Filed Pursuant to Rule 424(b)(5)
Registration No. 333-274083
PROSPECTUS SUPPLEMENT
(To Prospectus dated August 28, 2023)
1,146,000 Shares of Class A Common Stock

BioVie Inc.
We are offering 1,146,000 shares (the “Shares”)
of our Class A common stock, $0.0001 par value per share (our “Common Stock”), at an offering price of $2.83 per Share, pursuant
to this prospectus supplement and the accompanying base prospectus.
We have engaged ThinkEquity LLC (the “placement
agent”) to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use its reasonable
best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing or selling any
of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific number or dollar
amount of securities. We have agreed to pay placement agent fees to the placement agent as set forth in the table below, which assumes
that we sell all of the securities offered by this prospectus. Since we will deliver the securities to be issued in this offering upon
our receipt of investor funds, there is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum
offering requirement as a condition of closing of this offering. Because there is no minimum offering amount required as a condition to
closing this offering, we may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds
received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient
to pursue our business goals described in this prospectus. In addition, because there is no escrow account and no minimum offering amount,
investors could be in a position where they have invested in our company, but we are unable to fulfill all of our contemplated objectives
due to a lack of interest in this offering. Further, any proceeds from the sale of securities offered by us will be available for our
immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. See the
section entitled “Risk Factors” for more information. We will bear all costs associated with the offering. See “Plan
of Distribution” on page S-25 of this prospectus for more information regarding these arrangements.
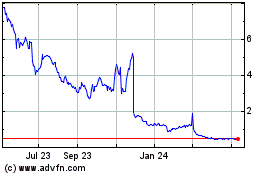
Our Common Stock is traded on The Nasdaq Capital
Market (“Nasdaq”) under the symbol “BIVI.” On October 28, 2024, the closing price for our Common Stock, as reported
on Nasdaq was $2.87 per share.
As of October 28, 2024, the aggregate market value of our outstanding Common Stock held by non-affiliates,
or public float, was approximately $47,737,637, based on 16,612,374 shares of outstanding Common Stock, of which approximately 2,404,744
shares were held by affiliates, and a price of $3.36 per share, which was the price at which our Common Stock was last sold on Nasdaq
on October 22, 2024. We have sold approximately $12.67 million of securities pursuant to General Instruction I.B.6 of Form S-3 during
the prior 12-calendar month period that ends on and includes the date of this prospectus supplement (excluding this offering). Accordingly,
based on the foregoing, we are currently eligible under General Instruction I.B.6 of Form S-3 to offer and sell shares of our Common Stock
having an aggregate offering price of up to approximately $3.25 million. Pursuant to General Instruction I.B.6 of Form S-3, in no event
will we sell securities in a public primary offering with a value exceeding one-third of our public float in any 12-month period so long
as our public float remains below $75.0 million.
Investing in these securities involves certain
risks. See “Risk Factors” on page S-5 of this prospectus supplement and the accompanying base prospectus, as well
as the risk factors incorporated by reference into this prospectus supplement and accompanying base prospectus for a discussion of the
factors you should carefully consider before deciding to purchase these securities.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or accompanying
base prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| |
|
Per Share of Common Stock |
|
|
Total |
|
| Public offering price |
|
$ |
2.83 |
|
|
$ |
3,243,180 |
|
| Placement agent fees (1) |
|
$ |
0.1981 |
|
|
$ |
227,023 |
|
| Proceeds to us, before expenses |
|
$ |
2.6319 |
|
|
$ |
3,016,157 |
|
| (1) | Does not include a non-accountable expense allowance of 1% of the gross proceeds. See “Plan of Distribution” beginning
on page S-25 of this prospectus supplement for additional information regarding placement agent fees and estimated expenses. |
The delivery to purchasers of the securities offered and sold in this offering
is expected to be made on or about October 29, 2024, subject to satisfaction of certain customary closing conditions.
ThinkEquity
The date of this prospectus supplement is October 28,
2024
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
PROSPECTUS
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying base
prospectus are part of a registration statement on Form S-3 (File No. 333-274083) that we filed with the Securities and Exchange Commission
(the “SEC”) on August 18, 2023, and that was declared effective by the SEC on August 28, 2023 using a “shelf”
registration process. This document is in two parts. The first part is this prospectus supplement, which describes the specific terms
of this offering and also adds to and updates information contained in the accompanying base prospectus and the documents incorporated
by reference herein. The second part, the accompanying base prospectus, provides more general information, some of which may not apply
to this offering. Generally, when we refer to this prospectus, we are referring to both the prospectus supplement and the accompanying
base prospectus combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information
contained in the accompanying base prospectus or any document incorporated by reference therein filed prior to the date of this prospectus
supplement, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent
with a statement in another document having a later date—for example, a document incorporated by reference in the accompanying base
prospectus—the statement in the document having the later date modifies or supersedes the earlier statement.
We further note that the representations, warranties
and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made
solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties
to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties
or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied
on as accurately representing the current state of our affairs.
You should rely only on the information contained
in this prospectus supplement or the accompanying base prospectus or incorporated by reference herein or therein. We have not authorized,
and the placement agent has not authorized, anyone to provide you with information that is different. The information contained in this
prospectus supplement or the accompanying base prospectus or incorporated by reference herein or therein is accurate only as of the respective
dates thereof, regardless of the time of delivery of this prospectus supplement and the accompanying base prospectus or of any sale of
our securities.
This prospectus supplement and the accompanying base
prospectus contain summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual
documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the
documents referred to herein have been filed, will be filed or will be incorporated herein by reference as exhibits to the registration
statement, and you may obtain copies of those documents as described below in the section entitled “Where You Can Find More Information.”
It is important for you to read and consider all information
contained in this prospectus supplement and the accompanying base prospectus, including the documents incorporated by reference herein
and therein, in making your investment decision. You should also read and consider the information in the documents to which we have referred
you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain Information By Reference”
in this prospectus supplement and in the accompanying base prospectus, respectively.
This prospectus supplement and the accompanying base
prospectus contain and incorporate by reference market data and industry statistics and forecasts that are based on independent industry
publications and other publicly-available information. Although we believe these sources are reliable, we do not guarantee the accuracy
or completeness of this information and we have not independently verified this information. Although we are not aware of any misstatements
regarding the market and industry data presented in this prospectus supplement, accompanying base prospectus or the documents incorporated
herein by reference, these estimates involve risks and uncertainties and are subject to change based on various factors, including those
discussed in the section entitled “Risk Factors” in this prospectus supplement and the accompanying base prospectus, and under
similar headings in the other documents that are incorporated by reference herein and therein. Accordingly, investors should not place
undue reliance on this information.
We are offering to sell, and seeking offers to buy,
the securities offered by this prospectus supplement only in jurisdictions where offers and sales are permitted. The distribution of this
prospectus supplement and the accompanying base prospectus and the offering of the securities offered by this prospectus supplement in
certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement
and the accompanying base prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities
and the distribution of this prospectus supplement and the accompanying base prospectus outside the United States. This prospectus supplement
and the accompanying base prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of
an offer to buy, any securities offered by this prospectus supplement and the accompanying base prospectus by any person in any jurisdiction
in which it is unlawful for such person to make such an offer or solicitation.
Unless the context otherwise indicates, references
in this prospectus to, “BioVie,” “the Company,” “we,” “our,” or “us” mean
BioVie Inc., a Nevada corporation.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights certain information about
us and certain information contained elsewhere in this prospectus supplement, in the accompanying base prospectus and in the documents
incorporated by reference herein and therein. This summary is not complete and does not contain all of the information that you should
consider before making an investment decision. For a more complete understanding of the Company, you should read and consider carefully
the more detailed information included in this prospectus supplement and the accompanying base prospectus, , including the factors described
under the heading “Risk Factors,” on page S-5 of this prospectus supplement, the “Risk Factors” section beginning
on page 5 of the accompanying base prospectus as well as the information incorporated herein and therein by reference, before making an
investment decision.
Overview of the Company
We are a clinical-stage company developing innovative
drug therapies to treat chronic debilitating conditions including liver disease and neurological and neuro-degenerative disorders.
Neurodegenerative Disease Program
The Company acquired the biopharmaceutical assets
of NeurMedix, Inc. (“NeurMedix”) a privately held clinical-stage pharmaceutical company and a related party in June 2021.
The acquired assets included NE3107. In April 2024, the Company announced that the United States Adopted Names Council, and the World
Health Organization International Nonproprietary Names expert committee had approved “bezisterim” as the non-proprietary (generic)
name for NE3107. Bezisterim (NE3107) is an investigational, novel, orally administered small molecule that is thought to inhibit inflammation-driven
insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus
that both inflammation and insulin resistance may play fundamental roles in the development of AD and PD, and bezisterim (NE3107) could,
if approved by FDA, represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million
Americans suffering from AD and 1 million Americans suffering from PD.
In neurodegenerative disease, bezisterim (NE3107)
inhibits activation of inflammatory extracellular signal-regulated kinase (“ERK”) and nuclear factor kappa-light-chain-enhancer
of activated B cells (“NFκB”) (including interactions with tumor necrosis factor (“TNF”) signaling and other
relevant inflammatory pathways) that lead to neuroinflammation and insulin resistance. Bezisterim (NE3107) does not interfere with their
homeostatic functions (e.g., insulin signaling and neuron growth and survival). Both inflammation and insulin resistance are drivers of
Alzheimer’s disease (“AD”) and Parkinson’s disease (“PD”).
A. Alzheimer’s Disease (NCT05083260)
On November 29, 2023, the Company announced topline
efficacy data from its Phase 3 clinical trial (NCT04669028) of bezisterim (NE3107) in the treatment of mild to moderate AD. The study
had co-primary endpoints looking at cognition using the Alzheimer’s Disease Assessment Scale-Cognitive Scale (ADAS-Cog 12) and function
using the Clinical Dementia Rating-Sum of Boxes. Patients were randomly assigned, 1:1 versus placebo, to receive sequentially 5 mg of
bezisterim (NE3107) orally twice a day for 14 days, then 10 mg orally twice a day for 14 days, followed by 26 weeks of 20 mg orally twice
daily.
Upon trial completion, as the Company began the process
of analyzing the trial data, the Company found significant deviation from protocol and current good clinical practices (“cGCPs”)
violations at 15 study sites (virtually all of which were from one geographic area). This highly unusual level of suspected improprieties
led the Company to exclude all patients from these sites and to refer the sites to the U.S. Food and Drug Administration (“FDA”)
Office of Scientific Investigations (“OSI”) for potential further action. After the patient exclusions, 81 patients remained
in the Modified Intent-to-Treat population, 57 of whom were in the Per-Protocol population which included those who completed the trial
and were verified to take study drug based on pharmacokinetic data.
The trial was originally designed to be 80% powered
with 125 patients in each of the treatment and placebo arms. The unplanned exclusion of so many patients left the trial underpowered for
its primary endpoints.
In the Per-Protocol population, which includes those
patients who completed the trial and who were further verified to have taken the study drug (based on pharmacokinetics data), an observed
but not statistically significant change from baseline appeared to suggest a slowing of cognitive loss; these same patients experienced
an advantage in age deceleration vs. placebo as measured by deoxyribonucleic acid (“DNA”) epigenetic change. Age deceleration
is used by longevity researchers to measure the difference between the patient’s biological age, in this case as measured by the
Horvath DNA methylation Skin Blood Clock, relative to the patient’s actual chronological age. This test was a non-primary/secondary
endpoint, other-outcome measure, done via blood test collected at week 30 (end of study).
Based on the efficacy signal seen in this trial, the
Company is exploring (1) a discussion with the FDA to potentially employ the adaptive trial feature of the protocol to continue enrolling
patients to achieve statistical significance; and/or (2) the design of a new Phase 3 study of bezisterim (NE3107) that leverages the most
recent data and understanding of the potential effects bezisterim (NE3107) may have in persons with AD.
B. Parkinson’s Disease (NCT05083260)
The Phase 2 study of bezisterim (NE3107) for the treatment
of PD (NCT05083260), completed in January 2023, was a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study
in PD participants treated with carbidopa/levodopa and NE3107. Forty-five patients with a defined L-dopa “off state” were
randomized 1:1 to placebo or bezisterim (NE3107) 20 mg twice daily for 28 days. This trial was launched with two design objectives: (1)
the primary objective was safety and drug-drug interaction, as requested by the FDA, to assess the potential for adverse interactions
between bezisterim (NE3107) and carbidopa/ levodopa; and (2) the secondary objective was to determine if preclinical indications of promotoric
activity and apparent enhancement of levodopa activity could be seen in humans. Both objectives were met.
To extend this Phase 2 data in progressed patients,
the Company has designed a new Phase 2 study of bezisterim (NE3107) as a potential first line therapy to treat patients with new onset
PD. In July 2024, the Company submitted the protocol for this new study to the FDA for regulatory review.
C. Long COVID Program
In April 2024, the Company announced the grant of
a clinical trial award of up to $13.1 million from the U.S. Department of Defense (“DOD”), awarded through the Peer Reviewed
Medical Research Program of the Congressionally Directed Medical Research Programs. In August 2024, U.S. Army Medical Research and Development
Command, Office of Human Research Oversight (“OHRO”) approved the Company’s plan to evaluate bezisterim (NE3107) for
the treatment of neurological symptoms that are associated with long COVID. The FDA had previously reviewed and approved the study as
“Safe to Proceed” in August 2024. The approval from OHRO is the last scientific review milestone needed for the Company to
receive the additional $12.6 million of the aggregate $13.1 million in grant funding from the DOD. The award can provide up to 2 years
of non-dilutive funding for a Phase 2 clinical trial that will assess bezisterim (NE3107) for the treatment of neurological symptoms that
are associated with long COVID. The Company anticipates the trial to commence by early 2025. The study protocol was finalized and submitted
to the FDA for regulatory review in July 2024 and on August 22, 2024 the FDA authorized our Investigational New Drug (“IND”)
application for bezisterim (NE3107) allowing us to study a novel, anti-inflammatory approach or the treatment of the debilitating neurocognitive
symptoms associated with long covid.
Long COVID is a condition in which symptoms of COVID-19,
the acute respiratory disease caused by the SARS-CoV-2 virus, persist for an extended period of time, generally three months or more.
The Centers for Disease Control recently reported that 6.8% of adults in the United States (more than 17 million individuals) currently
or previously had long COVID. Symptoms, which include fatigue, cognitive dysfunction and sleep disturbances, are debilitating. The loss
in quality of life and earnings and increased medical costs has an enormous economic impact estimated to be 3.7 trillion dollars. To date
there are no therapies proven effective for treatment.
Chronic inflammation is one of the main hypotheses
that researchers have proposed to explain the persistence of symptoms in long COVID. Specifically in individuals with “brain fog,”
sustained systemic inflammation and persistent localized blood-brain-barrier (“BBB”) dysfunction are key physiological features.
Bezisterim (NE3107) permeates the BBB and has been shown to modulate inflammation via the inhibition of NF-kB activation, thus representing
a novel oral treatment targeting an underlying cause of long COVID symptoms.
Chronic neuroinflammation, insulin resistance, and
oxidative stress are common features in the major neurodegenerative diseases, including AD, PD, frontotemporal lobar dementia, and Amyotrophic
lateral sclerosis. Bezisterim (NE3107) is an investigational oral small molecule, blood-brain permeable, compound with potential anti-inflammatory,
insulin sensitizing, and ERK-binding properties that may allow it to selectively inhibit ERK-, NFκB- and TNF-stimulated inflammation.
Bezisterim’s (NE3107) potential to inhibit neuroinflammation and insulin resistance forms the basis for the Company’s work
testing the molecule in AD, PD, and long COVID patients. Bezisterim (NE3107) is patented in the United States, Australia, Canada, Europe
and South Korea.
Liver Cirrhosis Program
In liver disease, our investigational drug candidate
BIV201 (continuous infusion terlipressin), which has been granted both FDA Fast Track designation status and FDA Orphan Drug status, is
being evaluated and discussed after receiving guidance from the FDA regarding the design of Phase 3 clinical testing for the treatment
of ascites due to chronic liver cirrhosis. BIV201 is administered as a patent-pending liquid formulation.
In June 2021, the Company initiated a Phase 2 study
(NCT04112199) designed to evaluate the efficacy of BIV201 (terlipressin, administered by continuous infusion for two 28-day treatment
cycles) combined with standard-of-care (“SOC”), compared to SOC alone, for the treatment of refractory ascites. The primary
endpoints of the study are the incidence of ascites-related complications and change in ascites fluid accumulation during treatment compared
to a pre-treatment period.
In March 2023, the Company announced enrollment was
paused and that data from the first 15 patients treated with BIV201 plus SOC appeared to show at least a 30% reduction in ascites fluid
during the 28 days after treatment initiation compared to the 28 days prior to treatment. The change in ascites volume was significantly
different from those patients receiving SOC treatment. Patients who completed the treatment with BIV201 experienced a 53% reduction in
ascites fluid, which was sustained (43% reduction) during the three months after treatment initiation as compared to the three-month pre-treatment
period.
In June 2023, the Company requested and subsequently
received guidance from the FDA regarding the design and endpoints for definitive clinical testing of BIV201 for the treatment of ascites
due to chronic liver cirrhosis. The Company is currently finalizing protocol designs for the Phase 3 study of BIV201 for the treatment
of ascites due to chronic liver cirrhosis.
While the active agent, terlipressin, is approved
in the U.S. and in about 40 countries for related complications of advanced liver cirrhosis, treatment of ascites is not included in these
authorizations. Patients with refractory ascites suffer from frequent life-threatening complications, generate more than $5 billion in
annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The FDA has not approved any drug to treat refractory
ascites.
The BIV201 development program was initiated by LAT
Pharma LLC. On April 11, 2016, the Company acquired LAT Pharma LLC and the rights to its BIV201 development program. The Company currently
owns all development and marketing rights to this drug candidate. Pursuant to the Agreement and Plan of Merger entered into on April 11,
2016, between our predecessor entities, LAT Pharma LLC and NanoAntibiotics, Inc., BioVie is obligated to pay a low single digit royalty
on net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT Pharma Members, PharmaIn Corporation, and The Barrett
Edge, Inc.
Recent Developments
At our Special Meeting of Stockholders (the “Special
Meeting”) on July 29, 2024, our stockholders approved a grant to our board of directors the authority, in its sole discretion, prior
to the one-year anniversary of this Special Meeting, to effect a reverse stock split of the outstanding shares of our Common Stock, at
a split ratio of between 1-for-6 and 1-for-10. Pursuant to such authority granted by our stockholders, our board of directors approved
a 1-for-10 reverse stock split of our Common Stock and the filing of a Certificate of Change (the “Certificate of Change”)
to effectuate the reverse stock split. The Certificate of Change was filed with the Secretary of State of the State of Nevada on July
31, 2024, and the reverse stock split became effective in accordance with the terms of the Certificate of Change at 12:01 a.m. Eastern
Time on August 6, 2024 (the “Effective Time”). The Certificate of Change provided that, at the Effective Time, every 10 shares
of our issued and outstanding Common Stock was automatically be combined into one issued and outstanding share of Common Stock, without
any change in par value per share, which will remain $0.0001. Unless the context expressly dictates otherwise, all reference to share
and per share amounts referred to herein reflect the one-for-ten reverse stock split.
On August 2, 2024, we filed the Certificate of Amendment
with the Secretary of State of the State of Nevada to correct the filing previously made on July 31, 2024 for the reverse stock split.
The Certificate of Amendment corrected a defect in the previously filed Certificate of Change. On August 5, 2024, we filed the Certificate
of Termination with the Secretary of State of the State of Nevada, which voided the previously filed Certificate of Change. No substantive
change was made to the terms or timing of the reverse stock split as provided above.
On October 22, 2024, we closed an offering of 4,443,000
shares of our Common Stock at a price of $1.50 per share. In a concurrent private placement, we also issued and sold warrants to purchase
up to 4,443,000 shares of our Common Stock, with each warrant exercisable for one share of Common Stock at an exercise price of $1.37
per share. Each such warrant will be exercisable beginning six months following issuance and will expire five years following the initial
exercise date. The gross proceeds received by us from this offering are approximately $5.7 million, after deducting the placement agent
fees and estimated offering expenses payable by us.
On October 24, 2024, we closed an offering of 2,667,000
shares of our Common Stock at a price of $2.25 per share. In a concurrent private placement, we also issued and sold warrants to purchase
up to 2,667,000 shares of our Common Stock, with each warrant exercisable for one share of Common Stock at an exercise price of $2.12
per share. Each such warrant will be exercisable beginning six months following issuance and will expire five years following the initial
exercise date. The gross proceeds received by us from this offering are approximately $5.3 million, after deducting the placement agent
fees and estimated offering expenses payable by us.
Corporate Information
Our principal executive office is located at 680 W.
Nye Lane, Suite 201, Carson City, Nevada 89703, and our phone number is (775) 888-3162.
THE OFFERING
| Common Stock offered by us |
|
1,146,000 shares of our Common Stock. |
| |
|
|
| Public Offering Price |
|
$2.83 per share of our Common Stock. |
| |
|
|
| Common Stock outstanding following this Offering |
|
17,758,374 shares of our Common Stock, assuming no exercise of the Placement Agent’s
Warrants (as defined below) to be issued upon consummation of this offering. |
| |
|
|
| Use of Proceeds |
|
We estimate that our net proceeds from
this offering will be approximately $2.7 million, after deducting the placement agent fees
and estimated offering expenses payable by us.
We intend to use the net proceeds from
this offering for working capital and general corporate purposes. See “Use of Proceeds” for a more complete description of
the intended use of proceeds from this offering. |
| |
|
|
| Dividend Policy |
|
We have never declared or paid cash dividends on our Common Stock and do not anticipate paying any cash dividends in the foreseeable future but intend to retain our capital resources for reinvestment in our business. |
| |
|
|
| Trading Market and Ticker Symbol for Common Stock |
|
Our Common Stock is listed on Nasdaq under the symbol “BIVI.” |
| |
|
|
| Risk Factors |
|
Investing in our securities involves a high degree of risk. For a discussion of factors to consider before deciding to invest in our Common Stock, you should carefully review and consider the “Risk Factors” section beginning on page S-5 of this prospectus supplement and in the documents incorporated by reference in this prospectus supplement. |
The number of shares of our Common Stock to be
outstanding immediately after this offering is based on 16,612,374 shares of
our Common Stock issued and outstanding as of October 28, 2024, and excludes:
| · | 517,916 shares of our Common Stock issuable upon the exercise of outstanding
stock options at a weighted average exercise price of $54.11 per share; |
| · | 10,140,202 shares of our Common Stock issuable upon the exercise of outstanding
warrants at a weighted average exercise price of $3.39 per share; |
| · | 34,567 shares of our Common Stock issuable upon vesting of restricted stock units issued under our equity
incentive plan; and |
| · | 57,300 shares of our Common Stock issuable upon exercise of the Placement Agent’s Warrants to be issued upon consummation of this
offering. |
Unless otherwise indicated, this prospectus supplement reflects and assumes
the following:
| · | no exercise of outstanding options or warrants; and |
| · | no exercise of the Placement Agent’s Warrants to be issued upon consummation of this offering at an exercise price equal to 125% of the offering price of our Common Stock. |
RISK FACTORS
Investing in our securities involves a high degree
of risk. Before deciding whether to invest in our securities, you should consider carefully the risks and uncertainties discussed under
the sections titled “Risk Factors” contained in our most recent Annual Report on Form 10-K and in any subsequently filed Quarterly
Report on Form 10-Q, as well as any amendments thereto reflected in our subsequent filings with the SEC, which are incorporated by reference
into this prospectus supplement, together with other information in this prospectus supplement, the documents incorporated by reference
herein, and any prospectus supplement and any free writing prospectus that we may authorize. Please also read carefully the section titled
“Cautionary Note Regarding Forward-Looking Statements.” Additional risks and uncertainties not presently known to us, or that
we currently deem immaterial, may also adversely affect our business. In addition, past financial performance may not be a reliable indicator
of future performance and historical trends should not be used to anticipate results or trends in future periods.
Risks Relating to Our Business and Industry
We rely and will continue to rely on third parties
to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines
or do not successfully perform and comply with regulatory requirements, we may not be able to obtain regulatory approval of or commercialize
our product candidates.
We depend, and will continue
to depend, on third parties, including, but not limited to, contract research organizations (“CROs”), clinical trial sites
and clinical trial principal investigators, contract laboratories, institutional review boards (“IRBs”), manufacturers, suppliers,
and other third parties to conduct our clinical trials, including those for our drug candidates bezisterim (NE3107) and BIV201. We rely
heavily on these third parties over the course of our clinical trials, and we control only certain aspects of their activities. Nevertheless,
we retain ultimate responsibility for ensuring that each of our studies is conducted in accordance with the protocol and applicable legal,
regulatory, and scientific standards and regulations, and our reliance on third parties does not relieve us of our regulatory responsibilities.
We and these third parties are required to comply with cGCPs, which are regulations and guidelines enforced by the FDA and comparable
foreign regulatory authorities for the conduct of clinical trials on product candidates in clinical development. Regulatory authorities
enforce cGCPs through periodic inspections and for-cause inspections of clinical trial principal investigators and trial sites. If, due
to the failure of either the Company or a third party, a clinical trial fails to comply with applicable cGCPs, FDA’s IND requirements,
other applicable regulatory requirements, or requirements set forth in the applicable IRB-approved protocol, the Company may be required
to conduct additional clinical trials to support our marketing applications, which would delay the regulatory approval process. For example,
our drug product candidate bezisterim (NE3107) was cleared by FDA for use in a Phase 3, randomized, double blind, placebo controlled,
parallel group, multicenter study in subjects who have mild to moderate AD. Enrollment in that trial began in August 2021, with a planned
primary completion in late 2022/early 2023. On November 29, 2023, the Company announced topline efficacy data from its Phase 3 clinical
trial (NCT04669028) of bezisterim (NE3107), in the treatment of mild to moderate AD. Upon trial completion, as the Company began the process
of analyzing the trial data, the Company found significant deviations from the protocol and cGCP violations at 15 study sites (virtually
all of which were from one geographic area). This highly unusual level of suspected improprieties led the Company to exclude all patients
from these sites. We subsequently notified FDA’s OSI of such significant deviations from study protocol, the suspected improprieties,
and the study sites involved. The identification of significant deviations from study protocol and numerous GCP violations at multiple
study sites raised questions regarding the validity and robustness of data from these study sites. The unplanned exclusion of so many
patients left the trial underpowered for its primary endpoints. However, based on the remaining dataset, a preliminary signal of efficacy
was detected. The Company is considering: (1) employing the adaptive trial feature of the protocol to continue enrolling patients to achieve
statistical significance; and/or (2) designing a new Phase 3 study of bezisterim (NE3107) that leverages the most recent scientific literature
relating to AD along with the company's understanding regarding the effects of bezisterim (NE3107) in persons with mild-moderate AD.
Although we design the clinical
trials for our product candidates, our CROs are tasked with facilitating and monitoring these trials. As a result, many aspects of our
clinical development programs, including site and investigator selection, and the conduct, timing, and monitoring of the study, is outside
our direct control, either partially or in whole. Our reliance on third parties to conduct clinical trials also results in less direct
control over the collection, management, and quality of data developed through clinical trials than would be the case if we were relying
entirely upon our own employees. Communicating with third parties can also be challenging, potentially leading to mistakes as well as
difficulties in coordinating activities. Our business may be impacted if any of these third parties violates applicable federal, state,
or foreign laws and/or regulations, including but not limited to FDA’s IND regulations, cGCPs, fraud and abuse or false claims laws,
healthcare privacy and data security laws, or provide us or government agencies with inaccurate, misleading, or incomplete data.
Successful development of biopharmaceuticals
is highly uncertain and is dependent on numerous factors, many of which are beyond our control.
Product candidates that appear promising in the early
phases of development may fail to reach the market for several reasons. Pre-clinical study results may show the product candidate to be
less effective than desired (e.g., the study failed to meet its primary endpoints) or to have harmful or problematic side effects. Product
candidates may fail to receive the necessary regulatory approvals or may be delayed in receiving such approvals. Among other things, such
delays may be caused by slow enrollment in clinical studies; length of time to achieve study endpoints; additional time requirements for
data analysis; IND and later new drug application preparation; discussions with the FDA; an FDA request for additional pre-clinical or
clinical data; unexpected safety or manufacturing issues; manufacturing costs; pricing or reimbursement issues; clinical sites deviating
from the trial protocol, committing scientific misconduct, or other violations of regulatory requirements - which can render data from
those sites unusable in support of regulatory approval; or other factors that make the product not economical. Proprietary rights of others
and their competing products and technologies may also prevent the product from being commercialized.
Success in pre-clinical and early clinical studies
does not ensure that large-scale clinical studies will be successful. Clinical results are frequently susceptible to varying interpretations
that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical studies and to submit an application
for marketing approval for a final decision by a regulatory authority varies significantly from one product to the next, and may be difficult
to predict. There can be no assurance that any of our products will develop successfully, and the failure to develop our products will
have a materially adverse effect on our business and will cause you to lose all of your investment.
The concentration of our assets within a certain
financial institution could have a material adverse effect on its business, financial condition and results of operations.
As of August 30, 2024, the Company had cash deposited
in a certain financial institution in excess of federally insured levels. The Company regularly monitors the financial stability of these
financial institutions and believes that it is not exposed to any significant credit risk in cash and cash equivalents. Bank failures,
events involving limited liquidity, defaults, non-performance, or other adverse developments that affect financial institutions, or concerns
or rumors about such events, may lead to liquidity constraints. In 2023, certain U.S. government banking regulators took steps to intervene
in the operations of certain financial institutions due to liquidity concerns, which caused general heightened uncertainties in financial
markets. While previous bank failures have not had a material direct impact on the Company’s operations, if further liquidity and
financial stability concerns arise with respect to banks and financial institutions, either nationally or in specific regions, the Company’s
ability to access cash or enter into new financing arrangements may be threatened, which could have a material adverse effect on its business,
financial condition and results of operations.
We are currently subject to securities class
action litigation and may be subject to similar or other litigation in the future, all of which will require significant management time
and attention, result in significant legal expenses and may result in unfavorable outcomes, which may have a material adverse effect on
our business, operating results and financial condition, and negatively affect the price of our Common Stock.
We are, and may in the future become, subject to various
legal proceedings and claims that arise in or outside the ordinary course of business. For example, On January 19, 2024, a purported shareholder
class action complaint, captioned Eric Olmstead v. BioVie Inc. et al., No. 3:24-cv-00035, was filed in the U.S. District Court
for the District of Nevada, naming the Company and certain of its officers as defendants. On February 22, 2024, a second, related putative
securities class action was filed in the same court asserting similar claims against the same defendants, captioned Way v. BioVie Inc.
et al., No. 2:24-cv-00361. On April 15, 2024, the court consolidated these two actions under the caption In re BioVie Inc. Securities
Litigation, No. 3:24-cv-00035, appointed the lead plaintiff, and approved selection of the lead counsel. On June 21, 2024, the lead
plaintiff filed an amended complaint, alleging that the defendants made material misrepresentations and/or omissions of material fact
relating to the Company’s business, operations, compliance, and prospects, including information related to the NM101 Phase 3 study
and trial of bezisterim (NE3107) in mild to moderate probable Alzheimer’s Disease, in violation
of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Rule 10b-5 promulgated
thereunder. The class action is on behalf of purchasers of the Company’s securities during the period from December 7, 2022 through
November 28, 2023 and seeks unspecified monetary damages on behalf of the putative class and an award of costs and expenses, including
attorney’s fees. The defendants filed a motion to dismiss the amended complaint on August 21, 2024. The defendants believe that
the claims are without merit and intend to defend vigorously against them, but there can be no assurances as to the outcome.
It is possible that additional lawsuits will be filed,
or allegations received from stockholders, with respect to these same or other matters and also naming us and/or our officers and directors
as defendants. Such lawsuits and any other related lawsuits are subject to inherent uncertainties, and the actual defense and disposition
costs will depend upon many unknown factors. The outcome of such lawsuits is necessarily uncertain. We could be forced to expend significant
resources in the defense of the pending lawsuit and any additional lawsuits, and we may not prevail. In addition, we may incur substantial
legal fees and costs in connection with such lawsuits. We currently are not able to estimate the possible cost to us from this matter,
as the pending lawsuit is currently at an early stage, and we cannot be certain how long it may take to resolve the pending lawsuit or
the possible amount of any damages that we may be required to pay. Monitoring, initiating and defending against legal actions is time-consuming
for our management, is likely to be expensive and may detract from our ability to fully focus our internal resources on our business activities.
We could be forced to expend significant resources in the settlement or defense of the pending lawsuit and any potential future lawsuits,
and we may not prevail in such lawsuits.
Although we have insurance coverage that we believe
applies to these actions, the coverage is subject to a $2 million deductible. That means that we are responsible for the first $2 million
of loss arising from these actions, which includes both defense costs and damages, before any insurance coverage will apply. Furthermore,
our insurance coverage may be insufficient, and our assets may be insufficient to cover any amounts that exceed our insurance coverage,
and we may have to pay damage awards or otherwise may enter into a settlement arrangement in connection with such claim. A decision adverse
to our interests in the pending lawsuit, or in similar or related litigation, could result in the payment of substantial damages, or possibly
fines, and could have a material adverse effect on our business, our stock price, cash flow, results of operations and financial condition.
We have not established any reserve for any potential liability relating to the pending lawsuit or any potential future lawsuits. Any
such payments or settlement arrangements in current or future litigation could have a material adverse effect on our business, operating
results or financial condition. In addition, such lawsuits may make it more difficult to finance our operations and affect our ability
to make payments for damages.
Risks Relating to This Offering and our Common
Stock
You may experience immediate and substantial
dilution in the net tangible book value per share of our Common Stock you purchase in the offering.
The offering price
per share in this offering may exceed the pro forma net tangible book value per share of our Common Stock outstanding prior to this offering.
After giving effect to the sale by us of the Shares at a price of $2.83 per Share, and after placement agent fees and estimated offering
expenses payable by us, you will experience immediate dilution of $0.51 per share,
representing the difference between our pro forma as adjusted net tangible book value per share as of June 30, 2024 after giving effect
to this offering and the assumed offering price. The exercise of outstanding warrants and stock options may also result in further
dilution of your investment. See the section entitled “Dilution” on page S-25
below for a more detailed illustration of the dilution you may incur if you participate in this offering.
Our management will have broad discretion over
the use of the net proceeds from this offering, may invest or spend the proceeds raised in this offering in ways with which you may not
agree and the proceeds may not yield a significant return.
Our management will have broad discretion over the
use of proceeds from this offering. We currently intend to use the net proceeds of this offering as described in the section entitled
“Use of Proceeds.” However, our management will have broad discretion in the application of the net proceeds from this offering
and could use them for purposes other than those contemplated at the time of this offering. Accordingly, you are relying on the judgment
of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision,
to assess whether the proceeds will be used appropriately. The failure by management to apply these funds effectively could result in
financial losses that could have a material adverse effect on our business, cause the price of our Common Stock to decline, and delay
the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in short-term, interest-bearing
instruments. These investments may not yield a favorable return, or any return, to us or our stockholders.
Our stock price is and may continue to be volatile
and you may not be able to resell our Common Stock at or above the price you paid.
The market price for our Common Stock is volatile
and may fluctuate significantly in response to a number of factors, many of which we cannot control, such as quarterly fluctuations in
financial results, the timing and our ability to advance the development of our product candidates or changes in securities analysts’
recommendations could cause the price of our stock to fluctuate substantially. In addition, stock markets generally have recently experienced
volatility. Our stock price is likely to experience significant volatility in the future. The price of our Common Stock may decline and
the value of any investment in our Common Stock may be reduced regardless of our performance. Further, the daily trading volume of our
Common Stock has historically been relatively low. As a result of the historically low volume, our stockholders may be unable to sell
significant quantities of Common Stock in the public trading markets without a significant reduction in the price of our shares of Common
Stock. Each of these factors, among others, could harm your investment in our Common Stock and could result in your being unable to resell
the shares of our Common Stock that you purchase at a price equal to or above the price you paid.
In the past, when the market price of a stock has
been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders
were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would
be diverted from the operation of our business.
We do not intend to pay dividends on our Common
Stock, so any returns will be limited to the value of our Common Stock.
We currently anticipate that we will retain any future
earnings to finance the continued development, operation and expansion of our business. As a result, we do not anticipate declaring or
paying any cash dividends or other distributions in the foreseeable future. If we do not pay dividends, our Common Stock may be less valuable
because stockholders must rely on sales of their Common Stock after price appreciation, which may never occur, to realize any gains on
their investment.
You may experience future dilution as a result
of future equity offerings or if we issue shares subject to options, warrants, stock awards or other arrangements.
In order to raise additional capital, we may in the
future offer additional shares of our Common Stock or other securities convertible into or exchangeable for our Common Stock in any other
offering at a price per share that is less than the current market price of our securities, and investors purchasing shares or other securities
in the future could have rights superior to existing stockholders. The sale of additional shares of our Common Stock or other securities
convertible into or exchangeable for our Common Stock would dilute all of our stockholders, and if such sales of convertible securities
into or exchangeable into our Common Stock occur at a deemed issuance price that is lower than the current exercise price of our outstanding
warrants sold to Acuitas Group Holdings, LLC (“Acuitas”) in August 2022 (the “Acuitas Warrants”), the exercise
price for those warrants would adjust downward to the deemed issuance price pursuant to price adjustment protection contained within those
warrants.
As of October 28, 2024, there were warrants outstanding to purchase an aggregate of 10,140,202 shares of our Common Stock at exercise prices ranging
from $1.37 to $125.00 per share and 517,916 shares issuable upon exercise of outstanding options at exercise prices ranging from $4.74
to $420.90 per share and restricted stock units totaling 34,567. In addition, pursuant to the Loan and Security Agreement and the Supplement
to the Loan and Security Agreement, each entered into on November 30, 2021 with Avenue Venture Opportunities Fund II, L.P. and Avenue
Venture Opportunities Fund, L.P., the lenders have the option to convert up to $5 million of the outstanding loan amount into shares of
our Common Stock at a conversion price of $58.20 per share. We may also grant additional options, warrants or equity awards. To the extent
such shares are issued, the interest of holders of our Common Stock will be diluted.
Moreover, we are obligated to issue shares of our
Common Stock upon achievement of certain clinical, regulatory and commercial milestones with respect to certain of our drug candidates
(i.e., bezisterim (NE3107), NE3291, NE3413, and NE3789) pursuant to the asset purchase agreement, dated April 27, 2021, by and among the
Company, NeurMedix and Acuitas, as amended on May 9, 2021. The achievement of these milestones could result in the issuance of up to 1.8
million shares of our Common Stock, further diluting the interest of holders of our Common Stock.
This is a best efforts offering, no minimum
number of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business plans.
The placement agent has agreed to use its reasonable
best efforts to solicit offers to purchase the Shares in this offering. The placement agent has no obligation to buy any of the securities
from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum
number of securities or amount of proceeds that must be sold as a condition to completion of this offering. Because there is no minimum
number of securities or amount of proceeds required as a condition to the closing of this offering, the actual offering amount, placement
agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We
may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors
in this offering will not receive a refund in the event that we do not sell all of the units offered in this offering. Thus, we may not
raise the amount of capital we believe is required for our operations in the short term and may need to raise additional funds, which
may not be available or available on terms acceptable to us.
Certain stockholder of the Company may have
significant control over our Company.
As of October 28, 2024, Acuitas beneficially owns 3,050,397 shares of our Common Stock,
which includes warrants to purchase 727,273 shares of our Common Stock and options to purchase 6,500 shares of our Common Stock that are
exercisable within 60 days of October 28, 2024 and currently constitutes 20.8% of our issued and outstanding Common Stock. As a
result, Acuitas has substantial influence over the management and affairs of our Company, as well as the ability to control the outcome
of matters submitted to our stockholders for approval, including the election of directors, the approval of significant corporate transactions,
including any merger, consolidation or sale of all or substantially all of our assets, the issuance or redemption of equity interests
in certain circumstances, and any other significant transaction. The interests of Acuitas may not always align with, and in some cases
may conflict with, our interests or the interests of our other stockholders. For instance, this concentration of ownership may have the
effect of delaying or preventing a change of control otherwise favored by our other stockholders and could deprive our other stockholders
of an opportunity to receive a premium for their Common Stock. This concentration of ownership may also negatively affect the prevailing
market price of our Common Stock due to investors’ perceptions that conflicts of interest may exist or arise. As a result, this
concentration of ownership may not be in your best interests.
We may, in the future, issue additional common
stock, which would reduce investors’ percent of ownership and may dilute our share value.
As of October 28, 2024, our Articles of Incorporation, as amended, authorize the issuance of 800,000,000
shares of Common Stock, and we had 16,638,700 shares of our Common Stock issued and 16,612,374 issued and outstanding. Accordingly,
we may issue up to an additional 783,361,300 shares of Common Stock. The future issuance of Common Stock may result in substantial dilution
in the percentage of our Common Stock held by our then existing stockholders. We may value any Common Stock in the future on an arbitrary
basis. The issuance of Common Stock for future services or acquisitions or other corporate actions may have the effect of diluting the
value of the shares held by our investors, might have an adverse effect on any trading market for our Common Stock and could impair our
ability to raise capital in the future through the sale of equity securities.
We effected a reverse stock split on August
6, 2024, and we cannot predict the effect that such reverse stock split will have on the market price for shares of our Common Stock.
Our board of directors approved a one-for-ten (1:10)
reverse stock split of our Common Stock, which became effective at 12:01 a.m. Eastern Time on August 6, 2024. We cannot predict the effect
that the reverse stock split will have on the market price for shares of our Common Stock, and the history of similar reverse stock splits
for companies in like circumstances has varied. Some investors may have a negative view of a reverse stock split. Even if the reverse
stock split has a positive effect on the market price for shares of our Common Stock, performance of our business and financial results,
general economic conditions and the market perception of our business, and other adverse factors which may not be in our control could
lead to a decrease in the price of our common stock following the reverse stock split.
Furthermore, even if the reverse stock split does
result in an increased market price per share of our Common Stock, the market price per share following the reverse stock split may not
increase in proportion to the reduction of the number of shares of our Common Stock outstanding before the implementation of the reverse
stock split. Accordingly, even with an increased market price per share, the total market capitalization of shares of our Common Stock
after a reverse stock split could be lower than the total market capitalization before the reverse stock split. Also, even if there is
an initial increase in the market price per share of our Common Stock after a reverse stock split, the market price may not remain at
that level.
If the market price of shares of our Common Stock
declines following the reverse stock split, the percentage decline as an absolute number and as a percentage of our overall market capitalization
may be greater than would occur in the absence of the reverse stock split due to decreased liquidity in the market for our Common Stock.
Accordingly, the total market capitalization of our Common Stock following the reverse stock split could be lower than the total market
capitalization before the reverse stock split.
Risks Relating to Our Intellectual Property
We may be unable to obtain or protect intellectual
property rights relating to our product candidates, which could have a materially adverse effect on our business.
Our ability to compete effectively will depend on
our ability to maintain the proprietary nature of our technologies. We cannot assure investors that we will continue to innovate and file
new patent applications, or that if filed any future patent applications will result in granted patents with respect to the technology
owned by us or licensed to us. Further, we cannot predict how long it will take for such patents to issue, if at all. The patent position
of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations
and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated
or circumvented.
We have pending patent applications for our
liquid formulations of terlipressen the following jurisdictions which claim priority to PCT/US2020/034269 filed on May 22, 2020 and
published as WO2020/237170: US, Europe, China, and Japan and 6 other jurisdictions. In two jurisdictions, we have patents for our
liquid formulations of terlipressen which claim priority to PCT/US2020/034269 filed on May 22, 2020 and published as WO2020/237170.
We also have thirteen (13) issued U.S. patents, six (6) pending U.S. applications, three (3) pending Patent Cooperation Treaty
applications, five (5) pending foreign patent applications, and six (6) issued foreign patents directed to protecting bezisterm
(NE3107) and related compounds and methods of making and using thereof. However, there can be no assurance that our pending patent
applications will result in issued patents, or that any issued patent claims from pending or future patent applications will be
sufficiently broad to protect BIV201, bezisterim (NE3107), or any other product candidates or to provide us with competitive
advantages.
We can provide no assurance that any issued patents
will provide us with any competitive advantage. We cannot be certain that there is no invalidating prior art of which we and the patent
examiner are unaware or that our interpretation of the relevance of prior art is correct. If a third-party patent or patent application
is determined to have an earlier priority date, it may prevent our patent applications from issuing at all or issuing in a form that provides
any competitive advantage for our drug candidates. Failure to obtain additional issued patents could have a material adverse effect on
our ability to develop and commercialize our drug candidates. Even if our patent applications do issue as patents, third parties may be
able to challenge the validity and enforceability of our patents on a variety of grounds, including that such third party’s patents
and patent applications have an earlier priority date, and if such challenges are successful, we may be required to obtain one or more
licenses from such third parties, if available on commercially reasonable terms, or be prohibited from commercializing our drug candidates.
We seek to protect our proprietary positions by, among
other things, filing patent applications in the United States and abroad related to our current drug candidates and other drug candidates
that we may identify. Obtaining, maintaining, defending and enforcing pharmaceutical patents is costly, time-consuming and complex, and
we may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents
that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we will fail to identify
patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, under certain of
our license or collaboration agreements, we may not have the right to control the preparation, filing, prosecution and maintenance of
patent applications, or to maintain the rights to patents licensed to or from third parties.
We currently are the assignee of a number of U.S.
provisional patent applications. U.S. provisional patent applications are not eligible to become issued patents until, among other things,
we file a non-provisional patent application within 12 months of filing one or more of our related provisional patent applications. With
regard to such U.S. provisional patent applications, if we do not timely file any non-provisional patent applications, we may lose our
priority dates with respect to our provisional patent applications and any patent protection on the inventions disclosed in our provisional
patent applications. Further, in the event that we do timely file non-provisional patent applications relating to our provisional patent
applications, we cannot predict whether any such patent applications will result in the issuance of patents or if such issued patents
will provide us with any competitive advantage.
As to our material inventions, trade secrets, and
intellectual property, our employees, consultants, and advisors execute confidentiality agreements and agree to disclose and assign to
us all inventions conceived during the workday, using our property, or which relate to our business. However, any of these parties may
breach these agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent
protection. Further, we may not be aware of all third-party intellectual property rights potentially relating to our drug candidates.
Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United
States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot
know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we
were the first to file for patent protection of such inventions.
The patent position of pharmaceutical companies generally
is highly uncertain, involves complex legal, technological and factual questions and has, in recent years, been the subject of much debate
and litigation throughout the world. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws
of the United States, or vice versa. The standards that the United States Patent and Trademark Office (the “USPTO”) (and foreign
countries) use to grant patents are not always applied predictably or uniformly and can change. There is also no uniform, worldwide policy
regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. Accordingly, we do not
know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to
us or to others. The issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain. The subject
matter claimed in a patent application can be significantly reduced or eliminated before the patent issues, if at all, and its scope can
be reinterpreted or narrowed after issuance. Therefore, our pending and future patent applications may not result in patents being issued
in relevant jurisdictions that protect our drug candidates, in whole or in part, or that effectively prevent others from commercializing
competitive drug candidates, and even if our patent applications issue as patents in relevant jurisdictions, they may not issue in a form
that will provide us with any meaningful protection for our drug candidates or technology, prevent competitors from competing with us
or otherwise provide us with any competitive advantage. Additionally, our competitors may be able to circumvent our patents by challenging
their validity or by developing similar or alternative drug candidates or technologies in a non-infringing manner.
The issuance of a patent is not conclusive as to its
inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States
and abroad. We may be subject to a third-party preissuance submission of prior art to the USPTO, or become involved in opposition, derivation,
reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights
of others, or other proceedings in the USPTO or applicable foreign offices that challenge priority of invention or other features of patentability.
An adverse determination in any such submission, proceeding or litigation could result in loss of exclusivity or ability to sell our products
free from infringing the patents of third parties, patent claims being narrowed, invalidated or held unenforceable, in whole or in part,
and limitation of the scope or duration of the patents directed to our drug candidates, all of which could limit our ability to stop others
from using or commercializing similar or identical drug candidates or technology to compete directly with us, without payment to us, or
result in our inability to manufacture or commercialize drug candidates or approved products (if any) without infringing third-party patent
rights. In addition, if the breadth or strength of the claims of our patents and patent applications is threatened, regardless of the
outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future drug candidates,
or could have a material adverse effect on our ability to raise funds necessary to continue our research programs or clinical trials.
Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual
outcome is favorable to us.
In addition, given the amount of time required for
the development, testing and regulatory review of new drug candidates, patents protecting such candidates might expire before or shortly
after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others
from commercializing products or technology similar or identical to ours for a meaningful amount of time, or at all. Moreover, some of
our licensed patents and owned or licensed patent applications may in the future be co-owned with third parties. If we are unable to obtain
exclusive licenses to any such co-owners’ interest in such patents or patent applications, such co-owners may be able to license
their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In
addition, we may need the cooperation of any such co-owners in order to enforce such patents against third parties, and such cooperation
may not be provided to us. Any of the foregoing could harm our competitive position, business, financial condition, results of operations
and prospects.
Further, we rely on a combination of trade secrets,
know-how, technology and nondisclosure, and other contractual agreements and technical measures to protect our rights in the technology.
If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor,
our business and financial condition could be materially and adversely affected. The laws of some foreign countries do not protect our
proprietary rights to the same extent as the laws of the U.S., and we may encounter significant problems in protecting our proprietary
rights in these countries.
Our success depends in significant part on our ability
to obtain, maintain, enforce and defend patents and other intellectual property rights with respect to our drug candidates and technology
and to operate our business without infringing, misappropriating, or otherwise violating the intellectual property rights of others. If
we are unable to obtain and maintain sufficient intellectual property protection for our drug candidates or other drug candidates that
we may identify, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors and other
third parties could develop and commercialize drug candidates similar or identical to ours, and our ability to successfully commercialize
our drug candidates and other drug candidates that we may pursue may be impaired.
Confidentiality agreements with employees and
others may not adequately prevent disclosure of trade secrets and other proprietary information and disclosure of our trade secrets or
proprietary information could compromise any competitive advantage that we have, which could have a materially adverse effect on our business.
Our success depends, in part, on our ability to protect
our proprietary rights to the technologies used in our product candidates. We depend heavily upon confidentiality agreements with our
officers, employees, consultants and subcontractors to maintain the proprietary nature of our technology. These measures may not afford
us complete or even sufficient protection, and may not afford an adequate remedy in the event of an unauthorized disclosure of confidential
information. If we fail to protect and/or maintain our intellectual property, third parties may be able to compete more effectively against
us, we may lose our technological or competitive advantage, and/or we may incur substantial litigation costs in our attempts to recover
or restrict use of our intellectual property. In addition, others may independently develop technology similar to ours, otherwise avoiding
the confidentiality agreements, or produce patents that would materially and adversely affect our business, prospects, financial condition
and results of operations, in which event you could lose all of your investment.
We may enter into licensing and collaboration
agreements with third parties. If we fail to comply with our obligations in the agreements under which we license intellectual property
rights to or from third parties, or these agreements are terminated, or we otherwise experience disruptions to our business relationships
with our licensors or licensees, our competitive position, business, financial condition, results of operations and prospects could be
harmed.
It may be necessary for us to use the patented or
proprietary technology of third parties to commercialize our products (if approved), in which case we would be required to obtain a license
from these third parties. The licensing of third-party intellectual property rights is a competitive area, and more established companies
may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. More
established companies may have a competitive advantage over us due to their size, capital resources and greater clinical development and
commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights
to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate
return on our investment or at all. If we are unable to license such technology, or if we are forced to license such technology on unfavorable
terms, our business could be materially harmed.
We may fail to obtain any of these licenses or intellectual
property rights on commercially reasonable terms. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our
competitors access to the same technologies licensed to us. Licenses may not provide us with exclusive rights to use the applicable intellectual
property and technology in all relevant fields of use and in all territories in which we may wish to develop or commercialize our drug
candidates, products (if approved) and technology in the future. In that event, we may be required to expend significant time and resources
to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products,
which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction
prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation. Conversely,
we may not always be able to successfully pursue our claims against others that infringe upon our technology. Thus, the proprietary nature
of our technology or technology licensed by us may not provide adequate protection against competitors, and we may not be able to prevent
competitors from developing and commercializing competitive products or technologies.
In addition, in some circumstances, we may not have
the right to control the preparation, filing and prosecution of patent applications or to maintain, defend and enforce the patents that
we license to or from third parties, and we may have to rely on our partners to fulfill these responsibilities. If our current or future
licensors, licensees or collaborators fail to prepare, file, prosecute, maintain, enforce, and defend licensed patents and other intellectual
property rights, such rights may be reduced or eliminated, and our right to develop and commercialize any of our drug candidates or technology
that are the subject of such licensed rights could be adversely affected. In addition, our licensors may own or control intellectual property
that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise
violating the licensor’s rights.
If we fail to comply with our obligations, including
the obligation to make various milestone payments and royalty payments, under any of the agreements under which we license intellectual
property rights from third parties, the licensor may have the right to terminate the license. If any of our license agreements is terminated,
the underlying licensed patents fail to provide the intended exclusivity or we otherwise experience disruptions to our business relationships
with our licensors, we could lose intellectual property rights that are important to our business or be prevented from developing and
commercializing our drug candidates, and competitors could have the freedom to seek regulatory approval of, and to market, products identical
to ours. Termination of these agreements or reduction or elimination of our rights under these agreements may also result in our having
to negotiate new or reinstated agreements with less favorable terms, cause us to lose our rights under these agreements, including our
rights to important intellectual property or technology, or impede, delay or prohibit the further development or commercialization of
one or more drug candidates that rely on such agreements. It is possible that we may be unable to obtain any additional licenses at a
reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to redesign
our drug candidates or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible
on a technical or commercial basis.
Licensing of intellectual property is of critical
importance to our business and involves complex legal, business and scientific issues and certain provisions in intellectual property
license agreements may be susceptible to multiple interpretations. Disputes may arise between us and our licensing partners regarding
intellectual property subject to a license agreement, including:
| · | the scope of rights granted under the license agreement and other interpretation-related issues; |
| · | whether and the extent to which technology and processes of one party infringe intellectual property of
the other party that are not subject to the licensing agreement; |
| · | rights to sublicense patent and other rights to third parties; |
| · | any diligence obligations with respect to the use of the licensed technology in relation to development
and commercialization of our drug candidates, and what activities satisfy those diligence obligations; |
| · | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property; |
| · | rights to transfer or assign the license; and |
| · | the effects of termination. |
The resolution of any contract interpretation disagreement
that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase
what we believe to be our financial or other obligations under the relevant agreement, either of which could harm our business, financial
condition, results of operations and prospects. If disputes over intellectual property that we have licensed prevent or impair our ability
to maintain our current licensing arrangements on acceptable terms or at all, we may be unable to successfully develop and commercialize
the affected drug candidates. Moreover, any dispute or disagreement with our licensing partners may result in the delay or termination
of the research, development or commercialization of our drug candidates or any future drug candidates, and may result in costly litigation
or arbitration that diverts management attention and resources away from our day-to-day activities, which may adversely affect our business,
financial condition, results of operations and prospects.
Furthermore, current and future collaborators or strategic
partners may develop, either alone or with others, products in related fields that are competitive with the products or potential products
that are the subject of these collaborations. Competing products, either developed by our collaborators or strategic partners or to which
the collaborators or strategic partners have rights, may result in the withdrawal of partner support for our drug candidates. Any of these
developments could harm our product development efforts.
In addition, if our licensors fail to abide by the
terms of the license, if the licensors fail to prevent infringement by third parties or if the licensed patents or other rights are found
to be invalid or unenforceable, our business, competitive position, financial condition, results of operations and prospects could be
materially harmed.
Some of our intellectual property may be subject
to federal regulations such as “march-in” rights, certain reporting requirements and a preference for U.S.-based companies
if it is determined that our intellectual property has been discovered through government-funded programs. Compliance with such regulations
may limit our exclusive rights, and limit our ability to contract with non-U.S. manufacturers.
Some of the intellectual property rights we have acquired
or licensed or may acquire or license in the future may have been generated through the use of U.S. government funding and may therefore
be subject to certain federal regulations. These U.S. government rights include a non-exclusive, non-transferable, irrevocable worldwide
license to use inventions for any governmental purpose. In addition, the U.S. government has the right, under certain limited circumstances,
to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines
that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health
or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred
to as “march-in rights”). The U.S. government also has the right to take title to these inventions if the grant recipient
fails to disclose the invention to the government or fails to file an application to register the intellectual property within specified
time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance
with which may require us to expend substantial resources. In addition, the U.S. government requires that any products embodying any of
these inventions or produced through the use of any of these inventions be manufactured substantially in the United States. This preference
for U.S. industry may be waived by the federal agency that provided the funding if the owner or assignee of the intellectual property
can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would
be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible.
This preference for U.S. industry may limit our ability to contract with non-U.S. product manufacturers for products relating to such
intellectual property. To the extent any of our future intellectual property is also generated through the use of U.S. government funding,
the provisions of the Bayh-Dole Act may similarly apply.
Patent terms may be inadequate to establish
our competitive position on our drug candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States,
if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional
filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents
directed to our drug candidates are obtained, once the patent life has expired for a drug candidate, we may be open to competition from
competitive medications, including generic versions. Given the amount of time required for the development, testing and regulatory review
of new drug candidates, patents directed towards such drug candidates might expire before or shortly after such drug candidates are commercialized.
As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing
drug candidates similar or identical to ours for a meaningful amount of time, or at all.
Depending upon the timing, duration and conditions
of any FDA marketing approval of our drug candidates, one or more of our owned or licensed U.S. patents may be eligible for limited patent
term extension under the Hatch-Waxman Act, and similar legislation in the European Union (the “EU”) and certain other countries.
The Hatch-Waxman Act permits a patent term extension of up to five years for a patent covering an approved product as compensation for
effective patent term lost during product development and the FDA regulatory review process. However, we may not receive an extension
if we fail to exercise due diligence during the testing phase or regulatory review process, fail to apply within applicable deadlines,
fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the
extension could be less than we request. Only one patent per approved product can be extended, the extension cannot extend the total patent
term beyond 14 years from approval and only those claims for the approved drug, a method for using it or a method for manufacturing it
may be extended. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period
during which we can enforce our patent rights for the applicable drug candidate will be shortened and our competitors may obtain approval
to market competing products sooner. As a result, our revenue from applicable products could be reduced. Further, if this occurs, our
competitors may take advantage of our investment in development and trials by referencing our clinical and nonclinical data and launch
their product earlier than might otherwise be the case, and our competitive position, business, financial condition, results of operations
and prospects could be materially harmed.
We may not be able to protect our intellectual
property rights throughout the world.
Filing, prosecuting, maintaining, defending and enforcing
patents on our drug candidates in all countries throughout the world would be prohibitively expensive, and consequently our intellectual
property rights in some countries outside the United States may be less extensive than those in the United States. In addition, the laws
of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States.
Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or
from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use
our technologies in jurisdictions where we have not obtained patents to develop their own products and may export otherwise infringing
products to territories where we have patents, but enforcement rights are not as strong as those in the United States. These products
may compete with our drug candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent
them from competing.
Many companies have encountered significant problems
in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries do not favor the
enforcement or protection of patents, trade secrets and other intellectual property, which could make it difficult for us to stop the
infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary rights generally.
Proceedings to enforce our intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts
and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent
applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that
we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful.
Many foreign countries, including some EU countries,
India, Japan and China, have compulsory licensing laws under which a patent owner may be compelled under specified circumstances to grant
licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors.
In those countries, we may have limited remedies if patents are infringed or if we are compelled to grant a license to a third party,
which could materially diminish the value of the applicable patents and limit our potential revenue opportunities. Accordingly, our efforts
to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual
property that we develop or license, which could adversely affect our business, financial condition, results of operations and prospects.
In 2012, the European Patent Package, or EU Patent
Package, regulations were passed with the goal of providing a single pan-European Unitary Patent and a new European Unified Patent Court
(“UPC”), for litigation involving European patents. Implementation of the EU Patent Package occurred in 2023. Under the UPC,
all European patents, including those issued prior to ratification of the European Patent Package, will by default automatically fall
under the jurisdiction of the UPC. The UPC will provide our competitors with a new forum to centrally revoke our European patents, and
allow for the possibility of a competitor to obtain pan-European injunctions. It will be several years before we will understand the scope
of patent rights that will be recognized and the strength of patent remedies that will be provided by the UPC. Under the EU Patent Package
as currently proposed, we will have the right to opt our patents out of the UPC over the first seven years of the court’s existence,
but doing so may preclude us from realizing the benefits of the new unified court.
Changes in patent law could diminish the value
of patents in general, thereby impairing our ability to protect our drug candidates.
Obtaining and enforcing patents in the pharmaceutical
industry is inherently uncertain, due in part to ongoing changes in the patent laws. For example, in the United States, depending on decisions
by Congress, the federal courts, and the USPTO, the laws and regulations governing patents, and interpretation thereof, could change in
unpredictable ways that could weaken our and our collaborators’ or licensors’ ability to obtain new patents or to enforce
existing or future patents. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the
scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. Therefore,
there is increased uncertainty with regard to our and our collaborators’ or licensors’ ability to obtain patents in the future,
as well as uncertainty with respect to the value of patents once obtained.
Patent reform legislation could increase the uncertainties
and costs surrounding the prosecution of our and our collaborators’ or licensors’ patent applications and the enforcement
or defense of our or our collaborators’ or licensors’ issued patents. For example, assuming that other requirements for patentability
are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside
the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America
Invents Act (the “Leahy-Smith Act”), enacted in September 2011, the United States transitioned to a first inventor to file
system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled
to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. The Leahy-Smith Act also
includes a number of significant changes that affect the way patent applications are prosecuted and may also affect patent litigation.
These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to challenge
the validity of a patent by USPTO-administered post-grant proceedings, including post-grant review, inter partes review and derivation
proceedings. The USPTO has developed regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive
changes to patent law associated with the Leahy-Smith Act, particularly the first inventor-to-file provisions. Accordingly, it is not
clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation
could increase the uncertainties and costs surrounding the prosecution of our or our licensors’ patent applications and the enforcement
or defense of our or our licensors’ issued patents. Similarly, statutory or judicial changes to the patent laws of other countries
may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents.
Any of the foregoing could harm our business, financial condition, results of operations and prospects.
We may become involved in lawsuits to protect
or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful, and issued patents directed
towards our technology and drug candidates could be found invalid or unenforceable if challenged.
We are not aware that our patents directed to either
BIV201 or bezisterim (NE3107), the product candidates we are currently developing, are infringed by third parties. However, there can
be no assurance that our patents will not be found in the future to be infringed by others. Any patents we do obtain may be challenged
by reexamination or otherwise invalidated or eventually found unenforceable. Both the patent application process and the process of managing
patent disputes can be time-consuming and expensive.
Significantly, our pending patent applications cannot
be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications.
Our ability to enforce patent rights also depends on our ability to identify infringement. It may be difficult to identify infringers
who do not advertise the components or methods that are used in connection with their products and services. Moreover, it may be difficult
or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product or service. Any claims
we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their
patents or that our patents are invalid or unenforceable. In a patent infringement proceeding, a court may decide that a patent of ours
is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using
the technology at issue on the grounds that our patents do not cover the technology. An adverse result in any litigation proceeding could
put one or more of our owned or licensed patents at risk of being invalidated, held unenforceable or interpreted narrowly. We may find
it impractical or undesirable to enforce our intellectual property against some third parties.
If we were to initiate legal proceedings against a
third party to enforce a patent directed to our drug candidates, or one of our future drug candidates, the defendant could counterclaim
that our patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or
unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements,
including lack of novelty, obviousness, non-enablement or insufficient written description. Grounds for a presentability assertion could
be an allegation that someone connected with prosecution of the patent withheld material information from the USPTO or made a misleading
statement during prosecution. Third parties may also raise similar claims before the USPTO or an equivalent foreign body, even outside
the context of litigation. Potential proceedings include reexamination, post-grant review, inter partes review, interference proceedings,
derivation proceedings and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result
in the revocation of, cancellation of, or amendment to our patents in such a way that they no longer cover our technology or any drug
candidates that we may develop. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect
to the validity question, for example, we cannot be certain that there is no invalidating prior art of which we and the patent examiner
were unaware during prosecution. These assertions may also be based on information known to us or the USPTO. If a defendant were to prevail
on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent rights directed towards
the applicable drug candidates or technology related to the patent rendered invalid or unenforceable. Such a loss of patent rights would
materially harm our business, financial condition, results of operations and prospects.
Interference proceedings provoked by third parties
or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent
applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from
the prevailing party. Our business could be materially harmed if the prevailing party does not offer us a license on commercially reasonable
terms or at all.
Furthermore, because of the substantial amount of
discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could
be compromised by disclosure during this type of litigation.
The pharmaceutical industry is characterized by extensive
litigation regarding patents and other intellectual property rights. Moreover, the cost to us of any litigation or other proceeding relating
to our patents and other intellectual property rights, even if resolved in our favor, could be substantial, and the litigation would divert
our management’s efforts. We may not have sufficient resources to bring any such action to a successful conclusion. Uncertainties
resulting from the initiation and continuation of any litigation could limit our ability to continue our operations and you could lose
all of your investment.
Some of our competitors are larger than we are and
have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex patent litigation or proceedings
more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios.
Accordingly, despite our efforts, we may not be able to prevent third parties from infringing, misappropriating or otherwise violating
our intellectual property. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims
could result in substantial costs and diversion of management resources, which could harm our business. In addition, the uncertainties
associated with litigation could compromise our ability to raise the funds necessary to continue our clinical trials, continue our internal
research programs, or in-license needed technology or other drug candidates. There could also be public announcements of the results of
the hearing, motions, or other interim proceedings or developments. If securities analysts or investors perceive those results to be negative,
it could cause the price of shares of our Common Stock to decline. Any of the foregoing events could harm our business, financial condition,
results of operation and prospects.
We may not identify relevant third-party patents
or may incorrectly interpret the relevance, scope or expiration of a third-party patent, which might subject us to infringement claims
or adversely affect our ability to develop and market our drug candidates.
We cannot guarantee that any of our or our licensors’
patent searches or analyses, including the identification of relevant patents, the scope of patent claims or the expiration of relevant
patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending patent
application in the United States and abroad that is relevant to or necessary for the commercialization of our drug candidates in any jurisdiction.
For example, U.S. patent applications filed before November 29, 2000 and certain U.S. patent applications filed after that date that will
not be filed outside the United States remain confidential until patents issue. As mentioned above, patent applications in the United
States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest
filing date being commonly referred to as the priority date. Therefore, patent applications covering our drug candidates could have been
filed by third parties without our knowledge. Additionally, pending patent applications that have been published can, subject to certain
limitations, be later amended in a manner that could cover our drug candidates or the use of our drug candidates. The scope of a patent
claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our
interpretation of the relevance or the scope of a patent or a pending application may be incorrect, which may negatively impact our ability
to market our drug candidates. We may incorrectly determine that our drug candidates are not covered by a third-party patent or may incorrectly
predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration
date of any patent in the United States or abroad that we consider relevant may be incorrect, which may negatively impact our ability
to develop and market our drug candidates. Our failure to identify and correctly interpret relevant patents may negatively impact our
ability to develop and market our drug candidates.
In addition, if we fail to identify and correctly
interpret relevant patents, we may be subject to infringement claims. We cannot guarantee that we will be able to successfully settle
or otherwise resolve such infringement claims. If we fail in any such dispute, in addition to being forced to pay damages, which may be
significant, we may be temporarily or permanently prohibited from commercializing any of our drug candidates that are held to be infringing.
We might, if possible, also be forced to redesign drug candidates so that they no longer infringe the third-party intellectual property
rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources
that we would otherwise be able to devote to our business and could adversely affect our business, financial condition, results of operations
and prospects.
Third parties may initiate legal proceedings
alleging that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would
be uncertain and could negatively impact the success of our business.
Our commercial success depends upon our ability to
develop, manufacture, market and sell our drug candidates and use our proprietary technologies without infringing, misappropriating or
otherwise violating the intellectual property and other proprietary rights of third parties. There is considerable intellectual property
litigation in the pharmaceutical industry. We may become party to, or be threatened with, future adversarial proceedings or litigation
regarding intellectual property rights with respect to our drug candidates and their manufacture and our other technology, including reexamination,
interference, post-grant review, inter partes review or derivation proceedings before the USPTO or an equivalent foreign body.
Numerous U.S.- and foreign-issued patents and pending patent applications owned by third parties exist in the fields in which we are developing
our drug candidates. Third parties may assert infringement claims against us based on existing patents or patents that may be granted
in the future, regardless of their merit.
We do not believe that either BIV201 or bezisterim
(NE3107), the product candidates we are currently developing, infringe the patents of any third parties. However, there can be no assurance
that our technology will not be found in the future to infringe the patents of others. Moreover, patent applications are in some cases
maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs
substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can
take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents
that our products or product candidates infringe. For example, pending applications may exist that provide support or can be amended to
provide support for a claim that results in an issued patent that our product infringes.
Even if we believe third-party intellectual property
claims are without merit, there is no assurance that a court would find in our favor on questions of claim scope, infringement, validity,
enforceability or priority. A court of competent jurisdiction could hold that third-party patents asserted against us are valid, enforceable
and infringed, which could materially and adversely affect our ability to commercialize any drug candidates we may develop and any other
drug candidates or technologies covered by the asserted third-party patents. In order to successfully challenge the validity of any such
U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present
clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction
would invalidate the claims of any such U.S. patent.
If we are found to infringe, misappropriate or otherwise
violate a third party’s intellectual property rights, and we are unsuccessful in demonstrating that such rights are invalid or unenforceable,
we could be required to obtain a license from such a third party in order to continue developing and marketing our products and technology.
However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain
a license, it could be or may become non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We
could be forced, including by court order, to cease commercializing the infringing technology or product. A finding of infringement could
prevent us from commercializing our drug candidates or force us to cease some of our business operations. In the event of a successful
claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful
infringement, pay royalties and other fees, redesign our infringing drug candidate or obtain one or more licenses from third parties,
which may be impossible or require substantial time and monetary expenditure. Claims that we have misappropriated the confidential information
or trade secrets of third parties could have a similar negative impact on our business. Any of the foregoing events would harm our business,
financial condition, results of operations and prospects.
We may be subject to claims by third parties
asserting that we or our employees have infringed, misappropriated or otherwise violated their intellectual property rights, or claiming
ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at
other biotechnology or pharmaceutical companies. Although we try to ensure that our employees, consultants and advisors do not use the
proprietary information or know-how of others in their work for us, we may be subject to claims that we or these individuals have used
or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s former employer.
We may also be subject to claims that patents and applications we have filed to protect inventions made on our behalf by our employees,
consultants and advisors, even those related to one or more of our drug candidates, are rightfully owned by their former or concurrent
employer. Litigation may be necessary to defend against these claims.
If we fail in prosecuting or defending any such claims,
in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting
or defending against such claims, litigation could result in substantial costs, delay development of our drug candidates and be a distraction
to management. Any of the foregoing events would harm our business, financial condition, results of operations and prospects.
We may be subject to claims challenging the
inventorship of our patents and other intellectual property.
We or our licensors may be subject to claims that
former employees, collaborators or other third parties have an interest (including co-ownership or ownership) in our owned or in-licensed
patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we or our licensors or collaborators
may have inventorship disputes arising from conflicting obligations of employees, consultants or others who are involved in developing
our drug candidates. While it is our policy to require our employees and contractors who may be involved in the development of intellectual
property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with
each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing
or may be breached, and litigation may be necessary to defend against these and other claims challenging inventorship or our or our licensors’
or collaborators’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors
or collaborators fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property
rights, such as exclusive ownership of, or right to use, intellectual property that is important to our drug candidates. Even if we are
successful in defending against such claims, these claims may create considerable distraction to management and other employees of the
company. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily
address all potential threats.
The degree of future protection, if any, afforded
by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect
our business or permit us to maintain our competitive advantage. For example:
| · | others may be able to make products that are similar to any drug candidates we may develop or utilize
similar technology but that are not covered by the claims of the patents that we license or may own in the future; |
| · | we, or our current or future licensors or collaborators, might not have been the first to make the inventions
covered by the issued patent or pending patent application that we license or may own in the future; |
| · | we, or our current or future licensors or collaborators might not have been the first to file patent applications
covering certain of our or their inventions; |
| · | others may independently develop similar or alternative technologies or duplicate any of our technologies
without infringing our owned or licensed intellectual property rights; |
| · | it is possible that our pending owned or licensed patent applications or those that we may own or license
in the future will not lead to issued patents; |
| · | issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal
challenges by our competitors; |
| · | our competitors might conduct research and development activities in countries where we do not have patent
rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| · | we may not develop additional proprietary technologies that are patentable; |
| · | the intellectual property rights of others may harm our business; and |
| · | we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third
party may subsequently file a patent directed to such intellectual property. |
Should any of these events occur, they could harm
our business, financial condition, results of operations and prospects.
Intellectual property
litigation may lead to unfavorable publicity that harms our reputation and causes the market price of shares of our Common Stock to decline.
During the course of any intellectual property litigation,
there could be public announcements of the initiation of the litigation as well as results of hearings, rulings on motions, and other
interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value
of our existing products, programs or intellectual property could be diminished. Accordingly, the market price of shares of our Common
Stock may decline. Such announcements could also harm our reputation or the market for our future products, which could have a material
adverse effect on our business.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement and any accompanying base
prospectus, including the documents incorporated by reference herein, contain “forward-looking statements” within the meaning
of Section 27A of the Securities Act, and Section 21E of the Exchange Act, that relate to future events or our future financial performance
and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance
or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by
these forward-looking statements. Such forward-looking statements concern our anticipated results and progress of our operations in future
periods, planned exploration and, if warranted, development of our properties, plans related to our business and other matters that may
occur in the future. These statements relate to analyses and other information that are based on forecasts of future results, estimates
of amounts not yet determinable and assumptions of management. All statements contained herein that are not clearly historical in nature
are forward-looking, and the words “anticipate,” “believe,” “expect,” “estimate,” “may,”
“will,” “could,” “leading,” “intend,” “contemplate,” “shall” and
similar expressions are generally intended to identify forward-looking statements. Forward-looking statements are subject to a variety
of known and unknown risks, uncertainties and other factors which could cause actual events or results to differ from those expressed
or implied by the forward-looking statements. The section in this prospectus supplement and accompanying prospectus entitled “Risk
Factors” and the sections in our periodic reports, including our Annual Report on Form 10-K for the fiscal year ended June 30, 2024
(the “2024 Form 10-K”) entitled “Business,” and in the 2024 Form 10-K and any future Quarterly Report on Form
10-Qs, entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as
other sections in this prospectus supplement, accompanying prospectus and the documents or reports incorporated by reference into this
prospectus supplement, discuss some of the factors that could contribute to these differences. Forward-looking statements in this prospectus
supplement, the accompanying prospectus and the documents incorporated by reference herein include, but are not limited to, statements
with respect to:
| · | our limited operating history and experience in developing and manufacturing drugs; |
| · | none of our products are approved for commercial sale; |
| · | our substantial capital needs; |
| · | product development risks; |
| · | our lack of sales and marketing personnel; |
| · | our reliance on third parties to conduct our clinical trials; |
| · | regulatory, competitive and contractual risks; |
| · | no assurance that our product candidates will obtain regulatory approval or that the results of clinical
studies will be favorable; |
| · | risks related to our intellectual property rights; |
| · | the volatility of the market price and trading volume in our Common Stock; |
| · | the absence of liquidity in our Common Stock; |
| · | the risk of substantial dilution from future issuances of our equity securities; and |
| · | the other risks set forth herein and in the documents incorporated by reference herein under the caption
“Risk Factors.” |
The foregoing does not represent an exhaustive list
of matters that may be covered by the forward-looking statements contained herein or risk factors that we are faced with. The factors
set forth above under “Risk Factors” and other cautionary statements made in this prospectus supplement and the accompanying
prospectus should be read and understood as being applicable to all related forward-looking statements wherever they appear in this prospectus
supplement and the accompanying prospectus. The forward-looking statements contained in this prospectus supplement and accompanying prospectus
represent our judgment as of the date of this prospectus supplement and the accompanying prospectus, as applicable. We caution readers
not to place undue reliance on such statements. You should read this prospectus supplement, the accompanying prospectus, and the documents
incorporated by reference herein and therein completely and with the understanding that our actual future results may be materially different
from the plans, intentions and expectations disclosed in the forward-looking statements we make. Except as required by law, we undertake
no obligation to update publicly any forward-looking statements for any reason, even if new information becomes available or other events
occur in the future. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are
expressly qualified in their entirety by the cautionary statements contained above and throughout this prospectus supplement and the accompanying
prospectus.
USE OF PROCEEDS
We estimate that the net proceeds from the sale
of our Common Stock in this offering will be approximately $2.7 million, after deducting placement agent fees and estimated offering
expenses payable by us. We intend to use the net proceeds from this offering for working capital and general corporate purposes.
This expected use of the net proceeds from this offering
and our existing cash represents our intentions based upon our current plans, financial condition and business conditions. The amount,
timing and nature of specific expenditures of net proceeds from this offering will depend on a number of factors, including the timing,
scope, progress and results of our development efforts and the timing and progress of any collaboration efforts. As of the date of this
prospectus supplement, we cannot specify with certainty all of the particular uses of the proceeds from this offering. Accordingly, we
will retain broad discretion over the use of such proceeds.
Pending our use of the net proceeds from this offering,
we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing
instruments, and government securities.
DIVIDEND POLICY
We have never declared or paid dividends on our Common
Stock and we do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. Payment of cash dividends, if
any, in the future will be at the discretion of our board of directors and will depend on applicable law and then-existing conditions,
including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors
our board of directors may deem relevant. We currently intend to retain all available funds and any future earnings to fund the development
and growth of our business.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
The following
description is a summary of some of the terms of our securities, our organizational documents and Nevada law. The descriptions in this
prospectus supplement and the accompanying prospectus of our securities and our organizational documents do not purport to be complete
and are subject to, and qualified in their entirety by reference to, our organizational documents, copies of which have been or will be
filed or incorporated by reference as exhibits to the registration statement of which this prospectus supplement and the accompanying
prospectus form a part. This summary supplements the description of our capital stock in the accompanying prospectus and, to the extent
it is inconsistent, replaces the description in the accompanying prospectus.
We are offering 1,146,000 shares of our Common Stock.
Common Stock
A description of our Common Stock that we are offering pursuant to this prospectus supplement
is set forth hereunder and under the heading “Description of Capital Stock” starting on page 8 of the accompanying prospectus.
On October 28, 2024, we had 16,612,374 shares of our Common Stock issued and
outstanding.
Anti-Takeover Effects of Nevada Law
Business Combinations
The “business combination” provisions
of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes (“NRS”) generally prohibit a Nevada corporation with
at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder for a period
of two years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved
by the board of directors prior to the date the interested stockholder obtained such status or the combination is approved by the board
of directors and thereafter is approved at a meeting of the stockholders by the affirmative vote of stockholders representing at least
60% of the outstanding voting power held by disinterested stockholders, such prohibition extends beyond the expiration of the two-year
period, unless:
| ● | the combination was approved by the board of directors prior to the person becoming an interested stockholder
or the transaction by which the person first became an interested stockholder was approved by the board of directors before the person
became an interested stockholder or the combination is later approved by a majority of the voting power held by disinterested stockholders;
or |
| ● | the combination meets specified statutory requirements. |
A “combination” is generally defined to
include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer, or other disposition, in one transaction or
a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the
aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value
of all outstanding shares of the corporation, (c) 10% or more of the earning power or net income of the corporation, and (d) certain other
transactions with an interested stockholder or an affiliate or associate of an interested stockholder.
In general, an “interested stockholder”
is a person who, together with affiliates and associates, owns (or within two years, did own) 10% or more of a corporation’s voting
stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts
to acquire our Company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above
the prevailing market price.
Control Share Acquisitions
The “control share” provisions of Sections
78.378 to 78.3793, inclusive, of the NRS apply to “issuing corporations” that are Nevada corporations with at least 200 stockholders,
including at least 100 stockholders of record who are Nevada residents, and that conduct business directly or indirectly in Nevada. The
control share statute prohibits an acquirer, under certain circumstances, from voting its shares of a target corporation’s stock
after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested
stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and
a majority or more, of the outstanding voting power. Generally, once an acquirer crosses one of the above thresholds, those shares in
an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of
the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded
full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote
in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance
with statutory procedures established for dissenters’ rights.
A corporation may elect to not be governed by, or
“opt out” of, the control share provisions by making an election in its articles of incorporation or bylaws, provided that
the opt-out election must be in place on the 10th day following the date an acquiring
person has acquired a controlling interest, that is, crossing any of the three thresholds described above. We have not opted out of the
control share statutes, and will be subject to these statutes if we are an “issuing corporation” as defined in such statutes.
The effect of the Nevada control share statutes is
that the acquiring person, and those acting in association with the acquiring person, will obtain only such voting rights in the control
shares as are conferred by a resolution of the stockholders at an annual or special meeting. The Nevada control share law, if applicable,
could have the effect of discouraging takeovers of our Company.
Anti-Takeover Effects of Our Articles of Incorporation
and Bylaws
Our Articles of Incorporation and Bylaws contain certain
provisions that may have anti-takeover effects, making it more difficult for or preventing a third party from acquiring control of us
or changing our board of directors and management. According to our Articles of Incorporation and Bylaws, neither the holders of our Common
Stock nor the holders of any preferred stock we may issue in the future have cumulative voting rights in the election of our directors.
The combination of the present ownership by a few stockholders of a significant portion of our issued and outstanding Common Stock and
lack of cumulative voting makes it more difficult for other stockholders to replace our board of directors or for a third party to obtain
control of us by replacing our board of directors.
DILUTION
If you invest in this offering, your ownership interest will be diluted to the extent of the difference
between the public offering price per share and the pro forma as adjusted net tangible book value per Common Stock immediately after this
offering.
Our net tangible book value as of June 30, 2024 was
approximately $14.3 million, or $2.32 per share of Common Stock. Net tangible book value per share is determined by dividing our total
tangible assets, less total liabilities, by the number of shares of our Common Stock outstanding as of June 30, 2024.
After giving effect to (i) the issuance of
1,360,800 shares of our Common Stock, pre-funded warrants to purchase 600,000
shares of our Common Stock, and warrants to purchase up to 1,960,800 shares of our Common Stock at a combined offering price of $1.53
per share, or pre-funded warrant, and the associated common warrant, pursuant to the Placement Agent Agreement, dated as of September
23, 2024, by and between the Company and ThinkEquity LLC (the “September 2024 Offering”), (ii) the issuance of 4,443,000 shares
of our Common Stock at an offering price of $1.50 per share, warrants to purchase up to 4,443,000 shares of our Common Stock in a
concurrent private placement, and placement agent warrants to purchase 222,150 shares of our Common Stock, pursuant to the Placement Agent Agreement, dated as of October 21, 2024,
by and between the Company and ThinkEquity LLC (the “First October 2024 Offering”), and (iii) the issuance of 2,667,000 shares
of our Common Stock at an offering price of $2.25 per share, warrants to purchase up to 2,667,000 shares of our Common Stock in a
concurrent private placement, and placement agent warrants to purchase 133,350 shares of Common Stock, pursuant to the Placement Agent Agreement, dated as of October 23, 2024,
by and between the Company and ThinkEquity LLC (the “Second October 2024 Offering”), our pro forma net tangible book value
as of June 30, 2024 would have been $27.7 million or $2.20 per share of Common Stock.
After giving effect to the sale of the Shares in this
offering at the public offering price of $2.83 per Share, and after deducting placement agent fees and estimated offering expenses payable
by us, our pro forma as adjusted net tangible book value as of June 30, 2024 would have been approximately $17.0 million, or approximately
$2.32 per share of our Common Stock. This represents an immediate decrease in pro forma as adjusted net tangible book value of approximately
$0.00 per share to our existing stockholders and an immediate dilution in pro forma as adjusted net tangible book value of approximately
$0.51 per share to new investors in this offering.
The following table illustrates this per share dilution.
| Public offering price per Share |
|
|
|
|
|
|
|
$ |
2.83 |
| Historical net tangible book value per share as of June 30, 2024 |
|
|
|
|
$ |
2.32 |
|
|
|
| Decrease in historical net tangible book value per share attributable to the September 2024 Offering, First
October 2024 Offering and Second October 2024 Offering |
|
$ |
(0.12 |
) |
|
|
|
|
|
| Pro forma net tangible book value per share as of June 30, 2024 |
|
$ |
2.20 |
|
|
|
|
|
|
| Decrease in net tangible book value per share attributable to this offering |
|
|
|
|
$ |
0.00 |
|
|
|
| Pro forma as adjusted net tangible book value per share as of June 30, 2024, after giving effect to this offering |
|
|
|
|
|
|
|
$ |
2.32 |
| Dilution per share to new investors in this offering |
|
|
|
|
|
|
|
$ |
0.51 |
The above table is based on 6,190,072 shares of our Common Stock outstanding
as of June 30, 2024, and excludes:
| · | 518,076 shares of our Common Stock issuable upon the exercise of outstanding
stock options at a weighted average exercise price of $54.11 per share; |
| · | 1,932,029 shares of our Common Stock issuable upon the exercise of outstanding
and exercisable warrants at a weighted average exercise price of $14.03 per share. (See “Risk
Factors – You may experience future dilution as a result of future equity offerings or if we issue shares subject to options, warrants,
stock awards or other arrangements.”); |
| · | 40,290 shares of our Common Stock issuable upon vesting of restricted stock
units issued under our equity incentive plan; |
| · | 2,143 shares of our Common Stock issued pursuant to the ATM Agreement
between the Company and Cantor since June 30, 2024; and |
| · | 57,300 shares of our Common Stock issuable upon exercise of the Placement
Agent’s Warrants. |
Except as otherwise indicated, all information in
this prospectus supplement assumes no exercise of the outstanding options or warrants described above. To the extent that any of these
outstanding warrants or options are exercised at prices per share below the public offering price per share in this offering or we issue
additional shares under our equity incentive plans at prices below the public offering price per share in this offering, you may experience
further dilution. In addition, to the extent that we raise additional capital by issuing equity or convertible debt securities, your ownership
will be further diluted.
PLAN OF DISTRIBUTION
We have engaged ThinkEquity LLC, or the placement
agent, to act as our exclusive placement agent to solicit offers to purchase the Shares offered by this prospectus supplement. The placement
agent is not purchasing or selling any such securities, nor is it required to arrange for the purchase and sale of any specific number
or dollar amount of such securities, other than to use its “reasonable best efforts” to arrange for the sale of such securities
by us. Therefore, we may not sell all of the Shares being offered. The terms of this offering are subject to market conditions and negotiations
between us, the placement agent and prospective investors. The placement agent will have no authority to bind us by virtue of their placement
agency agreement. This is a best efforts offering and there is no minimum offering amount required as a condition to the closing of this
offering. The placement agent may retain sub-agents and selected dealers in connection with this offering.
Investors purchasing securities offered hereby will
have the option to execute a securities purchase agreement with us. Investors who do not enter into a securities purchase agreement shall
rely solely on this prospectus supplement in connection with the purchase of our securities in this offering. In addition to rights and
remedies available to all purchasers in this offering under federal securities and state law, the purchasers which enter into a securities
purchase agreement will also be able to bring claims of breach of contract against us.
Delivery of the shares of our Common Stock offered
hereby is expected to occur on or about October 29, 2024, subject to satisfaction of certain customary closing conditions.
Fees and Expenses
The following table shows the per share price and
total cash fees we will pay to the placement agent in connection with the sale of the securities pursuant to this prospectus supplement.
| |
|
Per Share of Common Stock |
|
Total |
| Offering price |
|
$ |
2.83 |
|
|
$ |
3,243,180 |
|
| Placement agent commissions (7.0%) |
|
$ |
0.1981 |
|
|
$ |
227,023 |
|
| Proceeds, before expense, to us |
|
$ |
2.6319 |
|
|
$ |
3,016,157 |
|
We have agreed to pay a non-accountable expense allowance
to the placement agent equal to 1% of the gross proceeds received in this offering.
The out-of-pocket accountable expenses that will be
paid by us to the placement agent in connection with this offering, and will be reimbursed to us to the extent not actually incurred in
compliance with Rule 5110(g)(4)(A) of the Financial Industry Regulatory Authority (“FINRA”).
We have also agreed to pay certain of the placement
agent’s expenses relating to the offering, including: (a) all fees, expenses and disbursements relating to background checks of
our officers, directors and entities in an amount not to exceed $15,000 in the aggregate; (b) fees and expenses of the placement agent’s
legal counsel not to exceed $125,000; (c) a $29,500 cost associated with the placement agent use of Ipreo’s book-building, prospectus
tracking and compliance software for the offering; (d) $10,000 for data services and communications expenses; (e) up to $30,000 of market
making and trading, and clearing firm settlement expenses for the offering; (f) up to $10,000 of the placement agent’s actual accountable
“road show” expenses; and (g) the costs associated with bound volumes of the public offering materials as well as commemorative
mementos and lucite tombstones.
Our total estimated expenses of the offering, including
registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding placement agent fees and excluding
the non-accountable expense allowance, are approximately $450,000.
Placement Agent’s Warrants
Upon closing of this offering, we have agreed to issue the placement agent warrants (“Placement
Agent’s Warrants”) to purchase up to 57,300 shares of our Common Stock (5% of the aggregate number of shares of our Common
Stock sold in this offering). The Placement Agent’s Warrants will be exercisable at a per share exercise price equal $3.5375, which
is equal to 125% of the public offering price per share in this offering. The Placement Agent’s Warrants are exercisable at any
time and from time to time, in whole or in part, during the five-year period commencing 180 days from the commencement of sales of the
Shares in this offering.
The Placement Agent’s Warrants have been deemed
compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(e)(1)(A) of FINRA. The placement agent (or
permitted assignees under Rule 5110(e)(2)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying
these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective
economic disposition of the warrants or the underlying securities for a period of 180 days following the commencement of sales of the
securities issued in this offering. In addition, the Placement Agent’s Warrants provide for registration rights upon request, in
certain cases. The sole demand registration right provided will not be greater than five years from the commencement of sales of the securities
issued in this offering in compliance with FINRA Rule 5110(g)(8)(C). The piggyback registration rights provided will not be greater than
seven years from the commencement of sales of the securities issued in this offering in compliance with FINRA Rule 5110(g)(8)(D). We will
bear all fees and expenses attendant to registering the securities issuable on exercise of the warrants other than underwriting commissions
incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the Placement Agent’s Warrants
may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or
consolidation. However, the Placement Agent’s Warrant exercise price or underlying shares will not be adjusted for issuances of
shares of our Common Stock at a price below the warrant exercise price.
Right of First Refusal
We have granted the placement
agent an irrevocable right of first refusal (the “Right of First Refusal”), for a period of 12 months after the date the Offering
is completed for aggregate net proceeds of not less than $5 million, to act, except as set forth below, as sole investment banker, sole
book-runner and/or sole placement agent, at the placement agent’s sole discretion, for each and every future public and private
equity offering that is not an “at-the-market” offering (an “ATM”) (an ATM executed through a broker dealer as
sales agent), and sole investment banker, sole book-runner and/or sole placement agent, at the Placement Agent’s sole discretion,
for each and every future debt offering (such future public and private equity offering or debt offering, a “Future Offering”),
including all equity linked financings, during such twelve (12) month period for the Company, or any successor to or any subsidiary of
the Company, on reasonable and customary terms, provided a Future Offering shall not be deemed to include, and no Right of First Refusal
is granted to the placement agent in connection with, any of the following: (a) any equity securities directly issued by the Company pursuant
to acquisitions or strategic transactions, including as part of any grant funding from a third party, or (b) any offer or sale of equity
securities by the Company directly to non-U.S. persons domiciled in the following jurisdictions: China, Korea, Latin America (e.g. the
Caribbean and/or the Cayman Islands) and Middle East, in each case, in a private placement not otherwise involving a public offering.
Notwithstanding anything to the contrary set forth above, the placement agent acknowledges that the Company is subject to a pre-existing
agreement with a third party under which such third party has a right of first refusal to act as the co-lead bookrunning underwriter,
co-lead initial purchaser, co-lead placement agent or co-lead selling agent, as the case may be, on any financing involving equity securities
for the Company (the “Prior ROFR”). In case the placement agent wishes to exercise its Right of First Refusal hereunder, the
Company will use its commercially reasonable efforts to obtain a waiver of the Prior ROFR, subject to certain conditions.
Prior Relationships
The placement agent acted as the representative of
the underwriters for our public offering that closed in August 2021. The placement agent received a commission equal to 4% of the gross
proceeds of our initial public offering, and a 1% non-accountable expense allowance.
The placement agent acted as the placement agent for
our public offering that closed in March 2024 (the “March 2024 Offering”). The placement agent received a commission equal
to 7.5% of the gross proceeds of the March 2024 Offering, and a 1% non-accountable expense allowance.
The placement agent acted as the placement agent
for our public offering that closed in September 2024 (the “September 2024 Offering”). The placement agent received a commission
equal to 7.0% of the gross proceeds of the September 2024 Offering, and a 1% non-accountable expense allowance.
The placement agent acted as the placement agent for our public offering that closed on October 22, 2024
(the “First October 2024 Offering”). The placement agent received a commission equal to 7.0% of the gross proceeds of the
First October 2024 Offering, and a 1% non-accountable expense allowance.
The placement agent acted as the placement agent for
our public offering that closed on October 24, 2024 (the “Second October 2024 Offering”). The placement agent received a commission
equal to 7.0% of the gross proceeds of the Second October 2024 Offering, and a 1% non-accountable expense allowance.
Regulation M Compliance
The placement agent may be deemed to be an underwriter
within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the sale of
our securities offered hereby by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities
Act. The placement agent will be required to comply with the requirements of the Securities Act and the Exchange Act, including, without
limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales
of our securities by the placement agent. Under these rules and regulations, the placement agent may not (i) engage in any stabilization
activity in connection with our securities; and (ii) bid for or purchase any of our securities or attempt to induce any person to purchase
any of our securities, other than as permitted under the Exchange Act, until they have completed their participation in the distribution.
Indemnification
We have agreed to indemnify the placement agent against
certain liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties
contained in our placement agency agreement with the placement agent. We have also agreed to contribute to payments the placement agent
may be required to make in respect of such liabilities.
Nasdaq Capital Market Listing
Our Common Stock is listed on Nasdaq under the symbol
“BIVI.”
Other
From time to time, the placement agent and/or their
affiliates may in the future provide, various investment banking and other financial services for us for which they may receive customary
fees. In the course of their businesses, the placement agent and their affiliates may actively trade our securities or loans for their
own account or for the accounts of customers, and, accordingly, the placement agent and their affiliates may at any time hold long or
short positions in such securities or loans.
Except for services provided in connection with this offering, the March 2024 Offering, September
2024 Offering, Frist October 2024 Offering and Second October 2024 Offering in each of which the placement agent acted as the placement
agent, and our initial public offering in which the placement agent acted as sole underwriter, the placement agent has not provided any
investment banking or other financial services to us during the 180-day period preceding the date of this prospectus supplement.
LEGAL MATTERS
The validity of the shares Common Stock offered hereby
will be passed upon for us by Fennemore Craig, P.C. Certain legal matters related to the offering will be passed upon for the placement
agent by Sheppard, Mullin, Richter & Hampton LLP, New York, New York.
EXPERTS
The balance sheets of BioVie Inc. as of June 30, 2024
and 2023, and the related statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for each
of the years then ended, have been audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their report
which is incorporated by reference, which report includes an explanatory paragraph about the existence of substantial doubt concerning
the Company’s ability to continue as a going concern. Such financial statements have been incorporated by reference in reliance
on the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus supplement and accompanying base prospectus
is part of the registration statement on Form S-3 we filed with the SEC under the Securities Act and does not contain all the information
set forth in the registration statement. Whenever a reference is made in this prospectus supplement or the accompanying base prospectus
to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are
a part of the registration statement or the exhibits to the reports or other documents incorporated by reference into this prospectus
supplement for a copy of such contract, agreement or other document. Because we are subject to the information and reporting requirements
of the Exchange Act, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings
are available to the public over the internet at the SEC’s website at http://www.sec.gov.
You may also access our SEC filings at our website
https://bioviepharma.com/. Our website and the information contained on, or that can be accessed through, our website will not
be deemed to be incorporated by reference in, and are not considered part of, this prospectus supplement. You should not rely on our website
or any such information in making your decision whether to purchase our securities.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference into
this prospectus supplement the information contained in other documents we file with the SEC, which means that we can disclose important
information to you by referring you to those documents. Any statement contained in any document incorporated or deemed to be incorporated
by reference herein shall be deemed to be modified or superseded, for purposes of this prospectus supplement, to the extent that a statement
contained in or omitted from this prospectus supplement, or in any other subsequently filed document that also is or is deemed to be incorporated
by reference herein, modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as
so modified or superseded, to constitute a part of this prospectus supplement. We incorporate by reference the documents listed below
which have been filed by us:
| · | Our Current Reports on Form 8-K, filed with the SEC on October
25, 2023, November 13,
2023, November 29,
2023, January 19,
2024, January 25,
2024, March 1,
2024, March 4,
2024, March 6,
2024, March 11,
2024, April 19,
2024, July 30,
2024, August 1,
2024, August 6,
2024, August 21,
2024, September 24,
2024, September 25,
2024 (excluding Item 7.01 thereof), October
22, 2024 (with respect to Items 1.01 and 3.02 thereof only), October
22, 2024 (with respect to Item 8.01 thereof only), October 24, 2024 (with respect to Items 1.01 and 3.02 thereof only), and
October 24, 2024 (with respect to Item 8.01 thereof only); and |
All documents we file with the SEC pursuant to Sections
13(a), 13(c), 14 or 15(d) of the Exchange Act, except as to any portion of any report or documents that is not deemed filed under such
provisions, (1) on or after the date of filing of the registration statement containing this prospectus supplement and prior to the effectiveness
of the registration statement and (2) on or after the date of this prospectus supplement until the earlier of the date on which all of
the securities registered hereunder have been sold or the registration statement of which this prospectus supplement is a part has been
withdrawn, shall be deemed incorporated by reference in this prospectus supplement and to be a part of this prospectus supplement from
the date of filing of those documents and will be automatically updated and, to the extent described above, supersede information contained
or incorporated by reference in this prospectus supplement and previously filed documents that are incorporated by reference in this prospectus
supplement.
Nothing in this prospectus supplement shall be deemed
to incorporate information furnished but not filed with the SEC pursuant to Item 2.02, 7.01 or 9.01 of Form 8-K. Upon written or oral
request, we will provide without charge to each person, including any beneficial owner, to whom a copy of the prospectus is delivered
a copy of any or all of the reports or documents incorporated by reference herein (other than exhibits to such documents, unless such
exhibits are specifically incorporated by reference herein). You may request a copy of these filings, at no cost, by writing or telephoning
us at the following address: BioVie Inc., 680 W Nye Lane, Suite 204, Carson City, NV 89703.
PROSPECTUS

Primary Offering of
$300,000,000
Class A Common Stock
Preferred Stock
Warrants
Debt Securities
Rights
Units
and
Secondary Offering of
Up to 311,002 Shares of Class A Common Stock
Offered by the Selling Stockholders
This prospectus relates to the offer and sale,
from time to time, by BioVie Inc. (“we,” “us,” or the “Company”), in one or more offerings, any combination
of Class A common stock (as defined below), preferred stock, warrants, debt securities, rights to purchase Class A common stock or other
securities or units having a maximum aggregate offering price of $300,000,000. When we decide to sell a particular class or series of
securities, we will provide specific terms of the offered securities in a prospectus supplement.
This prospectus also relates to the offer and
resale, from time to time, by the selling stockholders named under the heading “Selling Stockholders” in this prospectus (the
“Selling Stockholders”), and their donees, pledgees, transferees or other successors-in-interest, of up to 311,002 shares
(the “Shares”) of common stock, par value $0.0001 per share (the “Class A common stock”), of the Company, issuable
upon the exercise of the warrants to purchase 311,002 shares of Class A common stock at an exercise price per share equal to $5.82 (the
“Lender Warrants”) held by the Selling Stockholders. We are registering the offer and sale of the Shares issuable upon exercise
of the Lender Warrants held by the Selling Stockholders to satisfy the registration rights they were granted by the Company pursuant to
the Loan and Security Agreement and the Supplement to the Loan and Security Agreement, each entered into on November 30, 2021 (together,
the “Loan Agreement”) with Avenue Venture Opportunities Fund II, L.P. (“AVOPII”) and Avenue Venture Opportunities
Fund, L.P. (“AVOPI” and, together with AVOPII, the “Lenders”).
Discounts, concessions, commissions and similar
selling expenses attributable to the sale of Shares covered by this prospectus will be borne by the Selling Stockholders. We will pay
all expenses (other than discounts, concessions, commissions and similar selling expenses) relating to the registration of the Shares
with the Securities and Exchange Commission (the “SEC”).
The prospectus supplements may also add, update
or change information contained in or incorporated by reference into this prospectus. However, no prospectus supplement shall offer a
security that is not registered and described in this prospectus at the time of its effectiveness. You should read this prospectus and
any prospectus supplement, as well as the documents incorporated by reference or deemed to be incorporated by reference into this prospectus,
carefully before you invest. This prospectus may not be used to offer or sell our securities unless accompanied by a prospectus supplement
relating to the offered securities.
Our Class A common stock is listed on the Nasdaq
Capital Market under the symbol “BIVI.” On August 25, 2023, the closing price for our Common Stock, as reported on The Nasdaq
Capital Market was $3.37 per share. Each prospectus supplement will contain information, where applicable, as to our listing on the Nasdaq
Capital Market or on any other securities exchange of the securities covered by the prospectus supplement.
These securities may be sold directly by us, through
dealers or agents designated from time to time, to or through underwriters or through a combination of these methods. Additionally, the
Selling Stockholders may sell or otherwise dispose of the Shares covered by this prospectus in a number of different ways and at varying
prices. See “Plan of Distribution” in this prospectus. We may also describe the plan of distribution for any particular
offering of our securities in a prospectus supplement. If any agents, underwriters or dealers are involved in the sale of any securities
in respect of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements with them in a
prospectus supplement. The net proceeds we expect to receive from any such sale will also be included in a prospectus supplement.
We will not receive any proceeds from the sales
of Shares by the Selling Stockholders. Upon any exercise of the Lender Warrants by payment of cash, we will receive the cash exercise
price paid by the holders of the Lender Warrants. We intend to use those proceeds, if any, for working capital and general corporate purposes.
An investment in our securities involves a
high degree of risk. Please carefully read the information under the headings “Risk Factors” beginning on page 5 of this prospectus,
the applicable prospectus supplement and “Item 1A - Risk Factors” of our most recent Annual Report on Form 10-K and in any
Quarterly Report on Form 10-Q that is incorporated by reference in this prospectus before you invest in our securities.
We may amend or supplement this prospectus
from time to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments or supplements
carefully before you make your investment decision.
Neither the SEC nor any state securities commission
has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary
is a criminal offense.
The date of this prospectus is August 28, 2023
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a Registration Statement
that we filed with the SEC using a “shelf” registration process. Under this shelf registration process, we may offer from
time to time securities having a maximum aggregate offering price of $300,000,000. In addition, the Selling Stockholders may from time
to time sell up to an aggregate of 311,002 shares of Class A common stock issuable upon exercise of the Lender Warrants. Each time we
or the Selling Stockholders offer any type or series of securities under this prospectus, we will prepare and file with the SEC a prospectus
supplement that contains more specific information about the terms of that offering. We may also authorize one or more free writing prospectuses
to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any related free
writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus
or the documents incorporated herein by reference. You should read carefully both this prospectus, any prospectus supplement and any related
free writing prospectuses we have authorized for use in connection with a specific offering, together with additional information described
below under the caption “Where You Can Find More Information,” before buying any of the securities being offered.
This prospectus does not contain all the information
provided in the Registration Statement we filed with the SEC. For further information about us or our securities offered hereby, you should
refer to that Registration Statement, which you can obtain from the SEC as described below under “Where You Can Find More Information.”
Neither we nor the Selling Stockholders have authorized
anyone to provide any information other than that contained or incorporated by reference in this prospectus or in any applicable prospectus
supplement or any applicable free writing prospectus that we have authorized. We take no responsibility for and can provide no assurance
as to the reliability of, any other information that others may give you. The securities offered hereby are not being offered in any jurisdiction
where the offer is not permitted. You should not assume that the information contained in or incorporated by reference in this prospectus
is accurate as of any date other than the respective dates of such document. Our business, financial condition, results of operations
and prospects may have changed since those dates.
We and the Selling Stockholders may sell securities
through underwriters or dealers, through agents, directly to purchasers or through any combination of these methods. We and our agents
reserve the sole right to accept or reject in whole or in part any proposed purchase of securities. The prospectus supplement, which we
will prepare and file with the SEC each time we offer securities, will set forth the names of any underwriters, agents or others involved
in the sale of securities, and any applicable fee, commission or discount arrangements with them. See “Plan of Distribution.”
Unless the context otherwise indicates, references
in this prospectus to, “BioVie,” “the Company,” “we,” “our,” or “us” mean
BioVie, Inc., a Nevada corporation. The term “Selling Stockholders” refers, collectively, to the selling stockholders named
under the heading “Selling Stockholders” in this prospectus and their donees, pledgees, transferees or other successors-in-interest.
PROSPECTUS SUMMARY
This prospectus summary highlights certain
information about our company and other information contained elsewhere in this prospectus or in documents incorporated by reference.
This summary does not contain all of the information that you should consider before making an investment decision. You should carefully
read the entire prospectus, any prospectus supplement, including the matters set forth under the section of this prospectus entitled “Risk
Factors” and the financial statements and related notes and other information that we incorporate by reference herein, including
our Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q, before making an investment decision.
Our Company
We are a clinical-stage company developing innovative
drug therapies for the treatment of neurological and neurodegenerative disorders and advanced liver disease.
Neurodegenerative Disease Program
In neurodegenerative disease, the Company’s
drug candidate NE3107 inhibits inflammatory activation of extracellular single-regulated kinase (“ERK”) and Nuclear factor
kappa-light-chain-enhancer of activated B cells (“NFkB”) (e.g., tumor necrosis factor (“TNF”) signaling) that
leads to neuroinflammation and insulin resistance, but not their homeostatic functions (e.g., insulin signaling and neuron growth and
survival). Both inflammation and insulin resistance are drivers of Alzheimer’s disease (“AD”) and Parkinson’s
disease (“PD”).
The Company is conducting a potentially pivotal
Phase 3 randomized, double blind, placebo controlled, parallel group, multicenter study to evaluate NE3107 in patients who have mild to
moderate AD (NCT04669028). The study has co-primary endpoints looking at cognition using the Alzheimer’s Disease Assessment Scale-Cognitive
Scale (ADAS-Cog 12) and function using the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGIC).
The program is fully enrolled and is targeting primary completion in the fourth quarter of the calendar 2023 year.
In December 2022, topline results were released
from the Company’s Phase 2 study assessing NE3107’s safety and tolerability and potential pro-motoric impact in PD patients.
The NM201 study (NCT05083260) was a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in PD participants
treated with carbidopa/levodopa and NE3107. Forty-five patients with a defined L-dopa “off state” were randomized 1:1 to placebo:NE3107
20 mg twice daily for 28 days. The trial was launched with two design objectives: 1) the primary objective was safety and a drug-drug
interaction study (as requested by the U.S. Food and Drug Administration (“FDA”)) to demonstrate the absence of adverse interactions
of NE3107 with levodopa; and 2) the secondary objective was to determine if preclinical indications of promotoric activity and apparent
enhancement of levodopa activity observed in a Parkinson’s disease model in monkeys can be seen in humans. Both objectives of the
study were met. Patients treated with NE3107 experienced greater motor control.
The Company provided the financial support and
the use of our NE3107 formulated drug product for an open-label phase 2, Investigator-Initiated Trial in mild cognitive impairment (“MCI”)
and Mild AD, NCT05227820, conducted by (“The Regenesis Project”) of Sheldon Jordan. The study received FDA authorization on
December 12, 2021, and was designed to measure NE3107’s effect on cognition, cerebral spinal fluid (“CSF”) and blood
biomarkers, and neuro-imagining endpoints. Topline results were released September 7, 2022, and additional data was presented at the Clinical
Trial in Alzheimer’s Disease (“CTAD”) annual conference in December 2022. The data showed that three months of treatment
with NE3107 in patients with MCI and mild AD enhanced cognition compared to baseline, as measured using multiple rating scales, had improvement
in daily function and improvements in inflammation correlated with improved cognition. No drug-related adverse events were observed.
The Company acquired the biopharmaceutical assets
of NeurMedix, Inc. (“NeurMedix”), from a related party privately held clinical-stage pharmaceutical company, in June 2021.
The acquired assets included NE3107, a potentially selective inhibitor of inflammatory ERK signaling that, based on animal studies and
Dr. Jordan’s study, is believed to reduce neuroinflammation. NE3107 is a novel orally administered small molecule that is thought
to inhibit inflammation-driven insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There
is emerging scientific consensus that both inflammation and insulin resistance may play fundamental roles in the development of AD and
PD, and NE3107 could, if approved by the FDA represent a new medical approach to treating these devastating conditions affecting an estimated
6 million Americans suffering from AD and 1 million Americans suffering from PD.
Inflammation-driven insulin resistance is believed
to be implicated in a broad range of serious diseases, and we plan to begin exploring these opportunities in the coming months using NE3107
or related compounds acquired in the NeurMedix asset purchase. NE3107 is patented in the United States (“U.S.”), Australia,
Canada, Europe and South Korea.
Liver Disease Program
In liver disease, our Orphan Drug candidate BIV201
(continuous infusion terlipressin), with FDA Fast Track status, has been evaluated in a U.S. Phase 2b study (NCT04112199) for the treatment
of refractory ascites due to liver cirrhosis. BIV201 is administered as a patent-pending liquid formulation. The study was closed before
full enrollment, without clinically meaningful adverse effects associated with BIV201 treatment and data that appeared to show that treatment
with BIV201 plus standard-of-care (“SOC”) resulted in a reduction in ascites fluid accumulation during treatment versus pre-treatment.
In June 2023, we requested guidance from the FDA regarding the design and endpoints for definitive clinical testing of BIV201 for the
treatment of ascites due to chronic liver cirrhosis.
While the active agent, terlipressin, is approved
in the U.S. and in about 40 countries for related complications of advanced liver cirrhosis, treatment of ascites is not included in these
authorizations. Patients with refractory ascites suffer from frequent life-threatening complications, generate more than $5 billion in
annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The U.S. FDA has not approved any drug to treat
refractory ascites.
The BIV201 development program was initiated by
LAT Pharma LLC. On April 11, 2016, the Company acquired LAT Pharma LLC and the rights to its BIV201 development program. The Company currently
owns all development and marketing rights to this drug candidate. Pursuant to the Agreement and Plan of Merger entered into on April 11,
2016, between our predecessor entities, LAT Pharma LLC and NanoAntibiotics, Inc., BioVie is obligated to pay a low single digit royalty
on net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT Pharma Members, PharmaIn Corporation, and The Barrett
Edge, Inc.
The Securities We May Offer
This prospectus is part of a Registration Statement
that we filed with the SEC utilizing a shelf registration process. Under this shelf registration process, we may sell any combination
of:
| ● | debt securities, in one or more series; |
| ● | right to purchase common stock or other securities; and/or |
in one or more offerings up to a total dollar
amount of $300,000,000. This prospectus provides you with a general description of the securities we may offer. Each time we sell securities,
we will provide a prospectus supplement that will contain specific information about the terms of that specific offering and include a
discussion of any risk factors or other special considerations that apply to those securities. The prospectus supplement may also add,
update or change information contained in this prospectus. You should read both this prospectus and any prospectus supplement together
with the additional information described under the heading “Where You Can Find More Information.”
Securities Offered by the Selling Securityholders
This prospectus also relates to the resale from
time to time by the Selling Stockholders identified in this prospectus of up to 311,002 shares of Class A common stock issuable upon the
exercise of the Lender Warrants held by the Selling Stockholders. We are registering the offer and sale of the Shares to satisfy the registration
rights they were granted by the Company pursuant to the Loan Agreement.
On November 30, 2021 (the “Loan Closing
Date”), the Company entered into the Loan Agreement with the Lenders for growth capital loans in an aggregate principal amount of
up to $20,000,000 (the “Loan”), with (i) $15,000,000 funded on the Loan Closing Date (“Tranche 1”) and (ii) up
to $5,000,000 to be made available to the Company on or prior to September 15, 2022, subject to the Company’s achievement of certain
milestones with respect to certain of its ongoing clinical trials. The Loan bears interest at an annual rate equal to the greater of (a)
the sum of 7.00% plus the prime rate as reported in The Wall Street Journal and (b) 10.75%. The Loan is secured by a lien upon and security
interest in all of the Company’s assets, including intellectual property, subject to agreed exceptions. The maturity date of the
Loan is December 1, 2024. Up to $5,000,000 of the principal amount of the Loan outstanding may be converted, at the option of the Lenders,
into shares of the Company’s Class A common stock at a conversion price of $6.98 per share.
In connection with the Loan, pursuant to the funding
of Tranche 1 on the Loan Closing Date, the Company issued 361,002 Lender Warrants. The Lender Warrants, which are exercisable until November
30, 2026, were offered and sold by the Company in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities
Act.
On March 31, 2023, the Company filed a Registration Statement on Form S-3 (File No. 333-271054), that was declared effective by the SEC
on April 10, 2023, which related in part to the offer and resale, from time to time, by the Selling Stockholders of up to 50,000 shares
of Class A common stock issuable upon exercise of the Lender Warrants.
The Lenders may exercise the Lender Warrants at
any time, or from time to time up to and including the Expiration Date, by making a cash payment equal to the exercise price multiplied
by the quantity of shares. The Lenders may also exercise the Lender Warrants on a cashless or “net issuance” basis by receiving
a net number of shares calculated pursuant to the formula set forth in the Lender Warrants. The Lender Warrants are subject to anti-dilution
adjustments for stock dividends, stock splits, and reverse stock splits. Pursuant to the terms of the Lender Warrants, the holders of
the Lender Warrants are entitled to piggyback registration rights if the Company proposes to file a new registration statement under the
Securities Act for purposes of effecting an underwritten offering of its equity securities, subject to certain limitations.
Use of Proceeds
Except as described in any applicable prospectus
supplement or in any free writing prospectuses we have authorized for use in connection with a specific offering, we currently intend
to use the net proceeds from the sale of the securities offered by us hereunder, if any, for working capital and general corporate purposes.
We will set forth in the applicable prospectus supplement or free writing prospectus our intended use for the net proceeds received from
the sale of any securities sold pursuant to the prospectus supplement or free writing prospectus. All of the securities offered by the
Selling Securityholders pursuant to this prospectus will be sold by the Selling Securityholders for their respective accounts. We will
not receive any of the proceeds from these sales.
Nasdaq Listing
Our Class A common stock is listed on the Nasdaq Capital Market under
the symbol “BIVI.”
Corporate Information
Our principal executive office is located at 680
W. Nye Lane, Suite 201, Carson City, Nevada 89703, and our phone number is (775) 888-3162. Our website address is http://www.bioviepharma.com/.
The inclusion of our website address does not include or incorporate by reference into this prospectus supplement or the accompanying
prospectus any information on, or accessible through, our website. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and
Current Reports on Form 8-K, together with amendments to these reports, are available on the “Investor Relations” section
of our website, free of charge, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the
SEC.
RISK FACTORS
Investing in our securities involves risk. The
prospectus supplement applicable to a particular offering of securities will contain a discussion of the risks applicable to an investment
in BioVie and to the particular types of securities that we are offering under that prospectus supplement. Before making an investment
decision, you should carefully consider the risks described under “Risk Factors” in the applicable prospectus supplement together
with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by
reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under “Part I-Item 1A-Risk
Factors” of our most recent Annual Report on Form 10-K and in “Part II-Item 1A-Risk Factors” in our
most recent Quarterly Report on Form 10-Q filed subsequent to such Form 10-K that are incorporated herein by reference, as
may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. Our business, financial
condition or results of operations could be materially adversely affected by any of these risks. The trading price of our securities could
decline due to any of these risks, and you may lose all or part of your investment.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, each prospectus supplement and
the documents incorporated by reference into this prospectus and each prospectus supplement contain “forward-looking statements”
within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as
amended, that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other
factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results,
levels of activity, performance or achievements expressed or implied by these forward-looking statements. Such forward-looking statements
concern our anticipated results and progress of our operations in future periods, planned exploration and, if warranted, development of
our properties, plans related to our business and other matters that may occur in the future. These statements relate to analyses and
other information that are based on forecasts of future results, estimates of amounts not yet determinable and assumptions of management.
All statements contained herein that are not clearly historical in nature are forward-looking, and the words “anticipate,”
“believe,” “expect,” “estimate,” “may,” “will,” “could,” “leading,”
“intend,” “contemplate,” “shall” and similar expressions are generally intended to identify forward-looking
statements. Forward-looking statements are subject to a variety of known and unknown risks, uncertainties and other factors which could
cause actual events or results to differ from those expressed or implied by the forward-looking statements. The section in this prospectus
entitled “Risk Factors” and the sections in our periodic reports, including the section in the 2023 Form 10-K entitled
“Business,” and the section in the 2023 Form 10-K and any future Quarterly Report on Form 10-Qs incorporated herein
by reference entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,”
as well as other sections in this prospectus and the documents or reports incorporated by reference into this prospectus, discuss some
of the factors that could contribute to these differences. Forward-looking statements in this prospectus, each prospectus supplement,
and the documents incorporated by reference herein and therein include, but are not limited to, statements with respect to:
| ● | our limited operating history and experience in developing and
manufacturing drugs; |
| ● | none of our products are approved for commercial sale; |
| ● | our substantial capital needs; |
| ● | product development risks; |
| ● | our lack of sales and marketing personnel; |
| ● | regulatory, competitive and contractual risks; |
| ● | no assurance that our product candidates will obtain regulatory
approval or that the results of clinical studies will be favorable; |
| ● | risks related to our intellectual property rights; |
| ● | the volatility of the market price and trading volume in our common
stock; |
| ● | the absence of liquidity in our common stock; |
| ● | the risk of substantial dilution from future issuances of our
equity securities; and |
| ● | the other risks set forth herein and in the documents incorporated
by reference herein under the caption “Risk Factors.” |
The foregoing does not represent an exhaustive
list of matters that may be covered by the forward-looking statements contained herein or risk factors that we are faced with. The factors
set forth above under “Risk Factors” and other cautionary statements made in this prospectus should be read and understood
as being applicable to all related forward-looking statements wherever they appear in this prospectus. The forward-looking statements
contained in this prospectus represent our judgment as of the date of this prospectus. We caution readers not to place undue reliance
on such statements. You should read this prospectus and the documents that we have filed as exhibits to this prospectus and incorporated
by reference herein completely and with the understanding that our actual future results may be materially different from the plans, intentions
and expectations disclosed in the forward-looking statements we make. Except as required by law, we undertake no obligation to update
publicly any forward-looking statements for any reason, even if new information becomes available or other events occur in the future.
All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified
in their entirety by the cautionary statements contained above and throughout this prospectus.
This prospectus and the documents incorporated
by reference in this prospectus may contain market data that we obtain from industry sources. These sources do not guarantee the accuracy
or completeness of the information. Although we believe that our industry sources are reliable, we do not independently verify the information.
The market data may include projections that are based on a number of other projections. While we believe these assumptions to be reasonable
and sound as of the date of this prospectus, actual results may differ from the projections.
DIVIDEND POLICY
We have never declared or paid dividends on our
common stock and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. Payment of cash dividends,
if any, in the future will be at the discretion of our board of directors and will depend on applicable law and then-existing conditions,
including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors
our board of directors may deem relevant. We currently intend to retain all available funds and any future earnings to fund the development
and growth of our business.
USE OF PROCEEDS
Except as otherwise provided in the applicable
prospectus supplement, we intend to use the net proceeds from the sale of the securities covered by this prospectus for general corporate
purposes, which may include, but is not limited to, working capital, capital expenditures, research and development expenditures and acquisitions
of new technologies or businesses. The precise amount, use and timing of the application of such proceeds will depend upon our funding
requirements and the availability and cost of other capital. Additional information on the use of net proceeds from an offering of securities
covered by this prospectus may be set forth in the prospectus supplement relating to the specific offering.
We will not receive any proceeds from the sales
of Shares by the Selling Stockholders.
Upon any exercise of the Lender Warrants by payment
of cash, we will receive the cash exercise price paid by the holders of the Lender Warrants. We cannot assure you that any of the Lender
Warrants will be exercised, or if exercised, of the quantity that will be exercised or the period in which such Lender Warrants will be
exercised.
DESCRIPTION OF CAPITAL STOCK
The following sections constitute a summary
as of the date of this prospectus and do not purport to be a complete description of our capital stock. We will describe in the applicable
prospectus supplement relating to a particular offering the specific terms of the securities offered by that prospectus supplement. We
will indicate in the applicable prospectus supplement if the terms of the securities differ from the terms we have summarized below. We
will also include in the prospectus supplement information, where applicable, material United States federal income tax considerations
relating to the securities.
General
The following description of common stock of the
Company (the “common stock”) and preferred stock of the Company (the “preferred stock”), together with the additional
information we include in any applicable prospectus supplement, summarizes the material terms and provisions of the common stock and preferred
stock that we may offer under this prospectus but is not complete. For the complete terms of our common stock and preferred stock, please
refer to our articles of incorporation, as may be amended from time to time (the “Articles of Incorporation”), any certificates
of designation for our preferred stock, that may be authorized from time to time, and our amended and restated bylaws, as amended from
time to time (the “Bylaws”). The Nevada General Corporation Law may also affect the terms of these securities. While the terms
we have summarized below will apply generally to any future common stock or preferred stock that we may offer, we will describe the specific
terms of any series of these securities in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement,
the terms of any common stock or preferred stock we offer under that prospectus supplement may differ from the terms we describe below.
As of August 17, 2023, our authorized capital
stock consists of 800,000,000 shares of Class A common stock, par value $0.0001 per share (the “Class A common stock”), of
which 36,826,648 shares of Common Stock were issued, and 36,803,768 shares were issued and outstanding; and 10,000,000 shares of preferred
stock, par value $0.001 per share, none of which were issued and outstanding. The authorized and unissued shares of Class A common stock
and preferred stock are available for issuance without further action by our stockholders, unless such action is required by applicable
law or the rules of any stock exchange on which our securities may be listed. Unless approval of our stockholders is so required, our
board of directors will not seek stockholder approval for the issuance and sale of our common stock.
Class A Common Stock
Each holder of Class A common stock is entitled
to one vote for each share of Class A common stock held on all matters submitted to a vote of the stockholders, including the election
of directors. Our Articles of Incorporation and Bylaws do not provide for cumulative voting rights. Subject to preferences that may be
applicable to any then outstanding preferred stock, the holders of our outstanding shares of Class A common stock are entitled to receive
dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. In the event of our
liquidation, dissolution or winding up, holders of Class A common stock will be entitled to share ratably in the net assets legally available
for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation
preference granted to the holders of any outstanding shares of preferred stock. Holders of our Class A common stock have no preemptive,
conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to the Class A common stock. The
rights, preferences and privileges of the holders of Class A common stock are subject to, and may be adversely affected by, the rights
of the holders of shares of any series of our preferred stock that we may designate and issue in the future. All of our outstanding shares
of Class A common stock are fully paid and nonassessable.
Our Class A common stock is listed on the Nasdaq
Capital Market under the symbol “BIVI.” The transfer agent and registrar for our Class A common stock is West Coast Stock
Transfer, Inc., Encinitas, California.
Options/Warrants/ Restricted Stock Units
As of August 17, 2023, we had outstanding options
to purchase 3,952,864 shares of our Class A common stock at a weighted average exercise price of $7.10 and outstanding warrants to purchase
7,770,285 shares of our Class A common stock at a weighted exercise price of $2.06 and restricted stock units totaling 557,727.
Anti-Takeover Effects of Our Articles of
Incorporation and Bylaws
Our Articles of Incorporation and Bylaws contain
certain provisions that may have anti-takeover effects, making it more difficult for or preventing a third party from acquiring control
of us or changing our Board of Directors and management. According to our Articles of Incorporation and Bylaws, neither the holders of
our common stock nor the holders of any preferred stock we may issue in the future have cumulative voting rights in the election of our
directors. The combination of the present ownership by a few stockholders of a significant portion of our issued and outstanding common
stock and lack of cumulative voting makes it more difficult for other stockholders to replace our Board of Directors or for a third party
to obtain control of us by replacing our Board of Directors.
Anti-Takeover Effects of Nevada Law
Business Combinations
The “business combination” provisions
of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes (“NRS”) generally prohibit a Nevada corporation with
at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder for a period
of two years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved
by the board of directors prior to the date the interested stockholder obtained such status or the combination is approved by the board
of directors and thereafter is approved at a meeting of the stockholders by the affirmative vote of stockholders representing at least
60% of the outstanding voting power held by disinterested stockholders, and extends beyond the expiration of the two-year period, unless:
| ● |
the combination was approved by the board of directors prior to the person becoming an interested stockholder or the transaction by which the person first became an interested stockholder was approved by the board of directors before the person became an interested stockholder or the combination is later approved by a majority of the voting power held by disinterested stockholders; or |
| ● |
if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the two years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is generally defined
to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer, or other disposition, in one transaction
or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of
the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market
value of all outstanding shares of the corporation, (c) 10% or more of the earning power or net income of the corporation, and (d) certain
other transactions with an interested stockholder or an affiliate or associate of an interested stockholder.
In general, an “interested stockholder”
is a person who, together with affiliates and associates, owns (or within two years, did own) 10% or more of a corporation’s voting
stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts
to acquire our Company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above
the prevailing market price.
Control Share Acquisitions
The “control share” provisions of
Sections 78.378 to 78.3793, inclusive, of the NRS apply to “issuing corporations” that are Nevada corporations with at least
200 stockholders, including at least 100 stockholders of record who are Nevada residents, and that conduct business directly or indirectly
in Nevada. The control share statute prohibits an acquirer, under certain circumstances, from voting its shares of a target corporation’s
stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested
stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and
a majority or more, of the outstanding voting power. Generally, once an acquirer crosses one of the above thresholds, those shares in
an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of
the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded
full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote
in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance
with statutory procedures established for dissenters’ rights.
A corporation may elect to not be governed by,
or “opt out” of, the control share provisions by making an election in its articles of incorporation or bylaws, provided that
the opt-out election must be in place on the 10th day following the date an acquiring person has acquired a controlling interest, that
is, crossing any of the three thresholds described above. We have not opted out of the control share statutes, and will be subject to
these statutes if we are an “issuing corporation” as defined in such statutes.
The effect of the Nevada control share statutes
is that the acquiring person, and those acting in association with the acquiring person, will obtain only such voting rights in the control
shares as are conferred by a resolution of the stockholders at an annual or special meeting. The Nevada control share law, if applicable,
could have the effect of discouraging takeovers of our Company.
DESCRIPTION OF WARRANTS
The following description,
together with the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions
of the warrants that we may offer under this prospectus and the related warrant agreements and warrant certificates. For the avoidance
of doubt, this section relates only to new warrants that we may issue and not any of our outstanding warrants, such as the Lender Warrants,
and we refer to such new warrants in this prospectus for the sake of simplicity as “warrants.”
While the terms summarized
below will apply generally to any warrants that we may offer, we will describe the particular terms of any series of warrants in more
detail in the applicable prospectus supplement. If we indicate in the prospectus supplement, the terms of any warrants offered under that
prospectus supplement may differ from the terms described below. Specific warrant agreements will contain additional important terms and
provisions and will be incorporated by reference as an exhibit to the registration statement, which includes this prospectus.
General
We may issue warrants for the
purchase of Class A common stock, preferred stock or debt securities, in one or more series. We may issue warrants independently or together
with Class A common stock, preferred stock and/or debt securities, and the warrants may be attached to or separate from these securities.
We plan to evidence each series
of warrants by warrant certificates that we will issue under a separate warrant agreement. We will enter into the warrant agreement with
a warrant agent. We will indicate the name and address of the warrant agent in the applicable prospectus supplement relating to a particular
series of warrants.
We will describe in the applicable
prospectus supplement the terms of the series of warrants, including:
| ● | the offering price and aggregate number of warrants offered; |
| ● | the currency for which the warrants may be purchased; |
| ● | if applicable, the designation and terms of the securities with
which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| ● | if applicable, the date on and after which the warrants and the
related securities will be separately transferable; |
| ● | the number of shares of Class A common stock purchasable upon
the exercise of one warrant and the price at which these shares may be purchased upon such exercise; |
| ● | the effect of any merger, consolidation, sale or other disposition
of our business on the warrant agreement and the warrants; |
| ● | the terms of any rights to redeem or call the warrants; |
| ● | any provisions for changes to or adjustments in the exercise price
or number of securities issuable upon exercise of the warrants; |
| ● | the periods during which, and places at which, the warrants are
exercisable; |
| ● | the dates on which the right to exercise the warrants will commence
and expire; |
| ● | the manner in which the warrant agreement and warrants may be
modified; |
| ● | if applicable, a discussion of certain material U.S. federal income
tax considerations of holding or exercising the warrants; and |
| ● | any other specific terms, preferences, rights or limitations of
or restrictions on the warrants. |
DESCRIPTION OF DEBT SECURITIES
The following description, together with the
additional information we include in any applicable prospectus supplements, summarizes the material terms and provisions of the debt securities
that we may offer under this prospectus. While the terms we have summarized below will generally apply to any future debt securities we
may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the
applicable prospectus supplement. The terms of any debt securities we offer under a prospectus supplement may differ from the terms we
describe below. As of the date of this prospectus, we have no outstanding registered debt securities.
We will issue senior notes under a senior indenture,
which we will enter into with the trustee to be named in the senior indenture. We will issue subordinated notes under a subordinated indenture,
which we will enter into with the trustee to be named in the subordinated indenture. We have filed forms of these documents as exhibits
to the registration statement of which this prospectus is a part. We use the term “indentures” to refer to both the senior
indenture and the subordinated indenture.
The indentures will be qualified under the Trust
Indenture Act of 1939, as amended (the “Trust Indenture Act”). We use the term “debenture trustee” to refer to
either the senior trustee or the subordinated trustee, as applicable.
The following summaries of material provisions
of the senior notes, the subordinated notes and the indentures are subject to, and qualified in their entirety by reference to, all the
provisions of the indenture applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements
related to the debt securities that we sell under this prospectus, as well as the complete indentures that contain the terms of the debt
securities. Except as we may otherwise indicate, the terms of the senior and the subordinated indentures are identical.
General
The terms of each series of debt securities will
be established by or pursuant to a resolution of our board of directors and set forth or determined in the manner provided in an officers’
certificate or by a supplemental indenture. Debt securities may be issued in separate series without limitation as to aggregate principal
amount. We may specify a maximum aggregate principal amount for the debt securities of any series. The particular terms of each series
of debt securities will be described in a prospectus supplement relating to such series, including any pricing supplement. The prospectus
supplement will set forth:
| ● | the principal amount being offered, and, if a series, the total
amount authorized and the total amount outstanding; |
| ● | any limit on the amount that may be issued; |
| ● | whether or not we will issue the series of debt securities in
global form and, if so, the terms and who the depositary will be; |
| ● | whether and under what circumstances, if any, we will pay additional
amounts on any debt securities held by a person who is not a U.S. person for tax purposes, and whether we can redeem the debt securities
if we have to pay such additional amounts; |
| ● | the annual interest rate, which may be fixed or variable, or the
method for determining the rate, the date interest will begin to accrue, the dates interest will be payable and the regular record dates
for interest payment dates or the method for determining such dates; |
| ● | whether or not the debt securities will be secured or unsecured,
and the terms of any secured debt; |
| ● | the terms of the subordination of any series of subordinated debt; |
| ● | the place where payments will be payable; |
| ● | restrictions on transfer, sale or other assignment, if any; |
| ● | our right, if any, to defer payment of interest and the maximum
length of any such deferral period; |
| ● | the date, if any, after which, the conditions upon which, and
the price at which we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions,
and any other applicable terms of those redemption provisions; |
| ● | the date, if any, on which, and the price at which we are obligated,
pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase,
the series of debt securities and the currency or currency unit in which the debt securities are payable; |
| ● | whether the indenture will restrict our ability and/or the ability
of our subsidiaries to, among other things: |
| ● | incur additional indebtedness; |
| ● | issue additional securities; |
| ● | pay dividends and make distributions in respect of our capital
stock and the capital stock of our subsidiaries; |
| ● | place restrictions on our subsidiaries’ ability to pay dividends,
make distributions or transfer assets; |
| ● | make investments or other restricted payments; |
| ● | sell or otherwise dispose of assets; |
| ● | enter into sale-leaseback transactions; |
| ● | engage in transactions with stockholders and affiliates; |
| ● | issue or sell stock of our subsidiaries; or |
| ● | effect a consolidation or merger; |
| ● | whether the indenture will require us to maintain any interest
coverage, fixed charge, cash flow-based, asset-based or other financial ratios; |
| ● | a discussion of any material or special U.S. federal income tax
considerations applicable to the debt securities; |
| ● | information describing any book-entry features; |
| ● | provisions for a sinking fund purchase or other analogous fund,
if any; |
| ● | whether the debt securities are to be offered at a price such
that they will be deemed to be offered at an “original issue discount” as defined in paragraph (a) of Section 1273 of the
Internal Revenue Code; |
| ● | the procedures for any auction and remarketing, if any; |
| ● | the denominations in which we will issue the series of debt securities,
if other than denominations of $1,000 and any integral multiple thereof; |
| ● | if other than dollars, the currency in which the series of debt
securities will be denominated; and |
| ● | any other specific terms, preferences, rights or limitations of,
or restrictions on, the debt securities, including any events of default that are in addition to those described in this prospectus or
any covenants provided with respect to the debt securities that are in addition to those described above, and any terms that may be required
by us or advisable under applicable laws or regulations or advisable in connection with the marketing of the debt securities. |
Conversion or Exchange Rights
We will set forth in the prospectus supplement
the terms on which a series of debt securities may be convertible into or exchangeable for Class A common stock or other securities of
ours or a third party, including the conversion or exchange rate, as applicable, or how it will be calculated, and the applicable conversion
or exchange period. We will include provisions as to whether conversion or exchange is mandatory, at the option of the holder or at our
option. We may include provisions pursuant to which the number of our securities or the securities of a third party that the holders of
the series of debt securities receive upon conversion or exchange would, under the circumstances described in those provisions, be subject
to adjustment, or pursuant to which those holders would, under those circumstances, receive other property upon conversion or exchange,
for example in the event of our merger or consolidation with another entity.
Consolidation, Merger or Sale
The indentures in the forms initially filed as
exhibits to the registration statement of which this prospectus is a part do not contain any covenant that restricts our ability to merge
or consolidate, or sell, convey, transfer or otherwise dispose of all or substantially all of our assets. However, any successor of ours
or the acquirer of such assets must assume all of our obligations under the indentures and the debt securities. If the debt securities
are convertible for our other securities, the person with whom we consolidate or merge or to whom we sell all of our property must make
provisions for the conversion of the debt securities into securities that the holders of the debt securities would have received if they
had converted the debt securities before the consolidation, merger or sale.
Events of Default Under the Indenture
The following are events of default under the
indentures in the forms initially filed as exhibits to the registration statement with respect to any series of debt securities that we
may issue:
| ● | if we fail to pay interest when due and payable and our failure
continues for 90 days and the time for payment has not been extended or deferred; |
| ● | if we fail to pay the principal, sinking fund payment or premium,
if any, when due and payable and the time for payment has not been extended or delayed; |
| ● | if we fail to observe or perform any other covenant contained
in the debt securities or the indentures, other than a covenant specifically relating to another series of debt securities, and our failure
continues for 90 days after we receive notice from the debenture trustee or holders of at least 25% in aggregate principal amount of
the outstanding debt securities of the applicable series; and |
| ● | if specified events of bankruptcy, insolvency or reorganization
occur. |
If an event of default with respect to debt securities
of any series occurs and is continuing, other than an event of default specified in the last bullet point above, the debenture trustee
or the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series, by notice to us in writing,
and to the debenture trustee if notice is given by such holders, may declare the unpaid principal of, premium, if any, and accrued interest,
if any, due and payable immediately. If an event of default specified in the last bullet point above occurs with respect to us, the principal
amount of and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice
or other action on the part of the debenture trustee or any holder.
The holders of a majority in principal amount
of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its
consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured
the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indentures, if an
event of default under an indenture shall occur and be continuing, the debenture trustee will be under no obligation to exercise any of
its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities,
unless such holders have offered the debenture trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding
debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available
to the debenture trustee, or exercising any trust or power conferred on the debenture trustee, with respect to the debt securities of
that series, provided that:
| ● | the direction so given by the holder is not in conflict with any
law or the applicable indenture; and |
| ● | subject to its duties under the Trust Indenture Act, the debenture
trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved
in the proceeding. |
A holder of the debt securities of any series
will only have the right to institute a proceeding under the indentures or to appoint a receiver or trustee, or to seek other remedies
if:
| ● | the holder has given written notice to the debenture trustee of
a continuing event of default with respect to that series; |
| ● | the holders of at least 25% in aggregate principal amount of the
outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity, to the debenture
trustee to institute the proceeding as trustee; and |
| ● | the debenture trustee does not institute the proceeding and does
not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting
directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted
by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the
debenture trustee regarding our compliance with specified covenants in the indentures.
Modification of Indenture; Waiver
We and the debenture trustee may change an indenture without the consent
of any holders with respect to specific matters, including:
| ● | to fix any ambiguity, defect or inconsistency in the indenture; |
| ● | to comply with the provisions described above under “Consolidation,
Merger or Sale”; |
| ● | to comply with any requirements of the SEC in connection with
the qualification of any indenture under the Trust Indenture Act; |
| ● | to evidence and provide for the acceptance of appointment hereunder
by a successor trustee; |
| ● | to provide for uncertificated debt securities and to make all
appropriate changes for such purpose; |
| ● | to add to, delete from, or revise the conditions, limitations
and restrictions on the authorized amount, terms or purposes of issuance, authorization and delivery of debt securities or any series,
as set forth in the indenture; |
| ● | to provide for the issuance of and establish the form and terms
and conditions of the debt securities of any series as provided under “General” to establish the form of any certifications
required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders
of any series of debt securities; |
| ● | to add to our covenants such new covenants, restrictions, conditions
or provisions for the protection of the holders, to make the occurrence, or the occurrence and the continuance, of a default in any such
additional covenants, restrictions, conditions or provisions an event of default, or to surrender any of our rights or powers under the
indenture; or |
| ● | to change anything that does not materially adversely affect the
interests of any holder of debt securities of any series. |
In addition, under the indentures, the rights
of holders of a series of debt securities may be changed by us and the debenture trustee with the written consent of the holders of at
least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, we and the
debenture trustee may only make the following changes with the consent of each holder of any outstanding debt securities affected:
| ● | extending the fixed maturity of the series of debt securities; |
| ● | reducing the principal amount, reducing the rate of or extending
the time of payment of interest, or reducing any premium payable upon the redemption of any debt securities; or |
| ● | reducing the percentage of debt securities, the holders of which
are required to consent to any amendment, supplement, modification or waiver. |
Discharge
Each indenture provides that we can elect to be
discharged from our obligations with respect to one or more series of debt securities, except that the following obligations survive until
the maturity date or the redemption date:
| ● | register the transfer or exchange of debt securities of the series; |
| ● | replace stolen, lost or mutilated debt securities of the series; |
| ● | maintain paying agencies; |
| ● | hold monies for payment in trust; and |
| ● | appoint any successor trustee; |
and the following obligations survive the maturity date or the redemption
date:
| ● | recover excess money held by the debenture trustee; and |
| ● | compensate and indemnify the debenture trustee. |
In order to exercise our rights to be discharged,
we must deposit with the debenture trustee money or government obligations sufficient to pay all the principal of, any premium, if any,
and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series
only in fully registered form without coupons and, unless we otherwise specify in the applicable prospectus supplement, in denominations
of $1,000 and any integral multiple thereof. The indentures provide that we may issue debt securities of a series in temporary or permanent
global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company, New York, New York,
known as DTC, or another depositary named by us and identified in a prospectus supplement with respect to that series.
At the option of the holder, subject to the terms
of the indentures and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of
the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination
and of like tenor and aggregate principal amount.
Subject to the terms of the indentures and the
limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present
the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed
if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated
by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will make
no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement
the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities.
We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office
through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt
securities of each series.
If we elect to redeem the debt securities of any series, we will not
be required to:
| ● | issue, register the transfer of, or exchange any debt securities
of any series being redeemed in part during a period beginning at the opening of business 15 days before the day of mailing of a notice
of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing;
or |
| ● | register the transfer of or exchange any debt securities so selected
for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Debenture Trustee
The debenture trustee, other than during the occurrence
and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically set forth in the
applicable indenture. Upon an event of default under an indenture, the debenture trustee must use the same degree of care as a prudent
person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the debenture trustee is under no obligation
to exercise any of the powers given it by the indentures at the request of any holder of debt securities unless it is offered reasonable
security and indemnity against the costs, expenses and liabilities that it might incur.
Payment and Paying Agents
Unless we otherwise indicate in the applicable
prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose
name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for
the interest.
We will pay principal of and any premium and interest
on the debt securities of a particular series at the office of the paying agents designated by us, except that, unless we otherwise indicate
in the applicable prospectus supplement, we may make interest payments by check that we will mail to the holder or by wire transfer to
certain holders. Unless we otherwise indicate in a prospectus supplement, we will designate the corporate office of the debenture trustee
in the State of Nevada as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable
prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain
a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the debenture
trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed at the end of two
years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter
may look only to us for payment thereof.
Governing Law
The indentures and the debt securities will be
governed by and construed in accordance with the laws of the State of Nevada, except to the extent that the Trust Indenture Act is applicable.
Subordination of Subordinated Debt Securities
The subordinated debt securities will be subordinate
and junior in priority of payment to certain of our other indebtedness to the extent described in a prospectus supplement. The indentures
in the forms initially filed as exhibits to the registration statement of which this prospectus is a part do not limit the amount of indebtedness
that we may incur, including senior indebtedness or subordinated indebtedness, and do not limit us from issuing any other debt, including
secured debt or unsecured debt.
DESCRIPTION OF RIGHTS
The complete terms of the rights will be contained
in the rights agreements we enter into with rights agents. These documents will be included or incorporated by reference as exhibits to
the registration statement of which this prospectus is a part. You should read the rights agreements and any related documents. You also
should read the prospectus supplement, which will contain additional information and which may update or change some of the information
below.
This section describes the general terms of the
rights to purchase Class A common stock or other securities that we may offer to stockholders using this prospectus. Further terms of
the rights will be stated in the applicable prospectus supplement (or applicable free writing prospectus). The following description and
any description of the rights in a prospectus supplement (or applicable free writing prospectus) may not be complete and is subject to
and qualified in its entirety by reference to the terms of any agreement relating to the rights.
Rights may be issued independently or together
with any other security and may or may not be transferable. As part of any rights offering, we may enter into a standby underwriting or
other arrangement under which the underwriters or any other person would purchase any securities that are not purchased in such rights
offering. If we issue rights, each series of rights will be issued under a separate rights agreement to be entered into between us and
a bank or trust company, as rights agent, that will be named in the applicable prospectus supplement. Further terms of the rights will
be stated in the applicable prospectus supplement. The rights agent will act solely as our agent and will not assume any obligation to
any holders of rights certificates or beneficial owners of rights. The rights agreements and rights certificates will be filed with the
SEC as an exhibit to the registration statement of which this prospectus is a part or as an exhibit to a filing incorporated by reference
in the registration statement. See “Where You Can Find Additional Information” for information on how to obtain copies
of the rights agreements and rights certificates.
The prospectus supplement relating to any rights
we offer will describe the specific terms of the offering and the rights, including the record date for stockholders entitled to the rights
distribution, the number of rights issued and the number of shares of Class A common stock that may be purchased upon exercise of the
rights, the exercise price of the rights, the date on which the rights will become effective and the date on which the rights will expire,
and any applicable U.S. federal income tax considerations.
In general, a right entitles the holder to purchase
for cash a specific number of shares of Class A common stock or other securities at a specified exercise price. The rights are normally
issued to stockholders as of a specific record date, may be exercised only for a limited period of time and become void following the
expiration of such period. If we determine to issue rights, we will accompany this prospectus with a prospectus supplement that will describe,
among other things:
| ● | the record date for stockholders entitled to receive the rights; |
| ● | the number of shares of Class A common stock or other securities
that may be purchased upon exercise of each right; |
| ● | the exercise price of the rights; |
| ● | the terms for changes to or adjustments in the exercise price,
if any; |
| ● | whether the rights are transferable; |
| ● | the period during which the rights may be exercised and when they
will expire; |
| ● | the steps required to exercise the rights; |
| ● | whether the rights include “oversubscription rights”
so that the holder may purchase more securities if other holders do not purchase their full allotments; |
| ● | whether we intend to sell the shares of Class A common stock or
other securities that are not purchased in the rights offering to an underwriter or other purchaser under a contractual “standby”
commitment or other arrangement; |
| ● | our ability to withdraw or terminate the rights offering; |
| ● | any material United States federal income tax consequences; and |
| ● | other material terms, including terms relating to transferability,
exchange, exercise or amendment of the rights. |
If fewer than all of the rights issued in any
rights offering are exercised, we may offer any unsubscribed securities directly to persons other than stockholders, to or through agents,
underwriters or dealers or through a combination of such methods, including pursuant to standby arrangements, as described in the applicable
prospectus supplement. After the close of business on the expiration date, all unexercised rights will become void.
DESCRIPTION OF UNITS
We may issue units comprised of shares of Class
A common stock, shares of preferred stock, debt securities, rights and warrants to purchase Class A common stock in any combination. We
may issue units in such amounts and in as many distinct series as we wish. This section outlines certain provisions of the units that
we may issue. If we issue units, they will be issued under one or more unit agreements to be entered into between us and a bank or other
financial institution, as unit agent. The information described in this section may not be complete in all respects and is qualified entirely
by reference to the unit agreement with respect to the units of any particular series. The specific terms of any series of units offered
will be described in the applicable prospectus supplement. If so described in a particular supplement, the specific terms of any series
of units may differ from the general description of terms presented below. We urge you to read any prospectus supplement related to any
series of units we may offer, as well as the complete unit agreement and unit certificate that contain the terms of the units. If we issue
units, forms of unit agreements and unit certificates relating to such units will be incorporated by reference as exhibits to the registration
statement, which includes this prospectus.
Each unit that we may issue will be issued so
that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights
and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities
included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
The applicable prospectus supplement may describe:
| ● | the designation and terms of the units and of the securities comprising
the units, including whether and under what circumstances those securities may be held or transferred separately; |
| ● | any provisions of the governing unit agreement; |
| ● | the price or prices at which such units will be issued; |
| ● | the applicable U.S. federal income tax considerations relating
to the units; |
| ● | any provisions for the issuance, payment, settlement, transfer
or exchange of the units or of the securities comprising the units; and |
| ● | any other terms of the units and of the securities comprising
the units. |
The provisions described in this section, as well
as those described under “Description of Capital Stock,” “Description of Debt Securities” and “Description
of Warrants” will apply to the securities included in each unit, to the extent relevant and as may be updated in any prospectus
supplements. We may issue units in such amounts and in as many distinct series as we wish. This section summarizes terms of the units
that apply generally to all series. Most of the financial and other specific terms of a particular series of units will be described in
the applicable prospectus supplement.
Unit Agreements
We will issue the units under one or more unit
agreements to be entered into between us and a bank or other financial institution, as unit agent. We may add, replace or terminate unit
agents from time to time. We will identify the unit agreement under which each series of units will be issued and the unit agent under
that agreement in the applicable prospectus supplement.
The following provisions will generally apply to all unit agreements
unless otherwise stated in the applicable prospectus supplement:
Modification Without Consent
We and the applicable unit agent may amend any unit or unit agreement
without the consent of any holder:
| ● | to cure any ambiguity in any provisions of the governing unit
agreement that differ from those described below; |
| ● | to correct or supplement any defective or inconsistent provision;
or |
| ● | to make any other change that we believe is necessary or desirable
and will not adversely affect the interests of the affected holders in any material respect. |
We do not need any approval to make changes that
affect only units to be issued after the changes take effect. We may also make changes that do not adversely affect a particular unit
in any material respect, even if they adversely affect other units in a material respect. In those cases, we do not need to obtain the
approval of the holder of the unaffected unit; we need only obtain any required approvals from the holders of the affected units.
Modification With Consent
We may not amend any particular unit or a unit agreement with respect
to any particular unit unless we obtain the consent of the holder of that unit, if the amendment would:
| ● | impair any right of the holder to exercise or enforce any right
under a security included in the unit if the terms of that security require the consent of the holder to any changes that would impair
the exercise or enforcement of that right; or |
| ● | reduce the percentage of outstanding units or any series or class
the consent of whose holders is required to amend that series or class, or the applicable unit agreement with respect to that series
or class, as described below. |
Any other change to a particular unit agreement and the units issued under that agreement would require the following approval:
| ● | if the change affects only the units of a particular series issued
under that agreement, the change must be approved by the holders of a majority of the outstanding units of that series; or |
| ● | if the change affects the units of more than one series issued
under that agreement, it must be approved by the holders of a majority of all outstanding units of all series affected by the change,
with the units of all the affected series voting together as one class for this purpose. |
These provisions regarding changes with majority
approval also apply to changes affecting any securities issued under a unit agreement, as the governing document.
In each case, the required approval must be given
by written consent.
Unit Agreements Will Not be Qualified Under
Trust Indenture Act
No unit agreement will be qualified as an indenture,
and no unit agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders of units issued under unit
agreements will not have the protections of the Trust Indenture Act with respect to their units.
Mergers and Similar Transactions Permitted;
No Restrictive Covenants or Events of Default
The unit agreements will not restrict our ability
to merge or consolidate with, or sell our assets to, another corporation or other entity or to engage in any other transactions. If at
any time we merge or consolidate with, or sell our assets substantially as an entirety to, another corporation or other entity, the successor
entity will succeed to and assume our obligations under the unit agreements. We will then be relieved of any further obligation under
these agreements.
The unit agreements will not include any restrictions
on our ability to put liens on our assets, nor will they restrict our ability to sell our assets. The unit agreements also will not provide
for any events of default or remedies upon the occurrence of any events of default.
Form, Exchange and Transfer
We will issue each unit in global (i.e., book-entry)
form only. Units in book-entry form will be represented by a global security registered in the name of a depositary, which will be the
holder of all the units represented by the global security. Those who own beneficial interests in a unit will do so through participants
in the depositary’s system, and the rights of these indirect owners will be governed solely by the applicable procedures of the
depositary and its participants. We will describe book-entry securities, and other terms regarding the issuance and registration of the
units in the applicable prospectus supplement.
Each unit and all securities comprising the unit will be issued in
the same form.
If we issue any units in registered, non-global form, the following
will apply to them:
| ● | The units will be issued in the denominations stated in the applicable
prospectus supplement. Holders may exchange their units for units of smaller denominations or combined into fewer units of larger denominations,
as long as the total amount is not changed. |
| ● | Holders may exchange or transfer their units at the office of
the unit agent. Holders may also replace lost, stolen, destroyed or mutilated units at that office. We may appoint another entity to
perform these functions or perform them ourselves. |
| ● | Holders will not be required to pay a service charge to transfer
or exchange their units, but they may be required to pay for any tax or other governmental charge associated with the transfer or exchange.
The transfer or exchange, and any replacement, will be made only if our transfer agent is satisfied with the holder’s proof of
legal ownership. The transfer agent may also require an indemnity before replacing any units. |
| ● | If we have the right to redeem, accelerate or settle any units
before their maturity, and we exercise our right as to less than all those units or other securities, we may block the exchange or transfer
of those units during the period beginning 15 days before the day we mail the notice of exercise and ending on the day of that mailing,
in order to freeze the list of holders to prepare the mailing. We may also refuse to register transfers of or exchange any unit selected
for early settlement, except that we will continue to permit transfers and exchanges of the unsettled portion of any unit being partially
settled. We may also block the transfer or exchange of any unit in this manner if the unit includes securities that are or may be selected
for early settlement. |
Only the depositary will be entitled to transfer
or exchange a unit in global form, since it will be the sole holder of the unit.
Payments and Notices
In making payments and giving notices with respect
to our units, we will follow the procedures as described in the applicable prospectus supplement.
SELLING STOCKHOLDERS
On November 30, 2021, we entered into the Loan
Agreement pursuant to which we issued and sold to the Selling Stockholders Lender Warrants to purchase 361,002 shares of Class A common
stock. This prospectus covers the sale or other disposition by the Selling Stockholders and their respective donees, pledgees or other
successors-in-interest of up to the total number of Shares registered on behalf of the Selling Stockholders in the manner contemplated
under “Plan of Distribution” below. Throughout this prospectus, when we refer to the Shares being registered on behalf
of the Selling Stockholders, we are referring to the Shares issuable upon the exercise of the Lender Warrants issued to the Selling Stockholders
in the Loan, and when we refer to the Selling Stockholders in this prospectus, we are referring to those investors set forth in the table
below.
On March 31, 2023, the Company filed a Registration
Statement on Form S-3 (File No. 333-271054), that was declared effective by the SEC on April 10, 2023, which related in part to the offer
and resale, from time to time, by the Selling Stockholders of up to 50,000 shares of Class A common stock issuable upon exercise of the
Lender Warrants.
In connection with the Loan Agreement, we granted
certain registration rights to the Selling Stockholders. The Loan Agreement also provide, among other things, certain indemnification
rights and reimbursement by the Company of certain fees and expenses.
We have agreed with the Selling Stockholders to
keep the registration statement of which this prospectus constitutes a part effective for a period of at least twelve (12) months after
the date that the Selling Stockholders are first given the opportunity to sell all of the Shares.
Except as otherwise disclosed herein and in the
footnotes below with respect to the Selling Stockholders, the Selling Stockholders do not, and within the past three years, have not had,
any position, office or other material relationship with us.
The following table sets forth the name of the
Selling Stockholders, the number of shares of Class A common stock beneficially owned by the Selling Stockholders, the number of Shares
that may be offered under this prospectus and the number of shares of our Class A common stock that will be owned by the Selling Stockholders
assuming all of the Shares covered hereby are sold. The number of Shares in the column “Number of Shares Being Offered” represents
all of the Shares that the Selling Stockholders may offer under this prospectus. Pursuant to Rules 13d-3 and 13d-5 of the Exchange Act
(“Rule 13(d)”), beneficial ownership includes all shares of our Class A common stock as to which a Selling Stockholder has
sole or shared voting power or investment power, and also any shares of our Class A common stock which the Selling Stockholder has the
right to acquire within 60 days of August 17, 2023, but without regard to the Beneficial Ownership Limitation included in the Lender Warrants
(described below). The actual beneficial ownership of certain Selling Stockholders (determined in accordance with Rule 13d) does not necessarily
correspond to the number of Shares reflected below in the column “Number of Shares Being Offered.”
Notwithstanding the presentation of Share ownership
in the table below, pursuant to the terms of the Lender Warrants, a holder of a Lender Warrant does not have the right to exercise any
portion of the Lender Warrant held by such holder to the extent (but only to the extent) that after giving effect to such issuance after
exercise, the holder (together with the holder’s affiliates, and any other persons acting as a group together with the holder or
any of the holder’s affiliates), would beneficially own in excess of 9.99% of the number of shares of Class A common stock outstanding
immediately after giving effect to the issuance of shares of Class A common stock issued upon exercise of the Lender Warrants (the “Beneficial
Ownership Limitation”). The holder of a Lender Warrant may, upon notice to the Company, increase or decrease the Beneficial Ownership
Limitation of its Warrant, provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of the
Class A common stock outstanding immediately after giving effect to the issuance of shares of Class A common stock upon exercise of the
Lender Warrant held by the holder. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st
day after such notice is delivered to the Company. No such increase notice has been provided to the Company as of the date of this prospectus.
The information set forth below is based upon
information obtained from the Selling Stockholders and upon information in our possession regarding the issuance of the Shares issuable
upon the exercise of the Lender Warrants to the Selling Stockholders. The percentages of shares of Class A common stock beneficially owned
before this offering are based on 36,803,768 shares of Class A common stock issued and outstanding as of August 17, 2023. The percentages
of shares of our Class A common stock owned after the offering are based on 37,114,770 shares of our Class A common stock outstanding
after this offering, including the 36,803,768 shares of Class A common stock outstanding as of August 17, 2023 plus 311,002 Shares issuable
upon the exercise of the Lender Warrants covered hereby.
The Shares covered hereby may be offered from time to time by the Selling
Stockholders. The Selling Stockholders may sell some, all or none of their respective Shares. We do not know how long the Selling Stockholders
will hold their Shares before selling them, and we currently have no agreements, arrangements or understandings with the Selling Stockholders
regarding the sale or other disposition of any of the Shares
| |
|
Shares of Class A common stock
Beneficially Owned
Prior To The
Offering |
|
|
Maximum Number of
Shares
Being Offered |
|
|
Shares of Class A common stock
Beneficially Owned
After The
Offering(1) |
|
| Name of Selling Stockholder |
|
Number |
|
Percentage |
|
|
Number |
|
|
Percent |
|
| Avenue Venture Opportunities Fund, LP(2) |
|
155,501 |
|
* |
|
|
155,501 |
|
|
0 |
|
|
- |
|
| Avenue Venture Opportunities Fund II, LP(3) |
|
155,501 |
|
* |
|
|
155,501 |
|
|
0 |
|
|
- |
|
Percentages denoted by * are less than 1%.
| (1) | Assumes that all Shares being registered in this prospectus are
resold to third parties and that the Selling Stockholders sell all Shares registered under this prospectus held by them. |
| (2) | The business address for Avenue Venture Opportunities Fund, LP
is 11 West 42nd St. 9th Floor, New York, NY, 10036. |
| (3) | The business address for Avenue Venture Opportunities Fund II,
LP is 11 West 42nd St. 9th Floor, New York, NY, 10036. |
PLAN OF DISTRIBUTION
We may sell the securities described herein, and
the Selling Stockholders may sell some or all of the Shares that they hold, from time to time in one or more offerings, by a variety of
methods, including the following:
| ● | on any national securities exchange or quotation service on which
our securities may be listed at the time of sale, including the Nasdaq Capital Market; |
| ● | in the over-the-counter market; |
| ● | in transactions otherwise than on such exchange or in the over-the-counter
market, which may include privately negotiated transactions and sales directly to one or more purchasers; |
| ● | through one or more agents, including an “at the market”
offering within the meaning of Rule 415(a)(4) under the Securities Act; |
| ● | through ordinary brokerage transactions and transactions in which
the broker-dealer solicits purchasers; |
| ● | through purchases by a broker-dealer as principal and resale by
the broker-dealer for its account; |
| ● | to or through underwriters, broker-dealers, agents, in privately
negotiated transactions, or any combination of these methods; |
| ● | through the writing or settlement of options or other hedging
transactions, whether through an options exchange or otherwise; |
| ● | by pledge to secure debts or other obligations; |
| ● | block trades in which the broker-dealer so engaged will attempt
to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction, or crosses
in which the same broker acts as agent on both sides of the trade; |
| ● | a combination of any of these methods; or |
| ● | by any other method permitted pursuant to applicable law. |
As used in this prospectus, “Selling Stockholders”
includes transferees, pledgees, donees, assignees or successors selling shares received after the date of this prospectus from a Selling
Stockholder as a gift, pledge, partnership distribution or other non-sale related transfer.
We will not receive any proceeds from the sale
of securities that may be sold from time to time pursuant to this prospectus by the Selling Stockholders. We will bear the costs associated
with this registration in accordance with the agreements granting registration rights to the Selling Stockholders. However, the Selling
Stockholders will bear any brokerage commissions, transfer taxes, or underwriting commissions and discounts attributable to their sale
of securities pursuant to this prospectus. To our knowledge, there are currently no plans, arrangements or understandings between any
Selling Stockholders and any underwriter, broker-dealer or agent regarding the sale of securities pursuant to this prospectus by the Selling
Stockholders.
We or the Selling Stockholders may sell the securities
to or through one or more underwriters or dealers (acting as principal or agent), through agents, or directly to one or more purchasers.
We or the Selling Stockholders may distribute the securities from time to time in one or more transactions:
| ● | at a fixed price or prices, which may be changed; |
| ● | at market prices prevailing at the time of sale; |
| ● | at prices related to such prevailing market prices; |
| ● | at varying prices determined at the time of sale; or |
We will describe the terms of the offering of
the securities and the specific plan of distribution in a prospectus supplement or supplements to this prospectus, any related free writing
prospectus that we may authorize to be provided to you, an amendment to the registration statement of which this prospectus is a part
or other filings we make with the SEC under the Exchange Act that are incorporated by reference. Such description may include, to the
extent applicable:
| ● | the name or names of any underwriters, dealers, agents or other
purchasers; |
| ● | the purchase price of the securities or other consideration therefor,
and the proceeds, if any, we or the Selling Stockholders will receive from the sale; |
| ● | any options to purchase additional shares or other options under
which underwriters, dealers, agents or other purchasers may purchase additional securities from us or the Selling Stockholders; |
| ● | any agency fees or underwriting discounts and other items constituting
agents’ or underwriters’ compensation; |
| ● | any public offering price; |
| ● | any discounts or concessions allowed or reallowed or paid to dealers;
and |
| ● | any securities exchange or market on which the securities may
be listed. |
Only underwriters named in the prospectus supplement
will be underwriters of the securities offered by the prospectus supplement. The Selling Stockholders who participate in the sale or distribution
of the securities offered by the Selling Stockholders and any dealers and agents participating in the distribution of the securities may
be deemed to be underwriters, and compensation received by them on resale of the securities may be deemed to be underwriting discounts.
Any Selling Stockholders identified as registered broker-dealers in the Selling Stockholders table in the section titled “Selling
Stockholders” are deemed to be underwriters. If such dealers or agents were deemed to be underwriters, they may be subject to
statutory liabilities under the Securities Act.
If underwriters are used in the sale, they will
acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public
offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will
be subject to the conditions set forth in the applicable underwriting agreement. We or the Selling Stockholders may offer the securities
to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to
certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement, other than
securities covered by any option to purchase additional shares or other option. If a dealer is used in the sale of securities, we or the
Selling Stockholders, or an underwriter, will sell the securities to the dealer, as principal. The dealer may then resell the securities
to the public at varying prices to be determined by the dealer at the time of resale. To the extent required, we will set forth in the
prospectus supplement the name of the dealer and the terms of the transaction. Any public offering price and any discounts or concessions
allowed or reallowed or paid to dealers may change from time to time. We or the Selling Stockholders may use underwriters, dealers or
agents with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, dealer or agent,
the nature of any such relationship.
We or the Selling Stockholders may sell securities
directly or through agents we designate from time to time. If required by applicable law, we will name any agent involved in the offering
and sale of securities and we will describe any commissions payable to the agent in the prospectus supplement. Unless the prospectus supplement
states otherwise, the agent will act on a best-efforts basis for the period of its appointment.
We may provide agents, dealers and underwriters
with indemnification against civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments
that the agents or dealers or underwriters may make with respect to these liabilities. Agents, dealers and underwriters or their affiliates
may engage in transactions with, or perform services for us in the ordinary course of business.
With respect to the offering and sale of securities
under this prospectus by the Selling Stockholders, we have agreed to indemnify each Selling Stockholder and any underwriter for such Selling
Stockholder (as determined in the Securities Act) against specified liabilities, including liabilities under the Securities Act. The Selling
Stockholders have agreed to indemnify us against specified liabilities, including liabilities under the Securities Act. In addition, we
have agreed to pay substantially all of the expenses incidental to the registration, offering and sale of securities pursuant to this
prospectus by the Selling Stockholders to the public, including the payment of federal securities law and state blue sky registration
fees and the reasonable fees and disbursements of one counsel for the Selling Stockholders, except that we will not bear any brokers’
or underwriters’ discounts and commissions, fees and expenses of counsel to underwriters or brokers, transfer taxes or transfer
fees relating to the sale of securities by the Selling Stockholders.
We may engage in at-the-market offerings into
an existing trading market in accordance with rule 415(a)(4) under the Securities Act. In addition, we or the Selling Stockholders may
enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately
negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those derivatives, the third parties
may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so,
the third party may use securities pledged by us or the Selling Stockholders or borrowed from us, the Selling Stockholders or others to
settle those sales or to close out any related open borrowings of Class A common stock, and may use securities received from us or the
Selling Stockholders in settlement of those derivatives to close out any related open borrowings of our Shares. In addition, we or the
Selling Stockholders may loan or pledge securities to a financial institution or other third party that in turn may sell the securities
using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer its economic
short position to investors in our securities or in connection with a concurrent offering of other securities.
The Selling Stockholders will act independently
of us in making decisions with respect to the timing, manner, and size of each resale or other transfer. There can be no assurance that
the Selling Stockholders will sell any or all of the securities under this prospectus. Further, we cannot assure you that the Selling
Stockholders will not transfer, distribute, devise or gift the securities by other means not described in this prospectus. In addition,
any securities covered by this prospectus that qualify for sale under Rule 144 of the Securities Act may be sold under Rule 144 rather
than under this prospectus. A Selling Stockholder that is an entity may elect to make an in-kind distribution of the securities to its
members, partners or shareholders pursuant to this prospectus by delivering a prospectus. To the extent that such members, partners or
shareholders are not affiliates of ours, such members, partners or stockholders would thereby receive freely tradable shares of the securities
pursuant to the distribution through this prospectus.
All securities we may offer, other than Class
A common stock and the Lender Warrants, will be new issues of securities with no established trading market. Any underwriters may make
a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We
cannot guarantee the liquidity of the trading markets for any securities.
Any underwriter may be granted an option to purchase
additional shares, and engage in stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation
M under the Exchange Act. An underwriter’s option to purchase additional shares involves sales in excess of the offering size, which
create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not
exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either
through exercise of the option to purchase additional shares or in the open market after the distribution is completed, to cover short
positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the
dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities
to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any underwriters, dealers or agents that are qualified
market makers on the Nasdaq may engage in passive market making transactions in our Class A common stock on the Nasdaq in accordance with
Regulation M under the Exchange Act, during the business day prior to the pricing of the offering, before the commencement of offers or
sales of the Class A common stock. Passive market makers must comply with applicable volume and price limitations and must be identified
as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent
bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s
bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities
at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
LEGAL MATTERS
Unless otherwise indicated in the applicable prospectus
supplement, the validity of the securities offered hereby will be passed upon for us by Sherman & Howard L.L.C. If the validity of
the securities offered hereby in connection with offerings made pursuant to this prospectus are passed upon by counsel for the underwriters,
dealers or agents, if any, such counsel will be named in the prospectus supplement relating to such offering.
EXPERTS
The balance sheets of BioVie Inc. as of June 30,
2023 and 2022, and the related statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows
for each of the years then ended, have been audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their
report which is incorporated by reference, which report includes an explanatory paragraph about the existence of substantial doubt concerning
the Company’s ability to continue as a going concern. Such financial statements have been incorporated by reference in reliance
on the report of such firm given upon their authority as experts in accounting and auditing.
LIMITATION ON LIABILITY AND DISCLOSURE OF COMMISSION
POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or
otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities
Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by
the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of
any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered,
we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction
the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the
final adjudication of such issue.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of the registration statement
on Form S-3 we filed with the SEC under the Securities Act and does not contain all the information set forth in the registration statement.
Whenever a reference is made in this prospectus to any of our contracts, agreements or other documents, the reference may not be complete
and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated
by reference into this prospectus for a copy of such contract, agreement or other document. Because we are subject to the information
and reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), we file annual, quarterly
and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet
at the SEC’s website at http://www.sec.gov.
You may also access our SEC filings at our website
https://bioviepharma.com/. Our website and the information contained on, or that can be accessed through, our website will not
be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely on our website or any
such information in making your decision whether to purchase our securities.
INFORMATION INCORPORATED BY REFERENCE
We have elected to incorporate certain information
by reference into this prospectus. By incorporating by reference, we can disclose important information to you by referring you to other
documents we have filed or will file with the SEC. The information incorporated by reference is deemed to be part of this prospectus,
except for information incorporated by reference that is superseded by information contained in this prospectus. This means that you must
look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously
incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below that
we have previously filed with the SEC under the Exchange Act:
| ● | Our Annual Report on Form 10-K for the year ended June 30, 2023,
filed with the SEC on August 16, 2023, including any amendments or supplements thereto; |
| ● | Our Current Reports on Form 8-K, filed with the SEC on October
5, 2022, November 10, 2022, December 6, 2022 (both filed on such date), December 7, 2022 December 15, 2022, December 23, 2022, March
6, 2023, March 13, 2023, March 23, 2023 and April 7, 2023; and |
| ● | The description of our Class A common stock contained in our registration
on Form 8-A (File No. 001-39015) filed with the SEC on August 25, 2020, including any amendment or report filed for the purpose of updating
such description. |
All documents subsequently filed by the Registrant
with the SEC pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of the initial filing of the registration
statement and prior to effectiveness of the registration statement that contains this prospectus and prior to the termination of the offering
(except in each case the information contained in such document to the extent “furnish” and not “filed”), shall
be deemed to be incorporated by reference herein and to be a part hereof from the date of filing of such documents.
Any statement contained in a document incorporated
or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes of this Registration Statement
to the extent that a statement contained herein, or in any other subsequently filed document which also is incorporated or deemed to be
incorporated by reference herein, modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed,
except as so modified or superseded, to constitute a part of this Registration Statement.
1,146,000 Shares of Class A Common Stock

PROSPECTUS SUPPLEMENT
ThinkEquity
October 28,
2024
BioVie (NASDAQ:BIVI)
Historical Stock Chart
From Oct 2024 to Nov 2024
BioVie (NASDAQ:BIVI)
Historical Stock Chart
From Nov 2023 to Nov 2024