As
filed with the Securities and Exchange Commission on December 29, 2023.
Registration
No. 333-_______
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
Palisade
Bio, Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2834 |
|
52-2007292 |
(State
or Other Jurisdiction of
Incorporation or Organization) |
|
(Primary
Standard Industrial
Classification Code Number) |
|
(I.R.S.
Employer
Identification Number) |
Palisade
Bio, Inc.
7750
El Camino Real, Suite 2A
Carlsbad,
CA 92009
(858)
704-4900
(Address,
including zip code, and telephone number, including area code of registrant’s principal executive offices)
Paracorp
Incorporated
2140
S Dupont highway
Camden,
DE 19934
(302)
697-4590
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Raul Silvestre
Dennis Gluck
2629 Townsgate Road #215
Westlake Village, CA 91361
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
|
☐ |
|
Accelerated
filer |
|
☐ |
| |
|
|
|
| Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
| |
|
|
|
| |
|
|
|
Emerging growth company |
|
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date
as the Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement
filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not
soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED DECEMBER 29, 2023
PRELIMINARY
PROSPECTUS

[*]
shares of Common Stock
We are offering shares
of our common stock, par value $0.01 per share (the “common stock”) at a public offering price of $
per share.
The
underwriter has the option to purchase up to additional shares
of common stock solely to cover over-allotments, if any, at the price to the public, less the underwriting discounts and commissions, but such purchases cannot
exceed an aggregate of % of the number of shares of common stock sold in this offering. The over-allotment option is exercisable for
days from the date of this prospectus.
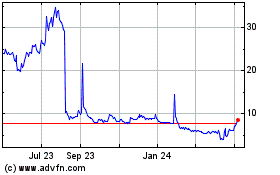
Our
common stock is listed on The Nasdaq Capital Market under the symbol “PALI”. On December 28, 2023, the last reported sale
price of our common stock was $0.6539 per share. The recent market price used throughout this prospectus may not be indicative
of the final offering price. The final public offering price will be determined through negotiation between us and the underwriter based
upon a number of factors, including our history and our prospects, the industry in which we operate, our past and present operating results
and the general condition of the securities markets at the time of this offering.
Investing
in our securities involves a high degree of risk. Before making an investment decision, please read the information under “Risk
Factors” beginning on page 6 of this prospectus and under similar headings in any amendment or supplement to this prospectus
or in any filing with the Securities and Exchange Commission that is incorporated by reference herein.
| | |
Per common share | | |
Total | |
| Public offering price | |
$ | | | |
$ | | |
| Underwriting discounts and commissions(1)(2) | |
$ | | | |
$ | | |
| Proceeds to us, before expenses | |
$ | | | |
$ | | |
| (1) |
We
have also agreed to reimburse the underwriter for certain expenses. See “Underwriting” for additional information. |
| (2) |
We
have granted a day option to the underwriter to purchase additional shares of common stock a (up to %
of the number of shares of common stock sold in this offering) solely to cover over-allotments, if any. |
The
underwriter expects to deliver the securities to purchasers in the offering on or about ,
2024
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED
IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
[NAME
OF UNDERWRITER]
The
date of this prospectus is , 2024.
TABLE
OF CONTENTS
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without
charge by following the instructions under “Incorporation of Certain Information by Reference.” You should carefully read
this prospectus as well as additional information described under “Incorporation of Certain Information by Reference,” before
deciding to invest in our securities.
Neither
we nor the underwriter have authorized anyone to provide you with additional information or information different from that contained
or incorporated by reference in this prospectus or in any free writing prospectus that we have authorized for use in connection with
this offering. We take no responsibility for and can provide no assurance as to the reliability of, any other information that others
may give you. This prospectus does not constitute an offer to sell to any person, or a solicitation of an offer to purchase from any
person, the securities offered by this prospectus in any jurisdiction in which it is unlawful to make such offer or solicitation of an
offer.
The
underwriter is offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted.
The information contained in this prospectus and any free writing prospectus that we have authorized for use in connection with this
offering is accurate only as of the respective dates thereof, and the information in the documents incorporated by reference in this
prospectus is accurate only as of the date of those respective documents, regardless of the time of delivery of this prospectus or of
any sale of our securities. Our business, financial condition, results of operations, and prospects may have changed since such dates.
It is important for you to read and consider all information contained or incorporated by reference in this prospectus in making your
investment decision. You should read both this prospectus, as well as the documents incorporated by reference into this prospectus and
the additional information described under “Incorporation of Certain Information by Reference” in this prospectus before
investing in our securities.
Unless
otherwise indicated, information contained in or incorporated by reference into this prospectus concerning our business and the industry
and markets in which we operate, including with respect to our business prospects, our market position and opportunity, and the competitive
landscape, is based on information from our management’s estimates, as well as from industry publications, surveys, and studies
conducted by third parties. Our management’s estimates are derived from publicly available information, their knowledge of our
business and industry, and assumptions based on such information and knowledge, which they believe to be reasonable. In addition, while
we believe that information contained in the industry publications, surveys, and studies has been obtained from reliable sources,
we have not independently verified any of the data contained in these third-party sources, and the accuracy and completeness of the information
contained in these sources is not guaranteed.
Although
we are not aware of any misstatements regarding the market and industry data presented in this prospectus and the documents incorporated
herein by reference, these estimates involve risks and uncertainties and are subject to change based on various factors, including those
discussed under the heading “Risk Factors” in this prospectus and any related free writing prospectus, and under similar
headings in the other documents that are incorporated by reference into this prospectus, including in our Annual Report on Form 10-K
filed with the Securities and Exchange Commission (the “SEC”) on March 22, 2023 and our Quarterly Report on Form 10-Q
filed with the SEC on November 9, 2023. Accordingly, you should not place undue reliance on this information.
For
investors outside the United States: We and the underwriter have not done anything that would permit this offering or the possession
or distribution of this prospectus in any jurisdiction where action for those purposes is required, other than in the United States.
Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions
relating to, the offering of the shares of common stock and the distribution of this prospectus outside of the United States.
PROSPECTUS
SUMMARY
This
summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated by reference
into this prospectus. This summary is not complete and does not contain all of the information that you should consider before making
an investment decision. For a more complete understanding of our company, you should read and consider carefully the more detailed information
included or incorporated by reference in this prospectus and any applicable prospectus supplement, including the factors described under
the heading “Risk Factors” beginning on page 6 of this prospectus, and in our Annual Report on Form 10-K filed with the
SEC on March 22, 2023 and our Quarterly Report on Form 10-Q filed with the SEC on November 9, 2023, together with any free writing
prospectus we have authorized for use in connection with this offering and the financial statements and all other information incorporated
by reference in this prospectus. When used in this prospectus, except where the context otherwise requires, the terms the “Company,”
“we,” “us,” “our,” “Palisade,” or similar terms refer to Palisade Bio, Inc.
Company
Overview
Palisade is a biopharmaceutical
company developing novel targeted therapeutics for serious chronic gastrointestinal diseases. The Company’s strategic focus is
the pre-clinical and clinical development of its lead product candidate, PALI-2108, which is at an early stage of development.
Our Lead Product Candidate
Our lead product candidate, PALI-2108,
is a precision orally administered, locally-restricted, colon-specific phosphodiesterase-4 (PDE4) inhibitor prodrug in early-stage development
for patients affected by moderate-to-severely active ulcerative colitis. We believe that PALI-2108 may be an effective treatment for
Crohn’s disease. PALI-2108 is currently undergoing investigational new drug approval in the United States (“IND”) and
clinical trial application in Canada (“CTA”) enabling studies including nonclinical safety and toxicology, chemistry, manufacturing
and controls and completing the validation of pharmacokinetic/pharmacodynamics assays. We believe we will be able to complete nonclinical
IND/CTA enabling activities by the end of the third quarter of 2024 and plan to submit our initial IND/CTA prior to the end of 2024.
Market
We
believe that if developed and approved for marketing, PALI-2108 could be an effective treatment for IBD. The Company’s initial
indications for PALI-2108 are:
| ● | Ulcerative
colitis. A condition involving inflammation and sores (ulcers) along the lining of the
large intestine (colon) and rectum; and |
| ● | Crohn’s
disease. A condition characterized by the inflammation of the lining of the digestive
tract, which often can involve the deeper layers of the digestive tract. Crohn’s disease
most commonly affects the small intestine. However, it can also affect the large intestine
and uncommonly, the upper gastrointestinal tract. |
Both
ulcerative colitis and Crohn’s disease are usually characterized by diarrhea, rectal bleeding, abdominal pain, fatigue and weight loss.
For some people, IBD is only a mild illness. For others, it’s a debilitating condition that can lead to life-threatening complications.
Based on statistics from the Centers for Disease Control and the United European Gastroenterology, it is estimated that globally, there
are approximately 3.6 million individuals suffering from IBD, resulting in a combined global market opportunity of $20 billion by 2031
(Source: Global Data).
Giiant
License Agreement
On
September 1, 2023, the Company entered into the Giiant License Agreement. Under the terms of the Giiant License Agreement, the Company
obtained the rights to develop, manufacture, and commercialize all compounds from Giiant, existing now and in the future, and any product
containing or delivering any licensed compound, in any formulation or dosage for all human and non-human therapeutic uses for any and
all indications worldwide, including those technologies that are the basis of PALI-2108. Pursuant to the terms of the Giiant License
Agreement, pre-clinical development PALI-2108 will be jointly undertaken by the Company and representatives of Giiant and the Company
will pay or reimburse a portion of the joint development costs. Upon the first approval of either an IND or CTA, the Company will assume
all development, manufacturing, regulatory and commercialization costs. Additionally, per the terms of the Giiant License Agreement,
the Company will pay (i) certain milestone payments (in cash or stock at the Company’s election) and (ii) royalty payments.
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage
of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We
will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our
shares of common stock held by non-affiliates does not equal or exceed $250.0 million as of the prior June 30th, or (2) our
annual revenues did not equal or exceed $100.0 million during such completed fiscal year and the market value of our shares of common
stock held by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of
any reduced disclosure obligations, it may make comparison of our financial statements with other public companies difficult or impossible.
Corporate
Information
We
were originally incorporated in 2001 in the State of Delaware under the name Neuralstem, Inc. In October 2019, we changed our name from
Neuralstem, Inc. to Seneca Biopharma, Inc., or Seneca. In April 2021, we effected a merger transaction with Leading Biosciences, Inc.,
or “LBS”, whereby LBS became a wholly owned subsidiary of Seneca. In April 2021, we changed our name from Seneca Biopharma,
Inc. to Palisade Bio, Inc. Our principal executive offices are located at 7750 El Camino Real, Suite 2A, Carlsbad, CA, 92009, our telephone
number is (858) 704-4900 and our website address is www.palisadebio.com. The information contained in or accessible through our website
does not constitute part of this prospectus supplement or the accompanying prospectus. As of December 28, 2023, we had nine full-time
employees.
Subsidiaries
We
primarily conduct our operations through LBS, our wholly owned subsidiary.
THE
OFFERING
| Securities
Offered |
|
Up
to shares of common stock. |
| |
|
| Use
of Proceeds |
|
We estimate that the net proceeds to us from this offering will be approximately $ million,
after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds
from this offering primarily for general corporate purposes, including research and development and working capital. See “Use of
Proceeds” for additional information |
| |
|
| Over-allotment
option |
|
The
underwriter has the option to purchase additional shares of common stock solely
to cover over-allotments, if any, at the price to the public less the underwriting discounts and commissions. The over-allotment
option may be used to purchase shares of common stock as determined by the underwriter, but such purchases cannot exceed an aggregate
of % of the number of shares of common stock sold in this offering. The over-allotment
option is exercisable for days from the date of this
prospectus. |
| |
|
| Common
stock outstanding before this offering |
|
shares.
|
| |
|
Common
stock to be outstanding immediately
after
this offering |
|
shares,
or shares if the underwriter exercises in full its option to purchase
additional shares of common stock. |
| |
|
|
| Risk
Factors |
|
This
investment involves a high degree of risk. See “Risk Factors” beginning on page 6 of this prospectus for a discussion
of factors you should consider carefully before buying our securities. |
| |
|
| Nasdaq
Symbol |
|
“PALI.” |
Unless otherwise indicated,
all information in this prospectus assumes no exercise of outstanding options or warrants, no conversion of the Series A 4.5% Convertible
Preferred Stock described.
Unless
otherwise indicated, the number of shares of common stock to be outstanding immediately after this offering is based on 9,270,894
shares of common stock outstanding as of December 28, 2023, which includes 9,210,751 shares outstanding as of September 30,
2023, as adjusted for the following subsequent issuances: (i) an aggregate of 26,467 shares that were issued to employees pursuant to
the vesting of restricted stock units, and (ii) 33,676 shares that were issued on November 20, 2023 to various employees pursuant to
the Company’s 2021 Employee Stock Purchase Plan, as amended, but excludes:
| ● |
8,430
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023 granted under the LBS 2013 Amended
and Restated Employee, Director, and Consultant Equity Incentive Plan, as amended and restated, or the 2013 Plan, with a weighted-average
exercise price of $1,054.60 per share; |
| |
|
| ● |
574,956
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023, granted under the 2021 Equity
Incentive Plan, as amended, or the 2021 Plan, with a weighted-average exercise price of $4.21 per share, which includes a total of
78,160 shares of common stock issuable upon exercise of outstanding stock options each with an exercise price of $0.59 that were
conditionally granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares
available under the 2021 Plan; |
| |
|
| ● |
82,086
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023 granted under the 2021 Inducement
Plan, with a weighted average exercise price of $4.90 per share; |
| |
|
| ● |
312,780
shares of common stock issuable upon vesting of restricted stock units outstanding as of December 28, 2023, granted under the 2021
Plan, which includes a total of 66,000 shares of common stock issuable upon vesting of restricted stock units that were conditionally
granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares available under
the 2021 Plan; |
| |
|
| ● |
50,144
shares of common stock issuable upon vesting of restricted stock units outstanding as of December 28, 2023, granted under the 2021
Inducement Plan; |
| |
|
| ● |
62,200
shares of common stock issuable upon vesting of restricted performance stock units outstanding as of December 28, 2023; all of which
were issued under the 2021 Plan which vest subject to certain milestones; |
| |
|
| ● |
5,397
shares of common stock reserved for future issuance under the 2021 Plan as of December 28, 2023, which excludes a total of 144,610
shares of common stock issuable upon exercise of outstanding stock options and outstanding restricted stock units that were conditionally
granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares being available
under the 2021 Plan, as well as any future automatic increases in the number of shares of common stock reserved for future issuance
under the 2021 Plan; |
| |
|
| ● |
110,871
shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan, or the ESPP, as of December 28,
2023, as well as any automatic increases in the number of shares of common stock reserved for future issuance under the ESPP; |
| |
|
| ● |
863,214
shares of common stock reserved for issuance under the 2021 Inducement Plan as of September 1, 2023; |
| |
|
| ● |
4,080,876
shares of common stock issuable upon exercise of outstanding warrants as of December 28, 2023 with a weighted-average exercise price
of $8.63 per share; and |
| |
|
| ● |
129
shares of common stock issuable upon conversion of the 200,000 outstanding shares of our Series A 4.5% Convertible Preferred Stock
as of December 28, 2023, as well as any future shares of common stock issuable upon conversion of additional shares of Series A 4.5%
Convertible Preferred Stock that may be issued as payment-in-kind dividends thereon in accordance with their terms. |
RISK
FACTORS SUMMARY
On
September 1, 2023, the Company announced that it had entered into a research collaboration and license agreement with Giiant Pharma Inc.
(“Giiant”) (the “Giiant License Agreement”) for the exclusive worldwide license to Giiant’s assets. As
a result, the Company changed its strategic focus. To the extent that the risk factors contained herein contradict any risk factors contained
in the Company’s recent periodic reports, the risk factors contained herein shall supersede. The risk factors contained in the
Company’s recent periodic reports remain applicable to the Company.
The
Company faces many risks and uncertainties, as more fully described in this Prospectus. Some of these risks and uncertainties are summarized
below. The summary below does not contain all of the information that may be important to you, and you should read this summary together
with the more detailed discussion of these risks and uncertainties contained in the Section entitled “Risk Factors.”
Risks
Related to This Offering and our Securities
| ● | You
will experience immediate and substantial dilution as a result of this offering and may experience
additional dilution in the future. |
| | | |
| ● | Management
will have broad discretion as to the use of the proceeds from this offering and may not use
the proceeds effectively |
| | | |
| ● | The
offering price will be set by the Company’s Board and does not necessarily indicate
the actual or market value of the Company’s common stock. |
| | | |
| ● | Future
sales of a significant number of our shares of common stock in the public markets, or the
perception that such sales could occur, could depress the market price of the Company’s
shares of common stock. |
| | | |
| ● | Terms
of subsequent financings may adversely impact the Company’s stockholders. |
| | | |
| ● | The
Company does not currently intend to pay dividends on our common stock, and any return to
investors is expected to come, if at all, only from potential increases in the price of the
Company’s common stock |
Risks
Related to the Company’s Development, Commercialization and Regulatory Approval of the Company’s Investigational Therapies
| ● | The
Company’s business depends on the successful pre-clinical and clinical development,
regulatory approval and commercialization of its recently licensed therapeutic compound,
PALI-2108. |
| | | |
| ● | There
are substantial risks inherent in drug development, and, as a result, the Company may not
be able to successfully develop PALI-2108 for commercial use. |
| | | |
| ● | The
Company depends on its license agreement with Giiant to permit the Company to use patents
and patent applications relating to PALI-2108. Termination of these rights or the failure
to comply with obligations under this agreement could materially harm the Company’s
business and prevent it from developing or commercializing its product candidates. |
| | | |
| ● | The
Company expects that its operations and development of PALI-2108 will require substantially
more capital than it currently has, and the Company cannot guarantee when or if it will be
able to secure such additional funding. |
| | | |
| ● | There
can be no assurance that the Company’s product candidates will obtain regulatory approval. |
| | | |
| ● | If
pre-clinical and clinical studies of PALI-2108 do not yield successful results, then the
Company will be unable to commercialize its product candidates. |
| | | |
| ● | Even
if the Company’s clinical studies are successful and achieve regulatory approval, the
approved product label may be more limited than the Company or analysts anticipate, which
could limit the commercial prospects of PALI-2108. |
| ● | The
Company may in the future conduct clinical trials for PALI-2108 outside the United States,
and the FDA and applicable foreign regulatory authorities may not accept data from such trials. |
| | | |
| ● | The
Company may rely on third-party CROs and other third parties to conduct and oversee its pre-clinical
studies and clinical trials. If these third parties do not meet the Company’s requirements
or otherwise conduct the studies or trials as required, the Company may not be able to satisfy
its contractual obligations or obtain regulatory approval for, or commercialize, its product
candidates. |
| | | |
| ● | The
Company has entered into a collaborative research agreement with Giiant related to pre-clinical
development, which will require the efforts of Giiant and its personnel, which are out of
the Company’s control. |
Risks
Related to the Company’s Business
| ● | The
Company has a very limited operating history and has never generated any revenues from product
sales. |
| | | |
| ● | The
Company’s business model assumes revenue from, among other activities, marketing or
out-licensing the products the Company develops. PALI-2108 is in the early stages of development
and because the Company has a short development history with PALI-2108, there is a limited
amount of information about the Company upon which you can evaluate its business and prospects. |
| | | |
| ● | The
Company has received a delisting notification from the Nasdaq Stock Market based on the Company’s
Bid Price being under $1.00 for thirty (30) consecutive trading days. If the Company is not
able to regain compliance with the applicable continued listing requirements or standards
of The Nasdaq Capital Market, Nasdaq could delist its common stock. |
| | | |
| ● | The
Company’s success depends on the attraction and retention of senior management and
scientists with relevant expertise. |
| | | |
| ● | The
Company may choose to discontinue developing or commercializing any of its product candidates,
or may choose to not commercialize product candidates in approved indications, at any time
during development or after approval, which could adversely affect the Company and its operations. |
| | | |
| ● | The
Company’s inability to successfully in-license, acquire, develop and market additional
product candidates or approved products would impair its ability to grow its business. |
Risks
Related to the Company’s Dependence on Third Parties
| ● | The
Company expects to rely on collaborations with third parties for the successful development
and commercialization of its product candidates. |
| | | |
| ● | The
Company anticipates relying completely on third-party contractors to supply, manufacture
and distribute clinical drug supplies for its product candidates. |
Risks
Related to the Company’s Financial Operations
| ● | The
Company has expressed substantial doubt about its ability to continue as a going concern. |
| | | |
| ● | The
Company has a history of net losses, and it expects to continue to incur net losses and may
not achieve or maintain profitability. |
| | | |
| ● | Failure
to remediate a material weakness in internal controls over financial reporting could result
in material misstatements in the Company’s consolidated financial statements. |
Risks
Related to the Company’s Intellectual Property
| ● | The
Company may not be able to obtain, maintain or enforce global patent rights or other intellectual
property rights that cover its product candidates and technologies that are of sufficient
breadth to prevent third parties from competing against the Company. |
| | | |
| ● | If
the Company fails to comply with its obligations under its intellectual property license
agreements, it could lose license rights that are important to its business. |
Other
Risks Related to the Company’s Securities
| ● | The
Company will need to raise additional financing in the future to fund its operations, which
may not be available to it on favorable terms or at all. |
| | | |
| ● | The
stock price of the Company may be highly volatile. |
| | | |
| ● | If
the Company fails to maintain proper and effective internal controls, its ability to produce
accurate financial statements on a timely basis could be impaired. |
| | | |
| ● | The
Company’s Board of Directors has broad discretion to issue additional securities, which
might dilute the net tangible book value per share of its common stock for existing stockholders. |
RISK
FACTORS
Investing
in the Company’s common stock involves a high degree of risk. The Company has described below a number of uncertainties and risks
which, in addition to uncertainties and risks presented elsewhere in this Prospectus, may adversely affect its business, operating results
and financial condition. This Prospectus does not describe all of those risks. You should carefully consider the risk factors described
in this prospectus under the caption “Risks Related to This Offering and Our Securities” below. The uncertainties and risks
enumerated below as well as those presented elsewhere in this Prospectus should be considered carefully when evaluating the Company,
its business and the value of its securities. To the extent the term “product candidate” or “product candidates”
are used, it refers to the current and potential future products of the Company. To the extent the term “clinical trial”
or “clinical trials” are used, it refers to the extent applicable, to pre-clinical and clinical trials of the Company. On
September 1, 2023, the Company announced that it had entered into the Giiant License Agreement with Giiant for the exclusive worldwide
license to Giiant’s assets. As a result, the Company changed its strategic focus. To the extent that the risk factors contained
herein contradict any risk factors contained in the Company’s recent periodic reports, the risk factors contained herein shall
supersede. The risk factors contained in the Company’s recent periodic reports remain applicable to the Company.
Risks
Related to This Offering and Our Securities
You
will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You
will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of the common shares
offered in this offering and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, investors
in this offering can expect an immediate dilution of approximately $ per share. In addition, our outstanding stock options, warrants
and shares of our Series A 4.5% Convertible Preferred Stock are convertible into or exercisable for shares of our common stock. To the extent that such securities are exercised or converted into
shares of our common stock, investors purchasing our securities in this offering may experience further dilution.
Moreover,
we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient
funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible
debt securities, the issuance of these securities could result in further dilution to our stockholders or result in downward pressure
on the price of our common stock. See the section titled “Dilution” for a more detailed discussion of the dilution you will
incur if you purchase common stock in this offering.
Management
will have broad discretion as to the use of the proceeds from this offering and may not use the proceeds effectively.
Our
management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways
that may not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively
could have a material adverse effect on our business and cause the price of our common stock to decline.
The
offering price will be set by the Company’s Board and does not necessarily indicate the actual or market value of the Company’s
common stock.
Our
Board (or a committee thereof) will approve the offering price and other terms of this offering after considering, among other things:
the number of shares authorized in our certificate of incorporation; the current market price of our common stock; trading prices of
our common stock over time; the volatility of our common stock; our current financial condition and the prospects for our future cash
flows; the availability of and likely cost of capital of other potential sources of capital; and market and economic conditions at the
time of the offering. The offering price is not intended to bear any relationship to the book value of our assets or our past operations,
cash flows, losses, financial condition, net worth or any other established criteria used to value securities. The offering price may
not be indicative of the fair value of the common stock.
Future
sales of a significant number of the Company’s shares of common stock in the public markets, or the perception that such sales
could occur, could depress the market price of the Company’s shares of common stock.
Sales
of a substantial number of our shares of common stock in the public markets, or the perception that such sales could occur, including
from the exercise of warrant or sales of common stock issuable thereunder, could depress the market price of our shares of common stock
and impair our ability to raise capital through the sale of additional equity securities. A substantial number of shares of common stock
are being offered by this prospectus. We cannot predict the number of these shares that might be sold nor the effect that future sales
of our shares of common stock, including shares issuable upon the exercise of warrants, would have on the market price of our shares
of common stock.
Terms
of subsequent financings may adversely impact the Company’s stockholders.
To
finance our future business plans and working capital needs, we will have to raise funds through the issuance of equity or debt securities
in addition to this offering. Depending on the type and the terms of any financing we pursue, stockholders’ rights and the value
of their investment in our common stock and warrants could be reduced. A financing could involve one or more types of securities including
common stock, convertible debt or warrants to acquire common stock. These securities could be issued at or below the then prevailing
market price for our common stock. In addition, if we issue secured debt securities, the holders of the debt would have a claim to our
assets that would be senior to the rights of stockholders until the debt is paid. Interest on these debt securities would increase costs
and negatively impact operating results. If the issuance of new securities results in diminished rights to holders of our common stock,
the market price of our common stock and the value of the warrants could be negatively impacted.
The
Company does not currently intend to pay dividends on the Company’s common stock, and any return to investors is expected to come,
if at all, only from potential increases in the price of the Company’s common stock.
At
the present time, we intend to use available funds to finance our operations. Accordingly, while payment of dividends rests within the
discretion of our Board, we have no intention of paying any such dividends in the foreseeable future. Any return to investors is expected
to come, if at all, only from potential increases in the price of our common stock.
Risks
Related to the Company’s Development, Commercialization and Regulatory Approval of the Company’s Investigational Therapies
The
Company’s business depends on the successful pre-clinical and clinical development, regulatory approval, and commercialization
of its recently licensed therapeutic compound, PALI-2108.
On
September 1, 2023, the Company announced that it had entered into a research collaboration and license agreement with Giiant, pursuant
to which the Company licensed all of Giiant’s current and future technologies, including PALI-2108. PALI-2108 is a pre-clinical
asset and the Company’s only asset being actively developed. The success of the Company depends on the development PALI-2108 which
is subject to a number of risks, including:
| ● | the
continued enforceability of the Company’s research collaboration and license
agreement with Giiant; |
| | | |
| ● | the
successful completion of pre-clinical and Individual New Drug Application (“IND”)
or Canadian Clinical Trial Application (“CTA”) enabling studies and research;
|
| | | |
| ● | the
submission and approval of an IND or CTA; |
| | | |
| ● | the
Company’s ability to develop clinical trial designs and protocols; |
| | | |
| ● | the
successful initiation and completion of its planned pre-clinical and clinical trials; |
| | | |
| ● | the
approval by the U.S. Food and Drug Administration (“FDA”) or other regulatory
authority to commence the marketing of the Company’s product candidates; |
| | | |
| ● | the
Company and its third-party contractors, if applicable, achieving and maintaining compliance
with their contractual obligations and with applicable regulatory requirements; |
| | | |
| ● | the
ability of the Company’s contract manufacturers to manufacture sufficient supply of
the Company’s product candidates to meet the required clinical trial and commercial
supplies; |
| | | |
| ● | the
ability of the Company’s contract manufacturers to remain in good standing with regulatory
agencies and to develop, validate and maintain commercially viable manufacturing facilities
and processes that are compliant with cGMP; |
| | | |
| ● | the
Company’s ability to obtain favorable labeling for its product candidates through regulators
that allows for successful commercialization; |
| ● | acceptance
by physicians, insurers and payors, and patients of the quality, benefits, safety and efficacy
of the Company’s product candidates, if approved, including relative to alternative
and competing treatments; |
| | | |
| ● | the
Company’s ability to price its product candidates to recover the Company’s development
costs and applicable milestone or royalty payments, and generate a satisfactory profit margin;
and |
| | | |
| ● | the
Company’s ability and its applicable collaboration and licensing partners’ ability
to establish and enforce intellectual property rights related to the product candidates and
technologies. |
If
the Company does not achieve one or more of these factors, many of which are beyond its control, in a timely manner or at all, the Company
could experience significant delays or an inability to obtain regulatory approvals or commercialize its proposed product candidate. Such
delays may result in increased costs and the failure to complete any required regulatory activity. Even if regulatory approvals are obtained,
the Company may never be able to successfully commercialize its product candidates. Accordingly, the Company cannot make assurances that
it will ever be able to generate sufficient revenue through the sale of any product candidates, if approved, to internally fund its business.
There
are substantial risks inherent in drug development, and, as a result, the Company may not be able to successfully develop PALI-2108 for
commercial use.
The
Company’s research and development efforts are focused on a therapeutic based on PDE4 inhibitors. The Company’s development
of PALI-2108 is in the early stages. However, such technology’s commercial feasibility and acceptance in the Company’s target
indication of inflammatory bowel disease (“IBD”) are unknown. Scientific research and development requires significant amounts
of capital and takes a long time to reach commercial viability, if it can be achieved at all. During the research and development process,
the Company may experience technological barriers that it may be unable to overcome. Further, certain underlying premises in the Company’s
development programs are not proven. Because of these and similar uncertainties, it is possible that the Company’s product candidates
will not reach commercialization. If the Company is unable to successfully develop and commercialize its product candidates, the Company
will be unable to generate revenue or build a sustainable or profitable business.
The
Company depends on its license agreement with Giiant to permit the Company to use patents and patent applications relating to PALI-2108.
Termination of these rights or the failure to comply with obligations under this agreement could materially harm the Company’s
business and prevent it from developing or commercializing its product candidates.
The
Company is a party to a license agreement with Giiant under which the Company is granted rights to patents and patent applications that
are important to its business. The Company relies on this license agreement in order to be able to use various proprietary technologies
that are material to its business, including certain patents and patent applications that cover PALI-2108. The Company’s rights
to use these patents and patent applications and employ the inventions claimed in these licensed patents are subject to the continuation
of and its compliance with the terms of its license agreement. If the Company fails to comply with any of its obligations under the license
agreement with Giiant, Giiant may have the right to terminate the license agreement, in which event the Company would not be able to
continue the development of PALI-2108. Additionally, disputes may arise under the license agreement regarding the intellectual property
that is subject to such license agreement. If disputes over intellectual property that the Company has licensed or in the future licenses,
prevent or impair its ability to maintain any of its license agreements on acceptable terms, the Company may be unable to successfully
develop and commercialize the affected product candidates and technologies.
Pre-clinical
and clinical drug development is very expensive, time-consuming and uncertain.
The
pre-clinical and clinical development of product candidates is very expensive, time-consuming, difficult to design and implement, and
the outcomes are inherently uncertain. Most product candidates that commence clinical trials are never approved by regulatory authorities
for commercialization and of those that are approved, many do not cover their costs of development. In addition, the Company, any partner
with which it may in the future collaborate, the FDA, or other regulatory authorities, including state and local agencies and counterpart
agencies in foreign countries, or institutional review boards (“IRB”) at the Company’s trial sites, may suspend, delay,
require modifications to or terminate the Company’s clinical trials, once begun, at any time.
The
Company expects that its operations and development of PALI-2108 will require substantially more capital than it currently has, and the
Company cannot guarantee when or if it will be able to secure such additional funding.
The
Company has historically funded its operations and prior development efforts through the sale of its securities. Based on the Company’s
existing cash resources and its current or future plan of operations, the Company may not have adequate capital to complete its anticipated
pre-clinical or clinical development or fund operations. Moreover, the Company cannot guarantee that its cash resources are sufficient
to provide for the Company’s working capital needs and complete any anticipated pre-clinical and clinical research and studies.
As a result, the Company may need to secure additional financing. If the Company is not able to obtain financing in the future or on
acceptable terms, it may have to curtail its research and development efforts as well as its operations.
There
can be no assurance that the Company’s product candidates will obtain regulatory approval.
The
sale of human therapeutic products in the U.S. and foreign jurisdictions is subject to extensive and time-consuming regulatory approval
which requires, among other things:
| ● | pre-clinical
data required for the submission of an IND or CTA; |
| | | |
| ● | controlled
research and human clinical testing; |
| | | |
| ● | establishment
of the safety and efficacy of the product; |
| | | |
| ● | government
review and approval of a submission containing manufacturing, pre-clinical and clinical data;
and |
| | | |
| ● | adherence
to cGMP regulations during production and storage. |
The
proposed product candidate the Company currently has under development, PALI-2108, will require significant development, pre-clinical
and clinical testing and the investment of significant funds to gain regulatory approval before it can be commercialized. The results
of the Company’s research and human clinical testing of PALI-2108 may not meet regulatory requirements. If approved, PALI-2108
may also require the completion of post-market studies. There can be no assurance that PALI-2108 will be successfully developed and approved.
The process of completing pre-clinical and clinical testing and obtaining required approvals is expected to take a number of years and
require the use of substantial resources. Further, there can be no assurance that PALI-2108 will be shown to be safe and effective in
clinical trials or receive applicable regulatory approvals. If the Company fails to obtain regulatory approvals, it will not be able
to market PALI-2108 and its operations may be adversely affected.
If
pre-clinical and clinical studies of PALI-2108 do not yield successful results, then the Company will be unable to commercialize its
product candidates.
The
Company must demonstrate that PALI-2108 is safe and efficacious in humans through extensive pre-clinical and clinical testing. The Company’s
research and development programs are at an early stage of development. The Company may experience numerous unforeseen events during,
or as a result of, the testing process that could delay or prevent commercialization of any products, including the following:
| ● | the
results of pre-clinical studies may be inconclusive, or they may not be indicative of results
that will be obtained in human clinical trials; |
| | | |
| ● | safety
and efficacy results attained in early human clinical trials, if approved, may not be indicative
of results that are obtained in later clinical trials; |
| | | |
| ● | after
reviewing test results, the Company may abandon projects that it previously believed to be
promising; |
| | | |
| ● | the
Company or its regulators may suspend or terminate clinical trials because the participating
subjects or patients are being exposed to unacceptable health risks; and |
| | | |
| ● | PALI-2108
may not have the desired effects or may include undesirable side effects or other characteristics
that preclude regulatory approval or limit their commercial use if approved. |
It
may take the Company longer than it projects to complete pre-clinical studies and clinical trials, and the Company may not be able to
complete them at all.
Although
for planning purposes the Company projects the commencement, continuation and completion of its pre-clinical studies and clinical trials;
a number of factors, including scheduling conflicts with participating researchers and/or clinicians and research or clinical institutions,
and difficulties in identifying or enrolling patients who meet trial eligibility criteria, may cause significant delays. The Company
may not commence or complete pre-clinical studies or clinical trials involving PALI-2108 as projected or may not conduct them successfully.
Even
if the Company’s clinical studies are successful and achieve regulatory approval, the approved product label may be more limited
than the Company or analysts anticipate, which could limit the commercial prospects of PALI-2108.
At
the time therapeutic drugs are approved for marketing, they are given a “product label” from the FDA or other regulatory
body. In most countries this label sets forth the approved indication for marketing, and identifies potential safety concerns for prescribing
physicians and patients. While the Company intends to seek as broad a product label as possible for PALI-2108, the Company may receive
a narrower label than is expected by either the Company or third parties, such as stockholders and securities analysts. For example,
any approved products may only be indicated to treat refractory patients (i.e., those who have failed some other first-line therapy).
Similarly, it is possible that only a specific sub-set of patients safely responds to PALI-2108. As a result, even if successful in clinical
trials, PALI-2108 could be approved only for a subset of patients. Additionally, safety considerations may result in contraindications
that could further limit the scope of an approved product label. Any of these or other safety and efficacy considerations could limit
the commercial prospects, including market size, of PALI-2108.
Even
if PALI-2108 is approved for commercialization, future regulatory reviews or inspections may result in its suspension or withdrawal,
closure of a facility or enforcement of substantial fines.
If
regulatory approval to sell PALI-2108 is received, regulatory agencies will subject PALI-2108, as well as the manufacturing facilities,
to continual review and periodic inspection. If previously unknown problems with a product or manufacturing and laboratory facility are
discovered, or the Company fails to comply with applicable regulatory approval requirements, a regulatory agency may impose restrictions
on PALI-2108 or the Company. The agency may require the withdrawal of PALI-2108 from the market, closure of the facility or enforcement
of substantial fines.
The
Company may in the future conduct clinical trials for PALI-2108 outside the United States, and the FDA and applicable foreign regulatory
authorities may not accept data from such trials.
The
Company may in the future choose to conduct clinical trials outside of the U.S. Although the FDA or applicable foreign regulatory authority
may accept data from clinical trials conducted outside the U.S. or the applicable jurisdiction, acceptance of such study data by the
FDA or applicable foreign regulatory authority may be subject to certain conditions or exclusion. Where data from foreign clinical trials
are intended to serve as the basis for marketing approval in the United States, the FDA will not approve the application on the basis
of foreign data alone unless such data are applicable to the U.S. population and U.S. medical practice; the studies were performed by
clinical investigators of recognized competence; and the data are considered valid without the need for an on-site inspection by the
FDA or, if the FDA considers such an inspection to be necessary, the FDA is able to validate the data through an on-site inspection or
other appropriate means. Many foreign regulatory bodies have similar requirements. In addition, such foreign studies would be subject
to the applicable local laws of the foreign jurisdictions where the studies are conducted. There can be no assurance the FDA or applicable
foreign regulatory authority will accept data from trials conducted outside of the United States or the applicable home country. If the
FDA or applicable foreign regulatory authority does not accept such data, it would likely result in the need for additional trials, which
would be costly and time-consuming and delay aspects of the Company’s business plan.
The
Company may rely on third-party CROs and other third parties to conduct and oversee its pre-clinical studies and clinical trials. If
these third parties do not meet the Company’s requirements or otherwise conduct the studies or trials as required, the Company
may not be able to satisfy its contractual obligations or obtain regulatory approval for, or commercialize, its product candidates.
The
Company may rely on third-party CROs to conduct and oversee its anticipated pre-clinical studies and clinical trials and other aspects
of product development. The Company also expects to rely on various medical institutions, clinical investigators and contract laboratories
to conduct its trials in accordance with the Company’s clinical protocols and all applicable regulatory requirements, including
the FDA’s regulations and good clinical practice (“GCP”) requirements, which are an international standard meant to
protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors, and state
regulations governing the handling, storage, security and recordkeeping for drug and biologic products. These CROs and other third parties
are expected to play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical
trials. The Company expects to rely heavily on these parties for the execution of its clinical trials and pre-clinical studies and will
control only certain aspects of their activities. The Company and its CROs and other third-party contractors will be required to comply
with GCP and good laboratory practice (“GLP”) requirements, which are regulations and guidelines enforced by the FDA and
comparable foreign regulatory authorities. Regulatory authorities enforce these GCP and GLP requirements through periodic inspections
of trial sponsors, principal investigators and trial sites. If the Company or any of these third parties fail to comply with applicable
GCP and GLP requirements, or reveal noncompliance from an audit or inspection, any clinical data generated in the Company’s clinical
trials may be deemed unreliable and the FDA or other regulatory authorities may require the Company to perform additional clinical trials
before approving the Company’s or the Company’s partners’ marketing applications. The Company cannot assure that upon
inspection by a given regulatory authority, such regulatory authority will determine whether or not any of the Company’s clinical
or pre-clinical trials comply with applicable GCP and GLP requirements. In addition, the Company’s clinical trials generally must
be conducted with compounds produced under cGMP regulations. The Company’s failure to comply with these regulations and policies
may require it to repeat clinical trials, which would be costly and delay the regulatory approval process. If any of the Company’s
CROs were to terminate their involvement with the Company, there is no assurance that the Company would be able to enter into arrangements
with alternative CROs or do so on commercially reasonable terms.
The
successful commercialization of PALI-2108, if approved, will depend in part on the extent to which government authorities and health
insurers establish adequate reimbursement levels and pricing policies.
Sales
of any approved drug candidate will depend in part on the availability of coverage and reimbursement from third-party payers such as
government insurance programs, including Medicare and Medicaid, private health insurers, health maintenance organizations and other health
care related organizations, who are increasingly challenging the price of medical products and services. Accordingly, coverage and reimbursement
may be uncertain. Adoption of any drug by the medical community may be limited if third-party payers will not offer coverage. Additionally,
significant uncertainty exists as to the reimbursement status of newly approved drugs. Cost control initiatives may decrease coverage
and payment levels for any drug and, in turn, the price that we will be able to charge and/or the volume of our sales. The Company is
unable to predict all changes to the coverage or reimbursement methodologies that will be applied by private or government payers. Any
denial of private or government payer coverage or inadequate reimbursement could harm the Company’s business or future revenues,
if any. If the Company partners with third parties with respect to any of its product candidates, the Company may be reliant on that
partner to obtain reimbursement from government and private payors for the drug, if approved, and any failure of that partner to establish
adequate reimbursement could have a negative impact on the Company’s revenues and profitability.
In
addition, both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation,
regulations, and policies affecting coverage and reimbursement rates, which are designed to contain or reduce the cost of health care.
Further federal and state proposals and healthcare reforms are likely, which could limit the prices that can be charged for the product
candidates that the Company develops and may further limit the Company’s commercial opportunity. There may be future changes that
result in reductions in potential coverage and reimbursement levels for the Company’s product candidates, if approved and commercialized,
and the Company cannot predict the scope of any future changes or the impact that those changes would have on its operations.
If
future reimbursement for PALI-2108, subject to approval, are substantially less than projected, or rebate obligations associated with
them are substantially greater than expected, the Company’s future net revenue and profitability, if any, could be materially diminished.
The
Company faces potential product liability exposure, and if successful claims are brought against the Company, it may incur substantial
liability for a product candidate and may have to limit its commercialization.
The
use of the Company’s product candidates in clinical trials and the sale of any products for which the Company obtains marketing
approval exposes it to the risk of product liability claims. Product liability claims might be brought against the Company by clinical
trial participants, consumers, health-care providers, pharmaceutical companies, or others selling the Company’s products. If the
Company cannot successfully defend itself against these claims, it may incur substantial liabilities. Regardless of merit or eventual
outcomes of such claims, product liability claims may result in:
| ● | decreased
demand for the Company’s product candidates; |
| | | |
| ● | impairment
of the Company’s business reputation; |
| | | |
| ● | withdrawal
of clinical trial participants; |
| | | |
| ● | costs
of litigation; |
| | | |
| ● | substantial
monetary awards to patients or other claimants; and |
| | | |
| ● | loss
of revenues. |
The
Company’s insurance coverage may not be sufficient to reimburse it for all expenses or losses it may suffer. Moreover, insurance
coverage is becoming increasingly expensive and, in the future, the Company may not be able to maintain insurance coverage at a reasonable
cost or in sufficient amounts to protect it against losses.
Even
if a product candidate obtains regulatory approval, it may fail to achieve the broad degree of physician and patient adoption and use
necessary for commercial success.
The
commercial success of the Company’s product candidates, if approved, will depend significantly on attaining broad adoption and
use of the drug by physicians and patients. The degree and rate of physician and patient adoption of a product, if approved, will depend
on a number of factors, including but not limited to:
| ● | patient
demand for approved products that treat the indication for which they are approved; |
| | | |
| ● | the
effectiveness of a product compared to other available therapies or treatment regimens; |
| | | |
| ● | the
availability of coverage and adequate reimbursement from managed care plans and other healthcare
payors; |
| | | |
| ● | the
cost of treatment in relation to alternative treatments and willingness to pay on the part
of patients; |
| | | |
| ● | insurers’
willingness to see the applicable indication as a disease worth treating; |
| | | |
| ● | proper
administration by physicians or patients; |
| | | |
| ● | patient
satisfaction with the results, administration and overall treatment experience; |
| ● | limitations
or contraindications, warnings, precautions or approved indications for use different than
those sought by the Company that are contained in the final FDA-approved labeling, or other
authoritative regulatory body approved labeling, for the applicable product; |
| | | |
| ● | any
FDA requirement, or other authoritative regulatory body requirement, to undertake a risk
evaluation and mitigation strategy; |
| | | |
| ● | the
effectiveness of the Company’s sales, marketing, pricing, reimbursement and access,
government affairs, and distribution efforts; |
| | | |
| ● | adverse
publicity about a product or favorable publicity about competitive products; |
| | | |
| ● | new
government regulations and programs, including price controls and/or limits or prohibitions
on ways to commercialize drugs, such as increased scrutiny on direct-to-consumer advertising
of pharmaceuticals; and |
| | | |
| ● | potential
product liability claims or other product-related litigation. |
If
any of the Company’s product candidates are approved for use but fail to achieve the broad degree of physician and patient adoption
necessary for commercial success, the Company’s operating results and financial condition will be adversely affected, which may
delay, prevent or limit its ability to generate revenue and continue its business.
The
Company has entered into a collaborative research agreement with Giiant related to pre-clinical development, which will require the efforts
of Giiant and its personnel, which are out of the Company’s control.
The
license agreement with Giiant provides for certain joint research and development of PALI-2108 related to pre-clinical studies and development.
The Company’s business strategy relies on such collaboration to shorten the time required to file and IND and accelerate the knowledge
transfer of trade secrets and other know-how associated with the licensed technologies. Overall, the success of the development PALI-2108
will depend on the Company’s ability to manage such relationship, and to a certain extent, to the efforts of Giiant, which are
beyond the Company’s control.
Risks
Related to the Company’s Business
The
Company has a very limited operating history and has never generated any revenues from product sales.
The
Company is a biopharmaceutical company with a very limited operating history that may make it difficult to evaluate the success of its
business to date and to assess its future viability. The Company was initially formed in 2001 and its operations, to date, have been
limited to business planning, raising capital and other research and development activities related to its product candidates. The Company
has not yet demonstrated an ability to successfully complete any clinical trials and has never completed the development of any product
candidate, nor has it ever generated any revenue from product sales or otherwise. Consequently, the Company has no meaningful operations
upon which to evaluate its business, and predictions about its future success or viability may not be as accurate as they could be if
it had a longer operating history or a history of successfully developing and commercializing biopharmaceutical products.
The
Company’s business model assumes revenue from, among other activities, marketing or out-licensing the products the Company develops.
PALI-2108 is in the early stages of development and because the Company has a short development history with PALI-2108, there is a limited
amount of information about the Company upon which you can evaluate its business and prospects.
The
Company has no approved drugs and thus have not begun to market or generate revenues from the commercialization of any products. The
Company recently in-licensed PALI-2108 and accordingly, has only a limited history upon which one can evaluate its ability to develop
PALI-2108 as it is still at an early stage of development. Thus, the Company has limited experience and has not yet demonstrated an ability
to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields,
particularly in the biopharmaceutical area. For example, to execute the Company’s business plan, it will need to successfully:
| ● | Execute
product development activities using unproven technologies; |
| | | |
| ● | Build,
maintain, and protect a strong intellectual property portfolio; |
| | | |
| ● | Demonstrate
safety and efficacy of the Company’s drug candidates in multiple human clinical studies; |
| | | |
| ● | Receive
FDA approval and approval from similar foreign regulatory bodies; |
| | | |
| ● | Gain
market acceptance for the development and commercialization of any drugs the Company develops; |
| | | |
| ● | Ensure
the Company’s products are reimbursed by commercial and/or government payors at a rate
that permits commercial viability; |
| | | |
| ● | Develop
and maintain successful strategic relationships with suppliers, distributors, and commercial
licensing partners; |
| | | |
| ● | Manage
the Company’s spending and cash requirements as its expenses will increase in the near
term if the Company adds programs and additional pre-clinical and clinical trials; and |
| | | |
| ● | Effectively
market any products for which the Company obtains marketing approval. |
If
the company is unsuccessful in accomplishing these objectives, it may not be able to develop products, raise capital, expand its business
or continue its operations.
The
Company has received a delisting notification from the Nasdaq Stock Market based on the Company’s Bid Price being under $1.00 for
thirty (30) consecutive trading days. If the Company is not able to regain compliance with the applicable continued listing requirements
or standards of The Nasdaq Capital Market, Nasdaq could delist its common stock.
The
Company’s ability to publicly or privately sell equity securities and the liquidity of its common stock could be adversely affected
if is delisted from the Nasdaq Capital Market or if it is unable to transfer its listing to another stock market. In order to maintain
this listing, it must satisfy minimum financial and other continued listing requirements and standards, including a requirement to maintain
a minimum bid price of the Company’s common stock of $1.00 per share (“Minimum Bid Price Requirement”). On October
19, 2023, the Company received notice (the “Notice”) from the Nasdaq Stock Market LLC (“Nasdaq”) advising the
Company that for 30 consecutive trading days preceding the date of the Notice, the bid price of the Company’s common stock had
closed below the $1.00 per share minimum required for continued listing on the Nasdaq Capital Market pursuant to Nasdaq Listing Rule
5550(a)(2). Under Nasdaq Listing Rule 5810(c)(3)(A), the Company has until April 16, 2024 to regain compliance with the Minimum Bid Price
Requirement. If at any time during this period the closing bid price of the Company’s common stock is at least $1.00 for a minimum
of 10 consecutive business days, the Company will regain compliance with the Minimum Bid Price Requirement and its common stock will
continue to be eligible for listing on The Nasdaq Capital Market absent noncompliance with any other requirement for continued listing.
In the event that the Company does not regain compliance by April 16, 2024, the Company may be eligible for an additional 180 calendar
day grace period if the Company meets the continued listing requirement for market value of publicly held shares and all other initial
listing standards for the Nasdaq Capital Market with the exception of bid price, and the Company provides written notice to Nasdaq of
its intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary.
If
the Company does not regain compliance within the allotted compliance period, including any extensions that may be granted by Nasdaq,
Nasdaq will provide notice that the Company’s common stock will be subject to delisting. The Company will then be entitled to appeal
the determination to a Nasdaq Listing Qualifications Panel and request a hearing. The Company cannot be sure that its share price will
comply with the requirements for continued listing of its shares on the Nasdaq Capital Market in the future or that it will comply with
the other continued listing requirements.
Notwithstanding,
the Company cannot assure you that, in the future, its securities will meet the continued listing requirements to be listed on Nasdaq.
If the Company’s common stock is delisted by Nasdaq, it could lead to a number of negative implications, including an adverse effect
on the price of its common stock, increased volatility in its common stock, reduced liquidity in its common stock, a limited availability
of market quotations for the Company’s common stock, the loss of federal preemption of state securities laws and greater difficulty
in obtaining financing. In addition, delisting of the Company’s common stock could deter broker-dealers from making a market in
or otherwise seeking or generating interest in its common stock, could result in a loss of current or future coverage by certain sell-side
analysts and might deter certain institutions and persons from investing in the Company’s securities at all. Delisting could also
cause a loss of confidence from the Company’s collaborators, vendors, suppliers and employees, which could harm its business and
future prospects.
If
the Company’s common stock is delisted by Nasdaq, its common stock may be eligible to trade on the OTC Bulletin Board, OTCQB or
another over-the-counter market. Any such alternative would likely result in it being more difficult for us to raise additional capital
through the public or private sale of equity securities and for investors to dispose of or obtain accurate quotations as to the market
value of, its common stock. In addition, there can be no assurance that the Company’s common stock would be eligible for trading
on any such alternative exchange or markets. Moreover, if the Company’s common stock is delisted, it may come within the definition
of “penny stock” under the Exchange Act, which imposes additional sales practice requirements on broker-dealers who sell
securities to persons other than established customers and accredited investors. For example, the Company and/or broker-dealers are required
to make a special suitability determination for purchases of such securities and must receive a purchaser’s written consent to
the transaction prior to any purchase. Additionally, unless exempt, prior to a transaction involving a penny stock, the penny stock rules
require the delivery of a disclosure schedule prescribed by the SEC relating to the penny stock market. The broker-dealer must also disclose
the commissions payable to the broker-dealer, current quotations for the securities and, if the broker-dealer is the sole market-maker
for the security, the fact that they are the sole market-maker and their presumed control over the market. Finally, monthly statements
disclosing recent price information on the limited market in penny stocks must be sent to holders of such penny stocks. These requirements
may reduce trading activity in the secondary market for the Company’s common stock and may impact the ability or willingness of
broker-dealers to sell its securities which could limit the ability of stockholders to sell their securities in the public market and
limit the
The
Company’s success depends on the attraction and retention of senior management and scientists with relevant expertise.
The
Company’s future success depends to a significant extent on the continued services of its key employees, including its senior scientific,
technical and managerial personnel. The Company does not maintain key person life insurance for any of its executives and it does not
maintain employment agreements with many senior employees. Competition for qualified employees in the pharmaceutical industry is high,
and the Company’s ability to execute its strategy will depend in part on our ability to continue to attract and retain qualified
scientists and management. If the Company is unable to find, hire and retain qualified individuals, it will have difficulty implementing
its business plan in a timely manner, or at all.
The
Company may choose to discontinue developing or commercializing any of its product candidates, or may choose to not commercialize product
candidates in approved indications, at any time during development or after approval, which could adversely affect the Company and its
operations.
At
any time, the Company may decide to discontinue the development of, or temporarily pause the development of, any of its product candidates
then in existence, for a variety of reasons, including the appearance of new technologies that make its product candidates obsolete,
competition from a competing product or changes in or failure to comply with applicable regulatory requirements. If the Company temporarily
pauses or terminates a program in which it has invested significant resources, the Company will not receive any return on its investment
and it will have missed the opportunity to have allocated those resources to potentially more productive uses which could have an adverse
effect on the Company and its business.
The
Company’s inability to successfully in-license, acquire, develop and market additional product candidates or approved products
would impair its ability to grow its business.
PALI-2108
is currently the Company’s only product candidate being actively developed. The Company may in-license, acquire, develop and market
additional products and product candidates. Since the Company’s internal research and development capabilities are limited, it
may be dependent on pharmaceutical companies, academic or government scientists and other researchers to sell or license products or
technology to it. The success of this strategy depends partly on the Company’s ability to identify and select promising pharmaceutical
product candidates and products, negotiate licensing or acquisition agreements with their current owners, and finance these arrangements.
The
process of identifying, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and
complex. Other companies, including some with substantially greater financial, marketing, sales and other resources, may compete with
the Company for the license or acquisition of product candidates and approved products. Moreover, the Company may devote resources to
potential acquisitions or licensing opportunities that are never completed, or the Company may fail to realize the anticipated benefits
of such efforts. The Company may not be able to acquire the rights to additional product candidates on terms that it finds acceptable
or at all.
Further,
any product candidate that the Company acquires or licenses may require additional development efforts prior to commercial sale, including
pre-clinical or clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone
to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown
to be sufficiently safe and effective for approval by regulatory authorities. In addition, the Company cannot provide assurance that
any approved products that it acquires will be manufactured or sold profitably or achieve market acceptance.
The
Company may seek to avail itself of mechanisms to expedite the development or approval for product candidates it may pursue in the future,
such as Fast Track or breakthrough designation, but such mechanisms may not actually lead to a faster development or regulatory review
or approval process.
The
Company may seek to avail itself of Fast Track designation, breakthrough designation, or priority review for product candidates it may
pursue in the future. For example, if a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates
the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA Fast Track designation. However,
the FDA has broad discretion with regard to these mechanisms, and even if the Company believes a particular product candidate is eligible
for any such mechanism, it cannot guarantee that the FDA would decide to grant it. Even if the Company believes a product candidate meets
the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. Even
if it does obtain Fast Track or priority review designation or pursue an accelerated approval pathway, the Company may not experience
a faster development process, review, or approval compared to conventional FDA procedures. The FDA may withdraw a particular designation
if it believes that the designation is no longer supported by data from the Company’s clinical development program.
Risks
Related to the Company’s Dependence on Third Parties
The
Company expects to rely on collaborations with third parties for the successful development and commercialization of its product candidates.
The
Company expects to rely upon the efforts of third parties for the successful development and commercialization of the Company’s
product candidates. The clinical and commercial success of the Company’s product candidates may depend upon maintaining successful
relationships with third-party partners which are subject to a number of significant risks, including the following:
| ● | the
Company’s partners’ ability to execute their responsibilities in a timely, cost-efficient
and compliant manner; |
| | | |
| ● | reduced
control over delivery and manufacturing schedules; |
| | | |
| ● | price
increases; |
| | | |
| ● | manufacturing
deviations from internal or regulatory specifications; |
| | | |
| ● | quality
incidents; |
| | | |
| ● | the
failure of partners to perform their obligations for technical, market or other reasons; |
| | | |
| ● | misappropriation
of the Company’s product candidates; and |
| | | |
| ● | other
risks in potentially meeting the Company’s product commercialization schedule or satisfying
the requirements of its end-users. |
The
Company cannot provide assurance that it will be able to establish or maintain third-party relationships in order to successfully develop
and commercialize its product candidates.
The
Company anticipates relying completely on third-party contractors to supply, manufacture and distribute clinical drug supplies for its
product candidates.
The
Company does not currently have, nor does it plan to acquire, the infrastructure or capability to supply, store, manufacture or distribute
pre-clinical, clinical or commercial quantities of drug substances or products. Additionally, the Company has not entered into a long-term
commercial supply agreement to provide it with such drug substances or products. As a result, the Company’s ability to develop
its product candidates is dependent, and the Company’s ability to supply its products commercially will depend, in part, on the
Company’s ability to obtain the active pharmaceutical ingredients (“APIs”) and other substances and materials used
in its product candidates successfully from third parties and to have finished products manufactured by third parties in accordance with
regulatory requirements and in sufficient quantities for pre-clinical and clinical testing and commercialization. If the Company fails
to develop and maintain supply and other technical relationships with these third parties, it may be unable to continue to develop or
commercialize its products and product candidates, which could adversely affect the Company and its business.
The
Company is dependent on its contract suppliers and manufacturers for day-to-day compliance with applicable laws and cGMPs for production
of both APIs and finished products. If the safety or quality of any product or product candidate or component is compromised due to a
failure to adhere to applicable laws or for other reasons, the Company may not be able to commercialize or obtain regulatory approval
for the affected product or product candidates successfully, and the Company may be held liable for injuries sustained as a result.
The
Company expects to continue to depend on third-party contract suppliers and manufacturers. The Company’s supply and manufacturing
agreements do not guarantee that a contract supplier or manufacturer will provide services adequate for its needs. Additionally, any
damage to or destruction of the Company’s third-party manufacturer’s or suppliers’ facilities or equipment, even by
force majeure, may significantly impair the Company’s ability to have its products and product candidates manufactured on a timely
basis. The Company’s reliance on contract manufacturers and suppliers further exposes it to the possibility that they, or third
parties with access to their facilities, will have access to and may misappropriate the Company’s trade secrets or other proprietary
information. In addition, the manufacturing facilities of certain of the Company’s suppliers may be located outside of the United
States. This may give rise to difficulties in importing the Company’s products or product candidates or their components into the
United States or other countries.
Risks
Related to the Company’s Financial Operations
The
Company has expressed substantial doubt about its ability to continue as a going concern.
Management
has determined that there is substantial doubt about the Company’s ability to continue as a going concern for a period of one year
following the date of its most recently quarterly report on Form 10-Q filed with the SEC on November 9, 2023. This determination
was based on conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue
as a going concern within one year after the date that the financial statements are issued, including: (i) the probability that significant
changes to the Company’s anticipated level of operations, due to factors that are within or outside of the Company’s control,
would cause the Company’s available cash as of the date of this filing to not be sufficient to fund its anticipated level of operations
for the next 12 months; and (ii) the uncertainties of the cost and timing of the Company’s efforts to in-license or acquire a new
product candidate. The Company’s future consolidated financial statements may include a similar qualification about its ability
to continue as a going concern. The Company’s year-end and interim consolidated financial statements were prepared assuming that
it will continue as a going concern and do not include any adjustments that may result from the outcome of this uncertainty.
If
the Company seeks additional financing to fund its business activities in the future and there remains substantial doubt about its ability
to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to the Company on
commercially reasonable terms or at all.
The
Company has a history of net losses, and it expects to continue to incur net losses and may not achieve or maintain profitability.
The
Company has incurred net losses since our inception, including net losses of $14.3 million and $9.3 million for the year ended December
31, 2022 and the nine months ended September 30, 2023, respectively. The Company expects that its operating losses will continue for
the foreseeable future as it continues its drug development and discovery efforts. To achieve profitability, it must, either directly
or through licensing and/or partnering relationships, meet certain milestones, successfully develop and obtain regulatory approval for
one or more drug candidates and effectively manufacture, market and sell any drugs we successfully develop. Even if the Company is able
to successfully commercialize product candidates that receive regulatory approval, it may not be able to realize revenues at a level
that would allow it to achieve or sustain profitability. Accordingly, the Company may never generate significant revenue and, even if
it does generate significant revenue, it may never achieve profitability.
Failure
to remediate a material weakness in internal controls over financial reporting could result in material misstatements in the Company’s
consolidated financial statements.
The
Company’s management has identified a material weakness in its internal control over financial reporting. The material weakness
was due to a lack of controls in the financial closing and reporting process, including a lack of segregation of duties and the documentation
and design of formalized processes and procedures surrounding the creation and posting of journal entries and account reconciliations.
Additionally, the Company’s management identified a material weakness in its internal control over the fair value calculation of
options granted during the quarter ended June 30, 2021, although management concluded that this material weakness has been remediated
in the year ended December 31, 2022.
If
the Company’s remaining material weakness, which management concluded is still present as of the date of these financial statements,
is not remediated, or if the Company identifies further material weaknesses in its internal controls, the Company’s failure to
establish and maintain effective disclosure controls and procedures and internal control over financial reporting could result in material
misstatements in its consolidated financial statements and a failure to meet its reporting and financial obligations.
Changing
circumstances and market conditions, some of which may be beyond the Company’s control, could impair its ability to access our
existing cash and cash equivalents and investments and to timely pay key vendors and others.
Changing
circumstances and market conditions, some of which may be beyond the Company’s control, could impair its ability to access its
existing cash and cash equivalents and investments and to timely pay key vendors and others. For example, on March 10, 2023, Silicon
Valley Bank (“SVB”) was placed into receivership with the Federal Deposit Insurance Corporation (“FDIC”), which
resulted in all funds held at SVB being temporarily inaccessible by SVB’s customers. Although the Company does not have any funds
at SVB, if other banks and financial institutions with whom the Company has banking relationships enter receivership or become insolvent
in the future in response to financial conditions affecting the banking system and financial markets, the Company may be unable to access,
and the Company may lose, some or all of its existing cash and cash equivalents to the extent those funds are not insured or otherwise
protected by the FDIC. In addition, in such circumstances the Company might not be able to timely pay key vendors and others. The Company
regularly maintains cash balances that are not insured or are in excess of the FDIC’s insurance limit. Any delay in the Company’s
ability to access its cash and cash equivalents (or the loss of some or all of such funds) or to timely pay key vendors and others could
have a material adverse effect on the Company’s operations and cause it to need to seek additional capital sooner than planned.
Risks
Related to the Company’s Intellectual Property
The
Company may not be able to obtain, maintain or enforce global patent rights or other intellectual property rights that cover its product
candidates and technologies that are of sufficient breadth to prevent third parties from competing against the Company.
The
Company’s success with respect to its current and future product candidates will depend, in part, on its ability to obtain and
maintain patent protection in both the U.S. and other countries, to preserve its trade secrets and to prevent third parties from infringing
on its proprietary rights. The Company’s ability to protect its product candidates from unauthorized or infringing use by third
parties depends in substantial part on its ability to obtain and maintain valid and enforceable patents around the world.
The
patent application process, also known as patent prosecution, is expensive and time-consuming, and the Company and its current or future
licensors and licensees may not be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable
cost or in a timely manner in all the countries that are desirable. It is also possible that the Company or its current licensors, or
any future licensors or licensees, will fail to identify patentable aspects of inventions made in the course of development and commercialization
activities before it is too late to obtain patent protection on them. Therefore, these and any of the Company’s patents and applications
may not be prosecuted and enforced in a manner consistent with the best interests of its business. Moreover, the Company’s competitors
independently may develop equivalent knowledge, methods and know-how or discover workarounds to the Company patents that would not constitute
infringement. Any of these outcomes could impair the Company’s ability to enforce the exclusivity of its patents effectively, which
may have an adverse impact on its business, financial condition and operating results.
The
Company’s ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions especially
across countries. Accordingly, rights under any existing patents or any patents the Company might obtain or license may not cover its
product candidates or may not provide the Company with sufficient protection for its product candidates to afford a sustainable commercial
advantage against competitive products or processes, including those from branded, generic and over-the-counter pharmaceutical companies.
In addition, the Company cannot guarantee that any patents or other intellectual property rights will issue from any pending or future
patent or other similar applications owned by or licensed to the Company. Even if patents or other intellectual property rights have
issued or will issue, the Company cannot guarantee that the claims of these patents and other rights are or will be held valid or enforceable
by the courts, through injunction or otherwise, or will provide the Company with any significant protection against competitive products
or otherwise be commercially valuable to the Company in every country of commercial significance that the Company may target.
The
Company’s ability to obtain and maintain valid and enforceable patents depends on whether the differences between its technology
and the prior art allow its technology to be patentable over the prior art. The Company does not have outstanding issued patents covering
all of the recent developments in its technology and is unsure of the patent protection that it will be successful in obtaining, if any.
Even if the patents do successfully issue, third parties may design around or challenge the validity, enforceability or scope of such
issued patents or any other issued patents the Company owns or licenses, which may result in such patents being narrowed, invalidated
or held unenforceable. If the breadth or strength of protection provided by the patents the Company holds or pursues with respect to
its product candidates is challenged, it could dissuade companies from collaborating with the Company to develop or threaten its ability
to commercialize or finance its product candidates.
The
laws of some foreign jurisdictions do not provide intellectual property rights to the same extent or duration as in the U.S., and many
companies have encountered significant difficulties in acquiring, maintaining, protecting, defending and especially enforcing such rights
in foreign jurisdictions. If the Company encounters such difficulties in protecting or are otherwise precluded from effectively protecting
its intellectual property in foreign jurisdictions, its business prospects could be substantially harmed, especially internationally.
Proprietary
trade secrets and unpatented know-how are also very important to the Company’s business. Although the Company has taken steps to
protect its trade secrets and unpatented know-how by entering into confidentiality agreements with third parties, and intellectual property
protection agreements with officers, directors, employees, and certain consultants and advisors, there can be no assurance that binding
agreements will not be breached or enforced by courts, that the Company would have adequate remedies for any breach, including injunctive
and other equitable relief, or that its trade secrets and unpatented know-how will not otherwise become known, inadvertently disclosed
by the Company or its agents and representatives, or be independently discovered by its competitors. If trade secrets are independently
discovered, the Company would not be able to prevent their use and if the Company and its agents or representatives inadvertently disclose
trade secrets and/or unpatented know-how, the Company may not be allowed to retrieve these trade secrets and/or unpatented know-how and
maintain the exclusivity it previously held.
The
Company may not be able to protect its intellectual property rights throughout the world.
Filing,
prosecuting and defending patents on the Company’s product candidates does not guarantee exclusivity. The requirements for patentability
differ in certain countries, particularly developing countries. In addition, the laws of some foreign countries do not protect intellectual
property rights to the same extent as laws in the United States, especially when it comes to granting use and other kinds of patents
and what kind of enforcement rights will be allowed, especially injunctive relief in a civil infringement proceeding. Consequently, the
Company may not be able to prevent third parties from practicing its inventions in all countries outside the United States and even in
launching an identical version of the Company’s product notwithstanding the Company has a valid patent in that country. Competitors
may use the Company’s technologies in jurisdictions where it has not obtained patent protection to develop their own products,
or produce copy products, and, further, may export otherwise infringing products to territories where the Company has patent protection
but enforcement on infringing activities is inadequate or where the Company has no patents. These products may compete with the Company’s
products, and the Company’s patents or other intellectual property rights may not be effective or sufficient to prevent them from
competing.
In
addition, certain countries in Europe and certain developing countries have compulsory licensing laws under which a patent owner may
be compelled to grant licenses to third parties, especially if the patent owner does not enforce or use its patents over a protracted
period of time. In some cases, the courts will force compulsory licenses on the patent holder even when finding the patent holder’s
patents are valid if the court believes it is in the best interests of the country to have widespread access to an essential product
covered by the patent. In these situations, the royalty the court requires to be paid by the license holder receiving the compulsory
license is not calculated at fair market value and can be inconsequential, thereby disaffecting the patentholder’s business. In
these countries, the Company may have limited remedies if its patents are infringed or if the Company is compelled to grant a license
to its patents to a third party, which could also materially diminish the value of those patents. This would limit its potential revenue
opportunities. Accordingly, the Company’s efforts to enforce its intellectual property rights around the world may be inadequate
to obtain a significant commercial advantage from the intellectual property that the Company owns or licenses, especially in comparison
to what it enjoys from enforcing its intellectual property rights in the United States. Finally, the Company’s ability to protect
and enforce its intellectual property rights may be adversely affected by unforeseen changes in both U.S. and foreign intellectual property
laws, or changes to the policies in various government agencies in these countries, including but not limited to the patent office issuing
patents and the health agency issuing pharmaceutical product approvals. Finally, many countries have large backlogs in patent prosecution,
and in some countries in Latin America it can take years, even decades, just to get a pharmaceutical patent application reviewed notwithstanding
the merits of the application.
Obtaining
and maintaining the Company’s patent protection depends on compliance with various procedural, document submission, fee payment,
and other requirements imposed by governmental patent agencies, and its patent protection could be reduced or eliminated for non-compliance
with these requirements.
Periodic
maintenance and annuity fees on any issued patent are due to be paid to the U.S. Patent and Trade Office (“USPTO”) and foreign
patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require
compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process.
While an inadvertent lapse can, in many cases, be cured by payment of a late fee or by other means in accordance with the applicable
rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting
in partial or complete loss of patent rights in the relevant jurisdiction just for failure to know about and/or timely pay a prosecution
fee. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to
official actions within prescribed time limits, non-payment of fees in prescribed time periods, and failure to properly legalize and
submit formal documents in the format and style the country requires. If the Company or its licensors fail to maintain the patents and
patent applications covering its product candidates for any reason, the Company’s competitors might be able to enter the market,
which would have an adverse effect on the Company’s business.
If
the Company fails to comply with its obligations under its intellectual property license agreements, it could lose license rights that
are important to its business.
The
Company has entered into an in-license agreement with respect to its current product candidate. This license agreement imposes various
diligence, milestone, royalty, insurance and other obligations on the Company. From time to time, the Company may be delayed in various
diligence or other obligations upon it. If the Company fails to comply with these obligations, the licensor may terminate the license.
The loss of such rights would materially adversely affect its business, financial condition, operating results and prospects.
The
Company may be subject to patent infringement claims, which could result in substantial costs and liability and prevent us from commercializing
our potential products.
Because
the intellectual property landscape in the fields in which the Company participates is rapidly evolving and interdisciplinary, it is
difficult to conclusively assess its freedom to operate without infringing on third-party rights. If any patent infringement claims are
brought against the Company, whether or not successful, it may incur significant expenses and divert the attention of its management
and key personnel from other business concerns. These could negatively affect the Company’s results of operations and prospects.
The Company cannot be certain that patents owned or licensed by it will not be challenged, potentially successfully, by others.
In
addition, if the Company’s product candidates are found to infringe the intellectual property rights of third parties, these third
parties may assert infringement claims against its customers, licensees and other parties with whom the Company has business relationships,
and it may be required to indemnify those parties for any damages they suffer as a result of these claims. The claims may require the
Company to initiate or defend protracted and costly litigation on behalf of customers, licensees, and other parties regardless of the
merits of these claims. If any of these claims succeed, the Company may be forced to pay damages on behalf of those parties or may be
required to obtain licenses for the products they use. If the Company cannot obtain all necessary licenses on commercially reasonable
terms, it may be unable to continue selling such products.
The
Company may be subject to claims that its officers, directors, employees, consultants or independent contractors have wrongfully used
or disclosed to us alleged trade secrets of their former employers or their former or current customers.
As
is common in the biotechnology and pharmaceutical industries, certain of the Company’s employees were formerly employed by other
biotechnology or pharmaceutical companies, including its competitors or potential competitors. Moreover, the Company engages the services
of consultants to assist us in the development of the Company’s product candidates, many of whom were previously employed at, or
may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including
the Company’s competitors or potential competitors. The Company may be subject to claims that these employees and consultants or
the Company has inadvertently or otherwise wrongfully used or disclosed trade secrets or other proprietary information of their former
employers or their former or current customers. Although the Company has no knowledge of any such claims being alleged to date, if such
claims were to arise, litigation may be necessary to defend against any such claims. Even if the Company is successful in defending against
any such claims, any such litigation could be protracted, expensive, a distraction to its management team, not viewed favorably by investors
and other third parties, and may potentially result in an unfavorable outcome.
Other
Risks Related to the Company Securities
The
Company will need to raise additional financing in the future to fund its operations, which may not be available to it on favorable terms
or at all.
The
Company will require substantial additional capital to fund its operations and conduct the costly and time-consuming clinical trials
necessary to pursue regulatory approval of product candidates. The Company’s future capital requirements will depend upon a number
of factors, including: the number and timing of product candidates in the pipeline; progress with and results from pre-clinical testing
and clinical trials; the ability to manufacture sufficient drug supplies to complete pre-clinical and clinical trials; the costs involved
in preparing, filing, acquiring, prosecuting, maintaining and enforcing patent and other intellectual property claims; and the time and
costs involved in obtaining regulatory approvals and favorable reimbursement or formulary acceptance. Raising additional capital may
be costly or difficult to obtain and could significantly dilute stockholders’ ownership interests or inhibit the Company’s
ability to achieve its business objectives. If the Company raises additional funds through public or private equity offerings, the terms
of these securities may include liquidation or other preferences that adversely impact the rights of its common stockholders. Further,
to the extent that the Company raises additional capital through the sale of common stock or securities convertible or exchangeable into
common stock, its stockholders’ ownership percentage in the Company will be diluted. In addition, any debt financing may subject
the Company to fixed payment obligations and covenants limiting or restricting its ability to take specific actions, such as incurring
additional debt, making capital expenditures or declaring dividends. If the Company raises additional capital through marketing and distribution
arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, the Company may have to relinquish
certain valuable intellectual property or other rights to its product candidates, technologies, future revenue streams or research programs
or grant licenses on terms that may not be favorable to it. Even if the Company were to obtain sufficient funding, there can be no assurance
that it will be available on terms acceptable to the Company or its stockholders.
The
stock price of the Company may be highly volatile.
Since
the completion of the Merger on April 27, 2021, the Company’s stock price has already been subject to significant fluctuation.
Market prices for securities of biotechnology and other life sciences companies historically have been particularly volatile subject
even to large daily price swings. Some of the factors that may cause the market price of shares of the Company to fluctuate include,
but are not limited to:
| ● | failure
of the Company product candidates to show safety and/or efficacy in its clinical trials; |
| | | |
| ● | the
ability of the Company to obtain timely regulatory approvals for its product candidates,
and delays or failures to obtain such approvals; |
| | | |
| ● | the
results of clinical trials of product candidates, including the Company’s decision
to pause or terminate any such trials; |
| | | |
| ● | failure
of the Company’s product candidates, if approved, to achieve commercial success; |
| | | |
| ● | the
entry into, or termination of, or breach by partners of key agreements, including key commercial
partner agreements; |
| | | |
| ● | the
initiation of, material developments in, or conclusion of any litigation to enforce or defend
any intellectual property rights or defend against the intellectual property rights of others; |
| | | |
| ● | announcements
of any financings; |
| | | |
| ● | announcements
by commercial partners or competitors of new commercial products, clinical progress or the
lack thereof, significant contracts, commercial relationships or capital commitments; |
| | | |
| ● | failure
to elicit meaningful stock analyst coverage and downgrades of the Company’s stock by
analysts; and |
| | | |
| ● | the
loss of key personnel. |
Moreover,
the stock markets in general have experienced substantial volatility in the biotechnology industry that has often been unrelated to the
operating performance of individual companies or a certain industry segment. These broad market fluctuations may also adversely affect
the trading price of the Company’s shares. In the past, following periods of volatility in the market price of a company’s
securities, shareholders have often instituted class action securities litigation against those companies. Such litigation, if instituted,
could result in substantial costs and diversion of management attention and resources, which could significantly harm the Company’s
profitability and reputation.
The
Company takes advantage of reduced disclosure and governance requirements applicable to smaller reporting companies, which could result
in its common stock being less attractive to investors.
As
of June 30, 2023, the last business day of the Company’s most recently completed second fiscal quarter, the public float of the
Company is less than $250 million and therefore, the Company qualifies as a smaller reporting company under SEC rules. As a smaller reporting
company, the Company is able to take advantage of reduced disclosure requirements, such as simplified executive compensation disclosures
and reduced financial statement disclosure requirements in its SEC filings. Decreased disclosures in the Company’s SEC filings
due to its status as a smaller reporting company may make it harder for investors to analyze its results of operations and financial
prospects. The Company cannot predict if investors will find the Company’s common stock less attractive if it relies on these exemptions.
If some investors find its common stock less attractive as a result, there may be a less active trading market for its common stock and
its stock price may be more volatile. The Company may take advantage of the reporting exemptions applicable to a smaller reporting company
until it is no longer a smaller reporting company, which status would end once it has a public float greater than $250 million. In that
event, the Company could still be a smaller reporting company if its annual revenues were below $100 million and it has a public float
of less than $700 million.
The
Company does not anticipate paying any dividends in the foreseeable future.
The
current expectation is that the Company will retain its future earnings to fund the development and growth of its business. As a result,
capital appreciation, if any, of the shares of the Company will be your sole source of gain, if any, for the foreseeable future.
If
equity research analysts do not publish research or reports, or publish unfavorable research or reports, about the Company, its business
or its market, its stock price and trading volume could decline.
The
trading market for the Company’s common stock is and will be influenced by the research and reports that equity research analysts
publish about it and its business. Equity research analysts may elect not to provide research coverage of the Company’s common
stock, and such lack of research coverage may adversely affect the market price of its common stock. In the event it does have equity
research analyst coverage, the Company will not have any control over the analysts, or the content and opinions included in their reports.
The price of the Company’s common stock could decline if one or more equity research analysts downgrade its stock or issue other
unfavorable commentary or research. If one or more equity research analysts ceases coverage of the Company or fails to publish reports
on it regularly, demand for its common stock could decrease, which in turn could cause its stock price or trading volume to decline.
Future
sales of substantial amounts of the Company’s common stock, or the possibility that such sales could occur, could adversely affect
the market price of its common stock.
Future
sales in the public market of shares of the Company’s common stock, including shares issued upon exercise of its outstanding stock
options, or the perception by the market that these sales could occur, could lower the market price of its common stock or make it difficult
for it to raise additional capital.
The
Company’s business could be negatively affected as a result of actions of activist stockholders, and such activism could impact
the trading value of its securities.
Stockholders
may, from time to time, engage in proxy solicitations or advance stockholder proposals, or otherwise attempt to effect changes and assert
influence on the Company’s Board of Directors (“Board”) and management. Activist campaigns that contest or conflict
with the Company’s strategic direction or seek changes in the composition of its Board could have an adverse effect on its operating
results and financial condition. A proxy contest would require the Company to incur significant legal and advisory fees, proxy solicitation
expenses and administrative and associated costs and require significant time and attention by the Company’s Board and management,
diverting their attention from the pursuit of its business strategy. Any perceived uncertainties as to the Company’s future direction
and control, its ability to execute on its strategy, or changes to the composition of its Board or senior management team arising from
a proxy contest could lead to the perception of a change in the direction of its business or instability which may result in the loss
of potential business opportunities, make it more difficult to pursue the Company’s strategic initiatives, or limit its ability
to attract and retain qualified personnel and business partners, any of which could adversely affect its business and operating results.
If individuals are ultimately elected to the Company’s Board with a specific agenda, it may adversely affect the Company’s
ability to effectively implement its business strategy and create additional value for our stockholders. The Company may choose to initiate,
or may become subject to, litigation as a result of the proxy contest or matters arising from the proxy contest, which would serve as
a further distraction to its Board and management and would require the Company to incur significant additional costs. In addition, actions
such as those described above could cause significant fluctuations in the Company’s stock price based upon temporary or speculative
market perceptions or other factors that do not necessarily reflect the underlying fundamentals and prospects of its business.
Securities
class action litigation could divert our management’s attention and harm our business and could subject us to significant liabilities.
The
stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the
equity securities of life sciences and biotechnology companies. These broad market fluctuations may cause the market price of the Company’s
common shares to decline. In the past, securities class action litigation has often been brought against a company following a decline
in the market price of its securities. This risk is especially relevant for the Company because biotechnology and biopharmaceutical companies
have experienced significant stock price volatility in recent years. Even if the Company is successful in defending claims that may be
brought in the future, such litigation could result in substantial costs and may be a distraction to the Company’s management and
may lead to an unfavorable outcome that could adversely impact its financial condition and prospects.
Anti-takeover
provisions in the Company’s charter documents and under Delaware law could make an acquisition of the Company more difficult and
may prevent attempts by the Company stockholders to replace or remove the Company management.
Provisions
in the Company’s certificate of incorporation and bylaws may delay or prevent an acquisition or a change in management. In addition,
because the Company is incorporated in Delaware, it is governed by the provisions of Section 203 of the DGCL, which prohibits stockholders
owning in excess of 15% of the outstanding Company voting stock from merging or combining with the Company. Although the Company believes
these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with
the Company’s Board, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions
may frustrate or prevent any attempts by the Company’s stockholders to replace or remove then current management by making it more
difficult for stockholders to replace members of the Board, which is responsible for appointing the members of management.
If
the Company fails to maintain proper and effective internal controls, its ability to produce accurate financial statements on a timely
basis could be impaired.
The
Company is subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of Nasdaq.
The Sarbanes-Oxley Act requires, among other things, that the Company maintain effective disclosure controls and procedures and internal
control over financial reporting. The Company must perform system and process evaluation and testing of its internal control over financial
reporting to allow management to report on the effectiveness of its internal controls over financial reporting in its Annual Report on
Form 10-K filing for that year, as required by Section 404 of the Sarbanes-Oxley Act. This has required that the Company incur substantial
professional fees and internal costs to expand its accounting and finance functions and that it expend significant management efforts.
The Company may experience difficulty in meeting these reporting requirements in a timely manner.
The
Company may discover weaknesses in its system of internal financial and accounting controls and procedures that could result in a material
misstatement of its consolidated financial statements. Prior to the Merger, LBS’s management identified a material weakness in
its internal control over financial reporting. The material weakness was due to a lack of controls in the financial closing and reporting
process for LBS, including a lack of segregation of duties and the documentation and design of formalized processes and procedures surrounding
the creation and posting of journal entries and account reconciliations. If the Company does not remediate this material weakness, or
if the Company identifies further material weaknesses in its internal controls, the Company’s failure to establish and maintain
effective internal financial and accounting controls and procedures could result in material misstatements in its consolidated financial
statements and a failure to meet its reporting and financial obligations.
If
the Company is not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act, or if it is unable to maintain proper
and effective internal controls, the Company may not be able to produce timely and accurate consolidated financial statements. If that
were to happen, the market price of its common stock could decline and it could be subject to sanctions or investigations by Nasdaq,
the SEC or other regulatory authorities.
The
Company’s Board of Directors has broad discretion to issue additional securities, which might dilute the net tangible book value
per share of its common stock for existing stockholders.
The
Company is entitled under its certificate of incorporation to issue up to 280,000,000 shares of common stock and 7,000,000 “blank
check” shares of preferred stock. Shares of the Company’s blank check preferred stock provide its Board with broad authority
to determine voting, dividend, conversion, and other rights. As of December 28, 2023, the Company had outstanding, common stock or securities
convertible into common stock, totaling 9,270,894 shares. As a result, the Company is authorized to issue up to an additional 270,729,106
shares of common stock or common stock equivalents under its certificate of incorporation as amended. Additionally, pursuant to the initial
issuance of (i) 1,000,000 shares of Series A 4.5% Convertible Preferred Stock, of which 200,000 shares are outstanding and (ii) 1,460
shares of Series B Convertible Preferred Stock, of which no shares are outstanding, the Company is authorized to issue up to an additional
6,800,000 shares of preferred stock. The Company expects that significant additional capital may be needed in the future to continue
its planned operations. To the extent the Company raises additional capital by issuing equity securities, its existing shareholders may
experience substantial dilution. The Company may sell common stock, convertible securities or other equity securities in one or more
transactions at prices and in a manner the Company determines from time to time. If the Company sells common stock, convertible securities
or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. These sales may also
result in material dilution to the Company’s existing shareholders, and new investors could gain rights superior to existing shareholders.
Pursuant to the Company’s equity incentive plans and employee stock purchase plan, management is authorized to grant stock options,
restricted stock units and other equity-based awards to employees, directors and consultants, and to sell common stock to employees,
respectively. Any increase in the number of shares outstanding as a result of the exercise of outstanding options, the vesting or settlement
of outstanding stock awards, or the purchase of shares pursuant to the employee stock purchase plan will cause shareholders to experience
additional dilution, which could cause the stock price to fall.
General
Risk Factors
The
COVID-19 pandemic, or a similar pandemic, epidemic, or outbreak of an infectious disease, may materially and adversely affect the Company’s
business and the Company’s financial results and could cause a disruption to the development of the Company’s product candidates.
Public
health crises, such as pandemics or similar outbreaks, could adversely impact the Company’s business. The impact of the COVID-19
pandemic and the efforts to mitigate it, resulted in and will likely continue to result in disruptions to the global economy, as well
as businesses and capital markets around the world. The Company experienced delays in its development activities as a result of the COVID-19
pandemic, primarily due to temporary and partial shutdowns at certain of the Company’s CROs and trial sites that have since resumed
operations, and due to governmental responses to the pandemic. Additionally, the emergence of new variants, which could prove resistant
to existing vaccines, could again result in major disruptions to businesses and markets worldwide. The extent to which the COVID-19 pandemic
will continue to impact the Company’s operations or those of its consultants and collaborators, will depend on future developments,
including the global macroeconomic effects of the virus.
Global,
market and economic conditions, including inflation, may negatively impact the Company’s business, financial condition and share
price.
Concerns
over inflation, geopolitical issues, the U.S. financial markets, foreign exchange rates, capital and exchange controls, unstable global
credit markets and financial conditions and the COVID-19 pandemic, have led to periods of significant economic instability, declines
in consumer confidence and discretionary spending, diminished expectations for the global economy and expectations of slower global economic
growth going forward, and increased unemployment rates. The Company’s general business strategy may be adversely affected by any
such economic downturns, volatile business environments and continued unstable or unpredictable economic and market conditions. If these
conditions continue to deteriorate or do not improve, it may make any necessary debt or equity financing more difficult to complete,
more costly and more dilutive. In addition, there is a risk that one or more of our current or future service providers, manufacturers,
suppliers and other partners could be negatively affected by difficult economic times, which could adversely affect the Company’s
ability to attain our operating goals on schedule and on budget or meet our business and financial objectives.
In
addition, the Company faces several risks associated with international business and are subject to global events beyond its control,
including war, public health crises, such as pandemics and epidemics, trade disputes, economic sanctions, trade wars and their collateral
impacts and other international events. Any of these changes could have a material adverse effect on the Company’s reputation,
business, financial condition or results of operations. There may be changes to the Company’s business if there is instability,
disruption or destruction in a significant geographic region, regardless of cause, including war, terrorism, riot, civil insurrection
or social unrest; and natural or man-made disasters, including famine, flood, fire, earthquake, storm or disease. In February 2022, armed
conflict escalated between Russia and Ukraine. The sanctions announced by the U.S. and other countries, following Russia’s invasion
of Ukraine against Russia to date include restrictions on selling or importing goods, services or technology in or from affected regions
and travel bans and asset freezes impacting connected individuals and political, military, business and financial organizations in Russia.
The U.S. and other countries could impose wider sanctions and take other actions should the conflict further escalate. It is not possible
to predict the broader consequences of this conflict, which could include further sanctions, embargoes, regional instability, geopolitical
shifts and adverse effects on macroeconomic conditions, currency exchange rates and financial markets, all of which could impact the
Company’s business, financial condition and results of operations.
The
Company may be adversely affected by natural disasters and other catastrophic events and by man-made problems such as terrorism that
could disrupt its business operations, and its business continuity and disaster recovery plans may not adequately protect it from a serious
disaster.
The
Company’s headquarters and main research facility are located in the greater San Diego area, which in the past has experienced
severe earthquakes and fires. If these earthquakes, fires, other natural disasters, health pandemics or epidemics, terrorism and similar
unforeseen events beyond its control, including for example the ongoing COVID-19 pandemic, prevented it from using all or a significant
portion of its headquarters or research facility, it may be difficult or, in certain cases, impossible for the Company to continue its
business for a substantial period of time. The Company does not have a disaster recovery or business continuity plan in place and may
incur substantial expenses as a result of the absence or limited nature of the Company’s internal or third-party service provider
disaster recovery and business continuity plans, which, particularly when taken together with its lack of earthquake insurance, could
have a material adverse effect on its business. Furthermore, integral parties in the Company’s supply chain are operating from
single sites, increasing their vulnerability to natural disasters or other sudden, unforeseen and severe adverse events. If such an event
were to affect its supply chain, it could have a material adverse effect on the Company’s ability to conduct clinical trials, its
development plans and its business.
If
the Company’s information systems or data, or those of third parties upon which it relies, are or were compromised, the Company
could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions;
litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; loss of customers
or sales; and other adverse consequences.
In
the ordinary course of the Company’s business, it may process, as defined above, proprietary, confidential, and sensitive data,
including personal data (such as health-related patient data), intellectual property, and trade secrets (collectively, sensitive information).
The Company may rely upon third-party service providers and technologies to operate critical business systems to process sensitive information
in a variety of contexts, including, without limitation, third-party providers of cloud-based infrastructure, employee email, CROs, and
other functions. The Company’s ability to monitor these third parties’ information security practices is limited, and these
third parties may not have adequate information security measures in place. The Company may share or receive sensitive information with
or from third parties.
The
risk of a security breach or disruption, particularly through cyber-attacks, cyber-intrusion, malicious internet-based activity, and
online and offline fraud, are prevalent and have generally increased as the number, intensity, and sophistication of attempted attacks
and intrusions from around the world have increased. These threats are becoming increasingly difficult to detect and come from a variety
of sources, including traditional computer hackers, threat actors, personnel (such as through theft or misuse), sophisticated nation
states, and nation-state-supported actors. Some actors now engage and are expected to continue to engage in cyber-attacks, including
without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During
times of war and other major conflicts, we and the third parties upon which we rely may be vulnerable to a heightened risk of these attacks,
including cyber-attacks that could materially disrupt the Company’s systems and operations, supply chain, and ability to produce,
sell and distribute the Company’s products.
The
Company and the third parties upon which the Company relies may be subject to a variety of evolving threats, including but not limited
to social engineering attacks (including through phishing attacks), malicious code (such as viruses and worms), malware (including as
a result of advanced persistent threat intrusions), denial-of-service attacks (such as credential stuffing), personnel misconduct or
error, ransomware attacks, supply-chain attacks, software bugs, server malfunctions, software or hardware failures, loss of data or other
information technology assets, adware, natural disasters, terrorism, war, and telecommunication and electrical failures. Ransomware attacks,
including by organized criminal threat actors, nation-states, and nation-state-supported actors, are becoming increasingly prevalent
and can lead to significant interruptions in our operations, loss of data and income, reputational harm, and diversion of funds. Extortion
payments may alleviate the negative impact of a ransomware attack, but the Company may be unwilling or unable to make such payments due
to, for example, applicable laws or regulations prohibiting such payments. Similarly, supply-chain attacks have increased in frequency
and severity.
Furthermore,
the COVID-19 pandemic and our remote workforce poses increased risks to the Company’s information technology systems and data,
as more of the Company’s employees work from home, utilizing network connections outside our premises.
Any
of the previously identified or similar threats could cause a security breach or disruption. While the Company has not experienced any
such security breach or other disruption to date, if such an event were to occur, it could result in unauthorized, unlawful, or accidental
acquisition, modification, destruction, loss, alteration, encryption, disclosure of, or access to our sensitive information and cause
interruptions in the Company’s operations, including material disruptions of its development programs and business operations.
The
Company may expend significant resources or modify its business activities (including our clinical trial activities) to try to protect
against security breaches and disruptions. Certain data privacy and security obligations may require the Company to implement and maintain
specific security measures, industry-standard or reasonable security measures to protect our information technology systems and sensitive
information. While the Company has implemented security measures designed to protect against security incidents, there can be no assurance
that these measures will be effective. The Company may be unable in the future to detect vulnerabilities in its information technology
systems because such threats and techniques change frequently, are often sophisticated in nature, and may not be detected until after
a security breach or disruption has occurred. Despite the Company’s efforts to identify and remediate vulnerabilities, if any,
in its information technology systems, its efforts may not be successful. Further, the Company may experience delays in developing and
deploying remedial measures designed to address any such identified vulnerabilities.
Applicable
data privacy and security obligations may require the Company to notify relevant stakeholders of certain security breaches and disruptions.
Such disclosures are costly, and the disclosure or the failure to comply with such requirements could lead to adverse consequences. If
the Company (or a third party upon whom it relies) experience a security breach or other disruption, or are perceived to have experienced
such events, the Company may experience adverse consequences, including: government enforcement actions (for example, investigations,
fines, penalties, audits, and inspections); additional reporting requirements and/or oversight; restrictions on processing sensitive
information (including personal data); litigation (including class claims); indemnification obligations; negative publicity; reputational
harm; monetary fund diversions; interruptions in the Company’s operations (including availability of data); financial loss; and
other similar harms. In particular, since the Company sponsors clinical trials, any breach or disruption that compromises patient data
and identities could generate significant reputational damage, which may affect trust in the Company and its ability to recruit for future
clinical trials. Additionally, the loss of clinical trial data from completed or future clinical trials could result in delays in the
Company’s regulatory approval efforts and significantly increase its costs to recover or reproduce the data.
The
Company’s contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations
of liability in its contracts are sufficient to protect it from liabilities, damages, or claims related to its data privacy and security
obligations. Furthermore, the Company cannot be sure that its insurance coverage will be adequate or sufficient to protect it from or
to mitigate liabilities arising out of its privacy and security practices, that such coverage will continue to be available on commercially
reasonable terms or at all, or that such coverage will pay future claims.
The
Company’s business and operations would suffer in the event of system failures, cyber-attacks or a deficiency in its cyber-security.
Despite
the implementation of security measures, the Company’s internal computer systems and those of its current and future CROs and other
contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and
telecommunication and electrical failures. Although the Company has not suffered any material incidents to date, the risk of a security
breach or disruption, particularly through cyber-attacks or cyber-intrusion, including by computer hackers, foreign governments, and
cyber-terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around
the world have increased. While the Company has not experienced any such material system failure, accident or security breach to date,
if such an event were to occur and cause interruptions in the Company’s operations, it could result in a material disruption of
its development programs and its business operations. In addition, since the Company sponsors clinical trials, any breach that compromises
patient data and identities causing a breach of privacy could generate significant reputational damage and legal liabilities and costs
to recover and repair, including affecting trust in the Company to recruit for future clinical trials. For example, the loss of clinical
trial data from completed or future clinical trials could result in delays in the Company’s regulatory approval efforts and significantly
increase its costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of,
or damage to, the Company’s data or applications or inappropriate disclosure of confidential or proprietary information, the Company
could incur liability and the further development and commercialization of its products and product candidates could be delayed.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and any applicable prospectus supplement or free writing prospectus, including the documents that we incorporate by reference
herein and therein, contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of
1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act.
These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties
and other factors which may cause our actual results, performance or achievements to be materially different from any future results,
performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are
not limited to, statements about:
| |
● |
estimates
about the size and growth potential of the markets for our product candidates, and our ability to serve those markets, including
any potential revenue generated; |
| |
|
|
| |
● |
future
regulatory, judicial, and legislative changes or developments in the United States (“U.S.”) and foreign countries and
the impact of these changes; |
| |
|
|
| |
● |
our
ability to successfully develop our licensed technologies; |
| |
● |
our
ability to build a commercial infrastructure in the U.S. and other markets; |
| |
|
|
| |
● |
our
ability to compete effectively in a competitive industry; |
| |
|
|
| |
● |
our
ability to identify and qualify additional manufacturers to provide API and manufacture drug product; |
| |
|
|
| |
● |
our
ability to enter into commercial supply agreements; |
| |
|
|
| |
● |
the
success of competing technologies that are or may become available; |
| |
|
|
| |
● |
our
ability to attract and retain key scientific or management personnel; |
| |
|
|
| |
● |
the
accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
| |
|
|
| |
● |
our
ability to obtain funding for our operations; |
| |
|
|
| |
● |
our
ability to attract collaborators and strategic partnerships; and |
| |
|
|
| |
● |
the
impact of the COVID-19 pandemic on our business, and operations, and supply. |
In
some cases, you can identify forward-looking statements by terms such as “may,” “will,” “intend,”
“should,” “could,” “would,” “expects,” “plans,” “anticipates,”
“believes,” “estimates,” “projects,” “predicts,” “potential” and similar
expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events
and are based on assumptions and are subject to risks and uncertainties. As such, our actual results may differ significantly from those
expressed in any forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking
statements.
These
forward-looking statements are based on the current beliefs and expectations of our management and are subject to significant risks and
uncertainties. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results may differ materially
from current expectations and projections. Factors that might cause such a difference include the risk factors identified under the caption
“Risk Factors” in this prospectus, as well as those identified under the caption “Risk Factors” in our Annual
Report on Form 10-K, filed with the SEC on March 22, 2023 and our Quarterly Report on Form 10-Q, filed with the SEC on November
9, 2023.
You
are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this prospectus or,
in the case of documents referred to or incorporated by reference, the date of those documents.
All
subsequent written or oral forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in
their entirety by the cautionary statements contained or referred to in this section. We do not undertake any obligation to release publicly
any revisions to these forward-looking statements to reflect events or circumstances after the date of this prospectus or to reflect
the occurrence of unanticipated events, except as may be required under applicable U.S. securities law. If we do update one or more forward-looking
statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
All
subsequent written or oral forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in
their entirety by the cautionary statements contained or referred to in this section. We do not undertake any obligation to release publicly
any revisions to these forward-looking statements to reflect events or circumstances after the date of this prospectus or to reflect
the occurrence of unanticipated events, except as may be required under applicable U.S. securities law. If we do update one or more forward-looking
statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
USE
OF PROCEEDS
We
estimate that the net proceeds from the sale of securities in this offering will be approximately $ million, after deducting
the underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriter exercises its over-allotment
option in full, we estimate that our net proceeds will be approximately $ million, after deducting the underwriting discounts and commissions
and estimated offering expenses payable by us.
Our
expected use of the net proceeds from this offering represents our current intentions based upon our present plans and business condition.
As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon
completion of this offering, or the amounts that we will actually spend on the uses set forth above. However, we currently plan to use
the net proceeds to us from this offering primarily for general corporate purposes, including research and development and working capital.
The
amounts and timing of our actual use of net proceeds will vary depending on numerous factors, including the relative success and cost
of our research and development programs and our ability to gain access to additional financing. As a result, our management will have
broad discretion in the application of the net proceeds, and investors will be relying on our management’s judgment regarding the
application of the net proceeds of this offering. In addition, we might decide to postpone or not pursue certain development activities
if the net proceeds from the offering and any other sources of cash are less than expected.
Pending
the uses described above, we plan to invest these net proceeds in short-term, interest-bearing investments, investment-grade instruments,
certificates of deposit or direct or guaranteed obligations of the United States. We also may use a portion of the net proceeds from
the offering to fund acquisitions or other business development opportunities. However, we have no current commitments or obligations
with respect to any such acquisitions or business development opportunities at this time.
MARKET
INFORMATION
Our
common stock is listed on The Nasdaq Capital Market under the symbol “PALI.” On December 28, 2023, the last reported
sale price for our common stock on The Nasdaq Capital Market was $0.6539 per share. As of December 28, 2023, we had approximately
155 stockholders of record.
DIVIDEND
POLICY
We
do not anticipate declaring or paying, in the foreseeable future, any cash dividends on our capital stock. We intend to retain all available
funds and future earnings, if any, to fund the development and expansion of our business. Any future determination regarding the declaration and payment of dividends, if any, will be at the discretion
of our Board and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions,
capital requirements, business prospects and other factors our Board may deem relevant.
CAPITALIZATION
The
following table sets forth our cash and cash equivalents and capitalization as of September 30, 2023 on:
| |
● |
an
actual basis; |
| |
|
|
| |
● |
A proforma basis after adjusting
for the following issuances subsequent to September 30, 2023: (i) an aggregate of 26,467 shares that were issued to employees pursuant
to the vesting of restricted stock units and (ii) 33,676 shares that were issued on November 20, 2023 to various employees pursuant
to the Company’s 2021 Employee Stock Purchase Plan, as amended; and |
| |
|
|
| |
● |
a
pro forma as adjusted basis, giving effect to the sale of common shares, at the assumed
public offering price of $ per common share and no exercise by the underwriter of its option
to purchase additional shares of common stock after deducting underwriting discounts and commissions and estimated offering expenses
payable by us. |
The
pro forma as adjusted information below is illustrative only, and our capitalization following the closing of this offering will be adjusted
based on the actual public offering price and other terms of this offering determined at pricing. You should read the following table
in conjunction with our consolidated financial statements, including the related notes, and “Management’s Discussion and
Analysis of Financial Condition and Results of Operations” from our Quarterly Report on Form 10-Q for the quarter ended September
30, 2023, which are incorporated by reference into this prospectus.
| | |
As of September 30, 2023 | |
| | |
(unaudited) (in thousands) | |
| | |
Actual | | |
Pro Forma | | |
Pro Forma as Adjusted | |
| Cash and cash equivalents | |
$ | 15,312 | | |
$ | 15,312 | | |
$ | | |
| Stockholders’ equity: | |
| | | |
| | | |
| | |
| Series A Convertible Preferred Stock, $0.01 par value per share, 7,000,000 shares authorized
as of September 30, 2023, actual, pro forma and pro forma as adjusted; 200,000 shares issued and outstanding as of September 30,
2023, actual, pro forma and pro forma as adjusted | |
| 2 | | |
| 2 | | |
| | |
| Common stock, $0.01 par value; 280,000,000 shares authorized as of September 30, 2023 actual, pro
forma, and adjusted pro forma, 9,270,894 shares issued and outstanding as of September 30, 2023, actual, shares issued and
outstanding as of September 30, 2023, proforma, and shares issued and outstanding as of September 30, 2023, pro forma as adjusted | |
| 92 | | |
| 92 | | |
| | |
| Additional paid-in capital | |
| 132,523 | | |
| 132,540 | | |
| | |
| Accumulated deficit | |
| (118,535 | ) | |
| (118,535 | ) | |
| ( | ) |
| Total stockholders’ equity | |
| 14,082 | | |
| 14,099 | | |
| | |
| Total capitalization | |
$ | 29,394 | | |
$ | 29,411 | | |
$ | | |
The
foregoing table excludes:
| ● |
8,430
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023 granted under the LBS 2013 Amended
and Restated Employee, Director, and Consultant Equity Incentive Plan, as amended and restated, or the 2013 Plan, with a weighted-average
exercise price of $1,054.60 per share; |
| |
|
| ● |
574,956
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023, granted under the 2021 Equity
Incentive Plan, as amended, or the 2021 Plan, with a weighted-average exercise price of $4.21 per share, which includes a total of
78,160 shares of common stock issuable upon exercise of outstanding stock options each with an exercise price of $0.59 that were
conditionally granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares
available under the 2021 Plan; |
| |
|
| ● |
82,086
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023 granted under the 2021 Inducement
Plan, with a weighted average exercise price of $4.90 per share; |
| |
|
| ● |
312,780
shares of common stock issuable upon vesting of restricted stock units outstanding as of December 28, 2023, granted under the 2021
Plan, which includes a total of 66,000 shares of common stock issuable upon vesting of restricted stock units that were conditionally
granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares available under
the 2021 Plan; |
| |
|
| ● |
50,144
shares of common stock issuable upon vesting of restricted stock units outstanding as of December 28, 2023, granted under the 2021
Inducement Plan; |
| |
|
| ● |
62,200
shares of common stock issuable upon vesting of restricted performance stock units outstanding as of December 28, 2023; all of which
were issued under the 2021 Plan which vest subject to certain milestones; |
| |
|
| ● |
5,397
shares of common stock reserved for future issuance under the 2021 Plan as of December 28, 2023, which excludes a total of 144,610
shares of common stock issuable upon exercise of outstanding stock options and outstanding restricted stock units that were conditionally
granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares being available
under the 2021 Plan, as well as any future automatic increases in the number of shares of common stock reserved for future issuance
under the 2021 Plan; |
| |
|
| ● |
110,871
shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan, or the ESPP, as of December 28,
2023, as well as any automatic increases in the number of shares of common stock reserved for future issuance under the ESPP; |
| ● |
863,214
shares of common stock reserved for issuance under the 2021 Inducement Plan as of September 1, 2023; |
| |
|
| ● |
4,080,876
shares of common stock issuable upon exercise of outstanding warrants as of December 28, 2023 with a weighted-average exercise price
of $8.63 per share; and |
| |
|
| ● |
129
shares of common stock issuable upon conversion of the 200,000 outstanding shares of our Series A 4.5% Convertible Preferred Stock
as of December 28, 2023, as well as any future shares of common stock issuable upon conversion of additional shares of Series A 4.5%
Convertible Preferred Stock that may be issued as payment-in-kind dividends thereon in accordance with their terms. |
DILUTION
If
you invest in our securities, your ownership interest will be diluted to the extent of the difference between the public offering price
per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after the
closing of this offering.
Our
historical net tangible book value as of September 30, 2023 was $14.1 million, or $1.53 per share. Our historical net tangible book value
is the amount of our total tangible assets less our liabilities. Our historical net tangible book value per share is our historical net
tangible book value divided by the number of shares of common stock outstanding on September 30, 2023. As of September 30, 2023, we had
pro forma net tangible book value of $ million, or $ per share. The dilution information set forth in the table below is illustrative
only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
Pro
forma as adjusted net tangible book value dilution per share to new investors represents the difference between the amount per share
paid by purchasers of common shares in this offering and the pro forma as adjusted net tangible book value per share of common
stock immediately after completion of this offering. After giving effect to the sale by us in this offering of common
shares at a public offering price of $ common share, and no exercise by the
underwriter of its option to purchase additional shares of common stock, and after deducting the estimated underwriting discounts and
commissions and estimated offering expenses that we will pay, our pro forma as adjusted net tangible book value as of September 30, 2023
would have been approximately $ million or $ per share of common stock. This
amount represents an immediate increase in net tangible book value of $ per share to existing shareholders
and an immediate dilution of $ per share to purchasers in this offering.
The
following table illustrates this dilution on a per share basis to new investors:
| Assumed public offering price per share of common stock | |
| | | |
$ | | |
| Pro Forma net tangible book value per share as of September 30, 2023 | |
$ | | | |
| | |
| Increase in pro forma net tangible book value per share attributable to new investors in this offering | |
$ | | | |
| | |
| Pro forma as adjusted net tangible book value per share after giving effect to this offering | |
| | | |
$ | | |
| Dilution per share to new investors participating in this offering | |
| | | |
$ | | |
The
discussion and table above are based on 9,270,894 shares of our common stock outstanding as of December 28, 2023, which includes
9,210,751 shares outstanding as of September 30, 2023, as adjusted for the following subsequent issuances: (i) an aggregate
of 26,467 shares that were issued to employees pursuant to the vesting of restricted stock units, and (ii) 33,676 shares that were issued
on November 20, 2023 to various employees pursuant to the Company’s 2021 Employee Stock Purchase Plan, as amended, but excludes:
| ● |
8,430
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023 granted under the LBS 2013 Amended
and Restated Employee, Director, and Consultant Equity Incentive Plan, as amended and restated, or the 2013 Plan, with a weighted-average
exercise price of $1,054 per share; |
| |
|
| ● |
574,956
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023, granted under the 2021 Equity
Incentive Plan, as amended, or the 2021 Plan, with a weighted-average exercise price of $4.21 per share, which includes a total of
78,160 shares of common stock issuable upon exercise of outstanding stock options each with an exercise price of $0.59 that were
conditionally granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares
available under the 2021 Plan; |
| ● |
82,086
shares of common stock issuable upon exercise of outstanding stock options as of December 28, 2023 granted under the 2021 Inducement
Plan, with a weighted average exercise price of $4.90 per share; |
| |
|
| ● |
312,780
shares of common stock issuable upon vesting of restricted stock units outstanding as of December 28, 2023, granted under the 2021
Plan, which includes a total of 66,000 shares of common stock issuable upon vesting of restricted stock units that were conditionally
granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares available under
the 2021 Plan; |
| |
|
| ● |
50,144
shares of common stock issuable upon vesting of restricted stock units outstanding as of December 28, 2023, granted under the 2021
Inducement Plan; |
| |
|
| ● |
62,200
shares of common stock issuable upon vesting of restricted performance stock units outstanding as of December 28, 2023; all of which
were issued under the 2021 Plan which vest subject to certain milestones; |
| |
|
| ● |
5,397
shares of common stock reserved for future issuance under the 2021 Plan as of December 28, 2023, which excludes a total of 144,610
shares of common stock issuable upon exercise of outstanding stock options and outstanding restricted stock units that were conditionally
granted to our Chief Executive Officer and Chief Medical Officer on November 21, 2023 subject to sufficient shares being available
under the 2021 Plan, as well as any future automatic increases in the number of shares of common stock reserved for future issuance
under the 2021 Plan; |
| |
|
| ● |
110,871
shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan, or the ESPP, as of December 28,
2023, as well as any automatic increases in the number of shares of common stock reserved for future issuance under the ESPP; |
| |
|
| ● |
863,214
shares of common stock reserved for issuance under the 2021 Inducement Plan as of September 1, 2023; |
| |
|
| ● |
4,080,876
shares of common stock issuable upon exercise of outstanding warrants as of December 28, 2023 with a weighted-average exercise price
of $8.63 per share; and |
| |
|
| ● |
129
shares of common stock issuable upon conversion of the 200,000 outstanding shares of our Series A 4.5% Convertible Preferred Stock
as of December 28, 2023, as well as any future shares of common stock issuable upon conversion of additional shares of Series A 4.5%
Convertible Preferred Stock that may be issued as payment-in-kind dividends thereon in accordance with their terms. |
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other
than compensation arrangements, including employment, termination of employment and change in control arrangements, with our directors
and executive officers, and the other transactions discussed in the sections titled “Executive Compensation”, “Director
Compensation”, and “Certain Relationships and Related Party Transactions” in our Definitive Proxy Statement on Schedule
14A filed with the SEC on April 21, 2023 and incorporated by reference herein, the following is a description of each transaction since
January 1, 2020 and each currently proposed transaction in which:
| (i) | the
amounts involved exceeded or will exceed the lesser of (a) $120,000 or (b) 1% of the average
of our total assets for the fiscal years ended December 31, 2022 or 2021; and |
| (ii) | any
of our directors, executive officers or holders of more than 5% of our capital stock, or
any member of the immediate family of, or person sharing the household with, the foregoing
persons, had or will have a direct or indirect material interest. |
●
Effective May 15, 2023, Robert McRae, the Company’s then Chief Operating Officer (“COO”) transitioned to an executive
strategic consultant. Upon the transition, Mr. McRae ceased his duties and responsibilities as COO. For his services, Mr. McRae receives
ongoing monthly compensation of $4,000 per month until terminated by either party, and Mr. McRae’s outstanding equity awards continue
to vest through the date his consulting services cease.
●
Effective June 1, 2023, the Company increased J.D. Finley’s base salary from $490,000 to $542,000 contemporaneous with his
appointment from interim CEO to CEO. Additionally, Mr. Finley’s target cash bonus was increased from 45% to 50% of his base
salary. Additionally, on June 11, 2023, the Company granted Mr. Finley: (i) options to purchase 148,500 shares of common stock with
a term of ten (10) years and an exercise price of $1.60 per share, valued at $151,978 on the grant date and (ii) 66,700 restricted
stock units valued at $106,720. Each of the options and restricted stock units granted to Mr. Finley vest in twelve (12) equal
installments on a quarterly basis over three (3) years. The equity grants were issued from the Company’s 2021 Equity Incentive
Plan, as amended (the “2021 Plan”)
●
On June 11, 2023, the Company granted to the non-employee members of the Board of Directors, as supplemental grants, an aggregate
of: (i) options to purchase 77,380 shares of common stock with a term of ten (10) years and an exercise price of $1.60 per share and
(ii) 36,240 restricted stock units. Each of the grants vests fully on the one (1) year anniversary of the grant date. The aggregate
options were valued at $78,136 and the aggregate restricted stock units were valued at $57,984. The equity grants were issued from
the 2021 Plan.
●
On September 5, 2023, pursuant to his appointment as Chief Medical Officer, the Company issued Mitchell Jones, M.D., Ph.D. (i)
options to purchase 75,000 shares of common stock with a term of ten (10) years and an exercise price of $0.6897 per share and (ii)
54,700 restricted stock units. The options vest quarterly over three (3) years from the grant date and the restricted stock units
vest as follows: (a) 4,556 shares on November 6, 2023, and (b) the remaining 51,144 shares vest over eleven (11) equal quarterly
periods after the initial vesting date. The options were valued at $33,267 and the restricted stock units were valued at $37,727,
respectively from the grant date. The equity grants were issued from the Company’s 2021 Inducement Plan, as amended
(“Inducement Plan”).
●
On November 21, 2023, the Company granted J.D. Finley, its CEO, on a conditional basis until such time as there are sufficient
shares available under the 2021 Plan: (i) options to purchase 45,000 shares of common stock with a term of ten (10) years and an
exercise price of $0.59 per share, valued at $22,114 on the grant date and (ii) 38,000 restricted stock units valued at $22,420.
Each of the options and restricted stock units granted to Mr. Finley vest in twelve (12) equal installments on a quarterly basis
over three (3) years.
●
On November 21, 2023, the Company granted Mitchell Jones, M.D., Ph.D., its Chief Medical Officer, on a conditional basis until such
time as there are sufficient shares available under the 2021 Plan: (i) options to purchase 33,160 shares of common stock with a term
of ten (10) years and an exercise price of $0.59 per share, valued at $16,296 on the grant date and (ii) 28,000 restricted stock
units valued at $16,520. Each of the options and restricted stock units granted to Dr. Jones vest in twelve (12) equal installments
on a quarterly basis over three (3) years.
●
On November 21, 2023, the Company granted to each of the non-employee members of the Board of Directors, as supplemental grants: (i)
options to purchase 6,880 shares of common stock with a term of ten (10) years and an exercise price of $0.59 per share and (ii)
5,820 restricted stock units. Each of the grants vests fully on the one (1) year anniversary of the grant date. The aggregate of all
options were valued at $23,494 and the aggregate of all restricted stock units were valued at $24,037. The equity grants were issued
from the 2021 Plan.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth information regarding beneficial ownership of our capital stock as of December 28, 2023 by:
| |
● |
each
person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; |
| |
● |
each
of our named executive officers; and |
| |
● |
all
of our current executive officers and directors as a group. |
The
information in the following table is calculated based on 9,270,894 shares of our common stock outstanding as of December 28, 2023. Beneficial
ownership is determined according to the rules of the SEC. Beneficial ownership means that a person has or shares voting or investment
power of a security and includes any securities that person or group has the right to acquire within 60 days after the measurement date,
including upon the exercise of common stock purchase options or warrants or the conversion of preferred stock.
| Name of Beneficial Owner(1) | |
Number of Shares Beneficially Owned | | |
Percentage of Shares Beneficially Owned | |
| Greater than 5% Stockholders | |
| | | |
| | |
| Lind Global Fund II L.P. (2) | |
| 743,313 | | |
| 7.88 | % |
| L1 Capital Global Opportunities Master Fund Ltd. (3) | |
| 584,958 | | |
| 6.31 | % |
| | |
| | | |
| | |
| Directors and Named Executive Officers | |
| | | |
| | |
| James R. Neal(4) | |
| 1,290 | | |
| * | |
| Thomas Hallam, Ph.D.(5) | |
| 831 | | |
| * | |
| Stephanie C. Diaz(6) | |
| 1,258 | | |
| * | |
| Donald Williams(7) | |
| 21,258 | | |
| * | |
| Mary Ann Gray, Ph.D.(8) | |
| 1,046 | | |
| * | |
| Cristina Csimma, Pharm.D., MHP(9) | |
| 1,010 | | |
| * | |
| Robert J. Trenschel, D.O.(10) | |
| 46,306 | | |
| * | |
| Binxian Wei(11) | |
| 1,006 | | |
| * | |
| J.D. Finley(12) | |
| 124,536 | | |
| * | |
| Michael Dawson, M.D.(13) | |
| 300 | | |
| * | |
| Herbert Slade, MD FAAAAI (14) | |
| - | | |
| * | |
| Robert McRae (14) | |
| 10,435 | | |
| * | |
| Mitchell Jones, M.D., Ph.D. (15) | |
| 16,067 | | |
| * | |
| All directors and executive officers as a group (13 persons)(16) | |
| 225,343 | | |
| 2.41 | % |
| |
* |
Represents
less than one percent |
| |
|
|
| |
(1) |
Except
as otherwise indicated in the footnotes to this table, this table is based upon information supplied by officers, directors and principal
stockholders and Schedules 13D and 13G, and Form 4s, filed with the SEC. Unless otherwise indicated in the footnotes to this table
and subject to community property laws where applicable, we believe that each of the stockholders named in this table has sole voting
and investment power with respect to the shares indicated as beneficially owned. Shares of our common stock underlying options, warrants
and convertible securities that are currently exercisable or exercisable within 60 days of December 28, 2023 are deemed to be outstanding
for the purpose of computing the number of shares held and the percent of total ownership of the person holding those options, warrants
or convertible securities, but are not treated as outstanding for the purpose of computing the percent of total ownership of any
other person. Applicable percentages are based on 9,270,894 shares of common stock outstanding on December 28, 2023, adjusted as
required by rules promulgated by the SEC. Unless otherwise indicated, the address of the beneficial owner is c/o Palisade Bio, Inc.
7750 El Camino Real, Suite 2A, Carlsbad, CA 92009. |
| |
|
|
| |
(2) |
Includes
584,958 shares of common stock and (ii) 158,355 shares of common stock that may be acquired pursuant to the exercise of outstanding
warrants. The address of beneficial owner is 444 Madison Avenue, 41th Floor, New York, NY 10022. The information with respect to
beneficial owner was taken from a Schedule 13G filing, filed with the SEC on September 15, 2023. |
| |
|
|
| |
(3) |
Includes
584,958 shares of common stock. Excludes 40,000 common shares underlying common stock warrants with a 4.99% ownership limitation.
The address of beneficial owner is 161A Shedden Road, A Artillery Court, PO Box 10085, Grand Cayman, Cayman Islands, KY1-1001. The
information with respect to beneficial owner was taken from a Schedule 13G filing, filed with the SEC on September 15, 2023. |
| |
|
|
| |
(4) |
Includes
1,290 shares of common stock underlying stock options. |
| |
|
|
| |
(5) |
Includes
(i) 31 shares of common stock and (ii) 800 shares of common stock underlying common stock purchase warrants. Dr. Hallam ceased to
be an officer and director of the Company effective October 11, 2022. |
| |
|
|
| |
(6) |
Includes
1,290 shares of common stock underlying stock options. |
| |
|
|
| |
(7) |
Includes
(i) 20,000 shares of common stock and (ii) 1,258 shares of common stock underlying stock options. |
| |
|
|
| |
(8) |
Includes
(i) 80 shares of common stock and (ii) 966 shares of common stock underlying stock options. |
| |
|
|
| |
(9) |
Includes
(i) 44 shares of common stock and (ii) 966 shares of common stock underlying stock options. |
| |
|
|
| |
(10) |
Includes
(i) 1,282 shares of common stock underlying stock options held by Dr. Trenschel and (ii) (a) 36,287 shares of common stock and (ii)
8,737 shares of common stock that may be acquired within 60 days pursuant to the exercise of outstanding warrants held by Yuma Regional
Medical Center. The board of directors of Yuma Regional Medical Center, acting by a majority vote, has the authority to direct the
vote and/or disposition of any and all shares of common stock and warrants held by Yuma Regional Medical Center. The address of Yuma
Regional Medical Center is 2400 South Avenue A, Yuma, Arizona, 85364. Dr. Trenschel is the President, Chief Executive Officer and
member of the board of directors of Yuma Regional Medical Center and shares voting and investment power over the shares held by Yuma
Regional Medical Center. Dr. Trenschel also serves on our board of directors. |
| |
(11) |
Includes
(i) 40 shares of common stock and (ii) 966 shares of common stock underlying stock options. |
| |
|
|
| |
(12) |
Consists
of (i)(a) 63,340 shares of common stock held by Mr. Finley, (b) 2,008 shares of common stock that may be acquired pursuant to the
exercise of outstanding warrants held by Mr. Finley, (c) 7,951 shares of common stock underlying restricted stock units (RSUs) held
by Mr. Finley, (d) 50,427 shares of common stock underlying options held by Mr. Finley, (ii)(a) 777 shares of common stock held by
FCW Investments LLC, and (b) 33 shares of common stock underlying warrants held by FCW Investments, LLC. The address for FCW Investments
LLC is 19 Cherrymoor Dr, Englewood, CO 80113. Does not include 32,500 performance stock units (PSUs), which vest based on volume
weighted average trading price of the Company’s common stock – see “Certain Related Party Transactions” in
this Registration Statement on Form S-1 for a further discussion of the vesting conditions. |
| |
|
|
| |
(13) |
Includes
300 shares of common stock. Dr. Dawson ceased to be an officer of the Company effective October 11, 2022. |
| |
|
|
| |
(14) |
Dr.
Slade was appointed to serve as our Chief Medical Officer on November 17, 2022 and cease to be our Chief Medical Officer on September
5, 2023. |
| |
|
|
| |
(15) |
Includes
(i) 3,694 shares of common stock held by Mr. McRae, (ii) 220 shares of common stock that may be acquired pursuant to the exercise
of outstanding warrants held by Mr. McRae, (iii) 1,321 shares of common stock underlying restricted stock units (RSUs) held by Mr.
McRae, (iv) 5,200 shares of common stock underlying options held by Mr. McRae. Does not include 17,900 performance stock units (PSUs),
which vest based on volume weighted average trading price of the Company’s common stock – see “Certain Related
Party Transactions” in this Registration Statement on Form S-1 for a further discussion of the vesting conditions. Mr. McRae
was appointed to serve as our Chief Operating Officer (“COO”) on February 2, 2023. Effective May 15, 2023, Mr. McRae
transitioned from the Company’s COO to an executive strategic consultant. For his services as a consultant, Mr. McRae receives
monthly compensation of $4,000. The term of his consulting agreement may be extended by the mutual consent of the Company and Mr.
McRae after the initial six (6) month term. During the term of this agreement, all of Mr. McRae equity awards continue to vest. |
| |
|
|
| |
(16) |
Dr.
Jones was appointed to serve as our Chief Operating Officer on September 5, 2023. Includes (i) 2,926 shares of common stock held
by Dr. Jones, (ii) 6,891 shares of common stock underlying restricted stock units held by Dr. Jones, and (iii) 6,250 shares of common
stock underlying options held by Dr. Jones. |
| |
|
|
| |
|
|
| |
(17) |
Includes
the securities described in footnotes (3)-(16) above |
DESCRIPTION
OF CAPITAL STOCK
The
following description of our capital stock, certain provisions of our Amended and Restated Certificate of Incorporation, as amended (“Certificate
of Incorporation”), Amended and Restated Bylaws (“Bylaws”), Certificate of Designation of Preferences, Rights and Limitations
of Series A 4.5% Convertible Preferred Stock (“Series A Certificate of Designation”), Certificate of Designation of Preferences,
Rights and Limitations of Series B Convertible Preferred Stock (“Series B Certificate of Designation”), and certain provisions
of Delaware law are summaries. The following description is not complete and is subject to and qualified in its entirety by our Certificate
of Incorporation, Bylaws, Series A Certificate of Designation, and Series B Certificate of Designation, which are filed as exhibits to
the registration statement of which this prospectus is a part, as well as the relevant provisions of the Delaware General Corporation
Law (“DGCL”).
As
of the date of this prospectus, our authorized capital stock consists of 280,000,000 shares of common stock, par value $0.01 per share,
and 7,000,000 shares of preferred stock, par value $0.01 per share.
Common
Stock
Fully
Paid and Non-Assessable
All
outstanding shares of common stock are duly authorized, validly issued, fully paid, and nonassessable. All authorized but unissued shares
of our common stock are available for issuance by our Board without any further stockholder action, except as required by the listing
standards of the Nasdaq Stock Market.
Voting
Rights
Our
common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including
the election of directors, and does not have cumulative voting rights. Our Bylaws establish a classified Board that is divided into three
classes with staggered three-year terms. Only the directors in one class will be subject to election by a plurality of the votes cast
at each annual meeting of our stockholders, with the directors in the other classes continuing for the remainder of their respective
three-year terms.
Economic
Rights
Except
as otherwise expressly provided in our Certificate of Incorporation or required by applicable law, all shares of common stock have the
same rights and privileges and rank equally, share ratably, and are identical in all respects for all matters, including those described
below.
Dividends
and Distributions. Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock
are entitled to receive ratably those dividends, if any, as may be declared from time to time by the Board out of funds legally available
for that purpose.
Liquidation
Rights. In the event of our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably
in the assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock then outstanding.
Holders
of our common stock have no preemptive or conversion rights or other subscription rights and there are no redemption or sinking funds
provisions applicable to our common stock. The rights, preferences, and privileges of holders of common stock are subject to and may
be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the
future.
Preferred
Stock
Under
the terms of our Certificate of Incorporation, our Board has the authority, without further action by our stockholders, to issue up to
7,000,000 shares of preferred stock in one or more series pursuant to a resolution or resolutions providing for such issue duly adopted
by our Board. Our Board is further authorized, subject to limitations prescribed by law, to fix by resolution or resolutions the designations,
powers, preferences and rights, and the qualifications, limitations or restrictions thereof, of any wholly unissued series of preferred
stock, including without limitation authority to fix by resolution or resolutions the dividend rights, dividend rate, conversion rights,
voting rights, rights and terms of redemption (including sinking fund provisions), redemption price or prices, and liquidation preferences
of any such series, and the number of shares constituting any such series and the designation thereof, or any of the foregoing.
Our
Board may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or
other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible
acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in
our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of our common
stock.
Series
A 4.5% Convertible Preferred Stock
In
December 2016, we designated a series of our preferred stock as Series A 4.5% Convertible Preferred Stock consisting of 1,000,000 designated
shares (which is subject to increase without the consent of all of the holders of the Series A 4.5% Convertible Preferred Stock in the
event such additional shares of Series A 4.5% Convertible Preferred Stock are issued solely to the holders as payment of accrued dividends).
As
of December 28, 2023, we had outstanding 200,000 shares of Series A 4.5% Convertible Preferred Stock with a stated value of $12.79
per share held by one holder and which are immediately convertible into an aggregate of 129 shares of common stock. The Series A 4.5%
Convertible Preferred Stock have no provisions regarding subsequent securities issuances or so called “price protection provisions.”
The holders of Series A 4.5% Convertible Preferred Stock shall be entitled to receive dividends in cash or additional shares of Series
A 4.5% Convertible Preferred Stock if and when declared by our Board in preference to the payment of any dividends on our common stock.
The holders of Series A 4.5% Convertible Preferred Stock shall have no voting rights but shall be entitled to appoint one member to our
Board. This right to appoint a member of the Board will terminate when there are less than 200,000 shares of Series A 4.5% Convertible
Preferred Stock outstanding. As long as any shares of Series A 4.5% Convertible Preferred Stock are outstanding, we shall not, without
the affirmative vote of the holders of a majority of the then outstanding shares of the Series A 4.5% Convertible Preferred Stock, alter
or change adversely the powers, preferences or rights given to the Series A 4.5% Convertible Preferred Stock or alter or amend the Certificate
of Designation, other than to authorize and issue additional shares of Series A 4.5% Convertible Preferred Stock. In addition, holders
of Series A 4.5% Convertible Preferred Stock are subject to beneficial ownership limitations.
Options
As
of December 28, 2023, we had outstanding stock options to purchase an aggregate of 665,472 shares of common stock issued pursuant to
(i) Leading BioSciences 2013 Amended and Restated, Employee, Director and Consultant Equity Incentive Plan, (ii) Palisade Bio 2021 Equity
Incentive Plan, and (iii) Palisade 2021 Inducement Plan, each as amended. The options have a remaining weighted average term of approximately
9.37 years and an average weighted exercise price of $17.60 per share. The amounts described herein include an aggregate of 78,160 options
that were issued on a conditional basis until such time as sufficient shares are available under the Company’s 2021 Equity Incentive
Plan.
Restricted
Stock Units
As
of December 28, 2023, we had an aggregate of 362,924 shares of common stock underlying restricted stock units. The restricted stock units
typically vest on a quarterly basis between one (1) to three (3) years and currently have a remaining average vesting term of approximately
2.17 years. The amounts described herein include an aggregate of 66,000 restricted stock units that were issued on a conditional basis
until such time as sufficient shares are available under the Company’s 2021 Equity Incentive Plan.
Performance
Restricted Stock Units
As
of December 28, 2023, we had an aggregate of 62,200 restricted performance stock units that vest (a) 50% when the volume weighted average
price of our Common Stock over 20 consecutive trading days is $3.20, and (b) 50% when such volume weighted average price of our Common
Stock over 20 consecutive trading days is $4.25.
Warrants
As
of December 28, 2023, we had outstanding common stock purchase warrants to purchase an aggregate of 4,080,876 shares of common stock,
with a remaining average term of 4.12 years and a weighted average exercise price of $8.63 per share.
Anti-Takeover
Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
Some
provisions of Delaware law, our Certificate of Incorporation and our Bylaws contain provisions that could make the following transactions
more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise; or the
removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could
deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions
which provide for payment of a premium over the market price for our shares.
These
provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids. These provisions are
also designed to encourage persons seeking to acquire control of us to first negotiate with our Board. We believe that the benefits of
the increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire
or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an
improvement of their terms.
Section
203 of the DGCL
We
are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in any business combination with any interested
stockholder for a period of three years after the date that such stockholder became an interested stockholder, subject to certain exceptions.
Amended
and Restated Bylaws
Board
Composition and Filling Vacancies. Our Bylaws provide for our Board to be divided into three classes with staggered three-year terms.
Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder
of their respective three-year terms. Because our stockholders do not have cumulative voting rights, the holders of a plurality of the
voting power of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors,
can elect all of the directors standing for election, if they so choose, other than any directors that holders of any preferred stock
we have or may issue may be entitled to elect. Our Bylaws also provide that subject to the rights of the holders of any series of preferred
stock then outstanding, any director or the entire Board may be removed from office at any time, with or without cause, by the affirmative
vote of the holders of at least a majority of the voting power of the issued and outstanding shares of capital stock of the Company then
entitled to vote in the election of directors.
Special
Meeting of Stockholders. Our Bylaws also provides that a special meeting of stockholders may be called only by our chairperson of
the Board, chief executive officer or president, the secretary or any two directors.
Advance
Notice Requirements. Our Bylaws also establish advance notice procedures with respect to certain stockholder proposals to be brought
before a stockholder meeting and the nomination of candidates for election as directors.
Amendment
to Bylaws. The Board is expressly empowered to adopt, amend or repeal the Bylaws. The stockholders shall also have power to adopt,
amend or repeal the Bylaws; provided, however, that, in addition to any vote of the holders of any class or series of stock of the Company
required by law or by the Certificate of Incorporation, the affirmative vote of the holders of at least a majority of the voting power
of all of the then outstanding shares of capital stock of the Company entitled to vote generally in the election of directors, voting
together as a single class, shall be required to adopt, amend or repeal any provision of the Bylaws.
The
provisions of Delaware law, our Certificate of Incorporation and our Bylaws could have the effect of discouraging others from attempting
hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often
result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in the composition
of our Board and management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders
may otherwise deem to be in their best interests.
Choice
of Forum
Our
Bylaws provide that unless we consent in writing to the selection of an alternative forum, the sole and exclusive forum for (i) any derivative
action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director,
officer or other employee of the Company to the Company or the Company’s stockholders, (iii) any action asserting a claim arising
pursuant to any provision of the DGCL or the Certificate of Incorporation or Bylaws (as either may be amended from time to time), or
(iv) any action asserting a claim governed by the internal affairs doctrine shall be the Court of Chancery in the State of Delaware (or,
if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware). If any action the subject
matter of which is within the scope of the preceding sentence is filed in a court other than a court located within the State of Delaware
(a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (i) the personal
jurisdiction of the state and federal courts located within the State of Delaware in connection with any action brought in any such court
to enforce the preceding sentence and (ii) having service of process made upon such stockholder in any such action by service upon such
stockholder’s counsel in the Foreign Action as agent for such stockholder.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC. We act as the transfer agent and
registrar for our Series A 4.5% Convertible Preferred Stock and Series B Convertible Preferred Stock to the extent any shares are outstanding.
Listing
on the Nasdaq Capital Market
Our
common stock is listed on The Nasdaq Capital Market under the symbol “PALI.”
DESCRIPTION
OF SECURITIES WE ARE OFFERING
The
following summary of certain terms and provisions of the securities that are being offered hereby is not complete and is subject to,
and qualified in its entirety by, the provisions of the underlying securities, the forms of which are filed as exhibits to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the forms
of securities for a complete description of the terms and conditions.
Common
Stock
The
material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common stock are
described under the caption “Description of Capital Stock” in this prospectus.
UNDERWRITING
We
have entered into an underwriting agreement dated , with (“ ,” “underwriter,” or “Representative”)
as the sole book-running manager. Subject to the terms and conditions of the underwriting agreement, the underwriter has agreed to purchase
the number of our securities set forth opposite its name below:
| Underwriter | |
Common Shares | |
| | |
| | |
| | |
| | |
| Total | |
| | |
A
copy of the underwriting agreement will be filed as an exhibit to the registration statement of which this prospectus forms a part.
Certain
investors in this offering may enter into leak-out agreements wherein each investor who is party thereto (together with certain of its
affiliates) will agree not to sell, dispose or otherwise transfer, directly or indirectly (including, without limitation, any sales,
short sales, swaps or any derivative transactions that would be equivalent to any sales or short positions), on any trading day, shares
of our common stock, including shares of common stock purchased in this offering in an amount more than a specified percentage of the
trading volume of the common stock on the principal trading market, subject to certain exceptions. This restriction will not apply to
sales or transfers of any such shares of common stock in transactions which do not need to be reported on the Nasdaq consolidated tape
so long as the purchaser or transferee executes and delivers a leak-out agreement. After such sale or transfer, future sales of the securities
covered by the leak-out agreement entered into by the original owner (together with certain of its affiliate) and the purchaser or transferee
will be aggregated to determine compliance with the terms of the leak-out agreement.
We
have been advised by the underwriter that it proposes to offer the common shares directly to the public at the public offering
price set forth on the cover page of this prospectus. Any securities sold by the underwriter to securities dealers will be sold at the
public offering price less a selling concession not in excess of $ per share.
The
underwriting agreement provides that subject to the satisfaction or waiver by the Representative of the conditions contained in the underwriting
agreement, the underwriter is obligated to purchase and pay for all of the common shares offered by this prospectus.
No
action has been taken by us or the underwriter that would permit a public offering of the shares of common stock in any jurisdiction
outside the United States where action for that purpose is required. None of our securities included in this offering may be offered
or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer
and sales of any of the securities offered hereby be distributed or published in any jurisdiction except under circumstances that will
result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised
to inform themselves about and to observe any restrictions relating to this offering of securities and the distribution of this prospectus.
This prospectus is neither an offer to sell nor a solicitation of any offer to buy the securities in any jurisdiction where that would
not be permitted or legal.
The
underwriter has advised us that it does not intend to confirm sales to any account over which it exercises discretionary authority.
Underwriting
Discount and Expenses
The
following table summarizes the underwriting discount and commission to be paid to the underwriter assuming no exercise of the over-allotment
option and assuming the full exercise of the over-allotment option.
| | |
Per Common Share(1) | | |
Total With No Exercise of the Over- Allotment Option | | |
Total With Full Exercise of the Over- Allotment Option | |
| Public offering price | |
| | | |
| | | |
| | |
| Underwriting discount to be paid to the underwriter by us(2)(3) | |
| | | |
| | | |
| | |
| Proceeds to us (before expenses) | |
| | | |
| | | |
| | |
| (1) |
The
public offering price and underwriting discount corresponds a public offering price per share of common stock of $ . |
| (2) |
We
have granted a day option to the underwriter to purchase additional shares of common stock (up to % of the number of shares of common
stock at the public offering price per share of common stock set forth above less the underwriting discounts and commissions, solely
to cover overallotments, if any. |
| (3) |
We
have agreed to pay an underwriter discount equal to % of the aggregate gross proceeds raised in this offering. In addition, we have
agreed to reimburse the reasonable out-of-pocket expenses of the Representative in an amount not to exceed $ . |
We
estimate the total expenses payable by us for this offering to be approximately $ million ($ million of the over-allotment option is
exercised in full), which amount includes (i) the underwriting discount of $ million ($ million if the over-allotment option is exercised
in full) and (ii) other estimated company expenses of approximately $ , which includes legal, accounting, printing costs and various
fees associated with the registration and listing of our shares.
The
securities we are offering are being offered by the underwriter subject to certain conditions specified in the underwriting agreement.
Overallotment
Option
We
have granted to the underwriter an option exercisable not later than days after the date of this prospectus to purchase up to a number
of additional shares of common stock not to exceed % of the number of shares of common stock sold in this offering (excluding any shares
of common stock issued upon any exercise of the underwriter’s overallotment option) at the public offering price per share of common
stock set forth on the cover page hereto less the underwriting discounts and commissions. The underwriter may exercise the option solely
to cover overallotments, if any, made in connection with this offering. If any additional shares of common stock are purchased pursuant
to the overallotment option, the underwriters will offer these shares of common stock on the same terms as those on which the other securities
are being offered.
Determination
of Offering Price
Our
common stock is currently traded on The Nasdaq Capital Market under the symbol “PALI.” On December 28, 2023, the closing
price of our common stock was $0.6539 per share.
The
public offering price of the securities offered by this prospectus will be determined by negotiation between us and the underwriter.
Among the factors that will be considered in determining the public offering price of the shares:
| |
● |
Our
history and our prospects; |
| |
● |
The
industry in which we operate; |
| |
● |
Our
past and present operating results; and |
| |
● |
The
general condition of the securities markets at this time of this offering. |
The
offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the shares of
common stock sold in this offering. That price is subject to change as a result of market conditions and other factors and we cannot
assure you that the shares of common stock sold in this offering can be resold at or above the public offering price.
Lock-up
Agreements
Our
officers and directors have agreed with the underwriter to be subject to a lock-up period of days following the date of this prospectus.
This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, distribute, grant any option,
right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or
any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted
during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed, in the underwriting agreement,
to similar lock-up restrictions on the issuance and sale of our securities for days following the effectiveness of the underwriting agreement,
although we will be permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. The
underwriter may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements. We have also agreed,
in the underwriting agreement, to similar lock-up restrictions on the issuance and sale of our securities from the date of this prospectus
until the later of (i) days following the closing of this offering. The underwriter may, in their sole discretion and without notice,
waive the terms of any of these lock-up agreements.
Certain
investors in this offering have agreed with the representative to enter into a lock-up and voting agreement whereby each such investor
will be subject to a lock-up period for three trading days following the pricing of this offering. This means that, during the applicable
lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase,
pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into,
or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if
the transferee agrees to these lock-up restrictions. Additionally, each such investor has agreed to vote all shares of common stock it
beneficially owns, including such common stock obtained in this offering, with respect to any proposals presented to the stockholders
of the Company at a meeting with a record date prior to the expiration of the lock-up.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC.
Stabilization,
Short Positions and Penalty Bids
The
underwriter may engage in syndicate covering transactions stabilizing transactions and penalty bids or purchases for the purpose of pegging,
fixing or maintaining the price of our common stock:
| |
● |
Syndicate
covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover
syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short
position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the
securities in the open market after pricing that could adversely affect investors who purchase in the offering. |
| |
● |
Stabilizing
transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific maximum and
are engaged in for the purpose of preventing or retarding a decline in the market price of the shares of common stock while this
offering is in progress. |
| |
● |
Penalty
bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate
member are purchased in a stabilizing or syndicate covering transaction to dover syndicate short positions. |
These
syndicate covering transactions, stabilizing transactions, and penalty bids may have the effect of raising or maintaining the market
prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result the price of our common
stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make any representation
or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may
be effected on The Nasdaq Capital Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued
at any time.
In
connection with this offering, the underwriter also may engage in passive market making transactions in our common stock in accordance
with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending
through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest
independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must
then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at
a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither
we, nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described
above may have on the prices of our securities. In addition, neither we nor the underwriters make any representation that the underwriter
will engage in these transactions or that any transactions, once commenced will not be discontinued without notice.
Indemnification
We
have agreed to indemnify the underwriter against certain liabilities, including certain liabilities arising under the Securities Act
or to contribute to payments that the underwriters may be required to make for these liabilities.
LEGAL
MATTERS
The
validity of the shares of common stock being offered hereby will be passed upon for us by Silvestre Law Group, P.C., Westlake Village,
California. The underwriter is being represented by .
EXPERTS
The
consolidated financial statements of Palisade Bio, Inc. as of December 31, 2022 and for the year then
ended, incorporated by reference in this Prospectus, have been audited by Baker Tilly US, LLP, an independent registered public
accounting firm, as stated in their report, which is incorporated herein by reference. Such consolidated financial statements have
been so incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and
auditing. The report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s
ability to continue as a going concern.
The consolidated financial statements of Palisade
Bio, Inc. (the “Company”) as of December 31, 2021 and for the year then ended incorporated by reference in this Prospectus
and in the Registration Statement have been so incorporated in reliance on the report of BDO USA, LLP (n/k/a BDO USA, P.C.), an
independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The
report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as
a going concern.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the securities being offered by
this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further
information with respect to us and the securities offered by this prospectus, we refer you to the registration statement and its exhibits.
Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete,
and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each
of these statements is qualified in all respects by this reference.
We
are subject to the information and periodic reporting requirements of the Exchange Act, and we file periodic reports, proxy statements
and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s
website at www.sec.gov. You may also request a copy of these filings, at no cost, by writing us at Palisade Bio, Inc., 7750 El Camino
Real, Suite 2A, Carlsbad, CA 92009 or telephoning us at (858) 704-4900. We also maintain a website at www.palisadebio.com, at which you
may access these materials free of charge after they are electronically filed with, or furnished to, the SEC. The information contained
in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus and any references
to this web site or any other web site are inactive textual references only.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information
to you by referring to those documents. The information incorporated by reference is an important part of this prospectus, and information
that we file later with the SEC will automatically update and supersede this information.
We
incorporate by reference the following documents we filed with the SEC pursuant to Section 13 of the Exchange Act and any future filings
we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of this prospectus until the termination
of the offering of the securities covered by this prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K):
| |
● |
our
Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 22, 2023; |
| |
|
|
| |
● |
Our
Quarterly Reports on Form 10-Q for the periods ended March 31, 2023, June 30, 2023, and September 30, 2023, filed with the SEC on May 11, 2023, August 10, 2023, and November 9, 2023, respectively. |
| |
|
|
| |
● |
Our
Current Reports Form 8-K filed with the SEC on January
4, 2023, February
8, 2023, March
13, 2023, April
5, 2023, June
9, 2023, September
8, 2023, September
11, 2023, September
11, 2023, October
20, 2023, and November 7, 2023. |
| |
|
|
| |
● |
our
Definitive Proxy Statement on Schedule 14A, filed with the SEC on April 21, 2023 (other than the portions thereof which are furnished
and not filed); and |
| |
|
|
| |
● |
the
description of our common stock which is registered under Section 12 of the Exchange Act, in our registration statement on Form 8-A
filed with the SEC on July 1, 2015, including any amendments or reports filed for the purpose of updating such description, including
Exhibit 4.2 to our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 17, 2022. |
You
may access the Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, Proxy Statements, and amendments,
if any, to those documents filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at the
SEC’s website (www.sec.gov) or our website (www.palisadebio.com) as soon as reasonably practicable after such material is electronically
filed with, or furnished to, the SEC. The reference to our website does not constitute incorporation by reference of the information
contained in our website. We do not consider information contained on, or that can be accessed through, our website to be part of this
prospectus or the related registration statement.
We
will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request,
a copy of any or all of the information that is incorporated by reference into this prospectus but not delivered with the prospectus,
including exhibits which are specifically incorporated by reference into such documents. You should direct any requests for documents
to 7750 El Camino Real, Suite 2A, Carlsbad, CA 92009, Attn: Secretary or may be made telephonically at (858) 704-4900.

[*]
shares of Common Stock
PRELIMINARY
PROSPECTUS
[UNDERWRITER]
You
should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information
that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities
in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is correct only as of the
date of this prospectus, regardless of the time of the delivery of this prospectus or any sale of these securities.
The
date of this prospectus is , 20__
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The
following table sets forth the expenses to be incurred in connection with the offering described in this registration statement, all
of which will be paid by the registrant. All amounts are estimates except the SEC registration fee.
| | |
Amount | |
| SEC registration fee | |
$ | 848.70 | |
| FINRA filing fee | |
$ | [*] | |
| Printing expenses | |
$ | [*] | |
| Legal fees and expenses | |
$ | [*] | |
| Accounting fees and expenses | |
$ | [*] | |
| Transfer agent fees and expenses | |
$ | [*] | |
| Miscellaneous fees and expenses | |
$ | [*] | |
| | |
| | |
| Total | |
$ | 848.70 | |
Item 14. Indemnification of Directors and Officers
We
are incorporated under the laws of the State of Delaware. Sections 145 and 102(b)(7) of the General Corporation Law of the State of Delaware
provide that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive
officer, employee or agent of the corporation or is or was serving at the request of the corporation against expenses (including attorneys’
fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action
if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the
corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful,
except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim
as to which such person is adjudged to be liable to the corporation.
Our
amended and restated certificate of incorporation contains provisions that eliminate, to the maximum extent permitted by the General
Corporation Law of the State of Delaware, the personal liability of directors and executive officers for monetary damages for breach
of their fiduciary duties as a director or officer. Our amended and restated certificate of incorporation and bylaws provide that we
shall indemnify our directors and executive officers and may indemnify our employees and other agents to the fullest extent permitted
by the General Corporation Law of the State of Delaware.
We
have purchased and intend to maintain insurance on behalf of any person who is or was a director or officer of our company against any
loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
We
have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers to
provide these directors and executive officers additional contractual assurances regarding the scope of the indemnification set forth
in the registrant’s amended and restated certificate of incorporation and amended and restated bylaws and to provide additional
procedural protections. At present, there is no pending litigation or proceeding involving a director or executive officer of the Company
regarding which indemnification is sought. The indemnification provisions in our amended and restated certificate of incorporation, amended
and restated bylaws and the indemnification agreements entered into or to be entered into between us and each of our directors and executive
officers may be sufficiently broad to permit indemnification of our directors and executive officers for liabilities arising under the
Securities Act. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers
or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the
Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable.
We carry liability insurance for our directors and officers.
Item
15. Recent Sales of Unregistered Securities
Set
forth below is information regarding securities sold by us since January 1, 2020 that were not registered under the Securities Act. Also
included is the consideration, if any, received by us for such shares and options and information relating to the section of the Securities
Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.
(a)
Issuances of Securities
| |
1. |
On
January 17, 2020, we entered into a letter agreement (“Letter Agreement”) with certain institutional holders warrant
holders of our Series M Warrants and Series N Warrants (each as defined below) (collectively, the “Holders”), pursuant
which each Holder will received: |
| |
a.
|
one
(1) Series P common stock replacement warrant (“Series P Warrant”) for every one (1) share purchased upon exercise of
outstanding Series M common stock purchase warrants (“Series M Warrants”) issued on July 30, 2019 in the Company’s
registered offering; and |
| |
b.
|
one
(1) Series Q common stock replacement warrant (“Series Q Warrant”) for every one (1) share purchased upon exercise of
outstanding Series N common stock purchase warrants (“Series N Warrants”) issued on July 30, 2019 in the Company’s
registered offering |
Pursuant
to the Letter Agreement, and as an inducement to exercise the Series M Warrants and Series N Warrants, the exercise price of the Holder’s
Series M Warrants and Series N Warrants are being reduced from $810 to $408 per share. The Holders collectively owned (i) 9,259 Series
M Warrants and (ii) 9,259 Series N Warrants. The Company received gross proceeds of approximately $7,555,553, not including closing costs
and placement agent fees. In connection with the transactions contemplated in the Letter Agreement, we entered into a letter agreement
(the “Wainwright Placement Agent Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”), pursuant to
which Wainwright was entitled to (i) a cash fee equal to 8% of the gross proceeds raised in the transactions contemplated by the Letter
Agreement, (ii) a common stock purchase warrant equal to 148 shares of common stock issued in the Offering with an exercise price of
$510, and a term of five (5) years (the “Placement Agent Warrant”), (iii) a management fee equal to 1.0% of the gross proceeds
raised, (iv) $35,000 for non-accountable expenses; and (v) up to $90,000 in expenses of legal counsel and other out-of-pocket closing
expenses. Additionally, Wainwright received a tail fee of 8% cash and 8% warrant coverage as described above with respect to any additional
financing completed with any investors that entered into the Letter Agreement within the 12-month period following date of the Letter
Agreement. Additionally, for a period of ten (10) months following the closing of the transaction contemplated by the Letter Agreement,
Wainwright will have a right of first refusal to act as the sole book-running manager, underwriter, or placement agent for any future
capital raising transactions.
| |
2. |
In
connection with our May 22, 2020 registered offering, we issued warrants to purchase up to 1,333 shares of common stock to Wainwright
Placement Agent, which were not registered at the time of issuance. |
| |
|
|
| |
3.
|
Immediately
prior to the Merger with Seneca Biopharma, Inc., we and LBS completed a private placement transaction (the “Pre-Merger Financing”)
with Altium Growth Fund, LP (“Altium”) pursuant to that certain Securities Purchase Agreement, by and among us, LBS and
the Investor, dated December 16, 2020, as amended (the “Securities Purchase Agreement”), for an aggregate purchase price
of $20.0 million. In connection with the closing of the Merger and the Pre-Merger Financing, we issued to Ecoban Securities, LLC
(i) a warrant to purchase 367 shares of the Company’s common stock at a price of $886 per share and (ii) 2,376 shares of common
stock, as payment for a success fee for closing the Merger and Pre-Merger Financing. |
| |
4.
|
On
May 20, 2021, we issued to Altium a warrant to purchase 99,917 shares of common stock at a price of $276.50 per share. The warrant
was immediately exercisable and has a term of five years from the date all of the shares underlying the warrant have been registered
for resale. |
| |
|
|
| |
5. |
In
2020, LBS sold to certain holders unsecured promissory notes in the aggregate principal amount of $0.6 million and warrants to purchase
an aggregate of 70,000 shares of common stock of LBS at an exercise price of $0.73 per share (the “Prior Warrants”).
In connection with the Merger, such warrants automatically converted into warrants to purchase an aggregate of 38 shares of our common
stock at a purchase price of $1,342 per share. On May 25, 2021, we amended such notes originally issued by LBS in 2020 in order to
extend the maturity date. In connection with such amendments, the Prior Warrants were canceled, and the Company issued warrants to
the noteholders to purchase an aggregate of 160 shares of Company common stock at a purchase price of $300 per share. |
| |
|
|
| |
6. |
Effective
July 21, 2021, we entered into a Waiver and Amendment Agreement with Altium pursuant to which Altium agreed to waive certain rights,
waive reset provisions with respect to the exercise price and number of shares subject to outstanding warrants held by Altium, eliminate
certain financing restrictions, and accelerate registration rights for the shares underlying the warrants. As consideration for the
foregoing, pursuant to the Waiver Agreement, we issued Altium an additional warrant to purchase up to 22,000 shares of our common
stock with an exercise price of $181.55 per share. The warrant is exercisable beginning six months following the date on which the
underlying shares are registered for resale and for five years thereafter. |
| |
|
|
| |
7.
|
On
August 19, 2021, we issued 30,197 shares of common stock and a warrant to purchase up to 7,549 shares of common stock to Yuma Regional
Medical Center for a total purchase price of $5,209,141.20. The shares were sold at a purchase price of $172.50 per share. The warrant
has an exercise price of $172.50 per share, subject to certain adjustments, and is exercisable for five years. |
| |
|
|
| |
8.
|
Effective
January 31, 2022, we and Altium entered into a Waiver and Amendment Agreement, pursuant to which Altium agreed to waive any adjustment
to the exercise price of the existing warrants held by it from and after such date for issuances of equity or equity-linked securities
at a price below the exercise price of the warrants. The Waiver Agreement also includes agreement to, among other things, (i) restrict
Altium’s ability to sell our securities through a “leak out” provision whereby sales are restricted by applying
a volume limitation, (ii) shorten the notice period for Altium’s participation rights related to certain future securities
offerings, (iii) restrict our ability to conduct a primary offering of our securities for a specified period of time, and (iv) provide
registration rights for the shares underlying the warrant issued to Altium in consideration of the foregoing. As consideration for
the foregoing, pursuant to the Waiver Agreement, we issued Altium an additional warrant to purchase up to 45,000 shares of our common
stock. The warrant is exercisable beginning six months following January 31, 2022 and the exercise price is $55 (the closing price
of our common stock on January 28, 2022), subject to customary adjustments for stock splits, stock dividends, stock combinations,
reclassifications and similar transactions. |
| |
9.
|
On
May 6, 2022, we entered into a securities purchase agreement with certain institutional and accredited investors, pursuant to which
we agreed to sell and issue, in a registered direct offering, an aggregate of 72,933 shares of our common stock, par value
$0.01 per share, at a purchase price per share of $27.50, for aggregate gross proceeds to us of approximately $2.0 million, before
deducting fees payable to the placement agent and other estimated offering expenses payable by us. The shares were offered pursuant
to an effective shelf registration statement on Form S-3. In connection with such offering, in a concurrent private placement, we
also agreed to sell and issue to such purchasers warrants to purchase up to 72,933 shares of common stock at an exercise price of
$35.525 per share, the closing bid price of our common stock on May 5, 2022. Such warrants are not exercisable until six months following
the date of issuance and expire five and a half years from the date of issuance. In addition, pursuant to a placement agency agreement
dated as of May 6, 2022, we engaged Ladenburg Thalmann & Co. Inc. (“Ladenburg”) to act as our exclusive placement
agent in connection with the aforementioned registered offering and private placement offering. We agreed to pay Ladenburg a cash
fee equal to 7.75% of the aggregate gross proceeds raised in the aforementioned registered offering and private placement offering
and to reimburse its expenses up to an aggregate of $85,000. In addition, we issued Ladenburg warrants on substantially the same
terms as the warrants issued to the purchasers in an amount equal to 6.0% of the aggregate number of shares sold in the offering,
or 4,376 shares of common stock, at an exercise price of $35.525 per share and a five-and-a-half year term. The placement agent warrants
are not exercisable until six months following the date of issuance. |
| |
10. |
On
December 30, 2022, we entered into securities purchase agreements with certain institutional and accredited investors, pursuant to
which we agreed to sell and issue, in a registered direct offering, an aggregate of (i) 476,842 shares of our common stock,
par value $0.01 per share, at a purchase price per share of $2.375 and (ii) registered prefunded warrants to purchase 37,000 shares
of common stock at an exercise price of $0.0001 per share. The transaction closed on January 4, 2023. The shares were offered pursuant
to an effective shelf registration statement on Form S-3. In connection with such offering, in a concurrent private placement, we
also agreed to sell and issue to such purchasers (i) Prefunded Warrants to purchase 538,789 shares of common stock at an exercise
price of $0.0001 per share and (ii) Warrants to purchase 1,052,631 shares of common stock at an exercise price of $2.375 per share.
Such Prefunded Warrants and Warrants are immediately exercisable and expire five years from the date of issuance. Gross proceeds
for the registered offering and concurrent private placement were approximately $2.5 million, before deducting fees payable to the
placement agent and other estimated offering expenses payable by us. In addition, pursuant to a placement agency agreement dated
as of December 30, 2022, we engaged Ladenburg to act as our exclusive placement agent in connection with the aforementioned registered
offering and private placement offering. We agreed to pay Ladenburg a cash fee equal to 7.75% of the aggregate gross proceeds raised
in the aforementioned registered offering and private placement offering and to reimburse its expenses up to an aggregate of $105,000.
In addition, we issued Ladenburg warrants on substantially the same terms as the warrants issued to the purchasers in an amount equal
to 6.0% of the aggregate number of shares sold in the offering, or 63,158 shares of common stock, at an exercise price of $2.9688
per share and a five-year term. |
| |
|
|
| |
11. |
On
April 3, 2023, we entered into securities purchase agreements with certain institutional and accredited investors, pursuant to which
we agreed to sell and issue, in the Registered Offering, an aggregate of 756,317 shares of our common stock. The transaction
closed on April 5, 2023. Concurrently, in the Private Offering we offered and sold an aggregate of: (i) 455,242 unregistered shares
of Common Stock, (ii) Prefunded Warrants to purchase 1,061,164 shares of common stock at an exercise price of $0.0001 per share and
(iii) Warrants to purchase 2,272,723 shares of common stock at an exercise price of $2.69 per share. Such Prefunded Warrants and
Warrants are immediately exercisable and expire five years from the date of issuance. In addition, pursuant to a placement agency
agreement dated as of April 3, 2023, we engaged Ladenburg to act as our exclusive placement agent in connection with the April 2023
Offering. We agreed to pay Ladenburg a cash fee equal to 7.75% of the aggregate gross proceeds received from the April 2023 Offering
or $465,000 and we reimbursed its expenses of $85,000. In addition, we issued Ladenburg 136,363 Placement Agent Warrants. |
| |
|
|
| |
12. |
On
September 7, 2023, we entered into securities purchase agreements with certain institutional and accredited investors, pursuant to
which we agreed to sell and issue, in a Registered Offering, an aggregate of 2,339,398 shares of our common stock. The transaction
closed on September 11, 2023. In addition, pursuant to a placement agency agreement dated as of September 7, 2023, we engaged Ladenburg
to act as our exclusive placement agent in connection with the September 2023 Offering. We agreed to pay Ladenburg a cash fee equal
to 7.75% of the aggregate gross proceeds received from the September 2023 Offering or $152,294 and we reimbursed its expenses of
$75,000. In addition, we issued Ladenburg 140,364 Placement Agent Warrants. The Placement Agent Warrants have a term of five (5)
years and an exercise price of $1.05 per share. |
| |
|
|
| |
13. |
On
December 15, 2023, we entered into an engagement letter with MDM Worldwide Solutions, Inc. for the purpose of providing us with business
advisory services. Pursuant to the agreement, in the event that we choose to renew the six (6) month term, we will be obligated to
issue the consultant $50,000 of common stock based on the closing price of the Company’s common stock on the date of the agreement,
or an aggregate of 84,048 common shares. |
The
offers, sales and issuances of the securities described in this section (a) of Item 15 were deemed to be exempt from registration under
the Securities Act in reliance on Section 4(a)(2) of the Securities Act or Rule 506 of Regulation D promulgated under the Securities
Act as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired
the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends
were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited
investor within the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business
or other relationships, to information about the Company.
(b)
Issuances of Equity Awards
| |
1. |
On
April 1, 2020, Seneca Biopharma entered into employment agreements with each of Dane Saglio and Matthew Kalnik, PhD. As an inducement
to Mr. Saglio’s employment, we granted him a non-qualified inducement option to purchase up to 235 shares of Common Stock on
such date. As an inducement to Dr. Kalnik’s employment, we granted him a non-qualified inducement option to purchase up to
942 shares of Common Stock on such date. These inducement options had an exercise price of $185.97 per share, a term of ten (10)
years, and vests as follows: (i) one quarter (1/4) of the options vest on April 1, 2020, and (ii) the remaining three-quarters (3/4)
of the options will vest on a monthly basis over the thirty-six (36) month period following such date. This inducement option was
issued from the Company’s Inducement Award Stock Option Plan (“Inducement Plan”). |
| |
|
|
| |
2.
|
Effective
April 2020, we granted Dr. Kenneth Carter, our then-Executive Chairman, a conditional option grant to purchase 1,571 shares of common
stock, subject to the receipt of shareholder approval as well as the forfeiture of all of his previously issued vested and unvested
grants. The option grant had a term of ten (10) years, and an exercise price of $185.97. The option vests (i) one quarter (1/4) on
the effective date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the effective
date, provided Dr. Carter remains a service provider to the Company over such period. For a period of nine (9) months, subject to
adjustment upon the Company’s issuance of common stock including by virtue of exercise, conversion or exchange of common stock
equivalents, the shares underlying the options are subject to adjustment to maintain the percentage ownership that the option grant
reflects on the date of grant. This resulted in the grant being increased to 2,882 shares of common stock through December 31, 2020.
This grant was approved by shareholders on September 9, 2020. |
| |
3. |
Effective
April 2020, our Senior Vice President of Research and Development received a conditional option grant to purchase 314 shares of common
stock, subject to the receipt of shareholder approval as well as the forfeiture of all of his previously issued vested and unvested
grants. The option grant has a term of ten (10) years, and an exercise price of $185.97. The option vests (i) one quarter (1/4) on
the effective date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the effective
date, provided that such individual remains a service provider to the Company over such period. For a period of nine (9) months,
subject to adjustment upon the Company’s issuance of common stock including by virtue of exercise, conversion or exchange of
common stock equivalents, the shares underlying the options are subject to adjustment to maintain the percentage ownership that the
option grant reflects on the date of grant. This resulted in the grant being increased to 576 shares of common stock through December
31, 2020. This grant was approved by shareholders on September 9, 2020. |
| |
|
|
| |
4.
|
On
April 3, 2020, we issued an aggregate of 80 restricted stock units (20 to each of our then-current four current directors) as partial
compensation for their service on the Board. |
| |
|
|
| |
5.
|
On
April 1, 2021, we issued an aggregate of 80 restricted stock units (20 to each of our then current four current directors) as partial
compensation for their service on the Board. |
| |
|
|
| |
6.
|
On
September 8, 2021, we issued 250 shares of common stock to a consultant in exchange for services to the Company. |
| |
|
|
| |
7. |
On
February 6, 2023, the Company granted J.D. Finley, our interim Chief Executive Officer and Chief Financial Officer: (i) an option
to purchase 57,200 shares of common stock valued at approximately $87,853, having an exercise price of $2.40 per share, a term of
10 years, and which vests quarterly over a three year period, (ii) 41,700 restricted stock units valued at approximately $100,080
which vests in 12 equal installments quarterly over a three year period, and (iii) 32,500 restricted performance stock units valued
at approximately $78,000, which vest (a) 50% when the volume weighted average price of the Company’s common stock over 20 consecutive
trading days is $3.20, and (b) 50% when such volume weighted average price of the Company’s common stock over 20 consecutive
trading days is $4.25. All of the grants issued to Mr. Finley as described in this paragraph were issued on a conditional basis,
and were subject to the receipt of shareholder approval of the grants, which was subsequently received on June 8, 2023. |
| |
|
|
| |
8. |
On
February 6, 2023, the Company granted Robert McRae, our Chief Operating Officer: (i) an option to purchase 12,000 shares of common
stock valued at approximately $18,431, having an exercise price of $2.40 per share, a term of 10 years, and which vests quarterly
over three years, (ii) 8,800 restricted stock units valued at approximately $21,120 which vests in 12 equal installments quarterly
over a three year period, and (iii) 17,900 restricted performance stock units valued at approximately $42,960, which vest (a) 50%
when the volume weighted average price of the Company’s common stock over 20 consecutive trading days is $3.20, and (b) 50%
when such volume weighted average price of the Company’s common stock over 20 consecutive trading days is $4.25. All of the
grants issued to Mr. McRae as described in this paragraph were issued on a conditional basis, and are subject to the receipt of shareholder
approval of the grants, which were subsequently received on June 8, 2023. |
| |
|
|
| |
9. |
On
February 6, 2023, the Company granted certain non-executive employees, an aggregate of (i) options to purchase 12,300 shares of common
stock valued at approximately $18,891, having an exercise price of $2.40 per share, a term of 10 years, and which vest quarterly
over three years, (ii) 9,000 restricted stock units valued at approximately $21,600 which vest in 12 equal installments quarterly
over a three year period, and (iii) 18,300 restricted performance stock units valued at approximately $43,920, which vest (a) 50%
when the volume weighted average price of the Company’s common stock over 20 consecutive trading days is $3.20, and (b) 50%
when such volume weighted average price of the Company’s common stock over 20 consecutive trading days is $4.25. All of the
grants issued to such employees as described in this paragraph were issued on a conditional basis, and are subject to the receipt
of shareholder approval of the grants, which was subsequently received on June 8, 2023. |
| |
|
|
| |
10. |
On
June 11, 2023, the Company granted Mr. Finley: (i) options to purchase 148,500 shares of common stock with a term of ten (10) years
and an exercise price of $1.60 per share, valued at $151,978 on the grant date and (ii) 66,700 restricted stock units valued at $106,720.
Each of the options and restricted stock units granted to Mr. Finley vest in twelve (12) equal installments on a quarterly basis
over three (3) years. The equity grants were issued from the Company’s 2021 Plan. |
| |
11. |
On
June 11, 2023, the Company granted to its non-executive employees an aggregate of: (i) options to purchase 97,160 shares of common
stock with a term of ten (10) years and an exercise price of $1.60 per share, valued in aggregate at $99,436 at the grant date and
(ii) 45,330 restricted stock units valued at $72,528. Each of the options and restricted stock units granted to the employees vest
in twelve (12) equal installments on a quarterly basis over three (3) years. The equity grants were issued from the Company’s
2021 Plan. |
| |
|
|
| |
12. |
On
June 11, 2023, the Company granted to the non-employee members of the Board of Directors, as supplemental grants, an aggregate of:
(i) options to purchase 77,380 shares of common stock with a term of ten (10) years and an exercise price of $1.60 per share and
(ii) 36,240 restricted stock units. Each of the grants vests fully on the one (1) year anniversary of the grant date. The aggregate
options were valued at $78,136 and the aggregate restricted stock units were valued at $57,984. The equity grants were issued from
the 2021 Plan |
| |
|
|
| |
14. |
On
September 5, 2023, pursuant to his appointment as Chief Medical Officer, the Company issued Mitchell Jones, M.D., Ph.D. (i) options
to purchase 75,000 shares of common stock with a term of ten (10) years and an exercise price of $0.6897 per share and (ii) 54,700
restricted stock units. The options vest quarterly over three (3) years from the grant date and the restricted stock units vest as
follows: (a) 4,556 shares on November 6, 2023, and (b) the remaining 51,144 shares vest over eleven (11) equal quarterly periods
after the initial vesting date. The options were valued at $33,267 and the restricted stock units were valued at $37,727, respectively
from the grant date. The equity grants were issued from the Inducement Plan. |
The
offers, sales and issuances of the securities described in this section (b) of Item 15 were deemed to be exempt from registration under
the Securities Act in reliance on Section 4(a)(2) of the Securities Act or Rule 506 of D promulgated under the Securities Act as transactions
by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for
investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to
the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor within
the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships,
to information about the Company.
Item
16. Exhibits and Financial Statement Schedules
(a)
Exhibits
Exhibit
Number |
|
Description
of document |
| 2.1& |
|
Form
of Underwriting Agreement |
| 2.1† |
|
Agreement and Plan of Merger, dated as of December 16, 2020, by and among Seneca Biopharma, Inc., Leading BioSciences, Inc. and Townsgate Acquisition Sub 1, Inc. (Incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 3.1 |
|
Amended and Restated Certificate of Incorporation of the Registrant (Incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 27, 2021). |
| 3.2 |
|
Certificate of Designation of Series A 4.5% Convertible Preferred Stock (Incorporated by reference to Exhibit 3.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 12, 2016). |
| 3.3 |
|
Amended and Restated Bylaws of the Registrant (Incorporated by reference to Exhibit 3.3 to the Registrant’s Registration Statement on Form S-1 Amendment 5, filed with the SEC on August 11, 2022). |
| 3.4 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series B Convertible Preferred Stock (Incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 16, 2022). |
| 3.5 |
|
Amendment to Amended and Restated Certificate of Incorporation of Palisade Bio, Inc., effective November 15, 2022 (Incorporated by reference to Exhibit 3.01(i) to the Registrant’s Current Report on Form 8-K, filed with the SEC on November 16, 2022). |
| 4.1 |
|
Reference
is made to Exhibits 3.1, 3.2 and 3.3. |
| 4.2 |
|
Description of Securities (incorporated by reference to Exhibit 4.2 to the Registrant’s Form 10-K, filed with the SEC on March 17, 2022). |
| 4.3 |
|
Specimen Common Stock Certificate. (Incorporated by reference to Exhibit 4.3 to the Registrant’s Annual Report on Form 10-K, filed with the SEC on March 17, 2022). |
| 4.4 |
|
Form of Series A Preferred Stock Certificate (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on September 12, 2016). |
| 4.5 |
|
Form of Consulting Warrant issued January 2011 and March 2012 (Incorporated by reference to Exhibit 4.01 to the Registrant’s Registration Statement on Form S-3 (File No. 333-188859) original filed with the SEC on May 24, 2013 |
| 4.6 |
|
Form of Common Stock Purchase Warrant from August 2017 Public Offering Dated August 1, 2017 (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on July 28, 2017). |
| 4.7 |
|
Form of Common Stock Purchase Warrant from October 2018 Offering (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on October 29, 2018) |
| 4.8 |
|
Form of Placement Agent Common Stock Purchase Warrant from October 2018 Offering (Incorporated by reference to Exhibit 4.02 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on October 29, 2018) |
| 4.9 |
|
Consultant Warrant for Hibiscus BioVentures, LLC issued January 2019 (Incorporated by reference to Exhibit 4.40 to the Registrant’s Form 10-Q, originally filed with the SEC on May 14, 2019) |
| 4.10 |
|
Form of Series M and Series N warrant from July 2019 Offering (Incorporated by reference to Exhibit 4.45 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-232273), filed with the SEC on July 24, 2019) |
| 4.11 |
|
Letter Agreement from January 2020 Offering (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on January 22, 2020) |
| 4.12 |
|
Form of Series O Pre-Funded Warrant from July 2019 Offering (Incorporated by reference to Exhibit 4.45 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-232273), filed with the SEC on July 24, 2019) |
| 4.13 |
|
Form of Series Q Replacement Warrant issued in January 2020 Offering (Incorporated by reference to Exhibit 4.02 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on January 22, 2020) |
| 4.14 |
|
Form of Placement Agent Agreement from January 2020 Offering (Incorporated by reference to Exhibit 10.02 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on January 22, 2020) |
| 4.15 |
|
Form of Placement Agent Warrant issued in January 2020 Offering (Incorporated by reference to Exhibit 4.03 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on January 22, 2020) |
| 4.16 |
|
Form of Placement Agent Warrant issued in May 2020 Offering (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on May 27, 2020) |
| 4.17 |
|
Form of Securities Purchase Agreement with Investors from May 2020 Offering (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, originally filed with the SEC on May 27, 2020) |
| 4.18 |
|
Form of Warrant to Purchase Shares of Common Stock of Leading BioSciences, Inc. (Incorporated by reference to Exhibit 4.30 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 4.19 |
|
Form of Bridge Warrant of Leading BioSciences, Inc. (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 4.20 |
|
Form of Equity Warrant of Leading BioSciences, Inc. (Incorporated by reference to Exhibit 4.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 4.21† |
|
Registration Rights Agreement, by and between Seneca Biopharma, Inc. and the investor party thereto, dated December 16, 2020 (Incorporated by reference to Exhibit 4.3 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 4.22 |
|
Waiver Agreement, dated as of July 21, 2021, by and between Palisade Bio, Inc. and Altium Growth Fund, LP (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on July 22, 2021). |
| 4.23 |
|
Warrant, dated as of July 21, 2021, issued to Altium Growth Fund, LP (Incorporated by reference to Exhibit 4.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on July 22, 2021). |
| 4.24 |
|
Waiver Agreement, dated as of January 31, 2022, by and between Palisade Bio, Inc. and Altium Growth Fund, LP (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on February 21, 2022). |
| 4.25 |
|
Warrant, dated as of January 31, 2022, issued to Altium Growth Fund, LP (Incorporated by reference to Exhibit 4.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on February 21, 2022). |
| 4.26 |
|
Securities Purchase Agreement, dated as of August 19, 2021, by and between Palisade Bio, Inc. and Yuma Regional Medical Center (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 24, 2021). |
| 4.27 |
|
Warrant, dated as of August 19, 2021, issued to Yuma Regional Medical Center (Incorporated by reference to Exhibit 4.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 24, 2021). |
| 4.28 |
|
Form of Common Stock Purchase Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 6, 2022). |
| 4.29 |
|
Form of Placement Agent Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 6, 2022). |
| 4.30 |
|
Form of Series 1 Common Stock Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 16, 2022). |
| 4.31 |
|
Form of Series 2 Common Stock Warrant (Incorporated by reference to Exhibit 4.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 16, 2022). |
| 4.32 |
|
Warrant Agency Agreement dated August 16, 2022, by and between Palisade Bio, Inc. and American Stock Transfer and Trust Company, LLC. (Incorporated by reference to Exhibit 4.3 to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 16, 2022). |
| 4.33 |
|
Form of Series B Preferred Stock Certificate of Registrant (Incorporated by reference to Exhibit 4.33 to the Registrant’s Registration Statement on Form S-1/A, filed with the SEC on August 9, 2022) |
| 4.34 |
|
Form of Underwriter Warrant issued August 16, 2022 (Incorporated by reference to Exhibit 4.33 to the Registrant’s Quarterly Report on Form 10-Q, filed with the SEC on November 14, 2022). |
| 4.35 |
|
Form of Registered Prefunded Warrant issued in January 2023 Registered Offering (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 4, 2023). |
| 4.36 |
|
Form of Prefunded Warrant issued in January 2023 Private Placement (Incorporated by reference to Exhibit 4.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 4, 2023). |
| 4.37 |
|
Form of Warrant issued in January 2023 Private Placement (Incorporated by reference to Exhibit 4.03 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 4, 2023). |
| 4.38 |
|
Form of Placement Agent Warrant issued in January 2023 Private Placement (Incorporated by reference to Exhibit 4.04 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 4, 2023). |
| 4.39 |
|
Form of Prefunded Warrant issued in April 2023 Private Placement (Incorporated by Reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 5, 2023). |
| 4.40 |
|
Form of Warrant issued in April 2023 Private Placement (Incorporated by Reference to Exhibit 4.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 5, 2023). |
| 4.41 |
|
Form of Placement Agent Warrant issued in April 2023 Private Placement (Incorporated by reference to Exhibit 4.03 to the Registrant’s Current Report on Form 8-K filed with the SEC on April 5, 2023). |
| 4.42 |
|
Form of Placement Agent Warrant issued in September 2023 Private Placement (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 11, 2023). |
| 5.1& |
|
Opinion
of Silvestre Law Group, P.C. |
| 10.1# |
|
Seneca Biopharma 2019 Equity Incentive Plan (Incorporated by reference to Appendix A to the Registrant’s Definitive Proxy Statement, originally filed with the SEC on April 29, 2019). |
| 10.2# |
|
Form of Restricted Option Grant from 2019 Equity Incentive Plan (Incorporated by reference to Exhibit 4.43 to the Registrant’s Registration Statement on Form S-1 (File No. 333-232273), originally filed with the SEC on June 21, 2019, originally filed with the SEC on June 21, 2019). |
| 10.3# |
|
License Agreement, by and between Leading BioSciences, Inc. and The Regents of the University of California, dated August 19, 2015, as amended on December 20, 2019 (Incorporated by reference to Exhibit 10.18 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.4# |
|
License Agreement, by and between Leading BioSciences, Inc. and The Regents of the University of California, dated April 1, 2020 (Incorporated by reference to Exhibit 10.19 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.5# |
|
License Agreement, by and between Palisade Bio, Inc. and The Regents of the University of California, dated July 6, 2021 (incorporated by reference to Exhibit 10.5 to the Registrant’s Form 10-K, filed with the SEC on March 17, 2022). |
| 10.6# |
|
Co-Development and Distribution Agreement, by and between Leading BioSciences, Inc. and Newsoara Biopharma Co., Ltd. (as successor-in-interest to Biolead Medical Technology Limited), dated February 17, 2018, as amended on November 27, 2018 (Incorporated by reference to Exhibit 10.20 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.7 |
|
Form of Seneca Biopharma, Inc. Support Agreement, dated as of December 16, 2020, by and between Leading BioSciences, Inc. and each of the parties named in each agreement therein (Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 10.8 |
|
Form of Leading BioSciences, Inc. Support Agreement, dated as of December 16, 2020, by and between Seneca Biopharma, Inc. and each of the parties named in each agreement therein(Incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 10.9† |
|
Securities Purchase Agreement, by and between Leading BioSciences, Inc. and the investor party thereto, dated December 16, 2020 (Incorporated by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 10.10† |
|
Securities Purchase Agreement, by and among Seneca Biopharma, Inc., Leading BioSciences, Inc. and the investor party thereto, dated December 16, 2020 (Incorporated by reference to Exhibit 10.6 to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 21, 2020). |
| 10.11 |
|
Amendment Agreement to Securities Purchase Agreement by and among, the Company, Leading BioSciences, Inc. and Altium Growth Fund, LP, dated May 3, 2021 (Incorporated by reference to Exhibit 10.03 to the Registrant’s Quarterly Report on Form 10-Q, filed with the SEC on May 14, 2021). |
| 10.12 |
|
Form of Separation Agreement with Seneca Biopharma, Inc. Executives (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on March 18, 2021). |
| 10.13† |
|
Contingent Value Rights Agreement, dated as of April 27, 2021, by and among the Company, American Stock Transfer & Trust Company, LLC and Raul Silvestre (Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 27, 2021). |
| 10.14+ |
|
Form of Indemnification Agreement (incorporated by reference from Exhibit 10.03 to the Registrant’s Current Report on Form 8-K filed with the SEC on December 18, 2018). |
| 10.15+ |
|
Leading BioSciences, Inc. Amended and Restated 2013 Employee, Director and Consultant Equity Incentive Plan and Forms of Stock Option Grant Notice, Stock Option Agreement and Notice of Exercise of Stock Option thereunder (Incorporated by reference to Exhibit 10.24 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.16+ |
|
Palisade Bio, Inc. 2021 Equity Incentive Plan, as amended (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 9, 2023). |
| 10.17+ |
|
Form of Stock Option Grant Notice, Stock Option Agreement and Notice of Exercise under the Palisade Bio, Inc. 2021 Equity Incentive Plan (Incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K, filed with the SEC on November 23, 2021). |
| 10.18+ |
|
Form of Non-Employee Director Stock Option Grant Notice, Stock Option Agreement and Notice of Exercise under the Palisade Bio, Inc. 2021 Equity Incentive Plan (Incorporated by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K, filed with the SEC on November 23, 2021). |
| 10.19+ |
|
Palisade Bio, Inc. Employee Stock Purchase Plan (Incorporated by reference to Exhibit 10.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 9, 2023). |
| 10.20+ |
|
Palisade Bio, Inc. 2021 Inducement Incentive Plan, as Amended August 7, 2023 (Incorporated by reference to Exhibit 10.20 to the Registrant’s Quarterly Report on Form 10-Q, filed with the SEC on August 10, 2023). |
| 10.21+ |
|
Form of Restricted Stock Unit Grant Notice and Award Agreement under the Palisade Bio, Inc. 2021 Inducement Incentive Plan (Incorporated by reference to Exhibit 99.1 to the Registrant’s Registration Statement on Form S-8 (File No. 333-261196), filed with the SEC on November 19, 2021). |
| 10.22+ |
|
Form of Stock Option Grant Notice and Award Agreement under the Palisade Bio, Inc. 2021 Inducement Incentive Plan (Incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 (File No. 333-261196), filed with the SEC on November 19, 2021). |
| 10.23+ |
|
Non-Employee Director Compensation Policy (Incorporated by reference to Exhibit 10.35 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 22, 2023). |
| 10.24+ |
|
Amended and Restated Executive Employment Agreement, by and between Leading BioSciences, Inc. and JD Finley, dated January 24, 2021(Incorporated by reference to Exhibit 10.23 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.25+ |
|
Executive Employment Agreement, by and between Leading BioSciences, Inc. and Thomas Hallam, Ph.D., dated December 16, 2020 (Incorporated by reference to Exhibit 10.22 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.26 |
|
Executive Employment Agreement, by and between Leading BioSciences, Inc. and Michael Dawson, M.D., dated December 16, 2020 (Incorporated by reference to Exhibit 10.21 to the Registrant’s Registration Statement on Form S-4 (File No. 333-251659), originally filed with the SEC on December 23, 2020, as amended). |
| 10.27† |
|
Asset Transfer Agreement, by and between Alto Neuroscience, Inc. and Palisade Bio, Inc., dated October 18, 2021 (incorporated by reference to Exhibit 10.27 to the Registrant’s Form 10-K, filed with the SEC on March 17, 2022). |
| 10.28 |
|
Office Lease Between AP Beacon Carlsbad, LP, and Palisade Bio, Inc., dated May 12, 2022 (Incorporate by reference to Exhibit 10.1 to the Registrant’s Form 10-Q filed with the SEC on May 13, 2022). |
| 10.29 |
|
First Amendment dated July 14, 2022 to the Office Lease Between AP Beacon Carlsbad, LP, and Palisade Bio, Inc., dated May 12, 2022 (Incorporated by reference to Exhibit 10.2 to the Registrants Form 10-Q filed with the SEC on August 15, 2022). |
| 10.30 |
|
Form of Securities Purchase Agreement, dated May 6, 2022, by and among the Company and the purchasers named therein (Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 6, 2022). |
| 10.31+ |
|
Separation Agreement and Release with former Chief Executive Officer (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 14, 2022). |
| 10.32 |
|
Form of Securities Purchase Agreement dated December 30, 2022, by and among the Company and the purchasers named therein (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current report on Form 8-K, filed with the SEC on January 4, 2023). |
| 10.33 |
|
Form of Registration Rights Agreement, dated December 30, 2022, by and among the Company and signatories named therein (Incorporated by reference to Exhibit 10.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 4, 2023). |
| 10.34 |
|
Form of Placement Agency Agreement, dated December 30, 2022, by and between the Company and Ladenburg Thalmann & Co Inc. (Incorporated by reference to Exhibit 10.03 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 4, 2023). |
| 10.35+ |
|
Form of First Amendment Consulting Agreement dated January 25, 2023 by and between Dr. Herbert Slade and the Company (Incorporated by reference to Exhibit 10.35 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 22, 2023). |
| 10.36+ |
|
Form of Consulting Agreement dated April 7, 2023 by and between Dr. Herbert Slade and the Company. (Incorporated by reference to Exhibit 10.36 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 22, 2023). |
| 10.37 |
|
Form of Securities Purchase Agreement dated April 3, 2023, by and among the Company and the purchasers named therein (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 5, 2023). |
| 10.38 |
|
Form of Registration Rights Agreement dated April 3, 2023, by and among the Company and the signatories named therein (Incorporated by reference to Exhibit 10.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 5, 2023). |
| 10.39 |
|
Form of Placement Agency Agreement dated April 3, 2023, by and among the Company and Ladenburg Thalmann & Co Inc. (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 5, 2023). |
| 10.40#† |
|
Form of Research, Collaboration, and License Agreement with Giiant Pharma (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on September 8, 2023. |
| 10.41 |
|
Form of Securities Purchase Agreement dated September 7, 2023, by and among the Company and the signatories named therein (Incorporated by Reference to Exhibit 10.01 to the Registrant’s Current report on Form 8-K, filed with the SEC on September 11, 2023). |
| 10.42 |
|
Form of Placement Agency Agreement dated September 7, 2023, by and among the Company and Ladenburg Thalmann & Co Inc. (Incorporated by reference to Exhibit 10.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on September 11, 2023). |
| 10.43 |
|
Form of Employment Agreement with Mitchell Jones, dated September 5, 2023 (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on September 11, 2023). |
| 19.1* |
|
Registrant’s Insider Trading Policy. |
| 21.1 |
|
Subsidiaries of the Registrant (Incorporated by reference to Exhibit 21.1 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 22, 2023). |
| 23.1* |
|
Consent
of BDO USA, P.C. Independent Registered Public Accounting Firm |
| 23.2* |
|
Consent
of Baker Tilly US, LLP, Independent Registered Public Accounting Firm |
| 23.3& |
|
Consent
of Silvestre Law Group, P.c. (included in Exhibit 5.1) |
| 107* |
|
Filing Fee Table |
*
Filed herewith
**
Furnished herewith.
&
To be Filed by Amendment.
+
Indicates management contract or compensatory plan.
#
Certain portions of this exhibit (indicated by “[***]”) have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K.
†
Certain schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. A copy of any omitted schedule and/or
exhibit will be furnished to the Securities and Exchange Commission upon request.
(b)
Financial Statement Schedules
No
financial statement schedules are provided because the information called for is not required or is shown either in the financial statements
or related notes, which are incorporated herein by reference.
Item 17. Undertakings
The
undersigned Registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume
and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement; and
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement.
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
(4)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: each prospectus filed pursuant to Rule
424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the
date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is
part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement
or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first
use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement
or made in any such document immediately prior to such date of first use.
(5)
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424;
(ii)
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant;
(iii)
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and
(iv)
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6)
That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report
pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee
benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference
in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the
offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(7)
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed
as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant
to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time
it was declared effective.
(8)
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of
prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons
of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities
and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the
event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid
by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted
by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the
opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question
whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of
such issue.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf
by the undersigned, thereunto duly authorized, in the City of Carlsbad, State of California on December 29, 2023.
| |
Palisade
Bio, Inc. |
| |
|
|
| |
By: |
/s/
J.D. Finley |
| |
|
J.D.
Finley |
| |
|
Chief
Executive Officer |
POWER
OF ATTORNEY
Each
person whose signature appears below constitutes and appoints J.D. Finley, as his or her true and lawful attorneys-in-fact and agent,
each acting alone, with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any
and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments (including post-effective
amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent registration statement
filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, together with all schedules and exhibits thereto, (ii) act
on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith,
(iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent
registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and (iv) take any and all actions
which may be necessary or appropriate to be done, as fully for all intents and purposes as he or she might or could do in person, hereby
ratifying and confirming all that said attorney-in-fact and agent, or his or her substitute or substitutes, may lawfully do or cause
to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities
indicated on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
| /s/
J.D. Finley |
|
Chief
Executive Officer and Director |
|
December
29, 2023 |
| J.D.
Finley |
|
(Principal
Executive Officer) |
|
|
| |
|
|
| /s/
J.D. Finley |
|
Chief
Financial Officer |
|
December
29, 2023 |
| J.D.
Finley |
|
(Principal
Financial and Accounting Officer) |
|
|
| |
|
|
| /s/
James R. Neal |
|
Chairman
of the Board of Directors |
|
December
29, 2023 |
| James
R. Neal |
|
|
|
|
| |
|
|
| /s/
Cristina Csimma, Pharm.D. |
|
Director |
|
December
29, 2023 |
| Cristina
Csimma, Pharm.D. |
|
|
|
|
| |
|
|
| /s/
Stephanie Diaz |
|
Director |
|
December
29, 2023 |
| Stephanie
Diaz |
|
|
|
|
| |
|
|
| /s/
Mary Ann Gray, Ph.D. |
|
Director |
|
December
29, 2023 |
| Mary
Ann Gray, Ph.D. |
|
|
|
|
| |
|
|
| /s/
Robert J. Trenschel, D.O. |
|
Director |
|
December
29, 2023 |
| Robert
J. Trenschel, D.O. |
|
|
|
|
| |
|
|
| /s/
Binxian Wei |
|
Director |
|
December
29, 2023 |
| Binxian
Wei |
|
|
|
|
| |
|
|
| /s/
Donald Williams |
|
Director |
|
December
29, 2023 |
| Donald
A. Williams |
|
|
|
|
Exhibit 19.1
Palisade
Bio, Inc.
Insider
Trading Policy
(As
Amended December 15, 2023)
Introduction
During
the course of your relationship with Palisade Bio, Inc. (the “Company”), you may receive material information
that is not yet publicly available (“material nonpublic information”) about the Company or other publicly traded
companies that the Company has business relationships with. Material nonpublic information may give you, or someone you pass that information
on to, an advantage over others when deciding whether to buy, sell or otherwise transact in the Company’s securities or the securities
of another publicly traded company. This policy sets forth guidelines with respect to transactions in Company securities by our employees,
directors and consultants who may become aware of material non-public information and the other persons subject to this policy as described
below.
Statement
of Policy
It
is the policy of the Company that employees, directors or consultants of the Company (or any other person subject to this policy) who
are aware of material nonpublic information relating to the Company may not, directly or indirectly:
| 1. | engage
in any transactions in the Company’s securities, except as otherwise specified under
the heading “Exceptions to this Policy” below; |
| | | |
| 2. | recommend
the purchase or sale of the Company’s securities; |
| | | |
| 3. | disclose
material nonpublic information to persons within the Company whose jobs do not require them
to have that information, or outside of the Company to other persons, such as family, friends,
business associates and investors, unless the disclosure is made in accordance with the Company’s
policies regarding the protection or authorized external disclosure of information regarding
the Company; or |
| | | |
| 4. | assist
anyone engaged in the above activities. |
The
prohibition against insider trading is absolute. It applies even if the decision to trade is not based on such material
nonpublic information. It also applies to transactions that may be necessary or justifiable for independent reasons (such as the need
to raise money for an emergency expenditure) and applies to very small transactions as well. All that matters is whether you are aware
of any material nonpublic information relating to the Company at the time of the transaction.
The
U.S. federal securities laws do not recognize any mitigating circumstances to insider trading. In addition, even the appearance of an
improper transaction must be avoided to preserve the Company’s reputation for adhering to the highest standards of conduct. In
some circumstances, you may need to forgo a planned transaction even if you planned it before becoming aware of the material nonpublic
information. So, even if you believe you may suffer an economic loss or sacrifice an anticipated profit by waiting to trade, you must
wait.
It
is also important to note that the laws prohibiting insider trading are not limited to trading by the insider alone; advising others
to trade based on material nonpublic information is illegal and prohibited by this policy and U.S. federal securities laws. Liability
in such cases can extend both to the “tippee”—the person to whom the insider disclosed material nonpublic information—and
to the “tipper,” the insider himself or herself. In such cases, you can be held liable for your own transactions, as well
as the transactions by a tippee and even the transactions of a tippee’s tippee. For these and other reasons, it is the policy of
the Company that no employee, director or consultant of the Company (or any other person subject to this policy) may either (a) recommend
to another person that they buy, hold or sell Company securities at any time or (b) disclose material nonpublic information to
persons within the Company whose jobs do not require them to have that information, or outside of the Company to other persons (unless
the disclosure is made in accordance with the Company’s policies regarding the protection or authorized external disclosure of
information).
In
addition, it is the policy of the Company that no employee, director or consultant of the Company (or any other person subject to this
policy) who, in the course of working for the Company, learns of or is otherwise aware of material nonpublic information about another
publicly traded company with which the Company does business, including a customer or supplier of the Company, may trade in that company’s
securities until the information becomes public or is no longer material.
There
are no exceptions to this policy, except as specifically noted above or below.
Transactions
Subject to this Policy
This
policy applies to all transactions in securities issued by the Company, as well as derivative securities that are not issued by the Company,
such as exchange-traded put or call options or swaps relating to the Company’s securities. Accordingly, for purposes of this policy,
the terms “trade,” “trading” and “transactions” include
not only purchases and sales of the Company’s common stock in the public market but also any other purchases, sales, transfers
or other acquisitions and dispositions of common or preferred equity, options, warrants and other securities (including debt securities)
and other arrangements or transactions that affect economic exposure to changes in the prices of these securities.
Persons
Subject to this Policy
This
policy applies to you and all other employees, directors and consultants of the Company and its subsidiaries. This policy also applies
to members of your immediate family, persons with whom you share a household, persons who are your economic dependents and any other
individuals or entities whose transactions in securities you influence, direct or control (including, e.g., a venture or other investment
fund, if you influence, direct or control transactions by the fund). The foregoing persons who are deemed subject to this policy
are referred to in this policy as “Related Persons.” You are responsible for making sure that your Related
Persons comply with this policy.
Material
Nonpublic Information
Material
information
It
is not always easy to figure out whether you are aware of material nonpublic information. But there is one important factor to determine
whether nonpublic information you know about a public company is material: whether the information could be expected to affect the market
price of that company’s securities or to be considered important by investors who are considering trading that company’s
securities. If the information makes you want to trade, it would probably have the same effect on others. Keep in mind that both positive
and negative information can be material.
There
is no bright-line standard for assessing materiality; rather, materiality is based on an assessment of all of the facts and circumstances,
and is often evaluated by relevant enforcement authorities with the benefit of hindsight. Depending on the specific details, the following
items may be considered material nonpublic information until publicly disclosed within the meaning of this policy. There may be other
types of information that would qualify as material information as well; use this list merely as a non-exhaustive guide:
| ● | financial
results or forecasts; |
| ● | status
of product or product candidate development or regulatory approvals; |
| ● | clinical
data relating to products or product candidates; |
| ● | timelines
for pre-clinical studies or clinical trials; |
| ● | acquisitions
or dispositions of assets, divisions or companies; |
| ● | timing
of public or private sales of debt or equity securities; |
| ● | timing
of stock splits, dividends or changes in dividend policy; |
| ● | the
establishment of a repurchase program for the Company’s securities; |
| ● | gain
or loss of a significant licensor, licensee or supplier; |
| ● | changes
or new corporate partner relationships or collaborations; |
| ● | notice
of issuance or denial of patents; |
| ● | regulatory
developments; |
| ● | management
or changes in control of the company; |
| ● | employee
layoffs; |
| ● | a
disruption in the Company’s operations or breach or unauthorized access of its property
or assets, including its facilities and information technology infrastructure; |
| ● | tender
offers or proxy fights; |
| ● | accounting
restatements; |
| ● | litigation
or settlements; and |
| ● | impending
bankruptcy. |
Also,
mere discussions of the foregoing or other factors that may result in material nonpublic information would also be covered.
When
information is considered public
The
prohibition on trading when you have material nonpublic information ends once that information becomes publicly disseminated. For information
to be considered publicly disseminated, it must be widely disseminated through a press release, a filing with the Securities and Exchange
Commission (the “SEC”), or other widely disseminated announcement. Once information is publicly disseminated,
it is still necessary to provide the investing public with sufficient time to absorb the information. Generally speaking, information
will be considered publicly disseminated for purposes of this policy after two full trading days have elapsed since the information was
publicly disclosed. For example, if we announce material nonpublic information before trading begins on Wednesday, then you may execute
a transaction in our securities on Friday; if we announce material nonpublic information after trading ends on Wednesday, then you may
execute a transaction in our securities on Monday. Depending on the particular circumstances, the Company may determine that a longer
or shorter waiting period should apply to the release of specific material nonpublic information. In such instance, the Company will
notify all you in writing of the longer or shorter waiting period.
STOCK
TRADING BY DIRECTORS, OFFICERS, EMPLOYEES AND OTHER SERVICE PROVIDERS
Pre-Clearance
and Advance Notice of Transactions
For
purposes of our Policy, members of our board of directors, officers, employees and employees of our consultants, as may be designated
by our Chief Executive officer from time to time, because of their access to sensitive information shall be deemed “Covered
Insiders”. Covered Insiders are required to notify and receive the written approval from the Company’s General Counsel
or his or her designee, which consent can be withheld for any reason, prior to engaging in transactions in the Company’s securities.
Also, such approval if granted, can be subject to other restrictions designed to minimize the risk of apparent or actual insider trading
as set forth in the section below titled “Pre-Clearance and Advance Notice of Transactions.” From time to time, we
may also require that certain persons limit their transactions in the Company’s securities to certain trading window periods as
described below.
Quarterly
Trading Blackouts
From
time to time, the Company may generally prohibit Covered Insiders from trading securities outside of a time period the Company designates
as an open trading window (such period when trading is allowed is referred to as a “trading window period”
and such period when trading is not allowed is referred to as a “trading blackout period”). As of the effective
date of this policy, the Company has not instituted a trading window period. In the event the Company institutes a trading window period
after the effective date of this policy, Covered Insiders will additionally be subject to these requirements.
In
the event the Company institutes a trading window period, except as described in this policy, all Covered Insiders will be able
to trade in Company securities only during limited open trading window periods that generally will begin after two full trading
days have elapsed since the public dissemination of the Company’s annual or quarterly financial results and end at the beginning
of the next quarterly trading blackout period. Of course, even during an open trading window period, you may not (unless an exception
applies) conduct any trades in Company securities if you are otherwise in possession of material nonpublic information. This window period
may be closed early or may not open at all if, in the judgment of the Chief Executive Officer, there exists undisclosed information that
would make trades inappropriate. In addition to a trading window period, the Company may close the trading window at any time and for
any duration pending the public release of material news. It is important to note that the fact that the trading window is closed should
itself be considered inside information.
A
Covered Insider who believes that special circumstances require him or her to trade during a quarterly trading blackout period should
consult the General Counsel. Permission to trade during a quarterly trading blackout period may be granted only where the circumstances
are extenuating, the General Counsel concludes that the person is not in fact aware of any material nonpublic information relating to
the Company or its securities, and there appears to be no significant risk that the trade may subsequently be questioned. Such determination
will be at the sole discretion of the Company.
Event-Specific
Trading Blackouts
From
time to time, an event may occur that is material to the Company and is known by only a few directors, officers and/or employees. So
long as the event remains material and nonpublic, the persons designated by the Chief Executive Officer may not trade in the Company’s
securities. In that situation, the Company will notify the designated individuals in writing that neither they nor their Related Persons
may trade in the Company’s securities. The existence of an event-specific trading blackout should also be considered material nonpublic
information and should not be communicated to any other person, including any other employee or consultant of the Company. Even if you
have not been designated as a person who should not trade due to an event-specific trading blackout, you should not trade while aware
of material nonpublic information. Exceptions will not be granted during an event-specific trading blackout.
The
quarterly and event-specific trading blackouts do not apply to those transactions to which this policy does not apply, as described under
the heading “Exceptions to this Policy” below.
Exceptions
to this Policy
This
policy does not apply in the case of the following transactions, except as specifically noted:
1. Option
Exercises. This policy does not apply to the exercise of options granted under the Company’s equity compensation plans
for cash. This policy does, however, apply to any sale of stock as part of a broker-assisted cashless exercise or any other market sale,
whether or not for the purpose of generating the cash needed to pay the exercise price or pay taxes.
2. Tax
Withholding Transactions. This policy does not apply to the surrender of shares directly to the Company to satisfy tax withholding
obligations as a result of the issuance of shares upon vesting or exercise of restricted stock units, options or other equity awards
granted under the Company’s equity compensation plans. Of course, any market sale of the stock received upon exercise or vesting
of any such equity awards remains subject to all provisions of this policy whether or not for the purpose of generating the cash needed
to pay the exercise price or pay taxes.
3. ESPP.
This policy does not apply to the purchase of stock by employees under the Company’s Employee Stock Purchase Plan (“ESPP”)
on periodic designated dates in accordance with the ESPP. This policy does, however, apply to any sale of stock acquired pursuant to
the ESPP.
4. 10b5-1
Automatic Trading Programs. Under Rule 10b5-1 of the Securities Exchange Act of 1934, as amended (“Exchange
Act”), employees, directors and consultants may establish a trading plan under which a broker is instructed to buy and
sell Company securities based on pre-determined criteria (a “Trading Plan”). So long as a Trading Plan is properly
established, purchases and sales of Company securities pursuant to that Trading Plan are not subject to this policy. To be properly established,
an employee’s, director’s or consultant’s Trading Plan must be established in compliance with the requirements of Rule
10b5-1 of the Exchange Act, including any cooling off periods, and any applicable 10b5-1 trading plan guidelines of the Company at a
time when such individual was unaware of any material nonpublic information relating to the Company and when the Company was not otherwise
in a trading blackout period. Moreover, all Trading Plans must be reviewed and approved by the Company before being established to confirm
that the Trading Plan complies with all pertinent company policies and applicable securities laws. It is the policy of the Company that
it will not verify the existence or non-existence of a trading blackout to any person adopting a Trading Plan unless the Company has
been permitted to review such Trading Plan in its entirety, and in no case will the Company approve any such Trading Plan unless the
Company is permitted to review such Trading Plan in its entirety prior to such Trading Plan being established.
5. Gifts.
This policy does not apply to bona fide gifts of Company securities that have been pre-cleared by the Company’s
General Counsel or his or her designee. Whether a gift is truly bona fide will depend on the facts and circumstances surrounding
each gift. Pre-clearance must be obtained at least two business days in advance of the proposed gift, and pre-cleared gifts not completed
within five business days will require new pre-clearance. The Company may choose to shorten this period. If such transaction is approved
by the General Counsel, the General Counsel or his or her designee will help comply with any required reporting requirements under Section
16(a) of the Exchange Act, if applicable.
6. 401(k)
Plan. This policy does not apply to purchases of the Company’s securities in the Company’s 401(k) plan resulting
from your periodic contribution of money to the plan pursuant to your payroll deduction election. This policy does apply, however, to
certain elections you may make under the 401(k) plan, to the extent permitted or provided for under the 401(k) plan, including: (a) an
election to increase or decrease the percentage of your periodic contributions that will be allocated to the Company stock fund; (b)
an election to make an intra-plan transfer of an existing account balance into or out of the Company stock fund; (c) an election to borrow
money against your 401(k) plan account if the loan will result in a liquidation of some or all of your the Company stock fund balance;
and (d) an election to pre-pay a plan loan if the pre- payment will result in allocation of loan proceeds to the Company stock fund.
Special
and Prohibited Transactions
1. Inherently
Speculative Transactions. No Company employee, director or consultant may engage in short sales, transactions in put options,
call options or other derivative securities on an exchange, any other organized market, through a private transaction or in any other
inherently speculative transactions with respect to the Company’s securities.
2. Hedging
Transactions. Hedging or monetization transactions can be accomplished through a number of possible mechanisms, including through
the use of financial instruments such as prepaid variable forwards, equity swaps, collars and exchange funds. Such hedging transactions
may permit a Company employee, director or consultant to continue to own the Company’s securities obtained through employee benefit
plans or otherwise, but without the full risks and rewards of ownership. When that occurs, the Company employee, director or consultant
may no longer have the same objectives as the Company’s other shareholders. Therefore, the Company employee, director and consultants
are prohibited from engaging in any such transactions.
3. Margin
Accounts and Pledged Securities. Securities held in a margin account as collateral for a margin loan may be sold by the broker
without the customer’s consent if the customer fails to meet a margin call. Similarly, securities pledged (or hypothecated) as
collateral for a loan may be sold in foreclosure if the borrower defaults on the loan. Because a margin sale or foreclosure sale may
occur at a time when the pledgor is aware of material nonpublic information or otherwise is not permitted to trade in the Company’s
securities, the Company employee, director and consultants are prohibited from holding Company securities in a margin account or otherwise
pledging the Company’s securities as collateral for a loan.
4. Standing
and Limit Orders. Standing and limit orders (except standing and limit orders under approved Trading Plans, as discussed above)
create heightened risks for insider trading violations. There is no control over the timing of purchases or sales that result from standing
instructions to a broker, and as a result the broker could execute a transaction when a Company employee, director or consultant is in
possession of material nonpublic information. The Company therefore discourages placing standing or limit orders on the Company’s
securities. If a person subject to this policy determines that they must use a standing order or limit order (other than under an approved
Trading Plan as discussed above), the order should be limited to short duration and the person using such standing order or limit order
is required to cancel such instructions immediately in the event restrictions are imposed on their ability to trade pursuant to the “Quarterly
Trading Blackouts” and “Event-Specific Trading Blackouts” provisions above.
Pre-Clearance
and Advance Notice of Transactions
In
addition to the requirements above, Covered Insiders may not engage in any transaction in the Company’s securities without first
obtaining pre-clearance of the transaction from the Company’s General Counsel or his or her designee at least two business days
in advance of the proposed transaction. The General Counsel or his or her designee will then determine whether the transaction may proceed
and, if so, the General Counsel or his or her designee will help comply with any required reporting requirements under Section 16(a)
of the Exchange Act, if applicable. Pre-cleared transactions not completed within five business days will require new pre-clearance.
The Company may choose to shorten this period.
Persons
subject to pre-clearance must also give advance notice of their plans to exercise an outstanding stock option to the General Counsel.
Once any transaction takes place, the officer, director or employee must immediately notify the General Counsel and any other individuals
identified under the heading “Notification of Execution of Transaction” in the Company’s Section 16 Compliance Program
so that the Company may assist in any Section 16 reporting obligations.
Short-Swing
Trading, Control Stock and Section 16 Reports
Officers
and directors subject to the reporting obligations under Section 16 of the Exchange Act should take care to avoid short-swing transactions
(within the meaning of Section 16(b) of the Exchange Act) and the restrictions on sales by control persons (Rule 144 under the Securities
Act of 1933, as amended), and should file all appropriate Section 16(a) reports (Forms 3, 4 and 5), which are described in the Company’s
Section 16 Compliance Program, and any notices of sale required by Rule 144.
Prohibition
of Trading During Pension Plan Blackouts
No
director or executive officer of the Company may, directly or indirectly, purchase, sell or otherwise transfer any equity security of
the Company (other than an exempt security) during any “blackout period’’ (as defined in Regulation BTR under the Exchange
Act) if a director or executive officer acquires or previously acquired such equity security in connection with his or her service or
employment as a director or executive officer. This prohibition does not apply to any transactions that are specifically exempted, including
but not limited to, purchases or sales of the Company’s securities made pursuant to, and in compliance with, a Trading Plan; compensatory
grants or awards of equity securities pursuant to a plan that, by its terms, permits executive officers and directors to receive automatic
grants or awards and specifies the terms of the grants and awards; or acquisitions or dispositions of equity securities involving a bona
fide gift or by will or the laws of descent or pursuant to a domestic relations order. The Company will notify each director and executive
officer of any blackout periods in accordance with the provisions of Regulation BTR. Because Regulation BTR is very complex, no director
or executive officer of the Company should engage in any transactions in the Company’s securities, even if believed to be exempt
from Regulation BTR, without first consulting with the General Counsel.
Policy’s
Duration
This
policy continues to apply to your transactions in the Company’s securities or the securities of other public companies engaged
in business transactions with the Company even after your relationship with the Company has ended. If you are aware of material nonpublic
information when your relationship with the Company ends, you may not trade the Company’s securities or the securities of other
applicable companies until the material nonpublic information has been publicly disseminated or is no longer material. Further, if you
leave the Company during a trading blackout period, then you may not trade the Company’s securities or the securities of other
applicable companies until the trading blackout period has ended.
Individual
Responsibility
Persons
subject to this policy have ethical and legal obligations to maintain the confidentiality of information about the Company and to not
engage in transactions in the Company’s securities while aware of material nonpublic information. Each individual is responsible
for making sure that he or she complies with this policy, and that any family member, household member or other person or entity whose
transactions are subject to this policy, as discussed under the heading “Persons Subject to this Policy” above, also comply
with this policy. In all cases, the responsibility for determining whether an individual is aware of material nonpublic information rests
with that individual, and any action on the part of the Company or any employee or director of the Company pursuant to this policy (or
otherwise) does not in any way constitute legal advice or insulate an individual from liability under applicable securities laws. You
could be subject to severe legal penalties and disciplinary action by the Company for any conduct prohibited by this policy or applicable
securities laws. See “Penalties” below.
Penalties
Anyone
who engages in insider trading or otherwise violates this policy may be subject to both civil liability and criminal penalties. Violators
also risk disciplinary action by the Company, including termination of employment. Anyone who has questions about this policy should
contact their attorney or the Company’s General Counsel. Please also see Frequently Asked Questions, which are attached as Exhibit
A.
Amendments
The
Company is committed to continuously reviewing and updating its policies and procedures. The Company therefore reserves the right to
amend, alter or terminate this policy at any time and for any reason. A current copy of the Company’s policies regarding insider
trading may be obtained by contacting the Company.
Exhibit
23.1
Consent
of Independent Registered Public Accounting Firm
We
hereby consent to the incorporation by reference in this Registration Statement of our report dated March 17, 2022, except for the immaterial
revision to previously issued financial statements as described in Note 3 and the impact of the reverse stock split on the 2021 financial
statements as described in Note 2, as to which the date is March 22, 2023, relating to the consolidated financial statements of Palisade
Bio, Inc. (the “Company”) appearing in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022.
Our report contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
We also
consent to the reference to us under the caption “Experts” in the Prospectus.
/s/
BDO USA, P.C.
San
Diego, California
December
29, 2023
Exhibit
23.2
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
hereby consent to the incorporation by reference in the Registration Statement on Form S-1 of our report dated March 22, 2023, relating
to the consolidated financial statements of Palisade Bio, Inc. Our report includes an explanatory paragraph relating to the Company’s
ability to continue as a going concern.
We
also consent to the reference to us under the heading “Experts” in such Registration Statement.
/s/
BAKER TILLY US, LLP
Tewksbury,
Massachusetts
December
29, 2023
Exhibit
107
Calculation
of Filing Fee Tables
Form
S-1
(Form
Type)
Palisade
Bio, Inc.
(Exact
Name of Registrant as Specified in its Charter)
Table
1: Newly Registered and Carry Forward Securities
| | |
Security
Type | |
Security Class Title | |
Fee
Calculation
or Carry
Forward
Rule | | |
Amount
Registered | | |
Proposed
Maximum
Offering
Price Per
Unit | | |
Maximum
Aggregate
Offering
Price(1) | | |
Fee Rate | | |
Amount of
Registration Fee | |
| | |
| |
| |
| | |
| | |
| | |
| | |
| | |
| |
| Fees to Be Paid | |
Equity | |
Shares of common stock, par value $0.0001 per share (2) | |
| 457(o) | | |
| | | |
| | | |
$ | 5,000,000 | | |
| 0.00014760 | | |
$ | 738.00 | |
| | |
| |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Total Offering Amounts | | |
| | | |
$ | 5,000,000 | | |
| | | |
$ | 738.00 | |
| | |
Total Fees Previously Paid | | |
| | | |
| | | |
| | | |
| | |
| | |
Total Fee Offsets | | |
| | | |
| | | |
| | | |
| — | |
| | |
Net Fee Due | | |
| | | |
| | | |
| | | |
$ | 738.00 | |
| (1) |
Estimated
solely for purposes of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended
(the “Securities Act”). |
| (2) |
Pursuant
to Rule 416 under the Securities Act, the securities registered hereby also include an indeterminate number of additional securities
as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations, or other similar transactions. |
Palisade Bio (NASDAQ:PALI)
Historical Stock Chart
From Mar 2024 to Apr 2024
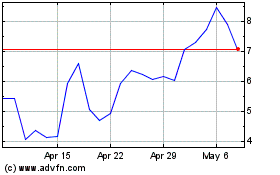
Palisade Bio (NASDAQ:PALI)
Historical Stock Chart
From Apr 2023 to Apr 2024