (1) | The risk-free rate is based on U.S. Treasury securities with
maturities equivalent to the expected term. |
| (2) | The expected term is the estimated length of time the grants
are expected to be outstanding before it is exercised or terminated. This number is calculated as the midpoint between the requisite
service period and the contractual term of the award, as the Company does not have any historical data that would provide a reasonable
basis to estimate the expected term for the option. |
| (3) | The expected price volatility is based on the average of
the historical volatility of comparable public companies over a period consistent with the expected term. |
| (4) | The Company historically made distributions to shareholder
but does not plan to declare dividends in the foreseeable future and therefore assumed a dividend yield of zero. |
Restricted Stock activity for the year ended
December 31, 2021 was as follows:
| | |
Shares | | |
Weighted
Average
Grant Date
Fair Value
per Share | |
| Outstanding as of January 1, 2021 | |
| — | | |
| — | |
| Granted | |
| 243,307 | | |
$ | 38.68 | |
| Exercised | |
| — | | |
| — | |
| Forfeited | |
| — | | |
| — | |
| Vested | |
| — | | |
| — | |
| Outstanding as of December 31, 2021 | |
| 243,307 | | |
$ | 38.68 | |
The Company’s equity
structure consists of a single class of common stock. On December 2, 2020 the Company amended and restated its Memorandum and Articles
of Association, authorizing 25,000,000 shares at $0.50 par value each. As of December 31, 2021, and 2020, the Company had 8,000,002
shares issued and outstanding. Refer to Note 1 for discussion of the Stock Split, which has been reflected retroactively in these consolidated
financial statements. Each share of common stock is entitled to one vote. The Company did not hold any shares as treasury shares as of
the periods presented in the accompanying consolidated financial statements.
The Company may, at the
discretion of its Directors, declare dividends and distributions out of the funds of the Company lawfully available therefor. Payments
of dividends and distributions are limited to realized or unrealized profits of the Company. In the years ended December 31, 2021,
2020, and 2019, the Company, through its wholly owned subsidiary, paid $2,000 (approximately $0.25 per share), $2,044 (approximately $0.26
per share), and $1,575 (approximately $0.20 per share) in dividends, respectively, to its shareholder.
| 12. | NET INCOME (LOSS) PER SHARE |
The following table sets
forth the computation of basic and diluted net income (loss) using the treasury stock method for the years ended December 31, 2021,
2020 and 2019 (in thousands, except for share and per share amounts):
| | |
Years Ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Net (loss) income | |
$ | (70 | ) | |
$ | (9,171 | ) | |
$ | 5,820 | |
| Weighted-average number of shares outstanding – basic and diluted | |
| 8,000,002 | | |
| 8,000,002 | | |
| 8,000,002 | |
| Net (loss) income per share – basic and diluted | |
$ | (0.01 | ) | |
$ | (1.14 | ) | |
$ | 0.73 | |
The following table represents
potential shares of common stock outstanding that were excluded from the computation of diluted net loss per share of common stock as
they are issuable contingent on the occurrence of a qualifying event, and for the periods presented, the necessary conditions have not
been satisfied (see Note 10):
| | |
Years Ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Stock options | |
| 800,000 | | |
| — | | |
| — | |
| Restricted Stock | |
| 243,307 | | |
| — | | |
| — | |
| Total | |
| 1,043,307 | | |
| — | | |
| — | |
| 13. | INCOME TAX EXPENSE (BENEFIT) |
The Company, domiciled
in the Cayman Islands, is subject to taxation in the U.S. and various states jurisdictions. ASC Topic 740, Income Taxes (“ASC 740”)
indicates that the federal statutory income tax rate of a foreign reporting entity be used when preparing the rate reconciliation disclosure.
As such, the Company and its wholly owned subsidiaries use the statutory income tax rate in the Cayman Islands, which is 0%. The Company’s
consolidated pretax income (loss) for the years ended December 31, 2021, 2020 and 2019 were generated by domestic and foreign operations
as follows (in thousands):
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Current provision: | |
| | | |
| | | |
| | |
| Federal | |
$ | — | | |
$ | (363 | ) | |
$ | 84 | |
| State | |
| 58 | | |
| (20 | ) | |
| (83 | ) |
| Foreign | |
| 169 | | |
| 32 | | |
| — | |
| | |
| 227 | | |
| (351 | ) | |
| 1 | |
| Deferred expense: | |
| | | |
| | | |
| | |
| Federal | |
| 9,039 | | |
| (3,915 | ) | |
| (1,471 | ) |
| State | |
| 2,035 | | |
| (828 | ) | |
| (119 | ) |
| Foreign | |
| — | | |
| — | | |
| — | |
| | |
| 11,074 | | |
| (4,743 | ) | |
| (1,590 | ) |
| Net income tax provision | |
$ | 11,301 | | |
$ | (5,094 | ) | |
$ | (1,589 | ) |
The provision for income
taxes for the years ended December 31, 2021, 2020 and 2019 consists of the following (in thousands):
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Income (loss) before income taxes: | |
| | | |
| | | |
| | |
| Federal | |
$ | (7,861 | ) | |
$ | (20,652 | ) | |
$ | (6,891 | ) |
| Foreign | |
| 19,092 | | |
| 6,387 | | |
| 11,122 | |
| Total | |
$ | 11,231 | | |
$ | (14,265 | ) | |
$ | 4,231 | |
The components of income tax expense relate
to the following (in thousands):
| | |
For the years Ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Income tax benefit at Cayman Islands statutory rate | |
| 0.0 | % | |
| 0.0 | % | |
| 0.0 | % |
| U.S./foreign tax rate differential | |
| (13.2 | )% | |
| 30.1 | % | |
| (34.1 | )% |
| State income tax benefit, net of federal benefit | |
| 0.2 | % | |
| 4.7 | % | |
| (4.0 | )% |
| Permanent Items | |
| 0.2 | % | |
| (0.6 | )% | |
| 2.4 | % |
| PPP Loan forgiveness | |
| (12.7 | )% | |
| 0.0 | % | |
| 0.0 | % |
| True-Ups | |
| (1.5 | )% | |
| 0.0 | % | |
| (0.5 | )% |
| Tax credits | |
| 0.0 | % | |
| 1.4 | % | |
| (2.4 | )% |
| Other | |
| 0.0 | % | |
| 0.0 | % | |
| 1.0 | % |
| Change in valuation allowance | |
| 127.6 | % | |
| 0.0 | % | |
| 0.0 | % |
| Total income tax expense (benefit) | |
| 100.6 | % | |
| 35.6 | % | |
| (37.6 | )% |
Deferred income taxes
reflect the net tax effects of (a) temporary differences between the carrying amounts of assets and liabilities for financial reporting
purposes and the amounts used for income tax purposes, and (b) operating losses and tax credit carryforwards. The tax effects of temporary
differences that give rise to portions of the deferred tax assets and deferred tax liabilities as of December 31, 2021 and 2020 are presented
below (in thousands):
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| Deferred tax assets: | |
| | |
| |
| Accrued interest to foreign related parties | |
$ | 581 | | |
$ | 509 | |
| Intangibles | |
| 4,110 | | |
| 2,434 | |
| Formation costs | |
| 1,629 | | |
| 1,807 | |
| Net operating losses | |
| 6,214 | | |
| 4,050 | |
| Other temporary differences | |
| 1,235 | | |
| 876 | |
| Accrued compensation | |
| 793 | | |
| 694 | |
| R&D tax credits | |
| 379 | | |
| 495 | |
| Non-deductible interest carryover | |
| 1,186 | | |
| 1,519 | |
| Transaction costs | |
| 1,230 | | |
| — | |
| Total deferred tax assets | |
| 17,357 | | |
| 12,384 | |
| Deferred tax liabilities: | |
| | | |
| | |
| Goodwill | |
| (2,795 | ) | |
| (1,475 | ) |
| Fixed asset basis | |
| (774 | ) | |
| (308 | ) |
| Capitalized software costs | |
| — | | |
| (75 | ) |
| Total deferred tax liabilities | |
| (3,569 | ) | |
| (1,858 | ) |
| Net deferred tax assets | |
| 13,788 | | |
| 10,526 | |
| Less: valuation allowance | |
| (14,336 | ) | |
| — | |
| Net deferred tax (liabilities) assets | |
$ | (548 | ) | |
$ | 10,526 | |
Management assesses the
available positive and negative evidence to estimate whether sufficient future taxable income will be generated to permit use of the existing
deferred tax assets. A significant piece of objective negative evidence evaluated was a cumulative loss incurred in our U.S. subsidiary
over the three-year period ended December 31, 2021. Such objective evidence limits the ability to consider other subjective evidence,
such as projections for future growth. On the basis of this evaluation, as of December 31, 2021, a valuation allowance of $14,336
has been recorded to recognize the portion of the deferred tax asset that is more likely than not to be realized. The amount of the deferred
tax asset considered realizable, however, could be adjusted if estimates of future taxable income during the carryforward period are reduced
or increased or if objective negative evidence in the form of cumulative losses is no longer present and additional weight is given to
subjective evidence such as our projections for growth.
The valuation allowance rollforward is summarize
below:
| | |
For the year
ended
December31, | |
| | |
2021 | |
| Balance at beginning of period | |
| — | |
| Charged to costs and expenses | |
| 14,336 | |
| Charged to other accounts | |
| — | |
| Deductions | |
| — | |
| Balance at end of period | |
| 14,336 | |
Net operating losses and tax credit carryforwards
as of December 31, 2021 were as follows (in thousands):
| | |
Amount | | |
Expiration Year |
| Net operating losses, federal | |
$ | 26,386 | | |
Do Not Expire |
| Net operating losses, state | |
$ | 27,466 | | |
2040-2041 |
| Tax Credits, federal | |
$ | 283 | | |
2040-2041 |
| Tax Credits, state | |
$ | 121 | | |
Do Not Expire |
Pursuant to IRC Sections
382 and 383, annual use of the Company’s net operating losses (“NOLs”) and R&D credit carryforwards may be limited
in the event a cumulative change in ownership of more than 50.0% occurs within a three-year period. The Company has not undergone an analysis
to determine whether this limitation would apply to the utilization of the net operating loss carryforward. However, as the federal NOLs
do not expire, the Company does not believe that any potential limitations to state NOL or federal credit carryforwards, if applicable,
would be material to the financial statements.
The Company recognizes
a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon examination, including
resolutions of any related appeals or litigation processes, based on the technical merits, and uncertain income tax positions must meet
a more likely than not recognition threshold to be recognized. The Company recognizes interest and penalties related to unrecognized tax
benefits within the income tax expense line in the consolidated statements of operations and comprehensive income (loss). There were no
unrecognized tax benefits as of December 31, 2021.
As of December 31,
2021, there were no active taxing authority examinations in any of the Company’s major tax jurisdictions. The Company remains subject
to examination for federal and state income tax purposes for the tax years ending 2018 through 2021.
| 14. | RELATED PARTY TRANSACTIONS |
There were no related party transactions during
the year ended December 31, 2021.
In 2020, the Company’s
shareholder paid $4,058 to register the Company’s products with the National Medical Products Administration in China. This non-cash
capital contribution was recorded as additional paid-in capital in the consolidated statements of shareholder’s equity.
In 2019, the Company
paid $1,959 in accrued interest to a shareholder related to a shareholder loan, the principal of which was repaid in 2018 upon refinancing
with a third-party syndicate of banks.
| 15. | COMMITMENTS AND CONTINGENCIES |
Legal Proceedings
From time to time, the
Company may be a party to litigation and subject to claims incidental to its business. Although the results of litigation and claims cannot
be predicted with certainty, the Company currently believes that the final outcome of these matters will not have a material adverse effect
on its business. Regardless of the outcome, litigation can have an adverse impact on the Company because of judgment, defense and settlement
costs, diversion of management resources, and other factors. At each reporting period, the Company evaluates whether or not a potential
loss amount or a potential range of loss is probable and reasonably estimable, requiring recognition of a loss accrual, or whether the
potential loss is reasonably possible, requiring potential disclosure. Legal fees are expensed as incurred.
Operating Lease Obligations
The Company leases office
space under three non-cancelable operating leases expiring between September 2023 and February 2032. Rent expense related to operating
leases was $1,177, $1,057 and $1,051 for the years ended December 31, 2021, 2020 and 2019, respectively.
As of December 31,
2021, future minimum lease payments under all noncancelable operating leases with an initial lease term in excess of one year were as
follows (in thousands):
| Year Ending December 31 | |
| |
| 2022 | |
$ | 1,544 | |
| 2023 | |
| 1,625 | |
| 2024 | |
| 1,456 | |
| 2025 | |
| 1,490 | |
| 2026 | |
| 844 | |
| Thereafter | |
| 1,911 | |
| | |
$ | 8,870 | |
The Company sponsors
a Section 401(k) retirement plan (“plan”) for employees. During the years ended December 31, 2021, 2020 and 2019, the
Company’s contributions to the plan were $612, $509 and $451, respectively.
The Company evaluated
subsequent events through April 26, 2022, the date on which the December 31, 2021 financial statements were originally available to be
issued, and June 15, 2022, the date on which the December 31, 2021 financial statements were available to be reissued.
In March 2022, the Company
entered into a lease agreement for approximately 16,470 square feet of office space in Woodlands, Texas. The initial lease term is ten
years and four months, with two optional five-year renewal periods. The future minimum rent commitment is approximately $5,500.
| 18. | CONDENSED FINANCIAL INFORMATION OF OBAGI GLOBAL HOLDINGS
LIMITED (PARENT COMPANY ONLY) |
Obagi Global is a holding
company with no material operations of its own that conducts substantially all of its activities through its wholly owned subsidiaries.
Obagi Global has no cash and, as a result, all expenditures and obligations of Obagi Global are allocated to and paid by its subsidiaries.
Obagi Cosmeceuticals, LLC is the borrower under the 2021 Credit Agreement and the since extinguished 2018 Credit Agreement. The terms
and conditions of the credit agreements limit the ability of Obagi Global and its wholly owned subsidiaries to declare dividends or make
other distributions, directly or indirectly, to the Company’s shareholder, subject to certain enumerated exceptions, including but
not limited to, dividends up to a specified amount if the Company is able to achieve certain “Consolidated Total Leverage Ratios”
thresholds. Due to the aforementioned restrictions, substantially all of the net assets of Obagi Global’s subsidiaries are restricted.
The following condensed
financial statements have been presented on a “parent-only” basis. Under a parent-only presentation, Obagi Global’s
investment in its subsidiaries is presented under the equity method of accounting. During the year ended December 31, 2020, Obagi
Global’s shareholder, on behalf of a wholly owned subsidiary, paid $4,058 to register the Company’s products with the National
Medical Products Administration in China. This non-cash capital contribution was recorded as additional paid-in capital. A condensed statement
of cash flows is not presented because Obagi Global has no cash, and, therefore, no material operating, investing, or financing cash flow
activities for the years ended December 31, 2021, 2020 and 2019. During the years ended December 31, 2021, 2020 and 2019, the
payments of dividends were non-cash transactions for Obagi Global, as the cash outflows associated with those transactions occurred at
the level of Obagi Holdings Company Limited, a wholly owned subsidiary of Obagi Global.
OBAGI GLOBAL HOLDINGS LIMITED
(PARENT COMPANY ONLY)
CONDENSED BALANCE SHEETS
(In thousands of U.S. dollars, except share
and per share data)
| | |
As of December 31, | |
| | |
2021 | | |
2020 | |
| ASSETS | |
| | |
| |
| Current assets | |
$ | — | | |
$ | — | |
| Noncurrent assets | |
| | | |
| | |
| Investment in subsidiaries | |
| 89,290 | | |
| 91,392 | |
| TOTAL ASSETS | |
$ | 89,290 | | |
$ | 91,392 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDER’S EQUITY | |
| | | |
| | |
| Current liabilities | |
$ | — | | |
$ | — | |
| Noncurrent liabilities | |
| — | | |
| — | |
| Total liabilities | |
| — | | |
| — | |
| | |
| | | |
| | |
| SHAREHOLDER’S EQUITY: | |
| | | |
| | |
| Common stock, 25,000,000 shares authorized; $0.50 par value; 8,000,002 shares issued and outstanding as of December 31, 2021, and 2020, respectively (Note 1) | |
| 4,000 | | |
| 4,000 | |
| Additional paid-in capital | |
| 100,113 | | |
| 100,113 | |
| Accumulated deficit | |
| (14,798 | ) | |
| (12,728 | ) |
| Accumulated other comprehensive (loss) income | |
| (25 | ) | |
| 7 | |
| TOTAL SHAREHOLDER’S EQUITY | |
$ | 89,290 | | |
$ | 91,392 | |
OBAGI GLOBAL HOLDINGS LIMITED
(PARENT COMPANY ONLY)
CONDENSED STATEMENTS OF OPERATIONS AND
COMPREHENSIVE INCOME (LOSS)
(In thousands of U.S. dollars, except share
and per share data)
| | |
For the years ended December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Net revenue | |
$ | — | | |
$ | — | | |
$ | — | |
| Selling, general and administrative | |
| — | | |
| — | | |
| — | |
| Total operating income | |
| — | | |
| — | | |
| — | |
| Other (loss) income | |
| — | | |
| — | | |
| — | |
| Equity earnings of consolidated subsidiaries | |
| (70 | ) | |
| (9,171 | ) | |
| 5,820 | |
| (Loss) income before income taxes | |
| (70 | ) | |
| (9,171 | ) | |
| 5,820 | |
| Income tax benefit | |
| — | | |
| — | | |
| — | |
| Net (loss) income | |
| (70 | ) | |
| (9,171 | ) | |
| 5,820 | |
| Other comprehensive (loss) income — Foreign currency translation adjustments, net of tax | |
| (32 | ) | |
| 16 | | |
| (9 | ) |
| Comprehensive (loss) income | |
$ | (102 | ) | |
$ | (9,155 | ) | |
$ | 5,811 | |
OBAGI
GLOBAL HOLDINGS LIMITED
CONDENSED
CONSOLIDATED BALANCE SHEETS (UNAUDITED)
(In
thousands of U.S. dollars, except share and per share data)
| | |
As of | |
| | |
June 30,
2022 | | |
December 31,
2021 | |
| ASSETS | |
| | |
| |
| CURRENT ASSETS: | |
| | |
| |
| Cash and cash equivalents | |
$ | 6,742 | | |
$ | 12,794 | |
| Restricted cash | |
| 650 | | |
| 650 | |
| Accounts and note receivable, net | |
| 77,665 | | |
| 63,367 | |
| Inventories | |
| 27,586 | | |
| 19,339 | |
| Prepaid expenses | |
| 8,086 | | |
| 7,944 | |
| Other current assets | |
| 374 | | |
| 335 | |
| Total current assets | |
| 121,103 | | |
| 104,429 | |
| | |
| | | |
| | |
| Property and equipment, net | |
| 3,777 | | |
| 3,584 | |
| Intangible assets, net | |
| 73,069 | | |
| 79,574 | |
| Goodwill | |
| 44,489 | | |
| 44,489 | |
| Other assets | |
| 1,274 | | |
| 1,497 | |
| TOTAL ASSETS | |
$ | 243,712 | | |
$ | 233,573 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDER’S EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payable | |
$ | 18,323 | | |
$ | 11,375 | |
| Current portion of long-term debt, net | |
| 22,603 | | |
| 15,382 | |
| Other current liabilities | |
| 14,417 | | |
| 12,983 | |
| Total current liabilities | |
| 55,343 | | |
| 39,740 | |
| | |
| | | |
| | |
| Long-term debt, net | |
| 100,764 | | |
| 103,423 | |
| Deferred income tax liabilities | |
| 548 | | |
| 548 | |
| Other liabilities | |
| 619 | | |
| 572 | |
| Total
liabilities | |
| 157,274 | | |
| 144,283 | |
| | |
| | | |
| | |
| COMMITMENTS AND CONTINGENCIES (Note 15) | |
| | | |
| | |
| SHAREHOLDER’S EQUITY: | |
| | | |
| | |
| Common stock, 25,000,000 shares authorized; $0.50 par value; 8,000,002 shares issued and outstanding as of June 30, 2022 and December 31, 2021 | |
| 4,000 | | |
| 4,000 | |
| Additional paid-in capital | |
| 100,113 | | |
| 100,113 | |
| Accumulated deficit | |
| (17,713 | ) | |
| (14,798 | ) |
| Accumulated other comprehensive income (loss) | |
| 38 | | |
| (25 | ) |
| Total shareholder’s equity | |
| 86,438 | | |
| 89,290 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES AND SHAREHOLDER’S EQUITY | |
$ | 243,712 | | |
$ | 233,573 | |
See
accompanying notes to condensed consolidated financial statements.
OBAGI
GLOBAL HOLDINGS LIMITED
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE (LOSS) INCOME (UNAUDITED)
(In
thousands of U.S. dollars, except share and per share data)
| | |
Six months ended | |
| | |
June 30, | |
| | |
2022 | | |
2021 | |
| Net revenue | |
$ | 106,440 | | |
$ | 94,204 | |
| Cost of goods sold (exclusive of depreciation and amortization shown separately below) | |
| 24,701 | | |
| 23,463 | |
| Selling, general and administrative | |
| 68,418 | | |
| 45,698 | |
| Research and development | |
| 3,262 | | |
| 2,534 | |
| Depreciation and amortization | |
| 7,369 | | |
| 6,936 | |
| Total operating expenses | |
| 103,750 | | |
| 78,631 | |
| Operating income | |
| 2,690 | | |
| 15,573 | |
| Interest expense | |
| 5,719 | | |
| 5,041 | |
| Loss on extinguishment of debt | |
| - | | |
| 2,317 | |
| Gain on PPP Loan forgiveness (Note 9) | |
| - | | |
| (6,824 | ) |
| Other (income) expense, net | |
| (74 | ) | |
| 145 | |
| (Loss) income before income taxes | |
| (2,955 | ) | |
| 14,894 | |
| Income tax (benefit) expense | |
| (40 | ) | |
| 1,948 | |
| Net (loss) income | |
| (2,915 | ) | |
| 12,946 | |
| Other comprehensive Income—Foreign currency translation adjustments, net of tax | |
| 63 | | |
| 1 | |
| Comprehensive (loss) income | |
$ | (2,852 | ) | |
$ | 12,947 | |
| | |
| | | |
| | |
| Net (loss) income per share of common stock — Basic and diluted | |
$ | (0.36 | ) | |
$ | 1.62 | |
| Weighted average shares of common stock outstanding — Basic and diluted (Note 12) | |
| 8,000,002 | | |
| 8,000,002 | |
See
accompanying notes to condensed consolidated financial statements.
OBAGI
GLOBAL HOLDINGS LIMITED
CONDENSED
CONSOLIDATED STATEMENTS OF SHAREHOLDER’S EQUITY (UNAUDITED)
FOR
THE SIX MONTHS ENDED JUNE 30, 2022 AND 2021
(In
thousands of U.S. dollars, except share data)
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| |
| | |
| | |
| | |
Additional | | |
| | |
Other | | |
Total | |
| | |
Common Stock | | |
Paid-In | | |
Accumulated | | |
Comprehensive | | |
Shareholder’s | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Income (Loss) | | |
Equity | |
| BALANCE—January 1, 2021 | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 100,113 | | |
$ | (12,728 | ) | |
$ | 5 | | |
$ | 91,390 | |
| Net income | |
| - | | |
| - | | |
| - | | |
| 12,946 | | |
| - | | |
| 12,946 | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1 | | |
| 1 | |
| Dividends | |
| - | | |
| - | | |
| - | | |
| (1,998 | ) | |
| - | | |
| (1,998 | ) |
| BALANCE—June 30, 2021 | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 100,113 | | |
$ | (1,780 | ) | |
$ | 6 | | |
$ | 102,339 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE—January 1, 2022 | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 100,113 | | |
$ | (14,798 | ) | |
$ | (25 | ) | |
$ | 89,290 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (2,915 | ) | |
| - | | |
| (2,915 | ) |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| 63 | | |
| 63 | |
| BALANCE—June 30, 2022 | |
| 8,000,002 | | |
$ | 4,000 | | |
$ | 100,113 | | |
$ | (17,713 | ) | |
$ | 38 | | |
$ | 86,438 | |
See
accompanying notes to condensed consolidated financial statements.
OBAGI
GLOBAL HOLDINGS LIMITED
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
(In
thousands of U.S. dollars)
| |
|
Six
months ended
June 30, |
|
| |
|
2022 |
|
|
2021 |
|
| CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
| Net (loss) income |
|
$ |
(2,915 |
) |
|
$ |
12,946 |
|
| Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
|
7,369 |
|
|
|
6,936 |
|
| Amortization of debt issuance costs |
|
|
680 |
|
|
|
538 |
|
| Loss on extinguishment of debt |
|
|
- |
|
|
|
2,317 |
|
| Gain on PPP loan forgiveness |
|
|
- |
|
|
|
(6,824 |
) |
| Loss on disposal of equipment |
|
|
- |
|
|
|
52 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Accounts receivable |
|
|
(14,298 |
) |
|
|
(14,276 |
) |
| Inventories |
|
|
(8,247 |
) |
|
|
3,809 |
|
| Prepaid expenses |
|
|
(142 |
) |
|
|
(3,564 |
) |
| Other current assets and other assets |
|
|
184 |
|
|
|
- |
|
| Accounts payable |
|
|
7,134 |
|
|
|
5,625 |
|
| Other current liabilities and other liabilities |
|
|
1,481 |
|
|
|
2,969 |
|
| Net cash (used in) provided by operating activities |
|
|
(8,754 |
) |
|
|
10,528 |
|
| CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
| Capital expenditure on intangible assets |
|
|
(261 |
) |
|
|
(363 |
) |
| Capital expenditure on property and equipment |
|
|
(920 |
) |
|
|
(507 |
) |
| Net cash used in investing activities |
|
|
(1,181 |
) |
|
|
(870 |
) |
| CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
| Payment of dividends |
|
|
- |
|
|
|
(750 |
) |
| Proceeds from term loan |
|
|
- |
|
|
|
110,000 |
|
| Proceeds from revolving credit facility |
|
|
6,000 |
|
|
|
20,000 |
|
| Repayment of revolving credit facility |
|
|
- |
|
|
|
(44,000 |
) |
| Repayment of term loan |
|
|
(1,375 |
) |
|
|
(71,905 |
) |
| Payment of debt issuance costs |
|
|
(742 |
) |
|
|
(6,383 |
) |
| Net cash provided by financing activities |
|
|
3,883 |
|
|
|
6,962 |
|
| CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH |
|
|
(6,052 |
) |
|
|
16,620 |
|
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH—Beginning of period |
|
|
13,444 |
|
|
|
8,572 |
|
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH—End of period |
|
|
7,392 |
|
|
|
25,192 |
|
See
accompanying notes to condensed consolidated financial statements.
OBAGI
Global HOldings LIMITED
NOTES
TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
AS
OF June 30, 2022 and December 31, 2021 AND FOR THE six months ENDED June 30, 2022 and 2021
(In
thousands of U.S. dollars, except share and per share data)
1. DESCRIPTION
OF BUSINESS
Obagi
Global Holdings Limited (“Obagi Global”) is a holding company incorporated in the Cayman Islands that conducts all operations
through its wholly owned subsidiaries (collectively the “Company” or “Obagi”). The Company is a global skincare
company that develops, markets, and sells proprietary-topical aesthetic and therapeutic prescription-strength skincare systems and related
products primarily in the physician-dispensed market. Obagi provides cosmetic, over-the-counter (“OTC”) and prescription
products designed to prevent or improve the most common and visible skin disorders in adult skin, including premature aging, photo damage,
hyperpigmentation (irregular or patchy discoloration of the skin), acne, sun damage, facial redness, and soft tissue deficits, such as
fine lines and wrinkles. The Company is headquartered in Long Beach, California.
On
July 15, 2021, ZhongHua Finance Acquisition Fund I, L.P., the Company’s sole shareholder, transferred its shares to its affiliate,
Cedarwalk Skincare Ltd. (“Cedarwalk”), which is now the sole shareholder of the Company. This transfer between affiliates
did not result in any change of control.
Business
Combination
On
November 15, 2021, Waldencast Acquisition Corp. (“Waldencast”) (NASDAQ: WALD), a special purpose acquisition company, entered
into an Agreement and Plan of Merger (the “Merger Agreement”) with the Company and Obagi Merger Sub, Inc., indirect wholly
owned subsidiaries of Waldencast (“Merger Sub”). On July 27, 2022 the merger was completed and the Merger Sub merged with
and into the Company (excluding its operations in the China Region, as defined below), the separate corporate existence of Merger Sub
ceased to exist, with the Company surviving as an indirect wholly owned subsidiary of Waldencast. Concurrently with the Merger Agreement,
Waldencast entered into an Equity Purchase Agreement with Milk Makeup LLC (“Milk”) (together, the transactions contemplated
in the Merger Agreement and Equity Purchase Agreement, the “Business Combination”) and acquired all of the issued and outstanding
membership units of Milk. Following the Business Combination, the combined company is organized in an “Up-C” structure, in
which the equity interests of the Company and Milk are held by Waldencast LP. Waldencast plc’s interests in the Company and Milk
are held through its wholly owned subsidiaries, Holdco 1 and Waldencast LP.
Waldencast
acquired the Company at an enterprise value of approximately $858,000 excluding 100% of the business of Obagi Hong Kong Limited and certain
related entities and assets (the “Obagi China Business”), consisting of approximately $317,000 in cash, and $246,000 in Class
A ordinary shares of the post-combination entity, Waldencast plc (as defined in the Plan of Domestication).
Immediately
prior to the closing of the Business Combination, the Company carved out and distributed the Note Receivable (as defined below) and
its operations in the People’s Republic of China, inclusive of the Hong Kong Special Administrative Region, the Macau Special
Administrative Region, and Taiwan (the “China Region”) to its shareholder, Cedarwalk.
In
connection with the distribution, and at the time of the Business Combination, the Company entered into an Intellectual Property License
Agreement (the “IP License Agreement”) with the Obagi China Business. Under the IP License Agreement, the Company will exclusively
license intellectual property relating to the Obagi brand to the Obagi China Business, and the Company retains the rights to such intellectual
property to conduct the Obagi-branded business worldwide except for the China Region. The Obagi China Business will pay the Company a
royalty of five and a half percent (5.5%) of gross sales of licensed products and services, less taxes and refunded returns.
In
connection with the distribution, and at the time of the Business Combination, the Company entered into a Global Supply Services Agreement
(the “Supply Agreement”) with the Obagi China Business, pursuant to which the Company will supply, or cause to be supplied
through certain CMOs (as defined in the Supply Agreement), products to the Obagi China Business and its affiliates, for the Obagi China
Business’s distribution and sale in the China Region. The term of the Supply Agreement is perpetual, subject to termination for
material breach and failure to cure or termination in the event that the IP License Agreement is terminated.
In
connection with the distribution, and at the time of the Business Combination, the Company entered into a Transition Services Agreement
(the “Transition Services Agreement”) with the Obagi China Business, for the provision of certain transition services to
enable the Obagi China Business to conduct Obagi-branded business as a going concern in the China Region. The transition services will
be provided for an initial term of up to twelve (12) months, with an option for the Obagi China Business to extend the service period
for up to an additional twelve (12) months solely as to certain research and development services. Services will be charged at the reasonable,
fully-loaded costs of providing the services, but such services will be provided at no charge for a certain period of time or up to a
specified amount of services.
2. SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation—These
condensed consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles (“GAAP”)
and applicable rules and regulations of the Securities and Exchange Commission (“SEC”) regarding interim financial reporting.
Certain information and note disclosures normally included in the financial statements prepared in accordance with GAAP have been condensed
or omitted pursuant to such rules and regulations. Accordingly, these interim condensed consolidated financial statements should be read
in conjunction with the audited financial statements and accompanying notes for the years ended December 31, 2021 and 2020, included in
Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022, and filed with the SEC on July 7, 2022. The consolidated balance
sheet as of December 31, 2021, included herein, was derived from the audited financial statements of Obagi Global Holdings Limited as
of that date.
The
unaudited interim condensed consolidated financial statements, in the opinion of management, reflect all adjustments, consisting only
of normal recurring adjustments, necessary to present fairly the Company’s financial position as of June 30, 2022, its results
of operations, comprehensive (loss) income, shareholder’s equity, and cash flows for the six months ended June 30, 2022 and 2021.
The results of the six months ended June 30, 2022 and 2021 are not necessarily indicative of the results to be expected for the year
ending December 31, 2022 or for any interim period or for any other future year.
There
were no changes to the significant accounting policies or recent accounting pronouncements as described in the Company’s financial
statements for the fiscal year ended December 31, 2021, except as noted below.
Emerging
Growth Company—Section 102(b)(1) of the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) exempts emerging
growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those
that have not had a registration statement declared effective under the Securities Act of 1933, as amended, or do not have a class of
securities registered under the Exchange Act of 1934, as amended) are required to comply with the new or revised financial accounting
standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with
the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected
not to opt out of such extended transition period, which means that when a standard is issued or revised, and it has different application
dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time
private companies adopt the new or revised standard.
This
may make comparison of the Company’s financial statement with another public company that is neither an emerging growth company
nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential
differences in accounting standards used.
Concentrations
of Credit Risk—Financial instruments that subject the Company to concentrations of credit risk consist primarily of cash and
cash equivalents and accounts receivable. The Company maintains its cash balances in accounts held by major banks and financial institutions
located primarily in the United States and considers such risk to be minimal. Such bank deposits from time to time may be exposed to
credit risk in excess of the Federal Deposit Insurance Corporation insurance limit.
The
Company’s accounts receivable primarily represent amounts due from wholesale distributors and group purchasing organizations located
both inside and outside the U.S. The Company mitigates its credit risks by performing ongoing credit evaluations of its customers’
financial conditions, requires customer advance payments in certain circumstances and has historically not had receipt of payment exceed
60 days. The Company generally does not require collateral.
As
of June 30, 2022, four customers accounted for 37%, 31%, 12% and 12% of accounts receivable, respectively. As of December 31, 2021, three
customers accounted for 35%, 33% and 13% of accounts receivable, respectively.
During
the six months ended June 30, 2022 and 2021, three vendors and two vendors, respectively, exceeded 10% of inventory purchases. In the
six months ended June 30, 2022, the Company purchased approximately 31%, 13%, and 12% of inventory, from the three vendors. In the six
months ended June 30, 2021, the Company purchased approximately 59% and 12% of inventory, respectively, from the two vendors. As of June
30, 2022, two vendors accounted for 19%, and 20%, respectively, of accounts payable. As of December 31, 2021, one vendor accounted for
40% of accounts payable.
Restricted
Cash—The Company’s restricted cash represents funds that are not accessible for general purpose cash needs due to contractual
limitations. As of June 30, 2022 and December 31, 2021, the Company had restricted cash of $650. The restricted cash balance represents
cash in a savings account held by a major bank located in the United States and provides collateral for corporate credit cards obtained
by the Company for its employees. The Company is required to hold the restricted cash in the bank’s savings account until August
2022. As of June 30, 2022, the Company’s cash, cash equivalents and restricted cash balance of $7,392 shown on the condensed consolidated
statements of cash flows consisted of $650 restricted cash and $6,742 in cash and cash equivalents. As of December 31, 2021, the Company’s
cash, cash equivalents and restricted cash balance of $13,444 shown on the condensed consolidated statements of cash flows consisted
of $650 restricted cash and $12,794 cash and cash equivalents.
Segments—The
Company operates as a single operating and reportable segment. Operating segments are defined as components of an enterprise for which
separate discrete financial information is evaluated regularly by the chief operating decision maker (“CODM”) in deciding
how to allocate resources and assess the Company’s financial and operational performance. The Company has determined that its Chief
Executive Officer is the CODM. To date, the Company’s CODM has made such decisions and assessed performance at the Company-level.
Recently
Issued Accounting Standards, Not Yet Adopted—
In
February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02,
Leases (Topic 842) and since that date has issued subsequent amendments to the initial guidance intended to clarify certain
aspects of the guidance and to provide certain practical expedients entities can elect upon adoption. ASU 2016-02 amends the guidance
for lease accounting to require lease assets and liabilities to be recognized on the balance sheet, along with additional disclosures
regarding key leasing arrangements. The amendments in this ASU are effective for fiscal years beginning after December 15, 2018, and
interim periods within those fiscal years, and early adoption is permitted. Entities must adopt the standard using a modified retrospective
transition and apply the guidance to the earliest comparative period presented, with certain practical expedients that entities may elect
to apply. In June 2020, the FASB issued ASU 2020-05, Revenue from Contracts with Customers (Topic 606) and Leases (Topic
842), which defers the effective date for one year for entities in the “all other” category and public not-for-profit entities
that have not yet issued their financial statements reflecting the adoption of Leases. Topic 842 is effective for the Company for the
annual period ending December 31, 2022, and interim periods within the annual period ending December 31, 2023, with earlier adoption
permitted. The Company will adopt Topic 842 during the annual period ending December 31, 2022 using the modified retrospective method
and as a result will not restate prior periods. The Company intends to elect the practical expedients provided in the new ASUs that (i)
allow historical lease classification of existing leases, (ii) allow the Company to not reassess whether any expired or existing contracts
contain leases, and (iii) allow the Company to not reassess initial direct costs for any existing leases. The Company is currently evaluating
its lease agreements and summarizing key contract terms and financial information associated with each lease agreement in order to assess
the impact the adoption of ASU 2016-02 will have on its consolidated financial statements. Based on the Company’s current lease
portfolio, the Company preliminarily expects Topic 842 to have a material impact on its consolidated balance sheet primarily related
to the recognition of operating lease assets and liabilities. The Company does not expect the adoption to have a material impact on the
Company’s consolidated statement of operations or on its consolidated statement of cash flows.
In
February 2016, the FASB issued ASU No. 2016-13, Financial Statements—Credit Losses (Topic 326): Measurement of Credit
Losses on Financial Instruments. This ASU adds an impairment model known as current expected credit loss that is based on expected
losses rather than incurred losses. It recognizes an allowance as its estimate of expected credit losses, which may result in more timely
recognition of such losses. In 2019, the FASB issued ASU 2019-10, Financial Instruments—Credit Losses (Topic 326), Derivatives
and Hedging (Topic 815), and Leases (Topic 842), which defers the effective date for entities in the “all other”
category and public not-for-profit entities that have not yet issued their financial statements reflecting the adoption of Credit Losses.
The amendments in this ASU are effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal
years, and early adoption is permitted. This guidance is effective for the Company for the annual period beginning on January 1, 2023,
and interim periods within the annual period beginning on January 1, 2023. The Company’s adoption of this standard is not expected
to have a material impact on the Company’s consolidated financial statements.
In
December 2019, the FASB issued ASU No. 2019-12, Income Taxes—Income Taxes (Topic 740): Simplifying the Accounting for Income
Taxes (“ASU 2019-12”). ASU 2019-12 simplifies the accounting for income taxes by removing certain exceptions to the general
principles in Topic 740. The amendments also improve consistent application of and simplify GAAP for other areas of Topic 740 by clarifying
and amending existing guidance. ASU 2019-12 is effective for the Company for annual period beginning on January 1, 2022, and interim
periods within the annual period beginning on January 1, 2023. The amendments do not create new accounting requirements. The adoption
of this standard is not expected to have a material impact on the Company’s consolidated financial statements.
In
March 2020, the FASB issued ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference
Rate Reform on Financial Reporting. This ASU provides relief that, if elected, will require less accounting analysis and less accounting
recognition for modifications related to reference rate reform. This update provides optional guidance for a limited period of time to
ease the potential burden in accounting for reference rate reform on financial reporting. The amendments in the update apply only to
contracts, hedging relationships, and other transactions that reference the London Inter-Bank Offered Rate (“LIBOR”) or another
reference rate expected to be discontinued because of reference rate reform. The amendments in this ASU were effective upon issuance
and may be applied through December 31, 2022. The Company is implementing a transition plan to modify its debt with interest rates that
are influenced by LIBOR. The Company is continuing to assess ASU 2020-04 and its impact on the Company’s transition away from LIBOR
for its debt.
3. REVENUE
The
Company disaggregates its revenue from contracts with customers by sales channel, as well as by revenue source and geographic region,
based on the location of the end customer, as it believes it best depicts how the nature, amount, timing and uncertainty of its revenue
and cash flows are affected by economic factors. Total revenue based on the disaggregation criteria was as follows (in thousands):
| | |
Six months ended June 30, | |
| | |
2022 | | |
2021 | |
| Revenue by Sales Channel | |
| | |
| |
| Medical | |
$ | 85,354 | | |
$ | 91,186 | |
| Clinical | |
| 20,477 | | |
| 2,330 | |
| Other | |
| 609 | | |
| 688 | |
| Total | |
$ | 106,440 | | |
$ | 94,204 | |
| | |
| | | |
| | |
| Revenue by Geographic Region | |
| | | |
| | |
| North America | |
$ | 46,615 | | |
$ | 46,008 | |
| Asia Pacific | |
| 50,275 | | |
| 36,041 | |
| Rest of the World | |
| 7,086 | | |
| 8,926 | |
| Net product sales | |
$ | 103,976 | | |
$ | 90,975 | |
| Asia Pacific Royalties | |
| 2,464 | | |
| 3,229 | |
| Total | |
$ | 106,440 | | |
$ | 94,204 | |
For
the six months ended June 30, 2022, the countries that accounted for more than 10% of the Company’s total revenue were the United
States and Vietnam, with net product sales amounting to $46,365 and $40,864, respectively. For the six months ended June 30, 2021, the
countries that accounted for more than 10% of the Company’s total revenue were the United States, Vietnam, and China, with net
product sales amounting to $46,008, $22,529, and 9,872 respectively.
For
the six months ended June 30, 2022, two customers accounted for 32% and 31%, respectively, of the Company’s total revenue.
For the six months ended June 30, 2021, two customers accounted for 40% and 22%, respectively,
of the Company’s total revenue.
Services
Provided by the Customer
Consideration
payable to a customer for distinct goods or services is treated as a purchase for an amount up to the fair value of those distinct
goods or services. The Company recognizes consideration payable to its customers as an operating
expense for distinct services which include marketing, technology support, program management, and regulatory compliance. When
consideration payable for the distinct services exceeds the fair value of services provided by the customer, the Company records those
excess amounts as a reduction of the transaction price in the arrangement. These services are provided by certain customers that
are distributors. Consideration payable to a customer for non-distinct services, mainly traditional
distribution services such as packing and shipping, is recorded as a reduction to the transaction price. In cases where the Company
cannot reasonably estimate the fair value of the goods or services received from the customer, it will recognize the consideration payable
as a reduction of the transaction price.
The expenses recognized relating to distinct services provided by customers
are as follows (in thousands):
| | |
Six months ended June 30, | |
| | |
2022 | | |
2021 | |
| Selling, general and administrative | |
$ | 16,383 | | |
$ | 10,481 | |
| Research and development | |
| 823 | | |
| 704 | |
| Total | |
$ | 17,206 | | |
$ | 11,185 | |
4. ACCOUNTS
AND NOTE RECEIVABLE, NET
As of June 30, 2022, accounts receivable, net consisted of accounts
receivable of $75,227, less allowance for doubtful accounts of $62. As of December 31, 2021, accounts receivable, net consisted of accounts
receivable of $60,929, less allowance for doubtful accounts of $62.
During the six months ended June 30, 2022 and 2021, there were no changes
to the Company’s allowance for doubtful accounts. The Company did not have any write-offs.
On July 30, 2021, and as amended on December 31, 2021 and April 30,
2022, Obagi Cosmeceuticals LLC, a wholly owned subsidiary of the Company, entered into a non-recourse, uncollateralized short-term promissory
note, not in the ordinary course of business, lending a third party $2,500 (the “Note Receivable”). This Note Receivable matures
on December 31, 2022 and carries an interest rate of 1.00% from July 30, 2021 to September 29, 2021 and 8.00% from September 30, 2021
through maturity. The outstanding principal and accrued interest are due upon maturity. In connection with the Business Combination described in Note 1, on July 27, 2022 the Note Receivable was carved out and distributed to
the Company’s shareholder, Cedarwalk.
5. INVENTORIES
The components of inventories were as follows (in thousands):
| | |
June 30,
2022 | | |
December 31,
2021 | |
| Work in process | |
$ | 1,420 | | |
$ | 1,619 | |
| Finished goods | |
| 26,166 | | |
| 17,720 | |
| Total | |
$ | 27,586 | | |
$ | 19,339 | |
6. PROPERTY
AND EQUIPMENT—NET
Property and equipment, net consisted of the following (in thousands):
| | |
June 30,
2022 | | |
December 31,
2021 | |
| Computer software and website development costs | |
$ | 4,576 | | |
$ | 4,334 | |
| Other property and equipment | |
| 647 | | |
| 83 | |
| Total property and equipment | |
| 5,223 | | |
| 4,417 | |
| Less: accumulated depreciation | |
| (1,446 | ) | |
| (833 | ) |
| Property and equipment, net | |
$ | 3,777 | | |
$ | 3,584 | |
Depreciation expense for property and equipment for the six months
ended June 30, 2022 and 2021 was $613 and $268, respectively. Depreciation expense during these periods pertain to property and equipment
utilized as part of the Company’s selling, general and administrative activities and therefore has not been allocated to cost of
goods sold.
7. INTANGIBLE
ASSETS, NET
Intangible assets, net consisted of the following as of June 30, 2022
(in thousands):
| | |
Weighted Average
Useful Lives
(Years) | | |
Gross Carrying
Amount | | |
Accumulated
Amortization | | |
Net Carrying
Amount | |
| Trademarks | |
| 10 | | |
$ | 46,216 | | |
$ | (20,176 | ) | |
$ | 26,040 | |
| Customer lists | |
| 10 | | |
| 39,370 | | |
| (18,373 | ) | |
| 20,997 | |
| Supply agreement | |
| 10 | | |
| 25,570 | | |
| (11,933 | ) | |
| 13,637 | |
| Developed technology | |
| 10 | | |
| 22,863 | | |
| (10,759 | ) | |
| 12,104 | |
| Patents | |
| 20 | | |
| 309 | | |
| (18 | ) | |
| 291 | |
| Total | |
| | | |
$ | 134,328 | | |
$ | (61,259 | ) | |
$ | 73,069 | |
Intangible assets, net consisted of the following as of December 31,
2021 (in thousands):
| | |
Weighted Average
Useful Lives
(Years) | | |
Gross Carrying
Amount | | |
Accumulated
Amortization | | |
Net Carrying
Amount | |
| Trademarks | |
| 10 | | |
$ | 46,004 | | |
$ | (17,842 | ) | |
$ | 28,162 | |
| Customer lists | |
| 10 | | |
| 39,370 | | |
| (16,404 | ) | |
| 22,966 | |
| Supply agreement | |
| 10 | | |
| 25,570 | | |
| (10,654 | ) | |
| 14,916 | |
| Developed technology | |
| 10 | | |
| 22,863 | | |
| (9,592 | ) | |
| 13,271 | |
| Patents | |
| 20 | | |
| 270 | | |
| (11 | ) | |
| 259 | |
| Total | |
| | | |
$ | 134,077 | | |
$ | (54,503 | ) | |
$ | 79,574 | |
No impairment loss was recognized for the six months ended June 30,
2022 and 2021. Amortization expense for the six months ended June 30, 2022 and 2021 was $6,756 and $6,668, respectively. Expected amortization
for each of the years between 2022 through 2026, and thereafter are as follows (in thousands):
Years
Ending December 31, | |
| |
| 2022 (remaining 6 months) | |
$ | 6,764 | |
| 2023 | |
| 13,531 | |
| 2024 | |
| 13,368 | |
| 2025 | |
| 13,325 | |
| 2026 | |
| 13,325 | |
| Thereafter | |
| 12,756 | |
| | |
$ | 73,069 | |
8. OTHER
CURRENT LIABILITIES
The major components of other current liabilities consisted
of the following (in thousands):
| | |
As of | |
| | |
June 30,
2022 | | |
December 31,
2021 | |
| Accrued salaries and related expenses | |
$ | 6,623 | | |
$ | 6,741 | |
| Accrued marketing expenses | |
| 4,031 | | |
| 2,963 | |
| Accrued distribution fees | |
| 2,930 | | |
| 1,926 | |
| Other | |
| 833 | | |
| 1,353 | |
| Total | |
$ | 14,417 | | |
$ | 12,983 | |
9. DEBT
Current
and long-term obligations consisted of the following (in thousands):
| | |
Maturity
Date | |
June 30,
2022 | | |
December 31,
2021 | |
| 2021 Term Loan | |
March 2026 | |
$ | 107,800 | | |
$ | 109,175 | |
| 2021 Revolving Credit Facility | |
March 2026 | |
| 21,000 | | |
| 15,000 | |
| Unamortized debt issuance costs | |
| |
| (5,433 | ) | |
| (5,370 | ) |
| Net carrying amount | |
| |
| 123,367 | | |
| 118,805 | |
| Less: Current portion of long-term debt | |
| |
| (22,603 | ) | |
| (15,382 | ) |
| Total long-term portion | |
| |
$ | 100,764 | | |
$ | 103,423 | |
2021 Credit Agreement
On March 16, 2021, and as amended on June 10, 2022, the Company
replaced the 2018 Credit Agreement (described below) for a new financing agreement with a syndicate of lenders, including TCW Asset Management
Company LLC as administrative agent for the lenders (the “2021 Credit Agreement”). The 2021 Credit Agreement included a term
loan of $110,000 (the “2021 Term Loan”) and a revolving credit facility with borrowing capacity of up to $40,000 (“2021
Revolving Credit Facility”). Both the 2021 Term Loan and the 2021 Revolving Credit Facility mature on March 16, 2026. The 2021
Term Loan and 2021 Revolving Credit Facility bear interest at the LIBOR plus an applicable margin, as determined by the Company’s
leverage ratios, and are subject to LIBOR succession provisions. If LIBOR becomes unavailable, the parties will establish an alternate
index rate that gives due consideration to the then prevailing market convention for determining a rate of interest for leveraged syndicated
loans in the United States. In connection with the issuance of the 2021 Credit Agreement, the Company incurred $6,383 of debt issuance
costs. The 2021 Credit Agreement is secured by the assets of the Company.
As of June 30, 2022, the Company had unpaid principal of $107,800 and
unamortized debt issuance costs of $3,984 on the 2021 Term Loan. The interest rate on the term loan was 8.50% and the accrued interest
was $0 as of June 30, 2022. The current portion of the 2021 Term Loan and 2021 Revolving Credit Facility is $4,125 and $21,000, respectively.
The current portion of the unamortized debt issuance costs on the 2021 Term Loan and 2021 Revolving Credit Facility is $1,073 and $1,449,
respectively. The outstanding balance under the 2021 Credit Agreement was settled upon the completion of the Business Combination, which
occurred on July 27, 2022.
2018 Credit Agreement
On December 13, 2018, and as amended on December 23, 2019 and
November 9, 2020, the Company entered into a credit agreement (the “2018 Credit Agreement”) with a syndicate of banks, including
Wells Fargo Bank, National Association as administrative agent for the banks (the “Syndicate of Banks”). The 2018 Credit Agreement
included a term loan of $90,000 (the “2018 Term Loan”) and a revolving credit facility with borrowing capacity of up to $35,000
(“2018 Revolving Credit Facility”). Both the 2018 Term Loan and the 2018 Revolving Credit Facility were due to mature on December 13,
2023. In connection with the issuance of the 2018 Credit Agreement, the Company incurred $2,914 of debt issuance costs. The 2018 Credit
Agreement was secured by the assets of the Company. Both the Term Loan and the Revolving Credit Facility carried an interest rate of LIBOR
plus applicable margin, as determined by the Company’s leverage ratios, and were subject to LIBOR succession provisions. On December 23,
2019 the 2018 Credit Agreement was amended to revise the definition of consolidated earnings before interest, taxes, depreciation, and
amortization (“Consolidated EBITDA”) to allow for certain additional adjustments, in relation to the debt covenants.
The Company recorded a loss on extinguishment of the 2018 Credit Agreement
of $2,317 to loss on extinguishment of debt in the accompanying condensed consolidated statement of operations and comprehensive (loss)
income during the six months ended June 30, 2021, which consisted of expensing unamortized debt issuance costs.
PPP Loan
On May 11, 2020, the Company received loan proceeds in the amount
of $6,750 under the Paycheck Protection Program (“PPP”) from MUFG Union Bank (the “PPP Loan”). The PPP, established
as part of the Coronavirus aid, Relief and Economic Security Act (“CARES Act”), provided for loans to qualifying businesses
for amounts up to 2.5 times of the average monthly payroll expenses of the qualifying business. The PPP Loan accrued interest at a rate
of 1%. The PPP Loan and accrued interest were forgivable after eight or twenty-four weeks as long as the borrower used the proceeds for
eligible purposes, including payroll, benefits, rent and utilities and maintains its payroll levels. The Company used the proceeds for
purposes consistent with the PPP, and in 2021, received approval from MUFG Union Bank and the Small Business Administration for forgiveness
of the full amount of its PPP Loan, inclusive of accrued interest of $74. The Company recognized a gain on PPP Loan forgiveness of $6,824
in the second quarter of 2021.
Scheduled Debt Maturities
Scheduled maturities under the Company’s 2021 Credit Agreement
(excluding unamortized debt issuance costs of $5,433) as of June 30, 2022 are as follows (in thousands):
Year Ending
December 31, | |
| |
| 2022 (remaining 6 months) | |
$ | 22,375 | |
| 2023 | |
| 5,500 | |
| 2024 | |
| 5,500 | |
| 2025 | |
| 5,500 | |
| 2026 | |
| 89,925 | |
| Total unpaid principal | |
$ | 128,800 | |
10. STOCK
BASED COMPENSATION
On January 26, 2021, the Company established a Stock Incentive Plan
(the “Stock Plan”), under which stock options, stock awards, and restricted stock units (“Restricted Stock”) of
the Company may be granted to eligible employees, directors, and consultants. Under the Stock Plan, the Company is authorized to issue
a maximum number of 1,500,000 shares of common stock. Incentive stock options must have an exercise price at or above the fair market
value of the stock on the date of the grant. Stock options and Restricted Stock granted by the Company have service-based and performance-based
vesting conditions.
The options vest over five years, with 25% of options vesting in four
equal quarterly installments at the end of each three-month period through the first anniversary of the grant, and the remaining 75% vesting
in a series of five equal annual installments over the five-year period measured from the grant date. The Restricted Stock vests in five
equal annual installments at the end of each year, over the five-year period from the grant date. Award holders have a ten-year period
to exercise the options before they expire. Notwithstanding achievement of the service-based condition, the options and Restricted Stock
do not vest or become exercisable until a qualifying transaction is consummated prior to the expiration date. A qualifying transaction
consists of either a change in control event or an underwritten initial public offering by the Company of its equity securities on a U.S.
or foreign exchange.
The weighted average fair value per share of
the Restricted Stock granted during the six months ended June 30, 2022 was $63.59. The weighted average fair value per share of the
awards granted during the six months ended June 30, 2021 was $15.55 and $38.86 for stock options and Restricted Stock, respectively.
The unrecognized compensation cost was $12,442 and $9,523 for stock options and Restricted Stock, respectively, as of June 30, 2022
and $12,442 and $9,411 for stock options and Restricted Stock, respectively, as of December 31, 2021. Immediately prior to the
consummation of the Business Combination, the qualifying transaction event became “probable” and previously unrecognized
stock-based compensation expense was recognized at the time of close based on the requisite service period through that date.
Stock option activity for the six months ended June 30, 2022 was as
follows:
| | |
Number of
Common
Stock
Options | | |
Weighted
Average
Exercise
Price | | |
Weighted
Average
Remaining
Contractual
Life | | |
Aggregate
Intrinsic
Value (in
thousands) | |
| Outstanding as of January 1, 2022 | |
| 800,000 | | |
$ | 41.10 | | |
| 9.1 | | |
$ | 16,456 | |
| Granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Forfeited | |
| - | | |
| - | | |
| - | | |
| - | |
| Vested | |
| - | | |
| - | | |
| - | | |
| - | |
| Outstanding as of June 30, 2022 | |
| 800,000 | | |
$ | 41.10 | | |
| 8.6 | | |
$ | 21,672 | |
Stock option activity for the six months ended June 30, 2021 was as
follows:
| | |
Number of
Common
Stock
Options | | |
Weighted
Average
Exercise
Price | | |
Weighted
Average
Remaining
Contractual
Life | | |
Aggregate
Intrinsic
Value (in
thousands) | |
| Outstanding as of January 1, 2021 | |
| - | | |
$ | - | | |
| - | | |
$ | - | |
| Granted | |
| 800,000 | | |
| 41.10 | | |
| 9.6 | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Forfeited | |
| - | | |
| - | | |
| - | | |
| - | |
| Vested | |
| - | | |
| - | | |
| - | | |
| - | |
| Outstanding as of June 30, 2021 | |
| 800,000 | | |
$ | 41.10 | | |
| 9.6 | | |
$ | (1,936 | ) |
Restricted Stock activity for the six months ended June 30, 2022 was
as follows:
| | |
Shares | | |
Weighted
Average
Grant Date
Fair Value
per Share | |
| Outstanding as of January 1, 2022 | |
| 243,307 | | |
$ | 38.68 | |
| Granted | |
| 1,754 | | |
$ | 63.59 | |
| Exercised | |
| - | | |
| - | |
| Forfeited | |
| - | | |
| - | |
| Vested | |
| - | | |
| - | |
| Outstanding as of June 30, 2022 | |
| 245,061 | | |
$ | 38.86 | |
Restricted Stock activity for the six months ended June 30, 2021 was
as follows:
| | |
Shares | | |
Weighted
Average
Grant Date
Fair Value
per Share | |
| Outstanding as of January 1, 2021 | |
| - | | |
| - | |
| Granted | |
| 243,307 | | |
$ | 38.68 | |
| Exercised | |
| - | | |
| - | |
| Forfeited | |
| - | | |
| - | |
| Vested | |
| - | | |
| - | |
| Outstanding as of June 30, 2021 | |
| 243,307 | | |
$ | 38.68 | |
11. SHAREHOLDER’S
EQUITY
The Company’s equity structure consists of a single class of
common stock. On December 2, 2020 the Company amended and restated its Memorandum and Articles of Association, authorizing 25,000,000
shares at $0.50 par value each. Each share of common stock is entitled to one vote. The Company did not hold any shares as treasury shares
as of the periods presented in the accompanying condensed consolidated financial statements.
The Company may, at the discretion of its Directors, declare dividends
and distributions out of the funds of the Company lawfully available therefor. Payments of dividends and distributions are limited to
realized or unrealized profits of the Company. The Company did not pay any dividends in the six months ended June 30, 2022. For the six
months ended June 30, 2021, the Company, through its wholly owned subsidiary, paid $1,998 (approximately $0.25 per share) in dividends
to its shareholder.
12. NET
(LOSS) INCOME PER SHARE
The
following table sets forth the computation of basic and diluted net (loss) income using the treasury stock method for the six months ended
June 30, 2022 and 2021 (in thousands, except for share and per share amounts):
| | |
Six Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Net (loss) income | |
$ | (2,915 | ) | |
$ | 12,946 | |
| Weighted-average number of shares outstanding – basic and diluted | |
| 8,000,002 | | |
| 8,000,002 | |
| Net (loss) income per share – basic and diluted | |
$ | (0.36 | ) | |
$ | 1.62 | |
The following table represents potential shares of common stock outstanding
that were excluded from the computation of diluted net (loss) income per share of common stock as they are issuable contingent on the
occurrence of a qualifying event, and for the periods presented, the necessary conditions have not been satisfied (see Note 10):
| | |
Six Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Stock options | |
| 800,000 | | |
| 800,000 | |
| Restricted Stock | |
| 245,061 | | |
| 243,307 | |
| Total | |
| 1,045,061 | | |
| 1,043,307 | |
13. INCOME
TAX (Benefit) expense
Income taxes are determined using an estimated
annual effective tax rate applied against income, and then adjusted for the tax impacts of certain significant and discrete items. The
table below sets forth information related to the Company’s income tax (benefit) expense:
| | |
Six Months Ended June 30, | |
| | |
2022 | | |
2021 | | |
Change | |
| Income tax (benefit) expense | |
$ | (40 | ) | |
$ | 1,948 | | |
$ | (1,988 | ) |
| Effective tax rate | |
| 1.4 | % | |
| 13.1 | % | |
| (11.7 | )% |
The Company, domiciled in the Cayman Islands, is subject to taxation
in the U.S. and various states jurisdictions. ASC Topic 740, Income Taxes (“ASC 740”) indicates that the federal statutory
income tax rate of a foreign reporting entity be used when preparing the rate reconciliation disclosure. As such, the Company and its
wholly owned subsidiaries use the statutory income tax rate in the Cayman Islands, which is 0%. The decrease in the effective tax rate
for the six months ended June 30, 2022 when compared to the six months ended June 30, 2021, was primarily attributable to the change in
pre-tax earnings and the change in valuation allowance recorded during the comparable periods. The Company’s future effective tax
rate may vary from the statutory tax rate due to the mix of earnings in tax jurisdictions with different statutory tax rates or due to
changes in valuation allowance.
The Company files income tax returns in the U.S. federal jurisdiction
and in various state and foreign jurisdictions. The Company remains subject to examination for federal and state income tax purposes for
the tax years ending 2017 and forward. The Company does not anticipate a significant change in its uncertain tax benefits over the next
12 months. Management believes it is more likely than not that all significant tax positions taken to date would be sustained by the relevant
taxing authorities.
14. RELATED
PARTY TRANSACTIONS
There were no related party transactions during the six months ended
June 30, 2022 and 2021.
15. commitments
and contingencies
Legal Proceedings
From time to time, the Company may be a party to litigation and subject
to claims incidental to its business. Although the results of litigation and claims cannot be predicted with certainty, the Company currently
believes that the final outcome of these matters will not have a material adverse effect on its business. Regardless of the outcome, litigation
can have an adverse impact on the Company because of judgment, defense and settlement costs, diversion of management resources, and other
factors. At each reporting period, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable
and reasonably estimable, requiring recognition of a loss accrual, or whether the potential loss is reasonably possible, requiring potential
disclosure. Legal fees are expensed as incurred.
Operating Lease Obligations
The Company leases office space under four non-cancelable operating
leases expiring between September 2023 and October 2032. Rent expense related to operating leases was $796 and $534 for the six months
ended June 30, 2022 and 2021, respectively.
As of June 30, 2022, future minimum lease payments under all noncancelable
operating leases with an initial lease term in excess of one year were as follows (in thousands):
Year
Ending December 31 | |
| |
| 2022 (remaining 6 months) | |
$ | 811 | |
| 2023 | |
| 2,075 | |
| 2024 | |
| 1,968 | |
| 2025 | |
| 2,012 | |
| 2026 | |
| 1,377 | |
| Thereafter | |
| 5,386 | |
| | |
$ | 13,629 | |
16. SUBSEQUENT
EVENTS
The Company has evaluated all subsequent events
through August 11, 2022, the date these condensed consolidated financial statements were issued.
On July 27, 2022 the Company carved out and distributed the Note Receivable and its operations in the China Region to its shareholder,
Cedarwalk, and completed the Business Combination with Waldencast as discussed in Note 1. Upon the completion of the Business Combination
the outstanding balance of the 2021 Credit Agreement inclusive of accrued interest was settled.
******
INDEPENDENT
AUDITOR’S REPORT
To
the Members,
Milk
Makeup LLC:
Report
on the Audit of the Financial Statements
Opinion
We
have audited the financial statements of Milk Makeup LLC (the “Company”), which comprise the balance sheets as of December
31, 2021 and 2020, and the related statements of operations and comprehensive loss, redeemable preferred units and members’ equity
and cash flows for the years then ended December 31, 2021, 2020 and 2019, and the related notes to financial statements.
In
our opinion, the accompanying financial statements present fairly, in all material respects, the financial position of the Company as
of December 31, 2021 and 2020, and the results of its operations and its cash flows for the years then ended in accordance with accounting
principles generally accepted in the United States of America.
Basis
of Opinion
We
conducted our audits in accordance with auditing standards generally accepted in the United States of America (GAAS). Our responsibilities
under those standards are further described in the Auditor’s Responsibilities for the Audit of the Financial Statements section
of our report. We are required to be independent of the Company and to meet our other ethical responsibilities, in accordance with the
relevant ethical requirements relating to our audits. We believe that the audit evidence we have obtained is sufficient and appropriate
to provide a basis for our audit opinion.
Responsibilities
of Management for the Financial Statements
Management
is responsible for the preparation and fair presentation of the financial statements in accordance with accounting principles generally
accepted in the United States of America, and for the design, implementation, and maintenance of internal control relevant to the preparation
and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
In
preparing the financial statements, management is required to evaluate whether there are conditions or events, considered in the aggregate,
that raise substantial doubt about the Company’s ability to continue as a going concern for one year after the date that the financial
statements are available to be issued.
Auditor’s
Responsibilities for the Audit of the Financial Statements
Our
objectives are to obtain reasonable assurance about whether the financial statements as a whole are free from material misstatement,
whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level
of assurance but is not absolute assurance and therefore is not a guarantee that an audit conducted in accordance with GAAS will always
detect a material misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than
for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of
internal control. Misstatements are considered material if there is a substantial likelihood that, individually or in the aggregate,
they would influence the judgment made by a reasonable user based on the financial statements.
In
performing an audit in accordance with GAAS, we:
| |
● |
Exercise professional judgment
and maintain professional skepticism throughout the audit. |
| |
● |
Identify
and assess the risks of material misstatement of the financial statements, whether due to fraud or error, and design and perform
audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and
disclosures in the financial statements. |
| |
● |
Obtain
an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances,
but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, no such
opinion is expressed. |
| |
● |
Evaluate
the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as
well as evaluate the overall presentation of the financial statements. |
| |
● |
Conclude
whether, in our judgment, there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s
ability to continue as a going concern for a reasonable period of time. |
We
are required to communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit,
significant audit findings, and certain internal control–related matters that we identified during the audit.
/s/
WithumSmith+Brown, P.C.
WithumSmith+Brown,
P.C.
New
York, NY
April
12, 2022
Milk
Makeup LLC
Statements
of Operations and Comprehensive Loss
| | |
Year
ended December 31, | |
| (In
thousands) | |
2021 | | |
2020 | | |
2019 | |
| Net
sales | |
$ | 47,076 | | |
$ | 39,515 | | |
$ | 50,811 | |
| Cost
of goods sold (exclusive of depreciation and amortization) | |
| 21,781 | | |
| 23,450 | | |
| 23,379 | |
| Selling,
general and administrative expenses | |
| 30,764 | | |
| 26,559 | | |
| 33,567 | |
| Depreciation
and amortization | |
| 1,975 | | |
| 1,746 | | |
| 2,536 | |
| Operating
loss | |
| (7,444 | ) | |
| (12,240 | ) | |
| (8,671 | ) |
| Interest
expense, net | |
| 18 | | |
| 301 | | |
| 1,369 | |
| Other
expense, net | |
| 385 | | |
| 393 | | |
| 918 | |
| Loss
before provision for income taxes | |
| (7,847 | ) | |
| (12,934 | ) | |
| (10,958 | ) |
| Income
tax provision | |
| — | | |
| — | | |
| — | |
| Net
loss | |
$ | (7,847 | ) | |
$ | (12,934 | ) | |
$ | (10,958 | ) |
| Comprehensive
loss | |
$ | (7,847 | ) | |
$ | (12,934 | ) | |
$ | (10,958 | ) |
The
accompanying notes are an integral part of the financial statements.
Milk
Makeup LLC
Balance
Sheets
| | |
December 31, | |
| (In thousands, except share data) | |
2021 | | |
2020 | |
| ASSETS | |
| | |
| |
| Current assets | |
| | |
| |
| Cash | |
$ | 4,417 | | |
$ | 7,207 | |
| Accounts receivable, net | |
| 1,802 | | |
| 5,529 | |
| Inventories | |
| 21,428 | | |
| 16,385 | |
| Prepaid expenses and other current assets | |
| 610 | | |
| 488 | |
| Prepaid supplier | |
| 1,051 | | |
| 1,039 | |
| Total current assets | |
| 29,308 | | |
| 30,648 | |
| Property, plant and equipment, net | |
| 4,970 | | |
| 5,492 | |
| Intangible assets, net | |
| — | | |
| 14 | |
| Due from officers | |
| 774 | | |
| 760 | |
| Total assets | |
$ | 35,052 | | |
$ | 36,914 | |
| | |
| | | |
| | |
| LIABILITIES, REDEEMABLE PREFERRED UNITS, AND MEMBERS’ EQUITY | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable | |
$ | 6,477 | | |
$ | 3,697 | |
| Tenant allowance liability – current | |
| 132 | | |
| 132 | |
| Accrued expenses and other current liabilities | |
| 6,735 | | |
| 3,624 | |
| Total current liabilities | |
| 13,344 | | |
| 7,453 | |
| Warrant liabilities | |
| 121 | | |
| 99 | |
| Deferred rent – non-current | |
| 1,063 | | |
| 936 | |
| Tenant allowance liability – non-current | |
| 1,044 | | |
| 1,176 | |
| Total liabilities | |
| 15,572 | | |
| 9,664 | |
| COMMITMENTS AND CONTINGENCIES | |
| | | |
| | |
| Redeemable Series A Preferred units, 3,843,750 units authorized, issued and outstanding as of December 31, 2021 and 2020 | |
| 23,601 | | |
| 15,375 | |
| Redeemable Series B Preferred units, 2,272,727 units authorized, issued and outstanding as of December 31, 2021 and 2020 | |
| 14,136 | | |
| 10,000 | |
| Redeemable Series C Preferred units, 3,515,352 units authorized as of December 31, 2021 and 2020, and 3,505,055 issued and outstanding as of December 31, 2021 and 2020 | |
| 34,210 | | |
| 21,276 | |
| Redeemable Series D Preferred units, 1,904,208 units authorized as of December 31, 2021 and 2020, and 1,898,069 units issued and outstanding as of December 31, 2021 and 2020 | |
| 27,335 | | |
| 20,330 | |
| Members’ Equity: | |
| | | |
| | |
| Common units, 23,086,766 units authorized as of December 31, 2021 and 2020, and 10,000,000 units issued and outstanding as of December 31, 2021 and 2020 | |
| — | | |
| 12,647 | |
| Members’ equity | |
| (79,802 | ) | |
| (52,378 | ) |
| Total members’ equity | |
| (79,802 | ) | |
| (39,731 | ) |
| TOTAL LIABILITIES, REDEEMABLE PREFERRED UNITS, AND MEMBERS’ EQUITY | |
$ | 35,052 | | |
$ | 36,914 | |
The
accompanying notes are an integral part of the financial statements.
Milk
Makeup LLC
Statements
of Redeemable Preferred Units and Members’ Equity
| |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| (In thousands, except | |
Redeemable
Series A
Preferred Units | | |
Redeemable
Series B
Preferred Units | | |
Redeemable
Series C
Preferred Units | | |
Redeemable
Series D
Preferred Units | | |
Common Units | | |
Members | | |
Total
Members’ | |
| share data) | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Equity | | |
Equity | |
| Balance as of December 31, 2018 | |
| 3,843,750 | | |
$ | 15,490 | | |
| 2,272,727 | | |
$ | 10,000 | | |
| — | | |
$ | — | | |
| — | | |
$ | — | | |
| 10,000,000 | | |
$ | 12,861 | | |
$ | (28,573 | ) | |
$ | (15,712 | ) |
| Issuance of Redeemable Series C Preferred Units | |
| — | | |
| — | | |
| — | | |
| — | | |
| 3,505,055 | | |
| 21,276 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Issuance of Redeemable Series D Preferred Units | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 915,751 | | |
| 10,000 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Change in redemption value of Redeemable Preferred Units | |
| — | | |
| (115 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 115 | | |
| — | | |
| 115 | |
| Equity based compensation expense | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 239 | | |
| 239 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (10,958 | ) | |
| (10,958 | ) |
| Balance as of December 31, 2019 | |
| 3,843,750 | | |
| 15,375 | | |
| 2,272,727 | | |
| 10,000 | | |
| 3,505,055 | | |
| 21,276 | | |
| 915,751 | | |
| 10,000 | | |
| 10,000,000 | | |
| 12,977 | | |
| (39,292 | ) | |
| (26,315 | ) |
| Issuance of Redeemable Series D Preferred Units | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 982,318 | | |
| 10,000 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Change in redemption value of Redeemable Preferred Units | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 330 | | |
| — | | |
| (330 | ) | |
| — | | |
| (330 | ) |
| Equity based compensation expense | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (152 | ) | |
| (152 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (12,934 | ) | |
| (12,934 | ) |
| Balance as of December 31, 2020 | |
| 3,843,750 | | |
| 15,375 | | |
| 2,272,727 | | |
| 10,000 | | |
| 3,505,055 | | |
| 21,276 | | |
| 1,898,069 | | |
| 20,330 | | |
| 10,000,000 | | |
| 12,647 | | |
| (52,378 | ) | |
| (39,731 | ) |
| Change in redemption value of Redeemable Preferred Units | |
| — | | |
| 8,226 | | |
| — | | |
| 4,136 | | |
| — | | |
| 12,934 | | |
| — | | |
| 7,005 | | |
| — | | |
| (12,647 | ) | |
| (19,654 | ) | |
| (32,301 | ) |
| Equity based compensation expense | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 77 | | |
| 77 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (7,847 | ) | |
| (7,847 | ) |
| Balance as of December 31, 2021 | |
| 3,843,750 | | |
$ | 23,601 | | |
| 2,272,727 | | |
$ | 14,136 | | |
| 3,505,055 | | |
$ | 34,210 | | |
| 1,898,069 | | |
$ | 27,335 | | |
| 10,000,000 | | |
$ | — | | |
$ | (79,802 | ) | |
$ | (79,802 | ) |
The
accompanying notes are an integral part of the financial statements.
Milk
Makeup LLC
Statements
of Cash Flows
| | |
Year ended December 31, | |
| (In thousands) | |
2021 | | |
2020 | | |
2019 | |
| Cash flows from operating activities: | |
| | |
| | |
| |
| Net loss | |
$ | (7,847 | ) | |
$ | (12,934 | ) | |
$ | (10,958 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | | |
| | |
| Depreciation and amortization | |
| 1,975 | | |
| 1,746 | | |
| 2,536 | |
| Non-cash interest (income) expense | |
| 8 | | |
| (24 | ) | |
| 1,370 | |
| Loss on disposal of assets | |
| 166 | | |
| 44 | | |
| 42 | |
| Equity-based compensation | |
| 77 | | |
| (152 | ) | |
| 239 | |
| Change in operating assets and liabilities: | |
| | | |
| | | |
| | |
| Accounts receivable, net | |
| 3,727 | | |
| (627 | ) | |
| (3,231 | ) |
| Inventories | |
| (5,043 | ) | |
| 11,539 | | |
| (16,509 | ) |
| Prepaid expenses and other current assets | |
| (134 | ) | |
| 2,360 | | |
| (2,992 | ) |
| Accounts payable | |
| 2,225 | | |
| (5,737 | ) | |
| 3,797 | |
| Other assets and liabilities | |
| 3,106 | | |
| (909 | ) | |
| 5,582 | |
| Net cash used in operating activities | |
| (1,740 | ) | |
| (4,694 | ) | |
| (20,124 | ) |
| Cash flows from investing activities: | |
| | | |
| | | |
| | |
| Purchases of property, plant, and equipment | |
| (1,050 | ) | |
| (5,851 | ) | |
| (896 | ) |
| Due from officers | |
| — | | |
| (150 | ) | |
| (210 | ) |
| Net cash used in investing activities | |
| (1,050 | ) | |
| (6,001 | ) | |
| (1,106 | ) |
| Cash flows from financing activities: | |
| | | |
| | | |
| | |
| Contributions from members | |
| — | | |
| 10,000 | | |
| 25,000 | |
| Net cash provided by financing activities | |
| — | | |
| 10,000 | | |
| 25,000 | |
| Net (decrease) increase in cash | |
| (2,790 | ) | |
| (695 | ) | |
| 3,770 | |
| Cash at beginning of year | |
| 7,207 | | |
| 7,902 | | |
| 4,132 | |
| Cash at end of year | |
$ | 4,417 | | |
$ | 7,207 | | |
$ | 7,902 | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | | |
| | |
| Cash paid during the year for interest | |
$ | 11 | | |
$ | 324 | | |
$ | — | |
| Supplemental disclosures of non-cash investing and financing activities | |
| | | |
| | | |
| | |
| Purchases of property, plant and equipment in accounts payable | |
$ | 555 | | |
$ | 237 | | |
$ | 588 | |
| Conversion of convertible promissory note into Redeemable Series C Preferred Units | |
$ | — | | |
$ | — | | |
$ | 5,021 | |
The
accompanying notes are an integral part of the financial statements.
Milk
Makeup LLC
Notes
to Financial Statements
| 1. | DESCRIPTION
OF BUSINESS |
Milk
Makeup LLC (the “Company”) develops and sells cosmetics, skin care and other beauty products.
The
Company was organized as a Limited Liability Company (LLC) under the provisions of the Delaware Limited Liability Company Act in 2014
and commenced operations in 2015. The term of the Company shall continue until terminated in accordance with the provisions of the law.
Members’ personal liability for debts is generally limited similar to stockholders’ liability for corporate debts.
On
November 15, 2021, Waldencast Acquisition Corp. (“Waldencast”), a special purpose acquisition company (“SPAC”),
entered into an Agreement and Plan of Merger (the “Obagi Merger Agreement”) with Obagi Global Holdings Limited (“Obagi”)
and an Equity Purchase Agreement (the “Milk Equity Purchase Agreement”) with the Company in transactions contemplated by
the agreements (the “Business Combinations”), resulting in the Company becoming a subsidiary of the combined company, subject
to the satisfaction or waiver of the conditions to closing of the transactions contemplated by the agreements.
| 2. | SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES |
Basis
of Presentation
The
Company’s financial statements are presented in U.S. dollars in accordance with accounting principles generally accepted in the United
States of America (“U.S. GAAP”).
Use
of Estimates
The
preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions in determining
the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements
and the reported amounts of revenues and expenses during the reporting period. These estimates include the allowance for doubtful accounts,
the reserve for inventory obsolescence, and depreciation. As future events and their effects cannot be determined with precision, actual
results could differ from those estimates and assumptions. Significant changes, if any, in those estimates and assumptions resulting
from continuing changes in the economic environment will be reflected in the financial statements in future periods.
Accounts
Receivable
Accounts
receivable, net is stated net of the allowance for doubtful accounts. Payment terms are short-term in nature and are generally less than
one year.
Receivables
are unsecured and are stated at the amount management expects to collect from outstanding balances with a majority due under normal trade
terms requiring payment within 30 days from the invoice date. Customer account balances considered delinquent are evaluated on a case-by-case
basis. Unpaid accounts receivable do not bear interest. Accounts receivable are stated at the amount billed to the customer. Payments
of accounts receivable are allocated to the specific invoices identified on the customer’s remittance advice or to the customer’s account,
if unspecified, until an invoice can be determined by the customer.
Management
provides for probable uncollectible amounts through a charge to earnings and a credit to a valuation allowance based on its assessment
of the current status of individual accounts. This assessment is done on a case-by-case basis as considered necessary. Balances that
are still outstanding after management has used reasonable collection efforts are written off through a charge to the valuation allowance
and a credit to trade accounts receivable. The Company did not have any allowance for doubtful accounts for 2021 and 2020.
Inventories
Inventories
are stated at the lower of cost or net realizable value, based on the average cost method. Included in inventories is an inventory reserve,
which represents the difference between the cost of the inventories and its estimated realizable value. This reserve is calculated using
an estimated obsolescence and excess percentage applied to the inventories based on age and historical results. In addition, and as necessary,
specific reserves for future known or anticipated events may be established.
During
the years ended December 31, 2021 and 2020, the Company identified obsolescence of non-finished goods, specifically manufacturing
components, labels, etc. The total charges during the years ended December 31, 2021 and 2020 as a result of this review was $511
thousand and $1,970, respectively, including destruction of inventories, destruction fees, adjustments to manufacturer and warehouse
reports, and old inventory liability and prepaid deposit write offs. The Company records inventory write-offs as a charge to Cost of
Goods Sold (“COGS”). During the year ended December 31, 2019, the Company did not record any inventory write-offs.
Prepaid
Supplier
Prepaid
supplier consists of down payments made by the Company on manufacturing purchase orders and are stated at the amount paid. Prepaid supplier
is relieved when the inventory related to the manufacturing purchase order, for which a down payment was made, is received by the Company.
As of December 31, 2021 and 2020, respectively, prepaid supplier deposits to amounted to $1,051 thousand and $1,039 thousand.
Property,
Plant and Equipment, net
Property,
plant and equipment is stated at cost less accumulated depreciation or amortization. Leasehold improvements are amortized on a straight-line
basis over the shorter of the lease term or the useful lives of the assets. The cost for maintenance and repairs is expensed as incurred.
Property, plant and equipment that is disposed of through sale, trade-in, or scrapping is written off and any gain or loss on the transaction,
net of costs to dispose, is recorded in Selling, general and administrative expense. Depreciation is computed using straight line method
over the estimated useful lives of the respective assets:
| Description |
|
Estimated
Useful Lives |
| Furniture and fixtures |
|
7 |
| Equipment |
|
3-7 |
| Computers |
|
5 |
| Leasehold improvements |
|
Shorter of estimated
useful life or lease term |
Intangible
Assets, net
Intangible
assets with finite lives, are tested for recoverability whenever events or changes in circumstances indicate that their carrying amount
may not be recoverable. When such events or changes in circumstances occur, a recoverability test is performed comparing projected undiscounted
cash flows from the use and eventual disposition of an asset or asset group to its carrying value. If the projected undiscounted cash
flows are less than the carrying value, an impairment charge would be recorded for the excess of the carrying value over the fair value.
The Company estimates fair value based on the best information available, including discounted cash flows and/or the use of third-party
valuations. There was no impairment adjustment in 2021, 2020, or 2019.
Intangible
assets with finite lives consist of internal use software and other intangibles. Amortization is computed using straight line method
over the useful lives of the respective assets:
| Description |
|
Estimated Useful Lives |
| Internal use software |
|
3-5 |
| Other intangible assets |
|
3 |
Revenue
Recognition
The
Company adopted the new revenue accounting standard, Accounting Standards Codification (ASC 606), under the modified retrospective method
to all contracts as of January 1, 2019. There was no significant impact on the Company’s results of operations or financial
condition upon adoption of the new standard.
The
Company generates revenue from the sale of cosmetics to retailers, including off-price retailers, and sales direct to consumer (“DTC”)
via the company’s website. For the years ended December 31, 2021, 2020, and 2019, the Company derived 93%, 89%, and 93%, respectively,
of its gross revenue from sales to retailers, with the remainder attributed to DTC sales.
The
Company’s revenue contracts represent a single performance obligation to sell its products to its customers. The Company recognizes revenue
at a point in time upon transfer of control, including passage of title to the customer and transfer of risk of loss related to the products,
in an amount that reflects the consideration the Company expects to be entitled to.
For
sales to retailers, transfer of control generally passes upon the pickup of goods by the retailer from the Company’s distribution center,
unless the Company is responsible for shipping the goods, in which case transfer of control passes upon delivery to the retailer. For
DTC sales, the Company charges credit cards in advance of shipment. Transfer of control passes upon delivery to the customer.
For
sales to retailers, the Company collects cash generally in 15 to 45 days depending on the retailer. The Company has not experienced issues
with collectability. For DTC sales, the Company collects cash in advance of shipment. Sales taxes imposed on DTC sales are recorded as
a sales tax liability on the Balance Sheet and do not impact Net revenue.
In
measuring revenue and determining the consideration the Company is entitled to as part of a contract with a customer, the Company takes
into account the related elements of variable consideration. Such elements of variable consideration include product returns and sales
incentives, such as volume rebates and discounts, markdowns, margin adjustments and early-payment discounts. For the sale of goods with
a right of return, the Company only recognizes revenue for the consideration it expects to be entitled to and records a sales return
reserve based on prior history, known events, and projections on sales in the current period. The Company estimates sales incentives
and other variable consideration using the most likely amount method and records a reserve when control of the related product is transferred
to the customer. Under this method, certain forms of variable consideration are based on expected sell-through results, which requires
subjective estimates. These estimates are supported by historical results as well as specific facts and circumstances related to the
current period. A reserve for expected returns or sales incentives is presented as part of Accrued expenses and other current liabilities
on the balance sheet. The reserve is trued up for actual results on an ongoing basis. The Company recorded a reserve for sales returns
and damages of approximately $4,726 thousand and $1,926 thousand as of December 31, 2021 and 2020, respectively.
The
Company’s contract assets consist of accounts receivable, net. The Company’s contract liabilities consist of cash collections
from its customers prior to the delivery of products purchased for DTC sales. These contract liabilities have not been material to the
Company’s financial statements. Accounts receivable decreased in 2021 primarily due to the Company’s strong efforts in collecting
past due balances. Accounts receivable increased in 2020 primarily due to sales to new customers toward the end of 2020 that remained
outstanding as of December 31, 2020.
| | |
Accounts Receivable, Net | | |
Contract Liabilities | |
| (In thousands) | |
2021 | | |
2020 | | |
2021 | | |
2020 | |
| Beginning of year | |
$ | 5,529 | | |
$ | 4,902 | | |
$ | 129 | | |
$ | 61 | |
| End of year | |
$ | 1,802 | | |
$ | 5,529 | | |
$ | 274 | | |
$ | 129 | |
Cost
of Goods Sold
Cost
of goods sold includes the aggregate costs to manufacture products, including the amounts invoice by third-party contract manufacturers
for finished goods, as well as costs related to inbound freight to distribution center and overhead. Cost of goods sold also includes
the effect of changes in the balance of reserves for excess and obsolete inventory.
Shipping
and Handling Expenses
Outbound
shipping and handling expenses, along with product processing fees, are included in selling expenses. Such amounts aggregated to approximately
$2,326 thousand, $2,761 thousand, and $2,567 thousand for the years ended December 31, 2021, 2020, and 2019, respectively.
Advertising
Expenses
Advertising
expenses aggregated approximately $4,142 thousand, $2,572 thousand, and $2,534 thousand for the years ended December 31, 2021, 2020,
and 2019, respectively, are recorded in Selling, general and administrative expenses in the accompanying statements of operations and
comprehensive loss and are expensed as incurred.
Research
and development
Research
and development costs of $139 thousand, $148 thousand, and $97 thousand during the years ended December 31, 2021, 2020, and 2019,
respectively, are recorded in Selling, general and administrative expenses in the accompanying statements of operations and comprehensive
loss and are expensed as incurred.
Income
Taxes
The
Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets (DTA) and
deferred tax liabilities (DTL) for the expected future tax consequences of events that have been included in the financial statements.
Under this method, the Company determined DTAs and DTLs on the basis of the differences between the financial statement and tax bases
of assets and liabilities by using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect
of a change in tax rates on DTAs and DTLs is recognized in income in the period that includes the enactment date.
The
Company recognizes DTAs to the extent the Company believes that these assets are more likely than not to be realized. In making such
a determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences,
projected future taxable income, tax-planning strategies, carryback potential if permitted under the tax law, and results of recent operations.
If the Company determines that we would be able to realize the DTAs in the future in excess of their net recorded amount, the Company
would make an adjustment to the DTA valuation allowance, which would reduce the provision for income taxes.
The
Company records uncertain tax positions in accordance with ASC 740 on the basis of a two-step process in which (1) we determine whether
it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (2) for
those tax positions that meet the more-likely-than-not recognition threshold, the Company recognizes the largest amount of tax benefit
that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority. The Company recognizes interest
and penalties related to UTBs in tax expense.
Fair
Value of Financial Instruments
The
Company accounts for financial instruments in accordance with the ASC 820 — Fair Value Measurements and Disclosures, which provides
a framework for measuring fair value and expands required disclosure about fair value measurements of assets and liabilities. ASC 820
defines fair value as the price that would be received for an asset or paid to transfer a liability in the principal or most advantageous
market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company applies
fair value accounting for all financial assets and liabilities that are recognized or disclosed at fair value in the financial statements
on a recurring basis. The topic also establishes a hierarchy for grouping these assets and liabilities based upon the lowest level of
input that is significant to the fair value measurement. The definition of each input is described below:
| |
● |
Level 1: Inputs based on
quoted market prices for identical assets or liabilities in active markets at the measurement date. |
| |
● |
Level 2: Observable inputs
other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted
prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can
be corroborated by observable market data. |
| |
● |
Level 3: Inputs reflect
management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date.
The inputs are unobservable in the market and significant to the instrument’s valuation. |
As
of December 31, 2021, and 2020, cash consisted of commercial bank account balances, Paypal balances, cash clearing and petty cash
balance. Other financial assets and liabilities including accounts receivable, accounts payable, accrued liabilities, and advances on
credit facility are carried at cost which approximates fair value because of the short-term nature of these instruments.
Concentrations
Sales
directly to Sephora accounts for approximately 69%, 56% and 78% of gross revenue and sales indirectly to Sephora through major retailer
distribution arrangements accounts for approximately 14%, 7% and 10% of gross revenue during the year ended December 31, 2021, 2020,
and 2019, respectively. Accounts receivable related to this same
customer amounted to approximately 27% and 47% of accounts receivable at December 31, 2021 and 2020, respectively. In addition,
sales to one other customer accounted for approximately 7%, 7%, and 10% of gross revenue for the years ended December 31, 2021,
2020, and 2019, respectively. Accounts receivable related to this other customer amounted to approximately 51% of accounts receivable
at December 31, 2021 and was in a credit balance at December 31, 2020, due to an overpayment in 2020, for which the balance
was subsequently reclassified to liabilities.
Purchases
of inventory from seven manufacturers accounted for approximately 70%, 77%, and 67% of total inventory receipts during the year ended
December 31, 2021, 2020, and 2019, respectively. Accounts payable related to these same manufacturers amounted to approximately
24% and 44% of accounts payable as of December 31, 2021 and 2020, respectively. There are no other concentration risks in expenditures
during the year ended December 31, 2021 or in accounts payable as of December 31, 2021.
The
Company imports a substantial portion of its purchases from suppliers in other countries. Although there are a number of suppliers who
could provide the Company’s products, a change in suppliers could cause a delay in purchasing and loss of sales, which could adversely
affect operating results. These suppliers are subject to various political, economic, and other risks and uncertainties inherent in the
countries in which they operate.
The
Company maintains its cash in two commercial banks. The amounts held in this account may, at times, exceed federally insured limits.
The Company believes there is no significant risk with respect to these deposits. The amount in excess as of December 31, 2021 and
2020 was approximately $3,917 thousand and $6,800 thousand, respectively.
Equity
Based Compensation
The
Company measures and recognizes the cost of employee services received in exchange for an award of equity instruments. Equity-based compensation
expenses are measured at the grant date, based on the fair value of the award, and recognized on a straight-line basis over the requisite
service period. The Company has elected to account for its graded vesting options on a straight-line basis over the requisite service
period for the entire award. Awards granted to non- employees are accounted for in accordance with the pronouncement addressing equity-based
compensation issued to non-employees. The Company elected to account for forfeitures as they occur and to utilize the practical expedient
to estimate the expected term of all awards.
Liquidity
During
the year ended December 31, 2021, the Company incurred a net loss of approximately $7,847 thousand and had cash used in operating
activities of approximately $1,740 thousand. To address future liquidity, the Company has a line of credit financing agreement and additional
equity financing in 2020 (see Notes 6 and 7). The novel coronavirus (COVID-19), that was declared a global pandemic, had a direct impact
on Company’s operations and financial performance in 2021 and 2020. Management took immediate action to secure financing and increased
the Company’s cash position in response to these events. Management has concluded that as of the financial statement issuance date, the
combination of these financing vehicles and the return to sales growth in 2022 will provide enough liquidity for a period not less than
one year from the date the financial statements were available to be issued. However, the extent to which COVID-19 impacts the Company’s
operations and financial performance depends on future developments that are uncertain and unpredictable, including the duration and
spread of the pandemic.
Recently
Adopted Accounting Pronouncements
No
new accounting pronouncement have been recently issued or newly effective which would have or be expected to have a material impact on
the Company’s financial statements.
Recently
Issued and Not Yet Adopted Accounting Pronouncements
In
June 2016, the Financial Accounting Standards Board (FASB) issued ASU 2016-13, Financial Instruments — Credit Losses: Measurement
of Credit Losses on Financial Instruments, and subsequent related amendments, which requires the use of a new current expected credit
loss (“CECL”) model in estimating allowances for doubtful accounts with respect to accounts receivable, and notes receivable.
Receivables from revenue transactions, or trade receivables, are recognized when the corresponding revenue is recognized under ASC 606,
Revenue from Contracts with Customers. The CECL model requires that the Company estimate its lifetime expected credit loss with respect
to these receivables and records allowances when deducted from
the balance of the receivables, which represent the estimated net amounts expected to be collected. Given the generally short-term nature
of trade receivables, the Company does not expect to apply a discounted cash flow methodology. However, the Company will consider whether
historical loss rates are consistent with expectations of forward-looking estimates for its trade receivables. This ASU (collectively
“ASC 326”) is effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal
years. The Company is in the process of evaluating the effects of adopting this ASU on its financial statements.
In
February 2016, the FASB issued ASU 2016-02, Leases (ASC 842), which requires lessees to recognize a right-of-use asset and
lease liability for all leases with terms of more than 12 months. Recognition, measurement and presentation of expenses will depend on
classification as finance or operating lease. ASU 2016-02 will be effective for fiscal years beginning after December 15, 2020.
On April 8, 2020, the F
ASB, pursuant
to ASU 2020-05, voted to defer the effective date for ASC 842 for one year. For private companies, the leasing standard will be effective
for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022.
Early adoption is permitted.
The
Company expects to adopt the new standard on January 1, 2022 in connection with the issuance of the annual financial statements
for the year ended December 31, 2022 using the modified retrospective approach. Therefore, upon adoption, the Company will recognize
and measure leases without revising comparative period information or disclosures.
The
Company plans to implement the transition package of three practical expedients permitted within the standard, which allows companies
not to reassess whether agreements contain leases, the classification of leases, and the capitalization of initial direct costs. The
Company will make an accounting policy election to recognize lease expense for leases with a term of 12 months or less on a straight-line
basis over the lease term and will not recognize any right-of-use assets or lease liabilities for those leases.
The
Company expects the standard to result in a material increase in long-term assets and long-term liabilities related to operating leases
currently not recorded on the balance sheet. The Company is currently completing its implementation efforts and is continuing to evaluate
other possible impacts of the adoption of this standard on its financial statements and related disclosures.
Reclassifications
Certain
reclassifications have been made to the financial statements that affected the presentation on the balance sheets as of December 31,
2021 and 2020, and statements of operations and comprehensive loss for the years ended 2021, 2020 and 2019. These reclassifications included
amounts previously presented within Accrued bonuses and Other current liabilities line items that are now combined and presented in Accrued
expenses and other current liabilities on the balance sheets as of December 31, 2021 and 2020, amounts related to the sales returns
and damages allowance previously presented as contra-assets within Accounts receivable, net that are reclassified to Accrued expenses
and other current liabilities on the balance sheets as of December 31, 2021 and 2020, amounts previously presented within Other
(income) expense that are reclassified to Cost of goods sold on the statement of operations for the year ended December 31, 2020,
and Depreciation and Amortization amounts that are reclassified from non-operating expense to operating expense on the statements of
operations and comprehensive loss for the years ended December 31, 2020 and 2019.
Inventory
is comprised of the following:
| | |
As of December 31, | |
| (In
thousands) | |
2021 | | |
2020 | |
| Components | |
$ | 6,149 | | |
$ | 4,704 | |
| Finished goods | |
| 15,619 | | |
| 12,451 | |
| | |
| 21,768 | | |
| 17,155 | |
| Less: Inventory reserve | |
| 340 | | |
| 770 | |
| Total inventories | |
$ | 21,428 | | |
$ | 16,385 | |
| 4. |
PROPERTY, PLANT AND
EQUIPMENT, NET |
Property,
plant and equipment are comprised of the following:
| | |
As of December 31, | |
| (In
thousands) | |
2021 | | |
2020 | |
| Furniture and fixtures | |
$ | 197 | | |
$ | 206 | |
| Equipment | |
| 5,578 | | |
| 4,724 | |
| Computers | |
| 297 | | |
| 255 | |
| Leasehold improvements | |
| 1,984 | | |
| 1,925 | |
| Construction in progress | |
| 342 | | |
| — | |
| Property, plant and equipment, gross | |
| 8,398 | | |
| 7,110 | |
| Less: accumulated depreciation | |
| 3,428 | | |
| 1,618 | |
| Property, plant and equipment, net | |
$ | 4,970 | | |
$ | 5,492 | |
Depreciation
expense of property, plant and equipment totaled $1,961 thousand, $1,451 thousand and $2,152 thousand for the year ended December 31,
2021, 2020, and 2019, respectively. Equipment predominantly includes retailer makeup gondolas. In 2019, it was determined that a majority
of the US gondolas would be replaced with new gondolas in early 2020. As a result, depreciation in the amount of $1,136 thousand was
accelerated in 2019 to reflect the new useful life of the gondolas. In 2020, the fully depreciated assets were written off after they
were replaced by the new gondolas, which were capitalized to Equipment. In 2021, the gondolas that were instated in 2020 were written-off
and replaced with larger gondolas that could hold more products. Loss on disposal of old gondolas totaled $166 thousand, $44 thousand
and $42 thousand for the year ended on December 31, 2021, 2020, and 2019, respectively.
| 5. |
INTANGIBLE ASSETS, NET |
The
following table presents the Company’s intangible assets and the related amortization:
| | |
December 31, 2021 | | |
December 31, 2020 | |
| (In
thousands) | |
Gross
Carrying
Amount | | |
Accumulated
Amortization | | |
Net | | |
Gross
Carrying
Amount | | |
Accumulated
Amortization | | |
Net | |
| Internal use software | |
$ | 1,180 | | |
$ | 1,180 | | |
$ | — | | |
$ | 1,180 | | |
$ | 1,166 | | |
$ | 14 | |
| Total | |
$ | 1,180 | | |
$ | 1,180 | | |
$ | — | | |
$ | 1,180 | | |
$ | 1,166 | | |
$ | 14 | |
Amortization
expense was $14 thousand, $295 thousand, and $384 thousand for the years ended December 31, 2021, 2020, and 2019, respectively.
As of December 31, 2021, all intangible assets subject to amortization were fully amortized.
The
Company evaluates the recoverability of identifiable intangible assets whenever events or changes in circumstances indicate that an intangible
asset’s carrying amount may not be recoverable. The evaluation of asset impairment requires the Company to make assumptions about future
cash flows over the life of the asset being evaluated. These assumptions require significant judgment and actual results may differ from
assumed and estimated amounts. During the years ended December 31, 2021, 2020, and 2019, no impairment loss was recognized.
| 6. |
ACCRUED EXPENSES AND OTHER
CURRENT LIABILITIES |
Accrued
expenses and other current liabilities consist of the following:
| | |
As of December 31, | |
| (In
thousands) | |
2021 | | |
2020 | |
| Accrued sales returns and damages | |
$ | 4,726 | | |
$ | 1,926 | |
| Accrued bonuses | |
| 1,350 | | |
| 657 | |
| Accrued payroll and other non-income taxes | |
| 296 | | |
| 288 | |
| Deferred revenue | |
| 274 | | |
| 548 | |
| Others | |
| 89 | | |
| 205 | |
| Accrued expenses and other current liabilities | |
$ | 6,735 | | |
$ | 3,624 | |
Convertible
Promissory Note
During
2018, the Company entered into a secured convertible promissory note in the amount of $5,000 thousand (the “Note”). The Note
bore interest at a rate of 2.76% per annum and had a maturity date on the earlier of (i) March 31, 2019, or (ii) an event
of default. It contained an automatic equity settlement following a qualified financing, as defined in the Note Agreement, such that
settlement would occur at 80% of the price per unit in the qualified financing transaction. The Note contained additional optional equity
settlement following a financing transaction that did not satisfy the requirements of a qualified financing. In such case, settlement
would also occur at 80% of the price per unit in that subsequent financing. The Note also contained a fixed price conversion such that
the holder may elect to receive Redeemable Series B Preferred Units at a price per share of $4.40. The Note may also be settled in cash
following a sale of the Company. In such case, the Note would be settled at two times the entire unpaid principal of the Note, together
with any accrued and unpaid interest. The Note was collateralized by all inventory assets and open accounts receivables. The Company
identified embedded derivatives related to the above-described variable equity settlements and the redemption following the sale of the
Company. Upon the closing of the Redeemable Series C Preferred Unit financing on January 24, 2019, the Note automatically converted
into 1,033,886 authorized Redeemable Series C Preferred Units. At the time of the conversion, the outstanding principal amount and all
accrued interest on the convertible note amounted to $5,021 thousand. During the year ended December 31, 2019 the Company recognized
approximately $1,276 thousand of non-cash interest expense related to the above-described embedded features. Upon conversion, the Note,
including all the above-described embedded derivatives were derecognized. The change in fair value of the embedded derivatives during
the year ended December 31, 2019 was not material.
Line
of Credit
In
October 2019, the Company entered into a Loan and Security Agreement, which provided the Company with a line of credit that would mature
on April 10, 2021. The Company may borrow advances in an aggregate outstanding amount not to exceed the lesser of $12,500 thousand
or the gross profit borrowing base, in each case inclusive of any amounts reserved under the Ancillary Services Sublimit as defined in
the Loan and Security Agreement. Advances under the line of credit bore interest on the outstanding daily balance at a variable rate
equal to or greater of 0.50% above the Prime Rate then in effect or 6.00%.
In
May 2020, the Company amended its Loan and Security Agreement (the “First Amendment”) to adjust the amount of advances that
the Company may borrow to the lesser of $8,000 thousand or the gross profit borrowing base, in each case inclusive of any amounts reserved
under the Ancillary Services Sublimit as defined in the First Amendment.
In
November 2020 and February 2021, the Company amended its Loan and Security Agreement and previous amendment (the “Second Amendment”
and the “Third Amendment” respectively) to revise the minimum EBITDA target for each reporting period under the financial
covenant.
In
April 2021, the Company amended its Loan and Security Agreement and previous amendments (the “Fourth Amendment”). The Fourth
Amendment extended the maturity date through April 10, 2022. The Company may borrow advances under a revolving line based on inventory
in an aggregate outstanding principal amount not to exceed the lesser of $8,000 thousand or the inventory borrowing base, as defined
in the Fourth Amendment, and in each case inclusive of any amounts reserved under the Ancillary Services Sublimit. The Company may also
borrow advances under a revolving line based on accounts receivable in an aggregate outstanding principal amount not to exceed the lesser
of $5,000 thousand or the accounts receivable borrowing base, as defined in the Fourth Amendment. The Company may borrow advances in
an aggregate outstanding amount, under both the inventory revolving line and the accounts receivable revolving line, not to exceed $8,000
thousand. Advances under the inventory revolving line of the Fourth Amendment bore interest at a variable annual rate equal to the greater
of 0.50% above the Prime Rate then in effect or 6.00%. Advances under the accounts receivable revolving line of the Fourth Amendment
bore interest at a variable annual rate equal to the greater of 0.25% above the Prime Rate then in effect or 5.50%. Prior to the Fourth
Amendment, advances under the Loan and Security Agreement bore interest at a variable annual rate equal to the greater of 0.5% above
the Prime Rate then in effect or 6.00%.
In
November 2021, the Company amended its Loan and Security Agreement and previous amendments (the “Fifth Amendment”) to adjust
the limit of amount that can be held in other bank accounts to $1,500 thousand.
In
March 2022, the Company amended its Loan and Security Agreement and previous amendments (the “Sixth Amendment”). The Sixth
Amendment extended the maturity date through April 10, 2023. The Company may borrow advances under a revolving line based on inventory
in an aggregate outstanding principal amount not to exceed the lesser of $15,000 thousand or the inventory borrowing base, as defined
in the Sixth Amendment, and in each case inclusive of any amounts reserved under the Ancillary Services Sublimit. The Company may also
borrow advances under a revolving line based on accounts receivable in an aggregate outstanding principal amount not to exceed the lesser
of $8,000 thousand or the accounts receivable borrowing base, as defined in the Sixth Amendment. The Company may borrow advances in an
aggregate outstanding amount, under both the inventory revolving line and the accounts receivable revolving line, not to exceed $15,000
thousand. Advances under the inventory revolving line of the Sixth Amendment bear interest at a variable annual rate equal to the greater
of 0.50% above the Prime Rate then in effect or 5.25%. Advances under the accounts receivable revolving line of the Sixth Amendment bear
interest at a variable annual rate equal to the greater of 0.25% above the Prime Rate then in effect or 4.50%.
As
of December 31, 2021, and 2020, the Company did not have any outstanding borrowings under the line of credit. For the year ended
December 31, 2021, 2020, and 2019, respectively, the Company incurred $0 thousand, $49 thousand, and $0 thousand
in interest on outstanding borrowings.
The
line of credit is subject to certain financial and restrictive covenants, including but not limited to, minimum monthly EBITDA amounts,
limitations on incurrence of indebtedness and liens, restrictions on affiliate transactions, restrictions on the sale or other disposition
of collateral, and maintaining cash in other bank accounts.
| 8. |
REDEEMABLE PREFERRED UNITS
AND MEMBERS’ EQUITY |
The
Company’s operating agreement as amended states:
| |
● |
The Founding
Member (as defined) acquired 10,000,000 Common Units for all contributions and considerations through December 31, 2016. The
Founding Member converted $1,276 thousand of due to affiliates to contributions to the Company during the year ended December 31,
2017. |
| |
● |
$10,000
thousand was contributed for the purchase of 2,500,000 authorized Redeemable Series A Preferred Units with the purchaser being admitted
as a member (Preferred Member #1) in 2016. Preferred Member #1 contributed $2,875 thousand for an additional 718,750 Redeemable Series
A Preferred Units and the Founding Member contributed $2,500 thousand for 625,000 Redeemable Series A Preferred Units during the
year ended December 31, 2017. |
| |
● |
Preferred
Member #1 contributed $10,000 thousand for 2,272,727 authorized Redeemable Series B Preferred Units during the year ended December 31,
2018 |
| |
● |
$15,000
thousand was contributed for the purchase of 2,471,169 authorized Redeemable Series C Preferred Units with the purchaser being admitted
as a member (Preferred Member #2) during the year ended December 31, 2019. The convertible promissory note issued during 2018
by Preferred Member #1 automatically converted into 1,033,886 authorized Redeemable Series C Preferred Units. At the time of the
conversion, the outstanding principal amount and all accrued interest on the convertible note amounted to $5,021 thousand. During
the year ended December 31, 2019 the Company recognized approximately $1,276 thousand of non-cash interest expense related to
the embedded features of variable equity settlements and the redemption following the sale of the Company, as described in Note 7. |
| |
● |
$10,000
thousand was contributed for the purchase of 915,751 authorized Redeemable Series D Preferred Units with the purchaser being admitted
as a member (Preferred Member #3) during the year ended December 31, 2019. Preferred Member #3 contributed an additional $10,000
thousand for the purchase of 982,318 authorized Redeemable Series D Preferred Units during the year ended December 31, 2020. |
Common
Units have the right to cast one vote per Unit. Redeemable Preferred Units have the right to vote the number of votes equal to the number
of the Common Units to which they are equivalent.
Redeemable
Preferred Units shall be convertible, at the option of the holders thereof, at any time and from time to time, and without the
payment of additional consideration by the holders thereof, into such number of Common Units as is determined by dividing the
original purchase price of such Redeemable Preferred Unit by the conversion price of such Redeemable Preferred Unit in effect at the
time of conversion. On or after December 23, 2021, the holders of Redeemable Preferred Units may request redemption of all
Preferred Units at the sole option of the holders holding a majority of the outstanding Preferred Units. The Company records
Redeemable Preferred Units in temporary
equity at an initial amount equal to the proceeds received. The Company subsequently remeasures Redeemable Preferred Units to redemption
value equal to fair value at each reporting date. Increases in the redemption value are recorded in full and decreases in the carrying
amount are only recorded to the extent that increases to the carrying value were previously recorded. In all cases, the offset to the
changes in Redeemable Preferred Unit carrying value is recorded against the carrying value of Common Units, as the Company is in an accumulated
loss position.
Net
profits and losses of the Company are allocated among the Members in such ratio or ratios as may be required to cause the balances of
the members’ economic capital accounts to equal, as nearly as possible, their Target Balances (as defined).
Company
distributions, including liquidating distributions, are distributed first to the Redeemable Preferred Units pro rata until each such
member has received an amount equal to their original purchase price and then pro rata to members holding the Common Units.
The
sale of the Company (as defined) requires gross proceeds received by holders of the Redeemable Preferred Units (when combined with all
other prior distributions) equal to at least three times the Redeemable Preferred Units’ original issue price.
The
Company may issue additional units or classes or series of Units, having such designations, preferences and relative, participating or
other special rights, powers and duties, as the Company shall determine. The Members understand and agree that rights afforded to any
additional classes or series of Units (including, without limitation, rights to Company distributions) may result in a reduction and/or
dilution in the rights of then outstanding Units.
The
complete respective rights, preferences, privileges and restrictions of the Redeemable Preferred Units and Common Units are set forth
in the agreement.
| 9. |
FAIR VALUE MEASUREMENTS |
The
Company records certain of its financial liabilities at fair value, which is defined as the price that would be paid to transfer a liability,
in the principal or most advantageous market for the liability, in an orderly transaction between market participants at the measurement
date. The accounting for fair value measurements must be applied to nonfinancial assets and nonfinancial liabilities that require initial
measurement or remeasurement at fair value, which principally consist of intangible assets and long-lived assets for the purposes of
calculating potential impairment. The Company is required to maximize the use of observable inputs and minimize the use of unobservable
inputs when measuring fair value.
In
2019, the Company issued Warrant Agreements giving the lender the option to purchase Redeemable Series C and Series D preferred
units. The Company measured and recorded the fair value of the warrant liabilities using the Black-Scholes model. The amount was recorded
as a long-term liability on the balance sheet due to the underlying redemption feature. Warrant liabilities are valued using unobservable
market inputs and, as such, are considered to be level 3 inputs.
The
following table presents the Company’s hierarchy for its financial liabilities measured at fair value on a recurring basis as of
December 31, 2021:
| (In
thousands) | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities: | |
| | |
| | |
| | |
| |
| Warrant Liabilities | |
| — | | |
| — | | |
$ | 121 | | |
$ | 121 | |
| Total | |
| — | | |
| — | | |
$ | 121 | | |
$ | 121 | |
The
following table presents the Company’s hierarchy for its financial liabilities measured at fair value on a recurring basis as of
December 31, 2020:
| (In
thousands) | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities: | |
| | |
| | |
| | |
| |
| Warrant Liabilities | |
| — | | |
| — | | |
$ | 99 | | |
$ | 99 | |
| Total | |
| — | | |
| — | | |
$ | 99 | | |
$ | 99 | |
The
following table presents the changes in fair value of the Company’s financial liabilities measured on a recurring basis, classified
as Level 3, during the 12 months ended December 31, 2021:
| (In
thousands) | |
Year ended December 31,
2021 | |
| Opening balance | |
$ | 99 | |
| Change in fair value | |
| 22 | |
| Ending balance | |
$ | 121 | |
| 10. |
EQUITY BASED COMPENSATION
EXPENSE |
The
Company’s Appreciation Rights Plan (“ARP”), Incentive Rights Plan (“IRP”), and Enterprise Management Incentive
(EMI) options for its employees and officers provides for granting of appreciation rights awards, incentive awards, and options at the
discretion of the Board of Directors. Appreciation rights awards, incentive awards, and options have no voting rights. The Company measures
awards using fair values as determined by the Black-Scholes model.
Under
the ARP, the total number of units that may be awarded is 2,371,856 common units. Awards granted during 2021 and 2020 totaled 99,000
and 1,385,206 units, respectively. Award terms are 10 years. Awards granted in 2021 vest 25% on vesting commencement date and the
remaining units vest in equal monthly installments over the following thirty-six months, contingent upon continued employment or service.
Awards granted in 2020 vest in four equal 25% installments on the anniversary date of the vesting commencement date, contingent upon
continued employment or service.
Under
the EMI, the total number of units that may be awarded is 161,607 common units. Awards granted during 2021 totaled 161,607 units. Award
terms are 10 years. Awards granted in 2021 vest 25% on the first anniversary of the vesting commencement date and the remaining
vest in equal monthly installments over the following three years, contingent upon continued employment or service.
The
appreciation right and option awards granted to employees and non-employees were accounted for as equity-classified awards. The awards
are issued as equity appreciation rights or options in accordance with the respective award letters and may be settled in cash or units
at the sole option of the Company. During the year ended December 31, 2021, 2020, and 2019, $77 thousand, $152 thousand and $238
thousand of equity-based compensation costs was recognized, respectively.
No
awards were reserved or granted under the IRP as of December 31, 2021.
The
following summarizes the Company’s ARP, EMI, and activity for the year ended December 31, 2021:
| (In
thousands, except per share data) | |
Appreciation Rights and
Options | | |
Weighted
Average Exercise
Price | | |
Aggregate Intrinsic
Value | | |
Weighted
Average Remaining Contractual
Term (Years) | |
| Balance at December 31, 2020 | |
| 2,071,728 | | |
$ | 4.52 | | |
$ | 168 | | |
| 8.89 | |
| Awards granted | |
| 260,607 | | |
| 5.63 | | |
| — | | |
| 9.69 | |
| Awards exercised | |
| — | | |
| — | | |
| — | | |
| — | |
| Awards forfeited | |
| — | | |
| — | | |
| — | | |
| — | |
| Balance at December 31, 2021 | |
| 2,332,335 | | |
$ | 4.65 | | |
$ | 168 | | |
| 8.10 | |
| Exercisable at December 31,2021 | |
| 918,350 | | |
$ | 3.26 | | |
$ | 168 | | |
| 7.61 | |
As
of December 31, 2021, the total unrecognized compensation cost related to unvested awards was $534 thousand and the related
weighted-average period over which it is expected to be recognized is approximately 3.5 years.
Valuation
Assumptions
The
Company estimates the fair value of each appreciation right and option grant using the Black-Scholes model with the following weighted-average
fair value per share at grant date and assumptions:
| | |
Year Ended
December 31, | |
| | |
2021 | | |
2020 | | |
2019 | |
| Weighted-average fair value per share at grant date | |
$ | 2.34 | | |
$ | 0.00 | | |
$ | 0.00 | |
| Expected term | |
| 10 years | | |
| 7 years | | |
| 7 years | |
| Volatility | |
| 57.5 | % | |
| 55.00 | % | |
| 47.69 | % |
| Risk-free interest rate | |
| 1.52 | % | |
| 0.65 | % | |
| 1.46 | % |
| Distributions yield | |
| 0 | % | |
| 0 | % | |
| 0 | % |
Expected
volatility: The volatility factor for the Company’s unit-based options was estimated using publicly available trading data, which was
used to estimate the Company’s volatility, had the Company been public.
Expected
distributions yield: The Company does not anticipate that distributions will be made in the near future.
Expected
term: The Company’s expected term represents the period that the awards are expected to be outstanding and was determined as a function
of contractual terms of the unit-based awards and vesting schedules. The Company uses the ;simplified method of calculation for estimating
expected term.
Risk-Free
Interest Rate: The Company bases the risk-free interest rate used in the Black-Scholes model on implied yield currently available on
U.S. Treasury zero-coupon issues with an equivalent remaining term.
In
conjunction with the Loan and Security Agreement (see Note 6) signed in 2019, the Company issued Warrant Agreements (the “warrants”)
giving the lender the option to purchase 10,297 of Redeemable Series C Preferred Units and 6,139 of Redeemable Series D Preferred Units.
The Company measured and recorded the fair value of the warrant liabilities using the Black-Scholes model. The fair value of the warrants
amounted to approximately $121 thousand and $99 thousand as of December 31, 2021 and 2020, respectively, and was recorded
as a long-term liability on the balance sheet due to the underlying redemption feature. In accordance with liability-classified award
accounting, the company remeasures the fair value of the warrant liabilities using the Black-Scholes model annually. Changes in fair
value of the warrant liabilities are recorded in Interest expense, net.
Valuation
Assumptions
The
Company estimated the fair value of the warrants using the Black-Scholes model.
| | |
Year Ended
December 31, | |
| | |
2021 | | |
2020 | |
| Expected term | |
| 8 years | | |
| 10 years | |
| Volatility | |
| 50.00 | % | |
| 55.00 | % |
| Risk-free interest rate | |
| 1.44 | % | |
| 0.93 | % |
| Distributions yield | |
| 0 | % | |
| 0 | % |
Expected
volatility: The volatility factor for the Company’s warrants was estimated using publicly available trading data, which was used to estimate
the Company’s volatility, had the Company been public.
Expected
distributions yield: The Company does not anticipate that distributions will be made in the near future.
Expected
term: The Company’s expected term represents the contractual term as stated in the warrant agreements.
Risk-Free
Interest Rate: The Company bases the risk-free interest rate used in the Black-Scholes model on implied yield currently available on
U.S. Treasury zero-coupon issues with an equivalent remaining term.
| 12. |
RELATED PARTY TRANSACTIONS |
The
Company receives certain administrative services from affiliated companies related through common ownership. Such amounts are included
in rent and management fee expenses and amounted to $60 thousand, $678 thousand, and $992 thousand for the years ended
December 31, 2021, 2020, and 2019, respectively. During 2020, the Company moved to a new office and will no longer incur rent related
fees from affiliated companies.
The
amounts due from officers are due and payable on July 30, 2026 or an earlier date (as defined) and July 31, 2029 or an earlier
date (as defined), and bear interest rate of 1.90% per year. One officer loan bears interest at 1.7%.
The
members of the company are committed to provide the necessary funding in the form of advances and capital contributions to fund the company’s
operations as needed.
The
Company maintains a 401(k) plan for the benefit of eligible employees. The plan allows participants to defer salary up to the maximum
amount for the year as specified in the Internal Revenue Code and regulations thereunder. The Company may make matching contributions
at its discretion. Company contributions were $221 thousand, $175 thousand, and $152 thousand for the years ended December 31,
2021, 2020, and 2019, respectively.
The
Company has operating leases primarily for offices with terms up to 10 years and rent escalations every 12 months. The Company incurred
rent expense of $1,479 thousand, $1,712 thousand and $872 thousand for the years ended December 31, 2021, 2020, and 2019, respectively.
Future
minimum lease payments for the company operating leases:
| (In
thousands) | |
Year Ended December 31, | |
| 2022 | |
$ | 1,291 | |
| 2023 | |
| 1,434 | |
| 2024 | |
| 1,463 | |
| 2025 | |
| 1,492 | |
| 2026 | |
| 1,522 | |
| Thereafter | |
| 6,260 | |
| Total future minimum lease payments | |
$ | 13,462 | |
| 15. |
COMMITMENTS & CONTINGENCIES |
The
Company is at times involved in various claims and legal actions. While the Company cannot predict any final outcomes relating thereto,
management believes that the outcome of current claims and legal actions will not have a material adverse impact on the financial position
of the Company or the results of its operations.
The
Company is treated as a partnership for U.S. federal income tax purposes and, as such, is not subject to any entity-level U.S. federal
income tax. Instead, for U.S. federal income tax purposes, taxable income of the Company is allocated to the Company’s unitholders.
The Company is subject to certain state, local and foreign income taxes.
Income
tax expense (benefit) was zero for the years ended December 31, 2021, 2020, and 2019, which is attributable to the partnership not
being subject to U.S. federal income tax and minimal foreign and state taxes generating losses that cannot be benefited due to having
a full valuation allowance.
As
of December 31, 2021, and 2020, the Company did not have material deferred tax assets. As of December 31, 2021, the Company
had a gross state operating loss carryforward of approximately $3,376 thousand (tax effected balance of $135 thousand), which will begin
to expire in 2035. As of December 31, 2021, the Company had a gross foreign operating loss carryforward of approximately $860 thousand
(tax effected balance of $215 thousand), which will be carried forward indefinitely. Management assesses the available positive and negative
evidence to estimate whether sufficient future taxable income will be generated to permit use of the existing DTAs. On the basis of this
evaluation, the Company has recorded a full valuation allowance for each period.
During
the years ended December 31, 2021, 2020, and 2019, the Company did not have any unrecognized tax benefits related to uncertain tax
positions. As of December 31, 2021, we are no longer subject to U.S. state, local, or foreign examinations by tax authorities for
years before 2018.
The
Company has evaluated subsequent events through April 12, 2022, the date the financial statements were available to be issued.
On
March 24, 2022, the Company amended its Loan and Security Agreement and previous amendments (the “Sixth Amendment”).
The Sixth Amendment extended the maturity date through April 10, 2023. See Note 7 — Debt for additional information.
Milk Makeup LLC
Unaudited Condensed Statements of Income and
Comprehensive Income
| | |
Six Months Ended
June 30, | |
| (In thousands) | |
2022 | | |
2021 | |
| Net sales | |
$ | 38,548 | | |
$ | 26,580 | |
| Cost of goods sold (exclusive of depreciation and amortization) | |
| 13,365 | | |
| 11,297 | |
| Selling, general and administrative | |
| 18,815 | | |
| 12,415 | |
| Depreciation and amortization | |
| 1,169 | | |
| 937 | |
| Operating income | |
| 5,199 | | |
| 1,931 | |
| Interest expense (income), net | |
| 21 | | |
| (25 | ) |
| Other expense, net | |
| 217 | | |
| (64 | ) |
| Income before provision for income taxes | |
| 4,961 | | |
| 2,020 | |
| Income tax provision | |
| - | | |
| - | |
| Net income | |
$ | 4,961 | | |
$ | 2,020 | |
| Comprehensive income | |
$ | 4,961 | | |
$ | 2,020 | |
The accompanying notes are an integral part of the unaudited
condensed financial statements.
Milk Makeup LLC
Condensed Balance Sheets
| | |
June 30, | | |
December 31, | |
| (In thousands, except share data) | |
2022 (Unaudited) | | |
2021
(Audited) | |
| ASSETS | |
| | |
| |
| Current assets | |
| | |
| |
| Cash | |
$ | 2,666 | | |
$ | 4,417 | |
| Accounts receivable, net | |
| 6,390 | | |
| 1,802 | |
| Inventories | |
| 24,835 | | |
| 21,428 | |
| Prepaid expenses and other current assets | |
| 453 | | |
| 610 | |
| Prepaid supplier | |
| 387 | | |
| 1,051 | |
| Total current assets | |
| 34,731 | | |
| 29,308 | |
| Property, plant and equipment, net | |
| 5,938 | | |
| 4,970 | |
| Due from officers | |
| 780 | | |
| 774 | |
| Total assets | |
$ | 41,449 | | |
$ | 35,052 | |
| | |
| | | |
| | |
| LIABILITIES, REDEEMABLE PREFERED UNITS, AND MEMBERS’ EQUITY | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable | |
$ | 6,590 | | |
$ | 6,477 | |
| Tenant allowance liability | |
| 161 | | |
| 132 | |
| Accrued expenses and other current liabilities | |
| 6,328 | | |
| 6,735 | |
| Line of credit | |
| 1,500 | | |
| - | |
| Total current liabilities | |
| 14,579 | | |
| 13,344 | |
| Warrant liabilities | |
| 148 | | |
| 121 | |
| Deferred rent – non-current | |
| 1,172 | | |
| 1,063 | |
| Tenant allowance liability – non-current | |
| 978 | | |
| 1,044 | |
| Total liabilities | |
| 16,877 | | |
| 15,572 | |
| COMMITMENTS AND CONTINGENCIES | |
| | | |
| | |
| Redeemable Series A Preferred units, 3,843,750 units authorized, issued and outstanding as of June 30, 2022 and December 31, 2021 | |
| 44,319 | | |
| 23,601 | |
| Redeemable Series B Preferred units, 2,272,727 units authorized, issued and outstanding as of June 30, 2022 and December 31, 2021 | |
| 26,227 | | |
| 14,136 | |
| Redeemable Series C Preferred units, 3,515,352 units authorized, and 3,505,055 issued and June 30, 2022 and December 31, 2021 | |
| 46,373 | | |
| 34,210 | |
| Redeemable Series D Preferred units, 1,904,208 units authorized as of June 30, 2022 and December 31, 2021, and 1,898,069 units issued and outstanding as of June 30, 2022 and December 31, 2021 | |
| 30,237 | | |
| 27,335 | |
| Members’ Equity: | |
| | | |
| | |
| Common units, 24,069,500 units authorized as of June 30, 2022 and December 31, 2021, and 10,000,000 units issued and outstanding as of June 30, 2022 and December 31, 2021 | |
| - | | |
| - | |
| Members’ (deficit) | |
| (122,584 | ) | |
| (79,802 | ) |
| Total members’ (deficit) | |
| (122,584 | ) | |
| (79,802 | ) |
| TOTAL LIABILITIES, REDEEMABLE PREFERRED UNITS, AND MEMBERS’ EQUITY | |
$ | 41,449 | | |
$ | 35,052 | |
The accompanying notes are an
integral part of the unaudited condensed financial statements.
Milk Makeup LLC
Unaudited Condensed Statements of Redeemable
Preferred Units and Members’ Equity
(In thousands, except share and per share data)
| | |
Redeemable Series A
Preferred Units | | |
Redeemable Series B
Preferred Units | | |
Redeemable Series C
Preferred Units | | |
Redeemable Series D
Preferred Units | | |
Common Units | | |
| | |
| |
| | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Members’ Equity | | |
Total Members’ Equity | |
| Balance as of December 31, 2021 (Audited) | |
| 3,843,750 | | |
$ | 23,601 | | |
| 2,272,727 | | |
$ | 14,136 | | |
| 3,505,055 | | |
$ | 34,210 | | |
| 1,898,069 | | |
$ | 27,335 | | |
| 10,000,000 | | |
$ | - | | |
$ | (79,802 | ) | |
$ | (79,802 | ) |
| Change in redemption value of Redeemable Preferred Units | |
| - | | |
| 20,718 | | |
| - | | |
| 12,091 | | |
| - | | |
| 12,163 | | |
| - | | |
| 2,902 | | |
| - | | |
| - | | |
| (47,874 | ) | |
| (47,874 | ) |
| Equity-based compensation expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 131 | | |
| 131 | |
| Net income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 4,961 | | |
| 4,961 | |
| Balance as of June 30, 2022 | |
| 3,843,750 | | |
$ | 44,319 | | |
| 2,272,727 | | |
$ | 26,227 | | |
| 3,505,055 | | |
$ | 46,373 | | |
| 1,898,069 | | |
$ | 30,237 | | |
| 10,000,000 | | |
$ | - | | |
$ | (122,584 | ) | |
$ | (122,584 | ) |
| | |
Redeemable Series A
Preferred Units | | |
Redeemable Series B
Preferred Units | | |
Redeemable Series C
Preferred Units | | |
Redeemable Series D
Preferred Units | | |
Common Units | | |
| | |
| |
| | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Units | | |
Amount | | |
Members’ Equity | | |
Total Members’ Equity | |
| Balance as of December 31, 2020 (Audited) | |
| 3,843,750 | | |
$ | 15,375 | | |
| 2,272,727 | | |
$ | 10,000 | | |
| 3,505,055 | | |
$ | 21,276 | | |
| 1,898,069 | | |
$ | 20,330 | | |
| 10,000,000 | | |
$ | 12,647 | | |
$ | (52,378 | ) | |
$ | (39,731 | ) |
| Change in redemption value of Redeemable Preferred Units | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,803 | | |
| - | | |
| (2,803 | ) | |
| - | | |
| (2,803 | ) |
| Equity-based compensation expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 6 | | |
| 6 | |
| Net income | |
| - | | |
| - | | |
| -- | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,020 | | |
| 2,020 | |
| Balance as of June 30, 2021 | |
| 3,843,750 | | |
$ | 15,375 | | |
| 2,272,727 | | |
$ | 10,000 | | |
| 3,505,055 | | |
$ | 21,276 | | |
| 1,898,069 | | |
$ | 23,133 | | |
| 10,000,000 | | |
$ | 9,844 | | |
$ | (50,352 | ) | |
$ | (40,508 | ) |
The accompanying notes are an integral part
of the unaudited condensed financial statements.
Milk Makeup LLC
Unaudited Condensed Statements of Cash Flows
| | |
Six Months Ended
June 30, | |
| (In thousands) | |
2022 | | |
2021 | |
| Cash flows from operating activities: | |
| | |
| |
| Net income | |
$ | 4,961 | | |
$ | 2,020 | |
| Adjustments to reconcile net income to net cash (used in) provided by operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 1,169 | | |
| 937 | |
| Non-cash interest (income) expense | |
| 21 | | |
| (7 | ) |
| Loss (gain) on disposal of assets | |
| 24 | | |
| (27 | ) |
| Equity-based compensation | |
| 131 | | |
| 6 | |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (4,588 | ) | |
| (281 | ) |
| Inventories | |
| (3,407 | ) | |
| 367 | |
| Prepaid expenses and other current assets | |
| 821 | | |
| (98 | ) |
| Accounts payable | |
| (1,900 | ) | |
| 121 | |
| Other assets and liabilities | |
| 1,165 | | |
| (1,768 | ) |
| Net cash (used in) provided by operating activities | |
| (1,603 | ) | |
| 1,270 | |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchases of property, plant, and equipment | |
| (148 | ) | |
| (38 | ) |
| Net cash used in investing activities | |
| (148 | ) | |
| (38 | ) |
| Net (decrease) increase in cash | |
| (1,751 | ) | |
| 1,232 | |
| Cash at beginning of period | |
| 4,417 | | |
| 7,207 | |
| Cash at end of period | |
$ | 2,666 | | |
$ | 8,439 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | 28 | | |
$ | 5 | |
| Supplemental disclosures of non-cash investing and financing activities | |
| | | |
| | |
| Purchases of property, plant and equipment in accounts payable | |
$ | 2,013 | | |
$ | 229 | |
The accompanying notes are an integral part
of the unaudited condensed financial statements.
1.
DESCRIPTION OF BUSINESS
Milk Makeup LLC (the “Company”) develops
and sells cosmetics, skin care and other beauty products.
The Company was organized as a Limited Liability
Company (LLC) under the provisions of the Delaware Limited Liability Company Act in 2014 and commenced operations in 2015. The term of
the Company shall continue until terminated in accordance with the provisions of the law. Members’ personal liability for debts is generally
limited similar to stockholders’ liability for corporate debts.
On November 15, 2021, Waldencast Acquisition Corp.
(“Waldencast”) (NASDAQ: WALD), a special purpose acquisition company (“SPAC”), entered into an Agreement and Plan
of Merger (the “Obagi Merger Agreement”) with Obagi Global Holdings Limited (“Obagi”) and an Equity Purchase Agreement
(the “Milk Equity Purchase Agreement”) with the Company in transactions contemplated by the agreements (the “Business
Combinations”). On July 27, 2022, the Business Combinations were completed resulting in the Company becoming a subsidiary of the
combined company. Following the Business Combination, the combined company is organized in an “Up-C” structure, in which the
equity interests of Obagi and the Company are held by Waldencast LP. Waldencast plc’s interests in Obagi and the Company are held
through its wholly owned subsidiaries, Holdco 1 and Waldencast LP.
Waldencast acquired the Company for total consideration
of approximately $310.8 million, consisting of approximately $112.5 million in cash, and $184.0 million in equity consideration, and $14.3
million in fair value of roll-over equity awards.
2.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The unaudited interim condensed financial statements
are presented in U.S. dollars in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for interim financial
information. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements.
The unaudited interim condensed financial statements furnished reflect all adjustments which are, in the opinion of management, necessary
for a fair statement of the results for the interim periods presented. The results of operations of any interim period are not necessarily
indicative of the results of operations to be expected for the full fiscal year. The unaudited interim condensed financial statements
should be read in conjunction with the financial statements and accompanying footnotes included in the Company’s annual financial
statements for the fiscal year ended December 31, 2021 included in Waldencast’s definitive proxy statement/final prospectus dated July 7, 2022,
and filed with the SEC on July 7, 2022. The interim results for the six months ended June 30, 2022 are not necessarily indicative
of the results to be expected for the year ending December 31, 2022, or for any future periods.
Use of Estimates
The preparation of financial statements in conformity
with U.S. GAAP requires management to make estimates and assumptions in determining the reported amounts of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses
during the reporting period. These estimates include the allowance for doubtful accounts, the reserve for inventory obsolescence, and
depreciation. As future events and their effects cannot be determined with precision, actual results could differ from those estimates
and assumptions. Significant changes, if any, in those estimates and assumptions resulting from continuing changes in the economic environment
will be reflected in the financial statements in future periods.
Revenue Recognition
The Company generates
revenue from the sale of cosmetics to retailers, including off-price retailers, and sales direct to consumer (“DTC”) via the
Company’s website. The Company derived 95% and 94% of its gross revenue from sales to retailers, with the remainder attributed to DTC
sales, during each of the six months ended June 30, 2022 and 2021.
The Company’s revenue contracts represent a single
performance obligation to sell its products to its customers. The Company recognizes revenue at a point in time upon transfer of control,
including passage of title to the customer and transfer of risk of loss related to the products, in an amount that reflects the consideration
the Company expects to be entitled to.
For sales to retailers, transfer of control generally
passes upon the pickup of goods by the retailer from the Company’s distribution center, unless the Company is responsible for shipping
the goods, in which case transfer of control passes upon delivery to the retailer. For DTC sales, the Company charges credit cards in
advance of shipment. Transfer of control passes upon delivery to the customer.
For sales to retailers, the Company collects cash
generally in 15 to 45 days depending on the retailer. The Company has not experienced issues with collectability. For DTC sales, the Company
collects cash in advance of shipment. Sales taxes imposed on DTC sales are recorded as a sales tax liability on the condensed Balance
Sheet and do not impact revenue.
In measuring revenue and determining the consideration
the Company is entitled to as part of a contract with a customer, the Company takes into account the related elements of variable consideration.
Such elements of variable consideration include product returns and sales incentives, such as volume rebates and discounts, markdowns,
margin adjustments and early-payment discounts. For the sale of goods with a right of return, the Company only recognizes revenue for
the consideration it expects to be entitled to and records a sales return reserve based on prior history, known events, and projections
on sales in the current period. The Company estimates sales incentives and other variable consideration using the most likely amount method
and records a reserve when control of the related product is transferred to the customer. Under this method, certain forms of variable
consideration are based on expected sell-through results, which requires subjective estimates. These estimates are supported by historical
results as well as specific facts and circumstances related to the current period. A reserve for expected returns or sales incentives
is presented as part of Accrued expenses and other current liabilities on the balance sheet. The reserve is trued up for actual results
on an ongoing basis. The Company recorded a reserve for sales returns and damages of approximately $4,668 thousand and $4,726 thousand
as of June 30, 2022 and December 31, 2021, respectively.
The Company’s contract assets consist of
accounts receivable, net. The Company’s contract liabilities consist of cash collections from its customers prior to the delivery
of products purchased for DTC sales. These contract liabilities are classified as deferred revenues and are included in the accompanying
condensed balance sheet, accrued expenses and other current liabilities (reference note 4). Accounts receivable increased in the six months
ended June 30, 2022 as significant amount of sales took place towards the end of the quarter. Accounts receivable decreased in 2021 primarily
due to the Company’s strong efforts in collecting past due balances.
| | |
Accounts Receivable, Net | | |
Contract Liabilities | |
| (In thousands) | |
June 30,
2022 | | |
December 31,
2021 | | |
June 30,
2022 | | |
December 31,
2021 | |
| Beginning of period | |
$ | 1,802 | | |
$ | 5,529 | | |
$ | 274 | | |
$ | 129 | |
| End of period | |
$ | 6,390 | | |
$ | 1,802 | | |
$ | 83 | | |
$ | 274 | |
Income Taxes
The Company is treated
as a partnership for U.S. federal income tax purposes and, as such, is not subject to any entity-level U.S. federal income tax. Instead,
for U.S. federal income tax purposes, taxable income of the Company is allocated to the Company’s unitholders. We are subject to
certain state, local and foreign income taxes, none of which are material for the six months ended June 30, 2022.
During the six months
ended June 30, 2022 and 2021, the Company did not have any unrecognized tax benefits related to uncertain tax positions. As of December
31, 2021, the Company is no longer subject to U.S. state, local, or foreign examinations by tax authorities for years before 2017.
Fair Value of Financial Instruments
The Company
accounts for financial instruments in accordance with the Accounting Standards Codification (“ASC”) 820 - Fair Value Measurements
and Disclosures, which provides a framework for measuring fair value and expands required disclosure about fair value measurements of
assets and liabilities. ASC 820 defines fair value as the price that would be received for an asset or paid to transfer a liability in
the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement
date. The Company applies fair value accounting for all financial assets and liabilities that are recognized or disclosed at fair value
in the financial statements on a recurring basis. The topic also establishes a hierarchy for grouping these assets and liabilities based
upon the lowest level of input that is significant to the fair value measurement. The definition of each input is described below:
| ● | Level 1: Inputs based on quoted market prices
for identical assets or liabilities in active markets at the measurement date. |
| ● | Level 2: Observable inputs other than quoted
prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or
similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable
market data. |
| ● | Level 3: Inputs reflect management’s best
estimate of what market participants would use in pricing the asset or liability at the measurement date. The inputs are unobservable
in the market and significant to the instrument’s valuation. |
As of June 30,
2022 and December 31, 2021, cash consisted of commercial bank account balances, Paypal balances, cash clearing and petty cash balance.
Other financial assets and liabilities including accounts receivable, accounts payable, accrued liabilities, and advances on credit facility
are carried at cost which approximates fair value because of the short-term nature of these instruments.
Concentrations
Sales directly to Sephora accounts for approximately
74% and 63% of gross revenue and sales indirectly to Sephora through major retailer distribution arrangements accounts for approximately
17% and 16% of gross revenue during the six months ended June 30, 2022 and 2021, respectively. Accounts receivable related directly to
Sephora amounted to approximately 69% and 27% of accounts receivable at June 30, 2022 and December 31, 2021, respectively. Accounts receivable
related indirectly to Sephora amounted to approximately 23% and 60% of accounts receivable at June 30, 2022 and December 31, 2021, respectively.
Purchases of inventory from seven manufacturers
accounted for approximately 65% and 79% of total inventory receipts during the six months ended June 30, 2022 and 2021, respectively.
Accounts payable related to these same manufacturers amounted to approximately 43% and 24% of accounts payable as of June 30, 2022 and
December 31, 2021, respectively. There are no other concentration risks in expenditures during the six months ended June 30, 2022 or in
accounts payable as of June 30, 2022.
The Company imports a substantial portion of its
purchases from suppliers in other countries. Although there are a number of suppliers who could provide the Company’s products,
a change in suppliers could cause a delay in purchasing and loss of sales, which could adversely affect operating results. These suppliers
are subject to various political, economic, and other risks and uncertainties inherent in the countries in which they operate.
The Company maintains its cash in two commercial
banks. The amounts held in these accounts may, at times, exceed federally insured limits. The Company believes there is no significant
risk with respect to these deposits. The amount in excess as of June 30, 2022 and December 31, 2021 was approximately $2,166 and $3,917,
respectively.
Equity Based Compensation
The Company measures and recognizes the cost of
employee services received in exchange for an award of equity instruments. Equity-based compensation expenses are measured at the grant
date, based on the fair value of the award, and recognized on a straight-line basis over the requisite service period. The Company has
elected to account for its graded vesting options on a straight-line basis over the requisite service period for the entire award. Awards
granted to non- employees are accounted for in accordance with the pronouncement addressing equity-based compensation issued to non-employees.
The Company elected to account for forfeitures as they occur and to utilize the practical expedient to estimate the expected term of all
awards.
Recently Adopted Accounting Pronouncements
In February 2016, the
FASB issued ASU 2016-02, Leases (ASC 842), which requires lessees to recognize a right-of-use asset and lease liability for all leases
with terms of more than 12 months. Recognition, measurement and presentation of expenses will depend on classification as finance or operating
lease. ASU 2016-02 will be effective for fiscal years beginning after December 15, 2020. On April 8, 2020, the FASB, pursuant
to ASU 2020-05, voted to defer the effective date for ASC 842 for one year. For private companies, the leasing standard will be effective
for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022.
Early adoption is permitted.
The Company adopted the
new standard on January 1, 2022 in connection with the issuance of the annual financial statements for the year ended December 31,
2022 using the modified retrospective approach. Therefore, upon adoption, the Company will recognize and measure leases without revising
comparative period information or disclosures.
The Company plans to
implement the transition package of three practical expedients permitted within the standard, which allows companies not to reassess whether
agreements contain leases, the classification of leases, and the capitalization of initial direct costs. The Company will make an accounting
policy election to recognize lease expense for leases with a term of 12 months or less on a straight-line basis over the lease term and
will not recognize any right-of-use assets or lease liabilities for those leases.
The Company expects the
standard to result in a material increase in long-term assets and long-term liabilities related to operating leases currently not recorded
on the balance sheet. The Company is currently completing its implementation efforts and is continuing to evaluate other possible impacts
of the adoption of this standard on its financial statements and related disclosures.
No other new accounting pronouncement have been
recently issued or newly effective which would have or be expected to have a material impact on the Company’s unaudited condensed
financial statements.
3.
INVENTORIES
Inventory is comprised of the following:
| | |
June 30, | | |
December 31, | |
| (In thousands) | |
2022 | | |
2021 | |
| Components | |
$ | 7,024 | | |
$ | 6,149 | |
| Finished goods | |
| 18,163 | | |
| 15,619 | |
| Inventory, gross | |
| 25,187 | | |
| 21,768 | |
| Less: Inventory reserve | |
| 352 | | |
| 340 | |
| Total inventories | |
$ | 24,835 | | |
$ | 21,428 | |
4.
ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other current liabilities
consist of the following:
| | |
June 30, | | |
December 31, | |
| (In thousands) | |
2022 | | |
2021 | |
| Accrued sales returns and damages | |
$ | 4,668 | | |
$ | 4,726 | |
| Accrued bonuses | |
| 1,113 | | |
| 1,350 | |
| Accrued payroll and other non-income taxes | |
| 419 | | |
| 296 | |
| Deferred revenue | |
| 83 | | |
| 274 | |
| Others | |
| 45 | | |
| 89 | |
| Accrued expenses and other current liabilities | |
$ | 6,328 | | |
$ | 6,735 | |
5.
DEBT
Line of Credit
In February 2021, the Company amended its Loan
and Security Agreement and previous amendments (the “Third Amendment”) to revise the minimum EBITDA target for each reporting
period under the financial covenant.
In April 2021, the Company amended its Loan and
Security Agreement and previous amendments (the “Fourth Amendment”). The Fourth Amendment extended the maturity date through
April 10, 2022. The Company may borrow advances under a revolving line based on inventory in an aggregate outstanding principal amount
not to exceed the lesser of $8,000 thousand or the inventory borrowing base, as defined in the Fourth Amendment, and in each case inclusive
of any amounts reserved under the Ancillary Services Sublimit. The Company may also borrow advances under a revolving line based on accounts
receivable in an aggregate outstanding principal amount not to exceed the lesser of $5,000 thousand or the accounts receivable borrowing
base, as defined in the Fourth Amendment. The Company may borrow advances in an aggregate outstanding amount, under both the inventory
revolving line and the accounts receivable revolving line, not to exceed $8,000 thousand. Advances under the inventory revolving line
of the Fourth Amendment bore interest at a variable annual rate equal to the greater of 0.50% above the Prime Rate then in effect or 6.00%.
Advances under the accounts receivable revolving line of the Fourth Amendment bore interest at a variable annual rate equal to the greater
of 0.25% above the Prime Rate then in effect or 5.50%. Prior to the Fourth Amendment, advances under the Loan and Security Agreement bore
interest at a variable annual rate equal to the greater of 0.5% above the Prime Rate then in effect or 6.00%.
In November 2021, the Company amended its Loan
and Security Agreement and previous amendments (the “Fifth Amendment”) to adjust the limit of amount that can be held in other
bank accounts to $1,500 thousand. In March 2022, the Company amended its Loan and Security Agreement and previous amendments (the “Sixth
Amendment”). The Sixth Amendment extended the maturity date through April 10, 2023. The Company may borrow advances under a revolving
line based on inventory in an aggregate outstanding principal amount not to exceed the lesser of $15,000 thousand or the inventory borrowing
base, as defined in the Sixth Amendment, and in each case inclusive of any amounts reserved under the Ancillary Services Sublimit. The
Company may also borrow advances under a revolving line based on accounts receivable in an aggregate outstanding principal amount not
to exceed the lesser of $8,000 thousand or the accounts receivable borrowing base, as defined in the Sixth Amendment. The Company may
borrow advances in an aggregate outstanding amount, under both the inventory revolving line and the accounts receivable revolving line,
not to exceed $15,000 thousand. Advances under the inventory revolving line of the Sixth Amendment bear interest at a variable annual
rate equal to the greater of 0.50% above the Prime Rate then in effect or 5.25%. Advances under the accounts receivable revolving line
of the Sixth Amendment bear interest at a variable annual rate equal to the greater of 0.25% above the Prime Rate then in effect or 4.50%.
As of June 30, 2022, the Company had outstanding
borrowings under the line of credit of $1,500 thousand that did not incur any interest. As of December 31, 2021, the Company did not have
any outstanding borrowings or incur any interest.
The line of credit is subject to certain financial
and restrictive covenants, including but not limited to, minimum monthly EBITDA amounts, limitations on incurrence of indebtedness and
liens, restrictions on affiliate transactions, restrictions on the sale or other disposition of collateral, and maintaining cash in other
bank accounts. As of June 30, 2022, the Company was in full compliance with all covenants.
6.
FAIR VALUE MEASUREMENTS
The Company records certain of its financial liabilities
at fair value, which is defined as the price that would be paid to transfer a liability, in the principal or most advantageous market
for the liability, in an orderly transaction between market participants at the measurement date. The accounting for fair value measurements
must be applied to nonfinancial assets and nonfinancial liabilities that require initial measurement or remeasurement at fair value, which
principally consist of intangible assets and long-lived assets for the purposes of calculating potential impairment. The Company is required
to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
In 2019, the Company issued Warrant Agreements
giving the lender the option to purchase Redeemable Series C and Series D preferred units. The Company measured and recorded the fair
value of the warrant liabilities using the Black-Scholes model. The amount was recorded as a long-term liability on the balance sheet
due to the underlying redemption feature. Warrant liabilities are valued using unobservable market inputs and, as such, are considered
to be level 3 inputs.
The following table presents the Company’s
hierarchy for its financial liabilities measured at fair value on a recurring basis as of June 30, 2022:
| (In thousands) | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities: | |
| | |
| | |
| | |
| |
| Warrant Liabilities | |
| - | | |
| - | | |
$ | 148 | | |
$ | 148 | |
| Total | |
| - | | |
| - | | |
$ | 148 | | |
$ | 148 | |
The following table presents the Company’s
hierarchy for its financial liabilities measured at fair value on a recurring basis as of December 31, 2021:
| (In thousands) | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities: | |
| | |
| | |
| | |
| |
| Warrant Liabilities | |
| - | | |
| - | | |
$ | 121 | | |
$ | 121 | |
| Total | |
| - | | |
| - | | |
$ | 121 | | |
$ | 121 | |
The following table presents the changes in fair
value of the Company’s financial liabilities measured on a recurring basis, classified as Level 3, during the six months ended June
30, 2022:
| (In thousands) | |
Six
months ended
June 30,
2022 | |
| Opening balance | |
$ | 121 | |
| Change in fair value | |
| 27 | |
| Ending balance | |
$ | 148 | |
| 7. | EQUITY BASED COMPENSATION EXPENSE |
The Company’s Appreciation Rights Plan (“ARP”),
Incentive Rights Plan (“IRP”), and Enterprise Management Incentive (“EMI”) options for its employees and officers
provide for granting of appreciation rights awards, incentive awards, and options at the discretion of the Board of Directors. Appreciation
rights awards, incentive awards, and options have no voting rights. The Company measures awards using fair values as determined by the
Black-Scholes model.
The total number of units that may be awarded
under the ARP and EMI are 2,371,856 and 161,607 common units, respectively. Under the ARP, no awards were granted during the six months
ended June 30, 2022 and 99,000 units of awards were granted during the six months ended June 30, 2021. No awards were granted under the
EMI during the six months ended June 30, 2022 and 2021.
The appreciation right and option awards granted
to employees and non-employees were accounted for as equity-classified awards. The awards are issued as equity appreciation rights or
options in accordance with the respective award letters and may be settled in cash or units at the sole option of the Company. During
the six months ended June 30, 2022 and 2021, $131 thousand and $6 thousand of equity-based compensation costs were recognized, respectively.
No awards were either reserved or granted
under the IRP as of June 30, 2022.
The following summarizes the Company’s ARP, EMI,
and activity for the six months ended June 30, 2022:
| (In thousands, except per share data) | |
Appreciation Rights and Options | | |
Weighted Average Exercise Price | | |
Aggregate Intrinsic Value | | |
Weighted Average Remaining Contractual Term (Years) | |
| Balance at December 31, 2021 | |
| 2,332,335 | | |
$ | 4.65 | | |
$ | 168 | | |
| 8.10 | |
| Awards granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Awards exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Awards forfeited | |
| - | | |
| - | | |
| - | | |
| - | |
| Balance at June 30, 2022 | |
| 2,332,335 | | |
$ | 4.65 | | |
$ | 168 | | |
| 7.60 | |
| Exercisable at June 30, 2022 | |
| - | | |
$ | - | | |
$ | - | | |
| - | |
As of June 30, 2022, the total unrecognized compensation
cost related to unvested awards was $403 thousand and the related weighted-average period over which it is expected to be recognized is
approximately 3.6 years.
8.
WARRANT LIABILITIES
In conjunction with the Loan and Security Agreement
(see Note 5) signed in 2019, the Company issued Warrant Agreements (the “warrants”) giving the lender the option to purchase
10,297 of Redeemable Series C Preferred Units and 6,139 of Redeemable Series D Preferred Units. The Company measured and recorded the
fair value of the warrant liabilities using the Black-Scholes model. The fair value of the warrants amounted to approximately $148 thousand
and $121 thousand as of June 30, 2022 and December 31, 2021, respectively, and was recorded as a long-term liability on the balance sheet
due to the underlying redemption feature. In accordance with liability-classified award accounting, the Company remeasures the fair value
of the warrant liabilities using the Black-Scholes model annually. Changes in fair value of the warrant liabilities are recorded in Interest
expense, net.
Valuation Assumptions
The Company estimated the fair value of the warrants
using the Black-Scholes model.
| | |
Six months
ended
June 30,
2022 | | |
Year
ended
December 31,
2021 | |
| Expected term | |
| 7 years | | |
| 8 years | |
| Volatility | |
| 35.20 | % | |
| 50.00 | % |
| Risk-free interest rate | |
| 3.04 | % | |
| 1.44 | % |
| Distributions yield | |
| 0 | % | |
| 0 | % |
Expected volatility: The volatility factor for
the Company’s warrants was estimated using publicly available trading data, which was used to estimate the Company’s volatility, had the
Company been public.
Expected distributions yield: The Company does
not anticipate that distributions will be made in the near future.
Expected term: The Company’s expected term represents
the contractual term as stated in the warrant agreements.
Risk-Free Interest Rate: The Company bases the
risk-free interest rate used in the Black-Scholes model on implied yield currently available on U.S. Treasury zero-coupon issues with
an equivalent remaining term.
9.
RELATED PARTY TRANSACTIONS
The Company receives certain administrative services
from affiliated companies related through common ownership. Such amounts are management fee expenses and amounted to $60 thousand each
for the six months ended June 30, 2022 and 2021.
The amounts due from officers are due and payable
on July 30, 2026 or an earlier date (as defined) and July 31, 2029 or an earlier date (as defined), and bear interest rate of 1.90% per
year. One officer loan bears interest at 1.7%.
The members of the company are committed to provide
the necessary funding in the form of advances and capital contributions to fund the company’s operations as needed.
10.
COMMITMENTS & CONTINGENCIES
The Company is at times involved in various claims
and legal actions. While the Company cannot predict any final outcomes relating thereto, management believes that the outcome of current
claims and legal actions will not have a material adverse impact on the financial position of the Company or the results of its operations.
11.
SUBSEQUENT EVENTS
The Company has evaluated subsequent events through
August 5, 2022, the date the financial statements were available to be issued.
On July 22, 2022, the Company granted 200,000
units under the Appreciation Rights Plan to an employee and were accounted for as equity-classified awards. These awards vest 25% upon
the first anniversary date of the vesting commencement date and the remaining vest in equal monthly installments over the following three
years, contingent upon continued employment or service.
On July 27, 2022, the Company completed the Business
Combinations with Waldencast as discussed in Note 1.

Waldencast
plc
Up
to 29,533,282 Class A Ordinary Shares (for issuance)
Up
to 121,120,063 Class A Ordinary Shares (for resale)
Up
to 18,033,332 Warrants to Purchase Class A Ordinary Shares (for resale)
PROSPECTUS
October 13, 2022
You
should rely only on the information contained in this prospectus or any supplement or amendment hereto. We have not authorized anyone
to provide you with different information. You should not assume that the information contained in this prospectus or any supplement
or amendment hereto is accurate as of any date other than the date of this prospectus or any such supplement or amendment. Neither the
Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Waldencast (NASDAQ:WALD)
Historical Stock Chart
From Dec 2024 to Jan 2025
Waldencast (NASDAQ:WALD)
Historical Stock Chart
From Jan 2024 to Jan 2025