Viridian Therapeutics, Inc.DE false 0001590750 --12-15 0001590750 2023-12-15 2023-12-15
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 15, 2023

VIRIDIAN THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-36483 |
|
47-1187261 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 221 Crescent Street, Suite 401 Waltham, MA |
|
02453 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (617) 272-4600
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.01 par value |
|
VRDN |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 5.03 |
Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. |
Effective December 15, 2023, the board of directors (the “Board”) of Viridian Therapeutics, Inc. (the “Company”) adopted the Fourth Amended and Restated Bylaws of the Company (the “Bylaws”). The Bylaws have been amended to designate the federal district courts of the United States as the sole and exclusive forum for any complaint asserting a cause of action arising under the Securities Act of 1933, as amended (the “Securities Act”).
The foregoing summary of the changes effected by the Bylaws does not purport to be complete and is qualified in its entirety by reference to the complete text of the Bylaws, which are attached hereto as Exhibit 3.1 and are incorporated herein by reference.
| Item 7.01 |
Regulation FD Disclosure. |
On December 18, 2023, the Company issued a press release announcing positive clinical data in its healthy volunteer study and the selection of VRDN-003 as a potential best-in-class subcutaneous (“SC”) anti insulin-like growth factor-1 receptor (“IGF-1R”) program with extended half-life for pivotal development in thyroid eye disease (“TED”). The Company will host a conference call and webcast today, Monday, December 18, 2023 at 8:00 a.m., Eastern Time, to discuss the data results and other updates.
A copy of the press release and the data presentation that will be referenced during the conference call are furnished as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K and are incorporated by reference herein. The exhibits furnished under Item 7.01 of this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act, regardless of any general incorporation language in such filing.
On December 18, 2023, the Company announced the selection of VRDN-003 as its lead SC program for TED based on positive data from a Phase 1 clinical study in healthy volunteers. The data showed that VRDN-003 has an extended half-life of 40-50 days, about 4-5 times longer than VRDN-001 and based upon comparisons of publicly disclosed data, significantly longer than other first generation anti IGF-1R antibodies. VRDN-003 was well tolerated and exhibited sustained pharmacodynamics supporting its development as a potential best-in-class, more convenient, less frequent, low-volume, self-administered, subcutaneous IGF-1R therapy for patients with TED.
VRDN-003 Results
VRDN-003 is a monoclonal antibody that acts as a full antagonist of IGF-1R, a clinically and commercially validated target for TED. VRDN-003 utilizes the same binding domain as VRDN-001 and is engineered with three amino acid changes to the Fc region designed to extend the half-life of the parent antibody, and therefore potentially enabling less frequent and more convenient dosing. VRDN-003 is designed to maintain the clinical response of VRDN-001 IV while significantly increasing patient convenience.
| |
• |
|
Study Design: VRDN-003 was dosed in four single dose cohorts of healthy volunteers at a concentration of 150 mg/ml receiving 5 mg/kg IV (n=4), 300 mg SC (n=6), 15 mg/kg IV (n=4) and 600 mg SC (n=6). A fifth cohort of two doses of VRDN-003 (n=4) is ongoing. |
| |
• |
|
Extended Half Life: VRDN-003 pharmacokinetics data showed an extended half-life of 40-50 days, which is a 4-to-5-fold increase over the half-life of VRDN-001 (which showed a half-life of 10-12 days). |
| |
• |
|
Prolonged Pharmacodynamics (“PD”): Following a single subcutaneous dose of VRDN-003, IGF-1 serum levels increased approximately 4-fold at peak. This was consistent with the increases in IGF-1 levels that have been shown in the clinic following a single dose of VRDN-001 SC and IV. IGF-1 is a PD biomarker for IGF-1R target engagement. IGF-1R inhibition by an anti-IGF-1R antibody demonstrated an increase in serum levels of its natural ligand IGF-1. |
| |
• |
|
Well Tolerated: VRDN-003 was well tolerated in all subjects with no serious adverse events. All treatment-related, treatment-emergent adverse events were grade 1 (mild). In the reported dataset, no antidrug antibodies (“ADAs”) were detected. |
| |
• |
|
Dosing Flexibility for Pivotal Development: VRDN-003 modeling demonstrates dosing flexibility for the program’s anticipated global pivotal development. |
| |
• |
|
Q8W (dosing once every 8 weeks): Modeled dose schedule is predicted to achieve Cmin and Cavg exposure achieved with 3 mg/kg VRDN-001 IV. |
| |
• |
|
Q4W (dosing once every 4 weeks): Modeled dose schedule is predicted to achieve Cmin exposures seen for 10 mg/kg VRDN-001 IV and exceed the Cavg exposure seen for 3 mg/kg by a factor of 2. |
| |
• |
|
Q2W (dosing once every 2 weeks): Modeled dose schedule is predicted to exceed Cmin exposures seen for 20 mg/kg VRDN-001 IV and the Cavg exposures seen for 10 mg/kg VRDN-001 IV. |
Based on the data reported, VRDN-003 has been selected as the Company’s go-forward candidate for subcutaneous development. The Company expects to initiate the VRDN-003 pivotal program in mid-2024, pending alignment with regulatory authorities. Based on these positive results and the expected development timeline for VRDN-003, both VRDN-001 SC and VRDN-002 SC development have been deprioritized.
VRDN-001 SC and VRDN-002 Results
VRDN-001 SC is a subcutaneous formulation of the same antibody being evaluated as an IV product candidate in the company’s ongoing Phase 3 THRIVE and THRIVE-2 clinical trials. The Phase 1 healthy volunteer study included a cohort of the SC formulation of VRDN-001 at a concentration of 150 mg/ml dosed in two single dose cohorts of healthy volunteers either receiving 3.5 mg/kg IV (n= 8) or 300mg SC (n=8). SC data showed an expected half-life of 10-12 days. Q1W dosing of VRDN-001 SC was modeled to achieve or exceed exposures shown for 10 mg/kg VRDN-001 IV and would be expected to achieve comparable clinical response to VRDN-001 10 mg/kg IV. VRDN-001 SC also showed an increase in IGF-1 levels similar to VRDN-001 IV and VRDN-003 SC. VRDN-001 SC was well tolerated in all subjects with no serious adverse events. All treatment-related, treatment-emergent adverse events were grade 1 (mild) and grade 2 (moderate).
VRDN-002 is a different and novel monoclonal antibody targeting IGF-1R as compared to VRDN-001 or VRDN-003. Similar to VRDN-003, VRDN-002 was engineered to have an extended half-life to allow for less frequent dosing. VRDN-002 SC was dosed at a concentration of 150 mg/ml in two single dose cohorts of healthy volunteers, either receiving 3.5mg/kg IV (n=8) or 300mg SC (n=8). Healthy volunteer data for VRDN-002 showed an extended half-life of 43 days as previously disclosed. VRDN-002 also showed a prolonged pharmacodynamic effect and increased IGF-1 levels, but the magnitude of increase was not as great as with VRDN-003 or VRDN-001. VRDN-002 was well tolerated in all subjects with no serious adverse events. All treatment-related, treatment-emergent adverse events were grade 1 (mild). No ADAs were detected.
Next Steps for the Development of VRDN-003 in TED
Based on the results reported, the company expects to initiate global pivotal clinical trials of VRDN-003 in mid-2024 with planned trials in both active and chronic TED patients pending alignment with regulatory authorities.
Forward-Looking Statements
This Current Report on Form 8-K and the press release contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as, but not limited to, “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or other similar terms or expressions that concern the Company’s expectations, plans and intentions. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on the Company’s current beliefs, expectations, and assumptions. Forward-looking statements include, without limitation, statements regarding: the therapeutic potential and utility, efficacy and clinical benefits of VRDN-003 for TED; the expected exposure levels of VRDN-003; the safety profile of VRDN-003; the potential dosing frequency for VRDN-003; trial designs, clinical development plans and timing for VRDN-003, including the global pivotal clinical trials of VRDN-003 in active and chronic TED patients; the expected attractiveness of VRDN-003 and convenience for patients; and anticipated work
with regulatory authorities. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including but not limited to: the potential efficacy and safety of VRDN-001, VRDN-002, and VRDN-003 for the treatment of TED; the potential for VRDN-006 and VRDN-008; the relationship between the results from the positive data from completed or ongoing clinical trials and the results of ongoing or future clinical trials; the timing, progress and plans for the Company’s ongoing or future research, pre-clinical and clinical development programs; trial protocols for ongoing clinical trials; expectations regarding the timing for IND filings; expectations regarding the timing for enrollment and data; uncertainty and potential delays related to clinical drug development; the duration and impact of regulatory delays in the Company’s clinical programs; the timing of and the Company’s ability to obtain and maintain regulatory approvals for its therapeutic candidates; manufacturing risks; competition from other therapies or products; estimates of market size; other matters that could affect the sufficiency of existing cash, cash equivalents and short-term investments to fund operations; the Company’s financial position and its projected cash runway; the Company’s future operating results and financial performance; the clinical utility of the Company’s therapeutic candidates and its intellectual property position; the timing of pre-clinical and clinical trial activities and reporting results from same, including those risks set forth under the caption “Risk Factors” in the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (“SEC”) on November 13, 2023 and other subsequent disclosure documents filed with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Neither the Company, nor its affiliates, advisors, or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof.
The following risk factor is provided to supplement the Company’s risk factor disclosure contained in the Company’s prior public filings, including those discussed under the heading “Item 1A. Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023.
Preliminary data from our clinical trials that we announce or publish are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we publish preliminary data from our clinical trials. On December 18, 2023, we reported clinical data from our Phase 1 clinical study in healthy volunteers and announced the selection of VRDN-003 as our lead subcutaneous program for TED. This data set includes preliminary data which was not subject to the standard quality control measures typically associated with final clinical trial results. Based on the comparable pharmacology of VRDN-003 and VRDN-001, we believe VRDN-003 can maintain the clinical response of VRDN-001 IV while significantly increasing patient convenience. However, the data from our Phase 2 trials for VRDN-001 may also not be fully reflective of topline results for our Phase 3 THRIVE and THRIVE -2 trials which are expected in 2024. Topline or preliminary data from our clinical trials that we announce or publish from time to time, including the data from our Phase 1 study in healthy volunteers and the data for VRDN-001 from our ongoing trials, may change as more patient data become available, and we become subject to audit and verification procedures that could result in material changes in the final data. This creates a risk that the final results could be materially different from the preliminary results reported to date. Significant adverse differences between preliminary data and final data could materially harm our reputation and business prospects.
| Item 9.01 |
Financial Statements and Exhibits |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Viridian Therapeutics, Inc. |
|
|
|
|
| Date: December 18, 2023 |
|
|
|
By: |
|
/s/ Stephen Mahoney |
|
|
|
|
|
|
Stephen Mahoney |
|
|
|
|
|
|
President, Chief Executive Officer, and Director |
Exhibit 3.1
FOURTH AMENDED AND RESTATED BYLAWS
OF
VIRIDIAN
THERAPEUTICS, INC.
ARTICLE 1
OFFICES
Section 1.01.
Registered Office. The registered office of Viridian Therapeutics, Inc. (hereinafter, the “Corporation”) shall be in the City of Wilmington, County of New Castle, State of Delaware 19810.
Section 1.02. Other Offices. The Corporation may also have offices at such other places both within and without the State of
Delaware as the board of directors of the Corporation (the “Board of Directors”) may from time to time determine or the business of the Corporation may require.
Section 1.03. Books. The books of the Corporation may be kept within or without the State of Delaware as the Board of Directors
may from time to time determine or the business of the Corporation may require.
ARTICLE 2
MEETINGS OF STOCKHOLDERS
Section 2.01. Time and Place of Meetings. All meetings of the stockholders shall be held at such place, if any, either within or
without the State of Delaware, on such date and at such time as may be determined from time to time by the Board of Directors (or the Chairman in the absence of a designation by the Board of Directors).
Section 2.02. Annual Meetings. An annual meeting of the stockholders, commencing with the year 2015, shall be held for the
election of directors and to transact such other business as may properly be brought before the meeting. Any other proper business may be transacted at the annual meeting. The Board of Directors may postpone, reschedule or cancel any annual meeting
of stockholders previously scheduled by the Board of Directors.
Section 2.03. Special Meetings. Special meetings of the
stockholders for any purpose or purposes may be called by the Board of Directors, the Chairman of the Board of Directors or the President of the Corporation, and may not be called by any other person. Business transacted at any special meeting of
the stockholders shall be limited to the purposes stated in the notice. The Board of Directors may postpone, reschedule or cancel any special meeting of stockholders previously scheduled by the Board of Directors. Notwithstanding the foregoing,
whenever holders of one or more classes or series of Preferred Stock shall have the right, voting separately as a class or series, to elect directors, such holders may call, pursuant to the terms of the resolution or resolutions adopted by the Board
of Directors pursuant to Article 4 hereto, special meetings of holders of such Preferred Stock.
Section 2.04. Notice of Meetings
and Adjourned Meetings; Waivers of Notice.
(a) Whenever stockholders are required or permitted to take any action at a meeting, a
written notice of the meeting shall be given which shall state the place, if any, date and hour of the meeting, the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at
such meeting, the record date for determining the stockholders entitled to vote at the meeting (if such date is different from the record date for stockholders entitled to notice of the meeting) and, in the case of a special meeting, the purpose or
purposes for which the meeting is called. Unless otherwise provided by the General Corporation Law of the State of Delaware as the same exists or may hereafter be amended (“Delaware Law”), the Certificate of Incorporation or these Bylaws,
such notice of any meeting shall be given not less than ten (10) nor more than sixty (60) days before the date of the meeting to each stockholder of record entitled to vote at such meeting. Except as otherwise provided herein or permitted
by applicable law, notice of stockholders shall be in writing and delivered personally or mailed to the stockholders at their address appearing on the books of the Corporation. Without limiting the manner by which notice otherwise may be given
effectively to stockholders, notice of meetings may be given to stockholders by means of electronic transmission in accordance with applicable law.
1
(b) Any meeting of the stockholders, annual or special, may be adjourned from time to time
to reconvene at the same or some other place, if any. Unless these Bylaws otherwise require, when a meeting is adjourned to another time or place (whether or not a quorum is present), notice need not be given of the adjourned meeting if the time,
place, if any, thereof and the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, are announced at the meeting at which the adjournment is taken. At the
adjourned meeting, the Corporation may transact any business which might have been transacted at the original meeting. If the adjournment is for more than thirty (30) days, or after the adjournment a new record date is fixed for the adjourned
meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting as of the record date for determining the stockholders entitled to notice of the meeting. If mailed, such notice shall be deemed
to be given when deposited in the United States mail, postage prepaid, directed to the stockholder at such stockholder’s address as it appears on the records of the Corporation.
(c) A written waiver of any such notice signed by the person entitled thereto, or a waiver by electronic transmission by the person entitled
to notice, whether before or after the time stated therein, shall be deemed equivalent to notice. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person attends the meeting for the express
purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Any stockholder so waiving notice of the meeting shall be bound by the proceedings of the meeting in
all respects as if due notice thereof had been given. Business transacted at any special meeting of stockholders shall be limited to the purposes stated in the notice.
Section 2.05. Quorum. Unless otherwise provided in the Certificate of Incorporation or these Bylaws, and subject to Delaware Law,
the presence, in person or by proxy, of the holders of a majority of the outstanding capital stock of the Corporation entitled to vote at a meeting of stockholders shall constitute a quorum for the transaction of business. If, however, such quorum
shall not be present or represented at any meeting of the stockholders, then the chairman of the meeting, or a majority of the voting power entitled to vote thereat, present in person or represented by proxy, shall have the power to adjourn the
meeting in the manner provided in Section 2.04, without notice other than announcement at the meeting, until a quorum shall be present or represented. A quorum once established, shall not be broken by the subsequent withdrawal of enough votes
to leave less than a quorum. At such adjourned meeting at which a quorum shall be present or represented, any business may be transacted which might have been transacted at the meeting as originally notified. Shares of stock of the Corporation
belonging to the Corporation or to another corporation, if a majority of the shares entitled to vote in the election of directors of such other corporation is held, directly or indirectly, by the Corporation, shall neither be entitled to vote nor be
counted for quorum purposes; provided, however, that the foregoing shall not limit the right of the Corporation, or any subsidiary of the Corporation, to vote stock, including but not limited to its own stock, held by it in a fiduciary capacity.
Section 2.06. Voting; Proxies.
(a) Unless otherwise provided in the Certificate of Incorporation and subject to Delaware Law, each stockholder shall be entitled to one vote
for each outstanding share of capital stock of the Corporation held by such stockholder. Any share of capital stock of the Corporation held by the Corporation shall have no voting rights. Except as otherwise provided by law, the Certificate of
Incorporation or these Bylaws, in all matters other than the election of directors, the affirmative vote of the majority of the votes cast at a meeting of the stockholders by the holders of stock entitled to vote on such matter shall be the act of
the stockholders. Subject to the rights of the holders of any series of preferred stock to elect additional directors under specific circumstances, directors shall be elected by a plurality of the votes cast at a meeting of the stockholders by the
holders of stock entitled to vote on the election of directors.
(b) Each stockholder entitled to vote at a meeting of stockholders, or to
express consent or dissent to a corporate action in writing without a meeting, may authorize another person or persons to act for such stockholder by proxy, appointed by an instrument in writing, subscribed by such stockholder or by his attorney
thereunto authorized, or by proxy sent by cable, telegram or by any means of electronic communication permitted by law, which results in a writing from such stockholder or by his attorney, and delivered to the secretary of the meeting. No proxy
shall be voted or acted upon after three (3) years from its date, unless said proxy provides for a
2
longer period. A proxy shall be irrevocable if it states that it is irrevocable and if, and only as long as, it is coupled with an interest sufficient in law to support an irrevocable power. A
stockholder may revoke any proxy which is not irrevocable by attending the meeting and voting in person or by delivering to the Secretary of the Corporation a revocation of the proxy or a new proxy bearing a later date.
(c) Voting at meetings of stockholders need not be by written ballot. Votes may be cast by any stockholder entitled to vote in person or by
his proxy. In determining the number of votes cast for or against a proposal or nominee, shares abstaining from voting on a matter (or votes withheld in the case of director elections) will not be treated as votes cast, but will be counted for
purposes of determining a quorum. A non-vote by a broker will be counted for purposes of determining a quorum but not for purposes of determining the number of votes cast.
Section 2.07. Inspector of Elections; Opening and Closing the Polls. The Board of Directors by resolution shall appoint one or
more inspectors, which inspector or inspectors may include individuals who serve the Corporation in other capacities, including, without limitation, as officers, employees, agents or representatives, to act at the meetings of stockholders and make a
written report thereof. One or more persons may be designated as alternate inspectors to replace any inspector who fails to act. If no inspector or alternate has been appointed to act or is able to act at a meeting of stockholders, the presiding
officer of the meeting shall appoint one or more inspectors to act at the meeting. Each inspector, before discharging his or her duties, shall take and sign an oath faithfully to execute the duties of inspector with strict impartiality and according
to the best of his or her ability. The inspectors shall have the duties prescribed by law. The presiding officer of the meeting shall fix and announce at the meeting the date and time of the opening and the closing of the polls for each matter upon
which the stockholders will vote at a meeting.
Section 2.08.[Reserved].
Section 2.09. Organization. At each meeting of stockholders, the Chairman of the Board, if one shall have been elected, or in the
Chairman’s absence or if one shall not have been elected, the director designated by the vote of the majority of the directors, shall act as chairman of, and preside at, the meeting. The Secretary, or in the Secretary’s absence or
inability to act, the person whom the chairman of the meeting shall appoint secretary of the meeting, shall act as secretary of the meeting and keep the minutes thereof.
Section 2.10. Order of Business. The order of business at all meetings of stockholders shall be as determined by the chairman of
the meeting.
Section 2.11. Nomination of Directors. Only persons who are nominated in accordance with the procedures set
forth in these Bylaws shall be eligible to serve as directors. Nominations of persons for election to the Board of Directors of the Corporation may be made at a meeting of stockholders (a) by or at the direction of the Board of Directors or
(b) by any stockholder of the Corporation who is a stockholder of record at the time of giving of notice provided for in this Section 2.11, who shall be entitled to vote for the election of directors at the meeting and who complies with
the notice procedures set forth in this Section 2.11. Such nominations, other than those made by or at the direction of the Board of Directors, shall be made pursuant to timely notice in writing to the secretary of the Corporation. To be
timely, a stockholder’s notice shall be delivered to or mailed and received at the principal executive offices of the Corporation not later than the close of business on the sixtieth (60th) day, nor earlier than the close of business on the
ninetieth (90th) day in advance of the first anniversary of the preceding year’s annual meeting of stockholders; provided, however, that in the event that the date of the annual meeting is advanced more than thirty (30) days prior to such
anniversary date or delayed more than sixty (60) days after such anniversary date then to be timely such notice must be received by the Corporation no later than the later of the close of business on the seventieth (70th) day prior to the date
of the meeting or the close of business on the tenth (10th) day following the day on which public announcement of the date of the meeting was made. In no event shall the public announcement of the new meeting date commence a new notice time period
(or extend any notice time period). Such stockholder’s notice shall set forth (a) as to each person whom the stockholder proposes to nominate for election or reelection as a director, all information relating to such person that is
required to be disclosed in solicitations of proxies for election of directors, or is otherwise required, in each case pursuant to Regulation 14A under the Securities Exchange Act of 1934 (including such person’s written consent to being named
in the proxy statement as a nominee and to serving as a director if elected); and (b) as to the stockholder giving the notice (i) the name and address, as they appear on the Corporation’s books, of such stockholder, and of the
beneficial owner, if any, on
3
whose behalf the nomination is being made, (ii) the class and number of shares of the Corporation which are owned (beneficially and of record) by such stockholder and owned by the beneficial
owner, if any, on whose behalf the nomination is being made, as of the date of the stockholder’s notice, and a representation that the stockholder will notify the Corporation in writing of the class and number of such shares owned of record and
beneficially as of the record date for the meeting promptly following the later of the record date or the date notice of the record date is first publicly disclosed, (iii) a description of any agreement, arrangement or understanding with
respect to such nomination between or among the stockholder and any of its affiliates or associates, and any others (including their names) acting in concert with any of the foregoing, and a representation that the stockholder will notify the
Corporation in writing of any such agreement, arrangement or understanding in effect as of the record date for the meeting promptly following the later of the record date or the date notice of the record date is first publicly disclosed, (iv) a
description of any agreement, arrangement or understanding (including, regardless of the form of settlement, any derivative, long or short positions, profit interests, forwards, futures, swaps, options, warrants, convertible securities, stock
appreciation or similar rights, hedging transactions and borrowed or loaned shares) that has been entered into as of the date of the proposing stockholder’s notice, by or on behalf of such stockholder with respect to the Corporation’s
securities, or any other agreement, arrangement or understanding that has been made, the effect or intent of which is to create or mitigate loss to, manage risk or benefit of share price changes for, or increase or decrease the voting power of, such
stockholder with respect to the Corporation’s securities, and a representation that the stockholder will notify the Corporation in writing of any such agreement, arrangement or understanding in effect as of the record date for the meeting
promptly following the later of the record date or the date notice of the record date is first publicly disclosed, (v) a representation that the proposing stockholder is a holder of record of the shares of the Corporation entitled to vote at
the meeting and intends to appear in person or by proxy at the meeting to nominate the person or persons specified in the notice, and (vi) a representation whether the proposing stockholder intends to deliver a proxy statement and/or form of
proxy to holders of at least the percentage of the Corporation’s outstanding capital stock required to approve the nomination and/or otherwise to solicit proxies from the stockholders in support of the nomination. The Corporation may require
any proposed nominee to furnish such other information as it may reasonably require to determine the eligibility of such proposed nominee to serve as an independent director of the Corporation or that could be material to a reasonable
stockholder’s understanding of the independence, or lack thereof, of such nominee. At the request of the Board of Directors, any person nominated by the Board of Directors for election as a director shall furnish to the secretary of the
Corporation that information required to be set forth in a stockholder’s notice of nomination that pertains to the nominee. No person shall be eligible to serve as a director of the Corporation unless nominated in accordance with the procedures
set forth in this bylaw. The chairman of the meeting shall, if the facts warrant, determine and declare to the meeting that a nomination was not made in accordance with the procedures prescribed by the Bylaws, and if he should so determine, he shall
so declare to the meeting and the defective nomination shall be disregarded. Notwithstanding the foregoing provisions of this Section 2.11, a stockholder shall also comply with all applicable requirements of the Securities Exchange Act of 1934,
and the rules and regulations thereunder with respect to the matters set forth in this Section 2.11.
Section 2.12. Notice of
Business. At any meeting of the stockholders, only such business shall be conducted as shall have been brought before the meeting (a) by or at the direction of the Board of Directors or (b) by any stockholder of the Corporation who is
a stockholder of record at the time of giving of the notice provided for in this Section 2.12, who shall be entitled to vote at such meeting and who complies with the notice procedures set forth in this Section 2.12. In addition, any
proposal of business must be a proper matter for stockholder action. For business to be properly brought before a stockholder meeting by a stockholder, the stockholder must have given timely notice thereof in writing to the secretary of the
Corporation. To be timely, a stockholder’s notice shall be delivered to or mailed and received at the principal executive offices of the Corporation not later than the close of business on the sixtieth (60th) day, nor earlier than the close of
business on the ninetieth (90th) day in advance of the first anniversary of the preceding year’s annual meeting of stockholders; provided, however, that in the event that the date of the annual meeting is advanced more than thirty
(30) days prior to such anniversary date or delayed more than sixty (60) days after such anniversary date then to be timely such notice must be received by the Corporation no later than the later of the close of business on the seventieth
(70th) day prior to the date of the meeting or the close of business on the tenth (10th) day following the day on which public announcement of the date of the meeting was made. In no event shall the public announcement of the new meeting date
commence a new notice time period (or extend any notice time period). A stockholder’s notice to the secretary shall set forth as to each matter the stockholder proposes to bring before the meeting (a) a brief description of the business
desired to be brought before the meeting and the reasons for conducting such business at the meeting, (b) the name and address, as they appear
4
on the Corporation’s books, of the stockholder proposing such business and of the beneficial owner, if any, on whose behalf the proposal is being made, (c) the class and number of
shares of the Corporation which are owned (beneficially and of record) by such stockholder and owned by the beneficial owner, if any, on whose behalf the proposal is being made, as of the date of the stockholder’s notice, and a representation
that the stockholder will notify the Corporation in writing of the class and number of such shares owned of record and beneficially as of the record date for the meeting promptly following the later of the record date or the date notice of the
record date is first publicly disclosed, (d) a description of any agreement, arrangement or understanding with respect to such nomination between or among the stockholder and any of its affiliates or associates, and any others (including their
names) acting in concert with any of the foregoing, and a representation that the stockholder will notify the Corporation in writing of any such agreement, arrangement or understanding in effect as of the record date for the meeting promptly
following the later of the record date or the date notice of the record date is first publicly disclosed, (e) a description of any agreement, arrangement or understanding (including, regardless of the form of settlement, any derivative, long or
short positions, profit interests, forwards, futures, swaps, options, warrants, convertible securities, stock appreciation or similar rights, hedging transactions and borrowed or loaned shares) that has been entered into as of the date of the
proposing stockholder’s notice, by or on behalf of such stockholder with respect to the Corporation’s securities, or any other agreement, arrangement or understanding that has been made, the effect or intent of which is to create or
mitigate loss to, manage risk or benefit of share price changes for, or increase or decrease the voting power of, such stockholder with respect to the Corporation’s securities, and a representation that the stockholder will notify the
Corporation in writing of any such agreement, arrangement or understanding in effect as of the record date for the meeting promptly following the later of the record date or the date notice of the record date is first publicly disclosed, (f) a
representation that the proposing stockholder is a holder of record of the shares of the Corporation entitled to vote at the meeting and intends to appear in person or by proxy at the meeting to propose the business specified in the notice,
(g) a representation whether the proposing stockholder intends to deliver a proxy statement and/or form of proxy to holders of at least the percentage of the Corporation’s outstanding capital stock required to approve the proposal and/or
otherwise to solicit proxies from the stockholders in support of the proposal, (h) any material interest of the stockholder in such business, and (i) any other information relating to such stockholder and beneficial owner, if any, on whose
behalf the proposal is being made, required to be disclosed in a proxy statement or other filings required to be made in connection with solicitations of proxies for the proposal and pursuant to and in accordance with Regulation 14A under the
Securities Exchange Act of 1934. Notwithstanding anything in the Bylaws to the contrary, no business shall be conducted at a stockholder meeting except in accordance with the procedures set forth in this Section 2.12. The chairman of the
meeting shall, if the facts warrant, determine and declare to the meeting that business was not properly brought before the meeting and in accordance with the provisions of the Bylaws, and if he should so determine, he shall so declare to the
meeting and any such business not properly brought before the meeting shall not be transacted. Notwithstanding the foregoing, provisions of this Section 2.12, a stockholder shall also comply with all applicable requirements of the Securities
Exchange Act of 1934, and the rules and regulations thereunder with respect to the matters set forth in this Section 2.12.
Section 2.13. Proxy Rules. The foregoing notice requirements of Section 2.12 shall be deemed satisfied by a stockholder with
respect to business other than a nomination if the stockholder has notified the Corporation of his, her or its intention to present a proposal at an annual meeting in compliance with the applicable rules and regulations promulgated under Regulation
14A under the Securities Exchange Act of 1934 and such stockholder’s proposal has been included in a proxy statement that has been prepared by the Corporation to solicit proxies for such annual meeting.
Section 2.14. Effect of Noncompliance. Notwithstanding anything in these Bylaws to the contrary: (i) no nominations shall be
made or business shall be conducted at any annual meeting except in accordance with the procedures set forth in this Article 2, and (ii) unless otherwise required by law, if a stockholder intending to propose business or make nominations at an
annual meeting pursuant to this Article 2 does not provide the information required under this Article 2 to the Corporation promptly following the later of the record date or the date notice of the record date is first publicly disclosed, or the
proposing stockholder (or a qualified representative of the proposing stockholder) does not appear at the meeting to present the proposed business or nominations, such business or nominations shall not be considered, notwithstanding that proxies in
respect of such business or nominations may have been received by the Corporation. The requirements of this Article 2 shall apply to any business or nominations to be brought before an annual meeting by a stockholder whether such business or
nomination are to be included in the Corporation’s proxy statement pursuant to Rule 14a-8 of the Exchange Act or presented to stockholders by
5
means of an independently financed proxy solicitation. The requirements of this Article 2 are included to provide the Corporation notice of a stockholder’s intention to bring business or
nominations before an annual meeting and shall in no event be construed as imposing upon any stockholder the requirement to seek approval from the Corporation as a condition precedent to bringing any such business or making such nominations before
an annual meeting.
ARTICLE 3
DIRECTORS
Section 3.01.
General Powers. Except as otherwise provided by Delaware Law or the Certificate of Incorporation, the business and affairs of the Corporation shall be managed by or under the direction of the Board of Directors. The Board of Directors may
adopt such rules and procedures, not inconsistent with the Certificate of Incorporation, these Bylaws, Delaware Law or other applicable law as it may deem proper for the conduct of its meetings and the management of the Corporation
Section 3.02. Number, Election and Term of office. The Board of Directors shall consist of not less than three (3) nor more
than eleven (11) directors, with the exact number of directors to be determined from time to time solely by resolution adopted by the affirmative vote of a majority of the entire Board of Directors. Except as otherwise provided in the
Certificate of Incorporation, each director shall serve for a term ending on the date of the annual meeting of stockholders next following the annual meeting at which such director was elected. Notwithstanding the foregoing, each director shall hold
office until such director’s successor shall have been duly elected and qualified or until such director’s earlier death, resignation or removal. Directors need not be stockholders.
Section 3.03. Quorum and Manner of Acting. Unless the Certificate of Incorporation or these Bylaws require a greater number, the
presence of a majority of the total number of directors shall constitute a quorum for the transaction of business, and the affirmative vote of a majority of the directors present at a meeting at which a quorum is present shall be the act of the
Board of Directors. When a meeting is adjourned to another time or place (whether or not a quorum is present), notice need not be given of the adjourned meeting if the time and place thereof are announced at the meeting at which the adjournment is
taken. At the adjourned meeting, the Board of Directors may transact any business which might have been transacted at the original meeting. If a quorum shall not be present at any meeting of the Board of Directors the directors present thereat shall
adjourn the meeting, from time to time, without notice other than announcement at the meeting, until a quorum shall be present.
Section 3.04. Time and Place of Meetings. The Board of Directors shall hold its meetings at such place, either within or without
the State of Delaware, and at such time as may be determined from time to time by the Board of Directors (or the Chairman in the absence of a determination by the Board of Directors).
Section 3.05. Annual Meeting. The Board of Directors shall meet for the purpose of organization, the election of officers and the
transaction of other business, as soon as practicable after each annual meeting of stockholders, on the same day and at the same place where such annual meeting shall be held. Notice of such meeting need not be given. In the event such annual
meeting is not so held, the annual meeting of the Board of Directors may be held at such place either within or without the State of Delaware, on such date and at such time as shall be specified in a notice thereof given as hereinafter provided in
Section 3.07 herein or in a waiver of notice thereof signed by any director who chooses to waive the requirement of notice.
Section 3.06. Regular Meetings. After the place and time of regular meetings of the Board of Directors shall have been determined
and notice thereof shall have been once given to each member of the Board of Directors, regular meetings may be held without further notice being given.
Section 3.07. Special Meetings. Special meetings of the Board of Directors may be called by the Chairman of the Board or the
President and shall be called by the Chairman of the Board, President or Secretary on the written request of three or more directors. Notice of special meetings of the Board of Directors shall be given to each director at least 24 hours before the
date of the meeting via email, in writing or in such other manner as is determined by the Board of Directors.
6
Section 3.08. Committees. The Board of Directors may designate one or more
committees, each committee to consist of one or more of the directors of the Corporation. The Board may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the
committee. In the absence or disqualification of a member of a committee, the member or members present at any meeting and not disqualified from voting, whether or not such member or members constitute a quorum, may unanimously appoint another
member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member. Any such committee, to the extent provided in the resolution of the Board of Directors or by applicable law, shall have and may exercise
all the powers and authority of the Board of Directors in the management of the business and affairs of the Corporation, and may authorize the seal of the Corporation to be affixed to all papers which may require it; but no such committee shall have
the power or authority in reference to the following matter: (a) approving or adopting, or recommending to the stockholders, any action or matter expressly required by Delaware Law to be submitted to the stockholders for approval or
(b) adopting, amending or repealing any bylaw of the Corporation. Unless the Board of Directors provides otherwise, at all meetings of such committee, a majority of the then authorized members of the committee shall constitute a quorum for the
transaction of business, and the vote of a majority of the members of the committee present at the meeting at which there is a quorum shall be the act of the Committee. Each committee shall keep regular minutes of its meetings and report the same to
the Board of Directors when required. Except as otherwise provided in the Certificate of Incorporation, these Bylaws, or the resolution of the Board of Directors designating the committee, a committee may create one or more subcommittees, each
subcommittee to consist of one or more members of the committee, and delegate to a subcommittee any or all of the powers and authority of the committee.
Section 3.09. Action by Consent. Unless otherwise restricted by the Certificate of Incorporation or these Bylaws, any action
required or permitted to be taken at any meeting of the Board of Directors or of any committee thereof may be taken without a meeting, if all members of the Board or such committee, as the case may be, consent thereto in writing or by electronic
transmission, and the writing or writings or electronic transmission or transmissions, are filed with the minutes of proceedings of the Board of Directors or such committee. Such filing shall be in paper form if the minutes are maintained in paper
form and shall be in electronic form if the minutes are maintained in electronic form.
Section 3.10. Telephonic Meetings.
Unless otherwise restricted by the Certificate of Incorporation or these Bylaws, members of the Board of Directors, or any committee designated by the Board of Directors, may participate in a meeting of the Board of Directors, or such committee,
as the case may be, by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and such participation in a meeting shall constitute presence in person at the
meeting.
Section 3.11. Resignation. Any director may resign at any time by giving notice in writing or by electronic
transmission to the Board of Directors or to the Secretary of the Corporation. The resignation of any director shall take effect upon receipt of notice thereof or at such later time as shall be specified in such notice; and unless otherwise
specified therein, the acceptance of such resignation shall not be necessary to make it effective.
Section 3.12. Vacancies.
Unless otherwise provided in the Certificate of Incorporation, vacancies on the Board of Directors resulting from death, resignation, removal or otherwise and newly created directorships resulting from any increase in the number of directors may
be filled solely by the affirmative vote of a majority of the remaining directors then in office (although less than a quorum) or by the sole remaining director. Each director so elected shall hold office until the earlier of the expiration of the
term of office of the director whom he or she has replaced, a successor is duly elected and qualified or the earlier of such director’s death, resignation or removal. If there are no directors in office, then an election of directors may be
held in accordance with Delaware Law. Unless otherwise provided in the Certificate of Incorporation, when one or more directors shall resign from the Board, effective at a future date, a majority of the directors then in office, including those who
have so resigned, shall have the power to fill such future vacancy or vacancies, the vote thereon to take effect when such resignation or resignations shall become effective, and each director so chosen shall hold office as provided in the filling
of the other vacancies.
Section 3.13. Removal. No director may be removed from office by the stockholders except for cause
with the affirmative vote of the holders of not less than a majority of the total voting power of all outstanding securities of the Corporation then entitled to vote generally in the election of directors, voting together as a single class.
7
Section 3.14. Compensation. Unless otherwise restricted by the Certificate of
Incorporation or these Bylaws, the Board of Directors shall have authority to fix the compensation of directors, including fees and reimbursement of expenses.
Section 3.15. Preferred Stock Directors. Notwithstanding anything else contained herein, whenever the holders of one or more
classes or series of Preferred Stock shall have the right, voting separately as a class or series, to elect directors, the election, term of office, filing of vacancies, removal and other features of such directorships shall be governed by the terms
of the resolutions applicable thereto adopted by the Board of Directors pursuant to the Certificate of Incorporation, and such directors so elected shall not be subject to the provisions of Sections 3.02, 3.12 and 3.13 of this Article 3 unless
otherwise provided therein.
ARTICLE 4
OFFICERS
Section 4.01.
Principal Officers. The officers of the Corporation shall be elected by the Board of Directors and shall include a president, a treasurer and a secretary. The Board of Directors, in its discretion, may also elect a chairman (who must be a
director), one or more vice chairmen (who must be directors) and one or more vice presidents, assistant treasurers, assistant secretaries and other officers. Any two or more offices may be held by the same person.
(a) President. The President shall have general responsibility for the management and control of the operations of the corporation. The
President shall have the power to affix the signature of the Corporation to all contracts that have been authorized by the Board of Directors. The President shall, when requested, counsel with and advise the other officers of the Corporation and
shall perform such other duties as such officer may agree to or as the Board of Directors may from time to time determine.
(b)
Treasurer. The Treasurer shall supervise and be responsible for all the funds and securities of the Corporation, the deposit of all moneys and other valuables to the credit of the Corporation in depositories of the Corporation, borrowings and
compliance with the provisions of all indentures, agreements and instruments governing such borrowings to which the Corporation is a party, the disbursement of funds of the Corporation and the investment of its funds, and in general shall perform
all of the duties incident to the office of the Treasurer. The Treasurer shall, when requested, counsel with and advise the other officers of the Corporation and shall perform such other duties as such officer may agree with the President or as the
Board of Directors may from time to time determine.
(c) Secretary. The powers and duties of the Secretary are: (i) to act as
Secretary at all meetings of the Board of Directors, of the committees of the Board of Directors and of the stockholders and to record the proceedings of such meetings in a book or books to be kept for that purpose; (ii) to see that all notices
required to be given by the Corporation are duly given and served; (iii) to act as custodian of the seal of the Corporation and affix the seal or cause it to be affixed to all certificates of stock of the Corporation and to all documents, the
execution of which on behalf of the Corporation under its seal is duly authorized in accordance with the provisions of these Bylaws; (iv) to have charge of the books, records and papers of the Corporation and see that the reports, statements
and other documents required by law to be kept and filed are properly kept and filed; and (AT) to perform all of the duties incident to the office of Secretary. The Secretary shall, when requested, counsel with and advise the other officers of the
Corporation and shall perform such other duties as such officer may agree with the President or as the Board of Directors may from time to time determine.
Section 4.02. Term of Office; Vacancy; and Remuneration. Each officer shall hold office until his or her successor is elected and
qualified, or until his or her earlier death, resignation or removal. Any vacancy in any office shall be filled in such manner as the Board of Directors shall determine. The remuneration of all officers of the Corporation shall be fixed by the Board
of Directors.
8
Section 4.03. Subordinate Officers. The Board of Directors may delegate to any
principal officer the power to appoint and to remove any such subordinate officers, agents or employees.
Section 4.04. Removal.
Except as otherwise permitted with respect to subordinate officers, any officer may be removed, with or without cause, at any time, by the majority vote of the members of the Board of Directors then in office.
Section 4.05. Resignations. Any officer may resign at any time by giving written notice to the Board of Directors (or to a
principal officer if the Board of Directors has delegated to such principal officer the power to appoint and to remove such officer) of such person’s resignation. The resignation of any officer shall take effect upon receipt of notice thereof
or at such later time as shall be specified in such notice; and unless otherwise specified therein, the acceptance of such resignation shall not be necessary to make it effective.
Section 4.06. Powers and Duties. The officers of the Corporation shall have such powers and perform such duties incident to each
of their respective offices and such other duties as may from time to time be conferred upon or assigned to them by the Board of Directors.
ARTICLE 5
CAPITAL STOCK
Section 5.01. Certificates for Stock; Uncertificated Shares. The shares of the Corporation shall be represented by certificates,
provided that the Board of Directors of the Corporation may provide by resolution or resolutions that some or all of any or all classes or series of its stock shall be uncertificated shares that may be evidenced by a book entry system maintained by
the registrar of such stock. Any such resolution shall not apply to shares represented by a certificate until such certificate is surrendered to the Corporation. Except as otherwise provided by law, the rights and obligations of the holders of
uncertificated shares and the rights and obligations of the holders of shares represented by certificates of the same class and series shall be identical. Every holder of stock represented by certificates shall be entitled to have a certificate
signed by, or in the name of the Corporation by the Chairman or Vice Chairman of the Board of Directors, or the President or Vice President, and by the Treasurer or an Assistant Treasurer or the Secretary or an assistant Secretary of such
Corporation representing the number of shares registered in certificate form. Any or all of the signatures on the certificate may be a facsimile. In case any officer, transfer agent or registrar who has signed or whose facsimile signature has been
placed upon a certificate shall have ceased to be such officer, transfer agent or registrar before such certificate is issued, it may be issued by the Corporation with the same effect as if such person were such officer, transfer agent or registrar
at the date of issue. A Corporation shall not have power to issue a certificate in bearer form.
Section 5.02. Transfer of Shares.
Shares of the stock of the Corporation may be transferred on the record of stockholders of the Corporation by the holder thereof or by such holder’s duly authorized attorney upon surrender of a certificate therefor properly endorsed or upon
receipt of proper transfer instructions from the registered holder of uncertificated shares or by such holder’s duly authorized attorney and upon compliance with appropriate procedures for transferring shares in uncertificated form, unless
waived by the Corporation.
Section 5.03. Authority for Additional Rules Regarding Transfer. The Board of Directors shall have
the power and authority to make all such rules and regulations as they may deem expedient concerning the issue, transfer and registration of certificated or uncertificated shares of the stock of the Corporation, as well as for the issuance of new
certificates in lieu of those which may be lost or destroyed, and may require of any stockholder requesting replacement of lost or destroyed certificates, bond in such amount and in such form as they may deem expedient to indemnify the Corporation,
and/or the transfer agents, and/or the registrars of its stock against any claims arising in connection therewith.
Section 5.04.
Lost, Stolen or Destroyed Stock Certificates; Issuance of New Certificates. The Corporation may issue a new certificate of stock in the place of any certificate theretofore issued by it, alleged to have been lost, stolen or destroyed, and the
Corporation may require the owner of the lost, stolen or destroyed certificate, or such owner’s legal representative, to give the Corporation a bond sufficient to indemnify it against any claim that may be made against it on account of the
alleged loss, theft or destruction of any such certificate or the issuance of such new certificate.
9
ARTICLE 6
INDEMNIFICATION AND ADVANCEMENT OF EXPENSES
Section 6.01. Right to Indemnification. The Corporation shall indemnify and hold harmless, to the fullest extent permitted by
applicable law as it presently exists or may hereafter be amended, any person (a “Covered Person”) who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal,
administrative or investigative (a “proceeding”), by reason of the fact that he or she, or a person for whom he or she is the legal representative, is or was a director or officer of the Corporation or, while a director or officer of the
Corporation, is or was serving at the request of the Corporation as a director, officer, employee or agent of another Corporation or of a partnership, joint venture, trust, enterprise or nonprofit entity, including service with respect to employee
benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees) reasonably incurred by such Covered Person. Notwithstanding the preceding sentence, except as otherwise provided in Section 6.3, the
Corporation shall be required to indemnify a Covered Person in connection with a proceeding (or part thereof) commenced by such Covered Person only if the commencement of such proceeding (or part thereof) by the Covered Person was authorized in the
specific case by the Board of Directors of the Corporation.
Section 6.02. Prepayment of Expenses. The Corporation shall to
the fullest extent not prohibited by applicable law pay the expenses (including attorneys’ fees) incurred by a Covered Person in defending any proceeding in advance of its final disposition, provided, however, that, to the extent required by
law, such payment of expenses in advance of the final disposition of the proceeding shall be made only upon receipt of an undertaking by the Covered Person to repay all amounts advanced if it should be ultimately determined that the Covered Person
is not entitled to be indemnified under this Article VI or otherwise.
Section 6.03. Nonexclusivity of Rights. The rights
conferred on any Covered Person by this Article VI shall not be exclusive of any other rights which such Covered Person may have or hereafter acquire under any statute, provision of the Certificate of Incorporation, these Bylaws, agreement, vote of
stockholders or disinterested directors or otherwise.
Section 6.04. Other Sources. The Corporation’s obligation, if any,
to indemnify or to advance expenses to any Covered Person who was or is serving at its request as a director, officer, employee or agent of another Corporation, partnership, joint venture, trust, enterprise or nonprofit entity shall be reduced by
any amount such Covered Person may collect as indemnification or advancement of expenses from such other Corporation, partnership, joint venture, trust, enterprise or non-profit enterprise.
Section 6.05. Amendment or Repeal. Any right to indemnification or to advancement of expenses of any Covered Person arising
hereunder shall not be eliminated or impaired by an amendment to or repeal of these Bylaws after the occurrence of the act or omission that is the subject of the civil, criminal, administrative or investigative action, suit or proceeding for which
indemnification or advancement of expenses is sought.
Section 6.06. Other Indemnification and Advancement of Expenses. This
Article VI shall not limit the right of the Corporation, to the extent and in the manner permitted by law, to indemnify and to advance expenses to persons other than Covered Persons when and as authorized by appropriate corporate action.
ARTICLE 7
GENERAL PROVISIONS
Section 7.01. Fixing the Record Date.
(a) In order that the Corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any
adjournment thereof, the Board of Directors may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which record date shall not be more than sixty
(60) nor less than ten (10) days before the date of such meeting. If the Board of Directors so fixes a date, such date shall also be the record date for determining the stockholders entitled to vote at such meeting unless the Board of
Directors determines, at the time it fixes the record date, that a
10
later date on or before the date of the meeting shall be the date for making such determination. If no record date is fixed by the Board of Directors, the record date for determining stockholders
entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given, or, if notice is waived, at the close of business on the day next preceding the day on which
the meeting is held. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided that the Board of Directors may fix a new record date for the
determination of stockholders entitled to vote at the adjourned meeting and in such case shall also fix as the record date for stockholders entitled to notice of such adjourned meeting the same or an earlier date as that fixed for the determination
of stockholders entitled to vote therewith at the adjourned meeting.
(b) In order that the Corporation may determine the stockholders
entitled to receive payment of any dividend or other distribution or allotment of any rights or the stockholders entitled to exercise any rights in respect of any change, conversion or exchange of stock, or for the purpose of any other lawful
action, the Board of Directors may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted, and which record date shall be not more than sixty (60) days prior to such action.
If no record date is fixed, the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board of Directors adopts the resolution relating thereto.
Section 7.02. Dividends. Subject to limitations contained in Delaware Law and the Certificate of Incorporation, the Board of
Directors may declare and pay dividends upon the shares of capital stock of the Corporation, which dividends may be paid either in cash, in property or in shares of the capital stock of the Corporation.
Section 7.03. Year. The fiscal year of the Corporation shall commence on January 1 and end on December 31 of each year.
The fiscal year of the Corporation may be changed by the Board of Directors.
Section 7.04. Corporate Seal. The corporate seal
shall have inscribed thereon the name of the Corporation, the year of its organization and the words “Corporate Seal, Delaware”. The seal may be used by causing it or a facsimile thereof to be impressed, affixed or otherwise reproduced.
Section 7.05. Voting of Stock Owned by the Corporation. The Board of Directors may authorize any person, on behalf of the
Corporation, to attend, vote at and grant proxies to be used at any meeting of stockholders of any Corporation (except this Corporation) in which the Corporation may hold stock.
Section 7.06. Form of Records. Any records maintained by the Corporation in the regular course of its business, including its
stock ledger, books of account, and minute books, may be kept on, or by means of, or be in the form of, any information storage device or method, provided that the records so kept can be converted into clearly legible paper form within a reasonable
time.
Section 7.07. Amendments. These Bylaws or any of them, may be altered, amended or repealed, or new Bylaws may be made,
by the stockholders entitled to vote thereon at any annual or special meeting thereof or by the Board of Directors. Unless a higher percentage is required by the Certificate of Incorporation as to any matter which is the subject of these Bylaws, all
such amendments must be approved by the affirmative vote of the holders of the majority of the total voting power of all outstanding securities of the Corporation then entitled to vote generally in the election of directors, voting together as a
single class or by a majority of the Board of Directors.
ARTICLE 8
FORUM FOR ADJUDICATION OF DISPUTES
Section 8.01. Forum for Adjudication of Disputes. Unless the Corporation consents in writing to the selection of an alternative
forum, (a) the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have, or declines to accept, jurisdiction, another state court or a federal court located within the State of Delaware) shall be the sole and
exclusive forum for (i) any derivative action or proceeding brought on behalf of the Corporation, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Corporation to the
Corporation or the Corporation’s stockholders, (iii) any action asserting
11
a claim arising pursuant to any provision of Delaware Law, the certificate of incorporation or the bylaws of the Corporation, or (iv) any action asserting a claim governed by the internal
affairs doctrine, and (b) the federal district courts of the United States of America shall be the sole and exclusive forum for any complaint asserting a cause of action arising under the Securities Act of 1933, to the fullest extent permitted
by law. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock of the Corporation shall be deemed to have notice of and consented to the provisions of this Section 8.01.
12
Exhibit 99.1

Viridian Therapeutics Announces Positive Clinical Data in Healthy Volunteer Study and Selects VRDN-003 as Potential Best-in-Class Subcutaneous
anti-IGF-1R Program with Extended Half-Life for Pivotal Development in Thyroid Eye Disease
- VRDN-003 clinical data exceeded expectations with extended half-life of 40-50 days, 4-5x longer than VRDN-001, supporting a potential
best-in-class, low-volume, less frequent, self-administered subcutaneous therapy for thyroid eye disease (TED) -
- Data support VRDN-003 dosing as infrequently as once every eight weeks and is expected to reach
exposure levels associated with robust clinical response in earlier trials of VRDN-001 in TED patients -
- Pivotal clinical development of subcutaneous VRDN-003 in TED patients expected to initiate mid-2024 pending alignment with regulatory authorities -
- Viridian to host investor conference
call and webcast today at 8:00am ET—
WALTHAM, Mass., December 18, 2023 — Viridian Therapeutics, Inc. (NASDAQ: VRDN), a biotechnology
company focused on discovering and developing potential best-in-class medicines for serious and rare diseases, today announced the selection of VRDN-003 as its lead subcutaneous (SC) program for thyroid eye disease (TED) based on positive data from a Phase 1 clinical study in healthy volunteers. The data showed that
VRDN-003 has an extended half-life of 40-50 days, about 4-5 times longer than VRDN-001
and based upon comparisons of publicly disclosed data, significantly longer than other first generation anti insulin-like growth factor-1 receptor (IGF-1R) antibodies. VRDN-003 was well tolerated and exhibited sustained pharmacodynamics supporting its development as a potential best-in-class, more
convenient, less frequent, low-volume, self-administered, subcutaneous IGF-1R therapy for patients with TED.
“The VRDN-003 data exceeded our expectations as a potential best-in-class treatment option for patients affected by TED and support advancing dosing regimens as infrequently as once every eight weeks, which we believe could be transformative for TED patients who
currently only have access to intravenous (IV) IGF-1R therapy,” said Steve Mahoney, President and CEO of Viridian Therapeutics. “The data reinforce our confidence in VRDN-003’s rapid development as a low-volume, self-administered, subcutaneous product. Given VRDN-003’s comparable
pharmacology with VRDN-001, which has already generated compelling clinical data in TED patients, we are excited about the clinical potential of this program for patients and our ability to rapidly move toward pivotal development.”
1
VRDN-003 Results
VRDN-003 is a monoclonal antibody that acts as a full antagonist of IGF-1R, a
clinically and commercially validated target for TED. VRDN-003 utilizes the same binding domain as VRDN-001 and is engineered with three amino acid changes to the Fc
region designed to extend the half-life of the parent antibody, and therefore potentially enabling less frequent and more convenient dosing. VRDN-003 is designed to maintain the clinical response of VRDN-001 IV while significantly increasing patient convenience.
| |
• |
|
Study Design: VRDN-003 was dosed in four single dose cohorts of
healthy volunteers at a concentration of 150 mg/ml receiving 5 mg/kg IV (n=4), 300 mg SC (n=6), 15 mg/kg IV (n=4) and 600 mg SC (n=6). A fifth cohort of two doses of VRDN-003 (n=4) is ongoing.
|
| |
• |
|
Extended Half Life: VRDN-003 pharmacokinetics data showed an
extended half-life of 40-50 days, which is a 4-to-5-fold increase over the half-life of VRDN-001 (which showed a half-life of 10-12 days). |
| |
• |
|
Prolonged Pharmacodynamics (PD): Following a single subcutaneous dose of
VRDN-003, IGF-1 serum levels increased approximately 4-fold at peak. This was consistent with the increases in IGF-1 levels that have been shown in the clinic following a single dose of VRDN-001 SC and IV. IGF-1 is a PD biomarker for IGF-1R target engagement. IGF-1R inhibition by an anti-IGF-1R antibody demonstrated an increase
in serum levels of its natural ligand IGF-1. |
| |
• |
|
Well Tolerated: VRDN-003 was well tolerated in all subjects
with no serious adverse events. All treatment-related, treatment-emergent adverse events were grade 1 (mild). In the reported dataset, no antidrug antibodies (ADAs) were detected. |
| |
• |
|
Dosing Flexibility for Pivotal Development: VRDN-003 modeling
demonstrates dosing flexibility for the program’s anticipated global pivotal development. |
| |
• |
|
Q8W (dosing once every 8 weeks): Modeled dose schedule is predicted to achieve Cmin and Cavg exposure achieved with 3 mg/kg VRDN-001 IV. |
2
| |
• |
|
Q4W (dosing once every 4 weeks): Modeled dose schedule is predicted to achieve Cmin exposures seen for 10 mg/kg VRDN-001 IV and exceed the Cavg exposure seen for 3 mg/kg
by a factor of 2. |
| |
• |
|
Q2W (dosing once every 2 weeks): Modeled dose schedule is predicted to exceed Cmin exposures seen for 20 mg/kg VRDN-001 IV and the Cavg exposures seen for 10 mg/kg VRDN-001 IV. |
“The shared binding domain and pharmacology of
VRDN-003 with VRDN-001 allow us to leverage the clinical activity of VRDN-001 IV at different exposure levels to confidently
select doses for VRDN-003 that we expect to demonstrate an impactful clinical response,” said Tom Ciulla, Viridian’s Chief Development Officer. “We are enthusiastic about delivering a product
with highly attractive, low-volume subcutaneous dosing schedules that we firmly believe will be preferred by patients.”
Based on the data being reported today, VRDN-003 has been selected as Viridian’s
go-forward candidate for subcutaneous development. We expect to initiate the VRDN-003 pivotal program in mid-2024, pending
alignment with regulatory authorities. Based on these positive results and the expected development timeline for VRDN-003, both VRDN-001 SC and VRDN-002 SC development have been deprioritized.
VRDN-001 SC and VRDN-002 Results
VRDN-001 SC is a subcutaneous formulation of the same
antibody being evaluated as an IV product candidate in the company’s ongoing Phase 3 THRIVE and THRIVE-2 clinical trials. The Phase 1 healthy volunteer study included a cohort of the SC formulation of VRDN-001 at a concentration of 150 mg/ml dosed in two single dose cohorts of healthy volunteers either receiving 3.5 mg/kg IV (n= 8) or 300mg SC (n=8). SC data showed an expected half-life of 10-12 days. Q1W dosing of VRDN-001 SC was modeled to achieve or exceed exposures shown for 10 mg/kg VRDN-001 IV and would be expected
to achieve comparable clinical response to VRDN-001 10 mg/kg IV. VRDN-001 SC also showed an increase in IGF-1 levels similar to VRDN-001 IV and VRDN-003 SC. VRDN-001 SC was well tolerated in all subjects with no serious adverse events. All treatment-related,
treatment-emergent adverse events were grade 1 (mild) and grade 2 (moderate).
3
VRDN-002 is a different and novel monoclonal antibody targeting IGF-1R as compared to VRDN-001 or VRDN-003. Similar to VRDN-003,
VRDN-002 was engineered to have an extended half-life to allow for less frequent dosing. VRDN-002 SC was dosed at a concentration of 150 mg/ml in two single dose cohorts
of healthy volunteers, either receiving 3.5mg/kg IV (n=8) or 300mg SC (n=8). Healthy volunteer data for VRDN-002 showed an extended half-life of 43 days as previously disclosed.
VRDN-002 also showed a prolonged pharmacodynamic effect and increased IGF-1 levels, but the magnitude of increase was not as great as with
VRDN-003 or VRDN-001. VRDN-002 was well tolerated in all subjects with no serious adverse events. All treatment-related,
treatment-emergent adverse events were grade 1 (mild). No ADAs were detected.
Next Steps for the Development of
VRDN-003 in TED
Based on the results reported today, the company expects to initiate global pivotal clinical
trials of VRDN-003 in mid-2024 with planned trials in both active and chronic TED patients pending alignment with regulatory authorities.
“We view the VRDN-003 data as a crucial step forward for TED patients,” said Mr. Mahoney.
“Based on the data, we expect that VRDN-003 could be a leader in the large TED commercial market that may be poised for expanded growth with a more convenient, less frequent, at-home, self-administered subcutaneous product. We plan to move forward rapidly and will provide additional details about our trial design and development plans in the first half of 2024.”
Conference call and webcast information
The Company will
host a conference call today at 8:00 a.m. ET to discuss the data for Viridian’s lead selection for the IGF-1R subcutaneous program. The dial-in number for the
conference call is 1-888-330-3622 for domestic participants and
1-646-960-0662 for international participants. The conference ID is 3961606.
A live webcast of the conference call can be accessed through the “Events” page in the Investors section of the Viridian Therapeutics website.
Following the live webcast, an archived version of the call will also be available on the website.
About Viridian’s Thyroid Eye Disease Pipeline (VRDN-001 and VRDN-003)
Viridian’s lead product candidate, VRDN-001, is a differentiated monoclonal antibody targeting insulin-like growth factor-1 receptor (IGF-1R), a clinically and
commercially validated target for the treatment of thyroid eye disease (TED). In preclinical studies, VRDN-001 was shown to be a full antagonist of IGF-1R, with more
complete receptor blockade than other anti-IGF-1R antibodies, including the only currently approved TED therapy. Data from the Phase 2 portion of the trial established
clinical proof-of-concept for VRDN-001 in patients with active and chronic TED. VRDN-001
was generally well tolerated in the trial.
4
The THRIVE and THRIVE-2 Phase 3 clinical trials in patients with
active and chronic TED are ongoing.
Viridian’s goal is to advance VRDN-001 as a best-in-class IV therapy followed by VRDN-003 as a first- and
best-in-class SC therapy for the treatment of TED. The company has selected VRDN-003 as its lead SC program for TED based on
positive data from its Phase 1 clinical study in healthy volunteers and expects to initiate global pivotal development of VRDN-003 with planned trials in both active and chronic TED patients pending alignment
with regulatory authorities.
About TED
TED is a
serious and debilitating rare autoimmune disease that causes inflammation within the orbit of the eye that can cause double vision, pain, and potential blindness. TED is a progressive disease consisting of an initial active phase, followed by a
transition to a secondary chronic phase. More than 50,000 and 200,000 people are estimated to suffer from active and chronic TED, respectively, in the United States and Europe.
About Viridian Therapeutics
Viridian is a
biopharmaceutical company focused on engineering and developing potential best-in-class medicines for patients with serious and rare diseases. Viridian’s expertise
in antibody discovery and engineering enables it to develop differentiated therapeutic candidates for previously validated drug targets in commercially established disease areas.
Viridian is advancing multiple candidates in the clinic for the treatment of patients with TED. The company is conducting two global Phase 3 clinical trials
(THRIVE and THRIVE-2) to evaluate the safety and efficacy of VRDN-001 in patients with active and chronic TED. Viridian’s goal is to advance VRDN-001 as a best-in-class IV therapy followed by VRDN-003 as a first- and best-in-class SC therapy for the treatment of TED. In addition to its TED portfolio, Viridian is advancing a novel portfolio of neonatal Fc receptor (FcRn) inhibitors, VRDN-006 and VRDN-008, which has the potential to be developed in multiple autoimmune diseases. Viridian is also developing additional preclinical assets in autoimmune and
rare diseases.
5
Viridian is based in Waltham, Massachusetts. For more information, please visit
www.viridiantherapeutics.com. Follow Viridian on LinkedIn and X.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be
identified by the use of words such as, but not limited to, “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,”
“plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or other similar terms or expressions that concern our expectations, plans and
intentions. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations, and assumptions. Forward-looking statements include, without limitation,
statements regarding: the therapeutic potential and utility, efficacy and clinical benefits of VRDN-003 for Thyroid Eye Disease (TED); the expected exposure levels of
VRDN-003; the safety profile of VRDN-003; the potential dosing frequency for VRDN-003; trial designs, clinical development plans
and timing for VRDN-003, including the global pivotal clinical trials of VRDN-003 in active and chronic TED patients; the expected attractiveness of VRDN-003 and convenience for patients; and anticipated work with regulatory authorities. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No
representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including but not limited to: the
potential efficacy and safety of VRDN-001, VRDN-002, and VRDN-003 for the treatment of TED; the potential for VRDN-006 and VRDN-008; the relationship between the results from the positive data from completed or ongoing clinical trials and the results of ongoing or future clinical
trials; the timing, progress and plans for our ongoing or future research, pre-clinical and clinical development programs; trial protocols for ongoing clinical trials; expectations regarding the timing for IND
filings; expectations regarding the timing for enrollment and data; uncertainty and potential delays related to clinical drug development; the duration and impact of regulatory delays in our clinical programs; the timing of and our ability to obtain
and maintain regulatory approvals for our therapeutic candidates; manufacturing risks; competition from other therapies or products; estimates of market size; other matters that could affect the sufficiency of existing cash, cash equivalents and
short-term investments to fund operations; our financial position and its projected cash runway; our future operating results and financial performance; the clinical utility of our therapeutic candidates and our intellectual property position; the
timing of pre-clinical and clinical trial activities and reporting results from same, including those risks set forth under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on
6
November 13, 2023 and other subsequent disclosure documents filed with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Neither the Company, nor its
affiliates, advisors, or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. These forward-looking
statements should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof.
Contact
Louisa Stone, 617-272-4604
Manager, Investor Relations
IR@viridiantherapeutics.com
Source: Viridian Therapeutics, Inc.
7

Exhibit 99.2 Subcutaneous IGF-1R Program Selection December 18,
2023

Cautionary note regarding forward-looking statements This presentation
contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as, but not limited to, “anticipate,” “believe,”
“continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,”
“should,” “target,” “will,” or “would” or other similar terms or expressions that concern our expectations, plans and intentions. Forward-looking statements are neither historical facts nor assurances
of future performance. Instead, they are based on our current beliefs, expectations, and assumptions. Forward-looking statements include, without limitation, statements regarding: the therapeutic potential and utility, efficacy and clinical benefits
of VRDN-003 for Thyroid Eye Disease (TED); the expected exposure levels of VRDN-003; the safety profile of VRDN- 003; the potential dosing frequency for VRDN-003; trial designs, clinical development plans and timing for VRDN-003, including the
global pivotal clinical trials of VRDN-003 in active and chronic TED patients; the expected attractiveness of VRDN-003 and convenience for patients; and anticipated work with regulatory authorities. New risks and uncertainties may emerge from time
to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a
number of material risks and uncertainties including but not limited to: the potential efficacy and safety of VRDN-001, VRDN-002, and VRDN-003 for the treatment of TED; the potential for VRDN-006 and VRDN-008; the relationship between the results
from the positive data from completed or ongoing clinical trials and the results of ongoing or future clinical trials; the timing, progress and plans for our ongoing or future research, pre-clinical and clinical development programs; trial protocols
for ongoing clinical trials; expectations regarding the timing for IND filings; expectations regarding the timing for enrollment and data; uncertainty and potential delays related to clinical drug development; the duration and impact of regulatory
delays in our clinical programs; the timing of and our ability to obtain and maintain regulatory approvals for our therapeutic candidates; manufacturing risks; competition from other therapies or products; estimates of market size; other matters
that could affect the sufficiency of existing cash, cash equivalents and short-term investments to fund operations; our financial position and its projected cash runway; our future operating results and financial performance; the clinical utility of
our therapeutic candidates and our intellectual property position; the timing of pre-clinical and clinical trial activities and reporting results from same, including those risks set forth under the caption “Risk Factors” in our
Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on November 13, 2023 and other subsequent disclosure documents filed with the SEC. The forward-looking statements in this presentation represent our views as of
the date of this presentation. Neither we, nor our affiliates, advisors, or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise,
except as required by law. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation also contains estimates and other statistical data made by
independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections,
assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. 2

Subcutaneous VRDN-003 selected and anticipated to advance to pivotal
trials in TED in mid-2024 VRDN-003 sets a potential new bar for best-in-class product in TED based on Phase 1 HV study • Extended half-life: VRDN-003 half-life exceeds expectations at 40-50 days – 4-5x increase compared to its parent
VRDN-001, significantly longer than first-generation anti-IGFRs including teprotumumab and lonigutamab – VRDN-003 IGF-1 increases at peak match VRDN-001 and demonstrates potential superiority to other disclosed data from IGF-1R antibodies
– Showed substantially prolonged durability of elevated IGF-1 levels compared to VRDN-001 • Convenient dosing: Dosing interval as long as Q8W is predicted to achieve clinically meaningful exposures • Best-in-class patient
experience expected: Anticipated 2mL low-volume (≥150mg/mL concentration), self-administered injection • Safety profile consistent with best-in-class profile: VRDN-003 was well tolerated with no SAEs or ADAs to date VRDN-003 to leverage
VRDN-001 IV experience to enable anticipated rapid and de-risked global pivotal development • Expedited regulatory path potential: Next steps expected to be two pivotal trials in patients with active and chronic TED • Optimized for speed
to market: Global pivotal program expected to begin in mid-2024 pending alignment with regulators VRDN-003 poised to enter a multi-billion-dollar TED market with a potentially best-in-class SC profile All data regarding third-party products in this
presentation are based on third-party studies and not our own. Conclusions are not based on head-to-head clinical trial results. 3 Source: Viridian clinical data on file. ADAs = antidrug antibodies, HV = healthy volunteers, IGF-1R = insulin-like
growth factor-1 receptor, IV = intravenous, Q8W = once every eight weeks, SAEs = serious adverse events, SC = subcutaneous, TED = thyroid eye disease.

VRDN-003 is nearly identical to its parent VRDN-001 and has a 4-5x
extended half-life VRDN-001 VRDN-003 VRDN-003 has half-life engineering via clinically validated, 3 amino acid (YTE) modification in Fc region VRDN-001 & VRDN-003 are nearly identical Differ in YTE change in Fc domain to enable extended
half-life 4 Source: Viridian data on file.

Thyroid eye disease represents a large market opportunity with potential
for growth Opportunities for growth Annualized Revenues ($) • Continued penetration into the active US TED market 1 ~$1.8B • Growth into chronic TED market • Market expansion from subcutaneous • Ex-US opportunity Validated
MoA Potential for differentiation • IGF-1R inhibition has demonstrated • Teprotumumab has eight-dose IV regimen robust clinical efficacy in TED • Anti-IGF-1R is the only approved • Other mAbs lack extended half-life targeted
therapy in TED • Small molecules risk off-target effects 2023 IGF-1R IV 5 1 Tepezza sales were $453M in Q3 2023. IGF-1R = insulin-like growth factor-1 receptor, IV = intravenous, mAbs = monoclonal antibodies, MoA = Mechanism of Action, SC =
subcutaneous, TED = thyroid eye disease.

Later-entrant SC therapies have the potential to significantly displace
incumbent IV and grow market IV to SC with same molecule IV to SC with new SC entrant 1 2 of IV market converted in 2 years 85% of new prescriptions converted in 3 years 30% Target: CD38 Target: CD20 • IV Launch: Nov 2015 by J&J for
multiple • IV Launch: Mar 2017 by Roche for multiple myeloma sclerosis • SC Launch: May 2020 by J&J• SC Launch: Aug 2020 by Novartis • Post-SC Sales Growth: 2X annual sales post-• Post-SC Sales Growth: 2x combined
CD20 1 3,4 SC launch sales post-Kesimpta SC launch Significant opportunity for a long-acting and convenient subcutaneous anti-IGF-1R therapy 1. https://www.fiercepharma.com/pharma/jjs-switch-iv-subcutaneous-darzalex-85-complete-us, 2. Novartis 2022
Q4 results, 3. Roche Earnings, 4. Novartis Q3 2023 Earnings; 6 1 IGF-1R = insulin-like growth factor-1 receptor, IV = intravenous, SC = subcutaneous, TED = thyroid eye disease.

Phase 1 clinical trials in healthy volunteers for VRDN-003, VRDN-002,
and VRDN-001 (N=34) (N=16) (N=16) VRDN-003 HV VRDN-002 HV VRDN-001 HV COHORT: COHORT: COHORT: Single dose of SC 300 mg Single dose of SC 300 mg Single dose of SC 300 mg 1 1 1 VRDN-003 (n=6), placebo (n=2) VRDN-002 (n=8) VRDN-001 (n=8) Low Dose
Single dose of IV 5.0 mg/kg Single dose of IV 3.5 mg/kg Single dose of IV 3.5 mg/kg 2 2 2 VRDN-003 (n=4), placebo (n=2) VRDN-002 (n=8) VRDN-001 (n=8) Single dose of SC 600 mg 3 VRDN-003 (n=6), placebo (n=2) High Dose Single dose of IV 15.0 mg/kg 4
VRDN-003 (n=4), placebo (n=2) Repeat dose of SC 600 mg/300 mg 5 VRDN-003 (n=4), placebo (n=2); data pending Multi-Dose 7 Source: Viridian data on file. HV = healthy volunteers, IV = intravenous, SC = subcutaneous.

Phase 1 healthy volunteer study pharmacokinetics and pharmacodynamics
VRDN-003 Phase 1 Pharmacokinetics (PK) VRDN-003 Phase 1 Pharmacodynamics (PD) VRDN-001 300 mg VRDN-001 300 mg SC 100 6 VRDN-002 300 mg VRDN-002 300 mg SC VRDN-003 300 mg VRDN-003 300 mg SC VRDN-003 600 mg SC VRDN-003 600 mg Lonigutamab 250 mg
Placebo 10 4 No public lonigutamab IGF-1 data available 1 2 0 0.1 0 20 40 60 80 100 0 20 40 60 80 100 Time (Days) Time (Days) VRDN-003 demonstrated superior IGF-1 increases relative to VRDN-003 half-life is 4-5x VRDN-001 VRDN-002 and was sustained
relative to VRDN-001 VRDN-003 shows potential best-in-class PK/PD and superior profile compared to all disclosed IGF-1R antibodies All data regarding third-party products in this presentation are based on third-party studies and not our own.
Conclusions are not based on head-to-head clinical trial results. 8 Source: 1. Viridian clinical data and modeling on file, 2. Keenan et al. NANOS 2023, 3. Xin et al., Clinical PK, 2021, 4. Pappos et al. Cancer 2014. IGF-1 = insulin-like growth
factor 1, SC = subcutaneous. Drug Concentration (μg/mL) Fold Change from Baseline IGF-1

VRDN-003 was well tolerated in healthy volunteers VRDN-003 (Cohorts 1-4)
VRDN-002 VRDN-001 Placebo IV (n=8) SC (n=12) IV (n=8) SC (n=8) IV (n=8) SC (n=8) (n=8) -- 3 (25%) -- 1 (12.5%) -- 3 (37.5%) ** 3 (37.5%) Treatment-Related AEs • No treatment -- -- -- -- -- 1 (12.5%) -- Muscle Spasms discontinuations -- -- -- 1
(12.5%) -- 3 (37.5%) -- Headaches • No hearing AEs -- 1 (8%) -- -- -- -- 1 (12.5%) Injection Site Reactions (ISRs) * • All treatment-related -- -- -- -- -- -- 1 (12.5%) Hyperglycemia AEs were grade 1 (mild) or grade 2 -- -- -- -- -- -- 1
(12.5%) Thrombocytopenia (moderate), no SAEs -- 1 (8%) -- -- -- -- -- Insomnia – VRDN-003: all grade 1 -- 1 (8%) -- -- -- -- -- Hepatic Enzyme Increased – VRDN-002: all grade 1 – VRDN-001: all grade 1 -- -- -- -- -- -- -- Severe
Adverse Events (SAEs) and grade 2 -- -- -- -- -- -- -- Hearing Impairment * • Hyperglycemia and -- -- -- -- -- -- -- Grade 3/4 Aes ISRs all resolved within the follow up period Low ADAs detected after Anti-Drug Antibodies (ADAs) None were
detected None were detected Day 43 with no neutralizing antibodies * Injection Site Reactions and Hearing Impairment each includes multiple MedDRA terms. 9 ** One participant experienced both muscle spasm and headache and was only counted once in
this row of total AEs, for the headache which was at a higher grade of 2; one additional participant experienced a grade 2 headache. AEs = Adverse Events, IV= intravenous, SAEs = serious adverse events, SC = subcutaneous.

VRDN-001 IV clinical experience informs exposures of VRDN-003 expected
to lead to clinical responses VRDN-001 IV Pharmacokinetics 250 200 • 20 mg/kg IV exposure range (C to C ) min avg 150 20 mg/kg IV C min 100 (20 mg/kg IV) • 10 mg/kg IV exposure range (C to C ) min avg 10 mg/kg IV • THRIVE and
THRIVE-2 dosing C min 50 (10 mg/kg IV) 3 mg/kg IV • 3 mg/kg IV exposure range (C to C ) min avg C min (3 mg/kg IV) 0 * VRDN-001 IV PK based on 5 doses and 15 weeks. 10 Source: Viridian clinical data and modeling on file. IV = intravenous.
Concentration (μg/mL)

VRDN-001 IV clinical experience informs exposures of VRDN-003 expected
to lead to clinical responses VRDN-001 IV Phase 2 Clinical Responses in Active TED VRDN-001 IV Pharmacokinetics 250 Proptosis: CAS*: Diplopia**: Responder Rate Score of 0 or 1 Complete resolution 200 % with ≥ 2mm reduction from % achieving CAS
of 0 or 1 % improved to a score of 0 baseline to week 6 at week 6 at week 6 150 20 mg/kg IV 67% 33% 75% C min 100 (20 mg/kg IV) 83% 83% 75% 10 mg/kg IV C min 50 (10 mg/kg IV) 67% 67% 20% 3 mg/kg IV C min (3 mg/kg IV) 0 All 3 cohorts: 71% 62% 54% 20
mg/kg IV exposure range (Cmin to Cavg) 10 mg/kg IV exposure range (Cmin to Cavg) Teprotumumab: 56% 22% 36% (cross-trial comparison) 3 mg/kg IV exposure range (Cmin to Cavg) * VRDN-001 IV PK based on 5 doses and 15 weeks. All data regarding
third-party products in this presentation are based on third-party studies and not our own. Conclusions are not based on head-to-head clinical trial results. 11 *Clinical Activity Score (CAS) = a composite 0-7 scale scoring signs and symptoms of
TED. **Diplopia was present at baseline in 13 out of 21 drug-treated patients; 4 in 10 and 20 mg/kg dose groups and 5 in the 3 mg/kg dose group. Source: Viridian clinical data and modeling on file. VRDN-001 Phase 2 Active TED data as of Dec 2022
data cut. IV = intravenous, TED = thyroid eye disease. Concentration (μg/mL)

VRDN-003 demonstrates exposures associated with VRDN- 001 IV clinical
response VRDN-003 PK Modeling Q8W dosing of VRDN-003 is predicted to 250 achieve 3 mg/kg VRDN-001 IV C and C min avg 003 Q8W 24 wks – Maximizes convenience (least frequent dosing) 003 Q4W 24 wks 003 Q2W 24 wks 200 – Lowest exposure,
potential to maximize safety 150 Q4W dosing of VRDN-003 is predicted to Clinically achieve 10 mg/kg VRDN-001 IV C and active min C min range of exceeds the 3 mg/kg IV C by 2-fold avg 100 (20 mg/kg IV) VRDN-001 – Exceptional convenience
exposures C min 50 (10 mg/kg IV) Q2W dosing of VRDN-003 is predicted to C min exceed 20 mg/kg VRDN-001 IV C and min (3 mg/kg IV) 0 10 mg/kg VRDN IV C avg 0 10 20 – Convenience of Dupixent-like Q2W dosing Convenient dosing regimens including
Q8W reach concentrations associated with VRDN-001 clinical response PK modeling used a 2-compartment model, loading dose of 600 mg, and subsequent doses of 300 mg/doses at specified intervals for 24 weeks. 12 Source: Viridian clinical data and
modeling on file. IV = intravenous, PK = pharmacokinetics, Q8W = every eight weeks, Q4W = every four weeks, Q2W = every two weeks. Concentration (μg/mL)

VRDN-003: potential pivotal development in TED with an established path
to BLA subject to alignment with regulators Two Global Possible Potential VRDN-003 Pivotal Studies Active Arms Endpoints To include at least one of: Primary: Goals: Proptosis Evaluating safety Q8W responder rate • Preserve compelling and
efficacy in IGF-1R efficacy from patients with Secondary: Q4W active and VRDN-001 Proptosis mean chronic TED change, CAS, and • Maximize convenience diplopia Q2W • Improve safety Next steps: plan to align with regulators and share
pivotal trial design prior to mid-2024 start BLA = Biologics License Application, CAS = Clinical Activity Score, IGF-1R = insulin-like growth factor-1 receptor, Q8W = every eight weeks, Q4W = every four weeks, 13 Q2W = every two weeks, TED = Thyroid
Eye Disease.

Viridian pipeline: registrational TED programs and differentiated
portfolio of Anti-FcRn therapies DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Thyroid Eye VRDN-001 Intravenous, five-dose regimen Disease (anti-IGF-1R) Pivotal program planned VRDN-003 Selected Subcutaneous Program to start in mid-2024 Potential
best-in-class subcutaneous program FcRn-targeted VRDN-006 FcRn-selective, albumin-sparing Fc fragment autoimmune franchise VRDN-008 Extended half-life anti-FcRn 14 FcRn = Fc receptor inhibitors, IGF-1R = insulin-like growth factor-1 receptor, TED =
thyroid eye disease.

Viridian is well funded through key catalysts into 2026 Cash Position
Select VRDN- Plan to initiate Phase 3 Phase 3 003 as potential pivotal SC THRIVE data in THRIVE-2 data • Ended Q3 with $313M in cash best-in-class program for active TED for in chronic TED and cash equivalents SC program VRDN-003 VRDN-001 IV
for VRDN-001 IV st • Closed $185M PIPE on Nov 1 TODAY Mid-2024 Mid-2024 YE2024 • Cash runway into 2026 ü 2023 2024 2025 2026 Q4 H1 H2 H1 H2 Q1 Additional VRDN-006 & VRDN-008 IND submission VRDN-006 program disclosures, including
for VRDN-006 HV data VRDN-008 NHP data (Fc fragment) 2024 YE2024 2H2025 15 SC = subcutaneous, IV = intravenous, TED = thyroid eye disease, IND = investigational new drug, HV = healthy volunteers. FcRn IGF-1R

Appendix: – VRDN-001 IV Phase 2 Clinical Data: Active and Chronic
TED – VRDN-001 and VRDN-003 PK Parameters – IGF-1 Pharmacodynamics Following IV Dosing 16 IGF-1 = insulin-like growth factor 1, IV = intravenous, PK = pharmacokinetics, TED = thyroid eye disease.
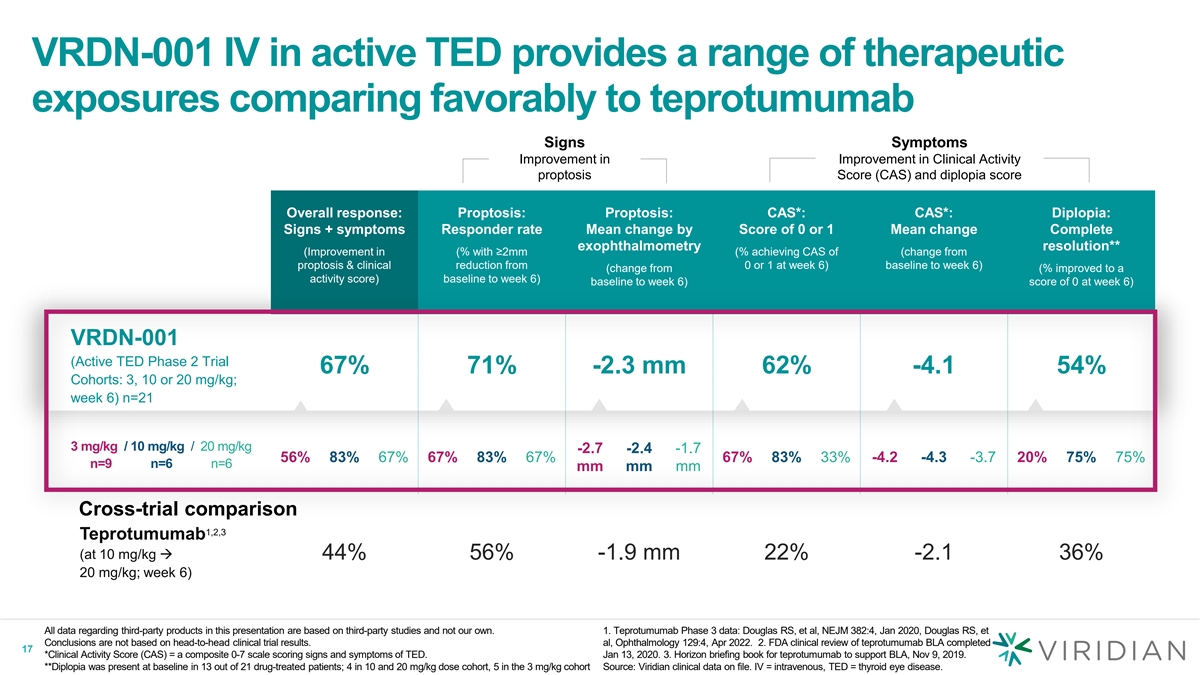
VRDN-001 IV in active TED provides a range of therapeutic exposures
comparing favorably to teprotumumab Signs Symptoms Improvement in Improvement in Clinical Activity proptosis Score (CAS) and diplopia score Overall response: Proptosis: Proptosis: CAS*: CAS*: Diplopia: Signs + symptoms Responder rate Mean change by
Score of 0 or 1 Mean change Complete exophthalmometry resolution** (Improvement in (% with ≥2mm (% achieving CAS of (change from proptosis & clinical reduction from 0 or 1 at week 6) baseline to week 6) (change from (% improved to a
activity score) baseline to week 6) baseline to week 6) score of 0 at week 6) VRDN-001 (Active TED Phase 2 Trial 67% 71% -2.3 mm 62% -4.1 54% Cohorts: 3, 10 or 20 mg/kg; week 6) n=21 3 mg/kg / 10 mg/kg / 20 mg/kg -2.7 -2.4 -1.7 56% 83% 67% 67% 83%
67% 67% 83% 33% -4.2 -4.3 -3.7 20% 75% 75% n=9 n=6 n=6 mm mm mm Cross-trial comparison 1,2,3 Teprotumumab (at 10 mg/kg à 44% 56% -1.9 mm 22% -2.1 36% 20 mg/kg; week 6) All data regarding third-party products in this presentation are based on
third-party studies and not our own. 1. Teprotumumab Phase 3 data: Douglas RS, et al, NEJM 382:4, Jan 2020, Douglas RS, et Conclusions are not based on head-to-head clinical trial results. al, Ophthalmology 129:4, Apr 2022. 2. FDA clinical review of
teprotumumab BLA completed 17 *Clinical Activity Score (CAS) = a composite 0-7 scale scoring signs and symptoms of TED. Jan 13, 2020. 3. Horizon briefing book for teprotumumab to support BLA, Nov 9, 2019. **Diplopia was present at baseline in 13 out
of 21 drug-treated patients; 4 in 10 and 20 mg/kg dose cohort, 5 in the 3 mg/kg cohort Source: Viridian clinical data on file. IV = intravenous, TED = thyroid eye disease.

VRDN-001 IV was well tolerated in active TED VRDN-001 3 mg/kg, 10
mg/kg, & 20 mg/kg TED cohorts VRDN-001 VRDN-001 VRDN-001 Placebo 3 mg/kg 10 mg/kg 20 mg/kg (n=5), n (n=9), n (n=6), n (n=6), n Adverse Reactions: Muscle spasms 2 2 2** - 2 - - - Nausea - - - 1 Alopecia No serious adverse Diarrhea 1 2** 1* -
events (SAEs), no - 1 - 3 Fatigue infusion reactions, and Hyperglycemia 1 - 1* - no discontinuations in patients treated with 1 1 - - Hearing impairment VRDN-001 - - 1 - Dysgeusia Headache 2 1 1 2** 1 - 1 - Dry skin Infusion reactions - - - - Safety
profile generally consistent across 3, 10, and 20 mg/kg cohorts; no SAEs or infusion reactions * Deemed unrelated to study drug by the masked investigator. ** One patient deemed related and one patient deemed unrelated to study drug by the masked
investigators. Source: Viridian clinical data on file. Data are as of data cut-off of December 19, 2022. Other AE that occurred in more than one patient and deemed related to study drug by masked investigators was acne (n=2). Instances were mild and
did not require intervention. Muscle spasms were mild and did not require intervention; hearing impairment (n=2) was “ringing in the ears” which resolved without intervention in both cases. Both 16 18 patients with hyperglycemia were
diabetic at baseline; in 1 case glucose variability was determined by masked PI to be unrelated to drug. IV = intravenous, TED = thyroid eye disease.

VRDN-001 IV in chronic TED showed robust clinical response at 3 and 10
mg/kg Symptoms Signs Improvement in Clinical Activity Improvement in proptosis Score (CAS) and diplopia score Proptosis: Proptosis: Proptosis: CAS: CAS: Diplopia: Responder rate Mean change by Mean change by Score of 0 or 1** Mean change** Complete
exophthalmometry MRI* resolution*** (% with ≥2 mm (% achieving CAS of (baseline to week 6) reduction baseline to 0 or 1 at week 6) (baseline to week 6) (baseline to week 6) (% improved to a Patients CAS>0 at week 6) score of 0 at week 6)
Excludes Patients baseline CAS=0 at baseline VRDN-001 (Chronic TED Phase 2 42% -1.6 mm -2.0 mm 40% -2.3 0% Trial Cohorts: 10 and 3 mg/kg; week 6) n=12 10 mg/kg / 3 mg/kg -1.8 -1.5 -1.5 -2.6 50% 33% 50% 33% -2.8 -2.0 0% 0% n=6 n=6 mm mm mm mm
Preliminary data - *MRI available for 4 of 6 VRDN-001 10 mg/kg treated patients, 4 of 6 VRDN-001 3 mg/kg treated patients. **2 patients with CAS of 0 at baseline excluded 19 from calculation. ***Includes only participants who had diplopia present at
baseline. Diplopia was present at baseline in 5 of 12 VRDN-001 treated patients; 2 in 3 mg/kg cohort, and 3 in 10 mg/kg cohort. Source: Viridian clinical data on file. IV = intravenous, TED = thyroid eye disease.

VRDN-001 IV was well tolerated in chronic TED Reported adverse events
occurring in ≥ 10% of patients VRDN-001 Placebo 10 & 3 mg/kg (n=5), n (n=13*), n 2 (15%) 0 (0%) Back pain 2 (15%) 0 (0%) Muscle spasms Headache 1 (8%) 2 (40%) 0 (0%) 1 (20%) Ear discomfort 0 (0%) 1 (20%) Fatigue 0 (0%) 1 (20%) Flatulence 0
(0%) 1 (20%) Pruritus No serious adverse events (SAEs); no hearing impairment or hyperglycemia events Preliminary data are as of data cut-off of May 30, 2023. th 20 *Though not evaluable at week 6 for clinical activity, the 7 patient randomized in
the 3 mg/kg cohort who discontinued the trial prior to week 6 due to leaving the country for a family emergency was followed for safety until their discontinuation. Source: Viridian clinical data on file. IV = intravenous, TED = thyroid eye disease.

VRDN-001 IV clinical experience to-date informs exposures of VRDN-003
expected to produce clinical benefit VRDN-001 IV Pharmacokinetics 250 Projected Projected Projected Projected 200 AUC AUC Dose C C min avg (µg*day/mL) (µg*day/mL) (µg/mL) (µg/mL) (5-doses) (8-doses) 150 20 mg/kg IV 20 mg/kg 105
219 23,000 38,000 C min 100 (20 mg/kg IV) 10 mg/kg IV C min 10 mg/kg 52 107 11,300 18,600 50 (10 mg/kg IV) 3 mg/kg IV C min (3 mg/kg IV) 0 3 mg/kg 13 30 3,200 5,200 20 mg/kg IV exposure range (Cmin to Cavg) 10 mg/kg IV exposure range (Cmin to Cavg)
3 mg/kg IV exposure range (Cmin to Cavg) * VRDN-001 IV PK based on 5 doses and 15 weeks. 21 Source: Viridian clinical and modeling data on file. AUC = area under the curve, IV = intravenous. Concentration (μg/mL)

VRDN-003 demonstrates exposures associated with VRDN- 001 IV clinical
response VRDN-003 PK Modeling 250 003 Q2W 24 wks Projected Projected Projected 003 Q4W 24 wks Dose AUC C (µg/mL) C (µg/mL) 200 min avg 003 Q8W 24 wks (µg*day/mL) 150 Clinically Q2W 136 116 19,500 active C min range of 100 (20 mg/kg
IV) VRDN-001 exposures Q4W 54 62 10,400 C min 50 (10 mg/kg IV) C min (3 mg/kg IV) 0 Q8W 14 35 5,800 0 10 20 Convenient dosing regimens including Q8W reach concentrations associated with VRDN-001 clinical efficacy PK modeling used a 2-compartment
model, loading dose of 600 mg, and subsequent doses of 300 mg/doses at specified intervals for 24 weeks. Source: Viridian clinical and modeling data on file. AUC = area under the curve, IV = intravenous, PK = pharmacokinetics, Q2W = every two weeks,
Q4W = every four 22 weeks, Q8W = every eight weeks. Concentration (μg/mL)

VRDN IGF-1 levels post-dosing VRDN-003 Phase 1 Healthy Volunteers
VRDN-001, VRDN-002, VRDN-003 (IV) (SC & IV) VRDN-001 3 mg/kg VRDN-001 10 mg/kg VRDN-001 20 mg/kg 6 6 VRDN-003 SC 300 mg Placebo VRDN-003 SC 600 mg VRDN-002 3 mg/kg VRDN-003 5 mg/kg IV 4 4 VRDN-002 10 mg/kg VRDN-003 15 mg/kg IV VRDN-002 20 mg/kg
Placebo PBO SC 2 2 PBO IV VRDN-003 5 mg/kg VRDN-003 15 mg/kg 0 0 Placebo 0 5 10 15 20 0 20 40 60 80 Time (day) Time (day) Data out to day 70 Data out to day 21 Source: Viridian clinical data on file. 23 IGF-1 = insulin-like growth factor 1, IV =
intravenous, PBO = placebo, SC = subcutaneous. Fold Change IGF-1 IGF-1 (Fold change from baseline)
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Viridian Therapeutics (NASDAQ:VRDN)
Historical Stock Chart
From Apr 2024 to May 2024
Viridian Therapeutics (NASDAQ:VRDN)
Historical Stock Chart
From May 2023 to May 2024