0001326706
false
0001326706
2023-08-22
2023-08-22
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of
the
Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): August 22, 2023
NanoVibronix,
Inc.
(Exact
name of registrant as specified in its charter)
Delaware
(State
or other jurisdiction of incorporation)
| 001-36445 |
|
01-0801232 |
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
525
Executive Blvd., Elmsford, NY 10523
(Address
of principal executive offices) (Zip Code)
Registrant’s
telephone number, including area code: (914) 233-3004
Not
Applicable
(Former
name or former address, if changed since last report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written communications
pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting material pursuant
to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common Stock, par value
$0.001 per share |
|
NAOV |
|
Nasdaq Capital Market |
| Item 1.01 |
Entry into a Material
Definitive Agreement. |
On
August 22, 2023, NanoVibronix, Inc. (the “Company”), entered into a second amendment (the “Amendment”)
to the Amended and Restated Distribution Agreement for private labeled products, dated December 10, 2020 (as amended by the Amendment,
the “Agreement”), between the Company and Ultra Pain Products Inc. (“UPPI”), effective
as of August 11, 2023.
Pursuant
to the Agreement, UPPI will continue to be the exclusive distributor of PainShield and PainShield Plus devices to the Durable Medical
Equipment distribution sector of the healthcare market in the United States. The Agreement also provides for an immediate re-stocking
order and minimum purchase guarantees through the end of 2023.
The
term of the Amendment began on the effective date of the Amendment, August 11, 2023, and will continue for twelve (12) months or until
the Centers for Medicare and Medicaid Services assigns a reimbursement value to the PainShield product, whichever comes first. At the
end of such term, the parties agreed to enter into good faith negotiations to enter into a new distribution agreement.
On
August 23, 2023, the Company issued a press release regarding entering into the Amendment described above under Item 1.01 of this Current
Report on Form 8-K. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
| Item 9.01 |
Financial
Statements and Exhibits. |
(d)
Exhibits.
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| Date: August 23, 2023 |
NANOVIBRONIX,
Inc. |
| |
|
|
| |
By: |
/s/ Stephen
Brown |
| |
Name: |
Stephen Brown |
| |
Title: |
Chief Financial Officer |
Exhibit
99.1
NanoVibronix
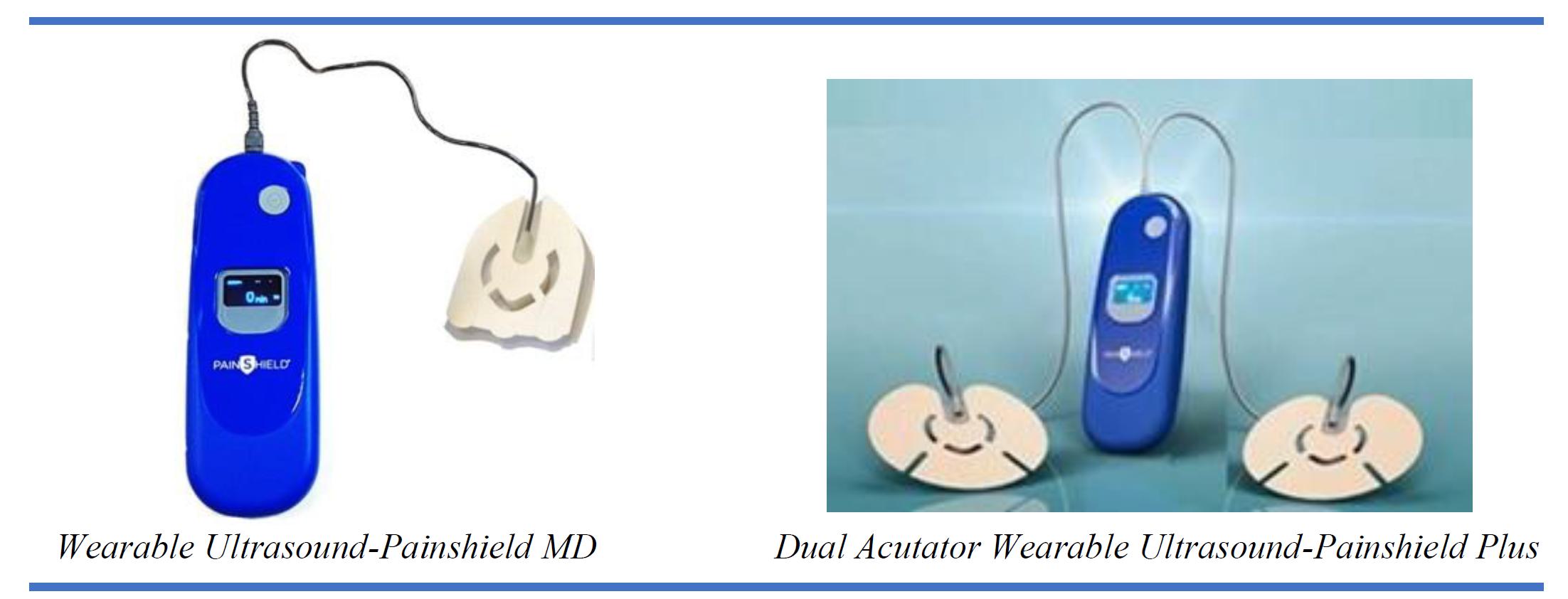
Extends Distribution Agreement with Its Largest Distributor for PainShield and PainShield Plus
Extension
Provides for Guaranteed Purchase Minimums
ELMSFORD,
N.Y., August 23, 2023 (Business Wire) — NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®,
PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that it has
conditionally extended its distribution agreement with Ultra Pain Products, Inc. (“UPPI”) for the company’s PainShield®
and PainShield Plus®.
Under
the terms of the extended agreement, UPPI will continue to be the exclusive distributor of PainShield and PainShield Plus devices to
the Durable Medical Equipment distribution sector of the healthcare market in the United States. The agreement provides for an immediate
re-stocking order and minumum purchase guarantees through the end of 2023.
Brian
Murphy, Chief Executive Officer of NanoVibronix, Inc., commented, “Since executing the original distribution agreement in 2020,
UPPI has grown to be our largest distributor of PainShield and PainShield Plus. The team at UPPI understands the importance of providing
clinicians and patients with non-narcotic/non-opiate clinincally-supported modalities to reduce the economic burden on the healthcare
system and limit the amount of lost working days for injured workers,1 which makes them a valuable channel for our products.
“Our
efforts to obtain full approval from the Centers for Medicare & Medicaid Services (‘CMS’) continue. We submitted the
final report with our application to CMS in March 2023, and we remain hopeful for a favorable outcome. If approved, reimbursements could
begin as early as October 1 of this year. The new agreement with UPPI takes into consideration approval from CMS and provides for modifications
that consider the opportunities afforded through reimbursement.”
1
https://ultrapainpro.com/about-us/
About
NanoVibronix, Inc.
NanoVibronix,
Inc. (NASDAQ: NAOV) is a medical device company headquartered in Elmsford, New York, with research and development in Nesher, Israel,
focused on developing medical devices utilizing its patented low intensity surface acoustic wave (SAW) technology. The proprietary technology
allows for the creation of low-frequency ultrasound waves that can be utilized for a variety of medical applications, including for disruption
of biofilms and bacterial colonization, as well as for pain relief. The devices can be administered at home without the assistance of
medical professionals. The Company’s primary products include PainShield® and UroShield®, which are portable devices suitable
for administration at home without assistance of medical professionals. Additional information about NanoVibronix is available at: www.nanovibronix.com
Forward-looking
Statements
This
press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,”
“may,” “will,” “plans,” “expects,” “anticipates,” “projects,”
“predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential”
or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject
to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or
quantified; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such
risks and uncertainties include, without limitation, risks and uncertainties associated with: (i) market acceptance of our existing and
new products or lengthy product delays in key markets; (ii) negative or unreliable clinical trial results; (iii) inability to secure
regulatory approvals for the sale of our products; (iv) intense competition in the medical device industry from much larger, multinational
companies; (v) product liability claims; (vi) product malfunctions; (vii) our limited manufacturing capabilities and reliance on subcontractor
assistance; (viii) insufficient or inadequate reimbursements by governmental and/or other third party payers for our products; (ix) our
ability to successfully obtain and maintain intellectual property protection covering our products; (x) legislative or regulatory reform
impacting the healthcare system in the U.S. or in foreign jurisdictions; (xi) our reliance on single suppliers for certain product components,
(xii) the need to raise additional capital to meet our future business requirements and obligations, given the fact that such capital
may not be available, or may be costly, dilutive or difficult to obtain; (xiii) our conducting business in foreign jurisdictions exposing
us to additional challenges, such as foreign currency exchange rate fluctuations, logistical and communications challenges, the burden
and cost of compliance with foreign laws, and political and/or economic instabilities in specific jurisdictions; and (xiv) market and
other conditions. More detailed information about the Company and the risk factors that may affect the realization of forward-looking
statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s
Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free
of charge on the SEC’s web site at: http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking
statements as a result of new information, future events, or otherwise, except as required by law.
Investor
Contacts:
Brett
Maas, Managing Principal, Hayden IR, LLC
brett@haydenir.com
(646)
536-7331
SOURCE:
NanoVibronix, Inc.
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
NanoVibronix (NASDAQ:NAOV)
Historical Stock Chart
From Apr 2024 to May 2024
NanoVibronix (NASDAQ:NAOV)
Historical Stock Chart
From May 2023 to May 2024