false
0000883975
0000883975
2024-01-26
2024-01-26
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d)
of
the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): January 26, 2024
MICROBOT
MEDICAL INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
000-19871 |
|
94-3078125 |
(State
or other jurisdiction
of
incorporation) |
|
(Commission
File
Number) |
|
(IRS
Employer
Identification
No.) |
288
Grove Street, Suite 388
Braintree,
MA 02184
(Address
of Principal Executive Offices) (Zip Code)
Registrant’s
telephone number, including area code: (781) 875-3605
(Former
Name or Former Address, if Changed Since Last Report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
Stock, $0.01 par value |
|
MBOT |
|
NASDAQ
Capital Market |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405)
or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging
Growth Company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item
1.01 |
Entry
into a Material Definitive Agreement |
As
of January 26, 2024 (the “Effective Date”), Microbot Medical Inc. (the “Company”) entered into a Settlement Agreement
and Release (the “Settlement Agreement”) with Empery Asset Master Ltd., Empery Tax Efficient, LP, Empery Tax Efficient III,
LP and Hudson Bay Master Fund Ltd. (collectively, “Plaintiffs”), which resolved and settled the below referenced litigation
between the Company and Plaintiffs. The Company previously announced that it was a defendant in a lawsuit captioned Empery Asset Master
Ltd., Empery Tax Efficient, LP, Empery Tax Efficient II, LP, Hudson Bay Master Fund Ltd., Plaintiffs, against Microbot Medical Inc.,
Defendant, in the Supreme Court of the State of New York, County of New York (Index No. 651182/2020) (the “Lawsuit”),
pursuant to which the Plaintiffs alleged, among other things, that the Company breached multiple representations and warranties contained
in the Securities Purchase Agreement (the “SPA”) related to the Company’s June 8, 2017 equity financing (the “Financing”),
of which the Plaintiffs participated, and fraudulently induced Plaintiffs into signing the SPA. The complaint sought rescission of the
SPA and return of the Plaintiffs’ $6.75 million purchase price with respect to the Financing.
Pursuant
to the Settlement Agreement, the Company agreed to pay Plaintiffs $2,154,000 (the “Total Settlement Amount”), consisting
of a cash payment covered by the Company’s insurance carrier of $1,100,000 and 1,005,965 shares of restricted Company common stock
(the “Shares”), which Shares represent the whole number of restricted shares of Company common stock calculated pursuant
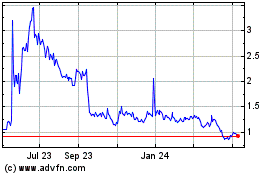
to the following formula: $1,054,000/[closing price of Company common stock on the Effective Date * 0.825]. Additionally, the Plaintiffs
and the Company each agreed to fully release the other from all claims arising out of the Financing, the SPA and/or the allegations and
claims asserted in the Lawsuit, subject to customary carve-outs.
The
Company also agreed, pursuant to a Registration Rights Agreement (the “Registration Rights Agreement”), to file a registration
statement on Form S-1 or Form S-3 covering the resale of the Shares (the “Resale Registration Statement”), within 30 calendar
days following the Effective Date, and to use reasonable best efforts to have such Resale Registration Statement declared effective by
the SEC within 60 days (or, in the event of a “full review” by the Securities and Exchange Commission, within 90 days) following
the Effective Date. The Company shall be required to make cash payments to the Plaintiffs in the event the Company fails to register
the Shares and keep the registration statement effective pursuant to the terms of the Registration Rights Agreement, and if the Company
fails to remove the restrictions on the Shares pursuant to the terms of the Settlement Agreement.
Within
three business days of Plaintiffs’ receipt of the Total Settlement Amount, Plaintiffs will file a stipulation discontinuing the
Lawsuit with prejudice.
The
Settlement Agreement and Registration Rights Agreement are attached as Exhibits 10.1 and 10.2, respectively, to this Current Report on
Form 8-K. The description of the terms of the Settlement Agreement and the Registration Rights Agreement is not intended to be complete
and is qualified in its entirety by reference to such exhibits.
| Item
3.02 |
Unregistered
Sales of Equity Securities |
The
Company issued the Shares pursuant to the exemption from the registration requirements of the Securities Act of 1933, as amended (the
“Securities Act”), available under Section 4(a)(2) as a transaction by an issuer not involving any public offering. The issuance
of the Shares has not been registered under the Securities Act and such securities may not be offered or sold in the United States absent
registration or an exemption from registration under the Securities Act and any applicable state securities laws.
| Item
7.01 |
Regulation
FD Disclosure |
On
January 30, 2024, the Company issued a press release announcing that it had entered into the Settlement Agreement.
The
press release, which is furnished as Exhibit 99.1 to this Current Report on Form 8-K, is incorporated herein by reference. The information
in this Item 7.01 and Exhibit 99.1 is being furnished and shall not be deemed to be “filed” for the purposes of Section 18
of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section. This report will not be
deemed an admission as to the materiality of any information in this Item 7.01 or Exhibit 99.1.
On
January 29, 2024, the Company submitted an Investigational Device Exemption (IDE) application with the U.S. Food and Drug Administration,
in order to commence its pivotal clinical trial in humans.
| Item
9.01 |
Financial
Statetments and Exhibits |
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned thereunto duly authorized.
| |
MICROBOT
MEDICAL INC. |
| |
|
|
| |
By: |
/s/
Harel Gadot |
| |
Name: |
Harel
Gadot |
| |
Title: |
Chief
Executive Officer, President and Chairman |
| |
|
|
| Date:
January 30, 2024 |
|
|
Exhibit 10.1
SETTLEMENT
AGREEMENT AND RELEASE
This
Settlement Agreement and Release (the “Agreement”) is made and entered into this 26th day of January, 2024 (the “Effective
Date”), by and between Empery Asset Master Ltd., Empery Tax Efficient, LP, Empery Tax Efficient III, LP (“Empery”)
and Hudson Bay Master Fund Ltd. (“Hudson Bay” together with Empery, “Plaintiffs”) on the one hand
and Microbot Medical Inc. (“Microbot” or the “Company”) on the other hand. Empery, Hudson Bay,
and Microbot are referred to herein collectively as the “Parties” and individually as a “Party.”
WHEREAS,
the Parties entered into a Securities Purchase Agreement dated June 5, 2017 (the SPA) whereby Empery and Hudson Bay each purchased
1,250,000 shares of Microbot stock for a purchase price of $3,375,000 (the “Offering”);
WHEREAS,
as part of the Offering, Plaintiffs received a Disclosure Schedule that contained a footnote in a “MBOT CAP TABLE” stating
that “Alpha Capital Anstalt, an affiliate of the Company,” was the holder of “‘toothless’ preferred stock
that converts into 11,916,000 shares of common stock subject to conversion limitations”;
WHEREAS,
Plaintiffs allege that Alpha Capital Anstalt (“Alpha”) was not an affiliate of Microbot at the time of the Offering
and that Microbot’s alleged representation that Alpha was an affiliate was a material misrepresentation upon which Plaintiffs relied;
WHEREAS,
after initially entering into a tolling and standstill agreement which thereafter expired, Plaintiffs filed a lawsuit on February 21,
2020 in the Supreme Court of the State of New York, New York County (the “Lawsuit”) alleging that Microbot materially
breached the SPA and sought, among other relief, rescission of the SPA;
WHEREAS,
Microbot denies the allegations in the Lawsuit and denies any liability to Plaintiffs with respect to the Lawsuit, the Offering,
and/or the SPA;
WHEREAS,
to avoid the uncertainty, expense, and burden of litigation, the Parties desire to resolve the dispute between them upon the terms and
in the manner provided in this Agreement, with no Party admitting nor acknowledging any fault or liability to any other Party.
NOW,
THEREFORE, in consideration of the covenants and agreements set forth in this Agreement and the RRA (as defined below) and for other
good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
1.
Settlement Amount. Microbot agrees to pay Plaintiffs
two million one hundred fifty four thousand dollars ($2,154,000) (the Total Settlement Amount), consisting of a cash payment of
one million one hundred thousand dollars ($1,100,000) (the “Cash Settlement Portion”) and the whole number of restricted
shares of Microbot common stock calculated pursuant to the following formula: $1,054,000/[closing price of Microbot common stock on the
Effective Date * 0.825] (the “Stock Settlement Portion”). For the avoidance of doubt, the Parties agree that the Stock Settlement
Portion equals one million five thousand nine hundred sixty-five (1,005,965) shares of restricted Microbot common stock (the “Shares”).
2.
Registration Rights Agreement. Contemporaneously with
the execution of this Agreement, each of the Parties will execute and delivery to the other Parties a registration rights agreement in
form and substance attached hereto as Exhibit A (the “RRA”) which governs the Company’s obligations to file
a registration statement covering the Shares and cause such registration statement to be declared effective and be maintained.
3.
Settlement Payment.
(a)
Within fifteen (15) business days from the Effective
Date, Microbot shall pay the Cash Settlement Portion to Plaintiffs as set forth on Schedule A attached hereto, all in accordance with
the wiring instructions set forth on Schedule A.
(b)
Within five (5) business days from the Effective Date,
Microbot shall issue the Stock Settlement Portion to Plaintiffs as set forth on Schedule A attached hereto. The Shares representing the
Stock Settlement Portion shall be delivered to Plaintiffs via delivery of a book entry statement issued by Microbot’s transfer
agent (the “Transfer Agent”). Microbot will pay all fees and expenses of its Transfer Agent in connection with the
delivery of the Stock Settlement Portion.
4.
Legends and Legend Removal.
(a)
The Plaintiffs agree to the imprinting, so long as is
required by this Section 4(a), of a legend on any of the Shares in substantially the following form:
THIS
SECURITY HAS NOT BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON
AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY
NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION
FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE
SECURITIES LAWS. THIS SECURITY MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT WITH A REGISTERED BROKER-DEALER OR OTHER
LOAN WITH A FINANCIAL INSTITUTION THAT IS AN “ACCREDITED INVESTOR” AS DEFINED IN RULE 501(a) UNDER THE SECURITIES ACT OR
OTHER LOAN SECURED BY SUCH SECURITIES.
(b)
Subject to applicable law, the Company acknowledges
and agrees that a Plaintiff may from time to time pledge pursuant to a bona fide margin agreement with a registered broker-dealer or
grant a security interest in some or all of the Shares to a financial institution that is an “accredited investor” as defined
in Rule 501(a) under the Securities Act and, if required under the terms of such arrangement, such Plaintiff may transfer pledged or
secured Shares to the pledgees or secured parties. Such a pledge or transfer would not be subject to approval of the Company and no legal
opinion of legal counsel of the pledgee, secured party or pledgor shall be required in connection therewith. Further, no notice shall
be required of such pledge. At the appropriate Plaintiff’s expense, and subject to applicable law, the Company will execute and
deliver such reasonable documentation as a pledgee or secured party of Shares may reasonably request in connection with a pledge or transfer
of the Shares, including, if the Shares are subject to registration pursuant to the Registration Rights Agreement, the preparation and
filing of any required prospectus supplement under Rule 424(b)(3) under the Securities Act or other applicable provision of the Securities
Act to appropriately amend the list of Selling Stockholders (as defined in the Registration Rights Agreement) thereunder.
(c)
Provided that the Plaintiffs are not affiliates of the
Company under applicable law, the Shares shall not contain any legend (including the legend set forth in Section 4(a) hereof), (i) while
a registration statement (including the Registration Statement (as defined in the RRA)) covering the resale of such security is effective
under the Securities Act, (ii) following any sale of such Shares pursuant to Rule 144 and the Company is then in compliance with the
current public information required under Rule 144, (iii) if such Shares are eligible for sale or may be sold under Rule 144, without
volume or manner-of-sale restrictions, or (iv) if such legend is not required under applicable requirements of the Securities Act (including
judicial interpretations and pronouncements issued by the staff of the Securities and Exchange Commission (the “Commission”))
(the earliest of such dates, the “Release Date”). The Company shall cause its counsel to issue a legal opinion to
the Transfer Agent or the Plaintiff promptly after the Release Date if required by the Transfer Agent to effect the removal of the legend
hereunder under applicable law, or if requested by a Plaintiff, respectively. In furtherance of the foregoing, the Company agrees that
following the earliest Release Date (for purposes of this Section 4(c), as to some or all Shares) or such time as such legend is no longer
required under this Section 4(c), it will, no later than the earlier of (i) two (2) Trading Days (as defined below) and (ii) the number
of Trading Days comprising the Standard Settlement Period (as defined below) following the delivery by a Plaintiff to the Company or
the Transfer Agent of instructions to cancel the Shares issued with a restrictive legend for de-legending (such date, the “Legend
Removal Date”), deliver or cause to be delivered to such Plaintiff such Shares that are free from all restrictive and other
legends. The Company may not make any notation on its records or give instructions to the Transfer Agent that enlarge the restrictions
on transfer set forth in this Section 4. Shares subject to legend removal hereunder shall be transmitted by the Transfer Agent to the
Plaintiff by crediting the account of the Plaintiff’s prime broker with the Depository Trust Company System as directed by such
Plaintiff. As used herein, (i) “Standard Settlement Period” means the standard settlement period, expressed in a number
of Trading Days, on the Company’s primary Trading Market with respect to the Shares as in effect on the date of delivery of a certificate
representing Shares issued with a restrictive legend, (ii) “Trading Day” means a day on which the principal Trading
Market is open for trading, and (ii) “Trading Market” means any of the following markets or exchanges on which the
Shares are listed or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market,
the Nasdaq Global Select Market, the New York Stock Exchange, the OTCQB, OTCQX, Pink Open Market (or any successors to any of the foregoing)
The Company will pay all fees and expenses of its Transfer Agent in connection with the removal of legends from the Shares under this
Section 4, including without limitation fees and expenses in connection with expedited actions by the Transfer Agent.
(d)
In addition to such Plaintiff’s other available
remedies, the Company shall pay to a Plaintiff, in cash, if the Company fails to (a) issue and deliver (or cause to be delivered) to
a Plaintiff by the Legend Removal Date the Shares (so delivered or instructed to be delivered to the Company or Transfer Agent by such
Plaintiff) that is free from all restrictive and other legends and (b) if after the Legend Removal Date such Plaintiff purchases (in
an open market transaction or otherwise) shares of Microbot common stock to deliver in satisfaction of a sale by such Plaintiff of all
or any portion of the number of shares of Microbot common stock, or a sale of a number of shares of Microbot common stock equal to all
or any portion of the number of shares of Microbot common stock that such Plaintiff anticipated receiving from the Company without any
restrictive legend, then, an amount equal to the excess of such Plaintiff’s total purchase price (including brokerage commissions
and other out-of-pocket expenses, if any) for the shares of Microbot common stock so purchased (including brokerage commissions and other
out-of-pocket expenses, if any) (the “Buy-In Price”) over the product of (A) such number of Shares that the Company
was required to deliver to such Plaintiff by the Legend Removal Date multiplied by (B) the lowest closing sale price of Microbot common
stock on any Trading Day during the period commencing on the date of the delivery by such Plaintiff to the Company of the applicable
Shares and ending on the date of such delivery and payment under this section. For the avoidance of doubt, no amounts shall be payable
by the Company under this Section 4(d) that relate to sales by a Plaintiff of Microbot common stock occurring prior to the earlier of
the date that (i) the Registration Statement (as defined in the RRA) is declared effective under the Securities Act, or (ii) the Shares
are eligible for resale pursuant to Rule 144.
(e)
The Company shall (a) by 9:00 am on January 30, 2024,
issue a press release disclosing the settlement, and (b) file a Current Report on Form 8-K, including this Agreement and the RRA as exhibits
thereto, with the Securities and Exchange Commission within the time required by the Securities Exchange Act of 1934, as amended. From
and after the issuance of such press release, the Company represents to the Plaintiffs that it shall have publicly disclosed all material,
non-public information delivered to any of the Plaintiffs by the Company or any of its subsidiaries, or any of their respective officers,
directors, employees, affiliates or agents in connection with the transactions contemplated hereby. In addition, effective upon the issuance
of such press release, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement,
whether written or oral, between the Company, any of its subsidiaries or any of their respective officers, directors, agents, employees
or affiliates, on the one hand, and any of the Plaintiffs or any of their affiliates on the other hand, shall terminate and be of no
further force or effect. The Company understands and confirms that each Plaintiff shall be relying on the foregoing covenant in effecting
transactions in securities of the Company. The Company further covenants and agrees that neither it, nor any other Person acting on its
behalf will provide any Plaintiff or its agents or counsel with any information that constitutes, or the Company reasonably believes
constitutes, material non-public information, unless prior thereto such Plaintiff shall have consented in writing to the receipt of such
information and agreed in writing with the Company to keep such information confidential.
5.
Mutual Releases.
(a)
Plaintiffs’ Releases of Microbot. Upon
receipt of an executed copy of this Agreement, the RRA and the Total Settlement Amount from Microbot, Plaintiffs on behalf of (i) themselves
and their current and former principals, members, shareholders, directors, managers, officers, employees, agents, representatives, partners,
joint venturers, consultants, beneficiaries, heirs, assigns, executors, administrators, trustees, attorneys and advisors, and (ii) each
of their predecessors, successors, parents, subsidiaries, affiliates, and each of their respective current and former principals, members,
shareholders, directors, managers, officers, employees, agents, representatives, partners, joint venturers, consultants, beneficiaries,
heirs, assigns, executors, administrators, trustees, attorneys and advisors (the “Plaintiff Releasing Parties”), fully
and irrevocably releases, settles, acquits and forever discharges (i) Microbot and its current and former principals, members, shareholders,
directors, managers, officers, employees, agents, representatives, partners, joint venturers, consultants, beneficiaries, heirs, assigns,
executors, administrators, trustees, attorneys and advisors, and (ii) each of their predecessors, successors, parents, subsidiaries,
affiliates and divisions, and each of their respective current and former principals, members, shareholders, directors, managers, officers,
employees, agents, representatives, partners, joint venturers, consultants, beneficiaries, heirs, assigns, executors, administrators,
trustees, attorneys and advisors (the “Microbot Released Parties”) to the fullest extent permitted by applicable law
from any and all claims, counterclaims, complaints, causes of action, suits, losses of every kind, demands, debts or expenses (including,
but not limited to, attorneys’ fees and costs actually incurred), liens, contractual obligations, undertakings, warranties, liabilities
or damages of whatever nature, at law, in equity, or otherwise, whether known or unknown, suspected or unsuspected, asserted or unasserted,
whether for equitable, declaratory, monetary, injunctive or any other type of relief whatsoever that the Plaintiff Releasing Parties
have, had or may have against the Microbot Released Parties, arising out of or relating to the Offering, the SPA, and/or the allegations
and claims asserted in the Lawsuit, from the beginning of the world through the date of signing this Agreement; provided that nothing
in this Section 5(a) releases Microbot from the obligations contained in this Agreement or the RRA nor are the Plaintiff Releasing Parties
releasing any claims or causes of action against Microbot for enforcement of this Agreement or the RRA.
(b)
Microbot Releases of Plaintiffs. Upon receipt
of an executed copy of this Agreement from Plaintiffs and payment of the Total Settlement Amount, Microbot, on behalf of (i) itself and
its current and former principals, members, shareholders, directors, managers, officers, employees, agents, representatives, partners,
joint venturers, consultants, beneficiaries, heirs, assigns, executors, administrators, trustees, attorneys and advisors, and (ii) each
of their predecessors, successors, parents, subsidiaries, affiliates and divisions, and each of their respective current and former principals,
members, shareholders, directors, managers, officers, employees, agents, representatives, partners, joint venturers, consultants, beneficiaries,
heirs, assigns, executors, administrators, trustees, attorneys and advisors (the “Microbot Releasing Parties”), fully
and irrevocably releases, settles, acquits and forever discharges (i) Plaintiffs and their current and former principals, members, shareholders,
directors, managers, officers, employees, agents, representatives, partners, joint venturers, consultants, beneficiaries, heirs, assigns,
attorneys and advisors, and (ii) each of Plaintiffs’ predecessors, successors, parents, subsidiaries, affiliates and divisions,
and each of their respective current and former principals, members, shareholders, directors, managers, officers, employees, agents,
representatives, partners, joint venturers, consultants, beneficiaries, heirs, assigns, executors, administrators, trustees, attorneys
and advisors (the “Plaintiff Released Parties”) to the fullest extent permitted by applicable law from any and all
claims, counterclaims, complaints, causes of action, suits, losses of every kind, demands, debts or expenses (including, but not limited
to, attorneys’ fees and costs actually incurred), liens, contractual obligations, undertakings, warranties, liabilities or damages
of whatever nature, at law, in equity, or otherwise, whether known or unknown, suspected or unsuspected, asserted or unasserted, whether
for equitable, declaratory, monetary, injunctive or any other type of relief whatsoever that the Microbot Releasing Parties have, had
or may have against the Plaintiff Released Parties, arising out of or relating to the Offering, the SPA, and/or the allegations and claims
asserted in the Lawsuit, from the beginning of the world through the date of signing this Agreement; provided that nothing in this Section
5(b) releases Plaintiffs from the obligations contained in this Agreement or the RRA nor are the Microbot Releasing Parties releasing
any claims or causes of action against the Plaintiffs for enforcement of this Agreement or the RRA.
(c)
The Plaintiff Releasing Parties and the Microbot Releasing
Parties each understand and agree that the Parties’ agreement to provide these general releases is a material condition of this
Agreement and to providing the Settlement Amount identified in this Agreement.
(d)
The Plaintiff Releasing Parties represent that they
have no other suits, claims, complaints or demands of any kind whatsoever currently pending against the Microbot Released Parties with
any local, state, or federal court or other tribunal, nor are they aware of any facts that would serve as the basis for any civil or
administrative proceeding against the Microbot Released Parties.
(e)
The Microbot Releasing Parties represent that they have
no other suits, claims, complaints or demands of any kind whatsoever currently pending against the Plaintiff Released Parties with any
local, state, or federal court or other tribunal, nor are they aware of any facts that would serve as the basis for any civil or administrative
proceeding against the Plaintiff Released Parties.
(f)
The Plaintiff Releasing Parties and Microbot Releasing
Parties hereby acknowledge and waive any and all rights and protections under California Civil Code Section 1542, or any similar provision
of state or federal law. The Parties each acknowledge that they have been advised by their attorneys of the contents and effects of Section
1542, which section has been duly explained and reads as follows:
A
GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR
AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE
DEBTOR OR RELEASED PARTY.
6.
No Release for Breach of this Agreement or the RRA.
Notwithstanding anything to the contrary in this Agreement or the RRA, the releases contained in this Agreement do not and are not intended
to release any claims that either Party may have against the other Party for a breach of this Agreement or the RRA.
7.
Covenant Not to Sue. Each Party covenants never
to institute, participate in, assist or encourage, either directly or indirectly, any suit, action, arbitration or proceeding, at law
or in equity, against the Microbot Released Parties or the Plaintiff Released Parties, as applicable, arising from or related to the
claims, counterclaims, complaints, causes of action, suits, losses, demands, debts or expenses (including, but not limited to, attorneys’
fees and costs actually incurred), liens, liabilities or damages that are released in this Agreement.
8.
Dismissal of the Action. Within three (3) business
days of Plaintiffs’ receipt of the Total Settlement Amount, Plaintiffs will file a stipulation discontinuing the Action with prejudice
that shall be executed by counsel for Plaintiffs and Microbot substantially in the same form attached to this Agreement as Exhibit
B.
9.
No Admission of Liability. The Parties have entered
into this Agreement and the RRA solely for the purposes of avoiding the expense and inconvenience of litigation. Neither the execution
of this Agreement and the RRA, nor the effectuation of the settlement as set forth herein and therein, shall constitute or be construed
in any manner whatsoever as an admission or concession of liability or wrongdoing, or lack of merit of any claims or defenses, on the
part of either Party. This Agreement, the RRA, and any other evidence of the terms of this settlement, shall not be offered or received
in evidence in any action or other proceeding as an admission or concession of liability or wrongdoing, or lack of merit of any claims
or defenses, on the part of either Party.
10.
Ownership of Claims. Each Party warrants and
represents that it is the sole and lawful owner of all rights, title and interests in and to any claims, counterclaims, complaints, causes
of action, suits, losses, demands, debts or expenses (including, but not limited to, attorneys’ fees and costs actually incurred),
liens, liabilities or damages that are the subject of this Agreement, including without limitation the releases set forth in this Agreement,
and that it has not sold, assigned, granted, transferred or hypothecated any right, title or interest in any such claims, counterclaims,
complaints, causes of action, suits, losses, demands, debts or expenses (including, but not limited to, attorneys’ fees and costs
actually incurred), liens, liabilities or damages to any other person or entity.
11.
Governing Law and Venue. This Agreement shall
be construed under and governed by the laws of the State of New York, without regard to choice-of-law principles. Any suit, action, or
proceeding between the Parties arising out of or related to this Agreement must be brought exclusively in the federal or state courts
located in New York, New York, and the Parties each hereby submit to the personal jurisdiction thereof and agree to such courts as the
appropriate venue, and expressly waive any objection to such jurisdiction or venue based on the doctrine of forum non conveniens.
Process in any action or proceeding referenced in this paragraph 13 may be served in accordance with the notice provisions set forth
in paragraph 22 of this Agreement.
12.
No Waiver. Any failure by a Party to pursue any
breach of any provision of this Agreement shall not constitute a waiver of that provision, or any other provision, of this Agreement.
The failure of a Party to insist upon the strict performance of any term or condition in this Agreement shall not be considered a waiver
or relinquishment of further compliance therewith.
13.
Entire Agreement; Amendments. This Agreement
and the RRA contains the entire agreement between the Parties concerning the subject matter hereof and supersedes all prior agreements,
understandings, discussions, negotiations and undertakings, whether written or oral, between the Parties with respect thereto. In entering
this Agreement and the RRA, no Party has relied upon any representation or warranty of the other Party that is not included in this Agreement
or the RRA.
14.
No Oral Modification. This Agreement may not
be amended, modified or terminated, except by a written instrument signed by each of the Parties hereto.
15.
Saving Clause and Severability. If any provision
of this Agreement is held to be invalid or unenforceable by any judicial or other competent authority, all other provisions of this Agreement
will remain in full force and effect and will not in any way be impaired, provided that no Party is deprived of the material benefits
of this Agreement. If owing to the invalidity or unenforceability of any provision of this Agreement any Party is deprived of the material
benefits of this Agreement, the Parties shall substitute for the invalid or unenforceable provision, a provision that will allow such
Party or Parties to enjoy such material benefits.
16.
Binding Effect. This Agreement shall inure to
the benefit of the Parties and shall be binding upon each of the Parties and their permitted assigns, successors, heirs, and representatives.
17.
Authority to Enter Into and Understanding of Agreement.
Each Party hereto represents and warrants as respects itself that: (i) the individual executing this Agreement on its behalf is duly
authorized to do so; (ii) such Party is entering this Agreement of its own free will, free of any duress, undue influence or compulsion
by any person or entity, and has the full authority and capacity necessary to do so; (iii) such Party is represented by counsel of its
own choosing in connection with this Agreement; and (iv) such Party has read this Agreement and understands all of the terms hereof.
18.
Agreement Jointly Drafted. The Parties agree
that each and every provision of this Agreement and the RRA shall be deemed to have been simultaneously drafted by each of the Parties,
and no laws or rules relating to the interpretation of contracts against the drafter of any particular clause should be applied to the
interpretation or enforcement of this Agreement or the RRA.
19.
Non-Inducement. The Parties warrant that no promise
or inducement has been made or offered, except as set forth herein, and that this Agreement is executed voluntarily to dispose of all
claims identified in this Agreement, without reliance upon any statement or representation by any attorney, agent or other representative
acting on behalf of any of the Parties.
20.
Attorneys’ Fees and Costs. Each Party to
this Agreement shall bear its own attorneys’ fees and costs arising out of or related to the dispute between the Parties, this
Agreement and the claims released herein, and no further claim shall be made therefore.
21.
No Third-Party Beneficiaries. The provisions
of this Agreement are for the sole benefit of the Parties and their successors and permitted assigns,
and they will not be construed as conferring any rights (including, without limitation, any third-party beneficiary rights) to any other
person.
22.
Notice. All notices, requests, and demands to
or upon a Party shall be in writing and sent by email and overnight courier as follows (or to such other address or email as either Party
may from time to time direct):
| If
to Empery: |
c/o
Empery Asset Management, LP
1
Rockefeller Plaza, Suite 1205
New
York, New York 10020
Attention:
Brett Director
E-mail:
notices@emperyam.com
With
a copy (which shall not constitute notice) to:
Schulte
Roth & Zabel, LLP
919
Third Avenue
New
York, New York 10022
Attention:
Andrew D. Gladstein
Email:
andrew.gladstein@srz.com |
| |
|
| If
to Hudson Bay: |
c/o
Hudson Bay Capital Management, LP
28
Havemeyer Place, 2nd Floor
Greenwich,
Connecticut 06830
Attention:
DITeam
Email:
diteam@hudsonbaycapital.com
With
a copy (which shall not constitute notice) to:
Schulte
Roth & Zabel LLP
919
Third Avenue
New
York, New York 10022
Attention:
Andrew D. Gladstein
Email:
andrew.gladstein@srz.com |
| |
|
| If
to Microbot |
Microbot
Medical Inc.
288
Grove Street, Suite 388
Braintree,
MA 02184
Attention:
Harel Gadot
Email:
info@microbotmedical.com
With
a copy (which shall not constitute notice) to:
Mintz
Levin Cohn Ferris
Glovsky
and Popeo, P.C.
One
Financial Center
Boston,
MA 02111
Attention:
John F. Sylvia
Email:
jfsylvia@mintz.com |
23.
No Assignment. This Agreement may not be assigned,
conveyed or otherwise transferred, in whole or in part, by either Party (other than by the operation of law in connection with a merger
or sale) without express written consent of the non-assigning Party.
24.
Counterparts. This Agreement may be executed
in counterparts, and each counterpart, when executed, shall have the efficacy of a signed original. Photographic, emailed, imaged and
facsimiled copies of such signed counterparts may be used in lieu of the originals for any purpose.
25.
Headings. The headings of the paragraphs of this
Agreement have been inserted for reference only and are not part of this Agreement and are not to be used in any way in the construction
or interpretation hereof.
IN
WITNESS WHEREOF, intending to be legally bound hereby, the undersigned have executed this Agreement as of the Effective Date.
[Signatures
on next page]
| |
EMPERY
ASSET MASTER LTD. |
| |
By:
|
Empery
Asset Management, LP, its authorized agent |
| |
|
|
| |
/s/
Brett Director |
| |
Name:
|
Brett
Director |
| |
Title: |
General
Counsel |
| |
|
|
| |
EMPERY
TAX EFFICIENT, LP |
| |
By:
|
Empery
Asset Management, LP, its authorized agent |
| |
|
|
| |
/s/
Brett Director |
| |
Name:
|
Brett
Director |
| |
Title: |
General
Counsel |
| |
|
|
| |
EMPERY
TAX EFFICIENT III, LP |
| |
By:
|
Empery
Asset Management, LP, its authorized agent |
| |
|
|
| |
/s/
Brett Director |
| |
Name:
|
Brett
Director |
| |
Title: |
General
Counsel |
| |
|
|
| |
HUDSON
BAY MASTER FUND LTD. |
| |
By: |
Hudson
Bay Capital Management, LP |
| |
|
|
| |
/s/
Richard Allison |
| |
Name: |
Richard
Allison |
| |
Title of Authorized Signatory: Authorized Signatory, Hudson Bay Capital Management LP not individually, but solely as Investment Advisor to Hudson Bay Master Fund Ltd. |
| |
|
|
| |
MICROBOT
MEDICAL INC. |
| |
|
|
| |
/s/
Harel Gadot |
| |
By: |
Harel
Gadot |
| |
Title of Authorized Signatory: CEO & President |
Schedule
A
| Name | |
Cash
Settlement Amount | | |
#
of Shares | |
| | |
| | |
| |
Empery
Asset Master, Ltd. | |
$ | 290,608.97 | | |
| 280,574 | |
| Wire Instructions: | |
| | | |
| | |
| | |
| | | |
| | |
Empery
Tax Efficient, LP | |
$ | 146,042.56 | | |
| 140,999 | |
| Wire Instructions: | |
| | | |
| | |
| | |
| | | |
| | |
Empery
Tax Efficient III, LP | |
$ | 188,515.67 | | |
| 182,006 | |
| Wire Instructions: | |
| | | |
| | |
| | |
| | | |
| | |
Hudson
Bay Master Fund Ltd. | |
$ | 474,832.80 | | |
| 402,386 | |
| Wire Instructions: | |
| | | |
| | |
| | |
| | | |
| | |
TOTAL
| |
$ | 1,100,000.00 | | |
| 1,005,965 | |
Exhibit
10.2
REGISTRATION
RIGHTS AGREEMENT
This
Registration Rights Agreement (this “Agreement”) is made and entered into as of January 26, 2024, between Microbot
Medical Inc., a Delaware corporation (the “Company”), and each of the several entities signatory hereto (each such
entity, a “Plaintiff” and, collectively, the “Plaintiffs”).
This
Agreement is made pursuant to the Settlement Agreement and Release, dated as of the date hereof, between the Company and each Plaintiff
(the “Settlement Agreement”).
The
Company and each Plaintiff hereby agree as follows:
1.
Definitions. Capitalized terms used and not otherwise defined herein that are defined in the Settlement Agreement shall have the
meanings given such terms in the Settlement Agreement. As used in this Agreement, the following terms shall have the following meanings:
“Advice”
shall have the meaning set forth in Section 6(b).
“Effectiveness
Date” means, with respect to the Registration Statement required to be filed hereunder, the 60th calendar day following
the date hereof (or, in the event of a “full review” by the Commission, the 90th calendar day following the date
hereof), provided, if such Effectiveness Date falls on a day that is not a Trading Day, then the Effectiveness Date shall be the
next succeeding Trading Day.
“Effectiveness
Period” shall have the meaning set forth in Section 2(a).
“Filing
Date” means the 30th calendar day following the date hereof.
“Holder”
or “Holders” means the holder or holders, as the case may be, from time to time of Registrable Securities.
“Indemnified
Party” shall have the meaning set forth in Section 5(c).
“Indemnifying
Party” shall have the meaning set forth in Section 5(c).
“Losses”
shall have the meaning set forth in Section 5(a).
“Prospectus”
means the prospectus included in a Registration Statement (including, without limitation, a prospectus that includes any information
previously omitted from a prospectus filed as part of an effective registration statement in reliance upon Rule 430A promulgated by the
Commission pursuant to the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the
offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to
the Prospectus, including post-effective amendments, and all material incorporated by reference or deemed to be incorporated by reference
in such Prospectus.
“Registrable
Securities” means, as of any date of determination, (a) all shares issued as the Stock Settlement Portion and (b) any securities
issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing;
provided, however, that any such Registrable Securities shall cease to be Registrable Securities (and the Company shall
not be required to maintain the effectiveness of any, or file another, Registration Statement hereunder with respect thereto) for so
long as (a) a Registration Statement with respect to the sale of such Registrable Securities is declared effective by the Commission
under the Securities Act and such Registrable Securities have been disposed of by the Holder in accordance with such effective Registration
Statement, (b) such Registrable Securities have been previously sold in accordance with Rule 144, or (c) such securities become eligible
for resale without volume or manner-of-sale restrictions and without current public information pursuant to Rule 144 as set forth in
a written opinion letter to such effect, addressed, delivered and acceptable to the Transfer Agent and the affected Holders (assuming
that such securities and any securities issuable upon exercise, conversion or exchange of which, or as a dividend upon which, such securities
were issued or are issuable, were at no time held by any Affiliate of the Company, as reasonably determined by the Company, upon the
advice of counsel to the Company.
“Registration
Statement” means any registration statement required to be filed hereunder pursuant to Section 2(a), including the Prospectus,
amendments and supplements to any such registration statement or Prospectus, including pre- and post-effective amendments, all exhibits
thereto, and all material incorporated by reference or deemed to be incorporated by reference in any such registration statement.
“Rule
415” means Rule 415 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted
from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“Rule
424” means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted
from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“Selling
Stockholder Questionnaire” shall have the meaning set forth in Section 3(a).
“SEC
Guidance” means (i) any publicly-available written or oral guidance of the Commission staff, or any comments, requirements
or requests of the Commission staff and (ii) the Securities Act.
2.
Shelf Registration.
(a)
On or prior to the Filing Date, the Company shall prepare and file with the Commission a Registration Statement covering the resale of
all of the Registrable Securities that are not then registered on an effective Registration Statement for an offering to be made on a
continuous basis pursuant to Rule 415. The Registration Statement filed hereunder shall be on Form S-3 or Form S-1 and shall contain
(unless otherwise directed by at least a majority in interest of the Holders) substantially the “Plan of Distribution”
attached hereto as Annex A and substantially the “Selling Stockholder” section attached hereto as Annex B;
provided, however, that no Holder shall be required to be named as an “underwriter” without such Holder’s
express prior written consent. Subject to the terms of this Agreement, the Company shall use its reasonable best efforts to cause a Registration
Statement filed under this Agreement to be declared effective under the Securities Act as promptly as possible after the filing thereof,
but in any event no later than the applicable Effectiveness Date, and shall use its reasonable best efforts to keep such Registration
Statement continuously effective under the Securities Act until the date that all Registrable Securities covered by such Registration
Statement (i) have been sold, thereunder or pursuant to Rule 144, or (ii) may be sold without volume or manner-of-sale restrictions pursuant
to Rule 144 and without the requirement for the Company to be in compliance with the current public information requirement under Rule
144, as determined by the counsel to the Company pursuant to a written opinion letter to such effect, addressed and acceptable to the
Transfer Agent and the affected Holders (the “Effectiveness Period”). The Company shall telephonically request effectiveness
of a Registration Statement as of 5:00 p.m. (New York City time) on a Trading Day. The Company shall promptly notify the Holders via
e-mail of the effectiveness of a Registration Statement on the same Trading Day that the Company telephonically confirms effectiveness
with the Commission, which shall be the date requested for effectiveness of such Registration Statement. The Company shall, by 9:30 a.m.
(New York City time) on the Trading Day after the effective date of such Registration Statement, file a final Prospectus with the Commission
as required by Rule 424. Failure to so notify the Holder within one (1) Trading Day of such notification of effectiveness or failure
to file a final Prospectus as aforesaid shall be deemed an Event under Section 2(c).
(b)
Notwithstanding the registration obligations set forth in Section 2(a), if the Commission informs the Company that all of the Registrable
Securities cannot, as a result of the application of Rule 415, be registered for resale as a secondary offering on a single registration
statement, the Company agrees to promptly inform each of the Holders thereof and use its reasonable best efforts to file amendments to
the Registration Statement as required by the Commission, covering the maximum number of Registrable Securities permitted to be registered
by the Commission, on Form S-3 or Form S-1 or such other form available to register for resale the Registrable Securities as a secondary
offering; provided, however, that prior to filing such amendment, the Company shall be obligated to use diligent efforts
to advocate with the Commission for the registration of all of the Registrable Securities in accordance with the SEC Guidance, including
without limitation, Compliance and Disclosure Interpretation 612.09.
(c)
If: (i) the Registration Statement is not filed on or prior to the Filing Date (if the Company files the Registration Statement without
affording the Holders the opportunity to review and comment on the same as required by Section 3(a) herein, the Company shall be deemed
to have not satisfied this clause (i)), or (ii) the Company fails to file with the Commission a request for acceleration of a Registration
Statement in accordance with Rule 461 promulgated by the Commission pursuant to the Securities Act, within five (5) Trading Days of the
date that the Company is notified (orally or in writing, whichever is earlier) by the Commission that such Registration Statement will
not be “reviewed” or will not be subject to further review, or (iii) prior to the effective date of a Registration Statement,
the Company fails to file a pre-effective amendment and otherwise respond in writing to comments made by the Commission in respect of
such Registration Statement within fifteen (15) calendar days after the receipt of comments by or notice from the Commission that such
amendment is required in order for such Registration Statement to be declared effective, or (iv) a Registration Statement registering
for resale all of the Registrable Securities is not declared effective by the Commission by the Effectiveness Date of the Registration
Statement, or (v) after the effective date of a Registration Statement, such Registration Statement ceases for any reason to remain continuously
effective as to all Registrable Securities included in such Registration Statement, or the Holders are otherwise not permitted to utilize
the Prospectus therein to resell such Registrable Securities, for more than twenty (20) consecutive calendar days or more than an aggregate
of thirty (30) calendar days (which need not be consecutive calendar days) during any 12-month period (any such failure or breach being
referred to as an “Event”, and for purposes of clauses (i) and (iv), the date on which such Event occurs, and for
purpose of clause (ii) the date on which such five (5) Trading Day period is exceeded, and for purpose of clause (iii) the date which
such fifteen (15) calendar day period is exceeded, and for purpose of clause (v) the date on which such twenty (20) or thirty (30) calendar
day period, as applicable, is exceeded being referred to as “Event Date”), then, in addition to any other rights the
Holders may have hereunder or under applicable law, on each such Event Date and on each monthly anniversary of each such Event Date (if
the applicable Event shall not have been cured by such date) until the applicable Event is cured, the Company shall pay to each Holder
an amount in cash, as partial liquidated damages and not as a penalty, equal to the product of 1.0% multiplied by the aggregate value
of such Holder’s pro-rata Stock Settlement Portion of the Total Settlement Amount attributable to such Holder pursuant to the Settlement
Agreement. If the Company fails to pay any partial liquidated damages pursuant to this Section in full within seven (7) days after the
date payable, the Company will pay interest thereon at a rate of 12% per annum (or such lesser maximum amount that is permitted to be
paid by applicable law) to the Holder, accruing daily from the date such partial liquidated damages are due until such amounts, plus
all such interest thereon, are paid in full. The partial liquidated damages pursuant to the terms hereof shall apply on a daily pro rata
basis for any portion of a month prior to the cure of an Event.
(d)
Notwithstanding anything to the contrary contained herein, in no event shall the Company be permitted to name any Holder or affiliate
of a Holder as any Underwriter without the prior written consent of such Holder.
3.
Registration Procedures. In connection with the Company’s registration obligations hereunder, the Company shall:
(a)
Not less than three (3) Trading Days prior to the filing of each Registration Statement and not less than one (1) Trading Day prior to
the filing of any related Prospectus or any amendment or supplement thereto (including any document that would be incorporated or deemed
to be incorporated therein by reference), the Company shall (i) furnish to each Holder copies of all such documents proposed to be filed,
which documents (other than those incorporated or deemed to be incorporated by reference) will be subject to the review of such Holders,
and (ii) cause its officers and directors, counsel and independent registered public accountants to respond to such inquiries as shall
be necessary, in the reasonable opinion of respective counsel to each Holder, to conduct a reasonable investigation within the meaning
of the Securities Act. Each Holder agrees to furnish to the Company a completed questionnaire in customary form (a “Selling
Stockholder Questionnaire”) on a date that is not less than one (1) Trading Day prior to the Filing Date.
(b)
(i) Prepare and file with the Commission such amendments, including post-effective amendments, to a Registration Statement and the Prospectus
used in connection therewith as may be necessary to keep a Registration Statement continuously effective as to the applicable Registrable
Securities for the Effectiveness Period and prepare and file with the Commission such additional Registration Statements in order to
register for resale under the Securities Act all of the Registrable Securities, (ii) cause the related Prospectus to be amended or supplemented
by any required Prospectus supplement (subject to the terms of this Agreement), and, as so supplemented or amended, to be filed pursuant
to Rule 424, (iii) respond as promptly as reasonably possible to any comments received from the Commission with respect to a Registration
Statement or any amendment thereto and provide as promptly as reasonably possible to the Holders true and complete copies of all correspondence
from and to the Commission relating to a Registration Statement (provided that, the Company shall excise any information contained therein
which would constitute material non-public information regarding the Company or any of its Subsidiaries), and (iv) comply in all material
respects with the applicable provisions of the Securities Act and the Exchange Act with respect to the disposition of all Registrable
Securities covered by a Registration Statement during the applicable period in accordance (subject to the terms of this Agreement) with
the intended methods of disposition by the Holders thereof set forth in such Registration Statement as so amended or in such Prospectus
as so supplemented.
(c)
Notify the Holders of Registrable Securities to be sold (which notice shall, pursuant to clauses (iii) through (vi) hereof, be accompanied
by an instruction to suspend the use of the Prospectus until the requisite changes have been made) as promptly as reasonably possible
(and, in the case of (i)(A) below, not less than one (1) Trading Day prior to such filing) and (if requested by any such Person) confirm
such notice in writing no later than one (1) Trading Day following the day (i)(A) when a Prospectus or any Prospectus supplement or post-effective
amendment to a Registration Statement is proposed to be filed, (B) when the Commission notifies the Company whether there will be a “review”
of such Registration Statement and whenever the Commission comments in writing on such Registration Statement, and (C) with respect to
a Registration Statement or any post-effective amendment, when the same has become effective, (ii) of any request by the Commission or
any other federal or state governmental authority for amendments or supplements to a Registration Statement or Prospectus or for additional
information, (iii) of the issuance by the Commission or any other federal or state governmental authority of any stop order suspending
the effectiveness of a Registration Statement covering any or all of the Registrable Securities or the initiation of any Proceedings
for that purpose, (iv) of the receipt by the Company of any notification with respect to the suspension of the qualification or exemption
from qualification of any of the Registrable Securities for sale in any jurisdiction, or the initiation or threatening of any Proceeding
for such purpose, (v) of the occurrence of any event or passage of time that makes the financial statements included in a Registration
Statement ineligible for inclusion therein or any statement made in a Registration Statement or Prospectus or any document incorporated
or deemed to be incorporated therein by reference untrue in any material respect or that requires any revisions to a Registration Statement,
Prospectus or other documents so that, in the case of a Registration Statement or the Prospectus, as the case may be, it will not contain
any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements
therein, in light of the circumstances under which they were made, not misleading, and (vi) of the occurrence or existence of any pending
corporate development with respect to the Company that the Company believes may be material and that, in the determination of the Company,
makes it not in the best interest of the Company to allow continued availability of a Registration Statement or Prospectus; provided,
however, that in no event shall any such notice contain any information which would constitute material, non-public information
regarding the Company or any of its Subsidiaries, and the Company agrees that the Holders shall not have any duty of confidentiality
to the Company or any of its Subsidiaries and shall not have any duty to the Company or any of its Subsidiaries not to trade on the basis
of such information.
(d)
Use its reasonable best efforts to avoid the issuance of, or, if issued, obtain the withdrawal of (i) any order stopping or suspending
the effectiveness of a Registration Statement, or (ii) any suspension of the qualification (or exemption from qualification) of any of
the Registrable Securities for sale in any jurisdiction, at the earliest practicable moment.
(e)
Furnish to each Holder, without charge, at least one conformed copy of each such Registration Statement and each amendment thereto, including
financial statements and schedules, all documents incorporated or deemed to be incorporated therein by reference to the extent requested
by such Person, and all exhibits to the extent requested by such Person (including those previously furnished or incorporated by reference)
promptly after the filing of such documents with the Commission, provided that any such item which is available on the EDGAR system (or
successor thereto) need not be furnished in physical form.
(f)
Subject to the terms of this Agreement, the Company hereby consents to the use of such Prospectus and each amendment or supplement thereto
by each of the selling Holders in connection with the offering and sale of the Registrable Securities covered by such Prospectus and
any amendment or supplement thereto, except after the giving of any notice pursuant to Section 3(c).
(g)
Prior to any resale of Registrable Securities by a Holder, use its commercially reasonable efforts to register or qualify or cooperate
with the selling Holders in connection with the registration or qualification (or exemption from the registration or qualification) of
such Registrable Securities for the resale by the Holder under the securities or Blue Sky laws of such jurisdictions within the United
States as any Holder reasonably requests in writing, to keep each registration or qualification (or exemption therefrom) effective during
the Effectiveness Period and to do any and all other acts or things reasonably necessary to enable the disposition in such jurisdictions
of the Registrable Securities covered by each Registration Statement, provided that the Company shall not be required to qualify generally
to do business in any jurisdiction where it is not then so qualified, subject the Company to any material tax in any such jurisdiction
where it is not then so subject or file a general consent to service of process in any such jurisdiction.
(h)
If requested by a Holder, cooperate with such Holder to facilitate the timely preparation and delivery of certificates representing Registrable
Securities to be delivered to a transferee pursuant to a Registration Statement, which certificates shall be free, to the extent permitted
by the Settlement Agreement, of all restrictive legends, and to enable such Registrable Securities to be in such denominations and registered
in such names as any such Holder may request.
(i)
Upon the occurrence of any event contemplated by Section 3(c), as promptly as reasonably possible under the circumstances taking into
account the Company’s good faith assessment of any adverse consequences to the Company and its stockholders of the premature disclosure
of such event, prepare a supplement or amendment, including a post-effective amendment, to a Registration Statement or a supplement to
the related Prospectus or any document incorporated or deemed to be incorporated therein by reference, and file any other required document
so that, as thereafter delivered, neither a Registration Statement nor such Prospectus will contain an untrue statement of a material
fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances
under which they were made, not misleading. If the Company notifies the Holders in accordance with
clauses (iii) through (vi) of Section 3(c) above to suspend the use of any Prospectus until the requisite changes to such Prospectus
have been made, then the Holders shall suspend use of such Prospectus. The Company will use its reasonable best efforts to ensure that
the use of the Prospectus may be resumed as promptly as is practicable. The Company shall
be entitled to exercise its right under this Section 3(i) to suspend the availability of a Registration Statement and Prospectus, subject
to the payment of partial liquidated damages otherwise required pursuant to Section 2(c), for a period not to exceed 60 calendar days
(which need not be consecutive days) in any 12-month period.
(j)
Otherwise use commercially reasonable efforts to comply with all applicable rules and regulations of the Commission under the Securities
Act and the Exchange Act, including, without limitation, Rule 172 under the Securities Act, file any final Prospectus, including any
supplement or amendment thereof, with the Commission pursuant to Rule 424 under the Securities Act, promptly inform the Holders in writing
if, at any time during the Effectiveness Period, the Company does not satisfy the conditions specified in Rule 172 and, as a result thereof,
the Holders are required to deliver a Prospectus in connection with any disposition of Registrable Securities and take such other actions
as may be reasonably necessary to facilitate the registration of the Registrable Securities hereunder.
(k)
The Company may require each selling Holder to furnish to the Company a certified statement as to the number of shares of Common Stock
beneficially owned by such Holder and, if required by the Commission, the natural persons thereof that have voting and dispositive control
over the shares. During any periods that the Company is unable to meet its obligations hereunder with respect to the registration of
the Registrable Securities solely because any Holder fails to furnish such information within three Trading Days of the Company’s
request, any liquidated damages that are accruing at such time as to such Holder only shall be tolled and any Event that may otherwise
occur solely because of such delay shall be suspended as to such Holder only, until such information is delivered to the Company.
4.
Registration Expenses. All fees and expenses incident to the performance of or compliance with, this Agreement by the Company
shall be borne by the Company whether or not any Registrable Securities are sold pursuant to a Registration Statement. The fees and expenses
referred to in the foregoing sentence shall include, without limitation, (i) all registration and filing fees (including, without limitation,
fees and expenses of the Company’s counsel and independent registered public accountants) (A) with respect to filings made with
the Commission, (B) with respect to filings required to be made with any Trading Market on which the Common Stock is then listed for
trading, and (C) in compliance with applicable state securities or Blue Sky laws reasonably agreed to by the Company in writing (including,
without limitation, fees and disbursements of counsel for the Company in connection with Blue Sky qualifications or exemptions of the
Registrable Securities), (ii) printing expenses (including, without limitation, expenses of printing certificates for Registrable Securities),
(iii) messenger, telephone and delivery expenses, (iv) fees and disbursements of counsel for the Company, (v) Securities Act liability
insurance, if the Company so desires such insurance, and (vi) fees and expenses of all other Persons retained by the Company in connection
with the consummation of the transactions contemplated by this Agreement. In addition, the Company shall be responsible for all of its
internal expenses incurred in connection with the consummation of the transactions contemplated by this Agreement (including, without
limitation, all salaries and expenses of its officers and employees performing legal or accounting duties), the expense of any annual
audit and the fees and expenses incurred in connection with the listing of the Registrable Securities on any securities exchange as required
hereunder. In no event shall the Company be responsible for any broker or similar commissions of any Holder or any legal fees or other
costs of the Holders.
5.
Indemnification.
(a)
Indemnification by the Company. The Company shall, notwithstanding any termination of this Agreement, indemnify and hold harmless
each Holder, the officers, directors, members, partners, agents, brokers (including brokers who offer and sell Registrable Securities
as principal as a result of a pledge or any failure to perform under a margin call of Common Stock), investment advisors and employees
(and any other Persons with a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any
other title) of each of them, each Person who controls any such Holder (within the meaning of Section 15 of the Securities Act or Section
20 of the Exchange Act) and the officers, directors, members, stockholders, partners, agents and employees (and any other Persons with
a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title) of each such
controlling Person, to the fullest extent permitted by applicable law, from and against any and all losses, claims, damages, liabilities,
costs (including, without limitation, reasonable attorneys’ fees) and expenses (collectively, “Losses”), as
incurred, arising out of or relating to (1) any untrue or alleged untrue statement of a material fact contained in a Registration Statement,
any Prospectus or any form of prospectus or in any amendment or supplement thereto or in any preliminary prospectus, or arising out of
or relating to any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements
therein (in the case of any Prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading
or (2) any violation or alleged violation by the Company of the Securities Act, the Exchange Act or any state securities law, or any
rule or regulation thereunder, in connection with the performance of its obligations under this Agreement, except to the extent, but
only to the extent, that (i) such untrue statements or omissions are based solely upon information regarding such Holder furnished in
writing to the Company by such Holder expressly for use therein, or to the extent that such information relates to such Holder or such
Holder’s proposed method of distribution of Registrable Securities and was reviewed and expressly approved in writing by such Holder
expressly for use in a Registration Statement, such Prospectus or in any amendment or supplement thereto (it being understood that the
Holder has approved Annex A hereto for this purpose) or (ii) in the case of an occurrence of an event of the type specified in Section
3(c)(iii)-(vi), the use by such Holder of an outdated, defective or otherwise unavailable Prospectus after the Company has notified such
Holder in writing (or otherwise in accordance with the notice provisions in the Settlement Agreement) that the Prospectus is outdated,
defective or otherwise unavailable for use by such Holder and prior to the receipt by such Holder of the Advice contemplated in Section
6(b). The Company shall notify the Holders promptly of the institution, threat or assertion of any Proceeding arising from or in connection
with the transactions contemplated by this Agreement of which the Company is aware. Such indemnity shall remain in full force and effect
regardless of any investigation made by or on behalf of such indemnified person and shall survive the transfer of any Registrable Securities
by any of the Holders in accordance with Section 6(f).
(b)
Indemnification by Holders. Each Holder shall, severally and not jointly, indemnify and hold harmless the Company, its directors,
officers, agents and employees, each Person who controls the Company (within the meaning of Section 15 of the Securities Act and Section
20 of the Exchange Act), and the directors, officers, agents or employees of such controlling Persons, to the fullest extent permitted
by applicable law, from and against all Losses, as incurred, to the extent arising out of or based solely upon: any untrue or alleged
untrue statement of a material fact contained in any Registration Statement, any Prospectus, or in any amendment or supplement thereto
or in any preliminary prospectus, or arising out of or relating to any omission or alleged omission of a material fact required to be
stated therein or necessary to make the statements therein (in the case of any Prospectus or supplement thereto, in light of the circumstances
under which they were made) not misleading (i) to the extent, but only to the extent, that such untrue statement or omission is contained
in any information so furnished in writing by such Holder to the Company expressly for inclusion in such Registration Statement or such
Prospectus or (ii) to the extent, but only to the extent, that such information relates to such Holder’s information provided in
the Selling Stockholder Questionnaire or the proposed method of distribution of Registrable Securities and was reviewed and expressly
approved in writing by such Holder expressly for use in a Registration Statement (it being understood that the Holder has approved Annex
A hereto for this purpose), such Prospectus or in any amendment or supplement thereto. In no event shall the liability of a selling Holder
be greater in amount than the dollar amount of the proceeds (net of all expenses paid by such Holder in connection with any claim relating
to this Section 5 and the amount of any damages such Holder has otherwise been required to pay by reason of such untrue statement or
omission) received by such Holder upon the sale of the Registrable Securities included in the Registration Statement giving rise to such
indemnification obligation.
(c)
Conduct of Indemnification Proceedings. If any Proceeding shall be brought or asserted against any Person entitled to indemnity
hereunder (an “Indemnified Party”), such Indemnified Party shall promptly notify the Person from whom indemnity is
sought (the “Indemnifying Party”) in writing, and the Indemnifying Party shall have the right to assume the defense
thereof, including the employment of counsel reasonably satisfactory to the Indemnified Party and the payment of all fees and expenses
incurred in connection with defense thereof, provided that the failure of any Indemnified Party to give such notice shall not relieve
the Indemnifying Party of its obligations or liabilities pursuant to this Agreement, except (and only) to the extent that it shall be
finally determined by a court of competent jurisdiction (which determination is not subject to appeal or further review) that such failure
shall have materially and adversely prejudiced the Indemnifying Party.
An
Indemnified Party shall have the right to employ separate counsel in any such Proceeding and to participate in the defense thereof, but
the fees and expenses of such counsel shall be at the expense of such Indemnified Party or Parties unless: (1) the Indemnifying Party
has agreed in writing to pay such fees and expenses, (2) the Indemnifying Party shall have failed promptly to assume the defense of such
Proceeding and to employ counsel reasonably satisfactory to such Indemnified Party in any such Proceeding, or (3) the named parties to
any such Proceeding (including any impleaded parties) include both such Indemnified Party and the Indemnifying Party, and counsel to
the Indemnified Party shall reasonably believe that a material conflict of interest is likely to exist if the same counsel were to represent
such Indemnified Party and the Indemnifying Party (in which case, if such Indemnified Party notifies the Indemnifying Party in writing
that it elects to employ separate counsel at the expense of the Indemnifying Party, the Indemnifying Party shall not have the right to
assume the defense thereof and the reasonable fees and expenses of no more than one separate counsel shall be at the expense of the Indemnifying
Party). The Indemnifying Party shall not be liable for any settlement of any such Proceeding effected without its written consent, which
consent shall not be unreasonably withheld or delayed. No Indemnifying Party shall, without the prior written consent of the Indemnified
Party, effect any settlement of any pending Proceeding in respect of which any Indemnified Party is a party, unless such settlement includes
an unconditional release of such Indemnified Party from all liability on claims that are the subject matter of such Proceeding.
Subject
to the terms of this Agreement, all reasonable fees and expenses of the Indemnified Party (including reasonable fees and expenses to
the extent incurred in connection with investigating or preparing to defend such Proceeding in a manner not inconsistent with this Section)
shall be paid to the Indemnified Party, as incurred, within ten Trading Days of written notice thereof to the Indemnifying Party, provided
that the Indemnified Party shall promptly reimburse the Indemnifying Party for that portion of such fees and expenses applicable to such
actions for which such Indemnified Party is finally determined by a court of competent jurisdiction (which determination is not subject
to appeal or further review) not to be entitled to indemnification hereunder.
(d)
Contribution. If the indemnification under Section 5(a) or 5(b) is unavailable to an Indemnified Party or insufficient to hold
an Indemnified Party harmless for any Losses, then each Indemnifying Party shall contribute to the amount paid or payable by such Indemnified
Party, in such proportion as is appropriate to reflect the relative fault of the Indemnifying Party and Indemnified Party in connection
with the actions, statements or omissions that resulted in such Losses as well as any other relevant equitable considerations. The relative
fault of such Indemnifying Party and Indemnified Party shall be determined by reference to, among other things, whether any action in
question, including any untrue or alleged untrue statement of a material fact or omission or alleged omission of a material fact, has
been taken or made by, or relates to information supplied by, such Indemnifying Party or Indemnified Party, and the parties’ relative
intent, knowledge, access to information and opportunity to correct or prevent such action, statement or omission. The amount paid or
payable by a party as a result of any Losses shall be deemed to include, subject to the limitations set forth in this Agreement, any
reasonable attorneys’ or other fees or expenses incurred by such party in connection with any Proceeding to the extent such party
would have been indemnified for such fees or expenses if the indemnification provided for in this Section was available to such party
in accordance with its terms.
The
parties hereto agree that it would not be just and equitable if contribution pursuant to this Section 5(d) were determined by pro rata
allocation or by any other method of allocation that does not take into account the equitable considerations referred to in the immediately
preceding paragraph. In no event shall the contribution obligation of a Holder of Registrable Securities be greater in amount than the
dollar amount of the proceeds (net of all expenses paid by such Holder in connection with any claim relating to this Section 5 and the
amount of any damages such Holder has otherwise been required to pay by reason of such untrue or alleged untrue statement or omission
or alleged omission) received by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
The
indemnity and contribution agreements contained in this Section are in addition to any liability that the Indemnifying Parties may have
to the Indemnified Parties.
6.
Miscellaneous.
(a)
Remedies. In the event of a breach by the Company or by a Holder of any of their respective obligations under this Agreement,
each Holder or the Company, as the case may be, in addition to being entitled to exercise all rights granted by law and under this Agreement,
including recovery of damages, shall be entitled to specific performance of its rights under this Agreement. Each of the Company and
each Holder agrees that monetary damages would not provide adequate compensation for any losses incurred by reason of a breach by it
of any of the provisions of this Agreement and hereby further agrees that, in the event of any action for specific performance in respect
of such breach, it shall not assert or shall waive the defense that a remedy at law would be adequate.
(b)
Discontinued Disposition. By its acquisition of Registrable Securities, each Holder agrees that, upon receipt of a notice from
the Company of the occurrence of any event of the kind described in Section 3(c)(iii) through (vi), such Holder will forthwith discontinue
disposition of such Registrable Securities under a Registration Statement until it is advised in writing (the “Advice”)
by the Company that the use of the applicable Prospectus (as it may have been supplemented or amended) may be resumed. The Company will
use its best efforts to ensure that the use of the Prospectus may be resumed as promptly as is practicable. The Company agrees and acknowledges
that any periods during which the Holder is required to discontinue the disposition of the Registrable Securities hereunder shall be
subject to the provisions of Section 2(c)
(c)
Amendments and Waivers. The provisions of this Agreement, including the provisions of this sentence, may not be amended, modified
or supplemented, and waivers or consents to departures from the provisions hereof may not be given, unless the same shall be in writing
and signed by the Company and the Holders of 50.1% or more of the then outstanding Registrable Securities, provided that, if any amendment,
modification or waiver by its terms disproportionately and adversely impacts a Holder (or group of Holders), the consent of such disproportionately
impacted Holder (or group of Holders) shall be required. If a Registration Statement does not register all of the Registrable Securities
pursuant to a waiver or amendment done in compliance with the previous sentence, then the number of Registrable Securities to be registered
for each Holder shall be reduced pro rata among all Holders and each Holder shall have the right to designate which of its Registrable
Securities shall be omitted from such Registration Statement. Notwithstanding the foregoing, a waiver or consent to depart from the provisions
hereof with respect to a matter that relates by its terms exclusively to the rights of a Holder or some Holders and that does not directly
or indirectly affect the rights of other Holders may be given only by such Holder or Holders of all of the Registrable Securities to
which such waiver or consent relates; provided, however, that the provisions of this sentence may not be amended, modified,
or supplemented except in accordance with the provisions of the first sentence of this Section 6(c). No consideration shall be offered
or paid to any Person to amend or consent to a waiver or modification of any provision of this Agreement unless the same consideration
also is offered to all of the parties to this Agreement.
(d)
Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be delivered
as set forth in the Settlement Agreement.
(e)
Successors and Assigns. This Agreement shall inure to the benefit of and be binding upon the successors and permitted assigns
of each of the parties and shall inure to the benefit of each Holder. The Company may not assign (except by merger) its rights or obligations
hereunder without the prior written consent of 50.1% or more of the Holders of the then outstanding Registrable Securities.
(f)
No Inconsistent Agreements. The Company has not entered, as of the date hereof, and shall not enter, on or after the date of this
Agreement, into any agreement with respect to its securities that would have the effect of impairing the rights granted to the Holders
in this Agreement or otherwise conflicts with the provisions hereof.
(g)
Execution and Counterparts. This Agreement may be executed in two or more counterparts, all of which when taken together shall
be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to
the other party, it being understood that both parties need not sign the same counterpart. In the event that any signature is delivered
by e-mail delivery of a “.pdf” format data file or any electronic signature complying with the U.S. federal ESIGN Act of
2000 (e.g., www.docusign.com), such signature shall create a valid and binding obligation of the party executing (or on whose behalf
such signature is executed) with the same force and effect as if such “.pdf” signature page were an original thereof.
(h)
Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be
determined in accordance with the provisions of the Settlement Agreement.
(i)
Cumulative Remedies. The remedies provided herein are cumulative and not exclusive of any other remedies provided by law.
(j)
Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to
be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall
remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially
reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated
by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would
have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared
invalid, illegal, void or unenforceable.
(k)
Headings. The headings in this Agreement are for convenience only, do not constitute a part of the Agreement and shall not be
deemed to limit or affect any of the provisions hereof.
(l)
Independent Nature of Holders’ Obligations and Rights. The obligations of each Holder hereunder are several and not joint
with the obligations of any other Holder hereunder, and no Holder shall be responsible in any way for the performance of the obligations
of any other Holder hereunder. Nothing contained herein or in any other agreement or document delivered at any closing, and no action
taken by any Holder pursuant hereto or thereto, shall be deemed to constitute the Holders as a partnership, an association, a joint venture
or any other kind of group or entity, or create a presumption that the Holders are in any way acting in concert or as a group or entity
with respect to such obligations or the transactions contemplated by this Agreement or any other matters, and the Company acknowledges
that the Holders are not acting in concert or as a group, and the Company shall not assert any such claim, with respect to such obligations
or transactions. Each Holder shall be entitled to protect and enforce its rights, including without limitation the rights arising out
of this Agreement, and it shall not be necessary for any other Holder to be joined as an additional party in any proceeding for such
purpose. The use of a single agreement with respect to the obligations of the Company contained was solely in the control of the Company,
not the action or decision of any Holder, and was done solely for the convenience of the Company and not because it was required or requested
to do so by any Holder. It is expressly understood and agreed that each provision contained in this Agreement is between the Company
and a Holder, solely, and not between the Company and the Holders collectively and not between and among Holders.
********************
(Signature
Pages Follow)
IN
WITNESS WHEREOF, the parties have executed this Registration Rights Agreement as of the date first written above.
| |
Microbot
Medical Inc. |
| |
|
| |
By:
|
/s/
Harel Gadot |
| |
Name:
|
Harel
Gadot |
| |
Title:
|
CEO
& President |
[SIGNATURE
PAGE OF HOLDERS FOLLOWS]
[SIGNATURE
PAGE OF HOLDERS TO MBOT RRA]
Name
of Holder: EMPERY ASSET MASTER, LTD.
| Signature of Authorized Signatory of Holder: |
/s/ Brett Director |
|
Name
of Authorized Signatory: Brett Director
Title
of Authorized Signatory: General Couunsel of Empery Asset Management, LP
[SIGNATURE
PAGES CONTINUE]
[SIGNATURE
PAGE OF HOLDERS TO MBOT RRA]
Name
of Holder: EMPERY TAX EFFICIENT, LP
| Signature of Authorized Signatory of Holder: |
/s/ Brett Director |
|
Name
of Authorized Signatory: Brett Director
Title
of Authorized Signatory: General Couunsel of Empery Asset Management, LP
[SIGNATURE
PAGES CONTINUE]
[SIGNATURE
PAGE OF HOLDERS TO MBOT RRA]
Name
of Holder: EMPERY TAX EFFICIENT III, LP
| Signature
of Authorized Signatory of Holder: |
/s/
Brett Director |
|
Name
of Authorized Signatory: Brett Director
Title
of Authorized Signatory: General Couunsel of Empery Asset Management, LP
[SIGNATURE
PAGES CONTINUE]
[SIGNATURE
PAGE OF HOLDERS TO MBOT RRA]
Name
of Holder: HUDSON BAY MASTER FUND LTD.
| Signature
of Authorized Signatory of Holder: |
/s/
Richard Allison |
|
Name
of Authorized Signatory: Richard Allison
Title
of Authorized Signatory: Authorized Signatory, Hudson Bay Capital Management LP not individually, but solely as Investment Advisor to
Hudson Bay Master Fund Ltd.
Annex
A
Plan
of Distribution
Each
Selling Stockholder (the “Selling Stockholders”) of the securities and any of their pledgees, assignees and successors-in-interest
may, from time to time, sell any or all of their securities covered hereby on the principal Trading Market or any other stock exchange,
market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices.
A Selling Stockholder may use any one or more of the following methods when selling securities:
| |
● |
ordinary brokerage transactions and transactions in which the
broker-dealer solicits purchasers; |
| |
|
|
| |
● |
block trades in which the broker-dealer will attempt to sell
the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| |
|
|
| |
● |
purchases by a broker-dealer as principal and resale by the
broker-dealer for its account; |
| |
|
|
| |
● |
an exchange distribution in accordance with the rules of the
applicable exchange; |
| |
|
|
| |
● |
privately negotiated transactions; |
| |
|
|
| |
● |
settlement of short sales; |
| |
|
|
| |
● |
in transactions through broker-dealers that agree with the
Selling Stockholders to sell a specified number of such securities at a stipulated price per security; |
| |
|
|
| |
● |
through the writing or settlement of options or other hedging
transactions, whether through an options exchange or otherwise; |
| |
|
|
| |
● |
a combination of any such methods of sale; or |
| |
|
|
| |
● |
any other method permitted pursuant to applicable law. |
The
Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933,
as amended (the “Securities Act”), if available, rather than under this prospectus.
Broker-dealers
engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions
or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchasers of securities, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or
markdown in compliance with FINRA Rule 2121.
In
connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
The
Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company
has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under
the Securities Act.
We
agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders
without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for
the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar
effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule
of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable
state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is
complied with.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the
Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the
common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders
and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
Annex
B
SELLING
SHAREHOLDERS
The
common stock being offered by the selling shareholders are those previously issued to the selling shareholders. For additional information
regarding the issuances of those shares of common stock, see “Litigation Settlement” above. We are registering the shares
of common stock in order to permit the selling shareholders to offer the shares for resale from time to time. Except for the ownership
of the shares of common stock and as described in “Litigation Settlement” described above, the selling shareholders have
not had any material relationship with us within the past three years.
The
table below lists the selling shareholders and other information regarding the beneficial ownership of the shares of common stock by
each of the selling shareholders. The second column lists the number of shares of common stock beneficially owned by each selling shareholder,
based on its ownership of the shares of common stock, as of ________, 2024.
The
third column lists the shares of common stock being offered by this prospectus by the selling shareholders.
In
accordance with the terms of a registration rights agreement with the selling shareholders, this prospectus generally covers the resale
of the sum of the number of shares of common stock issued to the selling shareholders in “Litigation Settlement” described
above The fourth column assumes the sale of all of the shares offered by the selling shareholders pursuant to this prospectus.
The
selling shareholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name of Selling Shareholder | |
Number of shares of Common Stock Owned Prior to Offering | |
Maximum Number of shares of Common Stock to be Sold Pursuant to this Prospectus | |
Number of shares of Common Stock Owned After Offering |
| | |
| | | |
| | | |
| | |
Exhibit
99.1

Microbot
Medical Announces Settlement and Release Agreement, Effectively Resolving all Associated Legal Matters
All
of the cash payable to the plaintiffs in the settlement will be covered by Microbot’s insurance carrier and will not impact company’s
cash position
BRAINTREE,
Mass., January 30, 2024 – Microbot Medical Inc. (Nasdaq: MBOT), developer of the innovative LIBERTY® Endovascular
Robotic Surgical System, today announced it has signed a Settlement Agreement and Release with Empery Asset Master Ltd., Empery Tax Efficient,
LP, Empery Tax Efficient II, LP, Hudson Bay Master Fund Ltd. that resolves all claims asserted against the Company by these entities
in a lawsuit filed in 2020 relating to a June 2017 securities purchase agreement.
All
of the cash payable to the plaintiffs in the settlement, representing over half of the settlement amount, will be covered by Microbot’s
insurance carrier. This arrangement ensures that the settlement will not impact the company’s cash position or its balance sheet.
“We
believe that resolving this matter while maintaining our cash position is in the best interest of our shareholders, as it allows us to
continue to move forward with focus on the upcoming milestones, including our IDE submission with the FDA and planned commencement of
our first in human clinical trial,” said Harel Gadot, CEO, President and Chairman of Microbot Medical.
About
Microbot Medical
Microbot
Medical Inc. (NASDAQ: MBOT) is a pre-clinical medical device company that specializes in transformational micro-robotic technologies,
with the goals of improving clinical outcomes for patients and increasing accessibility through the natural and artificial lumens within
the human body.
The
LIBERTY Endovascular Robotic Surgical System aims to improve the way surgical robotics are being used in endovascular procedures today,
by eliminating the need for large, cumbersome, and expensive capital equipment, while reducing radiation exposure and physician strain.
The Company believes the LIBERTY Endovascular Robotic Surgical System’s remote operation has the potential to be the first system
to democratize endovascular interventional procedures.
Further
information about Microbot Medical is available at http://www.microbotmedical.com.
Safe
Harbor
Statements
to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities
for Microbot Medical Inc. and its subsidiaries, along with other statements about the future expectations, beliefs, goals, plans, or
prospects expressed by management, constitute forward-looking statements within the meaning of the Private Securities Litigation Reform
Act of 1995 and the Federal securities laws. Any statements that are not historical fact (including, but not limited to statements that
contain words such as “will,” “believes,” “plans,” “anticipates,” “expects”
and “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and
uncertainties, including, without limitation, market conditions, risks inherent in the development and/or commercialization of potential
products, including LIBERTY, the outcome of its studies to evaluate LIBERTY, whether the Company’s core business focus program
and cost reduction plan are sufficient to enable the Company to continue to focus on its LIBERTY technology while it stabilizes its financial
condition and seeks additional working capital, any failure or inability to recruit physicians and clinicians to serve as primary investigators
to conduct regulatory studies which could adversely affect or delay such studies, uncertainty in the results of pre-clinical and clinical
trials or regulatory pathways and regulatory approvals, disruptions resulting from new and ongoing hostilities between Israel and the
Palestinians, any lingering uncertainty resulting from the COVID-19 pandemic, need and ability to obtain future capital, and maintenance
of intellectual property rights. Additional information on risks facing Microbot Medical can be found under the heading “Risk Factors”
in Microbot Medical’s periodic reports filed with the Securities and Exchange Commission (SEC), which are available on the SEC’s
web site at www.sec.gov. Microbot Medical disclaims any intent or obligation to update these forward-looking statements, except as required
by law.
Investor
Contact:
Michal
Efraty
+972-(0)52-3044404
IR@microbotmedical.com
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Microbot Medical (NASDAQ:MBOT)
Historical Stock Chart
From Mar 2024 to Apr 2024
Microbot Medical (NASDAQ:MBOT)
Historical Stock Chart
From Apr 2023 to Apr 2024