0001860657
false
0001860657
2023-11-13
2023-11-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
November 13, 2023
ALLARITY THERAPEUTICS, INC.
(Exact name of registrant as specified in our charter)
| Delaware |
|
001-41160 |
|
87-2147982 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
24 School Street, 2nd Floor,
Boston, MA |
|
02108 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
(401) 426-4664
(Registrant’s telephone number, including
area code)
Not applicable
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction
A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
ALLR |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange
Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure
On
November 13, 2023, Allarity Therapeutics, Inc. (the “Company”) is providing corporate presentation slides about the Company
(the “Corporate Presentation”). The Corporate Presentation
may be used in presentations to investors, analysts, and others. A copy of the Corporate Presentation is
attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The
information reported under Item 7.01 in this Current Report on Form 8-K, including Exhibit 99.1, is being “furnished” and
shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)
or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities
Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing. This Current Report on
Form 8-K will not be deemed an admission as to the materiality of any information contained herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit |
|
Exhibit Description |
| 99.1 |
|
Corporate Presentation |
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Allarity Therapeutics, Inc. |
| |
|
| |
By: |
/s/ James G. Cullem |
| |
|
James G. Cullem
Chief Executive Officer |
| |
|
|
| Dated: November 13, 2023 |
|
|
2
Exhibit 99.1

Copyright © 2023 Allarity Therapeutics. All rights reserved. Nasdaq: ALLR Personalized Cancer Care. Realized. James G. Cullem, CEO H2 2023

Legal Statement Allarity Therapeutics 2 This presentation is provided for informational purposes only and is subject to change. The information contained herein does not purport to be all - inclusive. The data contained herein is derived from various internal and external sources, and may rely upon assumptions, stated or otherwise, and forward - looking statements discussed below. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any forward - looking statements, modeling or any other information contained herein. Allarity Therapeutics, Inc. (collectively “Allarity Therapeutics,” “Allarity,” or the “Company”) assume no obligation to update the information in this presentation. This material is not for the benefit of, and does not convey any rights or remedies for the benefit of, any holder of securities or any other person. This material is not intended to provide the basis for evaluation of any transaction and does not purport to contain all information that may be required and should not be considered a recommendation or opinion of any kind with respect to any transaction. No Offer or Solicitation. This material shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Forward - Looking Statements. This presentation contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward - looking statements provide Allarity’s current expectations or forecasts of future events. The words “anticipates,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “would” and similar expressions may identify forward - looking statements, but the absence of these words does not mean that a statement is not forward - looking. These forward - looking statements include, but are not limited to, statements relating to estimated time periods for interim data read outs for our ongoing and prospective clinical trials and value inflection points, any resubmission of the NDA for dovitinib and PMA for the drug - specific DRP ® companion diagnostic for dovitinib, and ongoing clinical trials for stenoparib and IXEMPRA ® . Any forward - looking statements in this presentation are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward - looking statements. These risks and uncertainties include, but are not limited to, the risk that that the Company is unable to raise sufficient capital to fund its ongoing and prospective clinical trials and operations, the risk that results of a clinical study do not necessarily predict final results and that one or more of the clinical outcomes may materially change following more comprehensive reviews of the data, and as more patient data become available, the risk that results of a clinical study are subject to interpretation and additional analyses may be needed and/or may contradict such results, the receipt of regulatory approval for dovitinib, the drug - specific DRP ® companion diagnostic for dovitinib, or any of our other therapeutic candidates or, if approved, the successful commercialization of such products, the risk of cessation or delay of any of the ongoing or planned clinical trials and/or our development of our product candidates, the risk that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our therapeutic candidates, and the risk that the current COVID - 19 pandemic will impact the Company’s current and future clinical trials and the timing of the Company’s preclinical studies and other operations. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward - looking statements, see the section entitled “Risk Factors” in our annual report on Form 10 - K on file with the Securities and Exchange Commission, available at the Securities and Exchange Commission’s website at www . sec . gov , and as well as discussions of potential risks, uncertainties and other important factors in the Company’s subsequent filings with the Securities and Exchange Commission . All information in this presentation is as of the date of the presentation , and the Company undertakes no duty to update this information unless required by law . Any financial projections in this presentation are forward - looking statements that are based on assumptions that are inherently subject to significant uncertainties and contingencies, many of which are beyond Allarity’s control. While all projections are necessarily speculative, Allarity believes that the preparation of prospective financial information involves increasingly higher levels of uncertainty the further out the projection extends from the date of preparation. The assumptions and estimates underlying the projected results are inherently uncertain and are subject to a wide variety of significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those contained in the projections. The inclusion of projections in this communication should not be regarded as an indication that Allarity or their representatives, considered or consider the projections to be a reliable prediction of future events.

Revitalizing Former Big Pharma Therapeutics with Our DRP ® CDx Companion Diagnostics Low Average Patient Benefit High Average Patient Benefit Classical Drug Development Treat all of the patients Allarity Approach Treat only patients sensitive to therapy RIGHTS INDICATION PHASE 3 PHASE 2 PHASE 1/1b Global 2 rd + line Ovarian Cancer PARP & tankyrase inhibitor Stenoparib Global Solid Tumors PARP & tankyrase inhibitor + pan - tyrosine kinase Inhibitor Stenoparib + dovitinib Out - licensed to Allarity in Europe (US Approved, rights held by R - Pharm U.S.) 2 nd + line Metastatic Breast Cancer Microtubule inhibitor Ixempra ® Lead Program Secondary Program Each program is advancing with a drug - specific DRP ® companion diagnostic (CDx) to select and treat patients most likely to benefit from treatment. Allarity Therapeutics 3

Stenoparib: Lead Pipeline Program Phase 2 Monotherapy in Ovarian Cancer

5 0 5 10 15 20 25 2023 2028 $Bn • First - in - class small molecule targeted inhibitor of DNA damage repair enzymes (PARP) with dual Tankyrase inhibitor activity: • Tankyrases are involved in telomerase maintenance during tumor cell division and are active in the Wnt signaling pathway in tumor cells. • Dual inhibition of PARP & Tankyrase potentially yields improved anti - tumor activity , including in tumors that develop PARPi resistance . • Stenoparib has shown promising monotherapy activity against OC and pancreatic cancer in prior Phase 1 clinical trial. • Stenoparib - DRP ® companion diagnostic showed ability to identify patients that benefited in Phase I study. • Global rights, exclusively in - licensed from Eisai . GMP drug manufacturing contract in place with LONZA. • PARPi’s approved for use in ovarian, breast, prostate, pancreatic, fallopian tube and peritoneal cancers. Allarity Therapeutics 1. Global PARP Inhibitor Market Size, Share, Trends, COVID - 19 Impact & Growth Analysis Report – Segmented By Drug type, Application, Distribution Channel and Region (North America, Europe, APAC, Latin America, Middle East and Africa) – Industry Forecast (2023 to 2028); Data comes from Market Data Forecast Stenoparib: A Unique Dual PARP and Tankyrase Inhibitor Annual global PARP inhibitor sales 1 32.6% CAGR Ongoing Phase 2 Monotherapy Study in 2 nd Line Ovarian Cancer (OC) & Ongoing Phase 1b Combo Study with Dovitinib in Solid Tumors

6 Allarity Therapeutics Corporate Overview Stenoparib: Potentially Best - in - Class in the PARPi Landscape “… the identification of reliable biomarkers will be critical for the success of this targeted agent” 1 Strong maintenance opportunity BBB penetration PARP trapping Multi - targeted (PARP/ Tankyrase) Absence of Myelotoxicity (Myelotox) Export Resistance (PgP mediated) Response biomarker Stage Owner Product ض ض ض ض ض ض DRP ® Phase 2 Stenoparib ض ض ض غ غ غ Platinum response Approved Niraparib ض غ ض غ غ غ BRCA Approved Olaparib ض غ ض ض غ غ Platinum response Approved Rucaparib ض غ ض غ غ ض BRCA Approved Talazoparib ض – ض غ غ – BRCA Phase 3 Senaparib غ غ غ غ غ غ BRCA Phase 3 Veliparib ض ض ض غ غ ض BRCA Phase 2/3 Pamiparib Lars M. Wagner, “Profile of veliparib and its potential in the treatment of solid tumors.” Onco Targets Ther. 2015; 8: 1931 – 1939. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4524591 NCT03878849 Allarity PARP inhibitor (Stenoparib) with exclusive DRP®: a novel, multi - targeted drug (Tankyrase and PARP inhibition) with potential best - in - class efficacy and safety profiles, aimed at improving outcomes in hard - to - treat patient populations.

Withdrawals of Indications for Approved PARPi’s Create Opportunity • Manufacturers of the following PARP inhibitors have voluntarily withdrawn indications for heavily pretreated patients with BRCA - mutated advanced ovarian cancer due to safety concerns: • As a result of clinical trials evaluating niraparib as fourth - line or later therapy for BRCA - mutated advanced ovarian cancer patients. GSK/Tesaro has withdrawn this indication from its label due to a potential detrimental effect on overall survival. • AstraZeneca and Merck have voluntarily withdrawn accelerated approval for olaparib for adults with BRCA - mutated advanced ovarian cancer who have received at least 3 prior lines of chemotherapy following a safety analysis of the SOLO3 trial which suggested a detrimental effect on survival in certain subgroups. • Clovis pulled the indication for rucaparib for BRCA - mutated ovarian cancer after at least 2 prior chemotherapies in the United States and Europe, after the European Medicines Agency recommended restricting the use of rucaparib based on differences in overall survival compared to chemotherapy. Recent, partial withdrawals of indications for niraparib, olaparib, and rucaparib THE OPPORTUNITY Allarity Therapeutics 7 - Withdrawals highlight need for more effective PARP inhibitors. - Withdrawn indications create a market opening for Stenoparib targeting heavily pretreated ovarian cancer patients.

Plummer et al, ASCO 2018, Plummer et al, Br J Cancer 123(4):525 - 533, 2020 Stenoparib - DRP ® CDx Identifies Responsive Patients Response rate below DRP ® cutoff of 50: 0% (0 of 7) Response rate above DRP ® cutoff of 50: 33% (2 of 6) Response rate without DRP: 15% (2/13) All 8 histologies Stenoparib DRP ® CDx (414 mRNAs) Identifies Responsive Patients in the Eisai Phase 1 trial Results of Phase I Trial Allarity Therapeutics 8

Stenoparib: Phase 2 Monotherapy Study in 2 nd Line OC • Ongoing Phase 2 monotherapy study in advanced ovarian cancer (OC). • Patients with predicted high likelihood of responding to stenoparib using DRP ® CDx included in the study . • Open label, multicenter, single arm study evaluating stenoparib as single agent therapy in a 28 - days cycle in advanced OC patients. • Recruiting trial sites in both the U.S. and U.K; . expansion of trial sites in 2022 to accelerate enrollment. • Protocol amendment to twice daily (BID) dosing (200 mg, 400 mg) in effect from end of Q1 2023. • ClinicalTrials.gov ID: NCT03878849 Currently enrolling patients in US and UK; Interim Data Readout Anticipated in Late Q4 2023 THE OPPORTUNITY Allarity Therapeutics 9 - PARP inhibitors have gained market share in numerous indications - Resistance creates opportunities for additional lines of treatment - Tankyrase inhibition is a potent add - on to PARP inhibition - Stenoparib - DRP ® CDx identifies a broader pool of likely responder patients beyond BRCA status .

Stenoparib: Lead Pipeline Program Phase 1b Combination Study with Dovitinib in Solid Tumors

• Pan - Tyrosine Kinase Inhibitor (TKI), small molecule, targeted inhibitor of FGFR, VEGFR, and other RTKs . Unique, inhibitory profile. • Clinical activity demonstrated in monotherapy Phase 3 Renal Cell Carcinoma (mRCC) trial 2 , and in Phase 2 studies for gastrointestinal stromal tumors (GIST) 3 , liver (HCC) 4 , breast 5 , and endometrial cancers 6 • Potential tumor vascular normalization that can potentiate delivery and benefit of other anti - cancer agents. • Dovitinib - DRP ® CDx evaluated in 5 different cancers including mRCC, GIST, liver, metastatic breast, and endometrial. • Global rights, exclusively in - licensed from Novartis . GMP drug manufacturing contract in place with LONZA. • Pan - TKI’s approved for use in a number of solid tumors including HCC, RCC, thyroid cancer, GIST, and pNET. 1) Source: reported sales (2019A) and eValuatePharma (2026E) 2. NCT01223027 3. NCT01478373 4. NCT01232296 5. NCT01528345 and NCT00958971 6. NCT01379534 Dovitinib: A Unique Pan - Tyrosine Kinase Inhibitor (TKI) 0 10 20 30 40 50 60 US$ Bn 70 2019A Breast cancer Liver cancer Endometrial cancer 2026E Renal Cell Carcinoma GIST Annual sales 1 in 5 indications where Dovitinib has shown clinical activity Allarity Therapeutics 11 Ongoing Phase 1b Combo Study with Stenoparib in Solid Tumors. Dovitinib Has Shown Therapeutic Equivalence to Sorafenib in Prior Phase 3 mRCC Study

mechanisms: 1. Liu JF et al (2022). Olaparib With or Without Cediranib Versus Platinum - Based Chemotherapy in Recurrent Platinum - Sensitive Ovarian Cancer (NRG - GY004): A Randomized, Open - Label, Phase III Trial. J Clin Oncol. 2022 2. Mirza MR et al (2019) Niraparib plus bevacizumab versus niraparib alone for platinum - sensitive recurrent ovarian cancer (NSGO - AVANOVA2/ENGOT - ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019 Oct;20 Rationale for Stenoparib + Dovitinib Combination in Ovarian Cancer Potential Synergistic Benefit of Dovitinib + Stenoparib in 2 nd line or later HR Proficient Ovarian Cancer; Phase 1b Dosing Study Initiated in Q1 2023 • PARP inhibitors have traditionally been targeted at homologous repair deficient (HRD) tumors, including those carrying mutations in BRCA1/2. This is called synthetic lethality. • Homologous repair proficient (HRP) tumors have been excluded from PARPi treatment because of lower response rates - - yet constitute about 70% of ovarian cancer (OC ). • Dovitinib can turn an HRP tumor into an HRD tumor via two documented • VEGF inhibition is anti - angiogenic leading to increased hypoxia and down - regulation of HR. PDGFR inhibition also inhibits HR. 1 • Addition of a VEGF/PDGFR inhibitor (cediranib or bevacizumab) to a PARP inhibitor (olaparib or niraparib) increased ORR as well as PFS in BRCA wild type OC patients 1,2 • Dovitinib + Stenoparib can potentially offer unprecedented benefit in OC patients with HRP tumors for whom limited targeted agents are currently available. Allarity Therapeutics 12

Stenoparib: Phase 1b Combo Study with Dovitinib in Solid Tumors Ongoing Phase 1b Combination Study: Potential Synergistic Benefit of Dovitinib + Stenoparib in Solid Tumors; Data Readout Anticipated in Q2 2024 Allarity Therapeutics 13 • Ongoing Phase 1b combination therapy study in advanced solid tumors. • Enriched enrollment for endometrial, pancreatic, breast, colon, and prostate cancers as likely responsive indications. • Prior clinical trials have shown promising synergy between PARPi and TKI’s . • Stenoparib - DRP ® and Dovitinib - DRP ® CDx’s will be evaluated for ability to select likely responder patients. • Open label, multicenter to determine the Maximum Tolerated Dose (MTD) of stenoparib BID monotherapy and the MTD of dovitinib in combination with stenoparib. • Current escalated dosing of Stenoparib = BID (400 mg, 400 mg); 28 day cycle. • Starting dosing of Dovitinib = 500 mg QD (prior MTD established by Novartis); 5 days on, 2 days until progression. • ClinicalTrials.gov ID: NCT05571969 THE OPPORTUNITY - New treatments for Homologous Recombination Proficient (HRP) tumors remains an unmet need in ovarian cancer - Dovitinib + Stenoparib has potential broad benefit in numerous solid tumors. - Strong potential for this drug combination in 2 nd line endometrial cancer .

1. NCT01223027; 49 out of 135 patients that consented to give biopsy that passed QC were DRP ® positive (36%). If samples are macrodissected to remove necrosis the median increases to 24.2 mo in the Dovitinib - DRP positive arm. Dovitinib - DRP ® CDx Identifies Dovitinib - Responsive Patients Dovitinib - DRP ® CDx (58 mRNAs) Identified Patients with Overall Survival (OS) Benefit in Novartis Phase 3 in 3 rd line RCC 1 DRP selects survivors in dovitinib arm but not in sorafenib arm • Dovitinib - DRP ® positive: • median 15.0 mo . OS (95%CI 12.9 - 26.3) • Dovitinib - DRP ® negative: • median 9.1 mo . OS (95% CI 7.6 - 13,2) • Dovitinib - DRP ® CDx does not select Sorafenib responders. Allarity Therapeutics 14

Pediatric Clinical Development Collaboration with OncoHeroes Phase 1 Trial Start for Dovitinib in Pediatric Osteosarcoma anticipated Q3 2024 Allarity Therapeutics 15

16 Allarity Therapeutics Corporate Overview H2 2021

Dovitinib: Potential Combo Study with Immune Checkpoint Inhibitor Strong Pre - clinical Evidence and Clinical Rationale for Combination of Dovitinib + a PD - 1 Inhibitor. Potential Phase 1b/2 Combination Study in Solid Tumor(s) in H1 2024. • Treatment setting = front - line to 3 rd line treatment in solid tumors where PD - 1i’s are approved. • Pembrolizumab (KEYTRUDA ® ), for example, is currently standard - of - care in 14+ indications , both in mono - and combination therapy, from front - line to 3 rd line treatment. • Dovitinib tumor vasculature effects may potentiate tumor susceptibility to checkpoint inhibitors. • Pembrolizumab peak sales projections in adjuvant RCC alone = ~$1.2 billion. 1 • Mouse models (unpublished) have shown combo Rx benefit of Dovitinib and anti - PD - 1 . • Pembrolizumab has shown clinical synergy with other TKIs like lenvatinib (LENVIMA ® ). 2 • Dovitinib - DRP ® CDx can be used to select those patients most likely to benefit from the combination of Dovitinib and a PD - 1 inhibitor across any/all indications. • DRP ® on label as mandatory companion Dx allows for drug pricing premium . 1. SVB Leerink analyst Daina Graybosch (FiercePharma) 2. NCT02811861 and NCT03517449 Allarity Therapeutics 17

45 40 35 30 25 20 15 10 5 0 2023 2028 $Billion • There are over 400,000 new cases of Endometrial cancer (EC) annually. Incidence and mortality are increasing. • Immune checkpoint inhibitors have moved to front - line setting in EC, leaving only chemotherapy ( e.g., paclitaxel and carboplatin) for second line, which has low clinical benefit (56% overall survival at 24 months 1 ). • Dovitinib has previously shown Phase 2 mono - therapy benefit in EC . • European and U.S. guidelines now recommend testing EC for tumor DNA damage repair status for risk classification and potentially to help guide treatment . 2,3 1 Mirza, Mansoor et al (2023) Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer N Engl J Med 2023; 388:2145 - 2158 2 Oaknin A, Bosse TJ, Creutzberg CL et al; Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow - up. Ann Oncol. 2022 Sep;33(9):860 - 877. 3 Abu - Rustum NR, Yashar CM, Bradley K, et al. NCCN Guidelines. insights: uterine neoplasms, version 3.2021. J Natl Compr Canc Netw 2021;19:888 – 95. 4 https://www.researchandmarkets.com/report/endometrial - cancer - drug Stenoparib: Potential Combo Study with Dovitinib in Endometrial Cancer Endometrial cancer global sales growth estimate 4 4.9 % CAGR KOL’s Positive on Potential Synergistic Therapeutic Benefit of Dovitinib + Stenoparib in 2 nd Line Advanced Endometrial Cancer Allarity Therapeutics 18

IXEMPRA ® Program Phase 2 Study in Metastatic Breast Cancer

• I XEMPRA ® is a small molecule targeted inhibitor of microtubules ; interferes with tumor cell division. • Approved & marketed in U.S. for the treatment of mBC : • In combination with capecitabine for the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline and a taxane . • As monotherapy for the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline, a taxane, and capecitabine . • Added to 2023 NCCN Guidelines for treatment of platinum - resistant ovarian cancer (in combination with bevacizumab). • European rights, in - licensed from R - PHARM U.S. Originally developed and marketed by Bristol Myers Squibb . • R - PHARM has first buy - back option following Phase 2 completion at FMV. IXEMPRA ® (Ixabepilone): An Approved Microtubule Inhibitor Ongoing European Phase 2 Monotherapy Study in 2 nd Line Metastatic Breast Cancer (mBC) 450 400 350 300 250 200 150 100 50 0 2029 2036 $Million Allarity Therapeutics 20 Estimated European IXEMPRA ® + DRP ® CDx Sales 35% CAGR

Phase 2 Monotherapy Study in Metastatic Breast Cancer • Ongoing Phase 2 monotherapy study in metastatic breast cancer (mBC) • Patients with predicted high likelihood of responding using the IXEMPRA ® - DRP ® CDx included in the study. • Study is open label, multicenter, single arm study. Evaluates IXEMPRA ® as single agent therapy in a 3 - week cycle in advanced mBC patients after failure of an anthracycline and taxanes. • Numerous trial sites in both the EU and UK. • Trial protocol amendment in Q1 2023 to include more patients for dosing, by change of the IXEMPRA ® - DRP ® CDx inclusion cut - off score. • ClinicalTrials.gov ID: NCT04796324 • New enrollment is slowed until after additional capital is raised to support program. Interim Data Readout Anticipated in Late Q1 2024 THE OPPORTUNITY - Opening the European market to IXEMPRA for the 1 st time. - Potential to reinvigorate U . S . drug sales if DRP ® CDx goes on the label . Allarity Therapeutics 21 - Potential to expand drug label to include endometrial cancer.

IXEMPRA ® Arm 1) Perez et al (2007) in mBC patients resistant to anthracycline, taxane, and capecitabine 2) Thomas et al (2007) in mBC patients resistant to taxanes IXEMPRA ® - DRP ® CDx Identifies Responsive Patients Ixempra ® DRP ® selected patients ORR 20% (CBR* 41% 0% 20% 40% 60% Resistant to anthracycline, taxane and capecitabine Resistant to taxane With previous adjuvant anthracycline therapy No previous taxane therapy Estimated ORR in DRP+ patients DRP ® unselected patients ORR 11.5 1 - 12% 2 N=138, p=0.0004 * CBR refers to Clinical Benefit Rate (CR+PR+SD>6months) IXEMPRA ® - DRP ® CDx (190 mRNAs) Predicts Response to IXEMPRA ® Based upon Published Biopsy Data from a Trial of IXEMPRA ® (Ixabepilone) in Neoadjuvant BC Allarity Therapeutics 22

IXEMPRA ® Early Clinical Results (Phase 2) Potential to Substantially Improve Drug Benefit/Risk Ratio to Support Registration in Europe 36 mBC patients screened with DRP ® CDx (under prior higher cut - off score) and 5 treated with IXEMPRA ® : Observed in first 4 evaluable patients *: • Patient #1 partial responder: tumor shrinkage of 66% . • Patient #2 partial responder: tumor shrinkage of 59% . • Patient #3 has: 24 weeks of stable disease . • Patient #4 has: 19 weeks of stable disease . Additional DRP ® - positive patients have been dosed and are awaiting evaluation. Interim data readout anticipated in late Q1 2024. * Early data is not indicative of final results and investors should not rely on early data in making an investment decision. Source: Allarity Therapeutics Provides Updates for IXEMPRA and Stenoparib Phase 2 Monotherapy Clinical Studies [Press release]. https://www.globenewswire.com/news - release/2023/03/28/2635468/0/en/Allarity - Therapeutics - Provides - Updates - for - IXEMPRA - and - Stenop arib - Phase - 2 - Monotherapy - Clinical - Studies.htm l Allarity Therapeutics 23

Our DRP ® Companion Diagnostics Platform

DRP ® is Superior to Conventional Biomarker Technology Conventional Biomarker Approaches Largely Fail to Address Complex Tumor Biology and Mechanisms of Drug Response/Resistance Gene Mutation Sequencing Few drugs can be predicted with a single or a few mutations Drug Target Analysis Focus on drug with single target Artificial Intelligence or Machine Learning Approaches Lacks clinical validation Allarity’s DRP ® develops predictive biomarkers based upon complex gene expression analysis Broadly Applicable Extensively Published First - In - Class Retrospectively & Prospectively Validated Regulatory Acceptable Trusted By Clinicians * DRP® is based on comprehensive, tumor cell transcriptome data from actual cancer patients. * DRP® is not based on data mining, AI, or database analysis. Allarity Therapeutics 25

The DRP ® P latform Addresses the Complexity of Cancer Cancer is Very Complex “Systems biology” is used to analyze all genes (~25,000) expressed in a cancer cell/tumor, without bias towards current knowledge of relevant drug targets or pathways. The Tumor Tells us What is Important Input is generated by taking drug testing data from cancer cell lines. Our DRP ® engine then applies the system biology analysis as a “filter” of human tumor biopsy data, to yield a 50 to 400 gene DRP ® signature for that specific drug. Graph of all 680 non - redundant proteins Allarity Therapeutics 26

Compare patients’ tumor gene expression to DRP ® signature PATIENTS’ TUMORS DRP ® CDx: Predicting a Cancer Patient’s Drug Response STRONG WEAK Patients’ biopsy samples PATIENTS’ TUMORS Step 1 Step 2 Step 3 WEAK MODERATE STRONG Identify patients with high DRP ® score for a given drug DRP + Allarity Therapeutics 27

DRP ® Platform: Extensively Proven in 47 Clinical Trials Cisplatin/LiPlaCis® Stenoparib IXEMPRA® (+ dozens of other validations*) PROSPECTIVE CLINICAL TRIALS – PHASE 2 DRP® Clinical Impact 2 - 5 fold increase in ORR or TTP between predicted sensitive and predicted resistant RETROSPECTIVE (BLINDED) CLINICAL TRIALS – PHASE 2/3 Allarity Therapeutics 28 2 - 5 fold increase in ORR or TTP between predicted sensitive and predicted resistant Lung Lung – NSCLC Ovarian Renal RCC – metastatic AML Breast Breast – Metastatic Dovitinib Fulvestrant Belinostat 5 - FU Solid Tumors Breast – Neoadjuvant Colon Epirubicin Exemestane Phase 2 study (n=37) completed – late - stage metastatic BC Phase 2 study (n=30) underway – 3 rd line ovarian cancer Phase 2 study (n=60) underway – 2 nd line metastatic BC

ϭ͘ �ŚĞŶ � :͕ � Ğƚ � Ăů͘ � � � ϳϭ Ͳ ŐĞŶĞ � ƐŝŐŶĂƚƵƌĞ � ŽĨ � dZ�/> � ƐĞŶƐŝƚŝǀŝƚLJ � ŝŶ � ĐĂŶĐĞƌ � ĐĞůůƐ ͘ � ;KĐƚŽďĞƌ � Ϯϱ ͕ � ϮϬϭϭ Ϳ͖ � DŽů � �ĂŶĐĞƌ � dŚĞƌ ͕ � ϭϬ͘ϭϭϱϴ ͬ ϭϱϯϱ Ͳ ϳϭϲϯ ͘ Ϯ͘ tĂŶŐ�t͕ � Ğƚ � Ăů͘ � /ŶĚĞƉĞŶĚĞŶƚ � ǀĂůŝĚĂƚŝŽŶ � ŽĨ � Ă � ŵŽĚĞů � ƵƐŝŶŐ�ĐĞůů � ůŝŶĞ � ĐŚĞŵŽƐĞŶƐŝƚŝǀŝƚLJ � ƚŽ � ƉƌĞĚŝĐƚ�ƌĞƐƉŽŶƐĞ � ƚŽ � ƚŚĞƌĂƉLJ ͘ � : � EĂƚů � �ĂŶĐĞƌ � /ŶƐƚ͘� ϮϬϭϯ � ^ĞƉ� ϰ ͖ ϭϬϱ ; ϭϳ Ϳ͗ ϭϮϴϰ Ͳ ϵϭ ϯ͘ <ŶƵĚƐĞŶ � ^͕�Ğƚ � Ăů͘ � �ĞǀĞůŽƉŵĞŶƚ � ĂŶĚ � ǀĂůŝĚĂƚŝŽŶ � ŽĨ�Ă � ŐĞŶĞ � ĞdžƉƌĞƐƐŝŽŶ � ƐĐŽƌĞ � ƚŚĂƚ � ƉƌĞĚŝĐƚƐ � ƌĞƐƉŽŶƐĞ � ƚŽ � ĨƵůǀĞƐƚƌĂŶƚ � ŝŶ � ďƌĞĂƐƚ � ĐĂŶĐĞƌ � ƉĂƚŝĞŶƚƐ ͘ � W>Ž^ � KŶĞ ͘ � ϮϬϭϰ � &Ğď � ϱ ͖ ϵ ; Ϯ Ϳ͗Ğ ϴϳϰϭϱ ϰ͘ <ŶƵĚƐĞŶ � ^͕ � Ğƚ � Ăů͘ � �ĞǀĞůŽƉŵĞŶƚ � ĂŶĚ � ďůŝŶĚ � ĐůŝŶŝĐĂů � ǀĂůŝĚĂƚŝŽŶ � ŽĨ � Ă � ŵŝĐƌŽZE� � ďĂƐĞĚ � ƉƌĞĚŝĐƚŽƌ � ŽĨ � ƌĞƐƉŽŶƐĞ � ƚŽ � ƚƌĞĂƚŵĞŶƚ � ǁŝƚŚ � Z Ͳ �,K;�ͿW � ŝŶ � �>��> ͘ � W>Ž^ � KŶĞ ͘ � ϮϬϭϱ � &Ğď � ϭϴ ͖ ϭϬ ; Ϯ Ϳ͗Ğ Ϭϭϭϱϱϯϴ ϱ͘ �ƵŚů � /<͕ � Ğƚ � Ăů͘ � �Ğůů � >ŝŶĞ � �ĞƌŝǀĞĚ � ϱ Ͳ &h � ĂŶĚ � /ƌŝŶŽƚĞĐĂŶ � �ƌƵŐ Ͳ ^ĞŶƐŝƚŝǀŝƚLJ � WƌŽĨŝůĞƐ � �ǀĂůƵĂƚĞĚ � ŝŶ � �ĚũƵǀĂŶƚ � �ŽůŽŶ � �ĂŶĐĞƌ � dƌŝĂů � �ĂƚĂ ͘ � W>Ž^ � KŶĞ ͘ � ϮϬϭϲ ͖ � ϭϭ ; ϱ Ϳ͗ � Ğ ϬϭϱϱϭϮϯ ͘ ϲ͘ tŝŶƚŚĞƌ � D͕ � Ğƚ�Ăů͘ � �ůŝŶŝĐĂů � /ŵƉĂĐƚ � ŽĨ � Ă � EŽǀĞů � DŝĐƌŽZE� � �ŚĞŵŽ Ͳ ^ĞŶƐŝƚŝǀŝƚLJ � WƌĞĚŝĐƚŽƌ � ŝŶ � 'ĂƐƚƌŽŽĞƐŽƉŚĂŐĞĂů� �ĂŶĐĞƌ ͘ � W>Ž^ � KŶĞ ͘ � ϮϬϭϲ ͖ � ϭϭ ; Ϯ Ϳ͗ � Ğ ϬϭϰϴϬϳϬ ͘ ϳ͘ Z ƺ ĐŬĞƌ � &'͕ � Ğƚ � Ăů͘ � DŽůĞĐƵůĂƌ � ĚŝƐƐĞĐƚŝŽŶ � ŽĨ � ǀĂůƉƌŽŝĐ � ĂĐŝĚ � ĞĨĨĞĐƚƐ � ŝŶ � ĂĐƵƚĞ � ŵLJĞůŽŝĚ � ůĞƵŬĞŵŝĂ � ŝĚĞŶƚŝĨŝĞƐ � ƉƌĞĚŝĐƚŝǀĞ � ŶĞƚǁŽƌŬƐ ͘ � �ƉŝŐĞŶĞƚŝĐƐ ͘ � ϮϬϭϲ � :Ƶů � Ϯ ͖ ϭϭ ; ϳ Ϳ͗ ϱϭϳ Ͳ Ϯϱ ͘ ϴ͘ WƌĂŚŵ � <W͕ � Ğƚ � Ăů͘ � �ůŝŶŝĐĂů � ǀĂůŝĚĂƚŝŽŶ � ŽĨ�ĐŚĞŵŽƚŚĞƌĂƉLJ � ƉƌĞĚŝĐƚŽƌƐ � ĚĞǀĞůŽƉĞĚ � ŽŶ � ŐůŽďĂů � ŵŝĐƌŽZE� � ĞdžƉƌĞƐƐŝŽŶ� ŝŶ�ƚŚĞ � E�/ ϲϬ � ĐĞůů � ůŝŶĞ � ƉĂŶĞů � ƚĞƐƚĞĚ � ŝŶ � ŽǀĂƌŝĂŶ � ĐĂŶĐĞƌ ͘ � W>Ž^ � KE� � ϭϮ ; ϯ Ϳ͗ � Ğ ϬϭϳϰϯϬϬ ͘ ϵ͘ �ŽŚů � ^Z͕ � Ğƚ � Ăů͘ � 'ĞŶĞ � ĞdžƉƌĞƐƐŝŽŶ� ĂŶĂůLJƐŝƐ � ŽĨ�ĚĞĐŝƚĂďŝŶĞ � ƚƌĞĂƚĞĚ � �D>͗ � ŚŝŐŚ � ŝŵƉĂĐƚ � ŽĨ � ƚƵŵŽƌ � ƐƵƉƉƌĞƐƐŽƌ�ŐĞŶĞ � ĞdžƉƌĞƐƐŝŽŶ�ĐŚĂŶŐĞƐ ͘ � >ĞƵŬĞŵŝĂ � Θ � >LJŵƉŚŽŵĂ � sŽů͘ � ϱϴ � ͕ � /ƐƐ͘ � ϵ ͕ � ϮϮϲϰ Ͳ ϮϮϲϳ � ; ϮϬϭϳ Ϳ͘ ϭϬ͘ sĂŶŐƐƚĞĚ � �:͕ � Ğƚ � Ăů͘ � �ƌƵŐ � ƌĞƐƉŽŶƐĞ � ƉƌĞĚŝĐƚŝŽŶ � ŝŶ � ŚŝŐŚ Ͳ ƌŝƐŬ � ŵƵůƚŝƉůĞ � ŵLJĞůŽŵĂ͘ � 'ĞŶĞ � ϲϰϰ � ϴϬ Ͳ ϴϲ � ; ϮϬϭϴ Ϳ͘ ϭϭ͘ �ƵŚů � /<͕ � Ğƚ � Ăů͘ � DŽůĞĐƵůĂƌ � ƉƌĞĚŝĐƚŝŽŶ � ŽĨ � ĂĚũƵǀĂŶƚ � ĐŝƐƉůĂƚŝŶ � ĞĨĨŝĐĂĐLJ � ŝŶ � EŽŶ Ͳ ^ŵĂůů � �Ğůů � >ƵŶŐ � �ĂŶĐĞƌ � ;E^�>�Ϳ Ͷ sĂůŝĚĂƚŝŽŶ � ŝŶ�ƚǁŽ � ŝŶĚĞƉĞŶĚĞŶƚ � ĐŽŚŽƌƚƐ ͘ � W>Ž^ � KE� � ϭϯ ; ϯ Ϳ͗ � Ğ ϬϭϵϰϲϬϵ � ; ϮϬϭϴ Ϳ͘ ϭϮ͘ �ƵŚů � �^<͕ � Ğƚ � Ăů͘ � WƌĞĚŝĐƚŝŶŐ � ĞĨĨŝĐĂĐLJ � ŽĨ � ĞƉŝƌƵďŝĐŝŶ � ďLJ � Ă � ŵƵůƚŝŐĞŶĞ � ĂƐƐĂLJ�ŝŶ � ĂĚǀĂŶĐĞĚ � ďƌĞĂƐƚ � ĐĂŶĐĞƌ � ǁŝƚŚŝŶ � Ă � �ĂŶŝƐŚ � �ƌĞĂƐƚ � �ĂŶĐĞƌ � �ŽŽƉĞƌĂƚŝǀĞ � 'ƌŽƵƉ � ;���'Ϳ � ĐŽŚŽƌƚ͗ � Ă � ƌĞƚƌŽƐƉĞĐƚŝǀĞ Ͳ ƉƌŽƐƉĞĐƚŝǀĞ � ďůŝŶĚĞĚ � ƐƚƵĚLJ ͘ � �ƌĞĂƐƚ� �ĂŶĐĞƌ � ZĞƐ � dƌĞĂƚ ͘ � ϮϬϭϴ � �ƵŐ � ϭϭ ͘ ϭϯ͘ �ŚƌŝƐƚĞŶƐĞŶ � d�͕ � Ğƚ � Ăů͘ � WƌĞĚŝĐƚŝŽŶ � ŽĨ � ĨƵůǀĞƐƚƌĂŶƚ � ĞĨĨŝĐĂĐLJ � ŝŶ�ƉĂƚŝĞŶƚƐ � ǁŝƚŚ � ĂĚǀĂŶĐĞĚ � ďƌĞĂƐƚ � ĐĂŶĐĞƌ͗ � ƌĞƚƌŽƐƉĞĐƚŝǀĞ Ͳ ƉƌŽƐƉĞĐƚŝǀĞ � ĞǀĂůƵĂƚŝŽŶ � ŽĨ�ƚŚĞ � ƉƌĞĚŝĐƚŝǀĞ � ƉŽƚĞŶƚŝĂů � ŽĨ � Ă � ŵƵůƚŝŐĞŶĞ � ĞdžƉƌĞƐƐŝŽŶ � ĂƐƐĂLJ ͘ � �ƌĞĂƐƚ � �ĂŶĐĞƌ ͘� ϮϬϭϵ � KĐƚ � Ϯϱ ͘�ĚŽŝ͗ � ϭϬ͘ϭϬϬϳ ͬƐ ϭϮϮϴϮ Ͳ Ϭϭϵ Ͳ ϬϭϬϭϳ Ͳ ϳ � ; ϮϬϭϵ Ϳ͘ ϭϰ͘ WůƵŵŵĞƌ � Z͕ � Ğƚ � Ăů͘ � &ŝƌƐƚ Ͳ ŝŶ Ͳ ŚƵŵĂŶ � ƐƚƵĚLJ � ŽĨ�ƚŚĞ � W�ZWͬƚĂŶŬLJƌĂƐĞ � ŝŶŚŝďŝƚŽƌ � � ϳϰϰϵ � ŝŶ � ƉĂƚŝĞŶƚƐ � ǁŝƚŚ � ĂĚǀĂŶĐĞĚ � ƐŽůŝĚ � ƚƵŵŽƵƌƐ � ĂŶĚ � ĞǀĂůƵĂƚŝŽŶ � ŽĨ � Ă � ŶŽǀĞů � ĚƌƵŐ Ͳ ƌĞƐƉŽŶƐĞ � ƉƌĞĚŝĐƚŽƌ ͘ � �ƌ�:��ĂŶĐĞƌ � ; ϮϬϮϬ Ϳ � ϭϮϯ ; ϰ Ϳ͗ ϱϮϱ Ͳ ϱϯϯ ͘� ŚƚƚƉƐ͗ͬͬĚŽŝ͘ŽƌŐͬ ϭϬ͘ϭϬϯϴ ͬƐ ϰϭϰϭϲ Ͳ ϬϮϬ Ͳ Ϭϵϭϲ Ͳ ϱ ϭϱ͘ <ŶƵĚƐĞŶ � ^͕ � Ğƚ � Ăů͘ � � � ŶŽǀĞů � ĚƌƵŐ � ƐƉĞĐŝĨŝĐ � ŵZE� � ďŝŽŵĂƌŬĞƌ � ƉƌĞĚŝĐƚŽƌ � ĨŽƌ � ƐĞůĞĐƚŝŽŶ � ŽĨ � ƉĂƚŝĞŶƚƐ � ƌĞƐƉŽŶĚŝŶŐ � ƚŽ � ĚŽǀŝƚŝŶŝď � ƚƌĞĂƚŵĞŶƚ � ŽĨ�ĂĚǀĂŶĐĞĚ � ƌĞŶĂů � ĐĞůů � ĐĂƌĐŝŶŽŵĂ � ĂŶĚ � ŽƚŚĞƌ � ƐŽůŝĚ � ƚƵŵŽƌƐ ͘ � W>K^ � KE� ͘ � ϭϴ ; ϴ Ϳ͗ � Ğ ϬϮϵϬϲϴϭ� ŚƚƚƉƐ͗ͬͬĚŽŝ͘ŽƌŐͬ ϭϬ͘ϭϯϳϭ ͬũŽƵƌŶĂů͘ƉŽŶĞ͘ ϬϮϵϬϲϴϭ � ; ϮϬϮϯ Ϳ Allarity Therapeutics 29 Publications of Clinical/Method Validation for Drug - Specific DRP ® ’s

DRP ® CDx can Support External Drug Development Programs • Broad potential to partner with other oncology therapeutics developers (including Big Pharma) to develop drug - specific DRP ® companion Dx for their pipeline programs. • DRP ® companion Dx can be developed and used to support : • Phase 1/1b clinical trials, to identify responsive patients and generate early drug efficacy signals . • Phase 2 and 3 clinical trials, to enrich trials with identified likely responder patients . • Regulatory approval filings and post - approval use as a mandatory/label companion diagnostic. • DRP ® companion Dx can potentially open new markets ( e . g . EU) for approved drugs that failed to meet cost/benefit requirements in another country/region . • DRP ® Platform may potentially be acquired by a third party, similar to Roche’s 2018 ( $ 2 . 4 B) acquisition of Foundation Medicine Allarity Therapeutics 30

‡ '53 Š � &'[ � IRU � � � )8 � RXW � OLFHQVHG � WR � )LYHS+XVLRQ � ‡ &OLQLFDO � GHYHORSPHQW � SDUWQHUVKLS � WR � VXSSRUW � SDWLHQW � VHOHFWLRQ � IRU � 'HIOH[LIRO Œ � LQ� XQUHVHFWDEOH � PHWDVWDWLF � FRORUHFWDO � FDQFHU� � $OODULW � HOLJLEOH � WR � UHFHLYH � UHJXODWRU � PLOHVWRQH�SDPHQWV� ‡ $GGLWLRQDO � SURVSHFWLYH � YDOLGDWLRQ � RI � '53 Š � &'[� ‡ /L3OD&LV Š � RXW � OLFHQVHG � WR � &KRVD � 2QFRORJ � ‡ )RU � IXUWKHU � FOLQLFDO � � FRPPHUFLDO � GHYHORSPHQW�LQ � FRQQHFWLRQ � ZLWK � FLVSODWLQ � '53 Š � &'[� $OODULW � HOLJLEOH � WR � UHFHLYH � UHJXODWRU � PLOHVWRQH � SDPHQWV� ‡ $GGLWLRQDO � SURVSHFWLYH � YDOLGDWLRQ � RI � '53 Š � &'[� Allarity Therapeutics 31 Monetizing Deprioritized Assets Through External Partnerships Potential For Downstream Revenue from Deprioritized Assets

Our Leadership & SAB
Thomas Jensen SVP of Investor Relations, Co - Founder Our Senior Leadership Team 0DULH � )RHJK� � 0' &KLHI � 0HGLFDO � 2IILFHU Steen Knudsen, Ph.D. Chief Scientific Officer, Co - Founder James G. Cullem, J.D. Chief Executive Officer Joan Y. Brown, CPA Chief Financial Officer Allarity Therapeutics 33

Mansoor Raza Mirza, MD Chief Oncologist at the Dept. of Oncology, Rigshospitalet, Cph. University Hospital, Denmark, and Medical Director of the Nordic Society of Gynecologic Oncology - Clinical Trial Unit. He is also Vice - Chairman of the Danish Society of Gynecologic Oncology. Our Scientific Advisory Board 8UVXOD � $� � 0DWXORQLV� � 0' &KLHI � DQG � 'LUHFWRU � RI � WKH � 'LYLVLRQ � RI� *QHFRORJLF � 2QFRORJ � DW � ')&,� � 3URIHVVRU � RI � 0HGLFLQH � DW � +DUYDUG � 0HGLFDO� 6FKRRO� � )LUVW � UHFLSLHQW � RI � %URFN � :LOVRQ� )DPLO � &KDLU � DW � ')&,� Roberto Pili, MD Roberto Pili, MD, is Associate Dean for Cancer Research & Integrative Oncology and Professor and Chief of the Division of Hematology/Oncology in the Department of Medicine at University of Buffalo. He is also the founder of the UB Cancer Research Consortium. Dan von Hoff, JD Physician in Chief, Distinguished Professor at the Translational Genomics Research Institute (TGen) in Phoenix, Arizona. Virginia G. Piper Distinguished Chair for Innovative Cancer Research at HonorHealth. Chief Scientific Officer for US Oncology Research Joyce A. O’Shaughnessy, MD Co - Chair of Breast Cancer Research and Chair of Breast Cancer Prevention Research at Baylor - Sammons Cancer Center and for The US Oncology Network. Allarity Therapeutics 34

Our Board of Directors Jerry McLaughlin Chairman CEO of Life Biosciences, Inc. Prior CEO and Board Member of Neos Therapeutics as well as previous CEO, President and Board Member of AgeneBio, Inc. -RVHSK � 9D]]DQR 1RQ � ([HFXWLYH � 'LUHFWRU &)2 � RI�$EHRQD � 7KHUDSHXWLFV� � ,QF�� 3UHYLRXV � &)2 � DW � $YHQXH� 7KHUDSHXWLFV�� ,QF� � DQG � $VVLVWDQW� &RUSRUDWH � &RQWUROOHU � DW � ,QWHUFHSW� 3KDUPDFHXWLFDOV� � ,QF� � &3$ � LQ � 1-� Thomas Jensen SVP, Executive Director SVP, Investor Relations of Allarity Therapeutics. Prior roles at Allarity include SVP, Information Technology and CTO in both Allarity Therapeutics, and its predecessor. Laura Benjamin Non - Executive Director CEO of OncXerna Therapeutics. Former VP in Oncology at Eli Lilly, leading cancer discovery. Expertise in biomarker R&D. Ph.D. in Molecular Biology. James G. Cullem CEO, Executive Director CEO of Allarity Therapeutics. (interim). Prior roles include CBO, and SVP of Corporate Development in Allarity Therapeutics Inc., and its predecessor. Allarity Therapeutics 35

I.P. & Strategic Milestones

Broad Intellectual Property Coverage Allarity Therapeutics 37 DRP ® CDx Formulations Methods of Use &RPSRVLWLRQ � RI� 0DWWHU ض ض ض ض Stenoparib ض ض ض Dovitinib ض IXEMPRA ® ض ض ض ض Novel Combinations • In addition to patent coverage, regulatory market exclusivity is available for these programs by having a DRP ® CDx on the drug label: FDA (NCE exclusivity = 5 years); EMA (Drug data exclusivity = 8 years). • Allarity has patents on 71 drug - specific DRP ® CDx with 2 further DRP ® CDx patent applications pending.

Pediatric Osteosarcoma: Mono - therapy (with OncoHeroes ) Combo Rx Study #2: TBD with immune checkpoint inhibitor (subject to additional funding) Pipeline Development 2023 - 2025 Metastatic Breast Cancer: Mono - therapy Dovitinib Ixempra Phase 2 (N=100) P ha s I n e t e r 1 i m b D a ( t a N = 30) Phase 1b Readout Phase 2 Interim Data Readout (N=12) Extend (N=25) Readout Allarity Therapeutics 3388 2025 2024 2023 2022 Indication H2 H1 H2 H1 ata H2 Interim D H1 H2 H1 t Interim Data Readou N=25) Extend ( ta R R e e a a d d o o u u t t ing (N=10) Interim Da Ovarian Cancer: Mono - therapy Stenoparib BID Dos Phase 2 Phase 2 (N=120+) Phase 1b (N=<18) Ovarian Cancer (2 nd line or later): Combo Rx study with dovitinib 1b Phase Pediatric Indication TBD Mono - therapy (with OncoHeroes)
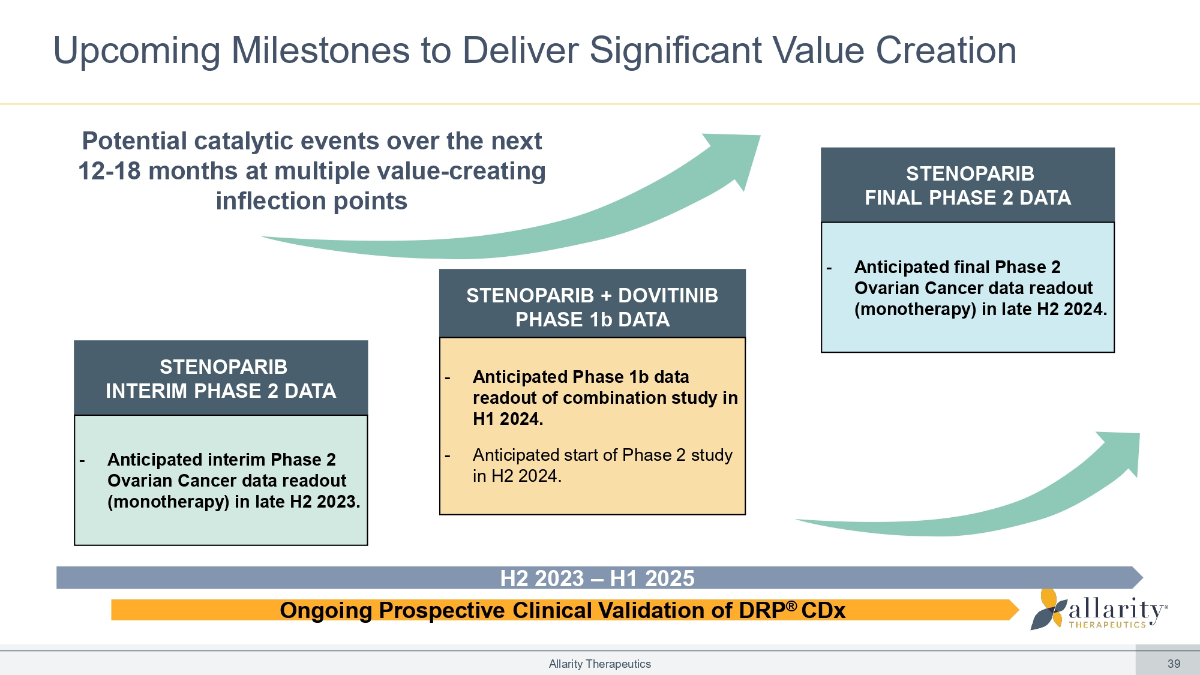
H2 2023 – H1 2025 Ongoing Prospective Clinical Validation of DRP ® CDx Upcoming Milestones to Deliver Significant Value Creation Potential catalytic events over the next 12 - 18 months at multiple value - creating inflection points STENOPARIB + DOVITINIB PHASE 1b DATA - Anticipated Phase 1b data readout of combination study in H1 2024. - Anticipated start of Phase 2 study in H2 2024. STENOPARIB INTERIM PHASE 2 DATA Allarity Therapeutics 39 - Anticipated interim Phase 2 Ovarian Cancer data readout (monotherapy) in late H2 2023. STENOPARIB FINAL PHASE 2 DATA - Anticipated final Phase 2 Ovarian Cancer data readout (monotherapy) in late H2 2024.

40 Comparable Public Equities $650M $120M $2M $44M $37M Alla rity Kura Oncology Acrivon The rapeutics Syros Pharma Lantern Pharma Phase 2 Phase 3 Phase 3 Phase 2 Phase 3 Most advanced stage 3 2 3 1 2 Number of assets in clinic 13 0 1 0 2 Peer Review Clinical validation publications Unlocking the Value of a Mid Clinical - Stage Oncology Pipeline Allarity Therapeutics * Peer group valuations based upon intraday stock prices as of October 17, 2023 U.S. Nasdaq Listed Equities

41 Allarity Therapeutics Multiple de - risked, former Big Pharma oncology assets in mid - clinical - stage pipeline with high development potential and opportunities for external partnering and/or program divestitures. Multiple value - driving inflection points within 12 - 18 months. Interim data readout for lead Phase 2 clinical program in late H2 2023. First - in - class, drug - specific companion diagnostic (CDx) platform with potential to drive acquisition value as stand - alone asset or in connection with clinical pipeline. Key Investment Highlights

James G. Cullem Chief Executive Officer +1 (978) 500 - 0863 jcullem@allarity.com allarity.com
v3.23.3
Cover
|
Nov. 13, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Nov. 13, 2023
|
| Entity File Number |
001-41160
|
| Entity Registrant Name |
ALLARITY THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001860657
|
| Entity Tax Identification Number |
87-2147982
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
24 School Street
|
| Entity Address, Address Line Two |
2nd Floor
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02108
|
| City Area Code |
401
|
| Local Phone Number |
426-4664
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
ALLR
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
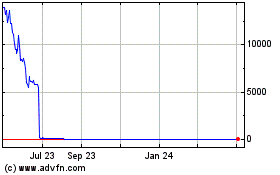
Allarity Therapeutics (NASDAQ:ALLR)
Historical Stock Chart
From Apr 2024 to May 2024
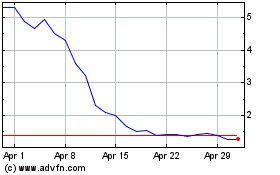
Allarity Therapeutics (NASDAQ:ALLR)
Historical Stock Chart
From May 2023 to May 2024