
Investor Presentation NASDAQ: SLP Q1 FY24 Update

With the exception of historical information, the matters discussed in this presentation are forward-looking statements that involve a number of risks and uncertainties. Words like “believe,” “expect” and “anticipate” mean that these are our best estimates as of this writing, but that there can be no assurances that expected or anticipated results or events will actually take place, so our actual future results could differ significantly from those statements. Factors that could cause or contribute to such differences include, but are not limited to: our ability to maintain our competitive advantages, acceptance of new software and improved versions of our existing software by our customers, the general economics of the pharmaceutical industry, our ability to finance growth, our ability to continue to attract and retain highly qualified technical staff, our ability to successfully integrate the recently acquired Immunetrics business with our own, as well as expenses we may incur in connection therewith, and a sustainable market. Further information on our risk factors is contained in our quarterly and annual reports and filed with the U.S. Securities and Exchange Commission. Safe Harbor Statement 2

Mission: Improve Health Through Innovative solutions To create value for customers by accelerating development timelines and reducing the cost of drug development R&D through innovative science-based software and consulting solutions that optimize treatment options and improve patient lives. 3

Leading Provider of Software and Consulting Services in the Biosimulation Market AI-powered technology solutions optimize the outcomes of drug discovery, development, research, and regulatory submissions processes. Our software-based technology both models and simulates how drugs and diseases behave in humans and in other species. 25+ YEARS OVER 25 YEARS IN BUSINESS AND CONTINUING THE COMMITMENT TO IMPROVE PUBLIC HEALTH THROUGH INNOVATIVE SOLUTIONS OUR CLIENTS TRUST OUR EXPERT CONSULTING THAT SUPPORTS DRUG RECOVERY, CLINICAL DEVELOPMENT RESEARCH AND REGULATORY SUBMISSIONS 300+ CLIENTS WE PROVIDE VALIDATED AI AND MACHINE LEARNING, MODELING AND SIMULATION SOFTWARE FOR NOVICE AND EXPERT USERS ALIKE 18+ SOFTWARE SOLUTIONS SLP At A Glance 4
Investment Highlights Industry Leader in Large and Growing Market Biosimulation Technology Leader Leveraging AI Compelling Customer Value Proposition Strong Competitive Position and Barriers to Entry Seasoned Management Team and Scientific Leadership Attractive Financial Profile +++ +++ 5

Biosimulation Overview Biosimulation is software-based technology that models and simulates how drugs and diseases behave in the human body Biosimulation combines core principles in biology, chemistry and pharmacology with proprietary mathematical algorithms to predict how biology and drugs interact with one another. Models can start in vitro (without animal or human testing) but are developed through the development cycle incorporating animal and human test results along the way. Model uses include lead optimization, dose regimens, clinical trial protocol development, clinical trial simulation, bioequivalence evaluation, toxicity assessment and many more. + + 6
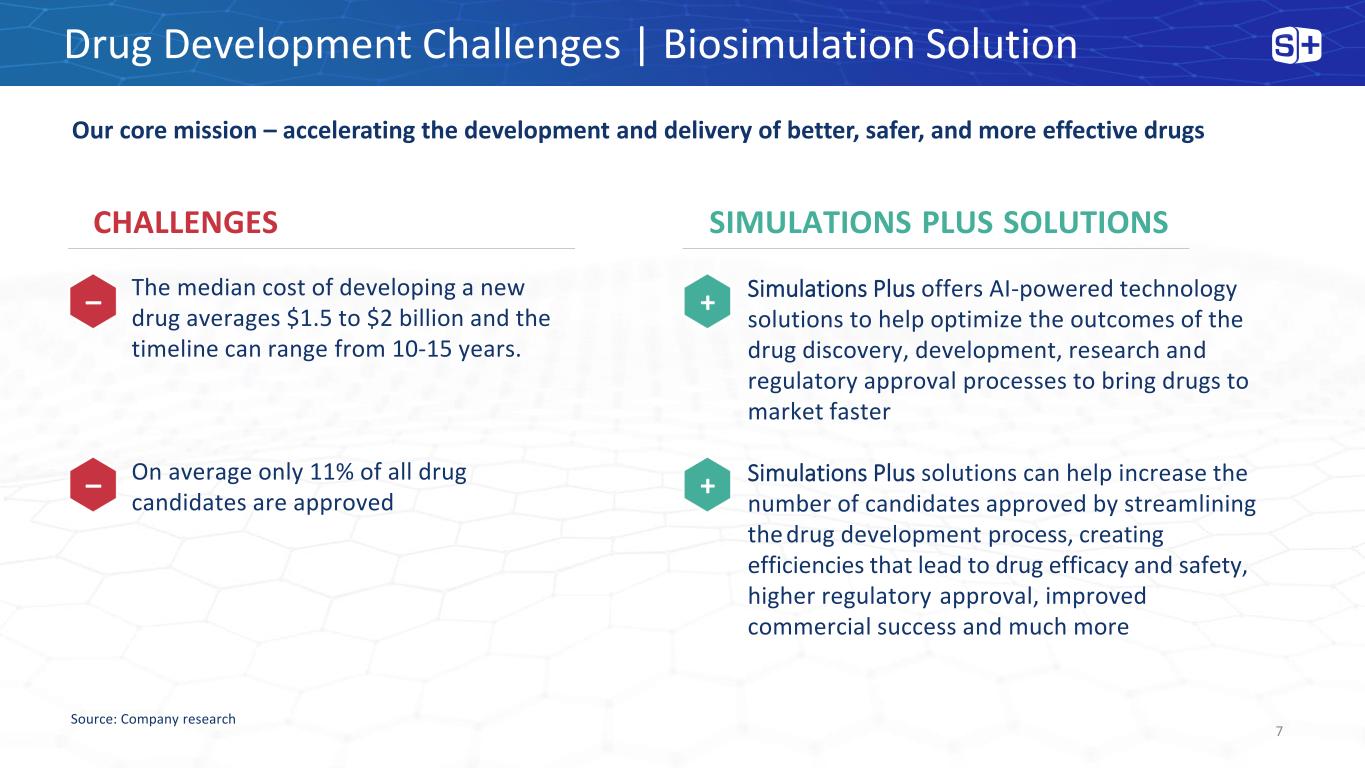
Drug Development Challenges | Biosimulation Solution Our core mission – accelerating the development and delivery of better, safer, and more effective drugs SIMULATIONS PLUS SOLUTIONS Simulations Plus offers AI-powered technology solutions to help optimize the outcomes of the drug discovery, development, research and regulatory approval processes to bring drugs to market faster Simulations Plus solutions can help increase the number of candidates approved by streamlining the drug development process, creating efficiencies that lead to drug efficacy and safety, higher regulatory approval, improved commercial success and much more CHALLENGES The median cost of developing a new drug averages $1.5 to $2 billion and the timeline can range from 10-15 years. On average only 11% of all drug candidates are approved Source: Company research – – + + 7

Leader in Large and Growing Market Spending for biosimulation products continues to increase given need to bring drugs to market faster Biosimulation market valued at $2.8B in 2022 and is expected to expand at a 16.9% CAGR from 2022 - 20301 Strategy to grow addressable market within the Biosimulation TAM through both internal R&D investment and strategic acquisitions • Biosimulation TAM growing 4-5x faster than global pharma and biotech spend • SLP is growing faster than the Biosimulation TAM Highly fragmented and underpenetrated market with only a few larger players • The global biosimulation market is segmented based on product, application, delivery model, and end users. 1 Company research, SkyQuest $200B GLOBAL PHARMA AND BIOTECH SPEND (3% CAGR)1 + + + $9.8B BIOSIMULATION1 TAM BY 2030 $700M-$800M SLP ADDRESSABLE 8

Compelling Customer Value Proposition Wide range of software solutions and consulting services Solutions offerings span the drug development process Saves time and money in drug development costs, improves likelihood of success and post approval returns Proven model improves probability and speed of clinical trial success HIGHLY EXPERIENCED SALES TEAM SUPPORTED BY SCIENTISTS AND ENGINEERS 9 • GastroPlus® • ADMET Predictor® • MonolixSuite (includes Monolix, Pkanalix, Simulix) • QSP/T Models (including DILISYM, RENASYM, IPFSYM, etc.) • Provides operational efficiencies and leads to accurate / timely decision making and regulatory reporting • Early drug discovery to preclinical • Clinical data analysis • Submission to regulatory agencies supporting product approval • Only ~7% of proposed new drug compounds pass Phase I trials • Only 53% of drugs that get to Phase III trials make it to market • 70% of drugs fail in Phase II or Phase III due to safety and efficacy issues
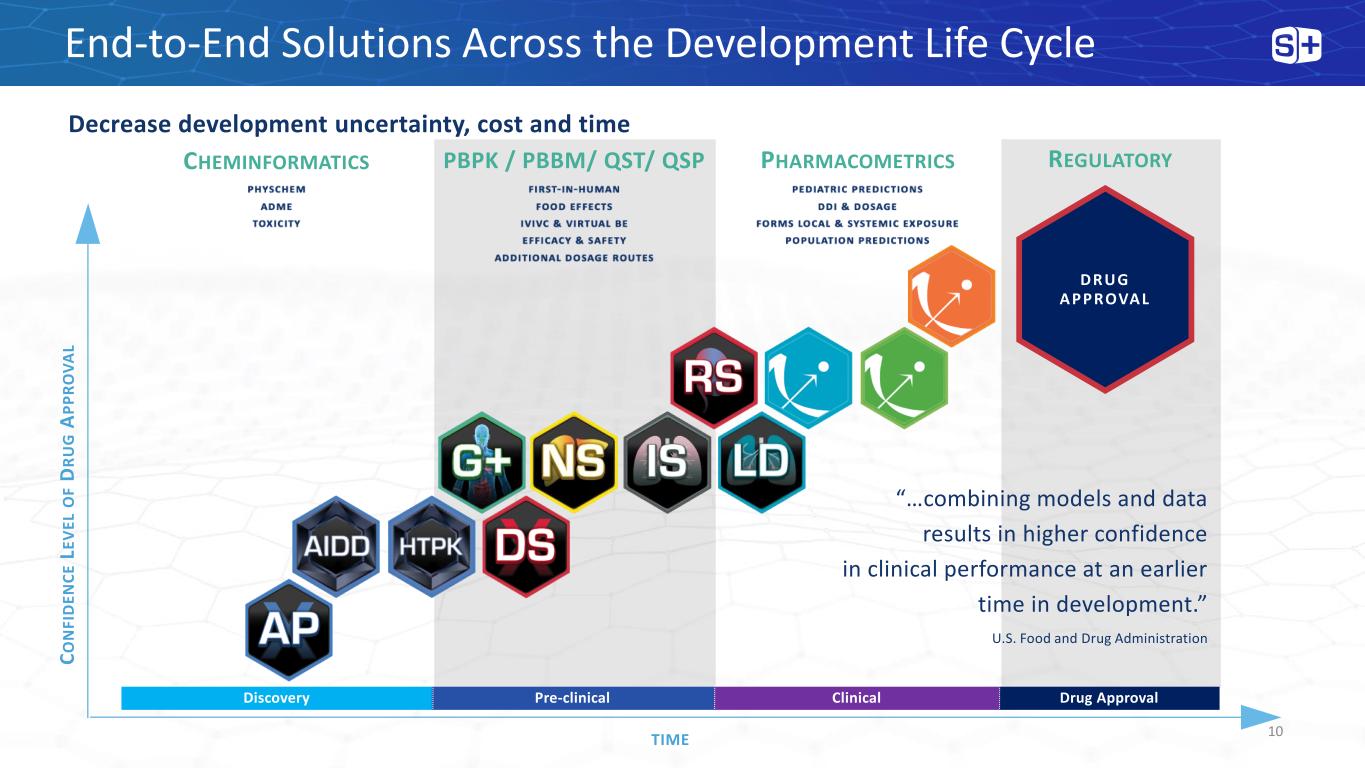
End-to-End Solutions Across the Development Life Cycle Decrease development uncertainty, cost and time C O N FI D E N C E LE V E L O F D R U G A P P R O V A L TIME DRUG A P P ROVA L Discovery Pre-clinical Clinical Drug Approval CHEMINFORMATICS PHARMACOMETRICSPBPK / PBBM/ QST/ QSP “…combining models and data results in higher confidence in clinical performance at an earlier time in development.” U.S. Food and Drug Administration REGULATORY 10

Strong Competitive Position LEADING POSITION IN LARGE, GROWING, FRAGMENTED AND UNDERPENETRATED MARKET LARGE, GROWING AND DIVERSE CUSTOMER BASE MOST COMPREHENSIVE AND WIDELY RECOGNIZED TOOLS IN THE INDUSTRY 300+ CUSTOMERS ACCUMULATED PUBLIC AND PRIVATE DATA THAT INFORMS OUR ALGORITHMS AND MODELS + + + + + 11
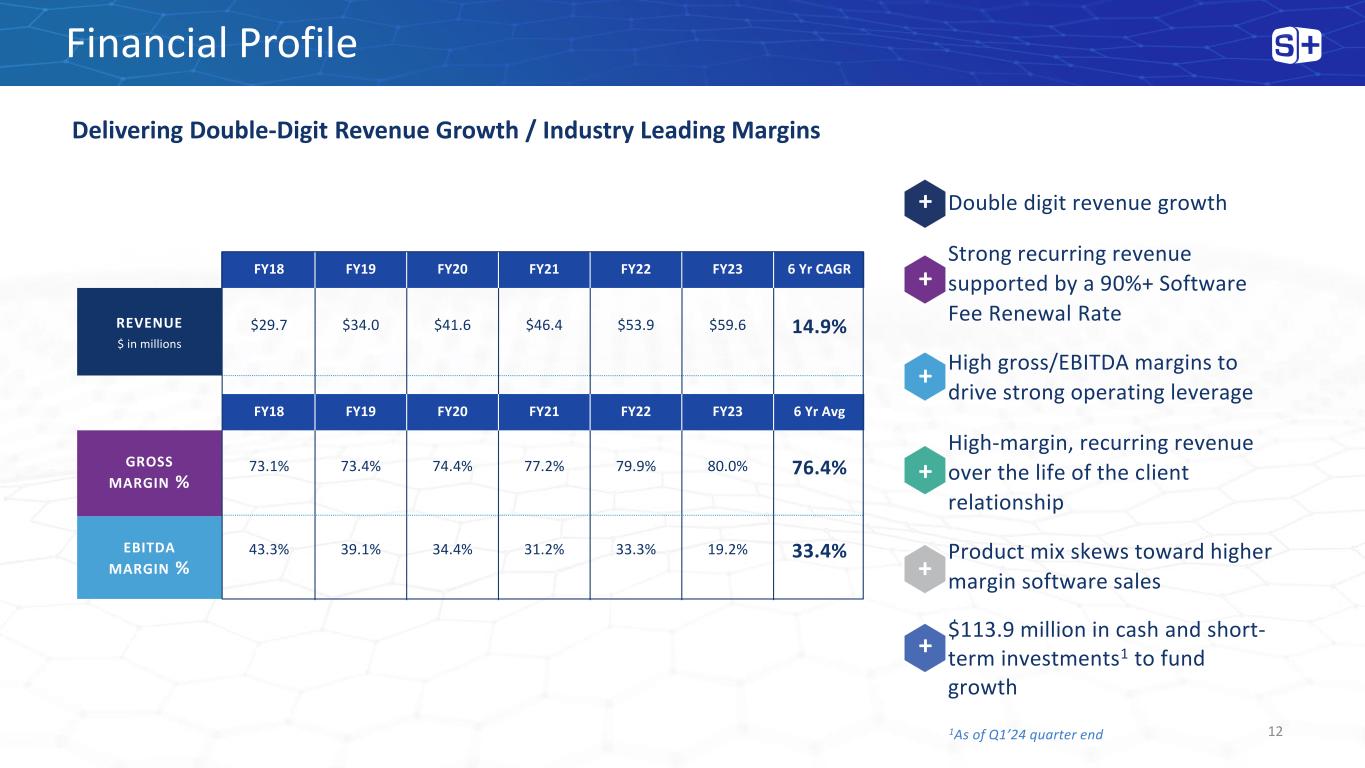
Financial Profile Delivering Double-Digit Revenue Growth / Industry Leading Margins REVENUE $ in millions $29.7 $34.0 $41.6 $46.4 $53.9 $59.6 14.9% GROSS MARGIN % EBITDA MARGIN % FY18 FY19 FY20 FY21 FY22 FY23 6 Yr CAGR FY18 FY19 FY20 FY21 FY22 FY23 6 Yr Avg 73.1% 73.4% 74.4% 77.2% 79.9% 80.0% 76.4% 43.3% 39.1% 34.4% 31.2% 33.3% 19.2% 33.4% Double digit revenue growth Strong recurring revenue supported by a 90%+ Software Fee Renewal Rate High gross/EBITDA margins to drive strong operating leverage High-margin, recurring revenue over the life of the client relationship Product mix skews toward higher margin software sales $113.9 million in cash and short- term investments1 to fund growth + + + + + + 121As of Q1’24 quarter end
▪ Quantitative Systems Pharmacology (QSP) is the discipline of building mathematical models to mechanistically stimulate the dynamics of diseases and treatments. These models incorporate public and proprietary data from in vitro, pre-clinical and clinical studies, and predict clinical outcomes across patient populations and treatment strategies ▪ Immunetrics has increased the range of therapeutic areas addressed by our QSP software and services offerings by more than 50% ▪ Ideal fit as the business leverages our existing infrastructure by expanding its therapeutic resources into largely underserved areas, including immunology and oncology ▪ Immunetrics chose Simulations Plus for its well-respected reputation ▪ Integration is going very well Integration and consolidation efficiencies M&A | Integration & Immunetrics Case Study 13
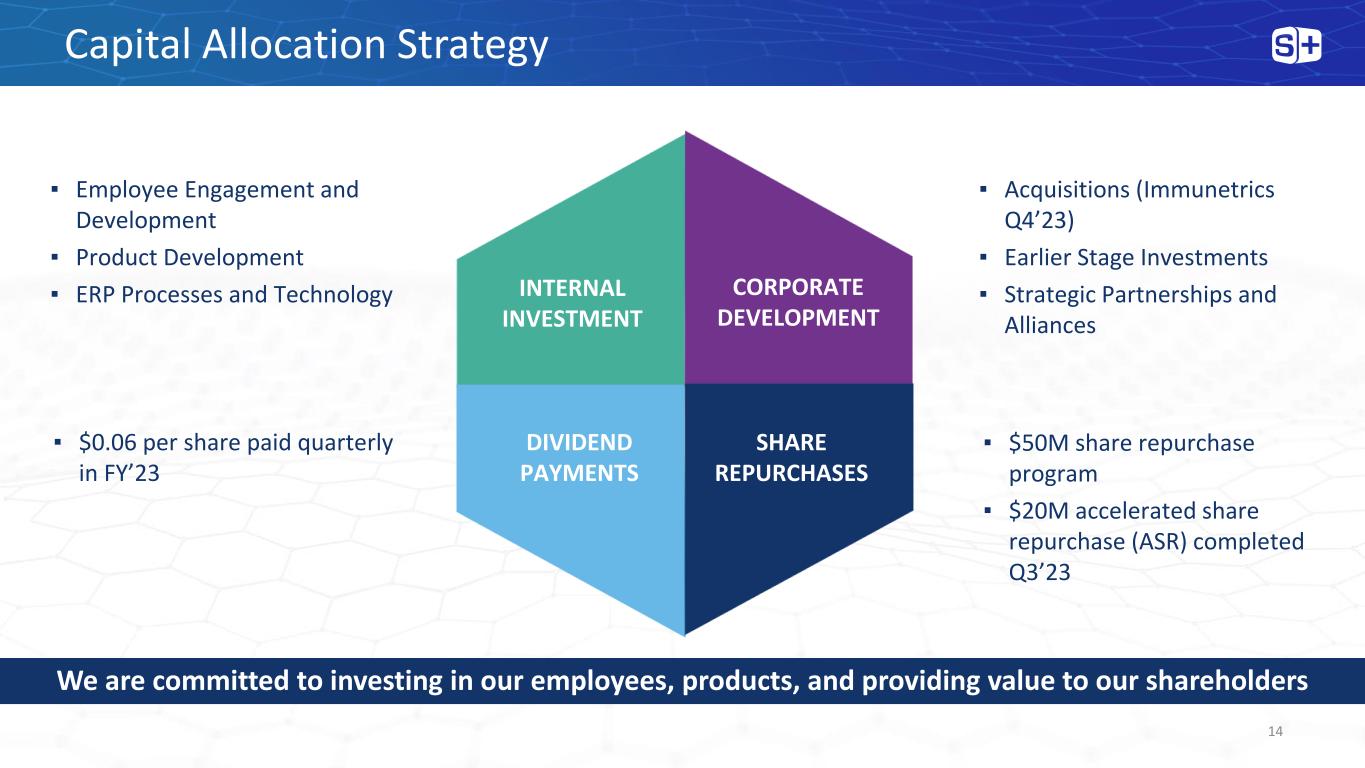
▪ Employee Engagement and Development ▪ Product Development ▪ ERP Processes and Technology ▪ Acquisitions (Immunetrics Q4’23) ▪ Earlier Stage Investments ▪ Strategic Partnerships and Alliances ▪ $0.06 per share paid quarterly in FY’23 ▪ $50M share repurchase program ▪ $20M accelerated share repurchase (ASR) completed Q3’23 INTERNAL INVESTMENT CORPORATE DEVELOPMENT DIVIDEND PAYMENTS SHARE REPURCHASES We are committed to investing in our employees, products, and providing value to our shareholders Capital Allocation Strategy 14
Corporate Development Initiative 15 Key investment objectives: ▪ Enhance Innovation and Adoption of Emerging Technologies: Explore software and services innovation and seek deeper visibility into evolving technologies, including artificial intelligence-driven drug design (AIDD) and development. ▪ Grow M&A Pipeline: Seed investments and partnerships in early-stage companies are expected to broaden the opportunity pipeline and total addressable market (TAM). ▪ Expand Revenue Opportunities: Broaden portfolio offerings through software technology and scientific service partnerships and explore new partner revenue models. ▪ Drive Shareholder Returns: Optimize the combination of organic growth, operating leverage, and strategic M&A to deliver long-term sustainable returns to our stakeholders. Strategic investments in early-stage companies to drive innovation and collaboration

Seasoned Management Team and Scientific Leadership Highly experienced management team with deep life science industry expertise, track record of growth and strong returns Shawn O’Connor Chief Executive Officer Will Frederick Chief Financial Officer Chief Operating Officer Brett Howell, Ph.D. President Quantitative Systems Pharmacology Solutions (QSP) Jill Fiedler-Kelly, M.S., FISoP President Clinical Pharmacology & Pharmacometrics Services Solutions (CPP) John DiBella, M.S President Physiologically Based Pharmacokinetic (PBPK) Solutions, Cheminformatics Solutions Jonathan Chauvin, Ph.D. President Clinical Pharmacology & Pharmacometrics Software Solutions (CPP) Dan Szot Chief Revenue Officer 16 Sandra Suarez-Sharp, Ph.D. President Regulatory Strategies
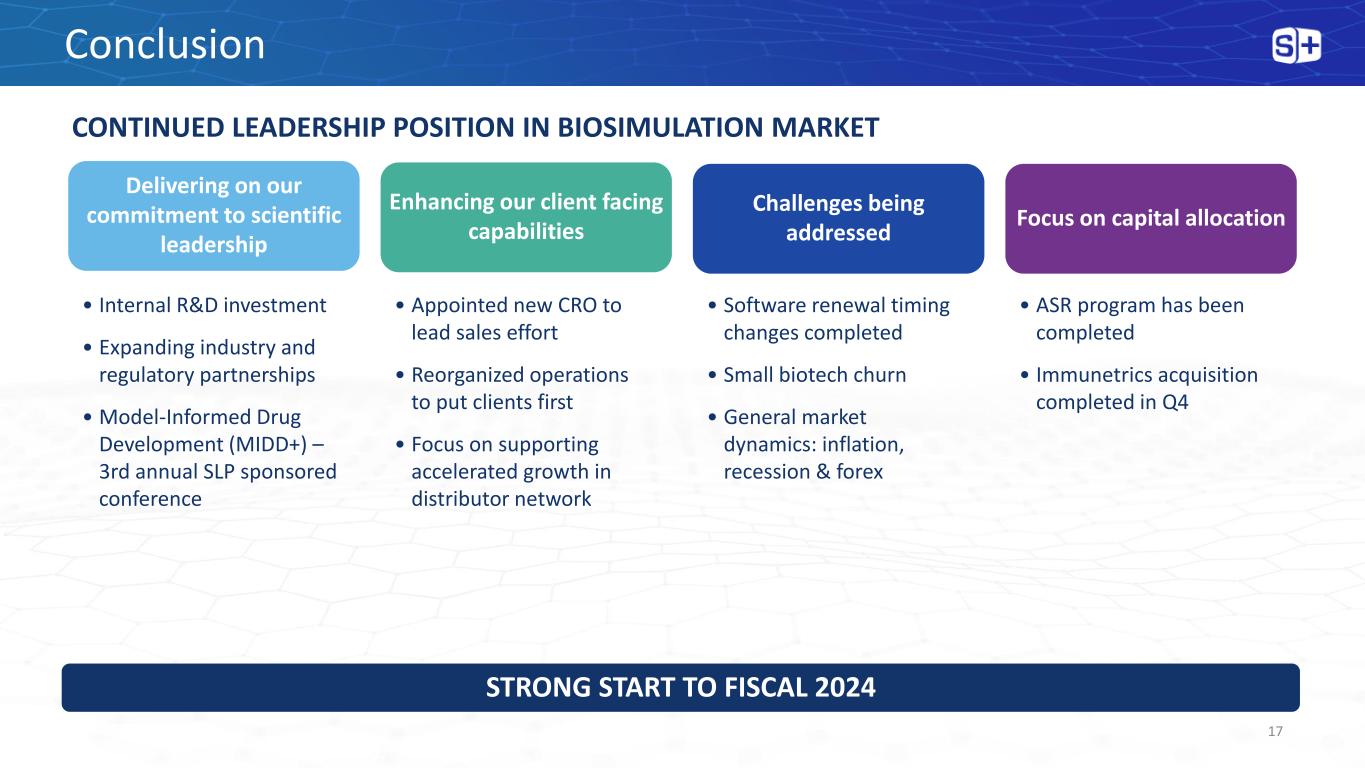
STRONG START TO FISCAL 2024 Delivering on our commitment to scientific leadership Challenges being addressed 17 • Internal R&D investment • Expanding industry and regulatory partnerships • Model-Informed Drug Development (MIDD+) – 3rd annual SLP sponsored conference Enhancing our client facing capabilities Focus on capital allocation CONTINUED LEADERSHIP POSITION IN BIOSIMULATION MARKET Conclusion • Appointed new CRO to lead sales effort • Reorganized operations to put clients first • Focus on supporting accelerated growth in distributor network • Software renewal timing changes completed • Small biotech churn • General market dynamics: inflation, recession & forex • ASR program has been completed • Immunetrics acquisition completed in Q4

Appendix

Secular Trends Driving Sustained Long-Term Growth Biosimulation taking greater percentage of drug development spend annually Scarcity of blockbuster drug opportunities drive industry focus to smaller market targets requiring increased development efficiency Increased scrutiny and rising cost of animal studies and human clinical Increased adoption of Modeling & Simulation by regulatory agencies and decision making based on Modeling & Simulation input Increasing use cases for application of Modeling & Simulation methods to impact decision making Drivers include: + + + + + 19
Drug Discovery & Development The Drug Discovery and Development Industry can be broken down into several subindustries, including: 1. TARGET DISCOVERY AND VALIDATION 2. LEAD COMPOUND IDENTIFICATION AND OPTIMIZATION 3. PRE-CLINICAL DEVELOPMENT 4. CLINICAL TRIALS 5. REGULATORY REVIEW & APPROVAL 6. COMMERCIALIZATION 7. POST-APPROVAL SURVEILLANCE AND LIFE CYCLE MANAGEMENT Each of these subindustries represents a different stage of the drug development process and involves different tasks, such as identifying targets for drug development, synthesizing and testing compounds, conducting clinical trials, and seeking regulatory approval for marketing and sales. The subindustries within drug discovery and development are highly interdependent, with progress in one stage often impacting the progress in other stages. 20
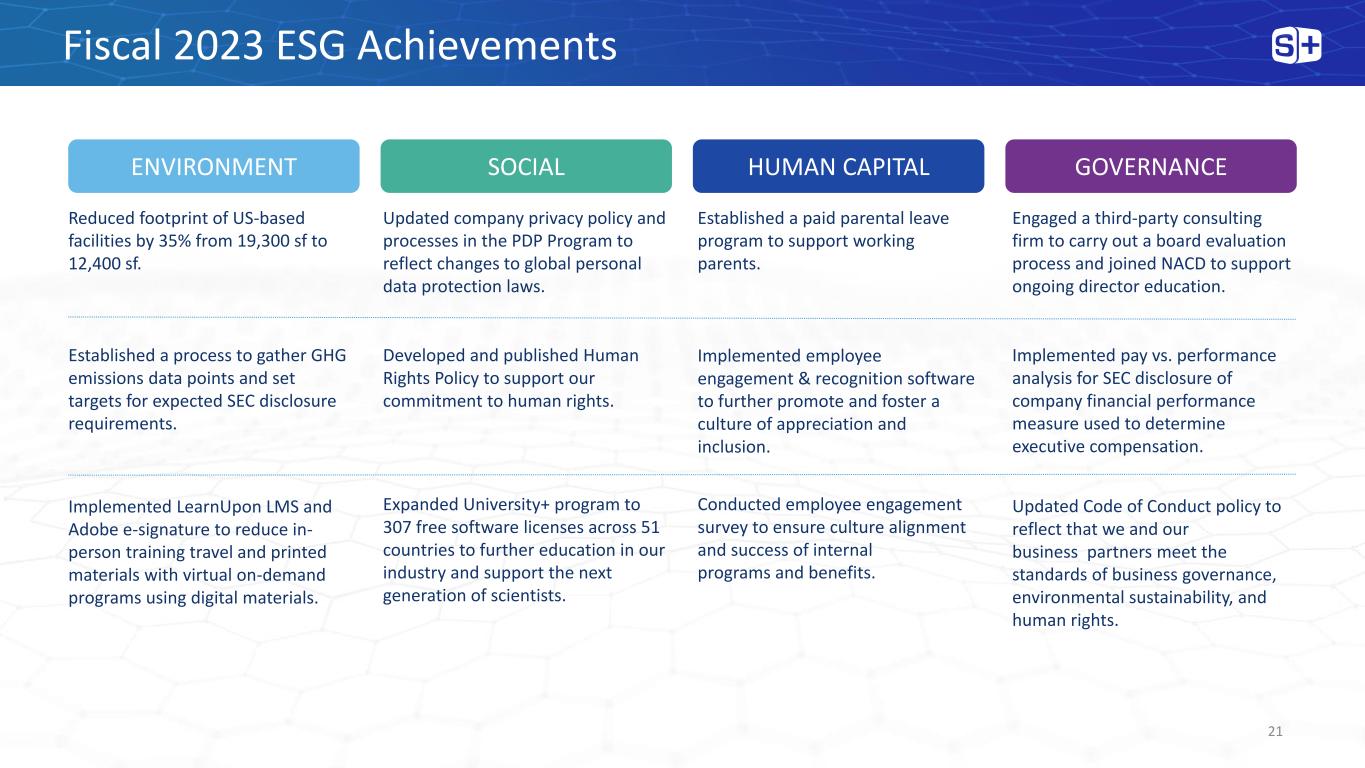
ENVIRONMENT HUMAN CAPITAL Reduced footprint of US-based facilities by 35% from 19,300 sf to 12,400 sf. Established a process to gather GHG emissions data points and set targets for expected SEC disclosure requirements. Implemented LearnUpon LMS and Adobe e-signature to reduce in- person training travel and printed materials with virtual on-demand programs using digital materials. SOCIAL GOVERNANCE Updated company privacy policy and processes in the PDP Program to reflect changes to global personal data protection laws. Developed and published Human Rights Policy to support our commitment to human rights. Expanded University+ program to 307 free software licenses across 51 countries to further education in our industry and support the next generation of scientists. Established a paid parental leave program to support working parents. Implemented employee engagement & recognition software to further promote and foster a culture of appreciation and inclusion. Conducted employee engagement survey to ensure culture alignment and success of internal programs and benefits. Engaged a third-party consulting firm to carry out a board evaluation process and joined NACD to support ongoing director education. Implemented pay vs. performance analysis for SEC disclosure of company financial performance measure used to determine executive compensation. Updated Code of Conduct policy to reflect that we and our business partners meet the standards of business governance, environmental sustainability, and human rights. Fiscal 2023 ESG Achievements 21

SLP software licensed to major pharma (19 of 20 largest) GastroPlus - mechanistically based simulation software package that simulates intravenous, oral, oral cavity, ocular, inhalation, dermal, subcutaneous, and intramuscular absorption, biopharmaceutics, pharmacokinetics, and pharmacodynamics in humans and animals. ADMET Predictor - the flagship machine learning platform for ADMET modeling with extended capabilities for data analysis, metabolism prediction, and AI-driven drug design. DILIsym is Quantitative Systems Toxicology (QST) - software designed to be used during drug development to provide an indication of the potential drug-induced liver injury (DILI) hazard posed by individual molecules and/or to provide deeper insight into the mechanisms responsible for observed DILI responses at various stages of the development process. MonolixSuite - built for model-informed drug development computing, MonolixSuite (including Monolix, Slmulix, Pkanalix) is a fast, easy-to-use, and powerful suite of applications for pharmacometrics analysis, modeling, and simulation. Intuitive, effective offering the most advanced calculation capabilities. QSP/T Models • ILDsym - model of interstitial lung disease (ILD) associated with systemic sclerosis (SSc). • RENAsym is quantitative systems toxicology (QST) software for predicting and understand drug -induced kidney injury. Software Products Overview Biosimulation Technology Leader Leveraging AI and Machine Learning + + + + + + 22

PBPK Software Solutions and AI Data LEAD SELECTION PHARMACOLOGY TREATMENT REGIMEN SAFETY CLINICAL EFFICACY ADMET DMPK SERVICES PBPK / PBBM Preclinical Regulatory Consulting 23

QSP/QST SolutionsMining LEAD SELECTION PHARMACOLOGY TREATMENT REGIMEN SAFETY CLINICAL EFFICACY ADMET DMPK SERVICES QSP Consulting QST Consulting 24

Pharmacometrics Solutions LEAD SELECTION PHARMACOLOGY TREATMENT REGIMEN SAFETY CLINICAL EFFICACY ADMET DMPK SERVICES Pharmacometrics Clinical Pharmacology Clinical Regulatory Consulting 25

Services Overview Provides customers with specialized knowledge and expertise Delivers complementary capabilities to software application solutions • Drives greater demand for its software solutions • Provides stable, steady revenue streams Helps clients minimize or eliminate questions posed by regulatory reviews while decreasing cost and time to develop detailed reports Drives operational efficiencies for customers leading to timelier and more streamlined decision-making and regulatory reporting Covers Pharmacometrics, PBPK, QSP/T and Regulatory Strategies disciplines Offers specialized therapeutic, modeling and regulatory knowledge Brings specialized knowledge and expertise as part of its consulting services, helping extend customers’ internal capabilities in the biosimulation modeling and the simulation space Average contract size of approximately ~$200k • Pharmacokinetic and Pharmacodynamic (PKPD) projects in the $100K - $300K range • Quantitative Systems Technology (QST) and Quantitative Systems Pharmacology (QSP) platform contracts can be in the $300K - $700K range • Customer contracts are sizable and provide steady revenue streams + + + + + + + + 26

Financial Results

Revenue - Trailing Twelve Months (TTM) (in millions) Software Revenue Growth1 Total Revenue Growth1 Services Revenue Growth1 +16% +21% +9% 1Q24 Revenue Mix 1Q23 Revenue Mix 1 TTM 1Q’24 vs. TTM 1Q’23 28
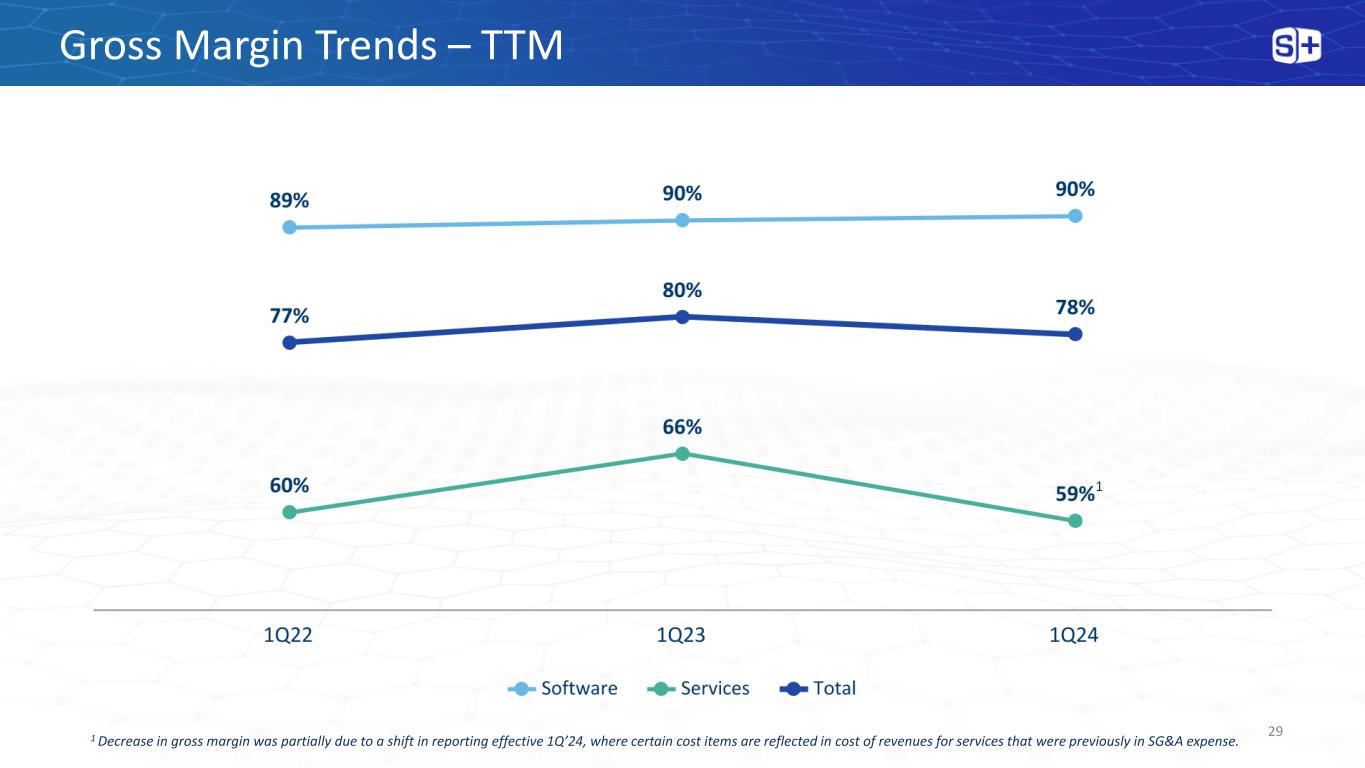
Gross Margin Trends – TTM 29 1 Decrease in gross margin was partially due to a shift in reporting effective 1Q’24, where certain cost items are reflected in cost of revenues for services that were previously in SG&A expense. 1
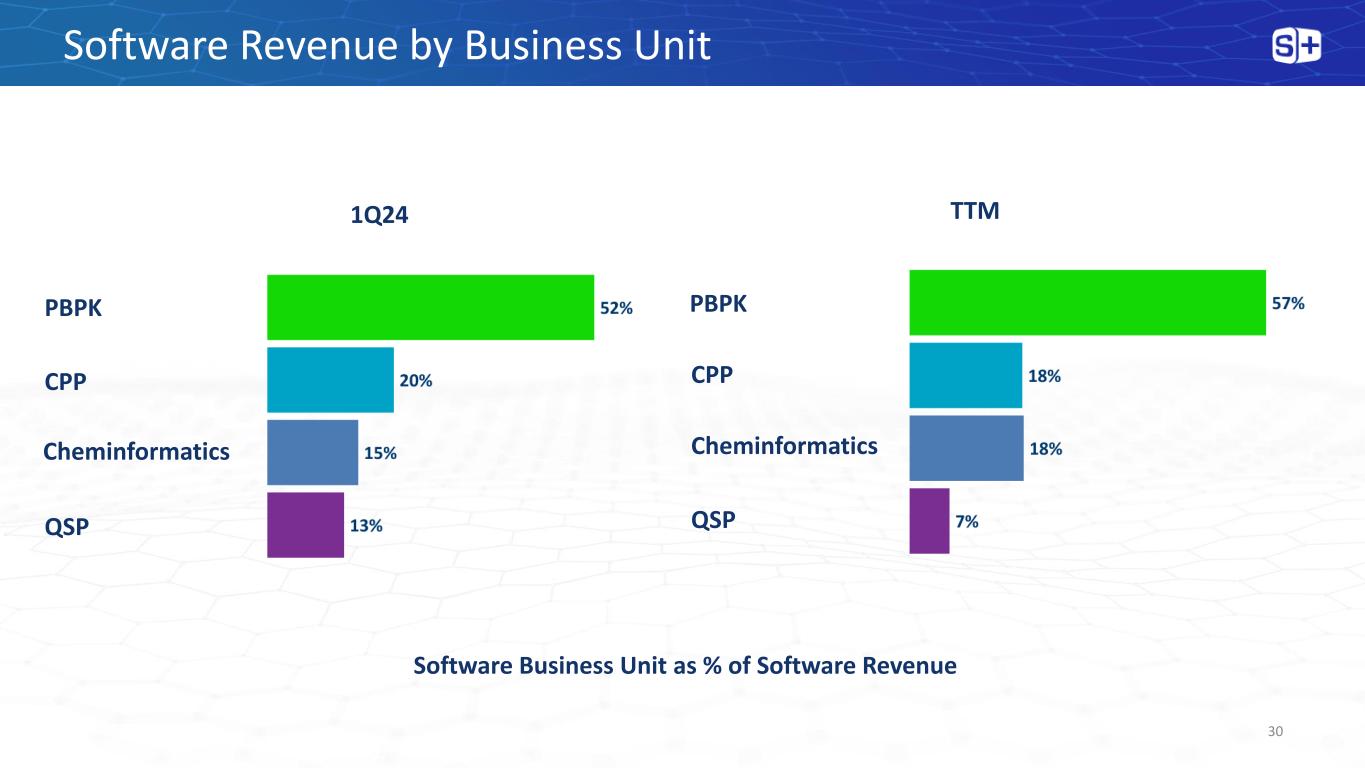
Software Revenue by Business Unit 30 Software Business Unit as % of Software Revenue 1Q24 TTM PBPK CPP Cheminformatics PBPK CPP Cheminformatics QSP QSP
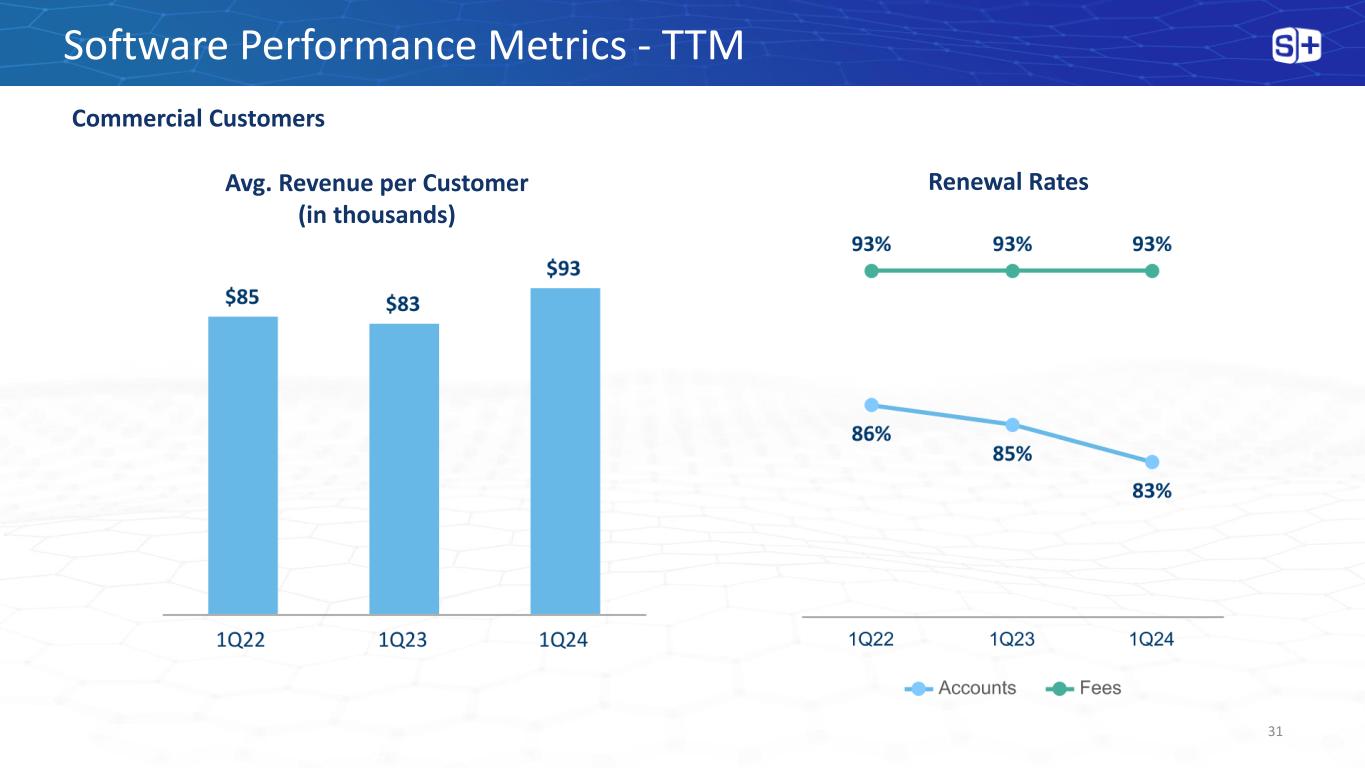
Avg. Revenue per Customer (in thousands) Software Performance Metrics - TTM Commercial Customers Renewal Rates 31

Services Revenue by Business Unit 32 Services Business Unit as % of Services Revenue Q1 FY24 TTM REG CPP QSP PBPK REG CPP QSP PBPK

Services Projects and Backlog Total Projects Backlog (in millions) 33
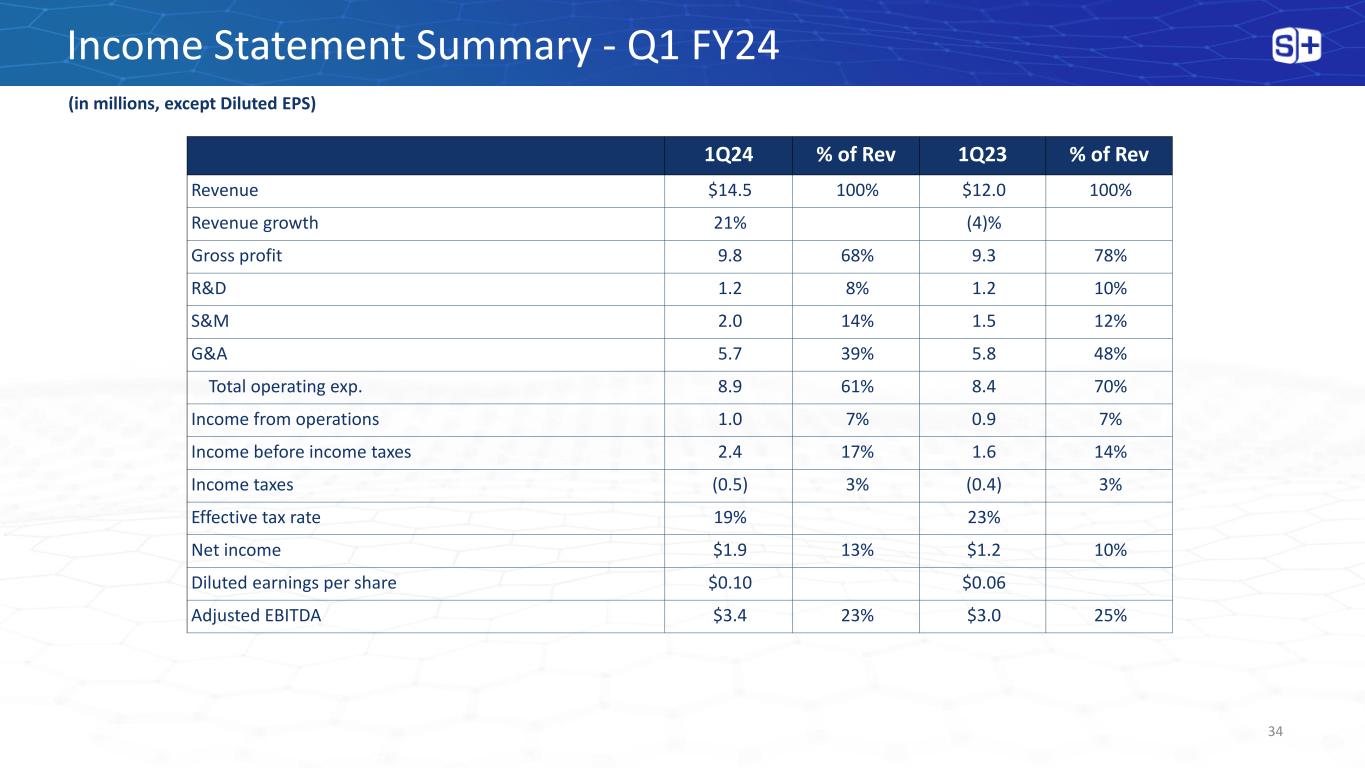
Income Statement Summary - Q1 FY24 34 (in millions, except Diluted EPS) 1Q24 % of Rev 1Q23 % of Rev Revenue $14.5 100% $12.0 100% Revenue growth 21% (4)% Gross profit 9.8 68% 9.3 78% R&D 1.2 8% 1.2 10% S&M 2.0 14% 1.5 12% G&A 5.7 39% 5.8 48% Total operating exp. 8.9 61% 8.4 70% Income from operations 1.0 7% 0.9 7% Income before income taxes 2.4 17% 1.6 14% Income taxes (0.5) 3% (0.4) 3% Effective tax rate 19% 23% Net income $1.9 13% $1.2 10% Diluted earnings per share $0.10 $0.06 Adjusted EBITDA $3.4 23% $3.0 25%

Balance Sheet Summary 35 November 30, 2023 August 31, 2023 Cash and short-term investments $113.9 $115.5 Total current assets 129.7 130.4 Total assets $185.8 $186.1 Current liabilities 8.6 12.0 Long-term liabilities 4.8 4.1 Total liabilities 13.4 16.1 Shareholders’ equity 172.3 170.0 Total liabilities and shareholders’ equity $185.8 $186.1 (in millions)

Fiscal 2024 Guidance 36 Guidance Total Revenue $66M to $69M Total Revenue Growth 10% to 15% Software Revenue Mix 55% to 60% Services Revenue Mix 40% to 45% Diluted EPS $0.66 to $0.68

Investor Relations Contacts: Lisa Fortuna Financial Profiles 310-622-8234 slp@finprofiles.com Renee Bouche Simulations Plus Investor Relations 661-723-7723 renee.bouche@simulations-plus.com